User login
Plan Aims to Make Cancer Radiotherapy Safer
The American Society for Radiation Oncology has released a six-point plan to improve patient safety and reduce the potential for medical errors during cancer radiotherapy.
The plan comes on the heels of two stories in the New York Times that detailed cases of excess radiation used in the treatment of specific patients, and raised alarms about errors in radiotherapy. Its announcement follows initiation of a systemic review of the society's patient safety and quality assurance projects at the ASTRO board of directors winter meeting in late January.
“We have been developing and refining many of these programs for years. … By committing to this plan, we are redoubling our efforts in this essential area of our specialty,” Dr. Timothy R. Williams, ASTRO board chairman, explained in a statement.
Dr. Williams, a practicing radiation oncologist in Boca Raton, Fla., acknowledged the stories, calling them “deeply troubling” to the society and noting that “in any area of medicine—and radiation oncology is no exception—even one error is too many. … Any errors, no matter how small, must be reported, understood, and used as a tool to further reduce the potential for future errors.”
The six key areas of effort include
▸ Creating a database for the reporting of linear-accelerator– and computer tomography–based medical errors.
▸ Enhancing ASTRO's practice accreditation program, starting with new accreditation models for advanced technologies, such as image-guided radiation therapy (IGRT), stereotactic body radiation therapy (SBRT), and brachytherapy.
▸ Expanding educational training programs to include courses on quality assurance and safety.
▸ Developing tools for cancer patients and caregivers to use in their discussions with their radiation oncologists to help understand quality and safety programs at the centers where they are being treated.
▸ Further developing the Integrating the Healthcare Enterprise–Radiation Oncology connectivity compliance program to ensure that medical technologies from different manufacturers can safely transfer information to reduce the chance of medical error.
▸ Advocating for the immediate passage of the Consistency, Accuracy, Responsibility and Excellence in Medical Imaging and Radiation Therapy (CARE) Act to require national standards for radiation therapy team members.
ASTRO and the American College of Radiology also jointly issued practice guidelines for IGRT (Int. J. Radiol. Oncol. Biol. Phys. 2010;76:319-25) and SBRT (Int. J. Radiol. Oncol. Biol. Phys. 2010;76:326-32), two developing radiation therapies. The guidelines were published in the February issue of the International Journal of Radiation Oncology, Biology, Physics, the official journal of ASTRO.
IGRT uses digitally reconstructed images of a treatment area to direct radiation to the tumor. New IGRT guidelines address the clinical implication of this technology, including personnel qualifications, quality assurance standards, and suggested documentation.
SBRT is a type of external beam radiation therapy that can be completed in 1-5 days, instead of several weeks. SBRT is most commonly used for small tumors. This technology requires greater levels of precision and accuracy than does conventional fractionated radiation therapy of intensity-modulated delivery.
The new guidelines provide guidance in administering this complex treatment, and define quality criteria in view of the high technical demands of SBRT.
The American Society for Radiation Oncology has released a six-point plan to improve patient safety and reduce the potential for medical errors during cancer radiotherapy.
The plan comes on the heels of two stories in the New York Times that detailed cases of excess radiation used in the treatment of specific patients, and raised alarms about errors in radiotherapy. Its announcement follows initiation of a systemic review of the society's patient safety and quality assurance projects at the ASTRO board of directors winter meeting in late January.
“We have been developing and refining many of these programs for years. … By committing to this plan, we are redoubling our efforts in this essential area of our specialty,” Dr. Timothy R. Williams, ASTRO board chairman, explained in a statement.
Dr. Williams, a practicing radiation oncologist in Boca Raton, Fla., acknowledged the stories, calling them “deeply troubling” to the society and noting that “in any area of medicine—and radiation oncology is no exception—even one error is too many. … Any errors, no matter how small, must be reported, understood, and used as a tool to further reduce the potential for future errors.”
The six key areas of effort include
▸ Creating a database for the reporting of linear-accelerator– and computer tomography–based medical errors.
▸ Enhancing ASTRO's practice accreditation program, starting with new accreditation models for advanced technologies, such as image-guided radiation therapy (IGRT), stereotactic body radiation therapy (SBRT), and brachytherapy.
▸ Expanding educational training programs to include courses on quality assurance and safety.
▸ Developing tools for cancer patients and caregivers to use in their discussions with their radiation oncologists to help understand quality and safety programs at the centers where they are being treated.
▸ Further developing the Integrating the Healthcare Enterprise–Radiation Oncology connectivity compliance program to ensure that medical technologies from different manufacturers can safely transfer information to reduce the chance of medical error.
▸ Advocating for the immediate passage of the Consistency, Accuracy, Responsibility and Excellence in Medical Imaging and Radiation Therapy (CARE) Act to require national standards for radiation therapy team members.
ASTRO and the American College of Radiology also jointly issued practice guidelines for IGRT (Int. J. Radiol. Oncol. Biol. Phys. 2010;76:319-25) and SBRT (Int. J. Radiol. Oncol. Biol. Phys. 2010;76:326-32), two developing radiation therapies. The guidelines were published in the February issue of the International Journal of Radiation Oncology, Biology, Physics, the official journal of ASTRO.
IGRT uses digitally reconstructed images of a treatment area to direct radiation to the tumor. New IGRT guidelines address the clinical implication of this technology, including personnel qualifications, quality assurance standards, and suggested documentation.
SBRT is a type of external beam radiation therapy that can be completed in 1-5 days, instead of several weeks. SBRT is most commonly used for small tumors. This technology requires greater levels of precision and accuracy than does conventional fractionated radiation therapy of intensity-modulated delivery.
The new guidelines provide guidance in administering this complex treatment, and define quality criteria in view of the high technical demands of SBRT.
The American Society for Radiation Oncology has released a six-point plan to improve patient safety and reduce the potential for medical errors during cancer radiotherapy.
The plan comes on the heels of two stories in the New York Times that detailed cases of excess radiation used in the treatment of specific patients, and raised alarms about errors in radiotherapy. Its announcement follows initiation of a systemic review of the society's patient safety and quality assurance projects at the ASTRO board of directors winter meeting in late January.
“We have been developing and refining many of these programs for years. … By committing to this plan, we are redoubling our efforts in this essential area of our specialty,” Dr. Timothy R. Williams, ASTRO board chairman, explained in a statement.
Dr. Williams, a practicing radiation oncologist in Boca Raton, Fla., acknowledged the stories, calling them “deeply troubling” to the society and noting that “in any area of medicine—and radiation oncology is no exception—even one error is too many. … Any errors, no matter how small, must be reported, understood, and used as a tool to further reduce the potential for future errors.”
The six key areas of effort include
▸ Creating a database for the reporting of linear-accelerator– and computer tomography–based medical errors.
▸ Enhancing ASTRO's practice accreditation program, starting with new accreditation models for advanced technologies, such as image-guided radiation therapy (IGRT), stereotactic body radiation therapy (SBRT), and brachytherapy.
▸ Expanding educational training programs to include courses on quality assurance and safety.
▸ Developing tools for cancer patients and caregivers to use in their discussions with their radiation oncologists to help understand quality and safety programs at the centers where they are being treated.
▸ Further developing the Integrating the Healthcare Enterprise–Radiation Oncology connectivity compliance program to ensure that medical technologies from different manufacturers can safely transfer information to reduce the chance of medical error.
▸ Advocating for the immediate passage of the Consistency, Accuracy, Responsibility and Excellence in Medical Imaging and Radiation Therapy (CARE) Act to require national standards for radiation therapy team members.
ASTRO and the American College of Radiology also jointly issued practice guidelines for IGRT (Int. J. Radiol. Oncol. Biol. Phys. 2010;76:319-25) and SBRT (Int. J. Radiol. Oncol. Biol. Phys. 2010;76:326-32), two developing radiation therapies. The guidelines were published in the February issue of the International Journal of Radiation Oncology, Biology, Physics, the official journal of ASTRO.
IGRT uses digitally reconstructed images of a treatment area to direct radiation to the tumor. New IGRT guidelines address the clinical implication of this technology, including personnel qualifications, quality assurance standards, and suggested documentation.
SBRT is a type of external beam radiation therapy that can be completed in 1-5 days, instead of several weeks. SBRT is most commonly used for small tumors. This technology requires greater levels of precision and accuracy than does conventional fractionated radiation therapy of intensity-modulated delivery.
The new guidelines provide guidance in administering this complex treatment, and define quality criteria in view of the high technical demands of SBRT.
Vigilance is Required to Avoid Misdiagnosed Melanomas
Misdiagnosis of melanoma is a major cause of litigation against both dermatologists and dermatopathologists.
The majority of claims filed between 1985 and 2001 involving the misdiagnosis of melanoma were because of a false negative diagnosis, which may translate to a reduced chance of survival for some patients, Dr. Ashfaq A. Marghoob reported at the seminar
Two important strategies can help minimize missing melanoma, he said. First, remain vigilant and remember that many melanomas lack the classic ABCD features.
"Questioning yourself and your pathologist regarding the diagnosis will help towards identifying many of these melanomas. In other words, remain skeptical of lesions lacking clinical-dermoscopy correlation or lesions lacking dermoscopy-histopathology correlation. Second, engage patients in their own care by having them share the responsibility of detecting early melanoma by encouraging them to examine their own skin on a regular basis," said Dr. Marghoob, who is a dermatologist at Memorial Sloan-Kettering Cancer Center in New York.
Some melanomas may not manifest concerning features, and can mimic benign lesions. To assure that a malignant melanoma will eventually be found, periodic total body examinations by a physician, and regular patient self-examinations, are key. He stressed that physician examinations and self-skin exams are complementary.
While it is widely accepted that early detection means better prognosis, modest delays of up to 6 months have not been shown to affect ultimate outcomes. However, there is one exception. Nodular melanoma can grow rapidly, and even small delays in diagnosis can have serious consequences.
The most common scenarios in melanoma litigation cases include nodular melanoma being misdiagnosed by a clinician or pathologist; a partial biopsy not capturing the most diagnostically relevant part of the lesion; malignant melanoma being misdiagnosed as a dysplastic or spitz nevus; unrecognized desmoplastic malignant melanoma; and metastatic malignant melanoma with an unknown primary or recurrence of melanoma (Am. J. Surg. Pathol. 2003;27:1278-83).
Dr. Marghoob discussed each of these cases in detail.
Misdiagnosis of nodular melanoma as nevus by a clinician or pathologist. Many nodular melanomas lack helpful diagnostic features, such as those in the ABCD criteria for malignant melanoma, which can lead to a misdiagnosis. However, the ABCDE criteria that take lesion evolution into account may be of some help, noted Dr. Marghoob.
In order to track lesion evolution, ask patients about the history of changes and symptoms. Total body photography may help on rare occasions to detect new lesions, some of which may be subtle. In addition, dermoscopy results may persuade the clinician to obtain a biopsy of a clinically banal appearing lesion that is in fact a nodular melanoma.
Partial biopsy issues. If a biopsy is performed of a lesion that clinically looks like melanoma and the pathology diagnosis is nevus, it is imperative that the clinician and pathologist reconcile the difference. In cases where there is discordance, consider asking for step-sectioning, special stains, or--in very rare instances--fluorescence in situ hybridization to look for signature chromosomal aberrations. In addition, a partial biopsy may not be representative of the rest of the lesion. If a partial biopsy was performed, re-excise the lesion, said Dr. Marghoob. Remember that a pathology report should never be read in a vacuum.
Excisional biopsy is the preferred method for melanocytic lesions, when possible, because partial biopsy may sample nondiagnostic areas or miss the prognostically-worse portion of the lesion.
"Partial biopsy assumes that a clinician can consistently predict the portion of a suspicious pigmented lesion that will have the worst representative histology," said Dr. Marghoob. In one study, 40% of excised melanomas had worse pathology, compared with initial punch biopsy, and 20% of melanomas revealed invasion, which was not seen in initial punch biopsy (Arch. Dermatol. 1996;132:1297-1302).
The ideal biopsy is excisional with a 2- to 3-mm margin, is oriented along the lines of lymphatic drainage, and is step sectioned. This limits sampling error, removes dysplastic nevus completely (preventing recurrence), and better predicts the Breslow depth if the lesion proves to be a melanoma, said Dr. Marghoob.
Even when step sectioned, less than 2% of the lesion is evaluated. In one study, thorough block sampling resulted in identification of increased tumor thickness in 43% of cases by a mean of 0.16 mm (Arch. Dermatol. 2005;141:734-6).
Misdiagnosis of a melanoma as dysplastic or spitz nevus. When a partial biopsy reveals dysplastic or spitz nevus, it is important to completely excise the lesion. Malignant melanoma can sometimes masquerade as a spitz nevus, and focus of malignant melanoma may have been missed on the biopsy. Many dermatologists are of the opinion that spitz nevi should be completely excised--at least in adults, said Dr. Marghoob.
Unrecognized desmoplastic malignant melanoma. Desmoplastic melanoma can be banal in appearance, with 70% appearing amelanotic, he said. These lesions may only present as firmness in the subcutaneous tissue. For "banal" appearing firm lesions on chronically sun-damaged skin, suspicion should be raised if the lesions are symptomatic, growing, are associated with a lentigo maligna, or reveal irregular vessels with dermoscopy, said Dr. Marghoob.
Metastatic melanoma with unknown primary or recurrence of melanoma. Whenever possible, do not remove seemingly benign lesions and discard them, he said. Also, be careful and selective about the use of liquid nitrogen or a laser on lesions that have not been confirmed to be benign through biopsy.
He noted that cases of assumed benign lesions that recur after ablation (via liquid nitrogen, curettage, or laser), may ultimately prove to be melanoma on histopathology. Furthermore, in the unlikely event that a patient develops metastatic melanoma with an unknown primary, it may be presumed that one of the ablated lesions may have been the primary.
Dr. Marghoob disclosed having no conflicts of interest. SDEF and this news organization are owned by Elsevier.
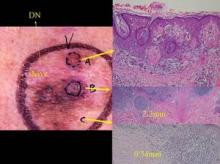
A partial biopsy was performed of this lesion which had clinical features of melanoma. The pathology analysis was reported as a Clark’s nevus (this area is the white scar on the left upper portion of the lesion). Due the clinical-pathology discordance the clinician decided to excise the lesion. As can be seen from the histology, this lesion was a melanoma and depending on the location of a partial biopsy the results can range from a Clark’s nevus to melanoma in situ to microinvasive to deeply invasive melanoma. Photo courtesy Dr. Ashfaq Marghoob.
