User login
Looking beyond the D-dimer
A 44-year-old woman sought care at the emergency department (ED) because she was having difficulty breathing and felt faint. She had been fine until that morning. Three days earlier the patient, who had a history of high blood pressure and elevated cholesterol levels, had driven from Connecticut to New York and back, spending a total of 4 hours in her car. The patient indicated that she’d been taking oral contraceptives (OCPs) for several years, but she did not smoke. There was no history of hemoptysis, recent surgery, or trauma. Neither blood clots nor cancer were part of her or her family’s history.
In the ED, the patient did not have any signs or symptoms of a deep venous thrombosis (DVT). She was obese, with a body mass index of 40.3 kg/m2; other vitals were: blood pressure (BP), 134/88 mm Hg; heart rate (HR), 64 beats per minute (bpm); respiratory rate (RR), 12; and O2 saturation, 99% with ambulation.
The ED physician strongly suspected a pulmonary embolism (PE), but the patient’s score on a clinical probability algorithm (using the Wells criteria) was a 3, indicating only “moderate probability“ of a PE (TABLE 1). (She scored a 3 because an “alternative diagnosis [was] less likely than PE.”) In addition, her D-dimer level was 160 ng/mL using the Triage D-Dimer Test by Biosite, Inc (normal <400 ng/mL), which ruled out a PE. (Many ED physicians at our institution are more cautious when using this D-dimer assay and use a lower cutoff value.)
Given these results, the ED physician did not order imaging studies because the expense and radiation exposure outweighed the probability of the patient having a PE. A subsequent coronary work-up was also negative. The patient was discharged to home and advised to follow up with her primary care physician a few days later.
Two days later we saw the patient at our office. Not only had her dyspnea gotten worse while the presyncope remained, but she now had left-sided pleuritic chest pain. She also reported mild pain in her right calf. On examination, the patient’s BP was 126/86 mm Hg, HR was 82 bpm, RR was 12, and O2 saturation was 96% with ambulation. Her Wells score was now 6, still a moderate probability for PE. (She received another 3 points for the new DVT symptoms—“clinically suspected DVT.”)
Although the patient did not also have signs of a DVT, her additional symptoms along with the original symptoms’ persistence and the existence of other risk factors (OCP use and obesity) led us to reconsider a PE diagnosis. These suspicions prompted us to send the patient back to the ED, where a Doppler ultrasound of the right lower extremity was negative, but the D-dimer was positive at 565 ng/mL.
A pulmonary computed tomography angiogram (CTA) showed 2 small pulmonary emboli within the distal left upper lobe pulmonary arteries.
The patient was treated with heparin and warfarin and discharged without complications.
TABLE 1
Calculating and interpreting the Wells score4,5,7,9,10
| Clinical parameter | Points |
|---|---|
| Clinically suspected DVT | 3.0 |
| Alternative diagnosis less likely than PE | 3.0 |
| Tachycardia | 1.5 |
| Immobilization/surgery (within 4 weeks) | 1.5 |
| History of DVT or PE | 1.5 |
| Hemoptysis | 1.0 |
| Malignancy (treatment within 6 months, palliative) | 1.0 |
| TOTAL | |
| Score | Traditional interpretation |
| <2.0 | Low probability of PE |
| 2.0-6.0 | Moderate probability of PE |
| >6.0 | High probability of PE |
| Score | Alternative classification scheme |
| ≤4.0 | PE unlikely |
| >4.0 | PE likely |
| DVT, deep venous thrombosis; PE, pulmonary embolism. | |
Discussion
The incidence of PE in the United States varies significantly: Individuals younger than 40 have a risk of 1 in 10,000 compared with 1 in 100 for those older than 80.1 Mortality associated with undiagnosed PE varies widely, from 9.2% to 51%.2 This percentage is significant given that half of all PEs go undiagnosed.3 In addition, when left untreated, PE will recur in 30% to 50% of patients, with a fatality rate of 10% to 45%.1 Further, up to 4% of patients with acute PE develop chronic PE and subsequent pulmonary hypertension.4,5 Given the consequences of failing to diagnose a PE, clinicians must consider this condition in patients who present with unexplained hypotension, dyspnea, or chest pain.6
Not an easy diagnosis
