User login
The liver transplant recipient: What you need to know for long-term care
- In general, long-term treatment of hypertension, diabetes, and obesity after liver transplantation is similar to that for the general population ( C ).
- Measure bone density within the first year after transplantation. Treat osteoporosis with standard agents. Joint replacement surgery appears safe in this group of patients ( B ).
- Resume standard screening for malignancy 2 to 3 years after transplantation, and repeat at intervals similar to that used with the general population. Given the high risk of skin cancer, transplant recipients should wear sunblock (SPF > 40) and have routine dermatologic examinations ( B ).
- Patients should wait at least 2 years before considering pregnancy and use barrier-type methods in this period ( C ).
- Vaccinate patients against hepatitis A and B, influenza, and pneumococcus. Avoid live vaccines ( C ).
Orthotopic liver transplantation (OLT) is the replacement of a whole diseased liver with a healthy donor liver. The number of persons receiving OLT is increasing. Though it is unlikely you will be involved in the care of a patient immediately after OLT, you ’ ll need to know about the complications that occur in this period as they may impact the long-term care of the patient.
Long-term issues — such as cardiovascular disease, bone disease, malignancy, anemia, psychiatric disorders, and financial stressors — put these patients at higher risk for problems more than the average patient. Perhaps the most important task is for you to keep in contact with the transplant center when questions or concerns arise. Over time, you will once again become the primary physician and advocate for these patients.
Complications after transplant (less than 1 year)
Within 1 month post-OLT, the most frequent complications are acute graft rejection, vascular thrombosis, biliary leak or stricture, and infection. Between 1 and 3 months, acute and chronic graft rejection can occur, but medication toxicity and opportunistic infections become more common (TABLE 1). 1
A broad range of infections may develop, including cytomegalovirus, Epstein-Barr virus, herpes simplex virus, varicella zoster virus, adenovirus, tuberculosis, Pneumocystis , toxoplasmosis, Listeria spp, Candida spp, Aspergillus spp, and Cryptococcus spp. During this time, doses of immunosuppressive agents are lowered and corticosteroids are discontinued in many patients.
Once patients are considered stable after OLT, they will likely come under your supervision again. While opportunistic infections, surgical issues, and acute rejection become less common between 3 and 12 months, other complications related to OLT may occur.
Graft reinfection with hepatitis C virus (HCV) is universal, and 50% to 80% patients will develop biopsy-proven hepatitis. 2 Many will require treatment for recurrent HCV to avoid progression to cirrhosis.
Recurrent hepatitis B infection is much less common due to prophylactic therapy with hepatitis B immunoglobulin and antiviral medications, although 10% of transplant recipients will develop hepatitis despite prophylaxis.
Other causes of recurrent liver disease post-OLT include liver injury due to recurrent drug or alcohol abuse, non-alcoholic steatohepatitis, cholestatic and autoimmune liver disease, and liver cancer.
Toxicity due to immunosuppressive medications is also common in this time frame (TABLE 2). 3 Be alert to the potential for hepatotoxicity and drug interactions with any new pharmacologic agent. Other drugs (eg, lipid-lowering agents, antibiotics, antifungals) may cause liver injury on their own and need to be closely monitored.
Lastly, even though patients are at increased risk for such common infections as influenza, pneumonia, and urinary tract infections, opportunistic infections are uncommon in this period. Keep in mind that patients usually develop infections that are community-acquired and not opportunistic, particularly as time goes on.
TABLE 1
Common complications immediately after liver transplantation
| COMPLICATION | SIGNS/SYMPTOMS | LABORATORY TESTS | INITIAL MANAGEMENT |
|---|---|---|---|
| Acute rejection | Usually nonspecific or asymptomatic; low-grade fever, malaise, RUQ pain | Early: high AP, GGT; mild AST/ALT | 1) Doppler U/S: exclude HAT, biliary obstruction |
| Severe: high AST/ALT (usually < 1000) and TB | 2) Liver biopsy | ||
| Biliary obstruction or leak | Nonspecific to cholangitis (high fever, jaundice, sepsis); often no abdominal pain | High TB, AP, GGT | 1) Doppler U/S: exclude HAT, evaluate bile duct dilation |
| Less common: elevations in AST/ALT | 2) T-tube cholangiogram | ||
| 3) ERCP or PTC; surgical revision if failure | |||
| Hepatic artery thrombosis (HAT) | High fever, RUQ pain, jaundice; may progress to liver failure rapidly | High AST/ALT, TB Prolonged INR | 1) Doppler U/S: evaluate artery flow, bile ducts, liver abscess, infarction; if HAT, urgent revascularization |
| 2) Equivocal presentation: arteriography | |||
| Hepatic vein or inferior vena cava obstruction | Hepatomegaly, ascites, lower extremity edema | Nonspecific liver test abnormalities | 1) Doppler U/S |
| 2) If positive or negative+high suspicion, contrast venogram; dilation/stent procedure if stenosis or thrombosis | |||
| Portal vein thrombosis | Hematemesis (variceal bleed), abdominal pain ± ascites | Nonspecific liver test abnormalities; rarely high liver enzymes | 1) Doppler U/S |
| 2) If positive or negative+high suspicion: arteriography with portal venous phase; treat with shunt or retransplantation | |||
| Calcineurin-inhibitor toxicity | Tremor, headache, seizure, gastrointestinal | Elevated creatinine | 1) Drug level and hold if high |
| Hyperkalemia | 2) Replete electrolytes/fluids | ||
| Hypomagnesemia | 3) Review other medications for interactions ( TABLE 2 ) | ||
| Anemia | |||
| ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; ERCP, endoscopic retrograde cholangiopancreatography; GGT, gamma glutamyl-transferase; HAT, hepatic artery thrombosis; INR, international normalized ratio; PTC, percutaneous transhepatic cholangiography, RUQ, right upper quadrant; TB, total bilirubin; U/S, ultrasound | |||
TABLE 2
Immunosuppressive medications and interactions after liver transplantation
| MEDICATION | SIDE EFFECTS | MONITORING | COMMON DRUG INTERACTIONS |
|---|---|---|---|
| Corticosteroids | Weight gain, diabetes, hypertension, high lipids, neurotoxic, cataracts, osteoporosis | Glucose | |
| Blood pressure | |||
| Lipids | |||
| Tacrolimus | Diabetes, hypertension, high lipids, nephrotoxic, neurotoxic, gastrointestinal, high potassium, low magnesium | As above | Increased levels with azole antifungals, macrolide antibiotics, diltiazem, verapamil, danazol, metoclopromide |
| Drug levels | Decreased levels with rifampicin, phenobarbital, phenytoin, carbamazepine, St. John ’ s wort | ||
| Renal function | |||
| Electrolytes | |||
| Cyclosporine | Same as tacrolimus+gingival hyperplasia, hirsutism, rare hepatotoxicity | As tacrolimus | As tacrolimus; increased levels with grapefruit juice and sirolimus |
| Mycophenylate mofetil | Anemia, leukopenia, thrombocytopenia, gastrointestinal | CBC | May increase acyclovir levels Antacids, cholestyramine: lower absorption |
| Azathioprine | Same as mycophenylate+pancreatitis, hepatotoxicity | CBC Liver function tests | Allopurinol, ACE inhibitors, sirolimus: may potentiate marrow toxicity |
| Liver function tests | May lower anticoagulation effect of warfarin | ||
| Sirolimus | Same as mycophenylate+hyperlipidemia, hypertension, hypokalemia, diarrhea | CBC | |
| Lipids | |||
| Abbreviations: ACE, angiotensin-converting enzyme; CBC, complete blood count. | |||
Long-term complications
Cardiovascular disease
Up to 20% of late deaths after OLT are caused by cardiovascular disease. 4 Uncontrollable factors, such as preexisting cardiac disease, male sex, family history of cardiac disease, and advanced age contribute to the incidence of cardiovascular disease. However, a number of potentially controllable factors, such as hypertension, hyperlipidemia, obesity, and diabetes are common after OLT and should be addressed.
Hypertension. Hypertension occurs in 40% to 75% of OLT patients. 5 Causes include calcineurin-inhibitor (cyclosporine, tacrolimus) therapy, high-dose corticosteroids, and renal insufficiency. Calcineurin inhibitors cause renal vasoconstriction, leading to sodium retention and hypertension. Reducing the doses of these medications by the transplant center typically improves blood pressure control.
Treatment of choice for hypertension depends on how recently the transplant was performed. In the first 6 months following the procedure, dihydropyridine calcium-channel blockers (eg, amlodipine) and alpha-blockers are the mainstay of therapy, although peripheral edema and orthostatic hypotension may affect their tolerability. Diuretics can also be used in volume-overloaded patients.
After 6 months, other pharmacologic agents, such as angiotensin-converting enzyme (ACE) inhibitors and beta-blockers, can be administered to patients with stable renal function and without other contraindications (strength of recommendation [SOR]: C ). Long-term management of hypertension does not differ significantly from that in non-transplant patients.
Hyperlipidemia/obesity. Obesity and hyperlipidemia may affect up to half of OLT patients. Factors that contribute to both disorders include immunosuppressive drugs, increased appetite, diabetes, pretransplant hyperlipidemia, and history of cholestatic liver disease.
For hyperlipidemia, lifestyle modifications, such as diet and exercise, are recommended. If these measures are ineffective, statins are first-line agents. Avoid bile acid binding resins, which may interfere with the absorption of all medications. For refractory cases, switching from cyclosporine to tacrolimus under the direction of the transplant center might be indicated.
Treatment of obesity following OLT should also focus on lifestyle changes, as the safety of pharmacotherapy and surgery for obesity is uncertain in these patients.
Glucose intolerance and diabetes. Many patients will have glucose intolerance that resolves after steroid withdrawal. Main risk factors are pre-OLT diabetes, episodes of steroid-resistant rejection, and obesity. Post-OLT onset of diabetes will persist for only a small percentage of patients. 6
More than 56,000 liver transplants have been performed since the United Network for Organ Sharing created a national database for liver transplantation in 1988. In 2002, more than 5000 liver transplants were performed and more than 17,000 patients were on the waiting list for transplantation. Approximately 70% to 80% of these patients will survive to 5 years after transplantation and sustain a high quality of life long-term.
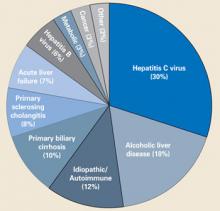
The most common indications for OLT in the US are shown in the FIGURE .7 Cirrhosis due to hepatitis C, chronic alcohol use, and idiopathic/autoimmune causes comprise almost 60% of the indications. Patients who meet minimal listing criteria may be placed on the waiting list for liver transplantation.
On February 27, 2002, a new nationwide system called MELD (Model for End-Stage Liver Disease) was adopted to rank patients on the waiting list based on the severity of liver disease and remove the subjectivity associated with the previous ranking system.8 The MELD score, which ranges from 6 to 40, is a mathematical computation based on the patient ’ s bilirubin, creatinine, and international normalized ratio (INR). Although early in use, the MELD system appears to be a good predictor of the need for transplantation and posttransplantation outcome.
Treatment of post-transplant diabetes is similar to that for any patient. Insulin is often required initially, but with reduction in immunosuppression and corticosteroids, patients can usually be switched to oral agents. Though there is no absolute contraindication to using any antidiabetes medications, most physicians try to avoid those with potential hepatotoxicity, such as the thiazolidinediones (SOR: C).
