User login
SARS-CoV-2 Seroprevalence Among Healthcare Workers by Job Function and Work Location in a New York Inner-City Hospital
SARS-CoV-2 has infected 141 million people worldwide and 31 million people in the United States as of April 20, 2021.1,2 The influx of hospital admissions and deaths has severely strained healthcare systems worldwide and placed healthcare workers (HCWs) at increased risk for acquiring COVID-19.3-5
Several studies have described the impact of COVID-19 on this heterogeneous group of HCWs. Shields et al reported a seroprevalence of 24.4% in HCWs at University Hospitals Birmingham (UK), with the highest rate, 34.5%, in housekeeping staff.6 Steensels et al reported a lower prevalence of 6.4% at a tertiary care center in Belgium, and showed no increased risk for HCWs when directly involved in clinical care.7 The authors attributed this to adequate use of personal protective equipment (PPE). Other studies have reported seroprevalences ranging from 1.6% to 18%.8-11 In the New York City (NYC) metro area, Jeremias et al reported a seroprevalence of 9.8% in HCWs and found no difference by job title or work location,12 whereas Moscola et al reported a seroprevalence of 13.7% and demonstrated a 3% increased risk for those working in service or maintenance.13 Antibody tests were conducted between March and April 2020 in all but two of these studies; testing in these two studies was performed between April 13 and June 23, 2020, with one reporting a seroprevalence of 6%11 and the other, 13.7%.13
NYC became the earliest pandemic epicenter in the United States following untracked transmission from ongoing circulation of SARS-CoV-2 in Europe.14 As a result, the COVID-19 surge in NYC commenced in March and largely subsided by the end of May 2020. Most HCW data reported to date do not reflect the situation at the end of the surge, and may underestimate true seroprevalence. We describe SARS-CoV-2 seroprevalence in HCWs in a large inner-city hospital in NYC, with antibody testing conducted from May 18 to June 26, 2020, at the subsidence of the surge. To further our understanding of occupational risk among different groups of HCWs, we examined associations of seroprevalence with HCWs’ job function and work location.
METHODS
This was a cross-sectional seroprevalence study conducted in the BronxCare Health System located in South and Central Bronx, an area that experienced one of the highest incidences of SARS-CoV-2 infections within NYC’s five boroughs.
HCWs were offered voluntary testing for serum antibodies to SARS-CoV-2 between May 18 and June 26, 2020. Testing occurred in the institution’s auditorium, a central and easily accessible location. Weekly emails were sent to all employees and department heads during the testing period, offering antibody testing and providing location and testing time information. The Elecsys Anti-SARS-CoV-2 (Roche) assay measuring total qualitative antibodies was used; the assay has a reported sensitivity of 97.1% 14 days after a positive SARS-CoV-2 RNA polymerase chain reaction (PCR) test result and a specificity of 100%.15
Demographic and work-related information was abstracted from electronic medical records, including all comorbid conditions that affected 30 or more HCWs. Pulmonary diagnoses, including asthma and chronic obstructive pulmonary disease, were grouped as chronic lung disease, and cardiovascular diseases, including hypertension, as chronic heart disease. Personal identifiers and data were delinked upon completion of data abstraction. The study was approved by the hospital’s institutional review board.
Job Function and Work Location
HCWs were grouped by job function as follows: physicians; nurses (including physician assistants and nurse practitioners); allied HCW I (medical assistants, patient care, and electrocardiogram, radiology, and ear, nose and throat technicians); allied HCW II (social workers, dieticians and nutritionists, registration clerks and unit associates, physical and occupational therapists); nonclinical staff (patient transporters, housekeeping staff, and security staff); pharmacists; engineering; and administrative staff. Respiratory therapists were considered as a separate group as their work placed them at high risk for respiratory diseases.
Work locations were as follows: clinics (including dental, outpatient, and satellite clinics), emergency departments (ED), inpatient units (including floors and intensive care units [ICU]), radiology suite, laboratory and pharmacy, and offices.
Statistical Analysis
Descriptive statistics were calculated using χ2 analyses. All demographic variables were tested against serology status (positive/negative). A binary logistic regression analysis was used to calculate odds ratios (ORs). Eight separate univariate unadjusted ORs were calculated by running each predictor variable against serology status (dependent variable), which included the six categorical variables—race, ethnicity, age, sex, body mass index (BMI), and prior SARS-CoV-2 PCR results—and the two main predictors—job function and work location. To obtain adjusted ORs, two final separate multivariable logistic regression analyses were executed including the six covariates listed. Due to high collinearity between job function and work location (χ2 = 3030.13, df = 35 [6 levels of work location – 1]*[8 levels of job function – 1]; P < .001), we included only one of the main predictors in each model. The regressions were specified such that the reference groups for the work location and job function variables were office work and administration, respectively. This choice was made based on the fact that their nonclinical functions do not confer an exposure risk in excess of that experienced by typical community populations. Sensitivity analyses were performed on the subset of HCWs whose address zip codes indicated residence within NYC to exclude the effect of different community seroprevalences in areas outside of NYC. The 95% CI for seroprevalence of antibodies within tested HCWs was estimated using the Clopper-Pearson binomial method.
RESULTS
Among all HCWs in the institution (N = 4,807), 2,749 (57.2%) underwent voluntary testing. Of those who underwent testing, 831 were positive for antibodies to SARS-CoV-2 (Figure 1), a seroprevalence of 30.2% (95% CI, 29%-32%). Among the age groups, the 45-to-64−year group had the highest seropositivity at 33% (400/1203), and those ≥75 years of age, the lowest at 16.7% (2/12) (P < .009). 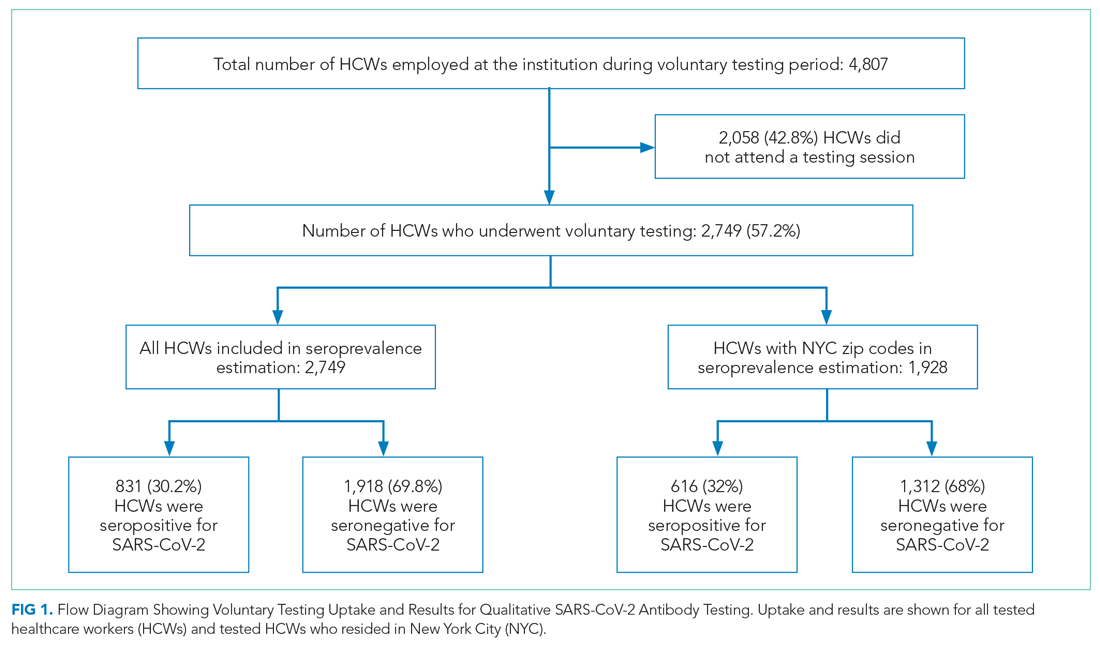
Among all tested HCWs, 70.1% (1,928/2,749) resided in NYC. SARS-CoV-2 seroprevalence in this subset was 32% (616/1,928) (Figure 1). Demographic and comorbid conditions in HCWs who lived in NYC were similar to those of the whole group (Appendix Table 1).
HCWs who underwent voluntary antibody testing (Appendix Table 2) had a higher percentage of persons in the 45-to-64−year age group (43.8% vs 40.9%) and a lower percentage of persons in the 65-to-74−year age group (3.3% vs 5.3%) compared with the group of HCWs that did not undergo testing (P < .001). Gender, race, ethnicity, comorbid conditions, SARS-CoV-2 PCR testing, and work locations were not different between groups. The tested group had higher proportions of clinicians (physicians, nurses, allied HCWs I and II) than the untested nonparticipant group (P = .014).
