User login
Enhanced recovery after surgery for the patient with chronic pain
CASE Chronic pelvic pain from endometriosis
A 40-year-old woman (G0) has a 20-year history of chronic pelvic pain. Stage III endometriosis is diagnosed on laparoscopic excision of endometriotic tissue. Postoperative pain symptoms include dysmenorrhea and deep dyspareunia, and the patient is feeling anxious. Physical examination reveals a retroverted uterus, right adnexal fullness and tenderness, and tenderness on palpation of the right levator ani and right obturator internus; rectovaginal examination findings are unremarkable. The patient, though now engaged in a pelvic floor physical therapy program, has yet to achieve the pain control she desires. After reviewing the treatment strategies for endometriosis with the patient, she elects definitive surgical management with minimally invasive hysterectomy and salpingo-oophorectomy. What pre-, intra-, and postoperative pain management plan do you devise for this patient?
Chronic pelvic pain presents a unique clinical challenge, as pain typically is multifactorial, and several peripheral pain generators may be involved. Although surgery can be performed to manage anatomically based disease processes, it does not address pain from musculoskeletal or neuropathic sources. A complete medical history and a physical examination are of utmost importance in developing a comprehensive multimodal management plan that may include surgery as treatment for the pain.
The standard of care for surgery is a minimally invasive approach (vaginal, laparoscopic, or robot-assisted laparoscopic), as it causes the least amount of trauma. Benefits of minimally invasive surgery include shorter hospitalization and faster recovery, likely owing to improved perioperative pain control, decreased blood loss, and fewer infections. Although this approach minimizes surgical trauma and thereby helps decrease the surgical stress response, the patient experience can be optimized with use of enhanced recovery pathways (ERPs), a multimodal approach to perioperative care.
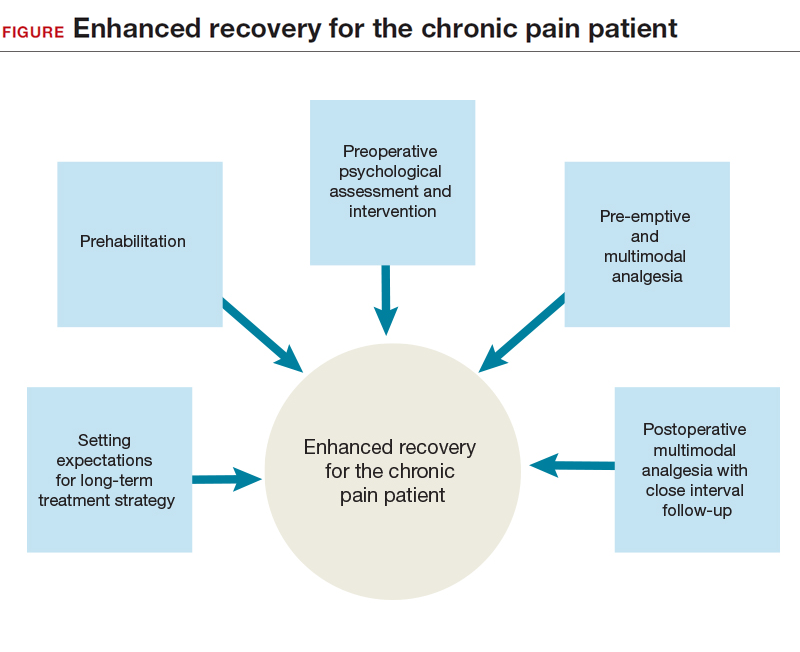
ERPs were initially proposed as a means of reducing the degree of surgical injury and the subsequent physiologic stress response.1 This multimodal approach begins in the outpatient setting, includes preoperative and intraoperative modalities, and continues postoperatively. In patients with chronic pain, ERPs are even more important. Assigning “prehabilitation” and setting expectations for surgery goals are the first step in improving the patient experience. Intraoperative use of opioid-sparing anesthetics or regional anesthesia can improve recovery. After surgery, patients with chronic pain and/or opioid dependence receive medications on a schedule, along with short-interval follow-up. Ultimately, reducing acute postoperative pain may lower the risk of developing chronic pain.
In this article on patients with chronic pelvic pain, we highlight elements of ERPs within the framework of enhanced recovery after surgery. Many of the interventions proposed here also can be used to improve the surgical experience of patients without chronic pain.
Preadmission education, expectations, and optimization
Preoperative counseling for elective procedures generally occurs in the outpatient setting. Although discussion traditionally has covered the type of procedure and its associated risks, benefits, and alternatives, new guidelines suggest a more mindful and comprehensive approach is warranted. Individualized patient-centered education programs have a positive impact on the perioperative course, effecting reductions in preoperative anxiety, opioid requirements, and hospital length of stay.2 From a pain management perspective, the clinician can take some time during preoperative counseling to inform the patient about the pain to be expected from surgery, the ways the pain will be managed intraoperatively and postoperatively, and the multimodal strategies that will be used throughout the patient’s stay2 and that may allow for early discharge. Although preadmission counseling still should address expectations for the surgery, it also presents an opportunity both to assess the patient’s ability to cope with the physical and psychological stress of surgery and to offer the patient appropriate need-based interventions, such as prehabilitation and cognitive-behavioral therapy (CBT).
