User login
Ovarian masses: Surgery or surveillance?
A meaningful evolution has occurred over the past 30 years in the evaluation of ovarian tumors. In the 1980s, any palpable ovarian tumor was recommended for surgical removal.1 In the early 2000s, studies showed that unilocular cysts were at very low risk for malignancy, and surveillance was recommended.2 In the following decade, septate cysts were added to the list of ovarian tumors unlikely to be malignant, and nonsurgical therapy was suggested.3 It is estimated that 10% of women will undergo surgery for an adnexal mass in their lifetime, despite the fact that only 1 in 6 (13%–21%) of these masses is found to be malignant.4,5
A comprehensive, morphology-based pelvic ultrasonography is the first and most important step in evaluating an ovarian tumor’s risk of malignancy to determine whether surgery or surveillance is required.
Ovarian cancer continues to be the leading cause of gynecologic cancer death. Despite achieving superior surgical and cancer outcomes, a gynecologic oncologist performs only 40% of the initial ovarian cancer surgeries.6 Premenopausal and menopausal ovarian tumors are different in cause and consequence. Only 15% of premenopausal tumors are malignant, most commonly germ cell tumors, borderline ovarian tumors, and epithelial ovarian cancers. Tumors in menopausal women are less common but are more likely to be malignant. In actuality, up to 50% of tumors in this population are malignant. The most common of these malignancies are epithelial ovarian cancers, cancers metastatic to the ovary, and malignant stromal tumors.
Effective and evidence-based preoperative evaluations are available to help the clinician estimate a tumor’s risk of malignancy and determine which tumors are appropriate for referral to a specialist for surgery.
The actual incidence and prevalence of ovarian tumors are not known. From a review of almost 40,000 ultrasonography scans performed in the University of Kentucky Ovarian Cancer Screening Program, the estimated incidence and prevalence of ovarian abnormalities are 8.2 per 100 women annuallyand 17%, respectively.7 Seventy percent of these abnormalities have a unilocular or simple septate morphology and are at low risk for malignancy.7 The remaining 30% of abnormalities are high risk, although this represents only 9% of the total population evaluated. Since the vast majority of these abnormalities are expected to be asymptomatic, most will go unrecognized in the general population. For women who have an ovarian abnormality on ultrasonography, the majority will be at low risk for malignancy and will not require surgery.
Ovarian ultrasonography plus morphologic scoring comprise a comprehensive approach
The recently published recommendations of the First International Consensus Conference report on adnexal masses are summarized in TABLE 1.8 The expert panel reviewed the evidence and concluded that effective ultrasonography strategies exist and are well validated, and that low-risk asymptomatic ovarian cysts do not require surgical removal.

While no single ultrasonographic findingcan differentiate a benign from a malignant mass, morphologic scoring systems improve our ability to estimate a tumor’s malignant potential. In the United States, most practitioners in women’s health have ready access to gynecologic ultrasonography, but individual training and proficiency vary. Since not everyone is an expert sonographer, it is useful to employ an objective strategy when evaluating an ovarian tumor. The focus of a comprehensive ovarian ultrasonography is to recognize morphologic patterns that reflect a tumor’s malignant potential. While tumor volume is useful, tumor morphology is the most prognostic feature.
International Ovarian Tumor Analysis group
The International Ovarian Tumor Analysis (IOTA) group has published extensively on sonographic definitions and patterns that categorize tumors based on appearance.9 Simple rules and the ADNEX risk model are 2 of the group’s approaches (FIGURE 1).10,11 Both methods have been validated as effective for differentiating benign from malignant ovarian tumors, but neither has been used to study serial changes in ovarian morphology.

