User login
Emergency Radiology: Current and advanced imaging techniques in the ED
As EDs have evolved to handle the increasing volume and complexity of patients requiring immediate care, so too, has the field of emergency radiology. Many EDs across the country now have multiple advanced imaging modalities available 24 hours a day, including Xray, computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI). While many emergency medicine physicians are now trained in performing and evaluating ultrasound images—similar to X-rays in the past—most are less comfortable with CT and MRI.
Emergency radiology, now a recognized subspecialty of diagnostic imaging, has proliferated to meet the demands for immediate interpretation of these images. This combination of around-the-clock access to equipment and expertise has brought cutting-edge, advanced imaging to the front lines of emergency care. In this special feature, we invited a group of emergency radiologists and an expert in cardiovascular imaging to discuss the applicability and utility of several of these techniques in the ED setting.
As illustrated by this panel, advanced imaging has become increasingly valuable and available in providing care to ED patients. In this presentation, however, you will notice the conspicuous absence of specific techniques that are utilized in diagnosis and evaluation of stroke, head/spine trauma, and other neurologic conditions that are commonplace in the ED. Advanced imaging has become critical in these instances and will be discussed in a future article.
Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and the executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center. He is a member of the EMER GENCY MEDICINE editorial board.
Faster than FAST: Single Pass Whole-Body Computed Tomography for Rapid Evaluation of Trauma Patients
Ashwin Asrani, MD
Dr Asrani is an assistant professor of radiology at Weill Cornell Medical College in New York City and an
assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center in New York City.
As part of initial assessment and resuscitation in the ED, severely injured trauma patients frequently undergo a bedside focused abdominal sonography for trauma (FAST) scan to assess for intraperitoneal hemorrhage from an underlying major abdominal visceral injury, as well as the presence of pleural and/or pericardial effusion. However, the use and role of a FAST scan in hemodynamically stable patients has recently been questioned since, based on its relatively low sensitivity for visceral injury, many of these cases eventually require computed tomography (CT) (See Case). In addition, patients in this subset with a positive FAST scan frequently have subsequent CT to further assist clinicians in understanding the nature of injury and to guide management recommendations (eg, operative versus nonoperative options).1
Advances in the speed of CT have made it possible to obtain images of the entire body with only a single dose of contrast injection. The elimination of segmental acquisition of torso images (eg, head, neck, chest, abdomen) eliminates the need to reposition the patient, repeat scout images, and postpone the reconstruction of reformats and other special views until the end of the examination. Depending on the original protocol, this in turn minimizes patient time in the CT-scan room by approximately 31% to 42%,2,3 and significantly reduces radiation exposure by 17% compared to conventional segmental acquisition of different body parts.2
Evidence suggests that direct single-pass whole-body CT improves outcomes in both hemodynamically stable and unstable patients and can reveal significant findings not apparent on initial clinical examination.4,5 Whole-body CT increases injury severity by detecting lesions that would not otherwise have been detected by conventional methods. While this information does not affect treatment options, it does artificially lower the ratio of observed-to-expected deaths.6 The Randomized Study of Early Assessment by CT Scanning in Trauma Patients (REACT-2), a recent international multicenter randomized clinical trial, is expected to provide evidence supporting the value of immediate total-body CT scanning during the primary evaluation of severely injured trauma patients; the results of this trial should become available in 2014.7
As EDs install and upgrade to newer multidetector CT scanners, single-pass whole-body scanning has the potential to save time in situations when it really matters.
References
1. Natarajan B, Gupta PK, Cemaj S, Sorensen M, Hatzoudis GI, Forse RA. FAST scan: Is it worth doing in hemodynamically stable blunt trauma patients? Surgery. 2010;148(4):695-700; discussion 700-701.
2. Fanucci E, Fiaschetti V, Rotili A, Floris R, Simonetti G. Whole body 16-row multislice CT in emergency room: Effects of different protocols on scanning time, image quality and radiation exposure. Emerg Radiol. 2007;13(5):251-257.
3. Nguyen D, Platon A, Shanmuganathan K, Mirvis SE, Becker CD, Poletti PA. Evaluation of a single-pass continuous whole-body 16-MDCT protocol for patients with polytrauma. AJR Am J Roentgenol. 2009;192(1):3-10.
4. Huber-Wagner S, Biberthaler P, Haberle S, et al. Whole-body CT in haemodynamically unstable severely injured patients – A retrospective, multicentre study. PLoS One. 2013;8(7):e68880.
5. Salim A, Sangthong B, Martin M, Brown C, Plurad D, Demetriades D. Whole body imaging in blunt multisystem trauma patients without obvious signs of injury: Results of a prospective study. Arch Surg. 2006;141(5):468-473; discussion 473-475.
6. Stengel D, Frank M, Matthes G, et al. Primary pan-computed tomography for blunt multiple trauma: Can the whole be better than its parts? Injury. 2009;40 Suppl 4:S36-S46.
7. Sierink JC, Saltzherr TP, Beenen LF, et al. A multicenter, randomized controlled trial of immediate total-body CT scanning in trauma patients (REACT-2). BMC Emerg Med. 2012;12:4-227X-12-4.
Obstructed Views: Imaging in Urinary Tract Obstruction
Lily M. Belfi, MD
Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center.
Urinary tract (UT) obstruction is one of the most common indications for genitourinary (GU) imaging in the ED. Until recently, diagnosis was made using intravenous (IV) pyelography. Today, improved imaging modalities, such as noncontrast computed tomography (CT), computed tomography urography (CTU), magnetic resonance urography (MRU), and magnetic resonance imaging (MRI), are available to better visualize and evaluate the underlying causes of obstruction.
Noncontrast Computed Tomography
|
|
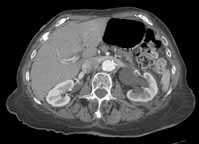
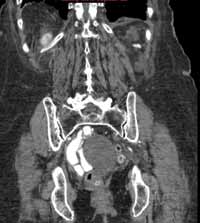
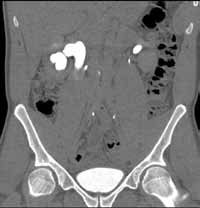
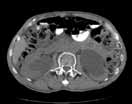
Noncontrast CT of the abdomen and pelvis is the imaging study of choice in evaluating acute GU obstruction, particularly when there is clinical suspicion of stone disease. CT is extremely sensitive and specific in detecting renal calculi and provides information about stone burden, size, location, and composition. In addition to renal calculi, noncontrast CT is also useful in identifying other, less common causes of UT obstruction such as ureteral herniation, which can occur in the inguinal, femoral, or sciatic region and result in acute obstruction. Patients with neuromuscular disorders that cause piriformis muscle atrophy (eg, multiple sclerosis) may be predisposed to ureteral sciatic herniation. CT reconstructions in the coronal plane are useful in detecting this condition (Figure 1).
|
|
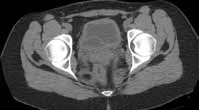
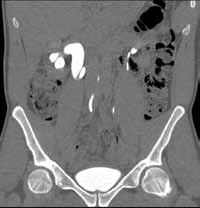
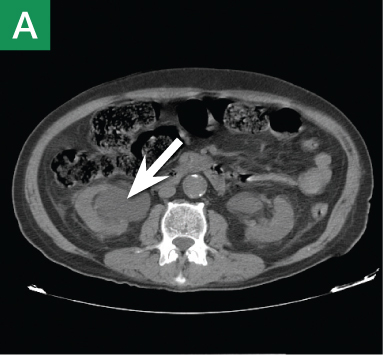
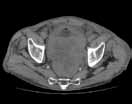
Reconstructed CT images can also reveal underlying congenital abnormalities that make a patient susceptible to obstruction. For example, CT can reveal the presence of a duplicated collecting system, which is often accompanied by an obstructing ureterocele associated with the upper pole moiety (Figure 2).
Computed Tomography Urography
|
| ||
| Figure 3. Computed tomography urography images (A and B) reveal "fish-hook" ureter (white arrows) classically seen in patients with with retrocaval ureter. | |||
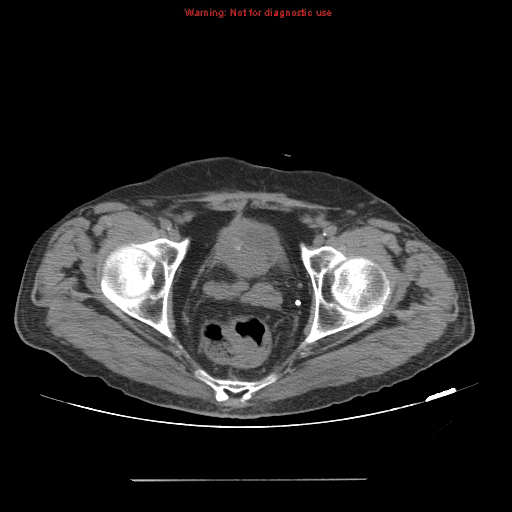
Other types of congenital anomalies may require additional imaging with CTU, in which IV iodinated contrast media is administered for increased sensitivity and visualization. For instance, patients with a retrocaval ureter often present with GU obstruction and hematuria; the classic “fish-hook” or “sickle-shaped” deformity of the ureter characterizing this disease is best visualized on CTU as the ureter is well-opacified by excreted contrast (Figure 3).
Magnetic Resonance Urography and Magnetic Resonance Imaging
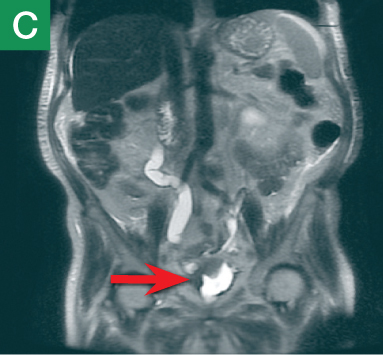
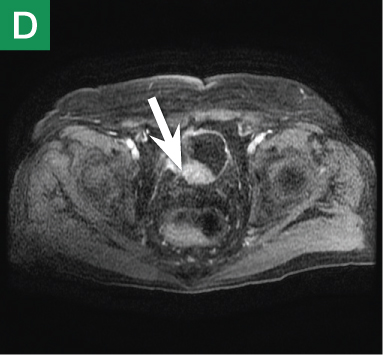
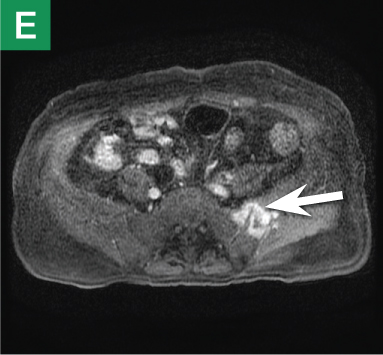
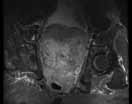
MRU and MRI are alternative modalities to CTU for patients in whom iodinated CT contrast is contraindicated or in cases where the avoidance of radiation exposure is indicated (eg, pregnant and pediatric patients). MRI and MRU are especially useful in further characterizing obstructive lesions in the GU tract. While initial noncontrast CT may suggest soft-tissue lesions within the ureter or urinary bladder, follow-up MRI clearly delineates the condition, helping clinicians quantify the extent of tumor involvement and determine disease stage (Figure 4). Pelvic MRI is also an essential tool in identifying causes of bladder outlet obstruction, especially in those involving the prostate gland (Figure 5).
|
|
|
|
|
Figure 4. Noncontrast pelvic computed tomography images (A and B) of a 72-year-old man with flank pain and difficulty voiding reveal hydronephrosis (white arrow) and bladder mass (red arrow). Magnetic resonance urography (C) further visualizes the bladder mass (red arrow), and postcontrast magnetic resonance images (D and E) show the extent of the tumor (red arrow) as well as a metastatic lesion (white arrow).
| ||||
Conclusion
|
|
| |||
| Figure 5. Noncontrast pelvic computed tomography images (A and B) of a 73-yearold man with pelvic pain demonstrate bilateral hydronephrosis. (white arrows) and a large mass in the expected location of the prostate (red arrow). Further evaluation of the large heterogenous mass with pelvic magnetic resonance imaging (C) reveals prostate carcinoma (red arrows). | |||||
Based on its sensitivity in detecting both common and uncommon causes of obstruction, noncontrast abdominal and pelvic CT is an excellent first-line imaging choice for evaluating patients presenting to the ED with UT obstruction. Moreover, noncontrast CT also helps guide clinicians in determining which, if any, additional studies with CTU, MRU, and/or MRI are warranted.
Sound Advice: Ultrasound in the Emergency Department
Kemi Babagbemi, MD
Dr Babagbemi is an assistant professor of radiology and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
In today’s evolving health-care environment, emphasis must be placed on providing accurate, safe, cost-effective, and timely diagnosis to the wide range of patients that present to the ED. To meet this need, emergency medicine physicians and radiologists have universally embraced the use of ultrasound in this setting.
Ultrasound is less expensive than computed tomography (CT) and magnetic resonance imaging (MRI), and its portability allows for bedside application, enabling its use in the most critical patients. It does not require extended preparation (eg, oral contrast) or carry the risk for adverse reactions associated with intravenously administered contrast. Also, since ultrasound does not use ionizing radiation, it is a particularly appropriate imaging option in susceptible populations such as pregnant and pediatric patients.1 Although a noninvasive modality, it can also be used to guide interventional procedures, including vascular access, thoracentesis/paracentesis, and specialized anesthesia.
Emergency physicians routinely perform ultrasound at bedside and are familiar with its utility in evaluating abdominal trauma, right upper quadrant pain, and acute biliary abnormalities; assessing vascular thrombosis or injury; and determining the etiology of pelvic pain and bleeding in both pregnant and nonpregnant patients. However, ultrasound is also increasingly being utilized to
diagnose other conditions for which CT and MRI were once thought the superior diagnostic tool.
Trauma (The Extended FAST Scan)
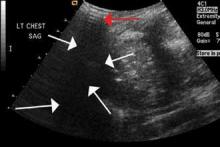
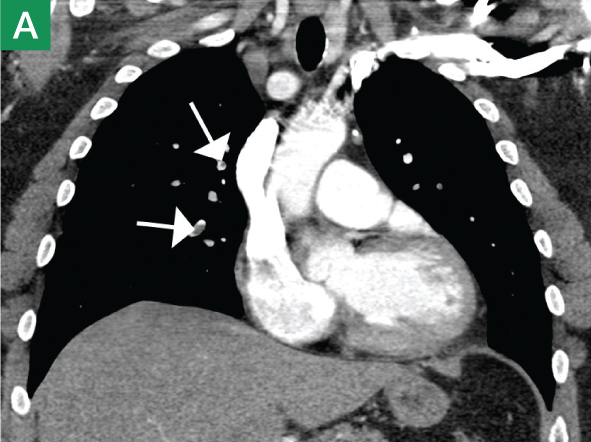
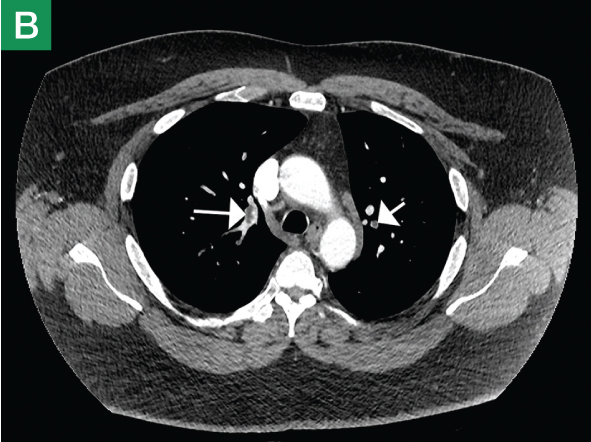
The focused abdominal sonography for trauma (FAST) scan has been used for rapid and immediate assessment of unstable trauma patients with suspected abdominal injury. In a recent study of 4,029 patients with blunt abdominal trauma, FAST scans had a sensitivity, specificity, and accuracy for detection of hemorrhage in patients with hypotension of 85%, 60%, and 77%, respectively.2 Other studies suggest that sonography of the chest may be useful in the rapid detection of additional life-threatening pathology such as pneumothorax (Figure 1), with data suggesting improved sensitivity over radiography.3 The inclusion of the pleural space in evaluation is increasingly more common and is referred to as the extended FAST (EFAST).
Appendicitis
The most common cause of abdominal pain requiring surgical intervention is appendicitis.4 Although certain clinically based prediction scores (eg, Alvarado scores) may be used, imaging is considered far superior in accurately diagnosing the condition.5 In a meta-analysis of data from 26 ultrasound and CT studies (15 prospective, 11 retrospective), there was a pooled 88% sensitivity and 94% specificity for ultrasound compared with CT, which exhibited a pooled sensitivity of 94% and specificity of 95%.6 As previously noted, because there is no ionizing radiation in ultrasound, it should be the preferred modality in both children and first-trimester pregnant patients.
Musculoskeletal Trauma
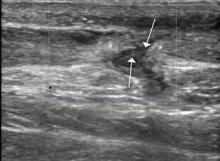
Ultrasound is an ideal imaging modality to evaluate the musculoskeletal system. Despite its widespread use in Europe for many years, musculoskeletal sonography is only now beginning to be adopted in the United States. Its ability to visualize soft-tissue structures makes it effective in evaluating for muscle, tendon, or ligament injury, and can even do so dynamically with stress maneuvers (Figure 2). With respect to fractures, sonography has also proved effective in evaluating for cortical disruption. For example, a recent study demonstrated overall 92% sensitivity and 100% sensitivity for fractures with high potential for complication in radiographically occult scaphoid fractures.7
Conclusion
As a true point-of-care imaging modality, ultrasound has an established place in the practice of emergency medicine. Future improvements in technology, including the ability to obtain true three-dimensional volumetric data sets, will expand its role even further.
