User login
Delirium and acute problematic behavior in LTC patients: What’s the best approach?
- What are the best methods to assess delirium and acute problematic behavior in the long-term care setting?
- What is the most appropriate treatment for these patients?
- Why is monitoring of interventions critical to patient outcomes?
The answers to these questions are summarized at right and in the 2008 edition of Delirium and Acute Problematic Behavior in the Long-Term Care Setting, published by the American Medical Directors Association (AMDA). This comprehensive guideline, developed to improve quality of care, features a 15-step systematic approach to recognizing, assessing, treating, and monitoring long-term care patients with delirium and acute problematic behavior. It includes a simple algorithm to guide the decision-making process.
Initially developed to fill a gap
Delirium and acute problematic behavior are common in the long-term care setting, but management guidelines have been limited. To assist physicians, advanced practice nurses, nurses, and allied health professionals in long-term care facilities, the AMDA developed the initial version of this guideline in 1998. A multidisciplinary workgroup used a process that combined evidence- and consensus-based approaches: An electronic literature search identified pertinent guidelines, research articles, and review articles, and the recommendations were based on the opinions of the expert workgroup.
Guideline revision expands its scope
The guideline update was completed in 2008, under the direction of the AMDA Clinical Practice Guideline Steering Committee. The new version incorporates information published in peer-reviewed journals after the original guideline was released; it has also been expanded to incorporate recommendations from seasoned practitioners in long-term care.
The AMDA facilitated peer review of the revised guideline, with input from 175 individuals outside of the steering committee. The result is a well-written, practical guide to dealing with long-term care residents with altered mental states.
Grade C Recommendations
Recognition/assessment
- Clearly identify the problematic behavior and altered mental function:
- Determine the urgency of the situation and the need for additional evaluation and testing.
- Identify the cause of the problematic behavior and altered mental function.
Management/treatment
- Initiate a plan for treatment.
- Provide both symptomatic and cause-specific management.
- Administer medications as needed, such as antipsychotics, antidepressants, cholinesterase inhibitors and memantine, anticonvulsants, and anxiolytics.
Monitoring
- Monitor and adjust interventions as indicated.
- Review the effectiveness and appropriateness of medications.
- Prevent, identify, and address any complications of the condition and treatment.
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A few limitations
This guideline does not include grades for individual recommendations. Since the recommendations are based on expert opinion, the evidence is rated C using the SORT taxonomy.1 The recommendations are easy to understand, but an executive summary would have been useful. The algorithm lacks detail, which weakens its clinical value.
In addition, this 36-page guideline is available only in print from the AMDA. Lack of Internet access limits its accessibility at the point of care.
Source for this guideline
American Medical Directors Association (AMDA). Delirium and acute problematic behavior in the long-term care setting. Columbia, Md: American Medical Directors Association (AMDA); 2008. 36 p. (36 references). Available from the American Medical Directors Association. (http://www.amda.com/tools/cpg/alteredmentalstates.cfm).
Other guideline on this topic
American Psychiatric Association. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999 May;156(5 suppl):S1-S20. [135 references]. Guideline Watch. August 2004. Available at: www.psychiatryonline.com/pracGuide/pracGuideChapToc_2.aspx.
The APA guideline is not current. The major recommendations focus on psychiatric management, environmental and supportive interventions, and somatic interventions. Although the guideline is based on a systematic review of the literature, the APA does not describe the methods used to review the evidence. Nor does it report ratings for the level of evidence.
CORRESPONDENCE Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177; [email protected]
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract. 2004;53:111-120.
2. Inouye S, van Dyck C, Alessi C, et al. Clarifying confusion: the confusion assessment method. Ann Intern Med. 1990;113:941-948.
- What are the best methods to assess delirium and acute problematic behavior in the long-term care setting?
- What is the most appropriate treatment for these patients?
- Why is monitoring of interventions critical to patient outcomes?
The answers to these questions are summarized at right and in the 2008 edition of Delirium and Acute Problematic Behavior in the Long-Term Care Setting, published by the American Medical Directors Association (AMDA). This comprehensive guideline, developed to improve quality of care, features a 15-step systematic approach to recognizing, assessing, treating, and monitoring long-term care patients with delirium and acute problematic behavior. It includes a simple algorithm to guide the decision-making process.
Initially developed to fill a gap
Delirium and acute problematic behavior are common in the long-term care setting, but management guidelines have been limited. To assist physicians, advanced practice nurses, nurses, and allied health professionals in long-term care facilities, the AMDA developed the initial version of this guideline in 1998. A multidisciplinary workgroup used a process that combined evidence- and consensus-based approaches: An electronic literature search identified pertinent guidelines, research articles, and review articles, and the recommendations were based on the opinions of the expert workgroup.
Guideline revision expands its scope
The guideline update was completed in 2008, under the direction of the AMDA Clinical Practice Guideline Steering Committee. The new version incorporates information published in peer-reviewed journals after the original guideline was released; it has also been expanded to incorporate recommendations from seasoned practitioners in long-term care.
The AMDA facilitated peer review of the revised guideline, with input from 175 individuals outside of the steering committee. The result is a well-written, practical guide to dealing with long-term care residents with altered mental states.
Grade C Recommendations
Recognition/assessment
- Clearly identify the problematic behavior and altered mental function:
- Determine the urgency of the situation and the need for additional evaluation and testing.
- Identify the cause of the problematic behavior and altered mental function.
Management/treatment
- Initiate a plan for treatment.
- Provide both symptomatic and cause-specific management.
- Administer medications as needed, such as antipsychotics, antidepressants, cholinesterase inhibitors and memantine, anticonvulsants, and anxiolytics.
Monitoring
- Monitor and adjust interventions as indicated.
- Review the effectiveness and appropriateness of medications.
- Prevent, identify, and address any complications of the condition and treatment.
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A few limitations
This guideline does not include grades for individual recommendations. Since the recommendations are based on expert opinion, the evidence is rated C using the SORT taxonomy.1 The recommendations are easy to understand, but an executive summary would have been useful. The algorithm lacks detail, which weakens its clinical value.
In addition, this 36-page guideline is available only in print from the AMDA. Lack of Internet access limits its accessibility at the point of care.
Source for this guideline
American Medical Directors Association (AMDA). Delirium and acute problematic behavior in the long-term care setting. Columbia, Md: American Medical Directors Association (AMDA); 2008. 36 p. (36 references). Available from the American Medical Directors Association. (http://www.amda.com/tools/cpg/alteredmentalstates.cfm).
Other guideline on this topic
American Psychiatric Association. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999 May;156(5 suppl):S1-S20. [135 references]. Guideline Watch. August 2004. Available at: www.psychiatryonline.com/pracGuide/pracGuideChapToc_2.aspx.
The APA guideline is not current. The major recommendations focus on psychiatric management, environmental and supportive interventions, and somatic interventions. Although the guideline is based on a systematic review of the literature, the APA does not describe the methods used to review the evidence. Nor does it report ratings for the level of evidence.
CORRESPONDENCE Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177; [email protected]
- What are the best methods to assess delirium and acute problematic behavior in the long-term care setting?
- What is the most appropriate treatment for these patients?
- Why is monitoring of interventions critical to patient outcomes?
The answers to these questions are summarized at right and in the 2008 edition of Delirium and Acute Problematic Behavior in the Long-Term Care Setting, published by the American Medical Directors Association (AMDA). This comprehensive guideline, developed to improve quality of care, features a 15-step systematic approach to recognizing, assessing, treating, and monitoring long-term care patients with delirium and acute problematic behavior. It includes a simple algorithm to guide the decision-making process.
Initially developed to fill a gap
Delirium and acute problematic behavior are common in the long-term care setting, but management guidelines have been limited. To assist physicians, advanced practice nurses, nurses, and allied health professionals in long-term care facilities, the AMDA developed the initial version of this guideline in 1998. A multidisciplinary workgroup used a process that combined evidence- and consensus-based approaches: An electronic literature search identified pertinent guidelines, research articles, and review articles, and the recommendations were based on the opinions of the expert workgroup.
Guideline revision expands its scope
The guideline update was completed in 2008, under the direction of the AMDA Clinical Practice Guideline Steering Committee. The new version incorporates information published in peer-reviewed journals after the original guideline was released; it has also been expanded to incorporate recommendations from seasoned practitioners in long-term care.
The AMDA facilitated peer review of the revised guideline, with input from 175 individuals outside of the steering committee. The result is a well-written, practical guide to dealing with long-term care residents with altered mental states.
Grade C Recommendations
Recognition/assessment
- Clearly identify the problematic behavior and altered mental function:
- Determine the urgency of the situation and the need for additional evaluation and testing.
- Identify the cause of the problematic behavior and altered mental function.
Management/treatment
- Initiate a plan for treatment.
- Provide both symptomatic and cause-specific management.
- Administer medications as needed, such as antipsychotics, antidepressants, cholinesterase inhibitors and memantine, anticonvulsants, and anxiolytics.
Monitoring
- Monitor and adjust interventions as indicated.
- Review the effectiveness and appropriateness of medications.
- Prevent, identify, and address any complications of the condition and treatment.
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A few limitations
This guideline does not include grades for individual recommendations. Since the recommendations are based on expert opinion, the evidence is rated C using the SORT taxonomy.1 The recommendations are easy to understand, but an executive summary would have been useful. The algorithm lacks detail, which weakens its clinical value.
In addition, this 36-page guideline is available only in print from the AMDA. Lack of Internet access limits its accessibility at the point of care.
Source for this guideline
American Medical Directors Association (AMDA). Delirium and acute problematic behavior in the long-term care setting. Columbia, Md: American Medical Directors Association (AMDA); 2008. 36 p. (36 references). Available from the American Medical Directors Association. (http://www.amda.com/tools/cpg/alteredmentalstates.cfm).
Other guideline on this topic
American Psychiatric Association. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999 May;156(5 suppl):S1-S20. [135 references]. Guideline Watch. August 2004. Available at: www.psychiatryonline.com/pracGuide/pracGuideChapToc_2.aspx.
The APA guideline is not current. The major recommendations focus on psychiatric management, environmental and supportive interventions, and somatic interventions. Although the guideline is based on a systematic review of the literature, the APA does not describe the methods used to review the evidence. Nor does it report ratings for the level of evidence.
CORRESPONDENCE Keith B. Holten, MD, 825 Locust Street, Wilmington, OH 45177; [email protected]
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract. 2004;53:111-120.
2. Inouye S, van Dyck C, Alessi C, et al. Clarifying confusion: the confusion assessment method. Ann Intern Med. 1990;113:941-948.
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Fam Pract. 2004;53:111-120.
2. Inouye S, van Dyck C, Alessi C, et al. Clarifying confusion: the confusion assessment method. Ann Intern Med. 1990;113:941-948.
Managing chronic pain: What’s the best approach?
Grade A recommendations
- Develop a physician-patient partnership. This should include a plan of care and realistic goal-setting.
- Begin physical rehabilitation and psychosocial management. This includes an exercise fitness program, cognitive-behavioral therapy, and self-management.
Grade B Recommendations
- Obtain a general history, including psychological assessment and spirituality evaluation, and identify barriers to treatment.
- Obtain a thorough pain history.
- Perform a physical examination, including a focused musculoskeletal and neurologic evaluation.
- Perform diagnostic testing as indicated. X-rays, computed tomography, magnetic resonance imaging, electromyography, and nerve conduction studies can help differentiate the biological mechanisms of pain.
- Teach patients to use pain scales for self-reporting.
Grade C recommendations
- Categorize the 4 biological mechanisms of pain (inflammatory, mechanical, musculoskeletal, or neuropathic).
- Consider the following pharmacologic options for Level I care:
Nonopioid analgesics
Nonsteroidal anti-inflammatory drugs
Antidepressants, including tricyclics
Anticonvulsants
Topical agents
Muscle relaxants
Anxiolytics
Drugs for insomnia
Opioids (last line) - Consider the following Level I therapeutic procedures:
Facet joint injection
Percutaneous radiofrequency neurotomy
Intradiscal electrothermal therapy
Epidural corticosteroid injections
Vertebroplasty and kyphoplasty
Acupuncture - Consider the following Level II interventions:
Referral to an interdisciplinary team and pain specialist
Surgery
Palliative interventions (nucleoplasty, spinal cord stimulation, intrathecal medication delivery systems)
Multidisciplinary pain rehabilitation
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
- What are the critical steps in assessing a patient with chronic pain?
- What are the 4 biological mechanisms of pain?
- When is referral to a pain specialist recommended?
The answers to these questions are summarized at right and in the 2008 edition of Assessment and Management of Chronic Pain. Originally developed in 2005, the guideline was funded and published by the Institute for Clinical Systems Improvement (ICSI), a collaboration of 57 medical groups sponsored by 6 Minnesota health plans. A 3rd edition, released in August, summarizes the current evidence in the assessment and treatment of chronic pain in mature adolescents (ages 16-18 years) and adults.
Chronic pain—a persistent, life-altering condition—is one of the most challenging clinical disorders for primary care physicians to treat. Unlike acute pain, where we seek to cure the underlying biological condition, the goal of chronic pain management is to improve patient function in the face of pain that may never completely resolve.
Achieving that goal, according to the new guideline, requires a patient-centered, multifaceted approach—often involving a health care team that includes specialists in behavioral health and physical rehabilitation—that is co-ordinated by a primary care physician. An effective treatment plan must address biopsychosocial factors as well as spiritual and cultural issues. Patients must be taught self-management skills focused on fitness, stress reduction, and maintaining a healthy lifestyle ( TABLE ). Medications may be part of the treatment plan but should not be the sole focus, according to the guideline. Opioids are an option when other therapies fail.
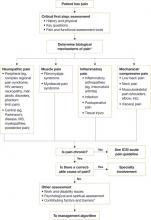
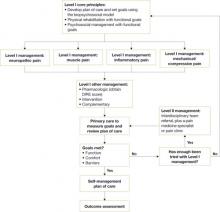
ICSI’s new guideline also addresses the effects of various therapies, the role of psychosocial factors, and the identification of barriers to treatment. The comprehensive guideline, which has 172 references and 9 appendices, also features 2 easy-to-use algorithms. One algorithm addresses the assessment of chronic pain ( FIGURE 1 ) and the other deals with chronic pain management ( FIGURE 2 ). Both algorithms identify level I and level II strategies. And both can be readily adapted to primary care practice, and are extremely helpful to physicians who are evaluating and developing care plans for patients with chronic pain.
FIGURE 1
Chronic pain assessment algorithm
HIV, human immunodeficiency virus; ICSI, Institute for Clinical Systems Improvement; MS, multiple sclerosis.
*Pain types and contributing factors are not mutually exclusive. Patients frequently have more than one type of pain, as well as overlapping contributing factors.
Source: Institute for Clinical Systems Improvement. Reprinted with permission.
4 objectives
This latest guideline was developed to:
- Improve the treatment of adult chronic pain patients by encouraging physicians to complete an appropriate biopsychosocial assessment (and reassessment).
- Improve patient function by recommending the development and use of a comprehensive treatment plan that includes a multispecialty team.
- Improve the use of Level I and Level II treatment approaches to chronic pain.
- Provide guidance on the most effective use of nonopioid and opioid medications in the treatment of chronic pain.
With these objectives in mind, the ICSI work group conducted a comprehensive literature review, giving priority to randomized controlled trials (RCTs), meta-analyses, and systematic reviews. The work group used a 7-tier grading system to rate the evidence and a 3-category system for the worksheets in the appendix. For this article, we have converted the evidence ratings into SORT taxonomy.1
What’s changed?
In addition to reflecting the latest research, the new guideline contains a number of clarifications. For example, the update states that medications are not the “sole” focus of treatment and should be used, when necessary, as part of an overall approach to pain management. The previous version noted that medications were not the “primary” focus.
The management algorithm ( FIGURE 2 ) now leads with “core principles”—a term suggesting greater importance than the former term, “general management,” implied. Clinical highlights, a synthesis of key recommendations, have been revised to better align with the guideline’s main components—assessment, functional goals, patient-centered/biopsychosocial care planning, Level I vs Level II approaches, and medication and patient selection.
Other changes in the guideline may contribute to clinicians’ understanding of chronic pain and its complex presentation. The guideline now includes a statement about allodynia and hyperalgesia to indicate that both may play an important role in any pain syndrome—not just complex regional pain syndrome. Information about fibromyalgia symptoms and myofascial pain has been added. The definitions page now has an entry for “biopsychosocial model,” as well as language designed to stress the differences between untreated acute pain and ongoing chronic pain.