This case report demonstrates the inherent difficulty in diagnosing a PE. Still, certain clinical symptoms/signs can aid in the decision-making process. Fever, crackles, and wheezes decrease the probability of PE, whereas syncope, hemodynamic shock, leg edema, and hemoptysis increase its likelihood.7 Despite the many commonly reported risk factors for PE, only malignancy, recent surgery, or a history of DVT/PE significantly increase the risk of developing a clot.8
The Wells criteria. This scoring system groups patients according to the probability of having a PE: low (score: <2), moderate (score: 2-6), and high (score: >6).6 An alternative classification scheme divides patients into 2 groups: likely to have a PE (score: >4) or unlikely to have a PE (score: ≤4).8
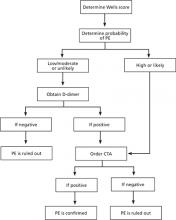
This case report illustrates a key problem with the Wells criteria—the somewhat subjective nature of the scoring. Some physicians find it questionable to award 3 points for “alternative diagnosis less likely than PE,” for example.4 Similarly, with respect to immobilization, some clinicians might have awarded our patient 1.5 points for her recent car trip to New York. We did not think that riding in a car for 2 uninterrupted hours for each leg of the trip was significant enough. However, awarding this patient 1.5 points could have made an important difference in her clinical management if the alternative classification scheme was used. Instead of having a score of 3, the patient would have had a score of 4.5, placing her in the “likely to have a PE” group and prompting us to perform a CTA sooner (FIGURE).
FIGURE
Diagnostic algorithm for pulmonary embolism6,7,10
CTA, computed tomography angiogram; PE, pulmonary embolism.
Inappropriate work-ups are common
Some physicians ignore algorithms when working up a PE and simply order a CTA. In fact, a large multicenter trial showed that 43% of patients suspected of having a PE were inappropriately managed diagnostically.9 Similarly, a meta-analysis of 4 studies including 1660 patients found that only 58% of those with a positive D-dimer had the requisite CTA, as did 7% of patients with a negative D-dimer.2
Physicians should not be concerned about ruling out a PE in the setting of a negative D-dimer, as a meta-analysis found that this diagnostic approach has a negative predictive value (NPV) of 99.7%.2 It is important to note that the NPV is significantly affected by the sensitivity of the D-dimer assay used. If the D-dimer assay is highly sensitive, a negative result in combination with a low, moderate, or unlikely probability Wells score rules out the diagnosis of PE. If the assay is moderately sensitive, however, only a low or unlikely probability Wells score rules out PE.10
The inappropriate work-up of this group of patients is significant and extends beyond the ultimate goal of preventing morbidity and mortality. The unnecessary use of pulmonary CTA is extremely expensive, exposes patients to unnecessary radiation, and results in contrast nephrotoxicity in about 4% of patients.9 Although pulmonary CTA is the standard diagnostic test for PE, other imaging modalities are more appropriate in some cases (TABLE 2).
TABLE 2
Alternative imaging modalities for diagnosing PE1,4,7,11
| Modality | Indication |
|---|---|
| Ventilation-perfusion scanning | Patients with contrast allergies or renal failure; test of choice for diagnosing chronic PE due to limited sensitivity of CT |
| Venous compression ultrasonography | Patients with symptoms of PE and signs/symptoms of DVT |
| Pulmonary angiography | Most invasive test. Should be used only in patients with high probability of PE who may need vascular intervention |
| CT, computed tomography; DVT, deep venous thrombosis; PE, pulmonary embolism. | |
The bottom line
This case report illustrates the importance of using sound clinical judgment when diagnosing a PE. Although our patient initially had a moderate probability Wells score and a negative D-dimer, her symptoms persisted. Her history of OCP use, persistent dyspnea, and new symptoms of a DVT prompted us to reinitiate the diagnostic algorithm and eventually diagnose a PE.
It is always essential to treat the patient and not simply react to laboratory values. To avoid unnecessary testing, however, adhering to the algorithm is equally important.
