User login
Some patients leave a scar on you
Every surgeon has experienced the anguish of an adverse outcome. The elective aneurysm that dies on the table, the asymptomatic carotid patient that has a stroke in the recovery room, the cosmetic varicose vein case that has a pulmonary embolus. Driving home alone, we tell ourselves that we did our “best,” but lingering in the dark shadows of our minds are the nagging questions: What should I have done differently? Am I really a safe surgeon? Should I quit and get a job with “industry”? What if I get sued? How should I deal with the family? Will I get fired?
Our houses are dark when we arrive home. We sit alone in living rooms silently mulling over the events of the day. Our spouses have seen this before and will offer sincere consolation, but will never really know how it feels. So we do what surgeons are trained to do – we suck it up and hide our feelings. As the Brits say: “Keep calm and carry on!”
A few years ago, I operated on a young woman with suspected median arcuate ligament syndrome. She had experienced temporary improvement after laparoscopic release of the median arcuate ligament at an outside hospital, but her symptoms returned after a few months.
Initially, I attempted to place a stent in the celiac artery from the groin but failed to establish a stable access sheath. Rather than choosing a brachial approach, I recommended open repair. The next day in the operating room, I was surprised to find a distinct blue tint to the adventitia of the celiac and hepatic arteries typical of dissection. After opening the common hepatic artery, I discovered that the dissection continued well into the bifurcation of the proper hepatic artery, forcing me to clamp the gastroduodenal artery, the primary collateral pathway to the liver. Within minutes, the liver turned a nauseating purple black.
I urgently constructed an aorto-hepatic bypass with vein using 8-0 suture to try to tack the dissection flap into place distally. I tried to ignore the dire appearance of the liver as I worked, but I was fearful that my distal anastomosis would be inadequate. When I took off the clamps, the liver improved slightly but remained bruised. The finding of a Doppler signal distal to my anastomosis gave me some hope but I remained fearful about the viability of the liver.
Postop, I found her husband in the waiting room with two small children. I explained the potentially catastrophic circumstances and prepared him for the possibility that she might need a liver transplant! He was stunned and angry but mostly silent. Her liver function tests (LFTs) deteriorated over the next 3 days, leaving me depressed, anxious, and sleepless. I hated making rounds on her. Her husband was invariably lying on a couch in her room, pictures of her children taped to her headboard. I reached out to hepatology and transplant surgery hoping for some encouragement. My partners patted me on the back and reminded me that they’d all been in similar binds. I swore to myself that I’d never do another operation on a patient with median arcuate ligament syndrome.
On the morning of the fourth postop day, her LFTs miraculously reversed course and she made an uneventful recovery. But I was scarred. To this day, when I see the diagnosis of median arcuate ligament syndrome on a chart in the office, I shudder. I remember the color of her liver – like the deep blackness of the abyss.
Some patients leave a scar on you. But how we, as surgeons, deal with adversity is largely unknown. Each of us has to discover through trial and error the most effective way to respond to unwanted outcomes. We model ourselves after our teachers, mentors, and chief residents. Some of us have enlightened, sympathetic partners to turn to for consolation, advice, and “competent critique.” But others may be isolated in solo practice or in shared-expense practice models where “partners” may actually be competitors.
Some of the same traits that make us effective surgeons – autonomy, courage, and leadership – also make us particularly unlikely to seek outside counsel. We fear that acknowledging our humanity will be perceived as a sign of weakness. While it has become common practice in most hospitals to have programs for so-called “second victims” – for example, emergency workers, nurses, and others caring for victims of the Boston Marathon bombing – it is uncommon for surgeons to take advantage of these resources. Surgeons tend to rely on each other, like soldiers in battle, for advice, consolation, and improvement. Professionals call it “peer support” and it forms the basis of some successful peer-to-peer rehabilitation programs.
Over the next few months, we hope to initiate a dialogue among members of the SVS community about how we can learn to best care for each other. Few of us have any formal training in how to ask for or provide assistance. We hope that you will share your stories, techniques, and best practices.
Who do you turn to for advice in times of adversity? A spouse, trusted senior partner, a mentor, a defense lawyer, a priest, a bottle? How do you respond to your partners facing adversity? How do you recognize in yourself, or your colleagues, that an adverse outcome has affected your ability to deliver safe, compassionate care? How do you listen for telltale signs that substance abuse, depression, or suicidal ideation have entered the equation? And what should you do next?