References
1. Image Gently and Ultrasound. Image Gently Campaign. The Alliance for Radiation Safety in Pediatric Imaging Web site. http://www.pedrad.org/associations/5364/ig/?page=787. Accessed September 19, 2013.
2. Lee BC, Ormsby EL, McGahan JP, Melendres GM, Richards JR. The utility of sonography for the triage of blunt abdominal trauma patients to exploratory laparotomy. AJR Am J Roentgenol. 2007;188(2):415-421.
3. Nandipati KC, Allamaneni S, Kakarla, et al. Extended focused assessment with sonography for trauma (EFAST) in the diagnosis of pneumothorax: experience at a community based level I trauma center. Injury. 2011;42(5):511-514.
4. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132(5):910-925.
5. Sun JS, Noh HW, Min YG, et al. Receiver operating characteristic analysis of the diagnostic performance of a computed tomographic examination and the Alvarado score for diagnosing acute appendicitis: emphasis on age and sex of the patients.
J Comput Assist Tomogr. 2008;32(3):386-391.
6. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for Diagnosis of Appendicitis in Children and Adults? A MetaAnalysis. Radiology. 2006;241(1):83-94.
7. Platon A, Poletti PA, Van Aaken J, Fusetti C, Della Santa D, Beaulieu JY, Becker CD. Occult fractures of the scaphoid: the role of Ultrasonography in the emergency department. Skeletal Radiol. 2011;40(7):869-875.
Stuck or Not? Noninvasive Vascular Imaging in the Emergency Setting
Michael L. Loftus, MD
Dr Loftus is assistant professor of radiology at New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
Conventional catheter-directed angiography has played an important role in the history of ED imaging, providing timely information about vessel integrity throughout the body and guiding potentially life-saving interventions. However, this imaging modality carries significant potential risks, including puncture-site hematoma or pseudoaneurysm, catheter-induced vasospasm, vascular occlusion or dissection, anesthesia-associated risks, and neurological deterioration or stroke.1 Moreover, emergent angiography is not universally available in all EDs.
Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) have opened new windows of opportunity for noninvasive vascular imaging to play a role in clinical decision-making in the ED. Cross-sectional angiography has virtually eliminated the need for acute catheter-directed angiography in several clinical settings, including pulmonary angiography, and the clinical applicability of CTA and MRA continues to expand as imaging techniques improve and achieve widespread acceptance and implementation. Multidetector CT is now widely available in most EDs, and the
accessibility of magnetic resonance imaging and MRA is expanding rapidly.
CTA allows evaluation of the vasculature on contrast-enhanced axial source images and also utilizes computer-generated maximum intensity projection reformations to create diagnostic images of the vascular region of interest. Similarly, MRA can be performed either with or without the administration of an intravenous gadolinium contrast agent, and provides additional information about directionality of flow, as well as detailed images of the surrounding soft tissues.
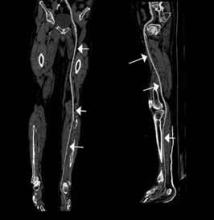
An emerging clinical situation in which contrast-enhanced cross-sectional imaging may supplant the need for arterial puncture and digital subtraction angiography is multiligament trauma to the knee. When significant kinetic force is applied to the knee, the joint is at risk for translocation and/or dislocation, with resultant injury to the surrounding soft-tissue envelope and potential trauma to the neurovascular structures around the knee. In this type of injury, cross-sectional imaging is routinely ordered to further evaluate and classify trauma, revealing potentially treatment-altering information concerning the integrity of the vascular structures with minimal risk to the patient (Figure 1).
The largest series of MRAs performed specifically in patients with knee dislocation reviewed 17 cases and found two cases of vascular pathology: one case of an intimal flap and one case of acute vasospasm. Digital subtraction angiography was performed on 6 of 17 cases and had 100% concordance with MRA findings.2 MRI is often performed in the setting of suspected multiligament knee injury to aid in preoperative planning, and the addition of MRA should be considered if trauma to the periarticular vasculature is suspected.
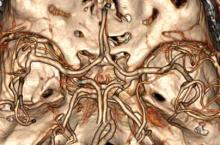
There is ample evidence that cross-sectional imaging performs well relative to conventional catheter angiography in the setting of peripheral vascular occlusion from atherosclerotic etiologies.3 Furthermore, there is precedence for utilizing contrast-enhanced CTA, as well as contrast-enhanced or three-dimensional time-of-flight MRA in other areas of the body—particularly the brain, head, and neck4 (Figure 2). Several MRA techniques are becoming available that will allow a high resolution angiographic without the use of contrast. Upcoming advances in CTA include dual-energy CT, which has the potential to allow angiography using very small amounts of contrast.
In general, when ordering cross-sectional imaging in the setting of trauma, consideration should be given to the potential for vascular compromise; thus, the addition of contrast-enhanced CTA or MRA can add valuable clinical information, with relatively little excess risk or time, sparing patients the risks of catheter-directed angiography.
References
1. Eisenberg, RL, Bank, WO, Hedgcock, MW. Neurologic complications of angiography for cerebrovascular disease. Neurology. 1980;30(8):895-897.
2. Potter HG, Weinstein M, Allen AA, Wickiewicz TL, Helfet DL. Magnetic resonance imaging of the multiple-ligament injured knee. J Orthop Trauma. 2002;16(5);330-339.
3. Chin AS, Rubin GD. CT angiography of peripheral arterial occlusive disease. Tech Vasc Interv Radiol. 2006;9(4):143-149.
4. Riles TS, Eidelman EM, Litt AW, Pinto RS, Oldford F, Schwartzenberg GW. Comparison of magnetic resonance angiography, conventional angiography, and duplex scanning. Stroke. 1992;23(3):341-346.
What’s Hip in 2013?
Roger J. Bartolotta, MD
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center.
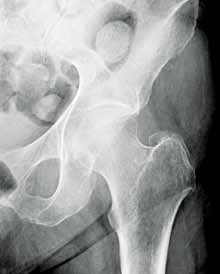
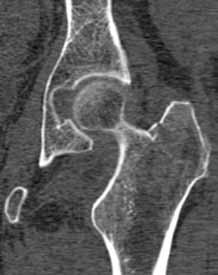
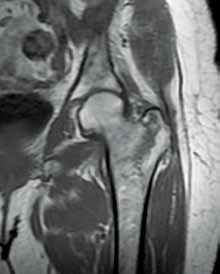
Post-traumatic hip pain is a common chief concern among ED patients. While routine radiography, the standard imaging modality for the initial evaluation of suspected hip fracture, detects most fractures, its sensitivity is decreased in the setting of osteoporosis—particularly with nondisplaced fractures. Thus, though elderly/osteoporotic patients are more likely to fracture, these fractures are harder to detect on radiography. In a study of 70 patients with negative radiographs but high clinical concern for fracture, magnetic resonance imaging (MRI) detected occult femoral fractures in 37% and occult pelvic fractures in 23%.1
Hip fractures in elderly patients are associated with substantial mortality and morbidity, the risks for which increase with delayed diagnosis.2 When there is high clinical suspicion for a radiographically occult hip fracture in this population, cross-sectional imaging should be considered for further evaluation (Figure). The decision of whether to use computed tomography (CT) or MRI for the cross-sectional examination must be made on both an institutional and patient-specific basis. CT is faster, less expensive, and more widely and temporally available. Although CT has increased sensitivity for fracture detection compared to radiography, studies have demonstrated false-negative CT examinations in the setting of nondisplaced proximal femoral fractures, especially in osteoporotic patients. Hakkarinen et al3 reported that among 235 hip fractures, 10% were occult radiographically; approximately 17% of these fractures (4 out of 24) were also occult on CT but visible on MRI. Moreover, while radiography and CT may demonstrate a seemingly isolated fracture at the femoral greater trochanter, a subset of these fractures exhibit intertrochanteric extension that is only evident on MRI (Figure). Isolated greater trochanteric fractures are typically treated conservatively, while some incomplete intertrochanteric fractures warrant internal fixation, especially fractures that cross the intertrochanteric midline on coronal MRI.4,5
|
|
| ||
Figure. Anteroposterior left hip radiograph (A) in a patient with hip pain (A) shows no definite evidence of fracture. Coronal reformatted image from left hip computed tomography (B) demonstrates nondisplaced, seemingly isolated fracture of the left femoral greater trochanter (white arrows). Coronal T1-weighted image from left hip magnetic resonance imaging (C) reveals intertrochanteric extension of the fracture spanning greater than 50% of the intertrochanteric diameter (red arrows). | ||||
In addition to improved fracture detection, MRI also provides superior evaluation of the underlying bone marrow for coexisting conditions, such as osteomyelitis, osteonecrosis, and primary or metastatic neoplasm in the setting of pathologic fracture. Additional benefits of MRI over radiography and CT include its lack of ionizing radiation and improved evaluation of adjacent soft tissue injuries, such as labral and/or musculotendinous tears.
MRI, however, does require a longer examination time in which the patient must remain still. This may be difficult for acutely post-traumatic patients, notably those with baseline dementia and/or claustrophobia. For patients in whom MRI is indicated (eg, patients who do not have an implantable device such as a cardiac pacemaker) and where it is institutionally available, the decision to utilize it over CT is largely rooted in health-care economics. MRI is more expensive than radiography and CT, and even in the largest medical centers, the examination requires substantially more time than CT, which inherently decreases patient throughput in the ED. Cannon et al6 present an evidence-based algorithm for patient stratification, in which patients at high-risk for osteoporosis and low-energy trauma should be considered for immediate MRI rather than CT. These risk factors optimize MRI utilization by selecting those patients with the greatest likelihood of nondisplaced, radiographically occult fracture.
References
1. Bogost GA, Lizerbram EK, Crues JV 3rd. MR imaging in evaluation of suspected hip fracture: frequency of unsuspected bone and soft-tissue injury. Radiology. 1995;197(1):263-267.
2. Zuckerman JD, Skovron ML, Koval KJ, Aharonoff G, Frankel VH. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77(10):1551-1556.
3. Hakkarinen DK, Banh KV, Hendey GW. Magnetic resonance imaging identifies occult hip fractures missed by 64-slice computed tomography. J Emerg Med. 2012;43(2):303-307.
4. Feldman F, Staron RB. MRI of seemingly isolated greater trochanteric fractures. AJR Am J Roentgenol. 2004;183(2):323-329.
5. Schultz E, Miller TT, Boruchov SD, Schmell EB, Toledano B. Incomplete intertrochanteric fractures: imaging features and clinical management. Radiology. 1999;211(1):237-240.
6. Cannon J, Silvestri S, Munro M. Imaging choices in occult hip fracture. J Emerg Med. 2009;37(2):144-152.
PE or Not PE: That Is the Question
Jessica Fisher, MD
Dr Fisher is an instructor of radiology at Weill Cornell Medical College in New York City.
Pulmonary embolism (PE) represents the third most common cause of death from cardiovascular disease after myocardial infarction and stroke.1 Given the potential for fatal outcome, prompt diagnosis and management are essential. To avoid overdiagnosis of PE and unnecessary treatment with anticoagulation therapy, diagnostic tests with both a high sensitivity and high specificity are essential.
Clinical stratification of patient risk for PE, in combination with D-dimer assays, is typically used to determine the need for imaging. Preliminary studies with chest X-ray are utilized to evaluate alternate causes of clinical symptoms but do not provide a definitive diagnosis. In the ED setting, advanced imaging options to detect PE include computed tomography angiography (CTA), ventilation perfusion scintigraphy (V/Q lung scan), and magnetic resonance angiography (MRA).
Computed Tomography Angiography
|
| ||
| Figure 1. Coronal (A) and axial (B) contrast-enhanced computed tomography angiography scans reveal pulmonary embolism (white arrows).. | |||
CTA has long been the standard technique for evaluating PE. In addition to reported sensitivities of 96% to 100% and specificities of 97% to 98% with multislice detectors,2 CTA also provides information on disease severity, such as clot burden, evidence of right heart strain, and the presence of pulmonary infarct (Figure 1). It also can reveal alternative etiology and diagnosis in negative cases (Figure 2).
CTA is readily available in today’s ED and can be performed quickly and efficiently. However, it does require intravenous (IV) contrast and emits a high-dose of radiation, which may be contraindicated in some patient populations (eg, patients with renal failure, allergy to contrast, and pregnant and pediatric patients). Also, to avoid degradation of images and accurately visualize peripheral branches, patient cooperation (ie, suspension of respiratory motion) during the examination is essential.
Ventilation Perfusion Scintigraphy
Before the advent of CTA, V/Q lung scan was the first-line imaging choice to assess for PE.3 This modality continues to have a role in modern practice, especially given its estimated 6-fold lower whole-body effective radiation dose compared to CTA.4 Current applications of this modality include patients with contraindications to IV contrast as well as pregnant and pediatric patients in whom perfusion-only studies should be used to significantly lower the radiation profile.
In patients with a clear chest X-ray, the negative predictive value of V/Q is not significantly different from CT.5 This suggests a growing role for V/Q scans in the younger, healthier patient population where limited/reduced exposure to radiation is particularly desired—eg, pediatric patients and women younger than age 20 years in whom there is an increased risk of malignancy associated with radiation exposure to the breast/chest area.6
The limitations of V/Q lung scan include a higher rate of indeterminate results compared to CTA (particularly in patients with an abnormal chest X-ray). Moreover, V/Q does not provide alternative diagnoses or qualify disease severity. Also, the longer imaging times compared with CTA make this modality impractical in critically ill patients.
Magnetic Resonance Angiography
MRA has emerged in recent years as a potential alternative to CTA. Since this modality does not use ionizing radiation or iodine-based contrast, it is a reasonable and appropriate option for pediatric patients and those with a contrast-dye allergy. Initial meta-analysis of MRA studies demonstrated sensitivity ranging from 77% to 100% and a specificity of 95% to 98%.7-9 However, subsequent studies have found unacceptably high rates of technically inadequate examinations resulting in nondiagnostic results.10 As progress to reduce respiratory and cardiac motion artifacts and improve spatial resolution continues, along with the growing availability of MRA in the ED, this modality may soon emerge as a useful diagnostic option.
Conclusion
New advances in the diagnosis of PE continue to emerge. Evidence suggests improved sensitivity of CTA with the use of dual-energy CT scanners.11 The recent advance of V/Q single photon emission CT has also shown promise in improving detection accuracy.12 Ongoing research will help continue to expand the emergency physician’s range of imaging choices in the diagnosis of PE.
References
1. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379(9828):1835-1846.
2. Burns SK, Haramati LB. Diagnostic imaging and risk stratification of patients with acute pulmonary embolism. Cardiol Rev. 2012;20(1):15-24.
3. Mos IC, Klok FA, Kroft LJ, de Roos A, Huisman MV. Imaging tests in the diagnosis of pulmonary embolism. Semin Respir Crit Care Med. 2012;33(2):138-143.
4. Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254-263.
5. Stein EG, Haramati LB, Chamarthy M, Sprayregen S, Davitt MM, Freeman LM. Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. Am J Roentgenol. 2010;194(2):392-397.
6. Hill DA, Preston-Martin S, Ross RK, Bernstein L. Medical radiation, family history of cancer, and benign breast disease in relation to breast cancer risk in young women, USA. Cancer Causes Control. 2002;13(8):711-718.
7. Oudkerk M, van Beek EJ, Wielopolski P, et al. Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet. 2002;359(9318):1643-1647.
8. Meaney JF, Weg JG, Chenevert TL, Stafford-Johnson D, Hamilton BH, Prince MR. Diagnosis of pulmonary embolism with magnetic resonance angiography. N Engl J Med 1997;336(20):1422-1427.
9. Gupta A, Frazer CK, Ferguson JM, et al. Acute pulmonary embolism: diagnosis with MR angiography. Radiology. 1999;210(2):353-359.
10. Stein PD, Chenevert TL, Fowler SE. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med. 2010;152(7):434-443, W142, W143.
11. Pontana F, Faivre JB, Remy-Jardin M. Lung perfusion with dual-energy multidetector-row CT (MDCT): feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad Radiol. 2008;15(12):1494-1504.
12. Gutte H, Mortensen J, Jensen CV, et al. Comparison of V/Q SPECT and planar V/Q lung scintigraphy in diagnosing acute pulmonary embolism. Nucl Med Commun. 2010;31(1):82-86.
Just MoRe Imaging?
MRI Evaluation of Acute Abdominal Pain
David A. Boyajian, MD
Dr Boyajian is clinical director of radiology at New York-Presbyterian/Lower Manhattan Hospital, and vice chairman and assistant professor of radiology, Department of Radiology, Weill Cornell Medical College, New York.
For the past few decades, the established cross-sectional imaging modalities used to evaluate acute abdominal pain in the ED have been computed tomography (CT) and ultrasound. Advantages of these modalities include the widespread availability of equipment and qualified technologists and radiologists, the ability to obtain images quickly, and relative cost-effectiveness. There are, however, several subsets of indications for which traditional cross-sectional imaging approaches are either undesirable or have a low sensitivity for accuracy (eg, suspected nonobstetric pathology in the pregnant patient, biliary ductal pathology).
