User login
Big heart, small ring
A 58-year-old man presents with a 1-year history of chronic daytime fatigue, low libido, and difficulty achieving erections. He is upset: his wife suspects him of having an extramarital affair because, in addition to problems with his sexual performance, he has not been wearing his wedding ring. The patient explains that the ring has become too small for his finger and that he has never cheated on his wife. His wife has also been complaining that he snores loudly at night.
The patient works as an accountant. He has no known allergies to medications and takes no medications or supplements. He has no surgical history. He has never smoked tobacco or abused illicit drugs. He drinks a glass of wine once a week.
His father died at age 78 of a myocardial infarction; his 86-year-old mother has hypertension. He has no siblings. His 28-year-old biological son is healthy.
Physical examination
His temperature is 97.9°F (36.6°C), blood pressure 150/90 mm Hg, heart rate 80 per minute, respiratory rate 12 per minute, and oxygen saturation 98% on room air. His height is 5 feet 11 inches (180 cm), weight 250 lb (113 kg), and body mass index 35 kg/m2.
His forehead is wide with deep creases, his jaw, nose, and lower lip are prominent, and his tongue, hands, and feet are large. He has mild thyromegaly with no palpable nodules.
On cardiac examination, his point of maximal impulse is 3 cm lateral to the left midclavicular line in the fifth intercostal space; he has normal S1 and S2 with no murmurs, rubs, or gallops. The lungs are clear on auscultation. His abdomen is soft, nontender, and nondistended; the liver is palpated 2 cm below the costal margin. His extremities are not edematous.
LABORATORY TESTING
1. In addition to a complete blood cell count and comprehensive metabolic panel, which is the most appropriate test to order?
- Growth hormone (GH) level
- Insulin-like growth factor 1 (IGF-1) level
- GH and IGF-1 levels
- IGF-2 level
Acromegaly, an overview
The patient’s history of snoring and daytime fatigue suggests obstructive sleep apnea, which together with his enlarging ring finger size, wide forehead with deep creases, prominent jaw, nose, and lower lip, and enlarged thyroid, heart, and liver suggests acromegaly.
In most cases, acromegaly is caused by a GH-secreting pituitary adenoma. Rare causes include hypothalamic tumors that secrete GH-releasing hormone (GHRH) and ectopic secretion of GHRH or GH.1 Pseudoacromegaly, a mimic, is characterized by acromegalic features without hypersecretion of GH and with normal IGF-1 levels.4
The prevalence of acromegaly is 36 to 60 cases per million, and its annual incidence is 3 to 4 per million.5
With this patient’s presentation, the most appropriate next step is to order an IGF-1 level to screen for acromegaly.
GH secretion is pulsatile, IGF-1 secretion is not
GH is synthesized and stored in somatotroph cells, which account for more than 50% of pituitary hormone-secreting cells.6 Three hormones regulate synthesis and secretion of GH: GHRH, ghrelin, and somatostatin.7 GH secretion is pulsatile, with minimal basal secretion dependent on sex, age, neurotransmitters, exercise, and stress.7 It exerts its physiologic effects through an interaction with the GH receptor, a single-chain transmembrane glycoprotein.8,9
A GH-secreting adenoma develops when pituitary somatotroph cells undergo a monoclonal expansion. Mutations of various genes such as GNAS, PRKAR1A, and AIP are suspected of triggering such expansion. Disruption of the MENIN gene leads to multiple endocrine neoplasia syndrome 1, a combination of pituitary adenoma, pancreatic tumor, and primary hyperparathyroidism.9 The pattern of cytoplasmic keratin in somatotroph cells defines 2 histologic subtypes: densely granulated and sparsely granulated. The latter subtype is associated with more-invasive lesions that are seen more often in younger patients and are less responsive to somatostatin ligand therapy.10
GH induces transcription of IGF-1, mostly in the liver. In contrast to GH, IGF-1 secretion is not pulsatile, and therefore IGF-1 can be measured more reliably in serum, and the results can be interpreted according to age- and sex-adjusted reference ranges.
The IGF-1 level is a very sensitive test, but it is not very specific. It can be falsely elevated in pregnancy, in patients on estrogen replacement therapy, and in late adolescence.11 In addition, it may be difficult to interpret the IGF-1 level in the setting of malnutrition, severe hyperglycemia, renal or hepatic failure, and hypothyroidism.11,12
Nonpulsatile secretion and high sensitivity make the IGF-1 level the screening test of choice for acromegaly.9,12 In contrast, because of the pulsatile nature of GH synthesis, one cannot rely on a random GH level alone to detect the hormone’s hypersecretion.
IGF-2 has no role in acromegaly
IGF-2, produced mainly by the liver, plays an important role in promoting fetal growth. IGF-2 may induce hypoglycemia when secreted by some mesenchymal tumors.13 This hormone has no role in the pathogenesis of acromegaly and should not be measured in this patient.
CASE CONTINUED: FURTHER TESTING
The patient’s IGF-1 level is 590 ng/mL; the reference range for his age and sex is 68 to 245 ng/mL.
A sleep study confirms obstructive sleep apnea, and the patient is started on continuous positive airway pressure at night, with some reduction of his fatigue.
2. What is the most appropriate next step?
- Order magnetic resonance imaging (MRI) of the pituitary with gadolinium contrast
- Perform a GH suppression test with a 75-g oral glucose load
- Perform a GH stimulation test
- Refer the patient to a neurosurgeon for a consultation
The most appropriate next step is a GH suppression test, performed by measuring the plasma GH level 2 hours after giving 75 g of glucose by mouth. This confirmatory test is necessary because the IGF-1 level can be falsely elevated. The normal response to an oral glucose challenge is suppression of the GH level to below 1 μg/L. Failure to suppress GH confirms the diagnosis of acromegaly.14
A GH stimulation test with insulin-induced hypoglycemia or with GHRH-arginine would be appropriate if GH deficiency were suspected rather than hypersecretion.
Imaging of the pituitary with MRI before obtaining biochemical confirmation of the diagnosis of acromegaly may mislead the physician because MRI does not determine the functional status of a pituitary tumor. Correct treatment of a pituitary tumor depends on whether the tumor causes hypersecretion or deficiency of any pituitary hormones.
Referral to a neurosurgeon for a consultation is premature until a biochemical diagnosis of acromegaly is made and a pituitary adenoma is subsequently demonstrated by imaging.
3. The patient’s GH level is 10 μg/L 2 hours after oral administration of 75 g of glucose. What is the most appropriate next step?
- Radiography of the skull to image the pituitary at a low cost
- MRI of the pituitary with contrast after making sure the patient’s renal function is normal
- MRI of the pituitary without contrast
- Computed tomography of the head
The next step is MRI of the pituitary with contrast (gadolinium) after obtaining blood urea nitrogen and creatinine measurements to make sure the patient’s renal function is normal.14
Gadolinium contrast is contraindicated in patients with severely reduced renal function (glomerular filtration rate < 30 mL/min/1.73 m2) because of the risk of nephrogenic systemic fibrosis. In such a case, MRI without contrast would be appropriate.
MRI is the most sensitive imaging test for detecting a pituitary adenoma, as it can detect tumors as small as 2 mm. A pituitary macroadenoma (> 10 mm in diameter) is detected in more than 75% of patients with acromegaly at diagnosis. The tumor often invades one or both cavernous sinuses or extends to the suprasellar region, possibly impinging on the optic chiasm.15
If MRI is contraindicated, computed tomography of the head should be performed.
CASE CONTINUED: IMAGING
The patient’s comprehensive metabolic panel is normal, but his fasting plasma glucose is 135 mg/dL (reference range 74–99). Pituitary MRI with contrast shows a 3-cm pituitary adenoma with suprasellar extension, impinging on the optic chiasm and invading the right cavernous sinus.
4. In addition to repeating the fasting plasma glucose and measuring hemoglobin A1c, what is the most appropriate next step in managing this patient?