Misdiagnosis of melanoma is a major cause of litigation against both dermatologists and dermatopathologists.
The majority of claims filed between 1985 and 2001 involving the misdiagnosis of melanoma were because of a false negative diagnosis, which may translate to a reduced chance of survival for some patients, Dr. Ashfaq A. Marghoob reported at the seminar
Two important strategies can help minimize missing melanoma, he said. First, remain vigilant and remember that many melanomas lack the classic ABCD features.
"Questioning yourself and your pathologist regarding the diagnosis will help towards identifying many of these melanomas. In other words, remain skeptical of lesions lacking clinical-dermoscopy correlation or lesions lacking dermoscopy-histopathology correlation. Second, engage patients in their own care by having them share the responsibility of detecting early melanoma by encouraging them to examine their own skin on a regular basis," said Dr. Marghoob, who is a dermatologist at Memorial Sloan-Kettering Cancer Center in New York.
Some melanomas may not manifest concerning features, and can mimic benign lesions. To assure that a malignant melanoma will eventually be found, periodic total body examinations by a physician, and regular patient self-examinations, are key. He stressed that physician examinations and self-skin exams are complementary.
While it is widely accepted that early detection means better prognosis, modest delays of up to 6 months have not been shown to affect ultimate outcomes. However, there is one exception. Nodular melanoma can grow rapidly, and even small delays in diagnosis can have serious consequences.
The most common scenarios in melanoma litigation cases include nodular melanoma being misdiagnosed by a clinician or pathologist; a partial biopsy not capturing the most diagnostically relevant part of the lesion; malignant melanoma being misdiagnosed as a dysplastic or spitz nevus; unrecognized desmoplastic malignant melanoma; and metastatic malignant melanoma with an unknown primary or recurrence of melanoma (Am. J. Surg. Pathol. 2003;27:1278-83).
Dr. Marghoob discussed each of these cases in detail.
Misdiagnosis of nodular melanoma as nevus by a clinician or pathologist. Many nodular melanomas lack helpful diagnostic features, such as those in the ABCD criteria for malignant melanoma, which can lead to a misdiagnosis. However, the ABCDE criteria that take lesion evolution into account may be of some help, noted Dr. Marghoob.
In order to track lesion evolution, ask patients about the history of changes and symptoms. Total body photography may help on rare occasions to detect new lesions, some of which may be subtle. In addition, dermoscopy results may persuade the clinician to obtain a biopsy of a clinically banal appearing lesion that is in fact a nodular melanoma.
Partial biopsy issues. If a biopsy is performed of a lesion that clinically looks like melanoma and the pathology diagnosis is nevus, it is imperative that the clinician and pathologist reconcile the difference. In cases where there is discordance, consider asking for step-sectioning, special stains, or--in very rare instances--fluorescence in situ hybridization to look for signature chromosomal aberrations. In addition, a partial biopsy may not be representative of the rest of the lesion. If a partial biopsy was performed, re-excise the lesion, said Dr. Marghoob. Remember that a pathology report should never be read in a vacuum.
Excisional biopsy is the preferred method for melanocytic lesions, when possible, because partial biopsy may sample nondiagnostic areas or miss the prognostically-worse portion of the lesion.
"Partial biopsy assumes that a clinician can consistently predict the portion of a suspicious pigmented lesion that will have the worst representative histology," said Dr. Marghoob. In one study, 40% of excised melanomas had worse pathology, compared with initial punch biopsy, and 20% of melanomas revealed invasion, which was not seen in initial punch biopsy (Arch. Dermatol. 1996;132:1297-1302).
The ideal biopsy is excisional with a 2- to 3-mm margin, is oriented along the lines of lymphatic drainage, and is step sectioned. This limits sampling error, removes dysplastic nevus completely (preventing recurrence), and better predicts the Breslow depth if the lesion proves to be a melanoma, said Dr. Marghoob.
Even when step sectioned, less than 2% of the lesion is evaluated. In one study, thorough block sampling resulted in identification of increased tumor thickness in 43% of cases by a mean of 0.16 mm (Arch. Dermatol. 2005;141:734-6).
Misdiagnosis of a melanoma as dysplastic or spitz nevus. When a partial biopsy reveals dysplastic or spitz nevus, it is important to completely excise the lesion. Malignant melanoma can sometimes masquerade as a spitz nevus, and focus of malignant melanoma may have been missed on the biopsy. Many dermatologists are of the opinion that spitz nevi should be completely excised--at least in adults, said Dr. Marghoob.
Unrecognized desmoplastic malignant melanoma. Desmoplastic melanoma can be banal in appearance, with 70% appearing amelanotic, he said. These lesions may only present as firmness in the subcutaneous tissue. For "banal" appearing firm lesions on chronically sun-damaged skin, suspicion should be raised if the lesions are symptomatic, growing, are associated with a lentigo maligna, or reveal irregular vessels with dermoscopy, said Dr. Marghoob.
Metastatic melanoma with unknown primary or recurrence of melanoma. Whenever possible, do not remove seemingly benign lesions and discard them, he said. Also, be careful and selective about the use of liquid nitrogen or a laser on lesions that have not been confirmed to be benign through biopsy.
He noted that cases of assumed benign lesions that recur after ablation (via liquid nitrogen, curettage, or laser), may ultimately prove to be melanoma on histopathology. Furthermore, in the unlikely event that a patient develops metastatic melanoma with an unknown primary, it may be presumed that one of the ablated lesions may have been the primary.
Dr. Marghoob disclosed having no conflicts of interest. SDEF and this news organization are owned by Elsevier.
A partial biopsy was performed of this lesion which had clinical features of melanoma. The pathology analysis was reported as a Clark’s nevus (this area is the white scar on the left upper portion of the lesion). Due the clinical-pathology discordance the clinician decided to excise the lesion. As can be seen from the histology, this lesion was a melanoma and depending on the location of a partial biopsy the results can range from a Clark’s nevus to melanoma in situ to microinvasive to deeply invasive melanoma. Photo courtesy Dr. Ashfaq Marghoob.
Misdiagnosis of melanoma is a major cause of litigation against both dermatologists and dermatopathologists.
The majority of claims filed between 1985 and 2001 involving the misdiagnosis of melanoma were because of a false negative diagnosis, which may translate to a reduced chance of survival for some patients, Dr. Ashfaq A. Marghoob reported at the seminar
Two important strategies can help minimize missing melanoma, he said. First, remain vigilant and remember that many melanomas lack the classic ABCD features.
"Questioning yourself and your pathologist regarding the diagnosis will help towards identifying many of these melanomas. In other words, remain skeptical of lesions lacking clinical-dermoscopy correlation or lesions lacking dermoscopy-histopathology correlation. Second, engage patients in their own care by having them share the responsibility of detecting early melanoma by encouraging them to examine their own skin on a regular basis," said Dr. Marghoob, who is a dermatologist at Memorial Sloan-Kettering Cancer Center in New York.
Some melanomas may not manifest concerning features, and can mimic benign lesions. To assure that a malignant melanoma will eventually be found, periodic total body examinations by a physician, and regular patient self-examinations, are key. He stressed that physician examinations and self-skin exams are complementary.
While it is widely accepted that early detection means better prognosis, modest delays of up to 6 months have not been shown to affect ultimate outcomes. However, there is one exception. Nodular melanoma can grow rapidly, and even small delays in diagnosis can have serious consequences.
The most common scenarios in melanoma litigation cases include nodular melanoma being misdiagnosed by a clinician or pathologist; a partial biopsy not capturing the most diagnostically relevant part of the lesion; malignant melanoma being misdiagnosed as a dysplastic or spitz nevus; unrecognized desmoplastic malignant melanoma; and metastatic malignant melanoma with an unknown primary or recurrence of melanoma (Am. J. Surg. Pathol. 2003;27:1278-83).
Dr. Marghoob discussed each of these cases in detail.
Misdiagnosis of nodular melanoma as nevus by a clinician or pathologist. Many nodular melanomas lack helpful diagnostic features, such as those in the ABCD criteria for malignant melanoma, which can lead to a misdiagnosis. However, the ABCDE criteria that take lesion evolution into account may be of some help, noted Dr. Marghoob.
In order to track lesion evolution, ask patients about the history of changes and symptoms. Total body photography may help on rare occasions to detect new lesions, some of which may be subtle. In addition, dermoscopy results may persuade the clinician to obtain a biopsy of a clinically banal appearing lesion that is in fact a nodular melanoma.
Partial biopsy issues. If a biopsy is performed of a lesion that clinically looks like melanoma and the pathology diagnosis is nevus, it is imperative that the clinician and pathologist reconcile the difference. In cases where there is discordance, consider asking for step-sectioning, special stains, or--in very rare instances--fluorescence in situ hybridization to look for signature chromosomal aberrations. In addition, a partial biopsy may not be representative of the rest of the lesion. If a partial biopsy was performed, re-excise the lesion, said Dr. Marghoob. Remember that a pathology report should never be read in a vacuum.
Excisional biopsy is the preferred method for melanocytic lesions, when possible, because partial biopsy may sample nondiagnostic areas or miss the prognostically-worse portion of the lesion.
"Partial biopsy assumes that a clinician can consistently predict the portion of a suspicious pigmented lesion that will have the worst representative histology," said Dr. Marghoob. In one study, 40% of excised melanomas had worse pathology, compared with initial punch biopsy, and 20% of melanomas revealed invasion, which was not seen in initial punch biopsy (Arch. Dermatol. 1996;132:1297-1302).
The ideal biopsy is excisional with a 2- to 3-mm margin, is oriented along the lines of lymphatic drainage, and is step sectioned. This limits sampling error, removes dysplastic nevus completely (preventing recurrence), and better predicts the Breslow depth if the lesion proves to be a melanoma, said Dr. Marghoob.
Even when step sectioned, less than 2% of the lesion is evaluated. In one study, thorough block sampling resulted in identification of increased tumor thickness in 43% of cases by a mean of 0.16 mm (Arch. Dermatol. 2005;141:734-6).
Misdiagnosis of a melanoma as dysplastic or spitz nevus. When a partial biopsy reveals dysplastic or spitz nevus, it is important to completely excise the lesion. Malignant melanoma can sometimes masquerade as a spitz nevus, and focus of malignant melanoma may have been missed on the biopsy. Many dermatologists are of the opinion that spitz nevi should be completely excised--at least in adults, said Dr. Marghoob.
Unrecognized desmoplastic malignant melanoma. Desmoplastic melanoma can be banal in appearance, with 70% appearing amelanotic, he said. These lesions may only present as firmness in the subcutaneous tissue. For "banal" appearing firm lesions on chronically sun-damaged skin, suspicion should be raised if the lesions are symptomatic, growing, are associated with a lentigo maligna, or reveal irregular vessels with dermoscopy, said Dr. Marghoob.
Metastatic melanoma with unknown primary or recurrence of melanoma. Whenever possible, do not remove seemingly benign lesions and discard them, he said. Also, be careful and selective about the use of liquid nitrogen or a laser on lesions that have not been confirmed to be benign through biopsy.
He noted that cases of assumed benign lesions that recur after ablation (via liquid nitrogen, curettage, or laser), may ultimately prove to be melanoma on histopathology. Furthermore, in the unlikely event that a patient develops metastatic melanoma with an unknown primary, it may be presumed that one of the ablated lesions may have been the primary.
Dr. Marghoob disclosed having no conflicts of interest. SDEF and this news organization are owned by Elsevier.
A partial biopsy was performed of this lesion which had clinical features of melanoma. The pathology analysis was reported as a Clark’s nevus (this area is the white scar on the left upper portion of the lesion). Due the clinical-pathology discordance the clinician decided to excise the lesion. As can be seen from the histology, this lesion was a melanoma and depending on the location of a partial biopsy the results can range from a Clark’s nevus to melanoma in situ to microinvasive to deeply invasive melanoma. Photo courtesy Dr. Ashfaq Marghoob.
Immunoassay May Identify Early-Stage Pancreatic Cancer
An investigational immunoassay can accurately identify pancreatic cancer, potentially giving clinicians the ability to identify and treat the disease in its early stages, according to research presented at the American Society of Clinical Oncology's annual gastrointestinal cancer symposium.
The assay uses a monoclonal antibody to hone in on malignant pancreatic cells; for added benefit, investigators are also employing the antibody to deliver targeted radiotherapy to tumor cells.
“We were able to identify the overwhelming majority of patients with early-stage disease,” lead author David V. Gold, Ph.D., said in a teleconference.
The PAM4 monoclonal antibody (clivatuzumab) quantifies blood levels of the PAM4 protein “that appears to be relatively unique to pancreatic cancer,” he said. The protein is not present in normal pancreatic tissue or in other types of malignancies. It also is rarely detected in pancreatitis, making it highly specific for pancreatic cancer.
In the small study, Dr. Gold and his associates evaluated 68 patients who had undergone surgery for pancreatic cancer. The investigators obtained information about disease stage from surgical notes about the spread of the disease. They also evaluated 19 healthy controls.
The sensitivity of the PAM4 blood test for detecting stage I pancreatic cancer (disease confined to the pancreas), stage II disease (disease that has spread to nearby organs), and stage III/IV cancers (disease with local and distant spread) was 62%, 86%, and 91%, respectively. Overall, the assay was 81% sensitive for detecting all stages of pancreatic cancer.
“The PAM4 blood test is very specific for pancreatic cancer. If the assay is positive, there is a high positive diagnostic likelihood that the patient has pancreatic cancer,” said Dr. Gold, a researcher at the Garden State Cancer Center in Belleville, N.J.
If validated, the assay would be valuable for the management of patients with the disease. Most patients do not have symptoms until the advanced stages of tumor growth, when cure is unlikely. Currently, only an estimated 5% of patients with pancreatic cancer survive to 5 years, according to the American Cancer Society.
The PAM4 antibody has the potential to be part of an effective therapy as well.
“Detection of the PAM4 antigen in the blood of these patients means that the cancer is producing the protein, and that this protein may act as a marker on the tumor for use of the antibody to target drugs and/or radioisotopes directly to the tumor,” Dr. Gold explained.
Researchers have already begun to explore attaching radioisotopes to the antibody in order to image tumors, or to target radiotherapy of the tumor cells in combination with chemotherapy. In a small related study, the researchers achieved a partial response rate (defined as at least a 30% reduction in the size of the tumor) of 23% and a stable disease rate of 45% in patients with stage III and IV pancreatic cancer.
