User login
DSCs ‘promising’ for severe acute GVHD
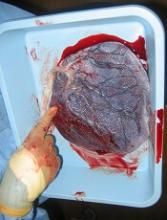
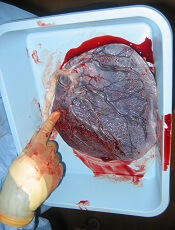
Placenta-derived decidua stromal cells (DSCs) can treat severe acute graft-versus-host disease (GVHD), according to a small study.
When given in the optimal way, DSCs produced GVHD responses in all patients, and the 1-year survival rate was 76%.
Steroid-refractory (SR) patients who received DSCs had superior 1-year survival rates compared to SR historical controls and SR patients who received mesenchymal stem cells (MSCs).
Olle Ringdén, MD, PhD, of Karolinska Institutet in Huddinge, Sweden, and his colleagues reported these findings in STEM CELLS Translational Medicine.
“There were a couple of things that led us to be curious about [DSCs as a treatment for GVHD],” Dr Ringdén said. “First, placenta plays an important role in helping the mother’s body tolerate the developing fetus.”
“[S]econd, placenta has been used in Africa for 100 years to successfully treat burn injuries. This speaks somewhat to its effectiveness and safety. We also found that placenta-derived DSCs are immunosuppressive in vitro and in vivo, which led us to wonder if they might cure severe acute GVHD.”
To test their theory, the team conducted a study of 38 patients with severe acute GVHD, including 25 SR patients.
The patients received DSCs in 1 of 2 groups. In group 1 (n=17), DSCs were infused in buffer supplemented with 10% AB plasma. In group 2 (n=21), the buffer was supplemented with 5% albumin.
Group 1 received significantly fewer infusions than group 2—1 (range, 1-5) and 2 (range, 1-6), respectively (P=0.002). But group 1 had a significantly higher median cell dose—2.0 x 106 DSCs/kg (range, 0.9-2.8) vs 1.2 x 106 DSCs/kg (range, 0.9-2.9; P<0.001).
Cell passage was significantly lower in group 1 than group 2—2 (range, 2-4) and 4 (range, 2-4), respectively (P<0.001). And cell viability was significantly lower in group 1 than 2—90% (range, 70-97) and 95% (range, 69-100), respectively (P<0.001).
Patients
There were no significant differences in baseline characteristics between groups 1 and 2. The median ages were 54.5 (range, 0.9-65.6) and 48.9 (range, 1.6-72.4), respectively.
Most patients were male (9 in group 1 and 16 in group 2), and most had malignant disease (14 and 17, respectively).
The most common graft source was peripheral blood stem cells (14 in group 1 and 16 in group 2), and most patients had a matched, unrelated donor (10 and 14, respectively).
Most patients received reduced-intensity conditioning (9 in group 1 and 17 in group 2) and GVHD prophylaxis with cyclosporine/methotrexate (13 in both groups).
All cases of GVHD were localized to the gut. Fifteen patients in each group had grade 3 GVHD. Two patients in group 1 and 6 in group 2 had grade 2 GVHD.
Results
Responses and survival rates were superior in group 2, but there was no significant difference in relapse or chronic GVHD between the groups.
In group 1, 7 patients did not respond, 5 had a partial response, and 5 had a complete response. In group 2, all patients responded, 10 with a partial response and 11 with a complete response (between-group difference, P=0.01).
The 1-year survival rate was 47% in group 1 and 76% in group 2 (P=0.016). The rate of GVHD-related mortality was 41% and 5%, respectively (P=0.003).
The cumulative incidence of chronic GVHD at 1.5 years was 36% in group 1 and 31% in group 2. The relapse rate was 29% and 18%, respectively.
The researchers compared SR patients in groups 1 (n=13) and 2 (n=11) to SR patients treated with bone marrow-derived MSCs (n=15) and SR historical controls (n=32).
The 1-year survival rate was 73% in SR group 2, which was significantly higher than the 31% survival rate in SR group 1 (P=0.02), the 20% rate in SR MSC recipients (P=0.0015), and the 3% rate in SR historical controls (P<0.001).
Severe adverse events in the DSC recipients included relapse (n=8), pneumonia (n=5), proven or probable invasive fungal infection (n=6), bacterial infection (n=2), graft failure (n=3), multiple organ failure (n=1), viral infection (n=2), central nervous system complications (n=2), septicemia (n=2), skin squamous cell carcinoma (n=2), and acute pancreatitis (n=1).
Causes of death in DSC recipients included acute GVHD (n=9), relapse (n=2), bacterial infection (n=2), invasive fungal infection (n=1), liver failure (n=1), hemorrhage (n=1), and secondary malignancy (n=1).
“Collectively, we think these data demonstrate that DSCs are a promising treatment for severe acute GVHD,” Dr Ringdén said. “But it was a small patient group, so, to further assess safety and efficacy, a larger, prospective trial will be necessary.”
“If an effective therapy for severe acute GVHD is indeed found and validated, it will increase the usefulness of stem cell transplantation with a possible broadening of indications.”
Placenta-derived decidua stromal cells (DSCs) can treat severe acute graft-versus-host disease (GVHD), according to a small study.
When given in the optimal way, DSCs produced GVHD responses in all patients, and the 1-year survival rate was 76%.
Steroid-refractory (SR) patients who received DSCs had superior 1-year survival rates compared to SR historical controls and SR patients who received mesenchymal stem cells (MSCs).
Olle Ringdén, MD, PhD, of Karolinska Institutet in Huddinge, Sweden, and his colleagues reported these findings in STEM CELLS Translational Medicine.
“There were a couple of things that led us to be curious about [DSCs as a treatment for GVHD],” Dr Ringdén said. “First, placenta plays an important role in helping the mother’s body tolerate the developing fetus.”
“[S]econd, placenta has been used in Africa for 100 years to successfully treat burn injuries. This speaks somewhat to its effectiveness and safety. We also found that placenta-derived DSCs are immunosuppressive in vitro and in vivo, which led us to wonder if they might cure severe acute GVHD.”
To test their theory, the team conducted a study of 38 patients with severe acute GVHD, including 25 SR patients.
The patients received DSCs in 1 of 2 groups. In group 1 (n=17), DSCs were infused in buffer supplemented with 10% AB plasma. In group 2 (n=21), the buffer was supplemented with 5% albumin.
Group 1 received significantly fewer infusions than group 2—1 (range, 1-5) and 2 (range, 1-6), respectively (P=0.002). But group 1 had a significantly higher median cell dose—2.0 x 106 DSCs/kg (range, 0.9-2.8) vs 1.2 x 106 DSCs/kg (range, 0.9-2.9; P<0.001).
Cell passage was significantly lower in group 1 than group 2—2 (range, 2-4) and 4 (range, 2-4), respectively (P<0.001). And cell viability was significantly lower in group 1 than 2—90% (range, 70-97) and 95% (range, 69-100), respectively (P<0.001).
Patients
There were no significant differences in baseline characteristics between groups 1 and 2. The median ages were 54.5 (range, 0.9-65.6) and 48.9 (range, 1.6-72.4), respectively.
Most patients were male (9 in group 1 and 16 in group 2), and most had malignant disease (14 and 17, respectively).
The most common graft source was peripheral blood stem cells (14 in group 1 and 16 in group 2), and most patients had a matched, unrelated donor (10 and 14, respectively).
Most patients received reduced-intensity conditioning (9 in group 1 and 17 in group 2) and GVHD prophylaxis with cyclosporine/methotrexate (13 in both groups).
All cases of GVHD were localized to the gut. Fifteen patients in each group had grade 3 GVHD. Two patients in group 1 and 6 in group 2 had grade 2 GVHD.
Results
Responses and survival rates were superior in group 2, but there was no significant difference in relapse or chronic GVHD between the groups.
In group 1, 7 patients did not respond, 5 had a partial response, and 5 had a complete response. In group 2, all patients responded, 10 with a partial response and 11 with a complete response (between-group difference, P=0.01).
The 1-year survival rate was 47% in group 1 and 76% in group 2 (P=0.016). The rate of GVHD-related mortality was 41% and 5%, respectively (P=0.003).
The cumulative incidence of chronic GVHD at 1.5 years was 36% in group 1 and 31% in group 2. The relapse rate was 29% and 18%, respectively.
The researchers compared SR patients in groups 1 (n=13) and 2 (n=11) to SR patients treated with bone marrow-derived MSCs (n=15) and SR historical controls (n=32).
The 1-year survival rate was 73% in SR group 2, which was significantly higher than the 31% survival rate in SR group 1 (P=0.02), the 20% rate in SR MSC recipients (P=0.0015), and the 3% rate in SR historical controls (P<0.001).
Severe adverse events in the DSC recipients included relapse (n=8), pneumonia (n=5), proven or probable invasive fungal infection (n=6), bacterial infection (n=2), graft failure (n=3), multiple organ failure (n=1), viral infection (n=2), central nervous system complications (n=2), septicemia (n=2), skin squamous cell carcinoma (n=2), and acute pancreatitis (n=1).
Causes of death in DSC recipients included acute GVHD (n=9), relapse (n=2), bacterial infection (n=2), invasive fungal infection (n=1), liver failure (n=1), hemorrhage (n=1), and secondary malignancy (n=1).
“Collectively, we think these data demonstrate that DSCs are a promising treatment for severe acute GVHD,” Dr Ringdén said. “But it was a small patient group, so, to further assess safety and efficacy, a larger, prospective trial will be necessary.”
“If an effective therapy for severe acute GVHD is indeed found and validated, it will increase the usefulness of stem cell transplantation with a possible broadening of indications.”
Placenta-derived decidua stromal cells (DSCs) can treat severe acute graft-versus-host disease (GVHD), according to a small study.
When given in the optimal way, DSCs produced GVHD responses in all patients, and the 1-year survival rate was 76%.
Steroid-refractory (SR) patients who received DSCs had superior 1-year survival rates compared to SR historical controls and SR patients who received mesenchymal stem cells (MSCs).
Olle Ringdén, MD, PhD, of Karolinska Institutet in Huddinge, Sweden, and his colleagues reported these findings in STEM CELLS Translational Medicine.
“There were a couple of things that led us to be curious about [DSCs as a treatment for GVHD],” Dr Ringdén said. “First, placenta plays an important role in helping the mother’s body tolerate the developing fetus.”
“[S]econd, placenta has been used in Africa for 100 years to successfully treat burn injuries. This speaks somewhat to its effectiveness and safety. We also found that placenta-derived DSCs are immunosuppressive in vitro and in vivo, which led us to wonder if they might cure severe acute GVHD.”
To test their theory, the team conducted a study of 38 patients with severe acute GVHD, including 25 SR patients.
The patients received DSCs in 1 of 2 groups. In group 1 (n=17), DSCs were infused in buffer supplemented with 10% AB plasma. In group 2 (n=21), the buffer was supplemented with 5% albumin.
Group 1 received significantly fewer infusions than group 2—1 (range, 1-5) and 2 (range, 1-6), respectively (P=0.002). But group 1 had a significantly higher median cell dose—2.0 x 106 DSCs/kg (range, 0.9-2.8) vs 1.2 x 106 DSCs/kg (range, 0.9-2.9; P<0.001).
Cell passage was significantly lower in group 1 than group 2—2 (range, 2-4) and 4 (range, 2-4), respectively (P<0.001). And cell viability was significantly lower in group 1 than 2—90% (range, 70-97) and 95% (range, 69-100), respectively (P<0.001).
Patients
There were no significant differences in baseline characteristics between groups 1 and 2. The median ages were 54.5 (range, 0.9-65.6) and 48.9 (range, 1.6-72.4), respectively.
Most patients were male (9 in group 1 and 16 in group 2), and most had malignant disease (14 and 17, respectively).
The most common graft source was peripheral blood stem cells (14 in group 1 and 16 in group 2), and most patients had a matched, unrelated donor (10 and 14, respectively).
Most patients received reduced-intensity conditioning (9 in group 1 and 17 in group 2) and GVHD prophylaxis with cyclosporine/methotrexate (13 in both groups).
All cases of GVHD were localized to the gut. Fifteen patients in each group had grade 3 GVHD. Two patients in group 1 and 6 in group 2 had grade 2 GVHD.
Results
Responses and survival rates were superior in group 2, but there was no significant difference in relapse or chronic GVHD between the groups.
In group 1, 7 patients did not respond, 5 had a partial response, and 5 had a complete response. In group 2, all patients responded, 10 with a partial response and 11 with a complete response (between-group difference, P=0.01).
The 1-year survival rate was 47% in group 1 and 76% in group 2 (P=0.016). The rate of GVHD-related mortality was 41% and 5%, respectively (P=0.003).
The cumulative incidence of chronic GVHD at 1.5 years was 36% in group 1 and 31% in group 2. The relapse rate was 29% and 18%, respectively.
The researchers compared SR patients in groups 1 (n=13) and 2 (n=11) to SR patients treated with bone marrow-derived MSCs (n=15) and SR historical controls (n=32).
The 1-year survival rate was 73% in SR group 2, which was significantly higher than the 31% survival rate in SR group 1 (P=0.02), the 20% rate in SR MSC recipients (P=0.0015), and the 3% rate in SR historical controls (P<0.001).
Severe adverse events in the DSC recipients included relapse (n=8), pneumonia (n=5), proven or probable invasive fungal infection (n=6), bacterial infection (n=2), graft failure (n=3), multiple organ failure (n=1), viral infection (n=2), central nervous system complications (n=2), septicemia (n=2), skin squamous cell carcinoma (n=2), and acute pancreatitis (n=1).
Causes of death in DSC recipients included acute GVHD (n=9), relapse (n=2), bacterial infection (n=2), invasive fungal infection (n=1), liver failure (n=1), hemorrhage (n=1), and secondary malignancy (n=1).