Weight loss is critical and often improves glucose tolerance. Transplant centers may switch patients from tacrolimus to cyclosporine to control hyperglycemia. Long-term screening for end organ complications (retinopathy, nephropathy, neuropathy) is as important for this population as it is for in non-transplant diabetics.
FIGURE
Indications for liver transplantation in the US
Renal disease
Up to 20% of OLT recipients develop end-stage renal disease, requiring hemodialysis or renal transplantation within 10 years after transplant. 5 If patients have renal dysfunction before OLT, lower-dose calcineurin inhibitors and using alternative immunosuppression post-OLT may improve renal function in the long term. A rise in creatinine in the first year after OLT is a strong risk factor for long-term development of renal insufficiency, while stable creatinine levels at 1 year usually indicate long-term maintenance of renal function.9,10
Closely monitor patients with early renal dysfunction, avoid nephrotoxic agents, and reduce or withdraw calcineurin inhibitors (as directed by the transplant center). Occasionally renal transplantation will be indicated.
Be aware that all OLT recipients need adequate hydration during acute illnesses (influenza, common colds, gastroenteritis), especially if they have renal dysfunction. Potential nephrotoxic agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs), aminoglycoside antibiotics, and intravenous contrast, should be avoided if possible.
Bone disease
Osteoporosis should be screened for and identified before OLT. Contributing factors for bone disease after transplant include preexisting osteoporosis, immobility, vitamin D deficiency, corticosteroid use, and hypogonadism. In the first 6 months after transplant, bone mineral density (BMD) significantly declines, often accelerated by immunosuppressive medications, corticosteroids, and immobility. 11 - 14 After 6 months, BMD increases rapidly and, by 12 months, approaches pre-OLT values. All patients should have bone densitometry performed before OLT or before hospital discharge and receive calcium (1500 mg/d) and vitamin D (800 IU/d) supplementation (SOR: C).
Unless significant risk factors for osteoporosis are present (eg, continued use of corticosteroids, history of bone loss, fracture, or cholestatic liver disease), it is unclear whether low-risk patients should have serial bone densitometry tests performed in the years following OLT. Patients with T-scores ≥ 2 standard deviations below mean should be considered for antiresorptive therapy. Given the recent concerns regarding estrogen use and cardiovascular disease, bisphosphonates and calcitonin are preferred. For patients who develop fractures or avascular necrosis from corticosteroids, joint replacement surgery appears to be safe and effective post-OLT (SOR: B).15,16
Malignancy
Of all of the complications following OLT, malignancy causes the highest morbidity and mortality. The overall incidence of malignancy is between 2.3% and 12.9% and may be up to 5 times higher than in the general population.17,18 The most common malignancies are post-transplant lymphoproliferative disorder (1% – 4.4%) and nonmelanoma skin cancer (0.5% – 4.3%); less common are gastrointestinal (0.4% – 1.0%), genitourinary (0.2% – 2.2%), lung (0.2% – 0.8%), and oropharyngeal (0.4% – 0.8%) malignancies.17 Many patients with small liver cancers (1 lesion < 5 cm or up to 3 lesions each < 3 cm) are receiving transplants and, despite the risk of recurrence post-OLT, have a similar survival rate as patients receiving OLT for other indications.
Though it is clear that OLT recipients are at higher risk than the general population for malignancy, there are no specific guidelines for screening. However, based on the risk of early malignancy, screening should resume within the first 2 to 3 years after OLT (SOR: B).19- 22 Most transplant centers will recommend either performing a more intense screening protocol than for non-transplant patients or individualizing screening protocols for each patient depending on risk factors.
Advise patients who spend time in the sun to wear sun block with a protective factor > 40 and to have routine skin examinations. It is unclear whether colorectal cancer screening in OLT recipients should occur more frequently than the general population. Colorectal adenomas may be more common among OLT recipients than among healthy controls, 23 but until more data are available, screening should mirror that of the general population (SOR: B). Since hepatocellular carcinoma may recur after OLT, transplant centers typically request imaging (computed tomography, ultrasound, magnetic resonance imaging) at regular intervals after OLT.
Use your discretion when screening for other common malignancies, such as breast, cervical, and prostate cancer. It is unclear whether screening specific groups of patients (such as tobacco smokers) for oropharyngeal, lung, and genitourinary cancer will be cost-effective or impact survival.
Anemia
The prevalence of anemia after OLT reportedly is between 4.3% and 28.2%, depending on the population studied and time after transplantation. 24 , 25 Blood loss, sepsis, medications, renal dysfunction, or hypersplenism can contribute to immediate postoperative anemia. Beyond the immediate postoperative period, anemia may be related to different causes (TABLE 3). 26 Medication-induced anemia is usually related to bone marrow suppression, although calcineurin inhibitors may cause microangiopathic hemolysis, hemolyticuremic syndrome, or pure redcell aplasia.
Viral infections often cause anemia in the first 12 weeks after transplantation. Aplastic anemia may be related to parvovirus B19 infection, although it is more commonly seen in patients who undergo liver transplantation for acute liver failure.24,27 Posttransplant lymphoproliferative disorder ranges from polyclonal B-cell hyperplasia (related to Epstein-Barr virus) that responds to reduction in immunosuppression to aggressive lymphoma treated with high dose chemotherapy.
Graft-versus-host disease is a rare but important cause of pancytopenia after OLT and is diagnosed by establishing chimerism, donor and recipient lymphocytes, in the blood and bone marrow; mortality is high.28
Lastly, renal failure and iron deficiency are other common causes of anemia after OLT that warrant investigation. Despite complete evaluation, half of adult patients do not have an identifiable cause of anemia and may respond to a therapeutic trial of erythropoietin (SOR: C).26
TABLE 3
Evaluation of anemia after liver transplantation
| CAUSE | TIME AFTER TRANSPLANT* | EVALUATION |
|---|---|---|
| Medications | ||
| Common : mycophenylate mofetil, azathioprine, sirolimus, tacrolimus, cyclosporine, interferon, ganciclovir | > 2 weeks | Alter immunosuppression, discontinue drug |
| Infrequent : dapsone, furosemide, trimethoprim/sulfamethoxazole | Discontinue drug | |
| Viral Infection | ||
| Parvovivurs B19 | 2 – 6 weeks | IgM titer, B19 DNA |
| Cytomegalovirus | 4 – 12 weeks | Rapid antigen, DNA |
| Epstein-Barr virus | 4 – 12 weeks | IgM titer, DNA |
| Aplastic anemia | 2 – 6 weeks | Bone marrow biopsy |
| Post-transplant lymphoproliferative disorder | > 6 weeks | Hemolysis indices (indirect bilirubin, haptoglobin, Coomb ’ s test), bone marrow biopsy |
| Graft-versus-host disease | 2 – 6 weeks | Demonstrate chimerism |
| Renal insufficiency | ||
| Common : tacrolimus, cyclosporine, diabetes, hypertension | > 2 weeks | Alter immunosuppression, treat diabetes/hypertension |
| Infrequent : HBV/HCV-related glomerulonephritis or cryoglobulinemia | Urinalysis, HBV DNA, HCV RNA, renal ultrasound/biopsy | |
| Iron-deficiency | > 6 weeks | Iron studies, evaluate for chronic blood loss (GI, GU) |
| Unknown cause | > 6 weeks | EPO trial |
| * These values represent the typical interval after transplantation | ||
| EPO, erythropoietin; GI, gastrointestinal; GU, genitourinary; HBV, hepatitis B virus; HCV, hepatitis C virus; IgM, immunoglobulin M. | ||
Psychosocial and socioeconomic concerns
Liver transplantation is a tremendously stressful and life-altering procedure affecting patients and their families. In the initial postoperative period, the stress of the operation and other factors (immunosuppression, infection, prolonged hospital stay) can lead to a variety of psychiatric disorders, such as delirium, anxiety, depression, mania, and psychosis. A multidisciplinary approach, including psychiatry, social work, and nursing care, is required to help the patients and families through this period, as expectations for full recovery may be delayed by psychiatric conditions.
Psychiatric problems
Many transplant recipients have long-term psychiatric problems. Depression and anxiety diminish quality of life, particularly for patients whose transplant was for hepatitis C and those with post-transplant viral recurrence.29,30 Most patients will respond to antidepressants and ongoing psychiatric care. The side-effect profile should be individualized for each patient, keeping in mind the potential interactions with the current medications.
Mania and hypomania, while less common than depression, are often related to higher doses of immunosuppression (eg, corticosteroids). Cyclosporine may increase lithium levels, leading to toxicity.31 Treatment with anticonvulsant medications, such as carbamazepine, may decrease calcineurin-inhibitor levels and should be monitored in coordination with the transplant team. Finally, some patients with encephalopathy prior to OLT have persistent cognitive deficits long after OLT.32
Drug and alcohol recidivism are common post-OLT and typically occurs in about 20% of patients. It is important that active steps are taken to avoid recidivism immediately after OLT. Long-term psychiatric care and continued attendance at support groups help maintain sobriety. The important contributions you can make are maintaining a heightened awareness for recidivism, communicating with patients regularly about drug and alcohol abuse, and providing support and referral services.
Socioeconomic problems
While most transplant recipients maintain a good quality of life, some have long-term socioeconomic problems. One study showed that only one third of OLT recipients returned successfully to work, just slightly higher than the percentage working before OLT.33 The economic situation improved in 11.9% of the recipients, worsened in 33.9%, and stayed the same in 54.2%. Concurrent illness, prolonged inactivity, psychiatric disorders, and the level of physical requirements at work are the main contributing factors to unemployment.
Another major stressor is medical cost. The average cost of immunosuppressive medications alone is $10,000 to $20,000 per year.3 Most of the charges are reimbursable, although this depends on the payer and time from transplantation. Medicare pays for immunosuppressive medications for only 36 months. Beyond that point, patients will require secondary insurance or other assistance. This expenditure is exacerbated by the cost of other medications, clinic visits with the transplant center, family physicians, and specialists, and time away from work.
Although patients are usually well informed of these concerns before OLT, they often do not appreciate the financial magnitude until after OLT. Encourage patients to return to work, stay active physically and mentally, and prepare for these financial considerations.
Sexual issues
Some patients have persistent sexual dysfunction that may have an organic basis (cardiovascular, renal, liver, endocrine) requiring investigation. The safety and efficacy of sildenafil (Viagra) in OLT recipients has not been investigated to date.
However, other patients regain their libido and gonadal function immediately after OLT; pregnancy may occur in this period. Advise patients to wait at least 2 years post-OLT before considering pregnancy (SOR: C). 34 Contraception, preferably barrier-type, should be used during sexual intercourse. Hormonal contraceptives are not contraindicated but should probably not be administered until the patient ’ s transplant status is stable.
If pregnancy does occur, apprise the patient of potential complications and adverse outcomes. Hypertension and preeclampsia are more common in pregnant OLT recipients; life-threatening infections and acute rejection are rare. Fortunately, most patients deliver healthy babies; miscarriages, stillbirths, and malformations are uncommon. An obstetrician specializing in high-risk pregnancy should follow all pregnant OLT recipients.
