User login
Ryan White Program Announces New Funding Grants
“The Ryan White HIV/AIDS Program plays an instrumental role in the United States’ public health response to HIV,” said HHS Secretary Sylvia Burwell, announcing nearly $2.3 billion in grants to the program to ease access to HIV/AIDS care and medications.
The program provides primary medical care, drug assistance, education and training, and a number of other essential support services to more than half a million people—more than50% of those living with diagnosed HIV infection in the U..S. The services are crucial in “preserving health, extending life expectancy, and reducing HIV transmission,” said HRSA Acting Administrator Jim Macrae. “In 2014, more than 80% of Ryan White HIV/AIDS Program clients who received HIV medical care were retained in care, and more than 81% of program clients who received HIV medical care were virally suppressed.”
About $627.8 million was awarded to 24 metropolitan areas and 28 transitional grant areas with the highest number of people living with HIV and AIDS or those experiencing increases in HIV and AIDS cases and emerging care needs. Another approximate $1.3 billion was awarded to 59 states and territories for core medical and support services and for the AIDS Drug Assistance Program.
Sixteen states received Emerging Community grants based on the number of AIDS cases over the most recent 5-year period. Thirty-two states and territories were awarded $10.4 million in Part B Minority AIDS Initiative grants.
Local community-based organizations and other groups across the country also were awarded funding to provide family-centered comprehensive care for women and children; technical assistance, clinical training, and oral health services; and education and training for health care professionals. Grant money will support the demonstration and evaluation of innovative models of care delivery for hard-to-reach populations as well as efforts to reduce new HIV infections.
“The Ryan White HIV/AIDS Program plays an instrumental role in the United States’ public health response to HIV,” said HHS Secretary Sylvia Burwell, announcing nearly $2.3 billion in grants to the program to ease access to HIV/AIDS care and medications.
The program provides primary medical care, drug assistance, education and training, and a number of other essential support services to more than half a million people—more than50% of those living with diagnosed HIV infection in the U..S. The services are crucial in “preserving health, extending life expectancy, and reducing HIV transmission,” said HRSA Acting Administrator Jim Macrae. “In 2014, more than 80% of Ryan White HIV/AIDS Program clients who received HIV medical care were retained in care, and more than 81% of program clients who received HIV medical care were virally suppressed.”
About $627.8 million was awarded to 24 metropolitan areas and 28 transitional grant areas with the highest number of people living with HIV and AIDS or those experiencing increases in HIV and AIDS cases and emerging care needs. Another approximate $1.3 billion was awarded to 59 states and territories for core medical and support services and for the AIDS Drug Assistance Program.
Sixteen states received Emerging Community grants based on the number of AIDS cases over the most recent 5-year period. Thirty-two states and territories were awarded $10.4 million in Part B Minority AIDS Initiative grants.
Local community-based organizations and other groups across the country also were awarded funding to provide family-centered comprehensive care for women and children; technical assistance, clinical training, and oral health services; and education and training for health care professionals. Grant money will support the demonstration and evaluation of innovative models of care delivery for hard-to-reach populations as well as efforts to reduce new HIV infections.
“The Ryan White HIV/AIDS Program plays an instrumental role in the United States’ public health response to HIV,” said HHS Secretary Sylvia Burwell, announcing nearly $2.3 billion in grants to the program to ease access to HIV/AIDS care and medications.
The program provides primary medical care, drug assistance, education and training, and a number of other essential support services to more than half a million people—more than50% of those living with diagnosed HIV infection in the U..S. The services are crucial in “preserving health, extending life expectancy, and reducing HIV transmission,” said HRSA Acting Administrator Jim Macrae. “In 2014, more than 80% of Ryan White HIV/AIDS Program clients who received HIV medical care were retained in care, and more than 81% of program clients who received HIV medical care were virally suppressed.”
About $627.8 million was awarded to 24 metropolitan areas and 28 transitional grant areas with the highest number of people living with HIV and AIDS or those experiencing increases in HIV and AIDS cases and emerging care needs. Another approximate $1.3 billion was awarded to 59 states and territories for core medical and support services and for the AIDS Drug Assistance Program.
Sixteen states received Emerging Community grants based on the number of AIDS cases over the most recent 5-year period. Thirty-two states and territories were awarded $10.4 million in Part B Minority AIDS Initiative grants.
Local community-based organizations and other groups across the country also were awarded funding to provide family-centered comprehensive care for women and children; technical assistance, clinical training, and oral health services; and education and training for health care professionals. Grant money will support the demonstration and evaluation of innovative models of care delivery for hard-to-reach populations as well as efforts to reduce new HIV infections.
Food Security Can Help Reduce Cardiovascular Risk Factors
Food insecurity has been linked to hypertension, diabetes, elevated cholesterol, and obesity—all cardiovascular risk factors and dangerous for pregnant women and infants. Researchers from Massachusetts General Hospital theorized that enrolling pregnant women in a program to ensure their access to food banks and other resources could help reduce their risks.
The researchers conducted a retrospective analysis of 1,295 women who visited the obstetrics clinic at a community health center. Of those, 145 (11%) were referred to Food for Families, which connects patients to resources such as the Supplemental Nutrition Assistance Program (SNAP) and the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC).
Two-thirds of referred women enrolled in Food for Families. A majority rated their health as good, very good, or excellent. Most had never used a free meal program, soup kitchen, or food pantry, although 43% were eligible for SNAP, and 87% were enrolled in WIC.
The primary outcomes measured were trends in blood pressure (BP) and blood glucose during pregnancy. Blood pressure numbers trended “modestly better” for women in the intervention program. They had a better systolic BP (0.2015 mm Hg/wk lower) and diastolic BP (0.1049 mm Hg/wk lower) than those who were not referred. The researchers found no differences in blood glucose trend.
