User login
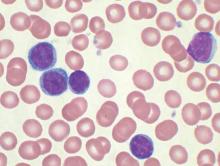
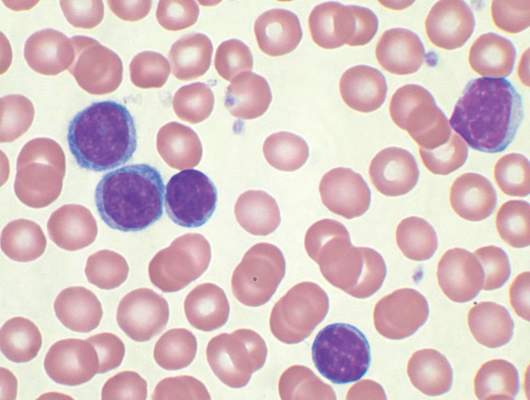
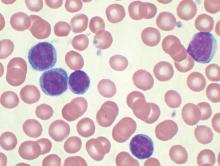
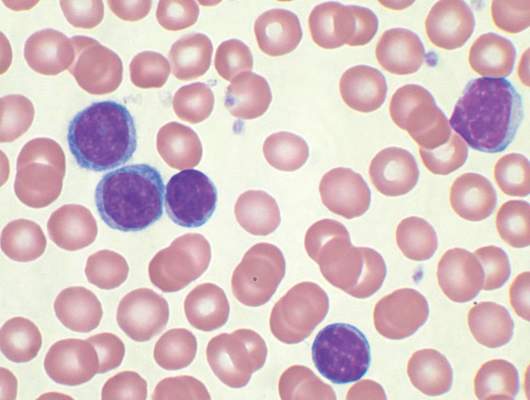
No evidence for CLL transmission via blood transfusion
Analysis of data from blood transfusions that took place in Sweden and Denmark over a 30-year period showed no indication that chronic lymphocytic leukemia (CLL) risk is higher among recipients of blood from donors who subsequently developed CLL, according to researchers.
The study compared 7,413 recipients of blood from 796 donors who subsequently developed CLL (exposed group), with 80,431 recipients from 7,477 donors free of CLL (unexposed group). In total, 12 recipients in the exposed group and 107 in the unexposed group were later diagnosed with CLL, for an incidence rate ratio of 0.94 (95% confidence interval, 0.52-1.71). When defining “exposed” as receiving blood less than 10 years before donor CLL diagnosis, the incidence rate ratio was 0.46 (95% CI, 0.12-1.85).
“The analyses provided little evidence that donor MBL [monoclonal B-cell lymphocytosis]/CLL transmission in blood products influences recipient CLL risk,” wrote Dr. Henrik Hjalgrim of the department of epidemiology research at Statens Serum Institut, Copenhagen, and his colleagues (Blood 2015 doi: 10.1182/blood-2015-03-632844).
MBL is fairly common in healthy individuals (estimated at 7.1% in a study of American blood donors aged 45-91 years) and may progress to CLL at various rates depending on the MBL cell count. Results from previous studies investigating the association between transfusion and risk of CLL or small lymphocytic lymphoma have been mixed, they noted.
Using a retrospective approach, Dr. Hjalgrim and his associates first identified donors subsequently diagnosed with CLL, then identified control donors free from CLL who were matched for age, sex, county, number of donations, and blood type.
In case MBL may have progressed in the recipient but not the donor, investigators also examined whether CLL clustered among recipients from an individual donor, regardless of donor CLL status, but found no such clusters.
Limiting the analysis was the lack of donor MBL status, for which postdonation CLL diagnosis substituted. Some recipients in the exposed group may have received blood drawn before the donor developed MBL.
Dr. Hjalgrim and his coauthors reported having no disclosures.
Analysis of data from blood transfusions that took place in Sweden and Denmark over a 30-year period showed no indication that chronic lymphocytic leukemia (CLL) risk is higher among recipients of blood from donors who subsequently developed CLL, according to researchers.
The study compared 7,413 recipients of blood from 796 donors who subsequently developed CLL (exposed group), with 80,431 recipients from 7,477 donors free of CLL (unexposed group). In total, 12 recipients in the exposed group and 107 in the unexposed group were later diagnosed with CLL, for an incidence rate ratio of 0.94 (95% confidence interval, 0.52-1.71). When defining “exposed” as receiving blood less than 10 years before donor CLL diagnosis, the incidence rate ratio was 0.46 (95% CI, 0.12-1.85).
“The analyses provided little evidence that donor MBL [monoclonal B-cell lymphocytosis]/CLL transmission in blood products influences recipient CLL risk,” wrote Dr. Henrik Hjalgrim of the department of epidemiology research at Statens Serum Institut, Copenhagen, and his colleagues (Blood 2015 doi: 10.1182/blood-2015-03-632844).
MBL is fairly common in healthy individuals (estimated at 7.1% in a study of American blood donors aged 45-91 years) and may progress to CLL at various rates depending on the MBL cell count. Results from previous studies investigating the association between transfusion and risk of CLL or small lymphocytic lymphoma have been mixed, they noted.
Using a retrospective approach, Dr. Hjalgrim and his associates first identified donors subsequently diagnosed with CLL, then identified control donors free from CLL who were matched for age, sex, county, number of donations, and blood type.
In case MBL may have progressed in the recipient but not the donor, investigators also examined whether CLL clustered among recipients from an individual donor, regardless of donor CLL status, but found no such clusters.
Limiting the analysis was the lack of donor MBL status, for which postdonation CLL diagnosis substituted. Some recipients in the exposed group may have received blood drawn before the donor developed MBL.
Dr. Hjalgrim and his coauthors reported having no disclosures.
Analysis of data from blood transfusions that took place in Sweden and Denmark over a 30-year period showed no indication that chronic lymphocytic leukemia (CLL) risk is higher among recipients of blood from donors who subsequently developed CLL, according to researchers.
The study compared 7,413 recipients of blood from 796 donors who subsequently developed CLL (exposed group), with 80,431 recipients from 7,477 donors free of CLL (unexposed group). In total, 12 recipients in the exposed group and 107 in the unexposed group were later diagnosed with CLL, for an incidence rate ratio of 0.94 (95% confidence interval, 0.52-1.71). When defining “exposed” as receiving blood less than 10 years before donor CLL diagnosis, the incidence rate ratio was 0.46 (95% CI, 0.12-1.85).
“The analyses provided little evidence that donor MBL [monoclonal B-cell lymphocytosis]/CLL transmission in blood products influences recipient CLL risk,” wrote Dr. Henrik Hjalgrim of the department of epidemiology research at Statens Serum Institut, Copenhagen, and his colleagues (Blood 2015 doi: 10.1182/blood-2015-03-632844).
MBL is fairly common in healthy individuals (estimated at 7.1% in a study of American blood donors aged 45-91 years) and may progress to CLL at various rates depending on the MBL cell count. Results from previous studies investigating the association between transfusion and risk of CLL or small lymphocytic lymphoma have been mixed, they noted.
Using a retrospective approach, Dr. Hjalgrim and his associates first identified donors subsequently diagnosed with CLL, then identified control donors free from CLL who were matched for age, sex, county, number of donations, and blood type.
In case MBL may have progressed in the recipient but not the donor, investigators also examined whether CLL clustered among recipients from an individual donor, regardless of donor CLL status, but found no such clusters.
Limiting the analysis was the lack of donor MBL status, for which postdonation CLL diagnosis substituted. Some recipients in the exposed group may have received blood drawn before the donor developed MBL.
Dr. Hjalgrim and his coauthors reported having no disclosures.
FROM BLOOD
Key clinical point: There is no evidence for higher risk of chronic lymphocytic leukemia (CLL) among recipients of blood products from donors who subsequently were diagnosed with CLL.
Major finding: Among exposed recipients (7,413 who received blood from 796 donors who subsequently developed CLL), 12 were diagnosed with CLL. Among unexposed recipients (80,431 who received blood from 7,477 donors free of CLL), 107 were diagnosed with CLL, for an incidence rate ratio of 0.94 (95% CI, 0.52-1.71).
Data source: The Scandinavian Donations and Transfusions (SCANDAT2) database comprises information, including donor and recipient health outcomes, for more than 20 million blood products handled by blood banks from 1968 to 2010.
Disclosures: Dr. Hjalgrim and his coauthors reported having no disclosures.
CLL exosomes promote stromal cell transition into cancer-associated fibroblasts
Exosomes released by chronic lymphocytic leukemia (CLL) cells induce stromal cells to adopt a cancer-associated fibroblast phenotype, thereby creating a microenvironment conducive to CLL cell adhesion, survival, and growth.
Although the role of exosomes in other cancers has been well studied, their role in hematologic malignancies has not been well characterized. Also, this study confirmed that exosomes are present in CLL lymph nodes and promote tumor growth in vivo.
“Our in vitro and in vivo data show that CLL exosomes harbor an oncogenic potential by stimulating stromal cells to induce an inflammatory and protumorigenic milieu, including increased angiogenesis, thus supporting the survival and outgrowth of CLL cells,” wrote Jerome Paggetti, Ph.D., of the laboratory of experimental hemato-oncology, Luxembourg Institute of Health (Blood 2015 Aug 27. doi:10.1182/blood-2014-12-618025).
Cells were obtained from 21 CLL patients; all patients had an absolute lymphocyte count of more than 30,000/mcL and were untreated for 3 months. The researchers established 30-day cocultures of bone marrow mesenchymal stem cells with primary CLL cells in culture inserts or they treated bone marrow mesenchymal stem cells weekly with exosomes. Similar experiments were performed with the Burkitt lymphoma cell line Namalwa to investigate whether the impact on stromal cells is CLL specific.
Based on gene expression analysis, CLL exosomes and CLL cells cocultured in inserts induced similar gene expression changes in bone marrow mesenchymal stem cells, highlighting the relevance of exosomes for microenvironment changes. “Importantly, lymphoma cells induced a distinct gene expression pattern in bone marrow mesenchymal stem cells, suggesting a specific response to CLL exosomes,” wrote Dr. Paggetti and coauthors.
The impact of CLL exosomes on tumor growth was studied in vivo by subcutaneously injecting cells with and without CLL exosomes into immunocompromised mice. Cells supplemented with exosomes resulted in an increased tumor size compared with tumor cells injected without additional exosomes. Also, the cells supplemented with exosomes accumulated in mice kidneys, confirming the renal involvement observed in CLL patients. “Our data demonstrate a protumorigenic effect of CLL-derived exosomes in vivo and their importance in the early onset of the disease when tumor cells impact the microenvironment to proliferate and promote angiogenesis,” the researchers concluded.
Chronic lymphocytic leukemia results in clonal expansion and invasive migration of cells that infiltrate the lymph nodes and bone marrow. Understanding the tumor microenvironment and the communication that occurs between malignant cells and their surroundings is imperative to improving cancer therapies.
Alongside well-studied signaling mechanisms involving cytokines, growth factors, and receptors, exosome shedding has emerged recently as a key player in cancer signaling. Paggetti et al. comprehensively analyzed CLL-derived exosomes and provided functional data illustrating the impact of exosomes on the tumor microenvironment by reprogramming healthy stromal cells into cancer-associated fibroblasts.
