User login
An Atypical Angiomyomatous Hamartoma With Unexplained Hepatosplenomegaly
Angiomyomatous hamartoma (AMH) of the lymph node is an extremely uncommon vascular disorder of unknown etiology, first described by Chan and colleagues in 1992.1-3 Angiomyomatous hamartoma particularly involves inguinal and femoral lymph nodes, with few cases reported in the cervical, popliteal, and submandibular lymph nodes.1 Angiomyomatous hamartoma can occasionally be associated with edema of the ipsilateral limb. To the authors’ knowledge, to date only 18 cases of AMH have been reported.4
Case Presentation
A 40-year-old white man started to have a left inguinal and scrotal pain along with left thigh swelling at age 22 while serving in the U.S. Army.
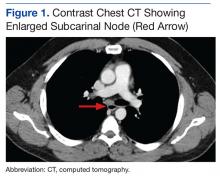
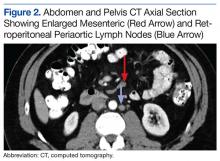
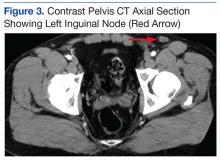
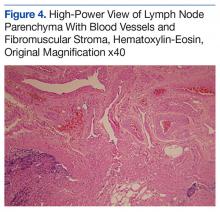
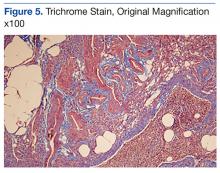
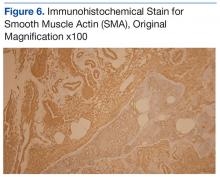
An abdominal Doppler ultrasound did not show any evidence of portal hypertension. A thoraco-abdomino-pelvic computed tomography (CT) scan showed bilateral axillary, subcarinal (Figure 1), mesenteric and retroperitoneal (Figure 2), and left inguinal (Figure 3) lymphadenopathy. Excisional biopsy of a 3.5 x 2.5 x 1.5 cm left inguinal lymph node was performed, and histopathology showed extensive smooth muscle and vascular proliferation replacing most of the lymph node (Figure 4), a finding consistent with AMH. A trichrome staining (Figure 5) and immunohistochemical study for smooth muscle actin (Figure 6) were performed and supported the diagnosis. Due to persistent pain in the scrotal area, the patient underwent a left spermatic cord denervation. Currently, the patient has persistent left thigh swelling. His condition remains stable with a regular follow-up CT scan showing unchanged lymphadenopathy.
Discussion
Angiomyomatous hamartoma is a rare, primary vascular tumor of the lymph nodes occurring almost exclusively in the inguinal and femoral lymph nodes and occasionally associated with edema of the ipsilateral limb.1 A few cases with popliteal and cervical lymph node involvement have been reported.1 There are no prior reports of cases with either generalized adenopathy or hepatosplenomegaly.
The histopathogenesis of AMH remains unclear. Chan and colleagues first reported this distinct clinicopathologic entity in 1992 as a primary vascular tumor of the lymph node.1-3 The hamartomatous nature of the disease was postulated by the authors on the basis of a disorganized growth pattern of smooth muscle cells and blood vessels noted on pathology.2,3 The AMH could represent a localized malformation in a congenitally damaged lymphatic vessel system.5 Other hypothesis suggests lymphedema as a possible etiology of AMH through continuous stimulation of lymphatic vessels, which triggers vasoproliferation and eventually the vascular transformation of the lymph nodes.5
Differential diagnoses of AMH include nodal lymphangiomyomatosis, which is most prevalent in women, particularly presenting with thoracic and intra-abdominal lymph nodes and plumper HMB45 (human melanoma black 45) -positive tumor cells6; leiomyomato
Treatment is either conservative or surgical, depending on clinical judgment. This is only the 19th case of AMH reported so far in the literature and the fifth reported case in which the patient presented with ipsilateral lymphedema of the limb. Importantly, it is the first reported case with generalized (axillary, subcarinal, mesenteric, inguinal and retroperitoneal) lymphadenopathy and unexplained hepatosplenomegaly.
Conclusion
Angiomyomatous hamartoma of the lymph nodes is an exceedingly rare diagnosis but should be considered when evaluating patients with lymphatic tumors. This patient remains relatively asymptomatic and on observation at this time and seems to have more extensive disease than prior reports in the literature.
1. Mridha AR, Ranjan R, Kinra P, Ray R, Khan SA, Shivanand G. Angiomyomatous hamartoma of popliteal lymph node: an unusual entity. J Pathol Transl Med. 2015;49(2):156-158.
2. Dargent JL, Lespagnard L, Verdebout JM, Bourgeois P, Munck D. Glomeruloid microvascular proliferation in angiomyomatous hamartoma of the lymph node. Virchows Arch. 2004;445(3):320-322.
3. Chan JK, Frizzera G, Fletcher CD, Rosai J. Primary vascular tumors of lymph nodes other than Kaposi’s sarcoma. Analysis of 39 cases and delineation of two new entities. Am J Surg Pathol. 1992;16(4):335-350.
4. Ram M, Alsanjari N, Ansari N. Angiomyomatous hamartoma: a rare case report with review of the literature. Rare Tumors. 2009;1(2):e25.
5. Piedimonte A, De Nictolis M, Lorenzini P, Sperti V, Bertani A. Angiomyomatous hamartoma of inguinal lymph nodes. Plast Reconstr Surg. 2006;117(2):714-716.
6. Lee CH, Chang TC, Ku JW. Angiomyomatous hamartoma in an inguinal lymph node with proliferating pericytes/smooth muscle cells, plexiform vessel tangles, and ectopic calcification. Indian J Pathol Microbiol. 2015;58(2):226-228.
Angiomyomatous hamartoma (AMH) of the lymph node is an extremely uncommon vascular disorder of unknown etiology, first described by Chan and colleagues in 1992.1-3 Angiomyomatous hamartoma particularly involves inguinal and femoral lymph nodes, with few cases reported in the cervical, popliteal, and submandibular lymph nodes.1 Angiomyomatous hamartoma can occasionally be associated with edema of the ipsilateral limb. To the authors’ knowledge, to date only 18 cases of AMH have been reported.4
Case Presentation
A 40-year-old white man started to have a left inguinal and scrotal pain along with left thigh swelling at age 22 while serving in the U.S. Army.
An abdominal Doppler ultrasound did not show any evidence of portal hypertension. A thoraco-abdomino-pelvic computed tomography (CT) scan showed bilateral axillary, subcarinal (Figure 1), mesenteric and retroperitoneal (Figure 2), and left inguinal (Figure 3) lymphadenopathy. Excisional biopsy of a 3.5 x 2.5 x 1.5 cm left inguinal lymph node was performed, and histopathology showed extensive smooth muscle and vascular proliferation replacing most of the lymph node (Figure 4), a finding consistent with AMH. A trichrome staining (Figure 5) and immunohistochemical study for smooth muscle actin (Figure 6) were performed and supported the diagnosis. Due to persistent pain in the scrotal area, the patient underwent a left spermatic cord denervation. Currently, the patient has persistent left thigh swelling. His condition remains stable with a regular follow-up CT scan showing unchanged lymphadenopathy.
Discussion
Angiomyomatous hamartoma is a rare, primary vascular tumor of the lymph nodes occurring almost exclusively in the inguinal and femoral lymph nodes and occasionally associated with edema of the ipsilateral limb.1 A few cases with popliteal and cervical lymph node involvement have been reported.1 There are no prior reports of cases with either generalized adenopathy or hepatosplenomegaly.
The histopathogenesis of AMH remains unclear. Chan and colleagues first reported this distinct clinicopathologic entity in 1992 as a primary vascular tumor of the lymph node.1-3 The hamartomatous nature of the disease was postulated by the authors on the basis of a disorganized growth pattern of smooth muscle cells and blood vessels noted on pathology.2,3 The AMH could represent a localized malformation in a congenitally damaged lymphatic vessel system.5 Other hypothesis suggests lymphedema as a possible etiology of AMH through continuous stimulation of lymphatic vessels, which triggers vasoproliferation and eventually the vascular transformation of the lymph nodes.5
Differential diagnoses of AMH include nodal lymphangiomyomatosis, which is most prevalent in women, particularly presenting with thoracic and intra-abdominal lymph nodes and plumper HMB45 (human melanoma black 45) -positive tumor cells6; leiomyomato
Treatment is either conservative or surgical, depending on clinical judgment. This is only the 19th case of AMH reported so far in the literature and the fifth reported case in which the patient presented with ipsilateral lymphedema of the limb. Importantly, it is the first reported case with generalized (axillary, subcarinal, mesenteric, inguinal and retroperitoneal) lymphadenopathy and unexplained hepatosplenomegaly.
Conclusion
Angiomyomatous hamartoma of the lymph nodes is an exceedingly rare diagnosis but should be considered when evaluating patients with lymphatic tumors. This patient remains relatively asymptomatic and on observation at this time and seems to have more extensive disease than prior reports in the literature.
