User login
Collaborative care
Reducing medical comorbidity and mortality in severe mental illness
People with serious mental illness (SMI) have a life expectancy that is 25 years less than the general population, according to the Centers for Disease Control and Prevention.1 This disparity is partially a consequence of the lack of primary and preventive medical care for those with psychiatric illness. Decades of research have shown that people with SMI experience higher medical morbidity and mortality in addition to facing the stigma of mental illness.
This article aims to advance the idea that longitudinal “cross education” between primary care providers (PCPs) and behavioral health providers (BHPs) is essential in addressing this problem. BHPs include psychiatry clinics, which often are part of a university or large health systems; county-based community mental health programs; and independent mental health clinics that contract with public and private health plans to provide mental health services.
Although suicide and injury account for 40% of the excess mortality in schizophrenia, 60% can be attributed to cardiovascular disease, diabetes, respiratory diseases, and infection.2 Patients with SMI have 2 to 3 times the risk of diabetes, dyslipidemia, hypertension, and obesity.3,4 Furthermore, those with SMI consume more than one-third of tobacco products,5 and 50% to 80% of people with SMI smoke tobacco, an important reversible risk factor for cardiovascular disease.
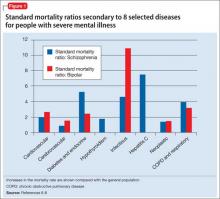
Figure 1 shows that people with SMI are at higher risk of dying from a chronic medical condition, such as cardiovascular disease, diabetes, chronic obstructive pulmonary disease, and hepatitis C6-8—many of which can be managed by primary and preventive medical interventions. These and other conditions often are not diagnosed or effectively managed in patients with SMI.
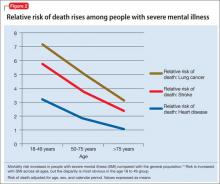
The high prevalence of metabolic syndrome and tobacco dependence among people with SMI accelerates development of cardiovascular disease, as shown by several studies. Bobes et al9 found that the prevalence of metabolic syndrome and cardiovascular risk among patients with SMI is similar to what is found in the general population at 10 to 15 years of greater age. Osborn et al10 demonstrated that people with SMI age 18 to 49 had a higher relative risk of death from coronary heart disease, stroke, and lung cancer than age-matched controls (Figure 2).
It can be said, therefore, that patients with SMI seem to “age” and die prematurely. To reduce this disparity, primary and preventive medical care—especially for cardiovascular disease—must be delivered earlier in life for those with SMI.
Iatrogenic causes of morbidity
Many psychiatric medications, especially second-generation antipsychotics (SGAs), could exacerbate cardiovascular and metabolic conditions by increasing the risk of weight gain, insulin resistance, and dyslipidemia. Antipsychotics that generally are considered to be more effective for refractory psychotic illness (eg, clozapine and olanzapine) are associated with the highest risk of metabolic syndrome. Simon et al11 found a dose-response relationship between olanzapine and clozapine serum concentrations and worsening metabolic outcomes. Valproic acid also can cause significant weight gain and could require monitoring similar to what is done with to SGAs, although there has been less clinical and research attention to this mood stabilizer.
The American Diabetes Association et al12 have published guidelines on monitoring antipsychotic-induced obesity and diabetes, but adoption of these guidelines has been slow. Mackin et al13 found that providers are slow to recognize the elevated rate of obesity and dyslipidemia among psychiatric patients, possibly because of “an alarmingly poor rate of monitoring of metabolic parameters.”
Treating adverse metabolic outcomes also seems to lag behind. The same study13 found that physical health parameters among psychiatric patients continue to become worse even when appropriate health care professionals were notified. Rates of nontreatment for diabetes, dyslipidemia, and hypertension were 30%, 60%, and 88% respectively, according to Nasrallah et al.14
Randomized controlled studies have shown that obesity and metabolic syndrome can be effectively managed using lifestyle and pharmacotherapeutic approaches,15,16 but more research is needed to test long-term outcomes and how to best incorporate these interventions. Newcomer et al17 found that gradually switching an antipsychotic with high risk of metabolic adverse effects to one with lower risk could reduce adverse metabolic outcomes; however, some patients returned to their prior antipsychotic because other medications did not effectively treat their schizophrenia symptoms. Therefore, physicians must pay careful attention to the trade-off between benefits and risks of antipsychotics and make treatment decisions on an individual basis.
Barriers to medical care
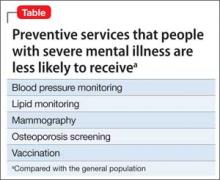
Research has demonstrated that patients with SMI receive less screening and fewer preventive medical services, especially blood pressure monitoring, vaccinations, mammography, lipid monitoring, and osteoporosis screening, compared with the general population (Table).18 Some barriers to preventive services could exist because of demographic factors and medical insurance coverage19 or medical providers’ discomfort with symptoms of SMI,20 although Mitchell et al21 found that disparities in mammography screening could not be explained by the presence of emotional distress in women with SMI.
DiMatteo et al22 reported that patients with SMI are 3 times more likely to be noncompliant with medical treatment. These patients also are less likely to receive sec ondary preventive medical care and invasive medical procedures. Those with SMI who experience acute myocardial infarction are less likely to receive drug therapy, such as a thrombolytic, aspirin, beta blocker, or angiotensin-converting enzyme inhibitor.23 They also are less likely to receive invasive cardiovascular procedures, including cardiac catheterization, angioplasty, and coronary artery bypass grafting.24
Therefore, not only are patients with SMI less likely to receive preventive care, they are also less likely to receive potentially lifesaving treatments for SMI. Because those with SMI might not be able to advocate for themselves in these matters, psychiatric clinicians can improve their patients’ lives by advocating for appropriate medical care despite multiple barriers.
Bridging the gap: Managing mental health in primary care
Research from the 1970s and 1980s demonstrated that most persons who sought help for depression or anxiety received treatment from their PCP, many of whom felt limited by their lack of behavioral health training. Moreover, many patients failed to receive a psychiatric diagnosis or adequate treatment, despite efforts to educate primary care physicians on appropriate diagnosis and treatment of mental illness.
Katon et al25 at the University of Washington developed the collaborative care model in the early 1990s to help improve treatment of depression in primary care settings. This model involved:
• case load review by psychiatrists
• use of nurses and other support staff to help monitor patients’ adherence and treatment response
• use of standardized tools such as the Patient Health Questionnaire to monitor symptoms
• enhancement of patient education with pamphlets or classes.
Studies evaluating the success of collaborative care models found overall improved outcomes, making it the only evidence-based model for integration of behavioral health and primary care.26 As a result, the collaborative care model has been implemented across the United States in primary care clinics and specialty care settings, such as obstetrics and gynecology.27
Regrettably, access to primary care has been hampered by:
• population growth
• a shortage of PCPs
• enrollment of a flood of new patients into the health care marketplace as a result of mandates of the Affordable Care Act (ACA).
In many settings, a psychiatrist might be the patient’s only consistent care provider, and could be thought of as a “primary care psychiatrist.”
To resolve this predicament, mental health professionals need to recognize the unique medical conditions faced by people with SMI, and also might need to provide treatment of common medical conditions, either directly or through collaborative arrangements. Psychiatrists who are capable of managing core medical issues likely will witness improved psychiatric and overall health outcomes in their patients. Consequently, psychiatrists and mental health professionals are increasingly called on to be advocates to improve access to medical services in patients with SMI and to participate in health systems reform.
Managing medical conditions in mental health settings
Although traditional collaborative care involves mental health providers working at primary care sites, other models have emerged that manage chronic disease in behavioral health settings. Federally funded grants for primary behavioral health care integration have allowed community mental health centers to partner with federally qualified health centers to provide on-site primary care services.28
In these models, care managers in mental health clinics:
• link patients to primary care services
• encourage lifestyle changes to improve their overall health
• identify and overcome barriers to receiving care
• track clinical outcomes in a registry format.
Currently, 126 mental health sites in the United States have received these grants and are working toward greater integration of primary care.
In addition, the ACA provided funding for “health homes” in non-primary care settings, which includes SMI. These health homes cannot provide direct primary care, but can deliver comprehensive care management, care coordination, health promotion, comprehensive transitional care services between facilities, individual and family support, and referral to community social support services. In these health homes, a PCP can act as a consultant to help establish priorities for disease management and improving health status.29 The PCP consultant also can support psychiatric staff and collaborate with providers who want to provide some direct care of medical conditions.30
Last, some behavioral health sites are choosing to apply for Federally Qualified Health Clinic status or add primary care services to their clinics, with the hope that sustainable funding will become available. Without additional funding to cover the limited reimbursement provided by public payers, such as Medicaid and Medicare, these models might be unsustainable. Current innovations in health care funding reform hopefully will offer solutions for sites to provide medical care in the natural “medical home” of the SMI population.
Bottom Line
Psychiatric providers are in a favorable position to develop and oversee a partnership with primary care physicians with the goal of addressing significant and often lethal health disparities among those with mental illness. Psychiatric providers must use evidence-based practices that include assessment and prevention of cardiopulmonary, metabolic, infectious, and oncologic disorders. True primary care–behavioral health integration must include longitudinal “cross education” and changes in health care policy, with an emphasis on decreasing morbidity and mortality in psychiatric patients.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
2. Parks J, Svendsen D, Singer P, et al, eds. Morbidity and mortality in people with serious mental illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006.
3. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
4. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trails of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
5. Compton MT, Daumit GL, Druss BG. Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: a preventive perspective. Harv Rev Psychiatry. 2006;14(2):212-222.
6. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
7. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156.
8. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based study. J Gen Intern Med. 2006;21(11):1133-1137.
9. Bobes J, Arango C, Aranda P, et al; CLAMORS Study Collaborative Group. Cardiovascular and metabolic risk in outpatients with schizoaffective disorder treated with antipsychotics; results from the CLAMORS study. Eur Psychiatry. 2012;27(4):267-274.
10. Osborn DP, Levy G, Nazareth I, et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database [Erratum in: Arch Gen Psychiatry. 2007;64(6):736]. Arch Gen Psychiatry. 2007;64(2):242-249.
11. Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70(7):1041-1050.
12. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. Mackin P, Bishop DR, Watkinson HM. A prospective study of monitoring practices for metabolic disease in antipsychotic-treated community psychiatric patients. BMC Psychiatry. 2007;7:28.
14. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
15. Alvarez-Jiménez M, Hetrick SE, González-Blanch C, et al. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomized controlled trials. Br J Psychiatry. 2008; 193(2):101-107.
16. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medication used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520-1530.
17. Newcomer JW, Weiden PJ, Buchanan RW. Switching antipsychotic medications to reduce adverse event burden in schizophrenia: establishing evidence-based practice. J Clin Psychiatry. 2013;74(11):1108-1120.
18. Lord O, Malone D, Mitchell AJ. Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry. 2010;32(5):519-543.
19. Xiong GL, Iosif AM, Bermudes RA, et al. Preventive medical services use among community mental health patients with severe mental illness: the influence of gender and insurance coverage. Prim Care Companion J Clin Psychiatry. 2010;12(5). doi: 10.4088/PCC.09m00927gre.
