User login
Two free, comprehensive drug reference apps for your practice
I understand that you as an ObGyn do not have the time or bandwidth to “vet” the available mobile apps for your practice. However, that does not mean you need to forgo using apps that could make your clinical life a little easier if possible. In this continuation of my “APP review” series, I focus on drug reference apps, which generally include the names of drugs, their indications, dosages, pharmacology, drug-drug interactions, contraindications, cost, and identifying characteristics.1 Drug reference apps, along with medical calculator and disease diagnosis apps, are reported as most useful by health care professionals and medical or nursing students.1 Drug reference apps are particularly popular among residents and medical students as the apps allow for rapid decision making.2
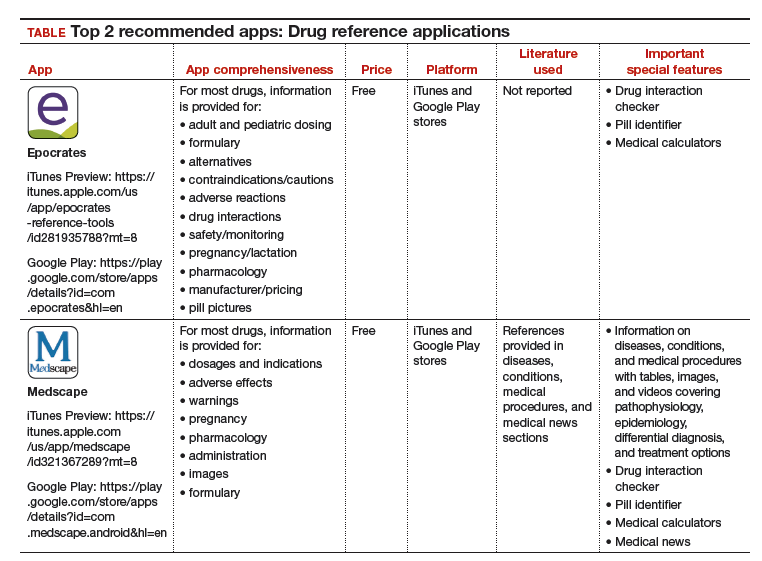
I have selected 2 drug reference apps—Epocrates and Medscape—to report here as both of these apps are free and are the only apps that appear in independent comprehensive studies.1,3 I particularly like Epocrates’ pill identification function for those patients who have forgotten the name of the medication they use but have the actual pill with them. I find Medscape’s additional information on diseases, conditions, and medical procedures especially useful for the times I have forgotten the condition that the medication is indicated for.
The recommended apps are listed in the TABLE alphabetically and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 Visit the OBG Management website to download the apps featured.
Watch for my next column in which I will recommend, according to APPLI, the top apps for patients to use to track their menstrual cycles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mosa AS, Yoo I, Sheets L. A systematic review of health care apps for smartphones. BMC Med Inform Decis Mak. 2012;12:67.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related app use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Med Inform Decis Mak. 2012;12:121.
- Aungst TD. Medical applications for pharmacists using mobile devices. Ann Pharmacother. 2013;47(7-8):1088-1095.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
I understand that you as an ObGyn do not have the time or bandwidth to “vet” the available mobile apps for your practice. However, that does not mean you need to forgo using apps that could make your clinical life a little easier if possible. In this continuation of my “APP review” series, I focus on drug reference apps, which generally include the names of drugs, their indications, dosages, pharmacology, drug-drug interactions, contraindications, cost, and identifying characteristics.1 Drug reference apps, along with medical calculator and disease diagnosis apps, are reported as most useful by health care professionals and medical or nursing students.1 Drug reference apps are particularly popular among residents and medical students as the apps allow for rapid decision making.2
I have selected 2 drug reference apps—Epocrates and Medscape—to report here as both of these apps are free and are the only apps that appear in independent comprehensive studies.1,3 I particularly like Epocrates’ pill identification function for those patients who have forgotten the name of the medication they use but have the actual pill with them. I find Medscape’s additional information on diseases, conditions, and medical procedures especially useful for the times I have forgotten the condition that the medication is indicated for.
The recommended apps are listed in the TABLE alphabetically and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 Visit the OBG Management website to download the apps featured.

Watch for my next column in which I will recommend, according to APPLI, the top apps for patients to use to track their menstrual cycles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
I understand that you as an ObGyn do not have the time or bandwidth to “vet” the available mobile apps for your practice. However, that does not mean you need to forgo using apps that could make your clinical life a little easier if possible. In this continuation of my “APP review” series, I focus on drug reference apps, which generally include the names of drugs, their indications, dosages, pharmacology, drug-drug interactions, contraindications, cost, and identifying characteristics.1 Drug reference apps, along with medical calculator and disease diagnosis apps, are reported as most useful by health care professionals and medical or nursing students.1 Drug reference apps are particularly popular among residents and medical students as the apps allow for rapid decision making.2
I have selected 2 drug reference apps—Epocrates and Medscape—to report here as both of these apps are free and are the only apps that appear in independent comprehensive studies.1,3 I particularly like Epocrates’ pill identification function for those patients who have forgotten the name of the medication they use but have the actual pill with them. I find Medscape’s additional information on diseases, conditions, and medical procedures especially useful for the times I have forgotten the condition that the medication is indicated for.
The recommended apps are listed in the TABLE alphabetically and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 Visit the OBG Management website to download the apps featured.

Watch for my next column in which I will recommend, according to APPLI, the top apps for patients to use to track their menstrual cycles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mosa AS, Yoo I, Sheets L. A systematic review of health care apps for smartphones. BMC Med Inform Decis Mak. 2012;12:67.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related app use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Med Inform Decis Mak. 2012;12:121.
- Aungst TD. Medical applications for pharmacists using mobile devices. Ann Pharmacother. 2013;47(7-8):1088-1095.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Mosa AS, Yoo I, Sheets L. A systematic review of health care apps for smartphones. BMC Med Inform Decis Mak. 2012;12:67.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related app use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Med Inform Decis Mak. 2012;12:121.
- Aungst TD. Medical applications for pharmacists using mobile devices. Ann Pharmacother. 2013;47(7-8):1088-1095.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
Three good apps for calculating the date of delivery
Technology has changed--and continues to change--the practice of medicine. Health care providers access word processing programs, e-mail, and electronic medical records using desktop and laptop computers. Now, providers are accessing these same tools with handheld devices such as smartphones, tablets, and "phablets" (a class of mobile devices designed to combine the form of a smartphone and a tablet).
Critical to the popularity and functionality of handheld devices are mobile applications, also known as "apps." An app is a self-contained program or piece of software designed to run on handheld devices to perform a specific purpose. App overload and app inaccuracy, however, are major problems. Health care providers do not have the time to search through thousands of medical apps in app stores to find specialty-related apps that might be useful in their practice--or to check the accuracy of those apps.
Vetted apps for ObGyns
My team's research has focused on identifying apps for ObGyns to use in clinical practice.1 In the process, we have developed the APPLICATIONS scoring system, which contains objective and subjective components to help differentiate among the accurate apps.2 This new quarterly "App review" series in OBG Management will showcase recommended apps for the busy ObGyn in the hope of improving work efficiency and the provider-patient relationship.
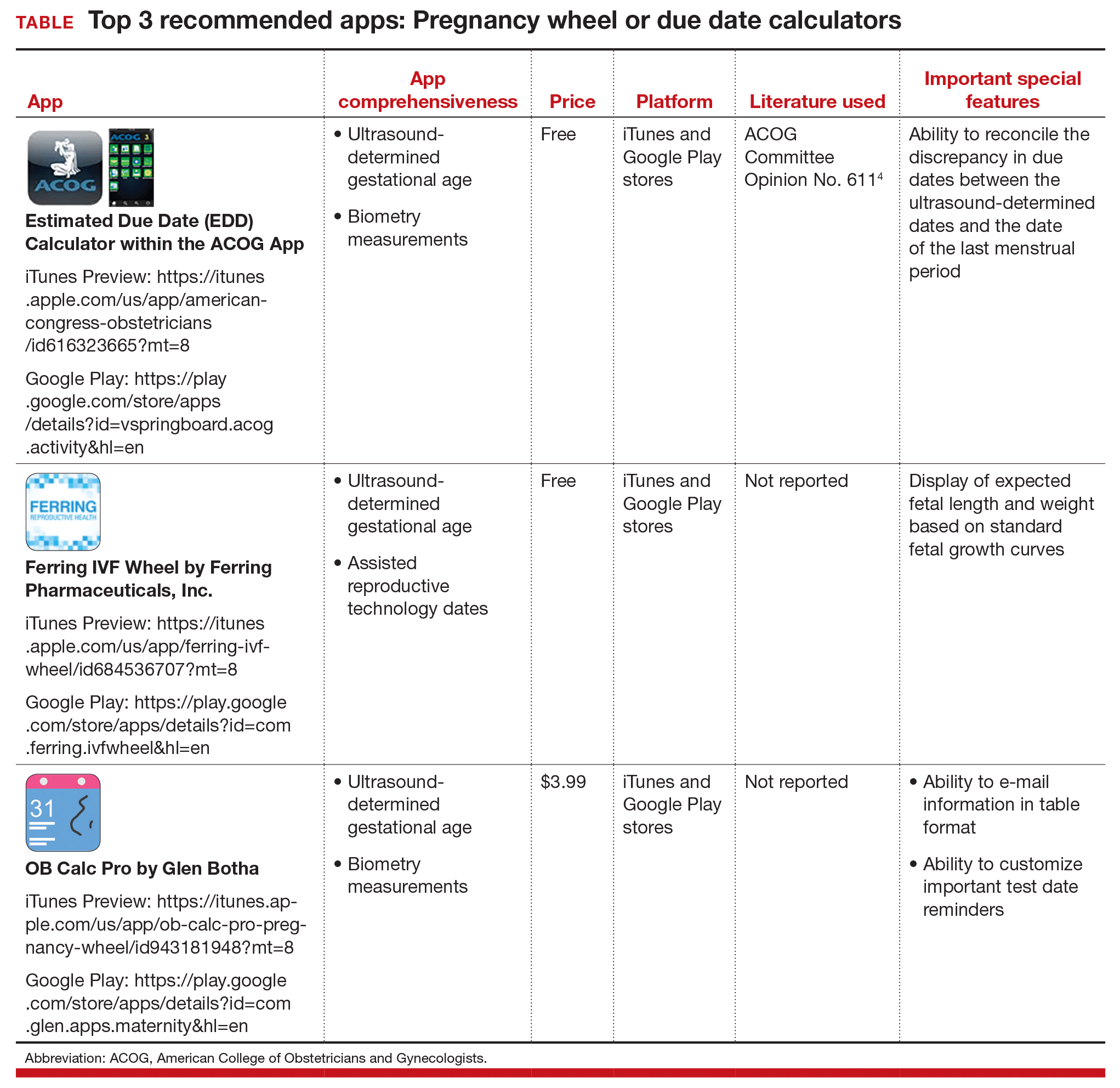
First up: Apps for calculating the date of delivery. This first app review focuses on pregnancy wheels, or due date calculators. Calculator apps are preferred over other types of apps such as procedure/case documentation apps, as providers use smartphones at point of care to allow rapid decision making.3 Calculating the estimated date of delivery (EDD) and gestational age (GA) is an important, vital task for providers of obstetric care. In fact, new guidelines for calculating EDD were recently developed by the American College of Obstetricians and Gynecologists (ACOG), the American Institute of Ultrasound in Medicine (AIUM), and the Society for Maternal-Fetal Medicine (SMFM).4 Notably, pregnancy wheel apps are more accurate than paper wheels.5 My team checked the accuracy of the pregnancy wheel apps by applying strict criteria to ensure the correct EDD and GA and then scored them in a systematic, nonbiased, conflict-of-interest-free manner.2
Related article:
Elective induction of labor at 39 (vs 41) weeks: Caveats and considerations
The TABLE below lists the top 3 recommended pregnancy wheel or due date calculator apps vetted by our research. The apps are listed alphabetically, and details for each app are provided based on a shortened version of the APPLICATIONS scoring system, APPLI--app comprehensiveness, price, platform, literature use, and important special features.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Farag S, Chyjek K, Chen KT. Identification of iPhone and iPad applications for obstetrics and gynecology providers. Obstet Gynecol. 2014;124(5):941-945.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Inform Decis Mak. 2012;12:121.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 611. Method for estimating due date. Obstet Gynecol. 2014;124(4):863-866.
- Chambliss LR, Clark SL. Paper gestational age wheels are generally inaccurate. Am J Obstet Gynecol. 2014;210(2):145.e1-e4.
Technology has changed--and continues to change--the practice of medicine. Health care providers access word processing programs, e-mail, and electronic medical records using desktop and laptop computers. Now, providers are accessing these same tools with handheld devices such as smartphones, tablets, and "phablets" (a class of mobile devices designed to combine the form of a smartphone and a tablet).
Critical to the popularity and functionality of handheld devices are mobile applications, also known as "apps." An app is a self-contained program or piece of software designed to run on handheld devices to perform a specific purpose. App overload and app inaccuracy, however, are major problems. Health care providers do not have the time to search through thousands of medical apps in app stores to find specialty-related apps that might be useful in their practice--or to check the accuracy of those apps.
Vetted apps for ObGyns
My team's research has focused on identifying apps for ObGyns to use in clinical practice.1 In the process, we have developed the APPLICATIONS scoring system, which contains objective and subjective components to help differentiate among the accurate apps.2 This new quarterly "App review" series in OBG Management will showcase recommended apps for the busy ObGyn in the hope of improving work efficiency and the provider-patient relationship.
First up: Apps for calculating the date of delivery. This first app review focuses on pregnancy wheels, or due date calculators. Calculator apps are preferred over other types of apps such as procedure/case documentation apps, as providers use smartphones at point of care to allow rapid decision making.3 Calculating the estimated date of delivery (EDD) and gestational age (GA) is an important, vital task for providers of obstetric care. In fact, new guidelines for calculating EDD were recently developed by the American College of Obstetricians and Gynecologists (ACOG), the American Institute of Ultrasound in Medicine (AIUM), and the Society for Maternal-Fetal Medicine (SMFM).4 Notably, pregnancy wheel apps are more accurate than paper wheels.5 My team checked the accuracy of the pregnancy wheel apps by applying strict criteria to ensure the correct EDD and GA and then scored them in a systematic, nonbiased, conflict-of-interest-free manner.2
Related article:
Elective induction of labor at 39 (vs 41) weeks: Caveats and considerations
The TABLE below lists the top 3 recommended pregnancy wheel or due date calculator apps vetted by our research. The apps are listed alphabetically, and details for each app are provided based on a shortened version of the APPLICATIONS scoring system, APPLI--app comprehensiveness, price, platform, literature use, and important special features.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Technology has changed--and continues to change--the practice of medicine. Health care providers access word processing programs, e-mail, and electronic medical records using desktop and laptop computers. Now, providers are accessing these same tools with handheld devices such as smartphones, tablets, and "phablets" (a class of mobile devices designed to combine the form of a smartphone and a tablet).
Critical to the popularity and functionality of handheld devices are mobile applications, also known as "apps." An app is a self-contained program or piece of software designed to run on handheld devices to perform a specific purpose. App overload and app inaccuracy, however, are major problems. Health care providers do not have the time to search through thousands of medical apps in app stores to find specialty-related apps that might be useful in their practice--or to check the accuracy of those apps.
Vetted apps for ObGyns
My team's research has focused on identifying apps for ObGyns to use in clinical practice.1 In the process, we have developed the APPLICATIONS scoring system, which contains objective and subjective components to help differentiate among the accurate apps.2 This new quarterly "App review" series in OBG Management will showcase recommended apps for the busy ObGyn in the hope of improving work efficiency and the provider-patient relationship.
