User login
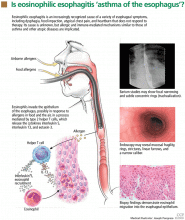
Eosinophilic esophagitis: An increasingly recognized cause of dysphagia, food impaction, and refractory heartburn
Abundant eosinophils in the esophagus were first described in 1977 in a 51-year-old man with dysphagia, chest pain, and a personal history of severe asthma and marked peripheral eosinophilia.1 In 1983, a similar case was reported in an adolescent with dysphagia.2 In both patients, large numbers of eosinophils were also noted in the duodenum, suggesting that these findings were part of a systemic hypereosinophilic syndrome.
Increased numbers of eosinophils in the gastrointestinal tract have been described in a number of diseases, including Crohn disease, connective tissue disorders, malignancy, various infections, and drug hypersensitivity reactions. However, not until 1993 was eosinophilic esophagitis described as a distinct clinical entity, consisting of isolated esophageal eosinophilia (typically more than 15 eosinophils per high-power field) in patients with dysphagia.3
Now, epidemiologic studies suggest that eosinophilic esophagitis may be as common as inflammatory bowel disease. In a study of children in Cincinnati, OH,4 the incidence was estimated at 10 per 100,000 children per year and the prevalence was estimated at 43 per 100,000. Of interest, 97% of cases were diagnosed after the year 2000.
RISING INCIDENCE, OR INCREASED RECOGNITION?
Over the last several years, the number of reported cases has increased substantially as interest in this disease has grown. The increase has been attributed in part to heightened awareness of this condition among clinicians and, hence, more esophageal biopsies being performed. Similarly, pathologists may have previously attributed esophageal eosinophilia to gastroesophageal reflux disease (GERD). However, the prevalence of eosinophilic esophagitis increased 10-fold between 1989 and 2003 in a fixed and stable adult population in Olten, Switzerland, suggesting that more than just increased awareness is responsible for this dramatic rise.5
PATHOGENESIS: SIMILAR TO OTHER ALLERGIC DISEASES?
The growing incidence of eosinophilic esophagitis parallels that of asthma, eczema, allergic rhinitis, and other atopic diseases, raising the possibility that these disorders share common environmental exposures and similar inflammatory pathways.6 The pathologic mechanisms of eosinophilic esophagitis are unknown, but emerging evidence suggests that, like other allergic diseases, it is an immune response mediated by type 2 T helper cells.
Several animal studies support this hypothesis. Mice sensitized and then exposed to aeroallergens developed both allergic airway inflammation and eosinophilic esophagitis. Interleukin 5, a cytokine involved in asthma, also helps recruit eosinophils into the esophagus, as transgenic mice deficient in interleukin 5 do not develop esophageal eosinophilia upon allergen exposure.7
Recently, eotaxin-3, a potent attractant for eosinophils, was shown to be markedly overexpressed in children with eosinophilic esophagitis compared with controls.8
Acid reflux does not appear to be a causative factor in most patients. However, reflux may play a secondary role, as some patients have experienced symptomatic, endoscopic, and histologic resolution of eosinophilic esophagitis after treatment with a proton pump inhibitor.9
GERD AND EOSINOPHILIC ESOPHAGITIS: WHAT IS THE RELATIONSHIP?
Given the high prevalence of GERD in the general population, much time and effort have been spent on comparing eosinophilic esophagitis with GERD. In fact, some endoscopic features typically seen in eosinophilic esophagitis were previously attributed to acid reflux.10
Both diseases share varying degrees of esophageal eosinophilia, and some have speculated on the relationship of eosinophilic esophagitis and GERD. Spechler et al11 recently suggested that the mucosal injury caused by acid reflux may allow swallowed allergens to penetrate an esophageal layer that is otherwise impermeable to most proteins, thereby causing mild eosinophilia. Conversely, the intense degranulation of activated eosinophils seen in eosinophilic esophagitis can trigger changes in the lower esophageal sphincter that could predispose to acid reflux.
Although their clinical and pathologic features may overlap, GERD and eosinophilic esophagitis appear to have different genetic profiles. In a recent pediatric study, Blanchard et al8 found that genes up-regulated in eosinophilic esophagitis were markedly different than those in chronic esophagitis. This suggests that while the two diseases share a constellation of symptoms, they have a different pathogenesis. Nevertheless, because of this possible overlap, the diagnosis of eosinophilic esophagitis should be made after acid reflux has been either treated or excluded with pH testing (see below).
THE ROLE OF ENVIRONMENTAL ALLERGENS AND GENETICS
Studies in children suggest that food allergies are a major contributor to eosinophilic esophagitis. In children, a strict amino-acid elemental diet has led to complete resolution of symptoms and a marked decrease in esophageal eosinophils. However, symptoms tend to recur once patients resume a regular diet.12
It is unclear if dietary modification is effective in adults. In six adults with eosinophilic esophagitis and a history of wheat and rye allergies, symptoms did not improve when these foods were eliminated and did not worsen when they were reintroduced.13
Of interest, there may be a seasonal variation of eosinophilic esophagitis, as suggested by a case report of a 21-year-old woman who had eosinophilic esophagitis that worsened symptomatically and histologically during the pollen season but resolved during winter. This is another example of the role aeroallergens may play in this disease.14
Evidence of a genetic predisposition to this disease is also growing, with a number of case reports describing multiple affected family members spanning generations.15
NEW CONSENSUS ON DIAGNOSTIC CRITERIA
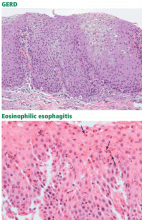
The diagnosis of eosinophilic esophagitis is made histologically, with “marked” eosinophilia on esophageal biopsies, ie, usually 15 or more eosinophils per high-power field. In contrast, a normal esophagus contains almost no eosinophils,16 and esophageal biopsies of patients with GERD usually have fewer than 10 eosinophils per high-power field, with eosinophils limited to the distal esophagus.17
However, a recent systematic review of the literature found 10 different histologic definitions of eosinophilic esophagitis, ranging from more than 5 to more than 30 eosinophils, and more than one-third of the articles included in the review did not contain any specific diagnostic criteria. Similarly, a lack of consensus on the size of a high-power field (ranging from 0.12 to 0.44 mm2) resulted in a 23-fold variability in the description of eosinophil density. Moreover, the biopsy protocols were reported in only 39% of the articles.18
In view of the growing interest in this disease, its increasing recognition, the diagnostic ambiguity described above, and concern about the role of acid reflux, consensus recommendations for its diagnosis and treatment in adults and children have recently been published.19 The current consensus definition for eosinophilic esophagitis is:
- Clinical symptoms of esophageal dysfunction (eg, dysphagia, food impaction);
- At least 15 eosinophils per high-power field; and
- Either no response to a high-dose proton pump inhibitor or normal results on pH monitoring of the distal esophagus.
CLINICAL PRESENTATION
Eosinophilic esophagitis predominantly affects men between the ages of 20 and 40, but cases in women and in younger and older patients have also been reported. Recent systematic reviews found a male-to-female ratio of approximately 3:1.
More than 90% of adults with eosinophilic esophagitis present with intermittent difficulty swallowing solids, while food impaction occurs in more than 60%. Heartburn is the only manifestation in 24% of patients. Noncardiac chest pain, vomiting, and abdominal pain have also been seen, but less frequently.