SARS-CoV-2 PCR Tests on HCWs
More than one-third (34.1%; 938/2,749) of HCWs had a documented nasopharyngeal PCR test between March 23 and June 26, 2020 (Table). Of all PCRs performed, 262 were positive, giving an overall PCR positivity rate of 27.9%. Positivity was 51.4% in March and 36.6% in April. The reasons for PCR testing were not available, but likely represent a combination of exposure-related testing among asymptomatic individuals and diagnostic testing of symptomatic HCWs. In contrast, serology testing was indicative of prior infection and yielded a cumulative seroprevalence at the end of the surge. Findings were similar among HCWs residing in NYC
Work Location and Job Function
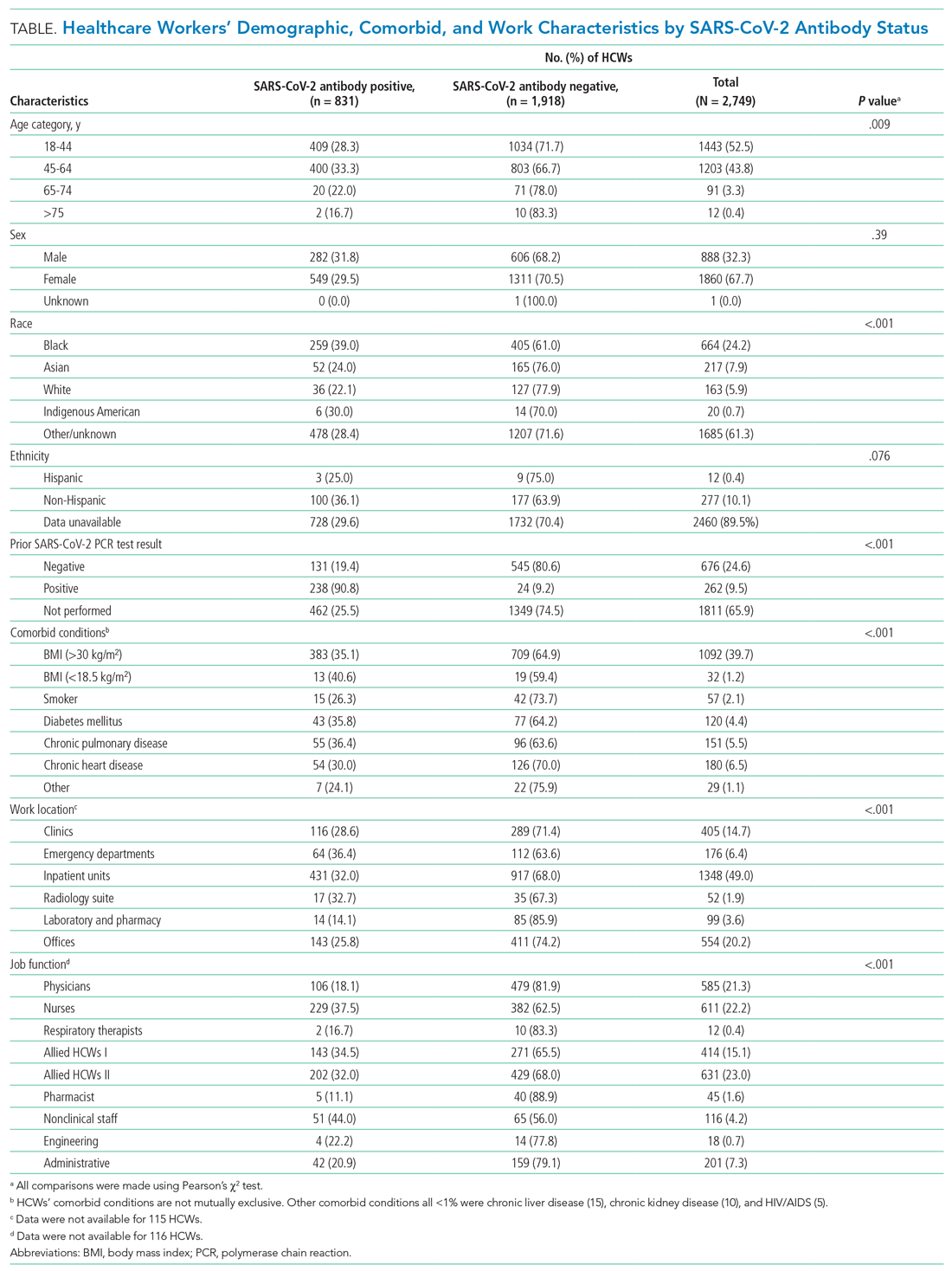
Among all HCWs (Table, Figure 2), there were differences in seropositivity by work location (P = .001). The largest number of HCWs worked in inpatient units (1,348/2,749, 49%), and the second largest in offices (554/2,749, 20%). The highest seropositivity rate was in the EDs, at 36.4% (64/176), followed by radiology suites, at 32.7% (17/52); the seropositivity rate in office locations was 25.8% (143/554). Among HCWs residing in NYC (Appendix Table 1, Appendix Figure 1), the rank order according to proportion seropositive by work location was similar to that of the whole group (P = .004), except that the second highest seropositivity rate was in the inpatient units (33.9% [323/953]). In the group of HCWs residing in NYC, office locations had a seropositivity of 27.4% (102/372). The seropositivity rates for both groups working in office locations were slightly higher than the 22% community seroprevalence in NYC reported for the same period.16
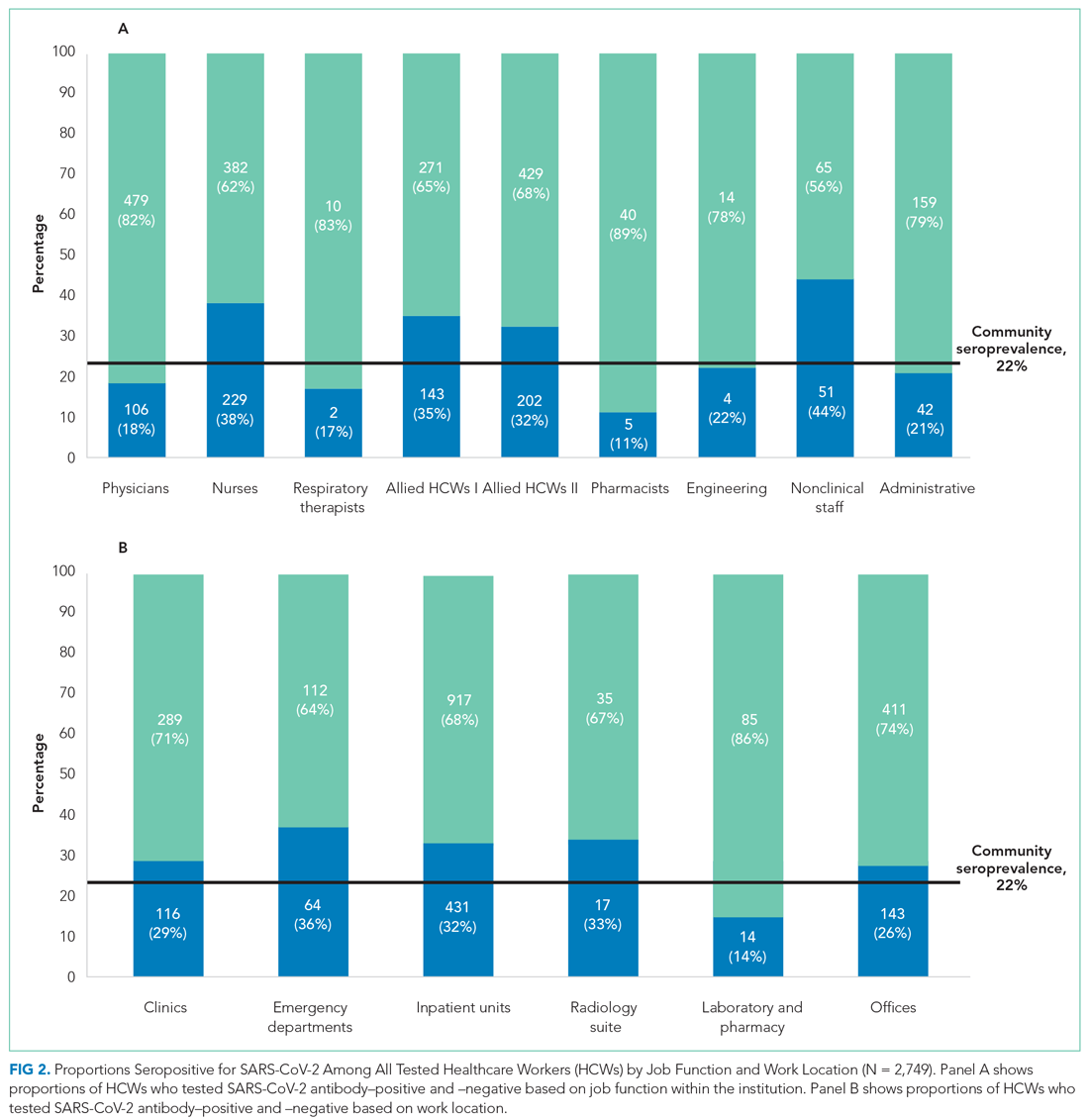
Among all HCWs, there were differences in seropositivity by job function (P = .001). The greatest proportion of HCWs were allied HCW II (23% [631/2,749]), followed by nurses (22.2% [611/2,749]) and physicians (21.3% [585/2,749] ). Seropositivity was highest for nonclinical staff (44.0% [51/116]), followed by nurses (37.5% [229/611]) and allied clinical HCW I and II (34.5% [143/414] and 32.0% [202/631], respectively). It was lowest for administrative staff (20.9% [42/201]) and pharmacists (11.1% [5/45]). Among HCWs residing in NYC, the rank order according to proportion seropositive by location was similar to that of the whole group. Administrative staff seropositivity was 18.3% (20/109). Administrative staff seropositivity for both groups was marginally lower than the 22% community seroprevalence in NYC for the same period.16
Odds Ratios for SARS-CoV-2 Seropositivity
For all HCWs, in unadjusted models (Appendix Table 3), age 45 to 64 years and Black race were associated with increased odds of being seropositive (1.26; 95% CI, 1.07-1.49 and 2.26; 95% CI, 1.51-3.37, respectively). Increased odds were seen for HCWs working in the ED (1.64; 95% CI, 1.14-2.36) and inpatient units (1.35; 95% CI, 1.08-1.69), and decreased odds were seen for those working in the laboratory and pharmacy (0.47; 95% CI, 0.26-0.86). Increased odds for seropositivity were found for nurses (2.27; 95% CI, 1.56-3.31), allied HCW I (2.00; 95% CI, 1.34-2.97), allied HCW II (1.78; 95% CI, 1.22-2.60), and nonclinical staff (2.97; 95% CI,1.80-4.90).
After adjusting for all covariates, HCWs who were Black remained at increased odds for being seropositive in the two final models (adjusted OR, 2.29; 95% CI, 1.38-3.81 and adjusted OR, 2.94; 95% CI, 1.78-4.85), as did those who had a BMI >30 kg/m2, with an adjusted OR of 1.36 (95% CI, 1.05-1.77) in one of the final models (Appendix Table 3). None of the other comorbid conditions had increased ORs. Those who worked in the ED and inpatient units also remained at increased odds after adjusting for covariates (2.27; 95% CI, 1.53-3.37 and 1.48; 95% CI, 1.14-1.92, respectively; Figure 3). Other job functions that had increased odds for seropositivity were nurses (2.54; 95% CI, 1.64-3.94), allied HCW I (1.83; 95% CI, 1.15-2.89) and II (1.70; 95% CI, 1.10-2.63), and nonclinical staff (2.51; 95% CI, 1.42-4.43).
