User login
2023 Update on contraception
More US women are using IUDs than ever before. With more use comes the potential for complications and more requests related to non-contraceptive benefits. New information provides contemporary insight into rare IUD complications and the use of hormonal IUDs for treatment of HMB.
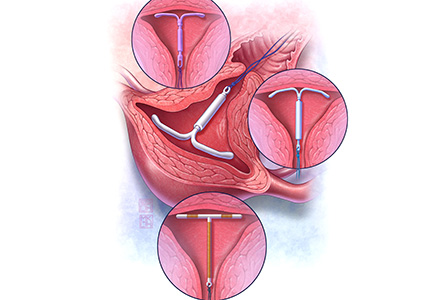
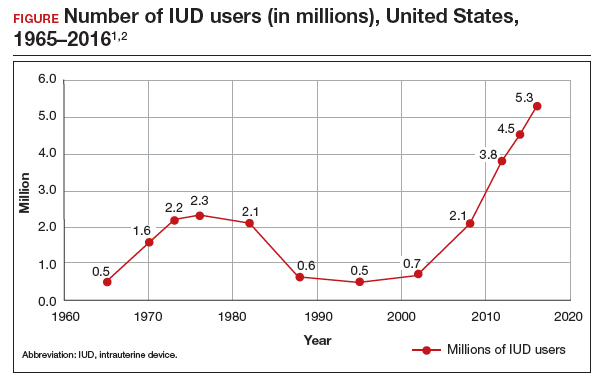
The first intrauterine device (IUD) to be approved in the United States, the Lippes Loop, became available in 1964. Sixty years later, more US women are using IUDs than ever before, and numbers are trending upward (FIGURE).1,2 Over the past year, contemporary information has become available to further inform IUD management when pregnancy occurs with an IUD in situ, as well as counseling about device breakage. Additionally, new data help clinicians expand which patients can use a levonorgestrel (LNG) 52-mg IUD for heavy menstrual bleeding (HMB) treatment.
As the total absolute number of IUD users increases, so do the absolute numbers of rare outcomes, such as pregnancy among IUD users. These highly effective contraceptives have a failure rate within the first year after placement ranging from 0.1% for the LNG 52-mg IUD to 0.8% for the copper 380-mm2 IUD.3 Although the possibility for extrauterine gestation is higher when pregnancy occurs while a patient is using an IUD as compared with most other contraceptive methods, most pregnancies that occur with an IUD in situ are intrauterine.4
The high contraceptive efficacy of IUDs make pregnancy with a retained IUD rare; therefore, it is difficult to perform a study with a large enough population to evaluate management of pregnancy complicated by an IUD in situ. Clinical management recommendations for these situations are 20 years old and are supported by limited data from case reports and series with fewer than 200 patients.5,6
Intrauterine device breakage is another rare event that is poorly understood due to the low absolute number of cases. Information about breakage has similarly been limited to case reports and case series.7,8 This past year, contemporary data were published to provide more insight into both intrauterine pregnancy with an IUD in situ and IUD breakage.
Beyond contraception, hormonal IUDs have become a popular and evidence-based treatment option for patients with HMB. The initial LNG 52-mg IUD (Mirena) regulatory approval studies for HMB treatment included data limited to parous patients and users with a body mass index (BMI) less than 35 kg/m2.9 Since that time, no studies have explored these populations. Although current practice has commonly extended use to include patients with these characteristics, we have lacked outcome data. New phase 3 data on the LNG 52-mg IUD (Liletta) included a broader range of participants and provide evidence to support this practice.
Removing retained copper 380-mm2 IUDs improves pregnancy outcomes
Panchal VR, Rau AR, Mandelbaum RS, et al. Pregnancy with retained intrauterine device: national-level assessment of characteristics and outcomes. Am J Obstet Gynecol MFM. 2023;5:101056. doi:10.1016/j.ajogmf.2023.101056
Karakuş SS, Karakuş R, Akalın EE, et al. Pregnancy outcomes with a copper 380 mm2 intrauterine device in place: a retrospective cohort study in Turkey, 2011-2021. Contraception. 2023;125:110090. doi:10.1016/j.contraception.2023.110090
To update our understanding of outcomes of pregnancy with an IUD in situ, Panchal and colleagues performed a cross-sectional study using the Healthcare Cost and Utilization Project’s National Inpatient Sample. This data set represents 85% of US hospital discharges. The population investigated included hospital deliveries from 2016 to 2020 with an ICD-10 (International Classification of Diseases, Tenth Revision) code of retained IUD. Those without the code were assigned to the comparison non-retained IUD group.
The primary outcome studied was the incidence rate of retained IUD, patient and pregnancy characteristics, and delivery outcomes including but not limited to gestational age at delivery, placental abnormalities, intrauterine fetal demise (IUFD), preterm premature rupture of membranes (PPROM), cesarean delivery, postpartum hemorrhage, and hysterectomy.
Outcomes were worse with retained IUD, regardless of IUD removal status
The authors found that an IUD in situ was reported in 1 out of 8,307 pregnancies and was associated with PPROM, fetal malpresentation, IUFD, placental abnormalities including abruption, accreta spectrum, retained placenta, and need for manual removal (TABLE 1). About three-quarters (76.3%) of patients had a term delivery (≥37 weeks).
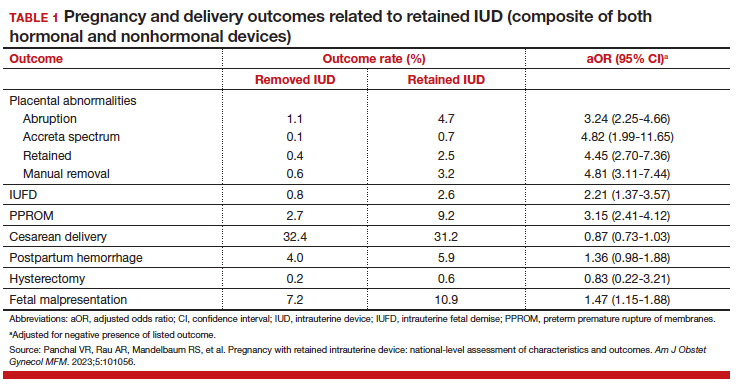
Retained IUD was associated with previable loss, defined as less than 22 weeks’ gestation (adjusted odds ratio [aOR], 5.49; 95% confidence interval [CI], 3.30–9.15) and periviable delivery, defined as 22 to 25 weeks’ gestation (aOR, 2.81; 95% CI, 1.63–4.85). Retained IUD was not associated with preterm delivery beyond 26 weeks’ gestation, cesarean delivery, postpartum hemorrhage, or hysterectomy.
Important limitations of this study are the lack of information on IUD type (copper vs hormonal) and the timing of removal or attempted removal in relation to measured pregnancy outcomes.
Continue to: Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes...
Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes
Karakus and colleagues conducted a retrospective cohort study of 233 patients in Turkey with pregnancies that occurred during copper 380-mm2 IUD use from 2011 to 2021. The authors reported that, at the time of first contact with the health system and diagnosis of retained IUD, 18.9% of the pregnancies were ectopic, 13.2% were first trimester losses, and 67.5% were ongoing pregnancies.
The authors assessed outcomes in patients with ongoing pregnancies based on whether or not the IUD was removed or retained. Outcomes included gestational age at delivery and adverse pregnancy outcomes, assessed as a composite of preterm delivery, PPROM, chorioamnionitis, placental abruption, and postpartum hemorrhage.
Of those with ongoing pregnancies, 13.3% chose to have an abortion, leaving 137 (86.7%) with continuing pregnancy. The IUD was able to be removed in 39.4% of the sample, with an average gestational age of 7 weeks at the time of removal.
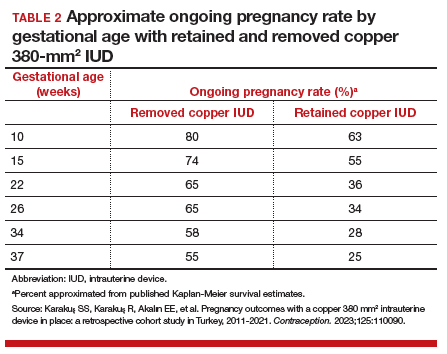
Compared with those with a retained IUD, patients in the removal group had a lower rate of pregnancy loss (33.3% vs 61.4%; P<.001) and a lower rate of the composite adverse pregnancy outcomes (53.1% vs 27.8%; P=.03). TABLE 2 shows the approximate rate of ongoing pregnancy by gestational age in patients with retained and removed copper 380-mm2 IUDs. Notably, the largest change occurred periviably, with the proportion of patients with an ongoing pregnancy after 26 weeks reducing to about half for patients with a retained IUD as compared with patients with a removed IUD; this proportion of ongoing pregnancies held through the remainder of gestation.
These studies confirm that a retained IUD is a rare outcome, occurring in about 1 in 8,000 pregnancies. Previous US national data from 2010 reported a similar incidence of 1 in 6,203 pregnancies (0.02%).10 Management and counseling depend on the patient’s desire to continue the pregnancy, gestational age, intrauterine IUD location, and ability to see the IUD strings. Contemporary data support management practices created from limited and outdated data, which include device removal (if able) and counseling those who desire to continue pregnancy about high-risk pregnancy complications. Those with a retained IUD should be counseled about increased risk of preterm or previable delivery, IUFD, and placental abnormalities (including accreta spectrum and retained placenta). Specifically, these contemporary data highlight that, beyond approximately 26 weeks’ gestation, the pregnancy loss rate is not different for those with a retained or removed IUD. Obstetric care providers should feel confident in using this more nuanced risk of extreme preterm delivery when counseling future patients. Implications for antepartum care and delivery timing with a retained IUD have not yet been defined.
Do national data reveal more breakage reports for copper 380-mm2 or LNG IUDs?
Latack KR, Nguyen BT. Trends in copper versus hormonal intrauterine device breakage reporting within the United States’ Food and Drug Administration Adverse Event Reporting System. Contraception. 2023;118:109909. doi:10.1016/j.contraception.2022.10.011
Latack and Nguyen reviewed postmarket surveillance data of IUD adverse events in the US Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) from 1998 to 2022. The FAERS is a voluntary, or passive, reporting system.
Study findings
Of the approximately 170,000 IUD-related adverse events reported to the agency during the 24-year timeframe, 25.4% were for copper IUDs and 74.6% were for hormonal IUDs. Slightly more than 4,000 reports were specific for device breakage, which the authors grouped into copper (copper 380-mm2)and hormonal (LNG 52 mg, 19.5 mg, and 13.5 mg) IUDs.
The copper 380-mm2 IUD was 6.19 times more likely to have a breakage report than hormonal IUDs (9.6% vs 1.7%; 95% CI, 5.87–6.53).
The overall proportion of IUD-related adverse events reported to the FDA was about 25% for copper and 75% for hormonal IUDs; this proportion is similar to sales figures, which show that about 15% of IUDs sold in the United States are copper and 85% are hormonal.11 However, the proportion of breakage events reported to the FDA is the inverse, with about 6 times more breakage reports with copper than with hormonal IUDs. Because these data come from a passive reporting system, the true incidence of IUD breakage cannot be assessed. However, these findings should remind clinicians to inform patients about this rare occurrence during counseling at the time of placement and, especially, when preparing for copper IUD removal. As the absolute number of IUD users increases, clinicians may be more likely to encounter this relatively rare event.
Management of IUD breakage is based on expert opinion, and recommendations are varied, ranging from observation to removal using an IUD hook, alligator forceps, manual vacuum aspiration, or hysteroscopy.7,10 Importantly, each individual patient situation will vary depending on the presence or absence of other symptoms and whether or not future pregnancy is desired.
Continue to: Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients...
Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients
Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi:10.1097AOG.0000000000005137
Creinin and colleagues conducted a study for US regulatory product approval of the LNG 52-mg IUD (Liletta) for HMB. This multicenter phase 3 open-label clinical trial recruited nonpregnant participants aged 18 to 50 years with HMB at 29 clinical sites in the United States. No BMI cutoff was used.
Baseline menstrual flow data were obtained over 2 to 3 screening cycles by collection of menstrual products and quantification of blood loss using alkaline hematin measurement. Patients with 2 cycles with a blood loss exceeding 80 mL had an IUD placement, with similar flow evaluations during the third and sixth postplacement cycles.
Treatment success was defined as a reduction in blood loss by more than 50% as compared with baseline (during screening) and measured blood loss of less than 80 mL. The enrolled population (n=105) included 28% nulliparous users, with 49% and 28% of participants having a BMI of 30 kg/m2 or higher and higher than 35 kg/m2, respectively.
Treatment highly successful in reducing blood loss
Participants in this trial had a 93% and a 98% reduction in blood loss at the third and sixth cycles of use, respectively. Additionally, during the sixth cycle of use, 19% of users had no bleeding. Treatment success occurred in about 80% of participants overall and occurred regardless of parity or BMI.
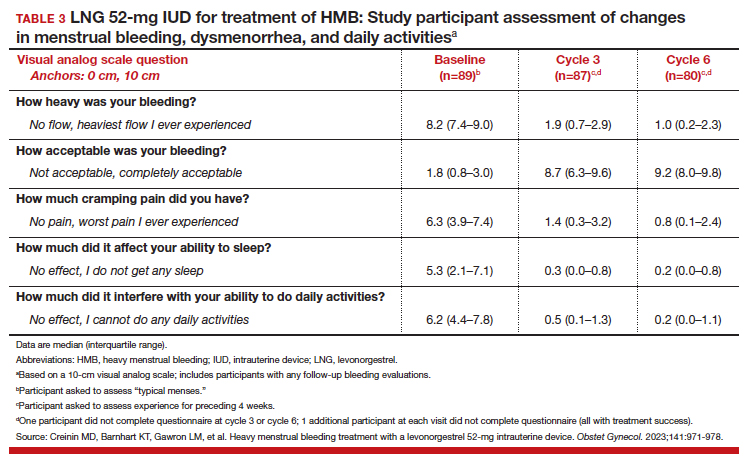
To assess a subjective measure of success, participants were asked to evaluate their menstrual bleeding and dysmenorrhea severity, acceptability, and overall impact on quality of life at 3 time points: during prior typical menses, cycle 3, and cycle 6. At cycle 6, all participants reported significantly improved acceptability of bleeding and uterine pain and, importantly, decreased overall menstrual interference with the ability to complete daily activities (TABLE 3).
IUD expulsion and replacement rates
Although bleeding greatly decreased in all participants, 13% (n=14) discontinued before cycle 6 due to expulsion or IUD-related symptoms, with the majority citing bleeding irregularities. Expulsion occurred in 9% (n=5) of users, with the majority (2/3) occurring in the first 3 months of use and more commonly in obese and/or parous users. About half of participants with expulsion had the IUD replaced during the study. ●
Interestingly, both LNG 52-mg IUDs have been approved in most countries throughout the world for HMB treatment, and only in the United States was one of the products (Liletta) not approved until this past year. The FDA required more stringent trials than had been previously performed for approval outside of the United States. However, a benefit for clinicians is that this phase 3 study provided data in a contemporary US population. Clinicians can feel confident in counseling and offering the LNG 52-mg IUD as a first-line treatment option for patients with HMB, including those who have never been pregnant or have a BMI greater than 35 kg/m2.
Importantly, though, clinicians should be realistic with all patients that this treatment, although highly effective, is not successful for about 20% of patients by about 6 months of use. For those in whom the treatment is beneficial, the quality-of-life improvement is dramatic. Additionally, this study reminds us that expulsion risk in a population primarily using the IUD for HMB, especially if also obese and/or parous, is higher in the first 6 months of use than patients using the method for contraception. Expulsion occurs in 1.6% of contraception users through 6 months of use.12 These data highlight that IUD expulsion risk is not a fixed number, but instead is modified by patient characteristics. Patients should be counseled regarding the appropriate expulsion risk and that the IUD can be safely replaced should expulsion occur.
- Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception. 2018;98:467470. doi:10.1016/j.contraception.2018.05.016
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93. doi:10.1016/j.xfre.2020.06.006
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:15.
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:185.
- Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430. doi:10.1016/j.contraception.2014.01.002
- Brahmi D, Steenland MW, Renner RM, et al. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131-139. doi:10.1016/j.contraception . 2011.06.010
- Wilson S, Tan G, Baylson M, et al. Controversies in family planning: how to manage a fractured IUD. Contraception. 2013;88:599-603. doi:10.1016/j.contraception.2013.07.007
- Fulkerson Schaeffer S, Gimovsky AC, Aly H, et al. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32:798-803. doi:10.1080/14767058.2017.1 391783
- Mirena. Prescribing information. Bayer HealthCare Pharmaceuticals. Accessed August 22, 2023. https://www .mirena-us.com/pi
- Myo MG, Nguyen BT. Intrauterine device complications and their management. Curr Obstet Gynecol Rep. 2023;12:88-95. doi.org/10.1007/s13669-023-00357-8
- National Center for Health Statistics (NCHS). 2017-2019 National Survey of Family Growth. Public-Use Data File Documentation. CDC National Center for Health Statistics. Accessed August 28, 2023. https://www.cdc.gov/nchs/data /nsfg/NSFG-2017-2019-UG-MainText-508.pdf
- Gilliam ML, Jensen JT, Eisenberg DL, et al. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception. 2021;103:444-449. doi: 10.1016/j.contraception.2021.02.013
More US women are using IUDs than ever before. With more use comes the potential for complications and more requests related to non-contraceptive benefits. New information provides contemporary insight into rare IUD complications and the use of hormonal IUDs for treatment of HMB.
The first intrauterine device (IUD) to be approved in the United States, the Lippes Loop, became available in 1964. Sixty years later, more US women are using IUDs than ever before, and numbers are trending upward (FIGURE).1,2 Over the past year, contemporary information has become available to further inform IUD management when pregnancy occurs with an IUD in situ, as well as counseling about device breakage. Additionally, new data help clinicians expand which patients can use a levonorgestrel (LNG) 52-mg IUD for heavy menstrual bleeding (HMB) treatment.

As the total absolute number of IUD users increases, so do the absolute numbers of rare outcomes, such as pregnancy among IUD users. These highly effective contraceptives have a failure rate within the first year after placement ranging from 0.1% for the LNG 52-mg IUD to 0.8% for the copper 380-mm2 IUD.3 Although the possibility for extrauterine gestation is higher when pregnancy occurs while a patient is using an IUD as compared with most other contraceptive methods, most pregnancies that occur with an IUD in situ are intrauterine.4
The high contraceptive efficacy of IUDs make pregnancy with a retained IUD rare; therefore, it is difficult to perform a study with a large enough population to evaluate management of pregnancy complicated by an IUD in situ. Clinical management recommendations for these situations are 20 years old and are supported by limited data from case reports and series with fewer than 200 patients.5,6
Intrauterine device breakage is another rare event that is poorly understood due to the low absolute number of cases. Information about breakage has similarly been limited to case reports and case series.7,8 This past year, contemporary data were published to provide more insight into both intrauterine pregnancy with an IUD in situ and IUD breakage.
Beyond contraception, hormonal IUDs have become a popular and evidence-based treatment option for patients with HMB. The initial LNG 52-mg IUD (Mirena) regulatory approval studies for HMB treatment included data limited to parous patients and users with a body mass index (BMI) less than 35 kg/m2.9 Since that time, no studies have explored these populations. Although current practice has commonly extended use to include patients with these characteristics, we have lacked outcome data. New phase 3 data on the LNG 52-mg IUD (Liletta) included a broader range of participants and provide evidence to support this practice.
Removing retained copper 380-mm2 IUDs improves pregnancy outcomes
Panchal VR, Rau AR, Mandelbaum RS, et al. Pregnancy with retained intrauterine device: national-level assessment of characteristics and outcomes. Am J Obstet Gynecol MFM. 2023;5:101056. doi:10.1016/j.ajogmf.2023.101056
Karakuş SS, Karakuş R, Akalın EE, et al. Pregnancy outcomes with a copper 380 mm2 intrauterine device in place: a retrospective cohort study in Turkey, 2011-2021. Contraception. 2023;125:110090. doi:10.1016/j.contraception.2023.110090
To update our understanding of outcomes of pregnancy with an IUD in situ, Panchal and colleagues performed a cross-sectional study using the Healthcare Cost and Utilization Project’s National Inpatient Sample. This data set represents 85% of US hospital discharges. The population investigated included hospital deliveries from 2016 to 2020 with an ICD-10 (International Classification of Diseases, Tenth Revision) code of retained IUD. Those without the code were assigned to the comparison non-retained IUD group.
The primary outcome studied was the incidence rate of retained IUD, patient and pregnancy characteristics, and delivery outcomes including but not limited to gestational age at delivery, placental abnormalities, intrauterine fetal demise (IUFD), preterm premature rupture of membranes (PPROM), cesarean delivery, postpartum hemorrhage, and hysterectomy.
Outcomes were worse with retained IUD, regardless of IUD removal status
The authors found that an IUD in situ was reported in 1 out of 8,307 pregnancies and was associated with PPROM, fetal malpresentation, IUFD, placental abnormalities including abruption, accreta spectrum, retained placenta, and need for manual removal (TABLE 1). About three-quarters (76.3%) of patients had a term delivery (≥37 weeks).

Retained IUD was associated with previable loss, defined as less than 22 weeks’ gestation (adjusted odds ratio [aOR], 5.49; 95% confidence interval [CI], 3.30–9.15) and periviable delivery, defined as 22 to 25 weeks’ gestation (aOR, 2.81; 95% CI, 1.63–4.85). Retained IUD was not associated with preterm delivery beyond 26 weeks’ gestation, cesarean delivery, postpartum hemorrhage, or hysterectomy.
Important limitations of this study are the lack of information on IUD type (copper vs hormonal) and the timing of removal or attempted removal in relation to measured pregnancy outcomes.
Continue to: Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes...
Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes
Karakus and colleagues conducted a retrospective cohort study of 233 patients in Turkey with pregnancies that occurred during copper 380-mm2 IUD use from 2011 to 2021. The authors reported that, at the time of first contact with the health system and diagnosis of retained IUD, 18.9% of the pregnancies were ectopic, 13.2% were first trimester losses, and 67.5% were ongoing pregnancies.
The authors assessed outcomes in patients with ongoing pregnancies based on whether or not the IUD was removed or retained. Outcomes included gestational age at delivery and adverse pregnancy outcomes, assessed as a composite of preterm delivery, PPROM, chorioamnionitis, placental abruption, and postpartum hemorrhage.
Of those with ongoing pregnancies, 13.3% chose to have an abortion, leaving 137 (86.7%) with continuing pregnancy. The IUD was able to be removed in 39.4% of the sample, with an average gestational age of 7 weeks at the time of removal.
Compared with those with a retained IUD, patients in the removal group had a lower rate of pregnancy loss (33.3% vs 61.4%; P<.001) and a lower rate of the composite adverse pregnancy outcomes (53.1% vs 27.8%; P=.03). TABLE 2 shows the approximate rate of ongoing pregnancy by gestational age in patients with retained and removed copper 380-mm2 IUDs. Notably, the largest change occurred periviably, with the proportion of patients with an ongoing pregnancy after 26 weeks reducing to about half for patients with a retained IUD as compared with patients with a removed IUD; this proportion of ongoing pregnancies held through the remainder of gestation.

These studies confirm that a retained IUD is a rare outcome, occurring in about 1 in 8,000 pregnancies. Previous US national data from 2010 reported a similar incidence of 1 in 6,203 pregnancies (0.02%).10 Management and counseling depend on the patient’s desire to continue the pregnancy, gestational age, intrauterine IUD location, and ability to see the IUD strings. Contemporary data support management practices created from limited and outdated data, which include device removal (if able) and counseling those who desire to continue pregnancy about high-risk pregnancy complications. Those with a retained IUD should be counseled about increased risk of preterm or previable delivery, IUFD, and placental abnormalities (including accreta spectrum and retained placenta). Specifically, these contemporary data highlight that, beyond approximately 26 weeks’ gestation, the pregnancy loss rate is not different for those with a retained or removed IUD. Obstetric care providers should feel confident in using this more nuanced risk of extreme preterm delivery when counseling future patients. Implications for antepartum care and delivery timing with a retained IUD have not yet been defined.
Do national data reveal more breakage reports for copper 380-mm2 or LNG IUDs?
Latack KR, Nguyen BT. Trends in copper versus hormonal intrauterine device breakage reporting within the United States’ Food and Drug Administration Adverse Event Reporting System. Contraception. 2023;118:109909. doi:10.1016/j.contraception.2022.10.011
Latack and Nguyen reviewed postmarket surveillance data of IUD adverse events in the US Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) from 1998 to 2022. The FAERS is a voluntary, or passive, reporting system.
Study findings
Of the approximately 170,000 IUD-related adverse events reported to the agency during the 24-year timeframe, 25.4% were for copper IUDs and 74.6% were for hormonal IUDs. Slightly more than 4,000 reports were specific for device breakage, which the authors grouped into copper (copper 380-mm2)and hormonal (LNG 52 mg, 19.5 mg, and 13.5 mg) IUDs.
The copper 380-mm2 IUD was 6.19 times more likely to have a breakage report than hormonal IUDs (9.6% vs 1.7%; 95% CI, 5.87–6.53).
The overall proportion of IUD-related adverse events reported to the FDA was about 25% for copper and 75% for hormonal IUDs; this proportion is similar to sales figures, which show that about 15% of IUDs sold in the United States are copper and 85% are hormonal.11 However, the proportion of breakage events reported to the FDA is the inverse, with about 6 times more breakage reports with copper than with hormonal IUDs. Because these data come from a passive reporting system, the true incidence of IUD breakage cannot be assessed. However, these findings should remind clinicians to inform patients about this rare occurrence during counseling at the time of placement and, especially, when preparing for copper IUD removal. As the absolute number of IUD users increases, clinicians may be more likely to encounter this relatively rare event.
Management of IUD breakage is based on expert opinion, and recommendations are varied, ranging from observation to removal using an IUD hook, alligator forceps, manual vacuum aspiration, or hysteroscopy.7,10 Importantly, each individual patient situation will vary depending on the presence or absence of other symptoms and whether or not future pregnancy is desired.
Continue to: Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients...
Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients
Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi:10.1097AOG.0000000000005137
Creinin and colleagues conducted a study for US regulatory product approval of the LNG 52-mg IUD (Liletta) for HMB. This multicenter phase 3 open-label clinical trial recruited nonpregnant participants aged 18 to 50 years with HMB at 29 clinical sites in the United States. No BMI cutoff was used.
Baseline menstrual flow data were obtained over 2 to 3 screening cycles by collection of menstrual products and quantification of blood loss using alkaline hematin measurement. Patients with 2 cycles with a blood loss exceeding 80 mL had an IUD placement, with similar flow evaluations during the third and sixth postplacement cycles.
Treatment success was defined as a reduction in blood loss by more than 50% as compared with baseline (during screening) and measured blood loss of less than 80 mL. The enrolled population (n=105) included 28% nulliparous users, with 49% and 28% of participants having a BMI of 30 kg/m2 or higher and higher than 35 kg/m2, respectively.
Treatment highly successful in reducing blood loss
Participants in this trial had a 93% and a 98% reduction in blood loss at the third and sixth cycles of use, respectively. Additionally, during the sixth cycle of use, 19% of users had no bleeding. Treatment success occurred in about 80% of participants overall and occurred regardless of parity or BMI.
To assess a subjective measure of success, participants were asked to evaluate their menstrual bleeding and dysmenorrhea severity, acceptability, and overall impact on quality of life at 3 time points: during prior typical menses, cycle 3, and cycle 6. At cycle 6, all participants reported significantly improved acceptability of bleeding and uterine pain and, importantly, decreased overall menstrual interference with the ability to complete daily activities (TABLE 3).

IUD expulsion and replacement rates
Although bleeding greatly decreased in all participants, 13% (n=14) discontinued before cycle 6 due to expulsion or IUD-related symptoms, with the majority citing bleeding irregularities. Expulsion occurred in 9% (n=5) of users, with the majority (2/3) occurring in the first 3 months of use and more commonly in obese and/or parous users. About half of participants with expulsion had the IUD replaced during the study. ●
Interestingly, both LNG 52-mg IUDs have been approved in most countries throughout the world for HMB treatment, and only in the United States was one of the products (Liletta) not approved until this past year. The FDA required more stringent trials than had been previously performed for approval outside of the United States. However, a benefit for clinicians is that this phase 3 study provided data in a contemporary US population. Clinicians can feel confident in counseling and offering the LNG 52-mg IUD as a first-line treatment option for patients with HMB, including those who have never been pregnant or have a BMI greater than 35 kg/m2.
Importantly, though, clinicians should be realistic with all patients that this treatment, although highly effective, is not successful for about 20% of patients by about 6 months of use. For those in whom the treatment is beneficial, the quality-of-life improvement is dramatic. Additionally, this study reminds us that expulsion risk in a population primarily using the IUD for HMB, especially if also obese and/or parous, is higher in the first 6 months of use than patients using the method for contraception. Expulsion occurs in 1.6% of contraception users through 6 months of use.12 These data highlight that IUD expulsion risk is not a fixed number, but instead is modified by patient characteristics. Patients should be counseled regarding the appropriate expulsion risk and that the IUD can be safely replaced should expulsion occur.
More US women are using IUDs than ever before. With more use comes the potential for complications and more requests related to non-contraceptive benefits. New information provides contemporary insight into rare IUD complications and the use of hormonal IUDs for treatment of HMB.
The first intrauterine device (IUD) to be approved in the United States, the Lippes Loop, became available in 1964. Sixty years later, more US women are using IUDs than ever before, and numbers are trending upward (FIGURE).1,2 Over the past year, contemporary information has become available to further inform IUD management when pregnancy occurs with an IUD in situ, as well as counseling about device breakage. Additionally, new data help clinicians expand which patients can use a levonorgestrel (LNG) 52-mg IUD for heavy menstrual bleeding (HMB) treatment.

As the total absolute number of IUD users increases, so do the absolute numbers of rare outcomes, such as pregnancy among IUD users. These highly effective contraceptives have a failure rate within the first year after placement ranging from 0.1% for the LNG 52-mg IUD to 0.8% for the copper 380-mm2 IUD.3 Although the possibility for extrauterine gestation is higher when pregnancy occurs while a patient is using an IUD as compared with most other contraceptive methods, most pregnancies that occur with an IUD in situ are intrauterine.4
The high contraceptive efficacy of IUDs make pregnancy with a retained IUD rare; therefore, it is difficult to perform a study with a large enough population to evaluate management of pregnancy complicated by an IUD in situ. Clinical management recommendations for these situations are 20 years old and are supported by limited data from case reports and series with fewer than 200 patients.5,6
Intrauterine device breakage is another rare event that is poorly understood due to the low absolute number of cases. Information about breakage has similarly been limited to case reports and case series.7,8 This past year, contemporary data were published to provide more insight into both intrauterine pregnancy with an IUD in situ and IUD breakage.
Beyond contraception, hormonal IUDs have become a popular and evidence-based treatment option for patients with HMB. The initial LNG 52-mg IUD (Mirena) regulatory approval studies for HMB treatment included data limited to parous patients and users with a body mass index (BMI) less than 35 kg/m2.9 Since that time, no studies have explored these populations. Although current practice has commonly extended use to include patients with these characteristics, we have lacked outcome data. New phase 3 data on the LNG 52-mg IUD (Liletta) included a broader range of participants and provide evidence to support this practice.
Removing retained copper 380-mm2 IUDs improves pregnancy outcomes
Panchal VR, Rau AR, Mandelbaum RS, et al. Pregnancy with retained intrauterine device: national-level assessment of characteristics and outcomes. Am J Obstet Gynecol MFM. 2023;5:101056. doi:10.1016/j.ajogmf.2023.101056
Karakuş SS, Karakuş R, Akalın EE, et al. Pregnancy outcomes with a copper 380 mm2 intrauterine device in place: a retrospective cohort study in Turkey, 2011-2021. Contraception. 2023;125:110090. doi:10.1016/j.contraception.2023.110090
To update our understanding of outcomes of pregnancy with an IUD in situ, Panchal and colleagues performed a cross-sectional study using the Healthcare Cost and Utilization Project’s National Inpatient Sample. This data set represents 85% of US hospital discharges. The population investigated included hospital deliveries from 2016 to 2020 with an ICD-10 (International Classification of Diseases, Tenth Revision) code of retained IUD. Those without the code were assigned to the comparison non-retained IUD group.
The primary outcome studied was the incidence rate of retained IUD, patient and pregnancy characteristics, and delivery outcomes including but not limited to gestational age at delivery, placental abnormalities, intrauterine fetal demise (IUFD), preterm premature rupture of membranes (PPROM), cesarean delivery, postpartum hemorrhage, and hysterectomy.
Outcomes were worse with retained IUD, regardless of IUD removal status
The authors found that an IUD in situ was reported in 1 out of 8,307 pregnancies and was associated with PPROM, fetal malpresentation, IUFD, placental abnormalities including abruption, accreta spectrum, retained placenta, and need for manual removal (TABLE 1). About three-quarters (76.3%) of patients had a term delivery (≥37 weeks).

Retained IUD was associated with previable loss, defined as less than 22 weeks’ gestation (adjusted odds ratio [aOR], 5.49; 95% confidence interval [CI], 3.30–9.15) and periviable delivery, defined as 22 to 25 weeks’ gestation (aOR, 2.81; 95% CI, 1.63–4.85). Retained IUD was not associated with preterm delivery beyond 26 weeks’ gestation, cesarean delivery, postpartum hemorrhage, or hysterectomy.
Important limitations of this study are the lack of information on IUD type (copper vs hormonal) and the timing of removal or attempted removal in relation to measured pregnancy outcomes.
Continue to: Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes...
Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes
Karakus and colleagues conducted a retrospective cohort study of 233 patients in Turkey with pregnancies that occurred during copper 380-mm2 IUD use from 2011 to 2021. The authors reported that, at the time of first contact with the health system and diagnosis of retained IUD, 18.9% of the pregnancies were ectopic, 13.2% were first trimester losses, and 67.5% were ongoing pregnancies.
The authors assessed outcomes in patients with ongoing pregnancies based on whether or not the IUD was removed or retained. Outcomes included gestational age at delivery and adverse pregnancy outcomes, assessed as a composite of preterm delivery, PPROM, chorioamnionitis, placental abruption, and postpartum hemorrhage.
Of those with ongoing pregnancies, 13.3% chose to have an abortion, leaving 137 (86.7%) with continuing pregnancy. The IUD was able to be removed in 39.4% of the sample, with an average gestational age of 7 weeks at the time of removal.
Compared with those with a retained IUD, patients in the removal group had a lower rate of pregnancy loss (33.3% vs 61.4%; P<.001) and a lower rate of the composite adverse pregnancy outcomes (53.1% vs 27.8%; P=.03). TABLE 2 shows the approximate rate of ongoing pregnancy by gestational age in patients with retained and removed copper 380-mm2 IUDs. Notably, the largest change occurred periviably, with the proportion of patients with an ongoing pregnancy after 26 weeks reducing to about half for patients with a retained IUD as compared with patients with a removed IUD; this proportion of ongoing pregnancies held through the remainder of gestation.

These studies confirm that a retained IUD is a rare outcome, occurring in about 1 in 8,000 pregnancies. Previous US national data from 2010 reported a similar incidence of 1 in 6,203 pregnancies (0.02%).10 Management and counseling depend on the patient’s desire to continue the pregnancy, gestational age, intrauterine IUD location, and ability to see the IUD strings. Contemporary data support management practices created from limited and outdated data, which include device removal (if able) and counseling those who desire to continue pregnancy about high-risk pregnancy complications. Those with a retained IUD should be counseled about increased risk of preterm or previable delivery, IUFD, and placental abnormalities (including accreta spectrum and retained placenta). Specifically, these contemporary data highlight that, beyond approximately 26 weeks’ gestation, the pregnancy loss rate is not different for those with a retained or removed IUD. Obstetric care providers should feel confident in using this more nuanced risk of extreme preterm delivery when counseling future patients. Implications for antepartum care and delivery timing with a retained IUD have not yet been defined.
Do national data reveal more breakage reports for copper 380-mm2 or LNG IUDs?
Latack KR, Nguyen BT. Trends in copper versus hormonal intrauterine device breakage reporting within the United States’ Food and Drug Administration Adverse Event Reporting System. Contraception. 2023;118:109909. doi:10.1016/j.contraception.2022.10.011
Latack and Nguyen reviewed postmarket surveillance data of IUD adverse events in the US Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) from 1998 to 2022. The FAERS is a voluntary, or passive, reporting system.
Study findings
Of the approximately 170,000 IUD-related adverse events reported to the agency during the 24-year timeframe, 25.4% were for copper IUDs and 74.6% were for hormonal IUDs. Slightly more than 4,000 reports were specific for device breakage, which the authors grouped into copper (copper 380-mm2)and hormonal (LNG 52 mg, 19.5 mg, and 13.5 mg) IUDs.
The copper 380-mm2 IUD was 6.19 times more likely to have a breakage report than hormonal IUDs (9.6% vs 1.7%; 95% CI, 5.87–6.53).
The overall proportion of IUD-related adverse events reported to the FDA was about 25% for copper and 75% for hormonal IUDs; this proportion is similar to sales figures, which show that about 15% of IUDs sold in the United States are copper and 85% are hormonal.11 However, the proportion of breakage events reported to the FDA is the inverse, with about 6 times more breakage reports with copper than with hormonal IUDs. Because these data come from a passive reporting system, the true incidence of IUD breakage cannot be assessed. However, these findings should remind clinicians to inform patients about this rare occurrence during counseling at the time of placement and, especially, when preparing for copper IUD removal. As the absolute number of IUD users increases, clinicians may be more likely to encounter this relatively rare event.
Management of IUD breakage is based on expert opinion, and recommendations are varied, ranging from observation to removal using an IUD hook, alligator forceps, manual vacuum aspiration, or hysteroscopy.7,10 Importantly, each individual patient situation will vary depending on the presence or absence of other symptoms and whether or not future pregnancy is desired.
Continue to: Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients...
Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients
Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi:10.1097AOG.0000000000005137
Creinin and colleagues conducted a study for US regulatory product approval of the LNG 52-mg IUD (Liletta) for HMB. This multicenter phase 3 open-label clinical trial recruited nonpregnant participants aged 18 to 50 years with HMB at 29 clinical sites in the United States. No BMI cutoff was used.
Baseline menstrual flow data were obtained over 2 to 3 screening cycles by collection of menstrual products and quantification of blood loss using alkaline hematin measurement. Patients with 2 cycles with a blood loss exceeding 80 mL had an IUD placement, with similar flow evaluations during the third and sixth postplacement cycles.
Treatment success was defined as a reduction in blood loss by more than 50% as compared with baseline (during screening) and measured blood loss of less than 80 mL. The enrolled population (n=105) included 28% nulliparous users, with 49% and 28% of participants having a BMI of 30 kg/m2 or higher and higher than 35 kg/m2, respectively.
Treatment highly successful in reducing blood loss
Participants in this trial had a 93% and a 98% reduction in blood loss at the third and sixth cycles of use, respectively. Additionally, during the sixth cycle of use, 19% of users had no bleeding. Treatment success occurred in about 80% of participants overall and occurred regardless of parity or BMI.
To assess a subjective measure of success, participants were asked to evaluate their menstrual bleeding and dysmenorrhea severity, acceptability, and overall impact on quality of life at 3 time points: during prior typical menses, cycle 3, and cycle 6. At cycle 6, all participants reported significantly improved acceptability of bleeding and uterine pain and, importantly, decreased overall menstrual interference with the ability to complete daily activities (TABLE 3).

