User login
Iatrogenic hyponatremia in a patient with bipolar disorder
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
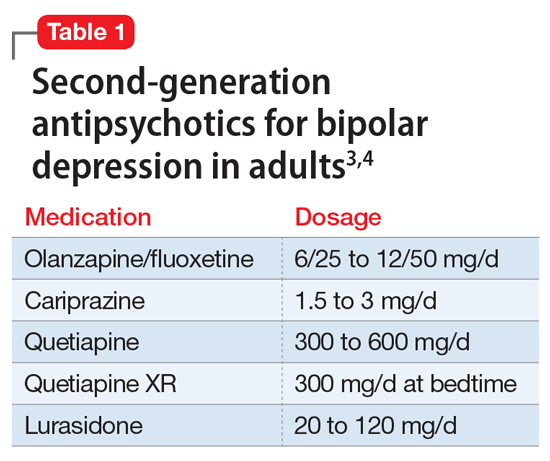
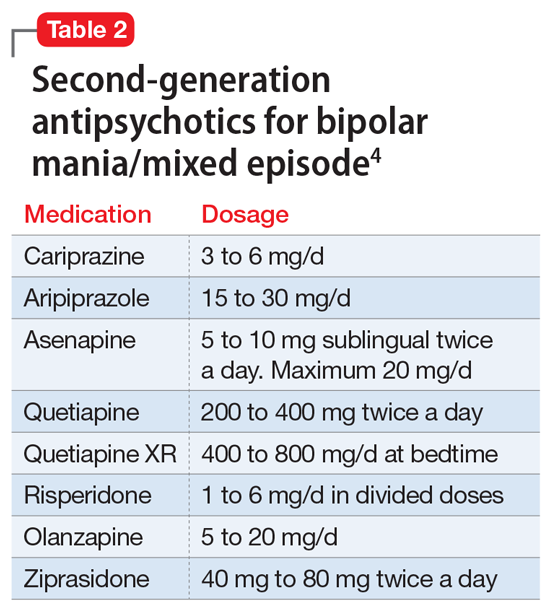
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.
The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
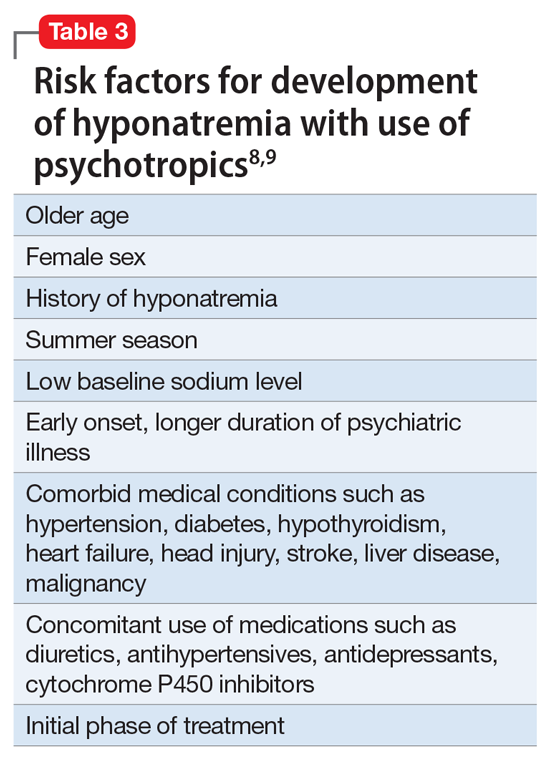
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.
Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.


The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.

Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.


The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.

Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.