User login
Lumateperone for major depressive episodes in bipolar I or bipolar II disorder
Among patients with bipolar I or II disorder (BD I or II), major depressive episodes represent the predominant mood state when not euthymic, and are disproportionately associated with the functional disability of BD and its suicide risk.1 Long-term naturalistic studies of weekly mood states in patients with BD I or II found that the proportion of time spent depressed greatly exceeded that spent in a mixed, hypomanic, or manic state during >12 years of follow-up (Figure 12and Figure 23). In the 20th century, traditional antidepressants represented the sole option for management of bipolar depression despite concerns of manic switching or lack of efficacy.4,5 Efficacy concerns were subsequently confirmed by placebo-controlled studies, such as the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial, which found limited effectiveness of adjunctive antidepressants for bipolar depression.6 Comprehensive reviews of randomized controlled trials and observational studies documented the risk of mood cycling and manic switching, especially in patients with BD I, even if antidepressants were used in the presence of mood-stabilizing medications.7,8
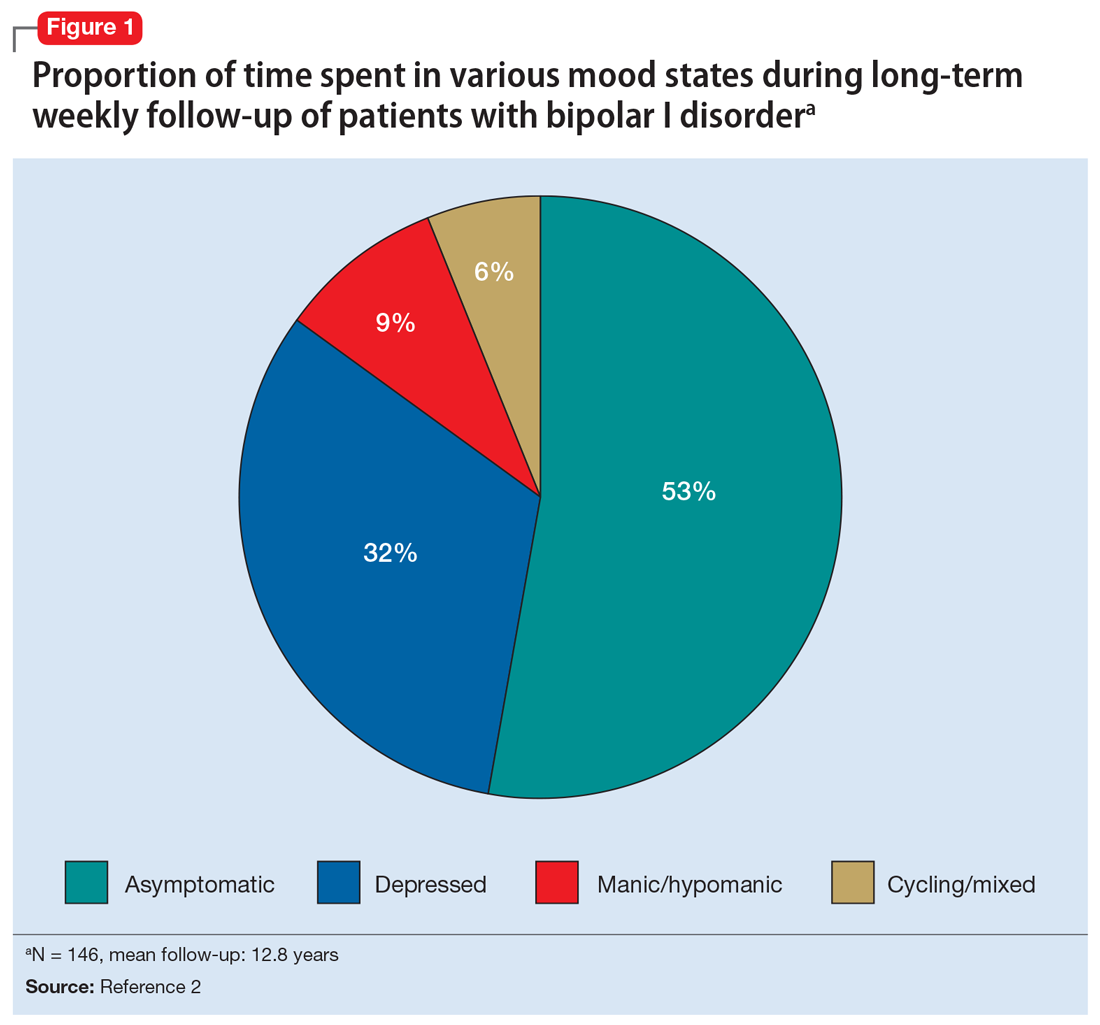
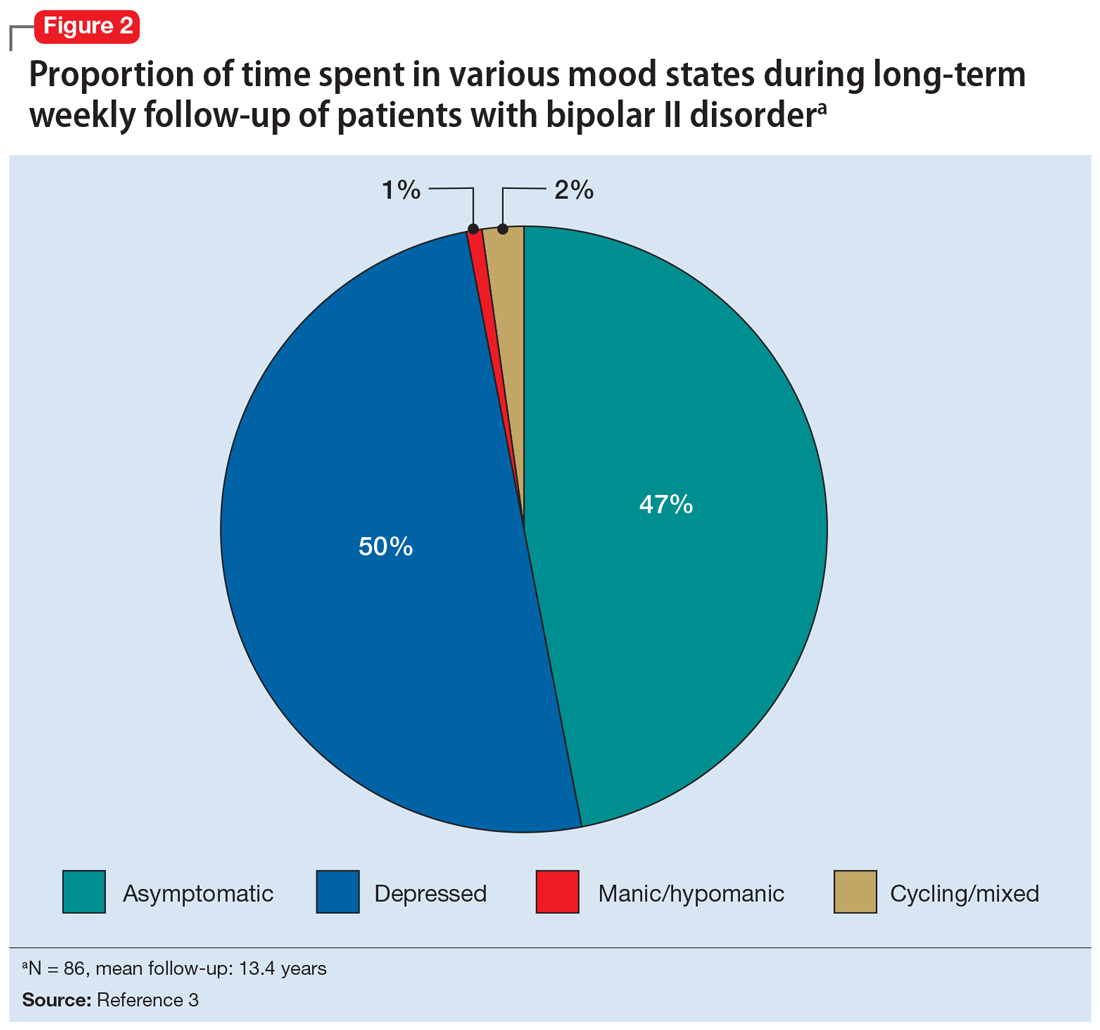
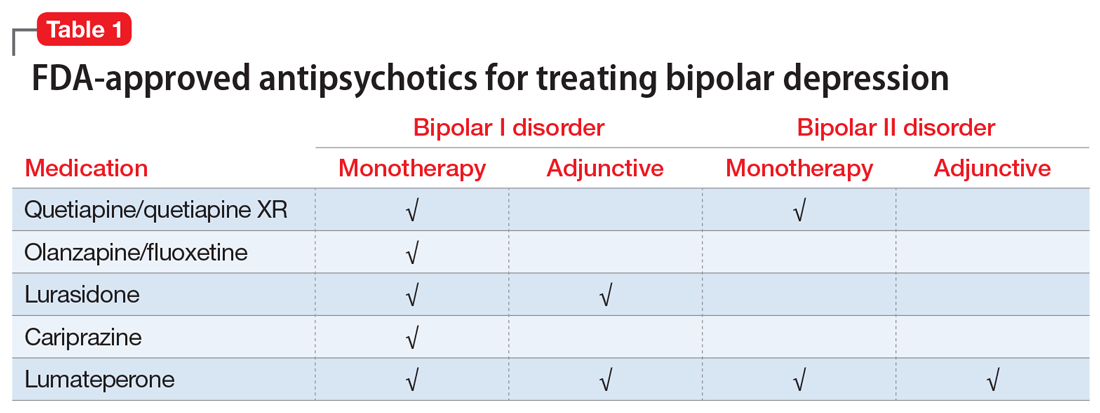
Several newer antipsychotics have been FDA-approved for treating depressive episodes associated with BD (Table 1). Approval of olanzapine/fluoxetine combination (OFC) in December 2003 for depressive episodes associated with BD I established that mechanisms exist which can effectively treat acute depressive episodes in patients with BD without an inordinate risk of mood instability. Subsequent approval of quetiapine in October 2006 for depression associated with BD I or II, lurasidone in June 2013, and cariprazine in May 2019 for major depression in BD I greatly expanded the options for management of acute bipolar depression. However, despite the array of molecules available, for certain patients these agents presented tolerability issues such as sedation, weight gain, akathisia, or parkinsonism that could hamper effective treatment.9 Safety and efficacy data in bipolar depression for adjunctive use with lithium or divalproex/valproate (VPA) also are lacking for quetiapine, OFC, and cariprazine.10,11 Moreover, despite the fact that BD II is as prevalent as BD I, and that patients with BD II have comparable rates of comorbidity, chronicity, disability, and suicidality,12 only quetiapine was approved for acute treatment of depression in patients with BD II. This omission is particularly problematic because the depressive episodes of BD II predominate over the time spent in hypomanic and cycling/mixed states (50.3% for depression vs 3.6% for hypomania/cycling/mixed combined), much more than is seen with BD I (31.9% for depression vs 14.8% for hypomania/cycling/mixed combined).2,3 The paucity of data for the use of newer antipsychotics in BD II depression presents a problem when patients cannot tolerate or refuse to consider quetiapine. This prevents clinicians from engaging in evidence-based efficacy discussions of other options, even if it is assumed that the tolerability profile for BD II depression patients may be similar to that seen when these agents are used for BD I depression.
Continue to: Table 1...
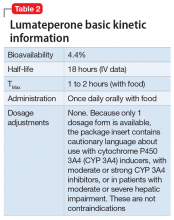
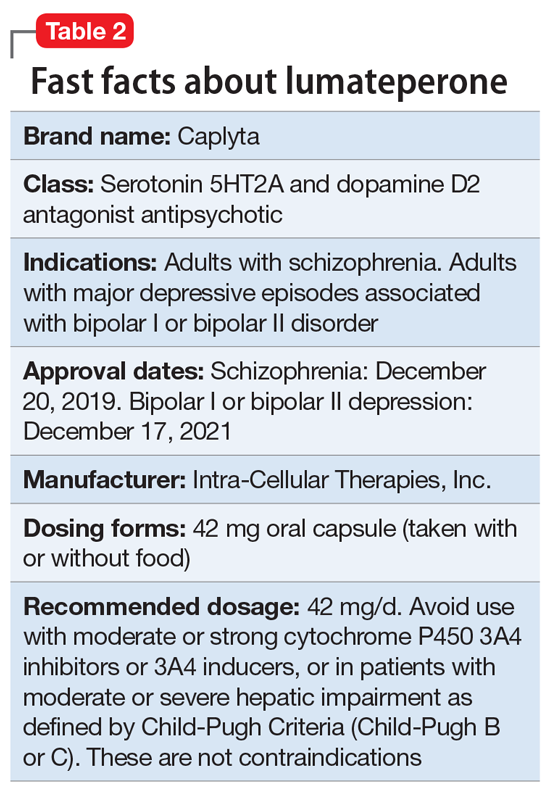
Lumateperone (Caplyta) is a novel oral antipsychotic initially approved in 2019 for the treatment of adult patients with schizophrenia. It was approved in December 2021 for the management of depression associated with BD I or II in adults as monotherapy or when used adjunctively with the mood stabilizers lithium or VPA (Table 2).13 Lumateperone possesses certain binding affinities not unlike those in other newer antipsychotics, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), low affinity for dopamine D2 receptors (Ki 32 nM), and low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).13,14 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that for other second-generation antipsychotics (SGAs) such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18).15 At steady state, D2 receptor occupancy remains <40%, and the corresponding rates of extrapyramidal side effects (EPS)/akathisia differed by only 0.4% for lumateperone vs placebo in short-term adult clinical schizophrenia trials,13,16 by 0.2% for lumateperone vs placebo in the monotherapy BD depression study, and by 1.7% in the adjunctive BD depression study.13,17,18 Lumateperone also exhibited no clinically significant impact on metabolic measures or serum prolactin during the 4-week schizophrenia trials, with mean weight gain ≤1 kg for the 42 mg dose across all studies.19 This favorable tolerability profile for endocrine and metabolic adverse effects was also seen in the BD depression studies. Across the 2 BD depression monotherapy trials and the single adjunctive study, the only adverse reactions occurring in ≥5% of lumateperone-treated patients and more than twice the rate of placebo were somnolence/sedation, dizziness, nausea, and dry mouth.13 There was also no single adverse reaction leading to discontinuation in the BD depression studies that occurred at a rate >2% in patients treated with lumateperone.13
In addition to the low risk of adverse events of all types, lumateperone has several pharmacologic features that distinguish it from other agents in its class. One unique aspect of lumateperone’s pharmacology is differential actions at presynaptic and postsynaptic dopamine D2 receptors noted in preclinical assays, a property that may explain its ability to act as an antipsychotic despite low D2 receptor occupancy.16 Preclinical assays also predicted that lumateperone was likely to have antidepressant effects.15,19,20 Unlike every SGA except ziprasidone, lumateperone also possesses moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM), with SERT occupancy of approximately 30% at 42 mg.21 Lumateperone facilitates dopamine D1-mediated neurotransmission, and this is associated with increased glutamate signaling in the prefrontal cortex and antidepressant actions.14,22 While the extent of SERT occupancy is significantly below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitors, it is hypothesized that near saturation of the 5HT2A receptor might act synergistically with modest 5HT reuptake inhibition and D1-mediated effects to promote the downstream glutamatergic effects that correlate with antidepressant activity (eg, changes in markers such as phosphorylation of glutamate N-methyl-D-aspartate receptor subunits, potentiation of AMPA receptor-mediated transmission).15,22
Continue to: Clinical implications...
Clinical implications
The approval of lumateperone for both BD I and BD II depression, and for its use as monotherapy and for adjunctive use with lithium or VPA, satisfies several unmet needs for the management of acute major depressive episodes in patients with BD. Clinicians now have both safety and tolerability data to present to their bipolar spectrum patients regardless of subtype, and regardless of whether the patient requires mood stabilizer therapy. The tolerability advantages for lumateperone seen in schizophrenia trials were replicated in a diagnostic group that is very sensitive to D2-related adverse effects, and for whom any signal of clinically significant weight gain or sedation often represents an insuperable barrier to patient acceptance.23
Efficacy in adults with BD I or II depression.
The efficacy of lumateperone for major depressive episodes has been established in 2 pivotal, double-blind, placebo-controlled trials in BD I or II patients: 1 monotherapy study,17 and 1 study when used adjunctively to lithium or VPA.18 The first study was a 6-week, double-blind, placebo-controlled monotherapy trial (study 404) in which 377 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode were randomized in a 1:1 manner to lumateperone 42 mg/d or placebo given once daily in the evening. Symptom entry criteria included a Montgomery-Åsberg Depression Rating Scale (MADRS) total score ≥20, and scores ≥4 on the depression and overall BD illness subscales of the Clinical Global Impressions Scale–Bipolar Version Severity scale (CGI-BP-S) at screening and at baseline.17 Study entry also required a score ≤12 on the Young Mania Rating Scale (YMRS) at screening and at baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS. Several secondary efficacy measures were examined, including the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS), or remission (MADRS score ≤12), and differential changes in MADRS scores from baseline for BD I and BD II subgroups.17
The patient population was 58% female and 91% White, with 79.9% diagnosed as BD I and 20.1% as BD II. The least squares mean changes on the MADRS total score from baseline to Day 43 were lumateperone 42 mg/d: -16.7 points; placebo: -12.1 points (P < .0001), and the effect size for this difference was moderate: 0.56. Secondary analyses indicated that 51.1% of those taking lumateperone 42 mg/d and 36.7% taking placebo met response criteria (P < .001), while 39.9% of those taking lumateperone 42 mg/d and 33.5% taking placebo met remission criteria (P = .018). Importantly, depression improvement was observed both in patients with BD I (effect size 0.49, P < .0001) and in those with BD II (effect size 0.81, P < .001).
The second pivotal trial (study 402) was a 6-week, double-blind, placebo-controlled adjunctive trial in which 528 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode despite treatment with lithium or VPA were randomized in a 1:1:1 manner to lumateperone 28 mg/d, lumateperone 42 mg/d, or placebo given once daily in the evening.18 Like the monotherapy trial, symptom entry criteria included a MADRS total score ≥20, and scores ≥4 on the depression and overall illness CGI-BP-S subscales at screening and baseline.18 Study entry also required a score ≤12 on the YMRS at screening and baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS for lumateperone 42 mg/d compared to placebo. Secondary efficacy measures included MADRS changes for lumateperone 28 mg/d and the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS) or remission (MADRS score ≤12).
The patient population was 58% female and 88% White, with 83.3% diagnosed as BD I, 16.7% diagnosed as BD II, and 28.6% treated with lithium vs 71.4% on VPA. The effect size for the difference in MADRS total score from baseline to Day 43 for lumateperone 42 mg/d was 0.27 (P < .05), while that for the lumateperone 28 mg/d dose did not reach statistical significance. Secondary analyses indicated that response rates for lumateperone 28 mg/d and lumateperone 42 mg/d were significantly higher than for placebo (both P < .05). Response rates were placebo: 39%; lumateperone 28 mg/d: 50%; and lumateperone 42 mg/d: 45%. Remission rates were similar at Day 43 in both lumateperone groups compared with placebo: placebo: 31%, lumateperone 28 mg/d: 31%, and lumateperone 42 mg/d: 28%.18 As of this writing, a secondary analysis by BD subtype has not yet been presented.
A third study examining lumateperone monotherapy failed to establish superiority of lumateperone over placebo (NCT02600494). The data regarding tolerability from that study were incorporated in product labeling describing adverse reactions.
Continue on to: Adverse reactions...
Adverse reactions
In the positive monotherapy trial, there were 376 patients in the modified intent-to-treat efficacy population to receive lumateperone (N = 188) or placebo (N = 188) with nearly identical completion rates in the active treatment and placebo cohorts: lumateperone, 88.8%; placebo, 88.3%.17 The proportion experiencing mania was low in both cohorts (lumateperone, 1.1%; placebo, 2.1%), and there was 1 case of hypomania in each group. One participant in the lumateperone group and 1 in the placebo group discontinued the study due to a serious adverse event of mania. There was no worsening of mania in either group as measured by mean change in the YMRS score. There was also no suicidal behavior in either cohort during the study. Pooling the 2 monotherapy trials, the adverse events that occurred at ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone 42 mg/d: 13%, placebo: 3%), dizziness (lumateperone 42 mg/d: 8%, placebo: 4%), and nausea (lumateperone 42 mg/d: 8%, placebo: 3%).13 Rates of EPS were low for both groups: lumateperone 42 mg/d: 1.3%, placebo: 1.1%.13 Mean weight change at Day 43 was +0.11 kg for lumateperone and +0.03 kg for placebo in the positive monotherapy trial.17 Moreover, compared to placebo, lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant. No patient exhibited a corrected QT interval >500 ms at any time, and increases ≥60 ms from baseline were similar between the lumateperone (n = 1, 0.6%) and placebo (n = 3, 1.8%) cohorts.
Complete safety and tolerability data for the adjunctive trial has not yet been published, but discontinuation rates due to treatment-emergent adverse effects for the 3 arms were: lumateperone 42 mg/d: 5.6%; lumateperone 28 mg/d: 1.7%; and placebo: 1.7%. Overall, 81.4% of patients completed the trial, with only 1 serious adverse event (lithium toxicity) occurring in a patient taking lumateperone 42 mg/d. While this led to study discontinuation, it was not considered related to lumateperone exposure by the investigator. There was no worsening of mania in either lumateperone dosage group or the placebo cohort as measured by mean change in YMRS score: -1.2 for placebo, -1.4 for lumateperone 28 mg/d, and -1.6 for lumateperone 42 mg/d. Suicidal behavior was not observed in any group during treatment. The adverse events that occurred at rates ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone, 13%; placebo, 3%), dizziness (lumateperone, 11%; placebo, 2%), and nausea (lumateperone, 9%; placebo, 4%).13 Rates of EPS were low for both groups: lumateperone, 4.0%, placebo, 2.3%.13 Mean weight changes at Day 43 were +0.23 kg for placebo, +0.02 kg for lumateperone 28 mg/d, and 0.00 kg for lumateperone 42 mg/d.18 Compared to placebo, both doses of lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant.18
Lastly, the package insert notes that in an uncontrolled, open-label trial of lumateperone for up to 6 months in patients with BD depression, the mean weight change was -0.01 ± 3.1 kg at Day 175.13
Continue on to: Pharmacologic profile...
Pharmacologic profile
Lumateperone’s preclinical discovery program found an impact on markers associated with increased glutamatergic neurotransmission, properties that were predicted to yield antidepressant benefit.14,15,24 This is hypothesized to be based on the complex pharmacology of lumateperone, including dopamine D1 agonism, modest SERT occupancy, and near saturation of the 5HT2A receptor.15,22 Dopamine D2 affinity is modest (32 nM), and the D2 receptor occupancy at the 42 mg dose is low. These properties translate to rates of EPS in clinical studies of schizophrenia and BD that are close to that of placebo. Lumateperone has very high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), which also helps mitigate D2-related adverse effects and may be part of the therapeutic antidepressant mechanism. Underlying the tolerability profile is the low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).
Clinical considerations
Data from the lumateperone BD depression trials led to it being only the second agent approved for acute major depression in BD II patients, and the only agent which has approvals as monotherapy and adjunctive therapy for both BD subtypes. The monotherapy trial results substantiate that lumateperone was robustly effective regardless of BD subtype, with significant improvement in depressive symptoms experienced by patients with BD I (effect size 0.49, P < .0001) and those with BD II (effect size 0.81, P < .001). Effect sizes in acute BD depression studies are much larger in monotherapy trials than in adjunctive trials, as the latter group represents patients who have already failed pretreatment with a mood stabilizer.25,26 In the lurasidone BD I depression trials, the effect size based on mean change in MADRS score over the course of 6 weeks was 0.51 in the monotherapy study compared to 0.34 when used adjunctively with lithium or VPA.25,26 In the lumateperone adjunctive study, the effect size for the difference in mean MADRS total score from baseline for lumateperone 42 mg/d, was 0.27 (P < .05). Subgroup analyses by BD subtype are not yet available for adjunctive use, but the data presented to FDA were sufficient to permit an indication for adjunctive use across both diagnostic groups.
The absence of clinically significant EPS, the minimal impact on metabolic or endocrine parameters, and the lack of a need for titration are all appealing properties. At the present there is only 1 marketed dose (42 mg capsules), so the package insert includes cautionary language regarding situations when a patient might encounter less drug exposure (concurrent use of cytochrome P450 [CYP] 3A4 inducers), or greater drug exposure due to concurrent use of moderate or strong CYP3A4 inhibitors, as well as in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include efficacy established as monotherapy for BD I and BD II patients, and efficacy for adjunctive use with lithium or VPA. Additionally, the extremely low rates of significant EPS and lack of clinically significant metabolic or endocrine adverse effects are unique properties of lumateperone.13
Why Rx? Reasons to prescribe lumateperone for adult BD depression patients include:
- data support efficacy for BD I and BD II patients, and for monotherapy or adjunctive use with lithium/VPA
- favorable tolerability profile, including no significant signal for EPS, endocrine or metabolic adverse effects, or QT prolongation
- no need for titration.
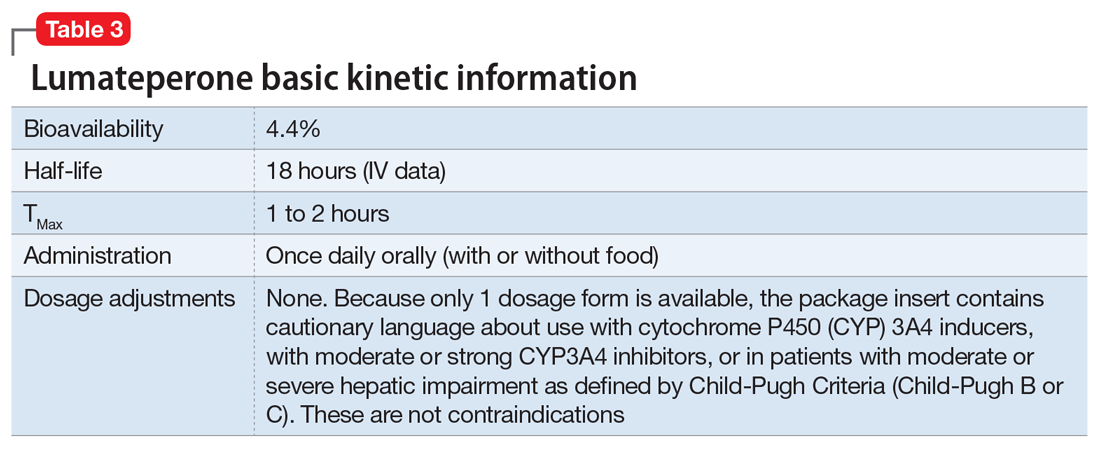
Dosing. There is only 1 dose available for lumateperone: 42 mg capsules (Table 3). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less drug exposure (use of CYP3A4 inducers), or greater drug exposure (use with moderate or strong CYP3A4 inhibitors or in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria [Child-Pugh B or C]). These are not contraindications. Based on newer pharmacokinetic studies, lumateperone does not need to be dosed with food, and there is no clinically significant interaction with UGT1A4 inhibitors such as VPA.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Bottom Line
Data support the efficacy of lumateperone for treating depressive episodes in adults with bipolar I or bipolar II disorder, either as monotherapy or adjunctive to lithium or divalproex/valproate. Potential advantages of lumateperone for this indication include a favorable tolerability profile and no need for titration.
1. Malhi GS, Bell E, Boyce P, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Bipolar Disord. 2020;22(8):805-821.
2. Judd LL, Akishal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akishal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Post RM. Treatment of bipolar depression: evolving recommendations. Psychiatr Clin North Am. 2016;39(1):11-33.
5. Pacchiarotti I, Verdolini N. Antidepressants in bipolar II depression: yes and no. Eur Neuropsychopharmacol 2021;47:48-50.
6. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
7. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
8. Gitlin MJ. Antidepressants in bipolar depression: an enduring controversy. Int J Bipolar Disord. 2018;6(1):25.
9. Verdolini N, Hidalgo-Mazzei D, Del Matto L, et al. Long-term treatment of bipolar disorder type I: a systematic and critical review of clinical guidelines with derived practice algorithms. Bipolar Disord. 2021;23(4):324-340.
10. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20(2):180-195.
11. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
12. Chakrabarty T, Hadijpavlou G, Bond DJ, et al. Bipolar II disorder in context: a review of its epidemiology, disability and economic burden. In: Parker G. Bipolar II Disorder: Modelling, Measuring and Managing. 3rd ed. Cambridge University Press; 2019:49-59.
13. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2021.
14. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
15. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232:605-621.
16. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
17. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry 2021;178(12):1098-1106.
18. Yatham LN, et al. Adjunctive lumateperone (ITI-007) in the treatment of bipolar depression: results from a randomized clinical trial. Poster presented at: American Psychiatric Association Annual Meeting. May 1-3, 2021; virtual conference.
19. Vanover K, Glass S, Kozauer S, et al. 30 Lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectrums. 2019;24(1):190-191.
20. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
21. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-72.
22. Björkholm C, Marcus MM, Konradsson-Geuken Å, et al. The novel antipsychotic drug brexpiprazole, alone and in combination with escitalopram, facilitates prefrontal glutamatergic transmission via a dopamine D1 receptor-dependent mechanism. Eur Neuropsychopharmacol. 2017;27(4):411-417.
23. Bai Y, Yang H, Chen G, et al. Acceptability of acute and maintenance pharmacotherapy of bipolar disorder: a systematic review of randomized, double-blind, placebo-controlled clinical trials. J Clin Psychopharmacol. 2020;40(2):167-179.
24. Vyas P, Hwang BJ, Braši´c JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
25. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
26. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169-77.
Among patients with bipolar I or II disorder (BD I or II), major depressive episodes represent the predominant mood state when not euthymic, and are disproportionately associated with the functional disability of BD and its suicide risk.1 Long-term naturalistic studies of weekly mood states in patients with BD I or II found that the proportion of time spent depressed greatly exceeded that spent in a mixed, hypomanic, or manic state during >12 years of follow-up (Figure 12and Figure 23). In the 20th century, traditional antidepressants represented the sole option for management of bipolar depression despite concerns of manic switching or lack of efficacy.4,5 Efficacy concerns were subsequently confirmed by placebo-controlled studies, such as the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial, which found limited effectiveness of adjunctive antidepressants for bipolar depression.6 Comprehensive reviews of randomized controlled trials and observational studies documented the risk of mood cycling and manic switching, especially in patients with BD I, even if antidepressants were used in the presence of mood-stabilizing medications.7,8


Several newer antipsychotics have been FDA-approved for treating depressive episodes associated with BD (Table 1). Approval of olanzapine/fluoxetine combination (OFC) in December 2003 for depressive episodes associated with BD I established that mechanisms exist which can effectively treat acute depressive episodes in patients with BD without an inordinate risk of mood instability. Subsequent approval of quetiapine in October 2006 for depression associated with BD I or II, lurasidone in June 2013, and cariprazine in May 2019 for major depression in BD I greatly expanded the options for management of acute bipolar depression. However, despite the array of molecules available, for certain patients these agents presented tolerability issues such as sedation, weight gain, akathisia, or parkinsonism that could hamper effective treatment.9 Safety and efficacy data in bipolar depression for adjunctive use with lithium or divalproex/valproate (VPA) also are lacking for quetiapine, OFC, and cariprazine.10,11 Moreover, despite the fact that BD II is as prevalent as BD I, and that patients with BD II have comparable rates of comorbidity, chronicity, disability, and suicidality,12 only quetiapine was approved for acute treatment of depression in patients with BD II. This omission is particularly problematic because the depressive episodes of BD II predominate over the time spent in hypomanic and cycling/mixed states (50.3% for depression vs 3.6% for hypomania/cycling/mixed combined), much more than is seen with BD I (31.9% for depression vs 14.8% for hypomania/cycling/mixed combined).2,3 The paucity of data for the use of newer antipsychotics in BD II depression presents a problem when patients cannot tolerate or refuse to consider quetiapine. This prevents clinicians from engaging in evidence-based efficacy discussions of other options, even if it is assumed that the tolerability profile for BD II depression patients may be similar to that seen when these agents are used for BD I depression.
Continue to: Table 1...

Lumateperone (Caplyta) is a novel oral antipsychotic initially approved in 2019 for the treatment of adult patients with schizophrenia. It was approved in December 2021 for the management of depression associated with BD I or II in adults as monotherapy or when used adjunctively with the mood stabilizers lithium or VPA (Table 2).13 Lumateperone possesses certain binding affinities not unlike those in other newer antipsychotics, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), low affinity for dopamine D2 receptors (Ki 32 nM), and low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).13,14 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that for other second-generation antipsychotics (SGAs) such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18).15 At steady state, D2 receptor occupancy remains <40%, and the corresponding rates of extrapyramidal side effects (EPS)/akathisia differed by only 0.4% for lumateperone vs placebo in short-term adult clinical schizophrenia trials,13,16 by 0.2% for lumateperone vs placebo in the monotherapy BD depression study, and by 1.7% in the adjunctive BD depression study.13,17,18 Lumateperone also exhibited no clinically significant impact on metabolic measures or serum prolactin during the 4-week schizophrenia trials, with mean weight gain ≤1 kg for the 42 mg dose across all studies.19 This favorable tolerability profile for endocrine and metabolic adverse effects was also seen in the BD depression studies. Across the 2 BD depression monotherapy trials and the single adjunctive study, the only adverse reactions occurring in ≥5% of lumateperone-treated patients and more than twice the rate of placebo were somnolence/sedation, dizziness, nausea, and dry mouth.13 There was also no single adverse reaction leading to discontinuation in the BD depression studies that occurred at a rate >2% in patients treated with lumateperone.13

In addition to the low risk of adverse events of all types, lumateperone has several pharmacologic features that distinguish it from other agents in its class. One unique aspect of lumateperone’s pharmacology is differential actions at presynaptic and postsynaptic dopamine D2 receptors noted in preclinical assays, a property that may explain its ability to act as an antipsychotic despite low D2 receptor occupancy.16 Preclinical assays also predicted that lumateperone was likely to have antidepressant effects.15,19,20 Unlike every SGA except ziprasidone, lumateperone also possesses moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM), with SERT occupancy of approximately 30% at 42 mg.21 Lumateperone facilitates dopamine D1-mediated neurotransmission, and this is associated with increased glutamate signaling in the prefrontal cortex and antidepressant actions.14,22 While the extent of SERT occupancy is significantly below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitors, it is hypothesized that near saturation of the 5HT2A receptor might act synergistically with modest 5HT reuptake inhibition and D1-mediated effects to promote the downstream glutamatergic effects that correlate with antidepressant activity (eg, changes in markers such as phosphorylation of glutamate N-methyl-D-aspartate receptor subunits, potentiation of AMPA receptor-mediated transmission).15,22
Continue to: Clinical implications...
Clinical implications
The approval of lumateperone for both BD I and BD II depression, and for its use as monotherapy and for adjunctive use with lithium or VPA, satisfies several unmet needs for the management of acute major depressive episodes in patients with BD. Clinicians now have both safety and tolerability data to present to their bipolar spectrum patients regardless of subtype, and regardless of whether the patient requires mood stabilizer therapy. The tolerability advantages for lumateperone seen in schizophrenia trials were replicated in a diagnostic group that is very sensitive to D2-related adverse effects, and for whom any signal of clinically significant weight gain or sedation often represents an insuperable barrier to patient acceptance.23
Efficacy in adults with BD I or II depression.
The efficacy of lumateperone for major depressive episodes has been established in 2 pivotal, double-blind, placebo-controlled trials in BD I or II patients: 1 monotherapy study,17 and 1 study when used adjunctively to lithium or VPA.18 The first study was a 6-week, double-blind, placebo-controlled monotherapy trial (study 404) in which 377 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode were randomized in a 1:1 manner to lumateperone 42 mg/d or placebo given once daily in the evening. Symptom entry criteria included a Montgomery-Åsberg Depression Rating Scale (MADRS) total score ≥20, and scores ≥4 on the depression and overall BD illness subscales of the Clinical Global Impressions Scale–Bipolar Version Severity scale (CGI-BP-S) at screening and at baseline.17 Study entry also required a score ≤12 on the Young Mania Rating Scale (YMRS) at screening and at baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS. Several secondary efficacy measures were examined, including the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS), or remission (MADRS score ≤12), and differential changes in MADRS scores from baseline for BD I and BD II subgroups.17
The patient population was 58% female and 91% White, with 79.9% diagnosed as BD I and 20.1% as BD II. The least squares mean changes on the MADRS total score from baseline to Day 43 were lumateperone 42 mg/d: -16.7 points; placebo: -12.1 points (P < .0001), and the effect size for this difference was moderate: 0.56. Secondary analyses indicated that 51.1% of those taking lumateperone 42 mg/d and 36.7% taking placebo met response criteria (P < .001), while 39.9% of those taking lumateperone 42 mg/d and 33.5% taking placebo met remission criteria (P = .018). Importantly, depression improvement was observed both in patients with BD I (effect size 0.49, P < .0001) and in those with BD II (effect size 0.81, P < .001).
The second pivotal trial (study 402) was a 6-week, double-blind, placebo-controlled adjunctive trial in which 528 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode despite treatment with lithium or VPA were randomized in a 1:1:1 manner to lumateperone 28 mg/d, lumateperone 42 mg/d, or placebo given once daily in the evening.18 Like the monotherapy trial, symptom entry criteria included a MADRS total score ≥20, and scores ≥4 on the depression and overall illness CGI-BP-S subscales at screening and baseline.18 Study entry also required a score ≤12 on the YMRS at screening and baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS for lumateperone 42 mg/d compared to placebo. Secondary efficacy measures included MADRS changes for lumateperone 28 mg/d and the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS) or remission (MADRS score ≤12).
The patient population was 58% female and 88% White, with 83.3% diagnosed as BD I, 16.7% diagnosed as BD II, and 28.6% treated with lithium vs 71.4% on VPA. The effect size for the difference in MADRS total score from baseline to Day 43 for lumateperone 42 mg/d was 0.27 (P < .05), while that for the lumateperone 28 mg/d dose did not reach statistical significance. Secondary analyses indicated that response rates for lumateperone 28 mg/d and lumateperone 42 mg/d were significantly higher than for placebo (both P < .05). Response rates were placebo: 39%; lumateperone 28 mg/d: 50%; and lumateperone 42 mg/d: 45%. Remission rates were similar at Day 43 in both lumateperone groups compared with placebo: placebo: 31%, lumateperone 28 mg/d: 31%, and lumateperone 42 mg/d: 28%.18 As of this writing, a secondary analysis by BD subtype has not yet been presented.
A third study examining lumateperone monotherapy failed to establish superiority of lumateperone over placebo (NCT02600494). The data regarding tolerability from that study were incorporated in product labeling describing adverse reactions.
Continue on to: Adverse reactions...
Adverse reactions
In the positive monotherapy trial, there were 376 patients in the modified intent-to-treat efficacy population to receive lumateperone (N = 188) or placebo (N = 188) with nearly identical completion rates in the active treatment and placebo cohorts: lumateperone, 88.8%; placebo, 88.3%.17 The proportion experiencing mania was low in both cohorts (lumateperone, 1.1%; placebo, 2.1%), and there was 1 case of hypomania in each group. One participant in the lumateperone group and 1 in the placebo group discontinued the study due to a serious adverse event of mania. There was no worsening of mania in either group as measured by mean change in the YMRS score. There was also no suicidal behavior in either cohort during the study. Pooling the 2 monotherapy trials, the adverse events that occurred at ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone 42 mg/d: 13%, placebo: 3%), dizziness (lumateperone 42 mg/d: 8%, placebo: 4%), and nausea (lumateperone 42 mg/d: 8%, placebo: 3%).13 Rates of EPS were low for both groups: lumateperone 42 mg/d: 1.3%, placebo: 1.1%.13 Mean weight change at Day 43 was +0.11 kg for lumateperone and +0.03 kg for placebo in the positive monotherapy trial.17 Moreover, compared to placebo, lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant. No patient exhibited a corrected QT interval >500 ms at any time, and increases ≥60 ms from baseline were similar between the lumateperone (n = 1, 0.6%) and placebo (n = 3, 1.8%) cohorts.
Complete safety and tolerability data for the adjunctive trial has not yet been published, but discontinuation rates due to treatment-emergent adverse effects for the 3 arms were: lumateperone 42 mg/d: 5.6%; lumateperone 28 mg/d: 1.7%; and placebo: 1.7%. Overall, 81.4% of patients completed the trial, with only 1 serious adverse event (lithium toxicity) occurring in a patient taking lumateperone 42 mg/d. While this led to study discontinuation, it was not considered related to lumateperone exposure by the investigator. There was no worsening of mania in either lumateperone dosage group or the placebo cohort as measured by mean change in YMRS score: -1.2 for placebo, -1.4 for lumateperone 28 mg/d, and -1.6 for lumateperone 42 mg/d. Suicidal behavior was not observed in any group during treatment. The adverse events that occurred at rates ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone, 13%; placebo, 3%), dizziness (lumateperone, 11%; placebo, 2%), and nausea (lumateperone, 9%; placebo, 4%).13 Rates of EPS were low for both groups: lumateperone, 4.0%, placebo, 2.3%.13 Mean weight changes at Day 43 were +0.23 kg for placebo, +0.02 kg for lumateperone 28 mg/d, and 0.00 kg for lumateperone 42 mg/d.18 Compared to placebo, both doses of lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant.18
Lastly, the package insert notes that in an uncontrolled, open-label trial of lumateperone for up to 6 months in patients with BD depression, the mean weight change was -0.01 ± 3.1 kg at Day 175.13
Continue on to: Pharmacologic profile...
Pharmacologic profile
Lumateperone’s preclinical discovery program found an impact on markers associated with increased glutamatergic neurotransmission, properties that were predicted to yield antidepressant benefit.14,15,24 This is hypothesized to be based on the complex pharmacology of lumateperone, including dopamine D1 agonism, modest SERT occupancy, and near saturation of the 5HT2A receptor.15,22 Dopamine D2 affinity is modest (32 nM), and the D2 receptor occupancy at the 42 mg dose is low. These properties translate to rates of EPS in clinical studies of schizophrenia and BD that are close to that of placebo. Lumateperone has very high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), which also helps mitigate D2-related adverse effects and may be part of the therapeutic antidepressant mechanism. Underlying the tolerability profile is the low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).
Clinical considerations
Data from the lumateperone BD depression trials led to it being only the second agent approved for acute major depression in BD II patients, and the only agent which has approvals as monotherapy and adjunctive therapy for both BD subtypes. The monotherapy trial results substantiate that lumateperone was robustly effective regardless of BD subtype, with significant improvement in depressive symptoms experienced by patients with BD I (effect size 0.49, P < .0001) and those with BD II (effect size 0.81, P < .001). Effect sizes in acute BD depression studies are much larger in monotherapy trials than in adjunctive trials, as the latter group represents patients who have already failed pretreatment with a mood stabilizer.25,26 In the lurasidone BD I depression trials, the effect size based on mean change in MADRS score over the course of 6 weeks was 0.51 in the monotherapy study compared to 0.34 when used adjunctively with lithium or VPA.25,26 In the lumateperone adjunctive study, the effect size for the difference in mean MADRS total score from baseline for lumateperone 42 mg/d, was 0.27 (P < .05). Subgroup analyses by BD subtype are not yet available for adjunctive use, but the data presented to FDA were sufficient to permit an indication for adjunctive use across both diagnostic groups.
The absence of clinically significant EPS, the minimal impact on metabolic or endocrine parameters, and the lack of a need for titration are all appealing properties. At the present there is only 1 marketed dose (42 mg capsules), so the package insert includes cautionary language regarding situations when a patient might encounter less drug exposure (concurrent use of cytochrome P450 [CYP] 3A4 inducers), or greater drug exposure due to concurrent use of moderate or strong CYP3A4 inhibitors, as well as in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include efficacy established as monotherapy for BD I and BD II patients, and efficacy for adjunctive use with lithium or VPA. Additionally, the extremely low rates of significant EPS and lack of clinically significant metabolic or endocrine adverse effects are unique properties of lumateperone.13
Why Rx? Reasons to prescribe lumateperone for adult BD depression patients include:
- data support efficacy for BD I and BD II patients, and for monotherapy or adjunctive use with lithium/VPA
- favorable tolerability profile, including no significant signal for EPS, endocrine or metabolic adverse effects, or QT prolongation
- no need for titration.
Dosing. There is only 1 dose available for lumateperone: 42 mg capsules (Table 3). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less drug exposure (use of CYP3A4 inducers), or greater drug exposure (use with moderate or strong CYP3A4 inhibitors or in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria [Child-Pugh B or C]). These are not contraindications. Based on newer pharmacokinetic studies, lumateperone does not need to be dosed with food, and there is no clinically significant interaction with UGT1A4 inhibitors such as VPA.

Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Bottom Line
Data support the efficacy of lumateperone for treating depressive episodes in adults with bipolar I or bipolar II disorder, either as monotherapy or adjunctive to lithium or divalproex/valproate. Potential advantages of lumateperone for this indication include a favorable tolerability profile and no need for titration.
Among patients with bipolar I or II disorder (BD I or II), major depressive episodes represent the predominant mood state when not euthymic, and are disproportionately associated with the functional disability of BD and its suicide risk.1 Long-term naturalistic studies of weekly mood states in patients with BD I or II found that the proportion of time spent depressed greatly exceeded that spent in a mixed, hypomanic, or manic state during >12 years of follow-up (Figure 12and Figure 23). In the 20th century, traditional antidepressants represented the sole option for management of bipolar depression despite concerns of manic switching or lack of efficacy.4,5 Efficacy concerns were subsequently confirmed by placebo-controlled studies, such as the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial, which found limited effectiveness of adjunctive antidepressants for bipolar depression.6 Comprehensive reviews of randomized controlled trials and observational studies documented the risk of mood cycling and manic switching, especially in patients with BD I, even if antidepressants were used in the presence of mood-stabilizing medications.7,8


Several newer antipsychotics have been FDA-approved for treating depressive episodes associated with BD (Table 1). Approval of olanzapine/fluoxetine combination (OFC) in December 2003 for depressive episodes associated with BD I established that mechanisms exist which can effectively treat acute depressive episodes in patients with BD without an inordinate risk of mood instability. Subsequent approval of quetiapine in October 2006 for depression associated with BD I or II, lurasidone in June 2013, and cariprazine in May 2019 for major depression in BD I greatly expanded the options for management of acute bipolar depression. However, despite the array of molecules available, for certain patients these agents presented tolerability issues such as sedation, weight gain, akathisia, or parkinsonism that could hamper effective treatment.9 Safety and efficacy data in bipolar depression for adjunctive use with lithium or divalproex/valproate (VPA) also are lacking for quetiapine, OFC, and cariprazine.10,11 Moreover, despite the fact that BD II is as prevalent as BD I, and that patients with BD II have comparable rates of comorbidity, chronicity, disability, and suicidality,12 only quetiapine was approved for acute treatment of depression in patients with BD II. This omission is particularly problematic because the depressive episodes of BD II predominate over the time spent in hypomanic and cycling/mixed states (50.3% for depression vs 3.6% for hypomania/cycling/mixed combined), much more than is seen with BD I (31.9% for depression vs 14.8% for hypomania/cycling/mixed combined).2,3 The paucity of data for the use of newer antipsychotics in BD II depression presents a problem when patients cannot tolerate or refuse to consider quetiapine. This prevents clinicians from engaging in evidence-based efficacy discussions of other options, even if it is assumed that the tolerability profile for BD II depression patients may be similar to that seen when these agents are used for BD I depression.
Continue to: Table 1...

Lumateperone (Caplyta) is a novel oral antipsychotic initially approved in 2019 for the treatment of adult patients with schizophrenia. It was approved in December 2021 for the management of depression associated with BD I or II in adults as monotherapy or when used adjunctively with the mood stabilizers lithium or VPA (Table 2).13 Lumateperone possesses certain binding affinities not unlike those in other newer antipsychotics, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), low affinity for dopamine D2 receptors (Ki 32 nM), and low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).13,14 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that for other second-generation antipsychotics (SGAs) such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18).15 At steady state, D2 receptor occupancy remains <40%, and the corresponding rates of extrapyramidal side effects (EPS)/akathisia differed by only 0.4% for lumateperone vs placebo in short-term adult clinical schizophrenia trials,13,16 by 0.2% for lumateperone vs placebo in the monotherapy BD depression study, and by 1.7% in the adjunctive BD depression study.13,17,18 Lumateperone also exhibited no clinically significant impact on metabolic measures or serum prolactin during the 4-week schizophrenia trials, with mean weight gain ≤1 kg for the 42 mg dose across all studies.19 This favorable tolerability profile for endocrine and metabolic adverse effects was also seen in the BD depression studies. Across the 2 BD depression monotherapy trials and the single adjunctive study, the only adverse reactions occurring in ≥5% of lumateperone-treated patients and more than twice the rate of placebo were somnolence/sedation, dizziness, nausea, and dry mouth.13 There was also no single adverse reaction leading to discontinuation in the BD depression studies that occurred at a rate >2% in patients treated with lumateperone.13

In addition to the low risk of adverse events of all types, lumateperone has several pharmacologic features that distinguish it from other agents in its class. One unique aspect of lumateperone’s pharmacology is differential actions at presynaptic and postsynaptic dopamine D2 receptors noted in preclinical assays, a property that may explain its ability to act as an antipsychotic despite low D2 receptor occupancy.16 Preclinical assays also predicted that lumateperone was likely to have antidepressant effects.15,19,20 Unlike every SGA except ziprasidone, lumateperone also possesses moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM), with SERT occupancy of approximately 30% at 42 mg.21 Lumateperone facilitates dopamine D1-mediated neurotransmission, and this is associated with increased glutamate signaling in the prefrontal cortex and antidepressant actions.14,22 While the extent of SERT occupancy is significantly below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitors, it is hypothesized that near saturation of the 5HT2A receptor might act synergistically with modest 5HT reuptake inhibition and D1-mediated effects to promote the downstream glutamatergic effects that correlate with antidepressant activity (eg, changes in markers such as phosphorylation of glutamate N-methyl-D-aspartate receptor subunits, potentiation of AMPA receptor-mediated transmission).15,22
Continue to: Clinical implications...
Clinical implications
The approval of lumateperone for both BD I and BD II depression, and for its use as monotherapy and for adjunctive use with lithium or VPA, satisfies several unmet needs for the management of acute major depressive episodes in patients with BD. Clinicians now have both safety and tolerability data to present to their bipolar spectrum patients regardless of subtype, and regardless of whether the patient requires mood stabilizer therapy. The tolerability advantages for lumateperone seen in schizophrenia trials were replicated in a diagnostic group that is very sensitive to D2-related adverse effects, and for whom any signal of clinically significant weight gain or sedation often represents an insuperable barrier to patient acceptance.23
Efficacy in adults with BD I or II depression.
The efficacy of lumateperone for major depressive episodes has been established in 2 pivotal, double-blind, placebo-controlled trials in BD I or II patients: 1 monotherapy study,17 and 1 study when used adjunctively to lithium or VPA.18 The first study was a 6-week, double-blind, placebo-controlled monotherapy trial (study 404) in which 377 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode were randomized in a 1:1 manner to lumateperone 42 mg/d or placebo given once daily in the evening. Symptom entry criteria included a Montgomery-Åsberg Depression Rating Scale (MADRS) total score ≥20, and scores ≥4 on the depression and overall BD illness subscales of the Clinical Global Impressions Scale–Bipolar Version Severity scale (CGI-BP-S) at screening and at baseline.17 Study entry also required a score ≤12 on the Young Mania Rating Scale (YMRS) at screening and at baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS. Several secondary efficacy measures were examined, including the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS), or remission (MADRS score ≤12), and differential changes in MADRS scores from baseline for BD I and BD II subgroups.17
The patient population was 58% female and 91% White, with 79.9% diagnosed as BD I and 20.1% as BD II. The least squares mean changes on the MADRS total score from baseline to Day 43 were lumateperone 42 mg/d: -16.7 points; placebo: -12.1 points (P < .0001), and the effect size for this difference was moderate: 0.56. Secondary analyses indicated that 51.1% of those taking lumateperone 42 mg/d and 36.7% taking placebo met response criteria (P < .001), while 39.9% of those taking lumateperone 42 mg/d and 33.5% taking placebo met remission criteria (P = .018). Importantly, depression improvement was observed both in patients with BD I (effect size 0.49, P < .0001) and in those with BD II (effect size 0.81, P < .001).
The second pivotal trial (study 402) was a 6-week, double-blind, placebo-controlled adjunctive trial in which 528 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode despite treatment with lithium or VPA were randomized in a 1:1:1 manner to lumateperone 28 mg/d, lumateperone 42 mg/d, or placebo given once daily in the evening.18 Like the monotherapy trial, symptom entry criteria included a MADRS total score ≥20, and scores ≥4 on the depression and overall illness CGI-BP-S subscales at screening and baseline.18 Study entry also required a score ≤12 on the YMRS at screening and baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS for lumateperone 42 mg/d compared to placebo. Secondary efficacy measures included MADRS changes for lumateperone 28 mg/d and the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS) or remission (MADRS score ≤12).
The patient population was 58% female and 88% White, with 83.3% diagnosed as BD I, 16.7% diagnosed as BD II, and 28.6% treated with lithium vs 71.4% on VPA. The effect size for the difference in MADRS total score from baseline to Day 43 for lumateperone 42 mg/d was 0.27 (P < .05), while that for the lumateperone 28 mg/d dose did not reach statistical significance. Secondary analyses indicated that response rates for lumateperone 28 mg/d and lumateperone 42 mg/d were significantly higher than for placebo (both P < .05). Response rates were placebo: 39%; lumateperone 28 mg/d: 50%; and lumateperone 42 mg/d: 45%. Remission rates were similar at Day 43 in both lumateperone groups compared with placebo: placebo: 31%, lumateperone 28 mg/d: 31%, and lumateperone 42 mg/d: 28%.18 As of this writing, a secondary analysis by BD subtype has not yet been presented.
A third study examining lumateperone monotherapy failed to establish superiority of lumateperone over placebo (NCT02600494). The data regarding tolerability from that study were incorporated in product labeling describing adverse reactions.
Continue on to: Adverse reactions...
Adverse reactions
In the positive monotherapy trial, there were 376 patients in the modified intent-to-treat efficacy population to receive lumateperone (N = 188) or placebo (N = 188) with nearly identical completion rates in the active treatment and placebo cohorts: lumateperone, 88.8%; placebo, 88.3%.17 The proportion experiencing mania was low in both cohorts (lumateperone, 1.1%; placebo, 2.1%), and there was 1 case of hypomania in each group. One participant in the lumateperone group and 1 in the placebo group discontinued the study due to a serious adverse event of mania. There was no worsening of mania in either group as measured by mean change in the YMRS score. There was also no suicidal behavior in either cohort during the study. Pooling the 2 monotherapy trials, the adverse events that occurred at ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone 42 mg/d: 13%, placebo: 3%), dizziness (lumateperone 42 mg/d: 8%, placebo: 4%), and nausea (lumateperone 42 mg/d: 8%, placebo: 3%).13 Rates of EPS were low for both groups: lumateperone 42 mg/d: 1.3%, placebo: 1.1%.13 Mean weight change at Day 43 was +0.11 kg for lumateperone and +0.03 kg for placebo in the positive monotherapy trial.17 Moreover, compared to placebo, lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant. No patient exhibited a corrected QT interval >500 ms at any time, and increases ≥60 ms from baseline were similar between the lumateperone (n = 1, 0.6%) and placebo (n = 3, 1.8%) cohorts.
Complete safety and tolerability data for the adjunctive trial has not yet been published, but discontinuation rates due to treatment-emergent adverse effects for the 3 arms were: lumateperone 42 mg/d: 5.6%; lumateperone 28 mg/d: 1.7%; and placebo: 1.7%. Overall, 81.4% of patients completed the trial, with only 1 serious adverse event (lithium toxicity) occurring in a patient taking lumateperone 42 mg/d. While this led to study discontinuation, it was not considered related to lumateperone exposure by the investigator. There was no worsening of mania in either lumateperone dosage group or the placebo cohort as measured by mean change in YMRS score: -1.2 for placebo, -1.4 for lumateperone 28 mg/d, and -1.6 for lumateperone 42 mg/d. Suicidal behavior was not observed in any group during treatment. The adverse events that occurred at rates ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone, 13%; placebo, 3%), dizziness (lumateperone, 11%; placebo, 2%), and nausea (lumateperone, 9%; placebo, 4%).13 Rates of EPS were low for both groups: lumateperone, 4.0%, placebo, 2.3%.13 Mean weight changes at Day 43 were +0.23 kg for placebo, +0.02 kg for lumateperone 28 mg/d, and 0.00 kg for lumateperone 42 mg/d.18 Compared to placebo, both doses of lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant.18
Lastly, the package insert notes that in an uncontrolled, open-label trial of lumateperone for up to 6 months in patients with BD depression, the mean weight change was -0.01 ± 3.1 kg at Day 175.13
Continue on to: Pharmacologic profile...
Pharmacologic profile
Lumateperone’s preclinical discovery program found an impact on markers associated with increased glutamatergic neurotransmission, properties that were predicted to yield antidepressant benefit.14,15,24 This is hypothesized to be based on the complex pharmacology of lumateperone, including dopamine D1 agonism, modest SERT occupancy, and near saturation of the 5HT2A receptor.15,22 Dopamine D2 affinity is modest (32 nM), and the D2 receptor occupancy at the 42 mg dose is low. These properties translate to rates of EPS in clinical studies of schizophrenia and BD that are close to that of placebo. Lumateperone has very high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), which also helps mitigate D2-related adverse effects and may be part of the therapeutic antidepressant mechanism. Underlying the tolerability profile is the low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).
Clinical considerations
Data from the lumateperone BD depression trials led to it being only the second agent approved for acute major depression in BD II patients, and the only agent which has approvals as monotherapy and adjunctive therapy for both BD subtypes. The monotherapy trial results substantiate that lumateperone was robustly effective regardless of BD subtype, with significant improvement in depressive symptoms experienced by patients with BD I (effect size 0.49, P < .0001) and those with BD II (effect size 0.81, P < .001). Effect sizes in acute BD depression studies are much larger in monotherapy trials than in adjunctive trials, as the latter group represents patients who have already failed pretreatment with a mood stabilizer.25,26 In the lurasidone BD I depression trials, the effect size based on mean change in MADRS score over the course of 6 weeks was 0.51 in the monotherapy study compared to 0.34 when used adjunctively with lithium or VPA.25,26 In the lumateperone adjunctive study, the effect size for the difference in mean MADRS total score from baseline for lumateperone 42 mg/d, was 0.27 (P < .05). Subgroup analyses by BD subtype are not yet available for adjunctive use, but the data presented to FDA were sufficient to permit an indication for adjunctive use across both diagnostic groups.
The absence of clinically significant EPS, the minimal impact on metabolic or endocrine parameters, and the lack of a need for titration are all appealing properties. At the present there is only 1 marketed dose (42 mg capsules), so the package insert includes cautionary language regarding situations when a patient might encounter less drug exposure (concurrent use of cytochrome P450 [CYP] 3A4 inducers), or greater drug exposure due to concurrent use of moderate or strong CYP3A4 inhibitors, as well as in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include efficacy established as monotherapy for BD I and BD II patients, and efficacy for adjunctive use with lithium or VPA. Additionally, the extremely low rates of significant EPS and lack of clinically significant metabolic or endocrine adverse effects are unique properties of lumateperone.13
Why Rx? Reasons to prescribe lumateperone for adult BD depression patients include:
- data support efficacy for BD I and BD II patients, and for monotherapy or adjunctive use with lithium/VPA
- favorable tolerability profile, including no significant signal for EPS, endocrine or metabolic adverse effects, or QT prolongation
- no need for titration.
Dosing. There is only 1 dose available for lumateperone: 42 mg capsules (Table 3). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less drug exposure (use of CYP3A4 inducers), or greater drug exposure (use with moderate or strong CYP3A4 inhibitors or in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria [Child-Pugh B or C]). These are not contraindications. Based on newer pharmacokinetic studies, lumateperone does not need to be dosed with food, and there is no clinically significant interaction with UGT1A4 inhibitors such as VPA.

Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Bottom Line
Data support the efficacy of lumateperone for treating depressive episodes in adults with bipolar I or bipolar II disorder, either as monotherapy or adjunctive to lithium or divalproex/valproate. Potential advantages of lumateperone for this indication include a favorable tolerability profile and no need for titration.
1. Malhi GS, Bell E, Boyce P, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Bipolar Disord. 2020;22(8):805-821.
2. Judd LL, Akishal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akishal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Post RM. Treatment of bipolar depression: evolving recommendations. Psychiatr Clin North Am. 2016;39(1):11-33.
5. Pacchiarotti I, Verdolini N. Antidepressants in bipolar II depression: yes and no. Eur Neuropsychopharmacol 2021;47:48-50.
6. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
7. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
8. Gitlin MJ. Antidepressants in bipolar depression: an enduring controversy. Int J Bipolar Disord. 2018;6(1):25.
9. Verdolini N, Hidalgo-Mazzei D, Del Matto L, et al. Long-term treatment of bipolar disorder type I: a systematic and critical review of clinical guidelines with derived practice algorithms. Bipolar Disord. 2021;23(4):324-340.
10. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20(2):180-195.
11. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
12. Chakrabarty T, Hadijpavlou G, Bond DJ, et al. Bipolar II disorder in context: a review of its epidemiology, disability and economic burden. In: Parker G. Bipolar II Disorder: Modelling, Measuring and Managing. 3rd ed. Cambridge University Press; 2019:49-59.
13. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2021.
14. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
15. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232:605-621.
16. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
17. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry 2021;178(12):1098-1106.
18. Yatham LN, et al. Adjunctive lumateperone (ITI-007) in the treatment of bipolar depression: results from a randomized clinical trial. Poster presented at: American Psychiatric Association Annual Meeting. May 1-3, 2021; virtual conference.
19. Vanover K, Glass S, Kozauer S, et al. 30 Lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectrums. 2019;24(1):190-191.
20. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
21. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-72.
22. Björkholm C, Marcus MM, Konradsson-Geuken Å, et al. The novel antipsychotic drug brexpiprazole, alone and in combination with escitalopram, facilitates prefrontal glutamatergic transmission via a dopamine D1 receptor-dependent mechanism. Eur Neuropsychopharmacol. 2017;27(4):411-417.
23. Bai Y, Yang H, Chen G, et al. Acceptability of acute and maintenance pharmacotherapy of bipolar disorder: a systematic review of randomized, double-blind, placebo-controlled clinical trials. J Clin Psychopharmacol. 2020;40(2):167-179.
24. Vyas P, Hwang BJ, Braši´c JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
25. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
26. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169-77.
1. Malhi GS, Bell E, Boyce P, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Bipolar Disord. 2020;22(8):805-821.
2. Judd LL, Akishal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akishal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Post RM. Treatment of bipolar depression: evolving recommendations. Psychiatr Clin North Am. 2016;39(1):11-33.
5. Pacchiarotti I, Verdolini N. Antidepressants in bipolar II depression: yes and no. Eur Neuropsychopharmacol 2021;47:48-50.
6. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
7. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
8. Gitlin MJ. Antidepressants in bipolar depression: an enduring controversy. Int J Bipolar Disord. 2018;6(1):25.
9. Verdolini N, Hidalgo-Mazzei D, Del Matto L, et al. Long-term treatment of bipolar disorder type I: a systematic and critical review of clinical guidelines with derived practice algorithms. Bipolar Disord. 2021;23(4):324-340.
10. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20(2):180-195.
11. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
12. Chakrabarty T, Hadijpavlou G, Bond DJ, et al. Bipolar II disorder in context: a review of its epidemiology, disability and economic burden. In: Parker G. Bipolar II Disorder: Modelling, Measuring and Managing. 3rd ed. Cambridge University Press; 2019:49-59.
13. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2021.
14. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
15. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232:605-621.
16. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
17. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry 2021;178(12):1098-1106.
18. Yatham LN, et al. Adjunctive lumateperone (ITI-007) in the treatment of bipolar depression: results from a randomized clinical trial. Poster presented at: American Psychiatric Association Annual Meeting. May 1-3, 2021; virtual conference.
19. Vanover K, Glass S, Kozauer S, et al. 30 Lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectrums. 2019;24(1):190-191.
20. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
21. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-72.
22. Björkholm C, Marcus MM, Konradsson-Geuken Å, et al. The novel antipsychotic drug brexpiprazole, alone and in combination with escitalopram, facilitates prefrontal glutamatergic transmission via a dopamine D1 receptor-dependent mechanism. Eur Neuropsychopharmacol. 2017;27(4):411-417.
23. Bai Y, Yang H, Chen G, et al. Acceptability of acute and maintenance pharmacotherapy of bipolar disorder: a systematic review of randomized, double-blind, placebo-controlled clinical trials. J Clin Psychopharmacol. 2020;40(2):167-179.
24. Vyas P, Hwang BJ, Braši´c JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
25. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
26. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169-77.
Lumateperone for schizophrenia
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9

How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9

How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9

How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
Risperidone extended-release injectable suspension
Oral antipsychotic nonadherence is a significant contributor to relapse in patients with schizophrenia spectrum disorders. Long-acting injectable (LAI) antipsychotics have been developed to provide sustained antipsychotic exposure, with evidence that use of LAIs significantly reduces hospitalization rates.1 One limiting factor in transitioning patients to certain LAIs is the need for prolonged oral coverage at the onset of treatment for agents that cannot be loaded. Nonadherence with this bridging oral therapy places the patient at risk for symptom exacerbation until effective antipsychotic plasma levels are achieved from the LAI.2 Although risperidone is one of the more widely used antipsychotics for treating schizophrenia, until recently the only available LAI preparation, risperidone microspheres (Risperdal Consta), required 3 weeks of oral coverage upon initiation.3

Clinical implications
Oral medication nonadherence remains a significant public health issue for patients with schizophrenia, with an estimated 50% of patients failing to achieve 80% adherence even when enrolled in clinical trials specifically designed to track adherence.5 Although LAI atypical antipsychotics have been available since the approval of Risperdal Consta, the LAI form of risperidone, and both LAI forms of aripiprazole, were not designed to be loaded. A 1-day initiation regimen for aripiprazole lauroxil has been developed to avoid the need for 3 weeks of oral medication coverage,6,7 but aripiprazole monohydrate and risperidone microspheres mandate oral bridging of 2 and 3 weeks, respectively.2 Because one of the primary indications for LAI antipsychotic therapy is oral medication nonadherence, this prolonged period of oral coverage creates a risk for symptom exacerbation when the bridging period occurs outside of a controlled setting, as is common when patients are discharged from inpatient hospitalization.
One solution to this problem has its antecedents in the development of the Atrigel biodegradable injectable polymer, which was designed to deliver prolonged medication exposure after subcutaneous injection.8 This biodegradable polymer drug delivery system suspends and dissolves the medication of interest (in this case, risperidone) in a poly DL-lactide-coglycolide gel and its biocompatible carrier.9 The viscous liquid undergoes a phase transition upon contact with tissue fluids after subcutaneous injection, resulting in an implant that releases risperidone in a controlled manner as it is resorbed. Importantly, the kinetic parameters of RBP-7000 are such that effective drug levels are seen within the first week without the need for oral coverage.10
Use in adults with schizophrenia. After establishing tolerability with oral risperidone, the recommended doses are 90 mg or 120 mg monthly, which correspond to oral daily risperidone doses of 3 mg or 4 mg. RBP-7000 must be administered as a subcutaneous abdominal injection by a health care professional. It is recommended that the patient be in the supine position for the injection and that the injection sites be rotated monthly among 4 quadrants in the abdominal region. The injection volumes for the 90 mg and 120 mg doses are 0.6 mL and 0.8 mL, respectively.10 As the gel implant becomes firmer, the patient will notice a lump for several weeks that will decrease in size over time. Patients should be advised not to rub or massage the injection site, and to be aware of the placement of any belts or clothing with waistbands.10
Pharmacologic profile, adverse reactions
Risperidone is an atypical antipsychotic that has been commercially available in the U.S. since December 29, 1993, and its adverse effect profile is well characterized. The most common adverse effects associated with risperidone include those related to dopamine D2 antagonism, metabolic adverse effects, and an increase in serum prolactin. In the 12-month long-term safety study of RBP-7000, 1-minute post-dose injection site pain scores (on a 100-point scale) were highest on Day 1 (mean of 25) and decreased over time with subsequent injections (14 to 16 following the last injection).10
Continue to: How the Atrigel system works
How the Atrigel system works. The Atrigel system was developed in the late 1980s and consists of a solution of a resorbable polymer in a biocompatible carrier.11 After in vivo administration (typically via subcutaneous injection), the polymer undergoes a phase change from a liquid to a formed implant (Figure 1). Being in liquid form, this system provides the advantage of placement by simple means, such as injection by syringes. The absorption rates of various polymers and the release rates for various drugs are tailored to the desired indication. Approved uses for Atrigel include the subgingival delivery of the antibiotic doxycycline for chronic adult periodontitis (approved September 1998), and the monthly subcutaneous injectable form of the anti-androgen leuprolide, which was approved in January 2002.8,12 Release periods up to 4 months have been achieved with Atrigel; 1 month is the most often desired release period. The biodegradable polymer used for RBP-7000 is designed to provide effective plasma drug levels during the first week of treatment, and sustained levels with a 1-month dosing interval. The small subcutaneous implant that is formed is gradually resorbed over the course of 1 month.
Pharmacokinetics. As with all LAI medications, the half-life with repeated dosing vastly exceeds that achieved with oral administration. Following oral administration, mean peak plasma levels of risperidone occur at 1 hour, and those for the active metabolite 9-OH risperidone occur at 3 hours.13 Oral risperidone has a mean half-life of 3 hours, while the active metabolite 9-OH risperidone has a mean half-life of 21 hours.14 Due to its longer half-life, the metabolite comprises 83% of the active drug levels at steady state.14 Although risperidone is susceptible to interactions via cytochrome P450 (CYP) inhibitors and inducers, particularly CYP2D6 (Table 210), the pharmacokinetics of the combined total of risperidone and 9-OH risperidone levels (deemed the active moiety) are similar in CYP2D6 extensive and poor metabolizers, with an overall mean elimination half-life of approximately 20 hours.13
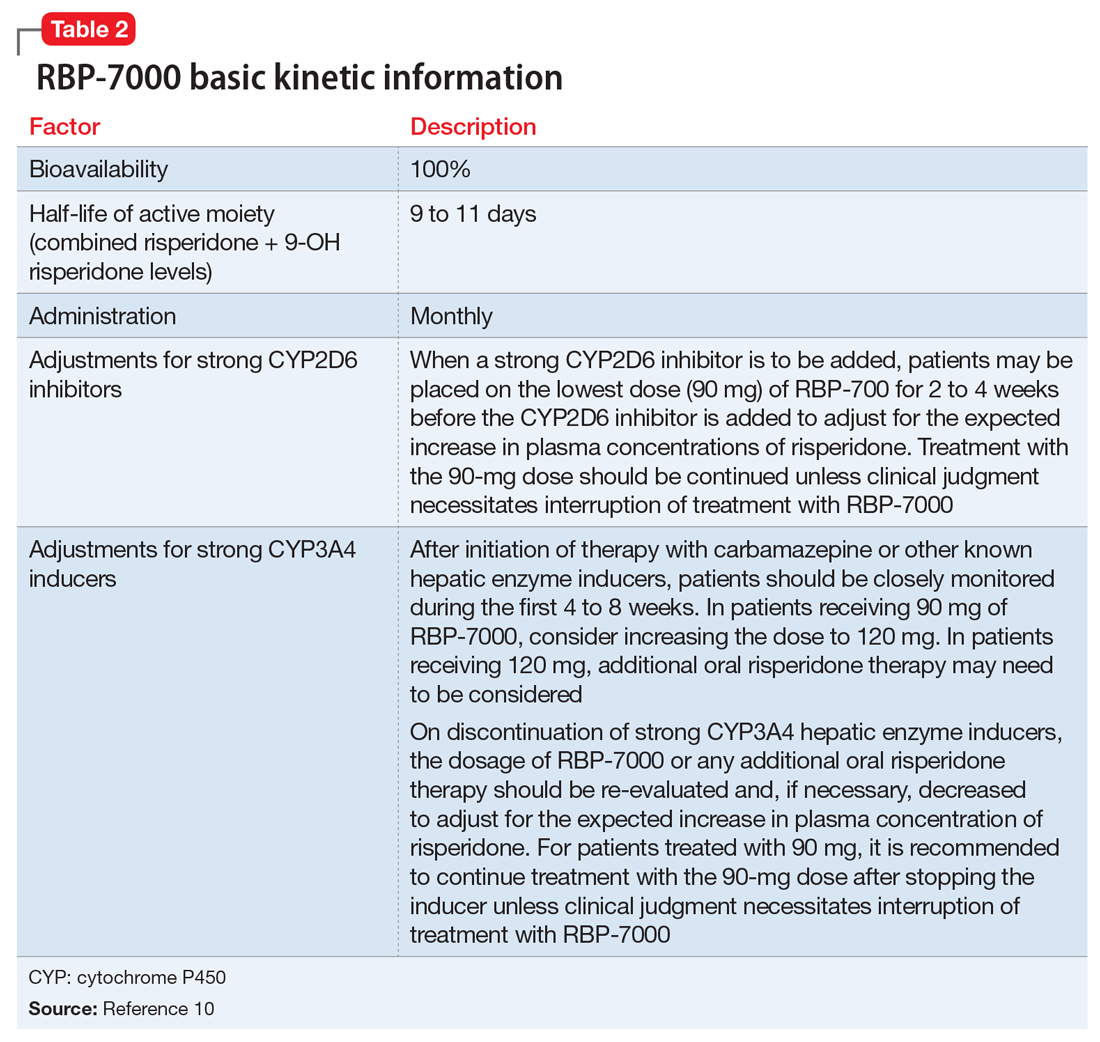
The kinetics for RBP-7000 are markedly different than those for oral risperidone (Figure 215). After a single subcutaneous injection, RBP-7000 shows 2 absorption peaks for risperidone. The first lower peak occurs with a Tmax of 4 to 6 hours due to initial release of risperidone during the implant formation process; a second risperidone peak occurs after 10 to 14 days and is associated with slow release from the subcutaneous depot.9,16,17 For both 9-OH risperidone levels and the total active moiety (risperidone plus 9-OH risperidone levels), the median Tmax of the first peak ranges from 4 to 48 hours and the second peak ranges from 7 to 11 days. Following a single subcutaneous injection of RBP-7000, the apparent terminal half-life of risperidone ranges from 9 to 11 days, on average. The mean apparent terminal half-life of the active moiety ranges from 8 to 9 days.9,16,17 Based on population pharmacokinetic modeling, the 90 mg and 120 mg doses of RBP-7000 are estimated to provide drug exposure equivalent to 3 mg/d and 4 mg/d of oral risperidone, respectively.9,16,17
Continue to: Efficacy of RBP-7000
Efficacy of RBP-7000 was established in an 8-week, double-blind, placebo-controlled trial of adult patients experiencing an acute exacerbation of schizophrenia (age 18 to 55).4 Eligible participants had:
- An acute exacerbation of schizophrenia that occurred ≤8 weeks before the screening visit and would have benefited from psychiatric hospitalization or continued hospitalization
- Positive and Negative Syndrome Scale (PANSS) total score between 80 and 120 at visit 1 and a score of >4 on at least 2 of the following 4 items: hallucinatory behavior, delusions, conceptual disorganization, or suspiciousness/persecution
- The diagnosis of acute exacerbation of schizophrenia and PANSS total score were confirmed through an independent video-conference interview conducted by an experienced rater.
Participants were excluded if they:
- Experienced a ≥20% improvement in PANSS total score between the initial screening visit and the first injection
- had been treated at any time with clozapine for treatment-resistant schizophrenia
- had met DSM-IV-TR criteria for substance dependence (with the exception of nicotine or caffeine) before screening.
During the initial screening visit, participants received a 0.25-mg tablet of oral risperidone on 2 consecutive days to assess the tolerability of risperidone.
Outcome. Participants were randomized in a 1:1:1 manner to placebo (n = 112) or 1 of 2 monthly doses of RBP-7000: 90 mg (n = 111) or 120 mg (n = 114). Using the least squares means of repeated-measures changes from baseline in PANSS total scores, there was a significant improvement in the difference in PANSS total scores from baseline to the end of the study compared with placebo: 90-mg RBP-7000, -6.148 points (95% confidence interval [CI], -9.982 to -2.314, P = .0004); 120-mg RBP-7000, -7.237 points (95% CI, -11.045 to -3.429, P < .0001). The absolute change from baseline in PANSS total score was -15.367 points for the 90-mg dose and -16.456 points for the 120-mg dose.4 Completion rates across all 3 arms were comparable: placebo 70.6%, RBP-7000 90 mg 77.6%, and RBP-7000 120 mg 71.4%.
Tolerability. In the 8-week phase III efficacy trial of RBP-7000, adverse effects occurring with an incidence ≥5% and at least twice the rate of placebo were weight gain (placebo 3.4%, 90 mg 13.0%, 120 mg 12.8%) and sedation (placebo 0%, 90 mg 7.0%, 120 mg 7.7%).10 Compared with baseline, participants had a mean weight gain at the end of the study of 2.83 kg in the placebo group, 5.15 kg in the 90-mg RBP-7000 group, and 4.69 kg in the 120-mg RBP-7000 group. There were no clinically significant differences at study endpoint in glucose and lipid parameters. Consistent with the known effects of risperidone, there were increases in mean prolactin levels during the 8-week study, the effects of which were greater for women. For men, mean prolactin levels from baseline to study end were: placebo: 9.8 ± 7.9 vs 9.9 ± 8.0 ng/mL; 90 mg: 8.9 ± 6.9 vs 22.4 ± 11.2 ng/mL; and 120 mg: 8.2 ± 5.2 vs 31.3 ± 14.8 ng/mL. For women, mean prolactin levels from baseline to study end were: placebo: 12.8 ± 11.7 vs 10.4 ± 8.0 ng/mL; 90 mg: 7.7 ± 5.3 vs 60.3 ± 46.9 ng/mL; and 120 mg: 10.9 ± 8.6 vs 85.5 ± 55.1 ng/mL. In the pivotal study, discontinuations due to adverse events were low across all treatment groups: 2.5% for placebo vs 0% for 90 mg and 1.7% for 120 mg.4 There was no single adverse reaction leading to discontinuation that occurred at a rate of ≥2% and greater than placebo in patients treated with RBP-7000.10 There were no clinically relevant differences in mean changes from baseline in corrected QT, QRS, and PR intervals, and in heart rate. Similarly, in the 12-month, long-term safety study, there were no clinically relevant changes in mean electrocardiography interval values from baseline to post-dose assessments.10
Using a 100-point visual analog scale (VAS), injection site pain scores 1 minute after the first dose decreased from a mean of 27 to the range of 3 to 7 for scores obtained 30 to 60 minutes post-dose. In the 12-month long-term safety study, 1-minute post-dose injection site pain VAS scores were highest on Day 1 (mean of 25) and decreased over time with subsequent injections (14 to 16 following last injection).10
Clinical considerations
Unique properties. RBP-7000 uses the established Atrigel system to provide effective antipsychotic levels in the first week of treatment, without the need for bridging oral coverage or a second loading injection. The abdominal subcutaneous injection volume is relatively small (0.6 mL or 0.8 mL).
Why Rx? The reasons to prescribe RBP-7000 for adult patients with schizophrenia include:
- no oral coverage required at the initiation of treatment
- effective plasma active moiety levels are seen within the first week without the need for a second loading injection
- monthly injection schedule.
Dosing. The recommended dosage of RBP-7000 is 90 mg or 120 mg once monthly, equivalent to 3 mg/d or 4 mg/d of oral risperidone, respectively. Oral risperidone tolerability should be established before the first injection. No oral risperidone coverage is required. RBP-7000 has not been studied in patients with renal or hepatic impairment and should be used with caution in these patients. Prior to initiating treatment in these patients, it is advised to carefully titrate up to at least 3 mg/d of oral risperidone. If a patient can tolerate 3 mg/d of oral risperidone and is psychiatrically stable, then the 90-mg dose of RBP-7000 can be considered.10
Contraindications. The only contraindications for RBP-7000 are known hypersensitivity to risperidone, paliperidone (9-OH risperidone), or other components of the injection.
Bottom Line
RBP-7000 (Perseris) is the second long-acting injectable (LAI) form of risperidone approved in the U.S. Unlike risperidone microspheres (Consta), RBP-7000 does not require any oral risperidone coverage at the beginning of therapy, provides effective drug levels within the first week of treatment with a single injection, and uses a monthly dosing interval. RBP-7000 does not require loading upon initiation. The monthly injection is <1 mL, is administered in abdominal subcutaneous tissue, and uses the Atrigel system.
Related Resource
- Carpenter J, Wong KK. Long-acting injectable antipsychotics: What to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Doxycycline • Atridox
Leuprolide acetate injectable suspension • Eligard
Paliperidone palmitate • Invega Sustenna
Risperidone • Risperdal
Risperidone extended-release injectable suspension • Perseris
Risperidone long-acting injection • Risperdal Consta
1. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619.
2. Meyer JM. Converting oral to long acting injectable antipsychotics: a guide for the perplexed. CNS Spectrums. 2017;22(S1):14-28.
3. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2018.
4. Nasser AF, Henderson DC, Fava M, et al. Efficacy, safety, and tolerability of RBP-7000 once-monthly risperidone for the treatment of acute schizophrenia: an 8-week, randomized, double-blind, placebo-controlled, multicenter phase 3 study. J Clin Psychopharmacol. 2016;36(2):130-140.
5. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263.
6. Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441.
7. Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469.
8. Southard GL, Dunn RL, Garrett S. The drug delivery and biomaterial attributes of the ATRIGEL technology in the treatment of periodontal disease. Expert Opin Investig Drugs. 1998;7(9):1483-1491.
9. Gomeni R, Heidbreder C, Fudala PJ, Nasser AF. A model-based approach to characterize the population pharmacokinetics and the relationship between the pharmacokinetic and safety profiles of RBP-7000, a new, long-acting, sustained-released formulation of risperidone. J Clin Pharmacol. 2013;53(10):1010-1019.
10. Perseris [package insert]. North Chesterfield, VA: Indivior Inc; 2018.
11. Malik K, Singh I, Nagpal M, et al. Atrigel: a potential parenteral controlled drug delivery system. Der Pharmacia Sinica. 2010;1(1):74-81.
12. Sartor O. Eligard: leuprolide acetate in a novel sustained-release delivery system. Urology. 2003;61(2 Suppl 1):25-31.
13. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2018.
14. de Leon J, Wynn G, Sandson NB. The pharmacokinetics of paliperidone versus risperidone. Psychosomatics. 2010;51(1):80-88.
15. Ivaturi V, Gopalakrishnan M, Gobburu JVS, et al. Exposure-response analysis after subcutaneous administration of RBP-7000, a once-a-month long-acting Atrigel formulation of risperidone. Br J Clin Pharmacol. 2017;83(7):1476-1498.
16. Laffont CM, Gomeni R, Zheng B, et al. Population pharmacokinetics and prediction of dopamine D2 receptor occupancy after multiple doses of RBP-7000, a new sustained-release formulation of risperidone, in schizophrenia patients on stable oral risperidone treatment. Clin Pharmacokinet. 2014;53(6):533-543.
17. Laffont CM, Gomeni R, Zheng B, et al. Population pharmacokinetic modeling and simulation to guide dose selection for RBP-7000, a new sustained-release formulation of risperidone. J Clin Pharmacol. 2015;55(1):93-103.
Oral antipsychotic nonadherence is a significant contributor to relapse in patients with schizophrenia spectrum disorders. Long-acting injectable (LAI) antipsychotics have been developed to provide sustained antipsychotic exposure, with evidence that use of LAIs significantly reduces hospitalization rates.1 One limiting factor in transitioning patients to certain LAIs is the need for prolonged oral coverage at the onset of treatment for agents that cannot be loaded. Nonadherence with this bridging oral therapy places the patient at risk for symptom exacerbation until effective antipsychotic plasma levels are achieved from the LAI.2 Although risperidone is one of the more widely used antipsychotics for treating schizophrenia, until recently the only available LAI preparation, risperidone microspheres (Risperdal Consta), required 3 weeks of oral coverage upon initiation.3

Clinical implications
Oral medication nonadherence remains a significant public health issue for patients with schizophrenia, with an estimated 50% of patients failing to achieve 80% adherence even when enrolled in clinical trials specifically designed to track adherence.5 Although LAI atypical antipsychotics have been available since the approval of Risperdal Consta, the LAI form of risperidone, and both LAI forms of aripiprazole, were not designed to be loaded. A 1-day initiation regimen for aripiprazole lauroxil has been developed to avoid the need for 3 weeks of oral medication coverage,6,7 but aripiprazole monohydrate and risperidone microspheres mandate oral bridging of 2 and 3 weeks, respectively.2 Because one of the primary indications for LAI antipsychotic therapy is oral medication nonadherence, this prolonged period of oral coverage creates a risk for symptom exacerbation when the bridging period occurs outside of a controlled setting, as is common when patients are discharged from inpatient hospitalization.
One solution to this problem has its antecedents in the development of the Atrigel biodegradable injectable polymer, which was designed to deliver prolonged medication exposure after subcutaneous injection.8 This biodegradable polymer drug delivery system suspends and dissolves the medication of interest (in this case, risperidone) in a poly DL-lactide-coglycolide gel and its biocompatible carrier.9 The viscous liquid undergoes a phase transition upon contact with tissue fluids after subcutaneous injection, resulting in an implant that releases risperidone in a controlled manner as it is resorbed. Importantly, the kinetic parameters of RBP-7000 are such that effective drug levels are seen within the first week without the need for oral coverage.10
Use in adults with schizophrenia. After establishing tolerability with oral risperidone, the recommended doses are 90 mg or 120 mg monthly, which correspond to oral daily risperidone doses of 3 mg or 4 mg. RBP-7000 must be administered as a subcutaneous abdominal injection by a health care professional. It is recommended that the patient be in the supine position for the injection and that the injection sites be rotated monthly among 4 quadrants in the abdominal region. The injection volumes for the 90 mg and 120 mg doses are 0.6 mL and 0.8 mL, respectively.10 As the gel implant becomes firmer, the patient will notice a lump for several weeks that will decrease in size over time. Patients should be advised not to rub or massage the injection site, and to be aware of the placement of any belts or clothing with waistbands.10
Pharmacologic profile, adverse reactions
Risperidone is an atypical antipsychotic that has been commercially available in the U.S. since December 29, 1993, and its adverse effect profile is well characterized. The most common adverse effects associated with risperidone include those related to dopamine D2 antagonism, metabolic adverse effects, and an increase in serum prolactin. In the 12-month long-term safety study of RBP-7000, 1-minute post-dose injection site pain scores (on a 100-point scale) were highest on Day 1 (mean of 25) and decreased over time with subsequent injections (14 to 16 following the last injection).10
Continue to: How the Atrigel system works
How the Atrigel system works. The Atrigel system was developed in the late 1980s and consists of a solution of a resorbable polymer in a biocompatible carrier.11 After in vivo administration (typically via subcutaneous injection), the polymer undergoes a phase change from a liquid to a formed implant (Figure 1). Being in liquid form, this system provides the advantage of placement by simple means, such as injection by syringes. The absorption rates of various polymers and the release rates for various drugs are tailored to the desired indication. Approved uses for Atrigel include the subgingival delivery of the antibiotic doxycycline for chronic adult periodontitis (approved September 1998), and the monthly subcutaneous injectable form of the anti-androgen leuprolide, which was approved in January 2002.8,12 Release periods up to 4 months have been achieved with Atrigel; 1 month is the most often desired release period. The biodegradable polymer used for RBP-7000 is designed to provide effective plasma drug levels during the first week of treatment, and sustained levels with a 1-month dosing interval. The small subcutaneous implant that is formed is gradually resorbed over the course of 1 month.

