User login
How should low-density lipoprotein cholesterol concentration be determined?
- The C-LDL-C remains the method of choice for LDL-C determination.
- The D-LDL-C has not been adequately standardized and was not used in the clinical trials which were the basis for the current NCEP-ATP III recommendations.
- Some D-LDL-C assays may give significantly different results from those of the C-LDL-C.
- Some D-LDL-C assays do not perform well in hypertriglyceridemia, the very situation for which they are advocated.
- Use of the D-LDL-C increases cost without evidence of benefit.
The National Cholesterol Education Program Adult Treatment Panel III Report (NCEP-ATP III) has identified low-density lipoprotein cholesterol (LDL-C) as the primary target of therapy.1,2The Friedewald calculated LDL-C (C-LDL-C) is the preferred method2,3 and is calculated with the following equation:
LDL-C =TC – HDL-C – TG/5
where TC is total cholesterol concentration, HDL-C is high-density lipoprotein cholesterol concentration, and TG is triglyceride concentration. The complete NCEP-ATP III report has indicated that methods to directly measure LDL-C (D-LDL-C) in the non-fasting state have been developed and will grow in use but require careful quality control.2Our VA hospital clinical laboratory is 1 of 10 hospitals in the South Central VA Health Care Network that routinely reports D-LDL-C rather than C-LDL-C levels to clinicians. Telephone calls to 4 other research and clinical laboratories found that all are using D-LDL-C to some extent.
D-LDL-C assays correlate variably with C-LDL-C measurements used in research studies.4-17The purported advantages of such measurements are that fasting is not required and that D-LDL-C may be determined in patients with serum triglyceride levels greater than 400 mg/dL when the C-HDL-C and are less reliable. However, clinical trials demonstrating benefit of lowering LDL-C with drug therapy used the C-LDL-C.18-22Only the recently reported Heart Protection Study used a non-fasting D-LDL-C.23Thus, it is important in practicing evidence-based medicine to demonstrate that the D-LDL-C measurements are comparable to those of the C-LDL-C. The present study determined how the D-LDL-C correlated with C-LDL-C and how such a correlation would affect treatment decisions based on the NCEP-ATP III guidelines.
Methods
Data from all patients with a lipid panel during a single week were analyzed. Patients with triglyceride levels above 1000 mg/dL were excluded. Thirty-four patients with triglyceride levels between 400 and 1000 mg/dL were analyzed separately. A C-LDL-C was determined and compared with the D-LDL-C in all 464 patients. Total cholesterol, triglyceride, and HDL-C measurements were done with an autoanalyzer. D-LDL-C was measured with Sigma Diagnostics EZ LDL Cholesterol, procedure 358 (Sigma, St. Louis, MO). Linear regression was performed using Microsoft Excel (Microsoft Corporation, Redmond, WA).
Results
The samples in this study represented the expected distribution of LDL-C concentrations seen in a clinical practice of predominantly male veterans. Of the 464 patient samples with triglyceride levels below 400 mg/dL, the mean C-LDL-C was 123 mg/dL. Twenty-eight percent had a C-LDL-C below 100 mg/dL, 32% had a C-LDL-C of 100 to 129.9 mg/dL, 24% had a C-LDL-C of 130 to 159.9 mg/dL, 12% had a C-LDL-C of 160 to 189.9 mg/dL, and 4% had a C-LDL-C above 190 mg/dL.
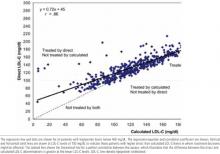
The Figure shows the correlation between the C-LDL-C and D-LDL-C in all patients with triglyceride levels below 400 mg/dL. Although there is a strong correlation between the C-LDL-C and D-LDL-C (r = .86), the regression line does not go through 0. A C-LDL-C of 100 mg/dL or lower is the NCEP-ATP III goal for patients with known coronary heart disease (CHD) and other clinical forms of atherosclerotic disease, diabetes, or multiple risk factors that confer a 10-year risk for CHD greater than 20%.1At this cutoff for C-LDL-C, the D-LDL-C derived from the regression line is 118 mg/dL. At a C-LDL-C of 160 mg/dL, the 2 values are comparable; at a C-LDL-C of 190 mg/dL, the D-LDL-C is slightly lower at 182 mg/dL. This is demonstrated graphically in the Figure by a dashed line indicating a perfect correlation between the 2 methods. The Figure also displays vertical and horizontal lines through an LDL-C of 100 mg/dL, the level above which drug therapy is likely to be started or increased in patients with CHD or CHD risk equivalents. This partition illustrates those patients who would require treatment when using the 100 mg/dL treatment goal by the C-LDL-C, the D-LDLC, neither, or both. This is also shown in the (Table, which shows the number of patients who would be treated with the LDL-C cutoffs for treatment recommended by NCEP-ATP III. At an LDL cutoff of 100 mg/dL, 60 patients (13% of total) would be treated with the D-LDL-C and not the C-LDL-C, whereas only 2 patients (<1%) would be treated with the C-LDL-C and not with the D-LDL-C. The results are similar when using a 130 mg/dL cutoff for treatment. Thus, treatment decisions based on the D-LDL-C results in many patients being treated who would not have been treated when using the C-LDL-C.