Angiomyomatous hamartoma (AMH) of the lymph node is an extremely uncommon vascular disorder of unknown etiology, first described by Chan and colleagues in 1992.1-3 Angiomyomatous hamartoma particularly involves inguinal and femoral lymph nodes, with few cases reported in the cervical, popliteal, and submandibular lymph nodes.1 Angiomyomatous hamartoma can occasionally be associated with edema of the ipsilateral limb. To the authors’ knowledge, to date only 18 cases of AMH have been reported.4
Case Presentation
A 40-year-old white man started to have a left inguinal and scrotal pain along with left thigh swelling at age 22 while serving in the U.S. Army.
An abdominal Doppler ultrasound did not show any evidence of portal hypertension. A thoraco-abdomino-pelvic computed tomography (CT) scan showed bilateral axillary, subcarinal (Figure 1), mesenteric and retroperitoneal (Figure 2), and left inguinal (Figure 3) lymphadenopathy. Excisional biopsy of a 3.5 x 2.5 x 1.5 cm left inguinal lymph node was performed, and histopathology showed extensive smooth muscle and vascular proliferation replacing most of the lymph node (Figure 4), a finding consistent with AMH. A trichrome staining (Figure 5) and immunohistochemical study for smooth muscle actin (Figure 6) were performed and supported the diagnosis. Due to persistent pain in the scrotal area, the patient underwent a left spermatic cord denervation. Currently, the patient has persistent left thigh swelling. His condition remains stable with a regular follow-up CT scan showing unchanged lymphadenopathy.
Discussion
Angiomyomatous hamartoma is a rare, primary vascular tumor of the lymph nodes occurring almost exclusively in the inguinal and femoral lymph nodes and occasionally associated with edema of the ipsilateral limb.1 A few cases with popliteal and cervical lymph node involvement have been reported.1 There are no prior reports of cases with either generalized adenopathy or hepatosplenomegaly.
The histopathogenesis of AMH remains unclear. Chan and colleagues first reported this distinct clinicopathologic entity in 1992 as a primary vascular tumor of the lymph node.1-3 The hamartomatous nature of the disease was postulated by the authors on the basis of a disorganized growth pattern of smooth muscle cells and blood vessels noted on pathology.2,3 The AMH could represent a localized malformation in a congenitally damaged lymphatic vessel system.5 Other hypothesis suggests lymphedema as a possible etiology of AMH through continuous stimulation of lymphatic vessels, which triggers vasoproliferation and eventually the vascular transformation of the lymph nodes.5
Differential diagnoses of AMH include nodal lymphangiomyomatosis, which is most prevalent in women, particularly presenting with thoracic and intra-abdominal lymph nodes and plumper HMB45 (human melanoma black 45) -positive tumor cells6; leiomyomato
Treatment is either conservative or surgical, depending on clinical judgment. This is only the 19th case of AMH reported so far in the literature and the fifth reported case in which the patient presented with ipsilateral lymphedema of the limb. Importantly, it is the first reported case with generalized (axillary, subcarinal, mesenteric, inguinal and retroperitoneal) lymphadenopathy and unexplained hepatosplenomegaly.
Conclusion
Angiomyomatous hamartoma of the lymph nodes is an exceedingly rare diagnosis but should be considered when evaluating patients with lymphatic tumors. This patient remains relatively asymptomatic and on observation at this time and seems to have more extensive disease than prior reports in the literature.
1. Mridha AR, Ranjan R, Kinra P, Ray R, Khan SA, Shivanand G. Angiomyomatous hamartoma of popliteal lymph node: an unusual entity. J Pathol Transl Med. 2015;49(2):156-158.
2. Dargent JL, Lespagnard L, Verdebout JM, Bourgeois P, Munck D. Glomeruloid microvascular proliferation in angiomyomatous hamartoma of the lymph node. Virchows Arch. 2004;445(3):320-322.
3. Chan JK, Frizzera G, Fletcher CD, Rosai J. Primary vascular tumors of lymph nodes other than Kaposi’s sarcoma. Analysis of 39 cases and delineation of two new entities. Am J Surg Pathol. 1992;16(4):335-350.
4. Ram M, Alsanjari N, Ansari N. Angiomyomatous hamartoma: a rare case report with review of the literature. Rare Tumors. 2009;1(2):e25.
5. Piedimonte A, De Nictolis M, Lorenzini P, Sperti V, Bertani A. Angiomyomatous hamartoma of inguinal lymph nodes. Plast Reconstr Surg. 2006;117(2):714-716.
6. Lee CH, Chang TC, Ku JW. Angiomyomatous hamartoma in an inguinal lymph node with proliferating pericytes/smooth muscle cells, plexiform vessel tangles, and ectopic calcification. Indian J Pathol Microbiol. 2015;58(2):226-228.
1. Mridha AR, Ranjan R, Kinra P, Ray R, Khan SA, Shivanand G. Angiomyomatous hamartoma of popliteal lymph node: an unusual entity. J Pathol Transl Med. 2015;49(2):156-158.
2. Dargent JL, Lespagnard L, Verdebout JM, Bourgeois P, Munck D. Glomeruloid microvascular proliferation in angiomyomatous hamartoma of the lymph node. Virchows Arch. 2004;445(3):320-322.
3. Chan JK, Frizzera G, Fletcher CD, Rosai J. Primary vascular tumors of lymph nodes other than Kaposi’s sarcoma. Analysis of 39 cases and delineation of two new entities. Am J Surg Pathol. 1992;16(4):335-350.
4. Ram M, Alsanjari N, Ansari N. Angiomyomatous hamartoma: a rare case report with review of the literature. Rare Tumors. 2009;1(2):e25.
5. Piedimonte A, De Nictolis M, Lorenzini P, Sperti V, Bertani A. Angiomyomatous hamartoma of inguinal lymph nodes. Plast Reconstr Surg. 2006;117(2):714-716.
6. Lee CH, Chang TC, Ku JW. Angiomyomatous hamartoma in an inguinal lymph node with proliferating pericytes/smooth muscle cells, plexiform vessel tangles, and ectopic calcification. Indian J Pathol Microbiol. 2015;58(2):226-228.
Beyond the bull's eye: Recognizing Lyme disease
› Consider the duration of a tick’s attachment and whether it was engorged when assessing an individual’s risk of acquiring Lyme disease. C
› Start treatment for Lyme disease without lab testing if a patient has the painless skin rash—erythema migrans—and a history of tick exposure. C
› Choose doxycycline as first-line treatment for early Lyme disease unless a patient has contraindications. Amoxicillin or cefuroxime axetil are suitable alternatives. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice L, a 39-year-old woman with an unremarkable medical history asks to see her primary care provider right away, concerned she might have contracted Lyme disease. She had been hiking the overgrown trails on her family-owned ranch in Florida, and the next day she noticed a black tick stuck to her forearm. Using tweezers, she pulled the whole tick off intact, put it in a plastic cup, and immediately sought medical attention. How should her family physician (FP) advise her?
Lyme disease is the most common tick-borne illness in the United States, with more than 25,000 cases confirmed in this country in 2014.1 It is concentrated mostly in the northeast and upper Midwest, and less frequently occurs in the Pacific coastal regions of Oregon and northern California. Cases have also increasingly been reported in the southwest region of the Appalachian Mountains and the mountainous regions of southern Virginia.2
In 2014, the only states reporting no incidence of Lyme disease were Colorado, Hawaii, Louisiana, New Mexico, and Oklahoma.1 Lyme disease is also endemic in several regions in Northern Europe, Eastern Asia, and Northern Africa.1,3-7 According to the Centers for Disease Control and Prevention (CDC), boys ages 5 to 9 years are most affected.1
Disease transmission: Duration of tick attachment is important
The spirochete that causes Lyme disease, Borrelia burgdorferi, is transmitted to humans by the Ixodes tick. The Ixodes scapularis (deer tick) is common in the eastern and northern midwestern states and I pacificus is common in the western United States.
The life cycle. These small, dark-colored ticks have a 2-year life cycle that is comprised of 4 developmental stages: egg, larva, nymph, and adult. Eggs are laid in spring and hatch into larvae during late summer. The larvae feed on small animals (eg, mice, chipmunks, birds) and can acquire B burgdorferi infection at this stage. The larvae then molt into nymphs (<2 mm, and difficult to see), which feed again the following spring to early summer and may transmit the infection to a new host. Nymphs become adult ticks in mid-October to early-November, when the females feed again, mainly on large animals.
Humans usually become infected from May through August, when both they and the nymph ticks are most active outdoors. The ticks are able to attach themselves to their host without being noticed because they secrete small amounts of saliva with anesthetic properties while feeding. Many ticks also secrete a cement-like substance that keeps them firmly attached.
Adult ticks can also transmit the disease and are larger and more easily recognized. Transmission of the spirochete requires that the tick be attached to the new host for 36 to 48 hours,1 allowing the spirochete to travel from the mid-gut of the tick to the salivary glands and into the host.
