User login
Treatment Needs of Older Adults With Newly Diagnosed Acute Myeloid Leukemia
Within the last 40 years, younger fit patients have benefited from intensive chemotherapy regimens for acute myeloid leukemia (AML) with improved survival, and the possibility of long-term disease-free survival (DFS) (“cure”).1 Older patients are often considered too unfit for standard curative treatment with intensive induction chemotherapy followed by consolidation chemotherapy, allogeneic hematopoietic cell transplantation (allo-HCT), or both.2-4 Higher induction mortality and poor overall survival (OS) are associated with worse performance status, organ impairment, significant comorbidities, and declining cognitive function, all of which are more common with advancing age. Although the suggested criteria for determining unfitness have not been validated (Table 1), they can provide guidance in clinical practice.2-5
The National Comprehensive Cancer Network (NCCN) panel recommends the consideration of a patient’s performance status and comorbid conditions in addition to their age to determine a patient’s fitness for intensive induction therapy.6 Adverse disease features should also be considered, because disease biology may make intensive chemotherapy futile or inappropriate. For example, the mutational driver tumor protein p53 (TP53) appears at a higher frequency in older adults than younger adults and is associated with dismal outcomes even with intensive chemotherapy. Likewise, the spliceosome and chromatin modifier gene mutations are more common in older patients with AML and confer a worse OS with intensive therapy.6,7 Older unfit patients faced a difficult decision: proceed with intensive therapy with some possibility of long-term survival but risk of early mortality and significant toxicity, or opt for supportive care and palliative chemotherapy, such as the hypomethylating agents (HMAs) or low-dose cytarabine, with much shorter survival.
Guidelines for Treating Older Unfit Patients
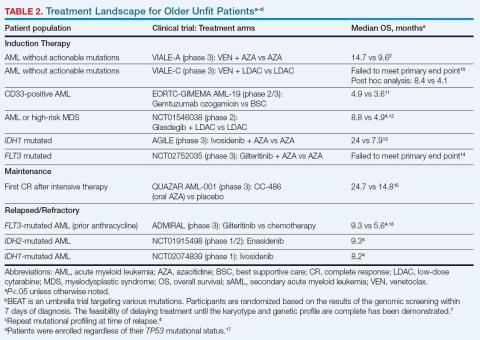
Evidence-based guidelines for managing older adults with newly diagnosed AML were developed by the American Society of Hematology in 2020; however, these guidelines were released prior to the results of several clinical trials involving older patients with AML (Table 2).8 In 2022, the European LeukemiaNet (ELN) recommendations were updated to include new therapeutic agents that target specific mutations in genes such as tyrosine kinase 3 (FLT3), isocitrate dehydrogenase 1 (IDH1), isocitrate dehydrogenase 2 (IDH2), and B-cell lymphoma 2 (BCL2). Given the important effects of genetic aberrations on disease phenotype, treatment options, and outcomes, screening for genetic aberrations at diagnosis is now essential.9
The potential for clonal evolution leading to new actionable targets that were not present at diagnosis highlights the importance of reevaluation of genetic aberrations throughout clinical progression. Actionable targets can include mutations in IDH1/IDH2, FLT3-internal tandem duplication or FLT3 tyrosine kinase domain.9
Treatment Landscape
Since 2018, several therapeutic agents have been added to the treatment armamentarium that can induce longer-term complete remission (CR) for older unfit patients with newly diagnosed AML (Table 2).4
Management of Primary AML With Less Intensive Induction Therapy
VIALE-A established a new standard of care for older unfit patients by demonstrating the benefit of adding the BCL2 inhibitor venetoclax (VEN) to azacitidine (AZA).2 VIALE-A demonstrated that the rate of CR plus CR with partial hematologic recovery (CRi) was 65% for VEN plus AZA and 18% for AZA. Most remissions in the AZA/VEN arm occurred rapidly in the first 2 cycles. The median survival improved from 9.6 months with AZA to 14.7 months with AZA/VEN. An improvement in survival with VEN and low-dose cytarabine also emerged in a 6-month post hoc analysis of the VIALE-C trial.10 Various other trials examining targeted therapies on specific mutations have provided mixed results in the front-line setting.13,14,18 It is important to note that a recent systematic review found that 12% to 25% of patients who were unfit for intensive therapy were successfully bridged to HCT.19
Management of Postremission Response
Patients with a longer duration of first remission have demonstrated better survival outcomes.15 Two trials have examined postremission therapy in the setting of prior intensive therapy. HOVON97 enrolled older patients who achieved CR/CRi after 2 cycles of intensive therapy to receive either AZA postremission or no further treatment. The proportion of patients with DFS at 12 months was greater in the AZA maintenance group than in the observation group (64% vs 42%), but significant DFS improvement did not translate into improved OS.20 QUAZAR AML-001 demonstrated that OS was longer for older patients receiving maintenance therapy with CC-486 (a non-bioequivalent oral formulation of AZA) vs placebo (24.7 vs 14.8 months).15 CC-486 was FDA-approved for maintenance therapy after intensive induction with or without consolidation in patients who are not candidates for allo-HCT. However, limited evidence exists specifically for postremission therapy in unfit patients who have received less intensive therapy. Continuation of the lower intensive therapy is recommended until disease progression.6 No data are available to support the use of oral AZA therapy alone for maintenance of remission following HMA/VEN-induced remissions.
