User login
Evaluation of the Effectiveness and Safety of Alirocumab Use in Statin-Intolerant Veterans
In 2016, 17.6 million deaths occurred globally due to cardiovascular disease (CVD) with coronary artery disease (CAD) and ischemic stroke as top contributors.1 Elevated low-density lipoprotein cholesterol (LDL-C) has been linked to greater risk of atherosclerotic cardiovascular disease (ASCVD); therefore, LDL-C reduction is imperative to decrease risk of cardiovascular (CV) morbidity and mortality.2 Since 1987, statin therapy has been the mainstay of treatment for hypercholesterolemia, and current practice guidelines recommend statins as first-line therapy given demonstrated reductions in LDL-C and CV mortality reduction in robust clinical trials.2-4 Although generally safe and well tolerated, muscle-related adverse events (AEs) limit optimal use of statins in up to 20% of individuals who have an indication for statin therapy.5 As a consequence, these patients receive suboptimal statin doses or no statin therapy and are at a higher risk for ASCVD.5
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been shown to significantly lower LDL-C when used as monotherapy or in combination with statins and/or other lipid-lowering therapies.5 These agents are currently approved by the US Food and Drug Administration as an adjunct to diet with or without other lipid-lowering therapies for the management of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia), homozygous familial hypercholesterolemia (evolocumab only), and for use in patients with established CVD unable to achieve their lipid-lowering goals with maximally tolerated statin doses and ezetimibe.4 With the ability to reduce LDL-C by up to 65%, PCSK9 inhibitors offer an alternative option for LDL-C and potentially CV risk reduction in statin-intolerant patients.5
Alirocumab, the formulary preferred PCSK9 inhibitor at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, has been increasingly used in high-risk statin-intolerant veterans. The primary objective of this case series was to assess LDL-C reduction associated with alirocumab use in statin-intolerant veterans at the MEDVAMC. The secondary objective was to assess the incidence of CV events. This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee.
Methods
In this single-center case series, a retrospective chart review was conducted to identify statin-intolerant veterans who were initiated on treatment with alirocumab for LDL-C and/or CV risk reduction between June 2017 and May 2019. Adult veterans with a diagnosis of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia) and/or CAD with documented statin intolerance were included in the study. Statin intolerance was defined in accordance with the National Lipid Association (NLA) definition as aninability to tolerate ≥ 2 statins with a trial of at least 1 statin at its lowest daily dose.5 Veterans who previously received treatment with evolocumab, those prescribed concurrent statin therapies, and those missing follow-up lipid panels at 24 weeks were excluded from the study. To assess LDL-C reduction, LDL-C at baseline was compared with LDL-C at 4 and 24 weeks. Incident CV events before and after alirocumab initiation were documented. The US Department of Veteran Affairs (VA) Computerized Patient Record System was used to collect patient data.
Data Collection, Measures, and Analysis
Electronic health records of all eligible patients who received alirocumab were reviewed, and basic demographics (patient age, sex, and race/ethnicity) as well as medical characteristics at baseline were collected. To confirm statin intolerance, each veteran’s history of statin use and use of additional lipid-lowering agents was documented. CV history was measured with an index of categorical measures for hypertension, confirmed CAD, hyperlipidemia, heart failure, arrhythmias, peripheral artery disease, stroke, diabetes mellitus, and hypothyroidism. Additionally, concomitant medications, such as aspirin, P2Y12 inhibitors, β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers that patients were taking also were collected. Each veteran’s lipid panel at baseline, and at 4 and 24 weeks posttreatment initiation, also was extracted. Continuous variables were summarized with means (SD), and categorical variables were summarized with frequencies and proportions. The paired Wilcoxon signed rank test was used to compare LDL-C at 4 and 24 weeks after alirocumab initiation with patients’ baseline LDL-C.
Results
Between June 2017 and May 2019, 122 veterans were initiated on alirocumab. Of these veterans, 98 were excluded: 35 concurrently received statin therapy, 33 missed follow-up lipid panels, 21 had previously received evolocumab, 6 failed to meet the NLA definition for statin intolerance, 2 did not fill active alirocumab prescriptions, and 1 had an incalculable LDL-C with a baseline triglyceride level of 3079 mg/dL. This resulted in 24 veterans included in the analysis.
Most participants were male (87.5%) and White veterans (79.2%) with a mean (SD) age of 66.0 (8.4) years and mean (SD) baseline LDL-C of 161.9 (74.3) mg/dL. At baseline, 21 veterans had a history of primary hyperlipidemia, 19 had a history of CAD, and 2 had a history of heterozygous familial hypercholesterolemia. Of the 24 patients included, the most trialed statins before alirocumab initiation were atorvastatin (95.8%), simvastatin (79.2%), rosuvastatin (79.2%), and pravastatin (62.5%) (Table).
LDL-C Reduction
Veterans were initially treated with alirocumab 75 mg administered subcutaneously every 2 weeks; however, 11 veterans required a dose increase to 150 mg every 2 weeks. At treatment week 4, the median LDL-C reduction was 78.5 mg/dL (IQR, 28.0-107.3; P < .01), and at treatment week 24, the median LDL-C reduction was 55.6 mg/dL (IQR, 18.6-85.3; P < .01). This equated to median LDL-C reductions from baseline of 48.5% at week 4 and 34.3% at week 24. A total of 3 veterans experienced LDL-C increases following initiation of alirocumab. At week 4, 9 veterans were noted to have an LDL-C reduction > 50%, 7 veterans had an LDL-C reduction between 30% and 50%, and 5 veterans had an LDL-C reduction of < 30%. At week 24, 6 had an LDL-C reduction > 50%, 9 veterans had an LDL-C reduction between 30% and 50%, and 6 had a LDL-C reduction < 30%.
Cardiovascular Events
Before alirocumab initiation, 22 CV events and interventions were reported in 16 veterans: 12 percutaneous coronary interventions, 5 coronary artery bypass surgeries (CABG), 4 myocardial infarctions, and 1 transient ischemic attack. One month following alirocumab initiation, 1 veteran underwent a CABG after a non-ST-elevation myocardial infarction (NSTEMI).
Safety and Tolerability
Alirocumab was discontinued in 5 veterans due to 4 cases of intolerance (reported memory loss, lethargy, myalgias, and body aches with dyspnea) and 1 case of persistent LDL-C of < 40 mg/dL. Alirocumab was discontinued after 1 year in 2 patients (persistent LDL-C < 40 mg/dL and reported memory loss) and after 6 months in the veteran who reported lethargy. Alirocumab was discontinued after 4 months in the veteran with myalgias and within 2 months in the veteran with body aches and dyspnea. No other AEs were reported.
Discussion
The Efficacy and Safety of Alirocumab vs Ezetimibe in Statin-Intolerant Veterans With a Statin Rechallenge Arm trial is the first clinical trial to examine the efficacy and safety of alirocumab use in statin-intolerant patients. In the trial, 314 patients were randomized to receive alirocumab, ezetimibe, or an atorvastatin rechallenge.6 At 24 weeks, alirocumab reduced mean (SE) LDL-C by 45.0% (2.2%) vs 14.6% (2.2%) with ezetimibe (mean difference 30.4% [3.1%], P < .01).6 Fewer skeletal-muscle-related events also were noted with alirocumab vs atorvastatin (hazard ratio, 0.61; 95% CI, 0.38-0.99; P = .04).6
In this case series, an LDL-C reduction of > 50% was observed in 9 veterans (42.9%) following 4 weeks of treatment; however, LDL-C reduction of > 50% compared with baseline was sustained in only 6 veterans (28.6%) at week 24. Additionally, LDL-C increases from baseline were observed in 3 veterans; the reasoning for the observed increase was unclear, but this may have been due to nonadherence and dietary factors.4 Although a majority of patients saw a significant and clinically meaningful reduction in LDL-C, the group of patients with an increase in the same may have benefitted from targeted intervention to improve medication and dietary adherence. PCSK9 inhibitor resistance also may have contributed to an increase in LDL-C during treatment.7
Of the 24 patients included, 4 reported AEs resulted in therapy discontinuation. Memory impairment, a rare AE of alirocumab, was reported 1 year following alirocumab initiation. Additionally, lethargy was reported after 6 months of treatment. Myalgia also was reported in a veteran 4 months following treatment, and 1 veteran experienced body aches and dyspnea < 2 months following treatment. The most common AEs associated with alirocumab, as noted in previous safety and efficacy clinical trials, included: nasopharyngitis, injection site reaction, influenza, urinary tract infection, and myalgias.8 Many of these more common AEs may be subclinical and underreported. This small case series, however, detected 4 events severe enough to lead to therapy discontinuation. Although this sample is not representative of all statin-intolerant patients who receive treatment with alirocumab, our findings suggest the need for patient education on potential AEs before therapy initiation and clinician monitoring at follow-up visits.
