User login
Tenalisib receives orphan designation for CTCL
The US Food and Drug Administration (FDA) has granted orphan drug designation to tenalisib for treatment of cutaneous T-cell lymphoma (CTCL).
Tenalisib (formerly RP6530) is a dual PI3K delta/gamma inhibitor under development by Rhizen Pharmaceuticals SA.
The FDA previously granted tenalisib fast track and orphan drug designations for the treatment of peripheral T-cell lymphoma (PTCL).
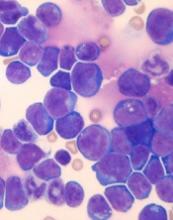
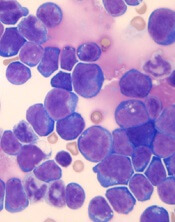
Tenalisib has been investigated in a phase 1 trial of patients with relapsed/refractory PTCL and CTCL. Results from this trial were presented at the 10th Annual T-cell Lymphoma Forum in February.
The data included 55 patients—28 with CTCL and 27 with PTCL—who received varying doses of tenalisib. The maximum tolerated dose was an 800 mg daily fasting dose.
Fourteen PTCL patients were evaluable for efficacy, and 7 responded (50%) to treatment. Three patients had a complete response, and 4 had a partial response.
Eighteen CTCL patients were evaluable for efficacy. Eight patients responded (44%), all with partial responses.
In the entire cohort, treatment-related adverse events (AEs) of grade 3 or higher included transaminitis (20%), rash (5%), neutropenia (2%), hypophosphatemia (2%), international normalized ratio increase (2%), sepsis (2%), pyrexia (2%), and diplopia secondary to neuropathy (2%).
Four CTCL patients stopped tenalisib due to a treatment-related AE—transaminitis, sepsis, diarrhea, and diplopia secondary to neuropathy. One PTCL patient stopped treatment due to a related AE, which was transaminitis.
About orphan and fast track designations
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s fast track drug development program is designed to expedite clinical development and submission of new drug applications for medicines with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss all aspects of development to support a drug’s approval, and also provides the opportunity to submit sections of a new drug application on a rolling basis as data become available.
The US Food and Drug Administration (FDA) has granted orphan drug designation to tenalisib for treatment of cutaneous T-cell lymphoma (CTCL).
Tenalisib (formerly RP6530) is a dual PI3K delta/gamma inhibitor under development by Rhizen Pharmaceuticals SA.
The FDA previously granted tenalisib fast track and orphan drug designations for the treatment of peripheral T-cell lymphoma (PTCL).
Tenalisib has been investigated in a phase 1 trial of patients with relapsed/refractory PTCL and CTCL. Results from this trial were presented at the 10th Annual T-cell Lymphoma Forum in February.
The data included 55 patients—28 with CTCL and 27 with PTCL—who received varying doses of tenalisib. The maximum tolerated dose was an 800 mg daily fasting dose.
Fourteen PTCL patients were evaluable for efficacy, and 7 responded (50%) to treatment. Three patients had a complete response, and 4 had a partial response.
Eighteen CTCL patients were evaluable for efficacy. Eight patients responded (44%), all with partial responses.
In the entire cohort, treatment-related adverse events (AEs) of grade 3 or higher included transaminitis (20%), rash (5%), neutropenia (2%), hypophosphatemia (2%), international normalized ratio increase (2%), sepsis (2%), pyrexia (2%), and diplopia secondary to neuropathy (2%).
Four CTCL patients stopped tenalisib due to a treatment-related AE—transaminitis, sepsis, diarrhea, and diplopia secondary to neuropathy. One PTCL patient stopped treatment due to a related AE, which was transaminitis.
About orphan and fast track designations
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s fast track drug development program is designed to expedite clinical development and submission of new drug applications for medicines with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss all aspects of development to support a drug’s approval, and also provides the opportunity to submit sections of a new drug application on a rolling basis as data become available.
The US Food and Drug Administration (FDA) has granted orphan drug designation to tenalisib for treatment of cutaneous T-cell lymphoma (CTCL).
Tenalisib (formerly RP6530) is a dual PI3K delta/gamma inhibitor under development by Rhizen Pharmaceuticals SA.
The FDA previously granted tenalisib fast track and orphan drug designations for the treatment of peripheral T-cell lymphoma (PTCL).
Tenalisib has been investigated in a phase 1 trial of patients with relapsed/refractory PTCL and CTCL. Results from this trial were presented at the 10th Annual T-cell Lymphoma Forum in February.
The data included 55 patients—28 with CTCL and 27 with PTCL—who received varying doses of tenalisib. The maximum tolerated dose was an 800 mg daily fasting dose.
Fourteen PTCL patients were evaluable for efficacy, and 7 responded (50%) to treatment. Three patients had a complete response, and 4 had a partial response.
Eighteen CTCL patients were evaluable for efficacy. Eight patients responded (44%), all with partial responses.
In the entire cohort, treatment-related adverse events (AEs) of grade 3 or higher included transaminitis (20%), rash (5%), neutropenia (2%), hypophosphatemia (2%), international normalized ratio increase (2%), sepsis (2%), pyrexia (2%), and diplopia secondary to neuropathy (2%).
Four CTCL patients stopped tenalisib due to a treatment-related AE—transaminitis, sepsis, diarrhea, and diplopia secondary to neuropathy. One PTCL patient stopped treatment due to a related AE, which was transaminitis.
About orphan and fast track designations
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s fast track drug development program is designed to expedite clinical development and submission of new drug applications for medicines with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss all aspects of development to support a drug’s approval, and also provides the opportunity to submit sections of a new drug application on a rolling basis as data become available.
Duvelisib NDA granted priority review
The US Food and Drug Administration (FDA) has accepted for priority review the new drug application (NDA) for duvelisib, a dual PI3K delta/gamma inhibitor.
With this NDA, Verastem, Inc., is seeking full approval of duvelisib for the treatment of relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and accelerated approval of the drug for the treatment of relapsed or refractory follicular lymphoma (FL).
The FDA expects to make a decision on the NDA by October 5, 2018.
The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The agency grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The application for duvelisib is supported by data from DUO™, a randomized, phase 3 study of patients with relapsed or refractory CLL/SLL, and DYNAMO™, a phase 2 study of patients with refractory indolent non-Hodgkin lymphoma.
Phase 3 DUO trial
Results from DUO were presented at the 2017 ASH Annual Meeting in December.
This study included 319 CLL/SLL patients who were randomized 1:1 to receive either duvelisib (25 mg orally twice daily) or ofatumumab (initial infusion of 300 mg followed by 7 weekly infusions and 4 monthly infusions of 2000 mg).
The study’s primary endpoint was met, as duvelisib conferred a significant improvement in median progression-free survival (PFS) over ofatumumab.
Per an independent review committee, the median PFS was 13.3 months with duvelisib and 9.9 months with ofatumumab (hazard ratio=0.52; P<0.0001). Duvelisib maintained a PFS advantage in all patient subgroups analyzed.
The overall response rate was 73.8% with duvelisib and 45.3% with ofatumumab (P<0.0001). The complete response rate was 0.6% in both arms.
Overall survival (OS) was similar in the duvelisib and ofatumumab arms (hazard ratio=0.99; P=0.4807). The median OS was not reached in either arm.
The most common grade 3 or higher adverse events (AEs)—in the duvelisib and ofatumumab arms, respectively—were neutropenia (30% vs 17%), anemia (13% vs 5%), diarrhea (15% vs 1%), pneumonia (14% vs 1%), and colitis (12% vs 1%).
Thirty-five percent of patients discontinued duvelisib due to an AE.
Severe opportunistic infections occurred in 6% of duvelisib recipients—bronchopulmonary aspergillosis (n=4), fungal infection (n=2), Pneumocystis jirovecii pneumonia (n=2), and cytomegalovirus colitis (n=1).
There were 4 deaths related to duvelisib—staphylococcal pneumonia (n=2), general physical health deterioration (n=1), and sepsis (n=1).
Phase 2 DYNAMO trial
Results from DYNAMO were presented at the 22nd EHA Congress (abstract S777) in June 2017.
This trial enrolled patients with indolent non-Hodgkin lymphoma whose disease was refractory to both rituximab and chemotherapy or radioimmunotherapy.
There were 83 patients with FL. They had a median of 3 prior anticancer regimens (range, 1-10).
The patients received duvelisib at 25 mg orally twice daily until disease progression or unacceptable toxicity.
The overall response rate, per an independent review committee, was 43%. One patient achieved a complete response, and 35 had a partial response. The median duration of response was 7.9 months.
The median PFS was 8.3 months, and the median OS was 27.8 months.
The most common grade 3 or higher AEs were neutropenia (22%), anemia (13%), diarrhea (16%), lipase increase (10%), and thrombocytopenia (9%).
There were 2 serious opportunistic infections—Pneumocystis pneumonia and fungal pneumonia.
There were 3 deaths attributed to duvelisib—toxic epidermal necrolysis/sepsis syndrome (n=1), drug reaction/eosinophilia/systemic symptoms (n=1), and pneumonitis/pneumonia (n=1).
The US Food and Drug Administration (FDA) has accepted for priority review the new drug application (NDA) for duvelisib, a dual PI3K delta/gamma inhibitor.
With this NDA, Verastem, Inc., is seeking full approval of duvelisib for the treatment of relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and accelerated approval of the drug for the treatment of relapsed or refractory follicular lymphoma (FL).