To determine whether triglyceride concentration influences treatment decisions by either method of LDL-C measurement, similar correlations and analyses were done on the data according to the following triglyceride groupings: <100 mg/dL, 100 to 199 mg/dL, 200 to 299 mg/dL, 300 to 399 mg/dL, and >400 mg/dL. This was further evaluated by plotting triglyceride vs D-LDL-C and triglyceride vs C-LDL-C (data not shown). Whereas the C-LDL-C showed no correlation with triglyceride, the DLDL-C showed a statistically significant correlation with triglyceride concentrations (r = .27), indicating that D-LDL-C increases at higher triglyceride levels. This suggested an influence of triglyceride on the D-LDL-C assay. This has been reported by others in 3 of 4 different D-LDL-C assays including the Sigma assay.15 However, alterations in treatment possibilities when using the D-LDL-C are present at all triglyceride concentrations.
FIGURE 1
Direct vs calculated LDL-C (mg/dL)
TABLE
Effect of LDL assay by LDL treatment cutoff*
| Patients who might be treated, n (%) | Additional patients who might be treated, n (%) | |||
|---|---|---|---|---|
| LDL cutoff for treatment, mg/dL | Calculated LDL | Direct LDL | Calculated, not direct, LDL | Direct, not calculated, LDL |
| >100 | 334 (72) | 393 (85) | 2 (<1) | 60 (13) |
| >130 | 185 (40) | 237 (51) | 2 (<1) | 55 (12) |
| >160 | 71 (15) | 87 (19) | 6 (1) | 21 (5) |
| *N = 464 patients. | ||||
| LDL, low-density lipoprotein. | ||||
Discussion
LDL-C has been identified in the NCEP-ATP III as the primary target of therapy. Treatment recommendations for high LDL-C are based on low, moderate, or high risk for CHD, with treatment goals of 160, 130, and 100 mg/dL, respectively.1,2
These evidence-based recommendations rely on data from clinical trials demonstrating prevention of CHD events by lowering LDL-C, all of which, with the exception of the recently reported Heart Protection Study, used the CLDL-C.18-23 Thus, important treatment decisions depend on this estimated LDL-C, and systematic deviations from the C-LDL-C will affect treatment decisions and cost.
The Sigma EZ LDL D-LDL-C assay in our hospital produces higher LDL-C levels than the C-LDL-C in a range of 100 to 160 mg/dL, the range of most common concern to clinicians. This results in inappropriate treatment or intensification in treatment according to the NCEP-ATP III guidelines. The DLDL-C was higher than the C-LDL-C at all triglyceride levels, but the error was greater for hypertriglyceridemia, the very situation for which it has been advocated.
Previous publications using a D-LDL-C assay have emphasized the correlation between the DLDL-C assay and research LDL-C determinations rather than the correlation with the C-LDL-C.5-8 Other investigators have observed a similar tendency for higher D-LDL-C than C-LDL-C measurements at an LDL-C of 100 mg/dL17 and a positive bias at higher triglyceride levels.14 Although C-LDLC was often performed, data similar to those shown in the Figure, ie, the simple correlation between the D-LDL-C and C-LDL-C, have not been presented. Two very recent reviews have suggested caution in routinely implementing the D-LDL-C assays and pointed out the considerable variation from one assay to another.14,15 Laboratories often change their assay method; in fact, our hospital laboratory has recently changed to a different DLDL-C method.
Physicians and institutions should be cautious about using a D-LDL-C method as a substitute for the C-LDL-C. First, it has not been standardized in large populations and, with the exception of the recent Heart Protection Study,23 has not been used in large clinical trials demonstrating the benefits of lowering LDL-C. Although the C-LDL-C has been recommended by the NCEP-ATP III,2 the Executive Summary of these guidelines did not address the method for measuring LDL-C.1 Second, cost is increased from the additional therapy and performing the D-LDL-C assay. Third, the major reasons proposed for using a D-LDL-C assay (lack of need for a fasting specimen and usefulness at triglyceride > 400 mg/dL) may not be valid or relevant. Variation in the LDL-C due to hypertriglyceridemia occurs with the D-LDL-C. In addition, the NCEP-ATP III report emphasized triglyceride and recommended a fasting lipid panel including total cholesterol, triglycerides, and HDL-C.1,2 One limitation of this study is the inclusion of predominantly male veterans. There may be populations, not considered in this study, that have an abnormal lipoprotein composition that significantly affects the C-LDL-C.
Conclusions
The C-LDL-C should remain the method of choice for LDL-C determinations because (1) this assay was used in clinical trials documenting the benefits of cholesterol-lowering therapy and (2) use of the D-LDL-C increases cost without evidence of benefit. Further studies are needed to standardize the direct LDL-C assays, and outcome trials using these assays need to be performed.
ACKNOWLEDGMENTS
This work was supported in part by the Biomedical Research Foundation of Arkansas and the Central Arkansas Veterans Healthcare System.
1. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97.
