User login
Uterine incision closure: Is it the culprit in the cesarean scar niche and related complications?
While its etiology remains uncertain, cesarean scar niche (CSN) is well publicized, as are its pathological clinical manifestations. In a future pregnancy, they include cesarean scar pregnancy (CSP), which in turn can lead to placenta accreta spectrum, and possible uterine rupture/dehiscence of a residual thin myometrial layer. CSP refers to the implantation of an early pregnancy on the scar or in the niche at the site of a prior cesarean delivery (CD); it has an incidence of 1 per 1,000 pregnancies. An estimated 52% of CSPs occur after even just one CD.1 CSP has been linked to placenta accreta spectrum and has been shown to be its precursor.2 Both CSP and placenta accreta spectrum can be consequences of CD and share a common histology of villous or placental attachment/invasion into the cesarean scar.3 The incidence of placenta accreta spectrum has risen from about 1 in 4,000 live births in the 1970s to 1 in 2,500 in the 1980s; in 2016, the incidence of placenta accreta spectrum was reported as 1 per 272 live births.4
Placenta accreta spectrum denotes the attachment of the placenta into and through the myometrium,5 and it can result in severe complications, including hemorrhage, hysterectomy, and intensive care treatment. The increasing rate of placenta accreta spectrum parallels the increasing CD rate, which rose from 5.8% in 1970 to 31.9% in 2016.6 Multiple repeat CDs are increasing in frequency as well. At the beginning of the century, placenta accreta spectrum mainly occurred after manual removal of the placenta, uterine curettage, or endometritis. Recently, experts are in agreement that the main determinant of placenta accreta spectrum is the uterine scar and niche formation after a previous CD.5 Larger niches are associated with an increased incidence of uterine rupture or dehiscence in a subsequent pregnancy.7
In the nonpregnant state, such niches are associated with intermenstrual bleeding, pelvic pain, painful intercourse, painful menses, and subfertility, becoming increasingly more severe in women with greater numbers of CDs.8-10 Conception rate with assisted reproductive treatment is notably reduced.11
Understanding its etiology
Monteagudo and colleagues first described a “niche” in 100% of 44 women evaluated for postmenopausal bleeding who had a prior CD.12 CSN has been the subject of well over 3,000 publications over the past 30 years. While the topic generates much interest among researchers, it is garnering little traction among practicing obstetricians. Such “niches,” also referred to as isthmocele, cesarean scar defect, or a diverticulum, was first described in 196113 and later defined on ultrasonography as a hypoechoic triangular-shaped uterine defect outlined by saline instillation sonohysterogram (SIS), reflecting a discontinuation of the myometrium at the site of a previous CD.12 In 2019, a European task force further defined a CSN as an “indentation at the site in the cesarean section scar with a depth of at least 2 mm” and extended the classification to include branches as extensions toward the anterior uterine serosa.14 Using this criterion, sonographic postoperative evaluation after one CD revealed a CSN in 68.9% of women with one single-layer uterine closure and in 73.6% of women after a double-layer closure.15 Larger niche sizes with thinner residual myometrial thickness appeared more frequently when a single-layer closure technique was used, without closure of the peritoneum. Its prevalence varies from 56% to 84%.16,17
Etiology of CSN formation: Our hypotheses
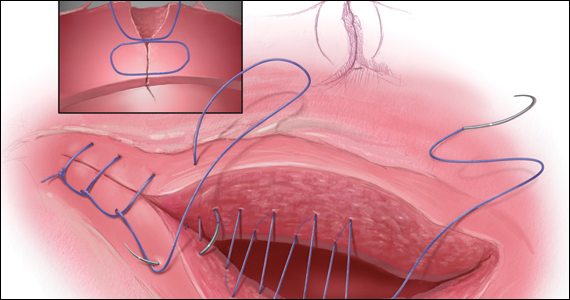
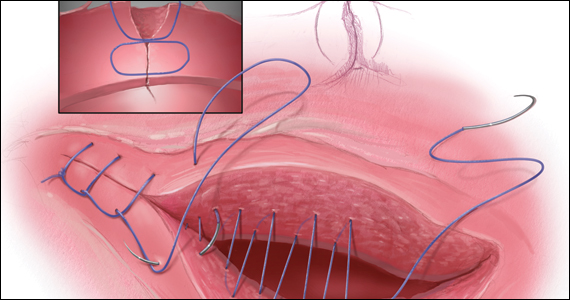
The precise pathophysiology of CSN remains elusive. Speculations attributed niche formation to numerous factors: timing of surgery, cervical incision, incomplete closure of the uterine incision, adhesion formation between the CD scar and the abdominal wall, and inherent maternal conditions which may impair healing, such as smoking, obesity, diabetes, maternal age, and labor status.18-20 Retroflexion of the uterus is reportedly associated with increased incidence and size of the niche, with CSN 50% more likely to develop in women with a retroflexed versus an anteverted uterus.21 We demonstrated the origin of niche formation in real-time from the start to the completion of uterine closure by a video capture of a single-layer closure followed by an immediate SIS of the ex vivo hysterectomized uterus, and histopathologic proof of the presence of endometrial cells defining the “niche.”22 This case exposes the misalignment of the uterine wall, while including the endometrium in the closure (FIGURE 1). Similarly, pathologic studies of hysteroscopy-resected isthmocele ridges of symptomatic women with niche-related subfertility revealed the tissue edges lined by endocervical, endometrial, or isthmic mucosa either combined or isolated in the scar.23 The presence of endometrial/cervical tissue in the myometrial closure has been debated for over a century.24,25
Continue to: Uterine closure techniques...
Uterine closure techniques: Historical perspective
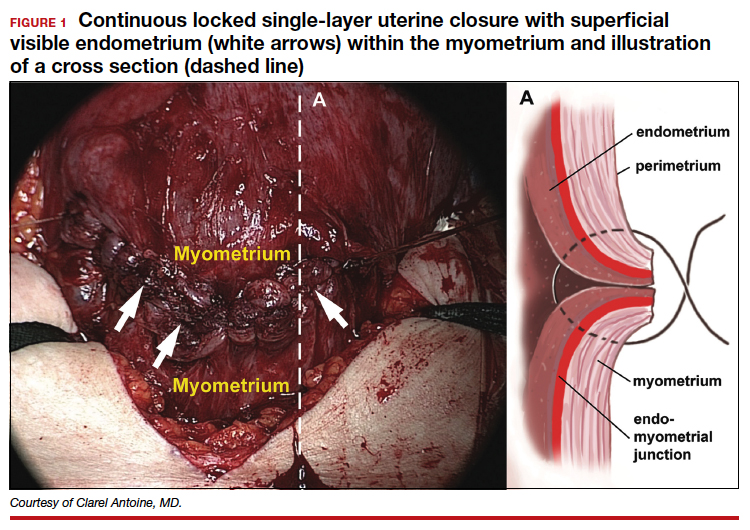
In 1882, Max Sanger introduced a vertical uterine closure of a classical cesarean operation in response to hysterectomy as the contemporaneous alternative to prevent infection, bleeding, and death.24 Dr. Sanger emphasized layer approximation, suturing, and the avoidance of decidua in the first layer (FIGURE 2). This became the teaching of the classical CD until the 1970s. In 1926, Munro Kerr addressed uterine rupture with labor after a classical CD by introducing the lower uterine segment transverse incision. He cautioned to maintain the decidua inside the uterine 2-layer closure of the cavity.25 These pioneers were joined by others to rally for endometrium exclusion while promoting layer approximation. These techniques became universally standard and were taught across teaching medical centers in the United States and abroad until about 50 years ago.
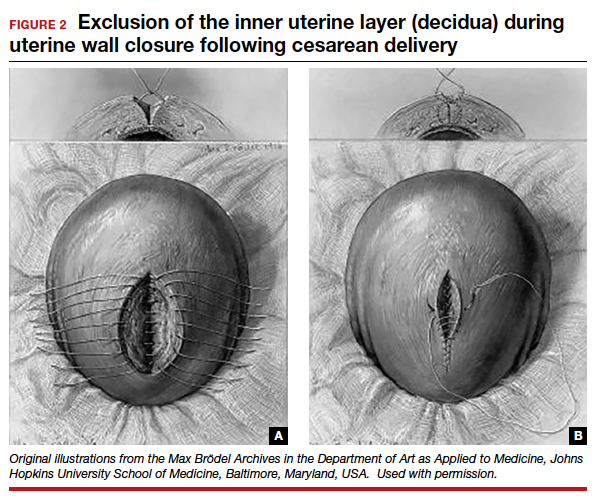
In the 1970s, newer developments brought significant changes to uterine closure techniques. Initiated by Joel-Cohen,26 blunt dissection of the abdominal incision was adapted by Michael Stark, creating what came to be known as the Misgav-Ladach cesarean technique.27 Stark emphasized blunt dissection and introduced single-layer closure. Thereby the exclusion of the endometrium, used for more than 70 years, was abandoned by the present-day single- or double-layer uterine closure in favor of cost and time savings. Systematic reviews and meta-analyses comparing the two contrasting techniques were inconclusive, noting that the niche prevalence and size were similar in both groups. These studies did not take into account the variety of individual techniques or the position of the endometrium in the final closures.28
Endometrium and uterine closure
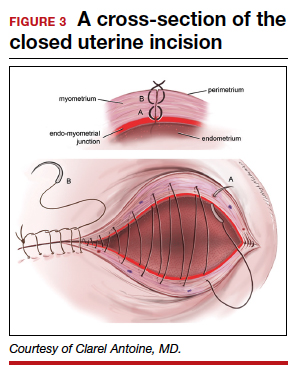
Our recent study examining uterine scar defect in women after one primary CD by SIS concluded that a specific endometrium-free closure technique (EFCT) (FIGURE 3) is associated with fewer and less severe defects and a thicker residual myometrial thickness when compared with closures with unknown or endometrium inclusion.29 The study found non-specific closure techniques to be 6 times more likely to form a niche of 2-mm deep or greater than the EFCT.
Furthermore, we surveyed the diversity of uterine closures and the location of the endometrium among obstetricians in one institution.30 Presence of endometrium on the surface of the final uterine closure was reported by 20% of respondents (see Figure 1). When asked for their opinion on the impact of CD techniques on placenta accreta spectrum, without available evidence 80% of the survey respondents reported no relationship to techniques, and only 20% suggested an association. This particular study demonstrates that the surgical techniques just described are random, unfettered, and applied without consideration of clinical outcomes.
Our recent retrospective study that spanned 30 years and examined the EFCT—performed anywhere between 3 to 9 consecutive CDs—revealed no abnormal placentation in any subsequent pregnancies.31 This was one of the few clinical studies of the long-term consequences of a uterine closure technique. In this study, the endometrium was excluded during the uterine closure, allowing its free edges to abut and heal. This step avoids scarring the endometrial-myometrial (EM) interface and unintentional inclusion of endometrium in the closed uterine wall. In this context, Jauniaux and colleagues cited the destruction of the EM interface as the main factor for placenta-adherent disorders.32 Sholapurkar and others highlight the need to further examine intrinsic details of uterine closure beyond single- and double-layer techniques to better understand the etiology of cesarean scar formation.19 The search for the pathophysiology of CSN continues to present significant challenges imposed by the variety of currently practiced uterine closures.
Continue to: Focus on prevention...
Research: Focus on prevention
Our research aims to address the endometrium, a specific layer that was the topic of concern in nascent CD techniques, as a renewed and contemporary one. The presence of the endometrium in ectopic locations or its destruction from intrauterine surgeries or infections has been implicated in abnormal placentation.13,24 Our approach, in theory, is to limit the position of the endometrium to its innermost location and avoid its iatrogenic suturing and inclusion into the uterine wall closure. The rationale of sparing the endometrium in a layer-by-layer approximation is to allow for a closer restoration to normal anatomy and physiology than a random “en masse” uterine wall closure would permit. For this reason, the EM junction, the perimetrium, and the serosa must be identified and realigned for a more effective closure that incorporates the entire myometrial thickness. As evidence supports technical impact on the development of uterine scar defect in women after one CD, future studies are needed to evaluate uterine integrity by saline infusion sonohysterography in multiparous women with a prior random closure technique or a prior EFCT.
The potential long-term risks of blunt dissection for opening the uterus have not been studied. There are no physiologic lines in the uterine wall to facilitate a regular-bordered uterine stretch. The tissue stretch, which depends on the individual surgeon’s strength applied during the procedure and patient’s labor status, may result in an irregular tear and a difficult repair. The EFCT technique shows a more optimized risk-benefit ratio for an anatomical repair and is replicable. The safety of uterine layer re-approximation has been demonstrated and can be studied in large populations using strict uniform criteria.
Current and future challenges
Residency training
Most recently, teachers of resident trainees are mostly familiar with blunt dissection, techniques of which are passed on unchallenged from resident to resident. The endometrium and peritoneum are neither identified nor treated as separate layers, thus becoming obsolete as surgical and anatomical landmarks.
Standardization of CD techniques
Front-line obstetricians are persuaded to practice a standardized approach that relies on the benefits of cost related to operating room turnover as well as surgeons’ time savings without consideration of outcomes in subsequent pregnancies. Sholapurkar has warned that “wrong standardization” is far worse than no standardization, worse for the training of junior obstetricians, as it can inhibit critical reasoning about safe surgical techniques that can optimize outcomes of the condition of the lower uterine segment.33
Emergence of cost and time savings in clinical practice
A time-cost savings argument is relatively negligeable in an estimated 40-minute CD. By contrast, deliberate surgical technique and carrying out the appropriate steps for the particular condition at hand to achieve the best outcomes assume more weight.32 Furthermore, this short-term cost benefit is challenged by the comparatively larger costs associated with the diagnosis, the treatment of post-CD adverse consequences (outlined above), as well as the emotional impact on women and their families. Additionally, the emphasis on time savings creates a generation of surgeons fixated with total operative time without consideration of long-term risks and adverse maternal outcomes.
Physician autonomy has led to the unmonitored freedom of obstetricians to choose their own technique for a CD, with some employing the commonly practiced culture of fastest turnaround even in nonurgent circumstances.
Documentation and terminology
Current documenting systems are not detail-oriented enough to assist in a thorough correlation between surgical techniques and outcomes. The use of single- or double-layer closure terminology is insufficient and has proven to be flawed, without describing the handling of the endometrium in terms of its inclusion or exclusion in the closure.
Quality improvement feedback
Long-term post-CD complications are often not reported to the physician or institution involved in the prior CD. In our opinion, some sort of registry would be of value. Perhaps then subsequent CD outcomes could be traced back and reported to the prior institution and surgeon. Feedback is critical to understanding the correlation between techniques and outcomes and more specifically to gathering learning points and using data for quality improvement of future cases.
Patient education
While women continue to have complications following the presently used surgical techniques, they often have expectations not discussed with their obstetricians. Women should be educated and empowered to realize the different approaches to all aspects and consequences of CDs.
Conclusion
The technique of excluding the endometrium in closing the uterine incision appears to reduce subsequent abnormal placentation and diminish the frequency and size of post-CD scar defect. The revival of the endometrium-free closure technique may allow significant change in the postoperative results. Currently, standardization of CD technique is being promoted on the basis of time- and cost-savings rather than clinical outcomes. Simultaneously, inroads are being made to better understand the risks and consequences of CD.
Emerging evidence suggests that a post-CD niche is the result of poor layer approximation as well as inclusion of the endometrium, which prevent healing of the uterine wall and often enables faulty implantation of the fertilized oocyte in the next pregnancy, potentially giving rise to placenta accreta spectrum. The prevalence and size of the defect can be minimized by techniques aimed at restoring the anatomy of the uterine wall and the physiology of the endometrium. Specialized training and education are necessary to stress the importance of anatomical assessment and decision making at the time of uterine closure. ●
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373-1381.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346-353. doi:10.1002/ uog.13426.
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383-395. doi: 10.1002/uog.13282.
- Mogos MF, Salemi JL, Ashley M, et al. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J Matern Fetal Neonatal Med. 2016;29:1077-1082.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75-87.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1-8.
- Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525-532.
- Chen YY, Tsai CC, Kung FT, et al. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwanese J Obstet Gynecol. 2019;58:541-544.
- Stegwee SI, Beij A, de Leeuw RA, et al. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res. 2020;29:1013-1025.
- Vissers J, Hehenkamp W, Lambalk CB, et al. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484-1494.
- Vissers J, Sluckin TC, van Driel-Delprat CCR, et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous caesarean section: a retrospective cohort study. Hum Reprod. 2020;35:595-604.
- Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105-1115.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67-71.
- Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107-115.
- Stegwee SI, van der Voet LF, Ben AJ, et al. Effect of single- versus double-layer uterine closure during caesarean section on postmenstrual spotting (2Close): multicentre, double-blind, randomised controlled superiority trial. BJOG. 2021;128:866-878.
- Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43:372-382.
- van der Voet LF, Bij de Vaate AM, Veersema S, et al. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236-244.
- Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, et al. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695-2702.
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J Clin Med Res. 2018;10:166-173.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of cesarean section. Ultrasound Obstet Gynecol. 2021;57:466-470.
- Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014;203:W117-124.
- Antoine C, Pimentel RN, Timor-Tritsch IE, et al. Origin of a post-cesarean delivery niche: diagnosis, pathophysiologic characteristics, and video documentation. J Ultrasound Med. 2021;40:205-208.
- AbdullGaffar B, Almulla A. A histopathologic approach to uterine niche: what to expect and to report in hysteroscopy-resected isthmocele specimens. Int J Surg Pathol. 2021:10668969211039415. doi: 10.1177/10668969211039415.
- Nagy S, Papp Z. Global approach of the cesarean section rates. J Perinatal Med. 2020;49:1-4.
- Kerr JM. The technic of cesarean section, with special reference to the lower uterine segment incision. Am J Obstet Gynecol. 1926;12:729-734.
- Joel-Cohen S. Abdominal and vaginal hysterectomy: new techniques based on time and motion studies. Lippincott Williams & Wilkins; 1977.
- Holmgren G, Sjoholm L, Stark M. The Misgav Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78:615-621.
- Abalos E, Addo V, Brocklehurst P, et al. Caesarean section surgical techniques: 3-year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016;388:62-72.
- Antoine C, Meyer JA, Silverstein JS, et al. The impact of uterine incision closure techniques on post-cesarean delivery niche formation and size: sonohysterographic examination of nonpregnant women. J Ultrasound Med. 2021. doi: 10.1002/ jum.15859.
- Antoine C AJ, Yaghoubian Y, Harary J. Variations in uterine closure technique: an institutional survey of obstetricians and implications for patient counseling and prevention of adverse sequelae [Abstract]. 2021.
- Antoine C, Pimentel RN, Reece EA, et al. Endometrium-free uterine closure technique and abnormal placental implantation in subsequent pregnancies. J Matern-Fetal Neonatal Med. 2019:1-9.
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33:244-251.
- Sholapurkar S. Review of unsafe changes in the practice of cesarean section with analysis of flaws in the interpretation of statistics and the evidence. Surgical Case Reports. 2021;4:2-6.

While its etiology remains uncertain, cesarean scar niche (CSN) is well publicized, as are its pathological clinical manifestations. In a future pregnancy, they include cesarean scar pregnancy (CSP), which in turn can lead to placenta accreta spectrum, and possible uterine rupture/dehiscence of a residual thin myometrial layer. CSP refers to the implantation of an early pregnancy on the scar or in the niche at the site of a prior cesarean delivery (CD); it has an incidence of 1 per 1,000 pregnancies. An estimated 52% of CSPs occur after even just one CD.1 CSP has been linked to placenta accreta spectrum and has been shown to be its precursor.2 Both CSP and placenta accreta spectrum can be consequences of CD and share a common histology of villous or placental attachment/invasion into the cesarean scar.3 The incidence of placenta accreta spectrum has risen from about 1 in 4,000 live births in the 1970s to 1 in 2,500 in the 1980s; in 2016, the incidence of placenta accreta spectrum was reported as 1 per 272 live births.4
Placenta accreta spectrum denotes the attachment of the placenta into and through the myometrium,5 and it can result in severe complications, including hemorrhage, hysterectomy, and intensive care treatment. The increasing rate of placenta accreta spectrum parallels the increasing CD rate, which rose from 5.8% in 1970 to 31.9% in 2016.6 Multiple repeat CDs are increasing in frequency as well. At the beginning of the century, placenta accreta spectrum mainly occurred after manual removal of the placenta, uterine curettage, or endometritis. Recently, experts are in agreement that the main determinant of placenta accreta spectrum is the uterine scar and niche formation after a previous CD.5 Larger niches are associated with an increased incidence of uterine rupture or dehiscence in a subsequent pregnancy.7
In the nonpregnant state, such niches are associated with intermenstrual bleeding, pelvic pain, painful intercourse, painful menses, and subfertility, becoming increasingly more severe in women with greater numbers of CDs.8-10 Conception rate with assisted reproductive treatment is notably reduced.11
Understanding its etiology
Monteagudo and colleagues first described a “niche” in 100% of 44 women evaluated for postmenopausal bleeding who had a prior CD.12 CSN has been the subject of well over 3,000 publications over the past 30 years. While the topic generates much interest among researchers, it is garnering little traction among practicing obstetricians. Such “niches,” also referred to as isthmocele, cesarean scar defect, or a diverticulum, was first described in 196113 and later defined on ultrasonography as a hypoechoic triangular-shaped uterine defect outlined by saline instillation sonohysterogram (SIS), reflecting a discontinuation of the myometrium at the site of a previous CD.12 In 2019, a European task force further defined a CSN as an “indentation at the site in the cesarean section scar with a depth of at least 2 mm” and extended the classification to include branches as extensions toward the anterior uterine serosa.14 Using this criterion, sonographic postoperative evaluation after one CD revealed a CSN in 68.9% of women with one single-layer uterine closure and in 73.6% of women after a double-layer closure.15 Larger niche sizes with thinner residual myometrial thickness appeared more frequently when a single-layer closure technique was used, without closure of the peritoneum. Its prevalence varies from 56% to 84%.16,17
Etiology of CSN formation: Our hypotheses
The precise pathophysiology of CSN remains elusive. Speculations attributed niche formation to numerous factors: timing of surgery, cervical incision, incomplete closure of the uterine incision, adhesion formation between the CD scar and the abdominal wall, and inherent maternal conditions which may impair healing, such as smoking, obesity, diabetes, maternal age, and labor status.18-20 Retroflexion of the uterus is reportedly associated with increased incidence and size of the niche, with CSN 50% more likely to develop in women with a retroflexed versus an anteverted uterus.21 We demonstrated the origin of niche formation in real-time from the start to the completion of uterine closure by a video capture of a single-layer closure followed by an immediate SIS of the ex vivo hysterectomized uterus, and histopathologic proof of the presence of endometrial cells defining the “niche.”22 This case exposes the misalignment of the uterine wall, while including the endometrium in the closure (FIGURE 1). Similarly, pathologic studies of hysteroscopy-resected isthmocele ridges of symptomatic women with niche-related subfertility revealed the tissue edges lined by endocervical, endometrial, or isthmic mucosa either combined or isolated in the scar.23 The presence of endometrial/cervical tissue in the myometrial closure has been debated for over a century.24,25

Continue to: Uterine closure techniques...
Uterine closure techniques: Historical perspective
In 1882, Max Sanger introduced a vertical uterine closure of a classical cesarean operation in response to hysterectomy as the contemporaneous alternative to prevent infection, bleeding, and death.24 Dr. Sanger emphasized layer approximation, suturing, and the avoidance of decidua in the first layer (FIGURE 2). This became the teaching of the classical CD until the 1970s. In 1926, Munro Kerr addressed uterine rupture with labor after a classical CD by introducing the lower uterine segment transverse incision. He cautioned to maintain the decidua inside the uterine 2-layer closure of the cavity.25 These pioneers were joined by others to rally for endometrium exclusion while promoting layer approximation. These techniques became universally standard and were taught across teaching medical centers in the United States and abroad until about 50 years ago.

In the 1970s, newer developments brought significant changes to uterine closure techniques. Initiated by Joel-Cohen,26 blunt dissection of the abdominal incision was adapted by Michael Stark, creating what came to be known as the Misgav-Ladach cesarean technique.27 Stark emphasized blunt dissection and introduced single-layer closure. Thereby the exclusion of the endometrium, used for more than 70 years, was abandoned by the present-day single- or double-layer uterine closure in favor of cost and time savings. Systematic reviews and meta-analyses comparing the two contrasting techniques were inconclusive, noting that the niche prevalence and size were similar in both groups. These studies did not take into account the variety of individual techniques or the position of the endometrium in the final closures.28
Endometrium and uterine closure
Our recent study examining uterine scar defect in women after one primary CD by SIS concluded that a specific endometrium-free closure technique (EFCT) (FIGURE 3) is associated with fewer and less severe defects and a thicker residual myometrial thickness when compared with closures with unknown or endometrium inclusion.29 The study found non-specific closure techniques to be 6 times more likely to form a niche of 2-mm deep or greater than the EFCT.

