User login
Approach to Peripheral Neuropathies
Early diagnosis of peripheral neuropathies can lead to life‐saving or limb‐saving intervention. While infrequently a cause for concern in the hospital setting, peripheral neuropathies are commonoccurring in up to 10% of the general population.1 The hospitalist needs to expeditiously identify acute and life‐threatening or limb‐threatening causes among an immense set of differentials. Fortunately, with an informed and careful approach, most neuropathies in need of urgent intervention can be readily identified. A thorough history and examination, with the addition of electrodiagnostic testing, comprise the mainstays of this process. Inpatient neurology consultation should be sought for any rapidly progressing or acute onset neuropathy. The aim of this review is to equip the general hospitalist with a solid framework for efficiently evaluating peripheral neuropathies in urgent cases.
Literature Review
Search Strategy
A PubMed search was conducted using the title word peripheral, the medical subject heading major topic peripheral nervous system diseases/diagnosis, and algorithm or diagnosis, differential or diagnostic techniques, neurological or neurologic examination or evaluation or evaluating. The search was limited to English language review articles published between January 2002 and November 2007. Articles were included in this review if they provided an overview of an approach to the diagnosis of peripheral neuropathies. References listed in these articles were cross‐checked and additional articles meeting these criteria were included. Articles specific to subtypes of neuropathies or diagnostic tools were excluded.
Search Results
No single guideline or algorithm has been widely endorsed for the approach to diagnosing peripheral neuropathies. Several are suggested in the literature, but none are directed at the hospitalist. In general, acute and multifocal neuropathies are characterized as neurologic emergencies requiring immediate evaluation.2, 3
Several articles underscore the importance of pattern recognition in diagnosing peripheral neuropathies.2, 4, 5 Many articles present essential questions in evaluating peripheral neuropathy; some suggest an ordered approach.13, 511 The nature of these questions and recommended order of inquiry varies among authors (Table 1). Three essentials common to all articles include: 1) noting the onset of symptoms; 2) determining the distribution of nerve involvement; and 3) identifying the pathology as axonal, demyelinating, or mixed. All articles underscore the importance of the physical examination in determining and confirming distribution and nerve type. A thorough examination evaluating for systemic signs of etiologic possibilities is strongly recommended. Electrodiagnostic testing provides confirmation of the distribution of nerve involvement and further characterizes a neuropathy as demyelinating, axonal, or mixed.
| Article (Publication Year) | Essentials of Recommended Approach |
|---|---|
| Lunn3 (2007) | Details 6 essential questions in the history, highlighting: 1. Temporal evolution; 2. Autonomic involvement; 3. Nerve involvement (sensory/motor); 4. Cranial nerve involvement; 5. Family history; and 6. Coexistent disease |
| Examination should confirm findings expected from history | |
| Acute and multifocal neuropathies merit urgent evaluation | |
| Electrodiagnostic testing and neurology consultation should ensue if no diagnosis identified from above | |
| Burns et al.6 (2006) | Focuses on evaluation of polyneuropathy |
| Poses 4 questions: 1. Nerve involvement (sensory/motor); 2. Distribution; 3. Onset; 4. Associated factors (family history, exposures, associated systemic symptoms) | |
| Recommends electrodiagnostic testing | |
| Laboratory testing as indicated | |
| Scott and Kothari5 (2005) | Highlights importance of pattern recognition in the history and on examination |
| Ordered approach: 1. Localize site of neuropathic lesion, 2. Perform electrodiagnostic testing to determine pathology | |
| Bromberg1 (2005) | Proposes 7 layers to consider in investigation: 1. Localizing to peripheral nervous system; 2. Distribution; 3. Onset; 4. Nerve involvement (sensory/motor); 5. Pathology (axonal/demyelinating); 6. Other associated features; and 7. Epidemiologic features |
| Kelly4 (2004) | Highlights pattern recognition and features distribution, onset, and pathology in developing the differential diagnosis |
| Younger10 (2004) | Several key elements, including: timing, nerve involvement (sensory/motor/autonomic), distribution, and pathology (axonal/demyelinating) |
| England and Asbury7 (2004) | Details to determine: 1. Distribution; 2. Pathology (axonal/demyelinating); and 3. Timing |
| Smith and Bromberg9 (2003) | Suggest an algorithm: 1. Confirm the localization (history, examination and electrodiagnostic testing); 2. Identify atypical patterns; and 3. Recognize prototypic neuropathy and perform focused laboratory testing |
| Bromberg and Smith11 (2002) | 4 basic steps: 1. Nerve involvement (sensory/motor); 2. Distribution; 3. Timing; and 4. Pathology (axonal/demyelinating) |
| Hughes2 (2002) | Pattern recognition |
| Suggests staged investigation: 1. Basic laboratory tests; 2. Electrodiagnostic testing and further laboratory tests; and 3. Additional laboratory tests, imaging, and specialized testing | |
| Pourmand8 (2002) | Offers 7 key questions/steps highlighting: 1. Onset; 2. Course; 3. Distribution; 4. Nerve involvement (sensory/motor); 5. Nerve fiber type (large/small); 6. Autonomic involvement; and 7. Pathology (axonal/demyelinating) |
A General Approach for the Hospitalist
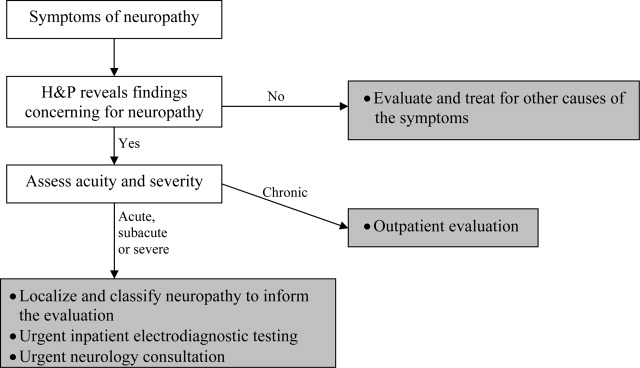
Pattern recognition and employing the essentials outlined above are key tools in the hospitalist's evaluation of peripheral neuropathy. Pattern recognition relies on a familiarity with the more common acute and severe neuropathies. For circumstances in which the diagnosis is not immediately recognizable, a systematic approach expedites evaluation. Figure 1 presents an algorithm for evaluating peripheral neuropathies in the acutely ill patient.
Pattern Recognition
In general, most acute or subacute and rapidly progressive neuropathies merit urgent neurology consultation. Patterns to be aware of in the acutely ill patient include Guillan‐Barr syndrome, vasculitis, ischemia, toxins, medication exposures, paraneoplastic syndromes, acute intermittent porphyria, diphtheria, and critical illness neuropathy. Any neuropathy presenting with associated respiratory symptoms or signs, such as shortness of breath, rapid shallow breathing, or hypoxia or hypercarbia, should also trigger urgent neurology consultation. As timely diagnosis of concerning entities relies heavily on pattern recognition, the typical presentation of more common etiologies and clues to their diagnosis are reviewed in Table 2.
| Etiology | Typical Presentation | Onset | Distribution | Electrodiagnostic Findings |
|---|---|---|---|---|
| ||||
| Traumatic neuropathy | Weakness and numbness in a limb following injury | Sudden | Asymmetric | Axonal |
| Guillan‐Barr syndrome | Acute inflammatory demyelinating polyneuropathy is most common but several variants exist; often follows URI or GI illness by 1‐3 weeks | Days to weeks | Ascending, symmetric | Usually demyelinating, largely motor |
| Diphtheria | Tonsillopharyngeal pseudomembrane | Days to weeks | Bulbar, descending, symmetric | Mostly demyelinating |
| Vasculitis | Waxing and waning, painful | Days to weeks | Asymmetric | Axonal |
| Acute intermittent porphyria | Can be associated with seizures/encephalopathy, abdominal pain | Days to weeks | Ascending, symmetric | Axonal, largely motor |
| Ischemic neuropathy | May follow vascular procedure by days to months; can be associated with poor peripheral pulses | Days to weeks | Asymmetric | Axonal |
| Toxins/drugs | Temporal association with offending agent: heavy metals: arsenic, lead, thallium; biologic toxins: ciguatera and shellfish poisoning. Medications: chemotherapies (ie, vincristine), colchicine, statins, nitrofurantoin, chloroquine | Days to months | Symmetric | Axonal |
| Critical illness neuropathy | Quadriparesis in the setting of sepsis/corticosteroids/neuromuscular blockade | Weeks | Symmetric | Axonal, largely motor |
| Paraneoplastic | Sensory ataxia most common; symptoms may precede cancer diagnosis; frequently associated tumors: small cell carcinoma of the lung; breast, ovarian, stomach cancers | Weeks | Symmetric | Axonal, largely sensory |
| Proximal diabetic neuropathy | Also known as diabetic lumbosacral plexopathy or Bruns‐Garland; leg pain followed by weakness/wasting | Weeks to months | Asymmetric | Axonal, largely motor |
For example, neuropathy from acute intermittent porphyria classically presents with pain in the back and limbs and progressive limb weakness (often more pronounced in the upper extremities). Respiratory failure may follow. A key to this history is that symptoms frequently follow within days of the colicky abdominal pain and encephalopathy of an attack. Additionally, attacks typically follow a precipitating event or drug exposure. These patients do not have the skin changes seen in other forms of porphyria. Treatment of this condition requires recognition and removal of any offending drug, correction of associated metabolic abnormalities, and the administration of hematin.12
Another, though rare, diagnosis that relies on pattern recognition is Bruns‐Garland syndrome (also known as proximal diabetic neuropathy). This condition is usually self‐limited, yet patients can be referred for unnecessary spinal surgery due to the severity of its symptoms. The clinical triad of severe thigh pain, absent knee jerk, and weakness in the lumbar vertebrae L3‐L4 distribution in a patient with diabetes should raise concern for this syndrome. The contralateral lower extremity can become involved in the following weeks. This syndrome is typified by a combination of injuries to the nerve root, the lumbar plexus, and the peripheral nerve. Electrodiagnostic testing confirms the syndrome, thus avoiding an unwarranted surgery.13
A Systematic Evaluation
When the etiology is not immediately evident, the essential questions identified in the review above are useful, and can be simplified for the hospitalist. First, understand the onset and timing of symptoms. Second, localize the symptoms to and within the peripheral nervous system (including classifying the distribution of nerve involvement). For acute, rapidly progressing or multifocal neuropathies urgent inpatient electrodiagnostic testing and neurology consultation should be obtained. Further testing, including laboratory testing, should be directed by these first steps.
Step 1
Delineating onset, timing and progression is of tremendous utility in establishing the diagnosis. Abrupt onset is typical of trauma, compression, thermal injury, and ischemia (due to vasculitis or other circulatory compromise). Guillan‐Barr syndrome, porphyria, critical illness neuropathy, and diphtheria can also present acutely with profound weakness. Neuropathies developing suddenly or over days to weeks merit urgent inpatient evaluation. Metabolic, paraneoplastic, and toxic causes tend to present with progressive symptoms over weeks to months. Chronic, insidious onset is most characteristic of hereditary neuropathies and some metabolic diseases such as diabetes mellitus. Evaluation of chronic neuropathies can be deferred to the outpatient setting.
Nonneuropathy causes of acute generalized weakness to consider in the differential diagnosis include: 1) muscle disorders such as periodic paralyses, metabolic defects, and myopathies (including acute viral and Lyme disease); 2) disorders of the neuromuscular junction such as myasthenia gravis, Eaton‐Lambert syndrome, organophosphate poisoning, and botulism; 3) central nervous system disorders such as brainstem ischemia, global ischemia, or multiple sclerosis; and 4) electrolyte disturbances such as hyperkalemia or hypercalcemia.14
Step 2
It is important to localize symptoms to the peripheral nervous system. Cortical lesions are unlikely to cause focal or positive sensory symptoms (ie, pain), and more frequently involve the face or upper and lower unilateral limb (ie, in the case of a stroke). Hyperreflexia can accompany cortical lesions. Conversely, peripheral nerve lesions often localize to a discrete region of a single limb or involve the contralateral limb in a symmetric fashion (ie, a stocking‐glove distribution or the ascending symmetric pattern seen in Guillan‐Barr syndrome).
With a thorough history and neurological examination the clinician can localize and classify the neuropathic lesion. Noting a motor or sensory predominance can narrow the diagnosis; for example, motor predominance is seen in Guillan‐Barr syndrome, critical illness neuropathy, and acute intermittent porphyria. Associated symptoms and signs discovered in a thorough review and physical examination of all systems can indicate the specific diagnosis. For example, a careful skin examination may find signs of vasculitis or Mees' lines (transverse white lines across the nails that can indicate heavy metal poisoning).12 Helpful tips for this evaluation are included in Table 3.
| History | Examination |
|---|---|
| |
| Ask the patient to outline the region involved | General findings |
| Dermatome radiculopathy | Screening for malignancy |
| Stocking‐glove polyneuropathy | Evaluate for vascular sufficiency |
| Single peripheral nerve mononeuropathy | Pes cavus suggests inherited disease |
| Asymmetry vasculitic neuropathy or other mononeuropathy multiplex | Skin exam for signs of vasculitis, Mees' lines |
| Associated symptoms | Neurologic findings: For each of the following, noting the distribution of abnormality will help classify the neuropathic lesion |
| Constitutional neoplasm | Decreased sensation (often the earliest sign) |
| Recent respiratory or GI illness GBS | Weakness without atrophy indicates recent axonal neuropathy or isolated demyelinating disease |
| Respiratory difficulties GBS | Marked atrophy indicates severe axonal damage |
| Autonomic symptoms GBS, porphyria | Decreased reflexes often present (except when only small sensory fibers are involved) |
| Colicky abdominal pain, encephalopathy | |
| Porphyria | |
The hospitalist should be able to classify the distribution as a mononeuropathy (involving a single nerve), a polyneuropathy (symmetric involvement of multiple nerves), or a mononeuropathy multiplex (asymmetric involvement of multiple nerves). Multifocal and proximal symmetric neuropathies commonly merit urgent evaluation.
The most devastating polyneuropathy is Guillan‐Barr syndrome, which can be fatal but is often reversible with early plasmapheresis. Vasculitis is another potentially treatable diagnosis that is critical to establish early; it most often presents as a mononeuropathy multiplex. Ischemic and traumatic mononeuropathies may be overshadowed by other illnesses and injuries, but finding these early can result in dramatically improved patient outcomes.
Step 3
Inpatient electrodiagnostic testing and neurology consultation should be ordered for any neuropathy with rapid onset, progression or severe symptoms or any neuropathy following one of the patterns described above. Electrodiagnostic testing characterizes the pathologic cause of the neuropathy as axonal, demyelinating, or mixed. It also assesses severity, chronicity, location, and symmetry of the neuropathy.15 It is imperative to have localized the neuropathy by history and examination prior to electrodiagnostic evaluation to ensure that the involved nerves are tested.