Regardless of the strategy employed, 25% of ovarian ultrasonography evaluations will be interpreted as “indeterminate” or “risk unknown.”10 The IOTA strategies have been successfully used in Europe for years, but they have not yet been studied or adopted in the United States.
Kentucky morphology index
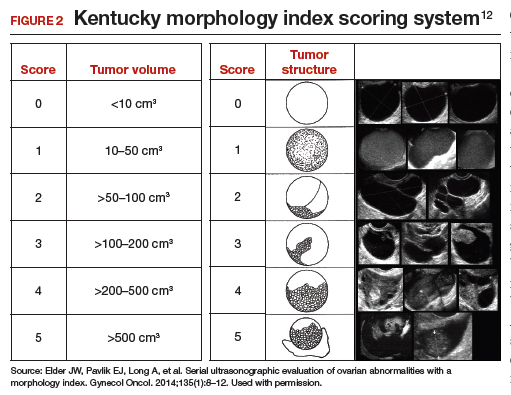
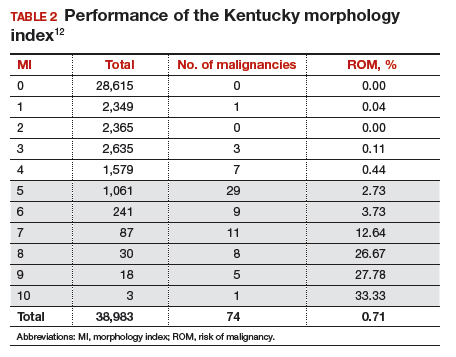
The morphology index (MI) from the University of Kentucky is an ultrasonography-based scoring system that combines tumor volume and tumor structure into a simple and effective index with a score ranging from 0 to 10 (FIGURE 2).12 A rising Kentucky MI score has a linear and predictable increase in the risk of ovarian malignancy. In a review of almost 40,000 sonograms, 85% of the malignancies had an MI score of 5 or greater (TABLE 2).12 Using this as a cutoff, the sensitivity and specificity for predicting malignancy was 86% and 98%, respectively.12
When comparing the ADNEX risk model with the Kentucky MI, investigators reviewed 45,000 ultrasound results and found that the majority of cancers were categorized by the ADNEX model in the lowest 4 of the 10 risk-of-malignancy groups, compared with only 15% for the MI.13 This clustering or skew is potentially problematic, since we expect higher scores to be more predictive of cancer than lower scores. It also infers that the ADNEX model may not be useful in serial surveillance strategies. Moreover, the ADNEX model identified only 30% of early stage cancers compared with identification of 80% with use of the MI.13
Serial ultrasonography
Serial ultrasonography is a concept similar to any longitudinal biomarker evaluation. In the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) program, the Risk of Ovarian Cancer Algorithm (ROCA) employs serial measurements of cancer antigen 125 (CA 125) to improve cancer detection. Serial ultrasonography similarly can be applied to better characterize a tumor’s physiology as well as its morphology. Over time, malignant ovarian tumors grow naturally in volume and complexity, and they do so at a rate faster than nonmalignant tumors. If this physical change can be measured objectively with ultrasonography, then serial sonography becomes a valuable diagnostic aid.
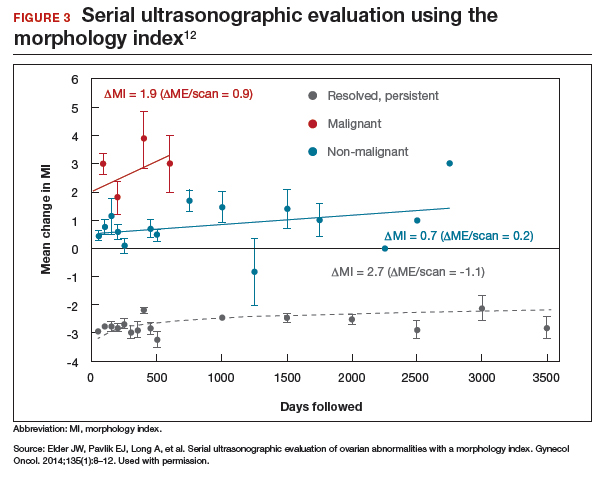
In comparing serial MI scores with clinical outcomes, studies have shown that malignant tumors exhibit a rapid increase, nonmalignant tumors have a stable or gradual rise, and resolving cysts show a decrease in MI score over time (FIGURE 3).12 An increase in the MI score of 1 or more per month (≥1 per month) is concerning for malignancy, and surgical removal should be considered. If the MI score of an asymptomatic ovarian tumor does not increase by 1 per month, it can be surveilled with intermittent ultrasonography.
Read about evaluating with serum biomarkers and sonography.
Serum biomarkers useful for determining risk, need for referral
Serum biomarkers can be used to complement an ultrasonographic evaluation. They are particularly useful when surgery is recommended but the sonographic evaluation is indeterminate for malignancy risk. Many serum biomarkers are commonly used for the preoperative evaluation of an ovarian tumor or for surveillance of a malignancy following diagnosis (TABLE 3).
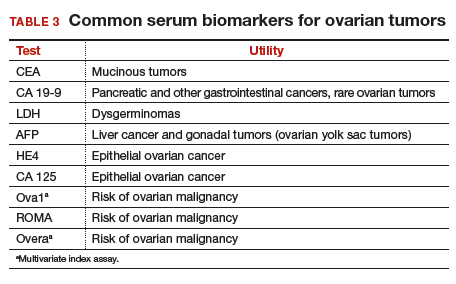
CA 125 is the most commonly ordered serum biomarker test for ovarian cancer. It is estimated that three‐quarters of CA 125 tests are ordered for preoperative use, which is not the US Food and Drug Administration (FDA) approved indication. Despite our clinical reliance on CA 125 as a diagnostic test prior to surgery, its utility is limited because of a low sensitivity for predicting cancer in premenopausal women and early stage disease.14,15 CA 125 specificity also varies widely, depending on patient age and other clinical factors, ranging from as low as 26% in premenopausal women to as high as 100% in postmenopausal women.16 Because CA 125 often is negative when early stage cancer is present, or positive when cancer is not, it is not recommended for preoperative use for determining whether an ovarian tumor is malignant or whether surgery is indicated. CA 125 should be used to monitor patients with a known ovarian malignancy.
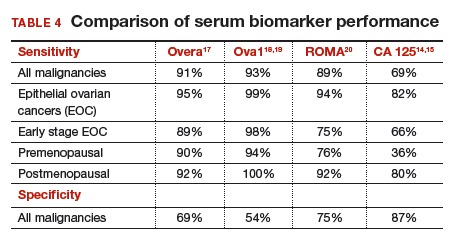
The new triage serum biomarkers, Overa, Ova1, and ROMA (Risk of Ovarian Malignancy Algorithm), are FDA cleared for preoperative use to help determine whether a woman needing surgery for an ovarian mass should be referred to a gynecologic oncologist.17–20 These tests should not be used to decide if surgery is indicated, but rather should be considered when the decision for surgery has already been made but the malignancy risk is unknown. A woman with a “high risk” result should be referred to a gynecologic oncologist, while one with a “low risk” score is very unlikely to have a malignancy and referral to a specialist is not necessary. TABLE 4 lists a comparison of the relative performance of these serum biomarkers.14,15,17–20 There are no published data on the use of serial triage biomarkers.
How to evaluate an ovarian tumor
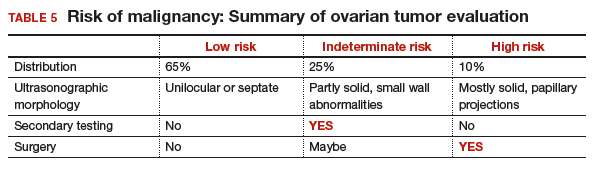
Approximately 65% of the time, ovarian cystic tumors can be identified accurately as low risk based on the initial sonographic evaluation (TABLE 5). In this scenario, the risk of malignancy is very low (<1%), no secondary testing is needed, and no surgery is recommended.1,3,21
About 10% of tumors are expected to have a high-risk morphology on ultrasonography, where the risk of malignancy exceeds 25% and referral to a gynecologic oncologist is required.
The remaining 25% of tumors cannot be accurately classified with a single ultrasonographic evaluation and are considered indeterminate.22 Indeterminate tumors require secondary testing to ascertain whether surgery is indicated. Secondary testing may consist of serial ultrasonography, magnetic resonance imaging (MRI), or serum triage biomarker testing if the decision for surgery has been made.
A 2-step process is recommended for evaluating an ovarian tumor.
Step 1. Perform a detailed ultrasonography study using a morphology-based system. Classify the tumor as:
- low risk (65%): unilocular, simple septate, no flow on color Doppler
- simple rules: benign
- MI score 0–3
- no secondary testing; no referral is recommended
- high risk (10%): irregular, mostly solid, papillary projections, very strong flow on color Doppler
- simple rules: malignant
- MI score ≥5
- no secondary testing; refer to a gynecologic oncologist
- indeterminate (25%): partly solid, small wall abnormalities, minimal or moderate flow on color Doppler
- simple rules: both M and B rules apply or no rule applies
- MI score usually 4–6
- perform secondary testing (step 2).
Step 2. Perform secondary testing as follows:
- serum triage biomarkers if surgery is planned (Ova1, ROMA, Overa), or
- MRI, or
- serial sonography.
The 3 case scenarios that follow illustrate how the ovarian tumor evaluation process may be applied in clinical practice, with referral to a gynecologic oncologist as appropriate.
CASE 1 Postmenopausal woman with urinary symptoms and pelvic pressure
A 61-year-old woman is referred with a newly identified ovarian tumor. She has had 1 month of urinary urgency, frequency, and pelvic pressure, but she denies vaginal bleeding or fever. She has no family history of cancer. The referring physician included results of a serum CA 125 (48 U/mL; normal, ≤35 U/mL). A pelvic examination reveals a palpable, irregular mass in the anterior pelvis with limited mobility.
What would be your next step in the evaluation of this patient?
Start with ultrasonography
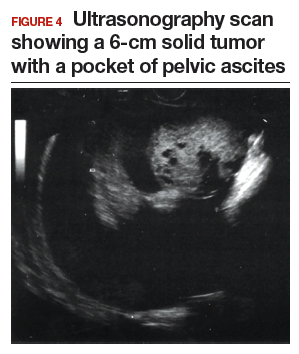
Step 1. Perform pelvic ultrasonography. In this patient, transvaginal sonography revealed a 6-cm (volume, 89 mL) mostly solid tumor (FIGURE 4). The maximum solid diameter of the tumor was 4.0 cm. There was a 20-mL pocket of pelvic ascites.
Results of morphology-based classification were as follows:
- simple rules: M1 and M5 positive; B rules: negative (malignant; high risk)
- ADNEX: 51.6% risk of malignancy (high risk)
- MI: 7 (high risk).
Step 2. Consider secondary testing. In this case, no secondary testing was recommended. Treatment plan. The patient was referred to a gynecologic oncologist for surgery and was found to have a stage IIA serous ovarian carcinoma.
CASE 2 Woman with history of pelvic symptoms and worsening pain
