User login
Metastatic Spinal Cord Compression: A Review
Case
A 60-year-old man with stage IV hormoneindependent prostate cancer, with widely metastatic disease to the bone, presents to the ED with increased weakness and new onset of numbness in the lower extremities, which he states began earlier that day. After failing several lines of chemotherapy, he is currently being treated with hormonal therapy alone. Patient first noted weakness in the left lower extremity 5 days before presentation, which progressed to bilateral involvement, making ambulation difficult and requiring the use of a walker. He denies back pain or urinary or fecal incontinence. Regarding pain management, he had been recently treated at one of the pain clinics in the hospital and has continued on opioid medication at another institution. Until the past week, he states he had back pain without neurological deficits.
His vital signs are stable at presentation. Patient is obese but in no acute distress. His cardiopulmonary examination is unremarkable; abdominal examination is benign; and back examination is normal. On neurological examination, iliopsoas flexion is 4/5 bilaterally; the rest of the motor examination is normal, with toes downgoing bilaterally upon plantar stimulation. Diminished sensation to light touch is noted at the T4-T6 sensory level and below; patient also has diminished proprioception in his lower extremities.
Patient had undergone a whole body scan one month prior to presentation, which revealed increased tracer uptake of Technetium-99m in multiple areas in the thoracic and lumbar spine. The radiologist also reported bilateral involvement in the wrists, femurs, tibias, and humeri—all in concordance with multifocal bone disease noted in previous computed tomography scans.
How should you approach this case?
Overview of Metastatic Spinal Cord Compression
Malignant or metastatic spinal cord compression (MSCC) of the thecal sac is an ominous complication of advanced cancer and an oncologic emergency presenting clinically in approximately 3% to 10% of cancer-related deaths.1,2 Cancer patients have a median survival of 3 to 6 months from diagnosis of MSCC.1,3,4 This disease causes significant disability due to paralysis, sensory loss, protracted pain, and sphincter dysfunction.5 If left untreated, MSCC has the potential to cause paraplegia in almost all affected patients; therefore, prompt recognition and treatment are essential to maintain mobility and neurological function. Generally speaking, any cancer patient who presents with new or worsening back pain—even in the absence of neurological deficits—merits evaluation for spinal cord compression.6 Nevertheless, individual risk assessment is warranted.7
Epidemiology
In the United States, more than 20,000 cases of MSCC are reported each year.8 According to postmortem studies, this condition affects 5% to 36% of cancer patients.9,10 In a US nationwide study of 15,367 cases of MSCC,2 the mean age at hospitalization was 62 years, with 37% of cases occurring in women. In approximately 20% of cases, MSCC was the initial presentation of cancer4; this has been reflected in our experience at MD Anderson Cancer Center.
Cancers of the breast, lung, prostate, and multiple myeloma are the most frequent underlying conditions in MSCC.2,8 Its prevalence varies depending on tumor type, occurring in 0.2% of pancreatic cancers; however, MSCC may affect up to 7.9% to 15% of myelomas1,2 and 13% of lymphomas.2 Interestingly, 5.5% of patients with prostate cancer develop MSCC.2 According to a study by Lu et al,11 historical risk factors include known nonvertebral bony metastases and stage IV disease at the time of diagnosis.
The most common location of MSCC is the thoracic spine (69% of cases); 29% of cases occur at the lumbosacral level and 10% at the cervical area.12 Most likely this pattern follows the lymphatic drainage, as metastases from breast and lung cancers tend to be found in the thoracic spine. Pelvic and intra-abdominal malignancies most commonly migrate to the lumbar spine. Multiple spinal epidural metastases were noted in 31% of those who underwent complete imaging of the spine.12
Pathophysiology
Most cases of MSCC are epidural in origin, arising from the vertebral column in 85% of patients.8 Epidural spread is caused mainly by hematogenous mechanism through the Batson venous plexus,13 debilitating the bone and eventually causing vertebral collapse with compression of the spinal canal. Epidural spread is less likely caused by direct tumor extension (ie, erosion through the bone) or by direct deposition of tumor cells into the epidural space.14 Ultimate neuronal injury is thought to involve vasogenic edema,15 leading to ischemia13 through venous infarction, but there has been debate regarding this last phenomenon.16 In cases of paralysis, demyelination is striking.16
Clinical Presentation
Even though cancer accounts for less than 1% of episodes of low back pain, it is the most common systemic disease affecting the spine.17 An important clinical inquiry is to determine whether back pain in an established cancer patient can be ruled out without extensive imaging. Unfortunately, clinical examination alone cannot exclude MSCC. Because of the high specificity (0.98), any cancer patient with new back pain should be considered to have metastasis until proven otherwise.17
Symptoms in MSCC at presentation can be motor, sensory, and/or autonomic. Back pain varies depending on the site of metastasis, which can be referred, local, radicular, or a combination of all three.18 The primary complaint is pain in 83% to 96% of cases,19,20 though this is a nonspecific sign.
Previous studies have shown 40% to 64% of patients were not ambulatory at the time of diagnosis.19,25 Recent case series, however, report an increased number of ambulatory patients—possibly due to increased clinician awareness.26 In other cases, only 9% of patients were able to walk independently without aid.27 Loss of sensation, dense paraplegia, and incontinence are late findings and likely signal some degree of permanent disability.19
Misdiagnosis is a common issue in the ED setting. In an interesting retrospective study of 63 patients with spinal cord compression28 (not necessarily malignant), 18 (29%) were misdiagnosed.28 Consequently, there was a significant delay in diagnosis despite obvious neurological deficits at presentation.
Evaluation and Imaging
A detailed physical examination is essential to diagnosing MSCC. A thorough neurological examination, including sensation, strength, and reflexes should be carefully documented. If spinal instability is suspected, range-of-motion testing is contraindicated. The modified Frankel classification,29 adapted from the traumatic spine cord injury work by Frankel, et al,30 may be used to assess the degree of disability (Table).
Lu et al11 noted hyperreflexia and upward going Babinski reflex as common findings. Moreover, risk factors of decreased rectal sphincter tone and bladder were determinant for poor outcomes.
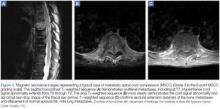
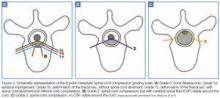
MRI studies should include the entire spine—not just the perceived area of interest— as up to 38% of patients have multiple-site metastases12 (Figure 1). Sensory deficits and mechanical pain may be present two to four vertebral levels away from the actual lesion.11 If MRI suggests cord compression, severity can be graded using the MSCC scale34 (Figure 2). Several scoring systems have been developed to aid in decision making concerning surgical treatment.