The ODYSSEY OUTCOMES trial established a CV benefit associated with alirocumab; however, patients included had a recent acute coronary syndrome event and were receiving a high-intensity statin.9 This case series is unique in that before alirocumab initiation, 22 CV events/interventions were reported in the sample of 24 patients. After therapy initiation, 1 patient underwent a CABG after an NSTEMI in the month following initiation. This suggests that cardiac complications are possible after PCSK-9 initiation; however, little information can be gained from 1 patient. Nevertheless, early therapy failure should be investigated in the context of real-world use in statin-intolerant patients. This is a complex task, however, given the difficulties of achieving a balanced study design. Statin intolerance is a clear source of selection bias into treatment with alirocumab as patients in this population have already initiated and failed statin therapy. The prevalence of prior CV events and the time-dependent association between prior and future CV events stand as another complex confounder. Although there is a clear and pressing need to understand the risks and benefits of PCSK9 therapy in statin-intolerant patients, future research in this area will need to cautiously address these important sources of bias.
Overall, the results of this case series support LDL-C reduction associated with alirocumab in the absence of statin therapy. Despite favorable results, use of alirocumab may be limited by cost and its subcutaneous route of administration. Bempedoic acid, an oral, once-daily lipid-lowering agent poses an alternative to PCSK9 inhibitors, but further data regarding CV outcomes with this agent is needed.10,11 Robust randomized controlled trials also are needed to evaluate CV outcomes for alirocumab use in statin-intolerant veterans.
Limitations
Only 24 veterans were included in the study, reflecting 20% of the charts reviewed (80% exclusion rate), and in this small sample, only 1 CV event was observed. Both of these serve as threats to external validity. As the study information was extracted from chart review, the results may be limited by coding or historical bias. Medical information from outside institutions may be missing from medical records. Additionally, results may be skewed by possible documentation errors. Furthermore, the period between previous CV events and alirocumab initiation is unclear as event dates were often not recorded if treatment was received at an outside institution.
Due to the short follow-up period, the case series is limited in its assessment of CV outcomes and safety outcomes. Larger studies over an extended period are needed to assess CV outcomes and safety of alirocumab use in statin-intolerant patients. Also, medication adherence was not assessed. Given the impact of medication adherence on LDL-C reduction, it is unclear what role medication adherence played in the LDL-C reduction observed in this study.4
Conclusions
Alirocumab use in 24 statin-intolerant veterans resulted in a significant reduction in LDL-C at 4 and 24 weeks after initiation. In addition, 1 CV event/intervention was observed following alirocumab initiation, although this should be interpreted with caution due to the retrospective nature of this case series, small sample size, and short follow-up period. Large, long-term studies would better evaluate the CV benefit associated with alirocumab therapy in a veteran population.
1. Benjamin EJ, Munter P, Alonso A, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi:10.1161/CIR.0000000000000659
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014 Jun 24;129(25)(suppl 2):S1-S45. doi:10.1016/j.jacc.2013.11.002
3. Hajar R. Statins: past and present. Heart Views. 2011;12(3): 121-127. doi:10.4103/1995-705X.95070
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73(4):3168-3209. doi:10.1016/j.jacc.2018.11.002
5. Toth PH, Patti AM, Giglio RV, et al. Management of statin intolerance in 2018: still more questions than answers. Am J Cardiovasc Drugs. 2018;18(3):157-173. doi:10.1007/s40256-017-0259-7
6. Moriarty PM, Thompson PD, Cannon CP, et al; ODYSSEY ALTERNATIVE Investigators. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
7. Shapiro MD, Miles J, Tavori H, Fazio S. Diagnosing resistance to a proprotein convertase subtilisin/kexin type 9 inhibitor. Ann Intern Med. 2018;168(5):376-379. doi:10.7326/M17-2485
8. Raedler LA. Praluent (alirocumab): first PCSK9 inhibitor approved by the FDA for hypercholesterolemia. Am Health Drug Benefits. 2016;9:123-126.
9. Schwartz GC, Steg PC, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
10. Nexletol. Package insert. Esperion Therapeutics Inc; 2020.
11. Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662. doi:10.1161/JAHA.118.011662
In 2016, 17.6 million deaths occurred globally due to cardiovascular disease (CVD) with coronary artery disease (CAD) and ischemic stroke as top contributors.1 Elevated low-density lipoprotein cholesterol (LDL-C) has been linked to greater risk of atherosclerotic cardiovascular disease (ASCVD); therefore, LDL-C reduction is imperative to decrease risk of cardiovascular (CV) morbidity and mortality.2 Since 1987, statin therapy has been the mainstay of treatment for hypercholesterolemia, and current practice guidelines recommend statins as first-line therapy given demonstrated reductions in LDL-C and CV mortality reduction in robust clinical trials.2-4 Although generally safe and well tolerated, muscle-related adverse events (AEs) limit optimal use of statins in up to 20% of individuals who have an indication for statin therapy.5 As a consequence, these patients receive suboptimal statin doses or no statin therapy and are at a higher risk for ASCVD.5
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been shown to significantly lower LDL-C when used as monotherapy or in combination with statins and/or other lipid-lowering therapies.5 These agents are currently approved by the US Food and Drug Administration as an adjunct to diet with or without other lipid-lowering therapies for the management of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia), homozygous familial hypercholesterolemia (evolocumab only), and for use in patients with established CVD unable to achieve their lipid-lowering goals with maximally tolerated statin doses and ezetimibe.4 With the ability to reduce LDL-C by up to 65%, PCSK9 inhibitors offer an alternative option for LDL-C and potentially CV risk reduction in statin-intolerant patients.5
Alirocumab, the formulary preferred PCSK9 inhibitor at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, has been increasingly used in high-risk statin-intolerant veterans. The primary objective of this case series was to assess LDL-C reduction associated with alirocumab use in statin-intolerant veterans at the MEDVAMC. The secondary objective was to assess the incidence of CV events. This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee.
Methods
In this single-center case series, a retrospective chart review was conducted to identify statin-intolerant veterans who were initiated on treatment with alirocumab for LDL-C and/or CV risk reduction between June 2017 and May 2019. Adult veterans with a diagnosis of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia) and/or CAD with documented statin intolerance were included in the study. Statin intolerance was defined in accordance with the National Lipid Association (NLA) definition as aninability to tolerate ≥ 2 statins with a trial of at least 1 statin at its lowest daily dose.5 Veterans who previously received treatment with evolocumab, those prescribed concurrent statin therapies, and those missing follow-up lipid panels at 24 weeks were excluded from the study. To assess LDL-C reduction, LDL-C at baseline was compared with LDL-C at 4 and 24 weeks. Incident CV events before and after alirocumab initiation were documented. The US Department of Veteran Affairs (VA) Computerized Patient Record System was used to collect patient data.
Data Collection, Measures, and Analysis
Electronic health records of all eligible patients who received alirocumab were reviewed, and basic demographics (patient age, sex, and race/ethnicity) as well as medical characteristics at baseline were collected. To confirm statin intolerance, each veteran’s history of statin use and use of additional lipid-lowering agents was documented. CV history was measured with an index of categorical measures for hypertension, confirmed CAD, hyperlipidemia, heart failure, arrhythmias, peripheral artery disease, stroke, diabetes mellitus, and hypothyroidism. Additionally, concomitant medications, such as aspirin, P2Y12 inhibitors, β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers that patients were taking also were collected. Each veteran’s lipid panel at baseline, and at 4 and 24 weeks posttreatment initiation, also was extracted. Continuous variables were summarized with means (SD), and categorical variables were summarized with frequencies and proportions. The paired Wilcoxon signed rank test was used to compare LDL-C at 4 and 24 weeks after alirocumab initiation with patients’ baseline LDL-C.
Results
Between June 2017 and May 2019, 122 veterans were initiated on alirocumab. Of these veterans, 98 were excluded: 35 concurrently received statin therapy, 33 missed follow-up lipid panels, 21 had previously received evolocumab, 6 failed to meet the NLA definition for statin intolerance, 2 did not fill active alirocumab prescriptions, and 1 had an incalculable LDL-C with a baseline triglyceride level of 3079 mg/dL. This resulted in 24 veterans included in the analysis.
Most participants were male (87.5%) and White veterans (79.2%) with a mean (SD) age of 66.0 (8.4) years and mean (SD) baseline LDL-C of 161.9 (74.3) mg/dL. At baseline, 21 veterans had a history of primary hyperlipidemia, 19 had a history of CAD, and 2 had a history of heterozygous familial hypercholesterolemia. Of the 24 patients included, the most trialed statins before alirocumab initiation were atorvastatin (95.8%), simvastatin (79.2%), rosuvastatin (79.2%), and pravastatin (62.5%) (Table).
LDL-C Reduction
Veterans were initially treated with alirocumab 75 mg administered subcutaneously every 2 weeks; however, 11 veterans required a dose increase to 150 mg every 2 weeks. At treatment week 4, the median LDL-C reduction was 78.5 mg/dL (IQR, 28.0-107.3; P < .01), and at treatment week 24, the median LDL-C reduction was 55.6 mg/dL (IQR, 18.6-85.3; P < .01). This equated to median LDL-C reductions from baseline of 48.5% at week 4 and 34.3% at week 24. A total of 3 veterans experienced LDL-C increases following initiation of alirocumab. At week 4, 9 veterans were noted to have an LDL-C reduction > 50%, 7 veterans had an LDL-C reduction between 30% and 50%, and 5 veterans had an LDL-C reduction of < 30%. At week 24, 6 had an LDL-C reduction > 50%, 9 veterans had an LDL-C reduction between 30% and 50%, and 6 had a LDL-C reduction < 30%.