Up to 80% of patients with eosinophilic esophagitis have a history of atopic disease such as asthma, allergic rhinitis, or allergies to food or medicine. One-third to one-half of patients have peripheral eosinophilia, and up to 55% have increased serum levels of immunoglobulin E (IgE).21
In children, presenting symptoms vary with age and include feeding disorders, vomiting, abdominal pain, and dysphagia. Moreover, children with eosinophilic esophagitis have a higher frequency of atopic symptoms and peripheral eosinophilia than do adults.5,22
Although motor abnormalities are common in patients with eosinophilic esophagitis (up to 40% of patients have esophageal manometric abnormalities, including uncoordinated contractions and ineffective peristalsis),21 esophageal manometry is of limited diagnostic value and so is not recommended as a routine test.19
These findings support the theory that inflammation can lead to submucosal fibrosis, remodeling, narrowing, and eventually symptoms. Furthermore, two recent studies found that children with eosinophilic esophagitis had increased subepithelial collagen deposition in their biopsy specimens,24 suggesting increased potential for fibrosis. Also increased are transforming growth factor beta (a profibrotic cytokine) and vascular cell adhesion molecule 1, which is implicated in angiogenesis.25
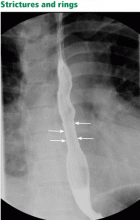
Although many patients with eosinophilic esophagitis have abnormal findings on barium radiography, the test is most useful before esophagogastroduodenoscopy to determine whether a stricture is present and potentially to guide endoscopic dilation.19
NATURAL HISTORY: CHRONIC, RELAPSING, AND MOST LIKELY BENIGN
Our understanding of the natural history of eosinophilic esophagitis is limited, but the available evidence suggests that its prognosis is favorable.
Thirty adults followed for up to 11.5 years remained in good health, maintained their weight, and had no evidence of nutritional deficiencies.26 However, all but 1 patient continued to have dysphagia, with the overall intensity of dysphagia increasing in 7 (23%), remaining stable and persistent in 11 (37%), and decreasing in the remainder. In over half of these patients, the disease impaired quality of life. The only treatment offered was endoscopic dilation, which 11 patients required. Patients with peripheral blood eosinophilia and those with more pronounced findings on endoscopy were more likely to have symptoms at follow-up.
Although dysphagia persisted, the number of eosinophils in esophageal biopsy specimens decreased significantly over time, suggesting that the intense eosinophilic infiltration seen earlier in the disease may evolve into fibrosis and remodeling, similar to that seen in asthma and other chronic atopic diseases. Unlike in Barrett esophagus, a premalignant complication of longstanding GERD, there appeared to be no increased risk of esophageal cancer in these patients with eosinophilic esophagitis during the follow-up period.26
TREATMENT
Dietary therapy
Strict elemental amino-acid diets have resulted in complete symptomatic and histologic resolution of eosinophilic esophagitis in children. However, these elemental diets often have to be given by nasogastric tube because they are unpalatable, and the disease tends to return once the diet is discontinued.27
Elimination diets, based either on avoiding the six foods most commonly associated with allergy (egg, wheat, soy, cow’s milk protein, seafood, peanuts) or on allergy testing such as skin prick testing or atopy patch testing, have shown promise in children.12,28 However, similar large-scale studies of elimination diets in adults have not been conducted.
Allergy evaluation
The recent consensus recommendations devoted considerable attention to the role of allergy evaluation.19 Between 50% and 80% of patients with eosinophilic esophagitis have a coexisting atopic disease such as atopic dermatitis, eczema, allergic rhinitis, or asthma, with a higher prevalence in children than in adults. In these patients, evidence suggests that allergy testing may predict response to therapy. Therefore, the current recommendation is for all patients with eosinophilic esophagitis to undergo a complete evaluation by an experienced allergist.
Checking the peripheral blood eosinophil count before and after treatment is reasonable, as many patients have elevated eosinophil counts that decrease after treatment.
Similarly, many patients with eosinophilic esophagitis have elevated serum total IgE levels, which suggests a concomitant atopic disease. Therefore, total IgE levels should also be checked before and after treatment. Checking for IgE against specific aeroallergens is recommended, but checking for IgE against specific food antigens has not proven beneficial at this time. Similarly, skin prick testing for aeroallergens may be useful, but not for food allergens.
Data on atopy patch testing in eosinophilic esophagitis are currently limited but promising.19
Medical therapy
Swallowed fluticasone (Flonase, using an inhaler) is the mainstay of therapy for both children and adults.
In one case series, 21 adult patients with eosinophilic esophagitis received a 6-week course of swallowed fluticasone 220 μg/puff, two to four puffs twice daily. Symptoms completely resolved in all patients for at least 4 months, and no patient needed endoscopic dilation.29
In another study, 19 patients treated with fluticasone for 4 weeks showed dramatic improvement both symptomatically and histologically. However, after 3 months, 14 (74%) of the 19 patients had a recurrence of symptoms, pointing to the chronic relapsing nature of this disease.30
The only randomized placebo-controlled trial of fluticasone to date has been in children. Konikoff et al31 found that a 3-month course of fluticasone induced remission, defined as less than one eosinophil per high-power field, in 50% of patients, compared with 9% in the placebo group.
Swallowed fluticasone is generally well tolerated, although cases of esophageal candidiasis have been reported.30
Acid suppression still has an unclear role in the treatment of eosinophilic esophagitis. As mentioned above, the disease is defined as the presence or persistence of esophageal eosinophilia after acid reflux has been maximally treated or ruled out. Most patients referred for further evaluation of eosinophilic esophagitis have tried twice-daily proton pump inhibitor therapy without success. The impact of concomitant therapy with a proton pump inhibitor has not yet been determined, but the recent guidelines suggest that these drugs are reasonable as co-therapy in patients who also have GERD symptoms.19
In patients whose symptoms do not improve with fluticasone, several other medications have been used:
Systemic corticosteroids have been used with success in both adults and children with hypereosinophilic syndromes, as well as in patients with refractory eosinophilic esophagitis, but adverse effects limit their routine and long-term use.
Cromolyn sodium (NasalCrom, Intal), a mast cell stabilizer, and montelukast (Singulair), a leukotriene inhibitor, have been used with limited success.32
Mepolizumab (Bosatria), a humanized monoclonal antibody to human interleukin 5, decreased the number of eosinophils in the esophagus and peripheral blood and improved clinical symptoms in patients with refractory eosinophilic esophagitis in a recent open-label trial.33 Further studies with mepolizumab and other biologic agents are expected.
Endoscopic dilation
Endoscopic dilation with either a guidewire or a balloon technique is often used to treat strictures and a diffusely narrowed esophagus in patients with eosinophilic esophagitis.
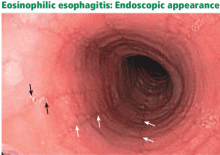
As mentioned above, a common endoscopic feature is mucosal fragility, which has been described as resembling crepe paper. Shearing and longitudinal splitting of this fragile mucosa may occur after dilation therapy.
Although esophageal dilation may be done safely in patients with eosinophilic esophagitis, the risk of perforation appears to be greater than in those with other indications for dilation.
Nevertheless, immediate symptomatic improvement has been reported in 83% of patients after dilation, with symptoms recurring in 20% within 3 to 8 months.34 Current recommendations suggest that dilation should be done cautiously in patients who have documented esophageal narrowing for which drug therapy has failed.
RECOMMENDED APPROACH
The approach to diagnosing and treating eosinophilic esophagitis begins with being aware of its prevalence. One should suspect it more in younger patients presenting with intermittent dysphagia, food impaction, or heartburn that does not respond to maximal doses of a proton pump inhibitor. Special attention should be paid to a personal or family history of allergic diseases or similar symptoms.
According to the consensus recommendations, barium esophagography is useful if the presentation suggests long-standing disease and associated esophageal stricture.
