User login
2021 Update on minimally invasive gynecologic surgery

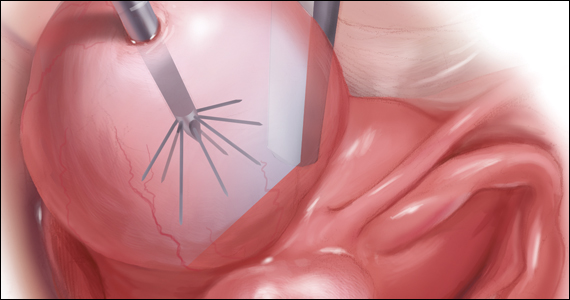
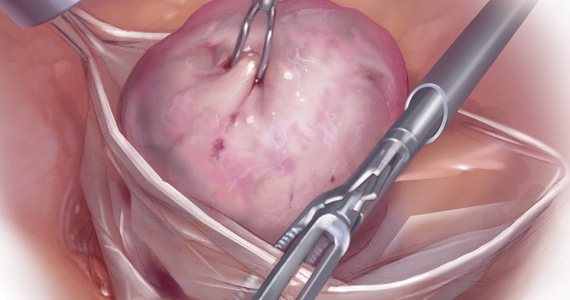
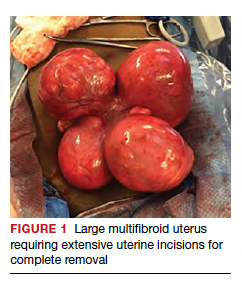
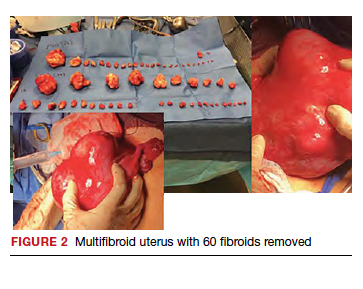
Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5
For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
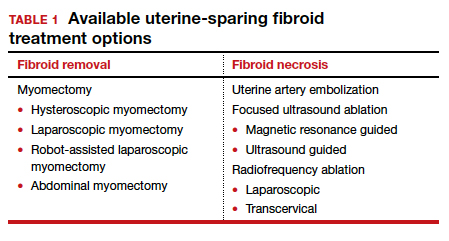
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.
Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
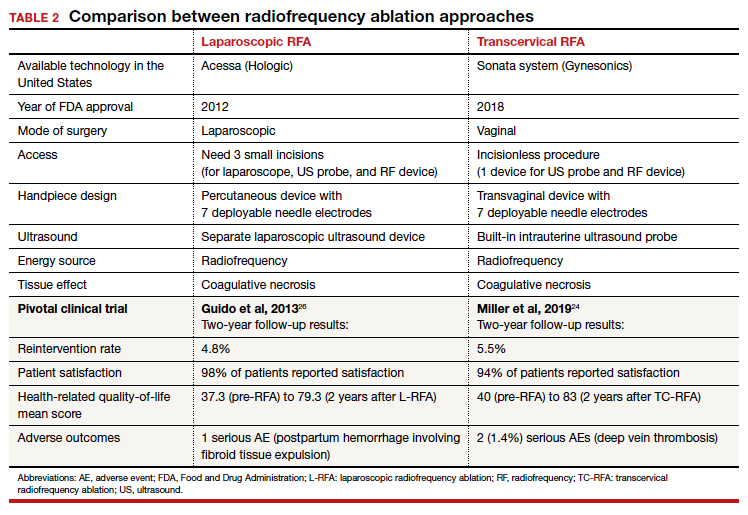
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.
Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5


For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.

Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.

Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5


For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.

Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.

Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.
Electrosurgical hysteroscopy: Principles and expert techniques for optimizing the resectoscope loop
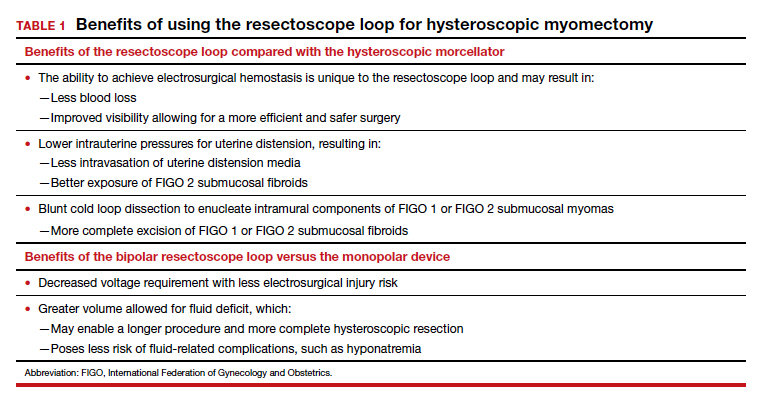
Hysteroscopic mechanical morcellators have gained popularity given their ease of use. Consequently, the resectoscope loop is being used less frequently, which has resulted in less familiarity with this device. The resectoscope loop, however, not only is cost effective but also allows for multiple distinct advantages, such as cold loop dissection of myomas and the ability to obtain electrosurgical hemostasis during operative hysteroscopy.
In this article, we review the basics of electrosurgical principles, compare outcomes associated with monopolar and bipolar resectoscopes, and discuss tips and tricks for optimizing surgical techniques when using the resectoscope loop for hysteroscopic myomectomy.
Evolution of hysteroscopy
The term hysteroscopy comes from the Greek words hystera, for uterus, and skopeo, meaning “to see.” The idea to investigate the uterus dates back to the year 1000 when physicians used a mirror with light to peer into the vaginal vault.
The first known successful hysteroscopy occurred in 1869 when Pantaleoni used an endoscope with a light source to identify uterine polyps in a 60-year-old woman with abnormal uterine bleeding. In 1898, Simon Duplay and Spiro Clado published the first textbook on hysteroscopy in which they described several models of hysteroscopic instruments and techniques.
In the 1950s, Harold Horace Hopkins and Karl Storz modified the shape and length of lenses within the endoscope by substituting longer cylindrical lenses for the old spherical lenses; this permitted improved image brightness and sharpness as well as a smaller diameter of the hysteroscope. Between the 1970s and 1980s, technological improvements allowed for the creation of practical and usable hysteroscopic instruments such as the resectoscope. The resectoscope, originally used in urology for transurethral resection of the prostate, was modified for hysteroscopy by incorporating the use of electrosurgical currents to aid in procedures.
Over the past few decades, continued refinements in technology have improved visualization and surgical techniques. For example, image clarity has been markedly improved, and narrow hysteroscope diameters, as small as 3 to 5 mm, require minimal to no cervical dilation.
Monopolar and bipolar resectoscopes
Electrosurgery is the application of an alternating electrical current to tissue to achieve the clinical effects of surgical cutting or hemostasis via cell vaporization or coagulation. Current runs from the electrosurgical unit (ESU) to the active electrode of the surgical instrument, then goes from the active electrode through the patient’s tissue to the return electrode, and then travels back to the ESU. This flow of current creates an electrical circuit (FIGURE).
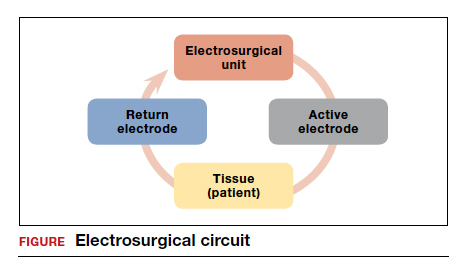
All electrosurgical devices have an active and a return electrode. The difference between monopolar and bipolar resectoscope devices lies in how the resectoscope loop is constructed. Bipolar resectoscope loops house the active and return electrodes on the same tip of the surgical device, which limits how much of the current flows through the patient. Alternatively, monopolar resectoscopes have only the active electrode on the tip of the device and the return electrode is off the surgical field, so the current flows through more of the patient. On monopolar electrosurgical devices, the current runs from the ESU to the active electrode (monopolar loop), which is then applied to tissue to produce the desired tissue effect. The current then travels via a path of least resistance from the surgical field through the patient to the return electrode, which is usually placed on the patient’s thigh, and then back to the ESU. The return electrode is often referred to as the grounding pad.
Continue to: How monopolar energy works...
How monopolar energy works
When first developed, all resectoscopes used monopolar energy. As such, throughout the 1990s, the monopolar resectoscope was the gold standard for performing electrosurgical hysteroscopy. Because the current travels a long distance between the active and the return electrode in a monopolar setup, a hypotonic, nonelectrolyte-rich medium (a poor conductor), such as glycine 1.5%, mannitol 5%, or sorbitol 3%, must be used. If an electrolyte-rich medium, such as normal saline, is used with a monopolar device, the current would be dispersed throughout the medium outside the operative field, causing unwanted tissue effects.
Although nonelectrolyte distension media improve visibility when encountering bleeding, they can be associated with hyponatremia, hyperglycemia, and even lifethreatening cerebral edema. Furthermore, glycine use is contraindicated in patients with renal or hepatic failure since oxidative deamination may cause hyperammonemia. Because of these numerous risk factors, the fluid deficit for hypotonic, nonelectrolyte distension media is limited to 1,000 mL, with a suggested maximum fluid deficit of 750 mL for elderly or fragile patients. Additionally, because the return electrode is off the surgical field in monopolar surgery, there is a risk of current diversion to the cervix, vagina, or vulva because the current travels between the active electrode on the surgical field to the return electrode on the patient’s thigh. The risk of current diversion is greater if there is damage to electrode insulation, loss of contact between the external sheath and the cervix, or direct coupling between the electrode and the surrounding tissue.
Advantages of the bipolar resectoscope
Because of the potential risks associated with the monopolar resectoscope, over the past 25 years the bipolar resectoscope emerged as an alternative due to its numerous benefits (TABLE 1).
Unlike monopolar resectoscopes, bipolar resectoscopes require an electrolyte-rich distension medium such as 0.9% normal saline or lactated Ringer’s. These isotonic distension media allow a much higher fluid deficit (2,500 mL for healthy patients, 1,500 mL for elderly patients or patients with comorbidities) as the isotonic solution is safer to use. Furthermore, it allows for lower voltage settings and decreased electrical spread compared to the monopolar resectoscope since the current stays between the 2 electrodes. Because isotonic media are miscible with blood, however, a potential drawback is that in cases with bleeding, visibility may be more limited compared to hypotonic distension media.
Evidence on fertility outcomes
Several studies have compared operative and fertility outcomes with the use of monopolar versus bipolar hysteroscopy.
In a randomized controlled trial (RCT) comparing outcomes after hysteroscopy with a monopolar (glycine 1.5%) versus bipolar (0.9% normal saline) 26 French resectoscope loop, Berg and colleagues found that the only significant difference between the 2 groups was that the change in serum sodium pre and postoperatively was greater in the monopolar group despite having a smaller mean fluid deficit (765 mL vs 1,227 mL).1
Similarly, in a study of fertility outcomes after monopolar versus bipolar hysteroscopic myomectomy with use of a 26 French resectoscope Collins knife, Roy and colleagues found no significant differences in postoperative pregnancy rates or successful pregnancy outcomes, operative time, fluid deficit, or improvement in menstrual symptoms.2 However, the monopolar group had a much higher incidence of postoperative hyponatremia (30% vs 0%) that required additional days of hospitalization despite similar fluid deficits of between 600 and 700 mL.2
Similar findings were noted in another RCT that compared operative outcomes between monopolar and bipolar resectoscope usage during metroplasty for infertility, with a postoperative hyponatremia incidence of 17.1% in the monopolar group versus 0% in the bipolar group despite similar fluid deficits.3 Energy type had no effect on reproductive outcomes in either group.3
Continue to: How does the resectoscope compare with mechanical tissue removal systems?...
How does the resectoscope compare with mechanical tissue removal systems?
In 2005, the first hysteroscopic mechanical tissue removal system was introduced in the United States, providing an additional treatment method for such intrauterine masses as fibroids and polyps.
Advantages. Rather than using an electrical current, these tissue removal systems use a rotating blade with suction that is introduced through a specially designed rigid hysteroscopic sheath. As the instrument incises the pathology, the tissue is removed from the intrauterine cavity and collected in a specimen bag inside the fluid management system. This immediate removal of tissue allows for insertion of the device only once during initial entry, decreasing both the risk of perforation and operative times. Furthermore, mechanical tissue removal systems can be used with isotonic media, negating the risks associated with hypotonic media. Currently, the 2 mechanical tissue removal systems available in the United States are the TruClear and the MyoSure hysteroscopic tissue removal systems.
Studies comparing mechanical tissue removal of polyps and myomas with conventional resectoscope resection have found that mechanical tissue removal is associated with reduced operative time, fluid deficit, and number of instrument insertions.4-8 However, studies have found no significant difference in postoperative patient satisfaction.7,9
Additionally, hysteroscopic tissue removal systems have an easier learning curve. Van Dongen and colleagues conducted an RCT to compare resident-in-training comfort levels when learning to use both a mechanical tissue removal system and a traditional resectoscope; they found increased comfort with the hysteroscopic tissue removal system, suggesting greater ease of use.10
Drawbacks. Despite their many benefits, mechanical tissue removal systems have some disadvantages when compared with the resectoscope. First, mechanical tissue removal systems are associated with higher instrument costs. In addition, they have extremely limited ability to achieve hemostasis when encountering blood vessels during resection, resulting in poor visibility especially when resecting large myomas with feeding vessels.
Hysteroscopic mechanical tissue removal systems typically use higher intrauterine pressures for uterine distension compared with the resectoscope, especially when trying to improve visibility in a bloody surgical field. Increasing the intrauterine pressure with the distension media allows for compression of the blood vessels. As a result, however, submucosal fibroids classified as FIGO 2 (International Federation of Gynecology and Obstetrics) may be less visible since the higher intrauterine pressure can compress both blood vessels and submucosal fibroids
Additionally, mechanical tissue removal systems have limited ability to resect the intramural component of FIGO 1 or FIGO 2 submucosal fibroids since the intramural portion is embedded in the myometrium. Use of the resectoscope loop instead allows for a technique called the cold loop dissection, which uses the resectoscope loop to bluntly dissect and enucleate the intramural component of FIGO 1 and FIGO 2 submucosal myomas from the surrounding myometrium without activating the current. This blunt cold loop dissection technique allows for a deeper and more thorough resection. Often, if the pseudocapsule plane is identified, even the intramural component of FIGO 1 or FIGO 2 submucosal fibroids can be resected, enabling complete removal.
Lastly, mechanical tissue removal systems are not always faster than resectoscopes for all pathology. We prefer using the resectoscope for larger myomas (>3 cm) as the resectoscope allows for resection and removal of larger myoma chips, helping to decrease operative times. Given the many benefits of the resectoscope, we argue that the resectoscope loop remains a crucial instrument in operative gynecology and that learners should continue to hone their hysteroscopic skills with both the resectoscope and mechanical tissue removal systems.
Tips and tricks for hysteroscopic myomectomy with the resectoscope loop
In the video below, "Bipolar resectoscope: Optimizing safe myomectomy," we review specific surgical techniques for optimizing outcomes and safety with the resectoscope loop. These include:
- bow-and-arrow technique
- identification of the fibroid anatomy (pseudocapsule plane)
- blunt cold loop dissection
- the push-and-tuck method
- efficient electrosurgical hemostasis (TABLE 2).
Although we use bipolar energy during this resection, the resection technique using the monopolar loop is the same.