Vaccination
Vaccination after OLT is controversial. Live vaccines are generally contraindicated post-OLT and their safety in patients with stable graft function and on low levels of immunosuppression is unclear (SOR: C). Patients should receive pneumococcal vaccination, hepatitis A and B vaccination if not already immune, and yearly influenza vaccination (SOR: C). For travel outside of the US or in uncertain situations or exposures, the best reference is the Centers for Disease Control web site: www.cdc.org.
Communication with the transplant center
Direct communication with the patient ’ s transplant center is extremely important. You and the transplant center should determine the most effective way (phone, fax or e-mail) to communicate.
When should you contact the transplant center? First, obtain the center ’ s approval for any new medications that may be used long-term or have the potential for nephrotoxicity, hepatotoxicity, or immunosuppression. Second, notify the transplant center in the event of new signs or symptoms, such as fever, weight loss, abdominal pain, or jaundice. Being cautious by communicating early is often the most prudent course. Third, alert the transplant center of any hospitalizations. Transfer to the transplant center for any transplant-related problem or prolonged hospitalization usually provides the best outcome for the patient.
On the flip side, you are the primary caretaker, and the transplant center should regularly communicate with you regarding general medical concerns and any new diagnoses, interventions, or treatments. The transplant center should also regularly communicate with you regarding general medical concerns and any new diagnoses, interventions, or treatments. A strong, mutual relationship between you and the transplant center will have great impact on the recipient ’ s long-term care.
CORRESPONDENCE
Josh Levitsky, MD, Northwestern Memorial Hospital, 675 North St. Clair St, Suite 15-250, Chicago, IL 60611. E-mail: [email protected]
1. Killenger PG, Clavien PA. Medical Care of the Liver Transplant Patient. 2nd ed. Malden, Mass: Blackwell Science, 2001: pp 183-232.
2. Fukumoto T, Berg T, Ku Y, Bechstein WO, et al. . Viral dynamics of hepatitis C early after orthotopic liver transplantation: Evidence for rapid turnover of serum virions. Hepatology 1996 ;24 :1351 -1354.
3. McCashland TM. Posttransplantation care: role of the primary care physician versus transplant center. Liver Transpl 2001 ;7(Suppl 1) :S2 -S12.
4. Romero M, Parera A, Salcedo M, et al. . Cardiovascular risk factors and late cardiovascular disease in liver transplantation. Transplantation Proc 1999 ;31 :2364 -2365.
5. Gonwa TA. Hypertension and renal dysfunction in long-term liver transplant recipients. Liver Transpl 2001 ;7(Suppl 1) :S22 -S26.
6. Reuben A. Long-term management of the liver transplant patient: diabetes, hyperlipidemia, and obesity. Liver Transpl 2001 ;7(Suppl 1) :S13 -S21.
7. Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transplantation 2004 ;10 :886 -897.
8. Kamath PS, Wiesner RH, Malinchoc M, et al. . A model to predict survival in patients with end-stage liver disease. Hepatology 2001 ;33 :464 -470.
9. Jain A, Reyes J, Kashyap R, et al. . What have we learned about primary liver transplantation under tacrolimus immunosuppression? Long-term follow-up of the first 1000 patients. Ann Surg 1999 ;230 :441 -448.
10. Gonwa TA, Mai ML, Melton LB, et al. . End stage renal disease (ESRD) following orthotopic liver transplantation (OLTX) utilizing calcineurin based immunotherapy: Risk of development and treatment. Transplantation 2001 ;72 :1934 -1939.
11. McDonald JA, Dunstan CR, Dilworth P, et al. . Bone loss after liver transplantation. Hepatology 1991 ;14 :613 -619.
12. Monegal A, Navasa M, Guanabens N, et al. . Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporosis Intl 2001 ;12 :484 -492.
13. Keogh JB, Tsalamandris C, Sewell RB, et al. . Bone loss at the proximal femur and reduced lean mass following liver transplantation: a longitudinal study. Nutrition 1999 ;15 :661 -664.
14. Crosbie OM, Freaney R, McKenna M, et al. . Predicting bone loss following orthotopic liver transplantation. Gut 1999 ;44 :430 -434.
15. Papagelopoulos PJ, Hay JE, Galanis EC, Morrey BF. Total joint arthroplasty in orthotopic liver transplant recipients. J Arthroplasty 1996 ;11 :889 -892.
16. Levitsky J, Te HS, Cohen SM. The safety and outcome of joint replacement surgery in liver transplant recipients. Liver Transpl 2003 ;9 :373 -376.
17. Fung JJ, Jain A, Kwak EJ, et al. . De novo malignancies after liver transplantation: A major cause of late death. Liver Transpl 2001 ;7 :S109 -S118.
18. Frezza EE, Fung JJ, van Thiel DH. Non-lymphoid cancer after liver transplantation. Hepatogastroenterology 1997 ;44 :1172 -1181.
19. Jain AB, Yee LD, Nalesnik MA, et al. . Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using Surveillance Epidemiologic End Result data. Transplantation 1998 ;66 :1193 -1200.
20. Sheil AGR. Malignancy following liver transplantation: A report from the Australian Combined Liver Transplant Registry. Transplant Proc 1995 ;27 :1247. -
21. Sheiner PA, Magliocca JF, Bodian CA, et al. . Long-term medical complications in patients surviving ≥ 5 years after liver transplantation. Transplantation 2000 ;69 :781 -789.
22. Levy M, Backman L, Husberg B, et al. . De novo malignancy following liver transplantation: A single center study. Transplant Proc 1993 ;25 :1397 -1399.
23. Atassi R, Thuluvath PJ. Risk of colorectal adenoma in liver transplant recipients compared to immunocompetent control population undergoing routine screening colonoscopy. J Clin Gastroenterol 2003 ;37 :72 -73.
24. Ndibmie OK, Frezza E, Jordan HA, Koch W, van Thiel DH. Parvovirus B19 in anemia liver transplant recipients. Clin Diagn Lab Immunol 1996 ;3 :756 -760.
25. Misra S, Moore TB, Ament ME, Vargas JH, Busitill RW, McDiarmid SV. Profile of anemia in children after liver transplantation. Transplantation 2000 ;70 :1459 -1463.
26. Maheshwari A, Mishra R, Thuluvath PJ. Post-liver transplant anemia: etiology and management. Liver Transpl 2004 ;10 :165 -173.
27. Tzakis AG, Arditi M, Whittington PF, et al. . Aplastic anemia complicating orthotopic liver transplantation for non-A, non-B hepatitis. N Engl J Med 1988 ;319 :393 -396.
28. Smith DM, Agura E, Netto G, et al. . Liver transplant-associated graft-versus-host disease. Transplantation 2003 ;75 :118 -126.
29. Singh N, Gayowski T, Wagener MM, Marino IR. Vulnerability to psychologic distress and depression in patients with end-stage liver disease due to hepatitis C virus. Clin Transplant 1997 ;11 :406 -411.
30. Paterson DL, Gayowski T, Wannstedt CF, et al. . Quality of life in long-term survivors after liver transplantation: impact of recurrent viral hepatitis C virus hepatitis. Clin Transplant 2000 ;14 :48 -54.
31. Trzepacz PT, DiMartini A, Tringali RD. Psychopharmacologic issues in organ transplantation. Part 2. Psychopharmacologic medications. Psychosomatics 1993 ;34 :290 -298.
32. Mechtcheriakov S, Graziadei IW, Mattedi M, et al. . Incomplete improvement of visuo-motor deficits in patients with minimal hepatic encephalopathy after liver transplantation. Liver Transpl 2004 ;10 :77 -83.
33. Loinaz C, Clemares M, Marqu é s E, et al. . Labor status of 137 patients with liver transplantation. Transplant Proc 1999 ;31 :2470 -2471.
34. Riely CA. Contraception and pregnancy after liver transplantation. Liver Transpl 2001 ;7(Suppl 1) :S74 -S76.
- In general, long-term treatment of hypertension, diabetes, and obesity after liver transplantation is similar to that for the general population ( C ).
- Measure bone density within the first year after transplantation. Treat osteoporosis with standard agents. Joint replacement surgery appears safe in this group of patients ( B ).
- Resume standard screening for malignancy 2 to 3 years after transplantation, and repeat at intervals similar to that used with the general population. Given the high risk of skin cancer, transplant recipients should wear sunblock (SPF > 40) and have routine dermatologic examinations ( B ).
- Patients should wait at least 2 years before considering pregnancy and use barrier-type methods in this period ( C ).
- Vaccinate patients against hepatitis A and B, influenza, and pneumococcus. Avoid live vaccines ( C ).
Orthotopic liver transplantation (OLT) is the replacement of a whole diseased liver with a healthy donor liver. The number of persons receiving OLT is increasing. Though it is unlikely you will be involved in the care of a patient immediately after OLT, you ’ ll need to know about the complications that occur in this period as they may impact the long-term care of the patient.
Long-term issues — such as cardiovascular disease, bone disease, malignancy, anemia, psychiatric disorders, and financial stressors — put these patients at higher risk for problems more than the average patient. Perhaps the most important task is for you to keep in contact with the transplant center when questions or concerns arise. Over time, you will once again become the primary physician and advocate for these patients.
Complications after transplant (less than 1 year)
Within 1 month post-OLT, the most frequent complications are acute graft rejection, vascular thrombosis, biliary leak or stricture, and infection. Between 1 and 3 months, acute and chronic graft rejection can occur, but medication toxicity and opportunistic infections become more common (TABLE 1). 1
A broad range of infections may develop, including cytomegalovirus, Epstein-Barr virus, herpes simplex virus, varicella zoster virus, adenovirus, tuberculosis, Pneumocystis , toxoplasmosis, Listeria spp, Candida spp, Aspergillus spp, and Cryptococcus spp. During this time, doses of immunosuppressive agents are lowered and corticosteroids are discontinued in many patients.
Once patients are considered stable after OLT, they will likely come under your supervision again. While opportunistic infections, surgical issues, and acute rejection become less common between 3 and 12 months, other complications related to OLT may occur.
Graft reinfection with hepatitis C virus (HCV) is universal, and 50% to 80% patients will develop biopsy-proven hepatitis. 2 Many will require treatment for recurrent HCV to avoid progression to cirrhosis.
Recurrent hepatitis B infection is much less common due to prophylactic therapy with hepatitis B immunoglobulin and antiviral medications, although 10% of transplant recipients will develop hepatitis despite prophylaxis.
Other causes of recurrent liver disease post-OLT include liver injury due to recurrent drug or alcohol abuse, non-alcoholic steatohepatitis, cholestatic and autoimmune liver disease, and liver cancer.
Toxicity due to immunosuppressive medications is also common in this time frame (TABLE 2). 3 Be alert to the potential for hepatotoxicity and drug interactions with any new pharmacologic agent. Other drugs (eg, lipid-lowering agents, antibiotics, antifungals) may cause liver injury on their own and need to be closely monitored.
Lastly, even though patients are at increased risk for such common infections as influenza, pneumonia, and urinary tract infections, opportunistic infections are uncommon in this period. Keep in mind that patients usually develop infections that are community-acquired and not opportunistic, particularly as time goes on.
