User login
Nine Things Chronic-Pain Specialists Think Hospitalists Need To Know
In This Edition
9 Things: At a Glance
An occasional series providing specialty-specific advice for hospitalists from experts in the field.
- Recognize the differential diagnosis for pain exacerbation in a chronic opioid therapy (COT) patient/chronic pain patient.
- Know where the opioids are going.
- Sometimes stopping pills, rather than adding them, can cure pain.
- Take time to educate patients about methadone and its risk of mortality if not used as prescribed.
- A little local anesthetic (and some steroid) goes a long way.
- Addiction to opioids is not rare.
- Safely changing opioid regimens requires good math and good judgment.
- For a low-risk chronic pain patient on low-dose opioids, don’t change the regimen, even if the indication for opioids isn’t clear.
- If a patient has pain all the time, they need to be on a medication that works all the time.
The differential diagnosis for pain exacerbation in a chronic opioid therapy (COT) patient/chronic pain patient is:
- Worsening medical problem;
- New medical problem;
- Nonopioid problem (side effect);
- Opioid problem (resistance/tolerance/side effect); and
- Opioid-induced hyperalgesia.
The search for an etiology and treatment for chronic pain should not end, even if a patient is labeled with “chronic pain syndrome.” The patient could simply be chronically undiagnosed or on an incorrect therapy.
Know where the opioids are going.
Whether it’s auditing a prescription-monitoring program (PMP), checking a urine drug screen, or calling a pharmacist, try to ensure that chronic pain patients are taking the opioids as prescribed. A phone call to the primary opioid prescriber or chronic pain provider could save a busy hospitalist a lot of time.
Using PMP data can consume a lot of time. Typically, only prescribing providers can access PMPs, so delegating this responsibility to someone else is not possible. If your state PMP does not help, simply call the patient’s pharmacy and ask for the last three fill dates on an opioid prescription. This also works well in case the patient’s pharmacy doesn’t participate in a PMP or is delayed in uploading recent prescriber data. Many COT patients have an opioid treatment agreement with their prescriber and must use only one pharmacy to fill opioids.
In January 2013, the University of North Carolina Injury Prevention Center published an analysis of three years of North Carolina PMP data.1 Patients followed by providers who consistently used the state PMP were five times more likely to receive treatment for opioid dependence compared with patients of providers who never used the state PMP.1
Why go through all this trouble if a chronic pain specialist is also doing it? It’s good documentation and good care, like monitoring levels of transplant meds or making sure hemoglobin A1Cs are up to date and trending toward goal. It may only take one misused or diverted opioid pill to result in a serious adverse event.
Sometimes stopping pills, rather than adding them, can cure pain.
Many chronic pain patients accumulate a patchwork of pills (e.g. benzodiazepines, opioids, muscle relaxants, and antidepressants). Many interpret noxious symptoms associated with the drug burden as “uncontrolled pain.” Two conditions that might afflict the pain sufferer who takes multiple medications are opioid-induced hyperalgesia (OIH) and medication-overuse headaches (MOH). They are uncommon but should be on a hospitalist’s differential for difficult-to-control chronic pain. Opioids commonly are implicated in causing MOH, a chronic headache occurring at least 15 days a month, four hours a day if untreated, and for at least three consecutive months. OIH is a nociceptive sensitization caused by opioids that can occur suddenly or insidiously.
If a drug isn’t absolutely necessary, stop it. If you and the patient start by agreeing to the shared goal of improving health, the conversation should go better. An axiom we learned from mentors at the University of Washington is: “There is no pain that cannot be made worse with inappropriate therapy.”
Take time to educate patients about methadone and its risk of mortality if not used as prescribed.
Methadone is less frequently prescribed than other opioids, yet it is more frequently associated with death from overdose. Though there is a risk of overdose and death with any opioid, managing methadone is more difficult. A desperate chronic pain patient may self-escalate their methadone without proper insight into the consequences.
Remember the logarithmic relationship methadone doses have with their morphine equivalency. The following highlights how deceiving the numbers are: 50 mg of methadone is about 100 mg of morphine-equivalent, but 100 mg of methadone is about 1,000 mg of morphine-equivalent, or 10 times as strong.
From 1999 to 2005, methadone-related deaths increased by 468%.2 If the patient doesn’t seem to understand these risks, they are not a good candidate for methadone treatment.
A little local anesthetic (and some steroid) goes a long way.
Hospitalists should offer an assortment of diagnostic and therapeutic injections to chronic pain patients. First, be sure you’ve done your due diligence:
- What procedures do you have privileges to do?
- Do you need to be proctored first?
- How do your local specialists feel about you doing injections?
In light of these considerations, hospitalists should be able to train and be credentialed to offer such procedures as trigger-point injections, joint injections (knees, shoulders), or even a peripheral nerve injection (e.g. lateral femoral cutaneous nerve or ilioinguinal nerve injection). Some hospitalists might even want to learn ultrasound-guided sacroiliac joint injections for chronic unexplained back pain.
Offering an indicated and effective injection is a good nonopioid option. And local anesthetic injections can help hospitalists establish an elusive diagnosis. For example, many patients spend years getting worked up for head and neck pain when dry-needling with a small volume (1 cc) of local anesthetic into a neck muscle trigger point can break their pain generator, eliminating their pain.
Addiction to opioids is not rare.
The use, misuse, and diversion of opioids and all the associated complications have appropriately received considerable media attention. A seminal paper by Porter and Jick titled “Addiction Is Rare in Patients Treated with Narcotics” is one of many tipping points associated with the boom in opioid prescribing.3 Whether it’s a three-day supply of hydrocodone, 24 hours on a PCA, or an opioid rotation, any exposure to opioids can put a patient on the runway to addiction.
There are only 3,071 board-certified addiction specialists certified by the American Board of Addiction Medicine, so access to an addiction specialist might be difficult.4
Nonetheless, do not become complacent and just continue the opioid therapy in a difficult opioid-addicted patient. Express your concerns to the primary opioid prescriber, or help patients who don’t have an opioid prescriber get access and treatment. Otherwise, you have no choice but to taper the opioids.
Ideally, chronic pain management should be delivered in the outpatient arena where long-term monitoring can take place.
Safely changing opioid regimens requires good math and good judgment.
During training and practice, hospitalists become accustomed to rapidly analyze objective data, such as ABGs, ECGs, anion gaps, and vent settings. A hospitalist should become similarly efficient at calculating morphine equivalencies (cautiously with methadone and fentanyl), making dose reductions, and rotating opioids. The more comfortable you are with morphine equivalencies, the faster and safer you will be at rotating opioids. Whatever morphine-equivalence table you feel comfortable with is the one you should use consistently.
We see many providers who unwittingly take, for example, a patient who has become resistant to hydrocodone/acetaminophen 10/325 mg PO TID (30 mg of morphine) and convert them to oxycodone/acetaminophen 10/325 mg PO QID (60 mg of morphine—a doubling), when doubling could cause respiratory depression or a faster path to addiction and dependency.
But there are cases in which judgment should trump math, such as when converting from an IV to an oral regimen. We frequently see patients in the clinic requesting refills for more than 100 mg of hydromorphone because “that’s what I was on when I was hospitalized and on the pump.” If the IV-to-oral conversion leaves you prescribing high doses of oral opioids, plan for a rapid taper and a smooth handoff to the outpatient setting.
One strategy to decrease an error in math and judgment is to use IV PCAs as infrequently as possible if a patient isn’t post-operative and they are able to take oral meds. And never hesitate to consult with your inpatient pharmacist or a chronic pain specialist.
For a low-risk chronic pain patient on low-dose opioids, don’t change the regimen, even if the indication for opioids isn’t clear.
Although it is tempting to become an opioid prohibitionist, if a patient has been taking an opioid for years and is functioning, working, compliant, and has no risk factors for complications from COT, it is likely fine to continue their current regimen. Touch base with the primary opioid prescriber, and if you’re concerned, use some of the monitoring instruments described earlier (PMP, urine drug screen, opioid treatment agreement, pill counts).
If a patient has pain all the time, they need to be on a medication that works all the time.
A good pain history followed by a good neurological and mental health assessment is of incalculable value, especially because physicians often underestimate a patient’s pain intensity and its impact on a patient’s quality of life.5,6 A patient’s pain intensity, quality of life, and function can be dramatically improved by starting a long-acting medication for “constant pain.”
If a patient hurts “24 hours a day” and cannot function on hydrocodone/acetaminophen 10/325 QID, it’s probably because they are constantly reacting to spikes in pain and using a “some of the time” medicine to treat “all the time” pain. Switching to a long-acting medication—and it doesn’t have to be an opioid—could improve control and decrease how much narcotic the patient needs.
If you choose a long-acting opioid (in this case, you could try morphine sulphate extended-release 15 mg BID and satisfy 50% of the hydrocodone need), then you could titrate slowly upwards to where the patient would not need hydrocodone. If the patient still had uncontrolled pain, then either morphine is the wrong compound for them or they are benefiting from the “nonanalgesic properties” of the opioids.
Give the patient the benefit of the doubt; because of genetic polymorphisms, a patient may need several opioid rotations before the right opioid compound is found.
Dr. Schultz is a hospitalist and assistant professor in the department of internal medicine at the University of Miami Miller School of Medicine. She is board-certified in hospice and palliative care and specializes in chronic pain management. Dr. Ajam is a hospitalist and a clinical assistant professor in the department of anesthesiology at Wake Forest University Baptist Medical Center and the Carolinas Pain Institute. He is board-certified in chronic pain management.
References
- Garrettson M, Ringwalt C. An evaluation of the North Carolina controlled substances reporting system: part II impact evaluation, January 2013. PDMP Center of Excellence website. Available at: http://pdmpexcellence.org/sites/all/pdfs/NC_control_sub_eval_pt_2.pdf. Accessed Sept. 3, 2013.
- Kung HC, Hoyert DL, Xu JQ, Murphy SL. Deaths: Final data for 2005, national vital statistics reports; Vol. 56 No. 10. Hyattsville, Md.: National Center for Health Statistics; 2008.
- Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980;302(2):123.
- American Society of Addiction Medicine; personal communication, 2013.
- Mäntyselkä P, Kumpusalo E, Ahonen R, Takala J. Patients’ versus general practitioners’ assessments of pain intensity in primary care patients with non-cancer pain. Br J Gen Pract. 2001;51(473):995-997.
- Petersen MA, Larsen H, Pedersen L, Sonne N, Groenvold M. Assessing health-related quality of life in palliative care: comparing patient and physician assessments. Eur J Cancer. 2006;42(8):1159-1166.
In This Edition
9 Things: At a Glance
An occasional series providing specialty-specific advice for hospitalists from experts in the field.
- Recognize the differential diagnosis for pain exacerbation in a chronic opioid therapy (COT) patient/chronic pain patient.
- Know where the opioids are going.
- Sometimes stopping pills, rather than adding them, can cure pain.
- Take time to educate patients about methadone and its risk of mortality if not used as prescribed.
- A little local anesthetic (and some steroid) goes a long way.
- Addiction to opioids is not rare.
- Safely changing opioid regimens requires good math and good judgment.
- For a low-risk chronic pain patient on low-dose opioids, don’t change the regimen, even if the indication for opioids isn’t clear.
- If a patient has pain all the time, they need to be on a medication that works all the time.
The differential diagnosis for pain exacerbation in a chronic opioid therapy (COT) patient/chronic pain patient is:
- Worsening medical problem;
- New medical problem;
- Nonopioid problem (side effect);
- Opioid problem (resistance/tolerance/side effect); and
- Opioid-induced hyperalgesia.
The search for an etiology and treatment for chronic pain should not end, even if a patient is labeled with “chronic pain syndrome.” The patient could simply be chronically undiagnosed or on an incorrect therapy.
Know where the opioids are going.
Whether it’s auditing a prescription-monitoring program (PMP), checking a urine drug screen, or calling a pharmacist, try to ensure that chronic pain patients are taking the opioids as prescribed. A phone call to the primary opioid prescriber or chronic pain provider could save a busy hospitalist a lot of time.
Using PMP data can consume a lot of time. Typically, only prescribing providers can access PMPs, so delegating this responsibility to someone else is not possible. If your state PMP does not help, simply call the patient’s pharmacy and ask for the last three fill dates on an opioid prescription. This also works well in case the patient’s pharmacy doesn’t participate in a PMP or is delayed in uploading recent prescriber data. Many COT patients have an opioid treatment agreement with their prescriber and must use only one pharmacy to fill opioids.