Prehabilitation is the process of increasing functional capacity before surgery in order to mitigate the stress of the surgery. Prehabilitation may involve aerobic exercise, strength training, or functional task training. The gynecologic surgery literature lacks prehabilitation data, but data in the colorectal literature support use of a prehabilitation program for patients having a scheduled colectomy, with improved postoperative recovery.3 Although the colectomy cohort predominantly included older men, the principle that guides program implementation is the same: improve recovery after the stress of abdominal surgery. Indeed, a patient who opts for an elective surgery may have to wait several weeks before undergoing the procedure, and during this period behavioral interventions can take effect. With postoperative complications occurring more often in patients with reduced functional capacity, the data support using prehabilitation to decrease the incidence of postoperative complications, particularly among the most vulnerable patients.4 However, a definitive recommendation on use of pelvic floor exercises as an adjunct to prehabilitation cannot be made.4 Successful prehabilitation takes at least 4 weeks and should be part of a multimodal program that addresses other behavioral risk factors that may negatively affect recovery.5 For example, current tobacco users have compromised pulmonary status and wound healing immediately after surgery, and use more opioids.6 Conversely, smoking cessation for as little as 4 weeks before surgery is associated with fewer complications.7 In addition, given that alcohol abuse may compromise the surgical stress response and increase the risk of opioid misuse, addressing alcohol abuse preoperatively may improve postoperative recovery.8
Treating mood disorders that coexist with chronic pain disorders is an important part of outpatient multimodal management—psychological intervention is a useful adjunct to prehabilitation in reducing perioperative anxiety and improving postoperative functional capacity.9 For patients who have chronic pain and are undergoing surgery, it is important to address any anxiety, depression, or poor coping skills (eg, pain catastrophizing) to try to reduce the postoperative pain experience and decrease the risk of chronic postsurgical pain (CPSP).10,11
Before surgery, patients with chronic pain syndromes should be evaluated for emotional distress and pain coping ability. When possible, they should be referred to a pain psychologist, who can initiate CBT and other interventions. In addition, pain coping skills can be developed or reinforced to address preoperative anxiety and pain catastrophizing. These interventions, which may include use of visual imagery, breathing exercises, and other relaxation techniques, are applicable to the management of postoperative anxiety as well.
Read about preoperative multimodal analgesia and intra- and postoperative management.
Preoperative multimodal analgesia
Multimodal analgesia has several benefits. Simultaneous effects can be generated on multiple pain-related neurotransmitters, and a synergistic effect (eg, of acetaminophen and a nonsteroidal anti-inflammatory drug [NSAID]) can improve pain management. In addition, small doses of multiple medications can be given, instead of a large dose of a single medication. Of course, this strategy must be modified in elderly and patients with impaired renal function, who are at high risk for polypharmacy.
Preoperative administration of 3 medications—a selective cyclooxygenase 2 (COX-2) inhibitor, acetaminophen, and a gabapentinoid—is increasingly accepted as part of multimodal analgesia. The selective COX-2 inhibitor targets inflammatory prostaglandins and has anti-inflammatory and analgesic effects; acetaminophen, an effective analgesic with an unclear mechanism of action, can reduce postoperative opioid consumption12 and works synergistically with NSAIDs13; and the gabapentinoid gabapentin has an analgesic effect likely contributing to decreased movement-related pain and subsequent improved functional recovery (data are mixed on whether continuing gabapentin after surgery prevents CPSP).14−16
Although serotonin and norepinephrine reuptake inhibitors (SNRIs) are commonly used in outpatient management of chronic pelvic pain, data suggest that their role in perioperative pain management is evolving. As SNRIs may reduce central nervous system (CNS) sensitization,17 their analgesic effect is thought to result from increased descending inhibitory tone in the CNS, which makes this class of medication ideal for patients with chronic neuropathic pain.15
Limited data also suggest a role for SNRIs in decreasing immediate postoperative pain and CPSP in high-risk patients. Studies of duloxetine use in the immediate perioperative period have found reduced postoperative acute pain and opioid use.18,19 In addition, a short course of low-dose (37.5 mg) venlafaxine both before and after surgery has demonstrated a reduction in postoperative opioid use and a reduction in movement-related pain 6 months after surgery.20
Intraoperative management
The surgical and anesthesia teams share the goal of optimizing both pain control and postoperative recovery. Surgical team members, who want longer-acting anesthetics for infiltration of incision sites, discuss with the anesthesiologist the appropriateness of using peripheral nerve blocks or neuraxial anesthesia, given the patient’s history and planned procedure. Anesthesia team members can improve anesthesia and minimize intraoperative opioid use through several methods, including total intravenous anesthesia,21 dexamethasone,22 ketorolac,23 and intravenous ketamine. Ketamine, in particular, has a wide range of surgical applications and has been found to reduce postoperative pain, postoperative pain medication use, and the risk of CPSP.2
Incision sites should be infiltrated before and after surgery. Lidocaine traditionally is used for its rapid onset of action in reducing surgical site pain, but its short half-life may limit its applicability to postoperative pain. Recently, bupivacaine (half-life, 3.5 hours) and liposomal bupivacaine (24–34 hours) have gained more attention. Both of these medications appear to be as effective as lidocaine in reducing surgical site pain.24
Transversus abdominis plane (TAP) blocks have been used as an adjunct in pain management during abdominopelvic surgery. Although initial data on postoperative pain and opioid use reductions with TAP blocks were inconclusive,25 more recent data showed a role for TAP blocks in a multimodal approach for reducing opioid use during laparoscopic and open surgery.26,27 Given the small number of studies on using liposomal bupivacaine for peripheral nerve blocks (eg, TAP blocks) in postoperative pain management, current data are inconclusive.28
Postoperative management
The ERP approach calls for continuing multimodal analgesia after surgery—in most cases, scheduling early use of oral acetaminophen and ibuprofen, and providing short-acting, low-dose opioid analgesia as needed. All patients should be given a bowel regimen. Similar to undergoing prehabilitation for surgery, patients should prepare themselves for recovery. They should be encouraged to engage in early ambulation and oral intake and, when clinically appropriate, be given same-day discharge for minimally invasive surgical procedures.
