User login
Condition Help: A patient- and family-initiated rapid response system
In recent years, rapid response teams (RRTs) have been widely implemented to improve patient safety and quality of care. RRTs traditionally are activated by providers to address a clinically deteriorating patient; trained nurses, respiratory care specialists, and physicians are brought bedside to assist in triage and management. After the Joint Commission1 endorsed patient engagement as a strategy for enhancing patient safety, new initiatives were developed to meet the challenge. Programs designed to enhance patient engagement have taken a variety of forms, including educational campaigns encouraging patients to report adverse events, requests for handwashing by providers, and the institution of patient- and family-activated RRTs.2 Patient involvement is viewed favorably and has been shown to increase patients’ perception of health care quality.3 Although these initiatives are presumed helpful in encouraging communication, there is limited evidence that more communication leads to safety improvements. Despite the increasing prevalence of patient-activated RRTs in the United States, they have gone largely unevaluated in the adult population, and their efficacy remains unclear.
CONDITION HELP
Condition Help (CH) is a patient- and family-initiated RRT designed to prevent medical errors and communication problems and improve patient safety. Patients and families are encouraged to call the CH hotline if they believe that there has been a breakdown in care or that their health is in imminent danger. This RRT was inspired by the case of Josie King, an 18-month-old girl who died of preventable causes at a large children’s hospital.4 After her daughter’s death, Sorrel King started the Josie King Foundation, an organization committed to preventing medical errors and creating a culture of patient safety. With the support of this foundation, CH was launched in 2005 at the Children’s Hospital of Pittsburgh at the University of Pittsburgh Medical Center (UPMC). Later it was implemented at the UPMC adult tertiary-care center, and now it is available in all UPMC facilities.
On admission, patients receive a brochure that details the purpose of CH and provides examples of when and how to call the CH hotline. In this brochure, patients are instructed to call CH in 3 situations: “1) There is an emergency and you cannot get the attention of hospital staff, 2) You see a change in the patient’s condition and the health care team is not recognizing the concern, or 3) There is breakdown in how care is given or uncertainty over what needs to be done.” These instructions are printed on bulletins placed in elevators and hallways throughout the hospital. Patients and families may activate the system at any time and can even do so from home.
When a patient or family member calls the hotline, an operator notifies the CH team. This team, which consists of a patient care liaison (or an on-duty administrator) and the unit charge nurse, convenes bedside to address the patient’s concern. The team was designed without a physician to ensure that the primary team remains in charge of the care plan. CH is kept separate from our traditional RRT and does not compete for resources (personnel, equipment, time) with the RRT, which is designed to address a clinically deteriorating patient.
In this article, we describe the characteristics of patients for whom CH was activated at our adult hospital. We also describe reasons for calls, whether changes in care were implemented, and outcomes, including traditional RRT activation, transfer to intensive care unit (ICU), and inpatient mortality. As CH was designed with patient safety as a goal, we tracked 2 types of calls, those involving safety issues and those involving nonsafety issues.
METHODS
This study was approved by the quality improvement committee at the University of Pittsburgh and was considered exempt from review by the university’s Institutional Review Board.
Our integrated health system consists of more than 20 hospitals serving a tristate region. UPMC Presbyterian and UPMC Montefiore are adult tertiary-care referral hospitals with more than 750 medical/surgical beds and 150 critical care beds and more than 30,000 annual inpatient admissions. These hospitals are physically connected and function as a single large medical center. We reviewed all CH events that occurred at this combined hospital during the period January 2012 through June 2015. The dates coincided with CH data acquisition.
CH was available 24 hours a day 7 days a week. A patient care liaison (or an on-duty administrator) and the unit charge nurse responded to CH calls. Data from all calls included date and time of call, day of week, primary service, patient location, unique patient identifiers, call initiator (patient or family), whether a call led to changes in care, and primary reason for call. Each call reason was sorted into 1 of 10 categories: pain control, staff problem, lack of communication between patient/family and care team, questions about patient management, care delays, delays in a particular service, questions about discharge, administrative issues, acute psychiatric needs, and unknown/other. In addition, after a call, we reviewed all charts to determine if a safety issue was involved; Dr. Eden and Dr. Bump independently reviewed calls for safety issues and discussed any differences until they reached consensus. We also recorded outcomes, including activation of a traditional RRT or transfer to ICU within 24 hours of CH call, inpatient mortality, and against medical advice (AMA) discharges. Given that many calls were made by patients who called more than once (during a single admission or over multiple admissions), we also sorted patients into one-time callers and repeat callers for comparison. Patient satisfaction data were unavailable for review.
Patient demographic data are presented as means, standard deviations, and percentages, and call characteristics as percentages. Chi-square tests and t tests were used for analyses except for comparisons having few observations. For those, Fisher exact test was used. All analyses were performed with SAS Version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
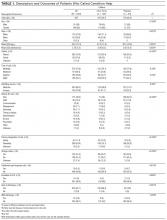
From January 2012 through June 2015, 367 CH calls were made, about 105 annually. During this period, there were about 33,000 admissions, 800 combined grievances and complaints, 170 AMA discharges, 155 cardiac arrests, 2300 traditional RRT activations, and 1200 inpatient deaths per year. The 367 CH calls were made by 240 patients (Table 1). Of these 240 patients, 43 (18%) activated the CH team with multiple calls; their calls accounted for (46.3%) of all calls (170/367). The majority of calls were made by patients (76.8%) rather than family members (21.8%). Mean (SD) patient age was 45.8 (17.4) years. Mean (SD) number of admissions per patient per year was 2.7 (3.5). More events were activated for patients admitted to medical services (66%) than surgical services (34%). Calls were evenly distributed between time of day and day of week.
The most common reason for CH calls was inadequate pain control (48.2%), followed by dissatisfaction with staff (12.5%); the remaining calls were evenly distributed among the other categories. The majority of calls involved nonsafety issues (83.4%) rather than safety issues (11.4%); in 5.2% of calls, the distinction could not be made because of lack of information (Table 2). In 152 (41.4%) of the 367 total calls, a change in care or alteration in management was made. Of these 152 calls, 99 (65.1%) involved distinct changes in the care plan, such as medication changes, imaging or additional testing, or consultation with other physicians; the other 53 calls (34.9%) involved additional patient counseling or nonmedical changes. Our traditional RRT was activated within 24 hours of CH in 19 cases (5.2%); of the 19 patients, 6 were transferred to ICU. Seven patients (2.9%) died during admission. Twelve (3.3%) were discharged AMA. We compared outcomes of patients who made safety-issue calls with those of patients who made nonsafety-issue calls. The composite outcome of RRT activation, ICU transfer, and mortality was found for 6 (14.3%) of the 42 safety-issue calls and 15 (4.9%) of the 306 nonsafety-issue calls (P = 0.0291).
The unexpected high rate of repeat calling prompted us to compare the characteristics of one-time and repeat callers. Repeat callers were younger: Mean age was 39.3 (12.8) years for repeat callers and 47.2 (17.9) years for one-time callers (P = 0.0012). Repeat callers had more admissions per year: Mean (SD) number of admissions was 5.67 (5.4) for repeat callers and 2.09 (2.5) for one-time callers (P = 0.0001). One-time and repeat callers did not differ with respect to race or sex. Compared with one-time callers, repeat callers were more often (P = 0.002) admitted to medical services (74.7%) than surgical services (58.9%). For repeat callers, a larger percentage of calls (P < 0.0001) were made by patients (93.5%) rather than families (62.4%). Calls about pain were more often (P < 0.0001) made by repeat callers (62.3%) than one-time callers (36%), calls involving safety issues were less often (P < 0.0001) made by repeat callers (5.9%) than one-time callers (16.2%), and changes in care were made less often (P < 0.0001) for repeat callers (32.9%) than one-time callers (48.7%). Between-group differences in rates of RRT activation, transfer to ICU, inpatient mortality, and AMA discharges were not significant.
DISCUSSION
Patient- and family-activated RRTs provide unique opportunities for patient and family engagement during inpatient hospital stays. Our study described the results obtained with use of a well-established patient-activated RRT over several years, one of the longer observation periods reported in the literature. We found that, with use of patient-activated RRTs, patient safety issues were identified, though these were far outnumbered by nonsafety issues.
Almost half of all CH events were related to pain. Pain as the primary driver for RRT activation may be attributable to several factors, including degree of illness, poor communication about pain management expectations, positive reinforcement of narcotic-seeking behavior as a result of CH activation, and high rate of opiate use in the catchment area. A striking finding of our analysis was repeat calling; only 43 (18%) of the 240 callers were repeat callers, but they made almost half of all the calls. In some cases, during a single admission, multiple calls were made because the first had no effect on care or management; more typically, though, multiple calls were made over several admissions. Repeat callers were admitted more often per year, and they used hospital services more. They should be further studied with a goal of designing programs that better meet their needs and that prospectively address expectations of pain control.
Our study was unique in describing several outcomes related to CH events. We found that traditional RRTs were seldom activated, level of care was seldom escalated, and mortality was rare, though these outcomes occurred more often for safety-issue calls than nonsafety-issue calls. We also found that activation of CH teams often led to changes in medical management, though we could not determine whether these changes in care led to different patient outcomes.
Patient-initiated RRTs are described in a limited number of pediatric and adult studies, all with findings differing from ours. In the pediatric models, most calls were initiated by family members, were less frequent, and tended to signal higher patient acuity.5,6 For example, in a pediatric RRT model,5 family members activated the RRT only twice within the study year, but both calls resulted in ICU transfer. Most descriptions of patient-activated RRTs in adult hospitals are from pilot studies, which similarly identified infrequent RRT calls but often did not identify call reasons or specific outcomes.7 A single-center study concluded that, after implementation of a mixed-model RRT8—a traditional practitioner-activated RRT later enhanced with a patient/family activation mechanism—non-ICU codes decreased, and there was a statistically significant drop in hospital-wide mortality rates. However, this RRT was patient-activated only 25 times over 2 years, and the specific outcomes of those events were not described.
Other initiatives have been designed to enhance patient care and communication. Purposeful rounding systems9 involve hourly rounding by bedside nurses and daily rounding by nurse leaders to improve timely patient care and provide proactive service. Such systems ideally preempt calls involving dissatisfaction and nonsafety issues. Although they would reduce the number of patient-dissatisfaction calls made in the CH system, they may not be any better than the CH system is in its main purpose, identifying safety issues. In addition, whether patient-activated RRTs or purposeful rounding systems are better at addressing patient dissatisfaction is unclear.
This study had its limitations. First, like other studies, it was a single-center observational study without a concurrent control group. Second, because CH was first implemented 10 years ago, we could not compare patient outcomes or traditional RRT use before and after program initiation. Third, our study cohort consisted of patients hospitalized at one academic tertiary-care center in one region, and the hospital is a training site for multiple residencies and fellowships. These factors likely affect the generalizability of our data to smaller or community-based centers. Fourth, some determinations were subjective (eg, whether calls involved safety or nonsafety issues). We tried to minimize bias by having 2 authors independently review cases, but the process did not reflect patient experience or perspective. Fifth, our hospital adopted its traditional RRT years before its CH system. The criteria used by hospital personnel for traditional RRT activation are designed to encourage staff to call for help at early signs of patient deterioration. Consequently, traditional RRT activations substantially outnumber CH calls. Whether this resulted in fewer CH safety calls is unclear. Sixth, we did not capture the financial implications of using CH teams.
Although patient-activated RRTs identified patient safety issues, questions about the utility or necessity of these RRTs remain. In our era of limited hospital resources, the case has not been definitively made that these teams are practical, based on patient outcomes, though other studies have found improved patient satisfaction.7 Most of the RRT calls in our study involved patient dissatisfaction and communication issues. CH may not be the ideal approach for managing these issues, but it represents the last line of patient advocacy once other systems have failed.
We think patient-activated RRTs have the potential to effect patient engagement in safe care. Given the importance of establishing a culture of patient safety and engagement, and increased detection of safety-related events, CH remains active throughout our hospital system. Newer iterations of CH may benefit from stricter language in defining appropriate occasions for calling RRTs, and from descriptions of other resources for patient advocacy within the hospital. These modifications could end up restricting RRT activations to patient complaints and preserving CH resources for patients with safety concerns. Our study lays the groundwork for other institutions that are considering similar interventions. Studies should now start evaluating how well patient- and family-activated RRTs improve patient satisfaction, staff satisfaction, and patient outcomes.
CONCLUSION
Patient- and family-activated RRTs were designed to engage patients and families in safe care. Although CH detects patient safety issues, these are far outnumbered by nonsafety issues. CH demonstrates a commitment to patient engagement and a culture that emphasizes patient safety.
Acknowledgements
This work was presented as a poster at the annual meeting of the Society of Hospital Medicine; March 6-9, 2016; San Diego, CA.
Disclosure
Nothing to report.
1. Joint Commission. Improving America’s Hospitals: The Joint Commission’s Annual Report on Quality and Safety 2008. http://www.jointcommission.org/assets/1/6/2008_Annual_Report.pdf. Published November 2008. Accessed May 4, 2016. PubMed
2. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
3. Weingart SN, Zhu J, Chiappetta L, et al. Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care. 2011;23(3):269-277. PubMed
4. Kennedy P, Pronovost P. Shepherding change: how the market, healthcare providers, and public policy can deliver quality care for the 21st century. Crit Care Med. 2006;34(3 suppl):S1-S6. PubMed
5. Ray EM, Smith R, Massie S, et al. Family alert: implementing direct family activation of a pediatric response team. Jt Comm J Qual Patient Saf. 2009;35(11):575-580. PubMed
6. Dean BS, Decker MJ, Hupp D, Urbach AH, Lewis E, Benes-Stickle J. Condition Help: a pediatric rapid response team triggered by patients and parents. J Healthc Qual. 2008;30(3):28-31. PubMed
7. Vorwerk J, King L. Consumer participation in early detection of the deteriorating patient and call activation to rapid response systems: a literature review. J Clin Nurs. 2015;25(1-2):38-52. PubMed
8. Gerdik C, Vallish RO, Miles K, Godwin SA, Wludyka PS, Panni MK. Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2010;81(12):1676-1681. PubMed
9. Hancock KK. From the bedside: purposeful rounding essential to patient experience. Association for Patient Experience website. http://www.patient-experience.org/Resources/Newsletter/Newsletters/Articles/2014/From-the-Bedside-Purposeful-Rounding-Essential-to.aspx. Published February 27, 2014. Accessed July 25, 2016.
In recent years, rapid response teams (RRTs) have been widely implemented to improve patient safety and quality of care. RRTs traditionally are activated by providers to address a clinically deteriorating patient; trained nurses, respiratory care specialists, and physicians are brought bedside to assist in triage and management. After the Joint Commission1 endorsed patient engagement as a strategy for enhancing patient safety, new initiatives were developed to meet the challenge. Programs designed to enhance patient engagement have taken a variety of forms, including educational campaigns encouraging patients to report adverse events, requests for handwashing by providers, and the institution of patient- and family-activated RRTs.2 Patient involvement is viewed favorably and has been shown to increase patients’ perception of health care quality.3 Although these initiatives are presumed helpful in encouraging communication, there is limited evidence that more communication leads to safety improvements. Despite the increasing prevalence of patient-activated RRTs in the United States, they have gone largely unevaluated in the adult population, and their efficacy remains unclear.
CONDITION HELP
Condition Help (CH) is a patient- and family-initiated RRT designed to prevent medical errors and communication problems and improve patient safety. Patients and families are encouraged to call the CH hotline if they believe that there has been a breakdown in care or that their health is in imminent danger. This RRT was inspired by the case of Josie King, an 18-month-old girl who died of preventable causes at a large children’s hospital.4 After her daughter’s death, Sorrel King started the Josie King Foundation, an organization committed to preventing medical errors and creating a culture of patient safety. With the support of this foundation, CH was launched in 2005 at the Children’s Hospital of Pittsburgh at the University of Pittsburgh Medical Center (UPMC). Later it was implemented at the UPMC adult tertiary-care center, and now it is available in all UPMC facilities.
On admission, patients receive a brochure that details the purpose of CH and provides examples of when and how to call the CH hotline. In this brochure, patients are instructed to call CH in 3 situations: “1) There is an emergency and you cannot get the attention of hospital staff, 2) You see a change in the patient’s condition and the health care team is not recognizing the concern, or 3) There is breakdown in how care is given or uncertainty over what needs to be done.” These instructions are printed on bulletins placed in elevators and hallways throughout the hospital. Patients and families may activate the system at any time and can even do so from home.
When a patient or family member calls the hotline, an operator notifies the CH team. This team, which consists of a patient care liaison (or an on-duty administrator) and the unit charge nurse, convenes bedside to address the patient’s concern. The team was designed without a physician to ensure that the primary team remains in charge of the care plan. CH is kept separate from our traditional RRT and does not compete for resources (personnel, equipment, time) with the RRT, which is designed to address a clinically deteriorating patient.
In this article, we describe the characteristics of patients for whom CH was activated at our adult hospital. We also describe reasons for calls, whether changes in care were implemented, and outcomes, including traditional RRT activation, transfer to intensive care unit (ICU), and inpatient mortality. As CH was designed with patient safety as a goal, we tracked 2 types of calls, those involving safety issues and those involving nonsafety issues.
METHODS
This study was approved by the quality improvement committee at the University of Pittsburgh and was considered exempt from review by the university’s Institutional Review Board.
Our integrated health system consists of more than 20 hospitals serving a tristate region. UPMC Presbyterian and UPMC Montefiore are adult tertiary-care referral hospitals with more than 750 medical/surgical beds and 150 critical care beds and more than 30,000 annual inpatient admissions. These hospitals are physically connected and function as a single large medical center. We reviewed all CH events that occurred at this combined hospital during the period January 2012 through June 2015. The dates coincided with CH data acquisition.
CH was available 24 hours a day 7 days a week. A patient care liaison (or an on-duty administrator) and the unit charge nurse responded to CH calls. Data from all calls included date and time of call, day of week, primary service, patient location, unique patient identifiers, call initiator (patient or family), whether a call led to changes in care, and primary reason for call. Each call reason was sorted into 1 of 10 categories: pain control, staff problem, lack of communication between patient/family and care team, questions about patient management, care delays, delays in a particular service, questions about discharge, administrative issues, acute psychiatric needs, and unknown/other. In addition, after a call, we reviewed all charts to determine if a safety issue was involved; Dr. Eden and Dr. Bump independently reviewed calls for safety issues and discussed any differences until they reached consensus. We also recorded outcomes, including activation of a traditional RRT or transfer to ICU within 24 hours of CH call, inpatient mortality, and against medical advice (AMA) discharges. Given that many calls were made by patients who called more than once (during a single admission or over multiple admissions), we also sorted patients into one-time callers and repeat callers for comparison. Patient satisfaction data were unavailable for review.
Patient demographic data are presented as means, standard deviations, and percentages, and call characteristics as percentages. Chi-square tests and t tests were used for analyses except for comparisons having few observations. For those, Fisher exact test was used. All analyses were performed with SAS Version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
From January 2012 through June 2015, 367 CH calls were made, about 105 annually. During this period, there were about 33,000 admissions, 800 combined grievances and complaints, 170 AMA discharges, 155 cardiac arrests, 2300 traditional RRT activations, and 1200 inpatient deaths per year. The 367 CH calls were made by 240 patients (Table 1). Of these 240 patients, 43 (18%) activated the CH team with multiple calls; their calls accounted for (46.3%) of all calls (170/367). The majority of calls were made by patients (76.8%) rather than family members (21.8%). Mean (SD) patient age was 45.8 (17.4) years. Mean (SD) number of admissions per patient per year was 2.7 (3.5). More events were activated for patients admitted to medical services (66%) than surgical services (34%). Calls were evenly distributed between time of day and day of week.
The most common reason for CH calls was inadequate pain control (48.2%), followed by dissatisfaction with staff (12.5%); the remaining calls were evenly distributed among the other categories. The majority of calls involved nonsafety issues (83.4%) rather than safety issues (11.4%); in 5.2% of calls, the distinction could not be made because of lack of information (Table 2). In 152 (41.4%) of the 367 total calls, a change in care or alteration in management was made. Of these 152 calls, 99 (65.1%) involved distinct changes in the care plan, such as medication changes, imaging or additional testing, or consultation with other physicians; the other 53 calls (34.9%) involved additional patient counseling or nonmedical changes. Our traditional RRT was activated within 24 hours of CH in 19 cases (5.2%); of the 19 patients, 6 were transferred to ICU. Seven patients (2.9%) died during admission. Twelve (3.3%) were discharged AMA. We compared outcomes of patients who made safety-issue calls with those of patients who made nonsafety-issue calls. The composite outcome of RRT activation, ICU transfer, and mortality was found for 6 (14.3%) of the 42 safety-issue calls and 15 (4.9%) of the 306 nonsafety-issue calls (P = 0.0291).
The unexpected high rate of repeat calling prompted us to compare the characteristics of one-time and repeat callers. Repeat callers were younger: Mean age was 39.3 (12.8) years for repeat callers and 47.2 (17.9) years for one-time callers (P = 0.0012). Repeat callers had more admissions per year: Mean (SD) number of admissions was 5.67 (5.4) for repeat callers and 2.09 (2.5) for one-time callers (P = 0.0001). One-time and repeat callers did not differ with respect to race or sex. Compared with one-time callers, repeat callers were more often (P = 0.002) admitted to medical services (74.7%) than surgical services (58.9%). For repeat callers, a larger percentage of calls (P < 0.0001) were made by patients (93.5%) rather than families (62.4%). Calls about pain were more often (P < 0.0001) made by repeat callers (62.3%) than one-time callers (36%), calls involving safety issues were less often (P < 0.0001) made by repeat callers (5.9%) than one-time callers (16.2%), and changes in care were made less often (P < 0.0001) for repeat callers (32.9%) than one-time callers (48.7%). Between-group differences in rates of RRT activation, transfer to ICU, inpatient mortality, and AMA discharges were not significant.
DISCUSSION
Patient- and family-activated RRTs provide unique opportunities for patient and family engagement during inpatient hospital stays. Our study described the results obtained with use of a well-established patient-activated RRT over several years, one of the longer observation periods reported in the literature. We found that, with use of patient-activated RRTs, patient safety issues were identified, though these were far outnumbered by nonsafety issues.
Almost half of all CH events were related to pain. Pain as the primary driver for RRT activation may be attributable to several factors, including degree of illness, poor communication about pain management expectations, positive reinforcement of narcotic-seeking behavior as a result of CH activation, and high rate of opiate use in the catchment area. A striking finding of our analysis was repeat calling; only 43 (18%) of the 240 callers were repeat callers, but they made almost half of all the calls. In some cases, during a single admission, multiple calls were made because the first had no effect on care or management; more typically, though, multiple calls were made over several admissions. Repeat callers were admitted more often per year, and they used hospital services more. They should be further studied with a goal of designing programs that better meet their needs and that prospectively address expectations of pain control.
Our study was unique in describing several outcomes related to CH events. We found that traditional RRTs were seldom activated, level of care was seldom escalated, and mortality was rare, though these outcomes occurred more often for safety-issue calls than nonsafety-issue calls. We also found that activation of CH teams often led to changes in medical management, though we could not determine whether these changes in care led to different patient outcomes.
Patient-initiated RRTs are described in a limited number of pediatric and adult studies, all with findings differing from ours. In the pediatric models, most calls were initiated by family members, were less frequent, and tended to signal higher patient acuity.5,6 For example, in a pediatric RRT model,5 family members activated the RRT only twice within the study year, but both calls resulted in ICU transfer. Most descriptions of patient-activated RRTs in adult hospitals are from pilot studies, which similarly identified infrequent RRT calls but often did not identify call reasons or specific outcomes.7 A single-center study concluded that, after implementation of a mixed-model RRT8—a traditional practitioner-activated RRT later enhanced with a patient/family activation mechanism—non-ICU codes decreased, and there was a statistically significant drop in hospital-wide mortality rates. However, this RRT was patient-activated only 25 times over 2 years, and the specific outcomes of those events were not described.
Other initiatives have been designed to enhance patient care and communication. Purposeful rounding systems9 involve hourly rounding by bedside nurses and daily rounding by nurse leaders to improve timely patient care and provide proactive service. Such systems ideally preempt calls involving dissatisfaction and nonsafety issues. Although they would reduce the number of patient-dissatisfaction calls made in the CH system, they may not be any better than the CH system is in its main purpose, identifying safety issues. In addition, whether patient-activated RRTs or purposeful rounding systems are better at addressing patient dissatisfaction is unclear.
This study had its limitations. First, like other studies, it was a single-center observational study without a concurrent control group. Second, because CH was first implemented 10 years ago, we could not compare patient outcomes or traditional RRT use before and after program initiation. Third, our study cohort consisted of patients hospitalized at one academic tertiary-care center in one region, and the hospital is a training site for multiple residencies and fellowships. These factors likely affect the generalizability of our data to smaller or community-based centers. Fourth, some determinations were subjective (eg, whether calls involved safety or nonsafety issues). We tried to minimize bias by having 2 authors independently review cases, but the process did not reflect patient experience or perspective. Fifth, our hospital adopted its traditional RRT years before its CH system. The criteria used by hospital personnel for traditional RRT activation are designed to encourage staff to call for help at early signs of patient deterioration. Consequently, traditional RRT activations substantially outnumber CH calls. Whether this resulted in fewer CH safety calls is unclear. Sixth, we did not capture the financial implications of using CH teams.
Although patient-activated RRTs identified patient safety issues, questions about the utility or necessity of these RRTs remain. In our era of limited hospital resources, the case has not been definitively made that these teams are practical, based on patient outcomes, though other studies have found improved patient satisfaction.7 Most of the RRT calls in our study involved patient dissatisfaction and communication issues. CH may not be the ideal approach for managing these issues, but it represents the last line of patient advocacy once other systems have failed.
We think patient-activated RRTs have the potential to effect patient engagement in safe care. Given the importance of establishing a culture of patient safety and engagement, and increased detection of safety-related events, CH remains active throughout our hospital system. Newer iterations of CH may benefit from stricter language in defining appropriate occasions for calling RRTs, and from descriptions of other resources for patient advocacy within the hospital. These modifications could end up restricting RRT activations to patient complaints and preserving CH resources for patients with safety concerns. Our study lays the groundwork for other institutions that are considering similar interventions. Studies should now start evaluating how well patient- and family-activated RRTs improve patient satisfaction, staff satisfaction, and patient outcomes.
CONCLUSION
Patient- and family-activated RRTs were designed to engage patients and families in safe care. Although CH detects patient safety issues, these are far outnumbered by nonsafety issues. CH demonstrates a commitment to patient engagement and a culture that emphasizes patient safety.
Acknowledgements
This work was presented as a poster at the annual meeting of the Society of Hospital Medicine; March 6-9, 2016; San Diego, CA.
Disclosure
Nothing to report.
In recent years, rapid response teams (RRTs) have been widely implemented to improve patient safety and quality of care. RRTs traditionally are activated by providers to address a clinically deteriorating patient; trained nurses, respiratory care specialists, and physicians are brought bedside to assist in triage and management. After the Joint Commission1 endorsed patient engagement as a strategy for enhancing patient safety, new initiatives were developed to meet the challenge. Programs designed to enhance patient engagement have taken a variety of forms, including educational campaigns encouraging patients to report adverse events, requests for handwashing by providers, and the institution of patient- and family-activated RRTs.2 Patient involvement is viewed favorably and has been shown to increase patients’ perception of health care quality.3 Although these initiatives are presumed helpful in encouraging communication, there is limited evidence that more communication leads to safety improvements. Despite the increasing prevalence of patient-activated RRTs in the United States, they have gone largely unevaluated in the adult population, and their efficacy remains unclear.
CONDITION HELP
Condition Help (CH) is a patient- and family-initiated RRT designed to prevent medical errors and communication problems and improve patient safety. Patients and families are encouraged to call the CH hotline if they believe that there has been a breakdown in care or that their health is in imminent danger. This RRT was inspired by the case of Josie King, an 18-month-old girl who died of preventable causes at a large children’s hospital.4 After her daughter’s death, Sorrel King started the Josie King Foundation, an organization committed to preventing medical errors and creating a culture of patient safety. With the support of this foundation, CH was launched in 2005 at the Children’s Hospital of Pittsburgh at the University of Pittsburgh Medical Center (UPMC). Later it was implemented at the UPMC adult tertiary-care center, and now it is available in all UPMC facilities.
On admission, patients receive a brochure that details the purpose of CH and provides examples of when and how to call the CH hotline. In this brochure, patients are instructed to call CH in 3 situations: “1) There is an emergency and you cannot get the attention of hospital staff, 2) You see a change in the patient’s condition and the health care team is not recognizing the concern, or 3) There is breakdown in how care is given or uncertainty over what needs to be done.” These instructions are printed on bulletins placed in elevators and hallways throughout the hospital. Patients and families may activate the system at any time and can even do so from home.
When a patient or family member calls the hotline, an operator notifies the CH team. This team, which consists of a patient care liaison (or an on-duty administrator) and the unit charge nurse, convenes bedside to address the patient’s concern. The team was designed without a physician to ensure that the primary team remains in charge of the care plan. CH is kept separate from our traditional RRT and does not compete for resources (personnel, equipment, time) with the RRT, which is designed to address a clinically deteriorating patient.
In this article, we describe the characteristics of patients for whom CH was activated at our adult hospital. We also describe reasons for calls, whether changes in care were implemented, and outcomes, including traditional RRT activation, transfer to intensive care unit (ICU), and inpatient mortality. As CH was designed with patient safety as a goal, we tracked 2 types of calls, those involving safety issues and those involving nonsafety issues.
METHODS
This study was approved by the quality improvement committee at the University of Pittsburgh and was considered exempt from review by the university’s Institutional Review Board.
Our integrated health system consists of more than 20 hospitals serving a tristate region. UPMC Presbyterian and UPMC Montefiore are adult tertiary-care referral hospitals with more than 750 medical/surgical beds and 150 critical care beds and more than 30,000 annual inpatient admissions. These hospitals are physically connected and function as a single large medical center. We reviewed all CH events that occurred at this combined hospital during the period January 2012 through June 2015. The dates coincided with CH data acquisition.
CH was available 24 hours a day 7 days a week. A patient care liaison (or an on-duty administrator) and the unit charge nurse responded to CH calls. Data from all calls included date and time of call, day of week, primary service, patient location, unique patient identifiers, call initiator (patient or family), whether a call led to changes in care, and primary reason for call. Each call reason was sorted into 1 of 10 categories: pain control, staff problem, lack of communication between patient/family and care team, questions about patient management, care delays, delays in a particular service, questions about discharge, administrative issues, acute psychiatric needs, and unknown/other. In addition, after a call, we reviewed all charts to determine if a safety issue was involved; Dr. Eden and Dr. Bump independently reviewed calls for safety issues and discussed any differences until they reached consensus. We also recorded outcomes, including activation of a traditional RRT or transfer to ICU within 24 hours of CH call, inpatient mortality, and against medical advice (AMA) discharges. Given that many calls were made by patients who called more than once (during a single admission or over multiple admissions), we also sorted patients into one-time callers and repeat callers for comparison. Patient satisfaction data were unavailable for review.
Patient demographic data are presented as means, standard deviations, and percentages, and call characteristics as percentages. Chi-square tests and t tests were used for analyses except for comparisons having few observations. For those, Fisher exact test was used. All analyses were performed with SAS Version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
From January 2012 through June 2015, 367 CH calls were made, about 105 annually. During this period, there were about 33,000 admissions, 800 combined grievances and complaints, 170 AMA discharges, 155 cardiac arrests, 2300 traditional RRT activations, and 1200 inpatient deaths per year. The 367 CH calls were made by 240 patients (Table 1). Of these 240 patients, 43 (18%) activated the CH team with multiple calls; their calls accounted for (46.3%) of all calls (170/367). The majority of calls were made by patients (76.8%) rather than family members (21.8%). Mean (SD) patient age was 45.8 (17.4) years. Mean (SD) number of admissions per patient per year was 2.7 (3.5). More events were activated for patients admitted to medical services (66%) than surgical services (34%). Calls were evenly distributed between time of day and day of week.
The most common reason for CH calls was inadequate pain control (48.2%), followed by dissatisfaction with staff (12.5%); the remaining calls were evenly distributed among the other categories. The majority of calls involved nonsafety issues (83.4%) rather than safety issues (11.4%); in 5.2% of calls, the distinction could not be made because of lack of information (Table 2). In 152 (41.4%) of the 367 total calls, a change in care or alteration in management was made. Of these 152 calls, 99 (65.1%) involved distinct changes in the care plan, such as medication changes, imaging or additional testing, or consultation with other physicians; the other 53 calls (34.9%) involved additional patient counseling or nonmedical changes. Our traditional RRT was activated within 24 hours of CH in 19 cases (5.2%); of the 19 patients, 6 were transferred to ICU. Seven patients (2.9%) died during admission. Twelve (3.3%) were discharged AMA. We compared outcomes of patients who made safety-issue calls with those of patients who made nonsafety-issue calls. The composite outcome of RRT activation, ICU transfer, and mortality was found for 6 (14.3%) of the 42 safety-issue calls and 15 (4.9%) of the 306 nonsafety-issue calls (P = 0.0291).
The unexpected high rate of repeat calling prompted us to compare the characteristics of one-time and repeat callers. Repeat callers were younger: Mean age was 39.3 (12.8) years for repeat callers and 47.2 (17.9) years for one-time callers (P = 0.0012). Repeat callers had more admissions per year: Mean (SD) number of admissions was 5.67 (5.4) for repeat callers and 2.09 (2.5) for one-time callers (P = 0.0001). One-time and repeat callers did not differ with respect to race or sex. Compared with one-time callers, repeat callers were more often (P = 0.002) admitted to medical services (74.7%) than surgical services (58.9%). For repeat callers, a larger percentage of calls (P < 0.0001) were made by patients (93.5%) rather than families (62.4%). Calls about pain were more often (P < 0.0001) made by repeat callers (62.3%) than one-time callers (36%), calls involving safety issues were less often (P < 0.0001) made by repeat callers (5.9%) than one-time callers (16.2%), and changes in care were made less often (P < 0.0001) for repeat callers (32.9%) than one-time callers (48.7%). Between-group differences in rates of RRT activation, transfer to ICU, inpatient mortality, and AMA discharges were not significant.
DISCUSSION
Patient- and family-activated RRTs provide unique opportunities for patient and family engagement during inpatient hospital stays. Our study described the results obtained with use of a well-established patient-activated RRT over several years, one of the longer observation periods reported in the literature. We found that, with use of patient-activated RRTs, patient safety issues were identified, though these were far outnumbered by nonsafety issues.
Almost half of all CH events were related to pain. Pain as the primary driver for RRT activation may be attributable to several factors, including degree of illness, poor communication about pain management expectations, positive reinforcement of narcotic-seeking behavior as a result of CH activation, and high rate of opiate use in the catchment area. A striking finding of our analysis was repeat calling; only 43 (18%) of the 240 callers were repeat callers, but they made almost half of all the calls. In some cases, during a single admission, multiple calls were made because the first had no effect on care or management; more typically, though, multiple calls were made over several admissions. Repeat callers were admitted more often per year, and they used hospital services more. They should be further studied with a goal of designing programs that better meet their needs and that prospectively address expectations of pain control.
Our study was unique in describing several outcomes related to CH events. We found that traditional RRTs were seldom activated, level of care was seldom escalated, and mortality was rare, though these outcomes occurred more often for safety-issue calls than nonsafety-issue calls. We also found that activation of CH teams often led to changes in medical management, though we could not determine whether these changes in care led to different patient outcomes.
Patient-initiated RRTs are described in a limited number of pediatric and adult studies, all with findings differing from ours. In the pediatric models, most calls were initiated by family members, were less frequent, and tended to signal higher patient acuity.5,6 For example, in a pediatric RRT model,5 family members activated the RRT only twice within the study year, but both calls resulted in ICU transfer. Most descriptions of patient-activated RRTs in adult hospitals are from pilot studies, which similarly identified infrequent RRT calls but often did not identify call reasons or specific outcomes.7 A single-center study concluded that, after implementation of a mixed-model RRT8—a traditional practitioner-activated RRT later enhanced with a patient/family activation mechanism—non-ICU codes decreased, and there was a statistically significant drop in hospital-wide mortality rates. However, this RRT was patient-activated only 25 times over 2 years, and the specific outcomes of those events were not described.
Other initiatives have been designed to enhance patient care and communication. Purposeful rounding systems9 involve hourly rounding by bedside nurses and daily rounding by nurse leaders to improve timely patient care and provide proactive service. Such systems ideally preempt calls involving dissatisfaction and nonsafety issues. Although they would reduce the number of patient-dissatisfaction calls made in the CH system, they may not be any better than the CH system is in its main purpose, identifying safety issues. In addition, whether patient-activated RRTs or purposeful rounding systems are better at addressing patient dissatisfaction is unclear.
This study had its limitations. First, like other studies, it was a single-center observational study without a concurrent control group. Second, because CH was first implemented 10 years ago, we could not compare patient outcomes or traditional RRT use before and after program initiation. Third, our study cohort consisted of patients hospitalized at one academic tertiary-care center in one region, and the hospital is a training site for multiple residencies and fellowships. These factors likely affect the generalizability of our data to smaller or community-based centers. Fourth, some determinations were subjective (eg, whether calls involved safety or nonsafety issues). We tried to minimize bias by having 2 authors independently review cases, but the process did not reflect patient experience or perspective. Fifth, our hospital adopted its traditional RRT years before its CH system. The criteria used by hospital personnel for traditional RRT activation are designed to encourage staff to call for help at early signs of patient deterioration. Consequently, traditional RRT activations substantially outnumber CH calls. Whether this resulted in fewer CH safety calls is unclear. Sixth, we did not capture the financial implications of using CH teams.
Although patient-activated RRTs identified patient safety issues, questions about the utility or necessity of these RRTs remain. In our era of limited hospital resources, the case has not been definitively made that these teams are practical, based on patient outcomes, though other studies have found improved patient satisfaction.7 Most of the RRT calls in our study involved patient dissatisfaction and communication issues. CH may not be the ideal approach for managing these issues, but it represents the last line of patient advocacy once other systems have failed.
We think patient-activated RRTs have the potential to effect patient engagement in safe care. Given the importance of establishing a culture of patient safety and engagement, and increased detection of safety-related events, CH remains active throughout our hospital system. Newer iterations of CH may benefit from stricter language in defining appropriate occasions for calling RRTs, and from descriptions of other resources for patient advocacy within the hospital. These modifications could end up restricting RRT activations to patient complaints and preserving CH resources for patients with safety concerns. Our study lays the groundwork for other institutions that are considering similar interventions. Studies should now start evaluating how well patient- and family-activated RRTs improve patient satisfaction, staff satisfaction, and patient outcomes.
CONCLUSION
Patient- and family-activated RRTs were designed to engage patients and families in safe care. Although CH detects patient safety issues, these are far outnumbered by nonsafety issues. CH demonstrates a commitment to patient engagement and a culture that emphasizes patient safety.
Acknowledgements
This work was presented as a poster at the annual meeting of the Society of Hospital Medicine; March 6-9, 2016; San Diego, CA.
Disclosure
Nothing to report.
1. Joint Commission. Improving America’s Hospitals: The Joint Commission’s Annual Report on Quality and Safety 2008. http://www.jointcommission.org/assets/1/6/2008_Annual_Report.pdf. Published November 2008. Accessed May 4, 2016. PubMed
2. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
3. Weingart SN, Zhu J, Chiappetta L, et al. Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care. 2011;23(3):269-277. PubMed
4. Kennedy P, Pronovost P. Shepherding change: how the market, healthcare providers, and public policy can deliver quality care for the 21st century. Crit Care Med. 2006;34(3 suppl):S1-S6. PubMed
5. Ray EM, Smith R, Massie S, et al. Family alert: implementing direct family activation of a pediatric response team. Jt Comm J Qual Patient Saf. 2009;35(11):575-580. PubMed
6. Dean BS, Decker MJ, Hupp D, Urbach AH, Lewis E, Benes-Stickle J. Condition Help: a pediatric rapid response team triggered by patients and parents. J Healthc Qual. 2008;30(3):28-31. PubMed
7. Vorwerk J, King L. Consumer participation in early detection of the deteriorating patient and call activation to rapid response systems: a literature review. J Clin Nurs. 2015;25(1-2):38-52. PubMed
8. Gerdik C, Vallish RO, Miles K, Godwin SA, Wludyka PS, Panni MK. Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2010;81(12):1676-1681. PubMed
9. Hancock KK. From the bedside: purposeful rounding essential to patient experience. Association for Patient Experience website. http://www.patient-experience.org/Resources/Newsletter/Newsletters/Articles/2014/From-the-Bedside-Purposeful-Rounding-Essential-to.aspx. Published February 27, 2014. Accessed July 25, 2016.
1. Joint Commission. Improving America’s Hospitals: The Joint Commission’s Annual Report on Quality and Safety 2008. http://www.jointcommission.org/assets/1/6/2008_Annual_Report.pdf. Published November 2008. Accessed May 4, 2016. PubMed
2. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
3. Weingart SN, Zhu J, Chiappetta L, et al. Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care. 2011;23(3):269-277. PubMed
4. Kennedy P, Pronovost P. Shepherding change: how the market, healthcare providers, and public policy can deliver quality care for the 21st century. Crit Care Med. 2006;34(3 suppl):S1-S6. PubMed
5. Ray EM, Smith R, Massie S, et al. Family alert: implementing direct family activation of a pediatric response team. Jt Comm J Qual Patient Saf. 2009;35(11):575-580. PubMed
6. Dean BS, Decker MJ, Hupp D, Urbach AH, Lewis E, Benes-Stickle J. Condition Help: a pediatric rapid response team triggered by patients and parents. J Healthc Qual. 2008;30(3):28-31. PubMed
7. Vorwerk J, King L. Consumer participation in early detection of the deteriorating patient and call activation to rapid response systems: a literature review. J Clin Nurs. 2015;25(1-2):38-52. PubMed
8. Gerdik C, Vallish RO, Miles K, Godwin SA, Wludyka PS, Panni MK. Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2010;81(12):1676-1681. PubMed
9. Hancock KK. From the bedside: purposeful rounding essential to patient experience. Association for Patient Experience website. http://www.patient-experience.org/Resources/Newsletter/Newsletters/Articles/2014/From-the-Bedside-Purposeful-Rounding-Essential-to.aspx. Published February 27, 2014. Accessed July 25, 2016.
© 2017 Society of Hospital Medicine
Taking the Detour
A 60‐year‐old woman presented to a community hospital's emergency department with 4 days of right‐sided abdominal pain and multiple episodes of black stools. She reported nausea without vomiting. She denied light‐headedness, chest pain, or shortness of breath. She also denied difficulty in swallowing, weight loss, jaundice, or other bleeding.
The first priority when assessing a patient with gastrointestinal (GI) bleeding is to ensure hemodynamic stability. Next, it is important to carefully characterize the stools to help narrow the differential diagnosis. As blood is a cathartic, frequent, loose, and black stools suggest vigorous bleeding. It is essential to establish that the stools are actually black, as some patients will mistake dark brown stools for melena. Using a visual aid like a black pen or shoes as a point of reference can help the patient differentiate between dark stool and melena. It is also important to obtain a thorough medication history because iron supplements or bismuth‐containing remedies can turn stool black. The use of any antiplatelet agents or anticoagulants should also be noted. The right‐sided abdominal pain should be characterized by establishing the frequency, severity, and association with eating, movement, and position. For this patient's presentation, increased pain with eating would rapidly heighten concern for mesenteric ischemia.
The patient reported having 1 to 2 semiformed, tarry, black bowel movements per day. The night prior to admission she had passed some bright red blood along with the melena. The abdominal pain had increased gradually over 4 days, was dull, constant, did not radiate, and there were no evident aggravating or relieving factors. She rated the pain as 4 out of 10 in intensity, worst in her right upper quadrant.
Her past medical history was notable for recurrent deep venous thromboses and pulmonary emboli that had occurred even while on oral anticoagulation. Inferior vena cava (IVC) filters had twice been placed many years prior; anticoagulation had been subsequently discontinued. Additionally, she was known to have chronic superior vena cava (SVC) occlusion, presumably related to hypercoagulability. Previous evaluation had identified only hyperhomocysteinemia as a risk factor for recurrent thromboses. Other medical problems included hemorrhoids, gastroesophageal reflux disease, and asthma. Her only surgical history was an abdominal hysterectomy and bilateral oophorectomy many years ago for nonmalignant disease. Home medications were omeprazole, ranitidine, albuterol, and fluticasone‐salmeterol. She denied using nonsteroidal anti‐inflammatory drugs, aspirin, or any dietary supplements. She denied smoking, alcohol, or recreational drug use.
Because melena is confirmed, an upper GI tract bleeding source is most likely. The more recent appearance of bright red blood is concerning for acceleration of bleeding, or may point to a distal small bowel or right colonic source. Given the history of thromboembolic disease and likely underlying hypercoagulability, vascular occlusion is a leading possibility. Thus, mesenteric arterial insufficiency or mesenteric venous thrombosis should be considered, even though the patient does not report the characteristic postprandial exacerbation of pain. Ischemic colitis due to arterial insufficiency typically presents with severe, acute pain, with or without hematochezia. This syndrome is typically manifested in vascular watershed areas such as the splenic flexure, but can also affect the right colon. Mesenteric venous thrombosis is a rare condition that most often occurs in patients with hypercoagulability. Patients present with variable degrees of abdominal pain and often with GI bleeding. Finally, portal venous thrombosis may be seen alongside thromboses of other mesenteric veins or may occur independently. Portal hypertension due to portal vein thrombosis can result in esophageal and/or gastric varices. Although variceal bleeding classically presents with dramatic hematemesis, the absence of hematemesis does not rule out a variceal bleed in this patient.
On physical examination, the patient had a temperature of 37.1C with a pulse of 90 beats per minute and blood pressure of 161/97 mm Hg. Orthostatics were not performed. No blood was seen on nasal and oropharyngeal exam. Respiratory and cardiovascular exams were normal. On abdominal exam, there was tenderness to palpation of the right upper quadrant without rebound or guarding. The spleen and the liver were not palpable. There was a lower midline incisional scar. Rectal exam revealed nonbleeding hemorrhoids and heme‐positive stool without gross blood. Bilateral lower extremities had trace pitting edema, hyperpigmentation, and superficial venous varicosities. On skin exam, there were distended subcutaneous veins radiating outward from around the umbilicus as well as prominent subcutaneous venous collaterals over the chest and lateral abdomen.
The collateral veins over the chest and lateral abdomen are consistent with central venous obstruction from the patient's known SVC thrombus. However, the presence of paraumbilical venous collaterals (caput medusa) is highly suggestive of portal hypertension. This evidence, in addition to the known central venous occlusion and history of thromboembolic disease, raises the suspicion for mesenteric thrombosis as a cause of her bleeding and pain. The first diagnostic procedure should be an esophagogastroduodenoscopy (EGD) to identify and potentially treat the source of bleeding, whether it is portal hypertension related (portal gastropathy, variceal bleed) or from a more common cause (peptic ulcer disease, stress gastritis). If the EGD is not diagnostic, the next step should be to obtain computed tomography (CT) of the abdomen and pelvis with intravenous (IV) and oral contrast. In many patients with GI bleed, a colonoscopy would typically be performed as the next diagnostic study after EGD. However, in this patient, a CT scan is likely to be of higher yield because it could help assess the mesenteric and portal vessels for patency and characterize the appearance of the small intestine and colon. Depending on the findings of the CT, additional dedicated vascular diagnostics might be needed.
Hemoglobin was 8.5 g/dL (12.4 g/dL 6 weeks prior) with a normal mean corpuscular volume and red cell distribution. The white cell count was normal, and the platelet count was 142,000/mm3. The blood urea nitrogen was 27 mg/dL, with a creatinine of 1.1 mg/dL. Routine chemistries, liver enzymes, bilirubin, and coagulation parameters were normal. Ferritin was 15 ng/mL (normal: 15200 ng/mL).
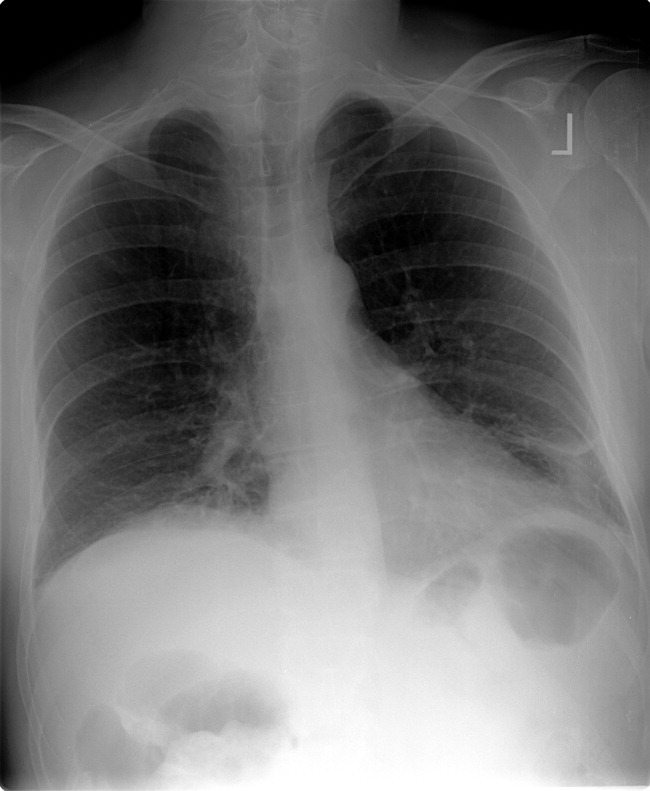
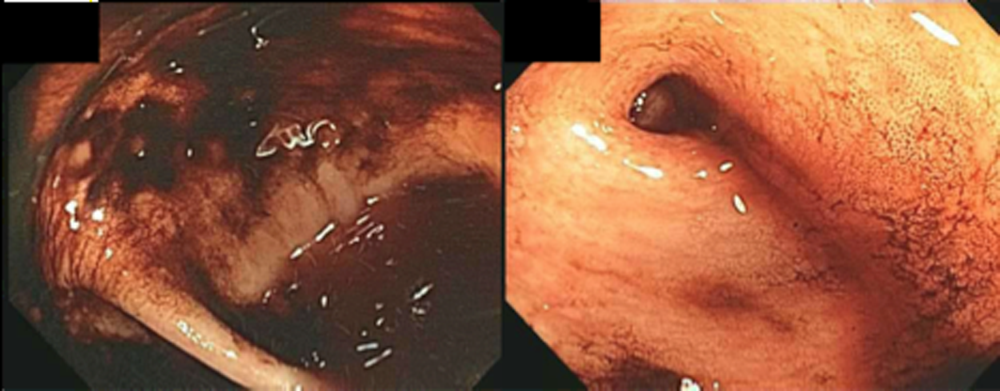
The patient was admitted to the intensive care unit. An EGD revealed a hiatal hernia and grade II nonbleeding esophageal varices with normal=appearing stomach and duodenum. The varices did not have stigmata of a recent bleed and were not ligated. The patient continued to bleed and received 2 U of packed red blood cells (RBCs), as her hemoglobin had decreased to 7.3 g/dL. On hospital day 3, a colonoscopy was done that showed blood clots in the ascending colon but was otherwise normal. The patient had ongoing abdominal pain, melena, and hematochezia, and continued to require blood transfusions every other day.
Esophageal varices were confirmed on EGD. However, no high‐risk stigmata were seen. Findings that suggest either recent bleeding or are risk factors for subsequent bleeding include large size of the varices, nipple sign referring to a protruding vessel from an underlying varix, or red wale sign, referring to a longitudinal red streak on a varix. The lack of evidence for an esophageal, gastric, or duodenal bleeding source correlates with lack of clinical signs of upper GI tract hemorrhage such as hematemesis or coffee ground emesis. Because the colonoscopy also did not identify a bleeding source, the bleeding remains unexplained. The absence of significant abnormalities in liver function or liver inflammation labs suggests that the patient does not have advanced cirrhosis and supports the suspicion of a vascular cause of the portal hypertension. At this point, it would be most useful to obtain a CT scan of the abdomen and pelvis.
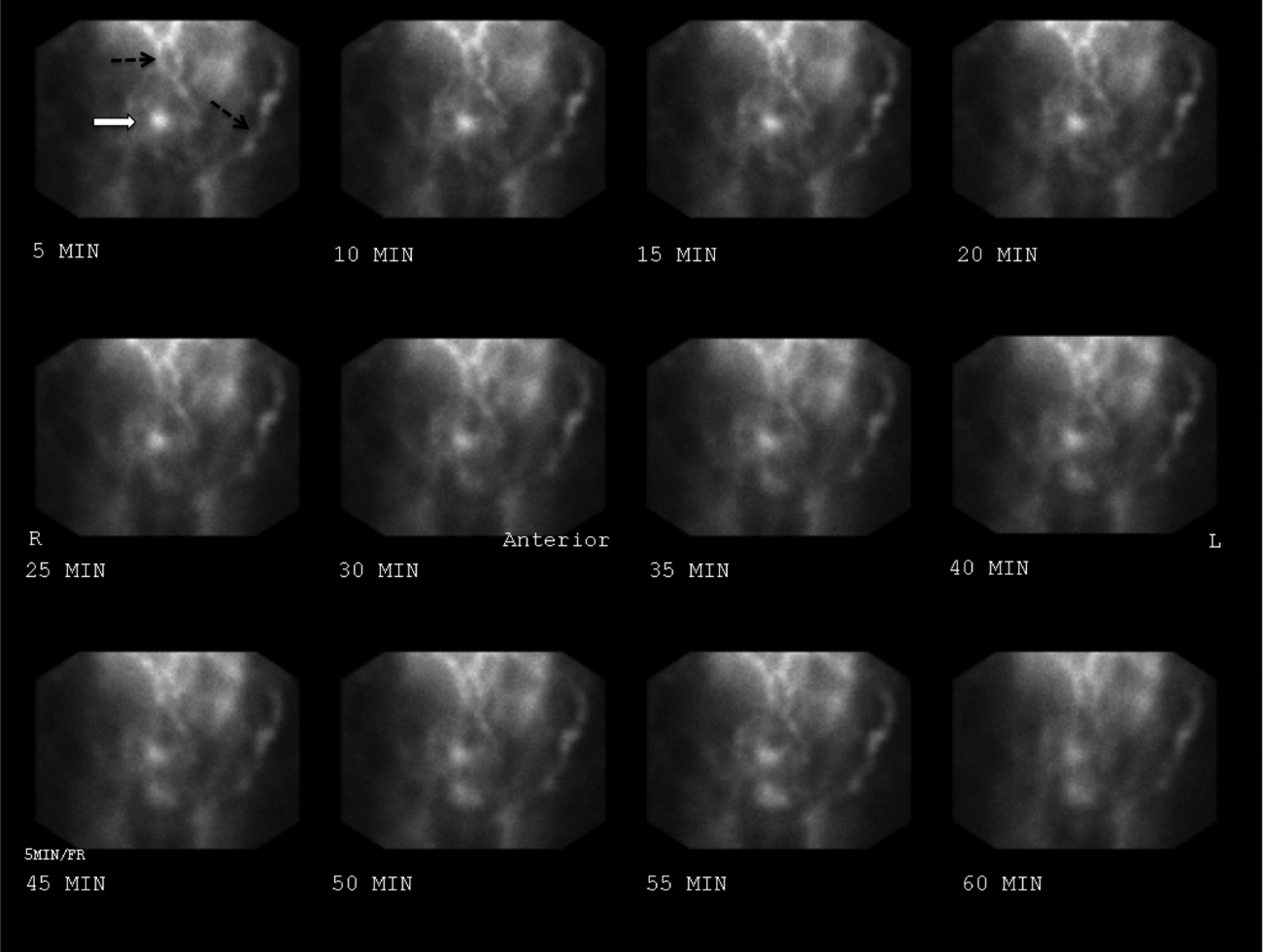
The patient continued to bleed, requiring a total of 7 U of packed RBCs over 7 days. On hospital day 4, a repeat EGD showed nonbleeding varices with a red wale sign that were banded. Despite this, the hemoglobin continued to drop. A technetium‐tagged RBC study showed a small area of subumbilical activity, which appeared to indicate transverse colonic or small bowel bleeding (Figure 1). A subsequent mesenteric angiogram failed to show active bleeding.

