User login
New frontiers in cardiovascular behavioral medicine: Comparative effectiveness of exercise and medication in treating depression
I am fortunate to be the recipient of the 2010 Bakken Institute Pioneer Award and feel especially honored to have my work recognized in this way. When informed that I was this year’s recipient, it prompted me to reflect on the meaning of the term “pioneer,” and how it related to me.
WHAT IS A PIONEER?
According to Merriam-Webster’s Collegiate Dictionary, a pioneer is one who (a) ventures into unknown or unclaimed territory to settle; and (b) opens up new areas of thought, research, or development. One requirement for any pioneer is that there be a frontier to explore. Thirty years ago, my colleagues and I began our investigations into cardiac rehabilitation (CR), which at the time we considered to be a new frontier for behavioral medicine.1
EXERCISE-BASED CARDIAC REHABILITATION
Historically, patients who suffered an acute myocardial infarction (AMI) were often discouraged from engaging in physical activity; patients were initially prescribed prolonged bed rest and told to avoid strenuous exercise.2 In the early 1950s, armchair therapy was proposed3 as an initial attempt to mobilize patients after a coronary event. Over the years, the value of physical exercise has been increasingly recognized and exercise is now considered to be the cornerstone of CR.4–7 Today, exercise-based CR, involving aerobic exercise supplemented by resistance training, is offered by virtually all CR programs in the United States.8 Proper medical management is also emphasized, along with dietary modification and smoking cessation, but exercise is the centerpiece of treatment.
Exercise has been shown to reduce traditional risk factors such as hypertension and hyperlipidemia,8 attenuate cardiovascular responses to mental stress,9 and reduce myocardial ischemia.10–12 Although no single study has demonstrated definitively that exercise reduces morbidity in patients with coronary heart disease (CHD), pooling data across clinical trials has shown that exercise may reduce risk of fatal CHD events by 25%.13 A recent, comprehensive meta-analysis by Jolliffe et al14 reported a 27% reduction in all-cause mortality and 31% reduction in cardiac mortality.
Not only is exercise considered beneficial for medical outcomes, but is also recognized as an important factor in improved quality of life. Indeed, there has been increased interest in the value of exercise for improving not just physical health, but also mental health.15–17 The mental health benefits of exercise are especially relevant for cardiac patients, as there is a growing literature documenting the importance of mental health, and, in particular the prognostic significance of depression, in patients with CHD.
PSYCHOSOCIAL RISK FACTORS: THE ROLE OF DEPRESSION IN CORONARY HEART DISEASE
There has long been an interest in psychosocial factors that contribute to the development and progression of CHD. More than three decades ago, researchers identified the type A behavior pattern as a risk factor for CHD.18 When subsequent studies failed to confirm the association of type A with adverse health outcomes, researchers turned their attention to other possible psychosocial risk factors, including anger and hostility,19 low social support,20 and most recently, depression.21 Indeed, the most consistent and compelling evidence is that clinical depression or elevated depressive symptoms in the presence of CHD increase the risk of fatal and nonfatal cardiac events and of all-cause mortality.22
Major depressive disorder (MDD) is a common and often chronic condition. Lifetime incidence estimates for MDD are approximately 12% in men and 20% in women.23 In addition, MDD is marked by high rates of relapse, with 22% to 50% of patients suffering recurrent episodes within 6 months after recovery.24 Furthermore, MDD is underrecognized and undertreated in older adults,25 CHD patients, and, especially, minorities.26–28
Cross-sectional studies have documented a higher prevalence of depression in CHD patients than in the general population. Point estimates range from 14% to as high as 47%, with higher rates recorded most often in patients with unstable angina, heart failure (HF), and patients awaiting coronary artery bypass graft (CABG) surgery.29–36
Depression associated with poor outcomes
A number of prospective studies have found that depression is associated with increased risk for mortality or nonfatal cardiac events in a variety of CHD populations. The most compelling evidence for depression as a risk factor has come from studies in Montreal, Canada. Frasure-Smith and colleagues31 assessed the impact of depression in 222 AMI patients, of whom 35 were diagnosed with MDD at the time of hospitalization. There were 12 deaths (six depressed and six nondepressed) over an initial 6-month followup period, representing more than a fivefold increased risk of death for depressed patients compared with nondepressed patients (hazard ratio, 5.7; 95% confidence interval [CI], 4.6 to 6.9). In a subsequent report,36 in which 896 AMI patients were followed for 1 year, the presence of elevated depressive symptoms was associated with more than a threefold increased risk in cardiac mortality after controlling for other multivariate predictors of mortality (odds ratio, 3.29 for women; 3.05 for men).
Studies of patients with stable CHD also have reported significant associations between the presence of depression and worse clinical outcomes. For example, Barefoot et al37 assessed 1,250 patients with documented CHD using the Zung self-report depression scale at the time of diagnostic coronary angiography and followed patients for up to 19.4 years. Results showed that patients with moderate to severe depression were at 69% greater risk for cardiac death and 78% greater risk for all-cause death.
Depression and heart failure outcomes
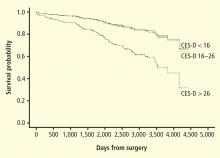
Patients with HF represent a particularly vulnerable group; a meta-analysis of depression in HF patients suggested that one in five patients are clinically depressed (range, 9% to 60%).41 Not only is depression in HF patients associated with worse outcomes,42–46 but recent evidence suggests that worsening of depressive symptoms, independent of clinical status, is related to worse outcomes. Sherwood et al46 demonstrated that increased symptoms of depression, as indicated by higher scores on the Beck Depression Inventory (BDI) over a 1-year interval (BDI change [1-point] hazard ratio, 1.07; 95% CI, 1.02 to 1.12; P = .007), were associated with higher risk of death or cardiovascular hospitalization after controlling for baseline depression (baseline BDI hazard ratio, 1.1; 95% CI, 1.06 to 1.14, P < .001) and established risk factors, including HF etiology, age, ejection fraction, N-terminal pro-B-type natriuretic peptides, and prior hospitalizations. Consequently, strategies to reduce depressive symptoms and prevent the worsening of depression may have important implications for improving cardiac health as well as for enhancing quality of life.
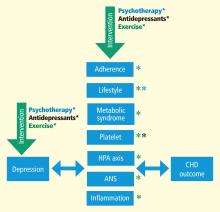
MECHANISMS LINKING DEPRESSION AND CHD
CONVENTIONAL APPROACHES TO TREATMENT OF DEPRESSION
Treatment of depression has focused on reduction of symptoms and restoration of function. Antidepressant medications are generally considered the treatment of choice.56 In particular, second-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs) are widely prescribed.57 Current treatment guidelines suggest 6 to 12 weeks of acute treatment followed by a continuation phase of 3 to 9 months to maintain therapeutic benefit.58 However, meta-analyses of antidepressant medications have reported only modest benefits over placebo treatments.59,60 In particular, active drug–placebo differences in antidepressant efficacy are positively correlated with depression severity: antidepressants are often comparable with placebo in patients with low levels of depression but may be superior to placebo among patients with more severe depression. However, the explanation for this relationship may be that placebo is less effective for more depressed patients rather than antidepressants being more effective for more depressed patients.59
For acute treatment of MDD, approximately 60% of patients respond to second-generation antidepressants,61 with a 40% relapse rate after 1 year.62 A recent meta-analysis60 of second-generation antidepressants summarized four comparative trials and 23 placebo-controlled trials and found that second-generation antidepressants were generally comparable with each other. Interestingly, despite the modest benefit of antidepressants, the percentage of patients treated for depression in the United States increased from 0.73% in 1987 to 2.33% in 1997. The proportion of those treated who received antidepressants increased from 37.3% in 1987 to 74.5% in 1997.63 The percentage of treated outpatients who used antidepressants has not increased significantly since 1997, but the use of psycho therapy as a sole treatment declined from 53.6% in 1998 to 43.1% in 2007.64 Moreover, the national expenditure for the outpatient treatment of depression increased from $10.05 billion in 1998 to $12.45 billion in 2007, primarily driven by an increase in expenditures for antidepressant medications.
Uncertainty about value of antidepressant therapy
Despite compelling reasons for treating depression in cardiac patients, the clinical significance of treating depression remains uncertain. To date, only the Enhancing Recovery in CHD Patients (ENRICHD) trial has examined the impact of treating depression in post-MI patients on “hard” clinical end points.65 Although more than 2,400 patients were enrolled in the trial, the results were disappointing. There were only modest differences (ie, two points on the Hamilton Depression Rating Scale [HAM-D]) in reductions of depressive symptoms in the group receiving cognitive behavior therapy (CBT) relative to usual-care controls and there were no treatment group differences in the primary outcome—all-cause mortality and nonfatal cardiac events. By the end of the follow-up period, 28.0% of patients in the CBT group and 20.6% of patients in usual care had received antidepressant medication. Although a subsequent reanalysis of the ENRICHD study revealed that antidepressant use was associated with improved clinical outcomes,66 because patients were not randomized to pharmacologic treatment it could not be concluded that SSRI use was responsible for the improved outcomes.
In a randomized trial of patients with acute coronary syndrome (the Sertraline Antidepressant Heart Attack Randomized Trial, or SADHART),67 almost 400 patients were treated with the SSRI sertraline or with placebo. Reductions in depressive symptoms were similar for patients receiving sertraline compared with placebo in the full sample, although a subgroup analysis revealed that patients with more severe depression (ie, those patients who reported two or more previous episodes) benefited more from sertraline compared with placebo. Interestingly, patients receiving sertraline tended to have more favorable cardiac outcomes, including a composite measure of both “hard” and “soft” clinical events, compared with placebo controls. These results suggested that antidepressant medication may improve underlying physiologic processes, such as platelet function, independent of changes in depression.68 However, because SADHART was not powered to detect differences in clinical events, there remain unanswered questions about the clinical value of treating depression in cardiac patients with antidepressant medication.
In a second sertraline trial, SADHART-HF,69 469 men and women with MDD and chronic systolic HF were randomized to receive either sertraline or placebo for 12 weeks. Participants were followed for a minimum of 6 months. Results showed that while sertraline was safe, its use did not result in greater reductions in depressive symptoms compared with placebo (−7.1 ± 0.5 vs −6.8 ± 0.5) and there were no differences in clinical event rates between patients receiving sertraline compared with those receiving placebo.
In an observational study of patients with HF,44 use of antidepressant medication was associated with increased risk of mortality or hospitalization. Although the potential harmful effects of antidepressant medication could not be ruled out, a more likely interpretation is that antidepressant medication use was a marker for individuals with more severe depression, and that the underlying depression may have contributed to their higher risk. Further, patients who are depressed, despite receiving treatment, may represent a subset of treatment-resistant patients who may be especially vulnerable to further cardiac events. Indeed, worsening depression is associated with worse outcomes in HF patients46; this is consistent with data from the ENRICHD trial, which showed that patients receiving CBT (and, in some cases, antidepressant medication) who failed to improve with treatment had higher mortality rates compared with patients who exhibited a positive response to treatment.70
A fourth randomized trial of CHD patients, the Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial,71 used a modified “2 by 2” design; 284 CHD patients with MDD and HAM-D rating scores of 20 or greater were randomized to receive 12 weeks of (a) interpersonal therapy (IPT) plus clinical management (CM) or (b) CM only and citalopram or matching placebo. Because the same interventionists delivered the CM and IPT, patients assigned to IPT received IPT plus CM within the same (extended) session. Patients receiving citalopram had greater reductions in depressive symptoms compared with placebo, with a small to medium effect size of 0.33, and better remission rates (35.9%) compared with placebo (22.5%). Unexpectedly, patients who received just CM tended to have greater improvements in depressive symptoms compared with patients who received IPT plus CM (P < .07); no clinical CHD end points were assessed, however.
Alternative approaches needed
Taken together, these data illustrate that antidepressant medications may reduce depressive symptoms for some patients; for other patients, however, medication fails to adequately relieve depressive symptoms and may perform no better than placebo. Adverse effects also may affect a subgroup of patients and may be relatively more common or more problematic in older persons with CHD.72 Thus, a need remains to identify alternative approaches for treating depression in cardiac patients. We believe that aerobic exercise, the cornerstone of traditional CR, may be one such approach. Exercise is safe for most cardiac patients,73,74 including patients with HF,75 and, if proven effective as a treatment for depression, exercise would hold several potential advantages over traditional medical therapies: it is relatively inexpensive, improves cardiovascular functioning, and avoids the side effects sometimes associated with medication use.
EXERCISE THERAPY FOR DEPRESSION
Some studies of exercise treatment for CHD patients have tracked depressive symptoms and thus have provided information regarding the potential efficacy of exercise as a treatment for depression in this population.76 –81 Although most previous studies have reported significant improvements in depression after completion of an exercise program, many studies had important methodologic limitations, including the absence of a control group.
In one of the few controlled studies in this field, Stern et al82 randomized 106 male patients who had a recent history of AMI along with elevated depression and anxiety or low fitness to 12 weeks of exercise training, group therapy, or a usual-care control group. At 1-year followup, both the exercise and counseling groups showed improvements in depression relative to controls.
Cross-sectional studies of non-CHD samples have reported that active individuals obtain significantly lower depression scores on self-report measures than sedentary persons.83 Studies also have shown that aerobic exercise may reduce self-reported depressive symptoms in nonclinical populations and in patients diagnosed with MDD.83 In 2001, a meta-analysis evaluating 11 randomized controlled trials of non-CHD patients with MDD84 noted that studies were limited because of self-selection bias, absence of control groups or nonrandom controls, and inadequate assessment of exercise training effects; the authors concluded that “the effectiveness of exercise in reducing symptoms of depression cannot be determined because of a lack of good quality research on clinical populations with adequate followup.”
Randomized controlled trials needed
A subsequent meta-analysis85 included 25 studies; for 23 trials (907 participants) that compared exercise with no treatment or a control intervention, the pooled standardized mean difference (SMD) was −0.82 (95% CI, −1.12, −0.51), indicating a large effect size. However, when only the three trials (216 participants) with adequate allocation concealment, intention to treat analysis, and blinded outcome assessment were included, the pooled SMD was −0.43 (95% CI, −0.88, 0.03), with a point estimate that was half the size of that with all trials. As a result, the authors concluded that “exercise seems to improve depressive symptoms in people with a diagnosis of depression, but when only the methodologically robust trials are included, the effect size is only moderate.”
To date, no randomized clinical trials (RCTs) have examined the effects of exercise on clinical outcomes in depressed cardiac patients. However, data from the ENRICHD trial suggest that exercise may reduce the rates of mortality and nonfatal reinfarction in patients with depression or in post-MI patients who are socially isolated.86 Self-report data were used to categorize participants as exercising regularly or not exercising regularly. After controlling for medical and demographic variables, the magnitude of reduction in risk associated with regular exercise was nearly 40% for nonfatal reinfarction and 50% for mortality. The evidence that exercise mitigates depression, reduces CHD risk factors, and improves CHD outcomes suggests that exercise may be a particularly promising intervention for depressed CHD patients.
COMPARATIVE EFFECTIVENESS OF EXERCISE AND ANTIDEPRESSANT MEDICATION
In 2008, an Institute of Medicine (IOM) report called for a national initiative of research that would provide a basis for better decision-making about how to best treat various medical conditions, including depression. In 2009, the American Reinvestment Recovery Act provided a major boost in funding for comparative effectiveness research (CER). The act allotted $1.1 billion to support this form of research. CER refers to the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition, or to improve the delivery of care. The purpose of CER is to assist consumers, clinicians, purchasers, and policy makers in reaching informed decisions that will improve health care at both the individual and population levels.87
Two research categories inform decision-making
Two broad categories of research have been used to inform decision-making:
- Epidemiologic studies provide evidence linking various treatments with patient outcomes. These sources of data are limited because they seldom specify the basis for medical decisions and they fail to consider patient characteristics that affect both clinical decisions and clinical outcomes. Indeed, it has been suggested that “overcoming the limitations of observational research is the most important frontier of research on study methods.”88
- RCTs address these limitations by randomly assigning patients to different treatment conditions. While this design may eliminate some of the uncertainty and potential confounders that characterize purely observational studies, most RCTs are efficacy studies; patients are carefully selected and a treatment is usually compared with a placebo or usual care.
The RCT design addresses the question of whether a given treatment is effective, but it does not necessarily address questions that many physicians want answers to: namely, is this treatment better than that treatment? Further, physicians want to know if one treatment is more effective than another for a given patient. For example, Hlatky et al89 showed that mortality associated with percutaneous coronary interventions (PCIs) and CABG surgery was comparable; however, mortality with CABG surgery was significantly lower for patients older than 65 years while PCI was superior for patients younger than 55 years. Thus, examination of individual differences may also help to inform clinicians about the optimal therapy for their particular patients.
Treatment of depression not necessarily a research priority
The IOM committee sought advice from a broad range of stakeholders and prioritized areas for research. The top-ranked topic was comparison of treatment strategies for atrial fibrillation, including surgery, catheter ablation, and pharmacologic treatment. Coming in at #98 was comparison of the effectiveness of different treatment strategies (eg, psychotherapy, antidepressants, combination treatment with case management) for depression after MI and their impact on medication adherence, cardiovascular events, hospitalization, and death.