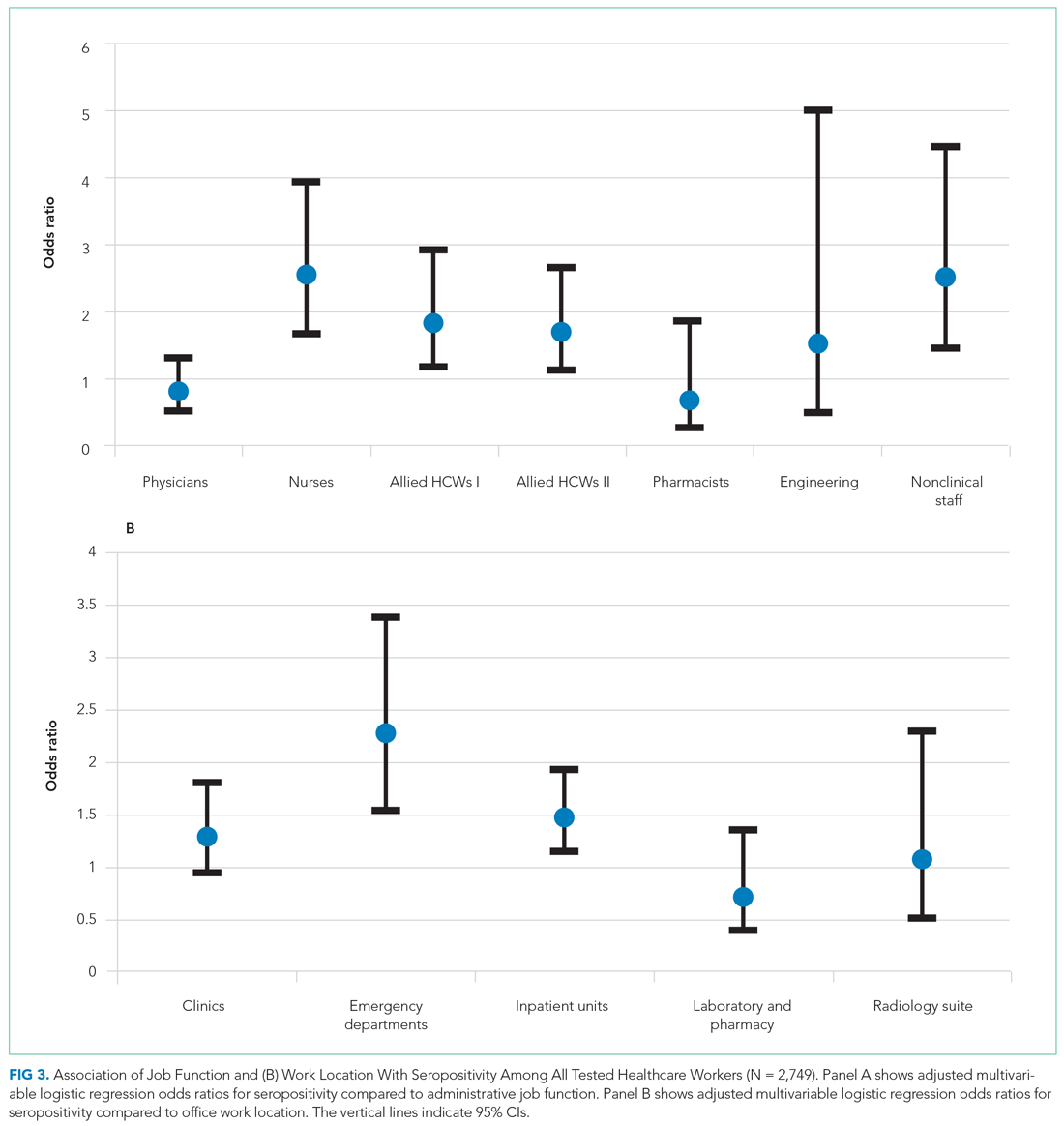
Having a positive PCR for SAR-CoV-2 on nasopharyngeal swabs was strongly associated with seropositivity (OR, 47.26; 95% CI, 29.30-76.23 and OR, 44.79; 95% CI, 27.87-72.00) in the two multivariate-adjusted models. These findings were confirmed when the analyses were performed on HCWs who resided in NYC (Appendix Table 4 and Appendix Figure 2).
DISCUSSION
In a large inner-city New York hospital, we report a cumulative SARS-CoV-2 seroprevalence of 30.2% in HCWs at the end of the first surge of SARS-CoV-2 infections in NYC. We identified the highest seropositivity rates for nonclinical staff and nurses, followed by allied HCWs, with the odds of being seropositive ranging from 1.7 to 2.5. The work locations with the highest seroprevalences were the ED and inpatient units, with 2.3-fold and 1.5-fold increased odds of seropositivity, respectively.
Serosurveillance studies have reported the trajectory of community prevalence in NYC over the first wave. A 6.3% prevalence was reported in samples collected between March 23 and April 1, 2020.17 In a study by Rosenberg et al18 with testing performed from April 9 through April 28, 2020, prevalence increased to 22.7%. Serosurveillance data from the NYC Department of Health show prevalence ranging from 20.1% to 23.3% (average 22%) during the study period.16 Compared to the estimated seroprevalence of 9.3% in the United States,19 these rates established NYC as an early epicenter for the COVID-19 pandemic, with our institution’s HCW seroprevalence considerably higher than NYC community serosurveillance rates, 2.2 times higher than reported in the earlier HCW study in the greater NYC area,13 and higher than the 27% rate during May 2020 recently reported in another NYC hospital.20
Data from studies of hospital transmission and effects of mitigation measures, such as a universal masking policy for HCWs and patients, clearly demonstrate the high effectiveness of these measures in reducing hospital transmissions.21,22 This suggests HCW seroprevalence in institutions with well-implemented infection control and universal masking policies may not be a consequence of workplace exposures, but rather may be reflective of community rates.23 Our institution’s response commenced February 3, 2020, with implementation of social distancing, a universal masking policy, transmission-based precautions, and use of fitted N95 masks. Mid-March, elective surgeries were canceled, and inpatient visitation suspended. During the surge, these measures were widely and consistently implemented for all categories of HCWs throughout the work environment, based on emerging guidelines from the Centers for Disease Control and Prevention (CDC) and NYC Department of Health. Our overall observed HCW seroprevalence, well above that of the community, with differences in categories of job function and work locations, is therefore an important finding. Our sample of 2,749 HCWs lived in NYC and its surrounding suburbs and nearby states. There is heterogeneity in community seroprevalence between areas outside of NYC and NYC (an epicenter) itself. We therefore analyzed our data in the subset with NYC zip codes, confirming a similar overall prevalence and increased odds of seropositivity in nurses, allied HCWs, and nonclinical staff.
Physicians and administrative and office staff had seropositivity rates of 18.1%, 20.9%, and 25.8%, respectively, consistent with community rates and illustrating the effectiveness of PPE in the hospital setting. Since PPE use was part of a universal policy applied to all HCWs in our institution, other possible reasons may explain the differences we found. We speculate that the close working relationship nurses have with their patients resulted in a longer duration and higher frequency of daily interactions, increasing the risk for transmission and causing breakthrough infections.24,25 This increased risk is reflected in a study in which 28% of hospitalized patients were nurses and 9% certified nursing assistants.26
The CDC recently redefined close contact with someone with COVID-19 as a cumulative total of >15 minutes over 24 hours.25 Thus, several multiple short periods of exposure can increase risk for infection with SARS-CoV-2; such exposure is characteristic of the job function of nurses, nursing staff, and nonclinical staff. Further, housekeeping, transportation, and security officers are all nonclinical staff with significant and multiple exposures to COVID-19 patients during the surge, and for security officers, to continuous public traffic in and out of the hospital. SARS-CoV-2 spreads by virus shedding in large droplets and aerosols, with droplet nuclei <5 microns in size efficiently dispersed in air, an important additional mode of transmission.27-30 Airborne transmission coupled with virus shedding in asymptomatic and presymptomatic persons, which has been shown to cause secondary attack rates of up to 32%, are other factors that likely contributed to the increased seroprevalence in this group.31 Our observation is consistent with the Birmingham study, which reported the highest rate in housekeeping staff, with a prevalence of 34.5%, compared to 44% in this study.6 Similar reasons for high seropositivity rates apply to the two groups of allied HCWs (eg, medical assistants and patient care technicians, social workers, nutritionists and therapists), whose job functions place them in intermittent but significant proximity with inpatients and outpatients.
Consistent with public health data showing that minorities are disproportionately affected by this disease, we found that Black HCWs were three times more likely to be seropositive.32 However, an unexpected observation was the association between obesity and SARS-CoV-2 seropositivity. A possible explanation for this association may be inability to achieve optimal fit testing for N95 masks, thereby increasing the risk of exposure to droplet nuclei. This is important given that obesity is associated with poorer outcomes from COVID-19.
During the height of the first wave in NYC, EDs and inpatient units handled a large volume of COVID-19 patients with high PCR positivity rates (peak of 51% in March in our hospital). It was not unexpected that we observed increased odds of seropositivity in these work locations. As ICUs were at capacity, inpatient units cared for critically ill patients they would not normally have. HCWs in these locations coped with an increased workload, increased demand on PPE supplies, and work fatigue, which contributed to increased risk for hospital-acquired SARS-CoV-2 infections.
Reporting seroprevalence at a single institution was a limitation of the study. Approximately 57% of the hospital’s total HCW population was tested for antibodies. It is possible their risk profile influenced their decision to volunteer for testing when it became available, introducing selection bias. A comparison between tested and untested HCWs showed similarity in all demographic measures, including nasopharyngeal PCR testing, except for age. We did not have information on symptoms that would prompt PCR testing. HCWs who underwent voluntary testing were younger compared to those who did not undergo testing. Current NYC serosurveillance data showed higher seropositivity in the 45-to-64–year age group (27.8%-28.6%) compared to the 65-to-74–year age group (24.3%), which suggests that the tested group may overestimate seroprevalence among HCWs relative to a randomly selected sample.33 Similarly, there were more nurses, allied HCWs, physicians, and administrative staff in the tested group, with the former two having higher SARS-CoV-2 seropositivity compared to community prevalence, which could also overestimate seroprevalence. Our large sample size provided us with the power to detect differences within several different job functions and work locations, a strength of this study. It was not possible to differentiate community- from hospital-acquired infection in our HCWs, a limitation in many observational HCW seroprevalence studies. However, when we analyzed data restricted only to HCWs in NYC, to reduce the effect of differing community prevalences outside the city, our results were unchanged. Since it is possible that nonclinical HCWs are of a lower socioeconomic status compared to others (nurses and allied HCWs), we cannot exclude the possibility that higher SARS-CoV-2 seroprevalence associated with lower status explains, partly or completely, the increased odds of seropositivity we observed.34 Due to the high proportion of missing data for race (61.3%), we advise caution in interpreting our finding that the odds of seropositivity were three times higher for Black race, even though consistent with prior literature.34 Healthcare organizations have similar job function and work location categories incorporated in their infrastructure, suggesting that our observations may be generalizable to other hospitals in the United States.
CONCLUSION
These findings show that during the first surge in NYC, with its increased burden of disease, hospitalizations, morbidity, and mortality, seroprevalences varied based on job function and work location within this institution. Nurses were at highest risk for SARS-CoV-2 infection, as were those who worked in the ED. In preparation for subsequent waves of SARS-CoV-2 and other highly contagious respiratory infections, major medical centers need to enhance efforts aimed at protecting HCWs, with particular attention to these groups. This study also strongly supports the recent CDC guideline prioritizing HCWs to receive COVID-19 mRNA and adenovirus vector vaccines that have obtained emergency use authorization by the US Food and Drug Administration.35
Acknowledgments
The authors thank all the residents, nurses, and staff of the Department of Family Medicine for their contribution to this work.
