User login
Dyspnea, pancytopenia, and splenomegaly
• Obtain a thorough history to aid in narrowing the list of potential causes in pancytopenia’s broad differential.
• Arrange for a peripheral smear and bone marrow biopsy to confirm the cause of pancytopenia.
• Screen any patient with hairy cell leukemia for a second primary malignancy.
• Base a treatment decision on the presence of symptoms and the severity of the pancytopenia.
CASE A 47-year-old man with a history of alcoholism came to our emergency department (ED) with a 3-week history of sore throat and dry cough. He said that for the past 2 months he had experienced worsening shortness of breath, increasing weakness, and episodes of light-headedness. He also said that his gums occasionally bled when he brushed his teeth.
Our patient owned a farm where he was exposed to pesticides and fertilizers, but he reported no contact with sick individuals, new medications, or recent travel. The patient had a 40 pack-year smoking history, but he had quit within the past year. His family history was negative for malignancies or rheumatologic diseases.
On physical exam, we noted splenomegaly (spleen was approximately 3 cm below costal margin); all other exam findings were within normal limits.
Lab results revealed pancytopenia: the patient’s serum white blood cell (WBC) was 900/mcL, the absolute neutrophil count (ANC) was 447/mcL, and the absolute lymphocyte count was 346/mcL. Hemoglobin was 5.4 g/dL and platelet count was 47,000/mcL. (Pancytopenia is defined as hemoglobin <13.5 g/dL [males] or 11.6 g/dL [females], platelet count <150,000/mcL, and WBC count <4000/mcL. Criteria for severe pancytopenia include an ANC <500/mcL, platelet count <20,000 mcL, and corrected reticulocyte count <1%.1,2)
A repeat complete blood count (CBC) showed similar results. Basic metabolic panel, chest x-ray film, and electrocardiogram results were all normal.
Based on the initial lab work, we ordered further testing for human immunodeficiency virus (HIV), Epstein-Barr virus (EBV), cytomegalovirus, hepatitis, parvovirus B19, and antinuclear antibodies. All results were negative. B12 and folate levels were low normal, and the reticulocyte count was 2.31%. We admitted the patient for evaluation of his pancytopenia.
WHAT ADDITIONAL TESTING WOULD YOU PURSUE AT THIS POINT?
We ordered a bone marrow biopsy and peripheral smear, which is protocol in a case such as this. Results showed leukemic and “hairy” cells.
Hairy cell leukemia
Hairy cell leukemia is an uncommon chronic B-cell lymphoproliferative disorder.3 It represents 2% of all adult leukemias; the median age of onset is 52 years, with a male predominance of 4:1. It is 3 times more common in Caucasians than in African Americans. There is still some controversy regarding its cell line, and its pathogenesis is still unknown. Exposures to ionizing radiation, EBV, organic chemicals, woodworking, and farming have been cited as possible causes.4
Symptoms of hairy cell leukemia include fatigue, weakness, and weight loss. Abdominal fullness or discomfort due to splenomegaly is seen in 25% of cases.5,6 Easy bruising and bleeding secondary to severe pancytopenia are also seen in about 25% of cases.5,6 However, 25% of patients are initially asymptomatic and are found to have abnormal lab values during a routine well visit.6
Narrowing in on a diagnosis
The etiology of pancytopenia is broad, but the diagnostic possibilities (TABLE) narrow depending on the pathogenesis of a patient’s condition: decreased bone marrow production, increased destruction or sequestration of cells, inherited/congenital, or idiopathic.
Testing. After an initial CBC, a second CBC should be obtained to confirm the pancytopenia before ordering further tests.1,7 Such testing includes a peripheral blood smear, a reticulocyte count, serum iron studies, and viral studies such as HIV, EBV, or parvovirus, along with an autoimmune profile and liver function tests.8 A hematology consult with probable bone marrow aspirate is also indicated.8
Diagnosis is made based on clinical findings and lab work revealing pancytopenia. A peripheral smear exhibiting leukemic cells and “hairy” cells suffices to make the diagnosis, although bone marrow biopsy—which reveals tartrate-resistant acid phosphatase– positive cells on cytochemical staining in 95% of cases3—is often use to corroborate the diagnosis. More recently, immunotyping using flow cytometry has become the standard for confirming the diagnosis of hairy cell leukemia.3
TABLE
Is it pancytopenia? The differential3
| Decreased bone marrow production | Increased destruction/sequestration of cells | Inherited/congenital |
|---|---|---|
Chemotherapy, radiotherapy Megaloblastic anemia Myelodysplastic syndromes Myelofibrosis Aplastic anemia Paroxysmal nocturnal hemoglobinuria Lymphoma with bone marrow infiltration Leukemia Plasma cell myeloma Infection (eg, parvovirus B19, CMV) Anorexia nervosa | Liver disease Portal hypertension Hypersplenism due to myelo- or lymphoproliferative disorders Evan’s syndrome Infection (eg, brucellosis, leishmaniasis) Heavy metal poisoning | Gaucher disease Fanconi’s anemia |
| Overlap | ||
| Connective tissue disorders (eg, SLE, RA) | ||
| AIDS | ||
| Mycobacterial infection; sepsis; acute viral infection (eg, CMV, HIV, EBV) | ||
| AIDS, acquired immune deficiency syndrome; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus. | ||
Adjust treatment to symptoms and severity of pancytopenia
Indications for treatment include the development of one or more the following: ANC <1000/mcL with repeat infections; symptomatic anemia with hemoglobin <11 g/dL; platelet count <100,000/mcL associated with bleeding, symptomatic splenomegaly, or adenopathy; or constitutional symptoms.9
First-line therapy is with one of the cytotoxic agents, either cladribine or pentostatin. Splenectomy is another effective option, with results that last up to 10 years in 50% of cases.9 Another option is interferon-alpha.10
Before the advent of cytotoxic agents, the 4-year survival rate for hairy cell leukemia was reported as 68%. Today, durable remission is attained for many patients. Even after relapse, retreatment yields good responses. The 5-year survival rate now is higher than 85%.11
Close follow-up of these patients is key
Potential complications of hairy cell leukemia include infections, bleeding, anemia, splenic rupture, and a second primary malignancy. There is a 2- to 3-fold increased risk of developing a second malignancy at a median interval of 40 months after initial diagnosis.10
This increased risk may be related to immunosuppression due to hairy cell leukemia or its treatment. The incidence of a second malignancy occurring before the diagnosis of hairy cell leukemia is 10.2%, and concurrently with the diagnosis is 2.6%.10 This finding suggests some pretreatment predisposition to cancer; further studies are being carried out to evaluate this matter. The most common solid tumors include prostate cancer, skin cancer, lung cancer, and gastrointestinal adenocarcinomas.10
Our patient’s outcome
During our patient’s hospital admission, he received 6 units of packed red blood cells, 3 daily injections of 480 mcg subcutaneous filgrastim for 5 days, and ongoing vitamin B12 supplementation to assist myeloid recovery. Upon discharge, his ANC level had increased to 1634/mcL. He began outpatient treatment with cladribine under the care of a hematologist. After almost a year of treatment, our patient is in complete remission.