Management and Outcomes
The goal of therapy is symptom control and preservation of function. This requires a multidisciplinary approach and may involve radiation therapy and surgery, as well as medical efforts. Upon diagnosis and initiation of therapy, serial neurological evaluation should be undertaken. Neurovital signs should be scheduled to coincide with other nursing efforts to ease the burden of care and minimize patient discomfort.
The mainstay of medical therapy is treatment with corticosteroids.35 Initial trials have demonstrated that corticosteroids improve functional status in MSCC, but controversy exists regarding the effective dose. In a randomized, controlled trial by Sorensen et al,36 which sought to evaluate functional outcomes of highdose corticosteroids as an adjunct to radiotherapy, 57 patients received either high-dose dexamethasone or no corticosteroid therapy. Fifty-nine percent of patients in the dexamethasone group were ambulatory 6 months after treatment compared to 39% in the group who did not receive steroids.36
A patient without a biopsy-confirmed cancer diagnosis in need of corticosteroid treatment presents a dilemma. Plasmacytomas, thymomas, lymphomas, multiple myeloma, germ-cell tumors are very sensitive to corticosteroid therapy in patients with MSCC.38 However, corticosteroids given before tissue samples are obtained may hinder proper diagnosis and complicate future management.39,40 In the absence of neurological deficit, corticosteroids may be withheld and emergent consultation with neurosurgery and oncology should be obtained. If there is any question regarding the nature of the lesion, tissue diagnosis must be obtained without delay.
Strict bed rest (including logroll and bedpan use) should be instituted if there is suspicion of spinal cord instability. Patients with suspected involvement of the cervical spine should have a Philadelphia collar placed until spinal stability has been confirmed. In the United Kingdom, the National Institutes for Health Care Excellence guidelines recommend all patients with suspected cord compression be nursed in a flat position.22 Other institutions, however, do not believe that strict bed rest is necessary, as it is presumed that MSCC is inherently different from that caused by trauma. Authors supporting this position contend that the increased incidence of deep vein thrombosis, infection (particularly from the urinary tract), and decubitus ulcers outweighs the benefit of bed rest. Patient preference should be taken into consideration as those with good functional status may be quite resistant to bed rest. In cases where cord compression is strongly suspected, these patients should be educated on proper bed rest. The greatest predictors of outcome are ambulatory and functional status at the time of diagnosis (generally based on an Eastern Cooperative Oncology Group scale). Patients with a good functional status, limited disease, and a life expectancy of greater than 3 to 6 months may benefit from surgery.41 However, emergent surgical evaluation is required in patients not responding to radiotherapy or who received received only limited doses of radiotherapy, as well as those with spinal instability, direct cord compression due to a bony fragment, impending sphincter dysfunction, unknown primary tumor, or no paraplegia for >48 hours.15
Unfortunately, surgery is only indicated in 10% to 15% of MSCC cases.42 In the past two decades, significant improvements regarding new aggressive surgical techniques have been made, and include circumferential decompression of the spine and staged or single stage anterior posterior surgery with stabilization. 43 Additionally, the combination of surgery with radiotherapy has improved outcomes.44
Most patients benefit from short-course radiotherapy45 even when given palliatively. 46 Longer courses of radiotherapy are highly recommended for patients with a more favorable prognosis.47 Up to 10% of patients diagnosed with spinal cord compression will require treatment for disease recurrence.42 There is a limited role for chemotherapy, and in seminomas and lymphomas, results can be quite dramatic.38
Prevention
Lu et al11 found that only 54% of patients were aware that back pain should be reported to their physician. Delays in diagnosis and treatment are common and well described in the literature.21 Patients should be instructed to call their physician within 24 hours from the development of any new or worsening back pain, and should be advised to seek immediate care if they develop any neurological symptoms. To facilitate appropriate and prompt management of MSCC, hospitals should develop diagnostic algorithms to minimize delays in referral to a comprehensive center for further treatment.
Case Conclusion
Based on this patient’s symptoms and status at presentation, the emergency team determined he was at high risk for MSCC. An initial dosage of 10 mg dexamethasone was administered intravenously (IV), followed by 4 mg IV every 6 hours prior to imaging. An MRI without contrast of the cervical, thoracic, and lumbar spine showed cord compression with mild cord edema at T4 level, along with diffused osseous metastasis.
Upon diagnosis, patient was referred to radiation oncology for radiotherapy of the T2-T6 vertebral bodies. Three days after initiation of radiation therapy, his neurological function deteriorated with paraplegia and incontinence, and he was emergently evaluated for neurosurgery. Although T4 laminectomy and decompression of the spinal cord were performed without complication, patient did not recover neurological function. His hospital course was complicated by Ogilvie syndrome and episodes of delirium, and he was discharged to a rehabilitation facility 23 days after admission; paraplegia and urinary and bowel incontinence remained unchanged.
- Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol). 2003;15(4):211-217.
- Mak KS, Lee LK, Mak RH, et al. Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998-2006. Int J Radiat Oncol Biol Phys. 2011;80(3):824-831.
- Constans JP, de Divitiis E, Donzelli R, Spaziante R, Meder JF, Haye C. Spinal metastases with
- neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59(1):111-118.
- Schiff D, O’Neill BP, Suman VJ. Spinal epidural metastasis as the initial manifestation of malignancy: clinical features and diagnostic approach. Neurology. 1997;49(2):452-456.
- Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative’s Neuro-Oncology Disease Site Group. J Clin Oncol. 2005;23(9):2028-2037.
- Levack P, Graham J, Collie D, et al. Don’t wait for a sensory level—listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol). 2002;14(6):472-480.
- Talcott JA, Stomper PC, Drislane FW, et al. Assessing suspected spinal cord compression: a multidisciplinary outcomes analysis of 342 episodes. Support Care Cancer. 1999;7(1):31-38.
- Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327(9):614-619.
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3(1):74-85.
- Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine (Phila Pa 1976). 1990;15(1):1-4.
- Lu C, Gonzalez RG, Jolesz FA, Wen PY, Talcott JA. Suspected spinal cord compression in cancer patients: a multidisciplinary risk assessment. J Support Oncol. 2005;3(4):305-312.
- Schiff D, O’Neill BP, Wang CH, O’Fallon JR. Neuroimaging and treatment implications of patients with multiple epidural spinal metastases. Cancer. 1998;83(8):1593-1601.
- Arguello F, Baggs RB, Duerst RE, Johnstone L, McQueen K, Frantz CN. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer. 1990;65(1):98-106.
- Schiff D. Spinal cord compression. Neurol Clin. 2003;21(1):67-86, viii.
- Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6(1):15-24.