1. Liu YC, Kuo RL, Shih SR. COVID-19: The first documented coronavirus pandemic in history. Biomed J. 2020;43(4):328-333. https://doi.org/10.1016/j.bj.2020.04.007
2. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Accessed April 12, 2021. https://covid19.who.int
3. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475-e483. https://doi.org/10.1016/S2468-2667(20)30164-X
4. Gupta S, Federman DG. Hospital preparedness for COVID-19 pandemic: experience from department of medicine at Veterans Affairs Connecticut Healthcare System. Postgrad Med. 2020:1-6. https://doi.org/10.1080/00325481.2020.1761668
5. Woolley K, Smith R, Arumugam S. Personal protective equipment (PPE) guidelines, adaptations and lessons during the COVID-19 pandemic. Ethics Med Public Health. 2020;14:100546. https://doi.org/10.1016/j.jemep.2020.100546
6. Shields A, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75(12):1089-1094. https://doi.org/10.1136/thoraxjnl-2020-215414
7. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. https://doi.org/10.1001/jama.2020.11160
8. Stubblefield WB, Talbot HK, Feldstein L, et al. Seroprevalence of SARS-CoV-2 Among frontline healthcare personnel during the first month of caring for COVID-19 patients - Nashville, Tennessee. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa936
9. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. https://doi.org/10.1016/j.jcv.2020.104437
10. Keeley AJ, Evans C, Colton H, et al. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill. 2020;25(14). https://doi.org/10.2807/1560-7917.ES.2020.25.14.2000433
11. Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network - 13 academic medical centers, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(35):1221-1226. https://doi.org/10.15585/mmwr.mm6935e2
12. Jeremias A, Nguyen J, Levine J, et al. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Intern Med. 2020 Aug 11:e204214. https://doi.org/10.1001/jamainternmed.2020.4214
13. Moscola J, Sembajwe G, Jarrett M, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324(9):893-895. https://doi.org/10.1001/jama.2020.14765
14. Gonzalez-Reiche AS, Hernandez MM, Sullivan MJ, et al. Introductions and early spread of SARS-CoV-2 in the New York City area. Science. 2020;369(6501):297-301. https://doi.org/10.1126/science.abc1917
15. Lau CS, Hoo SF, Yew SF, et al. Evaluation of the Roche Elecsys Anti-SARS-CoV-2 assay. Preprint. Posted online June 29, 2020. Accessed November 8, 2020. https://www.medrxiv.org/content/10.1101/2020.06.28.20142232v1 https://doi.org/10.1101/2020.06.28.20142232
16. New York City Department of Health. Covid-19: data. long-term trends. Antibody testing. Accessed March 5, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-trends.page#antibody
17. Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. Published online July 21, 2020. https://doi.org/10.1001/jamainternmed.2020.4130
18. Rosenberg ES, Tesoriero JM, Rosenthal EM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. Aug 2020;48:23-29 e4. https://doi.org/10.1016/j.annepidem.2020.06.004
19. Anand S, Montez-Rath M, Han J, et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet. 2020;396(10259):1335-1344. https://doi.org/10.1016/S0140-6736(20)32009-2
20. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: a cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2020;102:63-69. https://doi.org/10.1016/j.ijid.2020.10.036
21. Samaranayake LP, Fakhruddin KS, Ngo HC, Chang JWW, Panduwawala C. The effectiveness and efficacy of respiratory protective equipment (RPE) in dentistry and other health care settings: a systematic review. Acta Odontol Scand. 2020;78(8):626-639. https://doi.org/10.1080/00016357.2020.1810769
22. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
23. Richterman A, Meyerowitz EA, Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public health. JAMA. Published online November 13, 2020. https://doi.org/10.1001/jama.2020.21399
24. Degesys NF, Wang RC, Kwan E, Fahimi J, Noble JA, Raven MC. Correlation between n95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324(1):94-96. https://doi.org/10.1001/jama.2020.9843
25. Pringle JC, Leikauskas J, Ransom-Kelley S, et al. COVID-19 in a correctional facility employee following multiple brief exposures to persons with COVID-19 - Vermont, July-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1569-1570. https://doi.org/10.15585/mmwr.mm6943e1
26. Kambhampati AK, O’Halloran AC, Whitaker M, et al. COVID-19-associated hospitalizations among health care personnel - COVID-NET, 13 states, March 1-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1576-1583. https://doi.org/10.15585/mmwr.mm6943e3
27. Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117(26):14857-14863. https://doi.org/10.1073/pnas.2009637117
28. Setti L, Passarini F, De Gennaro G, et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not be enough. Int J Environ Res Public Health. 2020;17(8):2932. https://doi.org/doi:10.3390/ijerph17082932
29. Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324(5):441-442. https://doi.org/10.1001/jama.2020.12458
30. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323(18):1837-1838. https://doi.org/10.1001/jama.2020.4756
31. Qiu X, Nergiz A, Maraolo A, Bogoch I, Low N, Cevik M. The role of asymptomatic and pre-symptomatic infection in SARS-CoV-2 transmission – a living systematic review. Clin Mibrobiol Infect. 2021;20:S1198-743X(21)00038-0. Published online January 20, 2021. https://doi.org/10.1016/j.cmi.2021.01.011
32. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/doi:10.1056/NEJMsa2011686
33. New York City Department of Health. Covid-19: Data. Antibody testing by group - age. Accessed March 5, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-totals.page#antibody
34. Patel JA, Nielsen FBH, Badiani AA, et al. Poverty, inequality and COVID-19: the forgotten vulnerable. Public Health. 2020;183:110-111. https://doi.org/10.1016/j.puhe.2020.05.006
35. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. https://doi.org/10.1056/NEJMoa2034577
SARS-CoV-2 has infected 141 million people worldwide and 31 million people in the United States as of April 20, 2021.1,2 The influx of hospital admissions and deaths has severely strained healthcare systems worldwide and placed healthcare workers (HCWs) at increased risk for acquiring COVID-19.3-5
Several studies have described the impact of COVID-19 on this heterogeneous group of HCWs. Shields et al reported a seroprevalence of 24.4% in HCWs at University Hospitals Birmingham (UK), with the highest rate, 34.5%, in housekeeping staff.6 Steensels et al reported a lower prevalence of 6.4% at a tertiary care center in Belgium, and showed no increased risk for HCWs when directly involved in clinical care.7 The authors attributed this to adequate use of personal protective equipment (PPE). Other studies have reported seroprevalences ranging from 1.6% to 18%.8-11 In the New York City (NYC) metro area, Jeremias et al reported a seroprevalence of 9.8% in HCWs and found no difference by job title or work location,12 whereas Moscola et al reported a seroprevalence of 13.7% and demonstrated a 3% increased risk for those working in service or maintenance.13 Antibody tests were conducted between March and April 2020 in all but two of these studies; testing in these two studies was performed between April 13 and June 23, 2020, with one reporting a seroprevalence of 6%11 and the other, 13.7%.13
NYC became the earliest pandemic epicenter in the United States following untracked transmission from ongoing circulation of SARS-CoV-2 in Europe.14 As a result, the COVID-19 surge in NYC commenced in March and largely subsided by the end of May 2020. Most HCW data reported to date do not reflect the situation at the end of the surge, and may underestimate true seroprevalence. We describe SARS-CoV-2 seroprevalence in HCWs in a large inner-city hospital in NYC, with antibody testing conducted from May 18 to June 26, 2020, at the subsidence of the surge. To further our understanding of occupational risk among different groups of HCWs, we examined associations of seroprevalence with HCWs’ job function and work location.
METHODS
This was a cross-sectional seroprevalence study conducted in the BronxCare Health System located in South and Central Bronx, an area that experienced one of the highest incidences of SARS-CoV-2 infections within NYC’s five boroughs.
HCWs were offered voluntary testing for serum antibodies to SARS-CoV-2 between May 18 and June 26, 2020. Testing occurred in the institution’s auditorium, a central and easily accessible location. Weekly emails were sent to all employees and department heads during the testing period, offering antibody testing and providing location and testing time information. The Elecsys Anti-SARS-CoV-2 (Roche) assay measuring total qualitative antibodies was used; the assay has a reported sensitivity of 97.1% 14 days after a positive SARS-CoV-2 RNA polymerase chain reaction (PCR) test result and a specificity of 100%.15
Demographic and work-related information was abstracted from electronic medical records, including all comorbid conditions that affected 30 or more HCWs. Pulmonary diagnoses, including asthma and chronic obstructive pulmonary disease, were grouped as chronic lung disease, and cardiovascular diseases, including hypertension, as chronic heart disease. Personal identifiers and data were delinked upon completion of data abstraction. The study was approved by the hospital’s institutional review board.
Job Function and Work Location
HCWs were grouped by job function as follows: physicians; nurses (including physician assistants and nurse practitioners); allied HCW I (medical assistants, patient care, and electrocardiogram, radiology, and ear, nose and throat technicians); allied HCW II (social workers, dieticians and nutritionists, registration clerks and unit associates, physical and occupational therapists); nonclinical staff (patient transporters, housekeeping staff, and security staff); pharmacists; engineering; and administrative staff. Respiratory therapists were considered as a separate group as their work placed them at high risk for respiratory diseases.
Work locations were as follows: clinics (including dental, outpatient, and satellite clinics), emergency departments (ED), inpatient units (including floors and intensive care units [ICU]), radiology suite, laboratory and pharmacy, and offices.
Statistical Analysis
Descriptive statistics were calculated using χ2 analyses. All demographic variables were tested against serology status (positive/negative). A binary logistic regression analysis was used to calculate odds ratios (ORs). Eight separate univariate unadjusted ORs were calculated by running each predictor variable against serology status (dependent variable), which included the six categorical variables—race, ethnicity, age, sex, body mass index (BMI), and prior SARS-CoV-2 PCR results—and the two main predictors—job function and work location. To obtain adjusted ORs, two final separate multivariable logistic regression analyses were executed including the six covariates listed. Due to high collinearity between job function and work location (χ2 = 3030.13, df = 35 [6 levels of work location – 1]*[8 levels of job function – 1]; P < .001), we included only one of the main predictors in each model. The regressions were specified such that the reference groups for the work location and job function variables were office work and administration, respectively. This choice was made based on the fact that their nonclinical functions do not confer an exposure risk in excess of that experienced by typical community populations. Sensitivity analyses were performed on the subset of HCWs whose address zip codes indicated residence within NYC to exclude the effect of different community seroprevalences in areas outside of NYC. The 95% CI for seroprevalence of antibodies within tested HCWs was estimated using the Clopper-Pearson binomial method.