CORRESPONDENCE
Sheena Boury, MD, Christ Hospital Family Medicine, 830 Thomas More Parkway, Edgewood, KY 41017; [email protected]
1. Ishtiaq O, Baqai HZ, Anwer F, et al. Patterns of pancytopenia patients in a general medical ward and a proposed diagnostic approach. J Ayub Med Coll Abbottabad. 2004;16:8-13.
2. Wang ES, Berliner N. Hematopoiesis and hematopoietic failure. In: Andreoli TE, Carpenter CCJ, Griggs RC, eds. Cecil Essentials of Medicine. 6th ed. Philadelphia, Pa: WB Saunders; 2003:435–437.
3. Lembersky BC, Golomb HM. Hairy cell leukemia: clinical features and therapeutic advances. Cancer Metastasis Rev. 1987;6:283-300.
4. Oleske D, Golomb HM, Farber MD, et al. A case-control inquiry into the etiology of hairy cell leukemia. Am J Epidemiol. 1985;121:675-683.
5. Catovsky D. Hairy-cell leukaemia and prolymphocytic leukaemia. Clin Haematol. 1977;6:245-268.
6. Golomb HM, Catovsky D, Golde DW. Hairy cell leukemia: a clinical review based on 71 cases. Ann Intern Med. 1978;89:677-683.
7. Kar M, Ghosh A. Pancytopenia. J Indian Acad Clin Med. 2002;3:29-34.
8. Evaluation of pancytopenia. Epocrates online. Available at: https://online.epocrates.com/noFrame/showPage.do?method=diseases&MonographId=1024&ActiveSectionId=31. Accessed December 8, 2010.
9. Grever MR. How I treat hairy cell leukemia. Blood. 2010;115:21-28.
10. Au WY, Klasa RJ, Gallagher R, et al. Second malignancies in patients with hairy cell leukemia in British Columbia: a 20-year experience. Blood. 1998;92:1160-1164.
11. Zakarija A, Peterson LC, Tallman RS. Hairy cell leukemia. In: Hoffman R, Furie B, Benz EJ, et al, eds. Hematology: Basic Principals and Practice. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2009. Available at: http://www.mdconsult.com/books/page.do?eid=4-u1.0-B978-0-443-06715-0..50086-8&isbn=978-0-443-06715-0&type=bookPage&from=content&uniqId=279464872-2. Accessed December 8, 2010.
• Obtain a thorough history to aid in narrowing the list of potential causes in pancytopenia’s broad differential.
• Arrange for a peripheral smear and bone marrow biopsy to confirm the cause of pancytopenia.
• Screen any patient with hairy cell leukemia for a second primary malignancy.
• Base a treatment decision on the presence of symptoms and the severity of the pancytopenia.
CASE A 47-year-old man with a history of alcoholism came to our emergency department (ED) with a 3-week history of sore throat and dry cough. He said that for the past 2 months he had experienced worsening shortness of breath, increasing weakness, and episodes of light-headedness. He also said that his gums occasionally bled when he brushed his teeth.
Our patient owned a farm where he was exposed to pesticides and fertilizers, but he reported no contact with sick individuals, new medications, or recent travel. The patient had a 40 pack-year smoking history, but he had quit within the past year. His family history was negative for malignancies or rheumatologic diseases.
On physical exam, we noted splenomegaly (spleen was approximately 3 cm below costal margin); all other exam findings were within normal limits.
Lab results revealed pancytopenia: the patient’s serum white blood cell (WBC) was 900/mcL, the absolute neutrophil count (ANC) was 447/mcL, and the absolute lymphocyte count was 346/mcL. Hemoglobin was 5.4 g/dL and platelet count was 47,000/mcL. (Pancytopenia is defined as hemoglobin <13.5 g/dL [males] or 11.6 g/dL [females], platelet count <150,000/mcL, and WBC count <4000/mcL. Criteria for severe pancytopenia include an ANC <500/mcL, platelet count <20,000 mcL, and corrected reticulocyte count <1%.1,2)
A repeat complete blood count (CBC) showed similar results. Basic metabolic panel, chest x-ray film, and electrocardiogram results were all normal.
Based on the initial lab work, we ordered further testing for human immunodeficiency virus (HIV), Epstein-Barr virus (EBV), cytomegalovirus, hepatitis, parvovirus B19, and antinuclear antibodies. All results were negative. B12 and folate levels were low normal, and the reticulocyte count was 2.31%. We admitted the patient for evaluation of his pancytopenia.
WHAT ADDITIONAL TESTING WOULD YOU PURSUE AT THIS POINT?
We ordered a bone marrow biopsy and peripheral smear, which is protocol in a case such as this. Results showed leukemic and “hairy” cells.
Hairy cell leukemia
Hairy cell leukemia is an uncommon chronic B-cell lymphoproliferative disorder.3 It represents 2% of all adult leukemias; the median age of onset is 52 years, with a male predominance of 4:1. It is 3 times more common in Caucasians than in African Americans. There is still some controversy regarding its cell line, and its pathogenesis is still unknown. Exposures to ionizing radiation, EBV, organic chemicals, woodworking, and farming have been cited as possible causes.4
Symptoms of hairy cell leukemia include fatigue, weakness, and weight loss. Abdominal fullness or discomfort due to splenomegaly is seen in 25% of cases.5,6 Easy bruising and bleeding secondary to severe pancytopenia are also seen in about 25% of cases.5,6 However, 25% of patients are initially asymptomatic and are found to have abnormal lab values during a routine well visit.6
Narrowing in on a diagnosis
The etiology of pancytopenia is broad, but the diagnostic possibilities (TABLE) narrow depending on the pathogenesis of a patient’s condition: decreased bone marrow production, increased destruction or sequestration of cells, inherited/congenital, or idiopathic.
