User login
When a fetus survives methotrexate exposure
CASE A 28-year-old woman, gravida 4, para 3, requested medical termination of her pregnancy at approximately 7 weeks’ gestation. She was given intramuscular methotrexate 50 mg/m2 and oral misoprostol 400 mcg. Nine weeks later, she presented at our primary care clinic complaining of mild pelvic pain. Given her history, we ordered transvaginal ultrasound, which showed a viable pregnancy with an average ultrasound age of 16 weeks’ gestation and grossly normal fetal anatomy. We counseled the patient regarding the risks associated with maintaining the pregnancy after exposure to methotrexate, and she and her husband elected to proceed with the pregnancy.
Throughout the pregnancy, a perinatologist conducted serial 2-dimensional ultrasounds. At 30 weeks’ gestation, ultrasound revealed mild hydrocephalus but yielded poor visualization of the kidneys, heart, and spine. A repeat ultrasound at 34 weeks demonstrated unchanged hydrocephalus and normal fetal anatomy with appropriate interval growth. A fetal echocardiogram in utero showed no cardiac anomalies. At 39 weeks, the patient underwent induction of labor due to severe oligohydramnios. A viable female infant weighing 2456 g was delivered by spontaneous vaginal delivery, with Apgar scores of 8 and 9 at 1 and 5 minutes, respectively.
The infant was small for her gestational age. Her hands had 4 digits each, including the thumb (FIGURE 1A). The upper extremities were shorter than expected in relation to the infant’s torso and were locked in 90 degrees of flexion at the elbow (FIGURE 1B). Her lower extremities were both normal in length, but her left ankle joint was everted with considerable laxity at the tibiotalar joint. The infant’s mandible deviated to the right. An ultrasound of her head showed dilation of the lateral ventricle, consistent with nonobstructive hydrocephalus. A skeletal survey revealed bilateral radial-humeral synostosis at approximately 90 degrees of flexion. The second day after birth, the infant was transferred to a tertiary medical facility for further evaluation by pediatric subspecialists.
A pediatric orthopedic surgeon noted bilateral hip dysplasia and fitted the infant for a Pavlik harness. Ultrasound of the spine identified a dysmorphic sacrum with a thickened conus medullaris ending at the level of L2-L3, with increased risk for a tethered spinal cord. To correct the hydrocephalus, a neurosurgeon recommended intervention in the neonatal period. The parents were counseled to expect some degree of developmental disability, and karyotyping was performed to rule out potential genetically linked syndromes.
FIGURE 1
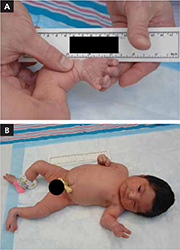
Methotrexate exposure led to these congenital anomalies
Methotrexate exposure at 7 weeks’ gestation resulted in this child having 4 digits on each hand (A), shortened arms locked in 90 degrees of flexion at the elbow, and an everted left ankle joint (B).
Risks associated with methotrexate
In the 1960s, methotrexate was commonly used as an abortifacient.1 Its use for that purpose became rare, however, after multiple reports from the 1950s to the 1970s of congenital malformations in infants exposed to the drug in utero, either inadvertently or after attempted abortion.1 In the 1990s, its use increased again in conjunction with misoprostol, primarily for medical management of suspected ectopic pregnancies and less often for elective terminations. Use of the combination resulted in fewer reports of congenital anomalies.1
The failure rate of medical abortion using methotrexate varies. In 1999, one study reported an 8% failure rate when methotrexate was used with misoprostol.2 In 2004, methotrexate alone led to a failure rate of 31% in medical termination of early pregnancy.3 In 2005, another study of methotrexate and misoprostol used in combination for elective termination reported a failure rate of 2% to 10%.4
Risk is not always foreseen
Methotrexate is widely used to treat such conditions as neoplastic disease and autoimmune disorders. Unintended exposure of a fetus to methotrexate is a very real possibility when the drug is used to treat a mother’s rheumatoid arthritis, psoriasis, or systemic lupus erythematosus. Methotrexate is a folic acid antagonist that produces its most teratogenic effects between 6 and 8 weeks postconception.4 Anomalies associated with methotrexate exposure include skull defects, central nervous system abnormalities, limb defects, gastrointestinal and cardiopulmonary defects, developmental delay, and cognitive impairment.4 Even at low doses and with short-term exposure, methotrexate can cause substantial fetal anomalies. In 2002, a woman who was unknowingly 3.5 weeks pregnant used oral methotrexate 7.5 mg/d for 2 days to treat her psoriasis.5 During a fetal anatomy sonogram at 18 weeks’ gestation, multiple anomalies were noted and later confirmed at fetopsy.