FIGURE 2
Chronic pain management algorithm
DIRE, diagnosis, intractability, risk, efficacy.
Source: Institute for Clinical System Improvement. Reprinted with permission.
A limitation, an improvement
A limitation of the guideline is the lack of studies addressing the effectiveness of a comprehensive, multidisciplinary treatment approach to chronic pain management. Most studies consider single therapies.
An improvement in this guideline is that the evidence levels of each strategy are now listed within the section describing it—a notable change that makes it far easier to identify the quality of individual recommendations.
As has been the case in the past, this latest edition of the guideline offers a number of tools for physicians. The assessment and management algorithms ( FIGURES 1 AND 2 , respectively) walk clinicians through the decision-making process. In addition, the following 9 appendices provide practical guidance to physicians in various aspects of patient evaluation and care:
- Brief Pain Inventory (Short Form)
- Patient Health Questionnaire (PHQ-9)
- Functional Ability Questionnaire
- Personal Care Plan for Chronic Pain
- DIRE (diagnosis, intractability, risk, efficacy) Score: Patient Selection for Chronic Opioid Analgesia
- Opioid Agreement Form
- Opioid Analgesics
- Pharmaceutical Interventions for Neuropathic Pain
- Neuropathic Pain Treatment Diagram.
Source for this guideline
Institute for Clinical Systems Improvement (ICSI). Assessment and Management of Chronic Pain. 3rd ed. Bloomington (Minn): Institute for Clinical Systems Improvement (ICSI); 2008 July. Available at: http://www.icsi.org/pain__chronic__assessment_and_management_of_14399/pain__chronic__assessment_and_management_of__guideline.html. Accessed September 9, 2008.
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract. 2004;53:111-120.
Grade A recommendations
- Develop a physician-patient partnership. This should include a plan of care and realistic goal-setting.
- Begin physical rehabilitation and psychosocial management. This includes an exercise fitness program, cognitive-behavioral therapy, and self-management.
Grade B Recommendations
- Obtain a general history, including psychological assessment and spirituality evaluation, and identify barriers to treatment.
- Obtain a thorough pain history.
- Perform a physical examination, including a focused musculoskeletal and neurologic evaluation.
- Perform diagnostic testing as indicated. X-rays, computed tomography, magnetic resonance imaging, electromyography, and nerve conduction studies can help differentiate the biological mechanisms of pain.
- Teach patients to use pain scales for self-reporting.
Grade C recommendations
- Categorize the 4 biological mechanisms of pain (inflammatory, mechanical, musculoskeletal, or neuropathic).
- Consider the following pharmacologic options for Level I care:
Nonopioid analgesics
Nonsteroidal anti-inflammatory drugs
Antidepressants, including tricyclics
Anticonvulsants
Topical agents
Muscle relaxants
Anxiolytics
Drugs for insomnia
Opioids (last line) - Consider the following Level I therapeutic procedures:
Facet joint injection
Percutaneous radiofrequency neurotomy
Intradiscal electrothermal therapy
Epidural corticosteroid injections
Vertebroplasty and kyphoplasty
Acupuncture - Consider the following Level II interventions:
Referral to an interdisciplinary team and pain specialist
Surgery
Palliative interventions (nucleoplasty, spinal cord stimulation, intrathecal medication delivery systems)
Multidisciplinary pain rehabilitation
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
- What are the critical steps in assessing a patient with chronic pain?
- What are the 4 biological mechanisms of pain?
- When is referral to a pain specialist recommended?
The answers to these questions are summarized at right and in the 2008 edition of Assessment and Management of Chronic Pain. Originally developed in 2005, the guideline was funded and published by the Institute for Clinical Systems Improvement (ICSI), a collaboration of 57 medical groups sponsored by 6 Minnesota health plans. A 3rd edition, released in August, summarizes the current evidence in the assessment and treatment of chronic pain in mature adolescents (ages 16-18 years) and adults.
Chronic pain—a persistent, life-altering condition—is one of the most challenging clinical disorders for primary care physicians to treat. Unlike acute pain, where we seek to cure the underlying biological condition, the goal of chronic pain management is to improve patient function in the face of pain that may never completely resolve.
Achieving that goal, according to the new guideline, requires a patient-centered, multifaceted approach—often involving a health care team that includes specialists in behavioral health and physical rehabilitation—that is co-ordinated by a primary care physician. An effective treatment plan must address biopsychosocial factors as well as spiritual and cultural issues. Patients must be taught self-management skills focused on fitness, stress reduction, and maintaining a healthy lifestyle ( TABLE ). Medications may be part of the treatment plan but should not be the sole focus, according to the guideline. Opioids are an option when other therapies fail.
ICSI’s new guideline also addresses the effects of various therapies, the role of psychosocial factors, and the identification of barriers to treatment. The comprehensive guideline, which has 172 references and 9 appendices, also features 2 easy-to-use algorithms. One algorithm addresses the assessment of chronic pain ( FIGURE 1 ) and the other deals with chronic pain management ( FIGURE 2 ). Both algorithms identify level I and level II strategies. And both can be readily adapted to primary care practice, and are extremely helpful to physicians who are evaluating and developing care plans for patients with chronic pain.
FIGURE 1
Chronic pain assessment algorithm
HIV, human immunodeficiency virus; ICSI, Institute for Clinical Systems Improvement; MS, multiple sclerosis.
*Pain types and contributing factors are not mutually exclusive. Patients frequently have more than one type of pain, as well as overlapping contributing factors.
Source: Institute for Clinical Systems Improvement. Reprinted with permission.
4 objectives
This latest guideline was developed to:
- Improve the treatment of adult chronic pain patients by encouraging physicians to complete an appropriate biopsychosocial assessment (and reassessment).
- Improve patient function by recommending the development and use of a comprehensive treatment plan that includes a multispecialty team.
- Improve the use of Level I and Level II treatment approaches to chronic pain.
- Provide guidance on the most effective use of nonopioid and opioid medications in the treatment of chronic pain.
With these objectives in mind, the ICSI work group conducted a comprehensive literature review, giving priority to randomized controlled trials (RCTs), meta-analyses, and systematic reviews. The work group used a 7-tier grading system to rate the evidence and a 3-category system for the worksheets in the appendix. For this article, we have converted the evidence ratings into SORT taxonomy.1
What’s changed?
In addition to reflecting the latest research, the new guideline contains a number of clarifications. For example, the update states that medications are not the “sole” focus of treatment and should be used, when necessary, as part of an overall approach to pain management. The previous version noted that medications were not the “primary” focus.
The management algorithm ( FIGURE 2 ) now leads with “core principles”—a term suggesting greater importance than the former term, “general management,” implied. Clinical highlights, a synthesis of key recommendations, have been revised to better align with the guideline’s main components—assessment, functional goals, patient-centered/biopsychosocial care planning, Level I vs Level II approaches, and medication and patient selection.
Other changes in the guideline may contribute to clinicians’ understanding of chronic pain and its complex presentation. The guideline now includes a statement about allodynia and hyperalgesia to indicate that both may play an important role in any pain syndrome—not just complex regional pain syndrome. Information about fibromyalgia symptoms and myofascial pain has been added. The definitions page now has an entry for “biopsychosocial model,” as well as language designed to stress the differences between untreated acute pain and ongoing chronic pain.
FIGURE 2
Chronic pain management algorithm
DIRE, diagnosis, intractability, risk, efficacy.
Source: Institute for Clinical System Improvement. Reprinted with permission.
A limitation, an improvement
A limitation of the guideline is the lack of studies addressing the effectiveness of a comprehensive, multidisciplinary treatment approach to chronic pain management. Most studies consider single therapies.
An improvement in this guideline is that the evidence levels of each strategy are now listed within the section describing it—a notable change that makes it far easier to identify the quality of individual recommendations.
As has been the case in the past, this latest edition of the guideline offers a number of tools for physicians. The assessment and management algorithms ( FIGURES 1 AND 2 , respectively) walk clinicians through the decision-making process. In addition, the following 9 appendices provide practical guidance to physicians in various aspects of patient evaluation and care:
- Brief Pain Inventory (Short Form)
- Patient Health Questionnaire (PHQ-9)
- Functional Ability Questionnaire
- Personal Care Plan for Chronic Pain
- DIRE (diagnosis, intractability, risk, efficacy) Score: Patient Selection for Chronic Opioid Analgesia
- Opioid Agreement Form
- Opioid Analgesics
- Pharmaceutical Interventions for Neuropathic Pain
- Neuropathic Pain Treatment Diagram.
Source for this guideline
Institute for Clinical Systems Improvement (ICSI). Assessment and Management of Chronic Pain. 3rd ed. Bloomington (Minn): Institute for Clinical Systems Improvement (ICSI); 2008 July. Available at: http://www.icsi.org/pain__chronic__assessment_and_management_of_14399/pain__chronic__assessment_and_management_of__guideline.html. Accessed September 9, 2008.
Grade A recommendations
- Develop a physician-patient partnership. This should include a plan of care and realistic goal-setting.
- Begin physical rehabilitation and psychosocial management. This includes an exercise fitness program, cognitive-behavioral therapy, and self-management.
Grade B Recommendations
- Obtain a general history, including psychological assessment and spirituality evaluation, and identify barriers to treatment.
- Obtain a thorough pain history.
- Perform a physical examination, including a focused musculoskeletal and neurologic evaluation.
- Perform diagnostic testing as indicated. X-rays, computed tomography, magnetic resonance imaging, electromyography, and nerve conduction studies can help differentiate the biological mechanisms of pain.
- Teach patients to use pain scales for self-reporting.
Grade C recommendations
- Categorize the 4 biological mechanisms of pain (inflammatory, mechanical, musculoskeletal, or neuropathic).
- Consider the following pharmacologic options for Level I care:
Nonopioid analgesics
Nonsteroidal anti-inflammatory drugs
Antidepressants, including tricyclics
Anticonvulsants
Topical agents
Muscle relaxants
Anxiolytics
Drugs for insomnia
Opioids (last line) - Consider the following Level I therapeutic procedures:
Facet joint injection
Percutaneous radiofrequency neurotomy
Intradiscal electrothermal therapy
Epidural corticosteroid injections
Vertebroplasty and kyphoplasty
Acupuncture - Consider the following Level II interventions:
Referral to an interdisciplinary team and pain specialist
Surgery
Palliative interventions (nucleoplasty, spinal cord stimulation, intrathecal medication delivery systems)
Multidisciplinary pain rehabilitation
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
- What are the critical steps in assessing a patient with chronic pain?
- What are the 4 biological mechanisms of pain?
- When is referral to a pain specialist recommended?
The answers to these questions are summarized at right and in the 2008 edition of Assessment and Management of Chronic Pain. Originally developed in 2005, the guideline was funded and published by the Institute for Clinical Systems Improvement (ICSI), a collaboration of 57 medical groups sponsored by 6 Minnesota health plans. A 3rd edition, released in August, summarizes the current evidence in the assessment and treatment of chronic pain in mature adolescents (ages 16-18 years) and adults.
Chronic pain—a persistent, life-altering condition—is one of the most challenging clinical disorders for primary care physicians to treat. Unlike acute pain, where we seek to cure the underlying biological condition, the goal of chronic pain management is to improve patient function in the face of pain that may never completely resolve.
Achieving that goal, according to the new guideline, requires a patient-centered, multifaceted approach—often involving a health care team that includes specialists in behavioral health and physical rehabilitation—that is co-ordinated by a primary care physician. An effective treatment plan must address biopsychosocial factors as well as spiritual and cultural issues. Patients must be taught self-management skills focused on fitness, stress reduction, and maintaining a healthy lifestyle ( TABLE ). Medications may be part of the treatment plan but should not be the sole focus, according to the guideline. Opioids are an option when other therapies fail.
ICSI’s new guideline also addresses the effects of various therapies, the role of psychosocial factors, and the identification of barriers to treatment. The comprehensive guideline, which has 172 references and 9 appendices, also features 2 easy-to-use algorithms. One algorithm addresses the assessment of chronic pain ( FIGURE 1 ) and the other deals with chronic pain management ( FIGURE 2 ). Both algorithms identify level I and level II strategies. And both can be readily adapted to primary care practice, and are extremely helpful to physicians who are evaluating and developing care plans for patients with chronic pain.
FIGURE 1
Chronic pain assessment algorithm
HIV, human immunodeficiency virus; ICSI, Institute for Clinical Systems Improvement; MS, multiple sclerosis.
*Pain types and contributing factors are not mutually exclusive. Patients frequently have more than one type of pain, as well as overlapping contributing factors.
Source: Institute for Clinical Systems Improvement. Reprinted with permission.
4 objectives
This latest guideline was developed to:
- Improve the treatment of adult chronic pain patients by encouraging physicians to complete an appropriate biopsychosocial assessment (and reassessment).
- Improve patient function by recommending the development and use of a comprehensive treatment plan that includes a multispecialty team.
- Improve the use of Level I and Level II treatment approaches to chronic pain.
- Provide guidance on the most effective use of nonopioid and opioid medications in the treatment of chronic pain.
With these objectives in mind, the ICSI work group conducted a comprehensive literature review, giving priority to randomized controlled trials (RCTs), meta-analyses, and systematic reviews. The work group used a 7-tier grading system to rate the evidence and a 3-category system for the worksheets in the appendix. For this article, we have converted the evidence ratings into SORT taxonomy.1
What’s changed?
In addition to reflecting the latest research, the new guideline contains a number of clarifications. For example, the update states that medications are not the “sole” focus of treatment and should be used, when necessary, as part of an overall approach to pain management. The previous version noted that medications were not the “primary” focus.
The management algorithm ( FIGURE 2 ) now leads with “core principles”—a term suggesting greater importance than the former term, “general management,” implied. Clinical highlights, a synthesis of key recommendations, have been revised to better align with the guideline’s main components—assessment, functional goals, patient-centered/biopsychosocial care planning, Level I vs Level II approaches, and medication and patient selection.
Other changes in the guideline may contribute to clinicians’ understanding of chronic pain and its complex presentation. The guideline now includes a statement about allodynia and hyperalgesia to indicate that both may play an important role in any pain syndrome—not just complex regional pain syndrome. Information about fibromyalgia symptoms and myofascial pain has been added. The definitions page now has an entry for “biopsychosocial model,” as well as language designed to stress the differences between untreated acute pain and ongoing chronic pain.
FIGURE 2
Chronic pain management algorithm
DIRE, diagnosis, intractability, risk, efficacy.
Source: Institute for Clinical System Improvement. Reprinted with permission.
A limitation, an improvement
A limitation of the guideline is the lack of studies addressing the effectiveness of a comprehensive, multidisciplinary treatment approach to chronic pain management. Most studies consider single therapies.
An improvement in this guideline is that the evidence levels of each strategy are now listed within the section describing it—a notable change that makes it far easier to identify the quality of individual recommendations.
As has been the case in the past, this latest edition of the guideline offers a number of tools for physicians. The assessment and management algorithms ( FIGURES 1 AND 2 , respectively) walk clinicians through the decision-making process. In addition, the following 9 appendices provide practical guidance to physicians in various aspects of patient evaluation and care:
- Brief Pain Inventory (Short Form)
- Patient Health Questionnaire (PHQ-9)
- Functional Ability Questionnaire
- Personal Care Plan for Chronic Pain
- DIRE (diagnosis, intractability, risk, efficacy) Score: Patient Selection for Chronic Opioid Analgesia
- Opioid Agreement Form
- Opioid Analgesics
- Pharmaceutical Interventions for Neuropathic Pain
- Neuropathic Pain Treatment Diagram.
Source for this guideline
Institute for Clinical Systems Improvement (ICSI). Assessment and Management of Chronic Pain. 3rd ed. Bloomington (Minn): Institute for Clinical Systems Improvement (ICSI); 2008 July. Available at: http://www.icsi.org/pain__chronic__assessment_and_management_of_14399/pain__chronic__assessment_and_management_of__guideline.html. Accessed September 9, 2008.
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract. 2004;53:111-120.
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract. 2004;53:111-120.
MANAGING CAP: Are you up-to-date?