Two of the most important factors to consider when assessing the risk of transmission is how long the tick was attached and whether it was engorged. Only about a quarter of individuals with Lyme disease recall having had a tick bite.1,3-6,8
Clinical presentation: Early and late findings
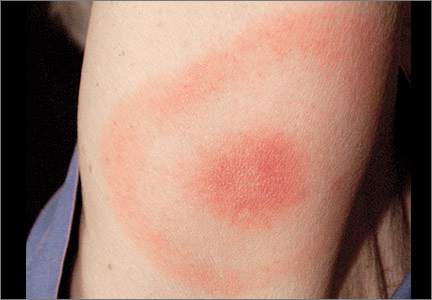
Symptoms of early Lyme disease usually start one to 2 weeks after a tick bite, but may start up to 30 days later. The most common presentation is a painless skin rash—erythema migrans (EM). It starts as a single red papule at the site of the bite (multiple lesions appear in 10% to 20% of cases9) and may progress to a painless erythematous lesion with red borders and a partial central clearing—the classic EM rash (FIGURE). Less commonly, the center of the lesion can appear vesicular or necrotic.
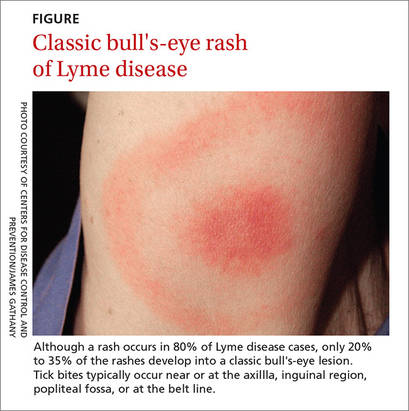
Although a rash occurs in 80% of Lyme disease cases, only 20% to 35% of the rashes develop into a classic bull's-eye lesion.3 Tick bites—and thus rashes—typically occur near or at the axilla, inguinal region, popliteal fossa, or at the belt line.
Individuals who don’t exhibit a rash may be asymptomatic or have nonspecific symptoms or flu-like symptoms of fatigue, fever, chills, myalgia, and headache.4 If Lyme disease continues untreated, the patient may experience extra-cutaneous complications, most often involving the joints and the nervous and cardiovascular systems.3-7
Ixodes ticks are also vectors for human granulocytic anaplasmosis (HGA) and babesiosis, which can cause a variety of symptoms. Keep these diseases in mind when a patient presents with severe or atypical features of Lyme disease.5 The benefit of antibiotics after a tick bite to reduce the incidence of HGA or babesiosis is unclear.10
Late manifestations of Lyme disease can occur within one to 2 months of infection or even months to years after tick exposure, often resulting in substantial morbidity.3-7,11 Musculoskeletal symptoms are the most common manifestations of late, disseminated disease, usually presenting as transient asymmetrical oligoarticular arthralgias or myalgia. Arthritis also occurs in 60% of untreated patients with late disease.4,5 Large joint effusions are typical, with synovial fluid studies showing high quantities of polymorphonuclear leukocytes (25,000/mm3).5 Joint symptoms that persist after antibiotic treatment are called antibiotic-refractory Lyme arthritis.4-7
Neurologic involvement affects 10% to 15% of untreated patients.3,4 It can present as lymphocytic meningitis (most common), cranial neuropathies, motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis. Late neurologic Lyme disease may also present as a subacute mild encephalopathy affecting memory and concentration. When cranial neuropathies are involved, it is usually as unilateral facial nerve palsy (but may be bilateral). Always consider Lyme disease in endemic areas when patients have severe Bell's palsy.
Patients may present with altered mental status, neck stiffness, pain, and headaches.4-7 The classic triad (known as Bannwarth syndrome) consists of lymphocytic meningitis, cranial neuritis, and radiculoneuritis. However, these conditions do not always occur together.3,4
Cardiovascular complications occur in 4% to 8% of untreated patients,4,5 usually one to 2 months following infection. Varying degrees of atrioventricular (AV) block can be seen, but third-degree block is most common. A less frequent complication is Lyme carditis, seen in 4% to 10% of patients.12 The pathophysiology of Lyme carditis is not well understood.11 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, often involving an AV block. Less frequent complications include myopericarditis, bundle branch block, and heart failure.
Post-treatment Lyme disease syndrome refers to the nonspecific symptoms of fatigue, sleep disorders, headaches, memory and concentration difficulties, myalgia, and arthralgias that may persist after successful antibiotic treatment. (We’ll discuss the specifics of treatment in a bit.) Post-treatment Lyme disease syndrome occurs in about 5% of patients properly treated for Lyme disease.13 The pathogenesis remains unknown, but some experts believe that lingering symptoms result from residual damage to tissues and the immune system. Education, rehabilitation, anti-inflammatory agents, antidepressants, a healthy diet, and plenty of rest have been recommended as treatment modalities.1,13
Chronic Lyme disease is also important to keep in mind. Although there is no standard definition for it, chronic Lyme disease refers broadly to chronic symptoms in patients who may or may not have Lyme disease—eg, an individual who may have been treated for presumed B burgdorferi infection without solid clinical or serologic confirmation.3-5 Chronic Lyme disease can often share somatic symptoms with other conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. Treatment often relies on a solid, trusting patient-doctor relationship, cognitive behavioral therapy, and regular counseling. Antibiotics are usually not necessary.
Dx: Serologic testing is preferred if clinical findings are insufficient
Lyme disease can be diagnosed clinically in patients who have an EM rash and a history of tick exposure. This is the only clinical presentation sufficient to make the diagnosis of Lyme disease without the need of confirmatory serologic testing.3,6 In the case of a tick bite but no rash, defer serologic testing unless associated symptoms arise (described earlier), at which time the accuracy of test results would be more trustworthy.10 Testing of ticks for infection with B burgdorferi is not recommended due to a lack of laboratory standardization.10
Two methods of laboratory testing are available to diagnose Lyme disease: direct, using cultures to detect B burgdorferi-specific proteins; and indirect, involving assays for antibodies.
Serologic testing. The CDC and Infectious Diseases Society of America recommend serology as the preferred initial diagnostic test. Tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer. However, these tests should not be used to screen individuals who have a low probability of infection, due to the tests’ poor positive predictive value.
The serologic tests used are the enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody (IFA) assay. These assays use a whole-cell sonicate of B burgdorferi and yield a significant number of false-positive results due to cross-reactive antigens such as flagellar and heat-shock proteins if other spirochetal infections are present. To increase testing specificity, both the CDC and European guidelines strongly recommend a 2-tier approach using an ELISA or IFA assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.1
The Western blot is interpreted using standardized criteria requiring at least 2 of 3 bands for a positive IgM diagnosis and 5 of 10 bands for a positive IgG diagnosis. Antibodies against Borrelia species are slow to develop. IgM generally is undetectable for the first one to 2 weeks after infection, and IgG often does not emerge for 4 to 6 weeks.
With patients who are seronegative at presentation, but for whom there is strong suspicion of Borrelia infection, it is advisable to obtain evidence of seroconversion, preferably within 8 to 14 days after presentation. Early antibiotic treatment may prevent the development of seropositivity.1,3-7,14
Past or newly acquired infection? IgM and IgG produced in response to B burgdorferi may persist for years following antimicrobial therapy, which makes it impossible to distinguish between past and newly acquired infections based on seropositivity alone. These persistently elevated levels are not an indication of ineffective treatment or chronic infection. Therefore, it is not recommended to repeat serologic testing for documentation of treatment effectiveness or cure.
