User login
2022 Update on contraception
On June 24, 2022, the US Supreme Court ruled in Dobbs v Jackson to overturn the landmark Roe v Wade decision, deeming that abortion is not protected by statutes that provide the right to privacy, liberty, or autonomy. With this historic ruling, other rights founded on the same principles, including the freedom to use contraception, may be called into question in the future. Clinics that provide abortion care typically play a vital role in providing contraception services. Due to abortion restriction across the country, many of these clinics are predicted to close and many have already closed. Within one month of the Dobbs decision, 43 clinics in 11 states had shut their doors to patients, reducing access to basic contraception services.1 It is more important now than ever that clinicians address barriers and lead the effort to improve and ensure that patients have access to contraceptive services.
In this Update, we review recent evidence that may help aid patients in obtaining contraception more easily and for longer periods of time. We review strategies demonstrated to improve contraceptive access, including how to increase prescribing rates of 1-year contraceptive supplies and pharmacist-prescribed contraception. We also review new data on extended use of the levonorgestrel 52 mg intrauterine device (LNG 52 mg IUD).
One-year prescribing of hormonal contraception decreases an access barrier
Uhm S, Chen MJ, Cutler ED, et al. Twelve-month prescribing of contraceptive pill, patch, and ring before and after a standardized electronic medical record order change. Contraception. 2021;103:60-63.
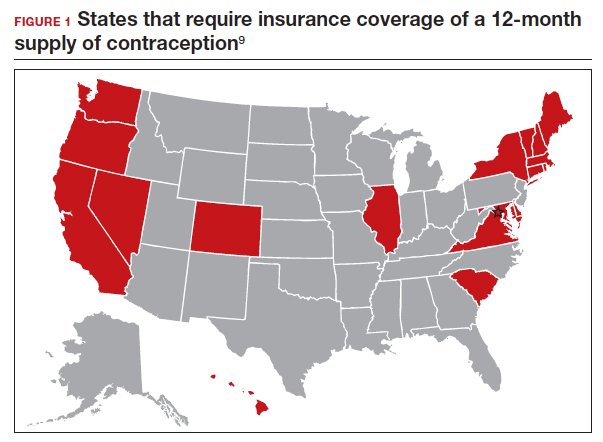
Providing a 1-year supply of self-administered contraception can lead to higher likelihood of continued use and is associated with reduced cost, unintended pregnancy, and abortion rates.2-4 Although some patients may not use a full year’s supply of pills, rings, or patches under such programs, the lower rates of unintended pregnancy result in significant cost savings as compared with the unused contraceptives.2,3 Accordingly, the Centers for Disease Control and Prevention (CDC) advises dispensing a 1-year supply of self-administered hormonal contraception.5 Insurance coverage and providers’ prescribing practices can be barriers to patients obtaining a year’s supply of hormonal contraception. Currently, 18 states and the District of Columbia legally require insurers to cover a 12-month supply of prescription contraceptives (FIGURE 1). Despite these laws and the CDC recommendation, studies show that most people continue to receive only a 1- to 3-month supply.6-8 One strategy to increase the number of 1-year supplies of self-administered contraception is institutional changes to default prescription orders.
Study design
In California, legislation enacted in January 2017 required commercial and medical assistance health plans to cover up to 12 months of US Food and Drug Administration (FDA)-approved self-administered hormonal contraceptives dispensed at 1 time as prescribed or requested. To better serve patients, a multidisciplinary team from the University of California Davis Health worked with the institution’s pharmacy to institute an electronic medical record (EMR) default order change from dispensing 1-month with refills to dispensing 12-month quantities for all combined and progestin-only pills, patches, and rings on formulary.
After this EMR order change in December 2019, Uhm and colleagues conducted a retrospective pre-post study using outpatient prescription data that included nearly 5,000 contraceptive pill, patch, and ring prescriptions over an 8-month period. They compared the frequency of 12-month prescriptions for each of these methods 4 months before and 4 months after the default order change. They compared the proportion of 12-month prescriptions by prescriber department affiliation and by clinic location. Department affiliation was categorized as obstetrics-gynecology or non–obstetrics-gynecology. Clinic location was categorized as medical center campus or community clinics.
Increase in 12-month prescriptions
The authors found an overall increase in 12-month prescriptions, from 11% to 27%, after the EMR order change. Prescribers at the medical center campus clinics more frequently ordered a 12-month supply compared with prescribers at community clinics both before (33% vs 4%, respectively) and after (53% vs 19%, respectively) the EMR change. The only group of providers without a significant increase in 12-month prescriptions was among obstetrics-gynecology providers at community clinics (4% before vs 6% after).