A red wale sign confers a higher risk of bleeding from esophageal varices. However, this finding can be subjective, and the endoscopist must individualize the decision for banding based on the size and appearance of the varices. It was reasonable to proceed with banding this time because the varices were large, had a red wale sign, and there was otherwise unexplained ongoing bleeding. Because her hemoglobin continued to drop after the banding and a tagged RBC study best localized the bleeding to the small intestine or transverse colon, it is unlikely that the varices are the primary source of bleeding. It is not surprising that the mesenteric angiogram did not show a source of bleeding, because this study requires active bleeding at a sufficient rate to radiographically identify the source.
The leading diagnosis remains an as yet uncharacterized small bowel bleeding source related to mesenteric thrombotic disease. Cross‐sectional imaging with IV contrast to identify significant vascular occlusion should be the next diagnostic step. Capsule endoscopy would be a more expensive and time‐consuming option, and although this could reveal the source of bleeding, it might not characterize the underlying vascular nature of the problem.
Due to persistent abdominal pain, a CT without intravenous contrast was done on hospital day 10. This showed extensive collateral vessels along the chest and abdominal wall with a distended azygos vein. The study was otherwise unrevealing. Her bloody stools cleared, so she was discharged with a plan for capsule endoscopy and outpatient follow‐up with her gastroenterologist. On the day of discharge (hospital day 11), hemoglobin was 7.5 g/dL and she received an eighth unit of packed RBCs. Overt bleeding was absent.
As an outpatient, intermittent hematochezia and melena recurred. The capsule endoscopy showed active bleeding approximately 45 minutes after the capsule exited the stomach. The lesion was not precisely located or characterized, but was believed to be in the distal small bowel.
The capsule finding supports the growing body of evidence implicating a small bowel source of bleeding. Furthermore, the ongoing but slow rate of blood loss makes a venous bleed more likely than an arterial bleed. A CT scan was performed prior to capsule study, but this was done without intravenous contrast. The brief description of the CT findings emphasizes the subcutaneous venous changes; a contraindication to IV contrast is not mentioned. Certainly IV contrast would have been very helpful to characterize the mesenteric arterial and venous vasculature. If there is no contraindication, a repeat CT scan with IV contrast should be performed. If there is a contraindication to IV contrast, it would be beneficial to revisit the noncontrast study with the specific purpose of searching for clues suggesting mesenteric or portal thrombosis. If the source still remains unclear, the next steps should be to perform push enteroscopy to assess the small intestine from the luminal side and magnetic resonance angiogram with venous phase imaging (or CT venogram if there is no contraindication to contrast) to evaluate the venous circulation.
The patient was readmitted 9 days after discharge with persistent melena and hematochezia. Her hemoglobin was 7.2 g/dL. Given the lack of a diagnosis, the patient was transferred to a tertiary care hospital, where a second colonoscopy and mesenteric angiogram were negative for bleeding. Small bowel enteroscopy showed no source of bleeding up to 60 cm past the pylorus. A third colonoscopy was performed due to recurrent bleeding; this showed a large amount of dark blood and clots throughout the entire colon including the cecum (Figure 2). After copious irrigation, the underlying mucosa was seen to be normal. At this point, a CT angiogram with both venous and arterial phases was done due to the high suspicion for a distal jejunal bleeding source. The CT angiogram showed numerous venous collaterals encasing a loop of midsmall bowel demonstrating progressive submucosal venous enhancement. In addition, a venous collateral ran down the right side of the sternum to the infraumbilical area and drained through the encasing collaterals into the portal venous system (Figure 3). The CT scan also revealed IVC obstruction below the distal IVC filter and an enlarged portal vein measuring 18 mm (normal 12 mm).
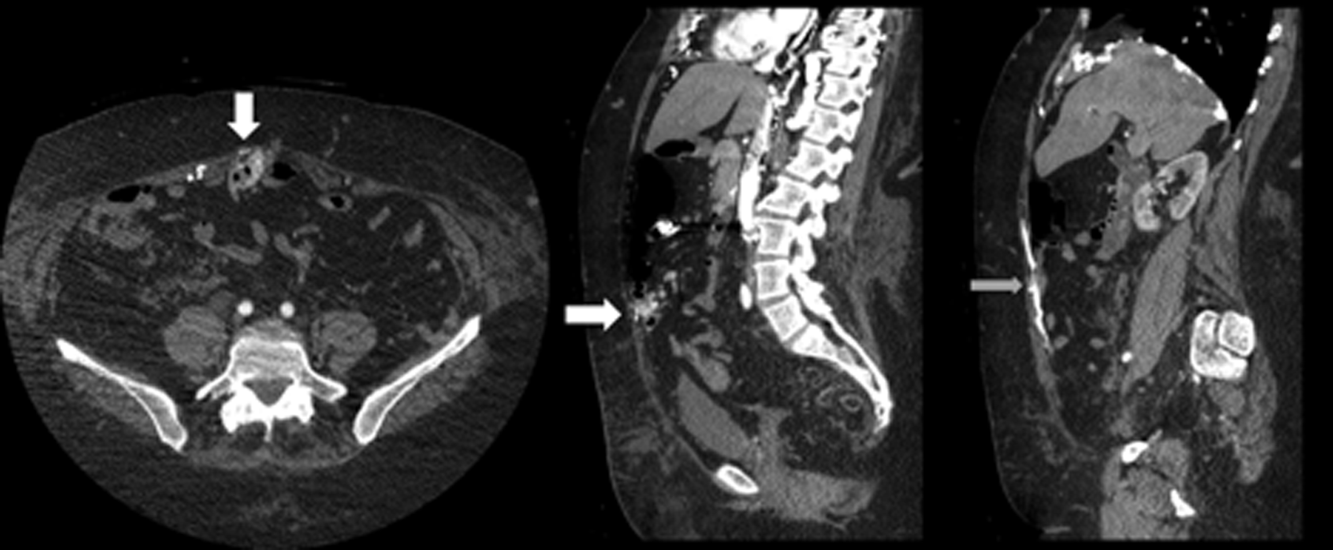
The CT angiogram provides much‐needed clarity. The continued bleeding is likely due to ectopic varices in the small bowel. The venous phase of the CT angiogram shows thrombosis of key venous structures and evidence of a dilated portal vein (indicating portal hypertension) leading to ectopic varices in the abdominal wall and jejunum. Given the prior studies that suggest a small bowel source of bleeding, jejunal varices are the most likely cause of recurrent GI bleeding in this patient.
The patient underwent exploratory laparotomy. Loops of small bowel were found to be adherent to the hysterectomy scar. There were many venous collaterals from the abdominal wall to these loops of bowel, dilating the veins both in intestinal walls and those in the adjacent mesentery. After clamping these veins, the small bowel was detached from the abdominal wall. On unclamping, the collaterals bled with a high venous pressure. Because these systemic‐portal shunts were responsible for the bleeding, the collaterals were sutured, stopping the bleeding. Thus, partial small bowel resection was not necessary. Postoperatively, her bleeding resolved completely and she maintained normal hemoglobin at 1‐year follow‐up.
COMMENTARY
The axiom common ailments are encountered most frequently underpins the classical stepwise approach to GI bleeding. First, a focused history helps localize the source of bleeding to the upper or lower GI tract. Next, endoscopy is performed to identify and treat the cause of bleeding. Finally, advanced tests such as angiography and capsule endoscopy are performed if needed. For this patient, following the usual algorithm failed to make the diagnosis or stop the bleeding. Despite historical and examination features suggesting that her case fell outside of the common patterns of GI bleeding, this patient underwent 3 upper endoscopies, 3 colonoscopies, a capsule endoscopy, a technetium‐tagged RBC study, 2 mesenteric angiograms, and a noncontrast CT scan before the study that was ultimately diagnostic was performed. The clinicians caring for this patient struggled to incorporate the atypical features of her history and presentation and failed to take an earlier detour from the usual algorithm. Instead, the same studies that had not previously led to the diagnosis were repeated multiple times.
Ectopic varices are enlarged portosystemic venous collaterals located anywhere outside the gastroesophageal region.[1] They occur in the setting of portal hypertension, surgical procedures involving abdominal viscera and vasculature, and venous occlusion. Ectopic varices account for 4% to 5% of all variceal bleeding episodes.[1] The most common sites include the anorectal junction (44%), duodenum (17%33%), jejunum/emleum (5%17%), colon (3.5%14%), and sites of previous abdominal surgery.[2, 3] Ectopic varices can cause either luminal or extraluminal (i.e., peritoneal) bleeding.[3] Luminal bleeding, seen in this case, is caused by venous protrusion into the submucosa. Ectopic varices present as a slow venous ooze, which explains this patient's ongoing requirement for recurrent blood transfusions.[4]
In this patient, submucosal ectopic varices developed as a result of a combination of known risk factors: portal hypertension in the setting of chronic venous occlusion from her hypercoagulability and a history of abdominal surgery (hysterectomy). [5] The apposition of her abdominal wall structures (drained by the systemic veins) to the bowel (drained by the portal veins) resulted in adhesion formation, detour of venous flow, collateralization, and submucosal varix formation.[1, 2, 6]
The key diagnostic study for this patient was a CT angiogram, with both arterial and venous phases. The prior 2 mesenteric angiograms had been limited to the arterial phase, which had missed identifying the venous abnormalities altogether. This highlights an important lesson from this case: contrast‐enhanced CT may have a higher yield in diagnosing ectopic varices compared to repeated endoscopiesespecially when captured in the late venous phaseand should strongly be considered for unexplained bleeding in patients with stigmata of liver disease or portal hypertension.[7, 8] Another clue for ectopic varices in a bleeding patient are nonbleeding esophageal or gastric varices, as was the case in this patient.[9]
The initial management of ectopic varices is similar to bleeding secondary to esophageal varices.[1] Definitive treatment includes endoscopic embolization or ligation, interventional radiological procedures such as portosystemic shunting or percutaneous embolization, and exploratory laparotomy to either resect the segment of bowel that is the source of bleeding or to decompress the collaterals surgically.[9] Although endoscopic ligation has been shown to have a lower rebleeding rate and mortality compared to endoscopic injection sclerotherapy in patients with esophageal varices, the data are too sparse in jejunal varices to recommend 1 treatment over another. Both have been used successfully either alone or in combination with each other, and can be useful alternatives for patients who are unable to undergo laparotomy.[9]
Diagnostic errors due to cognitive biases can be avoided by following diagnostic algorithms. However, over‐reliance on algorithms can result in vertical line failure, a form of cognitive bias in which the clinician subconsciously adheres to an inflexible diagnostic approach.[10] To overcome this bias, clinicians need to think laterally and consider alternative diagnoses when algorithms do not lead to expected outcomes. This case highlights the challenges of knowing when to break free of conventional approaches and the rewards of taking a well‐chosen detour that leads to the diagnosis.
KEY POINTS
- Recurrent, occult gastrointestinal bleeding should raise concern for a small bowel source, and clinicians may need to take a detour away from the usual workup to arrive at a diagnosis.
- CT angiography of the abdomen and pelvis may miss venous sources of bleeding, unless a venous phase is specifically requested.
- Ectopic varices can occur in patients with portal hypertension who have had a history of abdominal surgery; these patients can develop venous collaterals for decompression into the systemic circulation through the abdominal wall.
Disclosure
Nothing to report.
- , , . Updates in the pathogenesis, diagnosis and management of ectopic varices. Hepatol Int. 2008;2:322–334.
- , , . Management of ectopic varices. Hepatology. 1998;28:1154–1158.
- , , , et al. Current status of ectopic varices in Japan: results of a survey by the Japan Society for Portal Hypertension. Hepatol Res. 2010;40:763–766.
- , , . Stomal Varices: Management with decompression TIPS and transvenous obliteration or sclerosis. Tech Vasc Interv Radiol. 2013;16:126–134.
- , , , et al. Jejunal varices as a cause of massive gastrointestinal bleeding. Am J Gastroenterol. 1992;87:514–517.
- , . Ectopic varices in portal hypertension. Clin Gastroenterol. 1985;14:105–121.
- , , , et al. Ectopic varices in portal hypertension: computed tomographic angiography instead of repeated endoscopies for diagnosis. Eur J Gastroenterol Hepatol. 2011;23:620–622.
- , , , et al. ACR appropriateness criteria. Radiologic management of lower gastrointestinal tract bleeding. Reston, VA: American College of Radiology; 2011. Available at: http://www.acr.org/Quality‐Safety/Appropriateness‐Criteria/∼/media/5F9CB95C164E4DA19DCBCFBBA790BB3C.pdf. Accessed January 28, 2015.
- , . Diagnosis and management of ectopic varices. Gastrointest Interv. 2012;1:3–10.
- . Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9:1184–1204.
A 60‐year‐old woman presented to a community hospital's emergency department with 4 days of right‐sided abdominal pain and multiple episodes of black stools. She reported nausea without vomiting. She denied light‐headedness, chest pain, or shortness of breath. She also denied difficulty in swallowing, weight loss, jaundice, or other bleeding.
The first priority when assessing a patient with gastrointestinal (GI) bleeding is to ensure hemodynamic stability. Next, it is important to carefully characterize the stools to help narrow the differential diagnosis. As blood is a cathartic, frequent, loose, and black stools suggest vigorous bleeding. It is essential to establish that the stools are actually black, as some patients will mistake dark brown stools for melena. Using a visual aid like a black pen or shoes as a point of reference can help the patient differentiate between dark stool and melena. It is also important to obtain a thorough medication history because iron supplements or bismuth‐containing remedies can turn stool black. The use of any antiplatelet agents or anticoagulants should also be noted. The right‐sided abdominal pain should be characterized by establishing the frequency, severity, and association with eating, movement, and position. For this patient's presentation, increased pain with eating would rapidly heighten concern for mesenteric ischemia.
The patient reported having 1 to 2 semiformed, tarry, black bowel movements per day. The night prior to admission she had passed some bright red blood along with the melena. The abdominal pain had increased gradually over 4 days, was dull, constant, did not radiate, and there were no evident aggravating or relieving factors. She rated the pain as 4 out of 10 in intensity, worst in her right upper quadrant.
Her past medical history was notable for recurrent deep venous thromboses and pulmonary emboli that had occurred even while on oral anticoagulation. Inferior vena cava (IVC) filters had twice been placed many years prior; anticoagulation had been subsequently discontinued. Additionally, she was known to have chronic superior vena cava (SVC) occlusion, presumably related to hypercoagulability. Previous evaluation had identified only hyperhomocysteinemia as a risk factor for recurrent thromboses. Other medical problems included hemorrhoids, gastroesophageal reflux disease, and asthma. Her only surgical history was an abdominal hysterectomy and bilateral oophorectomy many years ago for nonmalignant disease. Home medications were omeprazole, ranitidine, albuterol, and fluticasone‐salmeterol. She denied using nonsteroidal anti‐inflammatory drugs, aspirin, or any dietary supplements. She denied smoking, alcohol, or recreational drug use.
Because melena is confirmed, an upper GI tract bleeding source is most likely. The more recent appearance of bright red blood is concerning for acceleration of bleeding, or may point to a distal small bowel or right colonic source. Given the history of thromboembolic disease and likely underlying hypercoagulability, vascular occlusion is a leading possibility. Thus, mesenteric arterial insufficiency or mesenteric venous thrombosis should be considered, even though the patient does not report the characteristic postprandial exacerbation of pain. Ischemic colitis due to arterial insufficiency typically presents with severe, acute pain, with or without hematochezia. This syndrome is typically manifested in vascular watershed areas such as the splenic flexure, but can also affect the right colon. Mesenteric venous thrombosis is a rare condition that most often occurs in patients with hypercoagulability. Patients present with variable degrees of abdominal pain and often with GI bleeding. Finally, portal venous thrombosis may be seen alongside thromboses of other mesenteric veins or may occur independently. Portal hypertension due to portal vein thrombosis can result in esophageal and/or gastric varices. Although variceal bleeding classically presents with dramatic hematemesis, the absence of hematemesis does not rule out a variceal bleed in this patient.
On physical examination, the patient had a temperature of 37.1C with a pulse of 90 beats per minute and blood pressure of 161/97 mm Hg. Orthostatics were not performed. No blood was seen on nasal and oropharyngeal exam. Respiratory and cardiovascular exams were normal. On abdominal exam, there was tenderness to palpation of the right upper quadrant without rebound or guarding. The spleen and the liver were not palpable. There was a lower midline incisional scar. Rectal exam revealed nonbleeding hemorrhoids and heme‐positive stool without gross blood. Bilateral lower extremities had trace pitting edema, hyperpigmentation, and superficial venous varicosities. On skin exam, there were distended subcutaneous veins radiating outward from around the umbilicus as well as prominent subcutaneous venous collaterals over the chest and lateral abdomen.
The collateral veins over the chest and lateral abdomen are consistent with central venous obstruction from the patient's known SVC thrombus. However, the presence of paraumbilical venous collaterals (caput medusa) is highly suggestive of portal hypertension. This evidence, in addition to the known central venous occlusion and history of thromboembolic disease, raises the suspicion for mesenteric thrombosis as a cause of her bleeding and pain. The first diagnostic procedure should be an esophagogastroduodenoscopy (EGD) to identify and potentially treat the source of bleeding, whether it is portal hypertension related (portal gastropathy, variceal bleed) or from a more common cause (peptic ulcer disease, stress gastritis). If the EGD is not diagnostic, the next step should be to obtain computed tomography (CT) of the abdomen and pelvis with intravenous (IV) and oral contrast. In many patients with GI bleed, a colonoscopy would typically be performed as the next diagnostic study after EGD. However, in this patient, a CT scan is likely to be of higher yield because it could help assess the mesenteric and portal vessels for patency and characterize the appearance of the small intestine and colon. Depending on the findings of the CT, additional dedicated vascular diagnostics might be needed.
Hemoglobin was 8.5 g/dL (12.4 g/dL 6 weeks prior) with a normal mean corpuscular volume and red cell distribution. The white cell count was normal, and the platelet count was 142,000/mm3. The blood urea nitrogen was 27 mg/dL, with a creatinine of 1.1 mg/dL. Routine chemistries, liver enzymes, bilirubin, and coagulation parameters were normal. Ferritin was 15 ng/mL (normal: 15200 ng/mL).
The patient was admitted to the intensive care unit. An EGD revealed a hiatal hernia and grade II nonbleeding esophageal varices with normal=appearing stomach and duodenum. The varices did not have stigmata of a recent bleed and were not ligated. The patient continued to bleed and received 2 U of packed red blood cells (RBCs), as her hemoglobin had decreased to 7.3 g/dL. On hospital day 3, a colonoscopy was done that showed blood clots in the ascending colon but was otherwise normal. The patient had ongoing abdominal pain, melena, and hematochezia, and continued to require blood transfusions every other day.
Esophageal varices were confirmed on EGD. However, no high‐risk stigmata were seen. Findings that suggest either recent bleeding or are risk factors for subsequent bleeding include large size of the varices, nipple sign referring to a protruding vessel from an underlying varix, or red wale sign, referring to a longitudinal red streak on a varix. The lack of evidence for an esophageal, gastric, or duodenal bleeding source correlates with lack of clinical signs of upper GI tract hemorrhage such as hematemesis or coffee ground emesis. Because the colonoscopy also did not identify a bleeding source, the bleeding remains unexplained. The absence of significant abnormalities in liver function or liver inflammation labs suggests that the patient does not have advanced cirrhosis and supports the suspicion of a vascular cause of the portal hypertension. At this point, it would be most useful to obtain a CT scan of the abdomen and pelvis.
The patient continued to bleed, requiring a total of 7 U of packed RBCs over 7 days. On hospital day 4, a repeat EGD showed nonbleeding varices with a red wale sign that were banded. Despite this, the hemoglobin continued to drop. A technetium‐tagged RBC study showed a small area of subumbilical activity, which appeared to indicate transverse colonic or small bowel bleeding (Figure 1). A subsequent mesenteric angiogram failed to show active bleeding.