Patients with chronic pain before surgery are at increased risk for suboptimal postoperative pain management, and those who are dependent on opioids require additional perioperative measures for adequate postoperative pain control. In these complicated cases, it is appropriate to enlist a pain specialist, potentially before surgery, to help plan perioperative and postoperative pain management.2 Postoperative pain management for opioid-dependent patients should include pharmacologic and nonpharmacologic interventions, such as use of nonopioid medications (eg, gabapentin) and continuation of CBT. Patients with chronic pain should be closely followed up for assessment of postoperative pain control and recovery.
CASE Resolved
Surgical management is one aspect of the longer term multimodal pain management strategy for this patient. After preoperative pelvic floor physical therapy, she is receptive to starting a trial of an SNRI for her pain and mood symptoms. Both interventions allow for optimization of her preoperative physical and psychological status. Expectations are set that she will be discharged the day of surgery and that the surgery is but one component of her multimodal treatment plan. In addition, before surgery, she takes oral acetaminophen, gabapentin, and celecoxib—previously having had no contraindications to these medications. During surgery, bupivacaine is used for infiltration of all incision sites, and the anesthesia team administers ketamine and a TAP block. After surgery, the patient is prepared for same-day discharge and given the NSAIDs and acetaminophen she is scheduled to take over the next 72 hours. She is also given a limited prescription for oxycodone for breakthrough pain. An office visit 1 to 2 weeks after surgery is scheduled.
ERP strategies for surgical management of endometriosis have not only improved this patient’s postoperative recovery but also reduced her surgical stress response and subsequent transition to chronic postoperative pain. Many of the strategies used in this case are applicable to patients without chronic pain.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606−617.
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131−157.
- Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150(3):505−514.
- Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. 2016;160(5):1189−1201.
- Tew GA, Ayyash R, Durrand J, Danjoux GR. Clinical guideline and recommendations on pre-operative exercise training in patients awaiting major non-cardiac surgery [published online ahead of print January 13, 2018]. Anaesthesia. doi:10.1111/anae.14177.
- Chiang HL, Chia YY, Lin HS, Chen CH. The implications of tobacco smoking on acute postoperative pain: a prospective observational study. Pain Res Manag. 2016;2016:9432493.
- Mastracci TM, Carli F, Finley RJ, Muccio S, Warner DO; Members of the Evidence-Based Reviews in Surgery Group. Effect of preoperative smoking cessation interventions on postoperative complications. J Am Coll Surg. 2011;212(6):1094−1096.
- Tonnesen H, Kehlet H. Preoperative alcoholism and postoperative morbidity. Br J Surg. 1999;86(7):869−874.
- Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937−947.
- Khan RS, Ahmed K, Blakeway E, et al. Catastrophizing: a predictive factor for postoperative pain. Am J Surg. 2011;201(1):122−131.
- Pinto PR, McIntyre T, Nogueira-Silva C, Almeida A, Araujo-Soares V. Risk factors for persistent postsurgical pain in women undergoing hysterectomy due to benign causes: a prospective predictive study. J Pain. 2012;13(11):1045−1057.
- Moon YE, Lee YK, Lee J, Moon DE. The effects of preoperative intravenous acetaminophen in patients undergoing abdominal hysterectomy. Arch Gynecol Obstet. 2011;284(6):1455−1460.
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170−1179.
- Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg. 2012;115(2):428−442.
- Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol. 2007;20(5):456−472.
- Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev. 2013;(7):CD008307.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 suppl):S2−S15.
- Castro-Alves LJ, Oliveira de Medeiros AC, Neves SP, et al. Perioperative duloxetine to improve postoperative recovery after abdominal hysterectomy: a prospective, randomized, double-blinded, placebo-controlled study. Anesth Analg. 2016;122(1):98−104.
- Bedin A, Caldart Bedin RA, Vieira JE, Ashmawi HA. Duloxetine as an analgesic reduces opioid consumption after spine surgery: a randomized, double-blind, controlled study. Clin J Pain. 2017;33(10):865−869.
- Amr YM, Yousef AA. Evaluation of efficacy of the perioperative administration of venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain. 2010;26(5):381–385.
- Marret E, Rolin M, Beaussier M, Bonnet F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg. 2008;95(11):1331–1338.
- De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588.
- De Oliveira GS Jr, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesth Analg. 2012;114(2):424–433.
- Hamilton TW, Athanassoglou V, Mellon S, et al. Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst Rev. 2017;(2):CD011419.
- Charlton S, Cyna AM, Middleton P, Griffiths JD. Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database Syst Rev. 2010;(12):CD007705.
- Hain E, Maggiori L, Prost À la Denise J, Panis Y. Transversus abdominis plane (TAP) block in laparoscopic colorectal surgery improves postoperative pain management: a meta-analysis [published online ahead of print January 30, 2018]. Colorectal Dis. doi:10.1111/codi.14037.
- Staker JJ, Liu D, Church R, et al. A triple-blind, placebo-controlled randomised trial of the ilioinguinal-transversus abdominis plane (I-TAP) nerve block for elective caesarean section [published online ahead of print January 29, 2018]. Anaesthesia. doi:10.1111/anae.14222.
- Hamilton TW, Athanassoglou V, Trivella M, et al. Liposomal bupivacaine peripheral nerve block for the management of postoperative pain. Cochrane Database Syst Rev. 2016;(8):CD011476.
CASE Chronic pelvic pain from endometriosis
A 40-year-old woman (G0) has a 20-year history of chronic pelvic pain. Stage III endometriosis is diagnosed on laparoscopic excision of endometriotic tissue. Postoperative pain symptoms include dysmenorrhea and deep dyspareunia, and the patient is feeling anxious. Physical examination reveals a retroverted uterus, right adnexal fullness and tenderness, and tenderness on palpation of the right levator ani and right obturator internus; rectovaginal examination findings are unremarkable. The patient, though now engaged in a pelvic floor physical therapy program, has yet to achieve the pain control she desires. After reviewing the treatment strategies for endometriosis with the patient, she elects definitive surgical management with minimally invasive hysterectomy and salpingo-oophorectomy. What pre-, intra-, and postoperative pain management plan do you devise for this patient?