First up: Apps for calculating the date of delivery. This first app review focuses on pregnancy wheels, or due date calculators. Calculator apps are preferred over other types of apps such as procedure/case documentation apps, as providers use smartphones at point of care to allow rapid decision making.3 Calculating the estimated date of delivery (EDD) and gestational age (GA) is an important, vital task for providers of obstetric care. In fact, new guidelines for calculating EDD were recently developed by the American College of Obstetricians and Gynecologists (ACOG), the American Institute of Ultrasound in Medicine (AIUM), and the Society for Maternal-Fetal Medicine (SMFM).4 Notably, pregnancy wheel apps are more accurate than paper wheels.5 My team checked the accuracy of the pregnancy wheel apps by applying strict criteria to ensure the correct EDD and GA and then scored them in a systematic, nonbiased, conflict-of-interest-free manner.2
Related article:
Elective induction of labor at 39 (vs 41) weeks: Caveats and considerations
The TABLE below lists the top 3 recommended pregnancy wheel or due date calculator apps vetted by our research. The apps are listed alphabetically, and details for each app are provided based on a shortened version of the APPLICATIONS scoring system, APPLI--app comprehensiveness, price, platform, literature use, and important special features.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Farag S, Chyjek K, Chen KT. Identification of iPhone and iPad applications for obstetrics and gynecology providers. Obstet Gynecol. 2014;124(5):941-945.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Inform Decis Mak. 2012;12:121.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 611. Method for estimating due date. Obstet Gynecol. 2014;124(4):863-866.
- Chambliss LR, Clark SL. Paper gestational age wheels are generally inaccurate. Am J Obstet Gynecol. 2014;210(2):145.e1-e4.
- Farag S, Chyjek K, Chen KT. Identification of iPhone and iPad applications for obstetrics and gynecology providers. Obstet Gynecol. 2014;124(5):941-945.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478-1483.
- Payne KB, Wharrad H, Watts K. Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Inform Decis Mak. 2012;12:121.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 611. Method for estimating due date. Obstet Gynecol. 2014;124(4):863-866.
- Chambliss LR, Clark SL. Paper gestational age wheels are generally inaccurate. Am J Obstet Gynecol. 2014;210(2):145.e1-e4.
UTI in pregnancy: 6 questions to guide therapy
A 29–year–old nullipara at 18 weeks’ gestation complains of fevers and back pain. She had a diagnosis of urinary tract infection with sulfonamide-resistant Escherichia coli at 9 weeks of gestation, which was treated with nitrofurantoin, 100 mg by mouth twice a day for 7 days. A test of cure by urine culture was negative.
Now her temperature is 101°F and she has right costovertebral angle tenderness.
How should you proceed?
Anatomy is destiny, in the case of susceptibility to urinary tract infection (UTI). The female urethra is only 3 cm to 4 cm long, and its proximity to the vagina, anus, and rectum facilitates colonization of normal gastrointestinal flora in the bladder.1
Sexual activity also facilitates migration of normal gastrointestinal flora to the female urethra.2
Anatomical features of pregnancy exacerbate the female predisposition to urinary tract infection. In pregnancy, hormonal and mechanical changes that occur in the urinary tract lead to urinary stasis and ureterovesical reflux—setting the stage for urinary tract infection (FIGURE 1).
Who should be screened?
All pregnant women should be screened for UTI early in pregnancy, according to the American College of Obstetricians and Gynecologists.3
I recommend a urine culture screening for all pregnant women at their first prenatal visit.
Screen often if she has risk factors
I recommend frequent screening (at least every trimester) by urine culture, in pregnant women with any of these risk factors:
- diabetes mellitus, including gestational diabetes4;
- urologic abnormalities—specifically, neurogenic bladder;
- prepregnancy (for example, 2 to 3 infections per year) and antepartum history of UTI prior to initiation of prenatal care5;
- sickle cell hemoglobinopathy.5
Which test is best?
The gold standard for detecting bacteria in urine is by culture.
Which threshold to use?
The standard definition of a positive urine culture from a clean-catch, midstream, voided specimen is ≥100,000 colony forming units (CFU) per mL of a single organism. However, in symptomatic patients, the test’s sensitivity is increased by lowering the cut-off to 100 CFU/mL of a single organism.6 In women with urinary symptoms, only 50% of patients had 100,000 CFU/mL by urine culture collected from clean-catch, midstream, voided specimens, though all of them had positive cultures from suprapubic taps.
The clean-catch, midstream, voided specimen is the specimen of choice for practical purposes, since it is noninvasive and easily obtained in the office setting.
For the record: The presence of any organism represents UTI in specimens obtained via suprapubic aspiration of the bladder; 100 CFU/mL of a single organism is positive for specimens obtained by urethral catheterization.
I recommend that, when obtaining urine cultures via clean-catch, midstream, voided specimens:
- for asymptomatic patients, use ≥100,000 CFU/mL of a single organism.
- in symptomatic patients, use ≥100 CFU/mL of a single organism.
What about rapid tests?
Urinary sediment analysis and urine dipstick testing offer speed and low cost, but with lower accuracy than urine cultures, which require 24 to 48 hours for results and cost more.
Urinary sediment analysis can diagnose pyuria, defined as a clean-catch, midstream, voided specimen, which is spun and which has >10 leukocytes per high-power field.
Pyuria can occur without infection due to:
- previous treatment with antibiotics,
- contamination of urine sample by sterilizing solution,
- contamination of urine sample with vaginal leukocytes,
- chronic interstitial nephritis (such as analgesic abuse),
- uroepithelial tumor, and
- nephrolithiasis.
Bacteria visualized on microscopic examination is more sensitive (75%) but less specific (60%).7
Urinary dipstick testing—fast, convenient, and low in cost—is considered positive if it identifies either leukocyte esterase or nitrite. Positive leukocyte esterase signifies pyuria. Positive nitrite indicates the presence of enteric organisms that convert urinary nitrate to nitrite.
With either finding, dipstick sensitivity is only 50%, although specificity is 97%.7
I recommend:
- If a symptomatic patient’s rapid test is positive, obtain a urine culture, empirically treat for UTI, and then use urine culture results to decide whether to continue treatment.
- If an asymptomatic patient’s rapid test is positive, obtain a urine culture and treat only if the culture is positive.
What urinary tract disorders occur in pregnancy?
First, determine if the patient has urinary tract symptoms and, if so, whether the symptoms are typical of upper or lower urinary tract infections.
Lower urinary tract symptoms:
- dysuria
- frequency
- urgency
- suprapubic pain
- hematuria in the absence of fever and systemic symptoms
Upper urinary tract symptoms:
- fever
- chills
- flank pain
- nausea and vomiting
- The patient may or may not have the symptoms of lower urinary tract infection, as well.
Positive culture and no symptoms
This profile is typical of asymptomatic bacteriuria, a lower urinary tract infection that occurs in 2% to 7% of pregnancies.1
Positive culture with symptoms
This profile probably reflects either:
- Acute cystitis, a lower urinary tract infection affecting 1% to 2% of pregnancies,8 or
- Acute pyelonephritis, an upper urinary tract infection affecting 2% of pregnancies.9
What are the consequences of UTI in pregnancy?
Maternal complications
Asymptomatic bacteriuria does not plague the patient with bothersome effects, but if left untreated, asymptomatic bacteriuria will progress to symptomatic UTI: 25% will develop acute pyelonephritis, compared to 3% to 4% of treated patients10; 20% of women with severe pyelonephritis develop serious complications,11 including:
- sepsis and septic shock,
- hemolysis and thrombocytopenia,12
- acute respiratory distress syndrome,13
- renal insufficiency.14
Adverse fetal outcomes
Untreated asymptomatic bacteriuria is associated with preterm delivery and low birthweight.15-17
Acute pyelonephritis is linked to preterm birth.18,19 Kaul et al,20 in an experimental model of pyelonephritis in mice, confirmed that E. coli plays an important role in the pathogenesis of preterm delivery and low birthweight.
What is the best treatment regimen?
Data are insufficient to recommend any specific regimen.21,22 The following strategies are based on evaluation of review articles.3,23
Asymptomatic bacteriuria and acute cystitis
Nitrofurantoin monohydrate macrocrystals is my first-line treatment. Nitrofurantoin has high concentrations in the urinary tract but induces minimal resistance in gram-negative organisms.
If nitrofurantoin is not effective, I change antibiotics based on urine culture antibiotic sensitivity profiles (FIGURE 2).
Keep in mind the current resistance of E. coli to antibiotics: ampicillin, 28% to 39%; trimethoprim-sulfamethoxazole, 31%; and first-generation cephalosporins, 9% to 19%.24
Single-dose treatment for pregnant women with asymptomatic bacteriuria has been evaluated, given its lower cost and better compliance. However, evidence is insufficient to determine whether single-dose or longer-duration regimens are more effective.25
I recommend longer-duration dosages for now, until a large randomized controlled trial can derive conclusive data.
Remember that treatment success is not contingent upon duration of therapy—just be sure that the test-of-cure urine culture is negative 1 to 2 weeks after treatment is completed.
Acute pyelonephritis
Management should include the following:
- hospitalization
- urine and blood cultures
- laboratory studies of complete blood cell count, electrolytes, creatinine, and liver function
- monitoring of vital signs and urine output
- intravenous (IV) crystalloid fluid to maintain urine output
- IV antibiotics (FIGURE 3).
Consider imaging by renal ultrasound to assess the presence of nephrolithiasis, perinephric abscess, or obstruction.
I recommend inpatient treatment for pregnant women with acute pyelonephritis at this time, until further studies are available.
To evaluate outpatient treatment, Millar and colleagues randomized 120 women under 24 weeks’ gestation either to inpatient IV cefazolin until 48 hours afebrile or to outpatient ceftriaxone intramuscularly. (Both treatment arms completed a course of oral cephalexin.) There were no differences in therapeutic response or birth outcomes, but 6 patients in the outpatient arm required hospitalization for IV therapy and 1 woman developed sepsis.26
The same researchers studied 92 patients of more than 24 weeks’ gestation who received 2 doses of ceftriaxone intramuscularly, then were randomized to either continued inpatient therapy until 48 hours afebrile or discharge with reevaluation as an outpatient in 48 to 72 hours. Again, there were no differences in therapeutic response or birth outcomes; however, almost two-thirds of patients were excluded from the study as they did not meet criteria for outpatient management, due to sepsis, preterm labor, or concurrent medical conditions.27
Adequate antibiotic coverage is crucial
To ensure adequate antibiotic coverage when treating UTI, it is important to understand which organisms cause these infections in pregnancy.
E. coli causes 75% to 90% of UTIs in nonpregnant women.28Staphylococcus saprophyticus causes 10% to 15% of UTIs in nonpregnant women, but less in pregnant women.
Group B Streptococcus (GBS)—another gram-positive organism—has important implications for pregnant women: Intrapartum prophylaxis is important, to prevent neonatal GBS disease.29
Klebsiella, Enterobacter, Proteus, and Enterococcus species28 infrequently cause UTI in pregnancy.
What about prophylaxis and follow-up cultures?
Expect recurrence
One third of pregnant women diagnosed with UTI will have recurrence.1 Recurrence is either relapse (same strain, within 2 weeks of completing initial treatment for the original infection) or reinfection (different strain or same strain after more than 2 weeks).
2 UTIs or pyelonephritis warrant suppressive therapy
I recommend suppressive therapy if a pregnant woman is diagnosed with 2 lower urinary tract infections or acute pyelonephritis (TABLE).
Nitrofurantoin is the preferred agent, as it has high concentrations in the urinary tract but induces minimal resistance in gram-negative organisms.
Start only after eradication of the acute infection, as evidenced by a negative test-of-cure urine culture at least 1 to 2 weeks after treatment is discontinued.23
Monthly urine cultures until delivery
I recommend monthly follow-up urine cultures until delivery.
Periodic follow-up screening is often recommended, but opinions differ on which test to use or how often to screen.
THE CASE: DIAGNOSIS, TREATMENT, FOLLOW-UP, AND OUTCOME
The patient with upper urinary tract symptoms had a white blood cell count of 15, a urine dipstick positive for leukocyte esterase and nitrites, and a urine sediment analysis indicating pyuria.
She was diagnosed with acute pyelonephritis and started on ampicillin and gentamicin intravenously. Her urine culture drawn upon admission grew >100,000 CFU/mL of sulfonamide-resistant E. coli.
Within 48 hours, she showed clinical improvement and was discharged home with a 10-day course of nitrofurantoin. One week after completing treatment, her test-of-cure urine culture was negative and she was started on nitrofurantoin 50 mg every night at bedtime.
For the rest of pregnancy, she underwent monthly screening urine cultures, which remained negative. She had an uncomplicated delivery at 38 weeks of gestation.
TABLE
Suppressive therapy to prevent UTI recurrence
| Suppressive therapy is recommended for any pregnant woman with: | |
| |
| Do not initiate suppressive therapy until a negative test-of-cure urine culture confirms eradication of the acute infection. | |
| ANTIBIOTIC | DOSE (ORAL) |
| Nitrofurantoin monohydrate macrocrystals | 50 mg at bedtime |
| or | |
| Cephalexin | 250 mg at bedtime |
1. Gilstrap LC, III, Ramin SM. Urinary tract infections during pregnancy. Obstet Gynecol Clin North Am. 2001;28:581-591.
2. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468-474.
3. American College of Obstetricians and Gynecologists. Antimicrobial therapy for obstetric patients. ACOG Technical Bulletin No. 245. Washington, DC: ACOG; 1998.
4. McMahon MJ, Ananth CV, Liston RM. Gestational diabetes mellitus. Risk factors, obstetric complications and infant outcomes. J Reprod Med. 1998;43:372-378.
5. Pastore LM, Savitz DA, Thorp JM, Jr. Predictors of urinary tract infection at the first prenatal visit. Epidemiology. 1999;10:282-287.
6. Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982;307:463-468.
7. Bachman JW, Heise RH, Naessens JM, Timmerman MG. A study of various tests to detect asymptomatic urinary tract infections in an obstetric population. JAMA. 1993;270:1971-1974.
8. Harris RE, Gilstrap LC, III. Cystitis during pregnancy: a distinct clinical entity. Obstet Gynecol. 1981;57:578-580.
9. Gilstrap LC, III, Cunningham FG, Whalley PJ. Acute pyelonephritis in pregnancy: an anterospective study. Obstet Gynecol. 1981;57:409-413.
10. Whalley P. Bacteriuria of pregnancy. Am J Obstet Gynecol. 1967;97:723-738.
11. Cunningham FG, Lucas MJ. Urinary tract infections complicating pregnancy. Baillieres Clin Obstet Gynaecol. 1994;8:353-373.
12. Cox SM, Shelburne P, Mason R, Guss S, Cunningham FG. Mechanisms of hemolysis and anemia associated with acute antepartum pyelonephritis. Am J Obstet Gynecol. 1991;164:587-590.
13. Cunningham FG, Lucas MJ, Hankins GD. Pulmonary injury complicating antepartum pyelonephritis. Am J Obstet Gynecol. 1987;156:797-807.
14. Whalley PJ, Cunningham FG, Martin FG. Transient renal dysfunction associated with acute pyelonephritis of pregnancy. Obstet Gynecol. 1975;46:174-177.
15. Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol. 1989;73:576-582.
16. Schieve LA, Handler A, Hershow R, Persky V, Davis F. Urinary tract infection during pregnancy: its association with maternal morbidity and perinatal outcome. Am J Public Health. 1994;84:405-410.
17. Mittendorf R, Williams MA, Kass EH. Prevention of preterm delivery and low birth weight associated with asymptomatic bacteriuria. Clin Infect Dis. 1992;14:927-932.
18. Gilstrap LC, Leveno KJ, Cunningham FG, Whalley PJ, Roark ML. Renal infection and pregnancy outcome. Am J Obstet Gynecol. 1981;141:709-716.
19. Millar LK, DeBuque L, Wing DA. Uterine contraction frequency during treatment of pyelonephritis in pregnancy and subsequent risk of preterm birth. J Perinatal Med. 2003;31(1):41-46.
20. Kaul AK, Khan S, Martens MG, Crosson JT, Lupo VR, Kaul R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect Immun. 1999;67:5958-5966.
21. Vazquez JC, Villar J. Treatments for symptomatic urinary tract infections during pregnancy. Cochrane Database Syst Rev. 2003(4);CD002256.-
22. Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2001(2);CD000490.-
23. Fihn SD. Clinical practice. Acute uncomplicated urinary tract infection in women. N Engl J Med. 2003;349:259-266.
24. Ovalle A, Levancini M. Urinary tract infections in pregnancy. Curr Opin Urol. 2001;11:55-59.
25. Villar J, Lydon-Rochelle MT, Gulmezoglu AM, Roganti A. Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database Syst Rev. 2000(2);CD000491.-
26. Millar LK, Wing DA, Paul RH, Grimes DA. Outpatient treatment of pyelonephritis in pregnancy: a randomized controlled trial. Obstet Gynecol. 1995;86:560-564.
27. Wing DA, Hendershott CM, DeBuque L, Millar LK. Outpatient treatment of acute pyelonephritis in pregnancy after 24 weeks. Obstet Gynecol. 1999;94:683-688.
28. Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2002;113(suppl 1A):14S-19S.
29. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51:1-22.
30. Delzell JE, Jr, Lefevre ML. Urinary tract infections during pregnancy. Am Fam Physician. 2000;61:713-721.
A 29–year–old nullipara at 18 weeks’ gestation complains of fevers and back pain. She had a diagnosis of urinary tract infection with sulfonamide-resistant Escherichia coli at 9 weeks of gestation, which was treated with nitrofurantoin, 100 mg by mouth twice a day for 7 days. A test of cure by urine culture was negative.
Now her temperature is 101°F and she has right costovertebral angle tenderness.
How should you proceed?
Anatomy is destiny, in the case of susceptibility to urinary tract infection (UTI). The female urethra is only 3 cm to 4 cm long, and its proximity to the vagina, anus, and rectum facilitates colonization of normal gastrointestinal flora in the bladder.1
Sexual activity also facilitates migration of normal gastrointestinal flora to the female urethra.2
Anatomical features of pregnancy exacerbate the female predisposition to urinary tract infection. In pregnancy, hormonal and mechanical changes that occur in the urinary tract lead to urinary stasis and ureterovesical reflux—setting the stage for urinary tract infection (FIGURE 1).
Who should be screened?
All pregnant women should be screened for UTI early in pregnancy, according to the American College of Obstetricians and Gynecologists.3
I recommend a urine culture screening for all pregnant women at their first prenatal visit.
Screen often if she has risk factors
I recommend frequent screening (at least every trimester) by urine culture, in pregnant women with any of these risk factors:
- diabetes mellitus, including gestational diabetes4;
- urologic abnormalities—specifically, neurogenic bladder;
- prepregnancy (for example, 2 to 3 infections per year) and antepartum history of UTI prior to initiation of prenatal care5;
- sickle cell hemoglobinopathy.5
Which test is best?
The gold standard for detecting bacteria in urine is by culture.
Which threshold to use?
The standard definition of a positive urine culture from a clean-catch, midstream, voided specimen is ≥100,000 colony forming units (CFU) per mL of a single organism. However, in symptomatic patients, the test’s sensitivity is increased by lowering the cut-off to 100 CFU/mL of a single organism.6 In women with urinary symptoms, only 50% of patients had 100,000 CFU/mL by urine culture collected from clean-catch, midstream, voided specimens, though all of them had positive cultures from suprapubic taps.
The clean-catch, midstream, voided specimen is the specimen of choice for practical purposes, since it is noninvasive and easily obtained in the office setting.
For the record: The presence of any organism represents UTI in specimens obtained via suprapubic aspiration of the bladder; 100 CFU/mL of a single organism is positive for specimens obtained by urethral catheterization.
I recommend that, when obtaining urine cultures via clean-catch, midstream, voided specimens:
- for asymptomatic patients, use ≥100,000 CFU/mL of a single organism.
- in symptomatic patients, use ≥100 CFU/mL of a single organism.
What about rapid tests?
Urinary sediment analysis and urine dipstick testing offer speed and low cost, but with lower accuracy than urine cultures, which require 24 to 48 hours for results and cost more.
Urinary sediment analysis can diagnose pyuria, defined as a clean-catch, midstream, voided specimen, which is spun and which has >10 leukocytes per high-power field.
Pyuria can occur without infection due to:
- previous treatment with antibiotics,
- contamination of urine sample by sterilizing solution,
- contamination of urine sample with vaginal leukocytes,
- chronic interstitial nephritis (such as analgesic abuse),
- uroepithelial tumor, and
- nephrolithiasis.
Bacteria visualized on microscopic examination is more sensitive (75%) but less specific (60%).7
Urinary dipstick testing—fast, convenient, and low in cost—is considered positive if it identifies either leukocyte esterase or nitrite. Positive leukocyte esterase signifies pyuria. Positive nitrite indicates the presence of enteric organisms that convert urinary nitrate to nitrite.
With either finding, dipstick sensitivity is only 50%, although specificity is 97%.7
I recommend:
- If a symptomatic patient’s rapid test is positive, obtain a urine culture, empirically treat for UTI, and then use urine culture results to decide whether to continue treatment.
- If an asymptomatic patient’s rapid test is positive, obtain a urine culture and treat only if the culture is positive.
What urinary tract disorders occur in pregnancy?
First, determine if the patient has urinary tract symptoms and, if so, whether the symptoms are typical of upper or lower urinary tract infections.
Lower urinary tract symptoms:
- dysuria
- frequency
- urgency
- suprapubic pain
- hematuria in the absence of fever and systemic symptoms
Upper urinary tract symptoms:
- fever
- chills
- flank pain
- nausea and vomiting
- The patient may or may not have the symptoms of lower urinary tract infection, as well.
Positive culture and no symptoms
This profile is typical of asymptomatic bacteriuria, a lower urinary tract infection that occurs in 2% to 7% of pregnancies.1
Positive culture with symptoms
This profile probably reflects either:
- Acute cystitis, a lower urinary tract infection affecting 1% to 2% of pregnancies,8 or
- Acute pyelonephritis, an upper urinary tract infection affecting 2% of pregnancies.9
What are the consequences of UTI in pregnancy?
Maternal complications
Asymptomatic bacteriuria does not plague the patient with bothersome effects, but if left untreated, asymptomatic bacteriuria will progress to symptomatic UTI: 25% will develop acute pyelonephritis, compared to 3% to 4% of treated patients10; 20% of women with severe pyelonephritis develop serious complications,11 including:
- sepsis and septic shock,
- hemolysis and thrombocytopenia,12
- acute respiratory distress syndrome,13
- renal insufficiency.14
Adverse fetal outcomes
Untreated asymptomatic bacteriuria is associated with preterm delivery and low birthweight.15-17
Acute pyelonephritis is linked to preterm birth.18,19 Kaul et al,20 in an experimental model of pyelonephritis in mice, confirmed that E. coli plays an important role in the pathogenesis of preterm delivery and low birthweight.
What is the best treatment regimen?
Data are insufficient to recommend any specific regimen.21,22 The following strategies are based on evaluation of review articles.3,23
Asymptomatic bacteriuria and acute cystitis
Nitrofurantoin monohydrate macrocrystals is my first-line treatment. Nitrofurantoin has high concentrations in the urinary tract but induces minimal resistance in gram-negative organisms.
If nitrofurantoin is not effective, I change antibiotics based on urine culture antibiotic sensitivity profiles (FIGURE 2).
Keep in mind the current resistance of E. coli to antibiotics: ampicillin, 28% to 39%; trimethoprim-sulfamethoxazole, 31%; and first-generation cephalosporins, 9% to 19%.24
Single-dose treatment for pregnant women with asymptomatic bacteriuria has been evaluated, given its lower cost and better compliance. However, evidence is insufficient to determine whether single-dose or longer-duration regimens are more effective.25
I recommend longer-duration dosages for now, until a large randomized controlled trial can derive conclusive data.
Remember that treatment success is not contingent upon duration of therapy—just be sure that the test-of-cure urine culture is negative 1 to 2 weeks after treatment is completed.
Acute pyelonephritis
Management should include the following:
- hospitalization
- urine and blood cultures
- laboratory studies of complete blood cell count, electrolytes, creatinine, and liver function
- monitoring of vital signs and urine output
- intravenous (IV) crystalloid fluid to maintain urine output
- IV antibiotics (FIGURE 3).
Consider imaging by renal ultrasound to assess the presence of nephrolithiasis, perinephric abscess, or obstruction.
I recommend inpatient treatment for pregnant women with acute pyelonephritis at this time, until further studies are available.
To evaluate outpatient treatment, Millar and colleagues randomized 120 women under 24 weeks’ gestation either to inpatient IV cefazolin until 48 hours afebrile or to outpatient ceftriaxone intramuscularly. (Both treatment arms completed a course of oral cephalexin.) There were no differences in therapeutic response or birth outcomes, but 6 patients in the outpatient arm required hospitalization for IV therapy and 1 woman developed sepsis.26
The same researchers studied 92 patients of more than 24 weeks’ gestation who received 2 doses of ceftriaxone intramuscularly, then were randomized to either continued inpatient therapy until 48 hours afebrile or discharge with reevaluation as an outpatient in 48 to 72 hours. Again, there were no differences in therapeutic response or birth outcomes; however, almost two-thirds of patients were excluded from the study as they did not meet criteria for outpatient management, due to sepsis, preterm labor, or concurrent medical conditions.27
Adequate antibiotic coverage is crucial
To ensure adequate antibiotic coverage when treating UTI, it is important to understand which organisms cause these infections in pregnancy.
E. coli causes 75% to 90% of UTIs in nonpregnant women.28Staphylococcus saprophyticus causes 10% to 15% of UTIs in nonpregnant women, but less in pregnant women.
Group B Streptococcus (GBS)—another gram-positive organism—has important implications for pregnant women: Intrapartum prophylaxis is important, to prevent neonatal GBS disease.29
Klebsiella, Enterobacter, Proteus, and Enterococcus species28 infrequently cause UTI in pregnancy.
What about prophylaxis and follow-up cultures?
Expect recurrence
One third of pregnant women diagnosed with UTI will have recurrence.1 Recurrence is either relapse (same strain, within 2 weeks of completing initial treatment for the original infection) or reinfection (different strain or same strain after more than 2 weeks).
2 UTIs or pyelonephritis warrant suppressive therapy
I recommend suppressive therapy if a pregnant woman is diagnosed with 2 lower urinary tract infections or acute pyelonephritis (TABLE).
Nitrofurantoin is the preferred agent, as it has high concentrations in the urinary tract but induces minimal resistance in gram-negative organisms.
Start only after eradication of the acute infection, as evidenced by a negative test-of-cure urine culture at least 1 to 2 weeks after treatment is discontinued.23
Monthly urine cultures until delivery
I recommend monthly follow-up urine cultures until delivery.
Periodic follow-up screening is often recommended, but opinions differ on which test to use or how often to screen.
THE CASE: DIAGNOSIS, TREATMENT, FOLLOW-UP, AND OUTCOME
The patient with upper urinary tract symptoms had a white blood cell count of 15, a urine dipstick positive for leukocyte esterase and nitrites, and a urine sediment analysis indicating pyuria.
She was diagnosed with acute pyelonephritis and started on ampicillin and gentamicin intravenously. Her urine culture drawn upon admission grew >100,000 CFU/mL of sulfonamide-resistant E. coli.
Within 48 hours, she showed clinical improvement and was discharged home with a 10-day course of nitrofurantoin. One week after completing treatment, her test-of-cure urine culture was negative and she was started on nitrofurantoin 50 mg every night at bedtime.
For the rest of pregnancy, she underwent monthly screening urine cultures, which remained negative. She had an uncomplicated delivery at 38 weeks of gestation.
TABLE
Suppressive therapy to prevent UTI recurrence
| Suppressive therapy is recommended for any pregnant woman with: | |
| |
| Do not initiate suppressive therapy until a negative test-of-cure urine culture confirms eradication of the acute infection. | |
| ANTIBIOTIC | DOSE (ORAL) |
| Nitrofurantoin monohydrate macrocrystals | 50 mg at bedtime |
| or | |
| Cephalexin | 250 mg at bedtime |
A 29–year–old nullipara at 18 weeks’ gestation complains of fevers and back pain. She had a diagnosis of urinary tract infection with sulfonamide-resistant Escherichia coli at 9 weeks of gestation, which was treated with nitrofurantoin, 100 mg by mouth twice a day for 7 days. A test of cure by urine culture was negative.
Now her temperature is 101°F and she has right costovertebral angle tenderness.
How should you proceed?
Anatomy is destiny, in the case of susceptibility to urinary tract infection (UTI). The female urethra is only 3 cm to 4 cm long, and its proximity to the vagina, anus, and rectum facilitates colonization of normal gastrointestinal flora in the bladder.1
Sexual activity also facilitates migration of normal gastrointestinal flora to the female urethra.2
Anatomical features of pregnancy exacerbate the female predisposition to urinary tract infection. In pregnancy, hormonal and mechanical changes that occur in the urinary tract lead to urinary stasis and ureterovesical reflux—setting the stage for urinary tract infection (FIGURE 1).
Who should be screened?
All pregnant women should be screened for UTI early in pregnancy, according to the American College of Obstetricians and Gynecologists.3
I recommend a urine culture screening for all pregnant women at their first prenatal visit.
Screen often if she has risk factors
I recommend frequent screening (at least every trimester) by urine culture, in pregnant women with any of these risk factors:
- diabetes mellitus, including gestational diabetes4;
- urologic abnormalities—specifically, neurogenic bladder;
- prepregnancy (for example, 2 to 3 infections per year) and antepartum history of UTI prior to initiation of prenatal care5;
- sickle cell hemoglobinopathy.5
Which test is best?
The gold standard for detecting bacteria in urine is by culture.
Which threshold to use?
The standard definition of a positive urine culture from a clean-catch, midstream, voided specimen is ≥100,000 colony forming units (CFU) per mL of a single organism. However, in symptomatic patients, the test’s sensitivity is increased by lowering the cut-off to 100 CFU/mL of a single organism.6 In women with urinary symptoms, only 50% of patients had 100,000 CFU/mL by urine culture collected from clean-catch, midstream, voided specimens, though all of them had positive cultures from suprapubic taps.
The clean-catch, midstream, voided specimen is the specimen of choice for practical purposes, since it is noninvasive and easily obtained in the office setting.
For the record: The presence of any organism represents UTI in specimens obtained via suprapubic aspiration of the bladder; 100 CFU/mL of a single organism is positive for specimens obtained by urethral catheterization.
I recommend that, when obtaining urine cultures via clean-catch, midstream, voided specimens:
- for asymptomatic patients, use ≥100,000 CFU/mL of a single organism.
- in symptomatic patients, use ≥100 CFU/mL of a single organism.
What about rapid tests?
Urinary sediment analysis and urine dipstick testing offer speed and low cost, but with lower accuracy than urine cultures, which require 24 to 48 hours for results and cost more.
Urinary sediment analysis can diagnose pyuria, defined as a clean-catch, midstream, voided specimen, which is spun and which has >10 leukocytes per high-power field.
Pyuria can occur without infection due to:
- previous treatment with antibiotics,
- contamination of urine sample by sterilizing solution,
- contamination of urine sample with vaginal leukocytes,
- chronic interstitial nephritis (such as analgesic abuse),
- uroepithelial tumor, and
- nephrolithiasis.
Bacteria visualized on microscopic examination is more sensitive (75%) but less specific (60%).7
Urinary dipstick testing—fast, convenient, and low in cost—is considered positive if it identifies either leukocyte esterase or nitrite. Positive leukocyte esterase signifies pyuria. Positive nitrite indicates the presence of enteric organisms that convert urinary nitrate to nitrite.
With either finding, dipstick sensitivity is only 50%, although specificity is 97%.7
I recommend:
- If a symptomatic patient’s rapid test is positive, obtain a urine culture, empirically treat for UTI, and then use urine culture results to decide whether to continue treatment.
- If an asymptomatic patient’s rapid test is positive, obtain a urine culture and treat only if the culture is positive.
What urinary tract disorders occur in pregnancy?
First, determine if the patient has urinary tract symptoms and, if so, whether the symptoms are typical of upper or lower urinary tract infections.
Lower urinary tract symptoms:
- dysuria
- frequency
- urgency
- suprapubic pain
- hematuria in the absence of fever and systemic symptoms
Upper urinary tract symptoms:
- fever
- chills
- flank pain
- nausea and vomiting
- The patient may or may not have the symptoms of lower urinary tract infection, as well.
Positive culture and no symptoms
This profile is typical of asymptomatic bacteriuria, a lower urinary tract infection that occurs in 2% to 7% of pregnancies.1
Positive culture with symptoms
This profile probably reflects either:
- Acute cystitis, a lower urinary tract infection affecting 1% to 2% of pregnancies,8 or
- Acute pyelonephritis, an upper urinary tract infection affecting 2% of pregnancies.9
What are the consequences of UTI in pregnancy?
Maternal complications
Asymptomatic bacteriuria does not plague the patient with bothersome effects, but if left untreated, asymptomatic bacteriuria will progress to symptomatic UTI: 25% will develop acute pyelonephritis, compared to 3% to 4% of treated patients10; 20% of women with severe pyelonephritis develop serious complications,11 including:
- sepsis and septic shock,
- hemolysis and thrombocytopenia,12
- acute respiratory distress syndrome,13
- renal insufficiency.14
Adverse fetal outcomes
Untreated asymptomatic bacteriuria is associated with preterm delivery and low birthweight.15-17
Acute pyelonephritis is linked to preterm birth.18,19 Kaul et al,20 in an experimental model of pyelonephritis in mice, confirmed that E. coli plays an important role in the pathogenesis of preterm delivery and low birthweight.