The takeaway
The resectoscope loop is a valuable tool that offers gynecologic surgeons a wider range of techniques for myomectomy. It also offers several surgical and clinical advantages. It is important to train residents in the use of both hysteroscopic mechanical tissue removal systems and resectoscope loops. ●
- Berg A, Sandvik L, Langebrekke A, et al. A randomized trial comparing monopolar electrodes using glycine 1.5% with two different types of bipolar electrodes (TCRis, Versapoint) using saline, in hysteroscopic surgery. Fertil Steril. 2009;91:1273- 1278.
- Roy KK, Metta S, Kansal Y, et al. A prospective randomized study comparing unipolar versus bipolar hysteroscopic myomectomy in infertile women. J Hum Reprod Sci. 2017;10:185-193.
- Roy KK, Kansal Y, Subbaiah M, et al. Hysteroscopic septal resection using unipolar resectoscope versus bipolar resectoscope: prospective, randomized study. J Obstet Gynaecol Res. 2015;41:952-956.
- Borg MH, Shehata A. Uterine morcellator versus resectoscopy in the management of heavy menstrual flow in reproductiveage women. J Gyn Res. 2016;2:1-8.
- Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12:62-66.
- Smith PP, Middleton LJ, Connor M, et al. Hysteroscopic morcellation compared with electrical resection of endometrial polyps: a randomized controlled trial. Obstet Gynecol. 2014;123:745-751.
- Vitale SG, Sapia F, Rapisarda AMC, et al. Hysteroscopic morcellation of submucous myomas: a systematic review. Biomed Res Int. 2017;2017:6848250.
- Stoll F, Lecointre L, Meyer N, et al. Randomized study comparing a reusable morcellator with a resectoscope in the hysteroscopic treatment of uterine polyps: the RESMO study. J Minimal Invasive Gyn. 2021;28:801-810.
- Lee MM, Matsuzono T. Hysteroscopic intrauterine morcellation of submucosal fibroids: preliminary results in Hong Kong and comparisons with conventional hysteroscopic monopolar loop resection. Hong Kong Med J. 2016;22:56-61.
- van Dongen H, Emanuel MH, Wolterbeek R, et al. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15:466-471.
Hysteroscopic mechanical morcellators have gained popularity given their ease of use. Consequently, the resectoscope loop is being used less frequently, which has resulted in less familiarity with this device. The resectoscope loop, however, not only is cost effective but also allows for multiple distinct advantages, such as cold loop dissection of myomas and the ability to obtain electrosurgical hemostasis during operative hysteroscopy.
In this article, we review the basics of electrosurgical principles, compare outcomes associated with monopolar and bipolar resectoscopes, and discuss tips and tricks for optimizing surgical techniques when using the resectoscope loop for hysteroscopic myomectomy.
Evolution of hysteroscopy
The term hysteroscopy comes from the Greek words hystera, for uterus, and skopeo, meaning “to see.” The idea to investigate the uterus dates back to the year 1000 when physicians used a mirror with light to peer into the vaginal vault.
The first known successful hysteroscopy occurred in 1869 when Pantaleoni used an endoscope with a light source to identify uterine polyps in a 60-year-old woman with abnormal uterine bleeding. In 1898, Simon Duplay and Spiro Clado published the first textbook on hysteroscopy in which they described several models of hysteroscopic instruments and techniques.
In the 1950s, Harold Horace Hopkins and Karl Storz modified the shape and length of lenses within the endoscope by substituting longer cylindrical lenses for the old spherical lenses; this permitted improved image brightness and sharpness as well as a smaller diameter of the hysteroscope. Between the 1970s and 1980s, technological improvements allowed for the creation of practical and usable hysteroscopic instruments such as the resectoscope. The resectoscope, originally used in urology for transurethral resection of the prostate, was modified for hysteroscopy by incorporating the use of electrosurgical currents to aid in procedures.
Over the past few decades, continued refinements in technology have improved visualization and surgical techniques. For example, image clarity has been markedly improved, and narrow hysteroscope diameters, as small as 3 to 5 mm, require minimal to no cervical dilation.
Monopolar and bipolar resectoscopes
Electrosurgery is the application of an alternating electrical current to tissue to achieve the clinical effects of surgical cutting or hemostasis via cell vaporization or coagulation. Current runs from the electrosurgical unit (ESU) to the active electrode of the surgical instrument, then goes from the active electrode through the patient’s tissue to the return electrode, and then travels back to the ESU. This flow of current creates an electrical circuit (FIGURE).

All electrosurgical devices have an active and a return electrode. The difference between monopolar and bipolar resectoscope devices lies in how the resectoscope loop is constructed. Bipolar resectoscope loops house the active and return electrodes on the same tip of the surgical device, which limits how much of the current flows through the patient. Alternatively, monopolar resectoscopes have only the active electrode on the tip of the device and the return electrode is off the surgical field, so the current flows through more of the patient. On monopolar electrosurgical devices, the current runs from the ESU to the active electrode (monopolar loop), which is then applied to tissue to produce the desired tissue effect. The current then travels via a path of least resistance from the surgical field through the patient to the return electrode, which is usually placed on the patient’s thigh, and then back to the ESU. The return electrode is often referred to as the grounding pad.
Continue to: How monopolar energy works...
How monopolar energy works
When first developed, all resectoscopes used monopolar energy. As such, throughout the 1990s, the monopolar resectoscope was the gold standard for performing electrosurgical hysteroscopy. Because the current travels a long distance between the active and the return electrode in a monopolar setup, a hypotonic, nonelectrolyte-rich medium (a poor conductor), such as glycine 1.5%, mannitol 5%, or sorbitol 3%, must be used. If an electrolyte-rich medium, such as normal saline, is used with a monopolar device, the current would be dispersed throughout the medium outside the operative field, causing unwanted tissue effects.
Although nonelectrolyte distension media improve visibility when encountering bleeding, they can be associated with hyponatremia, hyperglycemia, and even lifethreatening cerebral edema. Furthermore, glycine use is contraindicated in patients with renal or hepatic failure since oxidative deamination may cause hyperammonemia. Because of these numerous risk factors, the fluid deficit for hypotonic, nonelectrolyte distension media is limited to 1,000 mL, with a suggested maximum fluid deficit of 750 mL for elderly or fragile patients. Additionally, because the return electrode is off the surgical field in monopolar surgery, there is a risk of current diversion to the cervix, vagina, or vulva because the current travels between the active electrode on the surgical field to the return electrode on the patient’s thigh. The risk of current diversion is greater if there is damage to electrode insulation, loss of contact between the external sheath and the cervix, or direct coupling between the electrode and the surrounding tissue.
Advantages of the bipolar resectoscope
Because of the potential risks associated with the monopolar resectoscope, over the past 25 years the bipolar resectoscope emerged as an alternative due to its numerous benefits (TABLE 1).

Unlike monopolar resectoscopes, bipolar resectoscopes require an electrolyte-rich distension medium such as 0.9% normal saline or lactated Ringer’s. These isotonic distension media allow a much higher fluid deficit (2,500 mL for healthy patients, 1,500 mL for elderly patients or patients with comorbidities) as the isotonic solution is safer to use. Furthermore, it allows for lower voltage settings and decreased electrical spread compared to the monopolar resectoscope since the current stays between the 2 electrodes. Because isotonic media are miscible with blood, however, a potential drawback is that in cases with bleeding, visibility may be more limited compared to hypotonic distension media.
Evidence on fertility outcomes
Several studies have compared operative and fertility outcomes with the use of monopolar versus bipolar hysteroscopy.
In a randomized controlled trial (RCT) comparing outcomes after hysteroscopy with a monopolar (glycine 1.5%) versus bipolar (0.9% normal saline) 26 French resectoscope loop, Berg and colleagues found that the only significant difference between the 2 groups was that the change in serum sodium pre and postoperatively was greater in the monopolar group despite having a smaller mean fluid deficit (765 mL vs 1,227 mL).1
Similarly, in a study of fertility outcomes after monopolar versus bipolar hysteroscopic myomectomy with use of a 26 French resectoscope Collins knife, Roy and colleagues found no significant differences in postoperative pregnancy rates or successful pregnancy outcomes, operative time, fluid deficit, or improvement in menstrual symptoms.2 However, the monopolar group had a much higher incidence of postoperative hyponatremia (30% vs 0%) that required additional days of hospitalization despite similar fluid deficits of between 600 and 700 mL.2
Similar findings were noted in another RCT that compared operative outcomes between monopolar and bipolar resectoscope usage during metroplasty for infertility, with a postoperative hyponatremia incidence of 17.1% in the monopolar group versus 0% in the bipolar group despite similar fluid deficits.3 Energy type had no effect on reproductive outcomes in either group.3
Continue to: How does the resectoscope compare with mechanical tissue removal systems?...
How does the resectoscope compare with mechanical tissue removal systems?
In 2005, the first hysteroscopic mechanical tissue removal system was introduced in the United States, providing an additional treatment method for such intrauterine masses as fibroids and polyps.
Advantages. Rather than using an electrical current, these tissue removal systems use a rotating blade with suction that is introduced through a specially designed rigid hysteroscopic sheath. As the instrument incises the pathology, the tissue is removed from the intrauterine cavity and collected in a specimen bag inside the fluid management system. This immediate removal of tissue allows for insertion of the device only once during initial entry, decreasing both the risk of perforation and operative times. Furthermore, mechanical tissue removal systems can be used with isotonic media, negating the risks associated with hypotonic media. Currently, the 2 mechanical tissue removal systems available in the United States are the TruClear and the MyoSure hysteroscopic tissue removal systems.
Studies comparing mechanical tissue removal of polyps and myomas with conventional resectoscope resection have found that mechanical tissue removal is associated with reduced operative time, fluid deficit, and number of instrument insertions.4-8 However, studies have found no significant difference in postoperative patient satisfaction.7,9
Additionally, hysteroscopic tissue removal systems have an easier learning curve. Van Dongen and colleagues conducted an RCT to compare resident-in-training comfort levels when learning to use both a mechanical tissue removal system and a traditional resectoscope; they found increased comfort with the hysteroscopic tissue removal system, suggesting greater ease of use.10
Drawbacks. Despite their many benefits, mechanical tissue removal systems have some disadvantages when compared with the resectoscope. First, mechanical tissue removal systems are associated with higher instrument costs. In addition, they have extremely limited ability to achieve hemostasis when encountering blood vessels during resection, resulting in poor visibility especially when resecting large myomas with feeding vessels.
Hysteroscopic mechanical tissue removal systems typically use higher intrauterine pressures for uterine distension compared with the resectoscope, especially when trying to improve visibility in a bloody surgical field. Increasing the intrauterine pressure with the distension media allows for compression of the blood vessels. As a result, however, submucosal fibroids classified as FIGO 2 (International Federation of Gynecology and Obstetrics) may be less visible since the higher intrauterine pressure can compress both blood vessels and submucosal fibroids
Additionally, mechanical tissue removal systems have limited ability to resect the intramural component of FIGO 1 or FIGO 2 submucosal fibroids since the intramural portion is embedded in the myometrium. Use of the resectoscope loop instead allows for a technique called the cold loop dissection, which uses the resectoscope loop to bluntly dissect and enucleate the intramural component of FIGO 1 and FIGO 2 submucosal myomas from the surrounding myometrium without activating the current. This blunt cold loop dissection technique allows for a deeper and more thorough resection. Often, if the pseudocapsule plane is identified, even the intramural component of FIGO 1 or FIGO 2 submucosal fibroids can be resected, enabling complete removal.
Lastly, mechanical tissue removal systems are not always faster than resectoscopes for all pathology. We prefer using the resectoscope for larger myomas (>3 cm) as the resectoscope allows for resection and removal of larger myoma chips, helping to decrease operative times. Given the many benefits of the resectoscope, we argue that the resectoscope loop remains a crucial instrument in operative gynecology and that learners should continue to hone their hysteroscopic skills with both the resectoscope and mechanical tissue removal systems.
Tips and tricks for hysteroscopic myomectomy with the resectoscope loop
In the video below, "Bipolar resectoscope: Optimizing safe myomectomy," we review specific surgical techniques for optimizing outcomes and safety with the resectoscope loop. These include:
- bow-and-arrow technique
- identification of the fibroid anatomy (pseudocapsule plane)
- blunt cold loop dissection
- the push-and-tuck method
- efficient electrosurgical hemostasis (TABLE 2).
Although we use bipolar energy during this resection, the resection technique using the monopolar loop is the same.