A red wale sign confers a higher risk of bleeding from esophageal varices. However, this finding can be subjective, and the endoscopist must individualize the decision for banding based on the size and appearance of the varices. It was reasonable to proceed with banding this time because the varices were large, had a red wale sign, and there was otherwise unexplained ongoing bleeding. Because her hemoglobin continued to drop after the banding and a tagged RBC study best localized the bleeding to the small intestine or transverse colon, it is unlikely that the varices are the primary source of bleeding. It is not surprising that the mesenteric angiogram did not show a source of bleeding, because this study requires active bleeding at a sufficient rate to radiographically identify the source.
The leading diagnosis remains an as yet uncharacterized small bowel bleeding source related to mesenteric thrombotic disease. Cross‐sectional imaging with IV contrast to identify significant vascular occlusion should be the next diagnostic step. Capsule endoscopy would be a more expensive and time‐consuming option, and although this could reveal the source of bleeding, it might not characterize the underlying vascular nature of the problem.
Due to persistent abdominal pain, a CT without intravenous contrast was done on hospital day 10. This showed extensive collateral vessels along the chest and abdominal wall with a distended azygos vein. The study was otherwise unrevealing. Her bloody stools cleared, so she was discharged with a plan for capsule endoscopy and outpatient follow‐up with her gastroenterologist. On the day of discharge (hospital day 11), hemoglobin was 7.5 g/dL and she received an eighth unit of packed RBCs. Overt bleeding was absent.
As an outpatient, intermittent hematochezia and melena recurred. The capsule endoscopy showed active bleeding approximately 45 minutes after the capsule exited the stomach. The lesion was not precisely located or characterized, but was believed to be in the distal small bowel.
The capsule finding supports the growing body of evidence implicating a small bowel source of bleeding. Furthermore, the ongoing but slow rate of blood loss makes a venous bleed more likely than an arterial bleed. A CT scan was performed prior to capsule study, but this was done without intravenous contrast. The brief description of the CT findings emphasizes the subcutaneous venous changes; a contraindication to IV contrast is not mentioned. Certainly IV contrast would have been very helpful to characterize the mesenteric arterial and venous vasculature. If there is no contraindication, a repeat CT scan with IV contrast should be performed. If there is a contraindication to IV contrast, it would be beneficial to revisit the noncontrast study with the specific purpose of searching for clues suggesting mesenteric or portal thrombosis. If the source still remains unclear, the next steps should be to perform push enteroscopy to assess the small intestine from the luminal side and magnetic resonance angiogram with venous phase imaging (or CT venogram if there is no contraindication to contrast) to evaluate the venous circulation.
The patient was readmitted 9 days after discharge with persistent melena and hematochezia. Her hemoglobin was 7.2 g/dL. Given the lack of a diagnosis, the patient was transferred to a tertiary care hospital, where a second colonoscopy and mesenteric angiogram were negative for bleeding. Small bowel enteroscopy showed no source of bleeding up to 60 cm past the pylorus. A third colonoscopy was performed due to recurrent bleeding; this showed a large amount of dark blood and clots throughout the entire colon including the cecum (Figure 2). After copious irrigation, the underlying mucosa was seen to be normal. At this point, a CT angiogram with both venous and arterial phases was done due to the high suspicion for a distal jejunal bleeding source. The CT angiogram showed numerous venous collaterals encasing a loop of midsmall bowel demonstrating progressive submucosal venous enhancement. In addition, a venous collateral ran down the right side of the sternum to the infraumbilical area and drained through the encasing collaterals into the portal venous system (Figure 3). The CT scan also revealed IVC obstruction below the distal IVC filter and an enlarged portal vein measuring 18 mm (normal 12 mm).


The CT angiogram provides much‐needed clarity. The continued bleeding is likely due to ectopic varices in the small bowel. The venous phase of the CT angiogram shows thrombosis of key venous structures and evidence of a dilated portal vein (indicating portal hypertension) leading to ectopic varices in the abdominal wall and jejunum. Given the prior studies that suggest a small bowel source of bleeding, jejunal varices are the most likely cause of recurrent GI bleeding in this patient.
The patient underwent exploratory laparotomy. Loops of small bowel were found to be adherent to the hysterectomy scar. There were many venous collaterals from the abdominal wall to these loops of bowel, dilating the veins both in intestinal walls and those in the adjacent mesentery. After clamping these veins, the small bowel was detached from the abdominal wall. On unclamping, the collaterals bled with a high venous pressure. Because these systemic‐portal shunts were responsible for the bleeding, the collaterals were sutured, stopping the bleeding. Thus, partial small bowel resection was not necessary. Postoperatively, her bleeding resolved completely and she maintained normal hemoglobin at 1‐year follow‐up.
COMMENTARY
The axiom common ailments are encountered most frequently underpins the classical stepwise approach to GI bleeding. First, a focused history helps localize the source of bleeding to the upper or lower GI tract. Next, endoscopy is performed to identify and treat the cause of bleeding. Finally, advanced tests such as angiography and capsule endoscopy are performed if needed. For this patient, following the usual algorithm failed to make the diagnosis or stop the bleeding. Despite historical and examination features suggesting that her case fell outside of the common patterns of GI bleeding, this patient underwent 3 upper endoscopies, 3 colonoscopies, a capsule endoscopy, a technetium‐tagged RBC study, 2 mesenteric angiograms, and a noncontrast CT scan before the study that was ultimately diagnostic was performed. The clinicians caring for this patient struggled to incorporate the atypical features of her history and presentation and failed to take an earlier detour from the usual algorithm. Instead, the same studies that had not previously led to the diagnosis were repeated multiple times.
Ectopic varices are enlarged portosystemic venous collaterals located anywhere outside the gastroesophageal region.[1] They occur in the setting of portal hypertension, surgical procedures involving abdominal viscera and vasculature, and venous occlusion. Ectopic varices account for 4% to 5% of all variceal bleeding episodes.[1] The most common sites include the anorectal junction (44%), duodenum (17%33%), jejunum/emleum (5%17%), colon (3.5%14%), and sites of previous abdominal surgery.[2, 3] Ectopic varices can cause either luminal or extraluminal (i.e., peritoneal) bleeding.[3] Luminal bleeding, seen in this case, is caused by venous protrusion into the submucosa. Ectopic varices present as a slow venous ooze, which explains this patient's ongoing requirement for recurrent blood transfusions.[4]
In this patient, submucosal ectopic varices developed as a result of a combination of known risk factors: portal hypertension in the setting of chronic venous occlusion from her hypercoagulability and a history of abdominal surgery (hysterectomy). [5] The apposition of her abdominal wall structures (drained by the systemic veins) to the bowel (drained by the portal veins) resulted in adhesion formation, detour of venous flow, collateralization, and submucosal varix formation.[1, 2, 6]
The key diagnostic study for this patient was a CT angiogram, with both arterial and venous phases. The prior 2 mesenteric angiograms had been limited to the arterial phase, which had missed identifying the venous abnormalities altogether. This highlights an important lesson from this case: contrast‐enhanced CT may have a higher yield in diagnosing ectopic varices compared to repeated endoscopiesespecially when captured in the late venous phaseand should strongly be considered for unexplained bleeding in patients with stigmata of liver disease or portal hypertension.[7, 8] Another clue for ectopic varices in a bleeding patient are nonbleeding esophageal or gastric varices, as was the case in this patient.[9]
The initial management of ectopic varices is similar to bleeding secondary to esophageal varices.[1] Definitive treatment includes endoscopic embolization or ligation, interventional radiological procedures such as portosystemic shunting or percutaneous embolization, and exploratory laparotomy to either resect the segment of bowel that is the source of bleeding or to decompress the collaterals surgically.[9] Although endoscopic ligation has been shown to have a lower rebleeding rate and mortality compared to endoscopic injection sclerotherapy in patients with esophageal varices, the data are too sparse in jejunal varices to recommend 1 treatment over another. Both have been used successfully either alone or in combination with each other, and can be useful alternatives for patients who are unable to undergo laparotomy.[9]
Diagnostic errors due to cognitive biases can be avoided by following diagnostic algorithms. However, over‐reliance on algorithms can result in vertical line failure, a form of cognitive bias in which the clinician subconsciously adheres to an inflexible diagnostic approach.[10] To overcome this bias, clinicians need to think laterally and consider alternative diagnoses when algorithms do not lead to expected outcomes. This case highlights the challenges of knowing when to break free of conventional approaches and the rewards of taking a well‐chosen detour that leads to the diagnosis.
KEY POINTS
- Recurrent, occult gastrointestinal bleeding should raise concern for a small bowel source, and clinicians may need to take a detour away from the usual workup to arrive at a diagnosis.
- CT angiography of the abdomen and pelvis may miss venous sources of bleeding, unless a venous phase is specifically requested.
- Ectopic varices can occur in patients with portal hypertension who have had a history of abdominal surgery; these patients can develop venous collaterals for decompression into the systemic circulation through the abdominal wall.
Disclosure
Nothing to report.
A 60‐year‐old woman presented to a community hospital's emergency department with 4 days of right‐sided abdominal pain and multiple episodes of black stools. She reported nausea without vomiting. She denied light‐headedness, chest pain, or shortness of breath. She also denied difficulty in swallowing, weight loss, jaundice, or other bleeding.
The first priority when assessing a patient with gastrointestinal (GI) bleeding is to ensure hemodynamic stability. Next, it is important to carefully characterize the stools to help narrow the differential diagnosis. As blood is a cathartic, frequent, loose, and black stools suggest vigorous bleeding. It is essential to establish that the stools are actually black, as some patients will mistake dark brown stools for melena. Using a visual aid like a black pen or shoes as a point of reference can help the patient differentiate between dark stool and melena. It is also important to obtain a thorough medication history because iron supplements or bismuth‐containing remedies can turn stool black. The use of any antiplatelet agents or anticoagulants should also be noted. The right‐sided abdominal pain should be characterized by establishing the frequency, severity, and association with eating, movement, and position. For this patient's presentation, increased pain with eating would rapidly heighten concern for mesenteric ischemia.
The patient reported having 1 to 2 semiformed, tarry, black bowel movements per day. The night prior to admission she had passed some bright red blood along with the melena. The abdominal pain had increased gradually over 4 days, was dull, constant, did not radiate, and there were no evident aggravating or relieving factors. She rated the pain as 4 out of 10 in intensity, worst in her right upper quadrant.
Her past medical history was notable for recurrent deep venous thromboses and pulmonary emboli that had occurred even while on oral anticoagulation. Inferior vena cava (IVC) filters had twice been placed many years prior; anticoagulation had been subsequently discontinued. Additionally, she was known to have chronic superior vena cava (SVC) occlusion, presumably related to hypercoagulability. Previous evaluation had identified only hyperhomocysteinemia as a risk factor for recurrent thromboses. Other medical problems included hemorrhoids, gastroesophageal reflux disease, and asthma. Her only surgical history was an abdominal hysterectomy and bilateral oophorectomy many years ago for nonmalignant disease. Home medications were omeprazole, ranitidine, albuterol, and fluticasone‐salmeterol. She denied using nonsteroidal anti‐inflammatory drugs, aspirin, or any dietary supplements. She denied smoking, alcohol, or recreational drug use.
Because melena is confirmed, an upper GI tract bleeding source is most likely. The more recent appearance of bright red blood is concerning for acceleration of bleeding, or may point to a distal small bowel or right colonic source. Given the history of thromboembolic disease and likely underlying hypercoagulability, vascular occlusion is a leading possibility. Thus, mesenteric arterial insufficiency or mesenteric venous thrombosis should be considered, even though the patient does not report the characteristic postprandial exacerbation of pain. Ischemic colitis due to arterial insufficiency typically presents with severe, acute pain, with or without hematochezia. This syndrome is typically manifested in vascular watershed areas such as the splenic flexure, but can also affect the right colon. Mesenteric venous thrombosis is a rare condition that most often occurs in patients with hypercoagulability. Patients present with variable degrees of abdominal pain and often with GI bleeding. Finally, portal venous thrombosis may be seen alongside thromboses of other mesenteric veins or may occur independently. Portal hypertension due to portal vein thrombosis can result in esophageal and/or gastric varices. Although variceal bleeding classically presents with dramatic hematemesis, the absence of hematemesis does not rule out a variceal bleed in this patient.
On physical examination, the patient had a temperature of 37.1C with a pulse of 90 beats per minute and blood pressure of 161/97 mm Hg. Orthostatics were not performed. No blood was seen on nasal and oropharyngeal exam. Respiratory and cardiovascular exams were normal. On abdominal exam, there was tenderness to palpation of the right upper quadrant without rebound or guarding. The spleen and the liver were not palpable. There was a lower midline incisional scar. Rectal exam revealed nonbleeding hemorrhoids and heme‐positive stool without gross blood. Bilateral lower extremities had trace pitting edema, hyperpigmentation, and superficial venous varicosities. On skin exam, there were distended subcutaneous veins radiating outward from around the umbilicus as well as prominent subcutaneous venous collaterals over the chest and lateral abdomen.
The collateral veins over the chest and lateral abdomen are consistent with central venous obstruction from the patient's known SVC thrombus. However, the presence of paraumbilical venous collaterals (caput medusa) is highly suggestive of portal hypertension. This evidence, in addition to the known central venous occlusion and history of thromboembolic disease, raises the suspicion for mesenteric thrombosis as a cause of her bleeding and pain. The first diagnostic procedure should be an esophagogastroduodenoscopy (EGD) to identify and potentially treat the source of bleeding, whether it is portal hypertension related (portal gastropathy, variceal bleed) or from a more common cause (peptic ulcer disease, stress gastritis). If the EGD is not diagnostic, the next step should be to obtain computed tomography (CT) of the abdomen and pelvis with intravenous (IV) and oral contrast. In many patients with GI bleed, a colonoscopy would typically be performed as the next diagnostic study after EGD. However, in this patient, a CT scan is likely to be of higher yield because it could help assess the mesenteric and portal vessels for patency and characterize the appearance of the small intestine and colon. Depending on the findings of the CT, additional dedicated vascular diagnostics might be needed.
Hemoglobin was 8.5 g/dL (12.4 g/dL 6 weeks prior) with a normal mean corpuscular volume and red cell distribution. The white cell count was normal, and the platelet count was 142,000/mm3. The blood urea nitrogen was 27 mg/dL, with a creatinine of 1.1 mg/dL. Routine chemistries, liver enzymes, bilirubin, and coagulation parameters were normal. Ferritin was 15 ng/mL (normal: 15200 ng/mL).
The patient was admitted to the intensive care unit. An EGD revealed a hiatal hernia and grade II nonbleeding esophageal varices with normal=appearing stomach and duodenum. The varices did not have stigmata of a recent bleed and were not ligated. The patient continued to bleed and received 2 U of packed red blood cells (RBCs), as her hemoglobin had decreased to 7.3 g/dL. On hospital day 3, a colonoscopy was done that showed blood clots in the ascending colon but was otherwise normal. The patient had ongoing abdominal pain, melena, and hematochezia, and continued to require blood transfusions every other day.
Esophageal varices were confirmed on EGD. However, no high‐risk stigmata were seen. Findings that suggest either recent bleeding or are risk factors for subsequent bleeding include large size of the varices, nipple sign referring to a protruding vessel from an underlying varix, or red wale sign, referring to a longitudinal red streak on a varix. The lack of evidence for an esophageal, gastric, or duodenal bleeding source correlates with lack of clinical signs of upper GI tract hemorrhage such as hematemesis or coffee ground emesis. Because the colonoscopy also did not identify a bleeding source, the bleeding remains unexplained. The absence of significant abnormalities in liver function or liver inflammation labs suggests that the patient does not have advanced cirrhosis and supports the suspicion of a vascular cause of the portal hypertension. At this point, it would be most useful to obtain a CT scan of the abdomen and pelvis.
The patient continued to bleed, requiring a total of 7 U of packed RBCs over 7 days. On hospital day 4, a repeat EGD showed nonbleeding varices with a red wale sign that were banded. Despite this, the hemoglobin continued to drop. A technetium‐tagged RBC study showed a small area of subumbilical activity, which appeared to indicate transverse colonic or small bowel bleeding (Figure 1). A subsequent mesenteric angiogram failed to show active bleeding.

