User login
Recreational cannabinoid use: The hazards behind the “high”
PRACTICE RECOMMENDATIONS
› Screen all patients for use of addiction-prone substances. A
› Screen cannabis users with a validated secondary screen for problematic use. A
› Counsel patients that there is no evidence that use of recreational cannabis is safe; advise them that it can cause numerous physical, psychomotor, cognitive, and psychiatric effects. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Approximately 156 million Americans (49% of the population) have tried cannabis.1 About 5.7 million people ages 12 years and older use it daily or almost daily, a number that has nearly doubled since 2006.2 There are 6600 new users in the United States every day,2 and almost half of all high school students will have tried it by graduation.3
There is limited evidence that cannabis may have medical benefit in some circumstances.4 (See “Medical marijuana: A treatment worth trying?” J Fam Pract. 2016;65:178-185 or http://www.mdedge.com/jfponline/article/106836/medical-marijuana-treatment-worth-trying.) As a result, it is now legal for medical purposes in 25 states. Recreational use by adults is also legal in 4 states and the District of Columbia.5 The US Food and Drug Administration, however, has reaffirmed its stance that marijuana is a Schedule I drug on the basis of its “high potential for abuse” and the absence of “currently accepted medical uses.”6
The effects of legalizing the medical and recreational use of cannabis for individuals—and society as a whole—are uncertain. Debate is ongoing about the risks, benefits, and rights of individuals. Some argue it is safer than alcohol or that criminalization has been ineffective and even harmful. Others make the case for personal liberty and autonomy. Still, others are convinced legalization is a misdirected experiment that will result in diverse adverse outcomes. Regardless, it is important that primary care providers understand the ramifications of marijuana use. This evidence-based narrative highlights major negative consequences of non-medical cannabinoid use.
Potential adverse consequences of cannabis use
Although the potential adverse consequences are vast, the literature on this subject is limited for various reasons:
- Many studies are observational with a small sample size.
- Most studies examine smoked cannabis—not other routes of delivery.
- When smoked, the dose, frequency, duration, and smoking technique are variable.
- The quantity of Δ-9-tetrahydrocannabinol (THC), the primary psychoactive component in cannabis, is variable. (For more on the chemical properties of the marijuana plant, see “Cannabinoids: A diverse group of chemicals.”7)
- Most studies do not examine medical users, who are expected to use less cannabis or lower doses of THC.
- There are confounding effects of other drugs, notably tobacco, which is used by up to 90% of cannabis users.8
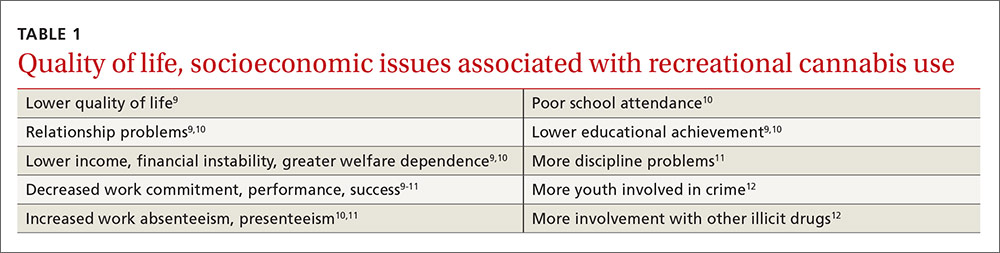
Lower quality of life. In general, regular non-medical cannabis use is associated with a lower quality of life and poorer socioeconomic outcomes (TABLE 1).9-12 Physical and mental health is ranked lower by heavy users as compared to extremely low users.9 Some who attempt butane extraction of THC from the plant have experienced explosions and severe burns.13
Studies regarding cannabis use and weight are conflicting. Appetite and weight may increase initially, and young adults who increase their use of the drug are more likely to find themselves on an increasing obesity trajectory.14 However, in an observational study of nearly 11,000 participants ages 20 to 59 years, cannabis users had a lower body mass index, better lipid parameters, and were less likely to have diabetes than non-using counterparts.15
Elevated rates of MI. Chronic effects may include oral health problems,16 gynecomastia, and changes in sexual function.17 Elevated rates of myocardial infarction, cardiomyopathy, limb arteritis, and stroke have been observed.18 Synthetic cannabinoids have been associated with heart attacks and acute renal injury in youth;19,20 however, plant-based marijuana does not affect the kidneys. In addition, high doses of plant-based marijuana can result in cannabinoid hyperemesis syndrome, characterized by cyclic vomiting and compulsive bathing that resolves with cessation of the drug.21
No major pulmonary effects. Interestingly, cannabis does not appear to have major negative pulmonary effects. Acutely, smoking marijuana causes bronchodilation.22 Chronic, low-level use over 20 years is associated with an increase in forced expiratory volume in one second (FEV1), but this upward trend diminishes and may reverse in high-level users.23 Although higher lung volumes are observed, cannabis does not appear to contribute to the development of chronic obstructive pulmonary disease, but can cause chronic bronchitis that resolves with smoking cessation.22 Chronic use has also been tied to airway infection. Lastly, fungal growth has been found on marijuana plants, which is concerning because of the potential to expose people to Aspergillus.22,24
Cannabis and cancer? The jury is out. Cannabis contains at least 33 carcinogens25 and may be contaminated with pesticides,26 but research about its relationship with cancer is incomplete. Although smoking results in histopathologic changes of the bronchial mucosa, evidence of lung cancer is mixed.22,25,27 Some studies have suggested associations with cancers of the brain, testis, prostate, and cervix,25,27 as well as certain rare cancers in children due to parental exposure.25,27
There are conflicting data about associations with head and neck squamous cell carcinoma,25,27,28 bladder cancer,25,29 and non-Hodgkin’s lymphoma.25,30 Some studies suggest marijuana offers protection against certain types of cancer. In fact, it appears that some cannabinoids found in marijuana, such as cannabidiol (CBD), may be antineoplastic.31 The potential oncogenic effects of edible and topical cannabinoid products have not been investigated.
Use linked to car accidents. More recent work indicates cannabis use is associated with injuries in motor vehicle,32 non-traffic,33 and workplace34 settings. In fact, a meta-analysis found a near-doubling of motor vehicle accidents with recent use.32 Risk is dose-dependent and heightened with alcohol.35-37 Psychomotor impairment persists for at least 6 hours after smoking cannabis,38 at least 10 hours after ingesting it,37 and may last up to 24 hours, as indicated by a study involving pilots using a flight simulator.39
In contrast to alcohol, there is a greater decrement in routine vs complex driving tasks in experimental studies.35,36 Behavioral strategies, like driving slowly, are employed to compensate for impairment, but the ability to do so is lost with alcohol co-ingestion.35 Importantly, individuals using marijuana may not recognize the presence or extent of the impairment they are experiencing,37,39 placing themselves and others in danger.
Data are insufficient to ascribe to marijuana an increase in overall mortality,40 and there have been no reported overdose deaths from respiratory depression. However, a few deaths and a greater number of hospitalizations, due mainly to central nervous system effects including agitation, depression, coma, delirium, and toxic psychosis, have been attributed to the use of synthetic cannabinoids.20
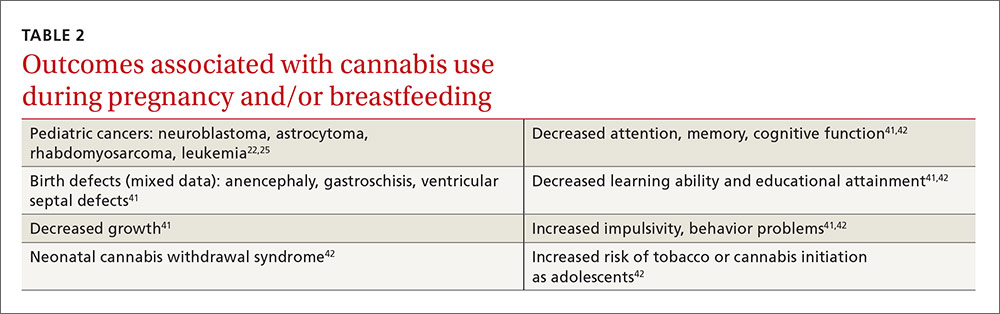
Cannabis use can pose a risk to the fetus. About 5% of pregnant women report recent marijuana use2 for recreational or medical reasons (eg, morning sickness), and there is concern about its effects on the developing fetus. Certain rare pediatric cancers22,25 and birth defects41 have been reported with cannabis use (TABLE 222,25,41,42). Neonatal withdrawal is minor, if present at all.42 Moderate evidence indicates prenatal and breastfeeding exposure can result in multiple developmental problems, as well as an increased likelihood of initiating tobacco and marijuana use as teens.41,42
Cognitive effects of cannabis are a concern. The central nervous system is not fully myelinated until the age of 18, and complete maturation continues beyond that. Due to neuroplasticity, life experiences and exogenous agents may result in further changes. Cannabis produces changes in brain structure and function that are evident on neuroimaging.43 Although accidental pediatric intoxication is alarming, negative consequences are likely to be of short duration.
Regular use by youth, on the other hand, negatively affects cognition and delays brain maturation, especially for younger initiates.9,38,44 With abstinence, deficits tend to normalize, but they may last indefinitely among young people who continue to use marijuana.44
Dyscognition is less severe and is more likely to resolve with abstinence in adults,44 which may tip the scale for adults weighing whether to use cannabis for a medical purpose.45 Keep in mind that individuals may not be aware of their cognitive deficits,46 even though nearly all domains (from basic motor coordination to more complex executive function tasks, such as the ability to control emotions and behavior) are affected.44 A possible exception may be improvement in attention with acute use in daily, but not occasional, users.44 Highly focused attention, however, is not always beneficial if it delays redirection toward a new urgent stimulus.
Mood benefit? Research suggests otherwise. The psychiatric effects of cannabis are not fully understood. Users may claim mood benefit, but research suggests marijuana prompts the development or worsening of anxiety, depression, and suicidality.12,47 Violence, paranoia, and borderline personality features have also been associated with use.38,47 Amotivational syndrome, a disorder that includes apathy, callousness, and antisocial behavior, has been described, but the interplay between cannabis and motivation beyond recent use is unclear.48
Lifetime cannabis use is related to panic,49 yet correlational studies suggest both benefit and problems for individuals who use cannabis for posttraumatic stress disorder.50 It is now well established that marijuana use is an independent causal risk factor for the development of psychosis, particularly in vulnerable youth, and that it worsens schizophrenia in those who suffer from it.51 Human experimental studies suggest this may be because the effect of THC is counteracted by CBD.52 Synthetic cannabinoids are even more potent anxiogenic and psychogenic agents than plant-based marijuana.19,20
Cannabis Use Disorder
About 9% of those who try cannabis develop Cannabis Use Disorder, which is characterized by continued use of the substance despite significant distress or impairment.53 Cannabis Use Disorder is essentially an addiction. Primary risk factors include male gender, younger age at marijuana initiation, and personal or family history of other substance or psychiatric problems.53
Although cannabis use often precedes use of other addiction-prone substances, it remains unclear if it is a “gateway” to the use of other illicit drugs.54 Marijuana withdrawal is relatively minor and is comparable to that for tobacco.55 While there are no known effective pharmacotherapies for discontinuing cannabis use, addiction therapy—including cognitive behavioral therapy and trigger management—is effective.56
SIDEBAR
Cannabinoids: A diverse group of chemicalsCannabis, the genus name for 3 species of marijuana plant (sativa, indica, ruderalis), has come to mean any psychoactive part of the plant and is used interchangeably with “marijuana.” There are at least 85 different cannabinoids in the native plant.7
Cannabinoids are a diverse group of chemicals that have activity at cannabinoid receptors. Δ-9-tetrahydrocannabinol (THC), a partial agonist of the CB1 receptor, is the primary psychoactive component and is found in larger quantities in Cannabis sativa, which is preferred by non-medical users. Cannabidiol (CBD), a weak partial CB1 antagonist, exhibits few, if any, psychotropic properties and is more plentiful in Cannabis indica.
Synthetic cannabinioids are a heterogeneous group of manufactured drugs that are full CB1 agonists and that are more potent than THC, yet are often assumed to be safe by users. Typically, they are dissolved in solvents, sprayed onto inert plant materials, and marketed as herbal products like “K2” and “spice.”
So how should the evidence inform your care?
Screen all patients for use of cannabinoids and other addiction-prone substances.57 Follow any affirmative answers to your questions about cannabis use by asking about potential negative consequences of use. For example, ask patients:
- How often during the past 6 months did you find that you were unable to stop using cannabis once you started?
- How often during the past 6 months did you fail to do what was expected of you because of using cannabis? (For more questions, see the Cannabis Use Disorder Identification Test available at: http://www.otago.ac.nz/nationaladdictioncentre/pdfs/cudit-r.pdf.)
Other validated screening tools include the Severity of Dependence Scale, the Cannabis Abuse Screening Test, and the Problematic Use of Marijuana.58
Counsel patients about possible adverse effects and inform them there is no evidence that recreational marijuana or synthetic cannabinoids can be used safely over time. Consider medical use requests only if there is a favorable risk/benefit balance, other recognized treatment options have been exhausted, and you have a strong understanding of the use of cannabis in the medical condition being considered.4
Since brief interventions using motivational interviewing to reduce or eliminate recreational use have not been found to be effective,59 referral to an addiction specialist may be indicated. If a diagnosis of cannabis use disorder is established, then abstinence from addiction-prone substances including marijuana, programs like Marijuana Anonymous (Available at: https://www.marijuana-anonymous.org/), and individualized addiction therapy scaled to the severity of the condition can be effective.56 Because psychiatric conditions frequently co-occur and complicate addiction,53 they should be screened for and managed, as well.
Drug testing. Cannabis Use Disorder has significant relapse potential.60 Abstinence and treatment adherence should be ascertained through regular follow-up that includes a clinical interview, exam, and body fluid drug testing. Point-of-care urine analysis for substances of potential addiction has limited utility. Definitive testing of urine with gas chromotography/mass spectrometry (GC/MS) or liquid chromatography (LC/MS-MS) can eliminate THC false-positives and false-negatives that can occur with point-of-care urine immunoassays. In addition, GCMS and LC/MS-MS can identify synthetic cannabinoids; in-office immunoassays cannot.
If the patient relapses, subsequent medical care should be coordinated with an addiction specialist with the goal of helping the patient to abstain from cannabis.
CORRESPONDENCE
Steven Wright, MD, FAAFP, 5325 Ridge Trail, Littleton, CO 80123; [email protected].
1. Pew Research Center. 6 facts about marijuana. Available at: http://www.pewresearch.org/fact-tank/2015/04/14/6-facts-about-marijuana/. Accessed September 27, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Pub # (SMA) 14-4863. 2014. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf. Accessed September 27, 2015.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future National Survey on Drug Use 1975-2015. Available at: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Accessed September 23, 2015.
4. Metts J, Wright S, Sundaram J, et al. Medical marijuana: a treatment worth trying? J Fam Pract. 2016;65:178-185.
5. Governing the states and localities. State marijuana laws map. Available at: http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html. Accessed October 12, 2016.
6. US Drug Enforcement Administration. Drug scheduling. Available at: https://www.dea.gov/druginfo/ds.shtml. Accessed October 12, 2016.
7. El-Alfy AT, Ivey K, Robinson K, et al. Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav. 2010;95:434-442.
8. Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107:1404-1417.