2. National Cholesterol Education Program. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), full report, manuscript version, 2001. Available at: www.nhlbi.nih.gov/guide-lines/cholesterol/atp3_rpt.htm. Accessed April 2, 2002.
3. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502.
4. Cobbaert C, Broodman I, Swart GR, et al. Performance of a direct, immunoseparation based LDL-cholesterol method compared to Friedewald calculation and a polyvinyl sulphate precipitation method. Eur J Clin Chem Clin Biochem. 1995;33:417-24.
5. Jialal I, Hirany SV, Devaraj S, et al. Comparison of an immunoprecipitation method for direct measurement of LDL- cholesterol with beta-quantification (ultracentrifugation). Am J Clin Pathol 1995;104:76-81.
6. Nauck M, Graziani MS, Bruton D. et al: Analytical and clinical performance of a detergent based homogeneous LDL-cholesterol assay: a multicenter evaluation Clin Chem 2000;46:506-14.
7. Hirany S, Li D, Jialal I. A more valid measurement of low-density lipoprotein cholesterol in diabetic patients Am J Med 1997;102:48-53.
8. Whiting MJ, Shephard MDS, Tallis GA. Measurement of plasma LDL cholesterol in patients with diabetes Diabetes Care. 1997;20:12-4.
9. McNamara JR, Cole TG, Contois JH, et al. Immunoseparation method for measuring low density lipoprotein cholesterol directly from serum evaluated. Clin Chem 1995;41:232-40.
10. Pisani T, Gebski CP, Leary ET, et al. Accurate direct determination of lowdensity lipoprotein cholesterol using an immunoseparation reagent and enzymatic cholesterol assay. Arch Pathol Lab Med 1995;119:1127-35.
11. Maitra A, Hirany SV, Jialal I. Comparison of two assays for measuring LDL cholesterol Clin Chem 1997;43:1040-7.
12. Yu HH, Markowitz R, De Ferranti SD, et al. Direct measurement of LDL-C in children performance of two surfactant-based methods in a general pediatric population. Clin Biochem 2000;95:89-95.
13. Sakaue T, Hirano T, Yoshino G, et al. Reactions of direct LDL-cho-lesterol assays with pure LDL fraction and IDL-comparison of three homogeneous methods. Clin Chim Acta 2000;295:97-106.
14. Nauck M, Warnick GR, Rifai N. Methods for measurement of LDL-cholesterol a critical assessment of direct measurement by homogeneous assays versus calculation. Clin Chem 2002;48:236-54.
15. Miller WG, Waymack PP, Anderson FP, Ethridge SF, Jayne EC. Performance of four homogeneous direct methods for LDL-cholesterol. Clin Chem 2002;48:489-98.
16. Smets EML, Pequerlaux NCV, Blaton V, Goldschmidt HMJ. Analytical performance of a direct assay for LDL-cholesterol. Clin Chem Lab Med 2001;39:270-80.
17. Yu HH, Ginsburg GS, Harris N, Rifai N. Evaluation and clinical application of a direct low-density lipoprotein cholesterol assay in normolipidemic and hyperlipidemic adults. Am J Cardiol 1997;80:1295-99.
18. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995;333:1301-7.
19. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279:1615-22.
20. Scandinavian Simvastatin Survival Study Group. Design and baseline results of the Scandinavian Simvastatin Survival Study of patients with stable angina and/or previous myocardial infarction Am J Cardiol 1993;71:393-400.
21. Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels N Engl J Med 1998;339:1349-57.
22. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-9.
23. MRC/BHF Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trial Lancet 2002;360:7-22.
- The C-LDL-C remains the method of choice for LDL-C determination.
- The D-LDL-C has not been adequately standardized and was not used in the clinical trials which were the basis for the current NCEP-ATP III recommendations.
- Some D-LDL-C assays may give significantly different results from those of the C-LDL-C.
- Some D-LDL-C assays do not perform well in hypertriglyceridemia, the very situation for which they are advocated.
- Use of the D-LDL-C increases cost without evidence of benefit.
The National Cholesterol Education Program Adult Treatment Panel III Report (NCEP-ATP III) has identified low-density lipoprotein cholesterol (LDL-C) as the primary target of therapy.1,2The Friedewald calculated LDL-C (C-LDL-C) is the preferred method2,3 and is calculated with the following equation:
LDL-C =TC – HDL-C – TG/5
where TC is total cholesterol concentration, HDL-C is high-density lipoprotein cholesterol concentration, and TG is triglyceride concentration. The complete NCEP-ATP III report has indicated that methods to directly measure LDL-C (D-LDL-C) in the non-fasting state have been developed and will grow in use but require careful quality control.2Our VA hospital clinical laboratory is 1 of 10 hospitals in the South Central VA Health Care Network that routinely reports D-LDL-C rather than C-LDL-C levels to clinicians. Telephone calls to 4 other research and clinical laboratories found that all are using D-LDL-C to some extent.