A red wale sign confers a higher risk of bleeding from esophageal varices. However, this finding can be subjective, and the endoscopist must individualize the decision for banding based on the size and appearance of the varices. It was reasonable to proceed with banding this time because the varices were large, had a red wale sign, and there was otherwise unexplained ongoing bleeding. Because her hemoglobin continued to drop after the banding and a tagged RBC study best localized the bleeding to the small intestine or transverse colon, it is unlikely that the varices are the primary source of bleeding. It is not surprising that the mesenteric angiogram did not show a source of bleeding, because this study requires active bleeding at a sufficient rate to radiographically identify the source.
The leading diagnosis remains an as yet uncharacterized small bowel bleeding source related to mesenteric thrombotic disease. Cross‐sectional imaging with IV contrast to identify significant vascular occlusion should be the next diagnostic step. Capsule endoscopy would be a more expensive and time‐consuming option, and although this could reveal the source of bleeding, it might not characterize the underlying vascular nature of the problem.
Due to persistent abdominal pain, a CT without intravenous contrast was done on hospital day 10. This showed extensive collateral vessels along the chest and abdominal wall with a distended azygos vein. The study was otherwise unrevealing. Her bloody stools cleared, so she was discharged with a plan for capsule endoscopy and outpatient follow‐up with her gastroenterologist. On the day of discharge (hospital day 11), hemoglobin was 7.5 g/dL and she received an eighth unit of packed RBCs. Overt bleeding was absent.
As an outpatient, intermittent hematochezia and melena recurred. The capsule endoscopy showed active bleeding approximately 45 minutes after the capsule exited the stomach. The lesion was not precisely located or characterized, but was believed to be in the distal small bowel.
The capsule finding supports the growing body of evidence implicating a small bowel source of bleeding. Furthermore, the ongoing but slow rate of blood loss makes a venous bleed more likely than an arterial bleed. A CT scan was performed prior to capsule study, but this was done without intravenous contrast. The brief description of the CT findings emphasizes the subcutaneous venous changes; a contraindication to IV contrast is not mentioned. Certainly IV contrast would have been very helpful to characterize the mesenteric arterial and venous vasculature. If there is no contraindication, a repeat CT scan with IV contrast should be performed. If there is a contraindication to IV contrast, it would be beneficial to revisit the noncontrast study with the specific purpose of searching for clues suggesting mesenteric or portal thrombosis. If the source still remains unclear, the next steps should be to perform push enteroscopy to assess the small intestine from the luminal side and magnetic resonance angiogram with venous phase imaging (or CT venogram if there is no contraindication to contrast) to evaluate the venous circulation.
The patient was readmitted 9 days after discharge with persistent melena and hematochezia. Her hemoglobin was 7.2 g/dL. Given the lack of a diagnosis, the patient was transferred to a tertiary care hospital, where a second colonoscopy and mesenteric angiogram were negative for bleeding. Small bowel enteroscopy showed no source of bleeding up to 60 cm past the pylorus. A third colonoscopy was performed due to recurrent bleeding; this showed a large amount of dark blood and clots throughout the entire colon including the cecum (Figure 2). After copious irrigation, the underlying mucosa was seen to be normal. At this point, a CT angiogram with both venous and arterial phases was done due to the high suspicion for a distal jejunal bleeding source. The CT angiogram showed numerous venous collaterals encasing a loop of midsmall bowel demonstrating progressive submucosal venous enhancement. In addition, a venous collateral ran down the right side of the sternum to the infraumbilical area and drained through the encasing collaterals into the portal venous system (Figure 3). The CT scan also revealed IVC obstruction below the distal IVC filter and an enlarged portal vein measuring 18 mm (normal 12 mm).


The CT angiogram provides much‐needed clarity. The continued bleeding is likely due to ectopic varices in the small bowel. The venous phase of the CT angiogram shows thrombosis of key venous structures and evidence of a dilated portal vein (indicating portal hypertension) leading to ectopic varices in the abdominal wall and jejunum. Given the prior studies that suggest a small bowel source of bleeding, jejunal varices are the most likely cause of recurrent GI bleeding in this patient.
The patient underwent exploratory laparotomy. Loops of small bowel were found to be adherent to the hysterectomy scar. There were many venous collaterals from the abdominal wall to these loops of bowel, dilating the veins both in intestinal walls and those in the adjacent mesentery. After clamping these veins, the small bowel was detached from the abdominal wall. On unclamping, the collaterals bled with a high venous pressure. Because these systemic‐portal shunts were responsible for the bleeding, the collaterals were sutured, stopping the bleeding. Thus, partial small bowel resection was not necessary. Postoperatively, her bleeding resolved completely and she maintained normal hemoglobin at 1‐year follow‐up.
COMMENTARY
The axiom common ailments are encountered most frequently underpins the classical stepwise approach to GI bleeding. First, a focused history helps localize the source of bleeding to the upper or lower GI tract. Next, endoscopy is performed to identify and treat the cause of bleeding. Finally, advanced tests such as angiography and capsule endoscopy are performed if needed. For this patient, following the usual algorithm failed to make the diagnosis or stop the bleeding. Despite historical and examination features suggesting that her case fell outside of the common patterns of GI bleeding, this patient underwent 3 upper endoscopies, 3 colonoscopies, a capsule endoscopy, a technetium‐tagged RBC study, 2 mesenteric angiograms, and a noncontrast CT scan before the study that was ultimately diagnostic was performed. The clinicians caring for this patient struggled to incorporate the atypical features of her history and presentation and failed to take an earlier detour from the usual algorithm. Instead, the same studies that had not previously led to the diagnosis were repeated multiple times.
Ectopic varices are enlarged portosystemic venous collaterals located anywhere outside the gastroesophageal region.[1] They occur in the setting of portal hypertension, surgical procedures involving abdominal viscera and vasculature, and venous occlusion. Ectopic varices account for 4% to 5% of all variceal bleeding episodes.[1] The most common sites include the anorectal junction (44%), duodenum (17%33%), jejunum/emleum (5%17%), colon (3.5%14%), and sites of previous abdominal surgery.[2, 3] Ectopic varices can cause either luminal or extraluminal (i.e., peritoneal) bleeding.[3] Luminal bleeding, seen in this case, is caused by venous protrusion into the submucosa. Ectopic varices present as a slow venous ooze, which explains this patient's ongoing requirement for recurrent blood transfusions.[4]
In this patient, submucosal ectopic varices developed as a result of a combination of known risk factors: portal hypertension in the setting of chronic venous occlusion from her hypercoagulability and a history of abdominal surgery (hysterectomy). [5] The apposition of her abdominal wall structures (drained by the systemic veins) to the bowel (drained by the portal veins) resulted in adhesion formation, detour of venous flow, collateralization, and submucosal varix formation.[1, 2, 6]
The key diagnostic study for this patient was a CT angiogram, with both arterial and venous phases. The prior 2 mesenteric angiograms had been limited to the arterial phase, which had missed identifying the venous abnormalities altogether. This highlights an important lesson from this case: contrast‐enhanced CT may have a higher yield in diagnosing ectopic varices compared to repeated endoscopiesespecially when captured in the late venous phaseand should strongly be considered for unexplained bleeding in patients with stigmata of liver disease or portal hypertension.[7, 8] Another clue for ectopic varices in a bleeding patient are nonbleeding esophageal or gastric varices, as was the case in this patient.[9]
The initial management of ectopic varices is similar to bleeding secondary to esophageal varices.[1] Definitive treatment includes endoscopic embolization or ligation, interventional radiological procedures such as portosystemic shunting or percutaneous embolization, and exploratory laparotomy to either resect the segment of bowel that is the source of bleeding or to decompress the collaterals surgically.[9] Although endoscopic ligation has been shown to have a lower rebleeding rate and mortality compared to endoscopic injection sclerotherapy in patients with esophageal varices, the data are too sparse in jejunal varices to recommend 1 treatment over another. Both have been used successfully either alone or in combination with each other, and can be useful alternatives for patients who are unable to undergo laparotomy.[9]
Diagnostic errors due to cognitive biases can be avoided by following diagnostic algorithms. However, over‐reliance on algorithms can result in vertical line failure, a form of cognitive bias in which the clinician subconsciously adheres to an inflexible diagnostic approach.[10] To overcome this bias, clinicians need to think laterally and consider alternative diagnoses when algorithms do not lead to expected outcomes. This case highlights the challenges of knowing when to break free of conventional approaches and the rewards of taking a well‐chosen detour that leads to the diagnosis.
KEY POINTS
- Recurrent, occult gastrointestinal bleeding should raise concern for a small bowel source, and clinicians may need to take a detour away from the usual workup to arrive at a diagnosis.
- CT angiography of the abdomen and pelvis may miss venous sources of bleeding, unless a venous phase is specifically requested.
- Ectopic varices can occur in patients with portal hypertension who have had a history of abdominal surgery; these patients can develop venous collaterals for decompression into the systemic circulation through the abdominal wall.
Disclosure
Nothing to report.
- , , . Updates in the pathogenesis, diagnosis and management of ectopic varices. Hepatol Int. 2008;2:322–334.
- , , . Management of ectopic varices. Hepatology. 1998;28:1154–1158.
- , , , et al. Current status of ectopic varices in Japan: results of a survey by the Japan Society for Portal Hypertension. Hepatol Res. 2010;40:763–766.
- , , . Stomal Varices: Management with decompression TIPS and transvenous obliteration or sclerosis. Tech Vasc Interv Radiol. 2013;16:126–134.
- , , , et al. Jejunal varices as a cause of massive gastrointestinal bleeding. Am J Gastroenterol. 1992;87:514–517.
- , . Ectopic varices in portal hypertension. Clin Gastroenterol. 1985;14:105–121.
- , , , et al. Ectopic varices in portal hypertension: computed tomographic angiography instead of repeated endoscopies for diagnosis. Eur J Gastroenterol Hepatol. 2011;23:620–622.
- , , , et al. ACR appropriateness criteria. Radiologic management of lower gastrointestinal tract bleeding. Reston, VA: American College of Radiology; 2011. Available at: http://www.acr.org/Quality‐Safety/Appropriateness‐Criteria/∼/media/5F9CB95C164E4DA19DCBCFBBA790BB3C.pdf. Accessed January 28, 2015.
- , . Diagnosis and management of ectopic varices. Gastrointest Interv. 2012;1:3–10.
- . Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9:1184–1204.
- , , . Updates in the pathogenesis, diagnosis and management of ectopic varices. Hepatol Int. 2008;2:322–334.
- , , . Management of ectopic varices. Hepatology. 1998;28:1154–1158.
- , , , et al. Current status of ectopic varices in Japan: results of a survey by the Japan Society for Portal Hypertension. Hepatol Res. 2010;40:763–766.
- , , . Stomal Varices: Management with decompression TIPS and transvenous obliteration or sclerosis. Tech Vasc Interv Radiol. 2013;16:126–134.
- , , , et al. Jejunal varices as a cause of massive gastrointestinal bleeding. Am J Gastroenterol. 1992;87:514–517.
- , . Ectopic varices in portal hypertension. Clin Gastroenterol. 1985;14:105–121.
- , , , et al. Ectopic varices in portal hypertension: computed tomographic angiography instead of repeated endoscopies for diagnosis. Eur J Gastroenterol Hepatol. 2011;23:620–622.
- , , , et al. ACR appropriateness criteria. Radiologic management of lower gastrointestinal tract bleeding. Reston, VA: American College of Radiology; 2011. Available at: http://www.acr.org/Quality‐Safety/Appropriateness‐Criteria/∼/media/5F9CB95C164E4DA19DCBCFBBA790BB3C.pdf. Accessed January 28, 2015.
- , . Diagnosis and management of ectopic varices. Gastrointest Interv. 2012;1:3–10.
- . Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9:1184–1204.
Evidence for Thromboembolism Prophylaxis
Deep venous thrombosis (DVT) and pulmonary embolism (PE), collectively referred to as venous thromboembolism (VTE), are common events in hospitalized patients and result in significant morbidity and mortality. Often silent and frequently unexpected, VTE is preventable. Accordingly, the American College of Chest Physicians recommends that pharmacologic prophylaxis be given to acutely ill medical patients admitted to the hospital with congestive heart failure or severe respiratory disease, or to patients who are confined to bed who have additional risk factors, such as cancer or previous VTE.1 Three recent meta‐analyses24 demonstrated significant reductions in VTE in general medicine patients with pharmacologic prophylaxis. Recently the National Quality Forum advocated that hospitals evaluate each patient upon admission and regularly thereafter, for the risk of developing DVT/VTE and utilize clinically appropriate methods to prevent DVT/VTE.5
Despite recommendations for prophylaxis, multiple studies demonstrate utilization in <50% of at‐risk general medical patients.68 Physicians' lack of awareness may partially explain this underutilization, but other likely factors include physicians' questions about the clinical importance of the outcome (eg, some studies have shown reductions primarily in asymptomatic distal DVT), doubt regarding the best form of prophylaxis (ie, unfractionated heparin [UFH] vs. low molecular weight heparin [LMWH]), uncertainty regarding optimal dosing regimens, and comparable uncertainty regarding which patients have sufficiently high risk for VTE to outweigh the risks of anticoagulation.
We undertook the current meta‐analysis to address questions about thromboembolism prevention in general medicine patients. Does pharmacologic prophylaxis prevent clinically relevant events? Is LMWH or UFH preferable in terms of either efficacy or safety?
MATERIALS AND METHODS
Search Strategy
We conducted an extensive search that included reviewing electronic databases (MEDLINE, EMBASE, and CINAHL) through June 2008, reviewing conference proceedings, and contacting drug manufacturers. The MEDLINE search combined the key words deep venous thrombosis, thromboembolism, AND pulmonary embolism with the terms primary prevention, prophylaxis, OR prevention. We limited the search results using the filter for randomized controlled trials in PubMed. Similar strategies (available on request) were used to search EMBASE, CINAHL, and the Cochrane Central Register of Controlled Trials. We also searched the Cochrane Database of Systematic Reviews to identify previous reviews on the same topic. We obtained translations of eligible, non‐English‐language articles.
The proceedings of annual meetings from the American Thoracic Society, the American Society of Hematology, and the Society for General Internal Medicine from 1994 to 2008 were hand‐searched for reports on DVT or PE prevention published in abstract form only. (Note: the American Society of Hematology was only available through 2007). We contacted the 3 main manufacturers of LMWHPfizer (dalteparin), Aventis (enoxaparin), Glaxo Smith Kline (nadoparin)and requested information on unpublished pharmaceutical sponsored trials. First authors from the trials included in this meta‐analysis were also contacted to determine if they knew of additional published or unpublished trials.
Inclusion and Exclusion Criteria
Studies were required to be prospective randomized controlled trials comparing UFH or LMWH to mechanical prophylaxis, placebo, or no intervention. We also included randomized head‐to‐head comparisons of UFH and LMWH. Eligible studies enrolled general medical patients. Trials including predominantly intensive care unit (ICU) patients; stroke, spinal cord, or acute myocardial infarction patients were excluded. We excluded trials focused on these populations because the risk for VTE may differ from that for general medical patients and because patients in these groups already commonly receive anticoagulants as a preventive measure or as active treatment (eg, for acute myocardial infarction [MI] care). Trials assessing thrombosis in patients with long‐term central venous access/catheters were also excluded. Articles focusing on long‐term rehabilitation patients were excluded.
Studies had to employ objective criteria for diagnosing VTE. For DVT these included duplex ultrasonography, venography, fibrinogen uptake scanning, impedance plethysmography, or autopsy as a primary or secondary outcome. Studies utilizing thermographic techniques were excluded.9 Eligible diagnostic modalities for PE consisted of pulmonary arteriogram, ventilation/perfusion scan, CT angiography, and autopsy.
After an initial review of article titles and abstracts, the full texts of all articles that potentially met our inclusion criteria were independently reviewed for eligibility by 2 authors (G.M.B., M.D.). In cases of disagreement, a third author (S.F.) independently reviewed the article and adjudicated decisions.
Quantitative Data Synthesis and Statistical Analysis
For all included articles, 2 reviewers independently abstracted data on key study features (including population size, trial design, modality of VTE diagnosis, and interventions delivered to treatment and control groups), results (including the rates of all DVT, proximal DVT, symptomatic DVT, PE, and death), as well as adverse events (such as bleeding and thrombocytopenia). We accepted the endpoint of DVT when assessed by duplex ultrasonography, venography, autopsy, or when diagnosed by fibrinogen uptake scanning or impedance plethysmography. For all endpoints we abstracted event rates as number of events based on intention to treat. Each study was assessed for quality using the Jadad scale.10 The Jadad scale is a validated tool for characterizing study quality that accounts for randomization, blinding, and description of withdrawals and dropouts in individual trials. The Jadad score ranges from 0 to 5 with higher numbers identifying trials of greater methodological rigor.
The trials were divided into 4 groups based on the prophylaxis agent used and the method of comparison (UFH vs. control, LMWH vs. control, LMWH vs. UFH, and LMWH/UFH combined vs. control). After combining trials for each group, we calculated a pooled relative risk (RR) and a 95% confidence interval (CI) based on both fixed and a random effects model using the DerSimonian and Laird method. Heterogeneity of the included studies was evaluated with a chi‐square statistic. The percentage of variation in the pooled RR attributable to heterogeneity was calculated and reported using the I‐squared statistic.11 Sensitivity analyses were performed and included repeating all analyses using high‐quality studies only (Jadad score 3 or higher). Publication bias was assessed using the methods developed by Egger et al.12 and Begg and Mazumdar.13 All analyses were performed using STATA SE version 9 (Stata Corp, College Station, TX).
RESULTS
Study Identification and Selection
The computerized literature search resulted in 5284 articles. Three additional citations were found by review of bibliographies. No additional trials were identified from reviews of abstracts from national meetings. Representatives from the 3 pharmaceutical companies reported no knowledge of additional published or unpublished data. Of the 5287 studies identified by the search, 14 studies met all eligibility criteria (Figure 1).
Study Characteristics
The 14 trials eligible for inclusion in the analysis consisted of 8 comparisons of UFH or LMWH vs. control (Table 1) and 6 head‐to‐head comparisons of UFH and LMWH (Table 2). The 14 studies included 8 multicenter trials and enrolled a total of 24,515 patients: 20,594 in the 8 trials that compared UFH or LMWH with placebo and 3921 in the 6 trials that compared LMWH with UFH. Two trials exclusively enrolled patients with either congestive heart failure or severe respiratory disease,14, 15 while 12 trials enrolled mixed populations. In 8 trials a period of immobility was necessary for study entry,14, 1621 while in 2 trials immobility was not required.22, 23 In the 4 remaining trials immobility was not explicitly discussed.15, 2426 One‐half of the trials required a length of stay greater than 3 days.1719, 2225
| Study (Year)Reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Belch et al. (1981)15 | 100 | 8 | Age 40‐80 years; CHF; chest infection | UFH TID | None | FUS | VQ | No | 1 |
| Dahan et al. (1986)23 | 270 | 10* | Age >65 years | Enoxaparin 60 mg | Placebo | FUS | Autopsy | Yes | 3 |
| Halkin et al. (1982)20 | 1358 | Not reported | Age >40 years; immobile | UFH BID | None | No | No | No | 1 |
| Mahe et al. (2005)16 | 2474 | 13.08 | Age >40 years; immobile | Nadroparin 7500 IU | Placebo | Autopsy | Autopsy | Yes | 5 |
| Gardlund (1996)21 | 11693 | 8.2 | Age >55 years; immobile | UFH BID | None | Autopsy | Autopsy | No | 2 |
| Samama et al. (1999)24 | 738 | 7 | Age >40 years; length of stay 6 days; CHF; respiratory failure or 1 additional risk factor | Enoxaparin 40 mg | Placebo | Venography | Composite | Yes | 4 |
| Leizorovicz et al. (2004)25 | 3681 | 12.6 | Age >40 years; length of stay 4 days; CHF; respiratory failure or 1 additional risk factor | Dalteparin 5000 IU | Placebo | DUS | Composite | Yes | 4 |
| Lederle et al. (2006)22 | 280 | 13.4 | Age >60 years; length of stay 3 days | Enoxaparin 40 mg | Placebo | DUS | Composite | Yes | 5 |
| Study (Year)reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug/Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Bergmann and Neuhart (1996)27 | 442 | 9.5 | Age >65 years; immobile | Enoxaparin 20 mg | UFH BID | FUS | Composite | Yes | 5 |
| Harenberg et al. (1990)19 | 166 | 10* | Age 40‐80 years; 1 week of bed rest | LMWH 1.500 aPTT units QD | UFH TID | IP | No | Yes | 3 |
| Kleber et al. (2003)14 | 665 | 9.8 | Age 18 years; severe CHF or respiratory disease; immobile | Enoxaparin 40 mg | UFH TID | Venography | Composite | No | 3 |
| Aquino et al. (1990)26 | 99 | Not reported | Age >70 years | Nadoparine 7500 IU | UFH BID | DUS | Composite | No | 1 |
| Harenberg et al. (1996)18 | 1590 | 10* | Age 50‐80 years; immobile + 1 additional risk factor | Nadoparine 36 mg | UFH TID | DUS | Composite | Yes | 4 |
| Lechler et al. (1996)17 | 959 | 7* | Age >18 years; immobile + 1 additional risk factor | Enoxaparin 40 mg | UFH TID | DUS | Composite | Yes | 3 |
While minimum age for study entry varied, the patient population predominantly ranged from 65 to 85 years of age. Many of the trials reported expected, not actual, treatment duration. The range of expected treatment was 7 to 21 days, with 10 days of treatment the most frequently mentioned. In the 8 trials1416, 21, 22, 24, 25, 27 that reported actual treatment duration, the range was 8 to 13.4 days. Most trials did not report number of VTE risk factors per patient, nor was there uniform acceptance of risk factors across trials.
UFH or LMWH vs. Control
DVT
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of all DVT (RR = 0.55; 95% CI: 0.36‐0.83) (Figure 2A). When stratified by methodological quality, 5 trials16, 2225 with Jadad scores of 3 or higher showed an RR reduction of 0.53 (95% CI: 0.38‐0.72) in reducing all DVT. All of the higher‐quality trials compared LMWH to placebo. Across 4 trials that reported data for symptomatic DVT there was a nonsignificant reduction in RR compared with placebo (RR = 0.73; 95% CI: 0.45‐1.16) (Figure 2B). Only 2 trials24, 25 (both LMWH trials) reported results for proximal DVT and demonstrated significant benefit of prophylaxis with a pooled RR of 0.46 (95% CI: 0.31‐0.69) (Figure 2C).
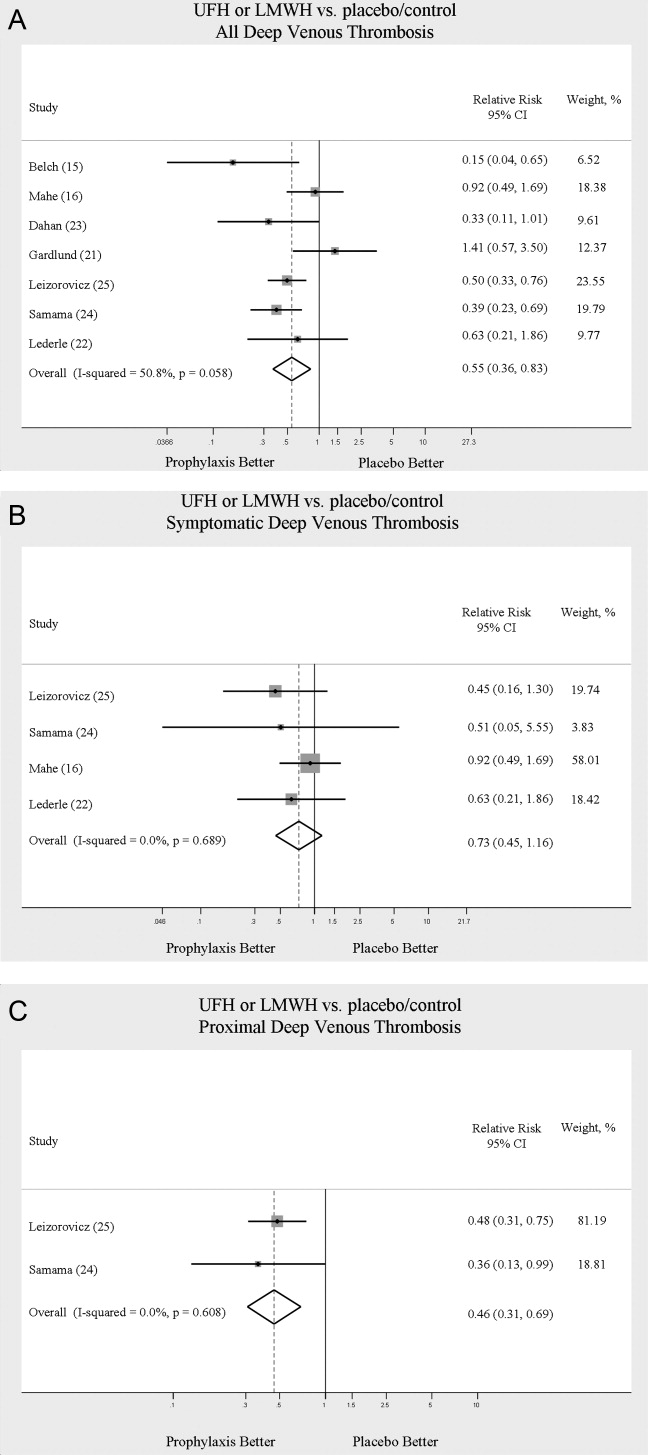
PE
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of PE (RR = 0.70; 95% CI: 0.53‐0.93) (Figure 3A). The 5 trials16, 2225 with Jadad scores of 3 or greater showed a similar relative risk reduction, but the result was no longer statistically significant (RR = 0.56; 95% CI: 0.31‐1.02). Two of the trials16, 21 relied solely on the results of autopsy to diagnose PE, which may have given rise to chance differences in detection due to generally low autopsy rates. Eliminating these 2 studies from the analysis resulted in loss of statistical significance for the reduction in risk for PE (RR = 0.48; 95% CI: 0.20‐1.15).
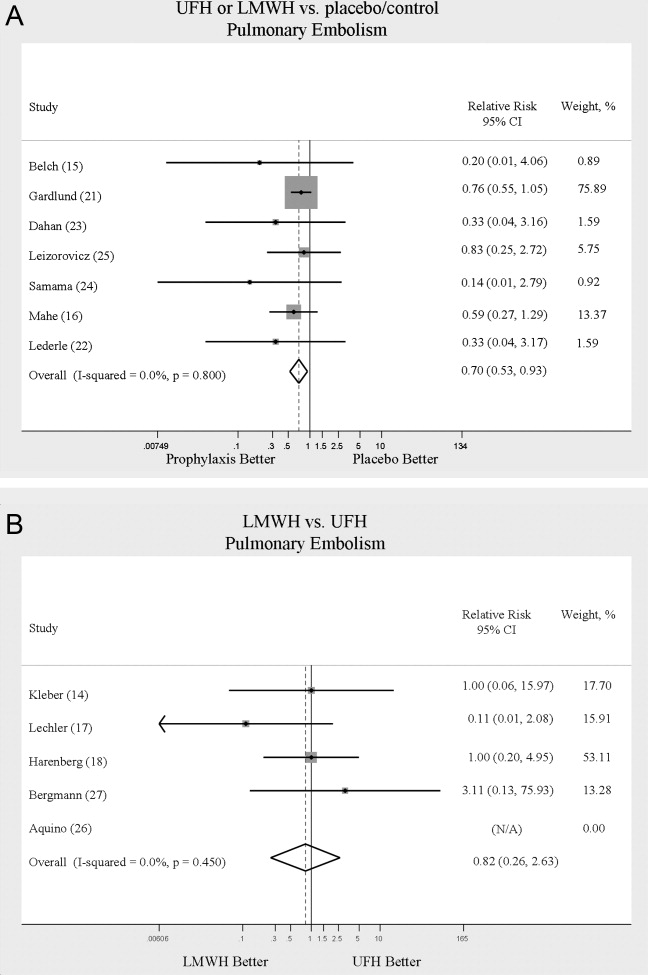
Death
Seven trials16, 2025 comparing either UFH or LMWH to control examined the impact of pharmacologic prophylaxis on death and found no significant difference between treated and untreated patients across all trials (RR = 0.92; 95% CI: 0.82‐1.03) and those limited to studies with Jadad scores of 3 or higher (RR = 0.97; 95% CI: 0.80‐1.17).
LMWH vs. UFH
DVT
In 6 trials14, 1719, 26, 27 comparing LMWH to UFH given either twice a day (BID) or 3 times a day (TID), there was no statistically significant difference in all DVT (RR = 0.90; 95% CI: 0.57‐1.43). (For all analyses RRs <1 favor LMWH, while RRs >1 favor UFH.) A total of 2 trials14, 18 reported results separately for proximal DVT with no statistically significant difference noted between UFH and LMWH (RR = 1.60; 95% CI: 0.53‐4.88). One small trial26 reported findings comparing UFH to LMWH for prevention of symptomatic DVT with no difference noted.
PE
Pooled data from the 5 trials14, 17, 18, 26, 27 comparing UFH to LMWH in the prevention of PE showed no statistically significant difference in rates of pulmonary embolism (RR = 0.82; 95% CI: 0.26‐2.63) (Figure 3B). In sensitivity analysis this result was not impacted by Jadad score.
Death
When UFH was compared to LMWH no statistically significant difference in the rate of death was found (RR = 0.96; 95% CI: 0.50‐1.85). Here again, no difference was noted when limited to studies with Jadad scores of 3 or higher.
Complications
We evaluated adverse events of heparin products used for prophylaxis and whether there were differences between UFH and LMWH. Reporting of complications was not uniform from study to study, making pooling more difficult. However, we were able to abstract data on any bleeding, major bleeding, and thrombocytopenia from several studies. In 5 studies15, 16, 2325 of either UFH or LMWH vs. control, a significantly increased risk of any bleeding (RR = 1.54; 95% CI: 1.15‐2.06) (Figure 4A) was found. When only major bleeding was evaluated, no statistically significant difference was noted (RR = 1.20; 95% CI: 0.55‐2.58) (Figure 4B). In 4 trials16, 22, 24, 25 the occurrence of thrombocytopenia was not significantly different when comparing UFH or LMWH to control (RR = 0.92; 95% CI: 0.46‐1.86).
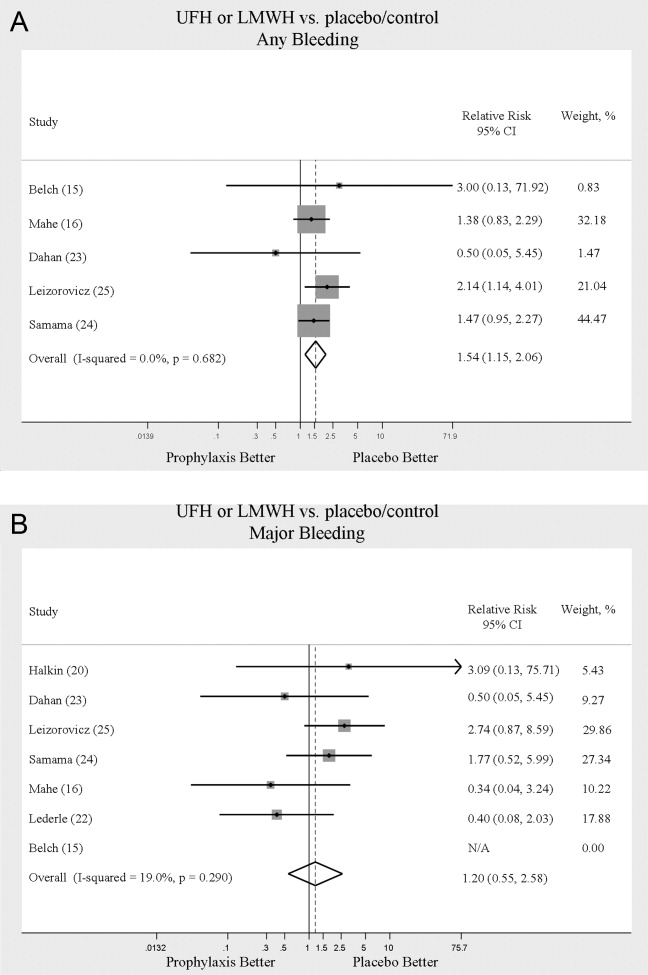
When LMWH was compared to UFH in 4 trials,14, 17, 18, 27 a nonsignificant trend toward a decrease in any bleeding was found in the LMWH group (RR = 0.72; 95% CI: 0.44‐1.16) (Figure 5A). A similar trend was seen favoring LMWH in rates of major bleeding (RR = 0.57; 95% CI: 0.25‐1.32) (Figure 5B). Neither trend was statistically significant. Three trials comparing LMWH to UFH reported on thrombocytopenia17, 18, 27 with no significant difference noted (RR = 0.52; 95% CI: 0.06‐4.18).
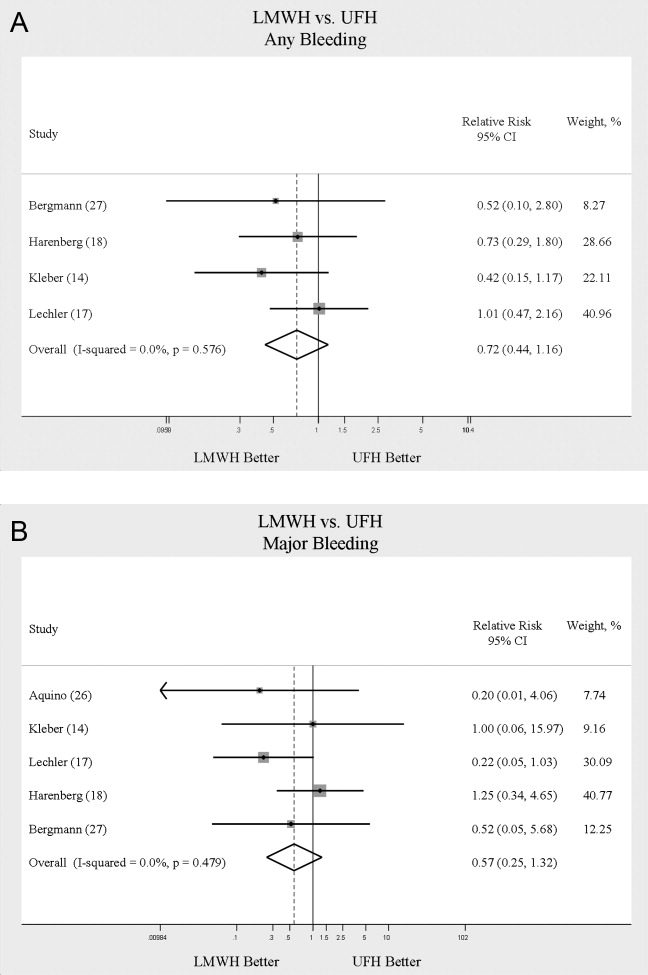
Heterogeneity and Publication Bias
No statistically significant heterogeneity was identified between trials for any outcomes. The highest I‐squared value was 54.5% (P = 0.14) for the endpoint of thrombocytopenia when UFH was compared to LMWH. In some cases, the nonsignificant results for tests of heterogeneity may have reflected small numbers of trials, but the values for I‐squared for all other endpoints were close to zero indicating that little nonrandom variation existed in the results across studies. All analyses were run using both random effects and fixed effects modeling. While we report results for random effects, no significant differences were observed using fixed effects.
We tested for publication bias using the methods developed by Egger et al.12 and Begg and Mazumdar.13 There was evidence of bias only for the outcome of PE when prophylaxis was compared to control, as the results for both tests were significant (Begg and Mazumdar:13 P = 0.035; Egger et al.:12 P = 0.010). For other outcomes tested, including all DVT (prophylaxis compared to control, and LMWH vs. UFH) as well as PE (LMWH vs. UFH), the P‐values were not significant.
DISCUSSION
When compared to control, LMWH or UFH decreased the risk of all DVT by 45% (RR = 0.55; 95% CI: 0.36‐0.83) and proximal DVT by 54% (RR = 0.46; 95% CI: 0.31‐0.69). PE was also decreased by 30% (RR = 0.70; 95% CI: 0.53‐0.93). Of note, when prophylaxis was compared with placebo all of the high‐quality studies showing a benefit were done using LMWH. The benefits of prophylaxis occurred at the cost of a 54% increased overall risk of bleeding (RR = 1.54; 95% CI 1.15‐2.06). However, the risk of major bleeding was not significantly increased. We did not find a mortality benefit to pharmacologic thromboembolism prophylaxis.
When comparing UFH to LMWH, we noted no difference in all DVT, symptomatic DVT, proximal DVT, PE, or death. While there was a trend toward less bleeding with LMWH, this was not statistically significant.
Taken in aggregate, our findings are in agreement with previous published meta‐analyses reporting net benefit for thromboembolism prophylaxis in medical patients.24, 22, 28, 29 Our meta‐analysis has several methodological strengths over the prior studies, including a comprehensive search of both the published and unpublished literature and assessment of the relationship between methodological quality of included trials and reported benefit. In contrast to previous reviews, our analysis highlights several limitations of the current evidence.
First, many of the studies are older, with predicted lengths of stay of greater than 1 week. The 8‐13‐day range of treatment duration we found in this study is longer than the average length of stay in today's hospitals. Second, there is variability in the diagnostic tests used to diagnose DVT, as well as variation in the definition of DVT among studies. Studies using fibrinogen uptake scanning reported rates of DVT as high as 26%15 while studies using venography reported DVT rates of almost 15% in the placebo arm.24 These rates are higher than most physicians' routine practice. One reason for this discrepancy is most studies did not distinguish below‐the‐knee DVT from more clinically relevant above‐the‐knee DVT. Systematic reviews of medical and surgical patients have found rates of proximal propagation from 0% to 29% in untreated patients.30, 31 Though controversial, below‐the‐knee DVT is believed less morbid than proximal DVT or symptomatic DVT. We addressed this by focusing specifically on clinically relevant endpoints of proximal and symptomatic DVT. When we restricted our analysis to proximal DVT we found a 54% RR reduction in 2 pooled trials of LMWH compared to placebo. In pooled analyses symptomatic DVT was not affected by prophylaxis. When compared head‐to‐head there were no differences between LMWH and UFH for proximal DVT or symptomatic DVT.
When considering PE, the utilization of autopsy as the sole diagnostic method in 2 large trials16, 21 is particularly problematic. In the trial by Garlund,21 the mortality rate was 5.4%, with an autopsy rate of 60.1%. Similarly, in the trial by Mahe et al.,16 the mortality rate was 10%, with an autopsy rate of 49%. Given the low absolute number of deaths and substantial proportion of decedents without autopsy, the potential for chance to produce an imbalance in detection of PE is high in these studies. When we excluded these 2 trials, we found that PE was no longer reduced to a statistically significant degree by prophylaxis. Loss of significance for PE in 2 sensitivity analyses (when excluding studies of lower quality, or using autopsy as a sole diagnostic study) is problematic and calls into question the true benefit of prophylaxis for prevention of PE.
Another limitation of the current literature centers on the variability of dosing used. We pooled trials of UFH whether given BID or TID. Given the small number of trials we did not do sensitivity analyses by dosage. A recent meta‐analysis3 found both doses are efficacious, while a recent review article32 suggested superiority of TID dosing. We believe the available literature does not clearly address this issue. Regarding comparisons of LMWH to UFH, dosing variability was also noted. The trial by Bergmann and Neuhart27 used enoxaparin 20 mg per day and found similar efficacy to UFH BID, while the Samama et al.24 trial found enoxaparin 20 mg per day no more efficacious than placebo. While the literature does not clearly define a best dose, we believe enoxaparin doses lower than 40 mg daily do not reflect the standard of care.
An additional limitation of the literature is publication bias. We assessed the possibility of publication bias by a variety of means. We did find statistical evidence of publication bias for the outcome of PE when prophylaxis was compared to control. Importantly, two meta‐analyses2, 4 on thromboembolism prophylaxis for general medicine patients suggested publication bias is present and our finding supports this conclusion. While no test for publication bias is foolproof, the best protection against publication bias, which we pursued in our study, consists of a thorough search for unpublished studies, including a search of conference proceedings, contact with experts in the field, and manufacturers of LMWH.
A final limitation of the current literature centers on risk assessment. All of the trials in this meta‐analysis included patients with an elevated level of risk. Unfortunately, risk was not clearly defined in many studies, and there was no minimum level of risk between trials. While immobility, age, and length of stay were reported for most studies, other risk factors such as personal history of thromboembolism and malignancy were not uniformly reported. Based on our analysis we are not confident our results can be extrapolated to all general medicine patients.
In conclusion, we found good evidence that pharmacologic prophylaxis significantly decreases the risk of all DVT and proximal DVT in at‐risk general medical patients. However, only LMWH was shown to prevent proximal DVT. We found inconclusive evidence that prophylaxis prevents PE. When compared directly we did not find clear superiority between UFH and LMWH, though several limitations of the current literature hamper decision‐making. Given the lower cost, it may seem justified to use UFH. However, there are other practical issues, such as the fact that LMWH is given once daily, and so potentially preferred by patients and more efficient for nurses. All of these results pertain to patients with elevated risk. While we did not find significant safety concerns with prophylaxis we do not know if these results can be extrapolated to lower‐risk patients. We believe that recommending widespread prophylaxis of all general medicine patients requires additional evidence about appropriate patient selection.
Acknowledgements
The authors thank Emmanuelle Williams, MD, for translating articles from French; Claudia Figueroa, MS, for translating articles from Spanish; Vikas Gulani, MD, for translating articles from German; and Rebecca Lee, MS, for translating articles from German, Dutch, and Italian. In addition, the authors thank Dr. Dilzer from Pfizer Global Pharmaceuticals, Kathleen E. Moigis from Aventis, and Carol McCullen from Glaxo Smith Kline for their search for unpublished pharmaceutical trials of low molecular weight heparins. Finally, the authors thank the Veterans Administration/University of Michigan Patient Safety Enhancement Program for research support.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(suppl):338S–400S.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- ,,,,.Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis.Chest.2007;131(2):507–516.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum. National Consensus Standards for the Prevention and Care of Venous Thromboembolism (including Deep Vein Thrombosis and Pulmonary Embolism). Available at: http://www.qualityforum.org/projects/completed/vte/index.asp. Accessed May2009.
- ,.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17(10):788–791.
- ,,,.[Effectiveness of low molecular weight heparin (Fragmin) in the prevention of thromboembolism in internal medicine patients. A randomized double‐blind study].Med Klin (Munich).1988;83(7):241–245, 278.
- ,,, et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials.1996;17(1):1–12.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21(11):1539–1558.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315(7109):629–634.
- ,.Operating characteristics of a rank correlation test for publication bias.Biometrics.1994;50(4):1088–1101.
- ,,,,,.Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease.Am Heart J.2003;145(4):614–621.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26(2):115–117.
- ,,,,.Lack of effect of a low‐molecular‐weight heparin (nadroparin) on mortality in bedridden medical in‐patients: a prospective randomised double‐blind study.Eur J Clin Pharmacol.2005;61(5‐6):347–351.
- ,,.The venous thrombotic risk in non‐surgical patients: epidemiological data and efficacy/safety profile of a low‐molecular‐weight heparin (enoxaparin). The Prime Study Group.Haemostasis.1996;26(suppl 2):49–56.
- ,,.Subcutaneous low‐molecular‐weight heparin versus standard heparin and the prevention of thromboembolism in medical inpatients. The Heparin Study in Internal Medicine Group.Haemostasis.1996;26(3):127–139.
- ,,, et al.Randomized controlled study of heparin and low molecular weight heparin for prevention of deep‐vein thrombosis in medical patients.Thromb Res.1990;59(3):639–650.
- ,,,.Reduction of mortality in general medical in‐patients by low‐dose heparin prophylaxis.Ann Intern Med.1982;96(5):561–565.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,, et al.The prophylaxis of medical patients for thromboembolism pilot study.Am J Med.2006;119(1):54–59.
- ,,, et al.Prevention of deep vein thrombosis in elderly medical in‐patients by a low molecular weight heparin: a randomized double‐blind trial.Haemostasis.1986;16(2):159–164.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- ,,.Prevention of thromboembolic accidents in elderly subjects with Fraxiparine. In: Bounameaux H, Samama MM, Ten Cate JW, eds.Fraxiaparine. 2nd International Symposium. Recent pharmacological and clinical data.New York:Schattauer;1990:51‐54.
- ,.A multicenter randomized double‐blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in‐patients bedridden for an acute medical illness. The Enoxaparin in Medicine Study Group.Thromb Haemost.1996;76(4):529–534.
- ,,, et al.Prevention of venous thromboembolism in internal medicine with unfractionated or low‐molecular‐weight heparins: a meta‐analysis of randomised clinical trials.Thromb Haemost.2000;83(1):14–19.
- ,,,,.Meta‐analysis of venous thromboembolism prophylaxis in medically Ill patients.Clin Ther.2007;29(11):2395–2405.
- ,,,,,.Clinical relevance of distal deep vein thrombosis. Review of literature data.Thromb Haemost.2006;95(1):56–64.
- .Natural history of venous thromboembolism.Circulation.2003;107(suppl 1):I22–I30.
- .Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients.N Engl J Med.2007;356(14):1438–1444.
Deep venous thrombosis (DVT) and pulmonary embolism (PE), collectively referred to as venous thromboembolism (VTE), are common events in hospitalized patients and result in significant morbidity and mortality. Often silent and frequently unexpected, VTE is preventable. Accordingly, the American College of Chest Physicians recommends that pharmacologic prophylaxis be given to acutely ill medical patients admitted to the hospital with congestive heart failure or severe respiratory disease, or to patients who are confined to bed who have additional risk factors, such as cancer or previous VTE.1 Three recent meta‐analyses24 demonstrated significant reductions in VTE in general medicine patients with pharmacologic prophylaxis. Recently the National Quality Forum advocated that hospitals evaluate each patient upon admission and regularly thereafter, for the risk of developing DVT/VTE and utilize clinically appropriate methods to prevent DVT/VTE.5
Despite recommendations for prophylaxis, multiple studies demonstrate utilization in <50% of at‐risk general medical patients.68 Physicians' lack of awareness may partially explain this underutilization, but other likely factors include physicians' questions about the clinical importance of the outcome (eg, some studies have shown reductions primarily in asymptomatic distal DVT), doubt regarding the best form of prophylaxis (ie, unfractionated heparin [UFH] vs. low molecular weight heparin [LMWH]), uncertainty regarding optimal dosing regimens, and comparable uncertainty regarding which patients have sufficiently high risk for VTE to outweigh the risks of anticoagulation.
We undertook the current meta‐analysis to address questions about thromboembolism prevention in general medicine patients. Does pharmacologic prophylaxis prevent clinically relevant events? Is LMWH or UFH preferable in terms of either efficacy or safety?
MATERIALS AND METHODS
Search Strategy
We conducted an extensive search that included reviewing electronic databases (MEDLINE, EMBASE, and CINAHL) through June 2008, reviewing conference proceedings, and contacting drug manufacturers. The MEDLINE search combined the key words deep venous thrombosis, thromboembolism, AND pulmonary embolism with the terms primary prevention, prophylaxis, OR prevention. We limited the search results using the filter for randomized controlled trials in PubMed. Similar strategies (available on request) were used to search EMBASE, CINAHL, and the Cochrane Central Register of Controlled Trials. We also searched the Cochrane Database of Systematic Reviews to identify previous reviews on the same topic. We obtained translations of eligible, non‐English‐language articles.
The proceedings of annual meetings from the American Thoracic Society, the American Society of Hematology, and the Society for General Internal Medicine from 1994 to 2008 were hand‐searched for reports on DVT or PE prevention published in abstract form only. (Note: the American Society of Hematology was only available through 2007). We contacted the 3 main manufacturers of LMWHPfizer (dalteparin), Aventis (enoxaparin), Glaxo Smith Kline (nadoparin)and requested information on unpublished pharmaceutical sponsored trials. First authors from the trials included in this meta‐analysis were also contacted to determine if they knew of additional published or unpublished trials.
Inclusion and Exclusion Criteria
Studies were required to be prospective randomized controlled trials comparing UFH or LMWH to mechanical prophylaxis, placebo, or no intervention. We also included randomized head‐to‐head comparisons of UFH and LMWH. Eligible studies enrolled general medical patients. Trials including predominantly intensive care unit (ICU) patients; stroke, spinal cord, or acute myocardial infarction patients were excluded. We excluded trials focused on these populations because the risk for VTE may differ from that for general medical patients and because patients in these groups already commonly receive anticoagulants as a preventive measure or as active treatment (eg, for acute myocardial infarction [MI] care). Trials assessing thrombosis in patients with long‐term central venous access/catheters were also excluded. Articles focusing on long‐term rehabilitation patients were excluded.
Studies had to employ objective criteria for diagnosing VTE. For DVT these included duplex ultrasonography, venography, fibrinogen uptake scanning, impedance plethysmography, or autopsy as a primary or secondary outcome. Studies utilizing thermographic techniques were excluded.9 Eligible diagnostic modalities for PE consisted of pulmonary arteriogram, ventilation/perfusion scan, CT angiography, and autopsy.
After an initial review of article titles and abstracts, the full texts of all articles that potentially met our inclusion criteria were independently reviewed for eligibility by 2 authors (G.M.B., M.D.). In cases of disagreement, a third author (S.F.) independently reviewed the article and adjudicated decisions.
Quantitative Data Synthesis and Statistical Analysis
For all included articles, 2 reviewers independently abstracted data on key study features (including population size, trial design, modality of VTE diagnosis, and interventions delivered to treatment and control groups), results (including the rates of all DVT, proximal DVT, symptomatic DVT, PE, and death), as well as adverse events (such as bleeding and thrombocytopenia). We accepted the endpoint of DVT when assessed by duplex ultrasonography, venography, autopsy, or when diagnosed by fibrinogen uptake scanning or impedance plethysmography. For all endpoints we abstracted event rates as number of events based on intention to treat. Each study was assessed for quality using the Jadad scale.10 The Jadad scale is a validated tool for characterizing study quality that accounts for randomization, blinding, and description of withdrawals and dropouts in individual trials. The Jadad score ranges from 0 to 5 with higher numbers identifying trials of greater methodological rigor.
The trials were divided into 4 groups based on the prophylaxis agent used and the method of comparison (UFH vs. control, LMWH vs. control, LMWH vs. UFH, and LMWH/UFH combined vs. control). After combining trials for each group, we calculated a pooled relative risk (RR) and a 95% confidence interval (CI) based on both fixed and a random effects model using the DerSimonian and Laird method. Heterogeneity of the included studies was evaluated with a chi‐square statistic. The percentage of variation in the pooled RR attributable to heterogeneity was calculated and reported using the I‐squared statistic.11 Sensitivity analyses were performed and included repeating all analyses using high‐quality studies only (Jadad score 3 or higher). Publication bias was assessed using the methods developed by Egger et al.12 and Begg and Mazumdar.13 All analyses were performed using STATA SE version 9 (Stata Corp, College Station, TX).
RESULTS
Study Identification and Selection
The computerized literature search resulted in 5284 articles. Three additional citations were found by review of bibliographies. No additional trials were identified from reviews of abstracts from national meetings. Representatives from the 3 pharmaceutical companies reported no knowledge of additional published or unpublished data. Of the 5287 studies identified by the search, 14 studies met all eligibility criteria (Figure 1).

