User login
Nab-paclitaxel marginally superior to dacarbazine for metastatic melanoma
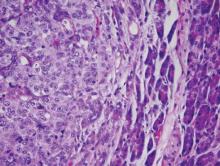
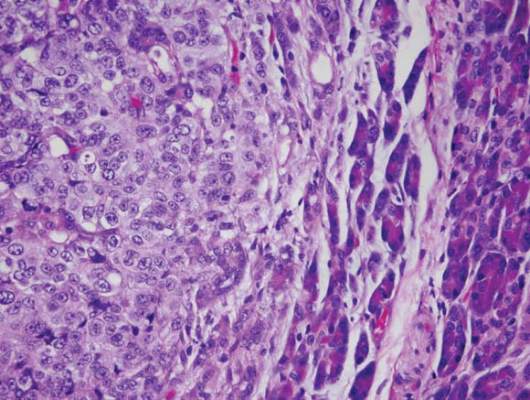
In chemotherapy-naive patients with metastatic melanoma, progression-free survival (PFS) was significantly longer with nab-paclitaxel, compared with dacarbazine, according to results from a phase III trial.
Median PFS for the nab-paclitaxel arm vs. the dacarbazine arm was 4.8 vs. 2.5 months, respectively (hazard ratio, 0.792; 95% confidence interval, 0.631-0.992; P = .044). Overall survival was similar in the two arms: 12.6 months for nab-paclitaxel vs. 10.5 months for dacarbazine (P = .27). The nab-paclitaxel group had an increased, but not significant, overall response rate (15% vs. 11%) and a significantly improved disease control rate (P = .004). The disease control rate reflects the number of complete responses plus partial responses plus stable disease for 16 or more weeks.
Nab-paclitaxel benefited patients regardless of BRAF mutation status, noted Dr. Evan Hersh, professor of medicine at the University of Arizona, Tucson, and colleagues.
“Additionally, in a post hoc analysis of this trial, nab-paclitaxel was shown to benefit a subgroup of patients with low or absent TILs [tumor-infiltrating lymphocytes], a poor prognostic factor in melanoma,” they wrote (Ann Oncol 2015 Sep 26. doi: 10.1093/annonc/mdv324).
The most common treatment-related adverse events of grade 3 or greater were neuropathy (25% vs. 0%), neutropenia (20% vs. 10%), and leukopenia (12% vs. 7%) for the nab-paclitaxel and dacarbazine arms, respectively. The median onset of grade 3 or greater peripheral neuropathy was 101 days after treatment began.
Between April 2009 and June 2011, the multicenter, phase III, randomized, controlled trial enrolled 529 adults with stage IV malignant melanoma who had no prior cytotoxic therapy.
A majority of participants from both treatment arms underwent post-study therapy, including newer agents such as BRAF inhibitors and ipilimumab. This may account for the diminished treatment affect of nab-paclitaxel on overall survival, compared with the more significant benefit in PFS observed early in the study.
A biomarker analysis found no correlation between tumor expression of the albumin-binding protein SPARC and PFS with nab-paclitaxel treatment. SPARC was hypothesized to enrich nab-paclitaxel in the tumor microenvironment thereby increasing its efficacy.
The study by Hersh et al. was conceived prior to the success of immune checkpoint inhibitors and targeted therapies for melanoma. The treatment landscape has changed rapidly, and the current role of chemotherapy, and nab-paclitaxel specifically, is uncertain.
In the last 5 years, four classes of therapies have significantly improved overall survival over dacarbazine: ipilimumab (HR, 0.72; P less than .001), vemurafenib (HR, 0.37; P less than .001), trametinib (HR, 0.54; P = .01), and nivolumab (HR, 0.42; P less than .001).
For the large majority of patients, chemotherapy would not be considered for the first line of therapy. Patients typically considered for chemotherapy would have failed or would have been intolerant to ipilimumab and an anti-PD1 agent, as well as BRAF/MEK inhibitors if the tumors were BRAF-mutation positive. In these heavily pretreated patients, however, toxicities associated with chemotherapy may outweigh benefits.
In patients with a history of autoimmune disease, some have suggested checkpoint inhibitors may exacerbate these conditions. However, recent retrospective studies suggest that ipilimumab is safe for patients with autoimmune disorders such as inflammatory bowel disease and rheumatoid arthritis.
The current study did not include quality of life data, and given the lack of improvement in overall survival, the superiority of nab-paclitaxel over dacarbazine has not been shown definitively.
Chemotherapy may have a role in combination with targeted or immunotherapeutic agents. Checkpoint inhibitors may be more active in patients with baseline tumor-infiltrating lymphocytes (TILs) and tumor PDL1 expression. Chemotherapy may induce TILs in tumors where the cells are low or absent, and nab-paclitaxel may be a good candidate to explore such combinations.
It remains to be determined if chemotherapy offers greater benefit than the many other strategies under consideration. This brings to the forefront the urgent need for preclinical models to enable comparisons of combination strategies.
Dr. Matteo Carlino is a clinical senior lecturer at the Sydney Medical School, University of Sydney and a medical oncologist at Westmead Hospital and Blacktown Hospital, Australia. Dr. Georgina Long is an associate professor and medical oncologist at Melanoma Institute Australia, University of Sydney. These remarks were part of an editorial accompanying the report (Ann Oncol. 2015 Sep 15. doi: 10.1093/annonc/mdv361). Dr. Carlino and Dr. Long reported having no disclosures.
The study by Hersh et al. was conceived prior to the success of immune checkpoint inhibitors and targeted therapies for melanoma. The treatment landscape has changed rapidly, and the current role of chemotherapy, and nab-paclitaxel specifically, is uncertain.
In the last 5 years, four classes of therapies have significantly improved overall survival over dacarbazine: ipilimumab (HR, 0.72; P less than .001), vemurafenib (HR, 0.37; P less than .001), trametinib (HR, 0.54; P = .01), and nivolumab (HR, 0.42; P less than .001).
For the large majority of patients, chemotherapy would not be considered for the first line of therapy. Patients typically considered for chemotherapy would have failed or would have been intolerant to ipilimumab and an anti-PD1 agent, as well as BRAF/MEK inhibitors if the tumors were BRAF-mutation positive. In these heavily pretreated patients, however, toxicities associated with chemotherapy may outweigh benefits.
In patients with a history of autoimmune disease, some have suggested checkpoint inhibitors may exacerbate these conditions. However, recent retrospective studies suggest that ipilimumab is safe for patients with autoimmune disorders such as inflammatory bowel disease and rheumatoid arthritis.
The current study did not include quality of life data, and given the lack of improvement in overall survival, the superiority of nab-paclitaxel over dacarbazine has not been shown definitively.
Chemotherapy may have a role in combination with targeted or immunotherapeutic agents. Checkpoint inhibitors may be more active in patients with baseline tumor-infiltrating lymphocytes (TILs) and tumor PDL1 expression. Chemotherapy may induce TILs in tumors where the cells are low or absent, and nab-paclitaxel may be a good candidate to explore such combinations.
It remains to be determined if chemotherapy offers greater benefit than the many other strategies under consideration. This brings to the forefront the urgent need for preclinical models to enable comparisons of combination strategies.
Dr. Matteo Carlino is a clinical senior lecturer at the Sydney Medical School, University of Sydney and a medical oncologist at Westmead Hospital and Blacktown Hospital, Australia. Dr. Georgina Long is an associate professor and medical oncologist at Melanoma Institute Australia, University of Sydney. These remarks were part of an editorial accompanying the report (Ann Oncol. 2015 Sep 15. doi: 10.1093/annonc/mdv361). Dr. Carlino and Dr. Long reported having no disclosures.
The study by Hersh et al. was conceived prior to the success of immune checkpoint inhibitors and targeted therapies for melanoma. The treatment landscape has changed rapidly, and the current role of chemotherapy, and nab-paclitaxel specifically, is uncertain.
In the last 5 years, four classes of therapies have significantly improved overall survival over dacarbazine: ipilimumab (HR, 0.72; P less than .001), vemurafenib (HR, 0.37; P less than .001), trametinib (HR, 0.54; P = .01), and nivolumab (HR, 0.42; P less than .001).
For the large majority of patients, chemotherapy would not be considered for the first line of therapy. Patients typically considered for chemotherapy would have failed or would have been intolerant to ipilimumab and an anti-PD1 agent, as well as BRAF/MEK inhibitors if the tumors were BRAF-mutation positive. In these heavily pretreated patients, however, toxicities associated with chemotherapy may outweigh benefits.
In patients with a history of autoimmune disease, some have suggested checkpoint inhibitors may exacerbate these conditions. However, recent retrospective studies suggest that ipilimumab is safe for patients with autoimmune disorders such as inflammatory bowel disease and rheumatoid arthritis.
The current study did not include quality of life data, and given the lack of improvement in overall survival, the superiority of nab-paclitaxel over dacarbazine has not been shown definitively.
Chemotherapy may have a role in combination with targeted or immunotherapeutic agents. Checkpoint inhibitors may be more active in patients with baseline tumor-infiltrating lymphocytes (TILs) and tumor PDL1 expression. Chemotherapy may induce TILs in tumors where the cells are low or absent, and nab-paclitaxel may be a good candidate to explore such combinations.
It remains to be determined if chemotherapy offers greater benefit than the many other strategies under consideration. This brings to the forefront the urgent need for preclinical models to enable comparisons of combination strategies.
Dr. Matteo Carlino is a clinical senior lecturer at the Sydney Medical School, University of Sydney and a medical oncologist at Westmead Hospital and Blacktown Hospital, Australia. Dr. Georgina Long is an associate professor and medical oncologist at Melanoma Institute Australia, University of Sydney. These remarks were part of an editorial accompanying the report (Ann Oncol. 2015 Sep 15. doi: 10.1093/annonc/mdv361). Dr. Carlino and Dr. Long reported having no disclosures.
In chemotherapy-naive patients with metastatic melanoma, progression-free survival (PFS) was significantly longer with nab-paclitaxel, compared with dacarbazine, according to results from a phase III trial.
Median PFS for the nab-paclitaxel arm vs. the dacarbazine arm was 4.8 vs. 2.5 months, respectively (hazard ratio, 0.792; 95% confidence interval, 0.631-0.992; P = .044). Overall survival was similar in the two arms: 12.6 months for nab-paclitaxel vs. 10.5 months for dacarbazine (P = .27). The nab-paclitaxel group had an increased, but not significant, overall response rate (15% vs. 11%) and a significantly improved disease control rate (P = .004). The disease control rate reflects the number of complete responses plus partial responses plus stable disease for 16 or more weeks.
Nab-paclitaxel benefited patients regardless of BRAF mutation status, noted Dr. Evan Hersh, professor of medicine at the University of Arizona, Tucson, and colleagues.
