User login
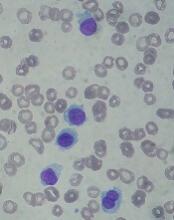
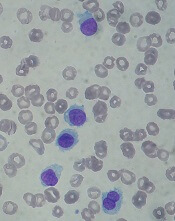
Drug receives priority review for HCL
The US Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for moxetumomab pasudotox, an investigational anti-CD22 recombinant immunotoxin.
With this BLA, AstraZeneca is seeking approval for moxetumomab pasudotox for the treatment of adults with hairy cell leukemia (HCL) who have received at least 2 prior lines of therapy.
The FDA expects to make a decision on the BLA in the third quarter of this year.
The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The agency grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
About moxetumomab pasudotox
Moxetumomab pasudotox (formerly CAT-8015 or HA22) is composed of a binding portion of an anti-CD22 antibody fused to a toxin. After binding to CD22, the molecule is internalized, processed, and releases its modified protein toxin, which inhibits protein translation and leads to apoptosis.
In addition to priority review, moxetumomab pasudotox has received orphan drug designation from the FDA.
Moxetumomab pasudotox has been tested in a phase 1 trial. Initial results from this trial were published in the Journal of Clinical Oncology in 2012. Long-term follow-up was presented at the 2017 ASH Annual Meeting.
The ASH data included 49 patients with relapsed/refractory HCL. Their median age was 57 (range, 40-77), most (n=41) were male, and they had a median of 35 (range, 1-60,444) circulating HCL cells/mm3 at baseline (in 48 evaluable patients).
Twenty-eight patients received moxetumomab pasudotox in the dose-escalation portion of the study—at 5, 10, 20, 30, 40, or 50 µg/kg—and 21 received the drug at 50 µg/kg for the extension portion of the study.
Among the 33 patients who received moxetumomab pasudotox at 50 µg/kg, the overall response rate was 88%, and the complete response (CR) rate was 64% (n=21). The median time to CR was 3.6 months, and the median duration of CR was 70.3 months.
The median follow-up was 75 months for the entire study population. At 72 months, the progression-free survival (PFS) rate was 77%.
The researchers found that minimal residual disease (MRD) negativity (via immunohistochemistry) was associated with extended response duration and prolonged PFS.
The MRD evaluation included 19 MRD+ patients and 18 MRD- patients. Forty-seven percent of the MRD+ patients (n=9) and 94% of the MRD- patients (n=17) had a CR as their best response.
The median duration of CR was 13.1 months among the MRD+ patients and was not reached among the MRD- patients (P=0.0002). The median PFS was 82.1 months among the MRD+ patients and not reached among the MRD- patients (P=0.0031).
Moxetumomab pasudotox did not undergo phase 2 testing but proceeded to a phase 3 trial. In this single-arm study, researchers evaluated the drug in HCL patients who had received at least 2 prior therapies.
According to AstraZeneca, the study’s primary endpoint—durable CR—was met. The company said the phase 3 results will be presented at an upcoming medical meeting.
The US Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for moxetumomab pasudotox, an investigational anti-CD22 recombinant immunotoxin.
With this BLA, AstraZeneca is seeking approval for moxetumomab pasudotox for the treatment of adults with hairy cell leukemia (HCL) who have received at least 2 prior lines of therapy.
The FDA expects to make a decision on the BLA in the third quarter of this year.
The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The agency grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
About moxetumomab pasudotox
Moxetumomab pasudotox (formerly CAT-8015 or HA22) is composed of a binding portion of an anti-CD22 antibody fused to a toxin. After binding to CD22, the molecule is internalized, processed, and releases its modified protein toxin, which inhibits protein translation and leads to apoptosis.
In addition to priority review, moxetumomab pasudotox has received orphan drug designation from the FDA.
Moxetumomab pasudotox has been tested in a phase 1 trial. Initial results from this trial were published in the Journal of Clinical Oncology in 2012. Long-term follow-up was presented at the 2017 ASH Annual Meeting.
The ASH data included 49 patients with relapsed/refractory HCL. Their median age was 57 (range, 40-77), most (n=41) were male, and they had a median of 35 (range, 1-60,444) circulating HCL cells/mm3 at baseline (in 48 evaluable patients).
Twenty-eight patients received moxetumomab pasudotox in the dose-escalation portion of the study—at 5, 10, 20, 30, 40, or 50 µg/kg—and 21 received the drug at 50 µg/kg for the extension portion of the study.
Among the 33 patients who received moxetumomab pasudotox at 50 µg/kg, the overall response rate was 88%, and the complete response (CR) rate was 64% (n=21). The median time to CR was 3.6 months, and the median duration of CR was 70.3 months.
The median follow-up was 75 months for the entire study population. At 72 months, the progression-free survival (PFS) rate was 77%.
The researchers found that minimal residual disease (MRD) negativity (via immunohistochemistry) was associated with extended response duration and prolonged PFS.
The MRD evaluation included 19 MRD+ patients and 18 MRD- patients. Forty-seven percent of the MRD+ patients (n=9) and 94% of the MRD- patients (n=17) had a CR as their best response.
The median duration of CR was 13.1 months among the MRD+ patients and was not reached among the MRD- patients (P=0.0002). The median PFS was 82.1 months among the MRD+ patients and not reached among the MRD- patients (P=0.0031).
Moxetumomab pasudotox did not undergo phase 2 testing but proceeded to a phase 3 trial. In this single-arm study, researchers evaluated the drug in HCL patients who had received at least 2 prior therapies.
According to AstraZeneca, the study’s primary endpoint—durable CR—was met. The company said the phase 3 results will be presented at an upcoming medical meeting.
The US Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for moxetumomab pasudotox, an investigational anti-CD22 recombinant immunotoxin.
With this BLA, AstraZeneca is seeking approval for moxetumomab pasudotox for the treatment of adults with hairy cell leukemia (HCL) who have received at least 2 prior lines of therapy.
The FDA expects to make a decision on the BLA in the third quarter of this year.
The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The agency grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
About moxetumomab pasudotox
Moxetumomab pasudotox (formerly CAT-8015 or HA22) is composed of a binding portion of an anti-CD22 antibody fused to a toxin. After binding to CD22, the molecule is internalized, processed, and releases its modified protein toxin, which inhibits protein translation and leads to apoptosis.
In addition to priority review, moxetumomab pasudotox has received orphan drug designation from the FDA.
Moxetumomab pasudotox has been tested in a phase 1 trial. Initial results from this trial were published in the Journal of Clinical Oncology in 2012. Long-term follow-up was presented at the 2017 ASH Annual Meeting.
The ASH data included 49 patients with relapsed/refractory HCL. Their median age was 57 (range, 40-77), most (n=41) were male, and they had a median of 35 (range, 1-60,444) circulating HCL cells/mm3 at baseline (in 48 evaluable patients).
Twenty-eight patients received moxetumomab pasudotox in the dose-escalation portion of the study—at 5, 10, 20, 30, 40, or 50 µg/kg—and 21 received the drug at 50 µg/kg for the extension portion of the study.
Among the 33 patients who received moxetumomab pasudotox at 50 µg/kg, the overall response rate was 88%, and the complete response (CR) rate was 64% (n=21). The median time to CR was 3.6 months, and the median duration of CR was 70.3 months.
The median follow-up was 75 months for the entire study population. At 72 months, the progression-free survival (PFS) rate was 77%.
The researchers found that minimal residual disease (MRD) negativity (via immunohistochemistry) was associated with extended response duration and prolonged PFS.
The MRD evaluation included 19 MRD+ patients and 18 MRD- patients. Forty-seven percent of the MRD+ patients (n=9) and 94% of the MRD- patients (n=17) had a CR as their best response.
The median duration of CR was 13.1 months among the MRD+ patients and was not reached among the MRD- patients (P=0.0002). The median PFS was 82.1 months among the MRD+ patients and not reached among the MRD- patients (P=0.0031).
Moxetumomab pasudotox did not undergo phase 2 testing but proceeded to a phase 3 trial. In this single-arm study, researchers evaluated the drug in HCL patients who had received at least 2 prior therapies.
According to AstraZeneca, the study’s primary endpoint—durable CR—was met. The company said the phase 3 results will be presented at an upcoming medical meeting.
Agent exhibits activity in leukemias, MDS
The experimental agent prexigebersen (formerly BP1001) was considered well-tolerated and demonstrated early evidence of activity against relapsed/refractory hematologic disorders in a phase 1/1b trial.
The drug reduced blasts in the bone marrow and peripheral blood for patients with acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and myelodysplastic syndrome (MDS).
When given in combination with low-dose cytarabine, prexigebersen produced complete responses (CRs) in patients with AML.
Researchers said that, overall, the toxic effects of prexigebersen were manageable.
There was 1 patient who had dose-limiting toxicities, 1 who discontinued treatment due to possible drug-related toxic effects, and 1 treatment-related death.
Still, the maximum tolerated dose of prexigebersen was not established.
These results were published in The Lancet Haematology. The study was sponsored by Bio-Path Holdings, Inc., the company developing prexigebersen.