A meta-analysis on the safety of methotrexate in treating rheumatoid arthritis concluded that, for doses typical in this setting, data were lacking regarding the safety and risks of the drug during conception, pregnancy, and lactation.6 The review said that rheumatologists should discourage patients from continuing methotrexate if they wish to become pregnant, and that any continuing pregnancy should be closely monitored.6
The exact malformation rate after in utero exposure to methotrexate is unknown.7 Kozlowski et al reported 10 pregnancies in which the fetus was exposed to low-dose methotrexate (5 mg orally every week) for the treatment of rheumatoid arthritis.8 Five of the pregnancies were carried to term and the newborns exhibited no abnormalities, thus illustrating the drug’s variability for teratogenicity. The risk is real, however, and methotrexate can remain in human tissue for up to 8 months, thereby putting a fetus at risk for exposure even after a mother has discontinued the drug.2
The importance of primary care counsel
Incidental exposure. Inform any pregnant patient who has used methotrexate of the potential for congenital anomalies. The capacity to make educated decisions about elective termination of pregnancy requires a full disclosure of risks. In particular, ultrasound may not identify teratogenic effects from methotrexate exposure, and antenatal diagnosis of congenital anomalies is uncommon.9 Diagnosis is usually made at delivery. Thorough counseling on this point is imperative to prevent a false reassurance of having a normal fetus.
Did medical termination fail? Despite methotrexate’s widespread use for pregnancy termination, insufficient published data exist to guide the counseling of patients who have experienced a failed termination. Nevertheless, primary care physicians are often called on to counsel such patients.
Only about half of women who undergo a medical termination procedure attend follow-up visits with the abortion provider.10 One reason is the distance some patients travel and the associated costs. A 2000 report showed that 87% of counties in the United States lack even a single abortion provider, and that approximately 25% of women travel 50 miles or more for their abortions.11
Financial hardship leads some women to opt for continuing a pregnancy after a failed elective termination.1 That was the case with our patient. When she began experiencing pelvic pain after the termination procedure, she did not return to the abortion clinic, but instead sought guidance from her primary care physician at our medical center. After learning that she was 16 weeks pregnant, she opted to proceed with the pregnancy because she couldn’t afford a second elective termination.
Primary care involvement makes sense for other reasons as well. Protocols requiring in-person follow-up appointments after elective termination may not make the best use of the medical system.10 The high proportion of “no shows” can lead to scheduling difficulties and reduce a provider’s availability to perform abortions. This in turn would lead to a loss of income for the provider and could possibly increase the total cost of medical care.
One proposed solution has been to teach women how to recognize the signs and symptoms of a successful abortion or possible complications. However, a study of methotrexate-misoprostol abortion in the United States showed that women were often unable to assess whether they had successfully aborted.10 Of 50 women, 28 thought they had aborted by day 9, and 13 of those (46%) were still pregnant.10 A patient’s overestimation of her ability to make such judgments is thought to be another reason for the low follow-up rates post termination.
When termination is performed—regardless of the modality used—it is imperative to confirm that it was successful. Primary care providers, who are usually accessible and offer cost-effective care, can provide such confirmation. In addition, primary care physicians may need to address the psychological stress caused by elective termination.
1. Wheeler M, O’Meara P, Stanford M. Fetal methotrexate and misoprostol exposure: the past revisited. Teratology. 2002;66:73-76.
2. Carbonell Esteve JL, Varela L, Velazco A, et al. 25 mg or 50 mg of oral methotrexate followed by vaginal misoprostol 7 days after for early abortion: a randomized trial. Gynecol Obstet Invest. 1999;47:182-187.
3. Addar MH. Methotrexate embryopathy in a surviving intrauterine fetus after presumed diagnosis of ectopic pregnancy: case report. J Obstet Gynecol Can. 2004;26:1001-1003.