“By using the combination of early detection and therapy improvements, we hope to be able to come up with a new paradigm for the management of the patient with pancreatic cancer,” Dr. Gold said.
The assay still needs to be validated in larger trials, however. He estimated that the assay and related therapies are still 2-3 years from clinical use.
Disclosures: Dr. Gold did not provide a disclosure statement. The study's senior author, Dr. David M. Goldenberg, disclosed that he is the chief scientific officer and chairman of the board of directors for Immunomedics Inc., a biopharmaceutical company that develops monoclonal, antibody-based products for the targeted treatment of cancer and other diseases.
My Take
Earlier Diagnosis Will Save Lives
Early diagnosis of pancreatic cancer can lead to a 10-fold improvement of survival (approximately 20% 5-year surgical survival for stage I disease versus 2% for stage IV disease). The problem has always been how to identify the patient with early disease since symptoms may occur late in those with pancreatic cancer.
The recent discovery that circulating blood levels of PAM4 (quantified through use of the monoclonal antibody clivatuzumab) are “relatively unique to pancreatic cancer” and positive in 68% of those with stage I pancreatic cancer raises hopes that we now have a tool that can lead to earlier diagnosis.
We do need to know more about the protein and its false-positive rates, ensuring that it is not prevalent in noncancer patients with chronic pancreatitis, diabetes mellitus, cigarette smoking, and other conditions that predispose to pancreatic cancer. That information and the development of a logical clinical algorithm of how to utilize circulating levels of PAM4 as a screening test will be important to determining its future clinical use.
An investigational immunoassay can accurately identify pancreatic cancer, potentially giving clinicians the ability to identify and treat the disease in its early stages, according to research presented at the American Society of Clinical Oncology's annual gastrointestinal cancer symposium.
The assay uses a monoclonal antibody to hone in on malignant pancreatic cells; for added benefit, investigators are also employing the antibody to deliver targeted radiotherapy to tumor cells.
“We were able to identify the overwhelming majority of patients with early-stage disease,” lead author David V. Gold, Ph.D., said in a teleconference.
The PAM4 monoclonal antibody (clivatuzumab) quantifies blood levels of the PAM4 protein “that appears to be relatively unique to pancreatic cancer,” he said. The protein is not present in normal pancreatic tissue or in other types of malignancies. It also is rarely detected in pancreatitis, making it highly specific for pancreatic cancer.
In the small study, Dr. Gold and his associates evaluated 68 patients who had undergone surgery for pancreatic cancer. The investigators obtained information about disease stage from surgical notes about the spread of the disease. They also evaluated 19 healthy controls.
The sensitivity of the PAM4 blood test for detecting stage I pancreatic cancer (disease confined to the pancreas), stage II disease (disease that has spread to nearby organs), and stage III/IV cancers (disease with local and distant spread) was 62%, 86%, and 91%, respectively. Overall, the assay was 81% sensitive for detecting all stages of pancreatic cancer.
“The PAM4 blood test is very specific for pancreatic cancer. If the assay is positive, there is a high positive diagnostic likelihood that the patient has pancreatic cancer,” said Dr. Gold, a researcher at the Garden State Cancer Center in Belleville, N.J.
If validated, the assay would be valuable for the management of patients with the disease. Most patients do not have symptoms until the advanced stages of tumor growth, when cure is unlikely. Currently, only an estimated 5% of patients with pancreatic cancer survive to 5 years, according to the American Cancer Society.
The PAM4 antibody has the potential to be part of an effective therapy as well.
“Detection of the PAM4 antigen in the blood of these patients means that the cancer is producing the protein, and that this protein may act as a marker on the tumor for use of the antibody to target drugs and/or radioisotopes directly to the tumor,” Dr. Gold explained.
Researchers have already begun to explore attaching radioisotopes to the antibody in order to image tumors, or to target radiotherapy of the tumor cells in combination with chemotherapy. In a small related study, the researchers achieved a partial response rate (defined as at least a 30% reduction in the size of the tumor) of 23% and a stable disease rate of 45% in patients with stage III and IV pancreatic cancer.
“By using the combination of early detection and therapy improvements, we hope to be able to come up with a new paradigm for the management of the patient with pancreatic cancer,” Dr. Gold said.
The assay still needs to be validated in larger trials, however. He estimated that the assay and related therapies are still 2-3 years from clinical use.
Disclosures: Dr. Gold did not provide a disclosure statement. The study's senior author, Dr. David M. Goldenberg, disclosed that he is the chief scientific officer and chairman of the board of directors for Immunomedics Inc., a biopharmaceutical company that develops monoclonal, antibody-based products for the targeted treatment of cancer and other diseases.
My Take
Earlier Diagnosis Will Save Lives
Early diagnosis of pancreatic cancer can lead to a 10-fold improvement of survival (approximately 20% 5-year surgical survival for stage I disease versus 2% for stage IV disease). The problem has always been how to identify the patient with early disease since symptoms may occur late in those with pancreatic cancer.
The recent discovery that circulating blood levels of PAM4 (quantified through use of the monoclonal antibody clivatuzumab) are “relatively unique to pancreatic cancer” and positive in 68% of those with stage I pancreatic cancer raises hopes that we now have a tool that can lead to earlier diagnosis.
We do need to know more about the protein and its false-positive rates, ensuring that it is not prevalent in noncancer patients with chronic pancreatitis, diabetes mellitus, cigarette smoking, and other conditions that predispose to pancreatic cancer. That information and the development of a logical clinical algorithm of how to utilize circulating levels of PAM4 as a screening test will be important to determining its future clinical use.
An investigational immunoassay can accurately identify pancreatic cancer, potentially giving clinicians the ability to identify and treat the disease in its early stages, according to research presented at the American Society of Clinical Oncology's annual gastrointestinal cancer symposium.
The assay uses a monoclonal antibody to hone in on malignant pancreatic cells; for added benefit, investigators are also employing the antibody to deliver targeted radiotherapy to tumor cells.
“We were able to identify the overwhelming majority of patients with early-stage disease,” lead author David V. Gold, Ph.D., said in a teleconference.
The PAM4 monoclonal antibody (clivatuzumab) quantifies blood levels of the PAM4 protein “that appears to be relatively unique to pancreatic cancer,” he said. The protein is not present in normal pancreatic tissue or in other types of malignancies. It also is rarely detected in pancreatitis, making it highly specific for pancreatic cancer.
In the small study, Dr. Gold and his associates evaluated 68 patients who had undergone surgery for pancreatic cancer. The investigators obtained information about disease stage from surgical notes about the spread of the disease. They also evaluated 19 healthy controls.
The sensitivity of the PAM4 blood test for detecting stage I pancreatic cancer (disease confined to the pancreas), stage II disease (disease that has spread to nearby organs), and stage III/IV cancers (disease with local and distant spread) was 62%, 86%, and 91%, respectively. Overall, the assay was 81% sensitive for detecting all stages of pancreatic cancer.
“The PAM4 blood test is very specific for pancreatic cancer. If the assay is positive, there is a high positive diagnostic likelihood that the patient has pancreatic cancer,” said Dr. Gold, a researcher at the Garden State Cancer Center in Belleville, N.J.
If validated, the assay would be valuable for the management of patients with the disease. Most patients do not have symptoms until the advanced stages of tumor growth, when cure is unlikely. Currently, only an estimated 5% of patients with pancreatic cancer survive to 5 years, according to the American Cancer Society.
The PAM4 antibody has the potential to be part of an effective therapy as well.
“Detection of the PAM4 antigen in the blood of these patients means that the cancer is producing the protein, and that this protein may act as a marker on the tumor for use of the antibody to target drugs and/or radioisotopes directly to the tumor,” Dr. Gold explained.
Researchers have already begun to explore attaching radioisotopes to the antibody in order to image tumors, or to target radiotherapy of the tumor cells in combination with chemotherapy. In a small related study, the researchers achieved a partial response rate (defined as at least a 30% reduction in the size of the tumor) of 23% and a stable disease rate of 45% in patients with stage III and IV pancreatic cancer.
“By using the combination of early detection and therapy improvements, we hope to be able to come up with a new paradigm for the management of the patient with pancreatic cancer,” Dr. Gold said.
The assay still needs to be validated in larger trials, however. He estimated that the assay and related therapies are still 2-3 years from clinical use.
Disclosures: Dr. Gold did not provide a disclosure statement. The study's senior author, Dr. David M. Goldenberg, disclosed that he is the chief scientific officer and chairman of the board of directors for Immunomedics Inc., a biopharmaceutical company that develops monoclonal, antibody-based products for the targeted treatment of cancer and other diseases.
My Take
Earlier Diagnosis Will Save Lives
Early diagnosis of pancreatic cancer can lead to a 10-fold improvement of survival (approximately 20% 5-year surgical survival for stage I disease versus 2% for stage IV disease). The problem has always been how to identify the patient with early disease since symptoms may occur late in those with pancreatic cancer.
The recent discovery that circulating blood levels of PAM4 (quantified through use of the monoclonal antibody clivatuzumab) are “relatively unique to pancreatic cancer” and positive in 68% of those with stage I pancreatic cancer raises hopes that we now have a tool that can lead to earlier diagnosis.
We do need to know more about the protein and its false-positive rates, ensuring that it is not prevalent in noncancer patients with chronic pancreatitis, diabetes mellitus, cigarette smoking, and other conditions that predispose to pancreatic cancer. That information and the development of a logical clinical algorithm of how to utilize circulating levels of PAM4 as a screening test will be important to determining its future clinical use.
IOM Urges Hepatitis Screening, Prevention
The Institute of Medicine is calling for increased awareness of hepatitis B and C among health care providers, social service providers, and at-risk communities as well as better surveillance and more stringent vaccination requirements nationwide in its newly released report on hepatitis and liver cancer.
“The committee believes that these recommendations will prevent further infections, improve the lives and health of infected individuals, and reduce the long-term burden of liver disease and liver cancer,” Dr. R. Palmer Beasley said during a teleconference sponsored by the National Academies of Science. Dr. Beasley chaired the IOM committee that wrote the report “Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C” (available at www.iom.edu/viralhepatitis
“Although hepatitis B and C are preventable, the rates of infection have not declined over the past several years, underscoring the conclusion that we have allowed gaps in screening, prevention, and treatment to go unchecked,” Dr. Beasley said in a statement.
“This report outlines the additional resources and actions needed to reduce the unacceptably high burden of liver disease and cancer associated with these viruses,” said Dr. Beasley, who is a professor of epidemiology and disease control at the University of Texas in Houston.
It's estimated that 800,000 to 1.4 million Americans have chronic hepatitis B, and between 2.7 million and 3.9 million have chronic hepatitis C. Most are unaware that they are infected until they develop symptoms of liver cancer or liver disease.
The committee identified three major factors that impede current efforts to prevent and control hepatitis B and C:
▸ Lack of knowledge and awareness about chronic viral hepatitis on the part of health care and social service providers.
▸ Inadequate knowledge and awareness about chronic viral hepatitis among at-risk populations, members of the public, and policy makers.
▸ Insufficient understanding about the extent and seriousness of this public health problem that has led to the inadequate allocation of resources for prevention, control, and surveillance programs.
Perhaps the greatest difficulty in diagnosing and treating patients with hepatitis B and C is that these diseases are often asymptomatic.
In addition, minority groups—Asians, Pacific Islanders, and blacks—are at greatest risk. Those most at risk for hepatitis B include people born in East and Southeast Asia or sub-Saharan Africa, infants born to women infected with the disease, and people who have sexual contact or share injection-drug equipment with an infected person. Those at greatest risk for hepatitis C include people who received a blood transfusion before 1992 (before implementation of blood screening for hepatitis C) and past or current injection drug users.
The committee noted that health care and social service providers generally have limited knowledge about the two conditions. Many of these providers fail to follow guidelines for screening patients and providing prevention, treatment, and follow-up services.
The committee made recommendations for improving surveillance, knowledge and awareness, immunization, and services for viral hepatitis. Highlights of these recommendations include:
▸ A complete evaluation by the Centers for Disease Control and Prevention of the national hepatitis B and C public health surveillance system.
▸ Coordination between CDC and key stakeholders to develop hepatitis B and C education programs for health care and social service personnel.
▸ Coordination between CDC and key stakeholders to develop innovative and effective programs to target at-risk populations and to increase awareness of hepatitis B and C among the general public.
▸ Vaccination of all neonates weighing at least 2,000 g and born to hepatitis B–positive women.
▸ Mandatory vaccination for hepatitis B as a requirement for school attendance.
▸ Studies to develop a vaccine for hepatitis C.
▸ Resources for the expansion of community-based programs that provide hepatitis B screening, testing, and vaccination services that target at-risk populations.
▸ A public awareness initiative, similar to the successful effort to increase recognition, prevention, and treatment of HIV/AIDS.
The report was developed in partnership with the Centers for Disease Control and Prevention Foundation, the Office of Minority Health, the Department of Veterans Affairs, and the National Viral Hepatitis Roundtable.
The Institute of Medicine is calling for increased awareness of hepatitis B and C among health care providers, social service providers, and at-risk communities as well as better surveillance and more stringent vaccination requirements nationwide in its newly released report on hepatitis and liver cancer.
“The committee believes that these recommendations will prevent further infections, improve the lives and health of infected individuals, and reduce the long-term burden of liver disease and liver cancer,” Dr. R. Palmer Beasley said during a teleconference sponsored by the National Academies of Science. Dr. Beasley chaired the IOM committee that wrote the report “Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C” (available at www.iom.edu/viralhepatitis
“Although hepatitis B and C are preventable, the rates of infection have not declined over the past several years, underscoring the conclusion that we have allowed gaps in screening, prevention, and treatment to go unchecked,” Dr. Beasley said in a statement.
“This report outlines the additional resources and actions needed to reduce the unacceptably high burden of liver disease and cancer associated with these viruses,” said Dr. Beasley, who is a professor of epidemiology and disease control at the University of Texas in Houston.
It's estimated that 800,000 to 1.4 million Americans have chronic hepatitis B, and between 2.7 million and 3.9 million have chronic hepatitis C. Most are unaware that they are infected until they develop symptoms of liver cancer or liver disease.
The committee identified three major factors that impede current efforts to prevent and control hepatitis B and C:
▸ Lack of knowledge and awareness about chronic viral hepatitis on the part of health care and social service providers.
▸ Inadequate knowledge and awareness about chronic viral hepatitis among at-risk populations, members of the public, and policy makers.