Furthermore, we surveyed the diversity of uterine closures and the location of the endometrium among obstetricians in one institution.30 Presence of endometrium on the surface of the final uterine closure was reported by 20% of respondents (see Figure 1). When asked for their opinion on the impact of CD techniques on placenta accreta spectrum, without available evidence 80% of the survey respondents reported no relationship to techniques, and only 20% suggested an association. This particular study demonstrates that the surgical techniques just described are random, unfettered, and applied without consideration of clinical outcomes.
Our recent retrospective study that spanned 30 years and examined the EFCT—performed anywhere between 3 to 9 consecutive CDs—revealed no abnormal placentation in any subsequent pregnancies.31 This was one of the few clinical studies of the long-term consequences of a uterine closure technique. In this study, the endometrium was excluded during the uterine closure, allowing its free edges to abut and heal. This step avoids scarring the endometrial-myometrial (EM) interface and unintentional inclusion of endometrium in the closed uterine wall. In this context, Jauniaux and colleagues cited the destruction of the EM interface as the main factor for placenta-adherent disorders.32 Sholapurkar and others highlight the need to further examine intrinsic details of uterine closure beyond single- and double-layer techniques to better understand the etiology of cesarean scar formation.19 The search for the pathophysiology of CSN continues to present significant challenges imposed by the variety of currently practiced uterine closures.
Continue to: Focus on prevention...
Research: Focus on prevention
Our research aims to address the endometrium, a specific layer that was the topic of concern in nascent CD techniques, as a renewed and contemporary one. The presence of the endometrium in ectopic locations or its destruction from intrauterine surgeries or infections has been implicated in abnormal placentation.13,24 Our approach, in theory, is to limit the position of the endometrium to its innermost location and avoid its iatrogenic suturing and inclusion into the uterine wall closure. The rationale of sparing the endometrium in a layer-by-layer approximation is to allow for a closer restoration to normal anatomy and physiology than a random “en masse” uterine wall closure would permit. For this reason, the EM junction, the perimetrium, and the serosa must be identified and realigned for a more effective closure that incorporates the entire myometrial thickness. As evidence supports technical impact on the development of uterine scar defect in women after one CD, future studies are needed to evaluate uterine integrity by saline infusion sonohysterography in multiparous women with a prior random closure technique or a prior EFCT.
The potential long-term risks of blunt dissection for opening the uterus have not been studied. There are no physiologic lines in the uterine wall to facilitate a regular-bordered uterine stretch. The tissue stretch, which depends on the individual surgeon’s strength applied during the procedure and patient’s labor status, may result in an irregular tear and a difficult repair. The EFCT technique shows a more optimized risk-benefit ratio for an anatomical repair and is replicable. The safety of uterine layer re-approximation has been demonstrated and can be studied in large populations using strict uniform criteria.
Current and future challenges
Residency training
Most recently, teachers of resident trainees are mostly familiar with blunt dissection, techniques of which are passed on unchallenged from resident to resident. The endometrium and peritoneum are neither identified nor treated as separate layers, thus becoming obsolete as surgical and anatomical landmarks.
Standardization of CD techniques
Front-line obstetricians are persuaded to practice a standardized approach that relies on the benefits of cost related to operating room turnover as well as surgeons’ time savings without consideration of outcomes in subsequent pregnancies. Sholapurkar has warned that “wrong standardization” is far worse than no standardization, worse for the training of junior obstetricians, as it can inhibit critical reasoning about safe surgical techniques that can optimize outcomes of the condition of the lower uterine segment.33
Emergence of cost and time savings in clinical practice
A time-cost savings argument is relatively negligeable in an estimated 40-minute CD. By contrast, deliberate surgical technique and carrying out the appropriate steps for the particular condition at hand to achieve the best outcomes assume more weight.32 Furthermore, this short-term cost benefit is challenged by the comparatively larger costs associated with the diagnosis, the treatment of post-CD adverse consequences (outlined above), as well as the emotional impact on women and their families. Additionally, the emphasis on time savings creates a generation of surgeons fixated with total operative time without consideration of long-term risks and adverse maternal outcomes.
Physician autonomy has led to the unmonitored freedom of obstetricians to choose their own technique for a CD, with some employing the commonly practiced culture of fastest turnaround even in nonurgent circumstances.
Documentation and terminology
Current documenting systems are not detail-oriented enough to assist in a thorough correlation between surgical techniques and outcomes. The use of single- or double-layer closure terminology is insufficient and has proven to be flawed, without describing the handling of the endometrium in terms of its inclusion or exclusion in the closure.
Quality improvement feedback
Long-term post-CD complications are often not reported to the physician or institution involved in the prior CD. In our opinion, some sort of registry would be of value. Perhaps then subsequent CD outcomes could be traced back and reported to the prior institution and surgeon. Feedback is critical to understanding the correlation between techniques and outcomes and more specifically to gathering learning points and using data for quality improvement of future cases.
Patient education
While women continue to have complications following the presently used surgical techniques, they often have expectations not discussed with their obstetricians. Women should be educated and empowered to realize the different approaches to all aspects and consequences of CDs.
Conclusion
The technique of excluding the endometrium in closing the uterine incision appears to reduce subsequent abnormal placentation and diminish the frequency and size of post-CD scar defect. The revival of the endometrium-free closure technique may allow significant change in the postoperative results. Currently, standardization of CD technique is being promoted on the basis of time- and cost-savings rather than clinical outcomes. Simultaneously, inroads are being made to better understand the risks and consequences of CD.
Emerging evidence suggests that a post-CD niche is the result of poor layer approximation as well as inclusion of the endometrium, which prevent healing of the uterine wall and often enables faulty implantation of the fertilized oocyte in the next pregnancy, potentially giving rise to placenta accreta spectrum. The prevalence and size of the defect can be minimized by techniques aimed at restoring the anatomy of the uterine wall and the physiology of the endometrium. Specialized training and education are necessary to stress the importance of anatomical assessment and decision making at the time of uterine closure. ●

While its etiology remains uncertain, cesarean scar niche (CSN) is well publicized, as are its pathological clinical manifestations. In a future pregnancy, they include cesarean scar pregnancy (CSP), which in turn can lead to placenta accreta spectrum, and possible uterine rupture/dehiscence of a residual thin myometrial layer. CSP refers to the implantation of an early pregnancy on the scar or in the niche at the site of a prior cesarean delivery (CD); it has an incidence of 1 per 1,000 pregnancies. An estimated 52% of CSPs occur after even just one CD.1 CSP has been linked to placenta accreta spectrum and has been shown to be its precursor.2 Both CSP and placenta accreta spectrum can be consequences of CD and share a common histology of villous or placental attachment/invasion into the cesarean scar.3 The incidence of placenta accreta spectrum has risen from about 1 in 4,000 live births in the 1970s to 1 in 2,500 in the 1980s; in 2016, the incidence of placenta accreta spectrum was reported as 1 per 272 live births.4
Placenta accreta spectrum denotes the attachment of the placenta into and through the myometrium,5 and it can result in severe complications, including hemorrhage, hysterectomy, and intensive care treatment. The increasing rate of placenta accreta spectrum parallels the increasing CD rate, which rose from 5.8% in 1970 to 31.9% in 2016.6 Multiple repeat CDs are increasing in frequency as well. At the beginning of the century, placenta accreta spectrum mainly occurred after manual removal of the placenta, uterine curettage, or endometritis. Recently, experts are in agreement that the main determinant of placenta accreta spectrum is the uterine scar and niche formation after a previous CD.5 Larger niches are associated with an increased incidence of uterine rupture or dehiscence in a subsequent pregnancy.7
In the nonpregnant state, such niches are associated with intermenstrual bleeding, pelvic pain, painful intercourse, painful menses, and subfertility, becoming increasingly more severe in women with greater numbers of CDs.8-10 Conception rate with assisted reproductive treatment is notably reduced.11
Understanding its etiology
Monteagudo and colleagues first described a “niche” in 100% of 44 women evaluated for postmenopausal bleeding who had a prior CD.12 CSN has been the subject of well over 3,000 publications over the past 30 years. While the topic generates much interest among researchers, it is garnering little traction among practicing obstetricians. Such “niches,” also referred to as isthmocele, cesarean scar defect, or a diverticulum, was first described in 196113 and later defined on ultrasonography as a hypoechoic triangular-shaped uterine defect outlined by saline instillation sonohysterogram (SIS), reflecting a discontinuation of the myometrium at the site of a previous CD.12 In 2019, a European task force further defined a CSN as an “indentation at the site in the cesarean section scar with a depth of at least 2 mm” and extended the classification to include branches as extensions toward the anterior uterine serosa.14 Using this criterion, sonographic postoperative evaluation after one CD revealed a CSN in 68.9% of women with one single-layer uterine closure and in 73.6% of women after a double-layer closure.15 Larger niche sizes with thinner residual myometrial thickness appeared more frequently when a single-layer closure technique was used, without closure of the peritoneum. Its prevalence varies from 56% to 84%.16,17
Etiology of CSN formation: Our hypotheses
The precise pathophysiology of CSN remains elusive. Speculations attributed niche formation to numerous factors: timing of surgery, cervical incision, incomplete closure of the uterine incision, adhesion formation between the CD scar and the abdominal wall, and inherent maternal conditions which may impair healing, such as smoking, obesity, diabetes, maternal age, and labor status.18-20 Retroflexion of the uterus is reportedly associated with increased incidence and size of the niche, with CSN 50% more likely to develop in women with a retroflexed versus an anteverted uterus.21 We demonstrated the origin of niche formation in real-time from the start to the completion of uterine closure by a video capture of a single-layer closure followed by an immediate SIS of the ex vivo hysterectomized uterus, and histopathologic proof of the presence of endometrial cells defining the “niche.”22 This case exposes the misalignment of the uterine wall, while including the endometrium in the closure (FIGURE 1). Similarly, pathologic studies of hysteroscopy-resected isthmocele ridges of symptomatic women with niche-related subfertility revealed the tissue edges lined by endocervical, endometrial, or isthmic mucosa either combined or isolated in the scar.23 The presence of endometrial/cervical tissue in the myometrial closure has been debated for over a century.24,25

Continue to: Uterine closure techniques...
Uterine closure techniques: Historical perspective
In 1882, Max Sanger introduced a vertical uterine closure of a classical cesarean operation in response to hysterectomy as the contemporaneous alternative to prevent infection, bleeding, and death.24 Dr. Sanger emphasized layer approximation, suturing, and the avoidance of decidua in the first layer (FIGURE 2). This became the teaching of the classical CD until the 1970s. In 1926, Munro Kerr addressed uterine rupture with labor after a classical CD by introducing the lower uterine segment transverse incision. He cautioned to maintain the decidua inside the uterine 2-layer closure of the cavity.25 These pioneers were joined by others to rally for endometrium exclusion while promoting layer approximation. These techniques became universally standard and were taught across teaching medical centers in the United States and abroad until about 50 years ago.

In the 1970s, newer developments brought significant changes to uterine closure techniques. Initiated by Joel-Cohen,26 blunt dissection of the abdominal incision was adapted by Michael Stark, creating what came to be known as the Misgav-Ladach cesarean technique.27 Stark emphasized blunt dissection and introduced single-layer closure. Thereby the exclusion of the endometrium, used for more than 70 years, was abandoned by the present-day single- or double-layer uterine closure in favor of cost and time savings. Systematic reviews and meta-analyses comparing the two contrasting techniques were inconclusive, noting that the niche prevalence and size were similar in both groups. These studies did not take into account the variety of individual techniques or the position of the endometrium in the final closures.28
Endometrium and uterine closure
Our recent study examining uterine scar defect in women after one primary CD by SIS concluded that a specific endometrium-free closure technique (EFCT) (FIGURE 3) is associated with fewer and less severe defects and a thicker residual myometrial thickness when compared with closures with unknown or endometrium inclusion.29 The study found non-specific closure techniques to be 6 times more likely to form a niche of 2-mm deep or greater than the EFCT.