Management of Relapsed and Refractory AML
Nearly 50% of patients with AML experience relapse and up to 40% may be refractory.19 Importantly, patients who were considered fit for intensive therapy may not remain so with relapsed or refractory AML (r/rAML), so patients should be evaluated for fitness for an intensive salvage regimen. Similar to assessing fitness for induction therapy, no standard definition of fitness exists for r/rAML.19
Disease control is the goal for patients with r/rAML who are unfit for intensive salvage therapy; however, treatment options remain limited and prognosis is poor.19 Depending on the patient’s cytogenetic profile, management can include HMA with or without VEN, glasdegib with LDAC, gilteritinib, ivosidenib or enasidenib, or gemtuzumab ozogamicin.9 Only a few studies have been published involving the r/rAML population not eligible for intensive salvage regimen, and guidelines are needed for this population.19 Thus, the ELN recommends that clinical trial enrollment be considered for patients with r/rAML.9
Management of Secondary AML or High-risk AML
Compared with de novo AML, both secondary AML (sAML) and therapy-related AML (tAML) have been associated with inferior outcomes. Factors that influence poor outcomes can include older age, comorbidities, persistent malignant disease or relapse of primary malignancy, treatment-induced depletion of hematopoietic reserves and/or prolonged myelosuppression, and genetic abnormalities, such as TP53 mutations.21
CPX-351 is a dual drug that contains cytarabine and daunorubicin.9,22 An open-label study (NCT01696084) compared CPX-351 with conventional cytarabine and daunorubicin (induction and consolidation therapy) in older patients (aged 60-75 years) with newly diagnosed high-risk/sAML who were considered fit for intensive therapy. The OS for CPX-351 was longer (9.56 vs 5.95 months) and the safety profiles were similar between the treatment groups.23 Patients achieving CR/CRi received up to 2 cycles of consolidation with CPX-351. An exploratory analysis of this subgroup revealed median OS was longer with CPX-351 consolidation (25.43 vs 8.53 months).22 Patients with TP53 mutations had poor treatment outcomes regardless of treatment arm, whereas patients with sAML-type mutations including spliceosome and chromatin modifier genes had longer OS with CPX-351 therapy.24 The 5-year results of this trial indicate that the survival benefit of CPX-351 was maintained.25 However, data from a retrospective review involving 136 patients with either sAML or AML with myelodysplasia-related changes revealed no difference in survival outcomes between patients treated with either HMA/VEN or CPX-351.26
Case Study: Elderly Woman With Newly Diagnosed AML
In 2018, Ms. W, age 69 years, was diagnosed with seropositive, non-erosive rheumatoid arthritis; she began methotrexate 17.5 mg per week split dosing in conjunction with oral folic acid 2 mg/d with varying doses based on symptoms. Her comorbidities included recurrent episodes of diverticulitis, hypertension, hypothyroidism, obstructive sleep apnea, and gastrointestinal reflux disease. On February 4, 2021, her methotrexate was increased to 20 mg and required intermittent prednisone tapers for flares. In November 2021, a blood test revealed she had a decreased white blood cell (WBC) count at 1.8 K/μL, and her methotrexate dose was decreased to 15 mg weekly. Despite the dose reduction, she had grade 3 neutropenia and anemia (WBC: 0.7 K/μL; HGB:10.5 g/dL) with a normal platelet count (PLT: 165,000/μL). Methotrexate was discontinued and leucovorin was initiated. She then had only modest improvement in her lab values and peripheral blood blasts.
On March 17, 2022, she underwent a bone marrow biopsy and aspirate, which resulted in a diagnosis of AML. She had 55% blasts in a 90% cellular bone marrow with mild reticulin fibrosis and numerous circulating blasts. She was classified as having AML without maturation (FAB AML-M1). Flow cytometry detected a phenotypically abnormal population with CD45 expression and side scatter/forward scatter features of small-to-medium sized blasts, accounting for 23% of total cells. The chromosome analysis demonstrated a normal female karyotype in all 19 available metaphases. Polymerase chain reaction analysis was negative for FLT3-ITD, FLT3-TKD, and NPM1 mutations and positive for an IDH1 R132C missense mutation. The myeloid gene panel identified only a single pathogenic variant, IDH1 R132C (variant allele frequency [VAF] 21.2%), and a variant of unknown significance DNMT3A A575P (VAF 25.7%).
Noting that she does not have favorable risk features, we discussed treatment options. Although she is a candidate for curative therapy, the patient was not interested in pursuing allo-HCT. Her history of diverticulitis is concerning for tolerating intensive chemotherapy. In addition, her immunosuppressive therapy increases her risk for opportunistic infections. Based on the available data from the AGILE and VIALE studies and associated potential adverse reactions, she opted for starting treatment with AZA and IVO.
On March 31, 2022, she began receiving AZA 75 mg/m2 intravenous (IV) once daily days 1-7 and oral IVO 500 mg once daily continuously. She has received 12 cycles and has not needed transfusion. She has not had febrile neutropenia or symptoms of differentiation syndrome. On March 24, 2023, she underwent laparoscopic cholecystectomy, because an ultrasound revealed cholelithiasis, abnormal gallbladder wall thickening, and pericholecystic fluid. She was discharged home the following day and is continuing with AZA/ivosidenib.