In a second Duke study that compared exercise and antidepressant medication,92 202 adults (153 women; 49 men) diagnosed with MDD were randomly assigned to one of four groups: supervised exercise in a group setting, home-based exercise, antidepressant medication (sertraline, 50 to 200 mg daily), or placebo pill for 16 weeks. Once again, patients underwent the Structured Clinical Interview for Depression and completed the HAM-D. After 4 months of treatment, 41% of participants achieved remission, defined as no longer meeting criteria for MDD and a HAM-D score of less than 8 points. Patients receiving active treatments tended to have higher remission rates than placebo controls: supervised exercise, 45%; home-based exercise, 40%; medication, 47%; placebo, 31% (P = .057). All treatment groups had lower HAM-D scores after treatment; scores for the active treatment groups were not significantly different from the placebo group (P = .23). However, when immediate responders (ie, those patients who reported more than 50% reduction in depressive symptoms after only 1 week of treatment) were excluded from the analysis, patients receiving active treatments (ie, either sertraline or exercise) had greater reductions in depressive symptoms compared with placebo controls (P = .048). There was no difference between the exercise and antidepressant groups. We concluded that the efficacy of exercise appears generally comparable with antidepressant medication and both tend to be better than placebo in patients with MDD. Placebo response rates were high, suggesting that a considerable portion of the therapeutic response could be determined by patient expectations, ongoing symptom monitoring, attention, and other nonspecific factors. Similar to our previous trial, participants who continued to exercise following the completion of the program were less likely to be depressed.93
Another RCT94 also demonstrated that exercise was associated with reduced depression, independent of group support. Participants exercised alone in a secluded setting, and the study included a no-treatment control group. Only 53 of 80 patients actually completed the 12-week trial, however, including only five of 13 no-treatment controls. Moreover, there was no active treatment comparison group, so that an estimate of comparative effectiveness could not be determined.
While these results are preliminary and should be interpreted with caution, it appears that exercise may be comparable with conventional antidepressant medication in reducing depressive symptoms, at least for patients who are willing to try it, and maintenance of exercise reduces the risk of relapse.
SUMMARY
Three decades ago, we recognized that CR was a new frontier for behavioral medicine. We now know that successful rehabilitation of patients with CHD involves modification of lifestyle behaviors, including smoking cessation, dietary modification, and exercise. Exercise is no longer considered unsafe for most cardiac patients, and exercise is currently the key component of CR services. Research also has provided strong evidence that depression is an important risk factor for CHD, although there is no consensus regarding the optimal way to treat depression in CHD patients.95 Research on comparative effectiveness of established and alternative treatments for depressed cardiac patients is a new frontier for future pioneers in heart-brain medicine.
- Blumenthal JA, Califf R, Williams RS, Hindman M. Cardiac rehabilitation: a new frontier for behavioral medicine. J Cardiac Rehabil 1983; 3:637–656.
- Lewis T. Diseases of the Heart. New York: Macmillan; 1933:41–49.
- Levine SA, Lown B. The “chair” treatment of acute thrombosis. Trans Assoc Am Physicians 1951; 64:316–327.
- Cain HD, Frasher WG, Stivelman R. Graded activity program for safe return to self-care after myocardial infarction. JAMA 1961; 177:111–115.
- Hellerstein HK, Ford AB. Rehabilitation of the cardiac patient. JAMA 1957; 14:225–231.
- Naughton J, Bruhn JG, Lategola MT. Effects of physical training on physiologic and behavioral characteristics of cardiac patients. Arch Phys Med Rehabil 1968; 49:131–137.
- O’Connor GT, Buring JE, Yusuf S, et al An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation 1989; 80:234–244.
- Wenger NK, Froelicher ES, Smith LK, et al Cardiac rehabilitation: clinical practice guideline no. 17. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, National Heart, Lung, and Blood Institute; October 1995; AHCPR publication 96-0672.
- Blumenthal JA, Emery CF, Cox DR, et al Exercise training in healthy type A middle-aged men: effects on behavioral and cardiovascular responses. Psychosom Med 1988; 50:418–433.
- Schuler G, Schlierf G, Wirth A, et al Low-fat diet and regular, supervised physical exercise in patients with symptomatic coronary artery disease: reduction of stress-induced myocardial ischemia. Circulation 1988; 77:172–181.
- Jiang W, Trauner MA, Coleman RE, et al Association of physical fitness and transient myocardial ischemia in patients with coronary artery disease. J Cardiopulm Rehabil 1995; 15:431–438.
- Blumenthal JA, Jiang W, Babyak MA, et al Stress management and exercise training in cardiac patients with myocardial ischemia: effects on prognosis and evaluation of mechanisms. Arch Int Med 1997; 157:2213–2223.
- Oldridge NB, Guyatt GH, Fisher ME, Rimm AA. Cardiac rehabilitation after myocardial infarction: combined experience of randomized clinical trials. JAMA 1988; 260:945–950.
- Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2001:CD001800.
- Folkins CH, Sime WE. Physical fitness training and mental health. Am Psychol 1981; 36:373–389.
- Hughes JR. Psychological effects of habitual aerobic exercise: a critical review. Prev Med 1984; 13:66–78.
- Plante TG, Rodin J. Physical fitness and enhanced psychological health. Curr Psychol: Res Rev 1990; 9:3–24.
- Review Panel on Coronary-Prone Behavior and Coronary Heart Disease. Coronary-prone behavior and coronary heart disease: a critical review. Circulation 1981; 63:1199–1215.
- Smith TW. Hostility and health: current status of a psychosomatic hypothesis. Health Psychol 1992; 11:139–150.
- Lett HS, Blumenthal JA, Babyak MA, Strauman TJ, Robins C, Sherwood A. Social support and coronary heart disease: epidemiologic evidence and implications for treatment. Psychosom Med 2005; 67:869–878.
- Lett HS, Blumenthal JA, Babyak MA, et al Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med 2004; 66:305–315.
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66:802–813.
- Kessler RC, Berglund P, Demler O, et al The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCR-R). JAMA 2003; 289:3095–3105.
- Belsher G, Costello CG. Relapse after recovery from unipolar depression: a critical review. Psychol Bull 1988; 104:84–96.
- Strothers HS, Rust G, Minor P, Fresh E, Druss B, Satcher D. Disparities in antidepressant treatment in Medicaid elderly diagnosed with depression. J Am Geriatr Soc 2005; 53:456–461.
- Simpson SM, Krishnan LL, Kunik ME, Ruiz P. Racial disparities in diagnosis and treatment of depression: a literature review. Psychiatr Q 2007; 78:3–14.
- Sclar DA, Robison LM, Skaer TL. Ethnicity/race and the diagnosis of depression and use of antidepressants by adults in the United States. Int Clin Psychopharmacol 2008; 23:106–109.
- Waldman SV, Blumenthal JA, Babyak MA, et al Ethnic differences in the treatment of depression in patients with ischemic heart disease. Am Heart J 2009; 57:77–83.
- Carney RM, Rich MW, Freedland KE, et al Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988; 50:627–633.
- Schleifer SJ, Macari-Hinson MM, Coyle DA, et al The nature and course of depression following myocardial infarction. Arch Intern Med 1989; 149:1785–1789.
- Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction: impact on 6-month survival. JAMA 1993; 270:1819–1825.
- Gonzalez MB, Snyderman TB, Colket JT, et al Depression in patients with coronary artery disease. Depression 1996; 4:57–62.
- Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet 2001; 358:1766–1771.
- Burker EJ, Blumenthal JA, Feldman M, et al Depression in male and female patients undergoing cardiac surgery. Br J Clin Psychol 1995; 34( Pt 1):119–128.
- Lespérance F, Frasure-Smith N, Juneau M, Théroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med 2000; 160:1354–1360.
- Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation 1995; 91:999–1005.
- Barefoot JC, Helms MJ, Mark DB, et al Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol 1996; 78:613–617.
- Burg MM, Benedetto C, Soufer R. Depressive symptoms and mortality two years after coronary artery bypass graft surgery (CABG) in men. Psychosom Med 2003; 65:508–510.
- Blumenthal JA, Lett HS, Babyak MA, et al Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003; 362:604–609.
- Connerney I, Sloan RP, Shapiro PA, Bagiella E, Seckman C. Depression is associated with increased mortality 10 years after coronary artery bypass surgery. Psychosom Med 2010; 72:874–881.
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006; 48:1527–1537.
- Murberg TA, Furze G. Depressive symptoms and mortality in patients with congestive heart failure: a six-year follow-up study. Med Sci Monit 2004; 10:CR643–CR648.
- Jiang W, Alexander J, Christopher E, et al Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856.
- Sherwood A, Blumenthal JA, Trivedi R, et al Relationship of depression to death or hospitalization in patients with heart failure. Arch Int Med 2007; 167:367–373.
- Frasure-Smith N, Lespérance F, Habra M, et al Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation 2009; 120:134–140.
- Sherwood A, Blumenthal JA, Hinderliter AL, et al Worsening depressive symptoms are associated with adverse clinical outcomes in patients with heart failure. J Am Coll Cardiol 2011; 57:418–423.
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001; 24:1069–1078.
- Thakore JH, Richards PJ, Reznek RH, Martin A, Dinan TG. Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biol Psychiatry 1997; 41:1140–1142.
- Musselman DL, Tomer A, Manatunga AK, et al Exaggerated platelet reactivity in major depression. Am J Psychiatry 1996; 153:1313–1317.
- Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry 2000; 61( suppl 1):5–12.
- Akil H, Haskett RF, Young EA, et al Multiple HPA profiles in endogenous depression: effect of age and sex on cortisol and beta-endorphin. Biol Psychiatry 1993; 33:73–85.
- Kop WJ, Gottdiener JS, Tangen CM, et al Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol 2002; 89:419–424.
- Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol 1995; 14:88–90.
- Lehto S, Koukkunen H, Hintikka J, Viinamäki H, Laakso M, Pyörälä K. Depression after coronary heart disease events. Scand Cardiovasc J 2000; 34:580–583.
- Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol 1991; 134:220–231.
- Clinical Practice Guideline Number 5: Depression in Primary Care, 2: Treatment of Major Depression. Rockville, MD: U.S. Department of Health and Human Services, Agency for Health Care Policy and Research; 1993. AHCPR publication 93-0551.
- Anderson IM, Ferrier IN, Baldwin RC, et al Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. J Psychopharmacol 2008; 22:343–396.
- American Psyciatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000; 157( suppl 4):1–45.
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 2008; 5:e45.
- Hansen R, Gaynes B, Thieda P, et al Meta-analysis of major depressive disorder relapse and recurrence with second-generation antidepressants. Psychiatr Serv 2008; 59:1121–1129.
- Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med 2005; 143:415–426.
- Rush AJ, Trivedi MH, Wisniewski SR, et al Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917.
- Olfson M, Marcus SC, Druss B, Elinson L, Tanielian T, Pincus HA. National trends in the outpatient treatment of depression. JAMA 2002; 287:203–209.
- Marcus SC, Olfson M. National trends in the treatment of depression from 1998 to 2007. Arch Gen Psychiatry 2010; 67:1265–1273.
- Berkman LF, Blumenthal J, Burg M, et al Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003; 289:3106–3116.
- Taylor CB, Youngblood ME, Catellier D, et al Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 2005; 62:792–798.
- Glassman AH, O’Connor CM, Califf RM, et al Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Serebruany VL, Glassman AH, Malinin AI, et al Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003; 108:939–44.
- O’Connor CM, Jiang W, Kuchibhatla M, et al Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol 2010; 56:692–699.
- Carney RM, Blumenthal JA, Freedland KE, et al Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosom Med 2004; 66:466–474.
- Lespérance F, Frasure-Smith N, Koszycki D, et al Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) Trial. JAMA 2007; 297:367–379.
- Salzman C, Schneider L, Alexopoulos GS. Pharmacological treatment of depression in late life. In:Bloon F, Kupfer D, eds. Psychopharmacology: Fourth Generation of Progress. New York: Raven Press; 1995.
- Franklin BA, Bonzheim K, Gordon S, Timmis GC. Safety of medically supervised outpatient cardiac rehabilitation exercise therapy: a 16-year follow-up. Chest 1998; 114:902–906.
- Vongvanich P, Paul-Labrador MJ, Merz CN. Safety of medically supervised exercise in a cardiac rehabilitation center. Am J Cardiol 1996; 77:1383–1385.
- O’Connor CM, Whellan DJ, Lee KL, et al Efficacy and safety of exercise training in patients with chronic heart failure: H-F ACTION randomized controlled trial. JAMA 2009; 301:1439–1540.
- Milani RV, Lavie CJ, Cassidy MM. Effects of cardiac rehabilitation and exercise training programs on depression in patients after major coronary events. Am Heart J 1996; 132:726–732.
- Beniamini Y, Rubenstein JJ, Zaichkowsky LD, Crim MC. Effects of high-intensity strength training on quality-of-life parameters in cardiac rehabilitation patients. Am J Cardiol 1997; 80:841–846.
- Maines TY, Lavie CJ, Milani RV, Cassidy MM, Gilliland YE, Murgo JP. Effects of cardiac rehabilitation and exercise programs on exercise capacity, coronary risk factors, behavior, and quality of life in patients with coronary artery disease. South Med J 1997; 90:43–49.
- Milani RV, Lavie CJ. Prevalence and effects of cardiac rehabilitation on depression in the elderly with coronary heart disease. Am J Cardiol 1998; 81:1233–1236.
- Blumenthal JA, Emery CF, Rejeski WJ. The effects of exercise training on psychosocial functioning after myocardial infarction. J Cardiopulmonary Rehabil 1988; 8:183–193.
- Taylor CB, Houston-Miller N, Ahn DK, Haskell W, DeBusk RF. The effects of exercise training programs on psychosocial improvement in uncomplicated postmyocardial infarction patients. J Psychosom Res 1986; 30:581–587.
- Stern MJ, Gorman PA, Kaslow L. The group counseling v exercise therapy study: a controlled intervention with subjects following myocardial infarction. Arch Intern Med 1983; 143:1719–1725.
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002; 32:741–760.
- Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ 2001; 322:763–767.
- Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database Syst Rev 2009:CD004366.
- Blumenthal JA, Babyak MA, Carney RM, et al Exercise, depression, and mortality after myocardial infarction in the ENRICHD trial. Med Sci Sports Exerc 2004; 36:746–755.
- Eden J, Wheatley B, McNeil B, Sox H, eds. Institute of Medicine. Knowing What Works in Health Care. A Roadmap for the Nation. Washington DC: National Academics Press; 2009.
- Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Intern Med 2009; 151:203–205.
- Hlatky MA, Boothroyd DB, Bravata DM, et al Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197.
- Blumenthal JA, Babyak MA, Moore KA, et al Effects of exercise training on older patients with major depression. Arch Intern Med 1999; 159:2349–2356.
- Babyak M, Blumenthal JA, Herman S. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000; 62:633–638.
- Blumenthal JA, Babyak MA, Doraiswamy PM, et al Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007; 69:587–596.
- Hoffman B, Babyak M, Craighead WE, et al Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosom Med 2010; 73:127–133.
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med 2005; 28:1–8.
- Lichtman JH, Bigger JT, Blumenthal JA, et al Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 2008; 118:1768–1775.
I am fortunate to be the recipient of the 2010 Bakken Institute Pioneer Award and feel especially honored to have my work recognized in this way. When informed that I was this year’s recipient, it prompted me to reflect on the meaning of the term “pioneer,” and how it related to me.
WHAT IS A PIONEER?
According to Merriam-Webster’s Collegiate Dictionary, a pioneer is one who (a) ventures into unknown or unclaimed territory to settle; and (b) opens up new areas of thought, research, or development. One requirement for any pioneer is that there be a frontier to explore. Thirty years ago, my colleagues and I began our investigations into cardiac rehabilitation (CR), which at the time we considered to be a new frontier for behavioral medicine.1
EXERCISE-BASED CARDIAC REHABILITATION
Historically, patients who suffered an acute myocardial infarction (AMI) were often discouraged from engaging in physical activity; patients were initially prescribed prolonged bed rest and told to avoid strenuous exercise.2 In the early 1950s, armchair therapy was proposed3 as an initial attempt to mobilize patients after a coronary event. Over the years, the value of physical exercise has been increasingly recognized and exercise is now considered to be the cornerstone of CR.4–7 Today, exercise-based CR, involving aerobic exercise supplemented by resistance training, is offered by virtually all CR programs in the United States.8 Proper medical management is also emphasized, along with dietary modification and smoking cessation, but exercise is the centerpiece of treatment.
Exercise has been shown to reduce traditional risk factors such as hypertension and hyperlipidemia,8 attenuate cardiovascular responses to mental stress,9 and reduce myocardial ischemia.10–12 Although no single study has demonstrated definitively that exercise reduces morbidity in patients with coronary heart disease (CHD), pooling data across clinical trials has shown that exercise may reduce risk of fatal CHD events by 25%.13 A recent, comprehensive meta-analysis by Jolliffe et al14 reported a 27% reduction in all-cause mortality and 31% reduction in cardiac mortality.
Not only is exercise considered beneficial for medical outcomes, but is also recognized as an important factor in improved quality of life. Indeed, there has been increased interest in the value of exercise for improving not just physical health, but also mental health.15–17 The mental health benefits of exercise are especially relevant for cardiac patients, as there is a growing literature documenting the importance of mental health, and, in particular the prognostic significance of depression, in patients with CHD.