IUD expulsion and replacement rates
Although bleeding greatly decreased in all participants, 13% (n=14) discontinued before cycle 6 due to expulsion or IUD-related symptoms, with the majority citing bleeding irregularities. Expulsion occurred in 9% (n=5) of users, with the majority (2/3) occurring in the first 3 months of use and more commonly in obese and/or parous users. About half of participants with expulsion had the IUD replaced during the study. ●
Interestingly, both LNG 52-mg IUDs have been approved in most countries throughout the world for HMB treatment, and only in the United States was one of the products (Liletta) not approved until this past year. The FDA required more stringent trials than had been previously performed for approval outside of the United States. However, a benefit for clinicians is that this phase 3 study provided data in a contemporary US population. Clinicians can feel confident in counseling and offering the LNG 52-mg IUD as a first-line treatment option for patients with HMB, including those who have never been pregnant or have a BMI greater than 35 kg/m2.
Importantly, though, clinicians should be realistic with all patients that this treatment, although highly effective, is not successful for about 20% of patients by about 6 months of use. For those in whom the treatment is beneficial, the quality-of-life improvement is dramatic. Additionally, this study reminds us that expulsion risk in a population primarily using the IUD for HMB, especially if also obese and/or parous, is higher in the first 6 months of use than patients using the method for contraception. Expulsion occurs in 1.6% of contraception users through 6 months of use.12 These data highlight that IUD expulsion risk is not a fixed number, but instead is modified by patient characteristics. Patients should be counseled regarding the appropriate expulsion risk and that the IUD can be safely replaced should expulsion occur.
- Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception. 2018;98:467470. doi:10.1016/j.contraception.2018.05.016
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93. doi:10.1016/j.xfre.2020.06.006
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:15.
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:185.
- Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430. doi:10.1016/j.contraception.2014.01.002
- Brahmi D, Steenland MW, Renner RM, et al. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131-139. doi:10.1016/j.contraception . 2011.06.010
- Wilson S, Tan G, Baylson M, et al. Controversies in family planning: how to manage a fractured IUD. Contraception. 2013;88:599-603. doi:10.1016/j.contraception.2013.07.007
- Fulkerson Schaeffer S, Gimovsky AC, Aly H, et al. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32:798-803. doi:10.1080/14767058.2017.1 391783
- Mirena. Prescribing information. Bayer HealthCare Pharmaceuticals. Accessed August 22, 2023. https://www .mirena-us.com/pi
- Myo MG, Nguyen BT. Intrauterine device complications and their management. Curr Obstet Gynecol Rep. 2023;12:88-95. doi.org/10.1007/s13669-023-00357-8
- National Center for Health Statistics (NCHS). 2017-2019 National Survey of Family Growth. Public-Use Data File Documentation. CDC National Center for Health Statistics. Accessed August 28, 2023. https://www.cdc.gov/nchs/data /nsfg/NSFG-2017-2019-UG-MainText-508.pdf
- Gilliam ML, Jensen JT, Eisenberg DL, et al. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception. 2021;103:444-449. doi: 10.1016/j.contraception.2021.02.013
- Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception. 2018;98:467470. doi:10.1016/j.contraception.2018.05.016
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93. doi:10.1016/j.xfre.2020.06.006
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:15.
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:185.
- Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430. doi:10.1016/j.contraception.2014.01.002
- Brahmi D, Steenland MW, Renner RM, et al. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131-139. doi:10.1016/j.contraception . 2011.06.010
- Wilson S, Tan G, Baylson M, et al. Controversies in family planning: how to manage a fractured IUD. Contraception. 2013;88:599-603. doi:10.1016/j.contraception.2013.07.007
- Fulkerson Schaeffer S, Gimovsky AC, Aly H, et al. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32:798-803. doi:10.1080/14767058.2017.1 391783
- Mirena. Prescribing information. Bayer HealthCare Pharmaceuticals. Accessed August 22, 2023. https://www .mirena-us.com/pi
- Myo MG, Nguyen BT. Intrauterine device complications and their management. Curr Obstet Gynecol Rep. 2023;12:88-95. doi.org/10.1007/s13669-023-00357-8
- National Center for Health Statistics (NCHS). 2017-2019 National Survey of Family Growth. Public-Use Data File Documentation. CDC National Center for Health Statistics. Accessed August 28, 2023. https://www.cdc.gov/nchs/data /nsfg/NSFG-2017-2019-UG-MainText-508.pdf
- Gilliam ML, Jensen JT, Eisenberg DL, et al. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception. 2021;103:444-449. doi: 10.1016/j.contraception.2021.02.013
2022 Update on contraception
On June 24, 2022, the US Supreme Court ruled in Dobbs v Jackson to overturn the landmark Roe v Wade decision, deeming that abortion is not protected by statutes that provide the right to privacy, liberty, or autonomy. With this historic ruling, other rights founded on the same principles, including the freedom to use contraception, may be called into question in the future. Clinics that provide abortion care typically play a vital role in providing contraception services. Due to abortion restriction across the country, many of these clinics are predicted to close and many have already closed. Within one month of the Dobbs decision, 43 clinics in 11 states had shut their doors to patients, reducing access to basic contraception services.1 It is more important now than ever that clinicians address barriers and lead the effort to improve and ensure that patients have access to contraceptive services.
In this Update, we review recent evidence that may help aid patients in obtaining contraception more easily and for longer periods of time. We review strategies demonstrated to improve contraceptive access, including how to increase prescribing rates of 1-year contraceptive supplies and pharmacist-prescribed contraception. We also review new data on extended use of the levonorgestrel 52 mg intrauterine device (LNG 52 mg IUD).
One-year prescribing of hormonal contraception decreases an access barrier
Uhm S, Chen MJ, Cutler ED, et al. Twelve-month prescribing of contraceptive pill, patch, and ring before and after a standardized electronic medical record order change. Contraception. 2021;103:60-63.
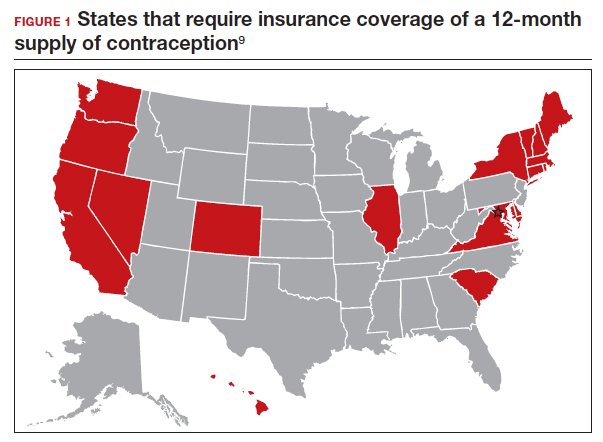
Providing a 1-year supply of self-administered contraception can lead to higher likelihood of continued use and is associated with reduced cost, unintended pregnancy, and abortion rates.2-4 Although some patients may not use a full year’s supply of pills, rings, or patches under such programs, the lower rates of unintended pregnancy result in significant cost savings as compared with the unused contraceptives.2,3 Accordingly, the Centers for Disease Control and Prevention (CDC) advises dispensing a 1-year supply of self-administered hormonal contraception.5 Insurance coverage and providers’ prescribing practices can be barriers to patients obtaining a year’s supply of hormonal contraception. Currently, 18 states and the District of Columbia legally require insurers to cover a 12-month supply of prescription contraceptives (FIGURE 1). Despite these laws and the CDC recommendation, studies show that most people continue to receive only a 1- to 3-month supply.6-8 One strategy to increase the number of 1-year supplies of self-administered contraception is institutional changes to default prescription orders.
Study design
In California, legislation enacted in January 2017 required commercial and medical assistance health plans to cover up to 12 months of US Food and Drug Administration (FDA)-approved self-administered hormonal contraceptives dispensed at 1 time as prescribed or requested. To better serve patients, a multidisciplinary team from the University of California Davis Health worked with the institution’s pharmacy to institute an electronic medical record (EMR) default order change from dispensing 1-month with refills to dispensing 12-month quantities for all combined and progestin-only pills, patches, and rings on formulary.
After this EMR order change in December 2019, Uhm and colleagues conducted a retrospective pre-post study using outpatient prescription data that included nearly 5,000 contraceptive pill, patch, and ring prescriptions over an 8-month period. They compared the frequency of 12-month prescriptions for each of these methods 4 months before and 4 months after the default order change. They compared the proportion of 12-month prescriptions by prescriber department affiliation and by clinic location. Department affiliation was categorized as obstetrics-gynecology or non–obstetrics-gynecology. Clinic location was categorized as medical center campus or community clinics.
Increase in 12-month prescriptions
The authors found an overall increase in 12-month prescriptions, from 11% to 27%, after the EMR order change. Prescribers at the medical center campus clinics more frequently ordered a 12-month supply compared with prescribers at community clinics both before (33% vs 4%, respectively) and after (53% vs 19%, respectively) the EMR change. The only group of providers without a significant increase in 12-month prescriptions was among obstetrics-gynecology providers at community clinics (4% before vs 6% after).
The system EMR change modified only the standard facility order settings and did not affect individual favorite orders, which may help explain the differences in prescribing practices. While this study found an increase in 12-month prescriptions, there were no data on the actual number of supplies a patient received or on reimbursement.
The study by Uhm and colleagues showed that making a relatively simple change to default EMR orders can increase 12-month contraception prescribing and lead to greater patient-centered care. Evidence shows that providers and pharmacists are not necessarily aware of laws that require 12-month supply coverage and routinely prescribe smaller supplies.6,7,9 For clinicians in states that have these laws (FIGURE 1), we urge you to provide as full a supply of contraceptives as possible as this approach is both evidence based and patient centered. Although this study shows the benefit of universal system change to the EMR, individual clinicians also must be sure to modify personal order preferences. In addition, pharmacists can play an important role by updating policies that comply with these laws and by increasing pharmacy stocks of contraception supplies.7 For those living in states that do not currently have these laws, we encourage you to reach out to your legislators to advocate for similar laws as the data show clear medical and cost benefits for patients and society.
Continue to: Pharmacist prescription of hormonal contraception is safe and promotes continuation...
Pharmacist prescription of hormonal contraception is safe and promotes continuation
Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
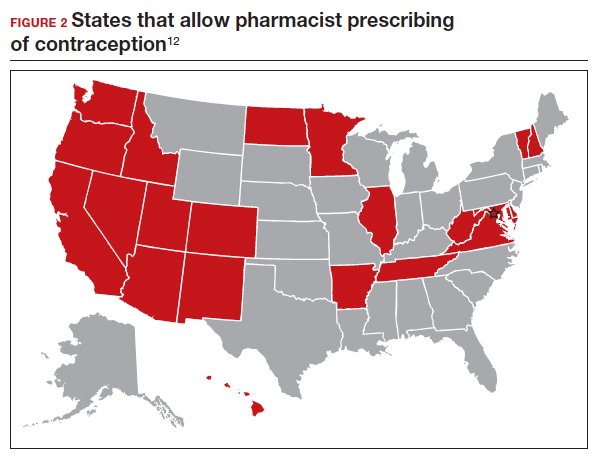
Patients often face difficulty obtaining both new and timely refills of self-administered contraception.10,11 To expand contraception access, Oregon became the first state (in 2016) to enact legislation to authorize direct pharmacist prescribing of hormonal contraceptives.12 Currently, 17 states and the District of Columbia have protocols for pharmacist prescribing privileges (FIGURE 2), and proposed legislation is pending in another 14 states.10,12 These protocols vary, but basic processes include screening, documentation, monitoring, and referrals when necessary. Typically, protocols require a pharmacist to review a patient’s medical history, pregnancy status, medication use, and blood pressure, followed by contraceptive counseling.10 Pharmacies are generally located in the community they serve, have extended hours, and usually do not require an appointment.8,13,14
Pharmacist prescribing increases the number of new contraceptive users, and pharmacists are more likely to prescribe a 6-month or longer supply of contraceptives compared with clinicians.8,13,15 Also, pharmacist prescribing is safe, with adherence rates to the CDC’s US Medical Eligibility Criteria for Contraceptive Use similar to those of prescriptions provided by a clinician.13
Authors of a recent multi-state study further assessed the impact of pharmacist prescribing by evaluating 12-month continuation and perfect use rates.
Study design
Rodriguez and colleagues evaluated the results of a 1-year prospective cohort study conducted in 2019 that included 388 participants who sought contraception in California, Colorado, Hawaii, and Oregon. All these states had laws permitting pharmacist prescribing and 12-month supply of hormonal contraception. Participants received prescriptions directly from a pharmacist at 1 of 139 pharmacies (n = 149) or filled a prescription provided by a clinician (n = 239). The primary outcomes were continuation of an effective method and perfect use of contraception across 12 months.
Participant demographics were similar between the 2 groups except for education and insurance status. Participants who received a prescription from a clinician reported higher levels of education. A greater proportion of uninsured participants received a prescription from a pharmacist (11%) compared with from a clinician (3%).
Contraceptive continuation rates
Participants were surveyed 3 times during the 12-month study about their current contraceptive method, if they had switched methods, or if they had any missed days of contraception.
Overall, 340 participants (88%) completed a full 12 months of follow-up. Continuation rates were similar between the 2 groups: 89% in the clinician-prescribed and 90% in the pharmacist-prescribed group (P=.86). Participants in the 2 groups also reported similar rates of perfect use (no missed days: 54% and 47%, respectively [P=.69]). Additionally, the authors reported that 29 participants changed from a tier 2 (pill, patch, ring, injection) to a tier 1 (intrauterine device or implant) method during follow-up, with no difference in switch rates for participants who received care from a clinician (10%) or a pharmacist (7%).
Patients have difficulties in obtaining both an initial contraceptive prescription and refills in time to avoid breaks in coverage.16 Pharmacist prescription of contraception is a proven strategy to increase access to contraception for new users or to promote continuation among current users. This practice is evidence based, decreases unintended pregnancy rates, and is safe.8,13,15,17
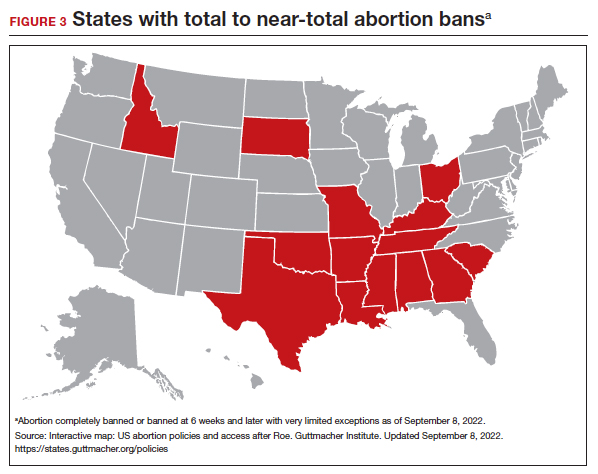
Promoting universal pharmacist prescribing is even more important given the overruling of Roe v Wade. With abortion restrictions, many family planning clinics that also play a vital role in providing contraception will close. Most states that are limiting abortion care (FIGURE 3) are the same states without pharmacist-prescribing provisions (FIGURE 2). As patient advocates, we need to continue to support this evidence-based practice in states where it is available and push legislators in states where it is not. Pharmacists should receive support to complete the training and certification needed to not only provide this service but also to receive appropriate reimbursements. Restrictions, such as requiring patients to be 18 years or older or to have prior consultation with a physician, should be limited as these are not necessary to provide self-administered contraception safely. Clinicians and pharmacists should inform patients, in states where this is available, that they can access initial or refill prescriptions at their local pharmacy if that is more convenient or their preference. Clinicians who live in states without these laws can advocate for their community by encouraging their legislators to pass laws that allow this evidence-based practice.
Continue to: LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use...
LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●
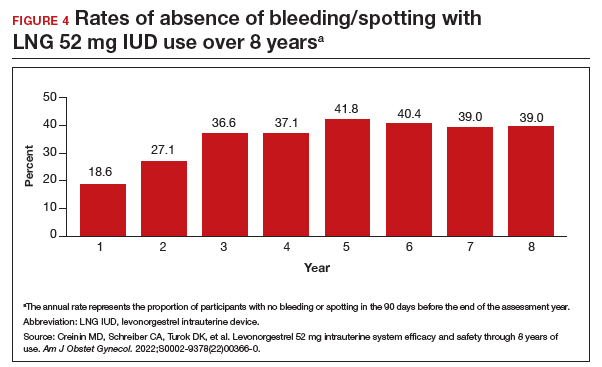
As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.
- Kirstein M, Jones RK, Philbin J. One month post-Roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. July 28, 2022. Accessed September 14, 2022. https://www.guttmacher.org /article/2022/07/one-month-post-roe-least-43-abortion-clinics-across -11-states-have-stopped-offering
- Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
- Foster DG, Parvataneni R, de Bocanegra HT, et al. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107-114.
- Niu F, Cornelius J, Aboubechara N, et al. Real world outcomes related to providing an annual supply of short-acting hormonal contraceptives. Contraception. 2022;107:58-61.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Women’s sexual and reproductive health services: key findings from the 2017 Kaiser Women’s Health Survey. KFF: Kaiser Family Foundation. March 13, 2018. Accessed September 14, 2022. https://www.kff.org/womens-health-policy /issue-brief/womens-sexual-and-reproductive-health-services-key-findings -from-the-2017-kaiser-womens-health-survey/
- Nikpour G, Allen A, Rafie S, et al. Pharmacy implementation of a new law allowing year-long hormonal contraception supplies. Pharmacy (Basel). 2020;8:E165.
- Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3:e205252.
- Insurance coverage of contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state-policy /explore/insurance-coverage-contraceptives
- Chim C, Sharma P. Pharmacists prescribing hormonal contraceptives: a status update. US Pharm. 2021;46:45-49.
- Rodriguez MI, Hersh A, Anderson LB, et al. Association of pharmacist prescription of hormonal contraception with unintended pregnancies and Medicaid costs. Obstet Gynecol. 2019;133:1238-1246.
- Pharmacist-prescribed contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state -policy/explore/pharmacist-prescribed-contraceptives
- Anderson L, Hartung DM, Middleton L, et al. Pharmacist provision of hormonal contraception in the Oregon Medicaid population. Obstet Gynecol. 2019;133:1231-1237.
- Rodriguez MI, Edelman AB, Skye M, et al. Reasons for and experience in obtaining pharmacist prescribed contraception. Contraception. 2020;102:259-261.
- Rodriguez MI, Manibusan B, Kaufman M, et al. Association of pharmacist prescription of contraception with breaks in coverage. Obstet Gynecol. 2022;139:781-787.
- Pittman ME, Secura GM, Allsworth JE, et al. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011;83:340-345.
- Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
- Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-7.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52 mg intrauterine system. Contraception. 2020;101:159-161.
On June 24, 2022, the US Supreme Court ruled in Dobbs v Jackson to overturn the landmark Roe v Wade decision, deeming that abortion is not protected by statutes that provide the right to privacy, liberty, or autonomy. With this historic ruling, other rights founded on the same principles, including the freedom to use contraception, may be called into question in the future. Clinics that provide abortion care typically play a vital role in providing contraception services. Due to abortion restriction across the country, many of these clinics are predicted to close and many have already closed. Within one month of the Dobbs decision, 43 clinics in 11 states had shut their doors to patients, reducing access to basic contraception services.1 It is more important now than ever that clinicians address barriers and lead the effort to improve and ensure that patients have access to contraceptive services.
In this Update, we review recent evidence that may help aid patients in obtaining contraception more easily and for longer periods of time. We review strategies demonstrated to improve contraceptive access, including how to increase prescribing rates of 1-year contraceptive supplies and pharmacist-prescribed contraception. We also review new data on extended use of the levonorgestrel 52 mg intrauterine device (LNG 52 mg IUD).
One-year prescribing of hormonal contraception decreases an access barrier
Uhm S, Chen MJ, Cutler ED, et al. Twelve-month prescribing of contraceptive pill, patch, and ring before and after a standardized electronic medical record order change. Contraception. 2021;103:60-63.
Providing a 1-year supply of self-administered contraception can lead to higher likelihood of continued use and is associated with reduced cost, unintended pregnancy, and abortion rates.2-4 Although some patients may not use a full year’s supply of pills, rings, or patches under such programs, the lower rates of unintended pregnancy result in significant cost savings as compared with the unused contraceptives.2,3 Accordingly, the Centers for Disease Control and Prevention (CDC) advises dispensing a 1-year supply of self-administered hormonal contraception.5 Insurance coverage and providers’ prescribing practices can be barriers to patients obtaining a year’s supply of hormonal contraception. Currently, 18 states and the District of Columbia legally require insurers to cover a 12-month supply of prescription contraceptives (FIGURE 1). Despite these laws and the CDC recommendation, studies show that most people continue to receive only a 1- to 3-month supply.6-8 One strategy to increase the number of 1-year supplies of self-administered contraception is institutional changes to default prescription orders.

Study design
In California, legislation enacted in January 2017 required commercial and medical assistance health plans to cover up to 12 months of US Food and Drug Administration (FDA)-approved self-administered hormonal contraceptives dispensed at 1 time as prescribed or requested. To better serve patients, a multidisciplinary team from the University of California Davis Health worked with the institution’s pharmacy to institute an electronic medical record (EMR) default order change from dispensing 1-month with refills to dispensing 12-month quantities for all combined and progestin-only pills, patches, and rings on formulary.
After this EMR order change in December 2019, Uhm and colleagues conducted a retrospective pre-post study using outpatient prescription data that included nearly 5,000 contraceptive pill, patch, and ring prescriptions over an 8-month period. They compared the frequency of 12-month prescriptions for each of these methods 4 months before and 4 months after the default order change. They compared the proportion of 12-month prescriptions by prescriber department affiliation and by clinic location. Department affiliation was categorized as obstetrics-gynecology or non–obstetrics-gynecology. Clinic location was categorized as medical center campus or community clinics.
Increase in 12-month prescriptions
The authors found an overall increase in 12-month prescriptions, from 11% to 27%, after the EMR order change. Prescribers at the medical center campus clinics more frequently ordered a 12-month supply compared with prescribers at community clinics both before (33% vs 4%, respectively) and after (53% vs 19%, respectively) the EMR change. The only group of providers without a significant increase in 12-month prescriptions was among obstetrics-gynecology providers at community clinics (4% before vs 6% after).
The system EMR change modified only the standard facility order settings and did not affect individual favorite orders, which may help explain the differences in prescribing practices. While this study found an increase in 12-month prescriptions, there were no data on the actual number of supplies a patient received or on reimbursement.
The study by Uhm and colleagues showed that making a relatively simple change to default EMR orders can increase 12-month contraception prescribing and lead to greater patient-centered care. Evidence shows that providers and pharmacists are not necessarily aware of laws that require 12-month supply coverage and routinely prescribe smaller supplies.6,7,9 For clinicians in states that have these laws (FIGURE 1), we urge you to provide as full a supply of contraceptives as possible as this approach is both evidence based and patient centered. Although this study shows the benefit of universal system change to the EMR, individual clinicians also must be sure to modify personal order preferences. In addition, pharmacists can play an important role by updating policies that comply with these laws and by increasing pharmacy stocks of contraception supplies.7 For those living in states that do not currently have these laws, we encourage you to reach out to your legislators to advocate for similar laws as the data show clear medical and cost benefits for patients and society.
Continue to: Pharmacist prescription of hormonal contraception is safe and promotes continuation...
Pharmacist prescription of hormonal contraception is safe and promotes continuation
Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
Patients often face difficulty obtaining both new and timely refills of self-administered contraception.10,11 To expand contraception access, Oregon became the first state (in 2016) to enact legislation to authorize direct pharmacist prescribing of hormonal contraceptives.12 Currently, 17 states and the District of Columbia have protocols for pharmacist prescribing privileges (FIGURE 2), and proposed legislation is pending in another 14 states.10,12 These protocols vary, but basic processes include screening, documentation, monitoring, and referrals when necessary. Typically, protocols require a pharmacist to review a patient’s medical history, pregnancy status, medication use, and blood pressure, followed by contraceptive counseling.10 Pharmacies are generally located in the community they serve, have extended hours, and usually do not require an appointment.8,13,14

Pharmacist prescribing increases the number of new contraceptive users, and pharmacists are more likely to prescribe a 6-month or longer supply of contraceptives compared with clinicians.8,13,15 Also, pharmacist prescribing is safe, with adherence rates to the CDC’s US Medical Eligibility Criteria for Contraceptive Use similar to those of prescriptions provided by a clinician.13
Authors of a recent multi-state study further assessed the impact of pharmacist prescribing by evaluating 12-month continuation and perfect use rates.
Study design
Rodriguez and colleagues evaluated the results of a 1-year prospective cohort study conducted in 2019 that included 388 participants who sought contraception in California, Colorado, Hawaii, and Oregon. All these states had laws permitting pharmacist prescribing and 12-month supply of hormonal contraception. Participants received prescriptions directly from a pharmacist at 1 of 139 pharmacies (n = 149) or filled a prescription provided by a clinician (n = 239). The primary outcomes were continuation of an effective method and perfect use of contraception across 12 months.
Participant demographics were similar between the 2 groups except for education and insurance status. Participants who received a prescription from a clinician reported higher levels of education. A greater proportion of uninsured participants received a prescription from a pharmacist (11%) compared with from a clinician (3%).
Contraceptive continuation rates
Participants were surveyed 3 times during the 12-month study about their current contraceptive method, if they had switched methods, or if they had any missed days of contraception.
Overall, 340 participants (88%) completed a full 12 months of follow-up. Continuation rates were similar between the 2 groups: 89% in the clinician-prescribed and 90% in the pharmacist-prescribed group (P=.86). Participants in the 2 groups also reported similar rates of perfect use (no missed days: 54% and 47%, respectively [P=.69]). Additionally, the authors reported that 29 participants changed from a tier 2 (pill, patch, ring, injection) to a tier 1 (intrauterine device or implant) method during follow-up, with no difference in switch rates for participants who received care from a clinician (10%) or a pharmacist (7%).

Patients have difficulties in obtaining both an initial contraceptive prescription and refills in time to avoid breaks in coverage.16 Pharmacist prescription of contraception is a proven strategy to increase access to contraception for new users or to promote continuation among current users. This practice is evidence based, decreases unintended pregnancy rates, and is safe.8,13,15,17
Promoting universal pharmacist prescribing is even more important given the overruling of Roe v Wade. With abortion restrictions, many family planning clinics that also play a vital role in providing contraception will close. Most states that are limiting abortion care (FIGURE 3) are the same states without pharmacist-prescribing provisions (FIGURE 2). As patient advocates, we need to continue to support this evidence-based practice in states where it is available and push legislators in states where it is not. Pharmacists should receive support to complete the training and certification needed to not only provide this service but also to receive appropriate reimbursements. Restrictions, such as requiring patients to be 18 years or older or to have prior consultation with a physician, should be limited as these are not necessary to provide self-administered contraception safely. Clinicians and pharmacists should inform patients, in states where this is available, that they can access initial or refill prescriptions at their local pharmacy if that is more convenient or their preference. Clinicians who live in states without these laws can advocate for their community by encouraging their legislators to pass laws that allow this evidence-based practice.
Continue to: LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use...
LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●

As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.
On June 24, 2022, the US Supreme Court ruled in Dobbs v Jackson to overturn the landmark Roe v Wade decision, deeming that abortion is not protected by statutes that provide the right to privacy, liberty, or autonomy. With this historic ruling, other rights founded on the same principles, including the freedom to use contraception, may be called into question in the future. Clinics that provide abortion care typically play a vital role in providing contraception services. Due to abortion restriction across the country, many of these clinics are predicted to close and many have already closed. Within one month of the Dobbs decision, 43 clinics in 11 states had shut their doors to patients, reducing access to basic contraception services.1 It is more important now than ever that clinicians address barriers and lead the effort to improve and ensure that patients have access to contraceptive services.
In this Update, we review recent evidence that may help aid patients in obtaining contraception more easily and for longer periods of time. We review strategies demonstrated to improve contraceptive access, including how to increase prescribing rates of 1-year contraceptive supplies and pharmacist-prescribed contraception. We also review new data on extended use of the levonorgestrel 52 mg intrauterine device (LNG 52 mg IUD).
One-year prescribing of hormonal contraception decreases an access barrier
Uhm S, Chen MJ, Cutler ED, et al. Twelve-month prescribing of contraceptive pill, patch, and ring before and after a standardized electronic medical record order change. Contraception. 2021;103:60-63.
Providing a 1-year supply of self-administered contraception can lead to higher likelihood of continued use and is associated with reduced cost, unintended pregnancy, and abortion rates.2-4 Although some patients may not use a full year’s supply of pills, rings, or patches under such programs, the lower rates of unintended pregnancy result in significant cost savings as compared with the unused contraceptives.2,3 Accordingly, the Centers for Disease Control and Prevention (CDC) advises dispensing a 1-year supply of self-administered hormonal contraception.5 Insurance coverage and providers’ prescribing practices can be barriers to patients obtaining a year’s supply of hormonal contraception. Currently, 18 states and the District of Columbia legally require insurers to cover a 12-month supply of prescription contraceptives (FIGURE 1). Despite these laws and the CDC recommendation, studies show that most people continue to receive only a 1- to 3-month supply.6-8 One strategy to increase the number of 1-year supplies of self-administered contraception is institutional changes to default prescription orders.

Study design
In California, legislation enacted in January 2017 required commercial and medical assistance health plans to cover up to 12 months of US Food and Drug Administration (FDA)-approved self-administered hormonal contraceptives dispensed at 1 time as prescribed or requested. To better serve patients, a multidisciplinary team from the University of California Davis Health worked with the institution’s pharmacy to institute an electronic medical record (EMR) default order change from dispensing 1-month with refills to dispensing 12-month quantities for all combined and progestin-only pills, patches, and rings on formulary.
After this EMR order change in December 2019, Uhm and colleagues conducted a retrospective pre-post study using outpatient prescription data that included nearly 5,000 contraceptive pill, patch, and ring prescriptions over an 8-month period. They compared the frequency of 12-month prescriptions for each of these methods 4 months before and 4 months after the default order change. They compared the proportion of 12-month prescriptions by prescriber department affiliation and by clinic location. Department affiliation was categorized as obstetrics-gynecology or non–obstetrics-gynecology. Clinic location was categorized as medical center campus or community clinics.
Increase in 12-month prescriptions
The authors found an overall increase in 12-month prescriptions, from 11% to 27%, after the EMR order change. Prescribers at the medical center campus clinics more frequently ordered a 12-month supply compared with prescribers at community clinics both before (33% vs 4%, respectively) and after (53% vs 19%, respectively) the EMR change. The only group of providers without a significant increase in 12-month prescriptions was among obstetrics-gynecology providers at community clinics (4% before vs 6% after).
The system EMR change modified only the standard facility order settings and did not affect individual favorite orders, which may help explain the differences in prescribing practices. While this study found an increase in 12-month prescriptions, there were no data on the actual number of supplies a patient received or on reimbursement.
The study by Uhm and colleagues showed that making a relatively simple change to default EMR orders can increase 12-month contraception prescribing and lead to greater patient-centered care. Evidence shows that providers and pharmacists are not necessarily aware of laws that require 12-month supply coverage and routinely prescribe smaller supplies.6,7,9 For clinicians in states that have these laws (FIGURE 1), we urge you to provide as full a supply of contraceptives as possible as this approach is both evidence based and patient centered. Although this study shows the benefit of universal system change to the EMR, individual clinicians also must be sure to modify personal order preferences. In addition, pharmacists can play an important role by updating policies that comply with these laws and by increasing pharmacy stocks of contraception supplies.7 For those living in states that do not currently have these laws, we encourage you to reach out to your legislators to advocate for similar laws as the data show clear medical and cost benefits for patients and society.
Continue to: Pharmacist prescription of hormonal contraception is safe and promotes continuation...
Pharmacist prescription of hormonal contraception is safe and promotes continuation
Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
Patients often face difficulty obtaining both new and timely refills of self-administered contraception.10,11 To expand contraception access, Oregon became the first state (in 2016) to enact legislation to authorize direct pharmacist prescribing of hormonal contraceptives.12 Currently, 17 states and the District of Columbia have protocols for pharmacist prescribing privileges (FIGURE 2), and proposed legislation is pending in another 14 states.10,12 These protocols vary, but basic processes include screening, documentation, monitoring, and referrals when necessary. Typically, protocols require a pharmacist to review a patient’s medical history, pregnancy status, medication use, and blood pressure, followed by contraceptive counseling.10 Pharmacies are generally located in the community they serve, have extended hours, and usually do not require an appointment.8,13,14

Pharmacist prescribing increases the number of new contraceptive users, and pharmacists are more likely to prescribe a 6-month or longer supply of contraceptives compared with clinicians.8,13,15 Also, pharmacist prescribing is safe, with adherence rates to the CDC’s US Medical Eligibility Criteria for Contraceptive Use similar to those of prescriptions provided by a clinician.13
Authors of a recent multi-state study further assessed the impact of pharmacist prescribing by evaluating 12-month continuation and perfect use rates.
Study design
Rodriguez and colleagues evaluated the results of a 1-year prospective cohort study conducted in 2019 that included 388 participants who sought contraception in California, Colorado, Hawaii, and Oregon. All these states had laws permitting pharmacist prescribing and 12-month supply of hormonal contraception. Participants received prescriptions directly from a pharmacist at 1 of 139 pharmacies (n = 149) or filled a prescription provided by a clinician (n = 239). The primary outcomes were continuation of an effective method and perfect use of contraception across 12 months.
Participant demographics were similar between the 2 groups except for education and insurance status. Participants who received a prescription from a clinician reported higher levels of education. A greater proportion of uninsured participants received a prescription from a pharmacist (11%) compared with from a clinician (3%).
Contraceptive continuation rates
Participants were surveyed 3 times during the 12-month study about their current contraceptive method, if they had switched methods, or if they had any missed days of contraception.
Overall, 340 participants (88%) completed a full 12 months of follow-up. Continuation rates were similar between the 2 groups: 89% in the clinician-prescribed and 90% in the pharmacist-prescribed group (P=.86). Participants in the 2 groups also reported similar rates of perfect use (no missed days: 54% and 47%, respectively [P=.69]). Additionally, the authors reported that 29 participants changed from a tier 2 (pill, patch, ring, injection) to a tier 1 (intrauterine device or implant) method during follow-up, with no difference in switch rates for participants who received care from a clinician (10%) or a pharmacist (7%).

Patients have difficulties in obtaining both an initial contraceptive prescription and refills in time to avoid breaks in coverage.16 Pharmacist prescription of contraception is a proven strategy to increase access to contraception for new users or to promote continuation among current users. This practice is evidence based, decreases unintended pregnancy rates, and is safe.8,13,15,17
Promoting universal pharmacist prescribing is even more important given the overruling of Roe v Wade. With abortion restrictions, many family planning clinics that also play a vital role in providing contraception will close. Most states that are limiting abortion care (FIGURE 3) are the same states without pharmacist-prescribing provisions (FIGURE 2). As patient advocates, we need to continue to support this evidence-based practice in states where it is available and push legislators in states where it is not. Pharmacists should receive support to complete the training and certification needed to not only provide this service but also to receive appropriate reimbursements. Restrictions, such as requiring patients to be 18 years or older or to have prior consultation with a physician, should be limited as these are not necessary to provide self-administered contraception safely. Clinicians and pharmacists should inform patients, in states where this is available, that they can access initial or refill prescriptions at their local pharmacy if that is more convenient or their preference. Clinicians who live in states without these laws can advocate for their community by encouraging their legislators to pass laws that allow this evidence-based practice.
Continue to: LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use...
LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●

As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.
- Kirstein M, Jones RK, Philbin J. One month post-Roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. July 28, 2022. Accessed September 14, 2022. https://www.guttmacher.org /article/2022/07/one-month-post-roe-least-43-abortion-clinics-across -11-states-have-stopped-offering
- Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
- Foster DG, Parvataneni R, de Bocanegra HT, et al. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107-114.
- Niu F, Cornelius J, Aboubechara N, et al. Real world outcomes related to providing an annual supply of short-acting hormonal contraceptives. Contraception. 2022;107:58-61.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Women’s sexual and reproductive health services: key findings from the 2017 Kaiser Women’s Health Survey. KFF: Kaiser Family Foundation. March 13, 2018. Accessed September 14, 2022. https://www.kff.org/womens-health-policy /issue-brief/womens-sexual-and-reproductive-health-services-key-findings -from-the-2017-kaiser-womens-health-survey/
- Nikpour G, Allen A, Rafie S, et al. Pharmacy implementation of a new law allowing year-long hormonal contraception supplies. Pharmacy (Basel). 2020;8:E165.
- Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3:e205252.
- Insurance coverage of contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state-policy /explore/insurance-coverage-contraceptives
- Chim C, Sharma P. Pharmacists prescribing hormonal contraceptives: a status update. US Pharm. 2021;46:45-49.
- Rodriguez MI, Hersh A, Anderson LB, et al. Association of pharmacist prescription of hormonal contraception with unintended pregnancies and Medicaid costs. Obstet Gynecol. 2019;133:1238-1246.
- Pharmacist-prescribed contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state -policy/explore/pharmacist-prescribed-contraceptives
- Anderson L, Hartung DM, Middleton L, et al. Pharmacist provision of hormonal contraception in the Oregon Medicaid population. Obstet Gynecol. 2019;133:1231-1237.
- Rodriguez MI, Edelman AB, Skye M, et al. Reasons for and experience in obtaining pharmacist prescribed contraception. Contraception. 2020;102:259-261.
- Rodriguez MI, Manibusan B, Kaufman M, et al. Association of pharmacist prescription of contraception with breaks in coverage. Obstet Gynecol. 2022;139:781-787.
- Pittman ME, Secura GM, Allsworth JE, et al. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011;83:340-345.
- Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
- Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-7.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52 mg intrauterine system. Contraception. 2020;101:159-161.
- Kirstein M, Jones RK, Philbin J. One month post-Roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. July 28, 2022. Accessed September 14, 2022. https://www.guttmacher.org /article/2022/07/one-month-post-roe-least-43-abortion-clinics-across -11-states-have-stopped-offering
- Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
- Foster DG, Parvataneni R, de Bocanegra HT, et al. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107-114.
- Niu F, Cornelius J, Aboubechara N, et al. Real world outcomes related to providing an annual supply of short-acting hormonal contraceptives. Contraception. 2022;107:58-61.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Women’s sexual and reproductive health services: key findings from the 2017 Kaiser Women’s Health Survey. KFF: Kaiser Family Foundation. March 13, 2018. Accessed September 14, 2022. https://www.kff.org/womens-health-policy /issue-brief/womens-sexual-and-reproductive-health-services-key-findings -from-the-2017-kaiser-womens-health-survey/
- Nikpour G, Allen A, Rafie S, et al. Pharmacy implementation of a new law allowing year-long hormonal contraception supplies. Pharmacy (Basel). 2020;8:E165.
- Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3:e205252.
- Insurance coverage of contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state-policy /explore/insurance-coverage-contraceptives
- Chim C, Sharma P. Pharmacists prescribing hormonal contraceptives: a status update. US Pharm. 2021;46:45-49.
- Rodriguez MI, Hersh A, Anderson LB, et al. Association of pharmacist prescription of hormonal contraception with unintended pregnancies and Medicaid costs. Obstet Gynecol. 2019;133:1238-1246.
- Pharmacist-prescribed contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state -policy/explore/pharmacist-prescribed-contraceptives
- Anderson L, Hartung DM, Middleton L, et al. Pharmacist provision of hormonal contraception in the Oregon Medicaid population. Obstet Gynecol. 2019;133:1231-1237.
- Rodriguez MI, Edelman AB, Skye M, et al. Reasons for and experience in obtaining pharmacist prescribed contraception. Contraception. 2020;102:259-261.
- Rodriguez MI, Manibusan B, Kaufman M, et al. Association of pharmacist prescription of contraception with breaks in coverage. Obstet Gynecol. 2022;139:781-787.
- Pittman ME, Secura GM, Allsworth JE, et al. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011;83:340-345.
- Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
- Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-7.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52 mg intrauterine system. Contraception. 2020;101:159-161.
2021 update on contraception
A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.
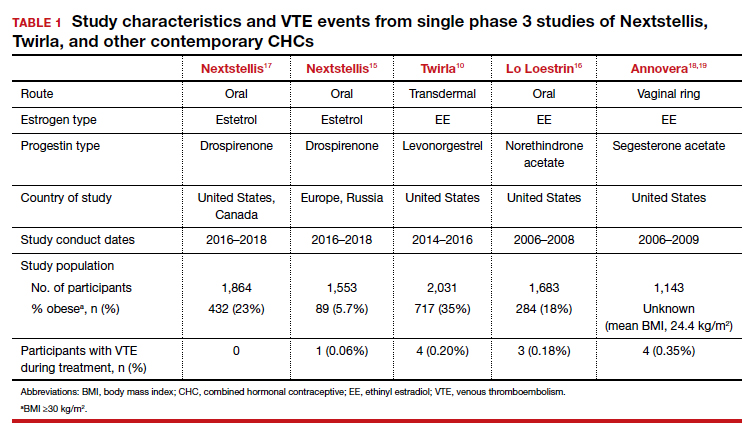
Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%
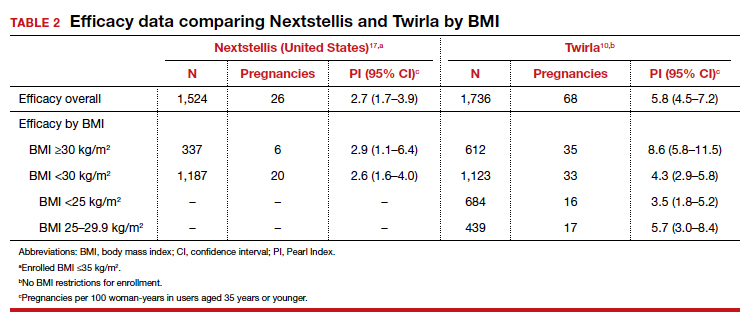
Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.
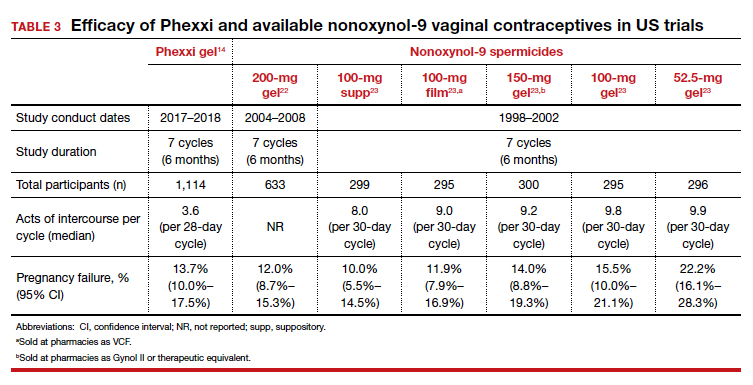
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.

Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%

Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.

A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.

Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%

Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.

- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
2020 Update on contraception
A vaginal ring that can be reused for up to 1 year and a progestin-only pill (POP) with a wider window for missed pills are 2 of the novel contraceptive products introduced to the market this year. In addition, an ongoing study of the levonorgestrel 52-mg intrauterine system (IUS) continues to provide evidence on its extended duration of use, now approved through 6 years.
The segesterone acetate (SA) and ethinyl estradiol (EE) vaginal ring (Annovera) is new among contraceptive options. Segesterone acetate is a novel progestin that can be used only via nonoral routes; it binds specifically to progesterone receptors without estrogenic or antiandrogen effects.1 Unlike the etonogestrel and ethinyl estradiol ring (NuvaRing; for which generic products became available this past year), which is used for 1 cycle and then thrown away, the SA/EE ring is effective for 13 consecutive cycles. It does not require refrigeration when not in use.2 Because a single ring can be used for 13 cycles, users in locations without laws that mandate a 12-month supply of pills, patches, and rings need less frequent visits to the pharmacy or clinic.
Progestin-only contraceptive pills are an important option for patients who desire hormonal contraception and have contraindications to estrogen, such as migraines with aura, cardiovascular risk factors, and being in the early postpartum period.3 In the United States, current POPs contain norethindrone, which has a 3-hour window for missed pills4; a desogestrel-only pill available outside the United States has a 12-hour window.5 Both are provided as a 28-day pill pack for continuous use, and both result in undesirable bleeding patterns in some users.
The prolonged half-life of drospirenone, another progestin, gives it the potential to increase reliability in the setting of missed or delayed pills and improve bleeding patterns. A new POP contraceptive contains drospirenone (Slynd) and is available in a 28-day pack with a 24-day supply of hormone and a 4-day supply of placebo; it provides a window for missed pill use similar to that for combined hormonal contraception (CHC) as well as a placebo period for a timed withdrawal bleed.6,7
Liletta is a well-known levonorgestrel 52-mg IUS that was first approved by the US Food and Drug Administration (FDA) in 2015. An ongoing clinical trial has been the basis for approval of this IUS for use in increasing durations, from 3 years initially to 4 and then 5 years. The newest data indicate efficacy up to 6 years.8
Continue to: Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile...
Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile
Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
Archer and colleagues reported the results of 2 pivotal multicenter, open-label, phase 3 trials, which included 2,265 users, conducted to evaluate efficacy and return to menses or pregnancy after use of the 1-year (13 cycles) SA/EE contraceptive vaginal system (CVS).
Details of the efficacy study
The study included 1,130 women in a US-only study and 1,135 women in an international study with sites in the United States, Australia, Brazil, Chile, Dominican Republic, Finland, Hungary, and Sweden. Participants used the CVS for 21 days followed by a 7-day use-free interval for up to 13 consecutive cycles; they were instructed not to remove the CVS for more than 2 hours during the 21 days of use.
Primary and secondary efficacy outcomes were calculated using the Pearl Index and an intention-to-treat Kaplan-Meier life table, respectively. At the end of the study, users who desired not to continue hormonal contraception or to become pregnant were followed up for 6 months to evaluate return to menses or pregnancy.
Year-long effectiveness
The investigators reported an overall Pearl index of 2.98 (95% confidence interval [CI], 2.13-4.06) and a Kaplan-Meier life table cumulative efficacy rate of 97.5% (FIGURE 1), consistent with other recently approved CHC methods. Women from non-European sites, who primarily were US participants, had a Pearl Index of 3.25 (95% CI, 2.35-4.37), and participants from the European sites had a Pearl Index of 0.47 (95% CI, 0.03-2.07). Importantly, CVS removal had a significant impact on efficacy, with a Pearl Index of 5.98 (95% CI, 2.46-9.27) in users reporting CVS removals for longer than 2 hours, suggesting escape ovulation with improper use. The Pearl Index was highest in users aged 18 to 19 years and was not affected by body mass index (BMI), although 91% of users had a BMI of 29.0 kg/m2 or lower.
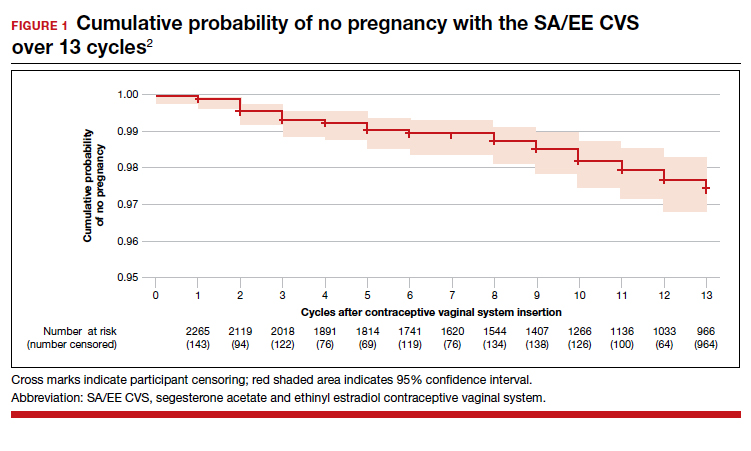
There was no trend for a change in pregnancy risk across 13 cycles, providing evidence of CVS efficacy throughout a full year's use. The follow-up portion of the study included 290 users who were not continuing hormonal contraception at study end; all follow-up participants reported return to menses after method discontinuation.
Clinical safety data
To evaluate safety outcomes from clinical studies on the CVS containing SA/EE, Gemzell-Danielsson and colleagues analyzed 9 studies. Most of the data were derived from 2 phase 3, multicenter trials (as discussed above), with supporting evidence from 7 other studies.
Adverse events reported
Among 2,308 CVS users in the phase 3 trials, 87% reported at least 1 adverse effect, with most of mild or moderate severity. These included headache, 26%; nausea, 18%; vaginal discharge, 10%; and metrorrhagia, 7%. Overall, 12% of CVS users discontinued use due to an adverse effect. Two percent of users experienced severe adverse effects, including venous thromboembolism (VTE), allergic reaction, gallbladder disease, and spontaneous abortion.
In the US-only phase 3 trial, 2 VTE events occurred in the first 6 months in women with baseline BMI greater than 29.0 kg/m2; therefore, enrollment of patients with a BMI greater than 29.0 kg/m2 was halted and current users meeting that criteria were discontinued. Notably, no cases of VTE occurred in studies with a segesterone acetate-only CVS; this suggests that risk can be attributed to the estrogen component. Overall, 4 nonfatal VTEs occurred, all among the 1,536 women enrolled in the phase 3 trials (4 of 1,536 [0.3%]); at least 3 of these cases occurred in users with VTE risk factors (TABLE 1). The estimated VTE rate in CVS users with a BMI greater than 29.0 kg/m2 is 10.8/10,000 women-years (95% CI, 8.9-13.1).
Complete expulsion of the CVS occurred in 7% of cycles and partial expulsion in 19.5% of cycles; users reported expulsion more frequently in the first cycle, most (about 70%) of which were partial expulsions. Of the laboratory values and vital signs studied, including weight, users had no clinically relevant changes from baseline.
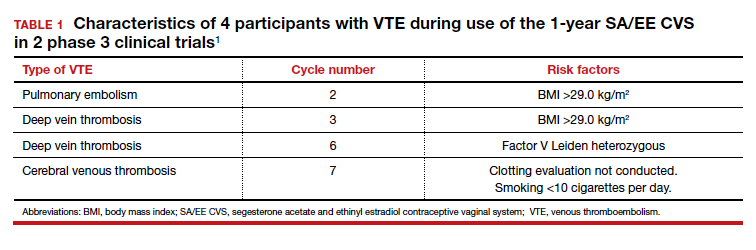
The 13-cycle efficacy and general adverse events rates of the new SA/EE CVS are consistent with those of other CHCs. However, the efficacy and safety findings are not necessarily generalizable to all patients. Because users with a BMI greater than 29.0 kg/m2 were excluded following 2 early VTE events in women with a BMI of 29.1 and 30.8 kg/m2 , only 9% of the phase 3 study population had a BMI greater than 29.0 kg/m2 . Clinicians may question whether the 1-year SA/EE CVS is an acceptable method for obese users. We know that EE causes similar changes in hemostatic factors regardless of oral or vaginal route,9 but these studies as well as pharmacokinetic studies typically include relatively few participants. While studies demonstrate that the SA/EE CVS delivers EE 13 µg daily,1 individual hormone absorption can vary. It is possible that the amount of EE in the CVS (17.4 mg) could, in a person predisposed to higher absorption, increase VTE risk. We do not know if this potential or actual risk is different for nonobese and obese users. To be fair, most of the EE-containing combined hormonal contraceptives were approved with study data that did not include obese women; the FDA first discussed the importance of including obese women in contraceptive approval studies in 2007.10 Thus, we do not know if this CVS has a significantly higher VTE risk in obese users than other methods.
All available information is based on cyclic CVS use (28-day cycles with a 7-day use-free interval). No data are available on drug levels, safety, or efficacy over extended periods of continuous use with the same CVS. During counseling, special emphasis should be placed on the increased pregnancy risk for patients who remove the ring for more than 2 hours.
Continue to: New drospirenone pill is an effective POP option...
New drospirenone pill is an effective POP option
Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
In a prospective, single-arm, multicenter phase 3 trial in the United States, Kimble and colleagues evaluated the efficacy and safety of an oral drospirenone POP in a cyclic 24-day hormone/4-day placebo regimen. The trial included 1,006 users. No BMI cutoff was used, and about one-third of study participants were obese (BMI >30.0 kg/m2). Women were instructed to take a missed tablet as soon as remembered if within 24 hours or with the next scheduled dose if more than 24 hours late.
Contraceptive effectiveness
The Pearl Index for nonbreastfeeding users aged 35 years or younger with pregnancies confirmed by a quantitative serum ß-human chorionic gonadotropin test (915 users) was 2.9 (95% CI, 1.5-5.1). Of note, 2 out of 15 on-treatment pregnancies were excluded from this calculation because of protocol site violations, as were 3 pregnancies that were unconfirmed. In the modified full analysis set of 915 users, 36% were obese (BMI≥30 kg/m2), and the Pearl Index was noted to be unaffected by BMI (TABLE 2).