A 46-year-old woman presents with worsening pelvic pain over the last month. She has a long-standing history of pelvic pain, dysmenorrhea, and dyspareunia from suspected endometriosis. She has no family history of cancer. The referring physician included the following serum biomarker results: CA 125, 48 U/mL (normal, ≤35 U/mL), and HE4, 60 pM (normal, ≤150 pM). On pelvic examination, there is a palpable mass with limited mobility in the posterior cul-de-sac.
Based on the patient’s available history, physical examination, and biomarker information, how would you proceed?
Follow the 2-step process
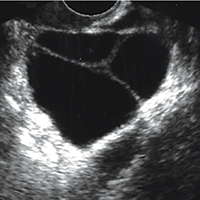
Step 1. Perform pelvic ultrasonography. Transvaginal sonography revealed a 6-cm (volume, 89 mL) partly solid tumor with regular internal borders (FIGURE 5). The maximum solid diameter of the tumor was 4.5 cm. There was no pelvic ascites.
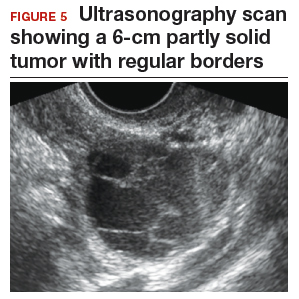
Morphology classification was as follows:
- simple rules: M5 equivocal; B4 positive (indeterminate risk)
- ADNEX: 42.7% risk of malignancy (high risk)
- MI: 6 (indeterminate risk).
Step 2. Secondary testing was recommended for this patient. Test results were:
- repeat ultrasonography in 4 weeks with MI of 7 (volume score increase from 2 to 3, structure score unchanged at 4). Change in MI score +1 per month (high risk)
- Overa: 5.2 (high risk)
- ROMA: 11.8% (low risk).
Treatment plan. The patient was referred to a gynecologic oncologist because of an increasing MI score on serial sonography. Surgery revealed a stage IA grade 2 endometrioid adenocarcinoma of the ovary with surrounding endometriosis.
Read about treating a woman with postmenstrual bleeding.
CASE 3 Woman with postmenopausal bleeding seeks medical care
A 62-year-old woman is referred with new-onset postmenopausal spotting for 1 month. She was recently prescribed antibiotics for diverticulitis. She has no family history of cancer. The referring physician included the results of a serum CA 125, which was 48 U/mL (normal, ≤35 U/mL). On pelvic examination, a mobile cystic mass is noted in the posterior cul-de-sac.
Use the stepwise protocol to sort out findings
Step 1. Pelvic ultrasonography. Transvaginal sonography suggested the presence of an endometrial polyp and revealed a 6-cm (volume, 89 mL) septate ovarian cyst (FIGURE 6).
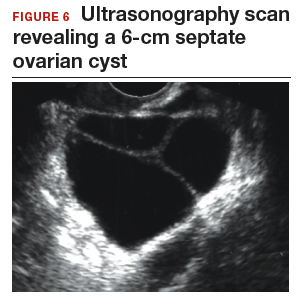
Based on morphology classification, risk was categorized as:
- simple rules: M rules negative; B2, B4, B5 positive (benign; low risk)
- ADNEX: 2.9% risk of malignancy (low risk)
- MI: 2 (low risk).
Step 2. No secondary testing was recommended in this case.
Treatment plan. The patient’s gynecologist performed a hysteroscopic polypectomy that revealed no cancer. Serial monitoring was recommended for the low-risk ovarian cyst. The next ultrasonography scan, at 6 months, was unchanged; a subsequent scan was ordered for 12 months later, and at that time the cyst had resolved.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Barber HR, Graber EA. The PMPO syndrome (postmenopausal palpable ovary syndrome). Obstet Gynecol. 1971;38(6):921–923.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR Jr. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol. 2003;102(3):594–599.
- Saunders BA, Podzielinski I, Ware RA, et al. Risk of malignancy in sonographically confirmed septated cystic ovarian tumors. Gynecol Oncol. 2010;118(3):278–282.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Jordan SM, Bristow RE. Ovarian cancer biomarkers as diagnostic triage tests. Current Biomarker Findings. 2013;3:35–42.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99(2):447–461.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 pt 1):210–217.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36(5):849–863.
- Timmerman D, Valentin L, Bourne TGH, Collins WP, Verrelst H, Vergote I; International Ovarian Tumor Analysis (IOTA) Group. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol. 2000;6(5):500–505.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31(6):681–690.
- Van Calser B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014;349:g5920.
- Elder JW, Pavlik EJ, Long A, et al. Serial ultrasonographic evaluation of ovarian abnormalities with a morphology index. Gynecol Oncol. 2014;135(1):8–12.
- Lefringhouse J, Ueland F, Ore R, et al. Comparing 2 sonographic scoring systems for distinguishing benign from malignant ovarian tumors [abstract]. Gynecol Oncol. 2016;141(suppl 1):57.
- Bast RC Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309(15):883–887.
- Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4(1):1–12.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Agency for Healthcare Research and Quality. https://archive.ahrq.gov/downloads/pub/evidence/pdf/adnexal/adnexal.pdf. Published February 2006. Accessed May 15, 2018.
- Coleman RL, Herzog TJ, Chan DW, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol. 2016;215(1):82.e1–e11.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol. 2011;117(6):1289–1297.
- Bristow RE, Smith A, Zhang Z, et al. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol. 2013;128(2):252–259.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Valentin L, Ameye L, Franchi D, et al. Risk of malignancy in unilocular cysts: a study of 1148 adnexal masses classified as unilocular cysts on transvaginal ultrasound and review of the literature. Ultrasound Obstet Gynecol. 2013;41(1):80–89.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ. 2010;341:c6839.
A meaningful evolution has occurred over the past 30 years in the evaluation of ovarian tumors. In the 1980s, any palpable ovarian tumor was recommended for surgical removal.1 In the early 2000s, studies showed that unilocular cysts were at very low risk for malignancy, and surveillance was recommended.2 In the following decade, septate cysts were added to the list of ovarian tumors unlikely to be malignant, and nonsurgical therapy was suggested.3 It is estimated that 10% of women will undergo surgery for an adnexal mass in their lifetime, despite the fact that only 1 in 6 (13%–21%) of these masses is found to be malignant.4,5
A comprehensive, morphology-based pelvic ultrasonography is the first and most important step in evaluating an ovarian tumor’s risk of malignancy to determine whether surgery or surveillance is required.
Ovarian cancer continues to be the leading cause of gynecologic cancer death. Despite achieving superior surgical and cancer outcomes, a gynecologic oncologist performs only 40% of the initial ovarian cancer surgeries.6 Premenopausal and menopausal ovarian tumors are different in cause and consequence. Only 15% of premenopausal tumors are malignant, most commonly germ cell tumors, borderline ovarian tumors, and epithelial ovarian cancers. Tumors in menopausal women are less common but are more likely to be malignant. In actuality, up to 50% of tumors in this population are malignant. The most common of these malignancies are epithelial ovarian cancers, cancers metastatic to the ovary, and malignant stromal tumors.
Effective and evidence-based preoperative evaluations are available to help the clinician estimate a tumor’s risk of malignancy and determine which tumors are appropriate for referral to a specialist for surgery.
The actual incidence and prevalence of ovarian tumors are not known. From a review of almost 40,000 ultrasonography scans performed in the University of Kentucky Ovarian Cancer Screening Program, the estimated incidence and prevalence of ovarian abnormalities are 8.2 per 100 women annuallyand 17%, respectively.7 Seventy percent of these abnormalities have a unilocular or simple septate morphology and are at low risk for malignancy.7 The remaining 30% of abnormalities are high risk, although this represents only 9% of the total population evaluated. Since the vast majority of these abnormalities are expected to be asymptomatic, most will go unrecognized in the general population. For women who have an ovarian abnormality on ultrasonography, the majority will be at low risk for malignancy and will not require surgery.
Ovarian ultrasonography plus morphologic scoring comprise a comprehensive approach
The recently published recommendations of the First International Consensus Conference report on adnexal masses are summarized in TABLE 1.8 The expert panel reviewed the evidence and concluded that effective ultrasonography strategies exist and are well validated, and that low-risk asymptomatic ovarian cysts do not require surgical removal.