- Schlenk RF. Acute myeloid leukemia: introduction to a series highlighting progress and ongoing challenges. Haematologica. 2023;108(2):306-307. doi:10.3324/haematol.2022.280803
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. doi:10.1056/NEJMoa2012971
- DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020;135(2):85-96. doi:10.1182/blood.2019001239
- Huerga-Domínguez S, Villar S, Prósper F, Alfonso-Piérola A. Updates on the management of acute myeloid leukemia. Cancers (Basel). 2022;14(19):4756. doi:10.3390/cancers14194756
- Ferrara F, Barosi G, Venditti A, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia. 2013;27(5):997-999. doi:10.1038/leu.2012.303
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(6):721-749. doi:10.6004/jnccn.2019.0028
- Burd A, Levine RL, Ruppert AS, et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med. 2020;26(12):1852-1858. doi:10.1038/s41591-020-1089-8
- Sekeres MA, Guyatt G, Abel G, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4(15):3528-3549. doi:10.1182/bloodadvances.2020001920
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377. doi:10.1182/blood.2022016867
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145. doi:10.1182/blood.2020004856
- Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979. doi:10.1200/JCO.2015.64.0060
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379-389. doi:10.1038/s41375-018-0312-9
- Montesinos P, Recher C, Vives S, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16):1519-1531. doi:10.1056/NEJMoa2117344
- Wang ES, Montesinos P, Minden MD, et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood. 2022;140(17):1845-1857. doi:10.1182/blood.2021014586
- Wei AH, Döhner H, Pocock C, et al; QUAZAR AML-001 Trial Investigators. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526-2537. doi:10.1056/NEJMoa2004444
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728-1740. doi:10.1056/NEJMoa1902688
- Konopleva MY, Röllig C, Cavenagh J, et al. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: results of the MIRROS trial. Blood Adv. 2022;6(14):4147-4156. doi:10.1182/bloodadvances.2021006303
- Pollyea DA, DiNardo CD, Arellano ML, et al. Impact of venetoclax and azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res. 2022;28(13):2753-2761. doi:10.1158/1078-0432.CCR-21-3467
- Russell-Smith TA, Gurskyte L, Muresan B, et al. Efficacy of non-intensive therapies approved for relapsed/refractory acute myeloid leukemia: a systematic literature review. Future Oncol. 2022;18(16):2029-2039. doi:10.2217/fon-2021-1355
- Huls G, Chitu DA, Havelange V, et al; Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON). Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457-1464. doi:10.1182/blood-2018-10-879866
- Granfeldt Østgård LS, Medeiros BC, Sengeløv H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641-3649. doi:10.1200/JCO.2014.60.0890
- Kolitz JE, Strickland SA, Cortes JE, et al. Consolidation outcomes in CPX-351 versus cytarabine/daunorubicin-treated older patients with high-risk/secondary acute myeloid leukemia. Leuk Lymphoma. 2020;61(3):631-640. doi:10.1080/1042819.2019.1688320
- Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684-2692. doi:10.1200/JCO.2017.77.6112
- Lindsley RC, Gibson CJ, Murdock HM, et al. Genetic characteristics and outcomes by mutation status in a phase 3 study of CPX-351 versus 7+3 in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (AML). Blood. 2019;134(suppl 1):15. doi:10.1182/blood-2019-124500
- Lancet JE, Uy GL, Newell LF, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481-e491. doi:10.1016/S2352-3026(21)00134-4
- Alharthy H, Alkaabba F, Williams M, et al. Outcomes of newly diagnosed therapy-related AML and AML with myelodysplasia-related changes treated with 7+3, hypomethylating agents with or without venetoclax and CPX-351: a retrospective cohort study. Blood. 2022;140(suppl 1):9025-9026. doi:10.1182/blood-2022-170688
Within the last 40 years, younger fit patients have benefited from intensive chemotherapy regimens for acute myeloid leukemia (AML) with improved survival, and the possibility of long-term disease-free survival (DFS) (“cure”).1 Older patients are often considered too unfit for standard curative treatment with intensive induction chemotherapy followed by consolidation chemotherapy, allogeneic hematopoietic cell transplantation (allo-HCT), or both.2-4 Higher induction mortality and poor overall survival (OS) are associated with worse performance status, organ impairment, significant comorbidities, and declining cognitive function, all of which are more common with advancing age. Although the suggested criteria for determining unfitness have not been validated (Table 1), they can provide guidance in clinical practice.2-5
The National Comprehensive Cancer Network (NCCN) panel recommends the consideration of a patient’s performance status and comorbid conditions in addition to their age to determine a patient’s fitness for intensive induction therapy.6 Adverse disease features should also be considered, because disease biology may make intensive chemotherapy futile or inappropriate. For example, the mutational driver tumor protein p53 (TP53) appears at a higher frequency in older adults than younger adults and is associated with dismal outcomes even with intensive chemotherapy. Likewise, the spliceosome and chromatin modifier gene mutations are more common in older patients with AML and confer a worse OS with intensive therapy.6,7 Older unfit patients faced a difficult decision: proceed with intensive therapy with some possibility of long-term survival but risk of early mortality and significant toxicity, or opt for supportive care and palliative chemotherapy, such as the hypomethylating agents (HMAs) or low-dose cytarabine, with much shorter survival.