Since no serologic test has sufficient specificity to be used alone, efforts are being made to develop testing that detects antibodies against the 26-mer peptide from the sixth invariant region (C6) of the VlsE lipoprotein (C6VlsE). In 2007, the US Food and Drug Administration (FDA) approved a C6 ELISA for first-tier testing; unfortunately, it still has the problem of cross-reactivity with other spirochetal and viral pathogens. The C6 ELISA may one day be approved as a single-tier test.4-7,14
Culture. The isolation of Borrelia species by culture is not routinely performed because it is expensive and requires special media and laboratory expertise, as well as a prolonged period of observation (6 to 12 weeks). Furthermore, this technique lacks sensitivity with samples taken from anywhere other than the rash site of patients with EM, in whom there is little need for laboratory diagnosis. Culture of cerebrospinal fluid has a positive yield of less than 10%,5 and it is extremely rare to isolate the spirochete from joint fluid. Therefore, negative results do not exclude a diagnosis of disease.4,5,14
The CDC recommends against cultures, immunofluorescence staining, and cell sorting of cell wall-deficient or cystic forms of B burgdorferi.1
Polymerase-chain reaction (PCR). This test is used to amplify genomic DNA of B burgdorferi and is most useful in patients with Lyme arthritis because of a high rate of DNA detection in synovial fluid samples (60% to 85%).5 In skin biopsies from EM lesions, PCR sensitivity can range from 25% to 90%.5 The PCR test is also used in cases of diagnostic uncertainty, but is generally performed only for research purposes. Negative findings do not exclude diagnosis of the disease.5,6,14
Urine antigen test. This test has a high false-positive rate and is generally not recommended.1,5
Treatment: Begin antibiotics ASAP
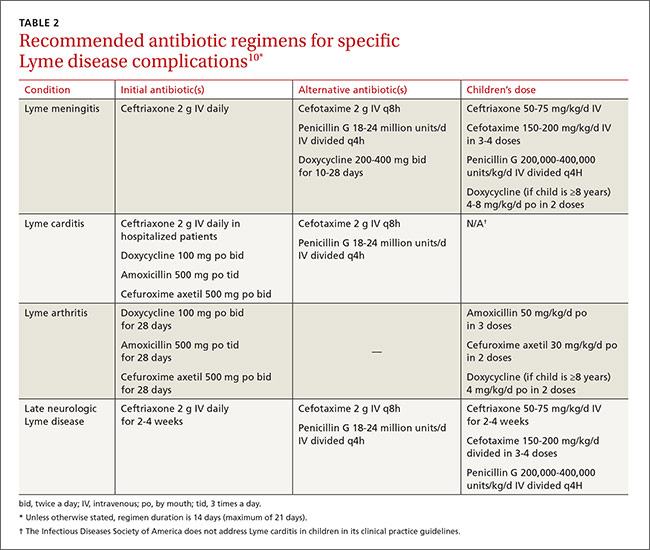
Treat Lyme disease with antibiotics as soon as the diagnosis is made. Early treatment hastens relief from symptoms and halts progression of later stages of the disease. The preferred antibiotics for early localized disease are doxycycline 100 mg orally twice daily; amoxicillin 500 mg orally 3 times a day; or cefuroxime axetil 500 mg orally twice a day (TABLE 1).10 Cefuroxime axetil is also appropriate if EM can’t be clearly distinguished from bacterial cellulitis. Reserve intravenous (IV) regimens for patients with more serious presentations (eg, neurologic symptoms and symptomatic cardiac disease) and for those with refractory Lyme arthritis (TABLE 2).10
Macrolides are not recommended as first-line therapy for early Lyme disease because they are less effective.10 However, macrolides may be used with patients unable to take the preferred antibiotics. Because there have been intermittent shortages of doxycycline, minocycline—another second-generation tetracycline with a similar chemical structure and antibacterial action—has been proposed as an alternative treatment.15
Ceftriaxone IV is preferred especially for patients presenting with an AV block or myopericarditis associated with early Lyme disease. The recommended course of treatment is usually 14 days. A temporary pacemaker may be required for patients with advanced blocks. Oral antibiotics may be started as soon as the AV block is resolved, or for outpatient therapy.
For adults who have early Lyme disease with acute neurologic manifestations such as meningitis or radiculopathy, IV antibiotics for 14 days are recommended. Cefotaxime has efficacy similar to ceftriaxone but requires multiple doses a day, making the latter the preferred treatment. Penicillin G 18 to 24 million units per day, divided into doses given every 4 hours, is also a satisfactory alternative.10,16 The American Academy of Neurology states that no definitive data exist to establish superiority, or lack thereof, of either oral or parenteral treatment.17
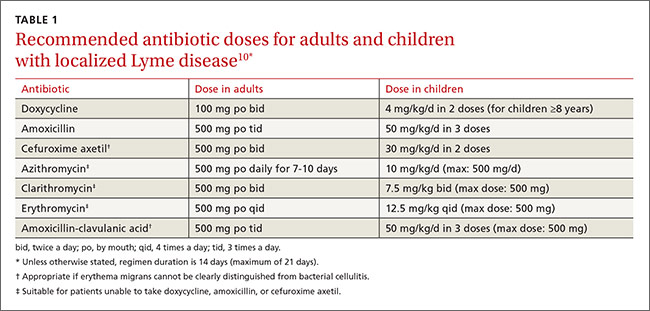
Lyme arthritis can be treated with oral doxycycline, amoxicillin, or cefuroxime axetil for 28 days. For patients with persistent or recurrent joint swelling who have been treated with a course of oral antibiotics, administer an additional 4 weeks of oral antibiotics or 2 to 4 weeks of IV ceftriaxone.10 A second 4-week course of oral antibiotics is also suggested for patients whose symptoms have greatly improved but not fully resolved.3,4,7,10
For post-Lyme syndromes, antibiotics have not proved useful and are not recommended for patients with chronic (>6 months) subjective symptoms.10,16 A recent study in Europe failed to show that antibiotic treatment for 12 weeks reduced symptoms or improved quality of life in patients with persistent symptoms associated with Lyme disease.18
Prognosis: It varies with specific complications
EM resolves within a few days or weeks (up to 8 weeks) after initiation of treatment. Generally, between 70% and 85% of patients with Lyme neuroborreliosis make a complete recovery, usually 6 to 12 months after initiation of therapy; and up to 90% of patients with facial palsy recover.6 Residual neurologic complications (facial nerve dysfunction, radiculopathies, vision or hearing loss, ataxia) have been documented in 5% to 28% of patients one year after therapy. Lyme arthritis resolves spontaneously, but it can take years and may require anti-inflammatory treatment.5,6
Prevention: Simple measures pay off
Advise patients to avoid ticks by avoiding brushy areas, especially at times when ticks are active. Wearing appropriate outdoor clothing (light-colored garments, long-sleeved shirts, and pants tucked into socks or boot tops) are key preventive steps. The possibility of transmission of B burgdorferi from an infected tick increases with time of attachment. Therefore, individuals spending time outdoors should apply insect repellent (N,N-diethyl-3-methylbenzamide (DEET) or permethrin), check for ticks daily, and remove them promptly if found. Applying pesticides and managing the landscape on one’s property also helps control tick populations.
Antimicrobial prophylaxis with a single 200-mg dose of oral doxycycline is appropriate for a patient who has no contraindications if there is known tick exposure and the patient lives in an area with at least a 20% incidence of Lyme disease, or for any patient who has a tick still attached (and it has been there for 36 hours).6,10 Prophylaxis can be started within 72 hours of tick removal.
Monitor all patients closely for up to 30 days for signs and symptoms of tick-borne diseases.
A Lyme-disease vaccine in humans was approved by the FDA in 1998, but was removed from the market in 2002 because of poor sales and theoretical concerns about triggering autoimmune arthritis.1,4-6,8,10,19
CASE › Ms. L’s FP opts to forgo doxycycline prophylaxis because she discovered the tick on her arm within 24 hours of the hiking expedition and removed it completely intact. In addition, the FP factored into her decision the fact that Lyme disease is not prevalent in Florida. The FP advised Ms. L about the signs and symptoms to watch for and made sure that a follow-up telephone appointment was scheduled for the next 4 to 6 weeks. Ms. L was also given ample educational pamphlets on the prevention of tick bites and Lyme disease.
CORRESPONDENCE
Sayed K. Ali, MD, FACP, Orlando Veterans Affairs Medical Center, 13800 Veterans Way, Orlando, FL, 32827; [email protected].
1. Centers for Disease Control and Prevention. Lyme disease data. Available at: http://www.cdc.gov/lyme/stats. Accessed April 19, 2016.
2. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000-2014. Open Forum Infect Dis. 2015;2:ofv143.
3. Gerstenblith TA, Stern TA. Lyme disease: a review of its epidemiology, evaluation and treatment. Psychosomatics. 2014;55:421-429.
4. Wright WF, Riedel DJ, Talwani R, et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85:1086-1093.
5. Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13-20.
6. Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115.
7. Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724-1731.
8. Cook MJ. Lyme borreliosis: a review of the data on transmission time after tick attachment. Int J Gen Med. 2014;8:1-8.
9. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;29:2617-2627.
10. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2006;43:1089-1134.
11. Khalil S, Padala SK, Hui CC, et al. Lyme carditis in the fast lane: from alternating bundle branch block to asystole in 12 hours. Conn Med. 2015;79:517-520.
12. Sigal LH. Early disseminated Lyme disease: cardiac manifestations. Am J Med. 1995;98:25S-28S.
13. Blaut-Jurkowska J, Jurkowski M. [Post-Lyme disease syndrome.] Pol Merkur Lekarski. 2016;40:129-133.
14. Steere AC, McHugh G, Damle N, et al. Prospective study of serologic test for lyme disease. Clin Infect Dis. 2008;47:188-195.
15. Carris NW, Pardo J, Montero J, et al. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2:ofv178.
16. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). 2015;21:1729-1744.
17. American Academy of Neurology. Treatment of nervous system Lyme disease. Available at: https://www.aan.com/Guidelines/home//241. Accessed May 13, 2016.
18. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Eng J Med. 2016;374:1209-1220.
19. Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med. 2015;35:883-899.
