User login
Postpartum IUD insertion: Best practices
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12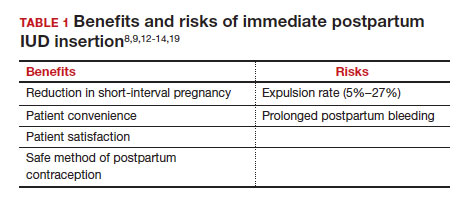
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
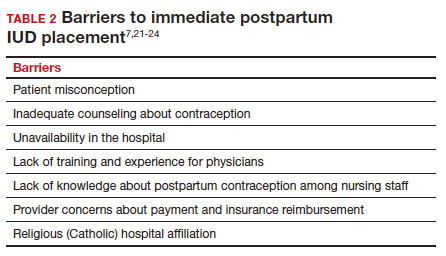
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21
System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
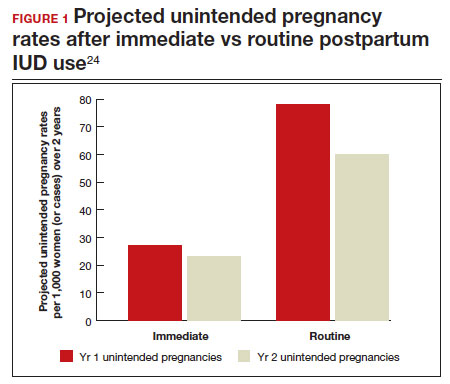
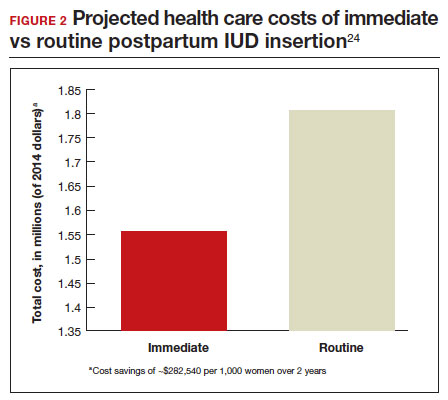
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).
Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
How to place an IUD with minimal patient discomfort
CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8
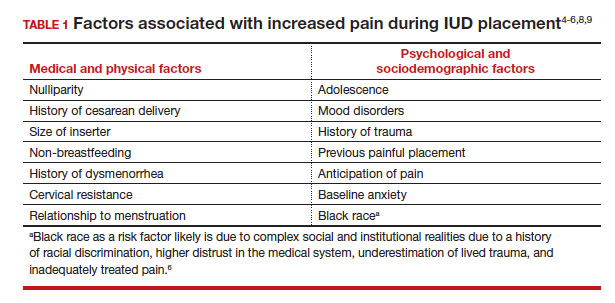
It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10
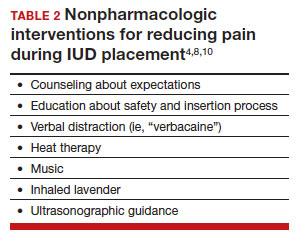
Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).
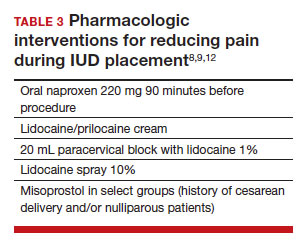
Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.

CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8

It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10

Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).

Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.

CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8

It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10

Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).

Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.
Should we rethink maternal monitoring of fetal movement through “kick counts”?

It is time to reconsider the recommendation for practicing fetal kick counts. A meta-analysis demonstrated no decrease in the outcome of stillbirth, but instead an increased risk of iatrogenic delivery.1
CASE 1 8 vs 10 fetal movements in 2 hours
Ms. M is 38 weeks pregnant with an uncomplicated pregnancy. She calls your practice with concerns about fetal kick counts. During her prenatal care, she was counseled to ensure that the baby moved 10 times over a period of 2 hours. This morning, however, she only perceived 8 movements in 2 hours. She is scheduled for evaluation with a nonstress test (NST) on the labor and delivery unit. The NST reveals a reassuring, reactive tracing. Ultrasonography evaluation demonstrates a normal amniotic fluid index and normal fetal growth. The patient is reassured, returns home, and goes on to deliver a healthy baby at 39 weeks and 5 days.
