User login
Remote-Onset Alopecia Areata Attributed to Ipilimumab
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) is a key co-stimulatory receptor expressed on activated T cells that negatively regulates T-cell activation.1-3 It exerts its effects in part by the prevention of IL-2 transcription and inhibition of cell-cycle progression.4 Cytotoxic T-lymphocyte–associated antigen 4 also is expressed by a subset of CD25+CD4+ regulatory T cells (Tregs), where it plays a role in immune tolerance.5 Blockade has demonstrated antitumor activity as well as immune activation, and CTLA-4 dysregulation has been implicated in autoimmune diseases such as alopecia areata (AA).6
Ipilimumab is a fully humanized monoclonal antibody against CTLA-4 and one of a growing class of immune checkpoint inhibitor therapies for metastatic melanoma. Phase 2 and 3 clinical trials have shown an improved survival effect of ipilimumab in patients with advanced melanoma,7-10 with 3-year survival rates ranging from 20.8% to 46.5%.10,11 The US Food and Drug Administration approved ipilimumab in 2011 for treatment of unresectable or metastatic melanoma.12 The most common toxicities of ipilimumab are immune-related adverse effects (irAEs), which represent loss of tolerance to self-antigens.13 Immune-related adverse effects occur in 64.2% of patients,14 with severe or life-threatening irAEs in 17.8% of patients.14 Rates of irAEs appear dose dependent but consistent across increased doses.15 Cutaneous irAEs occur in more than 47% of patients16 and commonly manifest as pruritus with or without a diffuse morbilliform rash,10,17 though less common skin reactions, including vitiligo, vasculitis, and Stevens-Johnson syndrome/toxic epidermal necrolysis, have been documented.9,18
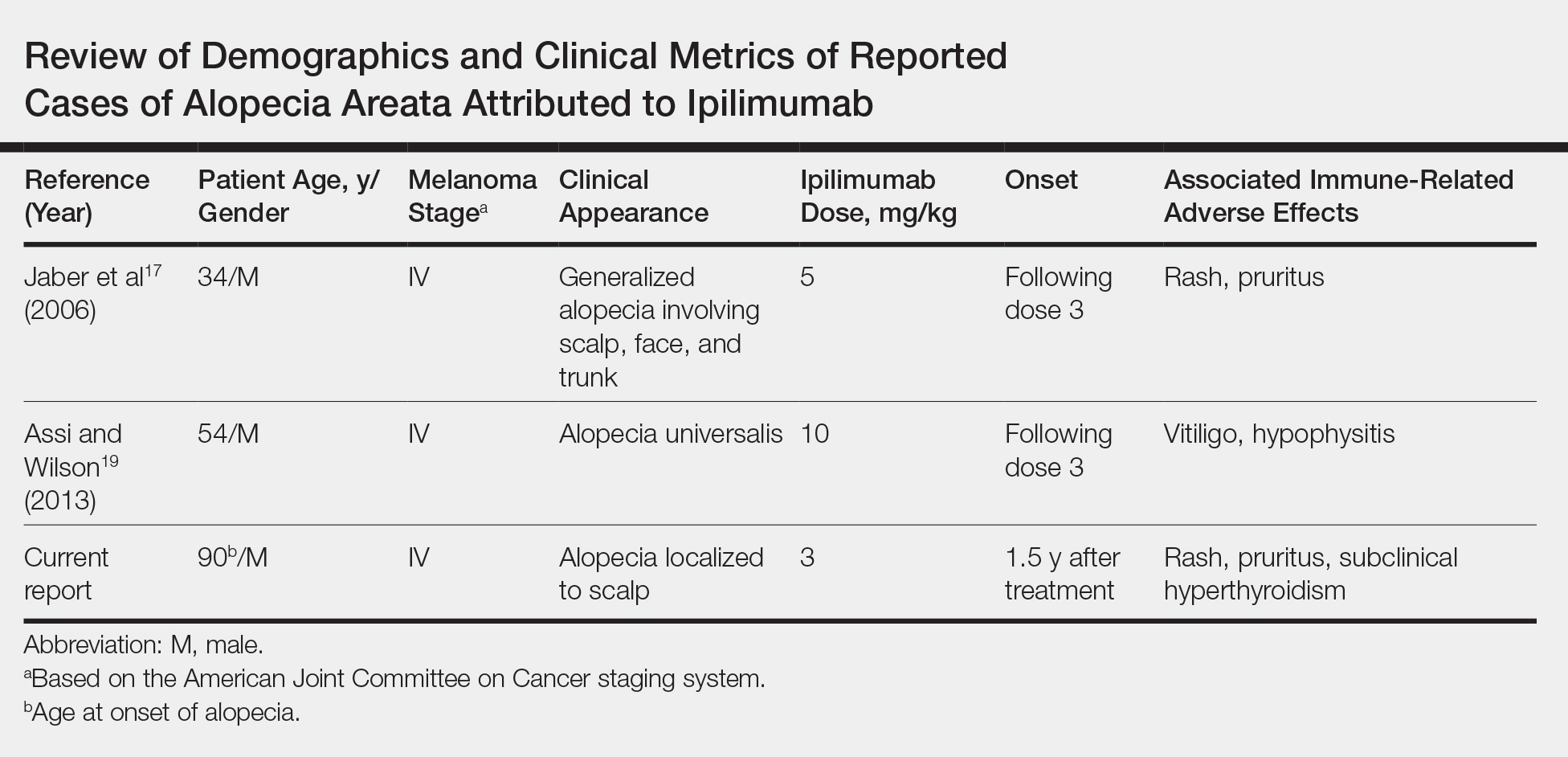