What is the best treatment regimen?
Data are insufficient to recommend any specific regimen.21,22 The following strategies are based on evaluation of review articles.3,23
Asymptomatic bacteriuria and acute cystitis
Nitrofurantoin monohydrate macrocrystals is my first-line treatment. Nitrofurantoin has high concentrations in the urinary tract but induces minimal resistance in gram-negative organisms.
If nitrofurantoin is not effective, I change antibiotics based on urine culture antibiotic sensitivity profiles (FIGURE 2).
Keep in mind the current resistance of E. coli to antibiotics: ampicillin, 28% to 39%; trimethoprim-sulfamethoxazole, 31%; and first-generation cephalosporins, 9% to 19%.24
Single-dose treatment for pregnant women with asymptomatic bacteriuria has been evaluated, given its lower cost and better compliance. However, evidence is insufficient to determine whether single-dose or longer-duration regimens are more effective.25
I recommend longer-duration dosages for now, until a large randomized controlled trial can derive conclusive data.
Remember that treatment success is not contingent upon duration of therapy—just be sure that the test-of-cure urine culture is negative 1 to 2 weeks after treatment is completed.
Acute pyelonephritis
Management should include the following:
- hospitalization
- urine and blood cultures
- laboratory studies of complete blood cell count, electrolytes, creatinine, and liver function
- monitoring of vital signs and urine output
- intravenous (IV) crystalloid fluid to maintain urine output
- IV antibiotics (FIGURE 3).
Consider imaging by renal ultrasound to assess the presence of nephrolithiasis, perinephric abscess, or obstruction.
I recommend inpatient treatment for pregnant women with acute pyelonephritis at this time, until further studies are available.
To evaluate outpatient treatment, Millar and colleagues randomized 120 women under 24 weeks’ gestation either to inpatient IV cefazolin until 48 hours afebrile or to outpatient ceftriaxone intramuscularly. (Both treatment arms completed a course of oral cephalexin.) There were no differences in therapeutic response or birth outcomes, but 6 patients in the outpatient arm required hospitalization for IV therapy and 1 woman developed sepsis.26
The same researchers studied 92 patients of more than 24 weeks’ gestation who received 2 doses of ceftriaxone intramuscularly, then were randomized to either continued inpatient therapy until 48 hours afebrile or discharge with reevaluation as an outpatient in 48 to 72 hours. Again, there were no differences in therapeutic response or birth outcomes; however, almost two-thirds of patients were excluded from the study as they did not meet criteria for outpatient management, due to sepsis, preterm labor, or concurrent medical conditions.27
Adequate antibiotic coverage is crucial
To ensure adequate antibiotic coverage when treating UTI, it is important to understand which organisms cause these infections in pregnancy.
E. coli causes 75% to 90% of UTIs in nonpregnant women.28Staphylococcus saprophyticus causes 10% to 15% of UTIs in nonpregnant women, but less in pregnant women.
Group B Streptococcus (GBS)—another gram-positive organism—has important implications for pregnant women: Intrapartum prophylaxis is important, to prevent neonatal GBS disease.29
Klebsiella, Enterobacter, Proteus, and Enterococcus species28 infrequently cause UTI in pregnancy.
What about prophylaxis and follow-up cultures?
Expect recurrence
One third of pregnant women diagnosed with UTI will have recurrence.1 Recurrence is either relapse (same strain, within 2 weeks of completing initial treatment for the original infection) or reinfection (different strain or same strain after more than 2 weeks).
2 UTIs or pyelonephritis warrant suppressive therapy
I recommend suppressive therapy if a pregnant woman is diagnosed with 2 lower urinary tract infections or acute pyelonephritis (TABLE).
Nitrofurantoin is the preferred agent, as it has high concentrations in the urinary tract but induces minimal resistance in gram-negative organisms.
Start only after eradication of the acute infection, as evidenced by a negative test-of-cure urine culture at least 1 to 2 weeks after treatment is discontinued.23
Monthly urine cultures until delivery
I recommend monthly follow-up urine cultures until delivery.
Periodic follow-up screening is often recommended, but opinions differ on which test to use or how often to screen.
THE CASE: DIAGNOSIS, TREATMENT, FOLLOW-UP, AND OUTCOME
The patient with upper urinary tract symptoms had a white blood cell count of 15, a urine dipstick positive for leukocyte esterase and nitrites, and a urine sediment analysis indicating pyuria.
She was diagnosed with acute pyelonephritis and started on ampicillin and gentamicin intravenously. Her urine culture drawn upon admission grew >100,000 CFU/mL of sulfonamide-resistant E. coli.
Within 48 hours, she showed clinical improvement and was discharged home with a 10-day course of nitrofurantoin. One week after completing treatment, her test-of-cure urine culture was negative and she was started on nitrofurantoin 50 mg every night at bedtime.
For the rest of pregnancy, she underwent monthly screening urine cultures, which remained negative. She had an uncomplicated delivery at 38 weeks of gestation.
TABLE
Suppressive therapy to prevent UTI recurrence
| Suppressive therapy is recommended for any pregnant woman with: | |
| |
| Do not initiate suppressive therapy until a negative test-of-cure urine culture confirms eradication of the acute infection. | |
| ANTIBIOTIC | DOSE (ORAL) |
| Nitrofurantoin monohydrate macrocrystals | 50 mg at bedtime |
| or | |
| Cephalexin | 250 mg at bedtime |
1. Gilstrap LC, III, Ramin SM. Urinary tract infections during pregnancy. Obstet Gynecol Clin North Am. 2001;28:581-591.
2. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468-474.
3. American College of Obstetricians and Gynecologists. Antimicrobial therapy for obstetric patients. ACOG Technical Bulletin No. 245. Washington, DC: ACOG; 1998.
4. McMahon MJ, Ananth CV, Liston RM. Gestational diabetes mellitus. Risk factors, obstetric complications and infant outcomes. J Reprod Med. 1998;43:372-378.
5. Pastore LM, Savitz DA, Thorp JM, Jr. Predictors of urinary tract infection at the first prenatal visit. Epidemiology. 1999;10:282-287.
6. Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982;307:463-468.
7. Bachman JW, Heise RH, Naessens JM, Timmerman MG. A study of various tests to detect asymptomatic urinary tract infections in an obstetric population. JAMA. 1993;270:1971-1974.
8. Harris RE, Gilstrap LC, III. Cystitis during pregnancy: a distinct clinical entity. Obstet Gynecol. 1981;57:578-580.
9. Gilstrap LC, III, Cunningham FG, Whalley PJ. Acute pyelonephritis in pregnancy: an anterospective study. Obstet Gynecol. 1981;57:409-413.
10. Whalley P. Bacteriuria of pregnancy. Am J Obstet Gynecol. 1967;97:723-738.
11. Cunningham FG, Lucas MJ. Urinary tract infections complicating pregnancy. Baillieres Clin Obstet Gynaecol. 1994;8:353-373.
12. Cox SM, Shelburne P, Mason R, Guss S, Cunningham FG. Mechanisms of hemolysis and anemia associated with acute antepartum pyelonephritis. Am J Obstet Gynecol. 1991;164:587-590.
13. Cunningham FG, Lucas MJ, Hankins GD. Pulmonary injury complicating antepartum pyelonephritis. Am J Obstet Gynecol. 1987;156:797-807.
14. Whalley PJ, Cunningham FG, Martin FG. Transient renal dysfunction associated with acute pyelonephritis of pregnancy. Obstet Gynecol. 1975;46:174-177.
15. Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol. 1989;73:576-582.
16. Schieve LA, Handler A, Hershow R, Persky V, Davis F. Urinary tract infection during pregnancy: its association with maternal morbidity and perinatal outcome. Am J Public Health. 1994;84:405-410.
17. Mittendorf R, Williams MA, Kass EH. Prevention of preterm delivery and low birth weight associated with asymptomatic bacteriuria. Clin Infect Dis. 1992;14:927-932.
18. Gilstrap LC, Leveno KJ, Cunningham FG, Whalley PJ, Roark ML. Renal infection and pregnancy outcome. Am J Obstet Gynecol. 1981;141:709-716.
19. Millar LK, DeBuque L, Wing DA. Uterine contraction frequency during treatment of pyelonephritis in pregnancy and subsequent risk of preterm birth. J Perinatal Med. 2003;31(1):41-46.
20. Kaul AK, Khan S, Martens MG, Crosson JT, Lupo VR, Kaul R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect Immun. 1999;67:5958-5966.
21. Vazquez JC, Villar J. Treatments for symptomatic urinary tract infections during pregnancy. Cochrane Database Syst Rev. 2003(4);CD002256.-
22. Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2001(2);CD000490.-
23. Fihn SD. Clinical practice. Acute uncomplicated urinary tract infection in women. N Engl J Med. 2003;349:259-266.
24. Ovalle A, Levancini M. Urinary tract infections in pregnancy. Curr Opin Urol. 2001;11:55-59.
25. Villar J, Lydon-Rochelle MT, Gulmezoglu AM, Roganti A. Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database Syst Rev. 2000(2);CD000491.-
26. Millar LK, Wing DA, Paul RH, Grimes DA. Outpatient treatment of pyelonephritis in pregnancy: a randomized controlled trial. Obstet Gynecol. 1995;86:560-564.
27. Wing DA, Hendershott CM, DeBuque L, Millar LK. Outpatient treatment of acute pyelonephritis in pregnancy after 24 weeks. Obstet Gynecol. 1999;94:683-688.
28. Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2002;113(suppl 1A):14S-19S.
29. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51:1-22.
30. Delzell JE, Jr, Lefevre ML. Urinary tract infections during pregnancy. Am Fam Physician. 2000;61:713-721.
1. Gilstrap LC, III, Ramin SM. Urinary tract infections during pregnancy. Obstet Gynecol Clin North Am. 2001;28:581-591.
2. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468-474.
3. American College of Obstetricians and Gynecologists. Antimicrobial therapy for obstetric patients. ACOG Technical Bulletin No. 245. Washington, DC: ACOG; 1998.
4. McMahon MJ, Ananth CV, Liston RM. Gestational diabetes mellitus. Risk factors, obstetric complications and infant outcomes. J Reprod Med. 1998;43:372-378.
5. Pastore LM, Savitz DA, Thorp JM, Jr. Predictors of urinary tract infection at the first prenatal visit. Epidemiology. 1999;10:282-287.
6. Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982;307:463-468.
7. Bachman JW, Heise RH, Naessens JM, Timmerman MG. A study of various tests to detect asymptomatic urinary tract infections in an obstetric population. JAMA. 1993;270:1971-1974.
8. Harris RE, Gilstrap LC, III. Cystitis during pregnancy: a distinct clinical entity. Obstet Gynecol. 1981;57:578-580.
9. Gilstrap LC, III, Cunningham FG, Whalley PJ. Acute pyelonephritis in pregnancy: an anterospective study. Obstet Gynecol. 1981;57:409-413.
10. Whalley P. Bacteriuria of pregnancy. Am J Obstet Gynecol. 1967;97:723-738.
11. Cunningham FG, Lucas MJ. Urinary tract infections complicating pregnancy. Baillieres Clin Obstet Gynaecol. 1994;8:353-373.
12. Cox SM, Shelburne P, Mason R, Guss S, Cunningham FG. Mechanisms of hemolysis and anemia associated with acute antepartum pyelonephritis. Am J Obstet Gynecol. 1991;164:587-590.
13. Cunningham FG, Lucas MJ, Hankins GD. Pulmonary injury complicating antepartum pyelonephritis. Am J Obstet Gynecol. 1987;156:797-807.
14. Whalley PJ, Cunningham FG, Martin FG. Transient renal dysfunction associated with acute pyelonephritis of pregnancy. Obstet Gynecol. 1975;46:174-177.
15. Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol. 1989;73:576-582.
16. Schieve LA, Handler A, Hershow R, Persky V, Davis F. Urinary tract infection during pregnancy: its association with maternal morbidity and perinatal outcome. Am J Public Health. 1994;84:405-410.
17. Mittendorf R, Williams MA, Kass EH. Prevention of preterm delivery and low birth weight associated with asymptomatic bacteriuria. Clin Infect Dis. 1992;14:927-932.
18. Gilstrap LC, Leveno KJ, Cunningham FG, Whalley PJ, Roark ML. Renal infection and pregnancy outcome. Am J Obstet Gynecol. 1981;141:709-716.
19. Millar LK, DeBuque L, Wing DA. Uterine contraction frequency during treatment of pyelonephritis in pregnancy and subsequent risk of preterm birth. J Perinatal Med. 2003;31(1):41-46.
20. Kaul AK, Khan S, Martens MG, Crosson JT, Lupo VR, Kaul R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect Immun. 1999;67:5958-5966.
21. Vazquez JC, Villar J. Treatments for symptomatic urinary tract infections during pregnancy. Cochrane Database Syst Rev. 2003(4);CD002256.-
22. Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2001(2);CD000490.-
23. Fihn SD. Clinical practice. Acute uncomplicated urinary tract infection in women. N Engl J Med. 2003;349:259-266.
24. Ovalle A, Levancini M. Urinary tract infections in pregnancy. Curr Opin Urol. 2001;11:55-59.
25. Villar J, Lydon-Rochelle MT, Gulmezoglu AM, Roganti A. Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database Syst Rev. 2000(2);CD000491.-
26. Millar LK, Wing DA, Paul RH, Grimes DA. Outpatient treatment of pyelonephritis in pregnancy: a randomized controlled trial. Obstet Gynecol. 1995;86:560-564.
27. Wing DA, Hendershott CM, DeBuque L, Millar LK. Outpatient treatment of acute pyelonephritis in pregnancy after 24 weeks. Obstet Gynecol. 1999;94:683-688.
28. Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2002;113(suppl 1A):14S-19S.
29. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51:1-22.
30. Delzell JE, Jr, Lefevre ML. Urinary tract infections during pregnancy. Am Fam Physician. 2000;61:713-721.
Hepatitis C: the silent epidemic
- The hepatitis C virus (HCV) is the leading cause of chronic liver disease and the leading indication for liver transplantation in the United States.
- In the United States, 3.9 million people have been infected with HCV, with an overall prevalence of 1.8%. In females, prevalence is highest during the childbearing years, peaking at age 35.
- Injection-drug use accounts for 60% of infections, while the transfusion of blood or blood products accounts for another 10%.
- About 15% of people with acute HCV infection clear the virus; the rest develop chronic infection.
- During chronic infection, most patients are asymptomatic or have mild, nonspecific symptoms such as fatigue.
- Combination therapy with interferon alpha and ribavirin elicits a sustained virologic response rate of 40%, and newer therapy with pegylated interferon alpha and ribavirin improves the rate to 54%.
- Perinatal transmission in women with chronic HCV infection occurs at an average rate of 5%.
The hepatitis C virus (HCV) was first identified in 1989 as the cause of non-A, non-B hepatitis infections. Since its discovery, HCV has become the most common chronic blood-borne infection in the United States: Approximately 2.7 to 3.5 million people have chronic HCV infection,1,2 as compared with 1.25 million people with chronic hepatitis B virus infection and 1 million with the human immunodeficiency virus (HIV). In addition, HCV infection is the leading cause of chronic liver disease and the leading indication for liver transplantation in the United States. Some call HCV infection “the silent epidemic,” since 75% of people infected are asymptomatic and chronic manifestations don’t appear for 1 to 2 decades.
In 1998, the Centers for Disease Control and Prevention (CDC) issued guidelines for screening for HCV infection ( Table 1).3 The American College of Obstetricians and Gynecologists (ACOG) advocates screening for HCV infection at the annual exam if the patient belongs to one of the CDC’s routine-screening categories.4 Although ACOG has not issued separate screening guidelines for obstetric patients, some practitioners have advocated screening based on risk factors, as listed in the CDC’s routine-screening categories.5
HCV accounts for about 20% of acute hepatitis cases in the U.S.
Because Ob/Gyns are increasingly likely to encounter patients with positive HCV blood-screening results, they should be prepared to answer the following inquiries: What is hepatitis C? How is it diagnosed and transmitted? What is the natural history of the infection? Is there a treatment for it? How will HCV infection affect pregnancy?
This review addresses those questions.