4. Yedlinsky NT, Morgan FC, Whitecar PW. Anomalies associated with failed methotrexate and misoprostol termination. Obstet Gynecol. 2005;105:1203-1205.
5. Nguyen C, Duhl AJ, Escallon CS, et al. Multiple anomalies in a fetus exposed to low-dose methotrexate in the first trimester. Obstet Gynecol. 2002;99:599-602.
6. Martinez Lopez JA, Loza E, Carmona L. Systemic review of the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy and breastfeeding). Clin Exp Rheumatol. 2009;27:678-684.
7. Goffman D, Cole DS, Bobby P, et al. Failed methotrexate termination of pregnancy: a case report. J Perinatol. 2006;26:645-647.
8. Kozlowski RD, Steinbrunner JV, MacKenzie AH, et al. Outcome of first trimester exposure to low dose methotrexate in eight patients with rheumatic disease. Am J Med. 1990;88:589-592.
9. Chapa JB, Hibbard JU, Weber EM, et al. Prenatal diagnosis of methotrexate embryopathy. Obstet Gynecol. 2003;101:1104-1107.
10. Grossman D, Ellertson C, Grimes DA, et al. Routine follow-up visits after first trimester induced abortion. Obstet Gynecol. 2004;103:738-745.
11. Finer LB, Henshaw SK. Abortion incidence and services in the United States in 2000. Perspect Sex Reprod Health. 2003;35:6-15.
CORRESPONDENCE Tammy Donoway, DO, Family Medicine, Womack Army Medical Center, 4-2817 Reilly Road, Fort Bragg, NC 28310; [email protected]
CASE A 28-year-old woman, gravida 4, para 3, requested medical termination of her pregnancy at approximately 7 weeks’ gestation. She was given intramuscular methotrexate 50 mg/m2 and oral misoprostol 400 mcg. Nine weeks later, she presented at our primary care clinic complaining of mild pelvic pain. Given her history, we ordered transvaginal ultrasound, which showed a viable pregnancy with an average ultrasound age of 16 weeks’ gestation and grossly normal fetal anatomy. We counseled the patient regarding the risks associated with maintaining the pregnancy after exposure to methotrexate, and she and her husband elected to proceed with the pregnancy.
Throughout the pregnancy, a perinatologist conducted serial 2-dimensional ultrasounds. At 30 weeks’ gestation, ultrasound revealed mild hydrocephalus but yielded poor visualization of the kidneys, heart, and spine. A repeat ultrasound at 34 weeks demonstrated unchanged hydrocephalus and normal fetal anatomy with appropriate interval growth. A fetal echocardiogram in utero showed no cardiac anomalies. At 39 weeks, the patient underwent induction of labor due to severe oligohydramnios. A viable female infant weighing 2456 g was delivered by spontaneous vaginal delivery, with Apgar scores of 8 and 9 at 1 and 5 minutes, respectively.
The infant was small for her gestational age. Her hands had 4 digits each, including the thumb (FIGURE 1A). The upper extremities were shorter than expected in relation to the infant’s torso and were locked in 90 degrees of flexion at the elbow (FIGURE 1B). Her lower extremities were both normal in length, but her left ankle joint was everted with considerable laxity at the tibiotalar joint. The infant’s mandible deviated to the right. An ultrasound of her head showed dilation of the lateral ventricle, consistent with nonobstructive hydrocephalus. A skeletal survey revealed bilateral radial-humeral synostosis at approximately 90 degrees of flexion. The second day after birth, the infant was transferred to a tertiary medical facility for further evaluation by pediatric subspecialists.
A pediatric orthopedic surgeon noted bilateral hip dysplasia and fitted the infant for a Pavlik harness. Ultrasound of the spine identified a dysmorphic sacrum with a thickened conus medullaris ending at the level of L2-L3, with increased risk for a tethered spinal cord. To correct the hydrocephalus, a neurosurgeon recommended intervention in the neonatal period. The parents were counseled to expect some degree of developmental disability, and karyotyping was performed to rule out potential genetically linked syndromes.
FIGURE 1

Methotrexate exposure led to these congenital anomalies
Methotrexate exposure at 7 weeks’ gestation resulted in this child having 4 digits on each hand (A), shortened arms locked in 90 degrees of flexion at the elbow, and an everted left ankle joint (B).