Prexigebersen is an anti-sense oligodeoxynucleotide developed to block Grb2 expression and function. Researchers tested the drug in a single-center, dose-escalation, phase 1/1b trial that enrolled and treated 39 patients.
In the phase 1 portion of the trial, patients received prexigebersen monotherapy. In the phase 1b portion, they received the drug in combination with low-dose cytarabine.
There were 32 patients in the phase 1 portion of the trial. Most (n=23) had AML, 5 had CML in blast phase, and 4 had MDS. The patients’ median age was 63 (range, 56-73), and they had received a median of 4 prior therapies.
All 7 patients in the phase 1b portion had AML. They had a median age of 72 (range, 70-76) and had all received 1 prior therapy.
For phase 1, prexigebersen was administered intravenously, twice weekly for 28 days at doses of 5 mg/m² in cohort 1 (n=13), 10 mg/m² in cohort 2 (n=6), 20 mg/m² in cohort 3 (n=3), 40 mg/m² in cohort 4 (n=3), 60 mg/m² in cohort 5 (n=3), and 90 mg/m² in cohort 6 (n=4).
In the phase 1b portion, patients received prexigebersen at 60 mg/m² (n=4) or 90 mg/m² (n=3) in combination with 20 mg of cytarabine (twice-daily subcutaneous injections).
Safety
Twenty-seven patients were evaluable for dose-limiting toxicity—21 from phase 1 and 6 from 1b.
One patient in cohort 1 developed mucositis and hand-foot syndrome, which were considered possibly related to prexigebersen and deemed dose-limiting toxicities. The patient was also receiving hydroxyurea (3 g/day) for CML and had a history of hydroxyurea-induced mucositis.
There were no other dose-limiting toxicities, and the researchers did not identify a maximum tolerated dose of prexigebersen.
The most common grade 3-4 adverse events (AEs) were cardiopulmonary disorders and fevers (including neutropenic fevers and infections).
In the monotherapy group, 17% of patients had grade 3-4 cardiopulmonary AEs, and 11% had fevers. In the prexigebersen-cytarabine combination group, 8% had grade 3-4 cardiopulmonary AEs, and 6% had fevers.
There were 5 grade 5 AEs in 4 patients, all of whom received monotherapy. These included cardiopulmonary disorders (n=2), fevers (n=2), and multi-organ failure (n=1). One patient had both fever (sepsis) and multi-organ failure.
Efficacy
According to the researchers’ assessments, 22% of phase 1 patients (7/32) benefited from prexigebersen monotherapy and therefore received more than 1 cycle of treatment. Five of these patients had AML, and 2 had MDS.
Single-agent activity was observed in other patients as well.
Thirty-three percent (9/27) of patients who had peripheral blood blasts at baseline saw their blasts reduced by 50% or more while receiving monotherapy. One of these patients had CML, and the rest had AML.
Ten percent (3/29) of patients with bone marrow blasts at baseline had a reduction in blasts of 50% or more while receiving monotherapy. Two of these patients had AML, and 1 had MDS.
Of the 7 patients receiving prexigebersen with cytarabine, 2 achieved a CR, and 1 had a CR with incomplete hematological recovery.
Two of the patients had stable disease, and the remaining 2 patients progressed. One of the patients with progressive disease withdrew from the study, and the other died.
Deaths
There were a total of 8 deaths.
One death was considered treatment-related. This patient had progressive CML in blast phase and died of multiple organ failure. This was the first patient treated on the trial, who also had the only dose-limiting toxicities.
Two patients with AML and 1 with MDS died of disease progression. Three AML patients died of sepsis, pneumonia, and cardiac arrest. And a CML patient died of respiratory distress.
The experimental agent prexigebersen (formerly BP1001) was considered well-tolerated and demonstrated early evidence of activity against relapsed/refractory hematologic disorders in a phase 1/1b trial.
The drug reduced blasts in the bone marrow and peripheral blood for patients with acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and myelodysplastic syndrome (MDS).
When given in combination with low-dose cytarabine, prexigebersen produced complete responses (CRs) in patients with AML.
Researchers said that, overall, the toxic effects of prexigebersen were manageable.
There was 1 patient who had dose-limiting toxicities, 1 who discontinued treatment due to possible drug-related toxic effects, and 1 treatment-related death.
Still, the maximum tolerated dose of prexigebersen was not established.
These results were published in The Lancet Haematology. The study was sponsored by Bio-Path Holdings, Inc., the company developing prexigebersen.
Prexigebersen is an anti-sense oligodeoxynucleotide developed to block Grb2 expression and function. Researchers tested the drug in a single-center, dose-escalation, phase 1/1b trial that enrolled and treated 39 patients.
In the phase 1 portion of the trial, patients received prexigebersen monotherapy. In the phase 1b portion, they received the drug in combination with low-dose cytarabine.
There were 32 patients in the phase 1 portion of the trial. Most (n=23) had AML, 5 had CML in blast phase, and 4 had MDS. The patients’ median age was 63 (range, 56-73), and they had received a median of 4 prior therapies.
All 7 patients in the phase 1b portion had AML. They had a median age of 72 (range, 70-76) and had all received 1 prior therapy.
For phase 1, prexigebersen was administered intravenously, twice weekly for 28 days at doses of 5 mg/m² in cohort 1 (n=13), 10 mg/m² in cohort 2 (n=6), 20 mg/m² in cohort 3 (n=3), 40 mg/m² in cohort 4 (n=3), 60 mg/m² in cohort 5 (n=3), and 90 mg/m² in cohort 6 (n=4).
In the phase 1b portion, patients received prexigebersen at 60 mg/m² (n=4) or 90 mg/m² (n=3) in combination with 20 mg of cytarabine (twice-daily subcutaneous injections).
Safety
Twenty-seven patients were evaluable for dose-limiting toxicity—21 from phase 1 and 6 from 1b.
One patient in cohort 1 developed mucositis and hand-foot syndrome, which were considered possibly related to prexigebersen and deemed dose-limiting toxicities. The patient was also receiving hydroxyurea (3 g/day) for CML and had a history of hydroxyurea-induced mucositis.
There were no other dose-limiting toxicities, and the researchers did not identify a maximum tolerated dose of prexigebersen.
The most common grade 3-4 adverse events (AEs) were cardiopulmonary disorders and fevers (including neutropenic fevers and infections).
In the monotherapy group, 17% of patients had grade 3-4 cardiopulmonary AEs, and 11% had fevers. In the prexigebersen-cytarabine combination group, 8% had grade 3-4 cardiopulmonary AEs, and 6% had fevers.
There were 5 grade 5 AEs in 4 patients, all of whom received monotherapy. These included cardiopulmonary disorders (n=2), fevers (n=2), and multi-organ failure (n=1). One patient had both fever (sepsis) and multi-organ failure.
Efficacy
According to the researchers’ assessments, 22% of phase 1 patients (7/32) benefited from prexigebersen monotherapy and therefore received more than 1 cycle of treatment. Five of these patients had AML, and 2 had MDS.
Single-agent activity was observed in other patients as well.
Thirty-three percent (9/27) of patients who had peripheral blood blasts at baseline saw their blasts reduced by 50% or more while receiving monotherapy. One of these patients had CML, and the rest had AML.
Ten percent (3/29) of patients with bone marrow blasts at baseline had a reduction in blasts of 50% or more while receiving monotherapy. Two of these patients had AML, and 1 had MDS.
Of the 7 patients receiving prexigebersen with cytarabine, 2 achieved a CR, and 1 had a CR with incomplete hematological recovery.
Two of the patients had stable disease, and the remaining 2 patients progressed. One of the patients with progressive disease withdrew from the study, and the other died.
Deaths
There were a total of 8 deaths.
One death was considered treatment-related. This patient had progressive CML in blast phase and died of multiple organ failure. This was the first patient treated on the trial, who also had the only dose-limiting toxicities.
Two patients with AML and 1 with MDS died of disease progression. Three AML patients died of sepsis, pneumonia, and cardiac arrest. And a CML patient died of respiratory distress.
The experimental agent prexigebersen (formerly BP1001) was considered well-tolerated and demonstrated early evidence of activity against relapsed/refractory hematologic disorders in a phase 1/1b trial.
The drug reduced blasts in the bone marrow and peripheral blood for patients with acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and myelodysplastic syndrome (MDS).
When given in combination with low-dose cytarabine, prexigebersen produced complete responses (CRs) in patients with AML.
Researchers said that, overall, the toxic effects of prexigebersen were manageable.
There was 1 patient who had dose-limiting toxicities, 1 who discontinued treatment due to possible drug-related toxic effects, and 1 treatment-related death.
Still, the maximum tolerated dose of prexigebersen was not established.
These results were published in The Lancet Haematology. The study was sponsored by Bio-Path Holdings, Inc., the company developing prexigebersen.
Prexigebersen is an anti-sense oligodeoxynucleotide developed to block Grb2 expression and function. Researchers tested the drug in a single-center, dose-escalation, phase 1/1b trial that enrolled and treated 39 patients.