PSYCHOSOCIAL RISK FACTORS: THE ROLE OF DEPRESSION IN CORONARY HEART DISEASE
There has long been an interest in psychosocial factors that contribute to the development and progression of CHD. More than three decades ago, researchers identified the type A behavior pattern as a risk factor for CHD.18 When subsequent studies failed to confirm the association of type A with adverse health outcomes, researchers turned their attention to other possible psychosocial risk factors, including anger and hostility,19 low social support,20 and most recently, depression.21 Indeed, the most consistent and compelling evidence is that clinical depression or elevated depressive symptoms in the presence of CHD increase the risk of fatal and nonfatal cardiac events and of all-cause mortality.22
Major depressive disorder (MDD) is a common and often chronic condition. Lifetime incidence estimates for MDD are approximately 12% in men and 20% in women.23 In addition, MDD is marked by high rates of relapse, with 22% to 50% of patients suffering recurrent episodes within 6 months after recovery.24 Furthermore, MDD is underrecognized and undertreated in older adults,25 CHD patients, and, especially, minorities.26–28
Cross-sectional studies have documented a higher prevalence of depression in CHD patients than in the general population. Point estimates range from 14% to as high as 47%, with higher rates recorded most often in patients with unstable angina, heart failure (HF), and patients awaiting coronary artery bypass graft (CABG) surgery.29–36
Depression associated with poor outcomes
A number of prospective studies have found that depression is associated with increased risk for mortality or nonfatal cardiac events in a variety of CHD populations. The most compelling evidence for depression as a risk factor has come from studies in Montreal, Canada. Frasure-Smith and colleagues31 assessed the impact of depression in 222 AMI patients, of whom 35 were diagnosed with MDD at the time of hospitalization. There were 12 deaths (six depressed and six nondepressed) over an initial 6-month followup period, representing more than a fivefold increased risk of death for depressed patients compared with nondepressed patients (hazard ratio, 5.7; 95% confidence interval [CI], 4.6 to 6.9). In a subsequent report,36 in which 896 AMI patients were followed for 1 year, the presence of elevated depressive symptoms was associated with more than a threefold increased risk in cardiac mortality after controlling for other multivariate predictors of mortality (odds ratio, 3.29 for women; 3.05 for men).
Studies of patients with stable CHD also have reported significant associations between the presence of depression and worse clinical outcomes. For example, Barefoot et al37 assessed 1,250 patients with documented CHD using the Zung self-report depression scale at the time of diagnostic coronary angiography and followed patients for up to 19.4 years. Results showed that patients with moderate to severe depression were at 69% greater risk for cardiac death and 78% greater risk for all-cause death.
Depression and heart failure outcomes
Patients with HF represent a particularly vulnerable group; a meta-analysis of depression in HF patients suggested that one in five patients are clinically depressed (range, 9% to 60%).41 Not only is depression in HF patients associated with worse outcomes,42–46 but recent evidence suggests that worsening of depressive symptoms, independent of clinical status, is related to worse outcomes. Sherwood et al46 demonstrated that increased symptoms of depression, as indicated by higher scores on the Beck Depression Inventory (BDI) over a 1-year interval (BDI change [1-point] hazard ratio, 1.07; 95% CI, 1.02 to 1.12; P = .007), were associated with higher risk of death or cardiovascular hospitalization after controlling for baseline depression (baseline BDI hazard ratio, 1.1; 95% CI, 1.06 to 1.14, P < .001) and established risk factors, including HF etiology, age, ejection fraction, N-terminal pro-B-type natriuretic peptides, and prior hospitalizations. Consequently, strategies to reduce depressive symptoms and prevent the worsening of depression may have important implications for improving cardiac health as well as for enhancing quality of life.
MECHANISMS LINKING DEPRESSION AND CHD
CONVENTIONAL APPROACHES TO TREATMENT OF DEPRESSION
Treatment of depression has focused on reduction of symptoms and restoration of function. Antidepressant medications are generally considered the treatment of choice.56 In particular, second-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs) are widely prescribed.57 Current treatment guidelines suggest 6 to 12 weeks of acute treatment followed by a continuation phase of 3 to 9 months to maintain therapeutic benefit.58 However, meta-analyses of antidepressant medications have reported only modest benefits over placebo treatments.59,60 In particular, active drug–placebo differences in antidepressant efficacy are positively correlated with depression severity: antidepressants are often comparable with placebo in patients with low levels of depression but may be superior to placebo among patients with more severe depression. However, the explanation for this relationship may be that placebo is less effective for more depressed patients rather than antidepressants being more effective for more depressed patients.59
For acute treatment of MDD, approximately 60% of patients respond to second-generation antidepressants,61 with a 40% relapse rate after 1 year.62 A recent meta-analysis60 of second-generation antidepressants summarized four comparative trials and 23 placebo-controlled trials and found that second-generation antidepressants were generally comparable with each other. Interestingly, despite the modest benefit of antidepressants, the percentage of patients treated for depression in the United States increased from 0.73% in 1987 to 2.33% in 1997. The proportion of those treated who received antidepressants increased from 37.3% in 1987 to 74.5% in 1997.63 The percentage of treated outpatients who used antidepressants has not increased significantly since 1997, but the use of psycho therapy as a sole treatment declined from 53.6% in 1998 to 43.1% in 2007.64 Moreover, the national expenditure for the outpatient treatment of depression increased from $10.05 billion in 1998 to $12.45 billion in 2007, primarily driven by an increase in expenditures for antidepressant medications.
Uncertainty about value of antidepressant therapy
Despite compelling reasons for treating depression in cardiac patients, the clinical significance of treating depression remains uncertain. To date, only the Enhancing Recovery in CHD Patients (ENRICHD) trial has examined the impact of treating depression in post-MI patients on “hard” clinical end points.65 Although more than 2,400 patients were enrolled in the trial, the results were disappointing. There were only modest differences (ie, two points on the Hamilton Depression Rating Scale [HAM-D]) in reductions of depressive symptoms in the group receiving cognitive behavior therapy (CBT) relative to usual-care controls and there were no treatment group differences in the primary outcome—all-cause mortality and nonfatal cardiac events. By the end of the follow-up period, 28.0% of patients in the CBT group and 20.6% of patients in usual care had received antidepressant medication. Although a subsequent reanalysis of the ENRICHD study revealed that antidepressant use was associated with improved clinical outcomes,66 because patients were not randomized to pharmacologic treatment it could not be concluded that SSRI use was responsible for the improved outcomes.
In a randomized trial of patients with acute coronary syndrome (the Sertraline Antidepressant Heart Attack Randomized Trial, or SADHART),67 almost 400 patients were treated with the SSRI sertraline or with placebo. Reductions in depressive symptoms were similar for patients receiving sertraline compared with placebo in the full sample, although a subgroup analysis revealed that patients with more severe depression (ie, those patients who reported two or more previous episodes) benefited more from sertraline compared with placebo. Interestingly, patients receiving sertraline tended to have more favorable cardiac outcomes, including a composite measure of both “hard” and “soft” clinical events, compared with placebo controls. These results suggested that antidepressant medication may improve underlying physiologic processes, such as platelet function, independent of changes in depression.68 However, because SADHART was not powered to detect differences in clinical events, there remain unanswered questions about the clinical value of treating depression in cardiac patients with antidepressant medication.
In a second sertraline trial, SADHART-HF,69 469 men and women with MDD and chronic systolic HF were randomized to receive either sertraline or placebo for 12 weeks. Participants were followed for a minimum of 6 months. Results showed that while sertraline was safe, its use did not result in greater reductions in depressive symptoms compared with placebo (−7.1 ± 0.5 vs −6.8 ± 0.5) and there were no differences in clinical event rates between patients receiving sertraline compared with those receiving placebo.
In an observational study of patients with HF,44 use of antidepressant medication was associated with increased risk of mortality or hospitalization. Although the potential harmful effects of antidepressant medication could not be ruled out, a more likely interpretation is that antidepressant medication use was a marker for individuals with more severe depression, and that the underlying depression may have contributed to their higher risk. Further, patients who are depressed, despite receiving treatment, may represent a subset of treatment-resistant patients who may be especially vulnerable to further cardiac events. Indeed, worsening depression is associated with worse outcomes in HF patients46; this is consistent with data from the ENRICHD trial, which showed that patients receiving CBT (and, in some cases, antidepressant medication) who failed to improve with treatment had higher mortality rates compared with patients who exhibited a positive response to treatment.70
A fourth randomized trial of CHD patients, the Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial,71 used a modified “2 by 2” design; 284 CHD patients with MDD and HAM-D rating scores of 20 or greater were randomized to receive 12 weeks of (a) interpersonal therapy (IPT) plus clinical management (CM) or (b) CM only and citalopram or matching placebo. Because the same interventionists delivered the CM and IPT, patients assigned to IPT received IPT plus CM within the same (extended) session. Patients receiving citalopram had greater reductions in depressive symptoms compared with placebo, with a small to medium effect size of 0.33, and better remission rates (35.9%) compared with placebo (22.5%). Unexpectedly, patients who received just CM tended to have greater improvements in depressive symptoms compared with patients who received IPT plus CM (P < .07); no clinical CHD end points were assessed, however.
Alternative approaches needed
Taken together, these data illustrate that antidepressant medications may reduce depressive symptoms for some patients; for other patients, however, medication fails to adequately relieve depressive symptoms and may perform no better than placebo. Adverse effects also may affect a subgroup of patients and may be relatively more common or more problematic in older persons with CHD.72 Thus, a need remains to identify alternative approaches for treating depression in cardiac patients. We believe that aerobic exercise, the cornerstone of traditional CR, may be one such approach. Exercise is safe for most cardiac patients,73,74 including patients with HF,75 and, if proven effective as a treatment for depression, exercise would hold several potential advantages over traditional medical therapies: it is relatively inexpensive, improves cardiovascular functioning, and avoids the side effects sometimes associated with medication use.
EXERCISE THERAPY FOR DEPRESSION
Some studies of exercise treatment for CHD patients have tracked depressive symptoms and thus have provided information regarding the potential efficacy of exercise as a treatment for depression in this population.76 –81 Although most previous studies have reported significant improvements in depression after completion of an exercise program, many studies had important methodologic limitations, including the absence of a control group.
In one of the few controlled studies in this field, Stern et al82 randomized 106 male patients who had a recent history of AMI along with elevated depression and anxiety or low fitness to 12 weeks of exercise training, group therapy, or a usual-care control group. At 1-year followup, both the exercise and counseling groups showed improvements in depression relative to controls.
Cross-sectional studies of non-CHD samples have reported that active individuals obtain significantly lower depression scores on self-report measures than sedentary persons.83 Studies also have shown that aerobic exercise may reduce self-reported depressive symptoms in nonclinical populations and in patients diagnosed with MDD.83 In 2001, a meta-analysis evaluating 11 randomized controlled trials of non-CHD patients with MDD84 noted that studies were limited because of self-selection bias, absence of control groups or nonrandom controls, and inadequate assessment of exercise training effects; the authors concluded that “the effectiveness of exercise in reducing symptoms of depression cannot be determined because of a lack of good quality research on clinical populations with adequate followup.”
Randomized controlled trials needed
A subsequent meta-analysis85 included 25 studies; for 23 trials (907 participants) that compared exercise with no treatment or a control intervention, the pooled standardized mean difference (SMD) was −0.82 (95% CI, −1.12, −0.51), indicating a large effect size. However, when only the three trials (216 participants) with adequate allocation concealment, intention to treat analysis, and blinded outcome assessment were included, the pooled SMD was −0.43 (95% CI, −0.88, 0.03), with a point estimate that was half the size of that with all trials. As a result, the authors concluded that “exercise seems to improve depressive symptoms in people with a diagnosis of depression, but when only the methodologically robust trials are included, the effect size is only moderate.”
To date, no randomized clinical trials (RCTs) have examined the effects of exercise on clinical outcomes in depressed cardiac patients. However, data from the ENRICHD trial suggest that exercise may reduce the rates of mortality and nonfatal reinfarction in patients with depression or in post-MI patients who are socially isolated.86 Self-report data were used to categorize participants as exercising regularly or not exercising regularly. After controlling for medical and demographic variables, the magnitude of reduction in risk associated with regular exercise was nearly 40% for nonfatal reinfarction and 50% for mortality. The evidence that exercise mitigates depression, reduces CHD risk factors, and improves CHD outcomes suggests that exercise may be a particularly promising intervention for depressed CHD patients.
COMPARATIVE EFFECTIVENESS OF EXERCISE AND ANTIDEPRESSANT MEDICATION
In 2008, an Institute of Medicine (IOM) report called for a national initiative of research that would provide a basis for better decision-making about how to best treat various medical conditions, including depression. In 2009, the American Reinvestment Recovery Act provided a major boost in funding for comparative effectiveness research (CER). The act allotted $1.1 billion to support this form of research. CER refers to the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition, or to improve the delivery of care. The purpose of CER is to assist consumers, clinicians, purchasers, and policy makers in reaching informed decisions that will improve health care at both the individual and population levels.87
Two research categories inform decision-making
Two broad categories of research have been used to inform decision-making:
- Epidemiologic studies provide evidence linking various treatments with patient outcomes. These sources of data are limited because they seldom specify the basis for medical decisions and they fail to consider patient characteristics that affect both clinical decisions and clinical outcomes. Indeed, it has been suggested that “overcoming the limitations of observational research is the most important frontier of research on study methods.”88
- RCTs address these limitations by randomly assigning patients to different treatment conditions. While this design may eliminate some of the uncertainty and potential confounders that characterize purely observational studies, most RCTs are efficacy studies; patients are carefully selected and a treatment is usually compared with a placebo or usual care.
The RCT design addresses the question of whether a given treatment is effective, but it does not necessarily address questions that many physicians want answers to: namely, is this treatment better than that treatment? Further, physicians want to know if one treatment is more effective than another for a given patient. For example, Hlatky et al89 showed that mortality associated with percutaneous coronary interventions (PCIs) and CABG surgery was comparable; however, mortality with CABG surgery was significantly lower for patients older than 65 years while PCI was superior for patients younger than 55 years. Thus, examination of individual differences may also help to inform clinicians about the optimal therapy for their particular patients.
Treatment of depression not necessarily a research priority
The IOM committee sought advice from a broad range of stakeholders and prioritized areas for research. The top-ranked topic was comparison of treatment strategies for atrial fibrillation, including surgery, catheter ablation, and pharmacologic treatment. Coming in at #98 was comparison of the effectiveness of different treatment strategies (eg, psychotherapy, antidepressants, combination treatment with case management) for depression after MI and their impact on medication adherence, cardiovascular events, hospitalization, and death.
In a second Duke study that compared exercise and antidepressant medication,92 202 adults (153 women; 49 men) diagnosed with MDD were randomly assigned to one of four groups: supervised exercise in a group setting, home-based exercise, antidepressant medication (sertraline, 50 to 200 mg daily), or placebo pill for 16 weeks. Once again, patients underwent the Structured Clinical Interview for Depression and completed the HAM-D. After 4 months of treatment, 41% of participants achieved remission, defined as no longer meeting criteria for MDD and a HAM-D score of less than 8 points. Patients receiving active treatments tended to have higher remission rates than placebo controls: supervised exercise, 45%; home-based exercise, 40%; medication, 47%; placebo, 31% (P = .057). All treatment groups had lower HAM-D scores after treatment; scores for the active treatment groups were not significantly different from the placebo group (P = .23). However, when immediate responders (ie, those patients who reported more than 50% reduction in depressive symptoms after only 1 week of treatment) were excluded from the analysis, patients receiving active treatments (ie, either sertraline or exercise) had greater reductions in depressive symptoms compared with placebo controls (P = .048). There was no difference between the exercise and antidepressant groups. We concluded that the efficacy of exercise appears generally comparable with antidepressant medication and both tend to be better than placebo in patients with MDD. Placebo response rates were high, suggesting that a considerable portion of the therapeutic response could be determined by patient expectations, ongoing symptom monitoring, attention, and other nonspecific factors. Similar to our previous trial, participants who continued to exercise following the completion of the program were less likely to be depressed.93
Another RCT94 also demonstrated that exercise was associated with reduced depression, independent of group support. Participants exercised alone in a secluded setting, and the study included a no-treatment control group. Only 53 of 80 patients actually completed the 12-week trial, however, including only five of 13 no-treatment controls. Moreover, there was no active treatment comparison group, so that an estimate of comparative effectiveness could not be determined.
While these results are preliminary and should be interpreted with caution, it appears that exercise may be comparable with conventional antidepressant medication in reducing depressive symptoms, at least for patients who are willing to try it, and maintenance of exercise reduces the risk of relapse.
SUMMARY
Three decades ago, we recognized that CR was a new frontier for behavioral medicine. We now know that successful rehabilitation of patients with CHD involves modification of lifestyle behaviors, including smoking cessation, dietary modification, and exercise. Exercise is no longer considered unsafe for most cardiac patients, and exercise is currently the key component of CR services. Research also has provided strong evidence that depression is an important risk factor for CHD, although there is no consensus regarding the optimal way to treat depression in CHD patients.95 Research on comparative effectiveness of established and alternative treatments for depressed cardiac patients is a new frontier for future pioneers in heart-brain medicine.
I am fortunate to be the recipient of the 2010 Bakken Institute Pioneer Award and feel especially honored to have my work recognized in this way. When informed that I was this year’s recipient, it prompted me to reflect on the meaning of the term “pioneer,” and how it related to me.
WHAT IS A PIONEER?
According to Merriam-Webster’s Collegiate Dictionary, a pioneer is one who (a) ventures into unknown or unclaimed territory to settle; and (b) opens up new areas of thought, research, or development. One requirement for any pioneer is that there be a frontier to explore. Thirty years ago, my colleagues and I began our investigations into cardiac rehabilitation (CR), which at the time we considered to be a new frontier for behavioral medicine.1
EXERCISE-BASED CARDIAC REHABILITATION
Historically, patients who suffered an acute myocardial infarction (AMI) were often discouraged from engaging in physical activity; patients were initially prescribed prolonged bed rest and told to avoid strenuous exercise.2 In the early 1950s, armchair therapy was proposed3 as an initial attempt to mobilize patients after a coronary event. Over the years, the value of physical exercise has been increasingly recognized and exercise is now considered to be the cornerstone of CR.4–7 Today, exercise-based CR, involving aerobic exercise supplemented by resistance training, is offered by virtually all CR programs in the United States.8 Proper medical management is also emphasized, along with dietary modification and smoking cessation, but exercise is the centerpiece of treatment.
