User login
Jennifer Smith is the editor of Oncology Practice, part of MDedge Hematology/Oncology. She was previously the editor of Hematology Times, an editor at Principal Investigators Association, and a reporter at The Oneida Daily Dispatch. She has a BS in journalism.
Next-generation sequencing test detects pathogens with high sensitivity
NEW ORLEANS – A next-generation sequencing (NGS) test for pathogen detection demonstrated higher sensitivity than conventional testing methods in a cohort of diverse pediatric patients, according to researchers.
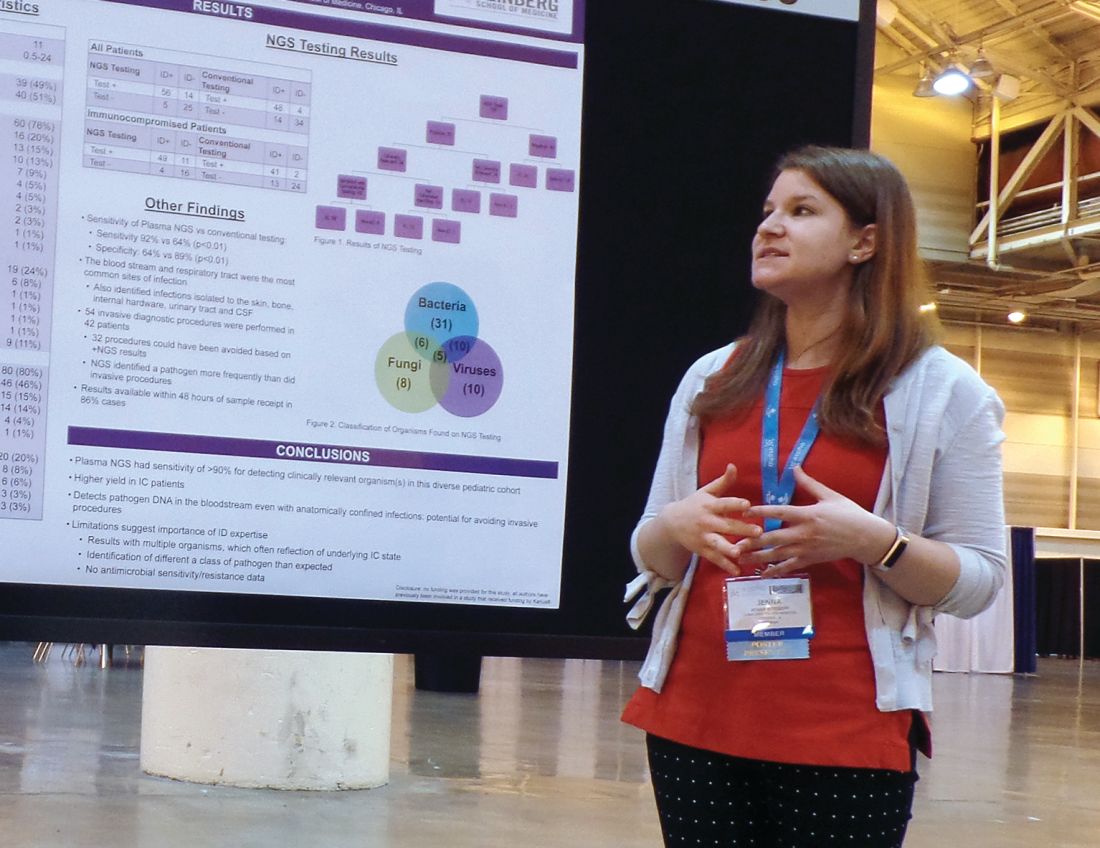
The NGS test, which detects sequences of circulating cell-free DNA in plasma, detected pathogens with 92% sensitivity, compared with 64% sensitivity for all conventional testing methods combined (P less than .01).
“While I think we can all recognize that specificity is important, I think sensitivity is more important to be able to get at sources of infection,” said Jenna Rossoff, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago.
Dr. Rossoff and her colleagues conducted this study and presented the results in a poster at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Lurie Children’s Hospital began using a commercially available NGS pathogen test, the Karius test, in 2016. Dr. Rossoff and her colleagues set out to evaluate how the test affected patient care by conducting a retrospective analysis of tests performed from December 2016 through August 2018.
The researchers studied 100 NGS tests performed for 79 pediatric patients. The patients had a median age of 11 years (range, 0.5-24 years).
Most patients (n = 60) were immunocompromised, largely due to a hematologic malignancy (n = 16), primary immune deficiency (n = 13), hematopoietic cell transplant (n = 10), or solid organ transplant (n = 7).
The remaining 19 patients were immunocompetent, and 9 of them had no underlying diagnosis. The most common diagnosis for this group was neurologic disorder (n = 6).
Results
Of the 100 NGS tests evaluated, 70 were positive for any organism, and 56 of these were deemed clinically relevant.
“What I think is quite remarkable is that, of those clinically relevant organisms, tests on 14, which is 25% of those, were able to identify clinically relevant or pathogenic organisms when no other conventional testing modality was able to identify them,” Dr. Rossoff said. “And these were often in patients who underwent invasive procedures to try to get at the source of their infectious disease.”
In fact, the study included 42 patients who underwent 54 invasive diagnostic procedures, and 32 of those procedures could have been avoided based on positive NGS results, according to Dr. Rossoff and her colleagues.
Dr. Rossoff noted that the most common sites of infection were the bloodstream and respiratory tract, but the NGS test was able to identify pathogens in the bone, skin, cerebrospinal fluid, and urinary tract. She also pointed out that NGS results were available “in a fairly timely manner,” as 86% of test results were available within 48 hours of sample receipt.
Dr. Rossoff and her colleagues did not receive any funding for this study, but they were previously involved in a study funded by Karius, the company that commercialized the NGS test.
SOURCE: Rossoff J et al. ASPHO 2019. Abstract 439.
NEW ORLEANS – A next-generation sequencing (NGS) test for pathogen detection demonstrated higher sensitivity than conventional testing methods in a cohort of diverse pediatric patients, according to researchers.
The NGS test, which detects sequences of circulating cell-free DNA in plasma, detected pathogens with 92% sensitivity, compared with 64% sensitivity for all conventional testing methods combined (P less than .01).
“While I think we can all recognize that specificity is important, I think sensitivity is more important to be able to get at sources of infection,” said Jenna Rossoff, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago.
Dr. Rossoff and her colleagues conducted this study and presented the results in a poster at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Lurie Children’s Hospital began using a commercially available NGS pathogen test, the Karius test, in 2016. Dr. Rossoff and her colleagues set out to evaluate how the test affected patient care by conducting a retrospective analysis of tests performed from December 2016 through August 2018.
The researchers studied 100 NGS tests performed for 79 pediatric patients. The patients had a median age of 11 years (range, 0.5-24 years).
Most patients (n = 60) were immunocompromised, largely due to a hematologic malignancy (n = 16), primary immune deficiency (n = 13), hematopoietic cell transplant (n = 10), or solid organ transplant (n = 7).
The remaining 19 patients were immunocompetent, and 9 of them had no underlying diagnosis. The most common diagnosis for this group was neurologic disorder (n = 6).
Results
Of the 100 NGS tests evaluated, 70 were positive for any organism, and 56 of these were deemed clinically relevant.
“What I think is quite remarkable is that, of those clinically relevant organisms, tests on 14, which is 25% of those, were able to identify clinically relevant or pathogenic organisms when no other conventional testing modality was able to identify them,” Dr. Rossoff said. “And these were often in patients who underwent invasive procedures to try to get at the source of their infectious disease.”
In fact, the study included 42 patients who underwent 54 invasive diagnostic procedures, and 32 of those procedures could have been avoided based on positive NGS results, according to Dr. Rossoff and her colleagues.
Dr. Rossoff noted that the most common sites of infection were the bloodstream and respiratory tract, but the NGS test was able to identify pathogens in the bone, skin, cerebrospinal fluid, and urinary tract. She also pointed out that NGS results were available “in a fairly timely manner,” as 86% of test results were available within 48 hours of sample receipt.
Dr. Rossoff and her colleagues did not receive any funding for this study, but they were previously involved in a study funded by Karius, the company that commercialized the NGS test.
SOURCE: Rossoff J et al. ASPHO 2019. Abstract 439.
NEW ORLEANS – A next-generation sequencing (NGS) test for pathogen detection demonstrated higher sensitivity than conventional testing methods in a cohort of diverse pediatric patients, according to researchers.
The NGS test, which detects sequences of circulating cell-free DNA in plasma, detected pathogens with 92% sensitivity, compared with 64% sensitivity for all conventional testing methods combined (P less than .01).
“While I think we can all recognize that specificity is important, I think sensitivity is more important to be able to get at sources of infection,” said Jenna Rossoff, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago.
Dr. Rossoff and her colleagues conducted this study and presented the results in a poster at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Lurie Children’s Hospital began using a commercially available NGS pathogen test, the Karius test, in 2016. Dr. Rossoff and her colleagues set out to evaluate how the test affected patient care by conducting a retrospective analysis of tests performed from December 2016 through August 2018.
The researchers studied 100 NGS tests performed for 79 pediatric patients. The patients had a median age of 11 years (range, 0.5-24 years).
Most patients (n = 60) were immunocompromised, largely due to a hematologic malignancy (n = 16), primary immune deficiency (n = 13), hematopoietic cell transplant (n = 10), or solid organ transplant (n = 7).
The remaining 19 patients were immunocompetent, and 9 of them had no underlying diagnosis. The most common diagnosis for this group was neurologic disorder (n = 6).
Results
Of the 100 NGS tests evaluated, 70 were positive for any organism, and 56 of these were deemed clinically relevant.
“What I think is quite remarkable is that, of those clinically relevant organisms, tests on 14, which is 25% of those, were able to identify clinically relevant or pathogenic organisms when no other conventional testing modality was able to identify them,” Dr. Rossoff said. “And these were often in patients who underwent invasive procedures to try to get at the source of their infectious disease.”
In fact, the study included 42 patients who underwent 54 invasive diagnostic procedures, and 32 of those procedures could have been avoided based on positive NGS results, according to Dr. Rossoff and her colleagues.
Dr. Rossoff noted that the most common sites of infection were the bloodstream and respiratory tract, but the NGS test was able to identify pathogens in the bone, skin, cerebrospinal fluid, and urinary tract. She also pointed out that NGS results were available “in a fairly timely manner,” as 86% of test results were available within 48 hours of sample receipt.
Dr. Rossoff and her colleagues did not receive any funding for this study, but they were previously involved in a study funded by Karius, the company that commercialized the NGS test.
SOURCE: Rossoff J et al. ASPHO 2019. Abstract 439.
REPORTING FROM 2019 ASPHO CONFERENCE
Inhibitor may overcome ibrutinib resistance in MCL
Investigators have identified a mechanism of ibrutinib resistance in mantle cell lymphoma (MCL) and showed that a small molecule can overcome that resistance in vitro and in vivo.
The team found that ibrutinib-resistant MCL cells rely on oxidative phosphorylation (OXPHOS) and glutaminolysis to survive.
Targeting the OXPHOS pathway with a small molecule, IACS-010759, inhibited the proliferation of ibrutinib-resistant cells in vitro.
IACS-010759 also decreased tumor volume and improved survival in mouse models of ibrutinib-resistant MCL and double-hit B-cell lymphoma.
Now, IACS-10759 is being tested in phase 1 trials of lymphoma and solid tumors (NCT03291938) as well as acute myeloid leukemia (NCT02882321).
Liang Zhang, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues conducted the preclinical research and described their findings in Science Translational Medicine.
The investigators sequenced samples from MCL patients with ibrutinib-sensitive and -resistant disease and found that “glutamine-fueled OXPHOS appears to be a prominent energy metabolism pathway in ibrutinib-resistant MCL cells.”
This finding prompted the team to test IACS-010759, an inhibitor of ETC complex I, in ibrutinib-resistant MCL. They theorized that the inhibitor would be effective because, during OXPHOS, electrons are transferred from electron donors to acceptors through the ETC in redox reactions that release energy to form ATP, and OXPHOS generates ATP to meet requirements for cell growth.
In experiments, IACS-010759 inhibited the proliferation of two ibrutinib-resistant MCL cell lines, Z-138 and Maver-1, in a dose-dependent manner.
The investigators also tested IACS-010759 in two mouse models of ibrutinib-resistant MCL. In both models, mice treated with IACS-010759 had a significant reduction in tumor volume, compared with controls. In one model, IACS-010759 extended survival by a median of 11 days.
Finally, the team tested IACS-010759 in a model of ibrutinib-resistant, double-hit (MYC and BCL-2) B-cell lymphoma with central nervous system involvement. Again, IACS-010759 significantly inhibited tumor growth. Compared to ibrutinib and vehicle control, IACS-010759 provided a median survival benefit of more than 20 days.