› Consider the duration of a tick’s attachment and whether it was engorged when assessing an individual’s risk of acquiring Lyme disease. C
› Start treatment for Lyme disease without lab testing if a patient has the painless skin rash—erythema migrans—and a history of tick exposure. C
› Choose doxycycline as first-line treatment for early Lyme disease unless a patient has contraindications. Amoxicillin or cefuroxime axetil are suitable alternatives. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice L, a 39-year-old woman with an unremarkable medical history asks to see her primary care provider right away, concerned she might have contracted Lyme disease. She had been hiking the overgrown trails on her family-owned ranch in Florida, and the next day she noticed a black tick stuck to her forearm. Using tweezers, she pulled the whole tick off intact, put it in a plastic cup, and immediately sought medical attention. How should her family physician (FP) advise her?
Lyme disease is the most common tick-borne illness in the United States, with more than 25,000 cases confirmed in this country in 2014.1 It is concentrated mostly in the northeast and upper Midwest, and less frequently occurs in the Pacific coastal regions of Oregon and northern California. Cases have also increasingly been reported in the southwest region of the Appalachian Mountains and the mountainous regions of southern Virginia.2
In 2014, the only states reporting no incidence of Lyme disease were Colorado, Hawaii, Louisiana, New Mexico, and Oklahoma.1 Lyme disease is also endemic in several regions in Northern Europe, Eastern Asia, and Northern Africa.1,3-7 According to the Centers for Disease Control and Prevention (CDC), boys ages 5 to 9 years are most affected.1
Disease transmission: Duration of tick attachment is important
The spirochete that causes Lyme disease, Borrelia burgdorferi, is transmitted to humans by the Ixodes tick. The Ixodes scapularis (deer tick) is common in the eastern and northern midwestern states and I pacificus is common in the western United States.
The life cycle. These small, dark-colored ticks have a 2-year life cycle that is comprised of 4 developmental stages: egg, larva, nymph, and adult. Eggs are laid in spring and hatch into larvae during late summer. The larvae feed on small animals (eg, mice, chipmunks, birds) and can acquire B burgdorferi infection at this stage. The larvae then molt into nymphs (<2 mm, and difficult to see), which feed again the following spring to early summer and may transmit the infection to a new host. Nymphs become adult ticks in mid-October to early-November, when the females feed again, mainly on large animals.
Humans usually become infected from May through August, when both they and the nymph ticks are most active outdoors. The ticks are able to attach themselves to their host without being noticed because they secrete small amounts of saliva with anesthetic properties while feeding. Many ticks also secrete a cement-like substance that keeps them firmly attached.
Adult ticks can also transmit the disease and are larger and more easily recognized. Transmission of the spirochete requires that the tick be attached to the new host for 36 to 48 hours,1 allowing the spirochete to travel from the mid-gut of the tick to the salivary glands and into the host.
Two of the most important factors to consider when assessing the risk of transmission is how long the tick was attached and whether it was engorged. Only about a quarter of individuals with Lyme disease recall having had a tick bite.1,3-6,8
Clinical presentation: Early and late findings
Symptoms of early Lyme disease usually start one to 2 weeks after a tick bite, but may start up to 30 days later. The most common presentation is a painless skin rash—erythema migrans (EM). It starts as a single red papule at the site of the bite (multiple lesions appear in 10% to 20% of cases9) and may progress to a painless erythematous lesion with red borders and a partial central clearing—the classic EM rash (FIGURE). Less commonly, the center of the lesion can appear vesicular or necrotic.

Although a rash occurs in 80% of Lyme disease cases, only 20% to 35% of the rashes develop into a classic bull's-eye lesion.3 Tick bites—and thus rashes—typically occur near or at the axilla, inguinal region, popliteal fossa, or at the belt line.
Individuals who don’t exhibit a rash may be asymptomatic or have nonspecific symptoms or flu-like symptoms of fatigue, fever, chills, myalgia, and headache.4 If Lyme disease continues untreated, the patient may experience extra-cutaneous complications, most often involving the joints and the nervous and cardiovascular systems.3-7
Ixodes ticks are also vectors for human granulocytic anaplasmosis (HGA) and babesiosis, which can cause a variety of symptoms. Keep these diseases in mind when a patient presents with severe or atypical features of Lyme disease.5 The benefit of antibiotics after a tick bite to reduce the incidence of HGA or babesiosis is unclear.10
Late manifestations of Lyme disease can occur within one to 2 months of infection or even months to years after tick exposure, often resulting in substantial morbidity.3-7,11 Musculoskeletal symptoms are the most common manifestations of late, disseminated disease, usually presenting as transient asymmetrical oligoarticular arthralgias or myalgia. Arthritis also occurs in 60% of untreated patients with late disease.4,5 Large joint effusions are typical, with synovial fluid studies showing high quantities of polymorphonuclear leukocytes (25,000/mm3).5 Joint symptoms that persist after antibiotic treatment are called antibiotic-refractory Lyme arthritis.4-7
Neurologic involvement affects 10% to 15% of untreated patients.3,4 It can present as lymphocytic meningitis (most common), cranial neuropathies, motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis. Late neurologic Lyme disease may also present as a subacute mild encephalopathy affecting memory and concentration. When cranial neuropathies are involved, it is usually as unilateral facial nerve palsy (but may be bilateral). Always consider Lyme disease in endemic areas when patients have severe Bell's palsy.
Patients may present with altered mental status, neck stiffness, pain, and headaches.4-7 The classic triad (known as Bannwarth syndrome) consists of lymphocytic meningitis, cranial neuritis, and radiculoneuritis. However, these conditions do not always occur together.3,4
Cardiovascular complications occur in 4% to 8% of untreated patients,4,5 usually one to 2 months following infection. Varying degrees of atrioventricular (AV) block can be seen, but third-degree block is most common. A less frequent complication is Lyme carditis, seen in 4% to 10% of patients.12 The pathophysiology of Lyme carditis is not well understood.11 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, often involving an AV block. Less frequent complications include myopericarditis, bundle branch block, and heart failure.
Post-treatment Lyme disease syndrome refers to the nonspecific symptoms of fatigue, sleep disorders, headaches, memory and concentration difficulties, myalgia, and arthralgias that may persist after successful antibiotic treatment. (We’ll discuss the specifics of treatment in a bit.) Post-treatment Lyme disease syndrome occurs in about 5% of patients properly treated for Lyme disease.13 The pathogenesis remains unknown, but some experts believe that lingering symptoms result from residual damage to tissues and the immune system. Education, rehabilitation, anti-inflammatory agents, antidepressants, a healthy diet, and plenty of rest have been recommended as treatment modalities.1,13
Chronic Lyme disease is also important to keep in mind. Although there is no standard definition for it, chronic Lyme disease refers broadly to chronic symptoms in patients who may or may not have Lyme disease—eg, an individual who may have been treated for presumed B burgdorferi infection without solid clinical or serologic confirmation.3-5 Chronic Lyme disease can often share somatic symptoms with other conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. Treatment often relies on a solid, trusting patient-doctor relationship, cognitive behavioral therapy, and regular counseling. Antibiotics are usually not necessary.
Dx: Serologic testing is preferred if clinical findings are insufficient
Lyme disease can be diagnosed clinically in patients who have an EM rash and a history of tick exposure. This is the only clinical presentation sufficient to make the diagnosis of Lyme disease without the need of confirmatory serologic testing.3,6 In the case of a tick bite but no rash, defer serologic testing unless associated symptoms arise (described earlier), at which time the accuracy of test results would be more trustworthy.10 Testing of ticks for infection with B burgdorferi is not recommended due to a lack of laboratory standardization.10
Two methods of laboratory testing are available to diagnose Lyme disease: direct, using cultures to detect B burgdorferi-specific proteins; and indirect, involving assays for antibodies.
Serologic testing. The CDC and Infectious Diseases Society of America recommend serology as the preferred initial diagnostic test. Tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer. However, these tests should not be used to screen individuals who have a low probability of infection, due to the tests’ poor positive predictive value.
The serologic tests used are the enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody (IFA) assay. These assays use a whole-cell sonicate of B burgdorferi and yield a significant number of false-positive results due to cross-reactive antigens such as flagellar and heat-shock proteins if other spirochetal infections are present. To increase testing specificity, both the CDC and European guidelines strongly recommend a 2-tier approach using an ELISA or IFA assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.1
The Western blot is interpreted using standardized criteria requiring at least 2 of 3 bands for a positive IgM diagnosis and 5 of 10 bands for a positive IgG diagnosis. Antibodies against Borrelia species are slow to develop. IgM generally is undetectable for the first one to 2 weeks after infection, and IgG often does not emerge for 4 to 6 weeks.
With patients who are seronegative at presentation, but for whom there is strong suspicion of Borrelia infection, it is advisable to obtain evidence of seroconversion, preferably within 8 to 14 days after presentation. Early antibiotic treatment may prevent the development of seropositivity.1,3-7,14
Past or newly acquired infection? IgM and IgG produced in response to B burgdorferi may persist for years following antimicrobial therapy, which makes it impossible to distinguish between past and newly acquired infections based on seropositivity alone. These persistently elevated levels are not an indication of ineffective treatment or chronic infection. Therefore, it is not recommended to repeat serologic testing for documentation of treatment effectiveness or cure.