D-LDL-C assays correlate variably with C-LDL-C measurements used in research studies.4-17The purported advantages of such measurements are that fasting is not required and that D-LDL-C may be determined in patients with serum triglyceride levels greater than 400 mg/dL when the C-HDL-C and are less reliable. However, clinical trials demonstrating benefit of lowering LDL-C with drug therapy used the C-LDL-C.18-22Only the recently reported Heart Protection Study used a non-fasting D-LDL-C.23Thus, it is important in practicing evidence-based medicine to demonstrate that the D-LDL-C measurements are comparable to those of the C-LDL-C. The present study determined how the D-LDL-C correlated with C-LDL-C and how such a correlation would affect treatment decisions based on the NCEP-ATP III guidelines.
Methods
Data from all patients with a lipid panel during a single week were analyzed. Patients with triglyceride levels above 1000 mg/dL were excluded. Thirty-four patients with triglyceride levels between 400 and 1000 mg/dL were analyzed separately. A C-LDL-C was determined and compared with the D-LDL-C in all 464 patients. Total cholesterol, triglyceride, and HDL-C measurements were done with an autoanalyzer. D-LDL-C was measured with Sigma Diagnostics EZ LDL Cholesterol, procedure 358 (Sigma, St. Louis, MO). Linear regression was performed using Microsoft Excel (Microsoft Corporation, Redmond, WA).
Results
The samples in this study represented the expected distribution of LDL-C concentrations seen in a clinical practice of predominantly male veterans. Of the 464 patient samples with triglyceride levels below 400 mg/dL, the mean C-LDL-C was 123 mg/dL. Twenty-eight percent had a C-LDL-C below 100 mg/dL, 32% had a C-LDL-C of 100 to 129.9 mg/dL, 24% had a C-LDL-C of 130 to 159.9 mg/dL, 12% had a C-LDL-C of 160 to 189.9 mg/dL, and 4% had a C-LDL-C above 190 mg/dL.
The Figure shows the correlation between the C-LDL-C and D-LDL-C in all patients with triglyceride levels below 400 mg/dL. Although there is a strong correlation between the C-LDL-C and D-LDL-C (r = .86), the regression line does not go through 0. A C-LDL-C of 100 mg/dL or lower is the NCEP-ATP III goal for patients with known coronary heart disease (CHD) and other clinical forms of atherosclerotic disease, diabetes, or multiple risk factors that confer a 10-year risk for CHD greater than 20%.1At this cutoff for C-LDL-C, the D-LDL-C derived from the regression line is 118 mg/dL. At a C-LDL-C of 160 mg/dL, the 2 values are comparable; at a C-LDL-C of 190 mg/dL, the D-LDL-C is slightly lower at 182 mg/dL. This is demonstrated graphically in the Figure by a dashed line indicating a perfect correlation between the 2 methods. The Figure also displays vertical and horizontal lines through an LDL-C of 100 mg/dL, the level above which drug therapy is likely to be started or increased in patients with CHD or CHD risk equivalents. This partition illustrates those patients who would require treatment when using the 100 mg/dL treatment goal by the C-LDL-C, the D-LDLC, neither, or both. This is also shown in the (Table, which shows the number of patients who would be treated with the LDL-C cutoffs for treatment recommended by NCEP-ATP III. At an LDL cutoff of 100 mg/dL, 60 patients (13% of total) would be treated with the D-LDL-C and not the C-LDL-C, whereas only 2 patients (<1%) would be treated with the C-LDL-C and not with the D-LDL-C. The results are similar when using a 130 mg/dL cutoff for treatment. Thus, treatment decisions based on the D-LDL-C results in many patients being treated who would not have been treated when using the C-LDL-C.
To determine whether triglyceride concentration influences treatment decisions by either method of LDL-C measurement, similar correlations and analyses were done on the data according to the following triglyceride groupings: <100 mg/dL, 100 to 199 mg/dL, 200 to 299 mg/dL, 300 to 399 mg/dL, and >400 mg/dL. This was further evaluated by plotting triglyceride vs D-LDL-C and triglyceride vs C-LDL-C (data not shown). Whereas the C-LDL-C showed no correlation with triglyceride, the DLDL-C showed a statistically significant correlation with triglyceride concentrations (r = .27), indicating that D-LDL-C increases at higher triglyceride levels. This suggested an influence of triglyceride on the D-LDL-C assay. This has been reported by others in 3 of 4 different D-LDL-C assays including the Sigma assay.15 However, alterations in treatment possibilities when using the D-LDL-C are present at all triglyceride concentrations.
FIGURE 1
Direct vs calculated LDL-C (mg/dL)
TABLE
Effect of LDL assay by LDL treatment cutoff*
| Patients who might be treated, n (%) | Additional patients who might be treated, n (%) | |||
|---|---|---|---|---|
| LDL cutoff for treatment, mg/dL | Calculated LDL | Direct LDL | Calculated, not direct, LDL | Direct, not calculated, LDL |
| >100 | 334 (72) | 393 (85) | 2 (<1) | 60 (13) |
| >130 | 185 (40) | 237 (51) | 2 (<1) | 55 (12) |
| >160 | 71 (15) | 87 (19) | 6 (1) | 21 (5) |
| *N = 464 patients. | ||||
| LDL, low-density lipoprotein. | ||||
Discussion
LDL-C has been identified in the NCEP-ATP III as the primary target of therapy. Treatment recommendations for high LDL-C are based on low, moderate, or high risk for CHD, with treatment goals of 160, 130, and 100 mg/dL, respectively.1,2
These evidence-based recommendations rely on data from clinical trials demonstrating prevention of CHD events by lowering LDL-C, all of which, with the exception of the recently reported Heart Protection Study, used the CLDL-C.18-23 Thus, important treatment decisions depend on this estimated LDL-C, and systematic deviations from the C-LDL-C will affect treatment decisions and cost.