Pharmacokinetics. As with all LAI medications, the half-life with repeated dosing vastly exceeds that achieved with oral administration. Following oral administration, mean peak plasma levels of risperidone occur at 1 hour, and those for the active metabolite 9-OH risperidone occur at 3 hours.13 Oral risperidone has a mean half-life of 3 hours, while the active metabolite 9-OH risperidone has a mean half-life of 21 hours.14 Due to its longer half-life, the metabolite comprises 83% of the active drug levels at steady state.14 Although risperidone is susceptible to interactions via cytochrome P450 (CYP) inhibitors and inducers, particularly CYP2D6 (Table 210), the pharmacokinetics of the combined total of risperidone and 9-OH risperidone levels (deemed the active moiety) are similar in CYP2D6 extensive and poor metabolizers, with an overall mean elimination half-life of approximately 20 hours.13

The kinetics for RBP-7000 are markedly different than those for oral risperidone (Figure 215). After a single subcutaneous injection, RBP-7000 shows 2 absorption peaks for risperidone. The first lower peak occurs with a Tmax of 4 to 6 hours due to initial release of risperidone during the implant formation process; a second risperidone peak occurs after 10 to 14 days and is associated with slow release from the subcutaneous depot.9,16,17 For both 9-OH risperidone levels and the total active moiety (risperidone plus 9-OH risperidone levels), the median Tmax of the first peak ranges from 4 to 48 hours and the second peak ranges from 7 to 11 days. Following a single subcutaneous injection of RBP-7000, the apparent terminal half-life of risperidone ranges from 9 to 11 days, on average. The mean apparent terminal half-life of the active moiety ranges from 8 to 9 days.9,16,17 Based on population pharmacokinetic modeling, the 90 mg and 120 mg doses of RBP-7000 are estimated to provide drug exposure equivalent to 3 mg/d and 4 mg/d of oral risperidone, respectively.9,16,17

Continue to: Efficacy of RBP-7000
Efficacy of RBP-7000 was established in an 8-week, double-blind, placebo-controlled trial of adult patients experiencing an acute exacerbation of schizophrenia (age 18 to 55).4 Eligible participants had:
- An acute exacerbation of schizophrenia that occurred ≤8 weeks before the screening visit and would have benefited from psychiatric hospitalization or continued hospitalization
- Positive and Negative Syndrome Scale (PANSS) total score between 80 and 120 at visit 1 and a score of >4 on at least 2 of the following 4 items: hallucinatory behavior, delusions, conceptual disorganization, or suspiciousness/persecution
- The diagnosis of acute exacerbation of schizophrenia and PANSS total score were confirmed through an independent video-conference interview conducted by an experienced rater.
Participants were excluded if they:
- Experienced a ≥20% improvement in PANSS total score between the initial screening visit and the first injection
- had been treated at any time with clozapine for treatment-resistant schizophrenia
- had met DSM-IV-TR criteria for substance dependence (with the exception of nicotine or caffeine) before screening.
During the initial screening visit, participants received a 0.25-mg tablet of oral risperidone on 2 consecutive days to assess the tolerability of risperidone.
Outcome. Participants were randomized in a 1:1:1 manner to placebo (n = 112) or 1 of 2 monthly doses of RBP-7000: 90 mg (n = 111) or 120 mg (n = 114). Using the least squares means of repeated-measures changes from baseline in PANSS total scores, there was a significant improvement in the difference in PANSS total scores from baseline to the end of the study compared with placebo: 90-mg RBP-7000, -6.148 points (95% confidence interval [CI], -9.982 to -2.314, P = .0004); 120-mg RBP-7000, -7.237 points (95% CI, -11.045 to -3.429, P < .0001). The absolute change from baseline in PANSS total score was -15.367 points for the 90-mg dose and -16.456 points for the 120-mg dose.4 Completion rates across all 3 arms were comparable: placebo 70.6%, RBP-7000 90 mg 77.6%, and RBP-7000 120 mg 71.4%.
Tolerability. In the 8-week phase III efficacy trial of RBP-7000, adverse effects occurring with an incidence ≥5% and at least twice the rate of placebo were weight gain (placebo 3.4%, 90 mg 13.0%, 120 mg 12.8%) and sedation (placebo 0%, 90 mg 7.0%, 120 mg 7.7%).10 Compared with baseline, participants had a mean weight gain at the end of the study of 2.83 kg in the placebo group, 5.15 kg in the 90-mg RBP-7000 group, and 4.69 kg in the 120-mg RBP-7000 group. There were no clinically significant differences at study endpoint in glucose and lipid parameters. Consistent with the known effects of risperidone, there were increases in mean prolactin levels during the 8-week study, the effects of which were greater for women. For men, mean prolactin levels from baseline to study end were: placebo: 9.8 ± 7.9 vs 9.9 ± 8.0 ng/mL; 90 mg: 8.9 ± 6.9 vs 22.4 ± 11.2 ng/mL; and 120 mg: 8.2 ± 5.2 vs 31.3 ± 14.8 ng/mL. For women, mean prolactin levels from baseline to study end were: placebo: 12.8 ± 11.7 vs 10.4 ± 8.0 ng/mL; 90 mg: 7.7 ± 5.3 vs 60.3 ± 46.9 ng/mL; and 120 mg: 10.9 ± 8.6 vs 85.5 ± 55.1 ng/mL. In the pivotal study, discontinuations due to adverse events were low across all treatment groups: 2.5% for placebo vs 0% for 90 mg and 1.7% for 120 mg.4 There was no single adverse reaction leading to discontinuation that occurred at a rate of ≥2% and greater than placebo in patients treated with RBP-7000.10 There were no clinically relevant differences in mean changes from baseline in corrected QT, QRS, and PR intervals, and in heart rate. Similarly, in the 12-month, long-term safety study, there were no clinically relevant changes in mean electrocardiography interval values from baseline to post-dose assessments.10
Using a 100-point visual analog scale (VAS), injection site pain scores 1 minute after the first dose decreased from a mean of 27 to the range of 3 to 7 for scores obtained 30 to 60 minutes post-dose. In the 12-month long-term safety study, 1-minute post-dose injection site pain VAS scores were highest on Day 1 (mean of 25) and decreased over time with subsequent injections (14 to 16 following last injection).10
Clinical considerations
Unique properties. RBP-7000 uses the established Atrigel system to provide effective antipsychotic levels in the first week of treatment, without the need for bridging oral coverage or a second loading injection. The abdominal subcutaneous injection volume is relatively small (0.6 mL or 0.8 mL).
Why Rx? The reasons to prescribe RBP-7000 for adult patients with schizophrenia include:
- no oral coverage required at the initiation of treatment
- effective plasma active moiety levels are seen within the first week without the need for a second loading injection
- monthly injection schedule.
Dosing. The recommended dosage of RBP-7000 is 90 mg or 120 mg once monthly, equivalent to 3 mg/d or 4 mg/d of oral risperidone, respectively. Oral risperidone tolerability should be established before the first injection. No oral risperidone coverage is required. RBP-7000 has not been studied in patients with renal or hepatic impairment and should be used with caution in these patients. Prior to initiating treatment in these patients, it is advised to carefully titrate up to at least 3 mg/d of oral risperidone. If a patient can tolerate 3 mg/d of oral risperidone and is psychiatrically stable, then the 90-mg dose of RBP-7000 can be considered.10
Contraindications. The only contraindications for RBP-7000 are known hypersensitivity to risperidone, paliperidone (9-OH risperidone), or other components of the injection.
Bottom Line
RBP-7000 (Perseris) is the second long-acting injectable (LAI) form of risperidone approved in the U.S. Unlike risperidone microspheres (Consta), RBP-7000 does not require any oral risperidone coverage at the beginning of therapy, provides effective drug levels within the first week of treatment with a single injection, and uses a monthly dosing interval. RBP-7000 does not require loading upon initiation. The monthly injection is <1 mL, is administered in abdominal subcutaneous tissue, and uses the Atrigel system.
Related Resource
- Carpenter J, Wong KK. Long-acting injectable antipsychotics: What to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Doxycycline • Atridox
Leuprolide acetate injectable suspension • Eligard
Paliperidone palmitate • Invega Sustenna
Risperidone • Risperdal
Risperidone extended-release injectable suspension • Perseris
Risperidone long-acting injection • Risperdal Consta
Oral antipsychotic nonadherence is a significant contributor to relapse in patients with schizophrenia spectrum disorders. Long-acting injectable (LAI) antipsychotics have been developed to provide sustained antipsychotic exposure, with evidence that use of LAIs significantly reduces hospitalization rates.1 One limiting factor in transitioning patients to certain LAIs is the need for prolonged oral coverage at the onset of treatment for agents that cannot be loaded. Nonadherence with this bridging oral therapy places the patient at risk for symptom exacerbation until effective antipsychotic plasma levels are achieved from the LAI.2 Although risperidone is one of the more widely used antipsychotics for treating schizophrenia, until recently the only available LAI preparation, risperidone microspheres (Risperdal Consta), required 3 weeks of oral coverage upon initiation.3

Clinical implications
Oral medication nonadherence remains a significant public health issue for patients with schizophrenia, with an estimated 50% of patients failing to achieve 80% adherence even when enrolled in clinical trials specifically designed to track adherence.5 Although LAI atypical antipsychotics have been available since the approval of Risperdal Consta, the LAI form of risperidone, and both LAI forms of aripiprazole, were not designed to be loaded. A 1-day initiation regimen for aripiprazole lauroxil has been developed to avoid the need for 3 weeks of oral medication coverage,6,7 but aripiprazole monohydrate and risperidone microspheres mandate oral bridging of 2 and 3 weeks, respectively.2 Because one of the primary indications for LAI antipsychotic therapy is oral medication nonadherence, this prolonged period of oral coverage creates a risk for symptom exacerbation when the bridging period occurs outside of a controlled setting, as is common when patients are discharged from inpatient hospitalization.
One solution to this problem has its antecedents in the development of the Atrigel biodegradable injectable polymer, which was designed to deliver prolonged medication exposure after subcutaneous injection.8 This biodegradable polymer drug delivery system suspends and dissolves the medication of interest (in this case, risperidone) in a poly DL-lactide-coglycolide gel and its biocompatible carrier.9 The viscous liquid undergoes a phase transition upon contact with tissue fluids after subcutaneous injection, resulting in an implant that releases risperidone in a controlled manner as it is resorbed. Importantly, the kinetic parameters of RBP-7000 are such that effective drug levels are seen within the first week without the need for oral coverage.10
Use in adults with schizophrenia. After establishing tolerability with oral risperidone, the recommended doses are 90 mg or 120 mg monthly, which correspond to oral daily risperidone doses of 3 mg or 4 mg. RBP-7000 must be administered as a subcutaneous abdominal injection by a health care professional. It is recommended that the patient be in the supine position for the injection and that the injection sites be rotated monthly among 4 quadrants in the abdominal region. The injection volumes for the 90 mg and 120 mg doses are 0.6 mL and 0.8 mL, respectively.10 As the gel implant becomes firmer, the patient will notice a lump for several weeks that will decrease in size over time. Patients should be advised not to rub or massage the injection site, and to be aware of the placement of any belts or clothing with waistbands.10
Pharmacologic profile, adverse reactions
Risperidone is an atypical antipsychotic that has been commercially available in the U.S. since December 29, 1993, and its adverse effect profile is well characterized. The most common adverse effects associated with risperidone include those related to dopamine D2 antagonism, metabolic adverse effects, and an increase in serum prolactin. In the 12-month long-term safety study of RBP-7000, 1-minute post-dose injection site pain scores (on a 100-point scale) were highest on Day 1 (mean of 25) and decreased over time with subsequent injections (14 to 16 following the last injection).10
Continue to: How the Atrigel system works
How the Atrigel system works. The Atrigel system was developed in the late 1980s and consists of a solution of a resorbable polymer in a biocompatible carrier.11 After in vivo administration (typically via subcutaneous injection), the polymer undergoes a phase change from a liquid to a formed implant (Figure 1). Being in liquid form, this system provides the advantage of placement by simple means, such as injection by syringes. The absorption rates of various polymers and the release rates for various drugs are tailored to the desired indication. Approved uses for Atrigel include the subgingival delivery of the antibiotic doxycycline for chronic adult periodontitis (approved September 1998), and the monthly subcutaneous injectable form of the anti-androgen leuprolide, which was approved in January 2002.8,12 Release periods up to 4 months have been achieved with Atrigel; 1 month is the most often desired release period. The biodegradable polymer used for RBP-7000 is designed to provide effective plasma drug levels during the first week of treatment, and sustained levels with a 1-month dosing interval. The small subcutaneous implant that is formed is gradually resorbed over the course of 1 month.

Pharmacokinetics. As with all LAI medications, the half-life with repeated dosing vastly exceeds that achieved with oral administration. Following oral administration, mean peak plasma levels of risperidone occur at 1 hour, and those for the active metabolite 9-OH risperidone occur at 3 hours.13 Oral risperidone has a mean half-life of 3 hours, while the active metabolite 9-OH risperidone has a mean half-life of 21 hours.14 Due to its longer half-life, the metabolite comprises 83% of the active drug levels at steady state.14 Although risperidone is susceptible to interactions via cytochrome P450 (CYP) inhibitors and inducers, particularly CYP2D6 (Table 210), the pharmacokinetics of the combined total of risperidone and 9-OH risperidone levels (deemed the active moiety) are similar in CYP2D6 extensive and poor metabolizers, with an overall mean elimination half-life of approximately 20 hours.13

The kinetics for RBP-7000 are markedly different than those for oral risperidone (Figure 215). After a single subcutaneous injection, RBP-7000 shows 2 absorption peaks for risperidone. The first lower peak occurs with a Tmax of 4 to 6 hours due to initial release of risperidone during the implant formation process; a second risperidone peak occurs after 10 to 14 days and is associated with slow release from the subcutaneous depot.9,16,17 For both 9-OH risperidone levels and the total active moiety (risperidone plus 9-OH risperidone levels), the median Tmax of the first peak ranges from 4 to 48 hours and the second peak ranges from 7 to 11 days. Following a single subcutaneous injection of RBP-7000, the apparent terminal half-life of risperidone ranges from 9 to 11 days, on average. The mean apparent terminal half-life of the active moiety ranges from 8 to 9 days.9,16,17 Based on population pharmacokinetic modeling, the 90 mg and 120 mg doses of RBP-7000 are estimated to provide drug exposure equivalent to 3 mg/d and 4 mg/d of oral risperidone, respectively.9,16,17

Continue to: Efficacy of RBP-7000
Efficacy of RBP-7000 was established in an 8-week, double-blind, placebo-controlled trial of adult patients experiencing an acute exacerbation of schizophrenia (age 18 to 55).4 Eligible participants had:
- An acute exacerbation of schizophrenia that occurred ≤8 weeks before the screening visit and would have benefited from psychiatric hospitalization or continued hospitalization
- Positive and Negative Syndrome Scale (PANSS) total score between 80 and 120 at visit 1 and a score of >4 on at least 2 of the following 4 items: hallucinatory behavior, delusions, conceptual disorganization, or suspiciousness/persecution
- The diagnosis of acute exacerbation of schizophrenia and PANSS total score were confirmed through an independent video-conference interview conducted by an experienced rater.
Participants were excluded if they:
- Experienced a ≥20% improvement in PANSS total score between the initial screening visit and the first injection
- had been treated at any time with clozapine for treatment-resistant schizophrenia
- had met DSM-IV-TR criteria for substance dependence (with the exception of nicotine or caffeine) before screening.
During the initial screening visit, participants received a 0.25-mg tablet of oral risperidone on 2 consecutive days to assess the tolerability of risperidone.
Outcome. Participants were randomized in a 1:1:1 manner to placebo (n = 112) or 1 of 2 monthly doses of RBP-7000: 90 mg (n = 111) or 120 mg (n = 114). Using the least squares means of repeated-measures changes from baseline in PANSS total scores, there was a significant improvement in the difference in PANSS total scores from baseline to the end of the study compared with placebo: 90-mg RBP-7000, -6.148 points (95% confidence interval [CI], -9.982 to -2.314, P = .0004); 120-mg RBP-7000, -7.237 points (95% CI, -11.045 to -3.429, P < .0001). The absolute change from baseline in PANSS total score was -15.367 points for the 90-mg dose and -16.456 points for the 120-mg dose.4 Completion rates across all 3 arms were comparable: placebo 70.6%, RBP-7000 90 mg 77.6%, and RBP-7000 120 mg 71.4%.
Tolerability. In the 8-week phase III efficacy trial of RBP-7000, adverse effects occurring with an incidence ≥5% and at least twice the rate of placebo were weight gain (placebo 3.4%, 90 mg 13.0%, 120 mg 12.8%) and sedation (placebo 0%, 90 mg 7.0%, 120 mg 7.7%).10 Compared with baseline, participants had a mean weight gain at the end of the study of 2.83 kg in the placebo group, 5.15 kg in the 90-mg RBP-7000 group, and 4.69 kg in the 120-mg RBP-7000 group. There were no clinically significant differences at study endpoint in glucose and lipid parameters. Consistent with the known effects of risperidone, there were increases in mean prolactin levels during the 8-week study, the effects of which were greater for women. For men, mean prolactin levels from baseline to study end were: placebo: 9.8 ± 7.9 vs 9.9 ± 8.0 ng/mL; 90 mg: 8.9 ± 6.9 vs 22.4 ± 11.2 ng/mL; and 120 mg: 8.2 ± 5.2 vs 31.3 ± 14.8 ng/mL. For women, mean prolactin levels from baseline to study end were: placebo: 12.8 ± 11.7 vs 10.4 ± 8.0 ng/mL; 90 mg: 7.7 ± 5.3 vs 60.3 ± 46.9 ng/mL; and 120 mg: 10.9 ± 8.6 vs 85.5 ± 55.1 ng/mL. In the pivotal study, discontinuations due to adverse events were low across all treatment groups: 2.5% for placebo vs 0% for 90 mg and 1.7% for 120 mg.4 There was no single adverse reaction leading to discontinuation that occurred at a rate of ≥2% and greater than placebo in patients treated with RBP-7000.10 There were no clinically relevant differences in mean changes from baseline in corrected QT, QRS, and PR intervals, and in heart rate. Similarly, in the 12-month, long-term safety study, there were no clinically relevant changes in mean electrocardiography interval values from baseline to post-dose assessments.10
Using a 100-point visual analog scale (VAS), injection site pain scores 1 minute after the first dose decreased from a mean of 27 to the range of 3 to 7 for scores obtained 30 to 60 minutes post-dose. In the 12-month long-term safety study, 1-minute post-dose injection site pain VAS scores were highest on Day 1 (mean of 25) and decreased over time with subsequent injections (14 to 16 following last injection).10
Clinical considerations
Unique properties. RBP-7000 uses the established Atrigel system to provide effective antipsychotic levels in the first week of treatment, without the need for bridging oral coverage or a second loading injection. The abdominal subcutaneous injection volume is relatively small (0.6 mL or 0.8 mL).
Why Rx? The reasons to prescribe RBP-7000 for adult patients with schizophrenia include:
- no oral coverage required at the initiation of treatment
- effective plasma active moiety levels are seen within the first week without the need for a second loading injection
- monthly injection schedule.
Dosing. The recommended dosage of RBP-7000 is 90 mg or 120 mg once monthly, equivalent to 3 mg/d or 4 mg/d of oral risperidone, respectively. Oral risperidone tolerability should be established before the first injection. No oral risperidone coverage is required. RBP-7000 has not been studied in patients with renal or hepatic impairment and should be used with caution in these patients. Prior to initiating treatment in these patients, it is advised to carefully titrate up to at least 3 mg/d of oral risperidone. If a patient can tolerate 3 mg/d of oral risperidone and is psychiatrically stable, then the 90-mg dose of RBP-7000 can be considered.10
Contraindications. The only contraindications for RBP-7000 are known hypersensitivity to risperidone, paliperidone (9-OH risperidone), or other components of the injection.
Bottom Line
RBP-7000 (Perseris) is the second long-acting injectable (LAI) form of risperidone approved in the U.S. Unlike risperidone microspheres (Consta), RBP-7000 does not require any oral risperidone coverage at the beginning of therapy, provides effective drug levels within the first week of treatment with a single injection, and uses a monthly dosing interval. RBP-7000 does not require loading upon initiation. The monthly injection is <1 mL, is administered in abdominal subcutaneous tissue, and uses the Atrigel system.
Related Resource
- Carpenter J, Wong KK. Long-acting injectable antipsychotics: What to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Carbatrol, Tegretol
Doxycycline • Atridox
Leuprolide acetate injectable suspension • Eligard
Paliperidone palmitate • Invega Sustenna
Risperidone • Risperdal
Risperidone extended-release injectable suspension • Perseris
Risperidone long-acting injection • Risperdal Consta
1. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619.
2. Meyer JM. Converting oral to long acting injectable antipsychotics: a guide for the perplexed. CNS Spectrums. 2017;22(S1):14-28.
3. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2018.
4. Nasser AF, Henderson DC, Fava M, et al. Efficacy, safety, and tolerability of RBP-7000 once-monthly risperidone for the treatment of acute schizophrenia: an 8-week, randomized, double-blind, placebo-controlled, multicenter phase 3 study. J Clin Psychopharmacol. 2016;36(2):130-140.
5. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263.
6. Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441.
7. Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469.
8. Southard GL, Dunn RL, Garrett S. The drug delivery and biomaterial attributes of the ATRIGEL technology in the treatment of periodontal disease. Expert Opin Investig Drugs. 1998;7(9):1483-1491.
9. Gomeni R, Heidbreder C, Fudala PJ, Nasser AF. A model-based approach to characterize the population pharmacokinetics and the relationship between the pharmacokinetic and safety profiles of RBP-7000, a new, long-acting, sustained-released formulation of risperidone. J Clin Pharmacol. 2013;53(10):1010-1019.
10. Perseris [package insert]. North Chesterfield, VA: Indivior Inc; 2018.
11. Malik K, Singh I, Nagpal M, et al. Atrigel: a potential parenteral controlled drug delivery system. Der Pharmacia Sinica. 2010;1(1):74-81.
12. Sartor O. Eligard: leuprolide acetate in a novel sustained-release delivery system. Urology. 2003;61(2 Suppl 1):25-31.
13. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2018.
14. de Leon J, Wynn G, Sandson NB. The pharmacokinetics of paliperidone versus risperidone. Psychosomatics. 2010;51(1):80-88.
15. Ivaturi V, Gopalakrishnan M, Gobburu JVS, et al. Exposure-response analysis after subcutaneous administration of RBP-7000, a once-a-month long-acting Atrigel formulation of risperidone. Br J Clin Pharmacol. 2017;83(7):1476-1498.
16. Laffont CM, Gomeni R, Zheng B, et al. Population pharmacokinetics and prediction of dopamine D2 receptor occupancy after multiple doses of RBP-7000, a new sustained-release formulation of risperidone, in schizophrenia patients on stable oral risperidone treatment. Clin Pharmacokinet. 2014;53(6):533-543.
17. Laffont CM, Gomeni R, Zheng B, et al. Population pharmacokinetic modeling and simulation to guide dose selection for RBP-7000, a new sustained-release formulation of risperidone. J Clin Pharmacol. 2015;55(1):93-103.
1. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619.
2. Meyer JM. Converting oral to long acting injectable antipsychotics: a guide for the perplexed. CNS Spectrums. 2017;22(S1):14-28.
3. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2018.
4. Nasser AF, Henderson DC, Fava M, et al. Efficacy, safety, and tolerability of RBP-7000 once-monthly risperidone for the treatment of acute schizophrenia: an 8-week, randomized, double-blind, placebo-controlled, multicenter phase 3 study. J Clin Psychopharmacol. 2016;36(2):130-140.
5. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263.
6. Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441.
7. Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469.
8. Southard GL, Dunn RL, Garrett S. The drug delivery and biomaterial attributes of the ATRIGEL technology in the treatment of periodontal disease. Expert Opin Investig Drugs. 1998;7(9):1483-1491.
9. Gomeni R, Heidbreder C, Fudala PJ, Nasser AF. A model-based approach to characterize the population pharmacokinetics and the relationship between the pharmacokinetic and safety profiles of RBP-7000, a new, long-acting, sustained-released formulation of risperidone. J Clin Pharmacol. 2013;53(10):1010-1019.
10. Perseris [package insert]. North Chesterfield, VA: Indivior Inc; 2018.
11. Malik K, Singh I, Nagpal M, et al. Atrigel: a potential parenteral controlled drug delivery system. Der Pharmacia Sinica. 2010;1(1):74-81.
12. Sartor O. Eligard: leuprolide acetate in a novel sustained-release delivery system. Urology. 2003;61(2 Suppl 1):25-31.
13. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2018.
14. de Leon J, Wynn G, Sandson NB. The pharmacokinetics of paliperidone versus risperidone. Psychosomatics. 2010;51(1):80-88.
15. Ivaturi V, Gopalakrishnan M, Gobburu JVS, et al. Exposure-response analysis after subcutaneous administration of RBP-7000, a once-a-month long-acting Atrigel formulation of risperidone. Br J Clin Pharmacol. 2017;83(7):1476-1498.
16. Laffont CM, Gomeni R, Zheng B, et al. Population pharmacokinetics and prediction of dopamine D2 receptor occupancy after multiple doses of RBP-7000, a new sustained-release formulation of risperidone, in schizophrenia patients on stable oral risperidone treatment. Clin Pharmacokinet. 2014;53(6):533-543.
17. Laffont CM, Gomeni R, Zheng B, et al. Population pharmacokinetic modeling and simulation to guide dose selection for RBP-7000, a new sustained-release formulation of risperidone. J Clin Pharmacol. 2015;55(1):93-103.
Aripiprazole lauroxil nanocrystal suspension

Clinical implications
Nonadherence with oral antipsychotics is a common problem for patients with schizophrenia, one that is often underappreciated by clinicians.5 Whether one uses 70% or 80% as the measure of oral medication adherence, at least 50% of schizophrenia patients are nonadherent, with resultant increased risks for symptom exacerbation and hospitalization.5,6 Although 2 LAI forms of aripiprazole have been introduced over the past few years, neither was designed to be loaded, resulting in the need for 2 or 3 weeks of oral antipsychotic coverage following the first injectable dose.1 The primary reason for LAI antipsychotic therapy is oral medication nonadherence, and thus the need for 14 to 21 days of oral coverage at the outset of treatment creates a risk for symptom exacerbation if the patient is nonadherent with this oral bridging therapy which is needed to achieve the necessary serum concentrations until the long-acting formulation takes over.
One approach was to create a new form of AL using smaller nanomolecular particles rather than the micron-sized particles used for maintenance AL injections.3,4 This nanocrystal suspension is called Aristada Initio (AL

Use in adults with schizophrenia. After establishing tolerability with oral aripiprazole, AL
Continue to: Pharmacologic profile, adverse reactions
Pharmacologic profile, adverse reactions
Aripiprazole is a dopamine partial agonist atypical antipsychotic that has been commercially available in the United States since November 15, 2002, and its adverse effect profile is well characterized. The LAI formulation AL was approved on October 5, 2015. In the pivotal, 12-week, fixed-dose, placebo-controlled clinical trial of AL 441 mg or 882 mg monthly for adults with an acute exacerbation of schizophrenia, the only adverse effect that occurred in ≥5% of AL-treated patients and a rate at least twice that of placebo was akathisia (441 mg: 11%; 882 mg: 11%; placebo: 4%).10 Only 2 of 415 AL-treated patients discontinued the study due to akathisia. Injection-site reactions were reported by 4%, 5%, and 2% of patients treated with AL 441 mg, AL 882 mg, and placebo, respectively. Most of these were injection-site pain associated with the first injection, and decreased with each subsequent injection. Other injection-site reactions (induration, swelling, and redness) occurred at rates <1%.11
Having established that the range of plasma aripiprazole levels consistent with effective treatment is bounded by levels seen with AL 441 mg or 882 mg monthly, the FDA did not require additional efficacy studies for new AL doses provided that pharmacokinetic (PK) studies could demonstrate levels within the effective range. This is consistent with how new doses of other LAI antipsychotic preparations have been addressed in the past. For example, the 37.5 mg dose of risperidone microspheres was approved based on PK data, although the pivotal efficacy trials included doses of 25 mg, 50 mg, and 75 mg.12 Based on PK studies, AL doses of 662 mg monthly, 882 mg every 6 weeks, and 1,064 mg every 8 weeks were previously approved.13 The approval process for AL
Pharmacokinetic outcomes. A comparative phase 1 PK study was performed to evaluate initiation regimens: either 21 days of oral aripiprazole (15 mg/d) and one AL dose (n = 81) or one injection of AL
Tolerability. In PK studies, the safety profile and incidences of injection site reactions of AL
Continue to: Clinical considerations
Clinical considerations
AL
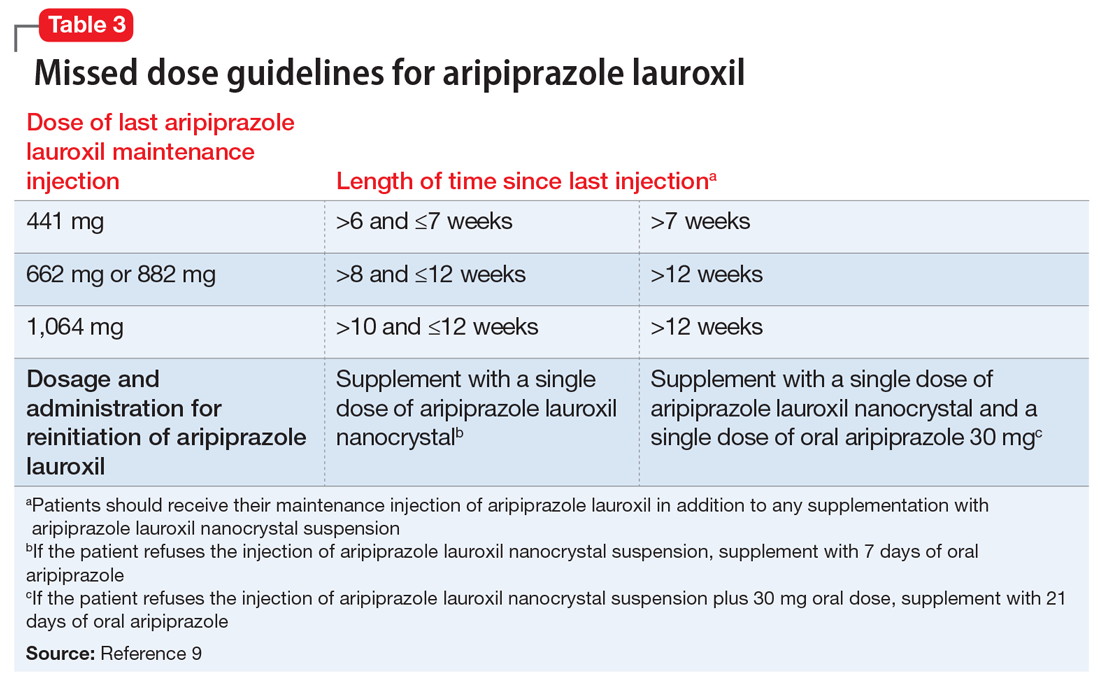
Unique properties. When combined with a single 30 mg oral dose, AL
Why Rx? The reasons to prescribe AL
- it obviates the need for 21 days of oral coverage previously required at the initiation of AL treatment
- clinically relevant plasma levels are seen within the first week when AL
ncd is combined with a single 30 mg oral aripiprazole dose - per the revised missed dose guidelines for AL, it can be used in those situations that previously demanded 7 days of oral coverage, and, when combined with a single 30 mg oral dose, can be used for resumption of therapy after prolonged absences that required 21 days of oral coverage. In all instances, the patient will also receive their usual maintenance dose of AL.
Dosing. There is only one dose available for AL
Contraindications. The only contraindication is a known hypersensitivity to aripiprazole.
Bottom Line
Aripiprazole lauroxil nanocrystal suspension (Aristada Initio) was specifically developed to obviate the need for 21 days of oral aripiprazole coverage when commencing treatment with aripiprazole lauroxil (Aristada). The plasma levels achieved when an injection of aripiprazole lauroxil nanocrystal suspension is combined with a single 30 mg oral dose are comparable to those achieved with 21 days of oral coverage. This initiation regimen, including a aripiprazole lauroxil nanocrystal injection and a 30 mg oral dose, should be administered on the same day as the maintenance aripiprazole lauroxil injection, although the latter can be administered on any of the next 10 days.
Related Resource
- Khan AY, Ovais DM. Long-acting injectable aripiprazole lauroxil for schizophrenia. Current Psychiatry. 2016;15(7):50-52,58.
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole lauroxil nanocrystal • Aristada Initio
Risperidone microspheres • Risperdal Consta
1. Meyer JM. Converting oral to long acting injectable antipsychotics: a guide for the perplexed. CNS Spectrums. 2017;22(S1):14-28.
2. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619.
3. Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469.
4. Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441.
5. Byerly MJ, Thompson A, Carmody T, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatric Services. 2007;58(6):844-847.
6. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263.
7. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
8. Hard ML, Mills RJ, Sadler BM, et al. Aripiprazole lauroxil: pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37(3):289-295.
9. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
10. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090.
11. Aristada [package insert]. Waltham, MA: Alkermes Inc; 2018.
12. Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry. 2003;64(10):1250-1257.
13. Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617-624.