▸ Insufficient understanding about the extent and seriousness of this public health problem that has led to the inadequate allocation of resources for prevention, control, and surveillance programs.
Perhaps the greatest difficulty in diagnosing and treating patients with hepatitis B and C is that these diseases are often asymptomatic.
In addition, minority groups—Asians, Pacific Islanders, and blacks—are at greatest risk. Those most at risk for hepatitis B include people born in East and Southeast Asia or sub-Saharan Africa, infants born to women infected with the disease, and people who have sexual contact or share injection-drug equipment with an infected person. Those at greatest risk for hepatitis C include people who received a blood transfusion before 1992 (before implementation of blood screening for hepatitis C) and past or current injection drug users.
The committee noted that health care and social service providers generally have limited knowledge about the two conditions. Many of these providers fail to follow guidelines for screening patients and providing prevention, treatment, and follow-up services.
The committee made recommendations for improving surveillance, knowledge and awareness, immunization, and services for viral hepatitis. Highlights of these recommendations include:
▸ A complete evaluation by the Centers for Disease Control and Prevention of the national hepatitis B and C public health surveillance system.
▸ Coordination between CDC and key stakeholders to develop hepatitis B and C education programs for health care and social service personnel.
▸ Coordination between CDC and key stakeholders to develop innovative and effective programs to target at-risk populations and to increase awareness of hepatitis B and C among the general public.
▸ Vaccination of all neonates weighing at least 2,000 g and born to hepatitis B–positive women.
▸ Mandatory vaccination for hepatitis B as a requirement for school attendance.
▸ Studies to develop a vaccine for hepatitis C.
▸ Resources for the expansion of community-based programs that provide hepatitis B screening, testing, and vaccination services that target at-risk populations.
▸ A public awareness initiative, similar to the successful effort to increase recognition, prevention, and treatment of HIV/AIDS.
The report was developed in partnership with the Centers for Disease Control and Prevention Foundation, the Office of Minority Health, the Department of Veterans Affairs, and the National Viral Hepatitis Roundtable.
The Institute of Medicine is calling for increased awareness of hepatitis B and C among health care providers, social service providers, and at-risk communities as well as better surveillance and more stringent vaccination requirements nationwide in its newly released report on hepatitis and liver cancer.
“The committee believes that these recommendations will prevent further infections, improve the lives and health of infected individuals, and reduce the long-term burden of liver disease and liver cancer,” Dr. R. Palmer Beasley said during a teleconference sponsored by the National Academies of Science. Dr. Beasley chaired the IOM committee that wrote the report “Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C” (available at www.iom.edu/viralhepatitis
“Although hepatitis B and C are preventable, the rates of infection have not declined over the past several years, underscoring the conclusion that we have allowed gaps in screening, prevention, and treatment to go unchecked,” Dr. Beasley said in a statement.
“This report outlines the additional resources and actions needed to reduce the unacceptably high burden of liver disease and cancer associated with these viruses,” said Dr. Beasley, who is a professor of epidemiology and disease control at the University of Texas in Houston.
It's estimated that 800,000 to 1.4 million Americans have chronic hepatitis B, and between 2.7 million and 3.9 million have chronic hepatitis C. Most are unaware that they are infected until they develop symptoms of liver cancer or liver disease.
The committee identified three major factors that impede current efforts to prevent and control hepatitis B and C:
▸ Lack of knowledge and awareness about chronic viral hepatitis on the part of health care and social service providers.
▸ Inadequate knowledge and awareness about chronic viral hepatitis among at-risk populations, members of the public, and policy makers.
▸ Insufficient understanding about the extent and seriousness of this public health problem that has led to the inadequate allocation of resources for prevention, control, and surveillance programs.
Perhaps the greatest difficulty in diagnosing and treating patients with hepatitis B and C is that these diseases are often asymptomatic.
In addition, minority groups—Asians, Pacific Islanders, and blacks—are at greatest risk. Those most at risk for hepatitis B include people born in East and Southeast Asia or sub-Saharan Africa, infants born to women infected with the disease, and people who have sexual contact or share injection-drug equipment with an infected person. Those at greatest risk for hepatitis C include people who received a blood transfusion before 1992 (before implementation of blood screening for hepatitis C) and past or current injection drug users.
The committee noted that health care and social service providers generally have limited knowledge about the two conditions. Many of these providers fail to follow guidelines for screening patients and providing prevention, treatment, and follow-up services.
The committee made recommendations for improving surveillance, knowledge and awareness, immunization, and services for viral hepatitis. Highlights of these recommendations include:
▸ A complete evaluation by the Centers for Disease Control and Prevention of the national hepatitis B and C public health surveillance system.
▸ Coordination between CDC and key stakeholders to develop hepatitis B and C education programs for health care and social service personnel.
▸ Coordination between CDC and key stakeholders to develop innovative and effective programs to target at-risk populations and to increase awareness of hepatitis B and C among the general public.
▸ Vaccination of all neonates weighing at least 2,000 g and born to hepatitis B–positive women.
▸ Mandatory vaccination for hepatitis B as a requirement for school attendance.
▸ Studies to develop a vaccine for hepatitis C.
▸ Resources for the expansion of community-based programs that provide hepatitis B screening, testing, and vaccination services that target at-risk populations.
▸ A public awareness initiative, similar to the successful effort to increase recognition, prevention, and treatment of HIV/AIDS.
The report was developed in partnership with the Centers for Disease Control and Prevention Foundation, the Office of Minority Health, the Department of Veterans Affairs, and the National Viral Hepatitis Roundtable.
PCI Raises Revascularization Risk in Diabetes
Major Finding: Endovascular treatment with paclitaxel-eluting stents significantly increased the risk of revascularization, compared with CABG treatment, in diabetic patients with left main and/or three-vessel disease but did not increase the rates of death, cerebrovascular accident, or MI in these patients.
Data Source: A diabetes subgroup analysis of the SYNTAX trial.
Disclosures: Funded by Boston Scientific. Dr. Banning and four coauthors have financial ties to the company. Dr. Banning is partially funded by the National Health Research Institute's Biomedical Research Center.
Similar mortality rates for endovascular and surgical treatment of complex coronary artery disease suggest that drug-eluting stents may be a viable treatment for selected diabetes patients, though revascularization rates are greater for these patients.
In the diabetes subgroup analysis of the SYNTAX trial, the 1-year major adverse cardiac and cerebrovascular event rate was significantly greater in medically treated diabetic patients with left main and/or three-vessel disease who underwent percutaneous coronary intervention with paclitaxel-eluting stents (PES), than in those who had coronary artery bypass grafting.
However, this increase in the primary end point of the trial appears to bedriven largely by an increased rate of repeat revascularization.
“For patients with medically treated diabetes, PES treatment was a significant predictor of repeat revascularization” but not death, cerebrovascular event, or MI, wrote Dr. Adrian P. Banning and his associates (J. Am. Coll. Cardiol. 2010 Jan. 13 [doi:10.1016/j.jacc.2009.09.057
In a related commentary, Dr. Harold L. Dauerman noted that many clinicians are already performing multivessel PCI in diabetic patients, “many of whom investigators believe could not be served at all with CABG because of a variety of comorbidities (risk of stroke) or anatomic challenges (diffuse distal vessel disease, poor conduits).
“The SYNTAX study diabetes analysis does not tell those clinicians to stop doing PCI in diabetic patients,” said Dr. Dauerman, professor of cardiology at the University of Vermont in Burlington. Instead, the results suggest that PCI is a viable option given the caveat that diabetic patients undergoing PCI with [drug-eluting stents] remain at greater risk for repeat revascularization with PCI versus CABG.
The SYNTAX (Synergy Between [PCI] With Taxus and Cardiac Surgery) study included 1,800 patients with de novo left main and/or three-vessel disease, with or without diabetes.
Patients were randomized to undergo CABG or PCI using paclitaxel-eluting stents (PES). The diabetes substudy included the 452 patients with medically treated diabetes, of whom 71% had three-vessel disease and 29% had left main disease.
Beyond that, 79% of patients with left main disease had concurrent two- or three-vessel disease. Of the 452 diabetes patients, 231 underwent PCI and 221 underwent CABG. Most (94%) of the patients with diabetes had type 2 disease.
The researchers used a composite end point of all-cause death, cerebrovascular accident, MI, or repeat revascularization (any subsequent PES of CABG procedure in any coronary vessel).
Among diabetic patients, the 1-year event rate was significantly greater after PES (26%), compared with CABG (14%), for a relative risk of 1.83.
However, among nondiabetic patients, the 1-year event rate was slightly higher for the PES group, though this was not significant—15% vs. 12%, relative risk 1.28.
“The number needed to treat CABG to avoid 1 MACCE event is 9 for diabetic patients and 31 for nondiabetic patients,” wrote Dr. Banning, a consultant cardiologist at the John Radcliffe Hospital in Oxford, England, and his coauthors.
There were no significant differences between CABG and PES in terms of the composite safety end point (death, cerebrovascular accident, or MI) for either diabetic or nondiabetic patients in SYNTAX. Neither was there a significant difference in terms of symptomatic graft occlusion or stent thrombosis for diabetic or nondiabetic patients.
Repeat revascularization appears to have driven the significantly greater event rate for diabetic patients treated with PES. Repeat revascularization was greater for the PES group, regardless of diabetes status. The PES revascularization rate for diabetic patients was 20% compared with 6% for diabetic patients who underwent CABG. Likewise, the PES revascularization rate for nondiabetic patients was 11% compared with 6% for nondiabetic patients who underwent CABG.
Repeat revascularization following PES was also greater for diabetic patients than for nondiabetic patients. This was not true for CABG patients.
“Medically treated diabetes was a significant independent predictor of revascularization the PES arm (odds ratio of 2.93) but not in the CABG arm,” the investigators wrote.
However, the degree of glycemic control was not a significant predictor of 1-year outcomes for patients with diabetes.
Among diabetic patients, there were no differences in death, MI, or cerebrovascular accident between PES and CABG groups in either those treated with insulin (182) or those treated with oral hypoglycemics (270).
The authors cautioned that the 1-year results may not yet reflect the true long-term differences between CABG and PES treatments of diabetic patients.
SYNTAX was funded by Boston Scientific Corporation, which makes the Taxus paclitaxel-eluting stent. Dr. Banning and two of his coauthors have received grant support from Boston Scientific and two other coauthors are employed by the company.
Dr. Banning also received funding from the National Health Research Institute's Biomedical Research Center in Oxford.
Dr. Dauerman indicated that he has significant financial relationships with Abbott Laboratories and Medtronic Inc.
Major Finding: Endovascular treatment with paclitaxel-eluting stents significantly increased the risk of revascularization, compared with CABG treatment, in diabetic patients with left main and/or three-vessel disease but did not increase the rates of death, cerebrovascular accident, or MI in these patients.
Data Source: A diabetes subgroup analysis of the SYNTAX trial.
Disclosures: Funded by Boston Scientific. Dr. Banning and four coauthors have financial ties to the company. Dr. Banning is partially funded by the National Health Research Institute's Biomedical Research Center.
Similar mortality rates for endovascular and surgical treatment of complex coronary artery disease suggest that drug-eluting stents may be a viable treatment for selected diabetes patients, though revascularization rates are greater for these patients.
In the diabetes subgroup analysis of the SYNTAX trial, the 1-year major adverse cardiac and cerebrovascular event rate was significantly greater in medically treated diabetic patients with left main and/or three-vessel disease who underwent percutaneous coronary intervention with paclitaxel-eluting stents (PES), than in those who had coronary artery bypass grafting.
However, this increase in the primary end point of the trial appears to bedriven largely by an increased rate of repeat revascularization.
“For patients with medically treated diabetes, PES treatment was a significant predictor of repeat revascularization” but not death, cerebrovascular event, or MI, wrote Dr. Adrian P. Banning and his associates (J. Am. Coll. Cardiol. 2010 Jan. 13 [doi:10.1016/j.jacc.2009.09.057
In a related commentary, Dr. Harold L. Dauerman noted that many clinicians are already performing multivessel PCI in diabetic patients, “many of whom investigators believe could not be served at all with CABG because of a variety of comorbidities (risk of stroke) or anatomic challenges (diffuse distal vessel disease, poor conduits).
“The SYNTAX study diabetes analysis does not tell those clinicians to stop doing PCI in diabetic patients,” said Dr. Dauerman, professor of cardiology at the University of Vermont in Burlington. Instead, the results suggest that PCI is a viable option given the caveat that diabetic patients undergoing PCI with [drug-eluting stents] remain at greater risk for repeat revascularization with PCI versus CABG.
The SYNTAX (Synergy Between [PCI] With Taxus and Cardiac Surgery) study included 1,800 patients with de novo left main and/or three-vessel disease, with or without diabetes.
Patients were randomized to undergo CABG or PCI using paclitaxel-eluting stents (PES). The diabetes substudy included the 452 patients with medically treated diabetes, of whom 71% had three-vessel disease and 29% had left main disease.
Beyond that, 79% of patients with left main disease had concurrent two- or three-vessel disease. Of the 452 diabetes patients, 231 underwent PCI and 221 underwent CABG. Most (94%) of the patients with diabetes had type 2 disease.
The researchers used a composite end point of all-cause death, cerebrovascular accident, MI, or repeat revascularization (any subsequent PES of CABG procedure in any coronary vessel).
Among diabetic patients, the 1-year event rate was significantly greater after PES (26%), compared with CABG (14%), for a relative risk of 1.83.
However, among nondiabetic patients, the 1-year event rate was slightly higher for the PES group, though this was not significant—15% vs. 12%, relative risk 1.28.
“The number needed to treat CABG to avoid 1 MACCE event is 9 for diabetic patients and 31 for nondiabetic patients,” wrote Dr. Banning, a consultant cardiologist at the John Radcliffe Hospital in Oxford, England, and his coauthors.
There were no significant differences between CABG and PES in terms of the composite safety end point (death, cerebrovascular accident, or MI) for either diabetic or nondiabetic patients in SYNTAX. Neither was there a significant difference in terms of symptomatic graft occlusion or stent thrombosis for diabetic or nondiabetic patients.
Repeat revascularization appears to have driven the significantly greater event rate for diabetic patients treated with PES. Repeat revascularization was greater for the PES group, regardless of diabetes status. The PES revascularization rate for diabetic patients was 20% compared with 6% for diabetic patients who underwent CABG. Likewise, the PES revascularization rate for nondiabetic patients was 11% compared with 6% for nondiabetic patients who underwent CABG.