TABLE 1
Common complications immediately after liver transplantation
| COMPLICATION | SIGNS/SYMPTOMS | LABORATORY TESTS | INITIAL MANAGEMENT |
|---|---|---|---|
| Acute rejection | Usually nonspecific or asymptomatic; low-grade fever, malaise, RUQ pain | Early: high AP, GGT; mild AST/ALT | 1) Doppler U/S: exclude HAT, biliary obstruction |
| Severe: high AST/ALT (usually < 1000) and TB | 2) Liver biopsy | ||
| Biliary obstruction or leak | Nonspecific to cholangitis (high fever, jaundice, sepsis); often no abdominal pain | High TB, AP, GGT | 1) Doppler U/S: exclude HAT, evaluate bile duct dilation |
| Less common: elevations in AST/ALT | 2) T-tube cholangiogram | ||
| 3) ERCP or PTC; surgical revision if failure | |||
| Hepatic artery thrombosis (HAT) | High fever, RUQ pain, jaundice; may progress to liver failure rapidly | High AST/ALT, TB Prolonged INR | 1) Doppler U/S: evaluate artery flow, bile ducts, liver abscess, infarction; if HAT, urgent revascularization |
| 2) Equivocal presentation: arteriography | |||
| Hepatic vein or inferior vena cava obstruction | Hepatomegaly, ascites, lower extremity edema | Nonspecific liver test abnormalities | 1) Doppler U/S |
| 2) If positive or negative+high suspicion, contrast venogram; dilation/stent procedure if stenosis or thrombosis | |||
| Portal vein thrombosis | Hematemesis (variceal bleed), abdominal pain ± ascites | Nonspecific liver test abnormalities; rarely high liver enzymes | 1) Doppler U/S |
| 2) If positive or negative+high suspicion: arteriography with portal venous phase; treat with shunt or retransplantation | |||
| Calcineurin-inhibitor toxicity | Tremor, headache, seizure, gastrointestinal | Elevated creatinine | 1) Drug level and hold if high |
| Hyperkalemia | 2) Replete electrolytes/fluids | ||
| Hypomagnesemia | 3) Review other medications for interactions ( TABLE 2 ) | ||
| Anemia | |||
| ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; ERCP, endoscopic retrograde cholangiopancreatography; GGT, gamma glutamyl-transferase; HAT, hepatic artery thrombosis; INR, international normalized ratio; PTC, percutaneous transhepatic cholangiography, RUQ, right upper quadrant; TB, total bilirubin; U/S, ultrasound | |||
TABLE 2
Immunosuppressive medications and interactions after liver transplantation
| MEDICATION | SIDE EFFECTS | MONITORING | COMMON DRUG INTERACTIONS |
|---|---|---|---|
| Corticosteroids | Weight gain, diabetes, hypertension, high lipids, neurotoxic, cataracts, osteoporosis | Glucose | |
| Blood pressure | |||
| Lipids | |||
| Tacrolimus | Diabetes, hypertension, high lipids, nephrotoxic, neurotoxic, gastrointestinal, high potassium, low magnesium | As above | Increased levels with azole antifungals, macrolide antibiotics, diltiazem, verapamil, danazol, metoclopromide |
| Drug levels | Decreased levels with rifampicin, phenobarbital, phenytoin, carbamazepine, St. John ’ s wort | ||
| Renal function | |||
| Electrolytes | |||
| Cyclosporine | Same as tacrolimus+gingival hyperplasia, hirsutism, rare hepatotoxicity | As tacrolimus | As tacrolimus; increased levels with grapefruit juice and sirolimus |
| Mycophenylate mofetil | Anemia, leukopenia, thrombocytopenia, gastrointestinal | CBC | May increase acyclovir levels Antacids, cholestyramine: lower absorption |
| Azathioprine | Same as mycophenylate+pancreatitis, hepatotoxicity | CBC Liver function tests | Allopurinol, ACE inhibitors, sirolimus: may potentiate marrow toxicity |
| Liver function tests | May lower anticoagulation effect of warfarin | ||
| Sirolimus | Same as mycophenylate+hyperlipidemia, hypertension, hypokalemia, diarrhea | CBC | |
| Lipids | |||
| Abbreviations: ACE, angiotensin-converting enzyme; CBC, complete blood count. | |||
Long-term complications
Cardiovascular disease
Up to 20% of late deaths after OLT are caused by cardiovascular disease. 4 Uncontrollable factors, such as preexisting cardiac disease, male sex, family history of cardiac disease, and advanced age contribute to the incidence of cardiovascular disease. However, a number of potentially controllable factors, such as hypertension, hyperlipidemia, obesity, and diabetes are common after OLT and should be addressed.
Hypertension. Hypertension occurs in 40% to 75% of OLT patients. 5 Causes include calcineurin-inhibitor (cyclosporine, tacrolimus) therapy, high-dose corticosteroids, and renal insufficiency. Calcineurin inhibitors cause renal vasoconstriction, leading to sodium retention and hypertension. Reducing the doses of these medications by the transplant center typically improves blood pressure control.
Treatment of choice for hypertension depends on how recently the transplant was performed. In the first 6 months following the procedure, dihydropyridine calcium-channel blockers (eg, amlodipine) and alpha-blockers are the mainstay of therapy, although peripheral edema and orthostatic hypotension may affect their tolerability. Diuretics can also be used in volume-overloaded patients.
After 6 months, other pharmacologic agents, such as angiotensin-converting enzyme (ACE) inhibitors and beta-blockers, can be administered to patients with stable renal function and without other contraindications (strength of recommendation [SOR]: C ). Long-term management of hypertension does not differ significantly from that in non-transplant patients.
Hyperlipidemia/obesity. Obesity and hyperlipidemia may affect up to half of OLT patients. Factors that contribute to both disorders include immunosuppressive drugs, increased appetite, diabetes, pretransplant hyperlipidemia, and history of cholestatic liver disease.
For hyperlipidemia, lifestyle modifications, such as diet and exercise, are recommended. If these measures are ineffective, statins are first-line agents. Avoid bile acid binding resins, which may interfere with the absorption of all medications. For refractory cases, switching from cyclosporine to tacrolimus under the direction of the transplant center might be indicated.
Treatment of obesity following OLT should also focus on lifestyle changes, as the safety of pharmacotherapy and surgery for obesity is uncertain in these patients.
Glucose intolerance and diabetes. Many patients will have glucose intolerance that resolves after steroid withdrawal. Main risk factors are pre-OLT diabetes, episodes of steroid-resistant rejection, and obesity. Post-OLT onset of diabetes will persist for only a small percentage of patients. 6
More than 56,000 liver transplants have been performed since the United Network for Organ Sharing created a national database for liver transplantation in 1988. In 2002, more than 5000 liver transplants were performed and more than 17,000 patients were on the waiting list for transplantation. Approximately 70% to 80% of these patients will survive to 5 years after transplantation and sustain a high quality of life long-term.
The most common indications for OLT in the US are shown in the FIGURE .7 Cirrhosis due to hepatitis C, chronic alcohol use, and idiopathic/autoimmune causes comprise almost 60% of the indications. Patients who meet minimal listing criteria may be placed on the waiting list for liver transplantation.
On February 27, 2002, a new nationwide system called MELD (Model for End-Stage Liver Disease) was adopted to rank patients on the waiting list based on the severity of liver disease and remove the subjectivity associated with the previous ranking system.8 The MELD score, which ranges from 6 to 40, is a mathematical computation based on the patient ’ s bilirubin, creatinine, and international normalized ratio (INR). Although early in use, the MELD system appears to be a good predictor of the need for transplantation and posttransplantation outcome.
Treatment of post-transplant diabetes is similar to that for any patient. Insulin is often required initially, but with reduction in immunosuppression and corticosteroids, patients can usually be switched to oral agents. Though there is no absolute contraindication to using any antidiabetes medications, most physicians try to avoid those with potential hepatotoxicity, such as the thiazolidinediones (SOR: C).
Weight loss is critical and often improves glucose tolerance. Transplant centers may switch patients from tacrolimus to cyclosporine to control hyperglycemia. Long-term screening for end organ complications (retinopathy, nephropathy, neuropathy) is as important for this population as it is for in non-transplant diabetics.
FIGURE
Indications for liver transplantation in the US
Renal disease
Up to 20% of OLT recipients develop end-stage renal disease, requiring hemodialysis or renal transplantation within 10 years after transplant. 5 If patients have renal dysfunction before OLT, lower-dose calcineurin inhibitors and using alternative immunosuppression post-OLT may improve renal function in the long term. A rise in creatinine in the first year after OLT is a strong risk factor for long-term development of renal insufficiency, while stable creatinine levels at 1 year usually indicate long-term maintenance of renal function.9,10
Closely monitor patients with early renal dysfunction, avoid nephrotoxic agents, and reduce or withdraw calcineurin inhibitors (as directed by the transplant center). Occasionally renal transplantation will be indicated.
Be aware that all OLT recipients need adequate hydration during acute illnesses (influenza, common colds, gastroenteritis), especially if they have renal dysfunction. Potential nephrotoxic agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs), aminoglycoside antibiotics, and intravenous contrast, should be avoided if possible.
Bone disease
Osteoporosis should be screened for and identified before OLT. Contributing factors for bone disease after transplant include preexisting osteoporosis, immobility, vitamin D deficiency, corticosteroid use, and hypogonadism. In the first 6 months after transplant, bone mineral density (BMD) significantly declines, often accelerated by immunosuppressive medications, corticosteroids, and immobility. 11 - 14 After 6 months, BMD increases rapidly and, by 12 months, approaches pre-OLT values. All patients should have bone densitometry performed before OLT or before hospital discharge and receive calcium (1500 mg/d) and vitamin D (800 IU/d) supplementation (SOR: C).
Unless significant risk factors for osteoporosis are present (eg, continued use of corticosteroids, history of bone loss, fracture, or cholestatic liver disease), it is unclear whether low-risk patients should have serial bone densitometry tests performed in the years following OLT. Patients with T-scores ≥ 2 standard deviations below mean should be considered for antiresorptive therapy. Given the recent concerns regarding estrogen use and cardiovascular disease, bisphosphonates and calcitonin are preferred. For patients who develop fractures or avascular necrosis from corticosteroids, joint replacement surgery appears to be safe and effective post-OLT (SOR: B).15,16
Malignancy
Of all of the complications following OLT, malignancy causes the highest morbidity and mortality. The overall incidence of malignancy is between 2.3% and 12.9% and may be up to 5 times higher than in the general population.17,18 The most common malignancies are post-transplant lymphoproliferative disorder (1% – 4.4%) and nonmelanoma skin cancer (0.5% – 4.3%); less common are gastrointestinal (0.4% – 1.0%), genitourinary (0.2% – 2.2%), lung (0.2% – 0.8%), and oropharyngeal (0.4% – 0.8%) malignancies.17 Many patients with small liver cancers (1 lesion < 5 cm or up to 3 lesions each < 3 cm) are receiving transplants and, despite the risk of recurrence post-OLT, have a similar survival rate as patients receiving OLT for other indications.
Though it is clear that OLT recipients are at higher risk than the general population for malignancy, there are no specific guidelines for screening. However, based on the risk of early malignancy, screening should resume within the first 2 to 3 years after OLT (SOR: B).19- 22 Most transplant centers will recommend either performing a more intense screening protocol than for non-transplant patients or individualizing screening protocols for each patient depending on risk factors.