The takeaway
The resectoscope loop is a valuable tool that offers gynecologic surgeons a wider range of techniques for myomectomy. It also offers several surgical and clinical advantages. It is important to train residents in the use of both hysteroscopic mechanical tissue removal systems and resectoscope loops. ●
Hysteroscopic mechanical morcellators have gained popularity given their ease of use. Consequently, the resectoscope loop is being used less frequently, which has resulted in less familiarity with this device. The resectoscope loop, however, not only is cost effective but also allows for multiple distinct advantages, such as cold loop dissection of myomas and the ability to obtain electrosurgical hemostasis during operative hysteroscopy.
In this article, we review the basics of electrosurgical principles, compare outcomes associated with monopolar and bipolar resectoscopes, and discuss tips and tricks for optimizing surgical techniques when using the resectoscope loop for hysteroscopic myomectomy.
Evolution of hysteroscopy
The term hysteroscopy comes from the Greek words hystera, for uterus, and skopeo, meaning “to see.” The idea to investigate the uterus dates back to the year 1000 when physicians used a mirror with light to peer into the vaginal vault.
The first known successful hysteroscopy occurred in 1869 when Pantaleoni used an endoscope with a light source to identify uterine polyps in a 60-year-old woman with abnormal uterine bleeding. In 1898, Simon Duplay and Spiro Clado published the first textbook on hysteroscopy in which they described several models of hysteroscopic instruments and techniques.
In the 1950s, Harold Horace Hopkins and Karl Storz modified the shape and length of lenses within the endoscope by substituting longer cylindrical lenses for the old spherical lenses; this permitted improved image brightness and sharpness as well as a smaller diameter of the hysteroscope. Between the 1970s and 1980s, technological improvements allowed for the creation of practical and usable hysteroscopic instruments such as the resectoscope. The resectoscope, originally used in urology for transurethral resection of the prostate, was modified for hysteroscopy by incorporating the use of electrosurgical currents to aid in procedures.
Over the past few decades, continued refinements in technology have improved visualization and surgical techniques. For example, image clarity has been markedly improved, and narrow hysteroscope diameters, as small as 3 to 5 mm, require minimal to no cervical dilation.
Monopolar and bipolar resectoscopes
Electrosurgery is the application of an alternating electrical current to tissue to achieve the clinical effects of surgical cutting or hemostasis via cell vaporization or coagulation. Current runs from the electrosurgical unit (ESU) to the active electrode of the surgical instrument, then goes from the active electrode through the patient’s tissue to the return electrode, and then travels back to the ESU. This flow of current creates an electrical circuit (FIGURE).

All electrosurgical devices have an active and a return electrode. The difference between monopolar and bipolar resectoscope devices lies in how the resectoscope loop is constructed. Bipolar resectoscope loops house the active and return electrodes on the same tip of the surgical device, which limits how much of the current flows through the patient. Alternatively, monopolar resectoscopes have only the active electrode on the tip of the device and the return electrode is off the surgical field, so the current flows through more of the patient. On monopolar electrosurgical devices, the current runs from the ESU to the active electrode (monopolar loop), which is then applied to tissue to produce the desired tissue effect. The current then travels via a path of least resistance from the surgical field through the patient to the return electrode, which is usually placed on the patient’s thigh, and then back to the ESU. The return electrode is often referred to as the grounding pad.
Continue to: How monopolar energy works...
How monopolar energy works
When first developed, all resectoscopes used monopolar energy. As such, throughout the 1990s, the monopolar resectoscope was the gold standard for performing electrosurgical hysteroscopy. Because the current travels a long distance between the active and the return electrode in a monopolar setup, a hypotonic, nonelectrolyte-rich medium (a poor conductor), such as glycine 1.5%, mannitol 5%, or sorbitol 3%, must be used. If an electrolyte-rich medium, such as normal saline, is used with a monopolar device, the current would be dispersed throughout the medium outside the operative field, causing unwanted tissue effects.
Although nonelectrolyte distension media improve visibility when encountering bleeding, they can be associated with hyponatremia, hyperglycemia, and even lifethreatening cerebral edema. Furthermore, glycine use is contraindicated in patients with renal or hepatic failure since oxidative deamination may cause hyperammonemia. Because of these numerous risk factors, the fluid deficit for hypotonic, nonelectrolyte distension media is limited to 1,000 mL, with a suggested maximum fluid deficit of 750 mL for elderly or fragile patients. Additionally, because the return electrode is off the surgical field in monopolar surgery, there is a risk of current diversion to the cervix, vagina, or vulva because the current travels between the active electrode on the surgical field to the return electrode on the patient’s thigh. The risk of current diversion is greater if there is damage to electrode insulation, loss of contact between the external sheath and the cervix, or direct coupling between the electrode and the surrounding tissue.
Advantages of the bipolar resectoscope
Because of the potential risks associated with the monopolar resectoscope, over the past 25 years the bipolar resectoscope emerged as an alternative due to its numerous benefits (TABLE 1).

Unlike monopolar resectoscopes, bipolar resectoscopes require an electrolyte-rich distension medium such as 0.9% normal saline or lactated Ringer’s. These isotonic distension media allow a much higher fluid deficit (2,500 mL for healthy patients, 1,500 mL for elderly patients or patients with comorbidities) as the isotonic solution is safer to use. Furthermore, it allows for lower voltage settings and decreased electrical spread compared to the monopolar resectoscope since the current stays between the 2 electrodes. Because isotonic media are miscible with blood, however, a potential drawback is that in cases with bleeding, visibility may be more limited compared to hypotonic distension media.
Evidence on fertility outcomes
Several studies have compared operative and fertility outcomes with the use of monopolar versus bipolar hysteroscopy.
In a randomized controlled trial (RCT) comparing outcomes after hysteroscopy with a monopolar (glycine 1.5%) versus bipolar (0.9% normal saline) 26 French resectoscope loop, Berg and colleagues found that the only significant difference between the 2 groups was that the change in serum sodium pre and postoperatively was greater in the monopolar group despite having a smaller mean fluid deficit (765 mL vs 1,227 mL).1
Similarly, in a study of fertility outcomes after monopolar versus bipolar hysteroscopic myomectomy with use of a 26 French resectoscope Collins knife, Roy and colleagues found no significant differences in postoperative pregnancy rates or successful pregnancy outcomes, operative time, fluid deficit, or improvement in menstrual symptoms.2 However, the monopolar group had a much higher incidence of postoperative hyponatremia (30% vs 0%) that required additional days of hospitalization despite similar fluid deficits of between 600 and 700 mL.2
Similar findings were noted in another RCT that compared operative outcomes between monopolar and bipolar resectoscope usage during metroplasty for infertility, with a postoperative hyponatremia incidence of 17.1% in the monopolar group versus 0% in the bipolar group despite similar fluid deficits.3 Energy type had no effect on reproductive outcomes in either group.3
Continue to: How does the resectoscope compare with mechanical tissue removal systems?...
How does the resectoscope compare with mechanical tissue removal systems?
In 2005, the first hysteroscopic mechanical tissue removal system was introduced in the United States, providing an additional treatment method for such intrauterine masses as fibroids and polyps.
Advantages. Rather than using an electrical current, these tissue removal systems use a rotating blade with suction that is introduced through a specially designed rigid hysteroscopic sheath. As the instrument incises the pathology, the tissue is removed from the intrauterine cavity and collected in a specimen bag inside the fluid management system. This immediate removal of tissue allows for insertion of the device only once during initial entry, decreasing both the risk of perforation and operative times. Furthermore, mechanical tissue removal systems can be used with isotonic media, negating the risks associated with hypotonic media. Currently, the 2 mechanical tissue removal systems available in the United States are the TruClear and the MyoSure hysteroscopic tissue removal systems.
Studies comparing mechanical tissue removal of polyps and myomas with conventional resectoscope resection have found that mechanical tissue removal is associated with reduced operative time, fluid deficit, and number of instrument insertions.4-8 However, studies have found no significant difference in postoperative patient satisfaction.7,9
Additionally, hysteroscopic tissue removal systems have an easier learning curve. Van Dongen and colleagues conducted an RCT to compare resident-in-training comfort levels when learning to use both a mechanical tissue removal system and a traditional resectoscope; they found increased comfort with the hysteroscopic tissue removal system, suggesting greater ease of use.10
Drawbacks. Despite their many benefits, mechanical tissue removal systems have some disadvantages when compared with the resectoscope. First, mechanical tissue removal systems are associated with higher instrument costs. In addition, they have extremely limited ability to achieve hemostasis when encountering blood vessels during resection, resulting in poor visibility especially when resecting large myomas with feeding vessels.
Hysteroscopic mechanical tissue removal systems typically use higher intrauterine pressures for uterine distension compared with the resectoscope, especially when trying to improve visibility in a bloody surgical field. Increasing the intrauterine pressure with the distension media allows for compression of the blood vessels. As a result, however, submucosal fibroids classified as FIGO 2 (International Federation of Gynecology and Obstetrics) may be less visible since the higher intrauterine pressure can compress both blood vessels and submucosal fibroids
Additionally, mechanical tissue removal systems have limited ability to resect the intramural component of FIGO 1 or FIGO 2 submucosal fibroids since the intramural portion is embedded in the myometrium. Use of the resectoscope loop instead allows for a technique called the cold loop dissection, which uses the resectoscope loop to bluntly dissect and enucleate the intramural component of FIGO 1 and FIGO 2 submucosal myomas from the surrounding myometrium without activating the current. This blunt cold loop dissection technique allows for a deeper and more thorough resection. Often, if the pseudocapsule plane is identified, even the intramural component of FIGO 1 or FIGO 2 submucosal fibroids can be resected, enabling complete removal.
Lastly, mechanical tissue removal systems are not always faster than resectoscopes for all pathology. We prefer using the resectoscope for larger myomas (>3 cm) as the resectoscope allows for resection and removal of larger myoma chips, helping to decrease operative times. Given the many benefits of the resectoscope, we argue that the resectoscope loop remains a crucial instrument in operative gynecology and that learners should continue to hone their hysteroscopic skills with both the resectoscope and mechanical tissue removal systems.
Tips and tricks for hysteroscopic myomectomy with the resectoscope loop
In the video below, "Bipolar resectoscope: Optimizing safe myomectomy," we review specific surgical techniques for optimizing outcomes and safety with the resectoscope loop. These include:
- bow-and-arrow technique
- identification of the fibroid anatomy (pseudocapsule plane)
- blunt cold loop dissection
- the push-and-tuck method
- efficient electrosurgical hemostasis (TABLE 2).
Although we use bipolar energy during this resection, the resection technique using the monopolar loop is the same.