Step 4
Focused, further testing may be ordered more efficiently subsequent to the above data collection. Directed laboratory examination should be performed when indicated rather than cast as an initial broad diagnostic net. Ultrasound, magnetic resonance imaging (MRI), computed tomographypositron emission tomography (CT‐PET), and nerve biopsy are diagnostic modalities available to the clinician. In general, nerve biopsy should be reserved for suspected vasculitis, sarcoidosis, lymphoma, leprosy, or amyloidosis.
In summary, symptoms and signs of multifocal or proximal nerve involvement, acute onset, or rapid progression demand immediate diagnostic attention. Pattern recognition and a systematic approach expedite the diagnostic process, focusing necessary testing and decreasing overall cost. Focused steps in a systematic approach include: (1) delineating timing and onset of symptoms; (2) localizing and classifying the neuropathy; (3) obtaining electrodiagnostic testing and neurology consultation; and (4) further testing as directed by the preceding steps. Early diagnosis of acute peripheral neuropathies can lead to life‐saving or limb‐saving therapy.
- .An approach to the evaluation of peripheral neuropathies.Semin Neurol.2005;25:153–159.
- .Peripheral neuropathy.BMJ.2002;324:466–469.
- .Pinpointing peripheral neuropathies.Practitioner.2007;251:67–68,71–74,6–7 passim.
- .The evaluation of peripheral neuropathy. Part I: Clinical and laboratory evidence.Rev Neurol Dis.2004;1:133–140.
- ,.Evaluating the patient with peripheral nervous system complaints.J Am Osteopath Assoc.2005;105:71–83.
- ,,.An easy approach to evaluating peripheral neuropathy.J Fam Pract.2006;55:853–861.
- ,.Peripheral neuropathy.Lancet.2004;363:2151–2161.
- .Evaluating patients with suspected peripheral neuropathy: do the right thing, not everything.Muscle Nerve.2002;26:288–290.
- ,.A rational diagnostic approach to peripheral neuropathy.J Clin Neuromuscul Dis.2003;4:190–198.
- .Peripheral nerve disorders.Prim Care.2004;31:67–83.
- ,.Toward an efficient method to evaluate peripheral neuropathies.J Clin Neuromuscul Dis.2002;3:172–182.
- .Peripheral neuropathies in clinical practice.Med Clin North Am.2003;87:697–724.
- .The evaluation of peripheral neuropathy. Part II: Identifying common clinical syndromes.Rev Neurol Dis.2004;1:190–201.
- .Acute generalized weakness due to thyrotoxic periodic paralysis.CMAJ.2005;172:471–472.
- ,.Electrodiagnostic testing of nerves and muscles: when, why, and how to order.Cleve Clin J Med.2005;72:37–48.
Early diagnosis of peripheral neuropathies can lead to life‐saving or limb‐saving intervention. While infrequently a cause for concern in the hospital setting, peripheral neuropathies are commonoccurring in up to 10% of the general population.1 The hospitalist needs to expeditiously identify acute and life‐threatening or limb‐threatening causes among an immense set of differentials. Fortunately, with an informed and careful approach, most neuropathies in need of urgent intervention can be readily identified. A thorough history and examination, with the addition of electrodiagnostic testing, comprise the mainstays of this process. Inpatient neurology consultation should be sought for any rapidly progressing or acute onset neuropathy. The aim of this review is to equip the general hospitalist with a solid framework for efficiently evaluating peripheral neuropathies in urgent cases.
Literature Review
Search Strategy
A PubMed search was conducted using the title word peripheral, the medical subject heading major topic peripheral nervous system diseases/diagnosis, and algorithm or diagnosis, differential or diagnostic techniques, neurological or neurologic examination or evaluation or evaluating. The search was limited to English language review articles published between January 2002 and November 2007. Articles were included in this review if they provided an overview of an approach to the diagnosis of peripheral neuropathies. References listed in these articles were cross‐checked and additional articles meeting these criteria were included. Articles specific to subtypes of neuropathies or diagnostic tools were excluded.
Search Results
No single guideline or algorithm has been widely endorsed for the approach to diagnosing peripheral neuropathies. Several are suggested in the literature, but none are directed at the hospitalist. In general, acute and multifocal neuropathies are characterized as neurologic emergencies requiring immediate evaluation.2, 3
Several articles underscore the importance of pattern recognition in diagnosing peripheral neuropathies.2, 4, 5 Many articles present essential questions in evaluating peripheral neuropathy; some suggest an ordered approach.13, 511 The nature of these questions and recommended order of inquiry varies among authors (Table 1). Three essentials common to all articles include: 1) noting the onset of symptoms; 2) determining the distribution of nerve involvement; and 3) identifying the pathology as axonal, demyelinating, or mixed. All articles underscore the importance of the physical examination in determining and confirming distribution and nerve type. A thorough examination evaluating for systemic signs of etiologic possibilities is strongly recommended. Electrodiagnostic testing provides confirmation of the distribution of nerve involvement and further characterizes a neuropathy as demyelinating, axonal, or mixed.
| Article (Publication Year) | Essentials of Recommended Approach |
|---|---|
| Lunn3 (2007) | Details 6 essential questions in the history, highlighting: 1. Temporal evolution; 2. Autonomic involvement; 3. Nerve involvement (sensory/motor); 4. Cranial nerve involvement; 5. Family history; and 6. Coexistent disease |
| Examination should confirm findings expected from history | |
| Acute and multifocal neuropathies merit urgent evaluation | |
| Electrodiagnostic testing and neurology consultation should ensue if no diagnosis identified from above | |
| Burns et al.6 (2006) | Focuses on evaluation of polyneuropathy |
| Poses 4 questions: 1. Nerve involvement (sensory/motor); 2. Distribution; 3. Onset; 4. Associated factors (family history, exposures, associated systemic symptoms) | |
| Recommends electrodiagnostic testing | |
| Laboratory testing as indicated | |
| Scott and Kothari5 (2005) | Highlights importance of pattern recognition in the history and on examination |
| Ordered approach: 1. Localize site of neuropathic lesion, 2. Perform electrodiagnostic testing to determine pathology | |
| Bromberg1 (2005) | Proposes 7 layers to consider in investigation: 1. Localizing to peripheral nervous system; 2. Distribution; 3. Onset; 4. Nerve involvement (sensory/motor); 5. Pathology (axonal/demyelinating); 6. Other associated features; and 7. Epidemiologic features |
| Kelly4 (2004) | Highlights pattern recognition and features distribution, onset, and pathology in developing the differential diagnosis |
| Younger10 (2004) | Several key elements, including: timing, nerve involvement (sensory/motor/autonomic), distribution, and pathology (axonal/demyelinating) |
| England and Asbury7 (2004) | Details to determine: 1. Distribution; 2. Pathology (axonal/demyelinating); and 3. Timing |
| Smith and Bromberg9 (2003) | Suggest an algorithm: 1. Confirm the localization (history, examination and electrodiagnostic testing); 2. Identify atypical patterns; and 3. Recognize prototypic neuropathy and perform focused laboratory testing |
| Bromberg and Smith11 (2002) | 4 basic steps: 1. Nerve involvement (sensory/motor); 2. Distribution; 3. Timing; and 4. Pathology (axonal/demyelinating) |
| Hughes2 (2002) | Pattern recognition |
| Suggests staged investigation: 1. Basic laboratory tests; 2. Electrodiagnostic testing and further laboratory tests; and 3. Additional laboratory tests, imaging, and specialized testing | |
| Pourmand8 (2002) | Offers 7 key questions/steps highlighting: 1. Onset; 2. Course; 3. Distribution; 4. Nerve involvement (sensory/motor); 5. Nerve fiber type (large/small); 6. Autonomic involvement; and 7. Pathology (axonal/demyelinating) |
A General Approach for the Hospitalist
Pattern recognition and employing the essentials outlined above are key tools in the hospitalist's evaluation of peripheral neuropathy. Pattern recognition relies on a familiarity with the more common acute and severe neuropathies. For circumstances in which the diagnosis is not immediately recognizable, a systematic approach expedites evaluation. Figure 1 presents an algorithm for evaluating peripheral neuropathies in the acutely ill patient.

Pattern Recognition
In general, most acute or subacute and rapidly progressive neuropathies merit urgent neurology consultation. Patterns to be aware of in the acutely ill patient include Guillan‐Barr syndrome, vasculitis, ischemia, toxins, medication exposures, paraneoplastic syndromes, acute intermittent porphyria, diphtheria, and critical illness neuropathy. Any neuropathy presenting with associated respiratory symptoms or signs, such as shortness of breath, rapid shallow breathing, or hypoxia or hypercarbia, should also trigger urgent neurology consultation. As timely diagnosis of concerning entities relies heavily on pattern recognition, the typical presentation of more common etiologies and clues to their diagnosis are reviewed in Table 2.
| Etiology | Typical Presentation | Onset | Distribution | Electrodiagnostic Findings |
|---|---|---|---|---|
| ||||
| Traumatic neuropathy | Weakness and numbness in a limb following injury | Sudden | Asymmetric | Axonal |
| Guillan‐Barr syndrome | Acute inflammatory demyelinating polyneuropathy is most common but several variants exist; often follows URI or GI illness by 1‐3 weeks | Days to weeks | Ascending, symmetric | Usually demyelinating, largely motor |
| Diphtheria | Tonsillopharyngeal pseudomembrane | Days to weeks | Bulbar, descending, symmetric | Mostly demyelinating |
| Vasculitis | Waxing and waning, painful | Days to weeks | Asymmetric | Axonal |
| Acute intermittent porphyria | Can be associated with seizures/encephalopathy, abdominal pain | Days to weeks | Ascending, symmetric | Axonal, largely motor |
| Ischemic neuropathy | May follow vascular procedure by days to months; can be associated with poor peripheral pulses | Days to weeks | Asymmetric | Axonal |
| Toxins/drugs | Temporal association with offending agent: heavy metals: arsenic, lead, thallium; biologic toxins: ciguatera and shellfish poisoning. Medications: chemotherapies (ie, vincristine), colchicine, statins, nitrofurantoin, chloroquine | Days to months | Symmetric | Axonal |
| Critical illness neuropathy | Quadriparesis in the setting of sepsis/corticosteroids/neuromuscular blockade | Weeks | Symmetric | Axonal, largely motor |
| Paraneoplastic | Sensory ataxia most common; symptoms may precede cancer diagnosis; frequently associated tumors: small cell carcinoma of the lung; breast, ovarian, stomach cancers | Weeks | Symmetric | Axonal, largely sensory |
| Proximal diabetic neuropathy | Also known as diabetic lumbosacral plexopathy or Bruns‐Garland; leg pain followed by weakness/wasting | Weeks to months | Asymmetric | Axonal, largely motor |
For example, neuropathy from acute intermittent porphyria classically presents with pain in the back and limbs and progressive limb weakness (often more pronounced in the upper extremities). Respiratory failure may follow. A key to this history is that symptoms frequently follow within days of the colicky abdominal pain and encephalopathy of an attack. Additionally, attacks typically follow a precipitating event or drug exposure. These patients do not have the skin changes seen in other forms of porphyria. Treatment of this condition requires recognition and removal of any offending drug, correction of associated metabolic abnormalities, and the administration of hematin.12
Another, though rare, diagnosis that relies on pattern recognition is Bruns‐Garland syndrome (also known as proximal diabetic neuropathy). This condition is usually self‐limited, yet patients can be referred for unnecessary spinal surgery due to the severity of its symptoms. The clinical triad of severe thigh pain, absent knee jerk, and weakness in the lumbar vertebrae L3‐L4 distribution in a patient with diabetes should raise concern for this syndrome. The contralateral lower extremity can become involved in the following weeks. This syndrome is typified by a combination of injuries to the nerve root, the lumbar plexus, and the peripheral nerve. Electrodiagnostic testing confirms the syndrome, thus avoiding an unwarranted surgery.13
A Systematic Evaluation
When the etiology is not immediately evident, the essential questions identified in the review above are useful, and can be simplified for the hospitalist. First, understand the onset and timing of symptoms. Second, localize the symptoms to and within the peripheral nervous system (including classifying the distribution of nerve involvement). For acute, rapidly progressing or multifocal neuropathies urgent inpatient electrodiagnostic testing and neurology consultation should be obtained. Further testing, including laboratory testing, should be directed by these first steps.
Step 1
Delineating onset, timing and progression is of tremendous utility in establishing the diagnosis. Abrupt onset is typical of trauma, compression, thermal injury, and ischemia (due to vasculitis or other circulatory compromise). Guillan‐Barr syndrome, porphyria, critical illness neuropathy, and diphtheria can also present acutely with profound weakness. Neuropathies developing suddenly or over days to weeks merit urgent inpatient evaluation. Metabolic, paraneoplastic, and toxic causes tend to present with progressive symptoms over weeks to months. Chronic, insidious onset is most characteristic of hereditary neuropathies and some metabolic diseases such as diabetes mellitus. Evaluation of chronic neuropathies can be deferred to the outpatient setting.
Nonneuropathy causes of acute generalized weakness to consider in the differential diagnosis include: 1) muscle disorders such as periodic paralyses, metabolic defects, and myopathies (including acute viral and Lyme disease); 2) disorders of the neuromuscular junction such as myasthenia gravis, Eaton‐Lambert syndrome, organophosphate poisoning, and botulism; 3) central nervous system disorders such as brainstem ischemia, global ischemia, or multiple sclerosis; and 4) electrolyte disturbances such as hyperkalemia or hypercalcemia.14
Step 2
It is important to localize symptoms to the peripheral nervous system. Cortical lesions are unlikely to cause focal or positive sensory symptoms (ie, pain), and more frequently involve the face or upper and lower unilateral limb (ie, in the case of a stroke). Hyperreflexia can accompany cortical lesions. Conversely, peripheral nerve lesions often localize to a discrete region of a single limb or involve the contralateral limb in a symmetric fashion (ie, a stocking‐glove distribution or the ascending symmetric pattern seen in Guillan‐Barr syndrome).