CORRESPONDENCE
Michael S. Kelleher, MD, University of Connecticut School of Medicine, 263 Farmington Avenue, Farmington, CT 06030; [email protected]
1. Meyer G, Roy PM, Gilberg S, et al. Pulmonary embolism. BMJ. 2010;340:1421.-
2. Pasha SM, Kiok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
3. Taira T, Taira BR, Carmen M, et al. Risk of venous thromboembolism in patients with borderline quantitative D-dimer levels. Am J Emerg Med. 2010;28:450-453.
4. Bounameaux H, Perrier A, Righini M. Diagnosis of venous thromboembolism: an update. Vasc Med. 2010;15:399-406.
5. Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257-2264.
6. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010;363:266-274.
7. Gandara E, Wells PS. Diagnosis: use of clinical probability algorithms. Clin Chest Med. 2010;31:629-639.
8. Drescher FS, Chandrika S, Weir ID, et al. Effectiveness and acceptability of a computerized decision support system using modified wells criteria for evaluation of suspected pulmonary embolism. Ann Emerg Med. 2011;57:613-621.
9. Gimber LH, Travis RI, Takahashi JM, et al. Computed tomography angiography in patients evaluated for acute pulmonary embolism with low serum D-dimer levels: a prospective study. Perm J. 2009;13:4-10.
10. Agency for Healthcare Research and Quality. Guidelines on the diagnosis and management of acute pulmonary embolism. Available at: http://www.guideline.gov/content.aspx?id=13410#Section420. Accessed February 12, 2011.
11. Kim NH. Chronic thromboembolic pulmonary hypertension: diagnosis. Medscape. Available at: http://www.medscape.org/viewarticle/556058_3. Accessed May 9, 2011.
A 44-year-old woman sought care at the emergency department (ED) because she was having difficulty breathing and felt faint. She had been fine until that morning. Three days earlier the patient, who had a history of high blood pressure and elevated cholesterol levels, had driven from Connecticut to New York and back, spending a total of 4 hours in her car. The patient indicated that she’d been taking oral contraceptives (OCPs) for several years, but she did not smoke. There was no history of hemoptysis, recent surgery, or trauma. Neither blood clots nor cancer were part of her or her family’s history.
In the ED, the patient did not have any signs or symptoms of a deep venous thrombosis (DVT). She was obese, with a body mass index of 40.3 kg/m2; other vitals were: blood pressure (BP), 134/88 mm Hg; heart rate (HR), 64 beats per minute (bpm); respiratory rate (RR), 12; and O2 saturation, 99% with ambulation.
The ED physician strongly suspected a pulmonary embolism (PE), but the patient’s score on a clinical probability algorithm (using the Wells criteria) was a 3, indicating only “moderate probability“ of a PE (TABLE 1). (She scored a 3 because an “alternative diagnosis [was] less likely than PE.”) In addition, her D-dimer level was 160 ng/mL using the Triage D-Dimer Test by Biosite, Inc (normal <400 ng/mL), which ruled out a PE. (Many ED physicians at our institution are more cautious when using this D-dimer assay and use a lower cutoff value.)
Given these results, the ED physician did not order imaging studies because the expense and radiation exposure outweighed the probability of the patient having a PE. A subsequent coronary work-up was also negative. The patient was discharged to home and advised to follow up with her primary care physician a few days later.
Two days later we saw the patient at our office. Not only had her dyspnea gotten worse while the presyncope remained, but she now had left-sided pleuritic chest pain. She also reported mild pain in her right calf. On examination, the patient’s BP was 126/86 mm Hg, HR was 82 bpm, RR was 12, and O2 saturation was 96% with ambulation. Her Wells score was now 6, still a moderate probability for PE. (She received another 3 points for the new DVT symptoms—“clinically suspected DVT.”)
Although the patient did not also have signs of a DVT, her additional symptoms along with the original symptoms’ persistence and the existence of other risk factors (OCP use and obesity) led us to reconsider a PE diagnosis. These suspicions prompted us to send the patient back to the ED, where a Doppler ultrasound of the right lower extremity was negative, but the D-dimer was positive at 565 ng/mL.
A pulmonary computed tomography angiogram (CTA) showed 2 small pulmonary emboli within the distal left upper lobe pulmonary arteries.
The patient was treated with heparin and warfarin and discharged without complications.
