User login
HCV Infection
The hepatitis C virus (HCV) is estimated to affect 180 million people worldwide, and the CDC estimates that approximately 3.2 million persons in the United States are chronically infected with HCV.1,2 In recent years, reported HCV-related deaths have outnumbered those attributed to HIV infection.3 Clinicians in almost any practice area are likely to encounter patients affected by HCV.
Infection with HCV is a major risk factor for cirrhosis, a disease associated with significant morbidity and mortality; HCV-associated cirrhosis is considered the leading cause of liver transplantation.4
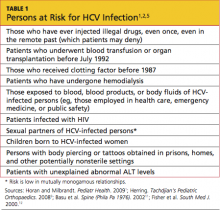
Screening for HCV is important in patients with known risk factors for the disease1 (see Table 11,2,5). Of note, however, the CDC is in the process of expanding its recommendations to one-time screening for all Americans born between 1945 and 1965—an age-group that accounts for more than 75% of cases of HCV infection among US adults.6 (Clinicians interested in viewing the draft document for public comment can refer to www.regulations.gov, docket #CDC-2-12-0005.)
PATIENT PRESENTATION/ PATIENT HISTORY
Most patients with hepatitis C present without signs or symptoms of their illness. If symptoms are present, they may include fatigue, pruritus, abdominal pain/ discomfort, arthralgias, or anorexia; results on routine liver function tests may be abnormal.1,7-9 Liver function may appear normal in patients with HCV, although 30% of patients with a normal alanine aminotransferase (ALT) level may have significant fibrosis.10 Lichen planus is commonly associated with HCV infection,9-11 and patients with this condition should be screened for HCV.
HCV infection most commonly presents between the fourth and sixth decades of life. Many patients have had the disease for as long as 20 years by the time they present for treatment—often after abnormal laboratory findings are discovered7 (but see "Can Some Patients Defer Treatment?"7,10,12).
Patients with acute HCV infection usually do not appear jaundiced or exhibit other signs of acute hepatitis. Symptomatic illness occurs in only 20% to 40% of patients with acute hepatitis C.7 Patients who present with acute illness (15% to 25% of patients with HCV) typically have an improved prognosis and are less likely to convert to chronicity if they survive the initial symptoms (ie, malaise, weakness, anorexia, jaundice).2,7 In many such patients, the body appears to mount a full immune response, and patients are often virus-free within weeks.
For 75% to 85% of patients, however, it is believed that the immune system fails to overcome the virus, and chronic infection, with progressive damage to the liver tissue, ensues.7 In chronic HCV infection, the rate of progression varies, depending on the HCV genotype, the infected host's genetic factors and lifestyle (including level of alcohol consumption), the extent of liver injury, and possible coinfection (as with HIV or hepatitis B virus [HBV]).1,7
Cirrhosis and Hepatocellular Carcinoma
Complications of cirrhosis include portal hypertension, ascites, hepatic encephalopathy, esophageal varices, and hepatocellular carcinoma (HCC).13 In patients with HCV-related cirrhosis, HCC develops at a rate of 1% to 4% per year, with a twofold to fourfold increased risk among black patients and Asian patients, respectively, compared with whites.7 The risk for HCC appears to be reduced in patients who undergo treatment leading to a sustained virologic response (ie, a viral load that is no longer detectable); and the risk is increased in patients with diabetes mellitus and those with HCV genotypes 1b and 3.14-16
HCC is difficult to treat unless detected in its early stages. It often results in death.13
PHYSICAL EXAMINATION
An appropriate physical exam is critical in detecting sequelae of chronic liver disease, which may reflect complications of long-term HCV infection. The patient should be evaluated for the presence of spider angiomas, palmar erythema, scleral icterus, ascites, caput medusae, and evidence of umbilical hernias—all possible signs of advanced liver disease.8,9
The initial physical exam is also an appropriate time to screen and treat patients for hypertension and diabetes, and to identify disorders that may make them poor candidates for HCV treatment. These conditions include coronary heart disease, untreated cancers or thyroid disease, kidney or autoimmune diseases, and psychiatric illness.1,17
Evaluation of the skin for "track marks," tattoos or body piercings that may have been applied in prisons, homes, or other nonsterile settings, or nonhealing lesions that may indicate immune compromise or diabetes is important.5,8 Visual acuity testing and fundoscopic exams are critical to establish a baseline, because treatment with pegylated interferon has the potential to cause visual changes and retinopathy.18
Laboratory Work-up
Initial testing for HCV includes an HCV antibody test. This serum test is commonly performed as part of a hepatitis panel—testing for hepatitis A virus (HAV), HBV, and HCV. Testing for hepatitis D and E is not routinely recommended unless the patient routinely travels to the Mediterranean Basin, the Middle East, Central Asia, or West Africa (where hepatitis D is most prevalent19) or the patient is pregnant (because during the third trimester, hepatitis E infection carries a mortality rate of 20% and can be transmitted to the fetus20).
If immunity to HAV and HBV are not shown on laboratory findings, it is critical to vaccinate the HCV-positive patient against these diseases. Coinfection with HAV and HCV can progress to a fulminant form of hepatitis and poor hepatic function; coinfection with HBV and HCV is associated with severe liver disease and an increased risk for HCC.21,22
The patient with positive results on the HCV antibody screen should be tested next with a quantitative HCV RNA assay to confirm the presence of active infection (ie, viral load).1 Detection of virus is considered indicative of active disease.
It is possible for a patient with positive HCV antibody test results to have no circulating virus detected on HCV RNA testing. Perhaps 15% to 25% of patients are able to clear HCV infection spontaneously within weeks to months after infection.7
Testing Considerations Before Treatment
Several assays are available to identify the patient's HCV genotype. It is essential to obtain this information before agents, dosing, and duration are determined, as the regimen will be tailored to the patient's HCV genotype.1 There are six major known HCV genotypes (numbered 1 through 6) and at least 50 minor subtypes (eg, 1a, 2b, 3a).1,23
Also to be considered before initiating HCV treatment is screening for autoimmune hepatitis.17 Appropriate tests include the anti-actin antibody, anti-smooth muscle antibody, antinuclear antibody, and anti-liver kidney microsomal antibody tests.24,25 Additionally, ceruloplasmin testing should be ordered along with the iron studies and ferritin levels obtained at the patient's first visit, to screen for Wilson's disease, a disorder of copper metabolism, and hemochromatosis, a disorder of iron metabolism.
The risks associated with HAV or HBV coinfection have been described; patients with HCV and HAV, HBV, or HIV are at greater risk for disease progression to cirrhosis and/or HCC than are mono-infected patients.1,21,22,26 HIV testing is routinely recommended for patients with HCV, with repeat testing during treatment if the patient maintains any risk factors for HIV exposure. Patients with HCV who are found to be coinfected with HIV must be treated urgently, but therapeutic components must be selected with considerable care: Serious interactions are possible among the effective agents, and antiretroviral drugs are associated with hepatotoxicity.1,27
Before HCV treatment begins, it is essential to obtain baseline values: liver function testing (aspartate aminotransferase, ALT, alkaline phosphatase, bilirubin), coagulation testing (prothrombin time and partial thromboplastin time), and thyroid testing (thyroid-stimulating hormone, triiodothyronine, thyroxine),1 as changes may occur as a result of anti-HCV therapy. It is also important to establish a baseline complete blood count (CBC) and electrolyte levels in order to identify, and later monitor, the presence of anemia, thrombocytopenia, and renal or other abnormalities.
Finally, it is important to screen the patient early for HCC by testing for serum alphafetoprotein (AFP, ie, tumor marker).28 Serum AFP must then be monitored at six-month intervals (with a recommended threshold of 400 ng/mL, but even readings as modest as 6 to 20 ng/mL) for elevations that may indicate HCC.29,30
Liver Studies
In addition to the recommended AFP monitoring, the patient should undergo an imaging study of the liver twice yearly. Ultrasound is the most cost-effective screening tool for most patients (with high specificity but varying sensitivity); when combined with a serum AFP reading of 10 ng/mL or greater, ultrasound has a sensitivity of 100% for detection of HCC.29 However, if lesions are found, or if a patient has known cirrhosis, CT or MRI is more accurate in detecting small lesions of the liver (specificity, 100%).29
Liver biopsy is recommended for certain patients to stage liver fibrosis; biopsy may not be necessary, for example, in patients with genotypes 2 and 3, as 80% of these patients react to standard therapy with sustained virologic response.1 Detection of advanced fibrosis or cirrhosis increases the urgency for treatment to avert associated complications.31
Thanks to radiologic guidance, the risk for liver biopsy-related complications has been reduced; less than 1% of patients, according to 2008 study results, required hospitalization because of postprocedural pain or bleeding32; these are risks for which patients should be prepared,1 in addition to possible perforation of the bowel or lung, bile leak, or hematoma formation.33 Less than 10% of patients require analgesia two hours after the procedure,34 although prophylactic analgesia (as with sublingual tramadol and oral lorazepam) has been found helpful in reducing pain and anxiety.35
TREATMENT/MANAGEMENT
According to information from the CDC and practice guidelines from the American Association for the Study of Liver Diseases (AASLD),1,2 early diagnosis of HCV and treatment with currently available interventions can lead to cures in 75% of cases; otherwise, serious, life-threatening complications can be expected. In patients treated for HCV infection, cure (or sustained virologic response) is defined by an undetectable viral load, 24 weeks after completion of therapy.1 Patients with these test results are no longer able to transmit the virus to others.
For the past decade, pegylated interferon (or peginterferon) combined with ribavirin has been considered the standard of care for HCV; treatment response rates, depending on race and genotype, can range from 35% to 80%.36,37 Two forms of peginterferon, alfa-2a and alfa-2b, are FDA approved for treatment of chronic hepatitis C; both forms are administered subcutaneously in combination with oral ribavirin.1,38
The choice between peginterferon alfa-2a and alfa-2b and the recommended duration of treatment are dependent on the patient's HCV genotype (see Table 21,39-45) and prior exposure to treatment. These complex regimens are best managed by specialists in gastroenterology, hepatology, or infectious disease—clinicians who are familiar with the agents' associated adverse effects, the elements of attentive monitoring, and protocols for dosing adjustments.46
As noted in the AASLD guidelines,1 alcohol consumption constitutes a risk for worsening fibrosis and possibly for HCC. Additionally, drinking in excess may facilitate replication of HCV RNA, interfering with therapy.