Cardiovascular Events
Before alirocumab initiation, 22 CV events and interventions were reported in 16 veterans: 12 percutaneous coronary interventions, 5 coronary artery bypass surgeries (CABG), 4 myocardial infarctions, and 1 transient ischemic attack. One month following alirocumab initiation, 1 veteran underwent a CABG after a non-ST-elevation myocardial infarction (NSTEMI).
Safety and Tolerability
Alirocumab was discontinued in 5 veterans due to 4 cases of intolerance (reported memory loss, lethargy, myalgias, and body aches with dyspnea) and 1 case of persistent LDL-C of < 40 mg/dL. Alirocumab was discontinued after 1 year in 2 patients (persistent LDL-C < 40 mg/dL and reported memory loss) and after 6 months in the veteran who reported lethargy. Alirocumab was discontinued after 4 months in the veteran with myalgias and within 2 months in the veteran with body aches and dyspnea. No other AEs were reported.
Discussion
The Efficacy and Safety of Alirocumab vs Ezetimibe in Statin-Intolerant Veterans With a Statin Rechallenge Arm trial is the first clinical trial to examine the efficacy and safety of alirocumab use in statin-intolerant patients. In the trial, 314 patients were randomized to receive alirocumab, ezetimibe, or an atorvastatin rechallenge.6 At 24 weeks, alirocumab reduced mean (SE) LDL-C by 45.0% (2.2%) vs 14.6% (2.2%) with ezetimibe (mean difference 30.4% [3.1%], P < .01).6 Fewer skeletal-muscle-related events also were noted with alirocumab vs atorvastatin (hazard ratio, 0.61; 95% CI, 0.38-0.99; P = .04).6
In this case series, an LDL-C reduction of > 50% was observed in 9 veterans (42.9%) following 4 weeks of treatment; however, LDL-C reduction of > 50% compared with baseline was sustained in only 6 veterans (28.6%) at week 24. Additionally, LDL-C increases from baseline were observed in 3 veterans; the reasoning for the observed increase was unclear, but this may have been due to nonadherence and dietary factors.4 Although a majority of patients saw a significant and clinically meaningful reduction in LDL-C, the group of patients with an increase in the same may have benefitted from targeted intervention to improve medication and dietary adherence. PCSK9 inhibitor resistance also may have contributed to an increase in LDL-C during treatment.7
Of the 24 patients included, 4 reported AEs resulted in therapy discontinuation. Memory impairment, a rare AE of alirocumab, was reported 1 year following alirocumab initiation. Additionally, lethargy was reported after 6 months of treatment. Myalgia also was reported in a veteran 4 months following treatment, and 1 veteran experienced body aches and dyspnea < 2 months following treatment. The most common AEs associated with alirocumab, as noted in previous safety and efficacy clinical trials, included: nasopharyngitis, injection site reaction, influenza, urinary tract infection, and myalgias.8 Many of these more common AEs may be subclinical and underreported. This small case series, however, detected 4 events severe enough to lead to therapy discontinuation. Although this sample is not representative of all statin-intolerant patients who receive treatment with alirocumab, our findings suggest the need for patient education on potential AEs before therapy initiation and clinician monitoring at follow-up visits.
The ODYSSEY OUTCOMES trial established a CV benefit associated with alirocumab; however, patients included had a recent acute coronary syndrome event and were receiving a high-intensity statin.9 This case series is unique in that before alirocumab initiation, 22 CV events/interventions were reported in the sample of 24 patients. After therapy initiation, 1 patient underwent a CABG after an NSTEMI in the month following initiation. This suggests that cardiac complications are possible after PCSK-9 initiation; however, little information can be gained from 1 patient. Nevertheless, early therapy failure should be investigated in the context of real-world use in statin-intolerant patients. This is a complex task, however, given the difficulties of achieving a balanced study design. Statin intolerance is a clear source of selection bias into treatment with alirocumab as patients in this population have already initiated and failed statin therapy. The prevalence of prior CV events and the time-dependent association between prior and future CV events stand as another complex confounder. Although there is a clear and pressing need to understand the risks and benefits of PCSK9 therapy in statin-intolerant patients, future research in this area will need to cautiously address these important sources of bias.
Overall, the results of this case series support LDL-C reduction associated with alirocumab in the absence of statin therapy. Despite favorable results, use of alirocumab may be limited by cost and its subcutaneous route of administration. Bempedoic acid, an oral, once-daily lipid-lowering agent poses an alternative to PCSK9 inhibitors, but further data regarding CV outcomes with this agent is needed.10,11 Robust randomized controlled trials also are needed to evaluate CV outcomes for alirocumab use in statin-intolerant veterans.
Limitations
Only 24 veterans were included in the study, reflecting 20% of the charts reviewed (80% exclusion rate), and in this small sample, only 1 CV event was observed. Both of these serve as threats to external validity. As the study information was extracted from chart review, the results may be limited by coding or historical bias. Medical information from outside institutions may be missing from medical records. Additionally, results may be skewed by possible documentation errors. Furthermore, the period between previous CV events and alirocumab initiation is unclear as event dates were often not recorded if treatment was received at an outside institution.
Due to the short follow-up period, the case series is limited in its assessment of CV outcomes and safety outcomes. Larger studies over an extended period are needed to assess CV outcomes and safety of alirocumab use in statin-intolerant patients. Also, medication adherence was not assessed. Given the impact of medication adherence on LDL-C reduction, it is unclear what role medication adherence played in the LDL-C reduction observed in this study.4
Conclusions
Alirocumab use in 24 statin-intolerant veterans resulted in a significant reduction in LDL-C at 4 and 24 weeks after initiation. In addition, 1 CV event/intervention was observed following alirocumab initiation, although this should be interpreted with caution due to the retrospective nature of this case series, small sample size, and short follow-up period. Large, long-term studies would better evaluate the CV benefit associated with alirocumab therapy in a veteran population.
In 2016, 17.6 million deaths occurred globally due to cardiovascular disease (CVD) with coronary artery disease (CAD) and ischemic stroke as top contributors.1 Elevated low-density lipoprotein cholesterol (LDL-C) has been linked to greater risk of atherosclerotic cardiovascular disease (ASCVD); therefore, LDL-C reduction is imperative to decrease risk of cardiovascular (CV) morbidity and mortality.2 Since 1987, statin therapy has been the mainstay of treatment for hypercholesterolemia, and current practice guidelines recommend statins as first-line therapy given demonstrated reductions in LDL-C and CV mortality reduction in robust clinical trials.2-4 Although generally safe and well tolerated, muscle-related adverse events (AEs) limit optimal use of statins in up to 20% of individuals who have an indication for statin therapy.5 As a consequence, these patients receive suboptimal statin doses or no statin therapy and are at a higher risk for ASCVD.5
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been shown to significantly lower LDL-C when used as monotherapy or in combination with statins and/or other lipid-lowering therapies.5 These agents are currently approved by the US Food and Drug Administration as an adjunct to diet with or without other lipid-lowering therapies for the management of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia), homozygous familial hypercholesterolemia (evolocumab only), and for use in patients with established CVD unable to achieve their lipid-lowering goals with maximally tolerated statin doses and ezetimibe.4 With the ability to reduce LDL-C by up to 65%, PCSK9 inhibitors offer an alternative option for LDL-C and potentially CV risk reduction in statin-intolerant patients.5
Alirocumab, the formulary preferred PCSK9 inhibitor at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, has been increasingly used in high-risk statin-intolerant veterans. The primary objective of this case series was to assess LDL-C reduction associated with alirocumab use in statin-intolerant veterans at the MEDVAMC. The secondary objective was to assess the incidence of CV events. This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee.
Methods
In this single-center case series, a retrospective chart review was conducted to identify statin-intolerant veterans who were initiated on treatment with alirocumab for LDL-C and/or CV risk reduction between June 2017 and May 2019. Adult veterans with a diagnosis of primary hypercholesterolemia (including heterozygous familial hypercholesterolemia) and/or CAD with documented statin intolerance were included in the study. Statin intolerance was defined in accordance with the National Lipid Association (NLA) definition as aninability to tolerate ≥ 2 statins with a trial of at least 1 statin at its lowest daily dose.5 Veterans who previously received treatment with evolocumab, those prescribed concurrent statin therapies, and those missing follow-up lipid panels at 24 weeks were excluded from the study. To assess LDL-C reduction, LDL-C at baseline was compared with LDL-C at 4 and 24 weeks. Incident CV events before and after alirocumab initiation were documented. The US Department of Veteran Affairs (VA) Computerized Patient Record System was used to collect patient data.