Upper endoscopy is performed, with biopsies obtained in the proximal, middle, and distal esophagus regardless of the appearance of the esophageal mucosa. Biopsies of the stomach and duodenum are also recommended to rule out eosinophilic gastroenteritis.19
After biopsy confirms the diagnosis, a trial of a proton pump inhibitor in maximum doses (usually twice daily) for 8 weeks is recommended if not already tried. If there is evidence of eosinophilic esophagitis on repeat endoscopy and biopsy studies after proton pump inhibitor therapy, the next step is swallowed fluticasone (220 μg, up to four puffs twice daily) for 6 to 8 weeks, with follow-up visits to confirm resolution of symptoms. Without a spacer, the fluticasone is swallowed after maximal expiration. Patients are instructed to avoid food and liquids for at least 30 minutes after use.
Optimal strategies for monitoring in adults have yet to be established, and following symptoms alone may or may not be sufficient.19 Our approach is to follow for symptomatic improvement after treatment is completed, and to consider repeat endoscopy with biopsy if the patient’s symptoms do not improve or if the patient has a recurrence after treatment.
In patients with evidence of long-standing esophageal narrowing or poor response to drug therapy, esophageal dilation can be performed after careful consideration.
Although data are limited as to the role of specific allergens in adult eosinophilic esophagitis, patients with eosinophilic esophagitis are referred to an allergist for allergy testing. Offending food or aeroallergens are removed for a period of time and patients are followed for changes in symptoms.
For patients who do not respond to swallowed fluticasone, proton pump inhibitors, or both, other medications such as systemic steroids, montelukast, or cromolyn can be considered. In the near future, anti-interleukin 5 therapy may be another option.
Patients are asked to return periodically for evaluation after treatment. Due to the chronic and relapsing nature of eosinophilic esophagitis, various therapies (especially fluticasone) are often restarted or continued because of symptom recurrence.
- Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology 1977; 72:1312–1316.
- Matzinger MA, Daneman A. Esophageal involvement in eosinophilic gastroenteritis. Pediatr Radiol 1983; 13:35–38.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. Dig Dis Sci 1993; 38:109–116.
- Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med 2004; 351:940–941.
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115:418–419.
- Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 2004; 113:11–28.
- Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 2003; 125:1419–1427.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Ngo P, Furuta G, Antonioli D, Fox V. Eosinophils in the esophagus—peptic or allergic eosinophilic esophagitis? Case series of three patients with esophageal eosinophilia. Am J Gastroenterol 2006; 101:1666–1670.
- Morrow JB, Vargo JJ, Goldblum JR, Richter JE. The ringed esophagus—histologic features of GERD. Am J Gastroenterol 2001; 96:984–989.
- Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol 2007; 102:1301–1306.
- Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol 2003; 98:777–782.
- Simon D, Straumann A, Wenk A, et al. Eosinophilic esophagitis in adults: no clinical relevance of wheat and rye sensitizations. Allergy 2006; 61:1480–1483.
- Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol 2003; 112:796–797.
- Zink DA, Amin M, Gebara S, Desai TK. Familial dysphagia and eosinophilia. Gastrointest Endoscop 2007; 65:330–334.
- Dellon ES, Aderoju A, Woosely JT, et al. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol 2007; 102:2300–2313.
- Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133:1342–1363.
- Parfitt JR, Gregor JC, Suskin NG, Jawa HA. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol 2006; 19:90–96.
- Kato M, Kephart GM, Talley NJ, et al. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec 1998; 242:418–425.
- Steiner SJ, Gupta SK, Croffie JM, Fitzgerald JF. Correlation between number of eosinophils and reflux index on same day esophageal biopsy and 24 hour esophageal pH monitoring. Am J Gastroenterol 2004; 99:801–805.
- Sgouros SN, Bergele C, Mantides A. Eosinophilic esophagitis in adults: a systematic review. Eur J Gastroenterol Hepatol 2006; 18:211–217.
- Liacouras CA, Spergel JM, Ruchelli E, Verma R. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol 2005; 3:1198–1206.
- Zimmerman SL, Levine MS, Rubesin SE, et al. Idiopathic eosino-philic esophagitis in adults: the ringed esophagus. Radiology 2005; 236:159–165.
- Chehade M, Sampson HA, Morotti RA, Magrid MS. Esophageal sub-epithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2007; 45:319–328.
- Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol 2007; 119:206–212.
- Straumann A, Spichtin HP, Grize L, et al. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125:1660–1669.
- Kelly KJ, Lazenby AJ, Rowe PC, et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 1995; 109:1503–1512.
- Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006; 4:1097–1102.
- Arora AS, Perrault J, Smyrk TC. Topical corticosteroid treatment of dysphagia due to eosinophilic esophagitis in adults. Mayo Clin Proc 2003; 78:830–835.
- Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endoscop 2006; 63:3–12.
- Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology 2006; 131:1381–1391.
- Attwood SE, Lewis CJ, Bronder CS, et al. Eosinophilic oesophagitis: a novel treatment using montelukast. Gut 2003; 52:181–185.
- Stein ML, Collins MH, Villanueva JM, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol 2006; 118:1312–1319.
- Sgouros SN, Bergele C, Mantides A. Eosinophilic esophagitis in adults: what is the clinical significance? Endoscopy 2006; 38:512–520.
Abundant eosinophils in the esophagus were first described in 1977 in a 51-year-old man with dysphagia, chest pain, and a personal history of severe asthma and marked peripheral eosinophilia.1 In 1983, a similar case was reported in an adolescent with dysphagia.2 In both patients, large numbers of eosinophils were also noted in the duodenum, suggesting that these findings were part of a systemic hypereosinophilic syndrome.
Increased numbers of eosinophils in the gastrointestinal tract have been described in a number of diseases, including Crohn disease, connective tissue disorders, malignancy, various infections, and drug hypersensitivity reactions. However, not until 1993 was eosinophilic esophagitis described as a distinct clinical entity, consisting of isolated esophageal eosinophilia (typically more than 15 eosinophils per high-power field) in patients with dysphagia.3
Now, epidemiologic studies suggest that eosinophilic esophagitis may be as common as inflammatory bowel disease. In a study of children in Cincinnati, OH,4 the incidence was estimated at 10 per 100,000 children per year and the prevalence was estimated at 43 per 100,000. Of interest, 97% of cases were diagnosed after the year 2000.
RISING INCIDENCE, OR INCREASED RECOGNITION?
Over the last several years, the number of reported cases has increased substantially as interest in this disease has grown. The increase has been attributed in part to heightened awareness of this condition among clinicians and, hence, more esophageal biopsies being performed. Similarly, pathologists may have previously attributed esophageal eosinophilia to gastroesophageal reflux disease (GERD). However, the prevalence of eosinophilic esophagitis increased 10-fold between 1989 and 2003 in a fixed and stable adult population in Olten, Switzerland, suggesting that more than just increased awareness is responsible for this dramatic rise.5
PATHOGENESIS: SIMILAR TO OTHER ALLERGIC DISEASES?
The growing incidence of eosinophilic esophagitis parallels that of asthma, eczema, allergic rhinitis, and other atopic diseases, raising the possibility that these disorders share common environmental exposures and similar inflammatory pathways.6 The pathologic mechanisms of eosinophilic esophagitis are unknown, but emerging evidence suggests that, like other allergic diseases, it is an immune response mediated by type 2 T helper cells.