20. Daub S. Turning toward treating the seriously mentally ill in primary care. Fam Syst Health. 2014;32(1):12-13.
21. Mitchell A, Pereira IE, Yadegarfar M, et al. Breast cancer screening in women with mental illness: comparative meta-analysis of mammography uptake. Br J Psychiatry. 2014;205(6):428-435.
22. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
23. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
24. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
25. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32(5):456-464.
26. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525.
27. Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: socioeconomic disadvantage and treatment response. Am J Psychiatry. 2015;172(1):32-40.
28. Substance Abuse and Mental Health Services Administration. Request for Applications (RFA) No. SM- 09-011. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009.
29. Parks J. Behavioral health homes. In: Integrated care: working at the interface of primary care and behavioral health. Raney LE, ed. Arlington, VA: American Psychiatric Publishing; 2015:195.
30. Raney L. Integrated care: the evolving role of psychiatry in the era of health care reform. Psychiatr Serv. 2013;64(11):1076-1078.
People with serious mental illness (SMI) have a life expectancy that is 25 years less than the general population, according to the Centers for Disease Control and Prevention.1 This disparity is partially a consequence of the lack of primary and preventive medical care for those with psychiatric illness. Decades of research have shown that people with SMI experience higher medical morbidity and mortality in addition to facing the stigma of mental illness.
This article aims to advance the idea that longitudinal “cross education” between primary care providers (PCPs) and behavioral health providers (BHPs) is essential in addressing this problem. BHPs include psychiatry clinics, which often are part of a university or large health systems; county-based community mental health programs; and independent mental health clinics that contract with public and private health plans to provide mental health services.
Although suicide and injury account for 40% of the excess mortality in schizophrenia, 60% can be attributed to cardiovascular disease, diabetes, respiratory diseases, and infection.2 Patients with SMI have 2 to 3 times the risk of diabetes, dyslipidemia, hypertension, and obesity.3,4 Furthermore, those with SMI consume more than one-third of tobacco products,5 and 50% to 80% of people with SMI smoke tobacco, an important reversible risk factor for cardiovascular disease.
Figure 1 shows that people with SMI are at higher risk of dying from a chronic medical condition, such as cardiovascular disease, diabetes, chronic obstructive pulmonary disease, and hepatitis C6-8—many of which can be managed by primary and preventive medical interventions. These and other conditions often are not diagnosed or effectively managed in patients with SMI.
The high prevalence of metabolic syndrome and tobacco dependence among people with SMI accelerates development of cardiovascular disease, as shown by several studies. Bobes et al9 found that the prevalence of metabolic syndrome and cardiovascular risk among patients with SMI is similar to what is found in the general population at 10 to 15 years of greater age. Osborn et al10 demonstrated that people with SMI age 18 to 49 had a higher relative risk of death from coronary heart disease, stroke, and lung cancer than age-matched controls (Figure 2).
It can be said, therefore, that patients with SMI seem to “age” and die prematurely. To reduce this disparity, primary and preventive medical care—especially for cardiovascular disease—must be delivered earlier in life for those with SMI.
Iatrogenic causes of morbidity
Many psychiatric medications, especially second-generation antipsychotics (SGAs), could exacerbate cardiovascular and metabolic conditions by increasing the risk of weight gain, insulin resistance, and dyslipidemia. Antipsychotics that generally are considered to be more effective for refractory psychotic illness (eg, clozapine and olanzapine) are associated with the highest risk of metabolic syndrome. Simon et al11 found a dose-response relationship between olanzapine and clozapine serum concentrations and worsening metabolic outcomes. Valproic acid also can cause significant weight gain and could require monitoring similar to what is done with to SGAs, although there has been less clinical and research attention to this mood stabilizer.
The American Diabetes Association et al12 have published guidelines on monitoring antipsychotic-induced obesity and diabetes, but adoption of these guidelines has been slow. Mackin et al13 found that providers are slow to recognize the elevated rate of obesity and dyslipidemia among psychiatric patients, possibly because of “an alarmingly poor rate of monitoring of metabolic parameters.”
Treating adverse metabolic outcomes also seems to lag behind. The same study13 found that physical health parameters among psychiatric patients continue to become worse even when appropriate health care professionals were notified. Rates of nontreatment for diabetes, dyslipidemia, and hypertension were 30%, 60%, and 88% respectively, according to Nasrallah et al.14
Randomized controlled studies have shown that obesity and metabolic syndrome can be effectively managed using lifestyle and pharmacotherapeutic approaches,15,16 but more research is needed to test long-term outcomes and how to best incorporate these interventions. Newcomer et al17 found that gradually switching an antipsychotic with high risk of metabolic adverse effects to one with lower risk could reduce adverse metabolic outcomes; however, some patients returned to their prior antipsychotic because other medications did not effectively treat their schizophrenia symptoms. Therefore, physicians must pay careful attention to the trade-off between benefits and risks of antipsychotics and make treatment decisions on an individual basis.
Barriers to medical care
Research has demonstrated that patients with SMI receive less screening and fewer preventive medical services, especially blood pressure monitoring, vaccinations, mammography, lipid monitoring, and osteoporosis screening, compared with the general population (Table).18 Some barriers to preventive services could exist because of demographic factors and medical insurance coverage19 or medical providers’ discomfort with symptoms of SMI,20 although Mitchell et al21 found that disparities in mammography screening could not be explained by the presence of emotional distress in women with SMI.
DiMatteo et al22 reported that patients with SMI are 3 times more likely to be noncompliant with medical treatment. These patients also are less likely to receive sec ondary preventive medical care and invasive medical procedures. Those with SMI who experience acute myocardial infarction are less likely to receive drug therapy, such as a thrombolytic, aspirin, beta blocker, or angiotensin-converting enzyme inhibitor.23 They also are less likely to receive invasive cardiovascular procedures, including cardiac catheterization, angioplasty, and coronary artery bypass grafting.24
Therefore, not only are patients with SMI less likely to receive preventive care, they are also less likely to receive potentially lifesaving treatments for SMI. Because those with SMI might not be able to advocate for themselves in these matters, psychiatric clinicians can improve their patients’ lives by advocating for appropriate medical care despite multiple barriers.
Bridging the gap: Managing mental health in primary care
Research from the 1970s and 1980s demonstrated that most persons who sought help for depression or anxiety received treatment from their PCP, many of whom felt limited by their lack of behavioral health training. Moreover, many patients failed to receive a psychiatric diagnosis or adequate treatment, despite efforts to educate primary care physicians on appropriate diagnosis and treatment of mental illness.
Katon et al25 at the University of Washington developed the collaborative care model in the early 1990s to help improve treatment of depression in primary care settings. This model involved:
• case load review by psychiatrists
• use of nurses and other support staff to help monitor patients’ adherence and treatment response
• use of standardized tools such as the Patient Health Questionnaire to monitor symptoms
• enhancement of patient education with pamphlets or classes.
Studies evaluating the success of collaborative care models found overall improved outcomes, making it the only evidence-based model for integration of behavioral health and primary care.26 As a result, the collaborative care model has been implemented across the United States in primary care clinics and specialty care settings, such as obstetrics and gynecology.27
Regrettably, access to primary care has been hampered by:
• population growth
• a shortage of PCPs
• enrollment of a flood of new patients into the health care marketplace as a result of mandates of the Affordable Care Act (ACA).
In many settings, a psychiatrist might be the patient’s only consistent care provider, and could be thought of as a “primary care psychiatrist.”
To resolve this predicament, mental health professionals need to recognize the unique medical conditions faced by people with SMI, and also might need to provide treatment of common medical conditions, either directly or through collaborative arrangements. Psychiatrists who are capable of managing core medical issues likely will witness improved psychiatric and overall health outcomes in their patients. Consequently, psychiatrists and mental health professionals are increasingly called on to be advocates to improve access to medical services in patients with SMI and to participate in health systems reform.
Managing medical conditions in mental health settings
Although traditional collaborative care involves mental health providers working at primary care sites, other models have emerged that manage chronic disease in behavioral health settings. Federally funded grants for primary behavioral health care integration have allowed community mental health centers to partner with federally qualified health centers to provide on-site primary care services.28
In these models, care managers in mental health clinics:
• link patients to primary care services
• encourage lifestyle changes to improve their overall health
• identify and overcome barriers to receiving care
• track clinical outcomes in a registry format.
Currently, 126 mental health sites in the United States have received these grants and are working toward greater integration of primary care.
In addition, the ACA provided funding for “health homes” in non-primary care settings, which includes SMI. These health homes cannot provide direct primary care, but can deliver comprehensive care management, care coordination, health promotion, comprehensive transitional care services between facilities, individual and family support, and referral to community social support services. In these health homes, a PCP can act as a consultant to help establish priorities for disease management and improving health status.29 The PCP consultant also can support psychiatric staff and collaborate with providers who want to provide some direct care of medical conditions.30
Last, some behavioral health sites are choosing to apply for Federally Qualified Health Clinic status or add primary care services to their clinics, with the hope that sustainable funding will become available. Without additional funding to cover the limited reimbursement provided by public payers, such as Medicaid and Medicare, these models might be unsustainable. Current innovations in health care funding reform hopefully will offer solutions for sites to provide medical care in the natural “medical home” of the SMI population.
Bottom Line
Psychiatric providers are in a favorable position to develop and oversee a partnership with primary care physicians with the goal of addressing significant and often lethal health disparities among those with mental illness. Psychiatric providers must use evidence-based practices that include assessment and prevention of cardiopulmonary, metabolic, infectious, and oncologic disorders. True primary care–behavioral health integration must include longitudinal “cross education” and changes in health care policy, with an emphasis on decreasing morbidity and mortality in psychiatric patients.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
People with serious mental illness (SMI) have a life expectancy that is 25 years less than the general population, according to the Centers for Disease Control and Prevention.1 This disparity is partially a consequence of the lack of primary and preventive medical care for those with psychiatric illness. Decades of research have shown that people with SMI experience higher medical morbidity and mortality in addition to facing the stigma of mental illness.
This article aims to advance the idea that longitudinal “cross education” between primary care providers (PCPs) and behavioral health providers (BHPs) is essential in addressing this problem. BHPs include psychiatry clinics, which often are part of a university or large health systems; county-based community mental health programs; and independent mental health clinics that contract with public and private health plans to provide mental health services.
Although suicide and injury account for 40% of the excess mortality in schizophrenia, 60% can be attributed to cardiovascular disease, diabetes, respiratory diseases, and infection.2 Patients with SMI have 2 to 3 times the risk of diabetes, dyslipidemia, hypertension, and obesity.3,4 Furthermore, those with SMI consume more than one-third of tobacco products,5 and 50% to 80% of people with SMI smoke tobacco, an important reversible risk factor for cardiovascular disease.
Figure 1 shows that people with SMI are at higher risk of dying from a chronic medical condition, such as cardiovascular disease, diabetes, chronic obstructive pulmonary disease, and hepatitis C6-8—many of which can be managed by primary and preventive medical interventions. These and other conditions often are not diagnosed or effectively managed in patients with SMI.