While 61% of women reported adverse effects, more than 95% of these were mild or moderate in intensity, including headache, nausea, dysmenorrhea, metrorrhagia, and breast pain. No VTE occurred. The frequency of hyperkalemia was 0.5%, and there was no evidence of hypotension, which is significant due to the antimineralocorticoid activity of drospirenone. All cases of hyperkalemia were considered mild, and all women were asymptomatic. There were no clinically relevant changes in body weight, gynecologic exam, or other laboratory values.
With increased cycles of use, the number of days with bleeding or spotting generally decreased and amenorrhea increased. However, in cycles 11 to 13, 41.6% of users still had unscheduled bleeding (reduced from 57.0% at cycles 2-4), and 29.0% had scheduled bleeding (decreased from 44% at cycles 2-4) (FIGURE 2). With these bleeding patterns, 86.2% of users agreed or strongly agreed that they were satisfied with the product.
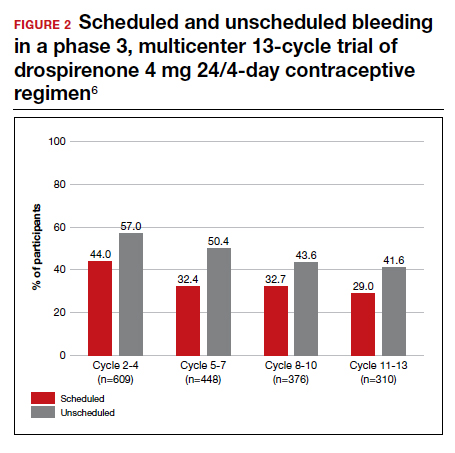
European multicenter study of drospironene
In a European investigation, Palacios and colleagues pooled and analyzed data from 2 phase 3 multicenter trials to assess the efficacy, tolerability, and safety of the same drospirenone-only pill (24 days of drospironene 4 mg and 4 days of placebo) in 1,571 users. No BMI cutoff was used, but overall only 71 participants (4.6%) were obese. One study included desogestrel 0.075 mg (in a regimen of 28 active pills) as a comparator for safety.
The overall Pearl Index for users 35 years or younger (1,251 users) was 1.0 (95% CI, 0.4-2.0). The "method failure Pearl Index" in users 35 years or younger, which included all pregnancies during "perfect medication cycles," was 1.3 (95% CI, 0.5-2.5).
The most common adverse effects were acne (6.6% in study 1 and 4.4% in study 2), headache (4.5% in study 1), and irregular bleeding (4.4% in study 2). No cases of VTE occurred; there was 1 case of asymptomatic hyperkalemia. Additional laboratory values and vital signs showed no significant changes. The trend in bleeding was similar to that in the US studies, but it is interesting to note that there were significantly lower rates of unscheduled bleeding or spotting in drospirenone users than in desogestrel users (67.9% vs 86.5%, respectively; P<.001).
In the US study, the higher Pearl Index compared with that found in the European study (2.9 vs 1.0) likely reflects an increased proportion of study participants with a BMI of 30 kg/m2 or higher, a younger average age of participants, and a historical tendency toward better contraceptive efficacy in European than in US study participants. Kimble and colleagues’ finding of a Pearl Index of 2.9 is similar to that seen with other CHCs and POPs, and the data from the US study are potentially more generalizable.
Among the 2,257 participants in 3 studies, 423 (19%) were obese. No VTE events occurred with drospirenone use, as compared with 4 events in the SA/EE CVS study with 2,308 participants in the phase 3 studies.
Historically, POPs were associated with more days of bleeding than CHCs and require stricter adherence to daily use within a narrow window for missed pills. The new drospirenone-only pill may provide women with more flexibility since it maintains contraceptive efficacy even with 24-hour delayed or missed-pill errors. Although intermenstrual bleeding rates are high, participants still had a very favorable assessment, and the profile may be more tolerable compared with other POPs. Clinicians prescribing this new POP should counsel patients that the cyclic regimen does not always result in regular bleeding patterns.
Continue to: Evidence supports 6 years' use of a levonorgestrel 52-mg IUS...
Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.
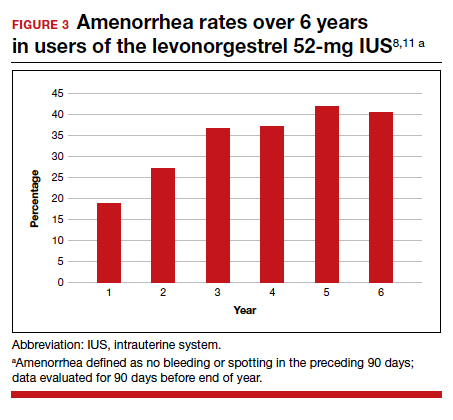
Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 206. Use of hormonal contraception in women with coexisiting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Ortho Micronor [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc; 2008.
- Cerazette [package insert]. Oss, Netherlands: Merck Sharp & Dohme Limited; 2019.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
- Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074-2079.
- Food and Drug Administration Advisory Committee for Reproductive Health Drugs meeting. Final summary minutes, January 23-24, 2007. https://wayback.archive-it.org/7993/20170404050830/https://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf. Accessed July 28, 2020.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
A vaginal ring that can be reused for up to 1 year and a progestin-only pill (POP) with a wider window for missed pills are 2 of the novel contraceptive products introduced to the market this year. In addition, an ongoing study of the levonorgestrel 52-mg intrauterine system (IUS) continues to provide evidence on its extended duration of use, now approved through 6 years.
The segesterone acetate (SA) and ethinyl estradiol (EE) vaginal ring (Annovera) is new among contraceptive options. Segesterone acetate is a novel progestin that can be used only via nonoral routes; it binds specifically to progesterone receptors without estrogenic or antiandrogen effects.1 Unlike the etonogestrel and ethinyl estradiol ring (NuvaRing; for which generic products became available this past year), which is used for 1 cycle and then thrown away, the SA/EE ring is effective for 13 consecutive cycles. It does not require refrigeration when not in use.2 Because a single ring can be used for 13 cycles, users in locations without laws that mandate a 12-month supply of pills, patches, and rings need less frequent visits to the pharmacy or clinic.
Progestin-only contraceptive pills are an important option for patients who desire hormonal contraception and have contraindications to estrogen, such as migraines with aura, cardiovascular risk factors, and being in the early postpartum period.3 In the United States, current POPs contain norethindrone, which has a 3-hour window for missed pills4; a desogestrel-only pill available outside the United States has a 12-hour window.5 Both are provided as a 28-day pill pack for continuous use, and both result in undesirable bleeding patterns in some users.
The prolonged half-life of drospirenone, another progestin, gives it the potential to increase reliability in the setting of missed or delayed pills and improve bleeding patterns. A new POP contraceptive contains drospirenone (Slynd) and is available in a 28-day pack with a 24-day supply of hormone and a 4-day supply of placebo; it provides a window for missed pill use similar to that for combined hormonal contraception (CHC) as well as a placebo period for a timed withdrawal bleed.6,7
Liletta is a well-known levonorgestrel 52-mg IUS that was first approved by the US Food and Drug Administration (FDA) in 2015. An ongoing clinical trial has been the basis for approval of this IUS for use in increasing durations, from 3 years initially to 4 and then 5 years. The newest data indicate efficacy up to 6 years.8
Continue to: Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile...
Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile
Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
Archer and colleagues reported the results of 2 pivotal multicenter, open-label, phase 3 trials, which included 2,265 users, conducted to evaluate efficacy and return to menses or pregnancy after use of the 1-year (13 cycles) SA/EE contraceptive vaginal system (CVS).
Details of the efficacy study
The study included 1,130 women in a US-only study and 1,135 women in an international study with sites in the United States, Australia, Brazil, Chile, Dominican Republic, Finland, Hungary, and Sweden. Participants used the CVS for 21 days followed by a 7-day use-free interval for up to 13 consecutive cycles; they were instructed not to remove the CVS for more than 2 hours during the 21 days of use.
Primary and secondary efficacy outcomes were calculated using the Pearl Index and an intention-to-treat Kaplan-Meier life table, respectively. At the end of the study, users who desired not to continue hormonal contraception or to become pregnant were followed up for 6 months to evaluate return to menses or pregnancy.
Year-long effectiveness
The investigators reported an overall Pearl index of 2.98 (95% confidence interval [CI], 2.13-4.06) and a Kaplan-Meier life table cumulative efficacy rate of 97.5% (FIGURE 1), consistent with other recently approved CHC methods. Women from non-European sites, who primarily were US participants, had a Pearl Index of 3.25 (95% CI, 2.35-4.37), and participants from the European sites had a Pearl Index of 0.47 (95% CI, 0.03-2.07). Importantly, CVS removal had a significant impact on efficacy, with a Pearl Index of 5.98 (95% CI, 2.46-9.27) in users reporting CVS removals for longer than 2 hours, suggesting escape ovulation with improper use. The Pearl Index was highest in users aged 18 to 19 years and was not affected by body mass index (BMI), although 91% of users had a BMI of 29.0 kg/m2 or lower.

There was no trend for a change in pregnancy risk across 13 cycles, providing evidence of CVS efficacy throughout a full year's use. The follow-up portion of the study included 290 users who were not continuing hormonal contraception at study end; all follow-up participants reported return to menses after method discontinuation.
Clinical safety data
To evaluate safety outcomes from clinical studies on the CVS containing SA/EE, Gemzell-Danielsson and colleagues analyzed 9 studies. Most of the data were derived from 2 phase 3, multicenter trials (as discussed above), with supporting evidence from 7 other studies.
Adverse events reported
Among 2,308 CVS users in the phase 3 trials, 87% reported at least 1 adverse effect, with most of mild or moderate severity. These included headache, 26%; nausea, 18%; vaginal discharge, 10%; and metrorrhagia, 7%. Overall, 12% of CVS users discontinued use due to an adverse effect. Two percent of users experienced severe adverse effects, including venous thromboembolism (VTE), allergic reaction, gallbladder disease, and spontaneous abortion.
In the US-only phase 3 trial, 2 VTE events occurred in the first 6 months in women with baseline BMI greater than 29.0 kg/m2; therefore, enrollment of patients with a BMI greater than 29.0 kg/m2 was halted and current users meeting that criteria were discontinued. Notably, no cases of VTE occurred in studies with a segesterone acetate-only CVS; this suggests that risk can be attributed to the estrogen component. Overall, 4 nonfatal VTEs occurred, all among the 1,536 women enrolled in the phase 3 trials (4 of 1,536 [0.3%]); at least 3 of these cases occurred in users with VTE risk factors (TABLE 1). The estimated VTE rate in CVS users with a BMI greater than 29.0 kg/m2 is 10.8/10,000 women-years (95% CI, 8.9-13.1).
Complete expulsion of the CVS occurred in 7% of cycles and partial expulsion in 19.5% of cycles; users reported expulsion more frequently in the first cycle, most (about 70%) of which were partial expulsions. Of the laboratory values and vital signs studied, including weight, users had no clinically relevant changes from baseline.

The 13-cycle efficacy and general adverse events rates of the new SA/EE CVS are consistent with those of other CHCs. However, the efficacy and safety findings are not necessarily generalizable to all patients. Because users with a BMI greater than 29.0 kg/m2 were excluded following 2 early VTE events in women with a BMI of 29.1 and 30.8 kg/m2 , only 9% of the phase 3 study population had a BMI greater than 29.0 kg/m2 . Clinicians may question whether the 1-year SA/EE CVS is an acceptable method for obese users. We know that EE causes similar changes in hemostatic factors regardless of oral or vaginal route,9 but these studies as well as pharmacokinetic studies typically include relatively few participants. While studies demonstrate that the SA/EE CVS delivers EE 13 µg daily,1 individual hormone absorption can vary. It is possible that the amount of EE in the CVS (17.4 mg) could, in a person predisposed to higher absorption, increase VTE risk. We do not know if this potential or actual risk is different for nonobese and obese users. To be fair, most of the EE-containing combined hormonal contraceptives were approved with study data that did not include obese women; the FDA first discussed the importance of including obese women in contraceptive approval studies in 2007.10 Thus, we do not know if this CVS has a significantly higher VTE risk in obese users than other methods.
All available information is based on cyclic CVS use (28-day cycles with a 7-day use-free interval). No data are available on drug levels, safety, or efficacy over extended periods of continuous use with the same CVS. During counseling, special emphasis should be placed on the increased pregnancy risk for patients who remove the ring for more than 2 hours.
Continue to: New drospirenone pill is an effective POP option...
New drospirenone pill is an effective POP option
Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
In a prospective, single-arm, multicenter phase 3 trial in the United States, Kimble and colleagues evaluated the efficacy and safety of an oral drospirenone POP in a cyclic 24-day hormone/4-day placebo regimen. The trial included 1,006 users. No BMI cutoff was used, and about one-third of study participants were obese (BMI >30.0 kg/m2). Women were instructed to take a missed tablet as soon as remembered if within 24 hours or with the next scheduled dose if more than 24 hours late.
Contraceptive effectiveness
The Pearl Index for nonbreastfeeding users aged 35 years or younger with pregnancies confirmed by a quantitative serum ß-human chorionic gonadotropin test (915 users) was 2.9 (95% CI, 1.5-5.1). Of note, 2 out of 15 on-treatment pregnancies were excluded from this calculation because of protocol site violations, as were 3 pregnancies that were unconfirmed. In the modified full analysis set of 915 users, 36% were obese (BMI≥30 kg/m2), and the Pearl Index was noted to be unaffected by BMI (TABLE 2).

While 61% of women reported adverse effects, more than 95% of these were mild or moderate in intensity, including headache, nausea, dysmenorrhea, metrorrhagia, and breast pain. No VTE occurred. The frequency of hyperkalemia was 0.5%, and there was no evidence of hypotension, which is significant due to the antimineralocorticoid activity of drospirenone. All cases of hyperkalemia were considered mild, and all women were asymptomatic. There were no clinically relevant changes in body weight, gynecologic exam, or other laboratory values.
With increased cycles of use, the number of days with bleeding or spotting generally decreased and amenorrhea increased. However, in cycles 11 to 13, 41.6% of users still had unscheduled bleeding (reduced from 57.0% at cycles 2-4), and 29.0% had scheduled bleeding (decreased from 44% at cycles 2-4) (FIGURE 2). With these bleeding patterns, 86.2% of users agreed or strongly agreed that they were satisfied with the product.

European multicenter study of drospironene
In a European investigation, Palacios and colleagues pooled and analyzed data from 2 phase 3 multicenter trials to assess the efficacy, tolerability, and safety of the same drospirenone-only pill (24 days of drospironene 4 mg and 4 days of placebo) in 1,571 users. No BMI cutoff was used, but overall only 71 participants (4.6%) were obese. One study included desogestrel 0.075 mg (in a regimen of 28 active pills) as a comparator for safety.
The overall Pearl Index for users 35 years or younger (1,251 users) was 1.0 (95% CI, 0.4-2.0). The "method failure Pearl Index" in users 35 years or younger, which included all pregnancies during "perfect medication cycles," was 1.3 (95% CI, 0.5-2.5).
The most common adverse effects were acne (6.6% in study 1 and 4.4% in study 2), headache (4.5% in study 1), and irregular bleeding (4.4% in study 2). No cases of VTE occurred; there was 1 case of asymptomatic hyperkalemia. Additional laboratory values and vital signs showed no significant changes. The trend in bleeding was similar to that in the US studies, but it is interesting to note that there were significantly lower rates of unscheduled bleeding or spotting in drospirenone users than in desogestrel users (67.9% vs 86.5%, respectively; P<.001).
In the US study, the higher Pearl Index compared with that found in the European study (2.9 vs 1.0) likely reflects an increased proportion of study participants with a BMI of 30 kg/m2 or higher, a younger average age of participants, and a historical tendency toward better contraceptive efficacy in European than in US study participants. Kimble and colleagues’ finding of a Pearl Index of 2.9 is similar to that seen with other CHCs and POPs, and the data from the US study are potentially more generalizable.
Among the 2,257 participants in 3 studies, 423 (19%) were obese. No VTE events occurred with drospirenone use, as compared with 4 events in the SA/EE CVS study with 2,308 participants in the phase 3 studies.
Historically, POPs were associated with more days of bleeding than CHCs and require stricter adherence to daily use within a narrow window for missed pills. The new drospirenone-only pill may provide women with more flexibility since it maintains contraceptive efficacy even with 24-hour delayed or missed-pill errors. Although intermenstrual bleeding rates are high, participants still had a very favorable assessment, and the profile may be more tolerable compared with other POPs. Clinicians prescribing this new POP should counsel patients that the cyclic regimen does not always result in regular bleeding patterns.
Continue to: Evidence supports 6 years' use of a levonorgestrel 52-mg IUS...
Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.

Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.
A vaginal ring that can be reused for up to 1 year and a progestin-only pill (POP) with a wider window for missed pills are 2 of the novel contraceptive products introduced to the market this year. In addition, an ongoing study of the levonorgestrel 52-mg intrauterine system (IUS) continues to provide evidence on its extended duration of use, now approved through 6 years.
The segesterone acetate (SA) and ethinyl estradiol (EE) vaginal ring (Annovera) is new among contraceptive options. Segesterone acetate is a novel progestin that can be used only via nonoral routes; it binds specifically to progesterone receptors without estrogenic or antiandrogen effects.1 Unlike the etonogestrel and ethinyl estradiol ring (NuvaRing; for which generic products became available this past year), which is used for 1 cycle and then thrown away, the SA/EE ring is effective for 13 consecutive cycles. It does not require refrigeration when not in use.2 Because a single ring can be used for 13 cycles, users in locations without laws that mandate a 12-month supply of pills, patches, and rings need less frequent visits to the pharmacy or clinic.
Progestin-only contraceptive pills are an important option for patients who desire hormonal contraception and have contraindications to estrogen, such as migraines with aura, cardiovascular risk factors, and being in the early postpartum period.3 In the United States, current POPs contain norethindrone, which has a 3-hour window for missed pills4; a desogestrel-only pill available outside the United States has a 12-hour window.5 Both are provided as a 28-day pill pack for continuous use, and both result in undesirable bleeding patterns in some users.
The prolonged half-life of drospirenone, another progestin, gives it the potential to increase reliability in the setting of missed or delayed pills and improve bleeding patterns. A new POP contraceptive contains drospirenone (Slynd) and is available in a 28-day pack with a 24-day supply of hormone and a 4-day supply of placebo; it provides a window for missed pill use similar to that for combined hormonal contraception (CHC) as well as a placebo period for a timed withdrawal bleed.6,7
Liletta is a well-known levonorgestrel 52-mg IUS that was first approved by the US Food and Drug Administration (FDA) in 2015. An ongoing clinical trial has been the basis for approval of this IUS for use in increasing durations, from 3 years initially to 4 and then 5 years. The newest data indicate efficacy up to 6 years.8
Continue to: Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile...
Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile
Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
Archer and colleagues reported the results of 2 pivotal multicenter, open-label, phase 3 trials, which included 2,265 users, conducted to evaluate efficacy and return to menses or pregnancy after use of the 1-year (13 cycles) SA/EE contraceptive vaginal system (CVS).
Details of the efficacy study
The study included 1,130 women in a US-only study and 1,135 women in an international study with sites in the United States, Australia, Brazil, Chile, Dominican Republic, Finland, Hungary, and Sweden. Participants used the CVS for 21 days followed by a 7-day use-free interval for up to 13 consecutive cycles; they were instructed not to remove the CVS for more than 2 hours during the 21 days of use.
Primary and secondary efficacy outcomes were calculated using the Pearl Index and an intention-to-treat Kaplan-Meier life table, respectively. At the end of the study, users who desired not to continue hormonal contraception or to become pregnant were followed up for 6 months to evaluate return to menses or pregnancy.
Year-long effectiveness
The investigators reported an overall Pearl index of 2.98 (95% confidence interval [CI], 2.13-4.06) and a Kaplan-Meier life table cumulative efficacy rate of 97.5% (FIGURE 1), consistent with other recently approved CHC methods. Women from non-European sites, who primarily were US participants, had a Pearl Index of 3.25 (95% CI, 2.35-4.37), and participants from the European sites had a Pearl Index of 0.47 (95% CI, 0.03-2.07). Importantly, CVS removal had a significant impact on efficacy, with a Pearl Index of 5.98 (95% CI, 2.46-9.27) in users reporting CVS removals for longer than 2 hours, suggesting escape ovulation with improper use. The Pearl Index was highest in users aged 18 to 19 years and was not affected by body mass index (BMI), although 91% of users had a BMI of 29.0 kg/m2 or lower.

There was no trend for a change in pregnancy risk across 13 cycles, providing evidence of CVS efficacy throughout a full year's use. The follow-up portion of the study included 290 users who were not continuing hormonal contraception at study end; all follow-up participants reported return to menses after method discontinuation.
Clinical safety data
To evaluate safety outcomes from clinical studies on the CVS containing SA/EE, Gemzell-Danielsson and colleagues analyzed 9 studies. Most of the data were derived from 2 phase 3, multicenter trials (as discussed above), with supporting evidence from 7 other studies.
Adverse events reported
Among 2,308 CVS users in the phase 3 trials, 87% reported at least 1 adverse effect, with most of mild or moderate severity. These included headache, 26%; nausea, 18%; vaginal discharge, 10%; and metrorrhagia, 7%. Overall, 12% of CVS users discontinued use due to an adverse effect. Two percent of users experienced severe adverse effects, including venous thromboembolism (VTE), allergic reaction, gallbladder disease, and spontaneous abortion.
In the US-only phase 3 trial, 2 VTE events occurred in the first 6 months in women with baseline BMI greater than 29.0 kg/m2; therefore, enrollment of patients with a BMI greater than 29.0 kg/m2 was halted and current users meeting that criteria were discontinued. Notably, no cases of VTE occurred in studies with a segesterone acetate-only CVS; this suggests that risk can be attributed to the estrogen component. Overall, 4 nonfatal VTEs occurred, all among the 1,536 women enrolled in the phase 3 trials (4 of 1,536 [0.3%]); at least 3 of these cases occurred in users with VTE risk factors (TABLE 1). The estimated VTE rate in CVS users with a BMI greater than 29.0 kg/m2 is 10.8/10,000 women-years (95% CI, 8.9-13.1).
Complete expulsion of the CVS occurred in 7% of cycles and partial expulsion in 19.5% of cycles; users reported expulsion more frequently in the first cycle, most (about 70%) of which were partial expulsions. Of the laboratory values and vital signs studied, including weight, users had no clinically relevant changes from baseline.

The 13-cycle efficacy and general adverse events rates of the new SA/EE CVS are consistent with those of other CHCs. However, the efficacy and safety findings are not necessarily generalizable to all patients. Because users with a BMI greater than 29.0 kg/m2 were excluded following 2 early VTE events in women with a BMI of 29.1 and 30.8 kg/m2 , only 9% of the phase 3 study population had a BMI greater than 29.0 kg/m2 . Clinicians may question whether the 1-year SA/EE CVS is an acceptable method for obese users. We know that EE causes similar changes in hemostatic factors regardless of oral or vaginal route,9 but these studies as well as pharmacokinetic studies typically include relatively few participants. While studies demonstrate that the SA/EE CVS delivers EE 13 µg daily,1 individual hormone absorption can vary. It is possible that the amount of EE in the CVS (17.4 mg) could, in a person predisposed to higher absorption, increase VTE risk. We do not know if this potential or actual risk is different for nonobese and obese users. To be fair, most of the EE-containing combined hormonal contraceptives were approved with study data that did not include obese women; the FDA first discussed the importance of including obese women in contraceptive approval studies in 2007.10 Thus, we do not know if this CVS has a significantly higher VTE risk in obese users than other methods.
All available information is based on cyclic CVS use (28-day cycles with a 7-day use-free interval). No data are available on drug levels, safety, or efficacy over extended periods of continuous use with the same CVS. During counseling, special emphasis should be placed on the increased pregnancy risk for patients who remove the ring for more than 2 hours.
Continue to: New drospirenone pill is an effective POP option...
New drospirenone pill is an effective POP option
Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
In a prospective, single-arm, multicenter phase 3 trial in the United States, Kimble and colleagues evaluated the efficacy and safety of an oral drospirenone POP in a cyclic 24-day hormone/4-day placebo regimen. The trial included 1,006 users. No BMI cutoff was used, and about one-third of study participants were obese (BMI >30.0 kg/m2). Women were instructed to take a missed tablet as soon as remembered if within 24 hours or with the next scheduled dose if more than 24 hours late.
Contraceptive effectiveness
The Pearl Index for nonbreastfeeding users aged 35 years or younger with pregnancies confirmed by a quantitative serum ß-human chorionic gonadotropin test (915 users) was 2.9 (95% CI, 1.5-5.1). Of note, 2 out of 15 on-treatment pregnancies were excluded from this calculation because of protocol site violations, as were 3 pregnancies that were unconfirmed. In the modified full analysis set of 915 users, 36% were obese (BMI≥30 kg/m2), and the Pearl Index was noted to be unaffected by BMI (TABLE 2).

While 61% of women reported adverse effects, more than 95% of these were mild or moderate in intensity, including headache, nausea, dysmenorrhea, metrorrhagia, and breast pain. No VTE occurred. The frequency of hyperkalemia was 0.5%, and there was no evidence of hypotension, which is significant due to the antimineralocorticoid activity of drospirenone. All cases of hyperkalemia were considered mild, and all women were asymptomatic. There were no clinically relevant changes in body weight, gynecologic exam, or other laboratory values.
With increased cycles of use, the number of days with bleeding or spotting generally decreased and amenorrhea increased. However, in cycles 11 to 13, 41.6% of users still had unscheduled bleeding (reduced from 57.0% at cycles 2-4), and 29.0% had scheduled bleeding (decreased from 44% at cycles 2-4) (FIGURE 2). With these bleeding patterns, 86.2% of users agreed or strongly agreed that they were satisfied with the product.

European multicenter study of drospironene
In a European investigation, Palacios and colleagues pooled and analyzed data from 2 phase 3 multicenter trials to assess the efficacy, tolerability, and safety of the same drospirenone-only pill (24 days of drospironene 4 mg and 4 days of placebo) in 1,571 users. No BMI cutoff was used, but overall only 71 participants (4.6%) were obese. One study included desogestrel 0.075 mg (in a regimen of 28 active pills) as a comparator for safety.
The overall Pearl Index for users 35 years or younger (1,251 users) was 1.0 (95% CI, 0.4-2.0). The "method failure Pearl Index" in users 35 years or younger, which included all pregnancies during "perfect medication cycles," was 1.3 (95% CI, 0.5-2.5).
The most common adverse effects were acne (6.6% in study 1 and 4.4% in study 2), headache (4.5% in study 1), and irregular bleeding (4.4% in study 2). No cases of VTE occurred; there was 1 case of asymptomatic hyperkalemia. Additional laboratory values and vital signs showed no significant changes. The trend in bleeding was similar to that in the US studies, but it is interesting to note that there were significantly lower rates of unscheduled bleeding or spotting in drospirenone users than in desogestrel users (67.9% vs 86.5%, respectively; P<.001).
In the US study, the higher Pearl Index compared with that found in the European study (2.9 vs 1.0) likely reflects an increased proportion of study participants with a BMI of 30 kg/m2 or higher, a younger average age of participants, and a historical tendency toward better contraceptive efficacy in European than in US study participants. Kimble and colleagues’ finding of a Pearl Index of 2.9 is similar to that seen with other CHCs and POPs, and the data from the US study are potentially more generalizable.
Among the 2,257 participants in 3 studies, 423 (19%) were obese. No VTE events occurred with drospirenone use, as compared with 4 events in the SA/EE CVS study with 2,308 participants in the phase 3 studies.
Historically, POPs were associated with more days of bleeding than CHCs and require stricter adherence to daily use within a narrow window for missed pills. The new drospirenone-only pill may provide women with more flexibility since it maintains contraceptive efficacy even with 24-hour delayed or missed-pill errors. Although intermenstrual bleeding rates are high, participants still had a very favorable assessment, and the profile may be more tolerable compared with other POPs. Clinicians prescribing this new POP should counsel patients that the cyclic regimen does not always result in regular bleeding patterns.
Continue to: Evidence supports 6 years' use of a levonorgestrel 52-mg IUS...
Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.

Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 206. Use of hormonal contraception in women with coexisiting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Ortho Micronor [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc; 2008.
- Cerazette [package insert]. Oss, Netherlands: Merck Sharp & Dohme Limited; 2019.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
- Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074-2079.
- Food and Drug Administration Advisory Committee for Reproductive Health Drugs meeting. Final summary minutes, January 23-24, 2007. https://wayback.archive-it.org/7993/20170404050830/https://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf. Accessed July 28, 2020.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 206. Use of hormonal contraception in women with coexisiting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Ortho Micronor [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc; 2008.
- Cerazette [package insert]. Oss, Netherlands: Merck Sharp & Dohme Limited; 2019.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
- Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074-2079.
- Food and Drug Administration Advisory Committee for Reproductive Health Drugs meeting. Final summary minutes, January 23-24, 2007. https://wayback.archive-it.org/7993/20170404050830/https://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf. Accessed July 28, 2020.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
2019 Update on contraception
Long-acting reversible contraception (LARC) use continues to increase in the United States. According to the most recent estimates from 2014, 14% of women use either an intrauterine device (IUD) or the etonogestrel implant.1 Forms of LARC currently available in the United States include:
- 4 hormone-releasing IUDs
- 1 nonhormonal copper IUD, and
- 1 hormonal subdermal implant.
The hormone-releasing IUDs all contain levonorgestrel (LNG). These include two 52-mg LNG products and a 19.5-mg LNG IUD, which are currently approved by the US Food and Drug Administration (FDA) for contraception for 5 continuous years of use. In addition, a 13.5-mg LNG IUD is FDA-approved for 3 years of use. The hormonal subdermal implant, which contains etonogestrel, is FDA-approved for 3 years of use. Although major complications with IUDs (perforation, expulsion, intrauterine infection)and implants (subfascial implantation, distant migration) are rare, adverse effects that can affect continuation—such as irregular bleeding—are more common.2,3
Contraceptive discontinuation due to bleeding concerns occurs more frequently with the etonogestrel implant than with LNG IUDs (TABLE 1). In a large prospective study in the United States, 13% of women discontinued the implant during 3 years of follow-up due to bleeding pattern changes.
Notably, it is important to use standardized definitions to understand and compare bleeding concerns with LARC use. The Belsey criteria of the World Health Organization (WHO), a standard used for decades, describe bleeding patterns using 90-day reference periods or intervals (TABLE 2).9 Bleeding patterns that decrease flow (amenorrhea, infrequent bleeding) often are considered favorable, and those that increase bleeding or irregularity often are considered unfavorable. These criteria are commonly used in package labeling to describe bleeding patterns with extended use.
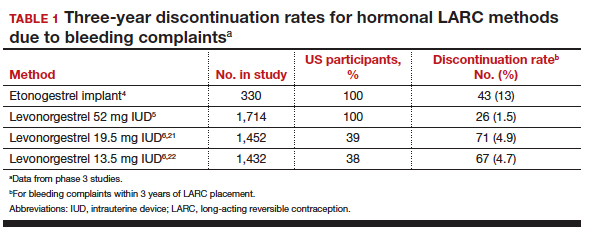
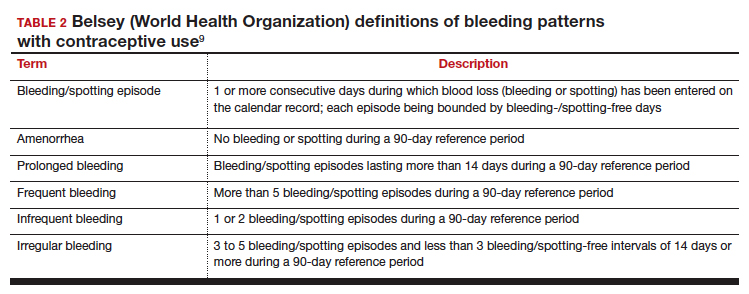
In this Update, we examine recent data evaluating differences in bleeding patterns with the 3 doses of the LNG IUD, predictors of abnormal bleeding with the etonogestrel implant, and the impact of timing on postpartum etonogestrel implant placement.
Continue to: Bleeding patterns with progestin-containing IUDs vary according to the LNG dose...
Bleeding patterns with progestin-containing IUDs vary according to the LNG dose
Goldthwaite LM, Creinin MD. Comparing bleeding patterns for the levonorgestrel 52 mg, 19.5 mg, and 13.5 mg intrauterine systems. Contraception. 2019;100:128-131.
Counseling on IUDs' different hormonal doses requires an understanding of patients' desires for contraceptive efficacy and bleeding expectations. A recent study provides guidance on what patients typically can expect for their bleeding patterns over the first few years with the 3 different doses of LNG IUDs.
Goldthwaite and Creinin used existing published or publicly available data to analyze differences in bleeding patterns associated with the 52-mg, 19.5-mg, and 13.5-mg LNG IUDs. Although two 52-mg LNG IUDs are available, published data using the WHO Belsey criteria are available only for one (Liletta; Allergan, Medicines360). The 2 products have been shown previously to have similar drug-release rates and LNG levels over 5 years.8
Comparing favorable bleeding patterns: Amenorrhea and infrequent bleeding
Among favorable bleeding patterns, amenorrhea was uncommon in the first 90 days and increased over time for all 3 IUDs. However, starting as soon as the second 90-day reference period, amenorrhea rates were significantly higher with the 52-mg LNG IUD compared with both of the lower-LNG dose IUDs, and this difference increased through 3 years of use (FIGURE 1).
Similarly, the 19.5-mg LNG IUD users had significantly higher rates of amenorrhea than the 13.5-mg LNG IUD users for all periods starting with the second 90-day reference period. At 3 years, 36% of women using the 52-mg LNG IUD had amenorrhea compared with 20% of those using the 19.5-mg LNG IUD (P<.0001) and 12% of those using the 13.5-mg LNG IUD (P<.0001).
Infrequent bleeding was similar for all 3 LNG IUDs in the first 90-day period, and it then increased most rapidly in the 52-mg LNG IUD users. At the end of year 1, 30% of the 52-mg LNG IUD users had infrequent bleeding compared with 26% of the 19.5-mg users (P = .01) and 20% of the 13.5-mg users (P<.0001). Although there was no difference in infrequent bleeding rates between the 52-mg and the 19.5-mg LNG IUD users at the end of year 1, those using a 52-mg LNG IUD had significantly higher rates of infrequent bleeding compared with the 13.5-mg LNG IUD at all time points.
Comparing unfavorable bleeding patterns: Frequent, prolonged, and irregular bleeding
Frequent and prolonged bleeding were uncommon with all LNG doses. Irregular bleeding rates declined for users of the 3 IUDs over time. However, significantly fewer users of the 52-mg LNG IUD reported irregular bleeding at 1 year (6%) compared with users of the 19.5-mg (16.5%, P<.0001) and 13.5-mg (23%, P<.0001) LNG IUD (FIGURE 2).
Study limitations
Comparing the data from different studies has limitations. For example, the data were collected from different populations, with the lower-dose LNG products tested in women who had a lower body mass index (BMI) and higher parity. However, prior analysis of the data on the 52-mg LNG IUD demonstrated that bleeding pattern changes did not vary based on these factors.10
When considering the different progestin-based IUD options, it is important to counsel patients according to their preferences for potential adverse effects. A randomized trial during product development found no difference in systemic adverse effects with the 3 doses of LNG IUD, likely because the systemic hormone levels are incredibly low for all 3 products.11 The summary data in this report helps explain why women using the lower-dose LNG products have slightly higher discontinuation rates for bleeding complaints, a fact we can explain to our patients during counseling.
Overall, the 52-mg LNG IUD is associated with a higher likelihood of favorable bleeding patterns over the first few years of use, with higher rates of amenorrhea and infrequent bleeding and lower rates of irregular bleeding. For women who prefer to not have periods or to have infrequent periods, the 52-mg LNG IUD is most likely to provide that outcome. For a patient who prefers to have periods, there is no evidence that the lower-dose IUDs result in “regular” or “normal” menstrual bleeding, even though they do result in more bleeding/spotting days overall. To the contrary, the available data show that these women have a significantly higher likelihood of experiencing prolonged, frequent, and irregular bleeding. In fact, no studies have reported rates of “normal” bleeding with the progestin IUDs, likely because women uncommonly have “normal” bleeding with these contraception methods. If a patient does not desire amenorrhea or strongly prefers to have “regular bleeding,” alternative methods such as a copper IUD should be considered rather than counseling her toward a lower-dose progestin IUD.
Continue to: Predicting long-term bleeding patterns after etonogestrel implant insertion...
Predicting long-term bleeding patterns after etonogestrel implant insertion
Mansour D, Fraser IS, Edelman A, et al. Can initial vaginal bleeding patterns in etonogestrel implant users predict subsequent bleeding in the first two years of use? Contraception. 2019. doi: 10.1016/j.contraception.2019.05.017.
Data from 2014 indicate that the etonogestrel implant was used by nearly 1 million women in the United States and by 3% of women using contraception.1 The primary reason women discontinue implant use is because of changes in bleeding patterns. Given the high prevalence of bleeding concerns with the etonogestrel implant, we need more data to help counsel our patients on how they can expect their bleeding to change with implant use.
Etonogestral implant and bleeding pattern trends
Mansour and colleagues completed a secondary analysis of 12 phase 3 studies to evaluate the correlation between bleeding patterns early after placement of the etonogestrel implant (days 29-118) compared with bleeding patterns through 90-day intervals during the rest of the first year of use. To account for differences in timing of etonogestrel implant placement relative to the menstrual cycle and discontinuation of other methods like oral contraceptives, bleeding outcomes on days 0-28 were excluded. They also sought to investigate the correlation between bleeding patterns in year 1 compared with those in year 2.
Overall, these studies included 923 individuals across 11 countries; however, for the current analysis, the researchers excluded women from Asian countries who comprised more than 28% of the study population. These women report significantly fewer bleeding/spotting days with the etonogestrel implant and have a lower average body weight compared with European and American women.12
A prior analysis of the same data set looked at the number of bleeding/spotting days in groups of users rather than trends in individual patients, and, as mentioned, it also included Asian women, which diluted the overall number of bleeding days.12 In this new analysis, Mansour and colleagues used the Belsey criteria to analyze individual bleeding patterns as favorable (amenorrhea, infrequent bleeding, normal bleeding) or unfavorable (prolonged and/or frequent bleeding) from a patient perspective. In this way, we can understand trends in bleeding patterns for each patient over time, rather than seeing a static (cross-sectional) report of bleeding patterns at one point in time. Data were analyzed from 537 women in year 1 and 428 women in year 2. During the first 90-day reference period (days 29-118 after implant insertion), 61% of women reported favorable bleeding, and 39% reported unfavorable bleeding.
Favorable bleeding correlates with favorable patterns later
A favorable bleeding pattern in this first reference period correlated with favorable bleeding patterns through year 1, with 85%, 80%, and 80% of these women having a favorable pattern in reference periods 2, 3, and 4, respectively. Overall, 61% of women with a favorable pattern in reference period 1 had favorable bleeding throughout the entire first year of use. Only 3.7% of women with favorable bleeding in the first reference period discontinued the implant for bleeding in year 1. Further, women with favorable bleeding at year 1 commonly continued to have favorable bleeding in year 2, with a low discontinuation rate (2.5%) in year 2.
Individual patients who have a favorable bleeding pattern initially with etonogestrel implant placement are highly likely to continue having favorable bleeding at year 1 and year 2. Notably, of women with a favorable bleeding pattern in any 90-day reference period, about 80% will continue to have a favorable bleeding pattern in the next reference period. These women can be counseled that, even if they have a 90-day period with unfavorable bleeding, about two-thirds will have a favorable pattern in the next reference period. For those with initial unfavorable patterns, about one-third to one-half change to a favorable pattern in subsequent 90-day reference periods. For women who require intervention for unfavorable bleeding but wish to keep their etonogestrel implant, prior data support use of combined oral contraceptive pills, although bleeding resolution seems to be temporary, with 86% of women having bleeding recurrence within 10 days after treatment.13
Initial unfavorable bleeding portends less favorable patterns later
Women who had an unfavorable bleeding pattern initially, however, had a less predictable course over the first year. For those with an initial unfavorable pattern, only 37%, 47%, and 51% reported a favorable pattern in reference periods 2, 3, and 4. Despite these relatively low rates of favorable bleeding, only 13% of the women with an initial unfavorable bleeding pattern discontinued implant use for a bleeding complaint by the end of year 1; this rate was significantly higher than that for women with a favorable initial bleeding pattern (P<.0001). The discontinuation rate for bleeding complaints also remained higher in year 2, at 16.5%.
Limitations and strengths to consider
Although the etonogestrel implant is FDA-approved for 3 years of use, the bleeding data from the combined trials included information for only up to 2 years after placement. The studies included also did not uniformly assess BMI, which makes it difficult to find correlations between bleeding patterns and BMI. Importantly, the studies did not include women who were more than 30% above their ideal body weight, so these assessments do not apply to obese users.12 Exclusion of women from Southeast Asia in this analysis makes this study's findings more generalizable to populations in the United States and Europe.
Continue to: Early versus delayed postpartum etonogestrel implant insertion...
Early versus delayed postpartum etonogestrel implant insertion: Similar impacts on 12-month bleeding patterns
Vieira CS, de Nadai MN, de Melo Pereira do Carmo LS, et al. Timing of postpartum etonogestrel-releasing implant insertion and bleeding patterns, weight change, 12-month continuation and satisfaction rates: a randomized controlled trial. Contraception. 2019. doi:10.1016/j.contraception.2019.05.007.
Initiation of a desired LARC method shortly after delivery is associated with significant reductions in short interpregnancy intervals.14 With that goal in mind, Vieira and colleagues compared bleeding patterns in women who received an etonogestrel implant within 48 hours of delivery with those who received an implant at 6 weeks postdelivery.
The study was a secondary analysis of data from a randomized controlled trial of early versus delayed postpartum insertion of the etonogestrel implant conducted in Sao Paulo, Brazil. That primary trial's goal was to examine the impact of early versus delayed implant insertion on infant growth (100 women were randomly assigned to the 2 implant groups); no difference in infant growth at 12 months was seen in the 2 groups.15 In the secondary analysis, bleeding patterns and BMI were evaluated every 90 days for 12 months. The mean BMI at enrollment postpartum was 29.4 kg/m2 in the early-insertion group and 30.2 kg/m2 for the delayed-insertion group.
Bleeding patterns with early or delayed implant insertion were similar
Vieira and colleagues found similar bleeding patterns between the groups over 12 months of follow-up. Amenorrhea was reported by 56% of the early-insertion group in the first 90 days and by 62% in the delayed-insertion group. During the last 90 days of the year, 52% of the early-insertion and 46% of the delayed-insertion group reported amenorrhea. Amenorrhea rates did not differ between women who were exclusively breastfeeding and those nonexclusively breastfeeding.
Continuation rates were high at 1 year
Prolonged bleeding episodes were uncommon in both groups, with only 2% of women reporting prolonged bleeding in any given reference period. Twelve-month implant continuation rates were high in both groups: 98% in the early- and 100% in the delayed-insertion group. Additionally, the investigators found that both groups experienced a BMI decrease, with no difference between groups (10.3% and 11% in the early- and delayed-insertion groups, respectively).
Study limitations and strengths
This study included a larger number of participants than prior randomized, controlled trials that evaluated bleeding patterns with postpartum etonogestrel implant insertion, and it had very low rates of loss to follow-up. The study's low rate of 12-month implant discontinuation (2%) is lower than that of other studies that reported rates of 6% to 14%.16,17 Although the authors stated that this low rate may be due to thorough anticipatory counseling prior to placement, it is also possible that this study population does not reflect all populations. Regardless, the data clearly show that placing an etonogestrel implant prior to hospital discharge, compared with waiting for later placement, does not impact bleeding patterns over the ensuing year.
For patients who desire an etonogestrel implant for contraception postpartum, we now have additional information to counsel about the impact of implant placement on postpartum bleeding patterns. Overall, bleeding patterns are highly favorable and do not vary whether the implant is placed in the hospital or later. Additionally, the timing of placement does not impact implant continuation rates or BMI changes over 1 year. Further, the primary study assessed infant growth in the early- versus delayed-placement groups and found no differences in infant growth. Although the data are limited, immediate postpartum etonogestrel implant placement does not seem to affect the rate of breastfeeding or the volume of breast milk.18,19 Timing of implant placement, assuming adequate resources, should be based primarily on patient preference. And, given the correlation of immediate postpartum LARC placement to increased interpregnancy interval, particular efforts should be made to provide the implant in the immediate postpartum period, if the patient desires.20
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97:14-21.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397-404.
- Odom EB, Eisenberg DL, Fox IK. Difficult removal of subdermal contraceptive implants: a multidisciplinary approach involving a peripheral nerve expert. Contraception. 2017;96: 89-95.
- Funk S, Miller MM, Mishell DR Jr, et al; Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
- Eisenberg DL, Schreiber CA, Turok DK, et al; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92:10-16.
- Nelson A, Apter D, Hauck B, et al. Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstet Gynecol. 2013;122:1205-1213.
- Beckert V, Ahlers C, Frenz AK, et al. Bleeding patterns with the 19.5mg LNG-IUS, with special focus on the first year of use: implications for counselling. Eur J Contracept Reprod Health Care. 2019;24:251-259.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
- Belsey EM, Machines D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. Contraception. 1986;34:253-260.
- Schreiber CA, Teal SB, Blumenthal PD, et al. Bleeding patterns for the Liletta® levonorgestrel 52mg intrauterine system. Eur J Contracept Reprod Health Care. 2018;23:116–120.
- Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril. 2012;97:616-22.e1-3.
- Mansour D, Korver T, Marintcheva-Petrova M, et al. The effects of Implanon on menstrual bleeding patterns. Eur J Contracept Reprod Health Care. 2008;13(suppl 1):13-28.
- Guiahi M, McBride M, Sheeder J, et al. Short-term treatment of bothersome bleeding for etonogestrel implant users using a 14-day oral contraceptive pill regimen: a randomized controlled trial. Obstet Gynecol. 2015;126:508-513.
- Brunson MR, Klein DA, Olsen CH, et al. Postpartum contraception: initiation and effectiveness in a large universal healthcare system. Am J Obstet Gynecol. 2017;217:55.e1-55.e9
- de Melo Pereira Carmo LS, Braga GC, Ferriani RA, et al. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017;130:100-107.
- Crockett AH, Pickell LB, Heberlein EC, et al. Six- and twelve-month documented removal rates among women electing postpartum inpatient compared to delayed or interval contraceptive implant insertions after Medicaid payment reform. Contraception. 2017;95:71-76.
- Wilson S, Tennant C, Sammel MD, et al. Immediate postpartum etonogestrel implant: a contraception option with long-term continuation. Contraception. 2014;90:259-264.
- Sothornwit J, Werawatakul Y, Kaewrudee S, et al. Immediate versus delayed postpartum insertion of contraceptive implant for contraception. Cochrane Database Syst Rev. 2017;4:CD011913.
- Braga GC, Ferriolli E, Quintana SM, et al. Immediate postpartum initiation of etonogestrel-releasing implant: a randomized controlled trial on breastfeeding impact. Contraception. 2015;92:536-542.
- Thiel de Bocanegra H, Chang R, Howell M, et al. Interpregnancy intervals: impact of postpartum contraceptive effectiveness and coverage. Am J Obstet Gynecol. 2014;210:311.e1-8.
- Kyleena [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc;2016.
- Skyla [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2016.
Long-acting reversible contraception (LARC) use continues to increase in the United States. According to the most recent estimates from 2014, 14% of women use either an intrauterine device (IUD) or the etonogestrel implant.1 Forms of LARC currently available in the United States include:
- 4 hormone-releasing IUDs
- 1 nonhormonal copper IUD, and
- 1 hormonal subdermal implant.
The hormone-releasing IUDs all contain levonorgestrel (LNG). These include two 52-mg LNG products and a 19.5-mg LNG IUD, which are currently approved by the US Food and Drug Administration (FDA) for contraception for 5 continuous years of use. In addition, a 13.5-mg LNG IUD is FDA-approved for 3 years of use. The hormonal subdermal implant, which contains etonogestrel, is FDA-approved for 3 years of use. Although major complications with IUDs (perforation, expulsion, intrauterine infection)and implants (subfascial implantation, distant migration) are rare, adverse effects that can affect continuation—such as irregular bleeding—are more common.2,3
Contraceptive discontinuation due to bleeding concerns occurs more frequently with the etonogestrel implant than with LNG IUDs (TABLE 1). In a large prospective study in the United States, 13% of women discontinued the implant during 3 years of follow-up due to bleeding pattern changes.
Notably, it is important to use standardized definitions to understand and compare bleeding concerns with LARC use. The Belsey criteria of the World Health Organization (WHO), a standard used for decades, describe bleeding patterns using 90-day reference periods or intervals (TABLE 2).9 Bleeding patterns that decrease flow (amenorrhea, infrequent bleeding) often are considered favorable, and those that increase bleeding or irregularity often are considered unfavorable. These criteria are commonly used in package labeling to describe bleeding patterns with extended use.


