User login
These 3 tools can help you streamline management of IBS
CASE › Amber S,* a 33-year-old woman who works on the production line at a bread factory, sought care at my health center with a several month history of non-bloody diarrhea that was increasing in frequency and urgency and was accompanied by painful abdominal bloating and cramping. She said that these symptoms were negatively impacting her interpersonal relationships, as well as her productivity at work. She reported that “almost everything” she ate upset her stomach and “goes right through her,” including fruits, vegetables, and meat, as well as greasy fast food. She had researched her symptoms on the Internet and was worried that she might have something serious like inflammatory bowel disease or cancer.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID) that negatively impacts the quality of life (QOL) of millions of people worldwide.1 In fact, one study of 179 people with IBS found that 76% of survey respondents reported some degree of IBS-related impairment in at least 5 domains of daily life: daily activities, comorbid psychiatric diagnoses, symptom severity, QOL, and symptom-specific cognitive affective factors related to IBS.2
Estimating prevalence and incidence is a formidable challenge given various diagnostic criteria, the influence of population selection, inclusion or exclusion of non-GI comorbidities, and various cultural influences.3 That said, it’s estimated that IBS impacts approximately 11% of the world’s population, and approximately 30% of these individuals seek treatment.1,4 While there are no significant differences in GI symptoms between those who consult physicians and those who do not, those who do seek treatment report higher pain scores, greater levels of anxiety, and a greater reduction in QOL.5
All ages affected. IBS has been reported in patients of all ages, including children and the elderly, with no definable difference reported in the frequency of subtypes (diarrhea- or constipation-predominant).
This article reviews the latest explanations, diagnostic criteria, and treatment guidelines for this challenging condition so that you can offer your patients confident care without needless testing or referral.
[polldaddy:9755564]
A lack of consensus among practicing physicians
Historically, IBS has been regarded by many primary care physicians (PCPs) as a diagnosis of exclusion. Lab tests would be ordered, nothing significant would be found, and the patient would be referred to the gastroenterologist for a definitive diagnosis.
Perceptions and misconceptions about IBS continue to abound to this day. Many are neither completely right nor wrong partly because so many triggers for IBS exist and partly because of the heretofore lack of simple, standardized criteria to diagnose the condition. Other factors contributing to the confusion are that the diagnosis of IBS is purely symptom-based and that proposals of its pathophysiology have traditionally been complex.
For example, a 2006 survey-based study of PCPs and gastroenterologists found that PCPs were less likely than gastroenterologists to believe that IBS was related to prior physical or sexual abuse, previous infection, or learned behavior, but were more likely to associate dietary factors or a linkable genetic etiology with IBS.6 Both sets of beliefs, however, may be considered correct.
Similarly, a 2009 qualitative study conducted in the Netherlands found that general practitioners (GPs) considered smoking, caffeine, diet, “hasty lifestyle,” and lack of exercise as potential triggers for IBS symptoms, while PCPs in the United Kingdom considered diet, infection, and travel to be possible triggers.7 Again, all play a role.
While GPs reported that patients should take responsibility for managing their IBS and for minimizing its impact on their daily lives, they admitted limited awareness of the extent to which IBS affected their patients’ daily living.7
A 2013 survey-based study in England determined that GPs understand the relationship between IBS and psychological symptoms including anxiety and stress, and posited that the majority of patients could be managed within primary care without referral for psychological interventions.8 Moreover, they reported that a dedicated risk assessment tool for patients with IBS would be helpful to stratify severity of disease. The study concluded that the reluctance of GPs to refer patients for evidence-based psychological treatments may prevent them from obtaining appropriate services and care.
Newer explanatory model shines light on IBS
A newer explanation that is based on 3 main hypotheses is elucidating the true nature of IBS and providing a pragmatic model for the clinical setting (FIGURE 1).9 According to the model, IBS entails the following 3 elements, which combined lead to the symptoms of IBS:
- Altered or abnormal peripheral regulation of gut function (including sensory and secretory mechanisms)
- Altered brain-gut signaling (including visceral hypersensitivity)
- Psychological distress.
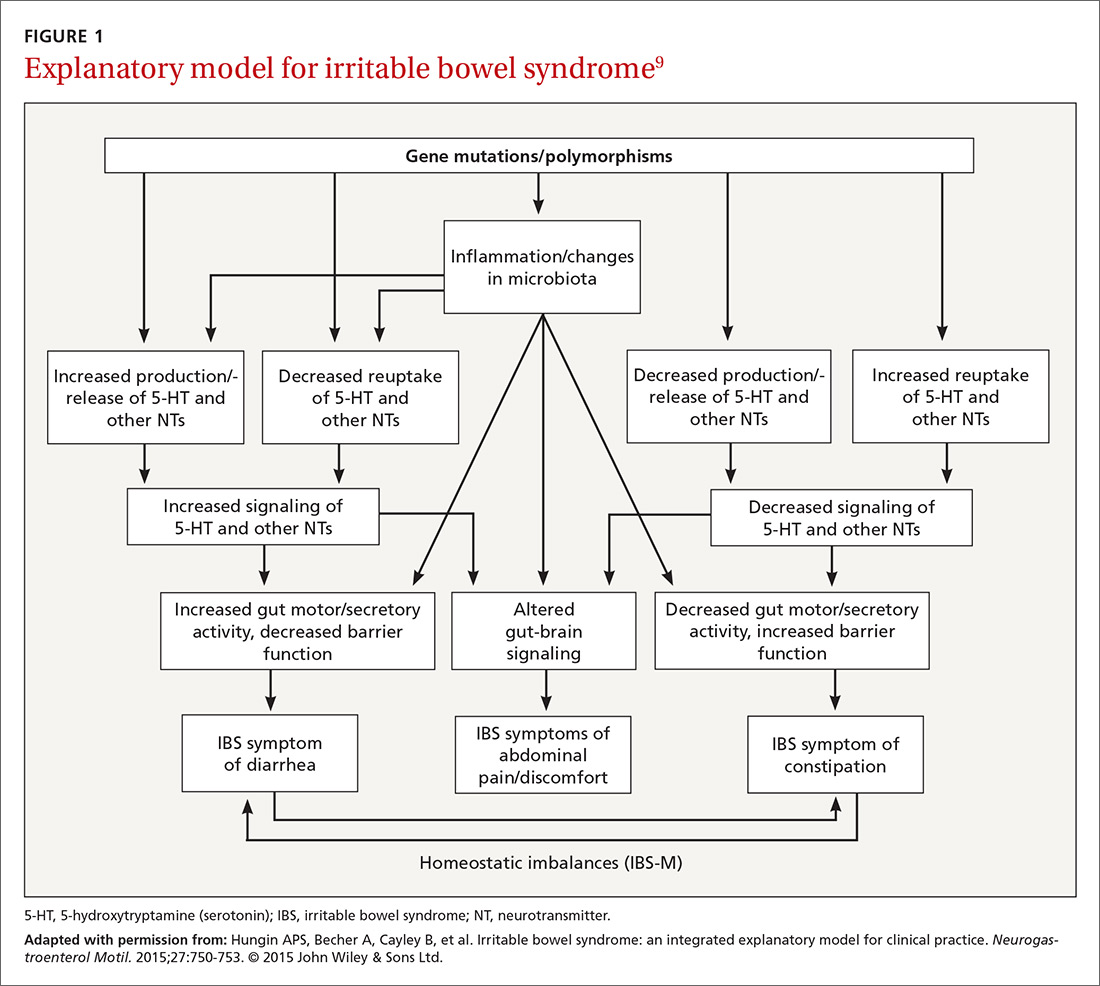
It is reasonable to consider that epigenetic changes may underlie the etiology and pathophysiology of IBS and could increase one’s susceptibility to developing the disorder. Additionally, it is presumed that IBS shares common pathophysiologic mechanisms, including visceral hypersensitivity, with other associated functional syndromes, such as functional dyspepsia.
New criteria make diagnosis on symptoms alone easier
In addition to a new explanatory model, clear criteria for diagnosing the disorder now exist, which should make it easier for PCPs to make the diagnosis without additional testing or referral. The 2016 Rome IV criteria3 provide guidelines for diagnosing the various subtypes of IBS including IBS-D (diarrhea predominant), IBS-C (constipation predominant), and IBS-M (mixed subtypes). A laboratory evaluation is really only needed for patients who fall outside the criteria or who have alarm symptoms, which include:
- age >50 years at onset of symptoms,
- new onset of constipation in the elderly,
- rectal bleeding,
- unexplained weight loss or anemia,
- family history of organic GI disease, and
- a palpable abdominal or rectal mass.
These symptoms should prompt referral to a gastroenterologist. Once alarm symptoms have been excluded, the diagnosis of IBS is based upon the presence of characteristic symptoms and changes in stool habits (FIGURE 23,10).

Patterns of migration. Over time, patients may migrate between subtypes, most commonly from IBS-C or IBS-D to IBS-M; switching between IBS-C and IBS-D occurs less commonly.11 Patients who meet criteria for IBS but whose bowel habits and symptoms cannot be grouped into any of these 3 categories are considered to have IBS unclassified. The Bristol Stool Form Scale (available at: https://www.niddk.nih.gov/health-information/health-communication-programs/bowel-control-awareness-campaign/Documents/Bristol_Stool_Form_Scale_508.pdf) should be used to gauge and track stool consistency.
A novel diagnostic test for IBS has been validated for differentiating patients with IBS-D from those with inflammatory bowel disease (IBD).12 The test focused on the beliefs that cytolethal distending toxin B (CdtB) is produced by bacteria that cause acute viral gastroenteritis (eg, norovirus, rotavirus), and that host antibodies to CdtB cross-react with the protein vinculin in the host gut, producing an “IBS-like phenotype.”
In a 2015 large-scale multicenter trial, both anti-CdtB and anti-vinculin antibodies were found to be significantly elevated in subjects with IBS-D compared to non-IBS subjects,12 providing evidence to support the long-held belief that viral gastroenteritis is often at the root of IBS.
Treatment aims to decrease symptoms and improve QOL
Treatment of IBS is directed at decreasing symptoms of abdominal pain and discomfort, bloating, diarrhea, and constipation while improving QOL. Therapeutic options for treatment of each symptom are listed in FIGURE 3
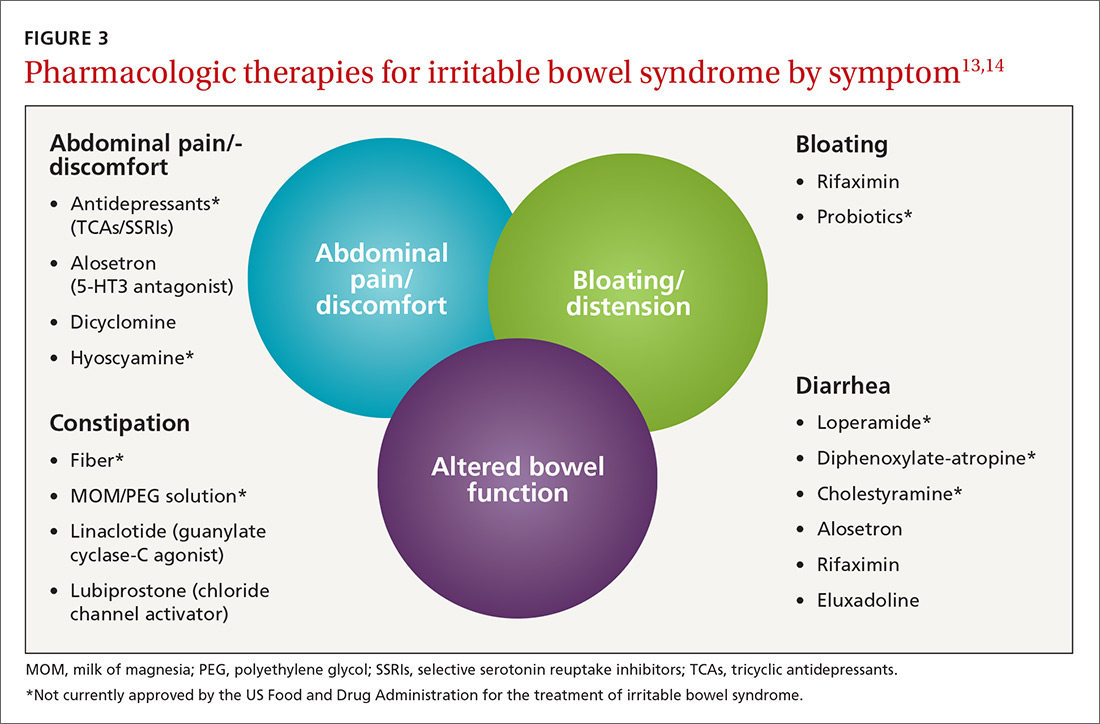
Current evidence-based pharmacologic guidelines from the American Gastroenterological Association (AGA) can be found at: https://www.guideline.gov/summaries/summary/49122?osrc=12. Figure 313,14 provides a few additional options not included in the AGA guidelines and presents the information in a simple schematic.
Pharmacologic therapies for IBS-D
Eluxadoline is a novel mixed mu opioid receptor agonist and delta opioid receptor antagonist developed for the treatment of IBS-D. It normalizes GI transit and defecation under conditions of environmental stress or post-inflammatory altered GI function.15 A 2016 study involving almost 2500 patients found that eluxadoline was significantly better than placebo at decreasing abdominal pain and improving stool consistency on the same day for at least half of a 26-week period.13 The most common adverse effects were nausea, constipation, and abdominal pain. Pancreatitis occurred rarely.
Rifaximin. Because GI flora play a central role in the pathophysiology of IBS, researchers have found that rifaximin, a minimally absorbed antibiotic, is a potentially important player in treatment. Two double-blind, placebo-controlled trials (TARGET 1 and TARGET 2) found that after 4 weeks of treatment, patients experienced significant improvement in global IBS symptoms including bloating, abdominal pain, and stool consistency on rifaximin vs placebo (40.7% vs 31.7%; P<.001 in the 2 studies combined).16 The incidence of adverse effects (headache, upper respiratory infection, nausea, abdominal pain, diarrhea, and urinary tract infection) was comparable to that with placebo.
Alosetron. Research has shown this selective 5-HT3 receptor antagonist to improve all IBS QOL measures, restriction of daily activities, and patient satisfaction significantly more than placebo in women.17 While initial use of alosetron in 2000 was widespread, the rare serious adverse event of ischemic colitis led to its withdrawal from the US market within a few months.18 Alosetron returned to the market in 2002 with restricted marketing (to treat only women with severe diarrhea-predominant IBS). (See Lotronex [alosetron hydrochloride] full prescribing information available at: https://lotronex.com/hcp/index.html.) Data from a 9-year risk management program subsequently found a cumulative incidence rate for ischemic colitis of 1.03 cases per 1000 patient/years.19
Other possible options include various antidepressants (tricyclics such as amitriptyline, imipramine, and nortriptyline; or selective serotonin reuptake inhibitors [SSRIs] such as citalopram, fluoxetine, and paroxetine) and antispasmodics such as dicyclomine and hyoscyamine.
Pharmacologic therapies for IBS-C
Linaclotide is a guanylate cyclase-C agonist with an indication for treatment of IBS-C. A double-blind, parallel-group, placebo-controlled trial found that the percentage of patients who experienced a decrease in abdominal pain was nearly 25%, with statistically significant improvements in bloating, straining, and stool consistency over a 26-week period.20 In a report on 2 phase 3 trials, researchers found that linaclotide improved global symptom scores and significantly decreased abdominal bloating and fullness, pain, cramping, and discomfort vs placebo. Diarrhea was the most commonly reported adverse event in patients with severe abdominal symptoms (18.8%-21%).21
Lubiprostone is a prostaglandin E1 analogue that activates type-2-chloride channels on the apical membrane of epithelial cells in the intestine. In a combined analysis of 2 phase 3 randomized trials, lubiprostone was administered twice daily for 12 weeks vs placebo and patients were asked to describe how they felt after the trial period. Survey responders reported significant improvements in global IBS-C symptoms (17.9% vs 10.1%; P=.001).22 A meta-analysis of studies on lubiprostone found that diarrhea, nausea, and abdominal pain were the most common adverse effects, but their occurrence was not that much greater than with placebo.23
Diet and probiotics can play a significant role
The role of dietary components in the treatment of IBS is gaining increasing attention. Such components can have a direct effect on gastric and intestinal motility, visceral sensation, immune activation, brain-gut interactions, and the microbiome. Current evidence suggests that targeted carbohydrate and gluten exclusion plays a favorable role in the treatment and symptomatic improvement of patients with IBS.24
A 2014 study conducted in Australia showed that a diet low in FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), which is characterized by avoiding foods containing gluten and those that are high in fructose, reduced overall GI symptom scores (including scores involving abdominal bloating, pain, and flatus) in patients with IBS compared to those consuming a normal Australian diet.25 The International Foundation for Functional Gastrointestinal Disorders’ Web site provides a detailed guide to low FODMAP foods and can be found at: http://www.aboutibs.org/low-fodmap-diet.html.
Probiotics are now commonly used in the symptomatic treatment of many upper and lower GI disorders. While much anecdotal evidence exists to support their benefit, there is a paucity of large-scale and rigorous research to provide substantial outcomes-based evidence. The theory for their use is that they support regulation of the gut microbiome, which in turn improves the imbalance between the intestinal microbiome and a dysfunctional intestinal barrier.