The system EMR change modified only the standard facility order settings and did not affect individual favorite orders, which may help explain the differences in prescribing practices. While this study found an increase in 12-month prescriptions, there were no data on the actual number of supplies a patient received or on reimbursement.
The study by Uhm and colleagues showed that making a relatively simple change to default EMR orders can increase 12-month contraception prescribing and lead to greater patient-centered care. Evidence shows that providers and pharmacists are not necessarily aware of laws that require 12-month supply coverage and routinely prescribe smaller supplies.6,7,9 For clinicians in states that have these laws (FIGURE 1), we urge you to provide as full a supply of contraceptives as possible as this approach is both evidence based and patient centered. Although this study shows the benefit of universal system change to the EMR, individual clinicians also must be sure to modify personal order preferences. In addition, pharmacists can play an important role by updating policies that comply with these laws and by increasing pharmacy stocks of contraception supplies.7 For those living in states that do not currently have these laws, we encourage you to reach out to your legislators to advocate for similar laws as the data show clear medical and cost benefits for patients and society.
Continue to: Pharmacist prescription of hormonal contraception is safe and promotes continuation...
Pharmacist prescription of hormonal contraception is safe and promotes continuation
Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
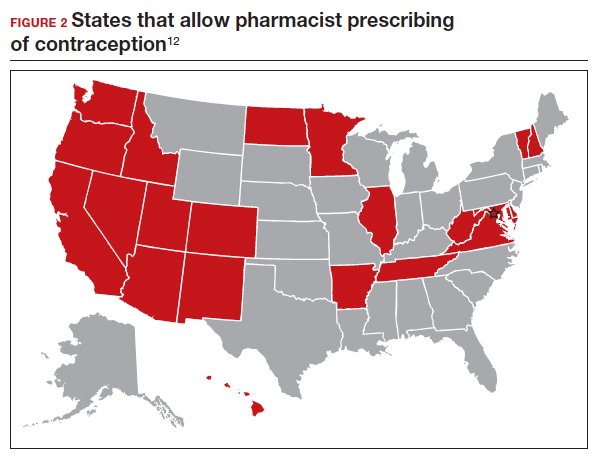
Patients often face difficulty obtaining both new and timely refills of self-administered contraception.10,11 To expand contraception access, Oregon became the first state (in 2016) to enact legislation to authorize direct pharmacist prescribing of hormonal contraceptives.12 Currently, 17 states and the District of Columbia have protocols for pharmacist prescribing privileges (FIGURE 2), and proposed legislation is pending in another 14 states.10,12 These protocols vary, but basic processes include screening, documentation, monitoring, and referrals when necessary. Typically, protocols require a pharmacist to review a patient’s medical history, pregnancy status, medication use, and blood pressure, followed by contraceptive counseling.10 Pharmacies are generally located in the community they serve, have extended hours, and usually do not require an appointment.8,13,14
Pharmacist prescribing increases the number of new contraceptive users, and pharmacists are more likely to prescribe a 6-month or longer supply of contraceptives compared with clinicians.8,13,15 Also, pharmacist prescribing is safe, with adherence rates to the CDC’s US Medical Eligibility Criteria for Contraceptive Use similar to those of prescriptions provided by a clinician.13
Authors of a recent multi-state study further assessed the impact of pharmacist prescribing by evaluating 12-month continuation and perfect use rates.
Study design
Rodriguez and colleagues evaluated the results of a 1-year prospective cohort study conducted in 2019 that included 388 participants who sought contraception in California, Colorado, Hawaii, and Oregon. All these states had laws permitting pharmacist prescribing and 12-month supply of hormonal contraception. Participants received prescriptions directly from a pharmacist at 1 of 139 pharmacies (n = 149) or filled a prescription provided by a clinician (n = 239). The primary outcomes were continuation of an effective method and perfect use of contraception across 12 months.
Participant demographics were similar between the 2 groups except for education and insurance status. Participants who received a prescription from a clinician reported higher levels of education. A greater proportion of uninsured participants received a prescription from a pharmacist (11%) compared with from a clinician (3%).
Contraceptive continuation rates
Participants were surveyed 3 times during the 12-month study about their current contraceptive method, if they had switched methods, or if they had any missed days of contraception.
Overall, 340 participants (88%) completed a full 12 months of follow-up. Continuation rates were similar between the 2 groups: 89% in the clinician-prescribed and 90% in the pharmacist-prescribed group (P=.86). Participants in the 2 groups also reported similar rates of perfect use (no missed days: 54% and 47%, respectively [P=.69]). Additionally, the authors reported that 29 participants changed from a tier 2 (pill, patch, ring, injection) to a tier 1 (intrauterine device or implant) method during follow-up, with no difference in switch rates for participants who received care from a clinician (10%) or a pharmacist (7%).