For our purposes, the evidence ratings are based on literature quality, not expert opinion, and are updated to comply with the SORT taxonomy*
Grade A Recommendations
- Severity-of-illness scores can be used to identify patients with CAP who are candidates for outpatient treatment.
- Appropriate outpatient antibiotic treatment for a previously healthy person, with no risk factors for drug-resistant S. pneumonia (DRSP) is a macrolide (azithromycin, clarithromycin, or erythromycin).
- High risk patients: those with co-morbidities (chronic heart, lung, liver, or renal disease), diabetes mellitus, alcoholism, malignancies, asplenia, immunosuppression, or antibiotics within 3 months should be treated with a respiratory flouroquinolone—moxifloxacin, gemifloxacin, or levofloxacin (750 mg dose).
- A beta-lactam (high-dose amoxicillin, amoxicillin clavulanate, ceftriaxone, cefpodoxime, of cefuroxime), plus a macrolide is an option for high risk patients.
- Blood cultures and sputum cultures are optional prior to treatment of outpatients.
- In geographic areas where >25% of pneumococcal organisms are macrolide resistant, a beta-lactam, plus docxycycline should be considered.
- Treat with antibiotics at least 5 days.
- Health care workers in inpatient and outpatient settings and long-term facilities should receive annual influenza immunization.
Grade B Recommendations
- Severity of illness scores should be supplemented with physician subjective opinion about individual patients. the ability to safely and reliably take oral medications and the availability of outpatient resources should be considered.
- Patients with Cap should be investigated for specific pathogens that would significantly alter standard (empirical) management decisions, when suspected on the basis of clinical assessment.
- A beta-lactam, plus doxycycline is an alternative to the beta-lactam, plus macrolide combination for high risk patients.
- Pneumococcal polysaccharide vaccine is recommended for persons >65 years of age and for those with selected high-risk concurrent diseases.
Grade C Recommendations
- In addition to clinical features, an infiltrate by chest radiograph or other imaging technique is required for the diagnosis of pneumonia.
- An appropriate outpatient treatment for previously healthy individuals with no risk factors for DRSP infection is doxycycline.
- Use respiratory hygiene measures (hand hygiene, masks, tissues) for patients with cough in outpatient settings.
*Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004; 53:111–120.
When is outpatient treatment appropriate for community-acquired pneumonia (CAP)? Which antibiotics are recommended for outpatient therapy? What are the best prevention strategies? The answers are in the consensus guidelines published earlier this year by the Infectious Diseases Society of America and the American Thoracic Society (IDSA/ATS). The new guidelines update an IDSA guideline published in 2003.
Background. Management (and prevention) of CAP is inconsistent, and there is also emerging resistance of pneumococcus to macrolides.
These guidelines were developed to hasten consistency among caregivers and hospitals in the care of patients with pneumonia. Appropriateness of outpatient care, severity of illness assessment, hospital treatment decisions, ICU care, and choice of antibiotics for high-risk patients and for drug-resistant S pneumonia were reviewed. The joint committee recommended that hospitals standardize care and create policies to increase the vaccination rate.
CASE 1
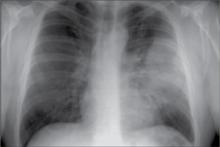
Your patient is a 45-year-old man with cough, fever, and chills. He has a history of metabolic syndrome, and a 40 pack-year smoking history. He was well until 1 week ago when he went camping in the rain. Over the last 2 days he has had shaking chills, cough productive of green phlegm, and he finds that he gets a bit short-winded when walking stairs. He wonders if he has pneumonia. He is overweight and in no acute distress.
T 101 • P 88 • RR 18 • WT 220 • HT 5 7
Exam Normal other than localized coarse rales in the left posterior lung field; spot O2 saturation is 96%
What is your diagnosis and initial management?
Which of the following statements are true regarding the outpatient management of pneumonia?
- If 2 or more CURB-65 criteria are present, the patient should be hospitalized
- A macrolide is an appropriate choice of treatment for a previously healthy person with no risk of drug resistance
- A positive chest x-ray or other imaging is required for the diagnosis
- Blood cultures and sputum cultures must be obtained
- Antibiotic treatment should be a minimum of 10 days
ANSWERS: A, B, AND C
Diagnosis Community acquired pneumonia—left lobar.
Initial management This patient can be treated as an outpatient based on the severity-of-illness scores in this guideline. He should be treated with antibiotics a minimum of 5 days. With his comorbidities, antibiotic choices include 1) a fluoroquinolone, 2) a beta-lactam plus macrolide, or 3) (in areas with high prevalence of macrolide resistance) a beta-lactam plus doxycycline.
CASE 2
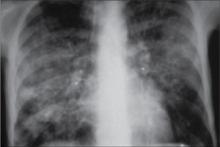
A 76-year-old man is brought into the office by his niece. “I just don’t feel well,” he says. The patient has been increasingly ill over the past week, and his niece is concerned that he seems to have trouble breathing. The patient minimizes his symptoms, but in relaying his history, he is obviously short of breath and cannot talk continuously. He needed a wheelchair to come in from the parking lot (and you know that he is usually spry and ambulatory). He has a history of congestive heart failure, hypertension, type 2 diabetes mellitus, depression, and osteoarthritis. He takes furosemide, potassium, enalapril, lantus insulin, sertraline, and PRN acetaminophen. He has never smoked. He denies PND and orthopnea. He is clearly short of breath and in some mild distress.
T 99 • P 102 • RR 36 • WT 260 • HT 5 9
Exam Remarkable for diffuse rhonchi and wheezing across all lung fields; spot O2 saturation is 89%
What is your diagnosis and initial management?
The differential diagnosis for this patient includes:
- Bacterial pneumonia
- Viral pneumonia
- Depression
- Congestive heart failure
- Pulmonary embolus
ANSWERS: A, B, D, E
Diagnosis This interstitial pattern on the chest x-ray is associated with multiple etiologies, both infectious and non-infectious. Examples include viral pneumonia, opportunistic infections in HIV patients, atypical infections such as mycoplasm, congestive heart failure, and pulmonary embolus.
Initial management Based on severity-of-illness scores, this patient should be admitted to the hospital. He should have further evaluation to identify the etiology.
By definition, CAP is acquired outside a hospital or long-term care facility. However, the new guidelines include ambulatory residents of nursing homes.
Adults with CAP are the focus of the guidelines, not immunocompromised patients, cancer patients receiving chemotherapy, patients on high-dose steroid therapy, or children under 18 years.
Epidemiology. There are about 5.6 million cases of CAP in the United States annually, and the cost is about $8.4 billion.1 Death rates increase with comorbidities and older age. There are no race or gender differences in morbidity.
Limitations of the guidelines. The decision whether to admit a patient with CAP is crucial, since the majority of the pneumonia care expenditures are the result of in-patient care.2 The guidelines do not state the outcomes that were considered or adverse events associated with therapy. It is weakened by lack of cost analysis and absence of clinical algorithms.
How the evidence was graded. Electronic databases were searched through June 2006. Experts considered reviews and meta-analyses and weighted the evidence according to a rating scheme. They graded each recommendation on the quality of the literature (levels I, II, or II) and by expert interpretation (strong, moderate, or weak). A strong recommendation required that more than 50% of the experts grade it as strong and the majority of the remainder grade it as moderate.
Most patients with CAP should receive a strongly rated intervention, and the rationale for variation should be apparent from the medical record. With a moderate or weak recommendation, the committee suggested, most physicians would follow the recommended management, but many would not.
1. Lutfiyya MN, Henley E, Chang LF, Reyburn SW. Diagnosis and treatment of community-acquired pneumonia. Am Fam Physician 2006;73:442-50.
2. Niederman MS, McCombs JS, Unger AN, Kumar A, Popovian R. The cost of treating community-acquired pneumonia. Clin Ther 1998;20:820-837.
Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–S72.
For our purposes, the evidence ratings are based on literature quality, not expert opinion, and are updated to comply with the SORT taxonomy*
Grade A Recommendations
- Severity-of-illness scores can be used to identify patients with CAP who are candidates for outpatient treatment.
- Appropriate outpatient antibiotic treatment for a previously healthy person, with no risk factors for drug-resistant S. pneumonia (DRSP) is a macrolide (azithromycin, clarithromycin, or erythromycin).
- High risk patients: those with co-morbidities (chronic heart, lung, liver, or renal disease), diabetes mellitus, alcoholism, malignancies, asplenia, immunosuppression, or antibiotics within 3 months should be treated with a respiratory flouroquinolone—moxifloxacin, gemifloxacin, or levofloxacin (750 mg dose).
- A beta-lactam (high-dose amoxicillin, amoxicillin clavulanate, ceftriaxone, cefpodoxime, of cefuroxime), plus a macrolide is an option for high risk patients.
- Blood cultures and sputum cultures are optional prior to treatment of outpatients.
- In geographic areas where >25% of pneumococcal organisms are macrolide resistant, a beta-lactam, plus docxycycline should be considered.
- Treat with antibiotics at least 5 days.
- Health care workers in inpatient and outpatient settings and long-term facilities should receive annual influenza immunization.
Grade B Recommendations
- Severity of illness scores should be supplemented with physician subjective opinion about individual patients. the ability to safely and reliably take oral medications and the availability of outpatient resources should be considered.
- Patients with Cap should be investigated for specific pathogens that would significantly alter standard (empirical) management decisions, when suspected on the basis of clinical assessment.
- A beta-lactam, plus doxycycline is an alternative to the beta-lactam, plus macrolide combination for high risk patients.
- Pneumococcal polysaccharide vaccine is recommended for persons >65 years of age and for those with selected high-risk concurrent diseases.
Grade C Recommendations
- In addition to clinical features, an infiltrate by chest radiograph or other imaging technique is required for the diagnosis of pneumonia.
- An appropriate outpatient treatment for previously healthy individuals with no risk factors for DRSP infection is doxycycline.
- Use respiratory hygiene measures (hand hygiene, masks, tissues) for patients with cough in outpatient settings.
*Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004; 53:111–120.
When is outpatient treatment appropriate for community-acquired pneumonia (CAP)? Which antibiotics are recommended for outpatient therapy? What are the best prevention strategies? The answers are in the consensus guidelines published earlier this year by the Infectious Diseases Society of America and the American Thoracic Society (IDSA/ATS). The new guidelines update an IDSA guideline published in 2003.
Background. Management (and prevention) of CAP is inconsistent, and there is also emerging resistance of pneumococcus to macrolides.
These guidelines were developed to hasten consistency among caregivers and hospitals in the care of patients with pneumonia. Appropriateness of outpatient care, severity of illness assessment, hospital treatment decisions, ICU care, and choice of antibiotics for high-risk patients and for drug-resistant S pneumonia were reviewed. The joint committee recommended that hospitals standardize care and create policies to increase the vaccination rate.
CASE 1
Your patient is a 45-year-old man with cough, fever, and chills. He has a history of metabolic syndrome, and a 40 pack-year smoking history. He was well until 1 week ago when he went camping in the rain. Over the last 2 days he has had shaking chills, cough productive of green phlegm, and he finds that he gets a bit short-winded when walking stairs. He wonders if he has pneumonia. He is overweight and in no acute distress.
T 101 • P 88 • RR 18 • WT 220 • HT 5 7
Exam Normal other than localized coarse rales in the left posterior lung field; spot O2 saturation is 96%
What is your diagnosis and initial management?
Which of the following statements are true regarding the outpatient management of pneumonia?
- If 2 or more CURB-65 criteria are present, the patient should be hospitalized
- A macrolide is an appropriate choice of treatment for a previously healthy person with no risk of drug resistance
- A positive chest x-ray or other imaging is required for the diagnosis
- Blood cultures and sputum cultures must be obtained
- Antibiotic treatment should be a minimum of 10 days
ANSWERS: A, B, AND C
Diagnosis Community acquired pneumonia—left lobar.
Initial management This patient can be treated as an outpatient based on the severity-of-illness scores in this guideline. He should be treated with antibiotics a minimum of 5 days. With his comorbidities, antibiotic choices include 1) a fluoroquinolone, 2) a beta-lactam plus macrolide, or 3) (in areas with high prevalence of macrolide resistance) a beta-lactam plus doxycycline.
CASE 2
A 76-year-old man is brought into the office by his niece. “I just don’t feel well,” he says. The patient has been increasingly ill over the past week, and his niece is concerned that he seems to have trouble breathing. The patient minimizes his symptoms, but in relaying his history, he is obviously short of breath and cannot talk continuously. He needed a wheelchair to come in from the parking lot (and you know that he is usually spry and ambulatory). He has a history of congestive heart failure, hypertension, type 2 diabetes mellitus, depression, and osteoarthritis. He takes furosemide, potassium, enalapril, lantus insulin, sertraline, and PRN acetaminophen. He has never smoked. He denies PND and orthopnea. He is clearly short of breath and in some mild distress.
T 99 • P 102 • RR 36 • WT 260 • HT 5 9
Exam Remarkable for diffuse rhonchi and wheezing across all lung fields; spot O2 saturation is 89%
What is your diagnosis and initial management?
The differential diagnosis for this patient includes:
- Bacterial pneumonia
- Viral pneumonia
- Depression
- Congestive heart failure
- Pulmonary embolus
ANSWERS: A, B, D, E
Diagnosis This interstitial pattern on the chest x-ray is associated with multiple etiologies, both infectious and non-infectious. Examples include viral pneumonia, opportunistic infections in HIV patients, atypical infections such as mycoplasm, congestive heart failure, and pulmonary embolus.
Initial management Based on severity-of-illness scores, this patient should be admitted to the hospital. He should have further evaluation to identify the etiology.
By definition, CAP is acquired outside a hospital or long-term care facility. However, the new guidelines include ambulatory residents of nursing homes.
Adults with CAP are the focus of the guidelines, not immunocompromised patients, cancer patients receiving chemotherapy, patients on high-dose steroid therapy, or children under 18 years.
Epidemiology. There are about 5.6 million cases of CAP in the United States annually, and the cost is about $8.4 billion.1 Death rates increase with comorbidities and older age. There are no race or gender differences in morbidity.
Limitations of the guidelines. The decision whether to admit a patient with CAP is crucial, since the majority of the pneumonia care expenditures are the result of in-patient care.2 The guidelines do not state the outcomes that were considered or adverse events associated with therapy. It is weakened by lack of cost analysis and absence of clinical algorithms.
How the evidence was graded. Electronic databases were searched through June 2006. Experts considered reviews and meta-analyses and weighted the evidence according to a rating scheme. They graded each recommendation on the quality of the literature (levels I, II, or II) and by expert interpretation (strong, moderate, or weak). A strong recommendation required that more than 50% of the experts grade it as strong and the majority of the remainder grade it as moderate.
Most patients with CAP should receive a strongly rated intervention, and the rationale for variation should be apparent from the medical record. With a moderate or weak recommendation, the committee suggested, most physicians would follow the recommended management, but many would not.
For our purposes, the evidence ratings are based on literature quality, not expert opinion, and are updated to comply with the SORT taxonomy*
Grade A Recommendations
- Severity-of-illness scores can be used to identify patients with CAP who are candidates for outpatient treatment.
- Appropriate outpatient antibiotic treatment for a previously healthy person, with no risk factors for drug-resistant S. pneumonia (DRSP) is a macrolide (azithromycin, clarithromycin, or erythromycin).
- High risk patients: those with co-morbidities (chronic heart, lung, liver, or renal disease), diabetes mellitus, alcoholism, malignancies, asplenia, immunosuppression, or antibiotics within 3 months should be treated with a respiratory flouroquinolone—moxifloxacin, gemifloxacin, or levofloxacin (750 mg dose).
- A beta-lactam (high-dose amoxicillin, amoxicillin clavulanate, ceftriaxone, cefpodoxime, of cefuroxime), plus a macrolide is an option for high risk patients.
- Blood cultures and sputum cultures are optional prior to treatment of outpatients.
- In geographic areas where >25% of pneumococcal organisms are macrolide resistant, a beta-lactam, plus docxycycline should be considered.
- Treat with antibiotics at least 5 days.
- Health care workers in inpatient and outpatient settings and long-term facilities should receive annual influenza immunization.
Grade B Recommendations
- Severity of illness scores should be supplemented with physician subjective opinion about individual patients. the ability to safely and reliably take oral medications and the availability of outpatient resources should be considered.
- Patients with Cap should be investigated for specific pathogens that would significantly alter standard (empirical) management decisions, when suspected on the basis of clinical assessment.