With a thorough history and neurological examination the clinician can localize and classify the neuropathic lesion. Noting a motor or sensory predominance can narrow the diagnosis; for example, motor predominance is seen in Guillan‐Barr syndrome, critical illness neuropathy, and acute intermittent porphyria. Associated symptoms and signs discovered in a thorough review and physical examination of all systems can indicate the specific diagnosis. For example, a careful skin examination may find signs of vasculitis or Mees' lines (transverse white lines across the nails that can indicate heavy metal poisoning).12 Helpful tips for this evaluation are included in Table 3.
| History | Examination |
|---|---|
| |
| Ask the patient to outline the region involved | General findings |
| Dermatome radiculopathy | Screening for malignancy |
| Stocking‐glove polyneuropathy | Evaluate for vascular sufficiency |
| Single peripheral nerve mononeuropathy | Pes cavus suggests inherited disease |
| Asymmetry vasculitic neuropathy or other mononeuropathy multiplex | Skin exam for signs of vasculitis, Mees' lines |
| Associated symptoms | Neurologic findings: For each of the following, noting the distribution of abnormality will help classify the neuropathic lesion |
| Constitutional neoplasm | Decreased sensation (often the earliest sign) |
| Recent respiratory or GI illness GBS | Weakness without atrophy indicates recent axonal neuropathy or isolated demyelinating disease |
| Respiratory difficulties GBS | Marked atrophy indicates severe axonal damage |
| Autonomic symptoms GBS, porphyria | Decreased reflexes often present (except when only small sensory fibers are involved) |
| Colicky abdominal pain, encephalopathy | |
| Porphyria | |
The hospitalist should be able to classify the distribution as a mononeuropathy (involving a single nerve), a polyneuropathy (symmetric involvement of multiple nerves), or a mononeuropathy multiplex (asymmetric involvement of multiple nerves). Multifocal and proximal symmetric neuropathies commonly merit urgent evaluation.
The most devastating polyneuropathy is Guillan‐Barr syndrome, which can be fatal but is often reversible with early plasmapheresis. Vasculitis is another potentially treatable diagnosis that is critical to establish early; it most often presents as a mononeuropathy multiplex. Ischemic and traumatic mononeuropathies may be overshadowed by other illnesses and injuries, but finding these early can result in dramatically improved patient outcomes.
Step 3
Inpatient electrodiagnostic testing and neurology consultation should be ordered for any neuropathy with rapid onset, progression or severe symptoms or any neuropathy following one of the patterns described above. Electrodiagnostic testing characterizes the pathologic cause of the neuropathy as axonal, demyelinating, or mixed. It also assesses severity, chronicity, location, and symmetry of the neuropathy.15 It is imperative to have localized the neuropathy by history and examination prior to electrodiagnostic evaluation to ensure that the involved nerves are tested.
Step 4
Focused, further testing may be ordered more efficiently subsequent to the above data collection. Directed laboratory examination should be performed when indicated rather than cast as an initial broad diagnostic net. Ultrasound, magnetic resonance imaging (MRI), computed tomographypositron emission tomography (CT‐PET), and nerve biopsy are diagnostic modalities available to the clinician. In general, nerve biopsy should be reserved for suspected vasculitis, sarcoidosis, lymphoma, leprosy, or amyloidosis.
In summary, symptoms and signs of multifocal or proximal nerve involvement, acute onset, or rapid progression demand immediate diagnostic attention. Pattern recognition and a systematic approach expedite the diagnostic process, focusing necessary testing and decreasing overall cost. Focused steps in a systematic approach include: (1) delineating timing and onset of symptoms; (2) localizing and classifying the neuropathy; (3) obtaining electrodiagnostic testing and neurology consultation; and (4) further testing as directed by the preceding steps. Early diagnosis of acute peripheral neuropathies can lead to life‐saving or limb‐saving therapy.
Early diagnosis of peripheral neuropathies can lead to life‐saving or limb‐saving intervention. While infrequently a cause for concern in the hospital setting, peripheral neuropathies are commonoccurring in up to 10% of the general population.1 The hospitalist needs to expeditiously identify acute and life‐threatening or limb‐threatening causes among an immense set of differentials. Fortunately, with an informed and careful approach, most neuropathies in need of urgent intervention can be readily identified. A thorough history and examination, with the addition of electrodiagnostic testing, comprise the mainstays of this process. Inpatient neurology consultation should be sought for any rapidly progressing or acute onset neuropathy. The aim of this review is to equip the general hospitalist with a solid framework for efficiently evaluating peripheral neuropathies in urgent cases.
Literature Review
Search Strategy
A PubMed search was conducted using the title word peripheral, the medical subject heading major topic peripheral nervous system diseases/diagnosis, and algorithm or diagnosis, differential or diagnostic techniques, neurological or neurologic examination or evaluation or evaluating. The search was limited to English language review articles published between January 2002 and November 2007. Articles were included in this review if they provided an overview of an approach to the diagnosis of peripheral neuropathies. References listed in these articles were cross‐checked and additional articles meeting these criteria were included. Articles specific to subtypes of neuropathies or diagnostic tools were excluded.
Search Results
No single guideline or algorithm has been widely endorsed for the approach to diagnosing peripheral neuropathies. Several are suggested in the literature, but none are directed at the hospitalist. In general, acute and multifocal neuropathies are characterized as neurologic emergencies requiring immediate evaluation.2, 3
Several articles underscore the importance of pattern recognition in diagnosing peripheral neuropathies.2, 4, 5 Many articles present essential questions in evaluating peripheral neuropathy; some suggest an ordered approach.13, 511 The nature of these questions and recommended order of inquiry varies among authors (Table 1). Three essentials common to all articles include: 1) noting the onset of symptoms; 2) determining the distribution of nerve involvement; and 3) identifying the pathology as axonal, demyelinating, or mixed. All articles underscore the importance of the physical examination in determining and confirming distribution and nerve type. A thorough examination evaluating for systemic signs of etiologic possibilities is strongly recommended. Electrodiagnostic testing provides confirmation of the distribution of nerve involvement and further characterizes a neuropathy as demyelinating, axonal, or mixed.
| Article (Publication Year) | Essentials of Recommended Approach |
|---|---|
| Lunn3 (2007) | Details 6 essential questions in the history, highlighting: 1. Temporal evolution; 2. Autonomic involvement; 3. Nerve involvement (sensory/motor); 4. Cranial nerve involvement; 5. Family history; and 6. Coexistent disease |
| Examination should confirm findings expected from history | |
| Acute and multifocal neuropathies merit urgent evaluation | |
| Electrodiagnostic testing and neurology consultation should ensue if no diagnosis identified from above | |
| Burns et al.6 (2006) | Focuses on evaluation of polyneuropathy |
| Poses 4 questions: 1. Nerve involvement (sensory/motor); 2. Distribution; 3. Onset; 4. Associated factors (family history, exposures, associated systemic symptoms) | |
| Recommends electrodiagnostic testing | |
| Laboratory testing as indicated | |
| Scott and Kothari5 (2005) | Highlights importance of pattern recognition in the history and on examination |
| Ordered approach: 1. Localize site of neuropathic lesion, 2. Perform electrodiagnostic testing to determine pathology | |
| Bromberg1 (2005) | Proposes 7 layers to consider in investigation: 1. Localizing to peripheral nervous system; 2. Distribution; 3. Onset; 4. Nerve involvement (sensory/motor); 5. Pathology (axonal/demyelinating); 6. Other associated features; and 7. Epidemiologic features |
| Kelly4 (2004) | Highlights pattern recognition and features distribution, onset, and pathology in developing the differential diagnosis |
| Younger10 (2004) | Several key elements, including: timing, nerve involvement (sensory/motor/autonomic), distribution, and pathology (axonal/demyelinating) |
| England and Asbury7 (2004) | Details to determine: 1. Distribution; 2. Pathology (axonal/demyelinating); and 3. Timing |
| Smith and Bromberg9 (2003) | Suggest an algorithm: 1. Confirm the localization (history, examination and electrodiagnostic testing); 2. Identify atypical patterns; and 3. Recognize prototypic neuropathy and perform focused laboratory testing |
| Bromberg and Smith11 (2002) | 4 basic steps: 1. Nerve involvement (sensory/motor); 2. Distribution; 3. Timing; and 4. Pathology (axonal/demyelinating) |
| Hughes2 (2002) | Pattern recognition |
| Suggests staged investigation: 1. Basic laboratory tests; 2. Electrodiagnostic testing and further laboratory tests; and 3. Additional laboratory tests, imaging, and specialized testing | |
| Pourmand8 (2002) | Offers 7 key questions/steps highlighting: 1. Onset; 2. Course; 3. Distribution; 4. Nerve involvement (sensory/motor); 5. Nerve fiber type (large/small); 6. Autonomic involvement; and 7. Pathology (axonal/demyelinating) |
A General Approach for the Hospitalist
Pattern recognition and employing the essentials outlined above are key tools in the hospitalist's evaluation of peripheral neuropathy. Pattern recognition relies on a familiarity with the more common acute and severe neuropathies. For circumstances in which the diagnosis is not immediately recognizable, a systematic approach expedites evaluation. Figure 1 presents an algorithm for evaluating peripheral neuropathies in the acutely ill patient.

Pattern Recognition
In general, most acute or subacute and rapidly progressive neuropathies merit urgent neurology consultation. Patterns to be aware of in the acutely ill patient include Guillan‐Barr syndrome, vasculitis, ischemia, toxins, medication exposures, paraneoplastic syndromes, acute intermittent porphyria, diphtheria, and critical illness neuropathy. Any neuropathy presenting with associated respiratory symptoms or signs, such as shortness of breath, rapid shallow breathing, or hypoxia or hypercarbia, should also trigger urgent neurology consultation. As timely diagnosis of concerning entities relies heavily on pattern recognition, the typical presentation of more common etiologies and clues to their diagnosis are reviewed in Table 2.
| Etiology | Typical Presentation | Onset | Distribution | Electrodiagnostic Findings |
|---|---|---|---|---|
| ||||
| Traumatic neuropathy | Weakness and numbness in a limb following injury | Sudden | Asymmetric | Axonal |
| Guillan‐Barr syndrome | Acute inflammatory demyelinating polyneuropathy is most common but several variants exist; often follows URI or GI illness by 1‐3 weeks | Days to weeks | Ascending, symmetric | Usually demyelinating, largely motor |
| Diphtheria | Tonsillopharyngeal pseudomembrane | Days to weeks | Bulbar, descending, symmetric | Mostly demyelinating |
| Vasculitis | Waxing and waning, painful | Days to weeks | Asymmetric | Axonal |
| Acute intermittent porphyria | Can be associated with seizures/encephalopathy, abdominal pain | Days to weeks | Ascending, symmetric | Axonal, largely motor |
| Ischemic neuropathy | May follow vascular procedure by days to months; can be associated with poor peripheral pulses | Days to weeks | Asymmetric | Axonal |
| Toxins/drugs | Temporal association with offending agent: heavy metals: arsenic, lead, thallium; biologic toxins: ciguatera and shellfish poisoning. Medications: chemotherapies (ie, vincristine), colchicine, statins, nitrofurantoin, chloroquine | Days to months | Symmetric | Axonal |
| Critical illness neuropathy | Quadriparesis in the setting of sepsis/corticosteroids/neuromuscular blockade | Weeks | Symmetric | Axonal, largely motor |
| Paraneoplastic | Sensory ataxia most common; symptoms may precede cancer diagnosis; frequently associated tumors: small cell carcinoma of the lung; breast, ovarian, stomach cancers | Weeks | Symmetric | Axonal, largely sensory |
| Proximal diabetic neuropathy | Also known as diabetic lumbosacral plexopathy or Bruns‐Garland; leg pain followed by weakness/wasting | Weeks to months | Asymmetric | Axonal, largely motor |
For example, neuropathy from acute intermittent porphyria classically presents with pain in the back and limbs and progressive limb weakness (often more pronounced in the upper extremities). Respiratory failure may follow. A key to this history is that symptoms frequently follow within days of the colicky abdominal pain and encephalopathy of an attack. Additionally, attacks typically follow a precipitating event or drug exposure. These patients do not have the skin changes seen in other forms of porphyria. Treatment of this condition requires recognition and removal of any offending drug, correction of associated metabolic abnormalities, and the administration of hematin.12
Another, though rare, diagnosis that relies on pattern recognition is Bruns‐Garland syndrome (also known as proximal diabetic neuropathy). This condition is usually self‐limited, yet patients can be referred for unnecessary spinal surgery due to the severity of its symptoms. The clinical triad of severe thigh pain, absent knee jerk, and weakness in the lumbar vertebrae L3‐L4 distribution in a patient with diabetes should raise concern for this syndrome. The contralateral lower extremity can become involved in the following weeks. This syndrome is typified by a combination of injuries to the nerve root, the lumbar plexus, and the peripheral nerve. Electrodiagnostic testing confirms the syndrome, thus avoiding an unwarranted surgery.13
A Systematic Evaluation
When the etiology is not immediately evident, the essential questions identified in the review above are useful, and can be simplified for the hospitalist. First, understand the onset and timing of symptoms. Second, localize the symptoms to and within the peripheral nervous system (including classifying the distribution of nerve involvement). For acute, rapidly progressing or multifocal neuropathies urgent inpatient electrodiagnostic testing and neurology consultation should be obtained. Further testing, including laboratory testing, should be directed by these first steps.
Step 1
Delineating onset, timing and progression is of tremendous utility in establishing the diagnosis. Abrupt onset is typical of trauma, compression, thermal injury, and ischemia (due to vasculitis or other circulatory compromise). Guillan‐Barr syndrome, porphyria, critical illness neuropathy, and diphtheria can also present acutely with profound weakness. Neuropathies developing suddenly or over days to weeks merit urgent inpatient evaluation. Metabolic, paraneoplastic, and toxic causes tend to present with progressive symptoms over weeks to months. Chronic, insidious onset is most characteristic of hereditary neuropathies and some metabolic diseases such as diabetes mellitus. Evaluation of chronic neuropathies can be deferred to the outpatient setting.
Nonneuropathy causes of acute generalized weakness to consider in the differential diagnosis include: 1) muscle disorders such as periodic paralyses, metabolic defects, and myopathies (including acute viral and Lyme disease); 2) disorders of the neuromuscular junction such as myasthenia gravis, Eaton‐Lambert syndrome, organophosphate poisoning, and botulism; 3) central nervous system disorders such as brainstem ischemia, global ischemia, or multiple sclerosis; and 4) electrolyte disturbances such as hyperkalemia or hypercalcemia.14
Step 2
It is important to localize symptoms to the peripheral nervous system. Cortical lesions are unlikely to cause focal or positive sensory symptoms (ie, pain), and more frequently involve the face or upper and lower unilateral limb (ie, in the case of a stroke). Hyperreflexia can accompany cortical lesions. Conversely, peripheral nerve lesions often localize to a discrete region of a single limb or involve the contralateral limb in a symmetric fashion (ie, a stocking‐glove distribution or the ascending symmetric pattern seen in Guillan‐Barr syndrome).