A 2014 randomized, double-blind, placebo-controlled trial involving multispecies probiotics (a mixture of Bifidobacterium longum, B. bifidum, B. lactis, Lactobacillus acidophilus, L. rhamnosus, and Streptococcus thermophilus) found that patients who received probiotics had significantly reduced symptoms of IBS after 4 weeks compared with placebo, and modest improvement in abdominal pain and discomfort as well as bloating.26 One study involving 122 patients from 2011 found that B. bifidum MIMBb75 reduced the global assessment of IBS symptoms by -88 points (95% CI, -1.07 to -0.69) when compared with only -0.16 (95% CI, -.32 to 0.00) points in the placebo group (P<.0001).27 MIMBb75 also significantly improved the IBS symptoms of pain/discomfort, distension/bloating, urgency, and digestive disorder. And one randomized, double-blind, placebo-controlled study involving 67 patients found that QOL scores improved two-fold when patients took Saccharomyces boulardii (15.4% vs 7.0%; P<.05).28
Dried plums or prunes have been used successfully for decades for the symptomatic treatment of constipation. A single-blinded, randomized, cross-over study compared prunes 50 g/d to psyllium fiber 11 g/d and found that prunes were more efficacious (P<.05) with spontaneous bowel movements and stool consistency scores.29
Peppermint oil has been studied as an alternative therapy for symptoms of IBS, but efficacy and tolerability are concerns. A meta-analysis of randomized controlled trials with a minimum duration of 2 weeks found that compared with placebo, peppermint oil provided improvement in abdominal pain, bloating, and global symptoms, but some patients reported transient heartburn.30 A 4-week, randomized, double-blind, placebo-controlled clinical trial sponsored by IM HealthScience found a novel oral formulation of triple-enteric-coated sustained-release peppermint oil microspheres caused less heartburn than was reported in the previous study, but still significantly improved abdominal symptoms and lessened pain on defecation and fecal urgency.31
CASE › Suspecting IBS-D, the FP ordered a complete blood count, tissue transglutaminase antibodies, and a stool culture, all of which were unremarkable. Ms. S has been trying to follow a low FODMAP diet and has been taking some over-the-counter probiotics with only minimal relief of abdominal bloating and cramping and no improvement in stool consistency. Her FP started her on eluxadoline 100 mg twice daily with food. After 12 weeks of therapy, she reports significant improvement in global IBS symptoms and nearly complete resolution of her diarrhea.
*Amber S is a real patient in my practice. Her name has been changed to protect her identity.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected].
1. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.
2. Ballou S, Keefer L. The impact of irritable bowel syndrome on daily functioning: characterizing and understanding daily consequences of IBS. Neurogastroenterol Motil. 2017;29. Epub 2016 Oct 25.
3. Heidelbaugh J, Hungin P, eds. ROME IV: Functional Gastrointestinal Disorders for Primary Care and Non-GI Clinicians. 1st ed. Raleigh, NC: Rome Foundation, Inc.; 2016.
4. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80.
5. Lee V, Guthrie E, Robinson A, et al. Functional bowel disorders in primary care: factors associated with health-related quality of life and doctor consultation. J Psychosom Res. 2008;64:129-138.
6. Lacy BE, Rosemore J, Robertson D, et al. Physicians’ attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Scand J Gastroenterol. 2006;41:892-902.
7. Casiday RE, Hungin AP, Cornford CS, et al. GPs’ explanatory models for irritable bowel syndrome: a mismatch with patient models? J Fam Pract. 2009;26:34-39.
8. Harkness EF, Harrington V, Hinder S, et al. GP perspectives of irritable bowel syndrome—an accepted illness, but management deviates from guidelines: a qualitative study. BMC Fam Pract. 2013;14:92.
9. Hungin AP, Becher A, Cayley B, et al. Irritable bowel syndrome: an integrated explanatory model for clinical practice. Neurogastroenterol Motil. 2015;27:750-753.
10. Lacy BE, Mearin F, Chang L, et al. Bowel Disorders. Gastroenterol. 2016;150:1393-1407.
11. Engsbro AL, Simren M, Bytzer P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment Pharmacol Ther. 2012;35:350-359.
12. Pimentel M, Morales W, Rezaie A, et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One. 2015;10:e0126438.
13. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242-253.
14. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561.
15. Fujita W, Gomes I, Dove LS, et al. Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers. Biochem Pharmacol. 2014;92:448-456.
16. Pimentel M, Lembo A, Chey WD, et al, for the TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32.
17. Cremonini F, Nicandro JP, Atkinson V, et al. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther. 2012;36:437-448.
18. Lewis JH. Alosetron for severe diarrhea-predominant irritable bowel syndrome: safety and efficacy in perspective. Expert Rev Gastroenterol Hepatol. 2010;4:13-29.
19. Tong K, Nicandro JP, Shringarpure R, et al. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Therap Adv Gastroenterol. 2013;6:344-357.
20. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702-1712.
21. Rao SS, Quigley EM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12:616-623.
22. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329-341.
23. Lacy BE, Chey WD. Lubiprostone: chronic constipation and irritable bowel syndrome with constipation. Expert Opin Pharmacother. 2009;10:143-152.
24. Spencer M, Chey WD, Eswaran S. Dietary Renaissance in IBS: has food replaced medications as a primary treatment strategy? Curr Treat Options Gastroenterol. 2014;12:424-440.
25. Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.
26. Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59.
27. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
28. Choi CH, Jo SY, Park HJ, et al. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45:679-683.
29. Attaluri A, Donahoe R, Valestin J, et al. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011;33:822-828.
30. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
31. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
CASE › Amber S,* a 33-year-old woman who works on the production line at a bread factory, sought care at my health center with a several month history of non-bloody diarrhea that was increasing in frequency and urgency and was accompanied by painful abdominal bloating and cramping. She said that these symptoms were negatively impacting her interpersonal relationships, as well as her productivity at work. She reported that “almost everything” she ate upset her stomach and “goes right through her,” including fruits, vegetables, and meat, as well as greasy fast food. She had researched her symptoms on the Internet and was worried that she might have something serious like inflammatory bowel disease or cancer.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID) that negatively impacts the quality of life (QOL) of millions of people worldwide.1 In fact, one study of 179 people with IBS found that 76% of survey respondents reported some degree of IBS-related impairment in at least 5 domains of daily life: daily activities, comorbid psychiatric diagnoses, symptom severity, QOL, and symptom-specific cognitive affective factors related to IBS.2
Estimating prevalence and incidence is a formidable challenge given various diagnostic criteria, the influence of population selection, inclusion or exclusion of non-GI comorbidities, and various cultural influences.3 That said, it’s estimated that IBS impacts approximately 11% of the world’s population, and approximately 30% of these individuals seek treatment.1,4 While there are no significant differences in GI symptoms between those who consult physicians and those who do not, those who do seek treatment report higher pain scores, greater levels of anxiety, and a greater reduction in QOL.5
All ages affected. IBS has been reported in patients of all ages, including children and the elderly, with no definable difference reported in the frequency of subtypes (diarrhea- or constipation-predominant).
This article reviews the latest explanations, diagnostic criteria, and treatment guidelines for this challenging condition so that you can offer your patients confident care without needless testing or referral.
[polldaddy:9755564]
A lack of consensus among practicing physicians
Historically, IBS has been regarded by many primary care physicians (PCPs) as a diagnosis of exclusion. Lab tests would be ordered, nothing significant would be found, and the patient would be referred to the gastroenterologist for a definitive diagnosis.
Perceptions and misconceptions about IBS continue to abound to this day. Many are neither completely right nor wrong partly because so many triggers for IBS exist and partly because of the heretofore lack of simple, standardized criteria to diagnose the condition. Other factors contributing to the confusion are that the diagnosis of IBS is purely symptom-based and that proposals of its pathophysiology have traditionally been complex.
For example, a 2006 survey-based study of PCPs and gastroenterologists found that PCPs were less likely than gastroenterologists to believe that IBS was related to prior physical or sexual abuse, previous infection, or learned behavior, but were more likely to associate dietary factors or a linkable genetic etiology with IBS.6 Both sets of beliefs, however, may be considered correct.
Similarly, a 2009 qualitative study conducted in the Netherlands found that general practitioners (GPs) considered smoking, caffeine, diet, “hasty lifestyle,” and lack of exercise as potential triggers for IBS symptoms, while PCPs in the United Kingdom considered diet, infection, and travel to be possible triggers.7 Again, all play a role.
While GPs reported that patients should take responsibility for managing their IBS and for minimizing its impact on their daily lives, they admitted limited awareness of the extent to which IBS affected their patients’ daily living.7
A 2013 survey-based study in England determined that GPs understand the relationship between IBS and psychological symptoms including anxiety and stress, and posited that the majority of patients could be managed within primary care without referral for psychological interventions.8 Moreover, they reported that a dedicated risk assessment tool for patients with IBS would be helpful to stratify severity of disease. The study concluded that the reluctance of GPs to refer patients for evidence-based psychological treatments may prevent them from obtaining appropriate services and care.
Newer explanatory model shines light on IBS
A newer explanation that is based on 3 main hypotheses is elucidating the true nature of IBS and providing a pragmatic model for the clinical setting (FIGURE 1).9 According to the model, IBS entails the following 3 elements, which combined lead to the symptoms of IBS:
- Altered or abnormal peripheral regulation of gut function (including sensory and secretory mechanisms)
- Altered brain-gut signaling (including visceral hypersensitivity)
- Psychological distress.

It is reasonable to consider that epigenetic changes may underlie the etiology and pathophysiology of IBS and could increase one’s susceptibility to developing the disorder. Additionally, it is presumed that IBS shares common pathophysiologic mechanisms, including visceral hypersensitivity, with other associated functional syndromes, such as functional dyspepsia.
New criteria make diagnosis on symptoms alone easier
In addition to a new explanatory model, clear criteria for diagnosing the disorder now exist, which should make it easier for PCPs to make the diagnosis without additional testing or referral. The 2016 Rome IV criteria3 provide guidelines for diagnosing the various subtypes of IBS including IBS-D (diarrhea predominant), IBS-C (constipation predominant), and IBS-M (mixed subtypes). A laboratory evaluation is really only needed for patients who fall outside the criteria or who have alarm symptoms, which include:
- age >50 years at onset of symptoms,
- new onset of constipation in the elderly,
- rectal bleeding,
- unexplained weight loss or anemia,
- family history of organic GI disease, and
- a palpable abdominal or rectal mass.
These symptoms should prompt referral to a gastroenterologist. Once alarm symptoms have been excluded, the diagnosis of IBS is based upon the presence of characteristic symptoms and changes in stool habits (FIGURE 23,10).

Patterns of migration. Over time, patients may migrate between subtypes, most commonly from IBS-C or IBS-D to IBS-M; switching between IBS-C and IBS-D occurs less commonly.11 Patients who meet criteria for IBS but whose bowel habits and symptoms cannot be grouped into any of these 3 categories are considered to have IBS unclassified. The Bristol Stool Form Scale (available at: https://www.niddk.nih.gov/health-information/health-communication-programs/bowel-control-awareness-campaign/Documents/Bristol_Stool_Form_Scale_508.pdf) should be used to gauge and track stool consistency.
A novel diagnostic test for IBS has been validated for differentiating patients with IBS-D from those with inflammatory bowel disease (IBD).12 The test focused on the beliefs that cytolethal distending toxin B (CdtB) is produced by bacteria that cause acute viral gastroenteritis (eg, norovirus, rotavirus), and that host antibodies to CdtB cross-react with the protein vinculin in the host gut, producing an “IBS-like phenotype.”
In a 2015 large-scale multicenter trial, both anti-CdtB and anti-vinculin antibodies were found to be significantly elevated in subjects with IBS-D compared to non-IBS subjects,12 providing evidence to support the long-held belief that viral gastroenteritis is often at the root of IBS.
Treatment aims to decrease symptoms and improve QOL
Treatment of IBS is directed at decreasing symptoms of abdominal pain and discomfort, bloating, diarrhea, and constipation while improving QOL. Therapeutic options for treatment of each symptom are listed in FIGURE 3

Current evidence-based pharmacologic guidelines from the American Gastroenterological Association (AGA) can be found at: https://www.guideline.gov/summaries/summary/49122?osrc=12. Figure 313,14 provides a few additional options not included in the AGA guidelines and presents the information in a simple schematic.
Pharmacologic therapies for IBS-D
Eluxadoline is a novel mixed mu opioid receptor agonist and delta opioid receptor antagonist developed for the treatment of IBS-D. It normalizes GI transit and defecation under conditions of environmental stress or post-inflammatory altered GI function.15 A 2016 study involving almost 2500 patients found that eluxadoline was significantly better than placebo at decreasing abdominal pain and improving stool consistency on the same day for at least half of a 26-week period.13 The most common adverse effects were nausea, constipation, and abdominal pain. Pancreatitis occurred rarely.
Rifaximin. Because GI flora play a central role in the pathophysiology of IBS, researchers have found that rifaximin, a minimally absorbed antibiotic, is a potentially important player in treatment. Two double-blind, placebo-controlled trials (TARGET 1 and TARGET 2) found that after 4 weeks of treatment, patients experienced significant improvement in global IBS symptoms including bloating, abdominal pain, and stool consistency on rifaximin vs placebo (40.7% vs 31.7%; P<.001 in the 2 studies combined).16 The incidence of adverse effects (headache, upper respiratory infection, nausea, abdominal pain, diarrhea, and urinary tract infection) was comparable to that with placebo.
Alosetron. Research has shown this selective 5-HT3 receptor antagonist to improve all IBS QOL measures, restriction of daily activities, and patient satisfaction significantly more than placebo in women.17 While initial use of alosetron in 2000 was widespread, the rare serious adverse event of ischemic colitis led to its withdrawal from the US market within a few months.18 Alosetron returned to the market in 2002 with restricted marketing (to treat only women with severe diarrhea-predominant IBS). (See Lotronex [alosetron hydrochloride] full prescribing information available at: https://lotronex.com/hcp/index.html.) Data from a 9-year risk management program subsequently found a cumulative incidence rate for ischemic colitis of 1.03 cases per 1000 patient/years.19
Other possible options include various antidepressants (tricyclics such as amitriptyline, imipramine, and nortriptyline; or selective serotonin reuptake inhibitors [SSRIs] such as citalopram, fluoxetine, and paroxetine) and antispasmodics such as dicyclomine and hyoscyamine.
Pharmacologic therapies for IBS-C
Linaclotide is a guanylate cyclase-C agonist with an indication for treatment of IBS-C. A double-blind, parallel-group, placebo-controlled trial found that the percentage of patients who experienced a decrease in abdominal pain was nearly 25%, with statistically significant improvements in bloating, straining, and stool consistency over a 26-week period.20 In a report on 2 phase 3 trials, researchers found that linaclotide improved global symptom scores and significantly decreased abdominal bloating and fullness, pain, cramping, and discomfort vs placebo. Diarrhea was the most commonly reported adverse event in patients with severe abdominal symptoms (18.8%-21%).21
Lubiprostone is a prostaglandin E1 analogue that activates type-2-chloride channels on the apical membrane of epithelial cells in the intestine. In a combined analysis of 2 phase 3 randomized trials, lubiprostone was administered twice daily for 12 weeks vs placebo and patients were asked to describe how they felt after the trial period. Survey responders reported significant improvements in global IBS-C symptoms (17.9% vs 10.1%; P=.001).22 A meta-analysis of studies on lubiprostone found that diarrhea, nausea, and abdominal pain were the most common adverse effects, but their occurrence was not that much greater than with placebo.23
Diet and probiotics can play a significant role
The role of dietary components in the treatment of IBS is gaining increasing attention. Such components can have a direct effect on gastric and intestinal motility, visceral sensation, immune activation, brain-gut interactions, and the microbiome. Current evidence suggests that targeted carbohydrate and gluten exclusion plays a favorable role in the treatment and symptomatic improvement of patients with IBS.24
A 2014 study conducted in Australia showed that a diet low in FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), which is characterized by avoiding foods containing gluten and those that are high in fructose, reduced overall GI symptom scores (including scores involving abdominal bloating, pain, and flatus) in patients with IBS compared to those consuming a normal Australian diet.25 The International Foundation for Functional Gastrointestinal Disorders’ Web site provides a detailed guide to low FODMAP foods and can be found at: http://www.aboutibs.org/low-fodmap-diet.html.
Probiotics are now commonly used in the symptomatic treatment of many upper and lower GI disorders. While much anecdotal evidence exists to support their benefit, there is a paucity of large-scale and rigorous research to provide substantial outcomes-based evidence. The theory for their use is that they support regulation of the gut microbiome, which in turn improves the imbalance between the intestinal microbiome and a dysfunctional intestinal barrier.
A 2014 randomized, double-blind, placebo-controlled trial involving multispecies probiotics (a mixture of Bifidobacterium longum, B. bifidum, B. lactis, Lactobacillus acidophilus, L. rhamnosus, and Streptococcus thermophilus) found that patients who received probiotics had significantly reduced symptoms of IBS after 4 weeks compared with placebo, and modest improvement in abdominal pain and discomfort as well as bloating.26 One study involving 122 patients from 2011 found that B. bifidum MIMBb75 reduced the global assessment of IBS symptoms by -88 points (95% CI, -1.07 to -0.69) when compared with only -0.16 (95% CI, -.32 to 0.00) points in the placebo group (P<.0001).27 MIMBb75 also significantly improved the IBS symptoms of pain/discomfort, distension/bloating, urgency, and digestive disorder. And one randomized, double-blind, placebo-controlled study involving 67 patients found that QOL scores improved two-fold when patients took Saccharomyces boulardii (15.4% vs 7.0%; P<.05).28
Dried plums or prunes have been used successfully for decades for the symptomatic treatment of constipation. A single-blinded, randomized, cross-over study compared prunes 50 g/d to psyllium fiber 11 g/d and found that prunes were more efficacious (P<.05) with spontaneous bowel movements and stool consistency scores.29
Peppermint oil has been studied as an alternative therapy for symptoms of IBS, but efficacy and tolerability are concerns. A meta-analysis of randomized controlled trials with a minimum duration of 2 weeks found that compared with placebo, peppermint oil provided improvement in abdominal pain, bloating, and global symptoms, but some patients reported transient heartburn.30 A 4-week, randomized, double-blind, placebo-controlled clinical trial sponsored by IM HealthScience found a novel oral formulation of triple-enteric-coated sustained-release peppermint oil microspheres caused less heartburn than was reported in the previous study, but still significantly improved abdominal symptoms and lessened pain on defecation and fecal urgency.31
CASE › Suspecting IBS-D, the FP ordered a complete blood count, tissue transglutaminase antibodies, and a stool culture, all of which were unremarkable. Ms. S has been trying to follow a low FODMAP diet and has been taking some over-the-counter probiotics with only minimal relief of abdominal bloating and cramping and no improvement in stool consistency. Her FP started her on eluxadoline 100 mg twice daily with food. After 12 weeks of therapy, she reports significant improvement in global IBS symptoms and nearly complete resolution of her diarrhea.