- Helweg-Larsen S, Laursen H. Clinical and autopsy findings in spinal cord compression due to metastatic disease. Eur J Neurol. 1998;5(6):587-592.
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 199218. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: "all I care about is walking and living my life." JAMA. 2008;299(8):937-946.
- Bach F, Larsen BH, Rohde K, et al. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien). 1990;107(1-2):37-43.
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3(1):40-51.
- Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21.
- Metastatic Spinal Cord Compression: Diagnosis and Management of Patients at Risk of or with Metastatic Spinal Cord Compression. Cardiff UK: National Collaborating Centre for Cancer; 2008.
- Shiue K, Sahgal A, Chow E, et al. Management of metastatic spinal cord compression. Expert Rev Anticancer Ther. 2010;10(5):697-708.
- Hammack JE. Spinal cord disease in patients with cancer. Continuum (Minneap Minn). 2012;18(2):312-327.
- Helweg-Larsen S. Clinical outcome in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol Scand. 1996;94(4):269-275.
- Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393.
- McLinton A, Hutchison C. Malignant spinal cord compression: a retrospective audit of clinical practice at a UK regional cancer centre. Br J Cancer. 2006;94(4):486-491.
- Dugas AF, Lucas JM, Edlow JA. Diagnosis of spinal cord compression in nontrauma patients in the emergency department. Acad Emerg Med. 2011;18(7):719-725.
- Ditunno JF, Jr, Young W, Donovan WH, Creasey metastatic spinal CORD compression 18 EMERGENCY MEDICINE I january 2014 www.emed-journal.com G. American Spinal Surgery Association. The international standards booklet for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Paraplegia. 1994;32(2):70-80.
- Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7(3):179-192.
- Portenoy RK, Galer BS, Salamon O, et al. Identification of epidural neoplasm. Radiography and bone scintigraphy in the symptomatic and asymptomatic spine. Cancer. 1989;64(11):2207-2213.
- Husband DJ, Grant KA, Romaniuk CS. MRI in the diagnosis and treatment of suspected malignant spinal cord compression. Br J Radiol. 2001;74(877):15-23.
- Carmody RF, Yang PJ, Seeley GW, Seeger JF, Unger EC, Johnson JE. Spinal cord compression due to metastatic disease: diagnosis with MR imaging versus myelography. Radiology. 1989;173(1):225-229.
- Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13(3):324-328.
- Loblaw DA, Mitera G, Ford M, Laperriere NJ. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys. 2012;84(2):312-317.
- Sorensen S, Helweg-Larsen S, Mouridsen H, Hansen HH. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer. 1994;30A(1):22-27.
- Heimdal K, Hirschberg H, Slettebo H, Watne K, Nome O. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-144.
- Posner JB, Howieson J, Cvitkovic E. "Disappearing" spinal cord compression: oncolytic effect of glucocorticoids (and other chemotherapeutic agents) on epidural metastases. Ann Neurol. 1977;2(5):409-413.
- Kan E, Levi I, Benharroch D. Alterations in the primary diagnosis of lymphomas pretreated with corticosteroid agents. Leuk Lymphoma. 2011;52(3):425-428.
- Borenstein SH, Gerstle T, Malkin D, Thorner P, Filler RM. The effects of prebiopsy cortico-steroid treatment on the diagnosis of mediastinal lymphoma. J Pediatr Surg. 2000;35(6):973-976.
- Akram H, Allibone J. Spinal surgery for palliation in malignant spinal cord compression. Clin Oncol (R Coll Radiol). 2010;22(9):792-800.
- Rades D, Abrahm JL. The role of radiotherapy for metastatic epidural spinal cord compression. Nat Rev Clin Oncol. 2010;7(10):590-598.
- Sundaresan N, Sachdev VP, Holland JF, et al. Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol. 1995;13(9):2330-2335.
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
- Rades D, Dahm-Daphi J, Rudat V, et al. Is shortcourse radiotherapy with high doses per fraction the appropriate regimen for metastatic spinal cord compression in colorectal cancer patients? Strahlenther Onkol. 2006;182(12):708-712.
- van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95(3):222-229.
- Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101-109.
- Rades D, Hueppe M, Schild SE. A score to identify patients with metastatic spinal cord compression who may be candidates for best supportive care. Cancer. 2013;119(4):897-903.
- Guo Y, Palmer JL, Bianty J, Konzen B, Shin K, Bruera E. Advance directives and do-not-resuscitate orders in patients with cancer with metastatic spinal cord compression: advanced care planning implications. J Palliat Med. 2010;13(5):513-517.
Case
A 60-year-old man with stage IV hormoneindependent prostate cancer, with widely metastatic disease to the bone, presents to the ED with increased weakness and new onset of numbness in the lower extremities, which he states began earlier that day. After failing several lines of chemotherapy, he is currently being treated with hormonal therapy alone. Patient first noted weakness in the left lower extremity 5 days before presentation, which progressed to bilateral involvement, making ambulation difficult and requiring the use of a walker. He denies back pain or urinary or fecal incontinence. Regarding pain management, he had been recently treated at one of the pain clinics in the hospital and has continued on opioid medication at another institution. Until the past week, he states he had back pain without neurological deficits.
His vital signs are stable at presentation. Patient is obese but in no acute distress. His cardiopulmonary examination is unremarkable; abdominal examination is benign; and back examination is normal. On neurological examination, iliopsoas flexion is 4/5 bilaterally; the rest of the motor examination is normal, with toes downgoing bilaterally upon plantar stimulation. Diminished sensation to light touch is noted at the T4-T6 sensory level and below; patient also has diminished proprioception in his lower extremities.
Patient had undergone a whole body scan one month prior to presentation, which revealed increased tracer uptake of Technetium-99m in multiple areas in the thoracic and lumbar spine. The radiologist also reported bilateral involvement in the wrists, femurs, tibias, and humeri—all in concordance with multifocal bone disease noted in previous computed tomography scans.
How should you approach this case?
Overview of Metastatic Spinal Cord Compression
Malignant or metastatic spinal cord compression (MSCC) of the thecal sac is an ominous complication of advanced cancer and an oncologic emergency presenting clinically in approximately 3% to 10% of cancer-related deaths.1,2 Cancer patients have a median survival of 3 to 6 months from diagnosis of MSCC.1,3,4 This disease causes significant disability due to paralysis, sensory loss, protracted pain, and sphincter dysfunction.5 If left untreated, MSCC has the potential to cause paraplegia in almost all affected patients; therefore, prompt recognition and treatment are essential to maintain mobility and neurological function. Generally speaking, any cancer patient who presents with new or worsening back pain—even in the absence of neurological deficits—merits evaluation for spinal cord compression.6 Nevertheless, individual risk assessment is warranted.7
Epidemiology
In the United States, more than 20,000 cases of MSCC are reported each year.8 According to postmortem studies, this condition affects 5% to 36% of cancer patients.9,10 In a US nationwide study of 15,367 cases of MSCC,2 the mean age at hospitalization was 62 years, with 37% of cases occurring in women. In approximately 20% of cases, MSCC was the initial presentation of cancer4; this has been reflected in our experience at MD Anderson Cancer Center.