Study Characteristics
The 14 trials eligible for inclusion in the analysis consisted of 8 comparisons of UFH or LMWH vs. control (Table 1) and 6 head‐to‐head comparisons of UFH and LMWH (Table 2). The 14 studies included 8 multicenter trials and enrolled a total of 24,515 patients: 20,594 in the 8 trials that compared UFH or LMWH with placebo and 3921 in the 6 trials that compared LMWH with UFH. Two trials exclusively enrolled patients with either congestive heart failure or severe respiratory disease,14, 15 while 12 trials enrolled mixed populations. In 8 trials a period of immobility was necessary for study entry,14, 1621 while in 2 trials immobility was not required.22, 23 In the 4 remaining trials immobility was not explicitly discussed.15, 2426 One‐half of the trials required a length of stay greater than 3 days.1719, 2225
| Study (Year)Reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Belch et al. (1981)15 | 100 | 8 | Age 40‐80 years; CHF; chest infection | UFH TID | None | FUS | VQ | No | 1 |
| Dahan et al. (1986)23 | 270 | 10* | Age >65 years | Enoxaparin 60 mg | Placebo | FUS | Autopsy | Yes | 3 |
| Halkin et al. (1982)20 | 1358 | Not reported | Age >40 years; immobile | UFH BID | None | No | No | No | 1 |
| Mahe et al. (2005)16 | 2474 | 13.08 | Age >40 years; immobile | Nadroparin 7500 IU | Placebo | Autopsy | Autopsy | Yes | 5 |
| Gardlund (1996)21 | 11693 | 8.2 | Age >55 years; immobile | UFH BID | None | Autopsy | Autopsy | No | 2 |
| Samama et al. (1999)24 | 738 | 7 | Age >40 years; length of stay 6 days; CHF; respiratory failure or 1 additional risk factor | Enoxaparin 40 mg | Placebo | Venography | Composite | Yes | 4 |
| Leizorovicz et al. (2004)25 | 3681 | 12.6 | Age >40 years; length of stay 4 days; CHF; respiratory failure or 1 additional risk factor | Dalteparin 5000 IU | Placebo | DUS | Composite | Yes | 4 |
| Lederle et al. (2006)22 | 280 | 13.4 | Age >60 years; length of stay 3 days | Enoxaparin 40 mg | Placebo | DUS | Composite | Yes | 5 |
| Study (Year)reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug/Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Bergmann and Neuhart (1996)27 | 442 | 9.5 | Age >65 years; immobile | Enoxaparin 20 mg | UFH BID | FUS | Composite | Yes | 5 |
| Harenberg et al. (1990)19 | 166 | 10* | Age 40‐80 years; 1 week of bed rest | LMWH 1.500 aPTT units QD | UFH TID | IP | No | Yes | 3 |
| Kleber et al. (2003)14 | 665 | 9.8 | Age 18 years; severe CHF or respiratory disease; immobile | Enoxaparin 40 mg | UFH TID | Venography | Composite | No | 3 |
| Aquino et al. (1990)26 | 99 | Not reported | Age >70 years | Nadoparine 7500 IU | UFH BID | DUS | Composite | No | 1 |
| Harenberg et al. (1996)18 | 1590 | 10* | Age 50‐80 years; immobile + 1 additional risk factor | Nadoparine 36 mg | UFH TID | DUS | Composite | Yes | 4 |
| Lechler et al. (1996)17 | 959 | 7* | Age >18 years; immobile + 1 additional risk factor | Enoxaparin 40 mg | UFH TID | DUS | Composite | Yes | 3 |
While minimum age for study entry varied, the patient population predominantly ranged from 65 to 85 years of age. Many of the trials reported expected, not actual, treatment duration. The range of expected treatment was 7 to 21 days, with 10 days of treatment the most frequently mentioned. In the 8 trials1416, 21, 22, 24, 25, 27 that reported actual treatment duration, the range was 8 to 13.4 days. Most trials did not report number of VTE risk factors per patient, nor was there uniform acceptance of risk factors across trials.
UFH or LMWH vs. Control
DVT
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of all DVT (RR = 0.55; 95% CI: 0.36‐0.83) (Figure 2A). When stratified by methodological quality, 5 trials16, 2225 with Jadad scores of 3 or higher showed an RR reduction of 0.53 (95% CI: 0.38‐0.72) in reducing all DVT. All of the higher‐quality trials compared LMWH to placebo. Across 4 trials that reported data for symptomatic DVT there was a nonsignificant reduction in RR compared with placebo (RR = 0.73; 95% CI: 0.45‐1.16) (Figure 2B). Only 2 trials24, 25 (both LMWH trials) reported results for proximal DVT and demonstrated significant benefit of prophylaxis with a pooled RR of 0.46 (95% CI: 0.31‐0.69) (Figure 2C).

PE
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of PE (RR = 0.70; 95% CI: 0.53‐0.93) (Figure 3A). The 5 trials16, 2225 with Jadad scores of 3 or greater showed a similar relative risk reduction, but the result was no longer statistically significant (RR = 0.56; 95% CI: 0.31‐1.02). Two of the trials16, 21 relied solely on the results of autopsy to diagnose PE, which may have given rise to chance differences in detection due to generally low autopsy rates. Eliminating these 2 studies from the analysis resulted in loss of statistical significance for the reduction in risk for PE (RR = 0.48; 95% CI: 0.20‐1.15).

Death
Seven trials16, 2025 comparing either UFH or LMWH to control examined the impact of pharmacologic prophylaxis on death and found no significant difference between treated and untreated patients across all trials (RR = 0.92; 95% CI: 0.82‐1.03) and those limited to studies with Jadad scores of 3 or higher (RR = 0.97; 95% CI: 0.80‐1.17).
LMWH vs. UFH
DVT
In 6 trials14, 1719, 26, 27 comparing LMWH to UFH given either twice a day (BID) or 3 times a day (TID), there was no statistically significant difference in all DVT (RR = 0.90; 95% CI: 0.57‐1.43). (For all analyses RRs <1 favor LMWH, while RRs >1 favor UFH.) A total of 2 trials14, 18 reported results separately for proximal DVT with no statistically significant difference noted between UFH and LMWH (RR = 1.60; 95% CI: 0.53‐4.88). One small trial26 reported findings comparing UFH to LMWH for prevention of symptomatic DVT with no difference noted.
PE
Pooled data from the 5 trials14, 17, 18, 26, 27 comparing UFH to LMWH in the prevention of PE showed no statistically significant difference in rates of pulmonary embolism (RR = 0.82; 95% CI: 0.26‐2.63) (Figure 3B). In sensitivity analysis this result was not impacted by Jadad score.
Death
When UFH was compared to LMWH no statistically significant difference in the rate of death was found (RR = 0.96; 95% CI: 0.50‐1.85). Here again, no difference was noted when limited to studies with Jadad scores of 3 or higher.
Complications
We evaluated adverse events of heparin products used for prophylaxis and whether there were differences between UFH and LMWH. Reporting of complications was not uniform from study to study, making pooling more difficult. However, we were able to abstract data on any bleeding, major bleeding, and thrombocytopenia from several studies. In 5 studies15, 16, 2325 of either UFH or LMWH vs. control, a significantly increased risk of any bleeding (RR = 1.54; 95% CI: 1.15‐2.06) (Figure 4A) was found. When only major bleeding was evaluated, no statistically significant difference was noted (RR = 1.20; 95% CI: 0.55‐2.58) (Figure 4B). In 4 trials16, 22, 24, 25 the occurrence of thrombocytopenia was not significantly different when comparing UFH or LMWH to control (RR = 0.92; 95% CI: 0.46‐1.86).

When LMWH was compared to UFH in 4 trials,14, 17, 18, 27 a nonsignificant trend toward a decrease in any bleeding was found in the LMWH group (RR = 0.72; 95% CI: 0.44‐1.16) (Figure 5A). A similar trend was seen favoring LMWH in rates of major bleeding (RR = 0.57; 95% CI: 0.25‐1.32) (Figure 5B). Neither trend was statistically significant. Three trials comparing LMWH to UFH reported on thrombocytopenia17, 18, 27 with no significant difference noted (RR = 0.52; 95% CI: 0.06‐4.18).