The FDA expects to make a decision on the NDA by October 5, 2018.
The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The agency grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The application for duvelisib is supported by data from DUO™, a randomized, phase 3 study of patients with relapsed or refractory CLL/SLL, and DYNAMO™, a phase 2 study of patients with refractory indolent non-Hodgkin lymphoma.
Phase 3 DUO trial
Results from DUO were presented at the 2017 ASH Annual Meeting in December.
This study included 319 CLL/SLL patients who were randomized 1:1 to receive either duvelisib (25 mg orally twice daily) or ofatumumab (initial infusion of 300 mg followed by 7 weekly infusions and 4 monthly infusions of 2000 mg).
The study’s primary endpoint was met, as duvelisib conferred a significant improvement in median progression-free survival (PFS) over ofatumumab.
Per an independent review committee, the median PFS was 13.3 months with duvelisib and 9.9 months with ofatumumab (hazard ratio=0.52; P<0.0001). Duvelisib maintained a PFS advantage in all patient subgroups analyzed.
The overall response rate was 73.8% with duvelisib and 45.3% with ofatumumab (P<0.0001). The complete response rate was 0.6% in both arms.
Overall survival (OS) was similar in the duvelisib and ofatumumab arms (hazard ratio=0.99; P=0.4807). The median OS was not reached in either arm.
The most common grade 3 or higher adverse events (AEs)—in the duvelisib and ofatumumab arms, respectively—were neutropenia (30% vs 17%), anemia (13% vs 5%), diarrhea (15% vs 1%), pneumonia (14% vs 1%), and colitis (12% vs 1%).
Thirty-five percent of patients discontinued duvelisib due to an AE.
Severe opportunistic infections occurred in 6% of duvelisib recipients—bronchopulmonary aspergillosis (n=4), fungal infection (n=2), Pneumocystis jirovecii pneumonia (n=2), and cytomegalovirus colitis (n=1).
There were 4 deaths related to duvelisib—staphylococcal pneumonia (n=2), general physical health deterioration (n=1), and sepsis (n=1).
Phase 2 DYNAMO trial
Results from DYNAMO were presented at the 22nd EHA Congress (abstract S777) in June 2017.
This trial enrolled patients with indolent non-Hodgkin lymphoma whose disease was refractory to both rituximab and chemotherapy or radioimmunotherapy.
There were 83 patients with FL. They had a median of 3 prior anticancer regimens (range, 1-10).
The patients received duvelisib at 25 mg orally twice daily until disease progression or unacceptable toxicity.
The overall response rate, per an independent review committee, was 43%. One patient achieved a complete response, and 35 had a partial response. The median duration of response was 7.9 months.
The median PFS was 8.3 months, and the median OS was 27.8 months.
The most common grade 3 or higher AEs were neutropenia (22%), anemia (13%), diarrhea (16%), lipase increase (10%), and thrombocytopenia (9%).
There were 2 serious opportunistic infections—Pneumocystis pneumonia and fungal pneumonia.
There were 3 deaths attributed to duvelisib—toxic epidermal necrolysis/sepsis syndrome (n=1), drug reaction/eosinophilia/systemic symptoms (n=1), and pneumonitis/pneumonia (n=1).
The US Food and Drug Administration (FDA) has accepted for priority review the new drug application (NDA) for duvelisib, a dual PI3K delta/gamma inhibitor.
With this NDA, Verastem, Inc., is seeking full approval of duvelisib for the treatment of relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and accelerated approval of the drug for the treatment of relapsed or refractory follicular lymphoma (FL).
The FDA expects to make a decision on the NDA by October 5, 2018.
The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The agency grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The application for duvelisib is supported by data from DUO™, a randomized, phase 3 study of patients with relapsed or refractory CLL/SLL, and DYNAMO™, a phase 2 study of patients with refractory indolent non-Hodgkin lymphoma.
Phase 3 DUO trial
Results from DUO were presented at the 2017 ASH Annual Meeting in December.
This study included 319 CLL/SLL patients who were randomized 1:1 to receive either duvelisib (25 mg orally twice daily) or ofatumumab (initial infusion of 300 mg followed by 7 weekly infusions and 4 monthly infusions of 2000 mg).
The study’s primary endpoint was met, as duvelisib conferred a significant improvement in median progression-free survival (PFS) over ofatumumab.
Per an independent review committee, the median PFS was 13.3 months with duvelisib and 9.9 months with ofatumumab (hazard ratio=0.52; P<0.0001). Duvelisib maintained a PFS advantage in all patient subgroups analyzed.
The overall response rate was 73.8% with duvelisib and 45.3% with ofatumumab (P<0.0001). The complete response rate was 0.6% in both arms.
Overall survival (OS) was similar in the duvelisib and ofatumumab arms (hazard ratio=0.99; P=0.4807). The median OS was not reached in either arm.
The most common grade 3 or higher adverse events (AEs)—in the duvelisib and ofatumumab arms, respectively—were neutropenia (30% vs 17%), anemia (13% vs 5%), diarrhea (15% vs 1%), pneumonia (14% vs 1%), and colitis (12% vs 1%).
Thirty-five percent of patients discontinued duvelisib due to an AE.
Severe opportunistic infections occurred in 6% of duvelisib recipients—bronchopulmonary aspergillosis (n=4), fungal infection (n=2), Pneumocystis jirovecii pneumonia (n=2), and cytomegalovirus colitis (n=1).
There were 4 deaths related to duvelisib—staphylococcal pneumonia (n=2), general physical health deterioration (n=1), and sepsis (n=1).
Phase 2 DYNAMO trial
Results from DYNAMO were presented at the 22nd EHA Congress (abstract S777) in June 2017.
This trial enrolled patients with indolent non-Hodgkin lymphoma whose disease was refractory to both rituximab and chemotherapy or radioimmunotherapy.
There were 83 patients with FL. They had a median of 3 prior anticancer regimens (range, 1-10).
The patients received duvelisib at 25 mg orally twice daily until disease progression or unacceptable toxicity.
The overall response rate, per an independent review committee, was 43%. One patient achieved a complete response, and 35 had a partial response. The median duration of response was 7.9 months.
The median PFS was 8.3 months, and the median OS was 27.8 months.
The most common grade 3 or higher AEs were neutropenia (22%), anemia (13%), diarrhea (16%), lipase increase (10%), and thrombocytopenia (9%).
There were 2 serious opportunistic infections—Pneumocystis pneumonia and fungal pneumonia.
There were 3 deaths attributed to duvelisib—toxic epidermal necrolysis/sepsis syndrome (n=1), drug reaction/eosinophilia/systemic symptoms (n=1), and pneumonitis/pneumonia (n=1).
Team maps genetic evolution of T-ALL subtype
Single-cell analysis has revealed key genetic events in a type of T-cell acute lymphoblastic leukemia (T-ALL), according to researchers.
The team tracked the branching pattern of evolution in STIL-TAL1-positive T-ALL and identified mutations that may trigger development of the disease.
The researchers believe their findings could be used in minimal residual disease assessments as well as for the development of new targeted drugs.
Caroline Furness, MD, of Institute of Cancer Research in London, UK, and her colleagues described this research in Leukemia.
To determine the sequence of mutational events in STIL-TAL1-positive T-ALL, the researchers examined individual leukemia cells from 19 children and young adults with the disease, as well as 1 cell line with the STIL-TAL1 rearrangement (RPMI 8402).
The team found the STIL-TAL1 gene fusion and inactivation of the CDKN2A gene occurred very early in leukemia development.
The researchers said it was difficult to tell whether STIL-TAL1 fusion or CDKN2A loss are initiating events in this disease, and it isn’t clear which mutational event occurs first.
However, the team believes the STIL-TAL1 fusion is likely a founder or truncal event for this type of leukemia, so targeting the TAL1 regulatory complex could be an effective way to treat the disease.
The researchers also identified mutations in NOTCH1 and PTEN as secondary subclonal events.
Half of the samples examined had errors affecting PTEN, which suggests these events are key to maintaining leukemia growth and survival in STIL-TAL1-positive T-ALL, according to the researchers.
“We need to understand how cancers evolve and unpick which mutations are key to triggering cancer development and which are important for driving its growth and spread,” Dr Furness said.
“Our study uncovered these crucial mutations in a type of leukemia that accounts for around a quarter of cases of T-cell leukemia in children and young adults. This will help us to develop more effective treatments, especially in those children who relapse, and kinder treatments that won’t cause life-long side effects.”
Single-cell analysis has revealed key genetic events in a type of T-cell acute lymphoblastic leukemia (T-ALL), according to researchers.
The team tracked the branching pattern of evolution in STIL-TAL1-positive T-ALL and identified mutations that may trigger development of the disease.
The researchers believe their findings could be used in minimal residual disease assessments as well as for the development of new targeted drugs.
Caroline Furness, MD, of Institute of Cancer Research in London, UK, and her colleagues described this research in Leukemia.
To determine the sequence of mutational events in STIL-TAL1-positive T-ALL, the researchers examined individual leukemia cells from 19 children and young adults with the disease, as well as 1 cell line with the STIL-TAL1 rearrangement (RPMI 8402).
The team found the STIL-TAL1 gene fusion and inactivation of the CDKN2A gene occurred very early in leukemia development.