Guidelines for Treating Older Unfit Patients
Evidence-based guidelines for managing older adults with newly diagnosed AML were developed by the American Society of Hematology in 2020; however, these guidelines were released prior to the results of several clinical trials involving older patients with AML (Table 2).8 In 2022, the European LeukemiaNet (ELN) recommendations were updated to include new therapeutic agents that target specific mutations in genes such as tyrosine kinase 3 (FLT3), isocitrate dehydrogenase 1 (IDH1), isocitrate dehydrogenase 2 (IDH2), and B-cell lymphoma 2 (BCL2). Given the important effects of genetic aberrations on disease phenotype, treatment options, and outcomes, screening for genetic aberrations at diagnosis is now essential.9
The potential for clonal evolution leading to new actionable targets that were not present at diagnosis highlights the importance of reevaluation of genetic aberrations throughout clinical progression. Actionable targets can include mutations in IDH1/IDH2, FLT3-internal tandem duplication or FLT3 tyrosine kinase domain.9
Treatment Landscape
Since 2018, several therapeutic agents have been added to the treatment armamentarium that can induce longer-term complete remission (CR) for older unfit patients with newly diagnosed AML (Table 2).4
Management of Primary AML With Less Intensive Induction Therapy
VIALE-A established a new standard of care for older unfit patients by demonstrating the benefit of adding the BCL2 inhibitor venetoclax (VEN) to azacitidine (AZA).2 VIALE-A demonstrated that the rate of CR plus CR with partial hematologic recovery (CRi) was 65% for VEN plus AZA and 18% for AZA. Most remissions in the AZA/VEN arm occurred rapidly in the first 2 cycles. The median survival improved from 9.6 months with AZA to 14.7 months with AZA/VEN. An improvement in survival with VEN and low-dose cytarabine also emerged in a 6-month post hoc analysis of the VIALE-C trial.10 Various other trials examining targeted therapies on specific mutations have provided mixed results in the front-line setting.13,14,18 It is important to note that a recent systematic review found that 12% to 25% of patients who were unfit for intensive therapy were successfully bridged to HCT.19
Management of Postremission Response
Patients with a longer duration of first remission have demonstrated better survival outcomes.15 Two trials have examined postremission therapy in the setting of prior intensive therapy. HOVON97 enrolled older patients who achieved CR/CRi after 2 cycles of intensive therapy to receive either AZA postremission or no further treatment. The proportion of patients with DFS at 12 months was greater in the AZA maintenance group than in the observation group (64% vs 42%), but significant DFS improvement did not translate into improved OS.20 QUAZAR AML-001 demonstrated that OS was longer for older patients receiving maintenance therapy with CC-486 (a non-bioequivalent oral formulation of AZA) vs placebo (24.7 vs 14.8 months).15 CC-486 was FDA-approved for maintenance therapy after intensive induction with or without consolidation in patients who are not candidates for allo-HCT. However, limited evidence exists specifically for postremission therapy in unfit patients who have received less intensive therapy. Continuation of the lower intensive therapy is recommended until disease progression.6 No data are available to support the use of oral AZA therapy alone for maintenance of remission following HMA/VEN-induced remissions.
Management of Relapsed and Refractory AML
Nearly 50% of patients with AML experience relapse and up to 40% may be refractory.19 Importantly, patients who were considered fit for intensive therapy may not remain so with relapsed or refractory AML (r/rAML), so patients should be evaluated for fitness for an intensive salvage regimen. Similar to assessing fitness for induction therapy, no standard definition of fitness exists for r/rAML.19
Disease control is the goal for patients with r/rAML who are unfit for intensive salvage therapy; however, treatment options remain limited and prognosis is poor.19 Depending on the patient’s cytogenetic profile, management can include HMA with or without VEN, glasdegib with LDAC, gilteritinib, ivosidenib or enasidenib, or gemtuzumab ozogamicin.9 Only a few studies have been published involving the r/rAML population not eligible for intensive salvage regimen, and guidelines are needed for this population.19 Thus, the ELN recommends that clinical trial enrollment be considered for patients with r/rAML.9
Management of Secondary AML or High-risk AML
Compared with de novo AML, both secondary AML (sAML) and therapy-related AML (tAML) have been associated with inferior outcomes. Factors that influence poor outcomes can include older age, comorbidities, persistent malignant disease or relapse of primary malignancy, treatment-induced depletion of hematopoietic reserves and/or prolonged myelosuppression, and genetic abnormalities, such as TP53 mutations.21