Magnetic resonance imaging (MRI) has become more widely available to ED practices across the country and an increasingly important tool in the arsenal of the emergency radiologist. Advantages of MRI include better soft-tissue characterization, ability to directly image in any plane, and the lack of ionizing radiation.
There are, however, barriers to using MRI in the emergency setting, including availability and location of the MRI scanners, long examination time, difficulty in monitoring unstable patients, and higher cost.1 In addition, relative to CT and ultrasound, there is poorer spatial resolution and greater potential for artifacts. Advances in MRI technology and workflow, including rapidly acquired imaging sequences and active radiologist management of such cases, can significantly reduce examination acquisition time and improve image quality. Due to the significant advantages in visualizing the brain and spine, MRI has become the gold standard in many emergent conditions such as suspected stroke and spinal cord compression. Increasingly, MRI is being used to diagnosis causes of abdominal pain, examples of such are discussed below.
Appendicitis in Pregnancy
Although acute appendicitis is the most common nonobstetric surgical condition during pregnancy, several factors such as physiological leukocytosis and appendiceal displacement (which reduces the sensitivity of ultrasound) confound the diagnosis. Due to the radiation conveyed by CT, MRI is considered a more appropriate imaging modality in this patient population.2 In a retrospective study of 23,290 pregnant patients, Pedrosa et al3 reported a sensitivity of 100%, specificity of 93.6%, and accuracy of 94% in detecting acute appendicitis in patients for whom ultrasound was inconclusive.
Biliary Pathology
Biliary pathology, particularly choledocholithiasis, may not be demonstrated to advantage with cross-sectional imaging. For example, CT sensitivity for detection is decreased in the absence of ductal dilatation and/or in poorly mineralized stones. Technical factors, such as obesity, patient positioning, and bowel gas, limit evaluation with ultrasound. MRI is able to depict both the intrahepatic and extrahepatic biliary ducts and identify any intraductal lesions (eg, masses, stones). Special MRI contrast agents that are excreted through the bile ducts have come to market in recent years and can assist in evaluation for biliary pathology. The sequences have replaced many of the diagnostic endoscopic retrograde cholangiopancreatograms (ERCP) that used to be performed. An advantage of magnetic resonance cholangiopancreatography (MRCP) is that structures outside of the biliary system may also be evaluated (eg, liver, kidneys).
|
|
Conclusion
The use of MRI for evaluation of abdominal pain continues to increase. While currently limited to specific vulnerable populations (eg, pregnant patients) or for specific conditions (eg, biliary disease), improving technology and availability will allow this technique to expand to more patients and conditions, including evaluation for inflammatory bowel conditions.
References
1. Saini S, Seltzer SE, Bramson RT, et al. Technical cost of radiologic examinations: Analysis across imaging modalities. Radiology. 2000; 216(1):269-272.
2. Appropriateness criteria. American College of Radiology Web site. http://www.acr.org/Quality-Safety/Appropriateness-Criteria. Accessed September 17, 2013.
3. Pedrosa I, Levine D, Eyvazzadeh AD, Siewert B, Ngo L, Rofsky NM. MR imaging evaluation of acute appendicitis in pregnancy. Radiology. 2006;238(3):891-899.
When a Picture Is Worth a Thousand Images:
3D Reconstruction in Emergency and Trauma Imaging
Jamlik-Omari Johnson, MD; Waqas Shuaib, MD
Dr Johnson is assistant professor of radiology and division director of radiology in the department of radiology and imaging services, division of emergency medicine at Emory University School of Medicine, Atlanta, Georgia. Dr Shuaib is a research associate in the department of radiology and imaging services, division of emergency medicine at Emory University School of Medicine, Atlanta, Georgia.
Three-dimensional (3D) reconstruction creates a digital image of a real-life object, capturing both its original shape and appearance. These techniques simulate reality through the use of lighting, color, and motion.1 Advantages of 3D reconstruction in the emergency and trauma setting include enhanced views of anatomy, making pathology that may be difficult to see in the axial plane easily visible (eg, rib fractures). 3D images may also increase interpretation efficiency, allowing rapid review of the large data sets associated with multidetector computed tomography (MDCT) of trauma patients. Since these images can be attached to the radiology report, this modality also enhances service to referring physicians. In addition, as 3D images succinctly illustrate a diagnosis, the treating physician may share them with patients to demonstrate a condition and explain treatment recommendations.
MDCT technology evolved rapidly over the past 25 years. Between 1992 and 2004, MDCT made quantum leaps from dual detectors to 64-slice scanners, and over the last decade, dual-energy source technology and 128-slice scanners have become integrated into clinical practice. These newer devices, along with technical enhancements, provide high temporal and superior spatial resolution, considerably improved image quality, and expanded clinical applications. With MDCT, scans are performed quickly, resulting in improved temporal resolution and reduced motion artifacts. Leverage of this technology in the emergency and trauma setting has expanded the spectrum of indications for use and increased the utility of CT in urgent care. Newer algorithms allow rapid and detailed examinations of the musculoskeletal and the vascular systems in submillimeter slices, without limitation of scan volume. The high-resolution source images are the basis for high quality 3D reconstructions.
The 3D reconstruction toolbox may benefit the surveillance of anatomy, detection of pathology, and planning of therapy. Examples of three such commonly used techniques are shaded surface display (SSD), maximum intensity projection (MIP), and volume rendering (VR).2
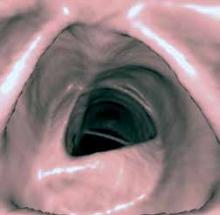
SSD provides a realistic 3D view of the surface of a structure within the acquired volume data set and is good at depicting aneurysms and other focal disease (Figure 1). This is particularly useful for evaluating long and tortuous structures, such as the arterial and venous structures. MIP is often used to detect lung nodules by making them stand-out from bronchi and vasculature (Figure 1). Adapted from the computer graphics field, VR creates a 2D image from a 3D model. VR is employed in the exploration of hollow structures, such as the airways or the colon (Figure 2).
Emergency and trauma environments are often fast-paced and high-stakes, and timely and accurate diagnosis is paramount. Therefore, to be useful, advanced 3D-processing tools should be embedded into the diagnostic viewing application (eg, PACS) to allow efficient reconstruction of images, simultaneous comparison of 2D and 3D images, and referencing of historical data in real time.
References
1. Blank M, Kalender WA. Medical volume exploration: gaining insights virtually. Eur J Radiol. 2000;33(3):161-169.
2. Philipp MO, Kubin K, Mang T, Hörmann M, Metz VM. Three-dimensional volume rendering of multidetector-row CT data: applicable for emergency radiology. Eur J Radiol. 2003;48(1):33-38.
After Imaging: Interventional Radiology in the ED
Joshua L. Weintraub, MD, and Thomas J. Ward, MD
Dr Weintraub is an interventional radiologist and executive vice chairman of the department of radiology
at New York Presbyterian/Columbia University Medical Center, New York. Dr Ward is a postgraduate fifth-year resident in interventional radiologist in the department of radiology at Mount Sinai Medical Center, New York, NY.
Interventional radiologists play an increasing role in treating patients in the ED setting. Simplistically, these roles are usually divided into one of three broad categories: to drain or aspirate infected fluid, to halt active bleeding, or to restore blood flow through an occluded vessel. While all of these procedures are critical, the newest advance in interventional radiology (IR) set to benefit the ED patient is not technical or procedural at all, but rather a philosophical one.
Fluid Aspiration and Drainage
In the ED, the need to drain or aspirate infected fluid is probably the most common indication for consult with an interventional radiologist. Perforated appendicitis or diverticulitis with an intra-abdominal abscess is generally managed with percutaneous drainage and antibiotics. If the clinical situation necessitates, and the location of the collection allows, this procedure may be performed at bedside with local anesthesia under ultrasound guidance. In the septic patient, the minimally invasive nature of this procedure confers significant benefits.
Nonoperative patients presenting with acute cholecystitis rapidly improve after the placement of a percutaneous cholecystostomy tube, and this procedure can be performed under ultrasound guidance. IR may also be indicated in cases of failed endoscopic retrograde cholangiopancreatography or cystoscopy in patients with cholangitis or urosepsis for benign or malignant obstruction. Percutaneous access is facilitated by a dilated system, and temporary decompression can be obtained until more definitive therapy is planned.
Active Bleeding
Active bleeding is another common presentation warranting an IR evaluation. Gastrointestinal, intracranial, posttraumatic, postsurgical, and postpartum bleeding, as well as massive hemoptysis, can all be managed endovascularly. Advances in microcatheter technology, covered vascular stents, and embolic agents have increased the efficacy of these interventions, and improved computed tomography angiography protocols facilitate accurate and timely diagnosis of active bleeding. With the availability of these techniques, waiting several hours for a tagged red blood cell nuclear scan is a thing of the past at many institutions. Embolization has become the mainstay of treating bleeding related to trauma in most major trauma centers.
Ischemia
An IR consult may be ordered for patients presenting with the sequelae of ischemia, which can range from a diabetic foot to an acute stroke. Percutaneous balloon angioplasty, endovascular stents, and catheter-directed thrombolysis were all monumental advances in the treatment of ischemia—conceptualized or introduced into clinical practice by interventional radiologists. This spirit of innovation continues. A variety of technical advances, atherectomy, and re-entry devices have been introduced to help recanalize chronically occluded vessels. New devices allow the interventional radiologist to quickly restore blood flow and function to patients suffering from cerebral embolus. There is increased interest in the use of catheter-assisted-embolectomy for submassive pulmonary embolism when intravenous fibrinolysis is unsuccessful or contraindicated.
Conclusion
The five decades of pioneering technical innovation highlighted in this article allow for minimally invasive treatment of the ED patient. The newest advance in IR is not technical, but rather philosophical, and a change in the role that the interventional radiologist plays. The American Board of Radiology has recently approved a new IR training pathway that more than doubles the amount of clinical training that graduating IR fellows now receive. This signals a renewed commitment to running a truly clinical service. Patients in the ED can be evaluated and treated by an interventional radiologist in the hospital and then discharged under the care of an interventional radiologist at an IR clinic. Several IR sections across the country currently practice in such a manner. Although this model is currently the exception and not the rule, the goal of the new training pathway hopes to change this, with increased advances and benefits to patients in the ED setting.
Suggested Reading
1. Gasior AC, Marty Knott E, Ostlie DJ, St Peter SD. To drain or not to drain: an analysis of abscess drains in the treatment of appendicitis with abscess. Pediatr Surg Int. 2013;29(5):455-458.
2. Sato KT. Percutaneous management of biliary emergencies. Semin Intervent Radiol. 2006;23(3):
249-257.
3. Funaki B. On-call treatment of acute gastrointestinal hemorrhage. Intervent Radiol. 2006;23(3):
215-222.
4. Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22(6):1395-1409.
5. Stead LG, Gilmore RM, Bellolio MF, Rabinstein AA, Decker WW. Percutaneous clot removal devices in acute ischemic stroke: a systematic review and meta-analysis. Arch Neurol. 2008;65(8):
1024-1030.
6. Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest. 2007;132(2):657-663.
7. Initial certification: vascular/interventional. American Board of Radiology Web site. http://www.theabr.org/ic-vir-landing. Accessed September 24, 2013.
Figure 2. Surface shaded display image of a virtual colonoscopy.
Evaluation of Chest Pain in the Emergency Department by Coronary CT Angiography
James K. Min, MD
Dr Min is director of the Institute of Cardiovascular Imaging at New York-Presbyterian Hospital/Weill-Cornell Medical College, New York.
In the United States each year, approximately 6 million patients present with complaints of chest pain suspicious for acute coronary syndromes (ACS), including unstable angina and myocardial infarction.1 Most of these patients are diagnosed with noncardiac conditions, and nearly half are due to noncardiac etiology. There are an array of diagnostic tests to identify and exclude patients with suspected ACS, such as medical history, cardiac enzyme measurements, electrocardiographic changes, and clinical risk scores. For those with nonnegligible risk for ACS for which this condition cannot be definitively diagnosed or excluded, many are often admitted to the hospital for further testing and observation. Among these patients, less than 30% are found to have ACS. And yet, despite these very careful clinical pathways, between 2% and 8% of patients with ACS are unknowingly discharged to home.2
In recent years, coronary computed tomography angiography (CCTA) has emerged as a noninvasive method that permits direct anatomic visualization of coronary atherosclerosis and luminal stenosis.3 Since the introduction of 64-multidetector row CT scanners in 2005, there have been significant advances in CT technology that now allow for reliable performance of CCTA with very low-dose radiation. Pertaining to the former, improvements in spatial resolution, temporal resolution, and volume coverage enable evaluation of coronary arteries at the submillimeter level, and can be performed in approximately 1 to 5 seconds. Concomitant to the progress in CT technology has been the parallel developments in radiation-dose reduction. As compared to the background radiation exposure of an individual living at sea level for 1 year (~3 millisieverts of radiation), current generation CCTA can be performed at doses <1 millisieverts, with doses approximating a screening mammogram now achievable.
|
|
| ||
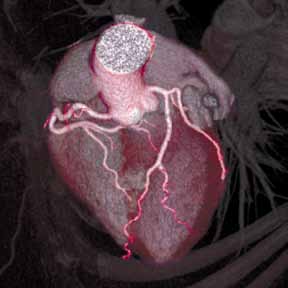
Figure. Volume-rendered coronary computed tomographic angiography showing the coronary vascular bed and myocardium (A); normal right coronary artery without evidence of atherosclerosis (B); and (C) left anterior descending artery with mild nonobstructive calcified plaque (white area). | ||||
The diagnostic accuracy of CCTA against invasive coronary angiography has been tested in several prospective multicenter trials.3 For patients without known but suspected coronary artery disease (CAD), the sensitivity and negative predictive value has ranged between 95% to 99%—that is, CCTA can exclude anatomically obstructive CAD with near 100% certainty (Figure). It is these diagnostic performance characteristics that have encouraged several investigators to evaluate the use of CCTA in the diagnostic algorithm of patients presenting to the ED with acute chest pain—the primary intent being to identify sufficiently low-risk patients without significant CAD who can be safely discharged home.
Since 2011, three prospective multicenter randomized controlled trials have evaluated the incorporation of CCTA into a diagnostic chest pain pathway, as compared to standard-of-care algorithms.4-6 Now comprising more than 3,000 patients, these trials have demonstrated remarkably consistent results, with a reduced time-to-diagnosis of 40% to 50%, reduced lengths of stay in the ED of 25%, and reduced ED costs of 20% to 40%. Importantly, these salient effects on resource utilization and economics were underscored by exceptionally safe outcomes, which never exceeded that of the standard of care. Several studies to date have subsequently assessed the duration of safety conferred by a normal CCTA, or its “warranty period.” These studies have shown the warranty period to last at least 7 years for major adverse cardiac events and mortality.7 Moreover, they also engender hope that the chest pain pathways used for millions of Americans annually can indeed be improved, with CCTA playing an essential role.
As its technology continues to iterate, the diagnostic and prognostic performance of CCTA will invariably continue to improve. However, even at present, CCTA is robust and more accurate for exclusion of CAD when compared to other traditional methods of evaluation, and its use for patients presenting to the ED with chest pain improves throughput, reduces costs, and maximizes patient safety.
References
1. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Surviva: 2006 emergency department summary. Natl Health Stat Report. 2008;7:1-38.
2. Pope JH, Aufderheidi TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163-1170.
3. Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography: a process in evolution. J Am Coll Cardiol. 2010;55(:957-965.
4. Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366(15):1393-403.
5. Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367(4):299-308.
6. Goldstein JA, Chinnaiyan KM, Abidov A, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58(14):1414-1422.
7. Andreini D, Pontone G, Mushtaq S, et al. A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc Imaging. 201
As EDs have evolved to handle the increasing volume and complexity of patients requiring immediate care, so too, has the field of emergency radiology. Many EDs across the country now have multiple advanced imaging modalities available 24 hours a day, including Xray, computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI). While many emergency medicine physicians are now trained in performing and evaluating ultrasound images—similar to X-rays in the past—most are less comfortable with CT and MRI.
Emergency radiology, now a recognized subspecialty of diagnostic imaging, has proliferated to meet the demands for immediate interpretation of these images. This combination of around-the-clock access to equipment and expertise has brought cutting-edge, advanced imaging to the front lines of emergency care. In this special feature, we invited a group of emergency radiologists and an expert in cardiovascular imaging to discuss the applicability and utility of several of these techniques in the ED setting.
As illustrated by this panel, advanced imaging has become increasingly valuable and available in providing care to ED patients. In this presentation, however, you will notice the conspicuous absence of specific techniques that are utilized in diagnosis and evaluation of stroke, head/spine trauma, and other neurologic conditions that are commonplace in the ED. Advanced imaging has become critical in these instances and will be discussed in a future article.
Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and the executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center. He is a member of the EMER GENCY MEDICINE editorial board.
Faster than FAST: Single Pass Whole-Body Computed Tomography for Rapid Evaluation of Trauma Patients
Ashwin Asrani, MD
Dr Asrani is an assistant professor of radiology at Weill Cornell Medical College in New York City and an
assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center in New York City.