RESULTS
Among all HCWs in the institution (N = 4,807), 2,749 (57.2%) underwent voluntary testing. Of those who underwent testing, 831 were positive for antibodies to SARS-CoV-2 (Figure 1), a seroprevalence of 30.2% (95% CI, 29%-32%). Among the age groups, the 45-to-64−year group had the highest seropositivity at 33% (400/1203), and those ≥75 years of age, the lowest at 16.7% (2/12) (P < .009). 

Among all tested HCWs, 70.1% (1,928/2,749) resided in NYC. SARS-CoV-2 seroprevalence in this subset was 32% (616/1,928) (Figure 1). Demographic and comorbid conditions in HCWs who lived in NYC were similar to those of the whole group (Appendix Table 1).
HCWs who underwent voluntary antibody testing (Appendix Table 2) had a higher percentage of persons in the 45-to-64−year age group (43.8% vs 40.9%) and a lower percentage of persons in the 65-to-74−year age group (3.3% vs 5.3%) compared with the group of HCWs that did not undergo testing (P < .001). Gender, race, ethnicity, comorbid conditions, SARS-CoV-2 PCR testing, and work locations were not different between groups. The tested group had higher proportions of clinicians (physicians, nurses, allied HCWs I and II) than the untested nonparticipant group (P = .014).
SARS-CoV-2 PCR Tests on HCWs
More than one-third (34.1%; 938/2,749) of HCWs had a documented nasopharyngeal PCR test between March 23 and June 26, 2020 (Table). Of all PCRs performed, 262 were positive, giving an overall PCR positivity rate of 27.9%. Positivity was 51.4% in March and 36.6% in April. The reasons for PCR testing were not available, but likely represent a combination of exposure-related testing among asymptomatic individuals and diagnostic testing of symptomatic HCWs. In contrast, serology testing was indicative of prior infection and yielded a cumulative seroprevalence at the end of the surge. Findings were similar among HCWs residing in NYC
Work Location and Job Function
Among all HCWs (Table, Figure 2), there were differences in seropositivity by work location (P = .001). The largest number of HCWs worked in inpatient units (1,348/2,749, 49%), and the second largest in offices (554/2,749, 20%). The highest seropositivity rate was in the EDs, at 36.4% (64/176), followed by radiology suites, at 32.7% (17/52); the seropositivity rate in office locations was 25.8% (143/554). Among HCWs residing in NYC (Appendix Table 1, Appendix Figure 1), the rank order according to proportion seropositive by work location was similar to that of the whole group (P = .004), except that the second highest seropositivity rate was in the inpatient units (33.9% [323/953]). In the group of HCWs residing in NYC, office locations had a seropositivity of 27.4% (102/372). The seropositivity rates for both groups working in office locations were slightly higher than the 22% community seroprevalence in NYC reported for the same period.16

Among all HCWs, there were differences in seropositivity by job function (P = .001). The greatest proportion of HCWs were allied HCW II (23% [631/2,749]), followed by nurses (22.2% [611/2,749]) and physicians (21.3% [585/2,749] ). Seropositivity was highest for nonclinical staff (44.0% [51/116]), followed by nurses (37.5% [229/611]) and allied clinical HCW I and II (34.5% [143/414] and 32.0% [202/631], respectively). It was lowest for administrative staff (20.9% [42/201]) and pharmacists (11.1% [5/45]). Among HCWs residing in NYC, the rank order according to proportion seropositive by location was similar to that of the whole group. Administrative staff seropositivity was 18.3% (20/109). Administrative staff seropositivity for both groups was marginally lower than the 22% community seroprevalence in NYC for the same period.16
Odds Ratios for SARS-CoV-2 Seropositivity
For all HCWs, in unadjusted models (Appendix Table 3), age 45 to 64 years and Black race were associated with increased odds of being seropositive (1.26; 95% CI, 1.07-1.49 and 2.26; 95% CI, 1.51-3.37, respectively). Increased odds were seen for HCWs working in the ED (1.64; 95% CI, 1.14-2.36) and inpatient units (1.35; 95% CI, 1.08-1.69), and decreased odds were seen for those working in the laboratory and pharmacy (0.47; 95% CI, 0.26-0.86). Increased odds for seropositivity were found for nurses (2.27; 95% CI, 1.56-3.31), allied HCW I (2.00; 95% CI, 1.34-2.97), allied HCW II (1.78; 95% CI, 1.22-2.60), and nonclinical staff (2.97; 95% CI,1.80-4.90).
After adjusting for all covariates, HCWs who were Black remained at increased odds for being seropositive in the two final models (adjusted OR, 2.29; 95% CI, 1.38-3.81 and adjusted OR, 2.94; 95% CI, 1.78-4.85), as did those who had a BMI >30 kg/m2, with an adjusted OR of 1.36 (95% CI, 1.05-1.77) in one of the final models (Appendix Table 3). None of the other comorbid conditions had increased ORs. Those who worked in the ED and inpatient units also remained at increased odds after adjusting for covariates (2.27; 95% CI, 1.53-3.37 and 1.48; 95% CI, 1.14-1.92, respectively; Figure 3). Other job functions that had increased odds for seropositivity were nurses (2.54; 95% CI, 1.64-3.94), allied HCW I (1.83; 95% CI, 1.15-2.89) and II (1.70; 95% CI, 1.10-2.63), and nonclinical staff (2.51; 95% CI, 1.42-4.43).

Having a positive PCR for SAR-CoV-2 on nasopharyngeal swabs was strongly associated with seropositivity (OR, 47.26; 95% CI, 29.30-76.23 and OR, 44.79; 95% CI, 27.87-72.00) in the two multivariate-adjusted models. These findings were confirmed when the analyses were performed on HCWs who resided in NYC (Appendix Table 4 and Appendix Figure 2).
DISCUSSION
In a large inner-city New York hospital, we report a cumulative SARS-CoV-2 seroprevalence of 30.2% in HCWs at the end of the first surge of SARS-CoV-2 infections in NYC. We identified the highest seropositivity rates for nonclinical staff and nurses, followed by allied HCWs, with the odds of being seropositive ranging from 1.7 to 2.5. The work locations with the highest seroprevalences were the ED and inpatient units, with 2.3-fold and 1.5-fold increased odds of seropositivity, respectively.
Serosurveillance studies have reported the trajectory of community prevalence in NYC over the first wave. A 6.3% prevalence was reported in samples collected between March 23 and April 1, 2020.17 In a study by Rosenberg et al18 with testing performed from April 9 through April 28, 2020, prevalence increased to 22.7%. Serosurveillance data from the NYC Department of Health show prevalence ranging from 20.1% to 23.3% (average 22%) during the study period.16 Compared to the estimated seroprevalence of 9.3% in the United States,19 these rates established NYC as an early epicenter for the COVID-19 pandemic, with our institution’s HCW seroprevalence considerably higher than NYC community serosurveillance rates, 2.2 times higher than reported in the earlier HCW study in the greater NYC area,13 and higher than the 27% rate during May 2020 recently reported in another NYC hospital.20
Data from studies of hospital transmission and effects of mitigation measures, such as a universal masking policy for HCWs and patients, clearly demonstrate the high effectiveness of these measures in reducing hospital transmissions.21,22 This suggests HCW seroprevalence in institutions with well-implemented infection control and universal masking policies may not be a consequence of workplace exposures, but rather may be reflective of community rates.23 Our institution’s response commenced February 3, 2020, with implementation of social distancing, a universal masking policy, transmission-based precautions, and use of fitted N95 masks. Mid-March, elective surgeries were canceled, and inpatient visitation suspended. During the surge, these measures were widely and consistently implemented for all categories of HCWs throughout the work environment, based on emerging guidelines from the Centers for Disease Control and Prevention (CDC) and NYC Department of Health. Our overall observed HCW seroprevalence, well above that of the community, with differences in categories of job function and work locations, is therefore an important finding. Our sample of 2,749 HCWs lived in NYC and its surrounding suburbs and nearby states. There is heterogeneity in community seroprevalence between areas outside of NYC and NYC (an epicenter) itself. We therefore analyzed our data in the subset with NYC zip codes, confirming a similar overall prevalence and increased odds of seropositivity in nurses, allied HCWs, and nonclinical staff.
Physicians and administrative and office staff had seropositivity rates of 18.1%, 20.9%, and 25.8%, respectively, consistent with community rates and illustrating the effectiveness of PPE in the hospital setting. Since PPE use was part of a universal policy applied to all HCWs in our institution, other possible reasons may explain the differences we found. We speculate that the close working relationship nurses have with their patients resulted in a longer duration and higher frequency of daily interactions, increasing the risk for transmission and causing breakthrough infections.24,25 This increased risk is reflected in a study in which 28% of hospitalized patients were nurses and 9% certified nursing assistants.26
The CDC recently redefined close contact with someone with COVID-19 as a cumulative total of >15 minutes over 24 hours.25 Thus, several multiple short periods of exposure can increase risk for infection with SARS-CoV-2; such exposure is characteristic of the job function of nurses, nursing staff, and nonclinical staff. Further, housekeeping, transportation, and security officers are all nonclinical staff with significant and multiple exposures to COVID-19 patients during the surge, and for security officers, to continuous public traffic in and out of the hospital. SARS-CoV-2 spreads by virus shedding in large droplets and aerosols, with droplet nuclei <5 microns in size efficiently dispersed in air, an important additional mode of transmission.27-30 Airborne transmission coupled with virus shedding in asymptomatic and presymptomatic persons, which has been shown to cause secondary attack rates of up to 32%, are other factors that likely contributed to the increased seroprevalence in this group.31 Our observation is consistent with the Birmingham study, which reported the highest rate in housekeeping staff, with a prevalence of 34.5%, compared to 44% in this study.6 Similar reasons for high seropositivity rates apply to the two groups of allied HCWs (eg, medical assistants and patient care technicians, social workers, nutritionists and therapists), whose job functions place them in intermittent but significant proximity with inpatients and outpatients.
Consistent with public health data showing that minorities are disproportionately affected by this disease, we found that Black HCWs were three times more likely to be seropositive.32 However, an unexpected observation was the association between obesity and SARS-CoV-2 seropositivity. A possible explanation for this association may be inability to achieve optimal fit testing for N95 masks, thereby increasing the risk of exposure to droplet nuclei. This is important given that obesity is associated with poorer outcomes from COVID-19.
During the height of the first wave in NYC, EDs and inpatient units handled a large volume of COVID-19 patients with high PCR positivity rates (peak of 51% in March in our hospital). It was not unexpected that we observed increased odds of seropositivity in these work locations. As ICUs were at capacity, inpatient units cared for critically ill patients they would not normally have. HCWs in these locations coped with an increased workload, increased demand on PPE supplies, and work fatigue, which contributed to increased risk for hospital-acquired SARS-CoV-2 infections.