The RNA and proteins delivered by exosomes to stromal cells induce an inflammatory phenotype characteristic of cancer-associated fibroblasts.
The work supports the theory that tumor cell induction of cancer-associated fibroblasts is a universal feature of progression in both solid and blood cancers. Continued research may identify novel therapies that reconfigure the tumor microenvironment for antitumorigenic effect.
Dr. Benedetta Apollonio is a researcher and Dr. Alan Ramsey is a senior lecturer in lymphoma biology at King’s College, London. Their remarks were part of an editorial accompanying the report (Blood 2015 Aug 27. doi:10.1182/blood-2015-07-655233). The authors had no disclosures to report.
Chronic lymphocytic leukemia results in clonal expansion and invasive migration of cells that infiltrate the lymph nodes and bone marrow. Understanding the tumor microenvironment and the communication that occurs between malignant cells and their surroundings is imperative to improving cancer therapies.
Alongside well-studied signaling mechanisms involving cytokines, growth factors, and receptors, exosome shedding has emerged recently as a key player in cancer signaling. Paggetti et al. comprehensively analyzed CLL-derived exosomes and provided functional data illustrating the impact of exosomes on the tumor microenvironment by reprogramming healthy stromal cells into cancer-associated fibroblasts.
The RNA and proteins delivered by exosomes to stromal cells induce an inflammatory phenotype characteristic of cancer-associated fibroblasts.
The work supports the theory that tumor cell induction of cancer-associated fibroblasts is a universal feature of progression in both solid and blood cancers. Continued research may identify novel therapies that reconfigure the tumor microenvironment for antitumorigenic effect.
Dr. Benedetta Apollonio is a researcher and Dr. Alan Ramsey is a senior lecturer in lymphoma biology at King’s College, London. Their remarks were part of an editorial accompanying the report (Blood 2015 Aug 27. doi:10.1182/blood-2015-07-655233). The authors had no disclosures to report.
Chronic lymphocytic leukemia results in clonal expansion and invasive migration of cells that infiltrate the lymph nodes and bone marrow. Understanding the tumor microenvironment and the communication that occurs between malignant cells and their surroundings is imperative to improving cancer therapies.
Alongside well-studied signaling mechanisms involving cytokines, growth factors, and receptors, exosome shedding has emerged recently as a key player in cancer signaling. Paggetti et al. comprehensively analyzed CLL-derived exosomes and provided functional data illustrating the impact of exosomes on the tumor microenvironment by reprogramming healthy stromal cells into cancer-associated fibroblasts.
The RNA and proteins delivered by exosomes to stromal cells induce an inflammatory phenotype characteristic of cancer-associated fibroblasts.
The work supports the theory that tumor cell induction of cancer-associated fibroblasts is a universal feature of progression in both solid and blood cancers. Continued research may identify novel therapies that reconfigure the tumor microenvironment for antitumorigenic effect.
Dr. Benedetta Apollonio is a researcher and Dr. Alan Ramsey is a senior lecturer in lymphoma biology at King’s College, London. Their remarks were part of an editorial accompanying the report (Blood 2015 Aug 27. doi:10.1182/blood-2015-07-655233). The authors had no disclosures to report.
Exosomes released by chronic lymphocytic leukemia (CLL) cells induce stromal cells to adopt a cancer-associated fibroblast phenotype, thereby creating a microenvironment conducive to CLL cell adhesion, survival, and growth.
Although the role of exosomes in other cancers has been well studied, their role in hematologic malignancies has not been well characterized. Also, this study confirmed that exosomes are present in CLL lymph nodes and promote tumor growth in vivo.
“Our in vitro and in vivo data show that CLL exosomes harbor an oncogenic potential by stimulating stromal cells to induce an inflammatory and protumorigenic milieu, including increased angiogenesis, thus supporting the survival and outgrowth of CLL cells,” wrote Jerome Paggetti, Ph.D., of the laboratory of experimental hemato-oncology, Luxembourg Institute of Health (Blood 2015 Aug 27. doi:10.1182/blood-2014-12-618025).
Cells were obtained from 21 CLL patients; all patients had an absolute lymphocyte count of more than 30,000/mcL and were untreated for 3 months. The researchers established 30-day cocultures of bone marrow mesenchymal stem cells with primary CLL cells in culture inserts or they treated bone marrow mesenchymal stem cells weekly with exosomes. Similar experiments were performed with the Burkitt lymphoma cell line Namalwa to investigate whether the impact on stromal cells is CLL specific.
Based on gene expression analysis, CLL exosomes and CLL cells cocultured in inserts induced similar gene expression changes in bone marrow mesenchymal stem cells, highlighting the relevance of exosomes for microenvironment changes. “Importantly, lymphoma cells induced a distinct gene expression pattern in bone marrow mesenchymal stem cells, suggesting a specific response to CLL exosomes,” wrote Dr. Paggetti and coauthors.
The impact of CLL exosomes on tumor growth was studied in vivo by subcutaneously injecting cells with and without CLL exosomes into immunocompromised mice. Cells supplemented with exosomes resulted in an increased tumor size compared with tumor cells injected without additional exosomes. Also, the cells supplemented with exosomes accumulated in mice kidneys, confirming the renal involvement observed in CLL patients. “Our data demonstrate a protumorigenic effect of CLL-derived exosomes in vivo and their importance in the early onset of the disease when tumor cells impact the microenvironment to proliferate and promote angiogenesis,” the researchers concluded.
Exosomes released by chronic lymphocytic leukemia (CLL) cells induce stromal cells to adopt a cancer-associated fibroblast phenotype, thereby creating a microenvironment conducive to CLL cell adhesion, survival, and growth.
Although the role of exosomes in other cancers has been well studied, their role in hematologic malignancies has not been well characterized. Also, this study confirmed that exosomes are present in CLL lymph nodes and promote tumor growth in vivo.
“Our in vitro and in vivo data show that CLL exosomes harbor an oncogenic potential by stimulating stromal cells to induce an inflammatory and protumorigenic milieu, including increased angiogenesis, thus supporting the survival and outgrowth of CLL cells,” wrote Jerome Paggetti, Ph.D., of the laboratory of experimental hemato-oncology, Luxembourg Institute of Health (Blood 2015 Aug 27. doi:10.1182/blood-2014-12-618025).
Cells were obtained from 21 CLL patients; all patients had an absolute lymphocyte count of more than 30,000/mcL and were untreated for 3 months. The researchers established 30-day cocultures of bone marrow mesenchymal stem cells with primary CLL cells in culture inserts or they treated bone marrow mesenchymal stem cells weekly with exosomes. Similar experiments were performed with the Burkitt lymphoma cell line Namalwa to investigate whether the impact on stromal cells is CLL specific.
Based on gene expression analysis, CLL exosomes and CLL cells cocultured in inserts induced similar gene expression changes in bone marrow mesenchymal stem cells, highlighting the relevance of exosomes for microenvironment changes. “Importantly, lymphoma cells induced a distinct gene expression pattern in bone marrow mesenchymal stem cells, suggesting a specific response to CLL exosomes,” wrote Dr. Paggetti and coauthors.
The impact of CLL exosomes on tumor growth was studied in vivo by subcutaneously injecting cells with and without CLL exosomes into immunocompromised mice. Cells supplemented with exosomes resulted in an increased tumor size compared with tumor cells injected without additional exosomes. Also, the cells supplemented with exosomes accumulated in mice kidneys, confirming the renal involvement observed in CLL patients. “Our data demonstrate a protumorigenic effect of CLL-derived exosomes in vivo and their importance in the early onset of the disease when tumor cells impact the microenvironment to proliferate and promote angiogenesis,” the researchers concluded.
FROM BLOOD
Key clinical point: Exosomes derived from chronic lymphocytic leukemia (CLL) cells induce stromal cell transition to cancer-associated fibroblasts.
Major finding: CLL exosomes and CLL cells cocultured in inserts induced similar gene expression changes in bone marrow mesenchymal stem cells.
Data source: In vitro and in vivo studies that used cells obtained from 21 CLL patients.
Disclosures: Jerome Paggetti, Ph.D., and coauthors reported having no disclosures.
Lenalidomide, thalidomide similar when combined with melphalan and prednisone for multiple myeloma
Combination treatment with melphalan, prednisone and either thalidomide or lenalidomide had comparable efficacy in elderly patients who were newly diagnosed with multiple myeloma. Lenalidomide was associated with less toxicity, however, and higher quality of life, according to results from a phase III trial.
The Eastern Cooperative Oncology Group (ECOG) E1a06 trial showed similar progression-free survival (PFS), overall survival (OS), and response rates for thalidomide and lenalidomide groups. PFS was 21 and 19 months, respectively (hazard ratio [HR], 0.84; 95% CI, 0.64-1.09; P = .186); OS was 53 and 48 months (HR, 0.88; 0.63-1.24; P = .476); partial response rates were 75% and 70%; and very good partial response rates were 25% and 32%.
Patients in the thalidomide group reported significantly more overall toxicity of grade 3 or greater, compared with the lenalidomide group, 73% vs. 58% (P = .007) (Blood 2015 Sep 17. doi:10.1182/blood-2014-12-613927).
The E1a06 trial included 306 patients, median age 76 years, with newly diagnosed multiple myeloma. The median follow up was 41 months, and 139 patients received maintenance therapy, with similar proportions from each arm.
The researchers compared melphalan, prednisone and thalidomide (MPT) with melphalan, prednisone, and lenalidomide (MPR), but administered lower doses of melphalan, with the hope that it would be better tolerated with less myelosuppression. Emerging data suggest benefit in using thalidomide or lenalidomide continuously, and the drugs were incorporated as ongoing maintenance therapy in both arms, MPT-T and MPR-R.
The data are similar to the recent Dutch-Belgium Hemato-Oncology Cooperative Group (HOVON) trial, which had median participant age of 73 years. Although response rates were higher in the HOVON study, possibly due to higher doses of melphalan and lenalidomide, PFS was essentially identical.
For reasons unclear but possibly due in part to higher median age, outcomes in this trial were inferior to the MM-015 trial, which examined continuous lenalidomide therapy and included patients of median age 71 years. In the MM-015 trial MPR-R arm, median PFS and OS were much higher at 31 and 56 months, respectively.
The difference was most obvious in younger patients, who are able to tolerate higher melphalan doses and myelosuppression, according to Dr. Keith Stewart of the division of hematology, Mayo Clinic Arizona, Scottsdale, and colleagues.
“We assume this explains the wide difference in the MM-015 and E1a06 and suggests that a dose-intense approach in younger patients receiving MPR-R is advisable,” they wrote.
In the this study’s MPR-R arm, patients older than 75 years had shorter PFS by 5 months than did younger patients, but toxicity reports were similar across ages.
The majority of patients experienced at least grade 3 toxicity: 58% of MPR-R and 73% of MPT-T patients. Only hematologic toxicities of grade 4 or higher were recorded, so estimates of overall hematologic toxicity are incomplete. Patients in the MPR-R group reported better quality of life, mostly attributable to lower neuropathy rates.