Furthermore, we surveyed the diversity of uterine closures and the location of the endometrium among obstetricians in one institution.30 Presence of endometrium on the surface of the final uterine closure was reported by 20% of respondents (see Figure 1). When asked for their opinion on the impact of CD techniques on placenta accreta spectrum, without available evidence 80% of the survey respondents reported no relationship to techniques, and only 20% suggested an association. This particular study demonstrates that the surgical techniques just described are random, unfettered, and applied without consideration of clinical outcomes.
Our recent retrospective study that spanned 30 years and examined the EFCT—performed anywhere between 3 to 9 consecutive CDs—revealed no abnormal placentation in any subsequent pregnancies.31 This was one of the few clinical studies of the long-term consequences of a uterine closure technique. In this study, the endometrium was excluded during the uterine closure, allowing its free edges to abut and heal. This step avoids scarring the endometrial-myometrial (EM) interface and unintentional inclusion of endometrium in the closed uterine wall. In this context, Jauniaux and colleagues cited the destruction of the EM interface as the main factor for placenta-adherent disorders.32 Sholapurkar and others highlight the need to further examine intrinsic details of uterine closure beyond single- and double-layer techniques to better understand the etiology of cesarean scar formation.19 The search for the pathophysiology of CSN continues to present significant challenges imposed by the variety of currently practiced uterine closures.
Continue to: Focus on prevention...
Research: Focus on prevention
Our research aims to address the endometrium, a specific layer that was the topic of concern in nascent CD techniques, as a renewed and contemporary one. The presence of the endometrium in ectopic locations or its destruction from intrauterine surgeries or infections has been implicated in abnormal placentation.13,24 Our approach, in theory, is to limit the position of the endometrium to its innermost location and avoid its iatrogenic suturing and inclusion into the uterine wall closure. The rationale of sparing the endometrium in a layer-by-layer approximation is to allow for a closer restoration to normal anatomy and physiology than a random “en masse” uterine wall closure would permit. For this reason, the EM junction, the perimetrium, and the serosa must be identified and realigned for a more effective closure that incorporates the entire myometrial thickness. As evidence supports technical impact on the development of uterine scar defect in women after one CD, future studies are needed to evaluate uterine integrity by saline infusion sonohysterography in multiparous women with a prior random closure technique or a prior EFCT.
The potential long-term risks of blunt dissection for opening the uterus have not been studied. There are no physiologic lines in the uterine wall to facilitate a regular-bordered uterine stretch. The tissue stretch, which depends on the individual surgeon’s strength applied during the procedure and patient’s labor status, may result in an irregular tear and a difficult repair. The EFCT technique shows a more optimized risk-benefit ratio for an anatomical repair and is replicable. The safety of uterine layer re-approximation has been demonstrated and can be studied in large populations using strict uniform criteria.
Current and future challenges
Residency training
Most recently, teachers of resident trainees are mostly familiar with blunt dissection, techniques of which are passed on unchallenged from resident to resident. The endometrium and peritoneum are neither identified nor treated as separate layers, thus becoming obsolete as surgical and anatomical landmarks.
Standardization of CD techniques
Front-line obstetricians are persuaded to practice a standardized approach that relies on the benefits of cost related to operating room turnover as well as surgeons’ time savings without consideration of outcomes in subsequent pregnancies. Sholapurkar has warned that “wrong standardization” is far worse than no standardization, worse for the training of junior obstetricians, as it can inhibit critical reasoning about safe surgical techniques that can optimize outcomes of the condition of the lower uterine segment.33
Emergence of cost and time savings in clinical practice
A time-cost savings argument is relatively negligeable in an estimated 40-minute CD. By contrast, deliberate surgical technique and carrying out the appropriate steps for the particular condition at hand to achieve the best outcomes assume more weight.32 Furthermore, this short-term cost benefit is challenged by the comparatively larger costs associated with the diagnosis, the treatment of post-CD adverse consequences (outlined above), as well as the emotional impact on women and their families. Additionally, the emphasis on time savings creates a generation of surgeons fixated with total operative time without consideration of long-term risks and adverse maternal outcomes.
Physician autonomy has led to the unmonitored freedom of obstetricians to choose their own technique for a CD, with some employing the commonly practiced culture of fastest turnaround even in nonurgent circumstances.
Documentation and terminology
Current documenting systems are not detail-oriented enough to assist in a thorough correlation between surgical techniques and outcomes. The use of single- or double-layer closure terminology is insufficient and has proven to be flawed, without describing the handling of the endometrium in terms of its inclusion or exclusion in the closure.
Quality improvement feedback
Long-term post-CD complications are often not reported to the physician or institution involved in the prior CD. In our opinion, some sort of registry would be of value. Perhaps then subsequent CD outcomes could be traced back and reported to the prior institution and surgeon. Feedback is critical to understanding the correlation between techniques and outcomes and more specifically to gathering learning points and using data for quality improvement of future cases.
Patient education
While women continue to have complications following the presently used surgical techniques, they often have expectations not discussed with their obstetricians. Women should be educated and empowered to realize the different approaches to all aspects and consequences of CDs.
Conclusion
The technique of excluding the endometrium in closing the uterine incision appears to reduce subsequent abnormal placentation and diminish the frequency and size of post-CD scar defect. The revival of the endometrium-free closure technique may allow significant change in the postoperative results. Currently, standardization of CD technique is being promoted on the basis of time- and cost-savings rather than clinical outcomes. Simultaneously, inroads are being made to better understand the risks and consequences of CD.
Emerging evidence suggests that a post-CD niche is the result of poor layer approximation as well as inclusion of the endometrium, which prevent healing of the uterine wall and often enables faulty implantation of the fertilized oocyte in the next pregnancy, potentially giving rise to placenta accreta spectrum. The prevalence and size of the defect can be minimized by techniques aimed at restoring the anatomy of the uterine wall and the physiology of the endometrium. Specialized training and education are necessary to stress the importance of anatomical assessment and decision making at the time of uterine closure. ●
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373-1381.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346-353. doi:10.1002/ uog.13426.
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383-395. doi: 10.1002/uog.13282.
- Mogos MF, Salemi JL, Ashley M, et al. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J Matern Fetal Neonatal Med. 2016;29:1077-1082.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75-87.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1-8.
- Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525-532.
- Chen YY, Tsai CC, Kung FT, et al. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwanese J Obstet Gynecol. 2019;58:541-544.
- Stegwee SI, Beij A, de Leeuw RA, et al. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res. 2020;29:1013-1025.
- Vissers J, Hehenkamp W, Lambalk CB, et al. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484-1494.
- Vissers J, Sluckin TC, van Driel-Delprat CCR, et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous caesarean section: a retrospective cohort study. Hum Reprod. 2020;35:595-604.
- Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105-1115.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67-71.
- Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107-115.
- Stegwee SI, van der Voet LF, Ben AJ, et al. Effect of single- versus double-layer uterine closure during caesarean section on postmenstrual spotting (2Close): multicentre, double-blind, randomised controlled superiority trial. BJOG. 2021;128:866-878.
- Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43:372-382.
- van der Voet LF, Bij de Vaate AM, Veersema S, et al. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236-244.
- Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, et al. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695-2702.
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J Clin Med Res. 2018;10:166-173.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of cesarean section. Ultrasound Obstet Gynecol. 2021;57:466-470.
- Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014;203:W117-124.
- Antoine C, Pimentel RN, Timor-Tritsch IE, et al. Origin of a post-cesarean delivery niche: diagnosis, pathophysiologic characteristics, and video documentation. J Ultrasound Med. 2021;40:205-208.
- AbdullGaffar B, Almulla A. A histopathologic approach to uterine niche: what to expect and to report in hysteroscopy-resected isthmocele specimens. Int J Surg Pathol. 2021:10668969211039415. doi: 10.1177/10668969211039415.
- Nagy S, Papp Z. Global approach of the cesarean section rates. J Perinatal Med. 2020;49:1-4.
- Kerr JM. The technic of cesarean section, with special reference to the lower uterine segment incision. Am J Obstet Gynecol. 1926;12:729-734.
- Joel-Cohen S. Abdominal and vaginal hysterectomy: new techniques based on time and motion studies. Lippincott Williams & Wilkins; 1977.
- Holmgren G, Sjoholm L, Stark M. The Misgav Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78:615-621.
- Abalos E, Addo V, Brocklehurst P, et al. Caesarean section surgical techniques: 3-year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016;388:62-72.
- Antoine C, Meyer JA, Silverstein JS, et al. The impact of uterine incision closure techniques on post-cesarean delivery niche formation and size: sonohysterographic examination of nonpregnant women. J Ultrasound Med. 2021. doi: 10.1002/ jum.15859.
- Antoine C AJ, Yaghoubian Y, Harary J. Variations in uterine closure technique: an institutional survey of obstetricians and implications for patient counseling and prevention of adverse sequelae [Abstract]. 2021.
- Antoine C, Pimentel RN, Reece EA, et al. Endometrium-free uterine closure technique and abnormal placental implantation in subsequent pregnancies. J Matern-Fetal Neonatal Med. 2019:1-9.
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33:244-251.
- Sholapurkar S. Review of unsafe changes in the practice of cesarean section with analysis of flaws in the interpretation of statistics and the evidence. Surgical Case Reports. 2021;4:2-6.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107:1373-1381.
- Timor-Tritsch IE, Monteagudo A, Calì G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol. 2014;44:346-353. doi:10.1002/ uog.13426.
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383-395. doi: 10.1002/uog.13282.
- Mogos MF, Salemi JL, Ashley M, et al. Recent trends in placenta accreta in the United States and its impact on maternal-fetal morbidity and healthcare-associated costs, 1998-2011. J Matern Fetal Neonatal Med. 2016;29:1077-1082.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75-87.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2016. NCHS Data Brief. 2017(287):1-8.
- Vikhareva Osser O, Valentin L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525-532.
- Chen YY, Tsai CC, Kung FT, et al. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwanese J Obstet Gynecol. 2019;58:541-544.
- Stegwee SI, Beij A, de Leeuw RA, et al. Niche-related outcomes after caesarean section and quality of life: a focus group study and review of literature. Qual Life Res. 2020;29:1013-1025.
- Vissers J, Hehenkamp W, Lambalk CB, et al. Post-caesarean section niche-related impaired fertility: hypothetical mechanisms. Hum Reprod. 2020;35:1484-1494.
- Vissers J, Sluckin TC, van Driel-Delprat CCR, et al. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous caesarean section: a retrospective cohort study. Hum Reprod. 2020;35:595-604.
- Monteagudo A, Carreno C, Timor-Tritsch IE. Saline infusion sonohysterography in nonpregnant women with previous cesarean delivery: the “niche” in the scar. J Ultrasound Med. 2001;20:1105-1115.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67-71.
- Jordans IPM, de Leeuw RA, Stegwee SI, et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet Gynecol. 2019;53:107-115.
- Stegwee SI, van der Voet LF, Ben AJ, et al. Effect of single- versus double-layer uterine closure during caesarean section on postmenstrual spotting (2Close): multicentre, double-blind, randomised controlled superiority trial. BJOG. 2021;128:866-878.
- Bij de Vaate AJ, van der Voet LF, Naji O, et al. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following cesarean section: systematic review. Ultrasound Obstet Gynecol. 2014;43:372-382.
- van der Voet LF, Bij de Vaate AM, Veersema S, et al. Long-term complications of caesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236-244.
- Vervoort AJ, Uittenbogaard LB, Hehenkamp WJ, et al. Why do niches develop in caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum Reprod. 2015;30:2695-2702.
- Sholapurkar SL. Etiology of cesarean uterine scar defect (niche): detailed critical analysis of hypotheses and prevention strategies and peritoneal closure debate. J Clin Med Res. 2018;10:166-173.
- Kamel R, Eissa T, Sharaf M, et al. Position and integrity of uterine scar are determined by degree of cervical dilatation at time of cesarean section. Ultrasound Obstet Gynecol. 2021;57:466-470.
- Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014;203:W117-124.
- Antoine C, Pimentel RN, Timor-Tritsch IE, et al. Origin of a post-cesarean delivery niche: diagnosis, pathophysiologic characteristics, and video documentation. J Ultrasound Med. 2021;40:205-208.
- AbdullGaffar B, Almulla A. A histopathologic approach to uterine niche: what to expect and to report in hysteroscopy-resected isthmocele specimens. Int J Surg Pathol. 2021:10668969211039415. doi: 10.1177/10668969211039415.
- Nagy S, Papp Z. Global approach of the cesarean section rates. J Perinatal Med. 2020;49:1-4.
- Kerr JM. The technic of cesarean section, with special reference to the lower uterine segment incision. Am J Obstet Gynecol. 1926;12:729-734.
- Joel-Cohen S. Abdominal and vaginal hysterectomy: new techniques based on time and motion studies. Lippincott Williams & Wilkins; 1977.
- Holmgren G, Sjoholm L, Stark M. The Misgav Ladach method for cesarean section: method description. Acta Obstet Gynecol Scand. 1999;78:615-621.
- Abalos E, Addo V, Brocklehurst P, et al. Caesarean section surgical techniques: 3-year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016;388:62-72.
- Antoine C, Meyer JA, Silverstein JS, et al. The impact of uterine incision closure techniques on post-cesarean delivery niche formation and size: sonohysterographic examination of nonpregnant women. J Ultrasound Med. 2021. doi: 10.1002/ jum.15859.
- Antoine C AJ, Yaghoubian Y, Harary J. Variations in uterine closure technique: an institutional survey of obstetricians and implications for patient counseling and prevention of adverse sequelae [Abstract]. 2021.
- Antoine C, Pimentel RN, Reece EA, et al. Endometrium-free uterine closure technique and abnormal placental implantation in subsequent pregnancies. J Matern-Fetal Neonatal Med. 2019:1-9.
- Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33:244-251.
- Sholapurkar S. Review of unsafe changes in the practice of cesarean section with analysis of flaws in the interpretation of statistics and the evidence. Surgical Case Reports. 2021;4:2-6.
How to identify and manage cesarean-scar pregnancy
Few ObGyn clinicians have faced a patient with a cesarean-scar pregnancy (CSP). Those few were confronted with a management dilemma. Continue the gestation, which would expose the mother to an elevated risk of heavy bleeding? Or terminate the pregnancy? And if termination is the patient’s choice, what is the most effective method?
The literature contains more than 750 reports of CSP, ranging from a single sporadic case to a series of one to two dozen cases. It is impossible to make sense of the numerous treatments used in the past, which were “tested” on extremely small numbers of patients (sometimes as few as one). In this article, we formulate a management plan for the diagnosis and treatment of CSP based on an in-depth review of the published literature and our personal experience in treating more than four dozen patients with CSP.
We’re all familiar with the “epidemic” of cesarean deliveries in this country, including late consequences of cesarean such as placenta previa and morbidly adherent placenta. One of the long-term consequences of cesarean delivery—the first-trimester CSP—is less well known and documented.
Our in-depth review of 751 CSP cases found no less than 30 published therapeutic approaches.1 No consensus exists as to management guidelines. We have formulated this clinical guide, based on the literature and our experience managing CSP, for clinicians who encounter this dangerous form of pregnancy.2
DIAGNOSIS REQUIRES TRANSVAGINAL SONOGRAPHY
Transvaginal sonography (TVS) is thought to be the best and first-line diagnostic tool, with magnetic resonance imaging (MRI) reserved for cases in which there is a diagnostic problem.
In making a diagnosis, consider two main differential diagnoses:
- Cervical pregnancy—This type of gestation is more likely to occur in women with no history of cesarean delivery
- Spontaneous miscarriage in progress—In a number of cases, the miscarriage happened to be caught on imaging as it passed the area where the CSP usually resides. Because there is no live embryo or fetus in spontaneous miscarriage, a heartbeat cannot be documented.
Components of diagnosis by TVS
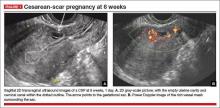
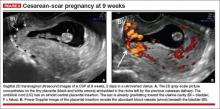
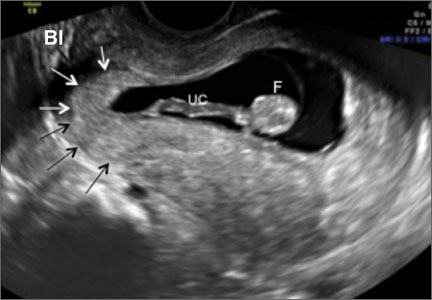
Accurate identification of CSP depends on the following sonographic criteria:
- empty uterine cavity and cervical canal (FIGURE 1A)
- close proximity of the gestational sac and the placenta to the anterior uterine surface within the scar or niche of the previous cesarean delivery (FIGURES 1B, 2A, and 2B)
- color flow signals between the posterior bladder wall and the gestation within the placenta (FIGURES 1B, 2B, and 3B)
- abundant blood flow around the gestational sac, at times morphing into an arteriovenous malformation with a high peak systolic velocity blood flow demonstrable on pulsed Doppler.
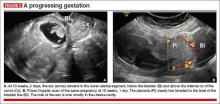
Our analysis of 751 cases of CSP found that almost a third—30%—were misdiagnosed, contributing to a large number of treatment complications. Most of these complications could have been avoided if diagnosis had been early and correct. The earlier the diagnosis, the better the outcome seemed to be. This was true even when treatment modalities with slightly higher complication rates were used in very early gestation.
Related articles:
• Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy? Andrew M. Kaunitz, MD (Examining the Evidence; February 2012)
• Can a single progesterone test distinguish viable and nonviable pregnancies accurately in women with pain or bleeding? Linda R. Chambliss, MD, MPH (Examining the Evidence; March 2013)
THOROUGH COUNSELING OF THE PATIENT IS PARAMOUNT
Once a diagnosis of CSP has been established, the patient should be counseled about her options. The presence of a live CSP requires immediate and decisive action to prevent further growth of the embryo or fetus. Literature from the past decade, particularly from the past several years, makes evidence-based counseling possible.
In general, treatment should be individualized, based on the patient’s age, number of previous cesarean deliveries, number of children, and the expertise of the clinicians managing her care. Options include:
- termination of the pregnancy
- continuation of the pregnancy with the possibility of delivering a live offspring, provided the patient understands that a morbidly adherent placenta may occur, often necessitating emergency hysterectomy.3,4
MANAGEMENT APPROACHES
Most treatment regimens and combinations thereof can be classified as one of the following:
- Surgical—requiring general anesthesia and either laparotomy with excision or hysterectomy, or laparoscopic or hysteroscopic excision followed by dilation and curettage (D&C).
- Minimally invasive—involving local injection of methotrexate or potassium chloride or systemic intervention, involving a major procedure such as uterine artery embolization in combination with a less complicated one: intramuscular injection of methotrexate in a single or a multidose regimen.
A variety of simultaneous as well as sequential combination treatments also were used. More recently, an ingenious adjunct to treatment is gaining attention: insertion and inflation of a Foley balloon catheter to prevent or tamponade bleeding.
A large number of treatments described in the literature—and their different combinations—have been reported as relatively small case series. Gynecologic surgeons generally perform D&C, laparoscopy, and hysteroscopy or laparotomy as the first-line approach. Obstetricians, radiologists, and in vitro fertilization specialists usually prefer systemic, parenteral administration of methotrexate or ultrasound-guided local methotrexate (or potassium chloride) as an injection into the gestational sac. On occasion, the help of an interventional radiologist was requested to embolize the area of the CSP through the uterine arteries.
POTENTIAL COMPLICATIONS
In our analysis of 751 cases of CSP, we used a rigorous definition of complication, which included an immediate or delayed need for a secondary treatment for blood loss exceeding 200 mL or requiring blood transfusion. If general anesthesia or major surgery was required, we classified that need as a complication.
Utilizing these criteria, we observed an overall complication rate of 44.1% (331 of 751 cases).1
Complications occurred most often when the following treatment modalities were used alone:
- single systemic dose of methotrexate
- D&C
- uterine artery embolization.
Of the 751 cases reviewed, 21.8% resulted in major surgery or interventional radiology procedures (primary or emergency). The total planned primary (nonemergency) interventions performed were 66 (8.7%), which included 3 hysterectomies, 14 laparotomies, and 49 uterine artery embolizations or ligations. There were 98 (13.0%) emergency interventions, which included 36 hysterectomies, 40 laparotomies, and 22 uterine artery embolizations or ligations.1
Related article: Eight tools for improving obstetric patient safety and unit performance. Henry M. Lerner, MD (Professional Liability; March 2014)
NINE TREATMENTS AND THEIR COMPLICATIONS
1. Systemic, single-dose methotrexate
The usual protocols were 1 mg/kg of body weight or 50 mg/m2 of body surface area. This treatment was associated with a complication rate of 64.6%, mostly because it required a second treatment when the fetal heart beat did not cease after several days.1
We speculate that the high failure rate with this treatment may be caused by its slow action and questionable ability to stop cardiac activity and placental expansion. The expected result can take days, and all the while the gestational sac, the embryo or fetus, and its vascularity are growing. Secondary treatment has to address a larger gestation with more abundant vascularization.
2. Systemic, multidose, sequential methotrexate
In this regimen, the amounts of methotrexate injected are similar to the dose for the single-dose regimen. Two to three intramuscular injections (1 mg/kg of body weight or 50 mg/mm2 of surface area) are given at an interval of 2 or 3 days over the course of a week. Be aware of the cumulative adverse effects of this drug on the liver and bone marrow—and the fact that even multidose treatment can fail.1
We found it impossible to assess the complication rate associated with this approach because it was often used in conjunction with another “first-line” treatment or after it. However, it is clear that methotrexate can be combined with other, mostly nonsurgical treatments.
3. Suction aspiration or D&C, alone or in combination
This option requires general anesthesia. The 305 cases involving this treatment had a mean complication rate of about 62% (range, 29%–86%).1 This approach caused the greatest number of bleeding complications, necessitating a third-line treatment that almost always was surgical.
At delivery or the time of spontaneous abortion, the multilayered myometrial grid in the uterine body is able to contain bleeding vessels after placental separation. However, in CSP, the exposed vessels in the cervical scar tissue bleed because there is no muscle grid to contract and contain the profuse bleeding.
If you choose D&C or aspiration, have blood products available and a Foley balloon catheter handy! In several reports, a Foley balloon catheter was used as backup after significant bleeding occurred following curettage.5,6
In one of the series involving 45 cases treated by methotrexate followed by suction curettage, mean blood loss was significant at 707 mL (standard deviation, 642 mL; range, 100–2,000 mL), and treatment failed in three patients despite insertion of a Foley balloon catheter.
4. Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. The complication rate was 47% among the 64 cases described in the literature.1 Uterine artery embolization appeared to work better when it was combined with other noninvasive treatments. It probably is not the best first-line treatment because the delay between treatment and effect allows the gestation to grow and vascularity to increase. And if uterine artery embolization fails, the clinician must contend with a larger gestation.
5. Excision by laparotomy, alone or in combination with hysteroscopy
General anesthesia is required. Of the 18 cases described in the literature, only five complications were reported—and only when used in an emergency situation.1
6. Laparoscopic excision
Again, general anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature involved complications, but only one of five cases (20%) experienced complications if hysteroscopy and laparoscopy were combined. Small numbers may not allow meaningful evaluation of the latter approach.1
7. Operative hysteroscopy, alone or in combination
General anesthesia is required. The overall complication rate for 108 cases was 13.8%. However, if hysteroscopy was combined with transabdominal ultrasound guidance (as it was in nine cases), no complications were noted. If hysteroscopy was combined with mifepristone, the complication rate was 17%.1 It appears that, when it is performed by an experienced clinician with ultrasound guidance, hysteroscopy may be a reasonable operative solution to CSP.
8. Intragestational-sac injection of methotrexate or potassium chloride, with ultrasound guidance
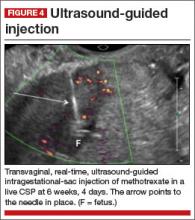
No anesthesia is required. This approach (FIGURE 4) had the fewest and least-involved complications. Of 83 cases, only 9 (10.8%) involved complications.
Cases performed with transabdominal sonography guidance had a slighter higher complication rate (15%) than those using TVS guidance.1
Because local injections are performed without general anesthesia and provide a final treatment by stopping heart activity, they appear to be the most effective intervention and may be especially useful when future fertility is desired.
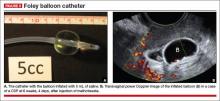
9. Use of a Foley balloon catheter
Inserting a Foley balloon catheter and inflating it at the site of the CSP is an ingenious, relatively new approach.1,2,5–7 The catheters come with balloons of different capacity (FIGURE 5A). They can be used alone (usually in gestations of 5–7 weeks) in the hope of stopping the evolution of the pregnancy by placing pressure on a small gestational sac. Even so, this approach is almost always used in a planned fashion in conjunction with another treatment or as backup if bleeding occurs.
Our impression of the value of the balloon catheters is positive. We suggest the French-12 size 10-mL silicone balloon catheter (prices range from $2 to $20), although we used a French-14 catheter with a 30-mL balloon successfully in a case of an 8-week CSP.
Insert the catheter using real-time transabdominal sonographic guidance when the patient has a comfortably full bladder. One also can switch to TVS guidance to allow for more precise placement and assess the pressure, avoiding overinflation of the balloon (FIGURE 5B).
There is no information in the literature about how long such a catheter should be kept in place. In our experience, 24 to 48 hours is the preferred duration, with the outer end of the catheter fastened to the patient’s thigh. We also provide antibiotic coverage and reevaluate the effect in 2 days or as needed. General anesthesia is not required.
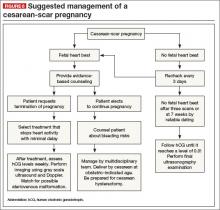
KEY TAKEAWAYS
Is there any single and effective treatment protocol? Probably not. Our management approach is presented as an algorithm (FIGURE 6).
We also offer the following guidelines:
- Do not confuse CSP with ectopic pregnancy. Such nomenclature has caused some referring physicians to simply use methotrexate protocols developed on “garden variety” tubal ectopic pregnancies, which not only failed but yielded disastrous results.
- Early diagnosis matters. TVS is the most effective and preferred diagnostic tool. Delay in the diagnosis delays treatment, increasing the possibility of complications.
- Cervical pregnancy is rare. In a patient who has had a cesarean delivery, a low chorionic sac is almost always a CSP.
- A key first step: Determine whether heart activity is present, and avoid methotrexate if no heart activity is observed.
- Counsel the patient. If heart activity is documented, provide evidence-based counseling about the patient’s options.
- Act fast. If continuation of the pregnancy is not desired, provide a reliable treatment that stops the embryonic or fetal heart beat without delay. Early treatment minimizes complications.
- Avoid single treatments unlikely to be effective, including D&C, suction curettage, single-dose intramuscular methotrexate, and uterine artery embolization applied alone.
- Keep a catheter at hand. Foley balloon tamponade to prevent or treat bleeding is a useful adjunct to have within easy reach.
- Consider combination treatments, as they may provide the best results.
- Fully inform the patient of the risks of pregnancy continuation. If a patient elects to continue the pregnancy, schedule an additional counseling session in which a more detailed overview of the anticipated clinical road is thoroughly explained.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to the Editor to: [email protected] Please include the city and state in which you practice.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. [published correction appears in Am J Obstet Gynecol. 2014;210(4):371–374.] Am J Obstet Gynecol. 2012;207(1):14–29.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–1841.
- Timor-Tritsch IE, Monteagudo A, Cali P, et al. Cesarean scar pregnancy and early placenta accreta share a common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: An analysis of 100 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–3189.
- Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–211.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
Few ObGyn clinicians have faced a patient with a cesarean-scar pregnancy (CSP). Those few were confronted with a management dilemma. Continue the gestation, which would expose the mother to an elevated risk of heavy bleeding? Or terminate the pregnancy? And if termination is the patient’s choice, what is the most effective method?
The literature contains more than 750 reports of CSP, ranging from a single sporadic case to a series of one to two dozen cases. It is impossible to make sense of the numerous treatments used in the past, which were “tested” on extremely small numbers of patients (sometimes as few as one). In this article, we formulate a management plan for the diagnosis and treatment of CSP based on an in-depth review of the published literature and our personal experience in treating more than four dozen patients with CSP.
We’re all familiar with the “epidemic” of cesarean deliveries in this country, including late consequences of cesarean such as placenta previa and morbidly adherent placenta. One of the long-term consequences of cesarean delivery—the first-trimester CSP—is less well known and documented.
Our in-depth review of 751 CSP cases found no less than 30 published therapeutic approaches.1 No consensus exists as to management guidelines. We have formulated this clinical guide, based on the literature and our experience managing CSP, for clinicians who encounter this dangerous form of pregnancy.2
DIAGNOSIS REQUIRES TRANSVAGINAL SONOGRAPHY
Transvaginal sonography (TVS) is thought to be the best and first-line diagnostic tool, with magnetic resonance imaging (MRI) reserved for cases in which there is a diagnostic problem.
In making a diagnosis, consider two main differential diagnoses:
- Cervical pregnancy—This type of gestation is more likely to occur in women with no history of cesarean delivery
- Spontaneous miscarriage in progress—In a number of cases, the miscarriage happened to be caught on imaging as it passed the area where the CSP usually resides. Because there is no live embryo or fetus in spontaneous miscarriage, a heartbeat cannot be documented.
Components of diagnosis by TVS
Accurate identification of CSP depends on the following sonographic criteria:
- empty uterine cavity and cervical canal (FIGURE 1A)
- close proximity of the gestational sac and the placenta to the anterior uterine surface within the scar or niche of the previous cesarean delivery (FIGURES 1B, 2A, and 2B)
- color flow signals between the posterior bladder wall and the gestation within the placenta (FIGURES 1B, 2B, and 3B)
- abundant blood flow around the gestational sac, at times morphing into an arteriovenous malformation with a high peak systolic velocity blood flow demonstrable on pulsed Doppler.
Our analysis of 751 cases of CSP found that almost a third—30%—were misdiagnosed, contributing to a large number of treatment complications. Most of these complications could have been avoided if diagnosis had been early and correct. The earlier the diagnosis, the better the outcome seemed to be. This was true even when treatment modalities with slightly higher complication rates were used in very early gestation.
Related articles:
• Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy? Andrew M. Kaunitz, MD (Examining the Evidence; February 2012)
• Can a single progesterone test distinguish viable and nonviable pregnancies accurately in women with pain or bleeding? Linda R. Chambliss, MD, MPH (Examining the Evidence; March 2013)
THOROUGH COUNSELING OF THE PATIENT IS PARAMOUNT
Once a diagnosis of CSP has been established, the patient should be counseled about her options. The presence of a live CSP requires immediate and decisive action to prevent further growth of the embryo or fetus. Literature from the past decade, particularly from the past several years, makes evidence-based counseling possible.
In general, treatment should be individualized, based on the patient’s age, number of previous cesarean deliveries, number of children, and the expertise of the clinicians managing her care. Options include:
- termination of the pregnancy
- continuation of the pregnancy with the possibility of delivering a live offspring, provided the patient understands that a morbidly adherent placenta may occur, often necessitating emergency hysterectomy.3,4
MANAGEMENT APPROACHES
Most treatment regimens and combinations thereof can be classified as one of the following:
- Surgical—requiring general anesthesia and either laparotomy with excision or hysterectomy, or laparoscopic or hysteroscopic excision followed by dilation and curettage (D&C).
- Minimally invasive—involving local injection of methotrexate or potassium chloride or systemic intervention, involving a major procedure such as uterine artery embolization in combination with a less complicated one: intramuscular injection of methotrexate in a single or a multidose regimen.
A variety of simultaneous as well as sequential combination treatments also were used. More recently, an ingenious adjunct to treatment is gaining attention: insertion and inflation of a Foley balloon catheter to prevent or tamponade bleeding.
A large number of treatments described in the literature—and their different combinations—have been reported as relatively small case series. Gynecologic surgeons generally perform D&C, laparoscopy, and hysteroscopy or laparotomy as the first-line approach. Obstetricians, radiologists, and in vitro fertilization specialists usually prefer systemic, parenteral administration of methotrexate or ultrasound-guided local methotrexate (or potassium chloride) as an injection into the gestational sac. On occasion, the help of an interventional radiologist was requested to embolize the area of the CSP through the uterine arteries.
POTENTIAL COMPLICATIONS
In our analysis of 751 cases of CSP, we used a rigorous definition of complication, which included an immediate or delayed need for a secondary treatment for blood loss exceeding 200 mL or requiring blood transfusion. If general anesthesia or major surgery was required, we classified that need as a complication.
Utilizing these criteria, we observed an overall complication rate of 44.1% (331 of 751 cases).1
Complications occurred most often when the following treatment modalities were used alone:
- single systemic dose of methotrexate
- D&C
- uterine artery embolization.
Of the 751 cases reviewed, 21.8% resulted in major surgery or interventional radiology procedures (primary or emergency). The total planned primary (nonemergency) interventions performed were 66 (8.7%), which included 3 hysterectomies, 14 laparotomies, and 49 uterine artery embolizations or ligations. There were 98 (13.0%) emergency interventions, which included 36 hysterectomies, 40 laparotomies, and 22 uterine artery embolizations or ligations.1
Related article: Eight tools for improving obstetric patient safety and unit performance. Henry M. Lerner, MD (Professional Liability; March 2014)
NINE TREATMENTS AND THEIR COMPLICATIONS
1. Systemic, single-dose methotrexate
The usual protocols were 1 mg/kg of body weight or 50 mg/m2 of body surface area. This treatment was associated with a complication rate of 64.6%, mostly because it required a second treatment when the fetal heart beat did not cease after several days.1
We speculate that the high failure rate with this treatment may be caused by its slow action and questionable ability to stop cardiac activity and placental expansion. The expected result can take days, and all the while the gestational sac, the embryo or fetus, and its vascularity are growing. Secondary treatment has to address a larger gestation with more abundant vascularization.
2. Systemic, multidose, sequential methotrexate
In this regimen, the amounts of methotrexate injected are similar to the dose for the single-dose regimen. Two to three intramuscular injections (1 mg/kg of body weight or 50 mg/mm2 of surface area) are given at an interval of 2 or 3 days over the course of a week. Be aware of the cumulative adverse effects of this drug on the liver and bone marrow—and the fact that even multidose treatment can fail.1
We found it impossible to assess the complication rate associated with this approach because it was often used in conjunction with another “first-line” treatment or after it. However, it is clear that methotrexate can be combined with other, mostly nonsurgical treatments.
3. Suction aspiration or D&C, alone or in combination
This option requires general anesthesia. The 305 cases involving this treatment had a mean complication rate of about 62% (range, 29%–86%).1 This approach caused the greatest number of bleeding complications, necessitating a third-line treatment that almost always was surgical.
At delivery or the time of spontaneous abortion, the multilayered myometrial grid in the uterine body is able to contain bleeding vessels after placental separation. However, in CSP, the exposed vessels in the cervical scar tissue bleed because there is no muscle grid to contract and contain the profuse bleeding.
If you choose D&C or aspiration, have blood products available and a Foley balloon catheter handy! In several reports, a Foley balloon catheter was used as backup after significant bleeding occurred following curettage.5,6
In one of the series involving 45 cases treated by methotrexate followed by suction curettage, mean blood loss was significant at 707 mL (standard deviation, 642 mL; range, 100–2,000 mL), and treatment failed in three patients despite insertion of a Foley balloon catheter.
4. Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. The complication rate was 47% among the 64 cases described in the literature.1 Uterine artery embolization appeared to work better when it was combined with other noninvasive treatments. It probably is not the best first-line treatment because the delay between treatment and effect allows the gestation to grow and vascularity to increase. And if uterine artery embolization fails, the clinician must contend with a larger gestation.
5. Excision by laparotomy, alone or in combination with hysteroscopy
General anesthesia is required. Of the 18 cases described in the literature, only five complications were reported—and only when used in an emergency situation.1
6. Laparoscopic excision
Again, general anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature involved complications, but only one of five cases (20%) experienced complications if hysteroscopy and laparoscopy were combined. Small numbers may not allow meaningful evaluation of the latter approach.1
7. Operative hysteroscopy, alone or in combination
General anesthesia is required. The overall complication rate for 108 cases was 13.8%. However, if hysteroscopy was combined with transabdominal ultrasound guidance (as it was in nine cases), no complications were noted. If hysteroscopy was combined with mifepristone, the complication rate was 17%.1 It appears that, when it is performed by an experienced clinician with ultrasound guidance, hysteroscopy may be a reasonable operative solution to CSP.
8. Intragestational-sac injection of methotrexate or potassium chloride, with ultrasound guidance
No anesthesia is required. This approach (FIGURE 4) had the fewest and least-involved complications. Of 83 cases, only 9 (10.8%) involved complications.
Cases performed with transabdominal sonography guidance had a slighter higher complication rate (15%) than those using TVS guidance.1
Because local injections are performed without general anesthesia and provide a final treatment by stopping heart activity, they appear to be the most effective intervention and may be especially useful when future fertility is desired.
9. Use of a Foley balloon catheter
Inserting a Foley balloon catheter and inflating it at the site of the CSP is an ingenious, relatively new approach.1,2,5–7 The catheters come with balloons of different capacity (FIGURE 5A). They can be used alone (usually in gestations of 5–7 weeks) in the hope of stopping the evolution of the pregnancy by placing pressure on a small gestational sac. Even so, this approach is almost always used in a planned fashion in conjunction with another treatment or as backup if bleeding occurs.
Our impression of the value of the balloon catheters is positive. We suggest the French-12 size 10-mL silicone balloon catheter (prices range from $2 to $20), although we used a French-14 catheter with a 30-mL balloon successfully in a case of an 8-week CSP.
Insert the catheter using real-time transabdominal sonographic guidance when the patient has a comfortably full bladder. One also can switch to TVS guidance to allow for more precise placement and assess the pressure, avoiding overinflation of the balloon (FIGURE 5B).
There is no information in the literature about how long such a catheter should be kept in place. In our experience, 24 to 48 hours is the preferred duration, with the outer end of the catheter fastened to the patient’s thigh. We also provide antibiotic coverage and reevaluate the effect in 2 days or as needed. General anesthesia is not required.
KEY TAKEAWAYS
Is there any single and effective treatment protocol? Probably not. Our management approach is presented as an algorithm (FIGURE 6).
We also offer the following guidelines:
- Do not confuse CSP with ectopic pregnancy. Such nomenclature has caused some referring physicians to simply use methotrexate protocols developed on “garden variety” tubal ectopic pregnancies, which not only failed but yielded disastrous results.
- Early diagnosis matters. TVS is the most effective and preferred diagnostic tool. Delay in the diagnosis delays treatment, increasing the possibility of complications.
- Cervical pregnancy is rare. In a patient who has had a cesarean delivery, a low chorionic sac is almost always a CSP.
- A key first step: Determine whether heart activity is present, and avoid methotrexate if no heart activity is observed.
- Counsel the patient. If heart activity is documented, provide evidence-based counseling about the patient’s options.
- Act fast. If continuation of the pregnancy is not desired, provide a reliable treatment that stops the embryonic or fetal heart beat without delay. Early treatment minimizes complications.
- Avoid single treatments unlikely to be effective, including D&C, suction curettage, single-dose intramuscular methotrexate, and uterine artery embolization applied alone.
- Keep a catheter at hand. Foley balloon tamponade to prevent or treat bleeding is a useful adjunct to have within easy reach.
- Consider combination treatments, as they may provide the best results.
- Fully inform the patient of the risks of pregnancy continuation. If a patient elects to continue the pregnancy, schedule an additional counseling session in which a more detailed overview of the anticipated clinical road is thoroughly explained.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to the Editor to: [email protected] Please include the city and state in which you practice.
Few ObGyn clinicians have faced a patient with a cesarean-scar pregnancy (CSP). Those few were confronted with a management dilemma. Continue the gestation, which would expose the mother to an elevated risk of heavy bleeding? Or terminate the pregnancy? And if termination is the patient’s choice, what is the most effective method?
The literature contains more than 750 reports of CSP, ranging from a single sporadic case to a series of one to two dozen cases. It is impossible to make sense of the numerous treatments used in the past, which were “tested” on extremely small numbers of patients (sometimes as few as one). In this article, we formulate a management plan for the diagnosis and treatment of CSP based on an in-depth review of the published literature and our personal experience in treating more than four dozen patients with CSP.
We’re all familiar with the “epidemic” of cesarean deliveries in this country, including late consequences of cesarean such as placenta previa and morbidly adherent placenta. One of the long-term consequences of cesarean delivery—the first-trimester CSP—is less well known and documented.
Our in-depth review of 751 CSP cases found no less than 30 published therapeutic approaches.1 No consensus exists as to management guidelines. We have formulated this clinical guide, based on the literature and our experience managing CSP, for clinicians who encounter this dangerous form of pregnancy.2
DIAGNOSIS REQUIRES TRANSVAGINAL SONOGRAPHY
Transvaginal sonography (TVS) is thought to be the best and first-line diagnostic tool, with magnetic resonance imaging (MRI) reserved for cases in which there is a diagnostic problem.
In making a diagnosis, consider two main differential diagnoses:
- Cervical pregnancy—This type of gestation is more likely to occur in women with no history of cesarean delivery
- Spontaneous miscarriage in progress—In a number of cases, the miscarriage happened to be caught on imaging as it passed the area where the CSP usually resides. Because there is no live embryo or fetus in spontaneous miscarriage, a heartbeat cannot be documented.
Components of diagnosis by TVS
Accurate identification of CSP depends on the following sonographic criteria:
- empty uterine cavity and cervical canal (FIGURE 1A)
- close proximity of the gestational sac and the placenta to the anterior uterine surface within the scar or niche of the previous cesarean delivery (FIGURES 1B, 2A, and 2B)
- color flow signals between the posterior bladder wall and the gestation within the placenta (FIGURES 1B, 2B, and 3B)
- abundant blood flow around the gestational sac, at times morphing into an arteriovenous malformation with a high peak systolic velocity blood flow demonstrable on pulsed Doppler.
Our analysis of 751 cases of CSP found that almost a third—30%—were misdiagnosed, contributing to a large number of treatment complications. Most of these complications could have been avoided if diagnosis had been early and correct. The earlier the diagnosis, the better the outcome seemed to be. This was true even when treatment modalities with slightly higher complication rates were used in very early gestation.
Related articles:
• Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy? Andrew M. Kaunitz, MD (Examining the Evidence; February 2012)
• Can a single progesterone test distinguish viable and nonviable pregnancies accurately in women with pain or bleeding? Linda R. Chambliss, MD, MPH (Examining the Evidence; March 2013)
THOROUGH COUNSELING OF THE PATIENT IS PARAMOUNT
Once a diagnosis of CSP has been established, the patient should be counseled about her options. The presence of a live CSP requires immediate and decisive action to prevent further growth of the embryo or fetus. Literature from the past decade, particularly from the past several years, makes evidence-based counseling possible.
In general, treatment should be individualized, based on the patient’s age, number of previous cesarean deliveries, number of children, and the expertise of the clinicians managing her care. Options include:
- termination of the pregnancy
- continuation of the pregnancy with the possibility of delivering a live offspring, provided the patient understands that a morbidly adherent placenta may occur, often necessitating emergency hysterectomy.3,4
MANAGEMENT APPROACHES
Most treatment regimens and combinations thereof can be classified as one of the following:
- Surgical—requiring general anesthesia and either laparotomy with excision or hysterectomy, or laparoscopic or hysteroscopic excision followed by dilation and curettage (D&C).
- Minimally invasive—involving local injection of methotrexate or potassium chloride or systemic intervention, involving a major procedure such as uterine artery embolization in combination with a less complicated one: intramuscular injection of methotrexate in a single or a multidose regimen.
A variety of simultaneous as well as sequential combination treatments also were used. More recently, an ingenious adjunct to treatment is gaining attention: insertion and inflation of a Foley balloon catheter to prevent or tamponade bleeding.
A large number of treatments described in the literature—and their different combinations—have been reported as relatively small case series. Gynecologic surgeons generally perform D&C, laparoscopy, and hysteroscopy or laparotomy as the first-line approach. Obstetricians, radiologists, and in vitro fertilization specialists usually prefer systemic, parenteral administration of methotrexate or ultrasound-guided local methotrexate (or potassium chloride) as an injection into the gestational sac. On occasion, the help of an interventional radiologist was requested to embolize the area of the CSP through the uterine arteries.
POTENTIAL COMPLICATIONS
In our analysis of 751 cases of CSP, we used a rigorous definition of complication, which included an immediate or delayed need for a secondary treatment for blood loss exceeding 200 mL or requiring blood transfusion. If general anesthesia or major surgery was required, we classified that need as a complication.
Utilizing these criteria, we observed an overall complication rate of 44.1% (331 of 751 cases).1
Complications occurred most often when the following treatment modalities were used alone:
- single systemic dose of methotrexate
- D&C
- uterine artery embolization.
Of the 751 cases reviewed, 21.8% resulted in major surgery or interventional radiology procedures (primary or emergency). The total planned primary (nonemergency) interventions performed were 66 (8.7%), which included 3 hysterectomies, 14 laparotomies, and 49 uterine artery embolizations or ligations. There were 98 (13.0%) emergency interventions, which included 36 hysterectomies, 40 laparotomies, and 22 uterine artery embolizations or ligations.1
Related article: Eight tools for improving obstetric patient safety and unit performance. Henry M. Lerner, MD (Professional Liability; March 2014)
NINE TREATMENTS AND THEIR COMPLICATIONS
1. Systemic, single-dose methotrexate
The usual protocols were 1 mg/kg of body weight or 50 mg/m2 of body surface area. This treatment was associated with a complication rate of 64.6%, mostly because it required a second treatment when the fetal heart beat did not cease after several days.1
We speculate that the high failure rate with this treatment may be caused by its slow action and questionable ability to stop cardiac activity and placental expansion. The expected result can take days, and all the while the gestational sac, the embryo or fetus, and its vascularity are growing. Secondary treatment has to address a larger gestation with more abundant vascularization.
2. Systemic, multidose, sequential methotrexate
In this regimen, the amounts of methotrexate injected are similar to the dose for the single-dose regimen. Two to three intramuscular injections (1 mg/kg of body weight or 50 mg/mm2 of surface area) are given at an interval of 2 or 3 days over the course of a week. Be aware of the cumulative adverse effects of this drug on the liver and bone marrow—and the fact that even multidose treatment can fail.1
We found it impossible to assess the complication rate associated with this approach because it was often used in conjunction with another “first-line” treatment or after it. However, it is clear that methotrexate can be combined with other, mostly nonsurgical treatments.
3. Suction aspiration or D&C, alone or in combination
This option requires general anesthesia. The 305 cases involving this treatment had a mean complication rate of about 62% (range, 29%–86%).1 This approach caused the greatest number of bleeding complications, necessitating a third-line treatment that almost always was surgical.
At delivery or the time of spontaneous abortion, the multilayered myometrial grid in the uterine body is able to contain bleeding vessels after placental separation. However, in CSP, the exposed vessels in the cervical scar tissue bleed because there is no muscle grid to contract and contain the profuse bleeding.
If you choose D&C or aspiration, have blood products available and a Foley balloon catheter handy! In several reports, a Foley balloon catheter was used as backup after significant bleeding occurred following curettage.5,6
In one of the series involving 45 cases treated by methotrexate followed by suction curettage, mean blood loss was significant at 707 mL (standard deviation, 642 mL; range, 100–2,000 mL), and treatment failed in three patients despite insertion of a Foley balloon catheter.
4. Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. The complication rate was 47% among the 64 cases described in the literature.1 Uterine artery embolization appeared to work better when it was combined with other noninvasive treatments. It probably is not the best first-line treatment because the delay between treatment and effect allows the gestation to grow and vascularity to increase. And if uterine artery embolization fails, the clinician must contend with a larger gestation.
5. Excision by laparotomy, alone or in combination with hysteroscopy
General anesthesia is required. Of the 18 cases described in the literature, only five complications were reported—and only when used in an emergency situation.1
6. Laparoscopic excision
Again, general anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature involved complications, but only one of five cases (20%) experienced complications if hysteroscopy and laparoscopy were combined. Small numbers may not allow meaningful evaluation of the latter approach.1
7. Operative hysteroscopy, alone or in combination
General anesthesia is required. The overall complication rate for 108 cases was 13.8%. However, if hysteroscopy was combined with transabdominal ultrasound guidance (as it was in nine cases), no complications were noted. If hysteroscopy was combined with mifepristone, the complication rate was 17%.1 It appears that, when it is performed by an experienced clinician with ultrasound guidance, hysteroscopy may be a reasonable operative solution to CSP.
8. Intragestational-sac injection of methotrexate or potassium chloride, with ultrasound guidance
No anesthesia is required. This approach (FIGURE 4) had the fewest and least-involved complications. Of 83 cases, only 9 (10.8%) involved complications.
Cases performed with transabdominal sonography guidance had a slighter higher complication rate (15%) than those using TVS guidance.1
Because local injections are performed without general anesthesia and provide a final treatment by stopping heart activity, they appear to be the most effective intervention and may be especially useful when future fertility is desired.
9. Use of a Foley balloon catheter
Inserting a Foley balloon catheter and inflating it at the site of the CSP is an ingenious, relatively new approach.1,2,5–7 The catheters come with balloons of different capacity (FIGURE 5A). They can be used alone (usually in gestations of 5–7 weeks) in the hope of stopping the evolution of the pregnancy by placing pressure on a small gestational sac. Even so, this approach is almost always used in a planned fashion in conjunction with another treatment or as backup if bleeding occurs.
Our impression of the value of the balloon catheters is positive. We suggest the French-12 size 10-mL silicone balloon catheter (prices range from $2 to $20), although we used a French-14 catheter with a 30-mL balloon successfully in a case of an 8-week CSP.
Insert the catheter using real-time transabdominal sonographic guidance when the patient has a comfortably full bladder. One also can switch to TVS guidance to allow for more precise placement and assess the pressure, avoiding overinflation of the balloon (FIGURE 5B).
There is no information in the literature about how long such a catheter should be kept in place. In our experience, 24 to 48 hours is the preferred duration, with the outer end of the catheter fastened to the patient’s thigh. We also provide antibiotic coverage and reevaluate the effect in 2 days or as needed. General anesthesia is not required.
KEY TAKEAWAYS
Is there any single and effective treatment protocol? Probably not. Our management approach is presented as an algorithm (FIGURE 6).
We also offer the following guidelines:
- Do not confuse CSP with ectopic pregnancy. Such nomenclature has caused some referring physicians to simply use methotrexate protocols developed on “garden variety” tubal ectopic pregnancies, which not only failed but yielded disastrous results.
- Early diagnosis matters. TVS is the most effective and preferred diagnostic tool. Delay in the diagnosis delays treatment, increasing the possibility of complications.
- Cervical pregnancy is rare. In a patient who has had a cesarean delivery, a low chorionic sac is almost always a CSP.
- A key first step: Determine whether heart activity is present, and avoid methotrexate if no heart activity is observed.
- Counsel the patient. If heart activity is documented, provide evidence-based counseling about the patient’s options.
- Act fast. If continuation of the pregnancy is not desired, provide a reliable treatment that stops the embryonic or fetal heart beat without delay. Early treatment minimizes complications.
- Avoid single treatments unlikely to be effective, including D&C, suction curettage, single-dose intramuscular methotrexate, and uterine artery embolization applied alone.
- Keep a catheter at hand. Foley balloon tamponade to prevent or treat bleeding is a useful adjunct to have within easy reach.
- Consider combination treatments, as they may provide the best results.
- Fully inform the patient of the risks of pregnancy continuation. If a patient elects to continue the pregnancy, schedule an additional counseling session in which a more detailed overview of the anticipated clinical road is thoroughly explained.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to the Editor to: [email protected] Please include the city and state in which you practice.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. [published correction appears in Am J Obstet Gynecol. 2014;210(4):371–374.] Am J Obstet Gynecol. 2012;207(1):14–29.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–1841.
- Timor-Tritsch IE, Monteagudo A, Cali P, et al. Cesarean scar pregnancy and early placenta accreta share a common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: An analysis of 100 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–3189.
- Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–211.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. [published correction appears in Am J Obstet Gynecol. 2014;210(4):371–374.] Am J Obstet Gynecol. 2012;207(1):14–29.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–1841.
- Timor-Tritsch IE, Monteagudo A, Cali P, et al. Cesarean scar pregnancy and early placenta accreta share a common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: An analysis of 100 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–3189.
- Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–211.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
Evolving applications of first-trimester ultrasound
The authors report no financial relationships relevant to this article.
In recent years, prenatal screening and testing have begun to shift from the second trimester to the first. Ultrasonographic evaluation—a large component of fetal testing—is also applied earlier in gestation to provide information to clinicians and patients about the integrity of the pregnancy. The shifting of the classic, “gold standard” anatomy scan to the first trimester was made possible by high-frequency transvaginal transducers and by greater understanding of the early signs of fetal pathology.
The authors report no financial relationships relevant to this article.
In recent years, prenatal screening and testing have begun to shift from the second trimester to the first. Ultrasonographic evaluation—a large component of fetal testing—is also applied earlier in gestation to provide information to clinicians and patients about the integrity of the pregnancy. The shifting of the classic, “gold standard” anatomy scan to the first trimester was made possible by high-frequency transvaginal transducers and by greater understanding of the early signs of fetal pathology.
The authors report no financial relationships relevant to this article.
In recent years, prenatal screening and testing have begun to shift from the second trimester to the first. Ultrasonographic evaluation—a large component of fetal testing—is also applied earlier in gestation to provide information to clinicians and patients about the integrity of the pregnancy. The shifting of the classic, “gold standard” anatomy scan to the first trimester was made possible by high-frequency transvaginal transducers and by greater understanding of the early signs of fetal pathology.
Skilled US imaging of the adnexae: The fallopian tubes
Part 1 A Starting Point (September 2010)
Part 2 The non-neoplastic ovarian mass (October 2010)
Part 3 Ovarian neoplasms (November 2010)
An imaging study of the adnexae would not be complete without thorough assessment of the fallopian tubes. Among the pathologies that may be identified or confirmed by ultrasonography are:
- ectopic pregnancy
- tubal inflammatory disease, or salpingitis
- chronic tubal disease, or hydrosalpinx
- tubo-ovarian complex
- tubo-ovarian abscess
- tubal and ovarian torsion
- cancer.
In this final installment of our four-part series on ultrasonographic (US) imaging of the adnexae, we take these entities as our focus.
Suspect ectopic pregnancy even if the hCG level is not yet available
A detailed discussion of ectopic pregnancy far exceeds the framework of this article. Suffice it to say that ectopic pregnancy should always be considered in a woman of reproductive age, especially one who complains of abdominal or pelvic pain, vaginal bleeding, or both. However, these signs and symptoms are present in only about 25% of women who have this condition. When these signs and symptoms are present, it is wise to be suspicious even if the results of human chorionic gonadotropin (hCG) measurement are not yet available.
A complete history is important in the diagnosis of ectopic pregnancy. Risk factors include a history of ectopic pregnancy, pelvic inflammatory disease (PID), or tubal surgery, or use of an intrauterine device.
Unequivocal US diagnosis of ectopic pregnancy is possible in only about 20% of cases, and depends on identification of an extrauterine pregnancy, which may not be visible in the early days of gestation. However, some grayscale ultrasonographic findings that may suggest ectopic pregnancy include:
- an empty uterus in a woman who has an hCG level above 1,000 to 1,500 mIu/mL (the discriminatory level)
- a thick, hyperechoic endometrial echo (decidualization)
- an adnexal mass other than a simple cyst
- echogenic fluid in the cul-de-sac (FIGURE 1A–1D).
Power Doppler can help the sonographer localize the ectopic pregnancy in the tubes by demonstrating the circular vascularization of the more or less typical “tubal ring” (FIGURE 1E–1G).
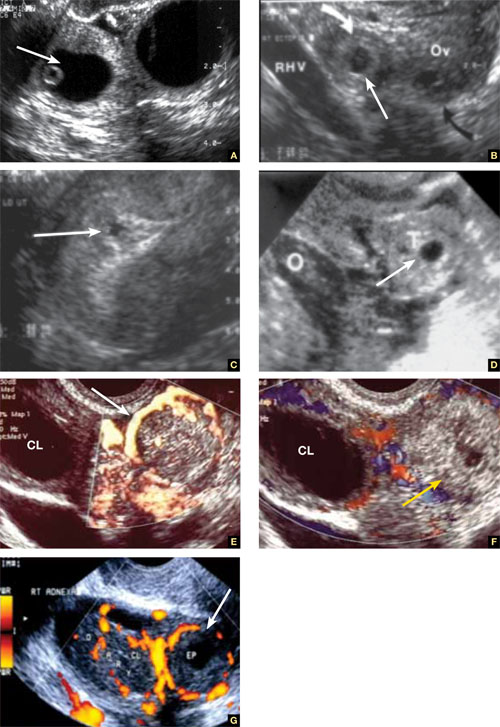
FIGURE 1 Ectopic pregnancy
A–D. Various cases of tubal ectopic gestation (arrows point to each gestation). E–G. Power Doppler localizes the ectopic pregnancy (arrows), side by side with the corpus luteum (CL).
A patient’s history may yield clues to tubal inflammatory disease
The diagnosis of acute salpingitis begins with a thorough patient history. Look for any report of PID, unexplained fever, foul vaginal discharge, sexually transmitted infection, or recent intrauterine procedures such as hysteroscopy, IUD insertion, endometrial biopsy, or saline infusion sonohysterography.
US diagnosis is based on the findings of a slightly dilated fallopian tube with low-level echogenic fluid content, thick tubal walls, and tenderness to the touch of the transvaginal probe.1
In cross section, the tube forms the “cogwheel sign” (FIGURE 2C). Power Doppler shows the subserosal blood vessels characteristic of this entity (FIGURE 2D).
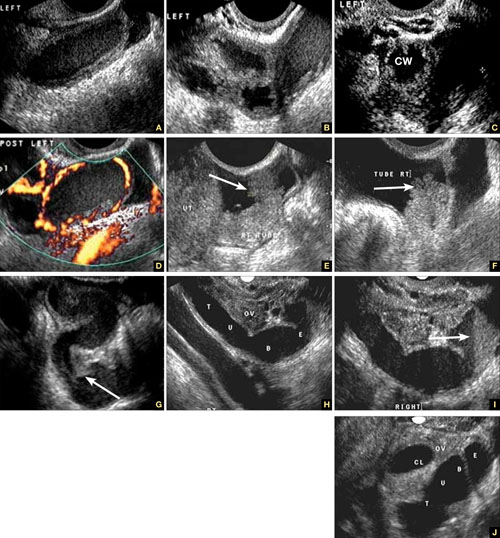
FIGURE 2 Tubal disease
A–C. Grayscale images showing thick walls, low-level echoic fluid (pus?) and the “cogwheel sign” (CW). D. Subserosal vascularization typical of an inflammatory response in hollow abdominal viscera. E,F. Edematous fimbrial end (arrow) of the inflamed tubes, floating in a small amount of free pelvic fluid. G. Low-level echoic, fluid-filled, thick-walled tubes with incomplete septae (arrow) are the hallmarks of hydrosalpinx. H–J. Bilateral hydrosalpinx. Note the thin walls and anechoic fluid-filled forms (sausage-shaped) (CL = corpus luteum; OV = ovary; UT = uterus).
Look for fluid dilating the tube in chronic tubal disease
Hydrosalpinx is characterized on US by thin tubal walls with a relatively anechoic but large amount of fluid dilating the tube (FIGURE 2G–2J). The interior wall is studded with shallow, echogenic, mural nodules (without blood vessels) that assume the appearance of a tube or sausage. The small, shallow internal papillae give the cross section of the tube the appearance of beads on a string.
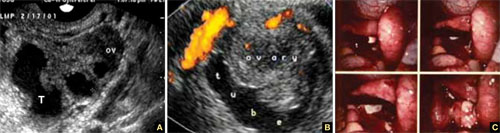
FIGURE 3 Tubo-ovarian complex
A. The tube (T) and ovary (OV) form an infectious conglomerate. B. Power Doppler appearance. C. Laparoscopic view.
Tubo-ovarian complex
When this complex arises, the anatomy and shape of the tube and the involved ovary are somewhat distorted but still largely discernible (FIGURE 3A AND 3B).
Tubo-ovarian abscess is a more advanced stage of a fast-progressing or neglected pelvic inflammatory process. In it, the tube and the ovary can barely be distinguished, and US signs of abscess appear, among them low-level echoic fluid and linear echogenicity (FIGURE 4).
FIGURE 4 Tubo-ovarian abscess
The tube and ovary are indistinguishable. The fluid is of low-level echogenicity (pus), and the walls are thick.
In ovarian torsion, the follicles press outward
Although torsion has distinct sonographic signs, it remains a clinical diagnosis that US findings may or may not support. Correct diagnosis often is the purview of expert sonographers and sonologists.
When ovarian torsion is present, the ovaries are enlarged and hyperechoic, their follicles pushed toward the surface (FIGURE 5A–5C). The ovaries are also tender to the touch and typically demonstrate no blood flow by Doppler interrogation. On occasion, when arterial flow is still present (venous flow is usually the first characteristic to vanish), a twisted arterial pattern may result, similar to the coil of a telephone cord. Some pelvic fluid may also appear.
Tubal torsion is harder to diagnose. US recognition depends on the finding of a normal ovary with intact blood flow beside a fluid-filled, thin-walled, tender, cystic structure with some of the previously mentioned sonomarkers of tubal occlusion such as the bead-on-a-string or cogwheel sign (FIGURE 5D–5G).
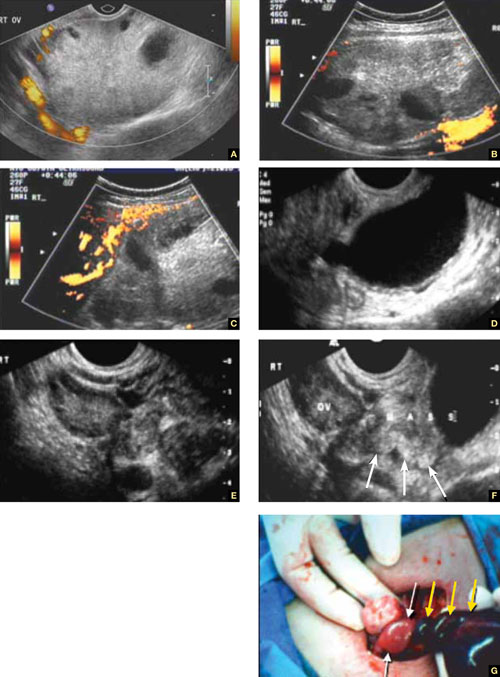
FIGURE 5 Torsion
A–C. Ovarian torsion. Hyperechoic, large ovary with follicles pushed toward the surface. Power Doppler reveals no blood flow in the ovary. D–F. Tubal torsion. Cystic dilatation with a small beak and a normal ovary. G. Intraoperative view of the tube (twisted three times; yellow arrows) and the normal ovary (white arrows).
Fluid in the cul-de-sac
In many cases, fluid may be present or trapped in the lesser pelvis, surrounded or blocked by the pelvic organs. If this fluid is the result or sequela of PID, thin, thread-like adhesive strands will be visible between the organs on US, betraying its pathogenesis (FIGURE 6). The “walls” of such loculated fluid are the pelvic wall itself and the surrounding organs.
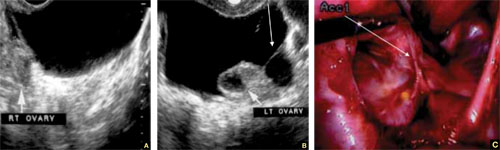
FIGURE 6 Fluid in the cul-de-sac
Sequelae of acute PID. A. Free pelvic fluid, also known as pelvic, peritoneal, loculated fluid. B. A normal ovary and an adhesive strand (arrow). C. Laparoscopic image of the adhesion (arrow).
Cancer of the tubes is unlikely, but it’s best to keep it in mind
Primary cancer of the fallopian tubes accounts for only 1% to 2% of all gynecologic cancers.2 Only 300 to 400 women are given this diagnosis each year in the United States— most of them postmenopausal.
Despite its rarity, fallopian-tube cancer is a major concern when a tubal mass is identified by palpation or imaging. In most cases, however, no palpable mass is found at the time of first examination, and tubal malignancy is diagnosed perioperatively or postoperatively.
US characteristics of tubal cancer are similar to those of ovarian cancer: a bizarre appearance, with extremely vascular tissue. At times, US attributes of tubal pathology, such as incomplete septae and tube-like fluid-filled structures, are apparent (FIGURE 7).
Consider cancer of the fallopian tube whenever an unexplained solid mass is palpated or imaged in the area of the tubes in conjunction with apparently normal ovaries.
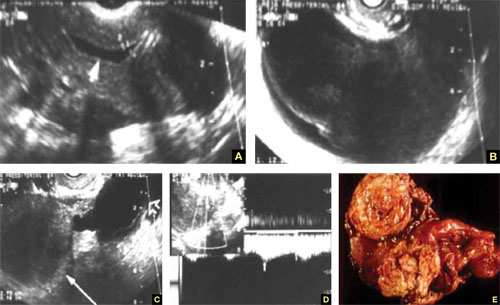
FIGURE 7 Fallopian tube cancer
A. Fluid-filled uterine cavity. B. Large cystic dilatation of the tube. C. A thickened tubal wall (arrow). D. Doppler interrogation reveals high diastolic flow (arrows). E. Macroscopic gross appearance.
The long view
As technology has advanced, so has ultrasonography. High-resolution transducers, color and power Doppler, and three-dimensional imaging make it possible for an experienced practitioner to identify and confirm the diagnosis of many adnexal masses and pathologies, from the corpus luteum to fallopian tube torsion. As the field continues to evolve, we expect that this modality will facilitate the diagnosis of adnexal abnormalities to an even greater degree.
In the meantime, this four-part tutorial offers guidance on the identification of adnexal masses. If we’ve helped ease coordination of care between the generalist ObGyn and the expert sonographer, we’ve accomplished our goal.
We want to hear from you! Tell us what you think.
1. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56-66.
2. Goswami PK, Kerr-Wilson R, McCarthy K. Cancer of the fallopian tube. The Obstetrician & Gynaecologist. 2006;8(3):147-152.doi: 10.1576/toag.8.3.147.27249.
Part 1 A Starting Point (September 2010)
Part 2 The non-neoplastic ovarian mass (October 2010)
Part 3 Ovarian neoplasms (November 2010)
An imaging study of the adnexae would not be complete without thorough assessment of the fallopian tubes. Among the pathologies that may be identified or confirmed by ultrasonography are:
- ectopic pregnancy
- tubal inflammatory disease, or salpingitis
- chronic tubal disease, or hydrosalpinx
- tubo-ovarian complex
- tubo-ovarian abscess
- tubal and ovarian torsion
- cancer.
In this final installment of our four-part series on ultrasonographic (US) imaging of the adnexae, we take these entities as our focus.
Suspect ectopic pregnancy even if the hCG level is not yet available
A detailed discussion of ectopic pregnancy far exceeds the framework of this article. Suffice it to say that ectopic pregnancy should always be considered in a woman of reproductive age, especially one who complains of abdominal or pelvic pain, vaginal bleeding, or both. However, these signs and symptoms are present in only about 25% of women who have this condition. When these signs and symptoms are present, it is wise to be suspicious even if the results of human chorionic gonadotropin (hCG) measurement are not yet available.
A complete history is important in the diagnosis of ectopic pregnancy. Risk factors include a history of ectopic pregnancy, pelvic inflammatory disease (PID), or tubal surgery, or use of an intrauterine device.
Unequivocal US diagnosis of ectopic pregnancy is possible in only about 20% of cases, and depends on identification of an extrauterine pregnancy, which may not be visible in the early days of gestation. However, some grayscale ultrasonographic findings that may suggest ectopic pregnancy include:
- an empty uterus in a woman who has an hCG level above 1,000 to 1,500 mIu/mL (the discriminatory level)
- a thick, hyperechoic endometrial echo (decidualization)
- an adnexal mass other than a simple cyst
- echogenic fluid in the cul-de-sac (FIGURE 1A–1D).
Power Doppler can help the sonographer localize the ectopic pregnancy in the tubes by demonstrating the circular vascularization of the more or less typical “tubal ring” (FIGURE 1E–1G).