“Collectively, we think these data demonstrate that DSCs are a promising treatment for severe acute GVHD,” Dr Ringdén said. “But it was a small patient group, so, to further assess safety and efficacy, a larger, prospective trial will be necessary.”
“If an effective therapy for severe acute GVHD is indeed found and validated, it will increase the usefulness of stem cell transplantation with a possible broadening of indications.”
Clopidogrel proves noninferior to Plavix
Generic clopidogrel is noninferior to the brand-name antiplatelet drug Plavix, according to research published in Circulation: Cardiovascular Quality and Outcomes.
When a Canadian health system switched from prescribing Plavix to generic clopidogrel, patients with acute coronary syndrome (ACS) were no more likely to die or experience ACS recurrence within a year.
In addition, there were no significant differences between the Plavix and clopidogrel groups in the percentage of patients who were hospitalized for any reason, had a stroke or transient ischemic attack, or experienced bleeding.
“People can safely use generic clopidogrel,” said study author Dennis T. Ko, MD, of the Institute for Clinical Evaluative Sciences in Toronto, Ontario, Canada.
“This large and real-world study should be reassuring to physicians and healthcare organizations who have been concerned about changing what is prescribed.”
Dr Ko and his colleagues compared outcomes in patients who were prescribed clopidogrel after hospitalization for ACS in Ontario, Canada, where the Ministry of Health began to automatically substitute generic clopidogrel for Plavix once the brand name drug’s patent expired in 2012.
Between 2009 and 2014, 12,643 patients were prescribed Plavix, and 11,887 were prescribed generic clopidogrel.
“There are quite a few different generic brands,” said study author Cynthia Jackevicius, PharmD, of Western University of Health Sciences in Pomona, California.
“In this study, we considered them as a group but later found no differences in outcome when we compared between different generics.”
Results
The study’s primary outcome—a composite of death and hospitalization for ACS at 1 year—was met by 17.6% of patients prescribed Plavix and 17.9% of patients prescribed clopidogrel. The hazard ratio (HR) was 1.02 (95% confidence interval [CI], 0.96–1.08; P=0.005 for noninferiority, P=0.619 for superiority).
The 1-year mortality rate was 10.5% in the Plavix group and 11.2% in the clopidogrel group (HR=1.07; 95% CI, 0.99–1.15, P=0.210 for noninferiority, P=0.114 for superiority).
And hospitalization for ACS occurred in 9.7% and 9.2%, respectively (HR=0.94; 95% CI, 0.87–1.03, P<0.001 for noninferiority, P=0.190 for superiority).
Hospitalization for any reason occurred in 39.4% of patients in the Plavix group and 39.8% of those in the clopidogrel group (HR=1.02; 95% CI, 0.97–1.06, P<0.001 for noninferiority, P=0.482 for superiority).
Hospitalization for stroke or transient ischemic attack occurred in 1.5% and 1.4%, respectively (HR=0.92; 95% CI, 0.74–1.15, P=0.056 for noninferiority, P=0.455 for superiority).
And hospitalization for bleeding occurred in 2.3% and 2.7%, respectively (HR=1.17; 95% CI, 0.99–1.39, P=0.772 for noninferiority, P=0.063 for superiority).
The researchers noted that, in 2010, Plavix cost about $2.58 Canadian dollars per pill. It was projected to cost the Ontario Drug Benefit Program $72.8 million by 2012.
By switching to generic clopidogrel, which costs $0.39 per pill in 2018, the expense was $19 million Canadian dollars.
“Plavix was one of the most commonly used drugs in cardiology,” Dr Ko said, “so switching to generics can reduce a lot of cost for individuals and health systems.”
Generic clopidogrel is noninferior to the brand-name antiplatelet drug Plavix, according to research published in Circulation: Cardiovascular Quality and Outcomes.
When a Canadian health system switched from prescribing Plavix to generic clopidogrel, patients with acute coronary syndrome (ACS) were no more likely to die or experience ACS recurrence within a year.
In addition, there were no significant differences between the Plavix and clopidogrel groups in the percentage of patients who were hospitalized for any reason, had a stroke or transient ischemic attack, or experienced bleeding.
“People can safely use generic clopidogrel,” said study author Dennis T. Ko, MD, of the Institute for Clinical Evaluative Sciences in Toronto, Ontario, Canada.
“This large and real-world study should be reassuring to physicians and healthcare organizations who have been concerned about changing what is prescribed.”
Dr Ko and his colleagues compared outcomes in patients who were prescribed clopidogrel after hospitalization for ACS in Ontario, Canada, where the Ministry of Health began to automatically substitute generic clopidogrel for Plavix once the brand name drug’s patent expired in 2012.
Between 2009 and 2014, 12,643 patients were prescribed Plavix, and 11,887 were prescribed generic clopidogrel.
“There are quite a few different generic brands,” said study author Cynthia Jackevicius, PharmD, of Western University of Health Sciences in Pomona, California.
“In this study, we considered them as a group but later found no differences in outcome when we compared between different generics.”
Results
The study’s primary outcome—a composite of death and hospitalization for ACS at 1 year—was met by 17.6% of patients prescribed Plavix and 17.9% of patients prescribed clopidogrel. The hazard ratio (HR) was 1.02 (95% confidence interval [CI], 0.96–1.08; P=0.005 for noninferiority, P=0.619 for superiority).
The 1-year mortality rate was 10.5% in the Plavix group and 11.2% in the clopidogrel group (HR=1.07; 95% CI, 0.99–1.15, P=0.210 for noninferiority, P=0.114 for superiority).
And hospitalization for ACS occurred in 9.7% and 9.2%, respectively (HR=0.94; 95% CI, 0.87–1.03, P<0.001 for noninferiority, P=0.190 for superiority).
Hospitalization for any reason occurred in 39.4% of patients in the Plavix group and 39.8% of those in the clopidogrel group (HR=1.02; 95% CI, 0.97–1.06, P<0.001 for noninferiority, P=0.482 for superiority).
Hospitalization for stroke or transient ischemic attack occurred in 1.5% and 1.4%, respectively (HR=0.92; 95% CI, 0.74–1.15, P=0.056 for noninferiority, P=0.455 for superiority).
And hospitalization for bleeding occurred in 2.3% and 2.7%, respectively (HR=1.17; 95% CI, 0.99–1.39, P=0.772 for noninferiority, P=0.063 for superiority).
The researchers noted that, in 2010, Plavix cost about $2.58 Canadian dollars per pill. It was projected to cost the Ontario Drug Benefit Program $72.8 million by 2012.
By switching to generic clopidogrel, which costs $0.39 per pill in 2018, the expense was $19 million Canadian dollars.
“Plavix was one of the most commonly used drugs in cardiology,” Dr Ko said, “so switching to generics can reduce a lot of cost for individuals and health systems.”
Generic clopidogrel is noninferior to the brand-name antiplatelet drug Plavix, according to research published in Circulation: Cardiovascular Quality and Outcomes.
When a Canadian health system switched from prescribing Plavix to generic clopidogrel, patients with acute coronary syndrome (ACS) were no more likely to die or experience ACS recurrence within a year.
In addition, there were no significant differences between the Plavix and clopidogrel groups in the percentage of patients who were hospitalized for any reason, had a stroke or transient ischemic attack, or experienced bleeding.
“People can safely use generic clopidogrel,” said study author Dennis T. Ko, MD, of the Institute for Clinical Evaluative Sciences in Toronto, Ontario, Canada.
“This large and real-world study should be reassuring to physicians and healthcare organizations who have been concerned about changing what is prescribed.”
Dr Ko and his colleagues compared outcomes in patients who were prescribed clopidogrel after hospitalization for ACS in Ontario, Canada, where the Ministry of Health began to automatically substitute generic clopidogrel for Plavix once the brand name drug’s patent expired in 2012.
Between 2009 and 2014, 12,643 patients were prescribed Plavix, and 11,887 were prescribed generic clopidogrel.
“There are quite a few different generic brands,” said study author Cynthia Jackevicius, PharmD, of Western University of Health Sciences in Pomona, California.
“In this study, we considered them as a group but later found no differences in outcome when we compared between different generics.”
Results
The study’s primary outcome—a composite of death and hospitalization for ACS at 1 year—was met by 17.6% of patients prescribed Plavix and 17.9% of patients prescribed clopidogrel. The hazard ratio (HR) was 1.02 (95% confidence interval [CI], 0.96–1.08; P=0.005 for noninferiority, P=0.619 for superiority).
The 1-year mortality rate was 10.5% in the Plavix group and 11.2% in the clopidogrel group (HR=1.07; 95% CI, 0.99–1.15, P=0.210 for noninferiority, P=0.114 for superiority).
And hospitalization for ACS occurred in 9.7% and 9.2%, respectively (HR=0.94; 95% CI, 0.87–1.03, P<0.001 for noninferiority, P=0.190 for superiority).
Hospitalization for any reason occurred in 39.4% of patients in the Plavix group and 39.8% of those in the clopidogrel group (HR=1.02; 95% CI, 0.97–1.06, P<0.001 for noninferiority, P=0.482 for superiority).
Hospitalization for stroke or transient ischemic attack occurred in 1.5% and 1.4%, respectively (HR=0.92; 95% CI, 0.74–1.15, P=0.056 for noninferiority, P=0.455 for superiority).
And hospitalization for bleeding occurred in 2.3% and 2.7%, respectively (HR=1.17; 95% CI, 0.99–1.39, P=0.772 for noninferiority, P=0.063 for superiority).
The researchers noted that, in 2010, Plavix cost about $2.58 Canadian dollars per pill. It was projected to cost the Ontario Drug Benefit Program $72.8 million by 2012.
By switching to generic clopidogrel, which costs $0.39 per pill in 2018, the expense was $19 million Canadian dollars.
“Plavix was one of the most commonly used drugs in cardiology,” Dr Ko said, “so switching to generics can reduce a lot of cost for individuals and health systems.”
Reversal agent exhibits efficacy in patients with major bleeding
ORLANDO—Interim trial results suggest andexanet alfa can reverse the activity of factor Xa inhibitors in patients with acute major bleeding.
Andexanet alfa reduced median anti-factor Xa inhibitor activity by 91% in patients taking apixaban, 88% in those taking rivaroxaban, and 75% in patients taking enoxaparin.
Eleven percent of patients experienced thrombotic events, and 12% of patients died.
Stuart J. Connolly, MD, of McMaster University in Hamilton, Ontario, Canada, presented these results at the American College of Cardiology’s 67th Annual Scientific Session & Expo (ACC.18, abstract 409-14).
The trial, known as ANNEXA-4, was sponsored by Portola Pharmaceuticals, Inc.
“These data are particularly compelling when you consider the high-risk profile of the ANNEXA-4 population, which includes a substantial number of elderly patients presenting with intracranial hemorrhage and anticoagulated for venous thromboembolism, and the lack of any FDA- or EMA-approved reversal agent for these patients,” Dr Connolly said.
“The interim efficacy and safety data continue to support the promising role of AndexXa [the brand name for andexanet alfa] as an antidote to reverse anticoagulation in factor Xa-associated bleeding.”
Andexanet alfa is a recombinant modified factor Xa molecule designed to bind to and disable factor Xa inhibitors, thereby allowing factor Xa produced by the body to play its normal role in the formation of blood clots.
In earlier trials of healthy volunteers, andexanet alfa reversed the anticoagulant effect of factor Xa inhibitors without any significant safety problems.
ANNEXA-4 is an ongoing trial of andexanet alfa in patients experiencing major bleeding while taking factor Xa inhibitors.
Dr Connolly presented safety outcomes for 227 trial subjects and adjudicated efficacy outcomes for 132 subjects. All subjects presented with acute major bleeding within 18 hours of taking a factor Xa inhibitor, including apixaban, rivaroxaban, enoxaparin, and edoxaban.
The patients received andexanet alfa given as a bolus dose over 20 to 30 minutes, followed by a 2-hour (120 minute) infusion. The dosage was determined based on the specific factor Xa inhibitor the patients were taking and how long it had been since their last dose.
The patients were evaluated for 30 days after andexanet alfa administration.
Efficacy
The median age of the efficacy population (n=137) was 77, and 51% (n=70) were male. Indications for anticoagulation included atrial fibrillation (Afib, 76%, n=104), venous thromboembolism (VTE, 28%, n=38), and both Afib and VTE (4%, n=6).
Patients were receiving apixaban (n=68), rivaroxaban (n=54), or enoxaparin (n=10). None of these patients had received edoxaban.
Types of bleeding included intracranial hemorrhage (ICH, 57%, n=78), gastrointestinal (GI) bleeding (31%, n=43), and “other” bleeding (12%, n=16).
The researchers assessed andexanet alfa’s efficacy in terms of 2 co-primary endpoints—reduction in anti-factor Xa inhibitor activity and achievement of clinical hemostasis by 12 hours after administration. Hemostatic efficacy was assessed by an independent endpoint adjudication committee as “excellent,” “good,” or “poor/none.”
After the bolus dose of andexanet alfa, the median anti-factor Xa inhibitor activity was reduced by 91% for patients taking apixaban, 88% for those taking rivaroxaban, and 75% for those taking enoxaparin.
For patients taking rivaroxaban, the median anti-factor Xa inhibitor activity was reduced by 88% at the end of the bolus dose, 87% at the end of the infusion, 42% at 4 hours, 49% at 8 hours, and 60% at 12 hours.
For patients taking apixaban, the median anti-factor Xa inhibitor activity was reduced by 91% at the end of the bolus dose, 91% at the end of the infusion, 36% at 4 hours, 30% at 8 hours, and 35% at 12 hours.
Overall, 83% of patients had “excellent” or “good” clinical hemostasis, and 17% had “poor/none.”