In the phase 1 portion of the trial, patients received prexigebersen monotherapy. In the phase 1b portion, they received the drug in combination with low-dose cytarabine.
There were 32 patients in the phase 1 portion of the trial. Most (n=23) had AML, 5 had CML in blast phase, and 4 had MDS. The patients’ median age was 63 (range, 56-73), and they had received a median of 4 prior therapies.
All 7 patients in the phase 1b portion had AML. They had a median age of 72 (range, 70-76) and had all received 1 prior therapy.
For phase 1, prexigebersen was administered intravenously, twice weekly for 28 days at doses of 5 mg/m² in cohort 1 (n=13), 10 mg/m² in cohort 2 (n=6), 20 mg/m² in cohort 3 (n=3), 40 mg/m² in cohort 4 (n=3), 60 mg/m² in cohort 5 (n=3), and 90 mg/m² in cohort 6 (n=4).
In the phase 1b portion, patients received prexigebersen at 60 mg/m² (n=4) or 90 mg/m² (n=3) in combination with 20 mg of cytarabine (twice-daily subcutaneous injections).
Safety
Twenty-seven patients were evaluable for dose-limiting toxicity—21 from phase 1 and 6 from 1b.
One patient in cohort 1 developed mucositis and hand-foot syndrome, which were considered possibly related to prexigebersen and deemed dose-limiting toxicities. The patient was also receiving hydroxyurea (3 g/day) for CML and had a history of hydroxyurea-induced mucositis.
There were no other dose-limiting toxicities, and the researchers did not identify a maximum tolerated dose of prexigebersen.
The most common grade 3-4 adverse events (AEs) were cardiopulmonary disorders and fevers (including neutropenic fevers and infections).
In the monotherapy group, 17% of patients had grade 3-4 cardiopulmonary AEs, and 11% had fevers. In the prexigebersen-cytarabine combination group, 8% had grade 3-4 cardiopulmonary AEs, and 6% had fevers.
There were 5 grade 5 AEs in 4 patients, all of whom received monotherapy. These included cardiopulmonary disorders (n=2), fevers (n=2), and multi-organ failure (n=1). One patient had both fever (sepsis) and multi-organ failure.
Efficacy
According to the researchers’ assessments, 22% of phase 1 patients (7/32) benefited from prexigebersen monotherapy and therefore received more than 1 cycle of treatment. Five of these patients had AML, and 2 had MDS.
Single-agent activity was observed in other patients as well.
Thirty-three percent (9/27) of patients who had peripheral blood blasts at baseline saw their blasts reduced by 50% or more while receiving monotherapy. One of these patients had CML, and the rest had AML.
Ten percent (3/29) of patients with bone marrow blasts at baseline had a reduction in blasts of 50% or more while receiving monotherapy. Two of these patients had AML, and 1 had MDS.
Of the 7 patients receiving prexigebersen with cytarabine, 2 achieved a CR, and 1 had a CR with incomplete hematological recovery.
Two of the patients had stable disease, and the remaining 2 patients progressed. One of the patients with progressive disease withdrew from the study, and the other died.
Deaths
There were a total of 8 deaths.
One death was considered treatment-related. This patient had progressive CML in blast phase and died of multiple organ failure. This was the first patient treated on the trial, who also had the only dose-limiting toxicities.
Two patients with AML and 1 with MDS died of disease progression. Three AML patients died of sepsis, pneumonia, and cardiac arrest. And a CML patient died of respiratory distress.
EC expands indication for denosumab to MM
The European Commission (EC) has approved an expanded indication for denosumab (Xgeva).
The drug is now approved for the prevention of skeletal-related events (SREs) in adults with advanced malignancies involving bone, which includes patients with multiple myeloma (MM).
Approval from the EC provides a centralized marketing authorization with unified labeling in all member countries of the European Union.
Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
Denosumab was previously approved by the EC to prevent SREs—defined as radiation to bone, pathologic fracture, surgery to bone, and spinal cord compression—in adults with bone metastases from solid tumors.
Denosumab also received EC approval for the treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity.
The EC’s approval for denosumab in MM patients is based on data from the ’482 study, which were recently published in The Lancet Oncology.
In this phase 3 trial, denosumab proved non-inferior to zoledronic acid for delaying SREs in patients with newly diagnosed MM and bone disease.
Researchers randomized 1718 patients to receive subcutaneous denosumab at 120 mg and intravenous placebo every 4 weeks (n=859) or intravenous zoledronic acid at 4 mg (adjusted for renal function at baseline) and subcutaneous placebo every 4 weeks (n=859).
All patients also received investigators’ choice of first-line MM therapy.
The median time to first on-study SRE was 22.8 months for patients in the denosumab arm and 24 months for those in the zoledronic acid arm (hazard ratio=0.98; 95% confidence interval: 0.85-1.14; P for non-inferiority=0.010).
There were fewer renal treatment-emergent adverse events in the denosumab arm than the zoledronic acid arm—10% and 17%, respectively.
But there were more hypocalcemia adverse events in the denosumab arm than the zoledronic acid arm—17% and 12%, respectively.
The European Commission (EC) has approved an expanded indication for denosumab (Xgeva).
The drug is now approved for the prevention of skeletal-related events (SREs) in adults with advanced malignancies involving bone, which includes patients with multiple myeloma (MM).
Approval from the EC provides a centralized marketing authorization with unified labeling in all member countries of the European Union.
Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
Denosumab was previously approved by the EC to prevent SREs—defined as radiation to bone, pathologic fracture, surgery to bone, and spinal cord compression—in adults with bone metastases from solid tumors.
Denosumab also received EC approval for the treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity.
The EC’s approval for denosumab in MM patients is based on data from the ’482 study, which were recently published in The Lancet Oncology.
In this phase 3 trial, denosumab proved non-inferior to zoledronic acid for delaying SREs in patients with newly diagnosed MM and bone disease.
Researchers randomized 1718 patients to receive subcutaneous denosumab at 120 mg and intravenous placebo every 4 weeks (n=859) or intravenous zoledronic acid at 4 mg (adjusted for renal function at baseline) and subcutaneous placebo every 4 weeks (n=859).
All patients also received investigators’ choice of first-line MM therapy.
The median time to first on-study SRE was 22.8 months for patients in the denosumab arm and 24 months for those in the zoledronic acid arm (hazard ratio=0.98; 95% confidence interval: 0.85-1.14; P for non-inferiority=0.010).
There were fewer renal treatment-emergent adverse events in the denosumab arm than the zoledronic acid arm—10% and 17%, respectively.
But there were more hypocalcemia adverse events in the denosumab arm than the zoledronic acid arm—17% and 12%, respectively.
The European Commission (EC) has approved an expanded indication for denosumab (Xgeva).
The drug is now approved for the prevention of skeletal-related events (SREs) in adults with advanced malignancies involving bone, which includes patients with multiple myeloma (MM).
Approval from the EC provides a centralized marketing authorization with unified labeling in all member countries of the European Union.
Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
Denosumab was previously approved by the EC to prevent SREs—defined as radiation to bone, pathologic fracture, surgery to bone, and spinal cord compression—in adults with bone metastases from solid tumors.
Denosumab also received EC approval for the treatment of adults and skeletally mature adolescents with giant cell tumor of bone that is unresectable or where surgical resection is likely to result in severe morbidity.
The EC’s approval for denosumab in MM patients is based on data from the ’482 study, which were recently published in The Lancet Oncology.
In this phase 3 trial, denosumab proved non-inferior to zoledronic acid for delaying SREs in patients with newly diagnosed MM and bone disease.
Researchers randomized 1718 patients to receive subcutaneous denosumab at 120 mg and intravenous placebo every 4 weeks (n=859) or intravenous zoledronic acid at 4 mg (adjusted for renal function at baseline) and subcutaneous placebo every 4 weeks (n=859).
All patients also received investigators’ choice of first-line MM therapy.
The median time to first on-study SRE was 22.8 months for patients in the denosumab arm and 24 months for those in the zoledronic acid arm (hazard ratio=0.98; 95% confidence interval: 0.85-1.14; P for non-inferiority=0.010).
There were fewer renal treatment-emergent adverse events in the denosumab arm than the zoledronic acid arm—10% and 17%, respectively.
But there were more hypocalcemia adverse events in the denosumab arm than the zoledronic acid arm—17% and 12%, respectively.
Drug nets orphan designation for SCD
The European Commission (EC) has granted orphan designation to Altemia (formerly SC411) for the treatment of pediatric patients with sickle cell disease (SCD).
Altemia gelatin capsules are designed to replenish the lipids destroyed by sickle hemoglobin.
Altemia is intended to be taken once daily to reduce vaso-occlusive crises, anemia, organ damage, and other complications of SCD.
Altemia consists of a mixture of fatty acids, primarily in the form of Ethyl Cervonate (a proprietary blend of docosahexaenoic acid and other omega-3 fatty acids), and surface active agents formulated using Advanced Lipid Technologies.
Advanced Lipid Technologies are proprietary formulation and manufacturing techniques used by Sancilio Pharmaceuticals Company, Inc. (SPCI) to create lipophilic drug products.