Perception of decreased movement triggers evaluation and monitoring
Maternal perception of normal fetal movement has conceivably been used throughout history as a means of reassurance of fetal well-being; it is highly predictive of fetal viability.2,3 When fetal movement is lacking or decreased, it can be an alarm sign and may result in concerns by the mother that her baby is unwell. Maternal perception of decreased fetal movements affects 5% to 15% of all pregnancies.2,4 While decreased fetal movement can be associated with poor perinatal outcomes such as fetal growth restriction, oligohydramnios, and neuro-developmental disability, it also can be reflective of more benign issues such as anterior placenta, maternal activity, maternal caffeine or sugar consumption, or maternal position.4,5
However, the definition of decreased fetal movement is subject to significant variation, from a total absence of movement over an entire day or what has commonly become accepted as the definition of fetal kick counts with Pearson’s Cardiff chart (which was defined in the 1970s as 10 movements within 12 hours).6,7 Today, women in the United States are commonly recommended to monitor their baby over a 2-hour period and to look for 10 movements during that time.8 Anything less is considered reduced fetal movement and results in recommendations to undergo assessment of previously known high-risk conditions or any possible underlying conditions, such as hypertension, gestational diabetes, or fetal growth restriction. Further evaluation with more objective measures such as electronic fetal monitoring or ultrasonography with biophysical profile are often recommended concurrently.9
It is estimated that up to 15% of women present reporting decreased fetal movement in the third trimester and, as such, require additional monitoring and evaluation. This is not without cost of time and money to the health care system and pregnant patients.
It is uncertain that fetal kick counting prevents stillbirth
Intrauterine fetal demise is neither an uncommon nor completely preventable outcome, despite advances in antenatal care. Many cases occur without evidence of fetal abnormality or other risk factors, and 30% to 55% of women who experience intrauterine fetal demise experience decreased fetal movement in the preceding week.10 It makes physiologic sense that a fetus’ adaptive response to decreased oxygenation is reduced fetal movement, resulting from the prioritization of blood to the fetal brain and other organs over skeletal muscle.4,9,11 Results of a 1976 small study of 61 low-risk pregnancies seemed to confirm that a decrease in fetal movement preceded intrauterine death by 3 to 4 days. Conversely, they found that a normal fetal movement count was generally associated with a good neonatal outcome.6 Thus, experts have long extrapolated that decreased fetal movement can be an indicator for utero-placental insufficiency and, in turn, chronic or acute hypoxia.
However, in larger studies, the ability of fetal movement counting to predict fetal death and fetal compromise appears limited.8,10,11 A meta-analysis of studies, including 5 randomized controlled trials and 468,000 fetuses, compared the incidence of stillbirth in women receiving instructions for fetal movement counting versus women who did not. Rates of stillbirth were the same for each group, demonstrating no advantage to fetal kick counts to prevent a poor perinatal outcome, including stillbirth.1
CASE 2 Reported reduced fetal movement over 4 weeks
Ms. E is a 20-year-old nullipara at 36 weeks’ and 6 days gestation who has come in to triage weekly for the last 4 weeks with concerns about decreased fetal movement. She states that she goes for several hours each day without feeling 10 movements in 2 hours. Recent fetal growth recorded 3 weeks ago was in the 45th percentile, and the amniotic fluid index has been above 10 cm on each weekly ultrasound. Her weekly NSTs have been reactive, and she has been normotensive. However, because she has had several weeks of persistent decreased fetal movement, the labor and delivery team opts to keep her for induction as she is “close to term.”