There were no toxicities associated with IACS-010759 treatment, according to the investigators.
This research was supported by the MD Anderson B Cell Lymphoma Moon Shot Project, Gary Rogers Foundation, Kinder Foundation, Cullen Foundation, Cancer Prevention Research Institute of Texas, and the National Institutes of Health. Most investigators reported having no competing interests, but two reported a patent (WO/2015/130790).
SOURCE: Zhang L et al. Sci Transl Med. 2019 May 8. doi: 10.1126/scitranslmed.aau1167.
Investigators have identified a mechanism of ibrutinib resistance in mantle cell lymphoma (MCL) and showed that a small molecule can overcome that resistance in vitro and in vivo.
The team found that ibrutinib-resistant MCL cells rely on oxidative phosphorylation (OXPHOS) and glutaminolysis to survive.
Targeting the OXPHOS pathway with a small molecule, IACS-010759, inhibited the proliferation of ibrutinib-resistant cells in vitro.
IACS-010759 also decreased tumor volume and improved survival in mouse models of ibrutinib-resistant MCL and double-hit B-cell lymphoma.
Now, IACS-10759 is being tested in phase 1 trials of lymphoma and solid tumors (NCT03291938) as well as acute myeloid leukemia (NCT02882321).
Liang Zhang, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues conducted the preclinical research and described their findings in Science Translational Medicine.
The investigators sequenced samples from MCL patients with ibrutinib-sensitive and -resistant disease and found that “glutamine-fueled OXPHOS appears to be a prominent energy metabolism pathway in ibrutinib-resistant MCL cells.”
This finding prompted the team to test IACS-010759, an inhibitor of ETC complex I, in ibrutinib-resistant MCL. They theorized that the inhibitor would be effective because, during OXPHOS, electrons are transferred from electron donors to acceptors through the ETC in redox reactions that release energy to form ATP, and OXPHOS generates ATP to meet requirements for cell growth.
In experiments, IACS-010759 inhibited the proliferation of two ibrutinib-resistant MCL cell lines, Z-138 and Maver-1, in a dose-dependent manner.
The investigators also tested IACS-010759 in two mouse models of ibrutinib-resistant MCL. In both models, mice treated with IACS-010759 had a significant reduction in tumor volume, compared with controls. In one model, IACS-010759 extended survival by a median of 11 days.
Finally, the team tested IACS-010759 in a model of ibrutinib-resistant, double-hit (MYC and BCL-2) B-cell lymphoma with central nervous system involvement. Again, IACS-010759 significantly inhibited tumor growth. Compared to ibrutinib and vehicle control, IACS-010759 provided a median survival benefit of more than 20 days.
There were no toxicities associated with IACS-010759 treatment, according to the investigators.
This research was supported by the MD Anderson B Cell Lymphoma Moon Shot Project, Gary Rogers Foundation, Kinder Foundation, Cullen Foundation, Cancer Prevention Research Institute of Texas, and the National Institutes of Health. Most investigators reported having no competing interests, but two reported a patent (WO/2015/130790).
SOURCE: Zhang L et al. Sci Transl Med. 2019 May 8. doi: 10.1126/scitranslmed.aau1167.
Investigators have identified a mechanism of ibrutinib resistance in mantle cell lymphoma (MCL) and showed that a small molecule can overcome that resistance in vitro and in vivo.
The team found that ibrutinib-resistant MCL cells rely on oxidative phosphorylation (OXPHOS) and glutaminolysis to survive.
Targeting the OXPHOS pathway with a small molecule, IACS-010759, inhibited the proliferation of ibrutinib-resistant cells in vitro.
IACS-010759 also decreased tumor volume and improved survival in mouse models of ibrutinib-resistant MCL and double-hit B-cell lymphoma.
Now, IACS-10759 is being tested in phase 1 trials of lymphoma and solid tumors (NCT03291938) as well as acute myeloid leukemia (NCT02882321).
Liang Zhang, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues conducted the preclinical research and described their findings in Science Translational Medicine.
The investigators sequenced samples from MCL patients with ibrutinib-sensitive and -resistant disease and found that “glutamine-fueled OXPHOS appears to be a prominent energy metabolism pathway in ibrutinib-resistant MCL cells.”
This finding prompted the team to test IACS-010759, an inhibitor of ETC complex I, in ibrutinib-resistant MCL. They theorized that the inhibitor would be effective because, during OXPHOS, electrons are transferred from electron donors to acceptors through the ETC in redox reactions that release energy to form ATP, and OXPHOS generates ATP to meet requirements for cell growth.
In experiments, IACS-010759 inhibited the proliferation of two ibrutinib-resistant MCL cell lines, Z-138 and Maver-1, in a dose-dependent manner.
The investigators also tested IACS-010759 in two mouse models of ibrutinib-resistant MCL. In both models, mice treated with IACS-010759 had a significant reduction in tumor volume, compared with controls. In one model, IACS-010759 extended survival by a median of 11 days.
Finally, the team tested IACS-010759 in a model of ibrutinib-resistant, double-hit (MYC and BCL-2) B-cell lymphoma with central nervous system involvement. Again, IACS-010759 significantly inhibited tumor growth. Compared to ibrutinib and vehicle control, IACS-010759 provided a median survival benefit of more than 20 days.
There were no toxicities associated with IACS-010759 treatment, according to the investigators.
This research was supported by the MD Anderson B Cell Lymphoma Moon Shot Project, Gary Rogers Foundation, Kinder Foundation, Cullen Foundation, Cancer Prevention Research Institute of Texas, and the National Institutes of Health. Most investigators reported having no competing interests, but two reported a patent (WO/2015/130790).
SOURCE: Zhang L et al. Sci Transl Med. 2019 May 8. doi: 10.1126/scitranslmed.aau1167.
FROM SCIENCE TRANSLATIONAL MEDICINE
Researchers propose new risk groups for NK-AML
NEWPORT BEACH, CALIF. – New research suggests patients with normal karyotype acute myeloid leukemia (NK-AML) can be divided into four risk groups associated with overall survival.
Investigators used machine learning algorithms to study the association between mutations and overall survival in 1,352 patients with NK-AML. The analysis revealed combinations of mutations that could be used to classify NK-AML patients into favorable, intermediate-1, intermediate-2, and unfavorable risk groups.
For example, patients who had NPM1 mutations but wild-type FLT3-ITD and DNMT3A, had a median overall survival of 99.1 months and could be classified as favorable risk. Conversely, patients who had NPM1, FLT3-ITD, and DNMT3A mutations, had a median overall survival of 13.4 months and could be classified as unfavorable risk.
Aziz Nazha, MD, of the Cleveland Clinic, and his colleagues conducted this research and presented the findings at the Acute Leukemia Forum of Hemedicus.
The investigators looked at genomic and clinical data from 1,352 patients with NK-AML. The patients were a median age of 55 years and had a median white blood cell count of 21.3 x 109/L, a median hemoglobin of 9.1 g/dL, and a median platelet count of 61 x 109/L. More than half of patients (57.3%) were male.
The patients were screened for 35 genes that are commonly mutated in AML and other myeloid malignancies. The investigators used machine learning algorithms, including random survival forest and recommender system algorithms, to study the association between mutations and overall survival in an “unbiased” way.
Dr. Nazha said there were a median of three mutations per patient sample, and “there are some competing interests between those mutations to impact the prognosis of the patient.”
The investigators used the mutations and their associations with overall survival to classify patients into the risk groups outlined in the table below.

These findings can improve the risk stratification of NK-AML and may aid physicians in making treatment decisions, according to Dr. Nazha and his colleagues. To move this work forward, the investigators are attempting to develop a personalized model that can make predictions specific to an individual patient based on that patient’s mutation information.
Dr. Nazha reported having no financial disclosures relevant to this research. Other investigators reported relationships with the Munich Leukemia Laboratory.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – New research suggests patients with normal karyotype acute myeloid leukemia (NK-AML) can be divided into four risk groups associated with overall survival.
Investigators used machine learning algorithms to study the association between mutations and overall survival in 1,352 patients with NK-AML. The analysis revealed combinations of mutations that could be used to classify NK-AML patients into favorable, intermediate-1, intermediate-2, and unfavorable risk groups.
For example, patients who had NPM1 mutations but wild-type FLT3-ITD and DNMT3A, had a median overall survival of 99.1 months and could be classified as favorable risk. Conversely, patients who had NPM1, FLT3-ITD, and DNMT3A mutations, had a median overall survival of 13.4 months and could be classified as unfavorable risk.
Aziz Nazha, MD, of the Cleveland Clinic, and his colleagues conducted this research and presented the findings at the Acute Leukemia Forum of Hemedicus.
The investigators looked at genomic and clinical data from 1,352 patients with NK-AML. The patients were a median age of 55 years and had a median white blood cell count of 21.3 x 109/L, a median hemoglobin of 9.1 g/dL, and a median platelet count of 61 x 109/L. More than half of patients (57.3%) were male.
The patients were screened for 35 genes that are commonly mutated in AML and other myeloid malignancies. The investigators used machine learning algorithms, including random survival forest and recommender system algorithms, to study the association between mutations and overall survival in an “unbiased” way.
Dr. Nazha said there were a median of three mutations per patient sample, and “there are some competing interests between those mutations to impact the prognosis of the patient.”
The investigators used the mutations and their associations with overall survival to classify patients into the risk groups outlined in the table below.

These findings can improve the risk stratification of NK-AML and may aid physicians in making treatment decisions, according to Dr. Nazha and his colleagues. To move this work forward, the investigators are attempting to develop a personalized model that can make predictions specific to an individual patient based on that patient’s mutation information.
Dr. Nazha reported having no financial disclosures relevant to this research. Other investigators reported relationships with the Munich Leukemia Laboratory.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – New research suggests patients with normal karyotype acute myeloid leukemia (NK-AML) can be divided into four risk groups associated with overall survival.
Investigators used machine learning algorithms to study the association between mutations and overall survival in 1,352 patients with NK-AML. The analysis revealed combinations of mutations that could be used to classify NK-AML patients into favorable, intermediate-1, intermediate-2, and unfavorable risk groups.
For example, patients who had NPM1 mutations but wild-type FLT3-ITD and DNMT3A, had a median overall survival of 99.1 months and could be classified as favorable risk. Conversely, patients who had NPM1, FLT3-ITD, and DNMT3A mutations, had a median overall survival of 13.4 months and could be classified as unfavorable risk.
Aziz Nazha, MD, of the Cleveland Clinic, and his colleagues conducted this research and presented the findings at the Acute Leukemia Forum of Hemedicus.
The investigators looked at genomic and clinical data from 1,352 patients with NK-AML. The patients were a median age of 55 years and had a median white blood cell count of 21.3 x 109/L, a median hemoglobin of 9.1 g/dL, and a median platelet count of 61 x 109/L. More than half of patients (57.3%) were male.
The patients were screened for 35 genes that are commonly mutated in AML and other myeloid malignancies. The investigators used machine learning algorithms, including random survival forest and recommender system algorithms, to study the association between mutations and overall survival in an “unbiased” way.
Dr. Nazha said there were a median of three mutations per patient sample, and “there are some competing interests between those mutations to impact the prognosis of the patient.”
The investigators used the mutations and their associations with overall survival to classify patients into the risk groups outlined in the table below.

These findings can improve the risk stratification of NK-AML and may aid physicians in making treatment decisions, according to Dr. Nazha and his colleagues. To move this work forward, the investigators are attempting to develop a personalized model that can make predictions specific to an individual patient based on that patient’s mutation information.
Dr. Nazha reported having no financial disclosures relevant to this research. Other investigators reported relationships with the Munich Leukemia Laboratory.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
Gabapentin falls short in treating sickle cell pain
NEW ORLEANS – Adding gabapentin to standard therapy did not significantly reduce vaso-occlusive pain in most patients with sickle cell disease enrolled in a phase 2 trial.
In the entire cohort, there were no significant differences in pain response between patients who received gabapentin and those who received placebo. However, patients with the HbSS genotype had a significantly greater decrease in pain score from baseline to discharge if they received gabapentin rather than placebo.
Additional studies are needed to confirm these findings because this trial was limited by a small sample size, according to study investigator Latika Puri, MD, of St. Jude Children’s Research Hospital in Memphis. Dr. Puri presented the trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial included 86 evaluable patients who had vaso-occlusive pain and a pain score of at least 4. All patients received standard therapy for vaso-occlusive pain and were randomized to receive placebo (n = 44) or a single oral dose of gabapentin at 15 mg/kg (n = 42).
Baseline characteristics were similar between the treatment arms. For the entire cohort, the mean age was 11.8 years (range, 1-21 years), and 51% of patients were male. Forty-four patients had the HbSS genotype, 25 had the HbSC genotype, 8 had HbS/beta0-thalassemia, and 9 had other genotypes.
The mean pain score at baseline was 7.8 for the entire cohort, 8.0 for the gabapentin arm, and 7.7 for the placebo arm.
