User login
Hand, foot, and mouth disease: Identifying and managing an acute viral syndrome
Hand, foot, and mouth disease (HFMD) is typically a benign childhood infection—except when it isn’t so benign or when it occurs in adults.
The usual presentation is in a child with fever, oral ulcerations, and papules on the palms of the hands and the soles of the feet.1 However, severe complications can occur, including central nervous system involvement and cardiopulmonary failure, and can lead to significant morbidity and even death.2 Fortunately, these complications are rare.
Less common in North America than in other regions, HFMD has recurrently broken out in many areas of Southern Asia and the surrounding Pacific region. However, several North American outbreaks have been documented in recent years and have affected unexpected numbers of immunocompetent adults, demonstrating that this disease is of worldwide importance in adults as well as children.3
Because HFMD has the potential to reach epidemic levels in the United States, early recognition is paramount, and primary care physicians need to be familiar with its common signs and symptoms.
USUALLY A SUMMER DISEASE
HFMD occurs all around the world, exhibiting seasonal variation in temperate climates. In these locations, individual cases and regional outbreaks usually occur in the spring, summer, and fall. No sexual predisposition has been documented. Most symptomatic cases are in children under the age of 10.
OUTBREAKS AROUND THE WORLD
The disease was first described more than 40 years ago, with several large outbreaks in the last 16 years.
1998—An outbreak in Taiwan affected more than 1.5 million people, mostly children. Severe cases numbered just over 400, and 78 children died.4
2008—China,5 Singapore,6 Vietnam,7 Mongolia,8 and Brunei9 were stricken with an outbreak that affected 30,000 people and led to more than 50 deaths.
2009—An outbreak in the Henan and Shandong provinces of eastern China killed 35 people.10
2010—In several southern Chinese regions, more than 70,000 people were infected, with almost 600 deaths.11
2011 to the present. The United States has had several outbreaks in the last 3 years. Although HFMD is not one of the diseases that must be reported to public health authorities in the United States, from November 2011 to February 2012 the US Centers for Disease Control and Prevention (CDC) received reports of 63 possible cases: 38 in Alabama, 17 in Nevada, 7 in California, and 1 in Connecticut.1 Fifteen of the patients were adults, and more than half had contacts who were sick.
The most recent US outbreak, in Alabama,12 was atypical because it occurred in the winter.
CAUSED BY ENTEROVIRUSES
HFMD is caused by infection with a variety of viruses in the genus Enterovirus, a large group that in turn is part of the larger Picornaviridae family.13 The taxonomy of this genus is complicated and subject to revision; species include coxsackieviruses, polioviruses, enteroviruses, and echoviruses. They are all small, nonenveloped, single-stranded RNA viruses.
The most common strains that cause HFMD are coxsackievirus A16 and enterovirus 71. In addition, coxsackievirus A6 may be emerging, and many other coxsackievirus strains have been directly implicated, including A5, A7, A9, A10, B2, and B5.
Coxsackievirus A16 is the leading cause of HFMD.
Enterovirus 71 is the second most common cause of HFMD and has also caused outbreaks. It usually results in benign disease. However, among the causes of HFMD, it is associated with more prominent central nervous system involvement14 and is the most common cause of viral meningoencephalitis in children.
Coxsackievirus A6. In December 2011, the California Department of Public Health isolated a strain of coxsackievirus A6 that caused extensive rash and nail shedding.15 Among the 63 possible cases of HFMD reported to the CDC from November 2011 to February 2012, specimens for clinical testing were obtained in 34, and 25 of those demonstrated coxsackievirus A6 infection.3
FEVER, ORAL ULCERS, RASH ON HANDS AND FEET
The typical clinical manifestations of HFMD are fever, stomatitis with oral ulcers, and an exanthem affecting the palms, soles, and other parts of the body. These last less than 7 to 10 days, usually occur during the spring to fall months, and have a benign course.
The incubation period is 3 to 5 days, with a prodrome that may include fever, malaise, abdominal pain, and myalgia before the onset of oral and cutaneous findings. Painful oral ulcers may precede the exanthem and can result in dehydration.16
The cutaneous manifestation of HFMD is typically a papulovesicular rash affecting the palms, soles, and buttocks (Figure 1). Other sites may include the knees, elbows, and the dorsal surfaces of the hands and feet. The lesions may be maculopapular and can be either asymptomatic or tender and painful. Desquamation can follow the exanthem, and lesions usually resolve without scarring or secondary infection.16,17
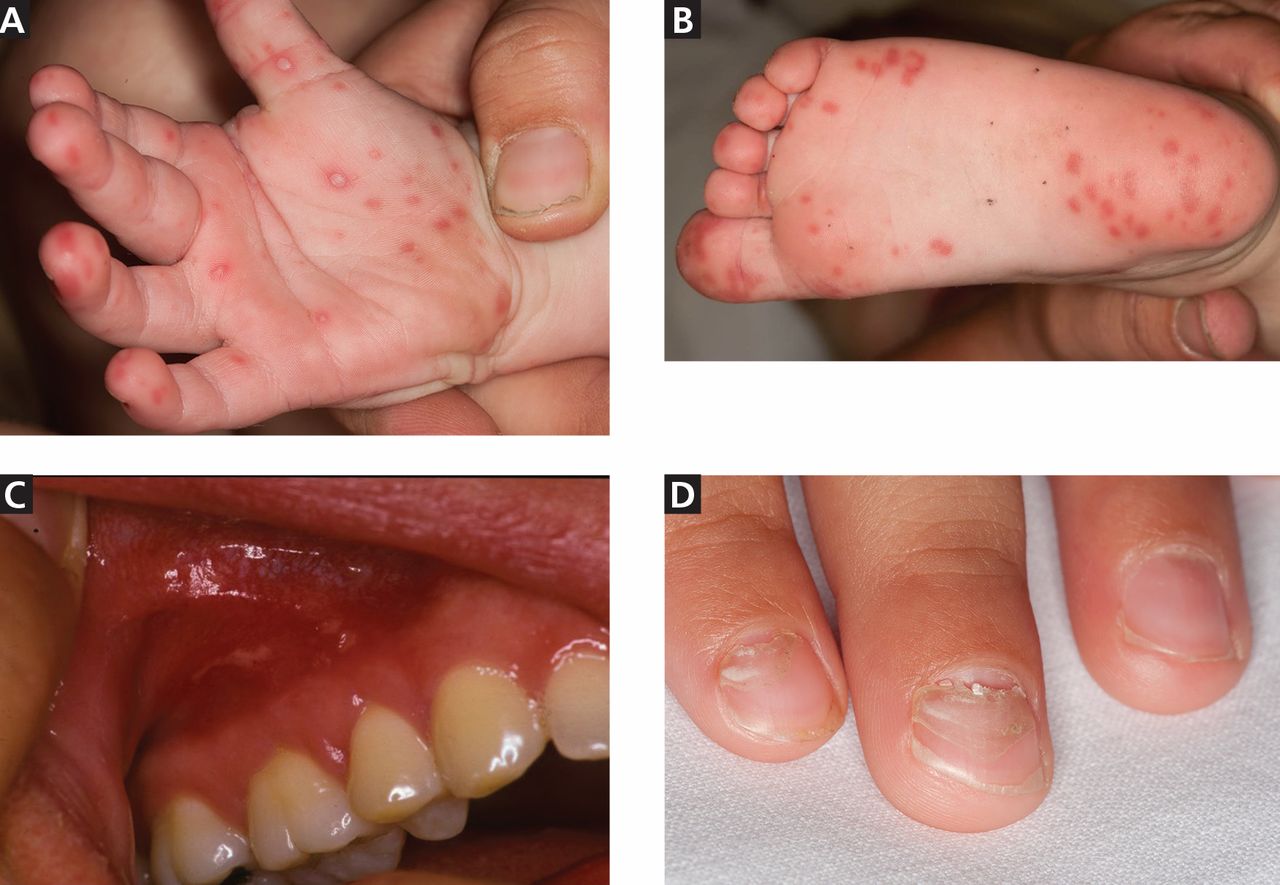
Table 1 and Table 2 compare HFMD with other common illnesses that can cause similar skin and mucosal findings. In particular, herpangina has the identical clinical presentation as HFMD except that it does not cause skin lesions. It is caused by many of the same enteroviruses linked to HFMD.