Chronic pelvic pain presents a unique clinical challenge, as pain typically is multifactorial, and several peripheral pain generators may be involved. Although surgery can be performed to manage anatomically based disease processes, it does not address pain from musculoskeletal or neuropathic sources. A complete medical history and a physical examination are of utmost importance in developing a comprehensive multimodal management plan that may include surgery as treatment for the pain.
The standard of care for surgery is a minimally invasive approach (vaginal, laparoscopic, or robot-assisted laparoscopic), as it causes the least amount of trauma. Benefits of minimally invasive surgery include shorter hospitalization and faster recovery, likely owing to improved perioperative pain control, decreased blood loss, and fewer infections. Although this approach minimizes surgical trauma and thereby helps decrease the surgical stress response, the patient experience can be optimized with use of enhanced recovery pathways (ERPs), a multimodal approach to perioperative care.
ERPs were initially proposed as a means of reducing the degree of surgical injury and the subsequent physiologic stress response.1 This multimodal approach begins in the outpatient setting, includes preoperative and intraoperative modalities, and continues postoperatively. In patients with chronic pain, ERPs are even more important. Assigning “prehabilitation” and setting expectations for surgery goals are the first step in improving the patient experience. Intraoperative use of opioid-sparing anesthetics or regional anesthesia can improve recovery. After surgery, patients with chronic pain and/or opioid dependence receive medications on a schedule, along with short-interval follow-up. Ultimately, reducing acute postoperative pain may lower the risk of developing chronic pain.
In this article on patients with chronic pelvic pain, we highlight elements of ERPs within the framework of enhanced recovery after surgery. Many of the interventions proposed here also can be used to improve the surgical experience of patients without chronic pain.

Preadmission education, expectations, and optimization
Preoperative counseling for elective procedures generally occurs in the outpatient setting. Although discussion traditionally has covered the type of procedure and its associated risks, benefits, and alternatives, new guidelines suggest a more mindful and comprehensive approach is warranted. Individualized patient-centered education programs have a positive impact on the perioperative course, effecting reductions in preoperative anxiety, opioid requirements, and hospital length of stay.2 From a pain management perspective, the clinician can take some time during preoperative counseling to inform the patient about the pain to be expected from surgery, the ways the pain will be managed intraoperatively and postoperatively, and the multimodal strategies that will be used throughout the patient’s stay2 and that may allow for early discharge. Although preadmission counseling still should address expectations for the surgery, it also presents an opportunity both to assess the patient’s ability to cope with the physical and psychological stress of surgery and to offer the patient appropriate need-based interventions, such as prehabilitation and cognitive-behavioral therapy (CBT).
Prehabilitation is the process of increasing functional capacity before surgery in order to mitigate the stress of the surgery. Prehabilitation may involve aerobic exercise, strength training, or functional task training. The gynecologic surgery literature lacks prehabilitation data, but data in the colorectal literature support use of a prehabilitation program for patients having a scheduled colectomy, with improved postoperative recovery.3 Although the colectomy cohort predominantly included older men, the principle that guides program implementation is the same: improve recovery after the stress of abdominal surgery. Indeed, a patient who opts for an elective surgery may have to wait several weeks before undergoing the procedure, and during this period behavioral interventions can take effect. With postoperative complications occurring more often in patients with reduced functional capacity, the data support using prehabilitation to decrease the incidence of postoperative complications, particularly among the most vulnerable patients.4 However, a definitive recommendation on use of pelvic floor exercises as an adjunct to prehabilitation cannot be made.4 Successful prehabilitation takes at least 4 weeks and should be part of a multimodal program that addresses other behavioral risk factors that may negatively affect recovery.5 For example, current tobacco users have compromised pulmonary status and wound healing immediately after surgery, and use more opioids.6 Conversely, smoking cessation for as little as 4 weeks before surgery is associated with fewer complications.7 In addition, given that alcohol abuse may compromise the surgical stress response and increase the risk of opioid misuse, addressing alcohol abuse preoperatively may improve postoperative recovery.8
Treating mood disorders that coexist with chronic pain disorders is an important part of outpatient multimodal management—psychological intervention is a useful adjunct to prehabilitation in reducing perioperative anxiety and improving postoperative functional capacity.9 For patients who have chronic pain and are undergoing surgery, it is important to address any anxiety, depression, or poor coping skills (eg, pain catastrophizing) to try to reduce the postoperative pain experience and decrease the risk of chronic postsurgical pain (CPSP).10,11
Before surgery, patients with chronic pain syndromes should be evaluated for emotional distress and pain coping ability. When possible, they should be referred to a pain psychologist, who can initiate CBT and other interventions. In addition, pain coping skills can be developed or reinforced to address preoperative anxiety and pain catastrophizing. These interventions, which may include use of visual imagery, breathing exercises, and other relaxation techniques, are applicable to the management of postoperative anxiety as well.
Read about preoperative multimodal analgesia and intra- and postoperative management.
Preoperative multimodal analgesia
Multimodal analgesia has several benefits. Simultaneous effects can be generated on multiple pain-related neurotransmitters, and a synergistic effect (eg, of acetaminophen and a nonsteroidal anti-inflammatory drug [NSAID]) can improve pain management. In addition, small doses of multiple medications can be given, instead of a large dose of a single medication. Of course, this strategy must be modified in elderly and patients with impaired renal function, who are at high risk for polypharmacy.