In January 2013, the University of North Carolina Injury Prevention Center published an analysis of three years of North Carolina PMP data.1 Patients followed by providers who consistently used the state PMP were five times more likely to receive treatment for opioid dependence compared with patients of providers who never used the state PMP.1
Why go through all this trouble if a chronic pain specialist is also doing it? It’s good documentation and good care, like monitoring levels of transplant meds or making sure hemoglobin A1Cs are up to date and trending toward goal. It may only take one misused or diverted opioid pill to result in a serious adverse event.
Sometimes stopping pills, rather than adding them, can cure pain.
Many chronic pain patients accumulate a patchwork of pills (e.g. benzodiazepines, opioids, muscle relaxants, and antidepressants). Many interpret noxious symptoms associated with the drug burden as “uncontrolled pain.” Two conditions that might afflict the pain sufferer who takes multiple medications are opioid-induced hyperalgesia (OIH) and medication-overuse headaches (MOH). They are uncommon but should be on a hospitalist’s differential for difficult-to-control chronic pain. Opioids commonly are implicated in causing MOH, a chronic headache occurring at least 15 days a month, four hours a day if untreated, and for at least three consecutive months. OIH is a nociceptive sensitization caused by opioids that can occur suddenly or insidiously.
If a drug isn’t absolutely necessary, stop it. If you and the patient start by agreeing to the shared goal of improving health, the conversation should go better. An axiom we learned from mentors at the University of Washington is: “There is no pain that cannot be made worse with inappropriate therapy.”
Take time to educate patients about methadone and its risk of mortality if not used as prescribed.
Methadone is less frequently prescribed than other opioids, yet it is more frequently associated with death from overdose. Though there is a risk of overdose and death with any opioid, managing methadone is more difficult. A desperate chronic pain patient may self-escalate their methadone without proper insight into the consequences.
Remember the logarithmic relationship methadone doses have with their morphine equivalency. The following highlights how deceiving the numbers are: 50 mg of methadone is about 100 mg of morphine-equivalent, but 100 mg of methadone is about 1,000 mg of morphine-equivalent, or 10 times as strong.
From 1999 to 2005, methadone-related deaths increased by 468%.2 If the patient doesn’t seem to understand these risks, they are not a good candidate for methadone treatment.
A little local anesthetic (and some steroid) goes a long way.
Hospitalists should offer an assortment of diagnostic and therapeutic injections to chronic pain patients. First, be sure you’ve done your due diligence:
- What procedures do you have privileges to do?
- Do you need to be proctored first?
- How do your local specialists feel about you doing injections?
In light of these considerations, hospitalists should be able to train and be credentialed to offer such procedures as trigger-point injections, joint injections (knees, shoulders), or even a peripheral nerve injection (e.g. lateral femoral cutaneous nerve or ilioinguinal nerve injection). Some hospitalists might even want to learn ultrasound-guided sacroiliac joint injections for chronic unexplained back pain.
Offering an indicated and effective injection is a good nonopioid option. And local anesthetic injections can help hospitalists establish an elusive diagnosis. For example, many patients spend years getting worked up for head and neck pain when dry-needling with a small volume (1 cc) of local anesthetic into a neck muscle trigger point can break their pain generator, eliminating their pain.
Addiction to opioids is not rare.
The use, misuse, and diversion of opioids and all the associated complications have appropriately received considerable media attention. A seminal paper by Porter and Jick titled “Addiction Is Rare in Patients Treated with Narcotics” is one of many tipping points associated with the boom in opioid prescribing.3 Whether it’s a three-day supply of hydrocodone, 24 hours on a PCA, or an opioid rotation, any exposure to opioids can put a patient on the runway to addiction.
There are only 3,071 board-certified addiction specialists certified by the American Board of Addiction Medicine, so access to an addiction specialist might be difficult.4
Nonetheless, do not become complacent and just continue the opioid therapy in a difficult opioid-addicted patient. Express your concerns to the primary opioid prescriber, or help patients who don’t have an opioid prescriber get access and treatment. Otherwise, you have no choice but to taper the opioids.
Ideally, chronic pain management should be delivered in the outpatient arena where long-term monitoring can take place.
Safely changing opioid regimens requires good math and good judgment.
During training and practice, hospitalists become accustomed to rapidly analyze objective data, such as ABGs, ECGs, anion gaps, and vent settings. A hospitalist should become similarly efficient at calculating morphine equivalencies (cautiously with methadone and fentanyl), making dose reductions, and rotating opioids. The more comfortable you are with morphine equivalencies, the faster and safer you will be at rotating opioids. Whatever morphine-equivalence table you feel comfortable with is the one you should use consistently.
We see many providers who unwittingly take, for example, a patient who has become resistant to hydrocodone/acetaminophen 10/325 mg PO TID (30 mg of morphine) and convert them to oxycodone/acetaminophen 10/325 mg PO QID (60 mg of morphine—a doubling), when doubling could cause respiratory depression or a faster path to addiction and dependency.
But there are cases in which judgment should trump math, such as when converting from an IV to an oral regimen. We frequently see patients in the clinic requesting refills for more than 100 mg of hydromorphone because “that’s what I was on when I was hospitalized and on the pump.” If the IV-to-oral conversion leaves you prescribing high doses of oral opioids, plan for a rapid taper and a smooth handoff to the outpatient setting.
One strategy to decrease an error in math and judgment is to use IV PCAs as infrequently as possible if a patient isn’t post-operative and they are able to take oral meds. And never hesitate to consult with your inpatient pharmacist or a chronic pain specialist.
For a low-risk chronic pain patient on low-dose opioids, don’t change the regimen, even if the indication for opioids isn’t clear.
Although it is tempting to become an opioid prohibitionist, if a patient has been taking an opioid for years and is functioning, working, compliant, and has no risk factors for complications from COT, it is likely fine to continue their current regimen. Touch base with the primary opioid prescriber, and if you’re concerned, use some of the monitoring instruments described earlier (PMP, urine drug screen, opioid treatment agreement, pill counts).
If a patient has pain all the time, they need to be on a medication that works all the time.
A good pain history followed by a good neurological and mental health assessment is of incalculable value, especially because physicians often underestimate a patient’s pain intensity and its impact on a patient’s quality of life.5,6 A patient’s pain intensity, quality of life, and function can be dramatically improved by starting a long-acting medication for “constant pain.”
If a patient hurts “24 hours a day” and cannot function on hydrocodone/acetaminophen 10/325 QID, it’s probably because they are constantly reacting to spikes in pain and using a “some of the time” medicine to treat “all the time” pain. Switching to a long-acting medication—and it doesn’t have to be an opioid—could improve control and decrease how much narcotic the patient needs.
If you choose a long-acting opioid (in this case, you could try morphine sulphate extended-release 15 mg BID and satisfy 50% of the hydrocodone need), then you could titrate slowly upwards to where the patient would not need hydrocodone. If the patient still had uncontrolled pain, then either morphine is the wrong compound for them or they are benefiting from the “nonanalgesic properties” of the opioids.
Give the patient the benefit of the doubt; because of genetic polymorphisms, a patient may need several opioid rotations before the right opioid compound is found.
Dr. Schultz is a hospitalist and assistant professor in the department of internal medicine at the University of Miami Miller School of Medicine. She is board-certified in hospice and palliative care and specializes in chronic pain management. Dr. Ajam is a hospitalist and a clinical assistant professor in the department of anesthesiology at Wake Forest University Baptist Medical Center and the Carolinas Pain Institute. He is board-certified in chronic pain management.
References
- Garrettson M, Ringwalt C. An evaluation of the North Carolina controlled substances reporting system: part II impact evaluation, January 2013. PDMP Center of Excellence website. Available at: http://pdmpexcellence.org/sites/all/pdfs/NC_control_sub_eval_pt_2.pdf. Accessed Sept. 3, 2013.
- Kung HC, Hoyert DL, Xu JQ, Murphy SL. Deaths: Final data for 2005, national vital statistics reports; Vol. 56 No. 10. Hyattsville, Md.: National Center for Health Statistics; 2008.
- Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980;302(2):123.
- American Society of Addiction Medicine; personal communication, 2013.
- Mäntyselkä P, Kumpusalo E, Ahonen R, Takala J. Patients’ versus general practitioners’ assessments of pain intensity in primary care patients with non-cancer pain. Br J Gen Pract. 2001;51(473):995-997.
- Petersen MA, Larsen H, Pedersen L, Sonne N, Groenvold M. Assessing health-related quality of life in palliative care: comparing patient and physician assessments. Eur J Cancer. 2006;42(8):1159-1166.
In This Edition
9 Things: At a Glance
An occasional series providing specialty-specific advice for hospitalists from experts in the field.
- Recognize the differential diagnosis for pain exacerbation in a chronic opioid therapy (COT) patient/chronic pain patient.
- Know where the opioids are going.
- Sometimes stopping pills, rather than adding them, can cure pain.
- Take time to educate patients about methadone and its risk of mortality if not used as prescribed.
- A little local anesthetic (and some steroid) goes a long way.
- Addiction to opioids is not rare.
- Safely changing opioid regimens requires good math and good judgment.
- For a low-risk chronic pain patient on low-dose opioids, don’t change the regimen, even if the indication for opioids isn’t clear.
- If a patient has pain all the time, they need to be on a medication that works all the time.
The differential diagnosis for pain exacerbation in a chronic opioid therapy (COT) patient/chronic pain patient is:
- Worsening medical problem;
- New medical problem;
- Nonopioid problem (side effect);
- Opioid problem (resistance/tolerance/side effect); and
- Opioid-induced hyperalgesia.
The search for an etiology and treatment for chronic pain should not end, even if a patient is labeled with “chronic pain syndrome.” The patient could simply be chronically undiagnosed or on an incorrect therapy.
Know where the opioids are going.
Whether it’s auditing a prescription-monitoring program (PMP), checking a urine drug screen, or calling a pharmacist, try to ensure that chronic pain patients are taking the opioids as prescribed. A phone call to the primary opioid prescriber or chronic pain provider could save a busy hospitalist a lot of time.
Using PMP data can consume a lot of time. Typically, only prescribing providers can access PMPs, so delegating this responsibility to someone else is not possible. If your state PMP does not help, simply call the patient’s pharmacy and ask for the last three fill dates on an opioid prescription. This also works well in case the patient’s pharmacy doesn’t participate in a PMP or is delayed in uploading recent prescriber data. Many COT patients have an opioid treatment agreement with their prescriber and must use only one pharmacy to fill opioids.
In January 2013, the University of North Carolina Injury Prevention Center published an analysis of three years of North Carolina PMP data.1 Patients followed by providers who consistently used the state PMP were five times more likely to receive treatment for opioid dependence compared with patients of providers who never used the state PMP.1
Why go through all this trouble if a chronic pain specialist is also doing it? It’s good documentation and good care, like monitoring levels of transplant meds or making sure hemoglobin A1Cs are up to date and trending toward goal. It may only take one misused or diverted opioid pill to result in a serious adverse event.
Sometimes stopping pills, rather than adding them, can cure pain.
Many chronic pain patients accumulate a patchwork of pills (e.g. benzodiazepines, opioids, muscle relaxants, and antidepressants). Many interpret noxious symptoms associated with the drug burden as “uncontrolled pain.” Two conditions that might afflict the pain sufferer who takes multiple medications are opioid-induced hyperalgesia (OIH) and medication-overuse headaches (MOH). They are uncommon but should be on a hospitalist’s differential for difficult-to-control chronic pain. Opioids commonly are implicated in causing MOH, a chronic headache occurring at least 15 days a month, four hours a day if untreated, and for at least three consecutive months. OIH is a nociceptive sensitization caused by opioids that can occur suddenly or insidiously.
If a drug isn’t absolutely necessary, stop it. If you and the patient start by agreeing to the shared goal of improving health, the conversation should go better. An axiom we learned from mentors at the University of Washington is: “There is no pain that cannot be made worse with inappropriate therapy.”
Take time to educate patients about methadone and its risk of mortality if not used as prescribed.
Methadone is less frequently prescribed than other opioids, yet it is more frequently associated with death from overdose. Though there is a risk of overdose and death with any opioid, managing methadone is more difficult. A desperate chronic pain patient may self-escalate their methadone without proper insight into the consequences.