As part of initial assessment and resuscitation in the ED, severely injured trauma patients frequently undergo a bedside focused abdominal sonography for trauma (FAST) scan to assess for intraperitoneal hemorrhage from an underlying major abdominal visceral injury, as well as the presence of pleural and/or pericardial effusion. However, the use and role of a FAST scan in hemodynamically stable patients has recently been questioned since, based on its relatively low sensitivity for visceral injury, many of these cases eventually require computed tomography (CT) (See Case). In addition, patients in this subset with a positive FAST scan frequently have subsequent CT to further assist clinicians in understanding the nature of injury and to guide management recommendations (eg, operative versus nonoperative options).1
Advances in the speed of CT have made it possible to obtain images of the entire body with only a single dose of contrast injection. The elimination of segmental acquisition of torso images (eg, head, neck, chest, abdomen) eliminates the need to reposition the patient, repeat scout images, and postpone the reconstruction of reformats and other special views until the end of the examination. Depending on the original protocol, this in turn minimizes patient time in the CT-scan room by approximately 31% to 42%,2,3 and significantly reduces radiation exposure by 17% compared to conventional segmental acquisition of different body parts.2
Evidence suggests that direct single-pass whole-body CT improves outcomes in both hemodynamically stable and unstable patients and can reveal significant findings not apparent on initial clinical examination.4,5 Whole-body CT increases injury severity by detecting lesions that would not otherwise have been detected by conventional methods. While this information does not affect treatment options, it does artificially lower the ratio of observed-to-expected deaths.6 The Randomized Study of Early Assessment by CT Scanning in Trauma Patients (REACT-2), a recent international multicenter randomized clinical trial, is expected to provide evidence supporting the value of immediate total-body CT scanning during the primary evaluation of severely injured trauma patients; the results of this trial should become available in 2014.7
As EDs install and upgrade to newer multidetector CT scanners, single-pass whole-body scanning has the potential to save time in situations when it really matters.
References
1. Natarajan B, Gupta PK, Cemaj S, Sorensen M, Hatzoudis GI, Forse RA. FAST scan: Is it worth doing in hemodynamically stable blunt trauma patients? Surgery. 2010;148(4):695-700; discussion 700-701.
2. Fanucci E, Fiaschetti V, Rotili A, Floris R, Simonetti G. Whole body 16-row multislice CT in emergency room: Effects of different protocols on scanning time, image quality and radiation exposure. Emerg Radiol. 2007;13(5):251-257.
3. Nguyen D, Platon A, Shanmuganathan K, Mirvis SE, Becker CD, Poletti PA. Evaluation of a single-pass continuous whole-body 16-MDCT protocol for patients with polytrauma. AJR Am J Roentgenol. 2009;192(1):3-10.
4. Huber-Wagner S, Biberthaler P, Haberle S, et al. Whole-body CT in haemodynamically unstable severely injured patients – A retrospective, multicentre study. PLoS One. 2013;8(7):e68880.
5. Salim A, Sangthong B, Martin M, Brown C, Plurad D, Demetriades D. Whole body imaging in blunt multisystem trauma patients without obvious signs of injury: Results of a prospective study. Arch Surg. 2006;141(5):468-473; discussion 473-475.
6. Stengel D, Frank M, Matthes G, et al. Primary pan-computed tomography for blunt multiple trauma: Can the whole be better than its parts? Injury. 2009;40 Suppl 4:S36-S46.
7. Sierink JC, Saltzherr TP, Beenen LF, et al. A multicenter, randomized controlled trial of immediate total-body CT scanning in trauma patients (REACT-2). BMC Emerg Med. 2012;12:4-227X-12-4.
Obstructed Views: Imaging in Urinary Tract Obstruction
Lily M. Belfi, MD
Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center.
Urinary tract (UT) obstruction is one of the most common indications for genitourinary (GU) imaging in the ED. Until recently, diagnosis was made using intravenous (IV) pyelography. Today, improved imaging modalities, such as noncontrast computed tomography (CT), computed tomography urography (CTU), magnetic resonance urography (MRU), and magnetic resonance imaging (MRI), are available to better visualize and evaluate the underlying causes of obstruction.
Noncontrast Computed Tomography
|
|
Noncontrast CT of the abdomen and pelvis is the imaging study of choice in evaluating acute GU obstruction, particularly when there is clinical suspicion of stone disease. CT is extremely sensitive and specific in detecting renal calculi and provides information about stone burden, size, location, and composition. In addition to renal calculi, noncontrast CT is also useful in identifying other, less common causes of UT obstruction such as ureteral herniation, which can occur in the inguinal, femoral, or sciatic region and result in acute obstruction. Patients with neuromuscular disorders that cause piriformis muscle atrophy (eg, multiple sclerosis) may be predisposed to ureteral sciatic herniation. CT reconstructions in the coronal plane are useful in detecting this condition (Figure 1).
|
|
Reconstructed CT images can also reveal underlying congenital abnormalities that make a patient susceptible to obstruction. For example, CT can reveal the presence of a duplicated collecting system, which is often accompanied by an obstructing ureterocele associated with the upper pole moiety (Figure 2).
Computed Tomography Urography
|
| ||
| Figure 3. Computed tomography urography images (A and B) reveal "fish-hook" ureter (white arrows) classically seen in patients with with retrocaval ureter. | |||
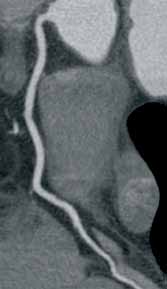
Other types of congenital anomalies may require additional imaging with CTU, in which IV iodinated contrast media is administered for increased sensitivity and visualization. For instance, patients with a retrocaval ureter often present with GU obstruction and hematuria; the classic “fish-hook” or “sickle-shaped” deformity of the ureter characterizing this disease is best visualized on CTU as the ureter is well-opacified by excreted contrast (Figure 3).
Magnetic Resonance Urography and Magnetic Resonance Imaging
MRU and MRI are alternative modalities to CTU for patients in whom iodinated CT contrast is contraindicated or in cases where the avoidance of radiation exposure is indicated (eg, pregnant and pediatric patients). MRI and MRU are especially useful in further characterizing obstructive lesions in the GU tract. While initial noncontrast CT may suggest soft-tissue lesions within the ureter or urinary bladder, follow-up MRI clearly delineates the condition, helping clinicians quantify the extent of tumor involvement and determine disease stage (Figure 4). Pelvic MRI is also an essential tool in identifying causes of bladder outlet obstruction, especially in those involving the prostate gland (Figure 5).
|
|
|
|
|
Figure 4. Noncontrast pelvic computed tomography images (A and B) of a 72-year-old man with flank pain and difficulty voiding reveal hydronephrosis (white arrow) and bladder mass (red arrow). Magnetic resonance urography (C) further visualizes the bladder mass (red arrow), and postcontrast magnetic resonance images (D and E) show the extent of the tumor (red arrow) as well as a metastatic lesion (white arrow).
| ||||
Conclusion
|
|
| |||
| Figure 5. Noncontrast pelvic computed tomography images (A and B) of a 73-yearold man with pelvic pain demonstrate bilateral hydronephrosis. (white arrows) and a large mass in the expected location of the prostate (red arrow). Further evaluation of the large heterogenous mass with pelvic magnetic resonance imaging (C) reveals prostate carcinoma (red arrows). | |||||
Based on its sensitivity in detecting both common and uncommon causes of obstruction, noncontrast abdominal and pelvic CT is an excellent first-line imaging choice for evaluating patients presenting to the ED with UT obstruction. Moreover, noncontrast CT also helps guide clinicians in determining which, if any, additional studies with CTU, MRU, and/or MRI are warranted.
Sound Advice: Ultrasound in the Emergency Department
Kemi Babagbemi, MD
Dr Babagbemi is an assistant professor of radiology and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
In today’s evolving health-care environment, emphasis must be placed on providing accurate, safe, cost-effective, and timely diagnosis to the wide range of patients that present to the ED. To meet this need, emergency medicine physicians and radiologists have universally embraced the use of ultrasound in this setting.
Ultrasound is less expensive than computed tomography (CT) and magnetic resonance imaging (MRI), and its portability allows for bedside application, enabling its use in the most critical patients. It does not require extended preparation (eg, oral contrast) or carry the risk for adverse reactions associated with intravenously administered contrast. Also, since ultrasound does not use ionizing radiation, it is a particularly appropriate imaging option in susceptible populations such as pregnant and pediatric patients.1 Although a noninvasive modality, it can also be used to guide interventional procedures, including vascular access, thoracentesis/paracentesis, and specialized anesthesia.
Emergency physicians routinely perform ultrasound at bedside and are familiar with its utility in evaluating abdominal trauma, right upper quadrant pain, and acute biliary abnormalities; assessing vascular thrombosis or injury; and determining the etiology of pelvic pain and bleeding in both pregnant and nonpregnant patients. However, ultrasound is also increasingly being utilized to
diagnose other conditions for which CT and MRI were once thought the superior diagnostic tool.
Trauma (The Extended FAST Scan)
The focused abdominal sonography for trauma (FAST) scan has been used for rapid and immediate assessment of unstable trauma patients with suspected abdominal injury. In a recent study of 4,029 patients with blunt abdominal trauma, FAST scans had a sensitivity, specificity, and accuracy for detection of hemorrhage in patients with hypotension of 85%, 60%, and 77%, respectively.2 Other studies suggest that sonography of the chest may be useful in the rapid detection of additional life-threatening pathology such as pneumothorax (Figure 1), with data suggesting improved sensitivity over radiography.3 The inclusion of the pleural space in evaluation is increasingly more common and is referred to as the extended FAST (EFAST).
Appendicitis
The most common cause of abdominal pain requiring surgical intervention is appendicitis.4 Although certain clinically based prediction scores (eg, Alvarado scores) may be used, imaging is considered far superior in accurately diagnosing the condition.5 In a meta-analysis of data from 26 ultrasound and CT studies (15 prospective, 11 retrospective), there was a pooled 88% sensitivity and 94% specificity for ultrasound compared with CT, which exhibited a pooled sensitivity of 94% and specificity of 95%.6 As previously noted, because there is no ionizing radiation in ultrasound, it should be the preferred modality in both children and first-trimester pregnant patients.
Musculoskeletal Trauma
Ultrasound is an ideal imaging modality to evaluate the musculoskeletal system. Despite its widespread use in Europe for many years, musculoskeletal sonography is only now beginning to be adopted in the United States. Its ability to visualize soft-tissue structures makes it effective in evaluating for muscle, tendon, or ligament injury, and can even do so dynamically with stress maneuvers (Figure 2). With respect to fractures, sonography has also proved effective in evaluating for cortical disruption. For example, a recent study demonstrated overall 92% sensitivity and 100% sensitivity for fractures with high potential for complication in radiographically occult scaphoid fractures.7
Conclusion
As a true point-of-care imaging modality, ultrasound has an established place in the practice of emergency medicine. Future improvements in technology, including the ability to obtain true three-dimensional volumetric data sets, will expand its role even further.
References
1. Image Gently and Ultrasound. Image Gently Campaign. The Alliance for Radiation Safety in Pediatric Imaging Web site. http://www.pedrad.org/associations/5364/ig/?page=787. Accessed September 19, 2013.
2. Lee BC, Ormsby EL, McGahan JP, Melendres GM, Richards JR. The utility of sonography for the triage of blunt abdominal trauma patients to exploratory laparotomy. AJR Am J Roentgenol. 2007;188(2):415-421.
3. Nandipati KC, Allamaneni S, Kakarla, et al. Extended focused assessment with sonography for trauma (EFAST) in the diagnosis of pneumothorax: experience at a community based level I trauma center. Injury. 2011;42(5):511-514.
4. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132(5):910-925.
5. Sun JS, Noh HW, Min YG, et al. Receiver operating characteristic analysis of the diagnostic performance of a computed tomographic examination and the Alvarado score for diagnosing acute appendicitis: emphasis on age and sex of the patients.
J Comput Assist Tomogr. 2008;32(3):386-391.
6. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for Diagnosis of Appendicitis in Children and Adults? A MetaAnalysis. Radiology. 2006;241(1):83-94.
7. Platon A, Poletti PA, Van Aaken J, Fusetti C, Della Santa D, Beaulieu JY, Becker CD. Occult fractures of the scaphoid: the role of Ultrasonography in the emergency department. Skeletal Radiol. 2011;40(7):869-875.
Stuck or Not? Noninvasive Vascular Imaging in the Emergency Setting
Michael L. Loftus, MD
Dr Loftus is assistant professor of radiology at New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
Conventional catheter-directed angiography has played an important role in the history of ED imaging, providing timely information about vessel integrity throughout the body and guiding potentially life-saving interventions. However, this imaging modality carries significant potential risks, including puncture-site hematoma or pseudoaneurysm, catheter-induced vasospasm, vascular occlusion or dissection, anesthesia-associated risks, and neurological deterioration or stroke.1 Moreover, emergent angiography is not universally available in all EDs.
Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) have opened new windows of opportunity for noninvasive vascular imaging to play a role in clinical decision-making in the ED. Cross-sectional angiography has virtually eliminated the need for acute catheter-directed angiography in several clinical settings, including pulmonary angiography, and the clinical applicability of CTA and MRA continues to expand as imaging techniques improve and achieve widespread acceptance and implementation. Multidetector CT is now widely available in most EDs, and the
accessibility of magnetic resonance imaging and MRA is expanding rapidly.
CTA allows evaluation of the vasculature on contrast-enhanced axial source images and also utilizes computer-generated maximum intensity projection reformations to create diagnostic images of the vascular region of interest. Similarly, MRA can be performed either with or without the administration of an intravenous gadolinium contrast agent, and provides additional information about directionality of flow, as well as detailed images of the surrounding soft tissues.
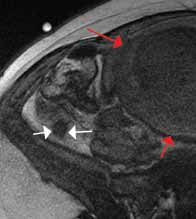
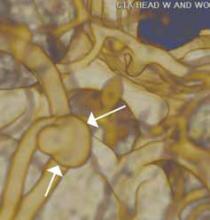
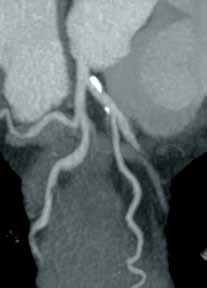
An emerging clinical situation in which contrast-enhanced cross-sectional imaging may supplant the need for arterial puncture and digital subtraction angiography is multiligament trauma to the knee. When significant kinetic force is applied to the knee, the joint is at risk for translocation and/or dislocation, with resultant injury to the surrounding soft-tissue envelope and potential trauma to the neurovascular structures around the knee. In this type of injury, cross-sectional imaging is routinely ordered to further evaluate and classify trauma, revealing potentially treatment-altering information concerning the integrity of the vascular structures with minimal risk to the patient (Figure 1).
The largest series of MRAs performed specifically in patients with knee dislocation reviewed 17 cases and found two cases of vascular pathology: one case of an intimal flap and one case of acute vasospasm. Digital subtraction angiography was performed on 6 of 17 cases and had 100% concordance with MRA findings.2 MRI is often performed in the setting of suspected multiligament knee injury to aid in preoperative planning, and the addition of MRA should be considered if trauma to the periarticular vasculature is suspected.
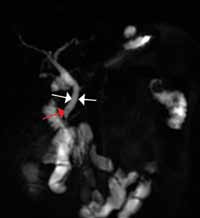
There is ample evidence that cross-sectional imaging performs well relative to conventional catheter angiography in the setting of peripheral vascular occlusion from atherosclerotic etiologies.3 Furthermore, there is precedence for utilizing contrast-enhanced CTA, as well as contrast-enhanced or three-dimensional time-of-flight MRA in other areas of the body—particularly the brain, head, and neck4 (Figure 2). Several MRA techniques are becoming available that will allow a high resolution angiographic without the use of contrast. Upcoming advances in CTA include dual-energy CT, which has the potential to allow angiography using very small amounts of contrast.
In general, when ordering cross-sectional imaging in the setting of trauma, consideration should be given to the potential for vascular compromise; thus, the addition of contrast-enhanced CTA or MRA can add valuable clinical information, with relatively little excess risk or time, sparing patients the risks of catheter-directed angiography.
References
1. Eisenberg, RL, Bank, WO, Hedgcock, MW. Neurologic complications of angiography for cerebrovascular disease. Neurology. 1980;30(8):895-897.
2. Potter HG, Weinstein M, Allen AA, Wickiewicz TL, Helfet DL. Magnetic resonance imaging of the multiple-ligament injured knee. J Orthop Trauma. 2002;16(5);330-339.
3. Chin AS, Rubin GD. CT angiography of peripheral arterial occlusive disease. Tech Vasc Interv Radiol. 2006;9(4):143-149.
4. Riles TS, Eidelman EM, Litt AW, Pinto RS, Oldford F, Schwartzenberg GW. Comparison of magnetic resonance angiography, conventional angiography, and duplex scanning. Stroke. 1992;23(3):341-346.
What’s Hip in 2013?
Roger J. Bartolotta, MD
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center.