Clinical implications
Nonadherence with oral antipsychotics is a common problem for patients with schizophrenia, one that is often underappreciated by clinicians.5 Whether one uses 70% or 80% as the measure of oral medication adherence, at least 50% of schizophrenia patients are nonadherent, with resultant increased risks for symptom exacerbation and hospitalization.5,6 Although 2 LAI forms of aripiprazole have been introduced over the past few years, neither was designed to be loaded, resulting in the need for 2 or 3 weeks of oral antipsychotic coverage following the first injectable dose.1 The primary reason for LAI antipsychotic therapy is oral medication nonadherence, and thus the need for 14 to 21 days of oral coverage at the outset of treatment creates a risk for symptom exacerbation if the patient is nonadherent with this oral bridging therapy which is needed to achieve the necessary serum concentrations until the long-acting formulation takes over.
One approach was to create a new form of AL using smaller nanomolecular particles rather than the micron-sized particles used for maintenance AL injections.3,4 This nanocrystal suspension is called Aristada Initio (AL

Use in adults with schizophrenia. After establishing tolerability with oral aripiprazole, AL
Continue to: Pharmacologic profile, adverse reactions
Pharmacologic profile, adverse reactions
Aripiprazole is a dopamine partial agonist atypical antipsychotic that has been commercially available in the United States since November 15, 2002, and its adverse effect profile is well characterized. The LAI formulation AL was approved on October 5, 2015. In the pivotal, 12-week, fixed-dose, placebo-controlled clinical trial of AL 441 mg or 882 mg monthly for adults with an acute exacerbation of schizophrenia, the only adverse effect that occurred in ≥5% of AL-treated patients and a rate at least twice that of placebo was akathisia (441 mg: 11%; 882 mg: 11%; placebo: 4%).10 Only 2 of 415 AL-treated patients discontinued the study due to akathisia. Injection-site reactions were reported by 4%, 5%, and 2% of patients treated with AL 441 mg, AL 882 mg, and placebo, respectively. Most of these were injection-site pain associated with the first injection, and decreased with each subsequent injection. Other injection-site reactions (induration, swelling, and redness) occurred at rates <1%.11
Having established that the range of plasma aripiprazole levels consistent with effective treatment is bounded by levels seen with AL 441 mg or 882 mg monthly, the FDA did not require additional efficacy studies for new AL doses provided that pharmacokinetic (PK) studies could demonstrate levels within the effective range. This is consistent with how new doses of other LAI antipsychotic preparations have been addressed in the past. For example, the 37.5 mg dose of risperidone microspheres was approved based on PK data, although the pivotal efficacy trials included doses of 25 mg, 50 mg, and 75 mg.12 Based on PK studies, AL doses of 662 mg monthly, 882 mg every 6 weeks, and 1,064 mg every 8 weeks were previously approved.13 The approval process for AL
Pharmacokinetic outcomes. A comparative phase 1 PK study was performed to evaluate initiation regimens: either 21 days of oral aripiprazole (15 mg/d) and one AL dose (n = 81) or one injection of AL

Tolerability. In PK studies, the safety profile and incidences of injection site reactions of AL

Continue to: Clinical considerations
Clinical considerations
AL

Unique properties. When combined with a single 30 mg oral dose, AL
Why Rx? The reasons to prescribe AL
- it obviates the need for 21 days of oral coverage previously required at the initiation of AL treatment
- clinically relevant plasma levels are seen within the first week when AL
ncd is combined with a single 30 mg oral aripiprazole dose - per the revised missed dose guidelines for AL, it can be used in those situations that previously demanded 7 days of oral coverage, and, when combined with a single 30 mg oral dose, can be used for resumption of therapy after prolonged absences that required 21 days of oral coverage. In all instances, the patient will also receive their usual maintenance dose of AL.
Dosing. There is only one dose available for AL
Contraindications. The only contraindication is a known hypersensitivity to aripiprazole.
Bottom Line
Aripiprazole lauroxil nanocrystal suspension (Aristada Initio) was specifically developed to obviate the need for 21 days of oral aripiprazole coverage when commencing treatment with aripiprazole lauroxil (Aristada). The plasma levels achieved when an injection of aripiprazole lauroxil nanocrystal suspension is combined with a single 30 mg oral dose are comparable to those achieved with 21 days of oral coverage. This initiation regimen, including a aripiprazole lauroxil nanocrystal injection and a 30 mg oral dose, should be administered on the same day as the maintenance aripiprazole lauroxil injection, although the latter can be administered on any of the next 10 days.
Related Resource
- Khan AY, Ovais DM. Long-acting injectable aripiprazole lauroxil for schizophrenia. Current Psychiatry. 2016;15(7):50-52,58.
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole lauroxil nanocrystal • Aristada Initio
Risperidone microspheres • Risperdal Consta

Clinical implications
Nonadherence with oral antipsychotics is a common problem for patients with schizophrenia, one that is often underappreciated by clinicians.5 Whether one uses 70% or 80% as the measure of oral medication adherence, at least 50% of schizophrenia patients are nonadherent, with resultant increased risks for symptom exacerbation and hospitalization.5,6 Although 2 LAI forms of aripiprazole have been introduced over the past few years, neither was designed to be loaded, resulting in the need for 2 or 3 weeks of oral antipsychotic coverage following the first injectable dose.1 The primary reason for LAI antipsychotic therapy is oral medication nonadherence, and thus the need for 14 to 21 days of oral coverage at the outset of treatment creates a risk for symptom exacerbation if the patient is nonadherent with this oral bridging therapy which is needed to achieve the necessary serum concentrations until the long-acting formulation takes over.
One approach was to create a new form of AL using smaller nanomolecular particles rather than the micron-sized particles used for maintenance AL injections.3,4 This nanocrystal suspension is called Aristada Initio (AL

Use in adults with schizophrenia. After establishing tolerability with oral aripiprazole, AL
Continue to: Pharmacologic profile, adverse reactions
Pharmacologic profile, adverse reactions
Aripiprazole is a dopamine partial agonist atypical antipsychotic that has been commercially available in the United States since November 15, 2002, and its adverse effect profile is well characterized. The LAI formulation AL was approved on October 5, 2015. In the pivotal, 12-week, fixed-dose, placebo-controlled clinical trial of AL 441 mg or 882 mg monthly for adults with an acute exacerbation of schizophrenia, the only adverse effect that occurred in ≥5% of AL-treated patients and a rate at least twice that of placebo was akathisia (441 mg: 11%; 882 mg: 11%; placebo: 4%).10 Only 2 of 415 AL-treated patients discontinued the study due to akathisia. Injection-site reactions were reported by 4%, 5%, and 2% of patients treated with AL 441 mg, AL 882 mg, and placebo, respectively. Most of these were injection-site pain associated with the first injection, and decreased with each subsequent injection. Other injection-site reactions (induration, swelling, and redness) occurred at rates <1%.11
Having established that the range of plasma aripiprazole levels consistent with effective treatment is bounded by levels seen with AL 441 mg or 882 mg monthly, the FDA did not require additional efficacy studies for new AL doses provided that pharmacokinetic (PK) studies could demonstrate levels within the effective range. This is consistent with how new doses of other LAI antipsychotic preparations have been addressed in the past. For example, the 37.5 mg dose of risperidone microspheres was approved based on PK data, although the pivotal efficacy trials included doses of 25 mg, 50 mg, and 75 mg.12 Based on PK studies, AL doses of 662 mg monthly, 882 mg every 6 weeks, and 1,064 mg every 8 weeks were previously approved.13 The approval process for AL
Pharmacokinetic outcomes. A comparative phase 1 PK study was performed to evaluate initiation regimens: either 21 days of oral aripiprazole (15 mg/d) and one AL dose (n = 81) or one injection of AL

Tolerability. In PK studies, the safety profile and incidences of injection site reactions of AL

Continue to: Clinical considerations
Clinical considerations
AL

Unique properties. When combined with a single 30 mg oral dose, AL
Why Rx? The reasons to prescribe AL
- it obviates the need for 21 days of oral coverage previously required at the initiation of AL treatment
- clinically relevant plasma levels are seen within the first week when AL
ncd is combined with a single 30 mg oral aripiprazole dose - per the revised missed dose guidelines for AL, it can be used in those situations that previously demanded 7 days of oral coverage, and, when combined with a single 30 mg oral dose, can be used for resumption of therapy after prolonged absences that required 21 days of oral coverage. In all instances, the patient will also receive their usual maintenance dose of AL.
Dosing. There is only one dose available for AL
Contraindications. The only contraindication is a known hypersensitivity to aripiprazole.
Bottom Line
Aripiprazole lauroxil nanocrystal suspension (Aristada Initio) was specifically developed to obviate the need for 21 days of oral aripiprazole coverage when commencing treatment with aripiprazole lauroxil (Aristada). The plasma levels achieved when an injection of aripiprazole lauroxil nanocrystal suspension is combined with a single 30 mg oral dose are comparable to those achieved with 21 days of oral coverage. This initiation regimen, including a aripiprazole lauroxil nanocrystal injection and a 30 mg oral dose, should be administered on the same day as the maintenance aripiprazole lauroxil injection, although the latter can be administered on any of the next 10 days.
Related Resource
- Khan AY, Ovais DM. Long-acting injectable aripiprazole lauroxil for schizophrenia. Current Psychiatry. 2016;15(7):50-52,58.
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole lauroxil nanocrystal • Aristada Initio
Risperidone microspheres • Risperdal Consta
1. Meyer JM. Converting oral to long acting injectable antipsychotics: a guide for the perplexed. CNS Spectrums. 2017;22(S1):14-28.
2. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619.
3. Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469.
4. Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441.
5. Byerly MJ, Thompson A, Carmody T, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatric Services. 2007;58(6):844-847.
6. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263.
7. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
8. Hard ML, Mills RJ, Sadler BM, et al. Aripiprazole lauroxil: pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37(3):289-295.
9. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
10. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090.
11. Aristada [package insert]. Waltham, MA: Alkermes Inc; 2018.
12. Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry. 2003;64(10):1250-1257.
13. Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617-624.
1. Meyer JM. Converting oral to long acting injectable antipsychotics: a guide for the perplexed. CNS Spectrums. 2017;22(S1):14-28.
2. Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619.
3. Hard ML, Wehr AY, Sadler BM, et al. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461-469.
4. Hard ML, Wehr AY, Du Y, et al. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435-441.
5. Byerly MJ, Thompson A, Carmody T, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatric Services. 2007;58(6):844-847.
6. Remington G, Teo C, Mann S, et al. Examining levels of antipsychotic adherence to better understand nonadherence. J Clin Psychopharmacol. 2013;33(2):261-263.
7. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
8. Hard ML, Mills RJ, Sadler BM, et al. Aripiprazole lauroxil: pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37(3):289-295.
9. Aristada Initio [package insert]. Waltham, MA: Alkermes Inc; 2018.
10. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090.
11. Aristada [package insert]. Waltham, MA: Alkermes Inc; 2018.
12. Fleischhacker WW, Eerdekens M, Karcher K, et al. Treatment of schizophrenia with long-acting injectable risperidone: a 12-month open-label trial of the first long-acting second-generation antipsychotic. J Clin Psychiatry. 2003;64(10):1250-1257.
13. Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617-624.
A concise guide to monoamine oxidase inhibitors: How to avoid drug interactions
Monoamine oxidase inhibitors (MAOIs) have well-established efficacy for treating depression, panic disorder, and social phobia. However, a lack of familiarity with these agents and misconceptions about the risks associated with their use have led to MAOIs being substantially underutilized. The goal of this 2-part guide to MAOIs is to educate clinicians about this often-overlooked class of medications. Part 1 (“A concise guide to monoamine inhibitors,”
MAOIs and potential drug interactions
One source of concern in patients receiving irreversible nonselective MAOIs is the development of excessive serotonergic neurotransmission resulting in SS. In the 1960s, researchers noted that administering large doses of
- mild symptoms: tremor, akathisia, inducible clonus
- moderate symptoms: spontaneous or sustained clonus, muscular hypertonicity
- severe symptoms: hyperthermia, diaphoresis.2
Although SS can be induced by significant exposure to individual agents that promote excess synaptic serotonin (eg, overdose of selective serotonin reuptake inhibitors [SSRIs]), the majority of fatal cases have occurred among patients taking MAOIs who were coadministered an agent that inhibited serotonin reuptake (Table 13). Animal studies have determined that excessive stimulation of the 5HT2A receptor is primarily responsible for SS,4 and that 5HT2A antagonists, such as mirtazapine, can block the development of SS in a mouse coadministered
Risk for SS. Most medications that promote serotonergic activity are well known for their use as antidepressants, but other agents that have 5HT reuptake properties (eg, the antihistamine chlorpheniramine) must be avoided. Although older literature suggests that the use of lower doses of certain tricyclic antidepressants concurrently with MAOIs may not be as dangerous as once believed,6 there are sufficient reports of serious outcomes that tricyclics should be avoided in patients taking MAOIs because of the risk of SS, and also because, in general, tricyclics are poorly tolerated.7
Desipramine, a potent norepinephrine transporter (NET) inhibitor, blocks the entry of tyramine into cells by NET, thereby preventing hypertensive events in animal models of tyramine overexposure. However, in some assays, the affinity for the serotonin transporter is not insignificant, so at higher doses desipramine may pose the same theoretical risk for SS as seen with other tricyclics.3
Lastly
Astute clinicians will recognize that antidepressants that lack 5HT reuptake (eg, bupropion, mirtazapine) are not on this list of agents that may increase SS risk when taken with an MAOI. Older papers often list mirtazapine, but as a 5HT2A antagonist, it does not possess a plausible mechanism by which it can induce 5HT toxicity.9,10 Most atypical antipsychotics have significant 5HT2A antagonism and can be combined with MAOIs, but ziprasidone is an exception: as a moderate SNRI, it has been associated with SS when administered with an MAOI.11
Pressor reactions. The only theoretical sources of concern for pressor effects are medications that act as norepinephrine releasers through interactions at the trace amine-associated receptor 1 (TAAR1) (for more information on TAAR1, see
Starting a patient on an MAOI

Contraindicated medications need to be tapered before beginning MAOI treatment. The duration of the washout period depends on the half-life of the medication and any active metabolites. Antidepressants with half-lives of approximately ≤24 hours should be tapered over 7 to 14 days (depending on the dose) to minimize the risk of withdrawal syndromes, while those with long half-lives (eg, fluoxetine,
Initiation of an MAOI is always based on whether the patient can reliably follow the basic dietary advice (see “A concise guide to monoamine inhibitors,”
The orthostasis management strategy is similar to that employed for
Augmentation options for patients taking MAOIs
For depressed patients who do not achieve remission of symptoms from MAOI therapy, augmentation options should be sought, as patients who respond but fail to remit are at increased risk of relapse.26 Lithium augmentation is one of the more common strategies, with abundant data supporting its use.27,28 Case reports dating back >12 years describe the concurrent use o
1. Krishnamoorthy S, Ma Z, Zhang G, et al. Involvement of 5-HT2A receptors in the serotonin (5-HT) syndrome caused by excessive 5-HT efflux in rat brain. Basic Clin Pharmacol Toxicol. 2010;107(4):830-841.
2. Sternbach H. The serotonin syndrome. Am J Psychiatry 1991;148(6):705-713.
3. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-90.
4. Haberzettl R, Fink H, Bert B. Role of 5-HT(1A)- and 5-HT(2A) receptors for the murine model of the serotonin syndrome. J Pharmacol Toxicol Methods. 2014;70(2):129-133.
5. Shioda K, Nisijima K, Yoshino T, et al. Mirtazapine abolishes hyperthermia in an animal model of serotonin syndrome. Neurosci Lett. 2010;482(3):216-219.
6. White K, Simpson G. Combined MAOI-tricyclic antidepressant treatment: a reevaluation. J Clin Psychopharmacol. 1981;1(5):264-282.
7. Otte W, Birkenhager TK, van den Broek WW. Fatal interaction between tranylcypromine and imipramine. Eur Psychiatry. 2003;18(5):264-265.
8. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
9. Gillman PK. Mirtazapine: unable to induce serotonin toxicity? Clin Neuropharmacol. 2003;26(6):288-289; author reply 289-290.
10. Gillman PK. A systematic review of the serotonergic effects of mirtazapine in humans: implications for its dual action status. Hum Psychopharmacol. 2006;21(2):117-125.
11. Meyer JM, Cummings MA, Proctor G. Augmentation of phenelzine with aripiprazole and quetiapine in a treatment resistant patient with psychotic unipolar depression: case report and literature review. CNS Spectr. 2017;22(5):391-396.
12. Feinberg SS. Combining stimulants with monoamine oxidase inhibitors: a review of uses and one possible additional indication. J Clin Psychiatry. 2004;65(11):1520-1524.
13. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6). doi: 10.4088/PCC.15br01836.
14. Simmler LD, Buchy D, Chaboz S, et al. In vitro characterization of psychoactive substances at rat, mouse, and human trace amine-associated receptor 1. J Pharmacol Exp Ther. 2016;357(1):134-144.
15. Froimowitz M, Gu Y, Dakin LA, et al. Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter. J Med Chem. 2007;50(2):219-232.
16. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
17. Hiemke C, Härtter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85(1):11-28.
18. Pristiq [package insert]. New York, NY: Pfizer Inc; 2016.
19. Savella [package insert]. Irvine, CA: Allergan USA Inc; 2016.
20. Viibryd [package insert]. Irvine, CA: Allergan USA Inc; 2016.
21. Trintellix [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; 2016.
22. Fetzima [package insert]. Irvine, CA: Allergan USA Inc; 2017.
23. Nardil [package insert]. New York, NY: Pfizer Inc; 2009.
24. Testani M Jr. Clozapine-induced orthostatic hypotension treated with fludrocortisone. J Clin Psychiatry. 1994;55(11):497-498.
25. Emsam [package insert]. Morgantown, WV: Somerset Pharmaceuticals Inc; 2015.
26. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
27. Tariot PN, Murphy DL, Sunderland T, et al. Rapid antidepressant effect of addition of lithium to tranylcypromine. J Clin Psychopharmacol. 1986;6(3):165-167.
28. Kok RM, Vink D, Heeren TJ, et al. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly: an open, randomized, controlled trial. J Clin Psychiatry. 2007;68(8):1177-1185.
29. Quante A, Zeugmann S. Tranylcypromine and bupropion combination therapy in treatment-resistant major depression: a report of 2 cases. J Clin Psychopharmacol. 2012;32(4):572-574.
30. Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry. 1988;49(10):409-410.
31. Hullett FJ, Bidder TG. Phenelzine plus triiodothyronine combination in a case of refractory depression. J Nerv Ment Dis. 1983;171(5):318-320.
Monoamine oxidase inhibitors (MAOIs) have well-established efficacy for treating depression, panic disorder, and social phobia. However, a lack of familiarity with these agents and misconceptions about the risks associated with their use have led to MAOIs being substantially underutilized. The goal of this 2-part guide to MAOIs is to educate clinicians about this often-overlooked class of medications. Part 1 (“A concise guide to monoamine inhibitors,”
MAOIs and potential drug interactions
One source of concern in patients receiving irreversible nonselective MAOIs is the development of excessive serotonergic neurotransmission resulting in SS. In the 1960s, researchers noted that administering large doses of
- mild symptoms: tremor, akathisia, inducible clonus
- moderate symptoms: spontaneous or sustained clonus, muscular hypertonicity
- severe symptoms: hyperthermia, diaphoresis.2
Although SS can be induced by significant exposure to individual agents that promote excess synaptic serotonin (eg, overdose of selective serotonin reuptake inhibitors [SSRIs]), the majority of fatal cases have occurred among patients taking MAOIs who were coadministered an agent that inhibited serotonin reuptake (Table 13). Animal studies have determined that excessive stimulation of the 5HT2A receptor is primarily responsible for SS,4 and that 5HT2A antagonists, such as mirtazapine, can block the development of SS in a mouse coadministered

Risk for SS. Most medications that promote serotonergic activity are well known for their use as antidepressants, but other agents that have 5HT reuptake properties (eg, the antihistamine chlorpheniramine) must be avoided. Although older literature suggests that the use of lower doses of certain tricyclic antidepressants concurrently with MAOIs may not be as dangerous as once believed,6 there are sufficient reports of serious outcomes that tricyclics should be avoided in patients taking MAOIs because of the risk of SS, and also because, in general, tricyclics are poorly tolerated.7
Desipramine, a potent norepinephrine transporter (NET) inhibitor, blocks the entry of tyramine into cells by NET, thereby preventing hypertensive events in animal models of tyramine overexposure. However, in some assays, the affinity for the serotonin transporter is not insignificant, so at higher doses desipramine may pose the same theoretical risk for SS as seen with other tricyclics.3
Lastly
Astute clinicians will recognize that antidepressants that lack 5HT reuptake (eg, bupropion, mirtazapine) are not on this list of agents that may increase SS risk when taken with an MAOI. Older papers often list mirtazapine, but as a 5HT2A antagonist, it does not possess a plausible mechanism by which it can induce 5HT toxicity.9,10 Most atypical antipsychotics have significant 5HT2A antagonism and can be combined with MAOIs, but ziprasidone is an exception: as a moderate SNRI, it has been associated with SS when administered with an MAOI.11
Pressor reactions. The only theoretical sources of concern for pressor effects are medications that act as norepinephrine releasers through interactions at the trace amine-associated receptor 1 (TAAR1) (for more information on TAAR1, see

Starting a patient on an MAOI

Contraindicated medications need to be tapered before beginning MAOI treatment. The duration of the washout period depends on the half-life of the medication and any active metabolites. Antidepressants with half-lives of approximately ≤24 hours should be tapered over 7 to 14 days (depending on the dose) to minimize the risk of withdrawal syndromes, while those with long half-lives (eg, fluoxetine,
Initiation of an MAOI is always based on whether the patient can reliably follow the basic dietary advice (see “A concise guide to monoamine inhibitors,”
The orthostasis management strategy is similar to that employed for
Augmentation options for patients taking MAOIs
For depressed patients who do not achieve remission of symptoms from MAOI therapy, augmentation options should be sought, as patients who respond but fail to remit are at increased risk of relapse.26 Lithium augmentation is one of the more common strategies, with abundant data supporting its use.27,28 Case reports dating back >12 years describe the concurrent use o
Monoamine oxidase inhibitors (MAOIs) have well-established efficacy for treating depression, panic disorder, and social phobia. However, a lack of familiarity with these agents and misconceptions about the risks associated with their use have led to MAOIs being substantially underutilized. The goal of this 2-part guide to MAOIs is to educate clinicians about this often-overlooked class of medications. Part 1 (“A concise guide to monoamine inhibitors,”
MAOIs and potential drug interactions
One source of concern in patients receiving irreversible nonselective MAOIs is the development of excessive serotonergic neurotransmission resulting in SS. In the 1960s, researchers noted that administering large doses of
- mild symptoms: tremor, akathisia, inducible clonus
- moderate symptoms: spontaneous or sustained clonus, muscular hypertonicity
- severe symptoms: hyperthermia, diaphoresis.2
Although SS can be induced by significant exposure to individual agents that promote excess synaptic serotonin (eg, overdose of selective serotonin reuptake inhibitors [SSRIs]), the majority of fatal cases have occurred among patients taking MAOIs who were coadministered an agent that inhibited serotonin reuptake (Table 13). Animal studies have determined that excessive stimulation of the 5HT2A receptor is primarily responsible for SS,4 and that 5HT2A antagonists, such as mirtazapine, can block the development of SS in a mouse coadministered

Risk for SS. Most medications that promote serotonergic activity are well known for their use as antidepressants, but other agents that have 5HT reuptake properties (eg, the antihistamine chlorpheniramine) must be avoided. Although older literature suggests that the use of lower doses of certain tricyclic antidepressants concurrently with MAOIs may not be as dangerous as once believed,6 there are sufficient reports of serious outcomes that tricyclics should be avoided in patients taking MAOIs because of the risk of SS, and also because, in general, tricyclics are poorly tolerated.7
Desipramine, a potent norepinephrine transporter (NET) inhibitor, blocks the entry of tyramine into cells by NET, thereby preventing hypertensive events in animal models of tyramine overexposure. However, in some assays, the affinity for the serotonin transporter is not insignificant, so at higher doses desipramine may pose the same theoretical risk for SS as seen with other tricyclics.3
Lastly
Astute clinicians will recognize that antidepressants that lack 5HT reuptake (eg, bupropion, mirtazapine) are not on this list of agents that may increase SS risk when taken with an MAOI. Older papers often list mirtazapine, but as a 5HT2A antagonist, it does not possess a plausible mechanism by which it can induce 5HT toxicity.9,10 Most atypical antipsychotics have significant 5HT2A antagonism and can be combined with MAOIs, but ziprasidone is an exception: as a moderate SNRI, it has been associated with SS when administered with an MAOI.11
Pressor reactions. The only theoretical sources of concern for pressor effects are medications that act as norepinephrine releasers through interactions at the trace amine-associated receptor 1 (TAAR1) (for more information on TAAR1, see

Starting a patient on an MAOI

Contraindicated medications need to be tapered before beginning MAOI treatment. The duration of the washout period depends on the half-life of the medication and any active metabolites. Antidepressants with half-lives of approximately ≤24 hours should be tapered over 7 to 14 days (depending on the dose) to minimize the risk of withdrawal syndromes, while those with long half-lives (eg, fluoxetine,
Initiation of an MAOI is always based on whether the patient can reliably follow the basic dietary advice (see “A concise guide to monoamine inhibitors,”
The orthostasis management strategy is similar to that employed for
Augmentation options for patients taking MAOIs
For depressed patients who do not achieve remission of symptoms from MAOI therapy, augmentation options should be sought, as patients who respond but fail to remit are at increased risk of relapse.26 Lithium augmentation is one of the more common strategies, with abundant data supporting its use.27,28 Case reports dating back >12 years describe the concurrent use o
1. Krishnamoorthy S, Ma Z, Zhang G, et al. Involvement of 5-HT2A receptors in the serotonin (5-HT) syndrome caused by excessive 5-HT efflux in rat brain. Basic Clin Pharmacol Toxicol. 2010;107(4):830-841.
2. Sternbach H. The serotonin syndrome. Am J Psychiatry 1991;148(6):705-713.
3. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-90.
4. Haberzettl R, Fink H, Bert B. Role of 5-HT(1A)- and 5-HT(2A) receptors for the murine model of the serotonin syndrome. J Pharmacol Toxicol Methods. 2014;70(2):129-133.
5. Shioda K, Nisijima K, Yoshino T, et al. Mirtazapine abolishes hyperthermia in an animal model of serotonin syndrome. Neurosci Lett. 2010;482(3):216-219.
6. White K, Simpson G. Combined MAOI-tricyclic antidepressant treatment: a reevaluation. J Clin Psychopharmacol. 1981;1(5):264-282.
7. Otte W, Birkenhager TK, van den Broek WW. Fatal interaction between tranylcypromine and imipramine. Eur Psychiatry. 2003;18(5):264-265.
8. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
9. Gillman PK. Mirtazapine: unable to induce serotonin toxicity? Clin Neuropharmacol. 2003;26(6):288-289; author reply 289-290.
10. Gillman PK. A systematic review of the serotonergic effects of mirtazapine in humans: implications for its dual action status. Hum Psychopharmacol. 2006;21(2):117-125.
11. Meyer JM, Cummings MA, Proctor G. Augmentation of phenelzine with aripiprazole and quetiapine in a treatment resistant patient with psychotic unipolar depression: case report and literature review. CNS Spectr. 2017;22(5):391-396.
12. Feinberg SS. Combining stimulants with monoamine oxidase inhibitors: a review of uses and one possible additional indication. J Clin Psychiatry. 2004;65(11):1520-1524.
13. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6). doi: 10.4088/PCC.15br01836.
14. Simmler LD, Buchy D, Chaboz S, et al. In vitro characterization of psychoactive substances at rat, mouse, and human trace amine-associated receptor 1. J Pharmacol Exp Ther. 2016;357(1):134-144.
15. Froimowitz M, Gu Y, Dakin LA, et al. Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter. J Med Chem. 2007;50(2):219-232.
16. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
17. Hiemke C, Härtter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85(1):11-28.
18. Pristiq [package insert]. New York, NY: Pfizer Inc; 2016.
19. Savella [package insert]. Irvine, CA: Allergan USA Inc; 2016.
20. Viibryd [package insert]. Irvine, CA: Allergan USA Inc; 2016.
21. Trintellix [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; 2016.
22. Fetzima [package insert]. Irvine, CA: Allergan USA Inc; 2017.
23. Nardil [package insert]. New York, NY: Pfizer Inc; 2009.
24. Testani M Jr. Clozapine-induced orthostatic hypotension treated with fludrocortisone. J Clin Psychiatry. 1994;55(11):497-498.
25. Emsam [package insert]. Morgantown, WV: Somerset Pharmaceuticals Inc; 2015.
26. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
27. Tariot PN, Murphy DL, Sunderland T, et al. Rapid antidepressant effect of addition of lithium to tranylcypromine. J Clin Psychopharmacol. 1986;6(3):165-167.
28. Kok RM, Vink D, Heeren TJ, et al. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly: an open, randomized, controlled trial. J Clin Psychiatry. 2007;68(8):1177-1185.
29. Quante A, Zeugmann S. Tranylcypromine and bupropion combination therapy in treatment-resistant major depression: a report of 2 cases. J Clin Psychopharmacol. 2012;32(4):572-574.
30. Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry. 1988;49(10):409-410.
31. Hullett FJ, Bidder TG. Phenelzine plus triiodothyronine combination in a case of refractory depression. J Nerv Ment Dis. 1983;171(5):318-320.
1. Krishnamoorthy S, Ma Z, Zhang G, et al. Involvement of 5-HT2A receptors in the serotonin (5-HT) syndrome caused by excessive 5-HT efflux in rat brain. Basic Clin Pharmacol Toxicol. 2010;107(4):830-841.
2. Sternbach H. The serotonin syndrome. Am J Psychiatry 1991;148(6):705-713.
3. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-90.
4. Haberzettl R, Fink H, Bert B. Role of 5-HT(1A)- and 5-HT(2A) receptors for the murine model of the serotonin syndrome. J Pharmacol Toxicol Methods. 2014;70(2):129-133.
5. Shioda K, Nisijima K, Yoshino T, et al. Mirtazapine abolishes hyperthermia in an animal model of serotonin syndrome. Neurosci Lett. 2010;482(3):216-219.
6. White K, Simpson G. Combined MAOI-tricyclic antidepressant treatment: a reevaluation. J Clin Psychopharmacol. 1981;1(5):264-282.
7. Otte W, Birkenhager TK, van den Broek WW. Fatal interaction between tranylcypromine and imipramine. Eur Psychiatry. 2003;18(5):264-265.
8. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
9. Gillman PK. Mirtazapine: unable to induce serotonin toxicity? Clin Neuropharmacol. 2003;26(6):288-289; author reply 289-290.
10. Gillman PK. A systematic review of the serotonergic effects of mirtazapine in humans: implications for its dual action status. Hum Psychopharmacol. 2006;21(2):117-125.
11. Meyer JM, Cummings MA, Proctor G. Augmentation of phenelzine with aripiprazole and quetiapine in a treatment resistant patient with psychotic unipolar depression: case report and literature review. CNS Spectr. 2017;22(5):391-396.
12. Feinberg SS. Combining stimulants with monoamine oxidase inhibitors: a review of uses and one possible additional indication. J Clin Psychiatry. 2004;65(11):1520-1524.
13. Israel JA. Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord. 2015;17(6). doi: 10.4088/PCC.15br01836.
14. Simmler LD, Buchy D, Chaboz S, et al. In vitro characterization of psychoactive substances at rat, mouse, and human trace amine-associated receptor 1. J Pharmacol Exp Ther. 2016;357(1):134-144.
15. Froimowitz M, Gu Y, Dakin LA, et al. Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter. J Med Chem. 2007;50(2):219-232.
16. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
17. Hiemke C, Härtter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85(1):11-28.
18. Pristiq [package insert]. New York, NY: Pfizer Inc; 2016.
19. Savella [package insert]. Irvine, CA: Allergan USA Inc; 2016.
20. Viibryd [package insert]. Irvine, CA: Allergan USA Inc; 2016.
21. Trintellix [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; 2016.
22. Fetzima [package insert]. Irvine, CA: Allergan USA Inc; 2017.
23. Nardil [package insert]. New York, NY: Pfizer Inc; 2009.
24. Testani M Jr. Clozapine-induced orthostatic hypotension treated with fludrocortisone. J Clin Psychiatry. 1994;55(11):497-498.
25. Emsam [package insert]. Morgantown, WV: Somerset Pharmaceuticals Inc; 2015.
26. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
27. Tariot PN, Murphy DL, Sunderland T, et al. Rapid antidepressant effect of addition of lithium to tranylcypromine. J Clin Psychopharmacol. 1986;6(3):165-167.
28. Kok RM, Vink D, Heeren TJ, et al. Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly: an open, randomized, controlled trial. J Clin Psychiatry. 2007;68(8):1177-1185.
29. Quante A, Zeugmann S. Tranylcypromine and bupropion combination therapy in treatment-resistant major depression: a report of 2 cases. J Clin Psychopharmacol. 2012;32(4):572-574.
30. Joffe RT. Triiodothyronine potentiation of the antidepressant effect of phenelzine. J Clin Psychiatry. 1988;49(10):409-410.
31. Hullett FJ, Bidder TG. Phenelzine plus triiodothyronine combination in a case of refractory depression. J Nerv Ment Dis. 1983;171(5):318-320.
A concise guide to monoamine oxidase inhibitors
Despite an abundance of evidenced-based literature supporting monoamine oxidase inhibitors (MAOIs) as an effective treatment for depression, use of these agents has decreased drastically in the past 3 decades. A lack of industry support and the ease of use of other agents are contributing factors, but the biggest impediments to routine use of MAOIs are unfamiliarity with their efficacy advantages and concerns about adverse effects, particularly the risk of hypertensive crises and serotonin syndrome. Many misconceptions regarding these medications are based on outdated data and studies that are no longer reliable.
The goal of this 2-part review is to provide clinicians with updated information regarding MAOIs. Part 1 provides a brief description of:
- the pharmacology of nonselective irreversible MAOIs
- the mechanism by which tyramine induces hypertension
- sources of clinically significant tyramine exposure
- what to tell patients about dietary restrictions and MAOIs.
Part 2 of this guide will cover the risk of serotonin syndrome when MAOIs are combined with inhibitors of serotonin reuptake, how to initiate MAOI therapy, and augmenting MAOIs with other agents.
The pharmacology of MAOIs
First used clinically in the 1950s to treat tuberculosis, MAOIs have a long and interesting history (see the Box “A brief history of monoamine oxidase inhibitors”). Table 11 lists MAOIs currently available in the United States, including the MAO-B–specific agent rasagiline, which is used for Parkinson’s disease.