The takeaway
The resectoscope loop is a valuable tool that offers gynecologic surgeons a wider range of techniques for myomectomy. It also offers several surgical and clinical advantages. It is important to train residents in the use of both hysteroscopic mechanical tissue removal systems and resectoscope loops. ●
- Berg A, Sandvik L, Langebrekke A, et al. A randomized trial comparing monopolar electrodes using glycine 1.5% with two different types of bipolar electrodes (TCRis, Versapoint) using saline, in hysteroscopic surgery. Fertil Steril. 2009;91:1273- 1278.
- Roy KK, Metta S, Kansal Y, et al. A prospective randomized study comparing unipolar versus bipolar hysteroscopic myomectomy in infertile women. J Hum Reprod Sci. 2017;10:185-193.
- Roy KK, Kansal Y, Subbaiah M, et al. Hysteroscopic septal resection using unipolar resectoscope versus bipolar resectoscope: prospective, randomized study. J Obstet Gynaecol Res. 2015;41:952-956.
- Borg MH, Shehata A. Uterine morcellator versus resectoscopy in the management of heavy menstrual flow in reproductiveage women. J Gyn Res. 2016;2:1-8.
- Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12:62-66.
- Smith PP, Middleton LJ, Connor M, et al. Hysteroscopic morcellation compared with electrical resection of endometrial polyps: a randomized controlled trial. Obstet Gynecol. 2014;123:745-751.
- Vitale SG, Sapia F, Rapisarda AMC, et al. Hysteroscopic morcellation of submucous myomas: a systematic review. Biomed Res Int. 2017;2017:6848250.
- Stoll F, Lecointre L, Meyer N, et al. Randomized study comparing a reusable morcellator with a resectoscope in the hysteroscopic treatment of uterine polyps: the RESMO study. J Minimal Invasive Gyn. 2021;28:801-810.
- Lee MM, Matsuzono T. Hysteroscopic intrauterine morcellation of submucosal fibroids: preliminary results in Hong Kong and comparisons with conventional hysteroscopic monopolar loop resection. Hong Kong Med J. 2016;22:56-61.
- van Dongen H, Emanuel MH, Wolterbeek R, et al. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15:466-471.
- Berg A, Sandvik L, Langebrekke A, et al. A randomized trial comparing monopolar electrodes using glycine 1.5% with two different types of bipolar electrodes (TCRis, Versapoint) using saline, in hysteroscopic surgery. Fertil Steril. 2009;91:1273- 1278.
- Roy KK, Metta S, Kansal Y, et al. A prospective randomized study comparing unipolar versus bipolar hysteroscopic myomectomy in infertile women. J Hum Reprod Sci. 2017;10:185-193.
- Roy KK, Kansal Y, Subbaiah M, et al. Hysteroscopic septal resection using unipolar resectoscope versus bipolar resectoscope: prospective, randomized study. J Obstet Gynaecol Res. 2015;41:952-956.
- Borg MH, Shehata A. Uterine morcellator versus resectoscopy in the management of heavy menstrual flow in reproductiveage women. J Gyn Res. 2016;2:1-8.
- Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12:62-66.
- Smith PP, Middleton LJ, Connor M, et al. Hysteroscopic morcellation compared with electrical resection of endometrial polyps: a randomized controlled trial. Obstet Gynecol. 2014;123:745-751.
- Vitale SG, Sapia F, Rapisarda AMC, et al. Hysteroscopic morcellation of submucous myomas: a systematic review. Biomed Res Int. 2017;2017:6848250.
- Stoll F, Lecointre L, Meyer N, et al. Randomized study comparing a reusable morcellator with a resectoscope in the hysteroscopic treatment of uterine polyps: the RESMO study. J Minimal Invasive Gyn. 2021;28:801-810.
- Lee MM, Matsuzono T. Hysteroscopic intrauterine morcellation of submucosal fibroids: preliminary results in Hong Kong and comparisons with conventional hysteroscopic monopolar loop resection. Hong Kong Med J. 2016;22:56-61.
- van Dongen H, Emanuel MH, Wolterbeek R, et al. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15:466-471.
Laparoscopic specimen retrieval bags in gyn surgery: Expert guidance on selection
The use of minimally invasive gynecologic surgery (MIGS) has grown rapidly over the past 20 years. MIGS, which includes vaginal hysterectomy and laparoscopic hysterectomy, is safe and has fewer complications and a more rapid recovery period than open abdominal surgery.1,2 In 2005, the role of MIGS was expanded further when the US Food and Drug Administration (FDA) approved robot-assisted surgery for the performance of gynecologic procedures.3 As knowledge and experience in the safe performance of MIGS progresses, the rates for MIGS procedures have skyrocketed and continue to grow. Between 2007 and 2010, laparoscopic hysterectomy rates rose from 23.5% to 30.5%, while robot-assisted laparoscopic hysterectomy rates increased from 0.5% to 9.5%, representing 40% of all hysterectomies.4 Due to the benefits of minimally invasive surgery over open abdominal surgery, patient and physician preference for minimally invasive procedures has grown significantly in popularity.1,5
Because incisions are small in minimally invasive surgery, surgeons have been challenged with removing large specimens through incisions that are much smaller than the presenting pathology. One approach is to use a specimen retrieval bag for specimen extraction. Once the dissection is completed, the specimen is placed within the retrieval bag for removal, thus minimizing exposure of the specimen and its contents to the abdominopelvic cavity and incision.
The use of specimen retrieval devices has been advocated to prevent infection, avoid spillage into the peritoneal cavity, and minimize the risk of port-site metastases in cases of potentially cancerous specimens. Devices include affordable and readily available products, such as nonpowdered gloves, and commercially produced bags.6
While the use of specimen containment systems for tissue extraction has been well described in gynecology, the available systems vary widely in construction, size, durability, and shape, potentially leading to confusion and suboptimal bag selection during surgery.7 In this article, we review the most common laparoscopic bags available in the United States, provide an overview of bag characteristics, offer practice guidelines for bag selection, and review bag terminology to highlight important concepts for bag selection.
Controversy spurs change
In April 2014, the FDA warned against the use of power morcellation for specimen removal during minimally invasive surgery, citing a prevalence of 1 in 352 unsuspected uterine sarcomas and 1 in 498 unsuspected uterine leiomyosarcomas among women undergoing hysterectomy or myomectomy for presumed benign leiomyoma.8 Since then, the risk of occult uterine sarcomas, including leiomyosarcoma, in women undergoing surgery for benign gynecologic indications has been determined to be much lower.
Nonetheless, the clinical importance of contained specimen removal was clearly highlighted and the role of specimen retrieval bags soared to the forefront. Open power morcellation is no longer commonly practiced, and national societies such as the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists (ACOG) recommend that containment systems be used for safer specimen retrieval during gynecologic surgery.9-11 After the specimen is placed inside the containment system (typically a specimen bag), the surgeon may deliver the bag through a vaginal colpotomy or through a slightly extended laparoscopic incision to remove bulky specimens using cold-cutting extraction techniques.12-15
Continue to: Know the pathology’s characteristics...
Know the pathology’s characteristics
In most cases, based on imaging studies and physical examination, surgeons have a good idea of what to expect before proceeding with surgery. The 2 most common characteristics used for surgical planning are the specimen size (dimensions) and the tissue type (solid, cystic, soft tissue, or mixed). The mass size can range from less than 1 cm to larger than a 20-week sized fibroid uterus. Assessing the specimen in 3 dimensions is important. Tissue type also is a consideration, as soft and squishy masses, such as ovarian cysts, are easier to deflate and manipulate within the bag compared with solid or calcified tumors, such as a large fibroid uterus or a large dermoid with solid components.
Specimen shape also is a critical determinant for bag selection. Most specimen retrieval bags are tapered to varying degrees, and some have an irregular shape. Long tubular structures, such as fallopian tubes that are composed of soft tissue, fit easily into most bags regardless of bag shape or extent of bag taper, whereas the round shape of a bulky myoma may render certain bags ineffective even if the bag’s entrance accommodates the greatest diameter of the myoma. Often, a round mass will not fully fit into a bag because there is a poor fit between the mass’s shape and the bag’s shape and taper. (We discuss the concept of a poor “fit” below.) Knowing the pathology before starting a procedure can help optimize bag selection, streamline operative flow, and reduce waste.
Overview of laparoscopic bag characteristics and clinical applications
The TABLE lists the most common laparoscopic bags available for purchase in the United States. Details include the trocar size, manufacturer, product name, mouth diameter, volume, bag shape, construction material, and best clinical application.
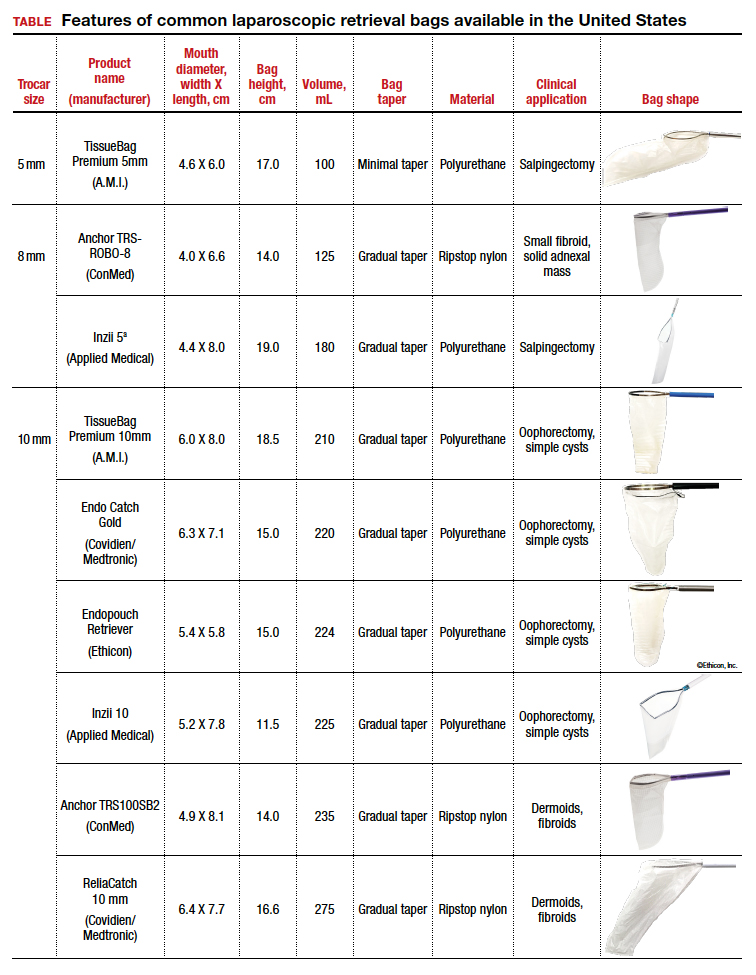
The following are terms used to refer to the components of a laparoscopic retrieval bag:
- Mouth diameter: diameter at the entrance of a fully opened bag (FIGURE 1)
- Bag volume: the total volume a bag can accommodate when completely full
- Bag rim: characteristics of the rim of the bag when opened (that is, rigid vs soft rim, complete vs partial rim mechanism to hold the bag open) (FIGURE 2)
- Bag shape: the shape of the bag when it is fully opened (square shaped vs cone shaped vs curved bag shape) (FIGURE 2)
- Bag taper (severity and type): extent the bag is tapered from the rim of the bag’s entrance to the base of the bag; categorized by taper severity (minimal, gradual, or steep taper) and type (continuous taper or curved taper) (FIGURE 3)
- Ball fit: the maximum spherical specimen size that completely fits into a bag and allows it to cinch closed (FIGURE 4)
- Bag strength: durability of a bag when placed on tension during specimen extraction (weak, moderate, or extremely durable).