Exercise has been shown to reduce traditional risk factors such as hypertension and hyperlipidemia,8 attenuate cardiovascular responses to mental stress,9 and reduce myocardial ischemia.10–12 Although no single study has demonstrated definitively that exercise reduces morbidity in patients with coronary heart disease (CHD), pooling data across clinical trials has shown that exercise may reduce risk of fatal CHD events by 25%.13 A recent, comprehensive meta-analysis by Jolliffe et al14 reported a 27% reduction in all-cause mortality and 31% reduction in cardiac mortality.
Not only is exercise considered beneficial for medical outcomes, but is also recognized as an important factor in improved quality of life. Indeed, there has been increased interest in the value of exercise for improving not just physical health, but also mental health.15–17 The mental health benefits of exercise are especially relevant for cardiac patients, as there is a growing literature documenting the importance of mental health, and, in particular the prognostic significance of depression, in patients with CHD.
PSYCHOSOCIAL RISK FACTORS: THE ROLE OF DEPRESSION IN CORONARY HEART DISEASE
There has long been an interest in psychosocial factors that contribute to the development and progression of CHD. More than three decades ago, researchers identified the type A behavior pattern as a risk factor for CHD.18 When subsequent studies failed to confirm the association of type A with adverse health outcomes, researchers turned their attention to other possible psychosocial risk factors, including anger and hostility,19 low social support,20 and most recently, depression.21 Indeed, the most consistent and compelling evidence is that clinical depression or elevated depressive symptoms in the presence of CHD increase the risk of fatal and nonfatal cardiac events and of all-cause mortality.22
Major depressive disorder (MDD) is a common and often chronic condition. Lifetime incidence estimates for MDD are approximately 12% in men and 20% in women.23 In addition, MDD is marked by high rates of relapse, with 22% to 50% of patients suffering recurrent episodes within 6 months after recovery.24 Furthermore, MDD is underrecognized and undertreated in older adults,25 CHD patients, and, especially, minorities.26–28
Cross-sectional studies have documented a higher prevalence of depression in CHD patients than in the general population. Point estimates range from 14% to as high as 47%, with higher rates recorded most often in patients with unstable angina, heart failure (HF), and patients awaiting coronary artery bypass graft (CABG) surgery.29–36
Depression associated with poor outcomes
A number of prospective studies have found that depression is associated with increased risk for mortality or nonfatal cardiac events in a variety of CHD populations. The most compelling evidence for depression as a risk factor has come from studies in Montreal, Canada. Frasure-Smith and colleagues31 assessed the impact of depression in 222 AMI patients, of whom 35 were diagnosed with MDD at the time of hospitalization. There were 12 deaths (six depressed and six nondepressed) over an initial 6-month followup period, representing more than a fivefold increased risk of death for depressed patients compared with nondepressed patients (hazard ratio, 5.7; 95% confidence interval [CI], 4.6 to 6.9). In a subsequent report,36 in which 896 AMI patients were followed for 1 year, the presence of elevated depressive symptoms was associated with more than a threefold increased risk in cardiac mortality after controlling for other multivariate predictors of mortality (odds ratio, 3.29 for women; 3.05 for men).
Studies of patients with stable CHD also have reported significant associations between the presence of depression and worse clinical outcomes. For example, Barefoot et al37 assessed 1,250 patients with documented CHD using the Zung self-report depression scale at the time of diagnostic coronary angiography and followed patients for up to 19.4 years. Results showed that patients with moderate to severe depression were at 69% greater risk for cardiac death and 78% greater risk for all-cause death.
Depression and heart failure outcomes
Patients with HF represent a particularly vulnerable group; a meta-analysis of depression in HF patients suggested that one in five patients are clinically depressed (range, 9% to 60%).41 Not only is depression in HF patients associated with worse outcomes,42–46 but recent evidence suggests that worsening of depressive symptoms, independent of clinical status, is related to worse outcomes. Sherwood et al46 demonstrated that increased symptoms of depression, as indicated by higher scores on the Beck Depression Inventory (BDI) over a 1-year interval (BDI change [1-point] hazard ratio, 1.07; 95% CI, 1.02 to 1.12; P = .007), were associated with higher risk of death or cardiovascular hospitalization after controlling for baseline depression (baseline BDI hazard ratio, 1.1; 95% CI, 1.06 to 1.14, P < .001) and established risk factors, including HF etiology, age, ejection fraction, N-terminal pro-B-type natriuretic peptides, and prior hospitalizations. Consequently, strategies to reduce depressive symptoms and prevent the worsening of depression may have important implications for improving cardiac health as well as for enhancing quality of life.
MECHANISMS LINKING DEPRESSION AND CHD
CONVENTIONAL APPROACHES TO TREATMENT OF DEPRESSION
Treatment of depression has focused on reduction of symptoms and restoration of function. Antidepressant medications are generally considered the treatment of choice.56 In particular, second-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs) are widely prescribed.57 Current treatment guidelines suggest 6 to 12 weeks of acute treatment followed by a continuation phase of 3 to 9 months to maintain therapeutic benefit.58 However, meta-analyses of antidepressant medications have reported only modest benefits over placebo treatments.59,60 In particular, active drug–placebo differences in antidepressant efficacy are positively correlated with depression severity: antidepressants are often comparable with placebo in patients with low levels of depression but may be superior to placebo among patients with more severe depression. However, the explanation for this relationship may be that placebo is less effective for more depressed patients rather than antidepressants being more effective for more depressed patients.59
For acute treatment of MDD, approximately 60% of patients respond to second-generation antidepressants,61 with a 40% relapse rate after 1 year.62 A recent meta-analysis60 of second-generation antidepressants summarized four comparative trials and 23 placebo-controlled trials and found that second-generation antidepressants were generally comparable with each other. Interestingly, despite the modest benefit of antidepressants, the percentage of patients treated for depression in the United States increased from 0.73% in 1987 to 2.33% in 1997. The proportion of those treated who received antidepressants increased from 37.3% in 1987 to 74.5% in 1997.63 The percentage of treated outpatients who used antidepressants has not increased significantly since 1997, but the use of psycho therapy as a sole treatment declined from 53.6% in 1998 to 43.1% in 2007.64 Moreover, the national expenditure for the outpatient treatment of depression increased from $10.05 billion in 1998 to $12.45 billion in 2007, primarily driven by an increase in expenditures for antidepressant medications.
Uncertainty about value of antidepressant therapy
Despite compelling reasons for treating depression in cardiac patients, the clinical significance of treating depression remains uncertain. To date, only the Enhancing Recovery in CHD Patients (ENRICHD) trial has examined the impact of treating depression in post-MI patients on “hard” clinical end points.65 Although more than 2,400 patients were enrolled in the trial, the results were disappointing. There were only modest differences (ie, two points on the Hamilton Depression Rating Scale [HAM-D]) in reductions of depressive symptoms in the group receiving cognitive behavior therapy (CBT) relative to usual-care controls and there were no treatment group differences in the primary outcome—all-cause mortality and nonfatal cardiac events. By the end of the follow-up period, 28.0% of patients in the CBT group and 20.6% of patients in usual care had received antidepressant medication. Although a subsequent reanalysis of the ENRICHD study revealed that antidepressant use was associated with improved clinical outcomes,66 because patients were not randomized to pharmacologic treatment it could not be concluded that SSRI use was responsible for the improved outcomes.
In a randomized trial of patients with acute coronary syndrome (the Sertraline Antidepressant Heart Attack Randomized Trial, or SADHART),67 almost 400 patients were treated with the SSRI sertraline or with placebo. Reductions in depressive symptoms were similar for patients receiving sertraline compared with placebo in the full sample, although a subgroup analysis revealed that patients with more severe depression (ie, those patients who reported two or more previous episodes) benefited more from sertraline compared with placebo. Interestingly, patients receiving sertraline tended to have more favorable cardiac outcomes, including a composite measure of both “hard” and “soft” clinical events, compared with placebo controls. These results suggested that antidepressant medication may improve underlying physiologic processes, such as platelet function, independent of changes in depression.68 However, because SADHART was not powered to detect differences in clinical events, there remain unanswered questions about the clinical value of treating depression in cardiac patients with antidepressant medication.
In a second sertraline trial, SADHART-HF,69 469 men and women with MDD and chronic systolic HF were randomized to receive either sertraline or placebo for 12 weeks. Participants were followed for a minimum of 6 months. Results showed that while sertraline was safe, its use did not result in greater reductions in depressive symptoms compared with placebo (−7.1 ± 0.5 vs −6.8 ± 0.5) and there were no differences in clinical event rates between patients receiving sertraline compared with those receiving placebo.
In an observational study of patients with HF,44 use of antidepressant medication was associated with increased risk of mortality or hospitalization. Although the potential harmful effects of antidepressant medication could not be ruled out, a more likely interpretation is that antidepressant medication use was a marker for individuals with more severe depression, and that the underlying depression may have contributed to their higher risk. Further, patients who are depressed, despite receiving treatment, may represent a subset of treatment-resistant patients who may be especially vulnerable to further cardiac events. Indeed, worsening depression is associated with worse outcomes in HF patients46; this is consistent with data from the ENRICHD trial, which showed that patients receiving CBT (and, in some cases, antidepressant medication) who failed to improve with treatment had higher mortality rates compared with patients who exhibited a positive response to treatment.70
A fourth randomized trial of CHD patients, the Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial,71 used a modified “2 by 2” design; 284 CHD patients with MDD and HAM-D rating scores of 20 or greater were randomized to receive 12 weeks of (a) interpersonal therapy (IPT) plus clinical management (CM) or (b) CM only and citalopram or matching placebo. Because the same interventionists delivered the CM and IPT, patients assigned to IPT received IPT plus CM within the same (extended) session. Patients receiving citalopram had greater reductions in depressive symptoms compared with placebo, with a small to medium effect size of 0.33, and better remission rates (35.9%) compared with placebo (22.5%). Unexpectedly, patients who received just CM tended to have greater improvements in depressive symptoms compared with patients who received IPT plus CM (P < .07); no clinical CHD end points were assessed, however.
Alternative approaches needed
Taken together, these data illustrate that antidepressant medications may reduce depressive symptoms for some patients; for other patients, however, medication fails to adequately relieve depressive symptoms and may perform no better than placebo. Adverse effects also may affect a subgroup of patients and may be relatively more common or more problematic in older persons with CHD.72 Thus, a need remains to identify alternative approaches for treating depression in cardiac patients. We believe that aerobic exercise, the cornerstone of traditional CR, may be one such approach. Exercise is safe for most cardiac patients,73,74 including patients with HF,75 and, if proven effective as a treatment for depression, exercise would hold several potential advantages over traditional medical therapies: it is relatively inexpensive, improves cardiovascular functioning, and avoids the side effects sometimes associated with medication use.
EXERCISE THERAPY FOR DEPRESSION
Some studies of exercise treatment for CHD patients have tracked depressive symptoms and thus have provided information regarding the potential efficacy of exercise as a treatment for depression in this population.76 –81 Although most previous studies have reported significant improvements in depression after completion of an exercise program, many studies had important methodologic limitations, including the absence of a control group.
In one of the few controlled studies in this field, Stern et al82 randomized 106 male patients who had a recent history of AMI along with elevated depression and anxiety or low fitness to 12 weeks of exercise training, group therapy, or a usual-care control group. At 1-year followup, both the exercise and counseling groups showed improvements in depression relative to controls.
Cross-sectional studies of non-CHD samples have reported that active individuals obtain significantly lower depression scores on self-report measures than sedentary persons.83 Studies also have shown that aerobic exercise may reduce self-reported depressive symptoms in nonclinical populations and in patients diagnosed with MDD.83 In 2001, a meta-analysis evaluating 11 randomized controlled trials of non-CHD patients with MDD84 noted that studies were limited because of self-selection bias, absence of control groups or nonrandom controls, and inadequate assessment of exercise training effects; the authors concluded that “the effectiveness of exercise in reducing symptoms of depression cannot be determined because of a lack of good quality research on clinical populations with adequate followup.”
Randomized controlled trials needed
A subsequent meta-analysis85 included 25 studies; for 23 trials (907 participants) that compared exercise with no treatment or a control intervention, the pooled standardized mean difference (SMD) was −0.82 (95% CI, −1.12, −0.51), indicating a large effect size. However, when only the three trials (216 participants) with adequate allocation concealment, intention to treat analysis, and blinded outcome assessment were included, the pooled SMD was −0.43 (95% CI, −0.88, 0.03), with a point estimate that was half the size of that with all trials. As a result, the authors concluded that “exercise seems to improve depressive symptoms in people with a diagnosis of depression, but when only the methodologically robust trials are included, the effect size is only moderate.”
To date, no randomized clinical trials (RCTs) have examined the effects of exercise on clinical outcomes in depressed cardiac patients. However, data from the ENRICHD trial suggest that exercise may reduce the rates of mortality and nonfatal reinfarction in patients with depression or in post-MI patients who are socially isolated.86 Self-report data were used to categorize participants as exercising regularly or not exercising regularly. After controlling for medical and demographic variables, the magnitude of reduction in risk associated with regular exercise was nearly 40% for nonfatal reinfarction and 50% for mortality. The evidence that exercise mitigates depression, reduces CHD risk factors, and improves CHD outcomes suggests that exercise may be a particularly promising intervention for depressed CHD patients.
COMPARATIVE EFFECTIVENESS OF EXERCISE AND ANTIDEPRESSANT MEDICATION
In 2008, an Institute of Medicine (IOM) report called for a national initiative of research that would provide a basis for better decision-making about how to best treat various medical conditions, including depression. In 2009, the American Reinvestment Recovery Act provided a major boost in funding for comparative effectiveness research (CER). The act allotted $1.1 billion to support this form of research. CER refers to the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition, or to improve the delivery of care. The purpose of CER is to assist consumers, clinicians, purchasers, and policy makers in reaching informed decisions that will improve health care at both the individual and population levels.87
Two research categories inform decision-making
Two broad categories of research have been used to inform decision-making:
- Epidemiologic studies provide evidence linking various treatments with patient outcomes. These sources of data are limited because they seldom specify the basis for medical decisions and they fail to consider patient characteristics that affect both clinical decisions and clinical outcomes. Indeed, it has been suggested that “overcoming the limitations of observational research is the most important frontier of research on study methods.”88
- RCTs address these limitations by randomly assigning patients to different treatment conditions. While this design may eliminate some of the uncertainty and potential confounders that characterize purely observational studies, most RCTs are efficacy studies; patients are carefully selected and a treatment is usually compared with a placebo or usual care.
The RCT design addresses the question of whether a given treatment is effective, but it does not necessarily address questions that many physicians want answers to: namely, is this treatment better than that treatment? Further, physicians want to know if one treatment is more effective than another for a given patient. For example, Hlatky et al89 showed that mortality associated with percutaneous coronary interventions (PCIs) and CABG surgery was comparable; however, mortality with CABG surgery was significantly lower for patients older than 65 years while PCI was superior for patients younger than 55 years. Thus, examination of individual differences may also help to inform clinicians about the optimal therapy for their particular patients.
Treatment of depression not necessarily a research priority
The IOM committee sought advice from a broad range of stakeholders and prioritized areas for research. The top-ranked topic was comparison of treatment strategies for atrial fibrillation, including surgery, catheter ablation, and pharmacologic treatment. Coming in at #98 was comparison of the effectiveness of different treatment strategies (eg, psychotherapy, antidepressants, combination treatment with case management) for depression after MI and their impact on medication adherence, cardiovascular events, hospitalization, and death.
In a second Duke study that compared exercise and antidepressant medication,92 202 adults (153 women; 49 men) diagnosed with MDD were randomly assigned to one of four groups: supervised exercise in a group setting, home-based exercise, antidepressant medication (sertraline, 50 to 200 mg daily), or placebo pill for 16 weeks. Once again, patients underwent the Structured Clinical Interview for Depression and completed the HAM-D. After 4 months of treatment, 41% of participants achieved remission, defined as no longer meeting criteria for MDD and a HAM-D score of less than 8 points. Patients receiving active treatments tended to have higher remission rates than placebo controls: supervised exercise, 45%; home-based exercise, 40%; medication, 47%; placebo, 31% (P = .057). All treatment groups had lower HAM-D scores after treatment; scores for the active treatment groups were not significantly different from the placebo group (P = .23). However, when immediate responders (ie, those patients who reported more than 50% reduction in depressive symptoms after only 1 week of treatment) were excluded from the analysis, patients receiving active treatments (ie, either sertraline or exercise) had greater reductions in depressive symptoms compared with placebo controls (P = .048). There was no difference between the exercise and antidepressant groups. We concluded that the efficacy of exercise appears generally comparable with antidepressant medication and both tend to be better than placebo in patients with MDD. Placebo response rates were high, suggesting that a considerable portion of the therapeutic response could be determined by patient expectations, ongoing symptom monitoring, attention, and other nonspecific factors. Similar to our previous trial, participants who continued to exercise following the completion of the program were less likely to be depressed.93
Another RCT94 also demonstrated that exercise was associated with reduced depression, independent of group support. Participants exercised alone in a secluded setting, and the study included a no-treatment control group. Only 53 of 80 patients actually completed the 12-week trial, however, including only five of 13 no-treatment controls. Moreover, there was no active treatment comparison group, so that an estimate of comparative effectiveness could not be determined.