Manipulation of the monoamines serotonin, norepinephrine, and dopamine is fundamental to managing major depressive disorder (MDD), yet only nonselective MAOIs directly promote neurotransmission of all 3 by inhibiting MAO-A and MAO-B.2 The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study demonstrated that <50% of MDD patients achieve remission in monotherapy trials of selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, mirtazapine, or bupropion, necessitating consideration of antidepressant combinations, augmentation options, and eventually irreversible, nonselective MAOIs such as phenelzine, tranylcypromine, or isocarboxazid.3,4 Nonselective MAOIs thus offer a therapeutic opportunity for patients who do not respond to single or dual-mechanism strategies; moreover, nonselective MAOIs have compelling effectiveness data for other conditions, including panic disorder and social phobia.5 Although MAOIs are among the most effective pharmacologic agents for MDD,6 they are underutilized because of an inadequate understanding of risk mechanisms and resultant fear of catastrophic outcomes. Because of the difficulties encountered in achieving clinical remission for MDD, the nonselective MAOIs deserve a second look.
Differentiation of MAO-A from MAO-B. It is essential to understand the mechanism of action of MAOIs, specifically the impact of MAO-A inhibition. Although the enzyme MAO was known in the 1950s, it wasn’t until 1968 that Johnston7 postulated the existence of >1 form. In 1971, Goridis and Neff8 used clorgyline to examine the deamination rate by MAO of tyramine and norepinephrine. They found that tyramine appeared to be a substrate of both MAO isoforms, but only 1 of the MAO types was sensitive to the inhibitory effects of clorgyline. They also discerned that norepinephrine was only a substrate for MAO-A, and that this form of MAO was sensitive to clorgyline inhibition. Thus, the forms of MAO were characterized by their preferred substrates (Table 29,10), and then later by their tissue distribution. Phenylethylamine is a naturally occurring compound found in foods, such as chocolate, and has an in vitro pharmacology similar to amphetamine but with 1 important difference: it has a short half-life of 5 to 10 minutes after oral ingestion, and therefore no appreciable CNS impact.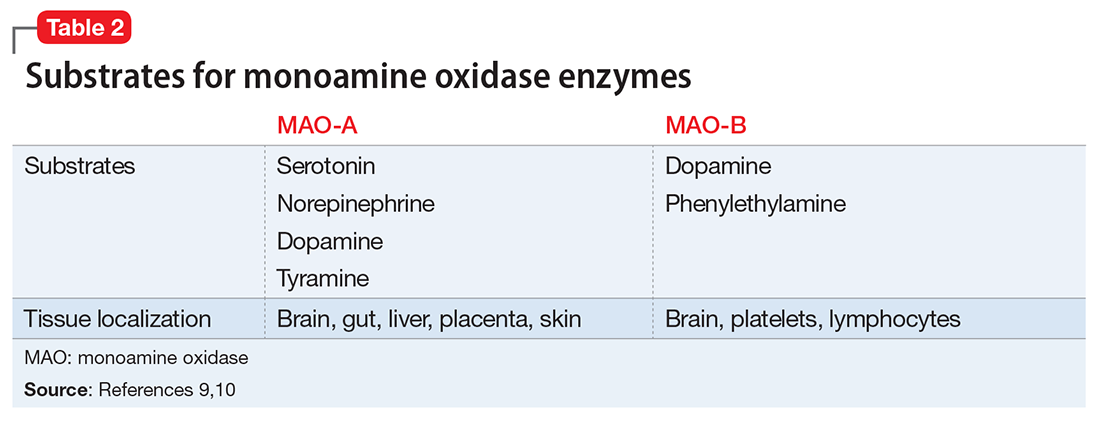
Within the CNS, norepinephrine and dopamine neurons possess both MAO forms, with the MAO-A content greater than MAO-B. Serotonergic neurons only contain MAO-B.11 Outside of the CNS, MAO-A predominates, with only platelets and lymphocytes possessing MAO-B activity.11 The overall relative tissue proportions of MAO-A to MAO-B activity are: brain, 25% MAO-A, 75% MAO-B; liver, 50% MAO-A, 50% MAO-B; intestine, 80% MAO-A, 20% MAO-B; and peripheral adrenergic neurons, 90% MAO-A, 10% MAO-B.
Because of its specificity for serotonin and norepinephrine, CNS MAO-A inhibition is necessary for antidepressant effects. MAO-B inhibition by itself does not appear to raise CNS dopamine levels unless exogenous dopamine is supplied.11 All MAOIs used in the United States to treat depression are irreversible, nonselective inhibitors of MAO-A and MAO-B.
Selegiline in oral form generates low plasma levels and primarily inhibits MAO-B. The transdermal form of selegiline achieves significantly greater systemic exposure, and at these higher plasma levels selegiline is a nonselective, irreversible MAOI effective for MDD (Figure 112). Administering selegiline systemically via a transdermal patch avoids clinically significant MAOI effects in the gut, so no dietary warnings exist for the lowest dose (6 mg/24 hours), although there are warnings for the higher dosages (9 mg/24 hours and 12 mg/24 hours).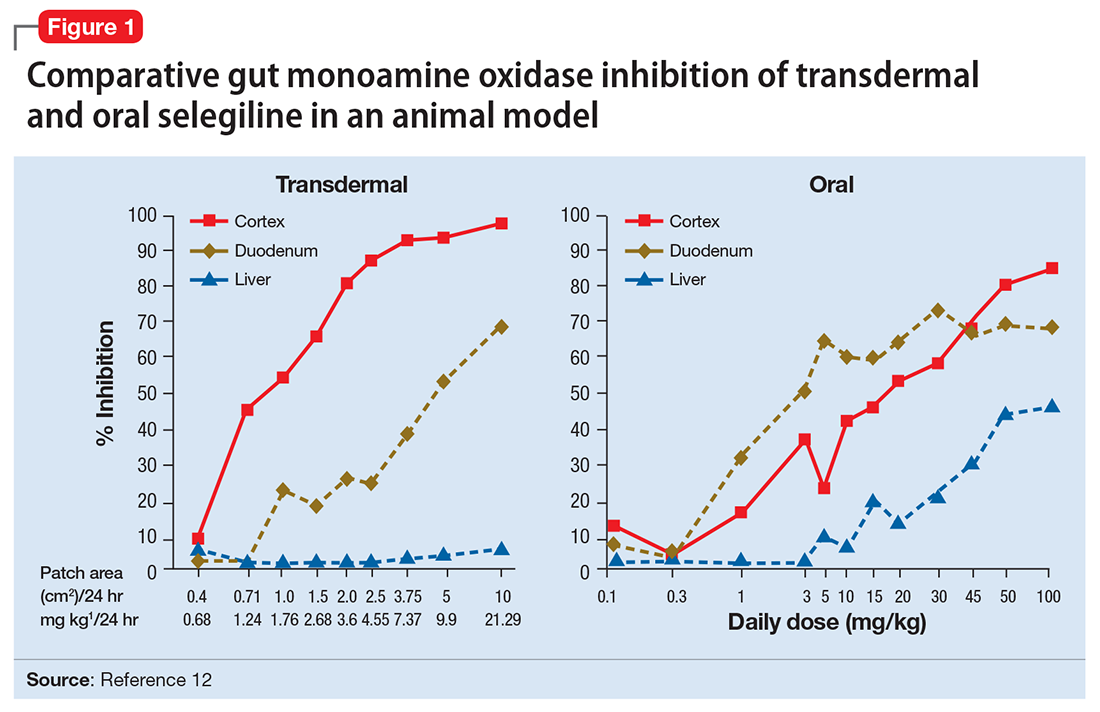
Differentiation of MAOIs by chemical class. The earliest MAOI, iproniazid, was a hydrazine derivative and exhibited hepatotoxicity,13 as did certain other hydrazine MAOIs. This lead to a search for safer hydrazine and nonhydrazine alternatives. Isocarboxazid and phenelzine are the 2 hydrazine MAOIs available in the United States, while tranylcypromine and selegiline transdermal are nonhydrazines (Figure 2).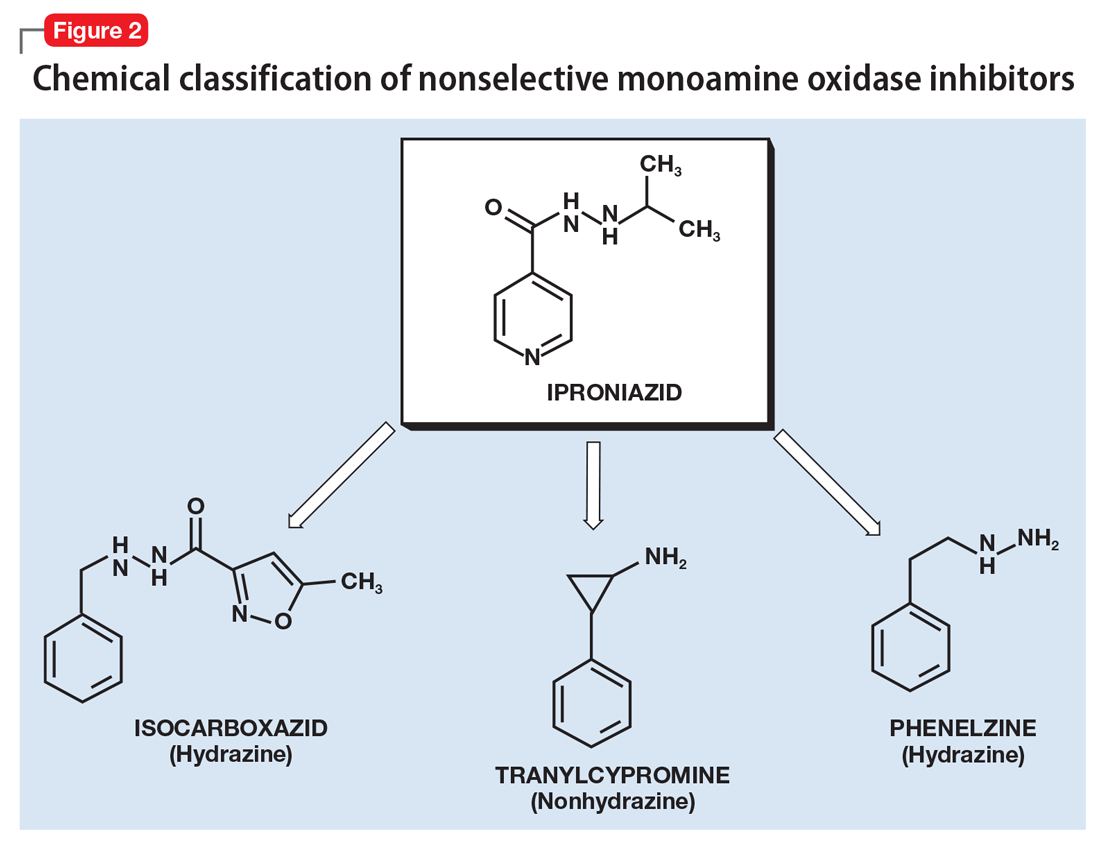
What distinguishes the nonhydrazine medication selegiline is that its metabolism generates L-amphetamine metabolites (Figure 314). This property was thought to be shared by other nonhydrazines, but recent studies indicate than neither tranylcypromine15 nor the MAO-B–selective rasagiline possess amphetamine metabolites.16 Unlike the dextro isomers, L-amphetamine structures do not inhibit dopamine reuptake or cause euphoria, but can cause stimulation (eg, sleep disturbance) by inhibiting norepinephrine reuptake, and also by interacting with the trace amine-associated receptor 1 (TAAR1), an intracellular receptor expressed within the presynaptic terminal of monoamine neurons. Activation of TAAR1 by tyramine is an important part of the hypertensive effects related to excessive tyramine exposure.17 (The importance of TAAR1 and the interaction with tyramine is discussed in the next section.) Importantly, patients taking selegiline must be warned that certain drug screens may not discriminate between levo and dextro isomers of amphetamines, and that the use of selegiline should be disclosed prior to drug testing procedures.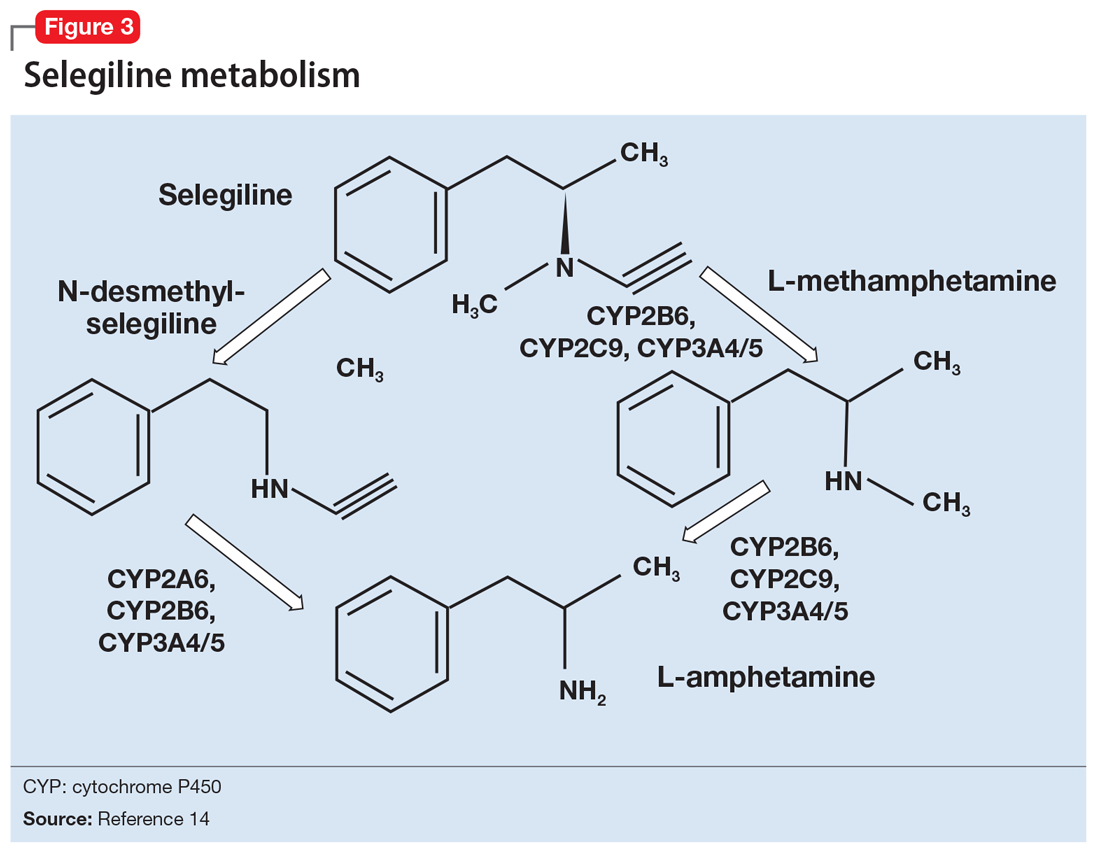
MAOIs and tyramine: Dietary requirements
Clinicians who are familiar with MAOIs recognize that there are dietary restrictions to minimize patients’ exposure to tyramine. As most clinicians know, significant tyramine ingestion may cause an increase in blood pressure (BP) in patients taking an MAOI, but many overestimate the prevalence of foods high in tyramine content since the original reports emerged in the early 1960s.18 In a recent monograph, one of the leading experts on MAOIs, Professor Ken Gillman, stated:
Very few foods now contain problematically high tyramine levels, that is a result of great changes in international food production methods and hygiene regulations. Cheese is the only food that, in the past, has been associated with documented fatalities resulting from hypertension. Nowadays most cheeses are quite safe, and even ‘matured’ cheeses are usually safe in healthy-sized portions. The variability of sensitivity to tyramine between individuals, and the sometimes unpredictable amount of tyramine content in foods, means a little knowledge and care are still required.19
What is tyramine? Tyramine is a biogenic amine that is virtually absent in fresh animal protein sources but is enriched after decay or fermentation.20 Modern food processing and handling methods have significantly limited the tyramine content in processed foods, with the exception of certain cheeses and sauces, as discussed below. Moreover, modern assaying techniques using high-performance liquid chromatography have generated extremely accurate assessments of the tyramine content of specific foods.21 Data published prior to 2000 are not reliable, because many of these publications employed outdated methods.17
When ingested, tyramine is metabolized by gut MAO-A, with doses up to 400 mg causing no known effects, although most people rarely ingest >25 mg during a meal.22 In addition to being a substrate for MAO-A, tyramine is also a substrate for the dopamine transporter, norepinephrine transporter (NET), the vesicular monoamine transporter 2, and TAAR1.23 Tyramine enters the cell via NET, where it interacts with TAAR1, a G protein-coupled receptor that is responsive to trace amines, such as tyramine, as well as amphetamines.20 The agonist properties at TAAR1 are the presumed site of action for the BP effects of tyramine, because binding results in potent release of norepinephrine.20,24 When tyramine is supplied to an animal in which MAO-A is inhibited, the decreased peripheral catabolism of tyramine results in markedly increased norepinephrine release by peripheral adrenergic neurons. Moreover, the absence of MAO-A activity in those neurons prevents any norepinephrine breakdown, resulting in robust synaptic norepinephrine delivery and peripheral effects.
All orally administered irreversible MAOIs potently inhibit gut and systemic MAO-A, and are susceptible to the impact of significant tyramine ingestion. The exception is selegiline transdermal (Figure 112), as appreciable gut MAO-A inhibition does not occur until doses >6 mg/24 hours are reached.22 No significant pressor response was seen in participants taking selegiline transdermal, 6 mg/24 hours for 13 days, who consumed a meal that provided 400 mg of tyramine.22 Conversely, for oral agents that produce gut MAO-A inhibition, tyramine doses as low as 8 to 10 mg (when administered as tyramine capsules) may increase systolic pressure by 30 mm Hg.25 The dietary warnings do not apply to rasagiline, which is a selective MAO-B inhibitor, although rasagiline may have an impact on resting BP; the prescribing information for rasagiline includes warnings about hypotension and hypertension.26
What to tell patients about tyramine. Although administering pure tyramine capsules can induce a measurable change in systolic BP, when ingested as food, tyramine doses <50 mg are unlikely to cause an increase in BP sufficient to warrant clinical intervention, although some individuals can be sensitive to 10 to 25 mg.19 When discussing with patients safety issues related to diet, there are a few important concepts to remember19:
- In an era when the tyramine content of foods was much higher (1960 to 1964) and MAOI users received no dietary guidance, only 14 deaths were reported among an estimated 1.5 million patients who took MAOIs.
- MAOIs do not raise BP, and their use is associated with orthostasis in some patients.
- Routine exercise or other vigorous activities (eg, weightlifting) can raise systolic pressure well above 200 mm Hg, and routine baseline systolic pressures, ranging from 180 to 220 mm Hg, do not increase the risk of subarachnoid hemorrhage.
- Hospital evaluation is needed only if a substantial amount of tyramine is ingested (eg, estimated ≥100 mg), and self-monitoring shows a systolic BP ≥220 mm Hg over a prolonged period (eg, 2 hours). Ingestion of 100 mg of tyramine would almost certainly have to be intentional, as it would require one to consume 3.5 oz of the most highly tyramine-laden cheeses.
Emphasize to patients that only a small number of highly aged cheeses, foods, and sauces contain high quantities of tyramine, and that even these foods can be enjoyed in small amounts. All patients who are prescribed an MAOI also should purchase a portable BP cuff for those rare instances when a dietary indiscretion may have occurred and the person experiences a headache within 1 to 2 hours after tyramine ingestion. Most reactions are self-limited and resolve over 2 to 4 hours.
Patients who ingest ≥100 mg of tyramine should be evaluated by a physician. Under no circumstances should a patient be given a prescription for nifedipine or other medications that can abruptly lower BP, because this may result in complications, including myocardial infarction.27,28 Counsel patients to remain calm. Some clinicians endorse the use of low doses of benzodiazepines (the equivalent of alprazolam 0.5 mg) to facilitate this, because anxiety elevates BP. A recent emergency room study of patients with an initial systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg without end organ damage demonstrated that alprazolam, 0.5 mg, was as effective as captopril, 25 mg, in lowering BP.29
Also, tell patients that if a food is unfamiliar and highly aged or fermented, they should avoid it until they can further inquire about it. In a review, Gillman19 provides the tyramine content of an exhaustive list of cheeses, aged meats, and sauces (see Related Resources). For other products, patients often can obtain information directly from the manufacturer. In many parts of the world, assays for tyramine content are required as a demonstration of adequate product safety procedures. Even the most highly aged cheeses with a tyramine content of 1,000 g/kg can be enjoyed in small amounts (<1 oz), and most products would require heroic intake to achieve clinically significant tyramine ingestion (Table 319).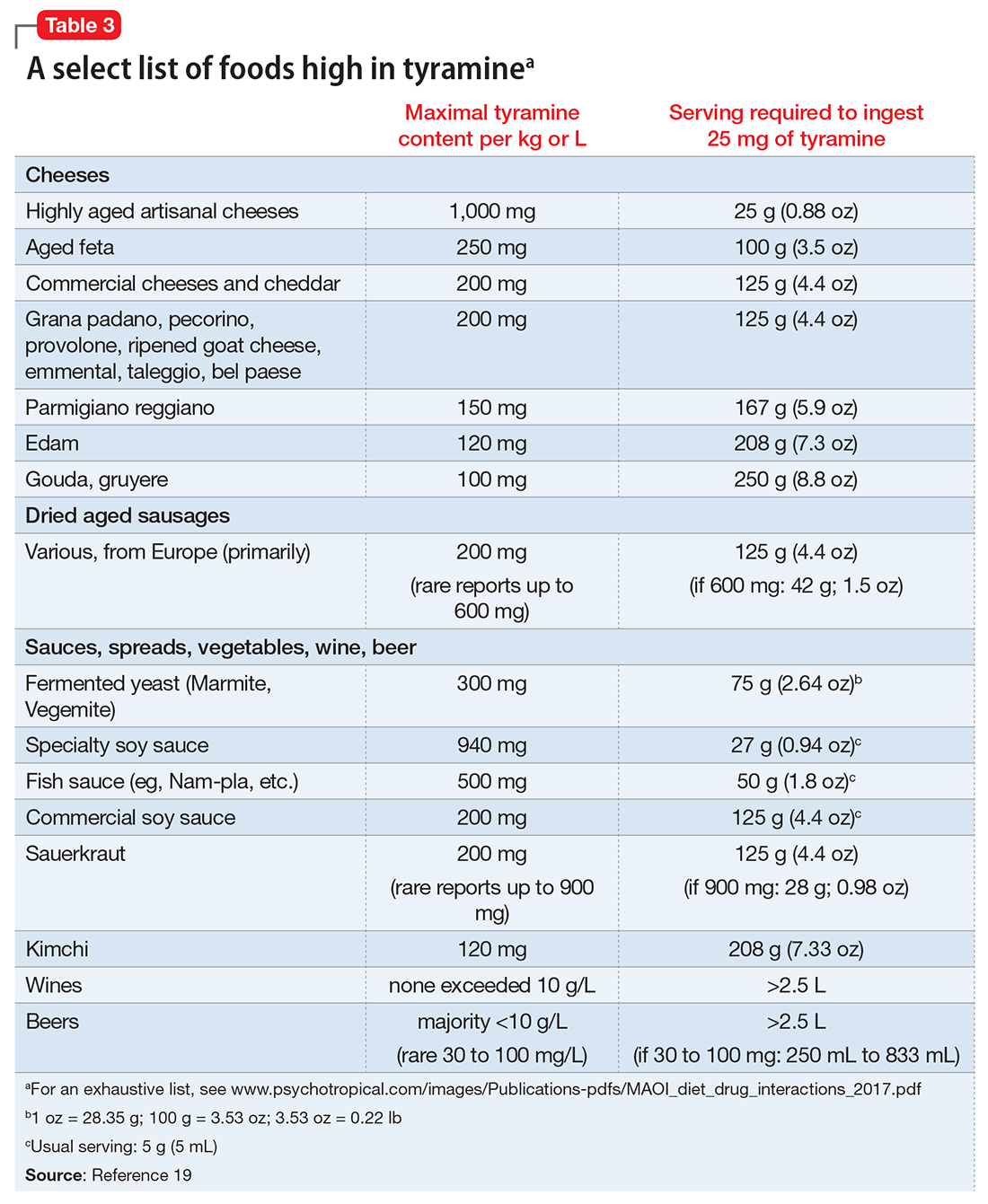
Improved education can clarify the risks
Medications such as lithium, clozapine, and MAOIs have a proven record of efficacy, yet often are underused due to fears engendered by lack of systematic training. A recent initiative in New York thus aimed to increase rates of
1. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
2. López-Muñoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15(14):1563-1586.
3. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
4. Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. New Engl J Med. 2006;354(12):1243-1252.
5. Bandelow B, Zohar J, Hollander E, et al; World Federation of Societies of Biological Psychiatry Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Posttraumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders. World J Biol Psychiatry. 2002;3(4):171-199.
6. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797.
7. Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17(7):1285-1297.
8. Goridis C, Neff NH. Monoamine oxidase in sympathetic nerves: a transmitter specific enzyme type. Br J Pharmacol. 1971;43(4):814-818.
9. Geha RM, Rebrin I, Chen K, et al. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276(13):9877-9882.
10. O’Carroll AM, Fowler CJ, Phillips JP, et al. The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. Naunyn Schmiedebergs Arch Pharmacol. 1983;322(3):198-202.
11. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
12. Mawhinney M, Cole D, Azzaro AJ. Daily transdermal administration of selegiline to guinea-pigs preferentially inhibits monoamine oxidase activity in brain when compared with intestinal and hepatic tissues. J Pharm Pharmacol. 2003;55(1):27-34.
13. Maille F, Duvoux C, Cherqui D, et al. Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France? [in French]. Gastroenterol Clin Biol. 1999;23(10):1083-1085.
14. Salonen JS, Nyman L, Boobis AR, et al. Comparative studies on the cytochrome p450-associated metabolism and interaction potential of selegiline between human liver-derived in vitro systems. Drug Metab Dispos. 2003;31(9):1093-1102.
15. Iwersen S, Schmoldt A. One fatal and one nonfatal intoxication with tranylcypromine. Absence of amphetamines as metabolites. J Anal Toxicol. 1996;20(5):301-304.
16. Müller T, Hoffmann JA, Dimpfel W, et al. Switch from selegiline to rasagiline is beneficial in patients with Parkinson’s disease. J Neural Transm (Vienna). 2013;120(5):761-765.
17. Lewin AH, Miller GM, Gilmour B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg Med Chem. 2011;19(23):7044-7048.
18. Blackwell B. Hypertensive crisis due to monoamine-oxidase inhibitors. Lancet. 1963;2(7313):849-850.
19. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-97.
20. Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front Neurosci. 2016;10:148.
21. Fiechter G, Sivec G, Mayer HK. Application of UHPLC for the simultaneous analysis of free amino acids and biogenic amines in ripened acid-curd cheeses. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;927:191-200.
22. Blob LF, Sharoky M, Campbell BJ, et al. Effects of a tyramine-enriched meal on blood pressure response in healthy male volunteers treated with selegiline transdermal system 6 mg/24 hour. CNS Spectr. 2007;12(1):25-34.
23. Partilla JS, Dempsey AG, Nagpal AS, et al. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther. 2006;319(1):237-246.
24. Borowsky B, Adham N, Jones KA, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98(16):8966-8971.
25. Azzaro AJ, Vandenberg CM, Blob LF, et al. Tyramine pressor sensitivity during treatment with the selegiline transdermal system 6 mg/24 h in healthy subjects. J Clin Pharmacol. 2006;46(8):933-944.
26. Azilect [package insert]. Overland Park, KS: Teva Neuroscience, Inc.; 2014.
27. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949-1962.
28. Burton TJ, Wilkinson IB. The dangers of immediate-release nifedipine in the emergency treatment of hypertension. J Hum Hypertens. 2008;22(4):301-302.
29. Yilmaz S, Pekdemir M, Tural U, et al. Comparison of alprazolam versus captopril in high blood pressure: a randomized controlled trial. Blood Press. 2011;20(4):239-243.
30. Carruthers J, Radigan M, Erlich MD, et al. An initiative to improve clozapine prescribing in New York State. Psychiatr Serv. 2016;67(4):369-371.
Despite an abundance of evidenced-based literature supporting monoamine oxidase inhibitors (MAOIs) as an effective treatment for depression, use of these agents has decreased drastically in the past 3 decades. A lack of industry support and the ease of use of other agents are contributing factors, but the biggest impediments to routine use of MAOIs are unfamiliarity with their efficacy advantages and concerns about adverse effects, particularly the risk of hypertensive crises and serotonin syndrome. Many misconceptions regarding these medications are based on outdated data and studies that are no longer reliable.
The goal of this 2-part review is to provide clinicians with updated information regarding MAOIs. Part 1 provides a brief description of:
- the pharmacology of nonselective irreversible MAOIs
- the mechanism by which tyramine induces hypertension
- sources of clinically significant tyramine exposure
- what to tell patients about dietary restrictions and MAOIs.
Part 2 of this guide will cover the risk of serotonin syndrome when MAOIs are combined with inhibitors of serotonin reuptake, how to initiate MAOI therapy, and augmenting MAOIs with other agents.
The pharmacology of MAOIs
First used clinically in the 1950s to treat tuberculosis, MAOIs have a long and interesting history (see the Box “A brief history of monoamine oxidase inhibitors”). Table 11 lists MAOIs currently available in the United States, including the MAO-B–specific agent rasagiline, which is used for Parkinson’s disease.

Manipulation of the monoamines serotonin, norepinephrine, and dopamine is fundamental to managing major depressive disorder (MDD), yet only nonselective MAOIs directly promote neurotransmission of all 3 by inhibiting MAO-A and MAO-B.2 The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study demonstrated that <50% of MDD patients achieve remission in monotherapy trials of selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, mirtazapine, or bupropion, necessitating consideration of antidepressant combinations, augmentation options, and eventually irreversible, nonselective MAOIs such as phenelzine, tranylcypromine, or isocarboxazid.3,4 Nonselective MAOIs thus offer a therapeutic opportunity for patients who do not respond to single or dual-mechanism strategies; moreover, nonselective MAOIs have compelling effectiveness data for other conditions, including panic disorder and social phobia.5 Although MAOIs are among the most effective pharmacologic agents for MDD,6 they are underutilized because of an inadequate understanding of risk mechanisms and resultant fear of catastrophic outcomes. Because of the difficulties encountered in achieving clinical remission for MDD, the nonselective MAOIs deserve a second look.

Differentiation of MAO-A from MAO-B. It is essential to understand the mechanism of action of MAOIs, specifically the impact of MAO-A inhibition. Although the enzyme MAO was known in the 1950s, it wasn’t until 1968 that Johnston7 postulated the existence of >1 form. In 1971, Goridis and Neff8 used clorgyline to examine the deamination rate by MAO of tyramine and norepinephrine. They found that tyramine appeared to be a substrate of both MAO isoforms, but only 1 of the MAO types was sensitive to the inhibitory effects of clorgyline. They also discerned that norepinephrine was only a substrate for MAO-A, and that this form of MAO was sensitive to clorgyline inhibition. Thus, the forms of MAO were characterized by their preferred substrates (Table 29,10), and then later by their tissue distribution. Phenylethylamine is a naturally occurring compound found in foods, such as chocolate, and has an in vitro pharmacology similar to amphetamine but with 1 important difference: it has a short half-life of 5 to 10 minutes after oral ingestion, and therefore no appreciable CNS impact.
Within the CNS, norepinephrine and dopamine neurons possess both MAO forms, with the MAO-A content greater than MAO-B. Serotonergic neurons only contain MAO-B.11 Outside of the CNS, MAO-A predominates, with only platelets and lymphocytes possessing MAO-B activity.11 The overall relative tissue proportions of MAO-A to MAO-B activity are: brain, 25% MAO-A, 75% MAO-B; liver, 50% MAO-A, 50% MAO-B; intestine, 80% MAO-A, 20% MAO-B; and peripheral adrenergic neurons, 90% MAO-A, 10% MAO-B.
Because of its specificity for serotonin and norepinephrine, CNS MAO-A inhibition is necessary for antidepressant effects. MAO-B inhibition by itself does not appear to raise CNS dopamine levels unless exogenous dopamine is supplied.11 All MAOIs used in the United States to treat depression are irreversible, nonselective inhibitors of MAO-A and MAO-B.
Selegiline in oral form generates low plasma levels and primarily inhibits MAO-B. The transdermal form of selegiline achieves significantly greater systemic exposure, and at these higher plasma levels selegiline is a nonselective, irreversible MAOI effective for MDD (Figure 112). Administering selegiline systemically via a transdermal patch avoids clinically significant MAOI effects in the gut, so no dietary warnings exist for the lowest dose (6 mg/24 hours), although there are warnings for the higher dosages (9 mg/24 hours and 12 mg/24 hours).
Differentiation of MAOIs by chemical class. The earliest MAOI, iproniazid, was a hydrazine derivative and exhibited hepatotoxicity,13 as did certain other hydrazine MAOIs. This lead to a search for safer hydrazine and nonhydrazine alternatives. Isocarboxazid and phenelzine are the 2 hydrazine MAOIs available in the United States, while tranylcypromine and selegiline transdermal are nonhydrazines (Figure 2).
What distinguishes the nonhydrazine medication selegiline is that its metabolism generates L-amphetamine metabolites (Figure 314). This property was thought to be shared by other nonhydrazines, but recent studies indicate than neither tranylcypromine15 nor the MAO-B–selective rasagiline possess amphetamine metabolites.16 Unlike the dextro isomers, L-amphetamine structures do not inhibit dopamine reuptake or cause euphoria, but can cause stimulation (eg, sleep disturbance) by inhibiting norepinephrine reuptake, and also by interacting with the trace amine-associated receptor 1 (TAAR1), an intracellular receptor expressed within the presynaptic terminal of monoamine neurons. Activation of TAAR1 by tyramine is an important part of the hypertensive effects related to excessive tyramine exposure.17 (The importance of TAAR1 and the interaction with tyramine is discussed in the next section.) Importantly, patients taking selegiline must be warned that certain drug screens may not discriminate between levo and dextro isomers of amphetamines, and that the use of selegiline should be disclosed prior to drug testing procedures.
MAOIs and tyramine: Dietary requirements
Clinicians who are familiar with MAOIs recognize that there are dietary restrictions to minimize patients’ exposure to tyramine. As most clinicians know, significant tyramine ingestion may cause an increase in blood pressure (BP) in patients taking an MAOI, but many overestimate the prevalence of foods high in tyramine content since the original reports emerged in the early 1960s.18 In a recent monograph, one of the leading experts on MAOIs, Professor Ken Gillman, stated:
Very few foods now contain problematically high tyramine levels, that is a result of great changes in international food production methods and hygiene regulations. Cheese is the only food that, in the past, has been associated with documented fatalities resulting from hypertension. Nowadays most cheeses are quite safe, and even ‘matured’ cheeses are usually safe in healthy-sized portions. The variability of sensitivity to tyramine between individuals, and the sometimes unpredictable amount of tyramine content in foods, means a little knowledge and care are still required.19
What is tyramine? Tyramine is a biogenic amine that is virtually absent in fresh animal protein sources but is enriched after decay or fermentation.20 Modern food processing and handling methods have significantly limited the tyramine content in processed foods, with the exception of certain cheeses and sauces, as discussed below. Moreover, modern assaying techniques using high-performance liquid chromatography have generated extremely accurate assessments of the tyramine content of specific foods.21 Data published prior to 2000 are not reliable, because many of these publications employed outdated methods.17
When ingested, tyramine is metabolized by gut MAO-A, with doses up to 400 mg causing no known effects, although most people rarely ingest >25 mg during a meal.22 In addition to being a substrate for MAO-A, tyramine is also a substrate for the dopamine transporter, norepinephrine transporter (NET), the vesicular monoamine transporter 2, and TAAR1.23 Tyramine enters the cell via NET, where it interacts with TAAR1, a G protein-coupled receptor that is responsive to trace amines, such as tyramine, as well as amphetamines.20 The agonist properties at TAAR1 are the presumed site of action for the BP effects of tyramine, because binding results in potent release of norepinephrine.20,24 When tyramine is supplied to an animal in which MAO-A is inhibited, the decreased peripheral catabolism of tyramine results in markedly increased norepinephrine release by peripheral adrenergic neurons. Moreover, the absence of MAO-A activity in those neurons prevents any norepinephrine breakdown, resulting in robust synaptic norepinephrine delivery and peripheral effects.
All orally administered irreversible MAOIs potently inhibit gut and systemic MAO-A, and are susceptible to the impact of significant tyramine ingestion. The exception is selegiline transdermal (Figure 112), as appreciable gut MAO-A inhibition does not occur until doses >6 mg/24 hours are reached.22 No significant pressor response was seen in participants taking selegiline transdermal, 6 mg/24 hours for 13 days, who consumed a meal that provided 400 mg of tyramine.22 Conversely, for oral agents that produce gut MAO-A inhibition, tyramine doses as low as 8 to 10 mg (when administered as tyramine capsules) may increase systolic pressure by 30 mm Hg.25 The dietary warnings do not apply to rasagiline, which is a selective MAO-B inhibitor, although rasagiline may have an impact on resting BP; the prescribing information for rasagiline includes warnings about hypotension and hypertension.26
What to tell patients about tyramine. Although administering pure tyramine capsules can induce a measurable change in systolic BP, when ingested as food, tyramine doses <50 mg are unlikely to cause an increase in BP sufficient to warrant clinical intervention, although some individuals can be sensitive to 10 to 25 mg.19 When discussing with patients safety issues related to diet, there are a few important concepts to remember19:
- In an era when the tyramine content of foods was much higher (1960 to 1964) and MAOI users received no dietary guidance, only 14 deaths were reported among an estimated 1.5 million patients who took MAOIs.
- MAOIs do not raise BP, and their use is associated with orthostasis in some patients.
- Routine exercise or other vigorous activities (eg, weightlifting) can raise systolic pressure well above 200 mm Hg, and routine baseline systolic pressures, ranging from 180 to 220 mm Hg, do not increase the risk of subarachnoid hemorrhage.
- Hospital evaluation is needed only if a substantial amount of tyramine is ingested (eg, estimated ≥100 mg), and self-monitoring shows a systolic BP ≥220 mm Hg over a prolonged period (eg, 2 hours). Ingestion of 100 mg of tyramine would almost certainly have to be intentional, as it would require one to consume 3.5 oz of the most highly tyramine-laden cheeses.
Emphasize to patients that only a small number of highly aged cheeses, foods, and sauces contain high quantities of tyramine, and that even these foods can be enjoyed in small amounts. All patients who are prescribed an MAOI also should purchase a portable BP cuff for those rare instances when a dietary indiscretion may have occurred and the person experiences a headache within 1 to 2 hours after tyramine ingestion. Most reactions are self-limited and resolve over 2 to 4 hours.
Patients who ingest ≥100 mg of tyramine should be evaluated by a physician. Under no circumstances should a patient be given a prescription for nifedipine or other medications that can abruptly lower BP, because this may result in complications, including myocardial infarction.27,28 Counsel patients to remain calm. Some clinicians endorse the use of low doses of benzodiazepines (the equivalent of alprazolam 0.5 mg) to facilitate this, because anxiety elevates BP. A recent emergency room study of patients with an initial systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg without end organ damage demonstrated that alprazolam, 0.5 mg, was as effective as captopril, 25 mg, in lowering BP.29
Also, tell patients that if a food is unfamiliar and highly aged or fermented, they should avoid it until they can further inquire about it. In a review, Gillman19 provides the tyramine content of an exhaustive list of cheeses, aged meats, and sauces (see Related Resources). For other products, patients often can obtain information directly from the manufacturer. In many parts of the world, assays for tyramine content are required as a demonstration of adequate product safety procedures. Even the most highly aged cheeses with a tyramine content of 1,000 g/kg can be enjoyed in small amounts (<1 oz), and most products would require heroic intake to achieve clinically significant tyramine ingestion (Table 319).
Improved education can clarify the risks
Medications such as lithium, clozapine, and MAOIs have a proven record of efficacy, yet often are underused due to fears engendered by lack of systematic training. A recent initiative in New York thus aimed to increase rates of
Despite an abundance of evidenced-based literature supporting monoamine oxidase inhibitors (MAOIs) as an effective treatment for depression, use of these agents has decreased drastically in the past 3 decades. A lack of industry support and the ease of use of other agents are contributing factors, but the biggest impediments to routine use of MAOIs are unfamiliarity with their efficacy advantages and concerns about adverse effects, particularly the risk of hypertensive crises and serotonin syndrome. Many misconceptions regarding these medications are based on outdated data and studies that are no longer reliable.
The goal of this 2-part review is to provide clinicians with updated information regarding MAOIs. Part 1 provides a brief description of:
- the pharmacology of nonselective irreversible MAOIs
- the mechanism by which tyramine induces hypertension
- sources of clinically significant tyramine exposure
- what to tell patients about dietary restrictions and MAOIs.
Part 2 of this guide will cover the risk of serotonin syndrome when MAOIs are combined with inhibitors of serotonin reuptake, how to initiate MAOI therapy, and augmenting MAOIs with other agents.
The pharmacology of MAOIs
First used clinically in the 1950s to treat tuberculosis, MAOIs have a long and interesting history (see the Box “A brief history of monoamine oxidase inhibitors”). Table 11 lists MAOIs currently available in the United States, including the MAO-B–specific agent rasagiline, which is used for Parkinson’s disease.

Manipulation of the monoamines serotonin, norepinephrine, and dopamine is fundamental to managing major depressive disorder (MDD), yet only nonselective MAOIs directly promote neurotransmission of all 3 by inhibiting MAO-A and MAO-B.2 The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study demonstrated that <50% of MDD patients achieve remission in monotherapy trials of selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, mirtazapine, or bupropion, necessitating consideration of antidepressant combinations, augmentation options, and eventually irreversible, nonselective MAOIs such as phenelzine, tranylcypromine, or isocarboxazid.3,4 Nonselective MAOIs thus offer a therapeutic opportunity for patients who do not respond to single or dual-mechanism strategies; moreover, nonselective MAOIs have compelling effectiveness data for other conditions, including panic disorder and social phobia.5 Although MAOIs are among the most effective pharmacologic agents for MDD,6 they are underutilized because of an inadequate understanding of risk mechanisms and resultant fear of catastrophic outcomes. Because of the difficulties encountered in achieving clinical remission for MDD, the nonselective MAOIs deserve a second look.