Since no serologic test has sufficient specificity to be used alone, efforts are being made to develop testing that detects antibodies against the 26-mer peptide from the sixth invariant region (C6) of the VlsE lipoprotein (C6VlsE). In 2007, the US Food and Drug Administration (FDA) approved a C6 ELISA for first-tier testing; unfortunately, it still has the problem of cross-reactivity with other spirochetal and viral pathogens. The C6 ELISA may one day be approved as a single-tier test.4-7,14
Culture. The isolation of Borrelia species by culture is not routinely performed because it is expensive and requires special media and laboratory expertise, as well as a prolonged period of observation (6 to 12 weeks). Furthermore, this technique lacks sensitivity with samples taken from anywhere other than the rash site of patients with EM, in whom there is little need for laboratory diagnosis. Culture of cerebrospinal fluid has a positive yield of less than 10%,5 and it is extremely rare to isolate the spirochete from joint fluid. Therefore, negative results do not exclude a diagnosis of disease.4,5,14
The CDC recommends against cultures, immunofluorescence staining, and cell sorting of cell wall-deficient or cystic forms of B burgdorferi.1
Polymerase-chain reaction (PCR). This test is used to amplify genomic DNA of B burgdorferi and is most useful in patients with Lyme arthritis because of a high rate of DNA detection in synovial fluid samples (60% to 85%).5 In skin biopsies from EM lesions, PCR sensitivity can range from 25% to 90%.5 The PCR test is also used in cases of diagnostic uncertainty, but is generally performed only for research purposes. Negative findings do not exclude diagnosis of the disease.5,6,14
Urine antigen test. This test has a high false-positive rate and is generally not recommended.1,5
Treatment: Begin antibiotics ASAP
Treat Lyme disease with antibiotics as soon as the diagnosis is made. Early treatment hastens relief from symptoms and halts progression of later stages of the disease. The preferred antibiotics for early localized disease are doxycycline 100 mg orally twice daily; amoxicillin 500 mg orally 3 times a day; or cefuroxime axetil 500 mg orally twice a day (TABLE 1).10 Cefuroxime axetil is also appropriate if EM can’t be clearly distinguished from bacterial cellulitis. Reserve intravenous (IV) regimens for patients with more serious presentations (eg, neurologic symptoms and symptomatic cardiac disease) and for those with refractory Lyme arthritis (TABLE 2).10
Macrolides are not recommended as first-line therapy for early Lyme disease because they are less effective.10 However, macrolides may be used with patients unable to take the preferred antibiotics. Because there have been intermittent shortages of doxycycline, minocycline—another second-generation tetracycline with a similar chemical structure and antibacterial action—has been proposed as an alternative treatment.15
Ceftriaxone IV is preferred especially for patients presenting with an AV block or myopericarditis associated with early Lyme disease. The recommended course of treatment is usually 14 days. A temporary pacemaker may be required for patients with advanced blocks. Oral antibiotics may be started as soon as the AV block is resolved, or for outpatient therapy.
For adults who have early Lyme disease with acute neurologic manifestations such as meningitis or radiculopathy, IV antibiotics for 14 days are recommended. Cefotaxime has efficacy similar to ceftriaxone but requires multiple doses a day, making the latter the preferred treatment. Penicillin G 18 to 24 million units per day, divided into doses given every 4 hours, is also a satisfactory alternative.10,16 The American Academy of Neurology states that no definitive data exist to establish superiority, or lack thereof, of either oral or parenteral treatment.17

Lyme arthritis can be treated with oral doxycycline, amoxicillin, or cefuroxime axetil for 28 days. For patients with persistent or recurrent joint swelling who have been treated with a course of oral antibiotics, administer an additional 4 weeks of oral antibiotics or 2 to 4 weeks of IV ceftriaxone.10 A second 4-week course of oral antibiotics is also suggested for patients whose symptoms have greatly improved but not fully resolved.3,4,7,10
For post-Lyme syndromes, antibiotics have not proved useful and are not recommended for patients with chronic (>6 months) subjective symptoms.10,16 A recent study in Europe failed to show that antibiotic treatment for 12 weeks reduced symptoms or improved quality of life in patients with persistent symptoms associated with Lyme disease.18

Prognosis: It varies with specific complications
EM resolves within a few days or weeks (up to 8 weeks) after initiation of treatment. Generally, between 70% and 85% of patients with Lyme neuroborreliosis make a complete recovery, usually 6 to 12 months after initiation of therapy; and up to 90% of patients with facial palsy recover.6 Residual neurologic complications (facial nerve dysfunction, radiculopathies, vision or hearing loss, ataxia) have been documented in 5% to 28% of patients one year after therapy. Lyme arthritis resolves spontaneously, but it can take years and may require anti-inflammatory treatment.5,6
Prevention: Simple measures pay off
Advise patients to avoid ticks by avoiding brushy areas, especially at times when ticks are active. Wearing appropriate outdoor clothing (light-colored garments, long-sleeved shirts, and pants tucked into socks or boot tops) are key preventive steps. The possibility of transmission of B burgdorferi from an infected tick increases with time of attachment. Therefore, individuals spending time outdoors should apply insect repellent (N,N-diethyl-3-methylbenzamide (DEET) or permethrin), check for ticks daily, and remove them promptly if found. Applying pesticides and managing the landscape on one’s property also helps control tick populations.
Antimicrobial prophylaxis with a single 200-mg dose of oral doxycycline is appropriate for a patient who has no contraindications if there is known tick exposure and the patient lives in an area with at least a 20% incidence of Lyme disease, or for any patient who has a tick still attached (and it has been there for 36 hours).6,10 Prophylaxis can be started within 72 hours of tick removal.
Monitor all patients closely for up to 30 days for signs and symptoms of tick-borne diseases.
A Lyme-disease vaccine in humans was approved by the FDA in 1998, but was removed from the market in 2002 because of poor sales and theoretical concerns about triggering autoimmune arthritis.1,4-6,8,10,19
CASE › Ms. L’s FP opts to forgo doxycycline prophylaxis because she discovered the tick on her arm within 24 hours of the hiking expedition and removed it completely intact. In addition, the FP factored into her decision the fact that Lyme disease is not prevalent in Florida. The FP advised Ms. L about the signs and symptoms to watch for and made sure that a follow-up telephone appointment was scheduled for the next 4 to 6 weeks. Ms. L was also given ample educational pamphlets on the prevention of tick bites and Lyme disease.
CORRESPONDENCE
Sayed K. Ali, MD, FACP, Orlando Veterans Affairs Medical Center, 13800 Veterans Way, Orlando, FL, 32827; [email protected].
› Consider the duration of a tick’s attachment and whether it was engorged when assessing an individual’s risk of acquiring Lyme disease. C
› Start treatment for Lyme disease without lab testing if a patient has the painless skin rash—erythema migrans—and a history of tick exposure. C
› Choose doxycycline as first-line treatment for early Lyme disease unless a patient has contraindications. Amoxicillin or cefuroxime axetil are suitable alternatives. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Alice L, a 39-year-old woman with an unremarkable medical history asks to see her primary care provider right away, concerned she might have contracted Lyme disease. She had been hiking the overgrown trails on her family-owned ranch in Florida, and the next day she noticed a black tick stuck to her forearm. Using tweezers, she pulled the whole tick off intact, put it in a plastic cup, and immediately sought medical attention. How should her family physician (FP) advise her?
Lyme disease is the most common tick-borne illness in the United States, with more than 25,000 cases confirmed in this country in 2014.1 It is concentrated mostly in the northeast and upper Midwest, and less frequently occurs in the Pacific coastal regions of Oregon and northern California. Cases have also increasingly been reported in the southwest region of the Appalachian Mountains and the mountainous regions of southern Virginia.2
In 2014, the only states reporting no incidence of Lyme disease were Colorado, Hawaii, Louisiana, New Mexico, and Oklahoma.1 Lyme disease is also endemic in several regions in Northern Europe, Eastern Asia, and Northern Africa.1,3-7 According to the Centers for Disease Control and Prevention (CDC), boys ages 5 to 9 years are most affected.1
Disease transmission: Duration of tick attachment is important
The spirochete that causes Lyme disease, Borrelia burgdorferi, is transmitted to humans by the Ixodes tick. The Ixodes scapularis (deer tick) is common in the eastern and northern midwestern states and I pacificus is common in the western United States.
The life cycle. These small, dark-colored ticks have a 2-year life cycle that is comprised of 4 developmental stages: egg, larva, nymph, and adult. Eggs are laid in spring and hatch into larvae during late summer. The larvae feed on small animals (eg, mice, chipmunks, birds) and can acquire B burgdorferi infection at this stage. The larvae then molt into nymphs (<2 mm, and difficult to see), which feed again the following spring to early summer and may transmit the infection to a new host. Nymphs become adult ticks in mid-October to early-November, when the females feed again, mainly on large animals.