Several animal studies support this hypothesis. Mice sensitized and then exposed to aeroallergens developed both allergic airway inflammation and eosinophilic esophagitis. Interleukin 5, a cytokine involved in asthma, also helps recruit eosinophils into the esophagus, as transgenic mice deficient in interleukin 5 do not develop esophageal eosinophilia upon allergen exposure.7
Recently, eotaxin-3, a potent attractant for eosinophils, was shown to be markedly overexpressed in children with eosinophilic esophagitis compared with controls.8
Acid reflux does not appear to be a causative factor in most patients. However, reflux may play a secondary role, as some patients have experienced symptomatic, endoscopic, and histologic resolution of eosinophilic esophagitis after treatment with a proton pump inhibitor.9
GERD AND EOSINOPHILIC ESOPHAGITIS: WHAT IS THE RELATIONSHIP?
Given the high prevalence of GERD in the general population, much time and effort have been spent on comparing eosinophilic esophagitis with GERD. In fact, some endoscopic features typically seen in eosinophilic esophagitis were previously attributed to acid reflux.10
Both diseases share varying degrees of esophageal eosinophilia, and some have speculated on the relationship of eosinophilic esophagitis and GERD. Spechler et al11 recently suggested that the mucosal injury caused by acid reflux may allow swallowed allergens to penetrate an esophageal layer that is otherwise impermeable to most proteins, thereby causing mild eosinophilia. Conversely, the intense degranulation of activated eosinophils seen in eosinophilic esophagitis can trigger changes in the lower esophageal sphincter that could predispose to acid reflux.
Although their clinical and pathologic features may overlap, GERD and eosinophilic esophagitis appear to have different genetic profiles. In a recent pediatric study, Blanchard et al8 found that genes up-regulated in eosinophilic esophagitis were markedly different than those in chronic esophagitis. This suggests that while the two diseases share a constellation of symptoms, they have a different pathogenesis. Nevertheless, because of this possible overlap, the diagnosis of eosinophilic esophagitis should be made after acid reflux has been either treated or excluded with pH testing (see below).
THE ROLE OF ENVIRONMENTAL ALLERGENS AND GENETICS
Studies in children suggest that food allergies are a major contributor to eosinophilic esophagitis. In children, a strict amino-acid elemental diet has led to complete resolution of symptoms and a marked decrease in esophageal eosinophils. However, symptoms tend to recur once patients resume a regular diet.12
It is unclear if dietary modification is effective in adults. In six adults with eosinophilic esophagitis and a history of wheat and rye allergies, symptoms did not improve when these foods were eliminated and did not worsen when they were reintroduced.13
Of interest, there may be a seasonal variation of eosinophilic esophagitis, as suggested by a case report of a 21-year-old woman who had eosinophilic esophagitis that worsened symptomatically and histologically during the pollen season but resolved during winter. This is another example of the role aeroallergens may play in this disease.14
Evidence of a genetic predisposition to this disease is also growing, with a number of case reports describing multiple affected family members spanning generations.15
NEW CONSENSUS ON DIAGNOSTIC CRITERIA
The diagnosis of eosinophilic esophagitis is made histologically, with “marked” eosinophilia on esophageal biopsies, ie, usually 15 or more eosinophils per high-power field. In contrast, a normal esophagus contains almost no eosinophils,16 and esophageal biopsies of patients with GERD usually have fewer than 10 eosinophils per high-power field, with eosinophils limited to the distal esophagus.17
However, a recent systematic review of the literature found 10 different histologic definitions of eosinophilic esophagitis, ranging from more than 5 to more than 30 eosinophils, and more than one-third of the articles included in the review did not contain any specific diagnostic criteria. Similarly, a lack of consensus on the size of a high-power field (ranging from 0.12 to 0.44 mm2) resulted in a 23-fold variability in the description of eosinophil density. Moreover, the biopsy protocols were reported in only 39% of the articles.18
In view of the growing interest in this disease, its increasing recognition, the diagnostic ambiguity described above, and concern about the role of acid reflux, consensus recommendations for its diagnosis and treatment in adults and children have recently been published.19 The current consensus definition for eosinophilic esophagitis is:
- Clinical symptoms of esophageal dysfunction (eg, dysphagia, food impaction);
- At least 15 eosinophils per high-power field; and
- Either no response to a high-dose proton pump inhibitor or normal results on pH monitoring of the distal esophagus.
CLINICAL PRESENTATION
Eosinophilic esophagitis predominantly affects men between the ages of 20 and 40, but cases in women and in younger and older patients have also been reported. Recent systematic reviews found a male-to-female ratio of approximately 3:1.
More than 90% of adults with eosinophilic esophagitis present with intermittent difficulty swallowing solids, while food impaction occurs in more than 60%. Heartburn is the only manifestation in 24% of patients. Noncardiac chest pain, vomiting, and abdominal pain have also been seen, but less frequently.
Up to 80% of patients with eosinophilic esophagitis have a history of atopic disease such as asthma, allergic rhinitis, or allergies to food or medicine. One-third to one-half of patients have peripheral eosinophilia, and up to 55% have increased serum levels of immunoglobulin E (IgE).21
In children, presenting symptoms vary with age and include feeding disorders, vomiting, abdominal pain, and dysphagia. Moreover, children with eosinophilic esophagitis have a higher frequency of atopic symptoms and peripheral eosinophilia than do adults.5,22
Although motor abnormalities are common in patients with eosinophilic esophagitis (up to 40% of patients have esophageal manometric abnormalities, including uncoordinated contractions and ineffective peristalsis),21 esophageal manometry is of limited diagnostic value and so is not recommended as a routine test.19
These findings support the theory that inflammation can lead to submucosal fibrosis, remodeling, narrowing, and eventually symptoms. Furthermore, two recent studies found that children with eosinophilic esophagitis had increased subepithelial collagen deposition in their biopsy specimens,24 suggesting increased potential for fibrosis. Also increased are transforming growth factor beta (a profibrotic cytokine) and vascular cell adhesion molecule 1, which is implicated in angiogenesis.25
Although many patients with eosinophilic esophagitis have abnormal findings on barium radiography, the test is most useful before esophagogastroduodenoscopy to determine whether a stricture is present and potentially to guide endoscopic dilation.19
NATURAL HISTORY: CHRONIC, RELAPSING, AND MOST LIKELY BENIGN
Our understanding of the natural history of eosinophilic esophagitis is limited, but the available evidence suggests that its prognosis is favorable.
Thirty adults followed for up to 11.5 years remained in good health, maintained their weight, and had no evidence of nutritional deficiencies.26 However, all but 1 patient continued to have dysphagia, with the overall intensity of dysphagia increasing in 7 (23%), remaining stable and persistent in 11 (37%), and decreasing in the remainder. In over half of these patients, the disease impaired quality of life. The only treatment offered was endoscopic dilation, which 11 patients required. Patients with peripheral blood eosinophilia and those with more pronounced findings on endoscopy were more likely to have symptoms at follow-up.
Although dysphagia persisted, the number of eosinophils in esophageal biopsy specimens decreased significantly over time, suggesting that the intense eosinophilic infiltration seen earlier in the disease may evolve into fibrosis and remodeling, similar to that seen in asthma and other chronic atopic diseases. Unlike in Barrett esophagus, a premalignant complication of longstanding GERD, there appeared to be no increased risk of esophageal cancer in these patients with eosinophilic esophagitis during the follow-up period.26
TREATMENT
Dietary therapy
Strict elemental amino-acid diets have resulted in complete symptomatic and histologic resolution of eosinophilic esophagitis in children. However, these elemental diets often have to be given by nasogastric tube because they are unpalatable, and the disease tends to return once the diet is discontinued.27
Elimination diets, based either on avoiding the six foods most commonly associated with allergy (egg, wheat, soy, cow’s milk protein, seafood, peanuts) or on allergy testing such as skin prick testing or atopy patch testing, have shown promise in children.12,28 However, similar large-scale studies of elimination diets in adults have not been conducted.