- A beta-lactam, plus doxycycline is an alternative to the beta-lactam, plus macrolide combination for high risk patients.
- Pneumococcal polysaccharide vaccine is recommended for persons >65 years of age and for those with selected high-risk concurrent diseases.
Grade C Recommendations
- In addition to clinical features, an infiltrate by chest radiograph or other imaging technique is required for the diagnosis of pneumonia.
- An appropriate outpatient treatment for previously healthy individuals with no risk factors for DRSP infection is doxycycline.
- Use respiratory hygiene measures (hand hygiene, masks, tissues) for patients with cough in outpatient settings.
*Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004; 53:111–120.
When is outpatient treatment appropriate for community-acquired pneumonia (CAP)? Which antibiotics are recommended for outpatient therapy? What are the best prevention strategies? The answers are in the consensus guidelines published earlier this year by the Infectious Diseases Society of America and the American Thoracic Society (IDSA/ATS). The new guidelines update an IDSA guideline published in 2003.
Background. Management (and prevention) of CAP is inconsistent, and there is also emerging resistance of pneumococcus to macrolides.
These guidelines were developed to hasten consistency among caregivers and hospitals in the care of patients with pneumonia. Appropriateness of outpatient care, severity of illness assessment, hospital treatment decisions, ICU care, and choice of antibiotics for high-risk patients and for drug-resistant S pneumonia were reviewed. The joint committee recommended that hospitals standardize care and create policies to increase the vaccination rate.
CASE 1
Your patient is a 45-year-old man with cough, fever, and chills. He has a history of metabolic syndrome, and a 40 pack-year smoking history. He was well until 1 week ago when he went camping in the rain. Over the last 2 days he has had shaking chills, cough productive of green phlegm, and he finds that he gets a bit short-winded when walking stairs. He wonders if he has pneumonia. He is overweight and in no acute distress.
T 101 • P 88 • RR 18 • WT 220 • HT 5 7
Exam Normal other than localized coarse rales in the left posterior lung field; spot O2 saturation is 96%
What is your diagnosis and initial management?
Which of the following statements are true regarding the outpatient management of pneumonia?
- If 2 or more CURB-65 criteria are present, the patient should be hospitalized
- A macrolide is an appropriate choice of treatment for a previously healthy person with no risk of drug resistance
- A positive chest x-ray or other imaging is required for the diagnosis
- Blood cultures and sputum cultures must be obtained
- Antibiotic treatment should be a minimum of 10 days
ANSWERS: A, B, AND C
Diagnosis Community acquired pneumonia—left lobar.
Initial management This patient can be treated as an outpatient based on the severity-of-illness scores in this guideline. He should be treated with antibiotics a minimum of 5 days. With his comorbidities, antibiotic choices include 1) a fluoroquinolone, 2) a beta-lactam plus macrolide, or 3) (in areas with high prevalence of macrolide resistance) a beta-lactam plus doxycycline.
CASE 2
A 76-year-old man is brought into the office by his niece. “I just don’t feel well,” he says. The patient has been increasingly ill over the past week, and his niece is concerned that he seems to have trouble breathing. The patient minimizes his symptoms, but in relaying his history, he is obviously short of breath and cannot talk continuously. He needed a wheelchair to come in from the parking lot (and you know that he is usually spry and ambulatory). He has a history of congestive heart failure, hypertension, type 2 diabetes mellitus, depression, and osteoarthritis. He takes furosemide, potassium, enalapril, lantus insulin, sertraline, and PRN acetaminophen. He has never smoked. He denies PND and orthopnea. He is clearly short of breath and in some mild distress.
T 99 • P 102 • RR 36 • WT 260 • HT 5 9
Exam Remarkable for diffuse rhonchi and wheezing across all lung fields; spot O2 saturation is 89%
What is your diagnosis and initial management?
The differential diagnosis for this patient includes:
- Bacterial pneumonia
- Viral pneumonia
- Depression
- Congestive heart failure
- Pulmonary embolus
ANSWERS: A, B, D, E
Diagnosis This interstitial pattern on the chest x-ray is associated with multiple etiologies, both infectious and non-infectious. Examples include viral pneumonia, opportunistic infections in HIV patients, atypical infections such as mycoplasm, congestive heart failure, and pulmonary embolus.
Initial management Based on severity-of-illness scores, this patient should be admitted to the hospital. He should have further evaluation to identify the etiology.
By definition, CAP is acquired outside a hospital or long-term care facility. However, the new guidelines include ambulatory residents of nursing homes.
Adults with CAP are the focus of the guidelines, not immunocompromised patients, cancer patients receiving chemotherapy, patients on high-dose steroid therapy, or children under 18 years.
Epidemiology. There are about 5.6 million cases of CAP in the United States annually, and the cost is about $8.4 billion.1 Death rates increase with comorbidities and older age. There are no race or gender differences in morbidity.
Limitations of the guidelines. The decision whether to admit a patient with CAP is crucial, since the majority of the pneumonia care expenditures are the result of in-patient care.2 The guidelines do not state the outcomes that were considered or adverse events associated with therapy. It is weakened by lack of cost analysis and absence of clinical algorithms.
How the evidence was graded. Electronic databases were searched through June 2006. Experts considered reviews and meta-analyses and weighted the evidence according to a rating scheme. They graded each recommendation on the quality of the literature (levels I, II, or II) and by expert interpretation (strong, moderate, or weak). A strong recommendation required that more than 50% of the experts grade it as strong and the majority of the remainder grade it as moderate.
Most patients with CAP should receive a strongly rated intervention, and the rationale for variation should be apparent from the medical record. With a moderate or weak recommendation, the committee suggested, most physicians would follow the recommended management, but many would not.
1. Lutfiyya MN, Henley E, Chang LF, Reyburn SW. Diagnosis and treatment of community-acquired pneumonia. Am Fam Physician 2006;73:442-50.
2. Niederman MS, McCombs JS, Unger AN, Kumar A, Popovian R. The cost of treating community-acquired pneumonia. Clin Ther 1998;20:820-837.
Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–S72.
1. Lutfiyya MN, Henley E, Chang LF, Reyburn SW. Diagnosis and treatment of community-acquired pneumonia. Am Fam Physician 2006;73:442-50.
2. Niederman MS, McCombs JS, Unger AN, Kumar A, Popovian R. The cost of treating community-acquired pneumonia. Clin Ther 1998;20:820-837.
Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–S72.
What’s the best approach to renal artery stenosis?
GRADE A RECOMMENDATIONS
- Blood pressure measurements improve after angioplasty—particularly in patients with bilateral disease.
- There is no difference in kidney function outcomes when medical and angioplasty treatments are compared.
- Worse baseline kidney function is associated with increased mortality and worse blood pressure measurements after angioplasty.
GRADE B RECOMMENDATIONS
- Patients with bilateral stenosis have larger decreases in blood pressure readings after angioplasty than with medical treatment. No such difference was found between treatment groups in patients with unilateral disease.
- There is no difference in mortality and cardiovascular event rates when medical and angioplasty treatments are compared.
- There is no difference in blood pressure and kidney outcomes between angioplasty patients with or without stent placement.
GRADE C RECOMMENDATIONS
- The evidence doesn’t support one treatment approach over the other (angioplasty with stent vs aggressive medical therapy) for the general population with atherosclerotic renal artery stenosis.
- The evidence is inconclusive about relative adverse events or complications from angioplasty compared with medical treatment.
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
- What treatment strategy is most effective at reducing mortality?
- What patient characteristics are associated with increased mortality?
- What are the indications for stent placement?
The answers to these questions are summarized below and in the Comparative Effectiveness Review: Comparative Effectiveness of Management Strategies for Renal Artery Stenosis, funded and published by Agency for Healthcare Research and Quality (AHRQ). The review summarizes the current evidence concerning the effectiveness and safety of angioplasty with stent placement compared with medical therapy in the treatment of atherosclerotic renal artery stenosis.
The review team accepted the patient population of original authors, without clearly defining the level of renal artery stenosis. “The population of interest for this report is adults with atherosclerotic renal artery stenosis that is of sufficient severity to warrant aggressive management, either due to resistant hypertension, evidence of kidney damage, or the high likelihood of poor outcomes.” The team considered the following outcomes: blood pressure control, preservation of kidney function, incidence of flash pulmonary edema, and survival rates. Adverse events associated with therapies were also considered.
Review is commissioned to tackle controversy
The Comparative Effectiveness Review notes that 12% to 14% of new dialysis patients in the United States have atherosclerotic renal artery stenosis. It also points out that the utilization of renal artery angioplasty has increased considerably over the last few years, from 7660 cases in 1996 to 18,520 in 2000. The review was commissioned because of the controversy regarding optimal strategies for the evaluation and management of patients with atherosclerotic renal artery stenosis. The Comparative Effectiveness Review is strengthened by excellent summary tables, a review of treatment-associated harm, and an extensive discussion of methods.
In addition to this review of the literature, the government is sponsoring a more definitive trial to determine which patients with atherosclerotic renal artery stenosis would most benefit from angioplasty with stent placement, as opposed to continued aggressive medical treatment. The results of the Cardiovascular Outcomes in Renal Atherosclerotic Lesion (CORAL) Trial, a large, multicenter trial sponsored by the National Institutes of Health, will not be available until 2010.
A review of nearly 40 years of research
The Tufts–New England Medical Center Evidence-Based Practice Center was commissioned by AHRQ to conduct the review. A comprehensive search of the literature included Medline from 1966 to September 6, 2005. A technical expert panel held teleconferences to refine key questions and define parameters for review of the evidence. Researchers gave priority to meta-analyses and systemic reviews. Abstracts of research presented at conferences and symposiums were not considered adequate to be considered. There were 76 references.
Quality assessment of the literature was designated by a 3-category grading system (A—good, B—fair/moderate, and C—poor). For our purposes, the evidence rating is updated to comply with the SORT taxonomy.1
A search of the literature did not identify any other guidelines for comparison.
Source for this guideline
Balk E, Raman G, Chung M, et al. Comparative Effectiveness Review: Comparative Effectiveness of Management Strategies for Renal Artery Stenosis. (Prepared by Tufts-New England Medical Center Evidence-based Practice Center under Contract No. 290-02-0022). Rockville, Md: Agency for Healthcare Research and Quality; October 2006. Available at: effectivehealthcare.ahrq.gov/repFiles/RAS_Final.pdf. Accessed on April 11, 2007.
Correspondence
Keith B. Holten, MD, 825 Locust Street, Wilmington, Ohio 45177; [email protected].
Reference
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
GRADE A RECOMMENDATIONS
- Blood pressure measurements improve after angioplasty—particularly in patients with bilateral disease.
- There is no difference in kidney function outcomes when medical and angioplasty treatments are compared.
- Worse baseline kidney function is associated with increased mortality and worse blood pressure measurements after angioplasty.
GRADE B RECOMMENDATIONS
- Patients with bilateral stenosis have larger decreases in blood pressure readings after angioplasty than with medical treatment. No such difference was found between treatment groups in patients with unilateral disease.
- There is no difference in mortality and cardiovascular event rates when medical and angioplasty treatments are compared.
- There is no difference in blood pressure and kidney outcomes between angioplasty patients with or without stent placement.
GRADE C RECOMMENDATIONS
- The evidence doesn’t support one treatment approach over the other (angioplasty with stent vs aggressive medical therapy) for the general population with atherosclerotic renal artery stenosis.
- The evidence is inconclusive about relative adverse events or complications from angioplasty compared with medical treatment.
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
- What treatment strategy is most effective at reducing mortality?
- What patient characteristics are associated with increased mortality?
- What are the indications for stent placement?
The answers to these questions are summarized below and in the Comparative Effectiveness Review: Comparative Effectiveness of Management Strategies for Renal Artery Stenosis, funded and published by Agency for Healthcare Research and Quality (AHRQ). The review summarizes the current evidence concerning the effectiveness and safety of angioplasty with stent placement compared with medical therapy in the treatment of atherosclerotic renal artery stenosis.
The review team accepted the patient population of original authors, without clearly defining the level of renal artery stenosis. “The population of interest for this report is adults with atherosclerotic renal artery stenosis that is of sufficient severity to warrant aggressive management, either due to resistant hypertension, evidence of kidney damage, or the high likelihood of poor outcomes.” The team considered the following outcomes: blood pressure control, preservation of kidney function, incidence of flash pulmonary edema, and survival rates. Adverse events associated with therapies were also considered.
Review is commissioned to tackle controversy
The Comparative Effectiveness Review notes that 12% to 14% of new dialysis patients in the United States have atherosclerotic renal artery stenosis. It also points out that the utilization of renal artery angioplasty has increased considerably over the last few years, from 7660 cases in 1996 to 18,520 in 2000. The review was commissioned because of the controversy regarding optimal strategies for the evaluation and management of patients with atherosclerotic renal artery stenosis. The Comparative Effectiveness Review is strengthened by excellent summary tables, a review of treatment-associated harm, and an extensive discussion of methods.
In addition to this review of the literature, the government is sponsoring a more definitive trial to determine which patients with atherosclerotic renal artery stenosis would most benefit from angioplasty with stent placement, as opposed to continued aggressive medical treatment. The results of the Cardiovascular Outcomes in Renal Atherosclerotic Lesion (CORAL) Trial, a large, multicenter trial sponsored by the National Institutes of Health, will not be available until 2010.
A review of nearly 40 years of research
The Tufts–New England Medical Center Evidence-Based Practice Center was commissioned by AHRQ to conduct the review. A comprehensive search of the literature included Medline from 1966 to September 6, 2005. A technical expert panel held teleconferences to refine key questions and define parameters for review of the evidence. Researchers gave priority to meta-analyses and systemic reviews. Abstracts of research presented at conferences and symposiums were not considered adequate to be considered. There were 76 references.
Quality assessment of the literature was designated by a 3-category grading system (A—good, B—fair/moderate, and C—poor). For our purposes, the evidence rating is updated to comply with the SORT taxonomy.1
A search of the literature did not identify any other guidelines for comparison.
Source for this guideline
Balk E, Raman G, Chung M, et al. Comparative Effectiveness Review: Comparative Effectiveness of Management Strategies for Renal Artery Stenosis. (Prepared by Tufts-New England Medical Center Evidence-based Practice Center under Contract No. 290-02-0022). Rockville, Md: Agency for Healthcare Research and Quality; October 2006. Available at: effectivehealthcare.ahrq.gov/repFiles/RAS_Final.pdf. Accessed on April 11, 2007.
Correspondence
Keith B. Holten, MD, 825 Locust Street, Wilmington, Ohio 45177; [email protected].
GRADE A RECOMMENDATIONS
- Blood pressure measurements improve after angioplasty—particularly in patients with bilateral disease.
- There is no difference in kidney function outcomes when medical and angioplasty treatments are compared.
- Worse baseline kidney function is associated with increased mortality and worse blood pressure measurements after angioplasty.
GRADE B RECOMMENDATIONS
- Patients with bilateral stenosis have larger decreases in blood pressure readings after angioplasty than with medical treatment. No such difference was found between treatment groups in patients with unilateral disease.
- There is no difference in mortality and cardiovascular event rates when medical and angioplasty treatments are compared.
- There is no difference in blood pressure and kidney outcomes between angioplasty patients with or without stent placement.
GRADE C RECOMMENDATIONS
- The evidence doesn’t support one treatment approach over the other (angioplasty with stent vs aggressive medical therapy) for the general population with atherosclerotic renal artery stenosis.
- The evidence is inconclusive about relative adverse events or complications from angioplasty compared with medical treatment.
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
- What treatment strategy is most effective at reducing mortality?
- What patient characteristics are associated with increased mortality?
- What are the indications for stent placement?
The answers to these questions are summarized below and in the Comparative Effectiveness Review: Comparative Effectiveness of Management Strategies for Renal Artery Stenosis, funded and published by Agency for Healthcare Research and Quality (AHRQ). The review summarizes the current evidence concerning the effectiveness and safety of angioplasty with stent placement compared with medical therapy in the treatment of atherosclerotic renal artery stenosis.
The review team accepted the patient population of original authors, without clearly defining the level of renal artery stenosis. “The population of interest for this report is adults with atherosclerotic renal artery stenosis that is of sufficient severity to warrant aggressive management, either due to resistant hypertension, evidence of kidney damage, or the high likelihood of poor outcomes.” The team considered the following outcomes: blood pressure control, preservation of kidney function, incidence of flash pulmonary edema, and survival rates. Adverse events associated with therapies were also considered.