9. Gruber AJ, Pope HG, Hudson JI, et al. Attributes of long-term heavy cannabis users: a case-control study. Psychol Med. 2003;33:1415-1422.
10. Palamar JJ, Fenstermaker M, Kamboukos D, et al. Adverse psychosocial outcomes associated with drug use among US high school seniors: a comparison of alcohol and marijuana. Am J Drug Alcohol Abuse. 2014;40:438-446.
11. Zwerling C, Ryan J, Orav EJ. The efficacy of preemployment drug screening for marijuana and cocaine in predicting employment outcome. JAMA. 1990;264:2639-2643.
12. Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123-1135.
13. Bell C, Slim J, Flaten HK, et al. Butane hash oil burns associated with marijuana liberalization in Colorado. J Med Toxicol. 2015;11:422-425.
14. Huang DYC, Lanza HI, Anglin MD. Association between adolescent substance use and obesity in young adulthood: a group-based dual trajectory analysis. Addict Behav. 2013;38:2653-2660.
15. Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494.
16. Cho CM, Hirsch R, Johnstone S. General and oral health implications of cannabis use. Aust Dent J. 2005;50:70-74.
17. Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav. 2010;58:91-99.
18. Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg. 2013;27:996-1005.
19. Mills B, Yepes A, Nugent K. Synthetic cannabinoids. Am J Med Sci. 2015;350:59-62.
20. Tuv SS, Strand MC, Karinen R, et al. Effect and occurrence of synthetic cannabinoids. Tidsskr Nor Laegeforen. 2012;132:2285-2288.
21. Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011;104:659-964.
22. Gates P, Jaffe A, Copeland J. Cannabis smoking and respiratory health: considerations of the literature. Respirology. 2014;19:655-662.
23. Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years: The Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2012;307:173-181.
24. Verweij PE, Kerremans JJ, Vos A, et al. Fungal contamination of tobacco and marijuana. JAMA. 2000;284:2875.
25. Office of Environmental Health Hazard Assessment. Evidence on the carcinogenicity of marijuana smoke. August 2009. Available at: http://oehha.ca.gov/media/downloads/crnr/finalmjsmokehid.pdf. Accessed September 5, 2015.
26. Stone D. Cannabis, pesticides and conflicting laws: the dilemma for legalized States and implications for public health. Regul Toxicol Pharmacol. 2014;69:284-288.
27. Hashibe M, Straif K, Tashkin DP, et al. Epidemiologic review of marijuana and cancer risk. Alcohol. 2005;35:265-275.
28. Liang C, McClean MD, Marsit C, et al. A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prev Res (Phila). 2009;2:759-768.
29. Thomas AA, Wallner LP, Quinn VP, et al. Association between cannabis use and the risk of bladder cancer: results from the California Men’s Health Study. Urology. 2015;85:388-392.
30. Holly EA, Lele C, Bracci PM, et al. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Francisco Bay area, California. Am J Epidemiol. 1999;150:375-389.
31. Massi P, Solinas M, Cinquina V, et al. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303-312.
32. Ashbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536.
33.Barrio G, Jimenez-Mejias E, Pulido J, et al. Association between cannabis use and non-traffic injuries. Accid Anal Prev. 2012;47:172-176.
34. MacDonald S, Hall W, Roman P, et al. Testing for cannabis in the work-place: a review of the evidence. Addiction. 2010;105:408-416.
35. Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict. 2009;18:185-193.
36. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109-119.
37. Menetrey A, Augsburger M, Favrat B, et al. Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoid levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Δ9-THC. J Anal Toxicol. 2005;29:327-338.
38. Raemakers JG, Kaurert G, van Ruitenbeek P, et al. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296-2303.
39. Leirer VO, Yesavage JA, Morrow DG. Marijuana carry-over effects on aircraft pilot performance. Aviat Space Environ Med. 1991;62:221-227.
40. Calabria B, Degenhardt L, Hall W, et al. Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010;29:318-330.
41. Colorado Department of Public Health and Environment. Monitoring health concerns related to marijuana in Colorado: 2014. Changes in marijuana use patterns, systematic literature review, and possible marijuana-related health effects. Available at: http://www2.cde.state.co.us/artemis/hemonos/he1282m332015internet/he1282m332015internet01.pdf. Accessed September 5, 2015.
42. Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn. Perinatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009-1024.
43. Batalla A, Bhattacharyya S, Yücel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821.
44. Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1-8.
45. Pavisian B, MacIntosh BJ, Szilagyi G, et al. Effects of cannabis on cognition in patients with multiple sclerosis: a psychometric and MRI study. Neurology. 2014;82:1879-1887.
46. Bartholomew J, Holroyd S, Heffernan TM. Does cannabis use affect prospective memory in young adults? J Psychopharmacol. 2010;24:241-246.
47. Copeland J, Rooke S, Swift W. Changes in cannabis use among young people: impact on mental health. Curr Opin Psychiatry. 2013;26:325-329.
48. Ari M, Sahpolat M, Kokacya H, et al. Amotivational syndrome: less known and diagnosed as a clinical. J Mood Disord. 2015;5:31-35.
49. Zvolensky MJ, Cougle JR, Johnson KA, et al. Marijuana use and panic psychopathology among a representative sample of adults. Exp Clin Psychopharmacol. 2010;18(2):129-134.
50. Yarnell S. The use of medicinal marijuana for posttraumatic stress disorder: a review of the current literature. Prim Care Companion CNS Disord. 2015;17(3).
51. Le Bec PY, Fatséas M, Denis C, et al. Cannabis and psychosis: search of a causal link through a critical and systematic review. Encephale. 2009;35:377-385.
52. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19-27.
53. Lopez-Quintero C, Perez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
54. Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108:84-97.
55. Vandrey RG, Budney AJ, Hughes JR, et al. A within subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92:48-54.
56.Budney AJ, Roffman R, Stephens RS, et al. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4-16.
57. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Phys. 2014;60:801-808.
58. Piontek D, Kraus L, Klempova D. Short scales to assess cannabis-related problems: a review of psychometric properties. Subst Abuse Treat Prev Policy. 2008;3:25.
59. Saitz R, Palfai TPA, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312:502-513.
60. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689-1695.
PRACTICE RECOMMENDATIONS
› Screen all patients for use of addiction-prone substances. A
› Screen cannabis users with a validated secondary screen for problematic use. A
› Counsel patients that there is no evidence that use of recreational cannabis is safe; advise them that it can cause numerous physical, psychomotor, cognitive, and psychiatric effects. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Approximately 156 million Americans (49% of the population) have tried cannabis.1 About 5.7 million people ages 12 years and older use it daily or almost daily, a number that has nearly doubled since 2006.2 There are 6600 new users in the United States every day,2 and almost half of all high school students will have tried it by graduation.3
There is limited evidence that cannabis may have medical benefit in some circumstances.4 (See “Medical marijuana: A treatment worth trying?” J Fam Pract. 2016;65:178-185 or http://www.mdedge.com/jfponline/article/106836/medical-marijuana-treatment-worth-trying.) As a result, it is now legal for medical purposes in 25 states. Recreational use by adults is also legal in 4 states and the District of Columbia.5 The US Food and Drug Administration, however, has reaffirmed its stance that marijuana is a Schedule I drug on the basis of its “high potential for abuse” and the absence of “currently accepted medical uses.”6
The effects of legalizing the medical and recreational use of cannabis for individuals—and society as a whole—are uncertain. Debate is ongoing about the risks, benefits, and rights of individuals. Some argue it is safer than alcohol or that criminalization has been ineffective and even harmful. Others make the case for personal liberty and autonomy. Still, others are convinced legalization is a misdirected experiment that will result in diverse adverse outcomes. Regardless, it is important that primary care providers understand the ramifications of marijuana use. This evidence-based narrative highlights major negative consequences of non-medical cannabinoid use.
Potential adverse consequences of cannabis use
Although the potential adverse consequences are vast, the literature on this subject is limited for various reasons:
- Many studies are observational with a small sample size.
- Most studies examine smoked cannabis—not other routes of delivery.
- When smoked, the dose, frequency, duration, and smoking technique are variable.
- The quantity of Δ-9-tetrahydrocannabinol (THC), the primary psychoactive component in cannabis, is variable. (For more on the chemical properties of the marijuana plant, see “Cannabinoids: A diverse group of chemicals.”7)
- Most studies do not examine medical users, who are expected to use less cannabis or lower doses of THC.
- There are confounding effects of other drugs, notably tobacco, which is used by up to 90% of cannabis users.8
Lower quality of life. In general, regular non-medical cannabis use is associated with a lower quality of life and poorer socioeconomic outcomes (TABLE 1).9-12 Physical and mental health is ranked lower by heavy users as compared to extremely low users.9 Some who attempt butane extraction of THC from the plant have experienced explosions and severe burns.13

Studies regarding cannabis use and weight are conflicting. Appetite and weight may increase initially, and young adults who increase their use of the drug are more likely to find themselves on an increasing obesity trajectory.14 However, in an observational study of nearly 11,000 participants ages 20 to 59 years, cannabis users had a lower body mass index, better lipid parameters, and were less likely to have diabetes than non-using counterparts.15
Elevated rates of MI. Chronic effects may include oral health problems,16 gynecomastia, and changes in sexual function.17 Elevated rates of myocardial infarction, cardiomyopathy, limb arteritis, and stroke have been observed.18 Synthetic cannabinoids have been associated with heart attacks and acute renal injury in youth;19,20 however, plant-based marijuana does not affect the kidneys. In addition, high doses of plant-based marijuana can result in cannabinoid hyperemesis syndrome, characterized by cyclic vomiting and compulsive bathing that resolves with cessation of the drug.21
No major pulmonary effects. Interestingly, cannabis does not appear to have major negative pulmonary effects. Acutely, smoking marijuana causes bronchodilation.22 Chronic, low-level use over 20 years is associated with an increase in forced expiratory volume in one second (FEV1), but this upward trend diminishes and may reverse in high-level users.23 Although higher lung volumes are observed, cannabis does not appear to contribute to the development of chronic obstructive pulmonary disease, but can cause chronic bronchitis that resolves with smoking cessation.22 Chronic use has also been tied to airway infection. Lastly, fungal growth has been found on marijuana plants, which is concerning because of the potential to expose people to Aspergillus.22,24
Cannabis and cancer? The jury is out. Cannabis contains at least 33 carcinogens25 and may be contaminated with pesticides,26 but research about its relationship with cancer is incomplete. Although smoking results in histopathologic changes of the bronchial mucosa, evidence of lung cancer is mixed.22,25,27 Some studies have suggested associations with cancers of the brain, testis, prostate, and cervix,25,27 as well as certain rare cancers in children due to parental exposure.25,27
There are conflicting data about associations with head and neck squamous cell carcinoma,25,27,28 bladder cancer,25,29 and non-Hodgkin’s lymphoma.25,30 Some studies suggest marijuana offers protection against certain types of cancer. In fact, it appears that some cannabinoids found in marijuana, such as cannabidiol (CBD), may be antineoplastic.31 The potential oncogenic effects of edible and topical cannabinoid products have not been investigated.
Use linked to car accidents. More recent work indicates cannabis use is associated with injuries in motor vehicle,32 non-traffic,33 and workplace34 settings. In fact, a meta-analysis found a near-doubling of motor vehicle accidents with recent use.32 Risk is dose-dependent and heightened with alcohol.35-37 Psychomotor impairment persists for at least 6 hours after smoking cannabis,38 at least 10 hours after ingesting it,37 and may last up to 24 hours, as indicated by a study involving pilots using a flight simulator.39
In contrast to alcohol, there is a greater decrement in routine vs complex driving tasks in experimental studies.35,36 Behavioral strategies, like driving slowly, are employed to compensate for impairment, but the ability to do so is lost with alcohol co-ingestion.35 Importantly, individuals using marijuana may not recognize the presence or extent of the impairment they are experiencing,37,39 placing themselves and others in danger.
Data are insufficient to ascribe to marijuana an increase in overall mortality,40 and there have been no reported overdose deaths from respiratory depression. However, a few deaths and a greater number of hospitalizations, due mainly to central nervous system effects including agitation, depression, coma, delirium, and toxic psychosis, have been attributed to the use of synthetic cannabinoids.20
Cannabis use can pose a risk to the fetus. About 5% of pregnant women report recent marijuana use2 for recreational or medical reasons (eg, morning sickness), and there is concern about its effects on the developing fetus. Certain rare pediatric cancers22,25 and birth defects41 have been reported with cannabis use (TABLE 222,25,41,42). Neonatal withdrawal is minor, if present at all.42 Moderate evidence indicates prenatal and breastfeeding exposure can result in multiple developmental problems, as well as an increased likelihood of initiating tobacco and marijuana use as teens.41,42

Cognitive effects of cannabis are a concern. The central nervous system is not fully myelinated until the age of 18, and complete maturation continues beyond that. Due to neuroplasticity, life experiences and exogenous agents may result in further changes. Cannabis produces changes in brain structure and function that are evident on neuroimaging.43 Although accidental pediatric intoxication is alarming, negative consequences are likely to be of short duration.
Regular use by youth, on the other hand, negatively affects cognition and delays brain maturation, especially for younger initiates.9,38,44 With abstinence, deficits tend to normalize, but they may last indefinitely among young people who continue to use marijuana.44
Dyscognition is less severe and is more likely to resolve with abstinence in adults,44 which may tip the scale for adults weighing whether to use cannabis for a medical purpose.45 Keep in mind that individuals may not be aware of their cognitive deficits,46 even though nearly all domains (from basic motor coordination to more complex executive function tasks, such as the ability to control emotions and behavior) are affected.44 A possible exception may be improvement in attention with acute use in daily, but not occasional, users.44 Highly focused attention, however, is not always beneficial if it delays redirection toward a new urgent stimulus.
Mood benefit? Research suggests otherwise. The psychiatric effects of cannabis are not fully understood. Users may claim mood benefit, but research suggests marijuana prompts the development or worsening of anxiety, depression, and suicidality.12,47 Violence, paranoia, and borderline personality features have also been associated with use.38,47 Amotivational syndrome, a disorder that includes apathy, callousness, and antisocial behavior, has been described, but the interplay between cannabis and motivation beyond recent use is unclear.48
Lifetime cannabis use is related to panic,49 yet correlational studies suggest both benefit and problems for individuals who use cannabis for posttraumatic stress disorder.50 It is now well established that marijuana use is an independent causal risk factor for the development of psychosis, particularly in vulnerable youth, and that it worsens schizophrenia in those who suffer from it.51 Human experimental studies suggest this may be because the effect of THC is counteracted by CBD.52 Synthetic cannabinoids are even more potent anxiogenic and psychogenic agents than plant-based marijuana.19,20
Cannabis Use Disorder
About 9% of those who try cannabis develop Cannabis Use Disorder, which is characterized by continued use of the substance despite significant distress or impairment.53 Cannabis Use Disorder is essentially an addiction. Primary risk factors include male gender, younger age at marijuana initiation, and personal or family history of other substance or psychiatric problems.53
Although cannabis use often precedes use of other addiction-prone substances, it remains unclear if it is a “gateway” to the use of other illicit drugs.54 Marijuana withdrawal is relatively minor and is comparable to that for tobacco.55 While there are no known effective pharmacotherapies for discontinuing cannabis use, addiction therapy—including cognitive behavioral therapy and trigger management—is effective.56
SIDEBAR
Cannabinoids: A diverse group of chemicalsCannabis, the genus name for 3 species of marijuana plant (sativa, indica, ruderalis), has come to mean any psychoactive part of the plant and is used interchangeably with “marijuana.” There are at least 85 different cannabinoids in the native plant.7
Cannabinoids are a diverse group of chemicals that have activity at cannabinoid receptors. Δ-9-tetrahydrocannabinol (THC), a partial agonist of the CB1 receptor, is the primary psychoactive component and is found in larger quantities in Cannabis sativa, which is preferred by non-medical users. Cannabidiol (CBD), a weak partial CB1 antagonist, exhibits few, if any, psychotropic properties and is more plentiful in Cannabis indica.