Dr. Stewart disclosed ties with Celgene, maker of lenalidomide (Revlimid); Novartis, Bristol Meyers Squib, Sanofi Aventis, and Janssen.
Combination treatment with melphalan, prednisone and either thalidomide or lenalidomide had comparable efficacy in elderly patients who were newly diagnosed with multiple myeloma. Lenalidomide was associated with less toxicity, however, and higher quality of life, according to results from a phase III trial.
The Eastern Cooperative Oncology Group (ECOG) E1a06 trial showed similar progression-free survival (PFS), overall survival (OS), and response rates for thalidomide and lenalidomide groups. PFS was 21 and 19 months, respectively (hazard ratio [HR], 0.84; 95% CI, 0.64-1.09; P = .186); OS was 53 and 48 months (HR, 0.88; 0.63-1.24; P = .476); partial response rates were 75% and 70%; and very good partial response rates were 25% and 32%.
Patients in the thalidomide group reported significantly more overall toxicity of grade 3 or greater, compared with the lenalidomide group, 73% vs. 58% (P = .007) (Blood 2015 Sep 17. doi:10.1182/blood-2014-12-613927).
The E1a06 trial included 306 patients, median age 76 years, with newly diagnosed multiple myeloma. The median follow up was 41 months, and 139 patients received maintenance therapy, with similar proportions from each arm.
The researchers compared melphalan, prednisone and thalidomide (MPT) with melphalan, prednisone, and lenalidomide (MPR), but administered lower doses of melphalan, with the hope that it would be better tolerated with less myelosuppression. Emerging data suggest benefit in using thalidomide or lenalidomide continuously, and the drugs were incorporated as ongoing maintenance therapy in both arms, MPT-T and MPR-R.
The data are similar to the recent Dutch-Belgium Hemato-Oncology Cooperative Group (HOVON) trial, which had median participant age of 73 years. Although response rates were higher in the HOVON study, possibly due to higher doses of melphalan and lenalidomide, PFS was essentially identical.
For reasons unclear but possibly due in part to higher median age, outcomes in this trial were inferior to the MM-015 trial, which examined continuous lenalidomide therapy and included patients of median age 71 years. In the MM-015 trial MPR-R arm, median PFS and OS were much higher at 31 and 56 months, respectively.
The difference was most obvious in younger patients, who are able to tolerate higher melphalan doses and myelosuppression, according to Dr. Keith Stewart of the division of hematology, Mayo Clinic Arizona, Scottsdale, and colleagues.
“We assume this explains the wide difference in the MM-015 and E1a06 and suggests that a dose-intense approach in younger patients receiving MPR-R is advisable,” they wrote.
In the this study’s MPR-R arm, patients older than 75 years had shorter PFS by 5 months than did younger patients, but toxicity reports were similar across ages.
The majority of patients experienced at least grade 3 toxicity: 58% of MPR-R and 73% of MPT-T patients. Only hematologic toxicities of grade 4 or higher were recorded, so estimates of overall hematologic toxicity are incomplete. Patients in the MPR-R group reported better quality of life, mostly attributable to lower neuropathy rates.
Dr. Stewart disclosed ties with Celgene, maker of lenalidomide (Revlimid); Novartis, Bristol Meyers Squib, Sanofi Aventis, and Janssen.
Combination treatment with melphalan, prednisone and either thalidomide or lenalidomide had comparable efficacy in elderly patients who were newly diagnosed with multiple myeloma. Lenalidomide was associated with less toxicity, however, and higher quality of life, according to results from a phase III trial.
The Eastern Cooperative Oncology Group (ECOG) E1a06 trial showed similar progression-free survival (PFS), overall survival (OS), and response rates for thalidomide and lenalidomide groups. PFS was 21 and 19 months, respectively (hazard ratio [HR], 0.84; 95% CI, 0.64-1.09; P = .186); OS was 53 and 48 months (HR, 0.88; 0.63-1.24; P = .476); partial response rates were 75% and 70%; and very good partial response rates were 25% and 32%.
Patients in the thalidomide group reported significantly more overall toxicity of grade 3 or greater, compared with the lenalidomide group, 73% vs. 58% (P = .007) (Blood 2015 Sep 17. doi:10.1182/blood-2014-12-613927).
The E1a06 trial included 306 patients, median age 76 years, with newly diagnosed multiple myeloma. The median follow up was 41 months, and 139 patients received maintenance therapy, with similar proportions from each arm.
The researchers compared melphalan, prednisone and thalidomide (MPT) with melphalan, prednisone, and lenalidomide (MPR), but administered lower doses of melphalan, with the hope that it would be better tolerated with less myelosuppression. Emerging data suggest benefit in using thalidomide or lenalidomide continuously, and the drugs were incorporated as ongoing maintenance therapy in both arms, MPT-T and MPR-R.
The data are similar to the recent Dutch-Belgium Hemato-Oncology Cooperative Group (HOVON) trial, which had median participant age of 73 years. Although response rates were higher in the HOVON study, possibly due to higher doses of melphalan and lenalidomide, PFS was essentially identical.
For reasons unclear but possibly due in part to higher median age, outcomes in this trial were inferior to the MM-015 trial, which examined continuous lenalidomide therapy and included patients of median age 71 years. In the MM-015 trial MPR-R arm, median PFS and OS were much higher at 31 and 56 months, respectively.
The difference was most obvious in younger patients, who are able to tolerate higher melphalan doses and myelosuppression, according to Dr. Keith Stewart of the division of hematology, Mayo Clinic Arizona, Scottsdale, and colleagues.
“We assume this explains the wide difference in the MM-015 and E1a06 and suggests that a dose-intense approach in younger patients receiving MPR-R is advisable,” they wrote.
In the this study’s MPR-R arm, patients older than 75 years had shorter PFS by 5 months than did younger patients, but toxicity reports were similar across ages.
The majority of patients experienced at least grade 3 toxicity: 58% of MPR-R and 73% of MPT-T patients. Only hematologic toxicities of grade 4 or higher were recorded, so estimates of overall hematologic toxicity are incomplete. Patients in the MPR-R group reported better quality of life, mostly attributable to lower neuropathy rates.
Dr. Stewart disclosed ties with Celgene, maker of lenalidomide (Revlimid); Novartis, Bristol Meyers Squib, Sanofi Aventis, and Janssen.
FROM BLOOD
Key clinical point: In elderly patients with multiple myeloma, outcomes were similar with melphalan and prednisone combined with either thalidomide or lenalidomide, but lenalidomide was associated with less toxicity and higher quality of life.
Major finding: Comparing thalidomide and lenalidomide arms, PFS was 21 and 19 months, respectively (hazard ratio [HR], 0.84; P = .186); OS was 53 and 48 months (HR, 0.88; P = .476); and overall toxicity of grade 3 or higher was 73% and 58% (P = .007).
Data source: A phase III trial (Eastern Cooperative Oncology Group E1a06) of 306 patients, median age 76 years, who had newly diagnosed multiple myeloma and received a combination of melphalan, prednisone and thalidomide (MPT) or melphalan, prednisone, and lenalidomide (MPR), with median follow up of 41 months.
Disclosures: Dr. Stewart disclosed ties with Celgene, maker of lenalidomide (Revlimid); Novartis, Bristol Meyers Squib, Sanofi Aventis, and Janssen.
Noncanonical Wnt signaling implicated in antiandrogen-resistant prostate cancer
Circulating tumor cells (CTCs) individually isolated from blood specimens of men with metastatic prostate cancer showed significant heterogeneity within individual patients, including androgen receptor mutations and multiple mRNA splicing variations, and significantly enriched Wnt signaling in enzalutamide-resistant patients, according to research published in Science.
More than half of the patients had multiple androgen receptor (AR) splice variants within different CTCs, and one in six CTCs simultaneously expressed several AR splice variants.
“The heterogeneity of CTCs in patients with CRPC [castration-resistant prostate cancer] stands in contrast to the striking homogeneity of AR signaling in single CTCs from untreated patients,” wrote Dr. David Miyamoto of the department of radiation oncology, Massachusetts General Hospital, Boston, and his colleagues (Science. 2015 Sept 16 [doi: 10.1126/science.aab0917]).
Researchers established single-cell RNA-sequencing profiles of tumor cells isolated after microfluidic enrichment from blood specimens of men with prostate cancer. Single-cell RNA-sequencing profiles were obtained from 77 CTCs isolated from 13 patients with metastatic prostate cancer, as well as comprehensive transcriptomes of primary prostate cancers from 12 patients.
Using the Pathway Interaction Database, the researchers identified 21 pathways upregulated in CTCs vs. primary tumors. The majority involved growth factor, cell adhesion, and hormone signaling.
Among the CTCs from patients with castration-resistant prostate cancer, 43% expressed at least one AR splice variant (8 of 11 CRPC patients), and 18% expressed more than one splice variant simultaneously (7 of 11 patients).
By contrast, no such alterations were found in the 12 primary prostate tumors analyzed, and one of four CTCs from patients with castration-sensitive prostate cancer that was previously untreated had low-level expression of an AR splice variant.
To evaluate heterogeneity in CTCs that may arise from additional selective pressure exerted by second-line therapies, the researchers retrospectively analyzed cells that were exposed (5 patients, 36 CTCs) or not exposed (8 patients, 41 CTCs) to enzalutamide, a potent AR inhibitor approved by the FDA for CRPC. CTCs from enzalutamide-resistant patients were significantly enriched in Wnt signaling, which mediates cell survival, proliferation, and motility.
Recent studies have shown that glucocorticoid receptor signaling contributes to antiandrogen resistance. Although GR transcripts were not significantly elevated among the enzalutamide exposed vs. nonexposed CTCs, there was an inverse relationship between GR expression and noncanonical Wnt signaling. The two AR-independent resistance pathways appear to predominate in different subsets of cells.
The study indicated that alterations conferring resistance to antiandrogen therapy, whether AR dependent or AR independent, varied considerably among CTCs. These results “point to complex and heterogeneous drug resistance mechanisms in advanced prostate cancer, which may affect therapeutic efficacy,” the authors noted.
Dr. Miyamoto reported having no relevant financial disclosures. Two of his coauthors reported financial ties to industry sources. The Massachusetts General Hospital has filed for patent protection on the CTC-iChip technology.
Through the use of a microfluidic device that isolates circulating tumor cells (CTCs) to allow molecular characterization, Miyamoto et al. (Science 2015 Sept 16) identified in certain patients a potential resistance mechanism to androgen-targeted therapies. The single-cell analysis offers several observations that would have been obscured by population averaging.
Contrary to the common notion that cancer cells often express several androgen receptor (AR) splice variants, and that these variants may homodimerize or compete with full-length AR for heterodimerization, the single CTC analysis indicated that just one in six CTCs expresses greater than one AR variant. Therapeutic strategies aimed at inhibiting AR-variant heterodimerization may need to be reconsidered, given that only a small minority of CTCs co-express AR splice variants.
The analysis showed significant upregulation in noncanonical Wnt signaling in CTCs from enzalutamide-resistant patients. Interestingly, expression of the glucocorticoid receptor signaling pathway, another AR-independent resistance pathway, appeared to be inversely related to noncanonical Wnt signaling.