The high prevalence of metabolic syndrome and tobacco dependence among people with SMI accelerates development of cardiovascular disease, as shown by several studies. Bobes et al9 found that the prevalence of metabolic syndrome and cardiovascular risk among patients with SMI is similar to what is found in the general population at 10 to 15 years of greater age. Osborn et al10 demonstrated that people with SMI age 18 to 49 had a higher relative risk of death from coronary heart disease, stroke, and lung cancer than age-matched controls (Figure 2).
It can be said, therefore, that patients with SMI seem to “age” and die prematurely. To reduce this disparity, primary and preventive medical care—especially for cardiovascular disease—must be delivered earlier in life for those with SMI.
Iatrogenic causes of morbidity
Many psychiatric medications, especially second-generation antipsychotics (SGAs), could exacerbate cardiovascular and metabolic conditions by increasing the risk of weight gain, insulin resistance, and dyslipidemia. Antipsychotics that generally are considered to be more effective for refractory psychotic illness (eg, clozapine and olanzapine) are associated with the highest risk of metabolic syndrome. Simon et al11 found a dose-response relationship between olanzapine and clozapine serum concentrations and worsening metabolic outcomes. Valproic acid also can cause significant weight gain and could require monitoring similar to what is done with to SGAs, although there has been less clinical and research attention to this mood stabilizer.
The American Diabetes Association et al12 have published guidelines on monitoring antipsychotic-induced obesity and diabetes, but adoption of these guidelines has been slow. Mackin et al13 found that providers are slow to recognize the elevated rate of obesity and dyslipidemia among psychiatric patients, possibly because of “an alarmingly poor rate of monitoring of metabolic parameters.”
Treating adverse metabolic outcomes also seems to lag behind. The same study13 found that physical health parameters among psychiatric patients continue to become worse even when appropriate health care professionals were notified. Rates of nontreatment for diabetes, dyslipidemia, and hypertension were 30%, 60%, and 88% respectively, according to Nasrallah et al.14
Randomized controlled studies have shown that obesity and metabolic syndrome can be effectively managed using lifestyle and pharmacotherapeutic approaches,15,16 but more research is needed to test long-term outcomes and how to best incorporate these interventions. Newcomer et al17 found that gradually switching an antipsychotic with high risk of metabolic adverse effects to one with lower risk could reduce adverse metabolic outcomes; however, some patients returned to their prior antipsychotic because other medications did not effectively treat their schizophrenia symptoms. Therefore, physicians must pay careful attention to the trade-off between benefits and risks of antipsychotics and make treatment decisions on an individual basis.
Barriers to medical care
Research has demonstrated that patients with SMI receive less screening and fewer preventive medical services, especially blood pressure monitoring, vaccinations, mammography, lipid monitoring, and osteoporosis screening, compared with the general population (Table).18 Some barriers to preventive services could exist because of demographic factors and medical insurance coverage19 or medical providers’ discomfort with symptoms of SMI,20 although Mitchell et al21 found that disparities in mammography screening could not be explained by the presence of emotional distress in women with SMI.
DiMatteo et al22 reported that patients with SMI are 3 times more likely to be noncompliant with medical treatment. These patients also are less likely to receive sec ondary preventive medical care and invasive medical procedures. Those with SMI who experience acute myocardial infarction are less likely to receive drug therapy, such as a thrombolytic, aspirin, beta blocker, or angiotensin-converting enzyme inhibitor.23 They also are less likely to receive invasive cardiovascular procedures, including cardiac catheterization, angioplasty, and coronary artery bypass grafting.24
Therefore, not only are patients with SMI less likely to receive preventive care, they are also less likely to receive potentially lifesaving treatments for SMI. Because those with SMI might not be able to advocate for themselves in these matters, psychiatric clinicians can improve their patients’ lives by advocating for appropriate medical care despite multiple barriers.
Bridging the gap: Managing mental health in primary care
Research from the 1970s and 1980s demonstrated that most persons who sought help for depression or anxiety received treatment from their PCP, many of whom felt limited by their lack of behavioral health training. Moreover, many patients failed to receive a psychiatric diagnosis or adequate treatment, despite efforts to educate primary care physicians on appropriate diagnosis and treatment of mental illness.
Katon et al25 at the University of Washington developed the collaborative care model in the early 1990s to help improve treatment of depression in primary care settings. This model involved:
• case load review by psychiatrists
• use of nurses and other support staff to help monitor patients’ adherence and treatment response
• use of standardized tools such as the Patient Health Questionnaire to monitor symptoms
• enhancement of patient education with pamphlets or classes.
Studies evaluating the success of collaborative care models found overall improved outcomes, making it the only evidence-based model for integration of behavioral health and primary care.26 As a result, the collaborative care model has been implemented across the United States in primary care clinics and specialty care settings, such as obstetrics and gynecology.27
Regrettably, access to primary care has been hampered by:
• population growth
• a shortage of PCPs
• enrollment of a flood of new patients into the health care marketplace as a result of mandates of the Affordable Care Act (ACA).
In many settings, a psychiatrist might be the patient’s only consistent care provider, and could be thought of as a “primary care psychiatrist.”
To resolve this predicament, mental health professionals need to recognize the unique medical conditions faced by people with SMI, and also might need to provide treatment of common medical conditions, either directly or through collaborative arrangements. Psychiatrists who are capable of managing core medical issues likely will witness improved psychiatric and overall health outcomes in their patients. Consequently, psychiatrists and mental health professionals are increasingly called on to be advocates to improve access to medical services in patients with SMI and to participate in health systems reform.
Managing medical conditions in mental health settings
Although traditional collaborative care involves mental health providers working at primary care sites, other models have emerged that manage chronic disease in behavioral health settings. Federally funded grants for primary behavioral health care integration have allowed community mental health centers to partner with federally qualified health centers to provide on-site primary care services.28
In these models, care managers in mental health clinics:
• link patients to primary care services
• encourage lifestyle changes to improve their overall health
• identify and overcome barriers to receiving care
• track clinical outcomes in a registry format.
Currently, 126 mental health sites in the United States have received these grants and are working toward greater integration of primary care.
In addition, the ACA provided funding for “health homes” in non-primary care settings, which includes SMI. These health homes cannot provide direct primary care, but can deliver comprehensive care management, care coordination, health promotion, comprehensive transitional care services between facilities, individual and family support, and referral to community social support services. In these health homes, a PCP can act as a consultant to help establish priorities for disease management and improving health status.29 The PCP consultant also can support psychiatric staff and collaborate with providers who want to provide some direct care of medical conditions.30
Last, some behavioral health sites are choosing to apply for Federally Qualified Health Clinic status or add primary care services to their clinics, with the hope that sustainable funding will become available. Without additional funding to cover the limited reimbursement provided by public payers, such as Medicaid and Medicare, these models might be unsustainable. Current innovations in health care funding reform hopefully will offer solutions for sites to provide medical care in the natural “medical home” of the SMI population.
Bottom Line
Psychiatric providers are in a favorable position to develop and oversee a partnership with primary care physicians with the goal of addressing significant and often lethal health disparities among those with mental illness. Psychiatric providers must use evidence-based practices that include assessment and prevention of cardiopulmonary, metabolic, infectious, and oncologic disorders. True primary care–behavioral health integration must include longitudinal “cross education” and changes in health care policy, with an emphasis on decreasing morbidity and mortality in psychiatric patients.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
2. Parks J, Svendsen D, Singer P, et al, eds. Morbidity and mortality in people with serious mental illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006.
3. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
4. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trails of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
5. Compton MT, Daumit GL, Druss BG. Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: a preventive perspective. Harv Rev Psychiatry. 2006;14(2):212-222.
6. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
7. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156.
8. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based study. J Gen Intern Med. 2006;21(11):1133-1137.
9. Bobes J, Arango C, Aranda P, et al; CLAMORS Study Collaborative Group. Cardiovascular and metabolic risk in outpatients with schizoaffective disorder treated with antipsychotics; results from the CLAMORS study. Eur Psychiatry. 2012;27(4):267-274.
10. Osborn DP, Levy G, Nazareth I, et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database [Erratum in: Arch Gen Psychiatry. 2007;64(6):736]. Arch Gen Psychiatry. 2007;64(2):242-249.
11. Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70(7):1041-1050.
12. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. Mackin P, Bishop DR, Watkinson HM. A prospective study of monitoring practices for metabolic disease in antipsychotic-treated community psychiatric patients. BMC Psychiatry. 2007;7:28.
14. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
15. Alvarez-Jiménez M, Hetrick SE, González-Blanch C, et al. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomized controlled trials. Br J Psychiatry. 2008; 193(2):101-107.
16. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medication used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520-1530.
17. Newcomer JW, Weiden PJ, Buchanan RW. Switching antipsychotic medications to reduce adverse event burden in schizophrenia: establishing evidence-based practice. J Clin Psychiatry. 2013;74(11):1108-1120.
18. Lord O, Malone D, Mitchell AJ. Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry. 2010;32(5):519-543.
19. Xiong GL, Iosif AM, Bermudes RA, et al. Preventive medical services use among community mental health patients with severe mental illness: the influence of gender and insurance coverage. Prim Care Companion J Clin Psychiatry. 2010;12(5). doi: 10.4088/PCC.09m00927gre.
20. Daub S. Turning toward treating the seriously mentally ill in primary care. Fam Syst Health. 2014;32(1):12-13.
21. Mitchell A, Pereira IE, Yadegarfar M, et al. Breast cancer screening in women with mental illness: comparative meta-analysis of mammography uptake. Br J Psychiatry. 2014;205(6):428-435.
22. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
23. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
24. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
25. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32(5):456-464.
26. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525.
27. Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: socioeconomic disadvantage and treatment response. Am J Psychiatry. 2015;172(1):32-40.
28. Substance Abuse and Mental Health Services Administration. Request for Applications (RFA) No. SM- 09-011. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009.
29. Parks J. Behavioral health homes. In: Integrated care: working at the interface of primary care and behavioral health. Raney LE, ed. Arlington, VA: American Psychiatric Publishing; 2015:195.
30. Raney L. Integrated care: the evolving role of psychiatry in the era of health care reform. Psychiatr Serv. 2013;64(11):1076-1078.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
2. Parks J, Svendsen D, Singer P, et al, eds. Morbidity and mortality in people with serious mental illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006.
3. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
4. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trails of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
5. Compton MT, Daumit GL, Druss BG. Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: a preventive perspective. Harv Rev Psychiatry. 2006;14(2):212-222.
6. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
7. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156.
8. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based study. J Gen Intern Med. 2006;21(11):1133-1137.
9. Bobes J, Arango C, Aranda P, et al; CLAMORS Study Collaborative Group. Cardiovascular and metabolic risk in outpatients with schizoaffective disorder treated with antipsychotics; results from the CLAMORS study. Eur Psychiatry. 2012;27(4):267-274.
10. Osborn DP, Levy G, Nazareth I, et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database [Erratum in: Arch Gen Psychiatry. 2007;64(6):736]. Arch Gen Psychiatry. 2007;64(2):242-249.
11. Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70(7):1041-1050.
12. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. Mackin P, Bishop DR, Watkinson HM. A prospective study of monitoring practices for metabolic disease in antipsychotic-treated community psychiatric patients. BMC Psychiatry. 2007;7:28.
14. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
15. Alvarez-Jiménez M, Hetrick SE, González-Blanch C, et al. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomized controlled trials. Br J Psychiatry. 2008; 193(2):101-107.
16. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medication used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520-1530.
17. Newcomer JW, Weiden PJ, Buchanan RW. Switching antipsychotic medications to reduce adverse event burden in schizophrenia: establishing evidence-based practice. J Clin Psychiatry. 2013;74(11):1108-1120.
18. Lord O, Malone D, Mitchell AJ. Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry. 2010;32(5):519-543.
19. Xiong GL, Iosif AM, Bermudes RA, et al. Preventive medical services use among community mental health patients with severe mental illness: the influence of gender and insurance coverage. Prim Care Companion J Clin Psychiatry. 2010;12(5). doi: 10.4088/PCC.09m00927gre.
20. Daub S. Turning toward treating the seriously mentally ill in primary care. Fam Syst Health. 2014;32(1):12-13.
21. Mitchell A, Pereira IE, Yadegarfar M, et al. Breast cancer screening in women with mental illness: comparative meta-analysis of mammography uptake. Br J Psychiatry. 2014;205(6):428-435.
22. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
23. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
24. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
25. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32(5):456-464.
26. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525.
27. Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: socioeconomic disadvantage and treatment response. Am J Psychiatry. 2015;172(1):32-40.
28. Substance Abuse and Mental Health Services Administration. Request for Applications (RFA) No. SM- 09-011. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009.
29. Parks J. Behavioral health homes. In: Integrated care: working at the interface of primary care and behavioral health. Raney LE, ed. Arlington, VA: American Psychiatric Publishing; 2015:195.
30. Raney L. Integrated care: the evolving role of psychiatry in the era of health care reform. Psychiatr Serv. 2013;64(11):1076-1078.
Take caution: Look for DISTURBED behaviors when you assess violence risk
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
Take caution: Look for DISTURBED behaviors when you assess violence risk
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impulsivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single (known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings— 2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
Take caution: Look for DISTURBED behaviors when you assess violence risk
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impusivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be aware of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings—2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impusivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be aware of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings—2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impusivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be aware of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings—2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
Deaf and self-signing
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
Take CAUTION in emergency and inpatient psychiatric settings
Mental health care professionals are at increased risk of being assaulted by patients, especially in emergency and inpatient settings. Less experienced clinicians are at an even higher risk: Studies estimate that up to 56% percent of psychiatric residents have been physically assaulted by a patient.1 Some researchers have examined systematic approaches to improving violence risk assessment2; however, the assessment and interview process itself poses a risk to residents and medical students. We recommend using the mnemonic CAUTION to remind clinicians about safety considerations when working in psychiatric settings.
Communication. Talking about safety should be a priority during daily rounds. Routinely ask staff and other personnel about safety concerns. In inpatient settings, post a safety board where hospital staff can record aggressive behaviors and other safety issues displayed by patients. Notify staff whenever you plan to interact with patients at risk for aggression or when a patient seems agitated.
Attire. Follow proper dress codes to ensure personal safety and improve your ability to quickly assist others in need. Avoid wearing necklaces, ties, and high heels in inpatient psychiatric units. Valuable accessories (eg, expensive wrist watches) should not be worn because they may be broken during a “take down.”
Untreated symptoms. Be aware of patients with untreated or undertreated symptoms, including psychosis or substance intoxication. Emergency room patients or newly admitted inpatients often present the greatest risk because of their untreated symptoms (eg, patients with paranoid delusions).
Threats. Patients who express threats are at significantly increased risk of assaulting someone.3 Patients who have recently voiced threats should not be engaged alone or without adequate staff support. Inform all residents and students about specific patients who have voiced threats. Agitated and threatening patients can pose a risk to everyone in the unit, regardless of whether they have worked directly with the clinician.
Impulsivity. Approach impulsive and aggressive patients with particular caution. Until the aggression is controlled, these patients are at risk for sudden assaults when they feel provoked. Warning signs include punching a wall or breaking objects on the unit, facial muscle tightening, clenching of fists, and pacing. If a patient does not respond to redirection from staff, he or she may require seclusion, emergency medications, or both.
Options. Whenever possible, provide patients with choices, especially when a patient requests discharge or demands a particular medication. Avoid taking an authoritarian stance by clarifying reasons why the patient’s requests are being denied and by providing alternatives and options. For instance, if discharge is not indicated, direct a patient to contact the patients’ rights advocate. You also can give some agitated patients the option of taking a voluntary “timeout” or going to an isolated area to calm down.
Navigate safely. Identify potential exits from the room before the encounter. Residents and medical students should have a clear understanding of where to escape from a potential assault. Also, it is important to point out to patients where the door is should they feel threatened. Do not block the door when interviewing paranoid patients.
We suggest that less experienced clinicians refer to this mnemonic before starting work in emergency and inpatient psychiatric settings. Safety is an important consideration. By considering these basic concepts, we believe that safety can be improved.
1. Antonius D, Fuchs L, Herbert F, et al. Psychiatric assessment of aggressive patients: a violent attack on a resident. Am J Psychiatry. 2010;167(3):253-259.
2.Teo AR, Holley SR, Leary M, et al. The relationship between level of training and accuracy of violence risk assessment. Psychiatr Serv. 2012;63(11):1089-1094.
3. Maier GJ. Managing threatening behavior: the role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
Mental health care professionals are at increased risk of being assaulted by patients, especially in emergency and inpatient settings. Less experienced clinicians are at an even higher risk: Studies estimate that up to 56% percent of psychiatric residents have been physically assaulted by a patient.1 Some researchers have examined systematic approaches to improving violence risk assessment2; however, the assessment and interview process itself poses a risk to residents and medical students. We recommend using the mnemonic CAUTION to remind clinicians about safety considerations when working in psychiatric settings.
Communication. Talking about safety should be a priority during daily rounds. Routinely ask staff and other personnel about safety concerns. In inpatient settings, post a safety board where hospital staff can record aggressive behaviors and other safety issues displayed by patients. Notify staff whenever you plan to interact with patients at risk for aggression or when a patient seems agitated.
Attire. Follow proper dress codes to ensure personal safety and improve your ability to quickly assist others in need. Avoid wearing necklaces, ties, and high heels in inpatient psychiatric units. Valuable accessories (eg, expensive wrist watches) should not be worn because they may be broken during a “take down.”
Untreated symptoms. Be aware of patients with untreated or undertreated symptoms, including psychosis or substance intoxication. Emergency room patients or newly admitted inpatients often present the greatest risk because of their untreated symptoms (eg, patients with paranoid delusions).
Threats. Patients who express threats are at significantly increased risk of assaulting someone.3 Patients who have recently voiced threats should not be engaged alone or without adequate staff support. Inform all residents and students about specific patients who have voiced threats. Agitated and threatening patients can pose a risk to everyone in the unit, regardless of whether they have worked directly with the clinician.
Impulsivity. Approach impulsive and aggressive patients with particular caution. Until the aggression is controlled, these patients are at risk for sudden assaults when they feel provoked. Warning signs include punching a wall or breaking objects on the unit, facial muscle tightening, clenching of fists, and pacing. If a patient does not respond to redirection from staff, he or she may require seclusion, emergency medications, or both.
Options. Whenever possible, provide patients with choices, especially when a patient requests discharge or demands a particular medication. Avoid taking an authoritarian stance by clarifying reasons why the patient’s requests are being denied and by providing alternatives and options. For instance, if discharge is not indicated, direct a patient to contact the patients’ rights advocate. You also can give some agitated patients the option of taking a voluntary “timeout” or going to an isolated area to calm down.
Navigate safely. Identify potential exits from the room before the encounter. Residents and medical students should have a clear understanding of where to escape from a potential assault. Also, it is important to point out to patients where the door is should they feel threatened. Do not block the door when interviewing paranoid patients.
We suggest that less experienced clinicians refer to this mnemonic before starting work in emergency and inpatient psychiatric settings. Safety is an important consideration. By considering these basic concepts, we believe that safety can be improved.
Mental health care professionals are at increased risk of being assaulted by patients, especially in emergency and inpatient settings. Less experienced clinicians are at an even higher risk: Studies estimate that up to 56% percent of psychiatric residents have been physically assaulted by a patient.1 Some researchers have examined systematic approaches to improving violence risk assessment2; however, the assessment and interview process itself poses a risk to residents and medical students. We recommend using the mnemonic CAUTION to remind clinicians about safety considerations when working in psychiatric settings.
Communication. Talking about safety should be a priority during daily rounds. Routinely ask staff and other personnel about safety concerns. In inpatient settings, post a safety board where hospital staff can record aggressive behaviors and other safety issues displayed by patients. Notify staff whenever you plan to interact with patients at risk for aggression or when a patient seems agitated.
Attire. Follow proper dress codes to ensure personal safety and improve your ability to quickly assist others in need. Avoid wearing necklaces, ties, and high heels in inpatient psychiatric units. Valuable accessories (eg, expensive wrist watches) should not be worn because they may be broken during a “take down.”
Untreated symptoms. Be aware of patients with untreated or undertreated symptoms, including psychosis or substance intoxication. Emergency room patients or newly admitted inpatients often present the greatest risk because of their untreated symptoms (eg, patients with paranoid delusions).
Threats. Patients who express threats are at significantly increased risk of assaulting someone.3 Patients who have recently voiced threats should not be engaged alone or without adequate staff support. Inform all residents and students about specific patients who have voiced threats. Agitated and threatening patients can pose a risk to everyone in the unit, regardless of whether they have worked directly with the clinician.
Impulsivity. Approach impulsive and aggressive patients with particular caution. Until the aggression is controlled, these patients are at risk for sudden assaults when they feel provoked. Warning signs include punching a wall or breaking objects on the unit, facial muscle tightening, clenching of fists, and pacing. If a patient does not respond to redirection from staff, he or she may require seclusion, emergency medications, or both.
Options. Whenever possible, provide patients with choices, especially when a patient requests discharge or demands a particular medication. Avoid taking an authoritarian stance by clarifying reasons why the patient’s requests are being denied and by providing alternatives and options. For instance, if discharge is not indicated, direct a patient to contact the patients’ rights advocate. You also can give some agitated patients the option of taking a voluntary “timeout” or going to an isolated area to calm down.
Navigate safely. Identify potential exits from the room before the encounter. Residents and medical students should have a clear understanding of where to escape from a potential assault. Also, it is important to point out to patients where the door is should they feel threatened. Do not block the door when interviewing paranoid patients.
We suggest that less experienced clinicians refer to this mnemonic before starting work in emergency and inpatient psychiatric settings. Safety is an important consideration. By considering these basic concepts, we believe that safety can be improved.
1. Antonius D, Fuchs L, Herbert F, et al. Psychiatric assessment of aggressive patients: a violent attack on a resident. Am J Psychiatry. 2010;167(3):253-259.