Cancers of the breast, lung, prostate, and multiple myeloma are the most frequent underlying conditions in MSCC.2,8 Its prevalence varies depending on tumor type, occurring in 0.2% of pancreatic cancers; however, MSCC may affect up to 7.9% to 15% of myelomas1,2 and 13% of lymphomas.2 Interestingly, 5.5% of patients with prostate cancer develop MSCC.2 According to a study by Lu et al,11 historical risk factors include known nonvertebral bony metastases and stage IV disease at the time of diagnosis.
The most common location of MSCC is the thoracic spine (69% of cases); 29% of cases occur at the lumbosacral level and 10% at the cervical area.12 Most likely this pattern follows the lymphatic drainage, as metastases from breast and lung cancers tend to be found in the thoracic spine. Pelvic and intra-abdominal malignancies most commonly migrate to the lumbar spine. Multiple spinal epidural metastases were noted in 31% of those who underwent complete imaging of the spine.12
Pathophysiology
Most cases of MSCC are epidural in origin, arising from the vertebral column in 85% of patients.8 Epidural spread is caused mainly by hematogenous mechanism through the Batson venous plexus,13 debilitating the bone and eventually causing vertebral collapse with compression of the spinal canal. Epidural spread is less likely caused by direct tumor extension (ie, erosion through the bone) or by direct deposition of tumor cells into the epidural space.14 Ultimate neuronal injury is thought to involve vasogenic edema,15 leading to ischemia13 through venous infarction, but there has been debate regarding this last phenomenon.16 In cases of paralysis, demyelination is striking.16
Clinical Presentation
Even though cancer accounts for less than 1% of episodes of low back pain, it is the most common systemic disease affecting the spine.17 An important clinical inquiry is to determine whether back pain in an established cancer patient can be ruled out without extensive imaging. Unfortunately, clinical examination alone cannot exclude MSCC. Because of the high specificity (0.98), any cancer patient with new back pain should be considered to have metastasis until proven otherwise.17
Symptoms in MSCC at presentation can be motor, sensory, and/or autonomic. Back pain varies depending on the site of metastasis, which can be referred, local, radicular, or a combination of all three.18 The primary complaint is pain in 83% to 96% of cases,19,20 though this is a nonspecific sign.
Previous studies have shown 40% to 64% of patients were not ambulatory at the time of diagnosis.19,25 Recent case series, however, report an increased number of ambulatory patients—possibly due to increased clinician awareness.26 In other cases, only 9% of patients were able to walk independently without aid.27 Loss of sensation, dense paraplegia, and incontinence are late findings and likely signal some degree of permanent disability.19
Misdiagnosis is a common issue in the ED setting. In an interesting retrospective study of 63 patients with spinal cord compression28 (not necessarily malignant), 18 (29%) were misdiagnosed.28 Consequently, there was a significant delay in diagnosis despite obvious neurological deficits at presentation.
Evaluation and Imaging
A detailed physical examination is essential to diagnosing MSCC. A thorough neurological examination, including sensation, strength, and reflexes should be carefully documented. If spinal instability is suspected, range-of-motion testing is contraindicated. The modified Frankel classification,29 adapted from the traumatic spine cord injury work by Frankel, et al,30 may be used to assess the degree of disability (Table).
Lu et al11 noted hyperreflexia and upward going Babinski reflex as common findings. Moreover, risk factors of decreased rectal sphincter tone and bladder were determinant for poor outcomes.
MRI studies should include the entire spine—not just the perceived area of interest— as up to 38% of patients have multiple-site metastases12 (Figure 1). Sensory deficits and mechanical pain may be present two to four vertebral levels away from the actual lesion.11 If MRI suggests cord compression, severity can be graded using the MSCC scale34 (Figure 2). Several scoring systems have been developed to aid in decision making concerning surgical treatment.
Management and Outcomes
The goal of therapy is symptom control and preservation of function. This requires a multidisciplinary approach and may involve radiation therapy and surgery, as well as medical efforts. Upon diagnosis and initiation of therapy, serial neurological evaluation should be undertaken. Neurovital signs should be scheduled to coincide with other nursing efforts to ease the burden of care and minimize patient discomfort.
The mainstay of medical therapy is treatment with corticosteroids.35 Initial trials have demonstrated that corticosteroids improve functional status in MSCC, but controversy exists regarding the effective dose. In a randomized, controlled trial by Sorensen et al,36 which sought to evaluate functional outcomes of highdose corticosteroids as an adjunct to radiotherapy, 57 patients received either high-dose dexamethasone or no corticosteroid therapy. Fifty-nine percent of patients in the dexamethasone group were ambulatory 6 months after treatment compared to 39% in the group who did not receive steroids.36
A patient without a biopsy-confirmed cancer diagnosis in need of corticosteroid treatment presents a dilemma. Plasmacytomas, thymomas, lymphomas, multiple myeloma, germ-cell tumors are very sensitive to corticosteroid therapy in patients with MSCC.38 However, corticosteroids given before tissue samples are obtained may hinder proper diagnosis and complicate future management.39,40 In the absence of neurological deficit, corticosteroids may be withheld and emergent consultation with neurosurgery and oncology should be obtained. If there is any question regarding the nature of the lesion, tissue diagnosis must be obtained without delay.