Remember the logarithmic relationship methadone doses have with their morphine equivalency. The following highlights how deceiving the numbers are: 50 mg of methadone is about 100 mg of morphine-equivalent, but 100 mg of methadone is about 1,000 mg of morphine-equivalent, or 10 times as strong.
From 1999 to 2005, methadone-related deaths increased by 468%.2 If the patient doesn’t seem to understand these risks, they are not a good candidate for methadone treatment.
A little local anesthetic (and some steroid) goes a long way.
Hospitalists should offer an assortment of diagnostic and therapeutic injections to chronic pain patients. First, be sure you’ve done your due diligence:
- What procedures do you have privileges to do?
- Do you need to be proctored first?
- How do your local specialists feel about you doing injections?
In light of these considerations, hospitalists should be able to train and be credentialed to offer such procedures as trigger-point injections, joint injections (knees, shoulders), or even a peripheral nerve injection (e.g. lateral femoral cutaneous nerve or ilioinguinal nerve injection). Some hospitalists might even want to learn ultrasound-guided sacroiliac joint injections for chronic unexplained back pain.
Offering an indicated and effective injection is a good nonopioid option. And local anesthetic injections can help hospitalists establish an elusive diagnosis. For example, many patients spend years getting worked up for head and neck pain when dry-needling with a small volume (1 cc) of local anesthetic into a neck muscle trigger point can break their pain generator, eliminating their pain.
Addiction to opioids is not rare.
The use, misuse, and diversion of opioids and all the associated complications have appropriately received considerable media attention. A seminal paper by Porter and Jick titled “Addiction Is Rare in Patients Treated with Narcotics” is one of many tipping points associated with the boom in opioid prescribing.3 Whether it’s a three-day supply of hydrocodone, 24 hours on a PCA, or an opioid rotation, any exposure to opioids can put a patient on the runway to addiction.
There are only 3,071 board-certified addiction specialists certified by the American Board of Addiction Medicine, so access to an addiction specialist might be difficult.4
Nonetheless, do not become complacent and just continue the opioid therapy in a difficult opioid-addicted patient. Express your concerns to the primary opioid prescriber, or help patients who don’t have an opioid prescriber get access and treatment. Otherwise, you have no choice but to taper the opioids.
Ideally, chronic pain management should be delivered in the outpatient arena where long-term monitoring can take place.
Safely changing opioid regimens requires good math and good judgment.
During training and practice, hospitalists become accustomed to rapidly analyze objective data, such as ABGs, ECGs, anion gaps, and vent settings. A hospitalist should become similarly efficient at calculating morphine equivalencies (cautiously with methadone and fentanyl), making dose reductions, and rotating opioids. The more comfortable you are with morphine equivalencies, the faster and safer you will be at rotating opioids. Whatever morphine-equivalence table you feel comfortable with is the one you should use consistently.
We see many providers who unwittingly take, for example, a patient who has become resistant to hydrocodone/acetaminophen 10/325 mg PO TID (30 mg of morphine) and convert them to oxycodone/acetaminophen 10/325 mg PO QID (60 mg of morphine—a doubling), when doubling could cause respiratory depression or a faster path to addiction and dependency.
But there are cases in which judgment should trump math, such as when converting from an IV to an oral regimen. We frequently see patients in the clinic requesting refills for more than 100 mg of hydromorphone because “that’s what I was on when I was hospitalized and on the pump.” If the IV-to-oral conversion leaves you prescribing high doses of oral opioids, plan for a rapid taper and a smooth handoff to the outpatient setting.
One strategy to decrease an error in math and judgment is to use IV PCAs as infrequently as possible if a patient isn’t post-operative and they are able to take oral meds. And never hesitate to consult with your inpatient pharmacist or a chronic pain specialist.
For a low-risk chronic pain patient on low-dose opioids, don’t change the regimen, even if the indication for opioids isn’t clear.
Although it is tempting to become an opioid prohibitionist, if a patient has been taking an opioid for years and is functioning, working, compliant, and has no risk factors for complications from COT, it is likely fine to continue their current regimen. Touch base with the primary opioid prescriber, and if you’re concerned, use some of the monitoring instruments described earlier (PMP, urine drug screen, opioid treatment agreement, pill counts).
If a patient has pain all the time, they need to be on a medication that works all the time.
A good pain history followed by a good neurological and mental health assessment is of incalculable value, especially because physicians often underestimate a patient’s pain intensity and its impact on a patient’s quality of life.5,6 A patient’s pain intensity, quality of life, and function can be dramatically improved by starting a long-acting medication for “constant pain.”
If a patient hurts “24 hours a day” and cannot function on hydrocodone/acetaminophen 10/325 QID, it’s probably because they are constantly reacting to spikes in pain and using a “some of the time” medicine to treat “all the time” pain. Switching to a long-acting medication—and it doesn’t have to be an opioid—could improve control and decrease how much narcotic the patient needs.
If you choose a long-acting opioid (in this case, you could try morphine sulphate extended-release 15 mg BID and satisfy 50% of the hydrocodone need), then you could titrate slowly upwards to where the patient would not need hydrocodone. If the patient still had uncontrolled pain, then either morphine is the wrong compound for them or they are benefiting from the “nonanalgesic properties” of the opioids.
Give the patient the benefit of the doubt; because of genetic polymorphisms, a patient may need several opioid rotations before the right opioid compound is found.
Dr. Schultz is a hospitalist and assistant professor in the department of internal medicine at the University of Miami Miller School of Medicine. She is board-certified in hospice and palliative care and specializes in chronic pain management. Dr. Ajam is a hospitalist and a clinical assistant professor in the department of anesthesiology at Wake Forest University Baptist Medical Center and the Carolinas Pain Institute. He is board-certified in chronic pain management.
References
- Garrettson M, Ringwalt C. An evaluation of the North Carolina controlled substances reporting system: part II impact evaluation, January 2013. PDMP Center of Excellence website. Available at: http://pdmpexcellence.org/sites/all/pdfs/NC_control_sub_eval_pt_2.pdf. Accessed Sept. 3, 2013.
- Kung HC, Hoyert DL, Xu JQ, Murphy SL. Deaths: Final data for 2005, national vital statistics reports; Vol. 56 No. 10. Hyattsville, Md.: National Center for Health Statistics; 2008.
- Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980;302(2):123.
- American Society of Addiction Medicine; personal communication, 2013.
- Mäntyselkä P, Kumpusalo E, Ahonen R, Takala J. Patients’ versus general practitioners’ assessments of pain intensity in primary care patients with non-cancer pain. Br J Gen Pract. 2001;51(473):995-997.
- Petersen MA, Larsen H, Pedersen L, Sonne N, Groenvold M. Assessing health-related quality of life in palliative care: comparing patient and physician assessments. Eur J Cancer. 2006;42(8):1159-1166.
Nerves of Steal
A 60 year‐old woman with advanced kidney disease presented with one month of progressively worsening, sharp burning pain and decreased sensation in her left hand. Cold air exacerbated the pain. She noted decreasing ability to utilize her left fingers, a weakened grip and that the muscles in her hand looked smaller.
Localized sensory and motor symptoms in a discrete region of a single limb suggest neuropathy. The lack of symptoms in the face or ipsilateral lower extremity would dissuade a clinician from considering central etiologies; the presence of neuropathic pain is uncommon for cortical lesions. The involvement of motor and sensory nerves indicates peripheral nerve involvement.
The general approach to patients with peripheral neuropathy begins with identifying the neuropathy as a mononeuropathy (involving a single nerve), a polyneuropathy (symmetric involvement of multiple nerves) or a mononeuropathy multiplex (asymmetric involvement of multiple nerves). The patient, in this case, described subacute neuropathic pain, sensory loss, and weakness in her left hand in a distribution consistent with mononeuropathy or mononeuropathy multiplex.
This patient could have carpal tunnel syndrome given its prevalence in patients with advanced renal disease. The differential diagnosis is broad, however, and includes ulnar mononeuropathy, nerve ischemia due to vasculitis or vasculopathy, lower cervical radiculopathy (though the patient does not describe neck or radicular pain), lower brachial plexopathy, and complex regional pain syndrome.
The patient was diagnosed with advanced kidney disease one year ago when biopsy revealed focal segmental glomerulosclerosis secondary to lithium. Since her diagnosis, two grafts were placed in the left upper arm in anticipation of dialysis: the first, placed seven months prior to this admission, failed to mature; the second, placed one month prior to this admission, was complicated by bleeding at the fistula site and was not yet mature. Prior to this admission she had not required hemodialysis. Her past history included hypertension, dyslipidemia, hypothyroidism, secondary hyperparathyroidism, a remote history of cervical cancer (stage unknown, recent PAP smear negative), microcytosis and schizoaffective disorder. Her medications were furosemide, amlodipine, lisinopril, atenolol, atorvastatin, pantoprazole, olanzapine, levothyroxine, iron, darbepoetin, sevelamer, multivitamin and docusate.
Given the history of procedures in the left arm one should consider ischemic injury to the left median nerve. Other local complications could include compressive lesions such as an abscess or hematoma or direct nerve injury from the procedure. Carpal tunnel syndrome remains high on the differential due to its prevalence and because renal failure and hypothyroidism increase the risk of carpal tunnel syndrome.
A careful physical examination would localize the nerve or nerves involved. Examination findings that would be consistent with carpal tunnel syndrome include sensory loss in the distribution of the left median nerve, weakness of muscles innervated by the median nerve, including the abductor pollicus brevis and opponens muscles, and a Tinels and Phalens sign of the left wrist. A proximal median neuropathy resulting from ischemia or compression might also involve median‐innervated forearm muscle such as the pronator teres (forearm pronation) and flexor carpi radialis (hand flexion and abduction) muscles. Complex regional pain syndrome can be seen after a traumatic injury or surgery and is typified by severe neuropathic pain in a limb, often in combination with trophic changes in the affected extremity. A cervical radiculopathy would affect muscles supplied by the injured nerve root. For example, a C8 radiculopathy would affect all of the intrinsic hand muscles, the wrist and finger extensors, and the triceps brachii. A lower brachial plexopathy would present similarly to a lower cervical radiculopathy on clinical examination; electrodiagnostic evaluation would be necessary to distinguish these two disorders.
The patient appeared fatigued and her left hand was wrapped in blankets. Her vital signs were stable. There was no thyroid enlargement or lymphadenopathy. There was marked thenar, hypothenar and forearm atrophy on the left. Strength testing of her left hand demonstrated a grip strength of 2/5, finger extension and interosseous strength of 1/5, left wrist flexion and extension of 3/5; left biceps and triceps were 5/5. Sensation was mildly decreased to light touch, temperature, pain and proprioception throughout the left hand. Strength and sensation were intact in the right upper and bilateral lower extremities. Reflexes were 2+ throughout. The left radial pulse was diminished compared to the left ulnar that was 1+. The left hand was dry and cool. Laboratory evaluation revealed an elevated white blood cell count of 15,300/mm3, a hematocrit of 33 percent, mean corpuscular volume of 73 fL, and a normal platelet count. Electrolytes were consistent with advanced renal disease. The thyroid stimulating hormone level was normal.
The patient's examination reveals an injury to the sensory and motor components of the left ulnar, median, and distal radial nerves. The volar forearm wasting suggests proximal median motor nerve injury in the forearm. Because the triceps is spared, the weakness of finger and wrist extension implies distal radial motor nerve injury. The interosseous weakness is consistent with injury to the ulnar motor nerve. Weakness of grip and wrist flexion is less specific and it may be explained by injury to either the median or ulnar motor nerves. The diffuse sensory loss over the palmar and dorsal aspect of the left hand is consistent with neuropathic injury to the left median, ulnar, and radial sensory nerves. The preserved deep tendon reflexes are controlled by the musculocutaneous nerve (the biceps reflex) and branches arising from the proximal radial nerve (the triceps and brachioradialis reflexes), both of which are proximal to the apparent level of neuropathic insult.
Carpal tunnel syndrome is excluded since abnormalities extend beyond the median nerve distribution. The presentation is consistent with a mononeuropathy multiplex. Axonal etiologies of mononeuropathy multiplexincluding vasculitis, ischemia, neoplastic infiltration, and infectious etiologies such as Lyme diseaseare more common than demyelinating causes. Vasculitic neuropathy commonly involves the lower extremities and may have systemic symptoms, not present in this case. Neoplasm or other compressive lesions such as hematoma could explain these findings. Abscess must be considered given the leukocytosis. A lower brachial plexopathy, technically a mononeuropathy multiplex involving the proximal arm at the level of the brachial plexus, is also in the differential diagnosis. Another axonal disorder to consider would be neuralgic amyotrophy, an idiopathic form of acute brachial plexopathy associated with pain that is a complication of surgery that typically presents within a few hours to weeks of the procedure.