Hemostasis was deemed excellent or good in 83% of patients on rivaroxaban, 82% of those on apixaban, and 80% of those on enoxaparin. It was excellent/good in 86% of patients with GI bleeding, 81% of patients with ICH, and 80% of patients with bleeding at other sites.
Safety
The median age of the safety population (n=227) was 77, and 52% (n=117) were male. Indications for anticoagulation included Afib (78%, n=178), VTE (23%, n=52), and both Afib and VTE (4%, n=8).
Patients were receiving apixaban (n=117), rivaroxaban (n=90), enoxaparin (n=17), and edoxaban (n=3). Types of bleeding included ICH (61%, n=139), GI (27%, n=62), and “other” (12%, n=26).
During the 30-day follow-up period, the rate of thrombotic events was 11% (n=24) for the entire population and 12% (n=17) among patients with ICH.
The mortality rate for all patients was 12% (n=27). Eleven deaths were due to cardiovascular causes.
According to Dr Connolly, these rates of adverse events are in line with what would be expected given the underlying medical condition of the patients in the trial and the fact that many (43%) had not resumed anticoagulant treatment in the 30-day follow-up period.
Two patients experienced an infusion reaction.
None of the patients developed antibodies to factor Xa or factor X, and there were no neutralizing antibodies to andexanet alfa.
ORLANDO—Interim trial results suggest andexanet alfa can reverse the activity of factor Xa inhibitors in patients with acute major bleeding.
Andexanet alfa reduced median anti-factor Xa inhibitor activity by 91% in patients taking apixaban, 88% in those taking rivaroxaban, and 75% in patients taking enoxaparin.
Eleven percent of patients experienced thrombotic events, and 12% of patients died.
Stuart J. Connolly, MD, of McMaster University in Hamilton, Ontario, Canada, presented these results at the American College of Cardiology’s 67th Annual Scientific Session & Expo (ACC.18, abstract 409-14).
The trial, known as ANNEXA-4, was sponsored by Portola Pharmaceuticals, Inc.
“These data are particularly compelling when you consider the high-risk profile of the ANNEXA-4 population, which includes a substantial number of elderly patients presenting with intracranial hemorrhage and anticoagulated for venous thromboembolism, and the lack of any FDA- or EMA-approved reversal agent for these patients,” Dr Connolly said.
“The interim efficacy and safety data continue to support the promising role of AndexXa [the brand name for andexanet alfa] as an antidote to reverse anticoagulation in factor Xa-associated bleeding.”
Andexanet alfa is a recombinant modified factor Xa molecule designed to bind to and disable factor Xa inhibitors, thereby allowing factor Xa produced by the body to play its normal role in the formation of blood clots.
In earlier trials of healthy volunteers, andexanet alfa reversed the anticoagulant effect of factor Xa inhibitors without any significant safety problems.
ANNEXA-4 is an ongoing trial of andexanet alfa in patients experiencing major bleeding while taking factor Xa inhibitors.
Dr Connolly presented safety outcomes for 227 trial subjects and adjudicated efficacy outcomes for 132 subjects. All subjects presented with acute major bleeding within 18 hours of taking a factor Xa inhibitor, including apixaban, rivaroxaban, enoxaparin, and edoxaban.
The patients received andexanet alfa given as a bolus dose over 20 to 30 minutes, followed by a 2-hour (120 minute) infusion. The dosage was determined based on the specific factor Xa inhibitor the patients were taking and how long it had been since their last dose.
The patients were evaluated for 30 days after andexanet alfa administration.
Efficacy
The median age of the efficacy population (n=137) was 77, and 51% (n=70) were male. Indications for anticoagulation included atrial fibrillation (Afib, 76%, n=104), venous thromboembolism (VTE, 28%, n=38), and both Afib and VTE (4%, n=6).
Patients were receiving apixaban (n=68), rivaroxaban (n=54), or enoxaparin (n=10). None of these patients had received edoxaban.
Types of bleeding included intracranial hemorrhage (ICH, 57%, n=78), gastrointestinal (GI) bleeding (31%, n=43), and “other” bleeding (12%, n=16).
The researchers assessed andexanet alfa’s efficacy in terms of 2 co-primary endpoints—reduction in anti-factor Xa inhibitor activity and achievement of clinical hemostasis by 12 hours after administration. Hemostatic efficacy was assessed by an independent endpoint adjudication committee as “excellent,” “good,” or “poor/none.”
After the bolus dose of andexanet alfa, the median anti-factor Xa inhibitor activity was reduced by 91% for patients taking apixaban, 88% for those taking rivaroxaban, and 75% for those taking enoxaparin.
For patients taking rivaroxaban, the median anti-factor Xa inhibitor activity was reduced by 88% at the end of the bolus dose, 87% at the end of the infusion, 42% at 4 hours, 49% at 8 hours, and 60% at 12 hours.
For patients taking apixaban, the median anti-factor Xa inhibitor activity was reduced by 91% at the end of the bolus dose, 91% at the end of the infusion, 36% at 4 hours, 30% at 8 hours, and 35% at 12 hours.
Overall, 83% of patients had “excellent” or “good” clinical hemostasis, and 17% had “poor/none.”
Hemostasis was deemed excellent or good in 83% of patients on rivaroxaban, 82% of those on apixaban, and 80% of those on enoxaparin. It was excellent/good in 86% of patients with GI bleeding, 81% of patients with ICH, and 80% of patients with bleeding at other sites.
Safety
The median age of the safety population (n=227) was 77, and 52% (n=117) were male. Indications for anticoagulation included Afib (78%, n=178), VTE (23%, n=52), and both Afib and VTE (4%, n=8).
Patients were receiving apixaban (n=117), rivaroxaban (n=90), enoxaparin (n=17), and edoxaban (n=3). Types of bleeding included ICH (61%, n=139), GI (27%, n=62), and “other” (12%, n=26).
During the 30-day follow-up period, the rate of thrombotic events was 11% (n=24) for the entire population and 12% (n=17) among patients with ICH.
The mortality rate for all patients was 12% (n=27). Eleven deaths were due to cardiovascular causes.
According to Dr Connolly, these rates of adverse events are in line with what would be expected given the underlying medical condition of the patients in the trial and the fact that many (43%) had not resumed anticoagulant treatment in the 30-day follow-up period.
Two patients experienced an infusion reaction.
None of the patients developed antibodies to factor Xa or factor X, and there were no neutralizing antibodies to andexanet alfa.
ORLANDO—Interim trial results suggest andexanet alfa can reverse the activity of factor Xa inhibitors in patients with acute major bleeding.
Andexanet alfa reduced median anti-factor Xa inhibitor activity by 91% in patients taking apixaban, 88% in those taking rivaroxaban, and 75% in patients taking enoxaparin.
Eleven percent of patients experienced thrombotic events, and 12% of patients died.
Stuart J. Connolly, MD, of McMaster University in Hamilton, Ontario, Canada, presented these results at the American College of Cardiology’s 67th Annual Scientific Session & Expo (ACC.18, abstract 409-14).
The trial, known as ANNEXA-4, was sponsored by Portola Pharmaceuticals, Inc.
“These data are particularly compelling when you consider the high-risk profile of the ANNEXA-4 population, which includes a substantial number of elderly patients presenting with intracranial hemorrhage and anticoagulated for venous thromboembolism, and the lack of any FDA- or EMA-approved reversal agent for these patients,” Dr Connolly said.
“The interim efficacy and safety data continue to support the promising role of AndexXa [the brand name for andexanet alfa] as an antidote to reverse anticoagulation in factor Xa-associated bleeding.”
Andexanet alfa is a recombinant modified factor Xa molecule designed to bind to and disable factor Xa inhibitors, thereby allowing factor Xa produced by the body to play its normal role in the formation of blood clots.
In earlier trials of healthy volunteers, andexanet alfa reversed the anticoagulant effect of factor Xa inhibitors without any significant safety problems.
ANNEXA-4 is an ongoing trial of andexanet alfa in patients experiencing major bleeding while taking factor Xa inhibitors.
Dr Connolly presented safety outcomes for 227 trial subjects and adjudicated efficacy outcomes for 132 subjects. All subjects presented with acute major bleeding within 18 hours of taking a factor Xa inhibitor, including apixaban, rivaroxaban, enoxaparin, and edoxaban.
The patients received andexanet alfa given as a bolus dose over 20 to 30 minutes, followed by a 2-hour (120 minute) infusion. The dosage was determined based on the specific factor Xa inhibitor the patients were taking and how long it had been since their last dose.
The patients were evaluated for 30 days after andexanet alfa administration.
Efficacy
The median age of the efficacy population (n=137) was 77, and 51% (n=70) were male. Indications for anticoagulation included atrial fibrillation (Afib, 76%, n=104), venous thromboembolism (VTE, 28%, n=38), and both Afib and VTE (4%, n=6).
Patients were receiving apixaban (n=68), rivaroxaban (n=54), or enoxaparin (n=10). None of these patients had received edoxaban.
Types of bleeding included intracranial hemorrhage (ICH, 57%, n=78), gastrointestinal (GI) bleeding (31%, n=43), and “other” bleeding (12%, n=16).
The researchers assessed andexanet alfa’s efficacy in terms of 2 co-primary endpoints—reduction in anti-factor Xa inhibitor activity and achievement of clinical hemostasis by 12 hours after administration. Hemostatic efficacy was assessed by an independent endpoint adjudication committee as “excellent,” “good,” or “poor/none.”
After the bolus dose of andexanet alfa, the median anti-factor Xa inhibitor activity was reduced by 91% for patients taking apixaban, 88% for those taking rivaroxaban, and 75% for those taking enoxaparin.
For patients taking rivaroxaban, the median anti-factor Xa inhibitor activity was reduced by 88% at the end of the bolus dose, 87% at the end of the infusion, 42% at 4 hours, 49% at 8 hours, and 60% at 12 hours.
For patients taking apixaban, the median anti-factor Xa inhibitor activity was reduced by 91% at the end of the bolus dose, 91% at the end of the infusion, 36% at 4 hours, 30% at 8 hours, and 35% at 12 hours.
Overall, 83% of patients had “excellent” or “good” clinical hemostasis, and 17% had “poor/none.”
Hemostasis was deemed excellent or good in 83% of patients on rivaroxaban, 82% of those on apixaban, and 80% of those on enoxaparin. It was excellent/good in 86% of patients with GI bleeding, 81% of patients with ICH, and 80% of patients with bleeding at other sites.
Safety
The median age of the safety population (n=227) was 77, and 52% (n=117) were male. Indications for anticoagulation included Afib (78%, n=178), VTE (23%, n=52), and both Afib and VTE (4%, n=8).
Patients were receiving apixaban (n=117), rivaroxaban (n=90), enoxaparin (n=17), and edoxaban (n=3). Types of bleeding included ICH (61%, n=139), GI (27%, n=62), and “other” (12%, n=26).
During the 30-day follow-up period, the rate of thrombotic events was 11% (n=24) for the entire population and 12% (n=17) among patients with ICH.
The mortality rate for all patients was 12% (n=27). Eleven deaths were due to cardiovascular causes.
According to Dr Connolly, these rates of adverse events are in line with what would be expected given the underlying medical condition of the patients in the trial and the fact that many (43%) had not resumed anticoagulant treatment in the 30-day follow-up period.
Two patients experienced an infusion reaction.
None of the patients developed antibodies to factor Xa or factor X, and there were no neutralizing antibodies to andexanet alfa.
Heme implicated in adverse transfusion outcomes
Free heme plays a key role in the adverse effects associated with massive transfusion of stored red blood cells (RBCs), according to researchers.
Experiments in a mouse model of trauma hemorrhage revealed a greater risk of mortality from bacterial pneumonia in mice that received transfusions of blood stored for 14 days, rather than fresh blood.
This greater risk was dependent upon free heme, which is released from RBCs during storage and upon transfusion.
In a study of human trauma patients, researchers found the amount of heme was proportional to the amount of blood transfused.
Brant M. Wagener, MD, PhD, of University of Alabama at Birmingham, and his colleagues detailed these findings in PLOS Medicine.
In the mouse model of trauma hemorrhage, the researchers resuscitated mice using either fresh blood (stored for 0 days) or blood stored for 2 weeks. (A 2-week storage of mouse blood approximates storage of human RBCs for 42 days.)
Two days after transfusion, the mice were challenged by instilling the lungs with the bacteria Pseudomonas aeruginosa.
Mice that received the stored blood had a significant increase in bacterial lung injury, as shown by higher mortality, and increased fluid accumulation and bacterial numbers in the lungs.
The researchers identified the connection between free heme and infection susceptibility/severity in 2 ways.
First, Pseudomonas aeruginosa-induced mortality was completely prevented by the addition of hemopexin, a scavenging protein that removes free heme from the blood.
Second, adding an inhibitor of toll-like receptor 4 (TLR4), or genetically removing TLR4 from mice, also prevented bacteria-induced mortality. Free heme—which is known to induce inflammatory injury to major organs in diseases like sickle cell or sepsis—acts, in part, by activating TLR4.
The researchers also found that transfusion with stored blood induced release of the inflammation mediator high mobility group box 1 (HMGB1). But an anti-HMGB1 antibody protected mice from bacteria-induced mortality.
The anti-HMGB1 antibody also restored macrophage-dependent phagocytosis of Pseudomonas aeruginosa in vitro.
Tissue culture experiments had revealed that free heme inhibits macrophages from ingesting Pseudomonas aeruginosa, and the addition of free heme increases permeability in endothelial cells.
Finally, in a 16-month study, the researchers found that human trauma-hemorrhage patients who received large amounts of transfused blood were also receiving amounts of free heme sufficient to overwhelm the normal amounts of hemopexin found in a person’s blood.