For the entire cohort, there was no significant difference in pain response between the gabapentin and placebo arms.
The proportion of patients who experienced a greater than 33% decrease in pain from baseline to 3 hours posttreatment was 67% in the gabapentin arm and 59% in the placebo arm (P = .23). The proportion of patients who experienced a greater than 33% decrease from baseline to discharge from the acute care clinic was 75% and 61%, respectively (P = .18).
In the entire cohort, decreases in pain scores from baseline to 3 hours posttreatment were not significantly different between the gabapentin and placebo arms, at 1.3 and 0.7, respectively (P = .74). Likewise, decreases in pain scores from baseline to discharge were not significantly different, at 1.6 and 0.8 (P = .38).
Among patients who had the HbSS genotype, there was a significantly greater decrease in pain score from baseline to discharge in the gabapentin arm than in the placebo arm, 5.9 versus 3.6 (P = .03). However, there were no other significant differences in pain response for the HbSS subgroup.
There were no significant differences in opioid consumption or hospitalization for the HbSS subgroup or the entire cohort. For the entire cohort, the mean morphine equivalent dose from baseline to 3 hours posttreatment was 0.16 mg/kg in the gabapentin arm and 0.17 mg/kg in the placebo arm (P = .89). For the HbSS subgroup, the mean dose was 0.16 mg/kg and 0.15 mg/kg, respectively (P = .93).
In the entire cohort, 24% of patients in the gabapentin arm and 27% of those in the placebo arm were hospitalized (P = .71). In the HbSS subgroup, hospitalizations occurred in 11% and 35% (P = .15).
Dr. Puri pointed out several challenges that led to limitations in this study. Specifically, the investigators had to obtain patient consent while delivering standard treatment, while patients were in pain and distress, and from patients who had already received opioids and were sleepy. Additionally, gabapentin had to be delivered within 1 hour of opioid administration, and a lack of after-hours staff limited enrollment.
“These challenges led to one of our biggest limitations, which was a small sample size, leading to a limited power to observe real differences,” Dr. Puri said. “We also defined a very short time period of evaluation for the primary outcomes; that was 3 hours from the gabapentin dose or placebo dose. This limited our capability to see real differences if they existed.”
Dr. Puri said additional studies with larger sample sizes are needed to confirm these findings. She added that efforts to better characterize pain in sickle cell disease could reveal patients who may benefit from gabapentin because they have a neuropathic component to their pain.
The trial was sponsored by St. Jude Children’s Research Hospital in collaboration with Scan|Design Foundation. Dr. Puri did not provide disclosure information at the meeting.
SOURCE: Puri L et al. ASPHO 2019, Abstract 2011.
NEW ORLEANS – Adding gabapentin to standard therapy did not significantly reduce vaso-occlusive pain in most patients with sickle cell disease enrolled in a phase 2 trial.
In the entire cohort, there were no significant differences in pain response between patients who received gabapentin and those who received placebo. However, patients with the HbSS genotype had a significantly greater decrease in pain score from baseline to discharge if they received gabapentin rather than placebo.
Additional studies are needed to confirm these findings because this trial was limited by a small sample size, according to study investigator Latika Puri, MD, of St. Jude Children’s Research Hospital in Memphis. Dr. Puri presented the trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial included 86 evaluable patients who had vaso-occlusive pain and a pain score of at least 4. All patients received standard therapy for vaso-occlusive pain and were randomized to receive placebo (n = 44) or a single oral dose of gabapentin at 15 mg/kg (n = 42).
Baseline characteristics were similar between the treatment arms. For the entire cohort, the mean age was 11.8 years (range, 1-21 years), and 51% of patients were male. Forty-four patients had the HbSS genotype, 25 had the HbSC genotype, 8 had HbS/beta0-thalassemia, and 9 had other genotypes.
The mean pain score at baseline was 7.8 for the entire cohort, 8.0 for the gabapentin arm, and 7.7 for the placebo arm.
For the entire cohort, there was no significant difference in pain response between the gabapentin and placebo arms.
The proportion of patients who experienced a greater than 33% decrease in pain from baseline to 3 hours posttreatment was 67% in the gabapentin arm and 59% in the placebo arm (P = .23). The proportion of patients who experienced a greater than 33% decrease from baseline to discharge from the acute care clinic was 75% and 61%, respectively (P = .18).
In the entire cohort, decreases in pain scores from baseline to 3 hours posttreatment were not significantly different between the gabapentin and placebo arms, at 1.3 and 0.7, respectively (P = .74). Likewise, decreases in pain scores from baseline to discharge were not significantly different, at 1.6 and 0.8 (P = .38).
Among patients who had the HbSS genotype, there was a significantly greater decrease in pain score from baseline to discharge in the gabapentin arm than in the placebo arm, 5.9 versus 3.6 (P = .03). However, there were no other significant differences in pain response for the HbSS subgroup.
There were no significant differences in opioid consumption or hospitalization for the HbSS subgroup or the entire cohort. For the entire cohort, the mean morphine equivalent dose from baseline to 3 hours posttreatment was 0.16 mg/kg in the gabapentin arm and 0.17 mg/kg in the placebo arm (P = .89). For the HbSS subgroup, the mean dose was 0.16 mg/kg and 0.15 mg/kg, respectively (P = .93).
In the entire cohort, 24% of patients in the gabapentin arm and 27% of those in the placebo arm were hospitalized (P = .71). In the HbSS subgroup, hospitalizations occurred in 11% and 35% (P = .15).
Dr. Puri pointed out several challenges that led to limitations in this study. Specifically, the investigators had to obtain patient consent while delivering standard treatment, while patients were in pain and distress, and from patients who had already received opioids and were sleepy. Additionally, gabapentin had to be delivered within 1 hour of opioid administration, and a lack of after-hours staff limited enrollment.
“These challenges led to one of our biggest limitations, which was a small sample size, leading to a limited power to observe real differences,” Dr. Puri said. “We also defined a very short time period of evaluation for the primary outcomes; that was 3 hours from the gabapentin dose or placebo dose. This limited our capability to see real differences if they existed.”
Dr. Puri said additional studies with larger sample sizes are needed to confirm these findings. She added that efforts to better characterize pain in sickle cell disease could reveal patients who may benefit from gabapentin because they have a neuropathic component to their pain.
The trial was sponsored by St. Jude Children’s Research Hospital in collaboration with Scan|Design Foundation. Dr. Puri did not provide disclosure information at the meeting.
SOURCE: Puri L et al. ASPHO 2019, Abstract 2011.
NEW ORLEANS – Adding gabapentin to standard therapy did not significantly reduce vaso-occlusive pain in most patients with sickle cell disease enrolled in a phase 2 trial.
In the entire cohort, there were no significant differences in pain response between patients who received gabapentin and those who received placebo. However, patients with the HbSS genotype had a significantly greater decrease in pain score from baseline to discharge if they received gabapentin rather than placebo.
Additional studies are needed to confirm these findings because this trial was limited by a small sample size, according to study investigator Latika Puri, MD, of St. Jude Children’s Research Hospital in Memphis. Dr. Puri presented the trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The trial included 86 evaluable patients who had vaso-occlusive pain and a pain score of at least 4. All patients received standard therapy for vaso-occlusive pain and were randomized to receive placebo (n = 44) or a single oral dose of gabapentin at 15 mg/kg (n = 42).
Baseline characteristics were similar between the treatment arms. For the entire cohort, the mean age was 11.8 years (range, 1-21 years), and 51% of patients were male. Forty-four patients had the HbSS genotype, 25 had the HbSC genotype, 8 had HbS/beta0-thalassemia, and 9 had other genotypes.
The mean pain score at baseline was 7.8 for the entire cohort, 8.0 for the gabapentin arm, and 7.7 for the placebo arm.
For the entire cohort, there was no significant difference in pain response between the gabapentin and placebo arms.
The proportion of patients who experienced a greater than 33% decrease in pain from baseline to 3 hours posttreatment was 67% in the gabapentin arm and 59% in the placebo arm (P = .23). The proportion of patients who experienced a greater than 33% decrease from baseline to discharge from the acute care clinic was 75% and 61%, respectively (P = .18).
In the entire cohort, decreases in pain scores from baseline to 3 hours posttreatment were not significantly different between the gabapentin and placebo arms, at 1.3 and 0.7, respectively (P = .74). Likewise, decreases in pain scores from baseline to discharge were not significantly different, at 1.6 and 0.8 (P = .38).
Among patients who had the HbSS genotype, there was a significantly greater decrease in pain score from baseline to discharge in the gabapentin arm than in the placebo arm, 5.9 versus 3.6 (P = .03). However, there were no other significant differences in pain response for the HbSS subgroup.
There were no significant differences in opioid consumption or hospitalization for the HbSS subgroup or the entire cohort. For the entire cohort, the mean morphine equivalent dose from baseline to 3 hours posttreatment was 0.16 mg/kg in the gabapentin arm and 0.17 mg/kg in the placebo arm (P = .89). For the HbSS subgroup, the mean dose was 0.16 mg/kg and 0.15 mg/kg, respectively (P = .93).
In the entire cohort, 24% of patients in the gabapentin arm and 27% of those in the placebo arm were hospitalized (P = .71). In the HbSS subgroup, hospitalizations occurred in 11% and 35% (P = .15).
Dr. Puri pointed out several challenges that led to limitations in this study. Specifically, the investigators had to obtain patient consent while delivering standard treatment, while patients were in pain and distress, and from patients who had already received opioids and were sleepy. Additionally, gabapentin had to be delivered within 1 hour of opioid administration, and a lack of after-hours staff limited enrollment.
“These challenges led to one of our biggest limitations, which was a small sample size, leading to a limited power to observe real differences,” Dr. Puri said. “We also defined a very short time period of evaluation for the primary outcomes; that was 3 hours from the gabapentin dose or placebo dose. This limited our capability to see real differences if they existed.”
Dr. Puri said additional studies with larger sample sizes are needed to confirm these findings. She added that efforts to better characterize pain in sickle cell disease could reveal patients who may benefit from gabapentin because they have a neuropathic component to their pain.
The trial was sponsored by St. Jude Children’s Research Hospital in collaboration with Scan|Design Foundation. Dr. Puri did not provide disclosure information at the meeting.
SOURCE: Puri L et al. ASPHO 2019, Abstract 2011.
REPORTING FROM THE 2019 ASPHO CONFERENCE
Key clinical point:
Major finding: The proportion of patients who experienced a greater than 33% decrease in pain from baseline to 3 hours posttreatment was 67% in the gabapentin arm and 59% in the placebo arm (P = .23).
Study details: A phase 2 trial of 86 evaluable patients.
Disclosures: The trial was sponsored by St. Jude Children’s Research Hospital in collaboration with Scan|Design Foundation. The speaker did not provide disclosure information at the meeting.
Source: Puri L et al. 2019 ASPHO Conference, Abstract 2011.
Researchers win Stand Up To Cancer grants
Stand Up To Cancer has awarded 1- and 2-year grants to five teams conducting cancer research.
Alan D’Andrea, MD, of the Dana-Farber Cancer Institute in Boston and Juan Cubillos-Ruiz, PhD, of Weill Cornell Medicine in New York won a 2-year grant totaling $250,000.
Dr. Cubillos-Ruiz and Dr. D’Andrea will use this funding to evaluate gene signatures controlled by phospholipid messengers and endoplasmic reticulum stress in responding and nonresponding ovarian cancer patients, and the team will use tumor organoids to test whether pharmacological inhibition of these pathways can fight ovarian cancer.
Denada Dibra, PhD, of the University of Texas MD Anderson Cancer Center in Houston and Peter Lee, MD, of City of Hope in Duarte, Calif., won a 1-year grant of $250,000.
Dr. Lee and Dr. Dibra will use mouse models to study how TP53 may influence the tumor microenvironment in triple-negative breast cancer and to characterize tumor cells and immune cells in the tumor tissue.
Maximilian Diehn, MD, PhD, of Stanford (Calif.) University and Aaron Hata, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston won a 2-year grant totaling $225,000.
Dr. Hata and Dr. Diehn will work on developing a novel method for analyzing cell-free RNA associated with resistance to tyrosine kinase inhibitors in patients with non–small cell lung cancer. The team’s goal is to develop a noninvasive approach to better characterize tumors and detect phenotypic changes during treatment.
Sarah Tasian, MD, of Children’s Hospital of Philadelphia and Kimberly Stegmaier, MD, of the Dana-Farber Cancer Institute won a 2-year grant totaling $250,000, which was funded with support from the Emily Whitehead Foundation.
Dr. Stegmaier and Dr. Tasian will test chimeric antigen receptor T cells targeting two or more neoantigens in preclinical models of childhood Down syndrome–associated acute lymphoblastic leukemia and Ph-like acute lymphoblastic leukemia.