What is hepatitis C?
Hepatitis C is a liver disease caused by the HCV, an RNA virus of the Flavivirus family, which includes the dengue and yellow fever viruses. Worldwide, approximately 170 million people are infected with HCV, with a prevalence ranging from a low of 0.15% in Scandinavia to a high of 38% in northern Egypt.6,7 In the United States, 3.9 million people have been infected with HCV, with an overall prevalence of 1.8%.1 U.S. prevalence rates by gender and age reveal that more males are infected than females and that, in females, prevalence is highest during the childbearing years, peaking at age 35.
TABLE 1
Recommendations for hepatitis C virus screening
| PEOPLE WHO SHOULD BE TESTED ROUTINELY |
|---|
|
| PEOPLE FOR WHOM ROUTINE TESTING IS OF UNCERTAIN NEED |
|
| PEOPLE FOR WHOM ROUTINE TESTING IS NOT RECOMMENDED* |
|
| * Except in cases where risk factors are present ALT=alanine aminotransferase; HCV=hepatitis C virus |
| Source: Centers for Disease Control and Prevention |
How is HCV infection diagnosed?
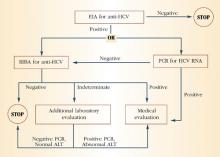
The initial screening test is an enzyme immunoassay (EIA) for the antibody to HCV. Currently, a third-generation EIA is used, with a sensitivity and specificity of 99% in immunocompetent people. If the EIA is positive, the practitioner may proceed to a confirmatory recombinant immunoblot assay (RIBA) in individuals with a low pretest probability, or to direct measurement of HCV RNA by reverse-transcription polymerase chain reaction (PCR) in individuals with a high pretest probability. Figure 1 depicts the HCV testing algorithm recommended by the CDC.
FIGURE 1 HCV infection testing algorithm
ALT=alanine aminotransferase; EIA=enzyme immunoassay; PCR=polymerase chain reaction; RIBA=recombinant immunoblast assay Source: Centers for Disease Control and Prevention
How is HCV transmitted?
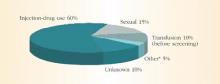
HCV spreads primarily through blood or fluids containing blood. Injection-drug use accounts for 60% of infections;1 a person who has used injection drugs for 5 years has a 60% to 90% chance of becoming infected with HCV. In fact, among injection-drug users, HCV infection is 4 times more common than HIV infection.8 The transfusion of blood or blood products accounts for another 10% of HCV cases. However, the transfusion-related risk has decreased markedly since the introduction of HCV screening in the blood banks in 1992. Figure 2 shows additional sources of HCV infection.
Although sexual transmission of HCV does occur, the virus is inefficiently spread in this manner. Evidence for this route of transmission has been accumulated from case-control and partner studies. Case-control studies have reported an association between HCV infection and exposure to a sex partner with a history of hepatitis or exposure to multiple sex partners.9,10 Cross-sectional studies suggest that the probability of HCV infection in the sexual partner of an HCV-positive patient is 0% to 3% in northern Europe or North America. Higher probabilities are found in southern Europe and the Far East.11 One prospective study showed no cases of sexual transmission of HCV from 94 HCV-positive females to their male part-ners.12 Another prospective study found a low incidence—12 infections per 1,000 per-son-years—among 449 sexual partners of HCV-positive individuals.13
Since the risk of spreading HCV through sexual contact is relatively low, individuals in long-term, stable relationships with an infected partner need not change their sexual practices. However, they may choose to use barrier methods to lower the chance of transmission even further. Individuals with high-risk sexual practices, such as having multiple sexual partners, should definitely use barrier methods.
FIGURE 2 Sources of infection for persons with hepatitis C
*Nosocomial, occupational, perinatal
Source: Centers for Disease Control and Prevention
What is the natural history of HCV infection?
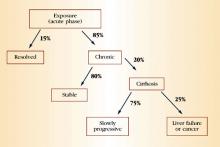
HCV accounts for approximately 20% of cases of acute hepatitis in the United States.14 The incubation period is approximately 6 weeks. Children and adults who acquire the infection usually are asymptomatic or have nonspecific symptoms of fatigue, malaise, anorexia, and weight loss. Some patients may present with jaundice. Serum aminotransferases can fluctuate during acute infection, and normalization occurs in 40% of individuals. However, this normalization does not necessarily represent a clearance of infection. Only 15% of patients clear the virus; the rest develop chronic infection.
Only 15% of patients clear the virus; the rest develop chronic infection.
During chronic infection, most patients are unaware that they are HCV-positive because, as with acute infection, they are asymptomatic or have mild, nonspecific symptoms such as fatigue. Again, serum aminotransferases typically fluctuate and are normal 30% of the time. Clinical progression to cirrhosis occurs in 20% of cases over a span of about 20 years. Once cirrhosis develops, the risk of decompensated cirrhosis or liver failure is about 3% to 4% per year. The annual risk of primary hepatocellular carcinoma in patients with cirrhosis is 1% to 4%. Data suggest that alcohol use is a predictive factor for progressive liver disease and liver cancer. Figure 3 summarizes the natural history of HCV infection.
FIGURE 3 Natural history of HCV infection
Is there a treatment for hepatitis C?
Over the past decade, there have been incremental improvements in hepatitis C therapy. The original mainstay of treatment was interferon alpha, with an initial response rate of 50% and a sustained response rate of only 10% to 15%.15-17 Later, combination therapy with interferon alpha and ribavirin raised the sustained response rate to 40%.18,19 However, both these therapies have substantial side effects and require frequent dosing schedules.
In January 2001, a new therapy, pegylated interferon alpha, was introduced. This modified form of traditional interferon alpha has fewer side effects and requires less frequent dosing. In addition, initial studies showed that it had a greater sustained-response rate than traditional interferon alpha alone.20 The Food and Drug Administration (FDA) recently approved it for the treatment of chronic hepatitis not previously treated with standard interferon alpha. A recent trial showed that the combination of pegylated interferon alpha with ribavirin had a sustained virologic response rate of 54%.21 None of these medications are approved for use in pregnancy or childhood.
To prevent progressive liver disease, patients chronically infected with HCV should avoid alcohol and other hepatotoxins and get vaccinated against hepatitis A and B. To reduce the risk of transmission to others, they should be advised not to donate blood and tissues; not to share toothbrushes, razors, or other personal-care items that may have blood on them; and to cover cuts and sores on the skin.
TABLE 2
Characteristics of hepatitis viral infection in the U.S. population
| HEPATITIS | HEPATITIS | HEPATITIS | HEPATITIS | HEPATITIS | |
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Source/route of transmission | Feces/Fecal-oral | Blood and blood-derived body fluids/Percutaneous, permucosal | Blood and blood-derived body fluids/Percutaneous, permucosal | Blood and blood-derived body fluids/Percutaneous, permucosal | Feces/Fecal-oral |
| No. of acute infections (per year) | 125,000-200,000 | 140,000-320,000 | 35,000-180,000 | 6,000-13,000 | Rare in the the United States |
| No. of chronic infections | 0 | 1-1.25 million | 3.5 million | 70,000 | 0 |
| Deaths from chronic liver disease (per year) | 0 | 5,000 | 8,000-10,000 | 1,000 | NA |
| Symptoms | Fatigue, nausea, pain near liver or in upper-abdominal area, dark urine, light stools, fever, jaundice | Same as hepatitis A. However, almost 50% of people infected with hepatitis B are asymptomatic. | Same as hepatitis A. However,most people are asymptomatic | Similar to other types of viral hepatitis | Similar to other types of viral hepatitis |
| Prevention | Good personal hygiene and proper sanitation. Also pre- or postexposure immunization | Pre- or post-exposure immunization | Blood donor screening; risk behavior modification | Pre- or post-exposure immunization | Ensure safe drinking water |
| Comments | Typically lasts about 3 weeks, but may persist for as long as 6 months | — | Originally defined in the 1970s as non-A non-B hepatitis. Renamed hepatitis C in 1989. | Requires the hepatitis B virus to exist* | Usually associated with fecally contaminated drinking water. U.S. cases usually involve a history of travel to areas with endemic hepatitis E infection |
| *Hepatitis D infection can be acquired either as a co-infection with the hepatitis B virus or as a superinfection in persons with chronic hepatitis B infection. Persons coinfected with the B and D viruses may have more severe acute disease and a higher risk of fulminant hepatitis (2% to 20%) compared with those infected with hepatitis B alone. However, chronic hepatitis B infection appears to occur less frequently in persons coinfected with the B and D viruses. Chronic hepatitis B carriers who acquire hepatitis D superinfection usually develop chronic hepatitis D infection. In long-term studies of chronic hepatitis B carriers with hepatitis D superinfection, 70% to 80% have developed evidence of chronic liver diseases with cirrhosis compared with 15% to 30% of patients with chronic hepatitis B infection alone. | |||||
| Source: American Liver Foundation, Centers for Disease Control and Prevention | |||||
How will hepatitis C affect my pregnancy?
Perinatal transmission occurs in women with chronic HCV infection at an average rate of 5%.22-24 Small studies suggest that the level of HCV viremia may affect the risk of viral transmission.22,25 However, in 2 large studies, no significant difference in HCV viremia levels was found between women who transmitted the virus to their children and women who did not.23,24 Women co-infected with HIV have 2 to 3 times the rate of HCV transmission compared with women infected only with HCV.26,27 The natural history of HCV infection in children is not well understood, but 50% to 80% of HCV-positive children develop chronic infection.28
Currently, no intervention is available to decrease perinatal HCV transmission.
Currently, no intervention is available to decrease perinatal HCV transmission, although an unclear association with mode of delivery exists. One study of 441 mother-infant pairs showed that elective cesarean delivery may be associated with a lower transmission risk.29 However, another study of 370 mother-infant pairs found no difference in risk.24
The effect of pregnancy on the progression of hepatitis C infection also is unclear. In studies of HCV-positive pregnant women—as compared to HCV-positive non-pregnant women—researchers reported a decrease in serum alanine aminotransferase (ALT) and an increase in HCV RNA during pregnancy,30 as well as a deterioration of hepatitis C disease after pregnancy, as confirmed by liver biopsy.31 In prospective cohort studies comparing infected and uninfected pregnant women, serum aminotrans-ferases flared postpartum in one study32 but remained normal in another.33
Not much is known regarding the effect of chronic hepatitis C infection on pregnancy outcome, although 1 case-control study reported an association between hepatitis C infection and cholestasis of pregnancy.34
Although HCV RNA has been detected in colostrum,35 studies of breastfed infants with HCV-positive mothers have found no cases of transmission secondary to breastfeeding.35,36 Thus, both the American Academy of Pediatrics and ACOG conclude that breastfeeding does not appreciably increase the risk of transmission to the neonate and should not be prohibited.37,38 However, pending further studies, practitioners may choose to advise against breastfeeding if nipples are cracked and bleeding.
- Approximately 25% of people with chronic HCV infection are asymptomatic with normal liver function tests and benign histology.
- Only 10% of people recognize acute infection when they acquire HCV. In the vast majority of cases, the disease is subclinical.
- The “epidemic” of HCV infection represents greater identification of chronic cases rather than increasing numbers of new outbreaks.
- Most new cases of hepatitis C occur in young adults, ages 25 to 40, who may not learn for years or even decades that they are infected.
- African Americans are twice as likely as non-Hispanic whites to be infected with HCV. Hispanics, too, are more likely than non-Hispanic whites to be infected.
- Infection with the human immunodeficiency virus (HIV) appears to accelerate the course of HCV, as does co-infection with the hepatitis B virus.
- Due to improvements in blood bank screening and a decrease in the use of IV drugs, the number of new cases of HCV diagnosed in 1997 was only about 37,000—an 80% decline. Nevertheless, over the next 1 to 2 decades, the HCV mortality rate is expected to double.
- Total expenditures for HCV therapy are thought to range from $10,000 to $12,000 when the costs of interferon, ribavirin, laboratory studies, and office visits are taken into account.
Acute infection with the hepatitis C virus (HCV) progresses to chronic disease in 50% to 84% of cases. Once HCV infection is chronic, combination therapy with interferon alfa-2b and ribavirin elicits a sustained virologic response in only 41% of cases. Even the latest therapy for chronic HCV—a combination of pegylated interferon alfa-2a or 2b and ribavirin—eradicates the virus in only 54% of patients.1 For these reasons, a team of researchers in Germany explored the effectiveness of treating HCV during the acute phase of infection using high-dose interferon alfa-2b to prevent progression.2
‘Peginterferons’ elicit a greater sustained virologic response.
Because the viral load of patients with HCV infection starts to rise within 24 hours after a single dose of inter-feron alfa-2b, investigators used a daily dosing regimen—rather than the standard thrice-weekly therapy—for the first 4 weeks of treatment. They then followed the standard schedule for another 20 weeks, measuring serum HCV RNA levels before, during, and 24 weeks after the termination of therapy.
All 44 patients in the study were given 5 million units of subcutaneous interferon alfa-2b daily for 4 weeks, after which time they received the same dosage 3 times a week. Twenty-four weeks after the end of therapy, 43 patients (98%) had normal serum alanine aminotransferase (ALT) and undetectable levels of serum HCV RNA. Side effects caused 1 patient to stop treatment after 12 weeks.
Although the investigators concluded that the treatment of acute HCV infection with interferon alfa-2b prevents progression to chronic disease, a number of issues remain unresolved. The primary one is whether all patients with acute disease should undergo treatment with a costly therapy that can be hard to tolerate, when many of these patients would recover without it. Further, since pegylated interferon has proven more effective than standard interferon in treating chronic HCV, it also may be more effective in treating acute disease. (“Peginterferons,” as they are called, can be administered weekly and elicit a greater sustained virologic response.) Finally, since acute infection is no longer common in the United States—dropping from 250,000 to 500,000 cases annually in the 1980s to less than 40,000 today—and since not all infections are clinically acute, this approach has limited applicability.3
These and other issues require further exploration. —Katherine Chen, MD, MPH
REFERENCES
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2b plus rib-avirin for initial treatment of chronic hepatitis C: results of a randomized trial. Lancet. 2001;358:958-965.
2. Jaeckel E, Cornberg M, Wedemeyer H, et al. for the German Acute Hepatitis C Therapy Group. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345(20):1452-1457.
3. Hoofnagle JH. Therapy for acute hepatitis C [editorial]. N Engl J Med. 2001;345(20):1495-1497.
Conclusion
Chronic HCV infection can lead to serious medical consequences, such as cirrhosis, liver failure, hepatocellular cancer, and death. Unfortunately, 75% of individuals infected with HCV are asymptomatic. By the time symptoms do occur, the disease often is in its advanced stages. Thus, both the CDC and ACOG recommend that Ob/Gyns screen women in high-risk groups. The early identification of chronic HCV infection is important, as it enables women to take advantage of increasingly effective treatments and alerts them to the need for preconception counseling.
Both the CDC and ACOG recommend that Ob/Gyns screen women in high-risk groups.
Although perinatal HCV transmission is a known risk, we lack strategies to prevent it. In addition, the effects of pregnancy on the progression of chronic HCV infection, and the effects of chronic HCV infection on pregnancy outcome, are unknown. However, the identification of HCV-positive pregnant women will lead to screening of their infants and earlier identification of infected children.
1. Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562.
2. National Center for Infectious Diseases. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncidod/dis-eases/hepatitis/slideset/intro/slide_5.htm. Accessed January 11, 2002.
3. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47:1-39.
4. American College of Obstetricians and Gynecologists. Primary and preventive care: periodic assessments. ACOG Committee Opinion #229. Washington, DC: ACOG; 1999.
5. Burns DN, Minkoff H. Hepatitis C: screening in pregnancy. Obstet Gynecol. 1999;94:1044-1048.
6. World Health Organization. Global prevalence of hepatitis C, 1999. Available at: http://www.who.int/emc/images/hepacmap.jpg. Accessed August 31, 2001.
7. Arthur RR, Hassan NF, Abdallah MY, et al. Hepatitis C antibody prevalence in blood donors in different governorates in Egypt. Trans R Soc Trop Med Hyg. 1997;91:271-274.
8. Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lym-photropic viruses. Am J Public Health. 1996;86:655-661.
9. Alter MJ, Gerety RJ, Smallwood LA, Sampliner RE, Tabor E, Deinhardt F, et al. Sporadic non-A, non-B hepatitis: frequency and epidemiology in an urban U.S. population. J Infect Dis. 1982;145:886-893.