*Amber S is a real patient in my practice. Her name has been changed to protect her identity.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected].
CASE › Amber S,* a 33-year-old woman who works on the production line at a bread factory, sought care at my health center with a several month history of non-bloody diarrhea that was increasing in frequency and urgency and was accompanied by painful abdominal bloating and cramping. She said that these symptoms were negatively impacting her interpersonal relationships, as well as her productivity at work. She reported that “almost everything” she ate upset her stomach and “goes right through her,” including fruits, vegetables, and meat, as well as greasy fast food. She had researched her symptoms on the Internet and was worried that she might have something serious like inflammatory bowel disease or cancer.
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID) that negatively impacts the quality of life (QOL) of millions of people worldwide.1 In fact, one study of 179 people with IBS found that 76% of survey respondents reported some degree of IBS-related impairment in at least 5 domains of daily life: daily activities, comorbid psychiatric diagnoses, symptom severity, QOL, and symptom-specific cognitive affective factors related to IBS.2
Estimating prevalence and incidence is a formidable challenge given various diagnostic criteria, the influence of population selection, inclusion or exclusion of non-GI comorbidities, and various cultural influences.3 That said, it’s estimated that IBS impacts approximately 11% of the world’s population, and approximately 30% of these individuals seek treatment.1,4 While there are no significant differences in GI symptoms between those who consult physicians and those who do not, those who do seek treatment report higher pain scores, greater levels of anxiety, and a greater reduction in QOL.5
All ages affected. IBS has been reported in patients of all ages, including children and the elderly, with no definable difference reported in the frequency of subtypes (diarrhea- or constipation-predominant).
This article reviews the latest explanations, diagnostic criteria, and treatment guidelines for this challenging condition so that you can offer your patients confident care without needless testing or referral.
[polldaddy:9755564]
A lack of consensus among practicing physicians
Historically, IBS has been regarded by many primary care physicians (PCPs) as a diagnosis of exclusion. Lab tests would be ordered, nothing significant would be found, and the patient would be referred to the gastroenterologist for a definitive diagnosis.
Perceptions and misconceptions about IBS continue to abound to this day. Many are neither completely right nor wrong partly because so many triggers for IBS exist and partly because of the heretofore lack of simple, standardized criteria to diagnose the condition. Other factors contributing to the confusion are that the diagnosis of IBS is purely symptom-based and that proposals of its pathophysiology have traditionally been complex.
For example, a 2006 survey-based study of PCPs and gastroenterologists found that PCPs were less likely than gastroenterologists to believe that IBS was related to prior physical or sexual abuse, previous infection, or learned behavior, but were more likely to associate dietary factors or a linkable genetic etiology with IBS.6 Both sets of beliefs, however, may be considered correct.
Similarly, a 2009 qualitative study conducted in the Netherlands found that general practitioners (GPs) considered smoking, caffeine, diet, “hasty lifestyle,” and lack of exercise as potential triggers for IBS symptoms, while PCPs in the United Kingdom considered diet, infection, and travel to be possible triggers.7 Again, all play a role.
While GPs reported that patients should take responsibility for managing their IBS and for minimizing its impact on their daily lives, they admitted limited awareness of the extent to which IBS affected their patients’ daily living.7
A 2013 survey-based study in England determined that GPs understand the relationship between IBS and psychological symptoms including anxiety and stress, and posited that the majority of patients could be managed within primary care without referral for psychological interventions.8 Moreover, they reported that a dedicated risk assessment tool for patients with IBS would be helpful to stratify severity of disease. The study concluded that the reluctance of GPs to refer patients for evidence-based psychological treatments may prevent them from obtaining appropriate services and care.
Newer explanatory model shines light on IBS
A newer explanation that is based on 3 main hypotheses is elucidating the true nature of IBS and providing a pragmatic model for the clinical setting (FIGURE 1).9 According to the model, IBS entails the following 3 elements, which combined lead to the symptoms of IBS:
- Altered or abnormal peripheral regulation of gut function (including sensory and secretory mechanisms)
- Altered brain-gut signaling (including visceral hypersensitivity)
- Psychological distress.

It is reasonable to consider that epigenetic changes may underlie the etiology and pathophysiology of IBS and could increase one’s susceptibility to developing the disorder. Additionally, it is presumed that IBS shares common pathophysiologic mechanisms, including visceral hypersensitivity, with other associated functional syndromes, such as functional dyspepsia.
New criteria make diagnosis on symptoms alone easier
In addition to a new explanatory model, clear criteria for diagnosing the disorder now exist, which should make it easier for PCPs to make the diagnosis without additional testing or referral. The 2016 Rome IV criteria3 provide guidelines for diagnosing the various subtypes of IBS including IBS-D (diarrhea predominant), IBS-C (constipation predominant), and IBS-M (mixed subtypes). A laboratory evaluation is really only needed for patients who fall outside the criteria or who have alarm symptoms, which include:
- age >50 years at onset of symptoms,
- new onset of constipation in the elderly,
- rectal bleeding,
- unexplained weight loss or anemia,
- family history of organic GI disease, and
- a palpable abdominal or rectal mass.
These symptoms should prompt referral to a gastroenterologist. Once alarm symptoms have been excluded, the diagnosis of IBS is based upon the presence of characteristic symptoms and changes in stool habits (FIGURE 23,10).

Patterns of migration. Over time, patients may migrate between subtypes, most commonly from IBS-C or IBS-D to IBS-M; switching between IBS-C and IBS-D occurs less commonly.11 Patients who meet criteria for IBS but whose bowel habits and symptoms cannot be grouped into any of these 3 categories are considered to have IBS unclassified. The Bristol Stool Form Scale (available at: https://www.niddk.nih.gov/health-information/health-communication-programs/bowel-control-awareness-campaign/Documents/Bristol_Stool_Form_Scale_508.pdf) should be used to gauge and track stool consistency.
A novel diagnostic test for IBS has been validated for differentiating patients with IBS-D from those with inflammatory bowel disease (IBD).12 The test focused on the beliefs that cytolethal distending toxin B (CdtB) is produced by bacteria that cause acute viral gastroenteritis (eg, norovirus, rotavirus), and that host antibodies to CdtB cross-react with the protein vinculin in the host gut, producing an “IBS-like phenotype.”
In a 2015 large-scale multicenter trial, both anti-CdtB and anti-vinculin antibodies were found to be significantly elevated in subjects with IBS-D compared to non-IBS subjects,12 providing evidence to support the long-held belief that viral gastroenteritis is often at the root of IBS.
Treatment aims to decrease symptoms and improve QOL
Treatment of IBS is directed at decreasing symptoms of abdominal pain and discomfort, bloating, diarrhea, and constipation while improving QOL. Therapeutic options for treatment of each symptom are listed in FIGURE 3

Current evidence-based pharmacologic guidelines from the American Gastroenterological Association (AGA) can be found at: https://www.guideline.gov/summaries/summary/49122?osrc=12. Figure 313,14 provides a few additional options not included in the AGA guidelines and presents the information in a simple schematic.
Pharmacologic therapies for IBS-D
Eluxadoline is a novel mixed mu opioid receptor agonist and delta opioid receptor antagonist developed for the treatment of IBS-D. It normalizes GI transit and defecation under conditions of environmental stress or post-inflammatory altered GI function.15 A 2016 study involving almost 2500 patients found that eluxadoline was significantly better than placebo at decreasing abdominal pain and improving stool consistency on the same day for at least half of a 26-week period.13 The most common adverse effects were nausea, constipation, and abdominal pain. Pancreatitis occurred rarely.
Rifaximin. Because GI flora play a central role in the pathophysiology of IBS, researchers have found that rifaximin, a minimally absorbed antibiotic, is a potentially important player in treatment. Two double-blind, placebo-controlled trials (TARGET 1 and TARGET 2) found that after 4 weeks of treatment, patients experienced significant improvement in global IBS symptoms including bloating, abdominal pain, and stool consistency on rifaximin vs placebo (40.7% vs 31.7%; P<.001 in the 2 studies combined).16 The incidence of adverse effects (headache, upper respiratory infection, nausea, abdominal pain, diarrhea, and urinary tract infection) was comparable to that with placebo.
Alosetron. Research has shown this selective 5-HT3 receptor antagonist to improve all IBS QOL measures, restriction of daily activities, and patient satisfaction significantly more than placebo in women.17 While initial use of alosetron in 2000 was widespread, the rare serious adverse event of ischemic colitis led to its withdrawal from the US market within a few months.18 Alosetron returned to the market in 2002 with restricted marketing (to treat only women with severe diarrhea-predominant IBS). (See Lotronex [alosetron hydrochloride] full prescribing information available at: https://lotronex.com/hcp/index.html.) Data from a 9-year risk management program subsequently found a cumulative incidence rate for ischemic colitis of 1.03 cases per 1000 patient/years.19
Other possible options include various antidepressants (tricyclics such as amitriptyline, imipramine, and nortriptyline; or selective serotonin reuptake inhibitors [SSRIs] such as citalopram, fluoxetine, and paroxetine) and antispasmodics such as dicyclomine and hyoscyamine.
Pharmacologic therapies for IBS-C
Linaclotide is a guanylate cyclase-C agonist with an indication for treatment of IBS-C. A double-blind, parallel-group, placebo-controlled trial found that the percentage of patients who experienced a decrease in abdominal pain was nearly 25%, with statistically significant improvements in bloating, straining, and stool consistency over a 26-week period.20 In a report on 2 phase 3 trials, researchers found that linaclotide improved global symptom scores and significantly decreased abdominal bloating and fullness, pain, cramping, and discomfort vs placebo. Diarrhea was the most commonly reported adverse event in patients with severe abdominal symptoms (18.8%-21%).21
Lubiprostone is a prostaglandin E1 analogue that activates type-2-chloride channels on the apical membrane of epithelial cells in the intestine. In a combined analysis of 2 phase 3 randomized trials, lubiprostone was administered twice daily for 12 weeks vs placebo and patients were asked to describe how they felt after the trial period. Survey responders reported significant improvements in global IBS-C symptoms (17.9% vs 10.1%; P=.001).22 A meta-analysis of studies on lubiprostone found that diarrhea, nausea, and abdominal pain were the most common adverse effects, but their occurrence was not that much greater than with placebo.23
Diet and probiotics can play a significant role
The role of dietary components in the treatment of IBS is gaining increasing attention. Such components can have a direct effect on gastric and intestinal motility, visceral sensation, immune activation, brain-gut interactions, and the microbiome. Current evidence suggests that targeted carbohydrate and gluten exclusion plays a favorable role in the treatment and symptomatic improvement of patients with IBS.24
A 2014 study conducted in Australia showed that a diet low in FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), which is characterized by avoiding foods containing gluten and those that are high in fructose, reduced overall GI symptom scores (including scores involving abdominal bloating, pain, and flatus) in patients with IBS compared to those consuming a normal Australian diet.25 The International Foundation for Functional Gastrointestinal Disorders’ Web site provides a detailed guide to low FODMAP foods and can be found at: http://www.aboutibs.org/low-fodmap-diet.html.
Probiotics are now commonly used in the symptomatic treatment of many upper and lower GI disorders. While much anecdotal evidence exists to support their benefit, there is a paucity of large-scale and rigorous research to provide substantial outcomes-based evidence. The theory for their use is that they support regulation of the gut microbiome, which in turn improves the imbalance between the intestinal microbiome and a dysfunctional intestinal barrier.
A 2014 randomized, double-blind, placebo-controlled trial involving multispecies probiotics (a mixture of Bifidobacterium longum, B. bifidum, B. lactis, Lactobacillus acidophilus, L. rhamnosus, and Streptococcus thermophilus) found that patients who received probiotics had significantly reduced symptoms of IBS after 4 weeks compared with placebo, and modest improvement in abdominal pain and discomfort as well as bloating.26 One study involving 122 patients from 2011 found that B. bifidum MIMBb75 reduced the global assessment of IBS symptoms by -88 points (95% CI, -1.07 to -0.69) when compared with only -0.16 (95% CI, -.32 to 0.00) points in the placebo group (P<.0001).27 MIMBb75 also significantly improved the IBS symptoms of pain/discomfort, distension/bloating, urgency, and digestive disorder. And one randomized, double-blind, placebo-controlled study involving 67 patients found that QOL scores improved two-fold when patients took Saccharomyces boulardii (15.4% vs 7.0%; P<.05).28
Dried plums or prunes have been used successfully for decades for the symptomatic treatment of constipation. A single-blinded, randomized, cross-over study compared prunes 50 g/d to psyllium fiber 11 g/d and found that prunes were more efficacious (P<.05) with spontaneous bowel movements and stool consistency scores.29
Peppermint oil has been studied as an alternative therapy for symptoms of IBS, but efficacy and tolerability are concerns. A meta-analysis of randomized controlled trials with a minimum duration of 2 weeks found that compared with placebo, peppermint oil provided improvement in abdominal pain, bloating, and global symptoms, but some patients reported transient heartburn.30 A 4-week, randomized, double-blind, placebo-controlled clinical trial sponsored by IM HealthScience found a novel oral formulation of triple-enteric-coated sustained-release peppermint oil microspheres caused less heartburn than was reported in the previous study, but still significantly improved abdominal symptoms and lessened pain on defecation and fecal urgency.31
CASE › Suspecting IBS-D, the FP ordered a complete blood count, tissue transglutaminase antibodies, and a stool culture, all of which were unremarkable. Ms. S has been trying to follow a low FODMAP diet and has been taking some over-the-counter probiotics with only minimal relief of abdominal bloating and cramping and no improvement in stool consistency. Her FP started her on eluxadoline 100 mg twice daily with food. After 12 weeks of therapy, she reports significant improvement in global IBS symptoms and nearly complete resolution of her diarrhea.
*Amber S is a real patient in my practice. Her name has been changed to protect her identity.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected].
1. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.
2. Ballou S, Keefer L. The impact of irritable bowel syndrome on daily functioning: characterizing and understanding daily consequences of IBS. Neurogastroenterol Motil. 2017;29. Epub 2016 Oct 25.
3. Heidelbaugh J, Hungin P, eds. ROME IV: Functional Gastrointestinal Disorders for Primary Care and Non-GI Clinicians. 1st ed. Raleigh, NC: Rome Foundation, Inc.; 2016.
4. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80.
5. Lee V, Guthrie E, Robinson A, et al. Functional bowel disorders in primary care: factors associated with health-related quality of life and doctor consultation. J Psychosom Res. 2008;64:129-138.
6. Lacy BE, Rosemore J, Robertson D, et al. Physicians’ attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Scand J Gastroenterol. 2006;41:892-902.
7. Casiday RE, Hungin AP, Cornford CS, et al. GPs’ explanatory models for irritable bowel syndrome: a mismatch with patient models? J Fam Pract. 2009;26:34-39.
8. Harkness EF, Harrington V, Hinder S, et al. GP perspectives of irritable bowel syndrome—an accepted illness, but management deviates from guidelines: a qualitative study. BMC Fam Pract. 2013;14:92.
9. Hungin AP, Becher A, Cayley B, et al. Irritable bowel syndrome: an integrated explanatory model for clinical practice. Neurogastroenterol Motil. 2015;27:750-753.
10. Lacy BE, Mearin F, Chang L, et al. Bowel Disorders. Gastroenterol. 2016;150:1393-1407.
11. Engsbro AL, Simren M, Bytzer P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment Pharmacol Ther. 2012;35:350-359.
12. Pimentel M, Morales W, Rezaie A, et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One. 2015;10:e0126438.
13. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242-253.
14. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561.
15. Fujita W, Gomes I, Dove LS, et al. Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers. Biochem Pharmacol. 2014;92:448-456.
16. Pimentel M, Lembo A, Chey WD, et al, for the TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32.
17. Cremonini F, Nicandro JP, Atkinson V, et al. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther. 2012;36:437-448.
18. Lewis JH. Alosetron for severe diarrhea-predominant irritable bowel syndrome: safety and efficacy in perspective. Expert Rev Gastroenterol Hepatol. 2010;4:13-29.
19. Tong K, Nicandro JP, Shringarpure R, et al. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Therap Adv Gastroenterol. 2013;6:344-357.
20. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702-1712.
21. Rao SS, Quigley EM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12:616-623.
22. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329-341.
23. Lacy BE, Chey WD. Lubiprostone: chronic constipation and irritable bowel syndrome with constipation. Expert Opin Pharmacother. 2009;10:143-152.
24. Spencer M, Chey WD, Eswaran S. Dietary Renaissance in IBS: has food replaced medications as a primary treatment strategy? Curr Treat Options Gastroenterol. 2014;12:424-440.
25. Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.
26. Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59.
27. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
28. Choi CH, Jo SY, Park HJ, et al. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45:679-683.
29. Attaluri A, Donahoe R, Valestin J, et al. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011;33:822-828.
30. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
31. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
1. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.
2. Ballou S, Keefer L. The impact of irritable bowel syndrome on daily functioning: characterizing and understanding daily consequences of IBS. Neurogastroenterol Motil. 2017;29. Epub 2016 Oct 25.
3. Heidelbaugh J, Hungin P, eds. ROME IV: Functional Gastrointestinal Disorders for Primary Care and Non-GI Clinicians. 1st ed. Raleigh, NC: Rome Foundation, Inc.; 2016.
4. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80.
5. Lee V, Guthrie E, Robinson A, et al. Functional bowel disorders in primary care: factors associated with health-related quality of life and doctor consultation. J Psychosom Res. 2008;64:129-138.
6. Lacy BE, Rosemore J, Robertson D, et al. Physicians’ attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Scand J Gastroenterol. 2006;41:892-902.
7. Casiday RE, Hungin AP, Cornford CS, et al. GPs’ explanatory models for irritable bowel syndrome: a mismatch with patient models? J Fam Pract. 2009;26:34-39.
8. Harkness EF, Harrington V, Hinder S, et al. GP perspectives of irritable bowel syndrome—an accepted illness, but management deviates from guidelines: a qualitative study. BMC Fam Pract. 2013;14:92.
9. Hungin AP, Becher A, Cayley B, et al. Irritable bowel syndrome: an integrated explanatory model for clinical practice. Neurogastroenterol Motil. 2015;27:750-753.
10. Lacy BE, Mearin F, Chang L, et al. Bowel Disorders. Gastroenterol. 2016;150:1393-1407.
11. Engsbro AL, Simren M, Bytzer P. Short-term stability of subtypes in the irritable bowel syndrome: prospective evaluation using the Rome III classification. Aliment Pharmacol Ther. 2012;35:350-359.
12. Pimentel M, Morales W, Rezaie A, et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One. 2015;10:e0126438.
13. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242-253.
14. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-1561.
15. Fujita W, Gomes I, Dove LS, et al. Molecular characterization of eluxadoline as a potential ligand targeting mu-delta opioid receptor heteromers. Biochem Pharmacol. 2014;92:448-456.
16. Pimentel M, Lembo A, Chey WD, et al, for the TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32.
17. Cremonini F, Nicandro JP, Atkinson V, et al. Randomised clinical trial: alosetron improves quality of life and reduces restriction of daily activities in women with severe diarrhoea-predominant IBS. Aliment Pharmacol Ther. 2012;36:437-448.
18. Lewis JH. Alosetron for severe diarrhea-predominant irritable bowel syndrome: safety and efficacy in perspective. Expert Rev Gastroenterol Hepatol. 2010;4:13-29.
19. Tong K, Nicandro JP, Shringarpure R, et al. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Therap Adv Gastroenterol. 2013;6:344-357.
20. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702-1712.
21. Rao SS, Quigley EM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12:616-623.
22. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329-341.
23. Lacy BE, Chey WD. Lubiprostone: chronic constipation and irritable bowel syndrome with constipation. Expert Opin Pharmacother. 2009;10:143-152.
24. Spencer M, Chey WD, Eswaran S. Dietary Renaissance in IBS: has food replaced medications as a primary treatment strategy? Curr Treat Options Gastroenterol. 2014;12:424-440.
25. Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.
26. Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59.
27. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
28. Choi CH, Jo SY, Park HJ, et al. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45:679-683.
29. Attaluri A, Donahoe R, Valestin J, et al. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011;33:822-828.
30. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
31. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
PRACTICE RECOMMENDATIONS
› Prescribe eluxadoline, rifaximin, or alosetron for diarrhea-predominant IBS because all 3 have proven efficacy with this diagnosis. A
› Prescribe linaclotide or lubiprostone for constipation-predominant IBS, as both have proven efficacy with this condition. A
› Suggest that patients with IBS follow a low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet; probiotics, prunes, and peppermint oil may also offer some improvement of IBS symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The perils of prescribing fluoroquinolones
• Evaluate liver function before initiating fluoroquinolone (FQ) therapy, and avoid prescribing these antibiotics for patients at increased risk for hepatotoxicity. C
• Avoid prescribing FQs for patients with a history of prolonged QT syndrome. C
• Closely monitor older patients being treated with FQs, particularly if they have atherosclerosis or epilepsy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
PATIENT HANDOUT
Taking a fluoroquinolone antibiotic?
CASE Sara Z, a 62-year-old patient with a history of chronic urinary tract infections, presents with a 3-day history of dysuria and urinary frequency. Her last 2 urine cultures found Escherichia coli resistant to trimethoprim-sulfamethoxazole, amoxicillin, and cephalosporins. So her family physician ordered a urine culture and prescribed a 7-day course of ciprofloxacin empirically.
Five days later, Ms. Z returned, suffering from nonbloody diarrhea and bilateral Achilles tendon pain.
If you were treating Ms. Z, what would your next step be?
Widely used to treat urinary tract, skin, and pulmonary infections and to fight infections resistant to other antibiotics, fluoroquinolones (FQ) are generally regarded as safe in both inpatient and outpatient settings. Yet these broad-spectrum antibiotics are associated with both common and rare adverse effects, as well as a number of drug-drug interactions.
The Centers for Disease Control and Prevention estimates that adverse events from FQs leading to emergency department (ED) visits occur at a rate of 9.2 for every 10,000 prescriptions. That’s higher than the ED rates for cephalosporins (6.1 per 10,000) and macrolides (5.1 per 10,000), but far lower than for penicillins (13 per 10,000), clindamycin (18.5 per 10,000), sulfonamides (18.9 per 10,000), and vancomycin (24.1 per 10,000).1
In fact, adverse events associated with FQs range from mild and self-limiting to rare and severe. This review discusses both. Relatively common adverse effects and drug-drug interactions are discussed in the text, while the TABLE2 includes a broader range of potential adverse effects. You’ll also find a handout for patients taking FQs on page 195 that clearly describes signs and symptoms that need to be reported right away.
TABLE
Fluoroquinolones: Adverse effects to guard against*2
Cardiovascular
| Immunologic
|
Dermatologic
| Musculoskeletal
|
Drug-drug interactions
| Neurologic
|
Endocrine/Metabolic
| Ocular
|
Gastrointestinal
| Psychiatric
|
Hematologic
| Respiratory
|
| *This is not a complete list of potential adverse effects associated with fluoroquinolones. †Fluoroquinolones may potentiate warfarin. | |
A black box warning of tendinopathies
FQs exhibit an affinity for connective tissue, with higher concentrations found in bone and cartilage than in serum. While FQs are therefore well suited for treating orthopedic-related infections,3 they also increase the risk of tendinopathies.
In the last 2 decades, numerous case reports linking tendinitis and FQs have been published.4-6 In 2008, the US Food and Drug Administration (FDA) issued a black box warning of tendinitis and tendon rupture. Patients on FQ therapy should be advised to stop taking the antibiotic at the first sign of pain, swelling, or inflammation in a tendon, the FDA advises.7
How common is this adverse effect? A case-control study of 22,194 patients with a diagnosis of nontraumatic tendiopathy determined that FQ use resulted in a 1.3-fold risk of tendon rupture and more than a 4-fold risk of rupture of the Achilles tendon. One Achilles tendon rupture would occur for every 5958 patients treated with FQs, the researchers estimated.8
The precise mechanism by which FQs lead to tendinopathies is not completely understood. Studies suggest that the antibiotics cause a decrease in the synthesis of type I collagen, elastin, fibronectin, and beta (1)-integrin, and time- and concentration-dependent increases of cellular apoptosis.9 In vitro studies have shown inhibition of both cell proliferation and fibroblast metabolism when tissue is exposed to FQs, which may impede tissue healing.10
Which patients are at higher risk? The risk of FQ-associated tendinopathies is greatest in patients older than 60 years; in kidney, heart, and lung transplant recipients; and in patients taking an FQ with concomitant corticosteroid therapy. Decreased renal clearance of the medication may play a role in the increased risk.11
GI problems are common, especially in kids and older patients
Gastrointestinal (GI) disturbances are common in patients taking FQs, and typically occur more frequently in children and older adults, and in those taking higher doses. Reactions attributable to ciprofloxacin, for example, include nausea (affecting 1.4%-4% of adults and 2.7% of children taking the drug), vomiting (1%-2% of adults and 4.8% of children), diarrhea (<1%-2% of adults and 4.8% of children), and abdominal pain or discomfort (<1%-1.7% of adults and 3.3% of children).12
C difficile and FQ resistance. The extent to which Clostridium difficile-associated diarrhea (CDAD) is attributable to FQs has been subject to controversy in recent years. A previously uncommon strain of C difficile (B1/NAP1) with variations that have become more resistant to FQs has been linked to an increased incidence of CDAD across both the United States and Europe.13 A systematic review suggested that FQs predispose patients to CDAD,14 while a retrospective case-control study of 174 adult inpatients with CDAD determined that FQ administration did not significantly increase the rate of complications from C difficile (odds ratio [OR]=1.37; 95% confidence interval [CI], 0.72-2.61).15
Factors that affect risk of hepatotoxicity
Hepatitis/transaminitis, pancreatitis, jaundice, liver injury, and hepatic failure have all been reported in patients taking FQs, with the extent of hepatotoxicity varying based on the particular FQ taken, the dosage, and the patient’s baseline hepatic function.16,17 Comorbidities, including renal failure, may increase the potential for FQ-associated hepatotoxicity, as well. Thus, some experts recommend that clinicians evaluate a patient’s liver function before initiating FQ therapy and avoid prescribing FQs for those at added risk.
The exact mechanism by which FQ-induced hepatotoxicity occurs is unknown. One theory posits that the drugs generate oxidative radicals involved in mitochondrial damage, RNA processing, transcription, and inflammation;18 another suggests that FQs generate oxidative radicals in the liver as a result of cytochrome P450 metabolism.16 Case reports have shown that hepatitis resolves when the drug is discontinued, but often recurs in patients who are switched to a different FQ.16,17
Torsades de pointes is the key cardiovascular risk
FQs prolong the QT interval by blocking voltage-gated potassium channels, causing a reduction of the rapid component of the delayed rectifier potassium current in a dose-dependent fashion.19 But the average QT interval prolongation caused by FQs over a 3- to 6-month period does not appear to have clinical significance, nor is it associated with any discernible cardiac symptoms or impairment.19
For most, risk is minimal. There appears to be considerable variation in QT interval prolongation among FQs. A retrospective database analysis of published case reports of patients who received FQs over a 15-year period found 25 cases of torsades de pointes; moxifloxacin (highest), levofloxacin, and gatifloxacin (which was taken off the market by the FDA in 2006)20 were associated with a higher incidence than ciprofloxacin.21 Ciprofloxacin appears to be the safest FQ for cardiovascular events, with the lowest reported risk of torsades de pointes.22 However, several small randomized controlled trials have found that levofloxacin, like ciprofloxacin, did not significantly affect the QT interval.23,24
These patients face a higher risk. Notably, individuals with abnormal baseline QT prolongation (>440 ms in men; >460 ms in women) are at increased risk of developing torsades de pointes from the use of FQs, regardless of the dose.19 In fact, anyone with a history of prolonged QT syndrome should avoid these antibiotics, particularly if he or she is taking class Ia (eg, procainamide, quinidine) or class III (eg, amiodarone, sotalol) antiarrhythmics.19 Patients taking warfarin may be candidates for FQ therapy, but because the antibiotics may potentiate the anticoagulant, close monitoring is required. (Other potential drug-drug interactions are detailed in the TABLE.)
Evaluation of risk vs benefit is imperative prior to prescribing FQs for patients with increased risk for adverse cardiovascular events. An electrocardiogram is advisable, as well.
Mild neurologic and psychiatric effects not uncommon
Studies examining central nervous system (CNS) effects have estimated that neurotoxicity occurs in approximately 1% to 4.4% of patients taking FQs, with serious adverse effects occurring less than 0.5% of the time.25 Common—and milder—CNS effects include headache, dizziness, and insomnia. More severe CNS effects include tremors, restlessness, anxiety, light-headedness, confusion, hallucinations, paranoia, depression, nightmares, insomnia, and suicidal thoughts or attempts.25,26 Case reports have documented FQ-induced psychosis, catatonia, seizures, and delirium, with a higher incidence associated with higher doses of the antibiotic.26
A literature review aimed at identifying case reports yielded reports of 232 adverse psychiatric and neurologic drug reactions attributable to FQs in 145 patients.27 Nearly half were related to ciprofloxacin, with psychiatric reactions such as mania and acute psychosis being the most common. Most adverse CNS events (eg, convulsion, confusional state, agitation) developed rather quickly—in some cases within a few minutes of FQ administration and in others, within the first one to 8 days. In most reported cases, the patients had no known underlying psychiatric diseases or concomitant medication likely to have precipitated the development of delirium, psychosis, or seizures.28
Monitor older adults taking FQs. Because the risk of psychiatric adverse events is greatest in older individuals, especially those with known atherosclerotic disease or epilepsy, FQ therapy should be used cautiously—and with close monitoring—in this patient population. Symptoms such as weakness, confusion, tremor, loss of appetite, and depression are often incorrectly attributed simply to age, and thus go unreported as potential adverse effects of FQs.29 The exact mechanism by which FQs may induce seizures is unknown, but it may be related to excitatory effects at GABA receptors in the hippocampus.30
FQs may affect glucose levels
FQs have been reported to have varying effects on glucose metabolism, and have been implicated in both hypo- and hyperglycemia. FQ-related hypoglycemia has been thought to occur as a result of an increase in insulin secretion through a sulfonylurea-like action on pancreatic beta cells,31 via drug-drug interactions in patients with renal impairment,32 or via cytochrome P450 interactions.33 The mechanism of action relating to hyperglycemia is less well understood.
One retrospective cohort study in outpatients at a Veterans Administration facility sought to identify outcomes of hospitalization with a primary diagnosis of either hypo- or hyperglycemia in patients with a new prescription for either an FQ or azithromycin.34 In patients with diabetes, the OR for FQ-associated hypoglycemia (compared with azithromycin) was 2.1 for levofloxacin (95% CI, 1.4-3.3) and 1.1 for ciprofloxacin (95% CI, 0.6-2.0). The ORs for hyperglycemia were 1.8 for levofloxacin (95%, CI 1.2-2.7) and 1.0 for ciprofloxacin (95% CI, 0.6-1.8).
A retrospective chart review of more than 17,000 hospitalized patients who were receiving either an FQ or ceftriaxone revealed that 101 patients had either high (>200 mg/dL) or low (<50 mg/dL) glucose levels within 72 hours of receiving the antibiotic.35 Nearly 89% of those studied had diabetes and 40% had prescriptions for oral hypoglycemic agents. While most of these patients had underlying renal insufficiency, rates of hyperglycemia were greater with levofloxacin than with ceftriaxone. (In this study and the VA study, gatifloxacin had greater effects on glucose levels than the non-FQ antibiotics they were compared with; as noted earlier, however, gatifloxacin was removed from the US market in 2006.)
Diplopia is the most common ophthalmologic effect
A database review found 171 case reports of diplopia associated with FQs; ciprofloxacin was the most commonly implicated FQ, with 75 cases. The median time between medication initiation and the development of diplopia was 9.6 days. Most FQ-associated diplopia is completely reversible upon cessation of drug therapy, as evidenced by 53 published reports in which that was the case.36
Adverse effects of intraocular FQs. Ocular keratitis, corneal infiltrates and precipitates, and delayed corneal epithelial healing have been linked to the administration of intraocular FQs.36-38 In addition, retinal detachment has been found to occur in 3.3% of patients being treated with intraocular FQs, compared with 0.6% of controls (adjusted rate ratio=4.50; number needed to harm= 2500).39
CASE Suspecting CDAD and Achilles tendinitis secondary to ciprofloxacin, you stop the medication. Ms. Z’s urine culture is positive for Klebsiella pneumoniae, which is also sensitive to nitrofurantoin, so a 7-day course is prescribed. And, because a stool test for C difficile is positive, you prescribe a 7-day course of metronidazole, as well. Within 4 weeks of stopping the ciprofloxacin, the Achilles tendinitis had completely resolved.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected]
1. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-743.
2. Poisindex System. Ann Arbor, Mich: Truven Health Analytics.
3. Melhus A, Apelqvist J, Larsson J, et al. Levofloxacin-associated Achilles tendon rupture and tendinopathy. Scand J Infect Dis. 2003;35:768-770.
4. Damuth E, Heidelbaugh JJ, Malani PN, et al. Case report: an elderly subject with fluoroquinolone-associated Achilles tendinitis. Am J Geriatr Pharmacother. 2008;6:264-268.
5. Gold L, Igra H. Levofloxacin-induced tendon rupture: a case report and review of the literature. J Am Board Fam Pract. 2003;16:458-460.
6. Haddow LJ, Chandra Sekhar M, Hajela V, et al. Spontaneous Achilles tendon rupture in patients treated with levofloxacin. J Antimicrob Chemother. 2003;51:747-748.
7. US Food and Drug Administration. Information for healthcare professionals: fluoroquinolone antimicrobial drugs Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126085.htm. Accessed September 30, 2012.
8. Corrao G, Zambon A, Bertu L, et al. Evidence of tendinitis provoked by fluoroquinolone treatment: a case-control study. Drug Saf. 2006;29:889-896.
9. Sendzik J, Shakibaei M, Schafer-Korting M, et al. Fluoroquinolones cause changes in extracellular matrix, signaling proteins, metalloproteinases and caspase-3 in cultured human tendon cells. Toxicology. 2005;212:24-36.
10. Corps AN, Harrall RL, Curry VA, et al. Ciprofloxacin enhances the stimulation of matrix metalloproteinase 3 expression by interleukin-1beta in human tendon-derived cells: a potential mechanism of fluoroquinolone-induced tendinopathy. Arthritis Rheum. 2002;46:3034-3040.
11. Muzi F, Gravanta G, Tati E, et al. Fluoroquinolones-induced tendinitis and tendon rupture in kidney transplant recipients: 2 cases and a review of the literature. Transplant Proc. 2007;39:1673-1675.
12. Cholongitas E, Georgousaki C, Spyrou S, et al. Ciprofloxacin-induced acute cholestatic hepatitis. Ann Hepatol. 2009;8:400-401.