Continue to: Mouth diameter...
Mouth diameter
Bag manufacturers often differentiate bag sizes by indicating “volume” in milliliters. Bag volume, however, offers little clinical value to surgeons, as pelvic mass dimensions are usually measured in centimeters on imaging. Rather, an important characteristic for bag selection is the diameter of the rim of the bag when it is fully opened—the so-called bag mouth diameter. For a specimen to fit, the 2 dimensions of the specimen must be smaller than the dimensions of the bag entrance.
Notably, the number often linked to the specimen bag—as, for example, in the 10-mm Endo Catch bag (Covidien/Medtronic)— describes the width of the shaft of the bag before it is opened rather than the mouth diameter of the opened bag. The number actually correlates with the trocar size necessary for bag insertion rather than with the specimen size that can fit into the bag. Therefore, a 10-mm Endo Catch bag cannot fit a 10-cm mass, but rather requires a trocar size of 10 mm or greater for insertion of the bag. Fully opened, the mouth diameters of the 10-mm Endo Catch bag are roughly 6 cm x 7 cm, which allows for delivery of a 6-cm mass.
Because 2 bags that use the same trocar size for insertion may have vastly differing bag dimensions, the surgeon must know the bag mouth diameters when selecting a bag to remove the presenting pathology. For example, the Inzii 12 (Applied Medical) laparoscopic bag has mouth diameters of 9.7 cm × 13.0 cm, whereas the Anchor TRSROBO-12 (ConMed) has mouth diameters of 6.7 cm × 7.6 cm (TABLE). Although both bags can be inserted through a 12-mm trocar, both bags cannot fit the same size mass for removal.
Shape and taper
Laparoscopic bags come in various shapes (curved, cone, or square shaped), with varying levels of bag taper (steep, gradual, or no taper) (FIGURES 2 and 3). While taper has little impact on long and skinny specimens, taper may hinder successful bagging of bulky or spherical specimens.
Each bag has different grades of taper regardless of mouth diameter or trocar size. For round masses, the steeper the taper, the smaller the mass that can comfortably fit within the bag. This concept is connected to the idea of “ball fit,” explained below.
In addition, bag shape may affect what mass size can fit into the bag. An irregularly shaped curved bag or a bag with a steep taper may be well suited for removal of multiple specimens of varying sizes or soft masses that are malleable enough to conform to the bag’s shape (such as a ruptured ovarian cyst). Alternatively, a square-shaped bag or a bag with minimal taper would better accommodate a round mass.
Ball fit
When thinking about large circular masses, such as myomas or ovarian cysts, one must consider the ball fit. This refers to the maximum spherical size of the specimen that fits completely within a bag while allowing the bag to cinch closed. Generally, this is an estimation that factors in the bag shape, extent of the bag taper, bag mouth diameter, and specimen shape and tissue type. At times, although a mass can fit through the bag’s mouth diameter, a steep taper may prevent the mass from being fully bagged and limit closure of the bag (FIGURE 4).
Curved bags like the Anchor TRSVATS-15 (ConMed), which have a very narrow bottom, are prone to a limited ball fit, and thus the bag mouth diameter will not correlate with the largest mass size that can be fitted within the bag. Therefore, if using a steeply tapered bag for removal of large round masses, do not rely on the bag’s mouth diameter for bag selection. The surgeon must visualize the ball fit within the bag, taking into account the specimen size and shape, bag shape, and bag taper. In these scenarios, using the diameter of the midportion of the opened bag may better reflect the mass size that can fit into that bag.
Bag strength
Bag strength depends on the material used for bag construction. Most laparoscopic bags in the United States are made of 3 different materials: polyurethane, polypropylene, and ripstop nylon.
Polyurethane and polypropylene are synthetic plastic polymers; in bag form they are stretchy and, under extreme force, may tear. They are best used for bagging fluid-filled cysts or soft pliable masses that will not require extensive bag or tissue handling, such as extraction of large leiomyomas. Polyurethane and polypropylene bags are more susceptible to puncture with sharp laparoscopic instruments or scalpels, and care must be taken to avoid accidentally cutting the bag during tissue extraction.
Alternatively, bags made of ripstop nylon are favored for their bag strength. Ripstop nylon is a synthetic fabric that is woven together in a crosshatch pattern that makes it resistant to tearing and ripping. It was developed originally during World War II as a replacement for silk parachutes. Modern applications include its use in sails, kites, and high-quality camping equipment. This material has a favorable strength-to-weight ratio, and, in case of a tear, it is less prone to extension of the tear. For surgical applications, these bags are best used for bagging specimens that will require a lot of bag manipulation and tissue extraction. However, the ripstop fabric takes up more space in the incision than polyurethane or polypropylene, leaving the surgeon with less space for tissue extraction. Thus, as a tradeoff for bag strength, the surgeon may need to extend the incision a little, and a small self-retracting wound retractor may be necessary to allow visibility for safe tissue extraction when using a ripstop nylon bag compared with others.
Continue to: Trocar selection is important...
Trocar selection is important
While considering bag selection, the surgeon also must consider trocar selection to allow for laparoscopic insertion of the bag. Trocar size for bag selection refers to the minimum trocar diameter needed to insert the laparoscopic bag. Most bags are designed to fit into a laparoscopic trocar or into the skin incision that previously housed the trocar. Trocar size does not directly correlate with bag mouth diameter; for example, a 10-mm laparoscopic bag that can be inserted through a 10- or 12-mm trocar size cannot fit a 10-cm mass (see the mouth diameter section above).
A tip to maximize operating room (OR) efficiency is to start off with a larger trocar, such as a 12-mm trocar, if it is known that a laparoscopic bag with a 12-mm trocar size will be used, rather than starting with a 5-mm trocar and upsizing the port site incision. This saves time and offers intraoperative flexibility, allowing for the use of larger instruments and quicker insufflation.
Furthermore, if the specimen has a solid component and tissue extraction is anticipated, consider starting off with a large trocar, one that is larger than the bag’s trocar size since the incision likely will be extended. For example, even if a myoma will fit within a 10-mm laparoscopic bag made of ripstop nylon, using a 15-mm trocar rather than a 10-mm trocar may be considered since the skin and fascial incisions will need to be extended to allow for cold-cut tissue extraction. Starting with the larger 15-mm trocar may offer surgical advantages, such as direct needle delivery of larger needles for myometrial closure after myomectomy or direct removal of smaller myomas through the trocar to avoid bagging multiple specimens.
Putting it all together
To optimize efficiency in the OR for specimen removal, we recommend streamlining OR flow and reducing waste by first considering the specimen size, tissue type, bag shape, and trocar selection. Choose a bag by taking into account the bag mouth diameter and the amount of taper you will need to obtain an appropriate ball fit. If the tissue type is soft and pliable, consider a polyurethane or polypropylene bag and the smallest bag size possible, even if it has a narrow bag shape and taper.
However, if the tissue type is solid, the shape is round, and the mass is large (requiring extensive tissue extraction for removal), consider a bag made of ripstop nylon and factor in the bag shape as well as the bag taper. Using a bag without a steep taper may allow a better fit.
After choosing a laparoscopic bag, select the appropriate trocars necessary for completion of the surgery. Consider starting off with a larger trocar rather than spending the time to upsize a trocar if you plan to use a large bag or intend to extend the trocar incision for a contained tissue extraction. These tips will help optimize efficiency, reduce equipment wastage, and prevent intra-abdominal spillage.
Keep in mind that all procedures, including specimen removal using containment systems, have inherent risks. For example, visualization of the mass within the bag and visualization of vital structures may be hindered by bulkiness of the bag or specimen. There is also a risk of bag compromise and leakage, whether through manipulation of the bag or puncture during specimen extraction. Lastly, even though removing a specimen within a containment system minimizes spillage and reports of in-bag cold-knife tissue extraction in women with histologically proven endometrial cancer have suggested that it is safe, laparoscopic bags have not been proven to prevent the dissemination of malignant tissue fragments.16,17
Overall, the inherent risks of specimen extraction during minimally invasive surgery are far outweighed by the well-established advantages of laparoscopic surgery, which carries lower risks of surgical complications such as bleeding and infection, shorter hospital stay, and quicker recovery time compared to laparotomy. There is no doubt minimally invasive surgery offers many benefits.
In summary, for best bag selection, it is equally important to know the characteristics of the pathology as it is to know the features of the specimen retrieval systems available at your institution. Understanding both the pathology and the equipment available will allow the surgeon to make the best surgical decisions for the case. ●
- Desai VB, Wright JD, Lin H, et al. Laparoscopic hysterectomy route, resource use, and outcomes: change after power morcellation warning. Obstet Gynecol. 2019;134:227-238.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
- Liu H, Lu D, Wang L, et al. Robotic surgery for benign gynecological disease. Cochrane Database Syst Rev. 2012;2:CD008978.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
- Turner LC, Shepherd JP, Wang L, et al. Hysterectomy surgery trends: a more accurate depiction of the last decade? Am J Obstet Gynecol. 2013;208:277.e1-7.
- Holme JB, Mortensen FV. A powder-free surgical glove bag for retraction of the gallbladder during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:209-211.
- Siedhoff MT, Cohen SL. Tissue extraction techniques for leiomyomas and uteri during minimally invasive surgery. Obstet Gynecol. 2017;130:1251-1260.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. https://wayback .archive-it.org/7993/20170722215731/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Accessed September 22, 2020.
- AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 770: uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
- Society of Gynecologic Oncology website. SGO position statement: morcellation. December 1, 2013. https://www .sgo.org/newsroom/position-statements-2/morcellation/. Accessed September 22, 2020.
- Advincula AP, Truong MD. ExCITE: minimally invasive tissue extraction made simple with simulation. OBG Manag. 2015;27(12):40-45.
- Solima E, Scagnelli G, Austoni V, et al. Vaginal uterine morcellation within a specimen containment system: a study of bag integrity. J Minim Invasive Gynecol. 2015;22:1244-1246.
- Ghezzi F, Casarin J, De Francesco G, et al. Transvaginal contained tissue extraction after laparoscopic myomectomy: a cohort study. BJOG. 2018;125:367-373.
- Dotson S, Landa A, Ehrisman J, et al. Safety and feasibility of contained uterine morcellation in women undergoing laparoscopic hysterectomy. Gynecol Oncol Res Pract. 2018;5:8.
- Favero G, Miglino G, Köhler C, et al. Vaginal morcellation inside protective pouch: a safe strategy for uterine extration in cases of bulky endometrial cancers: operative and oncological safety of the method. J Minim Invasive Gynecol. 2015;22:938-943.
- Montella F, Riboni F, Cosma S, et al. A safe method of vaginal longitudinal morcellation of bulky uterus with endometrial cancer in a bag at laparoscopy. Surg Endosc. 2014;28:1949-1953.
The use of minimally invasive gynecologic surgery (MIGS) has grown rapidly over the past 20 years. MIGS, which includes vaginal hysterectomy and laparoscopic hysterectomy, is safe and has fewer complications and a more rapid recovery period than open abdominal surgery.1,2 In 2005, the role of MIGS was expanded further when the US Food and Drug Administration (FDA) approved robot-assisted surgery for the performance of gynecologic procedures.3 As knowledge and experience in the safe performance of MIGS progresses, the rates for MIGS procedures have skyrocketed and continue to grow. Between 2007 and 2010, laparoscopic hysterectomy rates rose from 23.5% to 30.5%, while robot-assisted laparoscopic hysterectomy rates increased from 0.5% to 9.5%, representing 40% of all hysterectomies.4 Due to the benefits of minimally invasive surgery over open abdominal surgery, patient and physician preference for minimally invasive procedures has grown significantly in popularity.1,5
Because incisions are small in minimally invasive surgery, surgeons have been challenged with removing large specimens through incisions that are much smaller than the presenting pathology. One approach is to use a specimen retrieval bag for specimen extraction. Once the dissection is completed, the specimen is placed within the retrieval bag for removal, thus minimizing exposure of the specimen and its contents to the abdominopelvic cavity and incision.
The use of specimen retrieval devices has been advocated to prevent infection, avoid spillage into the peritoneal cavity, and minimize the risk of port-site metastases in cases of potentially cancerous specimens. Devices include affordable and readily available products, such as nonpowdered gloves, and commercially produced bags.6
While the use of specimen containment systems for tissue extraction has been well described in gynecology, the available systems vary widely in construction, size, durability, and shape, potentially leading to confusion and suboptimal bag selection during surgery.7 In this article, we review the most common laparoscopic bags available in the United States, provide an overview of bag characteristics, offer practice guidelines for bag selection, and review bag terminology to highlight important concepts for bag selection.

Controversy spurs change
In April 2014, the FDA warned against the use of power morcellation for specimen removal during minimally invasive surgery, citing a prevalence of 1 in 352 unsuspected uterine sarcomas and 1 in 498 unsuspected uterine leiomyosarcomas among women undergoing hysterectomy or myomectomy for presumed benign leiomyoma.8 Since then, the risk of occult uterine sarcomas, including leiomyosarcoma, in women undergoing surgery for benign gynecologic indications has been determined to be much lower.
Nonetheless, the clinical importance of contained specimen removal was clearly highlighted and the role of specimen retrieval bags soared to the forefront. Open power morcellation is no longer commonly practiced, and national societies such as the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists (ACOG) recommend that containment systems be used for safer specimen retrieval during gynecologic surgery.9-11 After the specimen is placed inside the containment system (typically a specimen bag), the surgeon may deliver the bag through a vaginal colpotomy or through a slightly extended laparoscopic incision to remove bulky specimens using cold-cutting extraction techniques.12-15
Continue to: Know the pathology’s characteristics...
Know the pathology’s characteristics
In most cases, based on imaging studies and physical examination, surgeons have a good idea of what to expect before proceeding with surgery. The 2 most common characteristics used for surgical planning are the specimen size (dimensions) and the tissue type (solid, cystic, soft tissue, or mixed). The mass size can range from less than 1 cm to larger than a 20-week sized fibroid uterus. Assessing the specimen in 3 dimensions is important. Tissue type also is a consideration, as soft and squishy masses, such as ovarian cysts, are easier to deflate and manipulate within the bag compared with solid or calcified tumors, such as a large fibroid uterus or a large dermoid with solid components.
Specimen shape also is a critical determinant for bag selection. Most specimen retrieval bags are tapered to varying degrees, and some have an irregular shape. Long tubular structures, such as fallopian tubes that are composed of soft tissue, fit easily into most bags regardless of bag shape or extent of bag taper, whereas the round shape of a bulky myoma may render certain bags ineffective even if the bag’s entrance accommodates the greatest diameter of the myoma. Often, a round mass will not fully fit into a bag because there is a poor fit between the mass’s shape and the bag’s shape and taper. (We discuss the concept of a poor “fit” below.) Knowing the pathology before starting a procedure can help optimize bag selection, streamline operative flow, and reduce waste.
Overview of laparoscopic bag characteristics and clinical applications
The TABLE lists the most common laparoscopic bags available for purchase in the United States. Details include the trocar size, manufacturer, product name, mouth diameter, volume, bag shape, construction material, and best clinical application.


The following are terms used to refer to the components of a laparoscopic retrieval bag:
- Mouth diameter: diameter at the entrance of a fully opened bag (FIGURE 1)
- Bag volume: the total volume a bag can accommodate when completely full
- Bag rim: characteristics of the rim of the bag when opened (that is, rigid vs soft rim, complete vs partial rim mechanism to hold the bag open) (FIGURE 2)
- Bag shape: the shape of the bag when it is fully opened (square shaped vs cone shaped vs curved bag shape) (FIGURE 2)
- Bag taper (severity and type): extent the bag is tapered from the rim of the bag’s entrance to the base of the bag; categorized by taper severity (minimal, gradual, or steep taper) and type (continuous taper or curved taper) (FIGURE 3)
- Ball fit: the maximum spherical specimen size that completely fits into a bag and allows it to cinch closed (FIGURE 4)
- Bag strength: durability of a bag when placed on tension during specimen extraction (weak, moderate, or extremely durable).