Thus, patients should be urged to discontinue alcohol use during treatment for HCV—or at least restrict it to an occasional drink.
New Agents to Improve Cure Rates
HCV genotype 1 accounts for 70% to 75% of cases in the US, but with current standard treatment, a sustained virologic response is achieved in only 45% to 50% of these patients37 (including 30% of black patients and 50% of whites).1,36,38 However, adding one of two new protease inhibitors to the current peginterferon/ribavirin regimen has the potential to increase sustained virologic response rates to as high as 90% to 95% in early virologic responders with genotype 1 infection, both treatment-naïve and previously treated; and in some patients, to shorten treatment to 24 weeks.41,42,44,45 Both boceprevir and telaprevir were approved by the FDA in May 2011.
Both agents are given for 12 weeks during standard peginterferon/ribavirin therapy. Depending on the patient's viral response, the overall regimen may be shortened or extended. Neither telaprevir nor boceprevir is administered as monotherapy,47,48 nor are they ever administered together. As with any protease inhibitor, resuming treatment after the agent has been stopped incurs a risk for drug resistance.40
Both new agents are taken every 7 to 9 hours, with a meal or snack.47,48 The telaprevir dose should be taken with non-low-fat food.47
Drug interactions are common. Patients also being treated for HIV and those being treated for HCV genotype 1 infection with either of the two new protease inhibitors must very carefully communicate any prescription drug changes to their treating provider. Substances of greatest concern act via the cytochrome P450 3A (CYP3A) pathway. Examples include ritonavir, St. John's wort, statins, and daily-dosed sildenafil (as used to treat patients with pulmonary hypertension).
Package inserts accompanying all agents used should be reviewed for monitoring parameters and associated recommendations.
Monitoring for Treatment Effectiveness and Complications
Patients are closely monitored for treatment effectiveness by repeated measurements of HCV RNA (ie, weeks 4, 12, 24, then at four- to 12-week intervals; at end of treatment; and 24 weeks after treatment ends). A viral load not detectable at week 4 indicates a good chance for a cure.1 If at week 12 the viral load remains detectable, the patient with HCV genotype 1 is unlikely to respond to triple therapy; similarly, dual therapy is not likely to produce a cure in patients with HCV genotype 2 or 3. Without a 2-log drop in viral load by week 12, the patient has only an 8% chance of achieving a sustained virologic response.49-51 In these cases, treatment should be discontinued or modified.51
Regular visits and frequent blood testing make it possible to avoid potential disease- and treatment-related complications. At a minimum, it is recommended that a CBC, serum creatinine, and ALT level be measured at weeks 2, 4, 8, 12, 16, 20, and 24 of therapy.1 If significant abnormalities are detected (eg, anemia, thrombocytopenia, neutropenia), the clinician may decide to monitor the patient more closely. Measures of liver and kidney function, electrolytes, and coagulation times are part of the recommended monitoring parameters at regular intervals. In addition, interferon therapy is associated with thyroid dysfunction, so relevant monitoring is advised every 12 weeks.1
Anemia, neutropenia, thrombocytopenia, and other hematologic complications are known adverse effects of HCV therapy—particularly in cirrhotic patients.1,52 Ribavirin use (especially aggressive use, as for patients with recurrent infection or post-liver transplantation53) is associated with hemolytic anemia,54 as is protease inhibitor use.41,45 In cases of profound anemia, dosing reduction or treatment discontinuation may be necessary. Severe cases of hemolysis may require transfusion, but treatment with epoietin alfa has been found helpful in some patients.52,53 Conventional iron supplementation is not an effective treatment for hemolytic anemia resulting from ribavirin use.
For patients with low absolute neutrophil counts, treatment dosing reduction was once standard management.45,52 More recently, the use of epoietin alfa or a granulocyte colony-stimulating factor (eg, filgrastim) has been found helpful in raising neutrophil counts.52
Intervention for thrombocytopenia is not typically undertaken unless the total platelet count indicates a risk for spontaneous bleeding. In some patients, a new oral thrombopoietin mimetic called eltrombopag may reduce the need for dose reduction or early termination of therapy.52
Ribavirin is a major teratogen. This agent is listed in pregnancy category X, and its label carries a black box warning advising patients to avoid pregnancy while undergoing therapy and for six months following therapy. It has such a high potential for teratogenicity that male patients are even advised not to impregnate their female partners while on therapy or six months following therapy.54 Pregnant women should never handle ribavirin. It is important to educate medical professionals and office staff about this risk.
For management of other adverse reactions to one or more components of HCV treatment, see Table 3.1,41,43,45,55-57
PATIENT EDUCATION/FOLLOW-UP DISCUSSION
Patient education is critical to successful treatment for HCV. Patients should be encouraged to avoid viral transmission to sexual partners by using condoms. They should also be advised to avoid sharing razors or toothbrushes with others in the home.1 Any patient with known HCV, HIV, or HBV should avoid undergoing body piercing or tattooing in any nonprofessional, nonsterile setting, where transmission may occur.5 Even successfully treated patients retain an HCV antibody and will test positive for HCV, making them ineligible to donate blood.
Six months after treatment, a viral load is drawn. If no active virus is found, patients will not need monitoring or follow-up labs unless they have known cirrhosis. Fibrosis is reversible in some patients following HCV treatment. However, if active disease is found at the six-month posttreatment follow-up visit, then the treatment was not effective, and the patient is considered a responder-relapser. Many such patients then consider enrolling in a drug trial or awaiting the potential approval of new treatments.
Herbal therapies, such as milk thistle and licorice root, have been widely tried by patients with HCV infection. The literature is mixed regarding the effectiveness of these substances or their potential for reducing liver function testing abnormalities. To date, no herbal compound has been proven to eradicate the virus. Most hepatologists encourage patients who take interferon, ribavirin, and/or protease inhibitors to avoid herbal or OTC substances with any potential for drug interactions or other complications.
ON THE HORIZON
Promising developments suggest a brightening future for patients with HCV infection. Researchers throughout the world are currently investigating new combinations of agents, effective against a wider range of genotypes and subtypes, that can control HCV infection without the emergence of drug-resistant strains. The near future may see more protease inhibitors added to current regimens and other direct-acting antivirals possibly replacing old treatment components. As is true of HIV, a vaccine for HCV will be difficult to develop; but in the meantime, new drug combinations will be created with a goal of sustained virologic response rates nearing 100% and ever-shorter treatment regimens.37,58
CONCLUSION
Since signs or symptoms are often not seen in patients infected with hepatitis C, it is important for primary care providers to question patients regarding known risk factors. At-risk patients should be screened using antibody testing, followed by confirmation of any positive results by HCV RNA testing. The patient's HCV genotype must be identified next, as it plays a key role in the therapeutic decisions to be made.
Because HCV therapy is complex, it is best managed by specialists who are experienced in all the aspects of each patient's regimen and who remain abreast of new therapeutic developments. Nevertheless, primary care providers can serve their patients well by acting as knowledgeable, supportive members of the health care team, accompanying their patients through the challenges of treatment and follow-up.
1. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
2. CDC. Hepatitis C FAQs for health professionals (2008). www.cdc.gov/hepatitis/HCV/HCVfaq. htm#section1. Accessed August 20, 2012.
3. Ly KN, Xing J, Klevens M, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271-278.
4. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2010 Annual Data Report (2011). Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation. www.srtr.org/ annual_reports/2010/pdf/00_intro_pgs_11.pdf. Accessed August 20, 2012.
5. Tohme RA, Holmberg SD. Transmission of hepatitis C virus infection through tattooing and piercing: a critical review. Clin Infect Dis. 2012;54(8):1167-1178.
6. CDC. Hepatitis C: proposed expansion of testing recommendations, 2012. www.cdc.gov/nchh-stp/newsroom/docs/HCV-TestingFactSheetNoEm-bargo508.pdf. Accessed August 20, 2012.
7. Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3(2):47-52.
8. Al-Ali J, Al-Mutari N, Ahmed el-SF. Hepatitis C virus and the skin. Hepatogastroenterology. 2011;58(107-108):880-886.
9. Raslan HM, Ezzat WM, Abd El Hamid MF, et al. Skin manifestations of chronic hepatitis C virus infection in Cairo, Egypt. East Mediterr Health J. 2009;15(3):692-700.
10. Niederau C, Hüppe D, Zehnter E, et al. Chronic hepatitis C: treat or wait? Medical decision making in clinical practice. World J Gastroenterol. 2012;18(12):1339-1347.
11. Mahboobi N, Agha-Hosseini F, Lankarani K. Hepatitis C virus and lichen planus: the real association. Hepat Mon. 2010;10(3):161-164.
12. Khokhar OS, Lewis JH. Reasons why patients infected with chronic hepatitis C virus choose to defer treatment: do they alter their decision with time? Dig Dis Sci. 2007;52(5):1168-1176.
13. Bruno S, Zuin M, Crosignani A, et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol. 2009;104(5):1147-1158.
14. Cheinquer N, Cheinquer H, Wolff FH, Coelho-Borges S. Effect of sustained virologic response on the incidence of hepatocellular carcinoma in patients with HCV cirrhosis. Braz J Infect Dis. 2010;14(5):457-461.
15. Veldt BJ, Chen W, Heathcote EJ, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47(6):1856-1862.
16. Nkontchou G, Ziol M, Aout M, et al. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011;18(10):e516-e522.
17. Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479-497.
18. Vujosevic S, Tempesta D, Noventa F, et al. Pegylated interferon-associated retinopathy is frequent in hepatitis C virus patients with hypertension and justifies ophthalmologic screening. Hepatology. 2012;56(2):455-463.
19. World Health Organization. Global alert and response: hepatitis D (2012). www.who.int/csr/ disease/hepatitis/whocdscsrncs20011/en/index3. html. Accessed August 20, 2012.
20. World Health Organization. Hepatitis E: fact sheet No 280 (July 2012). www.who.int/media centre/factsheets/fs280/en. Accessed August 20, 2012.