13. Denève C, Janoir C, Poilane I, et al. New trends in Clostridium difficile virulence and pathogenesis. Int J Antimicrob Agents. 2009;33(suppl 1):S24-S28.
14. Deshpande A, Pant C, Jain A, et al. Do fluoroquinolones predispose patients to Clostridium difficile associated disease? A review of the evidence. Curr Med Res Opin. 2008;24:329-333.
15. Novelle M, Morreale CA. The relationship between inpatient fluoroquinolone use and Clostridium difficile-associated diarrhea. Ann Pharmacother. 2010;44:826-831.
16. Adikwu E, Deo O. Fluoroquinolones reported hepatotoxicity. Pharmacology Pharmacy. 2012;3:328-336.
17. Nicholson SC, Webb CD, Moellering RC. Antimicrobial-associated acute hepatitis. Pharmacotherapy. 2002;22:794-797.
18. Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondria dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol. 2008;22:335-353.
19. Mitcheson JS, Chen J, Lim M, et al. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci. 2000;97:12329-12333.
20. US Food and Drug Administration. Determination that Tequin (gatifloxacin) was withdrawn from sale for reasons of safety or effectiveness. Fed Regist. 2008;73:52357-52358.
21. Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother. 2002;49:593-596.
22. Briasoulis A, Agarwal V, Pierce WJ. QT prolongation and torsade de pointes induced by fluoroquinolones: infrequent side effects from commonly used medications. Cardiology. 2011;120:103-110.
23. Prabhakar M, Krahn AD. Ciprofloxacin-induced acquired long QT syndrome. Heart Rhythm. 2004;1:624-626.
24. Demolis JL, Kubitza D, Tenneze L, et al. Effect of a single oral dose of moxifloxacin (400 and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther. 2000;68:658-666.
25. Tome AM, Filipe A. Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf. 2011;34:465-488.
26. Kiangkitiwan B. Levofloxacin-induced delirium with psychotic features. Gen Hosp Psychiatry. 2008;30:381-383.
27. Carbon C. Comparison of side effects of levofloxacin versus other fluoroquinolones. Chemotherapy. 2001;47(suppl 3):S9-S14.
28. LaSalvia EA, Domek GJ, Gitlin DF. Fluoroquinolone-induced suicidal ideation. Gen Hosp Psychiatry. 2010;32:108-110.
29. Stahlmann R, Lode H. Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging. 2010;27:193-209.
30. Owens RC, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl):S144-S157.
31. Smith KM, Lomaestro BM. What role do fluoroquinolone antimicrobial agents play in cardiac dysfunction and altered glycemic control? J Pharm Pract. 2003;16:349-360.
32. LeBlanc M, Belanger C, Cossette P. Severe and resistant hypoglycemia associated with concomitant gatifloxacin and glyburide therapy. Pharmacotherapy. 2004;24:926-931.
33. Graumlich JF, Habis S, Avelino RR, et al. Hypoglycemia in inpatients after gatifloxacin or levofloxacin therapy: nested case control study. Pharmacotherapy. 2005;25:1296-1302.
34. Aspinall SL, Good CB, Jiang R, et al. Severe dysglycemia with the fluoroquinolones: a class effect? Clin Infect Dis. 2009;49:402-408.
35. Mohr JF, McKinnon PS, Peymann PJ, et al. A retrospective, comparative evaluation of dysglycemias in hospitalized patients receiving gatifloxacin, levofloxacin, ciprofloxacin, or ceftriaxone. Pharmacotherapy. 2005;25:1303-1309.
36. Fraunfelder FW, Fraunfelder FT. Diplopia and fluoroquinolones. Ophthalmology. 2009;116:1814-1817.
37. Eiferman RA, Snyder JP, Nordquist RE. Ciprofloxacin microprecipitates and macroprecipitates in the human corneal epithelium. J Cataract Refract Surg. 2001;27:1701-1702.
38. Fraunfelder FW. Corneal toxicity from topical ocular and systemic medications. Cornea. 2006;25:1133-1138.
39. Etminan M, Forooghian F, Brophy JM, et al. Oral fluoroquinolones and the risk of retinal detachment. JAMA. 2012;307:1414-1419.
• Evaluate liver function before initiating fluoroquinolone (FQ) therapy, and avoid prescribing these antibiotics for patients at increased risk for hepatotoxicity. C
• Avoid prescribing FQs for patients with a history of prolonged QT syndrome. C
• Closely monitor older patients being treated with FQs, particularly if they have atherosclerosis or epilepsy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
PATIENT HANDOUT
Taking a fluoroquinolone antibiotic?
CASE Sara Z, a 62-year-old patient with a history of chronic urinary tract infections, presents with a 3-day history of dysuria and urinary frequency. Her last 2 urine cultures found Escherichia coli resistant to trimethoprim-sulfamethoxazole, amoxicillin, and cephalosporins. So her family physician ordered a urine culture and prescribed a 7-day course of ciprofloxacin empirically.
Five days later, Ms. Z returned, suffering from nonbloody diarrhea and bilateral Achilles tendon pain.
If you were treating Ms. Z, what would your next step be?
Widely used to treat urinary tract, skin, and pulmonary infections and to fight infections resistant to other antibiotics, fluoroquinolones (FQ) are generally regarded as safe in both inpatient and outpatient settings. Yet these broad-spectrum antibiotics are associated with both common and rare adverse effects, as well as a number of drug-drug interactions.
The Centers for Disease Control and Prevention estimates that adverse events from FQs leading to emergency department (ED) visits occur at a rate of 9.2 for every 10,000 prescriptions. That’s higher than the ED rates for cephalosporins (6.1 per 10,000) and macrolides (5.1 per 10,000), but far lower than for penicillins (13 per 10,000), clindamycin (18.5 per 10,000), sulfonamides (18.9 per 10,000), and vancomycin (24.1 per 10,000).1
In fact, adverse events associated with FQs range from mild and self-limiting to rare and severe. This review discusses both. Relatively common adverse effects and drug-drug interactions are discussed in the text, while the TABLE2 includes a broader range of potential adverse effects. You’ll also find a handout for patients taking FQs on page 195 that clearly describes signs and symptoms that need to be reported right away.
TABLE
Fluoroquinolones: Adverse effects to guard against*2
Cardiovascular
| Immunologic
|
Dermatologic
| Musculoskeletal
|
Drug-drug interactions
| Neurologic
|
Endocrine/Metabolic
| Ocular
|
Gastrointestinal
| Psychiatric
|
Hematologic
| Respiratory
|
| *This is not a complete list of potential adverse effects associated with fluoroquinolones. †Fluoroquinolones may potentiate warfarin. | |
A black box warning of tendinopathies
FQs exhibit an affinity for connective tissue, with higher concentrations found in bone and cartilage than in serum. While FQs are therefore well suited for treating orthopedic-related infections,3 they also increase the risk of tendinopathies.
In the last 2 decades, numerous case reports linking tendinitis and FQs have been published.4-6 In 2008, the US Food and Drug Administration (FDA) issued a black box warning of tendinitis and tendon rupture. Patients on FQ therapy should be advised to stop taking the antibiotic at the first sign of pain, swelling, or inflammation in a tendon, the FDA advises.7
How common is this adverse effect? A case-control study of 22,194 patients with a diagnosis of nontraumatic tendiopathy determined that FQ use resulted in a 1.3-fold risk of tendon rupture and more than a 4-fold risk of rupture of the Achilles tendon. One Achilles tendon rupture would occur for every 5958 patients treated with FQs, the researchers estimated.8
The precise mechanism by which FQs lead to tendinopathies is not completely understood. Studies suggest that the antibiotics cause a decrease in the synthesis of type I collagen, elastin, fibronectin, and beta (1)-integrin, and time- and concentration-dependent increases of cellular apoptosis.9 In vitro studies have shown inhibition of both cell proliferation and fibroblast metabolism when tissue is exposed to FQs, which may impede tissue healing.10
Which patients are at higher risk? The risk of FQ-associated tendinopathies is greatest in patients older than 60 years; in kidney, heart, and lung transplant recipients; and in patients taking an FQ with concomitant corticosteroid therapy. Decreased renal clearance of the medication may play a role in the increased risk.11
GI problems are common, especially in kids and older patients
Gastrointestinal (GI) disturbances are common in patients taking FQs, and typically occur more frequently in children and older adults, and in those taking higher doses. Reactions attributable to ciprofloxacin, for example, include nausea (affecting 1.4%-4% of adults and 2.7% of children taking the drug), vomiting (1%-2% of adults and 4.8% of children), diarrhea (<1%-2% of adults and 4.8% of children), and abdominal pain or discomfort (<1%-1.7% of adults and 3.3% of children).12
C difficile and FQ resistance. The extent to which Clostridium difficile-associated diarrhea (CDAD) is attributable to FQs has been subject to controversy in recent years. A previously uncommon strain of C difficile (B1/NAP1) with variations that have become more resistant to FQs has been linked to an increased incidence of CDAD across both the United States and Europe.13 A systematic review suggested that FQs predispose patients to CDAD,14 while a retrospective case-control study of 174 adult inpatients with CDAD determined that FQ administration did not significantly increase the rate of complications from C difficile (odds ratio [OR]=1.37; 95% confidence interval [CI], 0.72-2.61).15
Factors that affect risk of hepatotoxicity
Hepatitis/transaminitis, pancreatitis, jaundice, liver injury, and hepatic failure have all been reported in patients taking FQs, with the extent of hepatotoxicity varying based on the particular FQ taken, the dosage, and the patient’s baseline hepatic function.16,17 Comorbidities, including renal failure, may increase the potential for FQ-associated hepatotoxicity, as well. Thus, some experts recommend that clinicians evaluate a patient’s liver function before initiating FQ therapy and avoid prescribing FQs for those at added risk.
The exact mechanism by which FQ-induced hepatotoxicity occurs is unknown. One theory posits that the drugs generate oxidative radicals involved in mitochondrial damage, RNA processing, transcription, and inflammation;18 another suggests that FQs generate oxidative radicals in the liver as a result of cytochrome P450 metabolism.16 Case reports have shown that hepatitis resolves when the drug is discontinued, but often recurs in patients who are switched to a different FQ.16,17
Torsades de pointes is the key cardiovascular risk
FQs prolong the QT interval by blocking voltage-gated potassium channels, causing a reduction of the rapid component of the delayed rectifier potassium current in a dose-dependent fashion.19 But the average QT interval prolongation caused by FQs over a 3- to 6-month period does not appear to have clinical significance, nor is it associated with any discernible cardiac symptoms or impairment.19
For most, risk is minimal. There appears to be considerable variation in QT interval prolongation among FQs. A retrospective database analysis of published case reports of patients who received FQs over a 15-year period found 25 cases of torsades de pointes; moxifloxacin (highest), levofloxacin, and gatifloxacin (which was taken off the market by the FDA in 2006)20 were associated with a higher incidence than ciprofloxacin.21 Ciprofloxacin appears to be the safest FQ for cardiovascular events, with the lowest reported risk of torsades de pointes.22 However, several small randomized controlled trials have found that levofloxacin, like ciprofloxacin, did not significantly affect the QT interval.23,24
These patients face a higher risk. Notably, individuals with abnormal baseline QT prolongation (>440 ms in men; >460 ms in women) are at increased risk of developing torsades de pointes from the use of FQs, regardless of the dose.19 In fact, anyone with a history of prolonged QT syndrome should avoid these antibiotics, particularly if he or she is taking class Ia (eg, procainamide, quinidine) or class III (eg, amiodarone, sotalol) antiarrhythmics.19 Patients taking warfarin may be candidates for FQ therapy, but because the antibiotics may potentiate the anticoagulant, close monitoring is required. (Other potential drug-drug interactions are detailed in the TABLE.)
Evaluation of risk vs benefit is imperative prior to prescribing FQs for patients with increased risk for adverse cardiovascular events. An electrocardiogram is advisable, as well.
Mild neurologic and psychiatric effects not uncommon
Studies examining central nervous system (CNS) effects have estimated that neurotoxicity occurs in approximately 1% to 4.4% of patients taking FQs, with serious adverse effects occurring less than 0.5% of the time.25 Common—and milder—CNS effects include headache, dizziness, and insomnia. More severe CNS effects include tremors, restlessness, anxiety, light-headedness, confusion, hallucinations, paranoia, depression, nightmares, insomnia, and suicidal thoughts or attempts.25,26 Case reports have documented FQ-induced psychosis, catatonia, seizures, and delirium, with a higher incidence associated with higher doses of the antibiotic.26
A literature review aimed at identifying case reports yielded reports of 232 adverse psychiatric and neurologic drug reactions attributable to FQs in 145 patients.27 Nearly half were related to ciprofloxacin, with psychiatric reactions such as mania and acute psychosis being the most common. Most adverse CNS events (eg, convulsion, confusional state, agitation) developed rather quickly—in some cases within a few minutes of FQ administration and in others, within the first one to 8 days. In most reported cases, the patients had no known underlying psychiatric diseases or concomitant medication likely to have precipitated the development of delirium, psychosis, or seizures.28
Monitor older adults taking FQs. Because the risk of psychiatric adverse events is greatest in older individuals, especially those with known atherosclerotic disease or epilepsy, FQ therapy should be used cautiously—and with close monitoring—in this patient population. Symptoms such as weakness, confusion, tremor, loss of appetite, and depression are often incorrectly attributed simply to age, and thus go unreported as potential adverse effects of FQs.29 The exact mechanism by which FQs may induce seizures is unknown, but it may be related to excitatory effects at GABA receptors in the hippocampus.30
FQs may affect glucose levels
FQs have been reported to have varying effects on glucose metabolism, and have been implicated in both hypo- and hyperglycemia. FQ-related hypoglycemia has been thought to occur as a result of an increase in insulin secretion through a sulfonylurea-like action on pancreatic beta cells,31 via drug-drug interactions in patients with renal impairment,32 or via cytochrome P450 interactions.33 The mechanism of action relating to hyperglycemia is less well understood.
One retrospective cohort study in outpatients at a Veterans Administration facility sought to identify outcomes of hospitalization with a primary diagnosis of either hypo- or hyperglycemia in patients with a new prescription for either an FQ or azithromycin.34 In patients with diabetes, the OR for FQ-associated hypoglycemia (compared with azithromycin) was 2.1 for levofloxacin (95% CI, 1.4-3.3) and 1.1 for ciprofloxacin (95% CI, 0.6-2.0). The ORs for hyperglycemia were 1.8 for levofloxacin (95%, CI 1.2-2.7) and 1.0 for ciprofloxacin (95% CI, 0.6-1.8).
A retrospective chart review of more than 17,000 hospitalized patients who were receiving either an FQ or ceftriaxone revealed that 101 patients had either high (>200 mg/dL) or low (<50 mg/dL) glucose levels within 72 hours of receiving the antibiotic.35 Nearly 89% of those studied had diabetes and 40% had prescriptions for oral hypoglycemic agents. While most of these patients had underlying renal insufficiency, rates of hyperglycemia were greater with levofloxacin than with ceftriaxone. (In this study and the VA study, gatifloxacin had greater effects on glucose levels than the non-FQ antibiotics they were compared with; as noted earlier, however, gatifloxacin was removed from the US market in 2006.)
Diplopia is the most common ophthalmologic effect
A database review found 171 case reports of diplopia associated with FQs; ciprofloxacin was the most commonly implicated FQ, with 75 cases. The median time between medication initiation and the development of diplopia was 9.6 days. Most FQ-associated diplopia is completely reversible upon cessation of drug therapy, as evidenced by 53 published reports in which that was the case.36
Adverse effects of intraocular FQs. Ocular keratitis, corneal infiltrates and precipitates, and delayed corneal epithelial healing have been linked to the administration of intraocular FQs.36-38 In addition, retinal detachment has been found to occur in 3.3% of patients being treated with intraocular FQs, compared with 0.6% of controls (adjusted rate ratio=4.50; number needed to harm= 2500).39
CASE Suspecting CDAD and Achilles tendinitis secondary to ciprofloxacin, you stop the medication. Ms. Z’s urine culture is positive for Klebsiella pneumoniae, which is also sensitive to nitrofurantoin, so a 7-day course is prescribed. And, because a stool test for C difficile is positive, you prescribe a 7-day course of metronidazole, as well. Within 4 weeks of stopping the ciprofloxacin, the Achilles tendinitis had completely resolved.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected]
• Evaluate liver function before initiating fluoroquinolone (FQ) therapy, and avoid prescribing these antibiotics for patients at increased risk for hepatotoxicity. C
• Avoid prescribing FQs for patients with a history of prolonged QT syndrome. C
• Closely monitor older patients being treated with FQs, particularly if they have atherosclerosis or epilepsy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
PATIENT HANDOUT
Taking a fluoroquinolone antibiotic?
CASE Sara Z, a 62-year-old patient with a history of chronic urinary tract infections, presents with a 3-day history of dysuria and urinary frequency. Her last 2 urine cultures found Escherichia coli resistant to trimethoprim-sulfamethoxazole, amoxicillin, and cephalosporins. So her family physician ordered a urine culture and prescribed a 7-day course of ciprofloxacin empirically.
Five days later, Ms. Z returned, suffering from nonbloody diarrhea and bilateral Achilles tendon pain.
If you were treating Ms. Z, what would your next step be?
Widely used to treat urinary tract, skin, and pulmonary infections and to fight infections resistant to other antibiotics, fluoroquinolones (FQ) are generally regarded as safe in both inpatient and outpatient settings. Yet these broad-spectrum antibiotics are associated with both common and rare adverse effects, as well as a number of drug-drug interactions.