TABLE 1
Calculating and interpreting the Wells score4,5,7,9,10
| Clinical parameter | Points |
|---|---|
| Clinically suspected DVT | 3.0 |
| Alternative diagnosis less likely than PE | 3.0 |
| Tachycardia | 1.5 |
| Immobilization/surgery (within 4 weeks) | 1.5 |
| History of DVT or PE | 1.5 |
| Hemoptysis | 1.0 |
| Malignancy (treatment within 6 months, palliative) | 1.0 |
| TOTAL | |
| Score | Traditional interpretation |
| <2.0 | Low probability of PE |
| 2.0-6.0 | Moderate probability of PE |
| >6.0 | High probability of PE |
| Score | Alternative classification scheme |
| ≤4.0 | PE unlikely |
| >4.0 | PE likely |
| DVT, deep venous thrombosis; PE, pulmonary embolism. | |
Discussion
The incidence of PE in the United States varies significantly: Individuals younger than 40 have a risk of 1 in 10,000 compared with 1 in 100 for those older than 80.1 Mortality associated with undiagnosed PE varies widely, from 9.2% to 51%.2 This percentage is significant given that half of all PEs go undiagnosed.3 In addition, when left untreated, PE will recur in 30% to 50% of patients, with a fatality rate of 10% to 45%.1 Further, up to 4% of patients with acute PE develop chronic PE and subsequent pulmonary hypertension.4,5 Given the consequences of failing to diagnose a PE, clinicians must consider this condition in patients who present with unexplained hypotension, dyspnea, or chest pain.6
Not an easy diagnosis
This case report demonstrates the inherent difficulty in diagnosing a PE. Still, certain clinical symptoms/signs can aid in the decision-making process. Fever, crackles, and wheezes decrease the probability of PE, whereas syncope, hemodynamic shock, leg edema, and hemoptysis increase its likelihood.7 Despite the many commonly reported risk factors for PE, only malignancy, recent surgery, or a history of DVT/PE significantly increase the risk of developing a clot.8
The Wells criteria. This scoring system groups patients according to the probability of having a PE: low (score: <2), moderate (score: 2-6), and high (score: >6).6 An alternative classification scheme divides patients into 2 groups: likely to have a PE (score: >4) or unlikely to have a PE (score: ≤4).8
This case report illustrates a key problem with the Wells criteria—the somewhat subjective nature of the scoring. Some physicians find it questionable to award 3 points for “alternative diagnosis less likely than PE,” for example.4 Similarly, with respect to immobilization, some clinicians might have awarded our patient 1.5 points for her recent car trip to New York. We did not think that riding in a car for 2 uninterrupted hours for each leg of the trip was significant enough. However, awarding this patient 1.5 points could have made an important difference in her clinical management if the alternative classification scheme was used. Instead of having a score of 3, the patient would have had a score of 4.5, placing her in the “likely to have a PE” group and prompting us to perform a CTA sooner (FIGURE).
FIGURE
Diagnostic algorithm for pulmonary embolism6,7,10
CTA, computed tomography angiogram; PE, pulmonary embolism.
Inappropriate work-ups are common
Some physicians ignore algorithms when working up a PE and simply order a CTA. In fact, a large multicenter trial showed that 43% of patients suspected of having a PE were inappropriately managed diagnostically.9 Similarly, a meta-analysis of 4 studies including 1660 patients found that only 58% of those with a positive D-dimer had the requisite CTA, as did 7% of patients with a negative D-dimer.2
Physicians should not be concerned about ruling out a PE in the setting of a negative D-dimer, as a meta-analysis found that this diagnostic approach has a negative predictive value (NPV) of 99.7%.2 It is important to note that the NPV is significantly affected by the sensitivity of the D-dimer assay used. If the D-dimer assay is highly sensitive, a negative result in combination with a low, moderate, or unlikely probability Wells score rules out the diagnosis of PE. If the assay is moderately sensitive, however, only a low or unlikely probability Wells score rules out PE.10
The inappropriate work-up of this group of patients is significant and extends beyond the ultimate goal of preventing morbidity and mortality. The unnecessary use of pulmonary CTA is extremely expensive, exposes patients to unnecessary radiation, and results in contrast nephrotoxicity in about 4% of patients.9 Although pulmonary CTA is the standard diagnostic test for PE, other imaging modalities are more appropriate in some cases (TABLE 2).