Repeat revascularization following PES was also greater for diabetic patients than for nondiabetic patients. This was not true for CABG patients.
“Medically treated diabetes was a significant independent predictor of revascularization the PES arm (odds ratio of 2.93) but not in the CABG arm,” the investigators wrote.
However, the degree of glycemic control was not a significant predictor of 1-year outcomes for patients with diabetes.
Among diabetic patients, there were no differences in death, MI, or cerebrovascular accident between PES and CABG groups in either those treated with insulin (182) or those treated with oral hypoglycemics (270).
The authors cautioned that the 1-year results may not yet reflect the true long-term differences between CABG and PES treatments of diabetic patients.
SYNTAX was funded by Boston Scientific Corporation, which makes the Taxus paclitaxel-eluting stent. Dr. Banning and two of his coauthors have received grant support from Boston Scientific and two other coauthors are employed by the company.
Dr. Banning also received funding from the National Health Research Institute's Biomedical Research Center in Oxford.
Dr. Dauerman indicated that he has significant financial relationships with Abbott Laboratories and Medtronic Inc.
Major Finding: Endovascular treatment with paclitaxel-eluting stents significantly increased the risk of revascularization, compared with CABG treatment, in diabetic patients with left main and/or three-vessel disease but did not increase the rates of death, cerebrovascular accident, or MI in these patients.
Data Source: A diabetes subgroup analysis of the SYNTAX trial.
Disclosures: Funded by Boston Scientific. Dr. Banning and four coauthors have financial ties to the company. Dr. Banning is partially funded by the National Health Research Institute's Biomedical Research Center.
Similar mortality rates for endovascular and surgical treatment of complex coronary artery disease suggest that drug-eluting stents may be a viable treatment for selected diabetes patients, though revascularization rates are greater for these patients.
In the diabetes subgroup analysis of the SYNTAX trial, the 1-year major adverse cardiac and cerebrovascular event rate was significantly greater in medically treated diabetic patients with left main and/or three-vessel disease who underwent percutaneous coronary intervention with paclitaxel-eluting stents (PES), than in those who had coronary artery bypass grafting.
However, this increase in the primary end point of the trial appears to bedriven largely by an increased rate of repeat revascularization.
“For patients with medically treated diabetes, PES treatment was a significant predictor of repeat revascularization” but not death, cerebrovascular event, or MI, wrote Dr. Adrian P. Banning and his associates (J. Am. Coll. Cardiol. 2010 Jan. 13 [doi:10.1016/j.jacc.2009.09.057
In a related commentary, Dr. Harold L. Dauerman noted that many clinicians are already performing multivessel PCI in diabetic patients, “many of whom investigators believe could not be served at all with CABG because of a variety of comorbidities (risk of stroke) or anatomic challenges (diffuse distal vessel disease, poor conduits).
“The SYNTAX study diabetes analysis does not tell those clinicians to stop doing PCI in diabetic patients,” said Dr. Dauerman, professor of cardiology at the University of Vermont in Burlington. Instead, the results suggest that PCI is a viable option given the caveat that diabetic patients undergoing PCI with [drug-eluting stents] remain at greater risk for repeat revascularization with PCI versus CABG.
The SYNTAX (Synergy Between [PCI] With Taxus and Cardiac Surgery) study included 1,800 patients with de novo left main and/or three-vessel disease, with or without diabetes.
Patients were randomized to undergo CABG or PCI using paclitaxel-eluting stents (PES). The diabetes substudy included the 452 patients with medically treated diabetes, of whom 71% had three-vessel disease and 29% had left main disease.
Beyond that, 79% of patients with left main disease had concurrent two- or three-vessel disease. Of the 452 diabetes patients, 231 underwent PCI and 221 underwent CABG. Most (94%) of the patients with diabetes had type 2 disease.
The researchers used a composite end point of all-cause death, cerebrovascular accident, MI, or repeat revascularization (any subsequent PES of CABG procedure in any coronary vessel).
Among diabetic patients, the 1-year event rate was significantly greater after PES (26%), compared with CABG (14%), for a relative risk of 1.83.
However, among nondiabetic patients, the 1-year event rate was slightly higher for the PES group, though this was not significant—15% vs. 12%, relative risk 1.28.
“The number needed to treat CABG to avoid 1 MACCE event is 9 for diabetic patients and 31 for nondiabetic patients,” wrote Dr. Banning, a consultant cardiologist at the John Radcliffe Hospital in Oxford, England, and his coauthors.
There were no significant differences between CABG and PES in terms of the composite safety end point (death, cerebrovascular accident, or MI) for either diabetic or nondiabetic patients in SYNTAX. Neither was there a significant difference in terms of symptomatic graft occlusion or stent thrombosis for diabetic or nondiabetic patients.
Repeat revascularization appears to have driven the significantly greater event rate for diabetic patients treated with PES. Repeat revascularization was greater for the PES group, regardless of diabetes status. The PES revascularization rate for diabetic patients was 20% compared with 6% for diabetic patients who underwent CABG. Likewise, the PES revascularization rate for nondiabetic patients was 11% compared with 6% for nondiabetic patients who underwent CABG.
Repeat revascularization following PES was also greater for diabetic patients than for nondiabetic patients. This was not true for CABG patients.
“Medically treated diabetes was a significant independent predictor of revascularization the PES arm (odds ratio of 2.93) but not in the CABG arm,” the investigators wrote.
However, the degree of glycemic control was not a significant predictor of 1-year outcomes for patients with diabetes.
Among diabetic patients, there were no differences in death, MI, or cerebrovascular accident between PES and CABG groups in either those treated with insulin (182) or those treated with oral hypoglycemics (270).
The authors cautioned that the 1-year results may not yet reflect the true long-term differences between CABG and PES treatments of diabetic patients.
SYNTAX was funded by Boston Scientific Corporation, which makes the Taxus paclitaxel-eluting stent. Dr. Banning and two of his coauthors have received grant support from Boston Scientific and two other coauthors are employed by the company.
Dr. Banning also received funding from the National Health Research Institute's Biomedical Research Center in Oxford.
Dr. Dauerman indicated that he has significant financial relationships with Abbott Laboratories and Medtronic Inc.
Age, Steroid Use, Low BMD Predict Bisphosphonate Scrips
DENVER — Significant predictors of receiving a bisphosphonate prescription within 90 days of a fracture for women are a low bone mineral density score after a fracture, being aged 65-74 years, and oral corticosteroid use, according to the results of a study of 2,000 women.
Women with a bone mineral density (BMD) T score of −2.5 or less in the 90 days after a fracture were almost five times as likely to receive a bisphosphonate prescription than women with higher T scores, according to a poster presented by Carl Asche, Ph.D., at the annual meeting of the American Society for Bone Mineral Research.
Women who were aged 65-74 years at the time of fracture were almost twice as likely to receive a prescription, compared with women younger than 65. Similarly, women taking oral corticosteroids also were more likely to receive a bisphosphonate prescription wrote Dr. Asche of the pharmacotherapy department at the University of Utah, Salt Lake City.
Using electronic health records from Geisinger Health System from Jan. 1, 2000, to June 30, 2007, women 50 years of age and older who had had a fracture were included. They also had to have continuous electronic health record activity for at least 365 days before and after the date of the fracture. Women were excluded if they had a diagnosis of osteoporosis, a bone mineral density score of −2.5 or less at the time of the fracture, a fracture in the 6 months prior to the index date, or a diagnosis of conditions known to impact bone density and quality.
A total of 2,000 women met the inclusion criteria, but less than 10% (188) received a prescription for a bisphosphonate within 90 days of fracture. “Very few women aged [over] 50 receive treatment with an oral bisphosphonate after having a fracture, leaving them potentially vulnerable to future fractures,” he noted.
Disclosures: The study was supported by the Alliance for Better Bone Health—Procter & Gamble Pharmaceuticals and Sanofi-Aventis U.S., which co-promote Actonel. Dr. Asche reported that he has a significant financial relationship with Sanofi-Aventis U.S.
Elsevier Global Medical News
DENVER — Significant predictors of receiving a bisphosphonate prescription within 90 days of a fracture for women are a low bone mineral density score after a fracture, being aged 65-74 years, and oral corticosteroid use, according to the results of a study of 2,000 women.
Women with a bone mineral density (BMD) T score of −2.5 or less in the 90 days after a fracture were almost five times as likely to receive a bisphosphonate prescription than women with higher T scores, according to a poster presented by Carl Asche, Ph.D., at the annual meeting of the American Society for Bone Mineral Research.
Women who were aged 65-74 years at the time of fracture were almost twice as likely to receive a prescription, compared with women younger than 65. Similarly, women taking oral corticosteroids also were more likely to receive a bisphosphonate prescription wrote Dr. Asche of the pharmacotherapy department at the University of Utah, Salt Lake City.
Using electronic health records from Geisinger Health System from Jan. 1, 2000, to June 30, 2007, women 50 years of age and older who had had a fracture were included. They also had to have continuous electronic health record activity for at least 365 days before and after the date of the fracture. Women were excluded if they had a diagnosis of osteoporosis, a bone mineral density score of −2.5 or less at the time of the fracture, a fracture in the 6 months prior to the index date, or a diagnosis of conditions known to impact bone density and quality.
A total of 2,000 women met the inclusion criteria, but less than 10% (188) received a prescription for a bisphosphonate within 90 days of fracture. “Very few women aged [over] 50 receive treatment with an oral bisphosphonate after having a fracture, leaving them potentially vulnerable to future fractures,” he noted.
Disclosures: The study was supported by the Alliance for Better Bone Health—Procter & Gamble Pharmaceuticals and Sanofi-Aventis U.S., which co-promote Actonel. Dr. Asche reported that he has a significant financial relationship with Sanofi-Aventis U.S.
Elsevier Global Medical News
DENVER — Significant predictors of receiving a bisphosphonate prescription within 90 days of a fracture for women are a low bone mineral density score after a fracture, being aged 65-74 years, and oral corticosteroid use, according to the results of a study of 2,000 women.
Women with a bone mineral density (BMD) T score of −2.5 or less in the 90 days after a fracture were almost five times as likely to receive a bisphosphonate prescription than women with higher T scores, according to a poster presented by Carl Asche, Ph.D., at the annual meeting of the American Society for Bone Mineral Research.
Women who were aged 65-74 years at the time of fracture were almost twice as likely to receive a prescription, compared with women younger than 65. Similarly, women taking oral corticosteroids also were more likely to receive a bisphosphonate prescription wrote Dr. Asche of the pharmacotherapy department at the University of Utah, Salt Lake City.
Using electronic health records from Geisinger Health System from Jan. 1, 2000, to June 30, 2007, women 50 years of age and older who had had a fracture were included. They also had to have continuous electronic health record activity for at least 365 days before and after the date of the fracture. Women were excluded if they had a diagnosis of osteoporosis, a bone mineral density score of −2.5 or less at the time of the fracture, a fracture in the 6 months prior to the index date, or a diagnosis of conditions known to impact bone density and quality.
A total of 2,000 women met the inclusion criteria, but less than 10% (188) received a prescription for a bisphosphonate within 90 days of fracture. “Very few women aged [over] 50 receive treatment with an oral bisphosphonate after having a fracture, leaving them potentially vulnerable to future fractures,” he noted.
Disclosures: The study was supported by the Alliance for Better Bone Health—Procter & Gamble Pharmaceuticals and Sanofi-Aventis U.S., which co-promote Actonel. Dr. Asche reported that he has a significant financial relationship with Sanofi-Aventis U.S.
Elsevier Global Medical News
Contrast Agent Restrictions Curb NSF at Two Care Centers
No new cases of nephrogenic systemic fibrosis occurred at two large tertiary care facilities after more restrictive policies on the use of gadolinium-based contrast agents were introduced.
Before the changes, the incidence of nephrogenic systemic fibrosis (NSF) was 1 in 33 in high-risk patients. In patients on dialysis, the incidence of NSF was 1 in 35, according Dr. Ersan Altun, a radiologist at the University of North Carolina in Chapel Hill, and his coauthors.
“The absence of NSF cases in the postadoption period may reflect the effect of the use of different GBCAs [gadolinium-based contrast agents] and the adoption of restrictive GBCA policies on the incidence of NSF,” they wrote (Radiology 2009;253:689–96).
In 2006, reports to the Food and Drug Administration suggested a strong association between NSF and gadolinium-based contrast agents used in MRI. The exact mechanism remains unknown; however, gadolinium contrast agents vary in their dissociation rates and dissociation of the gadolinium ion from the chelating ligand may be a risk factor, the researchers said.
Cases of NSF were documented at two tertiary care centers for three periods: before the adoption of restrictive GBCA policies and a change in agents, during the transition period, and after the adoption of these changes.
The new policies included careful screening of patients for risk factors for NSF such as renal disease, hypertension, dialysis, and diabetes before they underwent gadolinium-enhanced MRI. If GBCA-enhanced imaging was unavoidable in a patient deemed to be at high risk, a half dose of gadobenate dimeglumine was used. The policies also specified greater use of other types of imaging that do not require contrast agents. In addition, gadolinium-enhanced MRI was not performed in pregnant women unless maternal survival was at stake, was not performed in any patient twice within 48 hours unless absolutely necessary, and was not done twice within 48 hours in any patient deemed to be at high risk of NSF.
The researchers used patient records at center A and ICD-9 codes for acute renal failure and chronic kidney disease (stages 3–5) to identify patients who were at risk for NSF and underwent gadolinium-enhanced MRI. At center B, the researchers used ICD-9 codes and patient records to identify dialysis patients who underwent gadolinium-enhanced MRI.
Before adoption of the changes, both centers used gadodiamide (Omniscan, GE Healthcare Inc.). After the adoption of the revised policies, both centers used either gadobenate dimeglumine (Multihance, Bracco Diagnostics Inc.) or gadopentetate dimeglumine (Magnevist, Bayer Healthcare Pharmaceuticals Inc.) Gadobenate was used for all MRI studies of adults, patients younger than 1 year, and pediatric patients at risk for the development of NSF. Gadopentetate was used for pediatric patients 1 year and older who were not at risk for NSF. Both agents have lower dissociation rates than gadodiamide.
NSF was diagnosed by clinical findings and histopathologic evaluation of deep-skin biopsy. The temporal relationship/interval between the administration of gadolinium-based contrast and onset of NSF was determined for each patient.