Generalized AA and its more widespread variant, alopecia universalis, have been reported as adverse effects of ipilimumab monotherapy in 2 prior cases in the English-language literature (Table).17,19 Alopecia areata also has been attributed to combination immune checkpoint inhibitor therapy.20,21 We report a case of AA attributable to ipilimumab monotherapy that was localized exclusively to the scalp and remote in onset following treatment.
Case Report
An 88-year-old man with pT3bpN3 nodular melanoma of the back demonstrated multiple lung metastases by positron emission tomography–computed tomography. Lactate dehydrogenase was within reference range, and his Eastern Cooperative Oncology Group performance status was 0 (fully active). One month later, he was started on ipilimumab 3 mg/kg intravenous infusion every 3 weeks for a total of 4 doses. At approximately week 6, his course was complicated by mild fatigue, a faintly erythematous morbilliform rash, and mild pruritus, with laboratory evidence of subclinical hyperthyroidism. Follow-up positron emission tomography–computed tomography at the conclusion of treatment demonstrated complete regression of previously noted hypermetabolic foci. His symptoms and subclinical hyperthyroidism resolved several months later.
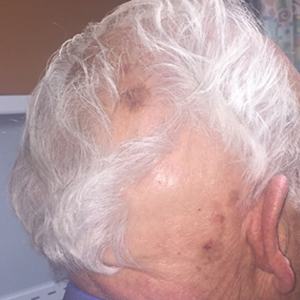
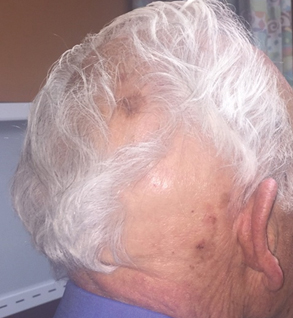
Seventeen months after completion of ipilimumab therapy (at age 90 years), the patient’s barber noted new-onset hair loss on the right occipital scalp. Physical examination demonstrated a well-circumscribed patch of nonscarring alopecia (approximately 6 cm) that was clinically consistent with AA (Figure). There were no associated symptoms or other involved areas of hair loss. He denied any personal or family history of AA. The patient’s melanoma has remained in remission to date.