Reporting seroprevalence at a single institution was a limitation of the study. Approximately 57% of the hospital’s total HCW population was tested for antibodies. It is possible their risk profile influenced their decision to volunteer for testing when it became available, introducing selection bias. A comparison between tested and untested HCWs showed similarity in all demographic measures, including nasopharyngeal PCR testing, except for age. We did not have information on symptoms that would prompt PCR testing. HCWs who underwent voluntary testing were younger compared to those who did not undergo testing. Current NYC serosurveillance data showed higher seropositivity in the 45-to-64–year age group (27.8%-28.6%) compared to the 65-to-74–year age group (24.3%), which suggests that the tested group may overestimate seroprevalence among HCWs relative to a randomly selected sample.33 Similarly, there were more nurses, allied HCWs, physicians, and administrative staff in the tested group, with the former two having higher SARS-CoV-2 seropositivity compared to community prevalence, which could also overestimate seroprevalence. Our large sample size provided us with the power to detect differences within several different job functions and work locations, a strength of this study. It was not possible to differentiate community- from hospital-acquired infection in our HCWs, a limitation in many observational HCW seroprevalence studies. However, when we analyzed data restricted only to HCWs in NYC, to reduce the effect of differing community prevalences outside the city, our results were unchanged. Since it is possible that nonclinical HCWs are of a lower socioeconomic status compared to others (nurses and allied HCWs), we cannot exclude the possibility that higher SARS-CoV-2 seroprevalence associated with lower status explains, partly or completely, the increased odds of seropositivity we observed.34 Due to the high proportion of missing data for race (61.3%), we advise caution in interpreting our finding that the odds of seropositivity were three times higher for Black race, even though consistent with prior literature.34 Healthcare organizations have similar job function and work location categories incorporated in their infrastructure, suggesting that our observations may be generalizable to other hospitals in the United States.
CONCLUSION
These findings show that during the first surge in NYC, with its increased burden of disease, hospitalizations, morbidity, and mortality, seroprevalences varied based on job function and work location within this institution. Nurses were at highest risk for SARS-CoV-2 infection, as were those who worked in the ED. In preparation for subsequent waves of SARS-CoV-2 and other highly contagious respiratory infections, major medical centers need to enhance efforts aimed at protecting HCWs, with particular attention to these groups. This study also strongly supports the recent CDC guideline prioritizing HCWs to receive COVID-19 mRNA and adenovirus vector vaccines that have obtained emergency use authorization by the US Food and Drug Administration.35
Acknowledgments
The authors thank all the residents, nurses, and staff of the Department of Family Medicine for their contribution to this work.
SARS-CoV-2 has infected 141 million people worldwide and 31 million people in the United States as of April 20, 2021.1,2 The influx of hospital admissions and deaths has severely strained healthcare systems worldwide and placed healthcare workers (HCWs) at increased risk for acquiring COVID-19.3-5
Several studies have described the impact of COVID-19 on this heterogeneous group of HCWs. Shields et al reported a seroprevalence of 24.4% in HCWs at University Hospitals Birmingham (UK), with the highest rate, 34.5%, in housekeeping staff.6 Steensels et al reported a lower prevalence of 6.4% at a tertiary care center in Belgium, and showed no increased risk for HCWs when directly involved in clinical care.7 The authors attributed this to adequate use of personal protective equipment (PPE). Other studies have reported seroprevalences ranging from 1.6% to 18%.8-11 In the New York City (NYC) metro area, Jeremias et al reported a seroprevalence of 9.8% in HCWs and found no difference by job title or work location,12 whereas Moscola et al reported a seroprevalence of 13.7% and demonstrated a 3% increased risk for those working in service or maintenance.13 Antibody tests were conducted between March and April 2020 in all but two of these studies; testing in these two studies was performed between April 13 and June 23, 2020, with one reporting a seroprevalence of 6%11 and the other, 13.7%.13
NYC became the earliest pandemic epicenter in the United States following untracked transmission from ongoing circulation of SARS-CoV-2 in Europe.14 As a result, the COVID-19 surge in NYC commenced in March and largely subsided by the end of May 2020. Most HCW data reported to date do not reflect the situation at the end of the surge, and may underestimate true seroprevalence. We describe SARS-CoV-2 seroprevalence in HCWs in a large inner-city hospital in NYC, with antibody testing conducted from May 18 to June 26, 2020, at the subsidence of the surge. To further our understanding of occupational risk among different groups of HCWs, we examined associations of seroprevalence with HCWs’ job function and work location.
METHODS
This was a cross-sectional seroprevalence study conducted in the BronxCare Health System located in South and Central Bronx, an area that experienced one of the highest incidences of SARS-CoV-2 infections within NYC’s five boroughs.
HCWs were offered voluntary testing for serum antibodies to SARS-CoV-2 between May 18 and June 26, 2020. Testing occurred in the institution’s auditorium, a central and easily accessible location. Weekly emails were sent to all employees and department heads during the testing period, offering antibody testing and providing location and testing time information. The Elecsys Anti-SARS-CoV-2 (Roche) assay measuring total qualitative antibodies was used; the assay has a reported sensitivity of 97.1% 14 days after a positive SARS-CoV-2 RNA polymerase chain reaction (PCR) test result and a specificity of 100%.15
Demographic and work-related information was abstracted from electronic medical records, including all comorbid conditions that affected 30 or more HCWs. Pulmonary diagnoses, including asthma and chronic obstructive pulmonary disease, were grouped as chronic lung disease, and cardiovascular diseases, including hypertension, as chronic heart disease. Personal identifiers and data were delinked upon completion of data abstraction. The study was approved by the hospital’s institutional review board.
Job Function and Work Location
HCWs were grouped by job function as follows: physicians; nurses (including physician assistants and nurse practitioners); allied HCW I (medical assistants, patient care, and electrocardiogram, radiology, and ear, nose and throat technicians); allied HCW II (social workers, dieticians and nutritionists, registration clerks and unit associates, physical and occupational therapists); nonclinical staff (patient transporters, housekeeping staff, and security staff); pharmacists; engineering; and administrative staff. Respiratory therapists were considered as a separate group as their work placed them at high risk for respiratory diseases.
Work locations were as follows: clinics (including dental, outpatient, and satellite clinics), emergency departments (ED), inpatient units (including floors and intensive care units [ICU]), radiology suite, laboratory and pharmacy, and offices.
Statistical Analysis
Descriptive statistics were calculated using χ2 analyses. All demographic variables were tested against serology status (positive/negative). A binary logistic regression analysis was used to calculate odds ratios (ORs). Eight separate univariate unadjusted ORs were calculated by running each predictor variable against serology status (dependent variable), which included the six categorical variables—race, ethnicity, age, sex, body mass index (BMI), and prior SARS-CoV-2 PCR results—and the two main predictors—job function and work location. To obtain adjusted ORs, two final separate multivariable logistic regression analyses were executed including the six covariates listed. Due to high collinearity between job function and work location (χ2 = 3030.13, df = 35 [6 levels of work location – 1]*[8 levels of job function – 1]; P < .001), we included only one of the main predictors in each model. The regressions were specified such that the reference groups for the work location and job function variables were office work and administration, respectively. This choice was made based on the fact that their nonclinical functions do not confer an exposure risk in excess of that experienced by typical community populations. Sensitivity analyses were performed on the subset of HCWs whose address zip codes indicated residence within NYC to exclude the effect of different community seroprevalences in areas outside of NYC. The 95% CI for seroprevalence of antibodies within tested HCWs was estimated using the Clopper-Pearson binomial method.
RESULTS
Among all HCWs in the institution (N = 4,807), 2,749 (57.2%) underwent voluntary testing. Of those who underwent testing, 831 were positive for antibodies to SARS-CoV-2 (Figure 1), a seroprevalence of 30.2% (95% CI, 29%-32%). Among the age groups, the 45-to-64−year group had the highest seropositivity at 33% (400/1203), and those ≥75 years of age, the lowest at 16.7% (2/12) (P < .009). 

Among all tested HCWs, 70.1% (1,928/2,749) resided in NYC. SARS-CoV-2 seroprevalence in this subset was 32% (616/1,928) (Figure 1). Demographic and comorbid conditions in HCWs who lived in NYC were similar to those of the whole group (Appendix Table 1).
HCWs who underwent voluntary antibody testing (Appendix Table 2) had a higher percentage of persons in the 45-to-64−year age group (43.8% vs 40.9%) and a lower percentage of persons in the 65-to-74−year age group (3.3% vs 5.3%) compared with the group of HCWs that did not undergo testing (P < .001). Gender, race, ethnicity, comorbid conditions, SARS-CoV-2 PCR testing, and work locations were not different between groups. The tested group had higher proportions of clinicians (physicians, nurses, allied HCWs I and II) than the untested nonparticipant group (P = .014).
SARS-CoV-2 PCR Tests on HCWs
More than one-third (34.1%; 938/2,749) of HCWs had a documented nasopharyngeal PCR test between March 23 and June 26, 2020 (Table). Of all PCRs performed, 262 were positive, giving an overall PCR positivity rate of 27.9%. Positivity was 51.4% in March and 36.6% in April. The reasons for PCR testing were not available, but likely represent a combination of exposure-related testing among asymptomatic individuals and diagnostic testing of symptomatic HCWs. In contrast, serology testing was indicative of prior infection and yielded a cumulative seroprevalence at the end of the surge. Findings were similar among HCWs residing in NYC
Work Location and Job Function
Among all HCWs (Table, Figure 2), there were differences in seropositivity by work location (P = .001). The largest number of HCWs worked in inpatient units (1,348/2,749, 49%), and the second largest in offices (554/2,749, 20%). The highest seropositivity rate was in the EDs, at 36.4% (64/176), followed by radiology suites, at 32.7% (17/52); the seropositivity rate in office locations was 25.8% (143/554). Among HCWs residing in NYC (Appendix Table 1, Appendix Figure 1), the rank order according to proportion seropositive by work location was similar to that of the whole group (P = .004), except that the second highest seropositivity rate was in the inpatient units (33.9% [323/953]). In the group of HCWs residing in NYC, office locations had a seropositivity of 27.4% (102/372). The seropositivity rates for both groups working in office locations were slightly higher than the 22% community seroprevalence in NYC reported for the same period.16

Among all HCWs, there were differences in seropositivity by job function (P = .001). The greatest proportion of HCWs were allied HCW II (23% [631/2,749]), followed by nurses (22.2% [611/2,749]) and physicians (21.3% [585/2,749] ). Seropositivity was highest for nonclinical staff (44.0% [51/116]), followed by nurses (37.5% [229/611]) and allied clinical HCW I and II (34.5% [143/414] and 32.0% [202/631], respectively). It was lowest for administrative staff (20.9% [42/201]) and pharmacists (11.1% [5/45]). Among HCWs residing in NYC, the rank order according to proportion seropositive by location was similar to that of the whole group. Administrative staff seropositivity was 18.3% (20/109). Administrative staff seropositivity for both groups was marginally lower than the 22% community seroprevalence in NYC for the same period.16
Odds Ratios for SARS-CoV-2 Seropositivity
For all HCWs, in unadjusted models (Appendix Table 3), age 45 to 64 years and Black race were associated with increased odds of being seropositive (1.26; 95% CI, 1.07-1.49 and 2.26; 95% CI, 1.51-3.37, respectively). Increased odds were seen for HCWs working in the ED (1.64; 95% CI, 1.14-2.36) and inpatient units (1.35; 95% CI, 1.08-1.69), and decreased odds were seen for those working in the laboratory and pharmacy (0.47; 95% CI, 0.26-0.86). Increased odds for seropositivity were found for nurses (2.27; 95% CI, 1.56-3.31), allied HCW I (2.00; 95% CI, 1.34-2.97), allied HCW II (1.78; 95% CI, 1.22-2.60), and nonclinical staff (2.97; 95% CI,1.80-4.90).