Different viruses, different signs?
The numerous viruses that cause HFMD usually cause similar signs and symptoms during bouts of typical, self-limited disease. However, neurologic and cardiopulmonary involvement, which are fortunately rare, are more often associated with enterovirus 71 infection.
Nail manifestations are common in HFMD. Nail separation from the nail matrix (onychomadesis) was associated with coxsackievirus A6 infection during a 2010 outbreak of HFMD in Taiwan and in a 2009 outbreak in Finland.18 Moreover, this virus was cultured from a nail specimen in one patient, suggesting viral infiltration as the cause of nail-matrix arrest.19
Perioral skin eruptions, desquamation, and Beau lines have also been associated with coxsackievirus A6.18 Beau lines are transverse depressions of the nail, most evident in the central nail plate; when seen on multiple nails, they imply a systemic illness causing disruption of nail matrix growth.20
Atypical HFMD and coxsackievirus A6
Atypical HFMD has recently been described in connection with coxsackievirus A6. Lott et al21 reported five cases of coxsackievirus A6-associated HFMD in 2013. Atypically, three of the affected patients presented in winter months, two were adults, and two had widespread skin involvement.21
Mathes et al22 reported a series of 80 cases of enteroviral infections in which the lesions had a predilection for the antecubital and popliteal fossae and were similar in severity and distribution to those seen in eczema herpeticum or Kaposi varicelliform eruption in patients with and patients without a history of atopic dermatitis. They named this find-clinical finding of pronounced coxsackievirus-associated skin disease at sites previously affected by atopic dermatitis.
Additional cutaneous findings of coxsackievirus A6 infection may include onychomadesis, Beau lines, and vesiculobullous lesions. Patients with atypical, coxsackievirus A6-associated HFMD may not have oral lesions.23
In the five cases reported by Lott et al,21 significant systemic symptoms (fever, chills, diarrhea, and myalgias) led all but one of the patients to seek care in an emergency department. However, atypical HFMD has not been associated with life-threatening illness.
Atypical HFMD associated with coxsackievirus A6 is an emerging entity in the United States, and the acuity of both cutaneous and systemic symptoms poses a diagnostic dilemma. Furthermore, infection has been documented in immunocompetent adults.23 Familiarity with the clinical findings may expedite appropriate care, prevent spread to contacts, and avoid unnecessary testing.
Neurologic and cardiopulmonary manifestations
Enteroviruses are the most common causes of viral meningoencephalitis in the United States. They mainly affect children and cause serious and potentially chronic disease in those with humoral immunodeficiencies.24 Neurologic and cardiopulmonary manifestations of HFMD are varied and extremely rare in the United States but should always be viewed clinically as signs of concern and severe disease.
Signs of potentially fatal disease that have been observed in young children include tachycardia, tachypnea, hypotension, hypertension, gastrointestinal bleeding, neurologic symptoms, leukocytosis, absence of oral lesions, and vomiting.2 Signs of dysautonomia, myoclonus, ataxia, and brainstem involvement may portend fatal disease in which rapid decompensation is the result of cardiogenic shock due to loss of ventricular contractility, causing pulmonary edema and end-organ dysfunction.16
Neurologic manifestations associated with enterovirus 71 infection include aseptic meningitis, a poliomyelitis-like syndrome, brainstem encephalitis, neurogenic pulmonary edema, opsoclonus-myoclonus syndrome, cerebellar ataxia, Guillain-Barré syndrome, and transverse myelitis.
Because some patients who have neurologic disease respond to treatment with high-dose methylprednisolone and intravenous immune globulin, there is reason to suspect that an autoimmune phenomenon triggered by the culprit enterovirus may be the cause of many of the neurologic symptoms.25
A 2012 meta-analysis26 found that an elevated white blood cell count and hyperglycemia could be clinically useful in distinguishing benign from severe HFMD. In patients with benign HFMD, white blood cell counts and blood glucose values were no different from those in healthy controls.26
DIAGNOSIS IS USUALLY CLINICAL
Most enteroviral infections are asymptomatic, but HFMD is a possibility if a patient has mild illness, fever, and a maculopapular or vesicular rash on the palms of the hands and soles of the feet, sometimes associated with oral ulcers (herpangina). Skin lesions can also be found on the legs, face, buttocks, and trunk.
In the United States, HFMD most commonly occurs in children under age 4 and is usually caused by coxsackievirus A16. Adults can also be affected, especially if they were in contact with children in child care, which was the case in approximately half of nonpediatric patients who tested positive for HFMD during an outbreak in several states between November 2011 and February 2012.3
The clinical characteristics of HFMD caused by enterovirus 71 may be somewhat different, with smaller vesicles, diffuse erythema of the trunk and limbs, and higher fever (temperature ≥ 39°C [102.2°F] for more than 3 days).27 However, the rash of coxsackievirus A16 HFMD may be more extensive and severe.
Other clinical manifestations of HFMD include nail dystrophies such as Beau lines and nail shedding, hyperglycemia, dehydration, and more serious and potentially life-threatening complications such as pulmonary edema28 and viral meningoencephalitis.29
Laboratory testing
In mild cases of HFMD, particularly in patients with a high probability of having the disease based on their clinical characteristics and sick contacts, laboratory testing is not necessary. Testing is usually reserved for severe cases and public health investigation of outbreaks.
Viral culture is the gold standard for diagnosing HFMD, but the final results can take nearly a week.
Polymerase chain reaction testing is faster, with a turnaround time of less than 1 day. It identifies viral RNA and is highly sensitive for detecting central nervous system infection.30
Where should samples be collected? Serum viremia precedes invasion of the skin and mucous membranes, so plasma can be tested. Inside the body, enteroviruses initially replicate in the gastrointestinal tract, although collecting a rectal swab or a stool sample is somewhat invasive. Further, in an enterovirus 71 epidemic in Taiwan, 93% of the patients had positive throat swabs, but only 30% tested positive by rectal swabs or analysis of the feces.27 At present, throat and vesicle specimens are considered to be the most useful sources for diagnostic purposes.16
ELISAs. Newly developed IgM-capture enzyme-linked immunosorbent assays (ELISAs) for coxsackievirus A16 and enterovirus 71 appear quite promising for diagnosing HFMD. These tests are inexpensive and detect IgM antibodies early and in a high percentage of patients. In the first week of the disease, the IgM detection rate was found to be 90.2% for enterovirus 71 and 68% for coxsackievirus A16.31
Cross-reactivity between these two viruses was a problem with ELISA testing in the past, causing false-positive results for enterovirus 71 in patients who in fact had coxsackievirus A16. The problem appears to be resolved in new versions that use specific enterovirus 71 proteins, eg, VP1.32
RECOGNITION AND PREVENTION ARE THE BEST MEDICINE
Recognizing HFMD early is crucial, because making the clinical diagnosis can identify patients who have signs of severe disease and can help protect future contacts and decrease the risk of an epidemic.
Infected patients continue to shed the virus for a long time, making hand hygiene and environmental control measures in health care settings and daycare centers of vital importance, to prevent spread of the infection.