Differentiation of MAO-A from MAO-B. It is essential to understand the mechanism of action of MAOIs, specifically the impact of MAO-A inhibition. Although the enzyme MAO was known in the 1950s, it wasn’t until 1968 that Johnston7 postulated the existence of >1 form. In 1971, Goridis and Neff8 used clorgyline to examine the deamination rate by MAO of tyramine and norepinephrine. They found that tyramine appeared to be a substrate of both MAO isoforms, but only 1 of the MAO types was sensitive to the inhibitory effects of clorgyline. They also discerned that norepinephrine was only a substrate for MAO-A, and that this form of MAO was sensitive to clorgyline inhibition. Thus, the forms of MAO were characterized by their preferred substrates (Table 29,10), and then later by their tissue distribution. Phenylethylamine is a naturally occurring compound found in foods, such as chocolate, and has an in vitro pharmacology similar to amphetamine but with 1 important difference: it has a short half-life of 5 to 10 minutes after oral ingestion, and therefore no appreciable CNS impact.
Within the CNS, norepinephrine and dopamine neurons possess both MAO forms, with the MAO-A content greater than MAO-B. Serotonergic neurons only contain MAO-B.11 Outside of the CNS, MAO-A predominates, with only platelets and lymphocytes possessing MAO-B activity.11 The overall relative tissue proportions of MAO-A to MAO-B activity are: brain, 25% MAO-A, 75% MAO-B; liver, 50% MAO-A, 50% MAO-B; intestine, 80% MAO-A, 20% MAO-B; and peripheral adrenergic neurons, 90% MAO-A, 10% MAO-B.
Because of its specificity for serotonin and norepinephrine, CNS MAO-A inhibition is necessary for antidepressant effects. MAO-B inhibition by itself does not appear to raise CNS dopamine levels unless exogenous dopamine is supplied.11 All MAOIs used in the United States to treat depression are irreversible, nonselective inhibitors of MAO-A and MAO-B.
Selegiline in oral form generates low plasma levels and primarily inhibits MAO-B. The transdermal form of selegiline achieves significantly greater systemic exposure, and at these higher plasma levels selegiline is a nonselective, irreversible MAOI effective for MDD (Figure 112). Administering selegiline systemically via a transdermal patch avoids clinically significant MAOI effects in the gut, so no dietary warnings exist for the lowest dose (6 mg/24 hours), although there are warnings for the higher dosages (9 mg/24 hours and 12 mg/24 hours).
Differentiation of MAOIs by chemical class. The earliest MAOI, iproniazid, was a hydrazine derivative and exhibited hepatotoxicity,13 as did certain other hydrazine MAOIs. This lead to a search for safer hydrazine and nonhydrazine alternatives. Isocarboxazid and phenelzine are the 2 hydrazine MAOIs available in the United States, while tranylcypromine and selegiline transdermal are nonhydrazines (Figure 2).
What distinguishes the nonhydrazine medication selegiline is that its metabolism generates L-amphetamine metabolites (Figure 314). This property was thought to be shared by other nonhydrazines, but recent studies indicate than neither tranylcypromine15 nor the MAO-B–selective rasagiline possess amphetamine metabolites.16 Unlike the dextro isomers, L-amphetamine structures do not inhibit dopamine reuptake or cause euphoria, but can cause stimulation (eg, sleep disturbance) by inhibiting norepinephrine reuptake, and also by interacting with the trace amine-associated receptor 1 (TAAR1), an intracellular receptor expressed within the presynaptic terminal of monoamine neurons. Activation of TAAR1 by tyramine is an important part of the hypertensive effects related to excessive tyramine exposure.17 (The importance of TAAR1 and the interaction with tyramine is discussed in the next section.) Importantly, patients taking selegiline must be warned that certain drug screens may not discriminate between levo and dextro isomers of amphetamines, and that the use of selegiline should be disclosed prior to drug testing procedures.
MAOIs and tyramine: Dietary requirements
Clinicians who are familiar with MAOIs recognize that there are dietary restrictions to minimize patients’ exposure to tyramine. As most clinicians know, significant tyramine ingestion may cause an increase in blood pressure (BP) in patients taking an MAOI, but many overestimate the prevalence of foods high in tyramine content since the original reports emerged in the early 1960s.18 In a recent monograph, one of the leading experts on MAOIs, Professor Ken Gillman, stated:
Very few foods now contain problematically high tyramine levels, that is a result of great changes in international food production methods and hygiene regulations. Cheese is the only food that, in the past, has been associated with documented fatalities resulting from hypertension. Nowadays most cheeses are quite safe, and even ‘matured’ cheeses are usually safe in healthy-sized portions. The variability of sensitivity to tyramine between individuals, and the sometimes unpredictable amount of tyramine content in foods, means a little knowledge and care are still required.19
What is tyramine? Tyramine is a biogenic amine that is virtually absent in fresh animal protein sources but is enriched after decay or fermentation.20 Modern food processing and handling methods have significantly limited the tyramine content in processed foods, with the exception of certain cheeses and sauces, as discussed below. Moreover, modern assaying techniques using high-performance liquid chromatography have generated extremely accurate assessments of the tyramine content of specific foods.21 Data published prior to 2000 are not reliable, because many of these publications employed outdated methods.17
When ingested, tyramine is metabolized by gut MAO-A, with doses up to 400 mg causing no known effects, although most people rarely ingest >25 mg during a meal.22 In addition to being a substrate for MAO-A, tyramine is also a substrate for the dopamine transporter, norepinephrine transporter (NET), the vesicular monoamine transporter 2, and TAAR1.23 Tyramine enters the cell via NET, where it interacts with TAAR1, a G protein-coupled receptor that is responsive to trace amines, such as tyramine, as well as amphetamines.20 The agonist properties at TAAR1 are the presumed site of action for the BP effects of tyramine, because binding results in potent release of norepinephrine.20,24 When tyramine is supplied to an animal in which MAO-A is inhibited, the decreased peripheral catabolism of tyramine results in markedly increased norepinephrine release by peripheral adrenergic neurons. Moreover, the absence of MAO-A activity in those neurons prevents any norepinephrine breakdown, resulting in robust synaptic norepinephrine delivery and peripheral effects.
All orally administered irreversible MAOIs potently inhibit gut and systemic MAO-A, and are susceptible to the impact of significant tyramine ingestion. The exception is selegiline transdermal (Figure 112), as appreciable gut MAO-A inhibition does not occur until doses >6 mg/24 hours are reached.22 No significant pressor response was seen in participants taking selegiline transdermal, 6 mg/24 hours for 13 days, who consumed a meal that provided 400 mg of tyramine.22 Conversely, for oral agents that produce gut MAO-A inhibition, tyramine doses as low as 8 to 10 mg (when administered as tyramine capsules) may increase systolic pressure by 30 mm Hg.25 The dietary warnings do not apply to rasagiline, which is a selective MAO-B inhibitor, although rasagiline may have an impact on resting BP; the prescribing information for rasagiline includes warnings about hypotension and hypertension.26
What to tell patients about tyramine. Although administering pure tyramine capsules can induce a measurable change in systolic BP, when ingested as food, tyramine doses <50 mg are unlikely to cause an increase in BP sufficient to warrant clinical intervention, although some individuals can be sensitive to 10 to 25 mg.19 When discussing with patients safety issues related to diet, there are a few important concepts to remember19:
- In an era when the tyramine content of foods was much higher (1960 to 1964) and MAOI users received no dietary guidance, only 14 deaths were reported among an estimated 1.5 million patients who took MAOIs.
- MAOIs do not raise BP, and their use is associated with orthostasis in some patients.
- Routine exercise or other vigorous activities (eg, weightlifting) can raise systolic pressure well above 200 mm Hg, and routine baseline systolic pressures, ranging from 180 to 220 mm Hg, do not increase the risk of subarachnoid hemorrhage.
- Hospital evaluation is needed only if a substantial amount of tyramine is ingested (eg, estimated ≥100 mg), and self-monitoring shows a systolic BP ≥220 mm Hg over a prolonged period (eg, 2 hours). Ingestion of 100 mg of tyramine would almost certainly have to be intentional, as it would require one to consume 3.5 oz of the most highly tyramine-laden cheeses.
Emphasize to patients that only a small number of highly aged cheeses, foods, and sauces contain high quantities of tyramine, and that even these foods can be enjoyed in small amounts. All patients who are prescribed an MAOI also should purchase a portable BP cuff for those rare instances when a dietary indiscretion may have occurred and the person experiences a headache within 1 to 2 hours after tyramine ingestion. Most reactions are self-limited and resolve over 2 to 4 hours.
Patients who ingest ≥100 mg of tyramine should be evaluated by a physician. Under no circumstances should a patient be given a prescription for nifedipine or other medications that can abruptly lower BP, because this may result in complications, including myocardial infarction.27,28 Counsel patients to remain calm. Some clinicians endorse the use of low doses of benzodiazepines (the equivalent of alprazolam 0.5 mg) to facilitate this, because anxiety elevates BP. A recent emergency room study of patients with an initial systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg without end organ damage demonstrated that alprazolam, 0.5 mg, was as effective as captopril, 25 mg, in lowering BP.29
Also, tell patients that if a food is unfamiliar and highly aged or fermented, they should avoid it until they can further inquire about it. In a review, Gillman19 provides the tyramine content of an exhaustive list of cheeses, aged meats, and sauces (see Related Resources). For other products, patients often can obtain information directly from the manufacturer. In many parts of the world, assays for tyramine content are required as a demonstration of adequate product safety procedures. Even the most highly aged cheeses with a tyramine content of 1,000 g/kg can be enjoyed in small amounts (<1 oz), and most products would require heroic intake to achieve clinically significant tyramine ingestion (Table 319).
Improved education can clarify the risks
Medications such as lithium, clozapine, and MAOIs have a proven record of efficacy, yet often are underused due to fears engendered by lack of systematic training. A recent initiative in New York thus aimed to increase rates of
1. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
2. López-Muñoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15(14):1563-1586.
3. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
4. Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. New Engl J Med. 2006;354(12):1243-1252.
5. Bandelow B, Zohar J, Hollander E, et al; World Federation of Societies of Biological Psychiatry Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Posttraumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders. World J Biol Psychiatry. 2002;3(4):171-199.
6. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797.
7. Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17(7):1285-1297.
8. Goridis C, Neff NH. Monoamine oxidase in sympathetic nerves: a transmitter specific enzyme type. Br J Pharmacol. 1971;43(4):814-818.
9. Geha RM, Rebrin I, Chen K, et al. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276(13):9877-9882.
10. O’Carroll AM, Fowler CJ, Phillips JP, et al. The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. Naunyn Schmiedebergs Arch Pharmacol. 1983;322(3):198-202.
11. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
12. Mawhinney M, Cole D, Azzaro AJ. Daily transdermal administration of selegiline to guinea-pigs preferentially inhibits monoamine oxidase activity in brain when compared with intestinal and hepatic tissues. J Pharm Pharmacol. 2003;55(1):27-34.
13. Maille F, Duvoux C, Cherqui D, et al. Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France? [in French]. Gastroenterol Clin Biol. 1999;23(10):1083-1085.
14. Salonen JS, Nyman L, Boobis AR, et al. Comparative studies on the cytochrome p450-associated metabolism and interaction potential of selegiline between human liver-derived in vitro systems. Drug Metab Dispos. 2003;31(9):1093-1102.
15. Iwersen S, Schmoldt A. One fatal and one nonfatal intoxication with tranylcypromine. Absence of amphetamines as metabolites. J Anal Toxicol. 1996;20(5):301-304.
16. Müller T, Hoffmann JA, Dimpfel W, et al. Switch from selegiline to rasagiline is beneficial in patients with Parkinson’s disease. J Neural Transm (Vienna). 2013;120(5):761-765.
17. Lewin AH, Miller GM, Gilmour B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg Med Chem. 2011;19(23):7044-7048.
18. Blackwell B. Hypertensive crisis due to monoamine-oxidase inhibitors. Lancet. 1963;2(7313):849-850.
19. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-97.
20. Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front Neurosci. 2016;10:148.
21. Fiechter G, Sivec G, Mayer HK. Application of UHPLC for the simultaneous analysis of free amino acids and biogenic amines in ripened acid-curd cheeses. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;927:191-200.
22. Blob LF, Sharoky M, Campbell BJ, et al. Effects of a tyramine-enriched meal on blood pressure response in healthy male volunteers treated with selegiline transdermal system 6 mg/24 hour. CNS Spectr. 2007;12(1):25-34.
23. Partilla JS, Dempsey AG, Nagpal AS, et al. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther. 2006;319(1):237-246.
24. Borowsky B, Adham N, Jones KA, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98(16):8966-8971.
25. Azzaro AJ, Vandenberg CM, Blob LF, et al. Tyramine pressor sensitivity during treatment with the selegiline transdermal system 6 mg/24 h in healthy subjects. J Clin Pharmacol. 2006;46(8):933-944.
26. Azilect [package insert]. Overland Park, KS: Teva Neuroscience, Inc.; 2014.
27. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949-1962.
28. Burton TJ, Wilkinson IB. The dangers of immediate-release nifedipine in the emergency treatment of hypertension. J Hum Hypertens. 2008;22(4):301-302.
29. Yilmaz S, Pekdemir M, Tural U, et al. Comparison of alprazolam versus captopril in high blood pressure: a randomized controlled trial. Blood Press. 2011;20(4):239-243.
30. Carruthers J, Radigan M, Erlich MD, et al. An initiative to improve clozapine prescribing in New York State. Psychiatr Serv. 2016;67(4):369-371.
1. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
2. López-Muñoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15(14):1563-1586.
3. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
4. Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. New Engl J Med. 2006;354(12):1243-1252.
5. Bandelow B, Zohar J, Hollander E, et al; World Federation of Societies of Biological Psychiatry Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Posttraumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders. World J Biol Psychiatry. 2002;3(4):171-199.
6. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797.
7. Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17(7):1285-1297.
8. Goridis C, Neff NH. Monoamine oxidase in sympathetic nerves: a transmitter specific enzyme type. Br J Pharmacol. 1971;43(4):814-818.
9. Geha RM, Rebrin I, Chen K, et al. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276(13):9877-9882.
10. O’Carroll AM, Fowler CJ, Phillips JP, et al. The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. Naunyn Schmiedebergs Arch Pharmacol. 1983;322(3):198-202.
11. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
12. Mawhinney M, Cole D, Azzaro AJ. Daily transdermal administration of selegiline to guinea-pigs preferentially inhibits monoamine oxidase activity in brain when compared with intestinal and hepatic tissues. J Pharm Pharmacol. 2003;55(1):27-34.
13. Maille F, Duvoux C, Cherqui D, et al. Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France? [in French]. Gastroenterol Clin Biol. 1999;23(10):1083-1085.
14. Salonen JS, Nyman L, Boobis AR, et al. Comparative studies on the cytochrome p450-associated metabolism and interaction potential of selegiline between human liver-derived in vitro systems. Drug Metab Dispos. 2003;31(9):1093-1102.
15. Iwersen S, Schmoldt A. One fatal and one nonfatal intoxication with tranylcypromine. Absence of amphetamines as metabolites. J Anal Toxicol. 1996;20(5):301-304.
16. Müller T, Hoffmann JA, Dimpfel W, et al. Switch from selegiline to rasagiline is beneficial in patients with Parkinson’s disease. J Neural Transm (Vienna). 2013;120(5):761-765.
17. Lewin AH, Miller GM, Gilmour B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg Med Chem. 2011;19(23):7044-7048.
18. Blackwell B. Hypertensive crisis due to monoamine-oxidase inhibitors. Lancet. 1963;2(7313):849-850.
19. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-97.
20. Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front Neurosci. 2016;10:148.
21. Fiechter G, Sivec G, Mayer HK. Application of UHPLC for the simultaneous analysis of free amino acids and biogenic amines in ripened acid-curd cheeses. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;927:191-200.
22. Blob LF, Sharoky M, Campbell BJ, et al. Effects of a tyramine-enriched meal on blood pressure response in healthy male volunteers treated with selegiline transdermal system 6 mg/24 hour. CNS Spectr. 2007;12(1):25-34.
23. Partilla JS, Dempsey AG, Nagpal AS, et al. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther. 2006;319(1):237-246.
24. Borowsky B, Adham N, Jones KA, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98(16):8966-8971.
25. Azzaro AJ, Vandenberg CM, Blob LF, et al. Tyramine pressor sensitivity during treatment with the selegiline transdermal system 6 mg/24 h in healthy subjects. J Clin Pharmacol. 2006;46(8):933-944.
26. Azilect [package insert]. Overland Park, KS: Teva Neuroscience, Inc.; 2014.
27. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949-1962.
28. Burton TJ, Wilkinson IB. The dangers of immediate-release nifedipine in the emergency treatment of hypertension. J Hum Hypertens. 2008;22(4):301-302.
29. Yilmaz S, Pekdemir M, Tural U, et al. Comparison of alprazolam versus captopril in high blood pressure: a randomized controlled trial. Blood Press. 2011;20(4):239-243.
30. Carruthers J, Radigan M, Erlich MD, et al. An initiative to improve clozapine prescribing in New York State. Psychiatr Serv. 2016;67(4):369-371.
Dietary restrictions with MAOIs
Deutetrabenazine for tardive dyskinesia
Compared with first-generation antipsychotics, second-generation antipsychotics (SGAs) have a lower risk for extrapyramidal symptoms. Yet tardive dyskinesia (TD) remains a concern because of the widespread use of SGAs for multiple indications.1 Prior to April 2017, clinicians had no FDA-approved TD treatment options. The most widely used agent worldwide, tetrabenazine, had positive efficacy data in TD trials over the past 45 years but was not available in the United States until 2008, and its sole indication was for chorea associated with Huntington’s disease.2 Moreover, the use of tetrabenazine involved slow titration, multiple daily dosing, cytochrome P450 (CYP) 2D6 genotyping for doses >50 mg/d, and tolerability issues.
Tetrabenazine is an inhibitor of vesicular monoamine transport type 2 (VMAT2), a transport protein located almost exclusively in the CNS whose role is to place monoamine neurotransmitters (dopamine, serotonin, norepinephrine) into presynaptic vesicles. By decreasing dopamine transport into these presynaptic vesicles, synaptic dopamine release is lessened, thus reducing postsynaptic dopamine D2 receptor activity and the severity of dyskinetic movements.1
In 2 pivotal 12-week clinical trials, deutetrabenazine significantly reduced TD severity as measured by Abnormal Involuntary Movement Scale (AIMS) scores (see Efficacy).6,7
Clinical implications
TD remains a substantial public health concern due to the increasing use of antipsychotics for mood and other disorders beyond the initial indications for schizophrenia.1 Although exposure to dopamine D2antagonism results in postsynaptic receptor upregulation and supersensitivity that underlies the development of dyskinesia, this process is often rapidly reversible in animal models.1 The persistence of TD symptoms in up to 80% of patients after dopamine receptor blocking agents (DRBAs) are stopped has led to hypotheses that the underlying pathophysiology of TD is also a problem with neuroplasticity. Aside from DRBA exposure, environmental factors (eg, oxidative stress) and genetic predisposition might contribute to TD risk.1
Before 2017, only 1 medication (branched-chain amino acids) had been FDA-approved for treating TD in the United States, and only a few existing medications (clonazepam, amantadine, and ginkgo biloba extract [EGb-761]) had positive results from controlled trials, most with small effect sizes.8 Moreover, there was only 1 controlled trial each for clonazepam and EGb-761.1 A branched-chain amino acid preparation received FDA approval for managing TD in male patients, but is no longer commercially available, except from compounding pharmacies.9
Tetrabenazine was developed in the mid-1950s to avoid orthostasis and sedation associated with reserpine.10 Both reserpine and tetrabenazine proved effective for TD,11 but tetrabenazine lacked reserpine’s peripheral adverse effects. However, the kinetics of tetrabenazine necessitated multiple daily doses, and CYP2D6 genotyping was required for doses >50 mg/d.2
Receptor blocking. The mechanism that distinguishes the clinical profiles of reserpine and tetrabenazine relates to their differential properties at VMAT.12 VMAT exists in 2 forms (VMAT1 and VMAT2) that vary in distribution, with VMAT1 expressed mainly in the peripheral nervous system and VMAT2 expressed mainly in monoaminergic cells of the CNS.13 Tetrabenazine is a specific and reversible VMAT2 inhibitor, whereas reserpine is an irreversible and nonselective antagonist of VMAT1 and VMAT2. It is reserpine’s VMAT1 inhibition that results in peripheral adverse effects such as orthostasis. Tetrabenazine is rapidly and extensively converted into 2 isomers, alpha-dihydrotetrabenazine (α-DHTBZ) and beta-dihydrotetrabenazine (β-DHTBZ), both of which are metabolized by CYP2D6, with a role for CYP3A4 in α-DHTBZ metabolism.1 These DHTBZ metabolites have a short half-life when generated from oral tetrabenazine, a feature that necessitates multiple daily dosing; moreover, the existence of 2D6 polymorphisms led to FDA-mandated CYP2D6 genotyping for tetrabenazine doses >50 mg/d when it was approved for Huntington’s chorea. The concern is that 2D6 poor metabolizers will have excessive exposure to the VMAT2 effects of DHTBZ, resulting in sedation, akathisia, parkinsonism, and mood symptoms.2
How deuterium impacts medication kinetics. Deuterium is a naturally occurring, stable, nontoxic isotope of hydrogen. Humans have 5 g of deuterium in their body at any time, mostly in the form of heavy water (D2O).14 When deuterium is used to replace selected hydrogen atoms, the resulting molecule will have similar configuration and receptor-binding properties but markedly different kinetics. Because the carbon–deuterium covalent bond requires 8 times more energy to break than a carbon–hydrogen bond, the half-life is prolonged.15 Utilizing this knowledge, a deuterated form of tetrabenazine, deutetrabenazine, was synthesized with such a purpose in mind. While the active metabolites of deutetrabenazine retain the VMAT2 affinity of non-deuterated tetrabenazine, the substitution of deuterium for hydrogen at specific positions slows the breakdown of metabolites, resulting in sustained duration of action, greater active drug exposure, and less impact of 2D6 genotype on drug exposure, thus eliminating the need for genotyping, unless one wants to exceed 36 mg/d.
Deutetrabenazine was first studied in Huntington’s chorea in a 13-week, double-blind, placebo-controlled, parallel-group study (N = 90).4 The maximum daily deutetrabenazine dose was 48 mg, but reduced to 36 mg in those taking strong CYP2D6 inhibitors (bupropion, fluoxetine, or paroxetine). Blinded 2D6 genotyping was performed, but there was no dose modification required based on 2D6 genotype. There was a 36.4% reduction in total maximal chorea score for deutetrabenazine compared with 14.4% for placebo (P < .001).4 Importantly, adverse effects were comparable between both groups, with 1 drop-out in the deutetrabenazine arm vs 2 in the placebo arm. The only adverse event occurring in ≥5% of deutetrabenazine participants and at a rate ≥2 times that of placebo was somnolence: 11.1% for deutetrabenazine vs 4.4% for placebo.4 The mean deutetrabenazine daily dose at the end of the treatment period was 39.7 ± 9.3 mg, and for those with impaired CYP2D6 function (poor metabolizers or those taking strong CYP2D6 inhibiting medications), the mean daily dose was 34.8 mg ± 3.8 mg.4
Use in tardive dyskinesia. The recommended starting dosage for TD treatment is 6 mg, twice daily with food. The dose may be increased at weekly intervals in increments of 6 mg/d to a maximum recommended daily dosage of 48 mg.5 The maximum daily dose is 36 mg (18 mg, twice daily) in patients receiving strong CYP2D6 inhibitors or who are 2D6 poor metabolizers.5
Deutetrabenazine has not been studied in those with moderate or severe hepatic impairment, and its use is contraindicated in these patients.5 No clinical studies have been conducted to assess the effect of renal impairment on the pharmacokinetics of deutetrabenazine.5
Pharmacologic profile, adverse reactions
When the data from the two 12-week, phase 3 placebo-controlled studies were pooled, the most common adverse reactions occurring in >3% of deutetrabenazine patients and greater than placebo were nasopharyngeal symptoms (4% vs 2% placebo) and insomnia (4% vs 1% placebo).5 Importantly, in neither TD study were there clinically significant changes in rating scales for depression, suicidal ideation and behavior, or parkinsonism. There also were no clinically significant changes in measures of schizophrenia symptoms. The mean QT prolongation for a single 24 mg dose of deutetrabenazine in healthy volunteers was 4.5 milliseconds, with the upper bound of the double-sided 90% confidence interval reaching 6.5 milliseconds.5 For tetrabenazine, single 50 mg doses administered to volunteers resulted in mean QT prolongation of 8 milliseconds.5 In patients requiring deutetrabenazine doses >24 mg/d who are taking other medications known to prolong QTc, assess the QTc interval before and after increasing the dose of deutetrabenazine or other medications that are known to prolong QTc.5
How it works
Tetrabenazine is the only agent that has demonstrated significant efficacy for TD management, but its use involves slow titration, multiple daily dosing, CYP2D6 genotyping for doses >50 mg/d, and tolerability issues. For example, the most common adverse effects in the pivotal tetrabenazine Huntington’s disease trial were sedation/somnolence (tetrabenazine 31% vs 3% for placebo), insomnia (tetrabenazine 22% vs 0% for placebo), depression (tetrabenazine 19% vs 0% for placebo), fatigue (tetrabenazine 22% vs 13% for placebo), and akathisia (tetrabenazine 19% vs 0% for placebo).2 For comparison, the only adverse event occurring in ≥5% of deutetrabenazine participants and at a rate ≥2 times that of placebo in the pivotal Huntington’s disease trial was somnolence (11.1% for deutetrabenazine vs 4.4% for placebo).4
Pharmacokinetics
Deutetrabenazine has 80% oral bioavailability, and is rapidly converted to its active metabolites after oral dosing (Table 2).5 Linear dose dependence of Cmax and area under the curve (AUC) was observed for the active metabolites following single or multiple doses of deutetrabenazine (6 to 24 mg and 7.5 to 22.5 mg, twice daily).15 Cmax of deuterated α-DHTBZ and β-DHTBZ is reached within 3 to 4 hours after dosing, with a steady state ratio of 3:1 for the α-DHTBZ vs the β-DHTBZ form. Food had no effect on AUC, but did increase Cmax by 50%.5
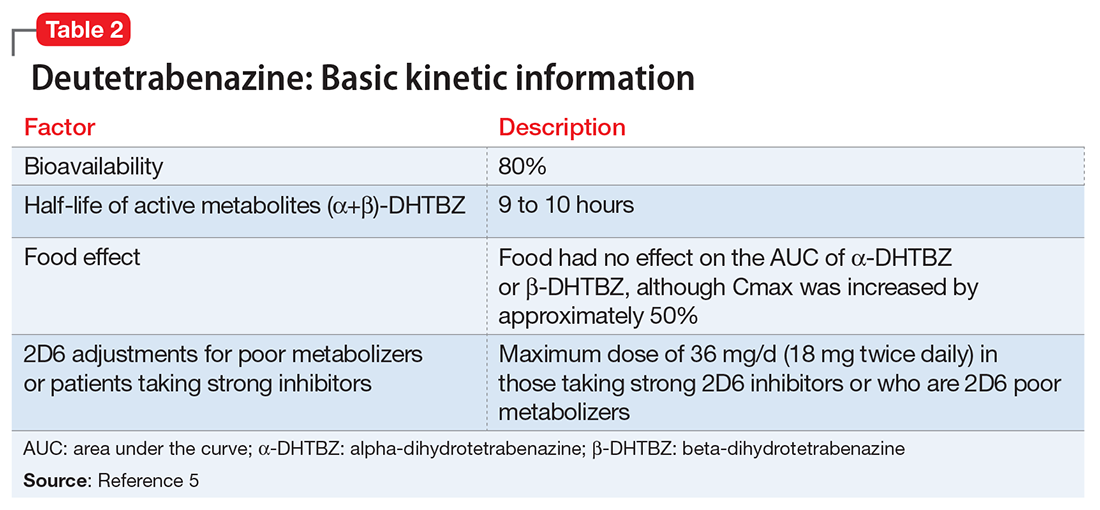
Deutetrabenazine is metabolized through carbonyl reductase enzymes to its active metabolites, and these are further metabolized through multiple CYP pathways, predominantly 2D6 and to a lesser extent 3A4. The effect of CYP2D6 inhibition on the pharmacokinetics of deutetrabenazine and its α-DHTBZ and β-DHTBZ metabolites was studied in 24 healthy participants following a single 22.5 mg dose of deutetrabenazine given after 8 days of administration of the strong CYP2D6 inhibitor paroxetine, 20 mg/d. In the presence of paroxetine, systemic exposure (AUC) of α-DHTBZ was 1.9-fold higher and β-DHTBZ was 6.5-fold higher, resulting in an approximately 3-fold increase in AUC for total (α+β)-DHTBZ, with corresponding increases in mean half-life of approximately 1.5-fold and 2.7-fold, respectively.5 Neither deutetrabenazine or its metabolites are inhibitors or inducers of major CYP enzymes. Aside from VMAT2, the results of in vitro studies suggest that deutetrabenazine and its active metabolites are unlikely to inhibit most major drug transporters at clinically relevant concentrations.
Efficacy
Efficacy was established in two 12-week, double-blind, placebo-controlled trials of adult patients with TD (ages 18 to 80).6,7 Eligible participants had:
- TD diagnosis for ≥3 months before screening and a history of DRBA treatment for ≥3 months (≥1 month if age ≥60)
- Total AIMS motor score ≥6 (items 1 to 7) at both screening and baseline, verified by a blinded central rater at screening via central video rating
- Patients with an underlying psychiatric illness had to be stable. Psychoactive medication use, including antipsychotics, was allowed if stable for ≥30 days before screening (antidepressants, ≥45 days).
Exclusion criteria included treatment with tetrabenazine, reserpine, α-methyl-p-tyrosine, strong anticholinergic medications, dopamine antagonizing antiemetics (eg, metoclopramide, prochlorperazine, promethazine), dopamine agonists, levodopa, stimulants, or a monoamine oxidase inhibitor (MAOI) within 30 days of screening or baseline, or treatment with botulinum toxin within 3 months of screening; and presence of a neurologic condition that could confound TD assessments, serious untreated or undertreated psychiatric illness, or unstable medical illness. Patients with a history of or active suicidal ideation or behavior within 6 months of screening or score ≥11 on the depression subscale of the Hospital Anxiety and Depression Scale were excluded. Those participants with Fridericia-corrected QT interval values >450 milliseconds in men, >460 milliseconds in women, or >480 milliseconds in patients with a right bundle branch block on electrocardiography at screening also were excluded.
The flexible-dose TD study was performed in 117 participants randomized in a 1:1 manner to deutetrabenazine or placebo, both administered twice daily, titrated to optimal dosage (12 to 48 mg/d) over 6 weeks, and then administered at that dose for another 6 weeks.7 The population demographics were: mean age, 54.6 ± 10.3 years, 52.1% female, 69.2% white, and 80.3% receiving ongoing dopamine antagonists, with a mean TD duration of 74.7 ± 81.5 months. Sixty-eight percent had schizophrenia spectrum disorders, and 30% had mood disorders. The primary outcome was change in total AIMS score (items 1 to 7) assessed by central, independent raters. The mean baseline AIMS score for items 1 to 7 was 9.6 ± 3.9, with 82.9% of participants with baseline AIMS scores ≥6. Study treatment retention was high: placebo 88.1%, deutetrabenazine 89.7%.7 There was a mean 3 point decrease in AIMS score for deutetrabenazine compared with 1.4 for placebo (P = .019). Among those with baseline AIMS scores ≥6, there was a 3.4 point decrease in AIMS scores for deutetrabenazine compared with a 1.9 point decrease for placebo (P = .027). The only adverse effects that occurred in ≥5% of deutetrabenazine participants and at a rate ≥2 times the rate in placebo were insomnia (deutetrabenazine 6.9% vs placebo 1.7%) and akathisia (deutetrabenazine 5.2% vs placebo 0%).
The fixed-dose TD study was performed in 293 participants randomized in 1:1:1:1 manner to 1 of 3 fixed doses of deutetrabenazine (12 mg/d, 24 mg/d, or 36 mg/d) or placebo, both administered twice daily.6 The starting dose of deutetrabenazine was 6 mg twice daily. During the dose escalation period (through Week 4), the dose of study drug was increased weekly in increments of 6 mg/d until the randomized dose was achieved. Patients continued to receive the dose they were assigned to over a maintenance period of 8 weeks.6 The population demographics were: mean age, 56.4 ± 11.3 years, 55% female, 79% white, 76% receiving ongoing dopamine antagonists, with a mean TD duration of 67.2 ± 66 months. Sixty percent had schizophrenia spectrum disorders, and 36% had mood disorders. The primary outcome was change in AIMS total score (items 1 to 7) assessed by central, independent raters. The mean AIMS score at baseline was 9.5 ± 2.7 in the placebo group, and for deutetrabenazine: 9.6 ± 2.4 in the 12 mg/d group, 9.4 ± 2.9 in the 24 mg/d group, and 10.1 ± 3.2 in the 36 mg/d group. The 24 mg/d and 36 mg/d doses significantly reduced AIMS scores from baseline vs placebo: 36 mg: −3.3 (0.42) vs −1.4 (0.41) (P = .001); 24 mg: −3.2 (0.45) vs −1.4 (0.41) (P = .003). Study treatment retention rates were high: placebo 90.5%, deutetrabenazine 88%. Across all doses, only 1 adverse effect occurred in ≥5% of deutetrabenazine participants: headache (5% deutetrabenazine vs 6% placebo). At the highest dose, 36 mg/d, the only adverse effects that occurred in ≥5% of participants were diarrhea (7% deutetrabenazine vs 3% placebo) and headache (7% deutetrabenazine vs 6% placebo).
Outcome. In the flexible-dose study (mean dose 38.8 ± 7.92 mg/d), the deutetrabenazine arm experienced a mean 30% reduction in AIMS scores from baseline at the Week 12 endpoint. Compared with placebo, the mean reduction in AIMS scores (standard error) was: −3.0 (0.45) deutetrabenazine vs −1.6 (0.46) placebo (P = .019).7 For the fixed-dose study, the 24 mg/d and 36 mg/d doses significantly reduced AIMS scores from baseline vs placebo: 36 mg: −3.3 (0.42) vs −1.4 (0.41) (P = .001); 24 mg: −3.2 (0.45) vs −1.4 (0.41) (P = .003). In addition to these mean changes from baseline, 35% of the 24 mg/d group and 33% of the 36 mg/d group demonstrated ≥50% reduction in AIMS scores.6
Tolerability
In the 2 phase 3 trials, there were no adverse effects occurring with an incidence ≥5% and at least twice the rate of placebo.5 Discontinuations because of adverse events were low in both pivotal studies across all treatment groups: 3.4% for placebo vs 1.7% for deutetrabenazine in the flexible-dose trial,7 and 3% for placebo vs 4% for deutetrabenazine in the fixed-dose study.6 In neither trial were there clinically significant changes in ratings of depression, suicidality, parkinsonism, or schizophrenia symptoms. The mean QT prolongation in healthy volunteers is described above.
Clinical considerations
Unique properties. Deutetrabenazine utilizes the greater bond strength of the carbon–deuterium bond to slow CYP metabolism, resulting in prolonged duration of action that is well tolerated, and provides significant efficacy.
Why Rx? The reasons to prescribe deutetrabenazine for TD patients include:
- only 1 of 2 agents with FDA approval for TD
- fewer tolerability issues than with tetrabenazine
- lower sedation rates in TD trials than with valbenazine
- no signal for effects on mood parameters or rates of parkinsonism when used for TD.
Dosing
The recommended starting dosage of deutetrabenazine is 6 mg twice daily taken with food, increasing by 6 mg/d weekly as needed, with a maximum dose of 48 mg/d or 36 mg/d in those taking strong CYP2D6 inhibitors or who are 2D6 poor metabolizers. Deutetrabenazine is contraindicated in patients with hepatic impairment (as determined by Child-Pugh criteria16). There are no data in patients with renal impairment. The combined efficacy and tolerability of dosages >48 mg/d has not been evaluated. Overdoses of tetrabenazine ranging from 100 to 1,000 mg have been reported in the literature and were associated with acute dystonia, oculogyric crisis, nausea and vomiting, sweating, sedation, hypotension, confusion, diarrhea, hallucinations, rubor, and tremor.5
Contraindications
When used for TD, deutetrabenazine is contraindicated for patients taking reserpine, tetrabenazine, valbenazine, or MAOIs, and for patients with hepatic impairment. As with most medications, there are no data on deutetrabenazine use in pregnant women; however, oral administration of deutetrabenazine (5, 10, or 30 mg/kg/d) or tetrabenazine (30 mg/kg/d) to pregnant rats during organogenesis had no clear effect on embryofetal development. The highest dose tested was 6 times the maximum recommended human dose of 48 mg/d on a body surface area (mg/m2) basis. There are no data on the presence of deutetrabenazine or its metabolites in human milk, the effects on the breastfed infant, or the effects of the drug on milk production.
1. Meyer JM. Forgotten but not gone: new developments in the understanding and treatment of tardive dyskinesia. CNS Spectr. 2016;21(S1):13-24.
2. Jankovic J, Clarence-Smith K. Tetrabenazine for the treatment of chorea and other hyperkinetic movement disorders. Expert Rev Neurother. 2011;11(11):1509-1523.
3. Meyer JM. Valbenazine for tardive dyskinesia. Current Psychiatry. 2017;16(5):40-46.
4. Huntington Study Group; Frank S, Testa CM, Stamler D, et al. Effect of deutetrabenazine on chorea among patients with Huntington disease: a randomized clinical trial. JAMA. 2016;316(1):40-50.
5. Austedo [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc.; 2017.
6. Anderson KE, Stamler D, Davis MD, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Psychiatry. 2017;4(8):595-604.
7. Fernandez HH, Factor SA, Hauser RA, et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia: the ARM-TD study. Neurology. 2017;88(21):2003-2010.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Richardson MA, Small AM, Read LL, et al. Branched chain amino acid treatment of tardive dyskinesia in children and adolescents. J Clin Psychiatry. 2004;65(1):92-96.
10. Quinn GP, Shore PA, Brodie BB. Biochemical and pharmacological studies of RO 1-9569 (tetrabenazine), a nonindole tranquilizing agent with reserpine-like effects. J Pharmacol Exp Ther. 1959;127:103-109.
11. Kazamatsuri H, Chien C, Cole JO. Treatment of tardive dyskinesia. I. Clinical efficacy of a dopamine-depleting agent, tetrabenazine. Arch Gen Psychiatry. 1972;27(1):95-99.
12. Scherman D, Weber MJ. Characterization of the vesicular monoamine transporter in cultured rat sympathetic neurons: persistence upon induction of cholinergic phenotypic traits. Dev Biol. 1987;119(1):68-74.
13. Erickson JD, Schafer MK, Bonner TI, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93(10):5166-5171.
14. Kushner DJ, Baker A, Dunstall TG. Pharmacological uses and perspectives of heavy water and deuterated compounds. Can J Physiol Pharmacol. 1999;77(2):79-88.
15. United States Securities and Exchange Commission. Form S-1 Registration Statement of Auspex Pharmaceuticals, Inc. https://www.sec.gov/Archives/edgar/data/1454189/000119312513481239/d627086ds1.htm. Published December 20, 2013. Accessed July 1, 2016.
16. Cholongitas E, Papatheodoridis GV, Vangeli M, et al. Systematic review: the model for end-stage liver disease—should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22(11-12):1079-1089.
Compared with first-generation antipsychotics, second-generation antipsychotics (SGAs) have a lower risk for extrapyramidal symptoms. Yet tardive dyskinesia (TD) remains a concern because of the widespread use of SGAs for multiple indications.1 Prior to April 2017, clinicians had no FDA-approved TD treatment options. The most widely used agent worldwide, tetrabenazine, had positive efficacy data in TD trials over the past 45 years but was not available in the United States until 2008, and its sole indication was for chorea associated with Huntington’s disease.2 Moreover, the use of tetrabenazine involved slow titration, multiple daily dosing, cytochrome P450 (CYP) 2D6 genotyping for doses >50 mg/d, and tolerability issues.
Tetrabenazine is an inhibitor of vesicular monoamine transport type 2 (VMAT2), a transport protein located almost exclusively in the CNS whose role is to place monoamine neurotransmitters (dopamine, serotonin, norepinephrine) into presynaptic vesicles. By decreasing dopamine transport into these presynaptic vesicles, synaptic dopamine release is lessened, thus reducing postsynaptic dopamine D2 receptor activity and the severity of dyskinetic movements.1
In 2 pivotal 12-week clinical trials, deutetrabenazine significantly reduced TD severity as measured by Abnormal Involuntary Movement Scale (AIMS) scores (see Efficacy).6,7
Clinical implications
TD remains a substantial public health concern due to the increasing use of antipsychotics for mood and other disorders beyond the initial indications for schizophrenia.1 Although exposure to dopamine D2antagonism results in postsynaptic receptor upregulation and supersensitivity that underlies the development of dyskinesia, this process is often rapidly reversible in animal models.1 The persistence of TD symptoms in up to 80% of patients after dopamine receptor blocking agents (DRBAs) are stopped has led to hypotheses that the underlying pathophysiology of TD is also a problem with neuroplasticity. Aside from DRBA exposure, environmental factors (eg, oxidative stress) and genetic predisposition might contribute to TD risk.1
Before 2017, only 1 medication (branched-chain amino acids) had been FDA-approved for treating TD in the United States, and only a few existing medications (clonazepam, amantadine, and ginkgo biloba extract [EGb-761]) had positive results from controlled trials, most with small effect sizes.8 Moreover, there was only 1 controlled trial each for clonazepam and EGb-761.1 A branched-chain amino acid preparation received FDA approval for managing TD in male patients, but is no longer commercially available, except from compounding pharmacies.9
Tetrabenazine was developed in the mid-1950s to avoid orthostasis and sedation associated with reserpine.10 Both reserpine and tetrabenazine proved effective for TD,11 but tetrabenazine lacked reserpine’s peripheral adverse effects. However, the kinetics of tetrabenazine necessitated multiple daily doses, and CYP2D6 genotyping was required for doses >50 mg/d.2
Receptor blocking. The mechanism that distinguishes the clinical profiles of reserpine and tetrabenazine relates to their differential properties at VMAT.12 VMAT exists in 2 forms (VMAT1 and VMAT2) that vary in distribution, with VMAT1 expressed mainly in the peripheral nervous system and VMAT2 expressed mainly in monoaminergic cells of the CNS.13 Tetrabenazine is a specific and reversible VMAT2 inhibitor, whereas reserpine is an irreversible and nonselective antagonist of VMAT1 and VMAT2. It is reserpine’s VMAT1 inhibition that results in peripheral adverse effects such as orthostasis. Tetrabenazine is rapidly and extensively converted into 2 isomers, alpha-dihydrotetrabenazine (α-DHTBZ) and beta-dihydrotetrabenazine (β-DHTBZ), both of which are metabolized by CYP2D6, with a role for CYP3A4 in α-DHTBZ metabolism.1 These DHTBZ metabolites have a short half-life when generated from oral tetrabenazine, a feature that necessitates multiple daily dosing; moreover, the existence of 2D6 polymorphisms led to FDA-mandated CYP2D6 genotyping for tetrabenazine doses >50 mg/d when it was approved for Huntington’s chorea. The concern is that 2D6 poor metabolizers will have excessive exposure to the VMAT2 effects of DHTBZ, resulting in sedation, akathisia, parkinsonism, and mood symptoms.2
How deuterium impacts medication kinetics. Deuterium is a naturally occurring, stable, nontoxic isotope of hydrogen. Humans have 5 g of deuterium in their body at any time, mostly in the form of heavy water (D2O).14 When deuterium is used to replace selected hydrogen atoms, the resulting molecule will have similar configuration and receptor-binding properties but markedly different kinetics. Because the carbon–deuterium covalent bond requires 8 times more energy to break than a carbon–hydrogen bond, the half-life is prolonged.15 Utilizing this knowledge, a deuterated form of tetrabenazine, deutetrabenazine, was synthesized with such a purpose in mind. While the active metabolites of deutetrabenazine retain the VMAT2 affinity of non-deuterated tetrabenazine, the substitution of deuterium for hydrogen at specific positions slows the breakdown of metabolites, resulting in sustained duration of action, greater active drug exposure, and less impact of 2D6 genotype on drug exposure, thus eliminating the need for genotyping, unless one wants to exceed 36 mg/d.
Deutetrabenazine was first studied in Huntington’s chorea in a 13-week, double-blind, placebo-controlled, parallel-group study (N = 90).4 The maximum daily deutetrabenazine dose was 48 mg, but reduced to 36 mg in those taking strong CYP2D6 inhibitors (bupropion, fluoxetine, or paroxetine). Blinded 2D6 genotyping was performed, but there was no dose modification required based on 2D6 genotype. There was a 36.4% reduction in total maximal chorea score for deutetrabenazine compared with 14.4% for placebo (P < .001).4 Importantly, adverse effects were comparable between both groups, with 1 drop-out in the deutetrabenazine arm vs 2 in the placebo arm. The only adverse event occurring in ≥5% of deutetrabenazine participants and at a rate ≥2 times that of placebo was somnolence: 11.1% for deutetrabenazine vs 4.4% for placebo.4 The mean deutetrabenazine daily dose at the end of the treatment period was 39.7 ± 9.3 mg, and for those with impaired CYP2D6 function (poor metabolizers or those taking strong CYP2D6 inhibiting medications), the mean daily dose was 34.8 mg ± 3.8 mg.4
Use in tardive dyskinesia. The recommended starting dosage for TD treatment is 6 mg, twice daily with food. The dose may be increased at weekly intervals in increments of 6 mg/d to a maximum recommended daily dosage of 48 mg.5 The maximum daily dose is 36 mg (18 mg, twice daily) in patients receiving strong CYP2D6 inhibitors or who are 2D6 poor metabolizers.5
Deutetrabenazine has not been studied in those with moderate or severe hepatic impairment, and its use is contraindicated in these patients.5 No clinical studies have been conducted to assess the effect of renal impairment on the pharmacokinetics of deutetrabenazine.5
Pharmacologic profile, adverse reactions
When the data from the two 12-week, phase 3 placebo-controlled studies were pooled, the most common adverse reactions occurring in >3% of deutetrabenazine patients and greater than placebo were nasopharyngeal symptoms (4% vs 2% placebo) and insomnia (4% vs 1% placebo).5 Importantly, in neither TD study were there clinically significant changes in rating scales for depression, suicidal ideation and behavior, or parkinsonism. There also were no clinically significant changes in measures of schizophrenia symptoms. The mean QT prolongation for a single 24 mg dose of deutetrabenazine in healthy volunteers was 4.5 milliseconds, with the upper bound of the double-sided 90% confidence interval reaching 6.5 milliseconds.5 For tetrabenazine, single 50 mg doses administered to volunteers resulted in mean QT prolongation of 8 milliseconds.5 In patients requiring deutetrabenazine doses >24 mg/d who are taking other medications known to prolong QTc, assess the QTc interval before and after increasing the dose of deutetrabenazine or other medications that are known to prolong QTc.5
How it works
Tetrabenazine is the only agent that has demonstrated significant efficacy for TD management, but its use involves slow titration, multiple daily dosing, CYP2D6 genotyping for doses >50 mg/d, and tolerability issues. For example, the most common adverse effects in the pivotal tetrabenazine Huntington’s disease trial were sedation/somnolence (tetrabenazine 31% vs 3% for placebo), insomnia (tetrabenazine 22% vs 0% for placebo), depression (tetrabenazine 19% vs 0% for placebo), fatigue (tetrabenazine 22% vs 13% for placebo), and akathisia (tetrabenazine 19% vs 0% for placebo).2 For comparison, the only adverse event occurring in ≥5% of deutetrabenazine participants and at a rate ≥2 times that of placebo in the pivotal Huntington’s disease trial was somnolence (11.1% for deutetrabenazine vs 4.4% for placebo).4
Pharmacokinetics
Deutetrabenazine has 80% oral bioavailability, and is rapidly converted to its active metabolites after oral dosing (Table 2).5 Linear dose dependence of Cmax and area under the curve (AUC) was observed for the active metabolites following single or multiple doses of deutetrabenazine (6 to 24 mg and 7.5 to 22.5 mg, twice daily).15 Cmax of deuterated α-DHTBZ and β-DHTBZ is reached within 3 to 4 hours after dosing, with a steady state ratio of 3:1 for the α-DHTBZ vs the β-DHTBZ form. Food had no effect on AUC, but did increase Cmax by 50%.5