After adjusting for all covariates, HCWs who were Black remained at increased odds for being seropositive in the two final models (adjusted OR, 2.29; 95% CI, 1.38-3.81 and adjusted OR, 2.94; 95% CI, 1.78-4.85), as did those who had a BMI >30 kg/m2, with an adjusted OR of 1.36 (95% CI, 1.05-1.77) in one of the final models (Appendix Table 3). None of the other comorbid conditions had increased ORs. Those who worked in the ED and inpatient units also remained at increased odds after adjusting for covariates (2.27; 95% CI, 1.53-3.37 and 1.48; 95% CI, 1.14-1.92, respectively; Figure 3). Other job functions that had increased odds for seropositivity were nurses (2.54; 95% CI, 1.64-3.94), allied HCW I (1.83; 95% CI, 1.15-2.89) and II (1.70; 95% CI, 1.10-2.63), and nonclinical staff (2.51; 95% CI, 1.42-4.43).

Having a positive PCR for SAR-CoV-2 on nasopharyngeal swabs was strongly associated with seropositivity (OR, 47.26; 95% CI, 29.30-76.23 and OR, 44.79; 95% CI, 27.87-72.00) in the two multivariate-adjusted models. These findings were confirmed when the analyses were performed on HCWs who resided in NYC (Appendix Table 4 and Appendix Figure 2).
DISCUSSION
In a large inner-city New York hospital, we report a cumulative SARS-CoV-2 seroprevalence of 30.2% in HCWs at the end of the first surge of SARS-CoV-2 infections in NYC. We identified the highest seropositivity rates for nonclinical staff and nurses, followed by allied HCWs, with the odds of being seropositive ranging from 1.7 to 2.5. The work locations with the highest seroprevalences were the ED and inpatient units, with 2.3-fold and 1.5-fold increased odds of seropositivity, respectively.
Serosurveillance studies have reported the trajectory of community prevalence in NYC over the first wave. A 6.3% prevalence was reported in samples collected between March 23 and April 1, 2020.17 In a study by Rosenberg et al18 with testing performed from April 9 through April 28, 2020, prevalence increased to 22.7%. Serosurveillance data from the NYC Department of Health show prevalence ranging from 20.1% to 23.3% (average 22%) during the study period.16 Compared to the estimated seroprevalence of 9.3% in the United States,19 these rates established NYC as an early epicenter for the COVID-19 pandemic, with our institution’s HCW seroprevalence considerably higher than NYC community serosurveillance rates, 2.2 times higher than reported in the earlier HCW study in the greater NYC area,13 and higher than the 27% rate during May 2020 recently reported in another NYC hospital.20
Data from studies of hospital transmission and effects of mitigation measures, such as a universal masking policy for HCWs and patients, clearly demonstrate the high effectiveness of these measures in reducing hospital transmissions.21,22 This suggests HCW seroprevalence in institutions with well-implemented infection control and universal masking policies may not be a consequence of workplace exposures, but rather may be reflective of community rates.23 Our institution’s response commenced February 3, 2020, with implementation of social distancing, a universal masking policy, transmission-based precautions, and use of fitted N95 masks. Mid-March, elective surgeries were canceled, and inpatient visitation suspended. During the surge, these measures were widely and consistently implemented for all categories of HCWs throughout the work environment, based on emerging guidelines from the Centers for Disease Control and Prevention (CDC) and NYC Department of Health. Our overall observed HCW seroprevalence, well above that of the community, with differences in categories of job function and work locations, is therefore an important finding. Our sample of 2,749 HCWs lived in NYC and its surrounding suburbs and nearby states. There is heterogeneity in community seroprevalence between areas outside of NYC and NYC (an epicenter) itself. We therefore analyzed our data in the subset with NYC zip codes, confirming a similar overall prevalence and increased odds of seropositivity in nurses, allied HCWs, and nonclinical staff.
Physicians and administrative and office staff had seropositivity rates of 18.1%, 20.9%, and 25.8%, respectively, consistent with community rates and illustrating the effectiveness of PPE in the hospital setting. Since PPE use was part of a universal policy applied to all HCWs in our institution, other possible reasons may explain the differences we found. We speculate that the close working relationship nurses have with their patients resulted in a longer duration and higher frequency of daily interactions, increasing the risk for transmission and causing breakthrough infections.24,25 This increased risk is reflected in a study in which 28% of hospitalized patients were nurses and 9% certified nursing assistants.26
The CDC recently redefined close contact with someone with COVID-19 as a cumulative total of >15 minutes over 24 hours.25 Thus, several multiple short periods of exposure can increase risk for infection with SARS-CoV-2; such exposure is characteristic of the job function of nurses, nursing staff, and nonclinical staff. Further, housekeeping, transportation, and security officers are all nonclinical staff with significant and multiple exposures to COVID-19 patients during the surge, and for security officers, to continuous public traffic in and out of the hospital. SARS-CoV-2 spreads by virus shedding in large droplets and aerosols, with droplet nuclei <5 microns in size efficiently dispersed in air, an important additional mode of transmission.27-30 Airborne transmission coupled with virus shedding in asymptomatic and presymptomatic persons, which has been shown to cause secondary attack rates of up to 32%, are other factors that likely contributed to the increased seroprevalence in this group.31 Our observation is consistent with the Birmingham study, which reported the highest rate in housekeeping staff, with a prevalence of 34.5%, compared to 44% in this study.6 Similar reasons for high seropositivity rates apply to the two groups of allied HCWs (eg, medical assistants and patient care technicians, social workers, nutritionists and therapists), whose job functions place them in intermittent but significant proximity with inpatients and outpatients.
Consistent with public health data showing that minorities are disproportionately affected by this disease, we found that Black HCWs were three times more likely to be seropositive.32 However, an unexpected observation was the association between obesity and SARS-CoV-2 seropositivity. A possible explanation for this association may be inability to achieve optimal fit testing for N95 masks, thereby increasing the risk of exposure to droplet nuclei. This is important given that obesity is associated with poorer outcomes from COVID-19.
During the height of the first wave in NYC, EDs and inpatient units handled a large volume of COVID-19 patients with high PCR positivity rates (peak of 51% in March in our hospital). It was not unexpected that we observed increased odds of seropositivity in these work locations. As ICUs were at capacity, inpatient units cared for critically ill patients they would not normally have. HCWs in these locations coped with an increased workload, increased demand on PPE supplies, and work fatigue, which contributed to increased risk for hospital-acquired SARS-CoV-2 infections.
Reporting seroprevalence at a single institution was a limitation of the study. Approximately 57% of the hospital’s total HCW population was tested for antibodies. It is possible their risk profile influenced their decision to volunteer for testing when it became available, introducing selection bias. A comparison between tested and untested HCWs showed similarity in all demographic measures, including nasopharyngeal PCR testing, except for age. We did not have information on symptoms that would prompt PCR testing. HCWs who underwent voluntary testing were younger compared to those who did not undergo testing. Current NYC serosurveillance data showed higher seropositivity in the 45-to-64–year age group (27.8%-28.6%) compared to the 65-to-74–year age group (24.3%), which suggests that the tested group may overestimate seroprevalence among HCWs relative to a randomly selected sample.33 Similarly, there were more nurses, allied HCWs, physicians, and administrative staff in the tested group, with the former two having higher SARS-CoV-2 seropositivity compared to community prevalence, which could also overestimate seroprevalence. Our large sample size provided us with the power to detect differences within several different job functions and work locations, a strength of this study. It was not possible to differentiate community- from hospital-acquired infection in our HCWs, a limitation in many observational HCW seroprevalence studies. However, when we analyzed data restricted only to HCWs in NYC, to reduce the effect of differing community prevalences outside the city, our results were unchanged. Since it is possible that nonclinical HCWs are of a lower socioeconomic status compared to others (nurses and allied HCWs), we cannot exclude the possibility that higher SARS-CoV-2 seroprevalence associated with lower status explains, partly or completely, the increased odds of seropositivity we observed.34 Due to the high proportion of missing data for race (61.3%), we advise caution in interpreting our finding that the odds of seropositivity were three times higher for Black race, even though consistent with prior literature.34 Healthcare organizations have similar job function and work location categories incorporated in their infrastructure, suggesting that our observations may be generalizable to other hospitals in the United States.
CONCLUSION
These findings show that during the first surge in NYC, with its increased burden of disease, hospitalizations, morbidity, and mortality, seroprevalences varied based on job function and work location within this institution. Nurses were at highest risk for SARS-CoV-2 infection, as were those who worked in the ED. In preparation for subsequent waves of SARS-CoV-2 and other highly contagious respiratory infections, major medical centers need to enhance efforts aimed at protecting HCWs, with particular attention to these groups. This study also strongly supports the recent CDC guideline prioritizing HCWs to receive COVID-19 mRNA and adenovirus vector vaccines that have obtained emergency use authorization by the US Food and Drug Administration.35
Acknowledgments
The authors thank all the residents, nurses, and staff of the Department of Family Medicine for their contribution to this work.