Allergy evaluation
The recent consensus recommendations devoted considerable attention to the role of allergy evaluation.19 Between 50% and 80% of patients with eosinophilic esophagitis have a coexisting atopic disease such as atopic dermatitis, eczema, allergic rhinitis, or asthma, with a higher prevalence in children than in adults. In these patients, evidence suggests that allergy testing may predict response to therapy. Therefore, the current recommendation is for all patients with eosinophilic esophagitis to undergo a complete evaluation by an experienced allergist.
Checking the peripheral blood eosinophil count before and after treatment is reasonable, as many patients have elevated eosinophil counts that decrease after treatment.
Similarly, many patients with eosinophilic esophagitis have elevated serum total IgE levels, which suggests a concomitant atopic disease. Therefore, total IgE levels should also be checked before and after treatment. Checking for IgE against specific aeroallergens is recommended, but checking for IgE against specific food antigens has not proven beneficial at this time. Similarly, skin prick testing for aeroallergens may be useful, but not for food allergens.
Data on atopy patch testing in eosinophilic esophagitis are currently limited but promising.19
Medical therapy
Swallowed fluticasone (Flonase, using an inhaler) is the mainstay of therapy for both children and adults.
In one case series, 21 adult patients with eosinophilic esophagitis received a 6-week course of swallowed fluticasone 220 μg/puff, two to four puffs twice daily. Symptoms completely resolved in all patients for at least 4 months, and no patient needed endoscopic dilation.29
In another study, 19 patients treated with fluticasone for 4 weeks showed dramatic improvement both symptomatically and histologically. However, after 3 months, 14 (74%) of the 19 patients had a recurrence of symptoms, pointing to the chronic relapsing nature of this disease.30
The only randomized placebo-controlled trial of fluticasone to date has been in children. Konikoff et al31 found that a 3-month course of fluticasone induced remission, defined as less than one eosinophil per high-power field, in 50% of patients, compared with 9% in the placebo group.
Swallowed fluticasone is generally well tolerated, although cases of esophageal candidiasis have been reported.30
Acid suppression still has an unclear role in the treatment of eosinophilic esophagitis. As mentioned above, the disease is defined as the presence or persistence of esophageal eosinophilia after acid reflux has been maximally treated or ruled out. Most patients referred for further evaluation of eosinophilic esophagitis have tried twice-daily proton pump inhibitor therapy without success. The impact of concomitant therapy with a proton pump inhibitor has not yet been determined, but the recent guidelines suggest that these drugs are reasonable as co-therapy in patients who also have GERD symptoms.19
In patients whose symptoms do not improve with fluticasone, several other medications have been used:
Systemic corticosteroids have been used with success in both adults and children with hypereosinophilic syndromes, as well as in patients with refractory eosinophilic esophagitis, but adverse effects limit their routine and long-term use.
Cromolyn sodium (NasalCrom, Intal), a mast cell stabilizer, and montelukast (Singulair), a leukotriene inhibitor, have been used with limited success.32
Mepolizumab (Bosatria), a humanized monoclonal antibody to human interleukin 5, decreased the number of eosinophils in the esophagus and peripheral blood and improved clinical symptoms in patients with refractory eosinophilic esophagitis in a recent open-label trial.33 Further studies with mepolizumab and other biologic agents are expected.
Endoscopic dilation
Endoscopic dilation with either a guidewire or a balloon technique is often used to treat strictures and a diffusely narrowed esophagus in patients with eosinophilic esophagitis.
As mentioned above, a common endoscopic feature is mucosal fragility, which has been described as resembling crepe paper. Shearing and longitudinal splitting of this fragile mucosa may occur after dilation therapy.
Although esophageal dilation may be done safely in patients with eosinophilic esophagitis, the risk of perforation appears to be greater than in those with other indications for dilation.
Nevertheless, immediate symptomatic improvement has been reported in 83% of patients after dilation, with symptoms recurring in 20% within 3 to 8 months.34 Current recommendations suggest that dilation should be done cautiously in patients who have documented esophageal narrowing for which drug therapy has failed.
RECOMMENDED APPROACH
The approach to diagnosing and treating eosinophilic esophagitis begins with being aware of its prevalence. One should suspect it more in younger patients presenting with intermittent dysphagia, food impaction, or heartburn that does not respond to maximal doses of a proton pump inhibitor. Special attention should be paid to a personal or family history of allergic diseases or similar symptoms.
According to the consensus recommendations, barium esophagography is useful if the presentation suggests long-standing disease and associated esophageal stricture.
Upper endoscopy is performed, with biopsies obtained in the proximal, middle, and distal esophagus regardless of the appearance of the esophageal mucosa. Biopsies of the stomach and duodenum are also recommended to rule out eosinophilic gastroenteritis.19
After biopsy confirms the diagnosis, a trial of a proton pump inhibitor in maximum doses (usually twice daily) for 8 weeks is recommended if not already tried. If there is evidence of eosinophilic esophagitis on repeat endoscopy and biopsy studies after proton pump inhibitor therapy, the next step is swallowed fluticasone (220 μg, up to four puffs twice daily) for 6 to 8 weeks, with follow-up visits to confirm resolution of symptoms. Without a spacer, the fluticasone is swallowed after maximal expiration. Patients are instructed to avoid food and liquids for at least 30 minutes after use.
Optimal strategies for monitoring in adults have yet to be established, and following symptoms alone may or may not be sufficient.19 Our approach is to follow for symptomatic improvement after treatment is completed, and to consider repeat endoscopy with biopsy if the patient’s symptoms do not improve or if the patient has a recurrence after treatment.
In patients with evidence of long-standing esophageal narrowing or poor response to drug therapy, esophageal dilation can be performed after careful consideration.
Although data are limited as to the role of specific allergens in adult eosinophilic esophagitis, patients with eosinophilic esophagitis are referred to an allergist for allergy testing. Offending food or aeroallergens are removed for a period of time and patients are followed for changes in symptoms.
For patients who do not respond to swallowed fluticasone, proton pump inhibitors, or both, other medications such as systemic steroids, montelukast, or cromolyn can be considered. In the near future, anti-interleukin 5 therapy may be another option.
Patients are asked to return periodically for evaluation after treatment. Due to the chronic and relapsing nature of eosinophilic esophagitis, various therapies (especially fluticasone) are often restarted or continued because of symptom recurrence.
Abundant eosinophils in the esophagus were first described in 1977 in a 51-year-old man with dysphagia, chest pain, and a personal history of severe asthma and marked peripheral eosinophilia.1 In 1983, a similar case was reported in an adolescent with dysphagia.2 In both patients, large numbers of eosinophils were also noted in the duodenum, suggesting that these findings were part of a systemic hypereosinophilic syndrome.
Increased numbers of eosinophils in the gastrointestinal tract have been described in a number of diseases, including Crohn disease, connective tissue disorders, malignancy, various infections, and drug hypersensitivity reactions. However, not until 1993 was eosinophilic esophagitis described as a distinct clinical entity, consisting of isolated esophageal eosinophilia (typically more than 15 eosinophils per high-power field) in patients with dysphagia.3
Now, epidemiologic studies suggest that eosinophilic esophagitis may be as common as inflammatory bowel disease. In a study of children in Cincinnati, OH,4 the incidence was estimated at 10 per 100,000 children per year and the prevalence was estimated at 43 per 100,000. Of interest, 97% of cases were diagnosed after the year 2000.