Last November, SPCI reported topline data from its phase 2 study of Altemia, the SCOT trial (NCT02973360). The trial included pediatric patients, ages 5 to 17, with SCD.
The study’s primary endpoint was the change from baseline in blood cell membranes’ fatty acids concentration. SPCI said Altemia showed a significant improvement in this endpoint, compared to placebo, within 4 weeks of treatment initiation.
Patients who received Altemia also had significant improvements in markers of coagulation (D-dimer), inflammation (C-reactive protein), and adhesion (E-selectin) after 8 weeks of treatment.
And patients treated with Altemia had a “clinically meaningful” reduction in vaso-occlusive events, according to SPCI.
There were no treatment-related serious adverse events reported.
Ninety-four percent of patients completed the study, and most have chosen to participate in the open-label extension phase, in which researchers will continue monitoring the safety and effectiveness of Altemia.
SPCI said additional analyses of SCOT data are ongoing, and the company plans to present detailed data from the study in journals and at upcoming scientific conferences.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure.
The European Commission (EC) has granted orphan designation to Altemia (formerly SC411) for the treatment of pediatric patients with sickle cell disease (SCD).
Altemia gelatin capsules are designed to replenish the lipids destroyed by sickle hemoglobin.
Altemia is intended to be taken once daily to reduce vaso-occlusive crises, anemia, organ damage, and other complications of SCD.
Altemia consists of a mixture of fatty acids, primarily in the form of Ethyl Cervonate (a proprietary blend of docosahexaenoic acid and other omega-3 fatty acids), and surface active agents formulated using Advanced Lipid Technologies.
Advanced Lipid Technologies are proprietary formulation and manufacturing techniques used by Sancilio Pharmaceuticals Company, Inc. (SPCI) to create lipophilic drug products.
Last November, SPCI reported topline data from its phase 2 study of Altemia, the SCOT trial (NCT02973360). The trial included pediatric patients, ages 5 to 17, with SCD.
The study’s primary endpoint was the change from baseline in blood cell membranes’ fatty acids concentration. SPCI said Altemia showed a significant improvement in this endpoint, compared to placebo, within 4 weeks of treatment initiation.
Patients who received Altemia also had significant improvements in markers of coagulation (D-dimer), inflammation (C-reactive protein), and adhesion (E-selectin) after 8 weeks of treatment.
And patients treated with Altemia had a “clinically meaningful” reduction in vaso-occlusive events, according to SPCI.
There were no treatment-related serious adverse events reported.
Ninety-four percent of patients completed the study, and most have chosen to participate in the open-label extension phase, in which researchers will continue monitoring the safety and effectiveness of Altemia.
SPCI said additional analyses of SCOT data are ongoing, and the company plans to present detailed data from the study in journals and at upcoming scientific conferences.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure.
The European Commission (EC) has granted orphan designation to Altemia (formerly SC411) for the treatment of pediatric patients with sickle cell disease (SCD).
Altemia gelatin capsules are designed to replenish the lipids destroyed by sickle hemoglobin.
Altemia is intended to be taken once daily to reduce vaso-occlusive crises, anemia, organ damage, and other complications of SCD.
Altemia consists of a mixture of fatty acids, primarily in the form of Ethyl Cervonate (a proprietary blend of docosahexaenoic acid and other omega-3 fatty acids), and surface active agents formulated using Advanced Lipid Technologies.
Advanced Lipid Technologies are proprietary formulation and manufacturing techniques used by Sancilio Pharmaceuticals Company, Inc. (SPCI) to create lipophilic drug products.
Last November, SPCI reported topline data from its phase 2 study of Altemia, the SCOT trial (NCT02973360). The trial included pediatric patients, ages 5 to 17, with SCD.
The study’s primary endpoint was the change from baseline in blood cell membranes’ fatty acids concentration. SPCI said Altemia showed a significant improvement in this endpoint, compared to placebo, within 4 weeks of treatment initiation.
Patients who received Altemia also had significant improvements in markers of coagulation (D-dimer), inflammation (C-reactive protein), and adhesion (E-selectin) after 8 weeks of treatment.
And patients treated with Altemia had a “clinically meaningful” reduction in vaso-occlusive events, according to SPCI.
There were no treatment-related serious adverse events reported.
Ninety-four percent of patients completed the study, and most have chosen to participate in the open-label extension phase, in which researchers will continue monitoring the safety and effectiveness of Altemia.
SPCI said additional analyses of SCOT data are ongoing, and the company plans to present detailed data from the study in journals and at upcoming scientific conferences.
About orphan designation
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval.
The designation also provides incentives for companies seeking protocol assistance from the European Medicines Agency during the product development phase and direct access to the centralized authorization procedure.
Test for HIT receives CE mark
HIT Confirm, a test used to diagnose heparin-induced thrombocytopenia (HIT), has received the CE mark.
This means the test meets regulatory requirements and health, safety, and environmental protection standards for products sold within the European Economic Area.
HIT Confirm is a flow cytometry-based test developed by Emosis. The test can be performed on any flow cytometer and provides results within 30 minutes.
HIT Confirm requires a low-volume sample (microliters) of platelet-rich plasma to confirm the presence of HIT antibodies that lead to the formation of a heparin-antibody-PF4 complex that will bind to platelets and activate them.
HIT Confirm requires a single incubation with 2 fluorophores against a marker of platelets (CD41) and a marker of activated platelets (CD62).
After 30 minutes of incubation with 2 dose levels of heparin (0.3 U/mL and 100 U/mL), the proportion of activated platelets is obtained via flow cytometry.
Results are interpreted using a platelet activation index called HEPLA, which was developed by Emosis.
Research presented at the 2017 ISTH Congress (abstract OC 34.3*) indicated that HIT Confirm produces results comparable to those provided by the serotonin release assay (SRA).
Researchers tested the sensitivity and specificity of SRA and HIT Confirm when analyzing plasma from 290 patients—131 of whom were deemed HIT-positive by experts.
When compared to expert opinion, HIT Confirm provided 90% sensitivity and 94% specificity. SRA provided 80% sensitivity and 94% specificity compared to expert opinion.
For more information on HIT Confirm, visit the Emosis website.
*Data in the abstract differ from data provided by Emosis. The data used here were provided by Emosis.
HIT Confirm, a test used to diagnose heparin-induced thrombocytopenia (HIT), has received the CE mark.
This means the test meets regulatory requirements and health, safety, and environmental protection standards for products sold within the European Economic Area.
HIT Confirm is a flow cytometry-based test developed by Emosis. The test can be performed on any flow cytometer and provides results within 30 minutes.
HIT Confirm requires a low-volume sample (microliters) of platelet-rich plasma to confirm the presence of HIT antibodies that lead to the formation of a heparin-antibody-PF4 complex that will bind to platelets and activate them.
HIT Confirm requires a single incubation with 2 fluorophores against a marker of platelets (CD41) and a marker of activated platelets (CD62).
After 30 minutes of incubation with 2 dose levels of heparin (0.3 U/mL and 100 U/mL), the proportion of activated platelets is obtained via flow cytometry.
Results are interpreted using a platelet activation index called HEPLA, which was developed by Emosis.
Research presented at the 2017 ISTH Congress (abstract OC 34.3*) indicated that HIT Confirm produces results comparable to those provided by the serotonin release assay (SRA).
Researchers tested the sensitivity and specificity of SRA and HIT Confirm when analyzing plasma from 290 patients—131 of whom were deemed HIT-positive by experts.
When compared to expert opinion, HIT Confirm provided 90% sensitivity and 94% specificity. SRA provided 80% sensitivity and 94% specificity compared to expert opinion.
For more information on HIT Confirm, visit the Emosis website.
*Data in the abstract differ from data provided by Emosis. The data used here were provided by Emosis.
HIT Confirm, a test used to diagnose heparin-induced thrombocytopenia (HIT), has received the CE mark.
This means the test meets regulatory requirements and health, safety, and environmental protection standards for products sold within the European Economic Area.
HIT Confirm is a flow cytometry-based test developed by Emosis. The test can be performed on any flow cytometer and provides results within 30 minutes.
HIT Confirm requires a low-volume sample (microliters) of platelet-rich plasma to confirm the presence of HIT antibodies that lead to the formation of a heparin-antibody-PF4 complex that will bind to platelets and activate them.
HIT Confirm requires a single incubation with 2 fluorophores against a marker of platelets (CD41) and a marker of activated platelets (CD62).
After 30 minutes of incubation with 2 dose levels of heparin (0.3 U/mL and 100 U/mL), the proportion of activated platelets is obtained via flow cytometry.
Results are interpreted using a platelet activation index called HEPLA, which was developed by Emosis.
Research presented at the 2017 ISTH Congress (abstract OC 34.3*) indicated that HIT Confirm produces results comparable to those provided by the serotonin release assay (SRA).
Researchers tested the sensitivity and specificity of SRA and HIT Confirm when analyzing plasma from 290 patients—131 of whom were deemed HIT-positive by experts.
When compared to expert opinion, HIT Confirm provided 90% sensitivity and 94% specificity. SRA provided 80% sensitivity and 94% specificity compared to expert opinion.