The researchers said it was difficult to tell whether STIL-TAL1 fusion or CDKN2A loss are initiating events in this disease, and it isn’t clear which mutational event occurs first.
However, the team believes the STIL-TAL1 fusion is likely a founder or truncal event for this type of leukemia, so targeting the TAL1 regulatory complex could be an effective way to treat the disease.
The researchers also identified mutations in NOTCH1 and PTEN as secondary subclonal events.
Half of the samples examined had errors affecting PTEN, which suggests these events are key to maintaining leukemia growth and survival in STIL-TAL1-positive T-ALL, according to the researchers.
“We need to understand how cancers evolve and unpick which mutations are key to triggering cancer development and which are important for driving its growth and spread,” Dr Furness said.
“Our study uncovered these crucial mutations in a type of leukemia that accounts for around a quarter of cases of T-cell leukemia in children and young adults. This will help us to develop more effective treatments, especially in those children who relapse, and kinder treatments that won’t cause life-long side effects.”
Single-cell analysis has revealed key genetic events in a type of T-cell acute lymphoblastic leukemia (T-ALL), according to researchers.
The team tracked the branching pattern of evolution in STIL-TAL1-positive T-ALL and identified mutations that may trigger development of the disease.
The researchers believe their findings could be used in minimal residual disease assessments as well as for the development of new targeted drugs.
Caroline Furness, MD, of Institute of Cancer Research in London, UK, and her colleagues described this research in Leukemia.
To determine the sequence of mutational events in STIL-TAL1-positive T-ALL, the researchers examined individual leukemia cells from 19 children and young adults with the disease, as well as 1 cell line with the STIL-TAL1 rearrangement (RPMI 8402).
The team found the STIL-TAL1 gene fusion and inactivation of the CDKN2A gene occurred very early in leukemia development.
The researchers said it was difficult to tell whether STIL-TAL1 fusion or CDKN2A loss are initiating events in this disease, and it isn’t clear which mutational event occurs first.
However, the team believes the STIL-TAL1 fusion is likely a founder or truncal event for this type of leukemia, so targeting the TAL1 regulatory complex could be an effective way to treat the disease.
The researchers also identified mutations in NOTCH1 and PTEN as secondary subclonal events.
Half of the samples examined had errors affecting PTEN, which suggests these events are key to maintaining leukemia growth and survival in STIL-TAL1-positive T-ALL, according to the researchers.
“We need to understand how cancers evolve and unpick which mutations are key to triggering cancer development and which are important for driving its growth and spread,” Dr Furness said.
“Our study uncovered these crucial mutations in a type of leukemia that accounts for around a quarter of cases of T-cell leukemia in children and young adults. This will help us to develop more effective treatments, especially in those children who relapse, and kinder treatments that won’t cause life-long side effects.”
Why iron can worsen malaria infection
Researchers believe they may have discovered why iron can sometimes worsen malaria infection.
By studying mice and samples from malaria patients, the researchers found that extra iron interferes with ferroportin, a protein that prevents a toxic buildup of iron in red blood cells and helps protect these cells against malaria infection.
The team also found a mutant form of ferroportin that occurs in African populations appears to protect against malaria.
These findings, published in Science, may help researchers and healthcare officials develop strategies to prevent and treat malaria.
“Our study helps solve a long-standing mystery,” said study author Tracey Rouault, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
“Iron supplements can sometimes worsen malaria infection and, conversely, iron deficiency can be protective in some cases. Our findings reveal that ferroportin—its function, as well as its regulation by iron levels—helps to explain these observations.”
The team found that red blood cells use ferroportin to remove excess iron, which malaria parasites consume as a food source.
In studies of mice, the researchers found the absence of ferroportin in erythroid cells caused iron to accumulate to toxic levels inside red blood cells. This, in turn, stressed the cells and shortened their life span.
In addition, the team found that mice lacking ferroportin had more parasites and worse outcomes when infected with malaria, compared to malaria-infected mice with intact ferroportin.
When they fed mice a high-iron diet, the researchers found that hepcidin regulated ferroportin in erythroid cells. The hormone, which is more abundant in high-iron environments, lowered ferroportin levels on erythroblasts and, subsequently, in red blood cells.
Additionally, hepcidin physically bound to ferroportin, preventing iron removal from the cells.
Next, the researchers sought to determine whether the ferroportin mutation Q248H, which is found in African populations, protects against malaria. This mutation shields ferroportin from hepcidin’s effects.
The team analyzed patient samples from 2 existing malaria studies. In one study, which enrolled children hospitalized for malaria in Zambia, 19.7% of the 66 patients had the Q248H mutation.
Children with the mutation tended to have fewer malarial parasites in their blood and tolerated their fevers for a longer period before coming to the hospital. While the trends were not statistically significant, they raise the possibility that Q248H reduces the iron available in the blood, therefore reducing the malaria parasite’s food source.
In the other study, which enrolled 290 pregnant women in Ghana, 8.6% had the Q248H mutation. Women with the mutation were significantly less likely to have pregnancy-associated malaria, in which parasites accumulate in the placenta and can cause adverse pregnancy and birth outcomes.
“Our findings suggest that Q248H does protect against malaria, possibly explaining why it occurs in people who live in malaria-endemic regions,” said study author De-Liang Zhang, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“Given the importance of iron metabolism overall, we will continue studying the ferroportin mutation and explore its other potential health effects.”
Researchers believe they may have discovered why iron can sometimes worsen malaria infection.
By studying mice and samples from malaria patients, the researchers found that extra iron interferes with ferroportin, a protein that prevents a toxic buildup of iron in red blood cells and helps protect these cells against malaria infection.
The team also found a mutant form of ferroportin that occurs in African populations appears to protect against malaria.
These findings, published in Science, may help researchers and healthcare officials develop strategies to prevent and treat malaria.
“Our study helps solve a long-standing mystery,” said study author Tracey Rouault, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
“Iron supplements can sometimes worsen malaria infection and, conversely, iron deficiency can be protective in some cases. Our findings reveal that ferroportin—its function, as well as its regulation by iron levels—helps to explain these observations.”
The team found that red blood cells use ferroportin to remove excess iron, which malaria parasites consume as a food source.
In studies of mice, the researchers found the absence of ferroportin in erythroid cells caused iron to accumulate to toxic levels inside red blood cells. This, in turn, stressed the cells and shortened their life span.
In addition, the team found that mice lacking ferroportin had more parasites and worse outcomes when infected with malaria, compared to malaria-infected mice with intact ferroportin.
When they fed mice a high-iron diet, the researchers found that hepcidin regulated ferroportin in erythroid cells. The hormone, which is more abundant in high-iron environments, lowered ferroportin levels on erythroblasts and, subsequently, in red blood cells.
Additionally, hepcidin physically bound to ferroportin, preventing iron removal from the cells.
Next, the researchers sought to determine whether the ferroportin mutation Q248H, which is found in African populations, protects against malaria. This mutation shields ferroportin from hepcidin’s effects.
The team analyzed patient samples from 2 existing malaria studies. In one study, which enrolled children hospitalized for malaria in Zambia, 19.7% of the 66 patients had the Q248H mutation.
Children with the mutation tended to have fewer malarial parasites in their blood and tolerated their fevers for a longer period before coming to the hospital. While the trends were not statistically significant, they raise the possibility that Q248H reduces the iron available in the blood, therefore reducing the malaria parasite’s food source.
In the other study, which enrolled 290 pregnant women in Ghana, 8.6% had the Q248H mutation. Women with the mutation were significantly less likely to have pregnancy-associated malaria, in which parasites accumulate in the placenta and can cause adverse pregnancy and birth outcomes.
“Our findings suggest that Q248H does protect against malaria, possibly explaining why it occurs in people who live in malaria-endemic regions,” said study author De-Liang Zhang, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“Given the importance of iron metabolism overall, we will continue studying the ferroportin mutation and explore its other potential health effects.”
Researchers believe they may have discovered why iron can sometimes worsen malaria infection.
By studying mice and samples from malaria patients, the researchers found that extra iron interferes with ferroportin, a protein that prevents a toxic buildup of iron in red blood cells and helps protect these cells against malaria infection.
The team also found a mutant form of ferroportin that occurs in African populations appears to protect against malaria.
These findings, published in Science, may help researchers and healthcare officials develop strategies to prevent and treat malaria.
“Our study helps solve a long-standing mystery,” said study author Tracey Rouault, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
“Iron supplements can sometimes worsen malaria infection and, conversely, iron deficiency can be protective in some cases. Our findings reveal that ferroportin—its function, as well as its regulation by iron levels—helps to explain these observations.”
The team found that red blood cells use ferroportin to remove excess iron, which malaria parasites consume as a food source.
In studies of mice, the researchers found the absence of ferroportin in erythroid cells caused iron to accumulate to toxic levels inside red blood cells. This, in turn, stressed the cells and shortened their life span.
In addition, the team found that mice lacking ferroportin had more parasites and worse outcomes when infected with malaria, compared to malaria-infected mice with intact ferroportin.
When they fed mice a high-iron diet, the researchers found that hepcidin regulated ferroportin in erythroid cells. The hormone, which is more abundant in high-iron environments, lowered ferroportin levels on erythroblasts and, subsequently, in red blood cells.
Additionally, hepcidin physically bound to ferroportin, preventing iron removal from the cells.
Next, the researchers sought to determine whether the ferroportin mutation Q248H, which is found in African populations, protects against malaria. This mutation shields ferroportin from hepcidin’s effects.