Advise patients who spend time in the sun to wear sun block with a protective factor > 40 and to have routine skin examinations. It is unclear whether colorectal cancer screening in OLT recipients should occur more frequently than the general population. Colorectal adenomas may be more common among OLT recipients than among healthy controls, 23 but until more data are available, screening should mirror that of the general population (SOR: B). Since hepatocellular carcinoma may recur after OLT, transplant centers typically request imaging (computed tomography, ultrasound, magnetic resonance imaging) at regular intervals after OLT.
Use your discretion when screening for other common malignancies, such as breast, cervical, and prostate cancer. It is unclear whether screening specific groups of patients (such as tobacco smokers) for oropharyngeal, lung, and genitourinary cancer will be cost-effective or impact survival.
Anemia
The prevalence of anemia after OLT reportedly is between 4.3% and 28.2%, depending on the population studied and time after transplantation. 24 , 25 Blood loss, sepsis, medications, renal dysfunction, or hypersplenism can contribute to immediate postoperative anemia. Beyond the immediate postoperative period, anemia may be related to different causes (TABLE 3). 26 Medication-induced anemia is usually related to bone marrow suppression, although calcineurin inhibitors may cause microangiopathic hemolysis, hemolyticuremic syndrome, or pure redcell aplasia.
Viral infections often cause anemia in the first 12 weeks after transplantation. Aplastic anemia may be related to parvovirus B19 infection, although it is more commonly seen in patients who undergo liver transplantation for acute liver failure.24,27 Posttransplant lymphoproliferative disorder ranges from polyclonal B-cell hyperplasia (related to Epstein-Barr virus) that responds to reduction in immunosuppression to aggressive lymphoma treated with high dose chemotherapy.
Graft-versus-host disease is a rare but important cause of pancytopenia after OLT and is diagnosed by establishing chimerism, donor and recipient lymphocytes, in the blood and bone marrow; mortality is high.28
Lastly, renal failure and iron deficiency are other common causes of anemia after OLT that warrant investigation. Despite complete evaluation, half of adult patients do not have an identifiable cause of anemia and may respond to a therapeutic trial of erythropoietin (SOR: C).26
TABLE 3
Evaluation of anemia after liver transplantation
| CAUSE | TIME AFTER TRANSPLANT* | EVALUATION |
|---|---|---|
| Medications | ||
| Common : mycophenylate mofetil, azathioprine, sirolimus, tacrolimus, cyclosporine, interferon, ganciclovir | > 2 weeks | Alter immunosuppression, discontinue drug |
| Infrequent : dapsone, furosemide, trimethoprim/sulfamethoxazole | Discontinue drug | |
| Viral Infection | ||
| Parvovivurs B19 | 2 – 6 weeks | IgM titer, B19 DNA |
| Cytomegalovirus | 4 – 12 weeks | Rapid antigen, DNA |
| Epstein-Barr virus | 4 – 12 weeks | IgM titer, DNA |
| Aplastic anemia | 2 – 6 weeks | Bone marrow biopsy |
| Post-transplant lymphoproliferative disorder | > 6 weeks | Hemolysis indices (indirect bilirubin, haptoglobin, Coomb ’ s test), bone marrow biopsy |
| Graft-versus-host disease | 2 – 6 weeks | Demonstrate chimerism |
| Renal insufficiency | ||
| Common : tacrolimus, cyclosporine, diabetes, hypertension | > 2 weeks | Alter immunosuppression, treat diabetes/hypertension |
| Infrequent : HBV/HCV-related glomerulonephritis or cryoglobulinemia | Urinalysis, HBV DNA, HCV RNA, renal ultrasound/biopsy | |
| Iron-deficiency | > 6 weeks | Iron studies, evaluate for chronic blood loss (GI, GU) |
| Unknown cause | > 6 weeks | EPO trial |
| * These values represent the typical interval after transplantation | ||
| EPO, erythropoietin; GI, gastrointestinal; GU, genitourinary; HBV, hepatitis B virus; HCV, hepatitis C virus; IgM, immunoglobulin M. | ||
Psychosocial and socioeconomic concerns
Liver transplantation is a tremendously stressful and life-altering procedure affecting patients and their families. In the initial postoperative period, the stress of the operation and other factors (immunosuppression, infection, prolonged hospital stay) can lead to a variety of psychiatric disorders, such as delirium, anxiety, depression, mania, and psychosis. A multidisciplinary approach, including psychiatry, social work, and nursing care, is required to help the patients and families through this period, as expectations for full recovery may be delayed by psychiatric conditions.
Psychiatric problems
Many transplant recipients have long-term psychiatric problems. Depression and anxiety diminish quality of life, particularly for patients whose transplant was for hepatitis C and those with post-transplant viral recurrence.29,30 Most patients will respond to antidepressants and ongoing psychiatric care. The side-effect profile should be individualized for each patient, keeping in mind the potential interactions with the current medications.
Mania and hypomania, while less common than depression, are often related to higher doses of immunosuppression (eg, corticosteroids). Cyclosporine may increase lithium levels, leading to toxicity.31 Treatment with anticonvulsant medications, such as carbamazepine, may decrease calcineurin-inhibitor levels and should be monitored in coordination with the transplant team. Finally, some patients with encephalopathy prior to OLT have persistent cognitive deficits long after OLT.32
Drug and alcohol recidivism are common post-OLT and typically occurs in about 20% of patients. It is important that active steps are taken to avoid recidivism immediately after OLT. Long-term psychiatric care and continued attendance at support groups help maintain sobriety. The important contributions you can make are maintaining a heightened awareness for recidivism, communicating with patients regularly about drug and alcohol abuse, and providing support and referral services.
Socioeconomic problems
While most transplant recipients maintain a good quality of life, some have long-term socioeconomic problems. One study showed that only one third of OLT recipients returned successfully to work, just slightly higher than the percentage working before OLT.33 The economic situation improved in 11.9% of the recipients, worsened in 33.9%, and stayed the same in 54.2%. Concurrent illness, prolonged inactivity, psychiatric disorders, and the level of physical requirements at work are the main contributing factors to unemployment.
Another major stressor is medical cost. The average cost of immunosuppressive medications alone is $10,000 to $20,000 per year.3 Most of the charges are reimbursable, although this depends on the payer and time from transplantation. Medicare pays for immunosuppressive medications for only 36 months. Beyond that point, patients will require secondary insurance or other assistance. This expenditure is exacerbated by the cost of other medications, clinic visits with the transplant center, family physicians, and specialists, and time away from work.
Although patients are usually well informed of these concerns before OLT, they often do not appreciate the financial magnitude until after OLT. Encourage patients to return to work, stay active physically and mentally, and prepare for these financial considerations.
Sexual issues
Some patients have persistent sexual dysfunction that may have an organic basis (cardiovascular, renal, liver, endocrine) requiring investigation. The safety and efficacy of sildenafil (Viagra) in OLT recipients has not been investigated to date.
However, other patients regain their libido and gonadal function immediately after OLT; pregnancy may occur in this period. Advise patients to wait at least 2 years post-OLT before considering pregnancy (SOR: C). 34 Contraception, preferably barrier-type, should be used during sexual intercourse. Hormonal contraceptives are not contraindicated but should probably not be administered until the patient ’ s transplant status is stable.
If pregnancy does occur, apprise the patient of potential complications and adverse outcomes. Hypertension and preeclampsia are more common in pregnant OLT recipients; life-threatening infections and acute rejection are rare. Fortunately, most patients deliver healthy babies; miscarriages, stillbirths, and malformations are uncommon. An obstetrician specializing in high-risk pregnancy should follow all pregnant OLT recipients.
Vaccination
Vaccination after OLT is controversial. Live vaccines are generally contraindicated post-OLT and their safety in patients with stable graft function and on low levels of immunosuppression is unclear (SOR: C). Patients should receive pneumococcal vaccination, hepatitis A and B vaccination if not already immune, and yearly influenza vaccination (SOR: C). For travel outside of the US or in uncertain situations or exposures, the best reference is the Centers for Disease Control web site: www.cdc.org.
Communication with the transplant center
Direct communication with the patient ’ s transplant center is extremely important. You and the transplant center should determine the most effective way (phone, fax or e-mail) to communicate.
When should you contact the transplant center? First, obtain the center ’ s approval for any new medications that may be used long-term or have the potential for nephrotoxicity, hepatotoxicity, or immunosuppression. Second, notify the transplant center in the event of new signs or symptoms, such as fever, weight loss, abdominal pain, or jaundice. Being cautious by communicating early is often the most prudent course. Third, alert the transplant center of any hospitalizations. Transfer to the transplant center for any transplant-related problem or prolonged hospitalization usually provides the best outcome for the patient.
On the flip side, you are the primary caretaker, and the transplant center should regularly communicate with you regarding general medical concerns and any new diagnoses, interventions, or treatments. The transplant center should also regularly communicate with you regarding general medical concerns and any new diagnoses, interventions, or treatments. A strong, mutual relationship between you and the transplant center will have great impact on the recipient ’ s long-term care.
CORRESPONDENCE
Josh Levitsky, MD, Northwestern Memorial Hospital, 675 North St. Clair St, Suite 15-250, Chicago, IL 60611. E-mail: [email protected]
- In general, long-term treatment of hypertension, diabetes, and obesity after liver transplantation is similar to that for the general population ( C ).
- Measure bone density within the first year after transplantation. Treat osteoporosis with standard agents. Joint replacement surgery appears safe in this group of patients ( B ).
- Resume standard screening for malignancy 2 to 3 years after transplantation, and repeat at intervals similar to that used with the general population. Given the high risk of skin cancer, transplant recipients should wear sunblock (SPF > 40) and have routine dermatologic examinations ( B ).
- Patients should wait at least 2 years before considering pregnancy and use barrier-type methods in this period ( C ).
- Vaccinate patients against hepatitis A and B, influenza, and pneumococcus. Avoid live vaccines ( C ).
Orthotopic liver transplantation (OLT) is the replacement of a whole diseased liver with a healthy donor liver. The number of persons receiving OLT is increasing. Though it is unlikely you will be involved in the care of a patient immediately after OLT, you ’ ll need to know about the complications that occur in this period as they may impact the long-term care of the patient.
Long-term issues — such as cardiovascular disease, bone disease, malignancy, anemia, psychiatric disorders, and financial stressors — put these patients at higher risk for problems more than the average patient. Perhaps the most important task is for you to keep in contact with the transplant center when questions or concerns arise. Over time, you will once again become the primary physician and advocate for these patients.
Complications after transplant (less than 1 year)
Within 1 month post-OLT, the most frequent complications are acute graft rejection, vascular thrombosis, biliary leak or stricture, and infection. Between 1 and 3 months, acute and chronic graft rejection can occur, but medication toxicity and opportunistic infections become more common (TABLE 1). 1
A broad range of infections may develop, including cytomegalovirus, Epstein-Barr virus, herpes simplex virus, varicella zoster virus, adenovirus, tuberculosis, Pneumocystis , toxoplasmosis, Listeria spp, Candida spp, Aspergillus spp, and Cryptococcus spp. During this time, doses of immunosuppressive agents are lowered and corticosteroids are discontinued in many patients.
Once patients are considered stable after OLT, they will likely come under your supervision again. While opportunistic infections, surgical issues, and acute rejection become less common between 3 and 12 months, other complications related to OLT may occur.
Graft reinfection with hepatitis C virus (HCV) is universal, and 50% to 80% patients will develop biopsy-proven hepatitis. 2 Many will require treatment for recurrent HCV to avoid progression to cirrhosis.