“Additionally, in a post hoc analysis of this trial, nab-paclitaxel was shown to benefit a subgroup of patients with low or absent TILs [tumor-infiltrating lymphocytes], a poor prognostic factor in melanoma,” they wrote (Ann Oncol 2015 Sep 26. doi: 10.1093/annonc/mdv324).
The most common treatment-related adverse events of grade 3 or greater were neuropathy (25% vs. 0%), neutropenia (20% vs. 10%), and leukopenia (12% vs. 7%) for the nab-paclitaxel and dacarbazine arms, respectively. The median onset of grade 3 or greater peripheral neuropathy was 101 days after treatment began.
Between April 2009 and June 2011, the multicenter, phase III, randomized, controlled trial enrolled 529 adults with stage IV malignant melanoma who had no prior cytotoxic therapy.
A majority of participants from both treatment arms underwent post-study therapy, including newer agents such as BRAF inhibitors and ipilimumab. This may account for the diminished treatment affect of nab-paclitaxel on overall survival, compared with the more significant benefit in PFS observed early in the study.
A biomarker analysis found no correlation between tumor expression of the albumin-binding protein SPARC and PFS with nab-paclitaxel treatment. SPARC was hypothesized to enrich nab-paclitaxel in the tumor microenvironment thereby increasing its efficacy.
In chemotherapy-naive patients with metastatic melanoma, progression-free survival (PFS) was significantly longer with nab-paclitaxel, compared with dacarbazine, according to results from a phase III trial.
Median PFS for the nab-paclitaxel arm vs. the dacarbazine arm was 4.8 vs. 2.5 months, respectively (hazard ratio, 0.792; 95% confidence interval, 0.631-0.992; P = .044). Overall survival was similar in the two arms: 12.6 months for nab-paclitaxel vs. 10.5 months for dacarbazine (P = .27). The nab-paclitaxel group had an increased, but not significant, overall response rate (15% vs. 11%) and a significantly improved disease control rate (P = .004). The disease control rate reflects the number of complete responses plus partial responses plus stable disease for 16 or more weeks.
Nab-paclitaxel benefited patients regardless of BRAF mutation status, noted Dr. Evan Hersh, professor of medicine at the University of Arizona, Tucson, and colleagues.
“Additionally, in a post hoc analysis of this trial, nab-paclitaxel was shown to benefit a subgroup of patients with low or absent TILs [tumor-infiltrating lymphocytes], a poor prognostic factor in melanoma,” they wrote (Ann Oncol 2015 Sep 26. doi: 10.1093/annonc/mdv324).
The most common treatment-related adverse events of grade 3 or greater were neuropathy (25% vs. 0%), neutropenia (20% vs. 10%), and leukopenia (12% vs. 7%) for the nab-paclitaxel and dacarbazine arms, respectively. The median onset of grade 3 or greater peripheral neuropathy was 101 days after treatment began.
Between April 2009 and June 2011, the multicenter, phase III, randomized, controlled trial enrolled 529 adults with stage IV malignant melanoma who had no prior cytotoxic therapy.
A majority of participants from both treatment arms underwent post-study therapy, including newer agents such as BRAF inhibitors and ipilimumab. This may account for the diminished treatment affect of nab-paclitaxel on overall survival, compared with the more significant benefit in PFS observed early in the study.
A biomarker analysis found no correlation between tumor expression of the albumin-binding protein SPARC and PFS with nab-paclitaxel treatment. SPARC was hypothesized to enrich nab-paclitaxel in the tumor microenvironment thereby increasing its efficacy.
FROM ANNALS OF ONCOLOGY
Key clinical point: Nab-pacilitaxel had significantly longer PFS than dacarbazine in chemotherapy-naive patients with metastatic melanoma.
Major finding: Median PFS in the nab-paclitaxel vs. dacarbazine arms was 4.8 vs. 2.5 months, respectively (HR, 0.792; 95% CI, 0.631-0.992; P = .044).
Data source: The phase III, randomized, controlled trial enrolled 529 patients between April 2009 and June 2011.
Disclosures: Dr. Hersh reported research funding from Celgene. His coauthors reported financial ties to several industry sources.
Noninvasive ventilation no better than oxygen alone in immunocompromised ICU patients
Early noninvasive ventilation, compared with oxygen therapy alone, did not reduce 28-day all-cause mortality in critically ill immunocompromised patients with acute respiratory failure, based on a randomized, parallel-group study of 374 patients conducted in 28 ICUs in France and Belgium.
Overall, 46 of 191 patients (24%) in the noninvasive ventilation group died, compared with 50 of 183 (27%) in the oxygen-alone group. A similar number of patients from each group required intubation – 38% in the noninvasive ventilation group and 45% in the oxygen group – with similar time to intubation. Nearly 85% of the patients were receiving treatment for hematologic malignancies or solid tumors, researchers reported in a study published online Oct. 7 in JAMA.
No significant differences between groups were observed in requirement for intubation, ICU or hospital length of stay, or duration of invasive mechanical ventilation. The study found no evidence that noninvasive ventilation influenced mortality estimates or was beneficial to any subgroup based on hypoxemia severity or underlying condition.
The study was limited, however, by a lower than expected mortality rate with oxygen alone, and as a result was not powered to detect significant between-group differences. Based on earlier studies, the researchers assumed a 35% mortality rate in the oxygen-alone group, but the actual rate was 27% (JAMA. 2015 Oct 7. doi: 10.1001/jama.2015.12402).
“Therefore, there remains uncertainty regarding our null finding, which may nonetheless fail to exclude a clinically important effect,” wrote Dr. Virginie Lemiale of Saint-Louis University Hospital, Paris, and colleagues.
Furthermore, high-flow nasal oxygen was used in about 40% of all patients, which may have decreased requirements for intubation as well as mortality rates. High-flow nasal oxygen was used more often in the oxygen group (44%) than in the noninvasive ventilation group (31%) (P = .01).
Dr. Lemiale and coauthors reported having no disclosures.
In contrast to reports from 10 years ago, the current study by Lemiale et al. failed to demonstrate a mortality benefit for noninvasive ventilation, compared with oxygen alone. However, the results should be interpreted in the context of recent advances in ICU care. Targeted chemotherapy, prophylactic use of antibiotics, and improved supportive care have contributed to overall mortality declines in the immunocompromised critically ill population. Dr. Lemiale and colleagues anticipated a higher baseline mortality rate (35% vs. 27% observed). The lower mortality rate limited the study’s power to detect a mortality difference between groups.
Second, patients in this trial may have had a lower acuity of illness, evidenced by less tachypnea, compared with that seen in earlier studies.
Third, the oxygen-alone group received more high-flow oxygen via nasal cannula than the noninvasive ventilation group, which may have diluted the benefits of noninvasive ventilation.
As efforts continue to reduce requirements for invasive mechanical ventilation, further examination of strategies for noninvasive ventilation, such as high-flow oxygen, compared with noninvasive ventilation, are warranted.
Dr. Bhakti Patel is a clinical instructor of medicine in the section of pulmonary and critical care, department of medicine, University of Chicago. Dr. John Kress is professor of medicine and director of the Medical Intensive Care Unit at University of Chicago Medicine. These remarks were part of an editorial accompanying the report (JAMA. 2015 Oct 7. doi: 10.1001/jama.2015.12401). Dr. Patel and Dr. Kress reported having no disclosures.
In contrast to reports from 10 years ago, the current study by Lemiale et al. failed to demonstrate a mortality benefit for noninvasive ventilation, compared with oxygen alone. However, the results should be interpreted in the context of recent advances in ICU care. Targeted chemotherapy, prophylactic use of antibiotics, and improved supportive care have contributed to overall mortality declines in the immunocompromised critically ill population. Dr. Lemiale and colleagues anticipated a higher baseline mortality rate (35% vs. 27% observed). The lower mortality rate limited the study’s power to detect a mortality difference between groups.
Second, patients in this trial may have had a lower acuity of illness, evidenced by less tachypnea, compared with that seen in earlier studies.
Third, the oxygen-alone group received more high-flow oxygen via nasal cannula than the noninvasive ventilation group, which may have diluted the benefits of noninvasive ventilation.
As efforts continue to reduce requirements for invasive mechanical ventilation, further examination of strategies for noninvasive ventilation, such as high-flow oxygen, compared with noninvasive ventilation, are warranted.
Dr. Bhakti Patel is a clinical instructor of medicine in the section of pulmonary and critical care, department of medicine, University of Chicago. Dr. John Kress is professor of medicine and director of the Medical Intensive Care Unit at University of Chicago Medicine. These remarks were part of an editorial accompanying the report (JAMA. 2015 Oct 7. doi: 10.1001/jama.2015.12401). Dr. Patel and Dr. Kress reported having no disclosures.
In contrast to reports from 10 years ago, the current study by Lemiale et al. failed to demonstrate a mortality benefit for noninvasive ventilation, compared with oxygen alone. However, the results should be interpreted in the context of recent advances in ICU care. Targeted chemotherapy, prophylactic use of antibiotics, and improved supportive care have contributed to overall mortality declines in the immunocompromised critically ill population. Dr. Lemiale and colleagues anticipated a higher baseline mortality rate (35% vs. 27% observed). The lower mortality rate limited the study’s power to detect a mortality difference between groups.
Second, patients in this trial may have had a lower acuity of illness, evidenced by less tachypnea, compared with that seen in earlier studies.
Third, the oxygen-alone group received more high-flow oxygen via nasal cannula than the noninvasive ventilation group, which may have diluted the benefits of noninvasive ventilation.
As efforts continue to reduce requirements for invasive mechanical ventilation, further examination of strategies for noninvasive ventilation, such as high-flow oxygen, compared with noninvasive ventilation, are warranted.
Dr. Bhakti Patel is a clinical instructor of medicine in the section of pulmonary and critical care, department of medicine, University of Chicago. Dr. John Kress is professor of medicine and director of the Medical Intensive Care Unit at University of Chicago Medicine. These remarks were part of an editorial accompanying the report (JAMA. 2015 Oct 7. doi: 10.1001/jama.2015.12401). Dr. Patel and Dr. Kress reported having no disclosures.
Early noninvasive ventilation, compared with oxygen therapy alone, did not reduce 28-day all-cause mortality in critically ill immunocompromised patients with acute respiratory failure, based on a randomized, parallel-group study of 374 patients conducted in 28 ICUs in France and Belgium.
Overall, 46 of 191 patients (24%) in the noninvasive ventilation group died, compared with 50 of 183 (27%) in the oxygen-alone group. A similar number of patients from each group required intubation – 38% in the noninvasive ventilation group and 45% in the oxygen group – with similar time to intubation. Nearly 85% of the patients were receiving treatment for hematologic malignancies or solid tumors, researchers reported in a study published online Oct. 7 in JAMA.
No significant differences between groups were observed in requirement for intubation, ICU or hospital length of stay, or duration of invasive mechanical ventilation. The study found no evidence that noninvasive ventilation influenced mortality estimates or was beneficial to any subgroup based on hypoxemia severity or underlying condition.