For more information on HIT Confirm, visit the Emosis website.
*Data in the abstract differ from data provided by Emosis. The data used here were provided by Emosis.
Team uses CRISPR to turn on fetal hemoglobin
Researchers have used CRISPR-Cas9 gene editing to reproduce naturally occurring mutations that boost the production of fetal hemoglobin.
The mutations are associated with hereditary persistence of fetal hemoglobin (HPFH), and the researchers believe that introducing these mutations into erythroid cells could be a safe way to treat β-hemoglobinopathies such as sickle cell disease (SCD) and β-thalassemia.
This research was published in Nature Genetics.
“Our new approach can be seen as a forerunner to ‘organic gene therapy’ for a range of common inherited blood disorders, including β-thalassemia and sickle cell anemia,” said study author Merlin Crossley, DPhil, of the University of New South Wales in Sydney, Australia.
“It is organic because no new DNA is introduced into the cells; rather, we engineer in naturally occurring, benign mutations that are known to be beneficial to people with these conditions. It should prove to be a safe and effective therapy, although more research would be needed to scale the processes up into effective treatments.”
Dr Crossley and his colleagues noted that reactivating fetal hemoglobin production has long been a therapeutic goal for SCD and β-thalassemia.
“The fetal hemoglobin gene is naturally silenced after birth,” Dr Crossley explained. “For 50 years, researchers have been competing furiously to find out how it is switched off, so it can be turned back on. Our study, which is the culmination of many years of work, solves that mystery.”
“We have found that 2 genes, called BCL11A and ZBTB7A, switch off the fetal hemoglobin gene by binding directly to it. And the beneficial mutations work by disrupting the 2 sites where these 2 genes bind.”
The “beneficial mutations,” which cause some forms of HPFH, are point mutations in the γ-globin gene promoter at -115 and -200 bp upstream of the transcription start site.
Dr Crossley and his colleagues found that BCL11A31 and ZBTB7A23—2 well-established fetal globin repressors—bind these regions of the γ-globin gene proximal promoter, and mutations at –115 and –200 bp disrupt the binding.
The researchers used CRISPR-Cas9 to introduce the HPFH-associated mutations into erythroid cells and observed both disruption of repressor binding and increased γ-globin gene expression.
“This landmark finding not only contributes to our appreciation of how these globin genes are regulated,” Dr Crossley said. “It means we can now shift our focus to developing therapies for these genetic diseases using CRISPR to target precise changes in the genome.”
Researchers have used CRISPR-Cas9 gene editing to reproduce naturally occurring mutations that boost the production of fetal hemoglobin.
The mutations are associated with hereditary persistence of fetal hemoglobin (HPFH), and the researchers believe that introducing these mutations into erythroid cells could be a safe way to treat β-hemoglobinopathies such as sickle cell disease (SCD) and β-thalassemia.
This research was published in Nature Genetics.
“Our new approach can be seen as a forerunner to ‘organic gene therapy’ for a range of common inherited blood disorders, including β-thalassemia and sickle cell anemia,” said study author Merlin Crossley, DPhil, of the University of New South Wales in Sydney, Australia.
“It is organic because no new DNA is introduced into the cells; rather, we engineer in naturally occurring, benign mutations that are known to be beneficial to people with these conditions. It should prove to be a safe and effective therapy, although more research would be needed to scale the processes up into effective treatments.”
Dr Crossley and his colleagues noted that reactivating fetal hemoglobin production has long been a therapeutic goal for SCD and β-thalassemia.
“The fetal hemoglobin gene is naturally silenced after birth,” Dr Crossley explained. “For 50 years, researchers have been competing furiously to find out how it is switched off, so it can be turned back on. Our study, which is the culmination of many years of work, solves that mystery.”
“We have found that 2 genes, called BCL11A and ZBTB7A, switch off the fetal hemoglobin gene by binding directly to it. And the beneficial mutations work by disrupting the 2 sites where these 2 genes bind.”
The “beneficial mutations,” which cause some forms of HPFH, are point mutations in the γ-globin gene promoter at -115 and -200 bp upstream of the transcription start site.
Dr Crossley and his colleagues found that BCL11A31 and ZBTB7A23—2 well-established fetal globin repressors—bind these regions of the γ-globin gene proximal promoter, and mutations at –115 and –200 bp disrupt the binding.
The researchers used CRISPR-Cas9 to introduce the HPFH-associated mutations into erythroid cells and observed both disruption of repressor binding and increased γ-globin gene expression.
“This landmark finding not only contributes to our appreciation of how these globin genes are regulated,” Dr Crossley said. “It means we can now shift our focus to developing therapies for these genetic diseases using CRISPR to target precise changes in the genome.”
Researchers have used CRISPR-Cas9 gene editing to reproduce naturally occurring mutations that boost the production of fetal hemoglobin.
The mutations are associated with hereditary persistence of fetal hemoglobin (HPFH), and the researchers believe that introducing these mutations into erythroid cells could be a safe way to treat β-hemoglobinopathies such as sickle cell disease (SCD) and β-thalassemia.
This research was published in Nature Genetics.
“Our new approach can be seen as a forerunner to ‘organic gene therapy’ for a range of common inherited blood disorders, including β-thalassemia and sickle cell anemia,” said study author Merlin Crossley, DPhil, of the University of New South Wales in Sydney, Australia.
“It is organic because no new DNA is introduced into the cells; rather, we engineer in naturally occurring, benign mutations that are known to be beneficial to people with these conditions. It should prove to be a safe and effective therapy, although more research would be needed to scale the processes up into effective treatments.”
Dr Crossley and his colleagues noted that reactivating fetal hemoglobin production has long been a therapeutic goal for SCD and β-thalassemia.
“The fetal hemoglobin gene is naturally silenced after birth,” Dr Crossley explained. “For 50 years, researchers have been competing furiously to find out how it is switched off, so it can be turned back on. Our study, which is the culmination of many years of work, solves that mystery.”
“We have found that 2 genes, called BCL11A and ZBTB7A, switch off the fetal hemoglobin gene by binding directly to it. And the beneficial mutations work by disrupting the 2 sites where these 2 genes bind.”
The “beneficial mutations,” which cause some forms of HPFH, are point mutations in the γ-globin gene promoter at -115 and -200 bp upstream of the transcription start site.
Dr Crossley and his colleagues found that BCL11A31 and ZBTB7A23—2 well-established fetal globin repressors—bind these regions of the γ-globin gene proximal promoter, and mutations at –115 and –200 bp disrupt the binding.
The researchers used CRISPR-Cas9 to introduce the HPFH-associated mutations into erythroid cells and observed both disruption of repressor binding and increased γ-globin gene expression.
“This landmark finding not only contributes to our appreciation of how these globin genes are regulated,” Dr Crossley said. “It means we can now shift our focus to developing therapies for these genetic diseases using CRISPR to target precise changes in the genome.”
FDA approves blinatumomab to treat MRD+ BCP-ALL
The US Food and Drug Administration (FDA) has expanded the approved indication for blinatumomab (Blincyto®).
The drug is now approved to treat adults and children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission (CR) with minimal residual disease (MRD) greater than or equal to 0.1%.
Blinatumomab received accelerated approval for this indication because the drug has not yet shown a clinical benefit in these patients.
The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition.
Accelerated approval is based on surrogate or intermediate endpoints—in this case, MRD response rate and hematologic relapse-free survival (RFS)—that are reasonably likely to predict clinical benefit.
Continued approval of blinatumomab for the aforementioned indication may be contingent upon verification of clinical benefit in confirmatory trials.
“This is the first FDA-approved treatment for patients with MRD-positive ALL,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence.
“Because patients who have MRD are more likely to relapse, having a treatment option that eliminates even very low amounts of residual leukemia cells may help keep the cancer in remission longer. We look forward to furthering our understanding about the reduction in MRD after treatment with Blincyto. Studies are being conducted to assess how Blincyto affects long-term survival outcomes in patients with MRD.”
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
In 2014, the FDA granted blinatumomab accelerated approval to treat adults with Philadelphia chromosome-negative (Ph-) relapsed/refractory BCP-ALL.
In 2016, the FDA granted the therapy accelerated approval for pediatric patients with Ph- relapsed/refractory BCP-ALL.
Last year, the FDA granted blinatumomab full approval for pediatric and adult patients with Ph- or Ph+ relapsed/refractory BCP-ALL.
The FDA-approved prescribing information for blinatumomab includes a boxed warning for cytokine release syndrome and neurologic toxicities. Blinatumomab is also under a risk evaluation and mitigation strategy program in the US.
BLAST study
The new accelerated approval for blinatumomab was supported by results from the phase 2 BLAST study, which were published in Blood in January.
The study enrolled adults with MRD-positive BCP-ALL in complete hematologic remission after 3 or more cycles of intensive chemotherapy.
Patients received continuous intravenous infusions of blinatumomab at 15 μg/m2/day for 4 weeks, followed by 2 weeks off. They received up to 4 cycles of treatment and could undergo hematopoietic stem cell transplant (HSCT) at any time after the first cycle.