CPX-351 is a dual drug that contains cytarabine and daunorubicin.9,22 An open-label study (NCT01696084) compared CPX-351 with conventional cytarabine and daunorubicin (induction and consolidation therapy) in older patients (aged 60-75 years) with newly diagnosed high-risk/sAML who were considered fit for intensive therapy. The OS for CPX-351 was longer (9.56 vs 5.95 months) and the safety profiles were similar between the treatment groups.23 Patients achieving CR/CRi received up to 2 cycles of consolidation with CPX-351. An exploratory analysis of this subgroup revealed median OS was longer with CPX-351 consolidation (25.43 vs 8.53 months).22 Patients with TP53 mutations had poor treatment outcomes regardless of treatment arm, whereas patients with sAML-type mutations including spliceosome and chromatin modifier genes had longer OS with CPX-351 therapy.24 The 5-year results of this trial indicate that the survival benefit of CPX-351 was maintained.25 However, data from a retrospective review involving 136 patients with either sAML or AML with myelodysplasia-related changes revealed no difference in survival outcomes between patients treated with either HMA/VEN or CPX-351.26
Case Study: Elderly Woman With Newly Diagnosed AML
In 2018, Ms. W, age 69 years, was diagnosed with seropositive, non-erosive rheumatoid arthritis; she began methotrexate 17.5 mg per week split dosing in conjunction with oral folic acid 2 mg/d with varying doses based on symptoms. Her comorbidities included recurrent episodes of diverticulitis, hypertension, hypothyroidism, obstructive sleep apnea, and gastrointestinal reflux disease. On February 4, 2021, her methotrexate was increased to 20 mg and required intermittent prednisone tapers for flares. In November 2021, a blood test revealed she had a decreased white blood cell (WBC) count at 1.8 K/μL, and her methotrexate dose was decreased to 15 mg weekly. Despite the dose reduction, she had grade 3 neutropenia and anemia (WBC: 0.7 K/μL; HGB:10.5 g/dL) with a normal platelet count (PLT: 165,000/μL). Methotrexate was discontinued and leucovorin was initiated. She then had only modest improvement in her lab values and peripheral blood blasts.
On March 17, 2022, she underwent a bone marrow biopsy and aspirate, which resulted in a diagnosis of AML. She had 55% blasts in a 90% cellular bone marrow with mild reticulin fibrosis and numerous circulating blasts. She was classified as having AML without maturation (FAB AML-M1). Flow cytometry detected a phenotypically abnormal population with CD45 expression and side scatter/forward scatter features of small-to-medium sized blasts, accounting for 23% of total cells. The chromosome analysis demonstrated a normal female karyotype in all 19 available metaphases. Polymerase chain reaction analysis was negative for FLT3-ITD, FLT3-TKD, and NPM1 mutations and positive for an IDH1 R132C missense mutation. The myeloid gene panel identified only a single pathogenic variant, IDH1 R132C (variant allele frequency [VAF] 21.2%), and a variant of unknown significance DNMT3A A575P (VAF 25.7%).
Noting that she does not have favorable risk features, we discussed treatment options. Although she is a candidate for curative therapy, the patient was not interested in pursuing allo-HCT. Her history of diverticulitis is concerning for tolerating intensive chemotherapy. In addition, her immunosuppressive therapy increases her risk for opportunistic infections. Based on the available data from the AGILE and VIALE studies and associated potential adverse reactions, she opted for starting treatment with AZA and IVO.
On March 31, 2022, she began receiving AZA 75 mg/m2 intravenous (IV) once daily days 1-7 and oral IVO 500 mg once daily continuously. She has received 12 cycles and has not needed transfusion. She has not had febrile neutropenia or symptoms of differentiation syndrome. On March 24, 2023, she underwent laparoscopic cholecystectomy, because an ultrasound revealed cholelithiasis, abnormal gallbladder wall thickening, and pericholecystic fluid. She was discharged home the following day and is continuing with AZA/ivosidenib.
Within the last 40 years, younger fit patients have benefited from intensive chemotherapy regimens for acute myeloid leukemia (AML) with improved survival, and the possibility of long-term disease-free survival (DFS) (“cure”).1 Older patients are often considered too unfit for standard curative treatment with intensive induction chemotherapy followed by consolidation chemotherapy, allogeneic hematopoietic cell transplantation (allo-HCT), or both.2-4 Higher induction mortality and poor overall survival (OS) are associated with worse performance status, organ impairment, significant comorbidities, and declining cognitive function, all of which are more common with advancing age. Although the suggested criteria for determining unfitness have not been validated (Table 1), they can provide guidance in clinical practice.2-5
The National Comprehensive Cancer Network (NCCN) panel recommends the consideration of a patient’s performance status and comorbid conditions in addition to their age to determine a patient’s fitness for intensive induction therapy.6 Adverse disease features should also be considered, because disease biology may make intensive chemotherapy futile or inappropriate. For example, the mutational driver tumor protein p53 (TP53) appears at a higher frequency in older adults than younger adults and is associated with dismal outcomes even with intensive chemotherapy. Likewise, the spliceosome and chromatin modifier gene mutations are more common in older patients with AML and confer a worse OS with intensive therapy.6,7 Older unfit patients faced a difficult decision: proceed with intensive therapy with some possibility of long-term survival but risk of early mortality and significant toxicity, or opt for supportive care and palliative chemotherapy, such as the hypomethylating agents (HMAs) or low-dose cytarabine, with much shorter survival.