The team analyzed patient samples from 2 existing malaria studies. In one study, which enrolled children hospitalized for malaria in Zambia, 19.7% of the 66 patients had the Q248H mutation.
Children with the mutation tended to have fewer malarial parasites in their blood and tolerated their fevers for a longer period before coming to the hospital. While the trends were not statistically significant, they raise the possibility that Q248H reduces the iron available in the blood, therefore reducing the malaria parasite’s food source.
In the other study, which enrolled 290 pregnant women in Ghana, 8.6% had the Q248H mutation. Women with the mutation were significantly less likely to have pregnancy-associated malaria, in which parasites accumulate in the placenta and can cause adverse pregnancy and birth outcomes.
“Our findings suggest that Q248H does protect against malaria, possibly explaining why it occurs in people who live in malaria-endemic regions,” said study author De-Liang Zhang, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“Given the importance of iron metabolism overall, we will continue studying the ferroportin mutation and explore its other potential health effects.”
MicroRNAs required for cGVHD development
New research suggests a family of microRNAs, miR-17-92, plays a key role in chronic graft-versus-host disease (cGVHD).
Researchers found miR-17-92 is responsible for the T- and B-cell pathogenicity that causes cGVHD.
The team also discovered that pharmacological inhibition of miR-17 alleviated the symptoms of cGVHD in mice.
Yongxia Wu, PhD, of the Medical University of South Carolina in Charleston, and her colleagues reported these findings in Blood.
The researchers previously found that miR-17-92 regulates CD4 T-cell proliferation and Th1 and Treg differentiation in acute (a) GVHD.
So the team set out to investigate whether miR-17-92 regulates T- and B-cell differentiation and function in the development of cGVHD.
“Chronic GVHD has a different pathophysiology and different target organs than aGVHD,” Dr Wu noted. “It’s been a big challenge to try to find a target for cGVHD therapies because of the more complex immune reaction in cGVHD and the fact that its cellular and molecular mechanisms are not as well understood.”
“We decided to extend our aGVHD study to cGVHD, but there’s no single, well-defined murine model that can reflect all of the clinical manifestations seen in cGVHD patients. So we decided to study 4 different cGVHD models to best understand how miR-17-92 contributes overall, across many clinical presentations.”
The team performed a series of experiments in murine models of cGVHD after allogeneic bone marrow transplant (BMT). This included models of scleroderma that had transitioned from aGVHD to cGVHD, classic cGVHD scleroderma, lung inflammation, and a lupus-like condition.
The experiments revealed shared mechanisms by which miR-17-92 mediates cGVHD progression—namely, by regulating T helper-cell differentiation, B-cell activation, germinal center responses, and autoantibody production.
“The mechanism for how miR-17-92 regulates T and B cells was very consistent,” Dr Wu said. “In other words, we did not find any big differences among the models.”
The researchers also assessed whether pharmacological inhibition of miR-17 or miR-19—“key members in the miR-17-92 cluster”—might be effective in the treatment of cGVHD.
The team tested antagomirs specific for miR-17 or miR-19 in the scleroderma cGVHD model and the lupus-like condition.
Anti-miR-17, but not anti-miR-19, reduced skin damage in the scleroderma model and alleviated proteinuria in the lupus-like condition.
“So we not only found a new mechanism for cGVHD development by demonstrating that miR-17-92 is heavily involved in the T- and B-cell responses that lead to cGVHD, but we also found that blocking miR-17 substantially reduced cGVHD symptoms in mice,” Dr Wu said.
“That’s exciting because it provides strong evidence that this miR may be a good target for controlling cGVHD after allogeneic BMT.”
Now, Dr Wu and her colleagues are investigating how other microRNAs may be involved in regulating T- and B-cell function during allogeneic BMT.
New research suggests a family of microRNAs, miR-17-92, plays a key role in chronic graft-versus-host disease (cGVHD).
Researchers found miR-17-92 is responsible for the T- and B-cell pathogenicity that causes cGVHD.
The team also discovered that pharmacological inhibition of miR-17 alleviated the symptoms of cGVHD in mice.
Yongxia Wu, PhD, of the Medical University of South Carolina in Charleston, and her colleagues reported these findings in Blood.
The researchers previously found that miR-17-92 regulates CD4 T-cell proliferation and Th1 and Treg differentiation in acute (a) GVHD.
So the team set out to investigate whether miR-17-92 regulates T- and B-cell differentiation and function in the development of cGVHD.
“Chronic GVHD has a different pathophysiology and different target organs than aGVHD,” Dr Wu noted. “It’s been a big challenge to try to find a target for cGVHD therapies because of the more complex immune reaction in cGVHD and the fact that its cellular and molecular mechanisms are not as well understood.”
“We decided to extend our aGVHD study to cGVHD, but there’s no single, well-defined murine model that can reflect all of the clinical manifestations seen in cGVHD patients. So we decided to study 4 different cGVHD models to best understand how miR-17-92 contributes overall, across many clinical presentations.”
The team performed a series of experiments in murine models of cGVHD after allogeneic bone marrow transplant (BMT). This included models of scleroderma that had transitioned from aGVHD to cGVHD, classic cGVHD scleroderma, lung inflammation, and a lupus-like condition.
The experiments revealed shared mechanisms by which miR-17-92 mediates cGVHD progression—namely, by regulating T helper-cell differentiation, B-cell activation, germinal center responses, and autoantibody production.
“The mechanism for how miR-17-92 regulates T and B cells was very consistent,” Dr Wu said. “In other words, we did not find any big differences among the models.”
The researchers also assessed whether pharmacological inhibition of miR-17 or miR-19—“key members in the miR-17-92 cluster”—might be effective in the treatment of cGVHD.
The team tested antagomirs specific for miR-17 or miR-19 in the scleroderma cGVHD model and the lupus-like condition.
Anti-miR-17, but not anti-miR-19, reduced skin damage in the scleroderma model and alleviated proteinuria in the lupus-like condition.
“So we not only found a new mechanism for cGVHD development by demonstrating that miR-17-92 is heavily involved in the T- and B-cell responses that lead to cGVHD, but we also found that blocking miR-17 substantially reduced cGVHD symptoms in mice,” Dr Wu said.
“That’s exciting because it provides strong evidence that this miR may be a good target for controlling cGVHD after allogeneic BMT.”
Now, Dr Wu and her colleagues are investigating how other microRNAs may be involved in regulating T- and B-cell function during allogeneic BMT.
New research suggests a family of microRNAs, miR-17-92, plays a key role in chronic graft-versus-host disease (cGVHD).
Researchers found miR-17-92 is responsible for the T- and B-cell pathogenicity that causes cGVHD.
The team also discovered that pharmacological inhibition of miR-17 alleviated the symptoms of cGVHD in mice.
Yongxia Wu, PhD, of the Medical University of South Carolina in Charleston, and her colleagues reported these findings in Blood.
The researchers previously found that miR-17-92 regulates CD4 T-cell proliferation and Th1 and Treg differentiation in acute (a) GVHD.
So the team set out to investigate whether miR-17-92 regulates T- and B-cell differentiation and function in the development of cGVHD.
“Chronic GVHD has a different pathophysiology and different target organs than aGVHD,” Dr Wu noted. “It’s been a big challenge to try to find a target for cGVHD therapies because of the more complex immune reaction in cGVHD and the fact that its cellular and molecular mechanisms are not as well understood.”
“We decided to extend our aGVHD study to cGVHD, but there’s no single, well-defined murine model that can reflect all of the clinical manifestations seen in cGVHD patients. So we decided to study 4 different cGVHD models to best understand how miR-17-92 contributes overall, across many clinical presentations.”
The team performed a series of experiments in murine models of cGVHD after allogeneic bone marrow transplant (BMT). This included models of scleroderma that had transitioned from aGVHD to cGVHD, classic cGVHD scleroderma, lung inflammation, and a lupus-like condition.
The experiments revealed shared mechanisms by which miR-17-92 mediates cGVHD progression—namely, by regulating T helper-cell differentiation, B-cell activation, germinal center responses, and autoantibody production.
“The mechanism for how miR-17-92 regulates T and B cells was very consistent,” Dr Wu said. “In other words, we did not find any big differences among the models.”
The researchers also assessed whether pharmacological inhibition of miR-17 or miR-19—“key members in the miR-17-92 cluster”—might be effective in the treatment of cGVHD.
The team tested antagomirs specific for miR-17 or miR-19 in the scleroderma cGVHD model and the lupus-like condition.
Anti-miR-17, but not anti-miR-19, reduced skin damage in the scleroderma model and alleviated proteinuria in the lupus-like condition.
“So we not only found a new mechanism for cGVHD development by demonstrating that miR-17-92 is heavily involved in the T- and B-cell responses that lead to cGVHD, but we also found that blocking miR-17 substantially reduced cGVHD symptoms in mice,” Dr Wu said.
“That’s exciting because it provides strong evidence that this miR may be a good target for controlling cGVHD after allogeneic BMT.”
Now, Dr Wu and her colleagues are investigating how other microRNAs may be involved in regulating T- and B-cell function during allogeneic BMT.
Project provides ‘unprecedented understanding’ of cancers
Through extensive analyses of data from The Cancer Genome Atlas (TCGA), researchers have produced a new resource known as the Pan-Cancer Atlas.