The Sigma EZ LDL D-LDL-C assay in our hospital produces higher LDL-C levels than the C-LDL-C in a range of 100 to 160 mg/dL, the range of most common concern to clinicians. This results in inappropriate treatment or intensification in treatment according to the NCEP-ATP III guidelines. The DLDL-C was higher than the C-LDL-C at all triglyceride levels, but the error was greater for hypertriglyceridemia, the very situation for which it has been advocated.
Previous publications using a D-LDL-C assay have emphasized the correlation between the DLDL-C assay and research LDL-C determinations rather than the correlation with the C-LDL-C.5-8 Other investigators have observed a similar tendency for higher D-LDL-C than C-LDL-C measurements at an LDL-C of 100 mg/dL17 and a positive bias at higher triglyceride levels.14 Although C-LDLC was often performed, data similar to those shown in the Figure, ie, the simple correlation between the D-LDL-C and C-LDL-C, have not been presented. Two very recent reviews have suggested caution in routinely implementing the D-LDL-C assays and pointed out the considerable variation from one assay to another.14,15 Laboratories often change their assay method; in fact, our hospital laboratory has recently changed to a different DLDL-C method.
Physicians and institutions should be cautious about using a D-LDL-C method as a substitute for the C-LDL-C. First, it has not been standardized in large populations and, with the exception of the recent Heart Protection Study,23 has not been used in large clinical trials demonstrating the benefits of lowering LDL-C. Although the C-LDL-C has been recommended by the NCEP-ATP III,2 the Executive Summary of these guidelines did not address the method for measuring LDL-C.1 Second, cost is increased from the additional therapy and performing the D-LDL-C assay. Third, the major reasons proposed for using a D-LDL-C assay (lack of need for a fasting specimen and usefulness at triglyceride > 400 mg/dL) may not be valid or relevant. Variation in the LDL-C due to hypertriglyceridemia occurs with the D-LDL-C. In addition, the NCEP-ATP III report emphasized triglyceride and recommended a fasting lipid panel including total cholesterol, triglycerides, and HDL-C.1,2 One limitation of this study is the inclusion of predominantly male veterans. There may be populations, not considered in this study, that have an abnormal lipoprotein composition that significantly affects the C-LDL-C.
Conclusions
The C-LDL-C should remain the method of choice for LDL-C determinations because (1) this assay was used in clinical trials documenting the benefits of cholesterol-lowering therapy and (2) use of the D-LDL-C increases cost without evidence of benefit. Further studies are needed to standardize the direct LDL-C assays, and outcome trials using these assays need to be performed.
ACKNOWLEDGMENTS
This work was supported in part by the Biomedical Research Foundation of Arkansas and the Central Arkansas Veterans Healthcare System.
- The C-LDL-C remains the method of choice for LDL-C determination.
- The D-LDL-C has not been adequately standardized and was not used in the clinical trials which were the basis for the current NCEP-ATP III recommendations.
- Some D-LDL-C assays may give significantly different results from those of the C-LDL-C.
- Some D-LDL-C assays do not perform well in hypertriglyceridemia, the very situation for which they are advocated.
- Use of the D-LDL-C increases cost without evidence of benefit.
The National Cholesterol Education Program Adult Treatment Panel III Report (NCEP-ATP III) has identified low-density lipoprotein cholesterol (LDL-C) as the primary target of therapy.1,2The Friedewald calculated LDL-C (C-LDL-C) is the preferred method2,3 and is calculated with the following equation:
LDL-C =TC – HDL-C – TG/5
where TC is total cholesterol concentration, HDL-C is high-density lipoprotein cholesterol concentration, and TG is triglyceride concentration. The complete NCEP-ATP III report has indicated that methods to directly measure LDL-C (D-LDL-C) in the non-fasting state have been developed and will grow in use but require careful quality control.2Our VA hospital clinical laboratory is 1 of 10 hospitals in the South Central VA Health Care Network that routinely reports D-LDL-C rather than C-LDL-C levels to clinicians. Telephone calls to 4 other research and clinical laboratories found that all are using D-LDL-C to some extent.
D-LDL-C assays correlate variably with C-LDL-C measurements used in research studies.4-17The purported advantages of such measurements are that fasting is not required and that D-LDL-C may be determined in patients with serum triglyceride levels greater than 400 mg/dL when the C-HDL-C and are less reliable. However, clinical trials demonstrating benefit of lowering LDL-C with drug therapy used the C-LDL-C.18-22Only the recently reported Heart Protection Study used a non-fasting D-LDL-C.23Thus, it is important in practicing evidence-based medicine to demonstrate that the D-LDL-C measurements are comparable to those of the C-LDL-C. The present study determined how the D-LDL-C correlated with C-LDL-C and how such a correlation would affect treatment decisions based on the NCEP-ATP III guidelines.