Data Collection, Measures, and Analysis
Electronic health records of all eligible patients who received alirocumab were reviewed, and basic demographics (patient age, sex, and race/ethnicity) as well as medical characteristics at baseline were collected. To confirm statin intolerance, each veteran’s history of statin use and use of additional lipid-lowering agents was documented. CV history was measured with an index of categorical measures for hypertension, confirmed CAD, hyperlipidemia, heart failure, arrhythmias, peripheral artery disease, stroke, diabetes mellitus, and hypothyroidism. Additionally, concomitant medications, such as aspirin, P2Y12 inhibitors, β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers that patients were taking also were collected. Each veteran’s lipid panel at baseline, and at 4 and 24 weeks posttreatment initiation, also was extracted. Continuous variables were summarized with means (SD), and categorical variables were summarized with frequencies and proportions. The paired Wilcoxon signed rank test was used to compare LDL-C at 4 and 24 weeks after alirocumab initiation with patients’ baseline LDL-C.
Results
Between June 2017 and May 2019, 122 veterans were initiated on alirocumab. Of these veterans, 98 were excluded: 35 concurrently received statin therapy, 33 missed follow-up lipid panels, 21 had previously received evolocumab, 6 failed to meet the NLA definition for statin intolerance, 2 did not fill active alirocumab prescriptions, and 1 had an incalculable LDL-C with a baseline triglyceride level of 3079 mg/dL. This resulted in 24 veterans included in the analysis.
Most participants were male (87.5%) and White veterans (79.2%) with a mean (SD) age of 66.0 (8.4) years and mean (SD) baseline LDL-C of 161.9 (74.3) mg/dL. At baseline, 21 veterans had a history of primary hyperlipidemia, 19 had a history of CAD, and 2 had a history of heterozygous familial hypercholesterolemia. Of the 24 patients included, the most trialed statins before alirocumab initiation were atorvastatin (95.8%), simvastatin (79.2%), rosuvastatin (79.2%), and pravastatin (62.5%) (Table).
LDL-C Reduction
Veterans were initially treated with alirocumab 75 mg administered subcutaneously every 2 weeks; however, 11 veterans required a dose increase to 150 mg every 2 weeks. At treatment week 4, the median LDL-C reduction was 78.5 mg/dL (IQR, 28.0-107.3; P < .01), and at treatment week 24, the median LDL-C reduction was 55.6 mg/dL (IQR, 18.6-85.3; P < .01). This equated to median LDL-C reductions from baseline of 48.5% at week 4 and 34.3% at week 24. A total of 3 veterans experienced LDL-C increases following initiation of alirocumab. At week 4, 9 veterans were noted to have an LDL-C reduction > 50%, 7 veterans had an LDL-C reduction between 30% and 50%, and 5 veterans had an LDL-C reduction of < 30%. At week 24, 6 had an LDL-C reduction > 50%, 9 veterans had an LDL-C reduction between 30% and 50%, and 6 had a LDL-C reduction < 30%.
Cardiovascular Events
Before alirocumab initiation, 22 CV events and interventions were reported in 16 veterans: 12 percutaneous coronary interventions, 5 coronary artery bypass surgeries (CABG), 4 myocardial infarctions, and 1 transient ischemic attack. One month following alirocumab initiation, 1 veteran underwent a CABG after a non-ST-elevation myocardial infarction (NSTEMI).
Safety and Tolerability
Alirocumab was discontinued in 5 veterans due to 4 cases of intolerance (reported memory loss, lethargy, myalgias, and body aches with dyspnea) and 1 case of persistent LDL-C of < 40 mg/dL. Alirocumab was discontinued after 1 year in 2 patients (persistent LDL-C < 40 mg/dL and reported memory loss) and after 6 months in the veteran who reported lethargy. Alirocumab was discontinued after 4 months in the veteran with myalgias and within 2 months in the veteran with body aches and dyspnea. No other AEs were reported.
Discussion
The Efficacy and Safety of Alirocumab vs Ezetimibe in Statin-Intolerant Veterans With a Statin Rechallenge Arm trial is the first clinical trial to examine the efficacy and safety of alirocumab use in statin-intolerant patients. In the trial, 314 patients were randomized to receive alirocumab, ezetimibe, or an atorvastatin rechallenge.6 At 24 weeks, alirocumab reduced mean (SE) LDL-C by 45.0% (2.2%) vs 14.6% (2.2%) with ezetimibe (mean difference 30.4% [3.1%], P < .01).6 Fewer skeletal-muscle-related events also were noted with alirocumab vs atorvastatin (hazard ratio, 0.61; 95% CI, 0.38-0.99; P = .04).6
In this case series, an LDL-C reduction of > 50% was observed in 9 veterans (42.9%) following 4 weeks of treatment; however, LDL-C reduction of > 50% compared with baseline was sustained in only 6 veterans (28.6%) at week 24. Additionally, LDL-C increases from baseline were observed in 3 veterans; the reasoning for the observed increase was unclear, but this may have been due to nonadherence and dietary factors.4 Although a majority of patients saw a significant and clinically meaningful reduction in LDL-C, the group of patients with an increase in the same may have benefitted from targeted intervention to improve medication and dietary adherence. PCSK9 inhibitor resistance also may have contributed to an increase in LDL-C during treatment.7
Of the 24 patients included, 4 reported AEs resulted in therapy discontinuation. Memory impairment, a rare AE of alirocumab, was reported 1 year following alirocumab initiation. Additionally, lethargy was reported after 6 months of treatment. Myalgia also was reported in a veteran 4 months following treatment, and 1 veteran experienced body aches and dyspnea < 2 months following treatment. The most common AEs associated with alirocumab, as noted in previous safety and efficacy clinical trials, included: nasopharyngitis, injection site reaction, influenza, urinary tract infection, and myalgias.8 Many of these more common AEs may be subclinical and underreported. This small case series, however, detected 4 events severe enough to lead to therapy discontinuation. Although this sample is not representative of all statin-intolerant patients who receive treatment with alirocumab, our findings suggest the need for patient education on potential AEs before therapy initiation and clinician monitoring at follow-up visits.
The ODYSSEY OUTCOMES trial established a CV benefit associated with alirocumab; however, patients included had a recent acute coronary syndrome event and were receiving a high-intensity statin.9 This case series is unique in that before alirocumab initiation, 22 CV events/interventions were reported in the sample of 24 patients. After therapy initiation, 1 patient underwent a CABG after an NSTEMI in the month following initiation. This suggests that cardiac complications are possible after PCSK-9 initiation; however, little information can be gained from 1 patient. Nevertheless, early therapy failure should be investigated in the context of real-world use in statin-intolerant patients. This is a complex task, however, given the difficulties of achieving a balanced study design. Statin intolerance is a clear source of selection bias into treatment with alirocumab as patients in this population have already initiated and failed statin therapy. The prevalence of prior CV events and the time-dependent association between prior and future CV events stand as another complex confounder. Although there is a clear and pressing need to understand the risks and benefits of PCSK9 therapy in statin-intolerant patients, future research in this area will need to cautiously address these important sources of bias.
Overall, the results of this case series support LDL-C reduction associated with alirocumab in the absence of statin therapy. Despite favorable results, use of alirocumab may be limited by cost and its subcutaneous route of administration. Bempedoic acid, an oral, once-daily lipid-lowering agent poses an alternative to PCSK9 inhibitors, but further data regarding CV outcomes with this agent is needed.10,11 Robust randomized controlled trials also are needed to evaluate CV outcomes for alirocumab use in statin-intolerant veterans.
Limitations
Only 24 veterans were included in the study, reflecting 20% of the charts reviewed (80% exclusion rate), and in this small sample, only 1 CV event was observed. Both of these serve as threats to external validity. As the study information was extracted from chart review, the results may be limited by coding or historical bias. Medical information from outside institutions may be missing from medical records. Additionally, results may be skewed by possible documentation errors. Furthermore, the period between previous CV events and alirocumab initiation is unclear as event dates were often not recorded if treatment was received at an outside institution.
Due to the short follow-up period, the case series is limited in its assessment of CV outcomes and safety outcomes. Larger studies over an extended period are needed to assess CV outcomes and safety of alirocumab use in statin-intolerant patients. Also, medication adherence was not assessed. Given the impact of medication adherence on LDL-C reduction, it is unclear what role medication adherence played in the LDL-C reduction observed in this study.4
Conclusions
Alirocumab use in 24 statin-intolerant veterans resulted in a significant reduction in LDL-C at 4 and 24 weeks after initiation. In addition, 1 CV event/intervention was observed following alirocumab initiation, although this should be interpreted with caution due to the retrospective nature of this case series, small sample size, and short follow-up period. Large, long-term studies would better evaluate the CV benefit associated with alirocumab therapy in a veteran population.