1. Liu YC, Kuo RL, Shih SR. COVID-19: The first documented coronavirus pandemic in history. Biomed J. 2020;43(4):328-333. https://doi.org/10.1016/j.bj.2020.04.007
2. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Accessed April 12, 2021. https://covid19.who.int
3. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475-e483. https://doi.org/10.1016/S2468-2667(20)30164-X
4. Gupta S, Federman DG. Hospital preparedness for COVID-19 pandemic: experience from department of medicine at Veterans Affairs Connecticut Healthcare System. Postgrad Med. 2020:1-6. https://doi.org/10.1080/00325481.2020.1761668
5. Woolley K, Smith R, Arumugam S. Personal protective equipment (PPE) guidelines, adaptations and lessons during the COVID-19 pandemic. Ethics Med Public Health. 2020;14:100546. https://doi.org/10.1016/j.jemep.2020.100546
6. Shields A, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75(12):1089-1094. https://doi.org/10.1136/thoraxjnl-2020-215414
7. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. https://doi.org/10.1001/jama.2020.11160
8. Stubblefield WB, Talbot HK, Feldstein L, et al. Seroprevalence of SARS-CoV-2 Among frontline healthcare personnel during the first month of caring for COVID-19 patients - Nashville, Tennessee. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa936
9. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. https://doi.org/10.1016/j.jcv.2020.104437
10. Keeley AJ, Evans C, Colton H, et al. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill. 2020;25(14). https://doi.org/10.2807/1560-7917.ES.2020.25.14.2000433
11. Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network - 13 academic medical centers, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(35):1221-1226. https://doi.org/10.15585/mmwr.mm6935e2
12. Jeremias A, Nguyen J, Levine J, et al. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Intern Med. 2020 Aug 11:e204214. https://doi.org/10.1001/jamainternmed.2020.4214
13. Moscola J, Sembajwe G, Jarrett M, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324(9):893-895. https://doi.org/10.1001/jama.2020.14765
14. Gonzalez-Reiche AS, Hernandez MM, Sullivan MJ, et al. Introductions and early spread of SARS-CoV-2 in the New York City area. Science. 2020;369(6501):297-301. https://doi.org/10.1126/science.abc1917
15. Lau CS, Hoo SF, Yew SF, et al. Evaluation of the Roche Elecsys Anti-SARS-CoV-2 assay. Preprint. Posted online June 29, 2020. Accessed November 8, 2020. https://www.medrxiv.org/content/10.1101/2020.06.28.20142232v1 https://doi.org/10.1101/2020.06.28.20142232
16. New York City Department of Health. Covid-19: data. long-term trends. Antibody testing. Accessed March 5, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-trends.page#antibody
17. Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. Published online July 21, 2020. https://doi.org/10.1001/jamainternmed.2020.4130
18. Rosenberg ES, Tesoriero JM, Rosenthal EM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. Aug 2020;48:23-29 e4. https://doi.org/10.1016/j.annepidem.2020.06.004
19. Anand S, Montez-Rath M, Han J, et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet. 2020;396(10259):1335-1344. https://doi.org/10.1016/S0140-6736(20)32009-2
20. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: a cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2020;102:63-69. https://doi.org/10.1016/j.ijid.2020.10.036
21. Samaranayake LP, Fakhruddin KS, Ngo HC, Chang JWW, Panduwawala C. The effectiveness and efficacy of respiratory protective equipment (RPE) in dentistry and other health care settings: a systematic review. Acta Odontol Scand. 2020;78(8):626-639. https://doi.org/10.1080/00016357.2020.1810769
22. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
23. Richterman A, Meyerowitz EA, Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public health. JAMA. Published online November 13, 2020. https://doi.org/10.1001/jama.2020.21399
24. Degesys NF, Wang RC, Kwan E, Fahimi J, Noble JA, Raven MC. Correlation between n95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324(1):94-96. https://doi.org/10.1001/jama.2020.9843
25. Pringle JC, Leikauskas J, Ransom-Kelley S, et al. COVID-19 in a correctional facility employee following multiple brief exposures to persons with COVID-19 - Vermont, July-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1569-1570. https://doi.org/10.15585/mmwr.mm6943e1
26. Kambhampati AK, O’Halloran AC, Whitaker M, et al. COVID-19-associated hospitalizations among health care personnel - COVID-NET, 13 states, March 1-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1576-1583. https://doi.org/10.15585/mmwr.mm6943e3
27. Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117(26):14857-14863. https://doi.org/10.1073/pnas.2009637117
28. Setti L, Passarini F, De Gennaro G, et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not be enough. Int J Environ Res Public Health. 2020;17(8):2932. https://doi.org/doi:10.3390/ijerph17082932
29. Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324(5):441-442. https://doi.org/10.1001/jama.2020.12458
30. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323(18):1837-1838. https://doi.org/10.1001/jama.2020.4756
31. Qiu X, Nergiz A, Maraolo A, Bogoch I, Low N, Cevik M. The role of asymptomatic and pre-symptomatic infection in SARS-CoV-2 transmission – a living systematic review. Clin Mibrobiol Infect. 2021;20:S1198-743X(21)00038-0. Published online January 20, 2021. https://doi.org/10.1016/j.cmi.2021.01.011
32. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/doi:10.1056/NEJMsa2011686
33. New York City Department of Health. Covid-19: Data. Antibody testing by group - age. Accessed March 5, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-totals.page#antibody
34. Patel JA, Nielsen FBH, Badiani AA, et al. Poverty, inequality and COVID-19: the forgotten vulnerable. Public Health. 2020;183:110-111. https://doi.org/10.1016/j.puhe.2020.05.006
35. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. https://doi.org/10.1056/NEJMoa2034577
1. Liu YC, Kuo RL, Shih SR. COVID-19: The first documented coronavirus pandemic in history. Biomed J. 2020;43(4):328-333. https://doi.org/10.1016/j.bj.2020.04.007
2. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Accessed April 12, 2021. https://covid19.who.int
3. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475-e483. https://doi.org/10.1016/S2468-2667(20)30164-X
4. Gupta S, Federman DG. Hospital preparedness for COVID-19 pandemic: experience from department of medicine at Veterans Affairs Connecticut Healthcare System. Postgrad Med. 2020:1-6. https://doi.org/10.1080/00325481.2020.1761668
5. Woolley K, Smith R, Arumugam S. Personal protective equipment (PPE) guidelines, adaptations and lessons during the COVID-19 pandemic. Ethics Med Public Health. 2020;14:100546. https://doi.org/10.1016/j.jemep.2020.100546
6. Shields A, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75(12):1089-1094. https://doi.org/10.1136/thoraxjnl-2020-215414
7. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. https://doi.org/10.1001/jama.2020.11160
8. Stubblefield WB, Talbot HK, Feldstein L, et al. Seroprevalence of SARS-CoV-2 Among frontline healthcare personnel during the first month of caring for COVID-19 patients - Nashville, Tennessee. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa936
9. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. https://doi.org/10.1016/j.jcv.2020.104437
10. Keeley AJ, Evans C, Colton H, et al. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill. 2020;25(14). https://doi.org/10.2807/1560-7917.ES.2020.25.14.2000433
11. Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network - 13 academic medical centers, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(35):1221-1226. https://doi.org/10.15585/mmwr.mm6935e2
12. Jeremias A, Nguyen J, Levine J, et al. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Intern Med. 2020 Aug 11:e204214. https://doi.org/10.1001/jamainternmed.2020.4214
13. Moscola J, Sembajwe G, Jarrett M, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324(9):893-895. https://doi.org/10.1001/jama.2020.14765
14. Gonzalez-Reiche AS, Hernandez MM, Sullivan MJ, et al. Introductions and early spread of SARS-CoV-2 in the New York City area. Science. 2020;369(6501):297-301. https://doi.org/10.1126/science.abc1917
15. Lau CS, Hoo SF, Yew SF, et al. Evaluation of the Roche Elecsys Anti-SARS-CoV-2 assay. Preprint. Posted online June 29, 2020. Accessed November 8, 2020. https://www.medrxiv.org/content/10.1101/2020.06.28.20142232v1 https://doi.org/10.1101/2020.06.28.20142232
16. New York City Department of Health. Covid-19: data. long-term trends. Antibody testing. Accessed March 5, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-trends.page#antibody
17. Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. Published online July 21, 2020. https://doi.org/10.1001/jamainternmed.2020.4130
18. Rosenberg ES, Tesoriero JM, Rosenthal EM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. Aug 2020;48:23-29 e4. https://doi.org/10.1016/j.annepidem.2020.06.004
19. Anand S, Montez-Rath M, Han J, et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet. 2020;396(10259):1335-1344. https://doi.org/10.1016/S0140-6736(20)32009-2
20. Venugopal U, Jilani N, Rabah S, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: a cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2020;102:63-69. https://doi.org/10.1016/j.ijid.2020.10.036
21. Samaranayake LP, Fakhruddin KS, Ngo HC, Chang JWW, Panduwawala C. The effectiveness and efficacy of respiratory protective equipment (RPE) in dentistry and other health care settings: a systematic review. Acta Odontol Scand. 2020;78(8):626-639. https://doi.org/10.1080/00016357.2020.1810769
22. Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
23. Richterman A, Meyerowitz EA, Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public health. JAMA. Published online November 13, 2020. https://doi.org/10.1001/jama.2020.21399
24. Degesys NF, Wang RC, Kwan E, Fahimi J, Noble JA, Raven MC. Correlation between n95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324(1):94-96. https://doi.org/10.1001/jama.2020.9843
25. Pringle JC, Leikauskas J, Ransom-Kelley S, et al. COVID-19 in a correctional facility employee following multiple brief exposures to persons with COVID-19 - Vermont, July-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1569-1570. https://doi.org/10.15585/mmwr.mm6943e1
26. Kambhampati AK, O’Halloran AC, Whitaker M, et al. COVID-19-associated hospitalizations among health care personnel - COVID-NET, 13 states, March 1-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1576-1583. https://doi.org/10.15585/mmwr.mm6943e3
27. Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117(26):14857-14863. https://doi.org/10.1073/pnas.2009637117
28. Setti L, Passarini F, De Gennaro G, et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not be enough. Int J Environ Res Public Health. 2020;17(8):2932. https://doi.org/doi:10.3390/ijerph17082932
29. Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324(5):441-442. https://doi.org/10.1001/jama.2020.12458
30. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323(18):1837-1838. https://doi.org/10.1001/jama.2020.4756
31. Qiu X, Nergiz A, Maraolo A, Bogoch I, Low N, Cevik M. The role of asymptomatic and pre-symptomatic infection in SARS-CoV-2 transmission – a living systematic review. Clin Mibrobiol Infect. 2021;20:S1198-743X(21)00038-0. Published online January 20, 2021. https://doi.org/10.1016/j.cmi.2021.01.011
32. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/doi:10.1056/NEJMsa2011686
33. New York City Department of Health. Covid-19: Data. Antibody testing by group - age. Accessed March 5, 2021. https://www1.nyc.gov/site/doh/covid/covid-19-data-totals.page#antibody
34. Patel JA, Nielsen FBH, Badiani AA, et al. Poverty, inequality and COVID-19: the forgotten vulnerable. Public Health. 2020;183:110-111. https://doi.org/10.1016/j.puhe.2020.05.006
35. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. https://doi.org/10.1056/NEJMoa2034577
© 2021 Society of Hospital Medicine