The researchers said this work underscores the need to confirm whether the storage age of transfused RBCs correlates with increasing levels of free heme after transfusion. The team would also like to establish whether patients with low ratios of hemopexin to free heme have a greater risk for adverse outcomes after massive transfusions.
Free heme plays a key role in the adverse effects associated with massive transfusion of stored red blood cells (RBCs), according to researchers.
Experiments in a mouse model of trauma hemorrhage revealed a greater risk of mortality from bacterial pneumonia in mice that received transfusions of blood stored for 14 days, rather than fresh blood.
This greater risk was dependent upon free heme, which is released from RBCs during storage and upon transfusion.
In a study of human trauma patients, researchers found the amount of heme was proportional to the amount of blood transfused.
Brant M. Wagener, MD, PhD, of University of Alabama at Birmingham, and his colleagues detailed these findings in PLOS Medicine.
In the mouse model of trauma hemorrhage, the researchers resuscitated mice using either fresh blood (stored for 0 days) or blood stored for 2 weeks. (A 2-week storage of mouse blood approximates storage of human RBCs for 42 days.)
Two days after transfusion, the mice were challenged by instilling the lungs with the bacteria Pseudomonas aeruginosa.
Mice that received the stored blood had a significant increase in bacterial lung injury, as shown by higher mortality, and increased fluid accumulation and bacterial numbers in the lungs.
The researchers identified the connection between free heme and infection susceptibility/severity in 2 ways.
First, Pseudomonas aeruginosa-induced mortality was completely prevented by the addition of hemopexin, a scavenging protein that removes free heme from the blood.
Second, adding an inhibitor of toll-like receptor 4 (TLR4), or genetically removing TLR4 from mice, also prevented bacteria-induced mortality. Free heme—which is known to induce inflammatory injury to major organs in diseases like sickle cell or sepsis—acts, in part, by activating TLR4.
The researchers also found that transfusion with stored blood induced release of the inflammation mediator high mobility group box 1 (HMGB1). But an anti-HMGB1 antibody protected mice from bacteria-induced mortality.
The anti-HMGB1 antibody also restored macrophage-dependent phagocytosis of Pseudomonas aeruginosa in vitro.
Tissue culture experiments had revealed that free heme inhibits macrophages from ingesting Pseudomonas aeruginosa, and the addition of free heme increases permeability in endothelial cells.
Finally, in a 16-month study, the researchers found that human trauma-hemorrhage patients who received large amounts of transfused blood were also receiving amounts of free heme sufficient to overwhelm the normal amounts of hemopexin found in a person’s blood.
The researchers said this work underscores the need to confirm whether the storage age of transfused RBCs correlates with increasing levels of free heme after transfusion. The team would also like to establish whether patients with low ratios of hemopexin to free heme have a greater risk for adverse outcomes after massive transfusions.
Free heme plays a key role in the adverse effects associated with massive transfusion of stored red blood cells (RBCs), according to researchers.
Experiments in a mouse model of trauma hemorrhage revealed a greater risk of mortality from bacterial pneumonia in mice that received transfusions of blood stored for 14 days, rather than fresh blood.
This greater risk was dependent upon free heme, which is released from RBCs during storage and upon transfusion.
In a study of human trauma patients, researchers found the amount of heme was proportional to the amount of blood transfused.
Brant M. Wagener, MD, PhD, of University of Alabama at Birmingham, and his colleagues detailed these findings in PLOS Medicine.
In the mouse model of trauma hemorrhage, the researchers resuscitated mice using either fresh blood (stored for 0 days) or blood stored for 2 weeks. (A 2-week storage of mouse blood approximates storage of human RBCs for 42 days.)
Two days after transfusion, the mice were challenged by instilling the lungs with the bacteria Pseudomonas aeruginosa.
Mice that received the stored blood had a significant increase in bacterial lung injury, as shown by higher mortality, and increased fluid accumulation and bacterial numbers in the lungs.
The researchers identified the connection between free heme and infection susceptibility/severity in 2 ways.
First, Pseudomonas aeruginosa-induced mortality was completely prevented by the addition of hemopexin, a scavenging protein that removes free heme from the blood.
Second, adding an inhibitor of toll-like receptor 4 (TLR4), or genetically removing TLR4 from mice, also prevented bacteria-induced mortality. Free heme—which is known to induce inflammatory injury to major organs in diseases like sickle cell or sepsis—acts, in part, by activating TLR4.
The researchers also found that transfusion with stored blood induced release of the inflammation mediator high mobility group box 1 (HMGB1). But an anti-HMGB1 antibody protected mice from bacteria-induced mortality.
The anti-HMGB1 antibody also restored macrophage-dependent phagocytosis of Pseudomonas aeruginosa in vitro.
Tissue culture experiments had revealed that free heme inhibits macrophages from ingesting Pseudomonas aeruginosa, and the addition of free heme increases permeability in endothelial cells.
Finally, in a 16-month study, the researchers found that human trauma-hemorrhage patients who received large amounts of transfused blood were also receiving amounts of free heme sufficient to overwhelm the normal amounts of hemopexin found in a person’s blood.
The researchers said this work underscores the need to confirm whether the storage age of transfused RBCs correlates with increasing levels of free heme after transfusion. The team would also like to establish whether patients with low ratios of hemopexin to free heme have a greater risk for adverse outcomes after massive transfusions.
Lymphoma, breast cancer survivors have greater risk of CHF
ORLANDO—Results of a retrospective study showed that survivors of lymphoma or breast cancer had a significantly greater risk of congestive heart failure (CHF) than patients who did not have cancer.
This increased risk was observed as early as a year after cancer diagnosis but was still present 20 years after diagnosis.
Overall, 1 in 10 cancer patients had CHF at the 20-year mark.
“The majority of patients do not develop heart failure, but our research helps us recognize the factors associated with it and the importance of appropriate heart care following cancer treatment,” said Carolyn Larsen, MD, of the Mayo Clinic in Rochester, Minnesota.
“Our research suggests that periodic cardiac imaging to monitor for heart damage may be needed for some cancer patients, even if they have no signs of heart damage initially after chemotherapy. Additionally, it emphasizes that working to live a heart-healthy lifestyle is important for cancer patients and survivors to reduce the overall risk of heart disease.”
Dr Larsen and her colleagues presented this research as a poster (abstract 1105-066) at the American College of Cardiology’s 67th Annual Scientific Session & Expo (ACC.18).
Patients
Using data from the Rochester Epidemiology Project, the researchers retrospectively tracked CHF cases in 900 cancer patients and 1550 non-cancer patients. Patients were treated in Olmsted County in Minnesota from 1985 to 2010.
For both patient groups, the median age at baseline was about 53, a little more than 90% of each group was white, and nearly 80% of each group was female.
Six to 7% of patients had diabetes, and about 30% of each group had hypertension. Thirty-eight percent of each group had hyperlipidemia, and 31% were obese.
Five percent of cancer patients and 2% of controls had coronary artery disease (P<0.001). This was the only significant difference in baseline characteristics.
Cancer patients had been diagnosed with non-Hodgkin lymphoma (28%), Hodgkin lymphoma (9%), or breast cancer (64%). Forty-seven percent had received radiation, including right chest (21%), left chest (23%), and mediastinal (4%).
Eighty-four percent of patients had received anthracycline therapy. The median doxorubicin isotoxic dose was 240 mg/m2.
At baseline, 12% of cancer patients were on beta-blockers, 8% were on angiotensin converting enzyme inhibitors, 4% were on angiotensin receptor blockers, and 11% were on statins.
Results
Cancer patients were more than 3 times as likely as controls to develop CHF. The hazard ratio (HR) was 3.6 (P<0.01) in an analysis adjusted for age, gender, diabetes, hypertension, coronary artery disease, dyslipidemia, and obesity at baseline.
The increased CHF risk among cancer patients was evident after the first year from cancer diagnosis and persisted at 20 years of follow-up.
“The risk of heart failure doesn’t go away after a couple of years,” Dr Larsen said. “It’s a long-term issue that patients need to discuss with their doctors and use as motivation to stay heart healthy.”
The incidence of CHF—in cancer patients and controls, respectively—was as follows:
- 1 year—1.5% vs 0.1%
- 5 years—3.1% vs 0.9%
- 10 years—5.0% vs 2%
- 20 years—10.1% vs 5.8%.
A multivariable analysis in the cancer patients revealed a few independent risk factors for CHF, including:
- Doxorubicin isotoxic dose ≥ 300 mg/m2 (HR=2.34, P=0.003)
- Age at diagnosis (HR=3.06 for age ≥ 80 vs 60-69, P=0.01)
- Coronary artery disease at diagnosis (HR=2.27, P=0.04)
- Diabetes mellitus at diagnosis (HR=2.39, P<0.01).
Dr Larsen said additional research is needed to determine why diabetes carries a greater risk than other traditional risk factors, such as high blood pressure, in this group.
Mitigating risk
These findings raise important questions about what the appropriate surveillance should be for heart problems post-cancer treatment, Dr Larsen said. She believes more frequent cardiac imaging may be warranted in some patients to detect signs of CHF earlier.
“It’s an area that needs to be better defined,” Dr Larsen said. “An echocardiogram is usually done 6 to 12 months after cancer treatment with an anthracycline, but how often should it be done after that? We need to be more vigilant in making sure we try to prevent or control heart issues post-cancer care, especially in light of the growing appreciation of the connection between some cancer treatments and heart disease.”
Dr Larsen also noted that patients themselves can play a role in decreasing their risk of CHF, even if they are starting at a disadvantage.
A heart-healthy lifestyle—maintaining a normal body weight, regular exercise, and controlling other risk factors such as high blood pressure, diabetes, and high cholesterol—can help lower the risk of heart disease and CHF.
“If patients know they have received a drug treatment that might increase their risk of heart failure, it’s even more important to take care of the aspects of their life that they can control to reduce their risk as much as possible and to work with their medical care team to detect issues as early as possible,” Dr Larsen said.
ORLANDO—Results of a retrospective study showed that survivors of lymphoma or breast cancer had a significantly greater risk of congestive heart failure (CHF) than patients who did not have cancer.
This increased risk was observed as early as a year after cancer diagnosis but was still present 20 years after diagnosis.
Overall, 1 in 10 cancer patients had CHF at the 20-year mark.
“The majority of patients do not develop heart failure, but our research helps us recognize the factors associated with it and the importance of appropriate heart care following cancer treatment,” said Carolyn Larsen, MD, of the Mayo Clinic in Rochester, Minnesota.
“Our research suggests that periodic cardiac imaging to monitor for heart damage may be needed for some cancer patients, even if they have no signs of heart damage initially after chemotherapy. Additionally, it emphasizes that working to live a heart-healthy lifestyle is important for cancer patients and survivors to reduce the overall risk of heart disease.”
Dr Larsen and her colleagues presented this research as a poster (abstract 1105-066) at the American College of Cardiology’s 67th Annual Scientific Session & Expo (ACC.18).
Patients
Using data from the Rochester Epidemiology Project, the researchers retrospectively tracked CHF cases in 900 cancer patients and 1550 non-cancer patients. Patients were treated in Olmsted County in Minnesota from 1985 to 2010.
For both patient groups, the median age at baseline was about 53, a little more than 90% of each group was white, and nearly 80% of each group was female.
Six to 7% of patients had diabetes, and about 30% of each group had hypertension. Thirty-eight percent of each group had hyperlipidemia, and 31% were obese.
Five percent of cancer patients and 2% of controls had coronary artery disease (P<0.001). This was the only significant difference in baseline characteristics.
Cancer patients had been diagnosed with non-Hodgkin lymphoma (28%), Hodgkin lymphoma (9%), or breast cancer (64%). Forty-seven percent had received radiation, including right chest (21%), left chest (23%), and mediastinal (4%).
Eighty-four percent of patients had received anthracycline therapy. The median doxorubicin isotoxic dose was 240 mg/m2.
At baseline, 12% of cancer patients were on beta-blockers, 8% were on angiotensin converting enzyme inhibitors, 4% were on angiotensin receptor blockers, and 11% were on statins.
Results
Cancer patients were more than 3 times as likely as controls to develop CHF. The hazard ratio (HR) was 3.6 (P<0.01) in an analysis adjusted for age, gender, diabetes, hypertension, coronary artery disease, dyslipidemia, and obesity at baseline.
The increased CHF risk among cancer patients was evident after the first year from cancer diagnosis and persisted at 20 years of follow-up.
“The risk of heart failure doesn’t go away after a couple of years,” Dr Larsen said. “It’s a long-term issue that patients need to discuss with their doctors and use as motivation to stay heart healthy.”
The incidence of CHF—in cancer patients and controls, respectively—was as follows:
- 1 year—1.5% vs 0.1%
- 5 years—3.1% vs 0.9%
- 10 years—5.0% vs 2%
- 20 years—10.1% vs 5.8%.
A multivariable analysis in the cancer patients revealed a few independent risk factors for CHF, including:
- Doxorubicin isotoxic dose ≥ 300 mg/m2 (HR=2.34, P=0.003)
- Age at diagnosis (HR=3.06 for age ≥ 80 vs 60-69, P=0.01)
- Coronary artery disease at diagnosis (HR=2.27, P=0.04)
- Diabetes mellitus at diagnosis (HR=2.39, P<0.01).
Dr Larsen said additional research is needed to determine why diabetes carries a greater risk than other traditional risk factors, such as high blood pressure, in this group.
Mitigating risk
These findings raise important questions about what the appropriate surveillance should be for heart problems post-cancer treatment, Dr Larsen said. She believes more frequent cardiac imaging may be warranted in some patients to detect signs of CHF earlier.