10. Alter MJ, Coleman PJ, Alexander WJ, et al. Importance of heterosexual activity in the transmission of hepatitis B and non-A, non-B hepatitis. JAMA. 1989;262:1201-1205.
11. Rooney G, Gilson RJ. Sexual transmission of hepatitis C virus infection. Sex Transm Infect. 1998;74:399-404.
12. Meisel H, Reip A, Faltus B, et al. Transmission of hepatitis C virus to children and husbands by women infected with contaminated anti-D immunoglobulin. Lancet. 1995;345:1209-1211.
13. Piazza M, Sagliocca L, Tosone G, et al. Sexual transmission of the hepatitis C virus and efficacy of prophylaxis with intramuscular immune serum globulin. A randomized controlled trial. Arch Intern Med. 1997;157:1537-1544.
14. Cheney CP, Chopra S, Graham C, Hepatitis C. Infect Dis Clin North Am. 2000;14:633-667.
15. Davis GL, Balart LA, Schiff ER, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989;321:1501-1506.
16. Di Bisceglie AM, Martin P, Kassianides C, et al. Recombinant interfer-on alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506-1510.
17. Marcellin P, Boyer N, Giostra E, et al. Recombinant human alpha-interferon in patients with chronic non-A, non-B hepatitis: a multicenter randomized controlled trial from France. Hepatology. 1991;13:393-397.
18. McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492.
19. Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha-2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426-1432.
20. Reddy KR, Wright TL, Pockros PJ, et al. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33:433-438.
21. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2b plus rib-avirin for initial treatment of chronic hepatitis C: results of a randomized trial. Lancet. 2001;358:958-965.
22. Ohto H, Terazawa S, Sasaki N, et al. Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group. N Engl J Med. 1994;30:744-750.
23. Resti M, Azzari C, Mannelli F, et al. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ. 1998;317:437-441.
24. Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
25. Lin HH, Kao JH, Hsu HY, et al. Possible role of high-titer maternal viremia in perinatal transmission of hepatitis C virus. J Infect Dis. 1994;169:638-641.
26. Thomas DL, et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1-infected mothers. Women and Infants Transmission Study. J Infect Dis. 1998;177:1480-1488.
27. Tovo PA, Palomba E, Ferraris G, et al. Increased risk of maternal-infant hepatitis C virus transmission for women coinfected with human immunodeficiency virus type 1. Italian Study Group for HCV Infection in Children. Clin Infect Dis. 1997;25:1121-1124.
28. Bortolotti F, Faggion S, Con P. Natural history of chronic viral hepatitis in childhood. Acta Gastroenterol Belg. 1998;61:198-201.
29. Lin HH, Kao JH, Hsu HY, et al. Absence of infection in breast-fed infants born to hepatitis C virus-infected mothers. J Pediatr. 1995;126:589-591.
30. Polywka S, Schroter M, Feucht HH, Zollner B, Laufs R. Low risk of vertical transmission of hepatitis C virus by breast milk. Clin Infect Dis. 1999;29:1327-1329.
31. Hepatitis C virus infection. American Academy of Pediatrics. Committee on Infectious Diseases. Pediatrics. 1998;101:481-485.
32. American College of Obstetricians and Gynecologists. Breastfeeding and the risk of hepatitis C virus transmission. ACOG Committee Opinion #220. Washington, DC: ACOG; 1999.
33. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
34. Gervais A, Bacq Y, Bernuau J, et al. Decrease in serum ALT and increase in serum HCV RNA during pregnancy in women with chronic hepatitis C. J Hepatol. 2000;32:293-299.
35. Fontaine H, Nalpas B, Carnot F, Brechot C, Pol S. Effect of pregnancy on chronic hepatitis C: a case-control study. Lancet. 2000;356:1328-1329.
36. Latt NC, Spencer JD, Beeby PJ, et al. Hepatitis C in injecting drug-using women during and after pregnancy. J Gastroenterol Hepatol. 2000;15:175-181.
37. Floreani A, Paternoster D, Zappala F, et al. Hepatitis C virus infection in pregnancy. Br J Obstet Gynaecol. 1996;103:325-329.
38. Locatelli A, Roncaglia N, Arreghini A, Bellini P, Vergani P, Ghidini A. Hepatitis C virus infection is associated with a higher incidence of cholestasis of pregnancy. Br J Obstet Gynaecol. 1999;106:498-500.
- The hepatitis C virus (HCV) is the leading cause of chronic liver disease and the leading indication for liver transplantation in the United States.
- In the United States, 3.9 million people have been infected with HCV, with an overall prevalence of 1.8%. In females, prevalence is highest during the childbearing years, peaking at age 35.
- Injection-drug use accounts for 60% of infections, while the transfusion of blood or blood products accounts for another 10%.
- About 15% of people with acute HCV infection clear the virus; the rest develop chronic infection.
- During chronic infection, most patients are asymptomatic or have mild, nonspecific symptoms such as fatigue.
- Combination therapy with interferon alpha and ribavirin elicits a sustained virologic response rate of 40%, and newer therapy with pegylated interferon alpha and ribavirin improves the rate to 54%.
- Perinatal transmission in women with chronic HCV infection occurs at an average rate of 5%.
The hepatitis C virus (HCV) was first identified in 1989 as the cause of non-A, non-B hepatitis infections. Since its discovery, HCV has become the most common chronic blood-borne infection in the United States: Approximately 2.7 to 3.5 million people have chronic HCV infection,1,2 as compared with 1.25 million people with chronic hepatitis B virus infection and 1 million with the human immunodeficiency virus (HIV). In addition, HCV infection is the leading cause of chronic liver disease and the leading indication for liver transplantation in the United States. Some call HCV infection “the silent epidemic,” since 75% of people infected are asymptomatic and chronic manifestations don’t appear for 1 to 2 decades.
In 1998, the Centers for Disease Control and Prevention (CDC) issued guidelines for screening for HCV infection ( Table 1).3 The American College of Obstetricians and Gynecologists (ACOG) advocates screening for HCV infection at the annual exam if the patient belongs to one of the CDC’s routine-screening categories.4 Although ACOG has not issued separate screening guidelines for obstetric patients, some practitioners have advocated screening based on risk factors, as listed in the CDC’s routine-screening categories.5
HCV accounts for about 20% of acute hepatitis cases in the U.S.
Because Ob/Gyns are increasingly likely to encounter patients with positive HCV blood-screening results, they should be prepared to answer the following inquiries: What is hepatitis C? How is it diagnosed and transmitted? What is the natural history of the infection? Is there a treatment for it? How will HCV infection affect pregnancy?
This review addresses those questions.
What is hepatitis C?
Hepatitis C is a liver disease caused by the HCV, an RNA virus of the Flavivirus family, which includes the dengue and yellow fever viruses. Worldwide, approximately 170 million people are infected with HCV, with a prevalence ranging from a low of 0.15% in Scandinavia to a high of 38% in northern Egypt.6,7 In the United States, 3.9 million people have been infected with HCV, with an overall prevalence of 1.8%.1 U.S. prevalence rates by gender and age reveal that more males are infected than females and that, in females, prevalence is highest during the childbearing years, peaking at age 35.
TABLE 1
Recommendations for hepatitis C virus screening
| PEOPLE WHO SHOULD BE TESTED ROUTINELY |
|---|
|
| PEOPLE FOR WHOM ROUTINE TESTING IS OF UNCERTAIN NEED |
|
| PEOPLE FOR WHOM ROUTINE TESTING IS NOT RECOMMENDED* |
|
| * Except in cases where risk factors are present ALT=alanine aminotransferase; HCV=hepatitis C virus |
| Source: Centers for Disease Control and Prevention |
How is HCV infection diagnosed?
The initial screening test is an enzyme immunoassay (EIA) for the antibody to HCV. Currently, a third-generation EIA is used, with a sensitivity and specificity of 99% in immunocompetent people. If the EIA is positive, the practitioner may proceed to a confirmatory recombinant immunoblot assay (RIBA) in individuals with a low pretest probability, or to direct measurement of HCV RNA by reverse-transcription polymerase chain reaction (PCR) in individuals with a high pretest probability. Figure 1 depicts the HCV testing algorithm recommended by the CDC.
FIGURE 1 HCV infection testing algorithm
ALT=alanine aminotransferase; EIA=enzyme immunoassay; PCR=polymerase chain reaction; RIBA=recombinant immunoblast assay Source: Centers for Disease Control and Prevention
How is HCV transmitted?
HCV spreads primarily through blood or fluids containing blood. Injection-drug use accounts for 60% of infections;1 a person who has used injection drugs for 5 years has a 60% to 90% chance of becoming infected with HCV. In fact, among injection-drug users, HCV infection is 4 times more common than HIV infection.8 The transfusion of blood or blood products accounts for another 10% of HCV cases. However, the transfusion-related risk has decreased markedly since the introduction of HCV screening in the blood banks in 1992. Figure 2 shows additional sources of HCV infection.
Although sexual transmission of HCV does occur, the virus is inefficiently spread in this manner. Evidence for this route of transmission has been accumulated from case-control and partner studies. Case-control studies have reported an association between HCV infection and exposure to a sex partner with a history of hepatitis or exposure to multiple sex partners.9,10 Cross-sectional studies suggest that the probability of HCV infection in the sexual partner of an HCV-positive patient is 0% to 3% in northern Europe or North America. Higher probabilities are found in southern Europe and the Far East.11 One prospective study showed no cases of sexual transmission of HCV from 94 HCV-positive females to their male part-ners.12 Another prospective study found a low incidence—12 infections per 1,000 per-son-years—among 449 sexual partners of HCV-positive individuals.13
Since the risk of spreading HCV through sexual contact is relatively low, individuals in long-term, stable relationships with an infected partner need not change their sexual practices. However, they may choose to use barrier methods to lower the chance of transmission even further. Individuals with high-risk sexual practices, such as having multiple sexual partners, should definitely use barrier methods.
FIGURE 2 Sources of infection for persons with hepatitis C
*Nosocomial, occupational, perinatal
Source: Centers for Disease Control and Prevention
What is the natural history of HCV infection?
HCV accounts for approximately 20% of cases of acute hepatitis in the United States.14 The incubation period is approximately 6 weeks. Children and adults who acquire the infection usually are asymptomatic or have nonspecific symptoms of fatigue, malaise, anorexia, and weight loss. Some patients may present with jaundice. Serum aminotransferases can fluctuate during acute infection, and normalization occurs in 40% of individuals. However, this normalization does not necessarily represent a clearance of infection. Only 15% of patients clear the virus; the rest develop chronic infection.
Only 15% of patients clear the virus; the rest develop chronic infection.
During chronic infection, most patients are unaware that they are HCV-positive because, as with acute infection, they are asymptomatic or have mild, nonspecific symptoms such as fatigue. Again, serum aminotransferases typically fluctuate and are normal 30% of the time. Clinical progression to cirrhosis occurs in 20% of cases over a span of about 20 years. Once cirrhosis develops, the risk of decompensated cirrhosis or liver failure is about 3% to 4% per year. The annual risk of primary hepatocellular carcinoma in patients with cirrhosis is 1% to 4%. Data suggest that alcohol use is a predictive factor for progressive liver disease and liver cancer. Figure 3 summarizes the natural history of HCV infection.
FIGURE 3 Natural history of HCV infection
Is there a treatment for hepatitis C?
Over the past decade, there have been incremental improvements in hepatitis C therapy. The original mainstay of treatment was interferon alpha, with an initial response rate of 50% and a sustained response rate of only 10% to 15%.15-17 Later, combination therapy with interferon alpha and ribavirin raised the sustained response rate to 40%.18,19 However, both these therapies have substantial side effects and require frequent dosing schedules.
In January 2001, a new therapy, pegylated interferon alpha, was introduced. This modified form of traditional interferon alpha has fewer side effects and requires less frequent dosing. In addition, initial studies showed that it had a greater sustained-response rate than traditional interferon alpha alone.20 The Food and Drug Administration (FDA) recently approved it for the treatment of chronic hepatitis not previously treated with standard interferon alpha. A recent trial showed that the combination of pegylated interferon alpha with ribavirin had a sustained virologic response rate of 54%.21 None of these medications are approved for use in pregnancy or childhood.
To prevent progressive liver disease, patients chronically infected with HCV should avoid alcohol and other hepatotoxins and get vaccinated against hepatitis A and B. To reduce the risk of transmission to others, they should be advised not to donate blood and tissues; not to share toothbrushes, razors, or other personal-care items that may have blood on them; and to cover cuts and sores on the skin.
TABLE 2
Characteristics of hepatitis viral infection in the U.S. population
| HEPATITIS | HEPATITIS | HEPATITIS | HEPATITIS | HEPATITIS | |
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Source/route of transmission | Feces/Fecal-oral | Blood and blood-derived body fluids/Percutaneous, permucosal | Blood and blood-derived body fluids/Percutaneous, permucosal | Blood and blood-derived body fluids/Percutaneous, permucosal | Feces/Fecal-oral |
| No. of acute infections (per year) | 125,000-200,000 | 140,000-320,000 | 35,000-180,000 | 6,000-13,000 | Rare in the the United States |
| No. of chronic infections | 0 | 1-1.25 million | 3.5 million | 70,000 | 0 |
| Deaths from chronic liver disease (per year) | 0 | 5,000 | 8,000-10,000 | 1,000 | NA |
| Symptoms | Fatigue, nausea, pain near liver or in upper-abdominal area, dark urine, light stools, fever, jaundice | Same as hepatitis A. However, almost 50% of people infected with hepatitis B are asymptomatic. | Same as hepatitis A. However,most people are asymptomatic | Similar to other types of viral hepatitis | Similar to other types of viral hepatitis |
| Prevention | Good personal hygiene and proper sanitation. Also pre- or postexposure immunization | Pre- or post-exposure immunization | Blood donor screening; risk behavior modification | Pre- or post-exposure immunization | Ensure safe drinking water |
| Comments | Typically lasts about 3 weeks, but may persist for as long as 6 months | — | Originally defined in the 1970s as non-A non-B hepatitis. Renamed hepatitis C in 1989. | Requires the hepatitis B virus to exist* | Usually associated with fecally contaminated drinking water. U.S. cases usually involve a history of travel to areas with endemic hepatitis E infection |
| *Hepatitis D infection can be acquired either as a co-infection with the hepatitis B virus or as a superinfection in persons with chronic hepatitis B infection. Persons coinfected with the B and D viruses may have more severe acute disease and a higher risk of fulminant hepatitis (2% to 20%) compared with those infected with hepatitis B alone. However, chronic hepatitis B infection appears to occur less frequently in persons coinfected with the B and D viruses. Chronic hepatitis B carriers who acquire hepatitis D superinfection usually develop chronic hepatitis D infection. In long-term studies of chronic hepatitis B carriers with hepatitis D superinfection, 70% to 80% have developed evidence of chronic liver diseases with cirrhosis compared with 15% to 30% of patients with chronic hepatitis B infection alone. | |||||
| Source: American Liver Foundation, Centers for Disease Control and Prevention | |||||
How will hepatitis C affect my pregnancy?
Perinatal transmission occurs in women with chronic HCV infection at an average rate of 5%.22-24 Small studies suggest that the level of HCV viremia may affect the risk of viral transmission.22,25 However, in 2 large studies, no significant difference in HCV viremia levels was found between women who transmitted the virus to their children and women who did not.23,24 Women co-infected with HIV have 2 to 3 times the rate of HCV transmission compared with women infected only with HCV.26,27 The natural history of HCV infection in children is not well understood, but 50% to 80% of HCV-positive children develop chronic infection.28
Currently, no intervention is available to decrease perinatal HCV transmission.
Currently, no intervention is available to decrease perinatal HCV transmission, although an unclear association with mode of delivery exists. One study of 441 mother-infant pairs showed that elective cesarean delivery may be associated with a lower transmission risk.29 However, another study of 370 mother-infant pairs found no difference in risk.24
The effect of pregnancy on the progression of hepatitis C infection also is unclear. In studies of HCV-positive pregnant women—as compared to HCV-positive non-pregnant women—researchers reported a decrease in serum alanine aminotransferase (ALT) and an increase in HCV RNA during pregnancy,30 as well as a deterioration of hepatitis C disease after pregnancy, as confirmed by liver biopsy.31 In prospective cohort studies comparing infected and uninfected pregnant women, serum aminotrans-ferases flared postpartum in one study32 but remained normal in another.33
Not much is known regarding the effect of chronic hepatitis C infection on pregnancy outcome, although 1 case-control study reported an association between hepatitis C infection and cholestasis of pregnancy.34
Although HCV RNA has been detected in colostrum,35 studies of breastfed infants with HCV-positive mothers have found no cases of transmission secondary to breastfeeding.35,36 Thus, both the American Academy of Pediatrics and ACOG conclude that breastfeeding does not appreciably increase the risk of transmission to the neonate and should not be prohibited.37,38 However, pending further studies, practitioners may choose to advise against breastfeeding if nipples are cracked and bleeding.