Investigation of noncanonical Wnt signaling in taxane resistance may be interesting. Taxanes disrupt microtubules and inhibit cell division. Given the involvement of Wnt signaling in actin cytoskeleton remodeling, and the interaction of AR with microtubules for translocation to the nucleus, examining the role of Wnt signaling in both prostate cancer and taxane resistance may be useful.
The new information provided by single-cell RNA profiling of CTCs may guide choices for therapy or lead to earlier use of combination therapies, bringing us closer to CTC-guided precision medicine.
Dr. David Nanus is professor of medicine and of urology, division of hematology and medical oncology at Cornell University, New York. Dr. Paraskevi Giannakakou is associate professor of pharmacology in medicine in the department of medicine, division of hematology and medical oncology at Cornell. These remarks were part of an editorial accompanying the report (Science. 2015 Sept 16 [doi: 10.1126/science.aad2448]).
Through the use of a microfluidic device that isolates circulating tumor cells (CTCs) to allow molecular characterization, Miyamoto et al. (Science 2015 Sept 16) identified in certain patients a potential resistance mechanism to androgen-targeted therapies. The single-cell analysis offers several observations that would have been obscured by population averaging.
Contrary to the common notion that cancer cells often express several androgen receptor (AR) splice variants, and that these variants may homodimerize or compete with full-length AR for heterodimerization, the single CTC analysis indicated that just one in six CTCs expresses greater than one AR variant. Therapeutic strategies aimed at inhibiting AR-variant heterodimerization may need to be reconsidered, given that only a small minority of CTCs co-express AR splice variants.
The analysis showed significant upregulation in noncanonical Wnt signaling in CTCs from enzalutamide-resistant patients. Interestingly, expression of the glucocorticoid receptor signaling pathway, another AR-independent resistance pathway, appeared to be inversely related to noncanonical Wnt signaling.
Investigation of noncanonical Wnt signaling in taxane resistance may be interesting. Taxanes disrupt microtubules and inhibit cell division. Given the involvement of Wnt signaling in actin cytoskeleton remodeling, and the interaction of AR with microtubules for translocation to the nucleus, examining the role of Wnt signaling in both prostate cancer and taxane resistance may be useful.
The new information provided by single-cell RNA profiling of CTCs may guide choices for therapy or lead to earlier use of combination therapies, bringing us closer to CTC-guided precision medicine.
Dr. David Nanus is professor of medicine and of urology, division of hematology and medical oncology at Cornell University, New York. Dr. Paraskevi Giannakakou is associate professor of pharmacology in medicine in the department of medicine, division of hematology and medical oncology at Cornell. These remarks were part of an editorial accompanying the report (Science. 2015 Sept 16 [doi: 10.1126/science.aad2448]).
Through the use of a microfluidic device that isolates circulating tumor cells (CTCs) to allow molecular characterization, Miyamoto et al. (Science 2015 Sept 16) identified in certain patients a potential resistance mechanism to androgen-targeted therapies. The single-cell analysis offers several observations that would have been obscured by population averaging.
Contrary to the common notion that cancer cells often express several androgen receptor (AR) splice variants, and that these variants may homodimerize or compete with full-length AR for heterodimerization, the single CTC analysis indicated that just one in six CTCs expresses greater than one AR variant. Therapeutic strategies aimed at inhibiting AR-variant heterodimerization may need to be reconsidered, given that only a small minority of CTCs co-express AR splice variants.
The analysis showed significant upregulation in noncanonical Wnt signaling in CTCs from enzalutamide-resistant patients. Interestingly, expression of the glucocorticoid receptor signaling pathway, another AR-independent resistance pathway, appeared to be inversely related to noncanonical Wnt signaling.
Investigation of noncanonical Wnt signaling in taxane resistance may be interesting. Taxanes disrupt microtubules and inhibit cell division. Given the involvement of Wnt signaling in actin cytoskeleton remodeling, and the interaction of AR with microtubules for translocation to the nucleus, examining the role of Wnt signaling in both prostate cancer and taxane resistance may be useful.
The new information provided by single-cell RNA profiling of CTCs may guide choices for therapy or lead to earlier use of combination therapies, bringing us closer to CTC-guided precision medicine.
Dr. David Nanus is professor of medicine and of urology, division of hematology and medical oncology at Cornell University, New York. Dr. Paraskevi Giannakakou is associate professor of pharmacology in medicine in the department of medicine, division of hematology and medical oncology at Cornell. These remarks were part of an editorial accompanying the report (Science. 2015 Sept 16 [doi: 10.1126/science.aad2448]).
Circulating tumor cells (CTCs) individually isolated from blood specimens of men with metastatic prostate cancer showed significant heterogeneity within individual patients, including androgen receptor mutations and multiple mRNA splicing variations, and significantly enriched Wnt signaling in enzalutamide-resistant patients, according to research published in Science.
More than half of the patients had multiple androgen receptor (AR) splice variants within different CTCs, and one in six CTCs simultaneously expressed several AR splice variants.
“The heterogeneity of CTCs in patients with CRPC [castration-resistant prostate cancer] stands in contrast to the striking homogeneity of AR signaling in single CTCs from untreated patients,” wrote Dr. David Miyamoto of the department of radiation oncology, Massachusetts General Hospital, Boston, and his colleagues (Science. 2015 Sept 16 [doi: 10.1126/science.aab0917]).
Researchers established single-cell RNA-sequencing profiles of tumor cells isolated after microfluidic enrichment from blood specimens of men with prostate cancer. Single-cell RNA-sequencing profiles were obtained from 77 CTCs isolated from 13 patients with metastatic prostate cancer, as well as comprehensive transcriptomes of primary prostate cancers from 12 patients.
Using the Pathway Interaction Database, the researchers identified 21 pathways upregulated in CTCs vs. primary tumors. The majority involved growth factor, cell adhesion, and hormone signaling.
Among the CTCs from patients with castration-resistant prostate cancer, 43% expressed at least one AR splice variant (8 of 11 CRPC patients), and 18% expressed more than one splice variant simultaneously (7 of 11 patients).
By contrast, no such alterations were found in the 12 primary prostate tumors analyzed, and one of four CTCs from patients with castration-sensitive prostate cancer that was previously untreated had low-level expression of an AR splice variant.
To evaluate heterogeneity in CTCs that may arise from additional selective pressure exerted by second-line therapies, the researchers retrospectively analyzed cells that were exposed (5 patients, 36 CTCs) or not exposed (8 patients, 41 CTCs) to enzalutamide, a potent AR inhibitor approved by the FDA for CRPC. CTCs from enzalutamide-resistant patients were significantly enriched in Wnt signaling, which mediates cell survival, proliferation, and motility.
Recent studies have shown that glucocorticoid receptor signaling contributes to antiandrogen resistance. Although GR transcripts were not significantly elevated among the enzalutamide exposed vs. nonexposed CTCs, there was an inverse relationship between GR expression and noncanonical Wnt signaling. The two AR-independent resistance pathways appear to predominate in different subsets of cells.
The study indicated that alterations conferring resistance to antiandrogen therapy, whether AR dependent or AR independent, varied considerably among CTCs. These results “point to complex and heterogeneous drug resistance mechanisms in advanced prostate cancer, which may affect therapeutic efficacy,” the authors noted.
Dr. Miyamoto reported having no relevant financial disclosures. Two of his coauthors reported financial ties to industry sources. The Massachusetts General Hospital has filed for patent protection on the CTC-iChip technology.
Circulating tumor cells (CTCs) individually isolated from blood specimens of men with metastatic prostate cancer showed significant heterogeneity within individual patients, including androgen receptor mutations and multiple mRNA splicing variations, and significantly enriched Wnt signaling in enzalutamide-resistant patients, according to research published in Science.
More than half of the patients had multiple androgen receptor (AR) splice variants within different CTCs, and one in six CTCs simultaneously expressed several AR splice variants.
“The heterogeneity of CTCs in patients with CRPC [castration-resistant prostate cancer] stands in contrast to the striking homogeneity of AR signaling in single CTCs from untreated patients,” wrote Dr. David Miyamoto of the department of radiation oncology, Massachusetts General Hospital, Boston, and his colleagues (Science. 2015 Sept 16 [doi: 10.1126/science.aab0917]).
Researchers established single-cell RNA-sequencing profiles of tumor cells isolated after microfluidic enrichment from blood specimens of men with prostate cancer. Single-cell RNA-sequencing profiles were obtained from 77 CTCs isolated from 13 patients with metastatic prostate cancer, as well as comprehensive transcriptomes of primary prostate cancers from 12 patients.
Using the Pathway Interaction Database, the researchers identified 21 pathways upregulated in CTCs vs. primary tumors. The majority involved growth factor, cell adhesion, and hormone signaling.
Among the CTCs from patients with castration-resistant prostate cancer, 43% expressed at least one AR splice variant (8 of 11 CRPC patients), and 18% expressed more than one splice variant simultaneously (7 of 11 patients).
By contrast, no such alterations were found in the 12 primary prostate tumors analyzed, and one of four CTCs from patients with castration-sensitive prostate cancer that was previously untreated had low-level expression of an AR splice variant.
To evaluate heterogeneity in CTCs that may arise from additional selective pressure exerted by second-line therapies, the researchers retrospectively analyzed cells that were exposed (5 patients, 36 CTCs) or not exposed (8 patients, 41 CTCs) to enzalutamide, a potent AR inhibitor approved by the FDA for CRPC. CTCs from enzalutamide-resistant patients were significantly enriched in Wnt signaling, which mediates cell survival, proliferation, and motility.
Recent studies have shown that glucocorticoid receptor signaling contributes to antiandrogen resistance. Although GR transcripts were not significantly elevated among the enzalutamide exposed vs. nonexposed CTCs, there was an inverse relationship between GR expression and noncanonical Wnt signaling. The two AR-independent resistance pathways appear to predominate in different subsets of cells.
The study indicated that alterations conferring resistance to antiandrogen therapy, whether AR dependent or AR independent, varied considerably among CTCs. These results “point to complex and heterogeneous drug resistance mechanisms in advanced prostate cancer, which may affect therapeutic efficacy,” the authors noted.
Dr. Miyamoto reported having no relevant financial disclosures. Two of his coauthors reported financial ties to industry sources. The Massachusetts General Hospital has filed for patent protection on the CTC-iChip technology.
FROM SCIENCE
Key clinical point: Noncanonical Wnt signaling was significantly enriched in single circulating tumor cells (CTCs) from patients resistant to the androgen receptor inhibitor enzalutamide.
Major finding: RNA-sequencing profiles of CTCs isolated from men with prostate cancer indicated heterogeneity of cancer cells from an individual, including androgen receptor point mutations and multiple mRNA splicing variations, and significantly enriched Wnt signaling in enzalutamide-resistant patients.
Data source: Single-cell RNA-sequencing profiles were obtained from 77 CTCs isolated from 13 patients with metastatic prostate cancer, as well as comprehensive transcriptomes of primary prostate cancers from 12 patients.