Synthetic cannabinioids are a heterogeneous group of manufactured drugs that are full CB1 agonists and that are more potent than THC, yet are often assumed to be safe by users. Typically, they are dissolved in solvents, sprayed onto inert plant materials, and marketed as herbal products like “K2” and “spice.”
So how should the evidence inform your care?
Screen all patients for use of cannabinoids and other addiction-prone substances.57 Follow any affirmative answers to your questions about cannabis use by asking about potential negative consequences of use. For example, ask patients:
- How often during the past 6 months did you find that you were unable to stop using cannabis once you started?
- How often during the past 6 months did you fail to do what was expected of you because of using cannabis? (For more questions, see the Cannabis Use Disorder Identification Test available at: http://www.otago.ac.nz/nationaladdictioncentre/pdfs/cudit-r.pdf.)
Other validated screening tools include the Severity of Dependence Scale, the Cannabis Abuse Screening Test, and the Problematic Use of Marijuana.58
Counsel patients about possible adverse effects and inform them there is no evidence that recreational marijuana or synthetic cannabinoids can be used safely over time. Consider medical use requests only if there is a favorable risk/benefit balance, other recognized treatment options have been exhausted, and you have a strong understanding of the use of cannabis in the medical condition being considered.4
Since brief interventions using motivational interviewing to reduce or eliminate recreational use have not been found to be effective,59 referral to an addiction specialist may be indicated. If a diagnosis of cannabis use disorder is established, then abstinence from addiction-prone substances including marijuana, programs like Marijuana Anonymous (Available at: https://www.marijuana-anonymous.org/), and individualized addiction therapy scaled to the severity of the condition can be effective.56 Because psychiatric conditions frequently co-occur and complicate addiction,53 they should be screened for and managed, as well.
Drug testing. Cannabis Use Disorder has significant relapse potential.60 Abstinence and treatment adherence should be ascertained through regular follow-up that includes a clinical interview, exam, and body fluid drug testing. Point-of-care urine analysis for substances of potential addiction has limited utility. Definitive testing of urine with gas chromotography/mass spectrometry (GC/MS) or liquid chromatography (LC/MS-MS) can eliminate THC false-positives and false-negatives that can occur with point-of-care urine immunoassays. In addition, GCMS and LC/MS-MS can identify synthetic cannabinoids; in-office immunoassays cannot.
If the patient relapses, subsequent medical care should be coordinated with an addiction specialist with the goal of helping the patient to abstain from cannabis.
CORRESPONDENCE
Steven Wright, MD, FAAFP, 5325 Ridge Trail, Littleton, CO 80123; [email protected].
PRACTICE RECOMMENDATIONS
› Screen all patients for use of addiction-prone substances. A
› Screen cannabis users with a validated secondary screen for problematic use. A
› Counsel patients that there is no evidence that use of recreational cannabis is safe; advise them that it can cause numerous physical, psychomotor, cognitive, and psychiatric effects. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Approximately 156 million Americans (49% of the population) have tried cannabis.1 About 5.7 million people ages 12 years and older use it daily or almost daily, a number that has nearly doubled since 2006.2 There are 6600 new users in the United States every day,2 and almost half of all high school students will have tried it by graduation.3
There is limited evidence that cannabis may have medical benefit in some circumstances.4 (See “Medical marijuana: A treatment worth trying?” J Fam Pract. 2016;65:178-185 or http://www.mdedge.com/jfponline/article/106836/medical-marijuana-treatment-worth-trying.) As a result, it is now legal for medical purposes in 25 states. Recreational use by adults is also legal in 4 states and the District of Columbia.5 The US Food and Drug Administration, however, has reaffirmed its stance that marijuana is a Schedule I drug on the basis of its “high potential for abuse” and the absence of “currently accepted medical uses.”6
The effects of legalizing the medical and recreational use of cannabis for individuals—and society as a whole—are uncertain. Debate is ongoing about the risks, benefits, and rights of individuals. Some argue it is safer than alcohol or that criminalization has been ineffective and even harmful. Others make the case for personal liberty and autonomy. Still, others are convinced legalization is a misdirected experiment that will result in diverse adverse outcomes. Regardless, it is important that primary care providers understand the ramifications of marijuana use. This evidence-based narrative highlights major negative consequences of non-medical cannabinoid use.
Potential adverse consequences of cannabis use
Although the potential adverse consequences are vast, the literature on this subject is limited for various reasons:
- Many studies are observational with a small sample size.
- Most studies examine smoked cannabis—not other routes of delivery.
- When smoked, the dose, frequency, duration, and smoking technique are variable.
- The quantity of Δ-9-tetrahydrocannabinol (THC), the primary psychoactive component in cannabis, is variable. (For more on the chemical properties of the marijuana plant, see “Cannabinoids: A diverse group of chemicals.”7)
- Most studies do not examine medical users, who are expected to use less cannabis or lower doses of THC.
- There are confounding effects of other drugs, notably tobacco, which is used by up to 90% of cannabis users.8
Lower quality of life. In general, regular non-medical cannabis use is associated with a lower quality of life and poorer socioeconomic outcomes (TABLE 1).9-12 Physical and mental health is ranked lower by heavy users as compared to extremely low users.9 Some who attempt butane extraction of THC from the plant have experienced explosions and severe burns.13

Studies regarding cannabis use and weight are conflicting. Appetite and weight may increase initially, and young adults who increase their use of the drug are more likely to find themselves on an increasing obesity trajectory.14 However, in an observational study of nearly 11,000 participants ages 20 to 59 years, cannabis users had a lower body mass index, better lipid parameters, and were less likely to have diabetes than non-using counterparts.15
Elevated rates of MI. Chronic effects may include oral health problems,16 gynecomastia, and changes in sexual function.17 Elevated rates of myocardial infarction, cardiomyopathy, limb arteritis, and stroke have been observed.18 Synthetic cannabinoids have been associated with heart attacks and acute renal injury in youth;19,20 however, plant-based marijuana does not affect the kidneys. In addition, high doses of plant-based marijuana can result in cannabinoid hyperemesis syndrome, characterized by cyclic vomiting and compulsive bathing that resolves with cessation of the drug.21
No major pulmonary effects. Interestingly, cannabis does not appear to have major negative pulmonary effects. Acutely, smoking marijuana causes bronchodilation.22 Chronic, low-level use over 20 years is associated with an increase in forced expiratory volume in one second (FEV1), but this upward trend diminishes and may reverse in high-level users.23 Although higher lung volumes are observed, cannabis does not appear to contribute to the development of chronic obstructive pulmonary disease, but can cause chronic bronchitis that resolves with smoking cessation.22 Chronic use has also been tied to airway infection. Lastly, fungal growth has been found on marijuana plants, which is concerning because of the potential to expose people to Aspergillus.22,24
Cannabis and cancer? The jury is out. Cannabis contains at least 33 carcinogens25 and may be contaminated with pesticides,26 but research about its relationship with cancer is incomplete. Although smoking results in histopathologic changes of the bronchial mucosa, evidence of lung cancer is mixed.22,25,27 Some studies have suggested associations with cancers of the brain, testis, prostate, and cervix,25,27 as well as certain rare cancers in children due to parental exposure.25,27
There are conflicting data about associations with head and neck squamous cell carcinoma,25,27,28 bladder cancer,25,29 and non-Hodgkin’s lymphoma.25,30 Some studies suggest marijuana offers protection against certain types of cancer. In fact, it appears that some cannabinoids found in marijuana, such as cannabidiol (CBD), may be antineoplastic.31 The potential oncogenic effects of edible and topical cannabinoid products have not been investigated.
Use linked to car accidents. More recent work indicates cannabis use is associated with injuries in motor vehicle,32 non-traffic,33 and workplace34 settings. In fact, a meta-analysis found a near-doubling of motor vehicle accidents with recent use.32 Risk is dose-dependent and heightened with alcohol.35-37 Psychomotor impairment persists for at least 6 hours after smoking cannabis,38 at least 10 hours after ingesting it,37 and may last up to 24 hours, as indicated by a study involving pilots using a flight simulator.39
In contrast to alcohol, there is a greater decrement in routine vs complex driving tasks in experimental studies.35,36 Behavioral strategies, like driving slowly, are employed to compensate for impairment, but the ability to do so is lost with alcohol co-ingestion.35 Importantly, individuals using marijuana may not recognize the presence or extent of the impairment they are experiencing,37,39 placing themselves and others in danger.
Data are insufficient to ascribe to marijuana an increase in overall mortality,40 and there have been no reported overdose deaths from respiratory depression. However, a few deaths and a greater number of hospitalizations, due mainly to central nervous system effects including agitation, depression, coma, delirium, and toxic psychosis, have been attributed to the use of synthetic cannabinoids.20
Cannabis use can pose a risk to the fetus. About 5% of pregnant women report recent marijuana use2 for recreational or medical reasons (eg, morning sickness), and there is concern about its effects on the developing fetus. Certain rare pediatric cancers22,25 and birth defects41 have been reported with cannabis use (TABLE 222,25,41,42). Neonatal withdrawal is minor, if present at all.42 Moderate evidence indicates prenatal and breastfeeding exposure can result in multiple developmental problems, as well as an increased likelihood of initiating tobacco and marijuana use as teens.41,42

Cognitive effects of cannabis are a concern. The central nervous system is not fully myelinated until the age of 18, and complete maturation continues beyond that. Due to neuroplasticity, life experiences and exogenous agents may result in further changes. Cannabis produces changes in brain structure and function that are evident on neuroimaging.43 Although accidental pediatric intoxication is alarming, negative consequences are likely to be of short duration.
Regular use by youth, on the other hand, negatively affects cognition and delays brain maturation, especially for younger initiates.9,38,44 With abstinence, deficits tend to normalize, but they may last indefinitely among young people who continue to use marijuana.44
Dyscognition is less severe and is more likely to resolve with abstinence in adults,44 which may tip the scale for adults weighing whether to use cannabis for a medical purpose.45 Keep in mind that individuals may not be aware of their cognitive deficits,46 even though nearly all domains (from basic motor coordination to more complex executive function tasks, such as the ability to control emotions and behavior) are affected.44 A possible exception may be improvement in attention with acute use in daily, but not occasional, users.44 Highly focused attention, however, is not always beneficial if it delays redirection toward a new urgent stimulus.
Mood benefit? Research suggests otherwise. The psychiatric effects of cannabis are not fully understood. Users may claim mood benefit, but research suggests marijuana prompts the development or worsening of anxiety, depression, and suicidality.12,47 Violence, paranoia, and borderline personality features have also been associated with use.38,47 Amotivational syndrome, a disorder that includes apathy, callousness, and antisocial behavior, has been described, but the interplay between cannabis and motivation beyond recent use is unclear.48
Lifetime cannabis use is related to panic,49 yet correlational studies suggest both benefit and problems for individuals who use cannabis for posttraumatic stress disorder.50 It is now well established that marijuana use is an independent causal risk factor for the development of psychosis, particularly in vulnerable youth, and that it worsens schizophrenia in those who suffer from it.51 Human experimental studies suggest this may be because the effect of THC is counteracted by CBD.52 Synthetic cannabinoids are even more potent anxiogenic and psychogenic agents than plant-based marijuana.19,20
Cannabis Use Disorder
About 9% of those who try cannabis develop Cannabis Use Disorder, which is characterized by continued use of the substance despite significant distress or impairment.53 Cannabis Use Disorder is essentially an addiction. Primary risk factors include male gender, younger age at marijuana initiation, and personal or family history of other substance or psychiatric problems.53
Although cannabis use often precedes use of other addiction-prone substances, it remains unclear if it is a “gateway” to the use of other illicit drugs.54 Marijuana withdrawal is relatively minor and is comparable to that for tobacco.55 While there are no known effective pharmacotherapies for discontinuing cannabis use, addiction therapy—including cognitive behavioral therapy and trigger management—is effective.56
SIDEBAR
Cannabinoids: A diverse group of chemicalsCannabis, the genus name for 3 species of marijuana plant (sativa, indica, ruderalis), has come to mean any psychoactive part of the plant and is used interchangeably with “marijuana.” There are at least 85 different cannabinoids in the native plant.7
Cannabinoids are a diverse group of chemicals that have activity at cannabinoid receptors. Δ-9-tetrahydrocannabinol (THC), a partial agonist of the CB1 receptor, is the primary psychoactive component and is found in larger quantities in Cannabis sativa, which is preferred by non-medical users. Cannabidiol (CBD), a weak partial CB1 antagonist, exhibits few, if any, psychotropic properties and is more plentiful in Cannabis indica.
Synthetic cannabinioids are a heterogeneous group of manufactured drugs that are full CB1 agonists and that are more potent than THC, yet are often assumed to be safe by users. Typically, they are dissolved in solvents, sprayed onto inert plant materials, and marketed as herbal products like “K2” and “spice.”
So how should the evidence inform your care?
Screen all patients for use of cannabinoids and other addiction-prone substances.57 Follow any affirmative answers to your questions about cannabis use by asking about potential negative consequences of use. For example, ask patients:
- How often during the past 6 months did you find that you were unable to stop using cannabis once you started?
- How often during the past 6 months did you fail to do what was expected of you because of using cannabis? (For more questions, see the Cannabis Use Disorder Identification Test available at: http://www.otago.ac.nz/nationaladdictioncentre/pdfs/cudit-r.pdf.)
Other validated screening tools include the Severity of Dependence Scale, the Cannabis Abuse Screening Test, and the Problematic Use of Marijuana.58
Counsel patients about possible adverse effects and inform them there is no evidence that recreational marijuana or synthetic cannabinoids can be used safely over time. Consider medical use requests only if there is a favorable risk/benefit balance, other recognized treatment options have been exhausted, and you have a strong understanding of the use of cannabis in the medical condition being considered.4
Since brief interventions using motivational interviewing to reduce or eliminate recreational use have not been found to be effective,59 referral to an addiction specialist may be indicated. If a diagnosis of cannabis use disorder is established, then abstinence from addiction-prone substances including marijuana, programs like Marijuana Anonymous (Available at: https://www.marijuana-anonymous.org/), and individualized addiction therapy scaled to the severity of the condition can be effective.56 Because psychiatric conditions frequently co-occur and complicate addiction,53 they should be screened for and managed, as well.
Drug testing. Cannabis Use Disorder has significant relapse potential.60 Abstinence and treatment adherence should be ascertained through regular follow-up that includes a clinical interview, exam, and body fluid drug testing. Point-of-care urine analysis for substances of potential addiction has limited utility. Definitive testing of urine with gas chromotography/mass spectrometry (GC/MS) or liquid chromatography (LC/MS-MS) can eliminate THC false-positives and false-negatives that can occur with point-of-care urine immunoassays. In addition, GCMS and LC/MS-MS can identify synthetic cannabinoids; in-office immunoassays cannot.