21. Bertino G, Ardiri AM, Bruno MC, et al. HAV infection in patients with chronic hepatitis C [in Italian]. Clin Ter. 2007;158(3):223-225.
22. Liu J, Hou J. Hepatitis B virus (HBV) and hepatitis C virus (HCV) dual infection. Int J Med Sci. 2006;3(2):57-62.
23. Schijman A, Colina R, Mukomolov S, et al. Comparison of hepatitis C viral loads in patients with or without coinfection with different genotypes. Clin Diagn Lab Immunol. 2004;11(2):433-435.
24. McClure JE, Shearer WT. Radioimmunoassay for anti-actin antibody: application in viral and autoimmune diseases. Mol Cell Probes. 1988;2(4):305-319.
25. Peakman M, Lobo-Yeo A, Mieli-Vergani, et al. Characterization of anti-liver kidney microsomal antibody in childhood autoimmune chronic active hepatitis: evidence for IgG1 subclass restriction, polyclonality and non cross-reactivity with hepatocyte surface antigens. Clin Exp Immunol. 1987;69(3):543-549.
26. Loko MA, Salmon D, Carrieri P, et al; ANRS CO 13 HEPAVIH Study Group. The French national prospective cohort of patients co-infected with HIV and HCV (ANRS CO13 HEPAVIH): early findings, 2006-2010. BMC Infect Dis. 2010 Oct 22;10:303.
27. Duclos-Vallée JC, Féray C, Sebagh M, et al; THEVIC Study Group. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2008;47(2):407-417.
28. Sato Y, Nakata K, Kato Y, et al. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med. 1993;328(25):1802-1806.
29. Gebo KA, Chander G, Jenckes MW, et al. Screening tests for hepatocellular carcinoma in patients with chronic hepatitis C: a systematic review. Hepatology. 2002;36(5 suppl 1):S84-S92.
30. Tateyama M, Yatsuhashi H, Taura N, et al. Alpha-fetoprotein above normal levels as a risk factor for the development of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol. 2011;46(1):92-100.
31. Bruno S, Shiffman ML, Roberts SK, et al. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology. 2010;51(2):388-397.
32. Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28(5):705-712.
33. van der Poorten D, Kwok A, Lam T, et al. Twenty-year audit of percutaneous liver biopsy in a major Australian teaching hospital. Intern Med J. 2006;36(11):692-699.
34. Howard R, Karageorge G, van Harselaar K, et al. Post-procedure surveillance in liver biopsy: how long is long enough? N Z Med J. 2008;121(1280):8-14.
35. Kramskay R, Tansky A, Eisenberg, et al. Prophylactic analgesia before percutaneous liver biopsy: a clinical comparative study. Eur J Gastroenterol Hepatol. 2011;23(9):782-786.
36. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
37. Lemon SM, McKeating JA, Pietschmann T, et al. Development of novel therapies for hepatitis C. Antiviral Res. 2010;86(1):79-92.
38. Shepherd J, Brodin H, Cave C, et al. Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2004;8(39):iii-iv, 1-125.
39. Kamal SM, El Kamary SS, Shardell MD, et al. Pegylated interferon alpha-2b plus ribavirin in patients with genotype 4 chronic hepatitis C: the role of rapid and early virologic response. Hepatology. 2007;46:1732-1740.
40. Hofmann WP, Sarrazin C, Zeuzem S. Current standards in the treatment of chronic hepatitis C. Dtsch Arztebl Int. 2012;109(19):352-358.
41. Kwo PY, Lawitz EJ, McCone J, et al; SPRINT-1 Investigators. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376(9742):705-716.
42. Klibanov OM, Vickery SB, Olin JL, et al. Boceprevir: a novel NS3/4 protease inhibitor for the treatment of hepatitis C. Pharmacotherapy. 2012;32(2):173-190.
43. Cunningham M, Foster GR. Efficacy and safety of telaprevir in patients with genotype 1 hepatitis C infection. Therap Adv Gastroenterol. 2012;5(2):139-151.
44. Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405-2416.
45. Zeuzem S, Andreone P, Pol S, et al; REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364(25):2417-2428.
46. Sagir A, Heintges T, Akyazi Z, et al. Therapy outcome in patients with chronic hepatitis C: role of therapy supervision by expert hepatologists. J Viral Hepat. 2007;14(9):633-638.
47. Highlights of prescribing information: INCIVEKTM (telaprevir) film coated tablets for oral use (2011). http://pi.vrtx.com/files/uspi_telaprevir. pdf. Accessed August 20, 2012.
48. Highlights of prescribing information: VICTRELISTM (boceprevir) capsules (2011). www .accessdata.fda.gov/drugsatfda_docs / label/2011/202258lbl.pdf. Accessed August 20, 2012.
49. Alavian SM, Tabatabaei SV, Behnava B, Mahboobi N. Optimal duration of treatment for HCV genotype 1 infection in slow responders: a meta-analysis. Hepat Mon. 2011;11(8):612-619.
50. Fried MW, Hadziyannis SJ, Shiffman ML, et al. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol. 2011;55(1):69-75.
51. Reau N, Satoskar R, Te H, et al. Evaluation of early null response to pegylated interferon and ribavirin as a predictor of therapeutic nonresponse in patients undergoing treatment for chronic hepatitis C. Am J Gastroenterol. 2011;106(3):452-458.
52. Mac Nicholas R, Norris S. Optimizing SVR and management of the haematological side effects of peginterferon/ribavirin antiviral therapy for HCV: the role of epoietin, G-CSF and novel agents. Aliment Pharmacol Ther. 2010;31(9):929-937.
53. Singhal A, Jain AB, Burke M, Black M. Aggressive use of ribavirin and prolonged course of peginterferon to improve the rate of viral response in liver transplant patients with recurrent hepatitis C viral infection. Exp Clin Transplant. 2010;8(3):214-219.
54. Highlights of prescribing information: COPEGUSTM (ribavirin) tablets (2002). http://www.gene .com/gene/products/information/pegasys/pdf/ copegus_pi.pdf. Accessed August 20, 2012.
55. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 suppl 1):S237-S244.
56. Dollarhide AW, Loh C, Leckband SG, et al. Psychiatric comorbidity does not predict interferon treatment completion rates in hepatitis C seropositive veterans. J Clin Gastroenterol. 2007;41(3):322-328.
57. Tavakoli-Tabasi S, Bagree A. A longitudinal cohort study of mucocutaneous drug eruptions during interferon and ribavirin treatment of hepatitis C. J Clin Gastroenterol. 2012;46(2):162-167.
58. Fox AN, Jacobson IM. Recent successes and noteworthy future prospects in the treatment of chronic hepatitis C. Clin Infect Dis. 2012;55 suppl 1:S16-S24.
The hepatitis C virus (HCV) is estimated to affect 180 million people worldwide, and the CDC estimates that approximately 3.2 million persons in the United States are chronically infected with HCV.1,2 In recent years, reported HCV-related deaths have outnumbered those attributed to HIV infection.3 Clinicians in almost any practice area are likely to encounter patients affected by HCV.
Infection with HCV is a major risk factor for cirrhosis, a disease associated with significant morbidity and mortality; HCV-associated cirrhosis is considered the leading cause of liver transplantation.4
Screening for HCV is important in patients with known risk factors for the disease1 (see Table 11,2,5). Of note, however, the CDC is in the process of expanding its recommendations to one-time screening for all Americans born between 1945 and 1965—an age-group that accounts for more than 75% of cases of HCV infection among US adults.6 (Clinicians interested in viewing the draft document for public comment can refer to www.regulations.gov, docket #CDC-2-12-0005.)
PATIENT PRESENTATION/ PATIENT HISTORY
Most patients with hepatitis C present without signs or symptoms of their illness. If symptoms are present, they may include fatigue, pruritus, abdominal pain/ discomfort, arthralgias, or anorexia; results on routine liver function tests may be abnormal.1,7-9 Liver function may appear normal in patients with HCV, although 30% of patients with a normal alanine aminotransferase (ALT) level may have significant fibrosis.10 Lichen planus is commonly associated with HCV infection,9-11 and patients with this condition should be screened for HCV.
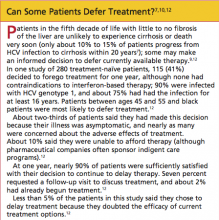
HCV infection most commonly presents between the fourth and sixth decades of life. Many patients have had the disease for as long as 20 years by the time they present for treatment—often after abnormal laboratory findings are discovered7 (but see "Can Some Patients Defer Treatment?"7,10,12).
Patients with acute HCV infection usually do not appear jaundiced or exhibit other signs of acute hepatitis. Symptomatic illness occurs in only 20% to 40% of patients with acute hepatitis C.7 Patients who present with acute illness (15% to 25% of patients with HCV) typically have an improved prognosis and are less likely to convert to chronicity if they survive the initial symptoms (ie, malaise, weakness, anorexia, jaundice).2,7 In many such patients, the body appears to mount a full immune response, and patients are often virus-free within weeks.