Testing. After an initial CBC, a second CBC should be obtained to confirm the pancytopenia before ordering further tests.1,7 Such testing includes a peripheral blood smear, a reticulocyte count, serum iron studies, and viral studies such as HIV, EBV, or parvovirus, along with an autoimmune profile and liver function tests.8 A hematology consult with probable bone marrow aspirate is also indicated.8
Diagnosis is made based on clinical findings and lab work revealing pancytopenia. A peripheral smear exhibiting leukemic cells and “hairy” cells suffices to make the diagnosis, although bone marrow biopsy—which reveals tartrate-resistant acid phosphatase– positive cells on cytochemical staining in 95% of cases3—is often use to corroborate the diagnosis. More recently, immunotyping using flow cytometry has become the standard for confirming the diagnosis of hairy cell leukemia.3
TABLE
Is it pancytopenia? The differential3
| Decreased bone marrow production | Increased destruction/sequestration of cells | Inherited/congenital |
|---|---|---|
Chemotherapy, radiotherapy Megaloblastic anemia Myelodysplastic syndromes Myelofibrosis Aplastic anemia Paroxysmal nocturnal hemoglobinuria Lymphoma with bone marrow infiltration Leukemia Plasma cell myeloma Infection (eg, parvovirus B19, CMV) Anorexia nervosa | Liver disease Portal hypertension Hypersplenism due to myelo- or lymphoproliferative disorders Evan’s syndrome Infection (eg, brucellosis, leishmaniasis) Heavy metal poisoning | Gaucher disease Fanconi’s anemia |
| Overlap | ||
| Connective tissue disorders (eg, SLE, RA) | ||
| AIDS | ||
| Mycobacterial infection; sepsis; acute viral infection (eg, CMV, HIV, EBV) | ||
| AIDS, acquired immune deficiency syndrome; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus. | ||
Adjust treatment to symptoms and severity of pancytopenia
Indications for treatment include the development of one or more the following: ANC <1000/mcL with repeat infections; symptomatic anemia with hemoglobin <11 g/dL; platelet count <100,000/mcL associated with bleeding, symptomatic splenomegaly, or adenopathy; or constitutional symptoms.9
First-line therapy is with one of the cytotoxic agents, either cladribine or pentostatin. Splenectomy is another effective option, with results that last up to 10 years in 50% of cases.9 Another option is interferon-alpha.10
Before the advent of cytotoxic agents, the 4-year survival rate for hairy cell leukemia was reported as 68%. Today, durable remission is attained for many patients. Even after relapse, retreatment yields good responses. The 5-year survival rate now is higher than 85%.11
Close follow-up of these patients is key
Potential complications of hairy cell leukemia include infections, bleeding, anemia, splenic rupture, and a second primary malignancy. There is a 2- to 3-fold increased risk of developing a second malignancy at a median interval of 40 months after initial diagnosis.10
This increased risk may be related to immunosuppression due to hairy cell leukemia or its treatment. The incidence of a second malignancy occurring before the diagnosis of hairy cell leukemia is 10.2%, and concurrently with the diagnosis is 2.6%.10 This finding suggests some pretreatment predisposition to cancer; further studies are being carried out to evaluate this matter. The most common solid tumors include prostate cancer, skin cancer, lung cancer, and gastrointestinal adenocarcinomas.10
Our patient’s outcome
During our patient’s hospital admission, he received 6 units of packed red blood cells, 3 daily injections of 480 mcg subcutaneous filgrastim for 5 days, and ongoing vitamin B12 supplementation to assist myeloid recovery. Upon discharge, his ANC level had increased to 1634/mcL. He began outpatient treatment with cladribine under the care of a hematologist. After almost a year of treatment, our patient is in complete remission.
CORRESPONDENCE
Sheena Boury, MD, Christ Hospital Family Medicine, 830 Thomas More Parkway, Edgewood, KY 41017; [email protected]
• Obtain a thorough history to aid in narrowing the list of potential causes in pancytopenia’s broad differential.
• Arrange for a peripheral smear and bone marrow biopsy to confirm the cause of pancytopenia.
• Screen any patient with hairy cell leukemia for a second primary malignancy.
• Base a treatment decision on the presence of symptoms and the severity of the pancytopenia.
CASE A 47-year-old man with a history of alcoholism came to our emergency department (ED) with a 3-week history of sore throat and dry cough. He said that for the past 2 months he had experienced worsening shortness of breath, increasing weakness, and episodes of light-headedness. He also said that his gums occasionally bled when he brushed his teeth.
Our patient owned a farm where he was exposed to pesticides and fertilizers, but he reported no contact with sick individuals, new medications, or recent travel. The patient had a 40 pack-year smoking history, but he had quit within the past year. His family history was negative for malignancies or rheumatologic diseases.
On physical exam, we noted splenomegaly (spleen was approximately 3 cm below costal margin); all other exam findings were within normal limits.
Lab results revealed pancytopenia: the patient’s serum white blood cell (WBC) was 900/mcL, the absolute neutrophil count (ANC) was 447/mcL, and the absolute lymphocyte count was 346/mcL. Hemoglobin was 5.4 g/dL and platelet count was 47,000/mcL. (Pancytopenia is defined as hemoglobin <13.5 g/dL [males] or 11.6 g/dL [females], platelet count <150,000/mcL, and WBC count <4000/mcL. Criteria for severe pancytopenia include an ANC <500/mcL, platelet count <20,000 mcL, and corrected reticulocyte count <1%.1,2)
A repeat complete blood count (CBC) showed similar results. Basic metabolic panel, chest x-ray film, and electrocardiogram results were all normal.
Based on the initial lab work, we ordered further testing for human immunodeficiency virus (HIV), Epstein-Barr virus (EBV), cytomegalovirus, hepatitis, parvovirus B19, and antinuclear antibodies. All results were negative. B12 and folate levels were low normal, and the reticulocyte count was 2.31%. We admitted the patient for evaluation of his pancytopenia.
WHAT ADDITIONAL TESTING WOULD YOU PURSUE AT THIS POINT?
We ordered a bone marrow biopsy and peripheral smear, which is protocol in a case such as this. Results showed leukemic and “hairy” cells.
Hairy cell leukemia
Hairy cell leukemia is an uncommon chronic B-cell lymphoproliferative disorder.3 It represents 2% of all adult leukemias; the median age of onset is 52 years, with a male predominance of 4:1. It is 3 times more common in Caucasians than in African Americans. There is still some controversy regarding its cell line, and its pathogenesis is still unknown. Exposures to ionizing radiation, EBV, organic chemicals, woodworking, and farming have been cited as possible causes.4
Symptoms of hairy cell leukemia include fatigue, weakness, and weight loss. Abdominal fullness or discomfort due to splenomegaly is seen in 25% of cases.5,6 Easy bruising and bleeding secondary to severe pancytopenia are also seen in about 25% of cases.5,6 However, 25% of patients are initially asymptomatic and are found to have abnormal lab values during a routine well visit.6
Narrowing in on a diagnosis
The etiology of pancytopenia is broad, but the diagnostic possibilities (TABLE) narrow depending on the pathogenesis of a patient’s condition: decreased bone marrow production, increased destruction or sequestration of cells, inherited/congenital, or idiopathic.