Comment
This case is unique in that AA was localized to a single circumscribed patch on the scalp and occurred nearly 1.5 years after treatment with ipilimumab, which may indicate a robust blockade of CTLA-4 given the remote development of autoimmunity in the setting of persistent remission of melanoma. Although the appearance of AA may be coincidental, onset at 90 years of age would be unusual. The mean age of onset of AA has been reported between 25.2 and 36.3 years,22,23 and its incidence in men older than 60 years is only 6.4 per 100,000 person-years.24
Although AA is a rare irAE of CTLA-4 blockade, the disease has been increasingly linked to CTLA-4 dysregulation in both animal models and humans.6,25,26 A genome-wide association study of 1054 patients with AA and 3278 controls implicated several genes controlling activation and proliferation of Tregs, including CTLA-4.27 More specifically, single-nucleotide polymorphisms of the CTLA-4 gene were found to be associated with AA in a study of 1196 unrelated patients and 1280 controls,28 and Megiorni et al
Given the role of CTLA-4 dysregulation in the pathogenesis of AA, the very low rates of AA in ipilimumab are somewhat surprising, which may represent a reporting bias. Alternatively, there may be sufficient Treg activity to prevent high rates of AA at a lower ipilimumab dose of 3 mg/kg but insufficient activity to prevent development of other irAEs. With US Food and Drug Administration approval of ipilimumab at a higher dose of 10 mg/kg for use as adjuvant therapy for stage III melanomas,12 less common cutaneous irAEs such as AA may be seen with increased frequency. Clinicians planning ipilimumab therapy should discuss this side effect and other potential irAEs with their patients before initiation of treatment.
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267-270.
- Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143-155.
- Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. 2015;125:3377-3383.
- Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813-5820.
- Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310.
- Carroll JM, McElwee KJ, E King L, et al. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392-402.
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591-5598.
- O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-1717.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
- Yervoy (ipilimumab)[package insert]. Princeton, NJ: Bristol-Myers Squibb; 2019.
- Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864-872.
- Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma [abstract]. J Clin Oncol. 2011;29(suppl):8583.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155-164.
- Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9:277-286.
- Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166-172.
- Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:E537545.
- Assi H, Wilson KS. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: two illustrative cases. Curr Oncol. 2013;20:E165-E169.
- Zarbo A, Belum VR, Sibaud V, et al. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br J Dermatol. 2017;176:1649-1652.
- Lakhmiri M, Cavelier-Balloy B, Lacoste C, et al. Nivolumab-induced alopecia areata: a reversible factor of good prognosis? JAAD Case Rep. 2018;4:761-765.
- Tan E, Tay YK, Goh CL, et al. The pattern and profile of alopecia areata in Singapore–a study of 219 Asians. Int J Dermatol. 2002;41:748-753.
- Goh C, Finkel M, Christos PJ, et al. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20:1055-1060.
- Mirzoyev SA, Schrum AG, Davis MD, et al. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Zöller M, McElwee KJ, Engel P, et al. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983-992.
- Zöller M, McElwee KJ, Vitacolonna M, et al. The progressive state, in contrast to the stable or regressive state of alopecia areata, is reflected in peripheral blood mononuclear cells. Exp Dermatol. 2004;13:435-444.
- Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113-117.
- John KK, Brockschmidt FF, Redler S, et al. Genetic variants in CTLA4 are strongly associated with alopecia areata. J Invest Dermatol. 2011;131:1169-1172.
- Megiorni F, Mora B, Maxia C, et al. Cytotoxic T-lymphocyte antigen 4 (CTLA4) +49AG and CT60 gene polymorphisms in alopecia areata: a case-control association study in the Italian population. Arch Dermatol Res. 2013;305:665-670
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) is a key co-stimulatory receptor expressed on activated T cells that negatively regulates T-cell activation.1-3 It exerts its effects in part by the prevention of IL-2 transcription and inhibition of cell-cycle progression.4 Cytotoxic T-lymphocyte–associated antigen 4 also is expressed by a subset of CD25+CD4+ regulatory T cells (Tregs), where it plays a role in immune tolerance.5 Blockade has demonstrated antitumor activity as well as immune activation, and CTLA-4 dysregulation has been implicated in autoimmune diseases such as alopecia areata (AA).6
Ipilimumab is a fully humanized monoclonal antibody against CTLA-4 and one of a growing class of immune checkpoint inhibitor therapies for metastatic melanoma. Phase 2 and 3 clinical trials have shown an improved survival effect of ipilimumab in patients with advanced melanoma,7-10 with 3-year survival rates ranging from 20.8% to 46.5%.10,11 The US Food and Drug Administration approved ipilimumab in 2011 for treatment of unresectable or metastatic melanoma.12 The most common toxicities of ipilimumab are immune-related adverse effects (irAEs), which represent loss of tolerance to self-antigens.13 Immune-related adverse effects occur in 64.2% of patients,14 with severe or life-threatening irAEs in 17.8% of patients.14 Rates of irAEs appear dose dependent but consistent across increased doses.15 Cutaneous irAEs occur in more than 47% of patients16 and commonly manifest as pruritus with or without a diffuse morbilliform rash,10,17 though less common skin reactions, including vitiligo, vasculitis, and Stevens-Johnson syndrome/toxic epidermal necrolysis, have been documented.9,18
Generalized AA and its more widespread variant, alopecia universalis, have been reported as adverse effects of ipilimumab monotherapy in 2 prior cases in the English-language literature (Table).17,19 Alopecia areata also has been attributed to combination immune checkpoint inhibitor therapy.20,21 We report a case of AA attributable to ipilimumab monotherapy that was localized exclusively to the scalp and remote in onset following treatment.