At center A, 35 patients with NSF were identified in the preadoption period; of these, 28 underwent gadolinium-enhanced MR only at center A and received only gadodiamide. The benchmark incidence of NSF at center A was 1/1,750 and the NSF incidence in high-risk patients was 1/33.
At center B, 14 patients with NSF were identified in the preadoption period; of these, 9 underwent gadolinium-enhanced MR only at center B and received only gadodiamide. The benchmark incidence of NSF at center B was 1/1,803 and the NSF incidence in dialysis patients was 1/35.
There were no cases of NSF in the transitional and postadoption periods at either center.
One coauthor received funding from GE Healthcare, Bayer Healthcare Pharmaceuticals, and Bracco
No new cases of nephrogenic systemic fibrosis occurred at two large tertiary care facilities after more restrictive policies on the use of gadolinium-based contrast agents were introduced.
Before the changes, the incidence of nephrogenic systemic fibrosis (NSF) was 1 in 33 in high-risk patients. In patients on dialysis, the incidence of NSF was 1 in 35, according Dr. Ersan Altun, a radiologist at the University of North Carolina in Chapel Hill, and his coauthors.
“The absence of NSF cases in the postadoption period may reflect the effect of the use of different GBCAs [gadolinium-based contrast agents] and the adoption of restrictive GBCA policies on the incidence of NSF,” they wrote (Radiology 2009;253:689–96).
In 2006, reports to the Food and Drug Administration suggested a strong association between NSF and gadolinium-based contrast agents used in MRI. The exact mechanism remains unknown; however, gadolinium contrast agents vary in their dissociation rates and dissociation of the gadolinium ion from the chelating ligand may be a risk factor, the researchers said.
Cases of NSF were documented at two tertiary care centers for three periods: before the adoption of restrictive GBCA policies and a change in agents, during the transition period, and after the adoption of these changes.
The new policies included careful screening of patients for risk factors for NSF such as renal disease, hypertension, dialysis, and diabetes before they underwent gadolinium-enhanced MRI. If GBCA-enhanced imaging was unavoidable in a patient deemed to be at high risk, a half dose of gadobenate dimeglumine was used. The policies also specified greater use of other types of imaging that do not require contrast agents. In addition, gadolinium-enhanced MRI was not performed in pregnant women unless maternal survival was at stake, was not performed in any patient twice within 48 hours unless absolutely necessary, and was not done twice within 48 hours in any patient deemed to be at high risk of NSF.
The researchers used patient records at center A and ICD-9 codes for acute renal failure and chronic kidney disease (stages 3–5) to identify patients who were at risk for NSF and underwent gadolinium-enhanced MRI. At center B, the researchers used ICD-9 codes and patient records to identify dialysis patients who underwent gadolinium-enhanced MRI.
Before adoption of the changes, both centers used gadodiamide (Omniscan, GE Healthcare Inc.). After the adoption of the revised policies, both centers used either gadobenate dimeglumine (Multihance, Bracco Diagnostics Inc.) or gadopentetate dimeglumine (Magnevist, Bayer Healthcare Pharmaceuticals Inc.) Gadobenate was used for all MRI studies of adults, patients younger than 1 year, and pediatric patients at risk for the development of NSF. Gadopentetate was used for pediatric patients 1 year and older who were not at risk for NSF. Both agents have lower dissociation rates than gadodiamide.
NSF was diagnosed by clinical findings and histopathologic evaluation of deep-skin biopsy. The temporal relationship/interval between the administration of gadolinium-based contrast and onset of NSF was determined for each patient.
At center A, 35 patients with NSF were identified in the preadoption period; of these, 28 underwent gadolinium-enhanced MR only at center A and received only gadodiamide. The benchmark incidence of NSF at center A was 1/1,750 and the NSF incidence in high-risk patients was 1/33.
At center B, 14 patients with NSF were identified in the preadoption period; of these, 9 underwent gadolinium-enhanced MR only at center B and received only gadodiamide. The benchmark incidence of NSF at center B was 1/1,803 and the NSF incidence in dialysis patients was 1/35.
There were no cases of NSF in the transitional and postadoption periods at either center.
One coauthor received funding from GE Healthcare, Bayer Healthcare Pharmaceuticals, and Bracco
No new cases of nephrogenic systemic fibrosis occurred at two large tertiary care facilities after more restrictive policies on the use of gadolinium-based contrast agents were introduced.
Before the changes, the incidence of nephrogenic systemic fibrosis (NSF) was 1 in 33 in high-risk patients. In patients on dialysis, the incidence of NSF was 1 in 35, according Dr. Ersan Altun, a radiologist at the University of North Carolina in Chapel Hill, and his coauthors.
“The absence of NSF cases in the postadoption period may reflect the effect of the use of different GBCAs [gadolinium-based contrast agents] and the adoption of restrictive GBCA policies on the incidence of NSF,” they wrote (Radiology 2009;253:689–96).
In 2006, reports to the Food and Drug Administration suggested a strong association between NSF and gadolinium-based contrast agents used in MRI. The exact mechanism remains unknown; however, gadolinium contrast agents vary in their dissociation rates and dissociation of the gadolinium ion from the chelating ligand may be a risk factor, the researchers said.
Cases of NSF were documented at two tertiary care centers for three periods: before the adoption of restrictive GBCA policies and a change in agents, during the transition period, and after the adoption of these changes.
The new policies included careful screening of patients for risk factors for NSF such as renal disease, hypertension, dialysis, and diabetes before they underwent gadolinium-enhanced MRI. If GBCA-enhanced imaging was unavoidable in a patient deemed to be at high risk, a half dose of gadobenate dimeglumine was used. The policies also specified greater use of other types of imaging that do not require contrast agents. In addition, gadolinium-enhanced MRI was not performed in pregnant women unless maternal survival was at stake, was not performed in any patient twice within 48 hours unless absolutely necessary, and was not done twice within 48 hours in any patient deemed to be at high risk of NSF.
The researchers used patient records at center A and ICD-9 codes for acute renal failure and chronic kidney disease (stages 3–5) to identify patients who were at risk for NSF and underwent gadolinium-enhanced MRI. At center B, the researchers used ICD-9 codes and patient records to identify dialysis patients who underwent gadolinium-enhanced MRI.
Before adoption of the changes, both centers used gadodiamide (Omniscan, GE Healthcare Inc.). After the adoption of the revised policies, both centers used either gadobenate dimeglumine (Multihance, Bracco Diagnostics Inc.) or gadopentetate dimeglumine (Magnevist, Bayer Healthcare Pharmaceuticals Inc.) Gadobenate was used for all MRI studies of adults, patients younger than 1 year, and pediatric patients at risk for the development of NSF. Gadopentetate was used for pediatric patients 1 year and older who were not at risk for NSF. Both agents have lower dissociation rates than gadodiamide.
NSF was diagnosed by clinical findings and histopathologic evaluation of deep-skin biopsy. The temporal relationship/interval between the administration of gadolinium-based contrast and onset of NSF was determined for each patient.
At center A, 35 patients with NSF were identified in the preadoption period; of these, 28 underwent gadolinium-enhanced MR only at center A and received only gadodiamide. The benchmark incidence of NSF at center A was 1/1,750 and the NSF incidence in high-risk patients was 1/33.
At center B, 14 patients with NSF were identified in the preadoption period; of these, 9 underwent gadolinium-enhanced MR only at center B and received only gadodiamide. The benchmark incidence of NSF at center B was 1/1,803 and the NSF incidence in dialysis patients was 1/35.
There were no cases of NSF in the transitional and postadoption periods at either center.
One coauthor received funding from GE Healthcare, Bayer Healthcare Pharmaceuticals, and Bracco
PCI for Complex CAD Ups Revascularization Risk in Diabetes
Major Finding: Endovascular treatment with paclitaxel-eluting stents significantly increased the risk of revascularization, compared with CABG treatment, in diabetic patients with left main and/or three-vessel disease but does not increase the rates of death, cerebrovascular accident, or MI in these patients.
Data Source: A diabetes subgroup analysis of the SYNTAX trial.
Disclosures: Funded by Boston Scientific, which makes the Taxus stent. Dr. Banning and four coauthors have financial ties to the company. Dr. Banning is partially funded by the National Health Research Institute's Biomedical Research Center in Oxford.
Similar mortality rates for endovascular and surgical treatment of complex coronary artery disease suggest that drug-eluting stents may be a viable treatment for selected diabetes patients, though revascularization rates are greater for these patients.
In the diabetes subgroup analysis of the SYNTAX trial, the 1-year major adverse cardiac and cerebrovascular event rate was significantly greater in medically treated diabetic patients with left main and/or three-vessel disease who underwent percutaneous coronary intervention with paclitaxel-eluting stents (PES), than in those who had coronary artery bypass grafting. However, this increase in the primary end point of the trial appears to be driven largely by an increased rate of repeat revascularization.
“For patients with medically treated diabetes, PES treatment was a significant predictor of repeat revascularization” but not death, cerebrovascular event, or MI, Dr. Adrian P. Banning and his associates wrote in a study published online Jan. 13 (J. Am. Coll. Cardiol. 2010 Jan. 13 [doi:10.1016/j.jacc.2009.09.057]).
In a related commentary, Dr. Harold L. Dauerman noted that many clinicians are already performing multivessel PCI in diabetic patients, “many of whom investigators believe could not be served at all with CABG because of a variety of comorbidities (risk of stroke) or anatomic challenges (diffuse distal vessel disease, poor conduits).
“The SYNTAX study diabetes analysis does not tell those clinicians to stop doing PCI in diabetic patients,” said Dr. Dauerman, professor of cardiology at the University of Vermont in Burlington. Instead, the results suggest that PCI is a viable option given the caveat that diabetic patients undergoing PCI with [drug-eluting stents] remain at greater risk for repeat revascularization with PCI versus CABG.
The SYNTAX (Synergy Between [PCI] With Taxus and Cardiac Surgery) study included 1,800 patients with de novo left main and/or three-vessel disease, with or without diabetes. Patients were randomized to undergo CABG or PCI using paclitaxel-eluting stents (PES). The diabetes substudy included the 452 patients with medically treated diabetes, of whom 71% had three-vessel disease and 29% had left main disease. Beyond that, 79% of patients with left main disease had concurrent two- or three-vessel disease. Of the 452 diabetes patients, 231 underwent PCI and 221 underwent CABG. Most (94%) of the diabetes patients had type 2 disease.
The researchers used a composite end point of all-cause death, cerebrovascular accident, MI, or repeat revascularization (any subsequent PES of CABG procedure in any coronary vessel). Among diabetic patients, the 1-year event rate was significantly greater after PES (26%), compared with CABG (14%), for a relative risk of 1.83. However, among nondiabetic patients, the 1-year event rate was slightly higher for the PES group, though this was not significant—15% vs. 12%, relative risk 1.28.
“The number needed to treat CABG to avoid 1 [major adverse coronary event] is 9 for diabetic patients and 31 for nondiabetic patients,” wrote Dr. Banning, a consultant cardiologist at the John Radcliffe Hospital in Oxford, England, and his coauthors.
There were no significant differences between CABG and PES in terms of the composite safety end point (death, cerebrovascular accident, or MI) for either diabetic or nondiabetic patients in SYNTAX. Neither was there a significant difference in terms of symptomatic graft occlusion or stent thrombosis for patients with or without diabetes.
Repeat revascularization appears to have driven the significantly greater event rate for diabetic patients treated with PES. Repeat revascularization was greater for the PES group, regardless of diabetes status. The PES revascularization rate for diabetic patients was 20% compared with 6% for diabetic patients who underwent CABG.
Likewise, the PES revascularization rate for nondiabetic patients was 11% compared with 6% for nondiabetic patients who underwent CABG. Repeat revascularization following PES was also greater for diabetic patients than for nondiabetic patients. This was not true for CABG patients.
“Medically treated diabetes was a significant independent predictor of revascularization the PES arm (odds ratio of 2.93) but not in the CABG arm,” the investigators wrote.
However, the degree of glycemic control was not a significant predictor of 1-year outcomes for diabetic patients.
Among diabetic patients, there were no differences in death, MI or cerebrovascular accident between PES and CABG groups in either those treated with insulin (182) or those treated with oral hypoglycemics (270).
The authors cautioned that the 1-year results may not yet reflect the true long-term differences between CABG and PES treatments of diabetic patients.
Dr. Dauerman reported significant financial relationships with Abbott Laboratories and Medtronic Inc.
Major Finding: Endovascular treatment with paclitaxel-eluting stents significantly increased the risk of revascularization, compared with CABG treatment, in diabetic patients with left main and/or three-vessel disease but does not increase the rates of death, cerebrovascular accident, or MI in these patients.
Data Source: A diabetes subgroup analysis of the SYNTAX trial.
Disclosures: Funded by Boston Scientific, which makes the Taxus stent. Dr. Banning and four coauthors have financial ties to the company. Dr. Banning is partially funded by the National Health Research Institute's Biomedical Research Center in Oxford.
Similar mortality rates for endovascular and surgical treatment of complex coronary artery disease suggest that drug-eluting stents may be a viable treatment for selected diabetes patients, though revascularization rates are greater for these patients.
In the diabetes subgroup analysis of the SYNTAX trial, the 1-year major adverse cardiac and cerebrovascular event rate was significantly greater in medically treated diabetic patients with left main and/or three-vessel disease who underwent percutaneous coronary intervention with paclitaxel-eluting stents (PES), than in those who had coronary artery bypass grafting. However, this increase in the primary end point of the trial appears to be driven largely by an increased rate of repeat revascularization.
“For patients with medically treated diabetes, PES treatment was a significant predictor of repeat revascularization” but not death, cerebrovascular event, or MI, Dr. Adrian P. Banning and his associates wrote in a study published online Jan. 13 (J. Am. Coll. Cardiol. 2010 Jan. 13 [doi:10.1016/j.jacc.2009.09.057]).
In a related commentary, Dr. Harold L. Dauerman noted that many clinicians are already performing multivessel PCI in diabetic patients, “many of whom investigators believe could not be served at all with CABG because of a variety of comorbidities (risk of stroke) or anatomic challenges (diffuse distal vessel disease, poor conduits).
“The SYNTAX study diabetes analysis does not tell those clinicians to stop doing PCI in diabetic patients,” said Dr. Dauerman, professor of cardiology at the University of Vermont in Burlington. Instead, the results suggest that PCI is a viable option given the caveat that diabetic patients undergoing PCI with [drug-eluting stents] remain at greater risk for repeat revascularization with PCI versus CABG.