FIGURE 1 Ectopic pregnancy
A–D. Various cases of tubal ectopic gestation (arrows point to each gestation). E–G. Power Doppler localizes the ectopic pregnancy (arrows), side by side with the corpus luteum (CL).
A patient’s history may yield clues to tubal inflammatory disease
The diagnosis of acute salpingitis begins with a thorough patient history. Look for any report of PID, unexplained fever, foul vaginal discharge, sexually transmitted infection, or recent intrauterine procedures such as hysteroscopy, IUD insertion, endometrial biopsy, or saline infusion sonohysterography.
US diagnosis is based on the findings of a slightly dilated fallopian tube with low-level echogenic fluid content, thick tubal walls, and tenderness to the touch of the transvaginal probe.1
In cross section, the tube forms the “cogwheel sign” (FIGURE 2C). Power Doppler shows the subserosal blood vessels characteristic of this entity (FIGURE 2D).

FIGURE 2 Tubal disease
A–C. Grayscale images showing thick walls, low-level echoic fluid (pus?) and the “cogwheel sign” (CW). D. Subserosal vascularization typical of an inflammatory response in hollow abdominal viscera. E,F. Edematous fimbrial end (arrow) of the inflamed tubes, floating in a small amount of free pelvic fluid. G. Low-level echoic, fluid-filled, thick-walled tubes with incomplete septae (arrow) are the hallmarks of hydrosalpinx. H–J. Bilateral hydrosalpinx. Note the thin walls and anechoic fluid-filled forms (sausage-shaped) (CL = corpus luteum; OV = ovary; UT = uterus).
Look for fluid dilating the tube in chronic tubal disease
Hydrosalpinx is characterized on US by thin tubal walls with a relatively anechoic but large amount of fluid dilating the tube (FIGURE 2G–2J). The interior wall is studded with shallow, echogenic, mural nodules (without blood vessels) that assume the appearance of a tube or sausage. The small, shallow internal papillae give the cross section of the tube the appearance of beads on a string.

FIGURE 3 Tubo-ovarian complex
A. The tube (T) and ovary (OV) form an infectious conglomerate. B. Power Doppler appearance. C. Laparoscopic view.
Tubo-ovarian complex
When this complex arises, the anatomy and shape of the tube and the involved ovary are somewhat distorted but still largely discernible (FIGURE 3A AND 3B).
Tubo-ovarian abscess is a more advanced stage of a fast-progressing or neglected pelvic inflammatory process. In it, the tube and the ovary can barely be distinguished, and US signs of abscess appear, among them low-level echoic fluid and linear echogenicity (FIGURE 4).

FIGURE 4 Tubo-ovarian abscess
The tube and ovary are indistinguishable. The fluid is of low-level echogenicity (pus), and the walls are thick.
In ovarian torsion, the follicles press outward
Although torsion has distinct sonographic signs, it remains a clinical diagnosis that US findings may or may not support. Correct diagnosis often is the purview of expert sonographers and sonologists.
When ovarian torsion is present, the ovaries are enlarged and hyperechoic, their follicles pushed toward the surface (FIGURE 5A–5C). The ovaries are also tender to the touch and typically demonstrate no blood flow by Doppler interrogation. On occasion, when arterial flow is still present (venous flow is usually the first characteristic to vanish), a twisted arterial pattern may result, similar to the coil of a telephone cord. Some pelvic fluid may also appear.
Tubal torsion is harder to diagnose. US recognition depends on the finding of a normal ovary with intact blood flow beside a fluid-filled, thin-walled, tender, cystic structure with some of the previously mentioned sonomarkers of tubal occlusion such as the bead-on-a-string or cogwheel sign (FIGURE 5D–5G).

FIGURE 5 Torsion
A–C. Ovarian torsion. Hyperechoic, large ovary with follicles pushed toward the surface. Power Doppler reveals no blood flow in the ovary. D–F. Tubal torsion. Cystic dilatation with a small beak and a normal ovary. G. Intraoperative view of the tube (twisted three times; yellow arrows) and the normal ovary (white arrows).
Fluid in the cul-de-sac
In many cases, fluid may be present or trapped in the lesser pelvis, surrounded or blocked by the pelvic organs. If this fluid is the result or sequela of PID, thin, thread-like adhesive strands will be visible between the organs on US, betraying its pathogenesis (FIGURE 6). The “walls” of such loculated fluid are the pelvic wall itself and the surrounding organs.

FIGURE 6 Fluid in the cul-de-sac
Sequelae of acute PID. A. Free pelvic fluid, also known as pelvic, peritoneal, loculated fluid. B. A normal ovary and an adhesive strand (arrow). C. Laparoscopic image of the adhesion (arrow).
Cancer of the tubes is unlikely, but it’s best to keep it in mind
Primary cancer of the fallopian tubes accounts for only 1% to 2% of all gynecologic cancers.2 Only 300 to 400 women are given this diagnosis each year in the United States— most of them postmenopausal.
Despite its rarity, fallopian-tube cancer is a major concern when a tubal mass is identified by palpation or imaging. In most cases, however, no palpable mass is found at the time of first examination, and tubal malignancy is diagnosed perioperatively or postoperatively.
US characteristics of tubal cancer are similar to those of ovarian cancer: a bizarre appearance, with extremely vascular tissue. At times, US attributes of tubal pathology, such as incomplete septae and tube-like fluid-filled structures, are apparent (FIGURE 7).
Consider cancer of the fallopian tube whenever an unexplained solid mass is palpated or imaged in the area of the tubes in conjunction with apparently normal ovaries.

FIGURE 7 Fallopian tube cancer
A. Fluid-filled uterine cavity. B. Large cystic dilatation of the tube. C. A thickened tubal wall (arrow). D. Doppler interrogation reveals high diastolic flow (arrows). E. Macroscopic gross appearance.
The long view
As technology has advanced, so has ultrasonography. High-resolution transducers, color and power Doppler, and three-dimensional imaging make it possible for an experienced practitioner to identify and confirm the diagnosis of many adnexal masses and pathologies, from the corpus luteum to fallopian tube torsion. As the field continues to evolve, we expect that this modality will facilitate the diagnosis of adnexal abnormalities to an even greater degree.
In the meantime, this four-part tutorial offers guidance on the identification of adnexal masses. If we’ve helped ease coordination of care between the generalist ObGyn and the expert sonographer, we’ve accomplished our goal.
We want to hear from you! Tell us what you think.
Part 1 A Starting Point (September 2010)
Part 2 The non-neoplastic ovarian mass (October 2010)
Part 3 Ovarian neoplasms (November 2010)
An imaging study of the adnexae would not be complete without thorough assessment of the fallopian tubes. Among the pathologies that may be identified or confirmed by ultrasonography are:
- ectopic pregnancy
- tubal inflammatory disease, or salpingitis
- chronic tubal disease, or hydrosalpinx
- tubo-ovarian complex
- tubo-ovarian abscess
- tubal and ovarian torsion
- cancer.
In this final installment of our four-part series on ultrasonographic (US) imaging of the adnexae, we take these entities as our focus.
Suspect ectopic pregnancy even if the hCG level is not yet available
A detailed discussion of ectopic pregnancy far exceeds the framework of this article. Suffice it to say that ectopic pregnancy should always be considered in a woman of reproductive age, especially one who complains of abdominal or pelvic pain, vaginal bleeding, or both. However, these signs and symptoms are present in only about 25% of women who have this condition. When these signs and symptoms are present, it is wise to be suspicious even if the results of human chorionic gonadotropin (hCG) measurement are not yet available.
A complete history is important in the diagnosis of ectopic pregnancy. Risk factors include a history of ectopic pregnancy, pelvic inflammatory disease (PID), or tubal surgery, or use of an intrauterine device.
Unequivocal US diagnosis of ectopic pregnancy is possible in only about 20% of cases, and depends on identification of an extrauterine pregnancy, which may not be visible in the early days of gestation. However, some grayscale ultrasonographic findings that may suggest ectopic pregnancy include:
- an empty uterus in a woman who has an hCG level above 1,000 to 1,500 mIu/mL (the discriminatory level)
- a thick, hyperechoic endometrial echo (decidualization)
- an adnexal mass other than a simple cyst
- echogenic fluid in the cul-de-sac (FIGURE 1A–1D).
Power Doppler can help the sonographer localize the ectopic pregnancy in the tubes by demonstrating the circular vascularization of the more or less typical “tubal ring” (FIGURE 1E–1G).

FIGURE 1 Ectopic pregnancy
A–D. Various cases of tubal ectopic gestation (arrows point to each gestation). E–G. Power Doppler localizes the ectopic pregnancy (arrows), side by side with the corpus luteum (CL).
A patient’s history may yield clues to tubal inflammatory disease
The diagnosis of acute salpingitis begins with a thorough patient history. Look for any report of PID, unexplained fever, foul vaginal discharge, sexually transmitted infection, or recent intrauterine procedures such as hysteroscopy, IUD insertion, endometrial biopsy, or saline infusion sonohysterography.
US diagnosis is based on the findings of a slightly dilated fallopian tube with low-level echogenic fluid content, thick tubal walls, and tenderness to the touch of the transvaginal probe.1
In cross section, the tube forms the “cogwheel sign” (FIGURE 2C). Power Doppler shows the subserosal blood vessels characteristic of this entity (FIGURE 2D).