- Measure the prolactin, morning serum cortisol, total testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and free thyroxine (T4) levels; refer the patient to an ophthalmologist for a formal evaluation of visual fields
- Measure these hormone levels; perform a gross evaluation of the visual fields and refer the patient to an ophthalmologist only if visual field deficits are found on the gross examination
- Measure these hormone levels; refer the patient to an ophthalmologist only if he complains of vision changes
- Do not order any additional tests; instruct the patient to call the office if he develops any vision changes
This patient should have all of these hormones measured. In addition, given that his macroadenoma is impinging on the optic chiasm, he should be referred to an ophthalmologist for a formal evaluation of visual fields even if the latter are intact on gross examination and even if the patient does not complain of any visual changes.
Abnormalities of hormones other than GH and IGF-1 in acromegaly
Secretion of pituitary hormones other than GH and IGF-1 must be assessed.
Prolactin. GH-secreting tumors also secrete prolactin in up to one-third of patients, with the resulting hyperprolactinemia contributing to hypogonadism.11 Prolactin hypersecretion should be distinguished from hyperprolactinemia caused by pituitary stalk compression, which may be evident on MRI.
Measuring the serum prolactin level with 1:100 dilution to counteract the “hook effect” may unmask severe hyperprolactinemia due to a large macroprolactinoma. (The hook effect occurs when the prolactin level is so high that there is not enough antibody in the assay to bind both ends of all the prolactin molecules present, causing the reading to be falsely low.).
Cortisol, T4, testosterone. Patients with acromegaly may develop central adrenal insufficiency, central hypothyroidism, and central hypogonadism; these hormonal deficits may occur in isolation or in combination.
Also, patients should be assessed for comorbidities such as colon cancer (all patients with acromegaly require a colonoscopy, as acromegaly raises the risk of colon cancer), diabetes mellitus, hypertension, cardiomyopathy, and sleep apnea.16
Visual field loss may be insidious
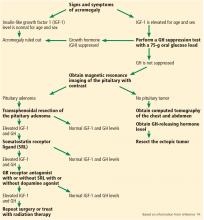
The diagnostic and treatment algorithm for acromegaly is summarized in Figure 1.
CASE CONTINUED: LABORATORY VALUES, TREATMENT OPTIONS
Our patient’s repeat fasting plasma glucose is 137 mg/dL; his hemoglobin A1c is 7.3%, consistent with diabetes mellitus secondary to acromegaly. Other laboratory values:
- Morning cortisol level 15 μg/dL (reference range 5.3–22.5),
- Prolactin 23 ng/mL, confirmed with 1:100 dilution (4.0–15.2)
- Total testosterone 59 ng/dL (193–824)
- LH 2.1 mIU/mL (1.8–10.8)
- FSH 3.0 mIU/mL (1.5–12.4)
- TSH 2.5 mIU/L (0.5–4.5)
- Free T4 1.3 ng/dL (0.9–1.7).
The patient is started on metformin 500 mg by mouth twice a day, counseled on a healthy diet, and informed that his diabetes may be a complication of his acromegaly. He is anxious to learn how his acromegaly can be treated.
5. What treatment would you recommend for the patient’s acromegaly?
- Medical treatment first, then transsphenoidal resection of the pituitary macroadenoma if medical treatment fails
- Medical treatment first, radiotherapy if medical treatment fails, and transsphenoidal resection of the pituitary macroadenoma as a last resort
- Transsphenoidal resection of the pituitary macroadenoma first, medical treatment if surgery fails, and radiotherapy if both surgery and medical treatment fail
- Taking a safe, conservative approach, monitoring IGF-1 levels frequently; starting medical treatment if acromegaly does not go into remission in 1 year
The initial treatment of choice for most patients with acromegaly is resection of the pituitary tumor.
A transsphenoidal approach is used for most patients; only rarely is craniotomy necessary. Endoscopic and microsurgical techniques reduce postoperative morbidity.17 Postoperative complications include symptoms related to the transsphenoidal approach (nasal congestion, sinusitis, epistaxis), cerebrospinal fluid leak, hemorrhage, meningitis, stroke, visual impairment, vascular damage, transient or permanent diabetes insipidus, and hypopituitarism. The surgical mortality rate is less than 0.5%.18,19
Successful resection of a pituitary tumor would lead to normalization of the IGF-1 level, a drop of the GH level to below 1 μg/L, and relief of the effect of the tumor pressing against other structures. An IGF-1 level and a random GH level should be obtained 12 weeks after the surgery.14 If the GH level is higher than 1 μg/L, a GH suppression test with a 75-g oral glucose load should be performed.14 MRI of the sella turcica should be done 12 weeks after surgery to visualize residual tumor and adjacent structures.14
A large tumor size, suprasellar extension, and high preoperative levels of IGF-1 and GH are associated with a lack of surgical success; however, surgical debulking should still be considered in patients with a low chance for surgical cure to improve the probability of achieving biochemical remission with postoperative medical and radiologic therapy.20
Medical therapy can be the initial treatment if the patient refuses surgery or if surgery is contraindicated because of severe comorbidities or because structural features of the tumor confer a high surgical risk (eg, if the adenoma encases the cavernous portion of a carotid artery).13 Medical therapy may shrink the tumor in some patients and may thereby make surgical resection easier and more likely to be successful.
Radiotherapy is usually reserved for patients whose tumors recur or persist postoperatively and who are resistant to or intolerant of medical therapy.14 The soft tissue changes caused by acromegaly may regress with treatment to some degree, but they are not likely to resolve completely; the bone changes do not regress.
CASE CONTINUED: MEDICAL TREATMENT
Three months after transsphenoidal resection of his pituitary macroadenoma, our patient’s laboratory values are as follows:
- IGF-1 400 ng/mL
- Morning cortisol 20 μg/dL
- Testosterone 95 ng/dL
- LH 2.1 mU/mL
- FSH 3.7 mU/mL
- Prolactin 12 ng/mL
- TSH 2.3 mIU/L
- Free T4 1.2 ng/dL
- Basic metabolic panel normal.
The patient denies frequent urination or increased thirst. Repeat MRI of the pituitary with contrast shows a residual 1.3-cm adenoma with no suprasellar extension.
6. What is the best next treatment choice for the patient?
- A GH receptor antagonist (pegvisomant)
- A somatostatin receptor ligand (SRL) such as octreotide
- Cabergoline (a dopamine agonist)
- A combination of an SRL and pegvisomant
An SRL such as octreotide would be the best choice for this patient.
The medical options for acromegaly are SRLs, pegvisomant, and cabergoline.21–23 The Endocrine Society guidelines recommend either an SRL or pegvisomant as the initial adjuvant medical therapy in patients with persistent disease after surgery.14 However, pegvisomant is much more expensive than any SRL, so an SRL would be a better choice in this patient. Also, pegvisomant does not suppress tumor growth, in contrast to SRLs, so SRLs are preferred in patients with large tumors abutting the optic chiasm.14
SRLs are used as primary therapy in patients who cannot be cured by surgery, have extensive cavernous sinus invasion, have no chiasmal compression, or are poor surgical candidates.
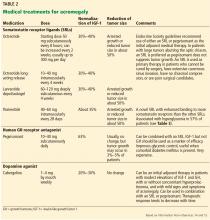
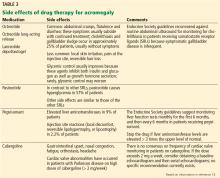
The medical treatment of acromegaly is summarized in Table 2.14,15 Side effects of the medications used to treat acromegaly are summarized in Table 3.14
CASE CONTINUED: RADIOTHERAPY
The patient is treated with octreotide, and the dose is subsequently titrated upward. His central hypogonadism is treated with testosterone gel. After 3 months, his IGF-1 level decreases to 190 ng/mL, the total testosterone increases to 450 ng/dL, and the hemoglobin A1c decreases to 5.9%.
The patient asks if stereotactic radiotherapy, which he read about on the Internet, can cure his acromegaly so that he can avoid the monthly octreotide injections.