Demyelinating causes of mononeuropathy multiplex are less likely and include a variant of chronic inflammatory demyelinating polyneuropathy (Lewis‐Sumner syndrome) and hereditary neuropathy with liability to pressure palsies. Both processes are typically indolent and usually not painful, though the latter may present with fulminant numbness and weakness.
Nerve conduction and needle electromyography of the arm and cervical paraspinal muscles would differentiate axonal degeneration from demyelination. It would identify the affected nerves and any nerve root involvement. Given the concern for abscess or hematoma, MR neurography focused on the surgical site would also be important.
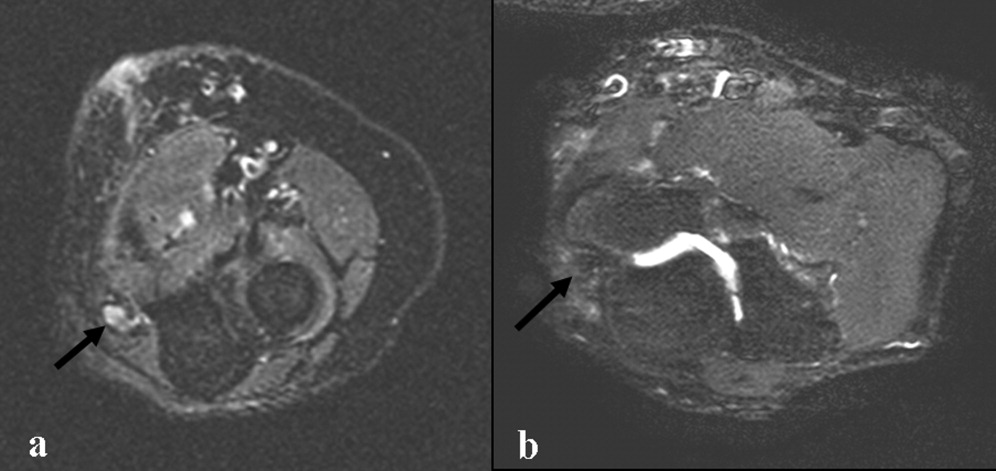
Electromyography and nerve conduction velocities demonstrated severe axonal loss of the left median, ulnar and distal radial sensory nerves consistent with acute denervation. A magnetic resonance neurogram following the course of these nerves revealed enlarged ulnar and median nerves with abnormal signal, but no compressive lesion (Fig. 1).
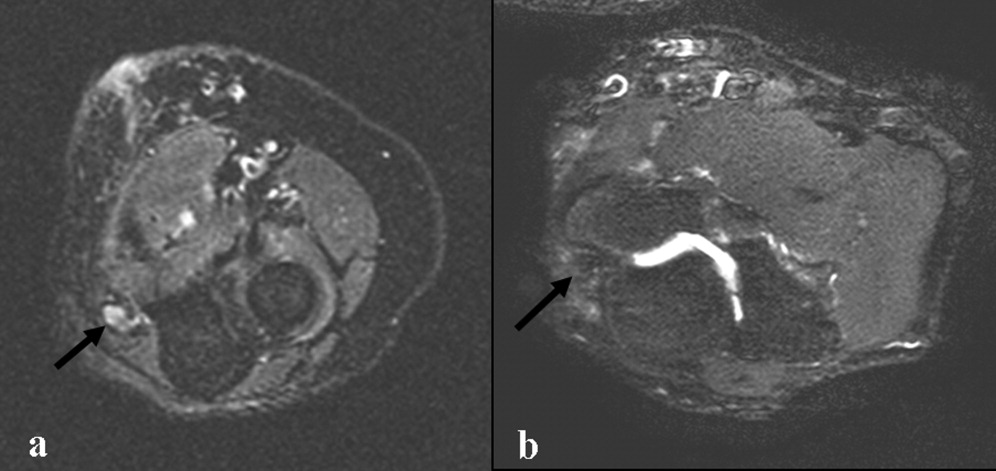
The electrodiagnostic testing is consistent with severe acute axonal injury to the left ulnar, median and radial nerves. This supports a diagnosis of mononeuropathy multiplex with axonal injury. Demyelinating causes are excluded at this point.
The MR neurogram demonstrates nonspecific nerve enlargement, which may be seen in ischemia, neoplastic processes (primary or metastatic), demyelinating disease, or, rarely, amyloidosis. Neoplastic involvement is unlikely in this case given the absence of a compressive mass lesion and the long segmental involvement of both the median and ulnar nerves. Compression from an abscess or hematoma is excluded. Neuralgic amyotrophy does not typically cause nerve enlargement.
Ischemia is the most likely diagnosis. Laboratory evaluation for vasculitis would be reasonable. Vasculitides that could present in this fashion include: polyarteritis nodosa, mixed connective tissue disease, Wegner's granulomatosis, Churg‐Strauss angiitis, Sjogren's, hepatitis C with serum cryoglobulinemia and possibly rheumatoid arthritis. Given the history of fistula placement in the affected limb, vascular sufficiency must be assessed.
Anti‐nuclear antibodies and anti‐neutrophilic cytoplasmic antibodies were negative, and a C‐ reactive protein was 5.9 mg/L (normal range, 0 to 10 mg/L). The erythrocyte sedimentation rate was 32mm/hr (normal range, 0 to 20) and serologies for hepatitis B and C were negative. There was no evidence of serum cryoglobulins.
A modestly elevated sedimentation rate and normal C‐reactive protein argue against a diagnosis of vasculitis. The negative ANA, ANCA, hepatitis serologies and cryoglobulin tests render unlikely the diagnoses of polyarteritis nodosa, Wegner's granulomatosis, Churg‐Strauss angiitis, or hepatitis related cryoglobulinemia. Eosinophilia (present in Churg‐Strauss), ENA (positive in mixed connective tissue disease), anti‐SSA and SSB (positive in Sjogren's) and a rheumatoid factor would round out this evaluation for vasculitis. A left radial sensory nerve biopsy could also be of value in diagnosing vasculitic neuropathy in this patient.
Given the evidence against vasculitis, the possibility of ischemia due to vascular insufficiency is concerning. Two ischemic complications of hemodialysis are known to cause distal multiple mononeuropathies. The first, ischemic monomelic neuropathy syndrome is seen almost exclusively in diabetics. It is characterized by the development of acute pain, weakness of the forearm and hand muscles, and sensory loss within minutes or hours of AV graft placement. Transient occlusion of the blood supply to the nerves of the forearm and hand induces nerve ischemia, but does not cause necrosis of other tissues. The nerve conduction findings in this patient are consistent with ischemic monomelic neuropathy syndrome. The delayed onset of her symptoms, however, makes this diagnosis unlikely.
The second ischemic complication of hemodialysis, and the likelier diagnosis, is vascular steal syndrome. This has a similar clinical and electrodiagnostic presentation to ischemic monomelic neuropathy syndrome, but has a latency period after surgery of days to months. Vascular steal occurs when a reversal of blood flow into the fistula steals flow from the palmar arch arteries and induces ischemia of the vasa nervorum. Vascular studies should be obtained urgently when this diagnosis is considered.
Evaluation of the arteriovenous graft and vascular surgery consultation were sought. Digital photoplethysmography revealed diminished waveforms in all fingers of the left hand. Arterial Doppler evaluation of the left upper extremity confirmed low‐velocity flow in the radial and ulnar arteries and failed to confirm flow in the brachial artery distal to the arteriovenous fistula. The patient underwent an angiogram of the left axillary and brachial arteries. There was normal flow until the level of the arteriovenous fistula but minimal flow distal to the fistula (Fig. 2).
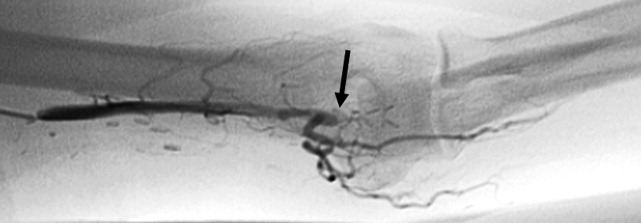
The diminished waveforms on digital photoplethysmography are consistent with poor perfusion distally. The angiogram suggests that the multiple mononeuropathies are a consequence of ischemia from impaired blood flow.
Consulting the vascular surgeons in this setting is essential because restoring adequate blood flow to the affected nerves can prevent further loss of function. Prognosis is dependent on many factors, including the severity of the functional loss and the duration of the symptoms prior to the restoration of blood flow. The patient's severe weakness and substantial muscle atrophy, manifestations of axonal degeneration, imply a poorer prognosis for recovery of function.
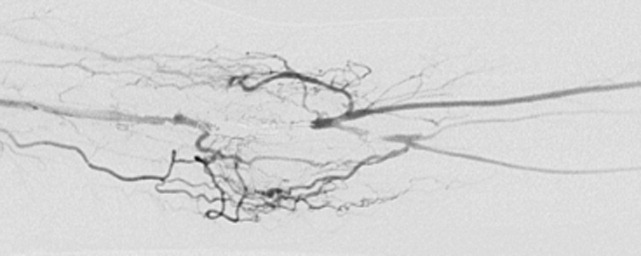
Embolization of the arteriovenous fistula was performed by interventional radiology. Post embolization angiograms demonstrated improved peripheral arterial flow (Fig. 3). One day later, the patient's finger flexion and extension improved. She reported mildly decreased dysesthesias and on examination her fingers were warmer to the touch. One month after discharge, her strength continued to be impaired, though improved and she still experienced pain.
COMMENTARY
Hospitalists must be equipped to recognize urgent and potentially reversible causes of neuropathy. The hospitalist should maintain a high index of suspicion for ischemia (either due to vasculitis or vascular compromise), traumatic nerve injury, nerve compression or entrapment, lymphoma or metastatic infiltration, hepatitis C with cryoglobulinemia, Guillain‐Barre syndrome and toxic exposures. Table 1 highlights important causes of mononeuropathy multiplex and summarizes associated findings and indicated diagnostic tests for specific evaluation.
| Diagnosis | Associated features | Specific evaluation |
|---|---|---|
| Axonal neuropathies | ||
| Ischemia (including vascular steal) | Poor arterial pulses, history of vascular surgery | Digital photoplethysmography, Doppler, angiography |
| Nerve compression and trauma | History of traumatic injury, mass, infection/abscess | MR neurography |
| Lymphoma or metastatic infiltration | History of known cancer, weight loss | PET, whole body CT, bone marrow biopsy |
| Vasculitis | Waxing and waning symptoms, association with connective tissue diseases, painful | CRP, ESR, Hepatitis C, cryoglobulins, ANA, ANCA, antibodies to SSA/SSB, ENA, eosinophil count, serum complement, SPEP/UPEP, RF, nerve biopsy |
| Neurosarcoidosis | Hilar lymphadenopathy, chronic cough | Chest CT, ACE, nerve biopsy |
| Lyme | Tick bite, erythema chronicum migrans | Lyme serology |
| Leprosy | Resident of southeast Asia, skin lesions | Skin smear for acid fast bacilli (mycobacterium), nerve biopsy |
| Demyelinating neuropathies | ||
| Lewis‐Sumner syndrome (i.e. asymmetric CIDP) | Relapsing remitting or chronic progressive course, areflexia | Lumbar puncture (increased spinal fluid protein common) |
| Hereditary neuropathy with liability to pressure palsy | Family history, recurrent episodes of entrapment/compression neuropathies | Genetic testing (deletion in the gene for peripheral myelin protein‐22) |
| Multifocal motor neuropathy with conduction block | Multifocal weakness in the distal arms/legs without sensory symptoms | Only motor abnormalities on nerve conduction including conduction block |
Another challenge for hospitalists is efficient evaluation of neuropathy. A systematic framework for creating a differential diagnosis and familiarity with available diagnostic tests is crucial. Hospitalists should be aware of three broad categories of neuropathy: mononeuropathy, polyneuropathy and mononeuropathy multiplex. Electrodiagnostic testing is essential to confirm the involved nerves and distinguishes axonal from demyelinating etiologies. Ultrasound, MR neurogram and, when indicated, nerve biopsy may be useful. Table 2 reviews these diagnostic tools as well as their indications and limitations.