2.Teo AR, Holley SR, Leary M, et al. The relationship between level of training and accuracy of violence risk assessment. Psychiatr Serv. 2012;63(11):1089-1094.
3. Maier GJ. Managing threatening behavior: the role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
1. Antonius D, Fuchs L, Herbert F, et al. Psychiatric assessment of aggressive patients: a violent attack on a resident. Am J Psychiatry. 2010;167(3):253-259.
2.Teo AR, Holley SR, Leary M, et al. The relationship between level of training and accuracy of violence risk assessment. Psychiatr Serv. 2012;63(11):1089-1094.
3. Maier GJ. Managing threatening behavior: the role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
Aspirin to prevent cardiovascular events: Weighing risks and benefits
Dr. Xiong is assistant clinical professor, departments of internal medicine and psychiatry and behavioral sciences, University of California, Davis. Dr. Kenedi is an adjunct professor of psychiatry at Duke University Medical Center in Durham, NC, and a consultant (attending physician) in internal medicine and liaison psychiatry, Auckland City Hospital, Auckland, New Zealand.
Principal Source: U.S. Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396-404.
- Consider discussing or recommending daily aspirin for men age 45 to 79 and women age 55 to 79 who are at risk for CVD, such as those who smoke or have diabetes.
- Psychiatric patients are at higher risk of CVD and often face systemic barriers to medical care. Collaborate with primary care physicians to determine which patients are good candidates for daily aspirin therapy.
- In psychiatric patients, watch for a potential drug-drug interaction between aspirin and valproate and increased risk of bleeding with selective serotonin reuptake inhibitors.
- Aspirin is associated with increased risk of serious gastrointestinal (GI) bleeding, hematuria, easy bruising, and epistaxis. Risk factors for GI bleeding include upper GI pain, history of GI ulcers, nonsteroidal anti-inflammatory drug (NSAID) use, alcohol dependence, and other anticoagulant use.
Cardiovascular disease (CVD) is the leading cause of death in the United States, accounting for >50% of all deaths. In persons age >40, the lifetime risk of death from CVD is 2 in 3 for men and more than 1 in 2 for women.1 Persons with severe mental illness have nearly twice the risk of death from CVD compared with the general population, which may be attributed to:
- lifestyle factors, including poor diet, lack of exercise, and tobacco dependence2
- antipsychotic medications, which have been shown to increase the risk of CVD3
- lower likelihood of undergoing cardiovascular procedures—including percutaneous transluminal coronary angioplasty and coronary artery bypass graft surgery—after myocardial infarction (MI).4
Psychiatrists are often the primary contact for patients with mental illness, giving us an opportunity to collaborate with primary care physicians and apply preventative measures that can reduce illness and improve patients’ morbidity and mortality. In addition to evaluating patients for possible hypercholesterolemia and diabetes, adding daily aspirin for primary prevention of heart attacks and strokes is an easily implementable option that could make a real difference in their health and quality of life.
New aspirin recommendations
The U.S. Preventive Services Task Force (USPSTF) found evidence that daily aspirin decreases the incidence of MI in men and ischemic strokes in women.1 However, total mortality for either gender was not significantly reduced.5 The USPSTF’s updated recommendations reflect results of the Women’s Health Study6 with different guidelines for men and women.
The USPSTF recommends daily aspirin for men age 45 to 79 and for women age 55 to 79 when the benefits of decreased MI for men and ischemic strokes for women outweigh the risks of increased GI bleeding ( Table 1 ).1 This grade A recommendation means there is high certainty of substantial net benefit.
Aspirin is not recommended for patients age ≥80 because of insufficient evidence of harm or benefit. The risks of MI in men age <45 and stroke in women age <55 are low, and daily aspirin generally is not indicated.
Optimal aspirin dose is unclear. The USPSTF recommends approximately 75 mg/d (effectively 81 mg/d or 1 “baby aspirin” in most U.S. settings). Higher aspirin doses might not be more effective for primary prevention and could increase the risk of GI bleeding. Note that some patients with a history of cardiovascular or cerebrovascular events might receive higher aspirin doses for secondary prevention of additional injury.
Risk assessment. In addition to age, other risk factors for CVD include:
- diabetes
- high total cholesterol (>240 mg/dL)
- low high-density lipoprotein cholesterol or so-called “good cholesterol” (<40 mg/dL for men, <50 mg/dL for women)
- hypertension
- smoking
- family history.
Several online tools—based on data from the Framingham Heart Study and other cohorts—can help estimate a patient’s CVD risk ( see Related Resources ), or consult with your patient’s primary care physician.
Potential harm of aspirin. USPSTF considers age and gender the most important risk factors for GI bleeding. GI bleeding is defined as serious hemorrhage, perforation, or other complications that could lead to hospitalization or death. Other risk factors include:
- upper GI pain
- history of gastric or duodenal ulcers
- NSAID use
- heavy, regular alcohol consumption.
In general, men have twice the risk of GI bleeding compared with women.1 The baseline number of GI bleeding events for individuals without a history of GI pain or bleeds taking daily aspirin is 4 per 10,000 person-years for women and 8 per 10,000 for men.1 Patients with preexisting GI ulcers who receive daily aspirin have more than 2 to 3 times the baseline risk of serious GI bleeding.7 NSAIDs taken with daily aspirin can quadruple the risk of GI bleeding compared with aspirin use alone, although antacid therapy can reduce this risk.8 Co-administered anticoagulants (eg, warfarin) also significantly increase the risk—especially when compliance with medication and monitoring is poor. Aspirin also increases the risk of hematuria, easy bruising, and epistaxis.
Because consuming >3 standard drinks a day also increases the risk of GI bleeding by up to 6 fold, patients with untreated chronic alcohol abuse or dependence might not be good candidates for daily aspirin therapy.9 Contrary to popular belief and pharmaceutical marketing, enteric-coated tablets do not seem to reduce the risk of bleeding because aspirin impacts platelet function, not the lining of the stomach.
Table 1
USPSTF recommendations for daily aspirin use
in primary prevention of cardiovascular disease
| Population | Recommendation |
|---|---|
| Men age 45 to 79 | Encourage aspirin use when potential benefit due to a reduction in myocardial infarctions outweighs potential increased risk of GI bleeding |
| Women age 55 to 79 | Encourage aspirin use when potential benefit of a reduction in ischemic strokes outweighs potential increased risk of GI bleeding |
| Men age <45 | Do not recommend aspirin use for cardiovascular prevention |
| Women age <55 | Do not recommend aspirin use for cardiovascular prevention |
| Men and women age ≥80 years | Insufficient evidence to make recommendations |
| GI: gastrointestinal; USPSTF: U.S. Preventive Services Task Force | |
| Source: Reference 1 | |
Aspirin for psychiatric patients
Patients who have serious mental illness are at increased risk for CVD and often experience systemic barriers to receiving appropriate medical care.10 Psychiatrists can provide and advocate for primary care services for our patients, including daily aspirin use to prevent CVD when appropriate, and encourage a closer relationship with a primary care physician before an adverse event occurs. Aspirin use in psychiatric patients is associated with:
- potential drug-drug interaction with valproate11
- mildly increased risk of bleeding as a result of reduced platelet function with the use of selective serotonin reuptake inhibitors.12
Balancing benefits vs risks. The USPSTF recommendation assumes that the value of preventing 1 MI or stroke is roughly equivalent to (or slightly less than) the cost of 1 GI bleeding event caused by aspirin. For example, among 1,000 men age 45 to 79 who have a ≥4% risk of MI over 10 years, 12.8 MIs would be prevented for every 8 GI bleeds caused by aspirin by following the current recommendations.1
The USPSTF guidelines are based on average levels of risk for age and gender. Some men age <45 may decide that it is more important to avoid a cardiovascular event rather than an episode of GI bleeding and might choose to begin daily aspirin. Aspirin use should be discouraged in most patients at high risk for GI bleeding. The point where the potential benefits outweigh the risks must be determined on an individual basis ( Table 2 ).
Table 2
Aspirin to prevent cardiovascular disease and stroke:
When benefits outweigh risks
| Men | Women | ||
|---|---|---|---|
| Age | 10-year CVD risk* | Age | 10-year stroke risk* |
| 45 to 59 | ≥4% | 55 to 59 | ≥3% |
| 60 to 69 | ≥9% | 60 to 69 | ≥8% |
| 70 to 79 | ≥12% | 70 to 79 | ≥11% |
| *Risk thresholds where aspirin should be started. Estimate risk using an online calculator based on the Framingham Heart Study at www.framinghamheartstudy.org/risk/coronary.html or consult with your patient’s primary care physician | |||
| CVD: cardiovascular disease | |||
| Source: Reference 1 | |||
- National Cholesterol Education Program. Online heart attack risk assessment tool. http://hp2010.nhlbihin.net/atpiii/calculator.asp.
- Framingham Heart study calculator. www.framinghamheartstudy.org/risk/coronary.html.
- National Cholesterol Education Program. www.nhlbi.nih.gov/health/public/heart/chol/wyntk.htm#risk.
- Colorado Neurological Institute. Online stroke risk assessment tool. www.thecni.org/Public/CNICenters/StrokeCenter/StrokeRiskCalculator/index.cfm.
- Agency for Healthcare Research and Quality. Fact sheet on aspirin to prevent cardiovascular disease. www.ahrq.gov/clinic/cvd/aspprovider.htm.
Drug brand names
- Valproate • Depacon
- Warfarin • Coumadin
Disclosures
Dr. Xiong reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Kenedi receives grant/research support from Duke University Medical Center and Auckland University.
1. U.S. Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2009;150:396-404.
2. Hert MD, Schreurs V, Vancampfort D, et al. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8:15-22.
3. Prevalence of metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III Schizophr Res. 2005;80:19-32.
4. Druss B, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;26:506-511.
5. Berger JS, Roncaglioni MC, Avanzini F, et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306-313.
6. Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293-1304.
7. Serrano P, Lanas A, Arroyo MT, et al. Risk of upper gastrointestinal bleeding in patients taking low-dose aspirin for the prevention of cardiovascular diseases. Aliment Pharmacol Ther. 2002;16:1945-1953.
8. Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006;55:1731-1738.
9. Kelly JP, Kaufman DW, Koff RS, et al. Alcohol consumption and the risk of major upper gastrointestinal bleeding. Am J Gastroenterol. 1995;90:1058-1064.
10. Druss BG, Marcu SC, Campbell J, et al. Medical services for clients in community mental health centers: results from a national survey. Psych Serv. 2008;59:917-920.
11. Sandson NB, Marcucci C, Bourke DL, et al. An interaction between aspirin and valproate: the relevance of plasma protein displacement drug-drug interactions. Am J Psychiatry. 2006;163:1891-1896.
12. de Abajo FJ, Rodriguez LA, Montero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ. 1999;319:1106-1109.
Dr. Xiong is assistant clinical professor, departments of internal medicine and psychiatry and behavioral sciences, University of California, Davis. Dr. Kenedi is an adjunct professor of psychiatry at Duke University Medical Center in Durham, NC, and a consultant (attending physician) in internal medicine and liaison psychiatry, Auckland City Hospital, Auckland, New Zealand.