1. Benjamin EJ, Munter P, Alonso A, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi:10.1161/CIR.0000000000000659
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014 Jun 24;129(25)(suppl 2):S1-S45. doi:10.1016/j.jacc.2013.11.002
3. Hajar R. Statins: past and present. Heart Views. 2011;12(3): 121-127. doi:10.4103/1995-705X.95070
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73(4):3168-3209. doi:10.1016/j.jacc.2018.11.002
5. Toth PH, Patti AM, Giglio RV, et al. Management of statin intolerance in 2018: still more questions than answers. Am J Cardiovasc Drugs. 2018;18(3):157-173. doi:10.1007/s40256-017-0259-7
6. Moriarty PM, Thompson PD, Cannon CP, et al; ODYSSEY ALTERNATIVE Investigators. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
7. Shapiro MD, Miles J, Tavori H, Fazio S. Diagnosing resistance to a proprotein convertase subtilisin/kexin type 9 inhibitor. Ann Intern Med. 2018;168(5):376-379. doi:10.7326/M17-2485
8. Raedler LA. Praluent (alirocumab): first PCSK9 inhibitor approved by the FDA for hypercholesterolemia. Am Health Drug Benefits. 2016;9:123-126.
9. Schwartz GC, Steg PC, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
10. Nexletol. Package insert. Esperion Therapeutics Inc; 2020.
11. Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662. doi:10.1161/JAHA.118.011662
1. Benjamin EJ, Munter P, Alonso A, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi:10.1161/CIR.0000000000000659
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014 Jun 24;129(25)(suppl 2):S1-S45. doi:10.1016/j.jacc.2013.11.002
3. Hajar R. Statins: past and present. Heart Views. 2011;12(3): 121-127. doi:10.4103/1995-705X.95070
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73(4):3168-3209. doi:10.1016/j.jacc.2018.11.002
5. Toth PH, Patti AM, Giglio RV, et al. Management of statin intolerance in 2018: still more questions than answers. Am J Cardiovasc Drugs. 2018;18(3):157-173. doi:10.1007/s40256-017-0259-7
6. Moriarty PM, Thompson PD, Cannon CP, et al; ODYSSEY ALTERNATIVE Investigators. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
7. Shapiro MD, Miles J, Tavori H, Fazio S. Diagnosing resistance to a proprotein convertase subtilisin/kexin type 9 inhibitor. Ann Intern Med. 2018;168(5):376-379. doi:10.7326/M17-2485
8. Raedler LA. Praluent (alirocumab): first PCSK9 inhibitor approved by the FDA for hypercholesterolemia. Am Health Drug Benefits. 2016;9:123-126.
9. Schwartz GC, Steg PC, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
10. Nexletol. Package insert. Esperion Therapeutics Inc; 2020.
11. Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662. doi:10.1161/JAHA.118.011662
Evaluation of Glycemic Control and Cost Savings Associated With Liraglutide Dose Reduction at a Veterans Affairs Hospital
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are injectable incretin hormones approved for the treatment of type 2 diabetes mellitus (T2DM). They are highly efficacious agents with hemoglobin A1c (HbA1c) reduction potential of approximately 0.8 to 1.6% and mechanisms of action that result in an average weight loss of 1 to 3 kg.1,2 Published in 2016, The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial established cardiovascular benefits associated with liraglutide, making it a preferred GLP-1 RA.3
In addition to HbA1c reduction, weight loss, and cardiovascular benefits, liraglutide also has shown insulin-sparing effects when used in combination with insulin. A trial by Lane and colleagues revealed a 34% decrease in total daily insulin dose 6 months after the addition of liraglutide to insulin in patients with T2DM receiving > 100 units of insulin daily.4 When used in combination with basal insulin analogues (glargine or detemir) similar findings also were shown.5
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, selected liraglutide as its preferred GLP-1 RA because of its favorable glycemic and cardiovascular outcomes. In addition, as part of a cost-savings initiative for fiscal year 2018, liraglutide 6 mg/mL injection 2-count pen packs was selected as the preferred liraglutide product. Before the availability of the 2-count pen packs, veterans previously received 3-count pen packs, which allowed for up to a 30-day supply of liraglutide 1.8 mg daily dosing. However, the cost-efficient 2-count pen packs allow for up to 1.2 mg daily dose of liraglutide for a 30-day supply. Due to these changes, veterans at MEDVAMC were converted from liraglutide 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018.
The primary objective of this study was to assess sustained glycemic control and cost savings that resulted from this change. The secondary objectives were to assess sustained weight loss and adverse effects (AEs).
Methods
This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee. In this single-center study, a retrospective chart review was conducted on veterans with T2DM who underwent a liraglutide dose reduction from 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018. Patients were included if they were aged ≥ 18 years with an active prescription for liraglutide 1.8 mg daily and insulin (with or without other antihyperglycemic agents) at the time of conversion. In addition, patients must have had ≥ 1 HbA1c reading within 3 months of the dose conversion and a follow-up HbA1c within 6 months after the dose conversion. To assess the primary objective of glycemic control that resulted from the liraglutide dose reduction, mean change of HbA1c at time of dose conversion was compared with mean HbA1c 6 months postconversion. To assess savings, cost information was obtained from the US Department of Veterans Affairs (VA) Drug Price Database and monthly and annual costs of liraglutide 6 mg/mL injection 2-count pen pack were compared with that of the 3-count pen pack. A chart review of patients’ electronic health records assessed secondary outcomes. The VA Computerized Patient Record System (CPRS) was used to collect patient data.
Patients and Characteristics
The following patient information was obtained from patients’ records: age, sex, race/ethnicity, diabetic medications (at time of conversion and 6 months after conversion), cardiovascular history and risk factors (hypertension, coronary artery disease, heart failure, arrhythmias, peripheral artery disease, obesity, etc), prescriber type (physician, nurse practitioner/physician assistant, pharmacist, etc), weight (at baseline, at time of conversion, and 6 months after conversion), HbA1c (at baseline, at time of conversion, and 6 months after conversion), average blood glucose (at baseline, at time of conversion, and 6 months after conversion), insulin dose (at time of conversion and 6 months after conversion), and reported AEs.
Statistical Analysis
The 2-tailed, paired t test was used to assess changes in HbA1c, average blood glucose, and body weight. Demographic data and other outcomes were assessed using descriptive statistics.
Results
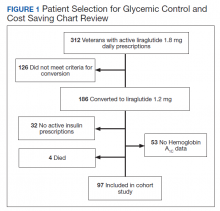
Prior to the dose reduction, 312 veterans had active prescriptions for liraglutide 1.8 mg daily. Due to lack of glycemic control benefit (failing to achieve a HbA1c reduction of at least 0.5% after at least 3 to 6 months following initiation of therapy) or nonadherence (assessed by medication refill history), 126 veterans did not meet the criteria for the dose conversion. As a result, liraglutide was discontinued, and veterans were sent patient letter notifications and health care providers were notified via medication review notes in the patient electronic health record “to make medication adjustments if warranted. A total of 186 veterans underwent a liraglutide dose reduction between May and August 2018. Thirty-two veterans were without active insulin prescriptions, 53 were without HbA1c results, and 4 veterans died; resulting in 97 veterans who were included in the study (Figure 1).
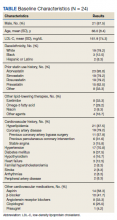
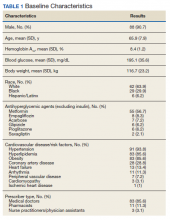
Most of the patients included in the study were male (90.7%) and White (63.9%) with an average (SD) age of 65.9 years (7.9) and a mean (SD) HbA1c at baseline of 8.4% (1.2). About 56.7% received concurrent T2DM treatment with metformin, and 8.3% received concurrent treatment with empagliflozin. The most common cardiovascular disease/risk factors included hypertension (93.8%), hyperlipidemia (85.6%), and obesity (85.6%) (Table 1).
Glycemic Control and Weight Loss
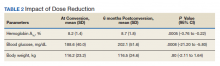
At the time of conversion, the average (SD) HbA1c was 8.2% (1.4) and increased to an average (SD) of 8.7% (1.8) (P =.0005) 6 months after the dose reduction (Table 2). The average (SD) body weight was 116.2 kg (23.2) at time of conversion and increased to 116.5 (24.6) 6 months following the dose reduction; however, the difference was not statistically significant (P = .8).
As a result of the HbA1c change, 41.2% of veterans underwent an insulin dose increase with dose increase of 5 to 200 units of total daily insulin during the 6-month period. Antihyperglycemic regimen remained unchanged for 40.2% of veterans, while additional glucose lowering agents were initiated in 6 veterans. Medications initiated included empagliflozin in 4 veterans and saxagliptin in 2 veterans.
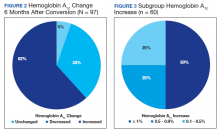
HbA1c reduction was noted in 33% of veterans (Figure 2) mostly due to improved diet and exercise habits. A majority of veterans, 62%, experienced an increase in HbA1c, whereas 5.2% of veterans maintained the same HbA1c. Of 60 veterans with HbA1c increases, 15 had an increase between 0.1% and 0.5%, another 15 with an increase between 0.5 to 0.9%, and half had HbA1c increases of at least 1% with a maximum increase of 5.1% (Figure 3).
Cost Savings
Cost information was obtained from the VA Drug Price Database. The estimated monthly cost savings per patient associated with the conversion from 3-count to 2-count injection pen packs of liraglutide 6 mg/mL was $103.46. With 186 veterans converted to the 2-count pen packs, MEDVAMC saved $115,461.36 in a 6-month period. The estimated annualized cost savings was estimated to be about $231,000 (Figure 4).