RISING INCIDENCE, OR INCREASED RECOGNITION?
Over the last several years, the number of reported cases has increased substantially as interest in this disease has grown. The increase has been attributed in part to heightened awareness of this condition among clinicians and, hence, more esophageal biopsies being performed. Similarly, pathologists may have previously attributed esophageal eosinophilia to gastroesophageal reflux disease (GERD). However, the prevalence of eosinophilic esophagitis increased 10-fold between 1989 and 2003 in a fixed and stable adult population in Olten, Switzerland, suggesting that more than just increased awareness is responsible for this dramatic rise.5
PATHOGENESIS: SIMILAR TO OTHER ALLERGIC DISEASES?
The growing incidence of eosinophilic esophagitis parallels that of asthma, eczema, allergic rhinitis, and other atopic diseases, raising the possibility that these disorders share common environmental exposures and similar inflammatory pathways.6 The pathologic mechanisms of eosinophilic esophagitis are unknown, but emerging evidence suggests that, like other allergic diseases, it is an immune response mediated by type 2 T helper cells.
Several animal studies support this hypothesis. Mice sensitized and then exposed to aeroallergens developed both allergic airway inflammation and eosinophilic esophagitis. Interleukin 5, a cytokine involved in asthma, also helps recruit eosinophils into the esophagus, as transgenic mice deficient in interleukin 5 do not develop esophageal eosinophilia upon allergen exposure.7
Recently, eotaxin-3, a potent attractant for eosinophils, was shown to be markedly overexpressed in children with eosinophilic esophagitis compared with controls.8
Acid reflux does not appear to be a causative factor in most patients. However, reflux may play a secondary role, as some patients have experienced symptomatic, endoscopic, and histologic resolution of eosinophilic esophagitis after treatment with a proton pump inhibitor.9
GERD AND EOSINOPHILIC ESOPHAGITIS: WHAT IS THE RELATIONSHIP?
Given the high prevalence of GERD in the general population, much time and effort have been spent on comparing eosinophilic esophagitis with GERD. In fact, some endoscopic features typically seen in eosinophilic esophagitis were previously attributed to acid reflux.10
Both diseases share varying degrees of esophageal eosinophilia, and some have speculated on the relationship of eosinophilic esophagitis and GERD. Spechler et al11 recently suggested that the mucosal injury caused by acid reflux may allow swallowed allergens to penetrate an esophageal layer that is otherwise impermeable to most proteins, thereby causing mild eosinophilia. Conversely, the intense degranulation of activated eosinophils seen in eosinophilic esophagitis can trigger changes in the lower esophageal sphincter that could predispose to acid reflux.
Although their clinical and pathologic features may overlap, GERD and eosinophilic esophagitis appear to have different genetic profiles. In a recent pediatric study, Blanchard et al8 found that genes up-regulated in eosinophilic esophagitis were markedly different than those in chronic esophagitis. This suggests that while the two diseases share a constellation of symptoms, they have a different pathogenesis. Nevertheless, because of this possible overlap, the diagnosis of eosinophilic esophagitis should be made after acid reflux has been either treated or excluded with pH testing (see below).
THE ROLE OF ENVIRONMENTAL ALLERGENS AND GENETICS
Studies in children suggest that food allergies are a major contributor to eosinophilic esophagitis. In children, a strict amino-acid elemental diet has led to complete resolution of symptoms and a marked decrease in esophageal eosinophils. However, symptoms tend to recur once patients resume a regular diet.12
It is unclear if dietary modification is effective in adults. In six adults with eosinophilic esophagitis and a history of wheat and rye allergies, symptoms did not improve when these foods were eliminated and did not worsen when they were reintroduced.13
Of interest, there may be a seasonal variation of eosinophilic esophagitis, as suggested by a case report of a 21-year-old woman who had eosinophilic esophagitis that worsened symptomatically and histologically during the pollen season but resolved during winter. This is another example of the role aeroallergens may play in this disease.14
Evidence of a genetic predisposition to this disease is also growing, with a number of case reports describing multiple affected family members spanning generations.15
NEW CONSENSUS ON DIAGNOSTIC CRITERIA
The diagnosis of eosinophilic esophagitis is made histologically, with “marked” eosinophilia on esophageal biopsies, ie, usually 15 or more eosinophils per high-power field. In contrast, a normal esophagus contains almost no eosinophils,16 and esophageal biopsies of patients with GERD usually have fewer than 10 eosinophils per high-power field, with eosinophils limited to the distal esophagus.17
However, a recent systematic review of the literature found 10 different histologic definitions of eosinophilic esophagitis, ranging from more than 5 to more than 30 eosinophils, and more than one-third of the articles included in the review did not contain any specific diagnostic criteria. Similarly, a lack of consensus on the size of a high-power field (ranging from 0.12 to 0.44 mm2) resulted in a 23-fold variability in the description of eosinophil density. Moreover, the biopsy protocols were reported in only 39% of the articles.18
In view of the growing interest in this disease, its increasing recognition, the diagnostic ambiguity described above, and concern about the role of acid reflux, consensus recommendations for its diagnosis and treatment in adults and children have recently been published.19 The current consensus definition for eosinophilic esophagitis is:
- Clinical symptoms of esophageal dysfunction (eg, dysphagia, food impaction);
- At least 15 eosinophils per high-power field; and
- Either no response to a high-dose proton pump inhibitor or normal results on pH monitoring of the distal esophagus.
CLINICAL PRESENTATION
Eosinophilic esophagitis predominantly affects men between the ages of 20 and 40, but cases in women and in younger and older patients have also been reported. Recent systematic reviews found a male-to-female ratio of approximately 3:1.
More than 90% of adults with eosinophilic esophagitis present with intermittent difficulty swallowing solids, while food impaction occurs in more than 60%. Heartburn is the only manifestation in 24% of patients. Noncardiac chest pain, vomiting, and abdominal pain have also been seen, but less frequently.
Up to 80% of patients with eosinophilic esophagitis have a history of atopic disease such as asthma, allergic rhinitis, or allergies to food or medicine. One-third to one-half of patients have peripheral eosinophilia, and up to 55% have increased serum levels of immunoglobulin E (IgE).21
In children, presenting symptoms vary with age and include feeding disorders, vomiting, abdominal pain, and dysphagia. Moreover, children with eosinophilic esophagitis have a higher frequency of atopic symptoms and peripheral eosinophilia than do adults.5,22
Although motor abnormalities are common in patients with eosinophilic esophagitis (up to 40% of patients have esophageal manometric abnormalities, including uncoordinated contractions and ineffective peristalsis),21 esophageal manometry is of limited diagnostic value and so is not recommended as a routine test.19
These findings support the theory that inflammation can lead to submucosal fibrosis, remodeling, narrowing, and eventually symptoms. Furthermore, two recent studies found that children with eosinophilic esophagitis had increased subepithelial collagen deposition in their biopsy specimens,24 suggesting increased potential for fibrosis. Also increased are transforming growth factor beta (a profibrotic cytokine) and vascular cell adhesion molecule 1, which is implicated in angiogenesis.25
Although many patients with eosinophilic esophagitis have abnormal findings on barium radiography, the test is most useful before esophagogastroduodenoscopy to determine whether a stricture is present and potentially to guide endoscopic dilation.19
NATURAL HISTORY: CHRONIC, RELAPSING, AND MOST LIKELY BENIGN
Our understanding of the natural history of eosinophilic esophagitis is limited, but the available evidence suggests that its prognosis is favorable.