Patients have difficulties in obtaining both an initial contraceptive prescription and refills in time to avoid breaks in coverage.16 Pharmacist prescription of contraception is a proven strategy to increase access to contraception for new users or to promote continuation among current users. This practice is evidence based, decreases unintended pregnancy rates, and is safe.8,13,15,17
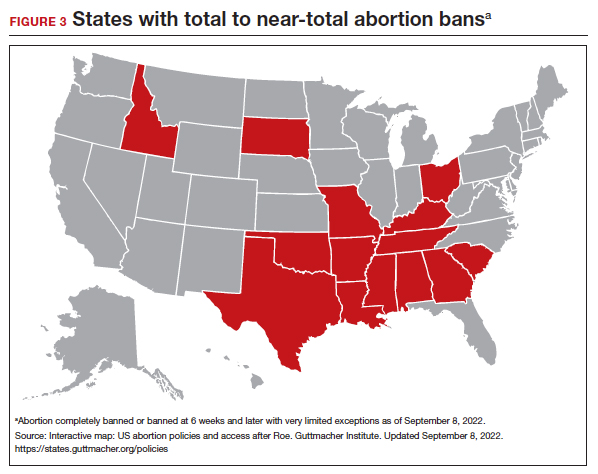
Promoting universal pharmacist prescribing is even more important given the overruling of Roe v Wade. With abortion restrictions, many family planning clinics that also play a vital role in providing contraception will close. Most states that are limiting abortion care (FIGURE 3) are the same states without pharmacist-prescribing provisions (FIGURE 2). As patient advocates, we need to continue to support this evidence-based practice in states where it is available and push legislators in states where it is not. Pharmacists should receive support to complete the training and certification needed to not only provide this service but also to receive appropriate reimbursements. Restrictions, such as requiring patients to be 18 years or older or to have prior consultation with a physician, should be limited as these are not necessary to provide self-administered contraception safely. Clinicians and pharmacists should inform patients, in states where this is available, that they can access initial or refill prescriptions at their local pharmacy if that is more convenient or their preference. Clinicians who live in states without these laws can advocate for their community by encouraging their legislators to pass laws that allow this evidence-based practice.
Continue to: LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use...
LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
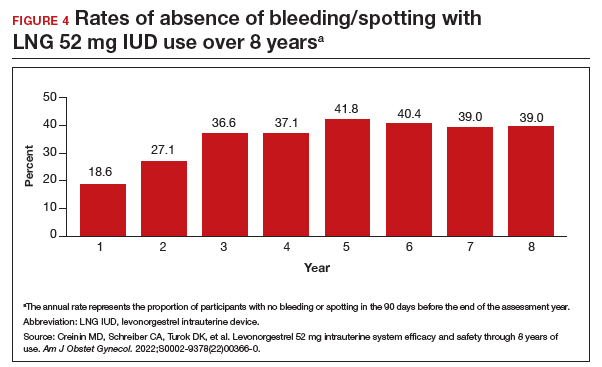
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●
As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.
- Kirstein M, Jones RK, Philbin J. One month post-Roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. July 28, 2022. Accessed September 14, 2022. https://www.guttmacher.org /article/2022/07/one-month-post-roe-least-43-abortion-clinics-across -11-states-have-stopped-offering
- Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
- Foster DG, Parvataneni R, de Bocanegra HT, et al. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107-114.
- Niu F, Cornelius J, Aboubechara N, et al. Real world outcomes related to providing an annual supply of short-acting hormonal contraceptives. Contraception. 2022;107:58-61.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Women’s sexual and reproductive health services: key findings from the 2017 Kaiser Women’s Health Survey. KFF: Kaiser Family Foundation. March 13, 2018. Accessed September 14, 2022. https://www.kff.org/womens-health-policy /issue-brief/womens-sexual-and-reproductive-health-services-key-findings -from-the-2017-kaiser-womens-health-survey/
- Nikpour G, Allen A, Rafie S, et al. Pharmacy implementation of a new law allowing year-long hormonal contraception supplies. Pharmacy (Basel). 2020;8:E165.
- Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3:e205252.
- Insurance coverage of contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state-policy /explore/insurance-coverage-contraceptives
- Chim C, Sharma P. Pharmacists prescribing hormonal contraceptives: a status update. US Pharm. 2021;46:45-49.
- Rodriguez MI, Hersh A, Anderson LB, et al. Association of pharmacist prescription of hormonal contraception with unintended pregnancies and Medicaid costs. Obstet Gynecol. 2019;133:1238-1246.