Recurrent hepatitis B infection is much less common due to prophylactic therapy with hepatitis B immunoglobulin and antiviral medications, although 10% of transplant recipients will develop hepatitis despite prophylaxis.
Other causes of recurrent liver disease post-OLT include liver injury due to recurrent drug or alcohol abuse, non-alcoholic steatohepatitis, cholestatic and autoimmune liver disease, and liver cancer.
Toxicity due to immunosuppressive medications is also common in this time frame (TABLE 2). 3 Be alert to the potential for hepatotoxicity and drug interactions with any new pharmacologic agent. Other drugs (eg, lipid-lowering agents, antibiotics, antifungals) may cause liver injury on their own and need to be closely monitored.
Lastly, even though patients are at increased risk for such common infections as influenza, pneumonia, and urinary tract infections, opportunistic infections are uncommon in this period. Keep in mind that patients usually develop infections that are community-acquired and not opportunistic, particularly as time goes on.
TABLE 1
Common complications immediately after liver transplantation
| COMPLICATION | SIGNS/SYMPTOMS | LABORATORY TESTS | INITIAL MANAGEMENT |
|---|---|---|---|
| Acute rejection | Usually nonspecific or asymptomatic; low-grade fever, malaise, RUQ pain | Early: high AP, GGT; mild AST/ALT | 1) Doppler U/S: exclude HAT, biliary obstruction |
| Severe: high AST/ALT (usually < 1000) and TB | 2) Liver biopsy | ||
| Biliary obstruction or leak | Nonspecific to cholangitis (high fever, jaundice, sepsis); often no abdominal pain | High TB, AP, GGT | 1) Doppler U/S: exclude HAT, evaluate bile duct dilation |
| Less common: elevations in AST/ALT | 2) T-tube cholangiogram | ||
| 3) ERCP or PTC; surgical revision if failure | |||
| Hepatic artery thrombosis (HAT) | High fever, RUQ pain, jaundice; may progress to liver failure rapidly | High AST/ALT, TB Prolonged INR | 1) Doppler U/S: evaluate artery flow, bile ducts, liver abscess, infarction; if HAT, urgent revascularization |
| 2) Equivocal presentation: arteriography | |||
| Hepatic vein or inferior vena cava obstruction | Hepatomegaly, ascites, lower extremity edema | Nonspecific liver test abnormalities | 1) Doppler U/S |
| 2) If positive or negative+high suspicion, contrast venogram; dilation/stent procedure if stenosis or thrombosis | |||
| Portal vein thrombosis | Hematemesis (variceal bleed), abdominal pain ± ascites | Nonspecific liver test abnormalities; rarely high liver enzymes | 1) Doppler U/S |
| 2) If positive or negative+high suspicion: arteriography with portal venous phase; treat with shunt or retransplantation | |||
| Calcineurin-inhibitor toxicity | Tremor, headache, seizure, gastrointestinal | Elevated creatinine | 1) Drug level and hold if high |
| Hyperkalemia | 2) Replete electrolytes/fluids | ||
| Hypomagnesemia | 3) Review other medications for interactions ( TABLE 2 ) | ||
| Anemia | |||
| ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; ERCP, endoscopic retrograde cholangiopancreatography; GGT, gamma glutamyl-transferase; HAT, hepatic artery thrombosis; INR, international normalized ratio; PTC, percutaneous transhepatic cholangiography, RUQ, right upper quadrant; TB, total bilirubin; U/S, ultrasound | |||
TABLE 2
Immunosuppressive medications and interactions after liver transplantation
| MEDICATION | SIDE EFFECTS | MONITORING | COMMON DRUG INTERACTIONS |
|---|---|---|---|
| Corticosteroids | Weight gain, diabetes, hypertension, high lipids, neurotoxic, cataracts, osteoporosis | Glucose | |
| Blood pressure | |||
| Lipids | |||
| Tacrolimus | Diabetes, hypertension, high lipids, nephrotoxic, neurotoxic, gastrointestinal, high potassium, low magnesium | As above | Increased levels with azole antifungals, macrolide antibiotics, diltiazem, verapamil, danazol, metoclopromide |
| Drug levels | Decreased levels with rifampicin, phenobarbital, phenytoin, carbamazepine, St. John ’ s wort | ||
| Renal function | |||
| Electrolytes | |||
| Cyclosporine | Same as tacrolimus+gingival hyperplasia, hirsutism, rare hepatotoxicity | As tacrolimus | As tacrolimus; increased levels with grapefruit juice and sirolimus |
| Mycophenylate mofetil | Anemia, leukopenia, thrombocytopenia, gastrointestinal | CBC | May increase acyclovir levels Antacids, cholestyramine: lower absorption |
| Azathioprine | Same as mycophenylate+pancreatitis, hepatotoxicity | CBC Liver function tests | Allopurinol, ACE inhibitors, sirolimus: may potentiate marrow toxicity |
| Liver function tests | May lower anticoagulation effect of warfarin | ||
| Sirolimus | Same as mycophenylate+hyperlipidemia, hypertension, hypokalemia, diarrhea | CBC | |
| Lipids | |||
| Abbreviations: ACE, angiotensin-converting enzyme; CBC, complete blood count. | |||
Long-term complications
Cardiovascular disease
Up to 20% of late deaths after OLT are caused by cardiovascular disease. 4 Uncontrollable factors, such as preexisting cardiac disease, male sex, family history of cardiac disease, and advanced age contribute to the incidence of cardiovascular disease. However, a number of potentially controllable factors, such as hypertension, hyperlipidemia, obesity, and diabetes are common after OLT and should be addressed.
Hypertension. Hypertension occurs in 40% to 75% of OLT patients. 5 Causes include calcineurin-inhibitor (cyclosporine, tacrolimus) therapy, high-dose corticosteroids, and renal insufficiency. Calcineurin inhibitors cause renal vasoconstriction, leading to sodium retention and hypertension. Reducing the doses of these medications by the transplant center typically improves blood pressure control.
Treatment of choice for hypertension depends on how recently the transplant was performed. In the first 6 months following the procedure, dihydropyridine calcium-channel blockers (eg, amlodipine) and alpha-blockers are the mainstay of therapy, although peripheral edema and orthostatic hypotension may affect their tolerability. Diuretics can also be used in volume-overloaded patients.
After 6 months, other pharmacologic agents, such as angiotensin-converting enzyme (ACE) inhibitors and beta-blockers, can be administered to patients with stable renal function and without other contraindications (strength of recommendation [SOR]: C ). Long-term management of hypertension does not differ significantly from that in non-transplant patients.
Hyperlipidemia/obesity. Obesity and hyperlipidemia may affect up to half of OLT patients. Factors that contribute to both disorders include immunosuppressive drugs, increased appetite, diabetes, pretransplant hyperlipidemia, and history of cholestatic liver disease.
For hyperlipidemia, lifestyle modifications, such as diet and exercise, are recommended. If these measures are ineffective, statins are first-line agents. Avoid bile acid binding resins, which may interfere with the absorption of all medications. For refractory cases, switching from cyclosporine to tacrolimus under the direction of the transplant center might be indicated.
Treatment of obesity following OLT should also focus on lifestyle changes, as the safety of pharmacotherapy and surgery for obesity is uncertain in these patients.
Glucose intolerance and diabetes. Many patients will have glucose intolerance that resolves after steroid withdrawal. Main risk factors are pre-OLT diabetes, episodes of steroid-resistant rejection, and obesity. Post-OLT onset of diabetes will persist for only a small percentage of patients. 6
More than 56,000 liver transplants have been performed since the United Network for Organ Sharing created a national database for liver transplantation in 1988. In 2002, more than 5000 liver transplants were performed and more than 17,000 patients were on the waiting list for transplantation. Approximately 70% to 80% of these patients will survive to 5 years after transplantation and sustain a high quality of life long-term.
The most common indications for OLT in the US are shown in the FIGURE .7 Cirrhosis due to hepatitis C, chronic alcohol use, and idiopathic/autoimmune causes comprise almost 60% of the indications. Patients who meet minimal listing criteria may be placed on the waiting list for liver transplantation.
On February 27, 2002, a new nationwide system called MELD (Model for End-Stage Liver Disease) was adopted to rank patients on the waiting list based on the severity of liver disease and remove the subjectivity associated with the previous ranking system.8 The MELD score, which ranges from 6 to 40, is a mathematical computation based on the patient ’ s bilirubin, creatinine, and international normalized ratio (INR). Although early in use, the MELD system appears to be a good predictor of the need for transplantation and posttransplantation outcome.
Treatment of post-transplant diabetes is similar to that for any patient. Insulin is often required initially, but with reduction in immunosuppression and corticosteroids, patients can usually be switched to oral agents. Though there is no absolute contraindication to using any antidiabetes medications, most physicians try to avoid those with potential hepatotoxicity, such as the thiazolidinediones (SOR: C).
Weight loss is critical and often improves glucose tolerance. Transplant centers may switch patients from tacrolimus to cyclosporine to control hyperglycemia. Long-term screening for end organ complications (retinopathy, nephropathy, neuropathy) is as important for this population as it is for in non-transplant diabetics.
FIGURE
Indications for liver transplantation in the US
Renal disease
Up to 20% of OLT recipients develop end-stage renal disease, requiring hemodialysis or renal transplantation within 10 years after transplant. 5 If patients have renal dysfunction before OLT, lower-dose calcineurin inhibitors and using alternative immunosuppression post-OLT may improve renal function in the long term. A rise in creatinine in the first year after OLT is a strong risk factor for long-term development of renal insufficiency, while stable creatinine levels at 1 year usually indicate long-term maintenance of renal function.9,10
Closely monitor patients with early renal dysfunction, avoid nephrotoxic agents, and reduce or withdraw calcineurin inhibitors (as directed by the transplant center). Occasionally renal transplantation will be indicated.
Be aware that all OLT recipients need adequate hydration during acute illnesses (influenza, common colds, gastroenteritis), especially if they have renal dysfunction. Potential nephrotoxic agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs), aminoglycoside antibiotics, and intravenous contrast, should be avoided if possible.
Bone disease
Osteoporosis should be screened for and identified before OLT. Contributing factors for bone disease after transplant include preexisting osteoporosis, immobility, vitamin D deficiency, corticosteroid use, and hypogonadism. In the first 6 months after transplant, bone mineral density (BMD) significantly declines, often accelerated by immunosuppressive medications, corticosteroids, and immobility. 11 - 14 After 6 months, BMD increases rapidly and, by 12 months, approaches pre-OLT values. All patients should have bone densitometry performed before OLT or before hospital discharge and receive calcium (1500 mg/d) and vitamin D (800 IU/d) supplementation (SOR: C).
Unless significant risk factors for osteoporosis are present (eg, continued use of corticosteroids, history of bone loss, fracture, or cholestatic liver disease), it is unclear whether low-risk patients should have serial bone densitometry tests performed in the years following OLT. Patients with T-scores ≥ 2 standard deviations below mean should be considered for antiresorptive therapy. Given the recent concerns regarding estrogen use and cardiovascular disease, bisphosphonates and calcitonin are preferred. For patients who develop fractures or avascular necrosis from corticosteroids, joint replacement surgery appears to be safe and effective post-OLT (SOR: B).15,16
Malignancy
Of all of the complications following OLT, malignancy causes the highest morbidity and mortality. The overall incidence of malignancy is between 2.3% and 12.9% and may be up to 5 times higher than in the general population.17,18 The most common malignancies are post-transplant lymphoproliferative disorder (1% – 4.4%) and nonmelanoma skin cancer (0.5% – 4.3%); less common are gastrointestinal (0.4% – 1.0%), genitourinary (0.2% – 2.2%), lung (0.2% – 0.8%), and oropharyngeal (0.4% – 0.8%) malignancies.17 Many patients with small liver cancers (1 lesion < 5 cm or up to 3 lesions each < 3 cm) are receiving transplants and, despite the risk of recurrence post-OLT, have a similar survival rate as patients receiving OLT for other indications.