Methods
Data from all patients with a lipid panel during a single week were analyzed. Patients with triglyceride levels above 1000 mg/dL were excluded. Thirty-four patients with triglyceride levels between 400 and 1000 mg/dL were analyzed separately. A C-LDL-C was determined and compared with the D-LDL-C in all 464 patients. Total cholesterol, triglyceride, and HDL-C measurements were done with an autoanalyzer. D-LDL-C was measured with Sigma Diagnostics EZ LDL Cholesterol, procedure 358 (Sigma, St. Louis, MO). Linear regression was performed using Microsoft Excel (Microsoft Corporation, Redmond, WA).
Results
The samples in this study represented the expected distribution of LDL-C concentrations seen in a clinical practice of predominantly male veterans. Of the 464 patient samples with triglyceride levels below 400 mg/dL, the mean C-LDL-C was 123 mg/dL. Twenty-eight percent had a C-LDL-C below 100 mg/dL, 32% had a C-LDL-C of 100 to 129.9 mg/dL, 24% had a C-LDL-C of 130 to 159.9 mg/dL, 12% had a C-LDL-C of 160 to 189.9 mg/dL, and 4% had a C-LDL-C above 190 mg/dL.
The Figure shows the correlation between the C-LDL-C and D-LDL-C in all patients with triglyceride levels below 400 mg/dL. Although there is a strong correlation between the C-LDL-C and D-LDL-C (r = .86), the regression line does not go through 0. A C-LDL-C of 100 mg/dL or lower is the NCEP-ATP III goal for patients with known coronary heart disease (CHD) and other clinical forms of atherosclerotic disease, diabetes, or multiple risk factors that confer a 10-year risk for CHD greater than 20%.1At this cutoff for C-LDL-C, the D-LDL-C derived from the regression line is 118 mg/dL. At a C-LDL-C of 160 mg/dL, the 2 values are comparable; at a C-LDL-C of 190 mg/dL, the D-LDL-C is slightly lower at 182 mg/dL. This is demonstrated graphically in the Figure by a dashed line indicating a perfect correlation between the 2 methods. The Figure also displays vertical and horizontal lines through an LDL-C of 100 mg/dL, the level above which drug therapy is likely to be started or increased in patients with CHD or CHD risk equivalents. This partition illustrates those patients who would require treatment when using the 100 mg/dL treatment goal by the C-LDL-C, the D-LDLC, neither, or both. This is also shown in the (Table, which shows the number of patients who would be treated with the LDL-C cutoffs for treatment recommended by NCEP-ATP III. At an LDL cutoff of 100 mg/dL, 60 patients (13% of total) would be treated with the D-LDL-C and not the C-LDL-C, whereas only 2 patients (<1%) would be treated with the C-LDL-C and not with the D-LDL-C. The results are similar when using a 130 mg/dL cutoff for treatment. Thus, treatment decisions based on the D-LDL-C results in many patients being treated who would not have been treated when using the C-LDL-C.
To determine whether triglyceride concentration influences treatment decisions by either method of LDL-C measurement, similar correlations and analyses were done on the data according to the following triglyceride groupings: <100 mg/dL, 100 to 199 mg/dL, 200 to 299 mg/dL, 300 to 399 mg/dL, and >400 mg/dL. This was further evaluated by plotting triglyceride vs D-LDL-C and triglyceride vs C-LDL-C (data not shown). Whereas the C-LDL-C showed no correlation with triglyceride, the DLDL-C showed a statistically significant correlation with triglyceride concentrations (r = .27), indicating that D-LDL-C increases at higher triglyceride levels. This suggested an influence of triglyceride on the D-LDL-C assay. This has been reported by others in 3 of 4 different D-LDL-C assays including the Sigma assay.15 However, alterations in treatment possibilities when using the D-LDL-C are present at all triglyceride concentrations.
FIGURE 1
Direct vs calculated LDL-C (mg/dL)
TABLE
Effect of LDL assay by LDL treatment cutoff*
| Patients who might be treated, n (%) | Additional patients who might be treated, n (%) | |||
|---|---|---|---|---|
| LDL cutoff for treatment, mg/dL | Calculated LDL | Direct LDL | Calculated, not direct, LDL | Direct, not calculated, LDL |
| >100 | 334 (72) | 393 (85) | 2 (<1) | 60 (13) |
| >130 | 185 (40) | 237 (51) | 2 (<1) | 55 (12) |
| >160 | 71 (15) | 87 (19) | 6 (1) | 21 (5) |
| *N = 464 patients. | ||||
| LDL, low-density lipoprotein. | ||||
Discussion
LDL-C has been identified in the NCEP-ATP III as the primary target of therapy. Treatment recommendations for high LDL-C are based on low, moderate, or high risk for CHD, with treatment goals of 160, 130, and 100 mg/dL, respectively.1,2
These evidence-based recommendations rely on data from clinical trials demonstrating prevention of CHD events by lowering LDL-C, all of which, with the exception of the recently reported Heart Protection Study, used the CLDL-C.18-23 Thus, important treatment decisions depend on this estimated LDL-C, and systematic deviations from the C-LDL-C will affect treatment decisions and cost.