TABLE 2
Alternative imaging modalities for diagnosing PE1,4,7,11
| Modality | Indication |
|---|---|
| Ventilation-perfusion scanning | Patients with contrast allergies or renal failure; test of choice for diagnosing chronic PE due to limited sensitivity of CT |
| Venous compression ultrasonography | Patients with symptoms of PE and signs/symptoms of DVT |
| Pulmonary angiography | Most invasive test. Should be used only in patients with high probability of PE who may need vascular intervention |
| CT, computed tomography; DVT, deep venous thrombosis; PE, pulmonary embolism. | |
The bottom line
This case report illustrates the importance of using sound clinical judgment when diagnosing a PE. Although our patient initially had a moderate probability Wells score and a negative D-dimer, her symptoms persisted. Her history of OCP use, persistent dyspnea, and new symptoms of a DVT prompted us to reinitiate the diagnostic algorithm and eventually diagnose a PE.
It is always essential to treat the patient and not simply react to laboratory values. To avoid unnecessary testing, however, adhering to the algorithm is equally important.
CORRESPONDENCE
Michael S. Kelleher, MD, University of Connecticut School of Medicine, 263 Farmington Avenue, Farmington, CT 06030; [email protected]
A 44-year-old woman sought care at the emergency department (ED) because she was having difficulty breathing and felt faint. She had been fine until that morning. Three days earlier the patient, who had a history of high blood pressure and elevated cholesterol levels, had driven from Connecticut to New York and back, spending a total of 4 hours in her car. The patient indicated that she’d been taking oral contraceptives (OCPs) for several years, but she did not smoke. There was no history of hemoptysis, recent surgery, or trauma. Neither blood clots nor cancer were part of her or her family’s history.
In the ED, the patient did not have any signs or symptoms of a deep venous thrombosis (DVT). She was obese, with a body mass index of 40.3 kg/m2; other vitals were: blood pressure (BP), 134/88 mm Hg; heart rate (HR), 64 beats per minute (bpm); respiratory rate (RR), 12; and O2 saturation, 99% with ambulation.
The ED physician strongly suspected a pulmonary embolism (PE), but the patient’s score on a clinical probability algorithm (using the Wells criteria) was a 3, indicating only “moderate probability“ of a PE (TABLE 1). (She scored a 3 because an “alternative diagnosis [was] less likely than PE.”) In addition, her D-dimer level was 160 ng/mL using the Triage D-Dimer Test by Biosite, Inc (normal <400 ng/mL), which ruled out a PE. (Many ED physicians at our institution are more cautious when using this D-dimer assay and use a lower cutoff value.)
Given these results, the ED physician did not order imaging studies because the expense and radiation exposure outweighed the probability of the patient having a PE. A subsequent coronary work-up was also negative. The patient was discharged to home and advised to follow up with her primary care physician a few days later.
Two days later we saw the patient at our office. Not only had her dyspnea gotten worse while the presyncope remained, but she now had left-sided pleuritic chest pain. She also reported mild pain in her right calf. On examination, the patient’s BP was 126/86 mm Hg, HR was 82 bpm, RR was 12, and O2 saturation was 96% with ambulation. Her Wells score was now 6, still a moderate probability for PE. (She received another 3 points for the new DVT symptoms—“clinically suspected DVT.”)
Although the patient did not also have signs of a DVT, her additional symptoms along with the original symptoms’ persistence and the existence of other risk factors (OCP use and obesity) led us to reconsider a PE diagnosis. These suspicions prompted us to send the patient back to the ED, where a Doppler ultrasound of the right lower extremity was negative, but the D-dimer was positive at 565 ng/mL.
A pulmonary computed tomography angiogram (CTA) showed 2 small pulmonary emboli within the distal left upper lobe pulmonary arteries.
The patient was treated with heparin and warfarin and discharged without complications.