While no single ultrasonographic findingcan differentiate a benign from a malignant mass, morphologic scoring systems improve our ability to estimate a tumor’s malignant potential. In the United States, most practitioners in women’s health have ready access to gynecologic ultrasonography, but individual training and proficiency vary. Since not everyone is an expert sonographer, it is useful to employ an objective strategy when evaluating an ovarian tumor. The focus of a comprehensive ovarian ultrasonography is to recognize morphologic patterns that reflect a tumor’s malignant potential. While tumor volume is useful, tumor morphology is the most prognostic feature.
International Ovarian Tumor Analysis group
The International Ovarian Tumor Analysis (IOTA) group has published extensively on sonographic definitions and patterns that categorize tumors based on appearance.9 Simple rules and the ADNEX risk model are 2 of the group’s approaches (FIGURE 1).10,11 Both methods have been validated as effective for differentiating benign from malignant ovarian tumors, but neither has been used to study serial changes in ovarian morphology.

Regardless of the strategy employed, 25% of ovarian ultrasonography evaluations will be interpreted as “indeterminate” or “risk unknown.”10 The IOTA strategies have been successfully used in Europe for years, but they have not yet been studied or adopted in the United States.
Kentucky morphology index
The morphology index (MI) from the University of Kentucky is an ultrasonography-based scoring system that combines tumor volume and tumor structure into a simple and effective index with a score ranging from 0 to 10 (FIGURE 2).12 A rising Kentucky MI score has a linear and predictable increase in the risk of ovarian malignancy. In a review of almost 40,000 sonograms, 85% of the malignancies had an MI score of 5 or greater (TABLE 2).12 Using this as a cutoff, the sensitivity and specificity for predicting malignancy was 86% and 98%, respectively.12


When comparing the ADNEX risk model with the Kentucky MI, investigators reviewed 45,000 ultrasound results and found that the majority of cancers were categorized by the ADNEX model in the lowest 4 of the 10 risk-of-malignancy groups, compared with only 15% for the MI.13 This clustering or skew is potentially problematic, since we expect higher scores to be more predictive of cancer than lower scores. It also infers that the ADNEX model may not be useful in serial surveillance strategies. Moreover, the ADNEX model identified only 30% of early stage cancers compared with identification of 80% with use of the MI.13
Serial ultrasonography
Serial ultrasonography is a concept similar to any longitudinal biomarker evaluation. In the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) program, the Risk of Ovarian Cancer Algorithm (ROCA) employs serial measurements of cancer antigen 125 (CA 125) to improve cancer detection. Serial ultrasonography similarly can be applied to better characterize a tumor’s physiology as well as its morphology. Over time, malignant ovarian tumors grow naturally in volume and complexity, and they do so at a rate faster than nonmalignant tumors. If this physical change can be measured objectively with ultrasonography, then serial sonography becomes a valuable diagnostic aid.
In comparing serial MI scores with clinical outcomes, studies have shown that malignant tumors exhibit a rapid increase, nonmalignant tumors have a stable or gradual rise, and resolving cysts show a decrease in MI score over time (FIGURE 3).12 An increase in the MI score of 1 or more per month (≥1 per month) is concerning for malignancy, and surgical removal should be considered. If the MI score of an asymptomatic ovarian tumor does not increase by 1 per month, it can be surveilled with intermittent ultrasonography.