Multiple research groups analyzed data on more than 10,000 tumors spanning 33 types of cancer, including acute myeloid leukemia and diffuse large B-cell lymphoma.
The work revealed new insights regarding cells of origin, oncogenic processes, and signaling pathways.
These insights make up the Pan-Cancer Atlas and are described in 27 papers published in Cell Press journals. The entire collection of papers is available through a portal on cell.com.
The Pan-Cancer Atlas is the final output of TCGA, a joint effort of the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI) to “collect, select, and analyze human tissues for genomic alterations on a very large scale.”
“This project is the culmination of more than a decade of ground-breaking work,” said Francis S. Collins, MD, PhD, director of the National Institutes of Health.
“This analysis provides cancer researchers with unprecedented understanding of how, where, and why tumors arise in humans, enabling better informed clinical trials and future treatments.”
The project focused on genome sequencing as well as other analyses, such as investigating gene and protein expression profiles and associating them with clinical and imaging data.
“The Pan-Cancer Atlas effort complements the over 30 tumor-specific papers that have been published by TCGA in the last decade and expands upon earlier pan-cancer work that was published in 2013,” said Jean Claude Zenklusen, PhD, director of the TCGA Program Office at NCI.
The Pan-Cancer Atlas is divided into 3 main categories—cell of origin, oncogenic processes, and signaling pathways—each anchored by a summary paper that recaps the core findings for the topic. Companion papers report in-depth explorations of individual topics within these categories.
Cell of origin
In the first Pan-Cancer Atlas summary paper, the authors review the findings from analyses using a technique called molecular clustering, which groups tumors by parameters such as genes being expressed, abnormality of chromosome numbers in tumor cells, and DNA modifications.
The analyses suggest that tumor types cluster by their possible cells of origin, a finding that has implications for the classification and treatment of various cancers.
“Rather than the organ of origin, we can now use molecular features to identify the cancer’s cell of origin,” said Li Ding, PhD, of Washington University School of Medicine in St. Louis, Missouri.
“We are looking at what genes are turned on in the tumor, and that brings us to a particular cell type. For example, squamous cell cancers can arise in the lung, bladder, cervix, and some tumors of the head and neck. We traditionally have treated cancers in these areas as completely different diseases, but, [by] studying their molecular features, we now know such cancers are closely related.”
“This new molecular-based classification system should greatly help in the clinic, where it is already explaining some of the similar clinical behavior of what we thought were different tumor types,” said Charles Perou, PhD, of UNC Lineberger Comprehensive Cancer Center in Chapel Hill, North Carolina.
“These findings also provide many new therapeutic opportunities, which can and will be tested in the next phase of human clinical trials.”
Oncogenic processes
The second Pan-Cancer Atlas summary paper presents a broad view of the TCGA findings on the processes that lead to cancer development and progression.
The research revealed insights into 3 critical oncogenic processes—germline and somatic mutations, the influence of the tumor’s underlying genome and epigenome on gene and protein expression, and the interplay of tumor and immune cells.
“For the 10,000 tumors we analyzed, we now know—in detail—the inherited mutations driving cancer and the genetic errors that accumulate as people age, increasing the risk of cancer,” Dr Ding said. “This is the first definitive summary of the genetics behind 33 major types of cancer.”
“TCGA has created a catalogue of alterations that occur in a variety of cancer types,” said Katherine Hoadley, PhD, of University of North Carolina at Chapel Hill.
“Having this catalogue of alterations is really important for us to look, in future studies, at why these alterations are there and to predict outcomes for patients.”
Signaling pathways
The final Pan-Cancer Atlas summary paper details TCGA research on the genomic alterations in the signaling pathways that control cell-cycle progression, cell death, and cell growth. The work highlights the similarities and differences in these processes across a range of cancers.
The researchers believe these studies have revealed new patterns of potential vulnerabilities that might aid the development of targeted and combination therapies.
Through extensive analyses of data from The Cancer Genome Atlas (TCGA), researchers have produced a new resource known as the Pan-Cancer Atlas.
Multiple research groups analyzed data on more than 10,000 tumors spanning 33 types of cancer, including acute myeloid leukemia and diffuse large B-cell lymphoma.
The work revealed new insights regarding cells of origin, oncogenic processes, and signaling pathways.
These insights make up the Pan-Cancer Atlas and are described in 27 papers published in Cell Press journals. The entire collection of papers is available through a portal on cell.com.
The Pan-Cancer Atlas is the final output of TCGA, a joint effort of the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI) to “collect, select, and analyze human tissues for genomic alterations on a very large scale.”
“This project is the culmination of more than a decade of ground-breaking work,” said Francis S. Collins, MD, PhD, director of the National Institutes of Health.
“This analysis provides cancer researchers with unprecedented understanding of how, where, and why tumors arise in humans, enabling better informed clinical trials and future treatments.”
The project focused on genome sequencing as well as other analyses, such as investigating gene and protein expression profiles and associating them with clinical and imaging data.
“The Pan-Cancer Atlas effort complements the over 30 tumor-specific papers that have been published by TCGA in the last decade and expands upon earlier pan-cancer work that was published in 2013,” said Jean Claude Zenklusen, PhD, director of the TCGA Program Office at NCI.
The Pan-Cancer Atlas is divided into 3 main categories—cell of origin, oncogenic processes, and signaling pathways—each anchored by a summary paper that recaps the core findings for the topic. Companion papers report in-depth explorations of individual topics within these categories.
Cell of origin
In the first Pan-Cancer Atlas summary paper, the authors review the findings from analyses using a technique called molecular clustering, which groups tumors by parameters such as genes being expressed, abnormality of chromosome numbers in tumor cells, and DNA modifications.
The analyses suggest that tumor types cluster by their possible cells of origin, a finding that has implications for the classification and treatment of various cancers.
“Rather than the organ of origin, we can now use molecular features to identify the cancer’s cell of origin,” said Li Ding, PhD, of Washington University School of Medicine in St. Louis, Missouri.
“We are looking at what genes are turned on in the tumor, and that brings us to a particular cell type. For example, squamous cell cancers can arise in the lung, bladder, cervix, and some tumors of the head and neck. We traditionally have treated cancers in these areas as completely different diseases, but, [by] studying their molecular features, we now know such cancers are closely related.”
“This new molecular-based classification system should greatly help in the clinic, where it is already explaining some of the similar clinical behavior of what we thought were different tumor types,” said Charles Perou, PhD, of UNC Lineberger Comprehensive Cancer Center in Chapel Hill, North Carolina.
“These findings also provide many new therapeutic opportunities, which can and will be tested in the next phase of human clinical trials.”
Oncogenic processes
The second Pan-Cancer Atlas summary paper presents a broad view of the TCGA findings on the processes that lead to cancer development and progression.
The research revealed insights into 3 critical oncogenic processes—germline and somatic mutations, the influence of the tumor’s underlying genome and epigenome on gene and protein expression, and the interplay of tumor and immune cells.
“For the 10,000 tumors we analyzed, we now know—in detail—the inherited mutations driving cancer and the genetic errors that accumulate as people age, increasing the risk of cancer,” Dr Ding said. “This is the first definitive summary of the genetics behind 33 major types of cancer.”
“TCGA has created a catalogue of alterations that occur in a variety of cancer types,” said Katherine Hoadley, PhD, of University of North Carolina at Chapel Hill.
“Having this catalogue of alterations is really important for us to look, in future studies, at why these alterations are there and to predict outcomes for patients.”
Signaling pathways
The final Pan-Cancer Atlas summary paper details TCGA research on the genomic alterations in the signaling pathways that control cell-cycle progression, cell death, and cell growth. The work highlights the similarities and differences in these processes across a range of cancers.
The researchers believe these studies have revealed new patterns of potential vulnerabilities that might aid the development of targeted and combination therapies.
Through extensive analyses of data from The Cancer Genome Atlas (TCGA), researchers have produced a new resource known as the Pan-Cancer Atlas.
Multiple research groups analyzed data on more than 10,000 tumors spanning 33 types of cancer, including acute myeloid leukemia and diffuse large B-cell lymphoma.
The work revealed new insights regarding cells of origin, oncogenic processes, and signaling pathways.
These insights make up the Pan-Cancer Atlas and are described in 27 papers published in Cell Press journals. The entire collection of papers is available through a portal on cell.com.
The Pan-Cancer Atlas is the final output of TCGA, a joint effort of the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI) to “collect, select, and analyze human tissues for genomic alterations on a very large scale.”
“This project is the culmination of more than a decade of ground-breaking work,” said Francis S. Collins, MD, PhD, director of the National Institutes of Health.
“This analysis provides cancer researchers with unprecedented understanding of how, where, and why tumors arise in humans, enabling better informed clinical trials and future treatments.”
The project focused on genome sequencing as well as other analyses, such as investigating gene and protein expression profiles and associating them with clinical and imaging data.
“The Pan-Cancer Atlas effort complements the over 30 tumor-specific papers that have been published by TCGA in the last decade and expands upon earlier pan-cancer work that was published in 2013,” said Jean Claude Zenklusen, PhD, director of the TCGA Program Office at NCI.
The Pan-Cancer Atlas is divided into 3 main categories—cell of origin, oncogenic processes, and signaling pathways—each anchored by a summary paper that recaps the core findings for the topic. Companion papers report in-depth explorations of individual topics within these categories.