- Approximately 25% of people with chronic HCV infection are asymptomatic with normal liver function tests and benign histology.
- Only 10% of people recognize acute infection when they acquire HCV. In the vast majority of cases, the disease is subclinical.
- The “epidemic” of HCV infection represents greater identification of chronic cases rather than increasing numbers of new outbreaks.
- Most new cases of hepatitis C occur in young adults, ages 25 to 40, who may not learn for years or even decades that they are infected.
- African Americans are twice as likely as non-Hispanic whites to be infected with HCV. Hispanics, too, are more likely than non-Hispanic whites to be infected.
- Infection with the human immunodeficiency virus (HIV) appears to accelerate the course of HCV, as does co-infection with the hepatitis B virus.
- Due to improvements in blood bank screening and a decrease in the use of IV drugs, the number of new cases of HCV diagnosed in 1997 was only about 37,000—an 80% decline. Nevertheless, over the next 1 to 2 decades, the HCV mortality rate is expected to double.
- Total expenditures for HCV therapy are thought to range from $10,000 to $12,000 when the costs of interferon, ribavirin, laboratory studies, and office visits are taken into account.
Acute infection with the hepatitis C virus (HCV) progresses to chronic disease in 50% to 84% of cases. Once HCV infection is chronic, combination therapy with interferon alfa-2b and ribavirin elicits a sustained virologic response in only 41% of cases. Even the latest therapy for chronic HCV—a combination of pegylated interferon alfa-2a or 2b and ribavirin—eradicates the virus in only 54% of patients.1 For these reasons, a team of researchers in Germany explored the effectiveness of treating HCV during the acute phase of infection using high-dose interferon alfa-2b to prevent progression.2
‘Peginterferons’ elicit a greater sustained virologic response.
Because the viral load of patients with HCV infection starts to rise within 24 hours after a single dose of inter-feron alfa-2b, investigators used a daily dosing regimen—rather than the standard thrice-weekly therapy—for the first 4 weeks of treatment. They then followed the standard schedule for another 20 weeks, measuring serum HCV RNA levels before, during, and 24 weeks after the termination of therapy.
All 44 patients in the study were given 5 million units of subcutaneous interferon alfa-2b daily for 4 weeks, after which time they received the same dosage 3 times a week. Twenty-four weeks after the end of therapy, 43 patients (98%) had normal serum alanine aminotransferase (ALT) and undetectable levels of serum HCV RNA. Side effects caused 1 patient to stop treatment after 12 weeks.
Although the investigators concluded that the treatment of acute HCV infection with interferon alfa-2b prevents progression to chronic disease, a number of issues remain unresolved. The primary one is whether all patients with acute disease should undergo treatment with a costly therapy that can be hard to tolerate, when many of these patients would recover without it. Further, since pegylated interferon has proven more effective than standard interferon in treating chronic HCV, it also may be more effective in treating acute disease. (“Peginterferons,” as they are called, can be administered weekly and elicit a greater sustained virologic response.) Finally, since acute infection is no longer common in the United States—dropping from 250,000 to 500,000 cases annually in the 1980s to less than 40,000 today—and since not all infections are clinically acute, this approach has limited applicability.3
These and other issues require further exploration. —Katherine Chen, MD, MPH
REFERENCES
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2b plus rib-avirin for initial treatment of chronic hepatitis C: results of a randomized trial. Lancet. 2001;358:958-965.
2. Jaeckel E, Cornberg M, Wedemeyer H, et al. for the German Acute Hepatitis C Therapy Group. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345(20):1452-1457.
3. Hoofnagle JH. Therapy for acute hepatitis C [editorial]. N Engl J Med. 2001;345(20):1495-1497.
Conclusion
Chronic HCV infection can lead to serious medical consequences, such as cirrhosis, liver failure, hepatocellular cancer, and death. Unfortunately, 75% of individuals infected with HCV are asymptomatic. By the time symptoms do occur, the disease often is in its advanced stages. Thus, both the CDC and ACOG recommend that Ob/Gyns screen women in high-risk groups. The early identification of chronic HCV infection is important, as it enables women to take advantage of increasingly effective treatments and alerts them to the need for preconception counseling.
Both the CDC and ACOG recommend that Ob/Gyns screen women in high-risk groups.
Although perinatal HCV transmission is a known risk, we lack strategies to prevent it. In addition, the effects of pregnancy on the progression of chronic HCV infection, and the effects of chronic HCV infection on pregnancy outcome, are unknown. However, the identification of HCV-positive pregnant women will lead to screening of their infants and earlier identification of infected children.
- The hepatitis C virus (HCV) is the leading cause of chronic liver disease and the leading indication for liver transplantation in the United States.
- In the United States, 3.9 million people have been infected with HCV, with an overall prevalence of 1.8%. In females, prevalence is highest during the childbearing years, peaking at age 35.
- Injection-drug use accounts for 60% of infections, while the transfusion of blood or blood products accounts for another 10%.
- About 15% of people with acute HCV infection clear the virus; the rest develop chronic infection.
- During chronic infection, most patients are asymptomatic or have mild, nonspecific symptoms such as fatigue.
- Combination therapy with interferon alpha and ribavirin elicits a sustained virologic response rate of 40%, and newer therapy with pegylated interferon alpha and ribavirin improves the rate to 54%.
- Perinatal transmission in women with chronic HCV infection occurs at an average rate of 5%.
The hepatitis C virus (HCV) was first identified in 1989 as the cause of non-A, non-B hepatitis infections. Since its discovery, HCV has become the most common chronic blood-borne infection in the United States: Approximately 2.7 to 3.5 million people have chronic HCV infection,1,2 as compared with 1.25 million people with chronic hepatitis B virus infection and 1 million with the human immunodeficiency virus (HIV). In addition, HCV infection is the leading cause of chronic liver disease and the leading indication for liver transplantation in the United States. Some call HCV infection “the silent epidemic,” since 75% of people infected are asymptomatic and chronic manifestations don’t appear for 1 to 2 decades.
In 1998, the Centers for Disease Control and Prevention (CDC) issued guidelines for screening for HCV infection ( Table 1).3 The American College of Obstetricians and Gynecologists (ACOG) advocates screening for HCV infection at the annual exam if the patient belongs to one of the CDC’s routine-screening categories.4 Although ACOG has not issued separate screening guidelines for obstetric patients, some practitioners have advocated screening based on risk factors, as listed in the CDC’s routine-screening categories.5
HCV accounts for about 20% of acute hepatitis cases in the U.S.
Because Ob/Gyns are increasingly likely to encounter patients with positive HCV blood-screening results, they should be prepared to answer the following inquiries: What is hepatitis C? How is it diagnosed and transmitted? What is the natural history of the infection? Is there a treatment for it? How will HCV infection affect pregnancy?
This review addresses those questions.
What is hepatitis C?
Hepatitis C is a liver disease caused by the HCV, an RNA virus of the Flavivirus family, which includes the dengue and yellow fever viruses. Worldwide, approximately 170 million people are infected with HCV, with a prevalence ranging from a low of 0.15% in Scandinavia to a high of 38% in northern Egypt.6,7 In the United States, 3.9 million people have been infected with HCV, with an overall prevalence of 1.8%.1 U.S. prevalence rates by gender and age reveal that more males are infected than females and that, in females, prevalence is highest during the childbearing years, peaking at age 35.
TABLE 1
Recommendations for hepatitis C virus screening
| PEOPLE WHO SHOULD BE TESTED ROUTINELY |
|---|
|
| PEOPLE FOR WHOM ROUTINE TESTING IS OF UNCERTAIN NEED |
|
| PEOPLE FOR WHOM ROUTINE TESTING IS NOT RECOMMENDED* |
|
| * Except in cases where risk factors are present ALT=alanine aminotransferase; HCV=hepatitis C virus |
| Source: Centers for Disease Control and Prevention |
How is HCV infection diagnosed?
The initial screening test is an enzyme immunoassay (EIA) for the antibody to HCV. Currently, a third-generation EIA is used, with a sensitivity and specificity of 99% in immunocompetent people. If the EIA is positive, the practitioner may proceed to a confirmatory recombinant immunoblot assay (RIBA) in individuals with a low pretest probability, or to direct measurement of HCV RNA by reverse-transcription polymerase chain reaction (PCR) in individuals with a high pretest probability. Figure 1 depicts the HCV testing algorithm recommended by the CDC.
FIGURE 1 HCV infection testing algorithm
ALT=alanine aminotransferase; EIA=enzyme immunoassay; PCR=polymerase chain reaction; RIBA=recombinant immunoblast assay Source: Centers for Disease Control and Prevention
How is HCV transmitted?
HCV spreads primarily through blood or fluids containing blood. Injection-drug use accounts for 60% of infections;1 a person who has used injection drugs for 5 years has a 60% to 90% chance of becoming infected with HCV. In fact, among injection-drug users, HCV infection is 4 times more common than HIV infection.8 The transfusion of blood or blood products accounts for another 10% of HCV cases. However, the transfusion-related risk has decreased markedly since the introduction of HCV screening in the blood banks in 1992. Figure 2 shows additional sources of HCV infection.
Although sexual transmission of HCV does occur, the virus is inefficiently spread in this manner. Evidence for this route of transmission has been accumulated from case-control and partner studies. Case-control studies have reported an association between HCV infection and exposure to a sex partner with a history of hepatitis or exposure to multiple sex partners.9,10 Cross-sectional studies suggest that the probability of HCV infection in the sexual partner of an HCV-positive patient is 0% to 3% in northern Europe or North America. Higher probabilities are found in southern Europe and the Far East.11 One prospective study showed no cases of sexual transmission of HCV from 94 HCV-positive females to their male part-ners.12 Another prospective study found a low incidence—12 infections per 1,000 per-son-years—among 449 sexual partners of HCV-positive individuals.13
Since the risk of spreading HCV through sexual contact is relatively low, individuals in long-term, stable relationships with an infected partner need not change their sexual practices. However, they may choose to use barrier methods to lower the chance of transmission even further. Individuals with high-risk sexual practices, such as having multiple sexual partners, should definitely use barrier methods.
FIGURE 2 Sources of infection for persons with hepatitis C
*Nosocomial, occupational, perinatal
Source: Centers for Disease Control and Prevention
What is the natural history of HCV infection?
HCV accounts for approximately 20% of cases of acute hepatitis in the United States.14 The incubation period is approximately 6 weeks. Children and adults who acquire the infection usually are asymptomatic or have nonspecific symptoms of fatigue, malaise, anorexia, and weight loss. Some patients may present with jaundice. Serum aminotransferases can fluctuate during acute infection, and normalization occurs in 40% of individuals. However, this normalization does not necessarily represent a clearance of infection. Only 15% of patients clear the virus; the rest develop chronic infection.
Only 15% of patients clear the virus; the rest develop chronic infection.
During chronic infection, most patients are unaware that they are HCV-positive because, as with acute infection, they are asymptomatic or have mild, nonspecific symptoms such as fatigue. Again, serum aminotransferases typically fluctuate and are normal 30% of the time. Clinical progression to cirrhosis occurs in 20% of cases over a span of about 20 years. Once cirrhosis develops, the risk of decompensated cirrhosis or liver failure is about 3% to 4% per year. The annual risk of primary hepatocellular carcinoma in patients with cirrhosis is 1% to 4%. Data suggest that alcohol use is a predictive factor for progressive liver disease and liver cancer. Figure 3 summarizes the natural history of HCV infection.
FIGURE 3 Natural history of HCV infection
Is there a treatment for hepatitis C?
Over the past decade, there have been incremental improvements in hepatitis C therapy. The original mainstay of treatment was interferon alpha, with an initial response rate of 50% and a sustained response rate of only 10% to 15%.15-17 Later, combination therapy with interferon alpha and ribavirin raised the sustained response rate to 40%.18,19 However, both these therapies have substantial side effects and require frequent dosing schedules.
In January 2001, a new therapy, pegylated interferon alpha, was introduced. This modified form of traditional interferon alpha has fewer side effects and requires less frequent dosing. In addition, initial studies showed that it had a greater sustained-response rate than traditional interferon alpha alone.20 The Food and Drug Administration (FDA) recently approved it for the treatment of chronic hepatitis not previously treated with standard interferon alpha. A recent trial showed that the combination of pegylated interferon alpha with ribavirin had a sustained virologic response rate of 54%.21 None of these medications are approved for use in pregnancy or childhood.
To prevent progressive liver disease, patients chronically infected with HCV should avoid alcohol and other hepatotoxins and get vaccinated against hepatitis A and B. To reduce the risk of transmission to others, they should be advised not to donate blood and tissues; not to share toothbrushes, razors, or other personal-care items that may have blood on them; and to cover cuts and sores on the skin.
TABLE 2
Characteristics of hepatitis viral infection in the U.S. population
| HEPATITIS | HEPATITIS | HEPATITIS | HEPATITIS | HEPATITIS | |
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Source/route of transmission | Feces/Fecal-oral | Blood and blood-derived body fluids/Percutaneous, permucosal | Blood and blood-derived body fluids/Percutaneous, permucosal | Blood and blood-derived body fluids/Percutaneous, permucosal | Feces/Fecal-oral |
| No. of acute infections (per year) | 125,000-200,000 | 140,000-320,000 | 35,000-180,000 | 6,000-13,000 | Rare in the the United States |
| No. of chronic infections | 0 | 1-1.25 million | 3.5 million | 70,000 | 0 |
| Deaths from chronic liver disease (per year) | 0 | 5,000 | 8,000-10,000 | 1,000 | NA |
| Symptoms | Fatigue, nausea, pain near liver or in upper-abdominal area, dark urine, light stools, fever, jaundice | Same as hepatitis A. However, almost 50% of people infected with hepatitis B are asymptomatic. | Same as hepatitis A. However,most people are asymptomatic | Similar to other types of viral hepatitis | Similar to other types of viral hepatitis |
| Prevention | Good personal hygiene and proper sanitation. Also pre- or postexposure immunization | Pre- or post-exposure immunization | Blood donor screening; risk behavior modification | Pre- or post-exposure immunization | Ensure safe drinking water |
| Comments | Typically lasts about 3 weeks, but may persist for as long as 6 months | — | Originally defined in the 1970s as non-A non-B hepatitis. Renamed hepatitis C in 1989. | Requires the hepatitis B virus to exist* | Usually associated with fecally contaminated drinking water. U.S. cases usually involve a history of travel to areas with endemic hepatitis E infection |
| *Hepatitis D infection can be acquired either as a co-infection with the hepatitis B virus or as a superinfection in persons with chronic hepatitis B infection. Persons coinfected with the B and D viruses may have more severe acute disease and a higher risk of fulminant hepatitis (2% to 20%) compared with those infected with hepatitis B alone. However, chronic hepatitis B infection appears to occur less frequently in persons coinfected with the B and D viruses. Chronic hepatitis B carriers who acquire hepatitis D superinfection usually develop chronic hepatitis D infection. In long-term studies of chronic hepatitis B carriers with hepatitis D superinfection, 70% to 80% have developed evidence of chronic liver diseases with cirrhosis compared with 15% to 30% of patients with chronic hepatitis B infection alone. | |||||
| Source: American Liver Foundation, Centers for Disease Control and Prevention | |||||
How will hepatitis C affect my pregnancy?
Perinatal transmission occurs in women with chronic HCV infection at an average rate of 5%.22-24 Small studies suggest that the level of HCV viremia may affect the risk of viral transmission.22,25 However, in 2 large studies, no significant difference in HCV viremia levels was found between women who transmitted the virus to their children and women who did not.23,24 Women co-infected with HIV have 2 to 3 times the rate of HCV transmission compared with women infected only with HCV.26,27 The natural history of HCV infection in children is not well understood, but 50% to 80% of HCV-positive children develop chronic infection.28
Currently, no intervention is available to decrease perinatal HCV transmission.