Strict bed rest (including logroll and bedpan use) should be instituted if there is suspicion of spinal cord instability. Patients with suspected involvement of the cervical spine should have a Philadelphia collar placed until spinal stability has been confirmed. In the United Kingdom, the National Institutes for Health Care Excellence guidelines recommend all patients with suspected cord compression be nursed in a flat position.22 Other institutions, however, do not believe that strict bed rest is necessary, as it is presumed that MSCC is inherently different from that caused by trauma. Authors supporting this position contend that the increased incidence of deep vein thrombosis, infection (particularly from the urinary tract), and decubitus ulcers outweighs the benefit of bed rest. Patient preference should be taken into consideration as those with good functional status may be quite resistant to bed rest. In cases where cord compression is strongly suspected, these patients should be educated on proper bed rest. The greatest predictors of outcome are ambulatory and functional status at the time of diagnosis (generally based on an Eastern Cooperative Oncology Group scale). Patients with a good functional status, limited disease, and a life expectancy of greater than 3 to 6 months may benefit from surgery.41 However, emergent surgical evaluation is required in patients not responding to radiotherapy or who received received only limited doses of radiotherapy, as well as those with spinal instability, direct cord compression due to a bony fragment, impending sphincter dysfunction, unknown primary tumor, or no paraplegia for >48 hours.15
Unfortunately, surgery is only indicated in 10% to 15% of MSCC cases.42 In the past two decades, significant improvements regarding new aggressive surgical techniques have been made, and include circumferential decompression of the spine and staged or single stage anterior posterior surgery with stabilization. 43 Additionally, the combination of surgery with radiotherapy has improved outcomes.44
Most patients benefit from short-course radiotherapy45 even when given palliatively. 46 Longer courses of radiotherapy are highly recommended for patients with a more favorable prognosis.47 Up to 10% of patients diagnosed with spinal cord compression will require treatment for disease recurrence.42 There is a limited role for chemotherapy, and in seminomas and lymphomas, results can be quite dramatic.38
Prevention
Lu et al11 found that only 54% of patients were aware that back pain should be reported to their physician. Delays in diagnosis and treatment are common and well described in the literature.21 Patients should be instructed to call their physician within 24 hours from the development of any new or worsening back pain, and should be advised to seek immediate care if they develop any neurological symptoms. To facilitate appropriate and prompt management of MSCC, hospitals should develop diagnostic algorithms to minimize delays in referral to a comprehensive center for further treatment.
Case Conclusion
Based on this patient’s symptoms and status at presentation, the emergency team determined he was at high risk for MSCC. An initial dosage of 10 mg dexamethasone was administered intravenously (IV), followed by 4 mg IV every 6 hours prior to imaging. An MRI without contrast of the cervical, thoracic, and lumbar spine showed cord compression with mild cord edema at T4 level, along with diffused osseous metastasis.
Upon diagnosis, patient was referred to radiation oncology for radiotherapy of the T2-T6 vertebral bodies. Three days after initiation of radiation therapy, his neurological function deteriorated with paraplegia and incontinence, and he was emergently evaluated for neurosurgery. Although T4 laminectomy and decompression of the spinal cord were performed without complication, patient did not recover neurological function. His hospital course was complicated by Ogilvie syndrome and episodes of delirium, and he was discharged to a rehabilitation facility 23 days after admission; paraplegia and urinary and bowel incontinence remained unchanged.
Case
A 60-year-old man with stage IV hormoneindependent prostate cancer, with widely metastatic disease to the bone, presents to the ED with increased weakness and new onset of numbness in the lower extremities, which he states began earlier that day. After failing several lines of chemotherapy, he is currently being treated with hormonal therapy alone. Patient first noted weakness in the left lower extremity 5 days before presentation, which progressed to bilateral involvement, making ambulation difficult and requiring the use of a walker. He denies back pain or urinary or fecal incontinence. Regarding pain management, he had been recently treated at one of the pain clinics in the hospital and has continued on opioid medication at another institution. Until the past week, he states he had back pain without neurological deficits.
His vital signs are stable at presentation. Patient is obese but in no acute distress. His cardiopulmonary examination is unremarkable; abdominal examination is benign; and back examination is normal. On neurological examination, iliopsoas flexion is 4/5 bilaterally; the rest of the motor examination is normal, with toes downgoing bilaterally upon plantar stimulation. Diminished sensation to light touch is noted at the T4-T6 sensory level and below; patient also has diminished proprioception in his lower extremities.
Patient had undergone a whole body scan one month prior to presentation, which revealed increased tracer uptake of Technetium-99m in multiple areas in the thoracic and lumbar spine. The radiologist also reported bilateral involvement in the wrists, femurs, tibias, and humeri—all in concordance with multifocal bone disease noted in previous computed tomography scans.
How should you approach this case?
Overview of Metastatic Spinal Cord Compression
Malignant or metastatic spinal cord compression (MSCC) of the thecal sac is an ominous complication of advanced cancer and an oncologic emergency presenting clinically in approximately 3% to 10% of cancer-related deaths.1,2 Cancer patients have a median survival of 3 to 6 months from diagnosis of MSCC.1,3,4 This disease causes significant disability due to paralysis, sensory loss, protracted pain, and sphincter dysfunction.5 If left untreated, MSCC has the potential to cause paraplegia in almost all affected patients; therefore, prompt recognition and treatment are essential to maintain mobility and neurological function. Generally speaking, any cancer patient who presents with new or worsening back pain—even in the absence of neurological deficits—merits evaluation for spinal cord compression.6 Nevertheless, individual risk assessment is warranted.7
Epidemiology
In the United States, more than 20,000 cases of MSCC are reported each year.8 According to postmortem studies, this condition affects 5% to 36% of cancer patients.9,10 In a US nationwide study of 15,367 cases of MSCC,2 the mean age at hospitalization was 62 years, with 37% of cases occurring in women. In approximately 20% of cases, MSCC was the initial presentation of cancer4; this has been reflected in our experience at MD Anderson Cancer Center.
Cancers of the breast, lung, prostate, and multiple myeloma are the most frequent underlying conditions in MSCC.2,8 Its prevalence varies depending on tumor type, occurring in 0.2% of pancreatic cancers; however, MSCC may affect up to 7.9% to 15% of myelomas1,2 and 13% of lymphomas.2 Interestingly, 5.5% of patients with prostate cancer develop MSCC.2 According to a study by Lu et al,11 historical risk factors include known nonvertebral bony metastases and stage IV disease at the time of diagnosis.
The most common location of MSCC is the thoracic spine (69% of cases); 29% of cases occur at the lumbosacral level and 10% at the cervical area.12 Most likely this pattern follows the lymphatic drainage, as metastases from breast and lung cancers tend to be found in the thoracic spine. Pelvic and intra-abdominal malignancies most commonly migrate to the lumbar spine. Multiple spinal epidural metastases were noted in 31% of those who underwent complete imaging of the spine.12
Pathophysiology
Most cases of MSCC are epidural in origin, arising from the vertebral column in 85% of patients.8 Epidural spread is caused mainly by hematogenous mechanism through the Batson venous plexus,13 debilitating the bone and eventually causing vertebral collapse with compression of the spinal canal. Epidural spread is less likely caused by direct tumor extension (ie, erosion through the bone) or by direct deposition of tumor cells into the epidural space.14 Ultimate neuronal injury is thought to involve vasogenic edema,15 leading to ischemia13 through venous infarction, but there has been debate regarding this last phenomenon.16 In cases of paralysis, demyelination is striking.16
Clinical Presentation
Even though cancer accounts for less than 1% of episodes of low back pain, it is the most common systemic disease affecting the spine.17 An important clinical inquiry is to determine whether back pain in an established cancer patient can be ruled out without extensive imaging. Unfortunately, clinical examination alone cannot exclude MSCC. Because of the high specificity (0.98), any cancer patient with new back pain should be considered to have metastasis until proven otherwise.17
Symptoms in MSCC at presentation can be motor, sensory, and/or autonomic. Back pain varies depending on the site of metastasis, which can be referred, local, radicular, or a combination of all three.18 The primary complaint is pain in 83% to 96% of cases,19,20 though this is a nonspecific sign.