Guidelines for Treating Older Unfit Patients
Evidence-based guidelines for managing older adults with newly diagnosed AML were developed by the American Society of Hematology in 2020; however, these guidelines were released prior to the results of several clinical trials involving older patients with AML (Table 2).8 In 2022, the European LeukemiaNet (ELN) recommendations were updated to include new therapeutic agents that target specific mutations in genes such as tyrosine kinase 3 (FLT3), isocitrate dehydrogenase 1 (IDH1), isocitrate dehydrogenase 2 (IDH2), and B-cell lymphoma 2 (BCL2). Given the important effects of genetic aberrations on disease phenotype, treatment options, and outcomes, screening for genetic aberrations at diagnosis is now essential.9
The potential for clonal evolution leading to new actionable targets that were not present at diagnosis highlights the importance of reevaluation of genetic aberrations throughout clinical progression. Actionable targets can include mutations in IDH1/IDH2, FLT3-internal tandem duplication or FLT3 tyrosine kinase domain.9
Treatment Landscape
Since 2018, several therapeutic agents have been added to the treatment armamentarium that can induce longer-term complete remission (CR) for older unfit patients with newly diagnosed AML (Table 2).4
Management of Primary AML With Less Intensive Induction Therapy
VIALE-A established a new standard of care for older unfit patients by demonstrating the benefit of adding the BCL2 inhibitor venetoclax (VEN) to azacitidine (AZA).2 VIALE-A demonstrated that the rate of CR plus CR with partial hematologic recovery (CRi) was 65% for VEN plus AZA and 18% for AZA. Most remissions in the AZA/VEN arm occurred rapidly in the first 2 cycles. The median survival improved from 9.6 months with AZA to 14.7 months with AZA/VEN. An improvement in survival with VEN and low-dose cytarabine also emerged in a 6-month post hoc analysis of the VIALE-C trial.10 Various other trials examining targeted therapies on specific mutations have provided mixed results in the front-line setting.13,14,18 It is important to note that a recent systematic review found that 12% to 25% of patients who were unfit for intensive therapy were successfully bridged to HCT.19
Management of Postremission Response
Patients with a longer duration of first remission have demonstrated better survival outcomes.15 Two trials have examined postremission therapy in the setting of prior intensive therapy. HOVON97 enrolled older patients who achieved CR/CRi after 2 cycles of intensive therapy to receive either AZA postremission or no further treatment. The proportion of patients with DFS at 12 months was greater in the AZA maintenance group than in the observation group (64% vs 42%), but significant DFS improvement did not translate into improved OS.20 QUAZAR AML-001 demonstrated that OS was longer for older patients receiving maintenance therapy with CC-486 (a non-bioequivalent oral formulation of AZA) vs placebo (24.7 vs 14.8 months).15 CC-486 was FDA-approved for maintenance therapy after intensive induction with or without consolidation in patients who are not candidates for allo-HCT. However, limited evidence exists specifically for postremission therapy in unfit patients who have received less intensive therapy. Continuation of the lower intensive therapy is recommended until disease progression.6 No data are available to support the use of oral AZA therapy alone for maintenance of remission following HMA/VEN-induced remissions.
Management of Relapsed and Refractory AML
Nearly 50% of patients with AML experience relapse and up to 40% may be refractory.19 Importantly, patients who were considered fit for intensive therapy may not remain so with relapsed or refractory AML (r/rAML), so patients should be evaluated for fitness for an intensive salvage regimen. Similar to assessing fitness for induction therapy, no standard definition of fitness exists for r/rAML.19
Disease control is the goal for patients with r/rAML who are unfit for intensive salvage therapy; however, treatment options remain limited and prognosis is poor.19 Depending on the patient’s cytogenetic profile, management can include HMA with or without VEN, glasdegib with LDAC, gilteritinib, ivosidenib or enasidenib, or gemtuzumab ozogamicin.9 Only a few studies have been published involving the r/rAML population not eligible for intensive salvage regimen, and guidelines are needed for this population.19 Thus, the ELN recommends that clinical trial enrollment be considered for patients with r/rAML.9
Management of Secondary AML or High-risk AML
Compared with de novo AML, both secondary AML (sAML) and therapy-related AML (tAML) have been associated with inferior outcomes. Factors that influence poor outcomes can include older age, comorbidities, persistent malignant disease or relapse of primary malignancy, treatment-induced depletion of hematopoietic reserves and/or prolonged myelosuppression, and genetic abnormalities, such as TP53 mutations.21
CPX-351 is a dual drug that contains cytarabine and daunorubicin.9,22 An open-label study (NCT01696084) compared CPX-351 with conventional cytarabine and daunorubicin (induction and consolidation therapy) in older patients (aged 60-75 years) with newly diagnosed high-risk/sAML who were considered fit for intensive therapy. The OS for CPX-351 was longer (9.56 vs 5.95 months) and the safety profiles were similar between the treatment groups.23 Patients achieving CR/CRi received up to 2 cycles of consolidation with CPX-351. An exploratory analysis of this subgroup revealed median OS was longer with CPX-351 consolidation (25.43 vs 8.53 months).22 Patients with TP53 mutations had poor treatment outcomes regardless of treatment arm, whereas patients with sAML-type mutations including spliceosome and chromatin modifier genes had longer OS with CPX-351 therapy.24 The 5-year results of this trial indicate that the survival benefit of CPX-351 was maintained.25 However, data from a retrospective review involving 136 patients with either sAML or AML with myelodysplasia-related changes revealed no difference in survival outcomes between patients treated with either HMA/VEN or CPX-351.26
Case Study: Elderly Woman With Newly Diagnosed AML
In 2018, Ms. W, age 69 years, was diagnosed with seropositive, non-erosive rheumatoid arthritis; she began methotrexate 17.5 mg per week split dosing in conjunction with oral folic acid 2 mg/d with varying doses based on symptoms. Her comorbidities included recurrent episodes of diverticulitis, hypertension, hypothyroidism, obstructive sleep apnea, and gastrointestinal reflux disease. On February 4, 2021, her methotrexate was increased to 20 mg and required intermittent prednisone tapers for flares. In November 2021, a blood test revealed she had a decreased white blood cell (WBC) count at 1.8 K/μL, and her methotrexate dose was decreased to 15 mg weekly. Despite the dose reduction, she had grade 3 neutropenia and anemia (WBC: 0.7 K/μL; HGB:10.5 g/dL) with a normal platelet count (PLT: 165,000/μL). Methotrexate was discontinued and leucovorin was initiated. She then had only modest improvement in her lab values and peripheral blood blasts.