The findings suggest that programs to reduce food insecurity can improve cardiovascular health in pregnant women, the researchers say. If so, screening for food insecurity in obstetric care may be a useful tool—particularly if the next step is to get patients the food they need
Food insecurity has been linked to hypertension, diabetes, elevated cholesterol, and obesity—all cardiovascular risk factors and dangerous for pregnant women and infants. Researchers from Massachusetts General Hospital theorized that enrolling pregnant women in a program to ensure their access to food banks and other resources could help reduce their risks.
The researchers conducted a retrospective analysis of 1,295 women who visited the obstetrics clinic at a community health center. Of those, 145 (11%) were referred to Food for Families, which connects patients to resources such as the Supplemental Nutrition Assistance Program (SNAP) and the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC).
Two-thirds of referred women enrolled in Food for Families. A majority rated their health as good, very good, or excellent. Most had never used a free meal program, soup kitchen, or food pantry, although 43% were eligible for SNAP, and 87% were enrolled in WIC.
The primary outcomes measured were trends in blood pressure (BP) and blood glucose during pregnancy. Blood pressure numbers trended “modestly better” for women in the intervention program. They had a better systolic BP (0.2015 mm Hg/wk lower) and diastolic BP (0.1049 mm Hg/wk lower) than those who were not referred. The researchers found no differences in blood glucose trend.
The findings suggest that programs to reduce food insecurity can improve cardiovascular health in pregnant women, the researchers say. If so, screening for food insecurity in obstetric care may be a useful tool—particularly if the next step is to get patients the food they need
Food insecurity has been linked to hypertension, diabetes, elevated cholesterol, and obesity—all cardiovascular risk factors and dangerous for pregnant women and infants. Researchers from Massachusetts General Hospital theorized that enrolling pregnant women in a program to ensure their access to food banks and other resources could help reduce their risks.
The researchers conducted a retrospective analysis of 1,295 women who visited the obstetrics clinic at a community health center. Of those, 145 (11%) were referred to Food for Families, which connects patients to resources such as the Supplemental Nutrition Assistance Program (SNAP) and the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC).
Two-thirds of referred women enrolled in Food for Families. A majority rated their health as good, very good, or excellent. Most had never used a free meal program, soup kitchen, or food pantry, although 43% were eligible for SNAP, and 87% were enrolled in WIC.
The primary outcomes measured were trends in blood pressure (BP) and blood glucose during pregnancy. Blood pressure numbers trended “modestly better” for women in the intervention program. They had a better systolic BP (0.2015 mm Hg/wk lower) and diastolic BP (0.1049 mm Hg/wk lower) than those who were not referred. The researchers found no differences in blood glucose trend.
The findings suggest that programs to reduce food insecurity can improve cardiovascular health in pregnant women, the researchers say. If so, screening for food insecurity in obstetric care may be a useful tool—particularly if the next step is to get patients the food they need
Preparing to Combat a New Resistant Threat
In 2009, Candida auris, an emerging invasive multidrug-resistant fungus, was isolated from external ear canal discharge of a patient in Japan. Since then, at least a dozen countries, including India, Israel, South Korea, and the United Kingdom, have published reports of C auris infections.
The emergence of C auris raises several serious concerns for public health, according to Morbidity and Mortality Weekly Report: Many isolates are multidrug-resistant; some strains have elevated minimum inhibitory concentrations to drugs in all 3 major classes of antifungal medications—a feature not found in other clinically relevant Candida species. Second, it’s challenging to identify, requiring specialized methods. C auris is often misidentified as other yeasts (most commonly Candida haemulonii). Finally, C auris has caused outbreaks in health care settings.
In June 2016, the CDC issued a clinical alert asking clinicians, public health authorities, and others to report C auris cases, or suspected cases, to state and local health departments and the CDC. Seven such US cases occurred between May 2013 and August 2016.
All patients had serious underlying medical conditions, including bone marrow transplantation. Four patients, all of whom had bloodstream infections, died during the weeks to months after C auris was identified.
The U.S. isolates were related to isolates from South America and South Asia, although available epidemiologic information suggests that most were acquired in the United States. Several findings suggested that transmission occurred in US health care settings.
Five isolates were resistant to fluconazole; 1 of those was resistant to amphotericin B and another to echinocandins. No isolate was resistant to all 3 classes of antifungal medications.
To reduce the risk of transmission, the CDC advises using Standard and Contact Precautions for patients colonized or infected with C auris. Facilities should ensure thorough daily and terminal cleaning of patients’ rooms with an EPA-registered disinfectant with a fungal claim. In nursing homes, providers should consider the level of patient care being provided and the presence of transmission risk factors when deciding on the level of precautions. When patients are transferred, receiving facilities should be notified of the presence of this multidrug-resistant organism to ensure continuity of precautions.
Five of 7 reported isolates were either misidentified initially as C haemulonii or not identified beyond Candida spp. The CDC urges local and state health departments to continue to report possible cases of C auris and of isolates of C haemulonii and Candida spp. that cannot be identified after routine testing.
In 2009, Candida auris, an emerging invasive multidrug-resistant fungus, was isolated from external ear canal discharge of a patient in Japan. Since then, at least a dozen countries, including India, Israel, South Korea, and the United Kingdom, have published reports of C auris infections.
The emergence of C auris raises several serious concerns for public health, according to Morbidity and Mortality Weekly Report: Many isolates are multidrug-resistant; some strains have elevated minimum inhibitory concentrations to drugs in all 3 major classes of antifungal medications—a feature not found in other clinically relevant Candida species. Second, it’s challenging to identify, requiring specialized methods. C auris is often misidentified as other yeasts (most commonly Candida haemulonii). Finally, C auris has caused outbreaks in health care settings.
In June 2016, the CDC issued a clinical alert asking clinicians, public health authorities, and others to report C auris cases, or suspected cases, to state and local health departments and the CDC. Seven such US cases occurred between May 2013 and August 2016.
All patients had serious underlying medical conditions, including bone marrow transplantation. Four patients, all of whom had bloodstream infections, died during the weeks to months after C auris was identified.