Cell of origin
In the first Pan-Cancer Atlas summary paper, the authors review the findings from analyses using a technique called molecular clustering, which groups tumors by parameters such as genes being expressed, abnormality of chromosome numbers in tumor cells, and DNA modifications.
The analyses suggest that tumor types cluster by their possible cells of origin, a finding that has implications for the classification and treatment of various cancers.
“Rather than the organ of origin, we can now use molecular features to identify the cancer’s cell of origin,” said Li Ding, PhD, of Washington University School of Medicine in St. Louis, Missouri.
“We are looking at what genes are turned on in the tumor, and that brings us to a particular cell type. For example, squamous cell cancers can arise in the lung, bladder, cervix, and some tumors of the head and neck. We traditionally have treated cancers in these areas as completely different diseases, but, [by] studying their molecular features, we now know such cancers are closely related.”
“This new molecular-based classification system should greatly help in the clinic, where it is already explaining some of the similar clinical behavior of what we thought were different tumor types,” said Charles Perou, PhD, of UNC Lineberger Comprehensive Cancer Center in Chapel Hill, North Carolina.
“These findings also provide many new therapeutic opportunities, which can and will be tested in the next phase of human clinical trials.”
Oncogenic processes
The second Pan-Cancer Atlas summary paper presents a broad view of the TCGA findings on the processes that lead to cancer development and progression.
The research revealed insights into 3 critical oncogenic processes—germline and somatic mutations, the influence of the tumor’s underlying genome and epigenome on gene and protein expression, and the interplay of tumor and immune cells.
“For the 10,000 tumors we analyzed, we now know—in detail—the inherited mutations driving cancer and the genetic errors that accumulate as people age, increasing the risk of cancer,” Dr Ding said. “This is the first definitive summary of the genetics behind 33 major types of cancer.”
“TCGA has created a catalogue of alterations that occur in a variety of cancer types,” said Katherine Hoadley, PhD, of University of North Carolina at Chapel Hill.
“Having this catalogue of alterations is really important for us to look, in future studies, at why these alterations are there and to predict outcomes for patients.”
Signaling pathways
The final Pan-Cancer Atlas summary paper details TCGA research on the genomic alterations in the signaling pathways that control cell-cycle progression, cell death, and cell growth. The work highlights the similarities and differences in these processes across a range of cancers.
The researchers believe these studies have revealed new patterns of potential vulnerabilities that might aid the development of targeted and combination therapies.
Injectable hydrogels stop bleeding, promote healing
Biomedical engineers have developed hydrogels that can act as an “injectable bandage,” according to preclinical research published in Acta Biomaterialia.
The team engineered injectable hydrogels that were able to promote hemostasis and facilitate wound healing in vitro.
“Injectable hydrogels are promising materials for achieving hemostasis in case of internal injuries and bleeding, as these biomaterials can be introduced into a wound site using minimally invasive approaches,” said study author Akhilesh K. Gaharwar, PhD, of Texas A&M University in College Station, Texas.
“An ideal injectable bandage should solidify after injection in the wound area and promote a natural clotting cascade. In addition, the injectable bandage should initiate wound healing response after achieving hemostasis.”
To create their injectable hydrogels, Dr Gaharwar and his colleagues used a thickening agent known as kappa-carrageenan, which is obtained from seaweed, as well as clay-based nanoparticles known as nanosilicates.
It is the charged characteristics of the nanosilicates that provide the hydrogels with hemostatic ability, according to the researchers.
Specifically, the team said adding nanosilicates to kappa-carrageenan increases protein adsorption on the hydrogels, which enhances cell adhesion and spreading, increases platelet binding, and reduces blood clotting time.
“Interestingly, we also found that these injectable bandages can show a prolonged release of therapeutics that can be used to heal the wound,” said study author Giriraj Lokhande, a graduate student in Dr Gaharwar’s lab.
“The negative surface charge of nanoparticles enabled electrostatic interactions with therapeutics, thus resulting in the slow release of therapeutics.”
The researchers encapsulated vascular endothelial growth factor (VEGF) in the hydrogels and observed sustained release of VEGF in experiments. The hydrogels containing VEGF promoted faster wound healing than control hydrogels.
The researchers said a “range of therapeutic biomacromolecules” could be delivered in the same way as the VEGF.
Biomedical engineers have developed hydrogels that can act as an “injectable bandage,” according to preclinical research published in Acta Biomaterialia.
The team engineered injectable hydrogels that were able to promote hemostasis and facilitate wound healing in vitro.
“Injectable hydrogels are promising materials for achieving hemostasis in case of internal injuries and bleeding, as these biomaterials can be introduced into a wound site using minimally invasive approaches,” said study author Akhilesh K. Gaharwar, PhD, of Texas A&M University in College Station, Texas.
“An ideal injectable bandage should solidify after injection in the wound area and promote a natural clotting cascade. In addition, the injectable bandage should initiate wound healing response after achieving hemostasis.”
To create their injectable hydrogels, Dr Gaharwar and his colleagues used a thickening agent known as kappa-carrageenan, which is obtained from seaweed, as well as clay-based nanoparticles known as nanosilicates.
It is the charged characteristics of the nanosilicates that provide the hydrogels with hemostatic ability, according to the researchers.
Specifically, the team said adding nanosilicates to kappa-carrageenan increases protein adsorption on the hydrogels, which enhances cell adhesion and spreading, increases platelet binding, and reduces blood clotting time.
“Interestingly, we also found that these injectable bandages can show a prolonged release of therapeutics that can be used to heal the wound,” said study author Giriraj Lokhande, a graduate student in Dr Gaharwar’s lab.
“The negative surface charge of nanoparticles enabled electrostatic interactions with therapeutics, thus resulting in the slow release of therapeutics.”
The researchers encapsulated vascular endothelial growth factor (VEGF) in the hydrogels and observed sustained release of VEGF in experiments. The hydrogels containing VEGF promoted faster wound healing than control hydrogels.
The researchers said a “range of therapeutic biomacromolecules” could be delivered in the same way as the VEGF.
Biomedical engineers have developed hydrogels that can act as an “injectable bandage,” according to preclinical research published in Acta Biomaterialia.
The team engineered injectable hydrogels that were able to promote hemostasis and facilitate wound healing in vitro.
“Injectable hydrogels are promising materials for achieving hemostasis in case of internal injuries and bleeding, as these biomaterials can be introduced into a wound site using minimally invasive approaches,” said study author Akhilesh K. Gaharwar, PhD, of Texas A&M University in College Station, Texas.
“An ideal injectable bandage should solidify after injection in the wound area and promote a natural clotting cascade. In addition, the injectable bandage should initiate wound healing response after achieving hemostasis.”
To create their injectable hydrogels, Dr Gaharwar and his colleagues used a thickening agent known as kappa-carrageenan, which is obtained from seaweed, as well as clay-based nanoparticles known as nanosilicates.
It is the charged characteristics of the nanosilicates that provide the hydrogels with hemostatic ability, according to the researchers.
Specifically, the team said adding nanosilicates to kappa-carrageenan increases protein adsorption on the hydrogels, which enhances cell adhesion and spreading, increases platelet binding, and reduces blood clotting time.
“Interestingly, we also found that these injectable bandages can show a prolonged release of therapeutics that can be used to heal the wound,” said study author Giriraj Lokhande, a graduate student in Dr Gaharwar’s lab.
“The negative surface charge of nanoparticles enabled electrostatic interactions with therapeutics, thus resulting in the slow release of therapeutics.”
The researchers encapsulated vascular endothelial growth factor (VEGF) in the hydrogels and observed sustained release of VEGF in experiments. The hydrogels containing VEGF promoted faster wound healing than control hydrogels.
The researchers said a “range of therapeutic biomacromolecules” could be delivered in the same way as the VEGF.
At-home measurement of WBCs
A portable device could be used to monitor patients’ white blood cell (WBC) levels at home, without the need for blood samples, according to researchers.
The team created a prototype that records video of blood cells flowing through capillaries just below the skin surface at the base of the fingernail.
The group is developing a computer algorithm that, in early testing, has been able to analyze the videos and determine if WBC levels are too low.
The researchers believe this type of device could be used to prevent infections among chemotherapy recipients.
“Our vision is that patients will have this portable device that they can take home, and they can monitor daily how they are reacting to the treatment,” said Carlos Castro-Gonzalez, PhD, of the Massachusetts Institute of Technology (MIT) in Cambridge, Massachusetts.
“If they go below the [safe WBC] threshold, then preventive treatment can be deployed.”
Dr Castro-Gonzalez and his colleagues described their prototype, and the testing of it, in Scientific Reports.
The device consists of a wide-field microscope that emits blue light, which penetrates about 50 to 150 microns below the skin and is reflected back to a video camera.
The researchers decided to image the skin at the base of the nail because the capillaries there are very close to the skin surface. These capillaries are so narrow that WBCs must squeeze through one at a time, making them easier to see.
The researchers tested the device in 11 patients at various points during their chemotherapy treatment.
The device does not provide precise WBC counts but allowed the team to differentiate cases of severe neutropenia (<500 neutrophils per μL) from non-neutropenic cases (>1500 neutrophils per μL).
To obtain enough data to make these classifications, the researchers recorded 1 minute of video per patient. Three blinded human assistants then watched the videos and noted whenever a WBC passed by.
However, since submitting their paper, the researchers have been developing a computer algorithm to perform the same task automatically.