Review is commissioned to tackle controversy
The Comparative Effectiveness Review notes that 12% to 14% of new dialysis patients in the United States have atherosclerotic renal artery stenosis. It also points out that the utilization of renal artery angioplasty has increased considerably over the last few years, from 7660 cases in 1996 to 18,520 in 2000. The review was commissioned because of the controversy regarding optimal strategies for the evaluation and management of patients with atherosclerotic renal artery stenosis. The Comparative Effectiveness Review is strengthened by excellent summary tables, a review of treatment-associated harm, and an extensive discussion of methods.
In addition to this review of the literature, the government is sponsoring a more definitive trial to determine which patients with atherosclerotic renal artery stenosis would most benefit from angioplasty with stent placement, as opposed to continued aggressive medical treatment. The results of the Cardiovascular Outcomes in Renal Atherosclerotic Lesion (CORAL) Trial, a large, multicenter trial sponsored by the National Institutes of Health, will not be available until 2010.
A review of nearly 40 years of research
The Tufts–New England Medical Center Evidence-Based Practice Center was commissioned by AHRQ to conduct the review. A comprehensive search of the literature included Medline from 1966 to September 6, 2005. A technical expert panel held teleconferences to refine key questions and define parameters for review of the evidence. Researchers gave priority to meta-analyses and systemic reviews. Abstracts of research presented at conferences and symposiums were not considered adequate to be considered. There were 76 references.
Quality assessment of the literature was designated by a 3-category grading system (A—good, B—fair/moderate, and C—poor). For our purposes, the evidence rating is updated to comply with the SORT taxonomy.1
A search of the literature did not identify any other guidelines for comparison.
Source for this guideline
Balk E, Raman G, Chung M, et al. Comparative Effectiveness Review: Comparative Effectiveness of Management Strategies for Renal Artery Stenosis. (Prepared by Tufts-New England Medical Center Evidence-based Practice Center under Contract No. 290-02-0022). Rockville, Md: Agency for Healthcare Research and Quality; October 2006. Available at: effectivehealthcare.ahrq.gov/repFiles/RAS_Final.pdf. Accessed on April 11, 2007.
Correspondence
Keith B. Holten, MD, 825 Locust Street, Wilmington, Ohio 45177; [email protected].
Reference
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
Reference
1. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. J Fam Pract 2004;53:111-120.
How should we evaluate a solitary pulmonary nodule found on chest x-ray?
- When is a CT scan indicated to examine a solitary pulmonary nodule found on a chest x-ray film?
- Is there an indication for positron-emission tomography scanning?
- When should a biopsy be performed?
- What is the best biopsy method?
In January 2003 the American College of Chest Physicians Expert Panel on Lung Cancer Guidelines released its guideline on evaluating a solitary pulmonary nodule (SPN), an intraparenchymal lung lesion <3 cm in diameter unassociated with atelectasis or adenopathy. The objectives of this guideline were to define appropriate evidence-based practices for imaging and diagnostic tests, as well as indications for obtaining a tissue evaluation for the patient with a SPN. This expert panel included physicians from nuclear medicine, oncology, pulmonary medicine, radiology, and thoracic surgery. The major recommendations were summarized in the National Guideline Clearinghouse (available at www.guideline.gov).
The evidence categories for this guideline are diagnosis and management. Outcomes considered were sensitivity and specificity of diagnostic tests and diagnostic yield. No cost analysis was performed.
The committee used a complex recommendation rating scheme (A, B, C, D, I) after comparing levels of evidence (good, fair, or poor) compared with net benefits (substantial, moderate, small/weak, or none). The scheme was then revised to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine.
Guideline relevance and limitations
Solitary pulmonary nodules are discovered in 150,000 patients per year, and a delay in performing diagnostic studies can have dire consequences for those whose nodule proves malignant.
The guideline is weakened by the lack of a cost-effectiveness analysis.
A lengthy bibliography accompanies the guideline, but the support document does not provide evidence tables.
Guideline development and evidence review
Computerized bibliographic databases including Medline, Cancerlit, CINAHL, HealthStar, the Cochrane Collaboration Database of Abstracts of Reviews of Effectiveness, the National Guideline Clearinghouse, and the National Cancer Institute Physician Data Query database were searched for existing evidence. Priority was given to secondary sources including guidelines, systematic reviews, and meta-analyses. Search terms were lung neoplasms or bronchial neoplasms. Reference lists of review articles were also studied for additional evidence. There were 55 references.
Source for this guideline
Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest 2003; 123(1 suppl):89S–96S.
Other guidelines on solitary pulmonary nodules
ACR Appropriateness Criteria™ for work-up of the solitary pulmonary nodule (SPN). 1995 (revised 2000). This guideline is one in a series of guidelines developed by the American College of Radiology. It ranks the utility of various diagnostic testing modalities based on evidence. This guideline is complex, because there are several “variants” based on the size of the lesion (≥1 cm or ≤1 cm) and the clinical suspicion of cancer (low, moderate to high). The clinical utility for primary care physicians is limited.
Source: Henschke CI, Yankelevitz D, Westcott J, et al. Work-up of the solitary pulmonary nodule. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000; 215(Suppl):607–609. (19 references)
Diagnosis
- A solitary pulmonary nodule (SPN) with benign central calcification does not require further diagnostic testing (A).
- Spiral chest computed tomography (CT) scan with contrast should be performed for new SPNs (B).
- Review all previous chest x-rays when a SPN is found (C).
- Magnetic resonance imaging (MRI) is not indicated (D).
- Positron-emission tomography (PET) scan is not recommended for SPN <1 cm in size (D).
Management and follow-up evaluations
- Lymph node dissection should be performed for all pulmonary resections (A).
- If a wedge resection is not possible, a diagnostic lobectomy is an acceptable alternative (A).
- SPN that does not change on chest x-ray after 2 years of follow-up requires no further evaluation (B).
- PET scan of the chest with 18-fluorodeoxyglucose, might be considered preoperatively for SPN patients who are surgical candidates and have a negative mediastinal chest CT (B).
- Chest x-ray and chest CT scanning at 3, 6, 12, and 24 months should be performed for patients who are not good surgical candidates (B).
- An alternative to surgical intervention is percutaneous transthoracic needle aspiration (TTNA) or transbronchial needle biopsy for patients who refuse surgery (B).
- High surgical risk patients may be candidates for TTNA (B).
- Wedge resection followed by lobectomy is appropriate for pathology positive for cancer (B).
- Wedge resection or segmentectomy may be appropriate for marginal surgical candidates (B).
- Without a definitive tissue diagnosis, follow-up for 2 years is recommended with chest x-ray and chest CT (at 3, 6, 12, and 24 months) (C).
- Marginal surgical candidates who have a negative PET scan should have a CT scan at least in 3 months (C).
- For patients who are surgical candidates, TTNA is not indicated (D).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- When is a CT scan indicated to examine a solitary pulmonary nodule found on a chest x-ray film?
- Is there an indication for positron-emission tomography scanning?
- When should a biopsy be performed?
- What is the best biopsy method?
In January 2003 the American College of Chest Physicians Expert Panel on Lung Cancer Guidelines released its guideline on evaluating a solitary pulmonary nodule (SPN), an intraparenchymal lung lesion <3 cm in diameter unassociated with atelectasis or adenopathy. The objectives of this guideline were to define appropriate evidence-based practices for imaging and diagnostic tests, as well as indications for obtaining a tissue evaluation for the patient with a SPN. This expert panel included physicians from nuclear medicine, oncology, pulmonary medicine, radiology, and thoracic surgery. The major recommendations were summarized in the National Guideline Clearinghouse (available at www.guideline.gov).
The evidence categories for this guideline are diagnosis and management. Outcomes considered were sensitivity and specificity of diagnostic tests and diagnostic yield. No cost analysis was performed.
The committee used a complex recommendation rating scheme (A, B, C, D, I) after comparing levels of evidence (good, fair, or poor) compared with net benefits (substantial, moderate, small/weak, or none). The scheme was then revised to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine.
Guideline relevance and limitations
Solitary pulmonary nodules are discovered in 150,000 patients per year, and a delay in performing diagnostic studies can have dire consequences for those whose nodule proves malignant.
The guideline is weakened by the lack of a cost-effectiveness analysis.
A lengthy bibliography accompanies the guideline, but the support document does not provide evidence tables.
Guideline development and evidence review
Computerized bibliographic databases including Medline, Cancerlit, CINAHL, HealthStar, the Cochrane Collaboration Database of Abstracts of Reviews of Effectiveness, the National Guideline Clearinghouse, and the National Cancer Institute Physician Data Query database were searched for existing evidence. Priority was given to secondary sources including guidelines, systematic reviews, and meta-analyses. Search terms were lung neoplasms or bronchial neoplasms. Reference lists of review articles were also studied for additional evidence. There were 55 references.
Source for this guideline
Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest 2003; 123(1 suppl):89S–96S.
Other guidelines on solitary pulmonary nodules
ACR Appropriateness Criteria™ for work-up of the solitary pulmonary nodule (SPN). 1995 (revised 2000). This guideline is one in a series of guidelines developed by the American College of Radiology. It ranks the utility of various diagnostic testing modalities based on evidence. This guideline is complex, because there are several “variants” based on the size of the lesion (≥1 cm or ≤1 cm) and the clinical suspicion of cancer (low, moderate to high). The clinical utility for primary care physicians is limited.
Source: Henschke CI, Yankelevitz D, Westcott J, et al. Work-up of the solitary pulmonary nodule. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000; 215(Suppl):607–609. (19 references)
Diagnosis
- A solitary pulmonary nodule (SPN) with benign central calcification does not require further diagnostic testing (A).
- Spiral chest computed tomography (CT) scan with contrast should be performed for new SPNs (B).
- Review all previous chest x-rays when a SPN is found (C).
- Magnetic resonance imaging (MRI) is not indicated (D).
- Positron-emission tomography (PET) scan is not recommended for SPN <1 cm in size (D).
Management and follow-up evaluations
- Lymph node dissection should be performed for all pulmonary resections (A).
- If a wedge resection is not possible, a diagnostic lobectomy is an acceptable alternative (A).
- SPN that does not change on chest x-ray after 2 years of follow-up requires no further evaluation (B).
- PET scan of the chest with 18-fluorodeoxyglucose, might be considered preoperatively for SPN patients who are surgical candidates and have a negative mediastinal chest CT (B).
- Chest x-ray and chest CT scanning at 3, 6, 12, and 24 months should be performed for patients who are not good surgical candidates (B).
- An alternative to surgical intervention is percutaneous transthoracic needle aspiration (TTNA) or transbronchial needle biopsy for patients who refuse surgery (B).
- High surgical risk patients may be candidates for TTNA (B).
- Wedge resection followed by lobectomy is appropriate for pathology positive for cancer (B).
- Wedge resection or segmentectomy may be appropriate for marginal surgical candidates (B).
- Without a definitive tissue diagnosis, follow-up for 2 years is recommended with chest x-ray and chest CT (at 3, 6, 12, and 24 months) (C).
- Marginal surgical candidates who have a negative PET scan should have a CT scan at least in 3 months (C).
- For patients who are surgical candidates, TTNA is not indicated (D).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- When is a CT scan indicated to examine a solitary pulmonary nodule found on a chest x-ray film?
- Is there an indication for positron-emission tomography scanning?
- When should a biopsy be performed?
- What is the best biopsy method?
In January 2003 the American College of Chest Physicians Expert Panel on Lung Cancer Guidelines released its guideline on evaluating a solitary pulmonary nodule (SPN), an intraparenchymal lung lesion <3 cm in diameter unassociated with atelectasis or adenopathy. The objectives of this guideline were to define appropriate evidence-based practices for imaging and diagnostic tests, as well as indications for obtaining a tissue evaluation for the patient with a SPN. This expert panel included physicians from nuclear medicine, oncology, pulmonary medicine, radiology, and thoracic surgery. The major recommendations were summarized in the National Guideline Clearinghouse (available at www.guideline.gov).
The evidence categories for this guideline are diagnosis and management. Outcomes considered were sensitivity and specificity of diagnostic tests and diagnostic yield. No cost analysis was performed.
The committee used a complex recommendation rating scheme (A, B, C, D, I) after comparing levels of evidence (good, fair, or poor) compared with net benefits (substantial, moderate, small/weak, or none). The scheme was then revised to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine.
Guideline relevance and limitations
Solitary pulmonary nodules are discovered in 150,000 patients per year, and a delay in performing diagnostic studies can have dire consequences for those whose nodule proves malignant.
The guideline is weakened by the lack of a cost-effectiveness analysis.
A lengthy bibliography accompanies the guideline, but the support document does not provide evidence tables.
Guideline development and evidence review
Computerized bibliographic databases including Medline, Cancerlit, CINAHL, HealthStar, the Cochrane Collaboration Database of Abstracts of Reviews of Effectiveness, the National Guideline Clearinghouse, and the National Cancer Institute Physician Data Query database were searched for existing evidence. Priority was given to secondary sources including guidelines, systematic reviews, and meta-analyses. Search terms were lung neoplasms or bronchial neoplasms. Reference lists of review articles were also studied for additional evidence. There were 55 references.
Source for this guideline
Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest 2003; 123(1 suppl):89S–96S.
Other guidelines on solitary pulmonary nodules
ACR Appropriateness Criteria™ for work-up of the solitary pulmonary nodule (SPN). 1995 (revised 2000). This guideline is one in a series of guidelines developed by the American College of Radiology. It ranks the utility of various diagnostic testing modalities based on evidence. This guideline is complex, because there are several “variants” based on the size of the lesion (≥1 cm or ≤1 cm) and the clinical suspicion of cancer (low, moderate to high). The clinical utility for primary care physicians is limited.
Source: Henschke CI, Yankelevitz D, Westcott J, et al. Work-up of the solitary pulmonary nodule. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000; 215(Suppl):607–609. (19 references)
Diagnosis
- A solitary pulmonary nodule (SPN) with benign central calcification does not require further diagnostic testing (A).
- Spiral chest computed tomography (CT) scan with contrast should be performed for new SPNs (B).
- Review all previous chest x-rays when a SPN is found (C).
- Magnetic resonance imaging (MRI) is not indicated (D).
- Positron-emission tomography (PET) scan is not recommended for SPN <1 cm in size (D).
Management and follow-up evaluations
- Lymph node dissection should be performed for all pulmonary resections (A).
- If a wedge resection is not possible, a diagnostic lobectomy is an acceptable alternative (A).
- SPN that does not change on chest x-ray after 2 years of follow-up requires no further evaluation (B).
- PET scan of the chest with 18-fluorodeoxyglucose, might be considered preoperatively for SPN patients who are surgical candidates and have a negative mediastinal chest CT (B).
- Chest x-ray and chest CT scanning at 3, 6, 12, and 24 months should be performed for patients who are not good surgical candidates (B).
- An alternative to surgical intervention is percutaneous transthoracic needle aspiration (TTNA) or transbronchial needle biopsy for patients who refuse surgery (B).
- High surgical risk patients may be candidates for TTNA (B).
- Wedge resection followed by lobectomy is appropriate for pathology positive for cancer (B).
- Wedge resection or segmentectomy may be appropriate for marginal surgical candidates (B).
- Without a definitive tissue diagnosis, follow-up for 2 years is recommended with chest x-ray and chest CT (at 3, 6, 12, and 24 months) (C).
- Marginal surgical candidates who have a negative PET scan should have a CT scan at least in 3 months (C).
- For patients who are surgical candidates, TTNA is not indicated (D).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
How should we diagnose and treat osteoarthritis of the knee?
- When are x-ray films indicated for a patient with knee pain?
- When should we prescribe selective cyclo-oxygenase-2 (COX-2) inhibitors, instead of nonsteroidal anti-inflammatory drugs (NSAIDs)?
- How often can intraarticular steroids be used?
- What is the role of viscosupplementation?
- When is total knee replacement appropriate?
Answers to these and other questions can be found in a guideline revised within the year by the Evidence-Based Practice Committee of the American Academy of Orthopedic Surgeons. The guideline—revised from a version developed and released in 1996—is divided into 2 phases: care provided by the first-contact primary care physician (the focus of this review), and recommendations for specialists (not addressed in this review).