7. Which statement best describes radiotherapy’s therapeutic effect in acromegaly?
- Stereotactic radiotherapy is more effective than medical therapy and should be used as a second-line treatment after surgery
- Stereotactic radiotherapy is less effective than conventional radiotherapy
- Stereotactic radiotherapy leads to stability or a decrease in the size of the GH-secreting tumor in 93% to 100% of patients in 5 to 10 years and to biochemical remission in 40% to 60% of patients at 5 years
- Stereotactic radiotherapy causes hypopituitarism in no more than 1% of patients
Stereotactic radiotherapy leads to stability or a decrease in the size of the GH-secreting tumor in 93% to 100% of patients in 5 to 10 years and biochemical remission in 40% to 60% of patients at 5 years.24,25
Hypopituitarism develops in up to 50% of patients at 5 years, and its incidence increases with the duration of follow-up.24 The risk of other complications is low (0% to 5% for new visual deficits, cranial nerve damage, or brain radionecrosis, and 0% to 1% for secondary brain tumors).24
Conventional radiotherapy has fallen out of favor because it is associated with an increased risk of death (mainly from stroke) independent of IGF-1 and GH levels, and a higher rate of complications than stereotactic radiotherapy.14,16 Radiotherapy is reserved for postsurgical treatment of patients with recurrent or persistent tumors who are resistant to or cannot tolerate medical therapy; it is the third-line treatment.24
Given that our patient responded to the medical therapy and tolerated it well and given the high risk of hypopituitarism associated with stereotactic radiotherapy, the latter would not be appropriate for the patient.
His fatigue has diminished further and his sexual performance has improved. He is still married and his wife no longer suspects him of infidelity.
KEY POINTS
- IGF-1 is the screening test of choice in a patient with signs and symptoms of acromegaly.
- A growth hormone suppression test with a 75-g oral glucose load is the gold standard test for confirmation of the diagnosis of acromegaly in patients with an elevated IGF-1 level.
- Transsphenoidal resection of the growth hormone-secreting pituitary macroadenoma is the initial treatment of choice for acromegaly.
- Patients with residual or recurrent growth hormone-secreting pituitary macroadenoma can be treated with somatostatin receptor ligands, a growth hormone receptor antagonist (pegvisomant), and a dopamine agonist cabergoline.
- Radiotherapy is reserved for postsurgical treatment of patients with recurrent or persistent tumors who are resistant to or intolerant of medical therapy. Stereotactic radiotherapy has largely replaced conventional radiotherapy.
- Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest 2009; 119:3189–3202.
- Molitch ME. Clinical manifestations of acromegaly. Endocrinol Metab Clin North Am 1992; 21:597–614.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017; 110:411–420.
- Yacub A, Yaqub N. Insulin-mediated pseudoacromegaly: a case report and review of the literature. W V Med J 2008; 104:12–15.
- Mestron A, Webb SM, Astorga R, et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol 2004; 151:439–446.
- Zhu X, Lin CR, Prefontaine CG, Tollkuhn J, Rosenfeld MG. Genetic control of pituitary development and hypopituitarism. Curr Opin Genet Dev 2005; 15:332–340.
- Tannenbaum GS, Epelbaum J, Bowers CY. Interrelationship between the novel peptide ghrelin and somatostatin/growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology 2003; 144:967–974.
- Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord 2006; 7:225–235.
- Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 2004; 25:102–152.
- Larkin S, Reddy R, Karavitaki N, Cudlip S, Wass J, Ansorge O. Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur J Endocrinol 2013; 168:491–499.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017; 110:411–420.
- Peacey SR, Toogood AA, Veldhuis JD, Thorner MO, Shalet SM. The relationship between 24-hour growth hormone secretion and insulin-like growth factor I in patients with successfully treated acromegaly: impact of surgery or radiotherapy. J Clin Endocrinol Metab 2001; 86:259–266.
- Livingstone C. IGF2 and cancer. Endocr Relat Cancer 2013; 20:R321–R339.
- Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2014; 99:3933–3951.
- Melmed S. Acromegaly. N Engl J Med 2006; 355:2558–2573.
- Melmed S, Casanueva FF, Klibanski A, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary 2013; 16:294–302.
- Marquez Y, Tuchman A, Zada G. Surgery and radiosurgery for acromegaly: a review of indications, operative techniques, outcomes, and complications. Int J Endocrinol 2012; 2012: 386401.
- Jane JA Jr, Starke RM, Elzoghby MA, et al. Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab 2011; 96:2732–2740.
- Cappabianca P, Cavallo LM, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg 2002; 97:293–298.
- Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical “cure.” Eur J Endocrinol 2005; 152:379–387.
- Howlett TA, Willis D, Walker G, Wass JA, Trainer PJ; UK Acromegaly Register Study Group (UKAR-3). Control of growth hormone and IGF1 in patients with acromegaly in the UK: responses to medical treatment with somatostatin analogues and dopamine agonists. Clin Endocrinol (Oxf) 2013; 79:689–699.
- Katznelson L. Pegvisomant for the treatment of acromegaly-translation of clinical trials into clinical practice. Nat Clin Pract Endocrinol Metab 2007; 3:514–515.
- Freda PU, Reyes CM, Nuruzzaman AT, Sundeen RE, Khandji AG, Post KD. Cabergoline therapy of growth hormone & growth hormone/prolactin secreting pituitary tumors. Pituitary 2004; 7:21–30.
- Castinetti F, Morange I, Dufour H, Regis J, Brue T. Radiotherapy and radiosurgery in acromegaly. Pituitary 2009; 12:3–10.
- Gheorghiu ML. Updates in outcomes of stereotactic radiation therapy in acromegaly. Pituitary 2017; 20:154–168.
A 58-year-old man presents with a 1-year history of chronic daytime fatigue, low libido, and difficulty achieving erections. He is upset: his wife suspects him of having an extramarital affair because, in addition to problems with his sexual performance, he has not been wearing his wedding ring. The patient explains that the ring has become too small for his finger and that he has never cheated on his wife. His wife has also been complaining that he snores loudly at night.
The patient works as an accountant. He has no known allergies to medications and takes no medications or supplements. He has no surgical history. He has never smoked tobacco or abused illicit drugs. He drinks a glass of wine once a week.
His father died at age 78 of a myocardial infarction; his 86-year-old mother has hypertension. He has no siblings. His 28-year-old biological son is healthy.
Physical examination
His temperature is 97.9°F (36.6°C), blood pressure 150/90 mm Hg, heart rate 80 per minute, respiratory rate 12 per minute, and oxygen saturation 98% on room air. His height is 5 feet 11 inches (180 cm), weight 250 lb (113 kg), and body mass index 35 kg/m2.
His forehead is wide with deep creases, his jaw, nose, and lower lip are prominent, and his tongue, hands, and feet are large. He has mild thyromegaly with no palpable nodules.
On cardiac examination, his point of maximal impulse is 3 cm lateral to the left midclavicular line in the fifth intercostal space; he has normal S1 and S2 with no murmurs, rubs, or gallops. The lungs are clear on auscultation. His abdomen is soft, nontender, and nondistended; the liver is palpated 2 cm below the costal margin. His extremities are not edematous.
LABORATORY TESTING
1. In addition to a complete blood cell count and comprehensive metabolic panel, which is the most appropriate test to order?
- Growth hormone (GH) level
- Insulin-like growth factor 1 (IGF-1) level
- GH and IGF-1 levels
- IGF-2 level
Acromegaly, an overview
The patient’s history of snoring and daytime fatigue suggests obstructive sleep apnea, which together with his enlarging ring finger size, wide forehead with deep creases, prominent jaw, nose, and lower lip, and enlarged thyroid, heart, and liver suggests acromegaly.
In most cases, acromegaly is caused by a GH-secreting pituitary adenoma. Rare causes include hypothalamic tumors that secrete GH-releasing hormone (GHRH) and ectopic secretion of GHRH or GH.1 Pseudoacromegaly, a mimic, is characterized by acromegalic features without hypersecretion of GH and with normal IGF-1 levels.4
The prevalence of acromegaly is 36 to 60 cases per million, and its annual incidence is 3 to 4 per million.5
With this patient’s presentation, the most appropriate next step is to order an IGF-1 level to screen for acromegaly.