If the patient relapses, subsequent medical care should be coordinated with an addiction specialist with the goal of helping the patient to abstain from cannabis.
CORRESPONDENCE
Steven Wright, MD, FAAFP, 5325 Ridge Trail, Littleton, CO 80123; [email protected].
1. Pew Research Center. 6 facts about marijuana. Available at: http://www.pewresearch.org/fact-tank/2015/04/14/6-facts-about-marijuana/. Accessed September 27, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Pub # (SMA) 14-4863. 2014. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf. Accessed September 27, 2015.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future National Survey on Drug Use 1975-2015. Available at: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Accessed September 23, 2015.
4. Metts J, Wright S, Sundaram J, et al. Medical marijuana: a treatment worth trying? J Fam Pract. 2016;65:178-185.
5. Governing the states and localities. State marijuana laws map. Available at: http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html. Accessed October 12, 2016.
6. US Drug Enforcement Administration. Drug scheduling. Available at: https://www.dea.gov/druginfo/ds.shtml. Accessed October 12, 2016.
7. El-Alfy AT, Ivey K, Robinson K, et al. Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav. 2010;95:434-442.
8. Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107:1404-1417.
9. Gruber AJ, Pope HG, Hudson JI, et al. Attributes of long-term heavy cannabis users: a case-control study. Psychol Med. 2003;33:1415-1422.
10. Palamar JJ, Fenstermaker M, Kamboukos D, et al. Adverse psychosocial outcomes associated with drug use among US high school seniors: a comparison of alcohol and marijuana. Am J Drug Alcohol Abuse. 2014;40:438-446.
11. Zwerling C, Ryan J, Orav EJ. The efficacy of preemployment drug screening for marijuana and cocaine in predicting employment outcome. JAMA. 1990;264:2639-2643.
12. Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123-1135.
13. Bell C, Slim J, Flaten HK, et al. Butane hash oil burns associated with marijuana liberalization in Colorado. J Med Toxicol. 2015;11:422-425.
14. Huang DYC, Lanza HI, Anglin MD. Association between adolescent substance use and obesity in young adulthood: a group-based dual trajectory analysis. Addict Behav. 2013;38:2653-2660.
15. Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494.
16. Cho CM, Hirsch R, Johnstone S. General and oral health implications of cannabis use. Aust Dent J. 2005;50:70-74.
17. Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav. 2010;58:91-99.
18. Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg. 2013;27:996-1005.
19. Mills B, Yepes A, Nugent K. Synthetic cannabinoids. Am J Med Sci. 2015;350:59-62.
20. Tuv SS, Strand MC, Karinen R, et al. Effect and occurrence of synthetic cannabinoids. Tidsskr Nor Laegeforen. 2012;132:2285-2288.
21. Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011;104:659-964.
22. Gates P, Jaffe A, Copeland J. Cannabis smoking and respiratory health: considerations of the literature. Respirology. 2014;19:655-662.
23. Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years: The Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2012;307:173-181.
24. Verweij PE, Kerremans JJ, Vos A, et al. Fungal contamination of tobacco and marijuana. JAMA. 2000;284:2875.
25. Office of Environmental Health Hazard Assessment. Evidence on the carcinogenicity of marijuana smoke. August 2009. Available at: http://oehha.ca.gov/media/downloads/crnr/finalmjsmokehid.pdf. Accessed September 5, 2015.
26. Stone D. Cannabis, pesticides and conflicting laws: the dilemma for legalized States and implications for public health. Regul Toxicol Pharmacol. 2014;69:284-288.
27. Hashibe M, Straif K, Tashkin DP, et al. Epidemiologic review of marijuana and cancer risk. Alcohol. 2005;35:265-275.
28. Liang C, McClean MD, Marsit C, et al. A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prev Res (Phila). 2009;2:759-768.
29. Thomas AA, Wallner LP, Quinn VP, et al. Association between cannabis use and the risk of bladder cancer: results from the California Men’s Health Study. Urology. 2015;85:388-392.
30. Holly EA, Lele C, Bracci PM, et al. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Francisco Bay area, California. Am J Epidemiol. 1999;150:375-389.
31. Massi P, Solinas M, Cinquina V, et al. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303-312.
32. Ashbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536.
33.Barrio G, Jimenez-Mejias E, Pulido J, et al. Association between cannabis use and non-traffic injuries. Accid Anal Prev. 2012;47:172-176.
34. MacDonald S, Hall W, Roman P, et al. Testing for cannabis in the work-place: a review of the evidence. Addiction. 2010;105:408-416.
35. Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict. 2009;18:185-193.
36. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109-119.
37. Menetrey A, Augsburger M, Favrat B, et al. Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoid levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Δ9-THC. J Anal Toxicol. 2005;29:327-338.
38. Raemakers JG, Kaurert G, van Ruitenbeek P, et al. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296-2303.
39. Leirer VO, Yesavage JA, Morrow DG. Marijuana carry-over effects on aircraft pilot performance. Aviat Space Environ Med. 1991;62:221-227.
40. Calabria B, Degenhardt L, Hall W, et al. Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010;29:318-330.
41. Colorado Department of Public Health and Environment. Monitoring health concerns related to marijuana in Colorado: 2014. Changes in marijuana use patterns, systematic literature review, and possible marijuana-related health effects. Available at: http://www2.cde.state.co.us/artemis/hemonos/he1282m332015internet/he1282m332015internet01.pdf. Accessed September 5, 2015.
42. Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn. Perinatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009-1024.
43. Batalla A, Bhattacharyya S, Yücel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821.
44. Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1-8.
45. Pavisian B, MacIntosh BJ, Szilagyi G, et al. Effects of cannabis on cognition in patients with multiple sclerosis: a psychometric and MRI study. Neurology. 2014;82:1879-1887.
46. Bartholomew J, Holroyd S, Heffernan TM. Does cannabis use affect prospective memory in young adults? J Psychopharmacol. 2010;24:241-246.
47. Copeland J, Rooke S, Swift W. Changes in cannabis use among young people: impact on mental health. Curr Opin Psychiatry. 2013;26:325-329.
48. Ari M, Sahpolat M, Kokacya H, et al. Amotivational syndrome: less known and diagnosed as a clinical. J Mood Disord. 2015;5:31-35.
49. Zvolensky MJ, Cougle JR, Johnson KA, et al. Marijuana use and panic psychopathology among a representative sample of adults. Exp Clin Psychopharmacol. 2010;18(2):129-134.
50. Yarnell S. The use of medicinal marijuana for posttraumatic stress disorder: a review of the current literature. Prim Care Companion CNS Disord. 2015;17(3).
51. Le Bec PY, Fatséas M, Denis C, et al. Cannabis and psychosis: search of a causal link through a critical and systematic review. Encephale. 2009;35:377-385.
52. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19-27.
53. Lopez-Quintero C, Perez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
54. Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108:84-97.
55. Vandrey RG, Budney AJ, Hughes JR, et al. A within subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92:48-54.
56.Budney AJ, Roffman R, Stephens RS, et al. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4-16.
57. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Phys. 2014;60:801-808.
58. Piontek D, Kraus L, Klempova D. Short scales to assess cannabis-related problems: a review of psychometric properties. Subst Abuse Treat Prev Policy. 2008;3:25.
59. Saitz R, Palfai TPA, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312:502-513.
60. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689-1695.
1. Pew Research Center. 6 facts about marijuana. Available at: http://www.pewresearch.org/fact-tank/2015/04/14/6-facts-about-marijuana/. Accessed September 27, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. HHS Pub # (SMA) 14-4863. 2014. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf. Accessed September 27, 2015.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future National Survey on Drug Use 1975-2015. Available at: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Accessed September 23, 2015.
4. Metts J, Wright S, Sundaram J, et al. Medical marijuana: a treatment worth trying? J Fam Pract. 2016;65:178-185.
5. Governing the states and localities. State marijuana laws map. Available at: http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html. Accessed October 12, 2016.
6. US Drug Enforcement Administration. Drug scheduling. Available at: https://www.dea.gov/druginfo/ds.shtml. Accessed October 12, 2016.
7. El-Alfy AT, Ivey K, Robinson K, et al. Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav. 2010;95:434-442.
8. Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107:1404-1417.
9. Gruber AJ, Pope HG, Hudson JI, et al. Attributes of long-term heavy cannabis users: a case-control study. Psychol Med. 2003;33:1415-1422.
10. Palamar JJ, Fenstermaker M, Kamboukos D, et al. Adverse psychosocial outcomes associated with drug use among US high school seniors: a comparison of alcohol and marijuana. Am J Drug Alcohol Abuse. 2014;40:438-446.
11. Zwerling C, Ryan J, Orav EJ. The efficacy of preemployment drug screening for marijuana and cocaine in predicting employment outcome. JAMA. 1990;264:2639-2643.
12. Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123-1135.
13. Bell C, Slim J, Flaten HK, et al. Butane hash oil burns associated with marijuana liberalization in Colorado. J Med Toxicol. 2015;11:422-425.
14. Huang DYC, Lanza HI, Anglin MD. Association between adolescent substance use and obesity in young adulthood: a group-based dual trajectory analysis. Addict Behav. 2013;38:2653-2660.
15. Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012;2:e000494.
16. Cho CM, Hirsch R, Johnstone S. General and oral health implications of cannabis use. Aust Dent J. 2005;50:70-74.
17. Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav. 2010;58:91-99.
18. Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg. 2013;27:996-1005.
19. Mills B, Yepes A, Nugent K. Synthetic cannabinoids. Am J Med Sci. 2015;350:59-62.
20. Tuv SS, Strand MC, Karinen R, et al. Effect and occurrence of synthetic cannabinoids. Tidsskr Nor Laegeforen. 2012;132:2285-2288.
21. Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011;104:659-964.
22. Gates P, Jaffe A, Copeland J. Cannabis smoking and respiratory health: considerations of the literature. Respirology. 2014;19:655-662.
23. Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years: The Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2012;307:173-181.
24. Verweij PE, Kerremans JJ, Vos A, et al. Fungal contamination of tobacco and marijuana. JAMA. 2000;284:2875.
25. Office of Environmental Health Hazard Assessment. Evidence on the carcinogenicity of marijuana smoke. August 2009. Available at: http://oehha.ca.gov/media/downloads/crnr/finalmjsmokehid.pdf. Accessed September 5, 2015.
26. Stone D. Cannabis, pesticides and conflicting laws: the dilemma for legalized States and implications for public health. Regul Toxicol Pharmacol. 2014;69:284-288.
27. Hashibe M, Straif K, Tashkin DP, et al. Epidemiologic review of marijuana and cancer risk. Alcohol. 2005;35:265-275.
28. Liang C, McClean MD, Marsit C, et al. A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prev Res (Phila). 2009;2:759-768.
29. Thomas AA, Wallner LP, Quinn VP, et al. Association between cannabis use and the risk of bladder cancer: results from the California Men’s Health Study. Urology. 2015;85:388-392.
30. Holly EA, Lele C, Bracci PM, et al. Case-control study of non-Hodgkin’s lymphoma among women and heterosexual men in the San Francisco Bay area, California. Am J Epidemiol. 1999;150:375-389.
31. Massi P, Solinas M, Cinquina V, et al. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303-312.
32. Ashbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536.
33.Barrio G, Jimenez-Mejias E, Pulido J, et al. Association between cannabis use and non-traffic injuries. Accid Anal Prev. 2012;47:172-176.
34. MacDonald S, Hall W, Roman P, et al. Testing for cannabis in the work-place: a review of the evidence. Addiction. 2010;105:408-416.
35. Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict. 2009;18:185-193.
36. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109-119.
37. Menetrey A, Augsburger M, Favrat B, et al. Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoid levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Δ9-THC. J Anal Toxicol. 2005;29:327-338.
38. Raemakers JG, Kaurert G, van Ruitenbeek P, et al. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296-2303.
39. Leirer VO, Yesavage JA, Morrow DG. Marijuana carry-over effects on aircraft pilot performance. Aviat Space Environ Med. 1991;62:221-227.
40. Calabria B, Degenhardt L, Hall W, et al. Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010;29:318-330.
41. Colorado Department of Public Health and Environment. Monitoring health concerns related to marijuana in Colorado: 2014. Changes in marijuana use patterns, systematic literature review, and possible marijuana-related health effects. Available at: http://www2.cde.state.co.us/artemis/hemonos/he1282m332015internet/he1282m332015internet01.pdf. Accessed September 5, 2015.
42. Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn. Perinatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131:e1009-1024.
43. Batalla A, Bhattacharyya S, Yücel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821.
44. Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1-8.
45. Pavisian B, MacIntosh BJ, Szilagyi G, et al. Effects of cannabis on cognition in patients with multiple sclerosis: a psychometric and MRI study. Neurology. 2014;82:1879-1887.
46. Bartholomew J, Holroyd S, Heffernan TM. Does cannabis use affect prospective memory in young adults? J Psychopharmacol. 2010;24:241-246.
47. Copeland J, Rooke S, Swift W. Changes in cannabis use among young people: impact on mental health. Curr Opin Psychiatry. 2013;26:325-329.
48. Ari M, Sahpolat M, Kokacya H, et al. Amotivational syndrome: less known and diagnosed as a clinical. J Mood Disord. 2015;5:31-35.
49. Zvolensky MJ, Cougle JR, Johnson KA, et al. Marijuana use and panic psychopathology among a representative sample of adults. Exp Clin Psychopharmacol. 2010;18(2):129-134.
50. Yarnell S. The use of medicinal marijuana for posttraumatic stress disorder: a review of the current literature. Prim Care Companion CNS Disord. 2015;17(3).
51. Le Bec PY, Fatséas M, Denis C, et al. Cannabis and psychosis: search of a causal link through a critical and systematic review. Encephale. 2009;35:377-385.
52. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27:19-27.
53. Lopez-Quintero C, Perez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
54. Degenhardt L, Dierker L, Chiu WT, et al. Evaluating the drug use “gateway” theory using cross-national data: consistency and associations of the order of initiation of drug use among participants in the WHO World Mental Health Surveys. Drug Alcohol Depend. 2010;108:84-97.
55. Vandrey RG, Budney AJ, Hughes JR, et al. A within subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92:48-54.
56.Budney AJ, Roffman R, Stephens RS, et al. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4-16.
57. Turner SD, Spithoff S, Kahan M. Approach to cannabis use disorder in primary care: focus on youth and other high-risk users. Can Fam Phys. 2014;60:801-808.
58. Piontek D, Kraus L, Klempova D. Short scales to assess cannabis-related problems: a review of psychometric properties. Subst Abuse Treat Prev Policy. 2008;3:25.
59. Saitz R, Palfai TPA, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312:502-513.
60. McLellan AT, Lewis DC, O’Brien CP, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689-1695.
Medical marijuana: A treatment worth trying?
› Consider recommending medical marijuana for conditions with evidence supporting its use only after other treatment options have been exhausted. B
› Thoroughly screen potential candidates for medical marijuana to rule out a history of substance abuse, mental illness, and other contraindications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Gladys B, a 68-year-old patient with a history of peripheral neuropathy related to chemotherapy she underwent years ago, has been treated alternately with acetaminophen with codeine, tramadol, gabapentin, and morphine. Each provided only minimal relief. Your state recently legalized medical marijuana, and she wants to know whether it might alleviate her pain.