Decreased kick count frequency may increase unnecessary interventions
Women with fewer kick counts are more likely to present with concerns about the well-being of their baby. In a survey of obstetricians and midwives, a large proportion of providers were more apt to recommend delivery or admission to the hospital for women presenting with decreased fetal movements.2 It stands to reason that recommendations for delivery or admission can lead to outcomes like preterm delivery or recommendations for cesarean delivery (CD). However, using fetal kick counts to portend stillbirth or other poor fetal and neonatal outcomes has been shown to be limited in its value with the AFFIRM trial.10 The results of this large study, which included more than 400,000 pregnancies from 37 hospitals, show the challenges of any study to address the use of management strategies for recent change in the frequency of fetal movements in the reduction of and cause of stillbirth. Additionally, the relatively low risk of stillbirth overall (4.06 stillbirths per 1,000 livebirths during the intervention period and 4.40 per 1,000 livebirths during the control period) but higher incidence of other outcomes, such as prolonged (>48 hours) antepartum admission (6.7% in the intervention period and 6.2% in the control period), induction of labor (40.7% in the intervention period and 35.9% in the control period), and CD (28.4% and 25.5%, respectively) may result in increased harm for many women rather than the intended benefit of preventing stillbirth.10,12
Mindfetalness may be a viable and valuable alternative to kick counts
Alternatives have been proposed as a measure of fetal movement without using kick counts specifically. Mindfetalness has been a method studied in Sweden; its purpose is to strengthen the mother’s awareness of her baby through developing an understanding of the fetal-movement pattern. It is practiced starting at 28 weeks’ gestation for 15 minutes a day, with the woman instructed to lie on her left side and discern the intensity and character of the movements, as well as frequency, without overtly counting the movements.12 In one small study, women felt more connected to their babies and felt less worried.12 In a much larger study of 13,000 women, the authors found no evidence of harm from generalized awareness of fetal movements in a population of pregnant women at or beyond 32 weeks; in fact, they did see significant reductions in iatrogenic outcomes such as CDs and labor inductions
The case for movement awareness over kick counts
Stillbirth risk does not appear to be modified by the use of methods to detect fetal movement.10,12 However, a perceived decrease in fetal kick counts has been shown to result in increased interventions and preterm deliveries. A more prudent approach appears to be educating mothers about general fetal movement, which appears to reduce potentially unnecessary visits and interventions without sacrificing the ability to reassure mothers about the well-being of their babies in utero. ●
- Haezell AEP, Green M, Wright C, et al. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica. 2008:87;331-339. doi: 10.1080/00016340801902034.
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13-24. doi: 10.1515/JPM.2004.003.
- Heazell AEP, Froen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28:147-154. doi: 10.1080/01443610801912618.
- Froen JF, Heazell AEP, Holm Tveit JV, et al. Fetal movement assessment. Semin Perinatal. 2008;32:243-246. doi: 10.1053/j.semperi.2008.04.004
- Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. British Med J. 1976;1:1305-1307. doi: 10.1136/bmj.1.6021.1305.
- Pearson JF. Fetal movements – a new approach to antenatal care. Nursing Mirror Midwives J. 1977;144:49-51.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197. doi: 10.1097/AOG.0000000000004407.
- Christensen FC, Rayburn WF. Fetal movement counts. Obstet Gynecol Clin North Am. 1999;26:4(607-621). doi: 10.1016/s0889-8545(05)70102-9.
- Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge cluster-randomized trial. Lancet. 2018;392:1629-1638. doi: 10.1016/S0140-6736(18)31543-5.
- Warrender LK, Batra G, Bernatavicius G, et al. Maternal perception of reduced fetal movement is associated with altered placental structure and function. PLoS One. 2012;7:4. doi: 10.1371/journal.pone.0034851.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality. A systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462. doi: 10.1097/AOG.0000000000003645.
- Akselsson A, Georgsson S, Lindgren H, et al. Women’s attitudes, experiences and compliance concerning the use of mindfetalness – a method for systematic observation of fetal movements in late pregnancy. BMC Pregnancy Childbirth. 2017;17:1-7. doi: 10.1186/s12884-017-1548-5.
- Akselsson A, Lindgren H, Skokic V, et al. A decrease in cesarean sections and labor inductions among Swedish women by awareness of fetal movements with the Mindfetalness method. BMC Pregnancy Childbirth. 2020;20;577:1-10.

It is time to reconsider the recommendation for practicing fetal kick counts. A meta-analysis demonstrated no decrease in the outcome of stillbirth, but instead an increased risk of iatrogenic delivery.1
CASE 1 8 vs 10 fetal movements in 2 hours
Ms. M is 38 weeks pregnant with an uncomplicated pregnancy. She calls your practice with concerns about fetal kick counts. During her prenatal care, she was counseled to ensure that the baby moved 10 times over a period of 2 hours. This morning, however, she only perceived 8 movements in 2 hours. She is scheduled for evaluation with a nonstress test (NST) on the labor and delivery unit. The NST reveals a reassuring, reactive tracing. Ultrasonography evaluation demonstrates a normal amniotic fluid index and normal fetal growth. The patient is reassured, returns home, and goes on to deliver a healthy baby at 39 weeks and 5 days.