Risks associated with methotrexate
In the 1960s, methotrexate was commonly used as an abortifacient.1 Its use for that purpose became rare, however, after multiple reports from the 1950s to the 1970s of congenital malformations in infants exposed to the drug in utero, either inadvertently or after attempted abortion.1 In the 1990s, its use increased again in conjunction with misoprostol, primarily for medical management of suspected ectopic pregnancies and less often for elective terminations. Use of the combination resulted in fewer reports of congenital anomalies.1
The failure rate of medical abortion using methotrexate varies. In 1999, one study reported an 8% failure rate when methotrexate was used with misoprostol.2 In 2004, methotrexate alone led to a failure rate of 31% in medical termination of early pregnancy.3 In 2005, another study of methotrexate and misoprostol used in combination for elective termination reported a failure rate of 2% to 10%.4
Risk is not always foreseen
Methotrexate is widely used to treat such conditions as neoplastic disease and autoimmune disorders. Unintended exposure of a fetus to methotrexate is a very real possibility when the drug is used to treat a mother’s rheumatoid arthritis, psoriasis, or systemic lupus erythematosus. Methotrexate is a folic acid antagonist that produces its most teratogenic effects between 6 and 8 weeks postconception.4 Anomalies associated with methotrexate exposure include skull defects, central nervous system abnormalities, limb defects, gastrointestinal and cardiopulmonary defects, developmental delay, and cognitive impairment.4 Even at low doses and with short-term exposure, methotrexate can cause substantial fetal anomalies. In 2002, a woman who was unknowingly 3.5 weeks pregnant used oral methotrexate 7.5 mg/d for 2 days to treat her psoriasis.5 During a fetal anatomy sonogram at 18 weeks’ gestation, multiple anomalies were noted and later confirmed at fetopsy.
A meta-analysis on the safety of methotrexate in treating rheumatoid arthritis concluded that, for doses typical in this setting, data were lacking regarding the safety and risks of the drug during conception, pregnancy, and lactation.6 The review said that rheumatologists should discourage patients from continuing methotrexate if they wish to become pregnant, and that any continuing pregnancy should be closely monitored.6
The exact malformation rate after in utero exposure to methotrexate is unknown.7 Kozlowski et al reported 10 pregnancies in which the fetus was exposed to low-dose methotrexate (5 mg orally every week) for the treatment of rheumatoid arthritis.8 Five of the pregnancies were carried to term and the newborns exhibited no abnormalities, thus illustrating the drug’s variability for teratogenicity. The risk is real, however, and methotrexate can remain in human tissue for up to 8 months, thereby putting a fetus at risk for exposure even after a mother has discontinued the drug.2
The importance of primary care counsel
Incidental exposure. Inform any pregnant patient who has used methotrexate of the potential for congenital anomalies. The capacity to make educated decisions about elective termination of pregnancy requires a full disclosure of risks. In particular, ultrasound may not identify teratogenic effects from methotrexate exposure, and antenatal diagnosis of congenital anomalies is uncommon.9 Diagnosis is usually made at delivery. Thorough counseling on this point is imperative to prevent a false reassurance of having a normal fetus.
Did medical termination fail? Despite methotrexate’s widespread use for pregnancy termination, insufficient published data exist to guide the counseling of patients who have experienced a failed termination. Nevertheless, primary care physicians are often called on to counsel such patients.
Only about half of women who undergo a medical termination procedure attend follow-up visits with the abortion provider.10 One reason is the distance some patients travel and the associated costs. A 2000 report showed that 87% of counties in the United States lack even a single abortion provider, and that approximately 25% of women travel 50 miles or more for their abortions.11
Financial hardship leads some women to opt for continuing a pregnancy after a failed elective termination.1 That was the case with our patient. When she began experiencing pelvic pain after the termination procedure, she did not return to the abortion clinic, but instead sought guidance from her primary care physician at our medical center. After learning that she was 16 weeks pregnant, she opted to proceed with the pregnancy because she couldn’t afford a second elective termination.
Primary care involvement makes sense for other reasons as well. Protocols requiring in-person follow-up appointments after elective termination may not make the best use of the medical system.10 The high proportion of “no shows” can lead to scheduling difficulties and reduce a provider’s availability to perform abortions. This in turn would lead to a loss of income for the provider and could possibly increase the total cost of medical care.