The study was limited, however, by a lower than expected mortality rate with oxygen alone, and as a result was not powered to detect significant between-group differences. Based on earlier studies, the researchers assumed a 35% mortality rate in the oxygen-alone group, but the actual rate was 27% (JAMA. 2015 Oct 7. doi: 10.1001/jama.2015.12402).
“Therefore, there remains uncertainty regarding our null finding, which may nonetheless fail to exclude a clinically important effect,” wrote Dr. Virginie Lemiale of Saint-Louis University Hospital, Paris, and colleagues.
Furthermore, high-flow nasal oxygen was used in about 40% of all patients, which may have decreased requirements for intubation as well as mortality rates. High-flow nasal oxygen was used more often in the oxygen group (44%) than in the noninvasive ventilation group (31%) (P = .01).
Dr. Lemiale and coauthors reported having no disclosures.
Early noninvasive ventilation, compared with oxygen therapy alone, did not reduce 28-day all-cause mortality in critically ill immunocompromised patients with acute respiratory failure, based on a randomized, parallel-group study of 374 patients conducted in 28 ICUs in France and Belgium.
Overall, 46 of 191 patients (24%) in the noninvasive ventilation group died, compared with 50 of 183 (27%) in the oxygen-alone group. A similar number of patients from each group required intubation – 38% in the noninvasive ventilation group and 45% in the oxygen group – with similar time to intubation. Nearly 85% of the patients were receiving treatment for hematologic malignancies or solid tumors, researchers reported in a study published online Oct. 7 in JAMA.
No significant differences between groups were observed in requirement for intubation, ICU or hospital length of stay, or duration of invasive mechanical ventilation. The study found no evidence that noninvasive ventilation influenced mortality estimates or was beneficial to any subgroup based on hypoxemia severity or underlying condition.
The study was limited, however, by a lower than expected mortality rate with oxygen alone, and as a result was not powered to detect significant between-group differences. Based on earlier studies, the researchers assumed a 35% mortality rate in the oxygen-alone group, but the actual rate was 27% (JAMA. 2015 Oct 7. doi: 10.1001/jama.2015.12402).
“Therefore, there remains uncertainty regarding our null finding, which may nonetheless fail to exclude a clinically important effect,” wrote Dr. Virginie Lemiale of Saint-Louis University Hospital, Paris, and colleagues.
Furthermore, high-flow nasal oxygen was used in about 40% of all patients, which may have decreased requirements for intubation as well as mortality rates. High-flow nasal oxygen was used more often in the oxygen group (44%) than in the noninvasive ventilation group (31%) (P = .01).
Dr. Lemiale and coauthors reported having no disclosures.
Key clinical point: Noninvasive ventilation, compared with oxygen therapy alone, did not reduce 28-day mortality among immunocompromised patients with acute respiratory failure.
Major finding: After 28 days, 46 of 191 patients (24%) in the noninvasive ventilation group had died, compared with 50 of 183 (27%) in the oxygen-alone group.
Data source: The randomized, parallel-group study was conducted in 28 ICUs in France and Belgium and included 374 immunocompromised patients with acute respiratory failure.
Disclosures: Dr. Lemiale and coauthors reported having no disclosures.
Aberrant miRNA levels may reflect progression to multiple myeloma
Levels of specific microRNAs (miRNAs) detected in the peripheral blood of patients with monoclonal gammopathy of undetermined significance (MGUS), and smoldering multiple myeloma may prove to predict progression to multiple myeloma, according to Weixin Wang of the Department of Laboratory Medicine, National Institutes of Health Clinical Center, Bethesda, Md., and colleagues (J Mol Diagn. 2015;17:669-78).
The research team analyzed bone marrow aspirates from 20 patients with multiple myeloma and 8 healthy controls and found 11 miRNAs with significantly lower expression. Serum was then analyzed in 17 patients with MGUS, 17 with smoldering multiple myeloma, 13 with multiple myeloma, and 12 healthy controls. Four of 11 miRNAs (let-7i, miR-15a, miR-16, and miR-106b) were significantly decreased in MGUS, suggesting that aberrant expression of these miRNAs may be associated with early neoplastic events. Eight of 11 miRNAs (let-7a, let-7b, let-7i, miR-15a, miR-15b, miR-16, miR-106b, and miR-20a) were decreased in smoldering disease. The other three miRNAs (miR-21, miR-223, and miR-361) were significantly decreased in multiple myeloma but not in MGUS/SMM, suggesting that down-regulation of this group of miRNAs may be related to later events in disease progression, including malignant transformation from precursor disease to myeloma, the researchers wrote.
Small, noncoding miRNA molecules function in posttranscriptional gene regulation through RNA silencing, and many of the gene targets of the 11 miRNAs encode proteins that regulate cell proliferation.
Previous studies have shown that the let-7 family of miRNAs are expressed at low levels in human cancer and stem cells, and these miRNAs were implicated in the current study as well. Let-7 miRNAs silence many genes involved in oncogenesis, cell cycle, proliferation, and apoptosis, including MYC. The researchers found that let-7a and let-7b expression was normal in MGUS and decreased in smoldering disease and multiple myeloma, which may be related to increased MYC expression in disease progression.
Dr. Wang and coauthors reported having no disclosures.
Levels of specific microRNAs (miRNAs) detected in the peripheral blood of patients with monoclonal gammopathy of undetermined significance (MGUS), and smoldering multiple myeloma may prove to predict progression to multiple myeloma, according to Weixin Wang of the Department of Laboratory Medicine, National Institutes of Health Clinical Center, Bethesda, Md., and colleagues (J Mol Diagn. 2015;17:669-78).
The research team analyzed bone marrow aspirates from 20 patients with multiple myeloma and 8 healthy controls and found 11 miRNAs with significantly lower expression. Serum was then analyzed in 17 patients with MGUS, 17 with smoldering multiple myeloma, 13 with multiple myeloma, and 12 healthy controls. Four of 11 miRNAs (let-7i, miR-15a, miR-16, and miR-106b) were significantly decreased in MGUS, suggesting that aberrant expression of these miRNAs may be associated with early neoplastic events. Eight of 11 miRNAs (let-7a, let-7b, let-7i, miR-15a, miR-15b, miR-16, miR-106b, and miR-20a) were decreased in smoldering disease. The other three miRNAs (miR-21, miR-223, and miR-361) were significantly decreased in multiple myeloma but not in MGUS/SMM, suggesting that down-regulation of this group of miRNAs may be related to later events in disease progression, including malignant transformation from precursor disease to myeloma, the researchers wrote.
Small, noncoding miRNA molecules function in posttranscriptional gene regulation through RNA silencing, and many of the gene targets of the 11 miRNAs encode proteins that regulate cell proliferation.
Previous studies have shown that the let-7 family of miRNAs are expressed at low levels in human cancer and stem cells, and these miRNAs were implicated in the current study as well. Let-7 miRNAs silence many genes involved in oncogenesis, cell cycle, proliferation, and apoptosis, including MYC. The researchers found that let-7a and let-7b expression was normal in MGUS and decreased in smoldering disease and multiple myeloma, which may be related to increased MYC expression in disease progression.
Dr. Wang and coauthors reported having no disclosures.
Levels of specific microRNAs (miRNAs) detected in the peripheral blood of patients with monoclonal gammopathy of undetermined significance (MGUS), and smoldering multiple myeloma may prove to predict progression to multiple myeloma, according to Weixin Wang of the Department of Laboratory Medicine, National Institutes of Health Clinical Center, Bethesda, Md., and colleagues (J Mol Diagn. 2015;17:669-78).
The research team analyzed bone marrow aspirates from 20 patients with multiple myeloma and 8 healthy controls and found 11 miRNAs with significantly lower expression. Serum was then analyzed in 17 patients with MGUS, 17 with smoldering multiple myeloma, 13 with multiple myeloma, and 12 healthy controls. Four of 11 miRNAs (let-7i, miR-15a, miR-16, and miR-106b) were significantly decreased in MGUS, suggesting that aberrant expression of these miRNAs may be associated with early neoplastic events. Eight of 11 miRNAs (let-7a, let-7b, let-7i, miR-15a, miR-15b, miR-16, miR-106b, and miR-20a) were decreased in smoldering disease. The other three miRNAs (miR-21, miR-223, and miR-361) were significantly decreased in multiple myeloma but not in MGUS/SMM, suggesting that down-regulation of this group of miRNAs may be related to later events in disease progression, including malignant transformation from precursor disease to myeloma, the researchers wrote.
Small, noncoding miRNA molecules function in posttranscriptional gene regulation through RNA silencing, and many of the gene targets of the 11 miRNAs encode proteins that regulate cell proliferation.
Previous studies have shown that the let-7 family of miRNAs are expressed at low levels in human cancer and stem cells, and these miRNAs were implicated in the current study as well. Let-7 miRNAs silence many genes involved in oncogenesis, cell cycle, proliferation, and apoptosis, including MYC. The researchers found that let-7a and let-7b expression was normal in MGUS and decreased in smoldering disease and multiple myeloma, which may be related to increased MYC expression in disease progression.
Dr. Wang and coauthors reported having no disclosures.
FROM THE JOURNAL OF MOLECULAR DIAGNOSTICS
Key clinical point: Low levels of specific miRNAs in peripheral blood may reflect disease progression from MGUS and smoldering disease to multiple myeloma.
Major finding: Of 11 miRNAs found to be significantly decreased in the serum of patients with multiple myeloma compared to healthy controls, 8 were decreased in smoldering multiple myeloma and 4 were decreased in MGUS.
Data source: Serum for miRNA analysis came from 13 patients with multiple myeloma, 17 with smoldering disease, 17 with MGUS, and 12 healthy controls.
Disclosures: Dr. Wang and coauthors reported having no disclosures.
Routine imaging for diffuse large B-cell lymphoma offers no survival benefit
A population-based comparison of patients with diffuse large B-cell lymphoma (DLBCL) in first complete remission indicated that routine imaging surveillance did not improve outcomes, researchers reported.
Overall survival was similar for Danish and Swedish populations who received similar follow-up care, except that routine imaging surveillance is the standard of care in Denmark, but not in Sweden. The 3-year overall survival for Danish and Swedish patients was 92% and 91%, respectively.
Outcomes grouped by international prognostic index (IPI) also showed no significant differences between populations (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.62.0229.).
“An imaging-based follow-up strategy does not improve postremission [overall survival] for DLBCL,” wrote Dr. Tarec Christoffer El-Galaly, of Aalborg University Hospital, Denmark, and colleagues.
They observed that aside from using IPI as risk stratification, the study “also points to baseline [lactate dehyrogenase] as a single discriminator of patients with high versus low risk of progression,” (Hazard ratio, 3.12; 95% CI, 1.78-5.48; P less than .01).