- Pharmacist-prescribed contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state -policy/explore/pharmacist-prescribed-contraceptives
- Anderson L, Hartung DM, Middleton L, et al. Pharmacist provision of hormonal contraception in the Oregon Medicaid population. Obstet Gynecol. 2019;133:1231-1237.
- Rodriguez MI, Edelman AB, Skye M, et al. Reasons for and experience in obtaining pharmacist prescribed contraception. Contraception. 2020;102:259-261.
- Rodriguez MI, Manibusan B, Kaufman M, et al. Association of pharmacist prescription of contraception with breaks in coverage. Obstet Gynecol. 2022;139:781-787.
- Pittman ME, Secura GM, Allsworth JE, et al. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011;83:340-345.
- Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
- Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-7.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52 mg intrauterine system. Contraception. 2020;101:159-161.
On June 24, 2022, the US Supreme Court ruled in Dobbs v Jackson to overturn the landmark Roe v Wade decision, deeming that abortion is not protected by statutes that provide the right to privacy, liberty, or autonomy. With this historic ruling, other rights founded on the same principles, including the freedom to use contraception, may be called into question in the future. Clinics that provide abortion care typically play a vital role in providing contraception services. Due to abortion restriction across the country, many of these clinics are predicted to close and many have already closed. Within one month of the Dobbs decision, 43 clinics in 11 states had shut their doors to patients, reducing access to basic contraception services.1 It is more important now than ever that clinicians address barriers and lead the effort to improve and ensure that patients have access to contraceptive services.
In this Update, we review recent evidence that may help aid patients in obtaining contraception more easily and for longer periods of time. We review strategies demonstrated to improve contraceptive access, including how to increase prescribing rates of 1-year contraceptive supplies and pharmacist-prescribed contraception. We also review new data on extended use of the levonorgestrel 52 mg intrauterine device (LNG 52 mg IUD).
One-year prescribing of hormonal contraception decreases an access barrier
Uhm S, Chen MJ, Cutler ED, et al. Twelve-month prescribing of contraceptive pill, patch, and ring before and after a standardized electronic medical record order change. Contraception. 2021;103:60-63.
Providing a 1-year supply of self-administered contraception can lead to higher likelihood of continued use and is associated with reduced cost, unintended pregnancy, and abortion rates.2-4 Although some patients may not use a full year’s supply of pills, rings, or patches under such programs, the lower rates of unintended pregnancy result in significant cost savings as compared with the unused contraceptives.2,3 Accordingly, the Centers for Disease Control and Prevention (CDC) advises dispensing a 1-year supply of self-administered hormonal contraception.5 Insurance coverage and providers’ prescribing practices can be barriers to patients obtaining a year’s supply of hormonal contraception. Currently, 18 states and the District of Columbia legally require insurers to cover a 12-month supply of prescription contraceptives (FIGURE 1). Despite these laws and the CDC recommendation, studies show that most people continue to receive only a 1- to 3-month supply.6-8 One strategy to increase the number of 1-year supplies of self-administered contraception is institutional changes to default prescription orders.

Study design
In California, legislation enacted in January 2017 required commercial and medical assistance health plans to cover up to 12 months of US Food and Drug Administration (FDA)-approved self-administered hormonal contraceptives dispensed at 1 time as prescribed or requested. To better serve patients, a multidisciplinary team from the University of California Davis Health worked with the institution’s pharmacy to institute an electronic medical record (EMR) default order change from dispensing 1-month with refills to dispensing 12-month quantities for all combined and progestin-only pills, patches, and rings on formulary.
After this EMR order change in December 2019, Uhm and colleagues conducted a retrospective pre-post study using outpatient prescription data that included nearly 5,000 contraceptive pill, patch, and ring prescriptions over an 8-month period. They compared the frequency of 12-month prescriptions for each of these methods 4 months before and 4 months after the default order change. They compared the proportion of 12-month prescriptions by prescriber department affiliation and by clinic location. Department affiliation was categorized as obstetrics-gynecology or non–obstetrics-gynecology. Clinic location was categorized as medical center campus or community clinics.
Increase in 12-month prescriptions
The authors found an overall increase in 12-month prescriptions, from 11% to 27%, after the EMR order change. Prescribers at the medical center campus clinics more frequently ordered a 12-month supply compared with prescribers at community clinics both before (33% vs 4%, respectively) and after (53% vs 19%, respectively) the EMR change. The only group of providers without a significant increase in 12-month prescriptions was among obstetrics-gynecology providers at community clinics (4% before vs 6% after).