If Ms. B were your patient, how would you respond?
Medical marijuana is now legal in 23 states and Washington, DC. Other states are considering legalization or have authorized particular components for use as medical treatment.1 As such laws proliferate and garner more media attention, it is increasingly likely that patients will turn to their primary care physicians with questions about the use of marijuana for medicinal purposes. What can you tell them?
Conversations about medical marijuana should be based on the understanding that while many claims have been made about the therapeutic effects of marijuana, only a few of these claims have evidence to back them up. Major medical organizations, including the American Academy of Family Physicians,2 the American College of Physicians,3 and the Institute of Medicine,4 recognize its potential as a treatment for various conditions, but emphasize the need for additional research rather than wholesale adoption.
Most commonly, medical marijuana is used to treat pain symptoms, but it is also used for a host of other conditions. A 2015 systematic review and meta-analysis5 found moderate-quality evidence to support its use for the treatment of chronic and neuropathic pain and spasticity associated with multiple sclerosis (MS), and low-quality evidence for the treatment of nausea and vomiting associated with chemotherapy, for weight gain in patients with human immunodeficiency virus (HIV), and to treat Tourette syndrome. (TABLE 1 lists the conditions for which medical marijuana has been found to be indicated.5-13) For most other conditions that qualify for the use of medical marijuana under state laws, however—insomnia, hepatitis C, Crohn’s disease, and anxiety and depression, among others—the evidence is either of very low quality or nonexistent.5
Evaluating marijuana is difficult
It is important to note that marijuana comprises more than 60 pharmacologically active cannabinoids, which makes it difficult to study. Both exogenous ligands, such as the cannabinoids from marijuana, and endogenous ligands (endocannabinoids), such as anandamide and 2-arachidonoylglycerol, act on cannabinoid receptors. These receptors are found throughout the body, but are primarily in the brain and spinal cord.14
The main cannabinoids contained in marijuana are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC produces the euphoria for which recreational marijuana is known, but can also induce psychosis. CBD is not psychoactive and is thought to have antianxiety and possibly antipsychotic properties. Thus, marijuana’s therapeutic effects depend on the concentration of THC in a given formulation. Because CBD has the ability to mitigate psychoactive effects, the ratio of THC to CBD is important, as well.15
What’s more, medical marijuana is available in various forms. It can be smoked—the most widely used route—or inhaled with an inhalation device, ingested in food or as a tea, taken orally, administered via an oromucosal spray, or even applied topically. Medical marijuana may be extracted naturally from the cannabis plant, produced by the isomerization of CBD, manufactured synthetically, or provided as an herbal formulation.
There are also cannabinoids that have been approved by the US Food and Drug Administration (FDA)—dronabinol (a synthetic version of THC) and nabilone (a synthetic cannabinoid). Nabiximols, a cannabis extract in the form of an oromucosal spray, is licensed in the UK for the treatment of symptoms associated with multiple sclerosis, but has not yet received FDA approval.16,17
As with any treatment or medication, the benefits must be weighed against the risks. Scientific studies have documented many adverse health effects associated with marijuana, including the risk of addiction and the potential for marijuana to be used as a gateway drug; its effect on brain development, school performance, and lifetime achievement; a potential relationship to mental illness; and the risk of cancer and motor vehicle accidents.1,16,18 Patients in clinical trials have reported dizziness, dysphoria, hallucinations, and paranoia, as well.12
What’s more, marijuana remains classified as a Schedule I agent.19 Because of its high potential for abuse, physicians in states where medical marijuana has been legalized should adhere to off-label prescribing principles: Recommend it only after standard medications, including FDA-approved cannabinoids, and nonpharmaceutical approaches have proven to be inadequate.6,20,21
Medical marijuana for your patient? A look at the evidence
The meta-analysis cited earlier included 79 randomized clinical trials (RCTs) of medical marijuana used for a variety of conditions in a number of delivery modes. However, only 4 were judged to be of low risk of bias.5 Nonetheless, here’s a look at this and other evidence.
Chronic and neuropathic pain
Twenty-eight of the 79 studies addressed chronic pain, with half assessing the oromucosal spray (nabiximols). Most others studied marijuana that was smoked or inhaled. Neuropathic pain was most frequently studied, but cancer pain, fibromyalgia, and musculoskeletal pain, among others, were also evaluated.5
The average number of patients who reported a reduction in pain of ≥30% was greater with marijuana compared with placebo (odds ratio=1.41; 95% confidence interval, .99-2.0). Delivery mode did not affect outcomes; different forms of administration were not associated with any significant difference in pain relief. Nor were there significant differences in results among the various pain conditions studied. Notably, however, quality of life measurements did not reflect any overall improvement.5
The authors of a literature review of marijuana for chronic and neuropathic pain and MS-induced spasticity did find high-quality evidence of its efficacy in several of the trials they assessed.6 And a review of well-conducted observational trials of smoked marijuana as a treatment for severe neuropathic pain revealed that it may be indicated for those who fail to respond to FDA-approved cannabinoids and standard analgesics.10 Neither functional status nor quality of life was evaluated, however, and none of the observational studies compared smoked cannabis to standard analgesics.
Notably, the authors did not recommend smoked marijuana for pain conditions such as low back pain and fibromyalgia, which are commonly seen in practice. That’s because the safety and efficacy of smoked cannabis has not been studied for these conditions and because evidence-based treatments for these disorders exist.10
CASE › Before considering medical marijuana for Ms. B, you suggest a trial of dronabinol. The patient agrees, and you prescribe 2.5 mg twice a day. You schedule a visit in 4 weeks to review the drug’s efficacy and tell her to call if she develops psychiatric symptoms, such as hallucinations or paranoia, or impaired cognition. You also advise her that dronabinol may increase the risk of auto accidents and caution her to avoid driving for 6 hours after taking the drug—or longer if she experiences an initial “high.”
MS symptoms
A comprehensive review of medical marijuana studies spanning nearly 7 decades revealed 12 trials focusing on MS—and found its use in treating MS-related spasticity supported by high-quality evidence.6
Two of the largest studies were done in the UK.7,8 One multicenter trial included 630 participants randomized to treatment with an oral cannabinoid extract, THC, or placebo for 6 weeks.7
There was no change in the primary outcome measure, the Ashworth spasticity scale. However, there was a treatment effect on patient-reported spasticity and pain, with improvement in spasticity reported by 61% of those treated with the cannabinoid extract, 60% of those treated with THC, and 46% of those treated with placebo.7
The other UK trial involved 22 centers and 279 patients, randomized to either oral cannabis extract or placebo. The primary outcome measure involved a category rating scale that reported on change in muscle stiffness since baseline and on body pain, spasms, and sleep quality. This study used a 2-week titration phase and a 10-week maintenance phase. The rate of relief from muscle stiffness after 12 weeks was almost twice as high in the cannabis extract group (29%) compared with placebo (16%).8
A systematic review of the efficacy and safety of medical marijuana by the American Academy of Neurology (AAN) concluded that oral cannabis extract, THC, and nabiximols are “probably effective” in reducing patient-centered measures of spasticity and pain associated with MS.9
Little help for other neurologic disorders. Studies of the efficacy and safety of medical marijuana for other neurologic disorders have been less encouraging. The AAN concluded that cannabinoids are probably ineffective for the treatment of tremors, and that oral cannabis extract is probably ineffective for treating levodopa-induced dyskinesias in patients with Parkinson’s disease.
A 2014 systematic review found that oral cannabinoids were of unknown efficacy in treating nonchorea-related symptoms of Huntington’s disease, Tourette syndrome, cervical dystonia, and epilepsy.9 The 2015 systematic review and meta-analysis cited earlier, however, suggests that there is low-quality evidence that cannabinoids improve symptoms associated with sleep disorders and Tourette symptoms.5
Cancer-related symptoms
In 1985, the FDA approved dronabinol for the treatment of chemotherapy-induced nausea and vomiting (CINV) not controlled by other medications. Nabilone followed, receiving FDA approval in 1992.11
Serotonin receptor antagonists (5-HT3 receptor antagonists) were also introduced in the early 1990s. In 2001, a systematic review of 30 RCTs with a total of 1366 patients looked at how cannabinoids—including oral dronabinol, oral nabilone, and intramuscular levonantradol, a synthetic drug that does not have FDA approval—compared with placebo or other antiemetics.12
The researchers found the FDA-approved cannabinoids to be more effective than prochlorperazine, metoclopramide, chlorpromazine, and other antiemetics for most patients. (The included studies did not compare cannabinoids with 5-HT3 agents.) That was not the case, however, for patients receiving either very low or very highly emetogenic chemotherapy.
In crossover studies, participants reported that they preferred cannabinoids for future CINV control. Although they cited the “high,” sedation, and euphoria as potential beneficial effects, those taking cannabinoids were also more likely than patients receiving other antiemetics to withdraw from studies due to adverse effects, including dizziness, dysphoria, depression, hallucinations, and paranoia. The authors concluded that cannabinoids might be useful as mood-enhancing adjuvants for controlling CINV, but that short-term adverse effects were likely to limit their widespread use.12
Recommended antiemetic regimens for patients with highly emetogenic regimens or those whose chemotherapy comes with a high risk of delayed CINV include the serotonin antagonist dexamethasone, with or without aprepitant or fosaprepitant. Because of the availability of safer and more effective agents, the National Comprehensive Cancer Network (NCCN) does not consider cannabinoids first-line treatment for the prevention of CINV. Instead, they are reserved for breakthrough symptoms or refractory nausea and vomiting.11
In fact, NCCN practice guidelines do not recommend medical marijuana for the management of CINV because of both medical and legal concerns. Even in states in which medical marijuana is legal, the organization states, its use is controversial.11
Combatting anorexia and cachexia. An estimated 50% of cancer patients develop anorexia and cachexia. The systemic inflammation and loss of protein, energy, and lean body mass is associated not only with a poor response to chemotherapy and decreased survival rates, but also with a lower quality of life. While therapies to alleviate these symptoms typically focus on palliation and reduction of distress rather than on prolonging life, some agents, such as megestrol and medroxyprogesterone, are reported to improve survival rates as well as quality of life.22
Cannabinoids have also been used to increase appetite and food intake and facilitate weight gain in cancer patients. The exact mechanism by which this effect occurs is not known; in fact, questions about the extent of the effect itself remain.
Two RCTs failed to show benefits in this regard compared with megestrol or placebo. One study of 469 patients with advanced cancer compared dronabinol, administered alone or in combination with megestrol, with megestrol alone. Using a Functional Assessment of Anorexia/Cachexia Therapy Questionnaire to assess quality of life, the researchers found that megestrol provided better palliation of anorexia than dronabinol alone and that the combination of dronabinol and megestrol showed no advantage over megestrol alone.13
The second study was a multicenter Phase III double-blind RCT comparing cannabis extract (CE), THC, and placebo in 289 cancer patients. The researchers found no differences in appetite, quality of life, or toxicity among those in the 3 arms of the study. A data review board subsequently recommended that study recruitment be stopped because of the absence of significant differences.23
HIV and AIDS-related morbidity and mortality
Evidence of the efficacy and safety of cannabinoid use among adult patients with HIV or acquired immune deficiency syndrome (AIDS) is lacking, according to a 2013 Cochrane review.24 The review looked at RCTs that compared any marijuana intervention in this patient population to either placebo or a known treatment, such as megestrol or medroxyprogesterone.Worth noting, however, is that the review included studies that were of short duration, involved small numbers of patients, and focused on short-term measures of efficacy.
Long-term studies indicating that cannabinoids have a sustained effect on AIDS-related morbidity and mortality in patients being treated with antiretroviral therapy have yet to be conducted.24 The systematic review and meta-analysis published in 2015, however, did find evidence suggesting that cannabinoids were associated with weight gain in patients with HIV.5 Dronabinol has had FDA approval for treatment of weight loss associated with AIDS-related anorexia since 1992.
Before you recommend medical marijuana…
Although medical marijuana is not actually “prescribed,” there are steps to take before recommending or facilitating its use for a particular patient (TABLE 2).25-29
After ensuring that he or she has a condition for which there is evidence to support it, you need to do a risk evaluation, drawing on the opioid-prescribing paradigm to look for contraindications to the use of a controlled substance or factors that indicate the need for additional precaution (TABLE 3).10,25,26
Take a thorough medical history and use screening tools
A thorough patient and family medical history, along with principles of Screening, Brief Intervention, and Referral for Treatment (SBIRT), can be used to identify addiction-prone substance use.28 You can also use a validated tool such as the Cannabis Use Disorder Test (CUDIT-R), available at http://sfmi.wufoo.com/forms/qulgngl12rydww/.Body fluid (usually urine) testing is also recommended.30 You may be able to access your state’s Prescription Drug Monitoring Program to check for worrisome prescribing, as well.
Stratify risk
The next step is to determine whether the patient is at low, intermediate, or high risk for use of a controlled substance based on your findings. Patients who are younger than 25 years, for example, have an increased risk.And high-risk patients—those with a history of substance abuse, psychiatric illness, or sexual trauma—are unlikely to be good candidates for medical marijuana10,25,26 and should be informed in a nonjudgmental manner that their problem is better addressed without it.
If the risk/benefit balance is favorable and the patient is willing to give medical marijuana a try, complete a signed certification of a medical condition for which medical marijuana is authorized in your state. Details of state laws are available at medicalmarijuana.procon.org/view.resource.php?resourceID=000881.
Because the individuals who dispense medical marijuana have varying skills, physicians should collaborate with clinicians judged to be knowledgeable about the best strains of marijuana, the best administration route, and the lowest effective dose—typically a pain management specialist or a physician experienced in recommending medical marijuana appropriately. Vaporization of marijuana, for use with an inhalation device, may prevent some of the potentially negative consequences of smoking it.31 Vaporizing is thought to eliminate some of the irritating—and possibly carcinogenic—materials contained in marijuana smoke.
Follow risk mitigation principles
Because marijuana is a controlled substance, you will need to talk to the patient about how to store and, if necessary, dispose of it to avoid the risk of diversion—a major concern about the legalization of marijuana.
You can cite a small study of adolescents in substance abuse treatment, in which 3 out of 4 reported having used someone else’s medical marijuana a median of 50 times.32 Adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana abuse, and more dependence and conduct disorder symptoms compared with teens who had not used medical marijuana.32
It is important, too, to obtain informed consent and draw up a controlled substance agreement, signed by the patient and you. The agreement should outline expected patient behavior, including regular monitoring and body fluid testing, and the consequences of a lack of adherence. (Using a certified laboratory for drug testing is important, as it avoids the possibility of actions based on inaccurate in-office screening.33) Regular follow-up also provides an opportunity to assess symptom and functional improvement.
If the patient fails to keep appointments and does not respond to efforts to address the problem, the marijuana recommendation may have to be rescinded. Adverse effects, continued aberrant behavior, or evidence of cannabis use disorder may necessitate immediate cessation of the drug. Depending on the scope of the problem, collaboration with addiction therapy may be necessary. Discharge from the practice, of course, should be the last resort.
CASE › At a subsequent visit—after a trial with the maximal dose of dronabinol—Ms. B states that although she had some relief, she continues to have a high degree of breakthrough pain. You suspect that medical marijuana may do more to alleviate her pain, and establish a regimen to quickly taper her off dronabinol.