I’m sure that, like me, most of you are burned out on burnout. And while I don’t diminish the importance of personal resilience, I also think that we as surgeons can learn to be better caregivers for each other. That we can learn from others how to ask the right questions, and how to be more attentive listeners. Vascular surgery is undoubtedly an immensely rewarding career, but it can bring with it very intense personal challenges. Through the resources of the SVS, we hope to raise awareness of the importance of peer support, to provide a forum to share our stories, and to develop programs that will assist each of us in acquiring the tools and skills to be better partners. Your comments are encouraged.
John F Eidt, MD, is a vascular surgeon at Baylor Scott & White Heart and Vascular Hospital, Dallas.
Every surgeon has experienced the anguish of an adverse outcome. The elective aneurysm that dies on the table, the asymptomatic carotid patient that has a stroke in the recovery room, the cosmetic varicose vein case that has a pulmonary embolus. Driving home alone, we tell ourselves that we did our “best,” but lingering in the dark shadows of our minds are the nagging questions: What should I have done differently? Am I really a safe surgeon? Should I quit and get a job with “industry”? What if I get sued? How should I deal with the family? Will I get fired?
Our houses are dark when we arrive home. We sit alone in living rooms silently mulling over the events of the day. Our spouses have seen this before and will offer sincere consolation, but will never really know how it feels. So we do what surgeons are trained to do – we suck it up and hide our feelings. As the Brits say: “Keep calm and carry on!”
A few years ago, I operated on a young woman with suspected median arcuate ligament syndrome. She had experienced temporary improvement after laparoscopic release of the median arcuate ligament at an outside hospital, but her symptoms returned after a few months.
Initially, I attempted to place a stent in the celiac artery from the groin but failed to establish a stable access sheath. Rather than choosing a brachial approach, I recommended open repair. The next day in the operating room, I was surprised to find a distinct blue tint to the adventitia of the celiac and hepatic arteries typical of dissection. After opening the common hepatic artery, I discovered that the dissection continued well into the bifurcation of the proper hepatic artery, forcing me to clamp the gastroduodenal artery, the primary collateral pathway to the liver. Within minutes, the liver turned a nauseating purple black.
I urgently constructed an aorto-hepatic bypass with vein using 8-0 suture to try to tack the dissection flap into place distally. I tried to ignore the dire appearance of the liver as I worked, but I was fearful that my distal anastomosis would be inadequate. When I took off the clamps, the liver improved slightly but remained bruised. The finding of a Doppler signal distal to my anastomosis gave me some hope but I remained fearful about the viability of the liver.
Postop, I found her husband in the waiting room with two small children. I explained the potentially catastrophic circumstances and prepared him for the possibility that she might need a liver transplant! He was stunned and angry but mostly silent. Her liver function tests (LFTs) deteriorated over the next 3 days, leaving me depressed, anxious, and sleepless. I hated making rounds on her. Her husband was invariably lying on a couch in her room, pictures of her children taped to her headboard. I reached out to hepatology and transplant surgery hoping for some encouragement. My partners patted me on the back and reminded me that they’d all been in similar binds. I swore to myself that I’d never do another operation on a patient with median arcuate ligament syndrome.
On the morning of the fourth postop day, her LFTs miraculously reversed course and she made an uneventful recovery. But I was scarred. To this day, when I see the diagnosis of median arcuate ligament syndrome on a chart in the office, I shudder. I remember the color of her liver – like the deep blackness of the abyss.
Some patients leave a scar on you. But how we, as surgeons, deal with adversity is largely unknown. Each of us has to discover through trial and error the most effective way to respond to unwanted outcomes. We model ourselves after our teachers, mentors, and chief residents. Some of us have enlightened, sympathetic partners to turn to for consolation, advice, and “competent critique.” But others may be isolated in solo practice or in shared-expense practice models where “partners” may actually be competitors.
Some of the same traits that make us effective surgeons – autonomy, courage, and leadership – also make us particularly unlikely to seek outside counsel. We fear that acknowledging our humanity will be perceived as a sign of weakness. While it has become common practice in most hospitals to have programs for so-called “second victims” – for example, emergency workers, nurses, and others caring for victims of the Boston Marathon bombing – it is uncommon for surgeons to take advantage of these resources. Surgeons tend to rely on each other, like soldiers in battle, for advice, consolation, and improvement. Professionals call it “peer support” and it forms the basis of some successful peer-to-peer rehabilitation programs.