While these results are preliminary and should be interpreted with caution, it appears that exercise may be comparable with conventional antidepressant medication in reducing depressive symptoms, at least for patients who are willing to try it, and maintenance of exercise reduces the risk of relapse.
SUMMARY
Three decades ago, we recognized that CR was a new frontier for behavioral medicine. We now know that successful rehabilitation of patients with CHD involves modification of lifestyle behaviors, including smoking cessation, dietary modification, and exercise. Exercise is no longer considered unsafe for most cardiac patients, and exercise is currently the key component of CR services. Research also has provided strong evidence that depression is an important risk factor for CHD, although there is no consensus regarding the optimal way to treat depression in CHD patients.95 Research on comparative effectiveness of established and alternative treatments for depressed cardiac patients is a new frontier for future pioneers in heart-brain medicine.
- Blumenthal JA, Califf R, Williams RS, Hindman M. Cardiac rehabilitation: a new frontier for behavioral medicine. J Cardiac Rehabil 1983; 3:637–656.
- Lewis T. Diseases of the Heart. New York: Macmillan; 1933:41–49.
- Levine SA, Lown B. The “chair” treatment of acute thrombosis. Trans Assoc Am Physicians 1951; 64:316–327.
- Cain HD, Frasher WG, Stivelman R. Graded activity program for safe return to self-care after myocardial infarction. JAMA 1961; 177:111–115.
- Hellerstein HK, Ford AB. Rehabilitation of the cardiac patient. JAMA 1957; 14:225–231.
- Naughton J, Bruhn JG, Lategola MT. Effects of physical training on physiologic and behavioral characteristics of cardiac patients. Arch Phys Med Rehabil 1968; 49:131–137.
- O’Connor GT, Buring JE, Yusuf S, et al An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation 1989; 80:234–244.
- Wenger NK, Froelicher ES, Smith LK, et al Cardiac rehabilitation: clinical practice guideline no. 17. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, National Heart, Lung, and Blood Institute; October 1995; AHCPR publication 96-0672.
- Blumenthal JA, Emery CF, Cox DR, et al Exercise training in healthy type A middle-aged men: effects on behavioral and cardiovascular responses. Psychosom Med 1988; 50:418–433.
- Schuler G, Schlierf G, Wirth A, et al Low-fat diet and regular, supervised physical exercise in patients with symptomatic coronary artery disease: reduction of stress-induced myocardial ischemia. Circulation 1988; 77:172–181.
- Jiang W, Trauner MA, Coleman RE, et al Association of physical fitness and transient myocardial ischemia in patients with coronary artery disease. J Cardiopulm Rehabil 1995; 15:431–438.
- Blumenthal JA, Jiang W, Babyak MA, et al Stress management and exercise training in cardiac patients with myocardial ischemia: effects on prognosis and evaluation of mechanisms. Arch Int Med 1997; 157:2213–2223.
- Oldridge NB, Guyatt GH, Fisher ME, Rimm AA. Cardiac rehabilitation after myocardial infarction: combined experience of randomized clinical trials. JAMA 1988; 260:945–950.
- Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2001:CD001800.
- Folkins CH, Sime WE. Physical fitness training and mental health. Am Psychol 1981; 36:373–389.
- Hughes JR. Psychological effects of habitual aerobic exercise: a critical review. Prev Med 1984; 13:66–78.
- Plante TG, Rodin J. Physical fitness and enhanced psychological health. Curr Psychol: Res Rev 1990; 9:3–24.
- Review Panel on Coronary-Prone Behavior and Coronary Heart Disease. Coronary-prone behavior and coronary heart disease: a critical review. Circulation 1981; 63:1199–1215.
- Smith TW. Hostility and health: current status of a psychosomatic hypothesis. Health Psychol 1992; 11:139–150.
- Lett HS, Blumenthal JA, Babyak MA, Strauman TJ, Robins C, Sherwood A. Social support and coronary heart disease: epidemiologic evidence and implications for treatment. Psychosom Med 2005; 67:869–878.
- Lett HS, Blumenthal JA, Babyak MA, et al Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med 2004; 66:305–315.
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66:802–813.
- Kessler RC, Berglund P, Demler O, et al The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCR-R). JAMA 2003; 289:3095–3105.
- Belsher G, Costello CG. Relapse after recovery from unipolar depression: a critical review. Psychol Bull 1988; 104:84–96.
- Strothers HS, Rust G, Minor P, Fresh E, Druss B, Satcher D. Disparities in antidepressant treatment in Medicaid elderly diagnosed with depression. J Am Geriatr Soc 2005; 53:456–461.
- Simpson SM, Krishnan LL, Kunik ME, Ruiz P. Racial disparities in diagnosis and treatment of depression: a literature review. Psychiatr Q 2007; 78:3–14.
- Sclar DA, Robison LM, Skaer TL. Ethnicity/race and the diagnosis of depression and use of antidepressants by adults in the United States. Int Clin Psychopharmacol 2008; 23:106–109.
- Waldman SV, Blumenthal JA, Babyak MA, et al Ethnic differences in the treatment of depression in patients with ischemic heart disease. Am Heart J 2009; 57:77–83.
- Carney RM, Rich MW, Freedland KE, et al Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988; 50:627–633.
- Schleifer SJ, Macari-Hinson MM, Coyle DA, et al The nature and course of depression following myocardial infarction. Arch Intern Med 1989; 149:1785–1789.
- Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction: impact on 6-month survival. JAMA 1993; 270:1819–1825.
- Gonzalez MB, Snyderman TB, Colket JT, et al Depression in patients with coronary artery disease. Depression 1996; 4:57–62.
- Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet 2001; 358:1766–1771.
- Burker EJ, Blumenthal JA, Feldman M, et al Depression in male and female patients undergoing cardiac surgery. Br J Clin Psychol 1995; 34( Pt 1):119–128.
- Lespérance F, Frasure-Smith N, Juneau M, Théroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med 2000; 160:1354–1360.
- Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation 1995; 91:999–1005.
- Barefoot JC, Helms MJ, Mark DB, et al Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol 1996; 78:613–617.
- Burg MM, Benedetto C, Soufer R. Depressive symptoms and mortality two years after coronary artery bypass graft surgery (CABG) in men. Psychosom Med 2003; 65:508–510.
- Blumenthal JA, Lett HS, Babyak MA, et al Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003; 362:604–609.
- Connerney I, Sloan RP, Shapiro PA, Bagiella E, Seckman C. Depression is associated with increased mortality 10 years after coronary artery bypass surgery. Psychosom Med 2010; 72:874–881.
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006; 48:1527–1537.
- Murberg TA, Furze G. Depressive symptoms and mortality in patients with congestive heart failure: a six-year follow-up study. Med Sci Monit 2004; 10:CR643–CR648.
- Jiang W, Alexander J, Christopher E, et al Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856.
- Sherwood A, Blumenthal JA, Trivedi R, et al Relationship of depression to death or hospitalization in patients with heart failure. Arch Int Med 2007; 167:367–373.
- Frasure-Smith N, Lespérance F, Habra M, et al Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation 2009; 120:134–140.
- Sherwood A, Blumenthal JA, Hinderliter AL, et al Worsening depressive symptoms are associated with adverse clinical outcomes in patients with heart failure. J Am Coll Cardiol 2011; 57:418–423.
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001; 24:1069–1078.
- Thakore JH, Richards PJ, Reznek RH, Martin A, Dinan TG. Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biol Psychiatry 1997; 41:1140–1142.
- Musselman DL, Tomer A, Manatunga AK, et al Exaggerated platelet reactivity in major depression. Am J Psychiatry 1996; 153:1313–1317.
- Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry 2000; 61( suppl 1):5–12.
- Akil H, Haskett RF, Young EA, et al Multiple HPA profiles in endogenous depression: effect of age and sex on cortisol and beta-endorphin. Biol Psychiatry 1993; 33:73–85.
- Kop WJ, Gottdiener JS, Tangen CM, et al Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol 2002; 89:419–424.
- Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol 1995; 14:88–90.
- Lehto S, Koukkunen H, Hintikka J, Viinamäki H, Laakso M, Pyörälä K. Depression after coronary heart disease events. Scand Cardiovasc J 2000; 34:580–583.
- Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol 1991; 134:220–231.
- Clinical Practice Guideline Number 5: Depression in Primary Care, 2: Treatment of Major Depression. Rockville, MD: U.S. Department of Health and Human Services, Agency for Health Care Policy and Research; 1993. AHCPR publication 93-0551.
- Anderson IM, Ferrier IN, Baldwin RC, et al Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. J Psychopharmacol 2008; 22:343–396.
- American Psyciatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000; 157( suppl 4):1–45.
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 2008; 5:e45.
- Hansen R, Gaynes B, Thieda P, et al Meta-analysis of major depressive disorder relapse and recurrence with second-generation antidepressants. Psychiatr Serv 2008; 59:1121–1129.
- Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med 2005; 143:415–426.
- Rush AJ, Trivedi MH, Wisniewski SR, et al Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917.
- Olfson M, Marcus SC, Druss B, Elinson L, Tanielian T, Pincus HA. National trends in the outpatient treatment of depression. JAMA 2002; 287:203–209.
- Marcus SC, Olfson M. National trends in the treatment of depression from 1998 to 2007. Arch Gen Psychiatry 2010; 67:1265–1273.
- Berkman LF, Blumenthal J, Burg M, et al Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003; 289:3106–3116.
- Taylor CB, Youngblood ME, Catellier D, et al Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 2005; 62:792–798.
- Glassman AH, O’Connor CM, Califf RM, et al Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Serebruany VL, Glassman AH, Malinin AI, et al Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003; 108:939–44.
- O’Connor CM, Jiang W, Kuchibhatla M, et al Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol 2010; 56:692–699.
- Carney RM, Blumenthal JA, Freedland KE, et al Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosom Med 2004; 66:466–474.
- Lespérance F, Frasure-Smith N, Koszycki D, et al Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) Trial. JAMA 2007; 297:367–379.
- Salzman C, Schneider L, Alexopoulos GS. Pharmacological treatment of depression in late life. In:Bloon F, Kupfer D, eds. Psychopharmacology: Fourth Generation of Progress. New York: Raven Press; 1995.
- Franklin BA, Bonzheim K, Gordon S, Timmis GC. Safety of medically supervised outpatient cardiac rehabilitation exercise therapy: a 16-year follow-up. Chest 1998; 114:902–906.
- Vongvanich P, Paul-Labrador MJ, Merz CN. Safety of medically supervised exercise in a cardiac rehabilitation center. Am J Cardiol 1996; 77:1383–1385.
- O’Connor CM, Whellan DJ, Lee KL, et al Efficacy and safety of exercise training in patients with chronic heart failure: H-F ACTION randomized controlled trial. JAMA 2009; 301:1439–1540.
- Milani RV, Lavie CJ, Cassidy MM. Effects of cardiac rehabilitation and exercise training programs on depression in patients after major coronary events. Am Heart J 1996; 132:726–732.
- Beniamini Y, Rubenstein JJ, Zaichkowsky LD, Crim MC. Effects of high-intensity strength training on quality-of-life parameters in cardiac rehabilitation patients. Am J Cardiol 1997; 80:841–846.
- Maines TY, Lavie CJ, Milani RV, Cassidy MM, Gilliland YE, Murgo JP. Effects of cardiac rehabilitation and exercise programs on exercise capacity, coronary risk factors, behavior, and quality of life in patients with coronary artery disease. South Med J 1997; 90:43–49.
- Milani RV, Lavie CJ. Prevalence and effects of cardiac rehabilitation on depression in the elderly with coronary heart disease. Am J Cardiol 1998; 81:1233–1236.
- Blumenthal JA, Emery CF, Rejeski WJ. The effects of exercise training on psychosocial functioning after myocardial infarction. J Cardiopulmonary Rehabil 1988; 8:183–193.
- Taylor CB, Houston-Miller N, Ahn DK, Haskell W, DeBusk RF. The effects of exercise training programs on psychosocial improvement in uncomplicated postmyocardial infarction patients. J Psychosom Res 1986; 30:581–587.
- Stern MJ, Gorman PA, Kaslow L. The group counseling v exercise therapy study: a controlled intervention with subjects following myocardial infarction. Arch Intern Med 1983; 143:1719–1725.
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002; 32:741–760.
- Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ 2001; 322:763–767.
- Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database Syst Rev 2009:CD004366.
- Blumenthal JA, Babyak MA, Carney RM, et al Exercise, depression, and mortality after myocardial infarction in the ENRICHD trial. Med Sci Sports Exerc 2004; 36:746–755.
- Eden J, Wheatley B, McNeil B, Sox H, eds. Institute of Medicine. Knowing What Works in Health Care. A Roadmap for the Nation. Washington DC: National Academics Press; 2009.
- Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Intern Med 2009; 151:203–205.
- Hlatky MA, Boothroyd DB, Bravata DM, et al Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197.
- Blumenthal JA, Babyak MA, Moore KA, et al Effects of exercise training on older patients with major depression. Arch Intern Med 1999; 159:2349–2356.
- Babyak M, Blumenthal JA, Herman S. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000; 62:633–638.
- Blumenthal JA, Babyak MA, Doraiswamy PM, et al Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007; 69:587–596.
- Hoffman B, Babyak M, Craighead WE, et al Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosom Med 2010; 73:127–133.
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med 2005; 28:1–8.
- Lichtman JH, Bigger JT, Blumenthal JA, et al Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 2008; 118:1768–1775.
- Blumenthal JA, Califf R, Williams RS, Hindman M. Cardiac rehabilitation: a new frontier for behavioral medicine. J Cardiac Rehabil 1983; 3:637–656.
- Lewis T. Diseases of the Heart. New York: Macmillan; 1933:41–49.
- Levine SA, Lown B. The “chair” treatment of acute thrombosis. Trans Assoc Am Physicians 1951; 64:316–327.
- Cain HD, Frasher WG, Stivelman R. Graded activity program for safe return to self-care after myocardial infarction. JAMA 1961; 177:111–115.
- Hellerstein HK, Ford AB. Rehabilitation of the cardiac patient. JAMA 1957; 14:225–231.
- Naughton J, Bruhn JG, Lategola MT. Effects of physical training on physiologic and behavioral characteristics of cardiac patients. Arch Phys Med Rehabil 1968; 49:131–137.
- O’Connor GT, Buring JE, Yusuf S, et al An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation 1989; 80:234–244.
- Wenger NK, Froelicher ES, Smith LK, et al Cardiac rehabilitation: clinical practice guideline no. 17. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, National Heart, Lung, and Blood Institute; October 1995; AHCPR publication 96-0672.
- Blumenthal JA, Emery CF, Cox DR, et al Exercise training in healthy type A middle-aged men: effects on behavioral and cardiovascular responses. Psychosom Med 1988; 50:418–433.
- Schuler G, Schlierf G, Wirth A, et al Low-fat diet and regular, supervised physical exercise in patients with symptomatic coronary artery disease: reduction of stress-induced myocardial ischemia. Circulation 1988; 77:172–181.
- Jiang W, Trauner MA, Coleman RE, et al Association of physical fitness and transient myocardial ischemia in patients with coronary artery disease. J Cardiopulm Rehabil 1995; 15:431–438.
- Blumenthal JA, Jiang W, Babyak MA, et al Stress management and exercise training in cardiac patients with myocardial ischemia: effects on prognosis and evaluation of mechanisms. Arch Int Med 1997; 157:2213–2223.
- Oldridge NB, Guyatt GH, Fisher ME, Rimm AA. Cardiac rehabilitation after myocardial infarction: combined experience of randomized clinical trials. JAMA 1988; 260:945–950.
- Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2001:CD001800.
- Folkins CH, Sime WE. Physical fitness training and mental health. Am Psychol 1981; 36:373–389.
- Hughes JR. Psychological effects of habitual aerobic exercise: a critical review. Prev Med 1984; 13:66–78.
- Plante TG, Rodin J. Physical fitness and enhanced psychological health. Curr Psychol: Res Rev 1990; 9:3–24.
- Review Panel on Coronary-Prone Behavior and Coronary Heart Disease. Coronary-prone behavior and coronary heart disease: a critical review. Circulation 1981; 63:1199–1215.
- Smith TW. Hostility and health: current status of a psychosomatic hypothesis. Health Psychol 1992; 11:139–150.
- Lett HS, Blumenthal JA, Babyak MA, Strauman TJ, Robins C, Sherwood A. Social support and coronary heart disease: epidemiologic evidence and implications for treatment. Psychosom Med 2005; 67:869–878.
- Lett HS, Blumenthal JA, Babyak MA, et al Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med 2004; 66:305–315.
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66:802–813.
- Kessler RC, Berglund P, Demler O, et al The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCR-R). JAMA 2003; 289:3095–3105.
- Belsher G, Costello CG. Relapse after recovery from unipolar depression: a critical review. Psychol Bull 1988; 104:84–96.
- Strothers HS, Rust G, Minor P, Fresh E, Druss B, Satcher D. Disparities in antidepressant treatment in Medicaid elderly diagnosed with depression. J Am Geriatr Soc 2005; 53:456–461.
- Simpson SM, Krishnan LL, Kunik ME, Ruiz P. Racial disparities in diagnosis and treatment of depression: a literature review. Psychiatr Q 2007; 78:3–14.
- Sclar DA, Robison LM, Skaer TL. Ethnicity/race and the diagnosis of depression and use of antidepressants by adults in the United States. Int Clin Psychopharmacol 2008; 23:106–109.
- Waldman SV, Blumenthal JA, Babyak MA, et al Ethnic differences in the treatment of depression in patients with ischemic heart disease. Am Heart J 2009; 57:77–83.
- Carney RM, Rich MW, Freedland KE, et al Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988; 50:627–633.