The Centers for Disease Control and Prevention estimates that adverse events from FQs leading to emergency department (ED) visits occur at a rate of 9.2 for every 10,000 prescriptions. That’s higher than the ED rates for cephalosporins (6.1 per 10,000) and macrolides (5.1 per 10,000), but far lower than for penicillins (13 per 10,000), clindamycin (18.5 per 10,000), sulfonamides (18.9 per 10,000), and vancomycin (24.1 per 10,000).1
In fact, adverse events associated with FQs range from mild and self-limiting to rare and severe. This review discusses both. Relatively common adverse effects and drug-drug interactions are discussed in the text, while the TABLE2 includes a broader range of potential adverse effects. You’ll also find a handout for patients taking FQs on page 195 that clearly describes signs and symptoms that need to be reported right away.
TABLE
Fluoroquinolones: Adverse effects to guard against*2
Cardiovascular
| Immunologic
|
Dermatologic
| Musculoskeletal
|
Drug-drug interactions
| Neurologic
|
Endocrine/Metabolic
| Ocular
|
Gastrointestinal
| Psychiatric
|
Hematologic
| Respiratory
|
| *This is not a complete list of potential adverse effects associated with fluoroquinolones. †Fluoroquinolones may potentiate warfarin. | |
A black box warning of tendinopathies
FQs exhibit an affinity for connective tissue, with higher concentrations found in bone and cartilage than in serum. While FQs are therefore well suited for treating orthopedic-related infections,3 they also increase the risk of tendinopathies.
In the last 2 decades, numerous case reports linking tendinitis and FQs have been published.4-6 In 2008, the US Food and Drug Administration (FDA) issued a black box warning of tendinitis and tendon rupture. Patients on FQ therapy should be advised to stop taking the antibiotic at the first sign of pain, swelling, or inflammation in a tendon, the FDA advises.7
How common is this adverse effect? A case-control study of 22,194 patients with a diagnosis of nontraumatic tendiopathy determined that FQ use resulted in a 1.3-fold risk of tendon rupture and more than a 4-fold risk of rupture of the Achilles tendon. One Achilles tendon rupture would occur for every 5958 patients treated with FQs, the researchers estimated.8
The precise mechanism by which FQs lead to tendinopathies is not completely understood. Studies suggest that the antibiotics cause a decrease in the synthesis of type I collagen, elastin, fibronectin, and beta (1)-integrin, and time- and concentration-dependent increases of cellular apoptosis.9 In vitro studies have shown inhibition of both cell proliferation and fibroblast metabolism when tissue is exposed to FQs, which may impede tissue healing.10
Which patients are at higher risk? The risk of FQ-associated tendinopathies is greatest in patients older than 60 years; in kidney, heart, and lung transplant recipients; and in patients taking an FQ with concomitant corticosteroid therapy. Decreased renal clearance of the medication may play a role in the increased risk.11
GI problems are common, especially in kids and older patients
Gastrointestinal (GI) disturbances are common in patients taking FQs, and typically occur more frequently in children and older adults, and in those taking higher doses. Reactions attributable to ciprofloxacin, for example, include nausea (affecting 1.4%-4% of adults and 2.7% of children taking the drug), vomiting (1%-2% of adults and 4.8% of children), diarrhea (<1%-2% of adults and 4.8% of children), and abdominal pain or discomfort (<1%-1.7% of adults and 3.3% of children).12
C difficile and FQ resistance. The extent to which Clostridium difficile-associated diarrhea (CDAD) is attributable to FQs has been subject to controversy in recent years. A previously uncommon strain of C difficile (B1/NAP1) with variations that have become more resistant to FQs has been linked to an increased incidence of CDAD across both the United States and Europe.13 A systematic review suggested that FQs predispose patients to CDAD,14 while a retrospective case-control study of 174 adult inpatients with CDAD determined that FQ administration did not significantly increase the rate of complications from C difficile (odds ratio [OR]=1.37; 95% confidence interval [CI], 0.72-2.61).15
Factors that affect risk of hepatotoxicity
Hepatitis/transaminitis, pancreatitis, jaundice, liver injury, and hepatic failure have all been reported in patients taking FQs, with the extent of hepatotoxicity varying based on the particular FQ taken, the dosage, and the patient’s baseline hepatic function.16,17 Comorbidities, including renal failure, may increase the potential for FQ-associated hepatotoxicity, as well. Thus, some experts recommend that clinicians evaluate a patient’s liver function before initiating FQ therapy and avoid prescribing FQs for those at added risk.
The exact mechanism by which FQ-induced hepatotoxicity occurs is unknown. One theory posits that the drugs generate oxidative radicals involved in mitochondrial damage, RNA processing, transcription, and inflammation;18 another suggests that FQs generate oxidative radicals in the liver as a result of cytochrome P450 metabolism.16 Case reports have shown that hepatitis resolves when the drug is discontinued, but often recurs in patients who are switched to a different FQ.16,17
Torsades de pointes is the key cardiovascular risk
FQs prolong the QT interval by blocking voltage-gated potassium channels, causing a reduction of the rapid component of the delayed rectifier potassium current in a dose-dependent fashion.19 But the average QT interval prolongation caused by FQs over a 3- to 6-month period does not appear to have clinical significance, nor is it associated with any discernible cardiac symptoms or impairment.19
For most, risk is minimal. There appears to be considerable variation in QT interval prolongation among FQs. A retrospective database analysis of published case reports of patients who received FQs over a 15-year period found 25 cases of torsades de pointes; moxifloxacin (highest), levofloxacin, and gatifloxacin (which was taken off the market by the FDA in 2006)20 were associated with a higher incidence than ciprofloxacin.21 Ciprofloxacin appears to be the safest FQ for cardiovascular events, with the lowest reported risk of torsades de pointes.22 However, several small randomized controlled trials have found that levofloxacin, like ciprofloxacin, did not significantly affect the QT interval.23,24
These patients face a higher risk. Notably, individuals with abnormal baseline QT prolongation (>440 ms in men; >460 ms in women) are at increased risk of developing torsades de pointes from the use of FQs, regardless of the dose.19 In fact, anyone with a history of prolonged QT syndrome should avoid these antibiotics, particularly if he or she is taking class Ia (eg, procainamide, quinidine) or class III (eg, amiodarone, sotalol) antiarrhythmics.19 Patients taking warfarin may be candidates for FQ therapy, but because the antibiotics may potentiate the anticoagulant, close monitoring is required. (Other potential drug-drug interactions are detailed in the TABLE.)
Evaluation of risk vs benefit is imperative prior to prescribing FQs for patients with increased risk for adverse cardiovascular events. An electrocardiogram is advisable, as well.
Mild neurologic and psychiatric effects not uncommon
Studies examining central nervous system (CNS) effects have estimated that neurotoxicity occurs in approximately 1% to 4.4% of patients taking FQs, with serious adverse effects occurring less than 0.5% of the time.25 Common—and milder—CNS effects include headache, dizziness, and insomnia. More severe CNS effects include tremors, restlessness, anxiety, light-headedness, confusion, hallucinations, paranoia, depression, nightmares, insomnia, and suicidal thoughts or attempts.25,26 Case reports have documented FQ-induced psychosis, catatonia, seizures, and delirium, with a higher incidence associated with higher doses of the antibiotic.26
A literature review aimed at identifying case reports yielded reports of 232 adverse psychiatric and neurologic drug reactions attributable to FQs in 145 patients.27 Nearly half were related to ciprofloxacin, with psychiatric reactions such as mania and acute psychosis being the most common. Most adverse CNS events (eg, convulsion, confusional state, agitation) developed rather quickly—in some cases within a few minutes of FQ administration and in others, within the first one to 8 days. In most reported cases, the patients had no known underlying psychiatric diseases or concomitant medication likely to have precipitated the development of delirium, psychosis, or seizures.28
Monitor older adults taking FQs. Because the risk of psychiatric adverse events is greatest in older individuals, especially those with known atherosclerotic disease or epilepsy, FQ therapy should be used cautiously—and with close monitoring—in this patient population. Symptoms such as weakness, confusion, tremor, loss of appetite, and depression are often incorrectly attributed simply to age, and thus go unreported as potential adverse effects of FQs.29 The exact mechanism by which FQs may induce seizures is unknown, but it may be related to excitatory effects at GABA receptors in the hippocampus.30
FQs may affect glucose levels
FQs have been reported to have varying effects on glucose metabolism, and have been implicated in both hypo- and hyperglycemia. FQ-related hypoglycemia has been thought to occur as a result of an increase in insulin secretion through a sulfonylurea-like action on pancreatic beta cells,31 via drug-drug interactions in patients with renal impairment,32 or via cytochrome P450 interactions.33 The mechanism of action relating to hyperglycemia is less well understood.
One retrospective cohort study in outpatients at a Veterans Administration facility sought to identify outcomes of hospitalization with a primary diagnosis of either hypo- or hyperglycemia in patients with a new prescription for either an FQ or azithromycin.34 In patients with diabetes, the OR for FQ-associated hypoglycemia (compared with azithromycin) was 2.1 for levofloxacin (95% CI, 1.4-3.3) and 1.1 for ciprofloxacin (95% CI, 0.6-2.0). The ORs for hyperglycemia were 1.8 for levofloxacin (95%, CI 1.2-2.7) and 1.0 for ciprofloxacin (95% CI, 0.6-1.8).
A retrospective chart review of more than 17,000 hospitalized patients who were receiving either an FQ or ceftriaxone revealed that 101 patients had either high (>200 mg/dL) or low (<50 mg/dL) glucose levels within 72 hours of receiving the antibiotic.35 Nearly 89% of those studied had diabetes and 40% had prescriptions for oral hypoglycemic agents. While most of these patients had underlying renal insufficiency, rates of hyperglycemia were greater with levofloxacin than with ceftriaxone. (In this study and the VA study, gatifloxacin had greater effects on glucose levels than the non-FQ antibiotics they were compared with; as noted earlier, however, gatifloxacin was removed from the US market in 2006.)
Diplopia is the most common ophthalmologic effect
A database review found 171 case reports of diplopia associated with FQs; ciprofloxacin was the most commonly implicated FQ, with 75 cases. The median time between medication initiation and the development of diplopia was 9.6 days. Most FQ-associated diplopia is completely reversible upon cessation of drug therapy, as evidenced by 53 published reports in which that was the case.36
Adverse effects of intraocular FQs. Ocular keratitis, corneal infiltrates and precipitates, and delayed corneal epithelial healing have been linked to the administration of intraocular FQs.36-38 In addition, retinal detachment has been found to occur in 3.3% of patients being treated with intraocular FQs, compared with 0.6% of controls (adjusted rate ratio=4.50; number needed to harm= 2500).39
CASE Suspecting CDAD and Achilles tendinitis secondary to ciprofloxacin, you stop the medication. Ms. Z’s urine culture is positive for Klebsiella pneumoniae, which is also sensitive to nitrofurantoin, so a 7-day course is prescribed. And, because a stool test for C difficile is positive, you prescribe a 7-day course of metronidazole, as well. Within 4 weeks of stopping the ciprofloxacin, the Achilles tendinitis had completely resolved.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected]
1. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-743.
2. Poisindex System. Ann Arbor, Mich: Truven Health Analytics.
3. Melhus A, Apelqvist J, Larsson J, et al. Levofloxacin-associated Achilles tendon rupture and tendinopathy. Scand J Infect Dis. 2003;35:768-770.
4. Damuth E, Heidelbaugh JJ, Malani PN, et al. Case report: an elderly subject with fluoroquinolone-associated Achilles tendinitis. Am J Geriatr Pharmacother. 2008;6:264-268.
5. Gold L, Igra H. Levofloxacin-induced tendon rupture: a case report and review of the literature. J Am Board Fam Pract. 2003;16:458-460.
6. Haddow LJ, Chandra Sekhar M, Hajela V, et al. Spontaneous Achilles tendon rupture in patients treated with levofloxacin. J Antimicrob Chemother. 2003;51:747-748.
7. US Food and Drug Administration. Information for healthcare professionals: fluoroquinolone antimicrobial drugs Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126085.htm. Accessed September 30, 2012.
8. Corrao G, Zambon A, Bertu L, et al. Evidence of tendinitis provoked by fluoroquinolone treatment: a case-control study. Drug Saf. 2006;29:889-896.
9. Sendzik J, Shakibaei M, Schafer-Korting M, et al. Fluoroquinolones cause changes in extracellular matrix, signaling proteins, metalloproteinases and caspase-3 in cultured human tendon cells. Toxicology. 2005;212:24-36.
10. Corps AN, Harrall RL, Curry VA, et al. Ciprofloxacin enhances the stimulation of matrix metalloproteinase 3 expression by interleukin-1beta in human tendon-derived cells: a potential mechanism of fluoroquinolone-induced tendinopathy. Arthritis Rheum. 2002;46:3034-3040.
11. Muzi F, Gravanta G, Tati E, et al. Fluoroquinolones-induced tendinitis and tendon rupture in kidney transplant recipients: 2 cases and a review of the literature. Transplant Proc. 2007;39:1673-1675.
12. Cholongitas E, Georgousaki C, Spyrou S, et al. Ciprofloxacin-induced acute cholestatic hepatitis. Ann Hepatol. 2009;8:400-401.
13. Denève C, Janoir C, Poilane I, et al. New trends in Clostridium difficile virulence and pathogenesis. Int J Antimicrob Agents. 2009;33(suppl 1):S24-S28.
14. Deshpande A, Pant C, Jain A, et al. Do fluoroquinolones predispose patients to Clostridium difficile associated disease? A review of the evidence. Curr Med Res Opin. 2008;24:329-333.
15. Novelle M, Morreale CA. The relationship between inpatient fluoroquinolone use and Clostridium difficile-associated diarrhea. Ann Pharmacother. 2010;44:826-831.
16. Adikwu E, Deo O. Fluoroquinolones reported hepatotoxicity. Pharmacology Pharmacy. 2012;3:328-336.
17. Nicholson SC, Webb CD, Moellering RC. Antimicrobial-associated acute hepatitis. Pharmacotherapy. 2002;22:794-797.
18. Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondria dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol. 2008;22:335-353.
19. Mitcheson JS, Chen J, Lim M, et al. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci. 2000;97:12329-12333.
20. US Food and Drug Administration. Determination that Tequin (gatifloxacin) was withdrawn from sale for reasons of safety or effectiveness. Fed Regist. 2008;73:52357-52358.
21. Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother. 2002;49:593-596.
22. Briasoulis A, Agarwal V, Pierce WJ. QT prolongation and torsade de pointes induced by fluoroquinolones: infrequent side effects from commonly used medications. Cardiology. 2011;120:103-110.
23. Prabhakar M, Krahn AD. Ciprofloxacin-induced acquired long QT syndrome. Heart Rhythm. 2004;1:624-626.
24. Demolis JL, Kubitza D, Tenneze L, et al. Effect of a single oral dose of moxifloxacin (400 and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther. 2000;68:658-666.
25. Tome AM, Filipe A. Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf. 2011;34:465-488.
26. Kiangkitiwan B. Levofloxacin-induced delirium with psychotic features. Gen Hosp Psychiatry. 2008;30:381-383.
27. Carbon C. Comparison of side effects of levofloxacin versus other fluoroquinolones. Chemotherapy. 2001;47(suppl 3):S9-S14.
28. LaSalvia EA, Domek GJ, Gitlin DF. Fluoroquinolone-induced suicidal ideation. Gen Hosp Psychiatry. 2010;32:108-110.
29. Stahlmann R, Lode H. Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging. 2010;27:193-209.
30. Owens RC, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl):S144-S157.
31. Smith KM, Lomaestro BM. What role do fluoroquinolone antimicrobial agents play in cardiac dysfunction and altered glycemic control? J Pharm Pract. 2003;16:349-360.
32. LeBlanc M, Belanger C, Cossette P. Severe and resistant hypoglycemia associated with concomitant gatifloxacin and glyburide therapy. Pharmacotherapy. 2004;24:926-931.
33. Graumlich JF, Habis S, Avelino RR, et al. Hypoglycemia in inpatients after gatifloxacin or levofloxacin therapy: nested case control study. Pharmacotherapy. 2005;25:1296-1302.
34. Aspinall SL, Good CB, Jiang R, et al. Severe dysglycemia with the fluoroquinolones: a class effect? Clin Infect Dis. 2009;49:402-408.
35. Mohr JF, McKinnon PS, Peymann PJ, et al. A retrospective, comparative evaluation of dysglycemias in hospitalized patients receiving gatifloxacin, levofloxacin, ciprofloxacin, or ceftriaxone. Pharmacotherapy. 2005;25:1303-1309.
36. Fraunfelder FW, Fraunfelder FT. Diplopia and fluoroquinolones. Ophthalmology. 2009;116:1814-1817.
37. Eiferman RA, Snyder JP, Nordquist RE. Ciprofloxacin microprecipitates and macroprecipitates in the human corneal epithelium. J Cataract Refract Surg. 2001;27:1701-1702.
38. Fraunfelder FW. Corneal toxicity from topical ocular and systemic medications. Cornea. 2006;25:1133-1138.
39. Etminan M, Forooghian F, Brophy JM, et al. Oral fluoroquinolones and the risk of retinal detachment. JAMA. 2012;307:1414-1419.
1. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-743.
2. Poisindex System. Ann Arbor, Mich: Truven Health Analytics.
3. Melhus A, Apelqvist J, Larsson J, et al. Levofloxacin-associated Achilles tendon rupture and tendinopathy. Scand J Infect Dis. 2003;35:768-770.
4. Damuth E, Heidelbaugh JJ, Malani PN, et al. Case report: an elderly subject with fluoroquinolone-associated Achilles tendinitis. Am J Geriatr Pharmacother. 2008;6:264-268.
5. Gold L, Igra H. Levofloxacin-induced tendon rupture: a case report and review of the literature. J Am Board Fam Pract. 2003;16:458-460.
6. Haddow LJ, Chandra Sekhar M, Hajela V, et al. Spontaneous Achilles tendon rupture in patients treated with levofloxacin. J Antimicrob Chemother. 2003;51:747-748.
7. US Food and Drug Administration. Information for healthcare professionals: fluoroquinolone antimicrobial drugs Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm126085.htm. Accessed September 30, 2012.