Though it is clear that OLT recipients are at higher risk than the general population for malignancy, there are no specific guidelines for screening. However, based on the risk of early malignancy, screening should resume within the first 2 to 3 years after OLT (SOR: B).19- 22 Most transplant centers will recommend either performing a more intense screening protocol than for non-transplant patients or individualizing screening protocols for each patient depending on risk factors.
Advise patients who spend time in the sun to wear sun block with a protective factor > 40 and to have routine skin examinations. It is unclear whether colorectal cancer screening in OLT recipients should occur more frequently than the general population. Colorectal adenomas may be more common among OLT recipients than among healthy controls, 23 but until more data are available, screening should mirror that of the general population (SOR: B). Since hepatocellular carcinoma may recur after OLT, transplant centers typically request imaging (computed tomography, ultrasound, magnetic resonance imaging) at regular intervals after OLT.
Use your discretion when screening for other common malignancies, such as breast, cervical, and prostate cancer. It is unclear whether screening specific groups of patients (such as tobacco smokers) for oropharyngeal, lung, and genitourinary cancer will be cost-effective or impact survival.
Anemia
The prevalence of anemia after OLT reportedly is between 4.3% and 28.2%, depending on the population studied and time after transplantation. 24 , 25 Blood loss, sepsis, medications, renal dysfunction, or hypersplenism can contribute to immediate postoperative anemia. Beyond the immediate postoperative period, anemia may be related to different causes (TABLE 3). 26 Medication-induced anemia is usually related to bone marrow suppression, although calcineurin inhibitors may cause microangiopathic hemolysis, hemolyticuremic syndrome, or pure redcell aplasia.
Viral infections often cause anemia in the first 12 weeks after transplantation. Aplastic anemia may be related to parvovirus B19 infection, although it is more commonly seen in patients who undergo liver transplantation for acute liver failure.24,27 Posttransplant lymphoproliferative disorder ranges from polyclonal B-cell hyperplasia (related to Epstein-Barr virus) that responds to reduction in immunosuppression to aggressive lymphoma treated with high dose chemotherapy.
Graft-versus-host disease is a rare but important cause of pancytopenia after OLT and is diagnosed by establishing chimerism, donor and recipient lymphocytes, in the blood and bone marrow; mortality is high.28
Lastly, renal failure and iron deficiency are other common causes of anemia after OLT that warrant investigation. Despite complete evaluation, half of adult patients do not have an identifiable cause of anemia and may respond to a therapeutic trial of erythropoietin (SOR: C).26
TABLE 3
Evaluation of anemia after liver transplantation
| CAUSE | TIME AFTER TRANSPLANT* | EVALUATION |
|---|---|---|
| Medications | ||
| Common : mycophenylate mofetil, azathioprine, sirolimus, tacrolimus, cyclosporine, interferon, ganciclovir | > 2 weeks | Alter immunosuppression, discontinue drug |
| Infrequent : dapsone, furosemide, trimethoprim/sulfamethoxazole | Discontinue drug | |
| Viral Infection | ||
| Parvovivurs B19 | 2 – 6 weeks | IgM titer, B19 DNA |
| Cytomegalovirus | 4 – 12 weeks | Rapid antigen, DNA |
| Epstein-Barr virus | 4 – 12 weeks | IgM titer, DNA |
| Aplastic anemia | 2 – 6 weeks | Bone marrow biopsy |
| Post-transplant lymphoproliferative disorder | > 6 weeks | Hemolysis indices (indirect bilirubin, haptoglobin, Coomb ’ s test), bone marrow biopsy |
| Graft-versus-host disease | 2 – 6 weeks | Demonstrate chimerism |
| Renal insufficiency | ||
| Common : tacrolimus, cyclosporine, diabetes, hypertension | > 2 weeks | Alter immunosuppression, treat diabetes/hypertension |
| Infrequent : HBV/HCV-related glomerulonephritis or cryoglobulinemia | Urinalysis, HBV DNA, HCV RNA, renal ultrasound/biopsy | |
| Iron-deficiency | > 6 weeks | Iron studies, evaluate for chronic blood loss (GI, GU) |
| Unknown cause | > 6 weeks | EPO trial |
| * These values represent the typical interval after transplantation | ||
| EPO, erythropoietin; GI, gastrointestinal; GU, genitourinary; HBV, hepatitis B virus; HCV, hepatitis C virus; IgM, immunoglobulin M. | ||
Psychosocial and socioeconomic concerns
Liver transplantation is a tremendously stressful and life-altering procedure affecting patients and their families. In the initial postoperative period, the stress of the operation and other factors (immunosuppression, infection, prolonged hospital stay) can lead to a variety of psychiatric disorders, such as delirium, anxiety, depression, mania, and psychosis. A multidisciplinary approach, including psychiatry, social work, and nursing care, is required to help the patients and families through this period, as expectations for full recovery may be delayed by psychiatric conditions.
Psychiatric problems
Many transplant recipients have long-term psychiatric problems. Depression and anxiety diminish quality of life, particularly for patients whose transplant was for hepatitis C and those with post-transplant viral recurrence.29,30 Most patients will respond to antidepressants and ongoing psychiatric care. The side-effect profile should be individualized for each patient, keeping in mind the potential interactions with the current medications.
Mania and hypomania, while less common than depression, are often related to higher doses of immunosuppression (eg, corticosteroids). Cyclosporine may increase lithium levels, leading to toxicity.31 Treatment with anticonvulsant medications, such as carbamazepine, may decrease calcineurin-inhibitor levels and should be monitored in coordination with the transplant team. Finally, some patients with encephalopathy prior to OLT have persistent cognitive deficits long after OLT.32
Drug and alcohol recidivism are common post-OLT and typically occurs in about 20% of patients. It is important that active steps are taken to avoid recidivism immediately after OLT. Long-term psychiatric care and continued attendance at support groups help maintain sobriety. The important contributions you can make are maintaining a heightened awareness for recidivism, communicating with patients regularly about drug and alcohol abuse, and providing support and referral services.
Socioeconomic problems
While most transplant recipients maintain a good quality of life, some have long-term socioeconomic problems. One study showed that only one third of OLT recipients returned successfully to work, just slightly higher than the percentage working before OLT.33 The economic situation improved in 11.9% of the recipients, worsened in 33.9%, and stayed the same in 54.2%. Concurrent illness, prolonged inactivity, psychiatric disorders, and the level of physical requirements at work are the main contributing factors to unemployment.
Another major stressor is medical cost. The average cost of immunosuppressive medications alone is $10,000 to $20,000 per year.3 Most of the charges are reimbursable, although this depends on the payer and time from transplantation. Medicare pays for immunosuppressive medications for only 36 months. Beyond that point, patients will require secondary insurance or other assistance. This expenditure is exacerbated by the cost of other medications, clinic visits with the transplant center, family physicians, and specialists, and time away from work.
Although patients are usually well informed of these concerns before OLT, they often do not appreciate the financial magnitude until after OLT. Encourage patients to return to work, stay active physically and mentally, and prepare for these financial considerations.
Sexual issues
Some patients have persistent sexual dysfunction that may have an organic basis (cardiovascular, renal, liver, endocrine) requiring investigation. The safety and efficacy of sildenafil (Viagra) in OLT recipients has not been investigated to date.
However, other patients regain their libido and gonadal function immediately after OLT; pregnancy may occur in this period. Advise patients to wait at least 2 years post-OLT before considering pregnancy (SOR: C). 34 Contraception, preferably barrier-type, should be used during sexual intercourse. Hormonal contraceptives are not contraindicated but should probably not be administered until the patient ’ s transplant status is stable.
If pregnancy does occur, apprise the patient of potential complications and adverse outcomes. Hypertension and preeclampsia are more common in pregnant OLT recipients; life-threatening infections and acute rejection are rare. Fortunately, most patients deliver healthy babies; miscarriages, stillbirths, and malformations are uncommon. An obstetrician specializing in high-risk pregnancy should follow all pregnant OLT recipients.
Vaccination
Vaccination after OLT is controversial. Live vaccines are generally contraindicated post-OLT and their safety in patients with stable graft function and on low levels of immunosuppression is unclear (SOR: C). Patients should receive pneumococcal vaccination, hepatitis A and B vaccination if not already immune, and yearly influenza vaccination (SOR: C). For travel outside of the US or in uncertain situations or exposures, the best reference is the Centers for Disease Control web site: www.cdc.org.
Communication with the transplant center
Direct communication with the patient ’ s transplant center is extremely important. You and the transplant center should determine the most effective way (phone, fax or e-mail) to communicate.
When should you contact the transplant center? First, obtain the center ’ s approval for any new medications that may be used long-term or have the potential for nephrotoxicity, hepatotoxicity, or immunosuppression. Second, notify the transplant center in the event of new signs or symptoms, such as fever, weight loss, abdominal pain, or jaundice. Being cautious by communicating early is often the most prudent course. Third, alert the transplant center of any hospitalizations. Transfer to the transplant center for any transplant-related problem or prolonged hospitalization usually provides the best outcome for the patient.
On the flip side, you are the primary caretaker, and the transplant center should regularly communicate with you regarding general medical concerns and any new diagnoses, interventions, or treatments. The transplant center should also regularly communicate with you regarding general medical concerns and any new diagnoses, interventions, or treatments. A strong, mutual relationship between you and the transplant center will have great impact on the recipient ’ s long-term care.
CORRESPONDENCE
Josh Levitsky, MD, Northwestern Memorial Hospital, 675 North St. Clair St, Suite 15-250, Chicago, IL 60611. E-mail: [email protected]
1. Killenger PG, Clavien PA. Medical Care of the Liver Transplant Patient. 2nd ed. Malden, Mass: Blackwell Science, 2001: pp 183-232.
2. Fukumoto T, Berg T, Ku Y, Bechstein WO, et al. . Viral dynamics of hepatitis C early after orthotopic liver transplantation: Evidence for rapid turnover of serum virions. Hepatology 1996 ;24 :1351 -1354.
3. McCashland TM. Posttransplantation care: role of the primary care physician versus transplant center. Liver Transpl 2001 ;7(Suppl 1) :S2 -S12.
4. Romero M, Parera A, Salcedo M, et al. . Cardiovascular risk factors and late cardiovascular disease in liver transplantation. Transplantation Proc 1999 ;31 :2364 -2365.
5. Gonwa TA. Hypertension and renal dysfunction in long-term liver transplant recipients. Liver Transpl 2001 ;7(Suppl 1) :S22 -S26.
6. Reuben A. Long-term management of the liver transplant patient: diabetes, hyperlipidemia, and obesity. Liver Transpl 2001 ;7(Suppl 1) :S13 -S21.
7. Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transplantation 2004 ;10 :886 -897.