Principal Source: U.S. Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396-404.
- Consider discussing or recommending daily aspirin for men age 45 to 79 and women age 55 to 79 who are at risk for CVD, such as those who smoke or have diabetes.
- Psychiatric patients are at higher risk of CVD and often face systemic barriers to medical care. Collaborate with primary care physicians to determine which patients are good candidates for daily aspirin therapy.
- In psychiatric patients, watch for a potential drug-drug interaction between aspirin and valproate and increased risk of bleeding with selective serotonin reuptake inhibitors.
- Aspirin is associated with increased risk of serious gastrointestinal (GI) bleeding, hematuria, easy bruising, and epistaxis. Risk factors for GI bleeding include upper GI pain, history of GI ulcers, nonsteroidal anti-inflammatory drug (NSAID) use, alcohol dependence, and other anticoagulant use.
Cardiovascular disease (CVD) is the leading cause of death in the United States, accounting for >50% of all deaths. In persons age >40, the lifetime risk of death from CVD is 2 in 3 for men and more than 1 in 2 for women.1 Persons with severe mental illness have nearly twice the risk of death from CVD compared with the general population, which may be attributed to:
- lifestyle factors, including poor diet, lack of exercise, and tobacco dependence2
- antipsychotic medications, which have been shown to increase the risk of CVD3
- lower likelihood of undergoing cardiovascular procedures—including percutaneous transluminal coronary angioplasty and coronary artery bypass graft surgery—after myocardial infarction (MI).4
Psychiatrists are often the primary contact for patients with mental illness, giving us an opportunity to collaborate with primary care physicians and apply preventative measures that can reduce illness and improve patients’ morbidity and mortality. In addition to evaluating patients for possible hypercholesterolemia and diabetes, adding daily aspirin for primary prevention of heart attacks and strokes is an easily implementable option that could make a real difference in their health and quality of life.
New aspirin recommendations
The U.S. Preventive Services Task Force (USPSTF) found evidence that daily aspirin decreases the incidence of MI in men and ischemic strokes in women.1 However, total mortality for either gender was not significantly reduced.5 The USPSTF’s updated recommendations reflect results of the Women’s Health Study6 with different guidelines for men and women.
The USPSTF recommends daily aspirin for men age 45 to 79 and for women age 55 to 79 when the benefits of decreased MI for men and ischemic strokes for women outweigh the risks of increased GI bleeding ( Table 1 ).1 This grade A recommendation means there is high certainty of substantial net benefit.
Aspirin is not recommended for patients age ≥80 because of insufficient evidence of harm or benefit. The risks of MI in men age <45 and stroke in women age <55 are low, and daily aspirin generally is not indicated.
Optimal aspirin dose is unclear. The USPSTF recommends approximately 75 mg/d (effectively 81 mg/d or 1 “baby aspirin” in most U.S. settings). Higher aspirin doses might not be more effective for primary prevention and could increase the risk of GI bleeding. Note that some patients with a history of cardiovascular or cerebrovascular events might receive higher aspirin doses for secondary prevention of additional injury.
Risk assessment. In addition to age, other risk factors for CVD include:
- diabetes
- high total cholesterol (>240 mg/dL)
- low high-density lipoprotein cholesterol or so-called “good cholesterol” (<40 mg/dL for men, <50 mg/dL for women)
- hypertension
- smoking
- family history.
Several online tools—based on data from the Framingham Heart Study and other cohorts—can help estimate a patient’s CVD risk ( see Related Resources ), or consult with your patient’s primary care physician.
Potential harm of aspirin. USPSTF considers age and gender the most important risk factors for GI bleeding. GI bleeding is defined as serious hemorrhage, perforation, or other complications that could lead to hospitalization or death. Other risk factors include:
- upper GI pain
- history of gastric or duodenal ulcers
- NSAID use
- heavy, regular alcohol consumption.
In general, men have twice the risk of GI bleeding compared with women.1 The baseline number of GI bleeding events for individuals without a history of GI pain or bleeds taking daily aspirin is 4 per 10,000 person-years for women and 8 per 10,000 for men.1 Patients with preexisting GI ulcers who receive daily aspirin have more than 2 to 3 times the baseline risk of serious GI bleeding.7 NSAIDs taken with daily aspirin can quadruple the risk of GI bleeding compared with aspirin use alone, although antacid therapy can reduce this risk.8 Co-administered anticoagulants (eg, warfarin) also significantly increase the risk—especially when compliance with medication and monitoring is poor. Aspirin also increases the risk of hematuria, easy bruising, and epistaxis.
Because consuming >3 standard drinks a day also increases the risk of GI bleeding by up to 6 fold, patients with untreated chronic alcohol abuse or dependence might not be good candidates for daily aspirin therapy.9 Contrary to popular belief and pharmaceutical marketing, enteric-coated tablets do not seem to reduce the risk of bleeding because aspirin impacts platelet function, not the lining of the stomach.
Table 1
USPSTF recommendations for daily aspirin use
in primary prevention of cardiovascular disease
| Population | Recommendation |
|---|---|
| Men age 45 to 79 | Encourage aspirin use when potential benefit due to a reduction in myocardial infarctions outweighs potential increased risk of GI bleeding |
| Women age 55 to 79 | Encourage aspirin use when potential benefit of a reduction in ischemic strokes outweighs potential increased risk of GI bleeding |
| Men age <45 | Do not recommend aspirin use for cardiovascular prevention |
| Women age <55 | Do not recommend aspirin use for cardiovascular prevention |
| Men and women age ≥80 years | Insufficient evidence to make recommendations |
| GI: gastrointestinal; USPSTF: U.S. Preventive Services Task Force | |
| Source: Reference 1 | |
Aspirin for psychiatric patients
Patients who have serious mental illness are at increased risk for CVD and often experience systemic barriers to receiving appropriate medical care.10 Psychiatrists can provide and advocate for primary care services for our patients, including daily aspirin use to prevent CVD when appropriate, and encourage a closer relationship with a primary care physician before an adverse event occurs. Aspirin use in psychiatric patients is associated with:
- potential drug-drug interaction with valproate11
- mildly increased risk of bleeding as a result of reduced platelet function with the use of selective serotonin reuptake inhibitors.12
Balancing benefits vs risks. The USPSTF recommendation assumes that the value of preventing 1 MI or stroke is roughly equivalent to (or slightly less than) the cost of 1 GI bleeding event caused by aspirin. For example, among 1,000 men age 45 to 79 who have a ≥4% risk of MI over 10 years, 12.8 MIs would be prevented for every 8 GI bleeds caused by aspirin by following the current recommendations.1
The USPSTF guidelines are based on average levels of risk for age and gender. Some men age <45 may decide that it is more important to avoid a cardiovascular event rather than an episode of GI bleeding and might choose to begin daily aspirin. Aspirin use should be discouraged in most patients at high risk for GI bleeding. The point where the potential benefits outweigh the risks must be determined on an individual basis ( Table 2 ).
Table 2
Aspirin to prevent cardiovascular disease and stroke:
When benefits outweigh risks
| Men | Women | ||
|---|---|---|---|
| Age | 10-year CVD risk* | Age | 10-year stroke risk* |
| 45 to 59 | ≥4% | 55 to 59 | ≥3% |
| 60 to 69 | ≥9% | 60 to 69 | ≥8% |
| 70 to 79 | ≥12% | 70 to 79 | ≥11% |
| *Risk thresholds where aspirin should be started. Estimate risk using an online calculator based on the Framingham Heart Study at www.framinghamheartstudy.org/risk/coronary.html or consult with your patient’s primary care physician | |||
| CVD: cardiovascular disease | |||
| Source: Reference 1 | |||
- National Cholesterol Education Program. Online heart attack risk assessment tool. http://hp2010.nhlbihin.net/atpiii/calculator.asp.
- Framingham Heart study calculator. www.framinghamheartstudy.org/risk/coronary.html.
- National Cholesterol Education Program. www.nhlbi.nih.gov/health/public/heart/chol/wyntk.htm#risk.
- Colorado Neurological Institute. Online stroke risk assessment tool. www.thecni.org/Public/CNICenters/StrokeCenter/StrokeRiskCalculator/index.cfm.
- Agency for Healthcare Research and Quality. Fact sheet on aspirin to prevent cardiovascular disease. www.ahrq.gov/clinic/cvd/aspprovider.htm.
Drug brand names
- Valproate • Depacon
- Warfarin • Coumadin
Disclosures
Dr. Xiong reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Kenedi receives grant/research support from Duke University Medical Center and Auckland University.
Dr. Xiong is assistant clinical professor, departments of internal medicine and psychiatry and behavioral sciences, University of California, Davis. Dr. Kenedi is an adjunct professor of psychiatry at Duke University Medical Center in Durham, NC, and a consultant (attending physician) in internal medicine and liaison psychiatry, Auckland City Hospital, Auckland, New Zealand.
Principal Source: U.S. Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396-404.
- Consider discussing or recommending daily aspirin for men age 45 to 79 and women age 55 to 79 who are at risk for CVD, such as those who smoke or have diabetes.
- Psychiatric patients are at higher risk of CVD and often face systemic barriers to medical care. Collaborate with primary care physicians to determine which patients are good candidates for daily aspirin therapy.
- In psychiatric patients, watch for a potential drug-drug interaction between aspirin and valproate and increased risk of bleeding with selective serotonin reuptake inhibitors.
- Aspirin is associated with increased risk of serious gastrointestinal (GI) bleeding, hematuria, easy bruising, and epistaxis. Risk factors for GI bleeding include upper GI pain, history of GI ulcers, nonsteroidal anti-inflammatory drug (NSAID) use, alcohol dependence, and other anticoagulant use.
Cardiovascular disease (CVD) is the leading cause of death in the United States, accounting for >50% of all deaths. In persons age >40, the lifetime risk of death from CVD is 2 in 3 for men and more than 1 in 2 for women.1 Persons with severe mental illness have nearly twice the risk of death from CVD compared with the general population, which may be attributed to:
- lifestyle factors, including poor diet, lack of exercise, and tobacco dependence2
- antipsychotic medications, which have been shown to increase the risk of CVD3
- lower likelihood of undergoing cardiovascular procedures—including percutaneous transluminal coronary angioplasty and coronary artery bypass graft surgery—after myocardial infarction (MI).4
Psychiatrists are often the primary contact for patients with mental illness, giving us an opportunity to collaborate with primary care physicians and apply preventative measures that can reduce illness and improve patients’ morbidity and mortality. In addition to evaluating patients for possible hypercholesterolemia and diabetes, adding daily aspirin for primary prevention of heart attacks and strokes is an easily implementable option that could make a real difference in their health and quality of life.
New aspirin recommendations
The U.S. Preventive Services Task Force (USPSTF) found evidence that daily aspirin decreases the incidence of MI in men and ischemic strokes in women.1 However, total mortality for either gender was not significantly reduced.5 The USPSTF’s updated recommendations reflect results of the Women’s Health Study6 with different guidelines for men and women.