| Test | Indications | Limitations |
|---|---|---|
| Electrodiagnostic Testing7 | Any peripheral neuropathy, muscle or neuromuscular junction disorder | Concomitant disease can reduce accuracy |
| Detects severity, chronicity, axonal v. demyelinating, diffuse v. focal, asymmetric v. symmetric | ||
| Electromyography (EMG) | EMG | EMG |
| Monopolar/concentric needle electrode inserted into the muscle belly | Differentiates axonal v. muscle damage; sensitive for even mild axon degeneration; localizes lesions. | Patient discomfort |
| Evaluates only motor fibers | ||
| Measures action potential at rest vs. during voluntary activation | Does not detect demyelination | |
| Might not be positive in first 21 days of symptoms | ||
| Nerve Conduction Studies (NCS) | NCS | NCS |
| Sensory | High sensitivity to differentiate axon loss from demyelination; localizes lesions. | Certain sensory responses lost with aging |
| Recording electrode placed over sensory nerve | ||
| Sensory nerve stimulated distally | Sensory localizes lesion to proximal vs. distal or to dorsal root ganglion | Less sensitive for mild axonal loss |
| Measures stimulus at proximal site | ||
| Motor | Motor amount of axonal loss | |
| Recording electrode placed over muscle belly | ||
| Motor nerve stimulated proximally | ||
| Measures stimulus at muscle | ||
| Specialized NCS tests | Specialized testing can identify radiculopathy, peripheral neuropathy, myasthenia gravis | |
| Ultrasound8 | ||
| Performed with typical ultrasound equipment | Suspected nerve entrapment | Doesn't show pathologic changes within nerves |
| Clinician must localize lesion for technician and explicitly guide test process | Evidence strongest for evaluation of median and ulnar nerves and Morton's neuroma | Difficult to visualize deep nerves or nerves surrounded by fat |
| Normal nerves appear tubular with linear echoes on a longitudinal scan; honeycomb on transverse scan | Detects lesions, nerve thickening, decreased echogenicity | Small field of view unless reconstructed |
| Results operator dependent | ||
| Less accurate than MRI for tumors | ||
| MRI9, 10 MR neurography | ||
| Standard MRI equipment | Concern regarding entrapment, trauma or mass lesions | Expense |
| Optimizes nerve resolution compared with surrounding tissues | To narrow differential when clinical and electrodiagnostic studies are inconclusive | Time (1560 minutes depending on scan requested) |
| When carpal tunnel syndrome does not respond to conservative management | ||
| Detects mass lesions compressing nerves, nerve enlargement and abnormal signal (neuritis, infiltration), increased signal in denervated muscle groups (once strength is 3 of 5). These changes can be seen as early at 4 days post trauma compared to 23 weeks on EMG. | ||
| Nerve Biopsy11 | ||
| Biopsy a nerve in the region of sensory loss or of a sensory nerve demonstrating electrophysiological abnormalities (decrease risk of adverse effects and to increase the likelihood of diagnosis | Rarely necessary | Painful, often for months |
| Concomitant muscle biopsy increases likelihood of diagnosing vasculitis or sarcoidosis | Use as last resort when evaluation not definitive | Risk of bleeding and infection |
| Greatest yield in multifocal neuropathies, or suspected amyloidotic polyneuropathy, vasculitis, sarcoidosis, lepromatous neuropathy, or rare hereditary disease where no genetic testing exists | ||
| Detects inflammation, amyloid deposits, tumor infiltration | ||
| Commonly targeted nerves include: LE sural, superficial peroneal, UE superficial radial |
Ischemic steal syndrome should be considered when neuropathy develops in a limb subsequent to arterio‐venous access procedures. Any vascular network, including the vertebral, carotid and coronary arteries, is at risk for steal. A feature common to all steal syndromes is the diversion of blood away from its original destination toward a lower pressure alternative. In some cases, this leads to a reversal of arterial flow and ischemia. Ischemic complications from AV access occur in 1‐9% of patients.1 Symptoms of steal can be mild, such as self‐limited dialysis induced pain, coldness and numbness, or severe, including severe pain, sensory and motor loss.2 If vascular compromise is sufficient, gangrene can ensue. Sensory deficits usually precede motor loss and the radial pulse is commonly absent or diminished. Other findings can include pallor of the fingers, muscle atrophy, resorption of the nail bed, and gangrene or ulcerations of the fingers. Risk factors for steal include atherosclerotic disease, female gender, age greater than 60 years, diabetes mellitus, previous surgery on the same arm, and use of the brachial artery as a donor.3 Symptoms of ischemic steal typically present within the first month after surgery, but can also be delayed; there is one report of a patient presenting one year postoperatively.4
Imaging studies such as doppler and angiography can be helpful in diagnosing ischemic steal syndrome. Fistulagrams may reveal a reversal of blood flow in the distal arm and hand, but these are reserved for cases with suspected proximal obstructive arterial disease.5 Vascular imaging studies can be misleading, however, as many patients will have physiologic but asymptomatic reversal of flow. Thus, a functional assessment such as digital plethysmography is recommended, especially in cases where clinical symptoms are vague. Digital pressures less than 60mmHg demonstrated 100% sensitivity and 87% specificity in one case control study of 40 patients.6 Treatment of ischemic steal syndrome is aimed at decreasing flow through the access shunt.
In conclusion, this case highlights the importance of timely and systematic evaluation of peripheral neuropathy in the hospital setting. Neuropathy with rapid progression and high potential for permanent damage necessitates early neurologic, or in this case, vascular consultation. Hospitalists should be facile in evaluating peripheral neuropathies and recognizing the appropriate indications for diagnostic tests and procedures.
- .Upper limb ischemia after vascular access surgery: differential diagnosis and management.Sem Dial2000;13:312–315.
- ,,,,,.Steal syndrome complicating hemodialysis access.Cardiovascular Surg (London, England)1997;5:648–653.
- ,,,,,.Onset of arterial ‘steal’ following proximal angioaccess: immediate and delayed types.Nephrol Dial Transplant2003;18:2387–2390.
- ,,,,.Incidence and characteristics of patients with hand ischemia after hemodialysis access procedure.J Surg Res1998;74:8–10
- ,,,,,.Ischemic steal syndrome: a case series and review of current management.Curr Surg2006;63:130–135.
- ,,,.Use of digital pressure measurements for the diagnosis of AV access‐induced hand ischemia.Vasc Med2006;11:227–231.
- ,.Electrodiagnostic testing of nerves and muscles: when, why, and how to order.Cleve Clin J Med2005;72:37–48.
- ,.High‐resolution sonography of the peripheral nervous system—a review of the literature.Eur J Neurol2004;11:305–314.
- ,,, et al.Role of magnetic resonance imaging in entrapment and compressive neuropathy‐what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 2. Upper extremity.Eur Radiol2007;17:509–522.
- ,,,,,.The utility of magnetic resonance imaging in evaluating peripheral nerve disorders.Muscle Nerve2002;25:314–331.
- .Indications and usefulness of nerve biopsy.Arch Neurol2002;59:1532–1535.
A 60 year‐old woman with advanced kidney disease presented with one month of progressively worsening, sharp burning pain and decreased sensation in her left hand. Cold air exacerbated the pain. She noted decreasing ability to utilize her left fingers, a weakened grip and that the muscles in her hand looked smaller.
Localized sensory and motor symptoms in a discrete region of a single limb suggest neuropathy. The lack of symptoms in the face or ipsilateral lower extremity would dissuade a clinician from considering central etiologies; the presence of neuropathic pain is uncommon for cortical lesions. The involvement of motor and sensory nerves indicates peripheral nerve involvement.
The general approach to patients with peripheral neuropathy begins with identifying the neuropathy as a mononeuropathy (involving a single nerve), a polyneuropathy (symmetric involvement of multiple nerves) or a mononeuropathy multiplex (asymmetric involvement of multiple nerves). The patient, in this case, described subacute neuropathic pain, sensory loss, and weakness in her left hand in a distribution consistent with mononeuropathy or mononeuropathy multiplex.
This patient could have carpal tunnel syndrome given its prevalence in patients with advanced renal disease. The differential diagnosis is broad, however, and includes ulnar mononeuropathy, nerve ischemia due to vasculitis or vasculopathy, lower cervical radiculopathy (though the patient does not describe neck or radicular pain), lower brachial plexopathy, and complex regional pain syndrome.
The patient was diagnosed with advanced kidney disease one year ago when biopsy revealed focal segmental glomerulosclerosis secondary to lithium. Since her diagnosis, two grafts were placed in the left upper arm in anticipation of dialysis: the first, placed seven months prior to this admission, failed to mature; the second, placed one month prior to this admission, was complicated by bleeding at the fistula site and was not yet mature. Prior to this admission she had not required hemodialysis. Her past history included hypertension, dyslipidemia, hypothyroidism, secondary hyperparathyroidism, a remote history of cervical cancer (stage unknown, recent PAP smear negative), microcytosis and schizoaffective disorder. Her medications were furosemide, amlodipine, lisinopril, atenolol, atorvastatin, pantoprazole, olanzapine, levothyroxine, iron, darbepoetin, sevelamer, multivitamin and docusate.
Given the history of procedures in the left arm one should consider ischemic injury to the left median nerve. Other local complications could include compressive lesions such as an abscess or hematoma or direct nerve injury from the procedure. Carpal tunnel syndrome remains high on the differential due to its prevalence and because renal failure and hypothyroidism increase the risk of carpal tunnel syndrome.
A careful physical examination would localize the nerve or nerves involved. Examination findings that would be consistent with carpal tunnel syndrome include sensory loss in the distribution of the left median nerve, weakness of muscles innervated by the median nerve, including the abductor pollicus brevis and opponens muscles, and a Tinels and Phalens sign of the left wrist. A proximal median neuropathy resulting from ischemia or compression might also involve median‐innervated forearm muscle such as the pronator teres (forearm pronation) and flexor carpi radialis (hand flexion and abduction) muscles. Complex regional pain syndrome can be seen after a traumatic injury or surgery and is typified by severe neuropathic pain in a limb, often in combination with trophic changes in the affected extremity. A cervical radiculopathy would affect muscles supplied by the injured nerve root. For example, a C8 radiculopathy would affect all of the intrinsic hand muscles, the wrist and finger extensors, and the triceps brachii. A lower brachial plexopathy would present similarly to a lower cervical radiculopathy on clinical examination; electrodiagnostic evaluation would be necessary to distinguish these two disorders.
The patient appeared fatigued and her left hand was wrapped in blankets. Her vital signs were stable. There was no thyroid enlargement or lymphadenopathy. There was marked thenar, hypothenar and forearm atrophy on the left. Strength testing of her left hand demonstrated a grip strength of 2/5, finger extension and interosseous strength of 1/5, left wrist flexion and extension of 3/5; left biceps and triceps were 5/5. Sensation was mildly decreased to light touch, temperature, pain and proprioception throughout the left hand. Strength and sensation were intact in the right upper and bilateral lower extremities. Reflexes were 2+ throughout. The left radial pulse was diminished compared to the left ulnar that was 1+. The left hand was dry and cool. Laboratory evaluation revealed an elevated white blood cell count of 15,300/mm3, a hematocrit of 33 percent, mean corpuscular volume of 73 fL, and a normal platelet count. Electrolytes were consistent with advanced renal disease. The thyroid stimulating hormone level was normal.
The patient's examination reveals an injury to the sensory and motor components of the left ulnar, median, and distal radial nerves. The volar forearm wasting suggests proximal median motor nerve injury in the forearm. Because the triceps is spared, the weakness of finger and wrist extension implies distal radial motor nerve injury. The interosseous weakness is consistent with injury to the ulnar motor nerve. Weakness of grip and wrist flexion is less specific and it may be explained by injury to either the median or ulnar motor nerves. The diffuse sensory loss over the palmar and dorsal aspect of the left hand is consistent with neuropathic injury to the left median, ulnar, and radial sensory nerves. The preserved deep tendon reflexes are controlled by the musculocutaneous nerve (the biceps reflex) and branches arising from the proximal radial nerve (the triceps and brachioradialis reflexes), both of which are proximal to the apparent level of neuropathic insult.