The system EMR change modified only the standard facility order settings and did not affect individual favorite orders, which may help explain the differences in prescribing practices. While this study found an increase in 12-month prescriptions, there were no data on the actual number of supplies a patient received or on reimbursement.
The study by Uhm and colleagues showed that making a relatively simple change to default EMR orders can increase 12-month contraception prescribing and lead to greater patient-centered care. Evidence shows that providers and pharmacists are not necessarily aware of laws that require 12-month supply coverage and routinely prescribe smaller supplies.6,7,9 For clinicians in states that have these laws (FIGURE 1), we urge you to provide as full a supply of contraceptives as possible as this approach is both evidence based and patient centered. Although this study shows the benefit of universal system change to the EMR, individual clinicians also must be sure to modify personal order preferences. In addition, pharmacists can play an important role by updating policies that comply with these laws and by increasing pharmacy stocks of contraception supplies.7 For those living in states that do not currently have these laws, we encourage you to reach out to your legislators to advocate for similar laws as the data show clear medical and cost benefits for patients and society.
Continue to: Pharmacist prescription of hormonal contraception is safe and promotes continuation...
Pharmacist prescription of hormonal contraception is safe and promotes continuation
Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
Patients often face difficulty obtaining both new and timely refills of self-administered contraception.10,11 To expand contraception access, Oregon became the first state (in 2016) to enact legislation to authorize direct pharmacist prescribing of hormonal contraceptives.12 Currently, 17 states and the District of Columbia have protocols for pharmacist prescribing privileges (FIGURE 2), and proposed legislation is pending in another 14 states.10,12 These protocols vary, but basic processes include screening, documentation, monitoring, and referrals when necessary. Typically, protocols require a pharmacist to review a patient’s medical history, pregnancy status, medication use, and blood pressure, followed by contraceptive counseling.10 Pharmacies are generally located in the community they serve, have extended hours, and usually do not require an appointment.8,13,14

Pharmacist prescribing increases the number of new contraceptive users, and pharmacists are more likely to prescribe a 6-month or longer supply of contraceptives compared with clinicians.8,13,15 Also, pharmacist prescribing is safe, with adherence rates to the CDC’s US Medical Eligibility Criteria for Contraceptive Use similar to those of prescriptions provided by a clinician.13
Authors of a recent multi-state study further assessed the impact of pharmacist prescribing by evaluating 12-month continuation and perfect use rates.
Study design
Rodriguez and colleagues evaluated the results of a 1-year prospective cohort study conducted in 2019 that included 388 participants who sought contraception in California, Colorado, Hawaii, and Oregon. All these states had laws permitting pharmacist prescribing and 12-month supply of hormonal contraception. Participants received prescriptions directly from a pharmacist at 1 of 139 pharmacies (n = 149) or filled a prescription provided by a clinician (n = 239). The primary outcomes were continuation of an effective method and perfect use of contraception across 12 months.
Participant demographics were similar between the 2 groups except for education and insurance status. Participants who received a prescription from a clinician reported higher levels of education. A greater proportion of uninsured participants received a prescription from a pharmacist (11%) compared with from a clinician (3%).
Contraceptive continuation rates
Participants were surveyed 3 times during the 12-month study about their current contraceptive method, if they had switched methods, or if they had any missed days of contraception.
Overall, 340 participants (88%) completed a full 12 months of follow-up. Continuation rates were similar between the 2 groups: 89% in the clinician-prescribed and 90% in the pharmacist-prescribed group (P=.86). Participants in the 2 groups also reported similar rates of perfect use (no missed days: 54% and 47%, respectively [P=.69]). Additionally, the authors reported that 29 participants changed from a tier 2 (pill, patch, ring, injection) to a tier 1 (intrauterine device or implant) method during follow-up, with no difference in switch rates for participants who received care from a clinician (10%) or a pharmacist (7%).

Patients have difficulties in obtaining both an initial contraceptive prescription and refills in time to avoid breaks in coverage.16 Pharmacist prescription of contraception is a proven strategy to increase access to contraception for new users or to promote continuation among current users. This practice is evidence based, decreases unintended pregnancy rates, and is safe.8,13,15,17
Promoting universal pharmacist prescribing is even more important given the overruling of Roe v Wade. With abortion restrictions, many family planning clinics that also play a vital role in providing contraception will close. Most states that are limiting abortion care (FIGURE 3) are the same states without pharmacist-prescribing provisions (FIGURE 2). As patient advocates, we need to continue to support this evidence-based practice in states where it is available and push legislators in states where it is not. Pharmacists should receive support to complete the training and certification needed to not only provide this service but also to receive appropriate reimbursements. Restrictions, such as requiring patients to be 18 years or older or to have prior consultation with a physician, should be limited as these are not necessary to provide self-administered contraception safely. Clinicians and pharmacists should inform patients, in states where this is available, that they can access initial or refill prescriptions at their local pharmacy if that is more convenient or their preference. Clinicians who live in states without these laws can advocate for their community by encouraging their legislators to pass laws that allow this evidence-based practice.