On March 17, 2022, she underwent a bone marrow biopsy and aspirate, which resulted in a diagnosis of AML. She had 55% blasts in a 90% cellular bone marrow with mild reticulin fibrosis and numerous circulating blasts. She was classified as having AML without maturation (FAB AML-M1). Flow cytometry detected a phenotypically abnormal population with CD45 expression and side scatter/forward scatter features of small-to-medium sized blasts, accounting for 23% of total cells. The chromosome analysis demonstrated a normal female karyotype in all 19 available metaphases. Polymerase chain reaction analysis was negative for FLT3-ITD, FLT3-TKD, and NPM1 mutations and positive for an IDH1 R132C missense mutation. The myeloid gene panel identified only a single pathogenic variant, IDH1 R132C (variant allele frequency [VAF] 21.2%), and a variant of unknown significance DNMT3A A575P (VAF 25.7%).
Noting that she does not have favorable risk features, we discussed treatment options. Although she is a candidate for curative therapy, the patient was not interested in pursuing allo-HCT. Her history of diverticulitis is concerning for tolerating intensive chemotherapy. In addition, her immunosuppressive therapy increases her risk for opportunistic infections. Based on the available data from the AGILE and VIALE studies and associated potential adverse reactions, she opted for starting treatment with AZA and IVO.
On March 31, 2022, she began receiving AZA 75 mg/m2 intravenous (IV) once daily days 1-7 and oral IVO 500 mg once daily continuously. She has received 12 cycles and has not needed transfusion. She has not had febrile neutropenia or symptoms of differentiation syndrome. On March 24, 2023, she underwent laparoscopic cholecystectomy, because an ultrasound revealed cholelithiasis, abnormal gallbladder wall thickening, and pericholecystic fluid. She was discharged home the following day and is continuing with AZA/ivosidenib.
- Schlenk RF. Acute myeloid leukemia: introduction to a series highlighting progress and ongoing challenges. Haematologica. 2023;108(2):306-307. doi:10.3324/haematol.2022.280803
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. doi:10.1056/NEJMoa2012971
- DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020;135(2):85-96. doi:10.1182/blood.2019001239
- Huerga-Domínguez S, Villar S, Prósper F, Alfonso-Piérola A. Updates on the management of acute myeloid leukemia. Cancers (Basel). 2022;14(19):4756. doi:10.3390/cancers14194756
- Ferrara F, Barosi G, Venditti A, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia. 2013;27(5):997-999. doi:10.1038/leu.2012.303
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(6):721-749. doi:10.6004/jnccn.2019.0028
- Burd A, Levine RL, Ruppert AS, et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med. 2020;26(12):1852-1858. doi:10.1038/s41591-020-1089-8
- Sekeres MA, Guyatt G, Abel G, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4(15):3528-3549. doi:10.1182/bloodadvances.2020001920
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377. doi:10.1182/blood.2022016867
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145. doi:10.1182/blood.2020004856
- Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979. doi:10.1200/JCO.2015.64.0060
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379-389. doi:10.1038/s41375-018-0312-9
- Montesinos P, Recher C, Vives S, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16):1519-1531. doi:10.1056/NEJMoa2117344
- Wang ES, Montesinos P, Minden MD, et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood. 2022;140(17):1845-1857. doi:10.1182/blood.2021014586
- Wei AH, Döhner H, Pocock C, et al; QUAZAR AML-001 Trial Investigators. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526-2537. doi:10.1056/NEJMoa2004444
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728-1740. doi:10.1056/NEJMoa1902688
- Konopleva MY, Röllig C, Cavenagh J, et al. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: results of the MIRROS trial. Blood Adv. 2022;6(14):4147-4156. doi:10.1182/bloodadvances.2021006303
- Pollyea DA, DiNardo CD, Arellano ML, et al. Impact of venetoclax and azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res. 2022;28(13):2753-2761. doi:10.1158/1078-0432.CCR-21-3467
- Russell-Smith TA, Gurskyte L, Muresan B, et al. Efficacy of non-intensive therapies approved for relapsed/refractory acute myeloid leukemia: a systematic literature review. Future Oncol. 2022;18(16):2029-2039. doi:10.2217/fon-2021-1355
- Huls G, Chitu DA, Havelange V, et al; Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON). Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457-1464. doi:10.1182/blood-2018-10-879866
- Granfeldt Østgård LS, Medeiros BC, Sengeløv H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641-3649. doi:10.1200/JCO.2014.60.0890