Heterogeneity and Publication Bias
No statistically significant heterogeneity was identified between trials for any outcomes. The highest I‐squared value was 54.5% (P = 0.14) for the endpoint of thrombocytopenia when UFH was compared to LMWH. In some cases, the nonsignificant results for tests of heterogeneity may have reflected small numbers of trials, but the values for I‐squared for all other endpoints were close to zero indicating that little nonrandom variation existed in the results across studies. All analyses were run using both random effects and fixed effects modeling. While we report results for random effects, no significant differences were observed using fixed effects.
We tested for publication bias using the methods developed by Egger et al.12 and Begg and Mazumdar.13 There was evidence of bias only for the outcome of PE when prophylaxis was compared to control, as the results for both tests were significant (Begg and Mazumdar:13 P = 0.035; Egger et al.:12 P = 0.010). For other outcomes tested, including all DVT (prophylaxis compared to control, and LMWH vs. UFH) as well as PE (LMWH vs. UFH), the P‐values were not significant.
DISCUSSION
When compared to control, LMWH or UFH decreased the risk of all DVT by 45% (RR = 0.55; 95% CI: 0.36‐0.83) and proximal DVT by 54% (RR = 0.46; 95% CI: 0.31‐0.69). PE was also decreased by 30% (RR = 0.70; 95% CI: 0.53‐0.93). Of note, when prophylaxis was compared with placebo all of the high‐quality studies showing a benefit were done using LMWH. The benefits of prophylaxis occurred at the cost of a 54% increased overall risk of bleeding (RR = 1.54; 95% CI 1.15‐2.06). However, the risk of major bleeding was not significantly increased. We did not find a mortality benefit to pharmacologic thromboembolism prophylaxis.
When comparing UFH to LMWH, we noted no difference in all DVT, symptomatic DVT, proximal DVT, PE, or death. While there was a trend toward less bleeding with LMWH, this was not statistically significant.
Taken in aggregate, our findings are in agreement with previous published meta‐analyses reporting net benefit for thromboembolism prophylaxis in medical patients.24, 22, 28, 29 Our meta‐analysis has several methodological strengths over the prior studies, including a comprehensive search of both the published and unpublished literature and assessment of the relationship between methodological quality of included trials and reported benefit. In contrast to previous reviews, our analysis highlights several limitations of the current evidence.
First, many of the studies are older, with predicted lengths of stay of greater than 1 week. The 8‐13‐day range of treatment duration we found in this study is longer than the average length of stay in today's hospitals. Second, there is variability in the diagnostic tests used to diagnose DVT, as well as variation in the definition of DVT among studies. Studies using fibrinogen uptake scanning reported rates of DVT as high as 26%15 while studies using venography reported DVT rates of almost 15% in the placebo arm.24 These rates are higher than most physicians' routine practice. One reason for this discrepancy is most studies did not distinguish below‐the‐knee DVT from more clinically relevant above‐the‐knee DVT. Systematic reviews of medical and surgical patients have found rates of proximal propagation from 0% to 29% in untreated patients.30, 31 Though controversial, below‐the‐knee DVT is believed less morbid than proximal DVT or symptomatic DVT. We addressed this by focusing specifically on clinically relevant endpoints of proximal and symptomatic DVT. When we restricted our analysis to proximal DVT we found a 54% RR reduction in 2 pooled trials of LMWH compared to placebo. In pooled analyses symptomatic DVT was not affected by prophylaxis. When compared head‐to‐head there were no differences between LMWH and UFH for proximal DVT or symptomatic DVT.
When considering PE, the utilization of autopsy as the sole diagnostic method in 2 large trials16, 21 is particularly problematic. In the trial by Garlund,21 the mortality rate was 5.4%, with an autopsy rate of 60.1%. Similarly, in the trial by Mahe et al.,16 the mortality rate was 10%, with an autopsy rate of 49%. Given the low absolute number of deaths and substantial proportion of decedents without autopsy, the potential for chance to produce an imbalance in detection of PE is high in these studies. When we excluded these 2 trials, we found that PE was no longer reduced to a statistically significant degree by prophylaxis. Loss of significance for PE in 2 sensitivity analyses (when excluding studies of lower quality, or using autopsy as a sole diagnostic study) is problematic and calls into question the true benefit of prophylaxis for prevention of PE.
Another limitation of the current literature centers on the variability of dosing used. We pooled trials of UFH whether given BID or TID. Given the small number of trials we did not do sensitivity analyses by dosage. A recent meta‐analysis3 found both doses are efficacious, while a recent review article32 suggested superiority of TID dosing. We believe the available literature does not clearly address this issue. Regarding comparisons of LMWH to UFH, dosing variability was also noted. The trial by Bergmann and Neuhart27 used enoxaparin 20 mg per day and found similar efficacy to UFH BID, while the Samama et al.24 trial found enoxaparin 20 mg per day no more efficacious than placebo. While the literature does not clearly define a best dose, we believe enoxaparin doses lower than 40 mg daily do not reflect the standard of care.
An additional limitation of the literature is publication bias. We assessed the possibility of publication bias by a variety of means. We did find statistical evidence of publication bias for the outcome of PE when prophylaxis was compared to control. Importantly, two meta‐analyses2, 4 on thromboembolism prophylaxis for general medicine patients suggested publication bias is present and our finding supports this conclusion. While no test for publication bias is foolproof, the best protection against publication bias, which we pursued in our study, consists of a thorough search for unpublished studies, including a search of conference proceedings, contact with experts in the field, and manufacturers of LMWH.
A final limitation of the current literature centers on risk assessment. All of the trials in this meta‐analysis included patients with an elevated level of risk. Unfortunately, risk was not clearly defined in many studies, and there was no minimum level of risk between trials. While immobility, age, and length of stay were reported for most studies, other risk factors such as personal history of thromboembolism and malignancy were not uniformly reported. Based on our analysis we are not confident our results can be extrapolated to all general medicine patients.
In conclusion, we found good evidence that pharmacologic prophylaxis significantly decreases the risk of all DVT and proximal DVT in at‐risk general medical patients. However, only LMWH was shown to prevent proximal DVT. We found inconclusive evidence that prophylaxis prevents PE. When compared directly we did not find clear superiority between UFH and LMWH, though several limitations of the current literature hamper decision‐making. Given the lower cost, it may seem justified to use UFH. However, there are other practical issues, such as the fact that LMWH is given once daily, and so potentially preferred by patients and more efficient for nurses. All of these results pertain to patients with elevated risk. While we did not find significant safety concerns with prophylaxis we do not know if these results can be extrapolated to lower‐risk patients. We believe that recommending widespread prophylaxis of all general medicine patients requires additional evidence about appropriate patient selection.
Acknowledgements
The authors thank Emmanuelle Williams, MD, for translating articles from French; Claudia Figueroa, MS, for translating articles from Spanish; Vikas Gulani, MD, for translating articles from German; and Rebecca Lee, MS, for translating articles from German, Dutch, and Italian. In addition, the authors thank Dr. Dilzer from Pfizer Global Pharmaceuticals, Kathleen E. Moigis from Aventis, and Carol McCullen from Glaxo Smith Kline for their search for unpublished pharmaceutical trials of low molecular weight heparins. Finally, the authors thank the Veterans Administration/University of Michigan Patient Safety Enhancement Program for research support.
Deep venous thrombosis (DVT) and pulmonary embolism (PE), collectively referred to as venous thromboembolism (VTE), are common events in hospitalized patients and result in significant morbidity and mortality. Often silent and frequently unexpected, VTE is preventable. Accordingly, the American College of Chest Physicians recommends that pharmacologic prophylaxis be given to acutely ill medical patients admitted to the hospital with congestive heart failure or severe respiratory disease, or to patients who are confined to bed who have additional risk factors, such as cancer or previous VTE.1 Three recent meta‐analyses24 demonstrated significant reductions in VTE in general medicine patients with pharmacologic prophylaxis. Recently the National Quality Forum advocated that hospitals evaluate each patient upon admission and regularly thereafter, for the risk of developing DVT/VTE and utilize clinically appropriate methods to prevent DVT/VTE.5
Despite recommendations for prophylaxis, multiple studies demonstrate utilization in <50% of at‐risk general medical patients.68 Physicians' lack of awareness may partially explain this underutilization, but other likely factors include physicians' questions about the clinical importance of the outcome (eg, some studies have shown reductions primarily in asymptomatic distal DVT), doubt regarding the best form of prophylaxis (ie, unfractionated heparin [UFH] vs. low molecular weight heparin [LMWH]), uncertainty regarding optimal dosing regimens, and comparable uncertainty regarding which patients have sufficiently high risk for VTE to outweigh the risks of anticoagulation.
We undertook the current meta‐analysis to address questions about thromboembolism prevention in general medicine patients. Does pharmacologic prophylaxis prevent clinically relevant events? Is LMWH or UFH preferable in terms of either efficacy or safety?
MATERIALS AND METHODS
Search Strategy
We conducted an extensive search that included reviewing electronic databases (MEDLINE, EMBASE, and CINAHL) through June 2008, reviewing conference proceedings, and contacting drug manufacturers. The MEDLINE search combined the key words deep venous thrombosis, thromboembolism, AND pulmonary embolism with the terms primary prevention, prophylaxis, OR prevention. We limited the search results using the filter for randomized controlled trials in PubMed. Similar strategies (available on request) were used to search EMBASE, CINAHL, and the Cochrane Central Register of Controlled Trials. We also searched the Cochrane Database of Systematic Reviews to identify previous reviews on the same topic. We obtained translations of eligible, non‐English‐language articles.
The proceedings of annual meetings from the American Thoracic Society, the American Society of Hematology, and the Society for General Internal Medicine from 1994 to 2008 were hand‐searched for reports on DVT or PE prevention published in abstract form only. (Note: the American Society of Hematology was only available through 2007). We contacted the 3 main manufacturers of LMWHPfizer (dalteparin), Aventis (enoxaparin), Glaxo Smith Kline (nadoparin)and requested information on unpublished pharmaceutical sponsored trials. First authors from the trials included in this meta‐analysis were also contacted to determine if they knew of additional published or unpublished trials.
Inclusion and Exclusion Criteria
Studies were required to be prospective randomized controlled trials comparing UFH or LMWH to mechanical prophylaxis, placebo, or no intervention. We also included randomized head‐to‐head comparisons of UFH and LMWH. Eligible studies enrolled general medical patients. Trials including predominantly intensive care unit (ICU) patients; stroke, spinal cord, or acute myocardial infarction patients were excluded. We excluded trials focused on these populations because the risk for VTE may differ from that for general medical patients and because patients in these groups already commonly receive anticoagulants as a preventive measure or as active treatment (eg, for acute myocardial infarction [MI] care). Trials assessing thrombosis in patients with long‐term central venous access/catheters were also excluded. Articles focusing on long‐term rehabilitation patients were excluded.
Studies had to employ objective criteria for diagnosing VTE. For DVT these included duplex ultrasonography, venography, fibrinogen uptake scanning, impedance plethysmography, or autopsy as a primary or secondary outcome. Studies utilizing thermographic techniques were excluded.9 Eligible diagnostic modalities for PE consisted of pulmonary arteriogram, ventilation/perfusion scan, CT angiography, and autopsy.
After an initial review of article titles and abstracts, the full texts of all articles that potentially met our inclusion criteria were independently reviewed for eligibility by 2 authors (G.M.B., M.D.). In cases of disagreement, a third author (S.F.) independently reviewed the article and adjudicated decisions.
Quantitative Data Synthesis and Statistical Analysis
For all included articles, 2 reviewers independently abstracted data on key study features (including population size, trial design, modality of VTE diagnosis, and interventions delivered to treatment and control groups), results (including the rates of all DVT, proximal DVT, symptomatic DVT, PE, and death), as well as adverse events (such as bleeding and thrombocytopenia). We accepted the endpoint of DVT when assessed by duplex ultrasonography, venography, autopsy, or when diagnosed by fibrinogen uptake scanning or impedance plethysmography. For all endpoints we abstracted event rates as number of events based on intention to treat. Each study was assessed for quality using the Jadad scale.10 The Jadad scale is a validated tool for characterizing study quality that accounts for randomization, blinding, and description of withdrawals and dropouts in individual trials. The Jadad score ranges from 0 to 5 with higher numbers identifying trials of greater methodological rigor.
The trials were divided into 4 groups based on the prophylaxis agent used and the method of comparison (UFH vs. control, LMWH vs. control, LMWH vs. UFH, and LMWH/UFH combined vs. control). After combining trials for each group, we calculated a pooled relative risk (RR) and a 95% confidence interval (CI) based on both fixed and a random effects model using the DerSimonian and Laird method. Heterogeneity of the included studies was evaluated with a chi‐square statistic. The percentage of variation in the pooled RR attributable to heterogeneity was calculated and reported using the I‐squared statistic.11 Sensitivity analyses were performed and included repeating all analyses using high‐quality studies only (Jadad score 3 or higher). Publication bias was assessed using the methods developed by Egger et al.12 and Begg and Mazumdar.13 All analyses were performed using STATA SE version 9 (Stata Corp, College Station, TX).
RESULTS
Study Identification and Selection
The computerized literature search resulted in 5284 articles. Three additional citations were found by review of bibliographies. No additional trials were identified from reviews of abstracts from national meetings. Representatives from the 3 pharmaceutical companies reported no knowledge of additional published or unpublished data. Of the 5287 studies identified by the search, 14 studies met all eligibility criteria (Figure 1).

Study Characteristics
The 14 trials eligible for inclusion in the analysis consisted of 8 comparisons of UFH or LMWH vs. control (Table 1) and 6 head‐to‐head comparisons of UFH and LMWH (Table 2). The 14 studies included 8 multicenter trials and enrolled a total of 24,515 patients: 20,594 in the 8 trials that compared UFH or LMWH with placebo and 3921 in the 6 trials that compared LMWH with UFH. Two trials exclusively enrolled patients with either congestive heart failure or severe respiratory disease,14, 15 while 12 trials enrolled mixed populations. In 8 trials a period of immobility was necessary for study entry,14, 1621 while in 2 trials immobility was not required.22, 23 In the 4 remaining trials immobility was not explicitly discussed.15, 2426 One‐half of the trials required a length of stay greater than 3 days.1719, 2225
| Study (Year)Reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Belch et al. (1981)15 | 100 | 8 | Age 40‐80 years; CHF; chest infection | UFH TID | None | FUS | VQ | No | 1 |
| Dahan et al. (1986)23 | 270 | 10* | Age >65 years | Enoxaparin 60 mg | Placebo | FUS | Autopsy | Yes | 3 |
| Halkin et al. (1982)20 | 1358 | Not reported | Age >40 years; immobile | UFH BID | None | No | No | No | 1 |
| Mahe et al. (2005)16 | 2474 | 13.08 | Age >40 years; immobile | Nadroparin 7500 IU | Placebo | Autopsy | Autopsy | Yes | 5 |
| Gardlund (1996)21 | 11693 | 8.2 | Age >55 years; immobile | UFH BID | None | Autopsy | Autopsy | No | 2 |
| Samama et al. (1999)24 | 738 | 7 | Age >40 years; length of stay 6 days; CHF; respiratory failure or 1 additional risk factor | Enoxaparin 40 mg | Placebo | Venography | Composite | Yes | 4 |
| Leizorovicz et al. (2004)25 | 3681 | 12.6 | Age >40 years; length of stay 4 days; CHF; respiratory failure or 1 additional risk factor | Dalteparin 5000 IU | Placebo | DUS | Composite | Yes | 4 |
| Lederle et al. (2006)22 | 280 | 13.4 | Age >60 years; length of stay 3 days | Enoxaparin 40 mg | Placebo | DUS | Composite | Yes | 5 |
| Study (Year)reference | Patients (n) | Duration (days) | VTE Risk Factors | Drug/Dose | Comparison | DVT Assessed | PE Assessed | Double Blind | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Bergmann and Neuhart (1996)27 | 442 | 9.5 | Age >65 years; immobile | Enoxaparin 20 mg | UFH BID | FUS | Composite | Yes | 5 |
| Harenberg et al. (1990)19 | 166 | 10* | Age 40‐80 years; 1 week of bed rest | LMWH 1.500 aPTT units QD | UFH TID | IP | No | Yes | 3 |
| Kleber et al. (2003)14 | 665 | 9.8 | Age 18 years; severe CHF or respiratory disease; immobile | Enoxaparin 40 mg | UFH TID | Venography | Composite | No | 3 |
| Aquino et al. (1990)26 | 99 | Not reported | Age >70 years | Nadoparine 7500 IU | UFH BID | DUS | Composite | No | 1 |
| Harenberg et al. (1996)18 | 1590 | 10* | Age 50‐80 years; immobile + 1 additional risk factor | Nadoparine 36 mg | UFH TID | DUS | Composite | Yes | 4 |
| Lechler et al. (1996)17 | 959 | 7* | Age >18 years; immobile + 1 additional risk factor | Enoxaparin 40 mg | UFH TID | DUS | Composite | Yes | 3 |
While minimum age for study entry varied, the patient population predominantly ranged from 65 to 85 years of age. Many of the trials reported expected, not actual, treatment duration. The range of expected treatment was 7 to 21 days, with 10 days of treatment the most frequently mentioned. In the 8 trials1416, 21, 22, 24, 25, 27 that reported actual treatment duration, the range was 8 to 13.4 days. Most trials did not report number of VTE risk factors per patient, nor was there uniform acceptance of risk factors across trials.
UFH or LMWH vs. Control
DVT
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of all DVT (RR = 0.55; 95% CI: 0.36‐0.83) (Figure 2A). When stratified by methodological quality, 5 trials16, 2225 with Jadad scores of 3 or higher showed an RR reduction of 0.53 (95% CI: 0.38‐0.72) in reducing all DVT. All of the higher‐quality trials compared LMWH to placebo. Across 4 trials that reported data for symptomatic DVT there was a nonsignificant reduction in RR compared with placebo (RR = 0.73; 95% CI: 0.45‐1.16) (Figure 2B). Only 2 trials24, 25 (both LMWH trials) reported results for proximal DVT and demonstrated significant benefit of prophylaxis with a pooled RR of 0.46 (95% CI: 0.31‐0.69) (Figure 2C).

PE
Across 7 trials comparing either UFH or LMWH to control, heparin products significantly decreased the risk of PE (RR = 0.70; 95% CI: 0.53‐0.93) (Figure 3A). The 5 trials16, 2225 with Jadad scores of 3 or greater showed a similar relative risk reduction, but the result was no longer statistically significant (RR = 0.56; 95% CI: 0.31‐1.02). Two of the trials16, 21 relied solely on the results of autopsy to diagnose PE, which may have given rise to chance differences in detection due to generally low autopsy rates. Eliminating these 2 studies from the analysis resulted in loss of statistical significance for the reduction in risk for PE (RR = 0.48; 95% CI: 0.20‐1.15).

Death
Seven trials16, 2025 comparing either UFH or LMWH to control examined the impact of pharmacologic prophylaxis on death and found no significant difference between treated and untreated patients across all trials (RR = 0.92; 95% CI: 0.82‐1.03) and those limited to studies with Jadad scores of 3 or higher (RR = 0.97; 95% CI: 0.80‐1.17).
LMWH vs. UFH
DVT
In 6 trials14, 1719, 26, 27 comparing LMWH to UFH given either twice a day (BID) or 3 times a day (TID), there was no statistically significant difference in all DVT (RR = 0.90; 95% CI: 0.57‐1.43). (For all analyses RRs <1 favor LMWH, while RRs >1 favor UFH.) A total of 2 trials14, 18 reported results separately for proximal DVT with no statistically significant difference noted between UFH and LMWH (RR = 1.60; 95% CI: 0.53‐4.88). One small trial26 reported findings comparing UFH to LMWH for prevention of symptomatic DVT with no difference noted.
PE
Pooled data from the 5 trials14, 17, 18, 26, 27 comparing UFH to LMWH in the prevention of PE showed no statistically significant difference in rates of pulmonary embolism (RR = 0.82; 95% CI: 0.26‐2.63) (Figure 3B). In sensitivity analysis this result was not impacted by Jadad score.
Death
When UFH was compared to LMWH no statistically significant difference in the rate of death was found (RR = 0.96; 95% CI: 0.50‐1.85). Here again, no difference was noted when limited to studies with Jadad scores of 3 or higher.
Complications
We evaluated adverse events of heparin products used for prophylaxis and whether there were differences between UFH and LMWH. Reporting of complications was not uniform from study to study, making pooling more difficult. However, we were able to abstract data on any bleeding, major bleeding, and thrombocytopenia from several studies. In 5 studies15, 16, 2325 of either UFH or LMWH vs. control, a significantly increased risk of any bleeding (RR = 1.54; 95% CI: 1.15‐2.06) (Figure 4A) was found. When only major bleeding was evaluated, no statistically significant difference was noted (RR = 1.20; 95% CI: 0.55‐2.58) (Figure 4B). In 4 trials16, 22, 24, 25 the occurrence of thrombocytopenia was not significantly different when comparing UFH or LMWH to control (RR = 0.92; 95% CI: 0.46‐1.86).

When LMWH was compared to UFH in 4 trials,14, 17, 18, 27 a nonsignificant trend toward a decrease in any bleeding was found in the LMWH group (RR = 0.72; 95% CI: 0.44‐1.16) (Figure 5A). A similar trend was seen favoring LMWH in rates of major bleeding (RR = 0.57; 95% CI: 0.25‐1.32) (Figure 5B). Neither trend was statistically significant. Three trials comparing LMWH to UFH reported on thrombocytopenia17, 18, 27 with no significant difference noted (RR = 0.52; 95% CI: 0.06‐4.18).