For 75% to 85% of patients, however, it is believed that the immune system fails to overcome the virus, and chronic infection, with progressive damage to the liver tissue, ensues.7 In chronic HCV infection, the rate of progression varies, depending on the HCV genotype, the infected host's genetic factors and lifestyle (including level of alcohol consumption), the extent of liver injury, and possible coinfection (as with HIV or hepatitis B virus [HBV]).1,7
Cirrhosis and Hepatocellular Carcinoma
Complications of cirrhosis include portal hypertension, ascites, hepatic encephalopathy, esophageal varices, and hepatocellular carcinoma (HCC).13 In patients with HCV-related cirrhosis, HCC develops at a rate of 1% to 4% per year, with a twofold to fourfold increased risk among black patients and Asian patients, respectively, compared with whites.7 The risk for HCC appears to be reduced in patients who undergo treatment leading to a sustained virologic response (ie, a viral load that is no longer detectable); and the risk is increased in patients with diabetes mellitus and those with HCV genotypes 1b and 3.14-16
HCC is difficult to treat unless detected in its early stages. It often results in death.13
PHYSICAL EXAMINATION
An appropriate physical exam is critical in detecting sequelae of chronic liver disease, which may reflect complications of long-term HCV infection. The patient should be evaluated for the presence of spider angiomas, palmar erythema, scleral icterus, ascites, caput medusae, and evidence of umbilical hernias—all possible signs of advanced liver disease.8,9
The initial physical exam is also an appropriate time to screen and treat patients for hypertension and diabetes, and to identify disorders that may make them poor candidates for HCV treatment. These conditions include coronary heart disease, untreated cancers or thyroid disease, kidney or autoimmune diseases, and psychiatric illness.1,17
Evaluation of the skin for "track marks," tattoos or body piercings that may have been applied in prisons, homes, or other nonsterile settings, or nonhealing lesions that may indicate immune compromise or diabetes is important.5,8 Visual acuity testing and fundoscopic exams are critical to establish a baseline, because treatment with pegylated interferon has the potential to cause visual changes and retinopathy.18
Laboratory Work-up
Initial testing for HCV includes an HCV antibody test. This serum test is commonly performed as part of a hepatitis panel—testing for hepatitis A virus (HAV), HBV, and HCV. Testing for hepatitis D and E is not routinely recommended unless the patient routinely travels to the Mediterranean Basin, the Middle East, Central Asia, or West Africa (where hepatitis D is most prevalent19) or the patient is pregnant (because during the third trimester, hepatitis E infection carries a mortality rate of 20% and can be transmitted to the fetus20).
If immunity to HAV and HBV are not shown on laboratory findings, it is critical to vaccinate the HCV-positive patient against these diseases. Coinfection with HAV and HCV can progress to a fulminant form of hepatitis and poor hepatic function; coinfection with HBV and HCV is associated with severe liver disease and an increased risk for HCC.21,22
The patient with positive results on the HCV antibody screen should be tested next with a quantitative HCV RNA assay to confirm the presence of active infection (ie, viral load).1 Detection of virus is considered indicative of active disease.
It is possible for a patient with positive HCV antibody test results to have no circulating virus detected on HCV RNA testing. Perhaps 15% to 25% of patients are able to clear HCV infection spontaneously within weeks to months after infection.7
Testing Considerations Before Treatment
Several assays are available to identify the patient's HCV genotype. It is essential to obtain this information before agents, dosing, and duration are determined, as the regimen will be tailored to the patient's HCV genotype.1 There are six major known HCV genotypes (numbered 1 through 6) and at least 50 minor subtypes (eg, 1a, 2b, 3a).1,23
Also to be considered before initiating HCV treatment is screening for autoimmune hepatitis.17 Appropriate tests include the anti-actin antibody, anti-smooth muscle antibody, antinuclear antibody, and anti-liver kidney microsomal antibody tests.24,25 Additionally, ceruloplasmin testing should be ordered along with the iron studies and ferritin levels obtained at the patient's first visit, to screen for Wilson's disease, a disorder of copper metabolism, and hemochromatosis, a disorder of iron metabolism.
The risks associated with HAV or HBV coinfection have been described; patients with HCV and HAV, HBV, or HIV are at greater risk for disease progression to cirrhosis and/or HCC than are mono-infected patients.1,21,22,26 HIV testing is routinely recommended for patients with HCV, with repeat testing during treatment if the patient maintains any risk factors for HIV exposure. Patients with HCV who are found to be coinfected with HIV must be treated urgently, but therapeutic components must be selected with considerable care: Serious interactions are possible among the effective agents, and antiretroviral drugs are associated with hepatotoxicity.1,27
Before HCV treatment begins, it is essential to obtain baseline values: liver function testing (aspartate aminotransferase, ALT, alkaline phosphatase, bilirubin), coagulation testing (prothrombin time and partial thromboplastin time), and thyroid testing (thyroid-stimulating hormone, triiodothyronine, thyroxine),1 as changes may occur as a result of anti-HCV therapy. It is also important to establish a baseline complete blood count (CBC) and electrolyte levels in order to identify, and later monitor, the presence of anemia, thrombocytopenia, and renal or other abnormalities.
Finally, it is important to screen the patient early for HCC by testing for serum alphafetoprotein (AFP, ie, tumor marker).28 Serum AFP must then be monitored at six-month intervals (with a recommended threshold of 400 ng/mL, but even readings as modest as 6 to 20 ng/mL) for elevations that may indicate HCC.29,30
Liver Studies
In addition to the recommended AFP monitoring, the patient should undergo an imaging study of the liver twice yearly. Ultrasound is the most cost-effective screening tool for most patients (with high specificity but varying sensitivity); when combined with a serum AFP reading of 10 ng/mL or greater, ultrasound has a sensitivity of 100% for detection of HCC.29 However, if lesions are found, or if a patient has known cirrhosis, CT or MRI is more accurate in detecting small lesions of the liver (specificity, 100%).29
Liver biopsy is recommended for certain patients to stage liver fibrosis; biopsy may not be necessary, for example, in patients with genotypes 2 and 3, as 80% of these patients react to standard therapy with sustained virologic response.1 Detection of advanced fibrosis or cirrhosis increases the urgency for treatment to avert associated complications.31
Thanks to radiologic guidance, the risk for liver biopsy-related complications has been reduced; less than 1% of patients, according to 2008 study results, required hospitalization because of postprocedural pain or bleeding32; these are risks for which patients should be prepared,1 in addition to possible perforation of the bowel or lung, bile leak, or hematoma formation.33 Less than 10% of patients require analgesia two hours after the procedure,34 although prophylactic analgesia (as with sublingual tramadol and oral lorazepam) has been found helpful in reducing pain and anxiety.35
TREATMENT/MANAGEMENT
According to information from the CDC and practice guidelines from the American Association for the Study of Liver Diseases (AASLD),1,2 early diagnosis of HCV and treatment with currently available interventions can lead to cures in 75% of cases; otherwise, serious, life-threatening complications can be expected. In patients treated for HCV infection, cure (or sustained virologic response) is defined by an undetectable viral load, 24 weeks after completion of therapy.1 Patients with these test results are no longer able to transmit the virus to others.
For the past decade, pegylated interferon (or peginterferon) combined with ribavirin has been considered the standard of care for HCV; treatment response rates, depending on race and genotype, can range from 35% to 80%.36,37 Two forms of peginterferon, alfa-2a and alfa-2b, are FDA approved for treatment of chronic hepatitis C; both forms are administered subcutaneously in combination with oral ribavirin.1,38
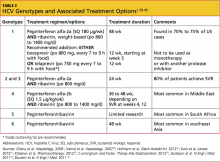
The choice between peginterferon alfa-2a and alfa-2b and the recommended duration of treatment are dependent on the patient's HCV genotype (see Table 21,39-45) and prior exposure to treatment. These complex regimens are best managed by specialists in gastroenterology, hepatology, or infectious disease—clinicians who are familiar with the agents' associated adverse effects, the elements of attentive monitoring, and protocols for dosing adjustments.46
As noted in the AASLD guidelines,1 alcohol consumption constitutes a risk for worsening fibrosis and possibly for HCC. Additionally, drinking in excess may facilitate replication of HCV RNA, interfering with therapy.
Thus, patients should be urged to discontinue alcohol use during treatment for HCV—or at least restrict it to an occasional drink.
New Agents to Improve Cure Rates
HCV genotype 1 accounts for 70% to 75% of cases in the US, but with current standard treatment, a sustained virologic response is achieved in only 45% to 50% of these patients37 (including 30% of black patients and 50% of whites).1,36,38 However, adding one of two new protease inhibitors to the current peginterferon/ribavirin regimen has the potential to increase sustained virologic response rates to as high as 90% to 95% in early virologic responders with genotype 1 infection, both treatment-naïve and previously treated; and in some patients, to shorten treatment to 24 weeks.41,42,44,45 Both boceprevir and telaprevir were approved by the FDA in May 2011.
Both agents are given for 12 weeks during standard peginterferon/ribavirin therapy. Depending on the patient's viral response, the overall regimen may be shortened or extended. Neither telaprevir nor boceprevir is administered as monotherapy,47,48 nor are they ever administered together. As with any protease inhibitor, resuming treatment after the agent has been stopped incurs a risk for drug resistance.40
Both new agents are taken every 7 to 9 hours, with a meal or snack.47,48 The telaprevir dose should be taken with non-low-fat food.47
Drug interactions are common. Patients also being treated for HIV and those being treated for HCV genotype 1 infection with either of the two new protease inhibitors must very carefully communicate any prescription drug changes to their treating provider. Substances of greatest concern act via the cytochrome P450 3A (CYP3A) pathway. Examples include ritonavir, St. John's wort, statins, and daily-dosed sildenafil (as used to treat patients with pulmonary hypertension).
Package inserts accompanying all agents used should be reviewed for monitoring parameters and associated recommendations.
Monitoring for Treatment Effectiveness and Complications
Patients are closely monitored for treatment effectiveness by repeated measurements of HCV RNA (ie, weeks 4, 12, 24, then at four- to 12-week intervals; at end of treatment; and 24 weeks after treatment ends). A viral load not detectable at week 4 indicates a good chance for a cure.1 If at week 12 the viral load remains detectable, the patient with HCV genotype 1 is unlikely to respond to triple therapy; similarly, dual therapy is not likely to produce a cure in patients with HCV genotype 2 or 3. Without a 2-log drop in viral load by week 12, the patient has only an 8% chance of achieving a sustained virologic response.49-51 In these cases, treatment should be discontinued or modified.51
Regular visits and frequent blood testing make it possible to avoid potential disease- and treatment-related complications. At a minimum, it is recommended that a CBC, serum creatinine, and ALT level be measured at weeks 2, 4, 8, 12, 16, 20, and 24 of therapy.1 If significant abnormalities are detected (eg, anemia, thrombocytopenia, neutropenia), the clinician may decide to monitor the patient more closely. Measures of liver and kidney function, electrolytes, and coagulation times are part of the recommended monitoring parameters at regular intervals. In addition, interferon therapy is associated with thyroid dysfunction, so relevant monitoring is advised every 12 weeks.1
Anemia, neutropenia, thrombocytopenia, and other hematologic complications are known adverse effects of HCV therapy—particularly in cirrhotic patients.1,52 Ribavirin use (especially aggressive use, as for patients with recurrent infection or post-liver transplantation53) is associated with hemolytic anemia,54 as is protease inhibitor use.41,45 In cases of profound anemia, dosing reduction or treatment discontinuation may be necessary. Severe cases of hemolysis may require transfusion, but treatment with epoietin alfa has been found helpful in some patients.52,53 Conventional iron supplementation is not an effective treatment for hemolytic anemia resulting from ribavirin use.