Enteroviruses are stable in the environment and therefore capable of fecal-oral and oral-oral transmission. Humans are the only known natural hosts. No chemoprophylaxis or vaccination has been established to prevent HFMD. The recurrence of large-scale epidemics in the developing world is perhaps explained by ineffective sewage treatment and limited access to clean drinking water, especially in light of the fecal-oral spread of the virus. Intrafamilial spread of HFMD has been shown to be an important means of disease transmission, and asymptomatic adult carriers of these viruses may spread it to young children.33
The different viruses that cause HFMD result in a similar clinical presentation in most patients. Therefore, identifying HFMD caused by enterovirus 71, which carries a risk of severe and even fatal disease in young children vs a virus such as coxsackievirus A16, can be very difficult in practice without virologic testing. Thus, when diagnosed with HFMD, patients should be counseled to control all variables that could lead to further spread of the disease.
An analysis of epidemics in Asia suggested that public health awareness may have averted deaths in successive epidemics, highlighting the need to identify HFMD epidemics in communities and to educate patients and families about measures to prevent further spread of the virus in addition to standard supportive care.34
The CDC recommends35:
- Frequent hand-washing after toileting and changing diapers
- Disinfecting frequently used surfaces and objects, including toys
- Avoiding close contact with infected individuals and sharing of personal items such as utensils and cups.
These measures should be recommended to all affected patients.35
NO PROVEN ANTIVIRAL TREATMENT
No proven antiviral treatment exists for HFMD. Thus, the goals of treatment are typically supportive, as for any self-limited viral syndrome.16
Does acyclovir help? Shelley et al36 treated 13 patients (12 children and 1 adult) with acyclovir within 1 to 2 days of the onset of the HFMD rash and reported that it was beneficial, with significant relief of fever and skin lesions within 24 hours of starting therapy. These anecdotal results have not been replicated, and acyclovir is not an established treatment for HFMD.
If acyclovir does help, how does it work? Acyclovir, like other common antiviral medications, inactivates thymidine kinase, an enzyme produced by herpesviruses but not by HFMD-causing viruses like coxsackievirus A16. Shelley et al proposed that acyclovir may enhance the antiviral effect of the patient’s own interferon.36
Intravenous immunoglobulin has been used in severe cases during outbreaks in Asia, with retrospective data showing a potential ability to halt disease progression if used before the development of cardiopulmonary failure. However, this has not been studied prospectively and is not currently recommended.16
Acknowledgment: We would like to thank Dr. Salvador Alvarez of the Mayo Clinic Department of Infectious Disease and Dr. Donald Lookingbill of the Mayo Clinic Department of Dermatology for their collaboration.
- Centers for Disease Control and Prevention (CDC). Hand, Foot, and Mouth Disease (HFMD). www.cdc.gov/hand-foot-mouth/index.html. Accessed June 10, 2014.
- Chatproedprai S, Theanboonlers A, Korkong S, Thongmee C, Wananukul S, Poovorawan Y. Clinical and molecular characterization of hand-foot-and-mouth disease in Thailand, 2008–2009. Jpn J Infect Dis 2010; 63:229–233.
- Centers for Disease Control and Prevention (CDC). Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011–February 2012. MMWR Morb Mortal Wkly Rep 2012; 61:213–214.
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 1999; 341:929–935.
- BBC News. China virus toll continues rise. May 5, 2008. http://news.bbc.co.uk/2/hi/asia-pacific/7383796.stm. Accessed February 5, 2014.
- Suhaimi ND. HFMD: 1,000 cases a week in Singapore is unusual, says doc. Straits Times April 20, 2008.
- Viet Nam News: HFMD cases prompt tighter health screening at airport. May 15, 2008.
- UBPOST. EV-71 virus continues dramatic rise. May 22, 2008.
- Begawan BS. 1,053 HFMD cases recorded. Brunei Times. November 7, 2008.
- Chinaview. Hand-foot-mouth disease death toll rises to 17 in East China’s Shandong Province. April 9, 2009.
- Chinaview. China reports 537 deaths from hand-foot-mouth disease this year. June 24, 2010.
- Wolfson H. Outbreak of hand, foot and mouth disease severe in Alabama. Birmingham News February 13, 2012.
- Centers for Disease Control and Prevention (CDC). Non-Polio Enterovirus Infections. www.cdc.gov/non-polio-enterovirus/. Accessed June 10, 2014.
- Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis 2003; 9:78–85.
- California Department of Public Health. Coxsackievirus A6 (CVA6). 2011. www.cdph.ca.gov/programs/cder/Pages/CVA6.aspx. Accessed June 10, 2014.
- World Health Organization: Western Pacific Region. A Guide to Clinical management and Public Health Response for Hand, Foot, and Mouth Disease (HFMD).
- Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol 2010; 22:216–218.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis 2011; 11:346.
- Osterback R, Vuorinen T, Linna M, Susi P, Hyypiä T, Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis 2009; 15:1485–1488.
- Tosti A, Piraccini BM. Nail Disorders. In:Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology JV. 3rded. Elsevier Limited; 2012:1129–1144.
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol 2013; 69:736–741.
- Mathes EF, Oza V, Frieden IJ, et al. ”Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics 2013; 132:e149–e157.
- Kaminska K, Martinetti G, Lucchini R, Kaya G, Mainetti C. Coxsackievirus A6 and hand, foot and mouth disease: three case reports of familial child-to-immunocompetent adult transmission and a literature review. Case Rep Dermatol 2013; 5:203–209.
- Romero JR. Diagnosis and management of enteroviral infections of the central nervous system. Curr Infect Dis Rep 2002; 4:309–316.
- Akiyama K, Imazeki R, Yoshii F, Koide T, Muto J. An adult case of hand, foot, and mouth disease caused by enterovirus 71 accompanied by opsoclonus myoclonica. Tokai J Exp Clin Med 2008; 33:143–145.
- Li Y, Zhu R, Qian Y, Deng J. The characteristics of blood glucose and WBC counts in peripheral blood of cases of hand foot and mouth disease in China: a systematic review. PLoS One 2012; 7:e29003.
- Chang LY, King CC, Hsu KH, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics 2002; 109:e88.
- Wang SM, Liu CC, Tseng HW, et al. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin Infect Dis 1999; 29:184–190.
- Chang LY, Lin TY, Hsu KH, et al. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 1999; 354:1682–1686.
- Mayo Clinic Laboratories. Enterovirus, Molecular Detection, PCR, Plasma. www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/89893. Accessed June 10, 2014.
- Yu N, Guo M, He SJ, et al. Evaluation of human enterovirus 71 and coxsackievirus A16 specific immunoglobulin M antibodies for diagnosis of hand-foot-and-mouth disease. Virol J 2012; 9:12.
- Wang C, You A, Tian X, et al. Analysis and solution of false-positives when testing CVA16 sera using an antibody assay against the EV71 virus. Virus Res 2013; 176:33–36.
- Liu MY, Liu W, Luo J, et al. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS One 2011; 6:e25287.
- Zhang J, Sun J, Chang Z, Zhang W, Wang Z, Feng Z. Characterization of hand, foot, and mouth disease in China between 2008 and 2009. Biomed Environ Sci 2011; 24:214–221.
- Centers for Disease Control and Prevention (CDC). Hand, Foot, and Mouth Disease: Prevention & Treatment. www.cdc.gov/hand-foot-mouth/about/prevention-treatment.html. Accessed June 10, 2014.
- Shelley WB, Hashim M, Shelley ED. Acyclovir in the treatment of hand-foot-and-mouth disease. Cutis 1996; 57:232–234.
Hand, foot, and mouth disease (HFMD) is typically a benign childhood infection—except when it isn’t so benign or when it occurs in adults.
The usual presentation is in a child with fever, oral ulcerations, and papules on the palms of the hands and the soles of the feet.1 However, severe complications can occur, including central nervous system involvement and cardiopulmonary failure, and can lead to significant morbidity and even death.2 Fortunately, these complications are rare.