The U.S. isolates were related to isolates from South America and South Asia, although available epidemiologic information suggests that most were acquired in the United States. Several findings suggested that transmission occurred in US health care settings.
Five isolates were resistant to fluconazole; 1 of those was resistant to amphotericin B and another to echinocandins. No isolate was resistant to all 3 classes of antifungal medications.
To reduce the risk of transmission, the CDC advises using Standard and Contact Precautions for patients colonized or infected with C auris. Facilities should ensure thorough daily and terminal cleaning of patients’ rooms with an EPA-registered disinfectant with a fungal claim. In nursing homes, providers should consider the level of patient care being provided and the presence of transmission risk factors when deciding on the level of precautions. When patients are transferred, receiving facilities should be notified of the presence of this multidrug-resistant organism to ensure continuity of precautions.
Five of 7 reported isolates were either misidentified initially as C haemulonii or not identified beyond Candida spp. The CDC urges local and state health departments to continue to report possible cases of C auris and of isolates of C haemulonii and Candida spp. that cannot be identified after routine testing.
In 2009, Candida auris, an emerging invasive multidrug-resistant fungus, was isolated from external ear canal discharge of a patient in Japan. Since then, at least a dozen countries, including India, Israel, South Korea, and the United Kingdom, have published reports of C auris infections.
The emergence of C auris raises several serious concerns for public health, according to Morbidity and Mortality Weekly Report: Many isolates are multidrug-resistant; some strains have elevated minimum inhibitory concentrations to drugs in all 3 major classes of antifungal medications—a feature not found in other clinically relevant Candida species. Second, it’s challenging to identify, requiring specialized methods. C auris is often misidentified as other yeasts (most commonly Candida haemulonii). Finally, C auris has caused outbreaks in health care settings.
In June 2016, the CDC issued a clinical alert asking clinicians, public health authorities, and others to report C auris cases, or suspected cases, to state and local health departments and the CDC. Seven such US cases occurred between May 2013 and August 2016.
All patients had serious underlying medical conditions, including bone marrow transplantation. Four patients, all of whom had bloodstream infections, died during the weeks to months after C auris was identified.
The U.S. isolates were related to isolates from South America and South Asia, although available epidemiologic information suggests that most were acquired in the United States. Several findings suggested that transmission occurred in US health care settings.
Five isolates were resistant to fluconazole; 1 of those was resistant to amphotericin B and another to echinocandins. No isolate was resistant to all 3 classes of antifungal medications.
To reduce the risk of transmission, the CDC advises using Standard and Contact Precautions for patients colonized or infected with C auris. Facilities should ensure thorough daily and terminal cleaning of patients’ rooms with an EPA-registered disinfectant with a fungal claim. In nursing homes, providers should consider the level of patient care being provided and the presence of transmission risk factors when deciding on the level of precautions. When patients are transferred, receiving facilities should be notified of the presence of this multidrug-resistant organism to ensure continuity of precautions.
Five of 7 reported isolates were either misidentified initially as C haemulonii or not identified beyond Candida spp. The CDC urges local and state health departments to continue to report possible cases of C auris and of isolates of C haemulonii and Candida spp. that cannot be identified after routine testing.
Primary Care Provider Programs Get New Funding
HHS has announced nearly $300 million in awards to primary care clinicians and students through the National Health Service Corps and NURSE Corps Scholarship and Loan Repayment Programs. The grants are awarded in exchange for providing primary care in areas of greatest need.
The funding includes:
- $42.8 million to the National Health Service Corps Scholarship Program for 205 new awards and 8 continuation awards to students who pursueprimary care training leading to a degree in medicine, dentistry, nurse-midwife, physician assistant, or nurse practitioner
- $164.9 million to National Health Service Corps Loan Repayment Program, providing 3,079 new awards and 2,111 continuation awards to fully trained primary care clinicians
Other funding went to the National Health Service Corps Students to Service Loan Repayment Program, the NURSE Corps Scholarship Program and Loan Repayment Program, the Faculty Loan Repayment Program, and the Native Hawaiian Health Scholarship Program.
HHS has announced nearly $300 million in awards to primary care clinicians and students through the National Health Service Corps and NURSE Corps Scholarship and Loan Repayment Programs. The grants are awarded in exchange for providing primary care in areas of greatest need.
The funding includes:
- $42.8 million to the National Health Service Corps Scholarship Program for 205 new awards and 8 continuation awards to students who pursueprimary care training leading to a degree in medicine, dentistry, nurse-midwife, physician assistant, or nurse practitioner
- $164.9 million to National Health Service Corps Loan Repayment Program, providing 3,079 new awards and 2,111 continuation awards to fully trained primary care clinicians
Other funding went to the National Health Service Corps Students to Service Loan Repayment Program, the NURSE Corps Scholarship Program and Loan Repayment Program, the Faculty Loan Repayment Program, and the Native Hawaiian Health Scholarship Program.
HHS has announced nearly $300 million in awards to primary care clinicians and students through the National Health Service Corps and NURSE Corps Scholarship and Loan Repayment Programs. The grants are awarded in exchange for providing primary care in areas of greatest need.
The funding includes:
- $42.8 million to the National Health Service Corps Scholarship Program for 205 new awards and 8 continuation awards to students who pursueprimary care training leading to a degree in medicine, dentistry, nurse-midwife, physician assistant, or nurse practitioner
- $164.9 million to National Health Service Corps Loan Repayment Program, providing 3,079 new awards and 2,111 continuation awards to fully trained primary care clinicians
Other funding went to the National Health Service Corps Students to Service Loan Repayment Program, the NURSE Corps Scholarship Program and Loan Repayment Program, the Faculty Loan Repayment Program, and the Native Hawaiian Health Scholarship Program.
Flu Vaccine Provides Substantial Benefits for Patients With Diabetes
Is it safe to give flu vaccinations to patients with an impaired immune response, such as those with diabetes? The evidence was both sparse and inconclusive, say researchers from Imperial College London. But their 7-year study of 124,503 patients with type 2 diabetes suggests “substantial benefits.”