You consult with a pain management specialist, who suggests that the patient begin with raw marijuana with a 10% THC content, smoking 0.6 gm tid. You obtain informed consent and ask her to sign a controlled substance agreement, explaining that you will need to monitor her closely for dizziness, dysphoria, and hallucinations, among other adverse effects. You instruct her not to drive for 6 hours after smoking marijuana, and you schedule a follow-up appointment in 2 weeks.
Before she leaves, Ms. B receives a copy of your clinic note and written recommendation that she can take to the state dispensary. The note indicates that she will use marijuana for neuropathic pain.
CORRESPONDENCE
Julius Metts, MD, California Substance Abuse Treatment Facility and State Prison, CDCR, 900 Quebec Avenue, Corcoran, CA 93212; [email protected].
1. Office of National Drug Control Policy. Marijuana Resource Center. State Laws Related to Marijuana. Available at: https://www.whitehouse.gov/ondcp/state-laws-related-to-marijuana. Accessed December 12, 2015.
2. American Academy of Family Physicians. AAFP policies: marijuana. Available at: http://www.aafp.org/about/policies/all/marijuana.html. Accessed January 16, 2016.
3. American College of Physicians. Supporting research into the therapeutic role of marijuana. Available at: https://www.acponline.org/acp_policy/policies/supporting_medmarijuana_2008.pdf. Accessed January 26, 2016.
4. Institute of Medicine. Marijuana and medicine: assessing the science base. Available at: http://iom.nationalacademies.org/reports/1999/marijuana-and-medicine-assessing-the-science-base.aspx. Accessed January 26, 2016.
5. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
6. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313:2474-2483.
7. Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo controlled trial. Lancet. 2003;362:1517-1526.
8. Zajicek JP, Hobart JC, Slade A, et al. MUSEC research group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83:1125–1132.
9. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556-1563.
10. Kahan M, Srivastava A, Spithoff S, et al. Smoked CB for chronic noncancer pain. Can Fam Physician. 2014;60:1083–1090.
11. Todaro B. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting. J Natl Compr Canc Network. 2012;10:487-492.
12. Tramer MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systemic review. BMJ. 2001;323:16-21.
13. Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567-573.
14. Hu SS, Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handbook Exp Pharmacol. 2015;231:59-93.
15. Bhattacharyya S, Morrison PD, Fusar-Poli P. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764-774.
16. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids – an international cross sectional survey on administration forms. J Psychoactive Drugs. 2013;45:199-210.
17. ProCon.org site. 10 pharmaceutical drugs based on cannabis. Available at: http://medicalmarijuana.procon.org/view.resource.php?resourceID=000883. Accessed January 28, 2016.
18. Cerda M, Wall M, Keyes KM, et al. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana, abuse and dependence. Drug Alcohol Depend. 2012;120:22-27.
19. US Food and Drug Administration. Inter-agency advisory regarding claims that smoked marijuana is a medicine. April 20, 2006. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108643. Accessed January 29, 2016.
20. Marinol dronabinol capsules. Available at: www.marinol.com. Accessed January 29, 2016.
21. Cesamet full prescribing information. Available at: http://www.cesamet.com/patient-home.asp. Accessed January 29, 2016.
22. Aoyagi T, Terracini KP, Raza A, et al. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7:17-29.
23. Strasser F, Laftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9 tetrahydrocannabinol (THC) in treating patients with cancer-related anorexia cachexia syndrome, a multicenter, randomized, double blind controlled clinical trial from the Cannabis-In Cachexia Study Group. Clin Oncol. 2006;24:3394 -3400.
24. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
25. Phillips JA, Holland MG, Baldwin DD. Marijuana in the workplace: guidance for occupational health professionals and employers: Joint Guidance Statement of the American Association of Occupational Health Nurses and the American College of Occupational and Environmental Medicine. J Occup Environ Med. 2015;57:459-475.
26. Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Phys. 2012;15:ES67-ES92.
27. Lopez-Quintero C, de los Cabos JP, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
28. Strobbe S. Prevention and screening, brief intervention and referral to treatment for substance use in primary care. Primary Care. 2014;41:185-213.
29. Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35:102-110.
30. American Society of Addiction Medicine. Drug testing: a white paper of the American Society of Addiction Medicine. Available at: http://www.asam.org/docs/default-source/publicy-policy-statements/drug-testing-a-white-paper-by-asam.pdf?sfvrsn=2. October 26, 2013. Accessed January 26, 2016.
31. Tomar RS, Beaumont J, Hsieh JCY. Evidence on the carcinogenicity of marijuana smoke. California EPA: Reproductive and Cancer Hazard Assessment Branch of the Office of Environmental Health Hazard Assessment. August 2009. Available at: http://oehha.ca.gov/prop65/hazard_ident/pdf_zip/FinalMJsmokeHID.pdf. Accessed January 29, 2016.
32. Salomonsen–Sautel S, Sakai JT, Thurstone C. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;7:694-702.
33. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1:937-952.
› Consider recommending medical marijuana for conditions with evidence supporting its use only after other treatment options have been exhausted. B
› Thoroughly screen potential candidates for medical marijuana to rule out a history of substance abuse, mental illness, and other contraindications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Gladys B, a 68-year-old patient with a history of peripheral neuropathy related to chemotherapy she underwent years ago, has been treated alternately with acetaminophen with codeine, tramadol, gabapentin, and morphine. Each provided only minimal relief. Your state recently legalized medical marijuana, and she wants to know whether it might alleviate her pain.
If Ms. B were your patient, how would you respond?
Medical marijuana is now legal in 23 states and Washington, DC. Other states are considering legalization or have authorized particular components for use as medical treatment.1 As such laws proliferate and garner more media attention, it is increasingly likely that patients will turn to their primary care physicians with questions about the use of marijuana for medicinal purposes. What can you tell them?
Conversations about medical marijuana should be based on the understanding that while many claims have been made about the therapeutic effects of marijuana, only a few of these claims have evidence to back them up. Major medical organizations, including the American Academy of Family Physicians,2 the American College of Physicians,3 and the Institute of Medicine,4 recognize its potential as a treatment for various conditions, but emphasize the need for additional research rather than wholesale adoption.
Most commonly, medical marijuana is used to treat pain symptoms, but it is also used for a host of other conditions. A 2015 systematic review and meta-analysis5 found moderate-quality evidence to support its use for the treatment of chronic and neuropathic pain and spasticity associated with multiple sclerosis (MS), and low-quality evidence for the treatment of nausea and vomiting associated with chemotherapy, for weight gain in patients with human immunodeficiency virus (HIV), and to treat Tourette syndrome. (TABLE 1 lists the conditions for which medical marijuana has been found to be indicated.5-13) For most other conditions that qualify for the use of medical marijuana under state laws, however—insomnia, hepatitis C, Crohn’s disease, and anxiety and depression, among others—the evidence is either of very low quality or nonexistent.5
Evaluating marijuana is difficult
It is important to note that marijuana comprises more than 60 pharmacologically active cannabinoids, which makes it difficult to study. Both exogenous ligands, such as the cannabinoids from marijuana, and endogenous ligands (endocannabinoids), such as anandamide and 2-arachidonoylglycerol, act on cannabinoid receptors. These receptors are found throughout the body, but are primarily in the brain and spinal cord.14
The main cannabinoids contained in marijuana are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC produces the euphoria for which recreational marijuana is known, but can also induce psychosis. CBD is not psychoactive and is thought to have antianxiety and possibly antipsychotic properties. Thus, marijuana’s therapeutic effects depend on the concentration of THC in a given formulation. Because CBD has the ability to mitigate psychoactive effects, the ratio of THC to CBD is important, as well.15
What’s more, medical marijuana is available in various forms. It can be smoked—the most widely used route—or inhaled with an inhalation device, ingested in food or as a tea, taken orally, administered via an oromucosal spray, or even applied topically. Medical marijuana may be extracted naturally from the cannabis plant, produced by the isomerization of CBD, manufactured synthetically, or provided as an herbal formulation.
There are also cannabinoids that have been approved by the US Food and Drug Administration (FDA)—dronabinol (a synthetic version of THC) and nabilone (a synthetic cannabinoid). Nabiximols, a cannabis extract in the form of an oromucosal spray, is licensed in the UK for the treatment of symptoms associated with multiple sclerosis, but has not yet received FDA approval.16,17
As with any treatment or medication, the benefits must be weighed against the risks. Scientific studies have documented many adverse health effects associated with marijuana, including the risk of addiction and the potential for marijuana to be used as a gateway drug; its effect on brain development, school performance, and lifetime achievement; a potential relationship to mental illness; and the risk of cancer and motor vehicle accidents.1,16,18 Patients in clinical trials have reported dizziness, dysphoria, hallucinations, and paranoia, as well.12
What’s more, marijuana remains classified as a Schedule I agent.19 Because of its high potential for abuse, physicians in states where medical marijuana has been legalized should adhere to off-label prescribing principles: Recommend it only after standard medications, including FDA-approved cannabinoids, and nonpharmaceutical approaches have proven to be inadequate.6,20,21
Medical marijuana for your patient? A look at the evidence
The meta-analysis cited earlier included 79 randomized clinical trials (RCTs) of medical marijuana used for a variety of conditions in a number of delivery modes. However, only 4 were judged to be of low risk of bias.5 Nonetheless, here’s a look at this and other evidence.
Chronic and neuropathic pain
Twenty-eight of the 79 studies addressed chronic pain, with half assessing the oromucosal spray (nabiximols). Most others studied marijuana that was smoked or inhaled. Neuropathic pain was most frequently studied, but cancer pain, fibromyalgia, and musculoskeletal pain, among others, were also evaluated.5
The average number of patients who reported a reduction in pain of ≥30% was greater with marijuana compared with placebo (odds ratio=1.41; 95% confidence interval, .99-2.0). Delivery mode did not affect outcomes; different forms of administration were not associated with any significant difference in pain relief. Nor were there significant differences in results among the various pain conditions studied. Notably, however, quality of life measurements did not reflect any overall improvement.5
The authors of a literature review of marijuana for chronic and neuropathic pain and MS-induced spasticity did find high-quality evidence of its efficacy in several of the trials they assessed.6 And a review of well-conducted observational trials of smoked marijuana as a treatment for severe neuropathic pain revealed that it may be indicated for those who fail to respond to FDA-approved cannabinoids and standard analgesics.10 Neither functional status nor quality of life was evaluated, however, and none of the observational studies compared smoked cannabis to standard analgesics.
Notably, the authors did not recommend smoked marijuana for pain conditions such as low back pain and fibromyalgia, which are commonly seen in practice. That’s because the safety and efficacy of smoked cannabis has not been studied for these conditions and because evidence-based treatments for these disorders exist.10
CASE › Before considering medical marijuana for Ms. B, you suggest a trial of dronabinol. The patient agrees, and you prescribe 2.5 mg twice a day. You schedule a visit in 4 weeks to review the drug’s efficacy and tell her to call if she develops psychiatric symptoms, such as hallucinations or paranoia, or impaired cognition. You also advise her that dronabinol may increase the risk of auto accidents and caution her to avoid driving for 6 hours after taking the drug—or longer if she experiences an initial “high.”
MS symptoms
A comprehensive review of medical marijuana studies spanning nearly 7 decades revealed 12 trials focusing on MS—and found its use in treating MS-related spasticity supported by high-quality evidence.6
Two of the largest studies were done in the UK.7,8 One multicenter trial included 630 participants randomized to treatment with an oral cannabinoid extract, THC, or placebo for 6 weeks.7
There was no change in the primary outcome measure, the Ashworth spasticity scale. However, there was a treatment effect on patient-reported spasticity and pain, with improvement in spasticity reported by 61% of those treated with the cannabinoid extract, 60% of those treated with THC, and 46% of those treated with placebo.7
The other UK trial involved 22 centers and 279 patients, randomized to either oral cannabis extract or placebo. The primary outcome measure involved a category rating scale that reported on change in muscle stiffness since baseline and on body pain, spasms, and sleep quality. This study used a 2-week titration phase and a 10-week maintenance phase. The rate of relief from muscle stiffness after 12 weeks was almost twice as high in the cannabis extract group (29%) compared with placebo (16%).8
A systematic review of the efficacy and safety of medical marijuana by the American Academy of Neurology (AAN) concluded that oral cannabis extract, THC, and nabiximols are “probably effective” in reducing patient-centered measures of spasticity and pain associated with MS.9
Little help for other neurologic disorders. Studies of the efficacy and safety of medical marijuana for other neurologic disorders have been less encouraging. The AAN concluded that cannabinoids are probably ineffective for the treatment of tremors, and that oral cannabis extract is probably ineffective for treating levodopa-induced dyskinesias in patients with Parkinson’s disease.
A 2014 systematic review found that oral cannabinoids were of unknown efficacy in treating nonchorea-related symptoms of Huntington’s disease, Tourette syndrome, cervical dystonia, and epilepsy.9 The 2015 systematic review and meta-analysis cited earlier, however, suggests that there is low-quality evidence that cannabinoids improve symptoms associated with sleep disorders and Tourette symptoms.5
Cancer-related symptoms
In 1985, the FDA approved dronabinol for the treatment of chemotherapy-induced nausea and vomiting (CINV) not controlled by other medications. Nabilone followed, receiving FDA approval in 1992.11
Serotonin receptor antagonists (5-HT3 receptor antagonists) were also introduced in the early 1990s. In 2001, a systematic review of 30 RCTs with a total of 1366 patients looked at how cannabinoids—including oral dronabinol, oral nabilone, and intramuscular levonantradol, a synthetic drug that does not have FDA approval—compared with placebo or other antiemetics.12
The researchers found the FDA-approved cannabinoids to be more effective than prochlorperazine, metoclopramide, chlorpromazine, and other antiemetics for most patients. (The included studies did not compare cannabinoids with 5-HT3 agents.) That was not the case, however, for patients receiving either very low or very highly emetogenic chemotherapy.
In crossover studies, participants reported that they preferred cannabinoids for future CINV control. Although they cited the “high,” sedation, and euphoria as potential beneficial effects, those taking cannabinoids were also more likely than patients receiving other antiemetics to withdraw from studies due to adverse effects, including dizziness, dysphoria, depression, hallucinations, and paranoia. The authors concluded that cannabinoids might be useful as mood-enhancing adjuvants for controlling CINV, but that short-term adverse effects were likely to limit their widespread use.12
Recommended antiemetic regimens for patients with highly emetogenic regimens or those whose chemotherapy comes with a high risk of delayed CINV include the serotonin antagonist dexamethasone, with or without aprepitant or fosaprepitant. Because of the availability of safer and more effective agents, the National Comprehensive Cancer Network (NCCN) does not consider cannabinoids first-line treatment for the prevention of CINV. Instead, they are reserved for breakthrough symptoms or refractory nausea and vomiting.11
In fact, NCCN practice guidelines do not recommend medical marijuana for the management of CINV because of both medical and legal concerns. Even in states in which medical marijuana is legal, the organization states, its use is controversial.11
Combatting anorexia and cachexia. An estimated 50% of cancer patients develop anorexia and cachexia. The systemic inflammation and loss of protein, energy, and lean body mass is associated not only with a poor response to chemotherapy and decreased survival rates, but also with a lower quality of life. While therapies to alleviate these symptoms typically focus on palliation and reduction of distress rather than on prolonging life, some agents, such as megestrol and medroxyprogesterone, are reported to improve survival rates as well as quality of life.22
Cannabinoids have also been used to increase appetite and food intake and facilitate weight gain in cancer patients. The exact mechanism by which this effect occurs is not known; in fact, questions about the extent of the effect itself remain.