Carpal tunnel syndrome is excluded since abnormalities extend beyond the median nerve distribution. The presentation is consistent with a mononeuropathy multiplex. Axonal etiologies of mononeuropathy multiplexincluding vasculitis, ischemia, neoplastic infiltration, and infectious etiologies such as Lyme diseaseare more common than demyelinating causes. Vasculitic neuropathy commonly involves the lower extremities and may have systemic symptoms, not present in this case. Neoplasm or other compressive lesions such as hematoma could explain these findings. Abscess must be considered given the leukocytosis. A lower brachial plexopathy, technically a mononeuropathy multiplex involving the proximal arm at the level of the brachial plexus, is also in the differential diagnosis. Another axonal disorder to consider would be neuralgic amyotrophy, an idiopathic form of acute brachial plexopathy associated with pain that is a complication of surgery that typically presents within a few hours to weeks of the procedure.
Demyelinating causes of mononeuropathy multiplex are less likely and include a variant of chronic inflammatory demyelinating polyneuropathy (Lewis‐Sumner syndrome) and hereditary neuropathy with liability to pressure palsies. Both processes are typically indolent and usually not painful, though the latter may present with fulminant numbness and weakness.
Nerve conduction and needle electromyography of the arm and cervical paraspinal muscles would differentiate axonal degeneration from demyelination. It would identify the affected nerves and any nerve root involvement. Given the concern for abscess or hematoma, MR neurography focused on the surgical site would also be important.
Electromyography and nerve conduction velocities demonstrated severe axonal loss of the left median, ulnar and distal radial sensory nerves consistent with acute denervation. A magnetic resonance neurogram following the course of these nerves revealed enlarged ulnar and median nerves with abnormal signal, but no compressive lesion (Fig. 1).

The electrodiagnostic testing is consistent with severe acute axonal injury to the left ulnar, median and radial nerves. This supports a diagnosis of mononeuropathy multiplex with axonal injury. Demyelinating causes are excluded at this point.
The MR neurogram demonstrates nonspecific nerve enlargement, which may be seen in ischemia, neoplastic processes (primary or metastatic), demyelinating disease, or, rarely, amyloidosis. Neoplastic involvement is unlikely in this case given the absence of a compressive mass lesion and the long segmental involvement of both the median and ulnar nerves. Compression from an abscess or hematoma is excluded. Neuralgic amyotrophy does not typically cause nerve enlargement.
Ischemia is the most likely diagnosis. Laboratory evaluation for vasculitis would be reasonable. Vasculitides that could present in this fashion include: polyarteritis nodosa, mixed connective tissue disease, Wegner's granulomatosis, Churg‐Strauss angiitis, Sjogren's, hepatitis C with serum cryoglobulinemia and possibly rheumatoid arthritis. Given the history of fistula placement in the affected limb, vascular sufficiency must be assessed.
Anti‐nuclear antibodies and anti‐neutrophilic cytoplasmic antibodies were negative, and a C‐ reactive protein was 5.9 mg/L (normal range, 0 to 10 mg/L). The erythrocyte sedimentation rate was 32mm/hr (normal range, 0 to 20) and serologies for hepatitis B and C were negative. There was no evidence of serum cryoglobulins.
A modestly elevated sedimentation rate and normal C‐reactive protein argue against a diagnosis of vasculitis. The negative ANA, ANCA, hepatitis serologies and cryoglobulin tests render unlikely the diagnoses of polyarteritis nodosa, Wegner's granulomatosis, Churg‐Strauss angiitis, or hepatitis related cryoglobulinemia. Eosinophilia (present in Churg‐Strauss), ENA (positive in mixed connective tissue disease), anti‐SSA and SSB (positive in Sjogren's) and a rheumatoid factor would round out this evaluation for vasculitis. A left radial sensory nerve biopsy could also be of value in diagnosing vasculitic neuropathy in this patient.
Given the evidence against vasculitis, the possibility of ischemia due to vascular insufficiency is concerning. Two ischemic complications of hemodialysis are known to cause distal multiple mononeuropathies. The first, ischemic monomelic neuropathy syndrome is seen almost exclusively in diabetics. It is characterized by the development of acute pain, weakness of the forearm and hand muscles, and sensory loss within minutes or hours of AV graft placement. Transient occlusion of the blood supply to the nerves of the forearm and hand induces nerve ischemia, but does not cause necrosis of other tissues. The nerve conduction findings in this patient are consistent with ischemic monomelic neuropathy syndrome. The delayed onset of her symptoms, however, makes this diagnosis unlikely.
The second ischemic complication of hemodialysis, and the likelier diagnosis, is vascular steal syndrome. This has a similar clinical and electrodiagnostic presentation to ischemic monomelic neuropathy syndrome, but has a latency period after surgery of days to months. Vascular steal occurs when a reversal of blood flow into the fistula steals flow from the palmar arch arteries and induces ischemia of the vasa nervorum. Vascular studies should be obtained urgently when this diagnosis is considered.
Evaluation of the arteriovenous graft and vascular surgery consultation were sought. Digital photoplethysmography revealed diminished waveforms in all fingers of the left hand. Arterial Doppler evaluation of the left upper extremity confirmed low‐velocity flow in the radial and ulnar arteries and failed to confirm flow in the brachial artery distal to the arteriovenous fistula. The patient underwent an angiogram of the left axillary and brachial arteries. There was normal flow until the level of the arteriovenous fistula but minimal flow distal to the fistula (Fig. 2).

The diminished waveforms on digital photoplethysmography are consistent with poor perfusion distally. The angiogram suggests that the multiple mononeuropathies are a consequence of ischemia from impaired blood flow.
Consulting the vascular surgeons in this setting is essential because restoring adequate blood flow to the affected nerves can prevent further loss of function. Prognosis is dependent on many factors, including the severity of the functional loss and the duration of the symptoms prior to the restoration of blood flow. The patient's severe weakness and substantial muscle atrophy, manifestations of axonal degeneration, imply a poorer prognosis for recovery of function.
Embolization of the arteriovenous fistula was performed by interventional radiology. Post embolization angiograms demonstrated improved peripheral arterial flow (Fig. 3). One day later, the patient's finger flexion and extension improved. She reported mildly decreased dysesthesias and on examination her fingers were warmer to the touch. One month after discharge, her strength continued to be impaired, though improved and she still experienced pain.

COMMENTARY
Hospitalists must be equipped to recognize urgent and potentially reversible causes of neuropathy. The hospitalist should maintain a high index of suspicion for ischemia (either due to vasculitis or vascular compromise), traumatic nerve injury, nerve compression or entrapment, lymphoma or metastatic infiltration, hepatitis C with cryoglobulinemia, Guillain‐Barre syndrome and toxic exposures. Table 1 highlights important causes of mononeuropathy multiplex and summarizes associated findings and indicated diagnostic tests for specific evaluation.
| Diagnosis | Associated features | Specific evaluation |
|---|---|---|
| Axonal neuropathies | ||
| Ischemia (including vascular steal) | Poor arterial pulses, history of vascular surgery | Digital photoplethysmography, Doppler, angiography |
| Nerve compression and trauma | History of traumatic injury, mass, infection/abscess | MR neurography |
| Lymphoma or metastatic infiltration | History of known cancer, weight loss | PET, whole body CT, bone marrow biopsy |
| Vasculitis | Waxing and waning symptoms, association with connective tissue diseases, painful | CRP, ESR, Hepatitis C, cryoglobulins, ANA, ANCA, antibodies to SSA/SSB, ENA, eosinophil count, serum complement, SPEP/UPEP, RF, nerve biopsy |
| Neurosarcoidosis | Hilar lymphadenopathy, chronic cough | Chest CT, ACE, nerve biopsy |
| Lyme | Tick bite, erythema chronicum migrans | Lyme serology |
| Leprosy | Resident of southeast Asia, skin lesions | Skin smear for acid fast bacilli (mycobacterium), nerve biopsy |
| Demyelinating neuropathies | ||
| Lewis‐Sumner syndrome (i.e. asymmetric CIDP) | Relapsing remitting or chronic progressive course, areflexia | Lumbar puncture (increased spinal fluid protein common) |
| Hereditary neuropathy with liability to pressure palsy | Family history, recurrent episodes of entrapment/compression neuropathies | Genetic testing (deletion in the gene for peripheral myelin protein‐22) |
| Multifocal motor neuropathy with conduction block | Multifocal weakness in the distal arms/legs without sensory symptoms | Only motor abnormalities on nerve conduction including conduction block |
Another challenge for hospitalists is efficient evaluation of neuropathy. A systematic framework for creating a differential diagnosis and familiarity with available diagnostic tests is crucial. Hospitalists should be aware of three broad categories of neuropathy: mononeuropathy, polyneuropathy and mononeuropathy multiplex. Electrodiagnostic testing is essential to confirm the involved nerves and distinguishes axonal from demyelinating etiologies. Ultrasound, MR neurogram and, when indicated, nerve biopsy may be useful. Table 2 reviews these diagnostic tools as well as their indications and limitations.
| Test | Indications | Limitations |
|---|---|---|
| Electrodiagnostic Testing7 | Any peripheral neuropathy, muscle or neuromuscular junction disorder | Concomitant disease can reduce accuracy |
| Detects severity, chronicity, axonal v. demyelinating, diffuse v. focal, asymmetric v. symmetric | ||
| Electromyography (EMG) | EMG | EMG |
| Monopolar/concentric needle electrode inserted into the muscle belly | Differentiates axonal v. muscle damage; sensitive for even mild axon degeneration; localizes lesions. | Patient discomfort |
| Evaluates only motor fibers | ||
| Measures action potential at rest vs. during voluntary activation | Does not detect demyelination | |
| Might not be positive in first 21 days of symptoms | ||
| Nerve Conduction Studies (NCS) | NCS | NCS |
| Sensory | High sensitivity to differentiate axon loss from demyelination; localizes lesions. | Certain sensory responses lost with aging |
| Recording electrode placed over sensory nerve | ||
| Sensory nerve stimulated distally | Sensory localizes lesion to proximal vs. distal or to dorsal root ganglion | Less sensitive for mild axonal loss |
| Measures stimulus at proximal site | ||
| Motor | Motor amount of axonal loss | |
| Recording electrode placed over muscle belly | ||
| Motor nerve stimulated proximally | ||
| Measures stimulus at muscle | ||
| Specialized NCS tests | Specialized testing can identify radiculopathy, peripheral neuropathy, myasthenia gravis | |
| Ultrasound8 | ||
| Performed with typical ultrasound equipment | Suspected nerve entrapment | Doesn't show pathologic changes within nerves |
| Clinician must localize lesion for technician and explicitly guide test process | Evidence strongest for evaluation of median and ulnar nerves and Morton's neuroma | Difficult to visualize deep nerves or nerves surrounded by fat |
| Normal nerves appear tubular with linear echoes on a longitudinal scan; honeycomb on transverse scan | Detects lesions, nerve thickening, decreased echogenicity | Small field of view unless reconstructed |
| Results operator dependent | ||
| Less accurate than MRI for tumors | ||
| MRI9, 10 MR neurography | ||
| Standard MRI equipment | Concern regarding entrapment, trauma or mass lesions | Expense |
| Optimizes nerve resolution compared with surrounding tissues | To narrow differential when clinical and electrodiagnostic studies are inconclusive | Time (1560 minutes depending on scan requested) |
| When carpal tunnel syndrome does not respond to conservative management | ||
| Detects mass lesions compressing nerves, nerve enlargement and abnormal signal (neuritis, infiltration), increased signal in denervated muscle groups (once strength is 3 of 5). These changes can be seen as early at 4 days post trauma compared to 23 weeks on EMG. | ||
| Nerve Biopsy11 | ||
| Biopsy a nerve in the region of sensory loss or of a sensory nerve demonstrating electrophysiological abnormalities (decrease risk of adverse effects and to increase the likelihood of diagnosis | Rarely necessary | Painful, often for months |
| Concomitant muscle biopsy increases likelihood of diagnosing vasculitis or sarcoidosis | Use as last resort when evaluation not definitive | Risk of bleeding and infection |
| Greatest yield in multifocal neuropathies, or suspected amyloidotic polyneuropathy, vasculitis, sarcoidosis, lepromatous neuropathy, or rare hereditary disease where no genetic testing exists | ||
| Detects inflammation, amyloid deposits, tumor infiltration | ||
| Commonly targeted nerves include: LE sural, superficial peroneal, UE superficial radial |
Ischemic steal syndrome should be considered when neuropathy develops in a limb subsequent to arterio‐venous access procedures. Any vascular network, including the vertebral, carotid and coronary arteries, is at risk for steal. A feature common to all steal syndromes is the diversion of blood away from its original destination toward a lower pressure alternative. In some cases, this leads to a reversal of arterial flow and ischemia. Ischemic complications from AV access occur in 1‐9% of patients.1 Symptoms of steal can be mild, such as self‐limited dialysis induced pain, coldness and numbness, or severe, including severe pain, sensory and motor loss.2 If vascular compromise is sufficient, gangrene can ensue. Sensory deficits usually precede motor loss and the radial pulse is commonly absent or diminished. Other findings can include pallor of the fingers, muscle atrophy, resorption of the nail bed, and gangrene or ulcerations of the fingers. Risk factors for steal include atherosclerotic disease, female gender, age greater than 60 years, diabetes mellitus, previous surgery on the same arm, and use of the brachial artery as a donor.3 Symptoms of ischemic steal typically present within the first month after surgery, but can also be delayed; there is one report of a patient presenting one year postoperatively.4
Imaging studies such as doppler and angiography can be helpful in diagnosing ischemic steal syndrome. Fistulagrams may reveal a reversal of blood flow in the distal arm and hand, but these are reserved for cases with suspected proximal obstructive arterial disease.5 Vascular imaging studies can be misleading, however, as many patients will have physiologic but asymptomatic reversal of flow. Thus, a functional assessment such as digital plethysmography is recommended, especially in cases where clinical symptoms are vague. Digital pressures less than 60mmHg demonstrated 100% sensitivity and 87% specificity in one case control study of 40 patients.6 Treatment of ischemic steal syndrome is aimed at decreasing flow through the access shunt.