TABLE 1
Calculating and interpreting the Wells score4,5,7,9,10
| Clinical parameter | Points |
|---|---|
| Clinically suspected DVT | 3.0 |
| Alternative diagnosis less likely than PE | 3.0 |
| Tachycardia | 1.5 |
| Immobilization/surgery (within 4 weeks) | 1.5 |
| History of DVT or PE | 1.5 |
| Hemoptysis | 1.0 |
| Malignancy (treatment within 6 months, palliative) | 1.0 |
| TOTAL | |
| Score | Traditional interpretation |
| <2.0 | Low probability of PE |
| 2.0-6.0 | Moderate probability of PE |
| >6.0 | High probability of PE |
| Score | Alternative classification scheme |
| ≤4.0 | PE unlikely |
| >4.0 | PE likely |
| DVT, deep venous thrombosis; PE, pulmonary embolism. | |
Discussion
The incidence of PE in the United States varies significantly: Individuals younger than 40 have a risk of 1 in 10,000 compared with 1 in 100 for those older than 80.1 Mortality associated with undiagnosed PE varies widely, from 9.2% to 51%.2 This percentage is significant given that half of all PEs go undiagnosed.3 In addition, when left untreated, PE will recur in 30% to 50% of patients, with a fatality rate of 10% to 45%.1 Further, up to 4% of patients with acute PE develop chronic PE and subsequent pulmonary hypertension.4,5 Given the consequences of failing to diagnose a PE, clinicians must consider this condition in patients who present with unexplained hypotension, dyspnea, or chest pain.6
Not an easy diagnosis
This case report demonstrates the inherent difficulty in diagnosing a PE. Still, certain clinical symptoms/signs can aid in the decision-making process. Fever, crackles, and wheezes decrease the probability of PE, whereas syncope, hemodynamic shock, leg edema, and hemoptysis increase its likelihood.7 Despite the many commonly reported risk factors for PE, only malignancy, recent surgery, or a history of DVT/PE significantly increase the risk of developing a clot.8
The Wells criteria. This scoring system groups patients according to the probability of having a PE: low (score: <2), moderate (score: 2-6), and high (score: >6).6 An alternative classification scheme divides patients into 2 groups: likely to have a PE (score: >4) or unlikely to have a PE (score: ≤4).8
This case report illustrates a key problem with the Wells criteria—the somewhat subjective nature of the scoring. Some physicians find it questionable to award 3 points for “alternative diagnosis less likely than PE,” for example.4 Similarly, with respect to immobilization, some clinicians might have awarded our patient 1.5 points for her recent car trip to New York. We did not think that riding in a car for 2 uninterrupted hours for each leg of the trip was significant enough. However, awarding this patient 1.5 points could have made an important difference in her clinical management if the alternative classification scheme was used. Instead of having a score of 3, the patient would have had a score of 4.5, placing her in the “likely to have a PE” group and prompting us to perform a CTA sooner (FIGURE).
FIGURE
Diagnostic algorithm for pulmonary embolism6,7,10
CTA, computed tomography angiogram; PE, pulmonary embolism.
Inappropriate work-ups are common
Some physicians ignore algorithms when working up a PE and simply order a CTA. In fact, a large multicenter trial showed that 43% of patients suspected of having a PE were inappropriately managed diagnostically.9 Similarly, a meta-analysis of 4 studies including 1660 patients found that only 58% of those with a positive D-dimer had the requisite CTA, as did 7% of patients with a negative D-dimer.2
Physicians should not be concerned about ruling out a PE in the setting of a negative D-dimer, as a meta-analysis found that this diagnostic approach has a negative predictive value (NPV) of 99.7%.2 It is important to note that the NPV is significantly affected by the sensitivity of the D-dimer assay used. If the D-dimer assay is highly sensitive, a negative result in combination with a low, moderate, or unlikely probability Wells score rules out the diagnosis of PE. If the assay is moderately sensitive, however, only a low or unlikely probability Wells score rules out PE.10
The inappropriate work-up of this group of patients is significant and extends beyond the ultimate goal of preventing morbidity and mortality. The unnecessary use of pulmonary CTA is extremely expensive, exposes patients to unnecessary radiation, and results in contrast nephrotoxicity in about 4% of patients.9 Although pulmonary CTA is the standard diagnostic test for PE, other imaging modalities are more appropriate in some cases (TABLE 2).