The study covered 4 periods in each cohort year: preinfluenza, influenza season, postinfluenza, and summer. The outcome measures were hospital admissions for acute myocardial infarction (MI), stroke, pneumonia, influenza, and heart failure as well as all-cause death.
During the study, there were 5,142 admissions for acute MI; 4,515 for stroke; 14,154 for pneumonia or influenza; 12,915 for heart failure; and 21,070 deaths.
Vaccine recipients were older and generally more ill; they had more coexisting conditions and were taking more medications than nonrecipients. However, vaccination was associated with significant reductions in all the outcomes during the flu season. After adjusting for residual confounding, the researchers found 19% lower rates of admissions for acute MI, 30% for stroke, 22% for heart failure, and 15% for pneumonia or influenza. The mortality rate for patients was 24% lower than that of nonrecipients.
That was during flu season, but vaccination also was associated with significantly fewer events during the pre- and postinfluenza seasons for all outcomes except for acute MI and pneumonia/influenza in the preinfluenza period.
Concerns about the benefits of influenza vaccination in older adults and patients with chronic illnesses affect the acceptance and uptake of vaccination, the researchers note. But their findings, they add, “underline the importance of influenza vaccination as part of comprehensive secondary prevention in this high-risk population.”
Source:
Vamos EP, Pape UJ, Curcin V, et al. CMAJ. 2016;188(14):E342-E351.
Is it safe to give flu vaccinations to patients with an impaired immune response, such as those with diabetes? The evidence was both sparse and inconclusive, say researchers from Imperial College London. But their 7-year study of 124,503 patients with type 2 diabetes suggests “substantial benefits.”
The study covered 4 periods in each cohort year: preinfluenza, influenza season, postinfluenza, and summer. The outcome measures were hospital admissions for acute myocardial infarction (MI), stroke, pneumonia, influenza, and heart failure as well as all-cause death.
During the study, there were 5,142 admissions for acute MI; 4,515 for stroke; 14,154 for pneumonia or influenza; 12,915 for heart failure; and 21,070 deaths.
Vaccine recipients were older and generally more ill; they had more coexisting conditions and were taking more medications than nonrecipients. However, vaccination was associated with significant reductions in all the outcomes during the flu season. After adjusting for residual confounding, the researchers found 19% lower rates of admissions for acute MI, 30% for stroke, 22% for heart failure, and 15% for pneumonia or influenza. The mortality rate for patients was 24% lower than that of nonrecipients.
That was during flu season, but vaccination also was associated with significantly fewer events during the pre- and postinfluenza seasons for all outcomes except for acute MI and pneumonia/influenza in the preinfluenza period.
Concerns about the benefits of influenza vaccination in older adults and patients with chronic illnesses affect the acceptance and uptake of vaccination, the researchers note. But their findings, they add, “underline the importance of influenza vaccination as part of comprehensive secondary prevention in this high-risk population.”
Source:
Vamos EP, Pape UJ, Curcin V, et al. CMAJ. 2016;188(14):E342-E351.
Is it safe to give flu vaccinations to patients with an impaired immune response, such as those with diabetes? The evidence was both sparse and inconclusive, say researchers from Imperial College London. But their 7-year study of 124,503 patients with type 2 diabetes suggests “substantial benefits.”
The study covered 4 periods in each cohort year: preinfluenza, influenza season, postinfluenza, and summer. The outcome measures were hospital admissions for acute myocardial infarction (MI), stroke, pneumonia, influenza, and heart failure as well as all-cause death.
During the study, there were 5,142 admissions for acute MI; 4,515 for stroke; 14,154 for pneumonia or influenza; 12,915 for heart failure; and 21,070 deaths.
Vaccine recipients were older and generally more ill; they had more coexisting conditions and were taking more medications than nonrecipients. However, vaccination was associated with significant reductions in all the outcomes during the flu season. After adjusting for residual confounding, the researchers found 19% lower rates of admissions for acute MI, 30% for stroke, 22% for heart failure, and 15% for pneumonia or influenza. The mortality rate for patients was 24% lower than that of nonrecipients.
That was during flu season, but vaccination also was associated with significantly fewer events during the pre- and postinfluenza seasons for all outcomes except for acute MI and pneumonia/influenza in the preinfluenza period.
Concerns about the benefits of influenza vaccination in older adults and patients with chronic illnesses affect the acceptance and uptake of vaccination, the researchers note. But their findings, they add, “underline the importance of influenza vaccination as part of comprehensive secondary prevention in this high-risk population.”
Source:
Vamos EP, Pape UJ, Curcin V, et al. CMAJ. 2016;188(14):E342-E351.
Predicting Flu Epidemics
Perhaps a seasonal link exists between “epidemic waves” of respiratory syncytial virus (RSV) infections and influenza,. but forecasting seasonal patterns with accuracy isn’t easy due to the many varieties of flu and environmental factors. However, researchers from the Public Health Center of Valencia and University of Valencia, Spain, say they have a way to predict an influenza epidemic 3 to 4 weeks in advance. And that could allow for more effective vaccination programs and influenza prophylaxis.
They used 2 epidemiologic surveillance systems: The first, the analysis of epidemiologicol surveillance system (AVE) in use in eastern Spain since 2004, collects real-time data from notifiable disease outbreaks and alerts. The second, Microbiologica surveillance network of the Comunitat Valenciana (RedMIVA), collects cases of RSV.
The researchers conducted a study that lasted from week 40 (2010) to week 8 (2014). During that time, 239,321 people reported cases of flu, and 19,676 cases of RSV were recorded, with 5,112 laboratory confirmed. Most (85%) of the RSV cases were children aged < 1 year .