- Schleifer SJ, Macari-Hinson MM, Coyle DA, et al The nature and course of depression following myocardial infarction. Arch Intern Med 1989; 149:1785–1789.
- Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction: impact on 6-month survival. JAMA 1993; 270:1819–1825.
- Gonzalez MB, Snyderman TB, Colket JT, et al Depression in patients with coronary artery disease. Depression 1996; 4:57–62.
- Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet 2001; 358:1766–1771.
- Burker EJ, Blumenthal JA, Feldman M, et al Depression in male and female patients undergoing cardiac surgery. Br J Clin Psychol 1995; 34( Pt 1):119–128.
- Lespérance F, Frasure-Smith N, Juneau M, Théroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med 2000; 160:1354–1360.
- Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation 1995; 91:999–1005.
- Barefoot JC, Helms MJ, Mark DB, et al Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol 1996; 78:613–617.
- Burg MM, Benedetto C, Soufer R. Depressive symptoms and mortality two years after coronary artery bypass graft surgery (CABG) in men. Psychosom Med 2003; 65:508–510.
- Blumenthal JA, Lett HS, Babyak MA, et al Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003; 362:604–609.
- Connerney I, Sloan RP, Shapiro PA, Bagiella E, Seckman C. Depression is associated with increased mortality 10 years after coronary artery bypass surgery. Psychosom Med 2010; 72:874–881.
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006; 48:1527–1537.
- Murberg TA, Furze G. Depressive symptoms and mortality in patients with congestive heart failure: a six-year follow-up study. Med Sci Monit 2004; 10:CR643–CR648.
- Jiang W, Alexander J, Christopher E, et al Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856.
- Sherwood A, Blumenthal JA, Trivedi R, et al Relationship of depression to death or hospitalization in patients with heart failure. Arch Int Med 2007; 167:367–373.
- Frasure-Smith N, Lespérance F, Habra M, et al Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation 2009; 120:134–140.
- Sherwood A, Blumenthal JA, Hinderliter AL, et al Worsening depressive symptoms are associated with adverse clinical outcomes in patients with heart failure. J Am Coll Cardiol 2011; 57:418–423.
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001; 24:1069–1078.
- Thakore JH, Richards PJ, Reznek RH, Martin A, Dinan TG. Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biol Psychiatry 1997; 41:1140–1142.
- Musselman DL, Tomer A, Manatunga AK, et al Exaggerated platelet reactivity in major depression. Am J Psychiatry 1996; 153:1313–1317.
- Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry 2000; 61( suppl 1):5–12.
- Akil H, Haskett RF, Young EA, et al Multiple HPA profiles in endogenous depression: effect of age and sex on cortisol and beta-endorphin. Biol Psychiatry 1993; 33:73–85.
- Kop WJ, Gottdiener JS, Tangen CM, et al Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol 2002; 89:419–424.
- Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol 1995; 14:88–90.
- Lehto S, Koukkunen H, Hintikka J, Viinamäki H, Laakso M, Pyörälä K. Depression after coronary heart disease events. Scand Cardiovasc J 2000; 34:580–583.
- Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol 1991; 134:220–231.
- Clinical Practice Guideline Number 5: Depression in Primary Care, 2: Treatment of Major Depression. Rockville, MD: U.S. Department of Health and Human Services, Agency for Health Care Policy and Research; 1993. AHCPR publication 93-0551.
- Anderson IM, Ferrier IN, Baldwin RC, et al Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. J Psychopharmacol 2008; 22:343–396.
- American Psyciatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000; 157( suppl 4):1–45.
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 2008; 5:e45.
- Hansen R, Gaynes B, Thieda P, et al Meta-analysis of major depressive disorder relapse and recurrence with second-generation antidepressants. Psychiatr Serv 2008; 59:1121–1129.
- Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med 2005; 143:415–426.
- Rush AJ, Trivedi MH, Wisniewski SR, et al Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917.
- Olfson M, Marcus SC, Druss B, Elinson L, Tanielian T, Pincus HA. National trends in the outpatient treatment of depression. JAMA 2002; 287:203–209.
- Marcus SC, Olfson M. National trends in the treatment of depression from 1998 to 2007. Arch Gen Psychiatry 2010; 67:1265–1273.
- Berkman LF, Blumenthal J, Burg M, et al Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003; 289:3106–3116.
- Taylor CB, Youngblood ME, Catellier D, et al Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 2005; 62:792–798.
- Glassman AH, O’Connor CM, Califf RM, et al Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Serebruany VL, Glassman AH, Malinin AI, et al Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003; 108:939–44.
- O’Connor CM, Jiang W, Kuchibhatla M, et al Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol 2010; 56:692–699.
- Carney RM, Blumenthal JA, Freedland KE, et al Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosom Med 2004; 66:466–474.
- Lespérance F, Frasure-Smith N, Koszycki D, et al Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) Trial. JAMA 2007; 297:367–379.
- Salzman C, Schneider L, Alexopoulos GS. Pharmacological treatment of depression in late life. In:Bloon F, Kupfer D, eds. Psychopharmacology: Fourth Generation of Progress. New York: Raven Press; 1995.
- Franklin BA, Bonzheim K, Gordon S, Timmis GC. Safety of medically supervised outpatient cardiac rehabilitation exercise therapy: a 16-year follow-up. Chest 1998; 114:902–906.
- Vongvanich P, Paul-Labrador MJ, Merz CN. Safety of medically supervised exercise in a cardiac rehabilitation center. Am J Cardiol 1996; 77:1383–1385.
- O’Connor CM, Whellan DJ, Lee KL, et al Efficacy and safety of exercise training in patients with chronic heart failure: H-F ACTION randomized controlled trial. JAMA 2009; 301:1439–1540.
- Milani RV, Lavie CJ, Cassidy MM. Effects of cardiac rehabilitation and exercise training programs on depression in patients after major coronary events. Am Heart J 1996; 132:726–732.
- Beniamini Y, Rubenstein JJ, Zaichkowsky LD, Crim MC. Effects of high-intensity strength training on quality-of-life parameters in cardiac rehabilitation patients. Am J Cardiol 1997; 80:841–846.
- Maines TY, Lavie CJ, Milani RV, Cassidy MM, Gilliland YE, Murgo JP. Effects of cardiac rehabilitation and exercise programs on exercise capacity, coronary risk factors, behavior, and quality of life in patients with coronary artery disease. South Med J 1997; 90:43–49.
- Milani RV, Lavie CJ. Prevalence and effects of cardiac rehabilitation on depression in the elderly with coronary heart disease. Am J Cardiol 1998; 81:1233–1236.
- Blumenthal JA, Emery CF, Rejeski WJ. The effects of exercise training on psychosocial functioning after myocardial infarction. J Cardiopulmonary Rehabil 1988; 8:183–193.
- Taylor CB, Houston-Miller N, Ahn DK, Haskell W, DeBusk RF. The effects of exercise training programs on psychosocial improvement in uncomplicated postmyocardial infarction patients. J Psychosom Res 1986; 30:581–587.
- Stern MJ, Gorman PA, Kaslow L. The group counseling v exercise therapy study: a controlled intervention with subjects following myocardial infarction. Arch Intern Med 1983; 143:1719–1725.
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002; 32:741–760.
- Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ 2001; 322:763–767.
- Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database Syst Rev 2009:CD004366.
- Blumenthal JA, Babyak MA, Carney RM, et al Exercise, depression, and mortality after myocardial infarction in the ENRICHD trial. Med Sci Sports Exerc 2004; 36:746–755.
- Eden J, Wheatley B, McNeil B, Sox H, eds. Institute of Medicine. Knowing What Works in Health Care. A Roadmap for the Nation. Washington DC: National Academics Press; 2009.
- Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Intern Med 2009; 151:203–205.
- Hlatky MA, Boothroyd DB, Bravata DM, et al Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197.
- Blumenthal JA, Babyak MA, Moore KA, et al Effects of exercise training on older patients with major depression. Arch Intern Med 1999; 159:2349–2356.
- Babyak M, Blumenthal JA, Herman S. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000; 62:633–638.
- Blumenthal JA, Babyak MA, Doraiswamy PM, et al Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007; 69:587–596.
- Hoffman B, Babyak M, Craighead WE, et al Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosom Med 2010; 73:127–133.
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med 2005; 28:1–8.
- Lichtman JH, Bigger JT, Blumenthal JA, et al Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 2008; 118:1768–1775.
Depression and coronary heart disease: Association and implications for treatment
Minor depressive disorder (mDD) is not an official DSM-IV diagnosis but is used for research purposes; it is similar to MDD in duration but requires that only two to four symptoms be present.
EPIDEMIOLOGY OF DEPRESSION
Depression is a widespread and often chronic condition. Lifetime prevalence estimates for MDD are approximately 15% to 20%;2,3 1-year prevalence estimates are 5% to 10%;2,4 and point prevalence estimates range from 4% to 7%.3,5 Moreover, MDD is characterized by high rates of relapse: 22% to 50% of patients suffer recurrent episodes within 6 months after recovery.6
Women are twice as likely as men to be diagnosed with MDD, with lifetime prevalence rates of 10% to 25% in women versus 5% to 12% in men.1
Although rates of depression do not appear to increase with age, MDD often goes undertreated in older adults3 and in cardiac patients.7
DIAGNOSING AND ASSESSING DEPRESSION
The gold standard for diagnosing MDD is a clinical interview. Commonly used instruments include the Diagnostic Interview Schedule8 and the Composite International Diagnostic Interview.9 The Structured Clinical Interview for DSM-IV Axis I Disorders10 and the Schedule for Affective Disorders and Schizophrenia11 are frequently used semistructured interviews.
The most common clinical instruments for assessing the severity of depressive symptoms are the Hamilton Rating Scale for Depression (HAM-D),12 which is a clinician-rated scale, and various psychometric questionnaires, including the Beck Depression Inventory (BDI)13,14 and the Center for Epidemiological Studies Depression Scale (CES-D).15
THE DEPRESSION–HEART DISEASE LINK
Depression as a primary risk factor
Depression as a secondary risk factor
Depression is an even stronger risk factor for cardiac events in patients with established CHD. Point estimates range from 14% to as high as 47%, with higher rates in patients with unstable angina and in patients awaiting coronary artery bypass graft (CABG) surgery; an additional 20% of patients exhibit elevated depressive symptoms or minor depression (mDD).19–25
Prospective studies have shown that depression increases the risk for death or nonfatal cardiac events approximately 2.5-fold in patients with CHD. For instance, Frasure-Smith et al followed 896 patients with a recent acute MI and found that the presence of depressive symptoms as indicated by an elevated BDI score was a significant predictor of cardiac mortality after controlling for multivariate predictors of mortality (odds ratio [OR] = 3.29 for women and 3.05 for men).26
Two recent meta-analyses confirmed the association between depression and adverse clinical outcomes in patients with CHD.27,28 For example, van Melle et al reported that post-MI depression was associated with a 2- to 2.5-fold increase in the risk of adverse health outcomes.28 In this analysis, depression’s effect on cardiac mortality and all-cause mortality was especially pronounced in older studies (before 1992) (OR = 3.2) compared with more recent studies (after 1992) (OR = 2.01).28
Duke University researchers have conducted several prospective studies in various cardiac populations.29–31 Barefoot et al assessed 1,250 patients with documented CHD using the Zung Self-Rating Depression Scale at the time of diagnostic coronary angiography and followed them for up to 19.4 years.29 Results showed that patients with moderate to severe depression were at 69% greater risk for cardiac death and 78% greater risk for all-cause death than were their nondepressed counterparts.
We also recently reported results from a prospective study that followed 204 patients with heart failure over a median interval of 3 years.31 Clinically significant symptoms of depression (BDI score ≥ 10) were associated with a hazard ratio of 1.56 (95% CI, 1.07 to 2.29) for the combined end point of death or cardiovascular hospitalization. These observations included adjustment for plasma NT-proBNP level, ejection fraction, and other established risk factors, suggesting that heightened risk of adverse clinical outcomes associated with depressive symptoms is not simply a reflection of the severity of heart failure.
In summary, a number of observational studies have demonstrated that depression is associated with increased risk of morbidity and mortality both in healthy populations and in a variety of populations with established cardiac disease.
BIOBEHAVIORAL MECHANISMS LINKING DEPRESSION AND CHD
A number of biobehavioral mechanisms have been hypothesized to underlie the relationship between depression and CHD. Most evidence is derived from cross-sectional studies and suggests that depression is associated with traditional risk factors for CHD, such as hypertension, diabetes, and insulin resistance,32,33 as well as changes in platelet reactivity,34 dysregulation of the autonomic nervous system35 and hypothalamic-pituitary-adrenal axis,36 and alterations in the immune response/inflammation.37 Depression is also associated with behavioral factors that are in turn associated with CHD risk, such as reduced treatment adherence,38 smoking,39 and physical inactivity.40
STUDIES OF DEPRESSION TREATMENT IN CARDIAC PATIENTS
Successful treatments for depression in patients with CHD may have the potential to improve not only quality of life but also cardiovascular and physical health. Several treatments for depression exist for use in the general population, such as antidepressant medication or psychotherapy.41 However, only three studies have tested the efficacy of these treatments in patients with CHD: SADHART, ENRICHD, and CREATE.42–44
SADHART (Sertraline Antidepressant Heart Attack Randomized Trial) was a safety and efficacy evaluation of antidepressant medication in patients with MDD and a recent MI or unstable angina.42 It showed only modest differences in reductions in depressive symptoms between sertraline recipients and placebo recipients, and it lacked statistical power to examine the impact of treatment on hard clinical end points.
ENRICHD (Enhancing Recovery in Coronary Heart Disease Patients) assessed the effect of psychosocial treatment on survival among more than than 2,400 post-MI patients.43 Although this trial found that cognitive behavior therapy resulted in significant, albeit small, improvements in depressive symptoms compared with usual care, it failed to demonstrate that treating depression and low social support was associated with increased survival.
CREATE (Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy), a recent placebo-controlled trial, assessed the value of antidepressant medication and clinical management in patients with CHD.44 The study’s 284 patients, all of whom had CHD as well as MDD and a HAM-D score of 20 or greater, underwent two separate randomizations: (1) to 12 weeks of interpersonal therapy plus clinical management or 12 weeks of clinical management alone, and (2) to 12 weeks of citalopram therapy or matching placebo. There was no difference between interpersonal therapy and clinical management alone; however, citalopram was superior to placebo in reducing HAM-D scores and demonstrated better remission rates (35.9% with citalopram vs 22.5% with placebo). The same therapists who provided interpersonal therapy also performed the clinical management, so it could be argued that this was why additional interpersonal therapist time did not result in greater reductions in depressive symptoms than did clinical management alone. Furthermore, this study did not examine the effects of depression therapy on clinical outcomes.
EXERCISE AS A TREATMENT FOR DEPRESSION
There is growing evidence that exercise may be an effective treatment for depression.45 Most of the existing studies of exercise for depression have focused on aerobic exercise.
In the relatively large SMILE study (Standard Medical Intervention and Long-term Exercise),46 conducted at Duke University, 156 adult noncardiac patients with MDD were randomized to 4 months of treatment with supervised aerobic exercise, antidepressant medication (sertraline), or a combination of exercise and medication. Although antidepressant medication was associated with faster reductions in depression in the first 4 weeks of treatment among mildly depressed patients, exercise was as effective as antidepressant medication in treating depression by the end of the 16-week intervention for all participants.
Exercise generally is considered safe for most patients with stable CHD.48 Some studies of exercise treatments for patients with CHD have tracked depressive symptoms and thus have provided insight into the potential efficacy of exercise as a treatment for depression in this population. Although most of these studies have reported significant improvements in depression after completion of an exercise program, many have had important methodologic limitations, including absence of a control group. In one of the few controlled studies in this area, Stern et al49 randomized 106 men who had a recent acute MI and elevated depression, anxiety, or low fitness to 12 weeks of exercise training, group therapy, or usual care (control). At 1-year follow-up, subjects in both the exercise and counseling groups showed improvements in depression relative to controls.
EFFECT OF EXERCISE ON CARDIOVASCULAR RISK FACTORS AND OUTCOMES
Exercise is a particularly promising intervention for depression in patients with CHD because it has well-documented cardiovascular benefits. In addition to the well-established role of exercise interventions in primary prevention, such interventions have been shown to improve outcomes for patients with CHD.50
Jolliffe et al conducted a meta-analysis comparing exercise-only interventions, comprehensive rehabilitation (including educational and behavioral components such as dietary changes and stress reduction in addition to exercise), and usual care.51 Exercise-only interventions were associated with reductions in both all-cause and cardiac mortality relative to usual care. Comprehensive rehabilitation, on the other hand, was not associated with statistically significant reductions in all-cause mortality relative to usual care, but it was associated with a decreased risk for cardiac mortality, to a slightly lesser extent than exercise-only interventions.
The evidence that exercise affects depression, CHD risk factors, and CHD outcomes suggests that exercise is a particularly promising intervention for depression in this population.
UPBEAT trial promises further insight
A new Duke University study known as UPBEAT (Understanding Prognostic Benefits of Exercise and Antidepressant Treatment) is randomizing 200 patients with elevated depressive symptoms to exercise, antidepressant therapy (sertraline), or placebo for 4 months.53 A variety of “biomarkers” of risk are being assessed, including measures of heart rate variability, vascular function, inflammation, and platelet aggregation. Results of this 5-year trial should be available by 2011.
CONCLUSIONS
Although depression has emerged as an important risk factor for CHD, there is no consensus on the optimal way to treat depression in patients with CHD. Interventions that are guided by an understanding of the mechanisms linking depression to CHD may prove to be most effective in improving both depression and physical health outcomes.