With a thorough history and neurological examination the clinician can localize and classify the neuropathic lesion. Noting a motor or sensory predominance can narrow the diagnosis; for example, motor predominance is seen in Guillan‐Barr syndrome, critical illness neuropathy, and acute intermittent porphyria. Associated symptoms and signs discovered in a thorough review and physical examination of all systems can indicate the specific diagnosis. For example, a careful skin examination may find signs of vasculitis or Mees' lines (transverse white lines across the nails that can indicate heavy metal poisoning).12 Helpful tips for this evaluation are included in Table 3.
| History | Examination |
|---|---|
| |
| Ask the patient to outline the region involved | General findings |
| Dermatome radiculopathy | Screening for malignancy |
| Stocking‐glove polyneuropathy | Evaluate for vascular sufficiency |
| Single peripheral nerve mononeuropathy | Pes cavus suggests inherited disease |
| Asymmetry vasculitic neuropathy or other mononeuropathy multiplex | Skin exam for signs of vasculitis, Mees' lines |
| Associated symptoms | Neurologic findings: For each of the following, noting the distribution of abnormality will help classify the neuropathic lesion |
| Constitutional neoplasm | Decreased sensation (often the earliest sign) |
| Recent respiratory or GI illness GBS | Weakness without atrophy indicates recent axonal neuropathy or isolated demyelinating disease |
| Respiratory difficulties GBS | Marked atrophy indicates severe axonal damage |
| Autonomic symptoms GBS, porphyria | Decreased reflexes often present (except when only small sensory fibers are involved) |
| Colicky abdominal pain, encephalopathy | |
| Porphyria | |
The hospitalist should be able to classify the distribution as a mononeuropathy (involving a single nerve), a polyneuropathy (symmetric involvement of multiple nerves), or a mononeuropathy multiplex (asymmetric involvement of multiple nerves). Multifocal and proximal symmetric neuropathies commonly merit urgent evaluation.
The most devastating polyneuropathy is Guillan‐Barr syndrome, which can be fatal but is often reversible with early plasmapheresis. Vasculitis is another potentially treatable diagnosis that is critical to establish early; it most often presents as a mononeuropathy multiplex. Ischemic and traumatic mononeuropathies may be overshadowed by other illnesses and injuries, but finding these early can result in dramatically improved patient outcomes.
Step 3
Inpatient electrodiagnostic testing and neurology consultation should be ordered for any neuropathy with rapid onset, progression or severe symptoms or any neuropathy following one of the patterns described above. Electrodiagnostic testing characterizes the pathologic cause of the neuropathy as axonal, demyelinating, or mixed. It also assesses severity, chronicity, location, and symmetry of the neuropathy.15 It is imperative to have localized the neuropathy by history and examination prior to electrodiagnostic evaluation to ensure that the involved nerves are tested.
Step 4
Focused, further testing may be ordered more efficiently subsequent to the above data collection. Directed laboratory examination should be performed when indicated rather than cast as an initial broad diagnostic net. Ultrasound, magnetic resonance imaging (MRI), computed tomographypositron emission tomography (CT‐PET), and nerve biopsy are diagnostic modalities available to the clinician. In general, nerve biopsy should be reserved for suspected vasculitis, sarcoidosis, lymphoma, leprosy, or amyloidosis.
In summary, symptoms and signs of multifocal or proximal nerve involvement, acute onset, or rapid progression demand immediate diagnostic attention. Pattern recognition and a systematic approach expedite the diagnostic process, focusing necessary testing and decreasing overall cost. Focused steps in a systematic approach include: (1) delineating timing and onset of symptoms; (2) localizing and classifying the neuropathy; (3) obtaining electrodiagnostic testing and neurology consultation; and (4) further testing as directed by the preceding steps. Early diagnosis of acute peripheral neuropathies can lead to life‐saving or limb‐saving therapy.
- .An approach to the evaluation of peripheral neuropathies.Semin Neurol.2005;25:153–159.
- .Peripheral neuropathy.BMJ.2002;324:466–469.
- .Pinpointing peripheral neuropathies.Practitioner.2007;251:67–68,71–74,6–7 passim.
- .The evaluation of peripheral neuropathy. Part I: Clinical and laboratory evidence.Rev Neurol Dis.2004;1:133–140.
- ,.Evaluating the patient with peripheral nervous system complaints.J Am Osteopath Assoc.2005;105:71–83.
- ,,.An easy approach to evaluating peripheral neuropathy.J Fam Pract.2006;55:853–861.
- ,.Peripheral neuropathy.Lancet.2004;363:2151–2161.
- .Evaluating patients with suspected peripheral neuropathy: do the right thing, not everything.Muscle Nerve.2002;26:288–290.
- ,.A rational diagnostic approach to peripheral neuropathy.J Clin Neuromuscul Dis.2003;4:190–198.
- .Peripheral nerve disorders.Prim Care.2004;31:67–83.
- ,.Toward an efficient method to evaluate peripheral neuropathies.J Clin Neuromuscul Dis.2002;3:172–182.
- .Peripheral neuropathies in clinical practice.Med Clin North Am.2003;87:697–724.
- .The evaluation of peripheral neuropathy. Part II: Identifying common clinical syndromes.Rev Neurol Dis.2004;1:190–201.
- .Acute generalized weakness due to thyrotoxic periodic paralysis.CMAJ.2005;172:471–472.
- ,.Electrodiagnostic testing of nerves and muscles: when, why, and how to order.Cleve Clin J Med.2005;72:37–48.
- .An approach to the evaluation of peripheral neuropathies.Semin Neurol.2005;25:153–159.
- .Peripheral neuropathy.BMJ.2002;324:466–469.
- .Pinpointing peripheral neuropathies.Practitioner.2007;251:67–68,71–74,6–7 passim.
- .The evaluation of peripheral neuropathy. Part I: Clinical and laboratory evidence.Rev Neurol Dis.2004;1:133–140.
- ,.Evaluating the patient with peripheral nervous system complaints.J Am Osteopath Assoc.2005;105:71–83.
- ,,.An easy approach to evaluating peripheral neuropathy.J Fam Pract.2006;55:853–861.
- ,.Peripheral neuropathy.Lancet.2004;363:2151–2161.
- .Evaluating patients with suspected peripheral neuropathy: do the right thing, not everything.Muscle Nerve.2002;26:288–290.
- ,.A rational diagnostic approach to peripheral neuropathy.J Clin Neuromuscul Dis.2003;4:190–198.
- .Peripheral nerve disorders.Prim Care.2004;31:67–83.
- ,.Toward an efficient method to evaluate peripheral neuropathies.J Clin Neuromuscul Dis.2002;3:172–182.
- .Peripheral neuropathies in clinical practice.Med Clin North Am.2003;87:697–724.
- .The evaluation of peripheral neuropathy. Part II: Identifying common clinical syndromes.Rev Neurol Dis.2004;1:190–201.
- .Acute generalized weakness due to thyrotoxic periodic paralysis.CMAJ.2005;172:471–472.
- ,.Electrodiagnostic testing of nerves and muscles: when, why, and how to order.Cleve Clin J Med.2005;72:37–48.
Nerves of Steal
A 60 year‐old woman with advanced kidney disease presented with one month of progressively worsening, sharp burning pain and decreased sensation in her left hand. Cold air exacerbated the pain. She noted decreasing ability to utilize her left fingers, a weakened grip and that the muscles in her hand looked smaller.
Localized sensory and motor symptoms in a discrete region of a single limb suggest neuropathy. The lack of symptoms in the face or ipsilateral lower extremity would dissuade a clinician from considering central etiologies; the presence of neuropathic pain is uncommon for cortical lesions. The involvement of motor and sensory nerves indicates peripheral nerve involvement.
The general approach to patients with peripheral neuropathy begins with identifying the neuropathy as a mononeuropathy (involving a single nerve), a polyneuropathy (symmetric involvement of multiple nerves) or a mononeuropathy multiplex (asymmetric involvement of multiple nerves). The patient, in this case, described subacute neuropathic pain, sensory loss, and weakness in her left hand in a distribution consistent with mononeuropathy or mononeuropathy multiplex.
This patient could have carpal tunnel syndrome given its prevalence in patients with advanced renal disease. The differential diagnosis is broad, however, and includes ulnar mononeuropathy, nerve ischemia due to vasculitis or vasculopathy, lower cervical radiculopathy (though the patient does not describe neck or radicular pain), lower brachial plexopathy, and complex regional pain syndrome.
The patient was diagnosed with advanced kidney disease one year ago when biopsy revealed focal segmental glomerulosclerosis secondary to lithium. Since her diagnosis, two grafts were placed in the left upper arm in anticipation of dialysis: the first, placed seven months prior to this admission, failed to mature; the second, placed one month prior to this admission, was complicated by bleeding at the fistula site and was not yet mature. Prior to this admission she had not required hemodialysis. Her past history included hypertension, dyslipidemia, hypothyroidism, secondary hyperparathyroidism, a remote history of cervical cancer (stage unknown, recent PAP smear negative), microcytosis and schizoaffective disorder. Her medications were furosemide, amlodipine, lisinopril, atenolol, atorvastatin, pantoprazole, olanzapine, levothyroxine, iron, darbepoetin, sevelamer, multivitamin and docusate.
Given the history of procedures in the left arm one should consider ischemic injury to the left median nerve. Other local complications could include compressive lesions such as an abscess or hematoma or direct nerve injury from the procedure. Carpal tunnel syndrome remains high on the differential due to its prevalence and because renal failure and hypothyroidism increase the risk of carpal tunnel syndrome.
A careful physical examination would localize the nerve or nerves involved. Examination findings that would be consistent with carpal tunnel syndrome include sensory loss in the distribution of the left median nerve, weakness of muscles innervated by the median nerve, including the abductor pollicus brevis and opponens muscles, and a Tinels and Phalens sign of the left wrist. A proximal median neuropathy resulting from ischemia or compression might also involve median‐innervated forearm muscle such as the pronator teres (forearm pronation) and flexor carpi radialis (hand flexion and abduction) muscles. Complex regional pain syndrome can be seen after a traumatic injury or surgery and is typified by severe neuropathic pain in a limb, often in combination with trophic changes in the affected extremity. A cervical radiculopathy would affect muscles supplied by the injured nerve root. For example, a C8 radiculopathy would affect all of the intrinsic hand muscles, the wrist and finger extensors, and the triceps brachii. A lower brachial plexopathy would present similarly to a lower cervical radiculopathy on clinical examination; electrodiagnostic evaluation would be necessary to distinguish these two disorders.
The patient appeared fatigued and her left hand was wrapped in blankets. Her vital signs were stable. There was no thyroid enlargement or lymphadenopathy. There was marked thenar, hypothenar and forearm atrophy on the left. Strength testing of her left hand demonstrated a grip strength of 2/5, finger extension and interosseous strength of 1/5, left wrist flexion and extension of 3/5; left biceps and triceps were 5/5. Sensation was mildly decreased to light touch, temperature, pain and proprioception throughout the left hand. Strength and sensation were intact in the right upper and bilateral lower extremities. Reflexes were 2+ throughout. The left radial pulse was diminished compared to the left ulnar that was 1+. The left hand was dry and cool. Laboratory evaluation revealed an elevated white blood cell count of 15,300/mm3, a hematocrit of 33 percent, mean corpuscular volume of 73 fL, and a normal platelet count. Electrolytes were consistent with advanced renal disease. The thyroid stimulating hormone level was normal.
The patient's examination reveals an injury to the sensory and motor components of the left ulnar, median, and distal radial nerves. The volar forearm wasting suggests proximal median motor nerve injury in the forearm. Because the triceps is spared, the weakness of finger and wrist extension implies distal radial motor nerve injury. The interosseous weakness is consistent with injury to the ulnar motor nerve. Weakness of grip and wrist flexion is less specific and it may be explained by injury to either the median or ulnar motor nerves. The diffuse sensory loss over the palmar and dorsal aspect of the left hand is consistent with neuropathic injury to the left median, ulnar, and radial sensory nerves. The preserved deep tendon reflexes are controlled by the musculocutaneous nerve (the biceps reflex) and branches arising from the proximal radial nerve (the triceps and brachioradialis reflexes), both of which are proximal to the apparent level of neuropathic insult.
Carpal tunnel syndrome is excluded since abnormalities extend beyond the median nerve distribution. The presentation is consistent with a mononeuropathy multiplex. Axonal etiologies of mononeuropathy multiplexincluding vasculitis, ischemia, neoplastic infiltration, and infectious etiologies such as Lyme diseaseare more common than demyelinating causes. Vasculitic neuropathy commonly involves the lower extremities and may have systemic symptoms, not present in this case. Neoplasm or other compressive lesions such as hematoma could explain these findings. Abscess must be considered given the leukocytosis. A lower brachial plexopathy, technically a mononeuropathy multiplex involving the proximal arm at the level of the brachial plexus, is also in the differential diagnosis. Another axonal disorder to consider would be neuralgic amyotrophy, an idiopathic form of acute brachial plexopathy associated with pain that is a complication of surgery that typically presents within a few hours to weeks of the procedure.
Demyelinating causes of mononeuropathy multiplex are less likely and include a variant of chronic inflammatory demyelinating polyneuropathy (Lewis‐Sumner syndrome) and hereditary neuropathy with liability to pressure palsies. Both processes are typically indolent and usually not painful, though the latter may present with fulminant numbness and weakness.
Nerve conduction and needle electromyography of the arm and cervical paraspinal muscles would differentiate axonal degeneration from demyelination. It would identify the affected nerves and any nerve root involvement. Given the concern for abscess or hematoma, MR neurography focused on the surgical site would also be important.
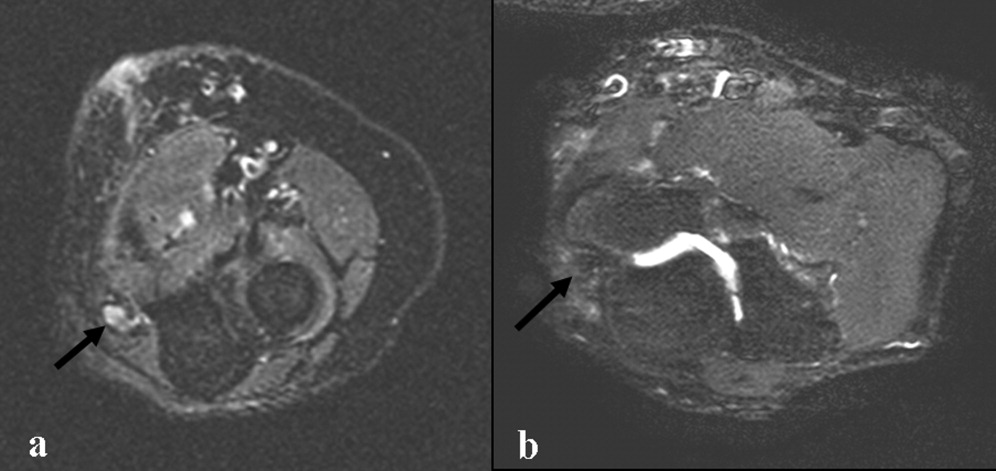
Electromyography and nerve conduction velocities demonstrated severe axonal loss of the left median, ulnar and distal radial sensory nerves consistent with acute denervation. A magnetic resonance neurogram following the course of these nerves revealed enlarged ulnar and median nerves with abnormal signal, but no compressive lesion (Fig. 1).