For patients with low absolute neutrophil counts, treatment dosing reduction was once standard management.45,52 More recently, the use of epoietin alfa or a granulocyte colony-stimulating factor (eg, filgrastim) has been found helpful in raising neutrophil counts.52
Intervention for thrombocytopenia is not typically undertaken unless the total platelet count indicates a risk for spontaneous bleeding. In some patients, a new oral thrombopoietin mimetic called eltrombopag may reduce the need for dose reduction or early termination of therapy.52
Ribavirin is a major teratogen. This agent is listed in pregnancy category X, and its label carries a black box warning advising patients to avoid pregnancy while undergoing therapy and for six months following therapy. It has such a high potential for teratogenicity that male patients are even advised not to impregnate their female partners while on therapy or six months following therapy.54 Pregnant women should never handle ribavirin. It is important to educate medical professionals and office staff about this risk.
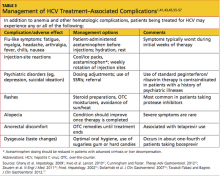
For management of other adverse reactions to one or more components of HCV treatment, see Table 3.1,41,43,45,55-57
PATIENT EDUCATION/FOLLOW-UP DISCUSSION
Patient education is critical to successful treatment for HCV. Patients should be encouraged to avoid viral transmission to sexual partners by using condoms. They should also be advised to avoid sharing razors or toothbrushes with others in the home.1 Any patient with known HCV, HIV, or HBV should avoid undergoing body piercing or tattooing in any nonprofessional, nonsterile setting, where transmission may occur.5 Even successfully treated patients retain an HCV antibody and will test positive for HCV, making them ineligible to donate blood.
Six months after treatment, a viral load is drawn. If no active virus is found, patients will not need monitoring or follow-up labs unless they have known cirrhosis. Fibrosis is reversible in some patients following HCV treatment. However, if active disease is found at the six-month posttreatment follow-up visit, then the treatment was not effective, and the patient is considered a responder-relapser. Many such patients then consider enrolling in a drug trial or awaiting the potential approval of new treatments.
Herbal therapies, such as milk thistle and licorice root, have been widely tried by patients with HCV infection. The literature is mixed regarding the effectiveness of these substances or their potential for reducing liver function testing abnormalities. To date, no herbal compound has been proven to eradicate the virus. Most hepatologists encourage patients who take interferon, ribavirin, and/or protease inhibitors to avoid herbal or OTC substances with any potential for drug interactions or other complications.
ON THE HORIZON
Promising developments suggest a brightening future for patients with HCV infection. Researchers throughout the world are currently investigating new combinations of agents, effective against a wider range of genotypes and subtypes, that can control HCV infection without the emergence of drug-resistant strains. The near future may see more protease inhibitors added to current regimens and other direct-acting antivirals possibly replacing old treatment components. As is true of HIV, a vaccine for HCV will be difficult to develop; but in the meantime, new drug combinations will be created with a goal of sustained virologic response rates nearing 100% and ever-shorter treatment regimens.37,58
CONCLUSION
Since signs or symptoms are often not seen in patients infected with hepatitis C, it is important for primary care providers to question patients regarding known risk factors. At-risk patients should be screened using antibody testing, followed by confirmation of any positive results by HCV RNA testing. The patient's HCV genotype must be identified next, as it plays a key role in the therapeutic decisions to be made.
Because HCV therapy is complex, it is best managed by specialists who are experienced in all the aspects of each patient's regimen and who remain abreast of new therapeutic developments. Nevertheless, primary care providers can serve their patients well by acting as knowledgeable, supportive members of the health care team, accompanying their patients through the challenges of treatment and follow-up.
The hepatitis C virus (HCV) is estimated to affect 180 million people worldwide, and the CDC estimates that approximately 3.2 million persons in the United States are chronically infected with HCV.1,2 In recent years, reported HCV-related deaths have outnumbered those attributed to HIV infection.3 Clinicians in almost any practice area are likely to encounter patients affected by HCV.
Infection with HCV is a major risk factor for cirrhosis, a disease associated with significant morbidity and mortality; HCV-associated cirrhosis is considered the leading cause of liver transplantation.4
Screening for HCV is important in patients with known risk factors for the disease1 (see Table 11,2,5). Of note, however, the CDC is in the process of expanding its recommendations to one-time screening for all Americans born between 1945 and 1965—an age-group that accounts for more than 75% of cases of HCV infection among US adults.6 (Clinicians interested in viewing the draft document for public comment can refer to www.regulations.gov, docket #CDC-2-12-0005.)
PATIENT PRESENTATION/ PATIENT HISTORY
Most patients with hepatitis C present without signs or symptoms of their illness. If symptoms are present, they may include fatigue, pruritus, abdominal pain/ discomfort, arthralgias, or anorexia; results on routine liver function tests may be abnormal.1,7-9 Liver function may appear normal in patients with HCV, although 30% of patients with a normal alanine aminotransferase (ALT) level may have significant fibrosis.10 Lichen planus is commonly associated with HCV infection,9-11 and patients with this condition should be screened for HCV.
HCV infection most commonly presents between the fourth and sixth decades of life. Many patients have had the disease for as long as 20 years by the time they present for treatment—often after abnormal laboratory findings are discovered7 (but see "Can Some Patients Defer Treatment?"7,10,12).
Patients with acute HCV infection usually do not appear jaundiced or exhibit other signs of acute hepatitis. Symptomatic illness occurs in only 20% to 40% of patients with acute hepatitis C.7 Patients who present with acute illness (15% to 25% of patients with HCV) typically have an improved prognosis and are less likely to convert to chronicity if they survive the initial symptoms (ie, malaise, weakness, anorexia, jaundice).2,7 In many such patients, the body appears to mount a full immune response, and patients are often virus-free within weeks.
For 75% to 85% of patients, however, it is believed that the immune system fails to overcome the virus, and chronic infection, with progressive damage to the liver tissue, ensues.7 In chronic HCV infection, the rate of progression varies, depending on the HCV genotype, the infected host's genetic factors and lifestyle (including level of alcohol consumption), the extent of liver injury, and possible coinfection (as with HIV or hepatitis B virus [HBV]).1,7
Cirrhosis and Hepatocellular Carcinoma
Complications of cirrhosis include portal hypertension, ascites, hepatic encephalopathy, esophageal varices, and hepatocellular carcinoma (HCC).13 In patients with HCV-related cirrhosis, HCC develops at a rate of 1% to 4% per year, with a twofold to fourfold increased risk among black patients and Asian patients, respectively, compared with whites.7 The risk for HCC appears to be reduced in patients who undergo treatment leading to a sustained virologic response (ie, a viral load that is no longer detectable); and the risk is increased in patients with diabetes mellitus and those with HCV genotypes 1b and 3.14-16
HCC is difficult to treat unless detected in its early stages. It often results in death.13
PHYSICAL EXAMINATION
An appropriate physical exam is critical in detecting sequelae of chronic liver disease, which may reflect complications of long-term HCV infection. The patient should be evaluated for the presence of spider angiomas, palmar erythema, scleral icterus, ascites, caput medusae, and evidence of umbilical hernias—all possible signs of advanced liver disease.8,9
The initial physical exam is also an appropriate time to screen and treat patients for hypertension and diabetes, and to identify disorders that may make them poor candidates for HCV treatment. These conditions include coronary heart disease, untreated cancers or thyroid disease, kidney or autoimmune diseases, and psychiatric illness.1,17
Evaluation of the skin for "track marks," tattoos or body piercings that may have been applied in prisons, homes, or other nonsterile settings, or nonhealing lesions that may indicate immune compromise or diabetes is important.5,8 Visual acuity testing and fundoscopic exams are critical to establish a baseline, because treatment with pegylated interferon has the potential to cause visual changes and retinopathy.18
Laboratory Work-up
Initial testing for HCV includes an HCV antibody test. This serum test is commonly performed as part of a hepatitis panel—testing for hepatitis A virus (HAV), HBV, and HCV. Testing for hepatitis D and E is not routinely recommended unless the patient routinely travels to the Mediterranean Basin, the Middle East, Central Asia, or West Africa (where hepatitis D is most prevalent19) or the patient is pregnant (because during the third trimester, hepatitis E infection carries a mortality rate of 20% and can be transmitted to the fetus20).
If immunity to HAV and HBV are not shown on laboratory findings, it is critical to vaccinate the HCV-positive patient against these diseases. Coinfection with HAV and HCV can progress to a fulminant form of hepatitis and poor hepatic function; coinfection with HBV and HCV is associated with severe liver disease and an increased risk for HCC.21,22
The patient with positive results on the HCV antibody screen should be tested next with a quantitative HCV RNA assay to confirm the presence of active infection (ie, viral load).1 Detection of virus is considered indicative of active disease.
It is possible for a patient with positive HCV antibody test results to have no circulating virus detected on HCV RNA testing. Perhaps 15% to 25% of patients are able to clear HCV infection spontaneously within weeks to months after infection.7
Testing Considerations Before Treatment
Several assays are available to identify the patient's HCV genotype. It is essential to obtain this information before agents, dosing, and duration are determined, as the regimen will be tailored to the patient's HCV genotype.1 There are six major known HCV genotypes (numbered 1 through 6) and at least 50 minor subtypes (eg, 1a, 2b, 3a).1,23
Also to be considered before initiating HCV treatment is screening for autoimmune hepatitis.17 Appropriate tests include the anti-actin antibody, anti-smooth muscle antibody, antinuclear antibody, and anti-liver kidney microsomal antibody tests.24,25 Additionally, ceruloplasmin testing should be ordered along with the iron studies and ferritin levels obtained at the patient's first visit, to screen for Wilson's disease, a disorder of copper metabolism, and hemochromatosis, a disorder of iron metabolism.