Preoperative administration of 3 medications—a selective cyclooxygenase 2 (COX-2) inhibitor, acetaminophen, and a gabapentinoid—is increasingly accepted as part of multimodal analgesia. The selective COX-2 inhibitor targets inflammatory prostaglandins and has anti-inflammatory and analgesic effects; acetaminophen, an effective analgesic with an unclear mechanism of action, can reduce postoperative opioid consumption12 and works synergistically with NSAIDs13; and the gabapentinoid gabapentin has an analgesic effect likely contributing to decreased movement-related pain and subsequent improved functional recovery (data are mixed on whether continuing gabapentin after surgery prevents CPSP).14−16
Although serotonin and norepinephrine reuptake inhibitors (SNRIs) are commonly used in outpatient management of chronic pelvic pain, data suggest that their role in perioperative pain management is evolving. As SNRIs may reduce central nervous system (CNS) sensitization,17 their analgesic effect is thought to result from increased descending inhibitory tone in the CNS, which makes this class of medication ideal for patients with chronic neuropathic pain.15
Limited data also suggest a role for SNRIs in decreasing immediate postoperative pain and CPSP in high-risk patients. Studies of duloxetine use in the immediate perioperative period have found reduced postoperative acute pain and opioid use.18,19 In addition, a short course of low-dose (37.5 mg) venlafaxine both before and after surgery has demonstrated a reduction in postoperative opioid use and a reduction in movement-related pain 6 months after surgery.20
Intraoperative management
The surgical and anesthesia teams share the goal of optimizing both pain control and postoperative recovery. Surgical team members, who want longer-acting anesthetics for infiltration of incision sites, discuss with the anesthesiologist the appropriateness of using peripheral nerve blocks or neuraxial anesthesia, given the patient’s history and planned procedure. Anesthesia team members can improve anesthesia and minimize intraoperative opioid use through several methods, including total intravenous anesthesia,21 dexamethasone,22 ketorolac,23 and intravenous ketamine. Ketamine, in particular, has a wide range of surgical applications and has been found to reduce postoperative pain, postoperative pain medication use, and the risk of CPSP.2
Incision sites should be infiltrated before and after surgery. Lidocaine traditionally is used for its rapid onset of action in reducing surgical site pain, but its short half-life may limit its applicability to postoperative pain. Recently, bupivacaine (half-life, 3.5 hours) and liposomal bupivacaine (24–34 hours) have gained more attention. Both of these medications appear to be as effective as lidocaine in reducing surgical site pain.24
Transversus abdominis plane (TAP) blocks have been used as an adjunct in pain management during abdominopelvic surgery. Although initial data on postoperative pain and opioid use reductions with TAP blocks were inconclusive,25 more recent data showed a role for TAP blocks in a multimodal approach for reducing opioid use during laparoscopic and open surgery.26,27 Given the small number of studies on using liposomal bupivacaine for peripheral nerve blocks (eg, TAP blocks) in postoperative pain management, current data are inconclusive.28
Postoperative management
The ERP approach calls for continuing multimodal analgesia after surgery—in most cases, scheduling early use of oral acetaminophen and ibuprofen, and providing short-acting, low-dose opioid analgesia as needed. All patients should be given a bowel regimen. Similar to undergoing prehabilitation for surgery, patients should prepare themselves for recovery. They should be encouraged to engage in early ambulation and oral intake and, when clinically appropriate, be given same-day discharge for minimally invasive surgical procedures.
Patients with chronic pain before surgery are at increased risk for suboptimal postoperative pain management, and those who are dependent on opioids require additional perioperative measures for adequate postoperative pain control. In these complicated cases, it is appropriate to enlist a pain specialist, potentially before surgery, to help plan perioperative and postoperative pain management.2 Postoperative pain management for opioid-dependent patients should include pharmacologic and nonpharmacologic interventions, such as use of nonopioid medications (eg, gabapentin) and continuation of CBT. Patients with chronic pain should be closely followed up for assessment of postoperative pain control and recovery.
CASE Resolved
Surgical management is one aspect of the longer term multimodal pain management strategy for this patient. After preoperative pelvic floor physical therapy, she is receptive to starting a trial of an SNRI for her pain and mood symptoms. Both interventions allow for optimization of her preoperative physical and psychological status. Expectations are set that she will be discharged the day of surgery and that the surgery is but one component of her multimodal treatment plan. In addition, before surgery, she takes oral acetaminophen, gabapentin, and celecoxib—previously having had no contraindications to these medications. During surgery, bupivacaine is used for infiltration of all incision sites, and the anesthesia team administers ketamine and a TAP block. After surgery, the patient is prepared for same-day discharge and given the NSAIDs and acetaminophen she is scheduled to take over the next 72 hours. She is also given a limited prescription for oxycodone for breakthrough pain. An office visit 1 to 2 weeks after surgery is scheduled.
ERP strategies for surgical management of endometriosis have not only improved this patient’s postoperative recovery but also reduced her surgical stress response and subsequent transition to chronic postoperative pain. Many of the strategies used in this case are applicable to patients without chronic pain.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Chronic pelvic pain from endometriosis
A 40-year-old woman (G0) has a 20-year history of chronic pelvic pain. Stage III endometriosis is diagnosed on laparoscopic excision of endometriotic tissue. Postoperative pain symptoms include dysmenorrhea and deep dyspareunia, and the patient is feeling anxious. Physical examination reveals a retroverted uterus, right adnexal fullness and tenderness, and tenderness on palpation of the right levator ani and right obturator internus; rectovaginal examination findings are unremarkable. The patient, though now engaged in a pelvic floor physical therapy program, has yet to achieve the pain control she desires. After reviewing the treatment strategies for endometriosis with the patient, she elects definitive surgical management with minimally invasive hysterectomy and salpingo-oophorectomy. What pre-, intra-, and postoperative pain management plan do you devise for this patient?
Chronic pelvic pain presents a unique clinical challenge, as pain typically is multifactorial, and several peripheral pain generators may be involved. Although surgery can be performed to manage anatomically based disease processes, it does not address pain from musculoskeletal or neuropathic sources. A complete medical history and a physical examination are of utmost importance in developing a comprehensive multimodal management plan that may include surgery as treatment for the pain.