“Based on the feature-set that our human raters identified, we are now developing an AI and machine-vision algorithm, with preliminary results that indicate the same accuracy as the raters,” said study author Aurélien Bourquard, PhD, of MIT.
The researchers have applied for patents on the technology and launched a company called Leuko, which is working on commercializing the technology with help from MIT.
To move the technology further toward commercialization, the researchers are building a new automated prototype.
“Automating the measurement process is key to making a viable home-use device,” said study author Ian Butterworth, of MIT. “The imaging needs to take place in the right spot on the patient’s finger, and the operation of the device must be straightforward.”
Using this new prototype, the researchers plan to test the device with additional cancer patients. And the team is investigating whether they can get accurate results with shorter lengths of video.
They also plan to adapt the technology so it can generate more precise WBC counts, which would make it useful for monitoring bone marrow transplant recipients or people with certain infectious diseases, Dr Castro-Gonzalez said.
This could also make it possible to determine whether chemotherapy patients can receive their next dose sooner than usual.
“There is a balancing act that oncologists must do,” said study author Alvaro Sanchez-Ferro, MD, of Centro Integral en Neurociencias A.C. HM CINAC in Madrid, Spain.
“Normally, doctors want to make chemotherapy as intensive as possible but without getting people too immunosuppressed. Current 21-day cycles are based on statistics of what most patients can take, but if you are ready early, then they can potentially bring you back early, and that can translate into better survival.”
A portable device could be used to monitor patients’ white blood cell (WBC) levels at home, without the need for blood samples, according to researchers.
The team created a prototype that records video of blood cells flowing through capillaries just below the skin surface at the base of the fingernail.
The group is developing a computer algorithm that, in early testing, has been able to analyze the videos and determine if WBC levels are too low.
The researchers believe this type of device could be used to prevent infections among chemotherapy recipients.
“Our vision is that patients will have this portable device that they can take home, and they can monitor daily how they are reacting to the treatment,” said Carlos Castro-Gonzalez, PhD, of the Massachusetts Institute of Technology (MIT) in Cambridge, Massachusetts.
“If they go below the [safe WBC] threshold, then preventive treatment can be deployed.”
Dr Castro-Gonzalez and his colleagues described their prototype, and the testing of it, in Scientific Reports.
The device consists of a wide-field microscope that emits blue light, which penetrates about 50 to 150 microns below the skin and is reflected back to a video camera.
The researchers decided to image the skin at the base of the nail because the capillaries there are very close to the skin surface. These capillaries are so narrow that WBCs must squeeze through one at a time, making them easier to see.
The researchers tested the device in 11 patients at various points during their chemotherapy treatment.
The device does not provide precise WBC counts but allowed the team to differentiate cases of severe neutropenia (<500 neutrophils per μL) from non-neutropenic cases (>1500 neutrophils per μL).
To obtain enough data to make these classifications, the researchers recorded 1 minute of video per patient. Three blinded human assistants then watched the videos and noted whenever a WBC passed by.
However, since submitting their paper, the researchers have been developing a computer algorithm to perform the same task automatically.
“Based on the feature-set that our human raters identified, we are now developing an AI and machine-vision algorithm, with preliminary results that indicate the same accuracy as the raters,” said study author Aurélien Bourquard, PhD, of MIT.
The researchers have applied for patents on the technology and launched a company called Leuko, which is working on commercializing the technology with help from MIT.
To move the technology further toward commercialization, the researchers are building a new automated prototype.
“Automating the measurement process is key to making a viable home-use device,” said study author Ian Butterworth, of MIT. “The imaging needs to take place in the right spot on the patient’s finger, and the operation of the device must be straightforward.”
Using this new prototype, the researchers plan to test the device with additional cancer patients. And the team is investigating whether they can get accurate results with shorter lengths of video.
They also plan to adapt the technology so it can generate more precise WBC counts, which would make it useful for monitoring bone marrow transplant recipients or people with certain infectious diseases, Dr Castro-Gonzalez said.
This could also make it possible to determine whether chemotherapy patients can receive their next dose sooner than usual.
“There is a balancing act that oncologists must do,” said study author Alvaro Sanchez-Ferro, MD, of Centro Integral en Neurociencias A.C. HM CINAC in Madrid, Spain.
“Normally, doctors want to make chemotherapy as intensive as possible but without getting people too immunosuppressed. Current 21-day cycles are based on statistics of what most patients can take, but if you are ready early, then they can potentially bring you back early, and that can translate into better survival.”
A portable device could be used to monitor patients’ white blood cell (WBC) levels at home, without the need for blood samples, according to researchers.
The team created a prototype that records video of blood cells flowing through capillaries just below the skin surface at the base of the fingernail.
The group is developing a computer algorithm that, in early testing, has been able to analyze the videos and determine if WBC levels are too low.
The researchers believe this type of device could be used to prevent infections among chemotherapy recipients.
“Our vision is that patients will have this portable device that they can take home, and they can monitor daily how they are reacting to the treatment,” said Carlos Castro-Gonzalez, PhD, of the Massachusetts Institute of Technology (MIT) in Cambridge, Massachusetts.
“If they go below the [safe WBC] threshold, then preventive treatment can be deployed.”
Dr Castro-Gonzalez and his colleagues described their prototype, and the testing of it, in Scientific Reports.
The device consists of a wide-field microscope that emits blue light, which penetrates about 50 to 150 microns below the skin and is reflected back to a video camera.
The researchers decided to image the skin at the base of the nail because the capillaries there are very close to the skin surface. These capillaries are so narrow that WBCs must squeeze through one at a time, making them easier to see.
The researchers tested the device in 11 patients at various points during their chemotherapy treatment.
The device does not provide precise WBC counts but allowed the team to differentiate cases of severe neutropenia (<500 neutrophils per μL) from non-neutropenic cases (>1500 neutrophils per μL).
To obtain enough data to make these classifications, the researchers recorded 1 minute of video per patient. Three blinded human assistants then watched the videos and noted whenever a WBC passed by.
However, since submitting their paper, the researchers have been developing a computer algorithm to perform the same task automatically.
“Based on the feature-set that our human raters identified, we are now developing an AI and machine-vision algorithm, with preliminary results that indicate the same accuracy as the raters,” said study author Aurélien Bourquard, PhD, of MIT.
The researchers have applied for patents on the technology and launched a company called Leuko, which is working on commercializing the technology with help from MIT.
To move the technology further toward commercialization, the researchers are building a new automated prototype.
“Automating the measurement process is key to making a viable home-use device,” said study author Ian Butterworth, of MIT. “The imaging needs to take place in the right spot on the patient’s finger, and the operation of the device must be straightforward.”
Using this new prototype, the researchers plan to test the device with additional cancer patients. And the team is investigating whether they can get accurate results with shorter lengths of video.
They also plan to adapt the technology so it can generate more precise WBC counts, which would make it useful for monitoring bone marrow transplant recipients or people with certain infectious diseases, Dr Castro-Gonzalez said.
This could also make it possible to determine whether chemotherapy patients can receive their next dose sooner than usual.
“There is a balancing act that oncologists must do,” said study author Alvaro Sanchez-Ferro, MD, of Centro Integral en Neurociencias A.C. HM CINAC in Madrid, Spain.
“Normally, doctors want to make chemotherapy as intensive as possible but without getting people too immunosuppressed. Current 21-day cycles are based on statistics of what most patients can take, but if you are ready early, then they can potentially bring you back early, and that can translate into better survival.”
How RBCs maintain their shape
New research indicates that non-muscle myosin II-A (NMIIA) plays a key role in maintaining red blood cell (RBC) shape and deformability.
Researchers found evidence to suggest that NMIIA forms filaments in RBCs, and specialized regions at both ends of the filaments can pull on actin to control the stiffness of the cell membrane.
“You need active contraction on the cell membrane, similar to how muscles contract,” explained study author Velia Fowler, PhD, of The Scripps Research Institute in La Jolla, California.
“The myosin pulls on the actin to provide tension in the membrane, and then that tension maintains the biconcave shape.”
Dr Fowler and her colleagues described these findings in PNAS.
The researchers noted that RBC shape and deformability depend upon the membrane skeleton—a network of actin filaments (F-actin) cross-linked by spectrin tetramers.
And although NMII motors are known to exert force on F-actin networks to control cell shapes, no one had investigated a function for NMII contractility in the spectrin–F-actin network of RBCs. So Dr Fowler and her colleagues did just that.
The researchers said their work suggests NMIIA is the predominant RBC NMII isoform, and NMIIA motor activity regulates interactions with the spectrin–F-actin network to control RBCs’ shape and deformability.
Specifically, NMIIA forms bipolar filaments in RBCs, and these filaments associate with F-actins via their motor domains. It is through these interactions that NMIIA contractile forces promote membrane tension to maintain RBC shape and deformability.
To test these findings, the researchers treated RBCs with a compound called blebbistatin, which inhibits NMII motor activity.
After treatment, the team observed a decrease in NMIIA filaments associated with the RBC membrane and evidence of decreased membrane tension. The treated RBCs became elongated and showed a reduction in biconcavity as well as an increase in deformability.
The researchers believe this work could have applications for diseases in which RBCs are deformed. In fact, the team thinks inhibiting NMIIA in RBCs might prove useful for treating patients with sickle cell disease, as it could restore some elasticity to the patients’ RBCs.
Going forward, the researchers hope to learn more about what regulates NMIIA’s activity in RBCs and other cells.