In this Update, we examine recent data evaluating differences in bleeding patterns with the 3 doses of the LNG IUD, predictors of abnormal bleeding with the etonogestrel implant, and the impact of timing on postpartum etonogestrel implant placement.
Continue to: Bleeding patterns with progestin-containing IUDs vary according to the LNG dose...
Bleeding patterns with progestin-containing IUDs vary according to the LNG dose
Goldthwaite LM, Creinin MD. Comparing bleeding patterns for the levonorgestrel 52 mg, 19.5 mg, and 13.5 mg intrauterine systems. Contraception. 2019;100:128-131.
Counseling on IUDs' different hormonal doses requires an understanding of patients' desires for contraceptive efficacy and bleeding expectations. A recent study provides guidance on what patients typically can expect for their bleeding patterns over the first few years with the 3 different doses of LNG IUDs.
Goldthwaite and Creinin used existing published or publicly available data to analyze differences in bleeding patterns associated with the 52-mg, 19.5-mg, and 13.5-mg LNG IUDs. Although two 52-mg LNG IUDs are available, published data using the WHO Belsey criteria are available only for one (Liletta; Allergan, Medicines360). The 2 products have been shown previously to have similar drug-release rates and LNG levels over 5 years.8
Comparing favorable bleeding patterns: Amenorrhea and infrequent bleeding
Among favorable bleeding patterns, amenorrhea was uncommon in the first 90 days and increased over time for all 3 IUDs. However, starting as soon as the second 90-day reference period, amenorrhea rates were significantly higher with the 52-mg LNG IUD compared with both of the lower-LNG dose IUDs, and this difference increased through 3 years of use (FIGURE 1).
Similarly, the 19.5-mg LNG IUD users had significantly higher rates of amenorrhea than the 13.5-mg LNG IUD users for all periods starting with the second 90-day reference period. At 3 years, 36% of women using the 52-mg LNG IUD had amenorrhea compared with 20% of those using the 19.5-mg LNG IUD (P<.0001) and 12% of those using the 13.5-mg LNG IUD (P<.0001).
Infrequent bleeding was similar for all 3 LNG IUDs in the first 90-day period, and it then increased most rapidly in the 52-mg LNG IUD users. At the end of year 1, 30% of the 52-mg LNG IUD users had infrequent bleeding compared with 26% of the 19.5-mg users (P = .01) and 20% of the 13.5-mg users (P<.0001). Although there was no difference in infrequent bleeding rates between the 52-mg and the 19.5-mg LNG IUD users at the end of year 1, those using a 52-mg LNG IUD had significantly higher rates of infrequent bleeding compared with the 13.5-mg LNG IUD at all time points.

Comparing unfavorable bleeding patterns: Frequent, prolonged, and irregular bleeding
Frequent and prolonged bleeding were uncommon with all LNG doses. Irregular bleeding rates declined for users of the 3 IUDs over time. However, significantly fewer users of the 52-mg LNG IUD reported irregular bleeding at 1 year (6%) compared with users of the 19.5-mg (16.5%, P<.0001) and 13.5-mg (23%, P<.0001) LNG IUD (FIGURE 2).

Study limitations
Comparing the data from different studies has limitations. For example, the data were collected from different populations, with the lower-dose LNG products tested in women who had a lower body mass index (BMI) and higher parity. However, prior analysis of the data on the 52-mg LNG IUD demonstrated that bleeding pattern changes did not vary based on these factors.10
When considering the different progestin-based IUD options, it is important to counsel patients according to their preferences for potential adverse effects. A randomized trial during product development found no difference in systemic adverse effects with the 3 doses of LNG IUD, likely because the systemic hormone levels are incredibly low for all 3 products.11 The summary data in this report helps explain why women using the lower-dose LNG products have slightly higher discontinuation rates for bleeding complaints, a fact we can explain to our patients during counseling.
Overall, the 52-mg LNG IUD is associated with a higher likelihood of favorable bleeding patterns over the first few years of use, with higher rates of amenorrhea and infrequent bleeding and lower rates of irregular bleeding. For women who prefer to not have periods or to have infrequent periods, the 52-mg LNG IUD is most likely to provide that outcome. For a patient who prefers to have periods, there is no evidence that the lower-dose IUDs result in “regular” or “normal” menstrual bleeding, even though they do result in more bleeding/spotting days overall. To the contrary, the available data show that these women have a significantly higher likelihood of experiencing prolonged, frequent, and irregular bleeding. In fact, no studies have reported rates of “normal” bleeding with the progestin IUDs, likely because women uncommonly have “normal” bleeding with these contraception methods. If a patient does not desire amenorrhea or strongly prefers to have “regular bleeding,” alternative methods such as a copper IUD should be considered rather than counseling her toward a lower-dose progestin IUD.
Continue to: Predicting long-term bleeding patterns after etonogestrel implant insertion...
Predicting long-term bleeding patterns after etonogestrel implant insertion
Mansour D, Fraser IS, Edelman A, et al. Can initial vaginal bleeding patterns in etonogestrel implant users predict subsequent bleeding in the first two years of use? Contraception. 2019. doi: 10.1016/j.contraception.2019.05.017.
Data from 2014 indicate that the etonogestrel implant was used by nearly 1 million women in the United States and by 3% of women using contraception.1 The primary reason women discontinue implant use is because of changes in bleeding patterns. Given the high prevalence of bleeding concerns with the etonogestrel implant, we need more data to help counsel our patients on how they can expect their bleeding to change with implant use.
Etonogestral implant and bleeding pattern trends
Mansour and colleagues completed a secondary analysis of 12 phase 3 studies to evaluate the correlation between bleeding patterns early after placement of the etonogestrel implant (days 29-118) compared with bleeding patterns through 90-day intervals during the rest of the first year of use. To account for differences in timing of etonogestrel implant placement relative to the menstrual cycle and discontinuation of other methods like oral contraceptives, bleeding outcomes on days 0-28 were excluded. They also sought to investigate the correlation between bleeding patterns in year 1 compared with those in year 2.
Overall, these studies included 923 individuals across 11 countries; however, for the current analysis, the researchers excluded women from Asian countries who comprised more than 28% of the study population. These women report significantly fewer bleeding/spotting days with the etonogestrel implant and have a lower average body weight compared with European and American women.12
A prior analysis of the same data set looked at the number of bleeding/spotting days in groups of users rather than trends in individual patients, and, as mentioned, it also included Asian women, which diluted the overall number of bleeding days.12 In this new analysis, Mansour and colleagues used the Belsey criteria to analyze individual bleeding patterns as favorable (amenorrhea, infrequent bleeding, normal bleeding) or unfavorable (prolonged and/or frequent bleeding) from a patient perspective. In this way, we can understand trends in bleeding patterns for each patient over time, rather than seeing a static (cross-sectional) report of bleeding patterns at one point in time. Data were analyzed from 537 women in year 1 and 428 women in year 2. During the first 90-day reference period (days 29-118 after implant insertion), 61% of women reported favorable bleeding, and 39% reported unfavorable bleeding.
Favorable bleeding correlates with favorable patterns later
A favorable bleeding pattern in this first reference period correlated with favorable bleeding patterns through year 1, with 85%, 80%, and 80% of these women having a favorable pattern in reference periods 2, 3, and 4, respectively. Overall, 61% of women with a favorable pattern in reference period 1 had favorable bleeding throughout the entire first year of use. Only 3.7% of women with favorable bleeding in the first reference period discontinued the implant for bleeding in year 1. Further, women with favorable bleeding at year 1 commonly continued to have favorable bleeding in year 2, with a low discontinuation rate (2.5%) in year 2.
Individual patients who have a favorable bleeding pattern initially with etonogestrel implant placement are highly likely to continue having favorable bleeding at year 1 and year 2. Notably, of women with a favorable bleeding pattern in any 90-day reference period, about 80% will continue to have a favorable bleeding pattern in the next reference period. These women can be counseled that, even if they have a 90-day period with unfavorable bleeding, about two-thirds will have a favorable pattern in the next reference period. For those with initial unfavorable patterns, about one-third to one-half change to a favorable pattern in subsequent 90-day reference periods. For women who require intervention for unfavorable bleeding but wish to keep their etonogestrel implant, prior data support use of combined oral contraceptive pills, although bleeding resolution seems to be temporary, with 86% of women having bleeding recurrence within 10 days after treatment.13
Initial unfavorable bleeding portends less favorable patterns later
Women who had an unfavorable bleeding pattern initially, however, had a less predictable course over the first year. For those with an initial unfavorable pattern, only 37%, 47%, and 51% reported a favorable pattern in reference periods 2, 3, and 4. Despite these relatively low rates of favorable bleeding, only 13% of the women with an initial unfavorable bleeding pattern discontinued implant use for a bleeding complaint by the end of year 1; this rate was significantly higher than that for women with a favorable initial bleeding pattern (P<.0001). The discontinuation rate for bleeding complaints also remained higher in year 2, at 16.5%.
Limitations and strengths to consider
Although the etonogestrel implant is FDA-approved for 3 years of use, the bleeding data from the combined trials included information for only up to 2 years after placement. The studies included also did not uniformly assess BMI, which makes it difficult to find correlations between bleeding patterns and BMI. Importantly, the studies did not include women who were more than 30% above their ideal body weight, so these assessments do not apply to obese users.12 Exclusion of women from Southeast Asia in this analysis makes this study's findings more generalizable to populations in the United States and Europe.
Continue to: Early versus delayed postpartum etonogestrel implant insertion...
Early versus delayed postpartum etonogestrel implant insertion: Similar impacts on 12-month bleeding patterns
Vieira CS, de Nadai MN, de Melo Pereira do Carmo LS, et al. Timing of postpartum etonogestrel-releasing implant insertion and bleeding patterns, weight change, 12-month continuation and satisfaction rates: a randomized controlled trial. Contraception. 2019. doi:10.1016/j.contraception.2019.05.007.
Initiation of a desired LARC method shortly after delivery is associated with significant reductions in short interpregnancy intervals.14 With that goal in mind, Vieira and colleagues compared bleeding patterns in women who received an etonogestrel implant within 48 hours of delivery with those who received an implant at 6 weeks postdelivery.
The study was a secondary analysis of data from a randomized controlled trial of early versus delayed postpartum insertion of the etonogestrel implant conducted in Sao Paulo, Brazil. That primary trial's goal was to examine the impact of early versus delayed implant insertion on infant growth (100 women were randomly assigned to the 2 implant groups); no difference in infant growth at 12 months was seen in the 2 groups.15 In the secondary analysis, bleeding patterns and BMI were evaluated every 90 days for 12 months. The mean BMI at enrollment postpartum was 29.4 kg/m2 in the early-insertion group and 30.2 kg/m2 for the delayed-insertion group.
Bleeding patterns with early or delayed implant insertion were similar
Vieira and colleagues found similar bleeding patterns between the groups over 12 months of follow-up. Amenorrhea was reported by 56% of the early-insertion group in the first 90 days and by 62% in the delayed-insertion group. During the last 90 days of the year, 52% of the early-insertion and 46% of the delayed-insertion group reported amenorrhea. Amenorrhea rates did not differ between women who were exclusively breastfeeding and those nonexclusively breastfeeding.
Continuation rates were high at 1 year
Prolonged bleeding episodes were uncommon in both groups, with only 2% of women reporting prolonged bleeding in any given reference period. Twelve-month implant continuation rates were high in both groups: 98% in the early- and 100% in the delayed-insertion group. Additionally, the investigators found that both groups experienced a BMI decrease, with no difference between groups (10.3% and 11% in the early- and delayed-insertion groups, respectively).
Study limitations and strengths
This study included a larger number of participants than prior randomized, controlled trials that evaluated bleeding patterns with postpartum etonogestrel implant insertion, and it had very low rates of loss to follow-up. The study's low rate of 12-month implant discontinuation (2%) is lower than that of other studies that reported rates of 6% to 14%.16,17 Although the authors stated that this low rate may be due to thorough anticipatory counseling prior to placement, it is also possible that this study population does not reflect all populations. Regardless, the data clearly show that placing an etonogestrel implant prior to hospital discharge, compared with waiting for later placement, does not impact bleeding patterns over the ensuing year.
For patients who desire an etonogestrel implant for contraception postpartum, we now have additional information to counsel about the impact of implant placement on postpartum bleeding patterns. Overall, bleeding patterns are highly favorable and do not vary whether the implant is placed in the hospital or later. Additionally, the timing of placement does not impact implant continuation rates or BMI changes over 1 year. Further, the primary study assessed infant growth in the early- versus delayed-placement groups and found no differences in infant growth. Although the data are limited, immediate postpartum etonogestrel implant placement does not seem to affect the rate of breastfeeding or the volume of breast milk.18,19 Timing of implant placement, assuming adequate resources, should be based primarily on patient preference. And, given the correlation of immediate postpartum LARC placement to increased interpregnancy interval, particular efforts should be made to provide the implant in the immediate postpartum period, if the patient desires.20
Long-acting reversible contraception (LARC) use continues to increase in the United States. According to the most recent estimates from 2014, 14% of women use either an intrauterine device (IUD) or the etonogestrel implant.1 Forms of LARC currently available in the United States include:
- 4 hormone-releasing IUDs
- 1 nonhormonal copper IUD, and
- 1 hormonal subdermal implant.
The hormone-releasing IUDs all contain levonorgestrel (LNG). These include two 52-mg LNG products and a 19.5-mg LNG IUD, which are currently approved by the US Food and Drug Administration (FDA) for contraception for 5 continuous years of use. In addition, a 13.5-mg LNG IUD is FDA-approved for 3 years of use. The hormonal subdermal implant, which contains etonogestrel, is FDA-approved for 3 years of use. Although major complications with IUDs (perforation, expulsion, intrauterine infection)and implants (subfascial implantation, distant migration) are rare, adverse effects that can affect continuation—such as irregular bleeding—are more common.2,3
Contraceptive discontinuation due to bleeding concerns occurs more frequently with the etonogestrel implant than with LNG IUDs (TABLE 1). In a large prospective study in the United States, 13% of women discontinued the implant during 3 years of follow-up due to bleeding pattern changes.
Notably, it is important to use standardized definitions to understand and compare bleeding concerns with LARC use. The Belsey criteria of the World Health Organization (WHO), a standard used for decades, describe bleeding patterns using 90-day reference periods or intervals (TABLE 2).9 Bleeding patterns that decrease flow (amenorrhea, infrequent bleeding) often are considered favorable, and those that increase bleeding or irregularity often are considered unfavorable. These criteria are commonly used in package labeling to describe bleeding patterns with extended use.


In this Update, we examine recent data evaluating differences in bleeding patterns with the 3 doses of the LNG IUD, predictors of abnormal bleeding with the etonogestrel implant, and the impact of timing on postpartum etonogestrel implant placement.
Continue to: Bleeding patterns with progestin-containing IUDs vary according to the LNG dose...
Bleeding patterns with progestin-containing IUDs vary according to the LNG dose
Goldthwaite LM, Creinin MD. Comparing bleeding patterns for the levonorgestrel 52 mg, 19.5 mg, and 13.5 mg intrauterine systems. Contraception. 2019;100:128-131.
Counseling on IUDs' different hormonal doses requires an understanding of patients' desires for contraceptive efficacy and bleeding expectations. A recent study provides guidance on what patients typically can expect for their bleeding patterns over the first few years with the 3 different doses of LNG IUDs.
Goldthwaite and Creinin used existing published or publicly available data to analyze differences in bleeding patterns associated with the 52-mg, 19.5-mg, and 13.5-mg LNG IUDs. Although two 52-mg LNG IUDs are available, published data using the WHO Belsey criteria are available only for one (Liletta; Allergan, Medicines360). The 2 products have been shown previously to have similar drug-release rates and LNG levels over 5 years.8
Comparing favorable bleeding patterns: Amenorrhea and infrequent bleeding
Among favorable bleeding patterns, amenorrhea was uncommon in the first 90 days and increased over time for all 3 IUDs. However, starting as soon as the second 90-day reference period, amenorrhea rates were significantly higher with the 52-mg LNG IUD compared with both of the lower-LNG dose IUDs, and this difference increased through 3 years of use (FIGURE 1).
Similarly, the 19.5-mg LNG IUD users had significantly higher rates of amenorrhea than the 13.5-mg LNG IUD users for all periods starting with the second 90-day reference period. At 3 years, 36% of women using the 52-mg LNG IUD had amenorrhea compared with 20% of those using the 19.5-mg LNG IUD (P<.0001) and 12% of those using the 13.5-mg LNG IUD (P<.0001).
Infrequent bleeding was similar for all 3 LNG IUDs in the first 90-day period, and it then increased most rapidly in the 52-mg LNG IUD users. At the end of year 1, 30% of the 52-mg LNG IUD users had infrequent bleeding compared with 26% of the 19.5-mg users (P = .01) and 20% of the 13.5-mg users (P<.0001). Although there was no difference in infrequent bleeding rates between the 52-mg and the 19.5-mg LNG IUD users at the end of year 1, those using a 52-mg LNG IUD had significantly higher rates of infrequent bleeding compared with the 13.5-mg LNG IUD at all time points.

Comparing unfavorable bleeding patterns: Frequent, prolonged, and irregular bleeding
Frequent and prolonged bleeding were uncommon with all LNG doses. Irregular bleeding rates declined for users of the 3 IUDs over time. However, significantly fewer users of the 52-mg LNG IUD reported irregular bleeding at 1 year (6%) compared with users of the 19.5-mg (16.5%, P<.0001) and 13.5-mg (23%, P<.0001) LNG IUD (FIGURE 2).

Study limitations
Comparing the data from different studies has limitations. For example, the data were collected from different populations, with the lower-dose LNG products tested in women who had a lower body mass index (BMI) and higher parity. However, prior analysis of the data on the 52-mg LNG IUD demonstrated that bleeding pattern changes did not vary based on these factors.10
When considering the different progestin-based IUD options, it is important to counsel patients according to their preferences for potential adverse effects. A randomized trial during product development found no difference in systemic adverse effects with the 3 doses of LNG IUD, likely because the systemic hormone levels are incredibly low for all 3 products.11 The summary data in this report helps explain why women using the lower-dose LNG products have slightly higher discontinuation rates for bleeding complaints, a fact we can explain to our patients during counseling.
Overall, the 52-mg LNG IUD is associated with a higher likelihood of favorable bleeding patterns over the first few years of use, with higher rates of amenorrhea and infrequent bleeding and lower rates of irregular bleeding. For women who prefer to not have periods or to have infrequent periods, the 52-mg LNG IUD is most likely to provide that outcome. For a patient who prefers to have periods, there is no evidence that the lower-dose IUDs result in “regular” or “normal” menstrual bleeding, even though they do result in more bleeding/spotting days overall. To the contrary, the available data show that these women have a significantly higher likelihood of experiencing prolonged, frequent, and irregular bleeding. In fact, no studies have reported rates of “normal” bleeding with the progestin IUDs, likely because women uncommonly have “normal” bleeding with these contraception methods. If a patient does not desire amenorrhea or strongly prefers to have “regular bleeding,” alternative methods such as a copper IUD should be considered rather than counseling her toward a lower-dose progestin IUD.
Continue to: Predicting long-term bleeding patterns after etonogestrel implant insertion...
Predicting long-term bleeding patterns after etonogestrel implant insertion
Mansour D, Fraser IS, Edelman A, et al. Can initial vaginal bleeding patterns in etonogestrel implant users predict subsequent bleeding in the first two years of use? Contraception. 2019. doi: 10.1016/j.contraception.2019.05.017.
Data from 2014 indicate that the etonogestrel implant was used by nearly 1 million women in the United States and by 3% of women using contraception.1 The primary reason women discontinue implant use is because of changes in bleeding patterns. Given the high prevalence of bleeding concerns with the etonogestrel implant, we need more data to help counsel our patients on how they can expect their bleeding to change with implant use.
Etonogestral implant and bleeding pattern trends
Mansour and colleagues completed a secondary analysis of 12 phase 3 studies to evaluate the correlation between bleeding patterns early after placement of the etonogestrel implant (days 29-118) compared with bleeding patterns through 90-day intervals during the rest of the first year of use. To account for differences in timing of etonogestrel implant placement relative to the menstrual cycle and discontinuation of other methods like oral contraceptives, bleeding outcomes on days 0-28 were excluded. They also sought to investigate the correlation between bleeding patterns in year 1 compared with those in year 2.
Overall, these studies included 923 individuals across 11 countries; however, for the current analysis, the researchers excluded women from Asian countries who comprised more than 28% of the study population. These women report significantly fewer bleeding/spotting days with the etonogestrel implant and have a lower average body weight compared with European and American women.12
A prior analysis of the same data set looked at the number of bleeding/spotting days in groups of users rather than trends in individual patients, and, as mentioned, it also included Asian women, which diluted the overall number of bleeding days.12 In this new analysis, Mansour and colleagues used the Belsey criteria to analyze individual bleeding patterns as favorable (amenorrhea, infrequent bleeding, normal bleeding) or unfavorable (prolonged and/or frequent bleeding) from a patient perspective. In this way, we can understand trends in bleeding patterns for each patient over time, rather than seeing a static (cross-sectional) report of bleeding patterns at one point in time. Data were analyzed from 537 women in year 1 and 428 women in year 2. During the first 90-day reference period (days 29-118 after implant insertion), 61% of women reported favorable bleeding, and 39% reported unfavorable bleeding.
Favorable bleeding correlates with favorable patterns later
A favorable bleeding pattern in this first reference period correlated with favorable bleeding patterns through year 1, with 85%, 80%, and 80% of these women having a favorable pattern in reference periods 2, 3, and 4, respectively. Overall, 61% of women with a favorable pattern in reference period 1 had favorable bleeding throughout the entire first year of use. Only 3.7% of women with favorable bleeding in the first reference period discontinued the implant for bleeding in year 1. Further, women with favorable bleeding at year 1 commonly continued to have favorable bleeding in year 2, with a low discontinuation rate (2.5%) in year 2.
Individual patients who have a favorable bleeding pattern initially with etonogestrel implant placement are highly likely to continue having favorable bleeding at year 1 and year 2. Notably, of women with a favorable bleeding pattern in any 90-day reference period, about 80% will continue to have a favorable bleeding pattern in the next reference period. These women can be counseled that, even if they have a 90-day period with unfavorable bleeding, about two-thirds will have a favorable pattern in the next reference period. For those with initial unfavorable patterns, about one-third to one-half change to a favorable pattern in subsequent 90-day reference periods. For women who require intervention for unfavorable bleeding but wish to keep their etonogestrel implant, prior data support use of combined oral contraceptive pills, although bleeding resolution seems to be temporary, with 86% of women having bleeding recurrence within 10 days after treatment.13
Initial unfavorable bleeding portends less favorable patterns later
Women who had an unfavorable bleeding pattern initially, however, had a less predictable course over the first year. For those with an initial unfavorable pattern, only 37%, 47%, and 51% reported a favorable pattern in reference periods 2, 3, and 4. Despite these relatively low rates of favorable bleeding, only 13% of the women with an initial unfavorable bleeding pattern discontinued implant use for a bleeding complaint by the end of year 1; this rate was significantly higher than that for women with a favorable initial bleeding pattern (P<.0001). The discontinuation rate for bleeding complaints also remained higher in year 2, at 16.5%.
Limitations and strengths to consider
Although the etonogestrel implant is FDA-approved for 3 years of use, the bleeding data from the combined trials included information for only up to 2 years after placement. The studies included also did not uniformly assess BMI, which makes it difficult to find correlations between bleeding patterns and BMI. Importantly, the studies did not include women who were more than 30% above their ideal body weight, so these assessments do not apply to obese users.12 Exclusion of women from Southeast Asia in this analysis makes this study's findings more generalizable to populations in the United States and Europe.
Continue to: Early versus delayed postpartum etonogestrel implant insertion...
Early versus delayed postpartum etonogestrel implant insertion: Similar impacts on 12-month bleeding patterns
Vieira CS, de Nadai MN, de Melo Pereira do Carmo LS, et al. Timing of postpartum etonogestrel-releasing implant insertion and bleeding patterns, weight change, 12-month continuation and satisfaction rates: a randomized controlled trial. Contraception. 2019. doi:10.1016/j.contraception.2019.05.007.
Initiation of a desired LARC method shortly after delivery is associated with significant reductions in short interpregnancy intervals.14 With that goal in mind, Vieira and colleagues compared bleeding patterns in women who received an etonogestrel implant within 48 hours of delivery with those who received an implant at 6 weeks postdelivery.
The study was a secondary analysis of data from a randomized controlled trial of early versus delayed postpartum insertion of the etonogestrel implant conducted in Sao Paulo, Brazil. That primary trial's goal was to examine the impact of early versus delayed implant insertion on infant growth (100 women were randomly assigned to the 2 implant groups); no difference in infant growth at 12 months was seen in the 2 groups.15 In the secondary analysis, bleeding patterns and BMI were evaluated every 90 days for 12 months. The mean BMI at enrollment postpartum was 29.4 kg/m2 in the early-insertion group and 30.2 kg/m2 for the delayed-insertion group.
Bleeding patterns with early or delayed implant insertion were similar
Vieira and colleagues found similar bleeding patterns between the groups over 12 months of follow-up. Amenorrhea was reported by 56% of the early-insertion group in the first 90 days and by 62% in the delayed-insertion group. During the last 90 days of the year, 52% of the early-insertion and 46% of the delayed-insertion group reported amenorrhea. Amenorrhea rates did not differ between women who were exclusively breastfeeding and those nonexclusively breastfeeding.
Continuation rates were high at 1 year
Prolonged bleeding episodes were uncommon in both groups, with only 2% of women reporting prolonged bleeding in any given reference period. Twelve-month implant continuation rates were high in both groups: 98% in the early- and 100% in the delayed-insertion group. Additionally, the investigators found that both groups experienced a BMI decrease, with no difference between groups (10.3% and 11% in the early- and delayed-insertion groups, respectively).
Study limitations and strengths
This study included a larger number of participants than prior randomized, controlled trials that evaluated bleeding patterns with postpartum etonogestrel implant insertion, and it had very low rates of loss to follow-up. The study's low rate of 12-month implant discontinuation (2%) is lower than that of other studies that reported rates of 6% to 14%.16,17 Although the authors stated that this low rate may be due to thorough anticipatory counseling prior to placement, it is also possible that this study population does not reflect all populations. Regardless, the data clearly show that placing an etonogestrel implant prior to hospital discharge, compared with waiting for later placement, does not impact bleeding patterns over the ensuing year.
For patients who desire an etonogestrel implant for contraception postpartum, we now have additional information to counsel about the impact of implant placement on postpartum bleeding patterns. Overall, bleeding patterns are highly favorable and do not vary whether the implant is placed in the hospital or later. Additionally, the timing of placement does not impact implant continuation rates or BMI changes over 1 year. Further, the primary study assessed infant growth in the early- versus delayed-placement groups and found no differences in infant growth. Although the data are limited, immediate postpartum etonogestrel implant placement does not seem to affect the rate of breastfeeding or the volume of breast milk.18,19 Timing of implant placement, assuming adequate resources, should be based primarily on patient preference. And, given the correlation of immediate postpartum LARC placement to increased interpregnancy interval, particular efforts should be made to provide the implant in the immediate postpartum period, if the patient desires.20
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97:14-21.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397-404.
- Odom EB, Eisenberg DL, Fox IK. Difficult removal of subdermal contraceptive implants: a multidisciplinary approach involving a peripheral nerve expert. Contraception. 2017;96: 89-95.
- Funk S, Miller MM, Mishell DR Jr, et al; Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
- Eisenberg DL, Schreiber CA, Turok DK, et al; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92:10-16.
- Nelson A, Apter D, Hauck B, et al. Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstet Gynecol. 2013;122:1205-1213.
- Beckert V, Ahlers C, Frenz AK, et al. Bleeding patterns with the 19.5mg LNG-IUS, with special focus on the first year of use: implications for counselling. Eur J Contracept Reprod Health Care. 2019;24:251-259.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
- Belsey EM, Machines D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. Contraception. 1986;34:253-260.
- Schreiber CA, Teal SB, Blumenthal PD, et al. Bleeding patterns for the Liletta® levonorgestrel 52mg intrauterine system. Eur J Contracept Reprod Health Care. 2018;23:116–120.
- Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril. 2012;97:616-22.e1-3.
- Mansour D, Korver T, Marintcheva-Petrova M, et al. The effects of Implanon on menstrual bleeding patterns. Eur J Contracept Reprod Health Care. 2008;13(suppl 1):13-28.
- Guiahi M, McBride M, Sheeder J, et al. Short-term treatment of bothersome bleeding for etonogestrel implant users using a 14-day oral contraceptive pill regimen: a randomized controlled trial. Obstet Gynecol. 2015;126:508-513.
- Brunson MR, Klein DA, Olsen CH, et al. Postpartum contraception: initiation and effectiveness in a large universal healthcare system. Am J Obstet Gynecol. 2017;217:55.e1-55.e9
- de Melo Pereira Carmo LS, Braga GC, Ferriani RA, et al. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017;130:100-107.
- Crockett AH, Pickell LB, Heberlein EC, et al. Six- and twelve-month documented removal rates among women electing postpartum inpatient compared to delayed or interval contraceptive implant insertions after Medicaid payment reform. Contraception. 2017;95:71-76.
- Wilson S, Tennant C, Sammel MD, et al. Immediate postpartum etonogestrel implant: a contraception option with long-term continuation. Contraception. 2014;90:259-264.
- Sothornwit J, Werawatakul Y, Kaewrudee S, et al. Immediate versus delayed postpartum insertion of contraceptive implant for contraception. Cochrane Database Syst Rev. 2017;4:CD011913.
- Braga GC, Ferriolli E, Quintana SM, et al. Immediate postpartum initiation of etonogestrel-releasing implant: a randomized controlled trial on breastfeeding impact. Contraception. 2015;92:536-542.
- Thiel de Bocanegra H, Chang R, Howell M, et al. Interpregnancy intervals: impact of postpartum contraceptive effectiveness and coverage. Am J Obstet Gynecol. 2014;210:311.e1-8.
- Kyleena [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc;2016.
- Skyla [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2016.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97:14-21.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397-404.
- Odom EB, Eisenberg DL, Fox IK. Difficult removal of subdermal contraceptive implants: a multidisciplinary approach involving a peripheral nerve expert. Contraception. 2017;96: 89-95.
- Funk S, Miller MM, Mishell DR Jr, et al; Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
- Eisenberg DL, Schreiber CA, Turok DK, et al; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92:10-16.
- Nelson A, Apter D, Hauck B, et al. Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstet Gynecol. 2013;122:1205-1213.
- Beckert V, Ahlers C, Frenz AK, et al. Bleeding patterns with the 19.5mg LNG-IUS, with special focus on the first year of use: implications for counselling. Eur J Contracept Reprod Health Care. 2019;24:251-259.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
- Belsey EM, Machines D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. Contraception. 1986;34:253-260.
- Schreiber CA, Teal SB, Blumenthal PD, et al. Bleeding patterns for the Liletta® levonorgestrel 52mg intrauterine system. Eur J Contracept Reprod Health Care. 2018;23:116–120.
- Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril. 2012;97:616-22.e1-3.
- Mansour D, Korver T, Marintcheva-Petrova M, et al. The effects of Implanon on menstrual bleeding patterns. Eur J Contracept Reprod Health Care. 2008;13(suppl 1):13-28.
- Guiahi M, McBride M, Sheeder J, et al. Short-term treatment of bothersome bleeding for etonogestrel implant users using a 14-day oral contraceptive pill regimen: a randomized controlled trial. Obstet Gynecol. 2015;126:508-513.
- Brunson MR, Klein DA, Olsen CH, et al. Postpartum contraception: initiation and effectiveness in a large universal healthcare system. Am J Obstet Gynecol. 2017;217:55.e1-55.e9
- de Melo Pereira Carmo LS, Braga GC, Ferriani RA, et al. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017;130:100-107.
- Crockett AH, Pickell LB, Heberlein EC, et al. Six- and twelve-month documented removal rates among women electing postpartum inpatient compared to delayed or interval contraceptive implant insertions after Medicaid payment reform. Contraception. 2017;95:71-76.
- Wilson S, Tennant C, Sammel MD, et al. Immediate postpartum etonogestrel implant: a contraception option with long-term continuation. Contraception. 2014;90:259-264.
- Sothornwit J, Werawatakul Y, Kaewrudee S, et al. Immediate versus delayed postpartum insertion of contraceptive implant for contraception. Cochrane Database Syst Rev. 2017;4:CD011913.
- Braga GC, Ferriolli E, Quintana SM, et al. Immediate postpartum initiation of etonogestrel-releasing implant: a randomized controlled trial on breastfeeding impact. Contraception. 2015;92:536-542.
- Thiel de Bocanegra H, Chang R, Howell M, et al. Interpregnancy intervals: impact of postpartum contraceptive effectiveness and coverage. Am J Obstet Gynecol. 2014;210:311.e1-8.
- Kyleena [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc;2016.
- Skyla [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2016.
2018 Update on contraception
Female permanent contraception is among the most widely used contraceptive methods worldwide. In the United States, more than 640,000 procedures are performed each year and it is used by 25% of women who use contraception.1–4 Female permanent contraception is achieved via salpingectomy, tubal interruption, or hysteroscopic techniques.
Essure, the only currently available hysteroscopic permanent contraception device, approved by the US Food and Drug Administration (FDA) in 2002,5,6 has been implanted in more than 750,000 women worldwide.7 Essure was developed by Conceptus Inc, a small medical device company that was acquired by Bayer in 2013. The greatest uptake has been in the United States, which accounts for approximately 80% of procedures worldwide.7,8
Essure placement involves insertion of a nickel-titanium alloy coil with a stainless-steel inner coil, polyethylene terephthalate fibers, platinum marker bands, and silver-tin solder.9 The insert is approximately 4 cm in length and expands to 2 mm in diameter once deployed.9
Potential advantages of a hysteroscopic approach are that intra-abdominal surgery can be avoided and the procedure can be performed in an office without the need for general anesthesia.7 Due to these potential benefits, hysteroscopic permanent contraception with Essure underwent expedited review and received FDA approval without any comparative trials.1,5,10 However, there also are disadvantages: the method is not always successfully placed on first attempt and it is not immediately effective. Successful placement rates range between 60% and 98%, most commonly around 90%.11–15 Additionally, if placement is successful, alternative contraception must be used until a confirmatory radiologic test is performed at least 3 months after the procedure.9,11 Initially, hysterosalpingography was required to demonstrate a satisfactory insert location and successful tubal occlusion.11,16 Compliance with this testing is variable, ranging in studies from 13% to 71%.11 As of 2015, transvaginal ultrasonography showing insert retention and location has been approved as an alternative confirmatory method.9,11,16,17 Evidence suggests that the less invasive ultrasound option increases follow-up rates; while limited, one study noted an increase in follow-up rates from 77.5% for hysterosalpingogram to 88% (P = .008) for transvaginal ultrasound.18
Recent concerns about potential medical and safety issues have impacted approval status and marketing of hysteroscopic permanent contraception worldwide. In response to safety concerns, the FDA added a boxed safety warning and patient decision checklist in 2016.19 Bayer withdrew the device from all markets outside of the United States as of May 2017.20–22 In April 2018, the FDA restricted Essure sales in the United States only to providers and facilities who utilized an FDA-approved checklist to ensure the device met standards for safety and effectiveness.19 Most recently, Bayer announced that Essure would no longer be sold or distributed in the United States after December 31, 2018 (See “FDA Press Release”).23
"The US Food and Drug Administration was notified by Bayer that the Essure permanent birth control device will no longer be sold or distributed after December 31, 2018... The decision today to halt Essure sales also follows a series of earlier actions that the FDA took to address the reports of serious adverse events associated with its use. For women who have received an Essure implant, the postmarket safety of Essure will continue to be a top priority for the FDA. We expect Bayer to meet its postmarket obligations concerning this device."
Reference
- Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; U.S. Food and Drug Administration. July 20, 2018.
So how did we get here? How did the promise of a “less invasive” approach for female permanent contraception get off course?
A search of the Manufacturer and User Facility Device Experience (MAUDE) database from Essure’s approval date in 2002 to December 2017 revealed 26,773 medical device reports, with more than 90% of those received in 2017 related to device removal.19 As more complications and complaints have been reported, the lack of comparative data has presented a problem for understanding the relative risk of the procedure as compared with laparoscopic techniques. Additionally, the approval studies lacked information about what happened to women who had an unsuccessful attempted hysteroscopic procedure. Without robust data sets or large trials, early research used evidence-based Markov modeling; findings suggested that hysteroscopic permanent contraception resulted in fewer women achieving successful permanent contraception and that the hysteroscopic procedure was not as effective as laparoscopic occlusion procedures with “typical” use.24,25
Over the past year, more clinical data have been published comparing hysteroscopic with laparoscopic permanent contraception procedures. In this article, we evaluate this information to help us better understand the relative efficacy and safety of the different permanent contraception methods and review recent articles describing removal techniques to further assist clinicians and patients considering such procedures.
Hysteroscopic versus laparoscopic procedures for permanent contraception
Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018:319(4):375-387.
Antoun L, Smith P, Gupta J, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J of Obstet Gynecol. 2017;217(5):570.e1-570.e6. doi:10.1016/j.ajog.2017.07.011.
Jokinen E, Heino A, Karipohja T, et al. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy, or laparotomy: a register based study. BJOG. 2017;124(12):1851-1857.
In this section, we present 3 recent studies that evaluate pregnancy outcomes and complications including reoperation or second permanent contraception procedure rates.
Data from France measure up to 3-year differences in adverse outcomes
Bouillon and colleagues aimed to identify differences in adverse outcome rates between hysteroscopic and laparoscopic permanent contraceptive methods. Utilizing national hospital discharge data in France, the researchers conducted a large database study review of records from more than 105,000 women aged 30 to 54 years receiving hysteroscopic or laparoscopic permanent contraception between 2010 and 2014. The database contains details based on the ICD-10 codes for all public and private hospitals in France, representing approximately 75% of the total population. Procedures were performed at 831 hospitals in 26 regions.
Adverse outcomes were divided into surgical, medical, and gynecologic complications (TABLE 1) and were assessed at 3 timepoints: at the time of procedure and at 1 and 3 years postprocedure.
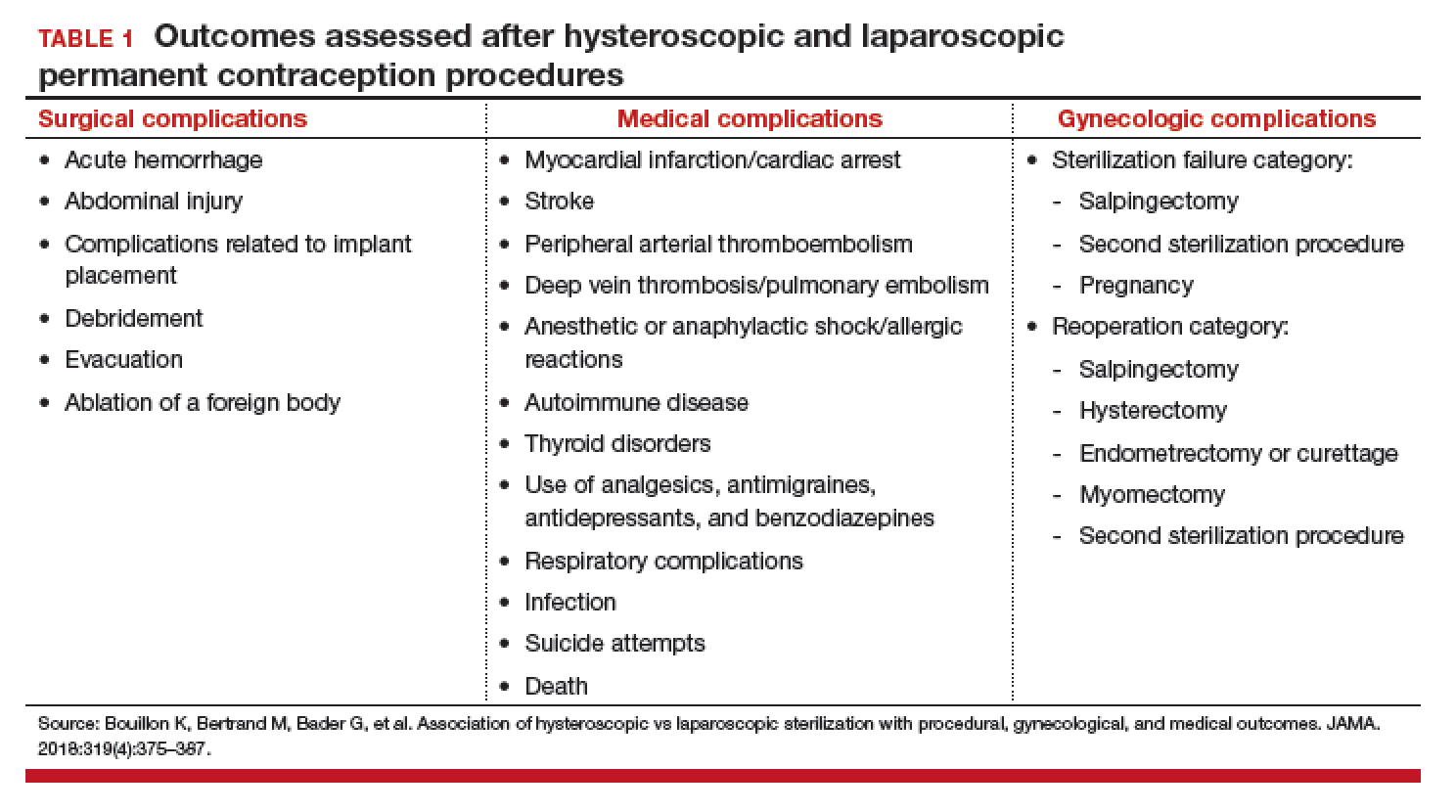
Overall, 71,303 women (67.7%) underwent hysteroscopic permanent contraception procedures and 34,054 women (32.3%) underwent laparoscopic permanent contraception procedures. Immediate surgical and medical complications were significantly less common for women having hysteroscopic compared with laparoscopic procedures. Surgical complications at the time of the procedure occurred in 96 (0.13%) and 265 (0.78%) women, respectively (adjusted odds ratio [aOR], 0.18; 95% confidence interval [CI], 0.14-0.23). Medical complications at the time of procedure occurred in 41 (0.06%) and 39 (0.11%) women, respectively (aOR, 0.51; 95% CI, 0.30-0.89).
However, gynecologic outcomes, including need for a second surgery to provide permanent contraception and overall failure rates (need for salpingectomy, a second permanent contraception procedure, or pregnancy) were significantly more common for women having hysteroscopic procedures. By 1 year after the procedure, 2,955 women (4.10%) who initially had a hysteroscopic procedure, and 56 women (0.16%) who had a laparoscopic procedure required a second permanent contraception surgery (adjusted hazard ratio [aHR], 25.99; 95% CI, 17.84-37.86). By the third year, additional procedures were performed in 3,230 (4.5%) and 97 (0.28%) women, respectively (aHR, 16.63; 95% CI, 12.50-22.20). Most (65%) of the repeat procedures were performed laparoscopically. Although pregnancy rates were significantly lower at 1 year among women who initially chose a hysteroscopic procedure (0.24% vs 0.41%; aHR, 0.70; 95% CI, 0.53-0.92), the rates did not differ at 3 years (0.48% vs 0.57%, respectively; aHR, 1.04; 95% CI, 0.83-1.30).
Most importantly, overall procedure failure rates were significantly higher at 1 year in women initially choosing a hysteroscopic approach compared with laparoscopic approach (3,446 [4.83%] vs 235 [0.69%] women; aHR, 7.11; 95% CI, 5.92-8.54). This difference persisted through 3 years (4,098 [5.75%] vs 438 [1.29%] women, respectively; aHR, 4.66; 95% CI, 4.06-5.34).
UK data indicate high reoperation rate for hysteroscopic procedures
Antoun and colleagues aimed to compare pregnancy rates, radiologic imaging follow-up rates, reoperations, and 30-day adverse outcomes, between hysteroscopic and laparoscopic permanent contraception methods. Conducted at a single teaching hospital in the United Kingdom, this study included 3,497 women who underwent procedures between 2005 and 2015. The data were collected prospectively for the 1,085 women who underwent hysteroscopic procedures and retrospectively for 2,412 women who had laparoscopic permanent contraception procedures with the Filshie clip.
Over the 10-year study period, hysteroscopic permanent contraception increased from 14.2% (40 of 280) of procedures in 2005 to 40.5% (150 of 350) of procedures in 2015 (P<.001). Overall, 2,400 women (99.5%) underwent successful laparoscopic permanent contraception, compared with 992 women (91.4%) in the hysteroscopic group (OR, 18.8; 95% CI, 10.2-34.4).
In the hysteroscopic group, 958 women (97%) returned for confirmatory testing, of whom 902 (91% of women with successful placement) underwent satisfactory confirmatory testing. There were 93 (8.6%) unsuccessful placements that were due to inability to visualize ostia or tubal stenosis (n = 72 [77.4%]), patient intolerance to procedure (n = 15 [16.1%]), or device failure (n = 6 [6.5%]).
The odds for reoperation were 6 times greater in the hysteroscopic group by 1 year after surgery (22 [2%] vs 8 [0.3%] women; OR, 6.2; 95% CI, 2.8-14.0). However, the 1-year pregnancy risk was similar between the 2 groups, with 3 reported pregnancies after hysteroscopic permanent contraception and 5 reported pregnancies after laparoscopic permanent contraception (OR, 1.3; 95% CI, 0.3-5.6).
Finnish researchers also find high reoperation rate
Jokinen and colleagues used linked national database registries in Finland to capture data on pregnancy rate and reoperations among 16,272 women who underwent permanent contraception procedures between 2009 and 2014. The authors compared outcomes following hysteroscopic (Essure), laparoscopic (Filshie clip), and postpartum minilaparotomy (Pomeroy) permanent contraception techniques. According to the investigators, the latter method was almost exclusively performed at the time of cesarean delivery. While there was no difference in pregnancy rates, second permanent contraception procedures were significantly greater in the hysteroscopic group compared with the laparoscopic group (TABLE 2).
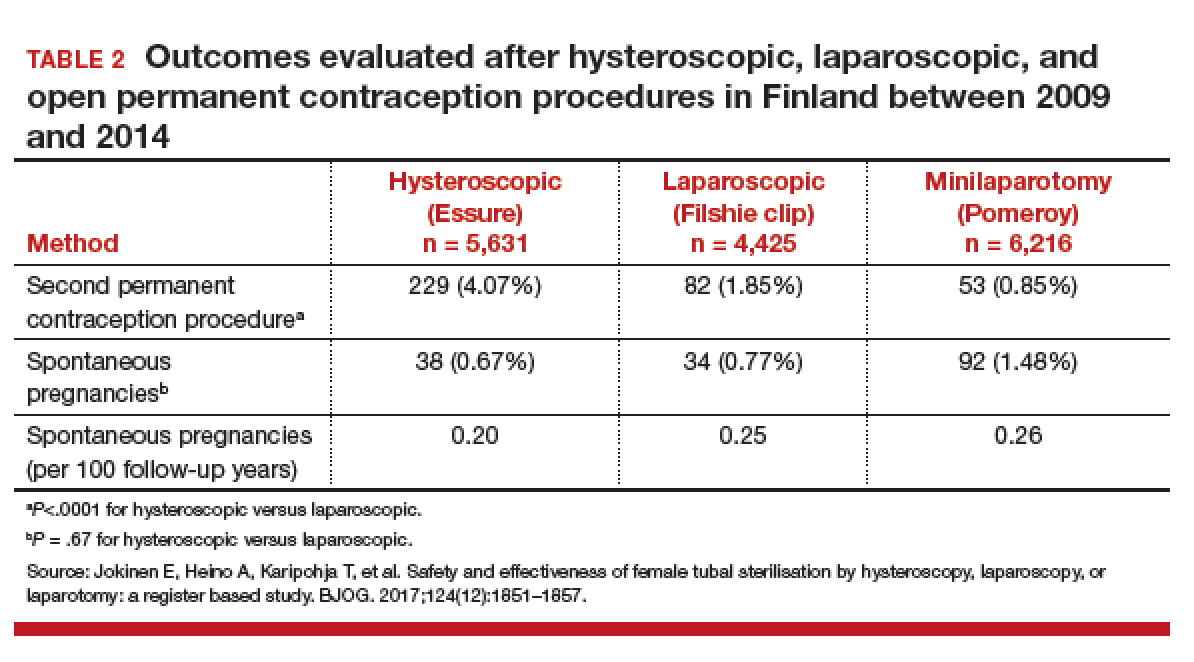
At a glance, these studies suggest that pregnancy rates are similar between hysteroscopic and laparoscopic permanent contraceptive approaches. But, these low failure rates were only achieved after including women who required reoperation or a second permanent contraceptive procedure. All 3 European studies showed a high follow-up rate; as method failure was identified, additional procedures were offered and performed when desired. These rates are higher than typically reported in US studies. None of the studies included discussion about the proportion of women with failed procedures who declined a second permanent contraceptive surgery. Bouillon et al26 reported a slight improvement in perioperative safety for a hysteroscopic procedure compared with a laparoscopic procedure. While severity of complications was not reported, the risk of reoperation for laparoscopic procedures remained <1%. By contrast, based on the evidence presented here, hysteroscopic permanent contraceptive methods required a second procedure for 4% to 8% of women, most of whom underwent a laparoscopic procedure. Thus, the slight potential improvement in safety of hysteroscopic procedures does not offset the significantly lower efficacy of the method.
Technique for hysteroscopic permanent contraception insert removal
Johal T, Kuruba N, Sule M, et al. Laparoscopic salpingectomy and removal of Essure hysteroscopic sterilisation device: a case series. Eur J Contracept Reprod Health Care. 2018;23(3):227-230.
Lazorwitz A, Tocce K. A case series of removal of nickel-titanium sterilization microinserts from the uterine cornua using laparoscopic electrocautery for salpingectomy. Contraception. 2017;96(2):96-98.
As reports of complications and concerns with hysteroscopic permanent contraception increase, there has been a rise in device removal procedures. We present 2 recent articles that review laparoscopic techniques for the removal of hysteroscopic permanent contraception devices and describe subsequent outcomes.
Laparoscopic salpingectomy without insert transection
In this descriptive retrospective study, Johal and colleagues reviewed hysteroscopic permanent contraception insert removal in 8 women between 2015 and 2017. The authors described their laparoscopic salpingectomy approach and perioperative complications. Overall safety and feasibility with laparoscopic salpingectomy were evaluated by identifying the number of procedures requiring intraoperative conversion to laparotomy, cornuectomy, or hysterectomy. The authors also measured operative time, estimated blood loss, length of stay, and incidence of implant fracture.
Indications for insert removal included pain (n = 4), dyspareunia (n = 2), abnormal uterine bleeding (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 4). The surgeons divided the mesosalpinx and then transected the fallopian tube approximately 1 cm distal to the cornua exposing the permanent contraception insert while avoiding direct electrosurgical application to the insert. The inserts were then removed intact with gentle traction. All 8 women underwent laparoscopic removal with salpingectomy. One patient had a surgical complication of serosal bowel injury due to laparoscopic entry that was repaired in the usual fashion. Operative time averaged 65 minutes (range, 30 to 100 minutes), blood loss was minimal, and there were no implant fractures.
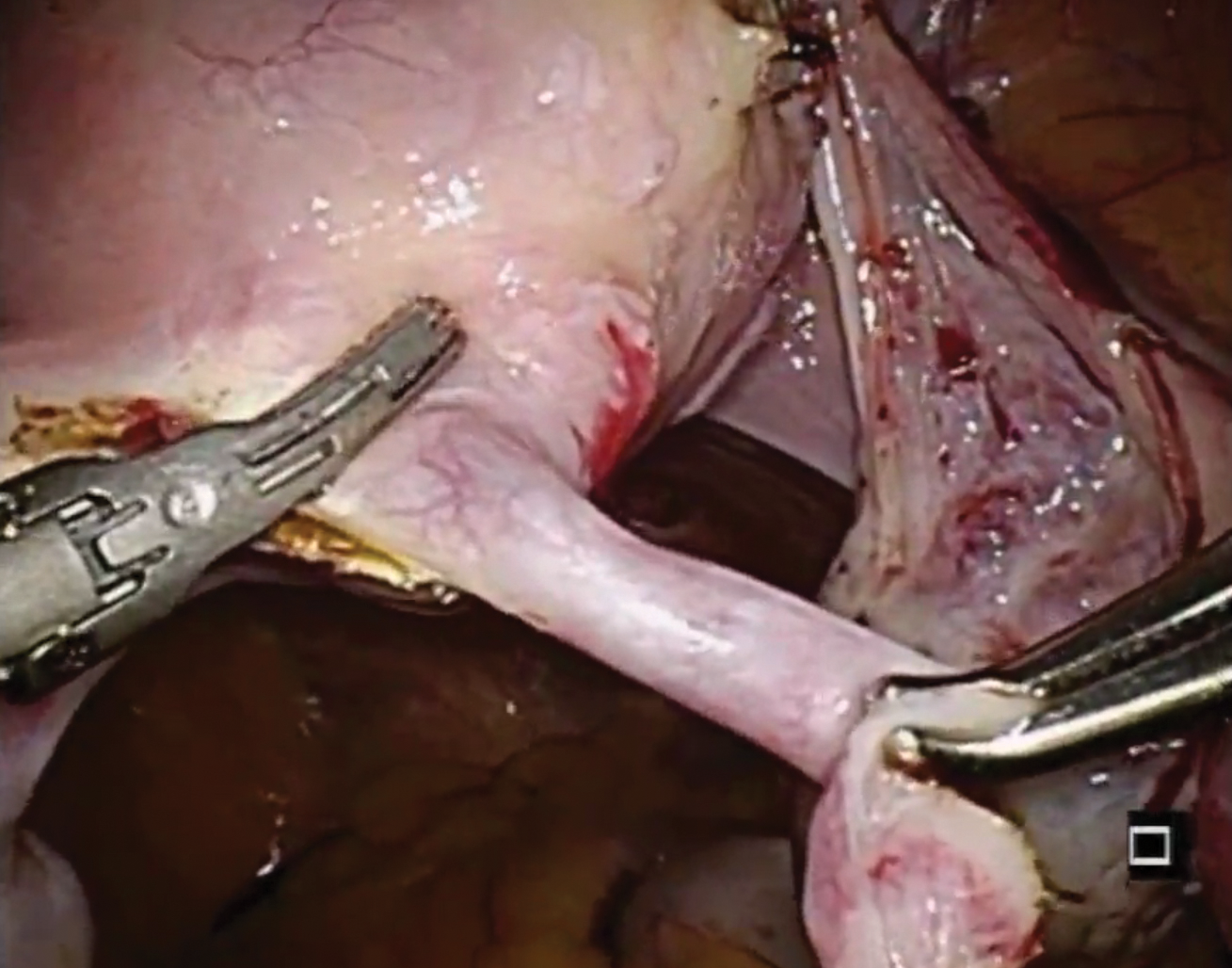
Laparoscopic salpingectomy with insert transection
In this case series, Lazorwitz and Tocce described the use of laparoscopic salpingectomy for hysteroscopic permanent contraception insert removal in 20 women between 2011 and 2017. The authors described their surgical technique, which included division of the mesosalpinx followed by transection of the fallopian tube about 0.5 to 1 cm distal to the cornua. This process often resulted in transection of the insert, and the remaining insert was grasped and removed with gentle traction. If removal of the insert was incomplete, hysteroscopy was performed to identify remaining parts.
Indications for removal included pelvic pain (n = 14), abnormal uterine bleeding (n = 2), rash (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 6). Three women underwent additional diagnostic hysteroscopy for retained implant fragments after laparoscopic salpingectomy. Fragments in all 3 women were 1 to 3 mm in size and left in situ as they were unable to be removed or located hysteroscopically. There were no reported postoperative complications including injury, infection, or readmission within 30 days of salpingectomy.
Shift in method use
Hysteroscopic permanent contraception procedures have low immediate surgical and medical complication rates but result in a high rate of reoperation to achieve the desired outcome. Notably, the largest available comparative trials are from Europe, which may affect the generalizability to US providers, patients, and health care systems.
Importantly, since the introduction of hysteroscopic permanent contraception in 2002, the landscape of contraception has changed in the United States. Contraception use has shifted to fewer permanent procedures and more high-efficacy reversible options. Overall, reliance on female permanent contraception has been declining in the United States, accounting for 17.8% of contracepting women in 1995 and 15.5% in 2013.27,28 Permanent contraception has begun shifting from tubal interruption to salpingectomy as mounting evidence has demonstrated up to a 65% reduction in a woman's lifetime risk of ovarian cancer.29-32 A recent study from a large Northern California integrated health system reported an increase in salpingectomy for permanent contraception from 1% of interval procedures in 2011 to 78% in 2016.33
Long-acting reversible contraceptive (LARC) methods are also becoming more prevalent and are used by 7.2% of women using contraception in the United States.28,34 Typical use pregnancy rates with the levonorgestrel 52-mg intrauterine system, etonogestrel implant, and copper T380A intrauterine device are 0.2%, 0.2%, and 0.4% in the first year, respectively.35,37 These rates are about the same as those reported for Essure in the articles presented here.13,26 Because these methods are easily placed in the office and are immediately effective, their increased availability over the past decade changes demand for a permanent contraceptive procedure.
Essure underwent expedited FDA review because it had the potential to fill a contraceptive void--it was considered permanent, highly efficacious, low risk, and accessible to women regardless of health comorbidities or access to hospital operating rooms. The removal of Essure from the market is not only the result of a collection of problem reports (relatively small given the overall number of women who have used the device) but also the aggregate result of a changing marketplace and the differential needs of pharmaceutical companies and patients.
For a hysteroscopic permanent contraception insert to survive as a marketed product, the company needs high volume use. However, the increase in LARC provision and permanent contraceptive procedures using opportunistic salpingectomy have matured the market away from the presently available hysteroscopic method. This technology, in its current form, is ideal for women desiring permanent contraception but who have a contraindication to laparoscopic surgery, or for women who can access an office procedure in their community but lack access to a hospital-based procedure. For a pharmaceutical company, that smaller market may not be enough. However, the technology itself is still vital, and future development should focus on what we have learned; the ideal product should be immediately effective, not require a follow-up confirmation test, and not leave permanent foreign body within the uterus or tube.
Although both case series were small in sample size, they demonstrated the feasibility of laparoscopic removal of hysteroscopic permanent contraceptive implants. These papers described techniques that can likely be performed by individuals with appropriate laparoscopic skill and experience. The indication for most removals in these reports was pain, unsuccessful placement, or the inability to confirm tubal occlusion by imaging. Importantly, most women do not have these issues, and for those who have been using it successfully, removal is not indicated.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2016(8):CD003034. doi:10.1002/14651858.CD003034.pub3.
- Daniels K, Daugherty J, Jones J, et al. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015(86):1-14.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
- Summary of safety and effectiveness data. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020014b.pdf. Accessed August 2, 2018.
- Shah V, Panay N, Williamson R, Hemingway A. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. Br J Radiol. 2011;84(1005):805-812.
- What is Essure? Bayer website. http://www.essure.com/what-is-essure. Accessed July 6, 2018.
- Stuart GS, Ramesh SS. Interval female sterilization. Obstet Gynecol. 2018;131(1):117-124.
- Essure permanent birth control: instructions for use. Bayer website. http://labeling.bayerhealthcare.com/html/products/pi/essure_ifu.pdf. Accessed July 16, 2018.
- Espey E, Hofler LG. Evaluating the long-term safety of hysteroscopic sterilization. JAMA. 2018;319(4). doi:10.1001/jama.2017.21268.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 pt 1):392-404.
- Cabezas-Palacios MN, Jiménez-Caraballo A, Tato-Varela S, et al. Safety and patients' satisfaction after hysteroscopic sterilisation. J Obstet Gynaecol. 2018;38(3):377-381.
- Antoun L, Smith P, Gupta JK, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J Obstet Gynecol. 2017;217(5):570.e571-570.e576.
- Franchini M, Zizolfi B, Coppola C, et al. Essure permanent birth control, effectiveness and safety: an Italian 11-year survey. J Minim Invasive Gynecol. 2017;24(4):640-645.
- Vleugels M, Cheng RF, Goldstein J, et al. Algorithm of transvaginal ultrasound and/or hysterosalpingogram for confirmation testing at 3 months after Essure placement. J Minim Invasive Gynecol. 2017;24(7):1128-1135.
- Essure confirmation test: Essure confirmation test overview. Bayer website. https://www.hcp.essure-us.com/essure-confirmation-test/. Accessed July 16, 2018.
- Casey J, Cedo-Cintron L, Pearce J, et al. Current techniques and outcomes in hysteroscopic sterilization: current evidence, considerations, and complications with hysteroscopic sterilization micro inserts. Curr Opin Obstet Gynecol. 2017;29(4):218-224.
- Jeirath N, Basinski CM, Hammond MA. Hysteroscopic sterilization device follow-up rate: hysterosalpingogram versus transvaginal ultrasound. J Minim Invasive Gynecol. 2018;25(5):836-841.
- US Department of Health and Human Services, US Food & Drug Administration. FDA Activities: Essure. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ucm452254.htm. Accessed July 6, 2018.
- Horwell DH. End of the road for Essure? J Fam Plann Reprod Health Care. 2017;43(3):240-241.
- Mackenzie J. Sterilisation implant withdrawn from non-US sale. BBC News Health. https://www.bbc.com/news/health-41331963. Accessed July 14, 2018.
- Federal Agency for Medicines and Health Products. ESSURE sterilisation device permanently withdrawn from the market in the European Union. Federal Agency for Medicines and Health Products. https://www.famhp.be/en/news/essure_sterilisation_device_permanently_withdrawn_from_the_market_in_the_european_union. Accessed July 9, 2018.
- Statement from FDA Commissioner Scott Gottlieb, MD, on manufacturer announcement to halt Essure sales in the US; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; US Food and Drug Administration. July 20, 2018.
- Gariepy AM, Creinin MD, Smith KJ, et al. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception. 2014;90(2):174-181.
- Gariepy AM, Creinin MD, Schwarz EB, et al. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2 pt 1):273-279.
- Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319(4):375-387.
- Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36.
- Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital Health Stat 23. 2010(29):1-44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2). pii: dju410.doi:10.1093/jnci/dju410.
- Madsen C, Baandrup L, Dehlendorff C, et al. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86-94.
- Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279-281.
- Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409-414.
- Powell CB, Alabaster A, Simmons S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011-2016. Obstet Gynecol. 2017;130(5):961-967.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014(173):1-8.
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969-980.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646-1653.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
Female permanent contraception is among the most widely used contraceptive methods worldwide. In the United States, more than 640,000 procedures are performed each year and it is used by 25% of women who use contraception.1–4 Female permanent contraception is achieved via salpingectomy, tubal interruption, or hysteroscopic techniques.
Essure, the only currently available hysteroscopic permanent contraception device, approved by the US Food and Drug Administration (FDA) in 2002,5,6 has been implanted in more than 750,000 women worldwide.7 Essure was developed by Conceptus Inc, a small medical device company that was acquired by Bayer in 2013. The greatest uptake has been in the United States, which accounts for approximately 80% of procedures worldwide.7,8
Essure placement involves insertion of a nickel-titanium alloy coil with a stainless-steel inner coil, polyethylene terephthalate fibers, platinum marker bands, and silver-tin solder.9 The insert is approximately 4 cm in length and expands to 2 mm in diameter once deployed.9
Potential advantages of a hysteroscopic approach are that intra-abdominal surgery can be avoided and the procedure can be performed in an office without the need for general anesthesia.7 Due to these potential benefits, hysteroscopic permanent contraception with Essure underwent expedited review and received FDA approval without any comparative trials.1,5,10 However, there also are disadvantages: the method is not always successfully placed on first attempt and it is not immediately effective. Successful placement rates range between 60% and 98%, most commonly around 90%.11–15 Additionally, if placement is successful, alternative contraception must be used until a confirmatory radiologic test is performed at least 3 months after the procedure.9,11 Initially, hysterosalpingography was required to demonstrate a satisfactory insert location and successful tubal occlusion.11,16 Compliance with this testing is variable, ranging in studies from 13% to 71%.11 As of 2015, transvaginal ultrasonography showing insert retention and location has been approved as an alternative confirmatory method.9,11,16,17 Evidence suggests that the less invasive ultrasound option increases follow-up rates; while limited, one study noted an increase in follow-up rates from 77.5% for hysterosalpingogram to 88% (P = .008) for transvaginal ultrasound.18
Recent concerns about potential medical and safety issues have impacted approval status and marketing of hysteroscopic permanent contraception worldwide. In response to safety concerns, the FDA added a boxed safety warning and patient decision checklist in 2016.19 Bayer withdrew the device from all markets outside of the United States as of May 2017.20–22 In April 2018, the FDA restricted Essure sales in the United States only to providers and facilities who utilized an FDA-approved checklist to ensure the device met standards for safety and effectiveness.19 Most recently, Bayer announced that Essure would no longer be sold or distributed in the United States after December 31, 2018 (See “FDA Press Release”).23
"The US Food and Drug Administration was notified by Bayer that the Essure permanent birth control device will no longer be sold or distributed after December 31, 2018... The decision today to halt Essure sales also follows a series of earlier actions that the FDA took to address the reports of serious adverse events associated with its use. For women who have received an Essure implant, the postmarket safety of Essure will continue to be a top priority for the FDA. We expect Bayer to meet its postmarket obligations concerning this device."
Reference
- Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; U.S. Food and Drug Administration. July 20, 2018.
So how did we get here? How did the promise of a “less invasive” approach for female permanent contraception get off course?
A search of the Manufacturer and User Facility Device Experience (MAUDE) database from Essure’s approval date in 2002 to December 2017 revealed 26,773 medical device reports, with more than 90% of those received in 2017 related to device removal.19 As more complications and complaints have been reported, the lack of comparative data has presented a problem for understanding the relative risk of the procedure as compared with laparoscopic techniques. Additionally, the approval studies lacked information about what happened to women who had an unsuccessful attempted hysteroscopic procedure. Without robust data sets or large trials, early research used evidence-based Markov modeling; findings suggested that hysteroscopic permanent contraception resulted in fewer women achieving successful permanent contraception and that the hysteroscopic procedure was not as effective as laparoscopic occlusion procedures with “typical” use.24,25
Over the past year, more clinical data have been published comparing hysteroscopic with laparoscopic permanent contraception procedures. In this article, we evaluate this information to help us better understand the relative efficacy and safety of the different permanent contraception methods and review recent articles describing removal techniques to further assist clinicians and patients considering such procedures.
Hysteroscopic versus laparoscopic procedures for permanent contraception
Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018:319(4):375-387.
Antoun L, Smith P, Gupta J, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J of Obstet Gynecol. 2017;217(5):570.e1-570.e6. doi:10.1016/j.ajog.2017.07.011.
Jokinen E, Heino A, Karipohja T, et al. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy, or laparotomy: a register based study. BJOG. 2017;124(12):1851-1857.
In this section, we present 3 recent studies that evaluate pregnancy outcomes and complications including reoperation or second permanent contraception procedure rates.
Data from France measure up to 3-year differences in adverse outcomes
Bouillon and colleagues aimed to identify differences in adverse outcome rates between hysteroscopic and laparoscopic permanent contraceptive methods. Utilizing national hospital discharge data in France, the researchers conducted a large database study review of records from more than 105,000 women aged 30 to 54 years receiving hysteroscopic or laparoscopic permanent contraception between 2010 and 2014. The database contains details based on the ICD-10 codes for all public and private hospitals in France, representing approximately 75% of the total population. Procedures were performed at 831 hospitals in 26 regions.
Adverse outcomes were divided into surgical, medical, and gynecologic complications (TABLE 1) and were assessed at 3 timepoints: at the time of procedure and at 1 and 3 years postprocedure.