Less common in North America than in other regions, HFMD has recurrently broken out in many areas of Southern Asia and the surrounding Pacific region. However, several North American outbreaks have been documented in recent years and have affected unexpected numbers of immunocompetent adults, demonstrating that this disease is of worldwide importance in adults as well as children.3
Because HFMD has the potential to reach epidemic levels in the United States, early recognition is paramount, and primary care physicians need to be familiar with its common signs and symptoms.
USUALLY A SUMMER DISEASE
HFMD occurs all around the world, exhibiting seasonal variation in temperate climates. In these locations, individual cases and regional outbreaks usually occur in the spring, summer, and fall. No sexual predisposition has been documented. Most symptomatic cases are in children under the age of 10.
OUTBREAKS AROUND THE WORLD
The disease was first described more than 40 years ago, with several large outbreaks in the last 16 years.
1998—An outbreak in Taiwan affected more than 1.5 million people, mostly children. Severe cases numbered just over 400, and 78 children died.4
2008—China,5 Singapore,6 Vietnam,7 Mongolia,8 and Brunei9 were stricken with an outbreak that affected 30,000 people and led to more than 50 deaths.
2009—An outbreak in the Henan and Shandong provinces of eastern China killed 35 people.10
2010—In several southern Chinese regions, more than 70,000 people were infected, with almost 600 deaths.11
2011 to the present. The United States has had several outbreaks in the last 3 years. Although HFMD is not one of the diseases that must be reported to public health authorities in the United States, from November 2011 to February 2012 the US Centers for Disease Control and Prevention (CDC) received reports of 63 possible cases: 38 in Alabama, 17 in Nevada, 7 in California, and 1 in Connecticut.1 Fifteen of the patients were adults, and more than half had contacts who were sick.
The most recent US outbreak, in Alabama,12 was atypical because it occurred in the winter.
CAUSED BY ENTEROVIRUSES
HFMD is caused by infection with a variety of viruses in the genus Enterovirus, a large group that in turn is part of the larger Picornaviridae family.13 The taxonomy of this genus is complicated and subject to revision; species include coxsackieviruses, polioviruses, enteroviruses, and echoviruses. They are all small, nonenveloped, single-stranded RNA viruses.
The most common strains that cause HFMD are coxsackievirus A16 and enterovirus 71. In addition, coxsackievirus A6 may be emerging, and many other coxsackievirus strains have been directly implicated, including A5, A7, A9, A10, B2, and B5.
Coxsackievirus A16 is the leading cause of HFMD.
Enterovirus 71 is the second most common cause of HFMD and has also caused outbreaks. It usually results in benign disease. However, among the causes of HFMD, it is associated with more prominent central nervous system involvement14 and is the most common cause of viral meningoencephalitis in children.
Coxsackievirus A6. In December 2011, the California Department of Public Health isolated a strain of coxsackievirus A6 that caused extensive rash and nail shedding.15 Among the 63 possible cases of HFMD reported to the CDC from November 2011 to February 2012, specimens for clinical testing were obtained in 34, and 25 of those demonstrated coxsackievirus A6 infection.3
FEVER, ORAL ULCERS, RASH ON HANDS AND FEET
The typical clinical manifestations of HFMD are fever, stomatitis with oral ulcers, and an exanthem affecting the palms, soles, and other parts of the body. These last less than 7 to 10 days, usually occur during the spring to fall months, and have a benign course.
The incubation period is 3 to 5 days, with a prodrome that may include fever, malaise, abdominal pain, and myalgia before the onset of oral and cutaneous findings. Painful oral ulcers may precede the exanthem and can result in dehydration.16
The cutaneous manifestation of HFMD is typically a papulovesicular rash affecting the palms, soles, and buttocks (Figure 1). Other sites may include the knees, elbows, and the dorsal surfaces of the hands and feet. The lesions may be maculopapular and can be either asymptomatic or tender and painful. Desquamation can follow the exanthem, and lesions usually resolve without scarring or secondary infection.16,17

Table 1 and Table 2 compare HFMD with other common illnesses that can cause similar skin and mucosal findings. In particular, herpangina has the identical clinical presentation as HFMD except that it does not cause skin lesions. It is caused by many of the same enteroviruses linked to HFMD.
Different viruses, different signs?
The numerous viruses that cause HFMD usually cause similar signs and symptoms during bouts of typical, self-limited disease. However, neurologic and cardiopulmonary involvement, which are fortunately rare, are more often associated with enterovirus 71 infection.
Nail manifestations are common in HFMD. Nail separation from the nail matrix (onychomadesis) was associated with coxsackievirus A6 infection during a 2010 outbreak of HFMD in Taiwan and in a 2009 outbreak in Finland.18 Moreover, this virus was cultured from a nail specimen in one patient, suggesting viral infiltration as the cause of nail-matrix arrest.19
Perioral skin eruptions, desquamation, and Beau lines have also been associated with coxsackievirus A6.18 Beau lines are transverse depressions of the nail, most evident in the central nail plate; when seen on multiple nails, they imply a systemic illness causing disruption of nail matrix growth.20
Atypical HFMD and coxsackievirus A6
Atypical HFMD has recently been described in connection with coxsackievirus A6. Lott et al21 reported five cases of coxsackievirus A6-associated HFMD in 2013. Atypically, three of the affected patients presented in winter months, two were adults, and two had widespread skin involvement.21
Mathes et al22 reported a series of 80 cases of enteroviral infections in which the lesions had a predilection for the antecubital and popliteal fossae and were similar in severity and distribution to those seen in eczema herpeticum or Kaposi varicelliform eruption in patients with and patients without a history of atopic dermatitis. They named this find-clinical finding of pronounced coxsackievirus-associated skin disease at sites previously affected by atopic dermatitis.
Additional cutaneous findings of coxsackievirus A6 infection may include onychomadesis, Beau lines, and vesiculobullous lesions. Patients with atypical, coxsackievirus A6-associated HFMD may not have oral lesions.23
In the five cases reported by Lott et al,21 significant systemic symptoms (fever, chills, diarrhea, and myalgias) led all but one of the patients to seek care in an emergency department. However, atypical HFMD has not been associated with life-threatening illness.
Atypical HFMD associated with coxsackievirus A6 is an emerging entity in the United States, and the acuity of both cutaneous and systemic symptoms poses a diagnostic dilemma. Furthermore, infection has been documented in immunocompetent adults.23 Familiarity with the clinical findings may expedite appropriate care, prevent spread to contacts, and avoid unnecessary testing.
Neurologic and cardiopulmonary manifestations
Enteroviruses are the most common causes of viral meningoencephalitis in the United States. They mainly affect children and cause serious and potentially chronic disease in those with humoral immunodeficiencies.24 Neurologic and cardiopulmonary manifestations of HFMD are varied and extremely rare in the United States but should always be viewed clinically as signs of concern and severe disease.
Signs of potentially fatal disease that have been observed in young children include tachycardia, tachypnea, hypotension, hypertension, gastrointestinal bleeding, neurologic symptoms, leukocytosis, absence of oral lesions, and vomiting.2 Signs of dysautonomia, myoclonus, ataxia, and brainstem involvement may portend fatal disease in which rapid decompensation is the result of cardiogenic shock due to loss of ventricular contractility, causing pulmonary edema and end-organ dysfunction.16
Neurologic manifestations associated with enterovirus 71 infection include aseptic meningitis, a poliomyelitis-like syndrome, brainstem encephalitis, neurogenic pulmonary edema, opsoclonus-myoclonus syndrome, cerebellar ataxia, Guillain-Barré syndrome, and transverse myelitis.