The SYNTAX (Synergy Between [PCI] With Taxus and Cardiac Surgery) study included 1,800 patients with de novo left main and/or three-vessel disease, with or without diabetes. Patients were randomized to undergo CABG or PCI using paclitaxel-eluting stents (PES). The diabetes substudy included the 452 patients with medically treated diabetes, of whom 71% had three-vessel disease and 29% had left main disease. Beyond that, 79% of patients with left main disease had concurrent two- or three-vessel disease. Of the 452 diabetes patients, 231 underwent PCI and 221 underwent CABG. Most (94%) of the diabetes patients had type 2 disease.
The researchers used a composite end point of all-cause death, cerebrovascular accident, MI, or repeat revascularization (any subsequent PES of CABG procedure in any coronary vessel). Among diabetic patients, the 1-year event rate was significantly greater after PES (26%), compared with CABG (14%), for a relative risk of 1.83. However, among nondiabetic patients, the 1-year event rate was slightly higher for the PES group, though this was not significant—15% vs. 12%, relative risk 1.28.
“The number needed to treat CABG to avoid 1 [major adverse coronary event] is 9 for diabetic patients and 31 for nondiabetic patients,” wrote Dr. Banning, a consultant cardiologist at the John Radcliffe Hospital in Oxford, England, and his coauthors.
There were no significant differences between CABG and PES in terms of the composite safety end point (death, cerebrovascular accident, or MI) for either diabetic or nondiabetic patients in SYNTAX. Neither was there a significant difference in terms of symptomatic graft occlusion or stent thrombosis for patients with or without diabetes.
Repeat revascularization appears to have driven the significantly greater event rate for diabetic patients treated with PES. Repeat revascularization was greater for the PES group, regardless of diabetes status. The PES revascularization rate for diabetic patients was 20% compared with 6% for diabetic patients who underwent CABG.
Likewise, the PES revascularization rate for nondiabetic patients was 11% compared with 6% for nondiabetic patients who underwent CABG. Repeat revascularization following PES was also greater for diabetic patients than for nondiabetic patients. This was not true for CABG patients.
“Medically treated diabetes was a significant independent predictor of revascularization the PES arm (odds ratio of 2.93) but not in the CABG arm,” the investigators wrote.
However, the degree of glycemic control was not a significant predictor of 1-year outcomes for diabetic patients.
Among diabetic patients, there were no differences in death, MI or cerebrovascular accident between PES and CABG groups in either those treated with insulin (182) or those treated with oral hypoglycemics (270).
The authors cautioned that the 1-year results may not yet reflect the true long-term differences between CABG and PES treatments of diabetic patients.
Dr. Dauerman reported significant financial relationships with Abbott Laboratories and Medtronic Inc.
Major Finding: Endovascular treatment with paclitaxel-eluting stents significantly increased the risk of revascularization, compared with CABG treatment, in diabetic patients with left main and/or three-vessel disease but does not increase the rates of death, cerebrovascular accident, or MI in these patients.
Data Source: A diabetes subgroup analysis of the SYNTAX trial.
Disclosures: Funded by Boston Scientific, which makes the Taxus stent. Dr. Banning and four coauthors have financial ties to the company. Dr. Banning is partially funded by the National Health Research Institute's Biomedical Research Center in Oxford.
Similar mortality rates for endovascular and surgical treatment of complex coronary artery disease suggest that drug-eluting stents may be a viable treatment for selected diabetes patients, though revascularization rates are greater for these patients.
In the diabetes subgroup analysis of the SYNTAX trial, the 1-year major adverse cardiac and cerebrovascular event rate was significantly greater in medically treated diabetic patients with left main and/or three-vessel disease who underwent percutaneous coronary intervention with paclitaxel-eluting stents (PES), than in those who had coronary artery bypass grafting. However, this increase in the primary end point of the trial appears to be driven largely by an increased rate of repeat revascularization.
“For patients with medically treated diabetes, PES treatment was a significant predictor of repeat revascularization” but not death, cerebrovascular event, or MI, Dr. Adrian P. Banning and his associates wrote in a study published online Jan. 13 (J. Am. Coll. Cardiol. 2010 Jan. 13 [doi:10.1016/j.jacc.2009.09.057]).
In a related commentary, Dr. Harold L. Dauerman noted that many clinicians are already performing multivessel PCI in diabetic patients, “many of whom investigators believe could not be served at all with CABG because of a variety of comorbidities (risk of stroke) or anatomic challenges (diffuse distal vessel disease, poor conduits).
“The SYNTAX study diabetes analysis does not tell those clinicians to stop doing PCI in diabetic patients,” said Dr. Dauerman, professor of cardiology at the University of Vermont in Burlington. Instead, the results suggest that PCI is a viable option given the caveat that diabetic patients undergoing PCI with [drug-eluting stents] remain at greater risk for repeat revascularization with PCI versus CABG.
The SYNTAX (Synergy Between [PCI] With Taxus and Cardiac Surgery) study included 1,800 patients with de novo left main and/or three-vessel disease, with or without diabetes. Patients were randomized to undergo CABG or PCI using paclitaxel-eluting stents (PES). The diabetes substudy included the 452 patients with medically treated diabetes, of whom 71% had three-vessel disease and 29% had left main disease. Beyond that, 79% of patients with left main disease had concurrent two- or three-vessel disease. Of the 452 diabetes patients, 231 underwent PCI and 221 underwent CABG. Most (94%) of the diabetes patients had type 2 disease.
The researchers used a composite end point of all-cause death, cerebrovascular accident, MI, or repeat revascularization (any subsequent PES of CABG procedure in any coronary vessel). Among diabetic patients, the 1-year event rate was significantly greater after PES (26%), compared with CABG (14%), for a relative risk of 1.83. However, among nondiabetic patients, the 1-year event rate was slightly higher for the PES group, though this was not significant—15% vs. 12%, relative risk 1.28.
“The number needed to treat CABG to avoid 1 [major adverse coronary event] is 9 for diabetic patients and 31 for nondiabetic patients,” wrote Dr. Banning, a consultant cardiologist at the John Radcliffe Hospital in Oxford, England, and his coauthors.
There were no significant differences between CABG and PES in terms of the composite safety end point (death, cerebrovascular accident, or MI) for either diabetic or nondiabetic patients in SYNTAX. Neither was there a significant difference in terms of symptomatic graft occlusion or stent thrombosis for patients with or without diabetes.
Repeat revascularization appears to have driven the significantly greater event rate for diabetic patients treated with PES. Repeat revascularization was greater for the PES group, regardless of diabetes status. The PES revascularization rate for diabetic patients was 20% compared with 6% for diabetic patients who underwent CABG.
Likewise, the PES revascularization rate for nondiabetic patients was 11% compared with 6% for nondiabetic patients who underwent CABG. Repeat revascularization following PES was also greater for diabetic patients than for nondiabetic patients. This was not true for CABG patients.
“Medically treated diabetes was a significant independent predictor of revascularization the PES arm (odds ratio of 2.93) but not in the CABG arm,” the investigators wrote.
However, the degree of glycemic control was not a significant predictor of 1-year outcomes for diabetic patients.
Among diabetic patients, there were no differences in death, MI or cerebrovascular accident between PES and CABG groups in either those treated with insulin (182) or those treated with oral hypoglycemics (270).
The authors cautioned that the 1-year results may not yet reflect the true long-term differences between CABG and PES treatments of diabetic patients.
Dr. Dauerman reported significant financial relationships with Abbott Laboratories and Medtronic Inc.
MDs Need to Understand E-Prescribing Benefits, Concerns
WASHINGTON — E-prescribing can help reduce medication errors, lower practice costs, and save physician time, but it's important to understand the limits and problems of this rapidly evolving technology, according to Dr. Kevin Johnson.
“There are enormously great advantages to using e-prescribing systems. [However,] there are issues that you need to be aware of,” said Dr. Johnson, vice chair of the biomedical informatics department at Vanderbilt University Medical Center in Nashville.
The primary reason for adopting e-prescribing is to prevent errors. “If all you ever did was just switch to Microsoft Word, you will decrease your risk [of prescription errors] by 15%,” Dr. Johnson said at the annual meeting of the American Academy of Pediatrics. Illegibility is one of the prime drivers of prescription errors, along with dose errors and unclear abbreviations. Dose errors in particular play a big role in pediatric medication errors.
Dr. Johnson cited the 10-fold error as an example of a common one. A pediatrician may include a trailing zero in the dose but if the pharmacist doesn't see the period, the dose will be increased 10-fold. “Believe it or not, that dose is probably a completely valid dose, depending on the age of the child,” he said. A similar mistake can occur with leading zeros. Another common error is the calculation of a dosage using the wrong units. For example, a pediatrician might use weight in pounds to calculate a dosage, when weight in kilograms should be used.
“These are all things that e-prescribing can potentially help in terms of making sure that the communication is clear between us and the pharmacy,” he said.
Other errors associated with paper-based prescriptions include omissions, dose or direction errors, unclear quantity to be dispensed, prescriptions for nonprescription products, incomplete directions, and unfulfilled legal requirements (J. Fam. Pract. 1989;29:290-5).
E-prescribing systems also can provide clinical decision support (such as drug-drug interaction checking), pharmacy benefit eligibility checking, formulary compliance, drug reference, medication history reporting, and prescription routing to retail or mail order pharmacies.
Electronic prescribing systems offer potential cost savings to both physicians and pharmacies by eliminating handwriting issues, reducing overtime and callback problems, and speeding up refill requests, according to Dr. Johnson.
However, if you're considering an e-prescribing system, he recommended considering an electronic health record system as well. “My opinion is that EHRs are as important as e-prescribing.” EHRs provide valuable patient information for e-prescribing, such as allergies and medication history, and solve some workflow issues.
Electronic prescribing systems can be integrated into existing electronic medical record systems or can be used as a stand-alone option. Stand-alone systems can involve either an application service provider or locally installed software. The use of an application service provider means that the system exists on the Web. Internet access is all that is needed to access the system. Physicians need to think about what setup will work best for them, said Dr. Johnson.
Connectivity is a key element of e-prescribing. For example, SureScripts is an e-prescribing network that is connected with approximately 95% of pharmacies in the United States, according to its Web site. This connectivity allows physicians and pharmacists to electronically exchange prescription information. Physicians can transmit new prescriptions and refill requests electronically to a pharmacy. Both pharmacists and physicians have access to aggregated information across the system about a patient's medication history and often can view formulary information from a patient's drug plan. In the future, “there will be some competitors, but at this moment there aren't,” he noted.
Reliability of prescription transmission remains a problem for some e-prescribing systems that rely on fax to transmit prescriptions. Although such a system may provide the physician office with confirmation that a prescription has been sent to a pharmacy, “on the pharmacy end, more than 20% of the time that prescription may not be received,” said Dr. Johnson. This can sometimes result in multiple prescriptions or multiple documentations in an e-prescribing system.
Another downside to e-prescribing is the number of alerts that a physician can receive, based on how many automatic checks have been activated. Physicians often end up turning off drug-drug interaction or drug allergy checks to decrease the number of alerts sent by the system. If all checks are activated, “it is actually not uncommon that almost every prescription generates something.”
Disclosures: Dr. Johnson reported that he receives royalties from ICA Corp.
Online Resources
The eHealth Initiative provides a set of guides and reports on e-prescribing (
www.ehealthinitiative.org/electronic-prescribing-resources.html
For more information on preventing pediatric medication errors, see the Pediatric Pharmacy Advocacy Group's 2001 publication, “Guidelines for Preventing Medication Errors” (J. Pediatr. Pharmacol. Ther. 2001;6:426-42;
WASHINGTON — E-prescribing can help reduce medication errors, lower practice costs, and save physician time, but it's important to understand the limits and problems of this rapidly evolving technology, according to Dr. Kevin Johnson.
“There are enormously great advantages to using e-prescribing systems. [However,] there are issues that you need to be aware of,” said Dr. Johnson, vice chair of the biomedical informatics department at Vanderbilt University Medical Center in Nashville.
The primary reason for adopting e-prescribing is to prevent errors. “If all you ever did was just switch to Microsoft Word, you will decrease your risk [of prescription errors] by 15%,” Dr. Johnson said at the annual meeting of the American Academy of Pediatrics. Illegibility is one of the prime drivers of prescription errors, along with dose errors and unclear abbreviations. Dose errors in particular play a big role in pediatric medication errors.
Dr. Johnson cited the 10-fold error as an example of a common one. A pediatrician may include a trailing zero in the dose but if the pharmacist doesn't see the period, the dose will be increased 10-fold. “Believe it or not, that dose is probably a completely valid dose, depending on the age of the child,” he said. A similar mistake can occur with leading zeros. Another common error is the calculation of a dosage using the wrong units. For example, a pediatrician might use weight in pounds to calculate a dosage, when weight in kilograms should be used.
“These are all things that e-prescribing can potentially help in terms of making sure that the communication is clear between us and the pharmacy,” he said.
Other errors associated with paper-based prescriptions include omissions, dose or direction errors, unclear quantity to be dispensed, prescriptions for nonprescription products, incomplete directions, and unfulfilled legal requirements (J. Fam. Pract. 1989;29:290-5).
E-prescribing systems also can provide clinical decision support (such as drug-drug interaction checking), pharmacy benefit eligibility checking, formulary compliance, drug reference, medication history reporting, and prescription routing to retail or mail order pharmacies.
Electronic prescribing systems offer potential cost savings to both physicians and pharmacies by eliminating handwriting issues, reducing overtime and callback problems, and speeding up refill requests, according to Dr. Johnson.
However, if you're considering an e-prescribing system, he recommended considering an electronic health record system as well. “My opinion is that EHRs are as important as e-prescribing.” EHRs provide valuable patient information for e-prescribing, such as allergies and medication history, and solve some workflow issues.
Electronic prescribing systems can be integrated into existing electronic medical record systems or can be used as a stand-alone option. Stand-alone systems can involve either an application service provider or locally installed software. The use of an application service provider means that the system exists on the Web. Internet access is all that is needed to access the system. Physicians need to think about what setup will work best for them, said Dr. Johnson.
Connectivity is a key element of e-prescribing. For example, SureScripts is an e-prescribing network that is connected with approximately 95% of pharmacies in the United States, according to its Web site. This connectivity allows physicians and pharmacists to electronically exchange prescription information. Physicians can transmit new prescriptions and refill requests electronically to a pharmacy. Both pharmacists and physicians have access to aggregated information across the system about a patient's medication history and often can view formulary information from a patient's drug plan. In the future, “there will be some competitors, but at this moment there aren't,” he noted.