Continue to: Mouth diameter...
Mouth diameter
Bag manufacturers often differentiate bag sizes by indicating “volume” in milliliters. Bag volume, however, offers little clinical value to surgeons, as pelvic mass dimensions are usually measured in centimeters on imaging. Rather, an important characteristic for bag selection is the diameter of the rim of the bag when it is fully opened—the so-called bag mouth diameter. For a specimen to fit, the 2 dimensions of the specimen must be smaller than the dimensions of the bag entrance.
Notably, the number often linked to the specimen bag—as, for example, in the 10-mm Endo Catch bag (Covidien/Medtronic)— describes the width of the shaft of the bag before it is opened rather than the mouth diameter of the opened bag. The number actually correlates with the trocar size necessary for bag insertion rather than with the specimen size that can fit into the bag. Therefore, a 10-mm Endo Catch bag cannot fit a 10-cm mass, but rather requires a trocar size of 10 mm or greater for insertion of the bag. Fully opened, the mouth diameters of the 10-mm Endo Catch bag are roughly 6 cm x 7 cm, which allows for delivery of a 6-cm mass.
Because 2 bags that use the same trocar size for insertion may have vastly differing bag dimensions, the surgeon must know the bag mouth diameters when selecting a bag to remove the presenting pathology. For example, the Inzii 12 (Applied Medical) laparoscopic bag has mouth diameters of 9.7 cm × 13.0 cm, whereas the Anchor TRSROBO-12 (ConMed) has mouth diameters of 6.7 cm × 7.6 cm (TABLE). Although both bags can be inserted through a 12-mm trocar, both bags cannot fit the same size mass for removal.
Shape and taper
Laparoscopic bags come in various shapes (curved, cone, or square shaped), with varying levels of bag taper (steep, gradual, or no taper) (FIGURES 2 and 3). While taper has little impact on long and skinny specimens, taper may hinder successful bagging of bulky or spherical specimens.
Each bag has different grades of taper regardless of mouth diameter or trocar size. For round masses, the steeper the taper, the smaller the mass that can comfortably fit within the bag. This concept is connected to the idea of “ball fit,” explained below.
In addition, bag shape may affect what mass size can fit into the bag. An irregularly shaped curved bag or a bag with a steep taper may be well suited for removal of multiple specimens of varying sizes or soft masses that are malleable enough to conform to the bag’s shape (such as a ruptured ovarian cyst). Alternatively, a square-shaped bag or a bag with minimal taper would better accommodate a round mass.
Ball fit
When thinking about large circular masses, such as myomas or ovarian cysts, one must consider the ball fit. This refers to the maximum spherical size of the specimen that fits completely within a bag while allowing the bag to cinch closed. Generally, this is an estimation that factors in the bag shape, extent of the bag taper, bag mouth diameter, and specimen shape and tissue type. At times, although a mass can fit through the bag’s mouth diameter, a steep taper may prevent the mass from being fully bagged and limit closure of the bag (FIGURE 4).
Curved bags like the Anchor TRSVATS-15 (ConMed), which have a very narrow bottom, are prone to a limited ball fit, and thus the bag mouth diameter will not correlate with the largest mass size that can be fitted within the bag. Therefore, if using a steeply tapered bag for removal of large round masses, do not rely on the bag’s mouth diameter for bag selection. The surgeon must visualize the ball fit within the bag, taking into account the specimen size and shape, bag shape, and bag taper. In these scenarios, using the diameter of the midportion of the opened bag may better reflect the mass size that can fit into that bag.
Bag strength
Bag strength depends on the material used for bag construction. Most laparoscopic bags in the United States are made of 3 different materials: polyurethane, polypropylene, and ripstop nylon.
Polyurethane and polypropylene are synthetic plastic polymers; in bag form they are stretchy and, under extreme force, may tear. They are best used for bagging fluid-filled cysts or soft pliable masses that will not require extensive bag or tissue handling, such as extraction of large leiomyomas. Polyurethane and polypropylene bags are more susceptible to puncture with sharp laparoscopic instruments or scalpels, and care must be taken to avoid accidentally cutting the bag during tissue extraction.
Alternatively, bags made of ripstop nylon are favored for their bag strength. Ripstop nylon is a synthetic fabric that is woven together in a crosshatch pattern that makes it resistant to tearing and ripping. It was developed originally during World War II as a replacement for silk parachutes. Modern applications include its use in sails, kites, and high-quality camping equipment. This material has a favorable strength-to-weight ratio, and, in case of a tear, it is less prone to extension of the tear. For surgical applications, these bags are best used for bagging specimens that will require a lot of bag manipulation and tissue extraction. However, the ripstop fabric takes up more space in the incision than polyurethane or polypropylene, leaving the surgeon with less space for tissue extraction. Thus, as a tradeoff for bag strength, the surgeon may need to extend the incision a little, and a small self-retracting wound retractor may be necessary to allow visibility for safe tissue extraction when using a ripstop nylon bag compared with others.
Continue to: Trocar selection is important...
Trocar selection is important
While considering bag selection, the surgeon also must consider trocar selection to allow for laparoscopic insertion of the bag. Trocar size for bag selection refers to the minimum trocar diameter needed to insert the laparoscopic bag. Most bags are designed to fit into a laparoscopic trocar or into the skin incision that previously housed the trocar. Trocar size does not directly correlate with bag mouth diameter; for example, a 10-mm laparoscopic bag that can be inserted through a 10- or 12-mm trocar size cannot fit a 10-cm mass (see the mouth diameter section above).
A tip to maximize operating room (OR) efficiency is to start off with a larger trocar, such as a 12-mm trocar, if it is known that a laparoscopic bag with a 12-mm trocar size will be used, rather than starting with a 5-mm trocar and upsizing the port site incision. This saves time and offers intraoperative flexibility, allowing for the use of larger instruments and quicker insufflation.
Furthermore, if the specimen has a solid component and tissue extraction is anticipated, consider starting off with a large trocar, one that is larger than the bag’s trocar size since the incision likely will be extended. For example, even if a myoma will fit within a 10-mm laparoscopic bag made of ripstop nylon, using a 15-mm trocar rather than a 10-mm trocar may be considered since the skin and fascial incisions will need to be extended to allow for cold-cut tissue extraction. Starting with the larger 15-mm trocar may offer surgical advantages, such as direct needle delivery of larger needles for myometrial closure after myomectomy or direct removal of smaller myomas through the trocar to avoid bagging multiple specimens.
Putting it all together
To optimize efficiency in the OR for specimen removal, we recommend streamlining OR flow and reducing waste by first considering the specimen size, tissue type, bag shape, and trocar selection. Choose a bag by taking into account the bag mouth diameter and the amount of taper you will need to obtain an appropriate ball fit. If the tissue type is soft and pliable, consider a polyurethane or polypropylene bag and the smallest bag size possible, even if it has a narrow bag shape and taper.
However, if the tissue type is solid, the shape is round, and the mass is large (requiring extensive tissue extraction for removal), consider a bag made of ripstop nylon and factor in the bag shape as well as the bag taper. Using a bag without a steep taper may allow a better fit.
After choosing a laparoscopic bag, select the appropriate trocars necessary for completion of the surgery. Consider starting off with a larger trocar rather than spending the time to upsize a trocar if you plan to use a large bag or intend to extend the trocar incision for a contained tissue extraction. These tips will help optimize efficiency, reduce equipment wastage, and prevent intra-abdominal spillage.
Keep in mind that all procedures, including specimen removal using containment systems, have inherent risks. For example, visualization of the mass within the bag and visualization of vital structures may be hindered by bulkiness of the bag or specimen. There is also a risk of bag compromise and leakage, whether through manipulation of the bag or puncture during specimen extraction. Lastly, even though removing a specimen within a containment system minimizes spillage and reports of in-bag cold-knife tissue extraction in women with histologically proven endometrial cancer have suggested that it is safe, laparoscopic bags have not been proven to prevent the dissemination of malignant tissue fragments.16,17
Overall, the inherent risks of specimen extraction during minimally invasive surgery are far outweighed by the well-established advantages of laparoscopic surgery, which carries lower risks of surgical complications such as bleeding and infection, shorter hospital stay, and quicker recovery time compared to laparotomy. There is no doubt minimally invasive surgery offers many benefits.
In summary, for best bag selection, it is equally important to know the characteristics of the pathology as it is to know the features of the specimen retrieval systems available at your institution. Understanding both the pathology and the equipment available will allow the surgeon to make the best surgical decisions for the case. ●
The use of minimally invasive gynecologic surgery (MIGS) has grown rapidly over the past 20 years. MIGS, which includes vaginal hysterectomy and laparoscopic hysterectomy, is safe and has fewer complications and a more rapid recovery period than open abdominal surgery.1,2 In 2005, the role of MIGS was expanded further when the US Food and Drug Administration (FDA) approved robot-assisted surgery for the performance of gynecologic procedures.3 As knowledge and experience in the safe performance of MIGS progresses, the rates for MIGS procedures have skyrocketed and continue to grow. Between 2007 and 2010, laparoscopic hysterectomy rates rose from 23.5% to 30.5%, while robot-assisted laparoscopic hysterectomy rates increased from 0.5% to 9.5%, representing 40% of all hysterectomies.4 Due to the benefits of minimally invasive surgery over open abdominal surgery, patient and physician preference for minimally invasive procedures has grown significantly in popularity.1,5
Because incisions are small in minimally invasive surgery, surgeons have been challenged with removing large specimens through incisions that are much smaller than the presenting pathology. One approach is to use a specimen retrieval bag for specimen extraction. Once the dissection is completed, the specimen is placed within the retrieval bag for removal, thus minimizing exposure of the specimen and its contents to the abdominopelvic cavity and incision.
The use of specimen retrieval devices has been advocated to prevent infection, avoid spillage into the peritoneal cavity, and minimize the risk of port-site metastases in cases of potentially cancerous specimens. Devices include affordable and readily available products, such as nonpowdered gloves, and commercially produced bags.6
While the use of specimen containment systems for tissue extraction has been well described in gynecology, the available systems vary widely in construction, size, durability, and shape, potentially leading to confusion and suboptimal bag selection during surgery.7 In this article, we review the most common laparoscopic bags available in the United States, provide an overview of bag characteristics, offer practice guidelines for bag selection, and review bag terminology to highlight important concepts for bag selection.

Controversy spurs change
In April 2014, the FDA warned against the use of power morcellation for specimen removal during minimally invasive surgery, citing a prevalence of 1 in 352 unsuspected uterine sarcomas and 1 in 498 unsuspected uterine leiomyosarcomas among women undergoing hysterectomy or myomectomy for presumed benign leiomyoma.8 Since then, the risk of occult uterine sarcomas, including leiomyosarcoma, in women undergoing surgery for benign gynecologic indications has been determined to be much lower.
Nonetheless, the clinical importance of contained specimen removal was clearly highlighted and the role of specimen retrieval bags soared to the forefront. Open power morcellation is no longer commonly practiced, and national societies such as the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists (ACOG) recommend that containment systems be used for safer specimen retrieval during gynecologic surgery.9-11 After the specimen is placed inside the containment system (typically a specimen bag), the surgeon may deliver the bag through a vaginal colpotomy or through a slightly extended laparoscopic incision to remove bulky specimens using cold-cutting extraction techniques.12-15
Continue to: Know the pathology’s characteristics...
Know the pathology’s characteristics
In most cases, based on imaging studies and physical examination, surgeons have a good idea of what to expect before proceeding with surgery. The 2 most common characteristics used for surgical planning are the specimen size (dimensions) and the tissue type (solid, cystic, soft tissue, or mixed). The mass size can range from less than 1 cm to larger than a 20-week sized fibroid uterus. Assessing the specimen in 3 dimensions is important. Tissue type also is a consideration, as soft and squishy masses, such as ovarian cysts, are easier to deflate and manipulate within the bag compared with solid or calcified tumors, such as a large fibroid uterus or a large dermoid with solid components.
Specimen shape also is a critical determinant for bag selection. Most specimen retrieval bags are tapered to varying degrees, and some have an irregular shape. Long tubular structures, such as fallopian tubes that are composed of soft tissue, fit easily into most bags regardless of bag shape or extent of bag taper, whereas the round shape of a bulky myoma may render certain bags ineffective even if the bag’s entrance accommodates the greatest diameter of the myoma. Often, a round mass will not fully fit into a bag because there is a poor fit between the mass’s shape and the bag’s shape and taper. (We discuss the concept of a poor “fit” below.) Knowing the pathology before starting a procedure can help optimize bag selection, streamline operative flow, and reduce waste.
Overview of laparoscopic bag characteristics and clinical applications
The TABLE lists the most common laparoscopic bags available for purchase in the United States. Details include the trocar size, manufacturer, product name, mouth diameter, volume, bag shape, construction material, and best clinical application.


The following are terms used to refer to the components of a laparoscopic retrieval bag:
- Mouth diameter: diameter at the entrance of a fully opened bag (FIGURE 1)
- Bag volume: the total volume a bag can accommodate when completely full
- Bag rim: characteristics of the rim of the bag when opened (that is, rigid vs soft rim, complete vs partial rim mechanism to hold the bag open) (FIGURE 2)
- Bag shape: the shape of the bag when it is fully opened (square shaped vs cone shaped vs curved bag shape) (FIGURE 2)
- Bag taper (severity and type): extent the bag is tapered from the rim of the bag’s entrance to the base of the bag; categorized by taper severity (minimal, gradual, or steep taper) and type (continuous taper or curved taper) (FIGURE 3)
- Ball fit: the maximum spherical specimen size that completely fits into a bag and allows it to cinch closed (FIGURE 4)
- Bag strength: durability of a bag when placed on tension during specimen extraction (weak, moderate, or extremely durable).