Over the next few months, we hope to initiate a dialogue among members of the SVS community about how we can learn to best care for each other. Few of us have any formal training in how to ask for or provide assistance. We hope that you will share your stories, techniques, and best practices.
Who do you turn to for advice in times of adversity? A spouse, trusted senior partner, a mentor, a defense lawyer, a priest, a bottle? How do you respond to your partners facing adversity? How do you recognize in yourself, or your colleagues, that an adverse outcome has affected your ability to deliver safe, compassionate care? How do you listen for telltale signs that substance abuse, depression, or suicidal ideation have entered the equation? And what should you do next?
I’m sure that, like me, most of you are burned out on burnout. And while I don’t diminish the importance of personal resilience, I also think that we as surgeons can learn to be better caregivers for each other. That we can learn from others how to ask the right questions, and how to be more attentive listeners. Vascular surgery is undoubtedly an immensely rewarding career, but it can bring with it very intense personal challenges. Through the resources of the SVS, we hope to raise awareness of the importance of peer support, to provide a forum to share our stories, and to develop programs that will assist each of us in acquiring the tools and skills to be better partners. Your comments are encouraged.
John F Eidt, MD, is a vascular surgeon at Baylor Scott & White Heart and Vascular Hospital, Dallas.
Every surgeon has experienced the anguish of an adverse outcome. The elective aneurysm that dies on the table, the asymptomatic carotid patient that has a stroke in the recovery room, the cosmetic varicose vein case that has a pulmonary embolus. Driving home alone, we tell ourselves that we did our “best,” but lingering in the dark shadows of our minds are the nagging questions: What should I have done differently? Am I really a safe surgeon? Should I quit and get a job with “industry”? What if I get sued? How should I deal with the family? Will I get fired?
Our houses are dark when we arrive home. We sit alone in living rooms silently mulling over the events of the day. Our spouses have seen this before and will offer sincere consolation, but will never really know how it feels. So we do what surgeons are trained to do – we suck it up and hide our feelings. As the Brits say: “Keep calm and carry on!”
A few years ago, I operated on a young woman with suspected median arcuate ligament syndrome. She had experienced temporary improvement after laparoscopic release of the median arcuate ligament at an outside hospital, but her symptoms returned after a few months.
Initially, I attempted to place a stent in the celiac artery from the groin but failed to establish a stable access sheath. Rather than choosing a brachial approach, I recommended open repair. The next day in the operating room, I was surprised to find a distinct blue tint to the adventitia of the celiac and hepatic arteries typical of dissection. After opening the common hepatic artery, I discovered that the dissection continued well into the bifurcation of the proper hepatic artery, forcing me to clamp the gastroduodenal artery, the primary collateral pathway to the liver. Within minutes, the liver turned a nauseating purple black.
I urgently constructed an aorto-hepatic bypass with vein using 8-0 suture to try to tack the dissection flap into place distally. I tried to ignore the dire appearance of the liver as I worked, but I was fearful that my distal anastomosis would be inadequate. When I took off the clamps, the liver improved slightly but remained bruised. The finding of a Doppler signal distal to my anastomosis gave me some hope but I remained fearful about the viability of the liver.
Postop, I found her husband in the waiting room with two small children. I explained the potentially catastrophic circumstances and prepared him for the possibility that she might need a liver transplant! He was stunned and angry but mostly silent. Her liver function tests (LFTs) deteriorated over the next 3 days, leaving me depressed, anxious, and sleepless. I hated making rounds on her. Her husband was invariably lying on a couch in her room, pictures of her children taped to her headboard. I reached out to hepatology and transplant surgery hoping for some encouragement. My partners patted me on the back and reminded me that they’d all been in similar binds. I swore to myself that I’d never do another operation on a patient with median arcuate ligament syndrome.
On the morning of the fourth postop day, her LFTs miraculously reversed course and she made an uneventful recovery. But I was scarred. To this day, when I see the diagnosis of median arcuate ligament syndrome on a chart in the office, I shudder. I remember the color of her liver – like the deep blackness of the abyss.
Some patients leave a scar on you. But how we, as surgeons, deal with adversity is largely unknown. Each of us has to discover through trial and error the most effective way to respond to unwanted outcomes. We model ourselves after our teachers, mentors, and chief residents. Some of us have enlightened, sympathetic partners to turn to for consolation, advice, and “competent critique.” But others may be isolated in solo practice or in shared-expense practice models where “partners” may actually be competitors.