GH secretion is pulsatile, IGF-1 secretion is not
GH is synthesized and stored in somatotroph cells, which account for more than 50% of pituitary hormone-secreting cells.6 Three hormones regulate synthesis and secretion of GH: GHRH, ghrelin, and somatostatin.7 GH secretion is pulsatile, with minimal basal secretion dependent on sex, age, neurotransmitters, exercise, and stress.7 It exerts its physiologic effects through an interaction with the GH receptor, a single-chain transmembrane glycoprotein.8,9
A GH-secreting adenoma develops when pituitary somatotroph cells undergo a monoclonal expansion. Mutations of various genes such as GNAS, PRKAR1A, and AIP are suspected of triggering such expansion. Disruption of the MENIN gene leads to multiple endocrine neoplasia syndrome 1, a combination of pituitary adenoma, pancreatic tumor, and primary hyperparathyroidism.9 The pattern of cytoplasmic keratin in somatotroph cells defines 2 histologic subtypes: densely granulated and sparsely granulated. The latter subtype is associated with more-invasive lesions that are seen more often in younger patients and are less responsive to somatostatin ligand therapy.10
GH induces transcription of IGF-1, mostly in the liver. In contrast to GH, IGF-1 secretion is not pulsatile, and therefore IGF-1 can be measured more reliably in serum, and the results can be interpreted according to age- and sex-adjusted reference ranges.
The IGF-1 level is a very sensitive test, but it is not very specific. It can be falsely elevated in pregnancy, in patients on estrogen replacement therapy, and in late adolescence.11 In addition, it may be difficult to interpret the IGF-1 level in the setting of malnutrition, severe hyperglycemia, renal or hepatic failure, and hypothyroidism.11,12
Nonpulsatile secretion and high sensitivity make the IGF-1 level the screening test of choice for acromegaly.9,12 In contrast, because of the pulsatile nature of GH synthesis, one cannot rely on a random GH level alone to detect the hormone’s hypersecretion.
IGF-2 has no role in acromegaly
IGF-2, produced mainly by the liver, plays an important role in promoting fetal growth. IGF-2 may induce hypoglycemia when secreted by some mesenchymal tumors.13 This hormone has no role in the pathogenesis of acromegaly and should not be measured in this patient.
CASE CONTINUED: FURTHER TESTING
The patient’s IGF-1 level is 590 ng/mL; the reference range for his age and sex is 68 to 245 ng/mL.
A sleep study confirms obstructive sleep apnea, and the patient is started on continuous positive airway pressure at night, with some reduction of his fatigue.
2. What is the most appropriate next step?
- Order magnetic resonance imaging (MRI) of the pituitary with gadolinium contrast
- Perform a GH suppression test with a 75-g oral glucose load
- Perform a GH stimulation test
- Refer the patient to a neurosurgeon for a consultation
The most appropriate next step is a GH suppression test, performed by measuring the plasma GH level 2 hours after giving 75 g of glucose by mouth. This confirmatory test is necessary because the IGF-1 level can be falsely elevated. The normal response to an oral glucose challenge is suppression of the GH level to below 1 μg/L. Failure to suppress GH confirms the diagnosis of acromegaly.14
A GH stimulation test with insulin-induced hypoglycemia or with GHRH-arginine would be appropriate if GH deficiency were suspected rather than hypersecretion.
Imaging of the pituitary with MRI before obtaining biochemical confirmation of the diagnosis of acromegaly may mislead the physician because MRI does not determine the functional status of a pituitary tumor. Correct treatment of a pituitary tumor depends on whether the tumor causes hypersecretion or deficiency of any pituitary hormones.
Referral to a neurosurgeon for a consultation is premature until a biochemical diagnosis of acromegaly is made and a pituitary adenoma is subsequently demonstrated by imaging.
3. The patient’s GH level is 10 μg/L 2 hours after oral administration of 75 g of glucose. What is the most appropriate next step?
- Radiography of the skull to image the pituitary at a low cost
- MRI of the pituitary with contrast after making sure the patient’s renal function is normal
- MRI of the pituitary without contrast
- Computed tomography of the head
The next step is MRI of the pituitary with contrast (gadolinium) after obtaining blood urea nitrogen and creatinine measurements to make sure the patient’s renal function is normal.14
Gadolinium contrast is contraindicated in patients with severely reduced renal function (glomerular filtration rate < 30 mL/min/1.73 m2) because of the risk of nephrogenic systemic fibrosis. In such a case, MRI without contrast would be appropriate.
MRI is the most sensitive imaging test for detecting a pituitary adenoma, as it can detect tumors as small as 2 mm. A pituitary macroadenoma (> 10 mm in diameter) is detected in more than 75% of patients with acromegaly at diagnosis. The tumor often invades one or both cavernous sinuses or extends to the suprasellar region, possibly impinging on the optic chiasm.15
If MRI is contraindicated, computed tomography of the head should be performed.
CASE CONTINUED: IMAGING
The patient’s comprehensive metabolic panel is normal, but his fasting plasma glucose is 135 mg/dL (reference range 74–99). Pituitary MRI with contrast shows a 3-cm pituitary adenoma with suprasellar extension, impinging on the optic chiasm and invading the right cavernous sinus.
4. In addition to repeating the fasting plasma glucose and measuring hemoglobin A1c, what is the most appropriate next step in managing this patient?
- Measure the prolactin, morning serum cortisol, total testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and free thyroxine (T4) levels; refer the patient to an ophthalmologist for a formal evaluation of visual fields
- Measure these hormone levels; perform a gross evaluation of the visual fields and refer the patient to an ophthalmologist only if visual field deficits are found on the gross examination
- Measure these hormone levels; refer the patient to an ophthalmologist only if he complains of vision changes
- Do not order any additional tests; instruct the patient to call the office if he develops any vision changes
This patient should have all of these hormones measured. In addition, given that his macroadenoma is impinging on the optic chiasm, he should be referred to an ophthalmologist for a formal evaluation of visual fields even if the latter are intact on gross examination and even if the patient does not complain of any visual changes.
Abnormalities of hormones other than GH and IGF-1 in acromegaly
Secretion of pituitary hormones other than GH and IGF-1 must be assessed.
Prolactin. GH-secreting tumors also secrete prolactin in up to one-third of patients, with the resulting hyperprolactinemia contributing to hypogonadism.11 Prolactin hypersecretion should be distinguished from hyperprolactinemia caused by pituitary stalk compression, which may be evident on MRI.
Measuring the serum prolactin level with 1:100 dilution to counteract the “hook effect” may unmask severe hyperprolactinemia due to a large macroprolactinoma. (The hook effect occurs when the prolactin level is so high that there is not enough antibody in the assay to bind both ends of all the prolactin molecules present, causing the reading to be falsely low.).
Cortisol, T4, testosterone. Patients with acromegaly may develop central adrenal insufficiency, central hypothyroidism, and central hypogonadism; these hormonal deficits may occur in isolation or in combination.
Also, patients should be assessed for comorbidities such as colon cancer (all patients with acromegaly require a colonoscopy, as acromegaly raises the risk of colon cancer), diabetes mellitus, hypertension, cardiomyopathy, and sleep apnea.16
Visual field loss may be insidious
The diagnostic and treatment algorithm for acromegaly is summarized in Figure 1.
CASE CONTINUED: LABORATORY VALUES, TREATMENT OPTIONS
Our patient’s repeat fasting plasma glucose is 137 mg/dL; his hemoglobin A1c is 7.3%, consistent with diabetes mellitus secondary to acromegaly. Other laboratory values:
- Morning cortisol level 15 μg/dL (reference range 5.3–22.5),
- Prolactin 23 ng/mL, confirmed with 1:100 dilution (4.0–15.2)
- Total testosterone 59 ng/dL (193–824)
- LH 2.1 mIU/mL (1.8–10.8)
- FSH 3.0 mIU/mL (1.5–12.4)
- TSH 2.5 mIU/L (0.5–4.5)
- Free T4 1.3 ng/dL (0.9–1.7).