Post-traumatic hip pain is a common chief concern among ED patients. While routine radiography, the standard imaging modality for the initial evaluation of suspected hip fracture, detects most fractures, its sensitivity is decreased in the setting of osteoporosis—particularly with nondisplaced fractures. Thus, though elderly/osteoporotic patients are more likely to fracture, these fractures are harder to detect on radiography. In a study of 70 patients with negative radiographs but high clinical concern for fracture, magnetic resonance imaging (MRI) detected occult femoral fractures in 37% and occult pelvic fractures in 23%.1
Hip fractures in elderly patients are associated with substantial mortality and morbidity, the risks for which increase with delayed diagnosis.2 When there is high clinical suspicion for a radiographically occult hip fracture in this population, cross-sectional imaging should be considered for further evaluation (Figure). The decision of whether to use computed tomography (CT) or MRI for the cross-sectional examination must be made on both an institutional and patient-specific basis. CT is faster, less expensive, and more widely and temporally available. Although CT has increased sensitivity for fracture detection compared to radiography, studies have demonstrated false-negative CT examinations in the setting of nondisplaced proximal femoral fractures, especially in osteoporotic patients. Hakkarinen et al3 reported that among 235 hip fractures, 10% were occult radiographically; approximately 17% of these fractures (4 out of 24) were also occult on CT but visible on MRI. Moreover, while radiography and CT may demonstrate a seemingly isolated fracture at the femoral greater trochanter, a subset of these fractures exhibit intertrochanteric extension that is only evident on MRI (Figure). Isolated greater trochanteric fractures are typically treated conservatively, while some incomplete intertrochanteric fractures warrant internal fixation, especially fractures that cross the intertrochanteric midline on coronal MRI.4,5
|
|
| ||
Figure. Anteroposterior left hip radiograph (A) in a patient with hip pain (A) shows no definite evidence of fracture. Coronal reformatted image from left hip computed tomography (B) demonstrates nondisplaced, seemingly isolated fracture of the left femoral greater trochanter (white arrows). Coronal T1-weighted image from left hip magnetic resonance imaging (C) reveals intertrochanteric extension of the fracture spanning greater than 50% of the intertrochanteric diameter (red arrows). | ||||
In addition to improved fracture detection, MRI also provides superior evaluation of the underlying bone marrow for coexisting conditions, such as osteomyelitis, osteonecrosis, and primary or metastatic neoplasm in the setting of pathologic fracture. Additional benefits of MRI over radiography and CT include its lack of ionizing radiation and improved evaluation of adjacent soft tissue injuries, such as labral and/or musculotendinous tears.
MRI, however, does require a longer examination time in which the patient must remain still. This may be difficult for acutely post-traumatic patients, notably those with baseline dementia and/or claustrophobia. For patients in whom MRI is indicated (eg, patients who do not have an implantable device such as a cardiac pacemaker) and where it is institutionally available, the decision to utilize it over CT is largely rooted in health-care economics. MRI is more expensive than radiography and CT, and even in the largest medical centers, the examination requires substantially more time than CT, which inherently decreases patient throughput in the ED. Cannon et al6 present an evidence-based algorithm for patient stratification, in which patients at high-risk for osteoporosis and low-energy trauma should be considered for immediate MRI rather than CT. These risk factors optimize MRI utilization by selecting those patients with the greatest likelihood of nondisplaced, radiographically occult fracture.
References
1. Bogost GA, Lizerbram EK, Crues JV 3rd. MR imaging in evaluation of suspected hip fracture: frequency of unsuspected bone and soft-tissue injury. Radiology. 1995;197(1):263-267.
2. Zuckerman JD, Skovron ML, Koval KJ, Aharonoff G, Frankel VH. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77(10):1551-1556.
3. Hakkarinen DK, Banh KV, Hendey GW. Magnetic resonance imaging identifies occult hip fractures missed by 64-slice computed tomography. J Emerg Med. 2012;43(2):303-307.
4. Feldman F, Staron RB. MRI of seemingly isolated greater trochanteric fractures. AJR Am J Roentgenol. 2004;183(2):323-329.
5. Schultz E, Miller TT, Boruchov SD, Schmell EB, Toledano B. Incomplete intertrochanteric fractures: imaging features and clinical management. Radiology. 1999;211(1):237-240.
6. Cannon J, Silvestri S, Munro M. Imaging choices in occult hip fracture. J Emerg Med. 2009;37(2):144-152.
PE or Not PE: That Is the Question
Jessica Fisher, MD
Dr Fisher is an instructor of radiology at Weill Cornell Medical College in New York City.
Pulmonary embolism (PE) represents the third most common cause of death from cardiovascular disease after myocardial infarction and stroke.1 Given the potential for fatal outcome, prompt diagnosis and management are essential. To avoid overdiagnosis of PE and unnecessary treatment with anticoagulation therapy, diagnostic tests with both a high sensitivity and high specificity are essential.
Clinical stratification of patient risk for PE, in combination with D-dimer assays, is typically used to determine the need for imaging. Preliminary studies with chest X-ray are utilized to evaluate alternate causes of clinical symptoms but do not provide a definitive diagnosis. In the ED setting, advanced imaging options to detect PE include computed tomography angiography (CTA), ventilation perfusion scintigraphy (V/Q lung scan), and magnetic resonance angiography (MRA).
Computed Tomography Angiography
|
| ||
| Figure 1. Coronal (A) and axial (B) contrast-enhanced computed tomography angiography scans reveal pulmonary embolism (white arrows).. | |||
CTA has long been the standard technique for evaluating PE. In addition to reported sensitivities of 96% to 100% and specificities of 97% to 98% with multislice detectors,2 CTA also provides information on disease severity, such as clot burden, evidence of right heart strain, and the presence of pulmonary infarct (Figure 1). It also can reveal alternative etiology and diagnosis in negative cases (Figure 2).
CTA is readily available in today’s ED and can be performed quickly and efficiently. However, it does require intravenous (IV) contrast and emits a high-dose of radiation, which may be contraindicated in some patient populations (eg, patients with renal failure, allergy to contrast, and pregnant and pediatric patients). Also, to avoid degradation of images and accurately visualize peripheral branches, patient cooperation (ie, suspension of respiratory motion) during the examination is essential.
Ventilation Perfusion Scintigraphy
Before the advent of CTA, V/Q lung scan was the first-line imaging choice to assess for PE.3 This modality continues to have a role in modern practice, especially given its estimated 6-fold lower whole-body effective radiation dose compared to CTA.4 Current applications of this modality include patients with contraindications to IV contrast as well as pregnant and pediatric patients in whom perfusion-only studies should be used to significantly lower the radiation profile.
In patients with a clear chest X-ray, the negative predictive value of V/Q is not significantly different from CT.5 This suggests a growing role for V/Q scans in the younger, healthier patient population where limited/reduced exposure to radiation is particularly desired—eg, pediatric patients and women younger than age 20 years in whom there is an increased risk of malignancy associated with radiation exposure to the breast/chest area.6
The limitations of V/Q lung scan include a higher rate of indeterminate results compared to CTA (particularly in patients with an abnormal chest X-ray). Moreover, V/Q does not provide alternative diagnoses or qualify disease severity. Also, the longer imaging times compared with CTA make this modality impractical in critically ill patients.
Magnetic Resonance Angiography
MRA has emerged in recent years as a potential alternative to CTA. Since this modality does not use ionizing radiation or iodine-based contrast, it is a reasonable and appropriate option for pediatric patients and those with a contrast-dye allergy. Initial meta-analysis of MRA studies demonstrated sensitivity ranging from 77% to 100% and a specificity of 95% to 98%.7-9 However, subsequent studies have found unacceptably high rates of technically inadequate examinations resulting in nondiagnostic results.10 As progress to reduce respiratory and cardiac motion artifacts and improve spatial resolution continues, along with the growing availability of MRA in the ED, this modality may soon emerge as a useful diagnostic option.
Conclusion
New advances in the diagnosis of PE continue to emerge. Evidence suggests improved sensitivity of CTA with the use of dual-energy CT scanners.11 The recent advance of V/Q single photon emission CT has also shown promise in improving detection accuracy.12 Ongoing research will help continue to expand the emergency physician’s range of imaging choices in the diagnosis of PE.
References
1. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379(9828):1835-1846.
2. Burns SK, Haramati LB. Diagnostic imaging and risk stratification of patients with acute pulmonary embolism. Cardiol Rev. 2012;20(1):15-24.
3. Mos IC, Klok FA, Kroft LJ, de Roos A, Huisman MV. Imaging tests in the diagnosis of pulmonary embolism. Semin Respir Crit Care Med. 2012;33(2):138-143.
4. Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254-263.
5. Stein EG, Haramati LB, Chamarthy M, Sprayregen S, Davitt MM, Freeman LM. Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. Am J Roentgenol. 2010;194(2):392-397.
6. Hill DA, Preston-Martin S, Ross RK, Bernstein L. Medical radiation, family history of cancer, and benign breast disease in relation to breast cancer risk in young women, USA. Cancer Causes Control. 2002;13(8):711-718.
7. Oudkerk M, van Beek EJ, Wielopolski P, et al. Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet. 2002;359(9318):1643-1647.
8. Meaney JF, Weg JG, Chenevert TL, Stafford-Johnson D, Hamilton BH, Prince MR. Diagnosis of pulmonary embolism with magnetic resonance angiography. N Engl J Med 1997;336(20):1422-1427.
9. Gupta A, Frazer CK, Ferguson JM, et al. Acute pulmonary embolism: diagnosis with MR angiography. Radiology. 1999;210(2):353-359.
10. Stein PD, Chenevert TL, Fowler SE. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med. 2010;152(7):434-443, W142, W143.
11. Pontana F, Faivre JB, Remy-Jardin M. Lung perfusion with dual-energy multidetector-row CT (MDCT): feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad Radiol. 2008;15(12):1494-1504.
12. Gutte H, Mortensen J, Jensen CV, et al. Comparison of V/Q SPECT and planar V/Q lung scintigraphy in diagnosing acute pulmonary embolism. Nucl Med Commun. 2010;31(1):82-86.
Just MoRe Imaging?
MRI Evaluation of Acute Abdominal Pain
David A. Boyajian, MD
Dr Boyajian is clinical director of radiology at New York-Presbyterian/Lower Manhattan Hospital, and vice chairman and assistant professor of radiology, Department of Radiology, Weill Cornell Medical College, New York.
For the past few decades, the established cross-sectional imaging modalities used to evaluate acute abdominal pain in the ED have been computed tomography (CT) and ultrasound. Advantages of these modalities include the widespread availability of equipment and qualified technologists and radiologists, the ability to obtain images quickly, and relative cost-effectiveness. There are, however, several subsets of indications for which traditional cross-sectional imaging approaches are either undesirable or have a low sensitivity for accuracy (eg, suspected nonobstetric pathology in the pregnant patient, biliary ductal pathology).
Magnetic resonance imaging (MRI) has become more widely available to ED practices across the country and an increasingly important tool in the arsenal of the emergency radiologist. Advantages of MRI include better soft-tissue characterization, ability to directly image in any plane, and the lack of ionizing radiation.
There are, however, barriers to using MRI in the emergency setting, including availability and location of the MRI scanners, long examination time, difficulty in monitoring unstable patients, and higher cost.1 In addition, relative to CT and ultrasound, there is poorer spatial resolution and greater potential for artifacts. Advances in MRI technology and workflow, including rapidly acquired imaging sequences and active radiologist management of such cases, can significantly reduce examination acquisition time and improve image quality. Due to the significant advantages in visualizing the brain and spine, MRI has become the gold standard in many emergent conditions such as suspected stroke and spinal cord compression. Increasingly, MRI is being used to diagnosis causes of abdominal pain, examples of such are discussed below.
Appendicitis in Pregnancy
Although acute appendicitis is the most common nonobstetric surgical condition during pregnancy, several factors such as physiological leukocytosis and appendiceal displacement (which reduces the sensitivity of ultrasound) confound the diagnosis. Due to the radiation conveyed by CT, MRI is considered a more appropriate imaging modality in this patient population.2 In a retrospective study of 23,290 pregnant patients, Pedrosa et al3 reported a sensitivity of 100%, specificity of 93.6%, and accuracy of 94% in detecting acute appendicitis in patients for whom ultrasound was inconclusive.
Biliary Pathology
Biliary pathology, particularly choledocholithiasis, may not be demonstrated to advantage with cross-sectional imaging. For example, CT sensitivity for detection is decreased in the absence of ductal dilatation and/or in poorly mineralized stones. Technical factors, such as obesity, patient positioning, and bowel gas, limit evaluation with ultrasound. MRI is able to depict both the intrahepatic and extrahepatic biliary ducts and identify any intraductal lesions (eg, masses, stones). Special MRI contrast agents that are excreted through the bile ducts have come to market in recent years and can assist in evaluation for biliary pathology. The sequences have replaced many of the diagnostic endoscopic retrograde cholangiopancreatograms (ERCP) that used to be performed. An advantage of magnetic resonance cholangiopancreatography (MRCP) is that structures outside of the biliary system may also be evaluated (eg, liver, kidneys).
|
|
Conclusion
The use of MRI for evaluation of abdominal pain continues to increase. While currently limited to specific vulnerable populations (eg, pregnant patients) or for specific conditions (eg, biliary disease), improving technology and availability will allow this technique to expand to more patients and conditions, including evaluation for inflammatory bowel conditions.
References
1. Saini S, Seltzer SE, Bramson RT, et al. Technical cost of radiologic examinations: Analysis across imaging modalities. Radiology. 2000; 216(1):269-272.
2. Appropriateness criteria. American College of Radiology Web site. http://www.acr.org/Quality-Safety/Appropriateness-Criteria. Accessed September 17, 2013.
3. Pedrosa I, Levine D, Eyvazzadeh AD, Siewert B, Ngo L, Rofsky NM. MR imaging evaluation of acute appendicitis in pregnancy. Radiology. 2006;238(3):891-899.
When a Picture Is Worth a Thousand Images:
3D Reconstruction in Emergency and Trauma Imaging
Jamlik-Omari Johnson, MD; Waqas Shuaib, MD
Dr Johnson is assistant professor of radiology and division director of radiology in the department of radiology and imaging services, division of emergency medicine at Emory University School of Medicine, Atlanta, Georgia. Dr Shuaib is a research associate in the department of radiology and imaging services, division of emergency medicine at Emory University School of Medicine, Atlanta, Georgia.
Three-dimensional (3D) reconstruction creates a digital image of a real-life object, capturing both its original shape and appearance. These techniques simulate reality through the use of lighting, color, and motion.1 Advantages of 3D reconstruction in the emergency and trauma setting include enhanced views of anatomy, making pathology that may be difficult to see in the axial plane easily visible (eg, rib fractures). 3D images may also increase interpretation efficiency, allowing rapid review of the large data sets associated with multidetector computed tomography (MDCT) of trauma patients. Since these images can be attached to the radiology report, this modality also enhances service to referring physicians. In addition, as 3D images succinctly illustrate a diagnosis, the treating physician may share them with patients to demonstrate a condition and explain treatment recommendations.
MDCT technology evolved rapidly over the past 25 years. Between 1992 and 2004, MDCT made quantum leaps from dual detectors to 64-slice scanners, and over the last decade, dual-energy source technology and 128-slice scanners have become integrated into clinical practice. These newer devices, along with technical enhancements, provide high temporal and superior spatial resolution, considerably improved image quality, and expanded clinical applications. With MDCT, scans are performed quickly, resulting in improved temporal resolution and reduced motion artifacts. Leverage of this technology in the emergency and trauma setting has expanded the spectrum of indications for use and increased the utility of CT in urgent care. Newer algorithms allow rapid and detailed examinations of the musculoskeletal and the vascular systems in submillimeter slices, without limitation of scan volume. The high-resolution source images are the basis for high quality 3D reconstructions.
The 3D reconstruction toolbox may benefit the surveillance of anatomy, detection of pathology, and planning of therapy. Examples of three such commonly used techniques are shaded surface display (SSD), maximum intensity projection (MIP), and volume rendering (VR).2
SSD provides a realistic 3D view of the surface of a structure within the acquired volume data set and is good at depicting aneurysms and other focal disease (Figure 1). This is particularly useful for evaluating long and tortuous structures, such as the arterial and venous structures. MIP is often used to detect lung nodules by making them stand-out from bronchi and vasculature (Figure 1). Adapted from the computer graphics field, VR creates a 2D image from a 3D model. VR is employed in the exploration of hollow structures, such as the airways or the colon (Figure 2).
Emergency and trauma environments are often fast-paced and high-stakes, and timely and accurate diagnosis is paramount. Therefore, to be useful, advanced 3D-processing tools should be embedded into the diagnostic viewing application (eg, PACS) to allow efficient reconstruction of images, simultaneous comparison of 2D and 3D images, and referencing of historical data in real time.
References
1. Blank M, Kalender WA. Medical volume exploration: gaining insights virtually. Eur J Radiol. 2000;33(3):161-169.
2. Philipp MO, Kubin K, Mang T, Hörmann M, Metz VM. Three-dimensional volume rendering of multidetector-row CT data: applicable for emergency radiology. Eur J Radiol. 2003;48(1):33-38.
After Imaging: Interventional Radiology in the ED
Joshua L. Weintraub, MD, and Thomas J. Ward, MD
Dr Weintraub is an interventional radiologist and executive vice chairman of the department of radiology
at New York Presbyterian/Columbia University Medical Center, New York. Dr Ward is a postgraduate fifth-year resident in interventional radiologist in the department of radiology at Mount Sinai Medical Center, New York, NY.
Interventional radiologists play an increasing role in treating patients in the ED setting. Simplistically, these roles are usually divided into one of three broad categories: to drain or aspirate infected fluid, to halt active bleeding, or to restore blood flow through an occluded vessel. While all of these procedures are critical, the newest advance in interventional radiology (IR) set to benefit the ED patient is not technical or procedural at all, but rather a philosophical one.