The major recommendations summarized in the National Guideline Clearinghouse (www.ngc.gov) did not include the excellent care algorithm. For this update, therefore, the source document was accessed. It summarizes the following recommendations for referral to a musculoskeletal specialist (orthopedist, physiatrist, or rheumatologist)—poor response to 12 weeks of treatment, suspected infection, or hemarthrosis.
The evidence categories for this guideline are diagnosis, evaluation, management, and treatment. Targeted patients were adults with longstanding knee pain. Outcomes measured were symptomatic pain relief, improved range of motion, better physical functioning, and complications associated with treatment.
The committee used a recommendation rating scheme of A to D, based on a review of the evidence. Ratings were altered to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine. (As explained on pages 111 to 120 of this issue, The journal of family practice and many other family-medicine publications will be using an evidence-rating system ranging from A to C. For this review, however, the scheme of A to D originally used by the guideline’s authors has been left intact.)
Limitations of guideline usefulness
Although this guideline was just published, the evidence is complete only through 2000. The bibliography is lengthy, but the support document does not provide evidence tables. The established outcomes set forth were not used to design the algorithm, which also lacks grades of evidence. The guideline is further weakened by the lack of cost-effectiveness analysis.
Guideline development and evidence review
The 1996 guideline was developed by a multidisciplinary group of American Academy of Orthopedic Surgeons, the American Association of Neurological Surgeons, the American College of Physical Medicine and Rehabilitation, and the American College of Rheumatology. The 2003 revision group performed a new literature search for 1990–2000 for human subjects aged 19 years and older. In all, 128 articles were reviewed, 114 references were cited, the evidence was graded, and the original guideline was revised based on the evidence.
Sources for this guideline
American Academy of Orthopaedic Surgeons. AAOS clinical practice guideline on osteoarthritis of the knee. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 2003.
Source document available at: www.aaos.org/wordhtml/pdfs_r/guidelin/suprt_04.pdf. Algorithm available at: www.aaos.org/word-html/pdfs_r/guidelin/chart_oakn.pdf. Accessed on December 30, 2003.
Grade A Recommendations
- Initial treatment with NSAIDs or acetaminophen. acetaminophen is as effective as NSAIDs
- Physical therapy, including conditioning, quadriceps strengthening, and range of motion exercises should be considered for patients with osteoarthritis (confirmed by radiographs) after 4 to 6 weeks of conservative therapy.
Grade B Recommendations
- COX-2 Inhibitors should be used only for patients at risk of adverse renal and gastrointestinal effects from NSAIDs.
- A tangential view of the patellofemoral joint and a standing posterior-anterior view of the knee flexed 20° should be obtained for patients who do not respond to treatment in 1 to 4 weeks or whose pain returns. Positive findings are narrowing of cartilage space, marginal osteophytes, subchondral sclerosis, and tibial spine beaking.
- If the patient is unresponsive to 1 NSAID, changing to another NSAID is an option.
- Use durable medical equipment assistive devices such as canes, fitted footwear, and braces.
- Educate patients regarding weight loss, support groups, and avoidance of activities that worsen knee pain.
Grade C Recommendations
- Viscosupplementation may be effective during the first 12 weeks of symptoms.
Grade D Recommendations
- Knee x-ray for patients with persistent pain (1–4 weeks) or return of pain after a symptom-free interval.
- With long-term NSAID use, monitor complete blood count, renal functions, liver functions, and stool guiac every 6 months.
- Arthrocentesis and intra-articular steroid injection are options for persistent pain (1–4 weeks).
- Chondroitin and glucosamine have not been studied adequately to make recommendations.
FIGURE
Osteoarthritis of the knee
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000; 43:1905–1915. Available at: www.rheumatology.org/publications/guide-lines/oa-knee/oa-knee.asp. Accessed on December 30, 2003.
- Knee pain or swelling: acute or chronic. University of Michigan Health System. Ann Arbor, Mich: University of Michigan Health System; 2002 Aug. Available at: cme.med.umich. edu/pdf/guideline/knee.pdf.
- Diagnosis and treatment of adult degenerative joint disease (DJD) of the knee. Institute for Clinical Systems Improvement (ICSI). Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2002 May. Available at: www.icsi.org/knowledge/browse_ category.asp?catID=29.
- Pain in osteoarthritis, rheumatoid arthritis, and juvenile chronic arthritis. Simon LS, Lipman AG, Jacox AK, et al. 2nd ed. Glenview, Ill: American Pain Society (APS); 2002. Not available on-line.
- Physical activity in the prevention, treatment, and rehabilitation of diseases. Finnish Medical Society Duodecim. Helsinki, Finland: Duodecim Medical Publications Ltd.; 2002 May 7. Available at: www.ebm-guidelines.com/home.html (fee for access).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- When are x-ray films indicated for a patient with knee pain?
- When should we prescribe selective cyclo-oxygenase-2 (COX-2) inhibitors, instead of nonsteroidal anti-inflammatory drugs (NSAIDs)?
- How often can intraarticular steroids be used?
- What is the role of viscosupplementation?
- When is total knee replacement appropriate?
Answers to these and other questions can be found in a guideline revised within the year by the Evidence-Based Practice Committee of the American Academy of Orthopedic Surgeons. The guideline—revised from a version developed and released in 1996—is divided into 2 phases: care provided by the first-contact primary care physician (the focus of this review), and recommendations for specialists (not addressed in this review).
The major recommendations summarized in the National Guideline Clearinghouse (www.ngc.gov) did not include the excellent care algorithm. For this update, therefore, the source document was accessed. It summarizes the following recommendations for referral to a musculoskeletal specialist (orthopedist, physiatrist, or rheumatologist)—poor response to 12 weeks of treatment, suspected infection, or hemarthrosis.
The evidence categories for this guideline are diagnosis, evaluation, management, and treatment. Targeted patients were adults with longstanding knee pain. Outcomes measured were symptomatic pain relief, improved range of motion, better physical functioning, and complications associated with treatment.
The committee used a recommendation rating scheme of A to D, based on a review of the evidence. Ratings were altered to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine. (As explained on pages 111 to 120 of this issue, The journal of family practice and many other family-medicine publications will be using an evidence-rating system ranging from A to C. For this review, however, the scheme of A to D originally used by the guideline’s authors has been left intact.)
Limitations of guideline usefulness
Although this guideline was just published, the evidence is complete only through 2000. The bibliography is lengthy, but the support document does not provide evidence tables. The established outcomes set forth were not used to design the algorithm, which also lacks grades of evidence. The guideline is further weakened by the lack of cost-effectiveness analysis.
Guideline development and evidence review
The 1996 guideline was developed by a multidisciplinary group of American Academy of Orthopedic Surgeons, the American Association of Neurological Surgeons, the American College of Physical Medicine and Rehabilitation, and the American College of Rheumatology. The 2003 revision group performed a new literature search for 1990–2000 for human subjects aged 19 years and older. In all, 128 articles were reviewed, 114 references were cited, the evidence was graded, and the original guideline was revised based on the evidence.
Sources for this guideline
American Academy of Orthopaedic Surgeons. AAOS clinical practice guideline on osteoarthritis of the knee. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 2003.
Source document available at: www.aaos.org/wordhtml/pdfs_r/guidelin/suprt_04.pdf. Algorithm available at: www.aaos.org/word-html/pdfs_r/guidelin/chart_oakn.pdf. Accessed on December 30, 2003.
Grade A Recommendations
- Initial treatment with NSAIDs or acetaminophen. acetaminophen is as effective as NSAIDs
- Physical therapy, including conditioning, quadriceps strengthening, and range of motion exercises should be considered for patients with osteoarthritis (confirmed by radiographs) after 4 to 6 weeks of conservative therapy.
Grade B Recommendations
- COX-2 Inhibitors should be used only for patients at risk of adverse renal and gastrointestinal effects from NSAIDs.
- A tangential view of the patellofemoral joint and a standing posterior-anterior view of the knee flexed 20° should be obtained for patients who do not respond to treatment in 1 to 4 weeks or whose pain returns. Positive findings are narrowing of cartilage space, marginal osteophytes, subchondral sclerosis, and tibial spine beaking.
- If the patient is unresponsive to 1 NSAID, changing to another NSAID is an option.
- Use durable medical equipment assistive devices such as canes, fitted footwear, and braces.
- Educate patients regarding weight loss, support groups, and avoidance of activities that worsen knee pain.
Grade C Recommendations
- Viscosupplementation may be effective during the first 12 weeks of symptoms.
Grade D Recommendations
- Knee x-ray for patients with persistent pain (1–4 weeks) or return of pain after a symptom-free interval.
- With long-term NSAID use, monitor complete blood count, renal functions, liver functions, and stool guiac every 6 months.
- Arthrocentesis and intra-articular steroid injection are options for persistent pain (1–4 weeks).
- Chondroitin and glucosamine have not been studied adequately to make recommendations.
FIGURE
Osteoarthritis of the knee
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000; 43:1905–1915. Available at: www.rheumatology.org/publications/guide-lines/oa-knee/oa-knee.asp. Accessed on December 30, 2003.
- Knee pain or swelling: acute or chronic. University of Michigan Health System. Ann Arbor, Mich: University of Michigan Health System; 2002 Aug. Available at: cme.med.umich. edu/pdf/guideline/knee.pdf.
- Diagnosis and treatment of adult degenerative joint disease (DJD) of the knee. Institute for Clinical Systems Improvement (ICSI). Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2002 May. Available at: www.icsi.org/knowledge/browse_ category.asp?catID=29.
- Pain in osteoarthritis, rheumatoid arthritis, and juvenile chronic arthritis. Simon LS, Lipman AG, Jacox AK, et al. 2nd ed. Glenview, Ill: American Pain Society (APS); 2002. Not available on-line.
- Physical activity in the prevention, treatment, and rehabilitation of diseases. Finnish Medical Society Duodecim. Helsinki, Finland: Duodecim Medical Publications Ltd.; 2002 May 7. Available at: www.ebm-guidelines.com/home.html (fee for access).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- When are x-ray films indicated for a patient with knee pain?
- When should we prescribe selective cyclo-oxygenase-2 (COX-2) inhibitors, instead of nonsteroidal anti-inflammatory drugs (NSAIDs)?
- How often can intraarticular steroids be used?
- What is the role of viscosupplementation?
- When is total knee replacement appropriate?
Answers to these and other questions can be found in a guideline revised within the year by the Evidence-Based Practice Committee of the American Academy of Orthopedic Surgeons. The guideline—revised from a version developed and released in 1996—is divided into 2 phases: care provided by the first-contact primary care physician (the focus of this review), and recommendations for specialists (not addressed in this review).
The major recommendations summarized in the National Guideline Clearinghouse (www.ngc.gov) did not include the excellent care algorithm. For this update, therefore, the source document was accessed. It summarizes the following recommendations for referral to a musculoskeletal specialist (orthopedist, physiatrist, or rheumatologist)—poor response to 12 weeks of treatment, suspected infection, or hemarthrosis.
The evidence categories for this guideline are diagnosis, evaluation, management, and treatment. Targeted patients were adults with longstanding knee pain. Outcomes measured were symptomatic pain relief, improved range of motion, better physical functioning, and complications associated with treatment.
The committee used a recommendation rating scheme of A to D, based on a review of the evidence. Ratings were altered to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine. (As explained on pages 111 to 120 of this issue, The journal of family practice and many other family-medicine publications will be using an evidence-rating system ranging from A to C. For this review, however, the scheme of A to D originally used by the guideline’s authors has been left intact.)
Limitations of guideline usefulness
Although this guideline was just published, the evidence is complete only through 2000. The bibliography is lengthy, but the support document does not provide evidence tables. The established outcomes set forth were not used to design the algorithm, which also lacks grades of evidence. The guideline is further weakened by the lack of cost-effectiveness analysis.
Guideline development and evidence review
The 1996 guideline was developed by a multidisciplinary group of American Academy of Orthopedic Surgeons, the American Association of Neurological Surgeons, the American College of Physical Medicine and Rehabilitation, and the American College of Rheumatology. The 2003 revision group performed a new literature search for 1990–2000 for human subjects aged 19 years and older. In all, 128 articles were reviewed, 114 references were cited, the evidence was graded, and the original guideline was revised based on the evidence.
Sources for this guideline
American Academy of Orthopaedic Surgeons. AAOS clinical practice guideline on osteoarthritis of the knee. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 2003.
Source document available at: www.aaos.org/wordhtml/pdfs_r/guidelin/suprt_04.pdf. Algorithm available at: www.aaos.org/word-html/pdfs_r/guidelin/chart_oakn.pdf. Accessed on December 30, 2003.
Grade A Recommendations
- Initial treatment with NSAIDs or acetaminophen. acetaminophen is as effective as NSAIDs
- Physical therapy, including conditioning, quadriceps strengthening, and range of motion exercises should be considered for patients with osteoarthritis (confirmed by radiographs) after 4 to 6 weeks of conservative therapy.
Grade B Recommendations
- COX-2 Inhibitors should be used only for patients at risk of adverse renal and gastrointestinal effects from NSAIDs.
- A tangential view of the patellofemoral joint and a standing posterior-anterior view of the knee flexed 20° should be obtained for patients who do not respond to treatment in 1 to 4 weeks or whose pain returns. Positive findings are narrowing of cartilage space, marginal osteophytes, subchondral sclerosis, and tibial spine beaking.
- If the patient is unresponsive to 1 NSAID, changing to another NSAID is an option.
- Use durable medical equipment assistive devices such as canes, fitted footwear, and braces.
- Educate patients regarding weight loss, support groups, and avoidance of activities that worsen knee pain.
Grade C Recommendations
- Viscosupplementation may be effective during the first 12 weeks of symptoms.
Grade D Recommendations
- Knee x-ray for patients with persistent pain (1–4 weeks) or return of pain after a symptom-free interval.
- With long-term NSAID use, monitor complete blood count, renal functions, liver functions, and stool guiac every 6 months.
- Arthrocentesis and intra-articular steroid injection are options for persistent pain (1–4 weeks).
- Chondroitin and glucosamine have not been studied adequately to make recommendations.
FIGURE
Osteoarthritis of the knee
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000; 43:1905–1915. Available at: www.rheumatology.org/publications/guide-lines/oa-knee/oa-knee.asp. Accessed on December 30, 2003.
- Knee pain or swelling: acute or chronic. University of Michigan Health System. Ann Arbor, Mich: University of Michigan Health System; 2002 Aug. Available at: cme.med.umich. edu/pdf/guideline/knee.pdf.
- Diagnosis and treatment of adult degenerative joint disease (DJD) of the knee. Institute for Clinical Systems Improvement (ICSI). Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2002 May. Available at: www.icsi.org/knowledge/browse_ category.asp?catID=29.
- Pain in osteoarthritis, rheumatoid arthritis, and juvenile chronic arthritis. Simon LS, Lipman AG, Jacox AK, et al. 2nd ed. Glenview, Ill: American Pain Society (APS); 2002. Not available on-line.
- Physical activity in the prevention, treatment, and rehabilitation of diseases. Finnish Medical Society Duodecim. Helsinki, Finland: Duodecim Medical Publications Ltd.; 2002 May 7. Available at: www.ebm-guidelines.com/home.html (fee for access).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
Preventing VTE in hospitalized patients
- How do we determine risk of venous thromboembolism (VTE) in patients scheduled for surgery?
- Do all surgical patients require VTE prevention?
- Is aspirin adequate to prevent VTE in low-risk hospitalized patients?
- Which anticoagulant is appropriate for a patient scheduled for total knee replacement?
These important questions are answered in a guideline developed by a committee of the American College of Chest Physicians, which considered the following prophylaxis recommendations: early ambulation, aspirin, graduated compression stockings, intermittent pneumatic compression, low-dose unfractionated heparin, low-molecular-weight heparin, or oral antithrombotic agents.
The committee categorized recommendations by type of surgical procedure and risk status. In this summary, the recommendations are reorganized by strength of recommendation.
Three outcomes were regarded:
- Efficacy of various prophylactic strategies
- Rates and relative risk of venous thromboembolism outcomes—ie, fatal pulmonary embolism, symptomatic deep vein thrombosis, pulmonary embolism, or asymptomatic proximal deep vein thrombosis
- Cost-effectiveness of prophylaxis.