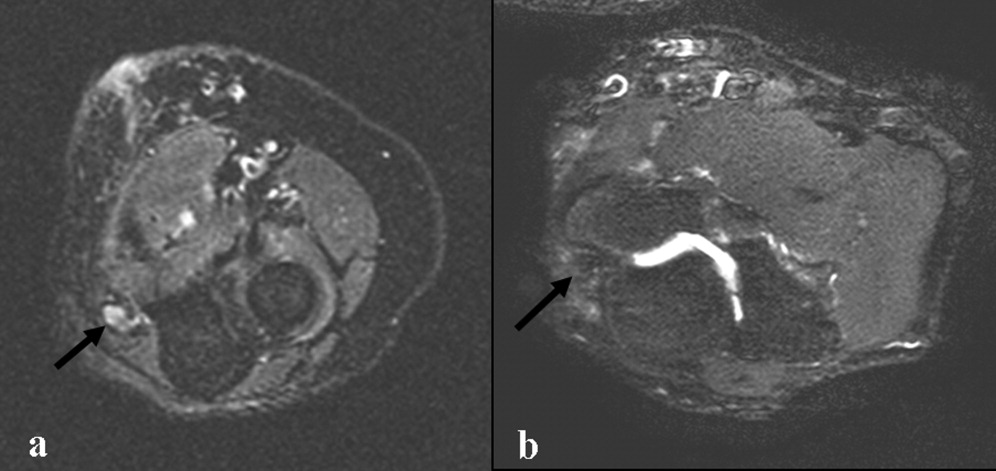
The electrodiagnostic testing is consistent with severe acute axonal injury to the left ulnar, median and radial nerves. This supports a diagnosis of mononeuropathy multiplex with axonal injury. Demyelinating causes are excluded at this point.
The MR neurogram demonstrates nonspecific nerve enlargement, which may be seen in ischemia, neoplastic processes (primary or metastatic), demyelinating disease, or, rarely, amyloidosis. Neoplastic involvement is unlikely in this case given the absence of a compressive mass lesion and the long segmental involvement of both the median and ulnar nerves. Compression from an abscess or hematoma is excluded. Neuralgic amyotrophy does not typically cause nerve enlargement.
Ischemia is the most likely diagnosis. Laboratory evaluation for vasculitis would be reasonable. Vasculitides that could present in this fashion include: polyarteritis nodosa, mixed connective tissue disease, Wegner's granulomatosis, Churg‐Strauss angiitis, Sjogren's, hepatitis C with serum cryoglobulinemia and possibly rheumatoid arthritis. Given the history of fistula placement in the affected limb, vascular sufficiency must be assessed.
Anti‐nuclear antibodies and anti‐neutrophilic cytoplasmic antibodies were negative, and a C‐ reactive protein was 5.9 mg/L (normal range, 0 to 10 mg/L). The erythrocyte sedimentation rate was 32mm/hr (normal range, 0 to 20) and serologies for hepatitis B and C were negative. There was no evidence of serum cryoglobulins.
A modestly elevated sedimentation rate and normal C‐reactive protein argue against a diagnosis of vasculitis. The negative ANA, ANCA, hepatitis serologies and cryoglobulin tests render unlikely the diagnoses of polyarteritis nodosa, Wegner's granulomatosis, Churg‐Strauss angiitis, or hepatitis related cryoglobulinemia. Eosinophilia (present in Churg‐Strauss), ENA (positive in mixed connective tissue disease), anti‐SSA and SSB (positive in Sjogren's) and a rheumatoid factor would round out this evaluation for vasculitis. A left radial sensory nerve biopsy could also be of value in diagnosing vasculitic neuropathy in this patient.
Given the evidence against vasculitis, the possibility of ischemia due to vascular insufficiency is concerning. Two ischemic complications of hemodialysis are known to cause distal multiple mononeuropathies. The first, ischemic monomelic neuropathy syndrome is seen almost exclusively in diabetics. It is characterized by the development of acute pain, weakness of the forearm and hand muscles, and sensory loss within minutes or hours of AV graft placement. Transient occlusion of the blood supply to the nerves of the forearm and hand induces nerve ischemia, but does not cause necrosis of other tissues. The nerve conduction findings in this patient are consistent with ischemic monomelic neuropathy syndrome. The delayed onset of her symptoms, however, makes this diagnosis unlikely.
The second ischemic complication of hemodialysis, and the likelier diagnosis, is vascular steal syndrome. This has a similar clinical and electrodiagnostic presentation to ischemic monomelic neuropathy syndrome, but has a latency period after surgery of days to months. Vascular steal occurs when a reversal of blood flow into the fistula steals flow from the palmar arch arteries and induces ischemia of the vasa nervorum. Vascular studies should be obtained urgently when this diagnosis is considered.
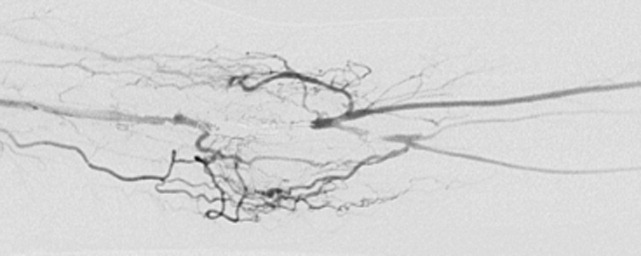
Evaluation of the arteriovenous graft and vascular surgery consultation were sought. Digital photoplethysmography revealed diminished waveforms in all fingers of the left hand. Arterial Doppler evaluation of the left upper extremity confirmed low‐velocity flow in the radial and ulnar arteries and failed to confirm flow in the brachial artery distal to the arteriovenous fistula. The patient underwent an angiogram of the left axillary and brachial arteries. There was normal flow until the level of the arteriovenous fistula but minimal flow distal to the fistula (Fig. 2).
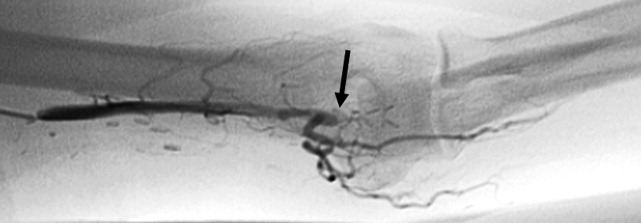
The diminished waveforms on digital photoplethysmography are consistent with poor perfusion distally. The angiogram suggests that the multiple mononeuropathies are a consequence of ischemia from impaired blood flow.
Consulting the vascular surgeons in this setting is essential because restoring adequate blood flow to the affected nerves can prevent further loss of function. Prognosis is dependent on many factors, including the severity of the functional loss and the duration of the symptoms prior to the restoration of blood flow. The patient's severe weakness and substantial muscle atrophy, manifestations of axonal degeneration, imply a poorer prognosis for recovery of function.
Embolization of the arteriovenous fistula was performed by interventional radiology. Post embolization angiograms demonstrated improved peripheral arterial flow (Fig. 3). One day later, the patient's finger flexion and extension improved. She reported mildly decreased dysesthesias and on examination her fingers were warmer to the touch. One month after discharge, her strength continued to be impaired, though improved and she still experienced pain.
COMMENTARY
Hospitalists must be equipped to recognize urgent and potentially reversible causes of neuropathy. The hospitalist should maintain a high index of suspicion for ischemia (either due to vasculitis or vascular compromise), traumatic nerve injury, nerve compression or entrapment, lymphoma or metastatic infiltration, hepatitis C with cryoglobulinemia, Guillain‐Barre syndrome and toxic exposures. Table 1 highlights important causes of mononeuropathy multiplex and summarizes associated findings and indicated diagnostic tests for specific evaluation.
| Diagnosis | Associated features | Specific evaluation |
|---|---|---|
| Axonal neuropathies | ||
| Ischemia (including vascular steal) | Poor arterial pulses, history of vascular surgery | Digital photoplethysmography, Doppler, angiography |
| Nerve compression and trauma | History of traumatic injury, mass, infection/abscess | MR neurography |
| Lymphoma or metastatic infiltration | History of known cancer, weight loss | PET, whole body CT, bone marrow biopsy |
| Vasculitis | Waxing and waning symptoms, association with connective tissue diseases, painful | CRP, ESR, Hepatitis C, cryoglobulins, ANA, ANCA, antibodies to SSA/SSB, ENA, eosinophil count, serum complement, SPEP/UPEP, RF, nerve biopsy |
| Neurosarcoidosis | Hilar lymphadenopathy, chronic cough | Chest CT, ACE, nerve biopsy |
| Lyme | Tick bite, erythema chronicum migrans | Lyme serology |
| Leprosy | Resident of southeast Asia, skin lesions | Skin smear for acid fast bacilli (mycobacterium), nerve biopsy |
| Demyelinating neuropathies | ||
| Lewis‐Sumner syndrome (i.e. asymmetric CIDP) | Relapsing remitting or chronic progressive course, areflexia | Lumbar puncture (increased spinal fluid protein common) |
| Hereditary neuropathy with liability to pressure palsy | Family history, recurrent episodes of entrapment/compression neuropathies | Genetic testing (deletion in the gene for peripheral myelin protein‐22) |
| Multifocal motor neuropathy with conduction block | Multifocal weakness in the distal arms/legs without sensory symptoms | Only motor abnormalities on nerve conduction including conduction block |
Another challenge for hospitalists is efficient evaluation of neuropathy. A systematic framework for creating a differential diagnosis and familiarity with available diagnostic tests is crucial. Hospitalists should be aware of three broad categories of neuropathy: mononeuropathy, polyneuropathy and mononeuropathy multiplex. Electrodiagnostic testing is essential to confirm the involved nerves and distinguishes axonal from demyelinating etiologies. Ultrasound, MR neurogram and, when indicated, nerve biopsy may be useful. Table 2 reviews these diagnostic tools as well as their indications and limitations.
| Test | Indications | Limitations |
|---|---|---|
| Electrodiagnostic Testing7 | Any peripheral neuropathy, muscle or neuromuscular junction disorder | Concomitant disease can reduce accuracy |
| Detects severity, chronicity, axonal v. demyelinating, diffuse v. focal, asymmetric v. symmetric | ||
| Electromyography (EMG) | EMG | EMG |
| Monopolar/concentric needle electrode inserted into the muscle belly | Differentiates axonal v. muscle damage; sensitive for even mild axon degeneration; localizes lesions. | Patient discomfort |
| Evaluates only motor fibers | ||
| Measures action potential at rest vs. during voluntary activation | Does not detect demyelination | |
| Might not be positive in first 21 days of symptoms | ||
| Nerve Conduction Studies (NCS) | NCS | NCS |
| Sensory | High sensitivity to differentiate axon loss from demyelination; localizes lesions. | Certain sensory responses lost with aging |
| Recording electrode placed over sensory nerve | ||
| Sensory nerve stimulated distally | Sensory localizes lesion to proximal vs. distal or to dorsal root ganglion | Less sensitive for mild axonal loss |
| Measures stimulus at proximal site | ||
| Motor | Motor amount of axonal loss | |
| Recording electrode placed over muscle belly | ||
| Motor nerve stimulated proximally | ||
| Measures stimulus at muscle | ||
| Specialized NCS tests | Specialized testing can identify radiculopathy, peripheral neuropathy, myasthenia gravis | |
| Ultrasound8 | ||
| Performed with typical ultrasound equipment | Suspected nerve entrapment | Doesn't show pathologic changes within nerves |
| Clinician must localize lesion for technician and explicitly guide test process | Evidence strongest for evaluation of median and ulnar nerves and Morton's neuroma | Difficult to visualize deep nerves or nerves surrounded by fat |
| Normal nerves appear tubular with linear echoes on a longitudinal scan; honeycomb on transverse scan | Detects lesions, nerve thickening, decreased echogenicity | Small field of view unless reconstructed |
| Results operator dependent | ||
| Less accurate than MRI for tumors | ||
| MRI9, 10 MR neurography | ||
| Standard MRI equipment | Concern regarding entrapment, trauma or mass lesions | Expense |
| Optimizes nerve resolution compared with surrounding tissues | To narrow differential when clinical and electrodiagnostic studies are inconclusive | Time (1560 minutes depending on scan requested) |
| When carpal tunnel syndrome does not respond to conservative management | ||
| Detects mass lesions compressing nerves, nerve enlargement and abnormal signal (neuritis, infiltration), increased signal in denervated muscle groups (once strength is 3 of 5). These changes can be seen as early at 4 days post trauma compared to 23 weeks on EMG. | ||
| Nerve Biopsy11 | ||
| Biopsy a nerve in the region of sensory loss or of a sensory nerve demonstrating electrophysiological abnormalities (decrease risk of adverse effects and to increase the likelihood of diagnosis | Rarely necessary | Painful, often for months |
| Concomitant muscle biopsy increases likelihood of diagnosing vasculitis or sarcoidosis | Use as last resort when evaluation not definitive | Risk of bleeding and infection |
| Greatest yield in multifocal neuropathies, or suspected amyloidotic polyneuropathy, vasculitis, sarcoidosis, lepromatous neuropathy, or rare hereditary disease where no genetic testing exists | ||
| Detects inflammation, amyloid deposits, tumor infiltration | ||
| Commonly targeted nerves include: LE sural, superficial peroneal, UE superficial radial |
Ischemic steal syndrome should be considered when neuropathy develops in a limb subsequent to arterio‐venous access procedures. Any vascular network, including the vertebral, carotid and coronary arteries, is at risk for steal. A feature common to all steal syndromes is the diversion of blood away from its original destination toward a lower pressure alternative. In some cases, this leads to a reversal of arterial flow and ischemia. Ischemic complications from AV access occur in 1‐9% of patients.1 Symptoms of steal can be mild, such as self‐limited dialysis induced pain, coldness and numbness, or severe, including severe pain, sensory and motor loss.2 If vascular compromise is sufficient, gangrene can ensue. Sensory deficits usually precede motor loss and the radial pulse is commonly absent or diminished. Other findings can include pallor of the fingers, muscle atrophy, resorption of the nail bed, and gangrene or ulcerations of the fingers. Risk factors for steal include atherosclerotic disease, female gender, age greater than 60 years, diabetes mellitus, previous surgery on the same arm, and use of the brachial artery as a donor.3 Symptoms of ischemic steal typically present within the first month after surgery, but can also be delayed; there is one report of a patient presenting one year postoperatively.4
Imaging studies such as doppler and angiography can be helpful in diagnosing ischemic steal syndrome. Fistulagrams may reveal a reversal of blood flow in the distal arm and hand, but these are reserved for cases with suspected proximal obstructive arterial disease.5 Vascular imaging studies can be misleading, however, as many patients will have physiologic but asymptomatic reversal of flow. Thus, a functional assessment such as digital plethysmography is recommended, especially in cases where clinical symptoms are vague. Digital pressures less than 60mmHg demonstrated 100% sensitivity and 87% specificity in one case control study of 40 patients.6 Treatment of ischemic steal syndrome is aimed at decreasing flow through the access shunt.