The retrospective study examined records of patients with DLBCL from Sweden (n=696) and Denmark (n=525) who achieved first complete remission after first-line therapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP-like regimens from 2007 to 2012. The proportion of patients with IPI greater than two were similar for both groups, though more Danish patients received radiotherapy compared with their Swedish counterparts (35% v. 9%).
Standard follow-up care after first complete remission is similar in Denmark and Sweden and typically includes symptom assessment, clinical examination, and blood tests at 3- to 4-month intervals for 2 years, and 6-month intervals in the third year. After 3 years, Swedish patients are seen annually for 2 years and then follow-up is ended for most patients. In Denmark, 6-month checks are continued until 5 years and then follow-up is usually ended. However, in Denmark guidelines support routine computerized tomography (CT) scans of the neck, abdomen, and thorax every 6 months for 2 years, which is not encouraged by guidelines in Sweden.
Early relapse detection aims to improve survival, and although low disease burden is associated with durable survival in patients treated for relapsed DLBCL, most studies show similar outcomes for imaging versus non-imaging detection. Additionally, previous retrospective studies that have reported survival differences based on relapse detection method are prone to lead-time bias, according to the researchers.
Given that a majority of patients with recurrent DLBCL experience symptoms before relapse, that elevated lactate dehyrogenase or abnormal physical examination may raise suspicion, and that exposure to ionizing radiation from medical imaging can lead to radiation-induced cancers, “routine imaging for DLBCL in first [complete remission] is not recommended,” the authors wrote.
The research was supported in part by the North Denmark Region. Dr. El-Galaly and coauthors reported having no financial disclosures.
The best way to determine the effectiveness of surveillance imaging would be a randomized trial including patients with diffuse large B-cell lymphoma (DLBCL) after first complete remission, but it is unlikely that such a study will be done. The study by El-Galaly et al may be the next best approach. Taking advantage of the fact that neighboring countries Denmark and Sweden have opposite policies for surveillance imaging but otherwise similar follow-up visit schedules and testing, the authors identified factors that predicted relapse (e.g., age greater than 60 years and elevated LDH), and they found that routine surveillance imaging had no impact on outcome. The study presents the strongest argument yet published against routine surveillance imaging.
The two other outstanding issues of routine surveillance are long-term safety and cost benefit. The study by El-Galaly et al, in combination with several other reports, suggests that routine surveillance imaging, in the absence of new or suspicious symptoms, physical findings, or change in laboratory results, is unlikely to benefit patients, may add to the patient’s stress, may cause long-term health problems, and incurs substantial economic cost.
Dr. James O. Armitage and Dr. Julie M. Vose are both at the University of Nebraska, Omaha. Dr. Armitage disclosed a leadership role with Tesaro and consulting or advisory roles with GlaxoSmithKline, Roche, Spectrum Pharmaceuticals, ZIOPHARM Oncology, Conatus, and Celgene. Dr. Vose reported honoraria from Sanofi-Aventis and Seattle Genetics; consulting or advisory roles with Bioconnections; and institutional research funding from Spectrum Pharmaceuticals, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte, Janssen Biotech, Pharmacyclics, Acerta, and Kite Pharma. These remarks were adapted from their accompanying editorial (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.63.5946).
The best way to determine the effectiveness of surveillance imaging would be a randomized trial including patients with diffuse large B-cell lymphoma (DLBCL) after first complete remission, but it is unlikely that such a study will be done. The study by El-Galaly et al may be the next best approach. Taking advantage of the fact that neighboring countries Denmark and Sweden have opposite policies for surveillance imaging but otherwise similar follow-up visit schedules and testing, the authors identified factors that predicted relapse (e.g., age greater than 60 years and elevated LDH), and they found that routine surveillance imaging had no impact on outcome. The study presents the strongest argument yet published against routine surveillance imaging.
The two other outstanding issues of routine surveillance are long-term safety and cost benefit. The study by El-Galaly et al, in combination with several other reports, suggests that routine surveillance imaging, in the absence of new or suspicious symptoms, physical findings, or change in laboratory results, is unlikely to benefit patients, may add to the patient’s stress, may cause long-term health problems, and incurs substantial economic cost.
Dr. James O. Armitage and Dr. Julie M. Vose are both at the University of Nebraska, Omaha. Dr. Armitage disclosed a leadership role with Tesaro and consulting or advisory roles with GlaxoSmithKline, Roche, Spectrum Pharmaceuticals, ZIOPHARM Oncology, Conatus, and Celgene. Dr. Vose reported honoraria from Sanofi-Aventis and Seattle Genetics; consulting or advisory roles with Bioconnections; and institutional research funding from Spectrum Pharmaceuticals, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte, Janssen Biotech, Pharmacyclics, Acerta, and Kite Pharma. These remarks were adapted from their accompanying editorial (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.63.5946).
The best way to determine the effectiveness of surveillance imaging would be a randomized trial including patients with diffuse large B-cell lymphoma (DLBCL) after first complete remission, but it is unlikely that such a study will be done. The study by El-Galaly et al may be the next best approach. Taking advantage of the fact that neighboring countries Denmark and Sweden have opposite policies for surveillance imaging but otherwise similar follow-up visit schedules and testing, the authors identified factors that predicted relapse (e.g., age greater than 60 years and elevated LDH), and they found that routine surveillance imaging had no impact on outcome. The study presents the strongest argument yet published against routine surveillance imaging.
The two other outstanding issues of routine surveillance are long-term safety and cost benefit. The study by El-Galaly et al, in combination with several other reports, suggests that routine surveillance imaging, in the absence of new or suspicious symptoms, physical findings, or change in laboratory results, is unlikely to benefit patients, may add to the patient’s stress, may cause long-term health problems, and incurs substantial economic cost.
Dr. James O. Armitage and Dr. Julie M. Vose are both at the University of Nebraska, Omaha. Dr. Armitage disclosed a leadership role with Tesaro and consulting or advisory roles with GlaxoSmithKline, Roche, Spectrum Pharmaceuticals, ZIOPHARM Oncology, Conatus, and Celgene. Dr. Vose reported honoraria from Sanofi-Aventis and Seattle Genetics; consulting or advisory roles with Bioconnections; and institutional research funding from Spectrum Pharmaceuticals, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte, Janssen Biotech, Pharmacyclics, Acerta, and Kite Pharma. These remarks were adapted from their accompanying editorial (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.63.5946).
A population-based comparison of patients with diffuse large B-cell lymphoma (DLBCL) in first complete remission indicated that routine imaging surveillance did not improve outcomes, researchers reported.
Overall survival was similar for Danish and Swedish populations who received similar follow-up care, except that routine imaging surveillance is the standard of care in Denmark, but not in Sweden. The 3-year overall survival for Danish and Swedish patients was 92% and 91%, respectively.
Outcomes grouped by international prognostic index (IPI) also showed no significant differences between populations (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.62.0229.).
“An imaging-based follow-up strategy does not improve postremission [overall survival] for DLBCL,” wrote Dr. Tarec Christoffer El-Galaly, of Aalborg University Hospital, Denmark, and colleagues.
They observed that aside from using IPI as risk stratification, the study “also points to baseline [lactate dehyrogenase] as a single discriminator of patients with high versus low risk of progression,” (Hazard ratio, 3.12; 95% CI, 1.78-5.48; P less than .01).
The retrospective study examined records of patients with DLBCL from Sweden (n=696) and Denmark (n=525) who achieved first complete remission after first-line therapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP-like regimens from 2007 to 2012. The proportion of patients with IPI greater than two were similar for both groups, though more Danish patients received radiotherapy compared with their Swedish counterparts (35% v. 9%).
Standard follow-up care after first complete remission is similar in Denmark and Sweden and typically includes symptom assessment, clinical examination, and blood tests at 3- to 4-month intervals for 2 years, and 6-month intervals in the third year. After 3 years, Swedish patients are seen annually for 2 years and then follow-up is ended for most patients. In Denmark, 6-month checks are continued until 5 years and then follow-up is usually ended. However, in Denmark guidelines support routine computerized tomography (CT) scans of the neck, abdomen, and thorax every 6 months for 2 years, which is not encouraged by guidelines in Sweden.
Early relapse detection aims to improve survival, and although low disease burden is associated with durable survival in patients treated for relapsed DLBCL, most studies show similar outcomes for imaging versus non-imaging detection. Additionally, previous retrospective studies that have reported survival differences based on relapse detection method are prone to lead-time bias, according to the researchers.
Given that a majority of patients with recurrent DLBCL experience symptoms before relapse, that elevated lactate dehyrogenase or abnormal physical examination may raise suspicion, and that exposure to ionizing radiation from medical imaging can lead to radiation-induced cancers, “routine imaging for DLBCL in first [complete remission] is not recommended,” the authors wrote.
The research was supported in part by the North Denmark Region. Dr. El-Galaly and coauthors reported having no financial disclosures.
A population-based comparison of patients with diffuse large B-cell lymphoma (DLBCL) in first complete remission indicated that routine imaging surveillance did not improve outcomes, researchers reported.
Overall survival was similar for Danish and Swedish populations who received similar follow-up care, except that routine imaging surveillance is the standard of care in Denmark, but not in Sweden. The 3-year overall survival for Danish and Swedish patients was 92% and 91%, respectively.
Outcomes grouped by international prognostic index (IPI) also showed no significant differences between populations (J Clin Oncol. 2015 Oct 5, doi:10.1200/jco.2015.62.0229.).
“An imaging-based follow-up strategy does not improve postremission [overall survival] for DLBCL,” wrote Dr. Tarec Christoffer El-Galaly, of Aalborg University Hospital, Denmark, and colleagues.
They observed that aside from using IPI as risk stratification, the study “also points to baseline [lactate dehyrogenase] as a single discriminator of patients with high versus low risk of progression,” (Hazard ratio, 3.12; 95% CI, 1.78-5.48; P less than .01).
The retrospective study examined records of patients with DLBCL from Sweden (n=696) and Denmark (n=525) who achieved first complete remission after first-line therapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP-like regimens from 2007 to 2012. The proportion of patients with IPI greater than two were similar for both groups, though more Danish patients received radiotherapy compared with their Swedish counterparts (35% v. 9%).
Standard follow-up care after first complete remission is similar in Denmark and Sweden and typically includes symptom assessment, clinical examination, and blood tests at 3- to 4-month intervals for 2 years, and 6-month intervals in the third year. After 3 years, Swedish patients are seen annually for 2 years and then follow-up is ended for most patients. In Denmark, 6-month checks are continued until 5 years and then follow-up is usually ended. However, in Denmark guidelines support routine computerized tomography (CT) scans of the neck, abdomen, and thorax every 6 months for 2 years, which is not encouraged by guidelines in Sweden.
Early relapse detection aims to improve survival, and although low disease burden is associated with durable survival in patients treated for relapsed DLBCL, most studies show similar outcomes for imaging versus non-imaging detection. Additionally, previous retrospective studies that have reported survival differences based on relapse detection method are prone to lead-time bias, according to the researchers.