The committee used a rating scheme that accounted for both the risk/benefit ratio (clear or unclear) and the strength of the supporting recommendation (A, B, C). The grades of evidence were altered to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine. (For an explanaton of these grades.)
Relevant recommendations
This guideline is clinically relevant because of the high mortality associated with pulmonary embolus complicating VTE.
It offers a practical, tabulated guide, listed by surgical procedure performed. It is pertinent to hospitalized patients under the care of family physicians. The rationale for each recommendation is clear and well supported by the referenced literature. The objectives of the guideline were met and the outcome measures were appropriate.
The guideline is weakened by the lack of cost-effectiveness considerations.
Determining surgical risk
| Surgery + | Patient age (yr) + | Risk factors = | Level of risk |
|---|---|---|---|
| Minor | < 40 | No | Low |
| Minor | Any | Yes* | Moderate |
| 40–60 | No | ||
| Major | < 40 | No | |
| Minor | > 60 | No | High |
| > 60 | Yes* | ||
| Major | > 40 | No | |
| > 40 | Yes* | ||
| Major | > 40 | Prior VTE, cancer, hypercoagulable states, hip/knee arthoplasty, hip fracture, major trauma, spinal injury | Very high |
*Additional risk factors: immobility, stroke, paralysis, trauma, obesity, varicose veins, cardiac dysfunction, indwelling central venous catheter, inflammatory bowel disease, nephrotic syndrome, pregnancy, estrogen use, congenital thrombophilic abnormalities
- For all risk groups of patients, aspirin is not recommended for prophylaxis (strength of recommendation [SOR]: A)
- Every hospital should have an appropriate thromboembolic event prevention strategy, determined by proper risk assessment (SOR: D)
- Antithrombotics should be used with caution before invasive spinal or epidural procedures (SOR: C)
Grade A Recommendations
- Low-dose unfractionated heparin (LDUH), low-molecular-weight heparin (LMWH), graduated compression stockings (GCS), or intermittent pneumatic compression (IPC) for moderate-risk surgery patients
- LDUH, LMWH, or IPC for higher-risk general surgery
- Twice-daily LDUH for major gynecological surgery for benign disease
- Three-times-daily dose LDUH for gynecological surgery for malignancy
- LMWH or warfarin for 7–10 days for total hip or total knee replacement surgery; continue for longer periods in higher-risk patients. Adjusted-dose intravenous heparin is an acceptable alternative, but more difficult to manage
- Aspirin alone is not acceptable for hip fracture patients
- IPC with GCS for intracranial surgery; LDUH or postoperative LMWH are acceptable alternatives
- LMWH or intravenous heparin for the acute myocardial infarction patient (for the VTE prevention indication)
- LDUH or LMWH for immobilized stroke patient. GCS if anticoagula tion is contraindicated
- LDUH or LMWH for medical patients with cancer, bedrest, congestive heart failure, or severe lung disease
Grade B Recommendations
- LDUH, GCF, IPC, or LMWH for open urologic procedures
- IPC for total knee replacement
- LMWH or warfarin for hip fracture; an alternative is IPC
- LMWH for acute spinal cord injury. Alternative GCS or IPC in combination with LMWH or LDUH, if LMWH is contraindicated
Grade C Recommendations
- Early ambulation (with no antithrombotic agents) for low-risk surgery patients or uncomplicated gynecologic procedures
- LDUH, LMWH, or IPC for higher-risk surgery patients
- For very-high-risk surgery patients, LDUH or LMWH combined with GCS or IPC.Some patients may benefit from post-hospital LMWH or warfarin
- Daily LDUH or IPC for major gynecologic procedures for benign disease
- LDUH plus GCS or LMWH for gynecologic surgery for malignancy
- Early ambulation for low risk urologic and gynecologic procedures
- High-risk urologic procedures GCS plus with LDUH or LMWH
- GCS or IPC added to antithrombotic drugs for total hip replacement
Guideline development and evidence review
Literature searches were performed for each patient group. Criteria for inclusion included relevant patient group, sample size of at least 10 patients per group, verified deep vein thrombosis, and patients with adequate outcome assessments.
In considering baseline risk of thrombosis, only either prospective cohort studies or control groups of randomized trials were considered. For prophylaxis efficacy recommendations, only randomized trials were considered. The consensus group analyzed data from 630 sources before making these recommendations.
Sources for this guideline
Sixth ACCP Consensus Conference on Antithrombotic Therapy
The Consensus Conference guidelines can be found at:
Geerts WH, et al. Prevention of thromboembolism. Chest 2001; 119:132S–175S. Available at: www.chestjournal.org/content/vol119/1_suppl/index. shtml. Accessed on December 16, 2003.
Tables illustrating these guideline, organized by type of surgical procedure can be accessed at: chestnet.safeserver.com/guidelines/antithrombotic/p8.php
In the same issue of this journal, there were reports on the mechanism of action for oral anticoagulants, managing oral anticoagulant therapy, platelet active drugs, mechanisms of action of heparin and low molecular weight heparin, hemorrhagic complications of anticoagulation, use of antithrombotic medications during pregnancy, antithrombotic therapy for heart disease and peripheral vascular disease, use of these for stroke, and their role in treating children.
OTHER GUIDELINES ON PREVENTION OF VTE
- Deep venous thrombosis. Finnish Medical Society Duodecim. Helsinki, Finland: Duodecim Publications Ltd; 2002. Available at: www.ngc.gov/guidelines/FTNGC-2610.html. Accessed on December 16, 2003.
- Practice paramenters for the prevention of venous thromboembolism. The Standards Task Force of the Society of Colon and Rectal Surgeons. Dis Colon Rectum 2000; 43:1037–47. [54 references.] Available at: www.fascrs.org/ascrspp-pvt.html. Accessed on December 16, 2003.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- How do we determine risk of venous thromboembolism (VTE) in patients scheduled for surgery?
- Do all surgical patients require VTE prevention?
- Is aspirin adequate to prevent VTE in low-risk hospitalized patients?
- Which anticoagulant is appropriate for a patient scheduled for total knee replacement?
These important questions are answered in a guideline developed by a committee of the American College of Chest Physicians, which considered the following prophylaxis recommendations: early ambulation, aspirin, graduated compression stockings, intermittent pneumatic compression, low-dose unfractionated heparin, low-molecular-weight heparin, or oral antithrombotic agents.
The committee categorized recommendations by type of surgical procedure and risk status. In this summary, the recommendations are reorganized by strength of recommendation.
Three outcomes were regarded:
- Efficacy of various prophylactic strategies
- Rates and relative risk of venous thromboembolism outcomes—ie, fatal pulmonary embolism, symptomatic deep vein thrombosis, pulmonary embolism, or asymptomatic proximal deep vein thrombosis
- Cost-effectiveness of prophylaxis.
The committee used a rating scheme that accounted for both the risk/benefit ratio (clear or unclear) and the strength of the supporting recommendation (A, B, C). The grades of evidence were altered to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine. (For an explanaton of these grades.)
Relevant recommendations
This guideline is clinically relevant because of the high mortality associated with pulmonary embolus complicating VTE.
It offers a practical, tabulated guide, listed by surgical procedure performed. It is pertinent to hospitalized patients under the care of family physicians. The rationale for each recommendation is clear and well supported by the referenced literature. The objectives of the guideline were met and the outcome measures were appropriate.
The guideline is weakened by the lack of cost-effectiveness considerations.
Determining surgical risk
| Surgery + | Patient age (yr) + | Risk factors = | Level of risk |
|---|---|---|---|
| Minor | < 40 | No | Low |
| Minor | Any | Yes* | Moderate |
| 40–60 | No | ||
| Major | < 40 | No | |
| Minor | > 60 | No | High |
| > 60 | Yes* | ||
| Major | > 40 | No | |
| > 40 | Yes* | ||
| Major | > 40 | Prior VTE, cancer, hypercoagulable states, hip/knee arthoplasty, hip fracture, major trauma, spinal injury | Very high |
*Additional risk factors: immobility, stroke, paralysis, trauma, obesity, varicose veins, cardiac dysfunction, indwelling central venous catheter, inflammatory bowel disease, nephrotic syndrome, pregnancy, estrogen use, congenital thrombophilic abnormalities
- For all risk groups of patients, aspirin is not recommended for prophylaxis (strength of recommendation [SOR]: A)
- Every hospital should have an appropriate thromboembolic event prevention strategy, determined by proper risk assessment (SOR: D)
- Antithrombotics should be used with caution before invasive spinal or epidural procedures (SOR: C)
Grade A Recommendations
- Low-dose unfractionated heparin (LDUH), low-molecular-weight heparin (LMWH), graduated compression stockings (GCS), or intermittent pneumatic compression (IPC) for moderate-risk surgery patients
- LDUH, LMWH, or IPC for higher-risk general surgery
- Twice-daily LDUH for major gynecological surgery for benign disease
- Three-times-daily dose LDUH for gynecological surgery for malignancy
- LMWH or warfarin for 7–10 days for total hip or total knee replacement surgery; continue for longer periods in higher-risk patients. Adjusted-dose intravenous heparin is an acceptable alternative, but more difficult to manage
- Aspirin alone is not acceptable for hip fracture patients
- IPC with GCS for intracranial surgery; LDUH or postoperative LMWH are acceptable alternatives
- LMWH or intravenous heparin for the acute myocardial infarction patient (for the VTE prevention indication)
- LDUH or LMWH for immobilized stroke patient. GCS if anticoagula tion is contraindicated
- LDUH or LMWH for medical patients with cancer, bedrest, congestive heart failure, or severe lung disease
Grade B Recommendations
- LDUH, GCF, IPC, or LMWH for open urologic procedures
- IPC for total knee replacement
- LMWH or warfarin for hip fracture; an alternative is IPC
- LMWH for acute spinal cord injury. Alternative GCS or IPC in combination with LMWH or LDUH, if LMWH is contraindicated
Grade C Recommendations
- Early ambulation (with no antithrombotic agents) for low-risk surgery patients or uncomplicated gynecologic procedures
- LDUH, LMWH, or IPC for higher-risk surgery patients
- For very-high-risk surgery patients, LDUH or LMWH combined with GCS or IPC.Some patients may benefit from post-hospital LMWH or warfarin
- Daily LDUH or IPC for major gynecologic procedures for benign disease
- LDUH plus GCS or LMWH for gynecologic surgery for malignancy
- Early ambulation for low risk urologic and gynecologic procedures
- High-risk urologic procedures GCS plus with LDUH or LMWH
- GCS or IPC added to antithrombotic drugs for total hip replacement
Guideline development and evidence review
Literature searches were performed for each patient group. Criteria for inclusion included relevant patient group, sample size of at least 10 patients per group, verified deep vein thrombosis, and patients with adequate outcome assessments.
In considering baseline risk of thrombosis, only either prospective cohort studies or control groups of randomized trials were considered. For prophylaxis efficacy recommendations, only randomized trials were considered. The consensus group analyzed data from 630 sources before making these recommendations.
Sources for this guideline
Sixth ACCP Consensus Conference on Antithrombotic Therapy
The Consensus Conference guidelines can be found at:
Geerts WH, et al. Prevention of thromboembolism. Chest 2001; 119:132S–175S. Available at: www.chestjournal.org/content/vol119/1_suppl/index. shtml. Accessed on December 16, 2003.
Tables illustrating these guideline, organized by type of surgical procedure can be accessed at: chestnet.safeserver.com/guidelines/antithrombotic/p8.php
In the same issue of this journal, there were reports on the mechanism of action for oral anticoagulants, managing oral anticoagulant therapy, platelet active drugs, mechanisms of action of heparin and low molecular weight heparin, hemorrhagic complications of anticoagulation, use of antithrombotic medications during pregnancy, antithrombotic therapy for heart disease and peripheral vascular disease, use of these for stroke, and their role in treating children.
OTHER GUIDELINES ON PREVENTION OF VTE
- Deep venous thrombosis. Finnish Medical Society Duodecim. Helsinki, Finland: Duodecim Publications Ltd; 2002. Available at: www.ngc.gov/guidelines/FTNGC-2610.html. Accessed on December 16, 2003.
- Practice paramenters for the prevention of venous thromboembolism. The Standards Task Force of the Society of Colon and Rectal Surgeons. Dis Colon Rectum 2000; 43:1037–47. [54 references.] Available at: www.fascrs.org/ascrspp-pvt.html. Accessed on December 16, 2003.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- How do we determine risk of venous thromboembolism (VTE) in patients scheduled for surgery?
- Do all surgical patients require VTE prevention?
- Is aspirin adequate to prevent VTE in low-risk hospitalized patients?
- Which anticoagulant is appropriate for a patient scheduled for total knee replacement?
These important questions are answered in a guideline developed by a committee of the American College of Chest Physicians, which considered the following prophylaxis recommendations: early ambulation, aspirin, graduated compression stockings, intermittent pneumatic compression, low-dose unfractionated heparin, low-molecular-weight heparin, or oral antithrombotic agents.
The committee categorized recommendations by type of surgical procedure and risk status. In this summary, the recommendations are reorganized by strength of recommendation.
Three outcomes were regarded:
- Efficacy of various prophylactic strategies
- Rates and relative risk of venous thromboembolism outcomes—ie, fatal pulmonary embolism, symptomatic deep vein thrombosis, pulmonary embolism, or asymptomatic proximal deep vein thrombosis
- Cost-effectiveness of prophylaxis.
The committee used a rating scheme that accounted for both the risk/benefit ratio (clear or unclear) and the strength of the supporting recommendation (A, B, C). The grades of evidence were altered to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine. (For an explanaton of these grades.)
Relevant recommendations
This guideline is clinically relevant because of the high mortality associated with pulmonary embolus complicating VTE.
It offers a practical, tabulated guide, listed by surgical procedure performed. It is pertinent to hospitalized patients under the care of family physicians. The rationale for each recommendation is clear and well supported by the referenced literature. The objectives of the guideline were met and the outcome measures were appropriate.
The guideline is weakened by the lack of cost-effectiveness considerations.
Determining surgical risk
| Surgery + | Patient age (yr) + | Risk factors = | Level of risk |
|---|---|---|---|
| Minor | < 40 | No | Low |
| Minor | Any | Yes* | Moderate |
| 40–60 | No | ||
| Major | < 40 | No | |
| Minor | > 60 | No | High |
| > 60 | Yes* | ||
| Major | > 40 | No | |
| > 40 | Yes* | ||
| Major | > 40 | Prior VTE, cancer, hypercoagulable states, hip/knee arthoplasty, hip fracture, major trauma, spinal injury | Very high |
*Additional risk factors: immobility, stroke, paralysis, trauma, obesity, varicose veins, cardiac dysfunction, indwelling central venous catheter, inflammatory bowel disease, nephrotic syndrome, pregnancy, estrogen use, congenital thrombophilic abnormalities
- For all risk groups of patients, aspirin is not recommended for prophylaxis (strength of recommendation [SOR]: A)
- Every hospital should have an appropriate thromboembolic event prevention strategy, determined by proper risk assessment (SOR: D)
- Antithrombotics should be used with caution before invasive spinal or epidural procedures (SOR: C)
Grade A Recommendations
- Low-dose unfractionated heparin (LDUH), low-molecular-weight heparin (LMWH), graduated compression stockings (GCS), or intermittent pneumatic compression (IPC) for moderate-risk surgery patients
- LDUH, LMWH, or IPC for higher-risk general surgery
- Twice-daily LDUH for major gynecological surgery for benign disease
- Three-times-daily dose LDUH for gynecological surgery for malignancy
- LMWH or warfarin for 7–10 days for total hip or total knee replacement surgery; continue for longer periods in higher-risk patients. Adjusted-dose intravenous heparin is an acceptable alternative, but more difficult to manage
- Aspirin alone is not acceptable for hip fracture patients
- IPC with GCS for intracranial surgery; LDUH or postoperative LMWH are acceptable alternatives
- LMWH or intravenous heparin for the acute myocardial infarction patient (for the VTE prevention indication)
- LDUH or LMWH for immobilized stroke patient. GCS if anticoagula tion is contraindicated
- LDUH or LMWH for medical patients with cancer, bedrest, congestive heart failure, or severe lung disease
Grade B Recommendations
- LDUH, GCF, IPC, or LMWH for open urologic procedures
- IPC for total knee replacement
- LMWH or warfarin for hip fracture; an alternative is IPC
- LMWH for acute spinal cord injury. Alternative GCS or IPC in combination with LMWH or LDUH, if LMWH is contraindicated
Grade C Recommendations
- Early ambulation (with no antithrombotic agents) for low-risk surgery patients or uncomplicated gynecologic procedures
- LDUH, LMWH, or IPC for higher-risk surgery patients
- For very-high-risk surgery patients, LDUH or LMWH combined with GCS or IPC.Some patients may benefit from post-hospital LMWH or warfarin
- Daily LDUH or IPC for major gynecologic procedures for benign disease
- LDUH plus GCS or LMWH for gynecologic surgery for malignancy
- Early ambulation for low risk urologic and gynecologic procedures
- High-risk urologic procedures GCS plus with LDUH or LMWH
- GCS or IPC added to antithrombotic drugs for total hip replacement
Guideline development and evidence review
Literature searches were performed for each patient group. Criteria for inclusion included relevant patient group, sample size of at least 10 patients per group, verified deep vein thrombosis, and patients with adequate outcome assessments.