Overall, 71,303 women (67.7%) underwent hysteroscopic permanent contraception procedures and 34,054 women (32.3%) underwent laparoscopic permanent contraception procedures. Immediate surgical and medical complications were significantly less common for women having hysteroscopic compared with laparoscopic procedures. Surgical complications at the time of the procedure occurred in 96 (0.13%) and 265 (0.78%) women, respectively (adjusted odds ratio [aOR], 0.18; 95% confidence interval [CI], 0.14-0.23). Medical complications at the time of procedure occurred in 41 (0.06%) and 39 (0.11%) women, respectively (aOR, 0.51; 95% CI, 0.30-0.89).
However, gynecologic outcomes, including need for a second surgery to provide permanent contraception and overall failure rates (need for salpingectomy, a second permanent contraception procedure, or pregnancy) were significantly more common for women having hysteroscopic procedures. By 1 year after the procedure, 2,955 women (4.10%) who initially had a hysteroscopic procedure, and 56 women (0.16%) who had a laparoscopic procedure required a second permanent contraception surgery (adjusted hazard ratio [aHR], 25.99; 95% CI, 17.84-37.86). By the third year, additional procedures were performed in 3,230 (4.5%) and 97 (0.28%) women, respectively (aHR, 16.63; 95% CI, 12.50-22.20). Most (65%) of the repeat procedures were performed laparoscopically. Although pregnancy rates were significantly lower at 1 year among women who initially chose a hysteroscopic procedure (0.24% vs 0.41%; aHR, 0.70; 95% CI, 0.53-0.92), the rates did not differ at 3 years (0.48% vs 0.57%, respectively; aHR, 1.04; 95% CI, 0.83-1.30).
Most importantly, overall procedure failure rates were significantly higher at 1 year in women initially choosing a hysteroscopic approach compared with laparoscopic approach (3,446 [4.83%] vs 235 [0.69%] women; aHR, 7.11; 95% CI, 5.92-8.54). This difference persisted through 3 years (4,098 [5.75%] vs 438 [1.29%] women, respectively; aHR, 4.66; 95% CI, 4.06-5.34).
UK data indicate high reoperation rate for hysteroscopic procedures
Antoun and colleagues aimed to compare pregnancy rates, radiologic imaging follow-up rates, reoperations, and 30-day adverse outcomes, between hysteroscopic and laparoscopic permanent contraception methods. Conducted at a single teaching hospital in the United Kingdom, this study included 3,497 women who underwent procedures between 2005 and 2015. The data were collected prospectively for the 1,085 women who underwent hysteroscopic procedures and retrospectively for 2,412 women who had laparoscopic permanent contraception procedures with the Filshie clip.
Over the 10-year study period, hysteroscopic permanent contraception increased from 14.2% (40 of 280) of procedures in 2005 to 40.5% (150 of 350) of procedures in 2015 (P<.001). Overall, 2,400 women (99.5%) underwent successful laparoscopic permanent contraception, compared with 992 women (91.4%) in the hysteroscopic group (OR, 18.8; 95% CI, 10.2-34.4).
In the hysteroscopic group, 958 women (97%) returned for confirmatory testing, of whom 902 (91% of women with successful placement) underwent satisfactory confirmatory testing. There were 93 (8.6%) unsuccessful placements that were due to inability to visualize ostia or tubal stenosis (n = 72 [77.4%]), patient intolerance to procedure (n = 15 [16.1%]), or device failure (n = 6 [6.5%]).
The odds for reoperation were 6 times greater in the hysteroscopic group by 1 year after surgery (22 [2%] vs 8 [0.3%] women; OR, 6.2; 95% CI, 2.8-14.0). However, the 1-year pregnancy risk was similar between the 2 groups, with 3 reported pregnancies after hysteroscopic permanent contraception and 5 reported pregnancies after laparoscopic permanent contraception (OR, 1.3; 95% CI, 0.3-5.6).
Finnish researchers also find high reoperation rate
Jokinen and colleagues used linked national database registries in Finland to capture data on pregnancy rate and reoperations among 16,272 women who underwent permanent contraception procedures between 2009 and 2014. The authors compared outcomes following hysteroscopic (Essure), laparoscopic (Filshie clip), and postpartum minilaparotomy (Pomeroy) permanent contraception techniques. According to the investigators, the latter method was almost exclusively performed at the time of cesarean delivery. While there was no difference in pregnancy rates, second permanent contraception procedures were significantly greater in the hysteroscopic group compared with the laparoscopic group (TABLE 2).

At a glance, these studies suggest that pregnancy rates are similar between hysteroscopic and laparoscopic permanent contraceptive approaches. But, these low failure rates were only achieved after including women who required reoperation or a second permanent contraceptive procedure. All 3 European studies showed a high follow-up rate; as method failure was identified, additional procedures were offered and performed when desired. These rates are higher than typically reported in US studies. None of the studies included discussion about the proportion of women with failed procedures who declined a second permanent contraceptive surgery. Bouillon et al26 reported a slight improvement in perioperative safety for a hysteroscopic procedure compared with a laparoscopic procedure. While severity of complications was not reported, the risk of reoperation for laparoscopic procedures remained <1%. By contrast, based on the evidence presented here, hysteroscopic permanent contraceptive methods required a second procedure for 4% to 8% of women, most of whom underwent a laparoscopic procedure. Thus, the slight potential improvement in safety of hysteroscopic procedures does not offset the significantly lower efficacy of the method.
Technique for hysteroscopic permanent contraception insert removal
Johal T, Kuruba N, Sule M, et al. Laparoscopic salpingectomy and removal of Essure hysteroscopic sterilisation device: a case series. Eur J Contracept Reprod Health Care. 2018;23(3):227-230.
Lazorwitz A, Tocce K. A case series of removal of nickel-titanium sterilization microinserts from the uterine cornua using laparoscopic electrocautery for salpingectomy. Contraception. 2017;96(2):96-98.
As reports of complications and concerns with hysteroscopic permanent contraception increase, there has been a rise in device removal procedures. We present 2 recent articles that review laparoscopic techniques for the removal of hysteroscopic permanent contraception devices and describe subsequent outcomes.
Laparoscopic salpingectomy without insert transection
In this descriptive retrospective study, Johal and colleagues reviewed hysteroscopic permanent contraception insert removal in 8 women between 2015 and 2017. The authors described their laparoscopic salpingectomy approach and perioperative complications. Overall safety and feasibility with laparoscopic salpingectomy were evaluated by identifying the number of procedures requiring intraoperative conversion to laparotomy, cornuectomy, or hysterectomy. The authors also measured operative time, estimated blood loss, length of stay, and incidence of implant fracture.
Indications for insert removal included pain (n = 4), dyspareunia (n = 2), abnormal uterine bleeding (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 4). The surgeons divided the mesosalpinx and then transected the fallopian tube approximately 1 cm distal to the cornua exposing the permanent contraception insert while avoiding direct electrosurgical application to the insert. The inserts were then removed intact with gentle traction. All 8 women underwent laparoscopic removal with salpingectomy. One patient had a surgical complication of serosal bowel injury due to laparoscopic entry that was repaired in the usual fashion. Operative time averaged 65 minutes (range, 30 to 100 minutes), blood loss was minimal, and there were no implant fractures.

Laparoscopic salpingectomy with insert transection
In this case series, Lazorwitz and Tocce described the use of laparoscopic salpingectomy for hysteroscopic permanent contraception insert removal in 20 women between 2011 and 2017. The authors described their surgical technique, which included division of the mesosalpinx followed by transection of the fallopian tube about 0.5 to 1 cm distal to the cornua. This process often resulted in transection of the insert, and the remaining insert was grasped and removed with gentle traction. If removal of the insert was incomplete, hysteroscopy was performed to identify remaining parts.
Indications for removal included pelvic pain (n = 14), abnormal uterine bleeding (n = 2), rash (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 6). Three women underwent additional diagnostic hysteroscopy for retained implant fragments after laparoscopic salpingectomy. Fragments in all 3 women were 1 to 3 mm in size and left in situ as they were unable to be removed or located hysteroscopically. There were no reported postoperative complications including injury, infection, or readmission within 30 days of salpingectomy.
Shift in method use
Hysteroscopic permanent contraception procedures have low immediate surgical and medical complication rates but result in a high rate of reoperation to achieve the desired outcome. Notably, the largest available comparative trials are from Europe, which may affect the generalizability to US providers, patients, and health care systems.
Importantly, since the introduction of hysteroscopic permanent contraception in 2002, the landscape of contraception has changed in the United States. Contraception use has shifted to fewer permanent procedures and more high-efficacy reversible options. Overall, reliance on female permanent contraception has been declining in the United States, accounting for 17.8% of contracepting women in 1995 and 15.5% in 2013.27,28 Permanent contraception has begun shifting from tubal interruption to salpingectomy as mounting evidence has demonstrated up to a 65% reduction in a woman's lifetime risk of ovarian cancer.29-32 A recent study from a large Northern California integrated health system reported an increase in salpingectomy for permanent contraception from 1% of interval procedures in 2011 to 78% in 2016.33
Long-acting reversible contraceptive (LARC) methods are also becoming more prevalent and are used by 7.2% of women using contraception in the United States.28,34 Typical use pregnancy rates with the levonorgestrel 52-mg intrauterine system, etonogestrel implant, and copper T380A intrauterine device are 0.2%, 0.2%, and 0.4% in the first year, respectively.35,37 These rates are about the same as those reported for Essure in the articles presented here.13,26 Because these methods are easily placed in the office and are immediately effective, their increased availability over the past decade changes demand for a permanent contraceptive procedure.
Essure underwent expedited FDA review because it had the potential to fill a contraceptive void--it was considered permanent, highly efficacious, low risk, and accessible to women regardless of health comorbidities or access to hospital operating rooms. The removal of Essure from the market is not only the result of a collection of problem reports (relatively small given the overall number of women who have used the device) but also the aggregate result of a changing marketplace and the differential needs of pharmaceutical companies and patients.
For a hysteroscopic permanent contraception insert to survive as a marketed product, the company needs high volume use. However, the increase in LARC provision and permanent contraceptive procedures using opportunistic salpingectomy have matured the market away from the presently available hysteroscopic method. This technology, in its current form, is ideal for women desiring permanent contraception but who have a contraindication to laparoscopic surgery, or for women who can access an office procedure in their community but lack access to a hospital-based procedure. For a pharmaceutical company, that smaller market may not be enough. However, the technology itself is still vital, and future development should focus on what we have learned; the ideal product should be immediately effective, not require a follow-up confirmation test, and not leave permanent foreign body within the uterus or tube.
Although both case series were small in sample size, they demonstrated the feasibility of laparoscopic removal of hysteroscopic permanent contraceptive implants. These papers described techniques that can likely be performed by individuals with appropriate laparoscopic skill and experience. The indication for most removals in these reports was pain, unsuccessful placement, or the inability to confirm tubal occlusion by imaging. Importantly, most women do not have these issues, and for those who have been using it successfully, removal is not indicated.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Female permanent contraception is among the most widely used contraceptive methods worldwide. In the United States, more than 640,000 procedures are performed each year and it is used by 25% of women who use contraception.1–4 Female permanent contraception is achieved via salpingectomy, tubal interruption, or hysteroscopic techniques.
Essure, the only currently available hysteroscopic permanent contraception device, approved by the US Food and Drug Administration (FDA) in 2002,5,6 has been implanted in more than 750,000 women worldwide.7 Essure was developed by Conceptus Inc, a small medical device company that was acquired by Bayer in 2013. The greatest uptake has been in the United States, which accounts for approximately 80% of procedures worldwide.7,8
Essure placement involves insertion of a nickel-titanium alloy coil with a stainless-steel inner coil, polyethylene terephthalate fibers, platinum marker bands, and silver-tin solder.9 The insert is approximately 4 cm in length and expands to 2 mm in diameter once deployed.9
Potential advantages of a hysteroscopic approach are that intra-abdominal surgery can be avoided and the procedure can be performed in an office without the need for general anesthesia.7 Due to these potential benefits, hysteroscopic permanent contraception with Essure underwent expedited review and received FDA approval without any comparative trials.1,5,10 However, there also are disadvantages: the method is not always successfully placed on first attempt and it is not immediately effective. Successful placement rates range between 60% and 98%, most commonly around 90%.11–15 Additionally, if placement is successful, alternative contraception must be used until a confirmatory radiologic test is performed at least 3 months after the procedure.9,11 Initially, hysterosalpingography was required to demonstrate a satisfactory insert location and successful tubal occlusion.11,16 Compliance with this testing is variable, ranging in studies from 13% to 71%.11 As of 2015, transvaginal ultrasonography showing insert retention and location has been approved as an alternative confirmatory method.9,11,16,17 Evidence suggests that the less invasive ultrasound option increases follow-up rates; while limited, one study noted an increase in follow-up rates from 77.5% for hysterosalpingogram to 88% (P = .008) for transvaginal ultrasound.18
Recent concerns about potential medical and safety issues have impacted approval status and marketing of hysteroscopic permanent contraception worldwide. In response to safety concerns, the FDA added a boxed safety warning and patient decision checklist in 2016.19 Bayer withdrew the device from all markets outside of the United States as of May 2017.20–22 In April 2018, the FDA restricted Essure sales in the United States only to providers and facilities who utilized an FDA-approved checklist to ensure the device met standards for safety and effectiveness.19 Most recently, Bayer announced that Essure would no longer be sold or distributed in the United States after December 31, 2018 (See “FDA Press Release”).23
"The US Food and Drug Administration was notified by Bayer that the Essure permanent birth control device will no longer be sold or distributed after December 31, 2018... The decision today to halt Essure sales also follows a series of earlier actions that the FDA took to address the reports of serious adverse events associated with its use. For women who have received an Essure implant, the postmarket safety of Essure will continue to be a top priority for the FDA. We expect Bayer to meet its postmarket obligations concerning this device."
Reference
- Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; U.S. Food and Drug Administration. July 20, 2018.
So how did we get here? How did the promise of a “less invasive” approach for female permanent contraception get off course?
A search of the Manufacturer and User Facility Device Experience (MAUDE) database from Essure’s approval date in 2002 to December 2017 revealed 26,773 medical device reports, with more than 90% of those received in 2017 related to device removal.19 As more complications and complaints have been reported, the lack of comparative data has presented a problem for understanding the relative risk of the procedure as compared with laparoscopic techniques. Additionally, the approval studies lacked information about what happened to women who had an unsuccessful attempted hysteroscopic procedure. Without robust data sets or large trials, early research used evidence-based Markov modeling; findings suggested that hysteroscopic permanent contraception resulted in fewer women achieving successful permanent contraception and that the hysteroscopic procedure was not as effective as laparoscopic occlusion procedures with “typical” use.24,25
Over the past year, more clinical data have been published comparing hysteroscopic with laparoscopic permanent contraception procedures. In this article, we evaluate this information to help us better understand the relative efficacy and safety of the different permanent contraception methods and review recent articles describing removal techniques to further assist clinicians and patients considering such procedures.
Hysteroscopic versus laparoscopic procedures for permanent contraception
Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018:319(4):375-387.
Antoun L, Smith P, Gupta J, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J of Obstet Gynecol. 2017;217(5):570.e1-570.e6. doi:10.1016/j.ajog.2017.07.011.
Jokinen E, Heino A, Karipohja T, et al. Safety and effectiveness of female tubal sterilisation by hysteroscopy, laparoscopy, or laparotomy: a register based study. BJOG. 2017;124(12):1851-1857.
In this section, we present 3 recent studies that evaluate pregnancy outcomes and complications including reoperation or second permanent contraception procedure rates.
Data from France measure up to 3-year differences in adverse outcomes
Bouillon and colleagues aimed to identify differences in adverse outcome rates between hysteroscopic and laparoscopic permanent contraceptive methods. Utilizing national hospital discharge data in France, the researchers conducted a large database study review of records from more than 105,000 women aged 30 to 54 years receiving hysteroscopic or laparoscopic permanent contraception between 2010 and 2014. The database contains details based on the ICD-10 codes for all public and private hospitals in France, representing approximately 75% of the total population. Procedures were performed at 831 hospitals in 26 regions.
Adverse outcomes were divided into surgical, medical, and gynecologic complications (TABLE 1) and were assessed at 3 timepoints: at the time of procedure and at 1 and 3 years postprocedure.

Overall, 71,303 women (67.7%) underwent hysteroscopic permanent contraception procedures and 34,054 women (32.3%) underwent laparoscopic permanent contraception procedures. Immediate surgical and medical complications were significantly less common for women having hysteroscopic compared with laparoscopic procedures. Surgical complications at the time of the procedure occurred in 96 (0.13%) and 265 (0.78%) women, respectively (adjusted odds ratio [aOR], 0.18; 95% confidence interval [CI], 0.14-0.23). Medical complications at the time of procedure occurred in 41 (0.06%) and 39 (0.11%) women, respectively (aOR, 0.51; 95% CI, 0.30-0.89).
However, gynecologic outcomes, including need for a second surgery to provide permanent contraception and overall failure rates (need for salpingectomy, a second permanent contraception procedure, or pregnancy) were significantly more common for women having hysteroscopic procedures. By 1 year after the procedure, 2,955 women (4.10%) who initially had a hysteroscopic procedure, and 56 women (0.16%) who had a laparoscopic procedure required a second permanent contraception surgery (adjusted hazard ratio [aHR], 25.99; 95% CI, 17.84-37.86). By the third year, additional procedures were performed in 3,230 (4.5%) and 97 (0.28%) women, respectively (aHR, 16.63; 95% CI, 12.50-22.20). Most (65%) of the repeat procedures were performed laparoscopically. Although pregnancy rates were significantly lower at 1 year among women who initially chose a hysteroscopic procedure (0.24% vs 0.41%; aHR, 0.70; 95% CI, 0.53-0.92), the rates did not differ at 3 years (0.48% vs 0.57%, respectively; aHR, 1.04; 95% CI, 0.83-1.30).
Most importantly, overall procedure failure rates were significantly higher at 1 year in women initially choosing a hysteroscopic approach compared with laparoscopic approach (3,446 [4.83%] vs 235 [0.69%] women; aHR, 7.11; 95% CI, 5.92-8.54). This difference persisted through 3 years (4,098 [5.75%] vs 438 [1.29%] women, respectively; aHR, 4.66; 95% CI, 4.06-5.34).
UK data indicate high reoperation rate for hysteroscopic procedures
Antoun and colleagues aimed to compare pregnancy rates, radiologic imaging follow-up rates, reoperations, and 30-day adverse outcomes, between hysteroscopic and laparoscopic permanent contraception methods. Conducted at a single teaching hospital in the United Kingdom, this study included 3,497 women who underwent procedures between 2005 and 2015. The data were collected prospectively for the 1,085 women who underwent hysteroscopic procedures and retrospectively for 2,412 women who had laparoscopic permanent contraception procedures with the Filshie clip.
Over the 10-year study period, hysteroscopic permanent contraception increased from 14.2% (40 of 280) of procedures in 2005 to 40.5% (150 of 350) of procedures in 2015 (P<.001). Overall, 2,400 women (99.5%) underwent successful laparoscopic permanent contraception, compared with 992 women (91.4%) in the hysteroscopic group (OR, 18.8; 95% CI, 10.2-34.4).
In the hysteroscopic group, 958 women (97%) returned for confirmatory testing, of whom 902 (91% of women with successful placement) underwent satisfactory confirmatory testing. There were 93 (8.6%) unsuccessful placements that were due to inability to visualize ostia or tubal stenosis (n = 72 [77.4%]), patient intolerance to procedure (n = 15 [16.1%]), or device failure (n = 6 [6.5%]).
The odds for reoperation were 6 times greater in the hysteroscopic group by 1 year after surgery (22 [2%] vs 8 [0.3%] women; OR, 6.2; 95% CI, 2.8-14.0). However, the 1-year pregnancy risk was similar between the 2 groups, with 3 reported pregnancies after hysteroscopic permanent contraception and 5 reported pregnancies after laparoscopic permanent contraception (OR, 1.3; 95% CI, 0.3-5.6).
Finnish researchers also find high reoperation rate
Jokinen and colleagues used linked national database registries in Finland to capture data on pregnancy rate and reoperations among 16,272 women who underwent permanent contraception procedures between 2009 and 2014. The authors compared outcomes following hysteroscopic (Essure), laparoscopic (Filshie clip), and postpartum minilaparotomy (Pomeroy) permanent contraception techniques. According to the investigators, the latter method was almost exclusively performed at the time of cesarean delivery. While there was no difference in pregnancy rates, second permanent contraception procedures were significantly greater in the hysteroscopic group compared with the laparoscopic group (TABLE 2).

At a glance, these studies suggest that pregnancy rates are similar between hysteroscopic and laparoscopic permanent contraceptive approaches. But, these low failure rates were only achieved after including women who required reoperation or a second permanent contraceptive procedure. All 3 European studies showed a high follow-up rate; as method failure was identified, additional procedures were offered and performed when desired. These rates are higher than typically reported in US studies. None of the studies included discussion about the proportion of women with failed procedures who declined a second permanent contraceptive surgery. Bouillon et al26 reported a slight improvement in perioperative safety for a hysteroscopic procedure compared with a laparoscopic procedure. While severity of complications was not reported, the risk of reoperation for laparoscopic procedures remained <1%. By contrast, based on the evidence presented here, hysteroscopic permanent contraceptive methods required a second procedure for 4% to 8% of women, most of whom underwent a laparoscopic procedure. Thus, the slight potential improvement in safety of hysteroscopic procedures does not offset the significantly lower efficacy of the method.
Technique for hysteroscopic permanent contraception insert removal
Johal T, Kuruba N, Sule M, et al. Laparoscopic salpingectomy and removal of Essure hysteroscopic sterilisation device: a case series. Eur J Contracept Reprod Health Care. 2018;23(3):227-230.
Lazorwitz A, Tocce K. A case series of removal of nickel-titanium sterilization microinserts from the uterine cornua using laparoscopic electrocautery for salpingectomy. Contraception. 2017;96(2):96-98.
As reports of complications and concerns with hysteroscopic permanent contraception increase, there has been a rise in device removal procedures. We present 2 recent articles that review laparoscopic techniques for the removal of hysteroscopic permanent contraception devices and describe subsequent outcomes.
Laparoscopic salpingectomy without insert transection
In this descriptive retrospective study, Johal and colleagues reviewed hysteroscopic permanent contraception insert removal in 8 women between 2015 and 2017. The authors described their laparoscopic salpingectomy approach and perioperative complications. Overall safety and feasibility with laparoscopic salpingectomy were evaluated by identifying the number of procedures requiring intraoperative conversion to laparotomy, cornuectomy, or hysterectomy. The authors also measured operative time, estimated blood loss, length of stay, and incidence of implant fracture.
Indications for insert removal included pain (n = 4), dyspareunia (n = 2), abnormal uterine bleeding (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 4). The surgeons divided the mesosalpinx and then transected the fallopian tube approximately 1 cm distal to the cornua exposing the permanent contraception insert while avoiding direct electrosurgical application to the insert. The inserts were then removed intact with gentle traction. All 8 women underwent laparoscopic removal with salpingectomy. One patient had a surgical complication of serosal bowel injury due to laparoscopic entry that was repaired in the usual fashion. Operative time averaged 65 minutes (range, 30 to 100 minutes), blood loss was minimal, and there were no implant fractures.