Adverse Effects During the 6-month period following the dose conversion, no major AEs associated with liraglutide were documented. Documented AEs included 3 cases of diarrhea, resulting in the discontinuation of metformin. Metformin also was discontinued in a veteran with worsened renal function and eGFR < 30 mL/min/1.73 m2.
Discussion
According to previous clinical trials, when used in combination with insulin, 1.2 mg and 1.8 mg daily liraglutide showed significant improvement in glycemic control and body weight and was associated with decreased insulin requirements.4-6 However, subgroup analyses were not performed to show differences in benefit between the liraglutide 1.8 mg and 1.2 mg groups.4-6 Similarly, cardiovascular benefit was observed in patients receiving liraglutide 1.2 mg daily and liraglutide 1.8 mg daily in the LEADER trial with no subgroup analysis or distinction between treatment doses.3 With this information and approval by the Veterans Integrated Services Network, the pharmacoeconomics team at MEDVAMC made the decision to select a more cost-efficient preparation and, hence, lower dose of liraglutide.
To ensure that patients only taking liraglutide for glycemic control were captured, patients without insulin therapies at baseline were excluded. Due to concerns of potential off-label use of liraglutide for weight loss, patients without active prescriptions for insulin at baseline were excluded.
A mean HbA1c increase of 0.5% was observed over the 6-month period, supporting findings of a dose-dependent HbA1c decrease observed in clinical trials. In the LEAD-3 MONO trial when used as monotherapy, liraglutide 1.8 mg was associated with significantly greater HbA1c reduction than liraglutide 1.2 mg (–0·29%; –0·50 to –0.09, P = .005) after 52 weeks of treatment.7 Liraglutide 1.8 mg was also associated with higher rates of AEs; particularly gastrointestinal. 7 To minimize these AEs, it is recommended to initiate liraglutide at 0.6 mg daily for a week then increase to 1.2 mg daily. If tolerated, liraglutide can be further titrated to 1.8 mg daily to optimize glycemic control.8 Unsurprisingly, no major AEs were noted in this study, as AEs are typically noted with increased doses.
Despite the observed trend of increased HbA1c, no changes were made to glucoselowering agents in 39 veterans. This group of veterans consisted primarily of those whose HbA1c remained unchanged during the 6-month period, those whose HbA1c improved (with no documented hypoglycemia), and older veterans with less stringent HbA1c goals. As a result, doses of glucose lowering agents were maintained as appropriate.
No significant difference was noted in body weight during the 6-month period. The slight weight gain observed may have been due to several factors. Lack of exercise and dietary changes may have contributed to weight gain. In addition, insulin doses were increased in 40 veterans, which may have contributed to the observed weight gain.
As expected, significant cost savings were achieved as a result of the liraglutide dose reduction. Of note, liraglutide was discontinued in 126 veterans (prior to the dose reduction) due to nonadherence or inadequate response to therapy, which also resulted in additional savings. Although cost savings was achieved, the long-term benefit of this initiative still remains unknown. The worsened glycemic control that was detected may increase the risk of microvascular and macrovascular complications, thereby negating cost savings achieved. To assess this effect, longterm prospective studies are warranted.
Limitations
A number of issues limit these finding, including its retrospective data review, small sample size, additional factors contributing to HbA1c increase, and missing documentation in some patient records. Only 97 patients were included in the study, reflecting less than half of the charts reviewed (52% exclusion rate). In addition, several confounding factors may have contributed to the increased HbA1c observed. Medication changes and lifestyle factors may have contributed to the observed change in HbA1c levels. Exclusion of patients without active prescriptions for insulin may have contributed to a selection bias, as most patients included in the study were veterans with uncontrolled T2DM requiring insulin. Finally, as a retrospective study involving patient records, investigators relied heavily on information provided in patients’ charts (HbA1c, body weight, insulin doses, adverse effects, etc), which may not entirely be accurate and may have been missing other pertinent information.
Conclusions
The daily dose reduction of liraglutide from 1.8 mg to 1.2 mg due to a cost-savings initiative resulted in a HbA1c increase of 0.5% in a 6-month period. Due to HbA1c increases, 41.2% of veterans underwent an insulin dose increase, negating the insulin-sparing role of liraglutide. Although this study further confirms the dose-dependent HbA1c reduction with liraglutide that has been noted in previous trials, long-term prospective studies and cost-effectiveness analyses are warranted to assess the overall clinical significance and other benefits of the change, including its effects on cardiovascular outcomes.
1. American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2019;42(suppl 1):S90-S102. doi:10.2337/dc19-S009
2. Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202-210. doi:10.2337/ds16-0026
3. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi:10.1056/NEJMoa1603827
4. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832. doi:10.1111/dom.12286
5. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056-1064. doi:10.1111/dom.12539
6. Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13(5):592-595. doi:10.1089/dia.2010.0221
7. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-481. doi:10.1016/S0140-6736(08)61246-5.
8. Victoza [package insert]. Princeton: Novo Nordisk Inc; 2020.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are injectable incretin hormones approved for the treatment of type 2 diabetes mellitus (T2DM). They are highly efficacious agents with hemoglobin A1c (HbA1c) reduction potential of approximately 0.8 to 1.6% and mechanisms of action that result in an average weight loss of 1 to 3 kg.1,2 Published in 2016, The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial established cardiovascular benefits associated with liraglutide, making it a preferred GLP-1 RA.3
In addition to HbA1c reduction, weight loss, and cardiovascular benefits, liraglutide also has shown insulin-sparing effects when used in combination with insulin. A trial by Lane and colleagues revealed a 34% decrease in total daily insulin dose 6 months after the addition of liraglutide to insulin in patients with T2DM receiving > 100 units of insulin daily.4 When used in combination with basal insulin analogues (glargine or detemir) similar findings also were shown.5
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, selected liraglutide as its preferred GLP-1 RA because of its favorable glycemic and cardiovascular outcomes. In addition, as part of a cost-savings initiative for fiscal year 2018, liraglutide 6 mg/mL injection 2-count pen packs was selected as the preferred liraglutide product. Before the availability of the 2-count pen packs, veterans previously received 3-count pen packs, which allowed for up to a 30-day supply of liraglutide 1.8 mg daily dosing. However, the cost-efficient 2-count pen packs allow for up to 1.2 mg daily dose of liraglutide for a 30-day supply. Due to these changes, veterans at MEDVAMC were converted from liraglutide 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018.
The primary objective of this study was to assess sustained glycemic control and cost savings that resulted from this change. The secondary objectives were to assess sustained weight loss and adverse effects (AEs).
Methods
This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee. In this single-center study, a retrospective chart review was conducted on veterans with T2DM who underwent a liraglutide dose reduction from 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018. Patients were included if they were aged ≥ 18 years with an active prescription for liraglutide 1.8 mg daily and insulin (with or without other antihyperglycemic agents) at the time of conversion. In addition, patients must have had ≥ 1 HbA1c reading within 3 months of the dose conversion and a follow-up HbA1c within 6 months after the dose conversion. To assess the primary objective of glycemic control that resulted from the liraglutide dose reduction, mean change of HbA1c at time of dose conversion was compared with mean HbA1c 6 months postconversion. To assess savings, cost information was obtained from the US Department of Veterans Affairs (VA) Drug Price Database and monthly and annual costs of liraglutide 6 mg/mL injection 2-count pen pack were compared with that of the 3-count pen pack. A chart review of patients’ electronic health records assessed secondary outcomes. The VA Computerized Patient Record System (CPRS) was used to collect patient data.
Patients and Characteristics
The following patient information was obtained from patients’ records: age, sex, race/ethnicity, diabetic medications (at time of conversion and 6 months after conversion), cardiovascular history and risk factors (hypertension, coronary artery disease, heart failure, arrhythmias, peripheral artery disease, obesity, etc), prescriber type (physician, nurse practitioner/physician assistant, pharmacist, etc), weight (at baseline, at time of conversion, and 6 months after conversion), HbA1c (at baseline, at time of conversion, and 6 months after conversion), average blood glucose (at baseline, at time of conversion, and 6 months after conversion), insulin dose (at time of conversion and 6 months after conversion), and reported AEs.
Statistical Analysis
The 2-tailed, paired t test was used to assess changes in HbA1c, average blood glucose, and body weight. Demographic data and other outcomes were assessed using descriptive statistics.
Results
Prior to the dose reduction, 312 veterans had active prescriptions for liraglutide 1.8 mg daily. Due to lack of glycemic control benefit (failing to achieve a HbA1c reduction of at least 0.5% after at least 3 to 6 months following initiation of therapy) or nonadherence (assessed by medication refill history), 126 veterans did not meet the criteria for the dose conversion. As a result, liraglutide was discontinued, and veterans were sent patient letter notifications and health care providers were notified via medication review notes in the patient electronic health record “to make medication adjustments if warranted. A total of 186 veterans underwent a liraglutide dose reduction between May and August 2018. Thirty-two veterans were without active insulin prescriptions, 53 were without HbA1c results, and 4 veterans died; resulting in 97 veterans who were included in the study (Figure 1).