Testing. After an initial CBC, a second CBC should be obtained to confirm the pancytopenia before ordering further tests.1,7 Such testing includes a peripheral blood smear, a reticulocyte count, serum iron studies, and viral studies such as HIV, EBV, or parvovirus, along with an autoimmune profile and liver function tests.8 A hematology consult with probable bone marrow aspirate is also indicated.8
Diagnosis is made based on clinical findings and lab work revealing pancytopenia. A peripheral smear exhibiting leukemic cells and “hairy” cells suffices to make the diagnosis, although bone marrow biopsy—which reveals tartrate-resistant acid phosphatase– positive cells on cytochemical staining in 95% of cases3—is often use to corroborate the diagnosis. More recently, immunotyping using flow cytometry has become the standard for confirming the diagnosis of hairy cell leukemia.3
TABLE
Is it pancytopenia? The differential3
| Decreased bone marrow production | Increased destruction/sequestration of cells | Inherited/congenital |
|---|---|---|
Chemotherapy, radiotherapy Megaloblastic anemia Myelodysplastic syndromes Myelofibrosis Aplastic anemia Paroxysmal nocturnal hemoglobinuria Lymphoma with bone marrow infiltration Leukemia Plasma cell myeloma Infection (eg, parvovirus B19, CMV) Anorexia nervosa | Liver disease Portal hypertension Hypersplenism due to myelo- or lymphoproliferative disorders Evan’s syndrome Infection (eg, brucellosis, leishmaniasis) Heavy metal poisoning | Gaucher disease Fanconi’s anemia |
| Overlap | ||
| Connective tissue disorders (eg, SLE, RA) | ||
| AIDS | ||
| Mycobacterial infection; sepsis; acute viral infection (eg, CMV, HIV, EBV) | ||
| AIDS, acquired immune deficiency syndrome; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus. | ||
Adjust treatment to symptoms and severity of pancytopenia
Indications for treatment include the development of one or more the following: ANC <1000/mcL with repeat infections; symptomatic anemia with hemoglobin <11 g/dL; platelet count <100,000/mcL associated with bleeding, symptomatic splenomegaly, or adenopathy; or constitutional symptoms.9
First-line therapy is with one of the cytotoxic agents, either cladribine or pentostatin. Splenectomy is another effective option, with results that last up to 10 years in 50% of cases.9 Another option is interferon-alpha.10
Before the advent of cytotoxic agents, the 4-year survival rate for hairy cell leukemia was reported as 68%. Today, durable remission is attained for many patients. Even after relapse, retreatment yields good responses. The 5-year survival rate now is higher than 85%.11
Close follow-up of these patients is key
Potential complications of hairy cell leukemia include infections, bleeding, anemia, splenic rupture, and a second primary malignancy. There is a 2- to 3-fold increased risk of developing a second malignancy at a median interval of 40 months after initial diagnosis.10
This increased risk may be related to immunosuppression due to hairy cell leukemia or its treatment. The incidence of a second malignancy occurring before the diagnosis of hairy cell leukemia is 10.2%, and concurrently with the diagnosis is 2.6%.10 This finding suggests some pretreatment predisposition to cancer; further studies are being carried out to evaluate this matter. The most common solid tumors include prostate cancer, skin cancer, lung cancer, and gastrointestinal adenocarcinomas.10
Our patient’s outcome
During our patient’s hospital admission, he received 6 units of packed red blood cells, 3 daily injections of 480 mcg subcutaneous filgrastim for 5 days, and ongoing vitamin B12 supplementation to assist myeloid recovery. Upon discharge, his ANC level had increased to 1634/mcL. He began outpatient treatment with cladribine under the care of a hematologist. After almost a year of treatment, our patient is in complete remission.
CORRESPONDENCE
Sheena Boury, MD, Christ Hospital Family Medicine, 830 Thomas More Parkway, Edgewood, KY 41017; [email protected]
1. Ishtiaq O, Baqai HZ, Anwer F, et al. Patterns of pancytopenia patients in a general medical ward and a proposed diagnostic approach. J Ayub Med Coll Abbottabad. 2004;16:8-13.
2. Wang ES, Berliner N. Hematopoiesis and hematopoietic failure. In: Andreoli TE, Carpenter CCJ, Griggs RC, eds. Cecil Essentials of Medicine. 6th ed. Philadelphia, Pa: WB Saunders; 2003:435–437.
3. Lembersky BC, Golomb HM. Hairy cell leukemia: clinical features and therapeutic advances. Cancer Metastasis Rev. 1987;6:283-300.
4. Oleske D, Golomb HM, Farber MD, et al. A case-control inquiry into the etiology of hairy cell leukemia. Am J Epidemiol. 1985;121:675-683.
5. Catovsky D. Hairy-cell leukaemia and prolymphocytic leukaemia. Clin Haematol. 1977;6:245-268.
6. Golomb HM, Catovsky D, Golde DW. Hairy cell leukemia: a clinical review based on 71 cases. Ann Intern Med. 1978;89:677-683.
7. Kar M, Ghosh A. Pancytopenia. J Indian Acad Clin Med. 2002;3:29-34.
8. Evaluation of pancytopenia. Epocrates online. Available at: https://online.epocrates.com/noFrame/showPage.do?method=diseases&MonographId=1024&ActiveSectionId=31. Accessed December 8, 2010.
9. Grever MR. How I treat hairy cell leukemia. Blood. 2010;115:21-28.
10. Au WY, Klasa RJ, Gallagher R, et al. Second malignancies in patients with hairy cell leukemia in British Columbia: a 20-year experience. Blood. 1998;92:1160-1164.
11. Zakarija A, Peterson LC, Tallman RS. Hairy cell leukemia. In: Hoffman R, Furie B, Benz EJ, et al, eds. Hematology: Basic Principals and Practice. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2009. Available at: http://www.mdconsult.com/books/page.do?eid=4-u1.0-B978-0-443-06715-0..50086-8&isbn=978-0-443-06715-0&type=bookPage&from=content&uniqId=279464872-2. Accessed December 8, 2010.
1. Ishtiaq O, Baqai HZ, Anwer F, et al. Patterns of pancytopenia patients in a general medical ward and a proposed diagnostic approach. J Ayub Med Coll Abbottabad. 2004;16:8-13.
2. Wang ES, Berliner N. Hematopoiesis and hematopoietic failure. In: Andreoli TE, Carpenter CCJ, Griggs RC, eds. Cecil Essentials of Medicine. 6th ed. Philadelphia, Pa: WB Saunders; 2003:435–437.
3. Lembersky BC, Golomb HM. Hairy cell leukemia: clinical features and therapeutic advances. Cancer Metastasis Rev. 1987;6:283-300.
4. Oleske D, Golomb HM, Farber MD, et al. A case-control inquiry into the etiology of hairy cell leukemia. Am J Epidemiol. 1985;121:675-683.
5. Catovsky D. Hairy-cell leukaemia and prolymphocytic leukaemia. Clin Haematol. 1977;6:245-268.
6. Golomb HM, Catovsky D, Golde DW. Hairy cell leukemia: a clinical review based on 71 cases. Ann Intern Med. 1978;89:677-683.
7. Kar M, Ghosh A. Pancytopenia. J Indian Acad Clin Med. 2002;3:29-34.
8. Evaluation of pancytopenia. Epocrates online. Available at: https://online.epocrates.com/noFrame/showPage.do?method=diseases&MonographId=1024&ActiveSectionId=31. Accessed December 8, 2010.