FIGURE 2 Tubal disease
A–C. Grayscale images showing thick walls, low-level echoic fluid (pus?) and the “cogwheel sign” (CW). D. Subserosal vascularization typical of an inflammatory response in hollow abdominal viscera. E,F. Edematous fimbrial end (arrow) of the inflamed tubes, floating in a small amount of free pelvic fluid. G. Low-level echoic, fluid-filled, thick-walled tubes with incomplete septae (arrow) are the hallmarks of hydrosalpinx. H–J. Bilateral hydrosalpinx. Note the thin walls and anechoic fluid-filled forms (sausage-shaped) (CL = corpus luteum; OV = ovary; UT = uterus).
Look for fluid dilating the tube in chronic tubal disease
Hydrosalpinx is characterized on US by thin tubal walls with a relatively anechoic but large amount of fluid dilating the tube (FIGURE 2G–2J). The interior wall is studded with shallow, echogenic, mural nodules (without blood vessels) that assume the appearance of a tube or sausage. The small, shallow internal papillae give the cross section of the tube the appearance of beads on a string.

FIGURE 3 Tubo-ovarian complex
A. The tube (T) and ovary (OV) form an infectious conglomerate. B. Power Doppler appearance. C. Laparoscopic view.
Tubo-ovarian complex
When this complex arises, the anatomy and shape of the tube and the involved ovary are somewhat distorted but still largely discernible (FIGURE 3A AND 3B).
Tubo-ovarian abscess is a more advanced stage of a fast-progressing or neglected pelvic inflammatory process. In it, the tube and the ovary can barely be distinguished, and US signs of abscess appear, among them low-level echoic fluid and linear echogenicity (FIGURE 4).

FIGURE 4 Tubo-ovarian abscess
The tube and ovary are indistinguishable. The fluid is of low-level echogenicity (pus), and the walls are thick.
In ovarian torsion, the follicles press outward
Although torsion has distinct sonographic signs, it remains a clinical diagnosis that US findings may or may not support. Correct diagnosis often is the purview of expert sonographers and sonologists.
When ovarian torsion is present, the ovaries are enlarged and hyperechoic, their follicles pushed toward the surface (FIGURE 5A–5C). The ovaries are also tender to the touch and typically demonstrate no blood flow by Doppler interrogation. On occasion, when arterial flow is still present (venous flow is usually the first characteristic to vanish), a twisted arterial pattern may result, similar to the coil of a telephone cord. Some pelvic fluid may also appear.
Tubal torsion is harder to diagnose. US recognition depends on the finding of a normal ovary with intact blood flow beside a fluid-filled, thin-walled, tender, cystic structure with some of the previously mentioned sonomarkers of tubal occlusion such as the bead-on-a-string or cogwheel sign (FIGURE 5D–5G).

FIGURE 5 Torsion
A–C. Ovarian torsion. Hyperechoic, large ovary with follicles pushed toward the surface. Power Doppler reveals no blood flow in the ovary. D–F. Tubal torsion. Cystic dilatation with a small beak and a normal ovary. G. Intraoperative view of the tube (twisted three times; yellow arrows) and the normal ovary (white arrows).
Fluid in the cul-de-sac
In many cases, fluid may be present or trapped in the lesser pelvis, surrounded or blocked by the pelvic organs. If this fluid is the result or sequela of PID, thin, thread-like adhesive strands will be visible between the organs on US, betraying its pathogenesis (FIGURE 6). The “walls” of such loculated fluid are the pelvic wall itself and the surrounding organs.

FIGURE 6 Fluid in the cul-de-sac
Sequelae of acute PID. A. Free pelvic fluid, also known as pelvic, peritoneal, loculated fluid. B. A normal ovary and an adhesive strand (arrow). C. Laparoscopic image of the adhesion (arrow).
Cancer of the tubes is unlikely, but it’s best to keep it in mind
Primary cancer of the fallopian tubes accounts for only 1% to 2% of all gynecologic cancers.2 Only 300 to 400 women are given this diagnosis each year in the United States— most of them postmenopausal.
Despite its rarity, fallopian-tube cancer is a major concern when a tubal mass is identified by palpation or imaging. In most cases, however, no palpable mass is found at the time of first examination, and tubal malignancy is diagnosed perioperatively or postoperatively.
US characteristics of tubal cancer are similar to those of ovarian cancer: a bizarre appearance, with extremely vascular tissue. At times, US attributes of tubal pathology, such as incomplete septae and tube-like fluid-filled structures, are apparent (FIGURE 7).
Consider cancer of the fallopian tube whenever an unexplained solid mass is palpated or imaged in the area of the tubes in conjunction with apparently normal ovaries.

FIGURE 7 Fallopian tube cancer
A. Fluid-filled uterine cavity. B. Large cystic dilatation of the tube. C. A thickened tubal wall (arrow). D. Doppler interrogation reveals high diastolic flow (arrows). E. Macroscopic gross appearance.
The long view
As technology has advanced, so has ultrasonography. High-resolution transducers, color and power Doppler, and three-dimensional imaging make it possible for an experienced practitioner to identify and confirm the diagnosis of many adnexal masses and pathologies, from the corpus luteum to fallopian tube torsion. As the field continues to evolve, we expect that this modality will facilitate the diagnosis of adnexal abnormalities to an even greater degree.
In the meantime, this four-part tutorial offers guidance on the identification of adnexal masses. If we’ve helped ease coordination of care between the generalist ObGyn and the expert sonographer, we’ve accomplished our goal.
We want to hear from you! Tell us what you think.
1. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56-66.
2. Goswami PK, Kerr-Wilson R, McCarthy K. Cancer of the fallopian tube. The Obstetrician & Gynaecologist. 2006;8(3):147-152.doi: 10.1576/toag.8.3.147.27249.
1. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56-66.
2. Goswami PK, Kerr-Wilson R, McCarthy K. Cancer of the fallopian tube. The Obstetrician & Gynaecologist. 2006;8(3):147-152.doi: 10.1576/toag.8.3.147.27249.
Skilled US imaging of the adnexae: Ovarian Neoplasms
Part 1: A Starting Point (September 2010)
Part 2: The non-neoplastic ovarian mass (October 2010)
Part 4: The fallopian tubes (December 2010)
Although roughly three quarters of ovarian neoplasms occur in premenopausal women, 87% of masses in this population are benign. The vast majority of malignant neoplasms—about 75%—are diagnosed in postmenopausal women.
These figures suggest that you have some discerning to do. Specifically, how do you identify the small percentage of masses in premenopausal women that are malignant—and winnow out the benign neoplasms in the postmenopausal population?
Now that we’ve equipped you with an understanding of the morphologic building blocks of adnexal masses, and how those masses are assessed using ultrasonography (US) (described in Part 2 of this four-part series), you can apply your skills of discernment to ovarian neoplasms. Specifically:
- teratoma (dermoid cyst)—one of the two most prevalent benign neoplasms of the ovary
- serous cystadenoma—the other most prevalent benign neoplasm
- hormone-secreting tumors
- malignant neoplasms.
Recall that Part 1 of this series offered a starting point for US imaging of the adnexae by describing (and showing) how basic pelvic structures appear in grayscale US and color and power Doppler. Part 2 focused on non-neoplastic ovarian masses. Part 4 will take as its subject tubal entities such as torsion, ectopic pregnancy, and cancer.
Teratomas present a variety of “faces”
Teratomas may appear to be solid, cystic, or both (FIGURE 1). At times, they have a bizarre or variable appearance. The overwhelming majority of teratomas can be recognized by shadowing, which may be extreme if the tumor contains a solid, echogenic central mass (FIGURE 1A). Such an echogenic core is sometimes called the “fried egg” sign when it is detected by transabdominal US.
FIGURE 1 Cystic and solid benign teratomas
A. Shadowing (small arrows) is apparent in a teratoma containing low-level echoic fluid. B. Several spherical “balls” floating in a cystic teratoma, with shadowing. C. Solid teratoma. D. A “typical” teratoma, with septation and multilocularity. E. Macroscopic view of an ovarian teratoma (arrow). F. Multiple sebaceous ball-shaped structures within a benign cystic teratoma (inset: macroscopic view).When the teratoma is cystic or partially cystic, it may contain a linear hyperechoic area consistent with sebaceous fluid and hair. Although magnetic resonance imaging (MRI) can confirm the fat content of a teratoma, US is very efficient in making the diagnosis, rendering MRI unnecessary.
As for blood vessels, teratomas are known to have scant or no apparent vascularity. A rule of thumb: If a bizarre adnexal structure with no vascularity is visible on US, and if it is cystic or solid in appearance (or both), benign teratoma should be included in the differential diagnosis.
Because an ovarian teratoma can assume almost any shape and form, three-dimensional (3D) US is almost useless in its evaluation.
Cystadenomas are relatively easy to identify on US
Benign cystadenomas—serous or mucinous—are extremely common. In at least 20% to 30% of cases, they are bilateral.
The US characteristics of these masses include:
- multilocularity, in many cases (although two thirds of simple unilocular cysts in postmenopausal women are serous cystadenomas)
- multiseptation, with the septae often fanning out from a central, apparently solid structure (FIGURE 2)
- anechoic nature when they contain fluid (in the serous variety) or with low-level echogenicity (in mucous cystadenomas).
FIGURE 2 Benign cystadenoma
A–C. Typical sonographic appearance of a benign cystadenoma, with septae fanning out from a solid area, creating an anechoic, fluid-filled, multilocular pattern. D. MRI appearance of the cyst (arrow points to solid area from which the septae fan out).As for vascularity, cystadenomas have a paucity of core vessels and have, if measured quantitatively, what we consider to be normal resistive and pulsatility indices and low peak systolic velocity. Histologically, they are benign. These neoplasms can be identified using US with relative ease and high confidence, rendering computed tomography (CT) and MRI (FIGURE 2D) virtually redundant.
When US characteristics overlap
Based on our 20 years of experience with US assessment of adnexal masses, and the potential overlap (on grayscale as well as color and power Doppler) between the US appearance of endometriomas, cystadenomas, and cystic teratomas, we recommend that, when a mass is not pathognomonic on US, this triad of entities be considered in the differential diagnosis. The entity that has the greatest number of relevant characteristics should be listed as the most likely and first possibility on the US report.
(For a description of the US appearance of endometriomas, see Part 2 of this series, which appeared in the October 2010 issue of OBG Management.)
Hormone-secreting tumors are small and symptomatic
Although hormone-secreting tumors are not malignant in the strict sense of the definition, they should be mentioned here because of the high probability that they can be diagnosed by transvaginal sonography (TVS). These tumors are small, hiding at times in an ovary of almost normal size. They are also vascular, featuring a characteristic ring-like pattern, much like that of the corpus luteum, on color or power Doppler. They also produce general and clear clinical symptoms and signs. For example, testosterone-like tumors cause male-pattern baldness, hirsutism, and voice changes.
Many providers suspect a hormone-secreting tumor based on its signs and symptoms, and seek US confirmation from us. In many of these cases, laboratory tests have been done and point to the possible diagnosis—e.g., a high testosterone level in the case of a Sertoli-Leydig cell tumor.
One typical estrogen-secreting tumor is the granulosa cell tumor (FIGURE 3). This tumor can usually be identified by the solid-appearing tissue surrounding multiple cysts of different sizes; it is typically richly supplied with blood vessels.
Another clue to the diagnosis is the state of the endometrium. Because a granulosa cell tumor secretes estrogen, it causes a thickened endometrial lining and, usually, abnormal uterine bleeding.
FIGURE 3 Granulosa cell tumor
A. Sagittal image of the uterus demonstrating a thick, hyperechoic endometrial echo under hormonal stimulation of the tumor. B. Multicystic and solid areas alternate in the enlarged uterus. Power Doppler demonstrates the typical increased vascularity. (The arrows point to the cystic area of the tumor.)
Malignant ovarian neoplasms are rare
As a rule, the larger the lesion, the more suspicious it is.
Malignant tumors usually have a complex appearance:
- thick walls (≥4 mm)
- heterogeneous texture
- multilocularity
- solid components
- papillary excrescences within the tumor as well as on the outer surface (FIGURE 4A and 4B).
FIGURE 4 Adenocarcinoma of the ovary
A. An enlarged right ovary containing several cystic structures. B. Right ovary and transverse section of the uterus. C, D. Power Doppler evaluation demonstrating rich vascularization. E. 3D orthogonal planes and volume calculation of the ovary (31.1 cc). F. 3D angiogram (lower right image) of the rich vascularization of the cancer. G. Relationship between the vascular right ovary and the uterus.Tumor vascularity is another marker suggestive of ovarian malignancy (FIGURE 4C and 4D). A fast-growing tumor requires a vascular “infrastructure,” a mesh of blood vessels that is laid down in expedited fashion and that is controlled by vascular growth factors. As explained in Part 2 of this series, the vessels in this vascular mesh lack the muscular layer of normal vessels. They frequently are intertwined, forming anastomoses and vascular lakes through which blood flows without much resistance. Look, therefore, for low resistance and high-velocity flow.
A new way to employ 3D US is to detect, measure, and quantify the blood supply to a tumor. FIGURE 4E shows how the vascularity and volume of an ovarian mass are calculated, with 3D angiographic display of the blood vessels contained within it demonstrated in FIGURE 4F. This vascular pattern can also be viewed in the context of the pelvic organs (FIGURE 4G), an approach that is useful in teaching.
Recently, Sladkevicus and colleagues used 3D US angiography to define tumor vascularity, identifying straight vessels, those that had changes in caliber, and bridging between vessels.1 They studied 104 patients who had 77 benign tumors, 6 borderline tumors, and 21 cancers. The researchers concluded that dense vessel patterns in the tumor made malignancy five times more likely. Widely dispersed straight vessels without branching were the strongest predictors of benign status, reducing the likelihood of malignancy by a factor of 10.1
We described the importance of a finding of blood vessels in an internal papillary structure as an accurate predictor of malignancy. We focused on a small volume of the mass, which was selected by a software program, and found that a preselected volume of 1 cc could reliably predict an increased, and pathological, vascular supply to an ovary containing cancer.2,3
Although ovarian cancer is rare, affecting 30 to 50 women of every 100,000, it is particularly deadly, with a 5-year survival rate (all stages) of 50%. If cancer is detected and treated during stage I, the 5-year survival rate rises substantially—to 95%. Sadly, only 25% of cases are detected while the cancer is still localized.
In stages III and IV, the 5-year survival rate is 28% or lower. It has been estimated that, if 75% of patients had their cancer detected during stage I, the mortality rate could be halved.
The lifetime risk of ovarian cancer in a woman who has no affected relative is 1.4% (1 case in every 70 women). When the patient has one affected first-degree relative, that risk rises to 5% (1 case in 20 women), and it rises to 7% (1 case in 14 women) when she has two or more affected first-degree relatives.
Stay tuned!
In the final installment of this series, coming next month, we discuss the use of US imaging to evaluate tubal anomalies, including torsion, ectopic pregnancy, and cancer.
We want to hear from you! Tell us what you think.
1. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnosis of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874.-
2. Kudla MJ, Timor-Tritsch IE, Hope JM, et al. Spherical tissue sampling in 3-dimensional power Doppler angiography: a new approach for evaluation of ovarian tumors. J Ultrasound Med. 2008;27(3):425-433.
3. Alcazar JL, Prka M. Evaluation of two different methods for vascular sampling by three-dimensional power Doppler angiography in solid and cystic-solid adnexal masses. Ultrasound Obstet Gynecol. 2009;33(3):349-354.
Part 1: A Starting Point (September 2010)
Part 2: The non-neoplastic ovarian mass (October 2010)
Part 4: The fallopian tubes (December 2010)
Although roughly three quarters of ovarian neoplasms occur in premenopausal women, 87% of masses in this population are benign. The vast majority of malignant neoplasms—about 75%—are diagnosed in postmenopausal women.
These figures suggest that you have some discerning to do. Specifically, how do you identify the small percentage of masses in premenopausal women that are malignant—and winnow out the benign neoplasms in the postmenopausal population?
Now that we’ve equipped you with an understanding of the morphologic building blocks of adnexal masses, and how those masses are assessed using ultrasonography (US) (described in Part 2 of this four-part series), you can apply your skills of discernment to ovarian neoplasms. Specifically:
- teratoma (dermoid cyst)—one of the two most prevalent benign neoplasms of the ovary
- serous cystadenoma—the other most prevalent benign neoplasm
- hormone-secreting tumors
- malignant neoplasms.
Recall that Part 1 of this series offered a starting point for US imaging of the adnexae by describing (and showing) how basic pelvic structures appear in grayscale US and color and power Doppler. Part 2 focused on non-neoplastic ovarian masses. Part 4 will take as its subject tubal entities such as torsion, ectopic pregnancy, and cancer.
Teratomas present a variety of “faces”
Teratomas may appear to be solid, cystic, or both (FIGURE 1). At times, they have a bizarre or variable appearance. The overwhelming majority of teratomas can be recognized by shadowing, which may be extreme if the tumor contains a solid, echogenic central mass (FIGURE 1A). Such an echogenic core is sometimes called the “fried egg” sign when it is detected by transabdominal US.
FIGURE 1 Cystic and solid benign teratomas
A. Shadowing (small arrows) is apparent in a teratoma containing low-level echoic fluid. B. Several spherical “balls” floating in a cystic teratoma, with shadowing. C. Solid teratoma. D. A “typical” teratoma, with septation and multilocularity. E. Macroscopic view of an ovarian teratoma (arrow). F. Multiple sebaceous ball-shaped structures within a benign cystic teratoma (inset: macroscopic view).When the teratoma is cystic or partially cystic, it may contain a linear hyperechoic area consistent with sebaceous fluid and hair. Although magnetic resonance imaging (MRI) can confirm the fat content of a teratoma, US is very efficient in making the diagnosis, rendering MRI unnecessary.
As for blood vessels, teratomas are known to have scant or no apparent vascularity. A rule of thumb: If a bizarre adnexal structure with no vascularity is visible on US, and if it is cystic or solid in appearance (or both), benign teratoma should be included in the differential diagnosis.
Because an ovarian teratoma can assume almost any shape and form, three-dimensional (3D) US is almost useless in its evaluation.
Cystadenomas are relatively easy to identify on US
Benign cystadenomas—serous or mucinous—are extremely common. In at least 20% to 30% of cases, they are bilateral.
The US characteristics of these masses include:
- multilocularity, in many cases (although two thirds of simple unilocular cysts in postmenopausal women are serous cystadenomas)
- multiseptation, with the septae often fanning out from a central, apparently solid structure (FIGURE 2)
- anechoic nature when they contain fluid (in the serous variety) or with low-level echogenicity (in mucous cystadenomas).
FIGURE 2 Benign cystadenoma
A–C. Typical sonographic appearance of a benign cystadenoma, with septae fanning out from a solid area, creating an anechoic, fluid-filled, multilocular pattern. D. MRI appearance of the cyst (arrow points to solid area from which the septae fan out).As for vascularity, cystadenomas have a paucity of core vessels and have, if measured quantitatively, what we consider to be normal resistive and pulsatility indices and low peak systolic velocity. Histologically, they are benign. These neoplasms can be identified using US with relative ease and high confidence, rendering computed tomography (CT) and MRI (FIGURE 2D) virtually redundant.
When US characteristics overlap
Based on our 20 years of experience with US assessment of adnexal masses, and the potential overlap (on grayscale as well as color and power Doppler) between the US appearance of endometriomas, cystadenomas, and cystic teratomas, we recommend that, when a mass is not pathognomonic on US, this triad of entities be considered in the differential diagnosis. The entity that has the greatest number of relevant characteristics should be listed as the most likely and first possibility on the US report.
(For a description of the US appearance of endometriomas, see Part 2 of this series, which appeared in the October 2010 issue of OBG Management.)
Hormone-secreting tumors are small and symptomatic
Although hormone-secreting tumors are not malignant in the strict sense of the definition, they should be mentioned here because of the high probability that they can be diagnosed by transvaginal sonography (TVS). These tumors are small, hiding at times in an ovary of almost normal size. They are also vascular, featuring a characteristic ring-like pattern, much like that of the corpus luteum, on color or power Doppler. They also produce general and clear clinical symptoms and signs. For example, testosterone-like tumors cause male-pattern baldness, hirsutism, and voice changes.
Many providers suspect a hormone-secreting tumor based on its signs and symptoms, and seek US confirmation from us. In many of these cases, laboratory tests have been done and point to the possible diagnosis—e.g., a high testosterone level in the case of a Sertoli-Leydig cell tumor.
One typical estrogen-secreting tumor is the granulosa cell tumor (FIGURE 3). This tumor can usually be identified by the solid-appearing tissue surrounding multiple cysts of different sizes; it is typically richly supplied with blood vessels.
Another clue to the diagnosis is the state of the endometrium. Because a granulosa cell tumor secretes estrogen, it causes a thickened endometrial lining and, usually, abnormal uterine bleeding.
FIGURE 3 Granulosa cell tumor
A. Sagittal image of the uterus demonstrating a thick, hyperechoic endometrial echo under hormonal stimulation of the tumor. B. Multicystic and solid areas alternate in the enlarged uterus. Power Doppler demonstrates the typical increased vascularity. (The arrows point to the cystic area of the tumor.)
Malignant ovarian neoplasms are rare
As a rule, the larger the lesion, the more suspicious it is.
Malignant tumors usually have a complex appearance:
- thick walls (≥4 mm)
- heterogeneous texture
- multilocularity
- solid components
- papillary excrescences within the tumor as well as on the outer surface (FIGURE 4A and 4B).
FIGURE 4 Adenocarcinoma of the ovary
A. An enlarged right ovary containing several cystic structures. B. Right ovary and transverse section of the uterus. C, D. Power Doppler evaluation demonstrating rich vascularization. E. 3D orthogonal planes and volume calculation of the ovary (31.1 cc). F. 3D angiogram (lower right image) of the rich vascularization of the cancer. G. Relationship between the vascular right ovary and the uterus.Tumor vascularity is another marker suggestive of ovarian malignancy (FIGURE 4C and 4D). A fast-growing tumor requires a vascular “infrastructure,” a mesh of blood vessels that is laid down in expedited fashion and that is controlled by vascular growth factors. As explained in Part 2 of this series, the vessels in this vascular mesh lack the muscular layer of normal vessels. They frequently are intertwined, forming anastomoses and vascular lakes through which blood flows without much resistance. Look, therefore, for low resistance and high-velocity flow.
A new way to employ 3D US is to detect, measure, and quantify the blood supply to a tumor. FIGURE 4E shows how the vascularity and volume of an ovarian mass are calculated, with 3D angiographic display of the blood vessels contained within it demonstrated in FIGURE 4F. This vascular pattern can also be viewed in the context of the pelvic organs (FIGURE 4G), an approach that is useful in teaching.
Recently, Sladkevicus and colleagues used 3D US angiography to define tumor vascularity, identifying straight vessels, those that had changes in caliber, and bridging between vessels.1 They studied 104 patients who had 77 benign tumors, 6 borderline tumors, and 21 cancers. The researchers concluded that dense vessel patterns in the tumor made malignancy five times more likely. Widely dispersed straight vessels without branching were the strongest predictors of benign status, reducing the likelihood of malignancy by a factor of 10.1
We described the importance of a finding of blood vessels in an internal papillary structure as an accurate predictor of malignancy. We focused on a small volume of the mass, which was selected by a software program, and found that a preselected volume of 1 cc could reliably predict an increased, and pathological, vascular supply to an ovary containing cancer.2,3
Although ovarian cancer is rare, affecting 30 to 50 women of every 100,000, it is particularly deadly, with a 5-year survival rate (all stages) of 50%. If cancer is detected and treated during stage I, the 5-year survival rate rises substantially—to 95%. Sadly, only 25% of cases are detected while the cancer is still localized.
In stages III and IV, the 5-year survival rate is 28% or lower. It has been estimated that, if 75% of patients had their cancer detected during stage I, the mortality rate could be halved.
The lifetime risk of ovarian cancer in a woman who has no affected relative is 1.4% (1 case in every 70 women). When the patient has one affected first-degree relative, that risk rises to 5% (1 case in 20 women), and it rises to 7% (1 case in 14 women) when she has two or more affected first-degree relatives.
Stay tuned!
In the final installment of this series, coming next month, we discuss the use of US imaging to evaluate tubal anomalies, including torsion, ectopic pregnancy, and cancer.
We want to hear from you! Tell us what you think.
Part 1: A Starting Point (September 2010)
Part 2: The non-neoplastic ovarian mass (October 2010)
Part 4: The fallopian tubes (December 2010)
Although roughly three quarters of ovarian neoplasms occur in premenopausal women, 87% of masses in this population are benign. The vast majority of malignant neoplasms—about 75%—are diagnosed in postmenopausal women.
These figures suggest that you have some discerning to do. Specifically, how do you identify the small percentage of masses in premenopausal women that are malignant—and winnow out the benign neoplasms in the postmenopausal population?
Now that we’ve equipped you with an understanding of the morphologic building blocks of adnexal masses, and how those masses are assessed using ultrasonography (US) (described in Part 2 of this four-part series), you can apply your skills of discernment to ovarian neoplasms. Specifically:
- teratoma (dermoid cyst)—one of the two most prevalent benign neoplasms of the ovary
- serous cystadenoma—the other most prevalent benign neoplasm
- hormone-secreting tumors
- malignant neoplasms.
Recall that Part 1 of this series offered a starting point for US imaging of the adnexae by describing (and showing) how basic pelvic structures appear in grayscale US and color and power Doppler. Part 2 focused on non-neoplastic ovarian masses. Part 4 will take as its subject tubal entities such as torsion, ectopic pregnancy, and cancer.
Teratomas present a variety of “faces”
Teratomas may appear to be solid, cystic, or both (FIGURE 1). At times, they have a bizarre or variable appearance. The overwhelming majority of teratomas can be recognized by shadowing, which may be extreme if the tumor contains a solid, echogenic central mass (FIGURE 1A). Such an echogenic core is sometimes called the “fried egg” sign when it is detected by transabdominal US.
FIGURE 1 Cystic and solid benign teratomas
A. Shadowing (small arrows) is apparent in a teratoma containing low-level echoic fluid. B. Several spherical “balls” floating in a cystic teratoma, with shadowing. C. Solid teratoma. D. A “typical” teratoma, with septation and multilocularity. E. Macroscopic view of an ovarian teratoma (arrow). F. Multiple sebaceous ball-shaped structures within a benign cystic teratoma (inset: macroscopic view).When the teratoma is cystic or partially cystic, it may contain a linear hyperechoic area consistent with sebaceous fluid and hair. Although magnetic resonance imaging (MRI) can confirm the fat content of a teratoma, US is very efficient in making the diagnosis, rendering MRI unnecessary.
As for blood vessels, teratomas are known to have scant or no apparent vascularity. A rule of thumb: If a bizarre adnexal structure with no vascularity is visible on US, and if it is cystic or solid in appearance (or both), benign teratoma should be included in the differential diagnosis.
Because an ovarian teratoma can assume almost any shape and form, three-dimensional (3D) US is almost useless in its evaluation.
Cystadenomas are relatively easy to identify on US
Benign cystadenomas—serous or mucinous—are extremely common. In at least 20% to 30% of cases, they are bilateral.
The US characteristics of these masses include:
- multilocularity, in many cases (although two thirds of simple unilocular cysts in postmenopausal women are serous cystadenomas)
- multiseptation, with the septae often fanning out from a central, apparently solid structure (FIGURE 2)
- anechoic nature when they contain fluid (in the serous variety) or with low-level echogenicity (in mucous cystadenomas).
FIGURE 2 Benign cystadenoma
A–C. Typical sonographic appearance of a benign cystadenoma, with septae fanning out from a solid area, creating an anechoic, fluid-filled, multilocular pattern. D. MRI appearance of the cyst (arrow points to solid area from which the septae fan out).As for vascularity, cystadenomas have a paucity of core vessels and have, if measured quantitatively, what we consider to be normal resistive and pulsatility indices and low peak systolic velocity. Histologically, they are benign. These neoplasms can be identified using US with relative ease and high confidence, rendering computed tomography (CT) and MRI (FIGURE 2D) virtually redundant.
When US characteristics overlap
Based on our 20 years of experience with US assessment of adnexal masses, and the potential overlap (on grayscale as well as color and power Doppler) between the US appearance of endometriomas, cystadenomas, and cystic teratomas, we recommend that, when a mass is not pathognomonic on US, this triad of entities be considered in the differential diagnosis. The entity that has the greatest number of relevant characteristics should be listed as the most likely and first possibility on the US report.
(For a description of the US appearance of endometriomas, see Part 2 of this series, which appeared in the October 2010 issue of OBG Management.)
Hormone-secreting tumors are small and symptomatic
Although hormone-secreting tumors are not malignant in the strict sense of the definition, they should be mentioned here because of the high probability that they can be diagnosed by transvaginal sonography (TVS). These tumors are small, hiding at times in an ovary of almost normal size. They are also vascular, featuring a characteristic ring-like pattern, much like that of the corpus luteum, on color or power Doppler. They also produce general and clear clinical symptoms and signs. For example, testosterone-like tumors cause male-pattern baldness, hirsutism, and voice changes.
Many providers suspect a hormone-secreting tumor based on its signs and symptoms, and seek US confirmation from us. In many of these cases, laboratory tests have been done and point to the possible diagnosis—e.g., a high testosterone level in the case of a Sertoli-Leydig cell tumor.
One typical estrogen-secreting tumor is the granulosa cell tumor (FIGURE 3). This tumor can usually be identified by the solid-appearing tissue surrounding multiple cysts of different sizes; it is typically richly supplied with blood vessels.
Another clue to the diagnosis is the state of the endometrium. Because a granulosa cell tumor secretes estrogen, it causes a thickened endometrial lining and, usually, abnormal uterine bleeding.
FIGURE 3 Granulosa cell tumor
A. Sagittal image of the uterus demonstrating a thick, hyperechoic endometrial echo under hormonal stimulation of the tumor. B. Multicystic and solid areas alternate in the enlarged uterus. Power Doppler demonstrates the typical increased vascularity. (The arrows point to the cystic area of the tumor.)
Malignant ovarian neoplasms are rare
As a rule, the larger the lesion, the more suspicious it is.
Malignant tumors usually have a complex appearance:
- thick walls (≥4 mm)
- heterogeneous texture
- multilocularity
- solid components
- papillary excrescences within the tumor as well as on the outer surface (FIGURE 4A and 4B).
FIGURE 4 Adenocarcinoma of the ovary
A. An enlarged right ovary containing several cystic structures. B. Right ovary and transverse section of the uterus. C, D. Power Doppler evaluation demonstrating rich vascularization. E. 3D orthogonal planes and volume calculation of the ovary (31.1 cc). F. 3D angiogram (lower right image) of the rich vascularization of the cancer. G. Relationship between the vascular right ovary and the uterus.Tumor vascularity is another marker suggestive of ovarian malignancy (FIGURE 4C and 4D). A fast-growing tumor requires a vascular “infrastructure,” a mesh of blood vessels that is laid down in expedited fashion and that is controlled by vascular growth factors. As explained in Part 2 of this series, the vessels in this vascular mesh lack the muscular layer of normal vessels. They frequently are intertwined, forming anastomoses and vascular lakes through which blood flows without much resistance. Look, therefore, for low resistance and high-velocity flow.
A new way to employ 3D US is to detect, measure, and quantify the blood supply to a tumor. FIGURE 4E shows how the vascularity and volume of an ovarian mass are calculated, with 3D angiographic display of the blood vessels contained within it demonstrated in FIGURE 4F. This vascular pattern can also be viewed in the context of the pelvic organs (FIGURE 4G), an approach that is useful in teaching.
Recently, Sladkevicus and colleagues used 3D US angiography to define tumor vascularity, identifying straight vessels, those that had changes in caliber, and bridging between vessels.1 They studied 104 patients who had 77 benign tumors, 6 borderline tumors, and 21 cancers. The researchers concluded that dense vessel patterns in the tumor made malignancy five times more likely. Widely dispersed straight vessels without branching were the strongest predictors of benign status, reducing the likelihood of malignancy by a factor of 10.1
We described the importance of a finding of blood vessels in an internal papillary structure as an accurate predictor of malignancy. We focused on a small volume of the mass, which was selected by a software program, and found that a preselected volume of 1 cc could reliably predict an increased, and pathological, vascular supply to an ovary containing cancer.2,3
Although ovarian cancer is rare, affecting 30 to 50 women of every 100,000, it is particularly deadly, with a 5-year survival rate (all stages) of 50%. If cancer is detected and treated during stage I, the 5-year survival rate rises substantially—to 95%. Sadly, only 25% of cases are detected while the cancer is still localized.
In stages III and IV, the 5-year survival rate is 28% or lower. It has been estimated that, if 75% of patients had their cancer detected during stage I, the mortality rate could be halved.
The lifetime risk of ovarian cancer in a woman who has no affected relative is 1.4% (1 case in every 70 women). When the patient has one affected first-degree relative, that risk rises to 5% (1 case in 20 women), and it rises to 7% (1 case in 14 women) when she has two or more affected first-degree relatives.
Stay tuned!
In the final installment of this series, coming next month, we discuss the use of US imaging to evaluate tubal anomalies, including torsion, ectopic pregnancy, and cancer.
We want to hear from you! Tell us what you think.
1. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnosis of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874.-
2. Kudla MJ, Timor-Tritsch IE, Hope JM, et al. Spherical tissue sampling in 3-dimensional power Doppler angiography: a new approach for evaluation of ovarian tumors. J Ultrasound Med. 2008;27(3):425-433.
3. Alcazar JL, Prka M. Evaluation of two different methods for vascular sampling by three-dimensional power Doppler angiography in solid and cystic-solid adnexal masses. Ultrasound Obstet Gynecol. 2009;33(3):349-354.
1. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnosis of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874.-
2. Kudla MJ, Timor-Tritsch IE, Hope JM, et al. Spherical tissue sampling in 3-dimensional power Doppler angiography: a new approach for evaluation of ovarian tumors. J Ultrasound Med. 2008;27(3):425-433.
3. Alcazar JL, Prka M. Evaluation of two different methods for vascular sampling by three-dimensional power Doppler angiography in solid and cystic-solid adnexal masses. Ultrasound Obstet Gynecol. 2009;33(3):349-354.
Skilled US imaging of the adnexae: The non-neoplastic mass
Part 1: A Starting Point (September 2010)
Part 3: Ovarian neoplasms (November 2010)
Part 4: The fallopian tubes (December 2010)
Scanning the ovaries is no simple task. As we mentioned in Part 1 of this four-part series, the practitioner must use the right equipment, take basic preparatory steps, be watchful for clues in the history, and reach a conclusion about what he or she sees. Not only that: The ultrasonographer must be extraordinarily vigilant, paying close attention to multiple characteristics of any mass, from thickness of the wall to the presence of papillations or a blood supply—signs of potential malignancy.
In this article, we detail the traits of various types of non-neoplastic ovarian masses, including:
- functional cysts—follicles, the corpus luteum, and theca lutein cysts
- nonfunctional cysts—serous masses and endometriomas
- cystadenofibromas. Although these masses are usually categorized histo-logically as neoplasms, we include them here due to their almost daily appearance in a busy gynecologic ultrasonographic (US) facility.
In Part 3, we will cover ovarian neoplasms, and in Part 4, our focus will be tubal entities such as ectopic pregnancy and torsion.
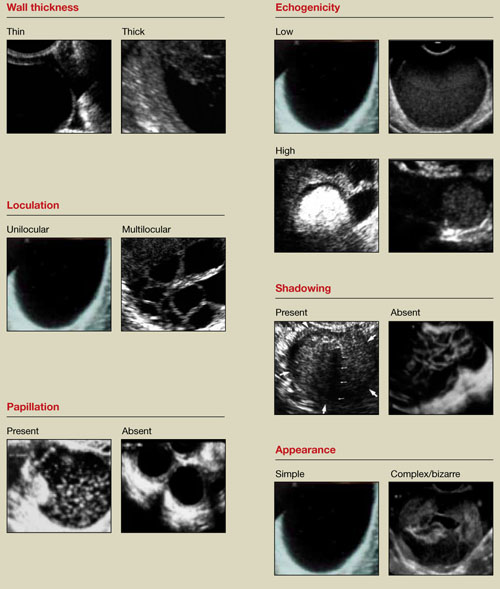
FIGURE 1 What is a mass made of? 6 morphologic building blocks
Take an inventory of the mass
Any adnexal mass should be assessed in light of its essential characteristics (Figure 1).
Wall structure. Pay attention to thickness. We use an arbitrary cutoff of 4 mm, giving extra scrutiny to thicknesses exceeding that measurement. In our experience, the thicker the wall, the more likely the mass is to be malignant.
Septation and loculation. A mass is typically unilocular or multilocular. Multilocularity is more common in tumors of low malignant potential and malignant neoplasms.
Papillation. Any internal or external papillae or excrescences should draw your attention. Papillarity in an ovarian mass renders that mass suspicious for malignancy.
Measure (height and width) any papillae that are identified, and document them. Because papillae are associated with ovarian malignancy, further assessment is warranted immediately. The first step is determining whether the papillations contain blood vessels—a task for which color and power Doppler are helpful. We prefer power Doppler because it is more sensitive, detecting blood-flow velocity in the lowest detectable range of 2 cm/s, and because it is not directionally influenced.
Papillae that contain blood vessels with detectable flow are suspicious for malignancy.
Exacoustos and colleagues found that papillae as large as 15 mm in height and 10 mm in width (base) were present in 48% of borderline ovarian tumors but in only 4% of benign and 4% of malignant tumors. However, when the intracystic solid tissue exceeded those dimensions, the lesions were present in 48% of invasive ovarian tumors, 18% of borderline ovarian tumors, and 7% of benign masses.1
Internal echo-structure. A mass can be anechoic, a finding that usually indicates the presence of clear fluid. Mostly solid masses are echogenic. And masses that contain particulate matter, such as blood, cellular matter, or even mucous material, usually have echogenicity of a low level, often described as a “ground-glass” appearance. A mass can also have mixed echo-genicity, a finding usually found in cases involving teratoma or malignancy.
Shadowing. If it is present, it may signify the presence of an extremely dense, solid tissue, such as bone or calcification. The diagnosis of a benign teratoma (i.e., dermoid cyst) should be entertained if shadowing is present in a hyperechoic nodule or mass. Malignant masses very rarely, if ever, display frank shadowing.
Overall appearance. On rare occasions, a bizarre shape or “complex” appearance (as it is termed in most radiology reports) may indicate a malignant mass. More likely it indicates the presence of a teratoma, cystadenoma, or even an atypical corpus luteum. In some reports generated by US laboratories, the term “complex” is applied to all structures other than simple cysts.
Size. The size of a mass can be misleading, as small ovarian lesions with the appropriate sonographic characteristics may be malignant and some larger ones without those characteristics may not be. However, it is understood that the larger an ovarian lesion, the more likely it is a tumor. One important distinction: The amount of fluid in a cystic structure or the amount of old blood in an endometrioma is not the disease process…it is the byproduct of the process. So an 8-cm endometrioma may create fewer pain or fertility issues than a 2- or 3-cm endometrioma. Similarly, the amount of “chocolate” fluid is not automatically indicative of the amount of active endometriotic glands or their sequelae!2
Ascites. If it is present, it should be recorded and investigated further because it may be caused by a malignant intra-abdominal tumor.
Motion tenderness. If the to-and-fro movement of the vaginal probe elicits any motion tenderness, it, too, should be documented. It may be a sign of pelvic peritonitis. In such cases, an “ominous appearing” adnexal finding may represent an inflammatory, rather than malignant, mass.
One of the components of extensive evaluation of the adnexae in general and ovaries in particular is color or power Doppler interrogation—or both.
Tumors contain a relatively large number of pathologic blood vessels that lack the muscular layer found in normal blood vessels and, as a result, demonstrate lower resistance to flow. Diastolic flow is high in these vessels, and resistance and pulsatility indices are low.
We also pay attention when these blood vessels have a tortuous appearance, changes in caliber, anastomoses, and vascular lakes.3 The more tortuous the vessels, with multiple inter-vessel connections and dilatations with changing calibers, the greater the risk of malignancy.4 No less important is the presence of a vessel within a “complex” ovarian mass. A centrally located vessel (also called a “lead vessel”) is suspicious for malignancy.5
A gallery of non-neoplastic ovarian masses
Non-neoplastic cysts are, by far, the most common structures of the ovary. They may be functional, as in the case of the follicles, corpus luteum, and theca lutein cysts, or they may be nonfunctional, as in serous cysts and endometriomas. (As we noted in Part 1, do not call the follicles and corpus luteum “cysts” because this designation suggests pathology.)6
FIGURE 2 Simple cyst
This cyst is anechoic and unilocular with thin walls and no papillae.
Functional cysts
Functional cysts, also known as “simple” cysts, may grow as large as 4 to 5 cm in diameter (Figure 2). They are typically unilocular, anechoic, and thin-walled, with no papillae, and almost never malignant. They usually resolve and require no treatment unless rupture or torsion occurs. Except for the corpus luteum, they have no increased blood flow, and need be viewed only by transvaginal ultrasonography (TVS).
The corpus luteum also can be recognized by TVS. It can exhibit any of a variety of internal structures and echo patterns, due to the multitude of shapes of the blood or clot that can be seen within it (Figure 3).
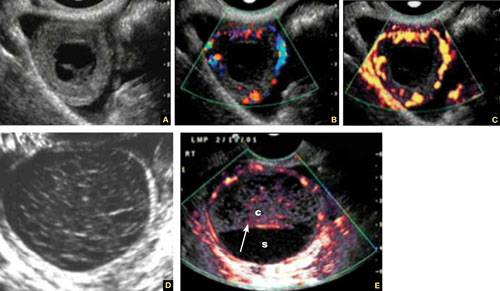
FIGURE 3 Corpus luteum
A–C. Gray-scale, color Doppler, and power Doppler images, respectively, of a typical corpus luteum. B and C show the enveloping vessels, or “ring of fire.” D. A rather typical gray-scale appearance with a mesh-like, linear internal texture. E. A common feature of the corpus luteum is a linear interphase (arrow) between the clot (c) and the liquified serum (s).
The corpus luteum is typically enveloped by blood vessels, visible on color Doppler as what is called a “ring of fire.” It regresses without intervention. In hyperstimulated ovaries, however, more than one may be present; this poses a real diagnostic challenge when ectopic pregnancy is suspected because it is difficult to differentiate the two entities.
Because the corpus luteum can sometimes resemble some types of ovarian tumors on TVS, imaging during the secretory phase of the cycle in a woman of reproductive age is not ideal. Instead, she should be scanned (or rescanned) between days 5 and 9 of the cycle.
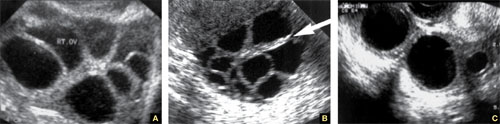
FIGURE 4 Hormonally stimulated ovaries
A, B. The right and left ovaries stimulated by follicle-stimulating hormone preparation (arrow points to hilus). C. An ovary stimulated by clomiphene.
Lutein cysts may reach 5 to 10 cm in diameter. They generally have a thick wall, are multilocular, and typically occur after hormonal induction of ovulation (Figure 4). They also can occur in diabetes, molar pregnancy, and hydrops fetalis. We have seen a unilateral theca lutein cyst in a normal pregnancy (Figure 5). No treatment is necessary unless rupture or torsion occurs.
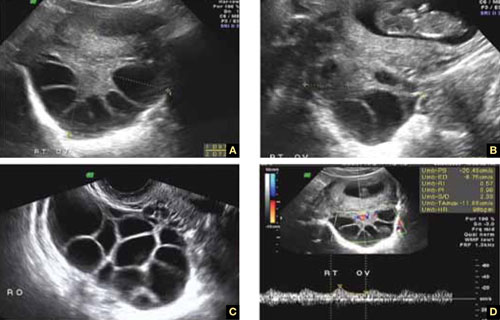
FIGURE 5 Lutein cysts
A–C. The typical “stained glass” appearance of three lutein cysts of the right ovary in a pregnant patient. D. Color Doppler image of the ovary demonstrating high-velocity flow (peak systolic velocity of 20.4 cm/s).
Serous cysts
These cysts can reach 4 cm in diameter, have smooth walls with no papillae, are unilocular, and occur most often during menopause. No pathological blood flow is visible in their walls. Most gynecologists follow them (Figure 6).1,7
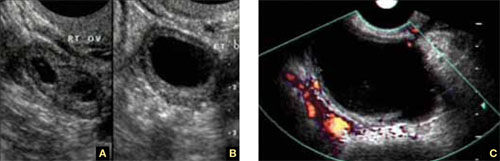
FIGURE 6 Serous cyst
A. Right ovary containing the cyst. B. Normal left ovary. C. Power Doppler interrogation showing no particular flow in the walls of a serous cyst.
Endometriomas
After the simple cyst, the endometrioma is the most prevalent ovarian or adnexal cyst (Figure 7). It usually has a thick wall and is filled with homogeneous fluid with low-level echo-genicity. It can reach 10 cm in size, and many are bilateral. It is sometimes called a “chocolate” cyst because of its dark blood content.
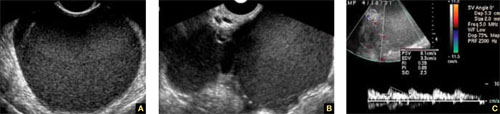
FIGURE 7 Endometriomas
Endometriomas have low echogenicity. A. Unilateral, unilocular cyst with thin walls. B. Bilateral endometriomas. C. Blood flow in a solid or papillary component of the endometrioma is an occasional finding. It should be investigated further because of the risk that it represents endometrioid cancer.
Endometriomas do not resolve; they usually require surgical excision, although very small ones wholly contained within an ovary are often managed medically or expectantly.
These masses rarely (<1%) give rise to endometrioid carcinoma. Should an endometrioma contain papillae with blood vessels, it is extremely suspicious for endometrioid cancer.
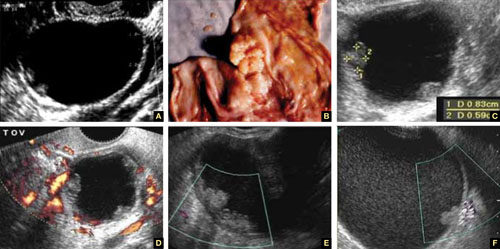
FIGURE 8 Cystic fibromas
A. Sonographic image shows a thin wall and hyperechoic, small mural nodules. B. Macroscopic appearance of an area of internal papillary excrescences. C. Measurement of the small, mural nodules. D. Lack of blood flow in the small papillae, a typical finding on color or power Doppler. E, F. Blood flow in the wall of the cyst and in the mural nodules.
Ovarian fibromas
A fibroma is a slow-growing, benign, solid ovarian tumor. It usually has a cystic component and then is called a cystadenofibroma.
The cystic variety is filled with anechoic fluid and has a thin wall. However, its pathognomonic feature is the small (2–3 mm), extremely hyperechoic mural nodules (papillae) it contains (Figure 8A–C). In the overwhelming majority of cases, no blood vessels are detectable, and the mass is unilocular (Figure 8D–E). It can be recognized in the ovary by the semilunar shape of the tissue surrounding it (crescent sign). The differential diagnosis includes the simple (serous) cyst.
The solid fibroma has a myometrium-like texture, with few or no detectable blood vessels in the stroma. The differential diagnosis includes the Brenner tumor and the Krukenberg tumor.
According to a technology assessment from the Agency for Healthcare Research and Quality (AHRQ), “conventional gray-scale ultrasonography is the most common imaging modality used to differentiate benign from malignant adnexal masses. Especially with the advent of high-frequency transvaginal probes, the quality of the images allows description of the gross anatomic features of the lesion.”8 This descriptive ability is limited, however, “by the great variability of macroscopic characteristics of both benign and malignant masses. Furthermore, the technique is operator dependent.”8
To overcome these challenges, some experts have developed ultrasonographic (US) morphologic scoring systems, which assign a value to individual characteristics. Lerner and colleagues devised a 4-point system:
| Characteristic | Points | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Wall structure | Smooth or small irregularities (<3 mm) | Solid or not applicable | Papillarities larger than 3 mm | |
| Shadowing | Yes | No | ||
| Septation | None or thin (<3 mm) | Thick (≥3 mm) | ||
| Echogenicity | Sonolucent or low-level echo or echogenic core | Mixed or high | ||
The mean point value for benign masses was 1.8; for tumors of low malignant potential it was 3.9; and for malignant tumors it was 5.6 (P < .0005). Lerner and associates proposed a cutoff of 3. A score of 3 or higher, they felt, would be most predictive of malignancy, with sensitivity of 96.8% and specificity of 77%. Positive and negative predictive values were 29.4% and 99.6%, respectively.9
Almost all published scoring systems are based upon or derived from one reported by Sassone and coworkers.10 The most important and practical feature of all scoring systems is their ability to rule out malignancy.
Morphology and Doppler: A synergistic combination
As the same AHRQ report points out, “all of the diagnostic tests and scoring systems we evaluated exhibited a trade-off between sensitivity and specificity—studies of a given test that reported higher sensitivity had lower specificity, and vice versa.”8 Among evaluation methods, the combination of US morphology scores and Doppler imaging achieved the highest pooled sensitivity and specificity scores in distinguishing benign and malignant adnexal masses in postmenopausal women: 86% and 91%, respectively, according to the AHRQ report.8
Compare these figures with those of:
- Bimanual pelvic examination (45% and 90%, respectively)
- Doppler resistance index (72% and 90%)
- Doppler pulsatility index (80% and 73%)
- presence of blood vessels (88% and 78%).
The combination of US morphology scores and Doppler was comparable to the pooled sensitivity and specificity of magnetic resonance imaging (91% and 88%, respectively) and superior to computed tomography (90% and 75%, respectively).
Why the need to know?
Discrimination between benign and malignant masses serves a number of purposes, depending on the setting.
For example, if a symptomatic woman is found to have an adnexal mass, it is important to identify the type of mass causing the symptoms to determine the best course of treatment. And because surgery may be one of the treatment options, it is helpful to know whether a mass is likely to be malignant so that the patient can be referred to a specialist or center that has optimal surgical expertise.8
Some asymptomatic masses may be identified during the annual bimanual pelvic examination recommended by ACOG or during pregnancy-related US imaging. In this setting, it is important to ascertain whether the mass is likely to be malignant so that the patient can be referred to a specialist, if necessary. In addition, thorough assessment of the mass can help “avoid unnecessary diagnostic procedures, including surgery, and anxiety in women with asymptomatic, nonmalignant conditions. In some cases, there may be a rationale for removing certain asymptomatic benign lesions, including prevention of malignant transformation; prevention of ovarian torsion”; and prevention of rupture. Surgery may also be appropriate to avert the need for more complicated surgery in the future or to enhance fertility.8 —Janelle Yates, Senior Editor
Stay tuned!
Next issue, in Part 3 of this series, we will review the use of imaging in the investigation of ovarian neoplasms, both benign and malignant, with an abundance of US images to accompany our discussion.
We want to hear from you! Tell us what you think.
1. Exacoustos C, Romanini ME, Rinaldo D, et al. Preoperative sonographic features of borderline ovarian tumors. Ultrasound Obstet Gynecol. 2004;25(1):50-59.
2. Rulin MC, Preston AL. Adnexal masses in postmenopausal women. Obstet Gynecol. 1987;70(4):578-581.
3. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultraound Med. 2005;24(3):255-258.
4. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnos is of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874-882.
5. Testa AC, Mancari R, Di Legge A, et al. The “lead vessel”: a vascular ultrasound feature of metastasis in the ovaries. Ultrasound Obstet Gynecol. 2008;31:218-221.
6. Goldstein SR. Postmenopausal adnexal cysts: how clinical management has evolved. Am J Obstet Gynecol. 1996;175(6):1496-1501.
7. Levine D, Gosink BB, Wolf S, Feldesman MR, Pretorius D. Simple adnexal cysts: the natural history in postmenopausal women. Radiology. 1992;184(3):653-659.
8. Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Evidence Report Technol Assess. 2006;Feb;(130):1-145.
9. Lerner JP, Timor-Tritsch IE, Federman A, Abramovich G. Transvaginal ultrasonographic characterization of ovarian masses with an improved, weighted scoring system. Am J Obstet Gynecol. 1994;170(1 Pt 1):81-85.
10. Sassone AM, Timor-Tritsch IE, Artner A, et al. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 2001;78:70-76.
Part 1: A Starting Point (September 2010)
Part 3: Ovarian neoplasms (November 2010)
Part 4: The fallopian tubes (December 2010)
Scanning the ovaries is no simple task. As we mentioned in Part 1 of this four-part series, the practitioner must use the right equipment, take basic preparatory steps, be watchful for clues in the history, and reach a conclusion about what he or she sees. Not only that: The ultrasonographer must be extraordinarily vigilant, paying close attention to multiple characteristics of any mass, from thickness of the wall to the presence of papillations or a blood supply—signs of potential malignancy.
In this article, we detail the traits of various types of non-neoplastic ovarian masses, including:
- functional cysts—follicles, the corpus luteum, and theca lutein cysts
- nonfunctional cysts—serous masses and endometriomas
- cystadenofibromas. Although these masses are usually categorized histo-logically as neoplasms, we include them here due to their almost daily appearance in a busy gynecologic ultrasonographic (US) facility.
In Part 3, we will cover ovarian neoplasms, and in Part 4, our focus will be tubal entities such as ectopic pregnancy and torsion.