In considering baseline risk of thrombosis, only either prospective cohort studies or control groups of randomized trials were considered. For prophylaxis efficacy recommendations, only randomized trials were considered. The consensus group analyzed data from 630 sources before making these recommendations.
Sources for this guideline
Sixth ACCP Consensus Conference on Antithrombotic Therapy
The Consensus Conference guidelines can be found at:
Geerts WH, et al. Prevention of thromboembolism. Chest 2001; 119:132S–175S. Available at: www.chestjournal.org/content/vol119/1_suppl/index. shtml. Accessed on December 16, 2003.
Tables illustrating these guideline, organized by type of surgical procedure can be accessed at: chestnet.safeserver.com/guidelines/antithrombotic/p8.php
In the same issue of this journal, there were reports on the mechanism of action for oral anticoagulants, managing oral anticoagulant therapy, platelet active drugs, mechanisms of action of heparin and low molecular weight heparin, hemorrhagic complications of anticoagulation, use of antithrombotic medications during pregnancy, antithrombotic therapy for heart disease and peripheral vascular disease, use of these for stroke, and their role in treating children.
OTHER GUIDELINES ON PREVENTION OF VTE
- Deep venous thrombosis. Finnish Medical Society Duodecim. Helsinki, Finland: Duodecim Publications Ltd; 2002. Available at: www.ngc.gov/guidelines/FTNGC-2610.html. Accessed on December 16, 2003.
- Practice paramenters for the prevention of venous thromboembolism. The Standards Task Force of the Society of Colon and Rectal Surgeons. Dis Colon Rectum 2000; 43:1037–47. [54 references.] Available at: www.fascrs.org/ascrspp-pvt.html. Accessed on December 16, 2003.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
How should we manage an acute exacerbation of COPD?
Diagnosis
- Chest radiography is useful (B).
- Spirometry should not be used to diagnose an exacerbation or to assess its severity (A).
- An arterial blood gas reading is helpful in gauging the severity of an exacerbation (A).
- There is little evidence regarding the contribution of additional laboratory testing, the predictive value of physical examination findings, or the usefulness of electrocardiography or echocardiography.
Treatment
- Inhaled short-acting beta-2 agonists and anticholinergic bronchodilators have positive effects. Since inhaled anti-cholinergic bronchodilators have fewer side effects, use them first. If improvement is slow with the initial bronchodilator, even at maximum dose, add a second bronchodilator (A).
- Parenteral agents (methyxanthines and sympathomimetics) are not as effective and have potential adverse effects (B).
- Mucolytic medications and chest physiotherapy are not effective (C).
- Systemic corticosteroids improve respiration and reduce relapse rate (A).
- Noninvasive positive-pressure ventilation decreases risk for invasive mechanical ventilation (A).
- Oxygen is beneficial for hypoxemic patients (B).
- Antibiotics are beneficial. Narrow-spectrum antibiotics (eg, amoxicillin, trimethoprimsulfamethoxazole, or tetracycline) are recommended as first-line agents. The more severe the episode, the more beneficial are antibiotics (A). There is no data regarding the optimal length of antibiotic treatment.
- Little evidence is available regarding the empiric use of diuretics.
Prognosis
- No methods reliably predict readmission to the hospital within 14 days after discharge (B).
- No methods reliably predict inpatient mortality (B).
Would you order a chest film to evaluate an acute exacerbation of chronic obstructive pulmonary disease (COPD)? Which medication would you first prescribe—a short-acting inhaled beta-2 agonist or an anticholinergic bronchodilator?
These are important questions for family physicians who commonly manage acute exacerbations of COPD.
The guideline summarized here was developed by a joint expert panel of the American College of Physicians–American Society of Internal Medicine and the American College of Chest Physicians. Three outcomes were considered: treatment efficacy, 6-month mortality, and relapse, as defined by return visit to the emergency department within 14 days of initial presentation. Systematic reviews with evidence tables were used to analyze data. The rationale for each recommendation is clear and well documented.
We added strength-of-recommendation ratings, which are not in the original guideline.
Limitations of the Guideline and Additional Evidence
Several weaknesses underlie this guideline. The authors found that, despite the importance of COPD, it has been the subject of very few high-quality studies. The highest-quality studies were few in number and had enrolled a small number of participants. The authors did not grade the strength of each recommendation in the summary document or in the detailed manuscripts, making it difficult to rapidly review.
Different diagnostic criteria are used in the source studies, making the context of treatment recommendations difficult to fully understand. Outcome endpoints also varied among studies. Goals for oxygen therapy were not addressed. Antibiotic treatment was based on studies before the emergence of multidrug-resistant organisms, particularly Streptococcus pneumoniae. It did not address tobacco use or smoking cessation, vaccine administration, outpatient management, management of stable COPD, or stratification of patients by severity.
Guideline Development and Evidence Review
Literature searches were performed using MEDLINE (1966–2000), EMBASE (1966– 2000), Health Star (1966–2000), and the Cochrane Controlled Trials Register (2000, Issue 1).
Search strategies included the index terms and text words chronic obstructive pulmonary disease and acute exacerbation and specific terms relating to interventions and outcomes. Variations on several search strategies were tested to locate the greatest number of relevant articles. Reference lists of retrieved articles were also examined. In all, 770 source articles were found.
Two other Guidelines for COPD
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, Md: Global Initiative for Chronic Obstructive Lung Disease, World Health Organization/National Heart, Lung, and Blood Institute; 2001. Various pagings. (Web access at: www.goldcopd.com.)
- Veterans Health Administration (VHA). Clinical practice guideline for the management of chronic obstructive pulmonary disease. Version 1.1a. Washington, DC: Department of Veterans Affairs (US), Veterans Health Administration; 1999 Aug. 116 p. (Web access at: www.oqp.med.va.gov/cpg/COPD/ COPD_base.htm).
FIGURE
Emphysematous dysfunction in COPD
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
GUIDELINE SOURCES
Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians–American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: A summary and appraisal of published evidence. Ann Intern Med 2001; 134:600-620. (Available at: www.annals.org/issues/ v134n7/full/200104030-00016.html. Accessed on September 5, 2003.)
McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001; 119:1190-1209.
Snow V, Lascher S, Mottur-Pilson C; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001; 134: 595-599. (Available at: www.annals.org/issues/v134n7/full/20010403000015.html. Accessed on September 5, 2003.)
Diagnosis
- Chest radiography is useful (B).
- Spirometry should not be used to diagnose an exacerbation or to assess its severity (A).
- An arterial blood gas reading is helpful in gauging the severity of an exacerbation (A).
- There is little evidence regarding the contribution of additional laboratory testing, the predictive value of physical examination findings, or the usefulness of electrocardiography or echocardiography.
Treatment
- Inhaled short-acting beta-2 agonists and anticholinergic bronchodilators have positive effects. Since inhaled anti-cholinergic bronchodilators have fewer side effects, use them first. If improvement is slow with the initial bronchodilator, even at maximum dose, add a second bronchodilator (A).
- Parenteral agents (methyxanthines and sympathomimetics) are not as effective and have potential adverse effects (B).
- Mucolytic medications and chest physiotherapy are not effective (C).
- Systemic corticosteroids improve respiration and reduce relapse rate (A).
- Noninvasive positive-pressure ventilation decreases risk for invasive mechanical ventilation (A).
- Oxygen is beneficial for hypoxemic patients (B).
- Antibiotics are beneficial. Narrow-spectrum antibiotics (eg, amoxicillin, trimethoprimsulfamethoxazole, or tetracycline) are recommended as first-line agents. The more severe the episode, the more beneficial are antibiotics (A). There is no data regarding the optimal length of antibiotic treatment.
- Little evidence is available regarding the empiric use of diuretics.
Prognosis
- No methods reliably predict readmission to the hospital within 14 days after discharge (B).
- No methods reliably predict inpatient mortality (B).
Would you order a chest film to evaluate an acute exacerbation of chronic obstructive pulmonary disease (COPD)? Which medication would you first prescribe—a short-acting inhaled beta-2 agonist or an anticholinergic bronchodilator?
These are important questions for family physicians who commonly manage acute exacerbations of COPD.
The guideline summarized here was developed by a joint expert panel of the American College of Physicians–American Society of Internal Medicine and the American College of Chest Physicians. Three outcomes were considered: treatment efficacy, 6-month mortality, and relapse, as defined by return visit to the emergency department within 14 days of initial presentation. Systematic reviews with evidence tables were used to analyze data. The rationale for each recommendation is clear and well documented.
We added strength-of-recommendation ratings, which are not in the original guideline.
Limitations of the Guideline and Additional Evidence
Several weaknesses underlie this guideline. The authors found that, despite the importance of COPD, it has been the subject of very few high-quality studies. The highest-quality studies were few in number and had enrolled a small number of participants. The authors did not grade the strength of each recommendation in the summary document or in the detailed manuscripts, making it difficult to rapidly review.
Different diagnostic criteria are used in the source studies, making the context of treatment recommendations difficult to fully understand. Outcome endpoints also varied among studies. Goals for oxygen therapy were not addressed. Antibiotic treatment was based on studies before the emergence of multidrug-resistant organisms, particularly Streptococcus pneumoniae. It did not address tobacco use or smoking cessation, vaccine administration, outpatient management, management of stable COPD, or stratification of patients by severity.
Guideline Development and Evidence Review
Literature searches were performed using MEDLINE (1966–2000), EMBASE (1966– 2000), Health Star (1966–2000), and the Cochrane Controlled Trials Register (2000, Issue 1).
Search strategies included the index terms and text words chronic obstructive pulmonary disease and acute exacerbation and specific terms relating to interventions and outcomes. Variations on several search strategies were tested to locate the greatest number of relevant articles. Reference lists of retrieved articles were also examined. In all, 770 source articles were found.
Two other Guidelines for COPD
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, Md: Global Initiative for Chronic Obstructive Lung Disease, World Health Organization/National Heart, Lung, and Blood Institute; 2001. Various pagings. (Web access at: www.goldcopd.com.)
- Veterans Health Administration (VHA). Clinical practice guideline for the management of chronic obstructive pulmonary disease. Version 1.1a. Washington, DC: Department of Veterans Affairs (US), Veterans Health Administration; 1999 Aug. 116 p. (Web access at: www.oqp.med.va.gov/cpg/COPD/ COPD_base.htm).
FIGURE
Emphysematous dysfunction in COPD
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
Diagnosis
- Chest radiography is useful (B).
- Spirometry should not be used to diagnose an exacerbation or to assess its severity (A).
- An arterial blood gas reading is helpful in gauging the severity of an exacerbation (A).
- There is little evidence regarding the contribution of additional laboratory testing, the predictive value of physical examination findings, or the usefulness of electrocardiography or echocardiography.
Treatment
- Inhaled short-acting beta-2 agonists and anticholinergic bronchodilators have positive effects. Since inhaled anti-cholinergic bronchodilators have fewer side effects, use them first. If improvement is slow with the initial bronchodilator, even at maximum dose, add a second bronchodilator (A).
- Parenteral agents (methyxanthines and sympathomimetics) are not as effective and have potential adverse effects (B).
- Mucolytic medications and chest physiotherapy are not effective (C).
- Systemic corticosteroids improve respiration and reduce relapse rate (A).
- Noninvasive positive-pressure ventilation decreases risk for invasive mechanical ventilation (A).
- Oxygen is beneficial for hypoxemic patients (B).
- Antibiotics are beneficial. Narrow-spectrum antibiotics (eg, amoxicillin, trimethoprimsulfamethoxazole, or tetracycline) are recommended as first-line agents. The more severe the episode, the more beneficial are antibiotics (A). There is no data regarding the optimal length of antibiotic treatment.
- Little evidence is available regarding the empiric use of diuretics.
Prognosis
- No methods reliably predict readmission to the hospital within 14 days after discharge (B).
- No methods reliably predict inpatient mortality (B).
Would you order a chest film to evaluate an acute exacerbation of chronic obstructive pulmonary disease (COPD)? Which medication would you first prescribe—a short-acting inhaled beta-2 agonist or an anticholinergic bronchodilator?
These are important questions for family physicians who commonly manage acute exacerbations of COPD.
The guideline summarized here was developed by a joint expert panel of the American College of Physicians–American Society of Internal Medicine and the American College of Chest Physicians. Three outcomes were considered: treatment efficacy, 6-month mortality, and relapse, as defined by return visit to the emergency department within 14 days of initial presentation. Systematic reviews with evidence tables were used to analyze data. The rationale for each recommendation is clear and well documented.
We added strength-of-recommendation ratings, which are not in the original guideline.
Limitations of the Guideline and Additional Evidence
Several weaknesses underlie this guideline. The authors found that, despite the importance of COPD, it has been the subject of very few high-quality studies. The highest-quality studies were few in number and had enrolled a small number of participants. The authors did not grade the strength of each recommendation in the summary document or in the detailed manuscripts, making it difficult to rapidly review.
Different diagnostic criteria are used in the source studies, making the context of treatment recommendations difficult to fully understand. Outcome endpoints also varied among studies. Goals for oxygen therapy were not addressed. Antibiotic treatment was based on studies before the emergence of multidrug-resistant organisms, particularly Streptococcus pneumoniae. It did not address tobacco use or smoking cessation, vaccine administration, outpatient management, management of stable COPD, or stratification of patients by severity.
Guideline Development and Evidence Review
Literature searches were performed using MEDLINE (1966–2000), EMBASE (1966– 2000), Health Star (1966–2000), and the Cochrane Controlled Trials Register (2000, Issue 1).
Search strategies included the index terms and text words chronic obstructive pulmonary disease and acute exacerbation and specific terms relating to interventions and outcomes. Variations on several search strategies were tested to locate the greatest number of relevant articles. Reference lists of retrieved articles were also examined. In all, 770 source articles were found.
Two other Guidelines for COPD
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, Md: Global Initiative for Chronic Obstructive Lung Disease, World Health Organization/National Heart, Lung, and Blood Institute; 2001. Various pagings. (Web access at: www.goldcopd.com.)
- Veterans Health Administration (VHA). Clinical practice guideline for the management of chronic obstructive pulmonary disease. Version 1.1a. Washington, DC: Department of Veterans Affairs (US), Veterans Health Administration; 1999 Aug. 116 p. (Web access at: www.oqp.med.va.gov/cpg/COPD/ COPD_base.htm).
FIGURE
Emphysematous dysfunction in COPD
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
GUIDELINE SOURCES
Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians–American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: A summary and appraisal of published evidence. Ann Intern Med 2001; 134:600-620. (Available at: www.annals.org/issues/ v134n7/full/200104030-00016.html. Accessed on September 5, 2003.)
McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001; 119:1190-1209.
Snow V, Lascher S, Mottur-Pilson C; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001; 134: 595-599. (Available at: www.annals.org/issues/v134n7/full/20010403000015.html. Accessed on September 5, 2003.)
GUIDELINE SOURCES
Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians–American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: A summary and appraisal of published evidence. Ann Intern Med 2001; 134:600-620. (Available at: www.annals.org/issues/ v134n7/full/200104030-00016.html. Accessed on September 5, 2003.)
McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001; 119:1190-1209.
Snow V, Lascher S, Mottur-Pilson C; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001; 134: 595-599. (Available at: www.annals.org/issues/v134n7/full/20010403000015.html. Accessed on September 5, 2003.)