Given that a majority of patients with recurrent DLBCL experience symptoms before relapse, that elevated lactate dehyrogenase or abnormal physical examination may raise suspicion, and that exposure to ionizing radiation from medical imaging can lead to radiation-induced cancers, “routine imaging for DLBCL in first [complete remission] is not recommended,” the authors wrote.
The research was supported in part by the North Denmark Region. Dr. El-Galaly and coauthors reported having no financial disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:Danish patients with diffuse large B-cell lymphoma (DLBCL) who received routine imaging during follow up had similar survival to Swedish patients who did not undergo routine imaging surveillance.
Major finding: After first complete remission, the 3-year overall survival for Danish and Swedish patients was 92% and 91%, respectively.
Data source: Population-based study of 525 Danish patients and 696 Swedish patients with DLBCL who achieved first complete remission after R-CHOP/CHOP-like first-line therapies from 2007 to 2012.
Disclosures: The research was supported in part by the North Denmark Region. Dr. El-Galaly and coauthors reported having no financial disclosures.
Adjuvant hormone therapy may improve survival in epithelial ovarian cancer
After a 19-year follow up, women who were diagnosed with ovarian cancer and randomly assigned to receive adjuvant hormone therapy (AHT) had significantly improved overall survival and relapse-free survival compared with control patients who did not receive AHT, researchers reported. The results were published online Sept. 28 in Journal of Clinical Oncology.
Despite the fact that 46 out of 72 patients discontinued taking the hormone therapy, (median 1.14 years of treatment), the benefits of treatment were apparent. Improved survival was observed in the AHT group as early as 5 years after assignment and persisted for 20 years.
Of 121 (81%) patients who died during follow up, 53 (71%) were in the AHT group and 68 (91%) in the control group (HR, 0.63; 95% CI, 0.44-0.90; P = .011) (J Clin Oncol. 2015 Sep 28. doi:10.1200/JCO.2015.60.9719).
“This trial has shown that women who have severe menopausal symptoms after ovarian cancer treatment can safely take HRT without compromising their survival by doing so,” wrote Dr. Rosalind Eeles, professor of oncogenetics and consultant in cancer genetics and clinical oncology at The Institute of Cancer Research, Sutton, London, and colleagues. Their findings from this prospective randomized trial confirmed the survival benefit previously reported in a retrospective study, “so administration of HRT for QOL [quality of life] and survival benefit should be considered in patients with ovarian cancer,” they said.
The phase III nonblinded, controlled trial randomized 150 patients, median age 59 years, who were diagnosed with epithelial ovarian cancer less than 9 months prior. In total, 77% of the patients were postmenopausal, and 63% were FIGO disease stage III or IV. Most of the patients (83%) were recruited from 17 centers in the United Kingdom, with 8% and 9% recruited from single centers in Spain and Hungary, respectively.
Long-term OS was superior in the AHT group. The hazard ratio, adjusted for stratification factors of treating center, menopausal status (pre vs. post), FIGO stage (I and II vs. III and IV), and additional prognostic factors, was 0.45 (95% CI, 0.30-0.69; P less than .001).
Relapse-free survival also was superior in the AHT group compared with the control group (HR, 0.67; 95% CI, 0.47-0.97; P = .032). The RFS benefit increased after adjustment for prognostic factors, as above (adjusted HR, 0.53; 95% CI, 0.34-0.81; P = .004).
Adverse event rates were low, and similar for both groups.
Dr. Eeles reported honoraria from Janssen-Cilag and expenses from Genprobe, Vista, and Illumina. Two of her coauthors reported ties to industry sources.
After a 19-year follow up, women who were diagnosed with ovarian cancer and randomly assigned to receive adjuvant hormone therapy (AHT) had significantly improved overall survival and relapse-free survival compared with control patients who did not receive AHT, researchers reported. The results were published online Sept. 28 in Journal of Clinical Oncology.
Despite the fact that 46 out of 72 patients discontinued taking the hormone therapy, (median 1.14 years of treatment), the benefits of treatment were apparent. Improved survival was observed in the AHT group as early as 5 years after assignment and persisted for 20 years.
Of 121 (81%) patients who died during follow up, 53 (71%) were in the AHT group and 68 (91%) in the control group (HR, 0.63; 95% CI, 0.44-0.90; P = .011) (J Clin Oncol. 2015 Sep 28. doi:10.1200/JCO.2015.60.9719).
“This trial has shown that women who have severe menopausal symptoms after ovarian cancer treatment can safely take HRT without compromising their survival by doing so,” wrote Dr. Rosalind Eeles, professor of oncogenetics and consultant in cancer genetics and clinical oncology at The Institute of Cancer Research, Sutton, London, and colleagues. Their findings from this prospective randomized trial confirmed the survival benefit previously reported in a retrospective study, “so administration of HRT for QOL [quality of life] and survival benefit should be considered in patients with ovarian cancer,” they said.
The phase III nonblinded, controlled trial randomized 150 patients, median age 59 years, who were diagnosed with epithelial ovarian cancer less than 9 months prior. In total, 77% of the patients were postmenopausal, and 63% were FIGO disease stage III or IV. Most of the patients (83%) were recruited from 17 centers in the United Kingdom, with 8% and 9% recruited from single centers in Spain and Hungary, respectively.
Long-term OS was superior in the AHT group. The hazard ratio, adjusted for stratification factors of treating center, menopausal status (pre vs. post), FIGO stage (I and II vs. III and IV), and additional prognostic factors, was 0.45 (95% CI, 0.30-0.69; P less than .001).
Relapse-free survival also was superior in the AHT group compared with the control group (HR, 0.67; 95% CI, 0.47-0.97; P = .032). The RFS benefit increased after adjustment for prognostic factors, as above (adjusted HR, 0.53; 95% CI, 0.34-0.81; P = .004).
Adverse event rates were low, and similar for both groups.
Dr. Eeles reported honoraria from Janssen-Cilag and expenses from Genprobe, Vista, and Illumina. Two of her coauthors reported ties to industry sources.
After a 19-year follow up, women who were diagnosed with ovarian cancer and randomly assigned to receive adjuvant hormone therapy (AHT) had significantly improved overall survival and relapse-free survival compared with control patients who did not receive AHT, researchers reported. The results were published online Sept. 28 in Journal of Clinical Oncology.
Despite the fact that 46 out of 72 patients discontinued taking the hormone therapy, (median 1.14 years of treatment), the benefits of treatment were apparent. Improved survival was observed in the AHT group as early as 5 years after assignment and persisted for 20 years.
Of 121 (81%) patients who died during follow up, 53 (71%) were in the AHT group and 68 (91%) in the control group (HR, 0.63; 95% CI, 0.44-0.90; P = .011) (J Clin Oncol. 2015 Sep 28. doi:10.1200/JCO.2015.60.9719).
“This trial has shown that women who have severe menopausal symptoms after ovarian cancer treatment can safely take HRT without compromising their survival by doing so,” wrote Dr. Rosalind Eeles, professor of oncogenetics and consultant in cancer genetics and clinical oncology at The Institute of Cancer Research, Sutton, London, and colleagues. Their findings from this prospective randomized trial confirmed the survival benefit previously reported in a retrospective study, “so administration of HRT for QOL [quality of life] and survival benefit should be considered in patients with ovarian cancer,” they said.
The phase III nonblinded, controlled trial randomized 150 patients, median age 59 years, who were diagnosed with epithelial ovarian cancer less than 9 months prior. In total, 77% of the patients were postmenopausal, and 63% were FIGO disease stage III or IV. Most of the patients (83%) were recruited from 17 centers in the United Kingdom, with 8% and 9% recruited from single centers in Spain and Hungary, respectively.
Long-term OS was superior in the AHT group. The hazard ratio, adjusted for stratification factors of treating center, menopausal status (pre vs. post), FIGO stage (I and II vs. III and IV), and additional prognostic factors, was 0.45 (95% CI, 0.30-0.69; P less than .001).
Relapse-free survival also was superior in the AHT group compared with the control group (HR, 0.67; 95% CI, 0.47-0.97; P = .032). The RFS benefit increased after adjustment for prognostic factors, as above (adjusted HR, 0.53; 95% CI, 0.34-0.81; P = .004).
Adverse event rates were low, and similar for both groups.
Dr. Eeles reported honoraria from Janssen-Cilag and expenses from Genprobe, Vista, and Illumina. Two of her coauthors reported ties to industry sources.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Epithelial ovarian cancer patients assigned to adjuvant hormone therapy had significantly better overall survival and relapse-free survival than did controls.
Major finding: After a median 19-year follow up, 71% of patients in the AHT group had died compared with 91% in the control group (HR, 0.63; 95% CI, 0.44-0.90; P = .011).
Data source: A phase III nonblinded, controlled trial that randomized 150 patients who were diagnosed with epithelial ovarian cancer less than 9 months prior to receive AHT or not to receive it.
Disclosures: Dr. Eeles reported honoraria from Janssen-Cilag and expenses from Genprobe, Vista, and Illumina. Two of her coauthors reported ties to industry sources.
Lung adenocarcinoma can harbor both ALK fusions and EGFR driver mutations
Microdissected tumors from patients with lung adenocarcinoma (LADC) showed considerable heterogeneity, and in two patients both ALK fusions and EGFR mutations coexisted, researchers reported.
The study used laser-capture microdissection to isolate spatially separated tumor-cell subpopulations according to adenocarcinoma subtypes. In 20 ALK-positive patients analyzed, nine patients had tumors that harbored cells with both ALK fusions and ALK wild-type. In 20 EGFR mutated tumors analyzed, intratumoral heterogeneity was observed in five, both between different adenocarcinoma subtypes and within the same adenocarcinoma subtype (J Clin Oncol. 2015 Sep 28. doi:10.1200/jco.2014.58.8293).
“Importantly, our findings may provide a rationale for differently treating patients with LADC that harbors dual drivers. It seems reasonable to treat patients who do harbor dual drivers with two different targeted inhibitors if the oncogenic drivers of tumor cells within the same primary tumor are not all the same,” wrote Dr. Weijing Cai of Shanghai (China) Pulmonary Hospital, Tongji University, and colleagues.
The ALK fusions did not coexist with the EGFR mutations in all of the tumor cells. Researchers observed that different tumor regions had one, both, or neither mutation. In cells that had neither mutation, search for a possible third oncogene using a capture-based targeted DNA sequencing panel did not uncover another oncogenic mutation.
From 2004 to 2010, 629 patients with lung adenocarcinoma underwent surgical resection at Shanghai Pulmonary Hospital: 364 were positive for EGFR mutations and 30 for ALK fusions. In 20 ALK-positive tumors evaluated, 2 concurrently harbored ALK fusions and EGFR mutations, for a 0.3% incidence rate. Only 18.4% (116 of 629) of patients showed a single mutation pattern.