The USPSTF recommends daily aspirin for men age 45 to 79 and for women age 55 to 79 when the benefits of decreased MI for men and ischemic strokes for women outweigh the risks of increased GI bleeding ( Table 1 ).1 This grade A recommendation means there is high certainty of substantial net benefit.
Aspirin is not recommended for patients age ≥80 because of insufficient evidence of harm or benefit. The risks of MI in men age <45 and stroke in women age <55 are low, and daily aspirin generally is not indicated.
Optimal aspirin dose is unclear. The USPSTF recommends approximately 75 mg/d (effectively 81 mg/d or 1 “baby aspirin” in most U.S. settings). Higher aspirin doses might not be more effective for primary prevention and could increase the risk of GI bleeding. Note that some patients with a history of cardiovascular or cerebrovascular events might receive higher aspirin doses for secondary prevention of additional injury.
Risk assessment. In addition to age, other risk factors for CVD include:
- diabetes
- high total cholesterol (>240 mg/dL)
- low high-density lipoprotein cholesterol or so-called “good cholesterol” (<40 mg/dL for men, <50 mg/dL for women)
- hypertension
- smoking
- family history.
Several online tools—based on data from the Framingham Heart Study and other cohorts—can help estimate a patient’s CVD risk ( see Related Resources ), or consult with your patient’s primary care physician.
Potential harm of aspirin. USPSTF considers age and gender the most important risk factors for GI bleeding. GI bleeding is defined as serious hemorrhage, perforation, or other complications that could lead to hospitalization or death. Other risk factors include:
- upper GI pain
- history of gastric or duodenal ulcers
- NSAID use
- heavy, regular alcohol consumption.
In general, men have twice the risk of GI bleeding compared with women.1 The baseline number of GI bleeding events for individuals without a history of GI pain or bleeds taking daily aspirin is 4 per 10,000 person-years for women and 8 per 10,000 for men.1 Patients with preexisting GI ulcers who receive daily aspirin have more than 2 to 3 times the baseline risk of serious GI bleeding.7 NSAIDs taken with daily aspirin can quadruple the risk of GI bleeding compared with aspirin use alone, although antacid therapy can reduce this risk.8 Co-administered anticoagulants (eg, warfarin) also significantly increase the risk—especially when compliance with medication and monitoring is poor. Aspirin also increases the risk of hematuria, easy bruising, and epistaxis.
Because consuming >3 standard drinks a day also increases the risk of GI bleeding by up to 6 fold, patients with untreated chronic alcohol abuse or dependence might not be good candidates for daily aspirin therapy.9 Contrary to popular belief and pharmaceutical marketing, enteric-coated tablets do not seem to reduce the risk of bleeding because aspirin impacts platelet function, not the lining of the stomach.
Table 1
USPSTF recommendations for daily aspirin use
in primary prevention of cardiovascular disease
| Population | Recommendation |
|---|---|
| Men age 45 to 79 | Encourage aspirin use when potential benefit due to a reduction in myocardial infarctions outweighs potential increased risk of GI bleeding |
| Women age 55 to 79 | Encourage aspirin use when potential benefit of a reduction in ischemic strokes outweighs potential increased risk of GI bleeding |
| Men age <45 | Do not recommend aspirin use for cardiovascular prevention |
| Women age <55 | Do not recommend aspirin use for cardiovascular prevention |
| Men and women age ≥80 years | Insufficient evidence to make recommendations |
| GI: gastrointestinal; USPSTF: U.S. Preventive Services Task Force | |
| Source: Reference 1 | |
Aspirin for psychiatric patients
Patients who have serious mental illness are at increased risk for CVD and often experience systemic barriers to receiving appropriate medical care.10 Psychiatrists can provide and advocate for primary care services for our patients, including daily aspirin use to prevent CVD when appropriate, and encourage a closer relationship with a primary care physician before an adverse event occurs. Aspirin use in psychiatric patients is associated with:
- potential drug-drug interaction with valproate11
- mildly increased risk of bleeding as a result of reduced platelet function with the use of selective serotonin reuptake inhibitors.12
Balancing benefits vs risks. The USPSTF recommendation assumes that the value of preventing 1 MI or stroke is roughly equivalent to (or slightly less than) the cost of 1 GI bleeding event caused by aspirin. For example, among 1,000 men age 45 to 79 who have a ≥4% risk of MI over 10 years, 12.8 MIs would be prevented for every 8 GI bleeds caused by aspirin by following the current recommendations.1
The USPSTF guidelines are based on average levels of risk for age and gender. Some men age <45 may decide that it is more important to avoid a cardiovascular event rather than an episode of GI bleeding and might choose to begin daily aspirin. Aspirin use should be discouraged in most patients at high risk for GI bleeding. The point where the potential benefits outweigh the risks must be determined on an individual basis ( Table 2 ).
Table 2
Aspirin to prevent cardiovascular disease and stroke:
When benefits outweigh risks
| Men | Women | ||
|---|---|---|---|
| Age | 10-year CVD risk* | Age | 10-year stroke risk* |
| 45 to 59 | ≥4% | 55 to 59 | ≥3% |
| 60 to 69 | ≥9% | 60 to 69 | ≥8% |
| 70 to 79 | ≥12% | 70 to 79 | ≥11% |
| *Risk thresholds where aspirin should be started. Estimate risk using an online calculator based on the Framingham Heart Study at www.framinghamheartstudy.org/risk/coronary.html or consult with your patient’s primary care physician | |||
| CVD: cardiovascular disease | |||
| Source: Reference 1 | |||
- National Cholesterol Education Program. Online heart attack risk assessment tool. http://hp2010.nhlbihin.net/atpiii/calculator.asp.
- Framingham Heart study calculator. www.framinghamheartstudy.org/risk/coronary.html.
- National Cholesterol Education Program. www.nhlbi.nih.gov/health/public/heart/chol/wyntk.htm#risk.
- Colorado Neurological Institute. Online stroke risk assessment tool. www.thecni.org/Public/CNICenters/StrokeCenter/StrokeRiskCalculator/index.cfm.
- Agency for Healthcare Research and Quality. Fact sheet on aspirin to prevent cardiovascular disease. www.ahrq.gov/clinic/cvd/aspprovider.htm.
Drug brand names
- Valproate • Depacon
- Warfarin • Coumadin
Disclosures
Dr. Xiong reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Kenedi receives grant/research support from Duke University Medical Center and Auckland University.
1. U.S. Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2009;150:396-404.
2. Hert MD, Schreurs V, Vancampfort D, et al. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8:15-22.
3. Prevalence of metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III Schizophr Res. 2005;80:19-32.
4. Druss B, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;26:506-511.
5. Berger JS, Roncaglioni MC, Avanzini F, et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306-313.
6. Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293-1304.
7. Serrano P, Lanas A, Arroyo MT, et al. Risk of upper gastrointestinal bleeding in patients taking low-dose aspirin for the prevention of cardiovascular diseases. Aliment Pharmacol Ther. 2002;16:1945-1953.
8. Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006;55:1731-1738.
9. Kelly JP, Kaufman DW, Koff RS, et al. Alcohol consumption and the risk of major upper gastrointestinal bleeding. Am J Gastroenterol. 1995;90:1058-1064.
10. Druss BG, Marcu SC, Campbell J, et al. Medical services for clients in community mental health centers: results from a national survey. Psych Serv. 2008;59:917-920.
11. Sandson NB, Marcucci C, Bourke DL, et al. An interaction between aspirin and valproate: the relevance of plasma protein displacement drug-drug interactions. Am J Psychiatry. 2006;163:1891-1896.
12. de Abajo FJ, Rodriguez LA, Montero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ. 1999;319:1106-1109.
1. U.S. Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2009;150:396-404.
2. Hert MD, Schreurs V, Vancampfort D, et al. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8:15-22.
3. Prevalence of metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III Schizophr Res. 2005;80:19-32.
4. Druss B, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;26:506-511.
5. Berger JS, Roncaglioni MC, Avanzini F, et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306-313.
6. Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293-1304.
7. Serrano P, Lanas A, Arroyo MT, et al. Risk of upper gastrointestinal bleeding in patients taking low-dose aspirin for the prevention of cardiovascular diseases. Aliment Pharmacol Ther. 2002;16:1945-1953.
8. Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006;55:1731-1738.
9. Kelly JP, Kaufman DW, Koff RS, et al. Alcohol consumption and the risk of major upper gastrointestinal bleeding. Am J Gastroenterol. 1995;90:1058-1064.
10. Druss BG, Marcu SC, Campbell J, et al. Medical services for clients in community mental health centers: results from a national survey. Psych Serv. 2008;59:917-920.
11. Sandson NB, Marcucci C, Bourke DL, et al. An interaction between aspirin and valproate: the relevance of plasma protein displacement drug-drug interactions. Am J Psychiatry. 2006;163:1891-1896.
12. de Abajo FJ, Rodriguez LA, Montero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ. 1999;319:1106-1109.
Make PROGRESS by supporting adherence
Medication nonadherence challenges psychiatrists in most clinical settings.1 Supportive psychotherapy techniques—outlined in the mnemonic PROGRESS—can improve adherence and strengthen the therapeutic relationship. They also can help restore adaptive living skills, promote patient autonomy, minimize relapse, and improve attitudes toward treatment.
Praise. Reinforce positive behavior with genuine praise. Build an inventory of phrases to congratulate patients when they meet their goals or demonstrate a new effort. Follow praise with a question that elicits feedback from patients about their behavior.
Reassure. Because patients may lose faith in medications’ efficacy, use reassurance to explain the time frame for drugs to reach therapeutic levels. Instill hope and remind patients that psychiatric illnesses can improve. Point out that although medications have limitations they do help reduce symptoms.
Optimize regimens. Find an appropriate dosing and frequency that minimizes side effects and facilitates a daily routine of taking medication.2 This will help alleviate patients’ anxiety and support confidence in managing their medications.
Guide. Provide verbal and written guidance about what patients can expect from their medications. Include information about side effects and explore supplemental treatment options such as healthy eating, psychotherapy, community rehabilitation programs, and refraining from substances. If patients are unsure about why they take medication, help identify their goals and point out how pharmacotherapy might improve their symptoms.
Remind. Brainstorm with patients about how they can set up ambient cues to help them remember to take medications. Environmental associations promote autonomous behavior that can become second nature. For example, patients may learn to associate breakfast with taking medications. If patients place their medications on the table where they eat their meals, this may reinforce breakfast as a cue to take their medications.
Encourage patients to complete tasks that could help them achieve their goals. This includes taking medications as prescribed and communicating with mental healthcare providers to report side effects or during times of crisis.
Solidify strengths. Build on patients’ adaptive skills and strengths. Identifying efforts to improve their illnesses may turn roadblocks into opportunities to build rapport.
Support self-efficacy. Commend patients, for example, when they can explain and break down into steps the complexities of refilling and taking their medications.
1. Dolder CR, Lacro JP, Leckband S, Jeste DV. Interventions to improve antipsychotic medication adherence: review of recent literature. J Clin Psychopharmacol 2003;23(4):389-99.
2. Heinssen RK. Improving medication compliance of a patient with schizophrenia through collaborative behavioral therapy. Psychiatr Serv 2002;53(3):255-7.