Most of the patients included in the study were male (90.7%) and White (63.9%) with an average (SD) age of 65.9 years (7.9) and a mean (SD) HbA1c at baseline of 8.4% (1.2). About 56.7% received concurrent T2DM treatment with metformin, and 8.3% received concurrent treatment with empagliflozin. The most common cardiovascular disease/risk factors included hypertension (93.8%), hyperlipidemia (85.6%), and obesity (85.6%) (Table 1).
Glycemic Control and Weight Loss
At the time of conversion, the average (SD) HbA1c was 8.2% (1.4) and increased to an average (SD) of 8.7% (1.8) (P =.0005) 6 months after the dose reduction (Table 2). The average (SD) body weight was 116.2 kg (23.2) at time of conversion and increased to 116.5 (24.6) 6 months following the dose reduction; however, the difference was not statistically significant (P = .8).
As a result of the HbA1c change, 41.2% of veterans underwent an insulin dose increase with dose increase of 5 to 200 units of total daily insulin during the 6-month period. Antihyperglycemic regimen remained unchanged for 40.2% of veterans, while additional glucose lowering agents were initiated in 6 veterans. Medications initiated included empagliflozin in 4 veterans and saxagliptin in 2 veterans.
HbA1c reduction was noted in 33% of veterans (Figure 2) mostly due to improved diet and exercise habits. A majority of veterans, 62%, experienced an increase in HbA1c, whereas 5.2% of veterans maintained the same HbA1c. Of 60 veterans with HbA1c increases, 15 had an increase between 0.1% and 0.5%, another 15 with an increase between 0.5 to 0.9%, and half had HbA1c increases of at least 1% with a maximum increase of 5.1% (Figure 3).
Cost Savings
Cost information was obtained from the VA Drug Price Database. The estimated monthly cost savings per patient associated with the conversion from 3-count to 2-count injection pen packs of liraglutide 6 mg/mL was $103.46. With 186 veterans converted to the 2-count pen packs, MEDVAMC saved $115,461.36 in a 6-month period. The estimated annualized cost savings was estimated to be about $231,000 (Figure 4).
Adverse Effects During the 6-month period following the dose conversion, no major AEs associated with liraglutide were documented. Documented AEs included 3 cases of diarrhea, resulting in the discontinuation of metformin. Metformin also was discontinued in a veteran with worsened renal function and eGFR < 30 mL/min/1.73 m2.
Discussion
According to previous clinical trials, when used in combination with insulin, 1.2 mg and 1.8 mg daily liraglutide showed significant improvement in glycemic control and body weight and was associated with decreased insulin requirements.4-6 However, subgroup analyses were not performed to show differences in benefit between the liraglutide 1.8 mg and 1.2 mg groups.4-6 Similarly, cardiovascular benefit was observed in patients receiving liraglutide 1.2 mg daily and liraglutide 1.8 mg daily in the LEADER trial with no subgroup analysis or distinction between treatment doses.3 With this information and approval by the Veterans Integrated Services Network, the pharmacoeconomics team at MEDVAMC made the decision to select a more cost-efficient preparation and, hence, lower dose of liraglutide.
To ensure that patients only taking liraglutide for glycemic control were captured, patients without insulin therapies at baseline were excluded. Due to concerns of potential off-label use of liraglutide for weight loss, patients without active prescriptions for insulin at baseline were excluded.
A mean HbA1c increase of 0.5% was observed over the 6-month period, supporting findings of a dose-dependent HbA1c decrease observed in clinical trials. In the LEAD-3 MONO trial when used as monotherapy, liraglutide 1.8 mg was associated with significantly greater HbA1c reduction than liraglutide 1.2 mg (–0·29%; –0·50 to –0.09, P = .005) after 52 weeks of treatment.7 Liraglutide 1.8 mg was also associated with higher rates of AEs; particularly gastrointestinal. 7 To minimize these AEs, it is recommended to initiate liraglutide at 0.6 mg daily for a week then increase to 1.2 mg daily. If tolerated, liraglutide can be further titrated to 1.8 mg daily to optimize glycemic control.8 Unsurprisingly, no major AEs were noted in this study, as AEs are typically noted with increased doses.
Despite the observed trend of increased HbA1c, no changes were made to glucoselowering agents in 39 veterans. This group of veterans consisted primarily of those whose HbA1c remained unchanged during the 6-month period, those whose HbA1c improved (with no documented hypoglycemia), and older veterans with less stringent HbA1c goals. As a result, doses of glucose lowering agents were maintained as appropriate.
No significant difference was noted in body weight during the 6-month period. The slight weight gain observed may have been due to several factors. Lack of exercise and dietary changes may have contributed to weight gain. In addition, insulin doses were increased in 40 veterans, which may have contributed to the observed weight gain.
As expected, significant cost savings were achieved as a result of the liraglutide dose reduction. Of note, liraglutide was discontinued in 126 veterans (prior to the dose reduction) due to nonadherence or inadequate response to therapy, which also resulted in additional savings. Although cost savings was achieved, the long-term benefit of this initiative still remains unknown. The worsened glycemic control that was detected may increase the risk of microvascular and macrovascular complications, thereby negating cost savings achieved. To assess this effect, longterm prospective studies are warranted.
Limitations
A number of issues limit these finding, including its retrospective data review, small sample size, additional factors contributing to HbA1c increase, and missing documentation in some patient records. Only 97 patients were included in the study, reflecting less than half of the charts reviewed (52% exclusion rate). In addition, several confounding factors may have contributed to the increased HbA1c observed. Medication changes and lifestyle factors may have contributed to the observed change in HbA1c levels. Exclusion of patients without active prescriptions for insulin may have contributed to a selection bias, as most patients included in the study were veterans with uncontrolled T2DM requiring insulin. Finally, as a retrospective study involving patient records, investigators relied heavily on information provided in patients’ charts (HbA1c, body weight, insulin doses, adverse effects, etc), which may not entirely be accurate and may have been missing other pertinent information.
Conclusions
The daily dose reduction of liraglutide from 1.8 mg to 1.2 mg due to a cost-savings initiative resulted in a HbA1c increase of 0.5% in a 6-month period. Due to HbA1c increases, 41.2% of veterans underwent an insulin dose increase, negating the insulin-sparing role of liraglutide. Although this study further confirms the dose-dependent HbA1c reduction with liraglutide that has been noted in previous trials, long-term prospective studies and cost-effectiveness analyses are warranted to assess the overall clinical significance and other benefits of the change, including its effects on cardiovascular outcomes.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are injectable incretin hormones approved for the treatment of type 2 diabetes mellitus (T2DM). They are highly efficacious agents with hemoglobin A1c (HbA1c) reduction potential of approximately 0.8 to 1.6% and mechanisms of action that result in an average weight loss of 1 to 3 kg.1,2 Published in 2016, The LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial established cardiovascular benefits associated with liraglutide, making it a preferred GLP-1 RA.3
In addition to HbA1c reduction, weight loss, and cardiovascular benefits, liraglutide also has shown insulin-sparing effects when used in combination with insulin. A trial by Lane and colleagues revealed a 34% decrease in total daily insulin dose 6 months after the addition of liraglutide to insulin in patients with T2DM receiving > 100 units of insulin daily.4 When used in combination with basal insulin analogues (glargine or detemir) similar findings also were shown.5
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, selected liraglutide as its preferred GLP-1 RA because of its favorable glycemic and cardiovascular outcomes. In addition, as part of a cost-savings initiative for fiscal year 2018, liraglutide 6 mg/mL injection 2-count pen packs was selected as the preferred liraglutide product. Before the availability of the 2-count pen packs, veterans previously received 3-count pen packs, which allowed for up to a 30-day supply of liraglutide 1.8 mg daily dosing. However, the cost-efficient 2-count pen packs allow for up to 1.2 mg daily dose of liraglutide for a 30-day supply. Due to these changes, veterans at MEDVAMC were converted from liraglutide 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018.
The primary objective of this study was to assess sustained glycemic control and cost savings that resulted from this change. The secondary objectives were to assess sustained weight loss and adverse effects (AEs).
Methods
This study was approved by the MEDVAMC Quality Assurance and Regulatory Affairs committee. In this single-center study, a retrospective chart review was conducted on veterans with T2DM who underwent a liraglutide dose reduction from 1.8 mg daily to 1.2 mg daily between May 2018 and August 2018. Patients were included if they were aged ≥ 18 years with an active prescription for liraglutide 1.8 mg daily and insulin (with or without other antihyperglycemic agents) at the time of conversion. In addition, patients must have had ≥ 1 HbA1c reading within 3 months of the dose conversion and a follow-up HbA1c within 6 months after the dose conversion. To assess the primary objective of glycemic control that resulted from the liraglutide dose reduction, mean change of HbA1c at time of dose conversion was compared with mean HbA1c 6 months postconversion. To assess savings, cost information was obtained from the US Department of Veterans Affairs (VA) Drug Price Database and monthly and annual costs of liraglutide 6 mg/mL injection 2-count pen pack were compared with that of the 3-count pen pack. A chart review of patients’ electronic health records assessed secondary outcomes. The VA Computerized Patient Record System (CPRS) was used to collect patient data.