- Kolitz JE, Strickland SA, Cortes JE, et al. Consolidation outcomes in CPX-351 versus cytarabine/daunorubicin-treated older patients with high-risk/secondary acute myeloid leukemia. Leuk Lymphoma. 2020;61(3):631-640. doi:10.1080/1042819.2019.1688320
- Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684-2692. doi:10.1200/JCO.2017.77.6112
- Lindsley RC, Gibson CJ, Murdock HM, et al. Genetic characteristics and outcomes by mutation status in a phase 3 study of CPX-351 versus 7+3 in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (AML). Blood. 2019;134(suppl 1):15. doi:10.1182/blood-2019-124500
- Lancet JE, Uy GL, Newell LF, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481-e491. doi:10.1016/S2352-3026(21)00134-4
- Alharthy H, Alkaabba F, Williams M, et al. Outcomes of newly diagnosed therapy-related AML and AML with myelodysplasia-related changes treated with 7+3, hypomethylating agents with or without venetoclax and CPX-351: a retrospective cohort study. Blood. 2022;140(suppl 1):9025-9026. doi:10.1182/blood-2022-170688
- Schlenk RF. Acute myeloid leukemia: introduction to a series highlighting progress and ongoing challenges. Haematologica. 2023;108(2):306-307. doi:10.3324/haematol.2022.280803
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. doi:10.1056/NEJMoa2012971
- DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood. 2020;135(2):85-96. doi:10.1182/blood.2019001239
- Huerga-Domínguez S, Villar S, Prósper F, Alfonso-Piérola A. Updates on the management of acute myeloid leukemia. Cancers (Basel). 2022;14(19):4756. doi:10.3390/cancers14194756
- Ferrara F, Barosi G, Venditti A, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia. 2013;27(5):997-999. doi:10.1038/leu.2012.303
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(6):721-749. doi:10.6004/jnccn.2019.0028
- Burd A, Levine RL, Ruppert AS, et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med. 2020;26(12):1852-1858. doi:10.1038/s41591-020-1089-8
- Sekeres MA, Guyatt G, Abel G, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020;4(15):3528-3549. doi:10.1182/bloodadvances.2020001920
- Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377. doi:10.1182/blood.2022016867
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145. doi:10.1182/blood.2020004856
- Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979. doi:10.1200/JCO.2015.64.0060
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379-389. doi:10.1038/s41375-018-0312-9
- Montesinos P, Recher C, Vives S, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16):1519-1531. doi:10.1056/NEJMoa2117344
- Wang ES, Montesinos P, Minden MD, et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood. 2022;140(17):1845-1857. doi:10.1182/blood.2021014586
- Wei AH, Döhner H, Pocock C, et al; QUAZAR AML-001 Trial Investigators. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526-2537. doi:10.1056/NEJMoa2004444
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728-1740. doi:10.1056/NEJMoa1902688
- Konopleva MY, Röllig C, Cavenagh J, et al. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: results of the MIRROS trial. Blood Adv. 2022;6(14):4147-4156. doi:10.1182/bloodadvances.2021006303
- Pollyea DA, DiNardo CD, Arellano ML, et al. Impact of venetoclax and azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res. 2022;28(13):2753-2761. doi:10.1158/1078-0432.CCR-21-3467
- Russell-Smith TA, Gurskyte L, Muresan B, et al. Efficacy of non-intensive therapies approved for relapsed/refractory acute myeloid leukemia: a systematic literature review. Future Oncol. 2022;18(16):2029-2039. doi:10.2217/fon-2021-1355
- Huls G, Chitu DA, Havelange V, et al; Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON). Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457-1464. doi:10.1182/blood-2018-10-879866
- Granfeldt Østgård LS, Medeiros BC, Sengeløv H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641-3649. doi:10.1200/JCO.2014.60.0890
- Kolitz JE, Strickland SA, Cortes JE, et al. Consolidation outcomes in CPX-351 versus cytarabine/daunorubicin-treated older patients with high-risk/secondary acute myeloid leukemia. Leuk Lymphoma. 2020;61(3):631-640. doi:10.1080/1042819.2019.1688320
- Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684-2692. doi:10.1200/JCO.2017.77.6112
- Lindsley RC, Gibson CJ, Murdock HM, et al. Genetic characteristics and outcomes by mutation status in a phase 3 study of CPX-351 versus 7+3 in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (AML). Blood. 2019;134(suppl 1):15. doi:10.1182/blood-2019-124500
- Lancet JE, Uy GL, Newell LF, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481-e491. doi:10.1016/S2352-3026(21)00134-4
- Alharthy H, Alkaabba F, Williams M, et al. Outcomes of newly diagnosed therapy-related AML and AML with myelodysplasia-related changes treated with 7+3, hypomethylating agents with or without venetoclax and CPX-351: a retrospective cohort study. Blood. 2022;140(suppl 1):9025-9026. doi:10.1182/blood-2022-170688