Case Report
An 88-year-old man with pT3bpN3 nodular melanoma of the back demonstrated multiple lung metastases by positron emission tomography–computed tomography. Lactate dehydrogenase was within reference range, and his Eastern Cooperative Oncology Group performance status was 0 (fully active). One month later, he was started on ipilimumab 3 mg/kg intravenous infusion every 3 weeks for a total of 4 doses. At approximately week 6, his course was complicated by mild fatigue, a faintly erythematous morbilliform rash, and mild pruritus, with laboratory evidence of subclinical hyperthyroidism. Follow-up positron emission tomography–computed tomography at the conclusion of treatment demonstrated complete regression of previously noted hypermetabolic foci. His symptoms and subclinical hyperthyroidism resolved several months later.
Seventeen months after completion of ipilimumab therapy (at age 90 years), the patient’s barber noted new-onset hair loss on the right occipital scalp. Physical examination demonstrated a well-circumscribed patch of nonscarring alopecia (approximately 6 cm) that was clinically consistent with AA (Figure). There were no associated symptoms or other involved areas of hair loss. He denied any personal or family history of AA. The patient’s melanoma has remained in remission to date.

Comment
This case is unique in that AA was localized to a single circumscribed patch on the scalp and occurred nearly 1.5 years after treatment with ipilimumab, which may indicate a robust blockade of CTLA-4 given the remote development of autoimmunity in the setting of persistent remission of melanoma. Although the appearance of AA may be coincidental, onset at 90 years of age would be unusual. The mean age of onset of AA has been reported between 25.2 and 36.3 years,22,23 and its incidence in men older than 60 years is only 6.4 per 100,000 person-years.24
Although AA is a rare irAE of CTLA-4 blockade, the disease has been increasingly linked to CTLA-4 dysregulation in both animal models and humans.6,25,26 A genome-wide association study of 1054 patients with AA and 3278 controls implicated several genes controlling activation and proliferation of Tregs, including CTLA-4.27 More specifically, single-nucleotide polymorphisms of the CTLA-4 gene were found to be associated with AA in a study of 1196 unrelated patients and 1280 controls,28 and Megiorni et al
Given the role of CTLA-4 dysregulation in the pathogenesis of AA, the very low rates of AA in ipilimumab are somewhat surprising, which may represent a reporting bias. Alternatively, there may be sufficient Treg activity to prevent high rates of AA at a lower ipilimumab dose of 3 mg/kg but insufficient activity to prevent development of other irAEs. With US Food and Drug Administration approval of ipilimumab at a higher dose of 10 mg/kg for use as adjuvant therapy for stage III melanomas,12 less common cutaneous irAEs such as AA may be seen with increased frequency. Clinicians planning ipilimumab therapy should discuss this side effect and other potential irAEs with their patients before initiation of treatment.