Patients and Characteristics
The following patient information was obtained from patients’ records: age, sex, race/ethnicity, diabetic medications (at time of conversion and 6 months after conversion), cardiovascular history and risk factors (hypertension, coronary artery disease, heart failure, arrhythmias, peripheral artery disease, obesity, etc), prescriber type (physician, nurse practitioner/physician assistant, pharmacist, etc), weight (at baseline, at time of conversion, and 6 months after conversion), HbA1c (at baseline, at time of conversion, and 6 months after conversion), average blood glucose (at baseline, at time of conversion, and 6 months after conversion), insulin dose (at time of conversion and 6 months after conversion), and reported AEs.
Statistical Analysis
The 2-tailed, paired t test was used to assess changes in HbA1c, average blood glucose, and body weight. Demographic data and other outcomes were assessed using descriptive statistics.
Results
Prior to the dose reduction, 312 veterans had active prescriptions for liraglutide 1.8 mg daily. Due to lack of glycemic control benefit (failing to achieve a HbA1c reduction of at least 0.5% after at least 3 to 6 months following initiation of therapy) or nonadherence (assessed by medication refill history), 126 veterans did not meet the criteria for the dose conversion. As a result, liraglutide was discontinued, and veterans were sent patient letter notifications and health care providers were notified via medication review notes in the patient electronic health record “to make medication adjustments if warranted. A total of 186 veterans underwent a liraglutide dose reduction between May and August 2018. Thirty-two veterans were without active insulin prescriptions, 53 were without HbA1c results, and 4 veterans died; resulting in 97 veterans who were included in the study (Figure 1).
Most of the patients included in the study were male (90.7%) and White (63.9%) with an average (SD) age of 65.9 years (7.9) and a mean (SD) HbA1c at baseline of 8.4% (1.2). About 56.7% received concurrent T2DM treatment with metformin, and 8.3% received concurrent treatment with empagliflozin. The most common cardiovascular disease/risk factors included hypertension (93.8%), hyperlipidemia (85.6%), and obesity (85.6%) (Table 1).
Glycemic Control and Weight Loss
At the time of conversion, the average (SD) HbA1c was 8.2% (1.4) and increased to an average (SD) of 8.7% (1.8) (P =.0005) 6 months after the dose reduction (Table 2). The average (SD) body weight was 116.2 kg (23.2) at time of conversion and increased to 116.5 (24.6) 6 months following the dose reduction; however, the difference was not statistically significant (P = .8).
As a result of the HbA1c change, 41.2% of veterans underwent an insulin dose increase with dose increase of 5 to 200 units of total daily insulin during the 6-month period. Antihyperglycemic regimen remained unchanged for 40.2% of veterans, while additional glucose lowering agents were initiated in 6 veterans. Medications initiated included empagliflozin in 4 veterans and saxagliptin in 2 veterans.
HbA1c reduction was noted in 33% of veterans (Figure 2) mostly due to improved diet and exercise habits. A majority of veterans, 62%, experienced an increase in HbA1c, whereas 5.2% of veterans maintained the same HbA1c. Of 60 veterans with HbA1c increases, 15 had an increase between 0.1% and 0.5%, another 15 with an increase between 0.5 to 0.9%, and half had HbA1c increases of at least 1% with a maximum increase of 5.1% (Figure 3).
Cost Savings
Cost information was obtained from the VA Drug Price Database. The estimated monthly cost savings per patient associated with the conversion from 3-count to 2-count injection pen packs of liraglutide 6 mg/mL was $103.46. With 186 veterans converted to the 2-count pen packs, MEDVAMC saved $115,461.36 in a 6-month period. The estimated annualized cost savings was estimated to be about $231,000 (Figure 4).
Adverse Effects During the 6-month period following the dose conversion, no major AEs associated with liraglutide were documented. Documented AEs included 3 cases of diarrhea, resulting in the discontinuation of metformin. Metformin also was discontinued in a veteran with worsened renal function and eGFR < 30 mL/min/1.73 m2.
Discussion
According to previous clinical trials, when used in combination with insulin, 1.2 mg and 1.8 mg daily liraglutide showed significant improvement in glycemic control and body weight and was associated with decreased insulin requirements.4-6 However, subgroup analyses were not performed to show differences in benefit between the liraglutide 1.8 mg and 1.2 mg groups.4-6 Similarly, cardiovascular benefit was observed in patients receiving liraglutide 1.2 mg daily and liraglutide 1.8 mg daily in the LEADER trial with no subgroup analysis or distinction between treatment doses.3 With this information and approval by the Veterans Integrated Services Network, the pharmacoeconomics team at MEDVAMC made the decision to select a more cost-efficient preparation and, hence, lower dose of liraglutide.
To ensure that patients only taking liraglutide for glycemic control were captured, patients without insulin therapies at baseline were excluded. Due to concerns of potential off-label use of liraglutide for weight loss, patients without active prescriptions for insulin at baseline were excluded.
A mean HbA1c increase of 0.5% was observed over the 6-month period, supporting findings of a dose-dependent HbA1c decrease observed in clinical trials. In the LEAD-3 MONO trial when used as monotherapy, liraglutide 1.8 mg was associated with significantly greater HbA1c reduction than liraglutide 1.2 mg (–0·29%; –0·50 to –0.09, P = .005) after 52 weeks of treatment.7 Liraglutide 1.8 mg was also associated with higher rates of AEs; particularly gastrointestinal. 7 To minimize these AEs, it is recommended to initiate liraglutide at 0.6 mg daily for a week then increase to 1.2 mg daily. If tolerated, liraglutide can be further titrated to 1.8 mg daily to optimize glycemic control.8 Unsurprisingly, no major AEs were noted in this study, as AEs are typically noted with increased doses.
Despite the observed trend of increased HbA1c, no changes were made to glucoselowering agents in 39 veterans. This group of veterans consisted primarily of those whose HbA1c remained unchanged during the 6-month period, those whose HbA1c improved (with no documented hypoglycemia), and older veterans with less stringent HbA1c goals. As a result, doses of glucose lowering agents were maintained as appropriate.
No significant difference was noted in body weight during the 6-month period. The slight weight gain observed may have been due to several factors. Lack of exercise and dietary changes may have contributed to weight gain. In addition, insulin doses were increased in 40 veterans, which may have contributed to the observed weight gain.
As expected, significant cost savings were achieved as a result of the liraglutide dose reduction. Of note, liraglutide was discontinued in 126 veterans (prior to the dose reduction) due to nonadherence or inadequate response to therapy, which also resulted in additional savings. Although cost savings was achieved, the long-term benefit of this initiative still remains unknown. The worsened glycemic control that was detected may increase the risk of microvascular and macrovascular complications, thereby negating cost savings achieved. To assess this effect, longterm prospective studies are warranted.
Limitations
A number of issues limit these finding, including its retrospective data review, small sample size, additional factors contributing to HbA1c increase, and missing documentation in some patient records. Only 97 patients were included in the study, reflecting less than half of the charts reviewed (52% exclusion rate). In addition, several confounding factors may have contributed to the increased HbA1c observed. Medication changes and lifestyle factors may have contributed to the observed change in HbA1c levels. Exclusion of patients without active prescriptions for insulin may have contributed to a selection bias, as most patients included in the study were veterans with uncontrolled T2DM requiring insulin. Finally, as a retrospective study involving patient records, investigators relied heavily on information provided in patients’ charts (HbA1c, body weight, insulin doses, adverse effects, etc), which may not entirely be accurate and may have been missing other pertinent information.
Conclusions
The daily dose reduction of liraglutide from 1.8 mg to 1.2 mg due to a cost-savings initiative resulted in a HbA1c increase of 0.5% in a 6-month period. Due to HbA1c increases, 41.2% of veterans underwent an insulin dose increase, negating the insulin-sparing role of liraglutide. Although this study further confirms the dose-dependent HbA1c reduction with liraglutide that has been noted in previous trials, long-term prospective studies and cost-effectiveness analyses are warranted to assess the overall clinical significance and other benefits of the change, including its effects on cardiovascular outcomes.
1. American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2019;42(suppl 1):S90-S102. doi:10.2337/dc19-S009
2. Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202-210. doi:10.2337/ds16-0026
3. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi:10.1056/NEJMoa1603827
4. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832. doi:10.1111/dom.12286
5. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056-1064. doi:10.1111/dom.12539
6. Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13(5):592-595. doi:10.1089/dia.2010.0221
7. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-481. doi:10.1016/S0140-6736(08)61246-5.
8. Victoza [package insert]. Princeton: Novo Nordisk Inc; 2020.
1. American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2019;42(suppl 1):S90-S102. doi:10.2337/dc19-S009
2. Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30(3):202-210. doi:10.2337/ds16-0026
3. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi:10.1056/NEJMoa1603827
4. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832. doi:10.1111/dom.12286
5. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17(11):1056-1064. doi:10.1111/dom.12539
6. Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13(5):592-595. doi:10.1089/dia.2010.0221
7. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473-481. doi:10.1016/S0140-6736(08)61246-5.
8. Victoza [package insert]. Princeton: Novo Nordisk Inc; 2020.