Robert Vonderheide, MD, of the University of Pennsylvania Abramson Cancer Center in Philadelphia and Vinod Balachandran, MD, of Memorial Sloan Kettering Cancer Center in New York won a 2-year grant totaling $225,000.
Dr. Balachandran and Dr. Vonderheide will analyze short- and long-term pancreatic cancer survivors, patients with primary resected pancreatic cancers and patients with mKRAS lung and colon cancers to evaluate how mKRAS immunogenicity may affect outcomes.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Stand Up To Cancer has awarded 1- and 2-year grants to five teams conducting cancer research.
Alan D’Andrea, MD, of the Dana-Farber Cancer Institute in Boston and Juan Cubillos-Ruiz, PhD, of Weill Cornell Medicine in New York won a 2-year grant totaling $250,000.
Dr. Cubillos-Ruiz and Dr. D’Andrea will use this funding to evaluate gene signatures controlled by phospholipid messengers and endoplasmic reticulum stress in responding and nonresponding ovarian cancer patients, and the team will use tumor organoids to test whether pharmacological inhibition of these pathways can fight ovarian cancer.
Denada Dibra, PhD, of the University of Texas MD Anderson Cancer Center in Houston and Peter Lee, MD, of City of Hope in Duarte, Calif., won a 1-year grant of $250,000.
Dr. Lee and Dr. Dibra will use mouse models to study how TP53 may influence the tumor microenvironment in triple-negative breast cancer and to characterize tumor cells and immune cells in the tumor tissue.
Maximilian Diehn, MD, PhD, of Stanford (Calif.) University and Aaron Hata, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston won a 2-year grant totaling $225,000.
Dr. Hata and Dr. Diehn will work on developing a novel method for analyzing cell-free RNA associated with resistance to tyrosine kinase inhibitors in patients with non–small cell lung cancer. The team’s goal is to develop a noninvasive approach to better characterize tumors and detect phenotypic changes during treatment.
Sarah Tasian, MD, of Children’s Hospital of Philadelphia and Kimberly Stegmaier, MD, of the Dana-Farber Cancer Institute won a 2-year grant totaling $250,000, which was funded with support from the Emily Whitehead Foundation.
Dr. Stegmaier and Dr. Tasian will test chimeric antigen receptor T cells targeting two or more neoantigens in preclinical models of childhood Down syndrome–associated acute lymphoblastic leukemia and Ph-like acute lymphoblastic leukemia.
Robert Vonderheide, MD, of the University of Pennsylvania Abramson Cancer Center in Philadelphia and Vinod Balachandran, MD, of Memorial Sloan Kettering Cancer Center in New York won a 2-year grant totaling $225,000.
Dr. Balachandran and Dr. Vonderheide will analyze short- and long-term pancreatic cancer survivors, patients with primary resected pancreatic cancers and patients with mKRAS lung and colon cancers to evaluate how mKRAS immunogenicity may affect outcomes.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Stand Up To Cancer has awarded 1- and 2-year grants to five teams conducting cancer research.
Alan D’Andrea, MD, of the Dana-Farber Cancer Institute in Boston and Juan Cubillos-Ruiz, PhD, of Weill Cornell Medicine in New York won a 2-year grant totaling $250,000.
Dr. Cubillos-Ruiz and Dr. D’Andrea will use this funding to evaluate gene signatures controlled by phospholipid messengers and endoplasmic reticulum stress in responding and nonresponding ovarian cancer patients, and the team will use tumor organoids to test whether pharmacological inhibition of these pathways can fight ovarian cancer.
Denada Dibra, PhD, of the University of Texas MD Anderson Cancer Center in Houston and Peter Lee, MD, of City of Hope in Duarte, Calif., won a 1-year grant of $250,000.
Dr. Lee and Dr. Dibra will use mouse models to study how TP53 may influence the tumor microenvironment in triple-negative breast cancer and to characterize tumor cells and immune cells in the tumor tissue.
Maximilian Diehn, MD, PhD, of Stanford (Calif.) University and Aaron Hata, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston won a 2-year grant totaling $225,000.
Dr. Hata and Dr. Diehn will work on developing a novel method for analyzing cell-free RNA associated with resistance to tyrosine kinase inhibitors in patients with non–small cell lung cancer. The team’s goal is to develop a noninvasive approach to better characterize tumors and detect phenotypic changes during treatment.
Sarah Tasian, MD, of Children’s Hospital of Philadelphia and Kimberly Stegmaier, MD, of the Dana-Farber Cancer Institute won a 2-year grant totaling $250,000, which was funded with support from the Emily Whitehead Foundation.
Dr. Stegmaier and Dr. Tasian will test chimeric antigen receptor T cells targeting two or more neoantigens in preclinical models of childhood Down syndrome–associated acute lymphoblastic leukemia and Ph-like acute lymphoblastic leukemia.
Robert Vonderheide, MD, of the University of Pennsylvania Abramson Cancer Center in Philadelphia and Vinod Balachandran, MD, of Memorial Sloan Kettering Cancer Center in New York won a 2-year grant totaling $225,000.
Dr. Balachandran and Dr. Vonderheide will analyze short- and long-term pancreatic cancer survivors, patients with primary resected pancreatic cancers and patients with mKRAS lung and colon cancers to evaluate how mKRAS immunogenicity may affect outcomes.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Combo proves most effective in HMA-naive, higher-risk MDS
NEWPORT BEACH, CALIF. – The combination of oral rigosertib and azacitidine is proceeding to a phase 3 trial in patients with myelodysplastic syndromes (MDS), but it isn’t clear if the combination will continue to be developed for acute myeloid leukemia (AML).
In a phase 1/2 trial, oral rigosertib plus azacitidine produced a 90% response rate in higher-risk MDS patients who were naive to hypomethylating agents (HMAs), a 54% response rate in higher-risk MDS patients who had failed HMA therapy, and a 50% response rate in patients with AML.
Genitourinary toxicities were initially a concern in this trial, but researchers found ways to mitigate the risk of these toxicities, according to Richard Woodman, MD, chief medical officer and senior vice president of research and development at Onconova Therapeutics, the company developing rigosertib.
Dr. Woodman and his colleagues presented results from the phase 1/2 trial in two posters at the Acute Leukemia Forum of Hemedicus.
Results in AML
The researchers reported phase 1 results in 17 patients with AML. Eleven patients had AML, according to investigator assessment, and six patients had refractory anemia with excess blasts in transformation, according to French American British criteria, as well as least 20% excess blasts at baseline.
The median age of the patients was 73 years, and 53% were men. Two patients had received no prior therapies, six patients had relapsed disease, and nine were refractory to their last therapy.
Patients received oral rigosertib at escalating doses twice daily on days 1-21 of a 28-day cycle. The recommended phase 2 dose was 840 mg daily (560 mg in the morning and 280 mg in the afternoon), but there were two expansion cohorts in which patients received 1,120 mg daily (560 mg twice a day or 840 mg in the morning and 280 mg in the afternoon). The patients also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
Patients received a median of three treatment cycles. Fifteen of the 17 patients (88%) discontinued treatment, most because of progressive disease (n = 5), toxicity (n = 4), or death (n = 3).
Twelve patients were evaluable for response, and six (50%) responded. One patient achieved a morphologic complete remission (CR), three achieved a morphologic leukemia-free state, and two had a partial response.
The most common treatment-emergent adverse events (TEAEs) were fatigue (53%), diarrhea (53%), nausea (53%), constipation (47%), back pain (41%), pyrexia (41%), and pneumonia (35%). Grade 3 or higher TEAEs included pneumonia (35%) and anemia (24%).
These results haven’t provided a clear way forward for oral rigosertib and azacitidine in AML. Dr. Woodman said the researchers will have to review past studies and evaluate how AML patients (with at least 20% blasts) have responded to intravenous rigosertib, consult experts in the field, and then decide how they will move forward with oral rigosertib and azacitidine in AML.
Results in MDS
Dr. Woodman and his colleagues presented data on 74 patients with higher-risk MDS. The median age was 69 years, and 59% were men. Most patients were high risk (n = 23) or very high risk (n = 33), according to the Revised International Prognostic Scoring System.
The patients received oral rigosertib at a dose of 840 mg/day or higher on days 1-21 of a 28-day cycle. They also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
The median duration of treatment was 7.8 months in patients who were HMA naive and 4.9 months in patients who failed HMA therapy. The most common reasons for treatment discontinuation in the HMA-naive patients were toxicity (n = 8), progression (n = 7), and patient request (n = 7). The most common reasons for discontinuation in patients who had failed HMA therapy were progression (n = 12), toxicity (n = 5), and investigator decision (n = 4).
In total, 55 patients were evaluable for response, 26 who had failed HMA therapy and 29 who were HMA naive.
“The best responses, not surprisingly, were in patients that were HMA naive,” Dr. Woodman said.
In the HMA-naive patients, the overall response rate was 90%. Ten patients had a CR, five had a marrow CR with hematologic improvement, three had hematologic improvement alone, eight had a marrow CR alone, and three patients had stable disease. None of the patients progressed.
In the patients who had failed HMA therapy, the overall response rate was 54%. One patient achieved a CR, one had a partial response, five had a marrow CR with hematologic improvement, two had hematologic improvement alone, five had a marrow CR alone, seven had stable disease, and five progressed.
The median duration of response was 10.8 months in patients who failed HMA therapy and 12.2 months in the HMA-naive patients.
The most common TEAEs in the entire MDS cohort were hematuria (45%), constipation (43%), diarrhea (42%), fatigue (42%), dysuria (38%), pyrexia (36%), nausea (35%), neutropenia (31%), and thrombocytopenia (30%).
Grade 3 or higher TEAEs were neutropenia (27%), thrombocytopenia (26%), hematuria (9%), dysuria (9%), diarrhea (5%), fatigue (4%), and pyrexia (1%).
Dr. Woodman said patients who were most likely to be at risk for genitourinary toxicities (hematuria and dysuria) were those who weren’t well hydrated, took rigosertib at night, and didn’t void their bladders before bedtime. He said the researchers’ hypothesis is that there is some local bladder irritation in that setting.
However, the researchers found ways to mitigate the risk of genitourinary toxicities, including:
- Requiring the second dose of rigosertib to be taken in the afternoon rather than evening (about 3 p.m.).
- Asking patients to consume at least 2 liters of fluid per day.
- Having patients empty their bladders before bedtime.
- Assessing urine pH roughly 2 hours after the morning dose of rigosertib and prescribing sodium bicarbonate if the pH is less than 7.5.
Dr. Woodman said the phase 2 results in MDS patients have prompted the development of a phase 3 trial in which researchers will compare oral rigosertib plus azacitidine to azacitidine plus placebo.
Dr. Woodman is employed by Onconova Therapeutics, which sponsored the phase 1/2 trial. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – The combination of oral rigosertib and azacitidine is proceeding to a phase 3 trial in patients with myelodysplastic syndromes (MDS), but it isn’t clear if the combination will continue to be developed for acute myeloid leukemia (AML).
In a phase 1/2 trial, oral rigosertib plus azacitidine produced a 90% response rate in higher-risk MDS patients who were naive to hypomethylating agents (HMAs), a 54% response rate in higher-risk MDS patients who had failed HMA therapy, and a 50% response rate in patients with AML.
Genitourinary toxicities were initially a concern in this trial, but researchers found ways to mitigate the risk of these toxicities, according to Richard Woodman, MD, chief medical officer and senior vice president of research and development at Onconova Therapeutics, the company developing rigosertib.
Dr. Woodman and his colleagues presented results from the phase 1/2 trial in two posters at the Acute Leukemia Forum of Hemedicus.
Results in AML
The researchers reported phase 1 results in 17 patients with AML. Eleven patients had AML, according to investigator assessment, and six patients had refractory anemia with excess blasts in transformation, according to French American British criteria, as well as least 20% excess blasts at baseline.
The median age of the patients was 73 years, and 53% were men. Two patients had received no prior therapies, six patients had relapsed disease, and nine were refractory to their last therapy.
Patients received oral rigosertib at escalating doses twice daily on days 1-21 of a 28-day cycle. The recommended phase 2 dose was 840 mg daily (560 mg in the morning and 280 mg in the afternoon), but there were two expansion cohorts in which patients received 1,120 mg daily (560 mg twice a day or 840 mg in the morning and 280 mg in the afternoon). The patients also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
Patients received a median of three treatment cycles. Fifteen of the 17 patients (88%) discontinued treatment, most because of progressive disease (n = 5), toxicity (n = 4), or death (n = 3).
Twelve patients were evaluable for response, and six (50%) responded. One patient achieved a morphologic complete remission (CR), three achieved a morphologic leukemia-free state, and two had a partial response.
The most common treatment-emergent adverse events (TEAEs) were fatigue (53%), diarrhea (53%), nausea (53%), constipation (47%), back pain (41%), pyrexia (41%), and pneumonia (35%). Grade 3 or higher TEAEs included pneumonia (35%) and anemia (24%).