Fluid Aspiration and Drainage
In the ED, the need to drain or aspirate infected fluid is probably the most common indication for consult with an interventional radiologist. Perforated appendicitis or diverticulitis with an intra-abdominal abscess is generally managed with percutaneous drainage and antibiotics. If the clinical situation necessitates, and the location of the collection allows, this procedure may be performed at bedside with local anesthesia under ultrasound guidance. In the septic patient, the minimally invasive nature of this procedure confers significant benefits.
Nonoperative patients presenting with acute cholecystitis rapidly improve after the placement of a percutaneous cholecystostomy tube, and this procedure can be performed under ultrasound guidance. IR may also be indicated in cases of failed endoscopic retrograde cholangiopancreatography or cystoscopy in patients with cholangitis or urosepsis for benign or malignant obstruction. Percutaneous access is facilitated by a dilated system, and temporary decompression can be obtained until more definitive therapy is planned.
Active Bleeding
Active bleeding is another common presentation warranting an IR evaluation. Gastrointestinal, intracranial, posttraumatic, postsurgical, and postpartum bleeding, as well as massive hemoptysis, can all be managed endovascularly. Advances in microcatheter technology, covered vascular stents, and embolic agents have increased the efficacy of these interventions, and improved computed tomography angiography protocols facilitate accurate and timely diagnosis of active bleeding. With the availability of these techniques, waiting several hours for a tagged red blood cell nuclear scan is a thing of the past at many institutions. Embolization has become the mainstay of treating bleeding related to trauma in most major trauma centers.
Ischemia
An IR consult may be ordered for patients presenting with the sequelae of ischemia, which can range from a diabetic foot to an acute stroke. Percutaneous balloon angioplasty, endovascular stents, and catheter-directed thrombolysis were all monumental advances in the treatment of ischemia—conceptualized or introduced into clinical practice by interventional radiologists. This spirit of innovation continues. A variety of technical advances, atherectomy, and re-entry devices have been introduced to help recanalize chronically occluded vessels. New devices allow the interventional radiologist to quickly restore blood flow and function to patients suffering from cerebral embolus. There is increased interest in the use of catheter-assisted-embolectomy for submassive pulmonary embolism when intravenous fibrinolysis is unsuccessful or contraindicated.
Conclusion
The five decades of pioneering technical innovation highlighted in this article allow for minimally invasive treatment of the ED patient. The newest advance in IR is not technical, but rather philosophical, and a change in the role that the interventional radiologist plays. The American Board of Radiology has recently approved a new IR training pathway that more than doubles the amount of clinical training that graduating IR fellows now receive. This signals a renewed commitment to running a truly clinical service. Patients in the ED can be evaluated and treated by an interventional radiologist in the hospital and then discharged under the care of an interventional radiologist at an IR clinic. Several IR sections across the country currently practice in such a manner. Although this model is currently the exception and not the rule, the goal of the new training pathway hopes to change this, with increased advances and benefits to patients in the ED setting.
Suggested Reading
1. Gasior AC, Marty Knott E, Ostlie DJ, St Peter SD. To drain or not to drain: an analysis of abscess drains in the treatment of appendicitis with abscess. Pediatr Surg Int. 2013;29(5):455-458.
2. Sato KT. Percutaneous management of biliary emergencies. Semin Intervent Radiol. 2006;23(3):
249-257.
3. Funaki B. On-call treatment of acute gastrointestinal hemorrhage. Intervent Radiol. 2006;23(3):
215-222.
4. Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22(6):1395-1409.
5. Stead LG, Gilmore RM, Bellolio MF, Rabinstein AA, Decker WW. Percutaneous clot removal devices in acute ischemic stroke: a systematic review and meta-analysis. Arch Neurol. 2008;65(8):
1024-1030.
6. Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest. 2007;132(2):657-663.
7. Initial certification: vascular/interventional. American Board of Radiology Web site. http://www.theabr.org/ic-vir-landing. Accessed September 24, 2013.
Figure 2. Surface shaded display image of a virtual colonoscopy.
Evaluation of Chest Pain in the Emergency Department by Coronary CT Angiography
James K. Min, MD
Dr Min is director of the Institute of Cardiovascular Imaging at New York-Presbyterian Hospital/Weill-Cornell Medical College, New York.
In the United States each year, approximately 6 million patients present with complaints of chest pain suspicious for acute coronary syndromes (ACS), including unstable angina and myocardial infarction.1 Most of these patients are diagnosed with noncardiac conditions, and nearly half are due to noncardiac etiology. There are an array of diagnostic tests to identify and exclude patients with suspected ACS, such as medical history, cardiac enzyme measurements, electrocardiographic changes, and clinical risk scores. For those with nonnegligible risk for ACS for which this condition cannot be definitively diagnosed or excluded, many are often admitted to the hospital for further testing and observation. Among these patients, less than 30% are found to have ACS. And yet, despite these very careful clinical pathways, between 2% and 8% of patients with ACS are unknowingly discharged to home.2
In recent years, coronary computed tomography angiography (CCTA) has emerged as a noninvasive method that permits direct anatomic visualization of coronary atherosclerosis and luminal stenosis.3 Since the introduction of 64-multidetector row CT scanners in 2005, there have been significant advances in CT technology that now allow for reliable performance of CCTA with very low-dose radiation. Pertaining to the former, improvements in spatial resolution, temporal resolution, and volume coverage enable evaluation of coronary arteries at the submillimeter level, and can be performed in approximately 1 to 5 seconds. Concomitant to the progress in CT technology has been the parallel developments in radiation-dose reduction. As compared to the background radiation exposure of an individual living at sea level for 1 year (~3 millisieverts of radiation), current generation CCTA can be performed at doses <1 millisieverts, with doses approximating a screening mammogram now achievable.
|
|
| ||
Figure. Volume-rendered coronary computed tomographic angiography showing the coronary vascular bed and myocardium (A); normal right coronary artery without evidence of atherosclerosis (B); and (C) left anterior descending artery with mild nonobstructive calcified plaque (white area). | ||||
The diagnostic accuracy of CCTA against invasive coronary angiography has been tested in several prospective multicenter trials.3 For patients without known but suspected coronary artery disease (CAD), the sensitivity and negative predictive value has ranged between 95% to 99%—that is, CCTA can exclude anatomically obstructive CAD with near 100% certainty (Figure). It is these diagnostic performance characteristics that have encouraged several investigators to evaluate the use of CCTA in the diagnostic algorithm of patients presenting to the ED with acute chest pain—the primary intent being to identify sufficiently low-risk patients without significant CAD who can be safely discharged home.
Since 2011, three prospective multicenter randomized controlled trials have evaluated the incorporation of CCTA into a diagnostic chest pain pathway, as compared to standard-of-care algorithms.4-6 Now comprising more than 3,000 patients, these trials have demonstrated remarkably consistent results, with a reduced time-to-diagnosis of 40% to 50%, reduced lengths of stay in the ED of 25%, and reduced ED costs of 20% to 40%. Importantly, these salient effects on resource utilization and economics were underscored by exceptionally safe outcomes, which never exceeded that of the standard of care. Several studies to date have subsequently assessed the duration of safety conferred by a normal CCTA, or its “warranty period.” These studies have shown the warranty period to last at least 7 years for major adverse cardiac events and mortality.7 Moreover, they also engender hope that the chest pain pathways used for millions of Americans annually can indeed be improved, with CCTA playing an essential role.
As its technology continues to iterate, the diagnostic and prognostic performance of CCTA will invariably continue to improve. However, even at present, CCTA is robust and more accurate for exclusion of CAD when compared to other traditional methods of evaluation, and its use for patients presenting to the ED with chest pain improves throughput, reduces costs, and maximizes patient safety.
References
1. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Surviva: 2006 emergency department summary. Natl Health Stat Report. 2008;7:1-38.
2. Pope JH, Aufderheidi TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163-1170.
3. Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography: a process in evolution. J Am Coll Cardiol. 2010;55(:957-965.
4. Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366(15):1393-403.
5. Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367(4):299-308.
6. Goldstein JA, Chinnaiyan KM, Abidov A, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58(14):1414-1422.
7. Andreini D, Pontone G, Mushtaq S, et al. A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc Imaging. 201
As EDs have evolved to handle the increasing volume and complexity of patients requiring immediate care, so too, has the field of emergency radiology. Many EDs across the country now have multiple advanced imaging modalities available 24 hours a day, including Xray, computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI). While many emergency medicine physicians are now trained in performing and evaluating ultrasound images—similar to X-rays in the past—most are less comfortable with CT and MRI.
Emergency radiology, now a recognized subspecialty of diagnostic imaging, has proliferated to meet the demands for immediate interpretation of these images. This combination of around-the-clock access to equipment and expertise has brought cutting-edge, advanced imaging to the front lines of emergency care. In this special feature, we invited a group of emergency radiologists and an expert in cardiovascular imaging to discuss the applicability and utility of several of these techniques in the ED setting.
As illustrated by this panel, advanced imaging has become increasingly valuable and available in providing care to ED patients. In this presentation, however, you will notice the conspicuous absence of specific techniques that are utilized in diagnosis and evaluation of stroke, head/spine trauma, and other neurologic conditions that are commonplace in the ED. Advanced imaging has become critical in these instances and will be discussed in a future article.
Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and the executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center. He is a member of the EMER GENCY MEDICINE editorial board.
Faster than FAST: Single Pass Whole-Body Computed Tomography for Rapid Evaluation of Trauma Patients
Ashwin Asrani, MD
Dr Asrani is an assistant professor of radiology at Weill Cornell Medical College in New York City and an
assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center in New York City.
As part of initial assessment and resuscitation in the ED, severely injured trauma patients frequently undergo a bedside focused abdominal sonography for trauma (FAST) scan to assess for intraperitoneal hemorrhage from an underlying major abdominal visceral injury, as well as the presence of pleural and/or pericardial effusion. However, the use and role of a FAST scan in hemodynamically stable patients has recently been questioned since, based on its relatively low sensitivity for visceral injury, many of these cases eventually require computed tomography (CT) (See Case). In addition, patients in this subset with a positive FAST scan frequently have subsequent CT to further assist clinicians in understanding the nature of injury and to guide management recommendations (eg, operative versus nonoperative options).1
Advances in the speed of CT have made it possible to obtain images of the entire body with only a single dose of contrast injection. The elimination of segmental acquisition of torso images (eg, head, neck, chest, abdomen) eliminates the need to reposition the patient, repeat scout images, and postpone the reconstruction of reformats and other special views until the end of the examination. Depending on the original protocol, this in turn minimizes patient time in the CT-scan room by approximately 31% to 42%,2,3 and significantly reduces radiation exposure by 17% compared to conventional segmental acquisition of different body parts.2
Evidence suggests that direct single-pass whole-body CT improves outcomes in both hemodynamically stable and unstable patients and can reveal significant findings not apparent on initial clinical examination.4,5 Whole-body CT increases injury severity by detecting lesions that would not otherwise have been detected by conventional methods. While this information does not affect treatment options, it does artificially lower the ratio of observed-to-expected deaths.6 The Randomized Study of Early Assessment by CT Scanning in Trauma Patients (REACT-2), a recent international multicenter randomized clinical trial, is expected to provide evidence supporting the value of immediate total-body CT scanning during the primary evaluation of severely injured trauma patients; the results of this trial should become available in 2014.7
As EDs install and upgrade to newer multidetector CT scanners, single-pass whole-body scanning has the potential to save time in situations when it really matters.
References
1. Natarajan B, Gupta PK, Cemaj S, Sorensen M, Hatzoudis GI, Forse RA. FAST scan: Is it worth doing in hemodynamically stable blunt trauma patients? Surgery. 2010;148(4):695-700; discussion 700-701.
2. Fanucci E, Fiaschetti V, Rotili A, Floris R, Simonetti G. Whole body 16-row multislice CT in emergency room: Effects of different protocols on scanning time, image quality and radiation exposure. Emerg Radiol. 2007;13(5):251-257.
3. Nguyen D, Platon A, Shanmuganathan K, Mirvis SE, Becker CD, Poletti PA. Evaluation of a single-pass continuous whole-body 16-MDCT protocol for patients with polytrauma. AJR Am J Roentgenol. 2009;192(1):3-10.
4. Huber-Wagner S, Biberthaler P, Haberle S, et al. Whole-body CT in haemodynamically unstable severely injured patients – A retrospective, multicentre study. PLoS One. 2013;8(7):e68880.
5. Salim A, Sangthong B, Martin M, Brown C, Plurad D, Demetriades D. Whole body imaging in blunt multisystem trauma patients without obvious signs of injury: Results of a prospective study. Arch Surg. 2006;141(5):468-473; discussion 473-475.
6. Stengel D, Frank M, Matthes G, et al. Primary pan-computed tomography for blunt multiple trauma: Can the whole be better than its parts? Injury. 2009;40 Suppl 4:S36-S46.
7. Sierink JC, Saltzherr TP, Beenen LF, et al. A multicenter, randomized controlled trial of immediate total-body CT scanning in trauma patients (REACT-2). BMC Emerg Med. 2012;12:4-227X-12-4.
Obstructed Views: Imaging in Urinary Tract Obstruction
Lily M. Belfi, MD
Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center.
Urinary tract (UT) obstruction is one of the most common indications for genitourinary (GU) imaging in the ED. Until recently, diagnosis was made using intravenous (IV) pyelography. Today, improved imaging modalities, such as noncontrast computed tomography (CT), computed tomography urography (CTU), magnetic resonance urography (MRU), and magnetic resonance imaging (MRI), are available to better visualize and evaluate the underlying causes of obstruction.
Noncontrast Computed Tomography
|
|
Noncontrast CT of the abdomen and pelvis is the imaging study of choice in evaluating acute GU obstruction, particularly when there is clinical suspicion of stone disease. CT is extremely sensitive and specific in detecting renal calculi and provides information about stone burden, size, location, and composition. In addition to renal calculi, noncontrast CT is also useful in identifying other, less common causes of UT obstruction such as ureteral herniation, which can occur in the inguinal, femoral, or sciatic region and result in acute obstruction. Patients with neuromuscular disorders that cause piriformis muscle atrophy (eg, multiple sclerosis) may be predisposed to ureteral sciatic herniation. CT reconstructions in the coronal plane are useful in detecting this condition (Figure 1).
|
|
Reconstructed CT images can also reveal underlying congenital abnormalities that make a patient susceptible to obstruction. For example, CT can reveal the presence of a duplicated collecting system, which is often accompanied by an obstructing ureterocele associated with the upper pole moiety (Figure 2).
Computed Tomography Urography
|
| ||
| Figure 3. Computed tomography urography images (A and B) reveal "fish-hook" ureter (white arrows) classically seen in patients with with retrocaval ureter. | |||
Other types of congenital anomalies may require additional imaging with CTU, in which IV iodinated contrast media is administered for increased sensitivity and visualization. For instance, patients with a retrocaval ureter often present with GU obstruction and hematuria; the classic “fish-hook” or “sickle-shaped” deformity of the ureter characterizing this disease is best visualized on CTU as the ureter is well-opacified by excreted contrast (Figure 3).
Magnetic Resonance Urography and Magnetic Resonance Imaging
MRU and MRI are alternative modalities to CTU for patients in whom iodinated CT contrast is contraindicated or in cases where the avoidance of radiation exposure is indicated (eg, pregnant and pediatric patients). MRI and MRU are especially useful in further characterizing obstructive lesions in the GU tract. While initial noncontrast CT may suggest soft-tissue lesions within the ureter or urinary bladder, follow-up MRI clearly delineates the condition, helping clinicians quantify the extent of tumor involvement and determine disease stage (Figure 4). Pelvic MRI is also an essential tool in identifying causes of bladder outlet obstruction, especially in those involving the prostate gland (Figure 5).
|
|
|
|
|
Figure 4. Noncontrast pelvic computed tomography images (A and B) of a 72-year-old man with flank pain and difficulty voiding reveal hydronephrosis (white arrow) and bladder mass (red arrow). Magnetic resonance urography (C) further visualizes the bladder mass (red arrow), and postcontrast magnetic resonance images (D and E) show the extent of the tumor (red arrow) as well as a metastatic lesion (white arrow).
| ||||
Conclusion
|
|
| |||
| Figure 5. Noncontrast pelvic computed tomography images (A and B) of a 73-yearold man with pelvic pain demonstrate bilateral hydronephrosis. (white arrows) and a large mass in the expected location of the prostate (red arrow). Further evaluation of the large heterogenous mass with pelvic magnetic resonance imaging (C) reveals prostate carcinoma (red arrows). | |||||
Based on its sensitivity in detecting both common and uncommon causes of obstruction, noncontrast abdominal and pelvic CT is an excellent first-line imaging choice for evaluating patients presenting to the ED with UT obstruction. Moreover, noncontrast CT also helps guide clinicians in determining which, if any, additional studies with CTU, MRU, and/or MRI are warranted.
Sound Advice: Ultrasound in the Emergency Department
Kemi Babagbemi, MD
Dr Babagbemi is an assistant professor of radiology and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
In today’s evolving health-care environment, emphasis must be placed on providing accurate, safe, cost-effective, and timely diagnosis to the wide range of patients that present to the ED. To meet this need, emergency medicine physicians and radiologists have universally embraced the use of ultrasound in this setting.