Laparoscopic salpingectomy with insert transection
In this case series, Lazorwitz and Tocce described the use of laparoscopic salpingectomy for hysteroscopic permanent contraception insert removal in 20 women between 2011 and 2017. The authors described their surgical technique, which included division of the mesosalpinx followed by transection of the fallopian tube about 0.5 to 1 cm distal to the cornua. This process often resulted in transection of the insert, and the remaining insert was grasped and removed with gentle traction. If removal of the insert was incomplete, hysteroscopy was performed to identify remaining parts.
Indications for removal included pelvic pain (n = 14), abnormal uterine bleeding (n = 2), rash (n = 1), and unsuccessful placement or evidence of tubal occlusion failure during confirmatory imaging (n = 6). Three women underwent additional diagnostic hysteroscopy for retained implant fragments after laparoscopic salpingectomy. Fragments in all 3 women were 1 to 3 mm in size and left in situ as they were unable to be removed or located hysteroscopically. There were no reported postoperative complications including injury, infection, or readmission within 30 days of salpingectomy.
Shift in method use
Hysteroscopic permanent contraception procedures have low immediate surgical and medical complication rates but result in a high rate of reoperation to achieve the desired outcome. Notably, the largest available comparative trials are from Europe, which may affect the generalizability to US providers, patients, and health care systems.
Importantly, since the introduction of hysteroscopic permanent contraception in 2002, the landscape of contraception has changed in the United States. Contraception use has shifted to fewer permanent procedures and more high-efficacy reversible options. Overall, reliance on female permanent contraception has been declining in the United States, accounting for 17.8% of contracepting women in 1995 and 15.5% in 2013.27,28 Permanent contraception has begun shifting from tubal interruption to salpingectomy as mounting evidence has demonstrated up to a 65% reduction in a woman's lifetime risk of ovarian cancer.29-32 A recent study from a large Northern California integrated health system reported an increase in salpingectomy for permanent contraception from 1% of interval procedures in 2011 to 78% in 2016.33
Long-acting reversible contraceptive (LARC) methods are also becoming more prevalent and are used by 7.2% of women using contraception in the United States.28,34 Typical use pregnancy rates with the levonorgestrel 52-mg intrauterine system, etonogestrel implant, and copper T380A intrauterine device are 0.2%, 0.2%, and 0.4% in the first year, respectively.35,37 These rates are about the same as those reported for Essure in the articles presented here.13,26 Because these methods are easily placed in the office and are immediately effective, their increased availability over the past decade changes demand for a permanent contraceptive procedure.
Essure underwent expedited FDA review because it had the potential to fill a contraceptive void--it was considered permanent, highly efficacious, low risk, and accessible to women regardless of health comorbidities or access to hospital operating rooms. The removal of Essure from the market is not only the result of a collection of problem reports (relatively small given the overall number of women who have used the device) but also the aggregate result of a changing marketplace and the differential needs of pharmaceutical companies and patients.
For a hysteroscopic permanent contraception insert to survive as a marketed product, the company needs high volume use. However, the increase in LARC provision and permanent contraceptive procedures using opportunistic salpingectomy have matured the market away from the presently available hysteroscopic method. This technology, in its current form, is ideal for women desiring permanent contraception but who have a contraindication to laparoscopic surgery, or for women who can access an office procedure in their community but lack access to a hospital-based procedure. For a pharmaceutical company, that smaller market may not be enough. However, the technology itself is still vital, and future development should focus on what we have learned; the ideal product should be immediately effective, not require a follow-up confirmation test, and not leave permanent foreign body within the uterus or tube.
Although both case series were small in sample size, they demonstrated the feasibility of laparoscopic removal of hysteroscopic permanent contraceptive implants. These papers described techniques that can likely be performed by individuals with appropriate laparoscopic skill and experience. The indication for most removals in these reports was pain, unsuccessful placement, or the inability to confirm tubal occlusion by imaging. Importantly, most women do not have these issues, and for those who have been using it successfully, removal is not indicated.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2016(8):CD003034. doi:10.1002/14651858.CD003034.pub3.
- Daniels K, Daugherty J, Jones J, et al. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015(86):1-14.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
- Summary of safety and effectiveness data. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020014b.pdf. Accessed August 2, 2018.
- Shah V, Panay N, Williamson R, Hemingway A. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. Br J Radiol. 2011;84(1005):805-812.
- What is Essure? Bayer website. http://www.essure.com/what-is-essure. Accessed July 6, 2018.
- Stuart GS, Ramesh SS. Interval female sterilization. Obstet Gynecol. 2018;131(1):117-124.
- Essure permanent birth control: instructions for use. Bayer website. http://labeling.bayerhealthcare.com/html/products/pi/essure_ifu.pdf. Accessed July 16, 2018.
- Espey E, Hofler LG. Evaluating the long-term safety of hysteroscopic sterilization. JAMA. 2018;319(4). doi:10.1001/jama.2017.21268.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 pt 1):392-404.
- Cabezas-Palacios MN, Jiménez-Caraballo A, Tato-Varela S, et al. Safety and patients' satisfaction after hysteroscopic sterilisation. J Obstet Gynaecol. 2018;38(3):377-381.
- Antoun L, Smith P, Gupta JK, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J Obstet Gynecol. 2017;217(5):570.e571-570.e576.
- Franchini M, Zizolfi B, Coppola C, et al. Essure permanent birth control, effectiveness and safety: an Italian 11-year survey. J Minim Invasive Gynecol. 2017;24(4):640-645.
- Vleugels M, Cheng RF, Goldstein J, et al. Algorithm of transvaginal ultrasound and/or hysterosalpingogram for confirmation testing at 3 months after Essure placement. J Minim Invasive Gynecol. 2017;24(7):1128-1135.
- Essure confirmation test: Essure confirmation test overview. Bayer website. https://www.hcp.essure-us.com/essure-confirmation-test/. Accessed July 16, 2018.
- Casey J, Cedo-Cintron L, Pearce J, et al. Current techniques and outcomes in hysteroscopic sterilization: current evidence, considerations, and complications with hysteroscopic sterilization micro inserts. Curr Opin Obstet Gynecol. 2017;29(4):218-224.
- Jeirath N, Basinski CM, Hammond MA. Hysteroscopic sterilization device follow-up rate: hysterosalpingogram versus transvaginal ultrasound. J Minim Invasive Gynecol. 2018;25(5):836-841.
- US Department of Health and Human Services, US Food & Drug Administration. FDA Activities: Essure. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ucm452254.htm. Accessed July 6, 2018.
- Horwell DH. End of the road for Essure? J Fam Plann Reprod Health Care. 2017;43(3):240-241.
- Mackenzie J. Sterilisation implant withdrawn from non-US sale. BBC News Health. https://www.bbc.com/news/health-41331963. Accessed July 14, 2018.
- Federal Agency for Medicines and Health Products. ESSURE sterilisation device permanently withdrawn from the market in the European Union. Federal Agency for Medicines and Health Products. https://www.famhp.be/en/news/essure_sterilisation_device_permanently_withdrawn_from_the_market_in_the_european_union. Accessed July 9, 2018.
- Statement from FDA Commissioner Scott Gottlieb, MD, on manufacturer announcement to halt Essure sales in the US; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; US Food and Drug Administration. July 20, 2018.
- Gariepy AM, Creinin MD, Smith KJ, et al. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception. 2014;90(2):174-181.
- Gariepy AM, Creinin MD, Schwarz EB, et al. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2 pt 1):273-279.
- Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319(4):375-387.
- Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36.
- Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital Health Stat 23. 2010(29):1-44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2). pii: dju410.doi:10.1093/jnci/dju410.
- Madsen C, Baandrup L, Dehlendorff C, et al. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86-94.
- Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279-281.
- Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409-414.
- Powell CB, Alabaster A, Simmons S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011-2016. Obstet Gynecol. 2017;130(5):961-967.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014(173):1-8.
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969-980.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646-1653.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
- Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2016(8):CD003034. doi:10.1002/14651858.CD003034.pub3.
- Daniels K, Daugherty J, Jones J, et al. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015(86):1-14.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1-6.
- Summary of safety and effectiveness data. FDA website. https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020014b.pdf. Accessed August 2, 2018.
- Shah V, Panay N, Williamson R, Hemingway A. Hysterosalpingogram: an essential examination following Essure hysteroscopic sterilisation. Br J Radiol. 2011;84(1005):805-812.
- What is Essure? Bayer website. http://www.essure.com/what-is-essure. Accessed July 6, 2018.
- Stuart GS, Ramesh SS. Interval female sterilization. Obstet Gynecol. 2018;131(1):117-124.
- Essure permanent birth control: instructions for use. Bayer website. http://labeling.bayerhealthcare.com/html/products/pi/essure_ifu.pdf. Accessed July 16, 2018.
- Espey E, Hofler LG. Evaluating the long-term safety of hysteroscopic sterilization. JAMA. 2018;319(4). doi:10.1001/jama.2017.21268.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 133: benefits and risks of sterilization. Obstet Gynecol. 2013;121(2 pt 1):392-404.
- Cabezas-Palacios MN, Jiménez-Caraballo A, Tato-Varela S, et al. Safety and patients' satisfaction after hysteroscopic sterilisation. J Obstet Gynaecol. 2018;38(3):377-381.
- Antoun L, Smith P, Gupta JK, et al. The feasibility, safety, and effectiveness of hysteroscopic sterilization compared with laparoscopic sterilization. Am J Obstet Gynecol. 2017;217(5):570.e571-570.e576.
- Franchini M, Zizolfi B, Coppola C, et al. Essure permanent birth control, effectiveness and safety: an Italian 11-year survey. J Minim Invasive Gynecol. 2017;24(4):640-645.
- Vleugels M, Cheng RF, Goldstein J, et al. Algorithm of transvaginal ultrasound and/or hysterosalpingogram for confirmation testing at 3 months after Essure placement. J Minim Invasive Gynecol. 2017;24(7):1128-1135.
- Essure confirmation test: Essure confirmation test overview. Bayer website. https://www.hcp.essure-us.com/essure-confirmation-test/. Accessed July 16, 2018.
- Casey J, Cedo-Cintron L, Pearce J, et al. Current techniques and outcomes in hysteroscopic sterilization: current evidence, considerations, and complications with hysteroscopic sterilization micro inserts. Curr Opin Obstet Gynecol. 2017;29(4):218-224.
- Jeirath N, Basinski CM, Hammond MA. Hysteroscopic sterilization device follow-up rate: hysterosalpingogram versus transvaginal ultrasound. J Minim Invasive Gynecol. 2018;25(5):836-841.
- US Department of Health and Human Services, US Food & Drug Administration. FDA Activities: Essure. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/ucm452254.htm. Accessed July 6, 2018.
- Horwell DH. End of the road for Essure? J Fam Plann Reprod Health Care. 2017;43(3):240-241.
- Mackenzie J. Sterilisation implant withdrawn from non-US sale. BBC News Health. https://www.bbc.com/news/health-41331963. Accessed July 14, 2018.
- Federal Agency for Medicines and Health Products. ESSURE sterilisation device permanently withdrawn from the market in the European Union. Federal Agency for Medicines and Health Products. https://www.famhp.be/en/news/essure_sterilisation_device_permanently_withdrawn_from_the_market_in_the_european_union. Accessed July 9, 2018.
- Statement from FDA Commissioner Scott Gottlieb, MD, on manufacturer announcement to halt Essure sales in the US; agency's continued commitment to postmarket review of Essure and keeping women informed [press release]. Silver Spring, MD; US Food and Drug Administration. July 20, 2018.
- Gariepy AM, Creinin MD, Smith KJ, et al. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception. 2014;90(2):174-181.
- Gariepy AM, Creinin MD, Schwarz EB, et al. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2 pt 1):273-279.
- Bouillon K, Bertrand M, Bader G, et al. Association of hysteroscopic vs laparoscopic sterilization with procedural, gynecological, and medical outcomes. JAMA. 2018;319(4):375-387.
- Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982-2002. Adv Data. 2004(350):1-36.
- Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital Health Stat 23. 2010(29):1-44.
- Falconer H, Yin L, Grönberg H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2). pii: dju410.doi:10.1093/jnci/dju410.
- Madsen C, Baandrup L, Dehlendorff C, et al. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86-94.
- Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279-281.
- Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409-414.
- Powell CB, Alabaster A, Simmons S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011-2016. Obstet Gynecol. 2017;130(5):961-967.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014(173):1-8.
- Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71(8):969-980.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646-1653.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341-352.
2017 Update on contraception
According to the most recent data (2011–2013), 62% of women of childbearing age (15–44 years) use some method of contraception. Of these “contracepting” women, about 25% reported relying on permanent contraception, making it one of the most common methods of contraception used by women in the United States (FIGURE 1).1,2 Women either can choose to have a permanent contraception procedure performed immediately postpartum, which occurs after approximately 9% of all hospital deliveries in the United States,3 or at a time separate from pregnancy.
The most common methods of permanent contraception include partial salpingectomy at the time of cesarean delivery or within 24 hours after vaginal delivery and laparoscopic occlusive procedures at a time unrelated to the postpartum period.3 Hysteroscopic occlusion of the tubal ostia is a newer option, introduced in 2002; its worldwide use is concentrated in the United States, which accounts for 80% of sales based on revenue.4
Historically, for procedures remote from pregnancy, the laparoscopic approach evolved with less sophisticated laparoscopic equipment and limited visualization, which resulted in efficiency and safety being the primary goals of the procedure.5 Accordingly, rapid occlusive procedures were commonplace. However, advancement of laparoscopic technology related to insufflation systems, surgical equipment, and video capabilities did not change this practice.
Recent literature has suggested that complete fallopian tube removal provides additional benefits. With increasing knowledge about the origin of ovarian cancer, as well as increasing data to support the hypothesis that complete tubal excision results in increased ovarian cancer protection when compared with occlusive or partial salpingectomies, both the American College of Obstetricians and Gynecologists (ACOG)6 and the Society of Gynecologic Oncology (SGO)7 recommend discussing bilateral total salpingectomy with patients desiring permanent contraception. Although occlusive procedures decrease a woman’s lifetime risk of ovarian cancer by 24% to 34%,8,9 total salpingectomy likely reduces this risk by 49% to 65%.10,11
With this new evidence, McAlpine and colleagues initiated an educational campaign, targeting all ObGyns in British Columbia, which outlined the role of the fallopian tube in ovarian cancer and urged the consideration of total salpingectomy for permanent contraception in place of occlusive or partial salpingectomy procedures. They found that this one-time targeted education increased the use of total salpingectomy for permanent contraception from 0.5% at 2 years before the intervention to 33.3% by 2 years afterwards.12 On average, laparoscopic bilateral salpingectomy took 10 minutes longer to complete than occlusive procedures. Most importantly, they found no significant differences in complication rates, including hospital readmissions or blood transfusions.
Although our community can be applauded for the rapid uptake of concomitant bilateral salpingectomy at the time of benign hysterectomy,12,13 offering total salpingectomy for permanent contraception is far from common practice. Similarly, while multiple studies have been published to support the practice of opportunistic salpingectomy at the time of hysterectomy, little has been published about the use of bilateral salpingectomy for permanent contraception until this past year.
In this article, we review some of the first publications to focus specifically on the feasibility and safety profile of performing either immediate postpartum total salpingectomy or interval total salpingectomy in women desiring permanent contraception.
Family Planning experts are now strongly discouraging the use of terms like “sterilization,” “permanent sterilization,” and “tubal ligation” due to sterilization abuses that affected vulnerable and marginalized populations in the United States during the early-to mid-20th century.
In 1907, Indiana was the first state to enact a eugenics-based permanent sterilization law, which initiated an aggressive eugenics movement across the United States. This movement lasted for approximately 70 years and resulted in the sterilization of more than 60,000 women, men, and children against their will or without their knowledge. One of the major contributors to this movement was the state of California, which sterilized more than 20,000 women, men, and children.
They defined sterilization as a prophylactic measure that could simultaneously defend public health, preserve precious fiscal resources, and mitigate menace of the “unfit and feebleminded.” The US eugenics movement even inspired Hitler and the Nazi eugenics movement in Germany.
Because of these reproductive rights atrocities, a large counter movement to protect the rights of women, men, and children resulted in the creation of the Medicaid permanent sterilization consents that we still use today. Although some experts question whether the current Medicaid protective policy should be reevaluated, many are focused on the use of less offensive language when discussing the topic.
Current recommendations are to use the phrase “permanent contraception” or simply refer to the procedure name (salpingectomy, vasectomy, tubal occlusion, etc.) to move away from the connection to the eugenics movement.
Read about a total salpingectomy at delivery
Total salpingectomy: A viable option for permanent contraception after vaginal or at cesarean delivery
Shinar S, Blecher Y, Alpern S, et al. Total bilateral salpingectomy versus partial bilateral salpingectomy for permanent sterilization during cesarean delivery. Arch Gynecol Obstet. 2017;295(5):1185-1189.
Danis RB, Della Badia CR, Richard SD. Postpartum permanent sterilization: could bilateral salpingectomy replace bilateral tubal ligation? J Minim Invasive Gynecol. 2016;23(6):928-932.
Shinar and colleagues presented a retrospective case series that included women undergoing permanent contraception procedures during cesarean delivery at a single tertiary medical center. The authors evaluated outcomes before and after a global hospital policy changed the preferred permanent contraception procedure from partial to total salpingectomy.
Details of the Shinar technique and outcomes
Of the 149 women included, 99 underwent partial salpingectomy via the modified Pomeroy technique and 50 underwent total salpingectomy using an electrothermal bipolar tissue-sealing instrument (Ligasure). The authors found no difference in operative times and similar rates of complications. Composite adverse outcomes, defined as surgery duration greater than 45 minutes, hemoglobin decline greater than 1.2 g/dL, need for blood transfusion, prolonged hospitalization, ICU admission, or re-laparotomy, were comparable and were reported as 30.3% and 36.0% in the partial and total salpingectomy groups, respectively, (P = .57).One major complication occurred in the total salpingectomy cohort; postoperatively the patient had hemodynamic instability and was found to have hemoperitoneum requiring exploratory laparotomy. Significant bleeding from the bilateral mesosalpinges was discovered, presumably directly related to the total salpingectomy.
Related article:
Hysteroscopic tubal occlusion: How new product labeling can be a resource for patient counseling
Details of Danis et al
Intuitively, performing salpingectomy at the time of cesarean delivery does not seem as significant a change in practice as would performing salpingectomy through a small periumbilical incision after vaginal delivery. However, Danis and colleagues did just that; they published a retrospective case series of total salpingectomy performed within 24 hours after a vaginal delivery at an urban, academic institution. They included all women admitted for full-term vaginal deliveries who desired permanent contraception, with no exclusion criteria related to body mass index (BMI). The authors reported on 80 women, including 64 (80%) who underwent partial salpingectomy via the modified Pomeroy or Parkland technique and 16 (20%) who underwent total salpingectomy. Most women had a BMI of less than 30 kg/m2; less than 15% of the women in each group had a BMI greater than 40 kg/m2.
The technique for total salpingectomy involved a 2- to 3-cm vertical incision at the level of the umbilicus, elevation of the entire fallopian tube with 2 Babcock clamps, followed by the development of 2 to 3 windows with monopolar electrocautery in the mesosalpinx and subsequent suture ligation with polyglactin 910 (Vicryl, Ethicon).
Major findings included slightly longer operative time in the total salpingectomy compared with the partial salpingectomy group (a finding consistent with other studies12,14,15) and no difference in complication rates. The average (SD) surgical time in the partial salpingectomy group was 59 (16) minutes, compared with 71 (6) minutes in the total salpingectomy group (P = .003). The authors reported 4 (6.3%) complications in the partial salpingectomy group--ileus, excessive bleeding from mesosalpinx, and incisional site hematoma--and no complications in the total salpingectomy group (P = .58).
These 2 studies, although small retrospective case series, demonstrate the feasibility of performing total salpingectomies with minimal operative time differences when compared with more traditional partial salpingectomy procedures. The re-laparotomy complication noted in the Shinar series cannot be dismissed, as this is a major morbidity, but it also should not dictate the conversation.
Overall, the need for blood transfusion or unintended major surgery after permanent contraception procedures is rare. In the U.S. Collaborative Review of Sterilization study, none of the 282 women who had a permanent contraception procedure performed via laparotomy experienced either of these outcomes.16 Only 1 of the 9,475 women (0.01%) having a laparoscopic procedure in this study required blood transfusion and 14 (0.15%) required reoperation secondary to a procedure complication.17 The complication reported in the Shinar study reminds us that the technique for salpingectomy in the postpartum period, whether partial or total, should be considered carefully, being mindful of the anatomical changes that occur in pregnancy.
While larger studies should be performed to confirm these initial findings, these 2 articles provide the reassurance that many providers may need before beginning to offer total salpingectomy procedures in the immediate postpartum period.
When women present for permanent contraception counseling, we must remember that our patients' needs are often far too diverse and dynamic to allow a universal counseling technique. Every provider likely has a counseling style, with a structure and language that has been altered and changed through years of practice, patient experiences, and new scientific technologies and data. Unfortunately, provider biases and past coercive practices also influence contraceptive counseling.
Historically, some providers used formulas related to a woman's age and parity to decide if she could have a permanent contraception procedure, possibly based on fears of patient regret. Such practices are an embarrassment to the principles of patient autonomy and empowerment, which should serve as the foundation for any contraceptive conversation. Studies of regret after permanent contraception procedures are often misinterpreted; although younger women experience higher rates of regret, the absolute rate still favors performing the procedure.1,2 When comparing women aged 30 or younger to those older than 30 years at the time of procedure, the vast majority (about 80%) of those 30 and younger do not express regret.1 Less than 5% of women who express regret access a reversal procedure.2,3 Our job as providers is to educate and allow women to understand the options--and with permanent contraception that also means explaining the potential for regret; however, empowering women does not mean limiting an opportunity for the majority to potentially impact the minority.
Our contraceptive counseling philosophy follows the shared decision-making model. This model informs the patient, tailors the conversation toward her priorities, and maintains patient autonomy, while empowering the patient to take control of her reproductive health and future. When a patient expresses the desire for permanent contraception, we ensure she understands the permanence of the procedure and offer information about other Tier 1 contraceptive options, including long-acting reversible methods and vasectomy. We use the evidence-based World Health Organization counseling table4,5 to assist with the discussion and provide vasectomy referral and further information about specific intrauterine devices or the contraceptive implant based on the woman's interests.
For women who desire a female permanent contraception procedure, we also provide information tables comparing laparoscopic tubal occlusion procedures, laparoscopic bilateral salpingectomy, and hysteroscopic tubal occlusion. These tables review how each procedure is performed; risks and benefits, including failure rates over time; and ovarian cancer protection estimates. Our office also has devised tables to inform women seeking permanent contraception immediately after delivery and unrelated to pregnancy. Ultimately, the woman can choose what makes the most sense for her at that specific time in her life, and as providers we must support and uphold that decision.
References
- Hills SD, Marchbanks PA, Tylor LR, Peterson HB. Poststerilization regret: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 1999;93(6):889-895.
- Curtis KM, Mohllajee AP, Peterson HB. Regret following female sterilization at a young age: a systematic review. Contraception. 2006;73(2):205-210.
- Schmidt JE, Hillis SD, Marchbanks PA, Jeng G, Peterson HB. Requesting information about and obtaining reversal after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization. Fertil Steril. 2000;74(5):892-898.
- Steiner MJ, Trussell J, Mehta N, Condon S, Subramaniam S, Bourne D. Communicating contraceptive effectiveness: a randomized controlled trial to inform a World Health Organization family planning handbook. Am J Obstet Gynecol. 2006;195(1):85-91.
- Steiner MJ, Trussell J, Johnson S. Communicating contraceptive effectiveness: an updated counseling chart. Am J Obstet Gynecol. 2007;197(1):118.
Read about interval permanent contraception
Feasibility of interval laparoscopic permanent contraception via bilateral salpingectomy
Westberg J, Scott F, Creinin MD. Safety outcomes of female sterilization by salpingectomy and tubal occlusion. Contraception. 2017;95(5):505-508.
In this retrospective study, authors used billing data to identify women undergoing interval laparoscopic permanent contraception at a single academic medical center. They educated physicians and patients about the potential benefits to ovarian cancer risk with total salpingectomy (similar to the educational initiative done in British Columbia) and discussed the requirement for the extra incision and more time for the surgery. From 2013 to 2015 use of salpingectomy for permanent contraception changed from 45% of the procedures to 85%, a fairly dramatic trend.18 With these data, the authors compared outcomes between the women receiving tubal occlusive procedures and women receiving bilateral salpingectomy.
Related article:
Risk-reducing salpingectomy at benign hysterectomy: Have surgeons embraced this practice?
Details of surgical time and complications
Tubal occlusion procedures were performed through 2 abdominal ports, and device placement was at the discretion of the provider. Bilateral salpingectomies were performed through 3 abdominal port sites with an electrothermal bipolar tissue-sealing instrument. A total of 149 procedures were identified, 68 tubal occlusions (19% Falope rings, 32% bipolar cautery, and 47% Filshie clips) and 81 bilateral salpingectomies.
The surgical time average (SD) was 6 minutes longer for the salpingectomies (44 [13] minutes vs 38 [15] minutes; P = .018). As would be expected, more experienced residents had shorter surgical times when compared with less experienced residents for both procedures (FIGURE 2).15 Similar rates of both immediate and short-term surgical complications were noted. One immediate complication was reported in each group, both of which were secondary to anesthesia issues.
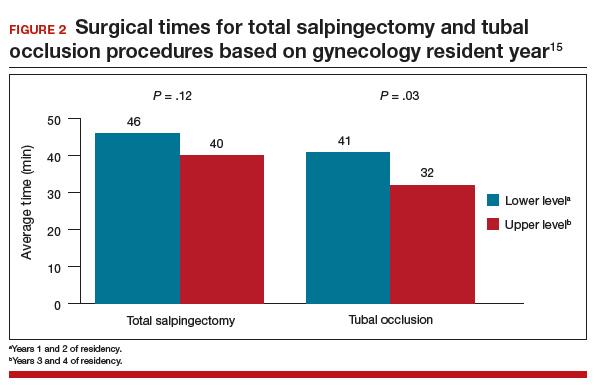
Interestingly, short-term complications were lower in the salpingectomy group (4.9%) versus the tubal occlusion group (14.7%), although this difference was barely not statistically significant (P = .051). These complications included 1 incisional site infection requiring oral antibiotics and 3 cases of increased pain in the salpingectomy group and 4 incisional site infections with 6 patients reporting increased pain in the tubal occlusion group.
This retrospective analysis provides further reassurance regarding the safety of offering bilateral salpingectomy to patients desiring permanent contraception. This study again consistently demonstrates that bilateral salpingectomy increases the operative time, but only minimally, which is unlikely clinically significant, especially when considering the potential benefits from total salpingectomy (increased ovarian cancer protection, higher contraceptive efficacy, decreased ectopic pregnancy rates, reduced risk of future surgeries for such tubal pathology as hydrosalpinx, etc). The study also shows that educational initiatives targeted at providers likely will increase acceptability as well as uptake of this practice for permanent contraception.
Read about tube removal and ovarian reserve
Does total removal of the tubes affect ovarian reserve?
Ganer Herman H, Gluck O, Keidar R, et al. Ovarian reserve following cesarean section with salpingectomy vs tubal ligation: a randomized trial. Am J Obstet Gynecol. 2017;doi: 10.1016/j.ajog.2017.04.028.
As acceptability of total salpingectomy for permanent contraception increases, one concern is that complete removal may alter blood supply to the ovary, resulting in decreased ovarian reserve and, subsequently, earlier menopause. Several studies have addressed the potential effect of salpingectomy on ovarian function when performed at the time of hysterectomy, most of which have noted no difference in anti-Müllerian hormone (AMH) levels and sonographic parameters following surgery.19 However, very little has been published to assess this same question when the salpingectomy is performed for the purpose of permanent contraception.
Ganer Herman and colleagues aimed to assess short-term ovarian reserve by measuring AMH levels preoperatively and 6 to 8 weeks postoperatively in patients undergoing partial or total salpingectomy at the time of elective cesarean delivery.
Related article:
Salpingectomy after vaginal hysterectomy: Technique, tips, and pearls
Details of the study
The study included women aged 18 to 45 who presented for elective cesarean delivery and who requested permanent contraception. Exclusion criteria included previous tubal surgery, emergent cesarean delivery, personal history of breast carcinoma, familial history of ovarian carcinoma, and BRCA carriage.
Women were randomly assigned at a 1:1 ratio to bilateral total salpingectomy or bilateral partial salpingectomy. A complete blood count and AMH level were drawn the night prior to surgery. Intraoperatively, after delivery and hysterotomy closure, partial salpingectomy, via the Parkland technique, or total salpingectomy, using a suture ligation technique, was performed.
Of the 46 women enrolled, follow-up was completed by 16 of 22 women (72%) in the total salpingectomy group and 18 of 24 women (75%) in the partial salpingectomy group. Patients in the total salpingectomy group were slightly older (mean age, 37 vs 34 years; P = .02), but otherwise all demographic and obstetric characteristics were comparable.
No differences were noted in preoperative and postoperative AMH levels between groups, with an average (SD) increase of 0.58 (0.98) ng/mL versus 0.39 (0.41) ng/mL in the total salpingectomy and partial salpingectomy groups, respectively (P = .45), consistent with known postpartum AMH level trends.
Other findings included an average 13-minute increase in operative time in the total salpingectomy cases, similar safety profile of the 2 methods as there were no postoperative complications during the study period, and no differences in postoperative hemoglobin levels.
This study was designed as a pilot trial to assess feasibility of enrollment, safety, and short-term ovarian reserve after salpingectomy for permanent contraception. Although the study is small and does not assess long-term effects, the findings are reassuring, especially in conjunction with other data.
A meta-analysis demonstrated no effect on ovarian reserve up to 18 months after salpingectomy based on AMH changes.19 A 5-year follow-up evaluation of 71 women undergoing total laparoscopic hysterectomy with bilateral salpingectomy also showed no effect on ovarian reserve as measured by multiple hormone levels including AMH and ultrasonographic findings.20 Thus, it is highly unlikely that a permanent contraception procedure that does not include removal of the uterus will have long-term ovarian reserve effects.
Additionally, consistent with other trials, Ganer Herman and colleagues demonstrate a slightly increased operative time and no increased complications. The surgical technique used in the study reflects the concern for postoperative bleeding from the mesosalpinx, and methods that ensure excellent hemostasis with suture ligation were used.
Conclusion
The studies reviewed in this article are some of the first to evaluate the feasibility and safety of opportunistic, or total, salpingectomy for permanent contraception since the ACOG and SGO recommendations were published. Just as our community has adopted the common practice of opportunistic salpingectomy at the time of hysterectomy, we should continue to advocate for a similar practice when discussing permanent contraception. Additionally, the Westberg study provides good evidence that educational initiatives can influence provider practices, which upholds the data published by McAlpine and colleagues in British Columbia. This information is promising and valuable.
Our universal goal as ObGyns is to provide the best reproductive health care possible based on the most recent evidence available. Continuing to advocate for opportunistic salpingectomy for permanent contraception purposes meets this goal and potentially provides significant noncontraceptive benefits.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015;86:1–14.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among U.S. women, 2009-2012. Obstet Gynecol. 2015;126(5):17–927.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1–6.
- Essure system for permanent birth control: Executive summary. Bayer Healthcare: Berlin, Germany; 2015:1–89. https://www.fda.gov/downloads/AdvisoryCommittees/UCM463460.pdf. Accessed July 19, 2017.
- Creinin MD, Zite N. Female tubal sterilization: the time has come to routinely consider removal. Obstet Gynecol. 2014;124(3):596–599.
- American College of Obstetrics and Gynecology Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279–281.
- Society of Gynecologic Oncology website. SGO clinical practice statement: salpingectomy for ovarian cancer. https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention/. Published November 2013. Accessed July 21, 2017.
- Cibula D, Widschwendter M, Majek O, Dusek L. Tubal ligation and the risk of ovarian cancer: review and meta-analysis. Hum Reprod Update. 2011;17(1): 55–67.
- Sieh W, Salvador S, McGuire V, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol. 2013;42(2): 579–589.
- Yoon SH, Kim SN, Shim SH, Kang SB, Lee SJ. Bilateral salpingectomy can reduce the risk of ovarian cancer in the general population: a meta-analysis. Eur J Cancer. 2016;55:38–46.
- Falconer H, Yin L, Grönberg H, Altman D. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2).
- McAlpine JN, Hanley GE, Woo MM, et al. Opportunistic salpingectomy: uptake, risks, and complications of a regional initiative for ovarian cancer prevention. Am J Obstet Gynecol. 2014;210(5):471e1–e11.
- Garcia C, Martin M, Tucker LY, et al. Experience with opportunistic salpingectomy in a large, community-based health system in the United States. Obstet Gynecol. 2016;128(2):277–283.
- Shinar S, Blecher Y, Alpern A, et al. Total bilateral salpingectomy versus partial bilateral salpingectomy for permanent sterilization during cesarean delivery. Arch Gynecol Obstet. 2017;295(5):1185–1189.
- Westberg J, Scott F, Creinin MD. Safety outcomes of female sterilization by salpingectomy and tubal occlusion. Contraception. 2017;95(5):505–508.
- Layde PM, Peterson HB, Dicker RC, DeStefano F, Rubin GL, Ory HW. Risk factors for complications of interval tubal sterilization by laparotomy. Obstet Gynecol. 1983;62(2):180–184.
- Jamieson DJ, Hillis SD, Duerr A, Marchbanks PA, Costello C, Peterson HB. Complications of interval laparoscopic tubal sterilization: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 2000;96(6):997–1002.
- Westberg JM, Scott F, Cansino C, Creinin MD. Recent trends in incidence of different permanent female sterilization methods. Obstet Gynecol. 2016;127(suppl):127S.
- Mohamed AA, Yosef AH, James C, Al-Hussaini TK, Bedaiwy MA, Amer SAKS. Ovarian reserve after salpingectomy: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2017;96(7):795–803.
- Venturella R, Lico D, Borelli M, et al. 3 to 5 years later: long-term effects of prophylactic bilateral salpingectomy on ovarian function. J Minim Invasive Gynecol. 2017;24(1):145–150.
According to the most recent data (2011–2013), 62% of women of childbearing age (15–44 years) use some method of contraception. Of these “contracepting” women, about 25% reported relying on permanent contraception, making it one of the most common methods of contraception used by women in the United States (FIGURE 1).1,2 Women either can choose to have a permanent contraception procedure performed immediately postpartum, which occurs after approximately 9% of all hospital deliveries in the United States,3 or at a time separate from pregnancy.

The most common methods of permanent contraception include partial salpingectomy at the time of cesarean delivery or within 24 hours after vaginal delivery and laparoscopic occlusive procedures at a time unrelated to the postpartum period.3 Hysteroscopic occlusion of the tubal ostia is a newer option, introduced in 2002; its worldwide use is concentrated in the United States, which accounts for 80% of sales based on revenue.4
Historically, for procedures remote from pregnancy, the laparoscopic approach evolved with less sophisticated laparoscopic equipment and limited visualization, which resulted in efficiency and safety being the primary goals of the procedure.5 Accordingly, rapid occlusive procedures were commonplace. However, advancement of laparoscopic technology related to insufflation systems, surgical equipment, and video capabilities did not change this practice.
Recent literature has suggested that complete fallopian tube removal provides additional benefits. With increasing knowledge about the origin of ovarian cancer, as well as increasing data to support the hypothesis that complete tubal excision results in increased ovarian cancer protection when compared with occlusive or partial salpingectomies, both the American College of Obstetricians and Gynecologists (ACOG)6 and the Society of Gynecologic Oncology (SGO)7 recommend discussing bilateral total salpingectomy with patients desiring permanent contraception. Although occlusive procedures decrease a woman’s lifetime risk of ovarian cancer by 24% to 34%,8,9 total salpingectomy likely reduces this risk by 49% to 65%.10,11
With this new evidence, McAlpine and colleagues initiated an educational campaign, targeting all ObGyns in British Columbia, which outlined the role of the fallopian tube in ovarian cancer and urged the consideration of total salpingectomy for permanent contraception in place of occlusive or partial salpingectomy procedures. They found that this one-time targeted education increased the use of total salpingectomy for permanent contraception from 0.5% at 2 years before the intervention to 33.3% by 2 years afterwards.12 On average, laparoscopic bilateral salpingectomy took 10 minutes longer to complete than occlusive procedures. Most importantly, they found no significant differences in complication rates, including hospital readmissions or blood transfusions.
Although our community can be applauded for the rapid uptake of concomitant bilateral salpingectomy at the time of benign hysterectomy,12,13 offering total salpingectomy for permanent contraception is far from common practice. Similarly, while multiple studies have been published to support the practice of opportunistic salpingectomy at the time of hysterectomy, little has been published about the use of bilateral salpingectomy for permanent contraception until this past year.
In this article, we review some of the first publications to focus specifically on the feasibility and safety profile of performing either immediate postpartum total salpingectomy or interval total salpingectomy in women desiring permanent contraception.
Family Planning experts are now strongly discouraging the use of terms like “sterilization,” “permanent sterilization,” and “tubal ligation” due to sterilization abuses that affected vulnerable and marginalized populations in the United States during the early-to mid-20th century.
In 1907, Indiana was the first state to enact a eugenics-based permanent sterilization law, which initiated an aggressive eugenics movement across the United States. This movement lasted for approximately 70 years and resulted in the sterilization of more than 60,000 women, men, and children against their will or without their knowledge. One of the major contributors to this movement was the state of California, which sterilized more than 20,000 women, men, and children.
They defined sterilization as a prophylactic measure that could simultaneously defend public health, preserve precious fiscal resources, and mitigate menace of the “unfit and feebleminded.” The US eugenics movement even inspired Hitler and the Nazi eugenics movement in Germany.
Because of these reproductive rights atrocities, a large counter movement to protect the rights of women, men, and children resulted in the creation of the Medicaid permanent sterilization consents that we still use today. Although some experts question whether the current Medicaid protective policy should be reevaluated, many are focused on the use of less offensive language when discussing the topic.
Current recommendations are to use the phrase “permanent contraception” or simply refer to the procedure name (salpingectomy, vasectomy, tubal occlusion, etc.) to move away from the connection to the eugenics movement.
Read about a total salpingectomy at delivery
Total salpingectomy: A viable option for permanent contraception after vaginal or at cesarean delivery
Shinar S, Blecher Y, Alpern S, et al. Total bilateral salpingectomy versus partial bilateral salpingectomy for permanent sterilization during cesarean delivery. Arch Gynecol Obstet. 2017;295(5):1185-1189.
Danis RB, Della Badia CR, Richard SD. Postpartum permanent sterilization: could bilateral salpingectomy replace bilateral tubal ligation? J Minim Invasive Gynecol. 2016;23(6):928-932.
Shinar and colleagues presented a retrospective case series that included women undergoing permanent contraception procedures during cesarean delivery at a single tertiary medical center. The authors evaluated outcomes before and after a global hospital policy changed the preferred permanent contraception procedure from partial to total salpingectomy.
Details of the Shinar technique and outcomes
Of the 149 women included, 99 underwent partial salpingectomy via the modified Pomeroy technique and 50 underwent total salpingectomy using an electrothermal bipolar tissue-sealing instrument (Ligasure). The authors found no difference in operative times and similar rates of complications. Composite adverse outcomes, defined as surgery duration greater than 45 minutes, hemoglobin decline greater than 1.2 g/dL, need for blood transfusion, prolonged hospitalization, ICU admission, or re-laparotomy, were comparable and were reported as 30.3% and 36.0% in the partial and total salpingectomy groups, respectively, (P = .57).One major complication occurred in the total salpingectomy cohort; postoperatively the patient had hemodynamic instability and was found to have hemoperitoneum requiring exploratory laparotomy. Significant bleeding from the bilateral mesosalpinges was discovered, presumably directly related to the total salpingectomy.
Related article:
Hysteroscopic tubal occlusion: How new product labeling can be a resource for patient counseling
Details of Danis et al
Intuitively, performing salpingectomy at the time of cesarean delivery does not seem as significant a change in practice as would performing salpingectomy through a small periumbilical incision after vaginal delivery. However, Danis and colleagues did just that; they published a retrospective case series of total salpingectomy performed within 24 hours after a vaginal delivery at an urban, academic institution. They included all women admitted for full-term vaginal deliveries who desired permanent contraception, with no exclusion criteria related to body mass index (BMI). The authors reported on 80 women, including 64 (80%) who underwent partial salpingectomy via the modified Pomeroy or Parkland technique and 16 (20%) who underwent total salpingectomy. Most women had a BMI of less than 30 kg/m2; less than 15% of the women in each group had a BMI greater than 40 kg/m2.
The technique for total salpingectomy involved a 2- to 3-cm vertical incision at the level of the umbilicus, elevation of the entire fallopian tube with 2 Babcock clamps, followed by the development of 2 to 3 windows with monopolar electrocautery in the mesosalpinx and subsequent suture ligation with polyglactin 910 (Vicryl, Ethicon).
Major findings included slightly longer operative time in the total salpingectomy compared with the partial salpingectomy group (a finding consistent with other studies12,14,15) and no difference in complication rates. The average (SD) surgical time in the partial salpingectomy group was 59 (16) minutes, compared with 71 (6) minutes in the total salpingectomy group (P = .003). The authors reported 4 (6.3%) complications in the partial salpingectomy group--ileus, excessive bleeding from mesosalpinx, and incisional site hematoma--and no complications in the total salpingectomy group (P = .58).
These 2 studies, although small retrospective case series, demonstrate the feasibility of performing total salpingectomies with minimal operative time differences when compared with more traditional partial salpingectomy procedures. The re-laparotomy complication noted in the Shinar series cannot be dismissed, as this is a major morbidity, but it also should not dictate the conversation.
Overall, the need for blood transfusion or unintended major surgery after permanent contraception procedures is rare. In the U.S. Collaborative Review of Sterilization study, none of the 282 women who had a permanent contraception procedure performed via laparotomy experienced either of these outcomes.16 Only 1 of the 9,475 women (0.01%) having a laparoscopic procedure in this study required blood transfusion and 14 (0.15%) required reoperation secondary to a procedure complication.17 The complication reported in the Shinar study reminds us that the technique for salpingectomy in the postpartum period, whether partial or total, should be considered carefully, being mindful of the anatomical changes that occur in pregnancy.
While larger studies should be performed to confirm these initial findings, these 2 articles provide the reassurance that many providers may need before beginning to offer total salpingectomy procedures in the immediate postpartum period.
When women present for permanent contraception counseling, we must remember that our patients' needs are often far too diverse and dynamic to allow a universal counseling technique. Every provider likely has a counseling style, with a structure and language that has been altered and changed through years of practice, patient experiences, and new scientific technologies and data. Unfortunately, provider biases and past coercive practices also influence contraceptive counseling.
Historically, some providers used formulas related to a woman's age and parity to decide if she could have a permanent contraception procedure, possibly based on fears of patient regret. Such practices are an embarrassment to the principles of patient autonomy and empowerment, which should serve as the foundation for any contraceptive conversation. Studies of regret after permanent contraception procedures are often misinterpreted; although younger women experience higher rates of regret, the absolute rate still favors performing the procedure.1,2 When comparing women aged 30 or younger to those older than 30 years at the time of procedure, the vast majority (about 80%) of those 30 and younger do not express regret.1 Less than 5% of women who express regret access a reversal procedure.2,3 Our job as providers is to educate and allow women to understand the options--and with permanent contraception that also means explaining the potential for regret; however, empowering women does not mean limiting an opportunity for the majority to potentially impact the minority.
Our contraceptive counseling philosophy follows the shared decision-making model. This model informs the patient, tailors the conversation toward her priorities, and maintains patient autonomy, while empowering the patient to take control of her reproductive health and future. When a patient expresses the desire for permanent contraception, we ensure she understands the permanence of the procedure and offer information about other Tier 1 contraceptive options, including long-acting reversible methods and vasectomy. We use the evidence-based World Health Organization counseling table4,5 to assist with the discussion and provide vasectomy referral and further information about specific intrauterine devices or the contraceptive implant based on the woman's interests.
For women who desire a female permanent contraception procedure, we also provide information tables comparing laparoscopic tubal occlusion procedures, laparoscopic bilateral salpingectomy, and hysteroscopic tubal occlusion. These tables review how each procedure is performed; risks and benefits, including failure rates over time; and ovarian cancer protection estimates. Our office also has devised tables to inform women seeking permanent contraception immediately after delivery and unrelated to pregnancy. Ultimately, the woman can choose what makes the most sense for her at that specific time in her life, and as providers we must support and uphold that decision.
References
- Hills SD, Marchbanks PA, Tylor LR, Peterson HB. Poststerilization regret: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 1999;93(6):889-895.
- Curtis KM, Mohllajee AP, Peterson HB. Regret following female sterilization at a young age: a systematic review. Contraception. 2006;73(2):205-210.
- Schmidt JE, Hillis SD, Marchbanks PA, Jeng G, Peterson HB. Requesting information about and obtaining reversal after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization. Fertil Steril. 2000;74(5):892-898.
- Steiner MJ, Trussell J, Mehta N, Condon S, Subramaniam S, Bourne D. Communicating contraceptive effectiveness: a randomized controlled trial to inform a World Health Organization family planning handbook. Am J Obstet Gynecol. 2006;195(1):85-91.
- Steiner MJ, Trussell J, Johnson S. Communicating contraceptive effectiveness: an updated counseling chart. Am J Obstet Gynecol. 2007;197(1):118.
Read about interval permanent contraception
Feasibility of interval laparoscopic permanent contraception via bilateral salpingectomy
Westberg J, Scott F, Creinin MD. Safety outcomes of female sterilization by salpingectomy and tubal occlusion. Contraception. 2017;95(5):505-508.
In this retrospective study, authors used billing data to identify women undergoing interval laparoscopic permanent contraception at a single academic medical center. They educated physicians and patients about the potential benefits to ovarian cancer risk with total salpingectomy (similar to the educational initiative done in British Columbia) and discussed the requirement for the extra incision and more time for the surgery. From 2013 to 2015 use of salpingectomy for permanent contraception changed from 45% of the procedures to 85%, a fairly dramatic trend.18 With these data, the authors compared outcomes between the women receiving tubal occlusive procedures and women receiving bilateral salpingectomy.
Related article:
Risk-reducing salpingectomy at benign hysterectomy: Have surgeons embraced this practice?
Details of surgical time and complications
Tubal occlusion procedures were performed through 2 abdominal ports, and device placement was at the discretion of the provider. Bilateral salpingectomies were performed through 3 abdominal port sites with an electrothermal bipolar tissue-sealing instrument. A total of 149 procedures were identified, 68 tubal occlusions (19% Falope rings, 32% bipolar cautery, and 47% Filshie clips) and 81 bilateral salpingectomies.
The surgical time average (SD) was 6 minutes longer for the salpingectomies (44 [13] minutes vs 38 [15] minutes; P = .018). As would be expected, more experienced residents had shorter surgical times when compared with less experienced residents for both procedures (FIGURE 2).15 Similar rates of both immediate and short-term surgical complications were noted. One immediate complication was reported in each group, both of which were secondary to anesthesia issues.