“It’s an area that needs to be better defined,” Dr Larsen said. “An echocardiogram is usually done 6 to 12 months after cancer treatment with an anthracycline, but how often should it be done after that? We need to be more vigilant in making sure we try to prevent or control heart issues post-cancer care, especially in light of the growing appreciation of the connection between some cancer treatments and heart disease.”
Dr Larsen also noted that patients themselves can play a role in decreasing their risk of CHF, even if they are starting at a disadvantage.
A heart-healthy lifestyle—maintaining a normal body weight, regular exercise, and controlling other risk factors such as high blood pressure, diabetes, and high cholesterol—can help lower the risk of heart disease and CHF.
“If patients know they have received a drug treatment that might increase their risk of heart failure, it’s even more important to take care of the aspects of their life that they can control to reduce their risk as much as possible and to work with their medical care team to detect issues as early as possible,” Dr Larsen said.
ORLANDO—Results of a retrospective study showed that survivors of lymphoma or breast cancer had a significantly greater risk of congestive heart failure (CHF) than patients who did not have cancer.
This increased risk was observed as early as a year after cancer diagnosis but was still present 20 years after diagnosis.
Overall, 1 in 10 cancer patients had CHF at the 20-year mark.
“The majority of patients do not develop heart failure, but our research helps us recognize the factors associated with it and the importance of appropriate heart care following cancer treatment,” said Carolyn Larsen, MD, of the Mayo Clinic in Rochester, Minnesota.
“Our research suggests that periodic cardiac imaging to monitor for heart damage may be needed for some cancer patients, even if they have no signs of heart damage initially after chemotherapy. Additionally, it emphasizes that working to live a heart-healthy lifestyle is important for cancer patients and survivors to reduce the overall risk of heart disease.”
Dr Larsen and her colleagues presented this research as a poster (abstract 1105-066) at the American College of Cardiology’s 67th Annual Scientific Session & Expo (ACC.18).
Patients
Using data from the Rochester Epidemiology Project, the researchers retrospectively tracked CHF cases in 900 cancer patients and 1550 non-cancer patients. Patients were treated in Olmsted County in Minnesota from 1985 to 2010.
For both patient groups, the median age at baseline was about 53, a little more than 90% of each group was white, and nearly 80% of each group was female.
Six to 7% of patients had diabetes, and about 30% of each group had hypertension. Thirty-eight percent of each group had hyperlipidemia, and 31% were obese.
Five percent of cancer patients and 2% of controls had coronary artery disease (P<0.001). This was the only significant difference in baseline characteristics.
Cancer patients had been diagnosed with non-Hodgkin lymphoma (28%), Hodgkin lymphoma (9%), or breast cancer (64%). Forty-seven percent had received radiation, including right chest (21%), left chest (23%), and mediastinal (4%).
Eighty-four percent of patients had received anthracycline therapy. The median doxorubicin isotoxic dose was 240 mg/m2.
At baseline, 12% of cancer patients were on beta-blockers, 8% were on angiotensin converting enzyme inhibitors, 4% were on angiotensin receptor blockers, and 11% were on statins.
Results
Cancer patients were more than 3 times as likely as controls to develop CHF. The hazard ratio (HR) was 3.6 (P<0.01) in an analysis adjusted for age, gender, diabetes, hypertension, coronary artery disease, dyslipidemia, and obesity at baseline.
The increased CHF risk among cancer patients was evident after the first year from cancer diagnosis and persisted at 20 years of follow-up.
“The risk of heart failure doesn’t go away after a couple of years,” Dr Larsen said. “It’s a long-term issue that patients need to discuss with their doctors and use as motivation to stay heart healthy.”
The incidence of CHF—in cancer patients and controls, respectively—was as follows:
- 1 year—1.5% vs 0.1%
- 5 years—3.1% vs 0.9%
- 10 years—5.0% vs 2%
- 20 years—10.1% vs 5.8%.
A multivariable analysis in the cancer patients revealed a few independent risk factors for CHF, including:
- Doxorubicin isotoxic dose ≥ 300 mg/m2 (HR=2.34, P=0.003)
- Age at diagnosis (HR=3.06 for age ≥ 80 vs 60-69, P=0.01)
- Coronary artery disease at diagnosis (HR=2.27, P=0.04)
- Diabetes mellitus at diagnosis (HR=2.39, P<0.01).
Dr Larsen said additional research is needed to determine why diabetes carries a greater risk than other traditional risk factors, such as high blood pressure, in this group.
Mitigating risk
These findings raise important questions about what the appropriate surveillance should be for heart problems post-cancer treatment, Dr Larsen said. She believes more frequent cardiac imaging may be warranted in some patients to detect signs of CHF earlier.
“It’s an area that needs to be better defined,” Dr Larsen said. “An echocardiogram is usually done 6 to 12 months after cancer treatment with an anthracycline, but how often should it be done after that? We need to be more vigilant in making sure we try to prevent or control heart issues post-cancer care, especially in light of the growing appreciation of the connection between some cancer treatments and heart disease.”
Dr Larsen also noted that patients themselves can play a role in decreasing their risk of CHF, even if they are starting at a disadvantage.
A heart-healthy lifestyle—maintaining a normal body weight, regular exercise, and controlling other risk factors such as high blood pressure, diabetes, and high cholesterol—can help lower the risk of heart disease and CHF.
“If patients know they have received a drug treatment that might increase their risk of heart failure, it’s even more important to take care of the aspects of their life that they can control to reduce their risk as much as possible and to work with their medical care team to detect issues as early as possible,” Dr Larsen said.
Pacritinib bests BAT, doesn’t seem to affect survival
Final results from the PERSIST-2 trial suggest pacritinib can be more effective than best available therapy (BAT) for patients with myelofibrosis and thrombocytopenia, and the drug has no significant effect on survival.
Patients who received pacritinib were more likely to experience at least a 35% reduction in spleen volume and a 50% reduction in total symptom score (TSS).
In addition, there was no significant difference in survival between patients who received pacritinib and those who received BAT.
Interim results from PERSIST-2 had indicated that pacritinib negatively impacted survival, which was consistent with results from PERSIST-1. Because of this, the US Food and Drug Administration placed pacritinib trials on clinical hold in February 2016. The hold was lifted in January 2017.
The final results from PERSIST-2 were published in JAMA Oncology. The study was sponsored by CTI BioPharma Corp.
In this phase 3 study, researchers compared 2 dosing schedules of pacritinib to BAT. The study enrolled patients with previously treated or untreated myelofibrosis (intermediate-1/2 or high-risk) and thrombocytopenia (platelet counts ≤ 100 x 109/L).
There were 311 patients randomized to receive pacritinib once daily (n=104), pacritinib twice daily (n=107), or BAT (n=100). Patients in the BAT arm received ruxolitinib (n=44), hydroxyurea (n=19), prednisone and/or prednisolone (n=13), as well as “watchful waiting” (n=19).
Patients could crossover from BAT to pacritinib after week 24 or for progression of splenomegaly. Fifty patients in the BAT arm did cross over.
All patients had discontinued treatment at a median of 23 weeks (pacritinib once daily), 25 weeks (twice daily), and 21 weeks (BAT) from the start of treatment.
Common reasons for discontinuation (in the once daily, twice daily, and BAT arms, respectively) were the clinical hold (59%, 71%, and 27%), adverse events (14%, 9%, and 4%), physician decision (5%, 3%, and 41%), progressive disease (5%, 7%, and 11%), and death (5%, 2%, and 5%).
Efficacy
The intention-to-treat efficacy population included 75 patients in the once-daily arm, 74 in the twice-daily arm, and 72 in the BAT arm. The researchers said baseline characteristics were balanced across the arms.
The co-primary endpoints were the rate of patients achieving a spleen volume reduction (SVR) of 35% or more and a 50% or more reduction in TSS at week 24.
Eighteen percent of patients in the combined pacritinib arms and 3% of patients in the BAT arm achieved an SVR of 35% or more (P=0.001). Fifteen percent of patients in the pacritinib once-daily arm and 22% of patients in the twice-daily arm achieved this endpoint (P values of 0.02 and 0.001, respectively, for comparison with BAT).
Twenty-five percent of patients in the combined pacritinib arms and 14% in the BAT arm had at least a 50% reduction in TSS (P=0.079). Seventeen percent of patients in the pacritinib once-daily arm and 32% of patients in the twice-daily arm achieved this endpoint (P values of 0.65 and 0.01, respectively, for comparison with BAT).
“Pacritinib was shown to reduce both spleen volume and total symptom score, 2 very important clinical measures in myelofibrosis patients with thrombocytopenia, including those patients who received prior treatment with ruxolitinib,” said study author John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York.
Survival
When the clinical hold was placed, there was no significant difference in overall survival between the 3 treatment arms.
The rates of death were 14% (n=14) in the pacritinib once-daily arm, 9% (n=10) in the twice-daily arm, and 14% (n=14) in the BAT arm. For patients in the BAT arm, the death rate was lower for those who crossed over to pacritinib (8%, n=4) than for those who did not (20%, n=10).
The hazard ratios for death were 1.18 in the once-daily pacritinib arm and 0.68 in the twice-daily pacritinib arm.
Safety
Dr Mascarenhas said pacritinib had “a generally manageable safety profile.”
Common adverse events—in the once daily, twice daily, and BAT arms, respectively—included:
- Diarrhea—67%, 48%, and 15%
- Nausea—38%, 32%, and 11%
- Thrombocytopenia—33%, 34%, and 23%
- Anemia—28%, 24%, and 15%
- Vomiting—21%, 19%, and 5%
- Fatigue—17%, 17%, and 16%
- Peripheral edema—13%, 2%, and 15%
- Dizziness—14%, 15%, and 5%
- Abdominal pain—19%, 9%, and 19%
- Pyrexia—11%, 15%, and 3%.
Grade 3/4 events—in the once daily, twice daily, and BAT arms, respectively—included:
- Thrombocytopenia—31%, 32%, and 18%
- Anemia—27%, 22%, and 14%
- Neutropenia—9%, 7%, and 5%
- Pneumonia—4%, 7%, and 3%
- Fatigue—7%, 3%, and 5%
- Diarrhea—5%, 4%, and 0%
- Epistaxis—2%, 5%, and 1%.
Serious adverse events—in the once daily, twice daily, and BAT arms, respectively—included:
- Anemia—5%, 8%, and 3%
- Thrombocytopenia—2%, 6%, and 2%
- Pneumonia—5%, 6%, and 4%
- Acute renal failure—5%, 2%, and 2%
- Congestive heart failure—1%, 4%, and 2%
- Atrial fibrillation—3%, 0%, and 3%
- Cardiac arrest—2%, 0%, and 0%
- Epistaxis—2%, 2%, and 1%
- Subdural hematoma—2%, 0%, and 0%.
Final results from the PERSIST-2 trial suggest pacritinib can be more effective than best available therapy (BAT) for patients with myelofibrosis and thrombocytopenia, and the drug has no significant effect on survival.
Patients who received pacritinib were more likely to experience at least a 35% reduction in spleen volume and a 50% reduction in total symptom score (TSS).
In addition, there was no significant difference in survival between patients who received pacritinib and those who received BAT.
Interim results from PERSIST-2 had indicated that pacritinib negatively impacted survival, which was consistent with results from PERSIST-1. Because of this, the US Food and Drug Administration placed pacritinib trials on clinical hold in February 2016. The hold was lifted in January 2017.
The final results from PERSIST-2 were published in JAMA Oncology. The study was sponsored by CTI BioPharma Corp.
In this phase 3 study, researchers compared 2 dosing schedules of pacritinib to BAT. The study enrolled patients with previously treated or untreated myelofibrosis (intermediate-1/2 or high-risk) and thrombocytopenia (platelet counts ≤ 100 x 109/L).
There were 311 patients randomized to receive pacritinib once daily (n=104), pacritinib twice daily (n=107), or BAT (n=100). Patients in the BAT arm received ruxolitinib (n=44), hydroxyurea (n=19), prednisone and/or prednisolone (n=13), as well as “watchful waiting” (n=19).
Patients could crossover from BAT to pacritinib after week 24 or for progression of splenomegaly. Fifty patients in the BAT arm did cross over.
All patients had discontinued treatment at a median of 23 weeks (pacritinib once daily), 25 weeks (twice daily), and 21 weeks (BAT) from the start of treatment.
Common reasons for discontinuation (in the once daily, twice daily, and BAT arms, respectively) were the clinical hold (59%, 71%, and 27%), adverse events (14%, 9%, and 4%), physician decision (5%, 3%, and 41%), progressive disease (5%, 7%, and 11%), and death (5%, 2%, and 5%).
Efficacy
The intention-to-treat efficacy population included 75 patients in the once-daily arm, 74 in the twice-daily arm, and 72 in the BAT arm. The researchers said baseline characteristics were balanced across the arms.
The co-primary endpoints were the rate of patients achieving a spleen volume reduction (SVR) of 35% or more and a 50% or more reduction in TSS at week 24.
Eighteen percent of patients in the combined pacritinib arms and 3% of patients in the BAT arm achieved an SVR of 35% or more (P=0.001). Fifteen percent of patients in the pacritinib once-daily arm and 22% of patients in the twice-daily arm achieved this endpoint (P values of 0.02 and 0.001, respectively, for comparison with BAT).
Twenty-five percent of patients in the combined pacritinib arms and 14% in the BAT arm had at least a 50% reduction in TSS (P=0.079). Seventeen percent of patients in the pacritinib once-daily arm and 32% of patients in the twice-daily arm achieved this endpoint (P values of 0.65 and 0.01, respectively, for comparison with BAT).
“Pacritinib was shown to reduce both spleen volume and total symptom score, 2 very important clinical measures in myelofibrosis patients with thrombocytopenia, including those patients who received prior treatment with ruxolitinib,” said study author John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York.
Survival
When the clinical hold was placed, there was no significant difference in overall survival between the 3 treatment arms.