Ultrasound is less expensive than computed tomography (CT) and magnetic resonance imaging (MRI), and its portability allows for bedside application, enabling its use in the most critical patients. It does not require extended preparation (eg, oral contrast) or carry the risk for adverse reactions associated with intravenously administered contrast. Also, since ultrasound does not use ionizing radiation, it is a particularly appropriate imaging option in susceptible populations such as pregnant and pediatric patients.1 Although a noninvasive modality, it can also be used to guide interventional procedures, including vascular access, thoracentesis/paracentesis, and specialized anesthesia.
Emergency physicians routinely perform ultrasound at bedside and are familiar with its utility in evaluating abdominal trauma, right upper quadrant pain, and acute biliary abnormalities; assessing vascular thrombosis or injury; and determining the etiology of pelvic pain and bleeding in both pregnant and nonpregnant patients. However, ultrasound is also increasingly being utilized to
diagnose other conditions for which CT and MRI were once thought the superior diagnostic tool.
Trauma (The Extended FAST Scan)
The focused abdominal sonography for trauma (FAST) scan has been used for rapid and immediate assessment of unstable trauma patients with suspected abdominal injury. In a recent study of 4,029 patients with blunt abdominal trauma, FAST scans had a sensitivity, specificity, and accuracy for detection of hemorrhage in patients with hypotension of 85%, 60%, and 77%, respectively.2 Other studies suggest that sonography of the chest may be useful in the rapid detection of additional life-threatening pathology such as pneumothorax (Figure 1), with data suggesting improved sensitivity over radiography.3 The inclusion of the pleural space in evaluation is increasingly more common and is referred to as the extended FAST (EFAST).
Appendicitis
The most common cause of abdominal pain requiring surgical intervention is appendicitis.4 Although certain clinically based prediction scores (eg, Alvarado scores) may be used, imaging is considered far superior in accurately diagnosing the condition.5 In a meta-analysis of data from 26 ultrasound and CT studies (15 prospective, 11 retrospective), there was a pooled 88% sensitivity and 94% specificity for ultrasound compared with CT, which exhibited a pooled sensitivity of 94% and specificity of 95%.6 As previously noted, because there is no ionizing radiation in ultrasound, it should be the preferred modality in both children and first-trimester pregnant patients.
Musculoskeletal Trauma
Ultrasound is an ideal imaging modality to evaluate the musculoskeletal system. Despite its widespread use in Europe for many years, musculoskeletal sonography is only now beginning to be adopted in the United States. Its ability to visualize soft-tissue structures makes it effective in evaluating for muscle, tendon, or ligament injury, and can even do so dynamically with stress maneuvers (Figure 2). With respect to fractures, sonography has also proved effective in evaluating for cortical disruption. For example, a recent study demonstrated overall 92% sensitivity and 100% sensitivity for fractures with high potential for complication in radiographically occult scaphoid fractures.7
Conclusion
As a true point-of-care imaging modality, ultrasound has an established place in the practice of emergency medicine. Future improvements in technology, including the ability to obtain true three-dimensional volumetric data sets, will expand its role even further.
References
1. Image Gently and Ultrasound. Image Gently Campaign. The Alliance for Radiation Safety in Pediatric Imaging Web site. http://www.pedrad.org/associations/5364/ig/?page=787. Accessed September 19, 2013.
2. Lee BC, Ormsby EL, McGahan JP, Melendres GM, Richards JR. The utility of sonography for the triage of blunt abdominal trauma patients to exploratory laparotomy. AJR Am J Roentgenol. 2007;188(2):415-421.
3. Nandipati KC, Allamaneni S, Kakarla, et al. Extended focused assessment with sonography for trauma (EFAST) in the diagnosis of pneumothorax: experience at a community based level I trauma center. Injury. 2011;42(5):511-514.
4. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132(5):910-925.
5. Sun JS, Noh HW, Min YG, et al. Receiver operating characteristic analysis of the diagnostic performance of a computed tomographic examination and the Alvarado score for diagnosing acute appendicitis: emphasis on age and sex of the patients.
J Comput Assist Tomogr. 2008;32(3):386-391.
6. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for Diagnosis of Appendicitis in Children and Adults? A MetaAnalysis. Radiology. 2006;241(1):83-94.
7. Platon A, Poletti PA, Van Aaken J, Fusetti C, Della Santa D, Beaulieu JY, Becker CD. Occult fractures of the scaphoid: the role of Ultrasonography in the emergency department. Skeletal Radiol. 2011;40(7):869-875.
Stuck or Not? Noninvasive Vascular Imaging in the Emergency Setting
Michael L. Loftus, MD
Dr Loftus is assistant professor of radiology at New York-Presbyterian Hospital/Weill Cornell Medical College, New York.
Conventional catheter-directed angiography has played an important role in the history of ED imaging, providing timely information about vessel integrity throughout the body and guiding potentially life-saving interventions. However, this imaging modality carries significant potential risks, including puncture-site hematoma or pseudoaneurysm, catheter-induced vasospasm, vascular occlusion or dissection, anesthesia-associated risks, and neurological deterioration or stroke.1 Moreover, emergent angiography is not universally available in all EDs.
Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) have opened new windows of opportunity for noninvasive vascular imaging to play a role in clinical decision-making in the ED. Cross-sectional angiography has virtually eliminated the need for acute catheter-directed angiography in several clinical settings, including pulmonary angiography, and the clinical applicability of CTA and MRA continues to expand as imaging techniques improve and achieve widespread acceptance and implementation. Multidetector CT is now widely available in most EDs, and the
accessibility of magnetic resonance imaging and MRA is expanding rapidly.
CTA allows evaluation of the vasculature on contrast-enhanced axial source images and also utilizes computer-generated maximum intensity projection reformations to create diagnostic images of the vascular region of interest. Similarly, MRA can be performed either with or without the administration of an intravenous gadolinium contrast agent, and provides additional information about directionality of flow, as well as detailed images of the surrounding soft tissues.
An emerging clinical situation in which contrast-enhanced cross-sectional imaging may supplant the need for arterial puncture and digital subtraction angiography is multiligament trauma to the knee. When significant kinetic force is applied to the knee, the joint is at risk for translocation and/or dislocation, with resultant injury to the surrounding soft-tissue envelope and potential trauma to the neurovascular structures around the knee. In this type of injury, cross-sectional imaging is routinely ordered to further evaluate and classify trauma, revealing potentially treatment-altering information concerning the integrity of the vascular structures with minimal risk to the patient (Figure 1).
The largest series of MRAs performed specifically in patients with knee dislocation reviewed 17 cases and found two cases of vascular pathology: one case of an intimal flap and one case of acute vasospasm. Digital subtraction angiography was performed on 6 of 17 cases and had 100% concordance with MRA findings.2 MRI is often performed in the setting of suspected multiligament knee injury to aid in preoperative planning, and the addition of MRA should be considered if trauma to the periarticular vasculature is suspected.
There is ample evidence that cross-sectional imaging performs well relative to conventional catheter angiography in the setting of peripheral vascular occlusion from atherosclerotic etiologies.3 Furthermore, there is precedence for utilizing contrast-enhanced CTA, as well as contrast-enhanced or three-dimensional time-of-flight MRA in other areas of the body—particularly the brain, head, and neck4 (Figure 2). Several MRA techniques are becoming available that will allow a high resolution angiographic without the use of contrast. Upcoming advances in CTA include dual-energy CT, which has the potential to allow angiography using very small amounts of contrast.
In general, when ordering cross-sectional imaging in the setting of trauma, consideration should be given to the potential for vascular compromise; thus, the addition of contrast-enhanced CTA or MRA can add valuable clinical information, with relatively little excess risk or time, sparing patients the risks of catheter-directed angiography.
References
1. Eisenberg, RL, Bank, WO, Hedgcock, MW. Neurologic complications of angiography for cerebrovascular disease. Neurology. 1980;30(8):895-897.
2. Potter HG, Weinstein M, Allen AA, Wickiewicz TL, Helfet DL. Magnetic resonance imaging of the multiple-ligament injured knee. J Orthop Trauma. 2002;16(5);330-339.
3. Chin AS, Rubin GD. CT angiography of peripheral arterial occlusive disease. Tech Vasc Interv Radiol. 2006;9(4):143-149.
4. Riles TS, Eidelman EM, Litt AW, Pinto RS, Oldford F, Schwartzenberg GW. Comparison of magnetic resonance angiography, conventional angiography, and duplex scanning. Stroke. 1992;23(3):341-346.
What’s Hip in 2013?
Roger J. Bartolotta, MD
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center.
Post-traumatic hip pain is a common chief concern among ED patients. While routine radiography, the standard imaging modality for the initial evaluation of suspected hip fracture, detects most fractures, its sensitivity is decreased in the setting of osteoporosis—particularly with nondisplaced fractures. Thus, though elderly/osteoporotic patients are more likely to fracture, these fractures are harder to detect on radiography. In a study of 70 patients with negative radiographs but high clinical concern for fracture, magnetic resonance imaging (MRI) detected occult femoral fractures in 37% and occult pelvic fractures in 23%.1
Hip fractures in elderly patients are associated with substantial mortality and morbidity, the risks for which increase with delayed diagnosis.2 When there is high clinical suspicion for a radiographically occult hip fracture in this population, cross-sectional imaging should be considered for further evaluation (Figure). The decision of whether to use computed tomography (CT) or MRI for the cross-sectional examination must be made on both an institutional and patient-specific basis. CT is faster, less expensive, and more widely and temporally available. Although CT has increased sensitivity for fracture detection compared to radiography, studies have demonstrated false-negative CT examinations in the setting of nondisplaced proximal femoral fractures, especially in osteoporotic patients. Hakkarinen et al3 reported that among 235 hip fractures, 10% were occult radiographically; approximately 17% of these fractures (4 out of 24) were also occult on CT but visible on MRI. Moreover, while radiography and CT may demonstrate a seemingly isolated fracture at the femoral greater trochanter, a subset of these fractures exhibit intertrochanteric extension that is only evident on MRI (Figure). Isolated greater trochanteric fractures are typically treated conservatively, while some incomplete intertrochanteric fractures warrant internal fixation, especially fractures that cross the intertrochanteric midline on coronal MRI.4,5
|
|
| ||
Figure. Anteroposterior left hip radiograph (A) in a patient with hip pain (A) shows no definite evidence of fracture. Coronal reformatted image from left hip computed tomography (B) demonstrates nondisplaced, seemingly isolated fracture of the left femoral greater trochanter (white arrows). Coronal T1-weighted image from left hip magnetic resonance imaging (C) reveals intertrochanteric extension of the fracture spanning greater than 50% of the intertrochanteric diameter (red arrows). | ||||
In addition to improved fracture detection, MRI also provides superior evaluation of the underlying bone marrow for coexisting conditions, such as osteomyelitis, osteonecrosis, and primary or metastatic neoplasm in the setting of pathologic fracture. Additional benefits of MRI over radiography and CT include its lack of ionizing radiation and improved evaluation of adjacent soft tissue injuries, such as labral and/or musculotendinous tears.
MRI, however, does require a longer examination time in which the patient must remain still. This may be difficult for acutely post-traumatic patients, notably those with baseline dementia and/or claustrophobia. For patients in whom MRI is indicated (eg, patients who do not have an implantable device such as a cardiac pacemaker) and where it is institutionally available, the decision to utilize it over CT is largely rooted in health-care economics. MRI is more expensive than radiography and CT, and even in the largest medical centers, the examination requires substantially more time than CT, which inherently decreases patient throughput in the ED. Cannon et al6 present an evidence-based algorithm for patient stratification, in which patients at high-risk for osteoporosis and low-energy trauma should be considered for immediate MRI rather than CT. These risk factors optimize MRI utilization by selecting those patients with the greatest likelihood of nondisplaced, radiographically occult fracture.
References
1. Bogost GA, Lizerbram EK, Crues JV 3rd. MR imaging in evaluation of suspected hip fracture: frequency of unsuspected bone and soft-tissue injury. Radiology. 1995;197(1):263-267.
2. Zuckerman JD, Skovron ML, Koval KJ, Aharonoff G, Frankel VH. Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip. J Bone Joint Surg Am. 1995;77(10):1551-1556.
3. Hakkarinen DK, Banh KV, Hendey GW. Magnetic resonance imaging identifies occult hip fractures missed by 64-slice computed tomography. J Emerg Med. 2012;43(2):303-307.
4. Feldman F, Staron RB. MRI of seemingly isolated greater trochanteric fractures. AJR Am J Roentgenol. 2004;183(2):323-329.
5. Schultz E, Miller TT, Boruchov SD, Schmell EB, Toledano B. Incomplete intertrochanteric fractures: imaging features and clinical management. Radiology. 1999;211(1):237-240.
6. Cannon J, Silvestri S, Munro M. Imaging choices in occult hip fracture. J Emerg Med. 2009;37(2):144-152.
PE or Not PE: That Is the Question
Jessica Fisher, MD
Dr Fisher is an instructor of radiology at Weill Cornell Medical College in New York City.
Pulmonary embolism (PE) represents the third most common cause of death from cardiovascular disease after myocardial infarction and stroke.1 Given the potential for fatal outcome, prompt diagnosis and management are essential. To avoid overdiagnosis of PE and unnecessary treatment with anticoagulation therapy, diagnostic tests with both a high sensitivity and high specificity are essential.
Clinical stratification of patient risk for PE, in combination with D-dimer assays, is typically used to determine the need for imaging. Preliminary studies with chest X-ray are utilized to evaluate alternate causes of clinical symptoms but do not provide a definitive diagnosis. In the ED setting, advanced imaging options to detect PE include computed tomography angiography (CTA), ventilation perfusion scintigraphy (V/Q lung scan), and magnetic resonance angiography (MRA).
Computed Tomography Angiography
|
| ||
| Figure 1. Coronal (A) and axial (B) contrast-enhanced computed tomography angiography scans reveal pulmonary embolism (white arrows).. | |||
CTA has long been the standard technique for evaluating PE. In addition to reported sensitivities of 96% to 100% and specificities of 97% to 98% with multislice detectors,2 CTA also provides information on disease severity, such as clot burden, evidence of right heart strain, and the presence of pulmonary infarct (Figure 1). It also can reveal alternative etiology and diagnosis in negative cases (Figure 2).
CTA is readily available in today’s ED and can be performed quickly and efficiently. However, it does require intravenous (IV) contrast and emits a high-dose of radiation, which may be contraindicated in some patient populations (eg, patients with renal failure, allergy to contrast, and pregnant and pediatric patients). Also, to avoid degradation of images and accurately visualize peripheral branches, patient cooperation (ie, suspension of respiratory motion) during the examination is essential.
Ventilation Perfusion Scintigraphy
Before the advent of CTA, V/Q lung scan was the first-line imaging choice to assess for PE.3 This modality continues to have a role in modern practice, especially given its estimated 6-fold lower whole-body effective radiation dose compared to CTA.4 Current applications of this modality include patients with contraindications to IV contrast as well as pregnant and pediatric patients in whom perfusion-only studies should be used to significantly lower the radiation profile.
In patients with a clear chest X-ray, the negative predictive value of V/Q is not significantly different from CT.5 This suggests a growing role for V/Q scans in the younger, healthier patient population where limited/reduced exposure to radiation is particularly desired—eg, pediatric patients and women younger than age 20 years in whom there is an increased risk of malignancy associated with radiation exposure to the breast/chest area.6
The limitations of V/Q lung scan include a higher rate of indeterminate results compared to CTA (particularly in patients with an abnormal chest X-ray). Moreover, V/Q does not provide alternative diagnoses or qualify disease severity. Also, the longer imaging times compared with CTA make this modality impractical in critically ill patients.
Magnetic Resonance Angiography
MRA has emerged in recent years as a potential alternative to CTA. Since this modality does not use ionizing radiation or iodine-based contrast, it is a reasonable and appropriate option for pediatric patients and those with a contrast-dye allergy. Initial meta-analysis of MRA studies demonstrated sensitivity ranging from 77% to 100% and a specificity of 95% to 98%.7-9 However, subsequent studies have found unacceptably high rates of technically inadequate examinations resulting in nondiagnostic results.10 As progress to reduce respiratory and cardiac motion artifacts and improve spatial resolution continues, along with the growing availability of MRA in the ED, this modality may soon emerge as a useful diagnostic option.
Conclusion
New advances in the diagnosis of PE continue to emerge. Evidence suggests improved sensitivity of CTA with the use of dual-energy CT scanners.11 The recent advance of V/Q single photon emission CT has also shown promise in improving detection accuracy.12 Ongoing research will help continue to expand the emergency physician’s range of imaging choices in the diagnosis of PE.
References
1. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379(9828):1835-1846.
2. Burns SK, Haramati LB. Diagnostic imaging and risk stratification of patients with acute pulmonary embolism. Cardiol Rev. 2012;20(1):15-24.
3. Mos IC, Klok FA, Kroft LJ, de Roos A, Huisman MV. Imaging tests in the diagnosis of pulmonary embolism. Semin Respir Crit Care Med. 2012;33(2):138-143.
4. Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254-263.
5. Stein EG, Haramati LB, Chamarthy M, Sprayregen S, Davitt MM, Freeman LM. Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. Am J Roentgenol. 2010;194(2):392-397.