In all, there were 116 patients who received at least 1 infusion of blinatumomab. Seventy-six patients went on to HSCT while in continuous CR after cycle 1 (n=27), 2 (n=36), or 3/4 (n=13).
The study’s primary endpoint was the rate of complete MRD response within the first treatment cycle, and 78% of evaluable patients (88/113) achieved this endpoint.
A key secondary endpoint was RFS at 18 months. There were 110 patients evaluable for this endpoint. They all had Ph- BCP-ALL and <5% blasts at baseline.
The estimated RFS at 18 months was 54%, and the median RFS was 18.9 months. The median RFS was 24.6 months for patients treated in first CR and 11.0 months for patients treated in a later CR (P=0.004).
Another key endpoint was overall survival (OS). The median OS was 36.5 months, both for the 110 patients in the RFS analysis and for the entire study population.
In a landmark analysis, complete MRD responders had longer OS than MRD nonresponders—38.9 months and 12.5 months, respectively (P=0.002). And complete MRD responders had longer RFS than nonresponders—23.6 months and 5.7 months, respectively (P=0.002).
All 116 patients who started cycle 1 had at least 1 adverse event (AE). The rate of grade 3 AEs was 33%, and the rate of grade 4 AEs was 27%. These AEs were considered treatment-related in 29% (grade 3) and 22% (grade 4) of patients.
Four (3%) patients developed cytokine release syndrome—2 with grade 1 and 2 with grade 3. All of these events occurred during cycle 1.
Fifty-three percent of patients (n=61) had neurologic events. In most cases (97%, n=59), these events resolved.
There were 2 fatal AEs during the treatment period, both in cycle 1. One of these events—atypical pneumonitis with H1N1 influenza—was considered treatment-related. The other event—subdural hemorrhage—was considered unrelated to treatment.
There were 4 fatal AEs reported after blinatumomab treatment. Two of these deaths—due to multifocal CNS lesions and graft-versus-host disease—occurred in HSCT recipients. The other 2 deaths—due to disease progression and multi-organ failure—occurred in nontransplanted patients after relapse.
The US Food and Drug Administration (FDA) has expanded the approved indication for blinatumomab (Blincyto®).
The drug is now approved to treat adults and children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission (CR) with minimal residual disease (MRD) greater than or equal to 0.1%.
Blinatumomab received accelerated approval for this indication because the drug has not yet shown a clinical benefit in these patients.
The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition.
Accelerated approval is based on surrogate or intermediate endpoints—in this case, MRD response rate and hematologic relapse-free survival (RFS)—that are reasonably likely to predict clinical benefit.
Continued approval of blinatumomab for the aforementioned indication may be contingent upon verification of clinical benefit in confirmatory trials.
“This is the first FDA-approved treatment for patients with MRD-positive ALL,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence.
“Because patients who have MRD are more likely to relapse, having a treatment option that eliminates even very low amounts of residual leukemia cells may help keep the cancer in remission longer. We look forward to furthering our understanding about the reduction in MRD after treatment with Blincyto. Studies are being conducted to assess how Blincyto affects long-term survival outcomes in patients with MRD.”
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
In 2014, the FDA granted blinatumomab accelerated approval to treat adults with Philadelphia chromosome-negative (Ph-) relapsed/refractory BCP-ALL.
In 2016, the FDA granted the therapy accelerated approval for pediatric patients with Ph- relapsed/refractory BCP-ALL.
Last year, the FDA granted blinatumomab full approval for pediatric and adult patients with Ph- or Ph+ relapsed/refractory BCP-ALL.
The FDA-approved prescribing information for blinatumomab includes a boxed warning for cytokine release syndrome and neurologic toxicities. Blinatumomab is also under a risk evaluation and mitigation strategy program in the US.
BLAST study
The new accelerated approval for blinatumomab was supported by results from the phase 2 BLAST study, which were published in Blood in January.
The study enrolled adults with MRD-positive BCP-ALL in complete hematologic remission after 3 or more cycles of intensive chemotherapy.
Patients received continuous intravenous infusions of blinatumomab at 15 μg/m2/day for 4 weeks, followed by 2 weeks off. They received up to 4 cycles of treatment and could undergo hematopoietic stem cell transplant (HSCT) at any time after the first cycle.
In all, there were 116 patients who received at least 1 infusion of blinatumomab. Seventy-six patients went on to HSCT while in continuous CR after cycle 1 (n=27), 2 (n=36), or 3/4 (n=13).
The study’s primary endpoint was the rate of complete MRD response within the first treatment cycle, and 78% of evaluable patients (88/113) achieved this endpoint.
A key secondary endpoint was RFS at 18 months. There were 110 patients evaluable for this endpoint. They all had Ph- BCP-ALL and <5% blasts at baseline.
The estimated RFS at 18 months was 54%, and the median RFS was 18.9 months. The median RFS was 24.6 months for patients treated in first CR and 11.0 months for patients treated in a later CR (P=0.004).
Another key endpoint was overall survival (OS). The median OS was 36.5 months, both for the 110 patients in the RFS analysis and for the entire study population.
In a landmark analysis, complete MRD responders had longer OS than MRD nonresponders—38.9 months and 12.5 months, respectively (P=0.002). And complete MRD responders had longer RFS than nonresponders—23.6 months and 5.7 months, respectively (P=0.002).
All 116 patients who started cycle 1 had at least 1 adverse event (AE). The rate of grade 3 AEs was 33%, and the rate of grade 4 AEs was 27%. These AEs were considered treatment-related in 29% (grade 3) and 22% (grade 4) of patients.
Four (3%) patients developed cytokine release syndrome—2 with grade 1 and 2 with grade 3. All of these events occurred during cycle 1.
Fifty-three percent of patients (n=61) had neurologic events. In most cases (97%, n=59), these events resolved.
There were 2 fatal AEs during the treatment period, both in cycle 1. One of these events—atypical pneumonitis with H1N1 influenza—was considered treatment-related. The other event—subdural hemorrhage—was considered unrelated to treatment.
There were 4 fatal AEs reported after blinatumomab treatment. Two of these deaths—due to multifocal CNS lesions and graft-versus-host disease—occurred in HSCT recipients. The other 2 deaths—due to disease progression and multi-organ failure—occurred in nontransplanted patients after relapse.
The US Food and Drug Administration (FDA) has expanded the approved indication for blinatumomab (Blincyto®).
The drug is now approved to treat adults and children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission (CR) with minimal residual disease (MRD) greater than or equal to 0.1%.
Blinatumomab received accelerated approval for this indication because the drug has not yet shown a clinical benefit in these patients.
The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition.
Accelerated approval is based on surrogate or intermediate endpoints—in this case, MRD response rate and hematologic relapse-free survival (RFS)—that are reasonably likely to predict clinical benefit.
Continued approval of blinatumomab for the aforementioned indication may be contingent upon verification of clinical benefit in confirmatory trials.
“This is the first FDA-approved treatment for patients with MRD-positive ALL,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence.
“Because patients who have MRD are more likely to relapse, having a treatment option that eliminates even very low amounts of residual leukemia cells may help keep the cancer in remission longer. We look forward to furthering our understanding about the reduction in MRD after treatment with Blincyto. Studies are being conducted to assess how Blincyto affects long-term survival outcomes in patients with MRD.”
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
In 2014, the FDA granted blinatumomab accelerated approval to treat adults with Philadelphia chromosome-negative (Ph-) relapsed/refractory BCP-ALL.
In 2016, the FDA granted the therapy accelerated approval for pediatric patients with Ph- relapsed/refractory BCP-ALL.
Last year, the FDA granted blinatumomab full approval for pediatric and adult patients with Ph- or Ph+ relapsed/refractory BCP-ALL.
The FDA-approved prescribing information for blinatumomab includes a boxed warning for cytokine release syndrome and neurologic toxicities. Blinatumomab is also under a risk evaluation and mitigation strategy program in the US.
BLAST study
The new accelerated approval for blinatumomab was supported by results from the phase 2 BLAST study, which were published in Blood in January.
The study enrolled adults with MRD-positive BCP-ALL in complete hematologic remission after 3 or more cycles of intensive chemotherapy.
Patients received continuous intravenous infusions of blinatumomab at 15 μg/m2/day for 4 weeks, followed by 2 weeks off. They received up to 4 cycles of treatment and could undergo hematopoietic stem cell transplant (HSCT) at any time after the first cycle.
In all, there were 116 patients who received at least 1 infusion of blinatumomab. Seventy-six patients went on to HSCT while in continuous CR after cycle 1 (n=27), 2 (n=36), or 3/4 (n=13).
The study’s primary endpoint was the rate of complete MRD response within the first treatment cycle, and 78% of evaluable patients (88/113) achieved this endpoint.
A key secondary endpoint was RFS at 18 months. There were 110 patients evaluable for this endpoint. They all had Ph- BCP-ALL and <5% blasts at baseline.
The estimated RFS at 18 months was 54%, and the median RFS was 18.9 months. The median RFS was 24.6 months for patients treated in first CR and 11.0 months for patients treated in a later CR (P=0.004).