Because some patients who have neurologic disease respond to treatment with high-dose methylprednisolone and intravenous immune globulin, there is reason to suspect that an autoimmune phenomenon triggered by the culprit enterovirus may be the cause of many of the neurologic symptoms.25
A 2012 meta-analysis26 found that an elevated white blood cell count and hyperglycemia could be clinically useful in distinguishing benign from severe HFMD. In patients with benign HFMD, white blood cell counts and blood glucose values were no different from those in healthy controls.26
DIAGNOSIS IS USUALLY CLINICAL
Most enteroviral infections are asymptomatic, but HFMD is a possibility if a patient has mild illness, fever, and a maculopapular or vesicular rash on the palms of the hands and soles of the feet, sometimes associated with oral ulcers (herpangina). Skin lesions can also be found on the legs, face, buttocks, and trunk.
In the United States, HFMD most commonly occurs in children under age 4 and is usually caused by coxsackievirus A16. Adults can also be affected, especially if they were in contact with children in child care, which was the case in approximately half of nonpediatric patients who tested positive for HFMD during an outbreak in several states between November 2011 and February 2012.3
The clinical characteristics of HFMD caused by enterovirus 71 may be somewhat different, with smaller vesicles, diffuse erythema of the trunk and limbs, and higher fever (temperature ≥ 39°C [102.2°F] for more than 3 days).27 However, the rash of coxsackievirus A16 HFMD may be more extensive and severe.
Other clinical manifestations of HFMD include nail dystrophies such as Beau lines and nail shedding, hyperglycemia, dehydration, and more serious and potentially life-threatening complications such as pulmonary edema28 and viral meningoencephalitis.29
Laboratory testing
In mild cases of HFMD, particularly in patients with a high probability of having the disease based on their clinical characteristics and sick contacts, laboratory testing is not necessary. Testing is usually reserved for severe cases and public health investigation of outbreaks.
Viral culture is the gold standard for diagnosing HFMD, but the final results can take nearly a week.
Polymerase chain reaction testing is faster, with a turnaround time of less than 1 day. It identifies viral RNA and is highly sensitive for detecting central nervous system infection.30
Where should samples be collected? Serum viremia precedes invasion of the skin and mucous membranes, so plasma can be tested. Inside the body, enteroviruses initially replicate in the gastrointestinal tract, although collecting a rectal swab or a stool sample is somewhat invasive. Further, in an enterovirus 71 epidemic in Taiwan, 93% of the patients had positive throat swabs, but only 30% tested positive by rectal swabs or analysis of the feces.27 At present, throat and vesicle specimens are considered to be the most useful sources for diagnostic purposes.16
ELISAs. Newly developed IgM-capture enzyme-linked immunosorbent assays (ELISAs) for coxsackievirus A16 and enterovirus 71 appear quite promising for diagnosing HFMD. These tests are inexpensive and detect IgM antibodies early and in a high percentage of patients. In the first week of the disease, the IgM detection rate was found to be 90.2% for enterovirus 71 and 68% for coxsackievirus A16.31
Cross-reactivity between these two viruses was a problem with ELISA testing in the past, causing false-positive results for enterovirus 71 in patients who in fact had coxsackievirus A16. The problem appears to be resolved in new versions that use specific enterovirus 71 proteins, eg, VP1.32
RECOGNITION AND PREVENTION ARE THE BEST MEDICINE
Recognizing HFMD early is crucial, because making the clinical diagnosis can identify patients who have signs of severe disease and can help protect future contacts and decrease the risk of an epidemic.
Infected patients continue to shed the virus for a long time, making hand hygiene and environmental control measures in health care settings and daycare centers of vital importance, to prevent spread of the infection.
Enteroviruses are stable in the environment and therefore capable of fecal-oral and oral-oral transmission. Humans are the only known natural hosts. No chemoprophylaxis or vaccination has been established to prevent HFMD. The recurrence of large-scale epidemics in the developing world is perhaps explained by ineffective sewage treatment and limited access to clean drinking water, especially in light of the fecal-oral spread of the virus. Intrafamilial spread of HFMD has been shown to be an important means of disease transmission, and asymptomatic adult carriers of these viruses may spread it to young children.33
The different viruses that cause HFMD result in a similar clinical presentation in most patients. Therefore, identifying HFMD caused by enterovirus 71, which carries a risk of severe and even fatal disease in young children vs a virus such as coxsackievirus A16, can be very difficult in practice without virologic testing. Thus, when diagnosed with HFMD, patients should be counseled to control all variables that could lead to further spread of the disease.
An analysis of epidemics in Asia suggested that public health awareness may have averted deaths in successive epidemics, highlighting the need to identify HFMD epidemics in communities and to educate patients and families about measures to prevent further spread of the virus in addition to standard supportive care.34
The CDC recommends35:
- Frequent hand-washing after toileting and changing diapers
- Disinfecting frequently used surfaces and objects, including toys
- Avoiding close contact with infected individuals and sharing of personal items such as utensils and cups.
These measures should be recommended to all affected patients.35
NO PROVEN ANTIVIRAL TREATMENT
No proven antiviral treatment exists for HFMD. Thus, the goals of treatment are typically supportive, as for any self-limited viral syndrome.16
Does acyclovir help? Shelley et al36 treated 13 patients (12 children and 1 adult) with acyclovir within 1 to 2 days of the onset of the HFMD rash and reported that it was beneficial, with significant relief of fever and skin lesions within 24 hours of starting therapy. These anecdotal results have not been replicated, and acyclovir is not an established treatment for HFMD.
If acyclovir does help, how does it work? Acyclovir, like other common antiviral medications, inactivates thymidine kinase, an enzyme produced by herpesviruses but not by HFMD-causing viruses like coxsackievirus A16. Shelley et al proposed that acyclovir may enhance the antiviral effect of the patient’s own interferon.36
Intravenous immunoglobulin has been used in severe cases during outbreaks in Asia, with retrospective data showing a potential ability to halt disease progression if used before the development of cardiopulmonary failure. However, this has not been studied prospectively and is not currently recommended.16
Acknowledgment: We would like to thank Dr. Salvador Alvarez of the Mayo Clinic Department of Infectious Disease and Dr. Donald Lookingbill of the Mayo Clinic Department of Dermatology for their collaboration.
Hand, foot, and mouth disease (HFMD) is typically a benign childhood infection—except when it isn’t so benign or when it occurs in adults.
The usual presentation is in a child with fever, oral ulcerations, and papules on the palms of the hands and the soles of the feet.1 However, severe complications can occur, including central nervous system involvement and cardiopulmonary failure, and can lead to significant morbidity and even death.2 Fortunately, these complications are rare.
Less common in North America than in other regions, HFMD has recurrently broken out in many areas of Southern Asia and the surrounding Pacific region. However, several North American outbreaks have been documented in recent years and have affected unexpected numbers of immunocompetent adults, demonstrating that this disease is of worldwide importance in adults as well as children.3
Because HFMD has the potential to reach epidemic levels in the United States, early recognition is paramount, and primary care physicians need to be familiar with its common signs and symptoms.
USUALLY A SUMMER DISEASE
HFMD occurs all around the world, exhibiting seasonal variation in temperate climates. In these locations, individual cases and regional outbreaks usually occur in the spring, summer, and fall. No sexual predisposition has been documented. Most symptomatic cases are in children under the age of 10.
OUTBREAKS AROUND THE WORLD
The disease was first described more than 40 years ago, with several large outbreaks in the last 16 years.
1998—An outbreak in Taiwan affected more than 1.5 million people, mostly children. Severe cases numbered just over 400, and 78 children died.4
2008—China,5 Singapore,6 Vietnam,7 Mongolia,8 and Brunei9 were stricken with an outbreak that affected 30,000 people and led to more than 50 deaths.