Using the data from the surveillance systems, the researchers found that the peak of maximum activity of the influenza virus appears at least 3 weeks after the RSV peak. Interestingly, they also found evidence suggesting that RSV infection has a short-term protective effect against human influenza type A (H1N1) infection. The seasons with the highest number of recorded cases of RSV coincided with the lowest number of influenza cases. In both seasons, the predominant influenza virus type was H1N1.
Source:
Míguez A, Iftimi A, Montes F. Epidemiol Infect. 2016;144(12):2621-2632.
Perhaps a seasonal link exists between “epidemic waves” of respiratory syncytial virus (RSV) infections and influenza,. but forecasting seasonal patterns with accuracy isn’t easy due to the many varieties of flu and environmental factors. However, researchers from the Public Health Center of Valencia and University of Valencia, Spain, say they have a way to predict an influenza epidemic 3 to 4 weeks in advance. And that could allow for more effective vaccination programs and influenza prophylaxis.
They used 2 epidemiologic surveillance systems: The first, the analysis of epidemiologicol surveillance system (AVE) in use in eastern Spain since 2004, collects real-time data from notifiable disease outbreaks and alerts. The second, Microbiologica surveillance network of the Comunitat Valenciana (RedMIVA), collects cases of RSV.
The researchers conducted a study that lasted from week 40 (2010) to week 8 (2014). During that time, 239,321 people reported cases of flu, and 19,676 cases of RSV were recorded, with 5,112 laboratory confirmed. Most (85%) of the RSV cases were children aged < 1 year .
Using the data from the surveillance systems, the researchers found that the peak of maximum activity of the influenza virus appears at least 3 weeks after the RSV peak. Interestingly, they also found evidence suggesting that RSV infection has a short-term protective effect against human influenza type A (H1N1) infection. The seasons with the highest number of recorded cases of RSV coincided with the lowest number of influenza cases. In both seasons, the predominant influenza virus type was H1N1.
Source:
Míguez A, Iftimi A, Montes F. Epidemiol Infect. 2016;144(12):2621-2632.
Perhaps a seasonal link exists between “epidemic waves” of respiratory syncytial virus (RSV) infections and influenza,. but forecasting seasonal patterns with accuracy isn’t easy due to the many varieties of flu and environmental factors. However, researchers from the Public Health Center of Valencia and University of Valencia, Spain, say they have a way to predict an influenza epidemic 3 to 4 weeks in advance. And that could allow for more effective vaccination programs and influenza prophylaxis.
They used 2 epidemiologic surveillance systems: The first, the analysis of epidemiologicol surveillance system (AVE) in use in eastern Spain since 2004, collects real-time data from notifiable disease outbreaks and alerts. The second, Microbiologica surveillance network of the Comunitat Valenciana (RedMIVA), collects cases of RSV.
The researchers conducted a study that lasted from week 40 (2010) to week 8 (2014). During that time, 239,321 people reported cases of flu, and 19,676 cases of RSV were recorded, with 5,112 laboratory confirmed. Most (85%) of the RSV cases were children aged < 1 year .
Using the data from the surveillance systems, the researchers found that the peak of maximum activity of the influenza virus appears at least 3 weeks after the RSV peak. Interestingly, they also found evidence suggesting that RSV infection has a short-term protective effect against human influenza type A (H1N1) infection. The seasons with the highest number of recorded cases of RSV coincided with the lowest number of influenza cases. In both seasons, the predominant influenza virus type was H1N1.
Source:
Míguez A, Iftimi A, Montes F. Epidemiol Infect. 2016;144(12):2621-2632.
Non-Hodgkin Lymphoma Death Rates Continue to Fall
The 5-year relative survival rate for non-Hodgkin lymphoma (NHL) climbed to 72.7% and is as high as 82.6% for localized NHL, according to the most recent SEER data. The number of new cases remains high at 19.1 per 100,000 people (all races) per year; however the number of deaths is relatively low at 5.7 deaths per 100,000 people (all races) per year. Death rates have been falling on average 2.4% each year from 2004 to 2013.
While the new cases represent 4.3% of all new cancer diagnoses, NHL deaths represent 3.4% of all cancer deaths. Based on 2011-2013 SEER data, about 2.1% of men and women will receive a NHL diagnosis at some point during their lifetime.
Patient diagnoses by stage:
- 28% are diagnosed at the local stage
- 15% are diagnosed with spread to regional lymph nodes
- 50% are diagnosed after distant cancer has metastasized
- 8% unknown/unstaged
As of 2013, there were an estimated 569,536 people living with NHL in the U.S.
Using statistical models for analysis, rates for new non-Hodgkin lymphoma cases have not changed significantly over the past 10 years.
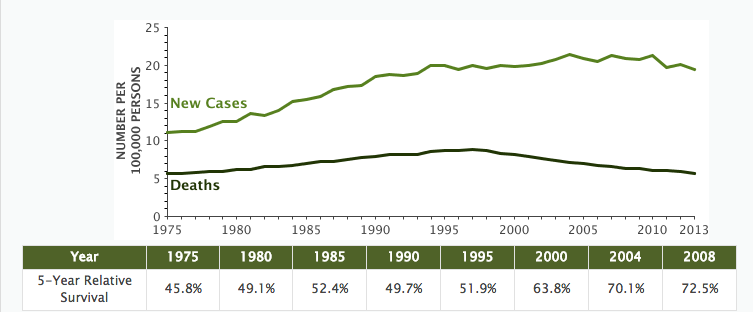
The 5-year relative survival rate for non-Hodgkin lymphoma (NHL) climbed to 72.7% and is as high as 82.6% for localized NHL, according to the most recent SEER data. The number of new cases remains high at 19.1 per 100,000 people (all races) per year; however the number of deaths is relatively low at 5.7 deaths per 100,000 people (all races) per year. Death rates have been falling on average 2.4% each year from 2004 to 2013.
While the new cases represent 4.3% of all new cancer diagnoses, NHL deaths represent 3.4% of all cancer deaths. Based on 2011-2013 SEER data, about 2.1% of men and women will receive a NHL diagnosis at some point during their lifetime.