These results haven’t provided a clear way forward for oral rigosertib and azacitidine in AML. Dr. Woodman said the researchers will have to review past studies and evaluate how AML patients (with at least 20% blasts) have responded to intravenous rigosertib, consult experts in the field, and then decide how they will move forward with oral rigosertib and azacitidine in AML.
Results in MDS
Dr. Woodman and his colleagues presented data on 74 patients with higher-risk MDS. The median age was 69 years, and 59% were men. Most patients were high risk (n = 23) or very high risk (n = 33), according to the Revised International Prognostic Scoring System.
The patients received oral rigosertib at a dose of 840 mg/day or higher on days 1-21 of a 28-day cycle. They also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
The median duration of treatment was 7.8 months in patients who were HMA naive and 4.9 months in patients who failed HMA therapy. The most common reasons for treatment discontinuation in the HMA-naive patients were toxicity (n = 8), progression (n = 7), and patient request (n = 7). The most common reasons for discontinuation in patients who had failed HMA therapy were progression (n = 12), toxicity (n = 5), and investigator decision (n = 4).
In total, 55 patients were evaluable for response, 26 who had failed HMA therapy and 29 who were HMA naive.
“The best responses, not surprisingly, were in patients that were HMA naive,” Dr. Woodman said.
In the HMA-naive patients, the overall response rate was 90%. Ten patients had a CR, five had a marrow CR with hematologic improvement, three had hematologic improvement alone, eight had a marrow CR alone, and three patients had stable disease. None of the patients progressed.
In the patients who had failed HMA therapy, the overall response rate was 54%. One patient achieved a CR, one had a partial response, five had a marrow CR with hematologic improvement, two had hematologic improvement alone, five had a marrow CR alone, seven had stable disease, and five progressed.
The median duration of response was 10.8 months in patients who failed HMA therapy and 12.2 months in the HMA-naive patients.
The most common TEAEs in the entire MDS cohort were hematuria (45%), constipation (43%), diarrhea (42%), fatigue (42%), dysuria (38%), pyrexia (36%), nausea (35%), neutropenia (31%), and thrombocytopenia (30%).
Grade 3 or higher TEAEs were neutropenia (27%), thrombocytopenia (26%), hematuria (9%), dysuria (9%), diarrhea (5%), fatigue (4%), and pyrexia (1%).
Dr. Woodman said patients who were most likely to be at risk for genitourinary toxicities (hematuria and dysuria) were those who weren’t well hydrated, took rigosertib at night, and didn’t void their bladders before bedtime. He said the researchers’ hypothesis is that there is some local bladder irritation in that setting.
However, the researchers found ways to mitigate the risk of genitourinary toxicities, including:
- Requiring the second dose of rigosertib to be taken in the afternoon rather than evening (about 3 p.m.).
- Asking patients to consume at least 2 liters of fluid per day.
- Having patients empty their bladders before bedtime.
- Assessing urine pH roughly 2 hours after the morning dose of rigosertib and prescribing sodium bicarbonate if the pH is less than 7.5.
Dr. Woodman said the phase 2 results in MDS patients have prompted the development of a phase 3 trial in which researchers will compare oral rigosertib plus azacitidine to azacitidine plus placebo.
Dr. Woodman is employed by Onconova Therapeutics, which sponsored the phase 1/2 trial. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – The combination of oral rigosertib and azacitidine is proceeding to a phase 3 trial in patients with myelodysplastic syndromes (MDS), but it isn’t clear if the combination will continue to be developed for acute myeloid leukemia (AML).
In a phase 1/2 trial, oral rigosertib plus azacitidine produced a 90% response rate in higher-risk MDS patients who were naive to hypomethylating agents (HMAs), a 54% response rate in higher-risk MDS patients who had failed HMA therapy, and a 50% response rate in patients with AML.
Genitourinary toxicities were initially a concern in this trial, but researchers found ways to mitigate the risk of these toxicities, according to Richard Woodman, MD, chief medical officer and senior vice president of research and development at Onconova Therapeutics, the company developing rigosertib.
Dr. Woodman and his colleagues presented results from the phase 1/2 trial in two posters at the Acute Leukemia Forum of Hemedicus.
Results in AML
The researchers reported phase 1 results in 17 patients with AML. Eleven patients had AML, according to investigator assessment, and six patients had refractory anemia with excess blasts in transformation, according to French American British criteria, as well as least 20% excess blasts at baseline.
The median age of the patients was 73 years, and 53% were men. Two patients had received no prior therapies, six patients had relapsed disease, and nine were refractory to their last therapy.
Patients received oral rigosertib at escalating doses twice daily on days 1-21 of a 28-day cycle. The recommended phase 2 dose was 840 mg daily (560 mg in the morning and 280 mg in the afternoon), but there were two expansion cohorts in which patients received 1,120 mg daily (560 mg twice a day or 840 mg in the morning and 280 mg in the afternoon). The patients also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
Patients received a median of three treatment cycles. Fifteen of the 17 patients (88%) discontinued treatment, most because of progressive disease (n = 5), toxicity (n = 4), or death (n = 3).
Twelve patients were evaluable for response, and six (50%) responded. One patient achieved a morphologic complete remission (CR), three achieved a morphologic leukemia-free state, and two had a partial response.
The most common treatment-emergent adverse events (TEAEs) were fatigue (53%), diarrhea (53%), nausea (53%), constipation (47%), back pain (41%), pyrexia (41%), and pneumonia (35%). Grade 3 or higher TEAEs included pneumonia (35%) and anemia (24%).
These results haven’t provided a clear way forward for oral rigosertib and azacitidine in AML. Dr. Woodman said the researchers will have to review past studies and evaluate how AML patients (with at least 20% blasts) have responded to intravenous rigosertib, consult experts in the field, and then decide how they will move forward with oral rigosertib and azacitidine in AML.
Results in MDS
Dr. Woodman and his colleagues presented data on 74 patients with higher-risk MDS. The median age was 69 years, and 59% were men. Most patients were high risk (n = 23) or very high risk (n = 33), according to the Revised International Prognostic Scoring System.
The patients received oral rigosertib at a dose of 840 mg/day or higher on days 1-21 of a 28-day cycle. They also received azacitidine at 75 mg/m2 per day subcutaneously or intravenously for 7 days starting on day 8.
The median duration of treatment was 7.8 months in patients who were HMA naive and 4.9 months in patients who failed HMA therapy. The most common reasons for treatment discontinuation in the HMA-naive patients were toxicity (n = 8), progression (n = 7), and patient request (n = 7). The most common reasons for discontinuation in patients who had failed HMA therapy were progression (n = 12), toxicity (n = 5), and investigator decision (n = 4).
In total, 55 patients were evaluable for response, 26 who had failed HMA therapy and 29 who were HMA naive.
“The best responses, not surprisingly, were in patients that were HMA naive,” Dr. Woodman said.
In the HMA-naive patients, the overall response rate was 90%. Ten patients had a CR, five had a marrow CR with hematologic improvement, three had hematologic improvement alone, eight had a marrow CR alone, and three patients had stable disease. None of the patients progressed.
In the patients who had failed HMA therapy, the overall response rate was 54%. One patient achieved a CR, one had a partial response, five had a marrow CR with hematologic improvement, two had hematologic improvement alone, five had a marrow CR alone, seven had stable disease, and five progressed.
The median duration of response was 10.8 months in patients who failed HMA therapy and 12.2 months in the HMA-naive patients.
The most common TEAEs in the entire MDS cohort were hematuria (45%), constipation (43%), diarrhea (42%), fatigue (42%), dysuria (38%), pyrexia (36%), nausea (35%), neutropenia (31%), and thrombocytopenia (30%).
Grade 3 or higher TEAEs were neutropenia (27%), thrombocytopenia (26%), hematuria (9%), dysuria (9%), diarrhea (5%), fatigue (4%), and pyrexia (1%).
Dr. Woodman said patients who were most likely to be at risk for genitourinary toxicities (hematuria and dysuria) were those who weren’t well hydrated, took rigosertib at night, and didn’t void their bladders before bedtime. He said the researchers’ hypothesis is that there is some local bladder irritation in that setting.
However, the researchers found ways to mitigate the risk of genitourinary toxicities, including:
- Requiring the second dose of rigosertib to be taken in the afternoon rather than evening (about 3 p.m.).
- Asking patients to consume at least 2 liters of fluid per day.
- Having patients empty their bladders before bedtime.
- Assessing urine pH roughly 2 hours after the morning dose of rigosertib and prescribing sodium bicarbonate if the pH is less than 7.5.
Dr. Woodman said the phase 2 results in MDS patients have prompted the development of a phase 3 trial in which researchers will compare oral rigosertib plus azacitidine to azacitidine plus placebo.
Dr. Woodman is employed by Onconova Therapeutics, which sponsored the phase 1/2 trial. The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
More abnormal cells linked to poorer ASCT outcomes in MDS
NEWPORT BEACH, CALIF. – Researchers say they’ve found an association between the percentage of cytogenetically abnormal cells at allogeneic stem cell transplant (ASCT) and posttransplant outcomes in patients with myelodysplastic syndromes (MDS).
Patients who had more than 60% cytogenetically abnormal cells at ASCT had significantly inferior overall survival (OS) and relapse-free survival (RFS), compared to patients with fewer abnormal cells.
Dipenkumar Modi, MD, of Barbara Ann Karmanos Cancer Institute at Wayne State University in Detroit, and his colleagues conducted this research and presented the results at the Acute Leukemia Forum of Hemedicus.
The researchers studied 109 adult MDS patients who underwent ASCT from January 2000 through December 2016. The patients were divided into three groups based on the percentage of cytogenetically abnormal cells at ASCT:
- Group 1 had less than 30% (n = 22)
- Group 2 had 30%-60% (n = 23)
- Group 3 had greater than 60% (n = 64).
Baseline characteristics were largely similar between the groups. However, patients in group 3 were significantly more likely than those in groups 1 and 2 to have del(5q) and monosomy 5+7 (P = .048).
Patients in group 1 had a significantly higher percentage of bone marrow transplants (as opposed to peripheral blood stem cell transplants) than patients in groups 2 and 3 (P = .039). And patients in group 1 had significantly fewer blasts at ASCT than patients in groups 2 and 3 (P = .011).
The researchers found no significant between-group differences in relapse and nonrelapse mortality, but there were significant differences in OS and RFS.
Patients in group 3 had inferior RFS compared to patients in group 1, which was the reference group. The hazard ratio (HR) was 2.503 (P = .013) in a univariable analysis and 2.196 (P = .049) in a multivariable analysis.
Group 3 also had inferior OS compared to group 1. The hazard ratio was 2.589 (P = .021) in a univariable analysis and 2.478 (P = .040) in a multivariable analysis.
There was no significant difference in RFS or OS between groups 1 and 2. The HR for RFS in group 2 was 1.879 (P = .148) in a univariable analysis and 1.365 (P = .506) in a multivariable analysis. The HR for OS was 1.997 (P = .155) and 1.413 (P = .511), respectively.
Dr. Modi said these results suggest patients with greater than 60% cytogenetically abnormal cells at ASCT should be monitored more closely after transplant, and their immunosuppressive medication should be tapered as soon as possible.
Dr. Modi and his colleagues reported having no conflicts of interest relevant to this research.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers say they’ve found an association between the percentage of cytogenetically abnormal cells at allogeneic stem cell transplant (ASCT) and posttransplant outcomes in patients with myelodysplastic syndromes (MDS).
Patients who had more than 60% cytogenetically abnormal cells at ASCT had significantly inferior overall survival (OS) and relapse-free survival (RFS), compared to patients with fewer abnormal cells.
Dipenkumar Modi, MD, of Barbara Ann Karmanos Cancer Institute at Wayne State University in Detroit, and his colleagues conducted this research and presented the results at the Acute Leukemia Forum of Hemedicus.
The researchers studied 109 adult MDS patients who underwent ASCT from January 2000 through December 2016. The patients were divided into three groups based on the percentage of cytogenetically abnormal cells at ASCT:
- Group 1 had less than 30% (n = 22)
- Group 2 had 30%-60% (n = 23)
- Group 3 had greater than 60% (n = 64).
Baseline characteristics were largely similar between the groups. However, patients in group 3 were significantly more likely than those in groups 1 and 2 to have del(5q) and monosomy 5+7 (P = .048).
Patients in group 1 had a significantly higher percentage of bone marrow transplants (as opposed to peripheral blood stem cell transplants) than patients in groups 2 and 3 (P = .039). And patients in group 1 had significantly fewer blasts at ASCT than patients in groups 2 and 3 (P = .011).
The researchers found no significant between-group differences in relapse and nonrelapse mortality, but there were significant differences in OS and RFS.
Patients in group 3 had inferior RFS compared to patients in group 1, which was the reference group. The hazard ratio (HR) was 2.503 (P = .013) in a univariable analysis and 2.196 (P = .049) in a multivariable analysis.