Reliability of prescription transmission remains a problem for some e-prescribing systems that rely on fax to transmit prescriptions. Although such a system may provide the physician office with confirmation that a prescription has been sent to a pharmacy, “on the pharmacy end, more than 20% of the time that prescription may not be received,” said Dr. Johnson. This can sometimes result in multiple prescriptions or multiple documentations in an e-prescribing system.
Another downside to e-prescribing is the number of alerts that a physician can receive, based on how many automatic checks have been activated. Physicians often end up turning off drug-drug interaction or drug allergy checks to decrease the number of alerts sent by the system. If all checks are activated, “it is actually not uncommon that almost every prescription generates something.”
Disclosures: Dr. Johnson reported that he receives royalties from ICA Corp.
Online Resources
The eHealth Initiative provides a set of guides and reports on e-prescribing (
www.ehealthinitiative.org/electronic-prescribing-resources.html
For more information on preventing pediatric medication errors, see the Pediatric Pharmacy Advocacy Group's 2001 publication, “Guidelines for Preventing Medication Errors” (J. Pediatr. Pharmacol. Ther. 2001;6:426-42;
WASHINGTON — E-prescribing can help reduce medication errors, lower practice costs, and save physician time, but it's important to understand the limits and problems of this rapidly evolving technology, according to Dr. Kevin Johnson.
“There are enormously great advantages to using e-prescribing systems. [However,] there are issues that you need to be aware of,” said Dr. Johnson, vice chair of the biomedical informatics department at Vanderbilt University Medical Center in Nashville.
The primary reason for adopting e-prescribing is to prevent errors. “If all you ever did was just switch to Microsoft Word, you will decrease your risk [of prescription errors] by 15%,” Dr. Johnson said at the annual meeting of the American Academy of Pediatrics. Illegibility is one of the prime drivers of prescription errors, along with dose errors and unclear abbreviations. Dose errors in particular play a big role in pediatric medication errors.
Dr. Johnson cited the 10-fold error as an example of a common one. A pediatrician may include a trailing zero in the dose but if the pharmacist doesn't see the period, the dose will be increased 10-fold. “Believe it or not, that dose is probably a completely valid dose, depending on the age of the child,” he said. A similar mistake can occur with leading zeros. Another common error is the calculation of a dosage using the wrong units. For example, a pediatrician might use weight in pounds to calculate a dosage, when weight in kilograms should be used.
“These are all things that e-prescribing can potentially help in terms of making sure that the communication is clear between us and the pharmacy,” he said.
Other errors associated with paper-based prescriptions include omissions, dose or direction errors, unclear quantity to be dispensed, prescriptions for nonprescription products, incomplete directions, and unfulfilled legal requirements (J. Fam. Pract. 1989;29:290-5).
E-prescribing systems also can provide clinical decision support (such as drug-drug interaction checking), pharmacy benefit eligibility checking, formulary compliance, drug reference, medication history reporting, and prescription routing to retail or mail order pharmacies.
Electronic prescribing systems offer potential cost savings to both physicians and pharmacies by eliminating handwriting issues, reducing overtime and callback problems, and speeding up refill requests, according to Dr. Johnson.
However, if you're considering an e-prescribing system, he recommended considering an electronic health record system as well. “My opinion is that EHRs are as important as e-prescribing.” EHRs provide valuable patient information for e-prescribing, such as allergies and medication history, and solve some workflow issues.
Electronic prescribing systems can be integrated into existing electronic medical record systems or can be used as a stand-alone option. Stand-alone systems can involve either an application service provider or locally installed software. The use of an application service provider means that the system exists on the Web. Internet access is all that is needed to access the system. Physicians need to think about what setup will work best for them, said Dr. Johnson.
Connectivity is a key element of e-prescribing. For example, SureScripts is an e-prescribing network that is connected with approximately 95% of pharmacies in the United States, according to its Web site. This connectivity allows physicians and pharmacists to electronically exchange prescription information. Physicians can transmit new prescriptions and refill requests electronically to a pharmacy. Both pharmacists and physicians have access to aggregated information across the system about a patient's medication history and often can view formulary information from a patient's drug plan. In the future, “there will be some competitors, but at this moment there aren't,” he noted.
Reliability of prescription transmission remains a problem for some e-prescribing systems that rely on fax to transmit prescriptions. Although such a system may provide the physician office with confirmation that a prescription has been sent to a pharmacy, “on the pharmacy end, more than 20% of the time that prescription may not be received,” said Dr. Johnson. This can sometimes result in multiple prescriptions or multiple documentations in an e-prescribing system.
Another downside to e-prescribing is the number of alerts that a physician can receive, based on how many automatic checks have been activated. Physicians often end up turning off drug-drug interaction or drug allergy checks to decrease the number of alerts sent by the system. If all checks are activated, “it is actually not uncommon that almost every prescription generates something.”
Disclosures: Dr. Johnson reported that he receives royalties from ICA Corp.
Online Resources
The eHealth Initiative provides a set of guides and reports on e-prescribing (
www.ehealthinitiative.org/electronic-prescribing-resources.html
For more information on preventing pediatric medication errors, see the Pediatric Pharmacy Advocacy Group's 2001 publication, “Guidelines for Preventing Medication Errors” (J. Pediatr. Pharmacol. Ther. 2001;6:426-42;
IOM Seeks Increased Awareness of Hepatitis
The Institute of Medicine is calling for increased awareness of hepatitis B and C among health care providers, social service providers, and at-risk communities as well as better surveillance and more stringent vaccination requirements nationwide in its newly released report on hepatitis and liver cancer.
“The committee believes that these recommendations will prevent further infections, improve the lives and health of infected individuals, and reduce the long-term burden of liver disease and liver cancer,” Dr. R. Palmer Beasley said during a teleconference sponsored by the National Academies of Science. Dr. Beasley chaired the IOM committee that wrote the report “Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C.”
“This report outlines the additional resources and actions needed to reduce the unacceptably high burden of liver disease and cancer associated with these viruses,” Dr. Beasley, professor of epidemiology and disease
The committee identified three major factors that impede current efforts to prevent and control hepatitis B and C:
▸ Lack of knowledge and awareness about chronic viral hepatitis on the part of health care and social service providers.
▸ Inadequate knowledge and awareness of chronic viral hepatitis among at-risk populations, members of the public, and policy makers.
▸ Insufficient understanding about the extent and seriousness of this public health problem that has led to the inadequate allocation of resources for prevention, control, and surveillance programs.
Perhaps the greatest difficulty in diagnosing and treating patients with hepatitis B and C is that these diseases are often asymptomatic.
In addition, minority groups—Asians, Pacific Islanders, and blacks—are at greatest risk. More specifically, those most at risk for hepatitis B include people born in East and Southeast Asia or sub-Saharan Africa, infants born to women infected with the disease, and people who have sexual contact or share injection-drug equipment with an infected person. Those at greatest risk for hepatitis C include people who received a blood transfusion before 1992—before the implementation of blood screening for hepatitis C—and past or current injection drug users.
The committee noted that manyoproviders fail to follow guidelines for screening patients and providing prevention, treatment, and follow-up services.
The committee made recommendations for improving surveillance, knowledge and awareness, immunization, and services for viral hepatitis. Highlights of these recommendations include:
▸ A complete evaluation by the Centers for Disease Control and Prevention of the national hepatitis B and C public health surveillance system.
▸ Coordination between CDC and key stakeholders to develop hepatitis B and C education programs for health care and social service personnel.
▸ Coordination between CDC and key stakeholders to develop innovative and effective programs to target at-risk populations and to increase awareness of hepatitis B and C among the general public.
▸ Vaccination of all neonates weighing at least 2,000 g and born to hepatitis B–positive women.
▸ Mandatory vaccination for hepatitis B as a requirement for school attendance.
▸ Studies to develop a hepatitis C vaccine.
Multiple tests are available to diagnose hepatitis B and C, but these tests each have different implications, Dr. Beasley said. “The issue is not whether there are tests but whether or not physicians and health care providers learn and understand how they should be used. [If] misinterpreted, they can lead to exactly the wrong action.”
The report was developed in partnership with the Centers for Disease Control and Prevention Foundation, the Office of Minority Health, the Department of Veterans Affairs, and the National Viral Hepatitis Roundtable.
Copies of the report can be obtained at www.iom.edu/viralhepatitis
The Institute of Medicine is calling for increased awareness of hepatitis B and C among health care providers, social service providers, and at-risk communities as well as better surveillance and more stringent vaccination requirements nationwide in its newly released report on hepatitis and liver cancer.
“The committee believes that these recommendations will prevent further infections, improve the lives and health of infected individuals, and reduce the long-term burden of liver disease and liver cancer,” Dr. R. Palmer Beasley said during a teleconference sponsored by the National Academies of Science. Dr. Beasley chaired the IOM committee that wrote the report “Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C.”
“This report outlines the additional resources and actions needed to reduce the unacceptably high burden of liver disease and cancer associated with these viruses,” Dr. Beasley, professor of epidemiology and disease
The committee identified three major factors that impede current efforts to prevent and control hepatitis B and C:
▸ Lack of knowledge and awareness about chronic viral hepatitis on the part of health care and social service providers.
▸ Inadequate knowledge and awareness of chronic viral hepatitis among at-risk populations, members of the public, and policy makers.
▸ Insufficient understanding about the extent and seriousness of this public health problem that has led to the inadequate allocation of resources for prevention, control, and surveillance programs.
Perhaps the greatest difficulty in diagnosing and treating patients with hepatitis B and C is that these diseases are often asymptomatic.
In addition, minority groups—Asians, Pacific Islanders, and blacks—are at greatest risk. More specifically, those most at risk for hepatitis B include people born in East and Southeast Asia or sub-Saharan Africa, infants born to women infected with the disease, and people who have sexual contact or share injection-drug equipment with an infected person. Those at greatest risk for hepatitis C include people who received a blood transfusion before 1992—before the implementation of blood screening for hepatitis C—and past or current injection drug users.
The committee noted that manyoproviders fail to follow guidelines for screening patients and providing prevention, treatment, and follow-up services.
The committee made recommendations for improving surveillance, knowledge and awareness, immunization, and services for viral hepatitis. Highlights of these recommendations include:
▸ A complete evaluation by the Centers for Disease Control and Prevention of the national hepatitis B and C public health surveillance system.
▸ Coordination between CDC and key stakeholders to develop hepatitis B and C education programs for health care and social service personnel.
▸ Coordination between CDC and key stakeholders to develop innovative and effective programs to target at-risk populations and to increase awareness of hepatitis B and C among the general public.
▸ Vaccination of all neonates weighing at least 2,000 g and born to hepatitis B–positive women.
▸ Mandatory vaccination for hepatitis B as a requirement for school attendance.
▸ Studies to develop a hepatitis C vaccine.
Multiple tests are available to diagnose hepatitis B and C, but these tests each have different implications, Dr. Beasley said. “The issue is not whether there are tests but whether or not physicians and health care providers learn and understand how they should be used. [If] misinterpreted, they can lead to exactly the wrong action.”
The report was developed in partnership with the Centers for Disease Control and Prevention Foundation, the Office of Minority Health, the Department of Veterans Affairs, and the National Viral Hepatitis Roundtable.
Copies of the report can be obtained at www.iom.edu/viralhepatitis
The Institute of Medicine is calling for increased awareness of hepatitis B and C among health care providers, social service providers, and at-risk communities as well as better surveillance and more stringent vaccination requirements nationwide in its newly released report on hepatitis and liver cancer.
“The committee believes that these recommendations will prevent further infections, improve the lives and health of infected individuals, and reduce the long-term burden of liver disease and liver cancer,” Dr. R. Palmer Beasley said during a teleconference sponsored by the National Academies of Science. Dr. Beasley chaired the IOM committee that wrote the report “Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C.”
“This report outlines the additional resources and actions needed to reduce the unacceptably high burden of liver disease and cancer associated with these viruses,” Dr. Beasley, professor of epidemiology and disease
The committee identified three major factors that impede current efforts to prevent and control hepatitis B and C:
▸ Lack of knowledge and awareness about chronic viral hepatitis on the part of health care and social service providers.
▸ Inadequate knowledge and awareness of chronic viral hepatitis among at-risk populations, members of the public, and policy makers.
▸ Insufficient understanding about the extent and seriousness of this public health problem that has led to the inadequate allocation of resources for prevention, control, and surveillance programs.
Perhaps the greatest difficulty in diagnosing and treating patients with hepatitis B and C is that these diseases are often asymptomatic.
In addition, minority groups—Asians, Pacific Islanders, and blacks—are at greatest risk. More specifically, those most at risk for hepatitis B include people born in East and Southeast Asia or sub-Saharan Africa, infants born to women infected with the disease, and people who have sexual contact or share injection-drug equipment with an infected person. Those at greatest risk for hepatitis C include people who received a blood transfusion before 1992—before the implementation of blood screening for hepatitis C—and past or current injection drug users.
The committee noted that manyoproviders fail to follow guidelines for screening patients and providing prevention, treatment, and follow-up services.
The committee made recommendations for improving surveillance, knowledge and awareness, immunization, and services for viral hepatitis. Highlights of these recommendations include:
▸ A complete evaluation by the Centers for Disease Control and Prevention of the national hepatitis B and C public health surveillance system.
▸ Coordination between CDC and key stakeholders to develop hepatitis B and C education programs for health care and social service personnel.
▸ Coordination between CDC and key stakeholders to develop innovative and effective programs to target at-risk populations and to increase awareness of hepatitis B and C among the general public.
▸ Vaccination of all neonates weighing at least 2,000 g and born to hepatitis B–positive women.
▸ Mandatory vaccination for hepatitis B as a requirement for school attendance.
▸ Studies to develop a hepatitis C vaccine.
Multiple tests are available to diagnose hepatitis B and C, but these tests each have different implications, Dr. Beasley said. “The issue is not whether there are tests but whether or not physicians and health care providers learn and understand how they should be used. [If] misinterpreted, they can lead to exactly the wrong action.”
The report was developed in partnership with the Centers for Disease Control and Prevention Foundation, the Office of Minority Health, the Department of Veterans Affairs, and the National Viral Hepatitis Roundtable.
Copies of the report can be obtained at www.iom.edu/viralhepatitis