Heterogeneity and Publication Bias
No statistically significant heterogeneity was identified between trials for any outcomes. The highest I‐squared value was 54.5% (P = 0.14) for the endpoint of thrombocytopenia when UFH was compared to LMWH. In some cases, the nonsignificant results for tests of heterogeneity may have reflected small numbers of trials, but the values for I‐squared for all other endpoints were close to zero indicating that little nonrandom variation existed in the results across studies. All analyses were run using both random effects and fixed effects modeling. While we report results for random effects, no significant differences were observed using fixed effects.
We tested for publication bias using the methods developed by Egger et al.12 and Begg and Mazumdar.13 There was evidence of bias only for the outcome of PE when prophylaxis was compared to control, as the results for both tests were significant (Begg and Mazumdar:13 P = 0.035; Egger et al.:12 P = 0.010). For other outcomes tested, including all DVT (prophylaxis compared to control, and LMWH vs. UFH) as well as PE (LMWH vs. UFH), the P‐values were not significant.
DISCUSSION
When compared to control, LMWH or UFH decreased the risk of all DVT by 45% (RR = 0.55; 95% CI: 0.36‐0.83) and proximal DVT by 54% (RR = 0.46; 95% CI: 0.31‐0.69). PE was also decreased by 30% (RR = 0.70; 95% CI: 0.53‐0.93). Of note, when prophylaxis was compared with placebo all of the high‐quality studies showing a benefit were done using LMWH. The benefits of prophylaxis occurred at the cost of a 54% increased overall risk of bleeding (RR = 1.54; 95% CI 1.15‐2.06). However, the risk of major bleeding was not significantly increased. We did not find a mortality benefit to pharmacologic thromboembolism prophylaxis.
When comparing UFH to LMWH, we noted no difference in all DVT, symptomatic DVT, proximal DVT, PE, or death. While there was a trend toward less bleeding with LMWH, this was not statistically significant.
Taken in aggregate, our findings are in agreement with previous published meta‐analyses reporting net benefit for thromboembolism prophylaxis in medical patients.24, 22, 28, 29 Our meta‐analysis has several methodological strengths over the prior studies, including a comprehensive search of both the published and unpublished literature and assessment of the relationship between methodological quality of included trials and reported benefit. In contrast to previous reviews, our analysis highlights several limitations of the current evidence.
First, many of the studies are older, with predicted lengths of stay of greater than 1 week. The 8‐13‐day range of treatment duration we found in this study is longer than the average length of stay in today's hospitals. Second, there is variability in the diagnostic tests used to diagnose DVT, as well as variation in the definition of DVT among studies. Studies using fibrinogen uptake scanning reported rates of DVT as high as 26%15 while studies using venography reported DVT rates of almost 15% in the placebo arm.24 These rates are higher than most physicians' routine practice. One reason for this discrepancy is most studies did not distinguish below‐the‐knee DVT from more clinically relevant above‐the‐knee DVT. Systematic reviews of medical and surgical patients have found rates of proximal propagation from 0% to 29% in untreated patients.30, 31 Though controversial, below‐the‐knee DVT is believed less morbid than proximal DVT or symptomatic DVT. We addressed this by focusing specifically on clinically relevant endpoints of proximal and symptomatic DVT. When we restricted our analysis to proximal DVT we found a 54% RR reduction in 2 pooled trials of LMWH compared to placebo. In pooled analyses symptomatic DVT was not affected by prophylaxis. When compared head‐to‐head there were no differences between LMWH and UFH for proximal DVT or symptomatic DVT.
When considering PE, the utilization of autopsy as the sole diagnostic method in 2 large trials16, 21 is particularly problematic. In the trial by Garlund,21 the mortality rate was 5.4%, with an autopsy rate of 60.1%. Similarly, in the trial by Mahe et al.,16 the mortality rate was 10%, with an autopsy rate of 49%. Given the low absolute number of deaths and substantial proportion of decedents without autopsy, the potential for chance to produce an imbalance in detection of PE is high in these studies. When we excluded these 2 trials, we found that PE was no longer reduced to a statistically significant degree by prophylaxis. Loss of significance for PE in 2 sensitivity analyses (when excluding studies of lower quality, or using autopsy as a sole diagnostic study) is problematic and calls into question the true benefit of prophylaxis for prevention of PE.
Another limitation of the current literature centers on the variability of dosing used. We pooled trials of UFH whether given BID or TID. Given the small number of trials we did not do sensitivity analyses by dosage. A recent meta‐analysis3 found both doses are efficacious, while a recent review article32 suggested superiority of TID dosing. We believe the available literature does not clearly address this issue. Regarding comparisons of LMWH to UFH, dosing variability was also noted. The trial by Bergmann and Neuhart27 used enoxaparin 20 mg per day and found similar efficacy to UFH BID, while the Samama et al.24 trial found enoxaparin 20 mg per day no more efficacious than placebo. While the literature does not clearly define a best dose, we believe enoxaparin doses lower than 40 mg daily do not reflect the standard of care.
An additional limitation of the literature is publication bias. We assessed the possibility of publication bias by a variety of means. We did find statistical evidence of publication bias for the outcome of PE when prophylaxis was compared to control. Importantly, two meta‐analyses2, 4 on thromboembolism prophylaxis for general medicine patients suggested publication bias is present and our finding supports this conclusion. While no test for publication bias is foolproof, the best protection against publication bias, which we pursued in our study, consists of a thorough search for unpublished studies, including a search of conference proceedings, contact with experts in the field, and manufacturers of LMWH.
A final limitation of the current literature centers on risk assessment. All of the trials in this meta‐analysis included patients with an elevated level of risk. Unfortunately, risk was not clearly defined in many studies, and there was no minimum level of risk between trials. While immobility, age, and length of stay were reported for most studies, other risk factors such as personal history of thromboembolism and malignancy were not uniformly reported. Based on our analysis we are not confident our results can be extrapolated to all general medicine patients.
In conclusion, we found good evidence that pharmacologic prophylaxis significantly decreases the risk of all DVT and proximal DVT in at‐risk general medical patients. However, only LMWH was shown to prevent proximal DVT. We found inconclusive evidence that prophylaxis prevents PE. When compared directly we did not find clear superiority between UFH and LMWH, though several limitations of the current literature hamper decision‐making. Given the lower cost, it may seem justified to use UFH. However, there are other practical issues, such as the fact that LMWH is given once daily, and so potentially preferred by patients and more efficient for nurses. All of these results pertain to patients with elevated risk. While we did not find significant safety concerns with prophylaxis we do not know if these results can be extrapolated to lower‐risk patients. We believe that recommending widespread prophylaxis of all general medicine patients requires additional evidence about appropriate patient selection.
Acknowledgements
The authors thank Emmanuelle Williams, MD, for translating articles from French; Claudia Figueroa, MS, for translating articles from Spanish; Vikas Gulani, MD, for translating articles from German; and Rebecca Lee, MS, for translating articles from German, Dutch, and Italian. In addition, the authors thank Dr. Dilzer from Pfizer Global Pharmaceuticals, Kathleen E. Moigis from Aventis, and Carol McCullen from Glaxo Smith Kline for their search for unpublished pharmaceutical trials of low molecular weight heparins. Finally, the authors thank the Veterans Administration/University of Michigan Patient Safety Enhancement Program for research support.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(suppl):338S–400S.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- ,,,,.Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis.Chest.2007;131(2):507–516.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum. National Consensus Standards for the Prevention and Care of Venous Thromboembolism (including Deep Vein Thrombosis and Pulmonary Embolism). Available at: http://www.qualityforum.org/projects/completed/vte/index.asp. Accessed May2009.
- ,.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17(10):788–791.
- ,,,.[Effectiveness of low molecular weight heparin (Fragmin) in the prevention of thromboembolism in internal medicine patients. A randomized double‐blind study].Med Klin (Munich).1988;83(7):241–245, 278.
- ,,, et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials.1996;17(1):1–12.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21(11):1539–1558.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315(7109):629–634.
- ,.Operating characteristics of a rank correlation test for publication bias.Biometrics.1994;50(4):1088–1101.
- ,,,,,.Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease.Am Heart J.2003;145(4):614–621.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26(2):115–117.
- ,,,,.Lack of effect of a low‐molecular‐weight heparin (nadroparin) on mortality in bedridden medical in‐patients: a prospective randomised double‐blind study.Eur J Clin Pharmacol.2005;61(5‐6):347–351.
- ,,.The venous thrombotic risk in non‐surgical patients: epidemiological data and efficacy/safety profile of a low‐molecular‐weight heparin (enoxaparin). The Prime Study Group.Haemostasis.1996;26(suppl 2):49–56.
- ,,.Subcutaneous low‐molecular‐weight heparin versus standard heparin and the prevention of thromboembolism in medical inpatients. The Heparin Study in Internal Medicine Group.Haemostasis.1996;26(3):127–139.
- ,,, et al.Randomized controlled study of heparin and low molecular weight heparin for prevention of deep‐vein thrombosis in medical patients.Thromb Res.1990;59(3):639–650.
- ,,,.Reduction of mortality in general medical in‐patients by low‐dose heparin prophylaxis.Ann Intern Med.1982;96(5):561–565.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,, et al.The prophylaxis of medical patients for thromboembolism pilot study.Am J Med.2006;119(1):54–59.
- ,,, et al.Prevention of deep vein thrombosis in elderly medical in‐patients by a low molecular weight heparin: a randomized double‐blind trial.Haemostasis.1986;16(2):159–164.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- ,,.Prevention of thromboembolic accidents in elderly subjects with Fraxiparine. In: Bounameaux H, Samama MM, Ten Cate JW, eds.Fraxiaparine. 2nd International Symposium. Recent pharmacological and clinical data.New York:Schattauer;1990:51‐54.
- ,.A multicenter randomized double‐blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in‐patients bedridden for an acute medical illness. The Enoxaparin in Medicine Study Group.Thromb Haemost.1996;76(4):529–534.
- ,,, et al.Prevention of venous thromboembolism in internal medicine with unfractionated or low‐molecular‐weight heparins: a meta‐analysis of randomised clinical trials.Thromb Haemost.2000;83(1):14–19.
- ,,,,.Meta‐analysis of venous thromboembolism prophylaxis in medically Ill patients.Clin Ther.2007;29(11):2395–2405.
- ,,,,,.Clinical relevance of distal deep vein thrombosis. Review of literature data.Thromb Haemost.2006;95(1):56–64.
- .Natural history of venous thromboembolism.Circulation.2003;107(suppl 1):I22–I30.
- .Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients.N Engl J Med.2007;356(14):1438–1444.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(suppl):338S–400S.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- ,,,,.Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: a metaanalysis.Chest.2007;131(2):507–516.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum. National Consensus Standards for the Prevention and Care of Venous Thromboembolism (including Deep Vein Thrombosis and Pulmonary Embolism). Available at: http://www.qualityforum.org/projects/completed/vte/index.asp. Accessed May2009.
- ,.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93(2):259–262.
- ,,, et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- ,,,.Pharmacological thromboembolic prophylaxis in a medical ward: room for improvement.J Gen Intern Med.2002;17(10):788–791.
- ,,,.[Effectiveness of low molecular weight heparin (Fragmin) in the prevention of thromboembolism in internal medicine patients. A randomized double‐blind study].Med Klin (Munich).1988;83(7):241–245, 278.
- ,,, et al.Assessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials.1996;17(1):1–12.
- ,.Quantifying heterogeneity in a meta‐analysis.Stat Med.2002;21(11):1539–1558.
- ,,,.Bias in meta‐analysis detected by a simple, graphical test.BMJ.1997;315(7109):629–634.
- ,.Operating characteristics of a rank correlation test for publication bias.Biometrics.1994;50(4):1088–1101.
- ,,,,,.Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease.Am Heart J.2003;145(4):614–621.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26(2):115–117.
- ,,,,.Lack of effect of a low‐molecular‐weight heparin (nadroparin) on mortality in bedridden medical in‐patients: a prospective randomised double‐blind study.Eur J Clin Pharmacol.2005;61(5‐6):347–351.
- ,,.The venous thrombotic risk in non‐surgical patients: epidemiological data and efficacy/safety profile of a low‐molecular‐weight heparin (enoxaparin). The Prime Study Group.Haemostasis.1996;26(suppl 2):49–56.
- ,,.Subcutaneous low‐molecular‐weight heparin versus standard heparin and the prevention of thromboembolism in medical inpatients. The Heparin Study in Internal Medicine Group.Haemostasis.1996;26(3):127–139.
- ,,, et al.Randomized controlled study of heparin and low molecular weight heparin for prevention of deep‐vein thrombosis in medical patients.Thromb Res.1990;59(3):639–650.
- ,,,.Reduction of mortality in general medical in‐patients by low‐dose heparin prophylaxis.Ann Intern Med.1982;96(5):561–565.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,, et al.The prophylaxis of medical patients for thromboembolism pilot study.Am J Med.2006;119(1):54–59.
- ,,, et al.Prevention of deep vein thrombosis in elderly medical in‐patients by a low molecular weight heparin: a randomized double‐blind trial.Haemostasis.1986;16(2):159–164.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- ,,.Prevention of thromboembolic accidents in elderly subjects with Fraxiparine. In: Bounameaux H, Samama MM, Ten Cate JW, eds.Fraxiaparine. 2nd International Symposium. Recent pharmacological and clinical data.New York:Schattauer;1990:51‐54.
- ,.A multicenter randomized double‐blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in‐patients bedridden for an acute medical illness. The Enoxaparin in Medicine Study Group.Thromb Haemost.1996;76(4):529–534.
- ,,, et al.Prevention of venous thromboembolism in internal medicine with unfractionated or low‐molecular‐weight heparins: a meta‐analysis of randomised clinical trials.Thromb Haemost.2000;83(1):14–19.
- ,,,,.Meta‐analysis of venous thromboembolism prophylaxis in medically Ill patients.Clin Ther.2007;29(11):2395–2405.
- ,,,,,.Clinical relevance of distal deep vein thrombosis. Review of literature data.Thromb Haemost.2006;95(1):56–64.
- .Natural history of venous thromboembolism.Circulation.2003;107(suppl 1):I22–I30.
- .Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients.N Engl J Med.2007;356(14):1438–1444.
Copyright © 2009 Society of Hospital Medicine
Clinical Conundrum
A 49‐year‐old man presented with 2 days of chills, fever, anorexia, and increased cough and dyspnea. The patient had a history of chronic obstructive pulmonary disease (COPD) and noted that his cough and dyspnea had increased above normal for several days. He was now dyspneic with minimal activity and had slept at a 45‐degree incline the night prior to evaluation due to dyspnea. He noted less improvement than usual with the use of his metered dose inhaler. His cough was occasionally productive of small amounts of white phlegm. He had vomited once. During a coughing episode the patient experienced a sudden onset of sharp right upper quadrant abdominal pain that worsened with coughing and sudden position changes. The patient denied a prior history of abdominal pain or surgery. The patient's last bowel movement was 2 days prior to admission. He denied melena or bright red blood per rectum.
My initial differential diagnosis for this patient's dyspnea and cough is pneumonia, acute exacerbation of COPD, or congestive heart failure. The presence of fever and anorexia increases the likelihood of infectious etiologies, whereas the presence of orthopnea points toward congestive heart failure. Noncardiac processessuch as a large pleural effusion or apical lung diseasecould also cause orthopnea. His abdominal pain could be a result of pneumonia alone (perhaps in the right lower lobe with diaphragmatic irritation), but I am also considering complications of pneumonia such as empyema. Although his abdominal pain, dyspnea, and cough could also be a result of hepatobiliary disease, a perforated viscus, or pancreatitis, we currently have little reason to suspect a direct abdominal etiology. My top diagnosis is community‐acquired pneumonia, perhaps accompanied by pleural effusion.
His medical history was significant for dilated cardiomyopathy and heavy alcohol use. His medications included various meter‐dosed inhalers, bupropion, digoxin, spironolactone, lisinopril, and metoprolol. He had never received corticosteroid therapy and had not previously been hospitalized for COPD‐related problems. He had smoked one pack of cigarettes daily for 40 years.
Heavy alcohol use is associated with an increased risk of several pulmonary infections such as gram‐negative necrotizing pneumonia (classically, Klebsiella pneumoniae), pneumococcal pneumonia, aspiration pneumonia, anaerobic lung abscesses, and tuberculosis. Given his right upper quadrant pain, acute alcoholic hepatitis and alcohol‐related pancreatitis enter the differential. His history of cardiomyopathy makes me consider congestive heart failure as more likely than before, and perhaps his abdominal pain is a result of hepatic congestion from right heart failure. His fever, however, cannot be attributed to cardiac failure. Less likely diagnoses include ischemic conditions related to his cardiomyopathy such as mesenteric ischemia from low perfusion or embolism from a cardiac thrombus. A pulmonary infection remains the most likely diagnosis.
He was an ill‐appearing man in moderate respiratory distress, looking older than his stated age. His temperature was 38.4C, heart rate 129 beats/minute, blood pressure 85/56 mm Hg, respiratory rate 24 breaths/minute, and oxygen saturation 92% on room air. A cardiovascular exam revealed no murmur, gallop, or rub. The jugular venous pulse was not elevated. His lungs were clear to auscultation. Abdominal exam revealed right‐sided abdominal tenderness that appeared to localize to the rectus sheath. Otherwise, the abdomen was soft, with normal bowel sounds and no organomegaly. Rectal examination revealed guaiac negative stool and no focal tenderness. His extremities were normal.
His vital signs are worrisome for impending cardiovascular collapse and shock, possibly due to sepsis. The relatively nonfocal cardiopulmonary exam is surprising given his initial symptoms and makes me wonder if his dyspnea is primarily related to an abdominal process leading to diaphragmatic irritation rather than to a thoracic process. Congestive heart failure seems unlikely given the lack of supportive physical examination findings. His abdominal exam findings are puzzling. Although his abdominal wall tenderness could be benignperhaps from muscular strain or a tear from coughingit could represent a more worrisome process such as infection or a hematoma in the abdominal wall muscles. Mesenteric ischemia is still possible, as the exam is often unimpressive. A hepatic abscess or subphrenic abscess should be considered, as physical exam findings in these conditions can be subtle.
My differential remains relatively unchanged, but I have now put consideration of a hepatic or subphrenic abscess higher on my list. Early empiric broad‐spectrum antibiotics seem necessary.
He had a white blood cell count of 26,700/mL with 92% neutrophils, a hemoglobin of 14.6 g/dL, and a platelet count of 312,000/mL. Sodium was 134 mmol/L, potassium was 4.3 mmol/L, chloride was 94 mmol/L, bicarbonate was 23 mmol/L, blood urea nitrogen was 23 mg/dL, and creatinine was 2.1 mg/dL. The results of the calcium, protein, albumin, and liver function tests were normal. Urinalysis was negative for protein and red blood cells. An electrocardiogram revealed sinus tachycardia. A chest radiograph at admission revealed mild opacities in both lower lobes and the right middle lobe consistent with either atelectasis or pneumonia (Fig. 1). A very small left effusion was also identified.
The additional data reinforce my clinical impression that this process is likely to be infectious. The chest radiograph is consistent with community‐acquired pneumonia, possibly from an atypical pathogen. Given his elevated creatinine, I am also considering a pulmonary‐renal syndrome such as vasculitis, though hematuria was not present. A subphrenic abscess, mesenteric ischemia, or an abdominal wall process (because his abdominal tenderness on exam still needs an explanation) remain possibilities; my suspicion would increase if he does not respond appropriately to therapy for community‐acquired pneumonia.
The clinical team's working diagnosis also was community‐acquired pneumonia. Blood and sputum cultures were obtained, and the patient was treated with intravenous ceftriaxone, azithromycin, and intravenous fluid. By the second day, his creatinine had normalized; however, his hypoxemia had worsened, and he now required supplemental oxygen. His temperature was 39.3C, and his heart rate was 150 beats/minute. The findings of an abdominal ultrasound of the kidneys, spleen, and right upper quadrant were normal.
It is too early to say the patient has failed therapy because a patient can get worse before getting better during the course of antibiotic therapy for community‐acquired pneumonia. Fever, for example, may take up to 7 days to resolve, depending on host factors and the pathogen. Though I typically wait about 72 hours before assuming a patient is not appropriately responding to therapy, the additional information has made me concerned. The degree of tachycardia is significant and warrants an EKG to exclude an arrthymia. I would also repeat the chest radiograph to evaluate for worsening infiltrates or increased pleural effusion.
On the third hospital day, the patient's abdominal pain had decreased with analgesia, but his fever, cough, and dyspnea remained largely unchanged. Antibiotics were changed to intravenous levofloxacin. A repeat chest radiograph revealed elevation of the right hemidiaphragm and bilateral effusions (Fig. 2). An electrocardiogram revealed sinus tachycardia. Blood cultures revealed no growth, and sputum cultures grew oral flora.
A significantly elevated right hemidiaphragm makes me reconsider the diagnosis of simple community‐acquired pneumonia. The differential diagnosis for an elevated hemidiaphragm is best considered by location in relation to the diaphragm. Causes above the diaphragm include rib fracture, atelectasis, pleural thickening, and volume loss of the lung for another reason (e.g., surgery, bronchial obstruction due to tumor or mucus plugging), as well as mimics such as a densely consolidated pneumonia, pulmonary infarction, or a subpulmonary effusion. Diaphragmatic causes include eventration, rupture, phrenic nerve weakness, and intrinsic weakness because of neuromuscular disease (usually bilateral). Causes below the diaphragm that must be considered are subphrenic or liver abscess, liver (and other abdominal) malignancy, pancreatic pseudocyst, and distended bowel. Given the clinical picture, I am focusing below the diaphragmespecially on a possible hepatic or subphrenic abscess (which could be missed on ultrasound) and mimics of it such as dense consolidation or a subpulmonary effusion. Given the lack of response to antibiotics, I need to consider an infection that is not being treated, either because of location (abscess, effusion) or microbiology (tuberculosis, a parasite, a fungus, resistant bacteria). After confirming that the patient has a substantive pleural effusion, he needs a thoracentesis.
On the fourth hospital day, his temperature was 38.8C, and his white blood cell count was 21,000/mL. A right‐sided thoracentesis was performed; approximately 250 cc of fluid was obtained. Pleural fluid analysis revealed bloody fluid, with a white blood cell count of 16,750/mL with 94% neutrophils, 40,000 red blood cells/mL, lactate dehydrogenase of 278 U/L (normal serum value 80200 U/L), protein of 3.7 g/dL, and glucose of 81 mg/dL. A pleural fluid pH was not obtained. A gram stain revealed many white blood cells with no organisms noted. Serum protein was 7.4 g/dL. These results were thought to represent an exudative parapneumonic effusion; levofloxacin and supplemental oxygen were continued.
The pleural fluid appears exudative, but I am not sure this man has a parapneumonic effusion because, despite clinical deterioration, an obvious infiltrate is not seen on interval chest radiography. We must look closely at the fluid because this is a bloody effusion and somewhat atypical for a parapneumonic effusion. Also, the effusion does not appear large enough to explain why he has not improved on the current antibiotics. We should thus reconsider our diagnosis and management. I would obtain additional imaging (such as an abdominal and chest computed tomography [CT]) and perhaps obtain a consultation from the pulmonary team regarding the postulated initial diagnosis of pneumonia with effusion.
On the fifth day of hospitalization, the patient's dyspnea and cough persisted but were improved. His abdominal pain was minimal and felt improved with flatus. Fever continued to 38.8C, and the white blood cell count was 20,000/mL. On examination the patient had decreased breath sounds at the right base and bibasilar crackles. His abdomen was soft, with tenderness in his right upper quadrant only with deep palpation; bowel sounds remained. An ultrasound of the chest was performed to look for a loculated effusion; however, no fluid was identified. The pulmonary consultant thought it likely that the patient had a subpulmonic effusion and recommended CT of the abdomen and chest.
His right upper quadrant tenderness is still unexplained. I would agree with the CT, primarily to evaluate other causes of his elevated diaphragm such as subphrenic or hepatic abscess. For now, I would make no change in antibiotic therapy.
On the sixth hospital day, the patient had an episode of bilious emesis. Chest and abdominal CT revealed collapse of the right middle and lower lobes with a small adjacent effusion, and a 6 6 16 cm abscess intimately opposed to the right lobe of the liver. Extending from the inferior extent of the abscess was a tubular thick‐walled structure connecting to the cecum that was suspicious of a thickened inflamed appendix. There was periappendiceal stranding suggesting inflammation. The small bowel was diffusely dilated up to 4.5 cm, suggesting a small bowel obstruction.
I suspect that his abscess is related to a perforated appendix and that the dilated small bowel is most likely a result of localized irritation of the bowel by the abscess and appendicitis. The collapsed lung is most likely due to local inflammation from the subdiaphragmatic abscess. Treatment should now be changed substantially. I would ask a surgeon to evaluate the patient because the most likely diagnosis is perforated appendicitis with abscess formation.
When the periappendiceal abscess was drained percutaneously, 190 mL of purulent fluid was removed. The cultures were positive for Klebsiella pneumonia, Enterococcus faecalis,and Streptococcus milleri. The patient was given 6 weeks of intravenous antibiotics with improvement in his clinical symptoms. During the interval the findings on his chest radiograph resolved completely. A laproscopic appendectomy 3 months later revealed significant right lower quadrant adhesions. The pathology specimen identified a distorted appendix with regeneration consistent with prior appendicitis. The patient was contacted 4 months after his surgery, and he reported that he was doing well, with no cardiopulmonary or gastrointestinal symptoms.
COMMENTARY
Community‐acquired pneumonia (CAP) is a common cause of acute illness and accounts for nearly 1 million admissions per year in the United States.1 The diagnosis of CAP is made when symptoms including dyspnea, fever, cough, or leukocytosis are present, with confirmation provided by a chest radiograph. Often the diagnosis is clear; however, there is no pathognomonic constellation of signs or symptoms that establish the diagnosis with certainty.2 Many physicians learn that pneumoniaespecially lower‐lobe pneumoniacan lead to abdominal findings such as upper quadrant pain, vomiting, and tenderness to palpation. Conversely, the patient discussed above illustrates that a primary abdominal process can also result in a symptom complex that mimics pneumonia.
The prevalence of CAP coupled with the inherent uncertainty of a clinical diagnosis of CAP leads to an important question: How long is too long before questioning the diagnosis? An analysis of the pneumonia Patient Outcomes Research Trial (PORT) limited to inpatients with CAP examined time to clinical stability. For the majority of patients, abnormal vital signs resolved within 23 days.3 In this study, 29% of patients had severe disease, and not surprisingly, these patients took longer to improve. Using the pneumonia severity index score, which accounts for age, comorbidity, abnormal vital signs, and laboratory data, the patient described in this article would be considered at high risk for death and complication with an estimated mortality of 9%.4 Using a combination of defervescence, resolution of tachycardia, tachypnea, and hypoxemia as markers of clinical stability, a patient like ours should respond within 4 days (with a range of 27 days). On the basis of these dataand the discrepancy between the patient's severe illness and relatively minor pulmonary infiltratesit seems reasonable to have considered this patient as failing CAP therapy as early as the fourth day of hospitalization.
In approximately 10% of hospitalized patients with CAP, the clinical course is protracted.5 When patients do not improve as quickly as expected, the reasons that could explain this should be investigated. In a cohort of 49 patients with CAP who failed therapy the most common reasons for failure to improve were severity of the pneumonia and drug resistance.6 A multicenter study found that the incidence of resistance to penicillin by Streptococcus pneumoniae, the most common bacterial pathogen in CAP, was 30%, with a 4% in vitro resistance rate to ceftriaxone.7 How well in vitro resistance predicts clinical response, however, is unclear. Risk factors for antibiotic resistance include close exposure to children, recent antibiotic use, and recent hospitalization. Immunosuppressive conditions should also be considered in patients who fail to improve. Suppurative complications of pneumoniasuch as empyema, parapneumonic effusion, and lung abscessalso delay recovery.
Another consideration in a patient with what appears to be a nonresolving pneumonia with pleural effusion is that the initial diagnosis is incorrect and the cause is extrathoracic. Pulmonary and cardiac diseases account for more than 90% of effusions, whereas less than 5% of pleural effusions result from intraabdominal causes.8 When should intraabdominal diseases be sought in patients with an effusion, fever, dyspnea, and cough? Light suggests that intraabdominal pathology should be investigated in patients who have pleural effusions without significant parenchymal disease.8 This point is underscored by the experience of our patient, whose chest radiographs showed, despite clinical decline, minimal airspace disease.
Several abdominal entities cause pleural effusion. Pancreatitis, either acute or chronic, with pseudocyst formation is the most common abdominal cause of exudative pleural effusions. Approximately 10% of patients with pancreatic disease will develop effusions, usually left‐sided.9 These left‐sided effusions are also seen in splenic abscesses, usually as a result of endocarditis. Intrahepatic abscess is associated with effusions in 20% of patients.10 A subphrenic abscess, as seen in our patient, is an uncommon cause of exudative pleural effusions. Historically, subphrenic abscesses resulted from a perforated viscus, with ruptured appendicitis the most common cause,11 followed by perforated peptic ulcers and biliary tract disease. With the advent of antibiotics, the causes of subphrenic abscess changed considerably, with the majority of current cases resulting from postsurgical complications.12 The findings of a chest radiograph are abnormal in 80% of patients with subphrenic abscess;1214 an elevated hemidiaphragm and pleural effusion are found in the majority of cases. The symptoms of a subphrenic abscess are nonspecific, and patient's complaints are equally split between predominantly thoracic and predomninantly abdominal complaints.15
Appendicitis, a common disease predominantly of the young, may lead to atypical presentations in older individuals. In a retrospective analysis of 113 patients older than 60 years with appendicitis, 70% presented in an atypical fashion.16 Typical symptoms include right lower quadrant pain, fever, anorexia and a white blood cell count greater than 10,000/mL. Fever was the most frequently absent symptom, seen in only 37% of older patients. In this cohort, approximately one third of older patients waited more than 48 hours prior to presentation. The time between symptom onset and clinical presentation is a strong predictor of perforation risk.17 As in this case, roughly 2% of patients with acute appendicitis will present with perforation and abscess formation.18 In such patients the management is initially conservative. Percutaneous drainage and broad spectrum antibiotics are the treatment of choice, followed by an interval appendectomy in 612 weeks.19 The rationale for delayed surgery is that earlier surgery may disseminate a localized inflammatory process.20
Community‐acquired pneumonia is a more frequent cause of hospital admission than is intraabdominal abscess. Physicians often face the dilemma of when to pursue alternative diagnoses after a patient who is thought to have an atypical presentation of a common disease (ie, CAP) fails to respond to conventional therapy. Although clinicians learn that right upper quadrant pain may be a symptom of pneumonia, our patient revealed that abdominal causes may mimic pneumonia and produce a pleural effusion. Determining whether the primary disease originates above or below the diaphragm is critical to guiding therapy. When patients fail to respond adequately to therapy, clinicians should set a low threshold for deciding to image the abdomen in a patient with modest pulmonary infiltrates, pleural effusion, and abdominal pain.
- ,,, et al.The cost of treating community‐acquired pneumonia.Clin Ther.1998;20:820–827.
- ,,.Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination.JAMA.1997;278:1440–1445.
- ,,, et al.Time to clinical stability in patients hospitalized with community acquired pneumonia. Implications for practice guidelines.JAMA.1998;279:1452–1457.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,, et al.Utility of fiberoptic bronchoscopy in non resolving pneumonia.Chest.1990;98:1322–1326.
- ,,, et al.Antimicrobial treatment failures in patients with community acquired pneumonia. Causes and prognostic implications.Am J Respir Crit Care Med.2000;162:154–160.
- ,,, et al.Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997–98.Emerg Infect Dis.1999;5:757–765.
- ,.Pleural effusion. In:Murray JF,Nadel JA, eds.Textbook of respiratory medicine. 3rd ed.Philadelphia:WB Saunders,2000:2013–2041.
- ,,.Significance of pleural effusion in patients with acute pancreatitis.Am J Gastroenterol.1992;87:871–874.
- .Exudative pleural effusions secondary to gastrointestinal diseases.Clin Chest Med.1985;6(1):103–111.
- .Subphrenic abscess.Ann Surg.1963;158:240–248.
- ,,,.Upper abdominal abscess: a continuing and deadly problem.Am J Roentgenol.1980;134:759–765.
- .Subphrenic abscess. A clinical study of 101 cases.Acta Chir Scand.1959;117:388–408.
- ,,.Subphrenic abscess a continuing hazard.Am J Surg.1969:117–122.
- ,.Subphrenic abscess: a thoracoabdominal clinical complex. The changing picture with antibiotics.Am J Surg.1964;108:165–172.
- ,.What have we learned over the past 20 years about appendicitis in the elderly.Am J Surg.2003;185:198–201.
- ,,, et al.Appendicitis: why so complicated? Analysis of 5755 consecutive appendectomies.Am Surg.2000;66:548–554.
- ,,.Appendicitis with a palpable mass.Ann Surg.1981;193:227–229.
- ,,, et al.Nonoperative management of perforated appendicitis without periappendiceal mass.Am J Surg.2000;179:177–181.
- ,,.Appendix. In:Townsend CM, ed.Sabiston textbook of surgery. The biologic basis of modern surgical practice. 16th ed.Philadelphia:W. B. Saunders,2001:917–928.
A 49‐year‐old man presented with 2 days of chills, fever, anorexia, and increased cough and dyspnea. The patient had a history of chronic obstructive pulmonary disease (COPD) and noted that his cough and dyspnea had increased above normal for several days. He was now dyspneic with minimal activity and had slept at a 45‐degree incline the night prior to evaluation due to dyspnea. He noted less improvement than usual with the use of his metered dose inhaler. His cough was occasionally productive of small amounts of white phlegm. He had vomited once. During a coughing episode the patient experienced a sudden onset of sharp right upper quadrant abdominal pain that worsened with coughing and sudden position changes. The patient denied a prior history of abdominal pain or surgery. The patient's last bowel movement was 2 days prior to admission. He denied melena or bright red blood per rectum.
My initial differential diagnosis for this patient's dyspnea and cough is pneumonia, acute exacerbation of COPD, or congestive heart failure. The presence of fever and anorexia increases the likelihood of infectious etiologies, whereas the presence of orthopnea points toward congestive heart failure. Noncardiac processessuch as a large pleural effusion or apical lung diseasecould also cause orthopnea. His abdominal pain could be a result of pneumonia alone (perhaps in the right lower lobe with diaphragmatic irritation), but I am also considering complications of pneumonia such as empyema. Although his abdominal pain, dyspnea, and cough could also be a result of hepatobiliary disease, a perforated viscus, or pancreatitis, we currently have little reason to suspect a direct abdominal etiology. My top diagnosis is community‐acquired pneumonia, perhaps accompanied by pleural effusion.
His medical history was significant for dilated cardiomyopathy and heavy alcohol use. His medications included various meter‐dosed inhalers, bupropion, digoxin, spironolactone, lisinopril, and metoprolol. He had never received corticosteroid therapy and had not previously been hospitalized for COPD‐related problems. He had smoked one pack of cigarettes daily for 40 years.
Heavy alcohol use is associated with an increased risk of several pulmonary infections such as gram‐negative necrotizing pneumonia (classically, Klebsiella pneumoniae), pneumococcal pneumonia, aspiration pneumonia, anaerobic lung abscesses, and tuberculosis. Given his right upper quadrant pain, acute alcoholic hepatitis and alcohol‐related pancreatitis enter the differential. His history of cardiomyopathy makes me consider congestive heart failure as more likely than before, and perhaps his abdominal pain is a result of hepatic congestion from right heart failure. His fever, however, cannot be attributed to cardiac failure. Less likely diagnoses include ischemic conditions related to his cardiomyopathy such as mesenteric ischemia from low perfusion or embolism from a cardiac thrombus. A pulmonary infection remains the most likely diagnosis.
He was an ill‐appearing man in moderate respiratory distress, looking older than his stated age. His temperature was 38.4C, heart rate 129 beats/minute, blood pressure 85/56 mm Hg, respiratory rate 24 breaths/minute, and oxygen saturation 92% on room air. A cardiovascular exam revealed no murmur, gallop, or rub. The jugular venous pulse was not elevated. His lungs were clear to auscultation. Abdominal exam revealed right‐sided abdominal tenderness that appeared to localize to the rectus sheath. Otherwise, the abdomen was soft, with normal bowel sounds and no organomegaly. Rectal examination revealed guaiac negative stool and no focal tenderness. His extremities were normal.
His vital signs are worrisome for impending cardiovascular collapse and shock, possibly due to sepsis. The relatively nonfocal cardiopulmonary exam is surprising given his initial symptoms and makes me wonder if his dyspnea is primarily related to an abdominal process leading to diaphragmatic irritation rather than to a thoracic process. Congestive heart failure seems unlikely given the lack of supportive physical examination findings. His abdominal exam findings are puzzling. Although his abdominal wall tenderness could be benignperhaps from muscular strain or a tear from coughingit could represent a more worrisome process such as infection or a hematoma in the abdominal wall muscles. Mesenteric ischemia is still possible, as the exam is often unimpressive. A hepatic abscess or subphrenic abscess should be considered, as physical exam findings in these conditions can be subtle.
My differential remains relatively unchanged, but I have now put consideration of a hepatic or subphrenic abscess higher on my list. Early empiric broad‐spectrum antibiotics seem necessary.
He had a white blood cell count of 26,700/mL with 92% neutrophils, a hemoglobin of 14.6 g/dL, and a platelet count of 312,000/mL. Sodium was 134 mmol/L, potassium was 4.3 mmol/L, chloride was 94 mmol/L, bicarbonate was 23 mmol/L, blood urea nitrogen was 23 mg/dL, and creatinine was 2.1 mg/dL. The results of the calcium, protein, albumin, and liver function tests were normal. Urinalysis was negative for protein and red blood cells. An electrocardiogram revealed sinus tachycardia. A chest radiograph at admission revealed mild opacities in both lower lobes and the right middle lobe consistent with either atelectasis or pneumonia (Fig. 1). A very small left effusion was also identified.

The additional data reinforce my clinical impression that this process is likely to be infectious. The chest radiograph is consistent with community‐acquired pneumonia, possibly from an atypical pathogen. Given his elevated creatinine, I am also considering a pulmonary‐renal syndrome such as vasculitis, though hematuria was not present. A subphrenic abscess, mesenteric ischemia, or an abdominal wall process (because his abdominal tenderness on exam still needs an explanation) remain possibilities; my suspicion would increase if he does not respond appropriately to therapy for community‐acquired pneumonia.
The clinical team's working diagnosis also was community‐acquired pneumonia. Blood and sputum cultures were obtained, and the patient was treated with intravenous ceftriaxone, azithromycin, and intravenous fluid. By the second day, his creatinine had normalized; however, his hypoxemia had worsened, and he now required supplemental oxygen. His temperature was 39.3C, and his heart rate was 150 beats/minute. The findings of an abdominal ultrasound of the kidneys, spleen, and right upper quadrant were normal.
It is too early to say the patient has failed therapy because a patient can get worse before getting better during the course of antibiotic therapy for community‐acquired pneumonia. Fever, for example, may take up to 7 days to resolve, depending on host factors and the pathogen. Though I typically wait about 72 hours before assuming a patient is not appropriately responding to therapy, the additional information has made me concerned. The degree of tachycardia is significant and warrants an EKG to exclude an arrthymia. I would also repeat the chest radiograph to evaluate for worsening infiltrates or increased pleural effusion.
On the third hospital day, the patient's abdominal pain had decreased with analgesia, but his fever, cough, and dyspnea remained largely unchanged. Antibiotics were changed to intravenous levofloxacin. A repeat chest radiograph revealed elevation of the right hemidiaphragm and bilateral effusions (Fig. 2). An electrocardiogram revealed sinus tachycardia. Blood cultures revealed no growth, and sputum cultures grew oral flora.