One proposed solution has been to teach women how to recognize the signs and symptoms of a successful abortion or possible complications. However, a study of methotrexate-misoprostol abortion in the United States showed that women were often unable to assess whether they had successfully aborted.10 Of 50 women, 28 thought they had aborted by day 9, and 13 of those (46%) were still pregnant.10 A patient’s overestimation of her ability to make such judgments is thought to be another reason for the low follow-up rates post termination.
When termination is performed—regardless of the modality used—it is imperative to confirm that it was successful. Primary care providers, who are usually accessible and offer cost-effective care, can provide such confirmation. In addition, primary care physicians may need to address the psychological stress caused by elective termination.
CASE A 28-year-old woman, gravida 4, para 3, requested medical termination of her pregnancy at approximately 7 weeks’ gestation. She was given intramuscular methotrexate 50 mg/m2 and oral misoprostol 400 mcg. Nine weeks later, she presented at our primary care clinic complaining of mild pelvic pain. Given her history, we ordered transvaginal ultrasound, which showed a viable pregnancy with an average ultrasound age of 16 weeks’ gestation and grossly normal fetal anatomy. We counseled the patient regarding the risks associated with maintaining the pregnancy after exposure to methotrexate, and she and her husband elected to proceed with the pregnancy.
Throughout the pregnancy, a perinatologist conducted serial 2-dimensional ultrasounds. At 30 weeks’ gestation, ultrasound revealed mild hydrocephalus but yielded poor visualization of the kidneys, heart, and spine. A repeat ultrasound at 34 weeks demonstrated unchanged hydrocephalus and normal fetal anatomy with appropriate interval growth. A fetal echocardiogram in utero showed no cardiac anomalies. At 39 weeks, the patient underwent induction of labor due to severe oligohydramnios. A viable female infant weighing 2456 g was delivered by spontaneous vaginal delivery, with Apgar scores of 8 and 9 at 1 and 5 minutes, respectively.
The infant was small for her gestational age. Her hands had 4 digits each, including the thumb (FIGURE 1A). The upper extremities were shorter than expected in relation to the infant’s torso and were locked in 90 degrees of flexion at the elbow (FIGURE 1B). Her lower extremities were both normal in length, but her left ankle joint was everted with considerable laxity at the tibiotalar joint. The infant’s mandible deviated to the right. An ultrasound of her head showed dilation of the lateral ventricle, consistent with nonobstructive hydrocephalus. A skeletal survey revealed bilateral radial-humeral synostosis at approximately 90 degrees of flexion. The second day after birth, the infant was transferred to a tertiary medical facility for further evaluation by pediatric subspecialists.
A pediatric orthopedic surgeon noted bilateral hip dysplasia and fitted the infant for a Pavlik harness. Ultrasound of the spine identified a dysmorphic sacrum with a thickened conus medullaris ending at the level of L2-L3, with increased risk for a tethered spinal cord. To correct the hydrocephalus, a neurosurgeon recommended intervention in the neonatal period. The parents were counseled to expect some degree of developmental disability, and karyotyping was performed to rule out potential genetically linked syndromes.
FIGURE 1

Methotrexate exposure led to these congenital anomalies
Methotrexate exposure at 7 weeks’ gestation resulted in this child having 4 digits on each hand (A), shortened arms locked in 90 degrees of flexion at the elbow, and an everted left ankle joint (B).