Disclosures: Dr. Miyamoto reported having no relevant financial disclosures. Two of his coauthors have financial ties to industry sources. The Massachusetts General Hospital has filed for patent protection on the CTC-iChip technology.
Radiation field optimization may preserve salivary gland function
Preservation of saliva production in patients undergoing radiotherapy for head and neck cancer may be improved by use of radiation technologies that spare regions of the salivary gland abundant in stem cells, according to researchers.
By tracking the stem cell marker c-Kit, investigators determined that stem cells were not uniformly distributed throughout the parotid gland, but were most abundant in the major ducts in the central region. In rats, irradiation of the center area of the parotid gland, compared with the exterior, resulted in a greater loss, and progressive loss, of saliva production after 1 year. Tissue morphology indicated clear differences in long-term regenerative capacities of the different regions of the gland (Sci Trans Med. 2015 Sep 16. doi:10.1126/scitranslmed.aac4441).
The findings suggest that irradiation localized to the central region where the major ducts are found may result in gland dysfunction. The radiation dose to this area predicted dysfunction.
Stem cell distribution and parotid gland regeneration studies were done in mice and rats. A retrospective study of 74 patients with head and neck cancer correlated radiotherapy dose by region with salivary gland function.
To further evaluate the effects of radiation to the central gland region, the researchers compared two treatment plans in 22 patients. The first plan used a minimum mean dose to the entire gland, and the second minimized the dose to the critical region. The results indicated the second treatment plan resulted in dose redistribution that spared the stem cell region and was predicted to result in better posttreatment gland function.
Radiotherapy of tumors in the head and neck area often leads to irreversible hyposalivation, which severely compromises quality of life for patients. Whether radiation field optimization will eventually result in less xerostomia, or dry mouth, among patients remains to be determined, and a clinical trial is currently underway.
These findings confirm the importance of stem cells in long-term salivary gland function, according to Peter van Luijk, Ph.D., of the department of radiation oncology, University Medical Center, Groningen, the Netherlands, and his colleagues.
“It also suggests that autologous transductal stem cell transplantation may be a viable treatment strategy in patients where sparing this specific subvolume of the salivary glands is not feasible,” they wrote.
Dr. van Luijk reported having no disclosures.
Preservation of saliva production in patients undergoing radiotherapy for head and neck cancer may be improved by use of radiation technologies that spare regions of the salivary gland abundant in stem cells, according to researchers.
By tracking the stem cell marker c-Kit, investigators determined that stem cells were not uniformly distributed throughout the parotid gland, but were most abundant in the major ducts in the central region. In rats, irradiation of the center area of the parotid gland, compared with the exterior, resulted in a greater loss, and progressive loss, of saliva production after 1 year. Tissue morphology indicated clear differences in long-term regenerative capacities of the different regions of the gland (Sci Trans Med. 2015 Sep 16. doi:10.1126/scitranslmed.aac4441).
The findings suggest that irradiation localized to the central region where the major ducts are found may result in gland dysfunction. The radiation dose to this area predicted dysfunction.
Stem cell distribution and parotid gland regeneration studies were done in mice and rats. A retrospective study of 74 patients with head and neck cancer correlated radiotherapy dose by region with salivary gland function.
To further evaluate the effects of radiation to the central gland region, the researchers compared two treatment plans in 22 patients. The first plan used a minimum mean dose to the entire gland, and the second minimized the dose to the critical region. The results indicated the second treatment plan resulted in dose redistribution that spared the stem cell region and was predicted to result in better posttreatment gland function.
Radiotherapy of tumors in the head and neck area often leads to irreversible hyposalivation, which severely compromises quality of life for patients. Whether radiation field optimization will eventually result in less xerostomia, or dry mouth, among patients remains to be determined, and a clinical trial is currently underway.
These findings confirm the importance of stem cells in long-term salivary gland function, according to Peter van Luijk, Ph.D., of the department of radiation oncology, University Medical Center, Groningen, the Netherlands, and his colleagues.
“It also suggests that autologous transductal stem cell transplantation may be a viable treatment strategy in patients where sparing this specific subvolume of the salivary glands is not feasible,” they wrote.
Dr. van Luijk reported having no disclosures.
Preservation of saliva production in patients undergoing radiotherapy for head and neck cancer may be improved by use of radiation technologies that spare regions of the salivary gland abundant in stem cells, according to researchers.
By tracking the stem cell marker c-Kit, investigators determined that stem cells were not uniformly distributed throughout the parotid gland, but were most abundant in the major ducts in the central region. In rats, irradiation of the center area of the parotid gland, compared with the exterior, resulted in a greater loss, and progressive loss, of saliva production after 1 year. Tissue morphology indicated clear differences in long-term regenerative capacities of the different regions of the gland (Sci Trans Med. 2015 Sep 16. doi:10.1126/scitranslmed.aac4441).
The findings suggest that irradiation localized to the central region where the major ducts are found may result in gland dysfunction. The radiation dose to this area predicted dysfunction.
Stem cell distribution and parotid gland regeneration studies were done in mice and rats. A retrospective study of 74 patients with head and neck cancer correlated radiotherapy dose by region with salivary gland function.
To further evaluate the effects of radiation to the central gland region, the researchers compared two treatment plans in 22 patients. The first plan used a minimum mean dose to the entire gland, and the second minimized the dose to the critical region. The results indicated the second treatment plan resulted in dose redistribution that spared the stem cell region and was predicted to result in better posttreatment gland function.
Radiotherapy of tumors in the head and neck area often leads to irreversible hyposalivation, which severely compromises quality of life for patients. Whether radiation field optimization will eventually result in less xerostomia, or dry mouth, among patients remains to be determined, and a clinical trial is currently underway.
These findings confirm the importance of stem cells in long-term salivary gland function, according to Peter van Luijk, Ph.D., of the department of radiation oncology, University Medical Center, Groningen, the Netherlands, and his colleagues.
“It also suggests that autologous transductal stem cell transplantation may be a viable treatment strategy in patients where sparing this specific subvolume of the salivary glands is not feasible,” they wrote.
Dr. van Luijk reported having no disclosures.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Optimized radiotherapy that spares salivary gland stem cells during treatment for head and neck cancer may preserve patients’ saliva production.
Major finding: Salivary gland stem cells carry long-term regenerative capacity, and the cells are most abundant in major ducts at the central part of the gland, which can be spared with current radiotherapy technology.
Data source: Stem cell distribution and parotid gland regeneration studies were done in mice and rats. A retrospective study of 74 patients with head and neck cancer correlated radiotherapy dose by region with salivary gland function. Radiation field optimization was prospectively studied in 22 patients.
Disclosures: Mr. van Luijk reported having no disclosures.
Individualized approach improves surveillance after renal cell carcinoma resection
A novel approach to oncologic surveillance after surgical resection for renal cell carcinoma (RCC) that incorporates patient age, Charlson comorbidity index, pathologic tumor stage, and relapse location–specific data, may provide better recommendations for surveillance duration than current guidelines, researchers reported.
“By providing more individualized recommendations and by eliminating oversimplified time thresholds for surveillance, this strategy, we believe, represents an improvement over current guidelines,” wrote Dr. Suzanne Stewart-Merrill of the department of urology, Mayo Clinic, Rochester, Minn., and colleagues (J Clin Oncol. 2015 Sep 7. doi: 10.1200/JCO.2015.61.8009).
Guidelines suggest surveillance durations based on cumulative incidence of recurrences, but do not account for individual patient risk factors. Adherence to the guidelines could miss up to one-third of all recurrences, the authors noted. The longest surveillance duration advocated by the National Comprehensive Cancer Network or the American Urological Association is 5 years.
Researchers used Weibull models for risk of recurrence, stratified by stage and relapse location, combined with risk estimates for non-RCC death, to estimate the optimum surveillance duration. For example, for patients aged 50-59 years with pT1Nx-0 disease and a Charlson comorbidity index of less than or equal to 1, risk of non-RCC death exceeded the risk of abdominal recurrence at 7 years, marking the recommended surveillance duration for that subgroup. The recommended surveillance duration for patients with similar characteristics aged 60-69 years is 2.5 years, and for patients aged 70-79 years it is 1.5 years.
When taking into account individual risk factors, the method produced a wide range of surveillance durations. For patients under 50 years old with pT1Nx-0 disease and a Charlson comorbidity index less than or equal to 1, the risk of non-RCC death did not exceed the risk of abdominal recurrence for more than 20 years. By contrast, patients with pT1Nx-0 disease and Charlson comorbidity index greater than or equal to 2, had a higher risk of non-RCC death than risk of abdominal recurrence at 30 days after surgery.
The retrospective study of 2,511 patients who underwent surgical resection for M0 sporadic RCC between 1990 and 2008 had a median follow up of 9.0 years (interquartile range 6.4-12.7 years).
The researchers noted that recurrences beyond 5 years are fairly common, with up to 15% of patients, by one study’s estimate, experiencing recurrence during the decade following surgery. Cumulative incidence of recurrence does not capture how a patient’s risk of recurrence changes with time or how comorbid conditions influence risk.
The proposed surveillance method results in shorter surveillance durations for some patients and longer surveillance durations than currently recommended for others. This diversity in stopping points “better reflects the range of disease courses appreciated in patients with RCC on the basis of the interplay that occurs between the risk of recurrence and other competing health factors,” Dr. Stewart-Merrill and associates explained.
Dr. Stewart-Merrill reported having no disclosures.
A novel approach to oncologic surveillance after surgical resection for renal cell carcinoma (RCC) that incorporates patient age, Charlson comorbidity index, pathologic tumor stage, and relapse location–specific data, may provide better recommendations for surveillance duration than current guidelines, researchers reported.
“By providing more individualized recommendations and by eliminating oversimplified time thresholds for surveillance, this strategy, we believe, represents an improvement over current guidelines,” wrote Dr. Suzanne Stewart-Merrill of the department of urology, Mayo Clinic, Rochester, Minn., and colleagues (J Clin Oncol. 2015 Sep 7. doi: 10.1200/JCO.2015.61.8009).
Guidelines suggest surveillance durations based on cumulative incidence of recurrences, but do not account for individual patient risk factors. Adherence to the guidelines could miss up to one-third of all recurrences, the authors noted. The longest surveillance duration advocated by the National Comprehensive Cancer Network or the American Urological Association is 5 years.
Researchers used Weibull models for risk of recurrence, stratified by stage and relapse location, combined with risk estimates for non-RCC death, to estimate the optimum surveillance duration. For example, for patients aged 50-59 years with pT1Nx-0 disease and a Charlson comorbidity index of less than or equal to 1, risk of non-RCC death exceeded the risk of abdominal recurrence at 7 years, marking the recommended surveillance duration for that subgroup. The recommended surveillance duration for patients with similar characteristics aged 60-69 years is 2.5 years, and for patients aged 70-79 years it is 1.5 years.
When taking into account individual risk factors, the method produced a wide range of surveillance durations. For patients under 50 years old with pT1Nx-0 disease and a Charlson comorbidity index less than or equal to 1, the risk of non-RCC death did not exceed the risk of abdominal recurrence for more than 20 years. By contrast, patients with pT1Nx-0 disease and Charlson comorbidity index greater than or equal to 2, had a higher risk of non-RCC death than risk of abdominal recurrence at 30 days after surgery.