The risks associated with HAV or HBV coinfection have been described; patients with HCV and HAV, HBV, or HIV are at greater risk for disease progression to cirrhosis and/or HCC than are mono-infected patients.1,21,22,26 HIV testing is routinely recommended for patients with HCV, with repeat testing during treatment if the patient maintains any risk factors for HIV exposure. Patients with HCV who are found to be coinfected with HIV must be treated urgently, but therapeutic components must be selected with considerable care: Serious interactions are possible among the effective agents, and antiretroviral drugs are associated with hepatotoxicity.1,27
Before HCV treatment begins, it is essential to obtain baseline values: liver function testing (aspartate aminotransferase, ALT, alkaline phosphatase, bilirubin), coagulation testing (prothrombin time and partial thromboplastin time), and thyroid testing (thyroid-stimulating hormone, triiodothyronine, thyroxine),1 as changes may occur as a result of anti-HCV therapy. It is also important to establish a baseline complete blood count (CBC) and electrolyte levels in order to identify, and later monitor, the presence of anemia, thrombocytopenia, and renal or other abnormalities.
Finally, it is important to screen the patient early for HCC by testing for serum alphafetoprotein (AFP, ie, tumor marker).28 Serum AFP must then be monitored at six-month intervals (with a recommended threshold of 400 ng/mL, but even readings as modest as 6 to 20 ng/mL) for elevations that may indicate HCC.29,30
Liver Studies
In addition to the recommended AFP monitoring, the patient should undergo an imaging study of the liver twice yearly. Ultrasound is the most cost-effective screening tool for most patients (with high specificity but varying sensitivity); when combined with a serum AFP reading of 10 ng/mL or greater, ultrasound has a sensitivity of 100% for detection of HCC.29 However, if lesions are found, or if a patient has known cirrhosis, CT or MRI is more accurate in detecting small lesions of the liver (specificity, 100%).29
Liver biopsy is recommended for certain patients to stage liver fibrosis; biopsy may not be necessary, for example, in patients with genotypes 2 and 3, as 80% of these patients react to standard therapy with sustained virologic response.1 Detection of advanced fibrosis or cirrhosis increases the urgency for treatment to avert associated complications.31
Thanks to radiologic guidance, the risk for liver biopsy-related complications has been reduced; less than 1% of patients, according to 2008 study results, required hospitalization because of postprocedural pain or bleeding32; these are risks for which patients should be prepared,1 in addition to possible perforation of the bowel or lung, bile leak, or hematoma formation.33 Less than 10% of patients require analgesia two hours after the procedure,34 although prophylactic analgesia (as with sublingual tramadol and oral lorazepam) has been found helpful in reducing pain and anxiety.35
TREATMENT/MANAGEMENT
According to information from the CDC and practice guidelines from the American Association for the Study of Liver Diseases (AASLD),1,2 early diagnosis of HCV and treatment with currently available interventions can lead to cures in 75% of cases; otherwise, serious, life-threatening complications can be expected. In patients treated for HCV infection, cure (or sustained virologic response) is defined by an undetectable viral load, 24 weeks after completion of therapy.1 Patients with these test results are no longer able to transmit the virus to others.
For the past decade, pegylated interferon (or peginterferon) combined with ribavirin has been considered the standard of care for HCV; treatment response rates, depending on race and genotype, can range from 35% to 80%.36,37 Two forms of peginterferon, alfa-2a and alfa-2b, are FDA approved for treatment of chronic hepatitis C; both forms are administered subcutaneously in combination with oral ribavirin.1,38
The choice between peginterferon alfa-2a and alfa-2b and the recommended duration of treatment are dependent on the patient's HCV genotype (see Table 21,39-45) and prior exposure to treatment. These complex regimens are best managed by specialists in gastroenterology, hepatology, or infectious disease—clinicians who are familiar with the agents' associated adverse effects, the elements of attentive monitoring, and protocols for dosing adjustments.46
As noted in the AASLD guidelines,1 alcohol consumption constitutes a risk for worsening fibrosis and possibly for HCC. Additionally, drinking in excess may facilitate replication of HCV RNA, interfering with therapy.
Thus, patients should be urged to discontinue alcohol use during treatment for HCV—or at least restrict it to an occasional drink.
New Agents to Improve Cure Rates
HCV genotype 1 accounts for 70% to 75% of cases in the US, but with current standard treatment, a sustained virologic response is achieved in only 45% to 50% of these patients37 (including 30% of black patients and 50% of whites).1,36,38 However, adding one of two new protease inhibitors to the current peginterferon/ribavirin regimen has the potential to increase sustained virologic response rates to as high as 90% to 95% in early virologic responders with genotype 1 infection, both treatment-naïve and previously treated; and in some patients, to shorten treatment to 24 weeks.41,42,44,45 Both boceprevir and telaprevir were approved by the FDA in May 2011.
Both agents are given for 12 weeks during standard peginterferon/ribavirin therapy. Depending on the patient's viral response, the overall regimen may be shortened or extended. Neither telaprevir nor boceprevir is administered as monotherapy,47,48 nor are they ever administered together. As with any protease inhibitor, resuming treatment after the agent has been stopped incurs a risk for drug resistance.40
Both new agents are taken every 7 to 9 hours, with a meal or snack.47,48 The telaprevir dose should be taken with non-low-fat food.47
Drug interactions are common. Patients also being treated for HIV and those being treated for HCV genotype 1 infection with either of the two new protease inhibitors must very carefully communicate any prescription drug changes to their treating provider. Substances of greatest concern act via the cytochrome P450 3A (CYP3A) pathway. Examples include ritonavir, St. John's wort, statins, and daily-dosed sildenafil (as used to treat patients with pulmonary hypertension).
Package inserts accompanying all agents used should be reviewed for monitoring parameters and associated recommendations.
Monitoring for Treatment Effectiveness and Complications
Patients are closely monitored for treatment effectiveness by repeated measurements of HCV RNA (ie, weeks 4, 12, 24, then at four- to 12-week intervals; at end of treatment; and 24 weeks after treatment ends). A viral load not detectable at week 4 indicates a good chance for a cure.1 If at week 12 the viral load remains detectable, the patient with HCV genotype 1 is unlikely to respond to triple therapy; similarly, dual therapy is not likely to produce a cure in patients with HCV genotype 2 or 3. Without a 2-log drop in viral load by week 12, the patient has only an 8% chance of achieving a sustained virologic response.49-51 In these cases, treatment should be discontinued or modified.51
Regular visits and frequent blood testing make it possible to avoid potential disease- and treatment-related complications. At a minimum, it is recommended that a CBC, serum creatinine, and ALT level be measured at weeks 2, 4, 8, 12, 16, 20, and 24 of therapy.1 If significant abnormalities are detected (eg, anemia, thrombocytopenia, neutropenia), the clinician may decide to monitor the patient more closely. Measures of liver and kidney function, electrolytes, and coagulation times are part of the recommended monitoring parameters at regular intervals. In addition, interferon therapy is associated with thyroid dysfunction, so relevant monitoring is advised every 12 weeks.1
Anemia, neutropenia, thrombocytopenia, and other hematologic complications are known adverse effects of HCV therapy—particularly in cirrhotic patients.1,52 Ribavirin use (especially aggressive use, as for patients with recurrent infection or post-liver transplantation53) is associated with hemolytic anemia,54 as is protease inhibitor use.41,45 In cases of profound anemia, dosing reduction or treatment discontinuation may be necessary. Severe cases of hemolysis may require transfusion, but treatment with epoietin alfa has been found helpful in some patients.52,53 Conventional iron supplementation is not an effective treatment for hemolytic anemia resulting from ribavirin use.
For patients with low absolute neutrophil counts, treatment dosing reduction was once standard management.45,52 More recently, the use of epoietin alfa or a granulocyte colony-stimulating factor (eg, filgrastim) has been found helpful in raising neutrophil counts.52
Intervention for thrombocytopenia is not typically undertaken unless the total platelet count indicates a risk for spontaneous bleeding. In some patients, a new oral thrombopoietin mimetic called eltrombopag may reduce the need for dose reduction or early termination of therapy.52
Ribavirin is a major teratogen. This agent is listed in pregnancy category X, and its label carries a black box warning advising patients to avoid pregnancy while undergoing therapy and for six months following therapy. It has such a high potential for teratogenicity that male patients are even advised not to impregnate their female partners while on therapy or six months following therapy.54 Pregnant women should never handle ribavirin. It is important to educate medical professionals and office staff about this risk.
For management of other adverse reactions to one or more components of HCV treatment, see Table 3.1,41,43,45,55-57
PATIENT EDUCATION/FOLLOW-UP DISCUSSION
Patient education is critical to successful treatment for HCV. Patients should be encouraged to avoid viral transmission to sexual partners by using condoms. They should also be advised to avoid sharing razors or toothbrushes with others in the home.1 Any patient with known HCV, HIV, or HBV should avoid undergoing body piercing or tattooing in any nonprofessional, nonsterile setting, where transmission may occur.5 Even successfully treated patients retain an HCV antibody and will test positive for HCV, making them ineligible to donate blood.
Six months after treatment, a viral load is drawn. If no active virus is found, patients will not need monitoring or follow-up labs unless they have known cirrhosis. Fibrosis is reversible in some patients following HCV treatment. However, if active disease is found at the six-month posttreatment follow-up visit, then the treatment was not effective, and the patient is considered a responder-relapser. Many such patients then consider enrolling in a drug trial or awaiting the potential approval of new treatments.
Herbal therapies, such as milk thistle and licorice root, have been widely tried by patients with HCV infection. The literature is mixed regarding the effectiveness of these substances or their potential for reducing liver function testing abnormalities. To date, no herbal compound has been proven to eradicate the virus. Most hepatologists encourage patients who take interferon, ribavirin, and/or protease inhibitors to avoid herbal or OTC substances with any potential for drug interactions or other complications.