In conclusion, this case highlights the importance of timely and systematic evaluation of peripheral neuropathy in the hospital setting. Neuropathy with rapid progression and high potential for permanent damage necessitates early neurologic, or in this case, vascular consultation. Hospitalists should be facile in evaluating peripheral neuropathies and recognizing the appropriate indications for diagnostic tests and procedures.
A 60 year‐old woman with advanced kidney disease presented with one month of progressively worsening, sharp burning pain and decreased sensation in her left hand. Cold air exacerbated the pain. She noted decreasing ability to utilize her left fingers, a weakened grip and that the muscles in her hand looked smaller.
Localized sensory and motor symptoms in a discrete region of a single limb suggest neuropathy. The lack of symptoms in the face or ipsilateral lower extremity would dissuade a clinician from considering central etiologies; the presence of neuropathic pain is uncommon for cortical lesions. The involvement of motor and sensory nerves indicates peripheral nerve involvement.
The general approach to patients with peripheral neuropathy begins with identifying the neuropathy as a mononeuropathy (involving a single nerve), a polyneuropathy (symmetric involvement of multiple nerves) or a mononeuropathy multiplex (asymmetric involvement of multiple nerves). The patient, in this case, described subacute neuropathic pain, sensory loss, and weakness in her left hand in a distribution consistent with mononeuropathy or mononeuropathy multiplex.
This patient could have carpal tunnel syndrome given its prevalence in patients with advanced renal disease. The differential diagnosis is broad, however, and includes ulnar mononeuropathy, nerve ischemia due to vasculitis or vasculopathy, lower cervical radiculopathy (though the patient does not describe neck or radicular pain), lower brachial plexopathy, and complex regional pain syndrome.
The patient was diagnosed with advanced kidney disease one year ago when biopsy revealed focal segmental glomerulosclerosis secondary to lithium. Since her diagnosis, two grafts were placed in the left upper arm in anticipation of dialysis: the first, placed seven months prior to this admission, failed to mature; the second, placed one month prior to this admission, was complicated by bleeding at the fistula site and was not yet mature. Prior to this admission she had not required hemodialysis. Her past history included hypertension, dyslipidemia, hypothyroidism, secondary hyperparathyroidism, a remote history of cervical cancer (stage unknown, recent PAP smear negative), microcytosis and schizoaffective disorder. Her medications were furosemide, amlodipine, lisinopril, atenolol, atorvastatin, pantoprazole, olanzapine, levothyroxine, iron, darbepoetin, sevelamer, multivitamin and docusate.
Given the history of procedures in the left arm one should consider ischemic injury to the left median nerve. Other local complications could include compressive lesions such as an abscess or hematoma or direct nerve injury from the procedure. Carpal tunnel syndrome remains high on the differential due to its prevalence and because renal failure and hypothyroidism increase the risk of carpal tunnel syndrome.
A careful physical examination would localize the nerve or nerves involved. Examination findings that would be consistent with carpal tunnel syndrome include sensory loss in the distribution of the left median nerve, weakness of muscles innervated by the median nerve, including the abductor pollicus brevis and opponens muscles, and a Tinels and Phalens sign of the left wrist. A proximal median neuropathy resulting from ischemia or compression might also involve median‐innervated forearm muscle such as the pronator teres (forearm pronation) and flexor carpi radialis (hand flexion and abduction) muscles. Complex regional pain syndrome can be seen after a traumatic injury or surgery and is typified by severe neuropathic pain in a limb, often in combination with trophic changes in the affected extremity. A cervical radiculopathy would affect muscles supplied by the injured nerve root. For example, a C8 radiculopathy would affect all of the intrinsic hand muscles, the wrist and finger extensors, and the triceps brachii. A lower brachial plexopathy would present similarly to a lower cervical radiculopathy on clinical examination; electrodiagnostic evaluation would be necessary to distinguish these two disorders.
The patient appeared fatigued and her left hand was wrapped in blankets. Her vital signs were stable. There was no thyroid enlargement or lymphadenopathy. There was marked thenar, hypothenar and forearm atrophy on the left. Strength testing of her left hand demonstrated a grip strength of 2/5, finger extension and interosseous strength of 1/5, left wrist flexion and extension of 3/5; left biceps and triceps were 5/5. Sensation was mildly decreased to light touch, temperature, pain and proprioception throughout the left hand. Strength and sensation were intact in the right upper and bilateral lower extremities. Reflexes were 2+ throughout. The left radial pulse was diminished compared to the left ulnar that was 1+. The left hand was dry and cool. Laboratory evaluation revealed an elevated white blood cell count of 15,300/mm3, a hematocrit of 33 percent, mean corpuscular volume of 73 fL, and a normal platelet count. Electrolytes were consistent with advanced renal disease. The thyroid stimulating hormone level was normal.
The patient's examination reveals an injury to the sensory and motor components of the left ulnar, median, and distal radial nerves. The volar forearm wasting suggests proximal median motor nerve injury in the forearm. Because the triceps is spared, the weakness of finger and wrist extension implies distal radial motor nerve injury. The interosseous weakness is consistent with injury to the ulnar motor nerve. Weakness of grip and wrist flexion is less specific and it may be explained by injury to either the median or ulnar motor nerves. The diffuse sensory loss over the palmar and dorsal aspect of the left hand is consistent with neuropathic injury to the left median, ulnar, and radial sensory nerves. The preserved deep tendon reflexes are controlled by the musculocutaneous nerve (the biceps reflex) and branches arising from the proximal radial nerve (the triceps and brachioradialis reflexes), both of which are proximal to the apparent level of neuropathic insult.
Carpal tunnel syndrome is excluded since abnormalities extend beyond the median nerve distribution. The presentation is consistent with a mononeuropathy multiplex. Axonal etiologies of mononeuropathy multiplexincluding vasculitis, ischemia, neoplastic infiltration, and infectious etiologies such as Lyme diseaseare more common than demyelinating causes. Vasculitic neuropathy commonly involves the lower extremities and may have systemic symptoms, not present in this case. Neoplasm or other compressive lesions such as hematoma could explain these findings. Abscess must be considered given the leukocytosis. A lower brachial plexopathy, technically a mononeuropathy multiplex involving the proximal arm at the level of the brachial plexus, is also in the differential diagnosis. Another axonal disorder to consider would be neuralgic amyotrophy, an idiopathic form of acute brachial plexopathy associated with pain that is a complication of surgery that typically presents within a few hours to weeks of the procedure.
Demyelinating causes of mononeuropathy multiplex are less likely and include a variant of chronic inflammatory demyelinating polyneuropathy (Lewis‐Sumner syndrome) and hereditary neuropathy with liability to pressure palsies. Both processes are typically indolent and usually not painful, though the latter may present with fulminant numbness and weakness.
Nerve conduction and needle electromyography of the arm and cervical paraspinal muscles would differentiate axonal degeneration from demyelination. It would identify the affected nerves and any nerve root involvement. Given the concern for abscess or hematoma, MR neurography focused on the surgical site would also be important.
Electromyography and nerve conduction velocities demonstrated severe axonal loss of the left median, ulnar and distal radial sensory nerves consistent with acute denervation. A magnetic resonance neurogram following the course of these nerves revealed enlarged ulnar and median nerves with abnormal signal, but no compressive lesion (Fig. 1).

The electrodiagnostic testing is consistent with severe acute axonal injury to the left ulnar, median and radial nerves. This supports a diagnosis of mononeuropathy multiplex with axonal injury. Demyelinating causes are excluded at this point.
The MR neurogram demonstrates nonspecific nerve enlargement, which may be seen in ischemia, neoplastic processes (primary or metastatic), demyelinating disease, or, rarely, amyloidosis. Neoplastic involvement is unlikely in this case given the absence of a compressive mass lesion and the long segmental involvement of both the median and ulnar nerves. Compression from an abscess or hematoma is excluded. Neuralgic amyotrophy does not typically cause nerve enlargement.
Ischemia is the most likely diagnosis. Laboratory evaluation for vasculitis would be reasonable. Vasculitides that could present in this fashion include: polyarteritis nodosa, mixed connective tissue disease, Wegner's granulomatosis, Churg‐Strauss angiitis, Sjogren's, hepatitis C with serum cryoglobulinemia and possibly rheumatoid arthritis. Given the history of fistula placement in the affected limb, vascular sufficiency must be assessed.
Anti‐nuclear antibodies and anti‐neutrophilic cytoplasmic antibodies were negative, and a C‐ reactive protein was 5.9 mg/L (normal range, 0 to 10 mg/L). The erythrocyte sedimentation rate was 32mm/hr (normal range, 0 to 20) and serologies for hepatitis B and C were negative. There was no evidence of serum cryoglobulins.
A modestly elevated sedimentation rate and normal C‐reactive protein argue against a diagnosis of vasculitis. The negative ANA, ANCA, hepatitis serologies and cryoglobulin tests render unlikely the diagnoses of polyarteritis nodosa, Wegner's granulomatosis, Churg‐Strauss angiitis, or hepatitis related cryoglobulinemia. Eosinophilia (present in Churg‐Strauss), ENA (positive in mixed connective tissue disease), anti‐SSA and SSB (positive in Sjogren's) and a rheumatoid factor would round out this evaluation for vasculitis. A left radial sensory nerve biopsy could also be of value in diagnosing vasculitic neuropathy in this patient.
Given the evidence against vasculitis, the possibility of ischemia due to vascular insufficiency is concerning. Two ischemic complications of hemodialysis are known to cause distal multiple mononeuropathies. The first, ischemic monomelic neuropathy syndrome is seen almost exclusively in diabetics. It is characterized by the development of acute pain, weakness of the forearm and hand muscles, and sensory loss within minutes or hours of AV graft placement. Transient occlusion of the blood supply to the nerves of the forearm and hand induces nerve ischemia, but does not cause necrosis of other tissues. The nerve conduction findings in this patient are consistent with ischemic monomelic neuropathy syndrome. The delayed onset of her symptoms, however, makes this diagnosis unlikely.
The second ischemic complication of hemodialysis, and the likelier diagnosis, is vascular steal syndrome. This has a similar clinical and electrodiagnostic presentation to ischemic monomelic neuropathy syndrome, but has a latency period after surgery of days to months. Vascular steal occurs when a reversal of blood flow into the fistula steals flow from the palmar arch arteries and induces ischemia of the vasa nervorum. Vascular studies should be obtained urgently when this diagnosis is considered.