Deutetrabenazine is metabolized through carbonyl reductase enzymes to its active metabolites, and these are further metabolized through multiple CYP pathways, predominantly 2D6 and to a lesser extent 3A4. The effect of CYP2D6 inhibition on the pharmacokinetics of deutetrabenazine and its α-DHTBZ and β-DHTBZ metabolites was studied in 24 healthy participants following a single 22.5 mg dose of deutetrabenazine given after 8 days of administration of the strong CYP2D6 inhibitor paroxetine, 20 mg/d. In the presence of paroxetine, systemic exposure (AUC) of α-DHTBZ was 1.9-fold higher and β-DHTBZ was 6.5-fold higher, resulting in an approximately 3-fold increase in AUC for total (α+β)-DHTBZ, with corresponding increases in mean half-life of approximately 1.5-fold and 2.7-fold, respectively.5 Neither deutetrabenazine or its metabolites are inhibitors or inducers of major CYP enzymes. Aside from VMAT2, the results of in vitro studies suggest that deutetrabenazine and its active metabolites are unlikely to inhibit most major drug transporters at clinically relevant concentrations.
Efficacy
Efficacy was established in two 12-week, double-blind, placebo-controlled trials of adult patients with TD (ages 18 to 80).6,7 Eligible participants had:
- TD diagnosis for ≥3 months before screening and a history of DRBA treatment for ≥3 months (≥1 month if age ≥60)
- Total AIMS motor score ≥6 (items 1 to 7) at both screening and baseline, verified by a blinded central rater at screening via central video rating
- Patients with an underlying psychiatric illness had to be stable. Psychoactive medication use, including antipsychotics, was allowed if stable for ≥30 days before screening (antidepressants, ≥45 days).
Exclusion criteria included treatment with tetrabenazine, reserpine, α-methyl-p-tyrosine, strong anticholinergic medications, dopamine antagonizing antiemetics (eg, metoclopramide, prochlorperazine, promethazine), dopamine agonists, levodopa, stimulants, or a monoamine oxidase inhibitor (MAOI) within 30 days of screening or baseline, or treatment with botulinum toxin within 3 months of screening; and presence of a neurologic condition that could confound TD assessments, serious untreated or undertreated psychiatric illness, or unstable medical illness. Patients with a history of or active suicidal ideation or behavior within 6 months of screening or score ≥11 on the depression subscale of the Hospital Anxiety and Depression Scale were excluded. Those participants with Fridericia-corrected QT interval values >450 milliseconds in men, >460 milliseconds in women, or >480 milliseconds in patients with a right bundle branch block on electrocardiography at screening also were excluded.
The flexible-dose TD study was performed in 117 participants randomized in a 1:1 manner to deutetrabenazine or placebo, both administered twice daily, titrated to optimal dosage (12 to 48 mg/d) over 6 weeks, and then administered at that dose for another 6 weeks.7 The population demographics were: mean age, 54.6 ± 10.3 years, 52.1% female, 69.2% white, and 80.3% receiving ongoing dopamine antagonists, with a mean TD duration of 74.7 ± 81.5 months. Sixty-eight percent had schizophrenia spectrum disorders, and 30% had mood disorders. The primary outcome was change in total AIMS score (items 1 to 7) assessed by central, independent raters. The mean baseline AIMS score for items 1 to 7 was 9.6 ± 3.9, with 82.9% of participants with baseline AIMS scores ≥6. Study treatment retention was high: placebo 88.1%, deutetrabenazine 89.7%.7 There was a mean 3 point decrease in AIMS score for deutetrabenazine compared with 1.4 for placebo (P = .019). Among those with baseline AIMS scores ≥6, there was a 3.4 point decrease in AIMS scores for deutetrabenazine compared with a 1.9 point decrease for placebo (P = .027). The only adverse effects that occurred in ≥5% of deutetrabenazine participants and at a rate ≥2 times the rate in placebo were insomnia (deutetrabenazine 6.9% vs placebo 1.7%) and akathisia (deutetrabenazine 5.2% vs placebo 0%).
The fixed-dose TD study was performed in 293 participants randomized in 1:1:1:1 manner to 1 of 3 fixed doses of deutetrabenazine (12 mg/d, 24 mg/d, or 36 mg/d) or placebo, both administered twice daily.6 The starting dose of deutetrabenazine was 6 mg twice daily. During the dose escalation period (through Week 4), the dose of study drug was increased weekly in increments of 6 mg/d until the randomized dose was achieved. Patients continued to receive the dose they were assigned to over a maintenance period of 8 weeks.6 The population demographics were: mean age, 56.4 ± 11.3 years, 55% female, 79% white, 76% receiving ongoing dopamine antagonists, with a mean TD duration of 67.2 ± 66 months. Sixty percent had schizophrenia spectrum disorders, and 36% had mood disorders. The primary outcome was change in AIMS total score (items 1 to 7) assessed by central, independent raters. The mean AIMS score at baseline was 9.5 ± 2.7 in the placebo group, and for deutetrabenazine: 9.6 ± 2.4 in the 12 mg/d group, 9.4 ± 2.9 in the 24 mg/d group, and 10.1 ± 3.2 in the 36 mg/d group. The 24 mg/d and 36 mg/d doses significantly reduced AIMS scores from baseline vs placebo: 36 mg: −3.3 (0.42) vs −1.4 (0.41) (P = .001); 24 mg: −3.2 (0.45) vs −1.4 (0.41) (P = .003). Study treatment retention rates were high: placebo 90.5%, deutetrabenazine 88%. Across all doses, only 1 adverse effect occurred in ≥5% of deutetrabenazine participants: headache (5% deutetrabenazine vs 6% placebo). At the highest dose, 36 mg/d, the only adverse effects that occurred in ≥5% of participants were diarrhea (7% deutetrabenazine vs 3% placebo) and headache (7% deutetrabenazine vs 6% placebo).
Outcome. In the flexible-dose study (mean dose 38.8 ± 7.92 mg/d), the deutetrabenazine arm experienced a mean 30% reduction in AIMS scores from baseline at the Week 12 endpoint. Compared with placebo, the mean reduction in AIMS scores (standard error) was: −3.0 (0.45) deutetrabenazine vs −1.6 (0.46) placebo (P = .019).7 For the fixed-dose study, the 24 mg/d and 36 mg/d doses significantly reduced AIMS scores from baseline vs placebo: 36 mg: −3.3 (0.42) vs −1.4 (0.41) (P = .001); 24 mg: −3.2 (0.45) vs −1.4 (0.41) (P = .003). In addition to these mean changes from baseline, 35% of the 24 mg/d group and 33% of the 36 mg/d group demonstrated ≥50% reduction in AIMS scores.6
Tolerability
In the 2 phase 3 trials, there were no adverse effects occurring with an incidence ≥5% and at least twice the rate of placebo.5 Discontinuations because of adverse events were low in both pivotal studies across all treatment groups: 3.4% for placebo vs 1.7% for deutetrabenazine in the flexible-dose trial,7 and 3% for placebo vs 4% for deutetrabenazine in the fixed-dose study.6 In neither trial were there clinically significant changes in ratings of depression, suicidality, parkinsonism, or schizophrenia symptoms. The mean QT prolongation in healthy volunteers is described above.
Clinical considerations
Unique properties. Deutetrabenazine utilizes the greater bond strength of the carbon–deuterium bond to slow CYP metabolism, resulting in prolonged duration of action that is well tolerated, and provides significant efficacy.
Why Rx? The reasons to prescribe deutetrabenazine for TD patients include:
- only 1 of 2 agents with FDA approval for TD
- fewer tolerability issues than with tetrabenazine
- lower sedation rates in TD trials than with valbenazine
- no signal for effects on mood parameters or rates of parkinsonism when used for TD.
Dosing
The recommended starting dosage of deutetrabenazine is 6 mg twice daily taken with food, increasing by 6 mg/d weekly as needed, with a maximum dose of 48 mg/d or 36 mg/d in those taking strong CYP2D6 inhibitors or who are 2D6 poor metabolizers. Deutetrabenazine is contraindicated in patients with hepatic impairment (as determined by Child-Pugh criteria16). There are no data in patients with renal impairment. The combined efficacy and tolerability of dosages >48 mg/d has not been evaluated. Overdoses of tetrabenazine ranging from 100 to 1,000 mg have been reported in the literature and were associated with acute dystonia, oculogyric crisis, nausea and vomiting, sweating, sedation, hypotension, confusion, diarrhea, hallucinations, rubor, and tremor.5
Contraindications
When used for TD, deutetrabenazine is contraindicated for patients taking reserpine, tetrabenazine, valbenazine, or MAOIs, and for patients with hepatic impairment. As with most medications, there are no data on deutetrabenazine use in pregnant women; however, oral administration of deutetrabenazine (5, 10, or 30 mg/kg/d) or tetrabenazine (30 mg/kg/d) to pregnant rats during organogenesis had no clear effect on embryofetal development. The highest dose tested was 6 times the maximum recommended human dose of 48 mg/d on a body surface area (mg/m2) basis. There are no data on the presence of deutetrabenazine or its metabolites in human milk, the effects on the breastfed infant, or the effects of the drug on milk production.
Compared with first-generation antipsychotics, second-generation antipsychotics (SGAs) have a lower risk for extrapyramidal symptoms. Yet tardive dyskinesia (TD) remains a concern because of the widespread use of SGAs for multiple indications.1 Prior to April 2017, clinicians had no FDA-approved TD treatment options. The most widely used agent worldwide, tetrabenazine, had positive efficacy data in TD trials over the past 45 years but was not available in the United States until 2008, and its sole indication was for chorea associated with Huntington’s disease.2 Moreover, the use of tetrabenazine involved slow titration, multiple daily dosing, cytochrome P450 (CYP) 2D6 genotyping for doses >50 mg/d, and tolerability issues.
Tetrabenazine is an inhibitor of vesicular monoamine transport type 2 (VMAT2), a transport protein located almost exclusively in the CNS whose role is to place monoamine neurotransmitters (dopamine, serotonin, norepinephrine) into presynaptic vesicles. By decreasing dopamine transport into these presynaptic vesicles, synaptic dopamine release is lessened, thus reducing postsynaptic dopamine D2 receptor activity and the severity of dyskinetic movements.1
In 2 pivotal 12-week clinical trials, deutetrabenazine significantly reduced TD severity as measured by Abnormal Involuntary Movement Scale (AIMS) scores (see Efficacy).6,7
Clinical implications
TD remains a substantial public health concern due to the increasing use of antipsychotics for mood and other disorders beyond the initial indications for schizophrenia.1 Although exposure to dopamine D2antagonism results in postsynaptic receptor upregulation and supersensitivity that underlies the development of dyskinesia, this process is often rapidly reversible in animal models.1 The persistence of TD symptoms in up to 80% of patients after dopamine receptor blocking agents (DRBAs) are stopped has led to hypotheses that the underlying pathophysiology of TD is also a problem with neuroplasticity. Aside from DRBA exposure, environmental factors (eg, oxidative stress) and genetic predisposition might contribute to TD risk.1
Before 2017, only 1 medication (branched-chain amino acids) had been FDA-approved for treating TD in the United States, and only a few existing medications (clonazepam, amantadine, and ginkgo biloba extract [EGb-761]) had positive results from controlled trials, most with small effect sizes.8 Moreover, there was only 1 controlled trial each for clonazepam and EGb-761.1 A branched-chain amino acid preparation received FDA approval for managing TD in male patients, but is no longer commercially available, except from compounding pharmacies.9
Tetrabenazine was developed in the mid-1950s to avoid orthostasis and sedation associated with reserpine.10 Both reserpine and tetrabenazine proved effective for TD,11 but tetrabenazine lacked reserpine’s peripheral adverse effects. However, the kinetics of tetrabenazine necessitated multiple daily doses, and CYP2D6 genotyping was required for doses >50 mg/d.2
Receptor blocking. The mechanism that distinguishes the clinical profiles of reserpine and tetrabenazine relates to their differential properties at VMAT.12 VMAT exists in 2 forms (VMAT1 and VMAT2) that vary in distribution, with VMAT1 expressed mainly in the peripheral nervous system and VMAT2 expressed mainly in monoaminergic cells of the CNS.13 Tetrabenazine is a specific and reversible VMAT2 inhibitor, whereas reserpine is an irreversible and nonselective antagonist of VMAT1 and VMAT2. It is reserpine’s VMAT1 inhibition that results in peripheral adverse effects such as orthostasis. Tetrabenazine is rapidly and extensively converted into 2 isomers, alpha-dihydrotetrabenazine (α-DHTBZ) and beta-dihydrotetrabenazine (β-DHTBZ), both of which are metabolized by CYP2D6, with a role for CYP3A4 in α-DHTBZ metabolism.1 These DHTBZ metabolites have a short half-life when generated from oral tetrabenazine, a feature that necessitates multiple daily dosing; moreover, the existence of 2D6 polymorphisms led to FDA-mandated CYP2D6 genotyping for tetrabenazine doses >50 mg/d when it was approved for Huntington’s chorea. The concern is that 2D6 poor metabolizers will have excessive exposure to the VMAT2 effects of DHTBZ, resulting in sedation, akathisia, parkinsonism, and mood symptoms.2
How deuterium impacts medication kinetics. Deuterium is a naturally occurring, stable, nontoxic isotope of hydrogen. Humans have 5 g of deuterium in their body at any time, mostly in the form of heavy water (D2O).14 When deuterium is used to replace selected hydrogen atoms, the resulting molecule will have similar configuration and receptor-binding properties but markedly different kinetics. Because the carbon–deuterium covalent bond requires 8 times more energy to break than a carbon–hydrogen bond, the half-life is prolonged.15 Utilizing this knowledge, a deuterated form of tetrabenazine, deutetrabenazine, was synthesized with such a purpose in mind. While the active metabolites of deutetrabenazine retain the VMAT2 affinity of non-deuterated tetrabenazine, the substitution of deuterium for hydrogen at specific positions slows the breakdown of metabolites, resulting in sustained duration of action, greater active drug exposure, and less impact of 2D6 genotype on drug exposure, thus eliminating the need for genotyping, unless one wants to exceed 36 mg/d.
Deutetrabenazine was first studied in Huntington’s chorea in a 13-week, double-blind, placebo-controlled, parallel-group study (N = 90).4 The maximum daily deutetrabenazine dose was 48 mg, but reduced to 36 mg in those taking strong CYP2D6 inhibitors (bupropion, fluoxetine, or paroxetine). Blinded 2D6 genotyping was performed, but there was no dose modification required based on 2D6 genotype. There was a 36.4% reduction in total maximal chorea score for deutetrabenazine compared with 14.4% for placebo (P < .001).4 Importantly, adverse effects were comparable between both groups, with 1 drop-out in the deutetrabenazine arm vs 2 in the placebo arm. The only adverse event occurring in ≥5% of deutetrabenazine participants and at a rate ≥2 times that of placebo was somnolence: 11.1% for deutetrabenazine vs 4.4% for placebo.4 The mean deutetrabenazine daily dose at the end of the treatment period was 39.7 ± 9.3 mg, and for those with impaired CYP2D6 function (poor metabolizers or those taking strong CYP2D6 inhibiting medications), the mean daily dose was 34.8 mg ± 3.8 mg.4
Use in tardive dyskinesia. The recommended starting dosage for TD treatment is 6 mg, twice daily with food. The dose may be increased at weekly intervals in increments of 6 mg/d to a maximum recommended daily dosage of 48 mg.5 The maximum daily dose is 36 mg (18 mg, twice daily) in patients receiving strong CYP2D6 inhibitors or who are 2D6 poor metabolizers.5
Deutetrabenazine has not been studied in those with moderate or severe hepatic impairment, and its use is contraindicated in these patients.5 No clinical studies have been conducted to assess the effect of renal impairment on the pharmacokinetics of deutetrabenazine.5
Pharmacologic profile, adverse reactions
When the data from the two 12-week, phase 3 placebo-controlled studies were pooled, the most common adverse reactions occurring in >3% of deutetrabenazine patients and greater than placebo were nasopharyngeal symptoms (4% vs 2% placebo) and insomnia (4% vs 1% placebo).5 Importantly, in neither TD study were there clinically significant changes in rating scales for depression, suicidal ideation and behavior, or parkinsonism. There also were no clinically significant changes in measures of schizophrenia symptoms. The mean QT prolongation for a single 24 mg dose of deutetrabenazine in healthy volunteers was 4.5 milliseconds, with the upper bound of the double-sided 90% confidence interval reaching 6.5 milliseconds.5 For tetrabenazine, single 50 mg doses administered to volunteers resulted in mean QT prolongation of 8 milliseconds.5 In patients requiring deutetrabenazine doses >24 mg/d who are taking other medications known to prolong QTc, assess the QTc interval before and after increasing the dose of deutetrabenazine or other medications that are known to prolong QTc.5
How it works
Tetrabenazine is the only agent that has demonstrated significant efficacy for TD management, but its use involves slow titration, multiple daily dosing, CYP2D6 genotyping for doses >50 mg/d, and tolerability issues. For example, the most common adverse effects in the pivotal tetrabenazine Huntington’s disease trial were sedation/somnolence (tetrabenazine 31% vs 3% for placebo), insomnia (tetrabenazine 22% vs 0% for placebo), depression (tetrabenazine 19% vs 0% for placebo), fatigue (tetrabenazine 22% vs 13% for placebo), and akathisia (tetrabenazine 19% vs 0% for placebo).2 For comparison, the only adverse event occurring in ≥5% of deutetrabenazine participants and at a rate ≥2 times that of placebo in the pivotal Huntington’s disease trial was somnolence (11.1% for deutetrabenazine vs 4.4% for placebo).4
Pharmacokinetics
Deutetrabenazine has 80% oral bioavailability, and is rapidly converted to its active metabolites after oral dosing (Table 2).5 Linear dose dependence of Cmax and area under the curve (AUC) was observed for the active metabolites following single or multiple doses of deutetrabenazine (6 to 24 mg and 7.5 to 22.5 mg, twice daily).15 Cmax of deuterated α-DHTBZ and β-DHTBZ is reached within 3 to 4 hours after dosing, with a steady state ratio of 3:1 for the α-DHTBZ vs the β-DHTBZ form. Food had no effect on AUC, but did increase Cmax by 50%.5

Deutetrabenazine is metabolized through carbonyl reductase enzymes to its active metabolites, and these are further metabolized through multiple CYP pathways, predominantly 2D6 and to a lesser extent 3A4. The effect of CYP2D6 inhibition on the pharmacokinetics of deutetrabenazine and its α-DHTBZ and β-DHTBZ metabolites was studied in 24 healthy participants following a single 22.5 mg dose of deutetrabenazine given after 8 days of administration of the strong CYP2D6 inhibitor paroxetine, 20 mg/d. In the presence of paroxetine, systemic exposure (AUC) of α-DHTBZ was 1.9-fold higher and β-DHTBZ was 6.5-fold higher, resulting in an approximately 3-fold increase in AUC for total (α+β)-DHTBZ, with corresponding increases in mean half-life of approximately 1.5-fold and 2.7-fold, respectively.5 Neither deutetrabenazine or its metabolites are inhibitors or inducers of major CYP enzymes. Aside from VMAT2, the results of in vitro studies suggest that deutetrabenazine and its active metabolites are unlikely to inhibit most major drug transporters at clinically relevant concentrations.
Efficacy
Efficacy was established in two 12-week, double-blind, placebo-controlled trials of adult patients with TD (ages 18 to 80).6,7 Eligible participants had:
- TD diagnosis for ≥3 months before screening and a history of DRBA treatment for ≥3 months (≥1 month if age ≥60)
- Total AIMS motor score ≥6 (items 1 to 7) at both screening and baseline, verified by a blinded central rater at screening via central video rating
- Patients with an underlying psychiatric illness had to be stable. Psychoactive medication use, including antipsychotics, was allowed if stable for ≥30 days before screening (antidepressants, ≥45 days).
Exclusion criteria included treatment with tetrabenazine, reserpine, α-methyl-p-tyrosine, strong anticholinergic medications, dopamine antagonizing antiemetics (eg, metoclopramide, prochlorperazine, promethazine), dopamine agonists, levodopa, stimulants, or a monoamine oxidase inhibitor (MAOI) within 30 days of screening or baseline, or treatment with botulinum toxin within 3 months of screening; and presence of a neurologic condition that could confound TD assessments, serious untreated or undertreated psychiatric illness, or unstable medical illness. Patients with a history of or active suicidal ideation or behavior within 6 months of screening or score ≥11 on the depression subscale of the Hospital Anxiety and Depression Scale were excluded. Those participants with Fridericia-corrected QT interval values >450 milliseconds in men, >460 milliseconds in women, or >480 milliseconds in patients with a right bundle branch block on electrocardiography at screening also were excluded.
The flexible-dose TD study was performed in 117 participants randomized in a 1:1 manner to deutetrabenazine or placebo, both administered twice daily, titrated to optimal dosage (12 to 48 mg/d) over 6 weeks, and then administered at that dose for another 6 weeks.7 The population demographics were: mean age, 54.6 ± 10.3 years, 52.1% female, 69.2% white, and 80.3% receiving ongoing dopamine antagonists, with a mean TD duration of 74.7 ± 81.5 months. Sixty-eight percent had schizophrenia spectrum disorders, and 30% had mood disorders. The primary outcome was change in total AIMS score (items 1 to 7) assessed by central, independent raters. The mean baseline AIMS score for items 1 to 7 was 9.6 ± 3.9, with 82.9% of participants with baseline AIMS scores ≥6. Study treatment retention was high: placebo 88.1%, deutetrabenazine 89.7%.7 There was a mean 3 point decrease in AIMS score for deutetrabenazine compared with 1.4 for placebo (P = .019). Among those with baseline AIMS scores ≥6, there was a 3.4 point decrease in AIMS scores for deutetrabenazine compared with a 1.9 point decrease for placebo (P = .027). The only adverse effects that occurred in ≥5% of deutetrabenazine participants and at a rate ≥2 times the rate in placebo were insomnia (deutetrabenazine 6.9% vs placebo 1.7%) and akathisia (deutetrabenazine 5.2% vs placebo 0%).
The fixed-dose TD study was performed in 293 participants randomized in 1:1:1:1 manner to 1 of 3 fixed doses of deutetrabenazine (12 mg/d, 24 mg/d, or 36 mg/d) or placebo, both administered twice daily.6 The starting dose of deutetrabenazine was 6 mg twice daily. During the dose escalation period (through Week 4), the dose of study drug was increased weekly in increments of 6 mg/d until the randomized dose was achieved. Patients continued to receive the dose they were assigned to over a maintenance period of 8 weeks.6 The population demographics were: mean age, 56.4 ± 11.3 years, 55% female, 79% white, 76% receiving ongoing dopamine antagonists, with a mean TD duration of 67.2 ± 66 months. Sixty percent had schizophrenia spectrum disorders, and 36% had mood disorders. The primary outcome was change in AIMS total score (items 1 to 7) assessed by central, independent raters. The mean AIMS score at baseline was 9.5 ± 2.7 in the placebo group, and for deutetrabenazine: 9.6 ± 2.4 in the 12 mg/d group, 9.4 ± 2.9 in the 24 mg/d group, and 10.1 ± 3.2 in the 36 mg/d group. The 24 mg/d and 36 mg/d doses significantly reduced AIMS scores from baseline vs placebo: 36 mg: −3.3 (0.42) vs −1.4 (0.41) (P = .001); 24 mg: −3.2 (0.45) vs −1.4 (0.41) (P = .003). Study treatment retention rates were high: placebo 90.5%, deutetrabenazine 88%. Across all doses, only 1 adverse effect occurred in ≥5% of deutetrabenazine participants: headache (5% deutetrabenazine vs 6% placebo). At the highest dose, 36 mg/d, the only adverse effects that occurred in ≥5% of participants were diarrhea (7% deutetrabenazine vs 3% placebo) and headache (7% deutetrabenazine vs 6% placebo).
Outcome. In the flexible-dose study (mean dose 38.8 ± 7.92 mg/d), the deutetrabenazine arm experienced a mean 30% reduction in AIMS scores from baseline at the Week 12 endpoint. Compared with placebo, the mean reduction in AIMS scores (standard error) was: −3.0 (0.45) deutetrabenazine vs −1.6 (0.46) placebo (P = .019).7 For the fixed-dose study, the 24 mg/d and 36 mg/d doses significantly reduced AIMS scores from baseline vs placebo: 36 mg: −3.3 (0.42) vs −1.4 (0.41) (P = .001); 24 mg: −3.2 (0.45) vs −1.4 (0.41) (P = .003). In addition to these mean changes from baseline, 35% of the 24 mg/d group and 33% of the 36 mg/d group demonstrated ≥50% reduction in AIMS scores.6
Tolerability
In the 2 phase 3 trials, there were no adverse effects occurring with an incidence ≥5% and at least twice the rate of placebo.5 Discontinuations because of adverse events were low in both pivotal studies across all treatment groups: 3.4% for placebo vs 1.7% for deutetrabenazine in the flexible-dose trial,7 and 3% for placebo vs 4% for deutetrabenazine in the fixed-dose study.6 In neither trial were there clinically significant changes in ratings of depression, suicidality, parkinsonism, or schizophrenia symptoms. The mean QT prolongation in healthy volunteers is described above.
Clinical considerations
Unique properties. Deutetrabenazine utilizes the greater bond strength of the carbon–deuterium bond to slow CYP metabolism, resulting in prolonged duration of action that is well tolerated, and provides significant efficacy.
Why Rx? The reasons to prescribe deutetrabenazine for TD patients include:
- only 1 of 2 agents with FDA approval for TD
- fewer tolerability issues than with tetrabenazine
- lower sedation rates in TD trials than with valbenazine
- no signal for effects on mood parameters or rates of parkinsonism when used for TD.
Dosing
The recommended starting dosage of deutetrabenazine is 6 mg twice daily taken with food, increasing by 6 mg/d weekly as needed, with a maximum dose of 48 mg/d or 36 mg/d in those taking strong CYP2D6 inhibitors or who are 2D6 poor metabolizers. Deutetrabenazine is contraindicated in patients with hepatic impairment (as determined by Child-Pugh criteria16). There are no data in patients with renal impairment. The combined efficacy and tolerability of dosages >48 mg/d has not been evaluated. Overdoses of tetrabenazine ranging from 100 to 1,000 mg have been reported in the literature and were associated with acute dystonia, oculogyric crisis, nausea and vomiting, sweating, sedation, hypotension, confusion, diarrhea, hallucinations, rubor, and tremor.5
Contraindications
When used for TD, deutetrabenazine is contraindicated for patients taking reserpine, tetrabenazine, valbenazine, or MAOIs, and for patients with hepatic impairment. As with most medications, there are no data on deutetrabenazine use in pregnant women; however, oral administration of deutetrabenazine (5, 10, or 30 mg/kg/d) or tetrabenazine (30 mg/kg/d) to pregnant rats during organogenesis had no clear effect on embryofetal development. The highest dose tested was 6 times the maximum recommended human dose of 48 mg/d on a body surface area (mg/m2) basis. There are no data on the presence of deutetrabenazine or its metabolites in human milk, the effects on the breastfed infant, or the effects of the drug on milk production.
1. Meyer JM. Forgotten but not gone: new developments in the understanding and treatment of tardive dyskinesia. CNS Spectr. 2016;21(S1):13-24.
2. Jankovic J, Clarence-Smith K. Tetrabenazine for the treatment of chorea and other hyperkinetic movement disorders. Expert Rev Neurother. 2011;11(11):1509-1523.
3. Meyer JM. Valbenazine for tardive dyskinesia. Current Psychiatry. 2017;16(5):40-46.
4. Huntington Study Group; Frank S, Testa CM, Stamler D, et al. Effect of deutetrabenazine on chorea among patients with Huntington disease: a randomized clinical trial. JAMA. 2016;316(1):40-50.
5. Austedo [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc.; 2017.
6. Anderson KE, Stamler D, Davis MD, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Psychiatry. 2017;4(8):595-604.
7. Fernandez HH, Factor SA, Hauser RA, et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia: the ARM-TD study. Neurology. 2017;88(21):2003-2010.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Richardson MA, Small AM, Read LL, et al. Branched chain amino acid treatment of tardive dyskinesia in children and adolescents. J Clin Psychiatry. 2004;65(1):92-96.
10. Quinn GP, Shore PA, Brodie BB. Biochemical and pharmacological studies of RO 1-9569 (tetrabenazine), a nonindole tranquilizing agent with reserpine-like effects. J Pharmacol Exp Ther. 1959;127:103-109.
11. Kazamatsuri H, Chien C, Cole JO. Treatment of tardive dyskinesia. I. Clinical efficacy of a dopamine-depleting agent, tetrabenazine. Arch Gen Psychiatry. 1972;27(1):95-99.
12. Scherman D, Weber MJ. Characterization of the vesicular monoamine transporter in cultured rat sympathetic neurons: persistence upon induction of cholinergic phenotypic traits. Dev Biol. 1987;119(1):68-74.
13. Erickson JD, Schafer MK, Bonner TI, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93(10):5166-5171.
14. Kushner DJ, Baker A, Dunstall TG. Pharmacological uses and perspectives of heavy water and deuterated compounds. Can J Physiol Pharmacol. 1999;77(2):79-88.
15. United States Securities and Exchange Commission. Form S-1 Registration Statement of Auspex Pharmaceuticals, Inc. https://www.sec.gov/Archives/edgar/data/1454189/000119312513481239/d627086ds1.htm. Published December 20, 2013. Accessed July 1, 2016.
16. Cholongitas E, Papatheodoridis GV, Vangeli M, et al. Systematic review: the model for end-stage liver disease—should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22(11-12):1079-1089.
1. Meyer JM. Forgotten but not gone: new developments in the understanding and treatment of tardive dyskinesia. CNS Spectr. 2016;21(S1):13-24.
2. Jankovic J, Clarence-Smith K. Tetrabenazine for the treatment of chorea and other hyperkinetic movement disorders. Expert Rev Neurother. 2011;11(11):1509-1523.
3. Meyer JM. Valbenazine for tardive dyskinesia. Current Psychiatry. 2017;16(5):40-46.
4. Huntington Study Group; Frank S, Testa CM, Stamler D, et al. Effect of deutetrabenazine on chorea among patients with Huntington disease: a randomized clinical trial. JAMA. 2016;316(1):40-50.
5. Austedo [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc.; 2017.
6. Anderson KE, Stamler D, Davis MD, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Psychiatry. 2017;4(8):595-604.
7. Fernandez HH, Factor SA, Hauser RA, et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia: the ARM-TD study. Neurology. 2017;88(21):2003-2010.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Richardson MA, Small AM, Read LL, et al. Branched chain amino acid treatment of tardive dyskinesia in children and adolescents. J Clin Psychiatry. 2004;65(1):92-96.
10. Quinn GP, Shore PA, Brodie BB. Biochemical and pharmacological studies of RO 1-9569 (tetrabenazine), a nonindole tranquilizing agent with reserpine-like effects. J Pharmacol Exp Ther. 1959;127:103-109.
11. Kazamatsuri H, Chien C, Cole JO. Treatment of tardive dyskinesia. I. Clinical efficacy of a dopamine-depleting agent, tetrabenazine. Arch Gen Psychiatry. 1972;27(1):95-99.
12. Scherman D, Weber MJ. Characterization of the vesicular monoamine transporter in cultured rat sympathetic neurons: persistence upon induction of cholinergic phenotypic traits. Dev Biol. 1987;119(1):68-74.
13. Erickson JD, Schafer MK, Bonner TI, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93(10):5166-5171.
14. Kushner DJ, Baker A, Dunstall TG. Pharmacological uses and perspectives of heavy water and deuterated compounds. Can J Physiol Pharmacol. 1999;77(2):79-88.
15. United States Securities and Exchange Commission. Form S-1 Registration Statement of Auspex Pharmaceuticals, Inc. https://www.sec.gov/Archives/edgar/data/1454189/000119312513481239/d627086ds1.htm. Published December 20, 2013. Accessed July 1, 2016.
16. Cholongitas E, Papatheodoridis GV, Vangeli M, et al. Systematic review: the model for end-stage liver disease—should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22(11-12):1079-1089.
Valbenazine for tardive dyskinesia
Despite improvements in the tolerability of antipsychotic medications, the development of tardive dyskinesia (TD) still is a significant area of concern; however, clinicians have had few treatment options. Valbenazine, a vesicular monoamine transport type 2 (VMAT2) inhibitor, is the only FDA-approved medication for TD (Table 1).1 By modulating dopamine transport into presynaptic vesicles, synaptic dopamine release is decreased, thereby reducing the postsynaptic stimulation of D2 receptors and the severity of dyskinetic movements.

In the pivotal 6-week clinical trial, valbenazine significantly reduced TD severity as measured by Abnormal Involuntary Movement Scale (AIMS) ratings.2 Study completion rates were high (87.6%), with only 2 dropouts because of adverse events in each of the placebo (n = 78) and 40-mg (n = 76) arms, and 3 in the 80-mg group (n = 80).
Before the development of valbenazine, tetrabenazine was the only effective option for treating TD. Despite tetrabenazine’s known efficacy for TD, it was not available in the United States until 2008 with the sole indication for movements related to Huntington’s disease. U.S. patients often were subjected to a litany of ineffective medications for TD, often at great expense. Moreover, tetrabenazine involved multiple daily dosing, required cytochrome P450 (CYP) 2D6 genotyping for doses >50 mg/d, had significant tolerability issues, and a monthly cost of $8,000 to $10,000. The availability of an agent that is effective for TD and does not have tetrabenazine’s kinetic limitations, adverse effect profile, or CYP2D6 monitoring requirements represents an enormous advance in the treatment of TD.
Clinical implications
Tardive dyskinesia remains a significant public health concern because of the increasing use of antipsychotics for disorders beyond the core indication for schizophrenia. Although exposure to dopamine D2 antagonism could result in postsynaptic receptor upregulation and supersensitivity, this process best explains what underlies withdrawal dyskinesia.3 The persistence of TD symptoms in 66% to 80% of patients after discontinuing offending agents has led to hypotheses that the underlying pathophysiology of TD might best be conceptualized as a problem with neuroplasticity. As with many disorders, environmental contributions (eg, oxidative stress) and genetic predisposition might play a role beyond that related to exposure to D2 antagonism.3
There have been trials of numerous agents, but no medication has been FDA-approved for treating TD, and limited data support the efficacy of a few existing medications (clonazepam, amantadine, and ginkgo biloba extract [EGb-761]),4 albeit with small effect sizes. A medical food, consisting of branched-chain amino acids, received FDA approval for the dietary management of TD in males, but is no longer commercially available except from compounding pharmacies.5
Tetrabenazine, a molecule developed in the mid-1950s to improve on the tolerability of reserpine, was associated with significant adverse effects such as orthostasis.6 Like reserpine, tetrabenazine subsequently was found to be effective for TD7 but without the peripheral adverse effects of reserpine. However, the kinetics of tetrabenazine necessitated multiple daily doses, and required CYP2D6 genotyping for doses >50 mg/d.8
Receptor blocking. The mechanism that differentiated reserpine’s and tetrabenazine’s clinical properties became clearer in the 1980s when researchers discovered that transporters were necessary to package neurotransmitters into the synaptic vesicles of presynaptic neurons.9 The vesicular monoamine transporter (VMAT) exists in 2 isoforms (VMAT1 and VMAT2) that vary in distribution, with VMAT1 expressed mainly in the peripheral nervous system and VMAT2 expressed mainly in monoaminergic cells of the central nervous system.10
Tetrabenazine’s improved tolerability profile was related to the fact that it is a specific and reversible VMAT2 inhibitor, while reserpine is an irreversible and nonselective antagonist of both VMAT isoforms. Investigation of tetrabenazine’s metabolism revealed that it is rapidly and extensively converted into 2 isomers, α-dihydrotetrabenazine (DH-TBZ) and β-DH-TBZ. The isomeric forms of DH-TBZ have multiple chiral centers, and therefore numerous forms of which only 2 are significantly active at VMAT2.3 The α–DH-TBZ isomer is metabolized via CYP2D6 and 3A4 into inactive metabolites, while β-DH-TBZ is metabolized solely via 2D6.3 Because of the short half-life of DH-TBZ when generated from oral tetrabenazine, the existence of 2D6 polymorphisms, and the predominant activity deriving from only 2 isomers, a molecule was synthesized (valbenazine), that when metabolized would slowly be converted into the most active isomer of α–DH-TBZ designated as NBI-98782 (Table 2). This slower conversion to NBI-98782 from valbenazine (compared with its formation from oral tetrabenazine) yielded improved kinetics and permitted once-daily dosing; moreover, because the metabolism of NBI-98782 is not solely dependent on CYP2D6, the need for genotyping was removed. Neither of the 2 metabolites from valbenazine NBI-98782 and NB-136110 have significant affinity for targets other than VMAT2.11
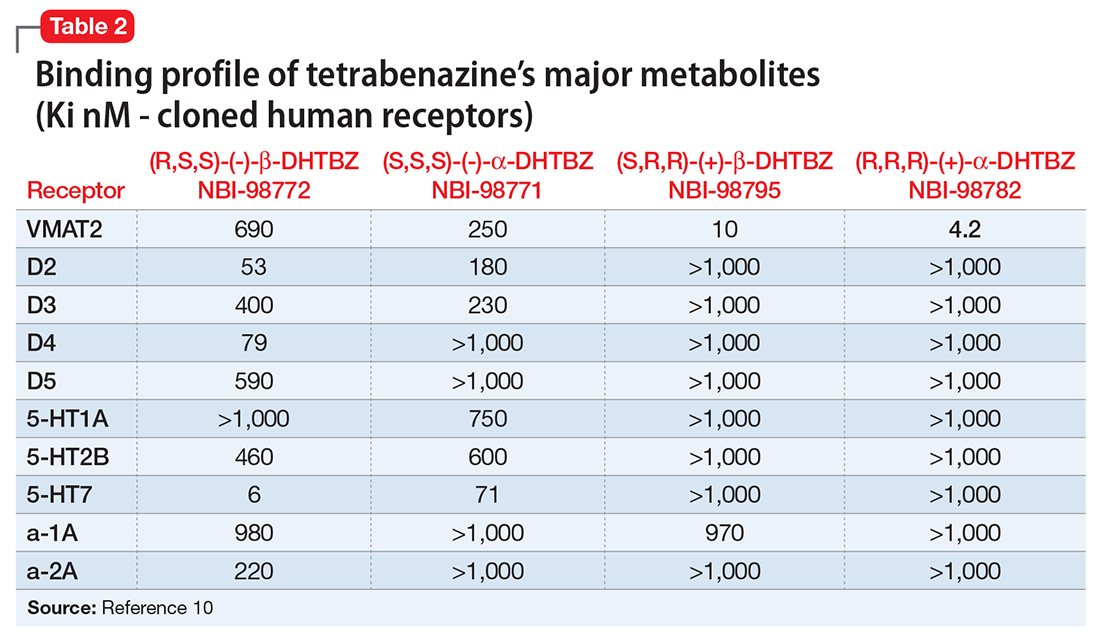
Use in tardive dyskinesia. Recommended starting dosage is 40 mg once daily with or without food, increased to 80 mg after 1 week, based on the design and results from the phase-III clinical trial.12 The FDA granted breakthrough therapy designation for this compound, and only 1 phase-III trial was performed. Valbenazine produced significant improvement on the AIMS, with a mean 30% reduction in AIMS scores at the Week 6 endpoint from baseline of 10.4 ± 3.6.2 The effect size was large (Cohen’s d = 0.90) for the 80-mg dosage. Continuation of 40 mg/d may be considered for some patients based on tolerability, including those who are known CYP2D6 poor metabolizers, and those taking strong CYP2D6 inhibitors. Patients taking strong 3A4 inhibitors should not exceed 40 mg/d. The maximum daily dose is 40 mg for those who have moderate or severe hepatic impairment (Child-Pugh score, 7 to 15). Dosage adjustment is not required for mild to moderate renal impairment (creatinine clearance, 30 to 90 mL/min).
Pharmacologic profile, adverse reactions
Valbenazine and its 2 metabolites lack affinity for receptors other than VMAT2, leading to an absence of orthostasis in clinical trials.1,2 In the phase-II trial, 76% of participants receiving valbenazine (n = 51) were titrated to the maximum dosage of 75 mg/d. Common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were headache (9.8% vs 4.1% placebo), fatigue (9.8% vs 4.1% placebo), and somnolence (5.9% vs 2% placebo).1 In the phase-III trial, participants were randomized 1:1:1 to valbenazine, 40 mg (n = 72), valbenazine, 80 mg (n = 79), or placebo (n = 76). In the clinical studies the most common diagnosis was schizophrenia or schizoaffective disorder, and 40% and 85% of participants in the phase-II and phase-III studies, respectively, remained on antipsychotics.1,2 There were no adverse effects with an incidence ≥5% and at least twice the rate of placebo in the phase-III trial.2
When data from all placebo-controlled studies were pooled, only 1 adverse effect occurred with an incidence ≥5% and twice that of placebo, somnolence with a rate of 10.9% for valbenazine vs 4.2% for placebo. The incidence of akathisia in the pooled analysis was 2.7% for valbenazine vs 0.5% for placebo. Importantly, in neither study was there a safety signal related to depression, suicidal ideation and behavior, or parkinsonism. There also were no clinically significant changes in measures of schizophrenia symptoms.
The mean QT prolongation for valbenazine in healthy participants was 6.7 milliseconds, with the upper bound of the double-sided 90% confidence interval reaching 8.4 milliseconds. For those taking strong 2D6 or 3A4 inhibitors, or known 2D6 poor metabolizers, the mean QT prolongation was 11.7 milliseconds (14.7 milliseconds upper bound of double-sided 90% CI). In the controlled trials, there was a dose-related increase in prolactin, alkaline phosphatase, and bilirubin. Overall, 3% of valbenazine-treated patients and 2% of placebo-treated patients discontinued because of adverse reactions.
As noted above, there were no adverse effects with an incidence ≥5% and at least twice the rate of placebo in the phase-III valbenazine trial. Aggregate data across all placebo-controlled studies found that somnolence was the only adverse effect that occurred with an incidence ≥5% and twice that of placebo (10.9% for valbenazine vs 4.2% for placebo).2 As a comparsion, rates of sedation and akathisia for tetrabenazine were higher in the pivotal Huntington’s disease trial: sedation/somnolence 31% vs 3% for placebo, and akathisia 19% vs 0% for placebo.8
How it works
Tetrabenazine, a selective VMAT2 inhibitor, is the only agent that has demonstrated significant efficacy and tolerability for TD management; however, its complex metabolism generates numerous isomers of the metabolites α-DH-TBZ and β-DH-TBZ, of which only 2 are significantly active (Table 3). By choosing an active isomer (NBI-98782) as the metabolite of interest because of its selective and potent activity at VMAT2 and having a metabolism not solely dependent on CYP2D6, a compound was generated (valbenazine) that when metabolized slowly converts into NBI-98782.
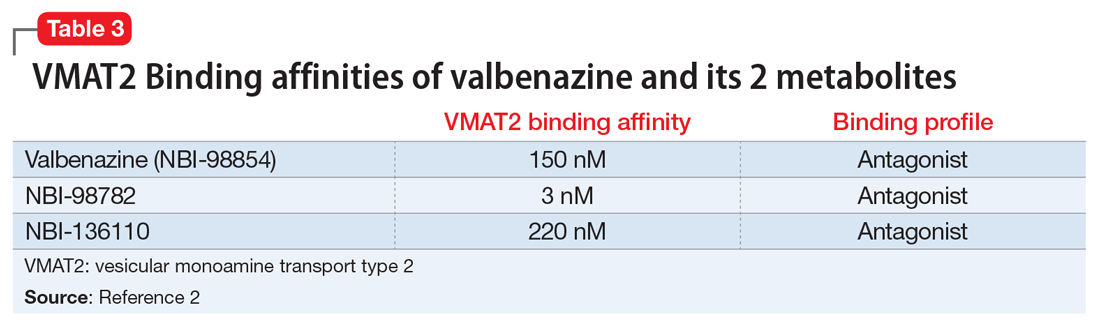
Pharmacokinetics
Valbenazine demonstrates dose-proportional pharmacokinetics after single oral dosages from 40 to 300 mg with no impact of food or fasting status on levels of the active metabolite. Valbenazine has a Tmax of 0.5 to 1.0 hours, with 49% oral bioavailability. The plasma half-life for valbenazine and for NBI-98782 ranges from 15 to 22 hours. The Tmax for NBI-98782 when formed from valbenazine occurs between 4 and 8 hours, with a Cmax of approximately 30 ng/mL. It should be noted that when NBI-98782 is generated from oral tetrabenazine, the mean half-life and Tmax are considerably shorter (6 hours and 1.5 hours, respectively), while the Cmax is much higher (approximately 77 ng/mL) (Table 4).
Valbenazine is metabolized through endogenous esterases to NBI-98782 and NBI-136110. NBI-98782, the active metabolite, is further metabolized through multiple CYP pathways, predominantly 3A4 and 2D6. Neither valbenazine nor its metabolites are inhibitors or inducers of major CYP enzymes. Aside from VMAT2, the results of in vitro studies suggest that valbenazine and its active metabolite are unlikely to inhibit most major drug transporters at clinically relevant concentrations. However, valbenazine increased digoxin levels because of inhibition of intestinal P-glycoprotein; therefore plasma digoxin level monitoring is recommended when these 2 are co-administered.
Efficacy
Efficacy was established in a 6-week, fixed-dosage, double-blind, placebo-controlled trial of adult patients with TD. Eligible participants had:
- DSM-IV diagnosis of antipsychotic-induced TD for ≥3 months before screening and moderate or severe TD, as indicated by AIMS item 8 (severity of abnormal movement), which was rated by a blinded, external reviewer using a video of the participant’s AIMS assessment at screening
- a DSM-IV diagnosis of schizophrenia or schizoaffective disorder or mood disorder (and stable per investigator)
- Brief Psychiatric Rating Scale score <50 at screening.
Exclusion criteria included clinically significant and unstable medical conditions within 1 month before screening; comorbid movement disorder (eg, parkinsonism, akathisia, truncal dystonia) that was more prominent than TD; and significant risk for active suicidal ideation, suicidal behavior, or violent behavior.2 Participants had a mean age of 56, 52% were male, and 65.7% of participants in the valbenazine 40-mg group had a schizophrenia spectrum disorder diagnosis, as did 65.8% in both the placebo and valbenazine 80-mg arms.
Antipsychotic treatments were permitted during the trial and >85% of participants continued taking these medications during the study. Participants (N = 234) were randomly allocated in a 1:1:1 manner to valbenazine 40 mg, 80 mg, or matched placebo. The primary outcome was change in AIMS total score (items 1 to 7) assessed by central, independent raters. Baseline AIMS scores were 9.9 ± 4.3 in the placebo group, and 9.8 ± 4.1 and 10.4 ± 3.6 in the valbenazine 40-mg and 80-mg arms, respectively.2
Outcome. A fixed-sequence testing procedure to control for family-wise error rate and multiplicity was employed, and the primary endpoint was change from baseline to Week 6 in AIMS total score (items 1 to 7) for valbenazine 80 mg vs placebo. Valbenazine, 40 mg, was associated with a 1.9 point decrease in AIMS score, while valbenazine, 80 mg, was associated with a 3.2 point decrease in AIMS score, compared with 0.1 point decrease for placebo (P < .05 for valbenazine, 40 mg, P < .001 for valbenazine, 80 mg). This difference for the 40-mg dosage did not meet the prespecified analysis endpoints; however, for the 80-mg valbenazine dosage, the effect size for this difference (Cohen’s d) was large 0.90. There also were statistically significant differences between 40 mg and 80 mg at weeks 2, 4, and 6 in the intent-to-treat population. Of the 79 participants, 43 taking the 80-mg dosage completed a 48-week extension. Efficacy was sustained in this group; however, when valbenazine was discontinued at Week 48, AIMS scores returned to baseline after 4 weeks.
Tolerability
Of the 234 randomized patients, 205 (87.6%) completed the 6-week trial. Discontinuations due to adverse events were low across all treatment groups: 2.6% and 2.8% in the placebo and valbenazine 40-mg arms, respectively, and 3.8% in valbenazine 80-mg cohort. There was no safety signal based on changes in depression, suicidality, parkinsonism rating, or changes in schizophrenia symptoms. Because valbenazine can cause somnolence, patients should not perform activities requiring mental alertness (eg, operating a vehicle or hazardous machinery) until they know how they will be affected by valbenazine.
Valbenazine should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, assess the QT interval before increasing the dosage.
Clinical considerations
Unique properties. Valbenazine is metabolized slowly to a potent, selective VMAT2 antagonist (NBI-98782) in a manner that permits once daily dosing, removes the need for CYP2D6 genotyping, and provides significant efficacy.
Why Rx? The reasons to prescribe valbenazine for TD patients include:
- currently the only agent with FDA approval for TD
- fewer tolerability issues seen with the only other effective agent, tetrabenazine
- no signal for effects on mood parameters or rates of parkinsonism
- lack of multiple daily dosing and possible need for 2D6 genotyping involved with TBZ prescribing.
Dosing
The recommended dosage of valbenazine is 80 mg/d administered as a single dose with or without food, starting at 40 mg once daily for 1 week. There is no dosage adjustment required in those with mild to moderate renal impairment; however, valbenazine is not recommended in those with severe renal impairment. The maximum dose is 40 mg/d for those who with moderate or severe hepatic impairment (Child-Pugh score, 7 to 15) however, valbenazine is not recommended for patients with severe renal impairment (creatinine clearance <30 mL/min) because the exposure to the active metabolite is reduced by approximately 75%. The combined efficacy and tolerability of dosages >80 mg/d has not been evaluated. Adverse effects seen with tetrabenazine at higher dosages include akathisia, anxiety, insomnia, parkinsonism, fatigue, and depression.
A daily dose of 40 mg may be considered for some patients based on tolerability, including those who are known CYP 2D6 poor metabolizers, and those taking strong CYP2D6 inhibitors.2 For those taking strong 3A4 inhibitors, the maximum daily dose is 40 mg. Concomitant use of valbenazine with strong 3A4 inducers is not recommended as the exposure to the active metabolite is reduced by approximately 75%.2 Lastly, because VMAT2 inhibition may alter synaptic levels of other monoamines, it is recommended that valbenazine not be administered with monoamine oxidase inhibitors, such as isocarboxazid, phenelzine, or selegiline.
Contraindications
There are no reported contraindications for valbenazine. As with most medications, there is limited available data on valbenazine use in pregnant women; however, administration of valbenazine to pregnant rats during organogenesis through lactation produced an increase in the number of stillborn pups and postnatal pup mortalities at doses under the maximum recommended human dose (MRHD) using body surface area based dosing (mg/m2). Pregnant women should be advised of the potential risk to a fetus. Valbenazine and its metabolites have been detected in rat milk at concentrations higher than in plasma after oral administration of valbenazine at doses 0.1 to 1.2 times the MRHD (based on mg/m2). Based on animal findings of increased perinatal mortality in exposed fetuses and pups, woman are advised not to breastfeed during valbenazine treatment and for 5 days after the final dose. No dosage adjustment is required for geriatric patients.
1. O’Brien CF, Jimenez R, Hauser RA, et al. NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Mov Disord. 2015;30(12):1681-1687.
2. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences Inc.; 2017.
3. Marder S, Knesevich MA, Hauser RA, et al. KINECT 3: A randomized, double-blind, placebo-controlled phase 3 trial of valbenazine (NBI-98854) for tardive dyskinesia. Poster presented at the American Psychiatric Association Annual Meeting; May 14-18, 2016; Atlanta, GA.
4. Kazamatsuri H, Chien C, Cole JO. Treatment of tardive dyskinesia. I. Clinical efficacy of a dopamine-depleting agent, tetrabenazine. Arch Gen Psychiatry. 1972;27(1):95-99.
5. Richardson MA, Bevans ML, Read LL, et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry. 2003;160(6):1117-1124.
6. Jankovic J, Clarence-Smith K. Tetrabenazine for the treatment of chorea and other hyperkinetic movement disorders. Expert Rev Neurother. 2011;11(11):1509-1523.
7. Meyer JM. Forgotten but not gone: new developments in the understanding and treatment of tardive dyskinesia. CNS Spectr. 2016;21(S1):13-24.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Quinn GP, Shore PA, Brodie BB. Biochemical and pharmacological studies of RO 1-9569 (tetrabenazine), a nonindole tranquilizing agent with reserpine-like effects. J Pharmacol Exp Ther. 1959;127:103-109.
10. Scherman D, Weber MJ. Characterization of the vesicular monoamine transporter in cultured rat sympathetic neurons: persistence upon induction of cholinergic phenotypic traits. Dev Biol. 1987;119(1):68-74.
11. Erickson JD, Schafer MK, Bonner TI, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93(10):5166-5171.
12. Grigoriadis DE, Smith E, Madan A, et al. Pharmacologic characteristics of valbenazine (NBI-98854) and its metabolites. Poster presented at the U.S. Psychiatric & Mental Health Congress, October 21-24, 2016; San Antonio, TX.
Despite improvements in the tolerability of antipsychotic medications, the development of tardive dyskinesia (TD) still is a significant area of concern; however, clinicians have had few treatment options. Valbenazine, a vesicular monoamine transport type 2 (VMAT2) inhibitor, is the only FDA-approved medication for TD (Table 1).1 By modulating dopamine transport into presynaptic vesicles, synaptic dopamine release is decreased, thereby reducing the postsynaptic stimulation of D2 receptors and the severity of dyskinetic movements.

