User login
A 46-year-old man with fever, ST-segment elevation
An otherwise healthy 46-year-old man presents with fever, chills, and rigors that began 1 day ago. He reports shortness of breath, nausea, neck pain, sore throat, and right-sided jaw pain, but no chest pain, headache, or photophobia.
His vital signs are within normal limits, except for a temperature of 38.5°C (101.3°F). His jugular venous pressure and heart sounds are normal, and no focal deficit or nuchal rigidity is elicited. Laboratory tests (hemography, biochemistry panel, and cardiac biomarkers) are normal. An enzyme immunoassay for influenza A and B is negative, as is a rapid antigen detection test for streptococcal infection.
Q: What is the most likely diagnosis?
- Acute myocardial infarction
- Coronary vasospasm
- Brugada syndrome
- Acute pericarditis
- Acute meningitis
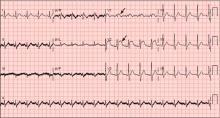
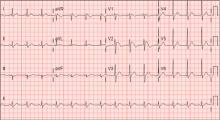
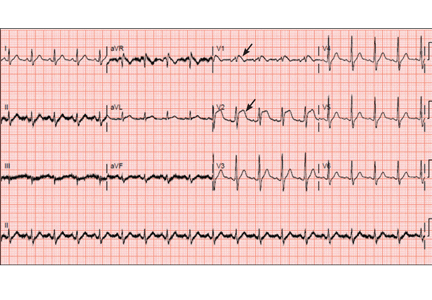
A: ST elevation commonly represents acute myocardial infarction, but it is associated with other conditions, including Prinzmetal angina, hyperkalemia, hypercalcemia, early repolarization, Brugada syndrome, and acute pericarditis.1 These conditions should be considered before an invasive intervention. The ECG findings (ST elevation in the right precordial leads) in this patient were consistent with those of Brugada syndrome.
WHAT IS BRUGADA SYNDROME?
Brugada syndrome is an arrhythmogenic disease characterized by ST-segment elevation in the right precordial leads, right bundle branch block, and a high incidence of sudden cardiac death in younger people.2 It accounts for 4% of all sudden deaths.3
Three different types of changes on ECG have been associated with Brugada syndrome. Type 1 is a coved ST-segment elevation of at least 2 mm, followed by a negative T wave, with little or no isoelectric separation, and present in more than one right precordial lead (from V1 to V3). Type 2 and type 3 patterns on ECG show the same 2-mm or greater J-point elevation, but a positive T wave gives the “saddleback” appearance to the ST-T portion.
Brugada syndrome is confirmed when a type 1 pattern is observed in conjunction with one of the following:
- Documented ventricular fibrillation
- Polymorphic ventricular tachycardia
- A family history of sudden cardiac death at 45 years of age or younger
- Type 1 pattern on ECG in family members
- Ventricular tachycardia that can be induced with programmed electrical stimulation
- Syncope
- Nocturnal agonal respiration.3
This patient had type 1 changes on ECG but none of the above findings.
Brugada syndrome is inherited as an autosomal dominant trait, and mutations in gene SCN5A account for 18% to 30% of cases.3 These mutations impair the function of the sodium channel current, leading to an unopposed outward shift of net transmembrane current at the end of phase 1 of the right ventricular epicardial action potential. Interestingly, changes on ECG that are associated with Brugada syndrome are often dynamic or concealed and are unmasked by sodium channel blockers, fever, vagotonic agents, adrenergic agonists or antagonists, and various electrolyte abnormalities.3
At temperatures above the physiologic range, the inward sodium current is reduced, either because of failure of expression of sodium channels or because of premature closing of the sodium channels in genetically susceptible individuals.4 Therefore, fever can unmask Brugada syndrome, as it did in our patient.
For patients with symptomatic Brugada syndrome, the only current treatment is implantation of a cardioverter-defibrillator.5 Patients without symptoms may benefit from an electrophysiologic study for risk stratification, and an implantable cardioverter-defibrillator is recommended for those in whom ventricular fibrillation can be induced.3,5
CASE CONTINUED
The patient remained febrile after catheterization and received vancomycin (Vancocin) and ceftriaxone (Rocephin) empirically for presumed meningitis. Multiple peripheral blood cultures grew gram-positive cocci in pairs and chains, which were identified as Streptococcus pneumoniae. His fever abated soon after the antibiotic therapy was started.
Lumbar puncture was not done. Transesophageal echocardiography revealed no vegetations, with preserved ejection fraction. The patient has no family history of sudden death and no personal history of syncope or presyncope.
On hospital day 3, although his fever was gone, ECG still showed a Brugada pattern. He was discharged home on a 3-week regimen of intravenous penicillin, with plans for appropriate follow-up, and he was counseled that his family should be screened. An electrophysiologic study was not done, and he had no symptoms 1 year later.