Continue to: LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use...
LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●

As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.
On June 24, 2022, the US Supreme Court ruled in Dobbs v Jackson to overturn the landmark Roe v Wade decision, deeming that abortion is not protected by statutes that provide the right to privacy, liberty, or autonomy. With this historic ruling, other rights founded on the same principles, including the freedom to use contraception, may be called into question in the future. Clinics that provide abortion care typically play a vital role in providing contraception services. Due to abortion restriction across the country, many of these clinics are predicted to close and many have already closed. Within one month of the Dobbs decision, 43 clinics in 11 states had shut their doors to patients, reducing access to basic contraception services.1 It is more important now than ever that clinicians address barriers and lead the effort to improve and ensure that patients have access to contraceptive services.
In this Update, we review recent evidence that may help aid patients in obtaining contraception more easily and for longer periods of time. We review strategies demonstrated to improve contraceptive access, including how to increase prescribing rates of 1-year contraceptive supplies and pharmacist-prescribed contraception. We also review new data on extended use of the levonorgestrel 52 mg intrauterine device (LNG 52 mg IUD).
One-year prescribing of hormonal contraception decreases an access barrier
Uhm S, Chen MJ, Cutler ED, et al. Twelve-month prescribing of contraceptive pill, patch, and ring before and after a standardized electronic medical record order change. Contraception. 2021;103:60-63.
Providing a 1-year supply of self-administered contraception can lead to higher likelihood of continued use and is associated with reduced cost, unintended pregnancy, and abortion rates.2-4 Although some patients may not use a full year’s supply of pills, rings, or patches under such programs, the lower rates of unintended pregnancy result in significant cost savings as compared with the unused contraceptives.2,3 Accordingly, the Centers for Disease Control and Prevention (CDC) advises dispensing a 1-year supply of self-administered hormonal contraception.5 Insurance coverage and providers’ prescribing practices can be barriers to patients obtaining a year’s supply of hormonal contraception. Currently, 18 states and the District of Columbia legally require insurers to cover a 12-month supply of prescription contraceptives (FIGURE 1). Despite these laws and the CDC recommendation, studies show that most people continue to receive only a 1- to 3-month supply.6-8 One strategy to increase the number of 1-year supplies of self-administered contraception is institutional changes to default prescription orders.

Study design
In California, legislation enacted in January 2017 required commercial and medical assistance health plans to cover up to 12 months of US Food and Drug Administration (FDA)-approved self-administered hormonal contraceptives dispensed at 1 time as prescribed or requested. To better serve patients, a multidisciplinary team from the University of California Davis Health worked with the institution’s pharmacy to institute an electronic medical record (EMR) default order change from dispensing 1-month with refills to dispensing 12-month quantities for all combined and progestin-only pills, patches, and rings on formulary.
After this EMR order change in December 2019, Uhm and colleagues conducted a retrospective pre-post study using outpatient prescription data that included nearly 5,000 contraceptive pill, patch, and ring prescriptions over an 8-month period. They compared the frequency of 12-month prescriptions for each of these methods 4 months before and 4 months after the default order change. They compared the proportion of 12-month prescriptions by prescriber department affiliation and by clinic location. Department affiliation was categorized as obstetrics-gynecology or non–obstetrics-gynecology. Clinic location was categorized as medical center campus or community clinics.
Increase in 12-month prescriptions
The authors found an overall increase in 12-month prescriptions, from 11% to 27%, after the EMR order change. Prescribers at the medical center campus clinics more frequently ordered a 12-month supply compared with prescribers at community clinics both before (33% vs 4%, respectively) and after (53% vs 19%, respectively) the EMR change. The only group of providers without a significant increase in 12-month prescriptions was among obstetrics-gynecology providers at community clinics (4% before vs 6% after).
The system EMR change modified only the standard facility order settings and did not affect individual favorite orders, which may help explain the differences in prescribing practices. While this study found an increase in 12-month prescriptions, there were no data on the actual number of supplies a patient received or on reimbursement.