Humans usually become infected from May through August, when both they and the nymph ticks are most active outdoors. The ticks are able to attach themselves to their host without being noticed because they secrete small amounts of saliva with anesthetic properties while feeding. Many ticks also secrete a cement-like substance that keeps them firmly attached.
Adult ticks can also transmit the disease and are larger and more easily recognized. Transmission of the spirochete requires that the tick be attached to the new host for 36 to 48 hours,1 allowing the spirochete to travel from the mid-gut of the tick to the salivary glands and into the host.
Two of the most important factors to consider when assessing the risk of transmission is how long the tick was attached and whether it was engorged. Only about a quarter of individuals with Lyme disease recall having had a tick bite.1,3-6,8
Clinical presentation: Early and late findings
Symptoms of early Lyme disease usually start one to 2 weeks after a tick bite, but may start up to 30 days later. The most common presentation is a painless skin rash—erythema migrans (EM). It starts as a single red papule at the site of the bite (multiple lesions appear in 10% to 20% of cases9) and may progress to a painless erythematous lesion with red borders and a partial central clearing—the classic EM rash (FIGURE). Less commonly, the center of the lesion can appear vesicular or necrotic.

Although a rash occurs in 80% of Lyme disease cases, only 20% to 35% of the rashes develop into a classic bull's-eye lesion.3 Tick bites—and thus rashes—typically occur near or at the axilla, inguinal region, popliteal fossa, or at the belt line.
Individuals who don’t exhibit a rash may be asymptomatic or have nonspecific symptoms or flu-like symptoms of fatigue, fever, chills, myalgia, and headache.4 If Lyme disease continues untreated, the patient may experience extra-cutaneous complications, most often involving the joints and the nervous and cardiovascular systems.3-7
Ixodes ticks are also vectors for human granulocytic anaplasmosis (HGA) and babesiosis, which can cause a variety of symptoms. Keep these diseases in mind when a patient presents with severe or atypical features of Lyme disease.5 The benefit of antibiotics after a tick bite to reduce the incidence of HGA or babesiosis is unclear.10
Late manifestations of Lyme disease can occur within one to 2 months of infection or even months to years after tick exposure, often resulting in substantial morbidity.3-7,11 Musculoskeletal symptoms are the most common manifestations of late, disseminated disease, usually presenting as transient asymmetrical oligoarticular arthralgias or myalgia. Arthritis also occurs in 60% of untreated patients with late disease.4,5 Large joint effusions are typical, with synovial fluid studies showing high quantities of polymorphonuclear leukocytes (25,000/mm3).5 Joint symptoms that persist after antibiotic treatment are called antibiotic-refractory Lyme arthritis.4-7
Neurologic involvement affects 10% to 15% of untreated patients.3,4 It can present as lymphocytic meningitis (most common), cranial neuropathies, motor or sensory radiculoneuropathy, mononeuritis multiplex, cerebellar ataxia, or myelitis. Late neurologic Lyme disease may also present as a subacute mild encephalopathy affecting memory and concentration. When cranial neuropathies are involved, it is usually as unilateral facial nerve palsy (but may be bilateral). Always consider Lyme disease in endemic areas when patients have severe Bell's palsy.
Patients may present with altered mental status, neck stiffness, pain, and headaches.4-7 The classic triad (known as Bannwarth syndrome) consists of lymphocytic meningitis, cranial neuritis, and radiculoneuritis. However, these conditions do not always occur together.3,4
Cardiovascular complications occur in 4% to 8% of untreated patients,4,5 usually one to 2 months following infection. Varying degrees of atrioventricular (AV) block can be seen, but third-degree block is most common. A less frequent complication is Lyme carditis, seen in 4% to 10% of patients.12 The pathophysiology of Lyme carditis is not well understood.11 It may present as chest pain, dyspnea on exertion, fatigue, palpitations, or syncope, often involving an AV block. Less frequent complications include myopericarditis, bundle branch block, and heart failure.
Post-treatment Lyme disease syndrome refers to the nonspecific symptoms of fatigue, sleep disorders, headaches, memory and concentration difficulties, myalgia, and arthralgias that may persist after successful antibiotic treatment. (We’ll discuss the specifics of treatment in a bit.) Post-treatment Lyme disease syndrome occurs in about 5% of patients properly treated for Lyme disease.13 The pathogenesis remains unknown, but some experts believe that lingering symptoms result from residual damage to tissues and the immune system. Education, rehabilitation, anti-inflammatory agents, antidepressants, a healthy diet, and plenty of rest have been recommended as treatment modalities.1,13
Chronic Lyme disease is also important to keep in mind. Although there is no standard definition for it, chronic Lyme disease refers broadly to chronic symptoms in patients who may or may not have Lyme disease—eg, an individual who may have been treated for presumed B burgdorferi infection without solid clinical or serologic confirmation.3-5 Chronic Lyme disease can often share somatic symptoms with other conditions such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. Treatment often relies on a solid, trusting patient-doctor relationship, cognitive behavioral therapy, and regular counseling. Antibiotics are usually not necessary.
Dx: Serologic testing is preferred if clinical findings are insufficient
Lyme disease can be diagnosed clinically in patients who have an EM rash and a history of tick exposure. This is the only clinical presentation sufficient to make the diagnosis of Lyme disease without the need of confirmatory serologic testing.3,6 In the case of a tick bite but no rash, defer serologic testing unless associated symptoms arise (described earlier), at which time the accuracy of test results would be more trustworthy.10 Testing of ticks for infection with B burgdorferi is not recommended due to a lack of laboratory standardization.10
Two methods of laboratory testing are available to diagnose Lyme disease: direct, using cultures to detect B burgdorferi-specific proteins; and indirect, involving assays for antibodies.
Serologic testing. The CDC and Infectious Diseases Society of America recommend serology as the preferred initial diagnostic test. Tests for antibodies have good sensitivity and specificity in patients who have had untreated infection for a month or longer. However, these tests should not be used to screen individuals who have a low probability of infection, due to the tests’ poor positive predictive value.
The serologic tests used are the enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody (IFA) assay. These assays use a whole-cell sonicate of B burgdorferi and yield a significant number of false-positive results due to cross-reactive antigens such as flagellar and heat-shock proteins if other spirochetal infections are present. To increase testing specificity, both the CDC and European guidelines strongly recommend a 2-tier approach using an ELISA or IFA assay initially, followed by the more specific Western blot to confirm the diagnosis when the assay samples are positive or equivocal.1
The Western blot is interpreted using standardized criteria requiring at least 2 of 3 bands for a positive IgM diagnosis and 5 of 10 bands for a positive IgG diagnosis. Antibodies against Borrelia species are slow to develop. IgM generally is undetectable for the first one to 2 weeks after infection, and IgG often does not emerge for 4 to 6 weeks.
With patients who are seronegative at presentation, but for whom there is strong suspicion of Borrelia infection, it is advisable to obtain evidence of seroconversion, preferably within 8 to 14 days after presentation. Early antibiotic treatment may prevent the development of seropositivity.1,3-7,14
Past or newly acquired infection? IgM and IgG produced in response to B burgdorferi may persist for years following antimicrobial therapy, which makes it impossible to distinguish between past and newly acquired infections based on seropositivity alone. These persistently elevated levels are not an indication of ineffective treatment or chronic infection. Therefore, it is not recommended to repeat serologic testing for documentation of treatment effectiveness or cure.
Since no serologic test has sufficient specificity to be used alone, efforts are being made to develop testing that detects antibodies against the 26-mer peptide from the sixth invariant region (C6) of the VlsE lipoprotein (C6VlsE). In 2007, the US Food and Drug Administration (FDA) approved a C6 ELISA for first-tier testing; unfortunately, it still has the problem of cross-reactivity with other spirochetal and viral pathogens. The C6 ELISA may one day be approved as a single-tier test.4-7,14
Culture. The isolation of Borrelia species by culture is not routinely performed because it is expensive and requires special media and laboratory expertise, as well as a prolonged period of observation (6 to 12 weeks). Furthermore, this technique lacks sensitivity with samples taken from anywhere other than the rash site of patients with EM, in whom there is little need for laboratory diagnosis. Culture of cerebrospinal fluid has a positive yield of less than 10%,5 and it is extremely rare to isolate the spirochete from joint fluid. Therefore, negative results do not exclude a diagnosis of disease.4,5,14
The CDC recommends against cultures, immunofluorescence staining, and cell sorting of cell wall-deficient or cystic forms of B burgdorferi.1
Polymerase-chain reaction (PCR). This test is used to amplify genomic DNA of B burgdorferi and is most useful in patients with Lyme arthritis because of a high rate of DNA detection in synovial fluid samples (60% to 85%).5 In skin biopsies from EM lesions, PCR sensitivity can range from 25% to 90%.5 The PCR test is also used in cases of diagnostic uncertainty, but is generally performed only for research purposes. Negative findings do not exclude diagnosis of the disease.5,6,14
Urine antigen test. This test has a high false-positive rate and is generally not recommended.1,5
Treatment: Begin antibiotics ASAP
Treat Lyme disease with antibiotics as soon as the diagnosis is made. Early treatment hastens relief from symptoms and halts progression of later stages of the disease. The preferred antibiotics for early localized disease are doxycycline 100 mg orally twice daily; amoxicillin 500 mg orally 3 times a day; or cefuroxime axetil 500 mg orally twice a day (TABLE 1).10 Cefuroxime axetil is also appropriate if EM can’t be clearly distinguished from bacterial cellulitis. Reserve intravenous (IV) regimens for patients with more serious presentations (eg, neurologic symptoms and symptomatic cardiac disease) and for those with refractory Lyme arthritis (TABLE 2).10
Macrolides are not recommended as first-line therapy for early Lyme disease because they are less effective.10 However, macrolides may be used with patients unable to take the preferred antibiotics. Because there have been intermittent shortages of doxycycline, minocycline—another second-generation tetracycline with a similar chemical structure and antibacterial action—has been proposed as an alternative treatment.15
Ceftriaxone IV is preferred especially for patients presenting with an AV block or myopericarditis associated with early Lyme disease. The recommended course of treatment is usually 14 days. A temporary pacemaker may be required for patients with advanced blocks. Oral antibiotics may be started as soon as the AV block is resolved, or for outpatient therapy.