In conclusion, this case highlights the importance of timely and systematic evaluation of peripheral neuropathy in the hospital setting. Neuropathy with rapid progression and high potential for permanent damage necessitates early neurologic, or in this case, vascular consultation. Hospitalists should be facile in evaluating peripheral neuropathies and recognizing the appropriate indications for diagnostic tests and procedures.
- .Upper limb ischemia after vascular access surgery: differential diagnosis and management.Sem Dial2000;13:312–315.
- ,,,,,.Steal syndrome complicating hemodialysis access.Cardiovascular Surg (London, England)1997;5:648–653.
- ,,,,,.Onset of arterial ‘steal’ following proximal angioaccess: immediate and delayed types.Nephrol Dial Transplant2003;18:2387–2390.
- ,,,,.Incidence and characteristics of patients with hand ischemia after hemodialysis access procedure.J Surg Res1998;74:8–10
- ,,,,,.Ischemic steal syndrome: a case series and review of current management.Curr Surg2006;63:130–135.
- ,,,.Use of digital pressure measurements for the diagnosis of AV access‐induced hand ischemia.Vasc Med2006;11:227–231.
- ,.Electrodiagnostic testing of nerves and muscles: when, why, and how to order.Cleve Clin J Med2005;72:37–48.
- ,.High‐resolution sonography of the peripheral nervous system—a review of the literature.Eur J Neurol2004;11:305–314.
- ,,, et al.Role of magnetic resonance imaging in entrapment and compressive neuropathy‐what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 2. Upper extremity.Eur Radiol2007;17:509–522.
- ,,,,,.The utility of magnetic resonance imaging in evaluating peripheral nerve disorders.Muscle Nerve2002;25:314–331.
- .Indications and usefulness of nerve biopsy.Arch Neurol2002;59:1532–1535.
A 60 year‐old woman with advanced kidney disease presented with one month of progressively worsening, sharp burning pain and decreased sensation in her left hand. Cold air exacerbated the pain. She noted decreasing ability to utilize her left fingers, a weakened grip and that the muscles in her hand looked smaller.
Localized sensory and motor symptoms in a discrete region of a single limb suggest neuropathy. The lack of symptoms in the face or ipsilateral lower extremity would dissuade a clinician from considering central etiologies; the presence of neuropathic pain is uncommon for cortical lesions. The involvement of motor and sensory nerves indicates peripheral nerve involvement.
The general approach to patients with peripheral neuropathy begins with identifying the neuropathy as a mononeuropathy (involving a single nerve), a polyneuropathy (symmetric involvement of multiple nerves) or a mononeuropathy multiplex (asymmetric involvement of multiple nerves). The patient, in this case, described subacute neuropathic pain, sensory loss, and weakness in her left hand in a distribution consistent with mononeuropathy or mononeuropathy multiplex.
This patient could have carpal tunnel syndrome given its prevalence in patients with advanced renal disease. The differential diagnosis is broad, however, and includes ulnar mononeuropathy, nerve ischemia due to vasculitis or vasculopathy, lower cervical radiculopathy (though the patient does not describe neck or radicular pain), lower brachial plexopathy, and complex regional pain syndrome.
The patient was diagnosed with advanced kidney disease one year ago when biopsy revealed focal segmental glomerulosclerosis secondary to lithium. Since her diagnosis, two grafts were placed in the left upper arm in anticipation of dialysis: the first, placed seven months prior to this admission, failed to mature; the second, placed one month prior to this admission, was complicated by bleeding at the fistula site and was not yet mature. Prior to this admission she had not required hemodialysis. Her past history included hypertension, dyslipidemia, hypothyroidism, secondary hyperparathyroidism, a remote history of cervical cancer (stage unknown, recent PAP smear negative), microcytosis and schizoaffective disorder. Her medications were furosemide, amlodipine, lisinopril, atenolol, atorvastatin, pantoprazole, olanzapine, levothyroxine, iron, darbepoetin, sevelamer, multivitamin and docusate.
Given the history of procedures in the left arm one should consider ischemic injury to the left median nerve. Other local complications could include compressive lesions such as an abscess or hematoma or direct nerve injury from the procedure. Carpal tunnel syndrome remains high on the differential due to its prevalence and because renal failure and hypothyroidism increase the risk of carpal tunnel syndrome.
A careful physical examination would localize the nerve or nerves involved. Examination findings that would be consistent with carpal tunnel syndrome include sensory loss in the distribution of the left median nerve, weakness of muscles innervated by the median nerve, including the abductor pollicus brevis and opponens muscles, and a Tinels and Phalens sign of the left wrist. A proximal median neuropathy resulting from ischemia or compression might also involve median‐innervated forearm muscle such as the pronator teres (forearm pronation) and flexor carpi radialis (hand flexion and abduction) muscles. Complex regional pain syndrome can be seen after a traumatic injury or surgery and is typified by severe neuropathic pain in a limb, often in combination with trophic changes in the affected extremity. A cervical radiculopathy would affect muscles supplied by the injured nerve root. For example, a C8 radiculopathy would affect all of the intrinsic hand muscles, the wrist and finger extensors, and the triceps brachii. A lower brachial plexopathy would present similarly to a lower cervical radiculopathy on clinical examination; electrodiagnostic evaluation would be necessary to distinguish these two disorders.
The patient appeared fatigued and her left hand was wrapped in blankets. Her vital signs were stable. There was no thyroid enlargement or lymphadenopathy. There was marked thenar, hypothenar and forearm atrophy on the left. Strength testing of her left hand demonstrated a grip strength of 2/5, finger extension and interosseous strength of 1/5, left wrist flexion and extension of 3/5; left biceps and triceps were 5/5. Sensation was mildly decreased to light touch, temperature, pain and proprioception throughout the left hand. Strength and sensation were intact in the right upper and bilateral lower extremities. Reflexes were 2+ throughout. The left radial pulse was diminished compared to the left ulnar that was 1+. The left hand was dry and cool. Laboratory evaluation revealed an elevated white blood cell count of 15,300/mm3, a hematocrit of 33 percent, mean corpuscular volume of 73 fL, and a normal platelet count. Electrolytes were consistent with advanced renal disease. The thyroid stimulating hormone level was normal.
The patient's examination reveals an injury to the sensory and motor components of the left ulnar, median, and distal radial nerves. The volar forearm wasting suggests proximal median motor nerve injury in the forearm. Because the triceps is spared, the weakness of finger and wrist extension implies distal radial motor nerve injury. The interosseous weakness is consistent with injury to the ulnar motor nerve. Weakness of grip and wrist flexion is less specific and it may be explained by injury to either the median or ulnar motor nerves. The diffuse sensory loss over the palmar and dorsal aspect of the left hand is consistent with neuropathic injury to the left median, ulnar, and radial sensory nerves. The preserved deep tendon reflexes are controlled by the musculocutaneous nerve (the biceps reflex) and branches arising from the proximal radial nerve (the triceps and brachioradialis reflexes), both of which are proximal to the apparent level of neuropathic insult.
Carpal tunnel syndrome is excluded since abnormalities extend beyond the median nerve distribution. The presentation is consistent with a mononeuropathy multiplex. Axonal etiologies of mononeuropathy multiplexincluding vasculitis, ischemia, neoplastic infiltration, and infectious etiologies such as Lyme diseaseare more common than demyelinating causes. Vasculitic neuropathy commonly involves the lower extremities and may have systemic symptoms, not present in this case. Neoplasm or other compressive lesions such as hematoma could explain these findings. Abscess must be considered given the leukocytosis. A lower brachial plexopathy, technically a mononeuropathy multiplex involving the proximal arm at the level of the brachial plexus, is also in the differential diagnosis. Another axonal disorder to consider would be neuralgic amyotrophy, an idiopathic form of acute brachial plexopathy associated with pain that is a complication of surgery that typically presents within a few hours to weeks of the procedure.
Demyelinating causes of mononeuropathy multiplex are less likely and include a variant of chronic inflammatory demyelinating polyneuropathy (Lewis‐Sumner syndrome) and hereditary neuropathy with liability to pressure palsies. Both processes are typically indolent and usually not painful, though the latter may present with fulminant numbness and weakness.
Nerve conduction and needle electromyography of the arm and cervical paraspinal muscles would differentiate axonal degeneration from demyelination. It would identify the affected nerves and any nerve root involvement. Given the concern for abscess or hematoma, MR neurography focused on the surgical site would also be important.
Electromyography and nerve conduction velocities demonstrated severe axonal loss of the left median, ulnar and distal radial sensory nerves consistent with acute denervation. A magnetic resonance neurogram following the course of these nerves revealed enlarged ulnar and median nerves with abnormal signal, but no compressive lesion (Fig. 1).

The electrodiagnostic testing is consistent with severe acute axonal injury to the left ulnar, median and radial nerves. This supports a diagnosis of mononeuropathy multiplex with axonal injury. Demyelinating causes are excluded at this point.
The MR neurogram demonstrates nonspecific nerve enlargement, which may be seen in ischemia, neoplastic processes (primary or metastatic), demyelinating disease, or, rarely, amyloidosis. Neoplastic involvement is unlikely in this case given the absence of a compressive mass lesion and the long segmental involvement of both the median and ulnar nerves. Compression from an abscess or hematoma is excluded. Neuralgic amyotrophy does not typically cause nerve enlargement.
Ischemia is the most likely diagnosis. Laboratory evaluation for vasculitis would be reasonable. Vasculitides that could present in this fashion include: polyarteritis nodosa, mixed connective tissue disease, Wegner's granulomatosis, Churg‐Strauss angiitis, Sjogren's, hepatitis C with serum cryoglobulinemia and possibly rheumatoid arthritis. Given the history of fistula placement in the affected limb, vascular sufficiency must be assessed.
Anti‐nuclear antibodies and anti‐neutrophilic cytoplasmic antibodies were negative, and a C‐ reactive protein was 5.9 mg/L (normal range, 0 to 10 mg/L). The erythrocyte sedimentation rate was 32mm/hr (normal range, 0 to 20) and serologies for hepatitis B and C were negative. There was no evidence of serum cryoglobulins.
A modestly elevated sedimentation rate and normal C‐reactive protein argue against a diagnosis of vasculitis. The negative ANA, ANCA, hepatitis serologies and cryoglobulin tests render unlikely the diagnoses of polyarteritis nodosa, Wegner's granulomatosis, Churg‐Strauss angiitis, or hepatitis related cryoglobulinemia. Eosinophilia (present in Churg‐Strauss), ENA (positive in mixed connective tissue disease), anti‐SSA and SSB (positive in Sjogren's) and a rheumatoid factor would round out this evaluation for vasculitis. A left radial sensory nerve biopsy could also be of value in diagnosing vasculitic neuropathy in this patient.
Given the evidence against vasculitis, the possibility of ischemia due to vascular insufficiency is concerning. Two ischemic complications of hemodialysis are known to cause distal multiple mononeuropathies. The first, ischemic monomelic neuropathy syndrome is seen almost exclusively in diabetics. It is characterized by the development of acute pain, weakness of the forearm and hand muscles, and sensory loss within minutes or hours of AV graft placement. Transient occlusion of the blood supply to the nerves of the forearm and hand induces nerve ischemia, but does not cause necrosis of other tissues. The nerve conduction findings in this patient are consistent with ischemic monomelic neuropathy syndrome. The delayed onset of her symptoms, however, makes this diagnosis unlikely.
The second ischemic complication of hemodialysis, and the likelier diagnosis, is vascular steal syndrome. This has a similar clinical and electrodiagnostic presentation to ischemic monomelic neuropathy syndrome, but has a latency period after surgery of days to months. Vascular steal occurs when a reversal of blood flow into the fistula steals flow from the palmar arch arteries and induces ischemia of the vasa nervorum. Vascular studies should be obtained urgently when this diagnosis is considered.
Evaluation of the arteriovenous graft and vascular surgery consultation were sought. Digital photoplethysmography revealed diminished waveforms in all fingers of the left hand. Arterial Doppler evaluation of the left upper extremity confirmed low‐velocity flow in the radial and ulnar arteries and failed to confirm flow in the brachial artery distal to the arteriovenous fistula. The patient underwent an angiogram of the left axillary and brachial arteries. There was normal flow until the level of the arteriovenous fistula but minimal flow distal to the fistula (Fig. 2).

The diminished waveforms on digital photoplethysmography are consistent with poor perfusion distally. The angiogram suggests that the multiple mononeuropathies are a consequence of ischemia from impaired blood flow.
Consulting the vascular surgeons in this setting is essential because restoring adequate blood flow to the affected nerves can prevent further loss of function. Prognosis is dependent on many factors, including the severity of the functional loss and the duration of the symptoms prior to the restoration of blood flow. The patient's severe weakness and substantial muscle atrophy, manifestations of axonal degeneration, imply a poorer prognosis for recovery of function.
Embolization of the arteriovenous fistula was performed by interventional radiology. Post embolization angiograms demonstrated improved peripheral arterial flow (Fig. 3). One day later, the patient's finger flexion and extension improved. She reported mildly decreased dysesthesias and on examination her fingers were warmer to the touch. One month after discharge, her strength continued to be impaired, though improved and she still experienced pain.