Group 3 also had inferior OS compared to group 1. The hazard ratio was 2.589 (P = .021) in a univariable analysis and 2.478 (P = .040) in a multivariable analysis.
There was no significant difference in RFS or OS between groups 1 and 2. The HR for RFS in group 2 was 1.879 (P = .148) in a univariable analysis and 1.365 (P = .506) in a multivariable analysis. The HR for OS was 1.997 (P = .155) and 1.413 (P = .511), respectively.
Dr. Modi said these results suggest patients with greater than 60% cytogenetically abnormal cells at ASCT should be monitored more closely after transplant, and their immunosuppressive medication should be tapered as soon as possible.
Dr. Modi and his colleagues reported having no conflicts of interest relevant to this research.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers say they’ve found an association between the percentage of cytogenetically abnormal cells at allogeneic stem cell transplant (ASCT) and posttransplant outcomes in patients with myelodysplastic syndromes (MDS).
Patients who had more than 60% cytogenetically abnormal cells at ASCT had significantly inferior overall survival (OS) and relapse-free survival (RFS), compared to patients with fewer abnormal cells.
Dipenkumar Modi, MD, of Barbara Ann Karmanos Cancer Institute at Wayne State University in Detroit, and his colleagues conducted this research and presented the results at the Acute Leukemia Forum of Hemedicus.
The researchers studied 109 adult MDS patients who underwent ASCT from January 2000 through December 2016. The patients were divided into three groups based on the percentage of cytogenetically abnormal cells at ASCT:
- Group 1 had less than 30% (n = 22)
- Group 2 had 30%-60% (n = 23)
- Group 3 had greater than 60% (n = 64).
Baseline characteristics were largely similar between the groups. However, patients in group 3 were significantly more likely than those in groups 1 and 2 to have del(5q) and monosomy 5+7 (P = .048).
Patients in group 1 had a significantly higher percentage of bone marrow transplants (as opposed to peripheral blood stem cell transplants) than patients in groups 2 and 3 (P = .039). And patients in group 1 had significantly fewer blasts at ASCT than patients in groups 2 and 3 (P = .011).
The researchers found no significant between-group differences in relapse and nonrelapse mortality, but there were significant differences in OS and RFS.
Patients in group 3 had inferior RFS compared to patients in group 1, which was the reference group. The hazard ratio (HR) was 2.503 (P = .013) in a univariable analysis and 2.196 (P = .049) in a multivariable analysis.
Group 3 also had inferior OS compared to group 1. The hazard ratio was 2.589 (P = .021) in a univariable analysis and 2.478 (P = .040) in a multivariable analysis.
There was no significant difference in RFS or OS between groups 1 and 2. The HR for RFS in group 2 was 1.879 (P = .148) in a univariable analysis and 1.365 (P = .506) in a multivariable analysis. The HR for OS was 1.997 (P = .155) and 1.413 (P = .511), respectively.
Dr. Modi said these results suggest patients with greater than 60% cytogenetically abnormal cells at ASCT should be monitored more closely after transplant, and their immunosuppressive medication should be tapered as soon as possible.
Dr. Modi and his colleagues reported having no conflicts of interest relevant to this research.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
LentiGlobin reduces transfusion dependence in young thalassemia patients
NEW ORLEANS – The gene therapy LentiGlobin can reduce transfusion dependence in children and young adults with non-beta0/beta0 thalassemia, according to two trials.
In a phase 1/2 trial, 8 of 10 of patients achieved transfusion independence at a median follow-up of 36.0 months. In a phase 3 trial, transfusion independence was achieved by 2 of 3 patients with follow-up of at least 12 months.
Timothy S. Olson, MD, PhD, of Children’s Hospital of Philadelphia, presented results from the phase 1/2 HGB-204 trial and the phase 3 HGB-207 trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Treatment
In both trials, patients received granulocyte colony-stimulating factor and plerixafor for hematopoietic stem cell mobilization. Their cells were collected via apheresis and transduced with the betibeglogene darolentivec (BB305) lentiviral vector. The patients received busulfan (for an average of 4 days) as conditioning and were infused with the transduced cells.
The manufacturing process for LentiGlobin was refined in the HGB-207 trial, which translated to a product with a higher vector copy number and higher proportion of CD34+ cells transduced, Dr. Olson said.
The median vector copy number was 3.1 in the HGB-207 trial and 0.7 in the HGB-204 trial. The median proportion of CD34+ cells transfused was 81% and 29%, respectively. The median cell dose was 7.7 x 106 CD34+ cells/kg and 7.1 x 106 CD34+ cells/kg, respectively.
HGB-204 patients and efficacy
The HGB-204 trial included 10 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 1 with beta+/beta0, 2 with beta+/beta+, and 1 with an “other” genotype.
The patients’ median age at consent was 19.5 years (range, 16-34). The annualized median prestudy red blood cell (RBC) transfusion volume was 151 mL/kg per year.
At a median follow-up of 36 months, 8 of the 10 patients achieved transfusion independence. The median duration of transfusion independence was 38 months. The median weighted average hemoglobin during transfusion independence was 10.2 g/dL.
“Two patients did not achieve transfusion independence, and both patients were on the lower end of the spectrum both in terms of vector copy number per cell and the percentage of CD34+ cells that were successfully transduced,” Dr. Olson said. “Both patients actually experienced a reduction in the annualized transfusion volume requirements of between 43% and 77%.”
HGB-207 patients and efficacy
The HGB-207 trial included 16 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 7 with beta+/beta0, and 3 with the beta+/beta+ genotype.
The patients’ median age at consent was 19 years . The annualized median prestudy RBC transfusion volume was 192 mL/kg per year.
The median follow-up in this trial is 9.3 months. Ten of 11 patients with at least 3 months of follow-up are transfusion-free with hemoglobin levels greater than 11 g/dL.
Two patients have achieved transfusion independence according to the protocol definition, which is weighted average hemoglobin of 9 g/dL or greater without any RBC transfusions for at least 12 months.
“In the one patient in this study who did not achieve transfusion independence, the vector-derived hemoglobin was quite low, and this correlated with a very low vector copy number seen in circulating peripheral blood mononuclear cells,” Dr. Olson said.
It isn’t clear why this occurred, however, as the vector copy number wasn’t especially low in the LentiGlobin product the patient received. Therefore, the researchers are still investigating why this patient failed to achieve transfusion independence.
Safety in both trials
“Very importantly, there were no deaths, there were no engraftment failures, there was no evidence of vector-mediated replication-competent lentivirus, and integration site analysis revealed no evidence of clonal dominance,” Dr. Olson said.
He added that most of the grade 3 or greater adverse events seen in both trials were directly attributable to busulfan-based myeloablative conditioning, including four episodes of veno-occlusive disease.
Nonhematologic grade 3 or higher adverse events in HGB-204 included stomatitis (n = 8), febrile neutropenia (n = 6), irregular menstruation (n = 3), pharyngeal inflammation (n = 2), and veno-occlusive liver disease (n = 1).
Nonhematologic grade 3 or higher adverse events in HGB-207 included stomatitis (n = 9), febrile neutropenia (n = 4), pharyngeal inflammation (n = 2), epistaxis (n = 3), pyrexia (n = 3), veno-occlusive liver disease (n = 3), ALT increase (n = 2), bilirubin increase (n = 2), and hypoxia (n = 2).
One patient in HGB-207 had grade 3 thrombocytopenia considered possibly related to LentiGlobin.
Dr. Olson reported advisory board engagement with bluebird bio, which sponsored both trials.
SOURCE: Olson TS et al. ASPHO 2019. Abstract 2002.
NEW ORLEANS – The gene therapy LentiGlobin can reduce transfusion dependence in children and young adults with non-beta0/beta0 thalassemia, according to two trials.
In a phase 1/2 trial, 8 of 10 of patients achieved transfusion independence at a median follow-up of 36.0 months. In a phase 3 trial, transfusion independence was achieved by 2 of 3 patients with follow-up of at least 12 months.
Timothy S. Olson, MD, PhD, of Children’s Hospital of Philadelphia, presented results from the phase 1/2 HGB-204 trial and the phase 3 HGB-207 trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Treatment
In both trials, patients received granulocyte colony-stimulating factor and plerixafor for hematopoietic stem cell mobilization. Their cells were collected via apheresis and transduced with the betibeglogene darolentivec (BB305) lentiviral vector. The patients received busulfan (for an average of 4 days) as conditioning and were infused with the transduced cells.
The manufacturing process for LentiGlobin was refined in the HGB-207 trial, which translated to a product with a higher vector copy number and higher proportion of CD34+ cells transduced, Dr. Olson said.
The median vector copy number was 3.1 in the HGB-207 trial and 0.7 in the HGB-204 trial. The median proportion of CD34+ cells transfused was 81% and 29%, respectively. The median cell dose was 7.7 x 106 CD34+ cells/kg and 7.1 x 106 CD34+ cells/kg, respectively.
HGB-204 patients and efficacy
The HGB-204 trial included 10 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 1 with beta+/beta0, 2 with beta+/beta+, and 1 with an “other” genotype.
The patients’ median age at consent was 19.5 years (range, 16-34). The annualized median prestudy red blood cell (RBC) transfusion volume was 151 mL/kg per year.
At a median follow-up of 36 months, 8 of the 10 patients achieved transfusion independence. The median duration of transfusion independence was 38 months. The median weighted average hemoglobin during transfusion independence was 10.2 g/dL.
“Two patients did not achieve transfusion independence, and both patients were on the lower end of the spectrum both in terms of vector copy number per cell and the percentage of CD34+ cells that were successfully transduced,” Dr. Olson said. “Both patients actually experienced a reduction in the annualized transfusion volume requirements of between 43% and 77%.”
HGB-207 patients and efficacy
The HGB-207 trial included 16 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 7 with beta+/beta0, and 3 with the beta+/beta+ genotype.
The patients’ median age at consent was 19 years . The annualized median prestudy RBC transfusion volume was 192 mL/kg per year.
The median follow-up in this trial is 9.3 months. Ten of 11 patients with at least 3 months of follow-up are transfusion-free with hemoglobin levels greater than 11 g/dL.
Two patients have achieved transfusion independence according to the protocol definition, which is weighted average hemoglobin of 9 g/dL or greater without any RBC transfusions for at least 12 months.
“In the one patient in this study who did not achieve transfusion independence, the vector-derived hemoglobin was quite low, and this correlated with a very low vector copy number seen in circulating peripheral blood mononuclear cells,” Dr. Olson said.
It isn’t clear why this occurred, however, as the vector copy number wasn’t especially low in the LentiGlobin product the patient received. Therefore, the researchers are still investigating why this patient failed to achieve transfusion independence.
Safety in both trials
“Very importantly, there were no deaths, there were no engraftment failures, there was no evidence of vector-mediated replication-competent lentivirus, and integration site analysis revealed no evidence of clonal dominance,” Dr. Olson said.
He added that most of the grade 3 or greater adverse events seen in both trials were directly attributable to busulfan-based myeloablative conditioning, including four episodes of veno-occlusive disease.
Nonhematologic grade 3 or higher adverse events in HGB-204 included stomatitis (n = 8), febrile neutropenia (n = 6), irregular menstruation (n = 3), pharyngeal inflammation (n = 2), and veno-occlusive liver disease (n = 1).
Nonhematologic grade 3 or higher adverse events in HGB-207 included stomatitis (n = 9), febrile neutropenia (n = 4), pharyngeal inflammation (n = 2), epistaxis (n = 3), pyrexia (n = 3), veno-occlusive liver disease (n = 3), ALT increase (n = 2), bilirubin increase (n = 2), and hypoxia (n = 2).
One patient in HGB-207 had grade 3 thrombocytopenia considered possibly related to LentiGlobin.
Dr. Olson reported advisory board engagement with bluebird bio, which sponsored both trials.
SOURCE: Olson TS et al. ASPHO 2019. Abstract 2002.
NEW ORLEANS – The gene therapy LentiGlobin can reduce transfusion dependence in children and young adults with non-beta0/beta0 thalassemia, according to two trials.
In a phase 1/2 trial, 8 of 10 of patients achieved transfusion independence at a median follow-up of 36.0 months. In a phase 3 trial, transfusion independence was achieved by 2 of 3 patients with follow-up of at least 12 months.
Timothy S. Olson, MD, PhD, of Children’s Hospital of Philadelphia, presented results from the phase 1/2 HGB-204 trial and the phase 3 HGB-207 trial at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Treatment
In both trials, patients received granulocyte colony-stimulating factor and plerixafor for hematopoietic stem cell mobilization. Their cells were collected via apheresis and transduced with the betibeglogene darolentivec (BB305) lentiviral vector. The patients received busulfan (for an average of 4 days) as conditioning and were infused with the transduced cells.
The manufacturing process for LentiGlobin was refined in the HGB-207 trial, which translated to a product with a higher vector copy number and higher proportion of CD34+ cells transduced, Dr. Olson said.