8. Corrao G, Zambon A, Bertu L, et al. Evidence of tendinitis provoked by fluoroquinolone treatment: a case-control study. Drug Saf. 2006;29:889-896.
9. Sendzik J, Shakibaei M, Schafer-Korting M, et al. Fluoroquinolones cause changes in extracellular matrix, signaling proteins, metalloproteinases and caspase-3 in cultured human tendon cells. Toxicology. 2005;212:24-36.
10. Corps AN, Harrall RL, Curry VA, et al. Ciprofloxacin enhances the stimulation of matrix metalloproteinase 3 expression by interleukin-1beta in human tendon-derived cells: a potential mechanism of fluoroquinolone-induced tendinopathy. Arthritis Rheum. 2002;46:3034-3040.
11. Muzi F, Gravanta G, Tati E, et al. Fluoroquinolones-induced tendinitis and tendon rupture in kidney transplant recipients: 2 cases and a review of the literature. Transplant Proc. 2007;39:1673-1675.
12. Cholongitas E, Georgousaki C, Spyrou S, et al. Ciprofloxacin-induced acute cholestatic hepatitis. Ann Hepatol. 2009;8:400-401.
13. Denève C, Janoir C, Poilane I, et al. New trends in Clostridium difficile virulence and pathogenesis. Int J Antimicrob Agents. 2009;33(suppl 1):S24-S28.
14. Deshpande A, Pant C, Jain A, et al. Do fluoroquinolones predispose patients to Clostridium difficile associated disease? A review of the evidence. Curr Med Res Opin. 2008;24:329-333.
15. Novelle M, Morreale CA. The relationship between inpatient fluoroquinolone use and Clostridium difficile-associated diarrhea. Ann Pharmacother. 2010;44:826-831.
16. Adikwu E, Deo O. Fluoroquinolones reported hepatotoxicity. Pharmacology Pharmacy. 2012;3:328-336.
17. Nicholson SC, Webb CD, Moellering RC. Antimicrobial-associated acute hepatitis. Pharmacotherapy. 2002;22:794-797.
18. Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondria dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol. 2008;22:335-353.
19. Mitcheson JS, Chen J, Lim M, et al. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci. 2000;97:12329-12333.
20. US Food and Drug Administration. Determination that Tequin (gatifloxacin) was withdrawn from sale for reasons of safety or effectiveness. Fed Regist. 2008;73:52357-52358.
21. Rubinstein E, Camm J. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother. 2002;49:593-596.
22. Briasoulis A, Agarwal V, Pierce WJ. QT prolongation and torsade de pointes induced by fluoroquinolones: infrequent side effects from commonly used medications. Cardiology. 2011;120:103-110.
23. Prabhakar M, Krahn AD. Ciprofloxacin-induced acquired long QT syndrome. Heart Rhythm. 2004;1:624-626.
24. Demolis JL, Kubitza D, Tenneze L, et al. Effect of a single oral dose of moxifloxacin (400 and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther. 2000;68:658-666.
25. Tome AM, Filipe A. Quinolones: review of psychiatric and neurological adverse reactions. Drug Saf. 2011;34:465-488.
26. Kiangkitiwan B. Levofloxacin-induced delirium with psychotic features. Gen Hosp Psychiatry. 2008;30:381-383.
27. Carbon C. Comparison of side effects of levofloxacin versus other fluoroquinolones. Chemotherapy. 2001;47(suppl 3):S9-S14.
28. LaSalvia EA, Domek GJ, Gitlin DF. Fluoroquinolone-induced suicidal ideation. Gen Hosp Psychiatry. 2010;32:108-110.
29. Stahlmann R, Lode H. Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging. 2010;27:193-209.
30. Owens RC, Ambrose PG. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis. 2005;41(suppl):S144-S157.
31. Smith KM, Lomaestro BM. What role do fluoroquinolone antimicrobial agents play in cardiac dysfunction and altered glycemic control? J Pharm Pract. 2003;16:349-360.
32. LeBlanc M, Belanger C, Cossette P. Severe and resistant hypoglycemia associated with concomitant gatifloxacin and glyburide therapy. Pharmacotherapy. 2004;24:926-931.
33. Graumlich JF, Habis S, Avelino RR, et al. Hypoglycemia in inpatients after gatifloxacin or levofloxacin therapy: nested case control study. Pharmacotherapy. 2005;25:1296-1302.
34. Aspinall SL, Good CB, Jiang R, et al. Severe dysglycemia with the fluoroquinolones: a class effect? Clin Infect Dis. 2009;49:402-408.
35. Mohr JF, McKinnon PS, Peymann PJ, et al. A retrospective, comparative evaluation of dysglycemias in hospitalized patients receiving gatifloxacin, levofloxacin, ciprofloxacin, or ceftriaxone. Pharmacotherapy. 2005;25:1303-1309.
36. Fraunfelder FW, Fraunfelder FT. Diplopia and fluoroquinolones. Ophthalmology. 2009;116:1814-1817.
37. Eiferman RA, Snyder JP, Nordquist RE. Ciprofloxacin microprecipitates and macroprecipitates in the human corneal epithelium. J Cataract Refract Surg. 2001;27:1701-1702.
38. Fraunfelder FW. Corneal toxicity from topical ocular and systemic medications. Cornea. 2006;25:1133-1138.
39. Etminan M, Forooghian F, Brophy JM, et al. Oral fluoroquinolones and the risk of retinal detachment. JAMA. 2012;307:1414-1419.
PPI therapy: When to worry about fracture risk
• For most patients with chronic heartburn and regurgitation, step-down therapy to the lowest effective dose of proton pump inhibitors (PPIs) or treatment with a histamine-2 receptor antagonist (H2RA) is a reasonable, cost-effective approach. A
• Advise elderly patients who require long-term, high-dose PPI therapy to increase their dietary and/or supplemental calcium intake. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Damian F,* a 39-year-old construction worker who takes omeprazole for chronic gastroesophageal reflux disease (GERD), comes in to request a refill. He’s had several accidents in recent years—he fell off a ladder on one occasion, and went down a flight of stairs on another—but none that resulted in significant trauma. Damian admits that he could better control his GERD symptoms by avoiding spicy and fatty foods, limiting alcohol consumption, and quitting smoking, but takes omeprazole nearly every day instead.
CASE 2 Estella G,* a 71-year-old retiree, has been on continuous proton pump inhibitor (PPI) therapy for chronic GERD and erosive esophagitis for nearly 20 years. The patient is a frail woman (body mass index=19.8 kg/m2) and a former smoker (1½ packs a day), both of which increase her risk of osteoporosis. But she has never had a dual energy x-ray absorptiometry (DEXA) scan.
*These cases are based on real patients in my practice, but their names and details have been changed to protect their identity.
Proton pump inhibitors (PPIs) are one of the most commonly used prescription drug categories in the United States,1 but they have been associated with an increase in fracture risk. A US Food and Drug Administration (FDA) safety update issued in March 2011 noted that there is little problem with the lower doses and shorter duration for which over-the-counter PPIs are intended, but patients who take higher-dose prescription PPIs or take prescription PPIs for more than a year may be at greater risk.2
If Damian and Estella were your patients, would you continue to prescribe PPI therapy or offer them alternatives? How should you treat other patients with chronic upper gastrointestinal (GI) distress? The evidence review that follows can help you answer those questions.
How high is the risk? Evidence is mixed (or lacking)
Several retrospective studies have demonstrated a modest increased risk for hip, spine, and wrist fractures in men and women taking PPIs, with the highest risk in patients who have taken higher than standard doses for >4 years.3-6 Concomitant risk factors (alcohol abuse, cigarette smoking, diabetes, and neurologic or renal disease) may increase fracture risk.6 But other retrospective studies, as well as prospective studies, have found no significant increase in fracture risk in patients taking PPIs,7-9 even after 5 years of therapy.7 However, some studies that failed to find an increased risk of osteoporosis with PPI use had a small number of subjects,8,9 resulting in a wide range in confidence intervals.
These findings, based on 6 retrospective case-control, cohort, and cross-sectional studies and 2 prospective cohort studies, are summarized in TABLE 1. No prospective randomized, blinded, controlled trials have examined the potential increased fracture risk associated with PPI use.
Do PPIs interfere with calcium metabolism?
Here, too, the findings are mixed. PPIs are known to inhibit the production and secretion of intragastric hydrochloric acid, which mediates small intestinal absorption of calcium,10 but evidence is conflicting about the role of intragastric hydrochloric acid in calcium absorption. Osteoclasts also have proton pumps, and some researchers have suggested that PPIs have the potential to limit the activity of these proton pumps, leading to reduced bone resorption.11
To date, the only studies that have examined the impact of PPIs on intestinal calcium absorption were limited by the health status of the participants—all either had renal failure and were on hemodialysis or had hypo- or achlorhydria, chronic conditions known to adversely affect calcium metabolism.12 Long-term randomized, double-blinded, placebo-controlled trials are needed to determine whether PPIs adversely affect intestinal calcium absorption and result in bone resorption abnormalities and increased fracture risk.
A closer look at the data
The varying responses associated with PPI dose and duration and the possibility that acid inhibition may decrease calcium absorption support a causal association between PPI use and fracture risk. But the low magnitude of the proposed association (most odds ratios <2) and the lack of data assessing potentially confounding factors limit evidence of causality.3,5,6,9 One key limitation of the earlier studies is that they were not designed to define the specific mechanism underlying the association between PPI therapy and fracture risk.
Older studies suggest a causal relationship
Two case-control studies3,4 found a causal association between PPI use and fracture risk, but one of them failed to identify either a dose-response or a duration-response effect.4 And neither study was designed to define underlying mechanisms to explain the potential association between fracture risk and PPI therapy.
A retrospective matched cohort study5 found an increase in the overall risk of fracture among patients with ≥7 years of PPI therapy and an in-creased risk of hip fracture with ≥5 years of therapy, but short-term risk of fracture was not found to be significant. The results of this study suggest that the risk of osteoporotic fracture increases with duration of exposure to PPI therapy, but not in a dose-dependent fashion.
Newer data are less worrisome
The results of a retrospective cross-sectional trial, published last year, are more reassuring. The researchers determined via univariate analysis that PPI use was associated with a lower risk of osteoporosis, both at the lumbar spine (for all levels of PPI use) and the hip (in patients who had taken more than 1500 standard PPI doses over the previous 5 years).7
This finding—that increasing intensity (both longer duration and higher dosage) of PPI exposure is not associated with an increased risk of osteoporosis—contrasts with results of the authors’ earlier study.5 This may be because they monitored annualized changes in BMD and were able to detect significant changes in other medications participants were taking that might affect bone loss or gain. That allowed them to validate their findings regarding a lack of true association between bone loss and PPI use, the authors reported.
A matched, nested case-control trial8 determined that the use of PPIs does not increase the risk of hip fracture in patients without associated major risk factors (ie, alcohol dependence, underlying neurologic disease, accidental falls, and senility). The researchers suggested that the difference between their findings and those of an earlier nested case-control study3 could mean that the increased risk of hip fracture found in the older study occurred only among PPI users with definable risk factors for hip fracture.
Recent results from the Women’s Health Initiative (WHI) suggest that in postmenopausal women, PPI use is not associated with hip fractures. The WHI did, however, find a modest association between PPI use and clinical spine, forearm, or wrist fracture, as well as total fractures.13 Compared with previous trials, this large cohort study had a large number of fracture events and assessed confounding factors that had not been addressed, including calcium intake. It also was the first trial to assess associations between BMD and fracture risk relative to PPI dosing. Although no specific conclusion was reported, the researchers did not find evidence of dose dependence.
A reasonable approach to PPI use
A consensus statement from the FDA2 and the authors of 2 meta-analyses14,15 recommend that PPIs be used only for appropriate indications—GERD, peptic ulcer disease, dyspepsia, and treatment of Helicobacter pylori—and not in higher doses or for longer periods than are necessary to achieve the desired results.
Whenever possible, implement step-down therapy to the lowest effective dose or prescribe an H2RA rather than a PPI. Both are cost-effective ways to treat most patients with upper GI symptoms.2 It is important, too, to advise elderly patients who require long-term, high-dose PPI therapy to increase their dietary and/or supplemental calcium intake, to recommend DEXA scans for individuals at risk for osteoporosis, and to counsel patients who suffer from GI distress to avoid foods that are known to exacerbate symptoms (TABLE 2).16
TABLE 2
GERD and diet: Foods that worsen symptoms16
| Alcohol |
| Caffeine-containing beverages |
| Citrus fruits |
| Chocolate |
| Fried and fatty foods |
| Garlic and onions |
| Mint flavorings |
| Spicy foods |
| Tomato-based foods (eg, chili, pizza, spaghetti sauce, salsa) |
CASE 1 Damian
You talk to Damian about the association between prolonged PPI therapy and fracture risk and stress the need for dietary changes and lifestyle modifications, particularly smoking cessation. On a return visit several months later, he reports that he has stopped smoking and cut way back on alcohol consumption, and eats fast food less frequently. As a result, he no longer requires chronic use of PPI therapy, and now takes omeprazole only when he has symptoms of GERD—usually, after indulging in fried or fatty foods.
CASE 2 Estella
Estella has severe GERD and erosive esophagitis and will probably need lifelong PPI therapy to adequately control her symptoms. After a detailed discussion of potential risks vs benefits of PPIs, she agrees to a DEXA scan to evaluate for osteoporosis. Her test results show osteopenia in the lumbar spine and femoral neck, but no evidence of osteoporosis. You advise her to increase her consumption of calcium and to undergo DEXA scanning in another 2 years.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected]
1. Bartholow M. Top 200 prescription drugs of 2009. May 11, 2010. Pharmacy Times. Available at: http://www.pharmacytimes. http://www.pharmacytimes.com/publications/issue/2010/May2010/RxFocusTopDrugs-0510. Accessed April 8, 2011.
2. US Food and Drug Administration. FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. May 25, 2010; March 23, 2011 update. Available at: http://www.fda.gov/Drugs/DrugSafety/postmarketdrugsafetyInformationforpatientsandproviders/ucm213206.htm#SafetyAnnouncement. Accessed March 24, 2011.
3. Yang YX, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-2953.
4. Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine h(2) receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76-83.
5. Targownik LE, Lix LM, Metge CJ. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179:319-326.
6. Corley DA, Kubo A, Zhao W, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93-101.
7. Targownik LE, Lix LM, Leung S, et al. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138:896-904.
8. Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008;28:951-959.
9. Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83:251-259.
10. Bo-Linn GW, Davis GR, Buddrus DJ, et al. An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J Clin Invest. 1984;73:640-647.
11. Farina C, Gagliardi S. Selective inhibition of osteoclast vacuolar H+-ATPase. Curr Pharm Des. 2002;8:2033-2048.
12. Insogna KL. The effect of proton pump-inhibiting drugs on mineral metabolism. Am J Gastroenterol. 2009;104(suppl 2):S2-S4.
13. Gray SL, LaCroix AZ, Larson L, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women. Arch Intern Med. 2010;170:765-771.
14. Laine L. Proton pump inhibitors and bone fractures? Am J Gastroenterol. 2009;104(suppl 2):S21-S26.
15. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk. Am J Gastroenterol.;2009;104(suppl 2):S27-S32.
16. National Digestive Diseases Information Clearinghouse. Heartburn, gastroesophageal reflux (GER), and gastroesophageal reflux disease (GERD). Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/gerd. Accessed April 18, 2011.
• For most patients with chronic heartburn and regurgitation, step-down therapy to the lowest effective dose of proton pump inhibitors (PPIs) or treatment with a histamine-2 receptor antagonist (H2RA) is a reasonable, cost-effective approach. A
• Advise elderly patients who require long-term, high-dose PPI therapy to increase their dietary and/or supplemental calcium intake. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Damian F,* a 39-year-old construction worker who takes omeprazole for chronic gastroesophageal reflux disease (GERD), comes in to request a refill. He’s had several accidents in recent years—he fell off a ladder on one occasion, and went down a flight of stairs on another—but none that resulted in significant trauma. Damian admits that he could better control his GERD symptoms by avoiding spicy and fatty foods, limiting alcohol consumption, and quitting smoking, but takes omeprazole nearly every day instead.
CASE 2 Estella G,* a 71-year-old retiree, has been on continuous proton pump inhibitor (PPI) therapy for chronic GERD and erosive esophagitis for nearly 20 years. The patient is a frail woman (body mass index=19.8 kg/m2) and a former smoker (1½ packs a day), both of which increase her risk of osteoporosis. But she has never had a dual energy x-ray absorptiometry (DEXA) scan.
*These cases are based on real patients in my practice, but their names and details have been changed to protect their identity.
Proton pump inhibitors (PPIs) are one of the most commonly used prescription drug categories in the United States,1 but they have been associated with an increase in fracture risk. A US Food and Drug Administration (FDA) safety update issued in March 2011 noted that there is little problem with the lower doses and shorter duration for which over-the-counter PPIs are intended, but patients who take higher-dose prescription PPIs or take prescription PPIs for more than a year may be at greater risk.2
If Damian and Estella were your patients, would you continue to prescribe PPI therapy or offer them alternatives? How should you treat other patients with chronic upper gastrointestinal (GI) distress? The evidence review that follows can help you answer those questions.
How high is the risk? Evidence is mixed (or lacking)
Several retrospective studies have demonstrated a modest increased risk for hip, spine, and wrist fractures in men and women taking PPIs, with the highest risk in patients who have taken higher than standard doses for >4 years.3-6 Concomitant risk factors (alcohol abuse, cigarette smoking, diabetes, and neurologic or renal disease) may increase fracture risk.6 But other retrospective studies, as well as prospective studies, have found no significant increase in fracture risk in patients taking PPIs,7-9 even after 5 years of therapy.7 However, some studies that failed to find an increased risk of osteoporosis with PPI use had a small number of subjects,8,9 resulting in a wide range in confidence intervals.