The study by Uhm and colleagues showed that making a relatively simple change to default EMR orders can increase 12-month contraception prescribing and lead to greater patient-centered care. Evidence shows that providers and pharmacists are not necessarily aware of laws that require 12-month supply coverage and routinely prescribe smaller supplies.6,7,9 For clinicians in states that have these laws (FIGURE 1), we urge you to provide as full a supply of contraceptives as possible as this approach is both evidence based and patient centered. Although this study shows the benefit of universal system change to the EMR, individual clinicians also must be sure to modify personal order preferences. In addition, pharmacists can play an important role by updating policies that comply with these laws and by increasing pharmacy stocks of contraception supplies.7 For those living in states that do not currently have these laws, we encourage you to reach out to your legislators to advocate for similar laws as the data show clear medical and cost benefits for patients and society.
Continue to: Pharmacist prescription of hormonal contraception is safe and promotes continuation...
Pharmacist prescription of hormonal contraception is safe and promotes continuation
Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
Patients often face difficulty obtaining both new and timely refills of self-administered contraception.10,11 To expand contraception access, Oregon became the first state (in 2016) to enact legislation to authorize direct pharmacist prescribing of hormonal contraceptives.12 Currently, 17 states and the District of Columbia have protocols for pharmacist prescribing privileges (FIGURE 2), and proposed legislation is pending in another 14 states.10,12 These protocols vary, but basic processes include screening, documentation, monitoring, and referrals when necessary. Typically, protocols require a pharmacist to review a patient’s medical history, pregnancy status, medication use, and blood pressure, followed by contraceptive counseling.10 Pharmacies are generally located in the community they serve, have extended hours, and usually do not require an appointment.8,13,14

Pharmacist prescribing increases the number of new contraceptive users, and pharmacists are more likely to prescribe a 6-month or longer supply of contraceptives compared with clinicians.8,13,15 Also, pharmacist prescribing is safe, with adherence rates to the CDC’s US Medical Eligibility Criteria for Contraceptive Use similar to those of prescriptions provided by a clinician.13
Authors of a recent multi-state study further assessed the impact of pharmacist prescribing by evaluating 12-month continuation and perfect use rates.
Study design
Rodriguez and colleagues evaluated the results of a 1-year prospective cohort study conducted in 2019 that included 388 participants who sought contraception in California, Colorado, Hawaii, and Oregon. All these states had laws permitting pharmacist prescribing and 12-month supply of hormonal contraception. Participants received prescriptions directly from a pharmacist at 1 of 139 pharmacies (n = 149) or filled a prescription provided by a clinician (n = 239). The primary outcomes were continuation of an effective method and perfect use of contraception across 12 months.
Participant demographics were similar between the 2 groups except for education and insurance status. Participants who received a prescription from a clinician reported higher levels of education. A greater proportion of uninsured participants received a prescription from a pharmacist (11%) compared with from a clinician (3%).
Contraceptive continuation rates
Participants were surveyed 3 times during the 12-month study about their current contraceptive method, if they had switched methods, or if they had any missed days of contraception.
Overall, 340 participants (88%) completed a full 12 months of follow-up. Continuation rates were similar between the 2 groups: 89% in the clinician-prescribed and 90% in the pharmacist-prescribed group (P=.86). Participants in the 2 groups also reported similar rates of perfect use (no missed days: 54% and 47%, respectively [P=.69]). Additionally, the authors reported that 29 participants changed from a tier 2 (pill, patch, ring, injection) to a tier 1 (intrauterine device or implant) method during follow-up, with no difference in switch rates for participants who received care from a clinician (10%) or a pharmacist (7%).

Patients have difficulties in obtaining both an initial contraceptive prescription and refills in time to avoid breaks in coverage.16 Pharmacist prescription of contraception is a proven strategy to increase access to contraception for new users or to promote continuation among current users. This practice is evidence based, decreases unintended pregnancy rates, and is safe.8,13,15,17
Promoting universal pharmacist prescribing is even more important given the overruling of Roe v Wade. With abortion restrictions, many family planning clinics that also play a vital role in providing contraception will close. Most states that are limiting abortion care (FIGURE 3) are the same states without pharmacist-prescribing provisions (FIGURE 2). As patient advocates, we need to continue to support this evidence-based practice in states where it is available and push legislators in states where it is not. Pharmacists should receive support to complete the training and certification needed to not only provide this service but also to receive appropriate reimbursements. Restrictions, such as requiring patients to be 18 years or older or to have prior consultation with a physician, should be limited as these are not necessary to provide self-administered contraception safely. Clinicians and pharmacists should inform patients, in states where this is available, that they can access initial or refill prescriptions at their local pharmacy if that is more convenient or their preference. Clinicians who live in states without these laws can advocate for their community by encouraging their legislators to pass laws that allow this evidence-based practice.
Continue to: LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use...
LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●

As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.