Perception of decreased movement triggers evaluation and monitoring
Maternal perception of normal fetal movement has conceivably been used throughout history as a means of reassurance of fetal well-being; it is highly predictive of fetal viability.2,3 When fetal movement is lacking or decreased, it can be an alarm sign and may result in concerns by the mother that her baby is unwell. Maternal perception of decreased fetal movements affects 5% to 15% of all pregnancies.2,4 While decreased fetal movement can be associated with poor perinatal outcomes such as fetal growth restriction, oligohydramnios, and neuro-developmental disability, it also can be reflective of more benign issues such as anterior placenta, maternal activity, maternal caffeine or sugar consumption, or maternal position.4,5
However, the definition of decreased fetal movement is subject to significant variation, from a total absence of movement over an entire day or what has commonly become accepted as the definition of fetal kick counts with Pearson’s Cardiff chart (which was defined in the 1970s as 10 movements within 12 hours).6,7 Today, women in the United States are commonly recommended to monitor their baby over a 2-hour period and to look for 10 movements during that time.8 Anything less is considered reduced fetal movement and results in recommendations to undergo assessment of previously known high-risk conditions or any possible underlying conditions, such as hypertension, gestational diabetes, or fetal growth restriction. Further evaluation with more objective measures such as electronic fetal monitoring or ultrasonography with biophysical profile are often recommended concurrently.9
It is estimated that up to 15% of women present reporting decreased fetal movement in the third trimester and, as such, require additional monitoring and evaluation. This is not without cost of time and money to the health care system and pregnant patients.
It is uncertain that fetal kick counting prevents stillbirth
Intrauterine fetal demise is neither an uncommon nor completely preventable outcome, despite advances in antenatal care. Many cases occur without evidence of fetal abnormality or other risk factors, and 30% to 55% of women who experience intrauterine fetal demise experience decreased fetal movement in the preceding week.10 It makes physiologic sense that a fetus’ adaptive response to decreased oxygenation is reduced fetal movement, resulting from the prioritization of blood to the fetal brain and other organs over skeletal muscle.4,9,11 Results of a 1976 small study of 61 low-risk pregnancies seemed to confirm that a decrease in fetal movement preceded intrauterine death by 3 to 4 days. Conversely, they found that a normal fetal movement count was generally associated with a good neonatal outcome.6 Thus, experts have long extrapolated that decreased fetal movement can be an indicator for utero-placental insufficiency and, in turn, chronic or acute hypoxia.
However, in larger studies, the ability of fetal movement counting to predict fetal death and fetal compromise appears limited.8,10,11 A meta-analysis of studies, including 5 randomized controlled trials and 468,000 fetuses, compared the incidence of stillbirth in women receiving instructions for fetal movement counting versus women who did not. Rates of stillbirth were the same for each group, demonstrating no advantage to fetal kick counts to prevent a poor perinatal outcome, including stillbirth.1
CASE 2 Reported reduced fetal movement over 4 weeks
Ms. E is a 20-year-old nullipara at 36 weeks’ and 6 days gestation who has come in to triage weekly for the last 4 weeks with concerns about decreased fetal movement. She states that she goes for several hours each day without feeling 10 movements in 2 hours. Recent fetal growth recorded 3 weeks ago was in the 45th percentile, and the amniotic fluid index has been above 10 cm on each weekly ultrasound. Her weekly NSTs have been reactive, and she has been normotensive. However, because she has had several weeks of persistent decreased fetal movement, the labor and delivery team opts to keep her for induction as she is “close to term.”