Although previous research found that intratumoral heterogeneity of EGFR mutations coincided with histologic subtypes in LADC, the current study showed that intratumoral heterogeneity exists in the same histologic subtype. Dr. Cai and associates speculated that clone evolution, not only histologic heterogeneity, may be responsible for molecular intratumoral heterogeneity.
Statistical analysis of 408 patients showed that ALK fusions were significantly more common in LADC with mucin production (P less than .001), they reported.
LADC shows high intratumoral and histologic heterogeneity, but the results of this study show that the correlation between the two has little significance for clinical diagnosis and treatment.
Dr. Cai reported having no disclosures. Several coauthors reported ties to industry sources.
In recent years it has become apparent that intratumoral heterogeneity is an inherent property of many types of cancer, including lung adenocarcinoma (LADC). Although previous studies have shown coexistence of ALK fusions with EGFR mutations in patients with LADC, Dr. Cai and associates have taken these observations further: In two patients with both ALK fusions and EGFR mutations, they examined four separate areas within one tumor. In different tumor regions, one, both, or neither mutation was present. In tumor regions with evidence of both mutations present, they found the relative abundance of ALK fusions and EGFR mutations differed, suggesting that at least some cancer cells did not harbor both mutations. It appears ALK fusions and EGFR mutations may not coexist in the same cancer cell population and may be later branched events in the evolution of the tumor. The specific EGFR mutations observed were inconsistent as well, either R832H or L858R.
If EGFR mutation–positive subclones within ALK-rearranged tumors cause relapse in a subset of patients, these patients may be candidates for subsequent treatment with anti-EGFR therapies. These findings support a role for repeated tumor analysis during the evolution of disease in patients who progress after ALK inhibitor therapy despite a prior EGFR-negative biopsy. Recognition of the limits of diagnostic assays and the influence that a minority of cells harboring oncogenic drivers may have on disease is important to advance precision medicine.
This study highlights the importance of evolutionary precision medicine in LADC and the need for genomic evaluations throughout the disease course. Advances in cell-free DNA detection may circumvent problems of tumor sampling and enable us to monitor evolving subclonal driver events in the tumor and in metastatic lesions so that therapy can be adapted accordingly.
Dr. Nicolai Juul Birkbak is a principal research associate at University College London. Dr. Crispin T. Hiley is a research fellow at University College London. Dr. Charles Swanton is a senior clinical research fellow and group leader of the translational cancer therapeutics laboratory at the London Research Institute and consultant medical oncologist at the Royal Marsden Hospital. These remarks were part of an editorial accompanying the report (J Clin Oncol. 2015 Sep 28. doi: 10.1200/jco.2014.58.8293). Dr. Birkbak disclosed stock or other ownership in Onxeo and royalties from Myriad Genetics. Dr. Hiley reported stock or other ownership in Bristol-Myers Squibb. Dr. Swanton reported stock or other ownership in Epic Sciences and Apogen Biotechnologies and honoraria or research funding from Roche, Boehringer Ingelheim, Novartis, and GlaxoSmithKline.
In recent years it has become apparent that intratumoral heterogeneity is an inherent property of many types of cancer, including lung adenocarcinoma (LADC). Although previous studies have shown coexistence of ALK fusions with EGFR mutations in patients with LADC, Dr. Cai and associates have taken these observations further: In two patients with both ALK fusions and EGFR mutations, they examined four separate areas within one tumor. In different tumor regions, one, both, or neither mutation was present. In tumor regions with evidence of both mutations present, they found the relative abundance of ALK fusions and EGFR mutations differed, suggesting that at least some cancer cells did not harbor both mutations. It appears ALK fusions and EGFR mutations may not coexist in the same cancer cell population and may be later branched events in the evolution of the tumor. The specific EGFR mutations observed were inconsistent as well, either R832H or L858R.
If EGFR mutation–positive subclones within ALK-rearranged tumors cause relapse in a subset of patients, these patients may be candidates for subsequent treatment with anti-EGFR therapies. These findings support a role for repeated tumor analysis during the evolution of disease in patients who progress after ALK inhibitor therapy despite a prior EGFR-negative biopsy. Recognition of the limits of diagnostic assays and the influence that a minority of cells harboring oncogenic drivers may have on disease is important to advance precision medicine.
This study highlights the importance of evolutionary precision medicine in LADC and the need for genomic evaluations throughout the disease course. Advances in cell-free DNA detection may circumvent problems of tumor sampling and enable us to monitor evolving subclonal driver events in the tumor and in metastatic lesions so that therapy can be adapted accordingly.
Dr. Nicolai Juul Birkbak is a principal research associate at University College London. Dr. Crispin T. Hiley is a research fellow at University College London. Dr. Charles Swanton is a senior clinical research fellow and group leader of the translational cancer therapeutics laboratory at the London Research Institute and consultant medical oncologist at the Royal Marsden Hospital. These remarks were part of an editorial accompanying the report (J Clin Oncol. 2015 Sep 28. doi: 10.1200/jco.2014.58.8293). Dr. Birkbak disclosed stock or other ownership in Onxeo and royalties from Myriad Genetics. Dr. Hiley reported stock or other ownership in Bristol-Myers Squibb. Dr. Swanton reported stock or other ownership in Epic Sciences and Apogen Biotechnologies and honoraria or research funding from Roche, Boehringer Ingelheim, Novartis, and GlaxoSmithKline.
In recent years it has become apparent that intratumoral heterogeneity is an inherent property of many types of cancer, including lung adenocarcinoma (LADC). Although previous studies have shown coexistence of ALK fusions with EGFR mutations in patients with LADC, Dr. Cai and associates have taken these observations further: In two patients with both ALK fusions and EGFR mutations, they examined four separate areas within one tumor. In different tumor regions, one, both, or neither mutation was present. In tumor regions with evidence of both mutations present, they found the relative abundance of ALK fusions and EGFR mutations differed, suggesting that at least some cancer cells did not harbor both mutations. It appears ALK fusions and EGFR mutations may not coexist in the same cancer cell population and may be later branched events in the evolution of the tumor. The specific EGFR mutations observed were inconsistent as well, either R832H or L858R.
If EGFR mutation–positive subclones within ALK-rearranged tumors cause relapse in a subset of patients, these patients may be candidates for subsequent treatment with anti-EGFR therapies. These findings support a role for repeated tumor analysis during the evolution of disease in patients who progress after ALK inhibitor therapy despite a prior EGFR-negative biopsy. Recognition of the limits of diagnostic assays and the influence that a minority of cells harboring oncogenic drivers may have on disease is important to advance precision medicine.
This study highlights the importance of evolutionary precision medicine in LADC and the need for genomic evaluations throughout the disease course. Advances in cell-free DNA detection may circumvent problems of tumor sampling and enable us to monitor evolving subclonal driver events in the tumor and in metastatic lesions so that therapy can be adapted accordingly.
Dr. Nicolai Juul Birkbak is a principal research associate at University College London. Dr. Crispin T. Hiley is a research fellow at University College London. Dr. Charles Swanton is a senior clinical research fellow and group leader of the translational cancer therapeutics laboratory at the London Research Institute and consultant medical oncologist at the Royal Marsden Hospital. These remarks were part of an editorial accompanying the report (J Clin Oncol. 2015 Sep 28. doi: 10.1200/jco.2014.58.8293). Dr. Birkbak disclosed stock or other ownership in Onxeo and royalties from Myriad Genetics. Dr. Hiley reported stock or other ownership in Bristol-Myers Squibb. Dr. Swanton reported stock or other ownership in Epic Sciences and Apogen Biotechnologies and honoraria or research funding from Roche, Boehringer Ingelheim, Novartis, and GlaxoSmithKline.
Microdissected tumors from patients with lung adenocarcinoma (LADC) showed considerable heterogeneity, and in two patients both ALK fusions and EGFR mutations coexisted, researchers reported.
The study used laser-capture microdissection to isolate spatially separated tumor-cell subpopulations according to adenocarcinoma subtypes. In 20 ALK-positive patients analyzed, nine patients had tumors that harbored cells with both ALK fusions and ALK wild-type. In 20 EGFR mutated tumors analyzed, intratumoral heterogeneity was observed in five, both between different adenocarcinoma subtypes and within the same adenocarcinoma subtype (J Clin Oncol. 2015 Sep 28. doi:10.1200/jco.2014.58.8293).
“Importantly, our findings may provide a rationale for differently treating patients with LADC that harbors dual drivers. It seems reasonable to treat patients who do harbor dual drivers with two different targeted inhibitors if the oncogenic drivers of tumor cells within the same primary tumor are not all the same,” wrote Dr. Weijing Cai of Shanghai (China) Pulmonary Hospital, Tongji University, and colleagues.
The ALK fusions did not coexist with the EGFR mutations in all of the tumor cells. Researchers observed that different tumor regions had one, both, or neither mutation. In cells that had neither mutation, search for a possible third oncogene using a capture-based targeted DNA sequencing panel did not uncover another oncogenic mutation.
From 2004 to 2010, 629 patients with lung adenocarcinoma underwent surgical resection at Shanghai Pulmonary Hospital: 364 were positive for EGFR mutations and 30 for ALK fusions. In 20 ALK-positive tumors evaluated, 2 concurrently harbored ALK fusions and EGFR mutations, for a 0.3% incidence rate. Only 18.4% (116 of 629) of patients showed a single mutation pattern.
Although previous research found that intratumoral heterogeneity of EGFR mutations coincided with histologic subtypes in LADC, the current study showed that intratumoral heterogeneity exists in the same histologic subtype. Dr. Cai and associates speculated that clone evolution, not only histologic heterogeneity, may be responsible for molecular intratumoral heterogeneity.
Statistical analysis of 408 patients showed that ALK fusions were significantly more common in LADC with mucin production (P less than .001), they reported.
LADC shows high intratumoral and histologic heterogeneity, but the results of this study show that the correlation between the two has little significance for clinical diagnosis and treatment.
Dr. Cai reported having no disclosures. Several coauthors reported ties to industry sources.
Microdissected tumors from patients with lung adenocarcinoma (LADC) showed considerable heterogeneity, and in two patients both ALK fusions and EGFR mutations coexisted, researchers reported.
The study used laser-capture microdissection to isolate spatially separated tumor-cell subpopulations according to adenocarcinoma subtypes. In 20 ALK-positive patients analyzed, nine patients had tumors that harbored cells with both ALK fusions and ALK wild-type. In 20 EGFR mutated tumors analyzed, intratumoral heterogeneity was observed in five, both between different adenocarcinoma subtypes and within the same adenocarcinoma subtype (J Clin Oncol. 2015 Sep 28. doi:10.1200/jco.2014.58.8293).
“Importantly, our findings may provide a rationale for differently treating patients with LADC that harbors dual drivers. It seems reasonable to treat patients who do harbor dual drivers with two different targeted inhibitors if the oncogenic drivers of tumor cells within the same primary tumor are not all the same,” wrote Dr. Weijing Cai of Shanghai (China) Pulmonary Hospital, Tongji University, and colleagues.