The patient is started on metformin 500 mg by mouth twice a day, counseled on a healthy diet, and informed that his diabetes may be a complication of his acromegaly. He is anxious to learn how his acromegaly can be treated.
5. What treatment would you recommend for the patient’s acromegaly?
- Medical treatment first, then transsphenoidal resection of the pituitary macroadenoma if medical treatment fails
- Medical treatment first, radiotherapy if medical treatment fails, and transsphenoidal resection of the pituitary macroadenoma as a last resort
- Transsphenoidal resection of the pituitary macroadenoma first, medical treatment if surgery fails, and radiotherapy if both surgery and medical treatment fail
- Taking a safe, conservative approach, monitoring IGF-1 levels frequently; starting medical treatment if acromegaly does not go into remission in 1 year
The initial treatment of choice for most patients with acromegaly is resection of the pituitary tumor.
A transsphenoidal approach is used for most patients; only rarely is craniotomy necessary. Endoscopic and microsurgical techniques reduce postoperative morbidity.17 Postoperative complications include symptoms related to the transsphenoidal approach (nasal congestion, sinusitis, epistaxis), cerebrospinal fluid leak, hemorrhage, meningitis, stroke, visual impairment, vascular damage, transient or permanent diabetes insipidus, and hypopituitarism. The surgical mortality rate is less than 0.5%.18,19
Successful resection of a pituitary tumor would lead to normalization of the IGF-1 level, a drop of the GH level to below 1 μg/L, and relief of the effect of the tumor pressing against other structures. An IGF-1 level and a random GH level should be obtained 12 weeks after the surgery.14 If the GH level is higher than 1 μg/L, a GH suppression test with a 75-g oral glucose load should be performed.14 MRI of the sella turcica should be done 12 weeks after surgery to visualize residual tumor and adjacent structures.14
A large tumor size, suprasellar extension, and high preoperative levels of IGF-1 and GH are associated with a lack of surgical success; however, surgical debulking should still be considered in patients with a low chance for surgical cure to improve the probability of achieving biochemical remission with postoperative medical and radiologic therapy.20
Medical therapy can be the initial treatment if the patient refuses surgery or if surgery is contraindicated because of severe comorbidities or because structural features of the tumor confer a high surgical risk (eg, if the adenoma encases the cavernous portion of a carotid artery).13 Medical therapy may shrink the tumor in some patients and may thereby make surgical resection easier and more likely to be successful.
Radiotherapy is usually reserved for patients whose tumors recur or persist postoperatively and who are resistant to or intolerant of medical therapy.14 The soft tissue changes caused by acromegaly may regress with treatment to some degree, but they are not likely to resolve completely; the bone changes do not regress.
CASE CONTINUED: MEDICAL TREATMENT
Three months after transsphenoidal resection of his pituitary macroadenoma, our patient’s laboratory values are as follows:
- IGF-1 400 ng/mL
- Morning cortisol 20 μg/dL
- Testosterone 95 ng/dL
- LH 2.1 mU/mL
- FSH 3.7 mU/mL
- Prolactin 12 ng/mL
- TSH 2.3 mIU/L
- Free T4 1.2 ng/dL
- Basic metabolic panel normal.
The patient denies frequent urination or increased thirst. Repeat MRI of the pituitary with contrast shows a residual 1.3-cm adenoma with no suprasellar extension.
6. What is the best next treatment choice for the patient?
- A GH receptor antagonist (pegvisomant)
- A somatostatin receptor ligand (SRL) such as octreotide
- Cabergoline (a dopamine agonist)
- A combination of an SRL and pegvisomant
An SRL such as octreotide would be the best choice for this patient.
The medical options for acromegaly are SRLs, pegvisomant, and cabergoline.21–23 The Endocrine Society guidelines recommend either an SRL or pegvisomant as the initial adjuvant medical therapy in patients with persistent disease after surgery.14 However, pegvisomant is much more expensive than any SRL, so an SRL would be a better choice in this patient. Also, pegvisomant does not suppress tumor growth, in contrast to SRLs, so SRLs are preferred in patients with large tumors abutting the optic chiasm.14
SRLs are used as primary therapy in patients who cannot be cured by surgery, have extensive cavernous sinus invasion, have no chiasmal compression, or are poor surgical candidates.
The medical treatment of acromegaly is summarized in Table 2.14,15 Side effects of the medications used to treat acromegaly are summarized in Table 3.14
CASE CONTINUED: RADIOTHERAPY
The patient is treated with octreotide, and the dose is subsequently titrated upward. His central hypogonadism is treated with testosterone gel. After 3 months, his IGF-1 level decreases to 190 ng/mL, the total testosterone increases to 450 ng/dL, and the hemoglobin A1c decreases to 5.9%.
The patient asks if stereotactic radiotherapy, which he read about on the Internet, can cure his acromegaly so that he can avoid the monthly octreotide injections.
7. Which statement best describes radiotherapy’s therapeutic effect in acromegaly?
- Stereotactic radiotherapy is more effective than medical therapy and should be used as a second-line treatment after surgery
- Stereotactic radiotherapy is less effective than conventional radiotherapy
- Stereotactic radiotherapy leads to stability or a decrease in the size of the GH-secreting tumor in 93% to 100% of patients in 5 to 10 years and to biochemical remission in 40% to 60% of patients at 5 years
- Stereotactic radiotherapy causes hypopituitarism in no more than 1% of patients
Stereotactic radiotherapy leads to stability or a decrease in the size of the GH-secreting tumor in 93% to 100% of patients in 5 to 10 years and biochemical remission in 40% to 60% of patients at 5 years.24,25
Hypopituitarism develops in up to 50% of patients at 5 years, and its incidence increases with the duration of follow-up.24 The risk of other complications is low (0% to 5% for new visual deficits, cranial nerve damage, or brain radionecrosis, and 0% to 1% for secondary brain tumors).24
Conventional radiotherapy has fallen out of favor because it is associated with an increased risk of death (mainly from stroke) independent of IGF-1 and GH levels, and a higher rate of complications than stereotactic radiotherapy.14,16 Radiotherapy is reserved for postsurgical treatment of patients with recurrent or persistent tumors who are resistant to or cannot tolerate medical therapy; it is the third-line treatment.24
Given that our patient responded to the medical therapy and tolerated it well and given the high risk of hypopituitarism associated with stereotactic radiotherapy, the latter would not be appropriate for the patient.
His fatigue has diminished further and his sexual performance has improved. He is still married and his wife no longer suspects him of infidelity.
KEY POINTS
- IGF-1 is the screening test of choice in a patient with signs and symptoms of acromegaly.
- A growth hormone suppression test with a 75-g oral glucose load is the gold standard test for confirmation of the diagnosis of acromegaly in patients with an elevated IGF-1 level.
- Transsphenoidal resection of the growth hormone-secreting pituitary macroadenoma is the initial treatment of choice for acromegaly.
- Patients with residual or recurrent growth hormone-secreting pituitary macroadenoma can be treated with somatostatin receptor ligands, a growth hormone receptor antagonist (pegvisomant), and a dopamine agonist cabergoline.
- Radiotherapy is reserved for postsurgical treatment of patients with recurrent or persistent tumors who are resistant to or intolerant of medical therapy. Stereotactic radiotherapy has largely replaced conventional radiotherapy.
A 58-year-old man presents with a 1-year history of chronic daytime fatigue, low libido, and difficulty achieving erections. He is upset: his wife suspects him of having an extramarital affair because, in addition to problems with his sexual performance, he has not been wearing his wedding ring. The patient explains that the ring has become too small for his finger and that he has never cheated on his wife. His wife has also been complaining that he snores loudly at night.
The patient works as an accountant. He has no known allergies to medications and takes no medications or supplements. He has no surgical history. He has never smoked tobacco or abused illicit drugs. He drinks a glass of wine once a week.