Two RCTs failed to show benefits in this regard compared with megestrol or placebo. One study of 469 patients with advanced cancer compared dronabinol, administered alone or in combination with megestrol, with megestrol alone. Using a Functional Assessment of Anorexia/Cachexia Therapy Questionnaire to assess quality of life, the researchers found that megestrol provided better palliation of anorexia than dronabinol alone and that the combination of dronabinol and megestrol showed no advantage over megestrol alone.13
The second study was a multicenter Phase III double-blind RCT comparing cannabis extract (CE), THC, and placebo in 289 cancer patients. The researchers found no differences in appetite, quality of life, or toxicity among those in the 3 arms of the study. A data review board subsequently recommended that study recruitment be stopped because of the absence of significant differences.23
HIV and AIDS-related morbidity and mortality
Evidence of the efficacy and safety of cannabinoid use among adult patients with HIV or acquired immune deficiency syndrome (AIDS) is lacking, according to a 2013 Cochrane review.24 The review looked at RCTs that compared any marijuana intervention in this patient population to either placebo or a known treatment, such as megestrol or medroxyprogesterone.Worth noting, however, is that the review included studies that were of short duration, involved small numbers of patients, and focused on short-term measures of efficacy.
Long-term studies indicating that cannabinoids have a sustained effect on AIDS-related morbidity and mortality in patients being treated with antiretroviral therapy have yet to be conducted.24 The systematic review and meta-analysis published in 2015, however, did find evidence suggesting that cannabinoids were associated with weight gain in patients with HIV.5 Dronabinol has had FDA approval for treatment of weight loss associated with AIDS-related anorexia since 1992.
Before you recommend medical marijuana…
Although medical marijuana is not actually “prescribed,” there are steps to take before recommending or facilitating its use for a particular patient (TABLE 2).25-29
After ensuring that he or she has a condition for which there is evidence to support it, you need to do a risk evaluation, drawing on the opioid-prescribing paradigm to look for contraindications to the use of a controlled substance or factors that indicate the need for additional precaution (TABLE 3).10,25,26
Take a thorough medical history and use screening tools
A thorough patient and family medical history, along with principles of Screening, Brief Intervention, and Referral for Treatment (SBIRT), can be used to identify addiction-prone substance use.28 You can also use a validated tool such as the Cannabis Use Disorder Test (CUDIT-R), available at http://sfmi.wufoo.com/forms/qulgngl12rydww/.Body fluid (usually urine) testing is also recommended.30 You may be able to access your state’s Prescription Drug Monitoring Program to check for worrisome prescribing, as well.
Stratify risk
The next step is to determine whether the patient is at low, intermediate, or high risk for use of a controlled substance based on your findings. Patients who are younger than 25 years, for example, have an increased risk.And high-risk patients—those with a history of substance abuse, psychiatric illness, or sexual trauma—are unlikely to be good candidates for medical marijuana10,25,26 and should be informed in a nonjudgmental manner that their problem is better addressed without it.
If the risk/benefit balance is favorable and the patient is willing to give medical marijuana a try, complete a signed certification of a medical condition for which medical marijuana is authorized in your state. Details of state laws are available at medicalmarijuana.procon.org/view.resource.php?resourceID=000881.
Because the individuals who dispense medical marijuana have varying skills, physicians should collaborate with clinicians judged to be knowledgeable about the best strains of marijuana, the best administration route, and the lowest effective dose—typically a pain management specialist or a physician experienced in recommending medical marijuana appropriately. Vaporization of marijuana, for use with an inhalation device, may prevent some of the potentially negative consequences of smoking it.31 Vaporizing is thought to eliminate some of the irritating—and possibly carcinogenic—materials contained in marijuana smoke.
Follow risk mitigation principles
Because marijuana is a controlled substance, you will need to talk to the patient about how to store and, if necessary, dispose of it to avoid the risk of diversion—a major concern about the legalization of marijuana.
You can cite a small study of adolescents in substance abuse treatment, in which 3 out of 4 reported having used someone else’s medical marijuana a median of 50 times.32 Adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana abuse, and more dependence and conduct disorder symptoms compared with teens who had not used medical marijuana.32
It is important, too, to obtain informed consent and draw up a controlled substance agreement, signed by the patient and you. The agreement should outline expected patient behavior, including regular monitoring and body fluid testing, and the consequences of a lack of adherence. (Using a certified laboratory for drug testing is important, as it avoids the possibility of actions based on inaccurate in-office screening.33) Regular follow-up also provides an opportunity to assess symptom and functional improvement.
If the patient fails to keep appointments and does not respond to efforts to address the problem, the marijuana recommendation may have to be rescinded. Adverse effects, continued aberrant behavior, or evidence of cannabis use disorder may necessitate immediate cessation of the drug. Depending on the scope of the problem, collaboration with addiction therapy may be necessary. Discharge from the practice, of course, should be the last resort.
CASE › At a subsequent visit—after a trial with the maximal dose of dronabinol—Ms. B states that although she had some relief, she continues to have a high degree of breakthrough pain. You suspect that medical marijuana may do more to alleviate her pain, and establish a regimen to quickly taper her off dronabinol.
You consult with a pain management specialist, who suggests that the patient begin with raw marijuana with a 10% THC content, smoking 0.6 gm tid. You obtain informed consent and ask her to sign a controlled substance agreement, explaining that you will need to monitor her closely for dizziness, dysphoria, and hallucinations, among other adverse effects. You instruct her not to drive for 6 hours after smoking marijuana, and you schedule a follow-up appointment in 2 weeks.
Before she leaves, Ms. B receives a copy of your clinic note and written recommendation that she can take to the state dispensary. The note indicates that she will use marijuana for neuropathic pain.
CORRESPONDENCE
Julius Metts, MD, California Substance Abuse Treatment Facility and State Prison, CDCR, 900 Quebec Avenue, Corcoran, CA 93212; [email protected].
› Consider recommending medical marijuana for conditions with evidence supporting its use only after other treatment options have been exhausted. B
› Thoroughly screen potential candidates for medical marijuana to rule out a history of substance abuse, mental illness, and other contraindications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Gladys B, a 68-year-old patient with a history of peripheral neuropathy related to chemotherapy she underwent years ago, has been treated alternately with acetaminophen with codeine, tramadol, gabapentin, and morphine. Each provided only minimal relief. Your state recently legalized medical marijuana, and she wants to know whether it might alleviate her pain.
If Ms. B were your patient, how would you respond?
Medical marijuana is now legal in 23 states and Washington, DC. Other states are considering legalization or have authorized particular components for use as medical treatment.1 As such laws proliferate and garner more media attention, it is increasingly likely that patients will turn to their primary care physicians with questions about the use of marijuana for medicinal purposes. What can you tell them?
Conversations about medical marijuana should be based on the understanding that while many claims have been made about the therapeutic effects of marijuana, only a few of these claims have evidence to back them up. Major medical organizations, including the American Academy of Family Physicians,2 the American College of Physicians,3 and the Institute of Medicine,4 recognize its potential as a treatment for various conditions, but emphasize the need for additional research rather than wholesale adoption.
Most commonly, medical marijuana is used to treat pain symptoms, but it is also used for a host of other conditions. A 2015 systematic review and meta-analysis5 found moderate-quality evidence to support its use for the treatment of chronic and neuropathic pain and spasticity associated with multiple sclerosis (MS), and low-quality evidence for the treatment of nausea and vomiting associated with chemotherapy, for weight gain in patients with human immunodeficiency virus (HIV), and to treat Tourette syndrome. (TABLE 1 lists the conditions for which medical marijuana has been found to be indicated.5-13) For most other conditions that qualify for the use of medical marijuana under state laws, however—insomnia, hepatitis C, Crohn’s disease, and anxiety and depression, among others—the evidence is either of very low quality or nonexistent.5
Evaluating marijuana is difficult
It is important to note that marijuana comprises more than 60 pharmacologically active cannabinoids, which makes it difficult to study. Both exogenous ligands, such as the cannabinoids from marijuana, and endogenous ligands (endocannabinoids), such as anandamide and 2-arachidonoylglycerol, act on cannabinoid receptors. These receptors are found throughout the body, but are primarily in the brain and spinal cord.14
The main cannabinoids contained in marijuana are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC produces the euphoria for which recreational marijuana is known, but can also induce psychosis. CBD is not psychoactive and is thought to have antianxiety and possibly antipsychotic properties. Thus, marijuana’s therapeutic effects depend on the concentration of THC in a given formulation. Because CBD has the ability to mitigate psychoactive effects, the ratio of THC to CBD is important, as well.15
What’s more, medical marijuana is available in various forms. It can be smoked—the most widely used route—or inhaled with an inhalation device, ingested in food or as a tea, taken orally, administered via an oromucosal spray, or even applied topically. Medical marijuana may be extracted naturally from the cannabis plant, produced by the isomerization of CBD, manufactured synthetically, or provided as an herbal formulation.
There are also cannabinoids that have been approved by the US Food and Drug Administration (FDA)—dronabinol (a synthetic version of THC) and nabilone (a synthetic cannabinoid). Nabiximols, a cannabis extract in the form of an oromucosal spray, is licensed in the UK for the treatment of symptoms associated with multiple sclerosis, but has not yet received FDA approval.16,17
As with any treatment or medication, the benefits must be weighed against the risks. Scientific studies have documented many adverse health effects associated with marijuana, including the risk of addiction and the potential for marijuana to be used as a gateway drug; its effect on brain development, school performance, and lifetime achievement; a potential relationship to mental illness; and the risk of cancer and motor vehicle accidents.1,16,18 Patients in clinical trials have reported dizziness, dysphoria, hallucinations, and paranoia, as well.12
What’s more, marijuana remains classified as a Schedule I agent.19 Because of its high potential for abuse, physicians in states where medical marijuana has been legalized should adhere to off-label prescribing principles: Recommend it only after standard medications, including FDA-approved cannabinoids, and nonpharmaceutical approaches have proven to be inadequate.6,20,21
Medical marijuana for your patient? A look at the evidence
The meta-analysis cited earlier included 79 randomized clinical trials (RCTs) of medical marijuana used for a variety of conditions in a number of delivery modes. However, only 4 were judged to be of low risk of bias.5 Nonetheless, here’s a look at this and other evidence.
Chronic and neuropathic pain
Twenty-eight of the 79 studies addressed chronic pain, with half assessing the oromucosal spray (nabiximols). Most others studied marijuana that was smoked or inhaled. Neuropathic pain was most frequently studied, but cancer pain, fibromyalgia, and musculoskeletal pain, among others, were also evaluated.5
The average number of patients who reported a reduction in pain of ≥30% was greater with marijuana compared with placebo (odds ratio=1.41; 95% confidence interval, .99-2.0). Delivery mode did not affect outcomes; different forms of administration were not associated with any significant difference in pain relief. Nor were there significant differences in results among the various pain conditions studied. Notably, however, quality of life measurements did not reflect any overall improvement.5
The authors of a literature review of marijuana for chronic and neuropathic pain and MS-induced spasticity did find high-quality evidence of its efficacy in several of the trials they assessed.6 And a review of well-conducted observational trials of smoked marijuana as a treatment for severe neuropathic pain revealed that it may be indicated for those who fail to respond to FDA-approved cannabinoids and standard analgesics.10 Neither functional status nor quality of life was evaluated, however, and none of the observational studies compared smoked cannabis to standard analgesics.
Notably, the authors did not recommend smoked marijuana for pain conditions such as low back pain and fibromyalgia, which are commonly seen in practice. That’s because the safety and efficacy of smoked cannabis has not been studied for these conditions and because evidence-based treatments for these disorders exist.10
CASE › Before considering medical marijuana for Ms. B, you suggest a trial of dronabinol. The patient agrees, and you prescribe 2.5 mg twice a day. You schedule a visit in 4 weeks to review the drug’s efficacy and tell her to call if she develops psychiatric symptoms, such as hallucinations or paranoia, or impaired cognition. You also advise her that dronabinol may increase the risk of auto accidents and caution her to avoid driving for 6 hours after taking the drug—or longer if she experiences an initial “high.”
MS symptoms
A comprehensive review of medical marijuana studies spanning nearly 7 decades revealed 12 trials focusing on MS—and found its use in treating MS-related spasticity supported by high-quality evidence.6
Two of the largest studies were done in the UK.7,8 One multicenter trial included 630 participants randomized to treatment with an oral cannabinoid extract, THC, or placebo for 6 weeks.7
There was no change in the primary outcome measure, the Ashworth spasticity scale. However, there was a treatment effect on patient-reported spasticity and pain, with improvement in spasticity reported by 61% of those treated with the cannabinoid extract, 60% of those treated with THC, and 46% of those treated with placebo.7
The other UK trial involved 22 centers and 279 patients, randomized to either oral cannabis extract or placebo. The primary outcome measure involved a category rating scale that reported on change in muscle stiffness since baseline and on body pain, spasms, and sleep quality. This study used a 2-week titration phase and a 10-week maintenance phase. The rate of relief from muscle stiffness after 12 weeks was almost twice as high in the cannabis extract group (29%) compared with placebo (16%).8
A systematic review of the efficacy and safety of medical marijuana by the American Academy of Neurology (AAN) concluded that oral cannabis extract, THC, and nabiximols are “probably effective” in reducing patient-centered measures of spasticity and pain associated with MS.9
Little help for other neurologic disorders. Studies of the efficacy and safety of medical marijuana for other neurologic disorders have been less encouraging. The AAN concluded that cannabinoids are probably ineffective for the treatment of tremors, and that oral cannabis extract is probably ineffective for treating levodopa-induced dyskinesias in patients with Parkinson’s disease.
A 2014 systematic review found that oral cannabinoids were of unknown efficacy in treating nonchorea-related symptoms of Huntington’s disease, Tourette syndrome, cervical dystonia, and epilepsy.9 The 2015 systematic review and meta-analysis cited earlier, however, suggests that there is low-quality evidence that cannabinoids improve symptoms associated with sleep disorders and Tourette symptoms.5
Cancer-related symptoms
In 1985, the FDA approved dronabinol for the treatment of chemotherapy-induced nausea and vomiting (CINV) not controlled by other medications. Nabilone followed, receiving FDA approval in 1992.11
Serotonin receptor antagonists (5-HT3 receptor antagonists) were also introduced in the early 1990s. In 2001, a systematic review of 30 RCTs with a total of 1366 patients looked at how cannabinoids—including oral dronabinol, oral nabilone, and intramuscular levonantradol, a synthetic drug that does not have FDA approval—compared with placebo or other antiemetics.12
The researchers found the FDA-approved cannabinoids to be more effective than prochlorperazine, metoclopramide, chlorpromazine, and other antiemetics for most patients. (The included studies did not compare cannabinoids with 5-HT3 agents.) That was not the case, however, for patients receiving either very low or very highly emetogenic chemotherapy.