The retrospective study of 2,511 patients who underwent surgical resection for M0 sporadic RCC between 1990 and 2008 had a median follow up of 9.0 years (interquartile range 6.4-12.7 years).
The researchers noted that recurrences beyond 5 years are fairly common, with up to 15% of patients, by one study’s estimate, experiencing recurrence during the decade following surgery. Cumulative incidence of recurrence does not capture how a patient’s risk of recurrence changes with time or how comorbid conditions influence risk.
The proposed surveillance method results in shorter surveillance durations for some patients and longer surveillance durations than currently recommended for others. This diversity in stopping points “better reflects the range of disease courses appreciated in patients with RCC on the basis of the interplay that occurs between the risk of recurrence and other competing health factors,” Dr. Stewart-Merrill and associates explained.
Dr. Stewart-Merrill reported having no disclosures.
A novel approach to oncologic surveillance after surgical resection for renal cell carcinoma (RCC) that incorporates patient age, Charlson comorbidity index, pathologic tumor stage, and relapse location–specific data, may provide better recommendations for surveillance duration than current guidelines, researchers reported.
“By providing more individualized recommendations and by eliminating oversimplified time thresholds for surveillance, this strategy, we believe, represents an improvement over current guidelines,” wrote Dr. Suzanne Stewart-Merrill of the department of urology, Mayo Clinic, Rochester, Minn., and colleagues (J Clin Oncol. 2015 Sep 7. doi: 10.1200/JCO.2015.61.8009).
Guidelines suggest surveillance durations based on cumulative incidence of recurrences, but do not account for individual patient risk factors. Adherence to the guidelines could miss up to one-third of all recurrences, the authors noted. The longest surveillance duration advocated by the National Comprehensive Cancer Network or the American Urological Association is 5 years.
Researchers used Weibull models for risk of recurrence, stratified by stage and relapse location, combined with risk estimates for non-RCC death, to estimate the optimum surveillance duration. For example, for patients aged 50-59 years with pT1Nx-0 disease and a Charlson comorbidity index of less than or equal to 1, risk of non-RCC death exceeded the risk of abdominal recurrence at 7 years, marking the recommended surveillance duration for that subgroup. The recommended surveillance duration for patients with similar characteristics aged 60-69 years is 2.5 years, and for patients aged 70-79 years it is 1.5 years.
When taking into account individual risk factors, the method produced a wide range of surveillance durations. For patients under 50 years old with pT1Nx-0 disease and a Charlson comorbidity index less than or equal to 1, the risk of non-RCC death did not exceed the risk of abdominal recurrence for more than 20 years. By contrast, patients with pT1Nx-0 disease and Charlson comorbidity index greater than or equal to 2, had a higher risk of non-RCC death than risk of abdominal recurrence at 30 days after surgery.
The retrospective study of 2,511 patients who underwent surgical resection for M0 sporadic RCC between 1990 and 2008 had a median follow up of 9.0 years (interquartile range 6.4-12.7 years).
The researchers noted that recurrences beyond 5 years are fairly common, with up to 15% of patients, by one study’s estimate, experiencing recurrence during the decade following surgery. Cumulative incidence of recurrence does not capture how a patient’s risk of recurrence changes with time or how comorbid conditions influence risk.
The proposed surveillance method results in shorter surveillance durations for some patients and longer surveillance durations than currently recommended for others. This diversity in stopping points “better reflects the range of disease courses appreciated in patients with RCC on the basis of the interplay that occurs between the risk of recurrence and other competing health factors,” Dr. Stewart-Merrill and associates explained.
Dr. Stewart-Merrill reported having no disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: An individualized approach to surveillance after surgical resection in patients with renal cell carcinoma may provide better patient benefit and resource allocation.
Major finding: Weibull models for risk of recurrence, stratified by stage and relapse location, combined with risk estimates for non-RCC death, provided the basis for recommendations on surveillance duration.
Data source: Retrospective study of 2,511 patients who underwent surgical resection for M0 sporadic RCC between 1990 and 2008, with a median follow up of 9.0 years (interquartile range 6.4-12.7 years).
Disclosures: Dr. Stewart-Merrill reported having no disclosures.
Ruxolitinib shows promise in refractory metastatic pancreatic cancer
Ruxolitinib showed clinical activity in patients with metastatic pancreatic cancer refractory to gemcitabine, particularly in those with elevated C-reactive protein (CRP) levels indicating systemic inflammation, according a study published online in Journal of Clinical Oncology.
Patients randomly assigned to receive ruxolitinib + capecitabine had overall survival similar to that of the placebo + capecitabine group; however, in the subgroup of patients with elevated CRP (greater than the median in the study of 13 mg/L), median overall survival was significantly greater with ruxolitinib: 2.7 vs. 1.8 months (HR, 0.47; 95% CI, 0.26-0.85; P = .011).
Ruxolitinib inhibits Janus kinases (JAK1/JAK2), part of the JAK/STAT pathway that is downstream from multiple receptor tyrosine kinases and involved in inflammatory responses of both tumor and normal tissue. JAK/STAT inhibition represents a novel cancer treatment strategy that may impact both cancer cells and the immune response to malignancy.
“These results additionally support the importance of cytokine signaling and JAK/STAT signaling in pancreatic cancer and highlight the potential role for JAK inhibition as a novel therapeutic strategy for these patients,” wrote Dr. Herbert Hurwitz of Duke University, Durham, N.C., and colleagues (J Clin Oncol. 2015 Sep 7. doi:10.1200/JCO.2015.61.4578).
Patients on ruxolitinib showed benefit in progression-free survival, tumor burden, and clinical benefit response (a composite of pain intensity, analgesic use, performance status, and body weight). Clinical benefit response was 12.5% in the ruxolitinib group compared with 1.5% for placebo (P = .017). These findings suggest ruxolitinib affects the host response to tumor as well as the tumor itself.
The double-blind phase II study comprised 127 patients from 41 U.S. centers; 64 received ruxolitinib + capecitabine and 63 received placebo + capecitabine.
Anemia was the most common hematologic adverse event, with grade 3 anemia occurring in 15.3% and 1.7% of the ruxolitinib and placebo groups, respectively. Nonhematologic grade 3 or greater adverse events that occurred more frequently for ruxolitinib included stomatitis, pneumonia, and pulmonary embolism.
Incyte Corporation funded the study. Dr. Hurwitz reported consulting or advisory roles with Incyte Corporation, Genentech, ImCone Systems, Bristol-Myers Squibb, Sanofi, Eli Lilly, Regeneron Pharmaceuticals, Amgen, Novartis, Bayer AG, TRACON Pharmaceuticals, Acceleron Pharma, and GlaxoSmithKline. All of his coauthors were employed by or received research funding from Incyte Corporation, as well as other industry sources.
Ruxolitinib showed clinical activity in patients with metastatic pancreatic cancer refractory to gemcitabine, particularly in those with elevated C-reactive protein (CRP) levels indicating systemic inflammation, according a study published online in Journal of Clinical Oncology.
Patients randomly assigned to receive ruxolitinib + capecitabine had overall survival similar to that of the placebo + capecitabine group; however, in the subgroup of patients with elevated CRP (greater than the median in the study of 13 mg/L), median overall survival was significantly greater with ruxolitinib: 2.7 vs. 1.8 months (HR, 0.47; 95% CI, 0.26-0.85; P = .011).
Ruxolitinib inhibits Janus kinases (JAK1/JAK2), part of the JAK/STAT pathway that is downstream from multiple receptor tyrosine kinases and involved in inflammatory responses of both tumor and normal tissue. JAK/STAT inhibition represents a novel cancer treatment strategy that may impact both cancer cells and the immune response to malignancy.
“These results additionally support the importance of cytokine signaling and JAK/STAT signaling in pancreatic cancer and highlight the potential role for JAK inhibition as a novel therapeutic strategy for these patients,” wrote Dr. Herbert Hurwitz of Duke University, Durham, N.C., and colleagues (J Clin Oncol. 2015 Sep 7. doi:10.1200/JCO.2015.61.4578).
Patients on ruxolitinib showed benefit in progression-free survival, tumor burden, and clinical benefit response (a composite of pain intensity, analgesic use, performance status, and body weight). Clinical benefit response was 12.5% in the ruxolitinib group compared with 1.5% for placebo (P = .017). These findings suggest ruxolitinib affects the host response to tumor as well as the tumor itself.
The double-blind phase II study comprised 127 patients from 41 U.S. centers; 64 received ruxolitinib + capecitabine and 63 received placebo + capecitabine.
Anemia was the most common hematologic adverse event, with grade 3 anemia occurring in 15.3% and 1.7% of the ruxolitinib and placebo groups, respectively. Nonhematologic grade 3 or greater adverse events that occurred more frequently for ruxolitinib included stomatitis, pneumonia, and pulmonary embolism.
Incyte Corporation funded the study. Dr. Hurwitz reported consulting or advisory roles with Incyte Corporation, Genentech, ImCone Systems, Bristol-Myers Squibb, Sanofi, Eli Lilly, Regeneron Pharmaceuticals, Amgen, Novartis, Bayer AG, TRACON Pharmaceuticals, Acceleron Pharma, and GlaxoSmithKline. All of his coauthors were employed by or received research funding from Incyte Corporation, as well as other industry sources.
Ruxolitinib showed clinical activity in patients with metastatic pancreatic cancer refractory to gemcitabine, particularly in those with elevated C-reactive protein (CRP) levels indicating systemic inflammation, according a study published online in Journal of Clinical Oncology.
Patients randomly assigned to receive ruxolitinib + capecitabine had overall survival similar to that of the placebo + capecitabine group; however, in the subgroup of patients with elevated CRP (greater than the median in the study of 13 mg/L), median overall survival was significantly greater with ruxolitinib: 2.7 vs. 1.8 months (HR, 0.47; 95% CI, 0.26-0.85; P = .011).
Ruxolitinib inhibits Janus kinases (JAK1/JAK2), part of the JAK/STAT pathway that is downstream from multiple receptor tyrosine kinases and involved in inflammatory responses of both tumor and normal tissue. JAK/STAT inhibition represents a novel cancer treatment strategy that may impact both cancer cells and the immune response to malignancy.
“These results additionally support the importance of cytokine signaling and JAK/STAT signaling in pancreatic cancer and highlight the potential role for JAK inhibition as a novel therapeutic strategy for these patients,” wrote Dr. Herbert Hurwitz of Duke University, Durham, N.C., and colleagues (J Clin Oncol. 2015 Sep 7. doi:10.1200/JCO.2015.61.4578).
Patients on ruxolitinib showed benefit in progression-free survival, tumor burden, and clinical benefit response (a composite of pain intensity, analgesic use, performance status, and body weight). Clinical benefit response was 12.5% in the ruxolitinib group compared with 1.5% for placebo (P = .017). These findings suggest ruxolitinib affects the host response to tumor as well as the tumor itself.