The Sigma EZ LDL D-LDL-C assay in our hospital produces higher LDL-C levels than the C-LDL-C in a range of 100 to 160 mg/dL, the range of most common concern to clinicians. This results in inappropriate treatment or intensification in treatment according to the NCEP-ATP III guidelines. The DLDL-C was higher than the C-LDL-C at all triglyceride levels, but the error was greater for hypertriglyceridemia, the very situation for which it has been advocated.
Previous publications using a D-LDL-C assay have emphasized the correlation between the DLDL-C assay and research LDL-C determinations rather than the correlation with the C-LDL-C.5-8 Other investigators have observed a similar tendency for higher D-LDL-C than C-LDL-C measurements at an LDL-C of 100 mg/dL17 and a positive bias at higher triglyceride levels.14 Although C-LDLC was often performed, data similar to those shown in the Figure, ie, the simple correlation between the D-LDL-C and C-LDL-C, have not been presented. Two very recent reviews have suggested caution in routinely implementing the D-LDL-C assays and pointed out the considerable variation from one assay to another.14,15 Laboratories often change their assay method; in fact, our hospital laboratory has recently changed to a different DLDL-C method.
Physicians and institutions should be cautious about using a D-LDL-C method as a substitute for the C-LDL-C. First, it has not been standardized in large populations and, with the exception of the recent Heart Protection Study,23 has not been used in large clinical trials demonstrating the benefits of lowering LDL-C. Although the C-LDL-C has been recommended by the NCEP-ATP III,2 the Executive Summary of these guidelines did not address the method for measuring LDL-C.1 Second, cost is increased from the additional therapy and performing the D-LDL-C assay. Third, the major reasons proposed for using a D-LDL-C assay (lack of need for a fasting specimen and usefulness at triglyceride > 400 mg/dL) may not be valid or relevant. Variation in the LDL-C due to hypertriglyceridemia occurs with the D-LDL-C. In addition, the NCEP-ATP III report emphasized triglyceride and recommended a fasting lipid panel including total cholesterol, triglycerides, and HDL-C.1,2 One limitation of this study is the inclusion of predominantly male veterans. There may be populations, not considered in this study, that have an abnormal lipoprotein composition that significantly affects the C-LDL-C.
Conclusions
The C-LDL-C should remain the method of choice for LDL-C determinations because (1) this assay was used in clinical trials documenting the benefits of cholesterol-lowering therapy and (2) use of the D-LDL-C increases cost without evidence of benefit. Further studies are needed to standardize the direct LDL-C assays, and outcome trials using these assays need to be performed.
ACKNOWLEDGMENTS
This work was supported in part by the Biomedical Research Foundation of Arkansas and the Central Arkansas Veterans Healthcare System.
1. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97.
2. National Cholesterol Education Program. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), full report, manuscript version, 2001. Available at: www.nhlbi.nih.gov/guide-lines/cholesterol/atp3_rpt.htm. Accessed April 2, 2002.
3. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502.
4. Cobbaert C, Broodman I, Swart GR, et al. Performance of a direct, immunoseparation based LDL-cholesterol method compared to Friedewald calculation and a polyvinyl sulphate precipitation method. Eur J Clin Chem Clin Biochem. 1995;33:417-24.
5. Jialal I, Hirany SV, Devaraj S, et al. Comparison of an immunoprecipitation method for direct measurement of LDL- cholesterol with beta-quantification (ultracentrifugation). Am J Clin Pathol 1995;104:76-81.
6. Nauck M, Graziani MS, Bruton D. et al: Analytical and clinical performance of a detergent based homogeneous LDL-cholesterol assay: a multicenter evaluation Clin Chem 2000;46:506-14.
7. Hirany S, Li D, Jialal I. A more valid measurement of low-density lipoprotein cholesterol in diabetic patients Am J Med 1997;102:48-53.
8. Whiting MJ, Shephard MDS, Tallis GA. Measurement of plasma LDL cholesterol in patients with diabetes Diabetes Care. 1997;20:12-4.
9. McNamara JR, Cole TG, Contois JH, et al. Immunoseparation method for measuring low density lipoprotein cholesterol directly from serum evaluated. Clin Chem 1995;41:232-40.
10. Pisani T, Gebski CP, Leary ET, et al. Accurate direct determination of lowdensity lipoprotein cholesterol using an immunoseparation reagent and enzymatic cholesterol assay. Arch Pathol Lab Med 1995;119:1127-35.
11. Maitra A, Hirany SV, Jialal I. Comparison of two assays for measuring LDL cholesterol Clin Chem 1997;43:1040-7.
12. Yu HH, Markowitz R, De Ferranti SD, et al. Direct measurement of LDL-C in children performance of two surfactant-based methods in a general pediatric population. Clin Biochem 2000;95:89-95.