FIGURE 1 What is a mass made of? 6 morphologic building blocks
Take an inventory of the mass
Any adnexal mass should be assessed in light of its essential characteristics (Figure 1).
Wall structure. Pay attention to thickness. We use an arbitrary cutoff of 4 mm, giving extra scrutiny to thicknesses exceeding that measurement. In our experience, the thicker the wall, the more likely the mass is to be malignant.
Septation and loculation. A mass is typically unilocular or multilocular. Multilocularity is more common in tumors of low malignant potential and malignant neoplasms.
Papillation. Any internal or external papillae or excrescences should draw your attention. Papillarity in an ovarian mass renders that mass suspicious for malignancy.
Measure (height and width) any papillae that are identified, and document them. Because papillae are associated with ovarian malignancy, further assessment is warranted immediately. The first step is determining whether the papillations contain blood vessels—a task for which color and power Doppler are helpful. We prefer power Doppler because it is more sensitive, detecting blood-flow velocity in the lowest detectable range of 2 cm/s, and because it is not directionally influenced.
Papillae that contain blood vessels with detectable flow are suspicious for malignancy.
Exacoustos and colleagues found that papillae as large as 15 mm in height and 10 mm in width (base) were present in 48% of borderline ovarian tumors but in only 4% of benign and 4% of malignant tumors. However, when the intracystic solid tissue exceeded those dimensions, the lesions were present in 48% of invasive ovarian tumors, 18% of borderline ovarian tumors, and 7% of benign masses.1
Internal echo-structure. A mass can be anechoic, a finding that usually indicates the presence of clear fluid. Mostly solid masses are echogenic. And masses that contain particulate matter, such as blood, cellular matter, or even mucous material, usually have echogenicity of a low level, often described as a “ground-glass” appearance. A mass can also have mixed echo-genicity, a finding usually found in cases involving teratoma or malignancy.
Shadowing. If it is present, it may signify the presence of an extremely dense, solid tissue, such as bone or calcification. The diagnosis of a benign teratoma (i.e., dermoid cyst) should be entertained if shadowing is present in a hyperechoic nodule or mass. Malignant masses very rarely, if ever, display frank shadowing.
Overall appearance. On rare occasions, a bizarre shape or “complex” appearance (as it is termed in most radiology reports) may indicate a malignant mass. More likely it indicates the presence of a teratoma, cystadenoma, or even an atypical corpus luteum. In some reports generated by US laboratories, the term “complex” is applied to all structures other than simple cysts.
Size. The size of a mass can be misleading, as small ovarian lesions with the appropriate sonographic characteristics may be malignant and some larger ones without those characteristics may not be. However, it is understood that the larger an ovarian lesion, the more likely it is a tumor. One important distinction: The amount of fluid in a cystic structure or the amount of old blood in an endometrioma is not the disease process…it is the byproduct of the process. So an 8-cm endometrioma may create fewer pain or fertility issues than a 2- or 3-cm endometrioma. Similarly, the amount of “chocolate” fluid is not automatically indicative of the amount of active endometriotic glands or their sequelae!2
Ascites. If it is present, it should be recorded and investigated further because it may be caused by a malignant intra-abdominal tumor.
Motion tenderness. If the to-and-fro movement of the vaginal probe elicits any motion tenderness, it, too, should be documented. It may be a sign of pelvic peritonitis. In such cases, an “ominous appearing” adnexal finding may represent an inflammatory, rather than malignant, mass.
One of the components of extensive evaluation of the adnexae in general and ovaries in particular is color or power Doppler interrogation—or both.
Tumors contain a relatively large number of pathologic blood vessels that lack the muscular layer found in normal blood vessels and, as a result, demonstrate lower resistance to flow. Diastolic flow is high in these vessels, and resistance and pulsatility indices are low.
We also pay attention when these blood vessels have a tortuous appearance, changes in caliber, anastomoses, and vascular lakes.3 The more tortuous the vessels, with multiple inter-vessel connections and dilatations with changing calibers, the greater the risk of malignancy.4 No less important is the presence of a vessel within a “complex” ovarian mass. A centrally located vessel (also called a “lead vessel”) is suspicious for malignancy.5
A gallery of non-neoplastic ovarian masses
Non-neoplastic cysts are, by far, the most common structures of the ovary. They may be functional, as in the case of the follicles, corpus luteum, and theca lutein cysts, or they may be nonfunctional, as in serous cysts and endometriomas. (As we noted in Part 1, do not call the follicles and corpus luteum “cysts” because this designation suggests pathology.)6

FIGURE 2 Simple cyst
This cyst is anechoic and unilocular with thin walls and no papillae.
Functional cysts
Functional cysts, also known as “simple” cysts, may grow as large as 4 to 5 cm in diameter (Figure 2). They are typically unilocular, anechoic, and thin-walled, with no papillae, and almost never malignant. They usually resolve and require no treatment unless rupture or torsion occurs. Except for the corpus luteum, they have no increased blood flow, and need be viewed only by transvaginal ultrasonography (TVS).
The corpus luteum also can be recognized by TVS. It can exhibit any of a variety of internal structures and echo patterns, due to the multitude of shapes of the blood or clot that can be seen within it (Figure 3).