The double-blind phase II study comprised 127 patients from 41 U.S. centers; 64 received ruxolitinib + capecitabine and 63 received placebo + capecitabine.
Anemia was the most common hematologic adverse event, with grade 3 anemia occurring in 15.3% and 1.7% of the ruxolitinib and placebo groups, respectively. Nonhematologic grade 3 or greater adverse events that occurred more frequently for ruxolitinib included stomatitis, pneumonia, and pulmonary embolism.
Incyte Corporation funded the study. Dr. Hurwitz reported consulting or advisory roles with Incyte Corporation, Genentech, ImCone Systems, Bristol-Myers Squibb, Sanofi, Eli Lilly, Regeneron Pharmaceuticals, Amgen, Novartis, Bayer AG, TRACON Pharmaceuticals, Acceleron Pharma, and GlaxoSmithKline. All of his coauthors were employed by or received research funding from Incyte Corporation, as well as other industry sources.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Ruxolitinib showed clinical activity in patients with refractory metastatic pancreatic cancer, particularly in those with signs of systemic inflammation.
Major finding: In patients with elevated C-reactive protein who received ruxolitinib vs. placebo, the median overall survival was 2.7 vs. 1.8 months (HR, 0.47; 95% CI, 0.26-0.85; P = .011).
Data source: A double-blind phase II study of 127 patients, 64 who received ruxolitinib + capecitabine and 63 who received placebo + capecitabine.
Disclosures: Incyte Corporation funded the study. Dr. Hurwitz reported consulting or advisory roles with Incyte Corporation, Genentech, ImCone Systems, Bristol-Myers Squibb, Sanofi, Eli Lilly, Regeneron Pharmaceuticals, Amgen, Novartis, Bayer AG, TRACON Pharmaceuticals, Acceleron Pharma, and GlaxoSmithKline. All of his coauthors were employed by or received research funding from Incyte Corporation, as well as other industry sources.
Myeloma precursor linked to Agent Orange exposure
Vietnam War veterans exposed to Agent Orange have a twofold higher prevalence of monoclonal gammopathy of undetermined significance (MGUS), compared with control veterans, providing the first scientific evidence for a link between the multiple myeloma precursor and Agent Orange exposure, researchers reported online in JAMA Oncology.
Serum samples from U.S. Air Force personnel who conducted aerial herbicide spray missions of Agent Orange in the Vietnam War from 1962 to 1971 (Operation Ranch Hand) were compared with samples from veterans who served in Vietnam during the same time period but were not involved in herbicide spray missions. The human carcinogen TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) was a contaminant found in variable amounts in Agent Orange, and levels of TCDD measured in the veteran’s serum samples were associated with cohort status. For example, TCDD levels greater than 10.92 parts per trillion were observed in 47.5% of the Ranch Hand cohort, compared with just 2.5% of the control veteran cohort. The risk of MGUS increased with increasing body burden of TCDD, although the trend was not significant.
MGUS prevalence in the Ranch Hand group was 7.1% (34 of 479) compared with 3.1% (15 of 479) in the control group (adjusted odds ratio, 2.37; 95% confidence interval, 1.27-4.44; P = .007).
“Our findings of increased MGUS risk among Ranch Hand veterans supports an association between Agent Orange exposure and multiple myeloma,” wrote Dr. Ola Landgren, Chief of Myeloma Service at Memorial Sloan Kettering, New York, and his colleagues (JAMA Oncol. 2015 Sep 3; [doi:10.1001/jamaoncol.2015.2938].
Serum samples were collected in 2002 from U.S. Air Force personnel who conducted aerial herbicide spray missions from 1962 to 1971 (n = 479) and control veterans who were not involved in the aerial missions (n = 479). The study was a follow-up of the Air Force Health Study. The first TCDD measurements were made in 1987, up to 25 years after Agent Orange exposure.
Ranch Hand veterans younger than 70 years had a significantly increased MGUS risk (OR, 3.4; 95% CI, 1.46-8.13; P = .004), but those older than 70 years had no increased MGUS risk.
Previous studies have pointed to an elevated risk of multiple myeloma among agricultural workers, and pesticides are thought to be responsible for the association.
The study was supported by the Agency for Toxic Substances and Disease Registry, the National Cancer Institute, and the Air Force Health Study Assets Research Program. Dr. Landgren reported having consulting or advisory roles with Onyx Pharmaceuticals/AMGEN, Celgene, Bristol-Myers Squibb, Jansen, and Millennium Pharmaceuticals/Takeda.
Given that all multiple myeloma cases originate from MGUS, the study by Landgren et al. provides the first scientific evidence for a direct link between multiple myeloma and exposure to Agent Orange. The study was based on well-characterized samples, a long follow-up period (25 years), and measurements of toxin exposure. The results are in line with an Agricultural Health Study that showed an almost twofold higher prevalence of MGUS among male pesticide applicators.
A weakness of this study was the variable time between exposure to Agent Orange and measurement of serum TCDD, which ranged from 16 to 25 years. It is unclear whether the peak level of TCDD exposure or the longer-term persistence of the substance in the body plays a role in MGUS transformation.
The study also highlights the importance of tissue banking to allow modern methods to investigate unanswered questions. Newer technologies can be applied to stored samples to better characterize the occurrence and progression of diseases. An important question that remains is whether TCDD exposure induces MGUS, which in turn requires additional mutations to undergo malignant transformation, or whether TCDD exposure creates the genomic instability that leads myeloma.
Dr. Nikhil Munshi is professor of medicine at the Dana-Farber Cancer Institute, Boston. These remarks were part of an editorial accompanying the report (JAMA Oncol. 2015 Sep 3; [doi:10.1001/jamaoncol.2015.2938]. Dr. Munshi had no disclosures to report.
Given that all multiple myeloma cases originate from MGUS, the study by Landgren et al. provides the first scientific evidence for a direct link between multiple myeloma and exposure to Agent Orange. The study was based on well-characterized samples, a long follow-up period (25 years), and measurements of toxin exposure. The results are in line with an Agricultural Health Study that showed an almost twofold higher prevalence of MGUS among male pesticide applicators.
A weakness of this study was the variable time between exposure to Agent Orange and measurement of serum TCDD, which ranged from 16 to 25 years. It is unclear whether the peak level of TCDD exposure or the longer-term persistence of the substance in the body plays a role in MGUS transformation.
The study also highlights the importance of tissue banking to allow modern methods to investigate unanswered questions. Newer technologies can be applied to stored samples to better characterize the occurrence and progression of diseases. An important question that remains is whether TCDD exposure induces MGUS, which in turn requires additional mutations to undergo malignant transformation, or whether TCDD exposure creates the genomic instability that leads myeloma.
Dr. Nikhil Munshi is professor of medicine at the Dana-Farber Cancer Institute, Boston. These remarks were part of an editorial accompanying the report (JAMA Oncol. 2015 Sep 3; [doi:10.1001/jamaoncol.2015.2938]. Dr. Munshi had no disclosures to report.
Given that all multiple myeloma cases originate from MGUS, the study by Landgren et al. provides the first scientific evidence for a direct link between multiple myeloma and exposure to Agent Orange. The study was based on well-characterized samples, a long follow-up period (25 years), and measurements of toxin exposure. The results are in line with an Agricultural Health Study that showed an almost twofold higher prevalence of MGUS among male pesticide applicators.
A weakness of this study was the variable time between exposure to Agent Orange and measurement of serum TCDD, which ranged from 16 to 25 years. It is unclear whether the peak level of TCDD exposure or the longer-term persistence of the substance in the body plays a role in MGUS transformation.
The study also highlights the importance of tissue banking to allow modern methods to investigate unanswered questions. Newer technologies can be applied to stored samples to better characterize the occurrence and progression of diseases. An important question that remains is whether TCDD exposure induces MGUS, which in turn requires additional mutations to undergo malignant transformation, or whether TCDD exposure creates the genomic instability that leads myeloma.
Dr. Nikhil Munshi is professor of medicine at the Dana-Farber Cancer Institute, Boston. These remarks were part of an editorial accompanying the report (JAMA Oncol. 2015 Sep 3; [doi:10.1001/jamaoncol.2015.2938]. Dr. Munshi had no disclosures to report.
Vietnam War veterans exposed to Agent Orange have a twofold higher prevalence of monoclonal gammopathy of undetermined significance (MGUS), compared with control veterans, providing the first scientific evidence for a link between the multiple myeloma precursor and Agent Orange exposure, researchers reported online in JAMA Oncology.
Serum samples from U.S. Air Force personnel who conducted aerial herbicide spray missions of Agent Orange in the Vietnam War from 1962 to 1971 (Operation Ranch Hand) were compared with samples from veterans who served in Vietnam during the same time period but were not involved in herbicide spray missions. The human carcinogen TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) was a contaminant found in variable amounts in Agent Orange, and levels of TCDD measured in the veteran’s serum samples were associated with cohort status. For example, TCDD levels greater than 10.92 parts per trillion were observed in 47.5% of the Ranch Hand cohort, compared with just 2.5% of the control veteran cohort. The risk of MGUS increased with increasing body burden of TCDD, although the trend was not significant.
MGUS prevalence in the Ranch Hand group was 7.1% (34 of 479) compared with 3.1% (15 of 479) in the control group (adjusted odds ratio, 2.37; 95% confidence interval, 1.27-4.44; P = .007).
“Our findings of increased MGUS risk among Ranch Hand veterans supports an association between Agent Orange exposure and multiple myeloma,” wrote Dr. Ola Landgren, Chief of Myeloma Service at Memorial Sloan Kettering, New York, and his colleagues (JAMA Oncol. 2015 Sep 3; [doi:10.1001/jamaoncol.2015.2938].
Serum samples were collected in 2002 from U.S. Air Force personnel who conducted aerial herbicide spray missions from 1962 to 1971 (n = 479) and control veterans who were not involved in the aerial missions (n = 479). The study was a follow-up of the Air Force Health Study. The first TCDD measurements were made in 1987, up to 25 years after Agent Orange exposure.
Ranch Hand veterans younger than 70 years had a significantly increased MGUS risk (OR, 3.4; 95% CI, 1.46-8.13; P = .004), but those older than 70 years had no increased MGUS risk.
Previous studies have pointed to an elevated risk of multiple myeloma among agricultural workers, and pesticides are thought to be responsible for the association.
The study was supported by the Agency for Toxic Substances and Disease Registry, the National Cancer Institute, and the Air Force Health Study Assets Research Program. Dr. Landgren reported having consulting or advisory roles with Onyx Pharmaceuticals/AMGEN, Celgene, Bristol-Myers Squibb, Jansen, and Millennium Pharmaceuticals/Takeda.
Vietnam War veterans exposed to Agent Orange have a twofold higher prevalence of monoclonal gammopathy of undetermined significance (MGUS), compared with control veterans, providing the first scientific evidence for a link between the multiple myeloma precursor and Agent Orange exposure, researchers reported online in JAMA Oncology.