COMMENTARY
Hospitalists must be equipped to recognize urgent and potentially reversible causes of neuropathy. The hospitalist should maintain a high index of suspicion for ischemia (either due to vasculitis or vascular compromise), traumatic nerve injury, nerve compression or entrapment, lymphoma or metastatic infiltration, hepatitis C with cryoglobulinemia, Guillain‐Barre syndrome and toxic exposures. Table 1 highlights important causes of mononeuropathy multiplex and summarizes associated findings and indicated diagnostic tests for specific evaluation.
| Diagnosis | Associated features | Specific evaluation |
|---|---|---|
| Axonal neuropathies | ||
| Ischemia (including vascular steal) | Poor arterial pulses, history of vascular surgery | Digital photoplethysmography, Doppler, angiography |
| Nerve compression and trauma | History of traumatic injury, mass, infection/abscess | MR neurography |
| Lymphoma or metastatic infiltration | History of known cancer, weight loss | PET, whole body CT, bone marrow biopsy |
| Vasculitis | Waxing and waning symptoms, association with connective tissue diseases, painful | CRP, ESR, Hepatitis C, cryoglobulins, ANA, ANCA, antibodies to SSA/SSB, ENA, eosinophil count, serum complement, SPEP/UPEP, RF, nerve biopsy |
| Neurosarcoidosis | Hilar lymphadenopathy, chronic cough | Chest CT, ACE, nerve biopsy |
| Lyme | Tick bite, erythema chronicum migrans | Lyme serology |
| Leprosy | Resident of southeast Asia, skin lesions | Skin smear for acid fast bacilli (mycobacterium), nerve biopsy |
| Demyelinating neuropathies | ||
| Lewis‐Sumner syndrome (i.e. asymmetric CIDP) | Relapsing remitting or chronic progressive course, areflexia | Lumbar puncture (increased spinal fluid protein common) |
| Hereditary neuropathy with liability to pressure palsy | Family history, recurrent episodes of entrapment/compression neuropathies | Genetic testing (deletion in the gene for peripheral myelin protein‐22) |
| Multifocal motor neuropathy with conduction block | Multifocal weakness in the distal arms/legs without sensory symptoms | Only motor abnormalities on nerve conduction including conduction block |
Another challenge for hospitalists is efficient evaluation of neuropathy. A systematic framework for creating a differential diagnosis and familiarity with available diagnostic tests is crucial. Hospitalists should be aware of three broad categories of neuropathy: mononeuropathy, polyneuropathy and mononeuropathy multiplex. Electrodiagnostic testing is essential to confirm the involved nerves and distinguishes axonal from demyelinating etiologies. Ultrasound, MR neurogram and, when indicated, nerve biopsy may be useful. Table 2 reviews these diagnostic tools as well as their indications and limitations.
| Test | Indications | Limitations |
|---|---|---|
| Electrodiagnostic Testing7 | Any peripheral neuropathy, muscle or neuromuscular junction disorder | Concomitant disease can reduce accuracy |
| Detects severity, chronicity, axonal v. demyelinating, diffuse v. focal, asymmetric v. symmetric | ||
| Electromyography (EMG) | EMG | EMG |
| Monopolar/concentric needle electrode inserted into the muscle belly | Differentiates axonal v. muscle damage; sensitive for even mild axon degeneration; localizes lesions. | Patient discomfort |
| Evaluates only motor fibers | ||
| Measures action potential at rest vs. during voluntary activation | Does not detect demyelination | |
| Might not be positive in first 21 days of symptoms | ||
| Nerve Conduction Studies (NCS) | NCS | NCS |
| Sensory | High sensitivity to differentiate axon loss from demyelination; localizes lesions. | Certain sensory responses lost with aging |
| Recording electrode placed over sensory nerve | ||
| Sensory nerve stimulated distally | Sensory localizes lesion to proximal vs. distal or to dorsal root ganglion | Less sensitive for mild axonal loss |
| Measures stimulus at proximal site | ||
| Motor | Motor amount of axonal loss | |
| Recording electrode placed over muscle belly | ||
| Motor nerve stimulated proximally | ||
| Measures stimulus at muscle | ||
| Specialized NCS tests | Specialized testing can identify radiculopathy, peripheral neuropathy, myasthenia gravis | |
| Ultrasound8 | ||
| Performed with typical ultrasound equipment | Suspected nerve entrapment | Doesn't show pathologic changes within nerves |
| Clinician must localize lesion for technician and explicitly guide test process | Evidence strongest for evaluation of median and ulnar nerves and Morton's neuroma | Difficult to visualize deep nerves or nerves surrounded by fat |
| Normal nerves appear tubular with linear echoes on a longitudinal scan; honeycomb on transverse scan | Detects lesions, nerve thickening, decreased echogenicity | Small field of view unless reconstructed |
| Results operator dependent | ||
| Less accurate than MRI for tumors | ||
| MRI9, 10 MR neurography | ||
| Standard MRI equipment | Concern regarding entrapment, trauma or mass lesions | Expense |
| Optimizes nerve resolution compared with surrounding tissues | To narrow differential when clinical and electrodiagnostic studies are inconclusive | Time (1560 minutes depending on scan requested) |
| When carpal tunnel syndrome does not respond to conservative management | ||
| Detects mass lesions compressing nerves, nerve enlargement and abnormal signal (neuritis, infiltration), increased signal in denervated muscle groups (once strength is 3 of 5). These changes can be seen as early at 4 days post trauma compared to 23 weeks on EMG. | ||
| Nerve Biopsy11 | ||
| Biopsy a nerve in the region of sensory loss or of a sensory nerve demonstrating electrophysiological abnormalities (decrease risk of adverse effects and to increase the likelihood of diagnosis | Rarely necessary | Painful, often for months |
| Concomitant muscle biopsy increases likelihood of diagnosing vasculitis or sarcoidosis | Use as last resort when evaluation not definitive | Risk of bleeding and infection |
| Greatest yield in multifocal neuropathies, or suspected amyloidotic polyneuropathy, vasculitis, sarcoidosis, lepromatous neuropathy, or rare hereditary disease where no genetic testing exists | ||
| Detects inflammation, amyloid deposits, tumor infiltration | ||
| Commonly targeted nerves include: LE sural, superficial peroneal, UE superficial radial |
Ischemic steal syndrome should be considered when neuropathy develops in a limb subsequent to arterio‐venous access procedures. Any vascular network, including the vertebral, carotid and coronary arteries, is at risk for steal. A feature common to all steal syndromes is the diversion of blood away from its original destination toward a lower pressure alternative. In some cases, this leads to a reversal of arterial flow and ischemia. Ischemic complications from AV access occur in 1‐9% of patients.1 Symptoms of steal can be mild, such as self‐limited dialysis induced pain, coldness and numbness, or severe, including severe pain, sensory and motor loss.2 If vascular compromise is sufficient, gangrene can ensue. Sensory deficits usually precede motor loss and the radial pulse is commonly absent or diminished. Other findings can include pallor of the fingers, muscle atrophy, resorption of the nail bed, and gangrene or ulcerations of the fingers. Risk factors for steal include atherosclerotic disease, female gender, age greater than 60 years, diabetes mellitus, previous surgery on the same arm, and use of the brachial artery as a donor.3 Symptoms of ischemic steal typically present within the first month after surgery, but can also be delayed; there is one report of a patient presenting one year postoperatively.4
Imaging studies such as doppler and angiography can be helpful in diagnosing ischemic steal syndrome. Fistulagrams may reveal a reversal of blood flow in the distal arm and hand, but these are reserved for cases with suspected proximal obstructive arterial disease.5 Vascular imaging studies can be misleading, however, as many patients will have physiologic but asymptomatic reversal of flow. Thus, a functional assessment such as digital plethysmography is recommended, especially in cases where clinical symptoms are vague. Digital pressures less than 60mmHg demonstrated 100% sensitivity and 87% specificity in one case control study of 40 patients.6 Treatment of ischemic steal syndrome is aimed at decreasing flow through the access shunt.
In conclusion, this case highlights the importance of timely and systematic evaluation of peripheral neuropathy in the hospital setting. Neuropathy with rapid progression and high potential for permanent damage necessitates early neurologic, or in this case, vascular consultation. Hospitalists should be facile in evaluating peripheral neuropathies and recognizing the appropriate indications for diagnostic tests and procedures.
A 60 year‐old woman with advanced kidney disease presented with one month of progressively worsening, sharp burning pain and decreased sensation in her left hand. Cold air exacerbated the pain. She noted decreasing ability to utilize her left fingers, a weakened grip and that the muscles in her hand looked smaller.
Localized sensory and motor symptoms in a discrete region of a single limb suggest neuropathy. The lack of symptoms in the face or ipsilateral lower extremity would dissuade a clinician from considering central etiologies; the presence of neuropathic pain is uncommon for cortical lesions. The involvement of motor and sensory nerves indicates peripheral nerve involvement.
The general approach to patients with peripheral neuropathy begins with identifying the neuropathy as a mononeuropathy (involving a single nerve), a polyneuropathy (symmetric involvement of multiple nerves) or a mononeuropathy multiplex (asymmetric involvement of multiple nerves). The patient, in this case, described subacute neuropathic pain, sensory loss, and weakness in her left hand in a distribution consistent with mononeuropathy or mononeuropathy multiplex.
This patient could have carpal tunnel syndrome given its prevalence in patients with advanced renal disease. The differential diagnosis is broad, however, and includes ulnar mononeuropathy, nerve ischemia due to vasculitis or vasculopathy, lower cervical radiculopathy (though the patient does not describe neck or radicular pain), lower brachial plexopathy, and complex regional pain syndrome.
The patient was diagnosed with advanced kidney disease one year ago when biopsy revealed focal segmental glomerulosclerosis secondary to lithium. Since her diagnosis, two grafts were placed in the left upper arm in anticipation of dialysis: the first, placed seven months prior to this admission, failed to mature; the second, placed one month prior to this admission, was complicated by bleeding at the fistula site and was not yet mature. Prior to this admission she had not required hemodialysis. Her past history included hypertension, dyslipidemia, hypothyroidism, secondary hyperparathyroidism, a remote history of cervical cancer (stage unknown, recent PAP smear negative), microcytosis and schizoaffective disorder. Her medications were furosemide, amlodipine, lisinopril, atenolol, atorvastatin, pantoprazole, olanzapine, levothyroxine, iron, darbepoetin, sevelamer, multivitamin and docusate.
Given the history of procedures in the left arm one should consider ischemic injury to the left median nerve. Other local complications could include compressive lesions such as an abscess or hematoma or direct nerve injury from the procedure. Carpal tunnel syndrome remains high on the differential due to its prevalence and because renal failure and hypothyroidism increase the risk of carpal tunnel syndrome.
A careful physical examination would localize the nerve or nerves involved. Examination findings that would be consistent with carpal tunnel syndrome include sensory loss in the distribution of the left median nerve, weakness of muscles innervated by the median nerve, including the abductor pollicus brevis and opponens muscles, and a Tinels and Phalens sign of the left wrist. A proximal median neuropathy resulting from ischemia or compression might also involve median‐innervated forearm muscle such as the pronator teres (forearm pronation) and flexor carpi radialis (hand flexion and abduction) muscles. Complex regional pain syndrome can be seen after a traumatic injury or surgery and is typified by severe neuropathic pain in a limb, often in combination with trophic changes in the affected extremity. A cervical radiculopathy would affect muscles supplied by the injured nerve root. For example, a C8 radiculopathy would affect all of the intrinsic hand muscles, the wrist and finger extensors, and the triceps brachii. A lower brachial plexopathy would present similarly to a lower cervical radiculopathy on clinical examination; electrodiagnostic evaluation would be necessary to distinguish these two disorders.
The patient appeared fatigued and her left hand was wrapped in blankets. Her vital signs were stable. There was no thyroid enlargement or lymphadenopathy. There was marked thenar, hypothenar and forearm atrophy on the left. Strength testing of her left hand demonstrated a grip strength of 2/5, finger extension and interosseous strength of 1/5, left wrist flexion and extension of 3/5; left biceps and triceps were 5/5. Sensation was mildly decreased to light touch, temperature, pain and proprioception throughout the left hand. Strength and sensation were intact in the right upper and bilateral lower extremities. Reflexes were 2+ throughout. The left radial pulse was diminished compared to the left ulnar that was 1+. The left hand was dry and cool. Laboratory evaluation revealed an elevated white blood cell count of 15,300/mm3, a hematocrit of 33 percent, mean corpuscular volume of 73 fL, and a normal platelet count. Electrolytes were consistent with advanced renal disease. The thyroid stimulating hormone level was normal.
The patient's examination reveals an injury to the sensory and motor components of the left ulnar, median, and distal radial nerves. The volar forearm wasting suggests proximal median motor nerve injury in the forearm. Because the triceps is spared, the weakness of finger and wrist extension implies distal radial motor nerve injury. The interosseous weakness is consistent with injury to the ulnar motor nerve. Weakness of grip and wrist flexion is less specific and it may be explained by injury to either the median or ulnar motor nerves. The diffuse sensory loss over the palmar and dorsal aspect of the left hand is consistent with neuropathic injury to the left median, ulnar, and radial sensory nerves. The preserved deep tendon reflexes are controlled by the musculocutaneous nerve (the biceps reflex) and branches arising from the proximal radial nerve (the triceps and brachioradialis reflexes), both of which are proximal to the apparent level of neuropathic insult.
Carpal tunnel syndrome is excluded since abnormalities extend beyond the median nerve distribution. The presentation is consistent with a mononeuropathy multiplex. Axonal etiologies of mononeuropathy multiplexincluding vasculitis, ischemia, neoplastic infiltration, and infectious etiologies such as Lyme diseaseare more common than demyelinating causes. Vasculitic neuropathy commonly involves the lower extremities and may have systemic symptoms, not present in this case. Neoplasm or other compressive lesions such as hematoma could explain these findings. Abscess must be considered given the leukocytosis. A lower brachial plexopathy, technically a mononeuropathy multiplex involving the proximal arm at the level of the brachial plexus, is also in the differential diagnosis. Another axonal disorder to consider would be neuralgic amyotrophy, an idiopathic form of acute brachial plexopathy associated with pain that is a complication of surgery that typically presents within a few hours to weeks of the procedure.
Demyelinating causes of mononeuropathy multiplex are less likely and include a variant of chronic inflammatory demyelinating polyneuropathy (Lewis‐Sumner syndrome) and hereditary neuropathy with liability to pressure palsies. Both processes are typically indolent and usually not painful, though the latter may present with fulminant numbness and weakness.
Nerve conduction and needle electromyography of the arm and cervical paraspinal muscles would differentiate axonal degeneration from demyelination. It would identify the affected nerves and any nerve root involvement. Given the concern for abscess or hematoma, MR neurography focused on the surgical site would also be important.
Electromyography and nerve conduction velocities demonstrated severe axonal loss of the left median, ulnar and distal radial sensory nerves consistent with acute denervation. A magnetic resonance neurogram following the course of these nerves revealed enlarged ulnar and median nerves with abnormal signal, but no compressive lesion (Fig. 1).

The electrodiagnostic testing is consistent with severe acute axonal injury to the left ulnar, median and radial nerves. This supports a diagnosis of mononeuropathy multiplex with axonal injury. Demyelinating causes are excluded at this point.
The MR neurogram demonstrates nonspecific nerve enlargement, which may be seen in ischemia, neoplastic processes (primary or metastatic), demyelinating disease, or, rarely, amyloidosis. Neoplastic involvement is unlikely in this case given the absence of a compressive mass lesion and the long segmental involvement of both the median and ulnar nerves. Compression from an abscess or hematoma is excluded. Neuralgic amyotrophy does not typically cause nerve enlargement.