A significantly elevated right hemidiaphragm makes me reconsider the diagnosis of simple community‐acquired pneumonia. The differential diagnosis for an elevated hemidiaphragm is best considered by location in relation to the diaphragm. Causes above the diaphragm include rib fracture, atelectasis, pleural thickening, and volume loss of the lung for another reason (e.g., surgery, bronchial obstruction due to tumor or mucus plugging), as well as mimics such as a densely consolidated pneumonia, pulmonary infarction, or a subpulmonary effusion. Diaphragmatic causes include eventration, rupture, phrenic nerve weakness, and intrinsic weakness because of neuromuscular disease (usually bilateral). Causes below the diaphragm that must be considered are subphrenic or liver abscess, liver (and other abdominal) malignancy, pancreatic pseudocyst, and distended bowel. Given the clinical picture, I am focusing below the diaphragmespecially on a possible hepatic or subphrenic abscess (which could be missed on ultrasound) and mimics of it such as dense consolidation or a subpulmonary effusion. Given the lack of response to antibiotics, I need to consider an infection that is not being treated, either because of location (abscess, effusion) or microbiology (tuberculosis, a parasite, a fungus, resistant bacteria). After confirming that the patient has a substantive pleural effusion, he needs a thoracentesis.
On the fourth hospital day, his temperature was 38.8C, and his white blood cell count was 21,000/mL. A right‐sided thoracentesis was performed; approximately 250 cc of fluid was obtained. Pleural fluid analysis revealed bloody fluid, with a white blood cell count of 16,750/mL with 94% neutrophils, 40,000 red blood cells/mL, lactate dehydrogenase of 278 U/L (normal serum value 80200 U/L), protein of 3.7 g/dL, and glucose of 81 mg/dL. A pleural fluid pH was not obtained. A gram stain revealed many white blood cells with no organisms noted. Serum protein was 7.4 g/dL. These results were thought to represent an exudative parapneumonic effusion; levofloxacin and supplemental oxygen were continued.
The pleural fluid appears exudative, but I am not sure this man has a parapneumonic effusion because, despite clinical deterioration, an obvious infiltrate is not seen on interval chest radiography. We must look closely at the fluid because this is a bloody effusion and somewhat atypical for a parapneumonic effusion. Also, the effusion does not appear large enough to explain why he has not improved on the current antibiotics. We should thus reconsider our diagnosis and management. I would obtain additional imaging (such as an abdominal and chest computed tomography [CT]) and perhaps obtain a consultation from the pulmonary team regarding the postulated initial diagnosis of pneumonia with effusion.
On the fifth day of hospitalization, the patient's dyspnea and cough persisted but were improved. His abdominal pain was minimal and felt improved with flatus. Fever continued to 38.8C, and the white blood cell count was 20,000/mL. On examination the patient had decreased breath sounds at the right base and bibasilar crackles. His abdomen was soft, with tenderness in his right upper quadrant only with deep palpation; bowel sounds remained. An ultrasound of the chest was performed to look for a loculated effusion; however, no fluid was identified. The pulmonary consultant thought it likely that the patient had a subpulmonic effusion and recommended CT of the abdomen and chest.
His right upper quadrant tenderness is still unexplained. I would agree with the CT, primarily to evaluate other causes of his elevated diaphragm such as subphrenic or hepatic abscess. For now, I would make no change in antibiotic therapy.
On the sixth hospital day, the patient had an episode of bilious emesis. Chest and abdominal CT revealed collapse of the right middle and lower lobes with a small adjacent effusion, and a 6 6 16 cm abscess intimately opposed to the right lobe of the liver. Extending from the inferior extent of the abscess was a tubular thick‐walled structure connecting to the cecum that was suspicious of a thickened inflamed appendix. There was periappendiceal stranding suggesting inflammation. The small bowel was diffusely dilated up to 4.5 cm, suggesting a small bowel obstruction.
I suspect that his abscess is related to a perforated appendix and that the dilated small bowel is most likely a result of localized irritation of the bowel by the abscess and appendicitis. The collapsed lung is most likely due to local inflammation from the subdiaphragmatic abscess. Treatment should now be changed substantially. I would ask a surgeon to evaluate the patient because the most likely diagnosis is perforated appendicitis with abscess formation.
When the periappendiceal abscess was drained percutaneously, 190 mL of purulent fluid was removed. The cultures were positive for Klebsiella pneumonia, Enterococcus faecalis,and Streptococcus milleri. The patient was given 6 weeks of intravenous antibiotics with improvement in his clinical symptoms. During the interval the findings on his chest radiograph resolved completely. A laproscopic appendectomy 3 months later revealed significant right lower quadrant adhesions. The pathology specimen identified a distorted appendix with regeneration consistent with prior appendicitis. The patient was contacted 4 months after his surgery, and he reported that he was doing well, with no cardiopulmonary or gastrointestinal symptoms.
COMMENTARY
Community‐acquired pneumonia (CAP) is a common cause of acute illness and accounts for nearly 1 million admissions per year in the United States.1 The diagnosis of CAP is made when symptoms including dyspnea, fever, cough, or leukocytosis are present, with confirmation provided by a chest radiograph. Often the diagnosis is clear; however, there is no pathognomonic constellation of signs or symptoms that establish the diagnosis with certainty.2 Many physicians learn that pneumoniaespecially lower‐lobe pneumoniacan lead to abdominal findings such as upper quadrant pain, vomiting, and tenderness to palpation. Conversely, the patient discussed above illustrates that a primary abdominal process can also result in a symptom complex that mimics pneumonia.
The prevalence of CAP coupled with the inherent uncertainty of a clinical diagnosis of CAP leads to an important question: How long is too long before questioning the diagnosis? An analysis of the pneumonia Patient Outcomes Research Trial (PORT) limited to inpatients with CAP examined time to clinical stability. For the majority of patients, abnormal vital signs resolved within 23 days.3 In this study, 29% of patients had severe disease, and not surprisingly, these patients took longer to improve. Using the pneumonia severity index score, which accounts for age, comorbidity, abnormal vital signs, and laboratory data, the patient described in this article would be considered at high risk for death and complication with an estimated mortality of 9%.4 Using a combination of defervescence, resolution of tachycardia, tachypnea, and hypoxemia as markers of clinical stability, a patient like ours should respond within 4 days (with a range of 27 days). On the basis of these dataand the discrepancy between the patient's severe illness and relatively minor pulmonary infiltratesit seems reasonable to have considered this patient as failing CAP therapy as early as the fourth day of hospitalization.
In approximately 10% of hospitalized patients with CAP, the clinical course is protracted.5 When patients do not improve as quickly as expected, the reasons that could explain this should be investigated. In a cohort of 49 patients with CAP who failed therapy the most common reasons for failure to improve were severity of the pneumonia and drug resistance.6 A multicenter study found that the incidence of resistance to penicillin by Streptococcus pneumoniae, the most common bacterial pathogen in CAP, was 30%, with a 4% in vitro resistance rate to ceftriaxone.7 How well in vitro resistance predicts clinical response, however, is unclear. Risk factors for antibiotic resistance include close exposure to children, recent antibiotic use, and recent hospitalization. Immunosuppressive conditions should also be considered in patients who fail to improve. Suppurative complications of pneumoniasuch as empyema, parapneumonic effusion, and lung abscessalso delay recovery.
Another consideration in a patient with what appears to be a nonresolving pneumonia with pleural effusion is that the initial diagnosis is incorrect and the cause is extrathoracic. Pulmonary and cardiac diseases account for more than 90% of effusions, whereas less than 5% of pleural effusions result from intraabdominal causes.8 When should intraabdominal diseases be sought in patients with an effusion, fever, dyspnea, and cough? Light suggests that intraabdominal pathology should be investigated in patients who have pleural effusions without significant parenchymal disease.8 This point is underscored by the experience of our patient, whose chest radiographs showed, despite clinical decline, minimal airspace disease.
Several abdominal entities cause pleural effusion. Pancreatitis, either acute or chronic, with pseudocyst formation is the most common abdominal cause of exudative pleural effusions. Approximately 10% of patients with pancreatic disease will develop effusions, usually left‐sided.9 These left‐sided effusions are also seen in splenic abscesses, usually as a result of endocarditis. Intrahepatic abscess is associated with effusions in 20% of patients.10 A subphrenic abscess, as seen in our patient, is an uncommon cause of exudative pleural effusions. Historically, subphrenic abscesses resulted from a perforated viscus, with ruptured appendicitis the most common cause,11 followed by perforated peptic ulcers and biliary tract disease. With the advent of antibiotics, the causes of subphrenic abscess changed considerably, with the majority of current cases resulting from postsurgical complications.12 The findings of a chest radiograph are abnormal in 80% of patients with subphrenic abscess;1214 an elevated hemidiaphragm and pleural effusion are found in the majority of cases. The symptoms of a subphrenic abscess are nonspecific, and patient's complaints are equally split between predominantly thoracic and predomninantly abdominal complaints.15
Appendicitis, a common disease predominantly of the young, may lead to atypical presentations in older individuals. In a retrospective analysis of 113 patients older than 60 years with appendicitis, 70% presented in an atypical fashion.16 Typical symptoms include right lower quadrant pain, fever, anorexia and a white blood cell count greater than 10,000/mL. Fever was the most frequently absent symptom, seen in only 37% of older patients. In this cohort, approximately one third of older patients waited more than 48 hours prior to presentation. The time between symptom onset and clinical presentation is a strong predictor of perforation risk.17 As in this case, roughly 2% of patients with acute appendicitis will present with perforation and abscess formation.18 In such patients the management is initially conservative. Percutaneous drainage and broad spectrum antibiotics are the treatment of choice, followed by an interval appendectomy in 612 weeks.19 The rationale for delayed surgery is that earlier surgery may disseminate a localized inflammatory process.20
Community‐acquired pneumonia is a more frequent cause of hospital admission than is intraabdominal abscess. Physicians often face the dilemma of when to pursue alternative diagnoses after a patient who is thought to have an atypical presentation of a common disease (ie, CAP) fails to respond to conventional therapy. Although clinicians learn that right upper quadrant pain may be a symptom of pneumonia, our patient revealed that abdominal causes may mimic pneumonia and produce a pleural effusion. Determining whether the primary disease originates above or below the diaphragm is critical to guiding therapy. When patients fail to respond adequately to therapy, clinicians should set a low threshold for deciding to image the abdomen in a patient with modest pulmonary infiltrates, pleural effusion, and abdominal pain.
A 49‐year‐old man presented with 2 days of chills, fever, anorexia, and increased cough and dyspnea. The patient had a history of chronic obstructive pulmonary disease (COPD) and noted that his cough and dyspnea had increased above normal for several days. He was now dyspneic with minimal activity and had slept at a 45‐degree incline the night prior to evaluation due to dyspnea. He noted less improvement than usual with the use of his metered dose inhaler. His cough was occasionally productive of small amounts of white phlegm. He had vomited once. During a coughing episode the patient experienced a sudden onset of sharp right upper quadrant abdominal pain that worsened with coughing and sudden position changes. The patient denied a prior history of abdominal pain or surgery. The patient's last bowel movement was 2 days prior to admission. He denied melena or bright red blood per rectum.
My initial differential diagnosis for this patient's dyspnea and cough is pneumonia, acute exacerbation of COPD, or congestive heart failure. The presence of fever and anorexia increases the likelihood of infectious etiologies, whereas the presence of orthopnea points toward congestive heart failure. Noncardiac processessuch as a large pleural effusion or apical lung diseasecould also cause orthopnea. His abdominal pain could be a result of pneumonia alone (perhaps in the right lower lobe with diaphragmatic irritation), but I am also considering complications of pneumonia such as empyema. Although his abdominal pain, dyspnea, and cough could also be a result of hepatobiliary disease, a perforated viscus, or pancreatitis, we currently have little reason to suspect a direct abdominal etiology. My top diagnosis is community‐acquired pneumonia, perhaps accompanied by pleural effusion.
His medical history was significant for dilated cardiomyopathy and heavy alcohol use. His medications included various meter‐dosed inhalers, bupropion, digoxin, spironolactone, lisinopril, and metoprolol. He had never received corticosteroid therapy and had not previously been hospitalized for COPD‐related problems. He had smoked one pack of cigarettes daily for 40 years.
Heavy alcohol use is associated with an increased risk of several pulmonary infections such as gram‐negative necrotizing pneumonia (classically, Klebsiella pneumoniae), pneumococcal pneumonia, aspiration pneumonia, anaerobic lung abscesses, and tuberculosis. Given his right upper quadrant pain, acute alcoholic hepatitis and alcohol‐related pancreatitis enter the differential. His history of cardiomyopathy makes me consider congestive heart failure as more likely than before, and perhaps his abdominal pain is a result of hepatic congestion from right heart failure. His fever, however, cannot be attributed to cardiac failure. Less likely diagnoses include ischemic conditions related to his cardiomyopathy such as mesenteric ischemia from low perfusion or embolism from a cardiac thrombus. A pulmonary infection remains the most likely diagnosis.
He was an ill‐appearing man in moderate respiratory distress, looking older than his stated age. His temperature was 38.4C, heart rate 129 beats/minute, blood pressure 85/56 mm Hg, respiratory rate 24 breaths/minute, and oxygen saturation 92% on room air. A cardiovascular exam revealed no murmur, gallop, or rub. The jugular venous pulse was not elevated. His lungs were clear to auscultation. Abdominal exam revealed right‐sided abdominal tenderness that appeared to localize to the rectus sheath. Otherwise, the abdomen was soft, with normal bowel sounds and no organomegaly. Rectal examination revealed guaiac negative stool and no focal tenderness. His extremities were normal.
His vital signs are worrisome for impending cardiovascular collapse and shock, possibly due to sepsis. The relatively nonfocal cardiopulmonary exam is surprising given his initial symptoms and makes me wonder if his dyspnea is primarily related to an abdominal process leading to diaphragmatic irritation rather than to a thoracic process. Congestive heart failure seems unlikely given the lack of supportive physical examination findings. His abdominal exam findings are puzzling. Although his abdominal wall tenderness could be benignperhaps from muscular strain or a tear from coughingit could represent a more worrisome process such as infection or a hematoma in the abdominal wall muscles. Mesenteric ischemia is still possible, as the exam is often unimpressive. A hepatic abscess or subphrenic abscess should be considered, as physical exam findings in these conditions can be subtle.
My differential remains relatively unchanged, but I have now put consideration of a hepatic or subphrenic abscess higher on my list. Early empiric broad‐spectrum antibiotics seem necessary.
He had a white blood cell count of 26,700/mL with 92% neutrophils, a hemoglobin of 14.6 g/dL, and a platelet count of 312,000/mL. Sodium was 134 mmol/L, potassium was 4.3 mmol/L, chloride was 94 mmol/L, bicarbonate was 23 mmol/L, blood urea nitrogen was 23 mg/dL, and creatinine was 2.1 mg/dL. The results of the calcium, protein, albumin, and liver function tests were normal. Urinalysis was negative for protein and red blood cells. An electrocardiogram revealed sinus tachycardia. A chest radiograph at admission revealed mild opacities in both lower lobes and the right middle lobe consistent with either atelectasis or pneumonia (Fig. 1). A very small left effusion was also identified.

The additional data reinforce my clinical impression that this process is likely to be infectious. The chest radiograph is consistent with community‐acquired pneumonia, possibly from an atypical pathogen. Given his elevated creatinine, I am also considering a pulmonary‐renal syndrome such as vasculitis, though hematuria was not present. A subphrenic abscess, mesenteric ischemia, or an abdominal wall process (because his abdominal tenderness on exam still needs an explanation) remain possibilities; my suspicion would increase if he does not respond appropriately to therapy for community‐acquired pneumonia.
The clinical team's working diagnosis also was community‐acquired pneumonia. Blood and sputum cultures were obtained, and the patient was treated with intravenous ceftriaxone, azithromycin, and intravenous fluid. By the second day, his creatinine had normalized; however, his hypoxemia had worsened, and he now required supplemental oxygen. His temperature was 39.3C, and his heart rate was 150 beats/minute. The findings of an abdominal ultrasound of the kidneys, spleen, and right upper quadrant were normal.
It is too early to say the patient has failed therapy because a patient can get worse before getting better during the course of antibiotic therapy for community‐acquired pneumonia. Fever, for example, may take up to 7 days to resolve, depending on host factors and the pathogen. Though I typically wait about 72 hours before assuming a patient is not appropriately responding to therapy, the additional information has made me concerned. The degree of tachycardia is significant and warrants an EKG to exclude an arrthymia. I would also repeat the chest radiograph to evaluate for worsening infiltrates or increased pleural effusion.
On the third hospital day, the patient's abdominal pain had decreased with analgesia, but his fever, cough, and dyspnea remained largely unchanged. Antibiotics were changed to intravenous levofloxacin. A repeat chest radiograph revealed elevation of the right hemidiaphragm and bilateral effusions (Fig. 2). An electrocardiogram revealed sinus tachycardia. Blood cultures revealed no growth, and sputum cultures grew oral flora.

A significantly elevated right hemidiaphragm makes me reconsider the diagnosis of simple community‐acquired pneumonia. The differential diagnosis for an elevated hemidiaphragm is best considered by location in relation to the diaphragm. Causes above the diaphragm include rib fracture, atelectasis, pleural thickening, and volume loss of the lung for another reason (e.g., surgery, bronchial obstruction due to tumor or mucus plugging), as well as mimics such as a densely consolidated pneumonia, pulmonary infarction, or a subpulmonary effusion. Diaphragmatic causes include eventration, rupture, phrenic nerve weakness, and intrinsic weakness because of neuromuscular disease (usually bilateral). Causes below the diaphragm that must be considered are subphrenic or liver abscess, liver (and other abdominal) malignancy, pancreatic pseudocyst, and distended bowel. Given the clinical picture, I am focusing below the diaphragmespecially on a possible hepatic or subphrenic abscess (which could be missed on ultrasound) and mimics of it such as dense consolidation or a subpulmonary effusion. Given the lack of response to antibiotics, I need to consider an infection that is not being treated, either because of location (abscess, effusion) or microbiology (tuberculosis, a parasite, a fungus, resistant bacteria). After confirming that the patient has a substantive pleural effusion, he needs a thoracentesis.
On the fourth hospital day, his temperature was 38.8C, and his white blood cell count was 21,000/mL. A right‐sided thoracentesis was performed; approximately 250 cc of fluid was obtained. Pleural fluid analysis revealed bloody fluid, with a white blood cell count of 16,750/mL with 94% neutrophils, 40,000 red blood cells/mL, lactate dehydrogenase of 278 U/L (normal serum value 80200 U/L), protein of 3.7 g/dL, and glucose of 81 mg/dL. A pleural fluid pH was not obtained. A gram stain revealed many white blood cells with no organisms noted. Serum protein was 7.4 g/dL. These results were thought to represent an exudative parapneumonic effusion; levofloxacin and supplemental oxygen were continued.
The pleural fluid appears exudative, but I am not sure this man has a parapneumonic effusion because, despite clinical deterioration, an obvious infiltrate is not seen on interval chest radiography. We must look closely at the fluid because this is a bloody effusion and somewhat atypical for a parapneumonic effusion. Also, the effusion does not appear large enough to explain why he has not improved on the current antibiotics. We should thus reconsider our diagnosis and management. I would obtain additional imaging (such as an abdominal and chest computed tomography [CT]) and perhaps obtain a consultation from the pulmonary team regarding the postulated initial diagnosis of pneumonia with effusion.
On the fifth day of hospitalization, the patient's dyspnea and cough persisted but were improved. His abdominal pain was minimal and felt improved with flatus. Fever continued to 38.8C, and the white blood cell count was 20,000/mL. On examination the patient had decreased breath sounds at the right base and bibasilar crackles. His abdomen was soft, with tenderness in his right upper quadrant only with deep palpation; bowel sounds remained. An ultrasound of the chest was performed to look for a loculated effusion; however, no fluid was identified. The pulmonary consultant thought it likely that the patient had a subpulmonic effusion and recommended CT of the abdomen and chest.
His right upper quadrant tenderness is still unexplained. I would agree with the CT, primarily to evaluate other causes of his elevated diaphragm such as subphrenic or hepatic abscess. For now, I would make no change in antibiotic therapy.
On the sixth hospital day, the patient had an episode of bilious emesis. Chest and abdominal CT revealed collapse of the right middle and lower lobes with a small adjacent effusion, and a 6 6 16 cm abscess intimately opposed to the right lobe of the liver. Extending from the inferior extent of the abscess was a tubular thick‐walled structure connecting to the cecum that was suspicious of a thickened inflamed appendix. There was periappendiceal stranding suggesting inflammation. The small bowel was diffusely dilated up to 4.5 cm, suggesting a small bowel obstruction.
I suspect that his abscess is related to a perforated appendix and that the dilated small bowel is most likely a result of localized irritation of the bowel by the abscess and appendicitis. The collapsed lung is most likely due to local inflammation from the subdiaphragmatic abscess. Treatment should now be changed substantially. I would ask a surgeon to evaluate the patient because the most likely diagnosis is perforated appendicitis with abscess formation.
When the periappendiceal abscess was drained percutaneously, 190 mL of purulent fluid was removed. The cultures were positive for Klebsiella pneumonia, Enterococcus faecalis,and Streptococcus milleri. The patient was given 6 weeks of intravenous antibiotics with improvement in his clinical symptoms. During the interval the findings on his chest radiograph resolved completely. A laproscopic appendectomy 3 months later revealed significant right lower quadrant adhesions. The pathology specimen identified a distorted appendix with regeneration consistent with prior appendicitis. The patient was contacted 4 months after his surgery, and he reported that he was doing well, with no cardiopulmonary or gastrointestinal symptoms.
COMMENTARY
Community‐acquired pneumonia (CAP) is a common cause of acute illness and accounts for nearly 1 million admissions per year in the United States.1 The diagnosis of CAP is made when symptoms including dyspnea, fever, cough, or leukocytosis are present, with confirmation provided by a chest radiograph. Often the diagnosis is clear; however, there is no pathognomonic constellation of signs or symptoms that establish the diagnosis with certainty.2 Many physicians learn that pneumoniaespecially lower‐lobe pneumoniacan lead to abdominal findings such as upper quadrant pain, vomiting, and tenderness to palpation. Conversely, the patient discussed above illustrates that a primary abdominal process can also result in a symptom complex that mimics pneumonia.
The prevalence of CAP coupled with the inherent uncertainty of a clinical diagnosis of CAP leads to an important question: How long is too long before questioning the diagnosis? An analysis of the pneumonia Patient Outcomes Research Trial (PORT) limited to inpatients with CAP examined time to clinical stability. For the majority of patients, abnormal vital signs resolved within 23 days.3 In this study, 29% of patients had severe disease, and not surprisingly, these patients took longer to improve. Using the pneumonia severity index score, which accounts for age, comorbidity, abnormal vital signs, and laboratory data, the patient described in this article would be considered at high risk for death and complication with an estimated mortality of 9%.4 Using a combination of defervescence, resolution of tachycardia, tachypnea, and hypoxemia as markers of clinical stability, a patient like ours should respond within 4 days (with a range of 27 days). On the basis of these dataand the discrepancy between the patient's severe illness and relatively minor pulmonary infiltratesit seems reasonable to have considered this patient as failing CAP therapy as early as the fourth day of hospitalization.
In approximately 10% of hospitalized patients with CAP, the clinical course is protracted.5 When patients do not improve as quickly as expected, the reasons that could explain this should be investigated. In a cohort of 49 patients with CAP who failed therapy the most common reasons for failure to improve were severity of the pneumonia and drug resistance.6 A multicenter study found that the incidence of resistance to penicillin by Streptococcus pneumoniae, the most common bacterial pathogen in CAP, was 30%, with a 4% in vitro resistance rate to ceftriaxone.7 How well in vitro resistance predicts clinical response, however, is unclear. Risk factors for antibiotic resistance include close exposure to children, recent antibiotic use, and recent hospitalization. Immunosuppressive conditions should also be considered in patients who fail to improve. Suppurative complications of pneumoniasuch as empyema, parapneumonic effusion, and lung abscessalso delay recovery.
Another consideration in a patient with what appears to be a nonresolving pneumonia with pleural effusion is that the initial diagnosis is incorrect and the cause is extrathoracic. Pulmonary and cardiac diseases account for more than 90% of effusions, whereas less than 5% of pleural effusions result from intraabdominal causes.8 When should intraabdominal diseases be sought in patients with an effusion, fever, dyspnea, and cough? Light suggests that intraabdominal pathology should be investigated in patients who have pleural effusions without significant parenchymal disease.8 This point is underscored by the experience of our patient, whose chest radiographs showed, despite clinical decline, minimal airspace disease.
Several abdominal entities cause pleural effusion. Pancreatitis, either acute or chronic, with pseudocyst formation is the most common abdominal cause of exudative pleural effusions. Approximately 10% of patients with pancreatic disease will develop effusions, usually left‐sided.9 These left‐sided effusions are also seen in splenic abscesses, usually as a result of endocarditis. Intrahepatic abscess is associated with effusions in 20% of patients.10 A subphrenic abscess, as seen in our patient, is an uncommon cause of exudative pleural effusions. Historically, subphrenic abscesses resulted from a perforated viscus, with ruptured appendicitis the most common cause,11 followed by perforated peptic ulcers and biliary tract disease. With the advent of antibiotics, the causes of subphrenic abscess changed considerably, with the majority of current cases resulting from postsurgical complications.12 The findings of a chest radiograph are abnormal in 80% of patients with subphrenic abscess;1214 an elevated hemidiaphragm and pleural effusion are found in the majority of cases. The symptoms of a subphrenic abscess are nonspecific, and patient's complaints are equally split between predominantly thoracic and predomninantly abdominal complaints.15
Appendicitis, a common disease predominantly of the young, may lead to atypical presentations in older individuals. In a retrospective analysis of 113 patients older than 60 years with appendicitis, 70% presented in an atypical fashion.16 Typical symptoms include right lower quadrant pain, fever, anorexia and a white blood cell count greater than 10,000/mL. Fever was the most frequently absent symptom, seen in only 37% of older patients. In this cohort, approximately one third of older patients waited more than 48 hours prior to presentation. The time between symptom onset and clinical presentation is a strong predictor of perforation risk.17 As in this case, roughly 2% of patients with acute appendicitis will present with perforation and abscess formation.18 In such patients the management is initially conservative. Percutaneous drainage and broad spectrum antibiotics are the treatment of choice, followed by an interval appendectomy in 612 weeks.19 The rationale for delayed surgery is that earlier surgery may disseminate a localized inflammatory process.20
Community‐acquired pneumonia is a more frequent cause of hospital admission than is intraabdominal abscess. Physicians often face the dilemma of when to pursue alternative diagnoses after a patient who is thought to have an atypical presentation of a common disease (ie, CAP) fails to respond to conventional therapy. Although clinicians learn that right upper quadrant pain may be a symptom of pneumonia, our patient revealed that abdominal causes may mimic pneumonia and produce a pleural effusion. Determining whether the primary disease originates above or below the diaphragm is critical to guiding therapy. When patients fail to respond adequately to therapy, clinicians should set a low threshold for deciding to image the abdomen in a patient with modest pulmonary infiltrates, pleural effusion, and abdominal pain.
- ,,, et al.The cost of treating community‐acquired pneumonia.Clin Ther.1998;20:820–827.
- ,,.Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination.JAMA.1997;278:1440–1445.
- ,,, et al.Time to clinical stability in patients hospitalized with community acquired pneumonia. Implications for practice guidelines.JAMA.1998;279:1452–1457.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,, et al.Utility of fiberoptic bronchoscopy in non resolving pneumonia.Chest.1990;98:1322–1326.
- ,,, et al.Antimicrobial treatment failures in patients with community acquired pneumonia. Causes and prognostic implications.Am J Respir Crit Care Med.2000;162:154–160.
- ,,, et al.Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997–98.Emerg Infect Dis.1999;5:757–765.
- ,.Pleural effusion. In:Murray JF,Nadel JA, eds.Textbook of respiratory medicine. 3rd ed.Philadelphia:WB Saunders,2000:2013–2041.
- ,,.Significance of pleural effusion in patients with acute pancreatitis.Am J Gastroenterol.1992;87:871–874.
- .Exudative pleural effusions secondary to gastrointestinal diseases.Clin Chest Med.1985;6(1):103–111.
- .Subphrenic abscess.Ann Surg.1963;158:240–248.
- ,,,.Upper abdominal abscess: a continuing and deadly problem.Am J Roentgenol.1980;134:759–765.
- .Subphrenic abscess. A clinical study of 101 cases.Acta Chir Scand.1959;117:388–408.
- ,,.Subphrenic abscess a continuing hazard.Am J Surg.1969:117–122.
- ,.Subphrenic abscess: a thoracoabdominal clinical complex. The changing picture with antibiotics.Am J Surg.1964;108:165–172.
- ,.What have we learned over the past 20 years about appendicitis in the elderly.Am J Surg.2003;185:198–201.
- ,,, et al.Appendicitis: why so complicated? Analysis of 5755 consecutive appendectomies.Am Surg.2000;66:548–554.
- ,,.Appendicitis with a palpable mass.Ann Surg.1981;193:227–229.
- ,,, et al.Nonoperative management of perforated appendicitis without periappendiceal mass.Am J Surg.2000;179:177–181.
- ,,.Appendix. In:Townsend CM, ed.Sabiston textbook of surgery. The biologic basis of modern surgical practice. 16th ed.Philadelphia:W. B. Saunders,2001:917–928.
- ,,, et al.The cost of treating community‐acquired pneumonia.Clin Ther.1998;20:820–827.
- ,,.Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination.JAMA.1997;278:1440–1445.
- ,,, et al.Time to clinical stability in patients hospitalized with community acquired pneumonia. Implications for practice guidelines.JAMA.1998;279:1452–1457.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,, et al.Utility of fiberoptic bronchoscopy in non resolving pneumonia.Chest.1990;98:1322–1326.
- ,,, et al.Antimicrobial treatment failures in patients with community acquired pneumonia. Causes and prognostic implications.Am J Respir Crit Care Med.2000;162:154–160.
- ,,, et al.Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997–98.Emerg Infect Dis.1999;5:757–765.
- ,.Pleural effusion. In:Murray JF,Nadel JA, eds.Textbook of respiratory medicine. 3rd ed.Philadelphia:WB Saunders,2000:2013–2041.
- ,,.Significance of pleural effusion in patients with acute pancreatitis.Am J Gastroenterol.1992;87:871–874.
- .Exudative pleural effusions secondary to gastrointestinal diseases.Clin Chest Med.1985;6(1):103–111.
- .Subphrenic abscess.Ann Surg.1963;158:240–248.
- ,,,.Upper abdominal abscess: a continuing and deadly problem.Am J Roentgenol.1980;134:759–765.
- .Subphrenic abscess. A clinical study of 101 cases.Acta Chir Scand.1959;117:388–408.
- ,,.Subphrenic abscess a continuing hazard.Am J Surg.1969:117–122.
- ,.Subphrenic abscess: a thoracoabdominal clinical complex. The changing picture with antibiotics.Am J Surg.1964;108:165–172.
- ,.What have we learned over the past 20 years about appendicitis in the elderly.Am J Surg.2003;185:198–201.
- ,,, et al.Appendicitis: why so complicated? Analysis of 5755 consecutive appendectomies.Am Surg.2000;66:548–554.
- ,,.Appendicitis with a palpable mass.Ann Surg.1981;193:227–229.
- ,,, et al.Nonoperative management of perforated appendicitis without periappendiceal mass.Am J Surg.2000;179:177–181.
- ,,.Appendix. In:Townsend CM, ed.Sabiston textbook of surgery. The biologic basis of modern surgical practice. 16th ed.Philadelphia:W. B. Saunders,2001:917–928.