Serum samples from U.S. Air Force personnel who conducted aerial herbicide spray missions of Agent Orange in the Vietnam War from 1962 to 1971 (Operation Ranch Hand) were compared with samples from veterans who served in Vietnam during the same time period but were not involved in herbicide spray missions. The human carcinogen TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) was a contaminant found in variable amounts in Agent Orange, and levels of TCDD measured in the veteran’s serum samples were associated with cohort status. For example, TCDD levels greater than 10.92 parts per trillion were observed in 47.5% of the Ranch Hand cohort, compared with just 2.5% of the control veteran cohort. The risk of MGUS increased with increasing body burden of TCDD, although the trend was not significant.
MGUS prevalence in the Ranch Hand group was 7.1% (34 of 479) compared with 3.1% (15 of 479) in the control group (adjusted odds ratio, 2.37; 95% confidence interval, 1.27-4.44; P = .007).
“Our findings of increased MGUS risk among Ranch Hand veterans supports an association between Agent Orange exposure and multiple myeloma,” wrote Dr. Ola Landgren, Chief of Myeloma Service at Memorial Sloan Kettering, New York, and his colleagues (JAMA Oncol. 2015 Sep 3; [doi:10.1001/jamaoncol.2015.2938].
Serum samples were collected in 2002 from U.S. Air Force personnel who conducted aerial herbicide spray missions from 1962 to 1971 (n = 479) and control veterans who were not involved in the aerial missions (n = 479). The study was a follow-up of the Air Force Health Study. The first TCDD measurements were made in 1987, up to 25 years after Agent Orange exposure.
Ranch Hand veterans younger than 70 years had a significantly increased MGUS risk (OR, 3.4; 95% CI, 1.46-8.13; P = .004), but those older than 70 years had no increased MGUS risk.
Previous studies have pointed to an elevated risk of multiple myeloma among agricultural workers, and pesticides are thought to be responsible for the association.
The study was supported by the Agency for Toxic Substances and Disease Registry, the National Cancer Institute, and the Air Force Health Study Assets Research Program. Dr. Landgren reported having consulting or advisory roles with Onyx Pharmaceuticals/AMGEN, Celgene, Bristol-Myers Squibb, Jansen, and Millennium Pharmaceuticals/Takeda.
FROM JAMA ONCOLOGY
Key clinical point: Prevalence of monoclonal gammopathy of undetermined significance (MGUS) was twofold higher in Vietnam War veterans exposed to Agent Orange, compared with veterans not exposed.
Major finding: Among veterans who conducted aerial spray missions using Agent Orange, MGUS prevalence was 7.1% (34 of 479), compared with 3.1% (15 of 479) among comparison veterans (adjusted odds ratio, 2.37; 95% CI, 1.27-4.44; P = .007).
Data source: Serum samples collected in 2002 from U.S. Air Force personnel exposed to Agent Orange from 1962 to 1971 (n = 479) and control veterans who were not involved in aerial spray missions (n = 479).
Disclosures: The study was supported by the Agency for Toxic Substances and Disease Registry, the National Cancer Institute, and the Air Force Health Study Assets Research Program. Dr. Landgren reported having consulting or advisory roles with Onyx Pharmaceuticals/AMGEN, Celgene, Bristol-Myers Squibb, Jansen, and Millennium Pharmaceuticals/Takeda.
Adjuvant erlotinib showed no benefit in NSCLC patients
Among patients with completely resected non–small-cell lung cancer (NSCLC) whose tumors expressed epidermal growth factor receptor (EGFR), disease-free survival (DFS) rates were similar between erlotinib and placebo groups, according to results from the RADIANT phase III trial.
EGFR-activating mutations (EGFRm) were observed in a subgroup of patients (16.5%, 89 with del19 and 72 with L858R mutations) but these patients were not stratified by mutation status, which limited interpretation of the results. For the erlotinib vs. placebo arms of the EGFRm-positive subgroup, median DFS was 46.4 and 28.5 months, respectively, with 2-year DFS rates of 75% and 54%. The results were not statistically significant due to hierarchical testing. There were between-arm imbalances of disease characteristics, and the placebo arm of the EGFRm-positive subgroup had substantially worse DFS than the intention-to-treat population.
“The trend toward improvement in DFS with erlotinib in the EGFRm-positive subgroup warrants further evaluation,” wrote Dr. Karen Kelly of UC Davis Comprehensive Cancer Center, Sacramento, California, and colleagues (J Clin Oncol. 2015 Aug 31. doi:10.1200/JCO.2015.61.8918).
The randomized, double-blind, placebo-controlled RADIANT trial included 973 patients with completely resected stage IB to IIIA NSCLC with EGFR-expressing tumors by immunohistochemistry (IHC) and EGFR high copy number or amplification by fluorescence in situ hybridization (FISH). Previous studies indicated that EGFR protein expression detected by IHC and FISH may be predictive of prolonged survival with EGFR tyrosine kinase inhibitors (TKIs) such as erlotinib. However, subsequent to activation of the RADIANT trial, two phase III studies failed to show that IHC and FISH results were predictive of EGFR-TKI efficacy. Emerging data show that EGFRm-positive status is the strongest predictor of EFGR-TKI sensitivity.
The duration of treatment in the RADIANT trial was 2 years, but the authors speculate that, based on results from the recent SELECT trial in which only four patients experienced relapse while taking erlotinib, a longer treatment duration “may be needed to achieve the goal of increasing the cure rate of early-stage NSCLC in an EGFRm-positive population.”
KRAS mutation status, tested in 828 patient samples, was not prognostic and did not predict erlotinib benefit.
The study was supported by Astellas Pharma Global Development, F. Hoffmann-La Roche, and Genentech. Dr. Kelly reported having no disclosures. Several of her coauthors were employed by or reported consulting or advisory roles with industry sources.
Among patients with completely resected non–small-cell lung cancer (NSCLC) whose tumors expressed epidermal growth factor receptor (EGFR), disease-free survival (DFS) rates were similar between erlotinib and placebo groups, according to results from the RADIANT phase III trial.
EGFR-activating mutations (EGFRm) were observed in a subgroup of patients (16.5%, 89 with del19 and 72 with L858R mutations) but these patients were not stratified by mutation status, which limited interpretation of the results. For the erlotinib vs. placebo arms of the EGFRm-positive subgroup, median DFS was 46.4 and 28.5 months, respectively, with 2-year DFS rates of 75% and 54%. The results were not statistically significant due to hierarchical testing. There were between-arm imbalances of disease characteristics, and the placebo arm of the EGFRm-positive subgroup had substantially worse DFS than the intention-to-treat population.
“The trend toward improvement in DFS with erlotinib in the EGFRm-positive subgroup warrants further evaluation,” wrote Dr. Karen Kelly of UC Davis Comprehensive Cancer Center, Sacramento, California, and colleagues (J Clin Oncol. 2015 Aug 31. doi:10.1200/JCO.2015.61.8918).
The randomized, double-blind, placebo-controlled RADIANT trial included 973 patients with completely resected stage IB to IIIA NSCLC with EGFR-expressing tumors by immunohistochemistry (IHC) and EGFR high copy number or amplification by fluorescence in situ hybridization (FISH). Previous studies indicated that EGFR protein expression detected by IHC and FISH may be predictive of prolonged survival with EGFR tyrosine kinase inhibitors (TKIs) such as erlotinib. However, subsequent to activation of the RADIANT trial, two phase III studies failed to show that IHC and FISH results were predictive of EGFR-TKI efficacy. Emerging data show that EGFRm-positive status is the strongest predictor of EFGR-TKI sensitivity.
The duration of treatment in the RADIANT trial was 2 years, but the authors speculate that, based on results from the recent SELECT trial in which only four patients experienced relapse while taking erlotinib, a longer treatment duration “may be needed to achieve the goal of increasing the cure rate of early-stage NSCLC in an EGFRm-positive population.”
KRAS mutation status, tested in 828 patient samples, was not prognostic and did not predict erlotinib benefit.
The study was supported by Astellas Pharma Global Development, F. Hoffmann-La Roche, and Genentech. Dr. Kelly reported having no disclosures. Several of her coauthors were employed by or reported consulting or advisory roles with industry sources.
Among patients with completely resected non–small-cell lung cancer (NSCLC) whose tumors expressed epidermal growth factor receptor (EGFR), disease-free survival (DFS) rates were similar between erlotinib and placebo groups, according to results from the RADIANT phase III trial.
EGFR-activating mutations (EGFRm) were observed in a subgroup of patients (16.5%, 89 with del19 and 72 with L858R mutations) but these patients were not stratified by mutation status, which limited interpretation of the results. For the erlotinib vs. placebo arms of the EGFRm-positive subgroup, median DFS was 46.4 and 28.5 months, respectively, with 2-year DFS rates of 75% and 54%. The results were not statistically significant due to hierarchical testing. There were between-arm imbalances of disease characteristics, and the placebo arm of the EGFRm-positive subgroup had substantially worse DFS than the intention-to-treat population.
“The trend toward improvement in DFS with erlotinib in the EGFRm-positive subgroup warrants further evaluation,” wrote Dr. Karen Kelly of UC Davis Comprehensive Cancer Center, Sacramento, California, and colleagues (J Clin Oncol. 2015 Aug 31. doi:10.1200/JCO.2015.61.8918).
The randomized, double-blind, placebo-controlled RADIANT trial included 973 patients with completely resected stage IB to IIIA NSCLC with EGFR-expressing tumors by immunohistochemistry (IHC) and EGFR high copy number or amplification by fluorescence in situ hybridization (FISH). Previous studies indicated that EGFR protein expression detected by IHC and FISH may be predictive of prolonged survival with EGFR tyrosine kinase inhibitors (TKIs) such as erlotinib. However, subsequent to activation of the RADIANT trial, two phase III studies failed to show that IHC and FISH results were predictive of EGFR-TKI efficacy. Emerging data show that EGFRm-positive status is the strongest predictor of EFGR-TKI sensitivity.
The duration of treatment in the RADIANT trial was 2 years, but the authors speculate that, based on results from the recent SELECT trial in which only four patients experienced relapse while taking erlotinib, a longer treatment duration “may be needed to achieve the goal of increasing the cure rate of early-stage NSCLC in an EGFRm-positive population.”
KRAS mutation status, tested in 828 patient samples, was not prognostic and did not predict erlotinib benefit.
The study was supported by Astellas Pharma Global Development, F. Hoffmann-La Roche, and Genentech. Dr. Kelly reported having no disclosures. Several of her coauthors were employed by or reported consulting or advisory roles with industry sources.
Key clinical point: Adjuvant erlotinib demonstrated no DFS benefit in patients with non–small-cell lung cancer (NSCLC) whose tumors expressed epidermal growth factor receptor (EGFR).
Major finding: After a median follow up of 47 months, the median DFS for patients who received erlotinib was 50.5 months compared with 48.2 months for placebo.
Data source: The randomized, double-blind, placebo-controlled RADIANT trial included 973 patients with completely resected stage IB to IIIA NSCLC with EGFR-expressing tumors by immunohistochemistry and EGFR high copy number or amplification by fluorescence in situ hybridization.
Disclosures: The study was supported by Astellas Pharma Global Development, F. Hoffmann-La Roche, and Genentech. Dr. Kelly reported having no disclosures. Several coauthors were employed by or reported consulting or advisory roles with industry sources.