As seen in this patient, Brugada syndrome is important to consider in the differential diagnosis in young patients who present with fever and ST elevations.
- Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med 2003; 349:2128–2135.
- Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol 1992; 20:1391–1396.
- Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005; 111:659–670.
- Dumaine R, Towbin JA, Brugada P, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res 1999; 85:803–809.
- Antzelevitch C, Nof E. Brugada syndrome: recent advances and controversies. Curr Cardiol Rep 2008; 10:376–383.
An otherwise healthy 46-year-old man presents with fever, chills, and rigors that began 1 day ago. He reports shortness of breath, nausea, neck pain, sore throat, and right-sided jaw pain, but no chest pain, headache, or photophobia.
His vital signs are within normal limits, except for a temperature of 38.5°C (101.3°F). His jugular venous pressure and heart sounds are normal, and no focal deficit or nuchal rigidity is elicited. Laboratory tests (hemography, biochemistry panel, and cardiac biomarkers) are normal. An enzyme immunoassay for influenza A and B is negative, as is a rapid antigen detection test for streptococcal infection.
Q: What is the most likely diagnosis?
- Acute myocardial infarction
- Coronary vasospasm
- Brugada syndrome
- Acute pericarditis
- Acute meningitis
A: ST elevation commonly represents acute myocardial infarction, but it is associated with other conditions, including Prinzmetal angina, hyperkalemia, hypercalcemia, early repolarization, Brugada syndrome, and acute pericarditis.1 These conditions should be considered before an invasive intervention. The ECG findings (ST elevation in the right precordial leads) in this patient were consistent with those of Brugada syndrome.
WHAT IS BRUGADA SYNDROME?
Brugada syndrome is an arrhythmogenic disease characterized by ST-segment elevation in the right precordial leads, right bundle branch block, and a high incidence of sudden cardiac death in younger people.2 It accounts for 4% of all sudden deaths.3
Three different types of changes on ECG have been associated with Brugada syndrome. Type 1 is a coved ST-segment elevation of at least 2 mm, followed by a negative T wave, with little or no isoelectric separation, and present in more than one right precordial lead (from V1 to V3). Type 2 and type 3 patterns on ECG show the same 2-mm or greater J-point elevation, but a positive T wave gives the “saddleback” appearance to the ST-T portion.
Brugada syndrome is confirmed when a type 1 pattern is observed in conjunction with one of the following:
- Documented ventricular fibrillation
- Polymorphic ventricular tachycardia
- A family history of sudden cardiac death at 45 years of age or younger
- Type 1 pattern on ECG in family members
- Ventricular tachycardia that can be induced with programmed electrical stimulation
- Syncope
- Nocturnal agonal respiration.3
This patient had type 1 changes on ECG but none of the above findings.
Brugada syndrome is inherited as an autosomal dominant trait, and mutations in gene SCN5A account for 18% to 30% of cases.3 These mutations impair the function of the sodium channel current, leading to an unopposed outward shift of net transmembrane current at the end of phase 1 of the right ventricular epicardial action potential. Interestingly, changes on ECG that are associated with Brugada syndrome are often dynamic or concealed and are unmasked by sodium channel blockers, fever, vagotonic agents, adrenergic agonists or antagonists, and various electrolyte abnormalities.3
At temperatures above the physiologic range, the inward sodium current is reduced, either because of failure of expression of sodium channels or because of premature closing of the sodium channels in genetically susceptible individuals.4 Therefore, fever can unmask Brugada syndrome, as it did in our patient.
For patients with symptomatic Brugada syndrome, the only current treatment is implantation of a cardioverter-defibrillator.5 Patients without symptoms may benefit from an electrophysiologic study for risk stratification, and an implantable cardioverter-defibrillator is recommended for those in whom ventricular fibrillation can be induced.3,5
CASE CONTINUED
The patient remained febrile after catheterization and received vancomycin (Vancocin) and ceftriaxone (Rocephin) empirically for presumed meningitis. Multiple peripheral blood cultures grew gram-positive cocci in pairs and chains, which were identified as Streptococcus pneumoniae. His fever abated soon after the antibiotic therapy was started.
Lumbar puncture was not done. Transesophageal echocardiography revealed no vegetations, with preserved ejection fraction. The patient has no family history of sudden death and no personal history of syncope or presyncope.
On hospital day 3, although his fever was gone, ECG still showed a Brugada pattern. He was discharged home on a 3-week regimen of intravenous penicillin, with plans for appropriate follow-up, and he was counseled that his family should be screened. An electrophysiologic study was not done, and he had no symptoms 1 year later.
As seen in this patient, Brugada syndrome is important to consider in the differential diagnosis in young patients who present with fever and ST elevations.