The standard of care for surgery is a minimally invasive approach (vaginal, laparoscopic, or robot-assisted laparoscopic), as it causes the least amount of trauma. Benefits of minimally invasive surgery include shorter hospitalization and faster recovery, likely owing to improved perioperative pain control, decreased blood loss, and fewer infections. Although this approach minimizes surgical trauma and thereby helps decrease the surgical stress response, the patient experience can be optimized with use of enhanced recovery pathways (ERPs), a multimodal approach to perioperative care.
ERPs were initially proposed as a means of reducing the degree of surgical injury and the subsequent physiologic stress response.1 This multimodal approach begins in the outpatient setting, includes preoperative and intraoperative modalities, and continues postoperatively. In patients with chronic pain, ERPs are even more important. Assigning “prehabilitation” and setting expectations for surgery goals are the first step in improving the patient experience. Intraoperative use of opioid-sparing anesthetics or regional anesthesia can improve recovery. After surgery, patients with chronic pain and/or opioid dependence receive medications on a schedule, along with short-interval follow-up. Ultimately, reducing acute postoperative pain may lower the risk of developing chronic pain.
In this article on patients with chronic pelvic pain, we highlight elements of ERPs within the framework of enhanced recovery after surgery. Many of the interventions proposed here also can be used to improve the surgical experience of patients without chronic pain.

Preadmission education, expectations, and optimization
Preoperative counseling for elective procedures generally occurs in the outpatient setting. Although discussion traditionally has covered the type of procedure and its associated risks, benefits, and alternatives, new guidelines suggest a more mindful and comprehensive approach is warranted. Individualized patient-centered education programs have a positive impact on the perioperative course, effecting reductions in preoperative anxiety, opioid requirements, and hospital length of stay.2 From a pain management perspective, the clinician can take some time during preoperative counseling to inform the patient about the pain to be expected from surgery, the ways the pain will be managed intraoperatively and postoperatively, and the multimodal strategies that will be used throughout the patient’s stay2 and that may allow for early discharge. Although preadmission counseling still should address expectations for the surgery, it also presents an opportunity both to assess the patient’s ability to cope with the physical and psychological stress of surgery and to offer the patient appropriate need-based interventions, such as prehabilitation and cognitive-behavioral therapy (CBT).
Prehabilitation is the process of increasing functional capacity before surgery in order to mitigate the stress of the surgery. Prehabilitation may involve aerobic exercise, strength training, or functional task training. The gynecologic surgery literature lacks prehabilitation data, but data in the colorectal literature support use of a prehabilitation program for patients having a scheduled colectomy, with improved postoperative recovery.3 Although the colectomy cohort predominantly included older men, the principle that guides program implementation is the same: improve recovery after the stress of abdominal surgery. Indeed, a patient who opts for an elective surgery may have to wait several weeks before undergoing the procedure, and during this period behavioral interventions can take effect. With postoperative complications occurring more often in patients with reduced functional capacity, the data support using prehabilitation to decrease the incidence of postoperative complications, particularly among the most vulnerable patients.4 However, a definitive recommendation on use of pelvic floor exercises as an adjunct to prehabilitation cannot be made.4 Successful prehabilitation takes at least 4 weeks and should be part of a multimodal program that addresses other behavioral risk factors that may negatively affect recovery.5 For example, current tobacco users have compromised pulmonary status and wound healing immediately after surgery, and use more opioids.6 Conversely, smoking cessation for as little as 4 weeks before surgery is associated with fewer complications.7 In addition, given that alcohol abuse may compromise the surgical stress response and increase the risk of opioid misuse, addressing alcohol abuse preoperatively may improve postoperative recovery.8
Treating mood disorders that coexist with chronic pain disorders is an important part of outpatient multimodal management—psychological intervention is a useful adjunct to prehabilitation in reducing perioperative anxiety and improving postoperative functional capacity.9 For patients who have chronic pain and are undergoing surgery, it is important to address any anxiety, depression, or poor coping skills (eg, pain catastrophizing) to try to reduce the postoperative pain experience and decrease the risk of chronic postsurgical pain (CPSP).10,11
Before surgery, patients with chronic pain syndromes should be evaluated for emotional distress and pain coping ability. When possible, they should be referred to a pain psychologist, who can initiate CBT and other interventions. In addition, pain coping skills can be developed or reinforced to address preoperative anxiety and pain catastrophizing. These interventions, which may include use of visual imagery, breathing exercises, and other relaxation techniques, are applicable to the management of postoperative anxiety as well.
Read about preoperative multimodal analgesia and intra- and postoperative management.
Preoperative multimodal analgesia
Multimodal analgesia has several benefits. Simultaneous effects can be generated on multiple pain-related neurotransmitters, and a synergistic effect (eg, of acetaminophen and a nonsteroidal anti-inflammatory drug [NSAID]) can improve pain management. In addition, small doses of multiple medications can be given, instead of a large dose of a single medication. Of course, this strategy must be modified in elderly and patients with impaired renal function, who are at high risk for polypharmacy.