The rates of death were 14% (n=14) in the pacritinib once-daily arm, 9% (n=10) in the twice-daily arm, and 14% (n=14) in the BAT arm. For patients in the BAT arm, the death rate was lower for those who crossed over to pacritinib (8%, n=4) than for those who did not (20%, n=10).
The hazard ratios for death were 1.18 in the once-daily pacritinib arm and 0.68 in the twice-daily pacritinib arm.
Safety
Dr Mascarenhas said pacritinib had “a generally manageable safety profile.”
Common adverse events—in the once daily, twice daily, and BAT arms, respectively—included:
- Diarrhea—67%, 48%, and 15%
- Nausea—38%, 32%, and 11%
- Thrombocytopenia—33%, 34%, and 23%
- Anemia—28%, 24%, and 15%
- Vomiting—21%, 19%, and 5%
- Fatigue—17%, 17%, and 16%
- Peripheral edema—13%, 2%, and 15%
- Dizziness—14%, 15%, and 5%
- Abdominal pain—19%, 9%, and 19%
- Pyrexia—11%, 15%, and 3%.
Grade 3/4 events—in the once daily, twice daily, and BAT arms, respectively—included:
- Thrombocytopenia—31%, 32%, and 18%
- Anemia—27%, 22%, and 14%
- Neutropenia—9%, 7%, and 5%
- Pneumonia—4%, 7%, and 3%
- Fatigue—7%, 3%, and 5%
- Diarrhea—5%, 4%, and 0%
- Epistaxis—2%, 5%, and 1%.
Serious adverse events—in the once daily, twice daily, and BAT arms, respectively—included:
- Anemia—5%, 8%, and 3%
- Thrombocytopenia—2%, 6%, and 2%
- Pneumonia—5%, 6%, and 4%
- Acute renal failure—5%, 2%, and 2%
- Congestive heart failure—1%, 4%, and 2%
- Atrial fibrillation—3%, 0%, and 3%
- Cardiac arrest—2%, 0%, and 0%
- Epistaxis—2%, 2%, and 1%
- Subdural hematoma—2%, 0%, and 0%.
Final results from the PERSIST-2 trial suggest pacritinib can be more effective than best available therapy (BAT) for patients with myelofibrosis and thrombocytopenia, and the drug has no significant effect on survival.
Patients who received pacritinib were more likely to experience at least a 35% reduction in spleen volume and a 50% reduction in total symptom score (TSS).
In addition, there was no significant difference in survival between patients who received pacritinib and those who received BAT.
Interim results from PERSIST-2 had indicated that pacritinib negatively impacted survival, which was consistent with results from PERSIST-1. Because of this, the US Food and Drug Administration placed pacritinib trials on clinical hold in February 2016. The hold was lifted in January 2017.
The final results from PERSIST-2 were published in JAMA Oncology. The study was sponsored by CTI BioPharma Corp.
In this phase 3 study, researchers compared 2 dosing schedules of pacritinib to BAT. The study enrolled patients with previously treated or untreated myelofibrosis (intermediate-1/2 or high-risk) and thrombocytopenia (platelet counts ≤ 100 x 109/L).
There were 311 patients randomized to receive pacritinib once daily (n=104), pacritinib twice daily (n=107), or BAT (n=100). Patients in the BAT arm received ruxolitinib (n=44), hydroxyurea (n=19), prednisone and/or prednisolone (n=13), as well as “watchful waiting” (n=19).
Patients could crossover from BAT to pacritinib after week 24 or for progression of splenomegaly. Fifty patients in the BAT arm did cross over.
All patients had discontinued treatment at a median of 23 weeks (pacritinib once daily), 25 weeks (twice daily), and 21 weeks (BAT) from the start of treatment.
Common reasons for discontinuation (in the once daily, twice daily, and BAT arms, respectively) were the clinical hold (59%, 71%, and 27%), adverse events (14%, 9%, and 4%), physician decision (5%, 3%, and 41%), progressive disease (5%, 7%, and 11%), and death (5%, 2%, and 5%).
Efficacy
The intention-to-treat efficacy population included 75 patients in the once-daily arm, 74 in the twice-daily arm, and 72 in the BAT arm. The researchers said baseline characteristics were balanced across the arms.
The co-primary endpoints were the rate of patients achieving a spleen volume reduction (SVR) of 35% or more and a 50% or more reduction in TSS at week 24.
Eighteen percent of patients in the combined pacritinib arms and 3% of patients in the BAT arm achieved an SVR of 35% or more (P=0.001). Fifteen percent of patients in the pacritinib once-daily arm and 22% of patients in the twice-daily arm achieved this endpoint (P values of 0.02 and 0.001, respectively, for comparison with BAT).
Twenty-five percent of patients in the combined pacritinib arms and 14% in the BAT arm had at least a 50% reduction in TSS (P=0.079). Seventeen percent of patients in the pacritinib once-daily arm and 32% of patients in the twice-daily arm achieved this endpoint (P values of 0.65 and 0.01, respectively, for comparison with BAT).
“Pacritinib was shown to reduce both spleen volume and total symptom score, 2 very important clinical measures in myelofibrosis patients with thrombocytopenia, including those patients who received prior treatment with ruxolitinib,” said study author John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York.
Survival
When the clinical hold was placed, there was no significant difference in overall survival between the 3 treatment arms.
The rates of death were 14% (n=14) in the pacritinib once-daily arm, 9% (n=10) in the twice-daily arm, and 14% (n=14) in the BAT arm. For patients in the BAT arm, the death rate was lower for those who crossed over to pacritinib (8%, n=4) than for those who did not (20%, n=10).
The hazard ratios for death were 1.18 in the once-daily pacritinib arm and 0.68 in the twice-daily pacritinib arm.
Safety
Dr Mascarenhas said pacritinib had “a generally manageable safety profile.”
Common adverse events—in the once daily, twice daily, and BAT arms, respectively—included:
- Diarrhea—67%, 48%, and 15%
- Nausea—38%, 32%, and 11%
- Thrombocytopenia—33%, 34%, and 23%
- Anemia—28%, 24%, and 15%
- Vomiting—21%, 19%, and 5%
- Fatigue—17%, 17%, and 16%
- Peripheral edema—13%, 2%, and 15%
- Dizziness—14%, 15%, and 5%
- Abdominal pain—19%, 9%, and 19%
- Pyrexia—11%, 15%, and 3%.
Grade 3/4 events—in the once daily, twice daily, and BAT arms, respectively—included:
- Thrombocytopenia—31%, 32%, and 18%
- Anemia—27%, 22%, and 14%
- Neutropenia—9%, 7%, and 5%
- Pneumonia—4%, 7%, and 3%
- Fatigue—7%, 3%, and 5%
- Diarrhea—5%, 4%, and 0%
- Epistaxis—2%, 5%, and 1%.
Serious adverse events—in the once daily, twice daily, and BAT arms, respectively—included:
- Anemia—5%, 8%, and 3%
- Thrombocytopenia—2%, 6%, and 2%
- Pneumonia—5%, 6%, and 4%
- Acute renal failure—5%, 2%, and 2%
- Congestive heart failure—1%, 4%, and 2%
- Atrial fibrillation—3%, 0%, and 3%
- Cardiac arrest—2%, 0%, and 0%
- Epistaxis—2%, 2%, and 1%
- Subdural hematoma—2%, 0%, and 0%.
New mutation linked to familial erythrocytosis
Researchers say they have discovered a mutation associated with hereditary erythrocytosis.
The mutation causes a messenger RNA (mRNA) that is not normally involved in the formation of proteins to be reprogrammed so that it produces erythropoietin (EPO), thereby abnormally increasing red blood cell production.
Radek Skoda, MD, of the University of Basel in Switzerland, and his colleagues described this discovery in NEJM.
The team found the mutation in a family with hereditary erythrocytosis. The researchers studied 10 affected family members spanning 4 generations.
Genome-wide linkage analysis and gene sequencing revealed a heterozygous single-base deletion in exon 2 of EPO (chromosome 7: 100,319,199 GG→G) in all 10 affected family members.
However, the researchers were initially puzzled. This c.32delG mutation should actually lead to a loss of function of the EPO gene because the absence of the base shifts the reading frame of the genetic code, meaning that no more EPO protein can be formed.
Despite this, the concentration of EPO hormone in the patients’ blood measurably increased rather than decreased.
To investigate this, the researchers used CRISPR to engineer cells carrying the c.32delG mutation. In this way, they found a second, hidden mRNA in the EPO gene that is not normally involved in the production of a protein.
The c.32delG mutation also leads to a shift in the reading frame of this second mRNA, with the result that more biologically active EPO hormone is produced.
“The mechanism is intriguing,” Dr Skoda said. “The mutation reprograms the gene product so that it gains a new function and is misused to overproduce EPO.”
Researchers say they have discovered a mutation associated with hereditary erythrocytosis.
The mutation causes a messenger RNA (mRNA) that is not normally involved in the formation of proteins to be reprogrammed so that it produces erythropoietin (EPO), thereby abnormally increasing red blood cell production.
Radek Skoda, MD, of the University of Basel in Switzerland, and his colleagues described this discovery in NEJM.
The team found the mutation in a family with hereditary erythrocytosis. The researchers studied 10 affected family members spanning 4 generations.
Genome-wide linkage analysis and gene sequencing revealed a heterozygous single-base deletion in exon 2 of EPO (chromosome 7: 100,319,199 GG→G) in all 10 affected family members.
However, the researchers were initially puzzled. This c.32delG mutation should actually lead to a loss of function of the EPO gene because the absence of the base shifts the reading frame of the genetic code, meaning that no more EPO protein can be formed.
Despite this, the concentration of EPO hormone in the patients’ blood measurably increased rather than decreased.
To investigate this, the researchers used CRISPR to engineer cells carrying the c.32delG mutation. In this way, they found a second, hidden mRNA in the EPO gene that is not normally involved in the production of a protein.
The c.32delG mutation also leads to a shift in the reading frame of this second mRNA, with the result that more biologically active EPO hormone is produced.
“The mechanism is intriguing,” Dr Skoda said. “The mutation reprograms the gene product so that it gains a new function and is misused to overproduce EPO.”
Researchers say they have discovered a mutation associated with hereditary erythrocytosis.
The mutation causes a messenger RNA (mRNA) that is not normally involved in the formation of proteins to be reprogrammed so that it produces erythropoietin (EPO), thereby abnormally increasing red blood cell production.
Radek Skoda, MD, of the University of Basel in Switzerland, and his colleagues described this discovery in NEJM.
The team found the mutation in a family with hereditary erythrocytosis. The researchers studied 10 affected family members spanning 4 generations.
Genome-wide linkage analysis and gene sequencing revealed a heterozygous single-base deletion in exon 2 of EPO (chromosome 7: 100,319,199 GG→G) in all 10 affected family members.
However, the researchers were initially puzzled. This c.32delG mutation should actually lead to a loss of function of the EPO gene because the absence of the base shifts the reading frame of the genetic code, meaning that no more EPO protein can be formed.
Despite this, the concentration of EPO hormone in the patients’ blood measurably increased rather than decreased.
To investigate this, the researchers used CRISPR to engineer cells carrying the c.32delG mutation. In this way, they found a second, hidden mRNA in the EPO gene that is not normally involved in the production of a protein.
The c.32delG mutation also leads to a shift in the reading frame of this second mRNA, with the result that more biologically active EPO hormone is produced.
“The mechanism is intriguing,” Dr Skoda said. “The mutation reprograms the gene product so that it gains a new function and is misused to overproduce EPO.”
AYA cancer survivors have better social support than peers
Researchers have developed a new method to measure social networks of adolescent and young adult (AYA) cancer survivors.
This method indicated that AYA cancer survivors often have stronger social networks than their non-cancer peers.
However, the strength of the social network varied by diagnosis, with the lymphoma and leukemia survivors having the greatest support.
These findings were published in Cancer.
“Cancer survivors need healthy social connections, and, to the best of our knowledge, this is the first published study to quantify social networks of adolescent and young adult cancer survivors compared to their peers,” said study author I-Chan Huang, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The study introduces a method we developed and validated for evaluating social networks of these cancer survivors.”
The method, called the functional social network index (FSNI), measures marital status, contact frequency with friends and relatives, and available resources for health support/advice, which includes emotional support, tangible support, physical activity advice, and weight management advice.
The researchers compared the FSNI to a pair of traditional social network indices—density and betweenness centrality.
Density represents the ratio of the existing relationships/connections within a network to all possible relationships/connections. And betweenness centrality represents the ratio of the existing shortest paths between 2 friends/relatives of the study participants to the shortest possible paths between 2 friends/relatives.
Subjects
The researchers used the 3 social network indices to analyze 102 AYA cancer survivors, ages 18 to 30, and 102 young adults with no cancer history who were matched to the survivors by age, sex, and race.
Subjects were recruited from a commercial national Internet survey panel. They reported detailed social connection information with up to 25 friends and relatives.
The cancer survivors were between 15 and 30 years old when their cancer was diagnosed, and all had completed treatment at least 5 years prior.
Results
Neither the density index nor the betweenness centrality index demonstrated significant differences between cancer survivors and controls (all P values were less than 0.05).
However, according to the FSNI, cancer survivors had more available resources for emotional support (beta [b]=3.02; P=0.003), tangible support (b=4.17; P<0.001), physical activity advice (b=3.94; P<0.001), and weight management advice (b=4.10; P<0.001).
“This makes sense,” Dr Huang said. “Because of their cancer, survivors often have strong networks of physicians, friends, and relatives to provide advice and support.”
However, the FSNI showed the strength of cancer survivors’ support network varied by diagnosis.