New research indicates that non-muscle myosin II-A (NMIIA) plays a key role in maintaining red blood cell (RBC) shape and deformability.
Researchers found evidence to suggest that NMIIA forms filaments in RBCs, and specialized regions at both ends of the filaments can pull on actin to control the stiffness of the cell membrane.
“You need active contraction on the cell membrane, similar to how muscles contract,” explained study author Velia Fowler, PhD, of The Scripps Research Institute in La Jolla, California.
“The myosin pulls on the actin to provide tension in the membrane, and then that tension maintains the biconcave shape.”
Dr Fowler and her colleagues described these findings in PNAS.
The researchers noted that RBC shape and deformability depend upon the membrane skeleton—a network of actin filaments (F-actin) cross-linked by spectrin tetramers.
And although NMII motors are known to exert force on F-actin networks to control cell shapes, no one had investigated a function for NMII contractility in the spectrin–F-actin network of RBCs. So Dr Fowler and her colleagues did just that.
The researchers said their work suggests NMIIA is the predominant RBC NMII isoform, and NMIIA motor activity regulates interactions with the spectrin–F-actin network to control RBCs’ shape and deformability.
Specifically, NMIIA forms bipolar filaments in RBCs, and these filaments associate with F-actins via their motor domains. It is through these interactions that NMIIA contractile forces promote membrane tension to maintain RBC shape and deformability.
To test these findings, the researchers treated RBCs with a compound called blebbistatin, which inhibits NMII motor activity.
After treatment, the team observed a decrease in NMIIA filaments associated with the RBC membrane and evidence of decreased membrane tension. The treated RBCs became elongated and showed a reduction in biconcavity as well as an increase in deformability.
The researchers believe this work could have applications for diseases in which RBCs are deformed. In fact, the team thinks inhibiting NMIIA in RBCs might prove useful for treating patients with sickle cell disease, as it could restore some elasticity to the patients’ RBCs.
Going forward, the researchers hope to learn more about what regulates NMIIA’s activity in RBCs and other cells.
New research indicates that non-muscle myosin II-A (NMIIA) plays a key role in maintaining red blood cell (RBC) shape and deformability.
Researchers found evidence to suggest that NMIIA forms filaments in RBCs, and specialized regions at both ends of the filaments can pull on actin to control the stiffness of the cell membrane.
“You need active contraction on the cell membrane, similar to how muscles contract,” explained study author Velia Fowler, PhD, of The Scripps Research Institute in La Jolla, California.
“The myosin pulls on the actin to provide tension in the membrane, and then that tension maintains the biconcave shape.”
Dr Fowler and her colleagues described these findings in PNAS.
The researchers noted that RBC shape and deformability depend upon the membrane skeleton—a network of actin filaments (F-actin) cross-linked by spectrin tetramers.
And although NMII motors are known to exert force on F-actin networks to control cell shapes, no one had investigated a function for NMII contractility in the spectrin–F-actin network of RBCs. So Dr Fowler and her colleagues did just that.
The researchers said their work suggests NMIIA is the predominant RBC NMII isoform, and NMIIA motor activity regulates interactions with the spectrin–F-actin network to control RBCs’ shape and deformability.
Specifically, NMIIA forms bipolar filaments in RBCs, and these filaments associate with F-actins via their motor domains. It is through these interactions that NMIIA contractile forces promote membrane tension to maintain RBC shape and deformability.
To test these findings, the researchers treated RBCs with a compound called blebbistatin, which inhibits NMII motor activity.
After treatment, the team observed a decrease in NMIIA filaments associated with the RBC membrane and evidence of decreased membrane tension. The treated RBCs became elongated and showed a reduction in biconcavity as well as an increase in deformability.
The researchers believe this work could have applications for diseases in which RBCs are deformed. In fact, the team thinks inhibiting NMIIA in RBCs might prove useful for treating patients with sickle cell disease, as it could restore some elasticity to the patients’ RBCs.
Going forward, the researchers hope to learn more about what regulates NMIIA’s activity in RBCs and other cells.
Screening may reduce prevalence of MM
Targeted screening could potentially reduce the prevalence of multiple myeloma (MM), according to research published in JCO Clinical Cancer Informatics.
Researchers found that screening for monoclonal gammopathy of undetermined significance (MGUS) might reduce the risk of MM in individuals with a high lifetime risk of MGUS, which includes men, African Americans, and people with a family history of MM.
The researchers said patients who screen positive for MGUS could seek medical care early and try strategies such as aspirin, metformin, or weight reduction to potentially reduce their risk of progression from MGUS to MM.
However, additional studies are needed to confirm the effectiveness of aspirin, metformin, and weight-loss strategies in preventing MGUS progression.
“Screening for MGUS may have significant benefits by lowering the incidence of multiple myeloma, provided that effective and non-toxic interventions can be identified,” said study author Philipp Altrock, PhD, of Moffitt Cancer Center in Tampa, Florida.
Dr Altrock and his colleagues performed a series of computational modeling experiments to determine the best screening strategies in different groups of patients. The goal was to determine when screening should begin, how often it should occur, and in which individuals it could be most effective.
The researchers designed their model to predict the progression of MGUS to MM, the changes in MGUS and MM prevalence, and the annual follow-up mortality due to disease.
The team found evidence to suggest that screening strategies could reduce the risk of progression and the prevalence of MM. This effect was more pronounced in individuals who had a higher risk of MGUS.
Modeling suggested the prevalence of MM could be reduced by 19% in patients who begin screening at age 55 and have follow-up screening every 6 years.
A similar reduction in prevalence could also be achieved by starting screening at age 65 and following up every 2 years.
“Regular screening of MGUS candidates should start as early as possible, with biannual follow-up, and focus on high-risk individuals, especially with a family history of multiple myeloma or in groups with a strong indication of MGUS progression,” Dr Altrock said.
Targeted screening could potentially reduce the prevalence of multiple myeloma (MM), according to research published in JCO Clinical Cancer Informatics.
Researchers found that screening for monoclonal gammopathy of undetermined significance (MGUS) might reduce the risk of MM in individuals with a high lifetime risk of MGUS, which includes men, African Americans, and people with a family history of MM.
The researchers said patients who screen positive for MGUS could seek medical care early and try strategies such as aspirin, metformin, or weight reduction to potentially reduce their risk of progression from MGUS to MM.
However, additional studies are needed to confirm the effectiveness of aspirin, metformin, and weight-loss strategies in preventing MGUS progression.
“Screening for MGUS may have significant benefits by lowering the incidence of multiple myeloma, provided that effective and non-toxic interventions can be identified,” said study author Philipp Altrock, PhD, of Moffitt Cancer Center in Tampa, Florida.
Dr Altrock and his colleagues performed a series of computational modeling experiments to determine the best screening strategies in different groups of patients. The goal was to determine when screening should begin, how often it should occur, and in which individuals it could be most effective.
The researchers designed their model to predict the progression of MGUS to MM, the changes in MGUS and MM prevalence, and the annual follow-up mortality due to disease.
The team found evidence to suggest that screening strategies could reduce the risk of progression and the prevalence of MM. This effect was more pronounced in individuals who had a higher risk of MGUS.
Modeling suggested the prevalence of MM could be reduced by 19% in patients who begin screening at age 55 and have follow-up screening every 6 years.
A similar reduction in prevalence could also be achieved by starting screening at age 65 and following up every 2 years.
“Regular screening of MGUS candidates should start as early as possible, with biannual follow-up, and focus on high-risk individuals, especially with a family history of multiple myeloma or in groups with a strong indication of MGUS progression,” Dr Altrock said.
Targeted screening could potentially reduce the prevalence of multiple myeloma (MM), according to research published in JCO Clinical Cancer Informatics.
Researchers found that screening for monoclonal gammopathy of undetermined significance (MGUS) might reduce the risk of MM in individuals with a high lifetime risk of MGUS, which includes men, African Americans, and people with a family history of MM.
The researchers said patients who screen positive for MGUS could seek medical care early and try strategies such as aspirin, metformin, or weight reduction to potentially reduce their risk of progression from MGUS to MM.
However, additional studies are needed to confirm the effectiveness of aspirin, metformin, and weight-loss strategies in preventing MGUS progression.
“Screening for MGUS may have significant benefits by lowering the incidence of multiple myeloma, provided that effective and non-toxic interventions can be identified,” said study author Philipp Altrock, PhD, of Moffitt Cancer Center in Tampa, Florida.
Dr Altrock and his colleagues performed a series of computational modeling experiments to determine the best screening strategies in different groups of patients. The goal was to determine when screening should begin, how often it should occur, and in which individuals it could be most effective.
The researchers designed their model to predict the progression of MGUS to MM, the changes in MGUS and MM prevalence, and the annual follow-up mortality due to disease.
The team found evidence to suggest that screening strategies could reduce the risk of progression and the prevalence of MM. This effect was more pronounced in individuals who had a higher risk of MGUS.
Modeling suggested the prevalence of MM could be reduced by 19% in patients who begin screening at age 55 and have follow-up screening every 6 years.
A similar reduction in prevalence could also be achieved by starting screening at age 65 and following up every 2 years.
“Regular screening of MGUS candidates should start as early as possible, with biannual follow-up, and focus on high-risk individuals, especially with a family history of multiple myeloma or in groups with a strong indication of MGUS progression,” Dr Altrock said.