His father died at age 78 of a myocardial infarction; his 86-year-old mother has hypertension. He has no siblings. His 28-year-old biological son is healthy.
Physical examination
His temperature is 97.9°F (36.6°C), blood pressure 150/90 mm Hg, heart rate 80 per minute, respiratory rate 12 per minute, and oxygen saturation 98% on room air. His height is 5 feet 11 inches (180 cm), weight 250 lb (113 kg), and body mass index 35 kg/m2.
His forehead is wide with deep creases, his jaw, nose, and lower lip are prominent, and his tongue, hands, and feet are large. He has mild thyromegaly with no palpable nodules.
On cardiac examination, his point of maximal impulse is 3 cm lateral to the left midclavicular line in the fifth intercostal space; he has normal S1 and S2 with no murmurs, rubs, or gallops. The lungs are clear on auscultation. His abdomen is soft, nontender, and nondistended; the liver is palpated 2 cm below the costal margin. His extremities are not edematous.
LABORATORY TESTING
1. In addition to a complete blood cell count and comprehensive metabolic panel, which is the most appropriate test to order?
- Growth hormone (GH) level
- Insulin-like growth factor 1 (IGF-1) level
- GH and IGF-1 levels
- IGF-2 level
Acromegaly, an overview
The patient’s history of snoring and daytime fatigue suggests obstructive sleep apnea, which together with his enlarging ring finger size, wide forehead with deep creases, prominent jaw, nose, and lower lip, and enlarged thyroid, heart, and liver suggests acromegaly.
In most cases, acromegaly is caused by a GH-secreting pituitary adenoma. Rare causes include hypothalamic tumors that secrete GH-releasing hormone (GHRH) and ectopic secretion of GHRH or GH.1 Pseudoacromegaly, a mimic, is characterized by acromegalic features without hypersecretion of GH and with normal IGF-1 levels.4
The prevalence of acromegaly is 36 to 60 cases per million, and its annual incidence is 3 to 4 per million.5
With this patient’s presentation, the most appropriate next step is to order an IGF-1 level to screen for acromegaly.
GH secretion is pulsatile, IGF-1 secretion is not
GH is synthesized and stored in somatotroph cells, which account for more than 50% of pituitary hormone-secreting cells.6 Three hormones regulate synthesis and secretion of GH: GHRH, ghrelin, and somatostatin.7 GH secretion is pulsatile, with minimal basal secretion dependent on sex, age, neurotransmitters, exercise, and stress.7 It exerts its physiologic effects through an interaction with the GH receptor, a single-chain transmembrane glycoprotein.8,9
A GH-secreting adenoma develops when pituitary somatotroph cells undergo a monoclonal expansion. Mutations of various genes such as GNAS, PRKAR1A, and AIP are suspected of triggering such expansion. Disruption of the MENIN gene leads to multiple endocrine neoplasia syndrome 1, a combination of pituitary adenoma, pancreatic tumor, and primary hyperparathyroidism.9 The pattern of cytoplasmic keratin in somatotroph cells defines 2 histologic subtypes: densely granulated and sparsely granulated. The latter subtype is associated with more-invasive lesions that are seen more often in younger patients and are less responsive to somatostatin ligand therapy.10
GH induces transcription of IGF-1, mostly in the liver. In contrast to GH, IGF-1 secretion is not pulsatile, and therefore IGF-1 can be measured more reliably in serum, and the results can be interpreted according to age- and sex-adjusted reference ranges.
The IGF-1 level is a very sensitive test, but it is not very specific. It can be falsely elevated in pregnancy, in patients on estrogen replacement therapy, and in late adolescence.11 In addition, it may be difficult to interpret the IGF-1 level in the setting of malnutrition, severe hyperglycemia, renal or hepatic failure, and hypothyroidism.11,12
Nonpulsatile secretion and high sensitivity make the IGF-1 level the screening test of choice for acromegaly.9,12 In contrast, because of the pulsatile nature of GH synthesis, one cannot rely on a random GH level alone to detect the hormone’s hypersecretion.
IGF-2 has no role in acromegaly
IGF-2, produced mainly by the liver, plays an important role in promoting fetal growth. IGF-2 may induce hypoglycemia when secreted by some mesenchymal tumors.13 This hormone has no role in the pathogenesis of acromegaly and should not be measured in this patient.
CASE CONTINUED: FURTHER TESTING
The patient’s IGF-1 level is 590 ng/mL; the reference range for his age and sex is 68 to 245 ng/mL.
A sleep study confirms obstructive sleep apnea, and the patient is started on continuous positive airway pressure at night, with some reduction of his fatigue.
2. What is the most appropriate next step?
- Order magnetic resonance imaging (MRI) of the pituitary with gadolinium contrast
- Perform a GH suppression test with a 75-g oral glucose load
- Perform a GH stimulation test
- Refer the patient to a neurosurgeon for a consultation
The most appropriate next step is a GH suppression test, performed by measuring the plasma GH level 2 hours after giving 75 g of glucose by mouth. This confirmatory test is necessary because the IGF-1 level can be falsely elevated. The normal response to an oral glucose challenge is suppression of the GH level to below 1 μg/L. Failure to suppress GH confirms the diagnosis of acromegaly.14
A GH stimulation test with insulin-induced hypoglycemia or with GHRH-arginine would be appropriate if GH deficiency were suspected rather than hypersecretion.
Imaging of the pituitary with MRI before obtaining biochemical confirmation of the diagnosis of acromegaly may mislead the physician because MRI does not determine the functional status of a pituitary tumor. Correct treatment of a pituitary tumor depends on whether the tumor causes hypersecretion or deficiency of any pituitary hormones.
Referral to a neurosurgeon for a consultation is premature until a biochemical diagnosis of acromegaly is made and a pituitary adenoma is subsequently demonstrated by imaging.
3. The patient’s GH level is 10 μg/L 2 hours after oral administration of 75 g of glucose. What is the most appropriate next step?
- Radiography of the skull to image the pituitary at a low cost
- MRI of the pituitary with contrast after making sure the patient’s renal function is normal
- MRI of the pituitary without contrast
- Computed tomography of the head
The next step is MRI of the pituitary with contrast (gadolinium) after obtaining blood urea nitrogen and creatinine measurements to make sure the patient’s renal function is normal.14
Gadolinium contrast is contraindicated in patients with severely reduced renal function (glomerular filtration rate < 30 mL/min/1.73 m2) because of the risk of nephrogenic systemic fibrosis. In such a case, MRI without contrast would be appropriate.
MRI is the most sensitive imaging test for detecting a pituitary adenoma, as it can detect tumors as small as 2 mm. A pituitary macroadenoma (> 10 mm in diameter) is detected in more than 75% of patients with acromegaly at diagnosis. The tumor often invades one or both cavernous sinuses or extends to the suprasellar region, possibly impinging on the optic chiasm.15
If MRI is contraindicated, computed tomography of the head should be performed.
CASE CONTINUED: IMAGING
The patient’s comprehensive metabolic panel is normal, but his fasting plasma glucose is 135 mg/dL (reference range 74–99). Pituitary MRI with contrast shows a 3-cm pituitary adenoma with suprasellar extension, impinging on the optic chiasm and invading the right cavernous sinus.
4. In addition to repeating the fasting plasma glucose and measuring hemoglobin A1c, what is the most appropriate next step in managing this patient?
- Measure the prolactin, morning serum cortisol, total testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and free thyroxine (T4) levels; refer the patient to an ophthalmologist for a formal evaluation of visual fields
- Measure these hormone levels; perform a gross evaluation of the visual fields and refer the patient to an ophthalmologist only if visual field deficits are found on the gross examination
- Measure these hormone levels; refer the patient to an ophthalmologist only if he complains of vision changes
- Do not order any additional tests; instruct the patient to call the office if he develops any vision changes
This patient should have all of these hormones measured. In addition, given that his macroadenoma is impinging on the optic chiasm, he should be referred to an ophthalmologist for a formal evaluation of visual fields even if the latter are intact on gross examination and even if the patient does not complain of any visual changes.