ON THE HORIZON
Promising developments suggest a brightening future for patients with HCV infection. Researchers throughout the world are currently investigating new combinations of agents, effective against a wider range of genotypes and subtypes, that can control HCV infection without the emergence of drug-resistant strains. The near future may see more protease inhibitors added to current regimens and other direct-acting antivirals possibly replacing old treatment components. As is true of HIV, a vaccine for HCV will be difficult to develop; but in the meantime, new drug combinations will be created with a goal of sustained virologic response rates nearing 100% and ever-shorter treatment regimens.37,58
CONCLUSION
Since signs or symptoms are often not seen in patients infected with hepatitis C, it is important for primary care providers to question patients regarding known risk factors. At-risk patients should be screened using antibody testing, followed by confirmation of any positive results by HCV RNA testing. The patient's HCV genotype must be identified next, as it plays a key role in the therapeutic decisions to be made.
Because HCV therapy is complex, it is best managed by specialists who are experienced in all the aspects of each patient's regimen and who remain abreast of new therapeutic developments. Nevertheless, primary care providers can serve their patients well by acting as knowledgeable, supportive members of the health care team, accompanying their patients through the challenges of treatment and follow-up.
1. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
2. CDC. Hepatitis C FAQs for health professionals (2008). www.cdc.gov/hepatitis/HCV/HCVfaq. htm#section1. Accessed August 20, 2012.
3. Ly KN, Xing J, Klevens M, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271-278.
4. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2010 Annual Data Report (2011). Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation. www.srtr.org/ annual_reports/2010/pdf/00_intro_pgs_11.pdf. Accessed August 20, 2012.
5. Tohme RA, Holmberg SD. Transmission of hepatitis C virus infection through tattooing and piercing: a critical review. Clin Infect Dis. 2012;54(8):1167-1178.
6. CDC. Hepatitis C: proposed expansion of testing recommendations, 2012. www.cdc.gov/nchh-stp/newsroom/docs/HCV-TestingFactSheetNoEm-bargo508.pdf. Accessed August 20, 2012.
7. Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3(2):47-52.
8. Al-Ali J, Al-Mutari N, Ahmed el-SF. Hepatitis C virus and the skin. Hepatogastroenterology. 2011;58(107-108):880-886.
9. Raslan HM, Ezzat WM, Abd El Hamid MF, et al. Skin manifestations of chronic hepatitis C virus infection in Cairo, Egypt. East Mediterr Health J. 2009;15(3):692-700.
10. Niederau C, Hüppe D, Zehnter E, et al. Chronic hepatitis C: treat or wait? Medical decision making in clinical practice. World J Gastroenterol. 2012;18(12):1339-1347.
11. Mahboobi N, Agha-Hosseini F, Lankarani K. Hepatitis C virus and lichen planus: the real association. Hepat Mon. 2010;10(3):161-164.
12. Khokhar OS, Lewis JH. Reasons why patients infected with chronic hepatitis C virus choose to defer treatment: do they alter their decision with time? Dig Dis Sci. 2007;52(5):1168-1176.
13. Bruno S, Zuin M, Crosignani A, et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol. 2009;104(5):1147-1158.
14. Cheinquer N, Cheinquer H, Wolff FH, Coelho-Borges S. Effect of sustained virologic response on the incidence of hepatocellular carcinoma in patients with HCV cirrhosis. Braz J Infect Dis. 2010;14(5):457-461.
15. Veldt BJ, Chen W, Heathcote EJ, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47(6):1856-1862.
16. Nkontchou G, Ziol M, Aout M, et al. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011;18(10):e516-e522.
17. Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479-497.
18. Vujosevic S, Tempesta D, Noventa F, et al. Pegylated interferon-associated retinopathy is frequent in hepatitis C virus patients with hypertension and justifies ophthalmologic screening. Hepatology. 2012;56(2):455-463.
19. World Health Organization. Global alert and response: hepatitis D (2012). www.who.int/csr/ disease/hepatitis/whocdscsrncs20011/en/index3. html. Accessed August 20, 2012.
20. World Health Organization. Hepatitis E: fact sheet No 280 (July 2012). www.who.int/media centre/factsheets/fs280/en. Accessed August 20, 2012.
21. Bertino G, Ardiri AM, Bruno MC, et al. HAV infection in patients with chronic hepatitis C [in Italian]. Clin Ter. 2007;158(3):223-225.
22. Liu J, Hou J. Hepatitis B virus (HBV) and hepatitis C virus (HCV) dual infection. Int J Med Sci. 2006;3(2):57-62.
23. Schijman A, Colina R, Mukomolov S, et al. Comparison of hepatitis C viral loads in patients with or without coinfection with different genotypes. Clin Diagn Lab Immunol. 2004;11(2):433-435.
24. McClure JE, Shearer WT. Radioimmunoassay for anti-actin antibody: application in viral and autoimmune diseases. Mol Cell Probes. 1988;2(4):305-319.
25. Peakman M, Lobo-Yeo A, Mieli-Vergani, et al. Characterization of anti-liver kidney microsomal antibody in childhood autoimmune chronic active hepatitis: evidence for IgG1 subclass restriction, polyclonality and non cross-reactivity with hepatocyte surface antigens. Clin Exp Immunol. 1987;69(3):543-549.
26. Loko MA, Salmon D, Carrieri P, et al; ANRS CO 13 HEPAVIH Study Group. The French national prospective cohort of patients co-infected with HIV and HCV (ANRS CO13 HEPAVIH): early findings, 2006-2010. BMC Infect Dis. 2010 Oct 22;10:303.
27. Duclos-Vallée JC, Féray C, Sebagh M, et al; THEVIC Study Group. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2008;47(2):407-417.
28. Sato Y, Nakata K, Kato Y, et al. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med. 1993;328(25):1802-1806.
29. Gebo KA, Chander G, Jenckes MW, et al. Screening tests for hepatocellular carcinoma in patients with chronic hepatitis C: a systematic review. Hepatology. 2002;36(5 suppl 1):S84-S92.
30. Tateyama M, Yatsuhashi H, Taura N, et al. Alpha-fetoprotein above normal levels as a risk factor for the development of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol. 2011;46(1):92-100.
31. Bruno S, Shiffman ML, Roberts SK, et al. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology. 2010;51(2):388-397.
32. Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28(5):705-712.
33. van der Poorten D, Kwok A, Lam T, et al. Twenty-year audit of percutaneous liver biopsy in a major Australian teaching hospital. Intern Med J. 2006;36(11):692-699.
34. Howard R, Karageorge G, van Harselaar K, et al. Post-procedure surveillance in liver biopsy: how long is long enough? N Z Med J. 2008;121(1280):8-14.
35. Kramskay R, Tansky A, Eisenberg, et al. Prophylactic analgesia before percutaneous liver biopsy: a clinical comparative study. Eur J Gastroenterol Hepatol. 2011;23(9):782-786.
36. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
37. Lemon SM, McKeating JA, Pietschmann T, et al. Development of novel therapies for hepatitis C. Antiviral Res. 2010;86(1):79-92.
38. Shepherd J, Brodin H, Cave C, et al. Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2004;8(39):iii-iv, 1-125.
39. Kamal SM, El Kamary SS, Shardell MD, et al. Pegylated interferon alpha-2b plus ribavirin in patients with genotype 4 chronic hepatitis C: the role of rapid and early virologic response. Hepatology. 2007;46:1732-1740.
40. Hofmann WP, Sarrazin C, Zeuzem S. Current standards in the treatment of chronic hepatitis C. Dtsch Arztebl Int. 2012;109(19):352-358.
41. Kwo PY, Lawitz EJ, McCone J, et al; SPRINT-1 Investigators. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376(9742):705-716.
42. Klibanov OM, Vickery SB, Olin JL, et al. Boceprevir: a novel NS3/4 protease inhibitor for the treatment of hepatitis C. Pharmacotherapy. 2012;32(2):173-190.
43. Cunningham M, Foster GR. Efficacy and safety of telaprevir in patients with genotype 1 hepatitis C infection. Therap Adv Gastroenterol. 2012;5(2):139-151.
44. Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405-2416.
45. Zeuzem S, Andreone P, Pol S, et al; REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364(25):2417-2428.
46. Sagir A, Heintges T, Akyazi Z, et al. Therapy outcome in patients with chronic hepatitis C: role of therapy supervision by expert hepatologists. J Viral Hepat. 2007;14(9):633-638.
47. Highlights of prescribing information: INCIVEKTM (telaprevir) film coated tablets for oral use (2011). http://pi.vrtx.com/files/uspi_telaprevir. pdf. Accessed August 20, 2012.
48. Highlights of prescribing information: VICTRELISTM (boceprevir) capsules (2011). www .accessdata.fda.gov/drugsatfda_docs / label/2011/202258lbl.pdf. Accessed August 20, 2012.
49. Alavian SM, Tabatabaei SV, Behnava B, Mahboobi N. Optimal duration of treatment for HCV genotype 1 infection in slow responders: a meta-analysis. Hepat Mon. 2011;11(8):612-619.
50. Fried MW, Hadziyannis SJ, Shiffman ML, et al. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol. 2011;55(1):69-75.
51. Reau N, Satoskar R, Te H, et al. Evaluation of early null response to pegylated interferon and ribavirin as a predictor of therapeutic nonresponse in patients undergoing treatment for chronic hepatitis C. Am J Gastroenterol. 2011;106(3):452-458.
52. Mac Nicholas R, Norris S. Optimizing SVR and management of the haematological side effects of peginterferon/ribavirin antiviral therapy for HCV: the role of epoietin, G-CSF and novel agents. Aliment Pharmacol Ther. 2010;31(9):929-937.
53. Singhal A, Jain AB, Burke M, Black M. Aggressive use of ribavirin and prolonged course of peginterferon to improve the rate of viral response in liver transplant patients with recurrent hepatitis C viral infection. Exp Clin Transplant. 2010;8(3):214-219.
54. Highlights of prescribing information: COPEGUSTM (ribavirin) tablets (2002). http://www.gene .com/gene/products/information/pegasys/pdf/ copegus_pi.pdf. Accessed August 20, 2012.
55. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 suppl 1):S237-S244.
56. Dollarhide AW, Loh C, Leckband SG, et al. Psychiatric comorbidity does not predict interferon treatment completion rates in hepatitis C seropositive veterans. J Clin Gastroenterol. 2007;41(3):322-328.