Another key endpoint was overall survival (OS). The median OS was 36.5 months, both for the 110 patients in the RFS analysis and for the entire study population.
In a landmark analysis, complete MRD responders had longer OS than MRD nonresponders—38.9 months and 12.5 months, respectively (P=0.002). And complete MRD responders had longer RFS than nonresponders—23.6 months and 5.7 months, respectively (P=0.002).
All 116 patients who started cycle 1 had at least 1 adverse event (AE). The rate of grade 3 AEs was 33%, and the rate of grade 4 AEs was 27%. These AEs were considered treatment-related in 29% (grade 3) and 22% (grade 4) of patients.
Four (3%) patients developed cytokine release syndrome—2 with grade 1 and 2 with grade 3. All of these events occurred during cycle 1.
Fifty-three percent of patients (n=61) had neurologic events. In most cases (97%, n=59), these events resolved.
There were 2 fatal AEs during the treatment period, both in cycle 1. One of these events—atypical pneumonitis with H1N1 influenza—was considered treatment-related. The other event—subdural hemorrhage—was considered unrelated to treatment.
There were 4 fatal AEs reported after blinatumomab treatment. Two of these deaths—due to multifocal CNS lesions and graft-versus-host disease—occurred in HSCT recipients. The other 2 deaths—due to disease progression and multi-organ failure—occurred in nontransplanted patients after relapse.
Drug receives orphan designation for AML
The US Food and Drug Administration (FDA) has granted orphan designation to MAX-40279 for the treatment of acute myeloid leukemia (AML).
MAX-40279 is a multi-target kinase inhibitor being developed by MaxiNovel Pharmaceuticals, Inc.
The drug mainly targets FMS-related tyrosine kinase 3 (FLT3) and fibroblast growth factor receptor (FGFR).
MAX-40279 demonstrated “potent” inhibition of FLT3 and FGFR in preclinical testing, according to MaxiNovel Pharmaceuticals, Inc.
The company is currently testing MAX-40279 in a phase 1 trial of patients with AML (NCT03412292).
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan designation to MAX-40279 for the treatment of acute myeloid leukemia (AML).
MAX-40279 is a multi-target kinase inhibitor being developed by MaxiNovel Pharmaceuticals, Inc.
The drug mainly targets FMS-related tyrosine kinase 3 (FLT3) and fibroblast growth factor receptor (FGFR).
MAX-40279 demonstrated “potent” inhibition of FLT3 and FGFR in preclinical testing, according to MaxiNovel Pharmaceuticals, Inc.
The company is currently testing MAX-40279 in a phase 1 trial of patients with AML (NCT03412292).
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan designation to MAX-40279 for the treatment of acute myeloid leukemia (AML).
MAX-40279 is a multi-target kinase inhibitor being developed by MaxiNovel Pharmaceuticals, Inc.
The drug mainly targets FMS-related tyrosine kinase 3 (FLT3) and fibroblast growth factor receptor (FGFR).
MAX-40279 demonstrated “potent” inhibition of FLT3 and FGFR in preclinical testing, according to MaxiNovel Pharmaceuticals, Inc.
The company is currently testing MAX-40279 in a phase 1 trial of patients with AML (NCT03412292).
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
Disease, genetics contribute to neurocognitive decline in ALL
Chemotherapeutic agents have been associated with neurocognitive side effects in survivors of pediatric acute lymphoblastic leukemia (ALL).
Now, researchers have found evidence to suggest that genetics and ALL itself can increase the risk for long-term problems with attention, organization, and related neurocognitive skills.
The researchers reported these findings in JAMA Oncology.
The team evaluated patients enrolled in the Total XV St. Jude clinical trial, analyzing the cerebrospinal fluid (CSF) of 235 pediatric ALL patients treated with chemotherapy alone.
The CSF had been collected at 5 times before and during treatment (between 2000 and 2010). The analysis included neurocognitive testing and brain imaging of 138 ALL survivors who were at least 8 years old and 5 years from their cancer diagnosis.
The researchers found that, even before treatment began, some patients had proteins in their CSF that suggested injury to glial cells.
“This was a surprise,” said study author Kevin Krull, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Until now, we had not suspected that leukemia by itself or the inflammatory response to the disease may lead to changes that leave ALL survivors at risk for problems with executive functioning and processing speed later.”
Previously, researchers had assumed neurocognitive problems were a side effect of ALL therapy, particularly treatment with methotrexate.
So finding elevated biomarkers in the CSF of some patients during methotrexate treatment was not surprising, but, previously, little was known about the neurotoxic mechanism involved. The biomarkers identified were indicative of injury to neurons, axons, and glial cells.
The researchers checked patients’ CSF for 5 proteins and other biomarkers of brain cell damage related to treatment with either high-dose intravenous methotrexate or intrathecal methotrexate.
The biomarkers were present early on but changed and varied throughout treatment. For example, biomarkers of demyelination were present in some patients newly diagnosed with ALL and then decreased during treatment.
Others, including biomarkers of inflammation and neuronal damage, increased as treatment progressed.
Overall, methotrexate treatment was associated with biomarkers that signaled as much as a 70% increased risk for reduced neurocognitive functioning in long-term ALL survivors.
The researchers also looked for evidence that genetic variation might influence the susceptibility of pediatric ALL patients to methotrexate injury.
The team checked patients’ DNA for 42 gene variants known to influence drug metabolism, neurodevelopment, and oxidative stress.
The analysis identified a variant of the COMT gene that was associated with higher biomarker levels following methotrexate treatment. The gene encodes instructions for a protein involved in processing the neurotransmitter dopamine in the frontal regions of the brain.
“Dopamine is the primary neurotransmitter in executive functioning,” Dr Krull noted. “This suggests that 2 independent processes might be coming together in some patients that influence their risk for diminished executive functioning.”
“Taken together, the results suggest that survivors’ neurocognitive deficits are multifactorial and reflect a complex interaction among genetics, treatment intensity, and other factors. Monitoring CSF biomarkers and screening for genetic mediators of brain injury may help identify and intervene with survivors at risk for neurocognitive problems.”
Chemotherapeutic agents have been associated with neurocognitive side effects in survivors of pediatric acute lymphoblastic leukemia (ALL).
Now, researchers have found evidence to suggest that genetics and ALL itself can increase the risk for long-term problems with attention, organization, and related neurocognitive skills.
The researchers reported these findings in JAMA Oncology.
The team evaluated patients enrolled in the Total XV St. Jude clinical trial, analyzing the cerebrospinal fluid (CSF) of 235 pediatric ALL patients treated with chemotherapy alone.
The CSF had been collected at 5 times before and during treatment (between 2000 and 2010). The analysis included neurocognitive testing and brain imaging of 138 ALL survivors who were at least 8 years old and 5 years from their cancer diagnosis.
The researchers found that, even before treatment began, some patients had proteins in their CSF that suggested injury to glial cells.
“This was a surprise,” said study author Kevin Krull, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Until now, we had not suspected that leukemia by itself or the inflammatory response to the disease may lead to changes that leave ALL survivors at risk for problems with executive functioning and processing speed later.”
Previously, researchers had assumed neurocognitive problems were a side effect of ALL therapy, particularly treatment with methotrexate.
So finding elevated biomarkers in the CSF of some patients during methotrexate treatment was not surprising, but, previously, little was known about the neurotoxic mechanism involved. The biomarkers identified were indicative of injury to neurons, axons, and glial cells.
The researchers checked patients’ CSF for 5 proteins and other biomarkers of brain cell damage related to treatment with either high-dose intravenous methotrexate or intrathecal methotrexate.
The biomarkers were present early on but changed and varied throughout treatment. For example, biomarkers of demyelination were present in some patients newly diagnosed with ALL and then decreased during treatment.
Others, including biomarkers of inflammation and neuronal damage, increased as treatment progressed.
Overall, methotrexate treatment was associated with biomarkers that signaled as much as a 70% increased risk for reduced neurocognitive functioning in long-term ALL survivors.
The researchers also looked for evidence that genetic variation might influence the susceptibility of pediatric ALL patients to methotrexate injury.
The team checked patients’ DNA for 42 gene variants known to influence drug metabolism, neurodevelopment, and oxidative stress.
The analysis identified a variant of the COMT gene that was associated with higher biomarker levels following methotrexate treatment. The gene encodes instructions for a protein involved in processing the neurotransmitter dopamine in the frontal regions of the brain.
“Dopamine is the primary neurotransmitter in executive functioning,” Dr Krull noted. “This suggests that 2 independent processes might be coming together in some patients that influence their risk for diminished executive functioning.”
“Taken together, the results suggest that survivors’ neurocognitive deficits are multifactorial and reflect a complex interaction among genetics, treatment intensity, and other factors. Monitoring CSF biomarkers and screening for genetic mediators of brain injury may help identify and intervene with survivors at risk for neurocognitive problems.”
Chemotherapeutic agents have been associated with neurocognitive side effects in survivors of pediatric acute lymphoblastic leukemia (ALL).