Risks associated with methotrexate
In the 1960s, methotrexate was commonly used as an abortifacient.1 Its use for that purpose became rare, however, after multiple reports from the 1950s to the 1970s of congenital malformations in infants exposed to the drug in utero, either inadvertently or after attempted abortion.1 In the 1990s, its use increased again in conjunction with misoprostol, primarily for medical management of suspected ectopic pregnancies and less often for elective terminations. Use of the combination resulted in fewer reports of congenital anomalies.1
The failure rate of medical abortion using methotrexate varies. In 1999, one study reported an 8% failure rate when methotrexate was used with misoprostol.2 In 2004, methotrexate alone led to a failure rate of 31% in medical termination of early pregnancy.3 In 2005, another study of methotrexate and misoprostol used in combination for elective termination reported a failure rate of 2% to 10%.4
Risk is not always foreseen
Methotrexate is widely used to treat such conditions as neoplastic disease and autoimmune disorders. Unintended exposure of a fetus to methotrexate is a very real possibility when the drug is used to treat a mother’s rheumatoid arthritis, psoriasis, or systemic lupus erythematosus. Methotrexate is a folic acid antagonist that produces its most teratogenic effects between 6 and 8 weeks postconception.4 Anomalies associated with methotrexate exposure include skull defects, central nervous system abnormalities, limb defects, gastrointestinal and cardiopulmonary defects, developmental delay, and cognitive impairment.4 Even at low doses and with short-term exposure, methotrexate can cause substantial fetal anomalies. In 2002, a woman who was unknowingly 3.5 weeks pregnant used oral methotrexate 7.5 mg/d for 2 days to treat her psoriasis.5 During a fetal anatomy sonogram at 18 weeks’ gestation, multiple anomalies were noted and later confirmed at fetopsy.
A meta-analysis on the safety of methotrexate in treating rheumatoid arthritis concluded that, for doses typical in this setting, data were lacking regarding the safety and risks of the drug during conception, pregnancy, and lactation.6 The review said that rheumatologists should discourage patients from continuing methotrexate if they wish to become pregnant, and that any continuing pregnancy should be closely monitored.6
The exact malformation rate after in utero exposure to methotrexate is unknown.7 Kozlowski et al reported 10 pregnancies in which the fetus was exposed to low-dose methotrexate (5 mg orally every week) for the treatment of rheumatoid arthritis.8 Five of the pregnancies were carried to term and the newborns exhibited no abnormalities, thus illustrating the drug’s variability for teratogenicity. The risk is real, however, and methotrexate can remain in human tissue for up to 8 months, thereby putting a fetus at risk for exposure even after a mother has discontinued the drug.2
The importance of primary care counsel
Incidental exposure. Inform any pregnant patient who has used methotrexate of the potential for congenital anomalies. The capacity to make educated decisions about elective termination of pregnancy requires a full disclosure of risks. In particular, ultrasound may not identify teratogenic effects from methotrexate exposure, and antenatal diagnosis of congenital anomalies is uncommon.9 Diagnosis is usually made at delivery. Thorough counseling on this point is imperative to prevent a false reassurance of having a normal fetus.
Did medical termination fail? Despite methotrexate’s widespread use for pregnancy termination, insufficient published data exist to guide the counseling of patients who have experienced a failed termination. Nevertheless, primary care physicians are often called on to counsel such patients.
Only about half of women who undergo a medical termination procedure attend follow-up visits with the abortion provider.10 One reason is the distance some patients travel and the associated costs. A 2000 report showed that 87% of counties in the United States lack even a single abortion provider, and that approximately 25% of women travel 50 miles or more for their abortions.11
Financial hardship leads some women to opt for continuing a pregnancy after a failed elective termination.1 That was the case with our patient. When she began experiencing pelvic pain after the termination procedure, she did not return to the abortion clinic, but instead sought guidance from her primary care physician at our medical center. After learning that she was 16 weeks pregnant, she opted to proceed with the pregnancy because she couldn’t afford a second elective termination.
Primary care involvement makes sense for other reasons as well. Protocols requiring in-person follow-up appointments after elective termination may not make the best use of the medical system.10 The high proportion of “no shows” can lead to scheduling difficulties and reduce a provider’s availability to perform abortions. This in turn would lead to a loss of income for the provider and could possibly increase the total cost of medical care.
One proposed solution has been to teach women how to recognize the signs and symptoms of a successful abortion or possible complications. However, a study of methotrexate-misoprostol abortion in the United States showed that women were often unable to assess whether they had successfully aborted.10 Of 50 women, 28 thought they had aborted by day 9, and 13 of those (46%) were still pregnant.10 A patient’s overestimation of her ability to make such judgments is thought to be another reason for the low follow-up rates post termination.
When termination is performed—regardless of the modality used—it is imperative to confirm that it was successful. Primary care providers, who are usually accessible and offer cost-effective care, can provide such confirmation. In addition, primary care physicians may need to address the psychological stress caused by elective termination.
1. Wheeler M, O’Meara P, Stanford M. Fetal methotrexate and misoprostol exposure: the past revisited. Teratology. 2002;66:73-76.