Decreased kick count frequency may increase unnecessary interventions
Women with fewer kick counts are more likely to present with concerns about the well-being of their baby. In a survey of obstetricians and midwives, a large proportion of providers were more apt to recommend delivery or admission to the hospital for women presenting with decreased fetal movements.2 It stands to reason that recommendations for delivery or admission can lead to outcomes like preterm delivery or recommendations for cesarean delivery (CD). However, using fetal kick counts to portend stillbirth or other poor fetal and neonatal outcomes has been shown to be limited in its value with the AFFIRM trial.10 The results of this large study, which included more than 400,000 pregnancies from 37 hospitals, show the challenges of any study to address the use of management strategies for recent change in the frequency of fetal movements in the reduction of and cause of stillbirth. Additionally, the relatively low risk of stillbirth overall (4.06 stillbirths per 1,000 livebirths during the intervention period and 4.40 per 1,000 livebirths during the control period) but higher incidence of other outcomes, such as prolonged (>48 hours) antepartum admission (6.7% in the intervention period and 6.2% in the control period), induction of labor (40.7% in the intervention period and 35.9% in the control period), and CD (28.4% and 25.5%, respectively) may result in increased harm for many women rather than the intended benefit of preventing stillbirth.10,12
Mindfetalness may be a viable and valuable alternative to kick counts
Alternatives have been proposed as a measure of fetal movement without using kick counts specifically. Mindfetalness has been a method studied in Sweden; its purpose is to strengthen the mother’s awareness of her baby through developing an understanding of the fetal-movement pattern. It is practiced starting at 28 weeks’ gestation for 15 minutes a day, with the woman instructed to lie on her left side and discern the intensity and character of the movements, as well as frequency, without overtly counting the movements.12 In one small study, women felt more connected to their babies and felt less worried.12 In a much larger study of 13,000 women, the authors found no evidence of harm from generalized awareness of fetal movements in a population of pregnant women at or beyond 32 weeks; in fact, they did see significant reductions in iatrogenic outcomes such as CDs and labor inductions
The case for movement awareness over kick counts
Stillbirth risk does not appear to be modified by the use of methods to detect fetal movement.10,12 However, a perceived decrease in fetal kick counts has been shown to result in increased interventions and preterm deliveries. A more prudent approach appears to be educating mothers about general fetal movement, which appears to reduce potentially unnecessary visits and interventions without sacrificing the ability to reassure mothers about the well-being of their babies in utero. ●

It is time to reconsider the recommendation for practicing fetal kick counts. A meta-analysis demonstrated no decrease in the outcome of stillbirth, but instead an increased risk of iatrogenic delivery.1
CASE 1 8 vs 10 fetal movements in 2 hours
Ms. M is 38 weeks pregnant with an uncomplicated pregnancy. She calls your practice with concerns about fetal kick counts. During her prenatal care, she was counseled to ensure that the baby moved 10 times over a period of 2 hours. This morning, however, she only perceived 8 movements in 2 hours. She is scheduled for evaluation with a nonstress test (NST) on the labor and delivery unit. The NST reveals a reassuring, reactive tracing. Ultrasonography evaluation demonstrates a normal amniotic fluid index and normal fetal growth. The patient is reassured, returns home, and goes on to deliver a healthy baby at 39 weeks and 5 days.
Perception of decreased movement triggers evaluation and monitoring
Maternal perception of normal fetal movement has conceivably been used throughout history as a means of reassurance of fetal well-being; it is highly predictive of fetal viability.2,3 When fetal movement is lacking or decreased, it can be an alarm sign and may result in concerns by the mother that her baby is unwell. Maternal perception of decreased fetal movements affects 5% to 15% of all pregnancies.2,4 While decreased fetal movement can be associated with poor perinatal outcomes such as fetal growth restriction, oligohydramnios, and neuro-developmental disability, it also can be reflective of more benign issues such as anterior placenta, maternal activity, maternal caffeine or sugar consumption, or maternal position.4,5
However, the definition of decreased fetal movement is subject to significant variation, from a total absence of movement over an entire day or what has commonly become accepted as the definition of fetal kick counts with Pearson’s Cardiff chart (which was defined in the 1970s as 10 movements within 12 hours).6,7 Today, women in the United States are commonly recommended to monitor their baby over a 2-hour period and to look for 10 movements during that time.8 Anything less is considered reduced fetal movement and results in recommendations to undergo assessment of previously known high-risk conditions or any possible underlying conditions, such as hypertension, gestational diabetes, or fetal growth restriction. Further evaluation with more objective measures such as electronic fetal monitoring or ultrasonography with biophysical profile are often recommended concurrently.9
It is estimated that up to 15% of women present reporting decreased fetal movement in the third trimester and, as such, require additional monitoring and evaluation. This is not without cost of time and money to the health care system and pregnant patients.