Patient diagnoses by stage:
- 28% are diagnosed at the local stage
- 15% are diagnosed with spread to regional lymph nodes
- 50% are diagnosed after distant cancer has metastasized
- 8% unknown/unstaged
As of 2013, there were an estimated 569,536 people living with NHL in the U.S.
Using statistical models for analysis, rates for new non-Hodgkin lymphoma cases have not changed significantly over the past 10 years.

The 5-year relative survival rate for non-Hodgkin lymphoma (NHL) climbed to 72.7% and is as high as 82.6% for localized NHL, according to the most recent SEER data. The number of new cases remains high at 19.1 per 100,000 people (all races) per year; however the number of deaths is relatively low at 5.7 deaths per 100,000 people (all races) per year. Death rates have been falling on average 2.4% each year from 2004 to 2013.
While the new cases represent 4.3% of all new cancer diagnoses, NHL deaths represent 3.4% of all cancer deaths. Based on 2011-2013 SEER data, about 2.1% of men and women will receive a NHL diagnosis at some point during their lifetime.
Patient diagnoses by stage:
- 28% are diagnosed at the local stage
- 15% are diagnosed with spread to regional lymph nodes
- 50% are diagnosed after distant cancer has metastasized
- 8% unknown/unstaged
As of 2013, there were an estimated 569,536 people living with NHL in the U.S.
Using statistical models for analysis, rates for new non-Hodgkin lymphoma cases have not changed significantly over the past 10 years.

IHS Funds Programs to Protect Native Youth from Substance Abuse
The IHS announced 42 new awards to promote best practice strategies for preventing suicide and substance abuse, incorporating culturally appropriate approaches that are effective for tribal communities.
The awards, totaling more than $7 million for 1 year, are specifically for Methamphetamine and Suicide Prevention Initiative (MSPI) funding. The award recipients focus on boosting positive youth development, fostering resiliency, and promoting family engagement among Native youth, the IHS says. “We know that protective factors provided through caring adults, traditional practices, and Native language and culture help offset negative outcomes and foster the long-term development of resilience,” said IHS Principal Deputy Director Mary Smith, in announcing the awards.
Current funded projects include the Ohkay Owingeh MSPI Project in New Mexico. The evidence- and practice-based prevention program, conducted by the local Boys and Girls Club, “strongly focuses” on the issues surrounding methamphetamine and other drugs and self-harm in Native communities.
Another funded program, Fresno American Indian Health Project, targets Native youth at risk for substance abuse and suicide in the San Francisco Bay Area. The Stronghold Project II after-school programs help to strengthen cultural systems and family capacity, addressing family violence and suicide due to substance abuse.
From 2009 through 2015, MSPI supported > 12,200 people entering treatment for methamphetamine abuse, plus > 16,560 substance use and mental health disorder encounters via telehealth. The funding also supported training nearly 17,000 professionals and community members in suicide crisis response, with nearly 700,000 encounters with youth through prevention activities. The recently announced awards build on the more than $13 million awarded in 2015.
The IHS announced 42 new awards to promote best practice strategies for preventing suicide and substance abuse, incorporating culturally appropriate approaches that are effective for tribal communities.
The awards, totaling more than $7 million for 1 year, are specifically for Methamphetamine and Suicide Prevention Initiative (MSPI) funding. The award recipients focus on boosting positive youth development, fostering resiliency, and promoting family engagement among Native youth, the IHS says. “We know that protective factors provided through caring adults, traditional practices, and Native language and culture help offset negative outcomes and foster the long-term development of resilience,” said IHS Principal Deputy Director Mary Smith, in announcing the awards.
Current funded projects include the Ohkay Owingeh MSPI Project in New Mexico. The evidence- and practice-based prevention program, conducted by the local Boys and Girls Club, “strongly focuses” on the issues surrounding methamphetamine and other drugs and self-harm in Native communities.
Another funded program, Fresno American Indian Health Project, targets Native youth at risk for substance abuse and suicide in the San Francisco Bay Area. The Stronghold Project II after-school programs help to strengthen cultural systems and family capacity, addressing family violence and suicide due to substance abuse.
From 2009 through 2015, MSPI supported > 12,200 people entering treatment for methamphetamine abuse, plus > 16,560 substance use and mental health disorder encounters via telehealth. The funding also supported training nearly 17,000 professionals and community members in suicide crisis response, with nearly 700,000 encounters with youth through prevention activities. The recently announced awards build on the more than $13 million awarded in 2015.
The IHS announced 42 new awards to promote best practice strategies for preventing suicide and substance abuse, incorporating culturally appropriate approaches that are effective for tribal communities.
The awards, totaling more than $7 million for 1 year, are specifically for Methamphetamine and Suicide Prevention Initiative (MSPI) funding. The award recipients focus on boosting positive youth development, fostering resiliency, and promoting family engagement among Native youth, the IHS says. “We know that protective factors provided through caring adults, traditional practices, and Native language and culture help offset negative outcomes and foster the long-term development of resilience,” said IHS Principal Deputy Director Mary Smith, in announcing the awards.
Current funded projects include the Ohkay Owingeh MSPI Project in New Mexico. The evidence- and practice-based prevention program, conducted by the local Boys and Girls Club, “strongly focuses” on the issues surrounding methamphetamine and other drugs and self-harm in Native communities.
Another funded program, Fresno American Indian Health Project, targets Native youth at risk for substance abuse and suicide in the San Francisco Bay Area. The Stronghold Project II after-school programs help to strengthen cultural systems and family capacity, addressing family violence and suicide due to substance abuse.
From 2009 through 2015, MSPI supported > 12,200 people entering treatment for methamphetamine abuse, plus > 16,560 substance use and mental health disorder encounters via telehealth. The funding also supported training nearly 17,000 professionals and community members in suicide crisis response, with nearly 700,000 encounters with youth through prevention activities. The recently announced awards build on the more than $13 million awarded in 2015.