9. Grever MR. How I treat hairy cell leukemia. Blood. 2010;115:21-28.
10. Au WY, Klasa RJ, Gallagher R, et al. Second malignancies in patients with hairy cell leukemia in British Columbia: a 20-year experience. Blood. 1998;92:1160-1164.
11. Zakarija A, Peterson LC, Tallman RS. Hairy cell leukemia. In: Hoffman R, Furie B, Benz EJ, et al, eds. Hematology: Basic Principals and Practice. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2009. Available at: http://www.mdconsult.com/books/page.do?eid=4-u1.0-B978-0-443-06715-0..50086-8&isbn=978-0-443-06715-0&type=bookPage&from=content&uniqId=279464872-2. Accessed December 8, 2010.
Recurrent pleural effusions and a normal cardiac CT
CASE An 84-year-old man was admitted to our family medicine inpatient service with increasing weakness, fatigue, nausea, jaundice, and anorexia after undergoing thoracentesis for recurrent large right-sided pleural effusion twice within the past 6 weeks. The patient had no fever, chills, night sweats, chest pain, or chronic cough.
His past medical history included coronary artery disease and hyperlipidemia, and he had undergone coronary artery bypass grafting 6 years prior to this admission.
Initial vital signs included a blood pressure of 116/68 mm hg and a pulse rate of 78 beats per minute. physical examination revealed a thin but otherwise active and functional man, with the presence of scleral icterus and a 2/6 systolic murmur that was loudest at the left upper sternal border, with no pericardial knocks or rubs. There was no jugular venous distension. The patient had decreased breath sounds at the right base, but no appreciable rales, rhonchi, or wheezes, and an enlarged liver with an irregular edge approximately 7 cm below the costal margin. he had bilateral trace pitting edema of the lower extremities and an erythematous chronic venous stasis ulcer on his left lower leg that was being treated with sulfamethoxazole and trimethoprim and rifampin.
Laboratory findings included a sodium level of 130 mEq/L; creatinine, 1.6 mg/dL; total bilirubin, 4.2 mg/dL; and an international normalized ratio (INR) of 1.6, with an otherwise normal liver profile. pleural fluid was transudative by light’s criteria, with negative pleural fluid cultures.
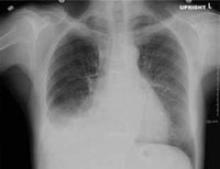
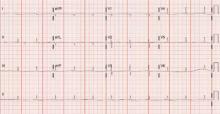
A chest x-ray showed right-sided airspace disease, with an associated small effusion (FIGURE 1), and an electrocardiogram (EKG) revealed a right axis deviation with flipped T waves in V3-V6 (FIGURE 2). A computed tomography (CT) scan of the chest performed 2 months prior to admission revealed a small right pleural effusion, moderate emphysema, and pleural plaques. A transthoracic echocardiogram performed a week earlier was significant for a normal ejection fraction without pericardial thickening, and mild dilation of the left and right atria and the right ventricle.
Our patient had recurrent transudative pleural effusions with no history of congestive heart failure, a normal thyroid-stimulating hormone, no signs of nephrotic syndrome, no risk factors for lung cancer, and a presentation that was not consistent with an acute pulmonary embolus. he had jaundice, but no risk factors for cirrhosis. A comprehensive hepatology consult suggested that the jaundice was associated with recent rifampin use, along with hepatic congestion likely due to cardiac etiology.
The patient also had a documented history of constrictive pericarditis. Although a cardiac CT scan 15 months prior to his hospital admission showed normal pericardial thickness, constrictive pericarditis remained high on our differential.
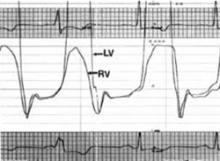
To further evaluate the patient’s cardiac function, a cardiologist performed a right-sided cardiac catheterization. in constrictive pericarditis, equalization of end-diastolic pressures occurs due to limitation of the total volume of the ventricles by a rigid pericardium. in our patient, these pressures did not equalize. his right and left ventricular pressure tracings did, however, have the classic square root sign often seen in constrictive pericarditis (FIGURE 3). This “dip and plateau” is the result of rapid filling of the ventricles during early diastole, with the inflexible pericardium causing an abrupt halt in ventricular filling.1
We considered restrictive cardiomyopathy in the differential, too, but the signs and symptoms weren’t a perfect fit there, either. equalization of left and right ventricular end-diastolic pressures in cardiac catheterization is usually not seen in restrictive cardiomyopathy, but neither is the square-root sign evident on ventricular pressure monitors.1
FIGURE 1
X-ray shows right-sided airspace disease
FIGURE 2
Right axis deviation with flipped T waves
FIGURE 3
Classic square root sign
WHAT IS THE MOST LIKELY EXPLANATION FOR HIS CONDITION?
Constrictive pericarditis
On the advice of a cardiologist who specializes in advanced cardiac imaging, our patient underwent tissue Doppler velocity echocardiography—a diagnostic test that provides evidence of a diseased myocardium (as in restrictive cardiomyopathy), as well as changes in diastolic blood flow that differentiate constrictive pericarditis from restrictive cardiomyopathy. Our patient’s tissue Doppler velocity echocardiogram revealed a normal myocardium with an abrupt cessation of early left ventricular filling, consistent with constrictive pericarditis. Along with his clinical presentation and history, this test was conclusive enough to diagnose constrictive pericarditis.
Making the diagnosis in more “typical” cases
Symptoms of constrictive pericarditis include fluid overload (eg, recurrent pleural effusions2-4) hepatic dysfunction, and peripheral edema. Studies show that 44% to 54% of patients with constrictive pericarditis present with pleural effusions;5 chest pain and dyspnea are other common symptoms.6 Past medical history is also important, considering the 3 most common risks for constrictive pericarditis: cardiac surgery, pericarditis, and irradiation of the mediastinum (TABLE).7
Typical physical exam findings in patients with constrictive pericarditis include an elevated jugular venous pressure, third spacing of fluids, a pericardial knock, and Kussmaul’s sign. Our patient had third spacing of fluids, including mild peripheral edema and the recurrent effusions as noted, as well as signs of hepatic dysfunction that were initially attributed to his rifampin use. While these symptoms raise the suspicion of constrictive pericarditis, none is specific to that condition alone.
Studies that help in the diagnosis of constrictive pericarditis include chest x-ray, cardiac CT, echocardiography, cardiac magnetic resonance imaging (MRI), and cardiac catheterization. EKG has no specific findings, but cardiac arrhythmias (eg, atrial fibrillation) are common.8 Pericardial thickening on cardiac CT scan is a definitive but not universal finding; in a Mayo Clinic study, such thickening was not evident in 26 of 143 patients with confirmed constrictive pericarditis.8 Cardiac MRI has been shown to have 88% sensitivity and 100% specificity,9 but will miss up to 18% of patients with constrictive pericarditis.8
Differentiating between restriction and constriction. It can be difficult to distinguish between constrictive pericarditis and restrictive cardiomyopathy. Although these conditions can present in a similar manner, they require different modes of treatment. Laboratory testing, cardiac catheterization, and tissue Doppler velocity echocardiography (which we relied on) can help to distinguish between them.