8. Kamath PS, Wiesner RH, Malinchoc M, et al. . A model to predict survival in patients with end-stage liver disease. Hepatology 2001 ;33 :464 -470.
9. Jain A, Reyes J, Kashyap R, et al. . What have we learned about primary liver transplantation under tacrolimus immunosuppression? Long-term follow-up of the first 1000 patients. Ann Surg 1999 ;230 :441 -448.
10. Gonwa TA, Mai ML, Melton LB, et al. . End stage renal disease (ESRD) following orthotopic liver transplantation (OLTX) utilizing calcineurin based immunotherapy: Risk of development and treatment. Transplantation 2001 ;72 :1934 -1939.
11. McDonald JA, Dunstan CR, Dilworth P, et al. . Bone loss after liver transplantation. Hepatology 1991 ;14 :613 -619.
12. Monegal A, Navasa M, Guanabens N, et al. . Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporosis Intl 2001 ;12 :484 -492.
13. Keogh JB, Tsalamandris C, Sewell RB, et al. . Bone loss at the proximal femur and reduced lean mass following liver transplantation: a longitudinal study. Nutrition 1999 ;15 :661 -664.
14. Crosbie OM, Freaney R, McKenna M, et al. . Predicting bone loss following orthotopic liver transplantation. Gut 1999 ;44 :430 -434.
15. Papagelopoulos PJ, Hay JE, Galanis EC, Morrey BF. Total joint arthroplasty in orthotopic liver transplant recipients. J Arthroplasty 1996 ;11 :889 -892.
16. Levitsky J, Te HS, Cohen SM. The safety and outcome of joint replacement surgery in liver transplant recipients. Liver Transpl 2003 ;9 :373 -376.
17. Fung JJ, Jain A, Kwak EJ, et al. . De novo malignancies after liver transplantation: A major cause of late death. Liver Transpl 2001 ;7 :S109 -S118.
18. Frezza EE, Fung JJ, van Thiel DH. Non-lymphoid cancer after liver transplantation. Hepatogastroenterology 1997 ;44 :1172 -1181.
19. Jain AB, Yee LD, Nalesnik MA, et al. . Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using Surveillance Epidemiologic End Result data. Transplantation 1998 ;66 :1193 -1200.
20. Sheil AGR. Malignancy following liver transplantation: A report from the Australian Combined Liver Transplant Registry. Transplant Proc 1995 ;27 :1247. -
21. Sheiner PA, Magliocca JF, Bodian CA, et al. . Long-term medical complications in patients surviving ≥ 5 years after liver transplantation. Transplantation 2000 ;69 :781 -789.
22. Levy M, Backman L, Husberg B, et al. . De novo malignancy following liver transplantation: A single center study. Transplant Proc 1993 ;25 :1397 -1399.
23. Atassi R, Thuluvath PJ. Risk of colorectal adenoma in liver transplant recipients compared to immunocompetent control population undergoing routine screening colonoscopy. J Clin Gastroenterol 2003 ;37 :72 -73.
24. Ndibmie OK, Frezza E, Jordan HA, Koch W, van Thiel DH. Parvovirus B19 in anemia liver transplant recipients. Clin Diagn Lab Immunol 1996 ;3 :756 -760.
25. Misra S, Moore TB, Ament ME, Vargas JH, Busitill RW, McDiarmid SV. Profile of anemia in children after liver transplantation. Transplantation 2000 ;70 :1459 -1463.
26. Maheshwari A, Mishra R, Thuluvath PJ. Post-liver transplant anemia: etiology and management. Liver Transpl 2004 ;10 :165 -173.
27. Tzakis AG, Arditi M, Whittington PF, et al. . Aplastic anemia complicating orthotopic liver transplantation for non-A, non-B hepatitis. N Engl J Med 1988 ;319 :393 -396.
28. Smith DM, Agura E, Netto G, et al. . Liver transplant-associated graft-versus-host disease. Transplantation 2003 ;75 :118 -126.
29. Singh N, Gayowski T, Wagener MM, Marino IR. Vulnerability to psychologic distress and depression in patients with end-stage liver disease due to hepatitis C virus. Clin Transplant 1997 ;11 :406 -411.
30. Paterson DL, Gayowski T, Wannstedt CF, et al. . Quality of life in long-term survivors after liver transplantation: impact of recurrent viral hepatitis C virus hepatitis. Clin Transplant 2000 ;14 :48 -54.
31. Trzepacz PT, DiMartini A, Tringali RD. Psychopharmacologic issues in organ transplantation. Part 2. Psychopharmacologic medications. Psychosomatics 1993 ;34 :290 -298.
32. Mechtcheriakov S, Graziadei IW, Mattedi M, et al. . Incomplete improvement of visuo-motor deficits in patients with minimal hepatic encephalopathy after liver transplantation. Liver Transpl 2004 ;10 :77 -83.
33. Loinaz C, Clemares M, Marqu é s E, et al. . Labor status of 137 patients with liver transplantation. Transplant Proc 1999 ;31 :2470 -2471.
34. Riely CA. Contraception and pregnancy after liver transplantation. Liver Transpl 2001 ;7(Suppl 1) :S74 -S76.
1. Killenger PG, Clavien PA. Medical Care of the Liver Transplant Patient. 2nd ed. Malden, Mass: Blackwell Science, 2001: pp 183-232.
2. Fukumoto T, Berg T, Ku Y, Bechstein WO, et al. . Viral dynamics of hepatitis C early after orthotopic liver transplantation: Evidence for rapid turnover of serum virions. Hepatology 1996 ;24 :1351 -1354.
3. McCashland TM. Posttransplantation care: role of the primary care physician versus transplant center. Liver Transpl 2001 ;7(Suppl 1) :S2 -S12.
4. Romero M, Parera A, Salcedo M, et al. . Cardiovascular risk factors and late cardiovascular disease in liver transplantation. Transplantation Proc 1999 ;31 :2364 -2365.
5. Gonwa TA. Hypertension and renal dysfunction in long-term liver transplant recipients. Liver Transpl 2001 ;7(Suppl 1) :S22 -S26.
6. Reuben A. Long-term management of the liver transplant patient: diabetes, hyperlipidemia, and obesity. Liver Transpl 2001 ;7(Suppl 1) :S13 -S21.
7. Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transplantation 2004 ;10 :886 -897.
8. Kamath PS, Wiesner RH, Malinchoc M, et al. . A model to predict survival in patients with end-stage liver disease. Hepatology 2001 ;33 :464 -470.
9. Jain A, Reyes J, Kashyap R, et al. . What have we learned about primary liver transplantation under tacrolimus immunosuppression? Long-term follow-up of the first 1000 patients. Ann Surg 1999 ;230 :441 -448.
10. Gonwa TA, Mai ML, Melton LB, et al. . End stage renal disease (ESRD) following orthotopic liver transplantation (OLTX) utilizing calcineurin based immunotherapy: Risk of development and treatment. Transplantation 2001 ;72 :1934 -1939.
11. McDonald JA, Dunstan CR, Dilworth P, et al. . Bone loss after liver transplantation. Hepatology 1991 ;14 :613 -619.
12. Monegal A, Navasa M, Guanabens N, et al. . Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporosis Intl 2001 ;12 :484 -492.
13. Keogh JB, Tsalamandris C, Sewell RB, et al. . Bone loss at the proximal femur and reduced lean mass following liver transplantation: a longitudinal study. Nutrition 1999 ;15 :661 -664.
14. Crosbie OM, Freaney R, McKenna M, et al. . Predicting bone loss following orthotopic liver transplantation. Gut 1999 ;44 :430 -434.
15. Papagelopoulos PJ, Hay JE, Galanis EC, Morrey BF. Total joint arthroplasty in orthotopic liver transplant recipients. J Arthroplasty 1996 ;11 :889 -892.
16. Levitsky J, Te HS, Cohen SM. The safety and outcome of joint replacement surgery in liver transplant recipients. Liver Transpl 2003 ;9 :373 -376.
17. Fung JJ, Jain A, Kwak EJ, et al. . De novo malignancies after liver transplantation: A major cause of late death. Liver Transpl 2001 ;7 :S109 -S118.
18. Frezza EE, Fung JJ, van Thiel DH. Non-lymphoid cancer after liver transplantation. Hepatogastroenterology 1997 ;44 :1172 -1181.
19. Jain AB, Yee LD, Nalesnik MA, et al. . Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using Surveillance Epidemiologic End Result data. Transplantation 1998 ;66 :1193 -1200.
20. Sheil AGR. Malignancy following liver transplantation: A report from the Australian Combined Liver Transplant Registry. Transplant Proc 1995 ;27 :1247. -
21. Sheiner PA, Magliocca JF, Bodian CA, et al. . Long-term medical complications in patients surviving ≥ 5 years after liver transplantation. Transplantation 2000 ;69 :781 -789.
22. Levy M, Backman L, Husberg B, et al. . De novo malignancy following liver transplantation: A single center study. Transplant Proc 1993 ;25 :1397 -1399.
23. Atassi R, Thuluvath PJ. Risk of colorectal adenoma in liver transplant recipients compared to immunocompetent control population undergoing routine screening colonoscopy. J Clin Gastroenterol 2003 ;37 :72 -73.
24. Ndibmie OK, Frezza E, Jordan HA, Koch W, van Thiel DH. Parvovirus B19 in anemia liver transplant recipients. Clin Diagn Lab Immunol 1996 ;3 :756 -760.
25. Misra S, Moore TB, Ament ME, Vargas JH, Busitill RW, McDiarmid SV. Profile of anemia in children after liver transplantation. Transplantation 2000 ;70 :1459 -1463.
26. Maheshwari A, Mishra R, Thuluvath PJ. Post-liver transplant anemia: etiology and management. Liver Transpl 2004 ;10 :165 -173.
27. Tzakis AG, Arditi M, Whittington PF, et al. . Aplastic anemia complicating orthotopic liver transplantation for non-A, non-B hepatitis. N Engl J Med 1988 ;319 :393 -396.
28. Smith DM, Agura E, Netto G, et al. . Liver transplant-associated graft-versus-host disease. Transplantation 2003 ;75 :118 -126.
29. Singh N, Gayowski T, Wagener MM, Marino IR. Vulnerability to psychologic distress and depression in patients with end-stage liver disease due to hepatitis C virus. Clin Transplant 1997 ;11 :406 -411.
30. Paterson DL, Gayowski T, Wannstedt CF, et al. . Quality of life in long-term survivors after liver transplantation: impact of recurrent viral hepatitis C virus hepatitis. Clin Transplant 2000 ;14 :48 -54.
31. Trzepacz PT, DiMartini A, Tringali RD. Psychopharmacologic issues in organ transplantation. Part 2. Psychopharmacologic medications. Psychosomatics 1993 ;34 :290 -298.
32. Mechtcheriakov S, Graziadei IW, Mattedi M, et al. . Incomplete improvement of visuo-motor deficits in patients with minimal hepatic encephalopathy after liver transplantation. Liver Transpl 2004 ;10 :77 -83.
33. Loinaz C, Clemares M, Marqu é s E, et al. . Labor status of 137 patients with liver transplantation. Transplant Proc 1999 ;31 :2470 -2471.
34. Riely CA. Contraception and pregnancy after liver transplantation. Liver Transpl 2001 ;7(Suppl 1) :S74 -S76.