IHS Program Targets HIV/AIDs in Young Natives
More than half of the new HIV diagnoses among American Indians and Alaska Natives are estimated to be among those aged < 35 years, according to the CDC. To improve HIV prevention and care outcomes, an ongoing collaboration between IHS and the CDC is funding cooperative agreements with First Nations Community HealthSource, Albuquerque, and Inter Tribal Council of Arizona, Phoenix. The groups will receive up to $100,000 a year for up to 5 years for community health care services. “These awards increase access to culturally appropriate, high-quality HIV treatment for our American Indian and Alaska Native communities,” said Mary L. Smith, IHS principal deputy director.
First Nations, New Mexico’s urban Indian health center and a Federally Qualified Health Center, operates 2 clinic sites and 3 school-based health centers. The Inter Tribal Council of Arizona, representing 21 tribal governments, operates more than 30 projects and provides technical assistance and training to tribal governments in program planning and development, research and data collection, resource development, management and evaluation.
The awards support activities in 5 main areas:
- Increasing access to comprehensive pre-exposure prophylaxis;
- Identifying local-level priorities for HIV care needs and creating tools and resources;
- Making it easier for people living with HIV and AIDS to stay in treatment;
- Teaching people who inject drugs about reducing risks and extending access to services for medication-assisted therapies for people with opioid use disorder in accordance with federal, state, tribal, and local laws; and
- Increasing age-appropriate prevention education at the local levels.
“This multiyear collaboration supports a sustained, in-depth HIV prevention program that will benefit not only tribes, but also American Indians and Alaska Natives in urban locations,” said Eugene McCray, MD, director of CDC’s Division of HIV/AIDS Prevention. “We are bringing services right to the local level, reaching American Indian and Alaska Native communities.”
More than half of the new HIV diagnoses among American Indians and Alaska Natives are estimated to be among those aged < 35 years, according to the CDC. To improve HIV prevention and care outcomes, an ongoing collaboration between IHS and the CDC is funding cooperative agreements with First Nations Community HealthSource, Albuquerque, and Inter Tribal Council of Arizona, Phoenix. The groups will receive up to $100,000 a year for up to 5 years for community health care services. “These awards increase access to culturally appropriate, high-quality HIV treatment for our American Indian and Alaska Native communities,” said Mary L. Smith, IHS principal deputy director.
First Nations, New Mexico’s urban Indian health center and a Federally Qualified Health Center, operates 2 clinic sites and 3 school-based health centers. The Inter Tribal Council of Arizona, representing 21 tribal governments, operates more than 30 projects and provides technical assistance and training to tribal governments in program planning and development, research and data collection, resource development, management and evaluation.
The awards support activities in 5 main areas:
- Increasing access to comprehensive pre-exposure prophylaxis;
- Identifying local-level priorities for HIV care needs and creating tools and resources;
- Making it easier for people living with HIV and AIDS to stay in treatment;
- Teaching people who inject drugs about reducing risks and extending access to services for medication-assisted therapies for people with opioid use disorder in accordance with federal, state, tribal, and local laws; and
- Increasing age-appropriate prevention education at the local levels.
“This multiyear collaboration supports a sustained, in-depth HIV prevention program that will benefit not only tribes, but also American Indians and Alaska Natives in urban locations,” said Eugene McCray, MD, director of CDC’s Division of HIV/AIDS Prevention. “We are bringing services right to the local level, reaching American Indian and Alaska Native communities.”
More than half of the new HIV diagnoses among American Indians and Alaska Natives are estimated to be among those aged < 35 years, according to the CDC. To improve HIV prevention and care outcomes, an ongoing collaboration between IHS and the CDC is funding cooperative agreements with First Nations Community HealthSource, Albuquerque, and Inter Tribal Council of Arizona, Phoenix. The groups will receive up to $100,000 a year for up to 5 years for community health care services. “These awards increase access to culturally appropriate, high-quality HIV treatment for our American Indian and Alaska Native communities,” said Mary L. Smith, IHS principal deputy director.
First Nations, New Mexico’s urban Indian health center and a Federally Qualified Health Center, operates 2 clinic sites and 3 school-based health centers. The Inter Tribal Council of Arizona, representing 21 tribal governments, operates more than 30 projects and provides technical assistance and training to tribal governments in program planning and development, research and data collection, resource development, management and evaluation.
The awards support activities in 5 main areas:
- Increasing access to comprehensive pre-exposure prophylaxis;
- Identifying local-level priorities for HIV care needs and creating tools and resources;
- Making it easier for people living with HIV and AIDS to stay in treatment;
- Teaching people who inject drugs about reducing risks and extending access to services for medication-assisted therapies for people with opioid use disorder in accordance with federal, state, tribal, and local laws; and
- Increasing age-appropriate prevention education at the local levels.
“This multiyear collaboration supports a sustained, in-depth HIV prevention program that will benefit not only tribes, but also American Indians and Alaska Natives in urban locations,” said Eugene McCray, MD, director of CDC’s Division of HIV/AIDS Prevention. “We are bringing services right to the local level, reaching American Indian and Alaska Native communities.”
Preventive Treatment for Posttraumatic Stress Disorder
Identifying people who might be at risk for posttraumatic stress disorder (PTSD) before the trauma—and teaching them preventive coping skills—could reduce or prevent long-term effects, according to University of Oxford in Oxford, United Kingdom, and King’s College London, United Kingdom, researchers.
They assessed 453 newly recruited paramedics every 4 months for 2 years. Of those, 386 paramedics participated in follow-up interviews.