FIGURE 3 Corpus luteum
A–C. Gray-scale, color Doppler, and power Doppler images, respectively, of a typical corpus luteum. B and C show the enveloping vessels, or “ring of fire.” D. A rather typical gray-scale appearance with a mesh-like, linear internal texture. E. A common feature of the corpus luteum is a linear interphase (arrow) between the clot (c) and the liquified serum (s).
The corpus luteum is typically enveloped by blood vessels, visible on color Doppler as what is called a “ring of fire.” It regresses without intervention. In hyperstimulated ovaries, however, more than one may be present; this poses a real diagnostic challenge when ectopic pregnancy is suspected because it is difficult to differentiate the two entities.
Because the corpus luteum can sometimes resemble some types of ovarian tumors on TVS, imaging during the secretory phase of the cycle in a woman of reproductive age is not ideal. Instead, she should be scanned (or rescanned) between days 5 and 9 of the cycle.

FIGURE 4 Hormonally stimulated ovaries
A, B. The right and left ovaries stimulated by follicle-stimulating hormone preparation (arrow points to hilus). C. An ovary stimulated by clomiphene.
Lutein cysts may reach 5 to 10 cm in diameter. They generally have a thick wall, are multilocular, and typically occur after hormonal induction of ovulation (Figure 4). They also can occur in diabetes, molar pregnancy, and hydrops fetalis. We have seen a unilateral theca lutein cyst in a normal pregnancy (Figure 5). No treatment is necessary unless rupture or torsion occurs.

FIGURE 5 Lutein cysts
A–C. The typical “stained glass” appearance of three lutein cysts of the right ovary in a pregnant patient. D. Color Doppler image of the ovary demonstrating high-velocity flow (peak systolic velocity of 20.4 cm/s).
Serous cysts
These cysts can reach 4 cm in diameter, have smooth walls with no papillae, are unilocular, and occur most often during menopause. No pathological blood flow is visible in their walls. Most gynecologists follow them (Figure 6).1,7

FIGURE 6 Serous cyst
A. Right ovary containing the cyst. B. Normal left ovary. C. Power Doppler interrogation showing no particular flow in the walls of a serous cyst.
Endometriomas
After the simple cyst, the endometrioma is the most prevalent ovarian or adnexal cyst (Figure 7). It usually has a thick wall and is filled with homogeneous fluid with low-level echo-genicity. It can reach 10 cm in size, and many are bilateral. It is sometimes called a “chocolate” cyst because of its dark blood content.

FIGURE 7 Endometriomas
Endometriomas have low echogenicity. A. Unilateral, unilocular cyst with thin walls. B. Bilateral endometriomas. C. Blood flow in a solid or papillary component of the endometrioma is an occasional finding. It should be investigated further because of the risk that it represents endometrioid cancer.
Endometriomas do not resolve; they usually require surgical excision, although very small ones wholly contained within an ovary are often managed medically or expectantly.
These masses rarely (<1%) give rise to endometrioid carcinoma. Should an endometrioma contain papillae with blood vessels, it is extremely suspicious for endometrioid cancer.

FIGURE 8 Cystic fibromas
A. Sonographic image shows a thin wall and hyperechoic, small mural nodules. B. Macroscopic appearance of an area of internal papillary excrescences. C. Measurement of the small, mural nodules. D. Lack of blood flow in the small papillae, a typical finding on color or power Doppler. E, F. Blood flow in the wall of the cyst and in the mural nodules.
Ovarian fibromas
A fibroma is a slow-growing, benign, solid ovarian tumor. It usually has a cystic component and then is called a cystadenofibroma.
The cystic variety is filled with anechoic fluid and has a thin wall. However, its pathognomonic feature is the small (2–3 mm), extremely hyperechoic mural nodules (papillae) it contains (Figure 8A–C). In the overwhelming majority of cases, no blood vessels are detectable, and the mass is unilocular (Figure 8D–E). It can be recognized in the ovary by the semilunar shape of the tissue surrounding it (crescent sign). The differential diagnosis includes the simple (serous) cyst.
The solid fibroma has a myometrium-like texture, with few or no detectable blood vessels in the stroma. The differential diagnosis includes the Brenner tumor and the Krukenberg tumor.
According to a technology assessment from the Agency for Healthcare Research and Quality (AHRQ), “conventional gray-scale ultrasonography is the most common imaging modality used to differentiate benign from malignant adnexal masses. Especially with the advent of high-frequency transvaginal probes, the quality of the images allows description of the gross anatomic features of the lesion.”8 This descriptive ability is limited, however, “by the great variability of macroscopic characteristics of both benign and malignant masses. Furthermore, the technique is operator dependent.”8
To overcome these challenges, some experts have developed ultrasonographic (US) morphologic scoring systems, which assign a value to individual characteristics. Lerner and colleagues devised a 4-point system:
| Characteristic | Points | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Wall structure | Smooth or small irregularities (<3 mm) | Solid or not applicable | Papillarities larger than 3 mm | |
| Shadowing | Yes | No | ||
| Septation | None or thin (<3 mm) | Thick (≥3 mm) | ||
| Echogenicity | Sonolucent or low-level echo or echogenic core | Mixed or high | ||
The mean point value for benign masses was 1.8; for tumors of low malignant potential it was 3.9; and for malignant tumors it was 5.6 (P < .0005). Lerner and associates proposed a cutoff of 3. A score of 3 or higher, they felt, would be most predictive of malignancy, with sensitivity of 96.8% and specificity of 77%. Positive and negative predictive values were 29.4% and 99.6%, respectively.9
Almost all published scoring systems are based upon or derived from one reported by Sassone and coworkers.10 The most important and practical feature of all scoring systems is their ability to rule out malignancy.
Morphology and Doppler: A synergistic combination
As the same AHRQ report points out, “all of the diagnostic tests and scoring systems we evaluated exhibited a trade-off between sensitivity and specificity—studies of a given test that reported higher sensitivity had lower specificity, and vice versa.”8 Among evaluation methods, the combination of US morphology scores and Doppler imaging achieved the highest pooled sensitivity and specificity scores in distinguishing benign and malignant adnexal masses in postmenopausal women: 86% and 91%, respectively, according to the AHRQ report.8
Compare these figures with those of:
- Bimanual pelvic examination (45% and 90%, respectively)
- Doppler resistance index (72% and 90%)
- Doppler pulsatility index (80% and 73%)
- presence of blood vessels (88% and 78%).
The combination of US morphology scores and Doppler was comparable to the pooled sensitivity and specificity of magnetic resonance imaging (91% and 88%, respectively) and superior to computed tomography (90% and 75%, respectively).
Why the need to know?
Discrimination between benign and malignant masses serves a number of purposes, depending on the setting.
For example, if a symptomatic woman is found to have an adnexal mass, it is important to identify the type of mass causing the symptoms to determine the best course of treatment. And because surgery may be one of the treatment options, it is helpful to know whether a mass is likely to be malignant so that the patient can be referred to a specialist or center that has optimal surgical expertise.8
Some asymptomatic masses may be identified during the annual bimanual pelvic examination recommended by ACOG or during pregnancy-related US imaging. In this setting, it is important to ascertain whether the mass is likely to be malignant so that the patient can be referred to a specialist, if necessary. In addition, thorough assessment of the mass can help “avoid unnecessary diagnostic procedures, including surgery, and anxiety in women with asymptomatic, nonmalignant conditions. In some cases, there may be a rationale for removing certain asymptomatic benign lesions, including prevention of malignant transformation; prevention of ovarian torsion”; and prevention of rupture. Surgery may also be appropriate to avert the need for more complicated surgery in the future or to enhance fertility.8 —Janelle Yates, Senior Editor
Stay tuned!
Next issue, in Part 3 of this series, we will review the use of imaging in the investigation of ovarian neoplasms, both benign and malignant, with an abundance of US images to accompany our discussion.
We want to hear from you! Tell us what you think.
Part 1: A Starting Point (September 2010)
Part 3: Ovarian neoplasms (November 2010)
Part 4: The fallopian tubes (December 2010)
Scanning the ovaries is no simple task. As we mentioned in Part 1 of this four-part series, the practitioner must use the right equipment, take basic preparatory steps, be watchful for clues in the history, and reach a conclusion about what he or she sees. Not only that: The ultrasonographer must be extraordinarily vigilant, paying close attention to multiple characteristics of any mass, from thickness of the wall to the presence of papillations or a blood supply—signs of potential malignancy.
In this article, we detail the traits of various types of non-neoplastic ovarian masses, including:
- functional cysts—follicles, the corpus luteum, and theca lutein cysts
- nonfunctional cysts—serous masses and endometriomas
- cystadenofibromas. Although these masses are usually categorized histo-logically as neoplasms, we include them here due to their almost daily appearance in a busy gynecologic ultrasonographic (US) facility.
In Part 3, we will cover ovarian neoplasms, and in Part 4, our focus will be tubal entities such as ectopic pregnancy and torsion.

FIGURE 1 What is a mass made of? 6 morphologic building blocks
Take an inventory of the mass
Any adnexal mass should be assessed in light of its essential characteristics (Figure 1).
Wall structure. Pay attention to thickness. We use an arbitrary cutoff of 4 mm, giving extra scrutiny to thicknesses exceeding that measurement. In our experience, the thicker the wall, the more likely the mass is to be malignant.
Septation and loculation. A mass is typically unilocular or multilocular. Multilocularity is more common in tumors of low malignant potential and malignant neoplasms.
Papillation. Any internal or external papillae or excrescences should draw your attention. Papillarity in an ovarian mass renders that mass suspicious for malignancy.
Measure (height and width) any papillae that are identified, and document them. Because papillae are associated with ovarian malignancy, further assessment is warranted immediately. The first step is determining whether the papillations contain blood vessels—a task for which color and power Doppler are helpful. We prefer power Doppler because it is more sensitive, detecting blood-flow velocity in the lowest detectable range of 2 cm/s, and because it is not directionally influenced.
Papillae that contain blood vessels with detectable flow are suspicious for malignancy.
Exacoustos and colleagues found that papillae as large as 15 mm in height and 10 mm in width (base) were present in 48% of borderline ovarian tumors but in only 4% of benign and 4% of malignant tumors. However, when the intracystic solid tissue exceeded those dimensions, the lesions were present in 48% of invasive ovarian tumors, 18% of borderline ovarian tumors, and 7% of benign masses.1
Internal echo-structure. A mass can be anechoic, a finding that usually indicates the presence of clear fluid. Mostly solid masses are echogenic. And masses that contain particulate matter, such as blood, cellular matter, or even mucous material, usually have echogenicity of a low level, often described as a “ground-glass” appearance. A mass can also have mixed echo-genicity, a finding usually found in cases involving teratoma or malignancy.
Shadowing. If it is present, it may signify the presence of an extremely dense, solid tissue, such as bone or calcification. The diagnosis of a benign teratoma (i.e., dermoid cyst) should be entertained if shadowing is present in a hyperechoic nodule or mass. Malignant masses very rarely, if ever, display frank shadowing.
Overall appearance. On rare occasions, a bizarre shape or “complex” appearance (as it is termed in most radiology reports) may indicate a malignant mass. More likely it indicates the presence of a teratoma, cystadenoma, or even an atypical corpus luteum. In some reports generated by US laboratories, the term “complex” is applied to all structures other than simple cysts.
Size. The size of a mass can be misleading, as small ovarian lesions with the appropriate sonographic characteristics may be malignant and some larger ones without those characteristics may not be. However, it is understood that the larger an ovarian lesion, the more likely it is a tumor. One important distinction: The amount of fluid in a cystic structure or the amount of old blood in an endometrioma is not the disease process…it is the byproduct of the process. So an 8-cm endometrioma may create fewer pain or fertility issues than a 2- or 3-cm endometrioma. Similarly, the amount of “chocolate” fluid is not automatically indicative of the amount of active endometriotic glands or their sequelae!2
Ascites. If it is present, it should be recorded and investigated further because it may be caused by a malignant intra-abdominal tumor.
Motion tenderness. If the to-and-fro movement of the vaginal probe elicits any motion tenderness, it, too, should be documented. It may be a sign of pelvic peritonitis. In such cases, an “ominous appearing” adnexal finding may represent an inflammatory, rather than malignant, mass.
One of the components of extensive evaluation of the adnexae in general and ovaries in particular is color or power Doppler interrogation—or both.
Tumors contain a relatively large number of pathologic blood vessels that lack the muscular layer found in normal blood vessels and, as a result, demonstrate lower resistance to flow. Diastolic flow is high in these vessels, and resistance and pulsatility indices are low.
We also pay attention when these blood vessels have a tortuous appearance, changes in caliber, anastomoses, and vascular lakes.3 The more tortuous the vessels, with multiple inter-vessel connections and dilatations with changing calibers, the greater the risk of malignancy.4 No less important is the presence of a vessel within a “complex” ovarian mass. A centrally located vessel (also called a “lead vessel”) is suspicious for malignancy.5
A gallery of non-neoplastic ovarian masses
Non-neoplastic cysts are, by far, the most common structures of the ovary. They may be functional, as in the case of the follicles, corpus luteum, and theca lutein cysts, or they may be nonfunctional, as in serous cysts and endometriomas. (As we noted in Part 1, do not call the follicles and corpus luteum “cysts” because this designation suggests pathology.)6

FIGURE 2 Simple cyst
This cyst is anechoic and unilocular with thin walls and no papillae.
Functional cysts
Functional cysts, also known as “simple” cysts, may grow as large as 4 to 5 cm in diameter (Figure 2). They are typically unilocular, anechoic, and thin-walled, with no papillae, and almost never malignant. They usually resolve and require no treatment unless rupture or torsion occurs. Except for the corpus luteum, they have no increased blood flow, and need be viewed only by transvaginal ultrasonography (TVS).
The corpus luteum also can be recognized by TVS. It can exhibit any of a variety of internal structures and echo patterns, due to the multitude of shapes of the blood or clot that can be seen within it (Figure 3).

FIGURE 3 Corpus luteum
A–C. Gray-scale, color Doppler, and power Doppler images, respectively, of a typical corpus luteum. B and C show the enveloping vessels, or “ring of fire.” D. A rather typical gray-scale appearance with a mesh-like, linear internal texture. E. A common feature of the corpus luteum is a linear interphase (arrow) between the clot (c) and the liquified serum (s).
The corpus luteum is typically enveloped by blood vessels, visible on color Doppler as what is called a “ring of fire.” It regresses without intervention. In hyperstimulated ovaries, however, more than one may be present; this poses a real diagnostic challenge when ectopic pregnancy is suspected because it is difficult to differentiate the two entities.
Because the corpus luteum can sometimes resemble some types of ovarian tumors on TVS, imaging during the secretory phase of the cycle in a woman of reproductive age is not ideal. Instead, she should be scanned (or rescanned) between days 5 and 9 of the cycle.

FIGURE 4 Hormonally stimulated ovaries
A, B. The right and left ovaries stimulated by follicle-stimulating hormone preparation (arrow points to hilus). C. An ovary stimulated by clomiphene.
Lutein cysts may reach 5 to 10 cm in diameter. They generally have a thick wall, are multilocular, and typically occur after hormonal induction of ovulation (Figure 4). They also can occur in diabetes, molar pregnancy, and hydrops fetalis. We have seen a unilateral theca lutein cyst in a normal pregnancy (Figure 5). No treatment is necessary unless rupture or torsion occurs.

FIGURE 5 Lutein cysts
A–C. The typical “stained glass” appearance of three lutein cysts of the right ovary in a pregnant patient. D. Color Doppler image of the ovary demonstrating high-velocity flow (peak systolic velocity of 20.4 cm/s).
Serous cysts
These cysts can reach 4 cm in diameter, have smooth walls with no papillae, are unilocular, and occur most often during menopause. No pathological blood flow is visible in their walls. Most gynecologists follow them (Figure 6).1,7

FIGURE 6 Serous cyst
A. Right ovary containing the cyst. B. Normal left ovary. C. Power Doppler interrogation showing no particular flow in the walls of a serous cyst.
Endometriomas
After the simple cyst, the endometrioma is the most prevalent ovarian or adnexal cyst (Figure 7). It usually has a thick wall and is filled with homogeneous fluid with low-level echo-genicity. It can reach 10 cm in size, and many are bilateral. It is sometimes called a “chocolate” cyst because of its dark blood content.

FIGURE 7 Endometriomas
Endometriomas have low echogenicity. A. Unilateral, unilocular cyst with thin walls. B. Bilateral endometriomas. C. Blood flow in a solid or papillary component of the endometrioma is an occasional finding. It should be investigated further because of the risk that it represents endometrioid cancer.
Endometriomas do not resolve; they usually require surgical excision, although very small ones wholly contained within an ovary are often managed medically or expectantly.
These masses rarely (<1%) give rise to endometrioid carcinoma. Should an endometrioma contain papillae with blood vessels, it is extremely suspicious for endometrioid cancer.

FIGURE 8 Cystic fibromas
A. Sonographic image shows a thin wall and hyperechoic, small mural nodules. B. Macroscopic appearance of an area of internal papillary excrescences. C. Measurement of the small, mural nodules. D. Lack of blood flow in the small papillae, a typical finding on color or power Doppler. E, F. Blood flow in the wall of the cyst and in the mural nodules.
Ovarian fibromas
A fibroma is a slow-growing, benign, solid ovarian tumor. It usually has a cystic component and then is called a cystadenofibroma.
The cystic variety is filled with anechoic fluid and has a thin wall. However, its pathognomonic feature is the small (2–3 mm), extremely hyperechoic mural nodules (papillae) it contains (Figure 8A–C). In the overwhelming majority of cases, no blood vessels are detectable, and the mass is unilocular (Figure 8D–E). It can be recognized in the ovary by the semilunar shape of the tissue surrounding it (crescent sign). The differential diagnosis includes the simple (serous) cyst.
The solid fibroma has a myometrium-like texture, with few or no detectable blood vessels in the stroma. The differential diagnosis includes the Brenner tumor and the Krukenberg tumor.
According to a technology assessment from the Agency for Healthcare Research and Quality (AHRQ), “conventional gray-scale ultrasonography is the most common imaging modality used to differentiate benign from malignant adnexal masses. Especially with the advent of high-frequency transvaginal probes, the quality of the images allows description of the gross anatomic features of the lesion.”8 This descriptive ability is limited, however, “by the great variability of macroscopic characteristics of both benign and malignant masses. Furthermore, the technique is operator dependent.”8
To overcome these challenges, some experts have developed ultrasonographic (US) morphologic scoring systems, which assign a value to individual characteristics. Lerner and colleagues devised a 4-point system:
| Characteristic | Points | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Wall structure | Smooth or small irregularities (<3 mm) | Solid or not applicable | Papillarities larger than 3 mm | |
| Shadowing | Yes | No | ||
| Septation | None or thin (<3 mm) | Thick (≥3 mm) | ||
| Echogenicity | Sonolucent or low-level echo or echogenic core | Mixed or high | ||
The mean point value for benign masses was 1.8; for tumors of low malignant potential it was 3.9; and for malignant tumors it was 5.6 (P < .0005). Lerner and associates proposed a cutoff of 3. A score of 3 or higher, they felt, would be most predictive of malignancy, with sensitivity of 96.8% and specificity of 77%. Positive and negative predictive values were 29.4% and 99.6%, respectively.9
Almost all published scoring systems are based upon or derived from one reported by Sassone and coworkers.10 The most important and practical feature of all scoring systems is their ability to rule out malignancy.
Morphology and Doppler: A synergistic combination
As the same AHRQ report points out, “all of the diagnostic tests and scoring systems we evaluated exhibited a trade-off between sensitivity and specificity—studies of a given test that reported higher sensitivity had lower specificity, and vice versa.”8 Among evaluation methods, the combination of US morphology scores and Doppler imaging achieved the highest pooled sensitivity and specificity scores in distinguishing benign and malignant adnexal masses in postmenopausal women: 86% and 91%, respectively, according to the AHRQ report.8
Compare these figures with those of:
- Bimanual pelvic examination (45% and 90%, respectively)
- Doppler resistance index (72% and 90%)
- Doppler pulsatility index (80% and 73%)
- presence of blood vessels (88% and 78%).
The combination of US morphology scores and Doppler was comparable to the pooled sensitivity and specificity of magnetic resonance imaging (91% and 88%, respectively) and superior to computed tomography (90% and 75%, respectively).
Why the need to know?
Discrimination between benign and malignant masses serves a number of purposes, depending on the setting.
For example, if a symptomatic woman is found to have an adnexal mass, it is important to identify the type of mass causing the symptoms to determine the best course of treatment. And because surgery may be one of the treatment options, it is helpful to know whether a mass is likely to be malignant so that the patient can be referred to a specialist or center that has optimal surgical expertise.8
Some asymptomatic masses may be identified during the annual bimanual pelvic examination recommended by ACOG or during pregnancy-related US imaging. In this setting, it is important to ascertain whether the mass is likely to be malignant so that the patient can be referred to a specialist, if necessary. In addition, thorough assessment of the mass can help “avoid unnecessary diagnostic procedures, including surgery, and anxiety in women with asymptomatic, nonmalignant conditions. In some cases, there may be a rationale for removing certain asymptomatic benign lesions, including prevention of malignant transformation; prevention of ovarian torsion”; and prevention of rupture. Surgery may also be appropriate to avert the need for more complicated surgery in the future or to enhance fertility.8 —Janelle Yates, Senior Editor
Stay tuned!
Next issue, in Part 3 of this series, we will review the use of imaging in the investigation of ovarian neoplasms, both benign and malignant, with an abundance of US images to accompany our discussion.
We want to hear from you! Tell us what you think.
1. Exacoustos C, Romanini ME, Rinaldo D, et al. Preoperative sonographic features of borderline ovarian tumors. Ultrasound Obstet Gynecol. 2004;25(1):50-59.
2. Rulin MC, Preston AL. Adnexal masses in postmenopausal women. Obstet Gynecol. 1987;70(4):578-581.
3. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultraound Med. 2005;24(3):255-258.
4. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnos is of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874-882.
5. Testa AC, Mancari R, Di Legge A, et al. The “lead vessel”: a vascular ultrasound feature of metastasis in the ovaries. Ultrasound Obstet Gynecol. 2008;31:218-221.
6. Goldstein SR. Postmenopausal adnexal cysts: how clinical management has evolved. Am J Obstet Gynecol. 1996;175(6):1496-1501.
7. Levine D, Gosink BB, Wolf S, Feldesman MR, Pretorius D. Simple adnexal cysts: the natural history in postmenopausal women. Radiology. 1992;184(3):653-659.
8. Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Evidence Report Technol Assess. 2006;Feb;(130):1-145.
9. Lerner JP, Timor-Tritsch IE, Federman A, Abramovich G. Transvaginal ultrasonographic characterization of ovarian masses with an improved, weighted scoring system. Am J Obstet Gynecol. 1994;170(1 Pt 1):81-85.
10. Sassone AM, Timor-Tritsch IE, Artner A, et al. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 2001;78:70-76.
1. Exacoustos C, Romanini ME, Rinaldo D, et al. Preoperative sonographic features of borderline ovarian tumors. Ultrasound Obstet Gynecol. 2004;25(1):50-59.
2. Rulin MC, Preston AL. Adnexal masses in postmenopausal women. Obstet Gynecol. 1987;70(4):578-581.
3. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultraound Med. 2005;24(3):255-258.
4. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnos is of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874-882.
5. Testa AC, Mancari R, Di Legge A, et al. The “lead vessel”: a vascular ultrasound feature of metastasis in the ovaries. Ultrasound Obstet Gynecol. 2008;31:218-221.
6. Goldstein SR. Postmenopausal adnexal cysts: how clinical management has evolved. Am J Obstet Gynecol. 1996;175(6):1496-1501.
7. Levine D, Gosink BB, Wolf S, Feldesman MR, Pretorius D. Simple adnexal cysts: the natural history in postmenopausal women. Radiology. 1992;184(3):653-659.
8. Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Evidence Report Technol Assess. 2006;Feb;(130):1-145.
9. Lerner JP, Timor-Tritsch IE, Federman A, Abramovich G. Transvaginal ultrasonographic characterization of ovarian masses with an improved, weighted scoring system. Am J Obstet Gynecol. 1994;170(1 Pt 1):81-85.
10. Sassone AM, Timor-Tritsch IE, Artner A, et al. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 2001;78:70-76.
Skilled US imaging of the adnexal mass: Starting point
No doubt about it: Scanning the adnexae is the most challenging task in gynecologic ultrasonography (US). There are many reasons for the difficulty, but probably none more important than the fact that you are expected to reach a conclusion about what you see—or at least narrow the differential diagnosis.
Some ultrasound laboratories try to hedge their bets, sending the referring physician a report that is nothing more than an exhaustive differential diagnosis, similar to what we see in textbooks. Such a list is useless to a referring clinician, who has probably already considered most of the possibilities and involved the lab to help narrow them down. Labs that send such reports are usually trying to protect themselves from litigation—typically involving cases in which ovarian cancer was missed—or attempting to accomplish a “self-referral” by encouraging further imaging.1
The referring physician is not perfect, either. In our practice, we often receive reports like the following terse description:
A complex cyst was seen in the adnexa. Ovarian malignancy cannot be ruled out.
That’s it. No description of the actual sonographic characteristics. No Doppler velocity flow studies. Yet, the few remarks include a mention of malignancy, and the provider often suggests that “additional imaging such as CT and MRI should be considered.”
When we scrutinize the sonographic images upon which these reports are based, we often discover a corpus luteum, cystic teratoma, benign cystadenoma, endometrioma, or, even, a simple cyst.
The need for competency is compelling
Now that gynecologic US has matured as a field in its own right, the referring physician should expect much more from a laboratory’s pelvic scan than a long recitation of potential diagnoses. And the lab should expect more basic information from the referring provider.
That is the primary reason for this four-part series—to help you identify some of the most prevalent adnexal masses, so that you can exclude cases that are no cause for concern, such as a corpus luteum, and refer patients who really do need additional imaging and expertise, providing as much information in the process as you can.
In Part 1 of the series, we introduce you to basic concepts, recommend equipment, and step you through numerous fundamental scans. Part 2 will focus on nonneoplastic ovarian masses, Part 3 on ovarian neoplasms, and Part 4 on tubal entities such as ectopic pregnancy and torsion.
As much as possible, we educate you by providing actual scans that represent real cases, pointing out the elements that should grab your attention. After all, a picture paints a thousand words.
Ultrasound reveals the polycystic nature of a patient’s ovary. The hilus is prominently hyperechoic.
A few fundamental practices enhance consistency and thoroughness
Before we shift our focus to scanning techniques and interpretation of images, we’d like to offer several basic pointers.
Establish, and document, the hormonal milieu. One of the most important requirements of US imaging, particularly during the reproductive years, is determining and documenting the date of the patient’s last menstrual period (LMP). The reason? Physiologic and pathologic processes involving the reproductive organs are driven by the menstrual cycle—or by therapeutic (or pathologic) hormonal stimulation. We mark each scan with the date of the LMP. If the patient is on hormone therapy, we also mark the scan “HT.” We make these marks on the screen in a way that prevents their erasure every time the picture is frozen and unfrozen. This makes it possible for us to look at the scan days, weeks, or even years later and know what day of the cycle it represents. Every finding must be judged in light of the patient’s hormonal status.
Use a transvaginal transducer. It provides a high-resolution view of any pathology. If need be, it can be combined with a trans-abdominal transducer to afford a more deeply penetrating, panoramic view of the pelvis. We use a variety of transducers to achieve depth, color, power Doppler, and three- dimensional (3D) US.
Take a history and examine the patient. Before scanning your own patient, take a short history and perform a bimanual, palpatory pelvic exam. You may need to examine her again after the scan to verify a sonographic finding.
It is doubly important to take a history if you are scanning a referred patient. Omitting this element is no excuse for overlooking a disease or pathology.
A bimanual, palpatory pelvic exam may also be recommended for some referred patients.
A transvaginal scan is not always possible. There are a number of reasons why the transvaginal approach may not be advisable for some patients, including virginal status, atrophic postmenopausal vagina, agenesis of the vagina, and transverse vaginal septae. In such cases, the best alternative is a transrectal scan, which makes it possible to image the pelvic organs from almost exactly the same vantage point as transvaginal US.2 With proper explanation (particularly with virginal patients), the initial reluctance and apprehension can usually be assuaged.
Don’t trust the referral slip. We recommend that you read, but do not overly trust, the referral slip. It often offers little useful information.
Helpful scanning techniques
Consider applying these maneuvers:
- place your non-scanning hand on the patient’s abdomen to help mobilize the pelvic contents as the transvaginal probe slides across the organs
- use the probe as an “eye” while your palpating finger touches the cervix, uterus, ovaries, and any adnexal mass. Observe the mobility of these structures in relation to each other and the pelvic wall. This technique yields what is often referred to as the “sliding organs” sign. It is possible to identify pelvic adhesions (if the structures do not slide freely) or rule them out (if they do)
- pinpoint the origin of any pain the patient may have by touching the ovary, cervix, and any adnexal mass. This technique is important in cases of ectopic pregnancy, adnexal torsion, or inflammatory disease of the pelvis or adnexae.
Start with a basic scan of key structures
On the way “in” toward the adnexae, take the time to look at the bladder and urethra (FIGURE 1). Some common pathologies of the bladder are diverticulae; calculi; and a thick and vascular bladder wall suggestive of cancer or endometrioma. Ask the patient whether she has experienced any hematuria if any of these pathologies are detected.
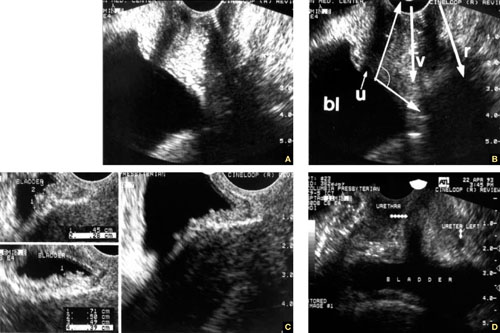
FIGURE 1 Imaging the bladder
(A, B) The bladder (bl), urethra (u), vagina (v), and rectum (r) appear in their proper relation in this sagittal view. The posterior angle of the bladder is also apparent (arrow closing an angle of about 110°). (C) Excessive thickness of the bladder wall suggests that this patient has cystitis. (D) Coronal view of the bladder and urethra (solid arrows).
Also take a look at the cervix, searching for Nabothian cysts, endocervical polyps, extreme vascularization (a possible indicator of cervical cancer), and prolapsing submucous myomas (FIGURE 2).
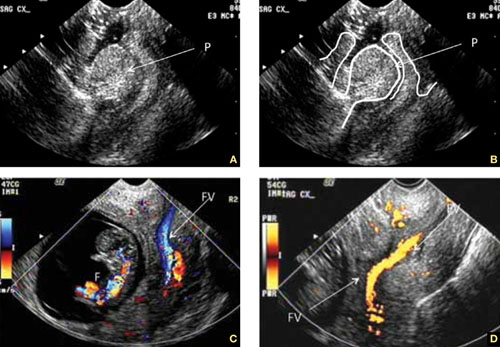
FIGURE 2 Uncommon pathology
A submucous myoma prolapses into the cervical canal in a 13-week intrauterine pregnancy. (A) Grayscale sagittal image and (B) outline view of the same image. (C,D) Color and power Doppler images show the blood supply to the myoma from the uterine cavity.
While you are looking, attempt to scan both kidneys and Morrison’s pouch. Large adnexal masses or fibroids of the uterus may put pressure on the ureter, causing various degrees of hydronephrosis.
Sometimes, when the right kidney is correctly imaged below the liver, you may detect fluid in the space between them (called Morrison’s space). This information has clear value that may aid in diagnosing the main pathology (i.e., ruptured tubal pregnancy, ascites, etc.).
Imaging of the ovaries
The best way to scan the ovaries is to use a high-frequency (4–9 MHz) transvaginal probe. In general, as the frequency of the probe increases, so does resolution of the image—but the ability to penetrate tissue diminishes. For this reason, for abdominal imaging, a 3-MHz probe is often used. For a transvaginal scan, in which the probe can be placed near an ovary, a 5-MHz probe is common. And for a scan of, say, the parathyroid gland, a 12-MHz probe is utilized.
During the reproductive years, the ovaries can be localized by their sonographic markers—the follicles (FIGURE 3A). The ovaries usually lie near the large hypogastric blood vessels (FIGURE 3B). During the secretory phase of the cycle, look for the corpus luteum, switching on the color or power Doppler mode to help locate it (FIGURES 3C, 3D).
The ovaries usually can be distinguished by their relative anechoic sono-texture in juxtaposition to the surrounding, constantly peristalsing small bowel. This strategy is the only help for spotting the ovaries in menopause, when they lose their follicles.
The size of the ovaries may be an important indicator of pathology. During the reproductive years, mean size is 8 mL (standard deviation [SD], 2–3 mL; range, 5–15 mL). Post-menopausal ovaries are small, with a mean size of 3.6 mL (SD, 1.4 mL; range, 1–14 mL).
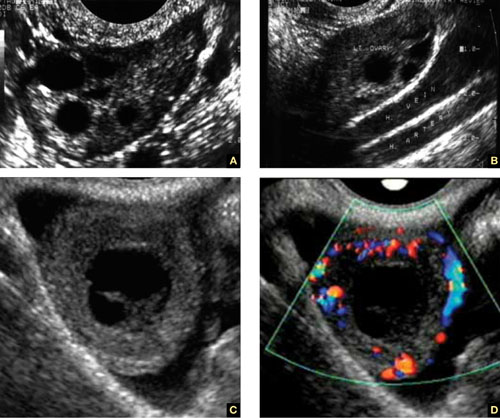
FIGURE 3 How to spot the ovaries
(A) Anechoic follicles are markers of the ovary during the reproductive years. (B) The ovaries in relation to the hypogastric vessels. (C) Gray-scale image of the corpus luteum and the same image in (D) color Doppler.
A word about terminology: Don’t call follicles “cysts”
During a normal menstrual cycle, one or more follicles mature, reaching about 2 to 2.5 cm in diameter around mid-cycle. Do not call these follicles “cysts” or “follicular cysts.” They are follicles. Calling them cysts, or even including the word cyst in their description, suggests to many gynecology and radiology providers—and to patients themselves—the idea of pathology.1
An exception to that rule: An ovary that is larger than 12 to 14 mL and has a hyperechoic hilus and more than 12 small (4–5 mm), peripherally pushed follicles is usually called “polycystic” (FIGURE 4).3 However, not every ovary that fulfills these sonographic criteria is indeed polycystic. At times normal ovaries may contain multiple follicles without any of the clinical or laboratory indications of a polycystic ovary. In these cases, the ovary may be of normal size and may lack a hyperechoic hilus with rich hilar vascularity. We term such ovaries “multicystic” in their appearance.