13. Sakaue T, Hirano T, Yoshino G, et al. Reactions of direct LDL-cho-lesterol assays with pure LDL fraction and IDL-comparison of three homogeneous methods. Clin Chim Acta 2000;295:97-106.
14. Nauck M, Warnick GR, Rifai N. Methods for measurement of LDL-cholesterol a critical assessment of direct measurement by homogeneous assays versus calculation. Clin Chem 2002;48:236-54.
15. Miller WG, Waymack PP, Anderson FP, Ethridge SF, Jayne EC. Performance of four homogeneous direct methods for LDL-cholesterol. Clin Chem 2002;48:489-98.
16. Smets EML, Pequerlaux NCV, Blaton V, Goldschmidt HMJ. Analytical performance of a direct assay for LDL-cholesterol. Clin Chem Lab Med 2001;39:270-80.
17. Yu HH, Ginsburg GS, Harris N, Rifai N. Evaluation and clinical application of a direct low-density lipoprotein cholesterol assay in normolipidemic and hyperlipidemic adults. Am J Cardiol 1997;80:1295-99.
18. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995;333:1301-7.
19. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279:1615-22.
20. Scandinavian Simvastatin Survival Study Group. Design and baseline results of the Scandinavian Simvastatin Survival Study of patients with stable angina and/or previous myocardial infarction Am J Cardiol 1993;71:393-400.
21. Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels N Engl J Med 1998;339:1349-57.
22. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-9.
23. MRC/BHF Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trial Lancet 2002;360:7-22.
1. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97.
2. National Cholesterol Education Program. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), full report, manuscript version, 2001. Available at: www.nhlbi.nih.gov/guide-lines/cholesterol/atp3_rpt.htm. Accessed April 2, 2002.
3. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502.
4. Cobbaert C, Broodman I, Swart GR, et al. Performance of a direct, immunoseparation based LDL-cholesterol method compared to Friedewald calculation and a polyvinyl sulphate precipitation method. Eur J Clin Chem Clin Biochem. 1995;33:417-24.
5. Jialal I, Hirany SV, Devaraj S, et al. Comparison of an immunoprecipitation method for direct measurement of LDL- cholesterol with beta-quantification (ultracentrifugation). Am J Clin Pathol 1995;104:76-81.
6. Nauck M, Graziani MS, Bruton D. et al: Analytical and clinical performance of a detergent based homogeneous LDL-cholesterol assay: a multicenter evaluation Clin Chem 2000;46:506-14.
7. Hirany S, Li D, Jialal I. A more valid measurement of low-density lipoprotein cholesterol in diabetic patients Am J Med 1997;102:48-53.
8. Whiting MJ, Shephard MDS, Tallis GA. Measurement of plasma LDL cholesterol in patients with diabetes Diabetes Care. 1997;20:12-4.
9. McNamara JR, Cole TG, Contois JH, et al. Immunoseparation method for measuring low density lipoprotein cholesterol directly from serum evaluated. Clin Chem 1995;41:232-40.
10. Pisani T, Gebski CP, Leary ET, et al. Accurate direct determination of lowdensity lipoprotein cholesterol using an immunoseparation reagent and enzymatic cholesterol assay. Arch Pathol Lab Med 1995;119:1127-35.
11. Maitra A, Hirany SV, Jialal I. Comparison of two assays for measuring LDL cholesterol Clin Chem 1997;43:1040-7.
12. Yu HH, Markowitz R, De Ferranti SD, et al. Direct measurement of LDL-C in children performance of two surfactant-based methods in a general pediatric population. Clin Biochem 2000;95:89-95.
13. Sakaue T, Hirano T, Yoshino G, et al. Reactions of direct LDL-cho-lesterol assays with pure LDL fraction and IDL-comparison of three homogeneous methods. Clin Chim Acta 2000;295:97-106.
14. Nauck M, Warnick GR, Rifai N. Methods for measurement of LDL-cholesterol a critical assessment of direct measurement by homogeneous assays versus calculation. Clin Chem 2002;48:236-54.
15. Miller WG, Waymack PP, Anderson FP, Ethridge SF, Jayne EC. Performance of four homogeneous direct methods for LDL-cholesterol. Clin Chem 2002;48:489-98.
16. Smets EML, Pequerlaux NCV, Blaton V, Goldschmidt HMJ. Analytical performance of a direct assay for LDL-cholesterol. Clin Chem Lab Med 2001;39:270-80.
17. Yu HH, Ginsburg GS, Harris N, Rifai N. Evaluation and clinical application of a direct low-density lipoprotein cholesterol assay in normolipidemic and hyperlipidemic adults. Am J Cardiol 1997;80:1295-99.
18. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 1995;333:1301-7.
19. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279:1615-22.
20. Scandinavian Simvastatin Survival Study Group. Design and baseline results of the Scandinavian Simvastatin Survival Study of patients with stable angina and/or previous myocardial infarction Am J Cardiol 1993;71:393-400.
21. Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels N Engl J Med 1998;339:1349-57.
22. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-9.
23. MRC/BHF Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trial Lancet 2002;360:7-22.