Related: Let’s Dance: A Holistic Approach to Treating Veterans With Posttraumatic Stress Disorder
Over the 2 years, 32 participants (8.3%) had an episode of PTSD, and 41 participants had (10.6%) an episode of major depression (MD). Most of the episodes were moderate and short lived. In most cases, the participant had recovered by the next 4-month assessment. However, at 2 years, those who had experienced episodes of PTSD or MD during the follow-up period reported more days off work, poorer sleep, poorer quality of life, and greater burn out as well as weight gain (mean gain, 6.9 kg) for those with P
Ten participants who developed PTSD received treatment during follow-up, as did 12 participants who developed MD. Five of 9 participants who had recurrent P
Related: Telehealth for Native Americans With PTSD
The researchers tested a number of possible pretrauma predictors of PTSD and MD. They correlated several: cognitive style (eg, suppression, rumination, intentional numbing), coping style (eg, avoidant styles, such as wishful thinking), and psychological traits (eg, neuroticism). However, they found rumination about memories of stressful events uniquely predicted an episode of PTSD. Perceived resilience uniquely predicted an episode of MD.
Interestingly, about 42% of the study participants had a psychiatric history before training—more than the general population. That might be a factor that draws them to emergency work, the researchers suggest.
Some predictors, such as psychiatric history, are fixed, the researchers note. But others, such as cognitive styles, can be modified or taught. Studies have shown that rumination can be redirected through training in concrete thinking, for instance, and psychoeducation and cognitive behavioral techniques (eg, modifying interpretations of stressful events) have been used to strengthen resilience. The predictors they identified in their study could serve as targets, the researchers suggest, for modifying future resilience programs.
Source:
Wild J, Smith KV, Thompson E, Béar F, Lommen MJ, Ehlers A. Psychol Med. 2016;46(12):2571-2582. doi: 10.1017/S0033291716000532.
Identifying people who might be at risk for posttraumatic stress disorder (PTSD) before the trauma—and teaching them preventive coping skills—could reduce or prevent long-term effects, according to University of Oxford in Oxford, United Kingdom, and King’s College London, United Kingdom, researchers.
They assessed 453 newly recruited paramedics every 4 months for 2 years. Of those, 386 paramedics participated in follow-up interviews.
Related: Let’s Dance: A Holistic Approach to Treating Veterans With Posttraumatic Stress Disorder
Over the 2 years, 32 participants (8.3%) had an episode of PTSD, and 41 participants had (10.6%) an episode of major depression (MD). Most of the episodes were moderate and short lived. In most cases, the participant had recovered by the next 4-month assessment. However, at 2 years, those who had experienced episodes of PTSD or MD during the follow-up period reported more days off work, poorer sleep, poorer quality of life, and greater burn out as well as weight gain (mean gain, 6.9 kg) for those with P
Ten participants who developed PTSD received treatment during follow-up, as did 12 participants who developed MD. Five of 9 participants who had recurrent P
Related: Telehealth for Native Americans With PTSD
The researchers tested a number of possible pretrauma predictors of PTSD and MD. They correlated several: cognitive style (eg, suppression, rumination, intentional numbing), coping style (eg, avoidant styles, such as wishful thinking), and psychological traits (eg, neuroticism). However, they found rumination about memories of stressful events uniquely predicted an episode of PTSD. Perceived resilience uniquely predicted an episode of MD.
Interestingly, about 42% of the study participants had a psychiatric history before training—more than the general population. That might be a factor that draws them to emergency work, the researchers suggest.
Some predictors, such as psychiatric history, are fixed, the researchers note. But others, such as cognitive styles, can be modified or taught. Studies have shown that rumination can be redirected through training in concrete thinking, for instance, and psychoeducation and cognitive behavioral techniques (eg, modifying interpretations of stressful events) have been used to strengthen resilience. The predictors they identified in their study could serve as targets, the researchers suggest, for modifying future resilience programs.
Source:
Wild J, Smith KV, Thompson E, Béar F, Lommen MJ, Ehlers A. Psychol Med. 2016;46(12):2571-2582. doi: 10.1017/S0033291716000532.
Identifying people who might be at risk for posttraumatic stress disorder (PTSD) before the trauma—and teaching them preventive coping skills—could reduce or prevent long-term effects, according to University of Oxford in Oxford, United Kingdom, and King’s College London, United Kingdom, researchers.
They assessed 453 newly recruited paramedics every 4 months for 2 years. Of those, 386 paramedics participated in follow-up interviews.
Related: Let’s Dance: A Holistic Approach to Treating Veterans With Posttraumatic Stress Disorder
Over the 2 years, 32 participants (8.3%) had an episode of PTSD, and 41 participants had (10.6%) an episode of major depression (MD). Most of the episodes were moderate and short lived. In most cases, the participant had recovered by the next 4-month assessment. However, at 2 years, those who had experienced episodes of PTSD or MD during the follow-up period reported more days off work, poorer sleep, poorer quality of life, and greater burn out as well as weight gain (mean gain, 6.9 kg) for those with P
Ten participants who developed PTSD received treatment during follow-up, as did 12 participants who developed MD. Five of 9 participants who had recurrent P
Related: Telehealth for Native Americans With PTSD
The researchers tested a number of possible pretrauma predictors of PTSD and MD. They correlated several: cognitive style (eg, suppression, rumination, intentional numbing), coping style (eg, avoidant styles, such as wishful thinking), and psychological traits (eg, neuroticism). However, they found rumination about memories of stressful events uniquely predicted an episode of PTSD. Perceived resilience uniquely predicted an episode of MD.
Interestingly, about 42% of the study participants had a psychiatric history before training—more than the general population. That might be a factor that draws them to emergency work, the researchers suggest.
Some predictors, such as psychiatric history, are fixed, the researchers note. But others, such as cognitive styles, can be modified or taught. Studies have shown that rumination can be redirected through training in concrete thinking, for instance, and psychoeducation and cognitive behavioral techniques (eg, modifying interpretations of stressful events) have been used to strengthen resilience. The predictors they identified in their study could serve as targets, the researchers suggest, for modifying future resilience programs.
Source:
Wild J, Smith KV, Thompson E, Béar F, Lommen MJ, Ehlers A. Psychol Med. 2016;46(12):2571-2582. doi: 10.1017/S0033291716000532.