Continue to: Mouth diameter...
Mouth diameter
Bag manufacturers often differentiate bag sizes by indicating “volume” in milliliters. Bag volume, however, offers little clinical value to surgeons, as pelvic mass dimensions are usually measured in centimeters on imaging. Rather, an important characteristic for bag selection is the diameter of the rim of the bag when it is fully opened—the so-called bag mouth diameter. For a specimen to fit, the 2 dimensions of the specimen must be smaller than the dimensions of the bag entrance.
Notably, the number often linked to the specimen bag—as, for example, in the 10-mm Endo Catch bag (Covidien/Medtronic)— describes the width of the shaft of the bag before it is opened rather than the mouth diameter of the opened bag. The number actually correlates with the trocar size necessary for bag insertion rather than with the specimen size that can fit into the bag. Therefore, a 10-mm Endo Catch bag cannot fit a 10-cm mass, but rather requires a trocar size of 10 mm or greater for insertion of the bag. Fully opened, the mouth diameters of the 10-mm Endo Catch bag are roughly 6 cm x 7 cm, which allows for delivery of a 6-cm mass.
Because 2 bags that use the same trocar size for insertion may have vastly differing bag dimensions, the surgeon must know the bag mouth diameters when selecting a bag to remove the presenting pathology. For example, the Inzii 12 (Applied Medical) laparoscopic bag has mouth diameters of 9.7 cm × 13.0 cm, whereas the Anchor TRSROBO-12 (ConMed) has mouth diameters of 6.7 cm × 7.6 cm (TABLE). Although both bags can be inserted through a 12-mm trocar, both bags cannot fit the same size mass for removal.
Shape and taper
Laparoscopic bags come in various shapes (curved, cone, or square shaped), with varying levels of bag taper (steep, gradual, or no taper) (FIGURES 2 and 3). While taper has little impact on long and skinny specimens, taper may hinder successful bagging of bulky or spherical specimens.
Each bag has different grades of taper regardless of mouth diameter or trocar size. For round masses, the steeper the taper, the smaller the mass that can comfortably fit within the bag. This concept is connected to the idea of “ball fit,” explained below.
In addition, bag shape may affect what mass size can fit into the bag. An irregularly shaped curved bag or a bag with a steep taper may be well suited for removal of multiple specimens of varying sizes or soft masses that are malleable enough to conform to the bag’s shape (such as a ruptured ovarian cyst). Alternatively, a square-shaped bag or a bag with minimal taper would better accommodate a round mass.
Ball fit
When thinking about large circular masses, such as myomas or ovarian cysts, one must consider the ball fit. This refers to the maximum spherical size of the specimen that fits completely within a bag while allowing the bag to cinch closed. Generally, this is an estimation that factors in the bag shape, extent of the bag taper, bag mouth diameter, and specimen shape and tissue type. At times, although a mass can fit through the bag’s mouth diameter, a steep taper may prevent the mass from being fully bagged and limit closure of the bag (FIGURE 4).
Curved bags like the Anchor TRSVATS-15 (ConMed), which have a very narrow bottom, are prone to a limited ball fit, and thus the bag mouth diameter will not correlate with the largest mass size that can be fitted within the bag. Therefore, if using a steeply tapered bag for removal of large round masses, do not rely on the bag’s mouth diameter for bag selection. The surgeon must visualize the ball fit within the bag, taking into account the specimen size and shape, bag shape, and bag taper. In these scenarios, using the diameter of the midportion of the opened bag may better reflect the mass size that can fit into that bag.
Bag strength
Bag strength depends on the material used for bag construction. Most laparoscopic bags in the United States are made of 3 different materials: polyurethane, polypropylene, and ripstop nylon.
Polyurethane and polypropylene are synthetic plastic polymers; in bag form they are stretchy and, under extreme force, may tear. They are best used for bagging fluid-filled cysts or soft pliable masses that will not require extensive bag or tissue handling, such as extraction of large leiomyomas. Polyurethane and polypropylene bags are more susceptible to puncture with sharp laparoscopic instruments or scalpels, and care must be taken to avoid accidentally cutting the bag during tissue extraction.
Alternatively, bags made of ripstop nylon are favored for their bag strength. Ripstop nylon is a synthetic fabric that is woven together in a crosshatch pattern that makes it resistant to tearing and ripping. It was developed originally during World War II as a replacement for silk parachutes. Modern applications include its use in sails, kites, and high-quality camping equipment. This material has a favorable strength-to-weight ratio, and, in case of a tear, it is less prone to extension of the tear. For surgical applications, these bags are best used for bagging specimens that will require a lot of bag manipulation and tissue extraction. However, the ripstop fabric takes up more space in the incision than polyurethane or polypropylene, leaving the surgeon with less space for tissue extraction. Thus, as a tradeoff for bag strength, the surgeon may need to extend the incision a little, and a small self-retracting wound retractor may be necessary to allow visibility for safe tissue extraction when using a ripstop nylon bag compared with others.
Continue to: Trocar selection is important...
Trocar selection is important
While considering bag selection, the surgeon also must consider trocar selection to allow for laparoscopic insertion of the bag. Trocar size for bag selection refers to the minimum trocar diameter needed to insert the laparoscopic bag. Most bags are designed to fit into a laparoscopic trocar or into the skin incision that previously housed the trocar. Trocar size does not directly correlate with bag mouth diameter; for example, a 10-mm laparoscopic bag that can be inserted through a 10- or 12-mm trocar size cannot fit a 10-cm mass (see the mouth diameter section above).
A tip to maximize operating room (OR) efficiency is to start off with a larger trocar, such as a 12-mm trocar, if it is known that a laparoscopic bag with a 12-mm trocar size will be used, rather than starting with a 5-mm trocar and upsizing the port site incision. This saves time and offers intraoperative flexibility, allowing for the use of larger instruments and quicker insufflation.
Furthermore, if the specimen has a solid component and tissue extraction is anticipated, consider starting off with a large trocar, one that is larger than the bag’s trocar size since the incision likely will be extended. For example, even if a myoma will fit within a 10-mm laparoscopic bag made of ripstop nylon, using a 15-mm trocar rather than a 10-mm trocar may be considered since the skin and fascial incisions will need to be extended to allow for cold-cut tissue extraction. Starting with the larger 15-mm trocar may offer surgical advantages, such as direct needle delivery of larger needles for myometrial closure after myomectomy or direct removal of smaller myomas through the trocar to avoid bagging multiple specimens.
Putting it all together
To optimize efficiency in the OR for specimen removal, we recommend streamlining OR flow and reducing waste by first considering the specimen size, tissue type, bag shape, and trocar selection. Choose a bag by taking into account the bag mouth diameter and the amount of taper you will need to obtain an appropriate ball fit. If the tissue type is soft and pliable, consider a polyurethane or polypropylene bag and the smallest bag size possible, even if it has a narrow bag shape and taper.
However, if the tissue type is solid, the shape is round, and the mass is large (requiring extensive tissue extraction for removal), consider a bag made of ripstop nylon and factor in the bag shape as well as the bag taper. Using a bag without a steep taper may allow a better fit.
After choosing a laparoscopic bag, select the appropriate trocars necessary for completion of the surgery. Consider starting off with a larger trocar rather than spending the time to upsize a trocar if you plan to use a large bag or intend to extend the trocar incision for a contained tissue extraction. These tips will help optimize efficiency, reduce equipment wastage, and prevent intra-abdominal spillage.
Keep in mind that all procedures, including specimen removal using containment systems, have inherent risks. For example, visualization of the mass within the bag and visualization of vital structures may be hindered by bulkiness of the bag or specimen. There is also a risk of bag compromise and leakage, whether through manipulation of the bag or puncture during specimen extraction. Lastly, even though removing a specimen within a containment system minimizes spillage and reports of in-bag cold-knife tissue extraction in women with histologically proven endometrial cancer have suggested that it is safe, laparoscopic bags have not been proven to prevent the dissemination of malignant tissue fragments.16,17
Overall, the inherent risks of specimen extraction during minimally invasive surgery are far outweighed by the well-established advantages of laparoscopic surgery, which carries lower risks of surgical complications such as bleeding and infection, shorter hospital stay, and quicker recovery time compared to laparotomy. There is no doubt minimally invasive surgery offers many benefits.
In summary, for best bag selection, it is equally important to know the characteristics of the pathology as it is to know the features of the specimen retrieval systems available at your institution. Understanding both the pathology and the equipment available will allow the surgeon to make the best surgical decisions for the case. ●
- Desai VB, Wright JD, Lin H, et al. Laparoscopic hysterectomy route, resource use, and outcomes: change after power morcellation warning. Obstet Gynecol. 2019;134:227-238.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
- Liu H, Lu D, Wang L, et al. Robotic surgery for benign gynecological disease. Cochrane Database Syst Rev. 2012;2:CD008978.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
- Turner LC, Shepherd JP, Wang L, et al. Hysterectomy surgery trends: a more accurate depiction of the last decade? Am J Obstet Gynecol. 2013;208:277.e1-7.
- Holme JB, Mortensen FV. A powder-free surgical glove bag for retraction of the gallbladder during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:209-211.
- Siedhoff MT, Cohen SL. Tissue extraction techniques for leiomyomas and uteri during minimally invasive surgery. Obstet Gynecol. 2017;130:1251-1260.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. https://wayback .archive-it.org/7993/20170722215731/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Accessed September 22, 2020.
- AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 770: uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
- Society of Gynecologic Oncology website. SGO position statement: morcellation. December 1, 2013. https://www .sgo.org/newsroom/position-statements-2/morcellation/. Accessed September 22, 2020.
- Advincula AP, Truong MD. ExCITE: minimally invasive tissue extraction made simple with simulation. OBG Manag. 2015;27(12):40-45.
- Solima E, Scagnelli G, Austoni V, et al. Vaginal uterine morcellation within a specimen containment system: a study of bag integrity. J Minim Invasive Gynecol. 2015;22:1244-1246.
- Ghezzi F, Casarin J, De Francesco G, et al. Transvaginal contained tissue extraction after laparoscopic myomectomy: a cohort study. BJOG. 2018;125:367-373.
- Dotson S, Landa A, Ehrisman J, et al. Safety and feasibility of contained uterine morcellation in women undergoing laparoscopic hysterectomy. Gynecol Oncol Res Pract. 2018;5:8.
- Favero G, Miglino G, Köhler C, et al. Vaginal morcellation inside protective pouch: a safe strategy for uterine extration in cases of bulky endometrial cancers: operative and oncological safety of the method. J Minim Invasive Gynecol. 2015;22:938-943.
- Montella F, Riboni F, Cosma S, et al. A safe method of vaginal longitudinal morcellation of bulky uterus with endometrial cancer in a bag at laparoscopy. Surg Endosc. 2014;28:1949-1953.
- Desai VB, Wright JD, Lin H, et al. Laparoscopic hysterectomy route, resource use, and outcomes: change after power morcellation warning. Obstet Gynecol. 2019;134:227-238.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
- Liu H, Lu D, Wang L, et al. Robotic surgery for benign gynecological disease. Cochrane Database Syst Rev. 2012;2:CD008978.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
- Turner LC, Shepherd JP, Wang L, et al. Hysterectomy surgery trends: a more accurate depiction of the last decade? Am J Obstet Gynecol. 2013;208:277.e1-7.
- Holme JB, Mortensen FV. A powder-free surgical glove bag for retraction of the gallbladder during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:209-211.
- Siedhoff MT, Cohen SL. Tissue extraction techniques for leiomyomas and uteri during minimally invasive surgery. Obstet Gynecol. 2017;130:1251-1260.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. https://wayback .archive-it.org/7993/20170722215731/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Accessed September 22, 2020.
- AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 770: uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
- Society of Gynecologic Oncology website. SGO position statement: morcellation. December 1, 2013. https://www .sgo.org/newsroom/position-statements-2/morcellation/. Accessed September 22, 2020.
- Advincula AP, Truong MD. ExCITE: minimally invasive tissue extraction made simple with simulation. OBG Manag. 2015;27(12):40-45.
- Solima E, Scagnelli G, Austoni V, et al. Vaginal uterine morcellation within a specimen containment system: a study of bag integrity. J Minim Invasive Gynecol. 2015;22:1244-1246.
- Ghezzi F, Casarin J, De Francesco G, et al. Transvaginal contained tissue extraction after laparoscopic myomectomy: a cohort study. BJOG. 2018;125:367-373.
- Dotson S, Landa A, Ehrisman J, et al. Safety and feasibility of contained uterine morcellation in women undergoing laparoscopic hysterectomy. Gynecol Oncol Res Pract. 2018;5:8.
- Favero G, Miglino G, Köhler C, et al. Vaginal morcellation inside protective pouch: a safe strategy for uterine extration in cases of bulky endometrial cancers: operative and oncological safety of the method. J Minim Invasive Gynecol. 2015;22:938-943.
- Montella F, Riboni F, Cosma S, et al. A safe method of vaginal longitudinal morcellation of bulky uterus with endometrial cancer in a bag at laparoscopy. Surg Endosc. 2014;28:1949-1953.
Endometriosis: Expert perspectives on medical and surgical management
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.
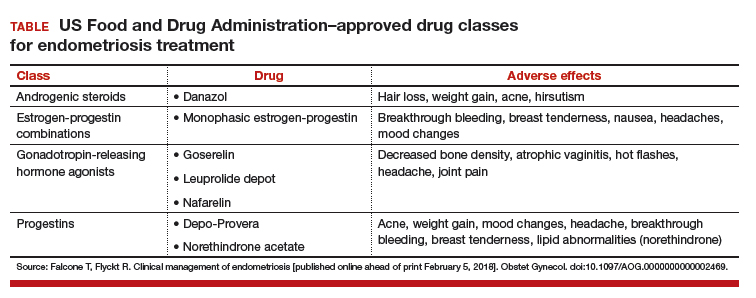
Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.

Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis is one of the more daunting diagnoses that gynecologists treat. In this roundtable discussion, moderated by
First-time evaluation
Arnold P. Advincula, MD: When a patient presents to your practice for the first time and you suspect endometriosis, what considerations tailor your evaluation, and what does that evaluation involve?
Hye-Chun Hur, MD, MPH: The diagnosis is contingent on a patient’s presenting profile. How symptomatic is she? How old is she? What are her reproductive goals? The gold standard for diagnosis is a histologic diagnosis, which is surgical. Depending on the age profile, however, and how close she is to menopause, the patient may be managed medically. Even women in the young reproductive age group may be managed medically if symptoms are responsive to medical treatment.
Douglas N. Brown, MD: I agree. When a patient presents without a laparoscopy, or a tissue diagnosis, but the symptoms are consistent with likely endometriosis (depending on where she is in her reproductive cycle and what her goals are), I think treating with a first-line therapy—hormonal treatments such as progestin-only oral contraceptive pills—is acceptable. I usually conduct a treatment trial period of 3 to 6 months to see if she obtains any symptom relief.
If that first-line treatment fails, generally you can move to a second-line treatment.
I have a discussion in which I either offer a second-line treatment, such as medroxyprogesterone (Depo-Provera) or leuprolide acetate (Lupron Depot), or get a tissue diagnosis, if possible, by performing laparoscopy. If first-line or even second-line therapy fails, you need to consider doing a diagnostic laparoscopy to confirm or deny the diagnosis.