For adults who have early Lyme disease with acute neurologic manifestations such as meningitis or radiculopathy, IV antibiotics for 14 days are recommended. Cefotaxime has efficacy similar to ceftriaxone but requires multiple doses a day, making the latter the preferred treatment. Penicillin G 18 to 24 million units per day, divided into doses given every 4 hours, is also a satisfactory alternative.10,16 The American Academy of Neurology states that no definitive data exist to establish superiority, or lack thereof, of either oral or parenteral treatment.17

Lyme arthritis can be treated with oral doxycycline, amoxicillin, or cefuroxime axetil for 28 days. For patients with persistent or recurrent joint swelling who have been treated with a course of oral antibiotics, administer an additional 4 weeks of oral antibiotics or 2 to 4 weeks of IV ceftriaxone.10 A second 4-week course of oral antibiotics is also suggested for patients whose symptoms have greatly improved but not fully resolved.3,4,7,10
For post-Lyme syndromes, antibiotics have not proved useful and are not recommended for patients with chronic (>6 months) subjective symptoms.10,16 A recent study in Europe failed to show that antibiotic treatment for 12 weeks reduced symptoms or improved quality of life in patients with persistent symptoms associated with Lyme disease.18

Prognosis: It varies with specific complications
EM resolves within a few days or weeks (up to 8 weeks) after initiation of treatment. Generally, between 70% and 85% of patients with Lyme neuroborreliosis make a complete recovery, usually 6 to 12 months after initiation of therapy; and up to 90% of patients with facial palsy recover.6 Residual neurologic complications (facial nerve dysfunction, radiculopathies, vision or hearing loss, ataxia) have been documented in 5% to 28% of patients one year after therapy. Lyme arthritis resolves spontaneously, but it can take years and may require anti-inflammatory treatment.5,6
Prevention: Simple measures pay off
Advise patients to avoid ticks by avoiding brushy areas, especially at times when ticks are active. Wearing appropriate outdoor clothing (light-colored garments, long-sleeved shirts, and pants tucked into socks or boot tops) are key preventive steps. The possibility of transmission of B burgdorferi from an infected tick increases with time of attachment. Therefore, individuals spending time outdoors should apply insect repellent (N,N-diethyl-3-methylbenzamide (DEET) or permethrin), check for ticks daily, and remove them promptly if found. Applying pesticides and managing the landscape on one’s property also helps control tick populations.
Antimicrobial prophylaxis with a single 200-mg dose of oral doxycycline is appropriate for a patient who has no contraindications if there is known tick exposure and the patient lives in an area with at least a 20% incidence of Lyme disease, or for any patient who has a tick still attached (and it has been there for 36 hours).6,10 Prophylaxis can be started within 72 hours of tick removal.
Monitor all patients closely for up to 30 days for signs and symptoms of tick-borne diseases.
A Lyme-disease vaccine in humans was approved by the FDA in 1998, but was removed from the market in 2002 because of poor sales and theoretical concerns about triggering autoimmune arthritis.1,4-6,8,10,19
CASE › Ms. L’s FP opts to forgo doxycycline prophylaxis because she discovered the tick on her arm within 24 hours of the hiking expedition and removed it completely intact. In addition, the FP factored into her decision the fact that Lyme disease is not prevalent in Florida. The FP advised Ms. L about the signs and symptoms to watch for and made sure that a follow-up telephone appointment was scheduled for the next 4 to 6 weeks. Ms. L was also given ample educational pamphlets on the prevention of tick bites and Lyme disease.
CORRESPONDENCE
Sayed K. Ali, MD, FACP, Orlando Veterans Affairs Medical Center, 13800 Veterans Way, Orlando, FL, 32827; [email protected].
1. Centers for Disease Control and Prevention. Lyme disease data. Available at: http://www.cdc.gov/lyme/stats. Accessed April 19, 2016.
2. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000-2014. Open Forum Infect Dis. 2015;2:ofv143.
3. Gerstenblith TA, Stern TA. Lyme disease: a review of its epidemiology, evaluation and treatment. Psychosomatics. 2014;55:421-429.
4. Wright WF, Riedel DJ, Talwani R, et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85:1086-1093.
5. Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13-20.
6. Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115.
7. Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724-1731.
8. Cook MJ. Lyme borreliosis: a review of the data on transmission time after tick attachment. Int J Gen Med. 2014;8:1-8.
9. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;29:2617-2627.
10. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2006;43:1089-1134.
11. Khalil S, Padala SK, Hui CC, et al. Lyme carditis in the fast lane: from alternating bundle branch block to asystole in 12 hours. Conn Med. 2015;79:517-520.
12. Sigal LH. Early disseminated Lyme disease: cardiac manifestations. Am J Med. 1995;98:25S-28S.
13. Blaut-Jurkowska J, Jurkowski M. [Post-Lyme disease syndrome.] Pol Merkur Lekarski. 2016;40:129-133.
14. Steere AC, McHugh G, Damle N, et al. Prospective study of serologic test for lyme disease. Clin Infect Dis. 2008;47:188-195.
15. Carris NW, Pardo J, Montero J, et al. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2:ofv178.
16. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). 2015;21:1729-1744.
17. American Academy of Neurology. Treatment of nervous system Lyme disease. Available at: https://www.aan.com/Guidelines/home//241. Accessed May 13, 2016.
18. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Eng J Med. 2016;374:1209-1220.
19. Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med. 2015;35:883-899.
1. Centers for Disease Control and Prevention. Lyme disease data. Available at: http://www.cdc.gov/lyme/stats. Accessed April 19, 2016.
2. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000-2014. Open Forum Infect Dis. 2015;2:ofv143.
3. Gerstenblith TA, Stern TA. Lyme disease: a review of its epidemiology, evaluation and treatment. Psychosomatics. 2014;55:421-429.
4. Wright WF, Riedel DJ, Talwani R, et al. Diagnosis and management of Lyme disease. Am Fam Physician. 2012;85:1086-1093.
5. Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13-20.
6. Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115.
7. Shapiro ED. Clinical practice. Lyme disease. N Engl J Med. 2014;370:1724-1731.
8. Cook MJ. Lyme borreliosis: a review of the data on transmission time after tick attachment. Int J Gen Med. 2014;8:1-8.
9. Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA. 2007;29:2617-2627.
10. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2006;43:1089-1134.
11. Khalil S, Padala SK, Hui CC, et al. Lyme carditis in the fast lane: from alternating bundle branch block to asystole in 12 hours. Conn Med. 2015;79:517-520.
12. Sigal LH. Early disseminated Lyme disease: cardiac manifestations. Am J Med. 1995;98:25S-28S.
13. Blaut-Jurkowska J, Jurkowski M. [Post-Lyme disease syndrome.] Pol Merkur Lekarski. 2016;40:129-133.
14. Steere AC, McHugh G, Damle N, et al. Prospective study of serologic test for lyme disease. Clin Infect Dis. 2008;47:188-195.
15. Carris NW, Pardo J, Montero J, et al. Minocycline as a substitute for doxycycline in targeted scenarios: a systematic review. Open Forum Infect Dis. 2015;2:ofv178.
16. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). 2015;21:1729-1744.
17. American Academy of Neurology. Treatment of nervous system Lyme disease. Available at: https://www.aan.com/Guidelines/home//241. Accessed May 13, 2016.
18. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Eng J Med. 2016;374:1209-1220.
19. Ogden NH, Lindsay LR, Schofield SW. Methods to prevent tick bites and Lyme disease. Clin Lab Med. 2015;35:883-899.
From The Journal of Family Practice | 2016;65(6):373-379.