Some of the same traits that make us effective surgeons – autonomy, courage, and leadership – also make us particularly unlikely to seek outside counsel. We fear that acknowledging our humanity will be perceived as a sign of weakness. While it has become common practice in most hospitals to have programs for so-called “second victims” – for example, emergency workers, nurses, and others caring for victims of the Boston Marathon bombing – it is uncommon for surgeons to take advantage of these resources. Surgeons tend to rely on each other, like soldiers in battle, for advice, consolation, and improvement. Professionals call it “peer support” and it forms the basis of some successful peer-to-peer rehabilitation programs.
Over the next few months, we hope to initiate a dialogue among members of the SVS community about how we can learn to best care for each other. Few of us have any formal training in how to ask for or provide assistance. We hope that you will share your stories, techniques, and best practices.
Who do you turn to for advice in times of adversity? A spouse, trusted senior partner, a mentor, a defense lawyer, a priest, a bottle? How do you respond to your partners facing adversity? How do you recognize in yourself, or your colleagues, that an adverse outcome has affected your ability to deliver safe, compassionate care? How do you listen for telltale signs that substance abuse, depression, or suicidal ideation have entered the equation? And what should you do next?
I’m sure that, like me, most of you are burned out on burnout. And while I don’t diminish the importance of personal resilience, I also think that we as surgeons can learn to be better caregivers for each other. That we can learn from others how to ask the right questions, and how to be more attentive listeners. Vascular surgery is undoubtedly an immensely rewarding career, but it can bring with it very intense personal challenges. Through the resources of the SVS, we hope to raise awareness of the importance of peer support, to provide a forum to share our stories, and to develop programs that will assist each of us in acquiring the tools and skills to be better partners. Your comments are encouraged.
John F Eidt, MD, is a vascular surgeon at Baylor Scott & White Heart and Vascular Hospital, Dallas.
Using the gracilis muscle flap
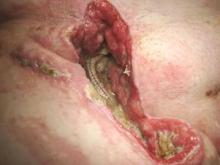
Muscle flaps have come to play an invaluable role in the management of complex groin wounds (Figure 1). We have found that the gracilis muscle offers significant advantages over other local muscle flaps. In comparison to the segmental blood supply of the sartorius muscle which can be disrupted during mobilization, the gracilis muscle has a single vascular pedicle that arises reliably from the medial femoral circumflex vessels. Unlike the sartorius muscle which may be damaged by the infectious/inflammatory process in the groin, the gracilis muscle is remote from the groin wound itself. In addition, the procedure is relatively simple and can be completed in less than thirty minutes.
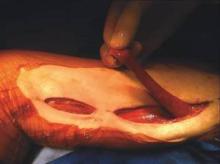
The procedure is performed with the patient in the supine position with the knee slightly flexed. The gracilis muscle can be palpated along the medial aspect of the thigh and the longitudinal incision is placed directly over the muscle . After the deep fascia is divided, the muscle can be easily freed from surrounding attachments (Figure 2). We completely mobilize the distal two-thirds of the muscle and divide it at the musculotendinous insertion on the femur. We do not mobilize the proximal one-third of the muscle to avoid injury to the vascular pedicle). The muscle is retroflexed into the groin wound using a ringed-forceps. The muscle provides excellent coverage of the femoral triangle. A vacuum dressing may be applied without concern for injury to the femoral vessels.
We used the gracilis flap to treat complex groin wounds in 68 limbs in 64 patients at the University of Arkansas for Medical Sciences, Little Rock. Complete healing was achieved in 91%. In six patients (9%), recurrent or persistent infection led to bleeding that required surgical management. Limb salvage was achieved in 86%.
The presence of autogenous vascular reconstruction was associated with a reduced risk of persistent/recurrent infection in comparison to synthetic grafts (2.3% vs 23.8%, P = .006). Age greater than 75 years was associated with worse outcomes overall. Wound problems (infection, hematoma, seroma) at the harvest site were rare.
We prefer the gracilis flap to the sartorius or other groin muscle flaps in the management of complex groin wounds. The procedure is simple enough for a vascular surgeon, the muscle is reliable in location and blood supply, and the results are satisfactory given the complexity of the problem.
Dr. Eidt is at Greenville Health System, University of South Carolina School of Medicine Greenville, and Dr. Ali is at University of Arkansas for Medical Sciences, Little Rock.