Dr. Advincula: Are there any points in the evaluation of a patient who visits your practice for the first time where you would immediately offer a surgical approach, as opposed to starting with medical management?
Dr. Hur: A large percentage of my patients undergo surgical evaluation, as surgical diagnosis is the gold standard. If you look at the literature, even among surgeons, the accuracy of visual diagnosis is not great.1,2 I target individuals who are either not responsive to medical treatment or who have never tried medical treatment but are trying to conceive, so they are not medical candidates, or individuals who genuinely want a diagnosis for surgical management—sometimes even before first-line medical treatment.
Dr. Brown: Your examination sometimes also dictates your approach. A patient may never have had a laparoscopy or hormone therapy, but if you find uterosacral ligament nodularity, extreme pain on examination, and suspicious findings on ultrasound or otherwise, a diagnostic laparoscopy may be warranted to confirm the diagnosis.
Endometrioma management
Dr. Advincula: Let’s jump ahead. You have decided to proceed with laparoscopy and you encounter an endometrioma. What is your management strategy, particularly in a fertility-desiring patient?
Dr. Hur: Even if a woman has not undergone first-line medical treatment, if she is trying to conceive or presents with infertility, it’s a different balancing act for approaching the patient. When a woman presents, either with an ultrasound finding or an intraoperative finding of an endometrioma, I am a strong advocate of treating symptomatic disease, which means complete cyst excision. Good clinical data suggest that reproductive outcomes are improved for spontaneous pregnancy rates when you excise an endometrioma.3-6
Dr. Advincula: What are the risks of excision of an endometrioma cyst that patients need to know about?
Dr. Brown: Current standard of care is cystectomy, stripping the cyst wall away from the ovarian cortex. There is some concern that the stripping process, depending on how long the endometrioma has been present within the ovary, can cause some destruction to the underlying oocytes and perhaps impact that ovary’s ability to produce viable eggs.
Some studies, from France in particular, have investigated different energy sources, such as plasma energy, that make it possible to remove part of the cyst and then use the plasma energy to vaporize the rest of the cyst wall that may be lying on the cortex. Researchers looked at anti-Müllerian hormone levels, and there does seem to be a difference in terms of how you remove the cyst.7-9 This energy source is not available to everyone; it’s similar to laser but does not have as much penetration. Standard of care is still ovarian stripping.
The conversation with the patient—if she is already infertile and this cyst is a problem—would be that it likely needs to be removed. There is a chance that she may need assisted reproduction; she might not be able to get pregnant on her own due either to the presence of the endometrioma or to the surgical process of removing it and stripping.
Dr. Advincula: How soon after surgery can a patient start to pursue trying to get pregnant?
Dr. Hur: I think there is no time restraint outside of recovery. As long as the patient has a routine postoperative course, she can try to conceive, spontaneously or with assisted reproduction. Some data suggest, however, that ovarian reserve is diminished immediately after surgery.10–12 If you look at the spontaneous clinical pregnancy outcomes, they are comparable 3 to 6 months postsurgery.4,12–14
Dr. Brown: I agree. Time is of the essence with a lot of patients, many of whom present after age 35.
Dr. Hur: It’s also important to highlight that there are 2 presentations with endometrioma: the symptomatic patient and the asymptomatic patient. In the asymptomatic patient, her age, reproductive goals, and the bilaterality (whether it is present on both sides or on one side) of the endometrioma are important in deciding on a patient-centered surgical plan. For someone with a smaller cyst, unilateral presentation, and maybe older age at presentation, it may or may not impact assisted reproductive outcomes.
If the patient is not symptomatic and she is older with bilateral endometriomas less than 4 cm, some data suggest that patient might be better served in a conservative fashion.6,15–17 Then, once she is done with assisted reproduction, we might be more aggressive surgically by treating the finding that would not resolve spontaneously without surgical management. It is important to highlight that endometriomas do not resolve on their own; they require surgical management.
Read about managing endometriosis for the patient not seeking fertility
Endometriosis management for the patient not seeking fertility
Dr. Advincula: Let’s now consider a patient on whom you have performed laparoscopy not only to diagnose and confirm the evidence of endometriosis but also to treat endometriosis, an endometrioma, and potentially deeply infiltrative disease. But this person is not trying to get pregnant. Postoperatively, what is your approach?
Dr. Brown: Suppressive therapy for this patient could be first-line or second-line therapy, such as a Lupron Depot or Depo-Provera. We keep the patient on suppressive therapy (whatever treatments work for her), until she’s ready to get pregnant; then we take her off. Hopefully she gets pregnant. After she delivers, we reinitiate suppressive therapy. I will follow these women throughout their reproductive cycle, and I think having a team of physicians who are all on the same page can help this patient manage her disease through her reproductive years.
Dr. Hur: If a patient presented warranting surgical management once, and she is not menopausal, the likelihood that disease will recur is quite high. Understanding the nature and the pathology of the disease, hormonal suppression would be warranted. Suppression is not just for between pregnancies, it’s until the patient reaches natural menopause. It’s also in the hopes of suppressing the disease so she does not need recurrent surgeries.
We typically do not operate unless patients have recurrence of symptoms that no longer respond to medical therapy. Our hope is to buy them more time closer to the age of natural menopause so that medical repercussions do not result in hysterectomy and ovary removal, which have other nongynecologic manifestations, including negative impact on bone and cardiac health.
Hye-Chun Hur, MD, MPH: I am a strong advocate of excision of endometriosis. I believe that it's essential to excise for 2 very important reasons. One reason is for diagnosis. Accurately diagnosing endometriosis through visualization alone is poor, even among gynecologic surgeons. It is very important to have an accurate diagnosis of endometriosis, since the diagnosis will then dictate the treatment for the rest of a patient's reproductive life.
The second reason that excision is essential is because you just do not know how much disease there is "behind the scenes." When you start to excise, you begin to appreciate the depth of the disease, and often fibrosis or inflammation is present even behind the endometriosis implant that is visualized.
Douglas N. Brown, MD: I approach endometriosis in the same way that an oncologist would approach cancer. I call it cytoreduction--reducing the disease. There is this iceberg phenomenon, where the tip of the iceberg is seen in the water, but you have no idea how deep it actually goes. That is very much deep, infiltrative endometriosis. Performing an ablation on the top does almost nothing for the patient and may actually complicate the situation by causing scar tissue. If a patient has symptoms, I firmly believe that you must resect the disease, whether it is on the peritoneum, bladder, bowel, or near the ureter. Now, these are radical surgeries, and not every patient should have a radical surgery. It is very much based on the patient's pain complaints and issues at that time, but excision of endometriosis really, in my opinion, should be the standard of care.
Risks of excision of endometriosis
Dr. Brown: The risks of disease excision depend on whether a patient has ureteral disease, bladder disease, or bowel disease, suggested through a preoperative or another operative report or imaging. If this is the case, we have a preoperative discussion with the patient about, "To what extent do you want me to go to remove the disease from your pelvis? If I remove it from your peritoneum and your bladder, there is the chance that you'll have to go home with a Foley catheter for a few days. If the bowel is involved, do you want me to try to resect the disease or shave it off the bowel? If we get into a problem, are you okay with me resecting that bowel?" These are the issues that we have to discuss, because there are potential complications, although known.
The role of the LNG-IUD
Dr. Advincula: Something that often comes up is the role of a levonorgestrel-releasing intrauterine device (LNG-IUD) as one therapy option, either preoperatively or postoperatively. What is your perspective?
Dr. Hur: I reserve the LNG-IUD as a second-line therapy for patients, predominantly because it allows direct delivery of the medication to the womb (rather than systemic exposure of the medication). For patients who experience adverse effects due to systemic exposure to first-line treatments, it might be a great option. However, I do not believe that it consistently suppresses the ovaries, which we understand feeds the pathology of the hormonal stimulation, and so typically I will reserve it as a second-line treatment.
Dr. Brown: I utilize the LNG-IUD in a similar fashion. I may have patients who have had a diagnostic laparoscopy somewhere else and were referred to me because they now have known stage 3 or 4 endometriosis without endometriomas. Those patients, if they are going to need suppressive therapy after surgery and are not ready to get pregnant, do very well with the LNG-IUD, and I will place it during surgery under anesthesia. If a patient has endometriomas seen at the time of surgery, we could still place an LNG-IUD at the time of surgery. We may need to add on an additional medication, however, like another oral progesterone. I do have patients that use both an IUD and either combined oral contraceptive pills and/or oral progestins. Those patients usually have complicated cases with very deep infiltrative disease.
Read about managing endometriosis involving the bowel
Managing endometriosis involving the bowel
Dr. Advincula: Patients often are quite concerned when the words “endometriosis” and “bowel” come together. How do you manage disease that involves the bowel?
Dr. Hur: A lot of patients with endometriosis have what I call neighboring disease—it’s not limited just to the pelvis, but it involves the neighboring organs including the bowel and bladder. Patients can present with symptoms related to those adjacent organs. However, not all disease involving the bowel or bladder manifests with symptoms, and patients with symptoms may not have visible disease.
Typically, when a patient presents with symptoms of bowel involvement, where the bowel lumen is narrowed to more than 50% and/or she has functional manifestations (signs of obstruction that result in abnormal bowel function), we have serious conversations about a bowel resection. If she has full-thickness disease without significant bowel dysfunction—other than blood in her stool—sometimes we talk about more conservative treatment because of the long-term manifestations that a bowel resection could have.
Dr. Brown: I agree completely. It is important to have a good relationship with our colorectal surgeons. If I suspect that the patient has narrowing of the lumen of the large bowel or she actually has symptoms such as bloody diarrhea during menstruation—which is suggestive of deep, infiltrative and penetrative disease—I will often order a colonoscopy ahead of time to get confirmed biopsies. Then the patient discussion occurs with our colorectal surgeon, who operates with me jointly if we decide to proceed with a bowel resection. It’s important to have subspecialty colleagues involved in this care, because a low anterior resection is a very big surgery and there can be down-the-stream complications.
The importance of multidisciplinary care
Dr. Advincula: What are your perspectives on a multidisciplinary or interdisciplinary approach to the patient with endometriosis?
Dr. Brown: As I previously mentioned, it is important to develop a good relationship with colorectal surgery/urology. In addition, behavioral therapists may be involved in the care of patients with endometriosis, for a number of reasons. The disease process is fluid. It will change during the patient’s reproductive years, and you need to manage it accordingly based on her symptoms. Sometimes the diagnosis is not made for 5 to 10 years, and that can lead to other issues: depression, fibromyalgia, or irritable bowel syndrome.
The patient may have multiple issues plus endometriosis. I think having specialists such as gastroenterologists and behavioral therapists on board, as well as colorectal and urological surgeons who can perform these complex surgeries, is very beneficial to the patient. That way, she benefits from the team’s focus and is cared for from start to finish.
Dr. Hur: I like to call the abdomen a studio. It does not have separate compartments for each organ system. It’s one big room, and often the neighboring organs are involved, including the bowel and bladder. I think Dr. Brown’s observation—the multidisciplinary approach to a patient’s comprehensive care—is critical. Like any surgery, preoperative planning and preoperative assessment are essential, and these steps should include the patient. The discussion should cover not only the surgical outcomes that the surgeons expect, but also what the patient expects to be improved. For example, for patients with extensive disease and bowel involvement, a bowel resection is not always the right approach because it can have potential long-term sequelae. Balancing the risks associated with surgery with the long-term benefits is an important part of the discussion.
Dr. Advincula: Those are both excellent perspectives. Endometriosis is a very complicated disease state, does require a multidisciplinary approach to management, and there are implications and strategies that involve both the medical approach to management and the surgical approach.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG. 2004;111(11):1204–1212.
- Fernando S, Soh PQ, Cooper M, et al. Reliability of visual diagnosis of endometriosis. J Minim Invasive Gynecol. 2013;20(6):783–789.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatments of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176–1180.
- Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2005;(3):CD004992.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Stochino-Loi E, Darwish B, Mircea O, et al. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil Steril. 2017;107(3):707–713.e3.
- Motte I, Roman H, Clavier B, et al. In vitro fertilization outcomes after ablation of endometriomas using plasma energy: A retrospective case-control study. Gynecol Obstet Fertil. 2016;44(10):541–547.
- Roman H, Bubenheim M, Auber M, Marpeau L, Puscasiu L. Antimullerian hormone level and endometrioma ablation using plasma energy. JSLS. 2014;18(3).
- Saito N, Okuda K, Yuguchi H, Yamashita Y, Terai Y, Ohmichi M. Compared with cystectomy, is ovarian vaporization of endometriotic cysts truly more effective in maintaining ovarian reserve? J Minim Invasive Gynecol. 2014;21(5):804–810.
- Giampaolino P, Bifulco G, Di Spiezio Sardo A, Mercorio A, Bruzzese D, Di Carlo C. Endometrioma size is a relevant factor in selection of the most appropriate surgical technique: a prospective randomized preliminary study. Eur J Obstet Gynecol Reprod Biol. 2015;195:88–93.
- Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-MTimes New Romanüllerian hormone levels. Fertil Steril. 2010;94(1):343–349.
- Ding Y, Yuan Y, Ding J, Chen Y, Zhang X, Hua K. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol. 2015;22(7):1252–1259.
- Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol. 2016;23(7):1138–1145.
- Ozaki R, Kumakiri J, Tinelli A, Grimbizis GF, Kitade M, Takeda S. Evaluation of factors predicting diminished ovarian reserve before and after laparoscopic cystectomy for ovarian endometriomas: a prospective cohort study. J Ovarian Res. 2016;9(1):37.
- Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: A prospective randomized study. Reprod Biomed Online. 2006;12(5):639–643.
- Kennedy S, Bergqvist A, Chapron C, et al; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–2704.
Take-home points
- Endometriosis management involves fluidity of care. Treatment approaches will change throughout a patient's reproductive life, depending on the patient's presenting symptoms and reproductive goals.
- Inform the patient of the disease process and how it may affect her menstrual pain symptoms and family planning.
- Educate patients so they may effectively participate in the management discussion. Hear the voice of the patient to make a tailored plan of care for each individual.
- Endometriosis can be a complex medical problem. Use a comprehensive multidisciplinary approach when appropriate.
Watch: Video roundtable–Endometriosis: Expert perspectives on medical and surgical management