Abnormalities of hormones other than GH and IGF-1 in acromegaly
Secretion of pituitary hormones other than GH and IGF-1 must be assessed.
Prolactin. GH-secreting tumors also secrete prolactin in up to one-third of patients, with the resulting hyperprolactinemia contributing to hypogonadism.11 Prolactin hypersecretion should be distinguished from hyperprolactinemia caused by pituitary stalk compression, which may be evident on MRI.
Measuring the serum prolactin level with 1:100 dilution to counteract the “hook effect” may unmask severe hyperprolactinemia due to a large macroprolactinoma. (The hook effect occurs when the prolactin level is so high that there is not enough antibody in the assay to bind both ends of all the prolactin molecules present, causing the reading to be falsely low.).
Cortisol, T4, testosterone. Patients with acromegaly may develop central adrenal insufficiency, central hypothyroidism, and central hypogonadism; these hormonal deficits may occur in isolation or in combination.
Also, patients should be assessed for comorbidities such as colon cancer (all patients with acromegaly require a colonoscopy, as acromegaly raises the risk of colon cancer), diabetes mellitus, hypertension, cardiomyopathy, and sleep apnea.16
Visual field loss may be insidious
The diagnostic and treatment algorithm for acromegaly is summarized in Figure 1.
CASE CONTINUED: LABORATORY VALUES, TREATMENT OPTIONS
Our patient’s repeat fasting plasma glucose is 137 mg/dL; his hemoglobin A1c is 7.3%, consistent with diabetes mellitus secondary to acromegaly. Other laboratory values:
- Morning cortisol level 15 μg/dL (reference range 5.3–22.5),
- Prolactin 23 ng/mL, confirmed with 1:100 dilution (4.0–15.2)
- Total testosterone 59 ng/dL (193–824)
- LH 2.1 mIU/mL (1.8–10.8)
- FSH 3.0 mIU/mL (1.5–12.4)
- TSH 2.5 mIU/L (0.5–4.5)
- Free T4 1.3 ng/dL (0.9–1.7).
The patient is started on metformin 500 mg by mouth twice a day, counseled on a healthy diet, and informed that his diabetes may be a complication of his acromegaly. He is anxious to learn how his acromegaly can be treated.
5. What treatment would you recommend for the patient’s acromegaly?
- Medical treatment first, then transsphenoidal resection of the pituitary macroadenoma if medical treatment fails
- Medical treatment first, radiotherapy if medical treatment fails, and transsphenoidal resection of the pituitary macroadenoma as a last resort
- Transsphenoidal resection of the pituitary macroadenoma first, medical treatment if surgery fails, and radiotherapy if both surgery and medical treatment fail
- Taking a safe, conservative approach, monitoring IGF-1 levels frequently; starting medical treatment if acromegaly does not go into remission in 1 year
The initial treatment of choice for most patients with acromegaly is resection of the pituitary tumor.
A transsphenoidal approach is used for most patients; only rarely is craniotomy necessary. Endoscopic and microsurgical techniques reduce postoperative morbidity.17 Postoperative complications include symptoms related to the transsphenoidal approach (nasal congestion, sinusitis, epistaxis), cerebrospinal fluid leak, hemorrhage, meningitis, stroke, visual impairment, vascular damage, transient or permanent diabetes insipidus, and hypopituitarism. The surgical mortality rate is less than 0.5%.18,19
Successful resection of a pituitary tumor would lead to normalization of the IGF-1 level, a drop of the GH level to below 1 μg/L, and relief of the effect of the tumor pressing against other structures. An IGF-1 level and a random GH level should be obtained 12 weeks after the surgery.14 If the GH level is higher than 1 μg/L, a GH suppression test with a 75-g oral glucose load should be performed.14 MRI of the sella turcica should be done 12 weeks after surgery to visualize residual tumor and adjacent structures.14
A large tumor size, suprasellar extension, and high preoperative levels of IGF-1 and GH are associated with a lack of surgical success; however, surgical debulking should still be considered in patients with a low chance for surgical cure to improve the probability of achieving biochemical remission with postoperative medical and radiologic therapy.20
Medical therapy can be the initial treatment if the patient refuses surgery or if surgery is contraindicated because of severe comorbidities or because structural features of the tumor confer a high surgical risk (eg, if the adenoma encases the cavernous portion of a carotid artery).13 Medical therapy may shrink the tumor in some patients and may thereby make surgical resection easier and more likely to be successful.
Radiotherapy is usually reserved for patients whose tumors recur or persist postoperatively and who are resistant to or intolerant of medical therapy.14 The soft tissue changes caused by acromegaly may regress with treatment to some degree, but they are not likely to resolve completely; the bone changes do not regress.
CASE CONTINUED: MEDICAL TREATMENT
Three months after transsphenoidal resection of his pituitary macroadenoma, our patient’s laboratory values are as follows:
- IGF-1 400 ng/mL
- Morning cortisol 20 μg/dL
- Testosterone 95 ng/dL
- LH 2.1 mU/mL
- FSH 3.7 mU/mL
- Prolactin 12 ng/mL
- TSH 2.3 mIU/L
- Free T4 1.2 ng/dL
- Basic metabolic panel normal.
The patient denies frequent urination or increased thirst. Repeat MRI of the pituitary with contrast shows a residual 1.3-cm adenoma with no suprasellar extension.
6. What is the best next treatment choice for the patient?
- A GH receptor antagonist (pegvisomant)
- A somatostatin receptor ligand (SRL) such as octreotide
- Cabergoline (a dopamine agonist)
- A combination of an SRL and pegvisomant
An SRL such as octreotide would be the best choice for this patient.
The medical options for acromegaly are SRLs, pegvisomant, and cabergoline.21–23 The Endocrine Society guidelines recommend either an SRL or pegvisomant as the initial adjuvant medical therapy in patients with persistent disease after surgery.14 However, pegvisomant is much more expensive than any SRL, so an SRL would be a better choice in this patient. Also, pegvisomant does not suppress tumor growth, in contrast to SRLs, so SRLs are preferred in patients with large tumors abutting the optic chiasm.14
SRLs are used as primary therapy in patients who cannot be cured by surgery, have extensive cavernous sinus invasion, have no chiasmal compression, or are poor surgical candidates.
The medical treatment of acromegaly is summarized in Table 2.14,15 Side effects of the medications used to treat acromegaly are summarized in Table 3.14
CASE CONTINUED: RADIOTHERAPY
The patient is treated with octreotide, and the dose is subsequently titrated upward. His central hypogonadism is treated with testosterone gel. After 3 months, his IGF-1 level decreases to 190 ng/mL, the total testosterone increases to 450 ng/dL, and the hemoglobin A1c decreases to 5.9%.
The patient asks if stereotactic radiotherapy, which he read about on the Internet, can cure his acromegaly so that he can avoid the monthly octreotide injections.
7. Which statement best describes radiotherapy’s therapeutic effect in acromegaly?
- Stereotactic radiotherapy is more effective than medical therapy and should be used as a second-line treatment after surgery
- Stereotactic radiotherapy is less effective than conventional radiotherapy
- Stereotactic radiotherapy leads to stability or a decrease in the size of the GH-secreting tumor in 93% to 100% of patients in 5 to 10 years and to biochemical remission in 40% to 60% of patients at 5 years
- Stereotactic radiotherapy causes hypopituitarism in no more than 1% of patients
Stereotactic radiotherapy leads to stability or a decrease in the size of the GH-secreting tumor in 93% to 100% of patients in 5 to 10 years and biochemical remission in 40% to 60% of patients at 5 years.24,25
Hypopituitarism develops in up to 50% of patients at 5 years, and its incidence increases with the duration of follow-up.24 The risk of other complications is low (0% to 5% for new visual deficits, cranial nerve damage, or brain radionecrosis, and 0% to 1% for secondary brain tumors).24
Conventional radiotherapy has fallen out of favor because it is associated with an increased risk of death (mainly from stroke) independent of IGF-1 and GH levels, and a higher rate of complications than stereotactic radiotherapy.14,16 Radiotherapy is reserved for postsurgical treatment of patients with recurrent or persistent tumors who are resistant to or cannot tolerate medical therapy; it is the third-line treatment.24
Given that our patient responded to the medical therapy and tolerated it well and given the high risk of hypopituitarism associated with stereotactic radiotherapy, the latter would not be appropriate for the patient.