Lymphoma survivors ranked highest on the FSNI (b=2.765; P=0.02), followed by survivors of leukemia (b=2.542; P=0.03) and solid tumors (b=2.178; P=0.047), with central nervous system malignancies as the reference.
The researchers also found a higher FSNI was associated with better coping skills, including using emotional support (b=0.08; P=0.04), using instrumental support (b=0.12; P<0.001), venting of emotions (b=0.10; P=0.004), positive reframing (b=0.12; P=0.003), planning for the future (b=0.08; P=0.03), participating in religious activities (b=0.16; P<0.001), and less denial (b=0.10; P=0.01) and destructive behavior (b=0.08; P=0.04).
The researchers said long-term follow-up is needed to understand how social networks and social support may change over time.
“Adolescents and young adult cancer survivors are in a transitory stage of independence from parents,” Dr Huang said. “While this study suggests that survivors often report strong social connections, our previous studies have reported that childhood cancer survivors are more likely than their peers to struggle mentally and physically and report issues like distress and loneliness.”
Dr Huang and his colleagues are working to streamline the FSNI to make it easier for healthcare providers to assess support available to cancer survivors of any age.
Meanwhile, researchers are working to better understand how social connections affect health outcomes in order to design interventions to foster those connections.
“A lack of social connections with friends and relatives is associated with poor quality of life, risky health behaviors, chronic health conditions, and premature death,” Dr Huang said. “Once we identify the mechanism between social connections and health outcomes, we can start designing interventions to use social networks to improve health outcomes of cancer survivors.”
Researchers have developed a new method to measure social networks of adolescent and young adult (AYA) cancer survivors.
This method indicated that AYA cancer survivors often have stronger social networks than their non-cancer peers.
However, the strength of the social network varied by diagnosis, with the lymphoma and leukemia survivors having the greatest support.
These findings were published in Cancer.
“Cancer survivors need healthy social connections, and, to the best of our knowledge, this is the first published study to quantify social networks of adolescent and young adult cancer survivors compared to their peers,” said study author I-Chan Huang, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The study introduces a method we developed and validated for evaluating social networks of these cancer survivors.”
The method, called the functional social network index (FSNI), measures marital status, contact frequency with friends and relatives, and available resources for health support/advice, which includes emotional support, tangible support, physical activity advice, and weight management advice.
The researchers compared the FSNI to a pair of traditional social network indices—density and betweenness centrality.
Density represents the ratio of the existing relationships/connections within a network to all possible relationships/connections. And betweenness centrality represents the ratio of the existing shortest paths between 2 friends/relatives of the study participants to the shortest possible paths between 2 friends/relatives.
Subjects
The researchers used the 3 social network indices to analyze 102 AYA cancer survivors, ages 18 to 30, and 102 young adults with no cancer history who were matched to the survivors by age, sex, and race.
Subjects were recruited from a commercial national Internet survey panel. They reported detailed social connection information with up to 25 friends and relatives.
The cancer survivors were between 15 and 30 years old when their cancer was diagnosed, and all had completed treatment at least 5 years prior.
Results
Neither the density index nor the betweenness centrality index demonstrated significant differences between cancer survivors and controls (all P values were less than 0.05).
However, according to the FSNI, cancer survivors had more available resources for emotional support (beta [b]=3.02; P=0.003), tangible support (b=4.17; P<0.001), physical activity advice (b=3.94; P<0.001), and weight management advice (b=4.10; P<0.001).
“This makes sense,” Dr Huang said. “Because of their cancer, survivors often have strong networks of physicians, friends, and relatives to provide advice and support.”
However, the FSNI showed the strength of cancer survivors’ support network varied by diagnosis.
Lymphoma survivors ranked highest on the FSNI (b=2.765; P=0.02), followed by survivors of leukemia (b=2.542; P=0.03) and solid tumors (b=2.178; P=0.047), with central nervous system malignancies as the reference.
The researchers also found a higher FSNI was associated with better coping skills, including using emotional support (b=0.08; P=0.04), using instrumental support (b=0.12; P<0.001), venting of emotions (b=0.10; P=0.004), positive reframing (b=0.12; P=0.003), planning for the future (b=0.08; P=0.03), participating in religious activities (b=0.16; P<0.001), and less denial (b=0.10; P=0.01) and destructive behavior (b=0.08; P=0.04).
The researchers said long-term follow-up is needed to understand how social networks and social support may change over time.
“Adolescents and young adult cancer survivors are in a transitory stage of independence from parents,” Dr Huang said. “While this study suggests that survivors often report strong social connections, our previous studies have reported that childhood cancer survivors are more likely than their peers to struggle mentally and physically and report issues like distress and loneliness.”
Dr Huang and his colleagues are working to streamline the FSNI to make it easier for healthcare providers to assess support available to cancer survivors of any age.
Meanwhile, researchers are working to better understand how social connections affect health outcomes in order to design interventions to foster those connections.
“A lack of social connections with friends and relatives is associated with poor quality of life, risky health behaviors, chronic health conditions, and premature death,” Dr Huang said. “Once we identify the mechanism between social connections and health outcomes, we can start designing interventions to use social networks to improve health outcomes of cancer survivors.”
Researchers have developed a new method to measure social networks of adolescent and young adult (AYA) cancer survivors.
This method indicated that AYA cancer survivors often have stronger social networks than their non-cancer peers.
However, the strength of the social network varied by diagnosis, with the lymphoma and leukemia survivors having the greatest support.
These findings were published in Cancer.
“Cancer survivors need healthy social connections, and, to the best of our knowledge, this is the first published study to quantify social networks of adolescent and young adult cancer survivors compared to their peers,” said study author I-Chan Huang, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The study introduces a method we developed and validated for evaluating social networks of these cancer survivors.”
The method, called the functional social network index (FSNI), measures marital status, contact frequency with friends and relatives, and available resources for health support/advice, which includes emotional support, tangible support, physical activity advice, and weight management advice.
The researchers compared the FSNI to a pair of traditional social network indices—density and betweenness centrality.
Density represents the ratio of the existing relationships/connections within a network to all possible relationships/connections. And betweenness centrality represents the ratio of the existing shortest paths between 2 friends/relatives of the study participants to the shortest possible paths between 2 friends/relatives.
Subjects
The researchers used the 3 social network indices to analyze 102 AYA cancer survivors, ages 18 to 30, and 102 young adults with no cancer history who were matched to the survivors by age, sex, and race.
Subjects were recruited from a commercial national Internet survey panel. They reported detailed social connection information with up to 25 friends and relatives.
The cancer survivors were between 15 and 30 years old when their cancer was diagnosed, and all had completed treatment at least 5 years prior.
Results
Neither the density index nor the betweenness centrality index demonstrated significant differences between cancer survivors and controls (all P values were less than 0.05).
However, according to the FSNI, cancer survivors had more available resources for emotional support (beta [b]=3.02; P=0.003), tangible support (b=4.17; P<0.001), physical activity advice (b=3.94; P<0.001), and weight management advice (b=4.10; P<0.001).
“This makes sense,” Dr Huang said. “Because of their cancer, survivors often have strong networks of physicians, friends, and relatives to provide advice and support.”
However, the FSNI showed the strength of cancer survivors’ support network varied by diagnosis.
Lymphoma survivors ranked highest on the FSNI (b=2.765; P=0.02), followed by survivors of leukemia (b=2.542; P=0.03) and solid tumors (b=2.178; P=0.047), with central nervous system malignancies as the reference.
The researchers also found a higher FSNI was associated with better coping skills, including using emotional support (b=0.08; P=0.04), using instrumental support (b=0.12; P<0.001), venting of emotions (b=0.10; P=0.004), positive reframing (b=0.12; P=0.003), planning for the future (b=0.08; P=0.03), participating in religious activities (b=0.16; P<0.001), and less denial (b=0.10; P=0.01) and destructive behavior (b=0.08; P=0.04).
The researchers said long-term follow-up is needed to understand how social networks and social support may change over time.
“Adolescents and young adult cancer survivors are in a transitory stage of independence from parents,” Dr Huang said. “While this study suggests that survivors often report strong social connections, our previous studies have reported that childhood cancer survivors are more likely than their peers to struggle mentally and physically and report issues like distress and loneliness.”
Dr Huang and his colleagues are working to streamline the FSNI to make it easier for healthcare providers to assess support available to cancer survivors of any age.
Meanwhile, researchers are working to better understand how social connections affect health outcomes in order to design interventions to foster those connections.
“A lack of social connections with friends and relatives is associated with poor quality of life, risky health behaviors, chronic health conditions, and premature death,” Dr Huang said. “Once we identify the mechanism between social connections and health outcomes, we can start designing interventions to use social networks to improve health outcomes of cancer survivors.”
CCSs have greater risk of cardiovascular disease
Childhood cancer survivors (CCSs) have an increased risk of premature cardiovascular disease in adulthood, according to a new study.
Researchers found a nearly 2-fold increased risk of cardiovascular diseases in CCSs compared to the general population.
Cardiovascular disease was identified in 4.5% of CCSs and occurred in most before they reached the age of 40, nearly 8 years earlier than in the general population.
“Our results show that these survivors of childhood cancer have a substantially elevated burden of prematurely occurring traditional cardiovascular risk factors and cardiovascular diseases,” said study author Joerg Faber, MD, PhD, of the Johannes Gutenberg University Mainz in Germany.
Dr Faber and his colleagues reported these results in the European Heart Journal.
The researchers evaluated 951 adult CCSs (ages 23 to 48), referred to as the CVSS cohort (Cardiac and Vascular Late Sequelae in Long-Term Survivors of Childhood Cancer Study).
The patients had been diagnosed with cancer between 1980 and 1990. The most common diagnoses were leukemia (43.5%), central nervous system tumors (12.8%), and lymphoma (9.9%).
For this study, the patients underwent standardized clinical and laboratory cardiovascular screening. The mean time from cancer diagnosis to cardiovascular screening was 28.4 years (range, 23–36).
The researchers compared the incidence of cardiovascular risk factors and cardiovascular disease in the CVSS cohort and subjects from the Gutenberg Health Study (GHS), a population-based study including more than 15,000 subjects.
Risk factors
The CVSS cohort had a greater risk of 2 cardiovascular risk factors—arterial hypertension and dyslipidemia—than the GHS cohort. In the CVSS cohort, the incidence of dyslipidemia was 28.3%, and the incidence of hypertension was 23.0%.
CVSS subjects had an age-adjusted 38% increase in risk for hypertension (relative risk [RR]=1.38) and a 26% increase in risk for dyslipidemia (RR=1.26).
Hypertension occurred about 6 years earlier in CVSS subjects than GHS subjects (rate advancement period estimator [RAP]=5.75). And dyslipidemia occurred about 8 years earlier in the CVSS cohort (RAP=8.16).
“[T]he premature onset of high blood pressure and blood lipid disorders may play an important role in the development of severe cardiovascular conditions, such as heart disease and stroke, in the long-term,” said study author Philipp S. Wild, MD, of the German Center for Cardiovascular Research (DZHK) in Mainz, Germany.
“We also found that a remarkable number [of CVSS subjects] attended their clinical examination for this study with previously unidentified cardiovascular risk factors and cardiovascular disease. For example, only 62 out of 269 were aware of having dyslipidemia. Consequently, 207, approximately 80%, were only diagnosed at that point.”
Disease
In the CVSS cohort, 4.5% of patients had at least 1 type of cardiovascular disease. This included venous thromboembolism (2.0%), congestive heart failure (1.2%), stroke (0.5%), peripheral artery disease (0.5%), atrial fibrillation (0.4%), and coronary heart disease (0.3%).
CVSS subjects had nearly twice the risk of cardiovascular disease as GHS subjects. The age-adjusted RR was 1.89. And CVSS subjects developed cardiovascular disease roughly 8 years earlier than GHS subjects (RAP=7.9).
In the CVSS cohort, the probability of developing cardiovascular disease was estimated as 2.9% at age 30 and 9.6% at age 45.
The researchers said these findings show that CCSs have a greater risk of cardiovascular disease that continues to increase with age. This, in turn, means CCSs may be more likely to die earlier. However, this might be preventable, according to Dr Wild.
“Early systematic screening, particularly focusing on blood pressure and lipid measurements, might be suggested in all childhood cancer survivors irrespective of the type of cancer or treatment they had had,” Dr Wild said. “This might help to prevent long-term cardiovascular diseases by intervening early—for instance, by modifying lifestyles and having treatment for high blood pressure.”
“Usually, survivors are followed for only 5 to 10 years after completion of therapy, and this is focused on the risk of the cancer returning and the acute adverse effects of their treatment, rather than on other conditions,” added Dr Faber.
“Current guidelines recommend cardiovascular assessments only for subgroups known to be at risk, such as for patients who were treated with anthracycline therapy and/or radiation therapy. However, further investigations are needed to answer questions about the best follow-up care.”
Childhood cancer survivors (CCSs) have an increased risk of premature cardiovascular disease in adulthood, according to a new study.
Researchers found a nearly 2-fold increased risk of cardiovascular diseases in CCSs compared to the general population.
Cardiovascular disease was identified in 4.5% of CCSs and occurred in most before they reached the age of 40, nearly 8 years earlier than in the general population.
“Our results show that these survivors of childhood cancer have a substantially elevated burden of prematurely occurring traditional cardiovascular risk factors and cardiovascular diseases,” said study author Joerg Faber, MD, PhD, of the Johannes Gutenberg University Mainz in Germany.
Dr Faber and his colleagues reported these results in the European Heart Journal.
The researchers evaluated 951 adult CCSs (ages 23 to 48), referred to as the CVSS cohort (Cardiac and Vascular Late Sequelae in Long-Term Survivors of Childhood Cancer Study).
The patients had been diagnosed with cancer between 1980 and 1990. The most common diagnoses were leukemia (43.5%), central nervous system tumors (12.8%), and lymphoma (9.9%).