It is uncertain that fetal kick counting prevents stillbirth
Intrauterine fetal demise is neither an uncommon nor completely preventable outcome, despite advances in antenatal care. Many cases occur without evidence of fetal abnormality or other risk factors, and 30% to 55% of women who experience intrauterine fetal demise experience decreased fetal movement in the preceding week.10 It makes physiologic sense that a fetus’ adaptive response to decreased oxygenation is reduced fetal movement, resulting from the prioritization of blood to the fetal brain and other organs over skeletal muscle.4,9,11 Results of a 1976 small study of 61 low-risk pregnancies seemed to confirm that a decrease in fetal movement preceded intrauterine death by 3 to 4 days. Conversely, they found that a normal fetal movement count was generally associated with a good neonatal outcome.6 Thus, experts have long extrapolated that decreased fetal movement can be an indicator for utero-placental insufficiency and, in turn, chronic or acute hypoxia.
However, in larger studies, the ability of fetal movement counting to predict fetal death and fetal compromise appears limited.8,10,11 A meta-analysis of studies, including 5 randomized controlled trials and 468,000 fetuses, compared the incidence of stillbirth in women receiving instructions for fetal movement counting versus women who did not. Rates of stillbirth were the same for each group, demonstrating no advantage to fetal kick counts to prevent a poor perinatal outcome, including stillbirth.1
CASE 2 Reported reduced fetal movement over 4 weeks
Ms. E is a 20-year-old nullipara at 36 weeks’ and 6 days gestation who has come in to triage weekly for the last 4 weeks with concerns about decreased fetal movement. She states that she goes for several hours each day without feeling 10 movements in 2 hours. Recent fetal growth recorded 3 weeks ago was in the 45th percentile, and the amniotic fluid index has been above 10 cm on each weekly ultrasound. Her weekly NSTs have been reactive, and she has been normotensive. However, because she has had several weeks of persistent decreased fetal movement, the labor and delivery team opts to keep her for induction as she is “close to term.”
Decreased kick count frequency may increase unnecessary interventions
Women with fewer kick counts are more likely to present with concerns about the well-being of their baby. In a survey of obstetricians and midwives, a large proportion of providers were more apt to recommend delivery or admission to the hospital for women presenting with decreased fetal movements.2 It stands to reason that recommendations for delivery or admission can lead to outcomes like preterm delivery or recommendations for cesarean delivery (CD). However, using fetal kick counts to portend stillbirth or other poor fetal and neonatal outcomes has been shown to be limited in its value with the AFFIRM trial.10 The results of this large study, which included more than 400,000 pregnancies from 37 hospitals, show the challenges of any study to address the use of management strategies for recent change in the frequency of fetal movements in the reduction of and cause of stillbirth. Additionally, the relatively low risk of stillbirth overall (4.06 stillbirths per 1,000 livebirths during the intervention period and 4.40 per 1,000 livebirths during the control period) but higher incidence of other outcomes, such as prolonged (>48 hours) antepartum admission (6.7% in the intervention period and 6.2% in the control period), induction of labor (40.7% in the intervention period and 35.9% in the control period), and CD (28.4% and 25.5%, respectively) may result in increased harm for many women rather than the intended benefit of preventing stillbirth.10,12
Mindfetalness may be a viable and valuable alternative to kick counts
Alternatives have been proposed as a measure of fetal movement without using kick counts specifically. Mindfetalness has been a method studied in Sweden; its purpose is to strengthen the mother’s awareness of her baby through developing an understanding of the fetal-movement pattern. It is practiced starting at 28 weeks’ gestation for 15 minutes a day, with the woman instructed to lie on her left side and discern the intensity and character of the movements, as well as frequency, without overtly counting the movements.12 In one small study, women felt more connected to their babies and felt less worried.12 In a much larger study of 13,000 women, the authors found no evidence of harm from generalized awareness of fetal movements in a population of pregnant women at or beyond 32 weeks; in fact, they did see significant reductions in iatrogenic outcomes such as CDs and labor inductions
The case for movement awareness over kick counts
Stillbirth risk does not appear to be modified by the use of methods to detect fetal movement.10,12 However, a perceived decrease in fetal kick counts has been shown to result in increased interventions and preterm deliveries. A more prudent approach appears to be educating mothers about general fetal movement, which appears to reduce potentially unnecessary visits and interventions without sacrificing the ability to reassure mothers about the well-being of their babies in utero. ●
- Haezell AEP, Green M, Wright C, et al. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica. 2008:87;331-339. doi: 10.1080/00016340801902034.