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) is a key co-stimulatory receptor expressed on activated T cells that negatively regulates T-cell activation.1-3 It exerts its effects in part by the prevention of IL-2 transcription and inhibition of cell-cycle progression.4 Cytotoxic T-lymphocyte–associated antigen 4 also is expressed by a subset of CD25+CD4+ regulatory T cells (Tregs), where it plays a role in immune tolerance.5 Blockade has demonstrated antitumor activity as well as immune activation, and CTLA-4 dysregulation has been implicated in autoimmune diseases such as alopecia areata (AA).6
Ipilimumab is a fully humanized monoclonal antibody against CTLA-4 and one of a growing class of immune checkpoint inhibitor therapies for metastatic melanoma. Phase 2 and 3 clinical trials have shown an improved survival effect of ipilimumab in patients with advanced melanoma,7-10 with 3-year survival rates ranging from 20.8% to 46.5%.10,11 The US Food and Drug Administration approved ipilimumab in 2011 for treatment of unresectable or metastatic melanoma.12 The most common toxicities of ipilimumab are immune-related adverse effects (irAEs), which represent loss of tolerance to self-antigens.13 Immune-related adverse effects occur in 64.2% of patients,14 with severe or life-threatening irAEs in 17.8% of patients.14 Rates of irAEs appear dose dependent but consistent across increased doses.15 Cutaneous irAEs occur in more than 47% of patients16 and commonly manifest as pruritus with or without a diffuse morbilliform rash,10,17 though less common skin reactions, including vitiligo, vasculitis, and Stevens-Johnson syndrome/toxic epidermal necrolysis, have been documented.9,18
Generalized AA and its more widespread variant, alopecia universalis, have been reported as adverse effects of ipilimumab monotherapy in 2 prior cases in the English-language literature (Table).17,19 Alopecia areata also has been attributed to combination immune checkpoint inhibitor therapy.20,21 We report a case of AA attributable to ipilimumab monotherapy that was localized exclusively to the scalp and remote in onset following treatment.

Case Report
An 88-year-old man with pT3bpN3 nodular melanoma of the back demonstrated multiple lung metastases by positron emission tomography–computed tomography. Lactate dehydrogenase was within reference range, and his Eastern Cooperative Oncology Group performance status was 0 (fully active). One month later, he was started on ipilimumab 3 mg/kg intravenous infusion every 3 weeks for a total of 4 doses. At approximately week 6, his course was complicated by mild fatigue, a faintly erythematous morbilliform rash, and mild pruritus, with laboratory evidence of subclinical hyperthyroidism. Follow-up positron emission tomography–computed tomography at the conclusion of treatment demonstrated complete regression of previously noted hypermetabolic foci. His symptoms and subclinical hyperthyroidism resolved several months later.
Seventeen months after completion of ipilimumab therapy (at age 90 years), the patient’s barber noted new-onset hair loss on the right occipital scalp. Physical examination demonstrated a well-circumscribed patch of nonscarring alopecia (approximately 6 cm) that was clinically consistent with AA (Figure). There were no associated symptoms or other involved areas of hair loss. He denied any personal or family history of AA. The patient’s melanoma has remained in remission to date.

Comment
This case is unique in that AA was localized to a single circumscribed patch on the scalp and occurred nearly 1.5 years after treatment with ipilimumab, which may indicate a robust blockade of CTLA-4 given the remote development of autoimmunity in the setting of persistent remission of melanoma. Although the appearance of AA may be coincidental, onset at 90 years of age would be unusual. The mean age of onset of AA has been reported between 25.2 and 36.3 years,22,23 and its incidence in men older than 60 years is only 6.4 per 100,000 person-years.24
Although AA is a rare irAE of CTLA-4 blockade, the disease has been increasingly linked to CTLA-4 dysregulation in both animal models and humans.6,25,26 A genome-wide association study of 1054 patients with AA and 3278 controls implicated several genes controlling activation and proliferation of Tregs, including CTLA-4.27 More specifically, single-nucleotide polymorphisms of the CTLA-4 gene were found to be associated with AA in a study of 1196 unrelated patients and 1280 controls,28 and Megiorni et al
Given the role of CTLA-4 dysregulation in the pathogenesis of AA, the very low rates of AA in ipilimumab are somewhat surprising, which may represent a reporting bias. Alternatively, there may be sufficient Treg activity to prevent high rates of AA at a lower ipilimumab dose of 3 mg/kg but insufficient activity to prevent development of other irAEs. With US Food and Drug Administration approval of ipilimumab at a higher dose of 10 mg/kg for use as adjuvant therapy for stage III melanomas,12 less common cutaneous irAEs such as AA may be seen with increased frequency. Clinicians planning ipilimumab therapy should discuss this side effect and other potential irAEs with their patients before initiation of treatment.
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267-270.
- Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143-155.
- Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. 2015;125:3377-3383.
- Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813-5820.
- Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310.
- Carroll JM, McElwee KJ, E King L, et al. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392-402.
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591-5598.