2009—An outbreak in the Henan and Shandong provinces of eastern China killed 35 people.10
2010—In several southern Chinese regions, more than 70,000 people were infected, with almost 600 deaths.11
2011 to the present. The United States has had several outbreaks in the last 3 years. Although HFMD is not one of the diseases that must be reported to public health authorities in the United States, from November 2011 to February 2012 the US Centers for Disease Control and Prevention (CDC) received reports of 63 possible cases: 38 in Alabama, 17 in Nevada, 7 in California, and 1 in Connecticut.1 Fifteen of the patients were adults, and more than half had contacts who were sick.
The most recent US outbreak, in Alabama,12 was atypical because it occurred in the winter.
CAUSED BY ENTEROVIRUSES
HFMD is caused by infection with a variety of viruses in the genus Enterovirus, a large group that in turn is part of the larger Picornaviridae family.13 The taxonomy of this genus is complicated and subject to revision; species include coxsackieviruses, polioviruses, enteroviruses, and echoviruses. They are all small, nonenveloped, single-stranded RNA viruses.
The most common strains that cause HFMD are coxsackievirus A16 and enterovirus 71. In addition, coxsackievirus A6 may be emerging, and many other coxsackievirus strains have been directly implicated, including A5, A7, A9, A10, B2, and B5.
Coxsackievirus A16 is the leading cause of HFMD.
Enterovirus 71 is the second most common cause of HFMD and has also caused outbreaks. It usually results in benign disease. However, among the causes of HFMD, it is associated with more prominent central nervous system involvement14 and is the most common cause of viral meningoencephalitis in children.
Coxsackievirus A6. In December 2011, the California Department of Public Health isolated a strain of coxsackievirus A6 that caused extensive rash and nail shedding.15 Among the 63 possible cases of HFMD reported to the CDC from November 2011 to February 2012, specimens for clinical testing were obtained in 34, and 25 of those demonstrated coxsackievirus A6 infection.3
FEVER, ORAL ULCERS, RASH ON HANDS AND FEET
The typical clinical manifestations of HFMD are fever, stomatitis with oral ulcers, and an exanthem affecting the palms, soles, and other parts of the body. These last less than 7 to 10 days, usually occur during the spring to fall months, and have a benign course.
The incubation period is 3 to 5 days, with a prodrome that may include fever, malaise, abdominal pain, and myalgia before the onset of oral and cutaneous findings. Painful oral ulcers may precede the exanthem and can result in dehydration.16
The cutaneous manifestation of HFMD is typically a papulovesicular rash affecting the palms, soles, and buttocks (Figure 1). Other sites may include the knees, elbows, and the dorsal surfaces of the hands and feet. The lesions may be maculopapular and can be either asymptomatic or tender and painful. Desquamation can follow the exanthem, and lesions usually resolve without scarring or secondary infection.16,17

Table 1 and Table 2 compare HFMD with other common illnesses that can cause similar skin and mucosal findings. In particular, herpangina has the identical clinical presentation as HFMD except that it does not cause skin lesions. It is caused by many of the same enteroviruses linked to HFMD.
Different viruses, different signs?
The numerous viruses that cause HFMD usually cause similar signs and symptoms during bouts of typical, self-limited disease. However, neurologic and cardiopulmonary involvement, which are fortunately rare, are more often associated with enterovirus 71 infection.
Nail manifestations are common in HFMD. Nail separation from the nail matrix (onychomadesis) was associated with coxsackievirus A6 infection during a 2010 outbreak of HFMD in Taiwan and in a 2009 outbreak in Finland.18 Moreover, this virus was cultured from a nail specimen in one patient, suggesting viral infiltration as the cause of nail-matrix arrest.19
Perioral skin eruptions, desquamation, and Beau lines have also been associated with coxsackievirus A6.18 Beau lines are transverse depressions of the nail, most evident in the central nail plate; when seen on multiple nails, they imply a systemic illness causing disruption of nail matrix growth.20
Atypical HFMD and coxsackievirus A6
Atypical HFMD has recently been described in connection with coxsackievirus A6. Lott et al21 reported five cases of coxsackievirus A6-associated HFMD in 2013. Atypically, three of the affected patients presented in winter months, two were adults, and two had widespread skin involvement.21
Mathes et al22 reported a series of 80 cases of enteroviral infections in which the lesions had a predilection for the antecubital and popliteal fossae and were similar in severity and distribution to those seen in eczema herpeticum or Kaposi varicelliform eruption in patients with and patients without a history of atopic dermatitis. They named this find-clinical finding of pronounced coxsackievirus-associated skin disease at sites previously affected by atopic dermatitis.
Additional cutaneous findings of coxsackievirus A6 infection may include onychomadesis, Beau lines, and vesiculobullous lesions. Patients with atypical, coxsackievirus A6-associated HFMD may not have oral lesions.23
In the five cases reported by Lott et al,21 significant systemic symptoms (fever, chills, diarrhea, and myalgias) led all but one of the patients to seek care in an emergency department. However, atypical HFMD has not been associated with life-threatening illness.
Atypical HFMD associated with coxsackievirus A6 is an emerging entity in the United States, and the acuity of both cutaneous and systemic symptoms poses a diagnostic dilemma. Furthermore, infection has been documented in immunocompetent adults.23 Familiarity with the clinical findings may expedite appropriate care, prevent spread to contacts, and avoid unnecessary testing.
Neurologic and cardiopulmonary manifestations
Enteroviruses are the most common causes of viral meningoencephalitis in the United States. They mainly affect children and cause serious and potentially chronic disease in those with humoral immunodeficiencies.24 Neurologic and cardiopulmonary manifestations of HFMD are varied and extremely rare in the United States but should always be viewed clinically as signs of concern and severe disease.
Signs of potentially fatal disease that have been observed in young children include tachycardia, tachypnea, hypotension, hypertension, gastrointestinal bleeding, neurologic symptoms, leukocytosis, absence of oral lesions, and vomiting.2 Signs of dysautonomia, myoclonus, ataxia, and brainstem involvement may portend fatal disease in which rapid decompensation is the result of cardiogenic shock due to loss of ventricular contractility, causing pulmonary edema and end-organ dysfunction.16
Neurologic manifestations associated with enterovirus 71 infection include aseptic meningitis, a poliomyelitis-like syndrome, brainstem encephalitis, neurogenic pulmonary edema, opsoclonus-myoclonus syndrome, cerebellar ataxia, Guillain-Barré syndrome, and transverse myelitis.
Because some patients who have neurologic disease respond to treatment with high-dose methylprednisolone and intravenous immune globulin, there is reason to suspect that an autoimmune phenomenon triggered by the culprit enterovirus may be the cause of many of the neurologic symptoms.25
A 2012 meta-analysis26 found that an elevated white blood cell count and hyperglycemia could be clinically useful in distinguishing benign from severe HFMD. In patients with benign HFMD, white blood cell counts and blood glucose values were no different from those in healthy controls.26
DIAGNOSIS IS USUALLY CLINICAL
Most enteroviral infections are asymptomatic, but HFMD is a possibility if a patient has mild illness, fever, and a maculopapular or vesicular rash on the palms of the hands and soles of the feet, sometimes associated with oral ulcers (herpangina). Skin lesions can also be found on the legs, face, buttocks, and trunk.
In the United States, HFMD most commonly occurs in children under age 4 and is usually caused by coxsackievirus A16. Adults can also be affected, especially if they were in contact with children in child care, which was the case in approximately half of nonpediatric patients who tested positive for HFMD during an outbreak in several states between November 2011 and February 2012.3
The clinical characteristics of HFMD caused by enterovirus 71 may be somewhat different, with smaller vesicles, diffuse erythema of the trunk and limbs, and higher fever (temperature ≥ 39°C [102.2°F] for more than 3 days).27 However, the rash of coxsackievirus A16 HFMD may be more extensive and severe.