For this study, the patients underwent standardized clinical and laboratory cardiovascular screening. The mean time from cancer diagnosis to cardiovascular screening was 28.4 years (range, 23–36).
The researchers compared the incidence of cardiovascular risk factors and cardiovascular disease in the CVSS cohort and subjects from the Gutenberg Health Study (GHS), a population-based study including more than 15,000 subjects.
Risk factors
The CVSS cohort had a greater risk of 2 cardiovascular risk factors—arterial hypertension and dyslipidemia—than the GHS cohort. In the CVSS cohort, the incidence of dyslipidemia was 28.3%, and the incidence of hypertension was 23.0%.
CVSS subjects had an age-adjusted 38% increase in risk for hypertension (relative risk [RR]=1.38) and a 26% increase in risk for dyslipidemia (RR=1.26).
Hypertension occurred about 6 years earlier in CVSS subjects than GHS subjects (rate advancement period estimator [RAP]=5.75). And dyslipidemia occurred about 8 years earlier in the CVSS cohort (RAP=8.16).
“[T]he premature onset of high blood pressure and blood lipid disorders may play an important role in the development of severe cardiovascular conditions, such as heart disease and stroke, in the long-term,” said study author Philipp S. Wild, MD, of the German Center for Cardiovascular Research (DZHK) in Mainz, Germany.
“We also found that a remarkable number [of CVSS subjects] attended their clinical examination for this study with previously unidentified cardiovascular risk factors and cardiovascular disease. For example, only 62 out of 269 were aware of having dyslipidemia. Consequently, 207, approximately 80%, were only diagnosed at that point.”
Disease
In the CVSS cohort, 4.5% of patients had at least 1 type of cardiovascular disease. This included venous thromboembolism (2.0%), congestive heart failure (1.2%), stroke (0.5%), peripheral artery disease (0.5%), atrial fibrillation (0.4%), and coronary heart disease (0.3%).
CVSS subjects had nearly twice the risk of cardiovascular disease as GHS subjects. The age-adjusted RR was 1.89. And CVSS subjects developed cardiovascular disease roughly 8 years earlier than GHS subjects (RAP=7.9).
In the CVSS cohort, the probability of developing cardiovascular disease was estimated as 2.9% at age 30 and 9.6% at age 45.
The researchers said these findings show that CCSs have a greater risk of cardiovascular disease that continues to increase with age. This, in turn, means CCSs may be more likely to die earlier. However, this might be preventable, according to Dr Wild.
“Early systematic screening, particularly focusing on blood pressure and lipid measurements, might be suggested in all childhood cancer survivors irrespective of the type of cancer or treatment they had had,” Dr Wild said. “This might help to prevent long-term cardiovascular diseases by intervening early—for instance, by modifying lifestyles and having treatment for high blood pressure.”
“Usually, survivors are followed for only 5 to 10 years after completion of therapy, and this is focused on the risk of the cancer returning and the acute adverse effects of their treatment, rather than on other conditions,” added Dr Faber.
“Current guidelines recommend cardiovascular assessments only for subgroups known to be at risk, such as for patients who were treated with anthracycline therapy and/or radiation therapy. However, further investigations are needed to answer questions about the best follow-up care.”
Childhood cancer survivors (CCSs) have an increased risk of premature cardiovascular disease in adulthood, according to a new study.
Researchers found a nearly 2-fold increased risk of cardiovascular diseases in CCSs compared to the general population.
Cardiovascular disease was identified in 4.5% of CCSs and occurred in most before they reached the age of 40, nearly 8 years earlier than in the general population.
“Our results show that these survivors of childhood cancer have a substantially elevated burden of prematurely occurring traditional cardiovascular risk factors and cardiovascular diseases,” said study author Joerg Faber, MD, PhD, of the Johannes Gutenberg University Mainz in Germany.
Dr Faber and his colleagues reported these results in the European Heart Journal.
The researchers evaluated 951 adult CCSs (ages 23 to 48), referred to as the CVSS cohort (Cardiac and Vascular Late Sequelae in Long-Term Survivors of Childhood Cancer Study).
The patients had been diagnosed with cancer between 1980 and 1990. The most common diagnoses were leukemia (43.5%), central nervous system tumors (12.8%), and lymphoma (9.9%).
For this study, the patients underwent standardized clinical and laboratory cardiovascular screening. The mean time from cancer diagnosis to cardiovascular screening was 28.4 years (range, 23–36).
The researchers compared the incidence of cardiovascular risk factors and cardiovascular disease in the CVSS cohort and subjects from the Gutenberg Health Study (GHS), a population-based study including more than 15,000 subjects.
Risk factors
The CVSS cohort had a greater risk of 2 cardiovascular risk factors—arterial hypertension and dyslipidemia—than the GHS cohort. In the CVSS cohort, the incidence of dyslipidemia was 28.3%, and the incidence of hypertension was 23.0%.
CVSS subjects had an age-adjusted 38% increase in risk for hypertension (relative risk [RR]=1.38) and a 26% increase in risk for dyslipidemia (RR=1.26).
Hypertension occurred about 6 years earlier in CVSS subjects than GHS subjects (rate advancement period estimator [RAP]=5.75). And dyslipidemia occurred about 8 years earlier in the CVSS cohort (RAP=8.16).
“[T]he premature onset of high blood pressure and blood lipid disorders may play an important role in the development of severe cardiovascular conditions, such as heart disease and stroke, in the long-term,” said study author Philipp S. Wild, MD, of the German Center for Cardiovascular Research (DZHK) in Mainz, Germany.
“We also found that a remarkable number [of CVSS subjects] attended their clinical examination for this study with previously unidentified cardiovascular risk factors and cardiovascular disease. For example, only 62 out of 269 were aware of having dyslipidemia. Consequently, 207, approximately 80%, were only diagnosed at that point.”
Disease
In the CVSS cohort, 4.5% of patients had at least 1 type of cardiovascular disease. This included venous thromboembolism (2.0%), congestive heart failure (1.2%), stroke (0.5%), peripheral artery disease (0.5%), atrial fibrillation (0.4%), and coronary heart disease (0.3%).
CVSS subjects had nearly twice the risk of cardiovascular disease as GHS subjects. The age-adjusted RR was 1.89. And CVSS subjects developed cardiovascular disease roughly 8 years earlier than GHS subjects (RAP=7.9).
In the CVSS cohort, the probability of developing cardiovascular disease was estimated as 2.9% at age 30 and 9.6% at age 45.
The researchers said these findings show that CCSs have a greater risk of cardiovascular disease that continues to increase with age. This, in turn, means CCSs may be more likely to die earlier. However, this might be preventable, according to Dr Wild.
“Early systematic screening, particularly focusing on blood pressure and lipid measurements, might be suggested in all childhood cancer survivors irrespective of the type of cancer or treatment they had had,” Dr Wild said. “This might help to prevent long-term cardiovascular diseases by intervening early—for instance, by modifying lifestyles and having treatment for high blood pressure.”
“Usually, survivors are followed for only 5 to 10 years after completion of therapy, and this is focused on the risk of the cancer returning and the acute adverse effects of their treatment, rather than on other conditions,” added Dr Faber.
“Current guidelines recommend cardiovascular assessments only for subgroups known to be at risk, such as for patients who were treated with anthracycline therapy and/or radiation therapy. However, further investigations are needed to answer questions about the best follow-up care.”
Team targets transcription factor in AML
Researchers say they have discovered a way to target the transcription factor MEF2C in acute myeloid leukemia (AML).
The team found they could stop the growth of MEF2C-driven AML cells by blocking either LKB1 or the salt-inducible kinases SIK3 and SIK2.
Christopher Vakoc, MD, PhD, of Cold Spring Harbor Laboratory in Cold Spring Harbor, New York, and his colleagues described this research in Molecular Cell.
The current discoveries are the result of a broad search for potential therapeutic strategies against AML that began several years ago in Dr Vakoc’s lab.
In 2013, his team devised a system based on CRISPR gene editing tools. They used this system to screen large numbers of genes, seeking to discover their impact on cancer cell survival.
Now, the system has revealed that LKB1 and SIK are critical for the survival of certain AML cells. These enzymes had not previously been linked to AML, but the researchers learned that LKB1 and SIK help control MEF2C.
The team observed overlapping LKB1, SIK, and MEF2C dependencies in AML cell lines, particularly MLL fusion lines (MOLM-13, MV4-11, NOMO-1, and THP-1). And the researchers found the transcriptional output of MEF2C could be suppressed by inhibition of LKB1 or SIK.
“At the end of project, we realized we’d actually discovered a way to control a transcription factor,” Dr Vakoc said.
He and his colleagues found that SIK3 inactivation had the strongest effect on MEF2C. Two hours of exposure to the SIK inhibitor HG-9-91-01 (100 nM) was enough to suppress the MEF2C signature.
The researchers also noted that the effect of SIK3 targeting on transcription was attenuated if it was performed in cells deficient in HDAC4. This and related findings suggested that LKB1-SIK3 signaling supports the transcriptional output of MEF2C through inhibition of HDAC4.
Dr Vakoc and his colleagues said the “potency and selectivity of AML growth arrest” they observed after targeting LKB1 or SIK2 and SIK3 resembles the effects of targeting other validated kinase oncogenes in AML, such as FLT3.
The team also said the sensitivity of AML cell lines to HG-9-91-01 “compares favorably” to the sensitivity of cancer cell lines to kinase inhibitors already approved for oncology indications. However, “additional optimization” of HG-9-91-01 is needed.
Researchers say they have discovered a way to target the transcription factor MEF2C in acute myeloid leukemia (AML).
The team found they could stop the growth of MEF2C-driven AML cells by blocking either LKB1 or the salt-inducible kinases SIK3 and SIK2.
Christopher Vakoc, MD, PhD, of Cold Spring Harbor Laboratory in Cold Spring Harbor, New York, and his colleagues described this research in Molecular Cell.
The current discoveries are the result of a broad search for potential therapeutic strategies against AML that began several years ago in Dr Vakoc’s lab.
In 2013, his team devised a system based on CRISPR gene editing tools. They used this system to screen large numbers of genes, seeking to discover their impact on cancer cell survival.
Now, the system has revealed that LKB1 and SIK are critical for the survival of certain AML cells. These enzymes had not previously been linked to AML, but the researchers learned that LKB1 and SIK help control MEF2C.
The team observed overlapping LKB1, SIK, and MEF2C dependencies in AML cell lines, particularly MLL fusion lines (MOLM-13, MV4-11, NOMO-1, and THP-1). And the researchers found the transcriptional output of MEF2C could be suppressed by inhibition of LKB1 or SIK.
“At the end of project, we realized we’d actually discovered a way to control a transcription factor,” Dr Vakoc said.
He and his colleagues found that SIK3 inactivation had the strongest effect on MEF2C. Two hours of exposure to the SIK inhibitor HG-9-91-01 (100 nM) was enough to suppress the MEF2C signature.
The researchers also noted that the effect of SIK3 targeting on transcription was attenuated if it was performed in cells deficient in HDAC4. This and related findings suggested that LKB1-SIK3 signaling supports the transcriptional output of MEF2C through inhibition of HDAC4.
Dr Vakoc and his colleagues said the “potency and selectivity of AML growth arrest” they observed after targeting LKB1 or SIK2 and SIK3 resembles the effects of targeting other validated kinase oncogenes in AML, such as FLT3.
The team also said the sensitivity of AML cell lines to HG-9-91-01 “compares favorably” to the sensitivity of cancer cell lines to kinase inhibitors already approved for oncology indications. However, “additional optimization” of HG-9-91-01 is needed.
Researchers say they have discovered a way to target the transcription factor MEF2C in acute myeloid leukemia (AML).
The team found they could stop the growth of MEF2C-driven AML cells by blocking either LKB1 or the salt-inducible kinases SIK3 and SIK2.
Christopher Vakoc, MD, PhD, of Cold Spring Harbor Laboratory in Cold Spring Harbor, New York, and his colleagues described this research in Molecular Cell.
The current discoveries are the result of a broad search for potential therapeutic strategies against AML that began several years ago in Dr Vakoc’s lab.
In 2013, his team devised a system based on CRISPR gene editing tools. They used this system to screen large numbers of genes, seeking to discover their impact on cancer cell survival.
Now, the system has revealed that LKB1 and SIK are critical for the survival of certain AML cells. These enzymes had not previously been linked to AML, but the researchers learned that LKB1 and SIK help control MEF2C.
The team observed overlapping LKB1, SIK, and MEF2C dependencies in AML cell lines, particularly MLL fusion lines (MOLM-13, MV4-11, NOMO-1, and THP-1). And the researchers found the transcriptional output of MEF2C could be suppressed by inhibition of LKB1 or SIK.
“At the end of project, we realized we’d actually discovered a way to control a transcription factor,” Dr Vakoc said.
He and his colleagues found that SIK3 inactivation had the strongest effect on MEF2C. Two hours of exposure to the SIK inhibitor HG-9-91-01 (100 nM) was enough to suppress the MEF2C signature.
The researchers also noted that the effect of SIK3 targeting on transcription was attenuated if it was performed in cells deficient in HDAC4. This and related findings suggested that LKB1-SIK3 signaling supports the transcriptional output of MEF2C through inhibition of HDAC4.
Dr Vakoc and his colleagues said the “potency and selectivity of AML growth arrest” they observed after targeting LKB1 or SIK2 and SIK3 resembles the effects of targeting other validated kinase oncogenes in AML, such as FLT3.
The team also said the sensitivity of AML cell lines to HG-9-91-01 “compares favorably” to the sensitivity of cancer cell lines to kinase inhibitors already approved for oncology indications. However, “additional optimization” of HG-9-91-01 is needed.