Now, researchers have found evidence to suggest that genetics and ALL itself can increase the risk for long-term problems with attention, organization, and related neurocognitive skills.
The researchers reported these findings in JAMA Oncology.
The team evaluated patients enrolled in the Total XV St. Jude clinical trial, analyzing the cerebrospinal fluid (CSF) of 235 pediatric ALL patients treated with chemotherapy alone.
The CSF had been collected at 5 times before and during treatment (between 2000 and 2010). The analysis included neurocognitive testing and brain imaging of 138 ALL survivors who were at least 8 years old and 5 years from their cancer diagnosis.
The researchers found that, even before treatment began, some patients had proteins in their CSF that suggested injury to glial cells.
“This was a surprise,” said study author Kevin Krull, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Until now, we had not suspected that leukemia by itself or the inflammatory response to the disease may lead to changes that leave ALL survivors at risk for problems with executive functioning and processing speed later.”
Previously, researchers had assumed neurocognitive problems were a side effect of ALL therapy, particularly treatment with methotrexate.
So finding elevated biomarkers in the CSF of some patients during methotrexate treatment was not surprising, but, previously, little was known about the neurotoxic mechanism involved. The biomarkers identified were indicative of injury to neurons, axons, and glial cells.
The researchers checked patients’ CSF for 5 proteins and other biomarkers of brain cell damage related to treatment with either high-dose intravenous methotrexate or intrathecal methotrexate.
The biomarkers were present early on but changed and varied throughout treatment. For example, biomarkers of demyelination were present in some patients newly diagnosed with ALL and then decreased during treatment.
Others, including biomarkers of inflammation and neuronal damage, increased as treatment progressed.
Overall, methotrexate treatment was associated with biomarkers that signaled as much as a 70% increased risk for reduced neurocognitive functioning in long-term ALL survivors.
The researchers also looked for evidence that genetic variation might influence the susceptibility of pediatric ALL patients to methotrexate injury.
The team checked patients’ DNA for 42 gene variants known to influence drug metabolism, neurodevelopment, and oxidative stress.
The analysis identified a variant of the COMT gene that was associated with higher biomarker levels following methotrexate treatment. The gene encodes instructions for a protein involved in processing the neurotransmitter dopamine in the frontal regions of the brain.
“Dopamine is the primary neurotransmitter in executive functioning,” Dr Krull noted. “This suggests that 2 independent processes might be coming together in some patients that influence their risk for diminished executive functioning.”
“Taken together, the results suggest that survivors’ neurocognitive deficits are multifactorial and reflect a complex interaction among genetics, treatment intensity, and other factors. Monitoring CSF biomarkers and screening for genetic mediators of brain injury may help identify and intervene with survivors at risk for neurocognitive problems.”
Drug approved to treat all adults with iron deficiency
The European Commission (EC) has extended the approved indication of ferric maltol (Feraccru) to include treatment of all adults with iron deficiency, with or without anemia.
The drug was previously only approved in Europe for the treatment of iron deficiency anemia in adults with inflammatory bowel disease.
The EC’s extended approval governs the marketing of ferric maltol in all 28 European Union member countries, as well as Iceland, Norway, and Liechtenstein.
“We are extremely pleased [the EC] has so rapidly ratified the expansion of the indication for Feraccru,” said Carl Sterritt, chief executive officer of Shield Therapeutics, the company developing the product.
“This decision confirms a significantly broader patient population target opportunity for Feraccru in Europe, where 40 million* people are estimated to be iron deficient.”
Ferric maltol is a stable, non-salt, oral formulation of ferric iron, which has a different mechanism of action than salt-based oral iron therapies.
When salt-based oral iron therapies are ingested, the iron must dissociate from the salt in the gastrointestinal tract to allow the iron to be absorbed and treat the iron deficiency or anemia. This free iron readily chelates to form insoluble clumps as well as producing free radicals that, together, can cause a range of mild-to-severe gastrointestinal adverse events, including nausea, bloating, and constipation.
Conversely, iron can be absorbed from the ferric maltol molecule. As a result, the treatment is less likely to cause gastrointestinal issues. Ferric maltol has been shown in clinical trials (AEGIS 1 and 2) to be well-tolerated by patients who previously failed treatment with salt-based oral iron therapies.
Prior to the extended approval of ferric maltol, the only treatment option for patients with iron deficiency, with or without anemia, who could not tolerate salt-based oral iron therapies, was intravenous iron therapy.
Intravenous iron quickly increases iron stores via direct administration of very large doses of iron, causing an increase in hemoglobin levels that is physiologically controlled and occurs over a period of weeks, as is the case with ferric maltol. However, intravenous iron therapies can be complex to administer and may produce spontaneous hypersensitivity reactions.
*Levi, M et al. Epidemiology of iron deficiency anaemia in four European countries: a population-based study in primary care. 2016, Eur J Haematol, 97: 583–593. doi:10.1111/ejh.12776
The European Commission (EC) has extended the approved indication of ferric maltol (Feraccru) to include treatment of all adults with iron deficiency, with or without anemia.
The drug was previously only approved in Europe for the treatment of iron deficiency anemia in adults with inflammatory bowel disease.
The EC’s extended approval governs the marketing of ferric maltol in all 28 European Union member countries, as well as Iceland, Norway, and Liechtenstein.
“We are extremely pleased [the EC] has so rapidly ratified the expansion of the indication for Feraccru,” said Carl Sterritt, chief executive officer of Shield Therapeutics, the company developing the product.
“This decision confirms a significantly broader patient population target opportunity for Feraccru in Europe, where 40 million* people are estimated to be iron deficient.”
Ferric maltol is a stable, non-salt, oral formulation of ferric iron, which has a different mechanism of action than salt-based oral iron therapies.
When salt-based oral iron therapies are ingested, the iron must dissociate from the salt in the gastrointestinal tract to allow the iron to be absorbed and treat the iron deficiency or anemia. This free iron readily chelates to form insoluble clumps as well as producing free radicals that, together, can cause a range of mild-to-severe gastrointestinal adverse events, including nausea, bloating, and constipation.
Conversely, iron can be absorbed from the ferric maltol molecule. As a result, the treatment is less likely to cause gastrointestinal issues. Ferric maltol has been shown in clinical trials (AEGIS 1 and 2) to be well-tolerated by patients who previously failed treatment with salt-based oral iron therapies.
Prior to the extended approval of ferric maltol, the only treatment option for patients with iron deficiency, with or without anemia, who could not tolerate salt-based oral iron therapies, was intravenous iron therapy.
Intravenous iron quickly increases iron stores via direct administration of very large doses of iron, causing an increase in hemoglobin levels that is physiologically controlled and occurs over a period of weeks, as is the case with ferric maltol. However, intravenous iron therapies can be complex to administer and may produce spontaneous hypersensitivity reactions.
*Levi, M et al. Epidemiology of iron deficiency anaemia in four European countries: a population-based study in primary care. 2016, Eur J Haematol, 97: 583–593. doi:10.1111/ejh.12776
The European Commission (EC) has extended the approved indication of ferric maltol (Feraccru) to include treatment of all adults with iron deficiency, with or without anemia.
The drug was previously only approved in Europe for the treatment of iron deficiency anemia in adults with inflammatory bowel disease.
The EC’s extended approval governs the marketing of ferric maltol in all 28 European Union member countries, as well as Iceland, Norway, and Liechtenstein.
“We are extremely pleased [the EC] has so rapidly ratified the expansion of the indication for Feraccru,” said Carl Sterritt, chief executive officer of Shield Therapeutics, the company developing the product.
“This decision confirms a significantly broader patient population target opportunity for Feraccru in Europe, where 40 million* people are estimated to be iron deficient.”
Ferric maltol is a stable, non-salt, oral formulation of ferric iron, which has a different mechanism of action than salt-based oral iron therapies.
When salt-based oral iron therapies are ingested, the iron must dissociate from the salt in the gastrointestinal tract to allow the iron to be absorbed and treat the iron deficiency or anemia. This free iron readily chelates to form insoluble clumps as well as producing free radicals that, together, can cause a range of mild-to-severe gastrointestinal adverse events, including nausea, bloating, and constipation.
Conversely, iron can be absorbed from the ferric maltol molecule. As a result, the treatment is less likely to cause gastrointestinal issues. Ferric maltol has been shown in clinical trials (AEGIS 1 and 2) to be well-tolerated by patients who previously failed treatment with salt-based oral iron therapies.
Prior to the extended approval of ferric maltol, the only treatment option for patients with iron deficiency, with or without anemia, who could not tolerate salt-based oral iron therapies, was intravenous iron therapy.
Intravenous iron quickly increases iron stores via direct administration of very large doses of iron, causing an increase in hemoglobin levels that is physiologically controlled and occurs over a period of weeks, as is the case with ferric maltol. However, intravenous iron therapies can be complex to administer and may produce spontaneous hypersensitivity reactions.
*Levi, M et al. Epidemiology of iron deficiency anaemia in four European countries: a population-based study in primary care. 2016, Eur J Haematol, 97: 583–593. doi:10.1111/ejh.12776