These findings, based on 6 retrospective case-control, cohort, and cross-sectional studies and 2 prospective cohort studies, are summarized in TABLE 1. No prospective randomized, blinded, controlled trials have examined the potential increased fracture risk associated with PPI use.
Do PPIs interfere with calcium metabolism?
Here, too, the findings are mixed. PPIs are known to inhibit the production and secretion of intragastric hydrochloric acid, which mediates small intestinal absorption of calcium,10 but evidence is conflicting about the role of intragastric hydrochloric acid in calcium absorption. Osteoclasts also have proton pumps, and some researchers have suggested that PPIs have the potential to limit the activity of these proton pumps, leading to reduced bone resorption.11
To date, the only studies that have examined the impact of PPIs on intestinal calcium absorption were limited by the health status of the participants—all either had renal failure and were on hemodialysis or had hypo- or achlorhydria, chronic conditions known to adversely affect calcium metabolism.12 Long-term randomized, double-blinded, placebo-controlled trials are needed to determine whether PPIs adversely affect intestinal calcium absorption and result in bone resorption abnormalities and increased fracture risk.
A closer look at the data
The varying responses associated with PPI dose and duration and the possibility that acid inhibition may decrease calcium absorption support a causal association between PPI use and fracture risk. But the low magnitude of the proposed association (most odds ratios <2) and the lack of data assessing potentially confounding factors limit evidence of causality.3,5,6,9 One key limitation of the earlier studies is that they were not designed to define the specific mechanism underlying the association between PPI therapy and fracture risk.
Older studies suggest a causal relationship
Two case-control studies3,4 found a causal association between PPI use and fracture risk, but one of them failed to identify either a dose-response or a duration-response effect.4 And neither study was designed to define underlying mechanisms to explain the potential association between fracture risk and PPI therapy.
A retrospective matched cohort study5 found an increase in the overall risk of fracture among patients with ≥7 years of PPI therapy and an in-creased risk of hip fracture with ≥5 years of therapy, but short-term risk of fracture was not found to be significant. The results of this study suggest that the risk of osteoporotic fracture increases with duration of exposure to PPI therapy, but not in a dose-dependent fashion.
Newer data are less worrisome
The results of a retrospective cross-sectional trial, published last year, are more reassuring. The researchers determined via univariate analysis that PPI use was associated with a lower risk of osteoporosis, both at the lumbar spine (for all levels of PPI use) and the hip (in patients who had taken more than 1500 standard PPI doses over the previous 5 years).7
This finding—that increasing intensity (both longer duration and higher dosage) of PPI exposure is not associated with an increased risk of osteoporosis—contrasts with results of the authors’ earlier study.5 This may be because they monitored annualized changes in BMD and were able to detect significant changes in other medications participants were taking that might affect bone loss or gain. That allowed them to validate their findings regarding a lack of true association between bone loss and PPI use, the authors reported.
A matched, nested case-control trial8 determined that the use of PPIs does not increase the risk of hip fracture in patients without associated major risk factors (ie, alcohol dependence, underlying neurologic disease, accidental falls, and senility). The researchers suggested that the difference between their findings and those of an earlier nested case-control study3 could mean that the increased risk of hip fracture found in the older study occurred only among PPI users with definable risk factors for hip fracture.
Recent results from the Women’s Health Initiative (WHI) suggest that in postmenopausal women, PPI use is not associated with hip fractures. The WHI did, however, find a modest association between PPI use and clinical spine, forearm, or wrist fracture, as well as total fractures.13 Compared with previous trials, this large cohort study had a large number of fracture events and assessed confounding factors that had not been addressed, including calcium intake. It also was the first trial to assess associations between BMD and fracture risk relative to PPI dosing. Although no specific conclusion was reported, the researchers did not find evidence of dose dependence.
A reasonable approach to PPI use
A consensus statement from the FDA2 and the authors of 2 meta-analyses14,15 recommend that PPIs be used only for appropriate indications—GERD, peptic ulcer disease, dyspepsia, and treatment of Helicobacter pylori—and not in higher doses or for longer periods than are necessary to achieve the desired results.
Whenever possible, implement step-down therapy to the lowest effective dose or prescribe an H2RA rather than a PPI. Both are cost-effective ways to treat most patients with upper GI symptoms.2 It is important, too, to advise elderly patients who require long-term, high-dose PPI therapy to increase their dietary and/or supplemental calcium intake, to recommend DEXA scans for individuals at risk for osteoporosis, and to counsel patients who suffer from GI distress to avoid foods that are known to exacerbate symptoms (TABLE 2).16
TABLE 2
GERD and diet: Foods that worsen symptoms16
| Alcohol |
| Caffeine-containing beverages |
| Citrus fruits |
| Chocolate |
| Fried and fatty foods |
| Garlic and onions |
| Mint flavorings |
| Spicy foods |
| Tomato-based foods (eg, chili, pizza, spaghetti sauce, salsa) |
CASE 1 Damian
You talk to Damian about the association between prolonged PPI therapy and fracture risk and stress the need for dietary changes and lifestyle modifications, particularly smoking cessation. On a return visit several months later, he reports that he has stopped smoking and cut way back on alcohol consumption, and eats fast food less frequently. As a result, he no longer requires chronic use of PPI therapy, and now takes omeprazole only when he has symptoms of GERD—usually, after indulging in fried or fatty foods.
CASE 2 Estella
Estella has severe GERD and erosive esophagitis and will probably need lifelong PPI therapy to adequately control her symptoms. After a detailed discussion of potential risks vs benefits of PPIs, she agrees to a DEXA scan to evaluate for osteoporosis. Her test results show osteopenia in the lumbar spine and femoral neck, but no evidence of osteoporosis. You advise her to increase her consumption of calcium and to undergo DEXA scanning in another 2 years.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected]
• For most patients with chronic heartburn and regurgitation, step-down therapy to the lowest effective dose of proton pump inhibitors (PPIs) or treatment with a histamine-2 receptor antagonist (H2RA) is a reasonable, cost-effective approach. A
• Advise elderly patients who require long-term, high-dose PPI therapy to increase their dietary and/or supplemental calcium intake. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Damian F,* a 39-year-old construction worker who takes omeprazole for chronic gastroesophageal reflux disease (GERD), comes in to request a refill. He’s had several accidents in recent years—he fell off a ladder on one occasion, and went down a flight of stairs on another—but none that resulted in significant trauma. Damian admits that he could better control his GERD symptoms by avoiding spicy and fatty foods, limiting alcohol consumption, and quitting smoking, but takes omeprazole nearly every day instead.
CASE 2 Estella G,* a 71-year-old retiree, has been on continuous proton pump inhibitor (PPI) therapy for chronic GERD and erosive esophagitis for nearly 20 years. The patient is a frail woman (body mass index=19.8 kg/m2) and a former smoker (1½ packs a day), both of which increase her risk of osteoporosis. But she has never had a dual energy x-ray absorptiometry (DEXA) scan.
*These cases are based on real patients in my practice, but their names and details have been changed to protect their identity.
Proton pump inhibitors (PPIs) are one of the most commonly used prescription drug categories in the United States,1 but they have been associated with an increase in fracture risk. A US Food and Drug Administration (FDA) safety update issued in March 2011 noted that there is little problem with the lower doses and shorter duration for which over-the-counter PPIs are intended, but patients who take higher-dose prescription PPIs or take prescription PPIs for more than a year may be at greater risk.2
If Damian and Estella were your patients, would you continue to prescribe PPI therapy or offer them alternatives? How should you treat other patients with chronic upper gastrointestinal (GI) distress? The evidence review that follows can help you answer those questions.
How high is the risk? Evidence is mixed (or lacking)
Several retrospective studies have demonstrated a modest increased risk for hip, spine, and wrist fractures in men and women taking PPIs, with the highest risk in patients who have taken higher than standard doses for >4 years.3-6 Concomitant risk factors (alcohol abuse, cigarette smoking, diabetes, and neurologic or renal disease) may increase fracture risk.6 But other retrospective studies, as well as prospective studies, have found no significant increase in fracture risk in patients taking PPIs,7-9 even after 5 years of therapy.7 However, some studies that failed to find an increased risk of osteoporosis with PPI use had a small number of subjects,8,9 resulting in a wide range in confidence intervals.
These findings, based on 6 retrospective case-control, cohort, and cross-sectional studies and 2 prospective cohort studies, are summarized in TABLE 1. No prospective randomized, blinded, controlled trials have examined the potential increased fracture risk associated with PPI use.
Do PPIs interfere with calcium metabolism?
Here, too, the findings are mixed. PPIs are known to inhibit the production and secretion of intragastric hydrochloric acid, which mediates small intestinal absorption of calcium,10 but evidence is conflicting about the role of intragastric hydrochloric acid in calcium absorption. Osteoclasts also have proton pumps, and some researchers have suggested that PPIs have the potential to limit the activity of these proton pumps, leading to reduced bone resorption.11
To date, the only studies that have examined the impact of PPIs on intestinal calcium absorption were limited by the health status of the participants—all either had renal failure and were on hemodialysis or had hypo- or achlorhydria, chronic conditions known to adversely affect calcium metabolism.12 Long-term randomized, double-blinded, placebo-controlled trials are needed to determine whether PPIs adversely affect intestinal calcium absorption and result in bone resorption abnormalities and increased fracture risk.
A closer look at the data
The varying responses associated with PPI dose and duration and the possibility that acid inhibition may decrease calcium absorption support a causal association between PPI use and fracture risk. But the low magnitude of the proposed association (most odds ratios <2) and the lack of data assessing potentially confounding factors limit evidence of causality.3,5,6,9 One key limitation of the earlier studies is that they were not designed to define the specific mechanism underlying the association between PPI therapy and fracture risk.
Older studies suggest a causal relationship
Two case-control studies3,4 found a causal association between PPI use and fracture risk, but one of them failed to identify either a dose-response or a duration-response effect.4 And neither study was designed to define underlying mechanisms to explain the potential association between fracture risk and PPI therapy.
A retrospective matched cohort study5 found an increase in the overall risk of fracture among patients with ≥7 years of PPI therapy and an in-creased risk of hip fracture with ≥5 years of therapy, but short-term risk of fracture was not found to be significant. The results of this study suggest that the risk of osteoporotic fracture increases with duration of exposure to PPI therapy, but not in a dose-dependent fashion.
Newer data are less worrisome
The results of a retrospective cross-sectional trial, published last year, are more reassuring. The researchers determined via univariate analysis that PPI use was associated with a lower risk of osteoporosis, both at the lumbar spine (for all levels of PPI use) and the hip (in patients who had taken more than 1500 standard PPI doses over the previous 5 years).7
This finding—that increasing intensity (both longer duration and higher dosage) of PPI exposure is not associated with an increased risk of osteoporosis—contrasts with results of the authors’ earlier study.5 This may be because they monitored annualized changes in BMD and were able to detect significant changes in other medications participants were taking that might affect bone loss or gain. That allowed them to validate their findings regarding a lack of true association between bone loss and PPI use, the authors reported.
A matched, nested case-control trial8 determined that the use of PPIs does not increase the risk of hip fracture in patients without associated major risk factors (ie, alcohol dependence, underlying neurologic disease, accidental falls, and senility). The researchers suggested that the difference between their findings and those of an earlier nested case-control study3 could mean that the increased risk of hip fracture found in the older study occurred only among PPI users with definable risk factors for hip fracture.
Recent results from the Women’s Health Initiative (WHI) suggest that in postmenopausal women, PPI use is not associated with hip fractures. The WHI did, however, find a modest association between PPI use and clinical spine, forearm, or wrist fracture, as well as total fractures.13 Compared with previous trials, this large cohort study had a large number of fracture events and assessed confounding factors that had not been addressed, including calcium intake. It also was the first trial to assess associations between BMD and fracture risk relative to PPI dosing. Although no specific conclusion was reported, the researchers did not find evidence of dose dependence.
A reasonable approach to PPI use
A consensus statement from the FDA2 and the authors of 2 meta-analyses14,15 recommend that PPIs be used only for appropriate indications—GERD, peptic ulcer disease, dyspepsia, and treatment of Helicobacter pylori—and not in higher doses or for longer periods than are necessary to achieve the desired results.
Whenever possible, implement step-down therapy to the lowest effective dose or prescribe an H2RA rather than a PPI. Both are cost-effective ways to treat most patients with upper GI symptoms.2 It is important, too, to advise elderly patients who require long-term, high-dose PPI therapy to increase their dietary and/or supplemental calcium intake, to recommend DEXA scans for individuals at risk for osteoporosis, and to counsel patients who suffer from GI distress to avoid foods that are known to exacerbate symptoms (TABLE 2).16
TABLE 2
GERD and diet: Foods that worsen symptoms16
| Alcohol |
| Caffeine-containing beverages |
| Citrus fruits |
| Chocolate |
| Fried and fatty foods |
| Garlic and onions |
| Mint flavorings |
| Spicy foods |
| Tomato-based foods (eg, chili, pizza, spaghetti sauce, salsa) |
CASE 1 Damian
You talk to Damian about the association between prolonged PPI therapy and fracture risk and stress the need for dietary changes and lifestyle modifications, particularly smoking cessation. On a return visit several months later, he reports that he has stopped smoking and cut way back on alcohol consumption, and eats fast food less frequently. As a result, he no longer requires chronic use of PPI therapy, and now takes omeprazole only when he has symptoms of GERD—usually, after indulging in fried or fatty foods.
CASE 2 Estella
Estella has severe GERD and erosive esophagitis and will probably need lifelong PPI therapy to adequately control her symptoms. After a detailed discussion of potential risks vs benefits of PPIs, she agrees to a DEXA scan to evaluate for osteoporosis. Her test results show osteopenia in the lumbar spine and femoral neck, but no evidence of osteoporosis. You advise her to increase her consumption of calcium and to undergo DEXA scanning in another 2 years.
CORRESPONDENCE
Joel J. Heidelbaugh, MD, FAAFP, FACG, Ypsilanti Health Center, 200 Arnet Suite 200, Ypsilanti, MI 48198; [email protected]
1. Bartholow M. Top 200 prescription drugs of 2009. May 11, 2010. Pharmacy Times. Available at: http://www.pharmacytimes. http://www.pharmacytimes.com/publications/issue/2010/May2010/RxFocusTopDrugs-0510. Accessed April 8, 2011.
2. US Food and Drug Administration. FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. May 25, 2010; March 23, 2011 update. Available at: http://www.fda.gov/Drugs/DrugSafety/postmarketdrugsafetyInformationforpatientsandproviders/ucm213206.htm#SafetyAnnouncement. Accessed March 24, 2011.
3. Yang YX, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-2953.
4. Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine h(2) receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76-83.
5. Targownik LE, Lix LM, Metge CJ. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179:319-326.
6. Corley DA, Kubo A, Zhao W, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93-101.
7. Targownik LE, Lix LM, Leung S, et al. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138:896-904.
8. Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008;28:951-959.
9. Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83:251-259.
10. Bo-Linn GW, Davis GR, Buddrus DJ, et al. An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J Clin Invest. 1984;73:640-647.
11. Farina C, Gagliardi S. Selective inhibition of osteoclast vacuolar H+-ATPase. Curr Pharm Des. 2002;8:2033-2048.
12. Insogna KL. The effect of proton pump-inhibiting drugs on mineral metabolism. Am J Gastroenterol. 2009;104(suppl 2):S2-S4.
13. Gray SL, LaCroix AZ, Larson L, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women. Arch Intern Med. 2010;170:765-771.
14. Laine L. Proton pump inhibitors and bone fractures? Am J Gastroenterol. 2009;104(suppl 2):S21-S26.
15. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk. Am J Gastroenterol.;2009;104(suppl 2):S27-S32.
16. National Digestive Diseases Information Clearinghouse. Heartburn, gastroesophageal reflux (GER), and gastroesophageal reflux disease (GERD). Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/gerd. Accessed April 18, 2011.
1. Bartholow M. Top 200 prescription drugs of 2009. May 11, 2010. Pharmacy Times. Available at: http://www.pharmacytimes. http://www.pharmacytimes.com/publications/issue/2010/May2010/RxFocusTopDrugs-0510. Accessed April 8, 2011.
2. US Food and Drug Administration. FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. May 25, 2010; March 23, 2011 update. Available at: http://www.fda.gov/Drugs/DrugSafety/postmarketdrugsafetyInformationforpatientsandproviders/ucm213206.htm#SafetyAnnouncement. Accessed March 24, 2011.
3. Yang YX, Lewis JD, Epstein S, et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-2953.
4. Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine h(2) receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76-83.
5. Targownik LE, Lix LM, Metge CJ. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179:319-326.
6. Corley DA, Kubo A, Zhao W, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93-101.
7. Targownik LE, Lix LM, Leung S, et al. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138:896-904.
8. Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008;28:951-959.
9. Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83:251-259.
10. Bo-Linn GW, Davis GR, Buddrus DJ, et al. An evaluation of the importance of gastric acid secretion in the absorption of dietary calcium. J Clin Invest. 1984;73:640-647.
11. Farina C, Gagliardi S. Selective inhibition of osteoclast vacuolar H+-ATPase. Curr Pharm Des. 2002;8:2033-2048.
12. Insogna KL. The effect of proton pump-inhibiting drugs on mineral metabolism. Am J Gastroenterol. 2009;104(suppl 2):S2-S4.
13. Gray SL, LaCroix AZ, Larson L, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women. Arch Intern Med. 2010;170:765-771.
14. Laine L. Proton pump inhibitors and bone fractures? Am J Gastroenterol. 2009;104(suppl 2):S21-S26.
15. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk. Am J Gastroenterol.;2009;104(suppl 2):S27-S32.
16. National Digestive Diseases Information Clearinghouse. Heartburn, gastroesophageal reflux (GER), and gastroesophageal reflux disease (GERD). Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/gerd. Accessed April 18, 2011.