2. Carbonell Esteve JL, Varela L, Velazco A, et al. 25 mg or 50 mg of oral methotrexate followed by vaginal misoprostol 7 days after for early abortion: a randomized trial. Gynecol Obstet Invest. 1999;47:182-187.
3. Addar MH. Methotrexate embryopathy in a surviving intrauterine fetus after presumed diagnosis of ectopic pregnancy: case report. J Obstet Gynecol Can. 2004;26:1001-1003.
4. Yedlinsky NT, Morgan FC, Whitecar PW. Anomalies associated with failed methotrexate and misoprostol termination. Obstet Gynecol. 2005;105:1203-1205.
5. Nguyen C, Duhl AJ, Escallon CS, et al. Multiple anomalies in a fetus exposed to low-dose methotrexate in the first trimester. Obstet Gynecol. 2002;99:599-602.
6. Martinez Lopez JA, Loza E, Carmona L. Systemic review of the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy and breastfeeding). Clin Exp Rheumatol. 2009;27:678-684.
7. Goffman D, Cole DS, Bobby P, et al. Failed methotrexate termination of pregnancy: a case report. J Perinatol. 2006;26:645-647.
8. Kozlowski RD, Steinbrunner JV, MacKenzie AH, et al. Outcome of first trimester exposure to low dose methotrexate in eight patients with rheumatic disease. Am J Med. 1990;88:589-592.
9. Chapa JB, Hibbard JU, Weber EM, et al. Prenatal diagnosis of methotrexate embryopathy. Obstet Gynecol. 2003;101:1104-1107.
10. Grossman D, Ellertson C, Grimes DA, et al. Routine follow-up visits after first trimester induced abortion. Obstet Gynecol. 2004;103:738-745.
11. Finer LB, Henshaw SK. Abortion incidence and services in the United States in 2000. Perspect Sex Reprod Health. 2003;35:6-15.
CORRESPONDENCE Tammy Donoway, DO, Family Medicine, Womack Army Medical Center, 4-2817 Reilly Road, Fort Bragg, NC 28310; [email protected]
1. Wheeler M, O’Meara P, Stanford M. Fetal methotrexate and misoprostol exposure: the past revisited. Teratology. 2002;66:73-76.
2. Carbonell Esteve JL, Varela L, Velazco A, et al. 25 mg or 50 mg of oral methotrexate followed by vaginal misoprostol 7 days after for early abortion: a randomized trial. Gynecol Obstet Invest. 1999;47:182-187.
3. Addar MH. Methotrexate embryopathy in a surviving intrauterine fetus after presumed diagnosis of ectopic pregnancy: case report. J Obstet Gynecol Can. 2004;26:1001-1003.
4. Yedlinsky NT, Morgan FC, Whitecar PW. Anomalies associated with failed methotrexate and misoprostol termination. Obstet Gynecol. 2005;105:1203-1205.
5. Nguyen C, Duhl AJ, Escallon CS, et al. Multiple anomalies in a fetus exposed to low-dose methotrexate in the first trimester. Obstet Gynecol. 2002;99:599-602.
6. Martinez Lopez JA, Loza E, Carmona L. Systemic review of the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy and breastfeeding). Clin Exp Rheumatol. 2009;27:678-684.
7. Goffman D, Cole DS, Bobby P, et al. Failed methotrexate termination of pregnancy: a case report. J Perinatol. 2006;26:645-647.
8. Kozlowski RD, Steinbrunner JV, MacKenzie AH, et al. Outcome of first trimester exposure to low dose methotrexate in eight patients with rheumatic disease. Am J Med. 1990;88:589-592.
9. Chapa JB, Hibbard JU, Weber EM, et al. Prenatal diagnosis of methotrexate embryopathy. Obstet Gynecol. 2003;101:1104-1107.
10. Grossman D, Ellertson C, Grimes DA, et al. Routine follow-up visits after first trimester induced abortion. Obstet Gynecol. 2004;103:738-745.
11. Finer LB, Henshaw SK. Abortion incidence and services in the United States in 2000. Perspect Sex Reprod Health. 2003;35:6-15.
CORRESPONDENCE Tammy Donoway, DO, Family Medicine, Womack Army Medical Center, 4-2817 Reilly Road, Fort Bragg, NC 28310; [email protected]