6. Hill DA, Preston-Martin S, Ross RK, Bernstein L. Medical radiation, family history of cancer, and benign breast disease in relation to breast cancer risk in young women, USA. Cancer Causes Control. 2002;13(8):711-718.
7. Oudkerk M, van Beek EJ, Wielopolski P, et al. Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet. 2002;359(9318):1643-1647.
8. Meaney JF, Weg JG, Chenevert TL, Stafford-Johnson D, Hamilton BH, Prince MR. Diagnosis of pulmonary embolism with magnetic resonance angiography. N Engl J Med 1997;336(20):1422-1427.
9. Gupta A, Frazer CK, Ferguson JM, et al. Acute pulmonary embolism: diagnosis with MR angiography. Radiology. 1999;210(2):353-359.
10. Stein PD, Chenevert TL, Fowler SE. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med. 2010;152(7):434-443, W142, W143.
11. Pontana F, Faivre JB, Remy-Jardin M. Lung perfusion with dual-energy multidetector-row CT (MDCT): feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad Radiol. 2008;15(12):1494-1504.
12. Gutte H, Mortensen J, Jensen CV, et al. Comparison of V/Q SPECT and planar V/Q lung scintigraphy in diagnosing acute pulmonary embolism. Nucl Med Commun. 2010;31(1):82-86.
Just MoRe Imaging?
MRI Evaluation of Acute Abdominal Pain
David A. Boyajian, MD
Dr Boyajian is clinical director of radiology at New York-Presbyterian/Lower Manhattan Hospital, and vice chairman and assistant professor of radiology, Department of Radiology, Weill Cornell Medical College, New York.
For the past few decades, the established cross-sectional imaging modalities used to evaluate acute abdominal pain in the ED have been computed tomography (CT) and ultrasound. Advantages of these modalities include the widespread availability of equipment and qualified technologists and radiologists, the ability to obtain images quickly, and relative cost-effectiveness. There are, however, several subsets of indications for which traditional cross-sectional imaging approaches are either undesirable or have a low sensitivity for accuracy (eg, suspected nonobstetric pathology in the pregnant patient, biliary ductal pathology).
Magnetic resonance imaging (MRI) has become more widely available to ED practices across the country and an increasingly important tool in the arsenal of the emergency radiologist. Advantages of MRI include better soft-tissue characterization, ability to directly image in any plane, and the lack of ionizing radiation.
There are, however, barriers to using MRI in the emergency setting, including availability and location of the MRI scanners, long examination time, difficulty in monitoring unstable patients, and higher cost.1 In addition, relative to CT and ultrasound, there is poorer spatial resolution and greater potential for artifacts. Advances in MRI technology and workflow, including rapidly acquired imaging sequences and active radiologist management of such cases, can significantly reduce examination acquisition time and improve image quality. Due to the significant advantages in visualizing the brain and spine, MRI has become the gold standard in many emergent conditions such as suspected stroke and spinal cord compression. Increasingly, MRI is being used to diagnosis causes of abdominal pain, examples of such are discussed below.
Appendicitis in Pregnancy
Although acute appendicitis is the most common nonobstetric surgical condition during pregnancy, several factors such as physiological leukocytosis and appendiceal displacement (which reduces the sensitivity of ultrasound) confound the diagnosis. Due to the radiation conveyed by CT, MRI is considered a more appropriate imaging modality in this patient population.2 In a retrospective study of 23,290 pregnant patients, Pedrosa et al3 reported a sensitivity of 100%, specificity of 93.6%, and accuracy of 94% in detecting acute appendicitis in patients for whom ultrasound was inconclusive.
Biliary Pathology
Biliary pathology, particularly choledocholithiasis, may not be demonstrated to advantage with cross-sectional imaging. For example, CT sensitivity for detection is decreased in the absence of ductal dilatation and/or in poorly mineralized stones. Technical factors, such as obesity, patient positioning, and bowel gas, limit evaluation with ultrasound. MRI is able to depict both the intrahepatic and extrahepatic biliary ducts and identify any intraductal lesions (eg, masses, stones). Special MRI contrast agents that are excreted through the bile ducts have come to market in recent years and can assist in evaluation for biliary pathology. The sequences have replaced many of the diagnostic endoscopic retrograde cholangiopancreatograms (ERCP) that used to be performed. An advantage of magnetic resonance cholangiopancreatography (MRCP) is that structures outside of the biliary system may also be evaluated (eg, liver, kidneys).
|
|
Conclusion
The use of MRI for evaluation of abdominal pain continues to increase. While currently limited to specific vulnerable populations (eg, pregnant patients) or for specific conditions (eg, biliary disease), improving technology and availability will allow this technique to expand to more patients and conditions, including evaluation for inflammatory bowel conditions.
References
1. Saini S, Seltzer SE, Bramson RT, et al. Technical cost of radiologic examinations: Analysis across imaging modalities. Radiology. 2000; 216(1):269-272.
2. Appropriateness criteria. American College of Radiology Web site. http://www.acr.org/Quality-Safety/Appropriateness-Criteria. Accessed September 17, 2013.
3. Pedrosa I, Levine D, Eyvazzadeh AD, Siewert B, Ngo L, Rofsky NM. MR imaging evaluation of acute appendicitis in pregnancy. Radiology. 2006;238(3):891-899.
When a Picture Is Worth a Thousand Images:
3D Reconstruction in Emergency and Trauma Imaging
Jamlik-Omari Johnson, MD; Waqas Shuaib, MD
Dr Johnson is assistant professor of radiology and division director of radiology in the department of radiology and imaging services, division of emergency medicine at Emory University School of Medicine, Atlanta, Georgia. Dr Shuaib is a research associate in the department of radiology and imaging services, division of emergency medicine at Emory University School of Medicine, Atlanta, Georgia.
Three-dimensional (3D) reconstruction creates a digital image of a real-life object, capturing both its original shape and appearance. These techniques simulate reality through the use of lighting, color, and motion.1 Advantages of 3D reconstruction in the emergency and trauma setting include enhanced views of anatomy, making pathology that may be difficult to see in the axial plane easily visible (eg, rib fractures). 3D images may also increase interpretation efficiency, allowing rapid review of the large data sets associated with multidetector computed tomography (MDCT) of trauma patients. Since these images can be attached to the radiology report, this modality also enhances service to referring physicians. In addition, as 3D images succinctly illustrate a diagnosis, the treating physician may share them with patients to demonstrate a condition and explain treatment recommendations.
MDCT technology evolved rapidly over the past 25 years. Between 1992 and 2004, MDCT made quantum leaps from dual detectors to 64-slice scanners, and over the last decade, dual-energy source technology and 128-slice scanners have become integrated into clinical practice. These newer devices, along with technical enhancements, provide high temporal and superior spatial resolution, considerably improved image quality, and expanded clinical applications. With MDCT, scans are performed quickly, resulting in improved temporal resolution and reduced motion artifacts. Leverage of this technology in the emergency and trauma setting has expanded the spectrum of indications for use and increased the utility of CT in urgent care. Newer algorithms allow rapid and detailed examinations of the musculoskeletal and the vascular systems in submillimeter slices, without limitation of scan volume. The high-resolution source images are the basis for high quality 3D reconstructions.
The 3D reconstruction toolbox may benefit the surveillance of anatomy, detection of pathology, and planning of therapy. Examples of three such commonly used techniques are shaded surface display (SSD), maximum intensity projection (MIP), and volume rendering (VR).2
SSD provides a realistic 3D view of the surface of a structure within the acquired volume data set and is good at depicting aneurysms and other focal disease (Figure 1). This is particularly useful for evaluating long and tortuous structures, such as the arterial and venous structures. MIP is often used to detect lung nodules by making them stand-out from bronchi and vasculature (Figure 1). Adapted from the computer graphics field, VR creates a 2D image from a 3D model. VR is employed in the exploration of hollow structures, such as the airways or the colon (Figure 2).
Emergency and trauma environments are often fast-paced and high-stakes, and timely and accurate diagnosis is paramount. Therefore, to be useful, advanced 3D-processing tools should be embedded into the diagnostic viewing application (eg, PACS) to allow efficient reconstruction of images, simultaneous comparison of 2D and 3D images, and referencing of historical data in real time.
References
1. Blank M, Kalender WA. Medical volume exploration: gaining insights virtually. Eur J Radiol. 2000;33(3):161-169.
2. Philipp MO, Kubin K, Mang T, Hörmann M, Metz VM. Three-dimensional volume rendering of multidetector-row CT data: applicable for emergency radiology. Eur J Radiol. 2003;48(1):33-38.
After Imaging: Interventional Radiology in the ED
Joshua L. Weintraub, MD, and Thomas J. Ward, MD
Dr Weintraub is an interventional radiologist and executive vice chairman of the department of radiology
at New York Presbyterian/Columbia University Medical Center, New York. Dr Ward is a postgraduate fifth-year resident in interventional radiologist in the department of radiology at Mount Sinai Medical Center, New York, NY.
Interventional radiologists play an increasing role in treating patients in the ED setting. Simplistically, these roles are usually divided into one of three broad categories: to drain or aspirate infected fluid, to halt active bleeding, or to restore blood flow through an occluded vessel. While all of these procedures are critical, the newest advance in interventional radiology (IR) set to benefit the ED patient is not technical or procedural at all, but rather a philosophical one.
Fluid Aspiration and Drainage
In the ED, the need to drain or aspirate infected fluid is probably the most common indication for consult with an interventional radiologist. Perforated appendicitis or diverticulitis with an intra-abdominal abscess is generally managed with percutaneous drainage and antibiotics. If the clinical situation necessitates, and the location of the collection allows, this procedure may be performed at bedside with local anesthesia under ultrasound guidance. In the septic patient, the minimally invasive nature of this procedure confers significant benefits.
Nonoperative patients presenting with acute cholecystitis rapidly improve after the placement of a percutaneous cholecystostomy tube, and this procedure can be performed under ultrasound guidance. IR may also be indicated in cases of failed endoscopic retrograde cholangiopancreatography or cystoscopy in patients with cholangitis or urosepsis for benign or malignant obstruction. Percutaneous access is facilitated by a dilated system, and temporary decompression can be obtained until more definitive therapy is planned.
Active Bleeding
Active bleeding is another common presentation warranting an IR evaluation. Gastrointestinal, intracranial, posttraumatic, postsurgical, and postpartum bleeding, as well as massive hemoptysis, can all be managed endovascularly. Advances in microcatheter technology, covered vascular stents, and embolic agents have increased the efficacy of these interventions, and improved computed tomography angiography protocols facilitate accurate and timely diagnosis of active bleeding. With the availability of these techniques, waiting several hours for a tagged red blood cell nuclear scan is a thing of the past at many institutions. Embolization has become the mainstay of treating bleeding related to trauma in most major trauma centers.
Ischemia
An IR consult may be ordered for patients presenting with the sequelae of ischemia, which can range from a diabetic foot to an acute stroke. Percutaneous balloon angioplasty, endovascular stents, and catheter-directed thrombolysis were all monumental advances in the treatment of ischemia—conceptualized or introduced into clinical practice by interventional radiologists. This spirit of innovation continues. A variety of technical advances, atherectomy, and re-entry devices have been introduced to help recanalize chronically occluded vessels. New devices allow the interventional radiologist to quickly restore blood flow and function to patients suffering from cerebral embolus. There is increased interest in the use of catheter-assisted-embolectomy for submassive pulmonary embolism when intravenous fibrinolysis is unsuccessful or contraindicated.
Conclusion
The five decades of pioneering technical innovation highlighted in this article allow for minimally invasive treatment of the ED patient. The newest advance in IR is not technical, but rather philosophical, and a change in the role that the interventional radiologist plays. The American Board of Radiology has recently approved a new IR training pathway that more than doubles the amount of clinical training that graduating IR fellows now receive. This signals a renewed commitment to running a truly clinical service. Patients in the ED can be evaluated and treated by an interventional radiologist in the hospital and then discharged under the care of an interventional radiologist at an IR clinic. Several IR sections across the country currently practice in such a manner. Although this model is currently the exception and not the rule, the goal of the new training pathway hopes to change this, with increased advances and benefits to patients in the ED setting.
Suggested Reading
1. Gasior AC, Marty Knott E, Ostlie DJ, St Peter SD. To drain or not to drain: an analysis of abscess drains in the treatment of appendicitis with abscess. Pediatr Surg Int. 2013;29(5):455-458.
2. Sato KT. Percutaneous management of biliary emergencies. Semin Intervent Radiol. 2006;23(3):
249-257.
3. Funaki B. On-call treatment of acute gastrointestinal hemorrhage. Intervent Radiol. 2006;23(3):
215-222.
4. Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics. 2002;22(6):1395-1409.
5. Stead LG, Gilmore RM, Bellolio MF, Rabinstein AA, Decker WW. Percutaneous clot removal devices in acute ischemic stroke: a systematic review and meta-analysis. Arch Neurol. 2008;65(8):
1024-1030.
6. Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest. 2007;132(2):657-663.
7. Initial certification: vascular/interventional. American Board of Radiology Web site. http://www.theabr.org/ic-vir-landing. Accessed September 24, 2013.
Figure 2. Surface shaded display image of a virtual colonoscopy.
Evaluation of Chest Pain in the Emergency Department by Coronary CT Angiography
James K. Min, MD
Dr Min is director of the Institute of Cardiovascular Imaging at New York-Presbyterian Hospital/Weill-Cornell Medical College, New York.
In the United States each year, approximately 6 million patients present with complaints of chest pain suspicious for acute coronary syndromes (ACS), including unstable angina and myocardial infarction.1 Most of these patients are diagnosed with noncardiac conditions, and nearly half are due to noncardiac etiology. There are an array of diagnostic tests to identify and exclude patients with suspected ACS, such as medical history, cardiac enzyme measurements, electrocardiographic changes, and clinical risk scores. For those with nonnegligible risk for ACS for which this condition cannot be definitively diagnosed or excluded, many are often admitted to the hospital for further testing and observation. Among these patients, less than 30% are found to have ACS. And yet, despite these very careful clinical pathways, between 2% and 8% of patients with ACS are unknowingly discharged to home.2
In recent years, coronary computed tomography angiography (CCTA) has emerged as a noninvasive method that permits direct anatomic visualization of coronary atherosclerosis and luminal stenosis.3 Since the introduction of 64-multidetector row CT scanners in 2005, there have been significant advances in CT technology that now allow for reliable performance of CCTA with very low-dose radiation. Pertaining to the former, improvements in spatial resolution, temporal resolution, and volume coverage enable evaluation of coronary arteries at the submillimeter level, and can be performed in approximately 1 to 5 seconds. Concomitant to the progress in CT technology has been the parallel developments in radiation-dose reduction. As compared to the background radiation exposure of an individual living at sea level for 1 year (~3 millisieverts of radiation), current generation CCTA can be performed at doses <1 millisieverts, with doses approximating a screening mammogram now achievable.
|
|
| ||
Figure. Volume-rendered coronary computed tomographic angiography showing the coronary vascular bed and myocardium (A); normal right coronary artery without evidence of atherosclerosis (B); and (C) left anterior descending artery with mild nonobstructive calcified plaque (white area). | ||||
The diagnostic accuracy of CCTA against invasive coronary angiography has been tested in several prospective multicenter trials.3 For patients without known but suspected coronary artery disease (CAD), the sensitivity and negative predictive value has ranged between 95% to 99%—that is, CCTA can exclude anatomically obstructive CAD with near 100% certainty (Figure). It is these diagnostic performance characteristics that have encouraged several investigators to evaluate the use of CCTA in the diagnostic algorithm of patients presenting to the ED with acute chest pain—the primary intent being to identify sufficiently low-risk patients without significant CAD who can be safely discharged home.
Since 2011, three prospective multicenter randomized controlled trials have evaluated the incorporation of CCTA into a diagnostic chest pain pathway, as compared to standard-of-care algorithms.4-6 Now comprising more than 3,000 patients, these trials have demonstrated remarkably consistent results, with a reduced time-to-diagnosis of 40% to 50%, reduced lengths of stay in the ED of 25%, and reduced ED costs of 20% to 40%. Importantly, these salient effects on resource utilization and economics were underscored by exceptionally safe outcomes, which never exceeded that of the standard of care. Several studies to date have subsequently assessed the duration of safety conferred by a normal CCTA, or its “warranty period.” These studies have shown the warranty period to last at least 7 years for major adverse cardiac events and mortality.7 Moreover, they also engender hope that the chest pain pathways used for millions of Americans annually can indeed be improved, with CCTA playing an essential role.
As its technology continues to iterate, the diagnostic and prognostic performance of CCTA will invariably continue to improve. However, even at present, CCTA is robust and more accurate for exclusion of CAD when compared to other traditional methods of evaluation, and its use for patients presenting to the ED with chest pain improves throughput, reduces costs, and maximizes patient safety.
References
1. Pitts SR, Niska RW, Xu J, Burt CW. National Hospital Ambulatory Medical Care Surviva: 2006 emergency department summary. Natl Health Stat Report. 2008;7:1-38.
2. Pope JH, Aufderheidi TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163-1170.
3. Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography: a process in evolution. J Am Coll Cardiol. 2010;55(:957-965.
4. Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366(15):1393-403.
5. Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367(4):299-308.
6. Goldstein JA, Chinnaiyan KM, Abidov A, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58(14):1414-1422.
7. Andreini D, Pontone G, Mushtaq S, et al. A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc Imaging. 201