Preoperative administration of 3 medications—a selective cyclooxygenase 2 (COX-2) inhibitor, acetaminophen, and a gabapentinoid—is increasingly accepted as part of multimodal analgesia. The selective COX-2 inhibitor targets inflammatory prostaglandins and has anti-inflammatory and analgesic effects; acetaminophen, an effective analgesic with an unclear mechanism of action, can reduce postoperative opioid consumption12 and works synergistically with NSAIDs13; and the gabapentinoid gabapentin has an analgesic effect likely contributing to decreased movement-related pain and subsequent improved functional recovery (data are mixed on whether continuing gabapentin after surgery prevents CPSP).14−16
Although serotonin and norepinephrine reuptake inhibitors (SNRIs) are commonly used in outpatient management of chronic pelvic pain, data suggest that their role in perioperative pain management is evolving. As SNRIs may reduce central nervous system (CNS) sensitization,17 their analgesic effect is thought to result from increased descending inhibitory tone in the CNS, which makes this class of medication ideal for patients with chronic neuropathic pain.15
Limited data also suggest a role for SNRIs in decreasing immediate postoperative pain and CPSP in high-risk patients. Studies of duloxetine use in the immediate perioperative period have found reduced postoperative acute pain and opioid use.18,19 In addition, a short course of low-dose (37.5 mg) venlafaxine both before and after surgery has demonstrated a reduction in postoperative opioid use and a reduction in movement-related pain 6 months after surgery.20
Intraoperative management
The surgical and anesthesia teams share the goal of optimizing both pain control and postoperative recovery. Surgical team members, who want longer-acting anesthetics for infiltration of incision sites, discuss with the anesthesiologist the appropriateness of using peripheral nerve blocks or neuraxial anesthesia, given the patient’s history and planned procedure. Anesthesia team members can improve anesthesia and minimize intraoperative opioid use through several methods, including total intravenous anesthesia,21 dexamethasone,22 ketorolac,23 and intravenous ketamine. Ketamine, in particular, has a wide range of surgical applications and has been found to reduce postoperative pain, postoperative pain medication use, and the risk of CPSP.2
Incision sites should be infiltrated before and after surgery. Lidocaine traditionally is used for its rapid onset of action in reducing surgical site pain, but its short half-life may limit its applicability to postoperative pain. Recently, bupivacaine (half-life, 3.5 hours) and liposomal bupivacaine (24–34 hours) have gained more attention. Both of these medications appear to be as effective as lidocaine in reducing surgical site pain.24
Transversus abdominis plane (TAP) blocks have been used as an adjunct in pain management during abdominopelvic surgery. Although initial data on postoperative pain and opioid use reductions with TAP blocks were inconclusive,25 more recent data showed a role for TAP blocks in a multimodal approach for reducing opioid use during laparoscopic and open surgery.26,27 Given the small number of studies on using liposomal bupivacaine for peripheral nerve blocks (eg, TAP blocks) in postoperative pain management, current data are inconclusive.28
Postoperative management
The ERP approach calls for continuing multimodal analgesia after surgery—in most cases, scheduling early use of oral acetaminophen and ibuprofen, and providing short-acting, low-dose opioid analgesia as needed. All patients should be given a bowel regimen. Similar to undergoing prehabilitation for surgery, patients should prepare themselves for recovery. They should be encouraged to engage in early ambulation and oral intake and, when clinically appropriate, be given same-day discharge for minimally invasive surgical procedures.
Patients with chronic pain before surgery are at increased risk for suboptimal postoperative pain management, and those who are dependent on opioids require additional perioperative measures for adequate postoperative pain control. In these complicated cases, it is appropriate to enlist a pain specialist, potentially before surgery, to help plan perioperative and postoperative pain management.2 Postoperative pain management for opioid-dependent patients should include pharmacologic and nonpharmacologic interventions, such as use of nonopioid medications (eg, gabapentin) and continuation of CBT. Patients with chronic pain should be closely followed up for assessment of postoperative pain control and recovery.
CASE Resolved
Surgical management is one aspect of the longer term multimodal pain management strategy for this patient. After preoperative pelvic floor physical therapy, she is receptive to starting a trial of an SNRI for her pain and mood symptoms. Both interventions allow for optimization of her preoperative physical and psychological status. Expectations are set that she will be discharged the day of surgery and that the surgery is but one component of her multimodal treatment plan. In addition, before surgery, she takes oral acetaminophen, gabapentin, and celecoxib—previously having had no contraindications to these medications. During surgery, bupivacaine is used for infiltration of all incision sites, and the anesthesia team administers ketamine and a TAP block. After surgery, the patient is prepared for same-day discharge and given the NSAIDs and acetaminophen she is scheduled to take over the next 72 hours. She is also given a limited prescription for oxycodone for breakthrough pain. An office visit 1 to 2 weeks after surgery is scheduled.
ERP strategies for surgical management of endometriosis have not only improved this patient’s postoperative recovery but also reduced her surgical stress response and subsequent transition to chronic postoperative pain. Many of the strategies used in this case are applicable to patients without chronic pain.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606−617.
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131−157.
- Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150(3):505−514.
- Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. 2016;160(5):1189−1201.
- Tew GA, Ayyash R, Durrand J, Danjoux GR. Clinical guideline and recommendations on pre-operative exercise training in patients awaiting major non-cardiac surgery [published online ahead of print January 13, 2018]. Anaesthesia. doi:10.1111/anae.14177.
- Chiang HL, Chia YY, Lin HS, Chen CH. The implications of tobacco smoking on acute postoperative pain: a prospective observational study. Pain Res Manag. 2016;2016:9432493.
- Mastracci TM, Carli F, Finley RJ, Muccio S, Warner DO; Members of the Evidence-Based Reviews in Surgery Group. Effect of preoperative smoking cessation interventions on postoperative complications. J Am Coll Surg. 2011;212(6):1094−1096.
- Tonnesen H, Kehlet H. Preoperative alcoholism and postoperative morbidity. Br J Surg. 1999;86(7):869−874.
- Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937−947.
- Khan RS, Ahmed K, Blakeway E, et al. Catastrophizing: a predictive factor for postoperative pain. Am J Surg. 2011;201(1):122−131.
- Pinto PR, McIntyre T, Nogueira-Silva C, Almeida A, Araujo-Soares V. Risk factors for persistent postsurgical pain in women undergoing hysterectomy due to benign causes: a prospective predictive study. J Pain. 2012;13(11):1045−1057.
- Moon YE, Lee YK, Lee J, Moon DE. The effects of preoperative intravenous acetaminophen in patients undergoing abdominal hysterectomy. Arch Gynecol Obstet. 2011;284(6):1455−1460.