TABLE
Causes of constrictive pericarditis7
| More common |
|---|
| Idiopathic Postcardiac surgery (coronary artery bypass grafting, valve replacement) Postradiation therapy (eg, for breast cancer, lymphoma) Viral (pericarditis) |
| Less common |
| Asbestosis Cancer and myeloproliferative disorders Connective tissue disorders (rheumatoid arthritis, systemic lupus erythematosus) Adverse drug reaction Infection (tuberculosis, fungal) Sarcoidosis Trauma Uremia |
Pericardiectomy is the treatment of choice
Pericardiectomy, the optimal treatment for most patients with constrictive pericarditis, carries a 30-day perioperative mortality of approximately 6%.5,7 Patients with minimal symptoms can be monitored for up to 2 months, but only 17% of cases are self-limited.6 Patients with end-stage disease or those who have radiation-induced constrictive pericarditis experience poor surgical outcomes, and may be better served by medical management.7
In light of our patient’s excellent baseline functional status, clinical presentation, and Doppler test results, a cardiothoracic surgeon performed a pericardiectomy. His symptoms improved postoperatively, and he has had no further pleural effusions. The patient’s fatigue, anorexia, weight loss, and dyspnea fully resolved, as well.
- Include constrictive pericarditis in the differential diagnosis of patients with recurrent pleural effusions, an important presenting symptom in 44% to 54% of patients with this condition.
- Consider multiple testing modalities to arrive at a diagnosis of constrictive pericarditis, including cardiac CT or MRI, tissue Doppler echocardiography, and cardiac catheterization.
- Do not rule out constrictive pericarditis if pericardial thickening is not found on cardiac CT scan; in 1 study, this finding was not present in 18% of patients with a confirmed diagnosis.
CORRESPONDENCE Jeffrey S. Morgeson, MD; 2123 Auburn Avenue, 340 MOB, Cincinnati, OH 45219; [email protected]
1. Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587-610.
2. Akhter MW, Nuño IN, Rahimtoola SH. Constrictive pericarditis masquerading as chronic idiopathic pleural effusion: importance of physical examination. Am J Med. 2006;119:e1-e4.
3. Ramar K, Daniels CA. Constrictive pericarditis presenting as unexplained dyspnea with recurrent pleural effusion. Respir Care. 2008;53:912-915.
4. Sadikot RT, Fredi JL, Light RW. A 43-year-old man with a large recurrent right-sided pleural effusion. Diagnosis: constrictive pericarditis. Chest. 2000;117:1191-1194.
5. Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43:1445-1452.
6. Haley JH, Tajik AJ, Danielson GK, et al. Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol. 2004;43:271-275.
7. Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380-1386.
8. Talreja DP, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852-1857.
9. Masui T, Finck S, Higgins CB. Constrictive pericarditis and restrictive cardiomyopathy: evaluation with MR imaging. Radiology. 1992;182:369-373.
CASE An 84-year-old man was admitted to our family medicine inpatient service with increasing weakness, fatigue, nausea, jaundice, and anorexia after undergoing thoracentesis for recurrent large right-sided pleural effusion twice within the past 6 weeks. The patient had no fever, chills, night sweats, chest pain, or chronic cough.
His past medical history included coronary artery disease and hyperlipidemia, and he had undergone coronary artery bypass grafting 6 years prior to this admission.
Initial vital signs included a blood pressure of 116/68 mm hg and a pulse rate of 78 beats per minute. physical examination revealed a thin but otherwise active and functional man, with the presence of scleral icterus and a 2/6 systolic murmur that was loudest at the left upper sternal border, with no pericardial knocks or rubs. There was no jugular venous distension. The patient had decreased breath sounds at the right base, but no appreciable rales, rhonchi, or wheezes, and an enlarged liver with an irregular edge approximately 7 cm below the costal margin. he had bilateral trace pitting edema of the lower extremities and an erythematous chronic venous stasis ulcer on his left lower leg that was being treated with sulfamethoxazole and trimethoprim and rifampin.
Laboratory findings included a sodium level of 130 mEq/L; creatinine, 1.6 mg/dL; total bilirubin, 4.2 mg/dL; and an international normalized ratio (INR) of 1.6, with an otherwise normal liver profile. pleural fluid was transudative by light’s criteria, with negative pleural fluid cultures.
A chest x-ray showed right-sided airspace disease, with an associated small effusion (FIGURE 1), and an electrocardiogram (EKG) revealed a right axis deviation with flipped T waves in V3-V6 (FIGURE 2). A computed tomography (CT) scan of the chest performed 2 months prior to admission revealed a small right pleural effusion, moderate emphysema, and pleural plaques. A transthoracic echocardiogram performed a week earlier was significant for a normal ejection fraction without pericardial thickening, and mild dilation of the left and right atria and the right ventricle.
Our patient had recurrent transudative pleural effusions with no history of congestive heart failure, a normal thyroid-stimulating hormone, no signs of nephrotic syndrome, no risk factors for lung cancer, and a presentation that was not consistent with an acute pulmonary embolus. he had jaundice, but no risk factors for cirrhosis. A comprehensive hepatology consult suggested that the jaundice was associated with recent rifampin use, along with hepatic congestion likely due to cardiac etiology.
The patient also had a documented history of constrictive pericarditis. Although a cardiac CT scan 15 months prior to his hospital admission showed normal pericardial thickness, constrictive pericarditis remained high on our differential.
To further evaluate the patient’s cardiac function, a cardiologist performed a right-sided cardiac catheterization. in constrictive pericarditis, equalization of end-diastolic pressures occurs due to limitation of the total volume of the ventricles by a rigid pericardium. in our patient, these pressures did not equalize. his right and left ventricular pressure tracings did, however, have the classic square root sign often seen in constrictive pericarditis (FIGURE 3). This “dip and plateau” is the result of rapid filling of the ventricles during early diastole, with the inflexible pericardium causing an abrupt halt in ventricular filling.1
We considered restrictive cardiomyopathy in the differential, too, but the signs and symptoms weren’t a perfect fit there, either. equalization of left and right ventricular end-diastolic pressures in cardiac catheterization is usually not seen in restrictive cardiomyopathy, but neither is the square-root sign evident on ventricular pressure monitors.1
FIGURE 1
X-ray shows right-sided airspace disease
FIGURE 2
Right axis deviation with flipped T waves
FIGURE 3
Classic square root sign
WHAT IS THE MOST LIKELY EXPLANATION FOR HIS CONDITION?