The median vector copy number was 3.1 in the HGB-207 trial and 0.7 in the HGB-204 trial. The median proportion of CD34+ cells transfused was 81% and 29%, respectively. The median cell dose was 7.7 x 106 CD34+ cells/kg and 7.1 x 106 CD34+ cells/kg, respectively.
HGB-204 patients and efficacy
The HGB-204 trial included 10 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 1 with beta+/beta0, 2 with beta+/beta+, and 1 with an “other” genotype.
The patients’ median age at consent was 19.5 years (range, 16-34). The annualized median prestudy red blood cell (RBC) transfusion volume was 151 mL/kg per year.
At a median follow-up of 36 months, 8 of the 10 patients achieved transfusion independence. The median duration of transfusion independence was 38 months. The median weighted average hemoglobin during transfusion independence was 10.2 g/dL.
“Two patients did not achieve transfusion independence, and both patients were on the lower end of the spectrum both in terms of vector copy number per cell and the percentage of CD34+ cells that were successfully transduced,” Dr. Olson said. “Both patients actually experienced a reduction in the annualized transfusion volume requirements of between 43% and 77%.”
HGB-207 patients and efficacy
The HGB-207 trial included 16 patients with non-beta0/beta0 genotypes – 6 with betaE/beta0, 7 with beta+/beta0, and 3 with the beta+/beta+ genotype.
The patients’ median age at consent was 19 years . The annualized median prestudy RBC transfusion volume was 192 mL/kg per year.
The median follow-up in this trial is 9.3 months. Ten of 11 patients with at least 3 months of follow-up are transfusion-free with hemoglobin levels greater than 11 g/dL.
Two patients have achieved transfusion independence according to the protocol definition, which is weighted average hemoglobin of 9 g/dL or greater without any RBC transfusions for at least 12 months.
“In the one patient in this study who did not achieve transfusion independence, the vector-derived hemoglobin was quite low, and this correlated with a very low vector copy number seen in circulating peripheral blood mononuclear cells,” Dr. Olson said.
It isn’t clear why this occurred, however, as the vector copy number wasn’t especially low in the LentiGlobin product the patient received. Therefore, the researchers are still investigating why this patient failed to achieve transfusion independence.
Safety in both trials
“Very importantly, there were no deaths, there were no engraftment failures, there was no evidence of vector-mediated replication-competent lentivirus, and integration site analysis revealed no evidence of clonal dominance,” Dr. Olson said.
He added that most of the grade 3 or greater adverse events seen in both trials were directly attributable to busulfan-based myeloablative conditioning, including four episodes of veno-occlusive disease.
Nonhematologic grade 3 or higher adverse events in HGB-204 included stomatitis (n = 8), febrile neutropenia (n = 6), irregular menstruation (n = 3), pharyngeal inflammation (n = 2), and veno-occlusive liver disease (n = 1).
Nonhematologic grade 3 or higher adverse events in HGB-207 included stomatitis (n = 9), febrile neutropenia (n = 4), pharyngeal inflammation (n = 2), epistaxis (n = 3), pyrexia (n = 3), veno-occlusive liver disease (n = 3), ALT increase (n = 2), bilirubin increase (n = 2), and hypoxia (n = 2).
One patient in HGB-207 had grade 3 thrombocytopenia considered possibly related to LentiGlobin.
Dr. Olson reported advisory board engagement with bluebird bio, which sponsored both trials.
SOURCE: Olson TS et al. ASPHO 2019. Abstract 2002.
REPORTING FROM 2019 ASPHO CONFERENCE
Biomarker testing may transform treatment of acute GVHD
NEWPORT BEACH, CALIF. – Researchers say they have identified biomarkers that may help guide early treatment decisions in patients with acute graft-versus-host disease (GVHD).
The biomarkers, ST2 and REG3-alpha, were measured during the first month of GVHD treatment and proved more accurate than clinical response for predicting 6-month nonrelapse mortality (NRM). In fact, biomarker assessment revealed patients who responded to treatment but had a high risk of NRM and nonresponders who had a low risk of NRM.
The researchers also found that biomarkers changed over the first month of treatment but remained significant predictors of NRM. This suggests that modifying treatment according to biomarker findings at various time points could result in better outcomes for patients.
“We think this is going to transform the way we treat graft-versus-host disease,” said James L.M. Ferrara, MD, DSc, of the Icahn School of Medicine at Mount Sinai, New York.
Dr. Ferrara and Hrishikesh Srinagesh, along with their colleagues at Mount Sinai, have conducted extensive research with these biomarkers and presented some of their findings at the Acute Leukemia Forum of Hemedicus.
Comparing biomarkers and response
In one study, the researchers evaluated 355 patients who had undergone allogeneic hematopoietic stem cell transplant at 1 of 20 Mount Sinai Acute GVHD International Consortium (MAGIC) centers between January 2016 and February 2018. All patients developed acute GVHD and received systemic steroids as treatment.
Patients provided blood samples weekly for the first month of treatment, and concentrations of ST2 and REG3-alpha were measured in each sample. Both biomarker concentrations were used to calculate the biomarker probability of NRM.
“The concentration of those two biomarkers are put into a computer, and we get … a single number, and that gives us the probability of mortality,” Dr. Ferrara said. “[W]e call this the MAGIC algorithm probability, or MAP. And when a MAP is low, the patient has a very low chance of dying from graft-versus-host disease, when it’s intermediate, they have an intermediate risk, and when it’s high, they have a high risk.”
The researchers then compared the MAP and clinical response for their ability to predict 6-month NRM throughout the first month of therapy for acute GVHD.
MAP bests response
After 1 month of therapy, the MAP was more accurate than clinical response for predicting 6-month NRM. The area under the curve was 0.84 and 0.65, respectively (P less than .001).
Likewise, the MAP after 1 week of therapy was more accurate than clinical response at 1 month for predicting 6-month NRM. The area under the curve was 0.80 and 0.65, respectively (P less than .001).
“[T]he clinical responses were good, but not great, at predicting long-term outcome, where the biomarker, the MAP, was significantly better,” Dr. Ferrara said. “[A]t every time point we tested, the biomarkers were better than the clinical responses.”
The researchers also identified subgroups of clinical responders and nonresponders for whom MAP more accurately predicted 6-month NRM.
The team found that 61% of clinical nonresponders were actually low risk according to MAP. And the incidence of 6-month NRM was significantly lower in the MAP-designated low-risk patients than in MAP-designated high-risk patients – 22% and 56%, respectively (P less than .001).
On the other hand, 10% of clinical responders were high risk according to MAP. The incidence of 6-month NRM was significantly higher in the high-risk patients than in the low-risk patients – 40% and 13%, respectively (P less than .001).
Assessing changes over time
The researchers found that patients who were initially high risk by MAP but had not experienced NRM by 6 months had significant decreases in their MAP after 4 weeks of treatment (P = .003). Patients who did experience NRM had a significant increase in their MAP whether their initial MAP was low (P = .007) or high (P = .024).
“What we found was that patients who lived tended to either have low biomarkers at the start of treatment and stay low or start out with high biomarkers and have reductions over the first month of therapy,” Mr. Srinagesh said. “Conversely, patients who tended to do worse were those who had either increases in their biomarkers or stayed high at all time points.”
The researchers identified a threshold – 0.290 – for separating patients by mortality risk.
“Patients who started out above the threshold and then went below it had a 5-fold reduction in mortality, whereas patients who started out below the threshold and rose above it had a 5-fold increase in mortality,” Mr. Srinagesh said.
MAP in clinical trials and practice
Based on these findings and results from related studies, the researchers theorize that MAP would be a better endpoint for clinical trials than clinical response.
At present, there are three trials in which researchers are using MAP as an endpoint to assess the efficacy of treatment for GVHD (NCT02133924, NCT03459040, and NCT03846479). Dr. Ferrara said a fourth trial is set to begin this summer.
Additionally, MAP is being used in clinical practice. A company called Viracor Eurofins Clinical Diagnostics licensed the MAGIC algorithm and provides three related tests for consumer use.
Viracor’s aGVHD Pre-Symptomatic Algorithm assigns patients to high- and low-risk groups based on results from samples collected 7 days after transplant. The aGVHD Symptomatic Onset Algorithm assigns patients to high-, intermediate-, and low-risk groups. The aGVHD Post-Treatment Algorithm, which can be used 7 days or more after GVHD treatment initiation, stratifies steroid-resistant patients into high- or low-risk groups for both NRM and overall survival.
“We are still in early days of figuring out how to use [the biomarker tests], but … what I’ve heard is that people are finding them to be useful in their clinical practice,” Dr. Ferrara said.
Dr. Ferrara has an ownership interest in and receives royalties from Viracor. Mr. Srinagesh reported having no relevant conflicts of interest. The research was supported by grants from the National Cancer Institute and the American Cancer Society.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers say they have identified biomarkers that may help guide early treatment decisions in patients with acute graft-versus-host disease (GVHD).
The biomarkers, ST2 and REG3-alpha, were measured during the first month of GVHD treatment and proved more accurate than clinical response for predicting 6-month nonrelapse mortality (NRM). In fact, biomarker assessment revealed patients who responded to treatment but had a high risk of NRM and nonresponders who had a low risk of NRM.
The researchers also found that biomarkers changed over the first month of treatment but remained significant predictors of NRM. This suggests that modifying treatment according to biomarker findings at various time points could result in better outcomes for patients.
“We think this is going to transform the way we treat graft-versus-host disease,” said James L.M. Ferrara, MD, DSc, of the Icahn School of Medicine at Mount Sinai, New York.
Dr. Ferrara and Hrishikesh Srinagesh, along with their colleagues at Mount Sinai, have conducted extensive research with these biomarkers and presented some of their findings at the Acute Leukemia Forum of Hemedicus.
Comparing biomarkers and response
In one study, the researchers evaluated 355 patients who had undergone allogeneic hematopoietic stem cell transplant at 1 of 20 Mount Sinai Acute GVHD International Consortium (MAGIC) centers between January 2016 and February 2018. All patients developed acute GVHD and received systemic steroids as treatment.
Patients provided blood samples weekly for the first month of treatment, and concentrations of ST2 and REG3-alpha were measured in each sample. Both biomarker concentrations were used to calculate the biomarker probability of NRM.
“The concentration of those two biomarkers are put into a computer, and we get … a single number, and that gives us the probability of mortality,” Dr. Ferrara said. “[W]e call this the MAGIC algorithm probability, or MAP. And when a MAP is low, the patient has a very low chance of dying from graft-versus-host disease, when it’s intermediate, they have an intermediate risk, and when it’s high, they have a high risk.”
The researchers then compared the MAP and clinical response for their ability to predict 6-month NRM throughout the first month of therapy for acute GVHD.
MAP bests response
After 1 month of therapy, the MAP was more accurate than clinical response for predicting 6-month NRM. The area under the curve was 0.84 and 0.65, respectively (P less than .001).
Likewise, the MAP after 1 week of therapy was more accurate than clinical response at 1 month for predicting 6-month NRM. The area under the curve was 0.80 and 0.65, respectively (P less than .001).
“[T]he clinical responses were good, but not great, at predicting long-term outcome, where the biomarker, the MAP, was significantly better,” Dr. Ferrara said. “[A]t every time point we tested, the biomarkers were better than the clinical responses.”
The researchers also identified subgroups of clinical responders and nonresponders for whom MAP more accurately predicted 6-month NRM.
The team found that 61% of clinical nonresponders were actually low risk according to MAP. And the incidence of 6-month NRM was significantly lower in the MAP-designated low-risk patients than in MAP-designated high-risk patients – 22% and 56%, respectively (P less than .001).
On the other hand, 10% of clinical responders were high risk according to MAP. The incidence of 6-month NRM was significantly higher in the high-risk patients than in the low-risk patients – 40% and 13%, respectively (P less than .001).
Assessing changes over time
The researchers found that patients who were initially high risk by MAP but had not experienced NRM by 6 months had significant decreases in their MAP after 4 weeks of treatment (P = .003). Patients who did experience NRM had a significant increase in their MAP whether their initial MAP was low (P = .007) or high (P = .024).
“What we found was that patients who lived tended to either have low biomarkers at the start of treatment and stay low or start out with high biomarkers and have reductions over the first month of therapy,” Mr. Srinagesh said. “Conversely, patients who tended to do worse were those who had either increases in their biomarkers or stayed high at all time points.”
The researchers identified a threshold – 0.290 – for separating patients by mortality risk.
“Patients who started out above the threshold and then went below it had a 5-fold reduction in mortality, whereas patients who started out below the threshold and rose above it had a 5-fold increase in mortality,” Mr. Srinagesh said.
MAP in clinical trials and practice
Based on these findings and results from related studies, the researchers theorize that MAP would be a better endpoint for clinical trials than clinical response.
At present, there are three trials in which researchers are using MAP as an endpoint to assess the efficacy of treatment for GVHD (NCT02133924, NCT03459040, and NCT03846479). Dr. Ferrara said a fourth trial is set to begin this summer.