Other clinical manifestations of HFMD include nail dystrophies such as Beau lines and nail shedding, hyperglycemia, dehydration, and more serious and potentially life-threatening complications such as pulmonary edema28 and viral meningoencephalitis.29
Laboratory testing
In mild cases of HFMD, particularly in patients with a high probability of having the disease based on their clinical characteristics and sick contacts, laboratory testing is not necessary. Testing is usually reserved for severe cases and public health investigation of outbreaks.
Viral culture is the gold standard for diagnosing HFMD, but the final results can take nearly a week.
Polymerase chain reaction testing is faster, with a turnaround time of less than 1 day. It identifies viral RNA and is highly sensitive for detecting central nervous system infection.30
Where should samples be collected? Serum viremia precedes invasion of the skin and mucous membranes, so plasma can be tested. Inside the body, enteroviruses initially replicate in the gastrointestinal tract, although collecting a rectal swab or a stool sample is somewhat invasive. Further, in an enterovirus 71 epidemic in Taiwan, 93% of the patients had positive throat swabs, but only 30% tested positive by rectal swabs or analysis of the feces.27 At present, throat and vesicle specimens are considered to be the most useful sources for diagnostic purposes.16
ELISAs. Newly developed IgM-capture enzyme-linked immunosorbent assays (ELISAs) for coxsackievirus A16 and enterovirus 71 appear quite promising for diagnosing HFMD. These tests are inexpensive and detect IgM antibodies early and in a high percentage of patients. In the first week of the disease, the IgM detection rate was found to be 90.2% for enterovirus 71 and 68% for coxsackievirus A16.31
Cross-reactivity between these two viruses was a problem with ELISA testing in the past, causing false-positive results for enterovirus 71 in patients who in fact had coxsackievirus A16. The problem appears to be resolved in new versions that use specific enterovirus 71 proteins, eg, VP1.32
RECOGNITION AND PREVENTION ARE THE BEST MEDICINE
Recognizing HFMD early is crucial, because making the clinical diagnosis can identify patients who have signs of severe disease and can help protect future contacts and decrease the risk of an epidemic.
Infected patients continue to shed the virus for a long time, making hand hygiene and environmental control measures in health care settings and daycare centers of vital importance, to prevent spread of the infection.
Enteroviruses are stable in the environment and therefore capable of fecal-oral and oral-oral transmission. Humans are the only known natural hosts. No chemoprophylaxis or vaccination has been established to prevent HFMD. The recurrence of large-scale epidemics in the developing world is perhaps explained by ineffective sewage treatment and limited access to clean drinking water, especially in light of the fecal-oral spread of the virus. Intrafamilial spread of HFMD has been shown to be an important means of disease transmission, and asymptomatic adult carriers of these viruses may spread it to young children.33
The different viruses that cause HFMD result in a similar clinical presentation in most patients. Therefore, identifying HFMD caused by enterovirus 71, which carries a risk of severe and even fatal disease in young children vs a virus such as coxsackievirus A16, can be very difficult in practice without virologic testing. Thus, when diagnosed with HFMD, patients should be counseled to control all variables that could lead to further spread of the disease.
An analysis of epidemics in Asia suggested that public health awareness may have averted deaths in successive epidemics, highlighting the need to identify HFMD epidemics in communities and to educate patients and families about measures to prevent further spread of the virus in addition to standard supportive care.34
The CDC recommends35:
- Frequent hand-washing after toileting and changing diapers
- Disinfecting frequently used surfaces and objects, including toys
- Avoiding close contact with infected individuals and sharing of personal items such as utensils and cups.
These measures should be recommended to all affected patients.35
NO PROVEN ANTIVIRAL TREATMENT
No proven antiviral treatment exists for HFMD. Thus, the goals of treatment are typically supportive, as for any self-limited viral syndrome.16
Does acyclovir help? Shelley et al36 treated 13 patients (12 children and 1 adult) with acyclovir within 1 to 2 days of the onset of the HFMD rash and reported that it was beneficial, with significant relief of fever and skin lesions within 24 hours of starting therapy. These anecdotal results have not been replicated, and acyclovir is not an established treatment for HFMD.
If acyclovir does help, how does it work? Acyclovir, like other common antiviral medications, inactivates thymidine kinase, an enzyme produced by herpesviruses but not by HFMD-causing viruses like coxsackievirus A16. Shelley et al proposed that acyclovir may enhance the antiviral effect of the patient’s own interferon.36
Intravenous immunoglobulin has been used in severe cases during outbreaks in Asia, with retrospective data showing a potential ability to halt disease progression if used before the development of cardiopulmonary failure. However, this has not been studied prospectively and is not currently recommended.16
Acknowledgment: We would like to thank Dr. Salvador Alvarez of the Mayo Clinic Department of Infectious Disease and Dr. Donald Lookingbill of the Mayo Clinic Department of Dermatology for their collaboration.
- Centers for Disease Control and Prevention (CDC). Hand, Foot, and Mouth Disease (HFMD). www.cdc.gov/hand-foot-mouth/index.html. Accessed June 10, 2014.
- Chatproedprai S, Theanboonlers A, Korkong S, Thongmee C, Wananukul S, Poovorawan Y. Clinical and molecular characterization of hand-foot-and-mouth disease in Thailand, 2008–2009. Jpn J Infect Dis 2010; 63:229–233.
- Centers for Disease Control and Prevention (CDC). Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011–February 2012. MMWR Morb Mortal Wkly Rep 2012; 61:213–214.
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 1999; 341:929–935.
- BBC News. China virus toll continues rise. May 5, 2008. http://news.bbc.co.uk/2/hi/asia-pacific/7383796.stm. Accessed February 5, 2014.
- Suhaimi ND. HFMD: 1,000 cases a week in Singapore is unusual, says doc. Straits Times April 20, 2008.
- Viet Nam News: HFMD cases prompt tighter health screening at airport. May 15, 2008.
- UBPOST. EV-71 virus continues dramatic rise. May 22, 2008.
- Begawan BS. 1,053 HFMD cases recorded. Brunei Times. November 7, 2008.
- Chinaview. Hand-foot-mouth disease death toll rises to 17 in East China’s Shandong Province. April 9, 2009.
- Chinaview. China reports 537 deaths from hand-foot-mouth disease this year. June 24, 2010.
- Wolfson H. Outbreak of hand, foot and mouth disease severe in Alabama. Birmingham News February 13, 2012.
- Centers for Disease Control and Prevention (CDC). Non-Polio Enterovirus Infections. www.cdc.gov/non-polio-enterovirus/. Accessed June 10, 2014.
- Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis 2003; 9:78–85.
- California Department of Public Health. Coxsackievirus A6 (CVA6). 2011. www.cdph.ca.gov/programs/cder/Pages/CVA6.aspx. Accessed June 10, 2014.
- World Health Organization: Western Pacific Region. A Guide to Clinical management and Public Health Response for Hand, Foot, and Mouth Disease (HFMD).
- Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol 2010; 22:216–218.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis 2011; 11:346.
- Osterback R, Vuorinen T, Linna M, Susi P, Hyypiä T, Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis 2009; 15:1485–1488.
- Tosti A, Piraccini BM. Nail Disorders. In:Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology JV. 3rded. Elsevier Limited; 2012:1129–1144.
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol 2013; 69:736–741.
- Mathes EF, Oza V, Frieden IJ, et al. ”Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics 2013; 132:e149–e157.
- Kaminska K, Martinetti G, Lucchini R, Kaya G, Mainetti C. Coxsackievirus A6 and hand, foot and mouth disease: three case reports of familial child-to-immunocompetent adult transmission and a literature review. Case Rep Dermatol 2013; 5:203–209.
- Romero JR. Diagnosis and management of enteroviral infections of the central nervous system. Curr Infect Dis Rep 2002; 4:309–316.
- Akiyama K, Imazeki R, Yoshii F, Koide T, Muto J. An adult case of hand, foot, and mouth disease caused by enterovirus 71 accompanied by opsoclonus myoclonica. Tokai J Exp Clin Med 2008; 33:143–145.