Constrictive pericarditis
On the advice of a cardiologist who specializes in advanced cardiac imaging, our patient underwent tissue Doppler velocity echocardiography—a diagnostic test that provides evidence of a diseased myocardium (as in restrictive cardiomyopathy), as well as changes in diastolic blood flow that differentiate constrictive pericarditis from restrictive cardiomyopathy. Our patient’s tissue Doppler velocity echocardiogram revealed a normal myocardium with an abrupt cessation of early left ventricular filling, consistent with constrictive pericarditis. Along with his clinical presentation and history, this test was conclusive enough to diagnose constrictive pericarditis.
Making the diagnosis in more “typical” cases
Symptoms of constrictive pericarditis include fluid overload (eg, recurrent pleural effusions2-4) hepatic dysfunction, and peripheral edema. Studies show that 44% to 54% of patients with constrictive pericarditis present with pleural effusions;5 chest pain and dyspnea are other common symptoms.6 Past medical history is also important, considering the 3 most common risks for constrictive pericarditis: cardiac surgery, pericarditis, and irradiation of the mediastinum (TABLE).7
Typical physical exam findings in patients with constrictive pericarditis include an elevated jugular venous pressure, third spacing of fluids, a pericardial knock, and Kussmaul’s sign. Our patient had third spacing of fluids, including mild peripheral edema and the recurrent effusions as noted, as well as signs of hepatic dysfunction that were initially attributed to his rifampin use. While these symptoms raise the suspicion of constrictive pericarditis, none is specific to that condition alone.
Studies that help in the diagnosis of constrictive pericarditis include chest x-ray, cardiac CT, echocardiography, cardiac magnetic resonance imaging (MRI), and cardiac catheterization. EKG has no specific findings, but cardiac arrhythmias (eg, atrial fibrillation) are common.8 Pericardial thickening on cardiac CT scan is a definitive but not universal finding; in a Mayo Clinic study, such thickening was not evident in 26 of 143 patients with confirmed constrictive pericarditis.8 Cardiac MRI has been shown to have 88% sensitivity and 100% specificity,9 but will miss up to 18% of patients with constrictive pericarditis.8
Differentiating between restriction and constriction. It can be difficult to distinguish between constrictive pericarditis and restrictive cardiomyopathy. Although these conditions can present in a similar manner, they require different modes of treatment. Laboratory testing, cardiac catheterization, and tissue Doppler velocity echocardiography (which we relied on) can help to distinguish between them.
TABLE
Causes of constrictive pericarditis7
| More common |
|---|
| Idiopathic Postcardiac surgery (coronary artery bypass grafting, valve replacement) Postradiation therapy (eg, for breast cancer, lymphoma) Viral (pericarditis) |
| Less common |
| Asbestosis Cancer and myeloproliferative disorders Connective tissue disorders (rheumatoid arthritis, systemic lupus erythematosus) Adverse drug reaction Infection (tuberculosis, fungal) Sarcoidosis Trauma Uremia |
Pericardiectomy is the treatment of choice
Pericardiectomy, the optimal treatment for most patients with constrictive pericarditis, carries a 30-day perioperative mortality of approximately 6%.5,7 Patients with minimal symptoms can be monitored for up to 2 months, but only 17% of cases are self-limited.6 Patients with end-stage disease or those who have radiation-induced constrictive pericarditis experience poor surgical outcomes, and may be better served by medical management.7
In light of our patient’s excellent baseline functional status, clinical presentation, and Doppler test results, a cardiothoracic surgeon performed a pericardiectomy. His symptoms improved postoperatively, and he has had no further pleural effusions. The patient’s fatigue, anorexia, weight loss, and dyspnea fully resolved, as well.
- Include constrictive pericarditis in the differential diagnosis of patients with recurrent pleural effusions, an important presenting symptom in 44% to 54% of patients with this condition.
- Consider multiple testing modalities to arrive at a diagnosis of constrictive pericarditis, including cardiac CT or MRI, tissue Doppler echocardiography, and cardiac catheterization.
- Do not rule out constrictive pericarditis if pericardial thickening is not found on cardiac CT scan; in 1 study, this finding was not present in 18% of patients with a confirmed diagnosis.
CORRESPONDENCE Jeffrey S. Morgeson, MD; 2123 Auburn Avenue, 340 MOB, Cincinnati, OH 45219; [email protected]
CASE An 84-year-old man was admitted to our family medicine inpatient service with increasing weakness, fatigue, nausea, jaundice, and anorexia after undergoing thoracentesis for recurrent large right-sided pleural effusion twice within the past 6 weeks. The patient had no fever, chills, night sweats, chest pain, or chronic cough.
His past medical history included coronary artery disease and hyperlipidemia, and he had undergone coronary artery bypass grafting 6 years prior to this admission.
Initial vital signs included a blood pressure of 116/68 mm hg and a pulse rate of 78 beats per minute. physical examination revealed a thin but otherwise active and functional man, with the presence of scleral icterus and a 2/6 systolic murmur that was loudest at the left upper sternal border, with no pericardial knocks or rubs. There was no jugular venous distension. The patient had decreased breath sounds at the right base, but no appreciable rales, rhonchi, or wheezes, and an enlarged liver with an irregular edge approximately 7 cm below the costal margin. he had bilateral trace pitting edema of the lower extremities and an erythematous chronic venous stasis ulcer on his left lower leg that was being treated with sulfamethoxazole and trimethoprim and rifampin.
Laboratory findings included a sodium level of 130 mEq/L; creatinine, 1.6 mg/dL; total bilirubin, 4.2 mg/dL; and an international normalized ratio (INR) of 1.6, with an otherwise normal liver profile. pleural fluid was transudative by light’s criteria, with negative pleural fluid cultures.
A chest x-ray showed right-sided airspace disease, with an associated small effusion (FIGURE 1), and an electrocardiogram (EKG) revealed a right axis deviation with flipped T waves in V3-V6 (FIGURE 2). A computed tomography (CT) scan of the chest performed 2 months prior to admission revealed a small right pleural effusion, moderate emphysema, and pleural plaques. A transthoracic echocardiogram performed a week earlier was significant for a normal ejection fraction without pericardial thickening, and mild dilation of the left and right atria and the right ventricle.
Our patient had recurrent transudative pleural effusions with no history of congestive heart failure, a normal thyroid-stimulating hormone, no signs of nephrotic syndrome, no risk factors for lung cancer, and a presentation that was not consistent with an acute pulmonary embolus. he had jaundice, but no risk factors for cirrhosis. A comprehensive hepatology consult suggested that the jaundice was associated with recent rifampin use, along with hepatic congestion likely due to cardiac etiology.
The patient also had a documented history of constrictive pericarditis. Although a cardiac CT scan 15 months prior to his hospital admission showed normal pericardial thickness, constrictive pericarditis remained high on our differential.