Read about evaluating with serum biomarkers and sonography.
Serum biomarkers useful for determining risk, need for referral
Serum biomarkers can be used to complement an ultrasonographic evaluation. They are particularly useful when surgery is recommended but the sonographic evaluation is indeterminate for malignancy risk. Many serum biomarkers are commonly used for the preoperative evaluation of an ovarian tumor or for surveillance of a malignancy following diagnosis (TABLE 3).

CA 125 is the most commonly ordered serum biomarker test for ovarian cancer. It is estimated that three‐quarters of CA 125 tests are ordered for preoperative use, which is not the US Food and Drug Administration (FDA) approved indication. Despite our clinical reliance on CA 125 as a diagnostic test prior to surgery, its utility is limited because of a low sensitivity for predicting cancer in premenopausal women and early stage disease.14,15 CA 125 specificity also varies widely, depending on patient age and other clinical factors, ranging from as low as 26% in premenopausal women to as high as 100% in postmenopausal women.16 Because CA 125 often is negative when early stage cancer is present, or positive when cancer is not, it is not recommended for preoperative use for determining whether an ovarian tumor is malignant or whether surgery is indicated. CA 125 should be used to monitor patients with a known ovarian malignancy.
The new triage serum biomarkers, Overa, Ova1, and ROMA (Risk of Ovarian Malignancy Algorithm), are FDA cleared for preoperative use to help determine whether a woman needing surgery for an ovarian mass should be referred to a gynecologic oncologist.17–20 These tests should not be used to decide if surgery is indicated, but rather should be considered when the decision for surgery has already been made but the malignancy risk is unknown. A woman with a “high risk” result should be referred to a gynecologic oncologist, while one with a “low risk” score is very unlikely to have a malignancy and referral to a specialist is not necessary. TABLE 4 lists a comparison of the relative performance of these serum biomarkers.14,15,17–20 There are no published data on the use of serial triage biomarkers.

How to evaluate an ovarian tumor
Approximately 65% of the time, ovarian cystic tumors can be identified accurately as low risk based on the initial sonographic evaluation (TABLE 5). In this scenario, the risk of malignancy is very low (<1%), no secondary testing is needed, and no surgery is recommended.1,3,21

About 10% of tumors are expected to have a high-risk morphology on ultrasonography, where the risk of malignancy exceeds 25% and referral to a gynecologic oncologist is required.
The remaining 25% of tumors cannot be accurately classified with a single ultrasonographic evaluation and are considered indeterminate.22 Indeterminate tumors require secondary testing to ascertain whether surgery is indicated. Secondary testing may consist of serial ultrasonography, magnetic resonance imaging (MRI), or serum triage biomarker testing if the decision for surgery has been made.
A 2-step process is recommended for evaluating an ovarian tumor.
Step 1. Perform a detailed ultrasonography study using a morphology-based system. Classify the tumor as:
- low risk (65%): unilocular, simple septate, no flow on color Doppler
- simple rules: benign
- MI score 0–3
- no secondary testing; no referral is recommended
- high risk (10%): irregular, mostly solid, papillary projections, very strong flow on color Doppler
- simple rules: malignant
- MI score ≥5
- no secondary testing; refer to a gynecologic oncologist
- indeterminate (25%): partly solid, small wall abnormalities, minimal or moderate flow on color Doppler
- simple rules: both M and B rules apply or no rule applies
- MI score usually 4–6
- perform secondary testing (step 2).
Step 2. Perform secondary testing as follows:
- serum triage biomarkers if surgery is planned (Ova1, ROMA, Overa), or
- MRI, or
- serial sonography.
The 3 case scenarios that follow illustrate how the ovarian tumor evaluation process may be applied in clinical practice, with referral to a gynecologic oncologist as appropriate.
CASE 1 Postmenopausal woman with urinary symptoms and pelvic pressure
A 61-year-old woman is referred with a newly identified ovarian tumor. She has had 1 month of urinary urgency, frequency, and pelvic pressure, but she denies vaginal bleeding or fever. She has no family history of cancer. The referring physician included results of a serum CA 125 (48 U/mL; normal, ≤35 U/mL). A pelvic examination reveals a palpable, irregular mass in the anterior pelvis with limited mobility.
What would be your next step in the evaluation of this patient?
Start with ultrasonography
Step 1. Perform pelvic ultrasonography. In this patient, transvaginal sonography revealed a 6-cm (volume, 89 mL) mostly solid tumor (FIGURE 4). The maximum solid diameter of the tumor was 4.0 cm. There was a 20-mL pocket of pelvic ascites.

Results of morphology-based classification were as follows:
- simple rules: M1 and M5 positive; B rules: negative (malignant; high risk)
- ADNEX: 51.6% risk of malignancy (high risk)
- MI: 7 (high risk).
Step 2. Consider secondary testing. In this case, no secondary testing was recommended. Treatment plan. The patient was referred to a gynecologic oncologist for surgery and was found to have a stage IIA serous ovarian carcinoma.
CASE 2 Woman with history of pelvic symptoms and worsening pain
A 46-year-old woman presents with worsening pelvic pain over the last month. She has a long-standing history of pelvic pain, dysmenorrhea, and dyspareunia from suspected endometriosis. She has no family history of cancer. The referring physician included the following serum biomarker results: CA 125, 48 U/mL (normal, ≤35 U/mL), and HE4, 60 pM (normal, ≤150 pM). On pelvic examination, there is a palpable mass with limited mobility in the posterior cul-de-sac.
Based on the patient’s available history, physical examination, and biomarker information, how would you proceed?
Follow the 2-step process
Step 1. Perform pelvic ultrasonography. Transvaginal sonography revealed a 6-cm (volume, 89 mL) partly solid tumor with regular internal borders (FIGURE 5). The maximum solid diameter of the tumor was 4.5 cm. There was no pelvic ascites.