Ischemia is the most likely diagnosis. Laboratory evaluation for vasculitis would be reasonable. Vasculitides that could present in this fashion include: polyarteritis nodosa, mixed connective tissue disease, Wegner's granulomatosis, Churg‐Strauss angiitis, Sjogren's, hepatitis C with serum cryoglobulinemia and possibly rheumatoid arthritis. Given the history of fistula placement in the affected limb, vascular sufficiency must be assessed.
Anti‐nuclear antibodies and anti‐neutrophilic cytoplasmic antibodies were negative, and a C‐ reactive protein was 5.9 mg/L (normal range, 0 to 10 mg/L). The erythrocyte sedimentation rate was 32mm/hr (normal range, 0 to 20) and serologies for hepatitis B and C were negative. There was no evidence of serum cryoglobulins.
A modestly elevated sedimentation rate and normal C‐reactive protein argue against a diagnosis of vasculitis. The negative ANA, ANCA, hepatitis serologies and cryoglobulin tests render unlikely the diagnoses of polyarteritis nodosa, Wegner's granulomatosis, Churg‐Strauss angiitis, or hepatitis related cryoglobulinemia. Eosinophilia (present in Churg‐Strauss), ENA (positive in mixed connective tissue disease), anti‐SSA and SSB (positive in Sjogren's) and a rheumatoid factor would round out this evaluation for vasculitis. A left radial sensory nerve biopsy could also be of value in diagnosing vasculitic neuropathy in this patient.
Given the evidence against vasculitis, the possibility of ischemia due to vascular insufficiency is concerning. Two ischemic complications of hemodialysis are known to cause distal multiple mononeuropathies. The first, ischemic monomelic neuropathy syndrome is seen almost exclusively in diabetics. It is characterized by the development of acute pain, weakness of the forearm and hand muscles, and sensory loss within minutes or hours of AV graft placement. Transient occlusion of the blood supply to the nerves of the forearm and hand induces nerve ischemia, but does not cause necrosis of other tissues. The nerve conduction findings in this patient are consistent with ischemic monomelic neuropathy syndrome. The delayed onset of her symptoms, however, makes this diagnosis unlikely.
The second ischemic complication of hemodialysis, and the likelier diagnosis, is vascular steal syndrome. This has a similar clinical and electrodiagnostic presentation to ischemic monomelic neuropathy syndrome, but has a latency period after surgery of days to months. Vascular steal occurs when a reversal of blood flow into the fistula steals flow from the palmar arch arteries and induces ischemia of the vasa nervorum. Vascular studies should be obtained urgently when this diagnosis is considered.
Evaluation of the arteriovenous graft and vascular surgery consultation were sought. Digital photoplethysmography revealed diminished waveforms in all fingers of the left hand. Arterial Doppler evaluation of the left upper extremity confirmed low‐velocity flow in the radial and ulnar arteries and failed to confirm flow in the brachial artery distal to the arteriovenous fistula. The patient underwent an angiogram of the left axillary and brachial arteries. There was normal flow until the level of the arteriovenous fistula but minimal flow distal to the fistula (Fig. 2).

The diminished waveforms on digital photoplethysmography are consistent with poor perfusion distally. The angiogram suggests that the multiple mononeuropathies are a consequence of ischemia from impaired blood flow.
Consulting the vascular surgeons in this setting is essential because restoring adequate blood flow to the affected nerves can prevent further loss of function. Prognosis is dependent on many factors, including the severity of the functional loss and the duration of the symptoms prior to the restoration of blood flow. The patient's severe weakness and substantial muscle atrophy, manifestations of axonal degeneration, imply a poorer prognosis for recovery of function.
Embolization of the arteriovenous fistula was performed by interventional radiology. Post embolization angiograms demonstrated improved peripheral arterial flow (Fig. 3). One day later, the patient's finger flexion and extension improved. She reported mildly decreased dysesthesias and on examination her fingers were warmer to the touch. One month after discharge, her strength continued to be impaired, though improved and she still experienced pain.

COMMENTARY
Hospitalists must be equipped to recognize urgent and potentially reversible causes of neuropathy. The hospitalist should maintain a high index of suspicion for ischemia (either due to vasculitis or vascular compromise), traumatic nerve injury, nerve compression or entrapment, lymphoma or metastatic infiltration, hepatitis C with cryoglobulinemia, Guillain‐Barre syndrome and toxic exposures. Table 1 highlights important causes of mononeuropathy multiplex and summarizes associated findings and indicated diagnostic tests for specific evaluation.
| Diagnosis | Associated features | Specific evaluation |
|---|---|---|
| Axonal neuropathies | ||
| Ischemia (including vascular steal) | Poor arterial pulses, history of vascular surgery | Digital photoplethysmography, Doppler, angiography |
| Nerve compression and trauma | History of traumatic injury, mass, infection/abscess | MR neurography |
| Lymphoma or metastatic infiltration | History of known cancer, weight loss | PET, whole body CT, bone marrow biopsy |
| Vasculitis | Waxing and waning symptoms, association with connective tissue diseases, painful | CRP, ESR, Hepatitis C, cryoglobulins, ANA, ANCA, antibodies to SSA/SSB, ENA, eosinophil count, serum complement, SPEP/UPEP, RF, nerve biopsy |
| Neurosarcoidosis | Hilar lymphadenopathy, chronic cough | Chest CT, ACE, nerve biopsy |
| Lyme | Tick bite, erythema chronicum migrans | Lyme serology |
| Leprosy | Resident of southeast Asia, skin lesions | Skin smear for acid fast bacilli (mycobacterium), nerve biopsy |
| Demyelinating neuropathies | ||
| Lewis‐Sumner syndrome (i.e. asymmetric CIDP) | Relapsing remitting or chronic progressive course, areflexia | Lumbar puncture (increased spinal fluid protein common) |
| Hereditary neuropathy with liability to pressure palsy | Family history, recurrent episodes of entrapment/compression neuropathies | Genetic testing (deletion in the gene for peripheral myelin protein‐22) |
| Multifocal motor neuropathy with conduction block | Multifocal weakness in the distal arms/legs without sensory symptoms | Only motor abnormalities on nerve conduction including conduction block |
Another challenge for hospitalists is efficient evaluation of neuropathy. A systematic framework for creating a differential diagnosis and familiarity with available diagnostic tests is crucial. Hospitalists should be aware of three broad categories of neuropathy: mononeuropathy, polyneuropathy and mononeuropathy multiplex. Electrodiagnostic testing is essential to confirm the involved nerves and distinguishes axonal from demyelinating etiologies. Ultrasound, MR neurogram and, when indicated, nerve biopsy may be useful. Table 2 reviews these diagnostic tools as well as their indications and limitations.
| Test | Indications | Limitations |
|---|---|---|
| Electrodiagnostic Testing7 | Any peripheral neuropathy, muscle or neuromuscular junction disorder | Concomitant disease can reduce accuracy |
| Detects severity, chronicity, axonal v. demyelinating, diffuse v. focal, asymmetric v. symmetric | ||
| Electromyography (EMG) | EMG | EMG |
| Monopolar/concentric needle electrode inserted into the muscle belly | Differentiates axonal v. muscle damage; sensitive for even mild axon degeneration; localizes lesions. | Patient discomfort |
| Evaluates only motor fibers | ||
| Measures action potential at rest vs. during voluntary activation | Does not detect demyelination | |
| Might not be positive in first 21 days of symptoms | ||
| Nerve Conduction Studies (NCS) | NCS | NCS |
| Sensory | High sensitivity to differentiate axon loss from demyelination; localizes lesions. | Certain sensory responses lost with aging |
| Recording electrode placed over sensory nerve | ||
| Sensory nerve stimulated distally | Sensory localizes lesion to proximal vs. distal or to dorsal root ganglion | Less sensitive for mild axonal loss |
| Measures stimulus at proximal site | ||
| Motor | Motor amount of axonal loss | |
| Recording electrode placed over muscle belly | ||
| Motor nerve stimulated proximally | ||
| Measures stimulus at muscle | ||
| Specialized NCS tests | Specialized testing can identify radiculopathy, peripheral neuropathy, myasthenia gravis | |
| Ultrasound8 | ||
| Performed with typical ultrasound equipment | Suspected nerve entrapment | Doesn't show pathologic changes within nerves |
| Clinician must localize lesion for technician and explicitly guide test process | Evidence strongest for evaluation of median and ulnar nerves and Morton's neuroma | Difficult to visualize deep nerves or nerves surrounded by fat |
| Normal nerves appear tubular with linear echoes on a longitudinal scan; honeycomb on transverse scan | Detects lesions, nerve thickening, decreased echogenicity | Small field of view unless reconstructed |
| Results operator dependent | ||
| Less accurate than MRI for tumors | ||
| MRI9, 10 MR neurography | ||
| Standard MRI equipment | Concern regarding entrapment, trauma or mass lesions | Expense |
| Optimizes nerve resolution compared with surrounding tissues | To narrow differential when clinical and electrodiagnostic studies are inconclusive | Time (1560 minutes depending on scan requested) |
| When carpal tunnel syndrome does not respond to conservative management | ||
| Detects mass lesions compressing nerves, nerve enlargement and abnormal signal (neuritis, infiltration), increased signal in denervated muscle groups (once strength is 3 of 5). These changes can be seen as early at 4 days post trauma compared to 23 weeks on EMG. | ||
| Nerve Biopsy11 | ||
| Biopsy a nerve in the region of sensory loss or of a sensory nerve demonstrating electrophysiological abnormalities (decrease risk of adverse effects and to increase the likelihood of diagnosis | Rarely necessary | Painful, often for months |
| Concomitant muscle biopsy increases likelihood of diagnosing vasculitis or sarcoidosis | Use as last resort when evaluation not definitive | Risk of bleeding and infection |
| Greatest yield in multifocal neuropathies, or suspected amyloidotic polyneuropathy, vasculitis, sarcoidosis, lepromatous neuropathy, or rare hereditary disease where no genetic testing exists | ||
| Detects inflammation, amyloid deposits, tumor infiltration | ||
| Commonly targeted nerves include: LE sural, superficial peroneal, UE superficial radial |
Ischemic steal syndrome should be considered when neuropathy develops in a limb subsequent to arterio‐venous access procedures. Any vascular network, including the vertebral, carotid and coronary arteries, is at risk for steal. A feature common to all steal syndromes is the diversion of blood away from its original destination toward a lower pressure alternative. In some cases, this leads to a reversal of arterial flow and ischemia. Ischemic complications from AV access occur in 1‐9% of patients.1 Symptoms of steal can be mild, such as self‐limited dialysis induced pain, coldness and numbness, or severe, including severe pain, sensory and motor loss.2 If vascular compromise is sufficient, gangrene can ensue. Sensory deficits usually precede motor loss and the radial pulse is commonly absent or diminished. Other findings can include pallor of the fingers, muscle atrophy, resorption of the nail bed, and gangrene or ulcerations of the fingers. Risk factors for steal include atherosclerotic disease, female gender, age greater than 60 years, diabetes mellitus, previous surgery on the same arm, and use of the brachial artery as a donor.3 Symptoms of ischemic steal typically present within the first month after surgery, but can also be delayed; there is one report of a patient presenting one year postoperatively.4
Imaging studies such as doppler and angiography can be helpful in diagnosing ischemic steal syndrome. Fistulagrams may reveal a reversal of blood flow in the distal arm and hand, but these are reserved for cases with suspected proximal obstructive arterial disease.5 Vascular imaging studies can be misleading, however, as many patients will have physiologic but asymptomatic reversal of flow. Thus, a functional assessment such as digital plethysmography is recommended, especially in cases where clinical symptoms are vague. Digital pressures less than 60mmHg demonstrated 100% sensitivity and 87% specificity in one case control study of 40 patients.6 Treatment of ischemic steal syndrome is aimed at decreasing flow through the access shunt.
In conclusion, this case highlights the importance of timely and systematic evaluation of peripheral neuropathy in the hospital setting. Neuropathy with rapid progression and high potential for permanent damage necessitates early neurologic, or in this case, vascular consultation. Hospitalists should be facile in evaluating peripheral neuropathies and recognizing the appropriate indications for diagnostic tests and procedures.
- .Upper limb ischemia after vascular access surgery: differential diagnosis and management.Sem Dial2000;13:312–315.
- ,,,,,.Steal syndrome complicating hemodialysis access.Cardiovascular Surg (London, England)1997;5:648–653.
- ,,,,,.Onset of arterial ‘steal’ following proximal angioaccess: immediate and delayed types.Nephrol Dial Transplant2003;18:2387–2390.
- ,,,,.Incidence and characteristics of patients with hand ischemia after hemodialysis access procedure.J Surg Res1998;74:8–10
- ,,,,,.Ischemic steal syndrome: a case series and review of current management.Curr Surg2006;63:130–135.
- ,,,.Use of digital pressure measurements for the diagnosis of AV access‐induced hand ischemia.Vasc Med2006;11:227–231.
- ,.Electrodiagnostic testing of nerves and muscles: when, why, and how to order.Cleve Clin J Med2005;72:37–48.
- ,.High‐resolution sonography of the peripheral nervous system—a review of the literature.Eur J Neurol2004;11:305–314.
- ,,, et al.Role of magnetic resonance imaging in entrapment and compressive neuropathy‐what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 2. Upper extremity.Eur Radiol2007;17:509–522.
- ,,,,,.The utility of magnetic resonance imaging in evaluating peripheral nerve disorders.Muscle Nerve2002;25:314–331.
- .Indications and usefulness of nerve biopsy.Arch Neurol2002;59:1532–1535.
- .Upper limb ischemia after vascular access surgery: differential diagnosis and management.Sem Dial2000;13:312–315.
- ,,,,,.Steal syndrome complicating hemodialysis access.Cardiovascular Surg (London, England)1997;5:648–653.
- ,,,,,.Onset of arterial ‘steal’ following proximal angioaccess: immediate and delayed types.Nephrol Dial Transplant2003;18:2387–2390.
- ,,,,.Incidence and characteristics of patients with hand ischemia after hemodialysis access procedure.J Surg Res1998;74:8–10
- ,,,,,.Ischemic steal syndrome: a case series and review of current management.Curr Surg2006;63:130–135.
- ,,,.Use of digital pressure measurements for the diagnosis of AV access‐induced hand ischemia.Vasc Med2006;11:227–231.
- ,.Electrodiagnostic testing of nerves and muscles: when, why, and how to order.Cleve Clin J Med2005;72:37–48.
- ,.High‐resolution sonography of the peripheral nervous system—a review of the literature.Eur J Neurol2004;11:305–314.
- ,,, et al.Role of magnetic resonance imaging in entrapment and compressive neuropathy‐what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 2. Upper extremity.Eur Radiol2007;17:509–522.
- ,,,,,.The utility of magnetic resonance imaging in evaluating peripheral nerve disorders.Muscle Nerve2002;25:314–331.
- .Indications and usefulness of nerve biopsy.Arch Neurol2002;59:1532–1535.