- O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-1717.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
- Yervoy (ipilimumab)[package insert]. Princeton, NJ: Bristol-Myers Squibb; 2019.
- Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864-872.
- Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma [abstract]. J Clin Oncol. 2011;29(suppl):8583.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155-164.
- Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9:277-286.
- Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166-172.
- Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:E537545.
- Assi H, Wilson KS. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: two illustrative cases. Curr Oncol. 2013;20:E165-E169.
- Zarbo A, Belum VR, Sibaud V, et al. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br J Dermatol. 2017;176:1649-1652.
- Lakhmiri M, Cavelier-Balloy B, Lacoste C, et al. Nivolumab-induced alopecia areata: a reversible factor of good prognosis? JAAD Case Rep. 2018;4:761-765.
- Tan E, Tay YK, Goh CL, et al. The pattern and profile of alopecia areata in Singapore–a study of 219 Asians. Int J Dermatol. 2002;41:748-753.
- Goh C, Finkel M, Christos PJ, et al. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20:1055-1060.
- Mirzoyev SA, Schrum AG, Davis MD, et al. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Zöller M, McElwee KJ, Engel P, et al. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983-992.
- Zöller M, McElwee KJ, Vitacolonna M, et al. The progressive state, in contrast to the stable or regressive state of alopecia areata, is reflected in peripheral blood mononuclear cells. Exp Dermatol. 2004;13:435-444.
- Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113-117.
- John KK, Brockschmidt FF, Redler S, et al. Genetic variants in CTLA4 are strongly associated with alopecia areata. J Invest Dermatol. 2011;131:1169-1172.
- Megiorni F, Mora B, Maxia C, et al. Cytotoxic T-lymphocyte antigen 4 (CTLA4) +49AG and CT60 gene polymorphisms in alopecia areata: a case-control association study in the Italian population. Arch Dermatol Res. 2013;305:665-670
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267-270.
- Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143-155.
- Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. 2015;125:3377-3383.
- Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813-5820.
- Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310.
- Carroll JM, McElwee KJ, E King L, et al. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392-402.
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591-5598.
- O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-1717.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
- Yervoy (ipilimumab)[package insert]. Princeton, NJ: Bristol-Myers Squibb; 2019.
- Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864-872.
- Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma [abstract]. J Clin Oncol. 2011;29(suppl):8583.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155-164.
- Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9:277-286.
- Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166-172.
- Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:E537545.
- Assi H, Wilson KS. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: two illustrative cases. Curr Oncol. 2013;20:E165-E169.
- Zarbo A, Belum VR, Sibaud V, et al. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br J Dermatol. 2017;176:1649-1652.
- Lakhmiri M, Cavelier-Balloy B, Lacoste C, et al. Nivolumab-induced alopecia areata: a reversible factor of good prognosis? JAAD Case Rep. 2018;4:761-765.
- Tan E, Tay YK, Goh CL, et al. The pattern and profile of alopecia areata in Singapore–a study of 219 Asians. Int J Dermatol. 2002;41:748-753.
- Goh C, Finkel M, Christos PJ, et al. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20:1055-1060.
- Mirzoyev SA, Schrum AG, Davis MD, et al. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Zöller M, McElwee KJ, Engel P, et al. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983-992.
- Zöller M, McElwee KJ, Vitacolonna M, et al. The progressive state, in contrast to the stable or regressive state of alopecia areata, is reflected in peripheral blood mononuclear cells. Exp Dermatol. 2004;13:435-444.
- Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113-117.
- John KK, Brockschmidt FF, Redler S, et al. Genetic variants in CTLA4 are strongly associated with alopecia areata. J Invest Dermatol. 2011;131:1169-1172.
- Megiorni F, Mora B, Maxia C, et al. Cytotoxic T-lymphocyte antigen 4 (CTLA4) +49AG and CT60 gene polymorphisms in alopecia areata: a case-control association study in the Italian population. Arch Dermatol Res. 2013;305:665-670
Practice Points
- Cutaneous immune-related adverse effects (irAEs) are among the most common adverse effects of ipilimumab, a fully humanized monoclonal antibody directed against cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) used to treat advanced-stage melanoma.
- Alopecia areata is a rarely reported irAE, but its connection to CTLA-4 dysregulation may mean that clinicians see an increased incidence at higher ipilimumab doses.