Morphology classification was as follows:
- simple rules: M5 equivocal; B4 positive (indeterminate risk)
- ADNEX: 42.7% risk of malignancy (high risk)
- MI: 6 (indeterminate risk).
Step 2. Secondary testing was recommended for this patient. Test results were:
- repeat ultrasonography in 4 weeks with MI of 7 (volume score increase from 2 to 3, structure score unchanged at 4). Change in MI score +1 per month (high risk)
- Overa: 5.2 (high risk)
- ROMA: 11.8% (low risk).
Treatment plan. The patient was referred to a gynecologic oncologist because of an increasing MI score on serial sonography. Surgery revealed a stage IA grade 2 endometrioid adenocarcinoma of the ovary with surrounding endometriosis.
Read about treating a woman with postmenstrual bleeding.
CASE 3 Woman with postmenopausal bleeding seeks medical care
A 62-year-old woman is referred with new-onset postmenopausal spotting for 1 month. She was recently prescribed antibiotics for diverticulitis. She has no family history of cancer. The referring physician included the results of a serum CA 125, which was 48 U/mL (normal, ≤35 U/mL). On pelvic examination, a mobile cystic mass is noted in the posterior cul-de-sac.
Use the stepwise protocol to sort out findings
Step 1. Pelvic ultrasonography. Transvaginal sonography suggested the presence of an endometrial polyp and revealed a 6-cm (volume, 89 mL) septate ovarian cyst (FIGURE 6).

Based on morphology classification, risk was categorized as:
- simple rules: M rules negative; B2, B4, B5 positive (benign; low risk)
- ADNEX: 2.9% risk of malignancy (low risk)
- MI: 2 (low risk).
Step 2. No secondary testing was recommended in this case.
Treatment plan. The patient’s gynecologist performed a hysteroscopic polypectomy that revealed no cancer. Serial monitoring was recommended for the low-risk ovarian cyst. The next ultrasonography scan, at 6 months, was unchanged; a subsequent scan was ordered for 12 months later, and at that time the cyst had resolved.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A meaningful evolution has occurred over the past 30 years in the evaluation of ovarian tumors. In the 1980s, any palpable ovarian tumor was recommended for surgical removal.1 In the early 2000s, studies showed that unilocular cysts were at very low risk for malignancy, and surveillance was recommended.2 In the following decade, septate cysts were added to the list of ovarian tumors unlikely to be malignant, and nonsurgical therapy was suggested.3 It is estimated that 10% of women will undergo surgery for an adnexal mass in their lifetime, despite the fact that only 1 in 6 (13%–21%) of these masses is found to be malignant.4,5
A comprehensive, morphology-based pelvic ultrasonography is the first and most important step in evaluating an ovarian tumor’s risk of malignancy to determine whether surgery or surveillance is required.
Ovarian cancer continues to be the leading cause of gynecologic cancer death. Despite achieving superior surgical and cancer outcomes, a gynecologic oncologist performs only 40% of the initial ovarian cancer surgeries.6 Premenopausal and menopausal ovarian tumors are different in cause and consequence. Only 15% of premenopausal tumors are malignant, most commonly germ cell tumors, borderline ovarian tumors, and epithelial ovarian cancers. Tumors in menopausal women are less common but are more likely to be malignant. In actuality, up to 50% of tumors in this population are malignant. The most common of these malignancies are epithelial ovarian cancers, cancers metastatic to the ovary, and malignant stromal tumors.
Effective and evidence-based preoperative evaluations are available to help the clinician estimate a tumor’s risk of malignancy and determine which tumors are appropriate for referral to a specialist for surgery.
The actual incidence and prevalence of ovarian tumors are not known. From a review of almost 40,000 ultrasonography scans performed in the University of Kentucky Ovarian Cancer Screening Program, the estimated incidence and prevalence of ovarian abnormalities are 8.2 per 100 women annuallyand 17%, respectively.7 Seventy percent of these abnormalities have a unilocular or simple septate morphology and are at low risk for malignancy.7 The remaining 30% of abnormalities are high risk, although this represents only 9% of the total population evaluated. Since the vast majority of these abnormalities are expected to be asymptomatic, most will go unrecognized in the general population. For women who have an ovarian abnormality on ultrasonography, the majority will be at low risk for malignancy and will not require surgery.
Ovarian ultrasonography plus morphologic scoring comprise a comprehensive approach
The recently published recommendations of the First International Consensus Conference report on adnexal masses are summarized in TABLE 1.8 The expert panel reviewed the evidence and concluded that effective ultrasonography strategies exist and are well validated, and that low-risk asymptomatic ovarian cysts do not require surgical removal.

While no single ultrasonographic findingcan differentiate a benign from a malignant mass, morphologic scoring systems improve our ability to estimate a tumor’s malignant potential. In the United States, most practitioners in women’s health have ready access to gynecologic ultrasonography, but individual training and proficiency vary. Since not everyone is an expert sonographer, it is useful to employ an objective strategy when evaluating an ovarian tumor. The focus of a comprehensive ovarian ultrasonography is to recognize morphologic patterns that reflect a tumor’s malignant potential. While tumor volume is useful, tumor morphology is the most prognostic feature.
International Ovarian Tumor Analysis group
The International Ovarian Tumor Analysis (IOTA) group has published extensively on sonographic definitions and patterns that categorize tumors based on appearance.9 Simple rules and the ADNEX risk model are 2 of the group’s approaches (FIGURE 1).10,11 Both methods have been validated as effective for differentiating benign from malignant ovarian tumors, but neither has been used to study serial changes in ovarian morphology.

Regardless of the strategy employed, 25% of ovarian ultrasonography evaluations will be interpreted as “indeterminate” or “risk unknown.”10 The IOTA strategies have been successfully used in Europe for years, but they have not yet been studied or adopted in the United States.
Kentucky morphology index
The morphology index (MI) from the University of Kentucky is an ultrasonography-based scoring system that combines tumor volume and tumor structure into a simple and effective index with a score ranging from 0 to 10 (FIGURE 2).12 A rising Kentucky MI score has a linear and predictable increase in the risk of ovarian malignancy. In a review of almost 40,000 sonograms, 85% of the malignancies had an MI score of 5 or greater (TABLE 2).12 Using this as a cutoff, the sensitivity and specificity for predicting malignancy was 86% and 98%, respectively.12


When comparing the ADNEX risk model with the Kentucky MI, investigators reviewed 45,000 ultrasound results and found that the majority of cancers were categorized by the ADNEX model in the lowest 4 of the 10 risk-of-malignancy groups, compared with only 15% for the MI.13 This clustering or skew is potentially problematic, since we expect higher scores to be more predictive of cancer than lower scores. It also infers that the ADNEX model may not be useful in serial surveillance strategies. Moreover, the ADNEX model identified only 30% of early stage cancers compared with identification of 80% with use of the MI.13
Serial ultrasonography
Serial ultrasonography is a concept similar to any longitudinal biomarker evaluation. In the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) program, the Risk of Ovarian Cancer Algorithm (ROCA) employs serial measurements of cancer antigen 125 (CA 125) to improve cancer detection. Serial ultrasonography similarly can be applied to better characterize a tumor’s physiology as well as its morphology. Over time, malignant ovarian tumors grow naturally in volume and complexity, and they do so at a rate faster than nonmalignant tumors. If this physical change can be measured objectively with ultrasonography, then serial sonography becomes a valuable diagnostic aid.
In comparing serial MI scores with clinical outcomes, studies have shown that malignant tumors exhibit a rapid increase, nonmalignant tumors have a stable or gradual rise, and resolving cysts show a decrease in MI score over time (FIGURE 3).12 An increase in the MI score of 1 or more per month (≥1 per month) is concerning for malignancy, and surgical removal should be considered. If the MI score of an asymptomatic ovarian tumor does not increase by 1 per month, it can be surveilled with intermittent ultrasonography.