To further evaluate the patient’s cardiac function, a cardiologist performed a right-sided cardiac catheterization. in constrictive pericarditis, equalization of end-diastolic pressures occurs due to limitation of the total volume of the ventricles by a rigid pericardium. in our patient, these pressures did not equalize. his right and left ventricular pressure tracings did, however, have the classic square root sign often seen in constrictive pericarditis (FIGURE 3). This “dip and plateau” is the result of rapid filling of the ventricles during early diastole, with the inflexible pericardium causing an abrupt halt in ventricular filling.1
We considered restrictive cardiomyopathy in the differential, too, but the signs and symptoms weren’t a perfect fit there, either. equalization of left and right ventricular end-diastolic pressures in cardiac catheterization is usually not seen in restrictive cardiomyopathy, but neither is the square-root sign evident on ventricular pressure monitors.1
FIGURE 1
X-ray shows right-sided airspace disease
FIGURE 2
Right axis deviation with flipped T waves
FIGURE 3
Classic square root sign
WHAT IS THE MOST LIKELY EXPLANATION FOR HIS CONDITION?
Constrictive pericarditis
On the advice of a cardiologist who specializes in advanced cardiac imaging, our patient underwent tissue Doppler velocity echocardiography—a diagnostic test that provides evidence of a diseased myocardium (as in restrictive cardiomyopathy), as well as changes in diastolic blood flow that differentiate constrictive pericarditis from restrictive cardiomyopathy. Our patient’s tissue Doppler velocity echocardiogram revealed a normal myocardium with an abrupt cessation of early left ventricular filling, consistent with constrictive pericarditis. Along with his clinical presentation and history, this test was conclusive enough to diagnose constrictive pericarditis.
Making the diagnosis in more “typical” cases
Symptoms of constrictive pericarditis include fluid overload (eg, recurrent pleural effusions2-4) hepatic dysfunction, and peripheral edema. Studies show that 44% to 54% of patients with constrictive pericarditis present with pleural effusions;5 chest pain and dyspnea are other common symptoms.6 Past medical history is also important, considering the 3 most common risks for constrictive pericarditis: cardiac surgery, pericarditis, and irradiation of the mediastinum (TABLE).7
Typical physical exam findings in patients with constrictive pericarditis include an elevated jugular venous pressure, third spacing of fluids, a pericardial knock, and Kussmaul’s sign. Our patient had third spacing of fluids, including mild peripheral edema and the recurrent effusions as noted, as well as signs of hepatic dysfunction that were initially attributed to his rifampin use. While these symptoms raise the suspicion of constrictive pericarditis, none is specific to that condition alone.
Studies that help in the diagnosis of constrictive pericarditis include chest x-ray, cardiac CT, echocardiography, cardiac magnetic resonance imaging (MRI), and cardiac catheterization. EKG has no specific findings, but cardiac arrhythmias (eg, atrial fibrillation) are common.8 Pericardial thickening on cardiac CT scan is a definitive but not universal finding; in a Mayo Clinic study, such thickening was not evident in 26 of 143 patients with confirmed constrictive pericarditis.8 Cardiac MRI has been shown to have 88% sensitivity and 100% specificity,9 but will miss up to 18% of patients with constrictive pericarditis.8
Differentiating between restriction and constriction. It can be difficult to distinguish between constrictive pericarditis and restrictive cardiomyopathy. Although these conditions can present in a similar manner, they require different modes of treatment. Laboratory testing, cardiac catheterization, and tissue Doppler velocity echocardiography (which we relied on) can help to distinguish between them.
TABLE
Causes of constrictive pericarditis7
| More common |
|---|
| Idiopathic Postcardiac surgery (coronary artery bypass grafting, valve replacement) Postradiation therapy (eg, for breast cancer, lymphoma) Viral (pericarditis) |
| Less common |
| Asbestosis Cancer and myeloproliferative disorders Connective tissue disorders (rheumatoid arthritis, systemic lupus erythematosus) Adverse drug reaction Infection (tuberculosis, fungal) Sarcoidosis Trauma Uremia |
Pericardiectomy is the treatment of choice
Pericardiectomy, the optimal treatment for most patients with constrictive pericarditis, carries a 30-day perioperative mortality of approximately 6%.5,7 Patients with minimal symptoms can be monitored for up to 2 months, but only 17% of cases are self-limited.6 Patients with end-stage disease or those who have radiation-induced constrictive pericarditis experience poor surgical outcomes, and may be better served by medical management.7
In light of our patient’s excellent baseline functional status, clinical presentation, and Doppler test results, a cardiothoracic surgeon performed a pericardiectomy. His symptoms improved postoperatively, and he has had no further pleural effusions. The patient’s fatigue, anorexia, weight loss, and dyspnea fully resolved, as well.
- Include constrictive pericarditis in the differential diagnosis of patients with recurrent pleural effusions, an important presenting symptom in 44% to 54% of patients with this condition.
- Consider multiple testing modalities to arrive at a diagnosis of constrictive pericarditis, including cardiac CT or MRI, tissue Doppler echocardiography, and cardiac catheterization.
- Do not rule out constrictive pericarditis if pericardial thickening is not found on cardiac CT scan; in 1 study, this finding was not present in 18% of patients with a confirmed diagnosis.
CORRESPONDENCE Jeffrey S. Morgeson, MD; 2123 Auburn Avenue, 340 MOB, Cincinnati, OH 45219; [email protected]
1. Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587-610.
2. Akhter MW, Nuño IN, Rahimtoola SH. Constrictive pericarditis masquerading as chronic idiopathic pleural effusion: importance of physical examination. Am J Med. 2006;119:e1-e4.
3. Ramar K, Daniels CA. Constrictive pericarditis presenting as unexplained dyspnea with recurrent pleural effusion. Respir Care. 2008;53:912-915.
4. Sadikot RT, Fredi JL, Light RW. A 43-year-old man with a large recurrent right-sided pleural effusion. Diagnosis: constrictive pericarditis. Chest. 2000;117:1191-1194.
5. Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43:1445-1452.
6. Haley JH, Tajik AJ, Danielson GK, et al. Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol. 2004;43:271-275.
7. Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380-1386.
8. Talreja DP, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852-1857.
9. Masui T, Finck S, Higgins CB. Constrictive pericarditis and restrictive cardiomyopathy: evaluation with MR imaging. Radiology. 1992;182:369-373.
1. Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587-610.
2. Akhter MW, Nuño IN, Rahimtoola SH. Constrictive pericarditis masquerading as chronic idiopathic pleural effusion: importance of physical examination. Am J Med. 2006;119:e1-e4.
3. Ramar K, Daniels CA. Constrictive pericarditis presenting as unexplained dyspnea with recurrent pleural effusion. Respir Care. 2008;53:912-915.
4. Sadikot RT, Fredi JL, Light RW. A 43-year-old man with a large recurrent right-sided pleural effusion. Diagnosis: constrictive pericarditis. Chest. 2000;117:1191-1194.
5. Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43:1445-1452.
6. Haley JH, Tajik AJ, Danielson GK, et al. Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol. 2004;43:271-275.
7. Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380-1386.
8. Talreja DP, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852-1857.
9. Masui T, Finck S, Higgins CB. Constrictive pericarditis and restrictive cardiomyopathy: evaluation with MR imaging. Radiology. 1992;182:369-373.