Muscle flaps have come to play an invaluable role in the management of complex groin wounds (Figure 1). We have found that the gracilis muscle offers significant advantages over other local muscle flaps. In comparison to the segmental blood supply of the sartorius muscle which can be disrupted during mobilization, the gracilis muscle has a single vascular pedicle that arises reliably from the medial femoral circumflex vessels. Unlike the sartorius muscle which may be damaged by the infectious/inflammatory process in the groin, the gracilis muscle is remote from the groin wound itself. In addition, the procedure is relatively simple and can be completed in less than thirty minutes.
The procedure is performed with the patient in the supine position with the knee slightly flexed. The gracilis muscle can be palpated along the medial aspect of the thigh and the longitudinal incision is placed directly over the muscle . After the deep fascia is divided, the muscle can be easily freed from surrounding attachments (Figure 2). We completely mobilize the distal two-thirds of the muscle and divide it at the musculotendinous insertion on the femur. We do not mobilize the proximal one-third of the muscle to avoid injury to the vascular pedicle). The muscle is retroflexed into the groin wound using a ringed-forceps. The muscle provides excellent coverage of the femoral triangle. A vacuum dressing may be applied without concern for injury to the femoral vessels.
We used the gracilis flap to treat complex groin wounds in 68 limbs in 64 patients at the University of Arkansas for Medical Sciences, Little Rock. Complete healing was achieved in 91%. In six patients (9%), recurrent or persistent infection led to bleeding that required surgical management. Limb salvage was achieved in 86%.
The presence of autogenous vascular reconstruction was associated with a reduced risk of persistent/recurrent infection in comparison to synthetic grafts (2.3% vs 23.8%, P = .006). Age greater than 75 years was associated with worse outcomes overall. Wound problems (infection, hematoma, seroma) at the harvest site were rare.
We prefer the gracilis flap to the sartorius or other groin muscle flaps in the management of complex groin wounds. The procedure is simple enough for a vascular surgeon, the muscle is reliable in location and blood supply, and the results are satisfactory given the complexity of the problem.
Dr. Eidt is at Greenville Health System, University of South Carolina School of Medicine Greenville, and Dr. Ali is at University of Arkansas for Medical Sciences, Little Rock.
Muscle flaps have come to play an invaluable role in the management of complex groin wounds (Figure 1). We have found that the gracilis muscle offers significant advantages over other local muscle flaps. In comparison to the segmental blood supply of the sartorius muscle which can be disrupted during mobilization, the gracilis muscle has a single vascular pedicle that arises reliably from the medial femoral circumflex vessels. Unlike the sartorius muscle which may be damaged by the infectious/inflammatory process in the groin, the gracilis muscle is remote from the groin wound itself. In addition, the procedure is relatively simple and can be completed in less than thirty minutes.
The procedure is performed with the patient in the supine position with the knee slightly flexed. The gracilis muscle can be palpated along the medial aspect of the thigh and the longitudinal incision is placed directly over the muscle . After the deep fascia is divided, the muscle can be easily freed from surrounding attachments (Figure 2). We completely mobilize the distal two-thirds of the muscle and divide it at the musculotendinous insertion on the femur. We do not mobilize the proximal one-third of the muscle to avoid injury to the vascular pedicle). The muscle is retroflexed into the groin wound using a ringed-forceps. The muscle provides excellent coverage of the femoral triangle. A vacuum dressing may be applied without concern for injury to the femoral vessels.
We used the gracilis flap to treat complex groin wounds in 68 limbs in 64 patients at the University of Arkansas for Medical Sciences, Little Rock. Complete healing was achieved in 91%. In six patients (9%), recurrent or persistent infection led to bleeding that required surgical management. Limb salvage was achieved in 86%.
The presence of autogenous vascular reconstruction was associated with a reduced risk of persistent/recurrent infection in comparison to synthetic grafts (2.3% vs 23.8%, P = .006). Age greater than 75 years was associated with worse outcomes overall. Wound problems (infection, hematoma, seroma) at the harvest site were rare.
We prefer the gracilis flap to the sartorius or other groin muscle flaps in the management of complex groin wounds. The procedure is simple enough for a vascular surgeon, the muscle is reliable in location and blood supply, and the results are satisfactory given the complexity of the problem.
Dr. Eidt is at Greenville Health System, University of South Carolina School of Medicine Greenville, and Dr. Ali is at University of Arkansas for Medical Sciences, Little Rock.