Exercise targets many of the mechanisms by which depression may be associated with increased risk, including autonomic nervous system activity, hypothalamic-pituitary-adrenal axis function, platelet activation, vascular function, and inflammation. Moreover, a growing body of evidence suggests that exercise is an effective treatment for depression that may be comparable in effect to antidepressant medication, at least in select subgroups (eg, patients who are receptive to exercise as a treatment for depression). The value of exercise training—not only for improving quality of life, but also for improving “biomarkers” of risk and other relevant health outcomes—is the focus of our current research efforts.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text Revision. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:8–19.
- Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry 2000; 57:601–617.
- Regier DA, Narrow WE, Rae DS, Manderscheid RW, Locke BZ, Goodwin FK. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry 1993; 50:85–94.
- Ayuso-Mateos JL, Vazquez-Barquero JL, Dowrick C, et al. Depressive disorders in Europe: prevalence figures from the ODIN study. Br J Psychiatry 2001; 179:308–316.
- Belsher G, Costello CG. Relapse after recovery from unipolar depression: a critical review. Psychol Bull 1988; 104:84–96.
- Carney RM, Rich MW, Tevelde A, Saini J, Clark K, Jaffe AS. Major depressive disorder in coronary artery disease. Am J Cardiol 1987; 60:1273–1275.
- Robins LN, Helzer JE, Crougham J, Ratliff K. National Institute of Mental Health diagnostic interview schedule. Arch Gen Psychiatry 1981; 38:381–389.
- World Health Organization. Composite International Diagnostic Interview. Geneva, Switzerland: World Health Organization; 1990.
- First M, Spitzer L, Gibbon M. Structured clinical interview for axis I DSM-IV disorders. Washington, DC: American Psychiatric Press; 1995.
- Endicott J, Spitzer RL. The schedule for affective disorders and schizophrenia. Arch Gen Psychiatry 1978; 35:837–844.
- Hamilton M. A rating scale for depression. J Neurol 1960; 23:56–61.
- Beck AT, Ward CH, Mendelsohn M. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571.
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd ed. San Antonio, TX: The Psychological Corporation; 1996.
- Hautzinger M. The CES-D scale: a depression-rating scale for research in the general population. Diagnostica 1988; 34:167–173.
- Lett H, Blumenthal J, Babyak M, Sherwood A, Strauman T, Robins C. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med 2004; 66:305–315.
- Rugulies R. Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med 2002; 23:51–61.
- Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol 2005; 45:637–651.
- Carney RM, Rich MW, Freedland KE, et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988; 50:627–633.
- Schleifer SJ, Macari-Hinson MM, Coyle DA, et al. The nature and course of depression following myocardial infarction. Arch Intern Med 1989; 149:1785–1789.
- Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993; 270:1819–1825.
- Lesperance F, Frasure-Smith N, Juneau M, Theroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med 2000; 160:1354–1360.
- Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet 2001; 358:1766–1771.
- Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856.
- Burker EJ, Blumenthal JA, Feldman M, et al. Depression in male and female patients undergoing cardiac surgery. Br J Clin Psychol 1995; 34:119–128.
- Frasure-Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom Med 1999; 61:26–37.
- Barth J, Schumacher M, Hermann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66:802–813.
- van Melle JP, de Jong P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822.
- Barefoot JC, Helms MJ, Mark DB, et al. Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol 1996; 78:613–617.
- Blumenthal JA, Lett H, Babyak M, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003; 362:604–609.
- Sherwood A, Blumenthal JA, Trivedi R, et al. Relationship of depression to mortality and hospitalization in patients with heart failure. Arch Intern Med 2007; 167:367–373.
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001; 24:1069–1078.
- Thakore JH, Richards PJ, Reznek RH, Martin A, Dinan TG. Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biol Psychiatry 1997; 41:1140–1142.
- Musselman DL, Tomer A, Manatunga AK, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry 1996; 153:1313–1317.
- Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry 2000; 61:5–12.
- Akil H, Haskett RF, Young EA, et al. Multiple HPA profiles in endogenous depression: effect of age and sex on cortisol and beta-endorphin. Biol Psychiatry 1993; 33:73–85.
- Kop WJ, Gottdiener JS, Tangen CM, et al. Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol 2002; 89:419–424.
- Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol 1995; 14:88–90.
- Lehto S, Koukkunen H, Hintikka J, Viinamaki H, Laakso M, Pyorala K. Depression after coronary heart disease events. Scand Cardiovasc J 2000; 34:580–583.
- Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol 1991; 134:220–231.
- Depression Guideline Panel. Depression in Primary Care: Volume 2. Treatment of Depression, Clinical Practice Guideline, No. 5. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, US Dept of Health and Human Services; 1993.
- Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003; 289:3106–3116.
- Lesperance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379.
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002; 32:741–760.
- Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999; 159:2349–2356.
- Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000; 62:633–638.
- Franklin BA, Bonzheim K, Gordon S, Timmis GC. Safety of medically supervised outpatient cardiac rehabilitation exercise therapy: a 16-year follow-up. Chest 1998; 114:902–906.
- Stern MJ, Gorman PA, Kaslow L. The group counseling v exercise therapy study. A controlled intervention with subjects following myocardial infarction. Arch Intern Med 1983; 143:1719–1725.
- Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA 1988; 260:945–950.
- Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2001; (1):CD001800.
- Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD trial. Med Sci Sports Exerc 2004; 36:746–755.
- Blumenthal JA, Sherwood A, Rogers SD, et al. Understanding prognostic benefits of exercise and antidepressant therapy for persons with depression and heart disease: the UPBEAT study—rationale, design, and methodological issues. Clin Trials 2007; 4:548–559.
Minor depressive disorder (mDD) is not an official DSM-IV diagnosis but is used for research purposes; it is similar to MDD in duration but requires that only two to four symptoms be present.
EPIDEMIOLOGY OF DEPRESSION
Depression is a widespread and often chronic condition. Lifetime prevalence estimates for MDD are approximately 15% to 20%;2,3 1-year prevalence estimates are 5% to 10%;2,4 and point prevalence estimates range from 4% to 7%.3,5 Moreover, MDD is characterized by high rates of relapse: 22% to 50% of patients suffer recurrent episodes within 6 months after recovery.6
Women are twice as likely as men to be diagnosed with MDD, with lifetime prevalence rates of 10% to 25% in women versus 5% to 12% in men.1
Although rates of depression do not appear to increase with age, MDD often goes undertreated in older adults3 and in cardiac patients.7
DIAGNOSING AND ASSESSING DEPRESSION
The gold standard for diagnosing MDD is a clinical interview. Commonly used instruments include the Diagnostic Interview Schedule8 and the Composite International Diagnostic Interview.9 The Structured Clinical Interview for DSM-IV Axis I Disorders10 and the Schedule for Affective Disorders and Schizophrenia11 are frequently used semistructured interviews.
The most common clinical instruments for assessing the severity of depressive symptoms are the Hamilton Rating Scale for Depression (HAM-D),12 which is a clinician-rated scale, and various psychometric questionnaires, including the Beck Depression Inventory (BDI)13,14 and the Center for Epidemiological Studies Depression Scale (CES-D).15
THE DEPRESSION–HEART DISEASE LINK
Depression as a primary risk factor
Depression as a secondary risk factor
Depression is an even stronger risk factor for cardiac events in patients with established CHD. Point estimates range from 14% to as high as 47%, with higher rates in patients with unstable angina and in patients awaiting coronary artery bypass graft (CABG) surgery; an additional 20% of patients exhibit elevated depressive symptoms or minor depression (mDD).19–25
Prospective studies have shown that depression increases the risk for death or nonfatal cardiac events approximately 2.5-fold in patients with CHD. For instance, Frasure-Smith et al followed 896 patients with a recent acute MI and found that the presence of depressive symptoms as indicated by an elevated BDI score was a significant predictor of cardiac mortality after controlling for multivariate predictors of mortality (odds ratio [OR] = 3.29 for women and 3.05 for men).26
Two recent meta-analyses confirmed the association between depression and adverse clinical outcomes in patients with CHD.27,28 For example, van Melle et al reported that post-MI depression was associated with a 2- to 2.5-fold increase in the risk of adverse health outcomes.28 In this analysis, depression’s effect on cardiac mortality and all-cause mortality was especially pronounced in older studies (before 1992) (OR = 3.2) compared with more recent studies (after 1992) (OR = 2.01).28
Duke University researchers have conducted several prospective studies in various cardiac populations.29–31 Barefoot et al assessed 1,250 patients with documented CHD using the Zung Self-Rating Depression Scale at the time of diagnostic coronary angiography and followed them for up to 19.4 years.29 Results showed that patients with moderate to severe depression were at 69% greater risk for cardiac death and 78% greater risk for all-cause death than were their nondepressed counterparts.
We also recently reported results from a prospective study that followed 204 patients with heart failure over a median interval of 3 years.31 Clinically significant symptoms of depression (BDI score ≥ 10) were associated with a hazard ratio of 1.56 (95% CI, 1.07 to 2.29) for the combined end point of death or cardiovascular hospitalization. These observations included adjustment for plasma NT-proBNP level, ejection fraction, and other established risk factors, suggesting that heightened risk of adverse clinical outcomes associated with depressive symptoms is not simply a reflection of the severity of heart failure.
In summary, a number of observational studies have demonstrated that depression is associated with increased risk of morbidity and mortality both in healthy populations and in a variety of populations with established cardiac disease.
BIOBEHAVIORAL MECHANISMS LINKING DEPRESSION AND CHD
A number of biobehavioral mechanisms have been hypothesized to underlie the relationship between depression and CHD. Most evidence is derived from cross-sectional studies and suggests that depression is associated with traditional risk factors for CHD, such as hypertension, diabetes, and insulin resistance,32,33 as well as changes in platelet reactivity,34 dysregulation of the autonomic nervous system35 and hypothalamic-pituitary-adrenal axis,36 and alterations in the immune response/inflammation.37 Depression is also associated with behavioral factors that are in turn associated with CHD risk, such as reduced treatment adherence,38 smoking,39 and physical inactivity.40
STUDIES OF DEPRESSION TREATMENT IN CARDIAC PATIENTS
Successful treatments for depression in patients with CHD may have the potential to improve not only quality of life but also cardiovascular and physical health. Several treatments for depression exist for use in the general population, such as antidepressant medication or psychotherapy.41 However, only three studies have tested the efficacy of these treatments in patients with CHD: SADHART, ENRICHD, and CREATE.42–44
SADHART (Sertraline Antidepressant Heart Attack Randomized Trial) was a safety and efficacy evaluation of antidepressant medication in patients with MDD and a recent MI or unstable angina.42 It showed only modest differences in reductions in depressive symptoms between sertraline recipients and placebo recipients, and it lacked statistical power to examine the impact of treatment on hard clinical end points.
ENRICHD (Enhancing Recovery in Coronary Heart Disease Patients) assessed the effect of psychosocial treatment on survival among more than than 2,400 post-MI patients.43 Although this trial found that cognitive behavior therapy resulted in significant, albeit small, improvements in depressive symptoms compared with usual care, it failed to demonstrate that treating depression and low social support was associated with increased survival.
CREATE (Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy), a recent placebo-controlled trial, assessed the value of antidepressant medication and clinical management in patients with CHD.44 The study’s 284 patients, all of whom had CHD as well as MDD and a HAM-D score of 20 or greater, underwent two separate randomizations: (1) to 12 weeks of interpersonal therapy plus clinical management or 12 weeks of clinical management alone, and (2) to 12 weeks of citalopram therapy or matching placebo. There was no difference between interpersonal therapy and clinical management alone; however, citalopram was superior to placebo in reducing HAM-D scores and demonstrated better remission rates (35.9% with citalopram vs 22.5% with placebo). The same therapists who provided interpersonal therapy also performed the clinical management, so it could be argued that this was why additional interpersonal therapist time did not result in greater reductions in depressive symptoms than did clinical management alone. Furthermore, this study did not examine the effects of depression therapy on clinical outcomes.
EXERCISE AS A TREATMENT FOR DEPRESSION
There is growing evidence that exercise may be an effective treatment for depression.45 Most of the existing studies of exercise for depression have focused on aerobic exercise.
In the relatively large SMILE study (Standard Medical Intervention and Long-term Exercise),46 conducted at Duke University, 156 adult noncardiac patients with MDD were randomized to 4 months of treatment with supervised aerobic exercise, antidepressant medication (sertraline), or a combination of exercise and medication. Although antidepressant medication was associated with faster reductions in depression in the first 4 weeks of treatment among mildly depressed patients, exercise was as effective as antidepressant medication in treating depression by the end of the 16-week intervention for all participants.
Exercise generally is considered safe for most patients with stable CHD.48 Some studies of exercise treatments for patients with CHD have tracked depressive symptoms and thus have provided insight into the potential efficacy of exercise as a treatment for depression in this population. Although most of these studies have reported significant improvements in depression after completion of an exercise program, many have had important methodologic limitations, including absence of a control group. In one of the few controlled studies in this area, Stern et al49 randomized 106 men who had a recent acute MI and elevated depression, anxiety, or low fitness to 12 weeks of exercise training, group therapy, or usual care (control). At 1-year follow-up, subjects in both the exercise and counseling groups showed improvements in depression relative to controls.
EFFECT OF EXERCISE ON CARDIOVASCULAR RISK FACTORS AND OUTCOMES
Exercise is a particularly promising intervention for depression in patients with CHD because it has well-documented cardiovascular benefits. In addition to the well-established role of exercise interventions in primary prevention, such interventions have been shown to improve outcomes for patients with CHD.50
Jolliffe et al conducted a meta-analysis comparing exercise-only interventions, comprehensive rehabilitation (including educational and behavioral components such as dietary changes and stress reduction in addition to exercise), and usual care.51 Exercise-only interventions were associated with reductions in both all-cause and cardiac mortality relative to usual care. Comprehensive rehabilitation, on the other hand, was not associated with statistically significant reductions in all-cause mortality relative to usual care, but it was associated with a decreased risk for cardiac mortality, to a slightly lesser extent than exercise-only interventions.
The evidence that exercise affects depression, CHD risk factors, and CHD outcomes suggests that exercise is a particularly promising intervention for depression in this population.
UPBEAT trial promises further insight
A new Duke University study known as UPBEAT (Understanding Prognostic Benefits of Exercise and Antidepressant Treatment) is randomizing 200 patients with elevated depressive symptoms to exercise, antidepressant therapy (sertraline), or placebo for 4 months.53 A variety of “biomarkers” of risk are being assessed, including measures of heart rate variability, vascular function, inflammation, and platelet aggregation. Results of this 5-year trial should be available by 2011.
CONCLUSIONS
Although depression has emerged as an important risk factor for CHD, there is no consensus on the optimal way to treat depression in patients with CHD. Interventions that are guided by an understanding of the mechanisms linking depression to CHD may prove to be most effective in improving both depression and physical health outcomes.
Exercise targets many of the mechanisms by which depression may be associated with increased risk, including autonomic nervous system activity, hypothalamic-pituitary-adrenal axis function, platelet activation, vascular function, and inflammation. Moreover, a growing body of evidence suggests that exercise is an effective treatment for depression that may be comparable in effect to antidepressant medication, at least in select subgroups (eg, patients who are receptive to exercise as a treatment for depression). The value of exercise training—not only for improving quality of life, but also for improving “biomarkers” of risk and other relevant health outcomes—is the focus of our current research efforts.
Minor depressive disorder (mDD) is not an official DSM-IV diagnosis but is used for research purposes; it is similar to MDD in duration but requires that only two to four symptoms be present.
EPIDEMIOLOGY OF DEPRESSION
Depression is a widespread and often chronic condition. Lifetime prevalence estimates for MDD are approximately 15% to 20%;2,3 1-year prevalence estimates are 5% to 10%;2,4 and point prevalence estimates range from 4% to 7%.3,5 Moreover, MDD is characterized by high rates of relapse: 22% to 50% of patients suffer recurrent episodes within 6 months after recovery.6
Women are twice as likely as men to be diagnosed with MDD, with lifetime prevalence rates of 10% to 25% in women versus 5% to 12% in men.1
Although rates of depression do not appear to increase with age, MDD often goes undertreated in older adults3 and in cardiac patients.7
DIAGNOSING AND ASSESSING DEPRESSION
The gold standard for diagnosing MDD is a clinical interview. Commonly used instruments include the Diagnostic Interview Schedule8 and the Composite International Diagnostic Interview.9 The Structured Clinical Interview for DSM-IV Axis I Disorders10 and the Schedule for Affective Disorders and Schizophrenia11 are frequently used semistructured interviews.
The most common clinical instruments for assessing the severity of depressive symptoms are the Hamilton Rating Scale for Depression (HAM-D),12 which is a clinician-rated scale, and various psychometric questionnaires, including the Beck Depression Inventory (BDI)13,14 and the Center for Epidemiological Studies Depression Scale (CES-D).15
THE DEPRESSION–HEART DISEASE LINK
Depression as a primary risk factor
Depression as a secondary risk factor
Depression is an even stronger risk factor for cardiac events in patients with established CHD. Point estimates range from 14% to as high as 47%, with higher rates in patients with unstable angina and in patients awaiting coronary artery bypass graft (CABG) surgery; an additional 20% of patients exhibit elevated depressive symptoms or minor depression (mDD).19–25
Prospective studies have shown that depression increases the risk for death or nonfatal cardiac events approximately 2.5-fold in patients with CHD. For instance, Frasure-Smith et al followed 896 patients with a recent acute MI and found that the presence of depressive symptoms as indicated by an elevated BDI score was a significant predictor of cardiac mortality after controlling for multivariate predictors of mortality (odds ratio [OR] = 3.29 for women and 3.05 for men).26
Two recent meta-analyses confirmed the association between depression and adverse clinical outcomes in patients with CHD.27,28 For example, van Melle et al reported that post-MI depression was associated with a 2- to 2.5-fold increase in the risk of adverse health outcomes.28 In this analysis, depression’s effect on cardiac mortality and all-cause mortality was especially pronounced in older studies (before 1992) (OR = 3.2) compared with more recent studies (after 1992) (OR = 2.01).28
Duke University researchers have conducted several prospective studies in various cardiac populations.29–31 Barefoot et al assessed 1,250 patients with documented CHD using the Zung Self-Rating Depression Scale at the time of diagnostic coronary angiography and followed them for up to 19.4 years.29 Results showed that patients with moderate to severe depression were at 69% greater risk for cardiac death and 78% greater risk for all-cause death than were their nondepressed counterparts.