The ALK fusions did not coexist with the EGFR mutations in all of the tumor cells. Researchers observed that different tumor regions had one, both, or neither mutation. In cells that had neither mutation, search for a possible third oncogene using a capture-based targeted DNA sequencing panel did not uncover another oncogenic mutation.
From 2004 to 2010, 629 patients with lung adenocarcinoma underwent surgical resection at Shanghai Pulmonary Hospital: 364 were positive for EGFR mutations and 30 for ALK fusions. In 20 ALK-positive tumors evaluated, 2 concurrently harbored ALK fusions and EGFR mutations, for a 0.3% incidence rate. Only 18.4% (116 of 629) of patients showed a single mutation pattern.
Although previous research found that intratumoral heterogeneity of EGFR mutations coincided with histologic subtypes in LADC, the current study showed that intratumoral heterogeneity exists in the same histologic subtype. Dr. Cai and associates speculated that clone evolution, not only histologic heterogeneity, may be responsible for molecular intratumoral heterogeneity.
Statistical analysis of 408 patients showed that ALK fusions were significantly more common in LADC with mucin production (P less than .001), they reported.
LADC shows high intratumoral and histologic heterogeneity, but the results of this study show that the correlation between the two has little significance for clinical diagnosis and treatment.
Dr. Cai reported having no disclosures. Several coauthors reported ties to industry sources.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Tumor cells within the same primary tumor were found to harbor both ALK fusions and EGFR driver mutations.
Major finding: In 20 patients with ALK-positive tumors, 2 concurrently harbored ALK fusions and EGFR mutations, for a 0.3% incidence rate.
Data source: From 2004 to 2010, 629 patients with lung adenocarcinoma underwent surgical resection in Shanghai, China: 364 were positive for EGFR mutations and 30 for ALK fusions.
Disclosures: Dr. Cai reported having no disclosures. Several coauthors reported ties to industry sources.
ASCO: Racial disparity in HER2+ breast cancer survival subsided after trastuzumab approval
Well-documented racial disparities in survival of patients with HER2+ breast cancer diminished after FDA approval of trastuzumab, according to research presented at the 2015 ASCO breast cancer symposium.
A retrospective study identified 32,597 cases of HER2+ breast cancer from the California Cancer Registry, and divided them into early (diagnosed 2000-2006) and late (diagnosed 2006-2012) cohorts. In the early cohort, diagnosed before trastuzumab was available, black patients had an increased risk of mortality (hazard ratio, 1.32; 95% confidence interval, 1.16-1.49), compared with whites. In the late cohort there were no survival differences based on race.
“Although we were unable to document use of anti-HER2 treatment, the era of adjuvant trastuzumab appears to have attenuated the black/white disparity in HER2-positive breast cancer,” wrote Dr. Vincent Caggiano, hematologist at Sutter Medical Center Sacramento, California, and colleagues in a meeting abstract.
The study analyzed risk of mortality, adjusted for stage, grade, age, and socioeconomic status, for African Americans, Hispanics, Asian/Pacific Islanders, and American Indians, compared with white patients. Considering estrogen-receptor and progesterone-receptor status, the combination of all HER2+ subtypes (ER+/PR+/HER2+, ER-/PR+/HER2+, ER+/PR-/HER2+, ER-/PR-/HER2+), showed increased risk for black patients before 2006. The ER-/PR-/HER2+ subtype showed no racial disparities for either cohort. The highest risk (HR, 1.43; 95% CI, 1.17-1.75) was observed for black patients of the ER+/PR+/HER2+ subtype diagnosed before 2006.
Well-documented racial disparities in survival of patients with HER2+ breast cancer diminished after FDA approval of trastuzumab, according to research presented at the 2015 ASCO breast cancer symposium.
A retrospective study identified 32,597 cases of HER2+ breast cancer from the California Cancer Registry, and divided them into early (diagnosed 2000-2006) and late (diagnosed 2006-2012) cohorts. In the early cohort, diagnosed before trastuzumab was available, black patients had an increased risk of mortality (hazard ratio, 1.32; 95% confidence interval, 1.16-1.49), compared with whites. In the late cohort there were no survival differences based on race.
“Although we were unable to document use of anti-HER2 treatment, the era of adjuvant trastuzumab appears to have attenuated the black/white disparity in HER2-positive breast cancer,” wrote Dr. Vincent Caggiano, hematologist at Sutter Medical Center Sacramento, California, and colleagues in a meeting abstract.
The study analyzed risk of mortality, adjusted for stage, grade, age, and socioeconomic status, for African Americans, Hispanics, Asian/Pacific Islanders, and American Indians, compared with white patients. Considering estrogen-receptor and progesterone-receptor status, the combination of all HER2+ subtypes (ER+/PR+/HER2+, ER-/PR+/HER2+, ER+/PR-/HER2+, ER-/PR-/HER2+), showed increased risk for black patients before 2006. The ER-/PR-/HER2+ subtype showed no racial disparities for either cohort. The highest risk (HR, 1.43; 95% CI, 1.17-1.75) was observed for black patients of the ER+/PR+/HER2+ subtype diagnosed before 2006.
Well-documented racial disparities in survival of patients with HER2+ breast cancer diminished after FDA approval of trastuzumab, according to research presented at the 2015 ASCO breast cancer symposium.
A retrospective study identified 32,597 cases of HER2+ breast cancer from the California Cancer Registry, and divided them into early (diagnosed 2000-2006) and late (diagnosed 2006-2012) cohorts. In the early cohort, diagnosed before trastuzumab was available, black patients had an increased risk of mortality (hazard ratio, 1.32; 95% confidence interval, 1.16-1.49), compared with whites. In the late cohort there were no survival differences based on race.
“Although we were unable to document use of anti-HER2 treatment, the era of adjuvant trastuzumab appears to have attenuated the black/white disparity in HER2-positive breast cancer,” wrote Dr. Vincent Caggiano, hematologist at Sutter Medical Center Sacramento, California, and colleagues in a meeting abstract.
The study analyzed risk of mortality, adjusted for stage, grade, age, and socioeconomic status, for African Americans, Hispanics, Asian/Pacific Islanders, and American Indians, compared with white patients. Considering estrogen-receptor and progesterone-receptor status, the combination of all HER2+ subtypes (ER+/PR+/HER2+, ER-/PR+/HER2+, ER+/PR-/HER2+, ER-/PR-/HER2+), showed increased risk for black patients before 2006. The ER-/PR-/HER2+ subtype showed no racial disparities for either cohort. The highest risk (HR, 1.43; 95% CI, 1.17-1.75) was observed for black patients of the ER+/PR+/HER2+ subtype diagnosed before 2006.
FROM THE 2015 ASCO BREAST CANCER SYMPOSIUM
Key clinical point: The well-documented disparity in survival between black and white patients with HER2+ breast cancer was not observed during a time period after 2006, when the FDA approved trastuzumab.
Major finding: Among patients diagnosed from 2000 to 2006, black patients had an increased risk of mortality, compared with white patients (HR, 1.32; 95% CI, 1.16-1.49); from 2006-2012 there were no survival differences based on race/ethnicity.
Data source: The retrospective study used the California Cancer Registry to identify 32,597 cases of HER2+ breast cancer diagnosed from 2000 to 2012, and divided them into early (2000-2006) and late (2006-2012) cohorts.
Disclosures: The authors reported having no disclosures.
ASCO: Overall survival similar regardless of distant relapse site in breast cancer
The initial distant relapse site had little influence on overall survival rates among women with metastatic breast cancer who underwent surgery and definitive radiotherapy, according to a study presented at the 2015 ASCO Breast Cancer Symposium.
In total, 3,417 patients with breast cancer treated with surgery and definitive radiotherapy in Tokyo between 1980 and 2014 were included in the study, with a median follow up of 113 months. Metastatic progression was a first relapse event in 370 patients, and median duration of OS after initial metastatic relapse was 69 months.
Tumors were classified as luminal A, luminal-human epidermal growth factor receptor 2 (HER2), luminal B, and triple negative. OS after distant relapse for patients whose initial tumor was luminal was significantly longer than triple negative and HER2 subtypes (P = .003).
OS was similar for all subtypes after initial bone or nonbone/brain relapse.
Despite the fact that bone metastasis as the initial distant relapse is commonly considered to have better prognosis than other sites in metastatic breast cancer, “OS rates of bone and non-bone/brain metastasis groups… were almost identical,” wrote Kenshiro Shiraishi of the University of Tokyo Hospital, Japan, and colleagues in a meeting abstract.
The initial distant relapse site had little influence on overall survival rates among women with metastatic breast cancer who underwent surgery and definitive radiotherapy, according to a study presented at the 2015 ASCO Breast Cancer Symposium.
In total, 3,417 patients with breast cancer treated with surgery and definitive radiotherapy in Tokyo between 1980 and 2014 were included in the study, with a median follow up of 113 months. Metastatic progression was a first relapse event in 370 patients, and median duration of OS after initial metastatic relapse was 69 months.
Tumors were classified as luminal A, luminal-human epidermal growth factor receptor 2 (HER2), luminal B, and triple negative. OS after distant relapse for patients whose initial tumor was luminal was significantly longer than triple negative and HER2 subtypes (P = .003).
OS was similar for all subtypes after initial bone or nonbone/brain relapse.
Despite the fact that bone metastasis as the initial distant relapse is commonly considered to have better prognosis than other sites in metastatic breast cancer, “OS rates of bone and non-bone/brain metastasis groups… were almost identical,” wrote Kenshiro Shiraishi of the University of Tokyo Hospital, Japan, and colleagues in a meeting abstract.
The initial distant relapse site had little influence on overall survival rates among women with metastatic breast cancer who underwent surgery and definitive radiotherapy, according to a study presented at the 2015 ASCO Breast Cancer Symposium.
In total, 3,417 patients with breast cancer treated with surgery and definitive radiotherapy in Tokyo between 1980 and 2014 were included in the study, with a median follow up of 113 months. Metastatic progression was a first relapse event in 370 patients, and median duration of OS after initial metastatic relapse was 69 months.
Tumors were classified as luminal A, luminal-human epidermal growth factor receptor 2 (HER2), luminal B, and triple negative. OS after distant relapse for patients whose initial tumor was luminal was significantly longer than triple negative and HER2 subtypes (P = .003).
OS was similar for all subtypes after initial bone or nonbone/brain relapse.
Despite the fact that bone metastasis as the initial distant relapse is commonly considered to have better prognosis than other sites in metastatic breast cancer, “OS rates of bone and non-bone/brain metastasis groups… were almost identical,” wrote Kenshiro Shiraishi of the University of Tokyo Hospital, Japan, and colleagues in a meeting abstract.
FROM THE 2015 ASCO BREAST CANCER SYMPOSIUM
Key clinical point: In patients with metastatic breast cancer, initial distant relapse site had little influence on overall survival rates.