In crossover studies, participants reported that they preferred cannabinoids for future CINV control. Although they cited the “high,” sedation, and euphoria as potential beneficial effects, those taking cannabinoids were also more likely than patients receiving other antiemetics to withdraw from studies due to adverse effects, including dizziness, dysphoria, depression, hallucinations, and paranoia. The authors concluded that cannabinoids might be useful as mood-enhancing adjuvants for controlling CINV, but that short-term adverse effects were likely to limit their widespread use.12
Recommended antiemetic regimens for patients with highly emetogenic regimens or those whose chemotherapy comes with a high risk of delayed CINV include the serotonin antagonist dexamethasone, with or without aprepitant or fosaprepitant. Because of the availability of safer and more effective agents, the National Comprehensive Cancer Network (NCCN) does not consider cannabinoids first-line treatment for the prevention of CINV. Instead, they are reserved for breakthrough symptoms or refractory nausea and vomiting.11
In fact, NCCN practice guidelines do not recommend medical marijuana for the management of CINV because of both medical and legal concerns. Even in states in which medical marijuana is legal, the organization states, its use is controversial.11
Combatting anorexia and cachexia. An estimated 50% of cancer patients develop anorexia and cachexia. The systemic inflammation and loss of protein, energy, and lean body mass is associated not only with a poor response to chemotherapy and decreased survival rates, but also with a lower quality of life. While therapies to alleviate these symptoms typically focus on palliation and reduction of distress rather than on prolonging life, some agents, such as megestrol and medroxyprogesterone, are reported to improve survival rates as well as quality of life.22
Cannabinoids have also been used to increase appetite and food intake and facilitate weight gain in cancer patients. The exact mechanism by which this effect occurs is not known; in fact, questions about the extent of the effect itself remain.
Two RCTs failed to show benefits in this regard compared with megestrol or placebo. One study of 469 patients with advanced cancer compared dronabinol, administered alone or in combination with megestrol, with megestrol alone. Using a Functional Assessment of Anorexia/Cachexia Therapy Questionnaire to assess quality of life, the researchers found that megestrol provided better palliation of anorexia than dronabinol alone and that the combination of dronabinol and megestrol showed no advantage over megestrol alone.13
The second study was a multicenter Phase III double-blind RCT comparing cannabis extract (CE), THC, and placebo in 289 cancer patients. The researchers found no differences in appetite, quality of life, or toxicity among those in the 3 arms of the study. A data review board subsequently recommended that study recruitment be stopped because of the absence of significant differences.23
HIV and AIDS-related morbidity and mortality
Evidence of the efficacy and safety of cannabinoid use among adult patients with HIV or acquired immune deficiency syndrome (AIDS) is lacking, according to a 2013 Cochrane review.24 The review looked at RCTs that compared any marijuana intervention in this patient population to either placebo or a known treatment, such as megestrol or medroxyprogesterone.Worth noting, however, is that the review included studies that were of short duration, involved small numbers of patients, and focused on short-term measures of efficacy.
Long-term studies indicating that cannabinoids have a sustained effect on AIDS-related morbidity and mortality in patients being treated with antiretroviral therapy have yet to be conducted.24 The systematic review and meta-analysis published in 2015, however, did find evidence suggesting that cannabinoids were associated with weight gain in patients with HIV.5 Dronabinol has had FDA approval for treatment of weight loss associated with AIDS-related anorexia since 1992.
Before you recommend medical marijuana…
Although medical marijuana is not actually “prescribed,” there are steps to take before recommending or facilitating its use for a particular patient (TABLE 2).25-29
After ensuring that he or she has a condition for which there is evidence to support it, you need to do a risk evaluation, drawing on the opioid-prescribing paradigm to look for contraindications to the use of a controlled substance or factors that indicate the need for additional precaution (TABLE 3).10,25,26
Take a thorough medical history and use screening tools
A thorough patient and family medical history, along with principles of Screening, Brief Intervention, and Referral for Treatment (SBIRT), can be used to identify addiction-prone substance use.28 You can also use a validated tool such as the Cannabis Use Disorder Test (CUDIT-R), available at http://sfmi.wufoo.com/forms/qulgngl12rydww/.Body fluid (usually urine) testing is also recommended.30 You may be able to access your state’s Prescription Drug Monitoring Program to check for worrisome prescribing, as well.
Stratify risk
The next step is to determine whether the patient is at low, intermediate, or high risk for use of a controlled substance based on your findings. Patients who are younger than 25 years, for example, have an increased risk.And high-risk patients—those with a history of substance abuse, psychiatric illness, or sexual trauma—are unlikely to be good candidates for medical marijuana10,25,26 and should be informed in a nonjudgmental manner that their problem is better addressed without it.
If the risk/benefit balance is favorable and the patient is willing to give medical marijuana a try, complete a signed certification of a medical condition for which medical marijuana is authorized in your state. Details of state laws are available at medicalmarijuana.procon.org/view.resource.php?resourceID=000881.
Because the individuals who dispense medical marijuana have varying skills, physicians should collaborate with clinicians judged to be knowledgeable about the best strains of marijuana, the best administration route, and the lowest effective dose—typically a pain management specialist or a physician experienced in recommending medical marijuana appropriately. Vaporization of marijuana, for use with an inhalation device, may prevent some of the potentially negative consequences of smoking it.31 Vaporizing is thought to eliminate some of the irritating—and possibly carcinogenic—materials contained in marijuana smoke.
Follow risk mitigation principles
Because marijuana is a controlled substance, you will need to talk to the patient about how to store and, if necessary, dispose of it to avoid the risk of diversion—a major concern about the legalization of marijuana.
You can cite a small study of adolescents in substance abuse treatment, in which 3 out of 4 reported having used someone else’s medical marijuana a median of 50 times.32 Adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana abuse, and more dependence and conduct disorder symptoms compared with teens who had not used medical marijuana.32
It is important, too, to obtain informed consent and draw up a controlled substance agreement, signed by the patient and you. The agreement should outline expected patient behavior, including regular monitoring and body fluid testing, and the consequences of a lack of adherence. (Using a certified laboratory for drug testing is important, as it avoids the possibility of actions based on inaccurate in-office screening.33) Regular follow-up also provides an opportunity to assess symptom and functional improvement.
If the patient fails to keep appointments and does not respond to efforts to address the problem, the marijuana recommendation may have to be rescinded. Adverse effects, continued aberrant behavior, or evidence of cannabis use disorder may necessitate immediate cessation of the drug. Depending on the scope of the problem, collaboration with addiction therapy may be necessary. Discharge from the practice, of course, should be the last resort.
CASE › At a subsequent visit—after a trial with the maximal dose of dronabinol—Ms. B states that although she had some relief, she continues to have a high degree of breakthrough pain. You suspect that medical marijuana may do more to alleviate her pain, and establish a regimen to quickly taper her off dronabinol.
You consult with a pain management specialist, who suggests that the patient begin with raw marijuana with a 10% THC content, smoking 0.6 gm tid. You obtain informed consent and ask her to sign a controlled substance agreement, explaining that you will need to monitor her closely for dizziness, dysphoria, and hallucinations, among other adverse effects. You instruct her not to drive for 6 hours after smoking marijuana, and you schedule a follow-up appointment in 2 weeks.
Before she leaves, Ms. B receives a copy of your clinic note and written recommendation that she can take to the state dispensary. The note indicates that she will use marijuana for neuropathic pain.
CORRESPONDENCE
Julius Metts, MD, California Substance Abuse Treatment Facility and State Prison, CDCR, 900 Quebec Avenue, Corcoran, CA 93212; [email protected].
1. Office of National Drug Control Policy. Marijuana Resource Center. State Laws Related to Marijuana. Available at: https://www.whitehouse.gov/ondcp/state-laws-related-to-marijuana. Accessed December 12, 2015.
2. American Academy of Family Physicians. AAFP policies: marijuana. Available at: http://www.aafp.org/about/policies/all/marijuana.html. Accessed January 16, 2016.
3. American College of Physicians. Supporting research into the therapeutic role of marijuana. Available at: https://www.acponline.org/acp_policy/policies/supporting_medmarijuana_2008.pdf. Accessed January 26, 2016.
4. Institute of Medicine. Marijuana and medicine: assessing the science base. Available at: http://iom.nationalacademies.org/reports/1999/marijuana-and-medicine-assessing-the-science-base.aspx. Accessed January 26, 2016.
5. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
6. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313:2474-2483.
7. Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo controlled trial. Lancet. 2003;362:1517-1526.
8. Zajicek JP, Hobart JC, Slade A, et al. MUSEC research group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83:1125–1132.
9. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556-1563.
10. Kahan M, Srivastava A, Spithoff S, et al. Smoked CB for chronic noncancer pain. Can Fam Physician. 2014;60:1083–1090.
11. Todaro B. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting. J Natl Compr Canc Network. 2012;10:487-492.
12. Tramer MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systemic review. BMJ. 2001;323:16-21.
13. Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567-573.
14. Hu SS, Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handbook Exp Pharmacol. 2015;231:59-93.
15. Bhattacharyya S, Morrison PD, Fusar-Poli P. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764-774.
16. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids – an international cross sectional survey on administration forms. J Psychoactive Drugs. 2013;45:199-210.
17. ProCon.org site. 10 pharmaceutical drugs based on cannabis. Available at: http://medicalmarijuana.procon.org/view.resource.php?resourceID=000883. Accessed January 28, 2016.
18. Cerda M, Wall M, Keyes KM, et al. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana, abuse and dependence. Drug Alcohol Depend. 2012;120:22-27.
19. US Food and Drug Administration. Inter-agency advisory regarding claims that smoked marijuana is a medicine. April 20, 2006. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108643. Accessed January 29, 2016.
20. Marinol dronabinol capsules. Available at: www.marinol.com. Accessed January 29, 2016.
21. Cesamet full prescribing information. Available at: http://www.cesamet.com/patient-home.asp. Accessed January 29, 2016.
22. Aoyagi T, Terracini KP, Raza A, et al. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7:17-29.
23. Strasser F, Laftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9 tetrahydrocannabinol (THC) in treating patients with cancer-related anorexia cachexia syndrome, a multicenter, randomized, double blind controlled clinical trial from the Cannabis-In Cachexia Study Group. Clin Oncol. 2006;24:3394 -3400.
24. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
25. Phillips JA, Holland MG, Baldwin DD. Marijuana in the workplace: guidance for occupational health professionals and employers: Joint Guidance Statement of the American Association of Occupational Health Nurses and the American College of Occupational and Environmental Medicine. J Occup Environ Med. 2015;57:459-475.
26. Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Phys. 2012;15:ES67-ES92.
27. Lopez-Quintero C, de los Cabos JP, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
28. Strobbe S. Prevention and screening, brief intervention and referral to treatment for substance use in primary care. Primary Care. 2014;41:185-213.
29. Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35:102-110.
30. American Society of Addiction Medicine. Drug testing: a white paper of the American Society of Addiction Medicine. Available at: http://www.asam.org/docs/default-source/publicy-policy-statements/drug-testing-a-white-paper-by-asam.pdf?sfvrsn=2. October 26, 2013. Accessed January 26, 2016.
31. Tomar RS, Beaumont J, Hsieh JCY. Evidence on the carcinogenicity of marijuana smoke. California EPA: Reproductive and Cancer Hazard Assessment Branch of the Office of Environmental Health Hazard Assessment. August 2009. Available at: http://oehha.ca.gov/prop65/hazard_ident/pdf_zip/FinalMJsmokeHID.pdf. Accessed January 29, 2016.
32. Salomonsen–Sautel S, Sakai JT, Thurstone C. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;7:694-702.
33. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1:937-952.
1. Office of National Drug Control Policy. Marijuana Resource Center. State Laws Related to Marijuana. Available at: https://www.whitehouse.gov/ondcp/state-laws-related-to-marijuana. Accessed December 12, 2015.
2. American Academy of Family Physicians. AAFP policies: marijuana. Available at: http://www.aafp.org/about/policies/all/marijuana.html. Accessed January 16, 2016.
3. American College of Physicians. Supporting research into the therapeutic role of marijuana. Available at: https://www.acponline.org/acp_policy/policies/supporting_medmarijuana_2008.pdf. Accessed January 26, 2016.
4. Institute of Medicine. Marijuana and medicine: assessing the science base. Available at: http://iom.nationalacademies.org/reports/1999/marijuana-and-medicine-assessing-the-science-base.aspx. Accessed January 26, 2016.
5. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
6. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313:2474-2483.
7. Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo controlled trial. Lancet. 2003;362:1517-1526.
8. Zajicek JP, Hobart JC, Slade A, et al. MUSEC research group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83:1125–1132.
9. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556-1563.
10. Kahan M, Srivastava A, Spithoff S, et al. Smoked CB for chronic noncancer pain. Can Fam Physician. 2014;60:1083–1090.
11. Todaro B. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting. J Natl Compr Canc Network. 2012;10:487-492.
12. Tramer MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systemic review. BMJ. 2001;323:16-21.
13. Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567-573.
14. Hu SS, Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handbook Exp Pharmacol. 2015;231:59-93.
15. Bhattacharyya S, Morrison PD, Fusar-Poli P. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764-774.
16. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids – an international cross sectional survey on administration forms. J Psychoactive Drugs. 2013;45:199-210.
17. ProCon.org site. 10 pharmaceutical drugs based on cannabis. Available at: http://medicalmarijuana.procon.org/view.resource.php?resourceID=000883. Accessed January 28, 2016.
18. Cerda M, Wall M, Keyes KM, et al. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana, abuse and dependence. Drug Alcohol Depend. 2012;120:22-27.
19. US Food and Drug Administration. Inter-agency advisory regarding claims that smoked marijuana is a medicine. April 20, 2006. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108643. Accessed January 29, 2016.
20. Marinol dronabinol capsules. Available at: www.marinol.com. Accessed January 29, 2016.
21. Cesamet full prescribing information. Available at: http://www.cesamet.com/patient-home.asp. Accessed January 29, 2016.
22. Aoyagi T, Terracini KP, Raza A, et al. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7:17-29.
23. Strasser F, Laftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9 tetrahydrocannabinol (THC) in treating patients with cancer-related anorexia cachexia syndrome, a multicenter, randomized, double blind controlled clinical trial from the Cannabis-In Cachexia Study Group. Clin Oncol. 2006;24:3394 -3400.
24. Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;(4):CD005175.
25. Phillips JA, Holland MG, Baldwin DD. Marijuana in the workplace: guidance for occupational health professionals and employers: Joint Guidance Statement of the American Association of Occupational Health Nurses and the American College of Occupational and Environmental Medicine. J Occup Environ Med. 2015;57:459-475.
26. Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Phys. 2012;15:ES67-ES92.
27. Lopez-Quintero C, de los Cabos JP, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011:115:120-130.
28. Strobbe S. Prevention and screening, brief intervention and referral to treatment for substance use in primary care. Primary Care. 2014;41:185-213.
29. Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35:102-110.
30. American Society of Addiction Medicine. Drug testing: a white paper of the American Society of Addiction Medicine. Available at: http://www.asam.org/docs/default-source/publicy-policy-statements/drug-testing-a-white-paper-by-asam.pdf?sfvrsn=2. October 26, 2013. Accessed January 26, 2016.
31. Tomar RS, Beaumont J, Hsieh JCY. Evidence on the carcinogenicity of marijuana smoke. California EPA: Reproductive and Cancer Hazard Assessment Branch of the Office of Environmental Health Hazard Assessment. August 2009. Available at: http://oehha.ca.gov/prop65/hazard_ident/pdf_zip/FinalMJsmokeHID.pdf. Accessed January 29, 2016.
32. Salomonsen–Sautel S, Sakai JT, Thurstone C. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;7:694-702.
33. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1:937-952.