57. Tavakoli-Tabasi S, Bagree A. A longitudinal cohort study of mucocutaneous drug eruptions during interferon and ribavirin treatment of hepatitis C. J Clin Gastroenterol. 2012;46(2):162-167.
58. Fox AN, Jacobson IM. Recent successes and noteworthy future prospects in the treatment of chronic hepatitis C. Clin Infect Dis. 2012;55 suppl 1:S16-S24.
1. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
2. CDC. Hepatitis C FAQs for health professionals (2008). www.cdc.gov/hepatitis/HCV/HCVfaq. htm#section1. Accessed August 20, 2012.
3. Ly KN, Xing J, Klevens M, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271-278.
4. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2010 Annual Data Report (2011). Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation. www.srtr.org/ annual_reports/2010/pdf/00_intro_pgs_11.pdf. Accessed August 20, 2012.
5. Tohme RA, Holmberg SD. Transmission of hepatitis C virus infection through tattooing and piercing: a critical review. Clin Infect Dis. 2012;54(8):1167-1178.
6. CDC. Hepatitis C: proposed expansion of testing recommendations, 2012. www.cdc.gov/nchh-stp/newsroom/docs/HCV-TestingFactSheetNoEm-bargo508.pdf. Accessed August 20, 2012.
7. Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3(2):47-52.
8. Al-Ali J, Al-Mutari N, Ahmed el-SF. Hepatitis C virus and the skin. Hepatogastroenterology. 2011;58(107-108):880-886.
9. Raslan HM, Ezzat WM, Abd El Hamid MF, et al. Skin manifestations of chronic hepatitis C virus infection in Cairo, Egypt. East Mediterr Health J. 2009;15(3):692-700.
10. Niederau C, Hüppe D, Zehnter E, et al. Chronic hepatitis C: treat or wait? Medical decision making in clinical practice. World J Gastroenterol. 2012;18(12):1339-1347.
11. Mahboobi N, Agha-Hosseini F, Lankarani K. Hepatitis C virus and lichen planus: the real association. Hepat Mon. 2010;10(3):161-164.
12. Khokhar OS, Lewis JH. Reasons why patients infected with chronic hepatitis C virus choose to defer treatment: do they alter their decision with time? Dig Dis Sci. 2007;52(5):1168-1176.
13. Bruno S, Zuin M, Crosignani A, et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol. 2009;104(5):1147-1158.
14. Cheinquer N, Cheinquer H, Wolff FH, Coelho-Borges S. Effect of sustained virologic response on the incidence of hepatocellular carcinoma in patients with HCV cirrhosis. Braz J Infect Dis. 2010;14(5):457-461.
15. Veldt BJ, Chen W, Heathcote EJ, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47(6):1856-1862.
16. Nkontchou G, Ziol M, Aout M, et al. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011;18(10):e516-e522.
17. Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479-497.
18. Vujosevic S, Tempesta D, Noventa F, et al. Pegylated interferon-associated retinopathy is frequent in hepatitis C virus patients with hypertension and justifies ophthalmologic screening. Hepatology. 2012;56(2):455-463.
19. World Health Organization. Global alert and response: hepatitis D (2012). www.who.int/csr/ disease/hepatitis/whocdscsrncs20011/en/index3. html. Accessed August 20, 2012.
20. World Health Organization. Hepatitis E: fact sheet No 280 (July 2012). www.who.int/media centre/factsheets/fs280/en. Accessed August 20, 2012.
21. Bertino G, Ardiri AM, Bruno MC, et al. HAV infection in patients with chronic hepatitis C [in Italian]. Clin Ter. 2007;158(3):223-225.
22. Liu J, Hou J. Hepatitis B virus (HBV) and hepatitis C virus (HCV) dual infection. Int J Med Sci. 2006;3(2):57-62.
23. Schijman A, Colina R, Mukomolov S, et al. Comparison of hepatitis C viral loads in patients with or without coinfection with different genotypes. Clin Diagn Lab Immunol. 2004;11(2):433-435.
24. McClure JE, Shearer WT. Radioimmunoassay for anti-actin antibody: application in viral and autoimmune diseases. Mol Cell Probes. 1988;2(4):305-319.
25. Peakman M, Lobo-Yeo A, Mieli-Vergani, et al. Characterization of anti-liver kidney microsomal antibody in childhood autoimmune chronic active hepatitis: evidence for IgG1 subclass restriction, polyclonality and non cross-reactivity with hepatocyte surface antigens. Clin Exp Immunol. 1987;69(3):543-549.
26. Loko MA, Salmon D, Carrieri P, et al; ANRS CO 13 HEPAVIH Study Group. The French national prospective cohort of patients co-infected with HIV and HCV (ANRS CO13 HEPAVIH): early findings, 2006-2010. BMC Infect Dis. 2010 Oct 22;10:303.
27. Duclos-Vallée JC, Féray C, Sebagh M, et al; THEVIC Study Group. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2008;47(2):407-417.
28. Sato Y, Nakata K, Kato Y, et al. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med. 1993;328(25):1802-1806.
29. Gebo KA, Chander G, Jenckes MW, et al. Screening tests for hepatocellular carcinoma in patients with chronic hepatitis C: a systematic review. Hepatology. 2002;36(5 suppl 1):S84-S92.
30. Tateyama M, Yatsuhashi H, Taura N, et al. Alpha-fetoprotein above normal levels as a risk factor for the development of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol. 2011;46(1):92-100.
31. Bruno S, Shiffman ML, Roberts SK, et al. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology. 2010;51(2):388-397.
32. Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28(5):705-712.
33. van der Poorten D, Kwok A, Lam T, et al. Twenty-year audit of percutaneous liver biopsy in a major Australian teaching hospital. Intern Med J. 2006;36(11):692-699.
34. Howard R, Karageorge G, van Harselaar K, et al. Post-procedure surveillance in liver biopsy: how long is long enough? N Z Med J. 2008;121(1280):8-14.
35. Kramskay R, Tansky A, Eisenberg, et al. Prophylactic analgesia before percutaneous liver biopsy: a clinical comparative study. Eur J Gastroenterol Hepatol. 2011;23(9):782-786.
36. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
37. Lemon SM, McKeating JA, Pietschmann T, et al. Development of novel therapies for hepatitis C. Antiviral Res. 2010;86(1):79-92.
38. Shepherd J, Brodin H, Cave C, et al. Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2004;8(39):iii-iv, 1-125.
39. Kamal SM, El Kamary SS, Shardell MD, et al. Pegylated interferon alpha-2b plus ribavirin in patients with genotype 4 chronic hepatitis C: the role of rapid and early virologic response. Hepatology. 2007;46:1732-1740.
40. Hofmann WP, Sarrazin C, Zeuzem S. Current standards in the treatment of chronic hepatitis C. Dtsch Arztebl Int. 2012;109(19):352-358.
41. Kwo PY, Lawitz EJ, McCone J, et al; SPRINT-1 Investigators. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376(9742):705-716.
42. Klibanov OM, Vickery SB, Olin JL, et al. Boceprevir: a novel NS3/4 protease inhibitor for the treatment of hepatitis C. Pharmacotherapy. 2012;32(2):173-190.
43. Cunningham M, Foster GR. Efficacy and safety of telaprevir in patients with genotype 1 hepatitis C infection. Therap Adv Gastroenterol. 2012;5(2):139-151.
44. Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405-2416.
45. Zeuzem S, Andreone P, Pol S, et al; REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364(25):2417-2428.
46. Sagir A, Heintges T, Akyazi Z, et al. Therapy outcome in patients with chronic hepatitis C: role of therapy supervision by expert hepatologists. J Viral Hepat. 2007;14(9):633-638.
47. Highlights of prescribing information: INCIVEKTM (telaprevir) film coated tablets for oral use (2011). http://pi.vrtx.com/files/uspi_telaprevir. pdf. Accessed August 20, 2012.
48. Highlights of prescribing information: VICTRELISTM (boceprevir) capsules (2011). www .accessdata.fda.gov/drugsatfda_docs / label/2011/202258lbl.pdf. Accessed August 20, 2012.
49. Alavian SM, Tabatabaei SV, Behnava B, Mahboobi N. Optimal duration of treatment for HCV genotype 1 infection in slow responders: a meta-analysis. Hepat Mon. 2011;11(8):612-619.
50. Fried MW, Hadziyannis SJ, Shiffman ML, et al. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol. 2011;55(1):69-75.
51. Reau N, Satoskar R, Te H, et al. Evaluation of early null response to pegylated interferon and ribavirin as a predictor of therapeutic nonresponse in patients undergoing treatment for chronic hepatitis C. Am J Gastroenterol. 2011;106(3):452-458.
52. Mac Nicholas R, Norris S. Optimizing SVR and management of the haematological side effects of peginterferon/ribavirin antiviral therapy for HCV: the role of epoietin, G-CSF and novel agents. Aliment Pharmacol Ther. 2010;31(9):929-937.
53. Singhal A, Jain AB, Burke M, Black M. Aggressive use of ribavirin and prolonged course of peginterferon to improve the rate of viral response in liver transplant patients with recurrent hepatitis C viral infection. Exp Clin Transplant. 2010;8(3):214-219.
54. Highlights of prescribing information: COPEGUSTM (ribavirin) tablets (2002). http://www.gene .com/gene/products/information/pegasys/pdf/ copegus_pi.pdf. Accessed August 20, 2012.
55. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 suppl 1):S237-S244.
56. Dollarhide AW, Loh C, Leckband SG, et al. Psychiatric comorbidity does not predict interferon treatment completion rates in hepatitis C seropositive veterans. J Clin Gastroenterol. 2007;41(3):322-328.
57. Tavakoli-Tabasi S, Bagree A. A longitudinal cohort study of mucocutaneous drug eruptions during interferon and ribavirin treatment of hepatitis C. J Clin Gastroenterol. 2012;46(2):162-167.
58. Fox AN, Jacobson IM. Recent successes and noteworthy future prospects in the treatment of chronic hepatitis C. Clin Infect Dis. 2012;55 suppl 1:S16-S24.