- Li Y, Zhu R, Qian Y, Deng J. The characteristics of blood glucose and WBC counts in peripheral blood of cases of hand foot and mouth disease in China: a systematic review. PLoS One 2012; 7:e29003.
- Chang LY, King CC, Hsu KH, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics 2002; 109:e88.
- Wang SM, Liu CC, Tseng HW, et al. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin Infect Dis 1999; 29:184–190.
- Chang LY, Lin TY, Hsu KH, et al. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 1999; 354:1682–1686.
- Mayo Clinic Laboratories. Enterovirus, Molecular Detection, PCR, Plasma. www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/89893. Accessed June 10, 2014.
- Yu N, Guo M, He SJ, et al. Evaluation of human enterovirus 71 and coxsackievirus A16 specific immunoglobulin M antibodies for diagnosis of hand-foot-and-mouth disease. Virol J 2012; 9:12.
- Wang C, You A, Tian X, et al. Analysis and solution of false-positives when testing CVA16 sera using an antibody assay against the EV71 virus. Virus Res 2013; 176:33–36.
- Liu MY, Liu W, Luo J, et al. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS One 2011; 6:e25287.
- Zhang J, Sun J, Chang Z, Zhang W, Wang Z, Feng Z. Characterization of hand, foot, and mouth disease in China between 2008 and 2009. Biomed Environ Sci 2011; 24:214–221.
- Centers for Disease Control and Prevention (CDC). Hand, Foot, and Mouth Disease: Prevention & Treatment. www.cdc.gov/hand-foot-mouth/about/prevention-treatment.html. Accessed June 10, 2014.
- Shelley WB, Hashim M, Shelley ED. Acyclovir in the treatment of hand-foot-and-mouth disease. Cutis 1996; 57:232–234.
- Centers for Disease Control and Prevention (CDC). Hand, Foot, and Mouth Disease (HFMD). www.cdc.gov/hand-foot-mouth/index.html. Accessed June 10, 2014.
- Chatproedprai S, Theanboonlers A, Korkong S, Thongmee C, Wananukul S, Poovorawan Y. Clinical and molecular characterization of hand-foot-and-mouth disease in Thailand, 2008–2009. Jpn J Infect Dis 2010; 63:229–233.
- Centers for Disease Control and Prevention (CDC). Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011–February 2012. MMWR Morb Mortal Wkly Rep 2012; 61:213–214.
- Ho M, Chen ER, Hsu KH, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 1999; 341:929–935.
- BBC News. China virus toll continues rise. May 5, 2008. http://news.bbc.co.uk/2/hi/asia-pacific/7383796.stm. Accessed February 5, 2014.
- Suhaimi ND. HFMD: 1,000 cases a week in Singapore is unusual, says doc. Straits Times April 20, 2008.
- Viet Nam News: HFMD cases prompt tighter health screening at airport. May 15, 2008.
- UBPOST. EV-71 virus continues dramatic rise. May 22, 2008.
- Begawan BS. 1,053 HFMD cases recorded. Brunei Times. November 7, 2008.
- Chinaview. Hand-foot-mouth disease death toll rises to 17 in East China’s Shandong Province. April 9, 2009.
- Chinaview. China reports 537 deaths from hand-foot-mouth disease this year. June 24, 2010.
- Wolfson H. Outbreak of hand, foot and mouth disease severe in Alabama. Birmingham News February 13, 2012.
- Centers for Disease Control and Prevention (CDC). Non-Polio Enterovirus Infections. www.cdc.gov/non-polio-enterovirus/. Accessed June 10, 2014.
- Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis 2003; 9:78–85.
- California Department of Public Health. Coxsackievirus A6 (CVA6). 2011. www.cdph.ca.gov/programs/cder/Pages/CVA6.aspx. Accessed June 10, 2014.
- World Health Organization: Western Pacific Region. A Guide to Clinical management and Public Health Response for Hand, Foot, and Mouth Disease (HFMD).
- Shin JU, Oh SH, Lee JH. A case of hand-foot-mouth disease in an immunocompetent adult. Ann Dermatol 2010; 22:216–218.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis 2011; 11:346.
- Osterback R, Vuorinen T, Linna M, Susi P, Hyypiä T, Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis 2009; 15:1485–1488.
- Tosti A, Piraccini BM. Nail Disorders. In:Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology JV. 3rded. Elsevier Limited; 2012:1129–1144.
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol 2013; 69:736–741.
- Mathes EF, Oza V, Frieden IJ, et al. ”Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics 2013; 132:e149–e157.
- Kaminska K, Martinetti G, Lucchini R, Kaya G, Mainetti C. Coxsackievirus A6 and hand, foot and mouth disease: three case reports of familial child-to-immunocompetent adult transmission and a literature review. Case Rep Dermatol 2013; 5:203–209.
- Romero JR. Diagnosis and management of enteroviral infections of the central nervous system. Curr Infect Dis Rep 2002; 4:309–316.
- Akiyama K, Imazeki R, Yoshii F, Koide T, Muto J. An adult case of hand, foot, and mouth disease caused by enterovirus 71 accompanied by opsoclonus myoclonica. Tokai J Exp Clin Med 2008; 33:143–145.
- Li Y, Zhu R, Qian Y, Deng J. The characteristics of blood glucose and WBC counts in peripheral blood of cases of hand foot and mouth disease in China: a systematic review. PLoS One 2012; 7:e29003.
- Chang LY, King CC, Hsu KH, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics 2002; 109:e88.
- Wang SM, Liu CC, Tseng HW, et al. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin Infect Dis 1999; 29:184–190.
- Chang LY, Lin TY, Hsu KH, et al. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 1999; 354:1682–1686.
- Mayo Clinic Laboratories. Enterovirus, Molecular Detection, PCR, Plasma. www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/89893. Accessed June 10, 2014.
- Yu N, Guo M, He SJ, et al. Evaluation of human enterovirus 71 and coxsackievirus A16 specific immunoglobulin M antibodies for diagnosis of hand-foot-and-mouth disease. Virol J 2012; 9:12.
- Wang C, You A, Tian X, et al. Analysis and solution of false-positives when testing CVA16 sera using an antibody assay against the EV71 virus. Virus Res 2013; 176:33–36.
- Liu MY, Liu W, Luo J, et al. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS One 2011; 6:e25287.
- Zhang J, Sun J, Chang Z, Zhang W, Wang Z, Feng Z. Characterization of hand, foot, and mouth disease in China between 2008 and 2009. Biomed Environ Sci 2011; 24:214–221.
- Centers for Disease Control and Prevention (CDC). Hand, Foot, and Mouth Disease: Prevention & Treatment. www.cdc.gov/hand-foot-mouth/about/prevention-treatment.html. Accessed June 10, 2014.
- Shelley WB, Hashim M, Shelley ED. Acyclovir in the treatment of hand-foot-and-mouth disease. Cutis 1996; 57:232–234.
KEY POINTS
- In Asian and Pacific nations, HFMD has been a significant public health concern since 1997, with recurrent epidemics and, in some cases, severe complications, including central nervous system disease, pulmonary edema, and death.
- Coxsackievirus A16 and enterovirus 71 are the most common agents of HFMD. In addition, coxsackievirus A6 seems to be emerging.
- Neurologic and cardiopulmonary involvement are more often associated with enterovirus 71 infection.
- In March 2012, 63 cases of severe HFMD were reported in Alabama, California, Connecticut, and Nevada. Fifteen of the patients were adults, and more than half had positive sick contacts. Of the 34 patients who underwent serologic testing, 25 were positive for coxsackievirus A6, an unusual pathogen for HFMD in the United States, associated with more severe skin findings.
- Treatment focuses on supportive care and prevention.