Evaluation of the arteriovenous graft and vascular surgery consultation were sought. Digital photoplethysmography revealed diminished waveforms in all fingers of the left hand. Arterial Doppler evaluation of the left upper extremity confirmed low‐velocity flow in the radial and ulnar arteries and failed to confirm flow in the brachial artery distal to the arteriovenous fistula. The patient underwent an angiogram of the left axillary and brachial arteries. There was normal flow until the level of the arteriovenous fistula but minimal flow distal to the fistula (Fig. 2).

The diminished waveforms on digital photoplethysmography are consistent with poor perfusion distally. The angiogram suggests that the multiple mononeuropathies are a consequence of ischemia from impaired blood flow.
Consulting the vascular surgeons in this setting is essential because restoring adequate blood flow to the affected nerves can prevent further loss of function. Prognosis is dependent on many factors, including the severity of the functional loss and the duration of the symptoms prior to the restoration of blood flow. The patient's severe weakness and substantial muscle atrophy, manifestations of axonal degeneration, imply a poorer prognosis for recovery of function.
Embolization of the arteriovenous fistula was performed by interventional radiology. Post embolization angiograms demonstrated improved peripheral arterial flow (Fig. 3). One day later, the patient's finger flexion and extension improved. She reported mildly decreased dysesthesias and on examination her fingers were warmer to the touch. One month after discharge, her strength continued to be impaired, though improved and she still experienced pain.

COMMENTARY
Hospitalists must be equipped to recognize urgent and potentially reversible causes of neuropathy. The hospitalist should maintain a high index of suspicion for ischemia (either due to vasculitis or vascular compromise), traumatic nerve injury, nerve compression or entrapment, lymphoma or metastatic infiltration, hepatitis C with cryoglobulinemia, Guillain‐Barre syndrome and toxic exposures. Table 1 highlights important causes of mononeuropathy multiplex and summarizes associated findings and indicated diagnostic tests for specific evaluation.
| Diagnosis | Associated features | Specific evaluation |
|---|---|---|
| Axonal neuropathies | ||
| Ischemia (including vascular steal) | Poor arterial pulses, history of vascular surgery | Digital photoplethysmography, Doppler, angiography |
| Nerve compression and trauma | History of traumatic injury, mass, infection/abscess | MR neurography |
| Lymphoma or metastatic infiltration | History of known cancer, weight loss | PET, whole body CT, bone marrow biopsy |
| Vasculitis | Waxing and waning symptoms, association with connective tissue diseases, painful | CRP, ESR, Hepatitis C, cryoglobulins, ANA, ANCA, antibodies to SSA/SSB, ENA, eosinophil count, serum complement, SPEP/UPEP, RF, nerve biopsy |
| Neurosarcoidosis | Hilar lymphadenopathy, chronic cough | Chest CT, ACE, nerve biopsy |
| Lyme | Tick bite, erythema chronicum migrans | Lyme serology |
| Leprosy | Resident of southeast Asia, skin lesions | Skin smear for acid fast bacilli (mycobacterium), nerve biopsy |
| Demyelinating neuropathies | ||
| Lewis‐Sumner syndrome (i.e. asymmetric CIDP) | Relapsing remitting or chronic progressive course, areflexia | Lumbar puncture (increased spinal fluid protein common) |
| Hereditary neuropathy with liability to pressure palsy | Family history, recurrent episodes of entrapment/compression neuropathies | Genetic testing (deletion in the gene for peripheral myelin protein‐22) |
| Multifocal motor neuropathy with conduction block | Multifocal weakness in the distal arms/legs without sensory symptoms | Only motor abnormalities on nerve conduction including conduction block |
Another challenge for hospitalists is efficient evaluation of neuropathy. A systematic framework for creating a differential diagnosis and familiarity with available diagnostic tests is crucial. Hospitalists should be aware of three broad categories of neuropathy: mononeuropathy, polyneuropathy and mononeuropathy multiplex. Electrodiagnostic testing is essential to confirm the involved nerves and distinguishes axonal from demyelinating etiologies. Ultrasound, MR neurogram and, when indicated, nerve biopsy may be useful. Table 2 reviews these diagnostic tools as well as their indications and limitations.
| Test | Indications | Limitations |
|---|---|---|
| Electrodiagnostic Testing7 | Any peripheral neuropathy, muscle or neuromuscular junction disorder | Concomitant disease can reduce accuracy |
| Detects severity, chronicity, axonal v. demyelinating, diffuse v. focal, asymmetric v. symmetric | ||
| Electromyography (EMG) | EMG | EMG |
| Monopolar/concentric needle electrode inserted into the muscle belly | Differentiates axonal v. muscle damage; sensitive for even mild axon degeneration; localizes lesions. | Patient discomfort |
| Evaluates only motor fibers | ||
| Measures action potential at rest vs. during voluntary activation | Does not detect demyelination | |
| Might not be positive in first 21 days of symptoms | ||
| Nerve Conduction Studies (NCS) | NCS | NCS |
| Sensory | High sensitivity to differentiate axon loss from demyelination; localizes lesions. | Certain sensory responses lost with aging |
| Recording electrode placed over sensory nerve | ||
| Sensory nerve stimulated distally | Sensory localizes lesion to proximal vs. distal or to dorsal root ganglion | Less sensitive for mild axonal loss |
| Measures stimulus at proximal site | ||
| Motor | Motor amount of axonal loss | |
| Recording electrode placed over muscle belly | ||
| Motor nerve stimulated proximally | ||
| Measures stimulus at muscle | ||
| Specialized NCS tests | Specialized testing can identify radiculopathy, peripheral neuropathy, myasthenia gravis | |
| Ultrasound8 | ||
| Performed with typical ultrasound equipment | Suspected nerve entrapment | Doesn't show pathologic changes within nerves |
| Clinician must localize lesion for technician and explicitly guide test process | Evidence strongest for evaluation of median and ulnar nerves and Morton's neuroma | Difficult to visualize deep nerves or nerves surrounded by fat |
| Normal nerves appear tubular with linear echoes on a longitudinal scan; honeycomb on transverse scan | Detects lesions, nerve thickening, decreased echogenicity | Small field of view unless reconstructed |
| Results operator dependent | ||
| Less accurate than MRI for tumors | ||
| MRI9, 10 MR neurography | ||
| Standard MRI equipment | Concern regarding entrapment, trauma or mass lesions | Expense |
| Optimizes nerve resolution compared with surrounding tissues | To narrow differential when clinical and electrodiagnostic studies are inconclusive | Time (1560 minutes depending on scan requested) |
| When carpal tunnel syndrome does not respond to conservative management | ||
| Detects mass lesions compressing nerves, nerve enlargement and abnormal signal (neuritis, infiltration), increased signal in denervated muscle groups (once strength is 3 of 5). These changes can be seen as early at 4 days post trauma compared to 23 weeks on EMG. | ||
| Nerve Biopsy11 | ||
| Biopsy a nerve in the region of sensory loss or of a sensory nerve demonstrating electrophysiological abnormalities (decrease risk of adverse effects and to increase the likelihood of diagnosis | Rarely necessary | Painful, often for months |
| Concomitant muscle biopsy increases likelihood of diagnosing vasculitis or sarcoidosis | Use as last resort when evaluation not definitive | Risk of bleeding and infection |
| Greatest yield in multifocal neuropathies, or suspected amyloidotic polyneuropathy, vasculitis, sarcoidosis, lepromatous neuropathy, or rare hereditary disease where no genetic testing exists | ||
| Detects inflammation, amyloid deposits, tumor infiltration | ||
| Commonly targeted nerves include: LE sural, superficial peroneal, UE superficial radial |
Ischemic steal syndrome should be considered when neuropathy develops in a limb subsequent to arterio‐venous access procedures. Any vascular network, including the vertebral, carotid and coronary arteries, is at risk for steal. A feature common to all steal syndromes is the diversion of blood away from its original destination toward a lower pressure alternative. In some cases, this leads to a reversal of arterial flow and ischemia. Ischemic complications from AV access occur in 1‐9% of patients.1 Symptoms of steal can be mild, such as self‐limited dialysis induced pain, coldness and numbness, or severe, including severe pain, sensory and motor loss.2 If vascular compromise is sufficient, gangrene can ensue. Sensory deficits usually precede motor loss and the radial pulse is commonly absent or diminished. Other findings can include pallor of the fingers, muscle atrophy, resorption of the nail bed, and gangrene or ulcerations of the fingers. Risk factors for steal include atherosclerotic disease, female gender, age greater than 60 years, diabetes mellitus, previous surgery on the same arm, and use of the brachial artery as a donor.3 Symptoms of ischemic steal typically present within the first month after surgery, but can also be delayed; there is one report of a patient presenting one year postoperatively.4
Imaging studies such as doppler and angiography can be helpful in diagnosing ischemic steal syndrome. Fistulagrams may reveal a reversal of blood flow in the distal arm and hand, but these are reserved for cases with suspected proximal obstructive arterial disease.5 Vascular imaging studies can be misleading, however, as many patients will have physiologic but asymptomatic reversal of flow. Thus, a functional assessment such as digital plethysmography is recommended, especially in cases where clinical symptoms are vague. Digital pressures less than 60mmHg demonstrated 100% sensitivity and 87% specificity in one case control study of 40 patients.6 Treatment of ischemic steal syndrome is aimed at decreasing flow through the access shunt.
In conclusion, this case highlights the importance of timely and systematic evaluation of peripheral neuropathy in the hospital setting. Neuropathy with rapid progression and high potential for permanent damage necessitates early neurologic, or in this case, vascular consultation. Hospitalists should be facile in evaluating peripheral neuropathies and recognizing the appropriate indications for diagnostic tests and procedures.
- .Upper limb ischemia after vascular access surgery: differential diagnosis and management.Sem Dial2000;13:312–315.
- ,,,,,.Steal syndrome complicating hemodialysis access.Cardiovascular Surg (London, England)1997;5:648–653.
- ,,,,,.Onset of arterial ‘steal’ following proximal angioaccess: immediate and delayed types.Nephrol Dial Transplant2003;18:2387–2390.
- ,,,,.Incidence and characteristics of patients with hand ischemia after hemodialysis access procedure.J Surg Res1998;74:8–10
- ,,,,,.Ischemic steal syndrome: a case series and review of current management.Curr Surg2006;63:130–135.
- ,,,.Use of digital pressure measurements for the diagnosis of AV access‐induced hand ischemia.Vasc Med2006;11:227–231.
- ,.Electrodiagnostic testing of nerves and muscles: when, why, and how to order.Cleve Clin J Med2005;72:37–48.
- ,.High‐resolution sonography of the peripheral nervous system—a review of the literature.Eur J Neurol2004;11:305–314.
- ,,, et al.Role of magnetic resonance imaging in entrapment and compressive neuropathy‐what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 2. Upper extremity.Eur Radiol2007;17:509–522.
- ,,,,,.The utility of magnetic resonance imaging in evaluating peripheral nerve disorders.Muscle Nerve2002;25:314–331.
- .Indications and usefulness of nerve biopsy.Arch Neurol2002;59:1532–1535.
- .Upper limb ischemia after vascular access surgery: differential diagnosis and management.Sem Dial2000;13:312–315.
- ,,,,,.Steal syndrome complicating hemodialysis access.Cardiovascular Surg (London, England)1997;5:648–653.
- ,,,,,.Onset of arterial ‘steal’ following proximal angioaccess: immediate and delayed types.Nephrol Dial Transplant2003;18:2387–2390.
- ,,,,.Incidence and characteristics of patients with hand ischemia after hemodialysis access procedure.J Surg Res1998;74:8–10
- ,,,,,.Ischemic steal syndrome: a case series and review of current management.Curr Surg2006;63:130–135.
- ,,,.Use of digital pressure measurements for the diagnosis of AV access‐induced hand ischemia.Vasc Med2006;11:227–231.
- ,.Electrodiagnostic testing of nerves and muscles: when, why, and how to order.Cleve Clin J Med2005;72:37–48.
- ,.High‐resolution sonography of the peripheral nervous system—a review of the literature.Eur J Neurol2004;11:305–314.
- ,,, et al.Role of magnetic resonance imaging in entrapment and compressive neuropathy‐what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 2. Upper extremity.Eur Radiol2007;17:509–522.
- ,,,,,.The utility of magnetic resonance imaging in evaluating peripheral nerve disorders.Muscle Nerve2002;25:314–331.
- .Indications and usefulness of nerve biopsy.Arch Neurol2002;59:1532–1535.