Additionally, MAP is being used in clinical practice. A company called Viracor Eurofins Clinical Diagnostics licensed the MAGIC algorithm and provides three related tests for consumer use.
Viracor’s aGVHD Pre-Symptomatic Algorithm assigns patients to high- and low-risk groups based on results from samples collected 7 days after transplant. The aGVHD Symptomatic Onset Algorithm assigns patients to high-, intermediate-, and low-risk groups. The aGVHD Post-Treatment Algorithm, which can be used 7 days or more after GVHD treatment initiation, stratifies steroid-resistant patients into high- or low-risk groups for both NRM and overall survival.
“We are still in early days of figuring out how to use [the biomarker tests], but … what I’ve heard is that people are finding them to be useful in their clinical practice,” Dr. Ferrara said.
Dr. Ferrara has an ownership interest in and receives royalties from Viracor. Mr. Srinagesh reported having no relevant conflicts of interest. The research was supported by grants from the National Cancer Institute and the American Cancer Society.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers say they have identified biomarkers that may help guide early treatment decisions in patients with acute graft-versus-host disease (GVHD).
The biomarkers, ST2 and REG3-alpha, were measured during the first month of GVHD treatment and proved more accurate than clinical response for predicting 6-month nonrelapse mortality (NRM). In fact, biomarker assessment revealed patients who responded to treatment but had a high risk of NRM and nonresponders who had a low risk of NRM.
The researchers also found that biomarkers changed over the first month of treatment but remained significant predictors of NRM. This suggests that modifying treatment according to biomarker findings at various time points could result in better outcomes for patients.
“We think this is going to transform the way we treat graft-versus-host disease,” said James L.M. Ferrara, MD, DSc, of the Icahn School of Medicine at Mount Sinai, New York.
Dr. Ferrara and Hrishikesh Srinagesh, along with their colleagues at Mount Sinai, have conducted extensive research with these biomarkers and presented some of their findings at the Acute Leukemia Forum of Hemedicus.
Comparing biomarkers and response
In one study, the researchers evaluated 355 patients who had undergone allogeneic hematopoietic stem cell transplant at 1 of 20 Mount Sinai Acute GVHD International Consortium (MAGIC) centers between January 2016 and February 2018. All patients developed acute GVHD and received systemic steroids as treatment.
Patients provided blood samples weekly for the first month of treatment, and concentrations of ST2 and REG3-alpha were measured in each sample. Both biomarker concentrations were used to calculate the biomarker probability of NRM.
“The concentration of those two biomarkers are put into a computer, and we get … a single number, and that gives us the probability of mortality,” Dr. Ferrara said. “[W]e call this the MAGIC algorithm probability, or MAP. And when a MAP is low, the patient has a very low chance of dying from graft-versus-host disease, when it’s intermediate, they have an intermediate risk, and when it’s high, they have a high risk.”
The researchers then compared the MAP and clinical response for their ability to predict 6-month NRM throughout the first month of therapy for acute GVHD.
MAP bests response
After 1 month of therapy, the MAP was more accurate than clinical response for predicting 6-month NRM. The area under the curve was 0.84 and 0.65, respectively (P less than .001).
Likewise, the MAP after 1 week of therapy was more accurate than clinical response at 1 month for predicting 6-month NRM. The area under the curve was 0.80 and 0.65, respectively (P less than .001).
“[T]he clinical responses were good, but not great, at predicting long-term outcome, where the biomarker, the MAP, was significantly better,” Dr. Ferrara said. “[A]t every time point we tested, the biomarkers were better than the clinical responses.”
The researchers also identified subgroups of clinical responders and nonresponders for whom MAP more accurately predicted 6-month NRM.
The team found that 61% of clinical nonresponders were actually low risk according to MAP. And the incidence of 6-month NRM was significantly lower in the MAP-designated low-risk patients than in MAP-designated high-risk patients – 22% and 56%, respectively (P less than .001).
On the other hand, 10% of clinical responders were high risk according to MAP. The incidence of 6-month NRM was significantly higher in the high-risk patients than in the low-risk patients – 40% and 13%, respectively (P less than .001).
Assessing changes over time
The researchers found that patients who were initially high risk by MAP but had not experienced NRM by 6 months had significant decreases in their MAP after 4 weeks of treatment (P = .003). Patients who did experience NRM had a significant increase in their MAP whether their initial MAP was low (P = .007) or high (P = .024).
“What we found was that patients who lived tended to either have low biomarkers at the start of treatment and stay low or start out with high biomarkers and have reductions over the first month of therapy,” Mr. Srinagesh said. “Conversely, patients who tended to do worse were those who had either increases in their biomarkers or stayed high at all time points.”
The researchers identified a threshold – 0.290 – for separating patients by mortality risk.
“Patients who started out above the threshold and then went below it had a 5-fold reduction in mortality, whereas patients who started out below the threshold and rose above it had a 5-fold increase in mortality,” Mr. Srinagesh said.
MAP in clinical trials and practice
Based on these findings and results from related studies, the researchers theorize that MAP would be a better endpoint for clinical trials than clinical response.
At present, there are three trials in which researchers are using MAP as an endpoint to assess the efficacy of treatment for GVHD (NCT02133924, NCT03459040, and NCT03846479). Dr. Ferrara said a fourth trial is set to begin this summer.
Additionally, MAP is being used in clinical practice. A company called Viracor Eurofins Clinical Diagnostics licensed the MAGIC algorithm and provides three related tests for consumer use.
Viracor’s aGVHD Pre-Symptomatic Algorithm assigns patients to high- and low-risk groups based on results from samples collected 7 days after transplant. The aGVHD Symptomatic Onset Algorithm assigns patients to high-, intermediate-, and low-risk groups. The aGVHD Post-Treatment Algorithm, which can be used 7 days or more after GVHD treatment initiation, stratifies steroid-resistant patients into high- or low-risk groups for both NRM and overall survival.
“We are still in early days of figuring out how to use [the biomarker tests], but … what I’ve heard is that people are finding them to be useful in their clinical practice,” Dr. Ferrara said.
Dr. Ferrara has an ownership interest in and receives royalties from Viracor. Mr. Srinagesh reported having no relevant conflicts of interest. The research was supported by grants from the National Cancer Institute and the American Cancer Society.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
Tagraxofusp produces high response rate in BPDCN
Tagraxofusp demonstrated efficacy in a phase 2 trial of patients with previously treated or untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
The researchers wrote that capillary leak syndrome (CLS) was an important adverse event in this trial, as it caused two deaths. However, the researchers developed strategies that appear to mitigate the risk of CLS in patients taking tagraxofusp.
Naveen Pemmaraju, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues conducted the trial and reported the results in the New England Journal of Medicine.
The trial included 47 patients – 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 years and 83% were men.
Three patients (all previously untreated) received tagraxofusp at 7 mcg/kg per day, and 44 patients received a 12 mcg/kg per day dose. All patients were treated on days 1-5 of a 21-day cycle.
Response and survival
In the 29 previously untreated patients who received the 12 mcg/kg dose of tagraxofusp, the overall response rate was 90%. The rate of complete response plus clinical complete response in these patients was 72%.
In the 15 patients with relapsed/refractory BPDCN, the overall response rate was 67%, and the rate of complete response plus clinical complete response was 33%.
A total of 14 patients, 13 of whom had previously untreated BPDCN, went on to transplant.
In the 29 previously untreated patients, the median overall survival was not reached at a median follow-up of 25 months. The overall survival rate was 62% at 12 months, 59% at 18 months, and 52% at 24 months.
In the 15 previously treated patients, the median overall survival was 8.5 months.
Safety
Common adverse events in this trial were ALT increase (64%), AST increase (60%), hypoalbuminemia (55%), peripheral edema (51%), thrombocytopenia (49%), nausea (45%), pyrexia (45%), and fatigue (45%).
Among the 44 patients who received the 12 mcg/kg dose of tagraxofusp, 8 (18%) developed CLS. Six patients had grade 2 CLS, one had grade 4, and one had grade 5. There was an additional CLS-related death in a patient who received tagraxofusp at 7 mcg/kg.
After the first death, the trial protocol was amended to reduce CLS risk. Inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and serum albumin of at least 3.2 g/dL. Additionally, the researchers began monitoring patients’ weight, albumin levels, and kidney function. The team withheld tagraxofusp if patients experienced rapid weight gain or if their serum albumin or systolic blood pressure fell too low.
The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
Tagraxofusp demonstrated efficacy in a phase 2 trial of patients with previously treated or untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
The researchers wrote that capillary leak syndrome (CLS) was an important adverse event in this trial, as it caused two deaths. However, the researchers developed strategies that appear to mitigate the risk of CLS in patients taking tagraxofusp.
Naveen Pemmaraju, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues conducted the trial and reported the results in the New England Journal of Medicine.
The trial included 47 patients – 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 years and 83% were men.
Three patients (all previously untreated) received tagraxofusp at 7 mcg/kg per day, and 44 patients received a 12 mcg/kg per day dose. All patients were treated on days 1-5 of a 21-day cycle.
Response and survival
In the 29 previously untreated patients who received the 12 mcg/kg dose of tagraxofusp, the overall response rate was 90%. The rate of complete response plus clinical complete response in these patients was 72%.
In the 15 patients with relapsed/refractory BPDCN, the overall response rate was 67%, and the rate of complete response plus clinical complete response was 33%.
A total of 14 patients, 13 of whom had previously untreated BPDCN, went on to transplant.
In the 29 previously untreated patients, the median overall survival was not reached at a median follow-up of 25 months. The overall survival rate was 62% at 12 months, 59% at 18 months, and 52% at 24 months.
In the 15 previously treated patients, the median overall survival was 8.5 months.
Safety
Common adverse events in this trial were ALT increase (64%), AST increase (60%), hypoalbuminemia (55%), peripheral edema (51%), thrombocytopenia (49%), nausea (45%), pyrexia (45%), and fatigue (45%).
Among the 44 patients who received the 12 mcg/kg dose of tagraxofusp, 8 (18%) developed CLS. Six patients had grade 2 CLS, one had grade 4, and one had grade 5. There was an additional CLS-related death in a patient who received tagraxofusp at 7 mcg/kg.
After the first death, the trial protocol was amended to reduce CLS risk. Inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and serum albumin of at least 3.2 g/dL. Additionally, the researchers began monitoring patients’ weight, albumin levels, and kidney function. The team withheld tagraxofusp if patients experienced rapid weight gain or if their serum albumin or systolic blood pressure fell too low.
The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
Tagraxofusp demonstrated efficacy in a phase 2 trial of patients with previously treated or untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
The researchers wrote that capillary leak syndrome (CLS) was an important adverse event in this trial, as it caused two deaths. However, the researchers developed strategies that appear to mitigate the risk of CLS in patients taking tagraxofusp.
Naveen Pemmaraju, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues conducted the trial and reported the results in the New England Journal of Medicine.
The trial included 47 patients – 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 years and 83% were men.
Three patients (all previously untreated) received tagraxofusp at 7 mcg/kg per day, and 44 patients received a 12 mcg/kg per day dose. All patients were treated on days 1-5 of a 21-day cycle.
Response and survival
In the 29 previously untreated patients who received the 12 mcg/kg dose of tagraxofusp, the overall response rate was 90%. The rate of complete response plus clinical complete response in these patients was 72%.
In the 15 patients with relapsed/refractory BPDCN, the overall response rate was 67%, and the rate of complete response plus clinical complete response was 33%.
A total of 14 patients, 13 of whom had previously untreated BPDCN, went on to transplant.
In the 29 previously untreated patients, the median overall survival was not reached at a median follow-up of 25 months. The overall survival rate was 62% at 12 months, 59% at 18 months, and 52% at 24 months.
In the 15 previously treated patients, the median overall survival was 8.5 months.
Safety
Common adverse events in this trial were ALT increase (64%), AST increase (60%), hypoalbuminemia (55%), peripheral edema (51%), thrombocytopenia (49%), nausea (45%), pyrexia (45%), and fatigue (45%).
Among the 44 patients who received the 12 mcg/kg dose of tagraxofusp, 8 (18%) developed CLS. Six patients had grade 2 CLS, one had grade 4, and one had grade 5. There was an additional CLS-related death in a patient who received tagraxofusp at 7 mcg/kg.
After the first death, the trial protocol was amended to reduce CLS risk. Inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and serum albumin of at least 3.2 g/dL. Additionally, the researchers began monitoring patients’ weight, albumin levels, and kidney function. The team withheld tagraxofusp if patients experienced rapid weight gain or if their serum albumin or systolic blood pressure fell too low.
The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Tagraxofusp produced responses in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Major finding: The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
Study details: A phase 2 trial of 47 patients, 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN.
Disclosures: The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
Source: Pemmaraju N et al. N Engl J Med. 2019;380:1628-37.