His fatigue has diminished further and his sexual performance has improved. He is still married and his wife no longer suspects him of infidelity.
KEY POINTS
- IGF-1 is the screening test of choice in a patient with signs and symptoms of acromegaly.
- A growth hormone suppression test with a 75-g oral glucose load is the gold standard test for confirmation of the diagnosis of acromegaly in patients with an elevated IGF-1 level.
- Transsphenoidal resection of the growth hormone-secreting pituitary macroadenoma is the initial treatment of choice for acromegaly.
- Patients with residual or recurrent growth hormone-secreting pituitary macroadenoma can be treated with somatostatin receptor ligands, a growth hormone receptor antagonist (pegvisomant), and a dopamine agonist cabergoline.
- Radiotherapy is reserved for postsurgical treatment of patients with recurrent or persistent tumors who are resistant to or intolerant of medical therapy. Stereotactic radiotherapy has largely replaced conventional radiotherapy.
- Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest 2009; 119:3189–3202.
- Molitch ME. Clinical manifestations of acromegaly. Endocrinol Metab Clin North Am 1992; 21:597–614.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017; 110:411–420.
- Yacub A, Yaqub N. Insulin-mediated pseudoacromegaly: a case report and review of the literature. W V Med J 2008; 104:12–15.
- Mestron A, Webb SM, Astorga R, et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol 2004; 151:439–446.
- Zhu X, Lin CR, Prefontaine CG, Tollkuhn J, Rosenfeld MG. Genetic control of pituitary development and hypopituitarism. Curr Opin Genet Dev 2005; 15:332–340.
- Tannenbaum GS, Epelbaum J, Bowers CY. Interrelationship between the novel peptide ghrelin and somatostatin/growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology 2003; 144:967–974.
- Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord 2006; 7:225–235.
- Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 2004; 25:102–152.
- Larkin S, Reddy R, Karavitaki N, Cudlip S, Wass J, Ansorge O. Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur J Endocrinol 2013; 168:491–499.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017; 110:411–420.
- Peacey SR, Toogood AA, Veldhuis JD, Thorner MO, Shalet SM. The relationship between 24-hour growth hormone secretion and insulin-like growth factor I in patients with successfully treated acromegaly: impact of surgery or radiotherapy. J Clin Endocrinol Metab 2001; 86:259–266.
- Livingstone C. IGF2 and cancer. Endocr Relat Cancer 2013; 20:R321–R339.
- Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2014; 99:3933–3951.
- Melmed S. Acromegaly. N Engl J Med 2006; 355:2558–2573.
- Melmed S, Casanueva FF, Klibanski A, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary 2013; 16:294–302.
- Marquez Y, Tuchman A, Zada G. Surgery and radiosurgery for acromegaly: a review of indications, operative techniques, outcomes, and complications. Int J Endocrinol 2012; 2012: 386401.
- Jane JA Jr, Starke RM, Elzoghby MA, et al. Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab 2011; 96:2732–2740.
- Cappabianca P, Cavallo LM, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg 2002; 97:293–298.
- Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical “cure.” Eur J Endocrinol 2005; 152:379–387.
- Howlett TA, Willis D, Walker G, Wass JA, Trainer PJ; UK Acromegaly Register Study Group (UKAR-3). Control of growth hormone and IGF1 in patients with acromegaly in the UK: responses to medical treatment with somatostatin analogues and dopamine agonists. Clin Endocrinol (Oxf) 2013; 79:689–699.
- Katznelson L. Pegvisomant for the treatment of acromegaly-translation of clinical trials into clinical practice. Nat Clin Pract Endocrinol Metab 2007; 3:514–515.
- Freda PU, Reyes CM, Nuruzzaman AT, Sundeen RE, Khandji AG, Post KD. Cabergoline therapy of growth hormone & growth hormone/prolactin secreting pituitary tumors. Pituitary 2004; 7:21–30.
- Castinetti F, Morange I, Dufour H, Regis J, Brue T. Radiotherapy and radiosurgery in acromegaly. Pituitary 2009; 12:3–10.
- Gheorghiu ML. Updates in outcomes of stereotactic radiation therapy in acromegaly. Pituitary 2017; 20:154–168.
- Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest 2009; 119:3189–3202.
- Molitch ME. Clinical manifestations of acromegaly. Endocrinol Metab Clin North Am 1992; 21:597–614.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017; 110:411–420.
- Yacub A, Yaqub N. Insulin-mediated pseudoacromegaly: a case report and review of the literature. W V Med J 2008; 104:12–15.
- Mestron A, Webb SM, Astorga R, et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol 2004; 151:439–446.
- Zhu X, Lin CR, Prefontaine CG, Tollkuhn J, Rosenfeld MG. Genetic control of pituitary development and hypopituitarism. Curr Opin Genet Dev 2005; 15:332–340.
- Tannenbaum GS, Epelbaum J, Bowers CY. Interrelationship between the novel peptide ghrelin and somatostatin/growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology 2003; 144:967–974.
- Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord 2006; 7:225–235.
- Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 2004; 25:102–152.
- Larkin S, Reddy R, Karavitaki N, Cudlip S, Wass J, Ansorge O. Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur J Endocrinol 2013; 168:491–499.
- Dineen R, Stewart PM, Sherlock M. Acromegaly. QJM 2017; 110:411–420.
- Peacey SR, Toogood AA, Veldhuis JD, Thorner MO, Shalet SM. The relationship between 24-hour growth hormone secretion and insulin-like growth factor I in patients with successfully treated acromegaly: impact of surgery or radiotherapy. J Clin Endocrinol Metab 2001; 86:259–266.
- Livingstone C. IGF2 and cancer. Endocr Relat Cancer 2013; 20:R321–R339.
- Katznelson L, Laws ER Jr, Melmed S, et al. Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2014; 99:3933–3951.
- Melmed S. Acromegaly. N Engl J Med 2006; 355:2558–2573.
- Melmed S, Casanueva FF, Klibanski A, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary 2013; 16:294–302.
- Marquez Y, Tuchman A, Zada G. Surgery and radiosurgery for acromegaly: a review of indications, operative techniques, outcomes, and complications. Int J Endocrinol 2012; 2012: 386401.
- Jane JA Jr, Starke RM, Elzoghby MA, et al. Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab 2011; 96:2732–2740.
- Cappabianca P, Cavallo LM, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg 2002; 97:293–298.
- Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical “cure.” Eur J Endocrinol 2005; 152:379–387.
- Howlett TA, Willis D, Walker G, Wass JA, Trainer PJ; UK Acromegaly Register Study Group (UKAR-3). Control of growth hormone and IGF1 in patients with acromegaly in the UK: responses to medical treatment with somatostatin analogues and dopamine agonists. Clin Endocrinol (Oxf) 2013; 79:689–699.
- Katznelson L. Pegvisomant for the treatment of acromegaly-translation of clinical trials into clinical practice. Nat Clin Pract Endocrinol Metab 2007; 3:514–515.
- Freda PU, Reyes CM, Nuruzzaman AT, Sundeen RE, Khandji AG, Post KD. Cabergoline therapy of growth hormone & growth hormone/prolactin secreting pituitary tumors. Pituitary 2004; 7:21–30.
- Castinetti F, Morange I, Dufour H, Regis J, Brue T. Radiotherapy and radiosurgery in acromegaly. Pituitary 2009; 12:3–10.
- Gheorghiu ML. Updates in outcomes of stereotactic radiation therapy in acromegaly. Pituitary 2017; 20:154–168.