Thirty adults followed for up to 11.5 years remained in good health, maintained their weight, and had no evidence of nutritional deficiencies.26 However, all but 1 patient continued to have dysphagia, with the overall intensity of dysphagia increasing in 7 (23%), remaining stable and persistent in 11 (37%), and decreasing in the remainder. In over half of these patients, the disease impaired quality of life. The only treatment offered was endoscopic dilation, which 11 patients required. Patients with peripheral blood eosinophilia and those with more pronounced findings on endoscopy were more likely to have symptoms at follow-up.
Although dysphagia persisted, the number of eosinophils in esophageal biopsy specimens decreased significantly over time, suggesting that the intense eosinophilic infiltration seen earlier in the disease may evolve into fibrosis and remodeling, similar to that seen in asthma and other chronic atopic diseases. Unlike in Barrett esophagus, a premalignant complication of longstanding GERD, there appeared to be no increased risk of esophageal cancer in these patients with eosinophilic esophagitis during the follow-up period.26
TREATMENT
Dietary therapy
Strict elemental amino-acid diets have resulted in complete symptomatic and histologic resolution of eosinophilic esophagitis in children. However, these elemental diets often have to be given by nasogastric tube because they are unpalatable, and the disease tends to return once the diet is discontinued.27
Elimination diets, based either on avoiding the six foods most commonly associated with allergy (egg, wheat, soy, cow’s milk protein, seafood, peanuts) or on allergy testing such as skin prick testing or atopy patch testing, have shown promise in children.12,28 However, similar large-scale studies of elimination diets in adults have not been conducted.
Allergy evaluation
The recent consensus recommendations devoted considerable attention to the role of allergy evaluation.19 Between 50% and 80% of patients with eosinophilic esophagitis have a coexisting atopic disease such as atopic dermatitis, eczema, allergic rhinitis, or asthma, with a higher prevalence in children than in adults. In these patients, evidence suggests that allergy testing may predict response to therapy. Therefore, the current recommendation is for all patients with eosinophilic esophagitis to undergo a complete evaluation by an experienced allergist.
Checking the peripheral blood eosinophil count before and after treatment is reasonable, as many patients have elevated eosinophil counts that decrease after treatment.
Similarly, many patients with eosinophilic esophagitis have elevated serum total IgE levels, which suggests a concomitant atopic disease. Therefore, total IgE levels should also be checked before and after treatment. Checking for IgE against specific aeroallergens is recommended, but checking for IgE against specific food antigens has not proven beneficial at this time. Similarly, skin prick testing for aeroallergens may be useful, but not for food allergens.
Data on atopy patch testing in eosinophilic esophagitis are currently limited but promising.19
Medical therapy
Swallowed fluticasone (Flonase, using an inhaler) is the mainstay of therapy for both children and adults.
In one case series, 21 adult patients with eosinophilic esophagitis received a 6-week course of swallowed fluticasone 220 μg/puff, two to four puffs twice daily. Symptoms completely resolved in all patients for at least 4 months, and no patient needed endoscopic dilation.29
In another study, 19 patients treated with fluticasone for 4 weeks showed dramatic improvement both symptomatically and histologically. However, after 3 months, 14 (74%) of the 19 patients had a recurrence of symptoms, pointing to the chronic relapsing nature of this disease.30
The only randomized placebo-controlled trial of fluticasone to date has been in children. Konikoff et al31 found that a 3-month course of fluticasone induced remission, defined as less than one eosinophil per high-power field, in 50% of patients, compared with 9% in the placebo group.
Swallowed fluticasone is generally well tolerated, although cases of esophageal candidiasis have been reported.30
Acid suppression still has an unclear role in the treatment of eosinophilic esophagitis. As mentioned above, the disease is defined as the presence or persistence of esophageal eosinophilia after acid reflux has been maximally treated or ruled out. Most patients referred for further evaluation of eosinophilic esophagitis have tried twice-daily proton pump inhibitor therapy without success. The impact of concomitant therapy with a proton pump inhibitor has not yet been determined, but the recent guidelines suggest that these drugs are reasonable as co-therapy in patients who also have GERD symptoms.19
In patients whose symptoms do not improve with fluticasone, several other medications have been used:
Systemic corticosteroids have been used with success in both adults and children with hypereosinophilic syndromes, as well as in patients with refractory eosinophilic esophagitis, but adverse effects limit their routine and long-term use.
Cromolyn sodium (NasalCrom, Intal), a mast cell stabilizer, and montelukast (Singulair), a leukotriene inhibitor, have been used with limited success.32
Mepolizumab (Bosatria), a humanized monoclonal antibody to human interleukin 5, decreased the number of eosinophils in the esophagus and peripheral blood and improved clinical symptoms in patients with refractory eosinophilic esophagitis in a recent open-label trial.33 Further studies with mepolizumab and other biologic agents are expected.
Endoscopic dilation
Endoscopic dilation with either a guidewire or a balloon technique is often used to treat strictures and a diffusely narrowed esophagus in patients with eosinophilic esophagitis.
As mentioned above, a common endoscopic feature is mucosal fragility, which has been described as resembling crepe paper. Shearing and longitudinal splitting of this fragile mucosa may occur after dilation therapy.
Although esophageal dilation may be done safely in patients with eosinophilic esophagitis, the risk of perforation appears to be greater than in those with other indications for dilation.
Nevertheless, immediate symptomatic improvement has been reported in 83% of patients after dilation, with symptoms recurring in 20% within 3 to 8 months.34 Current recommendations suggest that dilation should be done cautiously in patients who have documented esophageal narrowing for which drug therapy has failed.
RECOMMENDED APPROACH
The approach to diagnosing and treating eosinophilic esophagitis begins with being aware of its prevalence. One should suspect it more in younger patients presenting with intermittent dysphagia, food impaction, or heartburn that does not respond to maximal doses of a proton pump inhibitor. Special attention should be paid to a personal or family history of allergic diseases or similar symptoms.
According to the consensus recommendations, barium esophagography is useful if the presentation suggests long-standing disease and associated esophageal stricture.
Upper endoscopy is performed, with biopsies obtained in the proximal, middle, and distal esophagus regardless of the appearance of the esophageal mucosa. Biopsies of the stomach and duodenum are also recommended to rule out eosinophilic gastroenteritis.19
After biopsy confirms the diagnosis, a trial of a proton pump inhibitor in maximum doses (usually twice daily) for 8 weeks is recommended if not already tried. If there is evidence of eosinophilic esophagitis on repeat endoscopy and biopsy studies after proton pump inhibitor therapy, the next step is swallowed fluticasone (220 μg, up to four puffs twice daily) for 6 to 8 weeks, with follow-up visits to confirm resolution of symptoms. Without a spacer, the fluticasone is swallowed after maximal expiration. Patients are instructed to avoid food and liquids for at least 30 minutes after use.
Optimal strategies for monitoring in adults have yet to be established, and following symptoms alone may or may not be sufficient.19 Our approach is to follow for symptomatic improvement after treatment is completed, and to consider repeat endoscopy with biopsy if the patient’s symptoms do not improve or if the patient has a recurrence after treatment.
In patients with evidence of long-standing esophageal narrowing or poor response to drug therapy, esophageal dilation can be performed after careful consideration.
Although data are limited as to the role of specific allergens in adult eosinophilic esophagitis, patients with eosinophilic esophagitis are referred to an allergist for allergy testing. Offending food or aeroallergens are removed for a period of time and patients are followed for changes in symptoms.
For patients who do not respond to swallowed fluticasone, proton pump inhibitors, or both, other medications such as systemic steroids, montelukast, or cromolyn can be considered. In the near future, anti-interleukin 5 therapy may be another option.