Medication nonadherence challenges psychiatrists in most clinical settings.1 Supportive psychotherapy techniques—outlined in the mnemonic PROGRESS—can improve adherence and strengthen the therapeutic relationship. They also can help restore adaptive living skills, promote patient autonomy, minimize relapse, and improve attitudes toward treatment.
Praise. Reinforce positive behavior with genuine praise. Build an inventory of phrases to congratulate patients when they meet their goals or demonstrate a new effort. Follow praise with a question that elicits feedback from patients about their behavior.
Reassure. Because patients may lose faith in medications’ efficacy, use reassurance to explain the time frame for drugs to reach therapeutic levels. Instill hope and remind patients that psychiatric illnesses can improve. Point out that although medications have limitations they do help reduce symptoms.
Optimize regimens. Find an appropriate dosing and frequency that minimizes side effects and facilitates a daily routine of taking medication.2 This will help alleviate patients’ anxiety and support confidence in managing their medications.
Guide. Provide verbal and written guidance about what patients can expect from their medications. Include information about side effects and explore supplemental treatment options such as healthy eating, psychotherapy, community rehabilitation programs, and refraining from substances. If patients are unsure about why they take medication, help identify their goals and point out how pharmacotherapy might improve their symptoms.
Remind. Brainstorm with patients about how they can set up ambient cues to help them remember to take medications. Environmental associations promote autonomous behavior that can become second nature. For example, patients may learn to associate breakfast with taking medications. If patients place their medications on the table where they eat their meals, this may reinforce breakfast as a cue to take their medications.
Encourage patients to complete tasks that could help them achieve their goals. This includes taking medications as prescribed and communicating with mental healthcare providers to report side effects or during times of crisis.
Solidify strengths. Build on patients’ adaptive skills and strengths. Identifying efforts to improve their illnesses may turn roadblocks into opportunities to build rapport.
Support self-efficacy. Commend patients, for example, when they can explain and break down into steps the complexities of refilling and taking their medications.
Medication nonadherence challenges psychiatrists in most clinical settings.1 Supportive psychotherapy techniques—outlined in the mnemonic PROGRESS—can improve adherence and strengthen the therapeutic relationship. They also can help restore adaptive living skills, promote patient autonomy, minimize relapse, and improve attitudes toward treatment.
Praise. Reinforce positive behavior with genuine praise. Build an inventory of phrases to congratulate patients when they meet their goals or demonstrate a new effort. Follow praise with a question that elicits feedback from patients about their behavior.
Reassure. Because patients may lose faith in medications’ efficacy, use reassurance to explain the time frame for drugs to reach therapeutic levels. Instill hope and remind patients that psychiatric illnesses can improve. Point out that although medications have limitations they do help reduce symptoms.
Optimize regimens. Find an appropriate dosing and frequency that minimizes side effects and facilitates a daily routine of taking medication.2 This will help alleviate patients’ anxiety and support confidence in managing their medications.
Guide. Provide verbal and written guidance about what patients can expect from their medications. Include information about side effects and explore supplemental treatment options such as healthy eating, psychotherapy, community rehabilitation programs, and refraining from substances. If patients are unsure about why they take medication, help identify their goals and point out how pharmacotherapy might improve their symptoms.
Remind. Brainstorm with patients about how they can set up ambient cues to help them remember to take medications. Environmental associations promote autonomous behavior that can become second nature. For example, patients may learn to associate breakfast with taking medications. If patients place their medications on the table where they eat their meals, this may reinforce breakfast as a cue to take their medications.
Encourage patients to complete tasks that could help them achieve their goals. This includes taking medications as prescribed and communicating with mental healthcare providers to report side effects or during times of crisis.
Solidify strengths. Build on patients’ adaptive skills and strengths. Identifying efforts to improve their illnesses may turn roadblocks into opportunities to build rapport.
Support self-efficacy. Commend patients, for example, when they can explain and break down into steps the complexities of refilling and taking their medications.
1. Dolder CR, Lacro JP, Leckband S, Jeste DV. Interventions to improve antipsychotic medication adherence: review of recent literature. J Clin Psychopharmacol 2003;23(4):389-99.
2. Heinssen RK. Improving medication compliance of a patient with schizophrenia through collaborative behavioral therapy. Psychiatr Serv 2002;53(3):255-7.
1. Dolder CR, Lacro JP, Leckband S, Jeste DV. Interventions to improve antipsychotic medication adherence: review of recent literature. J Clin Psychopharmacol 2003;23(4):389-99.
2. Heinssen RK. Improving medication compliance of a patient with schizophrenia through collaborative behavioral therapy. Psychiatr Serv 2002;53(3):255-7.
FRISBEE: Does the study fly in the face of evidence-based medicine?
With the rapid emergence of novel therapies, psychiatrists face the challenge of deciphering the clinical application of published clinical trials. Although double-blind, randomized, placebo-controlled trials are the gold standard, their validity should be carefully examined.1 The FRISBEE mnemonic from Duke University’s psychiatry residency program can help you incorporate evidence-based medicine into your patient care.
Follow-up. Carefully interpret studies with inadequate follow-up or high drop-out rates. The reason for patient discontinuation might not be related to the studied intervention.
Randomization. To control for unknown confounding variables, patient assignment must be randomized.
Intent-to-treat analysis. ITT assumes that complete data are available during final analysis on every subject, but subjects often drop out. To compensate for drop-outs, researchers could:
- carry forward the last available measurement as the final result, known as last observation carried forward (LOCF).
- use data only from patients who complete entire study protocol (completer analysis method).
Both methods have statistical limitations, but LOCF generally is preferred because it accounts for every subject who enrolled in the study.2
Similar baseline. Compare known characteristics of the treatment and placebo groups at baseline. Confounding variables, such as illness severity or medical or psychiatric comorbidities, should appear equally among randomized patient groups. Not all variables will be similar because of random effects, however.
Blinding. With ineffective blinding, patients or researchers can tell which treatment was administered. If this occurs, the study’s outcome likely is biased by treatment expectations. To detect faulty blinding, some studies ask patients and/or providers if they can guess the intervention that was delivered.
Equal treatment. Even with proper randomization and blinding, other intervention-related treatments—such as blood work to monitor side effects or the duration or frequency of provider contact—might not be administered equally among patient groups. This can clue patients and researchers into which intervention was administered and create bias.
Equivalence to your patient. A typical study patient often has few medical and psychiatric comorbidities or psychosocial risk factors. Your patient might be substantially different. Carefully compare the patients in the study with the patient in your office before choosing a treatment.
1. Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 2000;284(3):357-62.
2. Lachin JM. Statistical considerations in the intent to treat principle. Control Clin Trials 2000;21:167-89.
Dr. Xiong is assistant clinical professor at the University of California, Davis. Dr. Adams is clinical associate at Duke University Medical Center, Durham, NC.
With the rapid emergence of novel therapies, psychiatrists face the challenge of deciphering the clinical application of published clinical trials. Although double-blind, randomized, placebo-controlled trials are the gold standard, their validity should be carefully examined.1 The FRISBEE mnemonic from Duke University’s psychiatry residency program can help you incorporate evidence-based medicine into your patient care.
Follow-up. Carefully interpret studies with inadequate follow-up or high drop-out rates. The reason for patient discontinuation might not be related to the studied intervention.
Randomization. To control for unknown confounding variables, patient assignment must be randomized.
Intent-to-treat analysis. ITT assumes that complete data are available during final analysis on every subject, but subjects often drop out. To compensate for drop-outs, researchers could:
- carry forward the last available measurement as the final result, known as last observation carried forward (LOCF).
- use data only from patients who complete entire study protocol (completer analysis method).
Both methods have statistical limitations, but LOCF generally is preferred because it accounts for every subject who enrolled in the study.2
Similar baseline. Compare known characteristics of the treatment and placebo groups at baseline. Confounding variables, such as illness severity or medical or psychiatric comorbidities, should appear equally among randomized patient groups. Not all variables will be similar because of random effects, however.
Blinding. With ineffective blinding, patients or researchers can tell which treatment was administered. If this occurs, the study’s outcome likely is biased by treatment expectations. To detect faulty blinding, some studies ask patients and/or providers if they can guess the intervention that was delivered.
Equal treatment. Even with proper randomization and blinding, other intervention-related treatments—such as blood work to monitor side effects or the duration or frequency of provider contact—might not be administered equally among patient groups. This can clue patients and researchers into which intervention was administered and create bias.
Equivalence to your patient. A typical study patient often has few medical and psychiatric comorbidities or psychosocial risk factors. Your patient might be substantially different. Carefully compare the patients in the study with the patient in your office before choosing a treatment.
With the rapid emergence of novel therapies, psychiatrists face the challenge of deciphering the clinical application of published clinical trials. Although double-blind, randomized, placebo-controlled trials are the gold standard, their validity should be carefully examined.1 The FRISBEE mnemonic from Duke University’s psychiatry residency program can help you incorporate evidence-based medicine into your patient care.
Follow-up. Carefully interpret studies with inadequate follow-up or high drop-out rates. The reason for patient discontinuation might not be related to the studied intervention.
Randomization. To control for unknown confounding variables, patient assignment must be randomized.
Intent-to-treat analysis. ITT assumes that complete data are available during final analysis on every subject, but subjects often drop out. To compensate for drop-outs, researchers could:
- carry forward the last available measurement as the final result, known as last observation carried forward (LOCF).
- use data only from patients who complete entire study protocol (completer analysis method).
Both methods have statistical limitations, but LOCF generally is preferred because it accounts for every subject who enrolled in the study.2
Similar baseline. Compare known characteristics of the treatment and placebo groups at baseline. Confounding variables, such as illness severity or medical or psychiatric comorbidities, should appear equally among randomized patient groups. Not all variables will be similar because of random effects, however.
Blinding. With ineffective blinding, patients or researchers can tell which treatment was administered. If this occurs, the study’s outcome likely is biased by treatment expectations. To detect faulty blinding, some studies ask patients and/or providers if they can guess the intervention that was delivered.
Equal treatment. Even with proper randomization and blinding, other intervention-related treatments—such as blood work to monitor side effects or the duration or frequency of provider contact—might not be administered equally among patient groups. This can clue patients and researchers into which intervention was administered and create bias.
Equivalence to your patient. A typical study patient often has few medical and psychiatric comorbidities or psychosocial risk factors. Your patient might be substantially different. Carefully compare the patients in the study with the patient in your office before choosing a treatment.
1. Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 2000;284(3):357-62.
2. Lachin JM. Statistical considerations in the intent to treat principle. Control Clin Trials 2000;21:167-89.
Dr. Xiong is assistant clinical professor at the University of California, Davis. Dr. Adams is clinical associate at Duke University Medical Center, Durham, NC.
1. Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 2000;284(3):357-62.
2. Lachin JM. Statistical considerations in the intent to treat principle. Control Clin Trials 2000;21:167-89.
Dr. Xiong is assistant clinical professor at the University of California, Davis. Dr. Adams is clinical associate at Duke University Medical Center, Durham, NC.