Read about evaluating with serum biomarkers and sonography.
Serum biomarkers useful for determining risk, need for referral
Serum biomarkers can be used to complement an ultrasonographic evaluation. They are particularly useful when surgery is recommended but the sonographic evaluation is indeterminate for malignancy risk. Many serum biomarkers are commonly used for the preoperative evaluation of an ovarian tumor or for surveillance of a malignancy following diagnosis (TABLE 3).

CA 125 is the most commonly ordered serum biomarker test for ovarian cancer. It is estimated that three‐quarters of CA 125 tests are ordered for preoperative use, which is not the US Food and Drug Administration (FDA) approved indication. Despite our clinical reliance on CA 125 as a diagnostic test prior to surgery, its utility is limited because of a low sensitivity for predicting cancer in premenopausal women and early stage disease.14,15 CA 125 specificity also varies widely, depending on patient age and other clinical factors, ranging from as low as 26% in premenopausal women to as high as 100% in postmenopausal women.16 Because CA 125 often is negative when early stage cancer is present, or positive when cancer is not, it is not recommended for preoperative use for determining whether an ovarian tumor is malignant or whether surgery is indicated. CA 125 should be used to monitor patients with a known ovarian malignancy.
The new triage serum biomarkers, Overa, Ova1, and ROMA (Risk of Ovarian Malignancy Algorithm), are FDA cleared for preoperative use to help determine whether a woman needing surgery for an ovarian mass should be referred to a gynecologic oncologist.17–20 These tests should not be used to decide if surgery is indicated, but rather should be considered when the decision for surgery has already been made but the malignancy risk is unknown. A woman with a “high risk” result should be referred to a gynecologic oncologist, while one with a “low risk” score is very unlikely to have a malignancy and referral to a specialist is not necessary. TABLE 4 lists a comparison of the relative performance of these serum biomarkers.14,15,17–20 There are no published data on the use of serial triage biomarkers.

How to evaluate an ovarian tumor
Approximately 65% of the time, ovarian cystic tumors can be identified accurately as low risk based on the initial sonographic evaluation (TABLE 5). In this scenario, the risk of malignancy is very low (<1%), no secondary testing is needed, and no surgery is recommended.1,3,21

About 10% of tumors are expected to have a high-risk morphology on ultrasonography, where the risk of malignancy exceeds 25% and referral to a gynecologic oncologist is required.
The remaining 25% of tumors cannot be accurately classified with a single ultrasonographic evaluation and are considered indeterminate.22 Indeterminate tumors require secondary testing to ascertain whether surgery is indicated. Secondary testing may consist of serial ultrasonography, magnetic resonance imaging (MRI), or serum triage biomarker testing if the decision for surgery has been made.
A 2-step process is recommended for evaluating an ovarian tumor.
Step 1. Perform a detailed ultrasonography study using a morphology-based system. Classify the tumor as:
- low risk (65%): unilocular, simple septate, no flow on color Doppler
- simple rules: benign
- MI score 0–3
- no secondary testing; no referral is recommended
- high risk (10%): irregular, mostly solid, papillary projections, very strong flow on color Doppler
- simple rules: malignant
- MI score ≥5
- no secondary testing; refer to a gynecologic oncologist
- indeterminate (25%): partly solid, small wall abnormalities, minimal or moderate flow on color Doppler
- simple rules: both M and B rules apply or no rule applies
- MI score usually 4–6
- perform secondary testing (step 2).
Step 2. Perform secondary testing as follows:
- serum triage biomarkers if surgery is planned (Ova1, ROMA, Overa), or
- MRI, or
- serial sonography.
The 3 case scenarios that follow illustrate how the ovarian tumor evaluation process may be applied in clinical practice, with referral to a gynecologic oncologist as appropriate.
CASE 1 Postmenopausal woman with urinary symptoms and pelvic pressure
A 61-year-old woman is referred with a newly identified ovarian tumor. She has had 1 month of urinary urgency, frequency, and pelvic pressure, but she denies vaginal bleeding or fever. She has no family history of cancer. The referring physician included results of a serum CA 125 (48 U/mL; normal, ≤35 U/mL). A pelvic examination reveals a palpable, irregular mass in the anterior pelvis with limited mobility.
What would be your next step in the evaluation of this patient?
Start with ultrasonography
Step 1. Perform pelvic ultrasonography. In this patient, transvaginal sonography revealed a 6-cm (volume, 89 mL) mostly solid tumor (FIGURE 4). The maximum solid diameter of the tumor was 4.0 cm. There was a 20-mL pocket of pelvic ascites.

Results of morphology-based classification were as follows:
- simple rules: M1 and M5 positive; B rules: negative (malignant; high risk)
- ADNEX: 51.6% risk of malignancy (high risk)
- MI: 7 (high risk).
Step 2. Consider secondary testing. In this case, no secondary testing was recommended. Treatment plan. The patient was referred to a gynecologic oncologist for surgery and was found to have a stage IIA serous ovarian carcinoma.
CASE 2 Woman with history of pelvic symptoms and worsening pain
A 46-year-old woman presents with worsening pelvic pain over the last month. She has a long-standing history of pelvic pain, dysmenorrhea, and dyspareunia from suspected endometriosis. She has no family history of cancer. The referring physician included the following serum biomarker results: CA 125, 48 U/mL (normal, ≤35 U/mL), and HE4, 60 pM (normal, ≤150 pM). On pelvic examination, there is a palpable mass with limited mobility in the posterior cul-de-sac.
Based on the patient’s available history, physical examination, and biomarker information, how would you proceed?
Follow the 2-step process
Step 1. Perform pelvic ultrasonography. Transvaginal sonography revealed a 6-cm (volume, 89 mL) partly solid tumor with regular internal borders (FIGURE 5). The maximum solid diameter of the tumor was 4.5 cm. There was no pelvic ascites.