TABLE 2
Alternative imaging modalities for diagnosing PE1,4,7,11
| Modality | Indication |
|---|---|
| Ventilation-perfusion scanning | Patients with contrast allergies or renal failure; test of choice for diagnosing chronic PE due to limited sensitivity of CT |
| Venous compression ultrasonography | Patients with symptoms of PE and signs/symptoms of DVT |
| Pulmonary angiography | Most invasive test. Should be used only in patients with high probability of PE who may need vascular intervention |
| CT, computed tomography; DVT, deep venous thrombosis; PE, pulmonary embolism. | |
The bottom line
This case report illustrates the importance of using sound clinical judgment when diagnosing a PE. Although our patient initially had a moderate probability Wells score and a negative D-dimer, her symptoms persisted. Her history of OCP use, persistent dyspnea, and new symptoms of a DVT prompted us to reinitiate the diagnostic algorithm and eventually diagnose a PE.
It is always essential to treat the patient and not simply react to laboratory values. To avoid unnecessary testing, however, adhering to the algorithm is equally important.
CORRESPONDENCE
Michael S. Kelleher, MD, University of Connecticut School of Medicine, 263 Farmington Avenue, Farmington, CT 06030; [email protected]
1. Meyer G, Roy PM, Gilberg S, et al. Pulmonary embolism. BMJ. 2010;340:1421.-
2. Pasha SM, Kiok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
3. Taira T, Taira BR, Carmen M, et al. Risk of venous thromboembolism in patients with borderline quantitative D-dimer levels. Am J Emerg Med. 2010;28:450-453.
4. Bounameaux H, Perrier A, Righini M. Diagnosis of venous thromboembolism: an update. Vasc Med. 2010;15:399-406.
5. Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257-2264.
6. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010;363:266-274.
7. Gandara E, Wells PS. Diagnosis: use of clinical probability algorithms. Clin Chest Med. 2010;31:629-639.
8. Drescher FS, Chandrika S, Weir ID, et al. Effectiveness and acceptability of a computerized decision support system using modified wells criteria for evaluation of suspected pulmonary embolism. Ann Emerg Med. 2011;57:613-621.
9. Gimber LH, Travis RI, Takahashi JM, et al. Computed tomography angiography in patients evaluated for acute pulmonary embolism with low serum D-dimer levels: a prospective study. Perm J. 2009;13:4-10.
10. Agency for Healthcare Research and Quality. Guidelines on the diagnosis and management of acute pulmonary embolism. Available at: http://www.guideline.gov/content.aspx?id=13410#Section420. Accessed February 12, 2011.
11. Kim NH. Chronic thromboembolic pulmonary hypertension: diagnosis. Medscape. Available at: http://www.medscape.org/viewarticle/556058_3. Accessed May 9, 2011.
1. Meyer G, Roy PM, Gilberg S, et al. Pulmonary embolism. BMJ. 2010;340:1421.-
2. Pasha SM, Kiok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
3. Taira T, Taira BR, Carmen M, et al. Risk of venous thromboembolism in patients with borderline quantitative D-dimer levels. Am J Emerg Med. 2010;28:450-453.
4. Bounameaux H, Perrier A, Righini M. Diagnosis of venous thromboembolism: an update. Vasc Med. 2010;15:399-406.
5. Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257-2264.
6. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010;363:266-274.
7. Gandara E, Wells PS. Diagnosis: use of clinical probability algorithms. Clin Chest Med. 2010;31:629-639.
8. Drescher FS, Chandrika S, Weir ID, et al. Effectiveness and acceptability of a computerized decision support system using modified wells criteria for evaluation of suspected pulmonary embolism. Ann Emerg Med. 2011;57:613-621.
9. Gimber LH, Travis RI, Takahashi JM, et al. Computed tomography angiography in patients evaluated for acute pulmonary embolism with low serum D-dimer levels: a prospective study. Perm J. 2009;13:4-10.
10. Agency for Healthcare Research and Quality. Guidelines on the diagnosis and management of acute pulmonary embolism. Available at: http://www.guideline.gov/content.aspx?id=13410#Section420. Accessed February 12, 2011.
11. Kim NH. Chronic thromboembolic pulmonary hypertension: diagnosis. Medscape. Available at: http://www.medscape.org/viewarticle/556058_3. Accessed May 9, 2011.