We also recently reported results from a prospective study that followed 204 patients with heart failure over a median interval of 3 years.31 Clinically significant symptoms of depression (BDI score ≥ 10) were associated with a hazard ratio of 1.56 (95% CI, 1.07 to 2.29) for the combined end point of death or cardiovascular hospitalization. These observations included adjustment for plasma NT-proBNP level, ejection fraction, and other established risk factors, suggesting that heightened risk of adverse clinical outcomes associated with depressive symptoms is not simply a reflection of the severity of heart failure.
In summary, a number of observational studies have demonstrated that depression is associated with increased risk of morbidity and mortality both in healthy populations and in a variety of populations with established cardiac disease.
BIOBEHAVIORAL MECHANISMS LINKING DEPRESSION AND CHD
A number of biobehavioral mechanisms have been hypothesized to underlie the relationship between depression and CHD. Most evidence is derived from cross-sectional studies and suggests that depression is associated with traditional risk factors for CHD, such as hypertension, diabetes, and insulin resistance,32,33 as well as changes in platelet reactivity,34 dysregulation of the autonomic nervous system35 and hypothalamic-pituitary-adrenal axis,36 and alterations in the immune response/inflammation.37 Depression is also associated with behavioral factors that are in turn associated with CHD risk, such as reduced treatment adherence,38 smoking,39 and physical inactivity.40
STUDIES OF DEPRESSION TREATMENT IN CARDIAC PATIENTS
Successful treatments for depression in patients with CHD may have the potential to improve not only quality of life but also cardiovascular and physical health. Several treatments for depression exist for use in the general population, such as antidepressant medication or psychotherapy.41 However, only three studies have tested the efficacy of these treatments in patients with CHD: SADHART, ENRICHD, and CREATE.42–44
SADHART (Sertraline Antidepressant Heart Attack Randomized Trial) was a safety and efficacy evaluation of antidepressant medication in patients with MDD and a recent MI or unstable angina.42 It showed only modest differences in reductions in depressive symptoms between sertraline recipients and placebo recipients, and it lacked statistical power to examine the impact of treatment on hard clinical end points.
ENRICHD (Enhancing Recovery in Coronary Heart Disease Patients) assessed the effect of psychosocial treatment on survival among more than than 2,400 post-MI patients.43 Although this trial found that cognitive behavior therapy resulted in significant, albeit small, improvements in depressive symptoms compared with usual care, it failed to demonstrate that treating depression and low social support was associated with increased survival.
CREATE (Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy), a recent placebo-controlled trial, assessed the value of antidepressant medication and clinical management in patients with CHD.44 The study’s 284 patients, all of whom had CHD as well as MDD and a HAM-D score of 20 or greater, underwent two separate randomizations: (1) to 12 weeks of interpersonal therapy plus clinical management or 12 weeks of clinical management alone, and (2) to 12 weeks of citalopram therapy or matching placebo. There was no difference between interpersonal therapy and clinical management alone; however, citalopram was superior to placebo in reducing HAM-D scores and demonstrated better remission rates (35.9% with citalopram vs 22.5% with placebo). The same therapists who provided interpersonal therapy also performed the clinical management, so it could be argued that this was why additional interpersonal therapist time did not result in greater reductions in depressive symptoms than did clinical management alone. Furthermore, this study did not examine the effects of depression therapy on clinical outcomes.
EXERCISE AS A TREATMENT FOR DEPRESSION
There is growing evidence that exercise may be an effective treatment for depression.45 Most of the existing studies of exercise for depression have focused on aerobic exercise.
In the relatively large SMILE study (Standard Medical Intervention and Long-term Exercise),46 conducted at Duke University, 156 adult noncardiac patients with MDD were randomized to 4 months of treatment with supervised aerobic exercise, antidepressant medication (sertraline), or a combination of exercise and medication. Although antidepressant medication was associated with faster reductions in depression in the first 4 weeks of treatment among mildly depressed patients, exercise was as effective as antidepressant medication in treating depression by the end of the 16-week intervention for all participants.
Exercise generally is considered safe for most patients with stable CHD.48 Some studies of exercise treatments for patients with CHD have tracked depressive symptoms and thus have provided insight into the potential efficacy of exercise as a treatment for depression in this population. Although most of these studies have reported significant improvements in depression after completion of an exercise program, many have had important methodologic limitations, including absence of a control group. In one of the few controlled studies in this area, Stern et al49 randomized 106 men who had a recent acute MI and elevated depression, anxiety, or low fitness to 12 weeks of exercise training, group therapy, or usual care (control). At 1-year follow-up, subjects in both the exercise and counseling groups showed improvements in depression relative to controls.
EFFECT OF EXERCISE ON CARDIOVASCULAR RISK FACTORS AND OUTCOMES
Exercise is a particularly promising intervention for depression in patients with CHD because it has well-documented cardiovascular benefits. In addition to the well-established role of exercise interventions in primary prevention, such interventions have been shown to improve outcomes for patients with CHD.50
Jolliffe et al conducted a meta-analysis comparing exercise-only interventions, comprehensive rehabilitation (including educational and behavioral components such as dietary changes and stress reduction in addition to exercise), and usual care.51 Exercise-only interventions were associated with reductions in both all-cause and cardiac mortality relative to usual care. Comprehensive rehabilitation, on the other hand, was not associated with statistically significant reductions in all-cause mortality relative to usual care, but it was associated with a decreased risk for cardiac mortality, to a slightly lesser extent than exercise-only interventions.
The evidence that exercise affects depression, CHD risk factors, and CHD outcomes suggests that exercise is a particularly promising intervention for depression in this population.
UPBEAT trial promises further insight
A new Duke University study known as UPBEAT (Understanding Prognostic Benefits of Exercise and Antidepressant Treatment) is randomizing 200 patients with elevated depressive symptoms to exercise, antidepressant therapy (sertraline), or placebo for 4 months.53 A variety of “biomarkers” of risk are being assessed, including measures of heart rate variability, vascular function, inflammation, and platelet aggregation. Results of this 5-year trial should be available by 2011.
CONCLUSIONS
Although depression has emerged as an important risk factor for CHD, there is no consensus on the optimal way to treat depression in patients with CHD. Interventions that are guided by an understanding of the mechanisms linking depression to CHD may prove to be most effective in improving both depression and physical health outcomes.
Exercise targets many of the mechanisms by which depression may be associated with increased risk, including autonomic nervous system activity, hypothalamic-pituitary-adrenal axis function, platelet activation, vascular function, and inflammation. Moreover, a growing body of evidence suggests that exercise is an effective treatment for depression that may be comparable in effect to antidepressant medication, at least in select subgroups (eg, patients who are receptive to exercise as a treatment for depression). The value of exercise training—not only for improving quality of life, but also for improving “biomarkers” of risk and other relevant health outcomes—is the focus of our current research efforts.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text Revision. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:8–19.
- Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry 2000; 57:601–617.
- Regier DA, Narrow WE, Rae DS, Manderscheid RW, Locke BZ, Goodwin FK. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry 1993; 50:85–94.
- Ayuso-Mateos JL, Vazquez-Barquero JL, Dowrick C, et al. Depressive disorders in Europe: prevalence figures from the ODIN study. Br J Psychiatry 2001; 179:308–316.
- Belsher G, Costello CG. Relapse after recovery from unipolar depression: a critical review. Psychol Bull 1988; 104:84–96.
- Carney RM, Rich MW, Tevelde A, Saini J, Clark K, Jaffe AS. Major depressive disorder in coronary artery disease. Am J Cardiol 1987; 60:1273–1275.
- Robins LN, Helzer JE, Crougham J, Ratliff K. National Institute of Mental Health diagnostic interview schedule. Arch Gen Psychiatry 1981; 38:381–389.
- World Health Organization. Composite International Diagnostic Interview. Geneva, Switzerland: World Health Organization; 1990.
- First M, Spitzer L, Gibbon M. Structured clinical interview for axis I DSM-IV disorders. Washington, DC: American Psychiatric Press; 1995.
- Endicott J, Spitzer RL. The schedule for affective disorders and schizophrenia. Arch Gen Psychiatry 1978; 35:837–844.
- Hamilton M. A rating scale for depression. J Neurol 1960; 23:56–61.
- Beck AT, Ward CH, Mendelsohn M. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571.
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd ed. San Antonio, TX: The Psychological Corporation; 1996.
- Hautzinger M. The CES-D scale: a depression-rating scale for research in the general population. Diagnostica 1988; 34:167–173.
- Lett H, Blumenthal J, Babyak M, Sherwood A, Strauman T, Robins C. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med 2004; 66:305–315.
- Rugulies R. Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med 2002; 23:51–61.
- Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol 2005; 45:637–651.
- Carney RM, Rich MW, Freedland KE, et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988; 50:627–633.
- Schleifer SJ, Macari-Hinson MM, Coyle DA, et al. The nature and course of depression following myocardial infarction. Arch Intern Med 1989; 149:1785–1789.
- Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993; 270:1819–1825.
- Lesperance F, Frasure-Smith N, Juneau M, Theroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med 2000; 160:1354–1360.
- Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet 2001; 358:1766–1771.
- Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856.
- Burker EJ, Blumenthal JA, Feldman M, et al. Depression in male and female patients undergoing cardiac surgery. Br J Clin Psychol 1995; 34:119–128.
- Frasure-Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom Med 1999; 61:26–37.
- Barth J, Schumacher M, Hermann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66:802–813.
- van Melle JP, de Jong P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822.
- Barefoot JC, Helms MJ, Mark DB, et al. Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol 1996; 78:613–617.
- Blumenthal JA, Lett H, Babyak M, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003; 362:604–609.
- Sherwood A, Blumenthal JA, Trivedi R, et al. Relationship of depression to mortality and hospitalization in patients with heart failure. Arch Intern Med 2007; 167:367–373.
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001; 24:1069–1078.
- Thakore JH, Richards PJ, Reznek RH, Martin A, Dinan TG. Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biol Psychiatry 1997; 41:1140–1142.
- Musselman DL, Tomer A, Manatunga AK, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry 1996; 153:1313–1317.
- Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry 2000; 61:5–12.
- Akil H, Haskett RF, Young EA, et al. Multiple HPA profiles in endogenous depression: effect of age and sex on cortisol and beta-endorphin. Biol Psychiatry 1993; 33:73–85.
- Kop WJ, Gottdiener JS, Tangen CM, et al. Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol 2002; 89:419–424.
- Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol 1995; 14:88–90.
- Lehto S, Koukkunen H, Hintikka J, Viinamaki H, Laakso M, Pyorala K. Depression after coronary heart disease events. Scand Cardiovasc J 2000; 34:580–583.
- Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol 1991; 134:220–231.
- Depression Guideline Panel. Depression in Primary Care: Volume 2. Treatment of Depression, Clinical Practice Guideline, No. 5. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, US Dept of Health and Human Services; 1993.
- Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003; 289:3106–3116.
- Lesperance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379.
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002; 32:741–760.
- Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999; 159:2349–2356.
- Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000; 62:633–638.
- Franklin BA, Bonzheim K, Gordon S, Timmis GC. Safety of medically supervised outpatient cardiac rehabilitation exercise therapy: a 16-year follow-up. Chest 1998; 114:902–906.
- Stern MJ, Gorman PA, Kaslow L. The group counseling v exercise therapy study. A controlled intervention with subjects following myocardial infarction. Arch Intern Med 1983; 143:1719–1725.
- Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA 1988; 260:945–950.
- Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2001; (1):CD001800.
- Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD trial. Med Sci Sports Exerc 2004; 36:746–755.
- Blumenthal JA, Sherwood A, Rogers SD, et al. Understanding prognostic benefits of exercise and antidepressant therapy for persons with depression and heart disease: the UPBEAT study—rationale, design, and methodological issues. Clin Trials 2007; 4:548–559.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text Revision. 4th ed. Washington, DC: American Psychiatric Association; 2000.
- Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:8–19.
- Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry 2000; 57:601–617.
- Regier DA, Narrow WE, Rae DS, Manderscheid RW, Locke BZ, Goodwin FK. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry 1993; 50:85–94.
- Ayuso-Mateos JL, Vazquez-Barquero JL, Dowrick C, et al. Depressive disorders in Europe: prevalence figures from the ODIN study. Br J Psychiatry 2001; 179:308–316.
- Belsher G, Costello CG. Relapse after recovery from unipolar depression: a critical review. Psychol Bull 1988; 104:84–96.
- Carney RM, Rich MW, Tevelde A, Saini J, Clark K, Jaffe AS. Major depressive disorder in coronary artery disease. Am J Cardiol 1987; 60:1273–1275.
- Robins LN, Helzer JE, Crougham J, Ratliff K. National Institute of Mental Health diagnostic interview schedule. Arch Gen Psychiatry 1981; 38:381–389.
- World Health Organization. Composite International Diagnostic Interview. Geneva, Switzerland: World Health Organization; 1990.
- First M, Spitzer L, Gibbon M. Structured clinical interview for axis I DSM-IV disorders. Washington, DC: American Psychiatric Press; 1995.
- Endicott J, Spitzer RL. The schedule for affective disorders and schizophrenia. Arch Gen Psychiatry 1978; 35:837–844.
- Hamilton M. A rating scale for depression. J Neurol 1960; 23:56–61.
- Beck AT, Ward CH, Mendelsohn M. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571.
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd ed. San Antonio, TX: The Psychological Corporation; 1996.
- Hautzinger M. The CES-D scale: a depression-rating scale for research in the general population. Diagnostica 1988; 34:167–173.
- Lett H, Blumenthal J, Babyak M, Sherwood A, Strauman T, Robins C. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med 2004; 66:305–315.
- Rugulies R. Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med 2002; 23:51–61.
- Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol 2005; 45:637–651.
- Carney RM, Rich MW, Freedland KE, et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988; 50:627–633.
- Schleifer SJ, Macari-Hinson MM, Coyle DA, et al. The nature and course of depression following myocardial infarction. Arch Intern Med 1989; 149:1785–1789.
- Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993; 270:1819–1825.
- Lesperance F, Frasure-Smith N, Juneau M, Theroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med 2000; 160:1354–1360.
- Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet 2001; 358:1766–1771.
- Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856.
- Burker EJ, Blumenthal JA, Feldman M, et al. Depression in male and female patients undergoing cardiac surgery. Br J Clin Psychol 1995; 34:119–128.
- Frasure-Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom Med 1999; 61:26–37.
- Barth J, Schumacher M, Hermann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66:802–813.
- van Melle JP, de Jong P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822.
- Barefoot JC, Helms MJ, Mark DB, et al. Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol 1996; 78:613–617.
- Blumenthal JA, Lett H, Babyak M, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003; 362:604–609.
- Sherwood A, Blumenthal JA, Trivedi R, et al. Relationship of depression to mortality and hospitalization in patients with heart failure. Arch Intern Med 2007; 167:367–373.
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001; 24:1069–1078.
- Thakore JH, Richards PJ, Reznek RH, Martin A, Dinan TG. Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biol Psychiatry 1997; 41:1140–1142.
- Musselman DL, Tomer A, Manatunga AK, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry 1996; 153:1313–1317.
- Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry 2000; 61:5–12.
- Akil H, Haskett RF, Young EA, et al. Multiple HPA profiles in endogenous depression: effect of age and sex on cortisol and beta-endorphin. Biol Psychiatry 1993; 33:73–85.
- Kop WJ, Gottdiener JS, Tangen CM, et al. Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol 2002; 89:419–424.
- Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol 1995; 14:88–90.
- Lehto S, Koukkunen H, Hintikka J, Viinamaki H, Laakso M, Pyorala K. Depression after coronary heart disease events. Scand Cardiovasc J 2000; 34:580–583.
- Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol 1991; 134:220–231.
- Depression Guideline Panel. Depression in Primary Care: Volume 2. Treatment of Depression, Clinical Practice Guideline, No. 5. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, US Dept of Health and Human Services; 1993.
- Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003; 289:3106–3116.
- Lesperance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379.
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002; 32:741–760.
- Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999; 159:2349–2356.
- Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000; 62:633–638.
- Franklin BA, Bonzheim K, Gordon S, Timmis GC. Safety of medically supervised outpatient cardiac rehabilitation exercise therapy: a 16-year follow-up. Chest 1998; 114:902–906.
- Stern MJ, Gorman PA, Kaslow L. The group counseling v exercise therapy study. A controlled intervention with subjects following myocardial infarction. Arch Intern Med 1983; 143:1719–1725.
- Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA 1988; 260:945–950.
- Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2001; (1):CD001800.
- Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD trial. Med Sci Sports Exerc 2004; 36:746–755.
- Blumenthal JA, Sherwood A, Rogers SD, et al. Understanding prognostic benefits of exercise and antidepressant therapy for persons with depression and heart disease: the UPBEAT study—rationale, design, and methodological issues. Clin Trials 2007; 4:548–559.