- Kirstein M, Jones RK, Philbin J. One month post-Roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. July 28, 2022. Accessed September 14, 2022. https://www.guttmacher.org /article/2022/07/one-month-post-roe-least-43-abortion-clinics-across -11-states-have-stopped-offering
- Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
- Foster DG, Parvataneni R, de Bocanegra HT, et al. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107-114.
- Niu F, Cornelius J, Aboubechara N, et al. Real world outcomes related to providing an annual supply of short-acting hormonal contraceptives. Contraception. 2022;107:58-61.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Women’s sexual and reproductive health services: key findings from the 2017 Kaiser Women’s Health Survey. KFF: Kaiser Family Foundation. March 13, 2018. Accessed September 14, 2022. https://www.kff.org/womens-health-policy /issue-brief/womens-sexual-and-reproductive-health-services-key-findings -from-the-2017-kaiser-womens-health-survey/
- Nikpour G, Allen A, Rafie S, et al. Pharmacy implementation of a new law allowing year-long hormonal contraception supplies. Pharmacy (Basel). 2020;8:E165.
- Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3:e205252.
- Insurance coverage of contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state-policy /explore/insurance-coverage-contraceptives
- Chim C, Sharma P. Pharmacists prescribing hormonal contraceptives: a status update. US Pharm. 2021;46:45-49.
- Rodriguez MI, Hersh A, Anderson LB, et al. Association of pharmacist prescription of hormonal contraception with unintended pregnancies and Medicaid costs. Obstet Gynecol. 2019;133:1238-1246.
- Pharmacist-prescribed contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state -policy/explore/pharmacist-prescribed-contraceptives
- Anderson L, Hartung DM, Middleton L, et al. Pharmacist provision of hormonal contraception in the Oregon Medicaid population. Obstet Gynecol. 2019;133:1231-1237.
- Rodriguez MI, Edelman AB, Skye M, et al. Reasons for and experience in obtaining pharmacist prescribed contraception. Contraception. 2020;102:259-261.
- Rodriguez MI, Manibusan B, Kaufman M, et al. Association of pharmacist prescription of contraception with breaks in coverage. Obstet Gynecol. 2022;139:781-787.
- Pittman ME, Secura GM, Allsworth JE, et al. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011;83:340-345.
- Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
- Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-7.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52 mg intrauterine system. Contraception. 2020;101:159-161.
- Kirstein M, Jones RK, Philbin J. One month post-Roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. July 28, 2022. Accessed September 14, 2022. https://www.guttmacher.org /article/2022/07/one-month-post-roe-least-43-abortion-clinics-across -11-states-have-stopped-offering
- Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
- Foster DG, Parvataneni R, de Bocanegra HT, et al. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107-114.
- Niu F, Cornelius J, Aboubechara N, et al. Real world outcomes related to providing an annual supply of short-acting hormonal contraceptives. Contraception. 2022;107:58-61.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Women’s sexual and reproductive health services: key findings from the 2017 Kaiser Women’s Health Survey. KFF: Kaiser Family Foundation. March 13, 2018. Accessed September 14, 2022. https://www.kff.org/womens-health-policy /issue-brief/womens-sexual-and-reproductive-health-services-key-findings -from-the-2017-kaiser-womens-health-survey/
- Nikpour G, Allen A, Rafie S, et al. Pharmacy implementation of a new law allowing year-long hormonal contraception supplies. Pharmacy (Basel). 2020;8:E165.
- Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3:e205252.
- Insurance coverage of contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state-policy /explore/insurance-coverage-contraceptives
- Chim C, Sharma P. Pharmacists prescribing hormonal contraceptives: a status update. US Pharm. 2021;46:45-49.
- Rodriguez MI, Hersh A, Anderson LB, et al. Association of pharmacist prescription of hormonal contraception with unintended pregnancies and Medicaid costs. Obstet Gynecol. 2019;133:1238-1246.
- Pharmacist-prescribed contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state -policy/explore/pharmacist-prescribed-contraceptives
- Anderson L, Hartung DM, Middleton L, et al. Pharmacist provision of hormonal contraception in the Oregon Medicaid population. Obstet Gynecol. 2019;133:1231-1237.
- Rodriguez MI, Edelman AB, Skye M, et al. Reasons for and experience in obtaining pharmacist prescribed contraception. Contraception. 2020;102:259-261.
- Rodriguez MI, Manibusan B, Kaufman M, et al. Association of pharmacist prescription of contraception with breaks in coverage. Obstet Gynecol. 2022;139:781-787.
- Pittman ME, Secura GM, Allsworth JE, et al. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011;83:340-345.
- Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
- Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-7.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52 mg intrauterine system. Contraception. 2020;101:159-161.