Morphology classification was as follows:
- simple rules: M5 equivocal; B4 positive (indeterminate risk)
- ADNEX: 42.7% risk of malignancy (high risk)
- MI: 6 (indeterminate risk).
Step 2. Secondary testing was recommended for this patient. Test results were:
- repeat ultrasonography in 4 weeks with MI of 7 (volume score increase from 2 to 3, structure score unchanged at 4). Change in MI score +1 per month (high risk)
- Overa: 5.2 (high risk)
- ROMA: 11.8% (low risk).
Treatment plan. The patient was referred to a gynecologic oncologist because of an increasing MI score on serial sonography. Surgery revealed a stage IA grade 2 endometrioid adenocarcinoma of the ovary with surrounding endometriosis.
Read about treating a woman with postmenstrual bleeding.
CASE 3 Woman with postmenopausal bleeding seeks medical care
A 62-year-old woman is referred with new-onset postmenopausal spotting for 1 month. She was recently prescribed antibiotics for diverticulitis. She has no family history of cancer. The referring physician included the results of a serum CA 125, which was 48 U/mL (normal, ≤35 U/mL). On pelvic examination, a mobile cystic mass is noted in the posterior cul-de-sac.
Use the stepwise protocol to sort out findings
Step 1. Pelvic ultrasonography. Transvaginal sonography suggested the presence of an endometrial polyp and revealed a 6-cm (volume, 89 mL) septate ovarian cyst (FIGURE 6).

Based on morphology classification, risk was categorized as:
- simple rules: M rules negative; B2, B4, B5 positive (benign; low risk)
- ADNEX: 2.9% risk of malignancy (low risk)
- MI: 2 (low risk).
Step 2. No secondary testing was recommended in this case.
Treatment plan. The patient’s gynecologist performed a hysteroscopic polypectomy that revealed no cancer. Serial monitoring was recommended for the low-risk ovarian cyst. The next ultrasonography scan, at 6 months, was unchanged; a subsequent scan was ordered for 12 months later, and at that time the cyst had resolved.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Barber HR, Graber EA. The PMPO syndrome (postmenopausal palpable ovary syndrome). Obstet Gynecol. 1971;38(6):921–923.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR Jr. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol. 2003;102(3):594–599.
- Saunders BA, Podzielinski I, Ware RA, et al. Risk of malignancy in sonographically confirmed septated cystic ovarian tumors. Gynecol Oncol. 2010;118(3):278–282.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Jordan SM, Bristow RE. Ovarian cancer biomarkers as diagnostic triage tests. Current Biomarker Findings. 2013;3:35–42.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99(2):447–461.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 pt 1):210–217.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36(5):849–863.
- Timmerman D, Valentin L, Bourne TGH, Collins WP, Verrelst H, Vergote I; International Ovarian Tumor Analysis (IOTA) Group. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol. 2000;6(5):500–505.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31(6):681–690.
- Van Calser B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014;349:g5920.
- Elder JW, Pavlik EJ, Long A, et al. Serial ultrasonographic evaluation of ovarian abnormalities with a morphology index. Gynecol Oncol. 2014;135(1):8–12.
- Lefringhouse J, Ueland F, Ore R, et al. Comparing 2 sonographic scoring systems for distinguishing benign from malignant ovarian tumors [abstract]. Gynecol Oncol. 2016;141(suppl 1):57.
- Bast RC Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309(15):883–887.
- Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4(1):1–12.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Agency for Healthcare Research and Quality. https://archive.ahrq.gov/downloads/pub/evidence/pdf/adnexal/adnexal.pdf. Published February 2006. Accessed May 15, 2018.
- Coleman RL, Herzog TJ, Chan DW, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol. 2016;215(1):82.e1–e11.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol. 2011;117(6):1289–1297.
- Bristow RE, Smith A, Zhang Z, et al. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol. 2013;128(2):252–259.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Valentin L, Ameye L, Franchi D, et al. Risk of malignancy in unilocular cysts: a study of 1148 adnexal masses classified as unilocular cysts on transvaginal ultrasound and review of the literature. Ultrasound Obstet Gynecol. 2013;41(1):80–89.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ. 2010;341:c6839.
- Barber HR, Graber EA. The PMPO syndrome (postmenopausal palpable ovary syndrome). Obstet Gynecol. 1971;38(6):921–923.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR Jr. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol. 2003;102(3):594–599.
- Saunders BA, Podzielinski I, Ware RA, et al. Risk of malignancy in sonographically confirmed septated cystic ovarian tumors. Gynecol Oncol. 2010;118(3):278–282.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Jordan SM, Bristow RE. Ovarian cancer biomarkers as diagnostic triage tests. Current Biomarker Findings. 2013;3:35–42.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99(2):447–461.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 pt 1):210–217.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36(5):849–863.
- Timmerman D, Valentin L, Bourne TGH, Collins WP, Verrelst H, Vergote I; International Ovarian Tumor Analysis (IOTA) Group. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol. 2000;6(5):500–505.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31(6):681–690.
- Van Calser B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014;349:g5920.
- Elder JW, Pavlik EJ, Long A, et al. Serial ultrasonographic evaluation of ovarian abnormalities with a morphology index. Gynecol Oncol. 2014;135(1):8–12.
- Lefringhouse J, Ueland F, Ore R, et al. Comparing 2 sonographic scoring systems for distinguishing benign from malignant ovarian tumors [abstract]. Gynecol Oncol. 2016;141(suppl 1):57.
- Bast RC Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309(15):883–887.
- Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4(1):1–12.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Agency for Healthcare Research and Quality. https://archive.ahrq.gov/downloads/pub/evidence/pdf/adnexal/adnexal.pdf. Published February 2006. Accessed May 15, 2018.
- Coleman RL, Herzog TJ, Chan DW, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol. 2016;215(1):82.e1–e11.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol. 2011;117(6):1289–1297.
- Bristow RE, Smith A, Zhang Z, et al. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol. 2013;128(2):252–259.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Valentin L, Ameye L, Franchi D, et al. Risk of malignancy in unilocular cysts: a study of 1148 adnexal masses classified as unilocular cysts on transvaginal ultrasound and review of the literature. Ultrasound Obstet Gynecol. 2013;41(1):80–89.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ. 2010;341:c6839.