Major finding: Overall survival (OS) rates for patients whose initial relapse site was bone were similar to those whose initial site was nonbone/brain. OS after distant relapse for patients whose initial tumor was luminal was significantly longer than triple negative and HER2 subtypes (P = .003).
Data source: In total, 3,417 patients with breast cancer treated with surgery and definitive radiotherapy in Tokyo between 1980 and 2014 were included in the study.
Disclosures: The authors reported having no disclosures.
Histologic information can’t replace recurrence score
Combining routine histologic information, including tumor size, grade, and cellular proliferation marker Ki67, failed to accurately reflect the recurrence score produced by the commercial diagnostic test, Oncotype Dx, according to researchers.
Although each variable individually was significantly correlated with Oncotype Dx, the combination of variables had a percentage of variance (R2) of 0.35 on the recurrence score and did not accurately predict the result generated by the 21-gene reverse-transcriptase polymerase chain reaction (RT-PCR) diagnostic test.
The single-institution study included 252 patients, (248 females and 4 males, median age 56 years) with early-stage, estrogen receptor–positive breast cancer. All patients had Oncotype Dx testing performed between 2007 and 2014. The median tumor size was 2.1 cm, and 49% were grade 2, 28% grade 3, and 23% grade 1.
Investigators examined baseline patient demographic data, such as age, race, and sex, and routine pathologic features, such as histologic grade, Ki-67, tumor size, and histologic type. The combination of three variables that individually were highly correlated to the Oncotype Dx score did not produce values that could substitute for the recurrence score.
Furthermore, the prediction capability of the three-variable combination decreased for tumors with high-recurrence scores.
“Therefore, we conclude the RS [recurrence score] can provide additional valuable information and should not be replaced by analysis of routine histologic variables alone,” wrote Dr. Kate Lathrop of the Cancer Therapy and Research Center at the University of Texas Health Science Center in San Antonio, and her colleagues.
Combining routine histologic information, including tumor size, grade, and cellular proliferation marker Ki67, failed to accurately reflect the recurrence score produced by the commercial diagnostic test, Oncotype Dx, according to researchers.
Although each variable individually was significantly correlated with Oncotype Dx, the combination of variables had a percentage of variance (R2) of 0.35 on the recurrence score and did not accurately predict the result generated by the 21-gene reverse-transcriptase polymerase chain reaction (RT-PCR) diagnostic test.
The single-institution study included 252 patients, (248 females and 4 males, median age 56 years) with early-stage, estrogen receptor–positive breast cancer. All patients had Oncotype Dx testing performed between 2007 and 2014. The median tumor size was 2.1 cm, and 49% were grade 2, 28% grade 3, and 23% grade 1.
Investigators examined baseline patient demographic data, such as age, race, and sex, and routine pathologic features, such as histologic grade, Ki-67, tumor size, and histologic type. The combination of three variables that individually were highly correlated to the Oncotype Dx score did not produce values that could substitute for the recurrence score.
Furthermore, the prediction capability of the three-variable combination decreased for tumors with high-recurrence scores.
“Therefore, we conclude the RS [recurrence score] can provide additional valuable information and should not be replaced by analysis of routine histologic variables alone,” wrote Dr. Kate Lathrop of the Cancer Therapy and Research Center at the University of Texas Health Science Center in San Antonio, and her colleagues.
Combining routine histologic information, including tumor size, grade, and cellular proliferation marker Ki67, failed to accurately reflect the recurrence score produced by the commercial diagnostic test, Oncotype Dx, according to researchers.
Although each variable individually was significantly correlated with Oncotype Dx, the combination of variables had a percentage of variance (R2) of 0.35 on the recurrence score and did not accurately predict the result generated by the 21-gene reverse-transcriptase polymerase chain reaction (RT-PCR) diagnostic test.
The single-institution study included 252 patients, (248 females and 4 males, median age 56 years) with early-stage, estrogen receptor–positive breast cancer. All patients had Oncotype Dx testing performed between 2007 and 2014. The median tumor size was 2.1 cm, and 49% were grade 2, 28% grade 3, and 23% grade 1.
Investigators examined baseline patient demographic data, such as age, race, and sex, and routine pathologic features, such as histologic grade, Ki-67, tumor size, and histologic type. The combination of three variables that individually were highly correlated to the Oncotype Dx score did not produce values that could substitute for the recurrence score.
Furthermore, the prediction capability of the three-variable combination decreased for tumors with high-recurrence scores.
“Therefore, we conclude the RS [recurrence score] can provide additional valuable information and should not be replaced by analysis of routine histologic variables alone,” wrote Dr. Kate Lathrop of the Cancer Therapy and Research Center at the University of Texas Health Science Center in San Antonio, and her colleagues.
FROM THE ASCO BREAST CANCER SYMPOSIUM
Key clinical point: A combination of three histologic variables (tumor size, grade, and cellular proliferation marker Ki67) failed to accurately predict the Oncotype Dx recurrence score.
Major finding: Based on the Oncotype Dx recurrence score, the score predicted by the three-variable combination had a percentage of variance (R2) of 0.35.
Data source: The retrospective, single-institution study evaluated data from 252 patients with early-stage estrogen receptor–positive breast cancer who had Oncotype Dx testing performed between 2007 and 2014.
Disclosures: The authors reported having no disclosures.
Topical resiquimod effective for early-stage cutaneous T-cell lymphoma
Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL), in some cases inducing regression in both treated and untreated lesions, according to researchers.
The mean number of prior unsuccessful therapies among the patients was 6, yet the majority of patients (11 of 12) experienced significant improvement, and 2 patients had complete clinical responses with no evidence of disease after treatment. One patient, despite a 15-year history of disease and 11 unsuccessful treatments, experienced a complete resolution of both treated and untreated skin lesions.
The open-label, phase I trial evaluated 12 patients with early-stage CTCL. Patients experienced minor adverse effects (all grade 1), which were primarily skin irritation. The trial evaluated 0.03% and 0.06% resiquimod, with complete and more rapid responses occurring at the higher dose. Both doses were equally well tolerated.
“These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL,” wrote Dr. Alain Rook of the Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, and colleagues.
Arising from T cells that traffic to the skin, CTCLs are non-Hodgkin lymphomas whose only potential cure is stem cell transplantation. Studies suggest that host antitumor immunity plays an important role in the disease, and in this study, high responders showed recruitment and expansion of benign T-cell clones and activation of T cells and natural killer cells in the skin.
In the absence of cell-surface markers to distinguish malignant from benign T cells in the lesion, the team used high throughput screening of the T-cell receptor–beta gene to quantify malignant cells and monitor response to therapy. Of the 10 patients with identified malignant cells, biopsied lesions showed that most had reduction of malignant T-cell clones and 3 had complete eradication. The results may not reflect responses in nonbiopsied lesions.
Resiquimod recruits T cells and other immune cells to the skin, causing inflammation that the researchers observed persisted after complete or nearly complete malignant T-cell eradication. Study results suggested that activation of CD4+ cells and expansion of tumor-specific T cells is critical for effectiveness of resiquimod.
Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL), in some cases inducing regression in both treated and untreated lesions, according to researchers.
The mean number of prior unsuccessful therapies among the patients was 6, yet the majority of patients (11 of 12) experienced significant improvement, and 2 patients had complete clinical responses with no evidence of disease after treatment. One patient, despite a 15-year history of disease and 11 unsuccessful treatments, experienced a complete resolution of both treated and untreated skin lesions.
The open-label, phase I trial evaluated 12 patients with early-stage CTCL. Patients experienced minor adverse effects (all grade 1), which were primarily skin irritation. The trial evaluated 0.03% and 0.06% resiquimod, with complete and more rapid responses occurring at the higher dose. Both doses were equally well tolerated.
“These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL,” wrote Dr. Alain Rook of the Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, and colleagues.
Arising from T cells that traffic to the skin, CTCLs are non-Hodgkin lymphomas whose only potential cure is stem cell transplantation. Studies suggest that host antitumor immunity plays an important role in the disease, and in this study, high responders showed recruitment and expansion of benign T-cell clones and activation of T cells and natural killer cells in the skin.
In the absence of cell-surface markers to distinguish malignant from benign T cells in the lesion, the team used high throughput screening of the T-cell receptor–beta gene to quantify malignant cells and monitor response to therapy. Of the 10 patients with identified malignant cells, biopsied lesions showed that most had reduction of malignant T-cell clones and 3 had complete eradication. The results may not reflect responses in nonbiopsied lesions.
Resiquimod recruits T cells and other immune cells to the skin, causing inflammation that the researchers observed persisted after complete or nearly complete malignant T-cell eradication. Study results suggested that activation of CD4+ cells and expansion of tumor-specific T cells is critical for effectiveness of resiquimod.
Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL), in some cases inducing regression in both treated and untreated lesions, according to researchers.
The mean number of prior unsuccessful therapies among the patients was 6, yet the majority of patients (11 of 12) experienced significant improvement, and 2 patients had complete clinical responses with no evidence of disease after treatment. One patient, despite a 15-year history of disease and 11 unsuccessful treatments, experienced a complete resolution of both treated and untreated skin lesions.
The open-label, phase I trial evaluated 12 patients with early-stage CTCL. Patients experienced minor adverse effects (all grade 1), which were primarily skin irritation. The trial evaluated 0.03% and 0.06% resiquimod, with complete and more rapid responses occurring at the higher dose. Both doses were equally well tolerated.
“These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL,” wrote Dr. Alain Rook of the Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, and colleagues.
Arising from T cells that traffic to the skin, CTCLs are non-Hodgkin lymphomas whose only potential cure is stem cell transplantation. Studies suggest that host antitumor immunity plays an important role in the disease, and in this study, high responders showed recruitment and expansion of benign T-cell clones and activation of T cells and natural killer cells in the skin.
In the absence of cell-surface markers to distinguish malignant from benign T cells in the lesion, the team used high throughput screening of the T-cell receptor–beta gene to quantify malignant cells and monitor response to therapy. Of the 10 patients with identified malignant cells, biopsied lesions showed that most had reduction of malignant T-cell clones and 3 had complete eradication. The results may not reflect responses in nonbiopsied lesions.
Resiquimod recruits T cells and other immune cells to the skin, causing inflammation that the researchers observed persisted after complete or nearly complete malignant T-cell eradication. Study results suggested that activation of CD4+ cells and expansion of tumor-specific T cells is critical for effectiveness of resiquimod.
FROM BLOOD
Key clinical point: Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL).
Major finding: In total, 11 of 12 patients had significant improvement: 2 had resolution of all evidence of disease, and 9 experienced improvement greater than or equal to 50%.
Data source: The open-label, phase I trial evaluated 12 patients with early stage CTCL.
Disclosures: Dr. Rook and one coauthor have patents pending on HTS in cutaneous lymphoma. His coauthor is employed by Adaptive Biotechnologies.