Currently, no intervention is available to decrease perinatal HCV transmission, although an unclear association with mode of delivery exists. One study of 441 mother-infant pairs showed that elective cesarean delivery may be associated with a lower transmission risk.29 However, another study of 370 mother-infant pairs found no difference in risk.24
The effect of pregnancy on the progression of hepatitis C infection also is unclear. In studies of HCV-positive pregnant women—as compared to HCV-positive non-pregnant women—researchers reported a decrease in serum alanine aminotransferase (ALT) and an increase in HCV RNA during pregnancy,30 as well as a deterioration of hepatitis C disease after pregnancy, as confirmed by liver biopsy.31 In prospective cohort studies comparing infected and uninfected pregnant women, serum aminotrans-ferases flared postpartum in one study32 but remained normal in another.33
Not much is known regarding the effect of chronic hepatitis C infection on pregnancy outcome, although 1 case-control study reported an association between hepatitis C infection and cholestasis of pregnancy.34
Although HCV RNA has been detected in colostrum,35 studies of breastfed infants with HCV-positive mothers have found no cases of transmission secondary to breastfeeding.35,36 Thus, both the American Academy of Pediatrics and ACOG conclude that breastfeeding does not appreciably increase the risk of transmission to the neonate and should not be prohibited.37,38 However, pending further studies, practitioners may choose to advise against breastfeeding if nipples are cracked and bleeding.
- Approximately 25% of people with chronic HCV infection are asymptomatic with normal liver function tests and benign histology.
- Only 10% of people recognize acute infection when they acquire HCV. In the vast majority of cases, the disease is subclinical.
- The “epidemic” of HCV infection represents greater identification of chronic cases rather than increasing numbers of new outbreaks.
- Most new cases of hepatitis C occur in young adults, ages 25 to 40, who may not learn for years or even decades that they are infected.
- African Americans are twice as likely as non-Hispanic whites to be infected with HCV. Hispanics, too, are more likely than non-Hispanic whites to be infected.
- Infection with the human immunodeficiency virus (HIV) appears to accelerate the course of HCV, as does co-infection with the hepatitis B virus.
- Due to improvements in blood bank screening and a decrease in the use of IV drugs, the number of new cases of HCV diagnosed in 1997 was only about 37,000—an 80% decline. Nevertheless, over the next 1 to 2 decades, the HCV mortality rate is expected to double.
- Total expenditures for HCV therapy are thought to range from $10,000 to $12,000 when the costs of interferon, ribavirin, laboratory studies, and office visits are taken into account.
Acute infection with the hepatitis C virus (HCV) progresses to chronic disease in 50% to 84% of cases. Once HCV infection is chronic, combination therapy with interferon alfa-2b and ribavirin elicits a sustained virologic response in only 41% of cases. Even the latest therapy for chronic HCV—a combination of pegylated interferon alfa-2a or 2b and ribavirin—eradicates the virus in only 54% of patients.1 For these reasons, a team of researchers in Germany explored the effectiveness of treating HCV during the acute phase of infection using high-dose interferon alfa-2b to prevent progression.2
‘Peginterferons’ elicit a greater sustained virologic response.
Because the viral load of patients with HCV infection starts to rise within 24 hours after a single dose of inter-feron alfa-2b, investigators used a daily dosing regimen—rather than the standard thrice-weekly therapy—for the first 4 weeks of treatment. They then followed the standard schedule for another 20 weeks, measuring serum HCV RNA levels before, during, and 24 weeks after the termination of therapy.
All 44 patients in the study were given 5 million units of subcutaneous interferon alfa-2b daily for 4 weeks, after which time they received the same dosage 3 times a week. Twenty-four weeks after the end of therapy, 43 patients (98%) had normal serum alanine aminotransferase (ALT) and undetectable levels of serum HCV RNA. Side effects caused 1 patient to stop treatment after 12 weeks.
Although the investigators concluded that the treatment of acute HCV infection with interferon alfa-2b prevents progression to chronic disease, a number of issues remain unresolved. The primary one is whether all patients with acute disease should undergo treatment with a costly therapy that can be hard to tolerate, when many of these patients would recover without it. Further, since pegylated interferon has proven more effective than standard interferon in treating chronic HCV, it also may be more effective in treating acute disease. (“Peginterferons,” as they are called, can be administered weekly and elicit a greater sustained virologic response.) Finally, since acute infection is no longer common in the United States—dropping from 250,000 to 500,000 cases annually in the 1980s to less than 40,000 today—and since not all infections are clinically acute, this approach has limited applicability.3
These and other issues require further exploration. —Katherine Chen, MD, MPH
REFERENCES
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2b plus rib-avirin for initial treatment of chronic hepatitis C: results of a randomized trial. Lancet. 2001;358:958-965.
2. Jaeckel E, Cornberg M, Wedemeyer H, et al. for the German Acute Hepatitis C Therapy Group. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345(20):1452-1457.
3. Hoofnagle JH. Therapy for acute hepatitis C [editorial]. N Engl J Med. 2001;345(20):1495-1497.
Conclusion
Chronic HCV infection can lead to serious medical consequences, such as cirrhosis, liver failure, hepatocellular cancer, and death. Unfortunately, 75% of individuals infected with HCV are asymptomatic. By the time symptoms do occur, the disease often is in its advanced stages. Thus, both the CDC and ACOG recommend that Ob/Gyns screen women in high-risk groups. The early identification of chronic HCV infection is important, as it enables women to take advantage of increasingly effective treatments and alerts them to the need for preconception counseling.
Both the CDC and ACOG recommend that Ob/Gyns screen women in high-risk groups.
Although perinatal HCV transmission is a known risk, we lack strategies to prevent it. In addition, the effects of pregnancy on the progression of chronic HCV infection, and the effects of chronic HCV infection on pregnancy outcome, are unknown. However, the identification of HCV-positive pregnant women will lead to screening of their infants and earlier identification of infected children.
1. Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562.
2. National Center for Infectious Diseases. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncidod/dis-eases/hepatitis/slideset/intro/slide_5.htm. Accessed January 11, 2002.
3. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47:1-39.
4. American College of Obstetricians and Gynecologists. Primary and preventive care: periodic assessments. ACOG Committee Opinion #229. Washington, DC: ACOG; 1999.
5. Burns DN, Minkoff H. Hepatitis C: screening in pregnancy. Obstet Gynecol. 1999;94:1044-1048.
6. World Health Organization. Global prevalence of hepatitis C, 1999. Available at: http://www.who.int/emc/images/hepacmap.jpg. Accessed August 31, 2001.
7. Arthur RR, Hassan NF, Abdallah MY, et al. Hepatitis C antibody prevalence in blood donors in different governorates in Egypt. Trans R Soc Trop Med Hyg. 1997;91:271-274.
8. Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lym-photropic viruses. Am J Public Health. 1996;86:655-661.
9. Alter MJ, Gerety RJ, Smallwood LA, Sampliner RE, Tabor E, Deinhardt F, et al. Sporadic non-A, non-B hepatitis: frequency and epidemiology in an urban U.S. population. J Infect Dis. 1982;145:886-893.
10. Alter MJ, Coleman PJ, Alexander WJ, et al. Importance of heterosexual activity in the transmission of hepatitis B and non-A, non-B hepatitis. JAMA. 1989;262:1201-1205.
11. Rooney G, Gilson RJ. Sexual transmission of hepatitis C virus infection. Sex Transm Infect. 1998;74:399-404.
12. Meisel H, Reip A, Faltus B, et al. Transmission of hepatitis C virus to children and husbands by women infected with contaminated anti-D immunoglobulin. Lancet. 1995;345:1209-1211.
13. Piazza M, Sagliocca L, Tosone G, et al. Sexual transmission of the hepatitis C virus and efficacy of prophylaxis with intramuscular immune serum globulin. A randomized controlled trial. Arch Intern Med. 1997;157:1537-1544.
14. Cheney CP, Chopra S, Graham C, Hepatitis C. Infect Dis Clin North Am. 2000;14:633-667.
15. Davis GL, Balart LA, Schiff ER, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989;321:1501-1506.
16. Di Bisceglie AM, Martin P, Kassianides C, et al. Recombinant interfer-on alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506-1510.
17. Marcellin P, Boyer N, Giostra E, et al. Recombinant human alpha-interferon in patients with chronic non-A, non-B hepatitis: a multicenter randomized controlled trial from France. Hepatology. 1991;13:393-397.
18. McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492.
19. Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha-2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426-1432.
20. Reddy KR, Wright TL, Pockros PJ, et al. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33:433-438.
21. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2b plus rib-avirin for initial treatment of chronic hepatitis C: results of a randomized trial. Lancet. 2001;358:958-965.
22. Ohto H, Terazawa S, Sasaki N, et al. Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group. N Engl J Med. 1994;30:744-750.
23. Resti M, Azzari C, Mannelli F, et al. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ. 1998;317:437-441.
24. Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
25. Lin HH, Kao JH, Hsu HY, et al. Possible role of high-titer maternal viremia in perinatal transmission of hepatitis C virus. J Infect Dis. 1994;169:638-641.
26. Thomas DL, et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1-infected mothers. Women and Infants Transmission Study. J Infect Dis. 1998;177:1480-1488.
27. Tovo PA, Palomba E, Ferraris G, et al. Increased risk of maternal-infant hepatitis C virus transmission for women coinfected with human immunodeficiency virus type 1. Italian Study Group for HCV Infection in Children. Clin Infect Dis. 1997;25:1121-1124.
28. Bortolotti F, Faggion S, Con P. Natural history of chronic viral hepatitis in childhood. Acta Gastroenterol Belg. 1998;61:198-201.
29. Lin HH, Kao JH, Hsu HY, et al. Absence of infection in breast-fed infants born to hepatitis C virus-infected mothers. J Pediatr. 1995;126:589-591.
30. Polywka S, Schroter M, Feucht HH, Zollner B, Laufs R. Low risk of vertical transmission of hepatitis C virus by breast milk. Clin Infect Dis. 1999;29:1327-1329.
31. Hepatitis C virus infection. American Academy of Pediatrics. Committee on Infectious Diseases. Pediatrics. 1998;101:481-485.
32. American College of Obstetricians and Gynecologists. Breastfeeding and the risk of hepatitis C virus transmission. ACOG Committee Opinion #220. Washington, DC: ACOG; 1999.
33. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
34. Gervais A, Bacq Y, Bernuau J, et al. Decrease in serum ALT and increase in serum HCV RNA during pregnancy in women with chronic hepatitis C. J Hepatol. 2000;32:293-299.
35. Fontaine H, Nalpas B, Carnot F, Brechot C, Pol S. Effect of pregnancy on chronic hepatitis C: a case-control study. Lancet. 2000;356:1328-1329.
36. Latt NC, Spencer JD, Beeby PJ, et al. Hepatitis C in injecting drug-using women during and after pregnancy. J Gastroenterol Hepatol. 2000;15:175-181.
37. Floreani A, Paternoster D, Zappala F, et al. Hepatitis C virus infection in pregnancy. Br J Obstet Gynaecol. 1996;103:325-329.
38. Locatelli A, Roncaglia N, Arreghini A, Bellini P, Vergani P, Ghidini A. Hepatitis C virus infection is associated with a higher incidence of cholestasis of pregnancy. Br J Obstet Gynaecol. 1999;106:498-500.
1. Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562.
2. National Center for Infectious Diseases. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncidod/dis-eases/hepatitis/slideset/intro/slide_5.htm. Accessed January 11, 2002.
3. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47:1-39.
4. American College of Obstetricians and Gynecologists. Primary and preventive care: periodic assessments. ACOG Committee Opinion #229. Washington, DC: ACOG; 1999.
5. Burns DN, Minkoff H. Hepatitis C: screening in pregnancy. Obstet Gynecol. 1999;94:1044-1048.
6. World Health Organization. Global prevalence of hepatitis C, 1999. Available at: http://www.who.int/emc/images/hepacmap.jpg. Accessed August 31, 2001.
7. Arthur RR, Hassan NF, Abdallah MY, et al. Hepatitis C antibody prevalence in blood donors in different governorates in Egypt. Trans R Soc Trop Med Hyg. 1997;91:271-274.
8. Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lym-photropic viruses. Am J Public Health. 1996;86:655-661.
9. Alter MJ, Gerety RJ, Smallwood LA, Sampliner RE, Tabor E, Deinhardt F, et al. Sporadic non-A, non-B hepatitis: frequency and epidemiology in an urban U.S. population. J Infect Dis. 1982;145:886-893.
10. Alter MJ, Coleman PJ, Alexander WJ, et al. Importance of heterosexual activity in the transmission of hepatitis B and non-A, non-B hepatitis. JAMA. 1989;262:1201-1205.
11. Rooney G, Gilson RJ. Sexual transmission of hepatitis C virus infection. Sex Transm Infect. 1998;74:399-404.
12. Meisel H, Reip A, Faltus B, et al. Transmission of hepatitis C virus to children and husbands by women infected with contaminated anti-D immunoglobulin. Lancet. 1995;345:1209-1211.
13. Piazza M, Sagliocca L, Tosone G, et al. Sexual transmission of the hepatitis C virus and efficacy of prophylaxis with intramuscular immune serum globulin. A randomized controlled trial. Arch Intern Med. 1997;157:1537-1544.
14. Cheney CP, Chopra S, Graham C, Hepatitis C. Infect Dis Clin North Am. 2000;14:633-667.
15. Davis GL, Balart LA, Schiff ER, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989;321:1501-1506.
16. Di Bisceglie AM, Martin P, Kassianides C, et al. Recombinant interfer-on alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506-1510.
17. Marcellin P, Boyer N, Giostra E, et al. Recombinant human alpha-interferon in patients with chronic non-A, non-B hepatitis: a multicenter randomized controlled trial from France. Hepatology. 1991;13:393-397.
18. McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492.
19. Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha-2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426-1432.
20. Reddy KR, Wright TL, Pockros PJ, et al. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33:433-438.
21. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2b plus rib-avirin for initial treatment of chronic hepatitis C: results of a randomized trial. Lancet. 2001;358:958-965.
22. Ohto H, Terazawa S, Sasaki N, et al. Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group. N Engl J Med. 1994;30:744-750.
23. Resti M, Azzari C, Mannelli F, et al. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ. 1998;317:437-441.
24. Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
25. Lin HH, Kao JH, Hsu HY, et al. Possible role of high-titer maternal viremia in perinatal transmission of hepatitis C virus. J Infect Dis. 1994;169:638-641.
26. Thomas DL, et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1-infected mothers. Women and Infants Transmission Study. J Infect Dis. 1998;177:1480-1488.
27. Tovo PA, Palomba E, Ferraris G, et al. Increased risk of maternal-infant hepatitis C virus transmission for women coinfected with human immunodeficiency virus type 1. Italian Study Group for HCV Infection in Children. Clin Infect Dis. 1997;25:1121-1124.
28. Bortolotti F, Faggion S, Con P. Natural history of chronic viral hepatitis in childhood. Acta Gastroenterol Belg. 1998;61:198-201.
29. Lin HH, Kao JH, Hsu HY, et al. Absence of infection in breast-fed infants born to hepatitis C virus-infected mothers. J Pediatr. 1995;126:589-591.
30. Polywka S, Schroter M, Feucht HH, Zollner B, Laufs R. Low risk of vertical transmission of hepatitis C virus by breast milk. Clin Infect Dis. 1999;29:1327-1329.
31. Hepatitis C virus infection. American Academy of Pediatrics. Committee on Infectious Diseases. Pediatrics. 1998;101:481-485.
32. American College of Obstetricians and Gynecologists. Breastfeeding and the risk of hepatitis C virus transmission. ACOG Committee Opinion #220. Washington, DC: ACOG; 1999.
33. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
34. Gervais A, Bacq Y, Bernuau J, et al. Decrease in serum ALT and increase in serum HCV RNA during pregnancy in women with chronic hepatitis C. J Hepatol. 2000;32:293-299.
35. Fontaine H, Nalpas B, Carnot F, Brechot C, Pol S. Effect of pregnancy on chronic hepatitis C: a case-control study. Lancet. 2000;356:1328-1329.
36. Latt NC, Spencer JD, Beeby PJ, et al. Hepatitis C in injecting drug-using women during and after pregnancy. J Gastroenterol Hepatol. 2000;15:175-181.
37. Floreani A, Paternoster D, Zappala F, et al. Hepatitis C virus infection in pregnancy. Br J Obstet Gynaecol. 1996;103:325-329.
38. Locatelli A, Roncaglia N, Arreghini A, Bellini P, Vergani P, Ghidini A. Hepatitis C virus infection is associated with a higher incidence of cholestasis of pregnancy. Br J Obstet Gynaecol. 1999;106:498-500.