Interestingly, short-term complications were lower in the salpingectomy group (4.9%) versus the tubal occlusion group (14.7%), although this difference was barely not statistically significant (P = .051). These complications included 1 incisional site infection requiring oral antibiotics and 3 cases of increased pain in the salpingectomy group and 4 incisional site infections with 6 patients reporting increased pain in the tubal occlusion group.
This retrospective analysis provides further reassurance regarding the safety of offering bilateral salpingectomy to patients desiring permanent contraception. This study again consistently demonstrates that bilateral salpingectomy increases the operative time, but only minimally, which is unlikely clinically significant, especially when considering the potential benefits from total salpingectomy (increased ovarian cancer protection, higher contraceptive efficacy, decreased ectopic pregnancy rates, reduced risk of future surgeries for such tubal pathology as hydrosalpinx, etc). The study also shows that educational initiatives targeted at providers likely will increase acceptability as well as uptake of this practice for permanent contraception.
Read about tube removal and ovarian reserve
Does total removal of the tubes affect ovarian reserve?
Ganer Herman H, Gluck O, Keidar R, et al. Ovarian reserve following cesarean section with salpingectomy vs tubal ligation: a randomized trial. Am J Obstet Gynecol. 2017;doi: 10.1016/j.ajog.2017.04.028.
As acceptability of total salpingectomy for permanent contraception increases, one concern is that complete removal may alter blood supply to the ovary, resulting in decreased ovarian reserve and, subsequently, earlier menopause. Several studies have addressed the potential effect of salpingectomy on ovarian function when performed at the time of hysterectomy, most of which have noted no difference in anti-Müllerian hormone (AMH) levels and sonographic parameters following surgery.19 However, very little has been published to assess this same question when the salpingectomy is performed for the purpose of permanent contraception.
Ganer Herman and colleagues aimed to assess short-term ovarian reserve by measuring AMH levels preoperatively and 6 to 8 weeks postoperatively in patients undergoing partial or total salpingectomy at the time of elective cesarean delivery.
Related article:
Salpingectomy after vaginal hysterectomy: Technique, tips, and pearls
Details of the study
The study included women aged 18 to 45 who presented for elective cesarean delivery and who requested permanent contraception. Exclusion criteria included previous tubal surgery, emergent cesarean delivery, personal history of breast carcinoma, familial history of ovarian carcinoma, and BRCA carriage.
Women were randomly assigned at a 1:1 ratio to bilateral total salpingectomy or bilateral partial salpingectomy. A complete blood count and AMH level were drawn the night prior to surgery. Intraoperatively, after delivery and hysterotomy closure, partial salpingectomy, via the Parkland technique, or total salpingectomy, using a suture ligation technique, was performed.
Of the 46 women enrolled, follow-up was completed by 16 of 22 women (72%) in the total salpingectomy group and 18 of 24 women (75%) in the partial salpingectomy group. Patients in the total salpingectomy group were slightly older (mean age, 37 vs 34 years; P = .02), but otherwise all demographic and obstetric characteristics were comparable.
No differences were noted in preoperative and postoperative AMH levels between groups, with an average (SD) increase of 0.58 (0.98) ng/mL versus 0.39 (0.41) ng/mL in the total salpingectomy and partial salpingectomy groups, respectively (P = .45), consistent with known postpartum AMH level trends.
Other findings included an average 13-minute increase in operative time in the total salpingectomy cases, similar safety profile of the 2 methods as there were no postoperative complications during the study period, and no differences in postoperative hemoglobin levels.
This study was designed as a pilot trial to assess feasibility of enrollment, safety, and short-term ovarian reserve after salpingectomy for permanent contraception. Although the study is small and does not assess long-term effects, the findings are reassuring, especially in conjunction with other data.
A meta-analysis demonstrated no effect on ovarian reserve up to 18 months after salpingectomy based on AMH changes.19 A 5-year follow-up evaluation of 71 women undergoing total laparoscopic hysterectomy with bilateral salpingectomy also showed no effect on ovarian reserve as measured by multiple hormone levels including AMH and ultrasonographic findings.20 Thus, it is highly unlikely that a permanent contraception procedure that does not include removal of the uterus will have long-term ovarian reserve effects.
Additionally, consistent with other trials, Ganer Herman and colleagues demonstrate a slightly increased operative time and no increased complications. The surgical technique used in the study reflects the concern for postoperative bleeding from the mesosalpinx, and methods that ensure excellent hemostasis with suture ligation were used.
Conclusion
The studies reviewed in this article are some of the first to evaluate the feasibility and safety of opportunistic, or total, salpingectomy for permanent contraception since the ACOG and SGO recommendations were published. Just as our community has adopted the common practice of opportunistic salpingectomy at the time of hysterectomy, we should continue to advocate for a similar practice when discussing permanent contraception. Additionally, the Westberg study provides good evidence that educational initiatives can influence provider practices, which upholds the data published by McAlpine and colleagues in British Columbia. This information is promising and valuable.
Our universal goal as ObGyns is to provide the best reproductive health care possible based on the most recent evidence available. Continuing to advocate for opportunistic salpingectomy for permanent contraception purposes meets this goal and potentially provides significant noncontraceptive benefits.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
According to the most recent data (2011–2013), 62% of women of childbearing age (15–44 years) use some method of contraception. Of these “contracepting” women, about 25% reported relying on permanent contraception, making it one of the most common methods of contraception used by women in the United States (FIGURE 1).1,2 Women either can choose to have a permanent contraception procedure performed immediately postpartum, which occurs after approximately 9% of all hospital deliveries in the United States,3 or at a time separate from pregnancy.

The most common methods of permanent contraception include partial salpingectomy at the time of cesarean delivery or within 24 hours after vaginal delivery and laparoscopic occlusive procedures at a time unrelated to the postpartum period.3 Hysteroscopic occlusion of the tubal ostia is a newer option, introduced in 2002; its worldwide use is concentrated in the United States, which accounts for 80% of sales based on revenue.4
Historically, for procedures remote from pregnancy, the laparoscopic approach evolved with less sophisticated laparoscopic equipment and limited visualization, which resulted in efficiency and safety being the primary goals of the procedure.5 Accordingly, rapid occlusive procedures were commonplace. However, advancement of laparoscopic technology related to insufflation systems, surgical equipment, and video capabilities did not change this practice.
Recent literature has suggested that complete fallopian tube removal provides additional benefits. With increasing knowledge about the origin of ovarian cancer, as well as increasing data to support the hypothesis that complete tubal excision results in increased ovarian cancer protection when compared with occlusive or partial salpingectomies, both the American College of Obstetricians and Gynecologists (ACOG)6 and the Society of Gynecologic Oncology (SGO)7 recommend discussing bilateral total salpingectomy with patients desiring permanent contraception. Although occlusive procedures decrease a woman’s lifetime risk of ovarian cancer by 24% to 34%,8,9 total salpingectomy likely reduces this risk by 49% to 65%.10,11
With this new evidence, McAlpine and colleagues initiated an educational campaign, targeting all ObGyns in British Columbia, which outlined the role of the fallopian tube in ovarian cancer and urged the consideration of total salpingectomy for permanent contraception in place of occlusive or partial salpingectomy procedures. They found that this one-time targeted education increased the use of total salpingectomy for permanent contraception from 0.5% at 2 years before the intervention to 33.3% by 2 years afterwards.12 On average, laparoscopic bilateral salpingectomy took 10 minutes longer to complete than occlusive procedures. Most importantly, they found no significant differences in complication rates, including hospital readmissions or blood transfusions.
Although our community can be applauded for the rapid uptake of concomitant bilateral salpingectomy at the time of benign hysterectomy,12,13 offering total salpingectomy for permanent contraception is far from common practice. Similarly, while multiple studies have been published to support the practice of opportunistic salpingectomy at the time of hysterectomy, little has been published about the use of bilateral salpingectomy for permanent contraception until this past year.
In this article, we review some of the first publications to focus specifically on the feasibility and safety profile of performing either immediate postpartum total salpingectomy or interval total salpingectomy in women desiring permanent contraception.
Family Planning experts are now strongly discouraging the use of terms like “sterilization,” “permanent sterilization,” and “tubal ligation” due to sterilization abuses that affected vulnerable and marginalized populations in the United States during the early-to mid-20th century.
In 1907, Indiana was the first state to enact a eugenics-based permanent sterilization law, which initiated an aggressive eugenics movement across the United States. This movement lasted for approximately 70 years and resulted in the sterilization of more than 60,000 women, men, and children against their will or without their knowledge. One of the major contributors to this movement was the state of California, which sterilized more than 20,000 women, men, and children.
They defined sterilization as a prophylactic measure that could simultaneously defend public health, preserve precious fiscal resources, and mitigate menace of the “unfit and feebleminded.” The US eugenics movement even inspired Hitler and the Nazi eugenics movement in Germany.
Because of these reproductive rights atrocities, a large counter movement to protect the rights of women, men, and children resulted in the creation of the Medicaid permanent sterilization consents that we still use today. Although some experts question whether the current Medicaid protective policy should be reevaluated, many are focused on the use of less offensive language when discussing the topic.
Current recommendations are to use the phrase “permanent contraception” or simply refer to the procedure name (salpingectomy, vasectomy, tubal occlusion, etc.) to move away from the connection to the eugenics movement.
Read about a total salpingectomy at delivery
Total salpingectomy: A viable option for permanent contraception after vaginal or at cesarean delivery
Shinar S, Blecher Y, Alpern S, et al. Total bilateral salpingectomy versus partial bilateral salpingectomy for permanent sterilization during cesarean delivery. Arch Gynecol Obstet. 2017;295(5):1185-1189.
Danis RB, Della Badia CR, Richard SD. Postpartum permanent sterilization: could bilateral salpingectomy replace bilateral tubal ligation? J Minim Invasive Gynecol. 2016;23(6):928-932.
Shinar and colleagues presented a retrospective case series that included women undergoing permanent contraception procedures during cesarean delivery at a single tertiary medical center. The authors evaluated outcomes before and after a global hospital policy changed the preferred permanent contraception procedure from partial to total salpingectomy.
Details of the Shinar technique and outcomes
Of the 149 women included, 99 underwent partial salpingectomy via the modified Pomeroy technique and 50 underwent total salpingectomy using an electrothermal bipolar tissue-sealing instrument (Ligasure). The authors found no difference in operative times and similar rates of complications. Composite adverse outcomes, defined as surgery duration greater than 45 minutes, hemoglobin decline greater than 1.2 g/dL, need for blood transfusion, prolonged hospitalization, ICU admission, or re-laparotomy, were comparable and were reported as 30.3% and 36.0% in the partial and total salpingectomy groups, respectively, (P = .57).One major complication occurred in the total salpingectomy cohort; postoperatively the patient had hemodynamic instability and was found to have hemoperitoneum requiring exploratory laparotomy. Significant bleeding from the bilateral mesosalpinges was discovered, presumably directly related to the total salpingectomy.
Related article:
Hysteroscopic tubal occlusion: How new product labeling can be a resource for patient counseling
Details of Danis et al
Intuitively, performing salpingectomy at the time of cesarean delivery does not seem as significant a change in practice as would performing salpingectomy through a small periumbilical incision after vaginal delivery. However, Danis and colleagues did just that; they published a retrospective case series of total salpingectomy performed within 24 hours after a vaginal delivery at an urban, academic institution. They included all women admitted for full-term vaginal deliveries who desired permanent contraception, with no exclusion criteria related to body mass index (BMI). The authors reported on 80 women, including 64 (80%) who underwent partial salpingectomy via the modified Pomeroy or Parkland technique and 16 (20%) who underwent total salpingectomy. Most women had a BMI of less than 30 kg/m2; less than 15% of the women in each group had a BMI greater than 40 kg/m2.
The technique for total salpingectomy involved a 2- to 3-cm vertical incision at the level of the umbilicus, elevation of the entire fallopian tube with 2 Babcock clamps, followed by the development of 2 to 3 windows with monopolar electrocautery in the mesosalpinx and subsequent suture ligation with polyglactin 910 (Vicryl, Ethicon).
Major findings included slightly longer operative time in the total salpingectomy compared with the partial salpingectomy group (a finding consistent with other studies12,14,15) and no difference in complication rates. The average (SD) surgical time in the partial salpingectomy group was 59 (16) minutes, compared with 71 (6) minutes in the total salpingectomy group (P = .003). The authors reported 4 (6.3%) complications in the partial salpingectomy group--ileus, excessive bleeding from mesosalpinx, and incisional site hematoma--and no complications in the total salpingectomy group (P = .58).
These 2 studies, although small retrospective case series, demonstrate the feasibility of performing total salpingectomies with minimal operative time differences when compared with more traditional partial salpingectomy procedures. The re-laparotomy complication noted in the Shinar series cannot be dismissed, as this is a major morbidity, but it also should not dictate the conversation.
Overall, the need for blood transfusion or unintended major surgery after permanent contraception procedures is rare. In the U.S. Collaborative Review of Sterilization study, none of the 282 women who had a permanent contraception procedure performed via laparotomy experienced either of these outcomes.16 Only 1 of the 9,475 women (0.01%) having a laparoscopic procedure in this study required blood transfusion and 14 (0.15%) required reoperation secondary to a procedure complication.17 The complication reported in the Shinar study reminds us that the technique for salpingectomy in the postpartum period, whether partial or total, should be considered carefully, being mindful of the anatomical changes that occur in pregnancy.
While larger studies should be performed to confirm these initial findings, these 2 articles provide the reassurance that many providers may need before beginning to offer total salpingectomy procedures in the immediate postpartum period.
When women present for permanent contraception counseling, we must remember that our patients' needs are often far too diverse and dynamic to allow a universal counseling technique. Every provider likely has a counseling style, with a structure and language that has been altered and changed through years of practice, patient experiences, and new scientific technologies and data. Unfortunately, provider biases and past coercive practices also influence contraceptive counseling.
Historically, some providers used formulas related to a woman's age and parity to decide if she could have a permanent contraception procedure, possibly based on fears of patient regret. Such practices are an embarrassment to the principles of patient autonomy and empowerment, which should serve as the foundation for any contraceptive conversation. Studies of regret after permanent contraception procedures are often misinterpreted; although younger women experience higher rates of regret, the absolute rate still favors performing the procedure.1,2 When comparing women aged 30 or younger to those older than 30 years at the time of procedure, the vast majority (about 80%) of those 30 and younger do not express regret.1 Less than 5% of women who express regret access a reversal procedure.2,3 Our job as providers is to educate and allow women to understand the options--and with permanent contraception that also means explaining the potential for regret; however, empowering women does not mean limiting an opportunity for the majority to potentially impact the minority.
Our contraceptive counseling philosophy follows the shared decision-making model. This model informs the patient, tailors the conversation toward her priorities, and maintains patient autonomy, while empowering the patient to take control of her reproductive health and future. When a patient expresses the desire for permanent contraception, we ensure she understands the permanence of the procedure and offer information about other Tier 1 contraceptive options, including long-acting reversible methods and vasectomy. We use the evidence-based World Health Organization counseling table4,5 to assist with the discussion and provide vasectomy referral and further information about specific intrauterine devices or the contraceptive implant based on the woman's interests.
For women who desire a female permanent contraception procedure, we also provide information tables comparing laparoscopic tubal occlusion procedures, laparoscopic bilateral salpingectomy, and hysteroscopic tubal occlusion. These tables review how each procedure is performed; risks and benefits, including failure rates over time; and ovarian cancer protection estimates. Our office also has devised tables to inform women seeking permanent contraception immediately after delivery and unrelated to pregnancy. Ultimately, the woman can choose what makes the most sense for her at that specific time in her life, and as providers we must support and uphold that decision.
References
- Hills SD, Marchbanks PA, Tylor LR, Peterson HB. Poststerilization regret: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 1999;93(6):889-895.
- Curtis KM, Mohllajee AP, Peterson HB. Regret following female sterilization at a young age: a systematic review. Contraception. 2006;73(2):205-210.
- Schmidt JE, Hillis SD, Marchbanks PA, Jeng G, Peterson HB. Requesting information about and obtaining reversal after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization. Fertil Steril. 2000;74(5):892-898.
- Steiner MJ, Trussell J, Mehta N, Condon S, Subramaniam S, Bourne D. Communicating contraceptive effectiveness: a randomized controlled trial to inform a World Health Organization family planning handbook. Am J Obstet Gynecol. 2006;195(1):85-91.
- Steiner MJ, Trussell J, Johnson S. Communicating contraceptive effectiveness: an updated counseling chart. Am J Obstet Gynecol. 2007;197(1):118.
Read about interval permanent contraception
Feasibility of interval laparoscopic permanent contraception via bilateral salpingectomy
Westberg J, Scott F, Creinin MD. Safety outcomes of female sterilization by salpingectomy and tubal occlusion. Contraception. 2017;95(5):505-508.
In this retrospective study, authors used billing data to identify women undergoing interval laparoscopic permanent contraception at a single academic medical center. They educated physicians and patients about the potential benefits to ovarian cancer risk with total salpingectomy (similar to the educational initiative done in British Columbia) and discussed the requirement for the extra incision and more time for the surgery. From 2013 to 2015 use of salpingectomy for permanent contraception changed from 45% of the procedures to 85%, a fairly dramatic trend.18 With these data, the authors compared outcomes between the women receiving tubal occlusive procedures and women receiving bilateral salpingectomy.
Related article:
Risk-reducing salpingectomy at benign hysterectomy: Have surgeons embraced this practice?
Details of surgical time and complications
Tubal occlusion procedures were performed through 2 abdominal ports, and device placement was at the discretion of the provider. Bilateral salpingectomies were performed through 3 abdominal port sites with an electrothermal bipolar tissue-sealing instrument. A total of 149 procedures were identified, 68 tubal occlusions (19% Falope rings, 32% bipolar cautery, and 47% Filshie clips) and 81 bilateral salpingectomies.
The surgical time average (SD) was 6 minutes longer for the salpingectomies (44 [13] minutes vs 38 [15] minutes; P = .018). As would be expected, more experienced residents had shorter surgical times when compared with less experienced residents for both procedures (FIGURE 2).15 Similar rates of both immediate and short-term surgical complications were noted. One immediate complication was reported in each group, both of which were secondary to anesthesia issues.

Interestingly, short-term complications were lower in the salpingectomy group (4.9%) versus the tubal occlusion group (14.7%), although this difference was barely not statistically significant (P = .051). These complications included 1 incisional site infection requiring oral antibiotics and 3 cases of increased pain in the salpingectomy group and 4 incisional site infections with 6 patients reporting increased pain in the tubal occlusion group.
This retrospective analysis provides further reassurance regarding the safety of offering bilateral salpingectomy to patients desiring permanent contraception. This study again consistently demonstrates that bilateral salpingectomy increases the operative time, but only minimally, which is unlikely clinically significant, especially when considering the potential benefits from total salpingectomy (increased ovarian cancer protection, higher contraceptive efficacy, decreased ectopic pregnancy rates, reduced risk of future surgeries for such tubal pathology as hydrosalpinx, etc). The study also shows that educational initiatives targeted at providers likely will increase acceptability as well as uptake of this practice for permanent contraception.
Read about tube removal and ovarian reserve
Does total removal of the tubes affect ovarian reserve?
Ganer Herman H, Gluck O, Keidar R, et al. Ovarian reserve following cesarean section with salpingectomy vs tubal ligation: a randomized trial. Am J Obstet Gynecol. 2017;doi: 10.1016/j.ajog.2017.04.028.
As acceptability of total salpingectomy for permanent contraception increases, one concern is that complete removal may alter blood supply to the ovary, resulting in decreased ovarian reserve and, subsequently, earlier menopause. Several studies have addressed the potential effect of salpingectomy on ovarian function when performed at the time of hysterectomy, most of which have noted no difference in anti-Müllerian hormone (AMH) levels and sonographic parameters following surgery.19 However, very little has been published to assess this same question when the salpingectomy is performed for the purpose of permanent contraception.
Ganer Herman and colleagues aimed to assess short-term ovarian reserve by measuring AMH levels preoperatively and 6 to 8 weeks postoperatively in patients undergoing partial or total salpingectomy at the time of elective cesarean delivery.
Related article:
Salpingectomy after vaginal hysterectomy: Technique, tips, and pearls
Details of the study
The study included women aged 18 to 45 who presented for elective cesarean delivery and who requested permanent contraception. Exclusion criteria included previous tubal surgery, emergent cesarean delivery, personal history of breast carcinoma, familial history of ovarian carcinoma, and BRCA carriage.
Women were randomly assigned at a 1:1 ratio to bilateral total salpingectomy or bilateral partial salpingectomy. A complete blood count and AMH level were drawn the night prior to surgery. Intraoperatively, after delivery and hysterotomy closure, partial salpingectomy, via the Parkland technique, or total salpingectomy, using a suture ligation technique, was performed.
Of the 46 women enrolled, follow-up was completed by 16 of 22 women (72%) in the total salpingectomy group and 18 of 24 women (75%) in the partial salpingectomy group. Patients in the total salpingectomy group were slightly older (mean age, 37 vs 34 years; P = .02), but otherwise all demographic and obstetric characteristics were comparable.
No differences were noted in preoperative and postoperative AMH levels between groups, with an average (SD) increase of 0.58 (0.98) ng/mL versus 0.39 (0.41) ng/mL in the total salpingectomy and partial salpingectomy groups, respectively (P = .45), consistent with known postpartum AMH level trends.
Other findings included an average 13-minute increase in operative time in the total salpingectomy cases, similar safety profile of the 2 methods as there were no postoperative complications during the study period, and no differences in postoperative hemoglobin levels.
This study was designed as a pilot trial to assess feasibility of enrollment, safety, and short-term ovarian reserve after salpingectomy for permanent contraception. Although the study is small and does not assess long-term effects, the findings are reassuring, especially in conjunction with other data.
A meta-analysis demonstrated no effect on ovarian reserve up to 18 months after salpingectomy based on AMH changes.19 A 5-year follow-up evaluation of 71 women undergoing total laparoscopic hysterectomy with bilateral salpingectomy also showed no effect on ovarian reserve as measured by multiple hormone levels including AMH and ultrasonographic findings.20 Thus, it is highly unlikely that a permanent contraception procedure that does not include removal of the uterus will have long-term ovarian reserve effects.
Additionally, consistent with other trials, Ganer Herman and colleagues demonstrate a slightly increased operative time and no increased complications. The surgical technique used in the study reflects the concern for postoperative bleeding from the mesosalpinx, and methods that ensure excellent hemostasis with suture ligation were used.
Conclusion
The studies reviewed in this article are some of the first to evaluate the feasibility and safety of opportunistic, or total, salpingectomy for permanent contraception since the ACOG and SGO recommendations were published. Just as our community has adopted the common practice of opportunistic salpingectomy at the time of hysterectomy, we should continue to advocate for a similar practice when discussing permanent contraception. Additionally, the Westberg study provides good evidence that educational initiatives can influence provider practices, which upholds the data published by McAlpine and colleagues in British Columbia. This information is promising and valuable.
Our universal goal as ObGyns is to provide the best reproductive health care possible based on the most recent evidence available. Continuing to advocate for opportunistic salpingectomy for permanent contraception purposes meets this goal and potentially provides significant noncontraceptive benefits.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015;86:1–14.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among U.S. women, 2009-2012. Obstet Gynecol. 2015;126(5):17–927.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1–6.
- Essure system for permanent birth control: Executive summary. Bayer Healthcare: Berlin, Germany; 2015:1–89. https://www.fda.gov/downloads/AdvisoryCommittees/UCM463460.pdf. Accessed July 19, 2017.
- Creinin MD, Zite N. Female tubal sterilization: the time has come to routinely consider removal. Obstet Gynecol. 2014;124(3):596–599.
- American College of Obstetrics and Gynecology Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279–281.
- Society of Gynecologic Oncology website. SGO clinical practice statement: salpingectomy for ovarian cancer. https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention/. Published November 2013. Accessed July 21, 2017.
- Cibula D, Widschwendter M, Majek O, Dusek L. Tubal ligation and the risk of ovarian cancer: review and meta-analysis. Hum Reprod Update. 2011;17(1): 55–67.
- Sieh W, Salvador S, McGuire V, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol. 2013;42(2): 579–589.
- Yoon SH, Kim SN, Shim SH, Kang SB, Lee SJ. Bilateral salpingectomy can reduce the risk of ovarian cancer in the general population: a meta-analysis. Eur J Cancer. 2016;55:38–46.
- Falconer H, Yin L, Grönberg H, Altman D. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2).
- McAlpine JN, Hanley GE, Woo MM, et al. Opportunistic salpingectomy: uptake, risks, and complications of a regional initiative for ovarian cancer prevention. Am J Obstet Gynecol. 2014;210(5):471e1–e11.
- Garcia C, Martin M, Tucker LY, et al. Experience with opportunistic salpingectomy in a large, community-based health system in the United States. Obstet Gynecol. 2016;128(2):277–283.
- Shinar S, Blecher Y, Alpern A, et al. Total bilateral salpingectomy versus partial bilateral salpingectomy for permanent sterilization during cesarean delivery. Arch Gynecol Obstet. 2017;295(5):1185–1189.
- Westberg J, Scott F, Creinin MD. Safety outcomes of female sterilization by salpingectomy and tubal occlusion. Contraception. 2017;95(5):505–508.
- Layde PM, Peterson HB, Dicker RC, DeStefano F, Rubin GL, Ory HW. Risk factors for complications of interval tubal sterilization by laparotomy. Obstet Gynecol. 1983;62(2):180–184.
- Jamieson DJ, Hillis SD, Duerr A, Marchbanks PA, Costello C, Peterson HB. Complications of interval laparoscopic tubal sterilization: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 2000;96(6):997–1002.
- Westberg JM, Scott F, Cansino C, Creinin MD. Recent trends in incidence of different permanent female sterilization methods. Obstet Gynecol. 2016;127(suppl):127S.
- Mohamed AA, Yosef AH, James C, Al-Hussaini TK, Bedaiwy MA, Amer SAKS. Ovarian reserve after salpingectomy: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2017;96(7):795–803.
- Venturella R, Lico D, Borelli M, et al. 3 to 5 years later: long-term effects of prophylactic bilateral salpingectomy on ovarian function. J Minim Invasive Gynecol. 2017;24(1):145–150.
- Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015;86:1–14.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among U.S. women, 2009-2012. Obstet Gynecol. 2015;126(5):17–927.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94(1):1–6.
- Essure system for permanent birth control: Executive summary. Bayer Healthcare: Berlin, Germany; 2015:1–89. https://www.fda.gov/downloads/AdvisoryCommittees/UCM463460.pdf. Accessed July 19, 2017.
- Creinin MD, Zite N. Female tubal sterilization: the time has come to routinely consider removal. Obstet Gynecol. 2014;124(3):596–599.
- American College of Obstetrics and Gynecology Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279–281.
- Society of Gynecologic Oncology website. SGO clinical practice statement: salpingectomy for ovarian cancer. https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention/. Published November 2013. Accessed July 21, 2017.
- Cibula D, Widschwendter M, Majek O, Dusek L. Tubal ligation and the risk of ovarian cancer: review and meta-analysis. Hum Reprod Update. 2011;17(1): 55–67.
- Sieh W, Salvador S, McGuire V, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol. 2013;42(2): 579–589.
- Yoon SH, Kim SN, Shim SH, Kang SB, Lee SJ. Bilateral salpingectomy can reduce the risk of ovarian cancer in the general population: a meta-analysis. Eur J Cancer. 2016;55:38–46.
- Falconer H, Yin L, Grönberg H, Altman D. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2).
- McAlpine JN, Hanley GE, Woo MM, et al. Opportunistic salpingectomy: uptake, risks, and complications of a regional initiative for ovarian cancer prevention. Am J Obstet Gynecol. 2014;210(5):471e1–e11.
- Garcia C, Martin M, Tucker LY, et al. Experience with opportunistic salpingectomy in a large, community-based health system in the United States. Obstet Gynecol. 2016;128(2):277–283.
- Shinar S, Blecher Y, Alpern A, et al. Total bilateral salpingectomy versus partial bilateral salpingectomy for permanent sterilization during cesarean delivery. Arch Gynecol Obstet. 2017;295(5):1185–1189.
- Westberg J, Scott F, Creinin MD. Safety outcomes of female sterilization by salpingectomy and tubal occlusion. Contraception. 2017;95(5):505–508.
- Layde PM, Peterson HB, Dicker RC, DeStefano F, Rubin GL, Ory HW. Risk factors for complications of interval tubal sterilization by laparotomy. Obstet Gynecol. 1983;62(2):180–184.
- Jamieson DJ, Hillis SD, Duerr A, Marchbanks PA, Costello C, Peterson HB. Complications of interval laparoscopic tubal sterilization: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 2000;96(6):997–1002.
- Westberg JM, Scott F, Cansino C, Creinin MD. Recent trends in incidence of different permanent female sterilization methods. Obstet Gynecol. 2016;127(suppl):127S.
- Mohamed AA, Yosef AH, James C, Al-Hussaini TK, Bedaiwy MA, Amer SAKS. Ovarian reserve after salpingectomy: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2017;96(7):795–803.
- Venturella R, Lico D, Borelli M, et al. 3 to 5 years later: long-term effects of prophylactic bilateral salpingectomy on ovarian function. J Minim Invasive Gynecol. 2017;24(1):145–150.
Liletta gets a new inserter: Steps for successful placement
A new single-handed inserter (SHI) for placement of Liletta has been introduced and is currently available (FIGURE 1). Liletta is a levonorgestrel 52-mg intrauterine system (IUS) that is currently approved for 3 years of use as a contraceptive in the United States. The same product in Europe, known as Levosert, is approved for both contraception and the treatment of heavy menstrual bleeding. Liletta, as a branded contraceptive, is being studied through a large Phase 3 clinical trial called ACCESS IUS, which will continue to evaluate the contraceptive efficacy of Liletta for at least 7 years of use.1 A recent publication from the study showed that the levonorgestrel release rates from Liletta are almost identical to the rates reported for the other levonorgestrel 52-mg IUS on the market (Mirena) through 5 years and supports the continued study of the IUS for at least 7 years.2
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
The initial marketing and distribution of Liletta used a two-handed inserter that allowed for successful placement but required multiple steps. The SHI will make loading and placement of Liletta easier for all clinicians. Features of the new SHI include:
- A double-slider mechanism in which the first slider moves into the second slider during IUS placement, providing a tactile sense for the user during the insertion process
- The ability to reload the inserter if needed before placement
- A firm but bendable tube (that is 2 cm longer than other IUS inserters on the market). This length can be helpful for obese patients or postprocedure placements.
- Depth markings to 12 cm on both sides of the insertion tube.
Related article:
2016 Update on contraception
Liletta is an important and unique product in the US market. For most public sector providers and clinics, Liletta costs only $50, significantly less than other long-acting reversible contraceptives. The price of the IUS is only one aspect of its overall cost, however, as women still need to pay for any office visit or insertion fees. Liletta is unique in its pricing. Sales of Liletta in the private sector support the low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we all can help poor women access affordable, effective contraception. For providers, Liletta is a highly effective and lower-cost alternative to currently available hormonal IUS products.
How to insert Liletta
Insertion can be performed using a no-touch technique. The loading and placement technique outlined below should occur after speculum and tenaculum placement, sounding of the uterus, and vaginal preparation as would be performed for any intrauterine contraceptive insertion. Never force the uterine sound or insertion tube through the cervix; if necessary, stop and dilate the cervical canal. The steps below are the main points for successful Liletta placement; see the full product label for more details.3
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
The key steps to remember are firm-gentle-firm
- Load the device. While pushing forward firmly on the blue slider (with the number 1), load Liletta by pulling down evenly on the threads. The IUS should be laid on a flat surface in the sterile tray to keep the arms parallel to the floor while pulling on the threads. Once loaded, the threads can be pulled up or down to be locked in the cleft. The tip of the IUS will form a hemispheric dome at the top of the insertion tube.
- Adjust the flange to the measured uterine depth based on sounding using the notch in the tray.
- Place inserter into the cervix. Apply gentle traction on the tenaculum to align the cervical canal and uterine cavity. While still holding firm forward pressure on the blue slider, place the tube through the cervical canal until the flange is approximately 1.5 to 2 cm from the cervix. (FIGURE 2.) Gently slide the blue slider downward until it is flush with the green slider (with the number 2; FIGURE 3). This step will allow the IUS arms to open in the lower uterine segment; wait 10 to 15 seconds for the arms to fully unfold. By sliding gently, you will feel when to stop moving the slider.
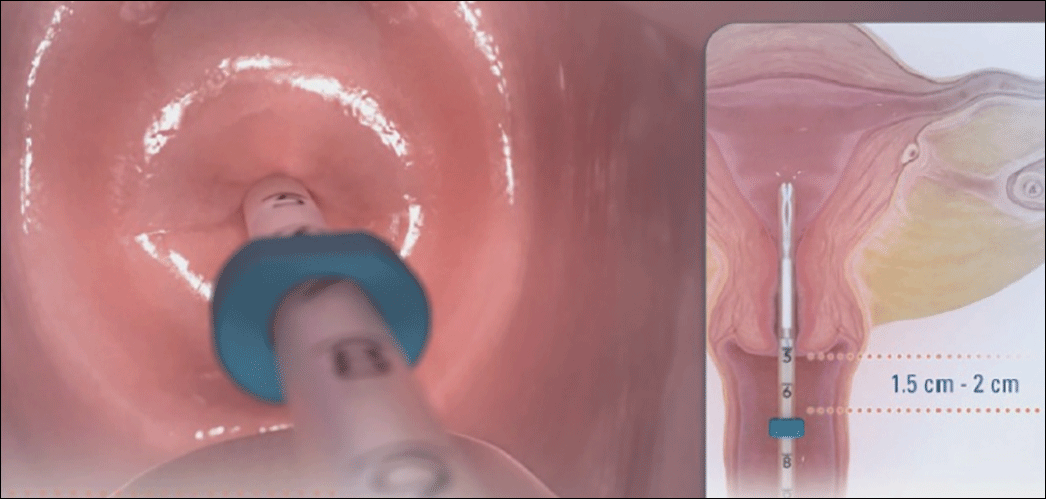 Image copyright Medicines360.
Image copyright Medicines360.Figure 2. Placing the inserter through the cervix  Image copyright Medicines360.
Image copyright Medicines360.Figure 3. Merging the blue and green sliders together to release the IUS arms - Advance the inserter. While holding traction on the tenaculum, advance the inserter to the fundus. You will feel slight resistance when the IUS is at the fundus and the flange should be flush against the cervix.
- Firmly slide both sliders together downward until a click is heard (FIGURE 4). A green indicator will be visible at the bottom of the handle to show that the threads have been ejected from the cleft.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 4. Moving the blue and green sliders downward together to release the IUS - Withdraw the inserter from the uterus and cut the threads with blunt tipped scissors approximately 3 cm from the cervical os.
Technique video
Allergan and Medicines360 offer a video demonstrating proper Liletta insertion technique at www.lilettahcp.com. The video includes proper loading of the inserter as well as correct uterine depth measurement and release of Liletta from the inserter. Clinicians are reminded to use aseptic technique during the entire insertion procedure.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10-16.
- Creinin MD, Jansen R, Starr R, Gobburu J, Gopalakrishnan M, Olariu A. Levonorgestrel release rates over 5 years with the Liletta® 52 mg intrauterine system. Contraception. 2016;94(4):353-356.
- Liletta [package insert]. Irvine, California: Allergan USA, Inc., and San Francisco, California: Medicines360; 2016.
A new single-handed inserter (SHI) for placement of Liletta has been introduced and is currently available (FIGURE 1). Liletta is a levonorgestrel 52-mg intrauterine system (IUS) that is currently approved for 3 years of use as a contraceptive in the United States. The same product in Europe, known as Levosert, is approved for both contraception and the treatment of heavy menstrual bleeding. Liletta, as a branded contraceptive, is being studied through a large Phase 3 clinical trial called ACCESS IUS, which will continue to evaluate the contraceptive efficacy of Liletta for at least 7 years of use.1 A recent publication from the study showed that the levonorgestrel release rates from Liletta are almost identical to the rates reported for the other levonorgestrel 52-mg IUS on the market (Mirena) through 5 years and supports the continued study of the IUS for at least 7 years.2

Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
The initial marketing and distribution of Liletta used a two-handed inserter that allowed for successful placement but required multiple steps. The SHI will make loading and placement of Liletta easier for all clinicians. Features of the new SHI include:
- A double-slider mechanism in which the first slider moves into the second slider during IUS placement, providing a tactile sense for the user during the insertion process
- The ability to reload the inserter if needed before placement
- A firm but bendable tube (that is 2 cm longer than other IUS inserters on the market). This length can be helpful for obese patients or postprocedure placements.
- Depth markings to 12 cm on both sides of the insertion tube.
Related article:
2016 Update on contraception
Liletta is an important and unique product in the US market. For most public sector providers and clinics, Liletta costs only $50, significantly less than other long-acting reversible contraceptives. The price of the IUS is only one aspect of its overall cost, however, as women still need to pay for any office visit or insertion fees. Liletta is unique in its pricing. Sales of Liletta in the private sector support the low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we all can help poor women access affordable, effective contraception. For providers, Liletta is a highly effective and lower-cost alternative to currently available hormonal IUS products.
How to insert Liletta
Insertion can be performed using a no-touch technique. The loading and placement technique outlined below should occur after speculum and tenaculum placement, sounding of the uterus, and vaginal preparation as would be performed for any intrauterine contraceptive insertion. Never force the uterine sound or insertion tube through the cervix; if necessary, stop and dilate the cervical canal. The steps below are the main points for successful Liletta placement; see the full product label for more details.3
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
The key steps to remember are firm-gentle-firm
- Load the device. While pushing forward firmly on the blue slider (with the number 1), load Liletta by pulling down evenly on the threads. The IUS should be laid on a flat surface in the sterile tray to keep the arms parallel to the floor while pulling on the threads. Once loaded, the threads can be pulled up or down to be locked in the cleft. The tip of the IUS will form a hemispheric dome at the top of the insertion tube.
- Adjust the flange to the measured uterine depth based on sounding using the notch in the tray.
- Place inserter into the cervix. Apply gentle traction on the tenaculum to align the cervical canal and uterine cavity. While still holding firm forward pressure on the blue slider, place the tube through the cervical canal until the flange is approximately 1.5 to 2 cm from the cervix. (FIGURE 2.) Gently slide the blue slider downward until it is flush with the green slider (with the number 2; FIGURE 3). This step will allow the IUS arms to open in the lower uterine segment; wait 10 to 15 seconds for the arms to fully unfold. By sliding gently, you will feel when to stop moving the slider.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 2. Placing the inserter through the cervix  Image copyright Medicines360.
Image copyright Medicines360.Figure 3. Merging the blue and green sliders together to release the IUS arms - Advance the inserter. While holding traction on the tenaculum, advance the inserter to the fundus. You will feel slight resistance when the IUS is at the fundus and the flange should be flush against the cervix.
- Firmly slide both sliders together downward until a click is heard (FIGURE 4). A green indicator will be visible at the bottom of the handle to show that the threads have been ejected from the cleft.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 4. Moving the blue and green sliders downward together to release the IUS - Withdraw the inserter from the uterus and cut the threads with blunt tipped scissors approximately 3 cm from the cervical os.
Technique video
Allergan and Medicines360 offer a video demonstrating proper Liletta insertion technique at www.lilettahcp.com. The video includes proper loading of the inserter as well as correct uterine depth measurement and release of Liletta from the inserter. Clinicians are reminded to use aseptic technique during the entire insertion procedure.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A new single-handed inserter (SHI) for placement of Liletta has been introduced and is currently available (FIGURE 1). Liletta is a levonorgestrel 52-mg intrauterine system (IUS) that is currently approved for 3 years of use as a contraceptive in the United States. The same product in Europe, known as Levosert, is approved for both contraception and the treatment of heavy menstrual bleeding. Liletta, as a branded contraceptive, is being studied through a large Phase 3 clinical trial called ACCESS IUS, which will continue to evaluate the contraceptive efficacy of Liletta for at least 7 years of use.1 A recent publication from the study showed that the levonorgestrel release rates from Liletta are almost identical to the rates reported for the other levonorgestrel 52-mg IUS on the market (Mirena) through 5 years and supports the continued study of the IUS for at least 7 years.2

Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
The initial marketing and distribution of Liletta used a two-handed inserter that allowed for successful placement but required multiple steps. The SHI will make loading and placement of Liletta easier for all clinicians. Features of the new SHI include:
- A double-slider mechanism in which the first slider moves into the second slider during IUS placement, providing a tactile sense for the user during the insertion process
- The ability to reload the inserter if needed before placement
- A firm but bendable tube (that is 2 cm longer than other IUS inserters on the market). This length can be helpful for obese patients or postprocedure placements.
- Depth markings to 12 cm on both sides of the insertion tube.
Related article:
2016 Update on contraception
Liletta is an important and unique product in the US market. For most public sector providers and clinics, Liletta costs only $50, significantly less than other long-acting reversible contraceptives. The price of the IUS is only one aspect of its overall cost, however, as women still need to pay for any office visit or insertion fees. Liletta is unique in its pricing. Sales of Liletta in the private sector support the low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we all can help poor women access affordable, effective contraception. For providers, Liletta is a highly effective and lower-cost alternative to currently available hormonal IUS products.
How to insert Liletta
Insertion can be performed using a no-touch technique. The loading and placement technique outlined below should occur after speculum and tenaculum placement, sounding of the uterus, and vaginal preparation as would be performed for any intrauterine contraceptive insertion. Never force the uterine sound or insertion tube through the cervix; if necessary, stop and dilate the cervical canal. The steps below are the main points for successful Liletta placement; see the full product label for more details.3
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
The key steps to remember are firm-gentle-firm
- Load the device. While pushing forward firmly on the blue slider (with the number 1), load Liletta by pulling down evenly on the threads. The IUS should be laid on a flat surface in the sterile tray to keep the arms parallel to the floor while pulling on the threads. Once loaded, the threads can be pulled up or down to be locked in the cleft. The tip of the IUS will form a hemispheric dome at the top of the insertion tube.
- Adjust the flange to the measured uterine depth based on sounding using the notch in the tray.
- Place inserter into the cervix. Apply gentle traction on the tenaculum to align the cervical canal and uterine cavity. While still holding firm forward pressure on the blue slider, place the tube through the cervical canal until the flange is approximately 1.5 to 2 cm from the cervix. (FIGURE 2.) Gently slide the blue slider downward until it is flush with the green slider (with the number 2; FIGURE 3). This step will allow the IUS arms to open in the lower uterine segment; wait 10 to 15 seconds for the arms to fully unfold. By sliding gently, you will feel when to stop moving the slider.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 2. Placing the inserter through the cervix  Image copyright Medicines360.
Image copyright Medicines360.Figure 3. Merging the blue and green sliders together to release the IUS arms - Advance the inserter. While holding traction on the tenaculum, advance the inserter to the fundus. You will feel slight resistance when the IUS is at the fundus and the flange should be flush against the cervix.
- Firmly slide both sliders together downward until a click is heard (FIGURE 4). A green indicator will be visible at the bottom of the handle to show that the threads have been ejected from the cleft.
 Image copyright Medicines360.
Image copyright Medicines360.Figure 4. Moving the blue and green sliders downward together to release the IUS - Withdraw the inserter from the uterus and cut the threads with blunt tipped scissors approximately 3 cm from the cervical os.
Technique video
Allergan and Medicines360 offer a video demonstrating proper Liletta insertion technique at www.lilettahcp.com. The video includes proper loading of the inserter as well as correct uterine depth measurement and release of Liletta from the inserter. Clinicians are reminded to use aseptic technique during the entire insertion procedure.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10-16.
- Creinin MD, Jansen R, Starr R, Gobburu J, Gopalakrishnan M, Olariu A. Levonorgestrel release rates over 5 years with the Liletta® 52 mg intrauterine system. Contraception. 2016;94(4):353-356.
- Liletta [package insert]. Irvine, California: Allergan USA, Inc., and San Francisco, California: Medicines360; 2016.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10-16.
- Creinin MD, Jansen R, Starr R, Gobburu J, Gopalakrishnan M, Olariu A. Levonorgestrel release rates over 5 years with the Liletta® 52 mg intrauterine system. Contraception. 2016;94(4):353-356.
- Liletta [package insert]. Irvine, California: Allergan USA, Inc., and San Francisco, California: Medicines360; 2016.
In this article
2016 Update on contraception
Contraception is an important tool that allows patients to carry out their reproductive-life plans. In the United States, the average woman desires 2 children.1 To achieve this goal, she will spend more than 30 years of her reproductive life avoiding pregnancy.1 The most effective reversible contraceptive methods, the intrauterine device (IUD) and the contraceptive implant, offer an efficient way to cover this significant period. Currently, American women more commonly choose an IUD than an implant by a factor 8 to 1.2 Between 2002 and 2012, the percentage of US contraceptive users aged 15 to 44 using the IUD rose from 2% to 10%.2
Significant barriers to contraceptive access still exist, however. Although widespread reports have lauded the decrease in unintended pregnancies in the United States, improvement only has been marginal for women who live below the poverty level. In fact, for unintended pregnancy the gap between women above and below the poverty level has increased from a 2.6-fold difference in 1994 to a 5.6-fold difference in 2011 (FIGURE).3−5 Since the decrease in the unintended pregnancy rate is most likely related to an increase in contraceptive use, particularly the IUD, we are not providing equal contraceptive access to all women.5
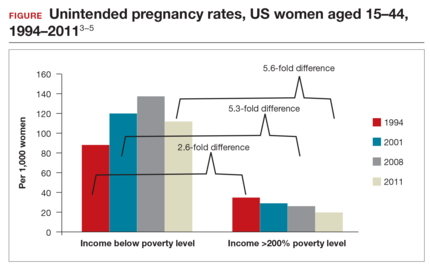
Both the copper IUD and the 3 available levonorgestrel (LNG)-releasing intrauterine system (IUS) products provide safe and effective contraception. As IUD research expands, it is imperative for providers to stay up to date so patients can have full access to these devices.
In this article, we present important updates regarding IUD use that will help break down some continuing barriers to contraceptive access, including:
- clinical trial data demonstrating efficacy of LNG 52-mg IUS for 7 years
- a novel emergency contraception (EC) regimen of same-day oral LNG and the LNG 52-mg IUS
- a large prospective trial demonstrating that women can safely have IUS placement without known sexually transmitted infection (STI) screening results and that pelvic infection rates are not higher in the time shortly after IUS placement.