Previous studies have shown 40% to 64% of patients were not ambulatory at the time of diagnosis.19,25 Recent case series, however, report an increased number of ambulatory patients—possibly due to increased clinician awareness.26 In other cases, only 9% of patients were able to walk independently without aid.27 Loss of sensation, dense paraplegia, and incontinence are late findings and likely signal some degree of permanent disability.19
Misdiagnosis is a common issue in the ED setting. In an interesting retrospective study of 63 patients with spinal cord compression28 (not necessarily malignant), 18 (29%) were misdiagnosed.28 Consequently, there was a significant delay in diagnosis despite obvious neurological deficits at presentation.
Evaluation and Imaging
A detailed physical examination is essential to diagnosing MSCC. A thorough neurological examination, including sensation, strength, and reflexes should be carefully documented. If spinal instability is suspected, range-of-motion testing is contraindicated. The modified Frankel classification,29 adapted from the traumatic spine cord injury work by Frankel, et al,30 may be used to assess the degree of disability (Table).
Lu et al11 noted hyperreflexia and upward going Babinski reflex as common findings. Moreover, risk factors of decreased rectal sphincter tone and bladder were determinant for poor outcomes.
MRI studies should include the entire spine—not just the perceived area of interest— as up to 38% of patients have multiple-site metastases12 (Figure 1). Sensory deficits and mechanical pain may be present two to four vertebral levels away from the actual lesion.11 If MRI suggests cord compression, severity can be graded using the MSCC scale34 (Figure 2). Several scoring systems have been developed to aid in decision making concerning surgical treatment.
Management and Outcomes
The goal of therapy is symptom control and preservation of function. This requires a multidisciplinary approach and may involve radiation therapy and surgery, as well as medical efforts. Upon diagnosis and initiation of therapy, serial neurological evaluation should be undertaken. Neurovital signs should be scheduled to coincide with other nursing efforts to ease the burden of care and minimize patient discomfort.
The mainstay of medical therapy is treatment with corticosteroids.35 Initial trials have demonstrated that corticosteroids improve functional status in MSCC, but controversy exists regarding the effective dose. In a randomized, controlled trial by Sorensen et al,36 which sought to evaluate functional outcomes of highdose corticosteroids as an adjunct to radiotherapy, 57 patients received either high-dose dexamethasone or no corticosteroid therapy. Fifty-nine percent of patients in the dexamethasone group were ambulatory 6 months after treatment compared to 39% in the group who did not receive steroids.36
A patient without a biopsy-confirmed cancer diagnosis in need of corticosteroid treatment presents a dilemma. Plasmacytomas, thymomas, lymphomas, multiple myeloma, germ-cell tumors are very sensitive to corticosteroid therapy in patients with MSCC.38 However, corticosteroids given before tissue samples are obtained may hinder proper diagnosis and complicate future management.39,40 In the absence of neurological deficit, corticosteroids may be withheld and emergent consultation with neurosurgery and oncology should be obtained. If there is any question regarding the nature of the lesion, tissue diagnosis must be obtained without delay.
Strict bed rest (including logroll and bedpan use) should be instituted if there is suspicion of spinal cord instability. Patients with suspected involvement of the cervical spine should have a Philadelphia collar placed until spinal stability has been confirmed. In the United Kingdom, the National Institutes for Health Care Excellence guidelines recommend all patients with suspected cord compression be nursed in a flat position.22 Other institutions, however, do not believe that strict bed rest is necessary, as it is presumed that MSCC is inherently different from that caused by trauma. Authors supporting this position contend that the increased incidence of deep vein thrombosis, infection (particularly from the urinary tract), and decubitus ulcers outweighs the benefit of bed rest. Patient preference should be taken into consideration as those with good functional status may be quite resistant to bed rest. In cases where cord compression is strongly suspected, these patients should be educated on proper bed rest. The greatest predictors of outcome are ambulatory and functional status at the time of diagnosis (generally based on an Eastern Cooperative Oncology Group scale). Patients with a good functional status, limited disease, and a life expectancy of greater than 3 to 6 months may benefit from surgery.41 However, emergent surgical evaluation is required in patients not responding to radiotherapy or who received received only limited doses of radiotherapy, as well as those with spinal instability, direct cord compression due to a bony fragment, impending sphincter dysfunction, unknown primary tumor, or no paraplegia for >48 hours.15
Unfortunately, surgery is only indicated in 10% to 15% of MSCC cases.42 In the past two decades, significant improvements regarding new aggressive surgical techniques have been made, and include circumferential decompression of the spine and staged or single stage anterior posterior surgery with stabilization. 43 Additionally, the combination of surgery with radiotherapy has improved outcomes.44
Most patients benefit from short-course radiotherapy45 even when given palliatively. 46 Longer courses of radiotherapy are highly recommended for patients with a more favorable prognosis.47 Up to 10% of patients diagnosed with spinal cord compression will require treatment for disease recurrence.42 There is a limited role for chemotherapy, and in seminomas and lymphomas, results can be quite dramatic.38
Prevention
Lu et al11 found that only 54% of patients were aware that back pain should be reported to their physician. Delays in diagnosis and treatment are common and well described in the literature.21 Patients should be instructed to call their physician within 24 hours from the development of any new or worsening back pain, and should be advised to seek immediate care if they develop any neurological symptoms. To facilitate appropriate and prompt management of MSCC, hospitals should develop diagnostic algorithms to minimize delays in referral to a comprehensive center for further treatment.
Case Conclusion
Based on this patient’s symptoms and status at presentation, the emergency team determined he was at high risk for MSCC. An initial dosage of 10 mg dexamethasone was administered intravenously (IV), followed by 4 mg IV every 6 hours prior to imaging. An MRI without contrast of the cervical, thoracic, and lumbar spine showed cord compression with mild cord edema at T4 level, along with diffused osseous metastasis.