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13-24. doi: 10.1515/JPM.2004.003.
- Heazell AEP, Froen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28:147-154. doi: 10.1080/01443610801912618.
- Froen JF, Heazell AEP, Holm Tveit JV, et al. Fetal movement assessment. Semin Perinatal. 2008;32:243-246. doi: 10.1053/j.semperi.2008.04.004
- Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. British Med J. 1976;1:1305-1307. doi: 10.1136/bmj.1.6021.1305.
- Pearson JF. Fetal movements – a new approach to antenatal care. Nursing Mirror Midwives J. 1977;144:49-51.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197. doi: 10.1097/AOG.0000000000004407.
- Christensen FC, Rayburn WF. Fetal movement counts. Obstet Gynecol Clin North Am. 1999;26:4(607-621). doi: 10.1016/s0889-8545(05)70102-9.
- Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge cluster-randomized trial. Lancet. 2018;392:1629-1638. doi: 10.1016/S0140-6736(18)31543-5.
- Warrender LK, Batra G, Bernatavicius G, et al. Maternal perception of reduced fetal movement is associated with altered placental structure and function. PLoS One. 2012;7:4. doi: 10.1371/journal.pone.0034851.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality. A systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462. doi: 10.1097/AOG.0000000000003645.
- Akselsson A, Georgsson S, Lindgren H, et al. Women’s attitudes, experiences and compliance concerning the use of mindfetalness – a method for systematic observation of fetal movements in late pregnancy. BMC Pregnancy Childbirth. 2017;17:1-7. doi: 10.1186/s12884-017-1548-5.
- Akselsson A, Lindgren H, Skokic V, et al. A decrease in cesarean sections and labor inductions among Swedish women by awareness of fetal movements with the Mindfetalness method. BMC Pregnancy Childbirth. 2020;20;577:1-10.
- Haezell AEP, Green M, Wright C, et al. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica. 2008:87;331-339. doi: 10.1080/00016340801902034.
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13-24. doi: 10.1515/JPM.2004.003.
- Heazell AEP, Froen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28:147-154. doi: 10.1080/01443610801912618.
- Froen JF, Heazell AEP, Holm Tveit JV, et al. Fetal movement assessment. Semin Perinatal. 2008;32:243-246. doi: 10.1053/j.semperi.2008.04.004
- Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. British Med J. 1976;1:1305-1307. doi: 10.1136/bmj.1.6021.1305.
- Pearson JF. Fetal movements – a new approach to antenatal care. Nursing Mirror Midwives J. 1977;144:49-51.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197. doi: 10.1097/AOG.0000000000004407.
- Christensen FC, Rayburn WF. Fetal movement counts. Obstet Gynecol Clin North Am. 1999;26:4(607-621). doi: 10.1016/s0889-8545(05)70102-9.
- Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge cluster-randomized trial. Lancet. 2018;392:1629-1638. doi: 10.1016/S0140-6736(18)31543-5.
- Warrender LK, Batra G, Bernatavicius G, et al. Maternal perception of reduced fetal movement is associated with altered placental structure and function. PLoS One. 2012;7:4. doi: 10.1371/journal.pone.0034851.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality. A systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462. doi: 10.1097/AOG.0000000000003645.
- Akselsson A, Georgsson S, Lindgren H, et al. Women’s attitudes, experiences and compliance concerning the use of mindfetalness – a method for systematic observation of fetal movements in late pregnancy. BMC Pregnancy Childbirth. 2017;17:1-7. doi: 10.1186/s12884-017-1548-5.
- Akselsson A, Lindgren H, Skokic V, et al. A decrease in cesarean sections and labor inductions among Swedish women by awareness of fetal movements with the Mindfetalness method. BMC Pregnancy Childbirth. 2020;20;577:1-10.