WHO study demonstrates LNG 52-mg IUS is highly effective for up to 7 years of use
Rowe P, Farley T, Peregoudov A, et al; IUD Research Group of the UNDP/UNFPA/WHO/World Bank Special Programme of Research; Development and Research Training in Human Reproduction. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498−506.
Currently, the 2 LNG 52-mg IUS products (Liletta, Mirena) approved by the US Food and Drug Administration (FDA) are available for use for 3 and 5 years, respectively. The pivotal approval trial for Liletta is still ongoing and is planned to continue for up to 7 years.6 The TCu380A (ParaGard) copper IUD is FDA approved for up to 10 years of use; however, this product initially was approved for only 4 years. The duration of use was expanded to 10 years based on continued clinical trials.
Based on current data, we will not need to wait for the Liletta pivotal trial to have clinical evidence of a longer duration of efficacy for the LNG 52-mg IUS. A collaborative group as part of the UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction performed a multicenter, open-label randomized controlled trial to evaluate outcomes through 7 years of use of the LNG 52-mg IUS and the TCu380A IUD.
Details of the study
A total of 3,836 women were enrolled at 20 centers in Europe, Asia, South America, and China and were randomly assigned to one of the 2 products. Eligible women were aged 16 to 40 years, parous, and without known leiomyoma or recent pelvic infection. After excluding 15 failed IUD insertions, 1,910 women received an LNG 52-mg IUS and 1,911 received a TCu380A. Ultimately, 398 women in the LNG 52-mg IUS group and 682 in the TCu380A group completed 7-year follow-up with the IUD in place. Women were surveyed regarding pregnancy and method discontinuation.
Lower pregnancy rate, higher discontinuation with LNG IUS
The cumulative 7-year pregnancy rate among LNG 52-mg IUS users was significantly lower than among TCu380A users (0.53 per 100 women vs 2.45 per 100 women, respectively). All pregnancies in the LNG 52-mg IUS group occurred in the first 5 years of study follow-up--with no pregnancies in years 6 through 7 (TABLE). The cumulative pregnancy rate in the TCu380A group in this study is consistent with that in a previous long-term trial of this IUD.7
Early removal was significantly higher in the LNG 52-mg IUS group, with a cumulative discontinuation rate of 70.6 per 100 women, compared with 40.8 per 100 women in the TCu380A group. Significant cultural variation existed when it came to both rate and reason for discontinuation. Most women at Chinese centers cited amenorrhea and decreased bleeding as the primary reason for discontinuation, and they did so at twice the rate of women at non-Chinese centers.
The patterns of method discontinuation in this study were different from those found among US women. By comparison, a recent study in the United States had lower overall discontinuation rates and did not find decreased bleeding to be among the main reasons for LNG 52-mg IUS removal.7 In fact, most women who discontinued the LNG 52-mg IUS cited concerns about upcoming expiration as their reason for removal.
The results of this large study also generally corroborated the low-risk profile of IUDs. Only 1 reported IUD perforation occurred, for a rate of 0.03 per 1,000 women. Device expulsion rates were similar between the IUDs and were uncommon overall with 7-year rates of 8 to 9 per 100 women. Pelvic infection was cited as reason for removal in only 7 women (0.18 per 100 women) over 7 years.
What this evidence means for practiceThis exciting study is the first large-scale clinical trial demonstrating continued high efficacy with LNG 52-mg IUS use through 7 years. This information affords women extended contraceptive coverage. While additional research will be welcome, particularly in younger women who will maintain greater fertility across the IUS’s 7-year life span, we are confident in extending the 7-year duration for this IUS to our patients.
Additionally, the method discontinuation findings in this study highlight the importance of discussing the expected menstrual changes of hormonal IUS use with women prior to insertion so they can determine if the potential changes would be satisfactory. As the acceptability of medical menstrual suppression may be new to many women, providers should frame the adverse effects in this context. Providers can use this opportunity to review the noncontraceptive benefits of the hormonal IUS as well.
Novel combination of LNG 52-mg IUS and oral LNG 1.5 mg is promising for emergency contraception
Turok DK, Sanders JN, Thompson IS, Royer PA, Eggebroten J, Gawron LM. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception. 2016;93(6):526−532.
The copper IUD is superior for EC relative to oral agents and has the added benefit of providing ongoing highly effective contraception after placement.8 Despite this strong evidence, the copper IUD remains underutilized for this indication. Turok and colleagues noted that women in their clinic seeking IUDs for non-EC purposes preferred the LNG 52-mg IUS over the copper IUD. It is understandable that women might carry these preferences into EC encounters as well. Thus, the investigators devised a novel combination of LNG 52-mg IUS and oral LNG 1.5 mg, which provided both known EC benefit and same-day access to a more popular contraceptive device.
Details of the study
Women presenting for EC who desired same-day IUD placement were enrolled in the prospective cohort study. Eligible women had a negative urine pregnancy test, known last menstrual period (LMP), regular menstrual cycle, and reported unprotected intercourse within 120 hours prior to presentation. Importantly, women with multiple episodes of unprotected intercourse in the weeks prior to presentation were also included to provide a population more comparable to that encountered clinically. The women were then offered the choice of a TCu380A copper IUD or oral LNG EC with LNG 52-mg IUS placement. They were counseled on the potential increased risk of pregnancy with the novel oral LNG EC plus LNG IUS combination compared with the copper IUD. Participants were given a home pregnancy test that they were to complete in 2 weeks and then report the results to the clinic.
Of the 1,004 women presenting to the clinic for EC over the 16-month study period, 188 (18%) desired same-day IUD insertion. Of these, more opted for the oral LNG EC plus LNG IUS combination (n = 121, 64%) than the copper IUD (n = 67, 36%), demonstrating that women were often willing to accept a possible decrease in EC efficacy with the goal of obtaining their preferred lUD type.
Excluding failed insertion, undiagnosed uterine didelphys, and patient withdrawal, 110 women received the oral LNG EC plus LNG IUS and 66 received the copper IUD. Demographics were comparable between groups except for body mass index (BMI). Of note, more than half (61%) of the women who opted for the oral LNG EC plus LNG IUS combination were overweight or obese.
Both EC methods are effective, broadening options
All women who received the copper IUD followed up at 2 weeks, and no pregnancies were reported. Of the women who received oral LNG EC plus the LNG IUS, 107 (97%) had follow-up at 2 weeks. In this group, there was 1 reported ectopic pregnancy that ultimately required surgical management. However, further review of the patient's coital history suggested that conception occurred prior to IUD insertion, and the case was not classified by the investigators as an EC failure.
Although this study was not powered to detect pregnancy rate differences between the traditional copper IUD and the oral LNG EC plus LNG IUS combination, the results are promising. An important strength of this study is the presence of 2 high-risk groups for EC failure in the oral LNG EC plus LNG IUS group (elevated BMI and multiple episodes of unprotected intercourse).
What this evidence means for practiceEncounters for EC are important opportunities in which to discuss a woman’s reproductive goals. For women who are interested in an IUD, this study opens up the option of same-day placement of the LNG 52-mg IUS. While larger trials need to be conducted to obtain more information regarding pregnancy rates with an oral EC plus LNG IUS combination versus the TCu380A, offering such a combination is reasonable. Given that ulipristal acetate (UPA) is a more effective EC product, especially for women who are overweight or obese,9 offering a UPA EC plus LNG IUS combination may be a better alternative.
It is time to remove the STI screening barrier to same-day IUD insertion
Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement [published online ahead of print May 12, 2016]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2016.05017.
Provider concerns regarding the presence of pelvic infection remain a significant barrier to same-day IUD insertion. Older studies suggested a higher risk of pelvic infection in the first 20 days after IUD placement and extrapolated that pelvic infection was related to inserting an IUD in a woman at risk for STI.10 As a result, patients may be restricted from same-day IUD insertion by providers who think that obtaining results of STI testing is required prior to placement. Recently, a systematic review suggested, based on limited evidence, that IUD placement does not increase the risk of pelvic infection in asymptomatic women compared with those without an IUD.11
Turok and colleagues (including M.D.C., coauthor of this article) reported results from a planned secondary analysis of A Comprehensive Contraceptive Efficacy and Safety Study of an IUS (ACCESS IUS), a component of the regulatory approval of the Liletta LNG 52-mg IUS. This analysis represents the first large-scale prospective investigation of pelvic infection rates during the first 2 years after IUD placement in US women.
Details of the study
Of the 1,751 women enrolled in the study, 1,714 had successful IUS insertions. Infection was assessed via baseline pelvic visual and bimanual examination and Chlamydia testing in all women. Gonorrhea testing was also performed in women who had not been tested with their current sexual partner. STI test results were not required for IUS insertion. Participants were assessed in person at 1, 3, 6, 12, and 24 months after insertion. Additional pelvic exams were performed as needed based on reported symptoms. At 6 months, 1 year, and 2 years, IUS continuation was reported in 1,553 (90.6%), 1,401 (81.7%), and 1,157 (67.3%) women, respectively.
Nearly all women received baseline STI testing (98.4%); however, results were not available prior to same-day IUS insertion for 79.6% of participants. Twenty-nine (1.7%) women had positive baseline STI tests (25 for Chlamydia, 3 for gonorrhea, 1 for both). Of these, only 6 women had results available prior to IUS placement. All women with a positive STI test were treated, and the IUS was left in place. Importantly, none of these women developed pelvic infection in the subsequent 2 years of follow-up and none requested IUS removal.
Infection risk is low with IUD placement
Among women with negative baseline STI tests, there were only 9 (0.5%) clinical diagnoses of pelvic infections over the first 2 years of follow-up. Diagnosis was typically made based on physical examination findings. Most women underwent repeat Chlamydia and gonorrhea testing at the time of pelvic infection diagnosis, and none had positive results. There were no medically recommended IUS removals; 2 women with pelvic infection requested IUS removal per their preference.
Three of the 9 women with pelvic infections were diagnosed within 1 week of IUS placement, 1 at 39 days after placement, and the remaining 5 more than 6 months after placement, suggesting that pelvic infection is not temporally related to IUS placement. Most women were successfully treated as outpatients.
What this evidence means for practiceThis study provides further reassurance regarding the low risk of pelvic infection among women with an LNG IUS. Insertion of an IUS should not be delayed to await results of Chlamydia or gonorrhea testing in a woman without clinical evidence of pelvic infection. Risk-based, as opposed to universal testing, is imperative.12 These recommendations are in agreement with current recommendations of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists.13,14 Practices that employ 2-visit protocols unnecessarily limit women’s access to the IUS, as research has shown that nearly half of women desiring an IUD do not return for device placement if a second encounter is required.15
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Office of Population Affairs, Department of Health and Human Services. Family planning program, FY 1999 service program grants by state. Bethesda, MD: Office of Population Affairs; 1999.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among US women, 2009-2012. Obstet Gynecol. 2015;126(5):917–927.
- Ventura SJ, Abma JC, Mosher WD, Henshaw S. Revised pregnancy rates, 1990-97, and new rates for 1998-99: United States. Natl Vital Stat Rep. 2003;52(7):1−14.
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health. 2014;104(suppl 1):S43–S48.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843–852.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
- United Nations Development Programme, United Nations Population Fund, World Health Organization, World Bank, Special Programme of Research, Development, and Research Training in Human Reproduction. Long-term reversible contraception: twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27(7):1994–2000.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339(8796):785–788.
- Jatlaoui TC, Simmons KB, Curtis KM. The safety of intrauterine contraception initiation among women with current asymptomatic cervical infections or at increased risk of sexually transmitted infections [published online ahead of print June 1, 2016]. Contraception. doi:10.1016/j.contracep tion.2016.05.013.
- Grentzer JM, Peipert JF, Zhao Q, McNicholas C, Secura GM, Madden T. Risk-based screening for Chlamydia trachomatis and Neisseria gonorrhoeae prior to intrauterine device insertion. Contraception. 2015;92(4):313–318.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Centers for Disease Control and Prevention. US selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd ed. MMWR Recomm Rep. 2013;62(RR05):1–46.
- Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two-visit IUD insertion protocols inhibit placement. Contraception. 2012;86(6):694–697.
Contraception is an important tool that allows patients to carry out their reproductive-life plans. In the United States, the average woman desires 2 children.1 To achieve this goal, she will spend more than 30 years of her reproductive life avoiding pregnancy.1 The most effective reversible contraceptive methods, the intrauterine device (IUD) and the contraceptive implant, offer an efficient way to cover this significant period. Currently, American women more commonly choose an IUD than an implant by a factor 8 to 1.2 Between 2002 and 2012, the percentage of US contraceptive users aged 15 to 44 using the IUD rose from 2% to 10%.2
Significant barriers to contraceptive access still exist, however. Although widespread reports have lauded the decrease in unintended pregnancies in the United States, improvement only has been marginal for women who live below the poverty level. In fact, for unintended pregnancy the gap between women above and below the poverty level has increased from a 2.6-fold difference in 1994 to a 5.6-fold difference in 2011 (FIGURE).3−5 Since the decrease in the unintended pregnancy rate is most likely related to an increase in contraceptive use, particularly the IUD, we are not providing equal contraceptive access to all women.5

Both the copper IUD and the 3 available levonorgestrel (LNG)-releasing intrauterine system (IUS) products provide safe and effective contraception. As IUD research expands, it is imperative for providers to stay up to date so patients can have full access to these devices.
In this article, we present important updates regarding IUD use that will help break down some continuing barriers to contraceptive access, including:
- clinical trial data demonstrating efficacy of LNG 52-mg IUS for 7 years
- a novel emergency contraception (EC) regimen of same-day oral LNG and the LNG 52-mg IUS
- a large prospective trial demonstrating that women can safely have IUS placement without known sexually transmitted infection (STI) screening results and that pelvic infection rates are not higher in the time shortly after IUS placement.

WHO study demonstrates LNG 52-mg IUS is highly effective for up to 7 years of use
Rowe P, Farley T, Peregoudov A, et al; IUD Research Group of the UNDP/UNFPA/WHO/World Bank Special Programme of Research; Development and Research Training in Human Reproduction. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498−506.
Currently, the 2 LNG 52-mg IUS products (Liletta, Mirena) approved by the US Food and Drug Administration (FDA) are available for use for 3 and 5 years, respectively. The pivotal approval trial for Liletta is still ongoing and is planned to continue for up to 7 years.6 The TCu380A (ParaGard) copper IUD is FDA approved for up to 10 years of use; however, this product initially was approved for only 4 years. The duration of use was expanded to 10 years based on continued clinical trials.
Based on current data, we will not need to wait for the Liletta pivotal trial to have clinical evidence of a longer duration of efficacy for the LNG 52-mg IUS. A collaborative group as part of the UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction performed a multicenter, open-label randomized controlled trial to evaluate outcomes through 7 years of use of the LNG 52-mg IUS and the TCu380A IUD.
Details of the study
A total of 3,836 women were enrolled at 20 centers in Europe, Asia, South America, and China and were randomly assigned to one of the 2 products. Eligible women were aged 16 to 40 years, parous, and without known leiomyoma or recent pelvic infection. After excluding 15 failed IUD insertions, 1,910 women received an LNG 52-mg IUS and 1,911 received a TCu380A. Ultimately, 398 women in the LNG 52-mg IUS group and 682 in the TCu380A group completed 7-year follow-up with the IUD in place. Women were surveyed regarding pregnancy and method discontinuation.
Lower pregnancy rate, higher discontinuation with LNG IUS
The cumulative 7-year pregnancy rate among LNG 52-mg IUS users was significantly lower than among TCu380A users (0.53 per 100 women vs 2.45 per 100 women, respectively). All pregnancies in the LNG 52-mg IUS group occurred in the first 5 years of study follow-up--with no pregnancies in years 6 through 7 (TABLE). The cumulative pregnancy rate in the TCu380A group in this study is consistent with that in a previous long-term trial of this IUD.7

Early removal was significantly higher in the LNG 52-mg IUS group, with a cumulative discontinuation rate of 70.6 per 100 women, compared with 40.8 per 100 women in the TCu380A group. Significant cultural variation existed when it came to both rate and reason for discontinuation. Most women at Chinese centers cited amenorrhea and decreased bleeding as the primary reason for discontinuation, and they did so at twice the rate of women at non-Chinese centers.
The patterns of method discontinuation in this study were different from those found among US women. By comparison, a recent study in the United States had lower overall discontinuation rates and did not find decreased bleeding to be among the main reasons for LNG 52-mg IUS removal.7 In fact, most women who discontinued the LNG 52-mg IUS cited concerns about upcoming expiration as their reason for removal.
The results of this large study also generally corroborated the low-risk profile of IUDs. Only 1 reported IUD perforation occurred, for a rate of 0.03 per 1,000 women. Device expulsion rates were similar between the IUDs and were uncommon overall with 7-year rates of 8 to 9 per 100 women. Pelvic infection was cited as reason for removal in only 7 women (0.18 per 100 women) over 7 years.
What this evidence means for practiceThis exciting study is the first large-scale clinical trial demonstrating continued high efficacy with LNG 52-mg IUS use through 7 years. This information affords women extended contraceptive coverage. While additional research will be welcome, particularly in younger women who will maintain greater fertility across the IUS’s 7-year life span, we are confident in extending the 7-year duration for this IUS to our patients.
Additionally, the method discontinuation findings in this study highlight the importance of discussing the expected menstrual changes of hormonal IUS use with women prior to insertion so they can determine if the potential changes would be satisfactory. As the acceptability of medical menstrual suppression may be new to many women, providers should frame the adverse effects in this context. Providers can use this opportunity to review the noncontraceptive benefits of the hormonal IUS as well.
Novel combination of LNG 52-mg IUS and oral LNG 1.5 mg is promising for emergency contraception
Turok DK, Sanders JN, Thompson IS, Royer PA, Eggebroten J, Gawron LM. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception. 2016;93(6):526−532.
The copper IUD is superior for EC relative to oral agents and has the added benefit of providing ongoing highly effective contraception after placement.8 Despite this strong evidence, the copper IUD remains underutilized for this indication. Turok and colleagues noted that women in their clinic seeking IUDs for non-EC purposes preferred the LNG 52-mg IUS over the copper IUD. It is understandable that women might carry these preferences into EC encounters as well. Thus, the investigators devised a novel combination of LNG 52-mg IUS and oral LNG 1.5 mg, which provided both known EC benefit and same-day access to a more popular contraceptive device.
Details of the study
Women presenting for EC who desired same-day IUD placement were enrolled in the prospective cohort study. Eligible women had a negative urine pregnancy test, known last menstrual period (LMP), regular menstrual cycle, and reported unprotected intercourse within 120 hours prior to presentation. Importantly, women with multiple episodes of unprotected intercourse in the weeks prior to presentation were also included to provide a population more comparable to that encountered clinically. The women were then offered the choice of a TCu380A copper IUD or oral LNG EC with LNG 52-mg IUS placement. They were counseled on the potential increased risk of pregnancy with the novel oral LNG EC plus LNG IUS combination compared with the copper IUD. Participants were given a home pregnancy test that they were to complete in 2 weeks and then report the results to the clinic.
Of the 1,004 women presenting to the clinic for EC over the 16-month study period, 188 (18%) desired same-day IUD insertion. Of these, more opted for the oral LNG EC plus LNG IUS combination (n = 121, 64%) than the copper IUD (n = 67, 36%), demonstrating that women were often willing to accept a possible decrease in EC efficacy with the goal of obtaining their preferred lUD type.
Excluding failed insertion, undiagnosed uterine didelphys, and patient withdrawal, 110 women received the oral LNG EC plus LNG IUS and 66 received the copper IUD. Demographics were comparable between groups except for body mass index (BMI). Of note, more than half (61%) of the women who opted for the oral LNG EC plus LNG IUS combination were overweight or obese.
Both EC methods are effective, broadening options
All women who received the copper IUD followed up at 2 weeks, and no pregnancies were reported. Of the women who received oral LNG EC plus the LNG IUS, 107 (97%) had follow-up at 2 weeks. In this group, there was 1 reported ectopic pregnancy that ultimately required surgical management. However, further review of the patient's coital history suggested that conception occurred prior to IUD insertion, and the case was not classified by the investigators as an EC failure.
Although this study was not powered to detect pregnancy rate differences between the traditional copper IUD and the oral LNG EC plus LNG IUS combination, the results are promising. An important strength of this study is the presence of 2 high-risk groups for EC failure in the oral LNG EC plus LNG IUS group (elevated BMI and multiple episodes of unprotected intercourse).
What this evidence means for practiceEncounters for EC are important opportunities in which to discuss a woman’s reproductive goals. For women who are interested in an IUD, this study opens up the option of same-day placement of the LNG 52-mg IUS. While larger trials need to be conducted to obtain more information regarding pregnancy rates with an oral EC plus LNG IUS combination versus the TCu380A, offering such a combination is reasonable. Given that ulipristal acetate (UPA) is a more effective EC product, especially for women who are overweight or obese,9 offering a UPA EC plus LNG IUS combination may be a better alternative.
It is time to remove the STI screening barrier to same-day IUD insertion
Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement [published online ahead of print May 12, 2016]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2016.05017.
Provider concerns regarding the presence of pelvic infection remain a significant barrier to same-day IUD insertion. Older studies suggested a higher risk of pelvic infection in the first 20 days after IUD placement and extrapolated that pelvic infection was related to inserting an IUD in a woman at risk for STI.10 As a result, patients may be restricted from same-day IUD insertion by providers who think that obtaining results of STI testing is required prior to placement. Recently, a systematic review suggested, based on limited evidence, that IUD placement does not increase the risk of pelvic infection in asymptomatic women compared with those without an IUD.11
Turok and colleagues (including M.D.C., coauthor of this article) reported results from a planned secondary analysis of A Comprehensive Contraceptive Efficacy and Safety Study of an IUS (ACCESS IUS), a component of the regulatory approval of the Liletta LNG 52-mg IUS. This analysis represents the first large-scale prospective investigation of pelvic infection rates during the first 2 years after IUD placement in US women.
Details of the study
Of the 1,751 women enrolled in the study, 1,714 had successful IUS insertions. Infection was assessed via baseline pelvic visual and bimanual examination and Chlamydia testing in all women. Gonorrhea testing was also performed in women who had not been tested with their current sexual partner. STI test results were not required for IUS insertion. Participants were assessed in person at 1, 3, 6, 12, and 24 months after insertion. Additional pelvic exams were performed as needed based on reported symptoms. At 6 months, 1 year, and 2 years, IUS continuation was reported in 1,553 (90.6%), 1,401 (81.7%), and 1,157 (67.3%) women, respectively.
Nearly all women received baseline STI testing (98.4%); however, results were not available prior to same-day IUS insertion for 79.6% of participants. Twenty-nine (1.7%) women had positive baseline STI tests (25 for Chlamydia, 3 for gonorrhea, 1 for both). Of these, only 6 women had results available prior to IUS placement. All women with a positive STI test were treated, and the IUS was left in place. Importantly, none of these women developed pelvic infection in the subsequent 2 years of follow-up and none requested IUS removal.
Infection risk is low with IUD placement
Among women with negative baseline STI tests, there were only 9 (0.5%) clinical diagnoses of pelvic infections over the first 2 years of follow-up. Diagnosis was typically made based on physical examination findings. Most women underwent repeat Chlamydia and gonorrhea testing at the time of pelvic infection diagnosis, and none had positive results. There were no medically recommended IUS removals; 2 women with pelvic infection requested IUS removal per their preference.
Three of the 9 women with pelvic infections were diagnosed within 1 week of IUS placement, 1 at 39 days after placement, and the remaining 5 more than 6 months after placement, suggesting that pelvic infection is not temporally related to IUS placement. Most women were successfully treated as outpatients.
What this evidence means for practiceThis study provides further reassurance regarding the low risk of pelvic infection among women with an LNG IUS. Insertion of an IUS should not be delayed to await results of Chlamydia or gonorrhea testing in a woman without clinical evidence of pelvic infection. Risk-based, as opposed to universal testing, is imperative.12 These recommendations are in agreement with current recommendations of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists.13,14 Practices that employ 2-visit protocols unnecessarily limit women’s access to the IUS, as research has shown that nearly half of women desiring an IUD do not return for device placement if a second encounter is required.15
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Contraception is an important tool that allows patients to carry out their reproductive-life plans. In the United States, the average woman desires 2 children.1 To achieve this goal, she will spend more than 30 years of her reproductive life avoiding pregnancy.1 The most effective reversible contraceptive methods, the intrauterine device (IUD) and the contraceptive implant, offer an efficient way to cover this significant period. Currently, American women more commonly choose an IUD than an implant by a factor 8 to 1.2 Between 2002 and 2012, the percentage of US contraceptive users aged 15 to 44 using the IUD rose from 2% to 10%.2
Significant barriers to contraceptive access still exist, however. Although widespread reports have lauded the decrease in unintended pregnancies in the United States, improvement only has been marginal for women who live below the poverty level. In fact, for unintended pregnancy the gap between women above and below the poverty level has increased from a 2.6-fold difference in 1994 to a 5.6-fold difference in 2011 (FIGURE).3−5 Since the decrease in the unintended pregnancy rate is most likely related to an increase in contraceptive use, particularly the IUD, we are not providing equal contraceptive access to all women.5

Both the copper IUD and the 3 available levonorgestrel (LNG)-releasing intrauterine system (IUS) products provide safe and effective contraception. As IUD research expands, it is imperative for providers to stay up to date so patients can have full access to these devices.
In this article, we present important updates regarding IUD use that will help break down some continuing barriers to contraceptive access, including:
- clinical trial data demonstrating efficacy of LNG 52-mg IUS for 7 years
- a novel emergency contraception (EC) regimen of same-day oral LNG and the LNG 52-mg IUS
- a large prospective trial demonstrating that women can safely have IUS placement without known sexually transmitted infection (STI) screening results and that pelvic infection rates are not higher in the time shortly after IUS placement.

WHO study demonstrates LNG 52-mg IUS is highly effective for up to 7 years of use
Rowe P, Farley T, Peregoudov A, et al; IUD Research Group of the UNDP/UNFPA/WHO/World Bank Special Programme of Research; Development and Research Training in Human Reproduction. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498−506.
Currently, the 2 LNG 52-mg IUS products (Liletta, Mirena) approved by the US Food and Drug Administration (FDA) are available for use for 3 and 5 years, respectively. The pivotal approval trial for Liletta is still ongoing and is planned to continue for up to 7 years.6 The TCu380A (ParaGard) copper IUD is FDA approved for up to 10 years of use; however, this product initially was approved for only 4 years. The duration of use was expanded to 10 years based on continued clinical trials.
Based on current data, we will not need to wait for the Liletta pivotal trial to have clinical evidence of a longer duration of efficacy for the LNG 52-mg IUS. A collaborative group as part of the UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction performed a multicenter, open-label randomized controlled trial to evaluate outcomes through 7 years of use of the LNG 52-mg IUS and the TCu380A IUD.
Details of the study
A total of 3,836 women were enrolled at 20 centers in Europe, Asia, South America, and China and were randomly assigned to one of the 2 products. Eligible women were aged 16 to 40 years, parous, and without known leiomyoma or recent pelvic infection. After excluding 15 failed IUD insertions, 1,910 women received an LNG 52-mg IUS and 1,911 received a TCu380A. Ultimately, 398 women in the LNG 52-mg IUS group and 682 in the TCu380A group completed 7-year follow-up with the IUD in place. Women were surveyed regarding pregnancy and method discontinuation.
Lower pregnancy rate, higher discontinuation with LNG IUS
The cumulative 7-year pregnancy rate among LNG 52-mg IUS users was significantly lower than among TCu380A users (0.53 per 100 women vs 2.45 per 100 women, respectively). All pregnancies in the LNG 52-mg IUS group occurred in the first 5 years of study follow-up--with no pregnancies in years 6 through 7 (TABLE). The cumulative pregnancy rate in the TCu380A group in this study is consistent with that in a previous long-term trial of this IUD.7

Early removal was significantly higher in the LNG 52-mg IUS group, with a cumulative discontinuation rate of 70.6 per 100 women, compared with 40.8 per 100 women in the TCu380A group. Significant cultural variation existed when it came to both rate and reason for discontinuation. Most women at Chinese centers cited amenorrhea and decreased bleeding as the primary reason for discontinuation, and they did so at twice the rate of women at non-Chinese centers.
The patterns of method discontinuation in this study were different from those found among US women. By comparison, a recent study in the United States had lower overall discontinuation rates and did not find decreased bleeding to be among the main reasons for LNG 52-mg IUS removal.7 In fact, most women who discontinued the LNG 52-mg IUS cited concerns about upcoming expiration as their reason for removal.
The results of this large study also generally corroborated the low-risk profile of IUDs. Only 1 reported IUD perforation occurred, for a rate of 0.03 per 1,000 women. Device expulsion rates were similar between the IUDs and were uncommon overall with 7-year rates of 8 to 9 per 100 women. Pelvic infection was cited as reason for removal in only 7 women (0.18 per 100 women) over 7 years.
What this evidence means for practiceThis exciting study is the first large-scale clinical trial demonstrating continued high efficacy with LNG 52-mg IUS use through 7 years. This information affords women extended contraceptive coverage. While additional research will be welcome, particularly in younger women who will maintain greater fertility across the IUS’s 7-year life span, we are confident in extending the 7-year duration for this IUS to our patients.
Additionally, the method discontinuation findings in this study highlight the importance of discussing the expected menstrual changes of hormonal IUS use with women prior to insertion so they can determine if the potential changes would be satisfactory. As the acceptability of medical menstrual suppression may be new to many women, providers should frame the adverse effects in this context. Providers can use this opportunity to review the noncontraceptive benefits of the hormonal IUS as well.
Novel combination of LNG 52-mg IUS and oral LNG 1.5 mg is promising for emergency contraception
Turok DK, Sanders JN, Thompson IS, Royer PA, Eggebroten J, Gawron LM. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception. 2016;93(6):526−532.
The copper IUD is superior for EC relative to oral agents and has the added benefit of providing ongoing highly effective contraception after placement.8 Despite this strong evidence, the copper IUD remains underutilized for this indication. Turok and colleagues noted that women in their clinic seeking IUDs for non-EC purposes preferred the LNG 52-mg IUS over the copper IUD. It is understandable that women might carry these preferences into EC encounters as well. Thus, the investigators devised a novel combination of LNG 52-mg IUS and oral LNG 1.5 mg, which provided both known EC benefit and same-day access to a more popular contraceptive device.
Details of the study
Women presenting for EC who desired same-day IUD placement were enrolled in the prospective cohort study. Eligible women had a negative urine pregnancy test, known last menstrual period (LMP), regular menstrual cycle, and reported unprotected intercourse within 120 hours prior to presentation. Importantly, women with multiple episodes of unprotected intercourse in the weeks prior to presentation were also included to provide a population more comparable to that encountered clinically. The women were then offered the choice of a TCu380A copper IUD or oral LNG EC with LNG 52-mg IUS placement. They were counseled on the potential increased risk of pregnancy with the novel oral LNG EC plus LNG IUS combination compared with the copper IUD. Participants were given a home pregnancy test that they were to complete in 2 weeks and then report the results to the clinic.
Of the 1,004 women presenting to the clinic for EC over the 16-month study period, 188 (18%) desired same-day IUD insertion. Of these, more opted for the oral LNG EC plus LNG IUS combination (n = 121, 64%) than the copper IUD (n = 67, 36%), demonstrating that women were often willing to accept a possible decrease in EC efficacy with the goal of obtaining their preferred lUD type.
Excluding failed insertion, undiagnosed uterine didelphys, and patient withdrawal, 110 women received the oral LNG EC plus LNG IUS and 66 received the copper IUD. Demographics were comparable between groups except for body mass index (BMI). Of note, more than half (61%) of the women who opted for the oral LNG EC plus LNG IUS combination were overweight or obese.
Both EC methods are effective, broadening options
All women who received the copper IUD followed up at 2 weeks, and no pregnancies were reported. Of the women who received oral LNG EC plus the LNG IUS, 107 (97%) had follow-up at 2 weeks. In this group, there was 1 reported ectopic pregnancy that ultimately required surgical management. However, further review of the patient's coital history suggested that conception occurred prior to IUD insertion, and the case was not classified by the investigators as an EC failure.
Although this study was not powered to detect pregnancy rate differences between the traditional copper IUD and the oral LNG EC plus LNG IUS combination, the results are promising. An important strength of this study is the presence of 2 high-risk groups for EC failure in the oral LNG EC plus LNG IUS group (elevated BMI and multiple episodes of unprotected intercourse).
What this evidence means for practiceEncounters for EC are important opportunities in which to discuss a woman’s reproductive goals. For women who are interested in an IUD, this study opens up the option of same-day placement of the LNG 52-mg IUS. While larger trials need to be conducted to obtain more information regarding pregnancy rates with an oral EC plus LNG IUS combination versus the TCu380A, offering such a combination is reasonable. Given that ulipristal acetate (UPA) is a more effective EC product, especially for women who are overweight or obese,9 offering a UPA EC plus LNG IUS combination may be a better alternative.
It is time to remove the STI screening barrier to same-day IUD insertion
Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement [published online ahead of print May 12, 2016]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2016.05017.
Provider concerns regarding the presence of pelvic infection remain a significant barrier to same-day IUD insertion. Older studies suggested a higher risk of pelvic infection in the first 20 days after IUD placement and extrapolated that pelvic infection was related to inserting an IUD in a woman at risk for STI.10 As a result, patients may be restricted from same-day IUD insertion by providers who think that obtaining results of STI testing is required prior to placement. Recently, a systematic review suggested, based on limited evidence, that IUD placement does not increase the risk of pelvic infection in asymptomatic women compared with those without an IUD.11
Turok and colleagues (including M.D.C., coauthor of this article) reported results from a planned secondary analysis of A Comprehensive Contraceptive Efficacy and Safety Study of an IUS (ACCESS IUS), a component of the regulatory approval of the Liletta LNG 52-mg IUS. This analysis represents the first large-scale prospective investigation of pelvic infection rates during the first 2 years after IUD placement in US women.
Details of the study
Of the 1,751 women enrolled in the study, 1,714 had successful IUS insertions. Infection was assessed via baseline pelvic visual and bimanual examination and Chlamydia testing in all women. Gonorrhea testing was also performed in women who had not been tested with their current sexual partner. STI test results were not required for IUS insertion. Participants were assessed in person at 1, 3, 6, 12, and 24 months after insertion. Additional pelvic exams were performed as needed based on reported symptoms. At 6 months, 1 year, and 2 years, IUS continuation was reported in 1,553 (90.6%), 1,401 (81.7%), and 1,157 (67.3%) women, respectively.
Nearly all women received baseline STI testing (98.4%); however, results were not available prior to same-day IUS insertion for 79.6% of participants. Twenty-nine (1.7%) women had positive baseline STI tests (25 for Chlamydia, 3 for gonorrhea, 1 for both). Of these, only 6 women had results available prior to IUS placement. All women with a positive STI test were treated, and the IUS was left in place. Importantly, none of these women developed pelvic infection in the subsequent 2 years of follow-up and none requested IUS removal.
Infection risk is low with IUD placement
Among women with negative baseline STI tests, there were only 9 (0.5%) clinical diagnoses of pelvic infections over the first 2 years of follow-up. Diagnosis was typically made based on physical examination findings. Most women underwent repeat Chlamydia and gonorrhea testing at the time of pelvic infection diagnosis, and none had positive results. There were no medically recommended IUS removals; 2 women with pelvic infection requested IUS removal per their preference.
Three of the 9 women with pelvic infections were diagnosed within 1 week of IUS placement, 1 at 39 days after placement, and the remaining 5 more than 6 months after placement, suggesting that pelvic infection is not temporally related to IUS placement. Most women were successfully treated as outpatients.
What this evidence means for practiceThis study provides further reassurance regarding the low risk of pelvic infection among women with an LNG IUS. Insertion of an IUS should not be delayed to await results of Chlamydia or gonorrhea testing in a woman without clinical evidence of pelvic infection. Risk-based, as opposed to universal testing, is imperative.12 These recommendations are in agreement with current recommendations of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists.13,14 Practices that employ 2-visit protocols unnecessarily limit women’s access to the IUS, as research has shown that nearly half of women desiring an IUD do not return for device placement if a second encounter is required.15
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Office of Population Affairs, Department of Health and Human Services. Family planning program, FY 1999 service program grants by state. Bethesda, MD: Office of Population Affairs; 1999.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among US women, 2009-2012. Obstet Gynecol. 2015;126(5):917–927.
- Ventura SJ, Abma JC, Mosher WD, Henshaw S. Revised pregnancy rates, 1990-97, and new rates for 1998-99: United States. Natl Vital Stat Rep. 2003;52(7):1−14.
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health. 2014;104(suppl 1):S43–S48.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843–852.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
- United Nations Development Programme, United Nations Population Fund, World Health Organization, World Bank, Special Programme of Research, Development, and Research Training in Human Reproduction. Long-term reversible contraception: twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27(7):1994–2000.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339(8796):785–788.
- Jatlaoui TC, Simmons KB, Curtis KM. The safety of intrauterine contraception initiation among women with current asymptomatic cervical infections or at increased risk of sexually transmitted infections [published online ahead of print June 1, 2016]. Contraception. doi:10.1016/j.contracep tion.2016.05.013.
- Grentzer JM, Peipert JF, Zhao Q, McNicholas C, Secura GM, Madden T. Risk-based screening for Chlamydia trachomatis and Neisseria gonorrhoeae prior to intrauterine device insertion. Contraception. 2015;92(4):313–318.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Centers for Disease Control and Prevention. US selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd ed. MMWR Recomm Rep. 2013;62(RR05):1–46.
- Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two-visit IUD insertion protocols inhibit placement. Contraception. 2012;86(6):694–697.
- Office of Population Affairs, Department of Health and Human Services. Family planning program, FY 1999 service program grants by state. Bethesda, MD: Office of Population Affairs; 1999.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among US women, 2009-2012. Obstet Gynecol. 2015;126(5):917–927.
- Ventura SJ, Abma JC, Mosher WD, Henshaw S. Revised pregnancy rates, 1990-97, and new rates for 1998-99: United States. Natl Vital Stat Rep. 2003;52(7):1−14.
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health. 2014;104(suppl 1):S43–S48.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843–852.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
- United Nations Development Programme, United Nations Population Fund, World Health Organization, World Bank, Special Programme of Research, Development, and Research Training in Human Reproduction. Long-term reversible contraception: twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27(7):1994–2000.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339(8796):785–788.
- Jatlaoui TC, Simmons KB, Curtis KM. The safety of intrauterine contraception initiation among women with current asymptomatic cervical infections or at increased risk of sexually transmitted infections [published online ahead of print June 1, 2016]. Contraception. doi:10.1016/j.contracep tion.2016.05.013.
- Grentzer JM, Peipert JF, Zhao Q, McNicholas C, Secura GM, Madden T. Risk-based screening for Chlamydia trachomatis and Neisseria gonorrhoeae prior to intrauterine device insertion. Contraception. 2015;92(4):313–318.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Centers for Disease Control and Prevention. US selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd ed. MMWR Recomm Rep. 2013;62(RR05):1–46.
- Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two-visit IUD insertion protocols inhibit placement. Contraception. 2012;86(6):694–697.
In this article
- Extended use of LNG IUS
- Oral LNG and LNG IUS combo for emergency contraception
- STI screening and same-day IUD placement
Is the levonorgestrel-releasing intrauterine system more effective than the copper IUD at preventing pregnancy?
Both the LNG-IUS and the copper IUD are highly effective at pregnancy prevention. However, large-scale comparative studies are lacking. These findings from the European Active Surveillance Study for Intrauterine Devices (EURAS IUD), an investigation of new users of the LNG-IUS (20 µg/day) and copper IUD (>30 different types) in Austria, Finland, Germany, Poland, Sweden, and the United Kingdom, confirm the low contraceptive failure rate for both devices.
The primary objective of this trial was to compare uterine perforation rates,1 but the results of a planned secondary analysis comparing contraceptive effectiveness may be of more interest to patients and providers.
Details of the study
Women who had a newly inserted IUD during the study period were eligible for recruitment. These women and their inserting health care provider then completed a follow-up questionnaire 12 months after enrollment to assess for pregnancy or any potential IUD complication.
In total, 61,448 women were enrolled, and 58,324 patients (41,001 using the LNG-IUS and 17,323 using the copper IUD) were included in the analysis. Only 1.7% of LNG-IUS users and 2.8% of copper IUD users were lost to follow-up. Women using the LNG-IUS were older than those using the copper IUD (mean age of 37.4 vs 33.3 years, respectively). About 43% and 24% of LNG-IUS and copper IUD users, respectively, were age 40 or older at the time of IUD insertion.
Strengths and limitations
The large sample size and low number of women lost to follow-up are strengths of this study. A major weakness: The indication for IUD insertion was not recorded. Nor was the risk of pregnancy assessed at enrollment.
Overall, the age of the study population was older than is typically found in a contraceptive efficacy trial, which generally covers the age range of 18 to 35 years.
Because women chose their type of IUD (as opposed to random allocation), variations in underlying fertility, age, and other confounders of efficacy cannot be accounted for fully with statistical analyses. The variation in age strongly suggests that women may have chosen the LNG-IUS for reasons other than contraception.
Furthermore, more than 30 types of copper IUDs were inserted during the study period, and small variations in contraceptive efficacy from one type to another may contribute to the overall difference in failure rates between the LNG-IUS and copper IUD. Although Heinemann and colleagues did perform an analysis of failure rates by copper content and found no differences between users of IUDs with less than 300 mm2 and those with at least 300 mm2 of copper, earlier prospective randomized trials show differences in contraceptive efficacy by device type and amount of copper.2
What this evidence means for practice
The LNG-IUS may be a more effective contraceptive than the copper IUD, but both possess excellent contraceptive efficacy. Prospective randomized trials, although much smaller than this nonrandomized cohort study, do not demonstrate differences in contraceptive efficacy between the LNG-IUS and copper IUD.3 The small difference in contraceptive failure rates (less than 1 in 200 women), if real, should not be the deciding factor for choosing one IUD over the other.
— Melissa J. Chen, MD, MPH, and Mitchell D. Creinin, MDShare your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Heinemann K, Reed S, Moehner S, Do Minh T. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91(4):274–279.
2. Thonneau PF, Almont T. Contraceptive efficacy of intrauterine devices. Am J Obstet Gynecol. 2008;198(3):248–253.
3. Sivin I, el Mahgoub S, McCarthy T, et al. Long-term contraception with the levonorgestrel 20 mcg/day (LNg 20) and the copper T380Ag intrauterine devices: a five-year randomized study. Contraception. 1990;42(4):361–378.
Both the LNG-IUS and the copper IUD are highly effective at pregnancy prevention. However, large-scale comparative studies are lacking. These findings from the European Active Surveillance Study for Intrauterine Devices (EURAS IUD), an investigation of new users of the LNG-IUS (20 µg/day) and copper IUD (>30 different types) in Austria, Finland, Germany, Poland, Sweden, and the United Kingdom, confirm the low contraceptive failure rate for both devices.
The primary objective of this trial was to compare uterine perforation rates,1 but the results of a planned secondary analysis comparing contraceptive effectiveness may be of more interest to patients and providers.
Details of the study
Women who had a newly inserted IUD during the study period were eligible for recruitment. These women and their inserting health care provider then completed a follow-up questionnaire 12 months after enrollment to assess for pregnancy or any potential IUD complication.
In total, 61,448 women were enrolled, and 58,324 patients (41,001 using the LNG-IUS and 17,323 using the copper IUD) were included in the analysis. Only 1.7% of LNG-IUS users and 2.8% of copper IUD users were lost to follow-up. Women using the LNG-IUS were older than those using the copper IUD (mean age of 37.4 vs 33.3 years, respectively). About 43% and 24% of LNG-IUS and copper IUD users, respectively, were age 40 or older at the time of IUD insertion.
Strengths and limitations
The large sample size and low number of women lost to follow-up are strengths of this study. A major weakness: The indication for IUD insertion was not recorded. Nor was the risk of pregnancy assessed at enrollment.
Overall, the age of the study population was older than is typically found in a contraceptive efficacy trial, which generally covers the age range of 18 to 35 years.
Because women chose their type of IUD (as opposed to random allocation), variations in underlying fertility, age, and other confounders of efficacy cannot be accounted for fully with statistical analyses. The variation in age strongly suggests that women may have chosen the LNG-IUS for reasons other than contraception.
Furthermore, more than 30 types of copper IUDs were inserted during the study period, and small variations in contraceptive efficacy from one type to another may contribute to the overall difference in failure rates between the LNG-IUS and copper IUD. Although Heinemann and colleagues did perform an analysis of failure rates by copper content and found no differences between users of IUDs with less than 300 mm2 and those with at least 300 mm2 of copper, earlier prospective randomized trials show differences in contraceptive efficacy by device type and amount of copper.2
What this evidence means for practice
The LNG-IUS may be a more effective contraceptive than the copper IUD, but both possess excellent contraceptive efficacy. Prospective randomized trials, although much smaller than this nonrandomized cohort study, do not demonstrate differences in contraceptive efficacy between the LNG-IUS and copper IUD.3 The small difference in contraceptive failure rates (less than 1 in 200 women), if real, should not be the deciding factor for choosing one IUD over the other.
— Melissa J. Chen, MD, MPH, and Mitchell D. Creinin, MDShare your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Both the LNG-IUS and the copper IUD are highly effective at pregnancy prevention. However, large-scale comparative studies are lacking. These findings from the European Active Surveillance Study for Intrauterine Devices (EURAS IUD), an investigation of new users of the LNG-IUS (20 µg/day) and copper IUD (>30 different types) in Austria, Finland, Germany, Poland, Sweden, and the United Kingdom, confirm the low contraceptive failure rate for both devices.
The primary objective of this trial was to compare uterine perforation rates,1 but the results of a planned secondary analysis comparing contraceptive effectiveness may be of more interest to patients and providers.
Details of the study
Women who had a newly inserted IUD during the study period were eligible for recruitment. These women and their inserting health care provider then completed a follow-up questionnaire 12 months after enrollment to assess for pregnancy or any potential IUD complication.
In total, 61,448 women were enrolled, and 58,324 patients (41,001 using the LNG-IUS and 17,323 using the copper IUD) were included in the analysis. Only 1.7% of LNG-IUS users and 2.8% of copper IUD users were lost to follow-up. Women using the LNG-IUS were older than those using the copper IUD (mean age of 37.4 vs 33.3 years, respectively). About 43% and 24% of LNG-IUS and copper IUD users, respectively, were age 40 or older at the time of IUD insertion.
Strengths and limitations
The large sample size and low number of women lost to follow-up are strengths of this study. A major weakness: The indication for IUD insertion was not recorded. Nor was the risk of pregnancy assessed at enrollment.
Overall, the age of the study population was older than is typically found in a contraceptive efficacy trial, which generally covers the age range of 18 to 35 years.
Because women chose their type of IUD (as opposed to random allocation), variations in underlying fertility, age, and other confounders of efficacy cannot be accounted for fully with statistical analyses. The variation in age strongly suggests that women may have chosen the LNG-IUS for reasons other than contraception.
Furthermore, more than 30 types of copper IUDs were inserted during the study period, and small variations in contraceptive efficacy from one type to another may contribute to the overall difference in failure rates between the LNG-IUS and copper IUD. Although Heinemann and colleagues did perform an analysis of failure rates by copper content and found no differences between users of IUDs with less than 300 mm2 and those with at least 300 mm2 of copper, earlier prospective randomized trials show differences in contraceptive efficacy by device type and amount of copper.2
What this evidence means for practice
The LNG-IUS may be a more effective contraceptive than the copper IUD, but both possess excellent contraceptive efficacy. Prospective randomized trials, although much smaller than this nonrandomized cohort study, do not demonstrate differences in contraceptive efficacy between the LNG-IUS and copper IUD.3 The small difference in contraceptive failure rates (less than 1 in 200 women), if real, should not be the deciding factor for choosing one IUD over the other.
— Melissa J. Chen, MD, MPH, and Mitchell D. Creinin, MDShare your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Heinemann K, Reed S, Moehner S, Do Minh T. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91(4):274–279.
2. Thonneau PF, Almont T. Contraceptive efficacy of intrauterine devices. Am J Obstet Gynecol. 2008;198(3):248–253.
3. Sivin I, el Mahgoub S, McCarthy T, et al. Long-term contraception with the levonorgestrel 20 mcg/day (LNg 20) and the copper T380Ag intrauterine devices: a five-year randomized study. Contraception. 1990;42(4):361–378.
1. Heinemann K, Reed S, Moehner S, Do Minh T. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91(4):274–279.
2. Thonneau PF, Almont T. Contraceptive efficacy of intrauterine devices. Am J Obstet Gynecol. 2008;198(3):248–253.
3. Sivin I, el Mahgoub S, McCarthy T, et al. Long-term contraception with the levonorgestrel 20 mcg/day (LNg 20) and the copper T380Ag intrauterine devices: a five-year randomized study. Contraception. 1990;42(4):361–378.