Upon diagnosis, patient was referred to radiation oncology for radiotherapy of the T2-T6 vertebral bodies. Three days after initiation of radiation therapy, his neurological function deteriorated with paraplegia and incontinence, and he was emergently evaluated for neurosurgery. Although T4 laminectomy and decompression of the spinal cord were performed without complication, patient did not recover neurological function. His hospital course was complicated by Ogilvie syndrome and episodes of delirium, and he was discharged to a rehabilitation facility 23 days after admission; paraplegia and urinary and bowel incontinence remained unchanged.
- Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol). 2003;15(4):211-217.
- Mak KS, Lee LK, Mak RH, et al. Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998-2006. Int J Radiat Oncol Biol Phys. 2011;80(3):824-831.
- Constans JP, de Divitiis E, Donzelli R, Spaziante R, Meder JF, Haye C. Spinal metastases with
- neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59(1):111-118.
- Schiff D, O’Neill BP, Suman VJ. Spinal epidural metastasis as the initial manifestation of malignancy: clinical features and diagnostic approach. Neurology. 1997;49(2):452-456.
- Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative’s Neuro-Oncology Disease Site Group. J Clin Oncol. 2005;23(9):2028-2037.
- Levack P, Graham J, Collie D, et al. Don’t wait for a sensory level—listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol). 2002;14(6):472-480.
- Talcott JA, Stomper PC, Drislane FW, et al. Assessing suspected spinal cord compression: a multidisciplinary outcomes analysis of 342 episodes. Support Care Cancer. 1999;7(1):31-38.
- Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327(9):614-619.
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3(1):74-85.
- Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine (Phila Pa 1976). 1990;15(1):1-4.
- Lu C, Gonzalez RG, Jolesz FA, Wen PY, Talcott JA. Suspected spinal cord compression in cancer patients: a multidisciplinary risk assessment. J Support Oncol. 2005;3(4):305-312.
- Schiff D, O’Neill BP, Wang CH, O’Fallon JR. Neuroimaging and treatment implications of patients with multiple epidural spinal metastases. Cancer. 1998;83(8):1593-1601.
- Arguello F, Baggs RB, Duerst RE, Johnstone L, McQueen K, Frantz CN. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer. 1990;65(1):98-106.
- Schiff D. Spinal cord compression. Neurol Clin. 2003;21(1):67-86, viii.
- Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6(1):15-24.
- Helweg-Larsen S, Laursen H. Clinical and autopsy findings in spinal cord compression due to metastatic disease. Eur J Neurol. 1998;5(6):587-592.
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 199218. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: "all I care about is walking and living my life." JAMA. 2008;299(8):937-946.
- Bach F, Larsen BH, Rohde K, et al. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien). 1990;107(1-2):37-43.
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3(1):40-51.
- Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21.
- Metastatic Spinal Cord Compression: Diagnosis and Management of Patients at Risk of or with Metastatic Spinal Cord Compression. Cardiff UK: National Collaborating Centre for Cancer; 2008.
- Shiue K, Sahgal A, Chow E, et al. Management of metastatic spinal cord compression. Expert Rev Anticancer Ther. 2010;10(5):697-708.
- Hammack JE. Spinal cord disease in patients with cancer. Continuum (Minneap Minn). 2012;18(2):312-327.
- Helweg-Larsen S. Clinical outcome in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol Scand. 1996;94(4):269-275.
- Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393.
- McLinton A, Hutchison C. Malignant spinal cord compression: a retrospective audit of clinical practice at a UK regional cancer centre. Br J Cancer. 2006;94(4):486-491.
- Dugas AF, Lucas JM, Edlow JA. Diagnosis of spinal cord compression in nontrauma patients in the emergency department. Acad Emerg Med. 2011;18(7):719-725.
- Ditunno JF, Jr, Young W, Donovan WH, Creasey metastatic spinal CORD compression 18 EMERGENCY MEDICINE I january 2014 www.emed-journal.com G. American Spinal Surgery Association. The international standards booklet for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Paraplegia. 1994;32(2):70-80.
- Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7(3):179-192.
- Portenoy RK, Galer BS, Salamon O, et al. Identification of epidural neoplasm. Radiography and bone scintigraphy in the symptomatic and asymptomatic spine. Cancer. 1989;64(11):2207-2213.
- Husband DJ, Grant KA, Romaniuk CS. MRI in the diagnosis and treatment of suspected malignant spinal cord compression. Br J Radiol. 2001;74(877):15-23.
- Carmody RF, Yang PJ, Seeley GW, Seeger JF, Unger EC, Johnson JE. Spinal cord compression due to metastatic disease: diagnosis with MR imaging versus myelography. Radiology. 1989;173(1):225-229.
- Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13(3):324-328.
- Loblaw DA, Mitera G, Ford M, Laperriere NJ. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys. 2012;84(2):312-317.
- Sorensen S, Helweg-Larsen S, Mouridsen H, Hansen HH. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer. 1994;30A(1):22-27.
- Heimdal K, Hirschberg H, Slettebo H, Watne K, Nome O. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-144.
- Posner JB, Howieson J, Cvitkovic E. "Disappearing" spinal cord compression: oncolytic effect of glucocorticoids (and other chemotherapeutic agents) on epidural metastases. Ann Neurol. 1977;2(5):409-413.
- Kan E, Levi I, Benharroch D. Alterations in the primary diagnosis of lymphomas pretreated with corticosteroid agents. Leuk Lymphoma. 2011;52(3):425-428.
- Borenstein SH, Gerstle T, Malkin D, Thorner P, Filler RM. The effects of prebiopsy cortico-steroid treatment on the diagnosis of mediastinal lymphoma. J Pediatr Surg. 2000;35(6):973-976.
- Akram H, Allibone J. Spinal surgery for palliation in malignant spinal cord compression. Clin Oncol (R Coll Radiol). 2010;22(9):792-800.
- Rades D, Abrahm JL. The role of radiotherapy for metastatic epidural spinal cord compression. Nat Rev Clin Oncol. 2010;7(10):590-598.
- Sundaresan N, Sachdev VP, Holland JF, et al. Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol. 1995;13(9):2330-2335.
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
- Rades D, Dahm-Daphi J, Rudat V, et al. Is shortcourse radiotherapy with high doses per fraction the appropriate regimen for metastatic spinal cord compression in colorectal cancer patients? Strahlenther Onkol. 2006;182(12):708-712.
- van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95(3):222-229.
- Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101-109.
- Rades D, Hueppe M, Schild SE. A score to identify patients with metastatic spinal cord compression who may be candidates for best supportive care. Cancer. 2013;119(4):897-903.
- Guo Y, Palmer JL, Bianty J, Konzen B, Shin K, Bruera E. Advance directives and do-not-resuscitate orders in patients with cancer with metastatic spinal cord compression: advanced care planning implications. J Palliat Med. 2010;13(5):513-517.
- Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol). 2003;15(4):211-217.
- Mak KS, Lee LK, Mak RH, et al. Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998-2006. Int J Radiat Oncol Biol Phys. 2011;80(3):824-831.
- Constans JP, de Divitiis E, Donzelli R, Spaziante R, Meder JF, Haye C. Spinal metastases with
- neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59(1):111-118.
- Schiff D, O’Neill BP, Suman VJ. Spinal epidural metastasis as the initial manifestation of malignancy: clinical features and diagnostic approach. Neurology. 1997;49(2):452-456.
- Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative’s Neuro-Oncology Disease Site Group. J Clin Oncol. 2005;23(9):2028-2037.
- Levack P, Graham J, Collie D, et al. Don’t wait for a sensory level—listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol). 2002;14(6):472-480.
- Talcott JA, Stomper PC, Drislane FW, et al. Assessing suspected spinal cord compression: a multidisciplinary outcomes analysis of 342 episodes. Support Care Cancer. 1999;7(1):31-38.
- Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327(9):614-619.
- Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3(1):74-85.
- Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine (Phila Pa 1976). 1990;15(1):1-4.
- Lu C, Gonzalez RG, Jolesz FA, Wen PY, Talcott JA. Suspected spinal cord compression in cancer patients: a multidisciplinary risk assessment. J Support Oncol. 2005;3(4):305-312.
- Schiff D, O’Neill BP, Wang CH, O’Fallon JR. Neuroimaging and treatment implications of patients with multiple epidural spinal metastases. Cancer. 1998;83(8):1593-1601.
- Arguello F, Baggs RB, Duerst RE, Johnstone L, McQueen K, Frantz CN. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer. 1990;65(1):98-106.
- Schiff D. Spinal cord compression. Neurol Clin. 2003;21(1):67-86, viii.
- Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6(1):15-24.
- Helweg-Larsen S, Laursen H. Clinical and autopsy findings in spinal cord compression due to metastatic disease. Eur J Neurol. 1998;5(6):587-592.
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 199218. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: "all I care about is walking and living my life." JAMA. 2008;299(8):937-946.
- Bach F, Larsen BH, Rohde K, et al. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien). 1990;107(1-2):37-43.
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3(1):40-51.
- Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21.
- Metastatic Spinal Cord Compression: Diagnosis and Management of Patients at Risk of or with Metastatic Spinal Cord Compression. Cardiff UK: National Collaborating Centre for Cancer; 2008.
- Shiue K, Sahgal A, Chow E, et al. Management of metastatic spinal cord compression. Expert Rev Anticancer Ther. 2010;10(5):697-708.
- Hammack JE. Spinal cord disease in patients with cancer. Continuum (Minneap Minn). 2012;18(2):312-327.
- Helweg-Larsen S. Clinical outcome in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol Scand. 1996;94(4):269-275.
- Rades D, Fehlauer F, Schulte R, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24(21):3388-3393.
- McLinton A, Hutchison C. Malignant spinal cord compression: a retrospective audit of clinical practice at a UK regional cancer centre. Br J Cancer. 2006;94(4):486-491.
- Dugas AF, Lucas JM, Edlow JA. Diagnosis of spinal cord compression in nontrauma patients in the emergency department. Acad Emerg Med. 2011;18(7):719-725.
- Ditunno JF, Jr, Young W, Donovan WH, Creasey metastatic spinal CORD compression 18 EMERGENCY MEDICINE I january 2014 www.emed-journal.com G. American Spinal Surgery Association. The international standards booklet for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Paraplegia. 1994;32(2):70-80.
- Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7(3):179-192.
- Portenoy RK, Galer BS, Salamon O, et al. Identification of epidural neoplasm. Radiography and bone scintigraphy in the symptomatic and asymptomatic spine. Cancer. 1989;64(11):2207-2213.
- Husband DJ, Grant KA, Romaniuk CS. MRI in the diagnosis and treatment of suspected malignant spinal cord compression. Br J Radiol. 2001;74(877):15-23.
- Carmody RF, Yang PJ, Seeley GW, Seeger JF, Unger EC, Johnson JE. Spinal cord compression due to metastatic disease: diagnosis with MR imaging versus myelography. Radiology. 1989;173(1):225-229.
- Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13(3):324-328.
- Loblaw DA, Mitera G, Ford M, Laperriere NJ. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys. 2012;84(2):312-317.
- Sorensen S, Helweg-Larsen S, Mouridsen H, Hansen HH. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer. 1994;30A(1):22-27.
- Heimdal K, Hirschberg H, Slettebo H, Watne K, Nome O. High incidence of serious side effects of high-dose dexamethasone treatment in patients with epidural spinal cord compression. J Neurooncol. 1992;12(2):141-144.
- Posner JB, Howieson J, Cvitkovic E. "Disappearing" spinal cord compression: oncolytic effect of glucocorticoids (and other chemotherapeutic agents) on epidural metastases. Ann Neurol. 1977;2(5):409-413.
- Kan E, Levi I, Benharroch D. Alterations in the primary diagnosis of lymphomas pretreated with corticosteroid agents. Leuk Lymphoma. 2011;52(3):425-428.
- Borenstein SH, Gerstle T, Malkin D, Thorner P, Filler RM. The effects of prebiopsy cortico-steroid treatment on the diagnosis of mediastinal lymphoma. J Pediatr Surg. 2000;35(6):973-976.
- Akram H, Allibone J. Spinal surgery for palliation in malignant spinal cord compression. Clin Oncol (R Coll Radiol). 2010;22(9):792-800.
- Rades D, Abrahm JL. The role of radiotherapy for metastatic epidural spinal cord compression. Nat Rev Clin Oncol. 2010;7(10):590-598.
- Sundaresan N, Sachdev VP, Holland JF, et al. Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol. 1995;13(9):2330-2335.
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
- Rades D, Dahm-Daphi J, Rudat V, et al. Is shortcourse radiotherapy with high doses per fraction the appropriate regimen for metastatic spinal cord compression in colorectal cancer patients? Strahlenther Onkol. 2006;182(12):708-712.
- van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95(3):222-229.
- Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52(2):101-109.
- Rades D, Hueppe M, Schild SE. A score to identify patients with metastatic spinal cord compression who may be candidates for best supportive care. Cancer. 2013;119(4):897-903.
- Guo Y, Palmer JL, Bianty J, Konzen B, Shin K, Bruera E. Advance directives and do-not-resuscitate orders in patients with cancer with metastatic spinal cord compression: advanced care planning implications. J Palliat Med. 2010;13(5):513-517.