An otherwise healthy 46-year-old man presents with fever, chills, and rigors that began 1 day ago. He reports shortness of breath, nausea, neck pain, sore throat, and right-sided jaw pain, but no chest pain, headache, or photophobia.
His vital signs are within normal limits, except for a temperature of 38.5°C (101.3°F). His jugular venous pressure and heart sounds are normal, and no focal deficit or nuchal rigidity is elicited. Laboratory tests (hemography, biochemistry panel, and cardiac biomarkers) are normal. An enzyme immunoassay for influenza A and B is negative, as is a rapid antigen detection test for streptococcal infection.
Q: What is the most likely diagnosis?
- Acute myocardial infarction
- Coronary vasospasm
- Brugada syndrome
- Acute pericarditis
- Acute meningitis
A: ST elevation commonly represents acute myocardial infarction, but it is associated with other conditions, including Prinzmetal angina, hyperkalemia, hypercalcemia, early repolarization, Brugada syndrome, and acute pericarditis.1 These conditions should be considered before an invasive intervention. The ECG findings (ST elevation in the right precordial leads) in this patient were consistent with those of Brugada syndrome.
WHAT IS BRUGADA SYNDROME?
Brugada syndrome is an arrhythmogenic disease characterized by ST-segment elevation in the right precordial leads, right bundle branch block, and a high incidence of sudden cardiac death in younger people.2 It accounts for 4% of all sudden deaths.3
Three different types of changes on ECG have been associated with Brugada syndrome. Type 1 is a coved ST-segment elevation of at least 2 mm, followed by a negative T wave, with little or no isoelectric separation, and present in more than one right precordial lead (from V1 to V3). Type 2 and type 3 patterns on ECG show the same 2-mm or greater J-point elevation, but a positive T wave gives the “saddleback” appearance to the ST-T portion.
Brugada syndrome is confirmed when a type 1 pattern is observed in conjunction with one of the following:
- Documented ventricular fibrillation
- Polymorphic ventricular tachycardia
- A family history of sudden cardiac death at 45 years of age or younger
- Type 1 pattern on ECG in family members
- Ventricular tachycardia that can be induced with programmed electrical stimulation
- Syncope
- Nocturnal agonal respiration.3
This patient had type 1 changes on ECG but none of the above findings.
Brugada syndrome is inherited as an autosomal dominant trait, and mutations in gene SCN5A account for 18% to 30% of cases.3 These mutations impair the function of the sodium channel current, leading to an unopposed outward shift of net transmembrane current at the end of phase 1 of the right ventricular epicardial action potential. Interestingly, changes on ECG that are associated with Brugada syndrome are often dynamic or concealed and are unmasked by sodium channel blockers, fever, vagotonic agents, adrenergic agonists or antagonists, and various electrolyte abnormalities.3
At temperatures above the physiologic range, the inward sodium current is reduced, either because of failure of expression of sodium channels or because of premature closing of the sodium channels in genetically susceptible individuals.4 Therefore, fever can unmask Brugada syndrome, as it did in our patient.
For patients with symptomatic Brugada syndrome, the only current treatment is implantation of a cardioverter-defibrillator.5 Patients without symptoms may benefit from an electrophysiologic study for risk stratification, and an implantable cardioverter-defibrillator is recommended for those in whom ventricular fibrillation can be induced.3,5
CASE CONTINUED
The patient remained febrile after catheterization and received vancomycin (Vancocin) and ceftriaxone (Rocephin) empirically for presumed meningitis. Multiple peripheral blood cultures grew gram-positive cocci in pairs and chains, which were identified as Streptococcus pneumoniae. His fever abated soon after the antibiotic therapy was started.
Lumbar puncture was not done. Transesophageal echocardiography revealed no vegetations, with preserved ejection fraction. The patient has no family history of sudden death and no personal history of syncope or presyncope.
On hospital day 3, although his fever was gone, ECG still showed a Brugada pattern. He was discharged home on a 3-week regimen of intravenous penicillin, with plans for appropriate follow-up, and he was counseled that his family should be screened. An electrophysiologic study was not done, and he had no symptoms 1 year later.
As seen in this patient, Brugada syndrome is important to consider in the differential diagnosis in young patients who present with fever and ST elevations.
- Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med 2003; 349:2128–2135.
- Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol 1992; 20:1391–1396.
- Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005; 111:659–670.
- Dumaine R, Towbin JA, Brugada P, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res 1999; 85:803–809.
- Antzelevitch C, Nof E. Brugada syndrome: recent advances and controversies. Curr Cardiol Rep 2008; 10:376–383.
- Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med 2003; 349:2128–2135.
- Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol 1992; 20:1391–1396.
- Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005; 111:659–670.
- Dumaine R, Towbin JA, Brugada P, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res 1999; 85:803–809.
- Antzelevitch C, Nof E. Brugada syndrome: recent advances and controversies. Curr Cardiol Rep 2008; 10:376–383.