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170−1179.
- Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg. 2012;115(2):428−442.
- Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol. 2007;20(5):456−472.
- Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev. 2013;(7):CD008307.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 suppl):S2−S15.
- Castro-Alves LJ, Oliveira de Medeiros AC, Neves SP, et al. Perioperative duloxetine to improve postoperative recovery after abdominal hysterectomy: a prospective, randomized, double-blinded, placebo-controlled study. Anesth Analg. 2016;122(1):98−104.
- Bedin A, Caldart Bedin RA, Vieira JE, Ashmawi HA. Duloxetine as an analgesic reduces opioid consumption after spine surgery: a randomized, double-blind, controlled study. Clin J Pain. 2017;33(10):865−869.
- Amr YM, Yousef AA. Evaluation of efficacy of the perioperative administration of venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain. 2010;26(5):381–385.
- Marret E, Rolin M, Beaussier M, Bonnet F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg. 2008;95(11):1331–1338.
- De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588.
- De Oliveira GS Jr, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesth Analg. 2012;114(2):424–433.
- Hamilton TW, Athanassoglou V, Mellon S, et al. Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst Rev. 2017;(2):CD011419.
- Charlton S, Cyna AM, Middleton P, Griffiths JD. Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database Syst Rev. 2010;(12):CD007705.
- Hain E, Maggiori L, Prost À la Denise J, Panis Y. Transversus abdominis plane (TAP) block in laparoscopic colorectal surgery improves postoperative pain management: a meta-analysis [published online ahead of print January 30, 2018]. Colorectal Dis. doi:10.1111/codi.14037.
- Staker JJ, Liu D, Church R, et al. A triple-blind, placebo-controlled randomised trial of the ilioinguinal-transversus abdominis plane (I-TAP) nerve block for elective caesarean section [published online ahead of print January 29, 2018]. Anaesthesia. doi:10.1111/anae.14222.
- Hamilton TW, Athanassoglou V, Trivella M, et al. Liposomal bupivacaine peripheral nerve block for the management of postoperative pain. Cochrane Database Syst Rev. 2016;(8):CD011476.
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606−617.
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131−157.
- Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150(3):505−514.
- Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. 2016;160(5):1189−1201.
- Tew GA, Ayyash R, Durrand J, Danjoux GR. Clinical guideline and recommendations on pre-operative exercise training in patients awaiting major non-cardiac surgery [published online ahead of print January 13, 2018]. Anaesthesia. doi:10.1111/anae.14177.
- Chiang HL, Chia YY, Lin HS, Chen CH. The implications of tobacco smoking on acute postoperative pain: a prospective observational study. Pain Res Manag. 2016;2016:9432493.
- Mastracci TM, Carli F, Finley RJ, Muccio S, Warner DO; Members of the Evidence-Based Reviews in Surgery Group. Effect of preoperative smoking cessation interventions on postoperative complications. J Am Coll Surg. 2011;212(6):1094−1096.
- Tonnesen H, Kehlet H. Preoperative alcoholism and postoperative morbidity. Br J Surg. 1999;86(7):869−874.
- Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937−947.
- Khan RS, Ahmed K, Blakeway E, et al. Catastrophizing: a predictive factor for postoperative pain. Am J Surg. 2011;201(1):122−131.
- Pinto PR, McIntyre T, Nogueira-Silva C, Almeida A, Araujo-Soares V. Risk factors for persistent postsurgical pain in women undergoing hysterectomy due to benign causes: a prospective predictive study. J Pain. 2012;13(11):1045−1057.
- Moon YE, Lee YK, Lee J, Moon DE. The effects of preoperative intravenous acetaminophen in patients undergoing abdominal hysterectomy. Arch Gynecol Obstet. 2011;284(6):1455−1460.
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170−1179.
- Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg. 2012;115(2):428−442.
- Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol. 2007;20(5):456−472.
- Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev. 2013;(7):CD008307.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 suppl):S2−S15.
- Castro-Alves LJ, Oliveira de Medeiros AC, Neves SP, et al. Perioperative duloxetine to improve postoperative recovery after abdominal hysterectomy: a prospective, randomized, double-blinded, placebo-controlled study. Anesth Analg. 2016;122(1):98−104.
- Bedin A, Caldart Bedin RA, Vieira JE, Ashmawi HA. Duloxetine as an analgesic reduces opioid consumption after spine surgery: a randomized, double-blind, controlled study. Clin J Pain. 2017;33(10):865−869.
- Amr YM, Yousef AA. Evaluation of efficacy of the perioperative administration of venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain. 2010;26(5):381–385.
- Marret E, Rolin M, Beaussier M, Bonnet F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg. 2008;95(11):1331–1338.
- De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588.
- De Oliveira GS Jr, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesth Analg. 2012;114(2):424–433.
- Hamilton TW, Athanassoglou V, Mellon S, et al. Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst Rev. 2017;(2):CD011419.
- Charlton S, Cyna AM, Middleton P, Griffiths JD. Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database Syst Rev. 2010;(12):CD007705.
- Hain E, Maggiori L, Prost À la Denise J, Panis Y. Transversus abdominis plane (TAP) block in laparoscopic colorectal surgery improves postoperative pain management: a meta-analysis [published online ahead of print January 30, 2018]. Colorectal Dis. doi:10.1111/codi.14037.
- Staker JJ, Liu D, Church R, et al. A triple-blind, placebo-controlled randomised trial of the ilioinguinal-transversus abdominis plane (I-TAP) nerve block for elective caesarean section [published online ahead of print January 29, 2018]. Anaesthesia. doi:10.1111/anae.14222.
- Hamilton TW, Athanassoglou V, Trivella M, et al. Liposomal bupivacaine peripheral nerve block for the management of postoperative pain. Cochrane Database Syst Rev. 2016;(8):CD011476.