FIGURE 4 The polycystic ovary
(A) Gray-scale image of a polycystic ovary. The typical hyperechoic hilus is evident (H). (B) Gross pathologic section of a polycystic ovary. (C) 3D orthogonal planes of a large ovary with a multitude of small follicles pushed peripherally by a voluminous hyperechoic hilus. (D) 3D inversion rendering of the same ovary.
We employ 3D inversion rendering to better see and count the number of follicles (FIGURE 4D).
An ovary can have a polycystic appearance in the following clinical situations:
- hyperthyroid state (36% of affected women)
- hyperprolactinemia (50%)
- hypothalamic hypogonadism (24%).
It also can appear polycystic for no apparent reason.
Stay tuned!
Next month, we continue our focus on adnexal imaging by describing (and showing) nonneoplastic ovarian masses.
We want to hear from you! Tell us what you think.
1. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultrasound Med. 2005;24(3):255-258.
2. Timor-Tritsch IE, Monteagudo A, Rebarber A, Goldstein SR, Tsymbal T. Transrectal scanning an alternative when transvaginal scanning is not feasible. Ultrasound Obstet Gynecol. 2003;21(5):443-479.
3. Abdel Gadir A, Khatim MS, Mowafi RS, Alnaser HM, Muharib NS, Shaw RW. Implications of ultrasonically diagnosed polycystic ovaries. II. Studies of dynamic and pulsatile hormonal patterns. Human Reprod. 1992;7(4):458-461.
No doubt about it: Scanning the adnexae is the most challenging task in gynecologic ultrasonography (US). There are many reasons for the difficulty, but probably none more important than the fact that you are expected to reach a conclusion about what you see—or at least narrow the differential diagnosis.
Some ultrasound laboratories try to hedge their bets, sending the referring physician a report that is nothing more than an exhaustive differential diagnosis, similar to what we see in textbooks. Such a list is useless to a referring clinician, who has probably already considered most of the possibilities and involved the lab to help narrow them down. Labs that send such reports are usually trying to protect themselves from litigation—typically involving cases in which ovarian cancer was missed—or attempting to accomplish a “self-referral” by encouraging further imaging.1
The referring physician is not perfect, either. In our practice, we often receive reports like the following terse description:
A complex cyst was seen in the adnexa. Ovarian malignancy cannot be ruled out.
That’s it. No description of the actual sonographic characteristics. No Doppler velocity flow studies. Yet, the few remarks include a mention of malignancy, and the provider often suggests that “additional imaging such as CT and MRI should be considered.”
When we scrutinize the sonographic images upon which these reports are based, we often discover a corpus luteum, cystic teratoma, benign cystadenoma, endometrioma, or, even, a simple cyst.
The need for competency is compelling
Now that gynecologic US has matured as a field in its own right, the referring physician should expect much more from a laboratory’s pelvic scan than a long recitation of potential diagnoses. And the lab should expect more basic information from the referring provider.
That is the primary reason for this four-part series—to help you identify some of the most prevalent adnexal masses, so that you can exclude cases that are no cause for concern, such as a corpus luteum, and refer patients who really do need additional imaging and expertise, providing as much information in the process as you can.
In Part 1 of the series, we introduce you to basic concepts, recommend equipment, and step you through numerous fundamental scans. Part 2 will focus on nonneoplastic ovarian masses, Part 3 on ovarian neoplasms, and Part 4 on tubal entities such as ectopic pregnancy and torsion.
As much as possible, we educate you by providing actual scans that represent real cases, pointing out the elements that should grab your attention. After all, a picture paints a thousand words.

Ultrasound reveals the polycystic nature of a patient’s ovary. The hilus is prominently hyperechoic.
A few fundamental practices enhance consistency and thoroughness
Before we shift our focus to scanning techniques and interpretation of images, we’d like to offer several basic pointers.
Establish, and document, the hormonal milieu. One of the most important requirements of US imaging, particularly during the reproductive years, is determining and documenting the date of the patient’s last menstrual period (LMP). The reason? Physiologic and pathologic processes involving the reproductive organs are driven by the menstrual cycle—or by therapeutic (or pathologic) hormonal stimulation. We mark each scan with the date of the LMP. If the patient is on hormone therapy, we also mark the scan “HT.” We make these marks on the screen in a way that prevents their erasure every time the picture is frozen and unfrozen. This makes it possible for us to look at the scan days, weeks, or even years later and know what day of the cycle it represents. Every finding must be judged in light of the patient’s hormonal status.
Use a transvaginal transducer. It provides a high-resolution view of any pathology. If need be, it can be combined with a trans-abdominal transducer to afford a more deeply penetrating, panoramic view of the pelvis. We use a variety of transducers to achieve depth, color, power Doppler, and three- dimensional (3D) US.
Take a history and examine the patient. Before scanning your own patient, take a short history and perform a bimanual, palpatory pelvic exam. You may need to examine her again after the scan to verify a sonographic finding.
It is doubly important to take a history if you are scanning a referred patient. Omitting this element is no excuse for overlooking a disease or pathology.
A bimanual, palpatory pelvic exam may also be recommended for some referred patients.
A transvaginal scan is not always possible. There are a number of reasons why the transvaginal approach may not be advisable for some patients, including virginal status, atrophic postmenopausal vagina, agenesis of the vagina, and transverse vaginal septae. In such cases, the best alternative is a transrectal scan, which makes it possible to image the pelvic organs from almost exactly the same vantage point as transvaginal US.2 With proper explanation (particularly with virginal patients), the initial reluctance and apprehension can usually be assuaged.
Don’t trust the referral slip. We recommend that you read, but do not overly trust, the referral slip. It often offers little useful information.
Helpful scanning techniques
Consider applying these maneuvers:
- place your non-scanning hand on the patient’s abdomen to help mobilize the pelvic contents as the transvaginal probe slides across the organs
- use the probe as an “eye” while your palpating finger touches the cervix, uterus, ovaries, and any adnexal mass. Observe the mobility of these structures in relation to each other and the pelvic wall. This technique yields what is often referred to as the “sliding organs” sign. It is possible to identify pelvic adhesions (if the structures do not slide freely) or rule them out (if they do)
- pinpoint the origin of any pain the patient may have by touching the ovary, cervix, and any adnexal mass. This technique is important in cases of ectopic pregnancy, adnexal torsion, or inflammatory disease of the pelvis or adnexae.
Start with a basic scan of key structures
On the way “in” toward the adnexae, take the time to look at the bladder and urethra (FIGURE 1). Some common pathologies of the bladder are diverticulae; calculi; and a thick and vascular bladder wall suggestive of cancer or endometrioma. Ask the patient whether she has experienced any hematuria if any of these pathologies are detected.

FIGURE 1 Imaging the bladder
(A, B) The bladder (bl), urethra (u), vagina (v), and rectum (r) appear in their proper relation in this sagittal view. The posterior angle of the bladder is also apparent (arrow closing an angle of about 110°). (C) Excessive thickness of the bladder wall suggests that this patient has cystitis. (D) Coronal view of the bladder and urethra (solid arrows).
Also take a look at the cervix, searching for Nabothian cysts, endocervical polyps, extreme vascularization (a possible indicator of cervical cancer), and prolapsing submucous myomas (FIGURE 2).

FIGURE 2 Uncommon pathology
A submucous myoma prolapses into the cervical canal in a 13-week intrauterine pregnancy. (A) Grayscale sagittal image and (B) outline view of the same image. (C,D) Color and power Doppler images show the blood supply to the myoma from the uterine cavity.
While you are looking, attempt to scan both kidneys and Morrison’s pouch. Large adnexal masses or fibroids of the uterus may put pressure on the ureter, causing various degrees of hydronephrosis.
Sometimes, when the right kidney is correctly imaged below the liver, you may detect fluid in the space between them (called Morrison’s space). This information has clear value that may aid in diagnosing the main pathology (i.e., ruptured tubal pregnancy, ascites, etc.).
Imaging of the ovaries
The best way to scan the ovaries is to use a high-frequency (4–9 MHz) transvaginal probe. In general, as the frequency of the probe increases, so does resolution of the image—but the ability to penetrate tissue diminishes. For this reason, for abdominal imaging, a 3-MHz probe is often used. For a transvaginal scan, in which the probe can be placed near an ovary, a 5-MHz probe is common. And for a scan of, say, the parathyroid gland, a 12-MHz probe is utilized.
During the reproductive years, the ovaries can be localized by their sonographic markers—the follicles (FIGURE 3A). The ovaries usually lie near the large hypogastric blood vessels (FIGURE 3B). During the secretory phase of the cycle, look for the corpus luteum, switching on the color or power Doppler mode to help locate it (FIGURES 3C, 3D).
The ovaries usually can be distinguished by their relative anechoic sono-texture in juxtaposition to the surrounding, constantly peristalsing small bowel. This strategy is the only help for spotting the ovaries in menopause, when they lose their follicles.
The size of the ovaries may be an important indicator of pathology. During the reproductive years, mean size is 8 mL (standard deviation [SD], 2–3 mL; range, 5–15 mL). Post-menopausal ovaries are small, with a mean size of 3.6 mL (SD, 1.4 mL; range, 1–14 mL).

FIGURE 3 How to spot the ovaries
(A) Anechoic follicles are markers of the ovary during the reproductive years. (B) The ovaries in relation to the hypogastric vessels. (C) Gray-scale image of the corpus luteum and the same image in (D) color Doppler.
A word about terminology: Don’t call follicles “cysts”
During a normal menstrual cycle, one or more follicles mature, reaching about 2 to 2.5 cm in diameter around mid-cycle. Do not call these follicles “cysts” or “follicular cysts.” They are follicles. Calling them cysts, or even including the word cyst in their description, suggests to many gynecology and radiology providers—and to patients themselves—the idea of pathology.1
An exception to that rule: An ovary that is larger than 12 to 14 mL and has a hyperechoic hilus and more than 12 small (4–5 mm), peripherally pushed follicles is usually called “polycystic” (FIGURE 4).3 However, not every ovary that fulfills these sonographic criteria is indeed polycystic. At times normal ovaries may contain multiple follicles without any of the clinical or laboratory indications of a polycystic ovary. In these cases, the ovary may be of normal size and may lack a hyperechoic hilus with rich hilar vascularity. We term such ovaries “multicystic” in their appearance.

FIGURE 4 The polycystic ovary
(A) Gray-scale image of a polycystic ovary. The typical hyperechoic hilus is evident (H). (B) Gross pathologic section of a polycystic ovary. (C) 3D orthogonal planes of a large ovary with a multitude of small follicles pushed peripherally by a voluminous hyperechoic hilus. (D) 3D inversion rendering of the same ovary.
We employ 3D inversion rendering to better see and count the number of follicles (FIGURE 4D).
An ovary can have a polycystic appearance in the following clinical situations:
- hyperthyroid state (36% of affected women)
- hyperprolactinemia (50%)
- hypothalamic hypogonadism (24%).
It also can appear polycystic for no apparent reason.
Stay tuned!
Next month, we continue our focus on adnexal imaging by describing (and showing) nonneoplastic ovarian masses.
We want to hear from you! Tell us what you think.
No doubt about it: Scanning the adnexae is the most challenging task in gynecologic ultrasonography (US). There are many reasons for the difficulty, but probably none more important than the fact that you are expected to reach a conclusion about what you see—or at least narrow the differential diagnosis.
Some ultrasound laboratories try to hedge their bets, sending the referring physician a report that is nothing more than an exhaustive differential diagnosis, similar to what we see in textbooks. Such a list is useless to a referring clinician, who has probably already considered most of the possibilities and involved the lab to help narrow them down. Labs that send such reports are usually trying to protect themselves from litigation—typically involving cases in which ovarian cancer was missed—or attempting to accomplish a “self-referral” by encouraging further imaging.1
The referring physician is not perfect, either. In our practice, we often receive reports like the following terse description:
A complex cyst was seen in the adnexa. Ovarian malignancy cannot be ruled out.
That’s it. No description of the actual sonographic characteristics. No Doppler velocity flow studies. Yet, the few remarks include a mention of malignancy, and the provider often suggests that “additional imaging such as CT and MRI should be considered.”
When we scrutinize the sonographic images upon which these reports are based, we often discover a corpus luteum, cystic teratoma, benign cystadenoma, endometrioma, or, even, a simple cyst.
The need for competency is compelling
Now that gynecologic US has matured as a field in its own right, the referring physician should expect much more from a laboratory’s pelvic scan than a long recitation of potential diagnoses. And the lab should expect more basic information from the referring provider.
That is the primary reason for this four-part series—to help you identify some of the most prevalent adnexal masses, so that you can exclude cases that are no cause for concern, such as a corpus luteum, and refer patients who really do need additional imaging and expertise, providing as much information in the process as you can.
In Part 1 of the series, we introduce you to basic concepts, recommend equipment, and step you through numerous fundamental scans. Part 2 will focus on nonneoplastic ovarian masses, Part 3 on ovarian neoplasms, and Part 4 on tubal entities such as ectopic pregnancy and torsion.
As much as possible, we educate you by providing actual scans that represent real cases, pointing out the elements that should grab your attention. After all, a picture paints a thousand words.

Ultrasound reveals the polycystic nature of a patient’s ovary. The hilus is prominently hyperechoic.
A few fundamental practices enhance consistency and thoroughness
Before we shift our focus to scanning techniques and interpretation of images, we’d like to offer several basic pointers.
Establish, and document, the hormonal milieu. One of the most important requirements of US imaging, particularly during the reproductive years, is determining and documenting the date of the patient’s last menstrual period (LMP). The reason? Physiologic and pathologic processes involving the reproductive organs are driven by the menstrual cycle—or by therapeutic (or pathologic) hormonal stimulation. We mark each scan with the date of the LMP. If the patient is on hormone therapy, we also mark the scan “HT.” We make these marks on the screen in a way that prevents their erasure every time the picture is frozen and unfrozen. This makes it possible for us to look at the scan days, weeks, or even years later and know what day of the cycle it represents. Every finding must be judged in light of the patient’s hormonal status.
Use a transvaginal transducer. It provides a high-resolution view of any pathology. If need be, it can be combined with a trans-abdominal transducer to afford a more deeply penetrating, panoramic view of the pelvis. We use a variety of transducers to achieve depth, color, power Doppler, and three- dimensional (3D) US.
Take a history and examine the patient. Before scanning your own patient, take a short history and perform a bimanual, palpatory pelvic exam. You may need to examine her again after the scan to verify a sonographic finding.
It is doubly important to take a history if you are scanning a referred patient. Omitting this element is no excuse for overlooking a disease or pathology.
A bimanual, palpatory pelvic exam may also be recommended for some referred patients.
A transvaginal scan is not always possible. There are a number of reasons why the transvaginal approach may not be advisable for some patients, including virginal status, atrophic postmenopausal vagina, agenesis of the vagina, and transverse vaginal septae. In such cases, the best alternative is a transrectal scan, which makes it possible to image the pelvic organs from almost exactly the same vantage point as transvaginal US.2 With proper explanation (particularly with virginal patients), the initial reluctance and apprehension can usually be assuaged.
Don’t trust the referral slip. We recommend that you read, but do not overly trust, the referral slip. It often offers little useful information.
Helpful scanning techniques
Consider applying these maneuvers:
- place your non-scanning hand on the patient’s abdomen to help mobilize the pelvic contents as the transvaginal probe slides across the organs
- use the probe as an “eye” while your palpating finger touches the cervix, uterus, ovaries, and any adnexal mass. Observe the mobility of these structures in relation to each other and the pelvic wall. This technique yields what is often referred to as the “sliding organs” sign. It is possible to identify pelvic adhesions (if the structures do not slide freely) or rule them out (if they do)
- pinpoint the origin of any pain the patient may have by touching the ovary, cervix, and any adnexal mass. This technique is important in cases of ectopic pregnancy, adnexal torsion, or inflammatory disease of the pelvis or adnexae.
Start with a basic scan of key structures
On the way “in” toward the adnexae, take the time to look at the bladder and urethra (FIGURE 1). Some common pathologies of the bladder are diverticulae; calculi; and a thick and vascular bladder wall suggestive of cancer or endometrioma. Ask the patient whether she has experienced any hematuria if any of these pathologies are detected.

FIGURE 1 Imaging the bladder
(A, B) The bladder (bl), urethra (u), vagina (v), and rectum (r) appear in their proper relation in this sagittal view. The posterior angle of the bladder is also apparent (arrow closing an angle of about 110°). (C) Excessive thickness of the bladder wall suggests that this patient has cystitis. (D) Coronal view of the bladder and urethra (solid arrows).
Also take a look at the cervix, searching for Nabothian cysts, endocervical polyps, extreme vascularization (a possible indicator of cervical cancer), and prolapsing submucous myomas (FIGURE 2).

FIGURE 2 Uncommon pathology
A submucous myoma prolapses into the cervical canal in a 13-week intrauterine pregnancy. (A) Grayscale sagittal image and (B) outline view of the same image. (C,D) Color and power Doppler images show the blood supply to the myoma from the uterine cavity.
While you are looking, attempt to scan both kidneys and Morrison’s pouch. Large adnexal masses or fibroids of the uterus may put pressure on the ureter, causing various degrees of hydronephrosis.
Sometimes, when the right kidney is correctly imaged below the liver, you may detect fluid in the space between them (called Morrison’s space). This information has clear value that may aid in diagnosing the main pathology (i.e., ruptured tubal pregnancy, ascites, etc.).
Imaging of the ovaries
The best way to scan the ovaries is to use a high-frequency (4–9 MHz) transvaginal probe. In general, as the frequency of the probe increases, so does resolution of the image—but the ability to penetrate tissue diminishes. For this reason, for abdominal imaging, a 3-MHz probe is often used. For a transvaginal scan, in which the probe can be placed near an ovary, a 5-MHz probe is common. And for a scan of, say, the parathyroid gland, a 12-MHz probe is utilized.
During the reproductive years, the ovaries can be localized by their sonographic markers—the follicles (FIGURE 3A). The ovaries usually lie near the large hypogastric blood vessels (FIGURE 3B). During the secretory phase of the cycle, look for the corpus luteum, switching on the color or power Doppler mode to help locate it (FIGURES 3C, 3D).
The ovaries usually can be distinguished by their relative anechoic sono-texture in juxtaposition to the surrounding, constantly peristalsing small bowel. This strategy is the only help for spotting the ovaries in menopause, when they lose their follicles.
The size of the ovaries may be an important indicator of pathology. During the reproductive years, mean size is 8 mL (standard deviation [SD], 2–3 mL; range, 5–15 mL). Post-menopausal ovaries are small, with a mean size of 3.6 mL (SD, 1.4 mL; range, 1–14 mL).

FIGURE 3 How to spot the ovaries
(A) Anechoic follicles are markers of the ovary during the reproductive years. (B) The ovaries in relation to the hypogastric vessels. (C) Gray-scale image of the corpus luteum and the same image in (D) color Doppler.
A word about terminology: Don’t call follicles “cysts”
During a normal menstrual cycle, one or more follicles mature, reaching about 2 to 2.5 cm in diameter around mid-cycle. Do not call these follicles “cysts” or “follicular cysts.” They are follicles. Calling them cysts, or even including the word cyst in their description, suggests to many gynecology and radiology providers—and to patients themselves—the idea of pathology.1
An exception to that rule: An ovary that is larger than 12 to 14 mL and has a hyperechoic hilus and more than 12 small (4–5 mm), peripherally pushed follicles is usually called “polycystic” (FIGURE 4).3 However, not every ovary that fulfills these sonographic criteria is indeed polycystic. At times normal ovaries may contain multiple follicles without any of the clinical or laboratory indications of a polycystic ovary. In these cases, the ovary may be of normal size and may lack a hyperechoic hilus with rich hilar vascularity. We term such ovaries “multicystic” in their appearance.

FIGURE 4 The polycystic ovary
(A) Gray-scale image of a polycystic ovary. The typical hyperechoic hilus is evident (H). (B) Gross pathologic section of a polycystic ovary. (C) 3D orthogonal planes of a large ovary with a multitude of small follicles pushed peripherally by a voluminous hyperechoic hilus. (D) 3D inversion rendering of the same ovary.
We employ 3D inversion rendering to better see and count the number of follicles (FIGURE 4D).
An ovary can have a polycystic appearance in the following clinical situations:
- hyperthyroid state (36% of affected women)
- hyperprolactinemia (50%)
- hypothalamic hypogonadism (24%).
It also can appear polycystic for no apparent reason.
Stay tuned!
Next month, we continue our focus on adnexal imaging by describing (and showing) nonneoplastic ovarian masses.
We want to hear from you! Tell us what you think.
1. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultrasound Med. 2005;24(3):255-258.
2. Timor-Tritsch IE, Monteagudo A, Rebarber A, Goldstein SR, Tsymbal T. Transrectal scanning an alternative when transvaginal scanning is not feasible. Ultrasound Obstet Gynecol. 2003;21(5):443-479.
3. Abdel Gadir A, Khatim MS, Mowafi RS, Alnaser HM, Muharib NS, Shaw RW. Implications of ultrasonically diagnosed polycystic ovaries. II. Studies of dynamic and pulsatile hormonal patterns. Human Reprod. 1992;7(4):458-461.
1. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultrasound Med. 2005;24(3):255-258.
2. Timor-Tritsch IE, Monteagudo A, Rebarber A, Goldstein SR, Tsymbal T. Transrectal scanning an alternative when transvaginal scanning is not feasible. Ultrasound Obstet Gynecol. 2003;21(5):443-479.
3. Abdel Gadir A, Khatim MS, Mowafi RS, Alnaser HM, Muharib NS, Shaw RW. Implications of ultrasonically diagnosed polycystic ovaries. II. Studies of dynamic and pulsatile hormonal patterns. Human Reprod. 1992;7(4):458-461.