Patients are asked to return periodically for evaluation after treatment. Due to the chronic and relapsing nature of eosinophilic esophagitis, various therapies (especially fluticasone) are often restarted or continued because of symptom recurrence.
- Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology 1977; 72:1312–1316.
- Matzinger MA, Daneman A. Esophageal involvement in eosinophilic gastroenteritis. Pediatr Radiol 1983; 13:35–38.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. Dig Dis Sci 1993; 38:109–116.
- Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med 2004; 351:940–941.
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115:418–419.
- Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 2004; 113:11–28.
- Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 2003; 125:1419–1427.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Ngo P, Furuta G, Antonioli D, Fox V. Eosinophils in the esophagus—peptic or allergic eosinophilic esophagitis? Case series of three patients with esophageal eosinophilia. Am J Gastroenterol 2006; 101:1666–1670.
- Morrow JB, Vargo JJ, Goldblum JR, Richter JE. The ringed esophagus—histologic features of GERD. Am J Gastroenterol 2001; 96:984–989.
- Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol 2007; 102:1301–1306.
- Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol 2003; 98:777–782.
- Simon D, Straumann A, Wenk A, et al. Eosinophilic esophagitis in adults: no clinical relevance of wheat and rye sensitizations. Allergy 2006; 61:1480–1483.
- Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol 2003; 112:796–797.
- Zink DA, Amin M, Gebara S, Desai TK. Familial dysphagia and eosinophilia. Gastrointest Endoscop 2007; 65:330–334.
- Dellon ES, Aderoju A, Woosely JT, et al. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol 2007; 102:2300–2313.
- Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133:1342–1363.
- Parfitt JR, Gregor JC, Suskin NG, Jawa HA. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol 2006; 19:90–96.
- Kato M, Kephart GM, Talley NJ, et al. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec 1998; 242:418–425.
- Steiner SJ, Gupta SK, Croffie JM, Fitzgerald JF. Correlation between number of eosinophils and reflux index on same day esophageal biopsy and 24 hour esophageal pH monitoring. Am J Gastroenterol 2004; 99:801–805.
- Sgouros SN, Bergele C, Mantides A. Eosinophilic esophagitis in adults: a systematic review. Eur J Gastroenterol Hepatol 2006; 18:211–217.
- Liacouras CA, Spergel JM, Ruchelli E, Verma R. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol 2005; 3:1198–1206.
- Zimmerman SL, Levine MS, Rubesin SE, et al. Idiopathic eosino-philic esophagitis in adults: the ringed esophagus. Radiology 2005; 236:159–165.
- Chehade M, Sampson HA, Morotti RA, Magrid MS. Esophageal sub-epithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2007; 45:319–328.
- Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol 2007; 119:206–212.
- Straumann A, Spichtin HP, Grize L, et al. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125:1660–1669.
- Kelly KJ, Lazenby AJ, Rowe PC, et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 1995; 109:1503–1512.
- Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006; 4:1097–1102.
- Arora AS, Perrault J, Smyrk TC. Topical corticosteroid treatment of dysphagia due to eosinophilic esophagitis in adults. Mayo Clin Proc 2003; 78:830–835.
- Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endoscop 2006; 63:3–12.
- Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology 2006; 131:1381–1391.
- Attwood SE, Lewis CJ, Bronder CS, et al. Eosinophilic oesophagitis: a novel treatment using montelukast. Gut 2003; 52:181–185.
- Stein ML, Collins MH, Villanueva JM, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol 2006; 118:1312–1319.
- Sgouros SN, Bergele C, Mantides A. Eosinophilic esophagitis in adults: what is the clinical significance? Endoscopy 2006; 38:512–520.
- Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology 1977; 72:1312–1316.
- Matzinger MA, Daneman A. Esophageal involvement in eosinophilic gastroenteritis. Pediatr Radiol 1983; 13:35–38.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. Dig Dis Sci 1993; 38:109–116.
- Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med 2004; 351:940–941.
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115:418–419.
- Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 2004; 113:11–28.
- Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 2003; 125:1419–1427.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Ngo P, Furuta G, Antonioli D, Fox V. Eosinophils in the esophagus—peptic or allergic eosinophilic esophagitis? Case series of three patients with esophageal eosinophilia. Am J Gastroenterol 2006; 101:1666–1670.
- Morrow JB, Vargo JJ, Goldblum JR, Richter JE. The ringed esophagus—histologic features of GERD. Am J Gastroenterol 2001; 96:984–989.
- Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol 2007; 102:1301–1306.
- Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol 2003; 98:777–782.
- Simon D, Straumann A, Wenk A, et al. Eosinophilic esophagitis in adults: no clinical relevance of wheat and rye sensitizations. Allergy 2006; 61:1480–1483.
- Fogg MI, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol 2003; 112:796–797.
- Zink DA, Amin M, Gebara S, Desai TK. Familial dysphagia and eosinophilia. Gastrointest Endoscop 2007; 65:330–334.
- Dellon ES, Aderoju A, Woosely JT, et al. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol 2007; 102:2300–2313.
- Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133:1342–1363.
- Parfitt JR, Gregor JC, Suskin NG, Jawa HA. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol 2006; 19:90–96.
- Kato M, Kephart GM, Talley NJ, et al. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec 1998; 242:418–425.
- Steiner SJ, Gupta SK, Croffie JM, Fitzgerald JF. Correlation between number of eosinophils and reflux index on same day esophageal biopsy and 24 hour esophageal pH monitoring. Am J Gastroenterol 2004; 99:801–805.
- Sgouros SN, Bergele C, Mantides A. Eosinophilic esophagitis in adults: a systematic review. Eur J Gastroenterol Hepatol 2006; 18:211–217.
- Liacouras CA, Spergel JM, Ruchelli E, Verma R. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol 2005; 3:1198–1206.
- Zimmerman SL, Levine MS, Rubesin SE, et al. Idiopathic eosino-philic esophagitis in adults: the ringed esophagus. Radiology 2005; 236:159–165.
- Chehade M, Sampson HA, Morotti RA, Magrid MS. Esophageal sub-epithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2007; 45:319–328.
- Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol 2007; 119:206–212.
- Straumann A, Spichtin HP, Grize L, et al. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125:1660–1669.
- Kelly KJ, Lazenby AJ, Rowe PC, et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 1995; 109:1503–1512.
- Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006; 4:1097–1102.
- Arora AS, Perrault J, Smyrk TC. Topical corticosteroid treatment of dysphagia due to eosinophilic esophagitis in adults. Mayo Clin Proc 2003; 78:830–835.
- Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endoscop 2006; 63:3–12.
- Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology 2006; 131:1381–1391.
- Attwood SE, Lewis CJ, Bronder CS, et al. Eosinophilic oesophagitis: a novel treatment using montelukast. Gut 2003; 52:181–185.
- Stein ML, Collins MH, Villanueva JM, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol 2006; 118:1312–1319.
- Sgouros SN, Bergele C, Mantides A. Eosinophilic esophagitis in adults: what is the clinical significance? Endoscopy 2006; 38:512–520.
KEY POINTS
- The diagnosis is made with upper endoscopy and esophageal biopsies that show diffuse infiltration of eosinophils.
- Current treatment in adults is limited and consists of either swallowed fluticasone (Flonase) or a proton pump inhibitor.
- Because many patients with eosinophilic esophagitis have atopic disease, a complete evaluation for dietary allergens and aeroallergens is recommended, as avoidance of these allergens may be helpful in some adults.
- Cautious endoscopic dilation is a treatment option in patients with evidence of esophageal stenosis. Systemic corticosteroids and novel biologic therapy have been used in refractory cases.