In the pivotal 6-week clinical trial, valbenazine significantly reduced TD severity as measured by Abnormal Involuntary Movement Scale (AIMS) ratings.2 Study completion rates were high (87.6%), with only 2 dropouts because of adverse events in each of the placebo (n = 78) and 40-mg (n = 76) arms, and 3 in the 80-mg group (n = 80).
Before the development of valbenazine, tetrabenazine was the only effective option for treating TD. Despite tetrabenazine’s known efficacy for TD, it was not available in the United States until 2008 with the sole indication for movements related to Huntington’s disease. U.S. patients often were subjected to a litany of ineffective medications for TD, often at great expense. Moreover, tetrabenazine involved multiple daily dosing, required cytochrome P450 (CYP) 2D6 genotyping for doses >50 mg/d, had significant tolerability issues, and a monthly cost of $8,000 to $10,000. The availability of an agent that is effective for TD and does not have tetrabenazine’s kinetic limitations, adverse effect profile, or CYP2D6 monitoring requirements represents an enormous advance in the treatment of TD.
Clinical implications
Tardive dyskinesia remains a significant public health concern because of the increasing use of antipsychotics for disorders beyond the core indication for schizophrenia. Although exposure to dopamine D2 antagonism could result in postsynaptic receptor upregulation and supersensitivity, this process best explains what underlies withdrawal dyskinesia.3 The persistence of TD symptoms in 66% to 80% of patients after discontinuing offending agents has led to hypotheses that the underlying pathophysiology of TD might best be conceptualized as a problem with neuroplasticity. As with many disorders, environmental contributions (eg, oxidative stress) and genetic predisposition might play a role beyond that related to exposure to D2 antagonism.3
There have been trials of numerous agents, but no medication has been FDA-approved for treating TD, and limited data support the efficacy of a few existing medications (clonazepam, amantadine, and ginkgo biloba extract [EGb-761]),4 albeit with small effect sizes. A medical food, consisting of branched-chain amino acids, received FDA approval for the dietary management of TD in males, but is no longer commercially available except from compounding pharmacies.5
Tetrabenazine, a molecule developed in the mid-1950s to improve on the tolerability of reserpine, was associated with significant adverse effects such as orthostasis.6 Like reserpine, tetrabenazine subsequently was found to be effective for TD7 but without the peripheral adverse effects of reserpine. However, the kinetics of tetrabenazine necessitated multiple daily doses, and required CYP2D6 genotyping for doses >50 mg/d.8
Receptor blocking. The mechanism that differentiated reserpine’s and tetrabenazine’s clinical properties became clearer in the 1980s when researchers discovered that transporters were necessary to package neurotransmitters into the synaptic vesicles of presynaptic neurons.9 The vesicular monoamine transporter (VMAT) exists in 2 isoforms (VMAT1 and VMAT2) that vary in distribution, with VMAT1 expressed mainly in the peripheral nervous system and VMAT2 expressed mainly in monoaminergic cells of the central nervous system.10
Tetrabenazine’s improved tolerability profile was related to the fact that it is a specific and reversible VMAT2 inhibitor, while reserpine is an irreversible and nonselective antagonist of both VMAT isoforms. Investigation of tetrabenazine’s metabolism revealed that it is rapidly and extensively converted into 2 isomers, α-dihydrotetrabenazine (DH-TBZ) and β-DH-TBZ. The isomeric forms of DH-TBZ have multiple chiral centers, and therefore numerous forms of which only 2 are significantly active at VMAT2.3 The α–DH-TBZ isomer is metabolized via CYP2D6 and 3A4 into inactive metabolites, while β-DH-TBZ is metabolized solely via 2D6.3 Because of the short half-life of DH-TBZ when generated from oral tetrabenazine, the existence of 2D6 polymorphisms, and the predominant activity deriving from only 2 isomers, a molecule was synthesized (valbenazine), that when metabolized would slowly be converted into the most active isomer of α–DH-TBZ designated as NBI-98782 (Table 2). This slower conversion to NBI-98782 from valbenazine (compared with its formation from oral tetrabenazine) yielded improved kinetics and permitted once-daily dosing; moreover, because the metabolism of NBI-98782 is not solely dependent on CYP2D6, the need for genotyping was removed. Neither of the 2 metabolites from valbenazine NBI-98782 and NB-136110 have significant affinity for targets other than VMAT2.11

Use in tardive dyskinesia. Recommended starting dosage is 40 mg once daily with or without food, increased to 80 mg after 1 week, based on the design and results from the phase-III clinical trial.12 The FDA granted breakthrough therapy designation for this compound, and only 1 phase-III trial was performed. Valbenazine produced significant improvement on the AIMS, with a mean 30% reduction in AIMS scores at the Week 6 endpoint from baseline of 10.4 ± 3.6.2 The effect size was large (Cohen’s d = 0.90) for the 80-mg dosage. Continuation of 40 mg/d may be considered for some patients based on tolerability, including those who are known CYP2D6 poor metabolizers, and those taking strong CYP2D6 inhibitors. Patients taking strong 3A4 inhibitors should not exceed 40 mg/d. The maximum daily dose is 40 mg for those who have moderate or severe hepatic impairment (Child-Pugh score, 7 to 15). Dosage adjustment is not required for mild to moderate renal impairment (creatinine clearance, 30 to 90 mL/min).
Pharmacologic profile, adverse reactions
Valbenazine and its 2 metabolites lack affinity for receptors other than VMAT2, leading to an absence of orthostasis in clinical trials.1,2 In the phase-II trial, 76% of participants receiving valbenazine (n = 51) were titrated to the maximum dosage of 75 mg/d. Common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were headache (9.8% vs 4.1% placebo), fatigue (9.8% vs 4.1% placebo), and somnolence (5.9% vs 2% placebo).1 In the phase-III trial, participants were randomized 1:1:1 to valbenazine, 40 mg (n = 72), valbenazine, 80 mg (n = 79), or placebo (n = 76). In the clinical studies the most common diagnosis was schizophrenia or schizoaffective disorder, and 40% and 85% of participants in the phase-II and phase-III studies, respectively, remained on antipsychotics.1,2 There were no adverse effects with an incidence ≥5% and at least twice the rate of placebo in the phase-III trial.2
When data from all placebo-controlled studies were pooled, only 1 adverse effect occurred with an incidence ≥5% and twice that of placebo, somnolence with a rate of 10.9% for valbenazine vs 4.2% for placebo. The incidence of akathisia in the pooled analysis was 2.7% for valbenazine vs 0.5% for placebo. Importantly, in neither study was there a safety signal related to depression, suicidal ideation and behavior, or parkinsonism. There also were no clinically significant changes in measures of schizophrenia symptoms.
The mean QT prolongation for valbenazine in healthy participants was 6.7 milliseconds, with the upper bound of the double-sided 90% confidence interval reaching 8.4 milliseconds. For those taking strong 2D6 or 3A4 inhibitors, or known 2D6 poor metabolizers, the mean QT prolongation was 11.7 milliseconds (14.7 milliseconds upper bound of double-sided 90% CI). In the controlled trials, there was a dose-related increase in prolactin, alkaline phosphatase, and bilirubin. Overall, 3% of valbenazine-treated patients and 2% of placebo-treated patients discontinued because of adverse reactions.
As noted above, there were no adverse effects with an incidence ≥5% and at least twice the rate of placebo in the phase-III valbenazine trial. Aggregate data across all placebo-controlled studies found that somnolence was the only adverse effect that occurred with an incidence ≥5% and twice that of placebo (10.9% for valbenazine vs 4.2% for placebo).2 As a comparsion, rates of sedation and akathisia for tetrabenazine were higher in the pivotal Huntington’s disease trial: sedation/somnolence 31% vs 3% for placebo, and akathisia 19% vs 0% for placebo.8
How it works
Tetrabenazine, a selective VMAT2 inhibitor, is the only agent that has demonstrated significant efficacy and tolerability for TD management; however, its complex metabolism generates numerous isomers of the metabolites α-DH-TBZ and β-DH-TBZ, of which only 2 are significantly active (Table 3). By choosing an active isomer (NBI-98782) as the metabolite of interest because of its selective and potent activity at VMAT2 and having a metabolism not solely dependent on CYP2D6, a compound was generated (valbenazine) that when metabolized slowly converts into NBI-98782.

Pharmacokinetics
Valbenazine demonstrates dose-proportional pharmacokinetics after single oral dosages from 40 to 300 mg with no impact of food or fasting status on levels of the active metabolite. Valbenazine has a Tmax of 0.5 to 1.0 hours, with 49% oral bioavailability. The plasma half-life for valbenazine and for NBI-98782 ranges from 15 to 22 hours. The Tmax for NBI-98782 when formed from valbenazine occurs between 4 and 8 hours, with a Cmax of approximately 30 ng/mL. It should be noted that when NBI-98782 is generated from oral tetrabenazine, the mean half-life and Tmax are considerably shorter (6 hours and 1.5 hours, respectively), while the Cmax is much higher (approximately 77 ng/mL) (Table 4).

Valbenazine is metabolized through endogenous esterases to NBI-98782 and NBI-136110. NBI-98782, the active metabolite, is further metabolized through multiple CYP pathways, predominantly 3A4 and 2D6. Neither valbenazine nor its metabolites are inhibitors or inducers of major CYP enzymes. Aside from VMAT2, the results of in vitro studies suggest that valbenazine and its active metabolite are unlikely to inhibit most major drug transporters at clinically relevant concentrations. However, valbenazine increased digoxin levels because of inhibition of intestinal P-glycoprotein; therefore plasma digoxin level monitoring is recommended when these 2 are co-administered.
Efficacy
Efficacy was established in a 6-week, fixed-dosage, double-blind, placebo-controlled trial of adult patients with TD. Eligible participants had:
- DSM-IV diagnosis of antipsychotic-induced TD for ≥3 months before screening and moderate or severe TD, as indicated by AIMS item 8 (severity of abnormal movement), which was rated by a blinded, external reviewer using a video of the participant’s AIMS assessment at screening
- a DSM-IV diagnosis of schizophrenia or schizoaffective disorder or mood disorder (and stable per investigator)
- Brief Psychiatric Rating Scale score <50 at screening.
Exclusion criteria included clinically significant and unstable medical conditions within 1 month before screening; comorbid movement disorder (eg, parkinsonism, akathisia, truncal dystonia) that was more prominent than TD; and significant risk for active suicidal ideation, suicidal behavior, or violent behavior.2 Participants had a mean age of 56, 52% were male, and 65.7% of participants in the valbenazine 40-mg group had a schizophrenia spectrum disorder diagnosis, as did 65.8% in both the placebo and valbenazine 80-mg arms.
Antipsychotic treatments were permitted during the trial and >85% of participants continued taking these medications during the study. Participants (N = 234) were randomly allocated in a 1:1:1 manner to valbenazine 40 mg, 80 mg, or matched placebo. The primary outcome was change in AIMS total score (items 1 to 7) assessed by central, independent raters. Baseline AIMS scores were 9.9 ± 4.3 in the placebo group, and 9.8 ± 4.1 and 10.4 ± 3.6 in the valbenazine 40-mg and 80-mg arms, respectively.2
Outcome. A fixed-sequence testing procedure to control for family-wise error rate and multiplicity was employed, and the primary endpoint was change from baseline to Week 6 in AIMS total score (items 1 to 7) for valbenazine 80 mg vs placebo. Valbenazine, 40 mg, was associated with a 1.9 point decrease in AIMS score, while valbenazine, 80 mg, was associated with a 3.2 point decrease in AIMS score, compared with 0.1 point decrease for placebo (P < .05 for valbenazine, 40 mg, P < .001 for valbenazine, 80 mg). This difference for the 40-mg dosage did not meet the prespecified analysis endpoints; however, for the 80-mg valbenazine dosage, the effect size for this difference (Cohen’s d) was large 0.90. There also were statistically significant differences between 40 mg and 80 mg at weeks 2, 4, and 6 in the intent-to-treat population. Of the 79 participants, 43 taking the 80-mg dosage completed a 48-week extension. Efficacy was sustained in this group; however, when valbenazine was discontinued at Week 48, AIMS scores returned to baseline after 4 weeks.
Tolerability
Of the 234 randomized patients, 205 (87.6%) completed the 6-week trial. Discontinuations due to adverse events were low across all treatment groups: 2.6% and 2.8% in the placebo and valbenazine 40-mg arms, respectively, and 3.8% in valbenazine 80-mg cohort. There was no safety signal based on changes in depression, suicidality, parkinsonism rating, or changes in schizophrenia symptoms. Because valbenazine can cause somnolence, patients should not perform activities requiring mental alertness (eg, operating a vehicle or hazardous machinery) until they know how they will be affected by valbenazine.
Valbenazine should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, assess the QT interval before increasing the dosage.
Clinical considerations
Unique properties. Valbenazine is metabolized slowly to a potent, selective VMAT2 antagonist (NBI-98782) in a manner that permits once daily dosing, removes the need for CYP2D6 genotyping, and provides significant efficacy.
Why Rx? The reasons to prescribe valbenazine for TD patients include:
- currently the only agent with FDA approval for TD
- fewer tolerability issues seen with the only other effective agent, tetrabenazine
- no signal for effects on mood parameters or rates of parkinsonism
- lack of multiple daily dosing and possible need for 2D6 genotyping involved with TBZ prescribing.
Dosing
The recommended dosage of valbenazine is 80 mg/d administered as a single dose with or without food, starting at 40 mg once daily for 1 week. There is no dosage adjustment required in those with mild to moderate renal impairment; however, valbenazine is not recommended in those with severe renal impairment. The maximum dose is 40 mg/d for those who with moderate or severe hepatic impairment (Child-Pugh score, 7 to 15) however, valbenazine is not recommended for patients with severe renal impairment (creatinine clearance <30 mL/min) because the exposure to the active metabolite is reduced by approximately 75%. The combined efficacy and tolerability of dosages >80 mg/d has not been evaluated. Adverse effects seen with tetrabenazine at higher dosages include akathisia, anxiety, insomnia, parkinsonism, fatigue, and depression.
A daily dose of 40 mg may be considered for some patients based on tolerability, including those who are known CYP 2D6 poor metabolizers, and those taking strong CYP2D6 inhibitors.2 For those taking strong 3A4 inhibitors, the maximum daily dose is 40 mg. Concomitant use of valbenazine with strong 3A4 inducers is not recommended as the exposure to the active metabolite is reduced by approximately 75%.2 Lastly, because VMAT2 inhibition may alter synaptic levels of other monoamines, it is recommended that valbenazine not be administered with monoamine oxidase inhibitors, such as isocarboxazid, phenelzine, or selegiline.
Contraindications
There are no reported contraindications for valbenazine. As with most medications, there is limited available data on valbenazine use in pregnant women; however, administration of valbenazine to pregnant rats during organogenesis through lactation produced an increase in the number of stillborn pups and postnatal pup mortalities at doses under the maximum recommended human dose (MRHD) using body surface area based dosing (mg/m2). Pregnant women should be advised of the potential risk to a fetus. Valbenazine and its metabolites have been detected in rat milk at concentrations higher than in plasma after oral administration of valbenazine at doses 0.1 to 1.2 times the MRHD (based on mg/m2). Based on animal findings of increased perinatal mortality in exposed fetuses and pups, woman are advised not to breastfeed during valbenazine treatment and for 5 days after the final dose. No dosage adjustment is required for geriatric patients.
Despite improvements in the tolerability of antipsychotic medications, the development of tardive dyskinesia (TD) still is a significant area of concern; however, clinicians have had few treatment options. Valbenazine, a vesicular monoamine transport type 2 (VMAT2) inhibitor, is the only FDA-approved medication for TD (Table 1).1 By modulating dopamine transport into presynaptic vesicles, synaptic dopamine release is decreased, thereby reducing the postsynaptic stimulation of D2 receptors and the severity of dyskinetic movements.

In the pivotal 6-week clinical trial, valbenazine significantly reduced TD severity as measured by Abnormal Involuntary Movement Scale (AIMS) ratings.2 Study completion rates were high (87.6%), with only 2 dropouts because of adverse events in each of the placebo (n = 78) and 40-mg (n = 76) arms, and 3 in the 80-mg group (n = 80).
Before the development of valbenazine, tetrabenazine was the only effective option for treating TD. Despite tetrabenazine’s known efficacy for TD, it was not available in the United States until 2008 with the sole indication for movements related to Huntington’s disease. U.S. patients often were subjected to a litany of ineffective medications for TD, often at great expense. Moreover, tetrabenazine involved multiple daily dosing, required cytochrome P450 (CYP) 2D6 genotyping for doses >50 mg/d, had significant tolerability issues, and a monthly cost of $8,000 to $10,000. The availability of an agent that is effective for TD and does not have tetrabenazine’s kinetic limitations, adverse effect profile, or CYP2D6 monitoring requirements represents an enormous advance in the treatment of TD.
Clinical implications
Tardive dyskinesia remains a significant public health concern because of the increasing use of antipsychotics for disorders beyond the core indication for schizophrenia. Although exposure to dopamine D2 antagonism could result in postsynaptic receptor upregulation and supersensitivity, this process best explains what underlies withdrawal dyskinesia.3 The persistence of TD symptoms in 66% to 80% of patients after discontinuing offending agents has led to hypotheses that the underlying pathophysiology of TD might best be conceptualized as a problem with neuroplasticity. As with many disorders, environmental contributions (eg, oxidative stress) and genetic predisposition might play a role beyond that related to exposure to D2 antagonism.3
There have been trials of numerous agents, but no medication has been FDA-approved for treating TD, and limited data support the efficacy of a few existing medications (clonazepam, amantadine, and ginkgo biloba extract [EGb-761]),4 albeit with small effect sizes. A medical food, consisting of branched-chain amino acids, received FDA approval for the dietary management of TD in males, but is no longer commercially available except from compounding pharmacies.5
Tetrabenazine, a molecule developed in the mid-1950s to improve on the tolerability of reserpine, was associated with significant adverse effects such as orthostasis.6 Like reserpine, tetrabenazine subsequently was found to be effective for TD7 but without the peripheral adverse effects of reserpine. However, the kinetics of tetrabenazine necessitated multiple daily doses, and required CYP2D6 genotyping for doses >50 mg/d.8
Receptor blocking. The mechanism that differentiated reserpine’s and tetrabenazine’s clinical properties became clearer in the 1980s when researchers discovered that transporters were necessary to package neurotransmitters into the synaptic vesicles of presynaptic neurons.9 The vesicular monoamine transporter (VMAT) exists in 2 isoforms (VMAT1 and VMAT2) that vary in distribution, with VMAT1 expressed mainly in the peripheral nervous system and VMAT2 expressed mainly in monoaminergic cells of the central nervous system.10
Tetrabenazine’s improved tolerability profile was related to the fact that it is a specific and reversible VMAT2 inhibitor, while reserpine is an irreversible and nonselective antagonist of both VMAT isoforms. Investigation of tetrabenazine’s metabolism revealed that it is rapidly and extensively converted into 2 isomers, α-dihydrotetrabenazine (DH-TBZ) and β-DH-TBZ. The isomeric forms of DH-TBZ have multiple chiral centers, and therefore numerous forms of which only 2 are significantly active at VMAT2.3 The α–DH-TBZ isomer is metabolized via CYP2D6 and 3A4 into inactive metabolites, while β-DH-TBZ is metabolized solely via 2D6.3 Because of the short half-life of DH-TBZ when generated from oral tetrabenazine, the existence of 2D6 polymorphisms, and the predominant activity deriving from only 2 isomers, a molecule was synthesized (valbenazine), that when metabolized would slowly be converted into the most active isomer of α–DH-TBZ designated as NBI-98782 (Table 2). This slower conversion to NBI-98782 from valbenazine (compared with its formation from oral tetrabenazine) yielded improved kinetics and permitted once-daily dosing; moreover, because the metabolism of NBI-98782 is not solely dependent on CYP2D6, the need for genotyping was removed. Neither of the 2 metabolites from valbenazine NBI-98782 and NB-136110 have significant affinity for targets other than VMAT2.11

Use in tardive dyskinesia. Recommended starting dosage is 40 mg once daily with or without food, increased to 80 mg after 1 week, based on the design and results from the phase-III clinical trial.12 The FDA granted breakthrough therapy designation for this compound, and only 1 phase-III trial was performed. Valbenazine produced significant improvement on the AIMS, with a mean 30% reduction in AIMS scores at the Week 6 endpoint from baseline of 10.4 ± 3.6.2 The effect size was large (Cohen’s d = 0.90) for the 80-mg dosage. Continuation of 40 mg/d may be considered for some patients based on tolerability, including those who are known CYP2D6 poor metabolizers, and those taking strong CYP2D6 inhibitors. Patients taking strong 3A4 inhibitors should not exceed 40 mg/d. The maximum daily dose is 40 mg for those who have moderate or severe hepatic impairment (Child-Pugh score, 7 to 15). Dosage adjustment is not required for mild to moderate renal impairment (creatinine clearance, 30 to 90 mL/min).
Pharmacologic profile, adverse reactions
Valbenazine and its 2 metabolites lack affinity for receptors other than VMAT2, leading to an absence of orthostasis in clinical trials.1,2 In the phase-II trial, 76% of participants receiving valbenazine (n = 51) were titrated to the maximum dosage of 75 mg/d. Common adverse reactions (incidence ≥5% and at least twice the rate of placebo) were headache (9.8% vs 4.1% placebo), fatigue (9.8% vs 4.1% placebo), and somnolence (5.9% vs 2% placebo).1 In the phase-III trial, participants were randomized 1:1:1 to valbenazine, 40 mg (n = 72), valbenazine, 80 mg (n = 79), or placebo (n = 76). In the clinical studies the most common diagnosis was schizophrenia or schizoaffective disorder, and 40% and 85% of participants in the phase-II and phase-III studies, respectively, remained on antipsychotics.1,2 There were no adverse effects with an incidence ≥5% and at least twice the rate of placebo in the phase-III trial.2
When data from all placebo-controlled studies were pooled, only 1 adverse effect occurred with an incidence ≥5% and twice that of placebo, somnolence with a rate of 10.9% for valbenazine vs 4.2% for placebo. The incidence of akathisia in the pooled analysis was 2.7% for valbenazine vs 0.5% for placebo. Importantly, in neither study was there a safety signal related to depression, suicidal ideation and behavior, or parkinsonism. There also were no clinically significant changes in measures of schizophrenia symptoms.
The mean QT prolongation for valbenazine in healthy participants was 6.7 milliseconds, with the upper bound of the double-sided 90% confidence interval reaching 8.4 milliseconds. For those taking strong 2D6 or 3A4 inhibitors, or known 2D6 poor metabolizers, the mean QT prolongation was 11.7 milliseconds (14.7 milliseconds upper bound of double-sided 90% CI). In the controlled trials, there was a dose-related increase in prolactin, alkaline phosphatase, and bilirubin. Overall, 3% of valbenazine-treated patients and 2% of placebo-treated patients discontinued because of adverse reactions.
As noted above, there were no adverse effects with an incidence ≥5% and at least twice the rate of placebo in the phase-III valbenazine trial. Aggregate data across all placebo-controlled studies found that somnolence was the only adverse effect that occurred with an incidence ≥5% and twice that of placebo (10.9% for valbenazine vs 4.2% for placebo).2 As a comparsion, rates of sedation and akathisia for tetrabenazine were higher in the pivotal Huntington’s disease trial: sedation/somnolence 31% vs 3% for placebo, and akathisia 19% vs 0% for placebo.8
How it works
Tetrabenazine, a selective VMAT2 inhibitor, is the only agent that has demonstrated significant efficacy and tolerability for TD management; however, its complex metabolism generates numerous isomers of the metabolites α-DH-TBZ and β-DH-TBZ, of which only 2 are significantly active (Table 3). By choosing an active isomer (NBI-98782) as the metabolite of interest because of its selective and potent activity at VMAT2 and having a metabolism not solely dependent on CYP2D6, a compound was generated (valbenazine) that when metabolized slowly converts into NBI-98782.

Pharmacokinetics
Valbenazine demonstrates dose-proportional pharmacokinetics after single oral dosages from 40 to 300 mg with no impact of food or fasting status on levels of the active metabolite. Valbenazine has a Tmax of 0.5 to 1.0 hours, with 49% oral bioavailability. The plasma half-life for valbenazine and for NBI-98782 ranges from 15 to 22 hours. The Tmax for NBI-98782 when formed from valbenazine occurs between 4 and 8 hours, with a Cmax of approximately 30 ng/mL. It should be noted that when NBI-98782 is generated from oral tetrabenazine, the mean half-life and Tmax are considerably shorter (6 hours and 1.5 hours, respectively), while the Cmax is much higher (approximately 77 ng/mL) (Table 4).

Valbenazine is metabolized through endogenous esterases to NBI-98782 and NBI-136110. NBI-98782, the active metabolite, is further metabolized through multiple CYP pathways, predominantly 3A4 and 2D6. Neither valbenazine nor its metabolites are inhibitors or inducers of major CYP enzymes. Aside from VMAT2, the results of in vitro studies suggest that valbenazine and its active metabolite are unlikely to inhibit most major drug transporters at clinically relevant concentrations. However, valbenazine increased digoxin levels because of inhibition of intestinal P-glycoprotein; therefore plasma digoxin level monitoring is recommended when these 2 are co-administered.
Efficacy
Efficacy was established in a 6-week, fixed-dosage, double-blind, placebo-controlled trial of adult patients with TD. Eligible participants had:
- DSM-IV diagnosis of antipsychotic-induced TD for ≥3 months before screening and moderate or severe TD, as indicated by AIMS item 8 (severity of abnormal movement), which was rated by a blinded, external reviewer using a video of the participant’s AIMS assessment at screening
- a DSM-IV diagnosis of schizophrenia or schizoaffective disorder or mood disorder (and stable per investigator)
- Brief Psychiatric Rating Scale score <50 at screening.
Exclusion criteria included clinically significant and unstable medical conditions within 1 month before screening; comorbid movement disorder (eg, parkinsonism, akathisia, truncal dystonia) that was more prominent than TD; and significant risk for active suicidal ideation, suicidal behavior, or violent behavior.2 Participants had a mean age of 56, 52% were male, and 65.7% of participants in the valbenazine 40-mg group had a schizophrenia spectrum disorder diagnosis, as did 65.8% in both the placebo and valbenazine 80-mg arms.
Antipsychotic treatments were permitted during the trial and >85% of participants continued taking these medications during the study. Participants (N = 234) were randomly allocated in a 1:1:1 manner to valbenazine 40 mg, 80 mg, or matched placebo. The primary outcome was change in AIMS total score (items 1 to 7) assessed by central, independent raters. Baseline AIMS scores were 9.9 ± 4.3 in the placebo group, and 9.8 ± 4.1 and 10.4 ± 3.6 in the valbenazine 40-mg and 80-mg arms, respectively.2
Outcome. A fixed-sequence testing procedure to control for family-wise error rate and multiplicity was employed, and the primary endpoint was change from baseline to Week 6 in AIMS total score (items 1 to 7) for valbenazine 80 mg vs placebo. Valbenazine, 40 mg, was associated with a 1.9 point decrease in AIMS score, while valbenazine, 80 mg, was associated with a 3.2 point decrease in AIMS score, compared with 0.1 point decrease for placebo (P < .05 for valbenazine, 40 mg, P < .001 for valbenazine, 80 mg). This difference for the 40-mg dosage did not meet the prespecified analysis endpoints; however, for the 80-mg valbenazine dosage, the effect size for this difference (Cohen’s d) was large 0.90. There also were statistically significant differences between 40 mg and 80 mg at weeks 2, 4, and 6 in the intent-to-treat population. Of the 79 participants, 43 taking the 80-mg dosage completed a 48-week extension. Efficacy was sustained in this group; however, when valbenazine was discontinued at Week 48, AIMS scores returned to baseline after 4 weeks.
Tolerability
Of the 234 randomized patients, 205 (87.6%) completed the 6-week trial. Discontinuations due to adverse events were low across all treatment groups: 2.6% and 2.8% in the placebo and valbenazine 40-mg arms, respectively, and 3.8% in valbenazine 80-mg cohort. There was no safety signal based on changes in depression, suicidality, parkinsonism rating, or changes in schizophrenia symptoms. Because valbenazine can cause somnolence, patients should not perform activities requiring mental alertness (eg, operating a vehicle or hazardous machinery) until they know how they will be affected by valbenazine.
Valbenazine should be avoided in patients with congenital long QT syndrome or with arrhythmias associated with a prolonged QT interval. For patients at increased risk of a prolonged QT interval, assess the QT interval before increasing the dosage.
Clinical considerations
Unique properties. Valbenazine is metabolized slowly to a potent, selective VMAT2 antagonist (NBI-98782) in a manner that permits once daily dosing, removes the need for CYP2D6 genotyping, and provides significant efficacy.
Why Rx? The reasons to prescribe valbenazine for TD patients include:
- currently the only agent with FDA approval for TD
- fewer tolerability issues seen with the only other effective agent, tetrabenazine
- no signal for effects on mood parameters or rates of parkinsonism
- lack of multiple daily dosing and possible need for 2D6 genotyping involved with TBZ prescribing.
Dosing
The recommended dosage of valbenazine is 80 mg/d administered as a single dose with or without food, starting at 40 mg once daily for 1 week. There is no dosage adjustment required in those with mild to moderate renal impairment; however, valbenazine is not recommended in those with severe renal impairment. The maximum dose is 40 mg/d for those who with moderate or severe hepatic impairment (Child-Pugh score, 7 to 15) however, valbenazine is not recommended for patients with severe renal impairment (creatinine clearance <30 mL/min) because the exposure to the active metabolite is reduced by approximately 75%. The combined efficacy and tolerability of dosages >80 mg/d has not been evaluated. Adverse effects seen with tetrabenazine at higher dosages include akathisia, anxiety, insomnia, parkinsonism, fatigue, and depression.
A daily dose of 40 mg may be considered for some patients based on tolerability, including those who are known CYP 2D6 poor metabolizers, and those taking strong CYP2D6 inhibitors.2 For those taking strong 3A4 inhibitors, the maximum daily dose is 40 mg. Concomitant use of valbenazine with strong 3A4 inducers is not recommended as the exposure to the active metabolite is reduced by approximately 75%.2 Lastly, because VMAT2 inhibition may alter synaptic levels of other monoamines, it is recommended that valbenazine not be administered with monoamine oxidase inhibitors, such as isocarboxazid, phenelzine, or selegiline.
Contraindications
There are no reported contraindications for valbenazine. As with most medications, there is limited available data on valbenazine use in pregnant women; however, administration of valbenazine to pregnant rats during organogenesis through lactation produced an increase in the number of stillborn pups and postnatal pup mortalities at doses under the maximum recommended human dose (MRHD) using body surface area based dosing (mg/m2). Pregnant women should be advised of the potential risk to a fetus. Valbenazine and its metabolites have been detected in rat milk at concentrations higher than in plasma after oral administration of valbenazine at doses 0.1 to 1.2 times the MRHD (based on mg/m2). Based on animal findings of increased perinatal mortality in exposed fetuses and pups, woman are advised not to breastfeed during valbenazine treatment and for 5 days after the final dose. No dosage adjustment is required for geriatric patients.
1. O’Brien CF, Jimenez R, Hauser RA, et al. NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Mov Disord. 2015;30(12):1681-1687.
2. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences Inc.; 2017.
3. Marder S, Knesevich MA, Hauser RA, et al. KINECT 3: A randomized, double-blind, placebo-controlled phase 3 trial of valbenazine (NBI-98854) for tardive dyskinesia. Poster presented at the American Psychiatric Association Annual Meeting; May 14-18, 2016; Atlanta, GA.
4. Kazamatsuri H, Chien C, Cole JO. Treatment of tardive dyskinesia. I. Clinical efficacy of a dopamine-depleting agent, tetrabenazine. Arch Gen Psychiatry. 1972;27(1):95-99.
5. Richardson MA, Bevans ML, Read LL, et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry. 2003;160(6):1117-1124.
6. Jankovic J, Clarence-Smith K. Tetrabenazine for the treatment of chorea and other hyperkinetic movement disorders. Expert Rev Neurother. 2011;11(11):1509-1523.
7. Meyer JM. Forgotten but not gone: new developments in the understanding and treatment of tardive dyskinesia. CNS Spectr. 2016;21(S1):13-24.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Quinn GP, Shore PA, Brodie BB. Biochemical and pharmacological studies of RO 1-9569 (tetrabenazine), a nonindole tranquilizing agent with reserpine-like effects. J Pharmacol Exp Ther. 1959;127:103-109.
10. Scherman D, Weber MJ. Characterization of the vesicular monoamine transporter in cultured rat sympathetic neurons: persistence upon induction of cholinergic phenotypic traits. Dev Biol. 1987;119(1):68-74.
11. Erickson JD, Schafer MK, Bonner TI, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93(10):5166-5171.
12. Grigoriadis DE, Smith E, Madan A, et al. Pharmacologic characteristics of valbenazine (NBI-98854) and its metabolites. Poster presented at the U.S. Psychiatric & Mental Health Congress, October 21-24, 2016; San Antonio, TX.
1. O’Brien CF, Jimenez R, Hauser RA, et al. NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Mov Disord. 2015;30(12):1681-1687.
2. Ingrezza [package insert]. San Diego, CA: Neurocrine Biosciences Inc.; 2017.
3. Marder S, Knesevich MA, Hauser RA, et al. KINECT 3: A randomized, double-blind, placebo-controlled phase 3 trial of valbenazine (NBI-98854) for tardive dyskinesia. Poster presented at the American Psychiatric Association Annual Meeting; May 14-18, 2016; Atlanta, GA.
4. Kazamatsuri H, Chien C, Cole JO. Treatment of tardive dyskinesia. I. Clinical efficacy of a dopamine-depleting agent, tetrabenazine. Arch Gen Psychiatry. 1972;27(1):95-99.
5. Richardson MA, Bevans ML, Read LL, et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry. 2003;160(6):1117-1124.
6. Jankovic J, Clarence-Smith K. Tetrabenazine for the treatment of chorea and other hyperkinetic movement disorders. Expert Rev Neurother. 2011;11(11):1509-1523.
7. Meyer JM. Forgotten but not gone: new developments in the understanding and treatment of tardive dyskinesia. CNS Spectr. 2016;21(S1):13-24.
8. Bhidayasiri R, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
9. Quinn GP, Shore PA, Brodie BB. Biochemical and pharmacological studies of RO 1-9569 (tetrabenazine), a nonindole tranquilizing agent with reserpine-like effects. J Pharmacol Exp Ther. 1959;127:103-109.
10. Scherman D, Weber MJ. Characterization of the vesicular monoamine transporter in cultured rat sympathetic neurons: persistence upon induction of cholinergic phenotypic traits. Dev Biol. 1987;119(1):68-74.
11. Erickson JD, Schafer MK, Bonner TI, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93(10):5166-5171.
12. Grigoriadis DE, Smith E, Madan A, et al. Pharmacologic characteristics of valbenazine (NBI-98854) and its metabolites. Poster presented at the U.S. Psychiatric & Mental Health Congress, October 21-24, 2016; San Antonio, TX.