User login
Methylnaltrexone for Acute OIC
The management of postoperative pain is essential to perioperative care, and adequate postoperative analgesia has been associated with several key clinical benefits, including fewer postoperative complications, earlier patient ambulation, reduced costs due to shorter hospital stays, and improved rehabilitation.1, 2 While opioids have long been central to postoperative analgesia, they have been associated with various adverse effects, including sedation, dizziness, nausea, vomiting, constipation, dependence, tolerance, and respiratory depression.2, 3 Constipation, one of the most common adverse effects resulting from opioid therapy, can be debilitating. Indeed, opioid effects on gut motility can occur even after a single dose.3 The consequences of opioid‐induced constipation (OIC) may be severe enough to warrant a dosage reduction of the opioid; however, this may lead to compromised analgesia, which can hinder recovery.4, 5 Thus, effective treatment of OIC is an important clinical consideration in patients undergoing pain management with opioids. Unfortunately, laxatives and other treatment strategies can have unpredictable or suboptimal results for many patients with OIC; therefore, other options are needed for the treatment of OIC.6, 7
Opioid receptor agonists cause constipation by adversely altering many aspects of intestinal function, including fluid dynamics, gastric emptying, propulsive motor activity, and transit time.3 Opioid receptors are widely distributed in the central nervous system and throughout the intestinal system. The mechanism of OIC may have both peripherally and centrally mediated components.8 Nonselective opioid receptor antagonists block the undesired effects on the gut, but because they cross the blood‐brain barrier, they also interfere with analgesia and may lead to symptoms of withdrawal. Methylnaltrexone is a selective, peripherally acting mu‐opioid receptor antagonist,9 formed by the addition of a methyl group to the amine ring of the mu‐opioid receptor antagonist naltrexone. The resulting quarternary amine has greater polarity, lower lipid solubility, and restricted ability to cross the blood‐brain barrier.10 Thus, methylnaltrexone was designed to decrease the peripheral adverse effects of opioids without interfering with centrally mediated analgesia.
Investigations of methylnaltrexone effects in healthy volunteers showed that methylnaltrexone attenuated morphine‐induced delays in gastric emptying and oral‐cecal transit without affecting analgesia.1113 Further studies of methylnaltrexone for the treatment of constipation due to methadone use demonstrated rapid laxation response.1416 Two randomized, double‐blind, placebo‐controlled studies of methylnaltrexone in 288 patients with advanced illness and OIC showed that methylnaltrexone rapidly induced laxation without compromising analgesia.17, 18 Methylnaltrexone is currently approved for the treatment of OIC in patients with advanced illness who are receiving palliative care, when response to laxative therapy has not been sufficient.19
Recently, the use of methylnaltrexone for the treatment of OIC in patients with chronic, nonmalignant pain was assessed in a randomized, double‐blind, placebo‐controlled trial of more than 400 patients. Investigators found that methylnaltrexone induced laxation and was generally well tolerated (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA; Duerden et al., 29th Annual Scientific Meeting of the American Pain Society, May 6‐10, 2010, Baltimore, MD), supporting the safety and efficacy of methylnaltrexone in the setting of OIC resulting from chronic opioid treatment. The present study aimed to assess the activity of methylnaltrexone in patients receiving mu‐agonist opioid analgesics during rehabilitation, following an orthopedic surgical procedure, who were experiencing acute OIC.
METHODS
Patients
Patients who had undergone orthopedic procedures within 4 to 10 days were screened for eligibility. Adults aged 18 years or older were considered eligible if they were acutely constipated, were receiving mu‐agonist opioid analgesics, and were expected to require daily opioid analgesics for at least 7 days following randomization. Acute constipation was defined as having no bowel movement for at least 48 hours prior to randomization, difficulty in having a spontaneous bowel movement (straining or sensation of incomplete evacuation or hard, lumpy stools), or the inability to have a spontaneous bowel movement. Exclusion criteria included fecal impaction, mechanical bowel obstruction, constipation not attributed to postprocedure opioid use, calculated creatinine clearance less than 50 mL/min, and corrected QT interval greater than 500 msec on a 12‐lead screening electrocardiogram (ECG). Patients with a known hypersensitivity to methylnaltrexone, naltrexone, or naloxone, who were pregnant or lactating, who had a history of alcohol or drug abuse within the past 2 years, or who had a spinal cord injury or gastrointestinal ostomy were also excluded. Any laxatives, enemas, and/or promotility agents being used must have been discontinued at least 48 hours prior to first dose of study medication and were not permitted during the study, but stool softener use was permitted if it had been administered at least 24 hours prior to screening and a stable dose was maintained throughout the study.
Study Design
This randomized, double‐blind, placebo‐controlled, parallel‐group, hypothesis‐generating phase 2 study was conducted from October 2007 to January 2009 at 16 US hospitals and rehabilitation facilities in accordance with the International Conference on Harmonisation Good Clinical Practice Guidelines and the Declaration of Helsinki, and was approved by the Institutional Review Board and/or Independent Ethics Committee at each of the participating investigational centers. All patients provided written informed consent prior to study participation.
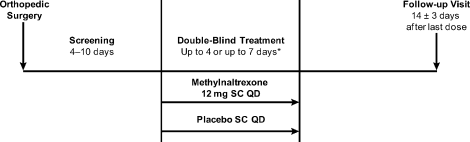
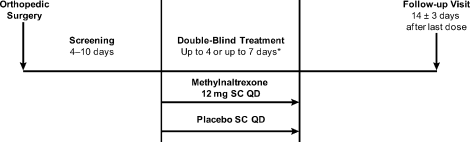
Eligible patients were randomized by interactive voice response system in a 1:1 ratio to receive once‐daily subcutaneous (SC) injections of either 12 mg methylnaltrexone or placebo (Figure 1). The chosen 12‐mg unit dosing corresponds to approximately 0.15 mg/kg (assuming an 80‐kg patient) and was found to be both efficacious and well tolerated in the treatment of OIC in prior studies, including studies in advanced‐illness patients17, 18 and in patients with chronic, nonmalignant pain (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA; Duerden et al., 29th Annual Scientific Meeting of the American Pain Society, May 6‐10, 2010, Baltimore, MD.20 The first dose of study medication was administered on the day of randomization or on the next calendar day. Once enrolled, the patient received once‐daily doses of methylnaltrexone for up to 4 or 7 days. Dosing continued until the patient received the maximum number of doses allowed, no longer needed opioid medication, or was discharged from the medical facility. Each patient completed a follow‐up safety visit at 14 3 days following the last dose.
Evaluations
All efficacy variables were considered exploratory and included the occurrence of laxation within 2 and 4 hours of the first dose of study drug, time to laxation, and a questionnaire assessing patient global satisfaction. Patients recorded the date, time, and assessment of each bowel movement in diaries.
Safety variables included adverse events (AEs), serious AEs (SAEs), clinical laboratory parameters, physical examinations, vital signs, ECGs, concomitant medications, Objective and Subjective Opioid Withdrawal Scales (OOWS and SOWS),21 and Numeric Rating Scales for Pain ([NRSP] 0 = no pain, 10 = worst pain possible).
Statistical Analysis
Enrolled patients were defined as all patients who consented to participate in the study. Both the modified intent‐to‐treat (mITT) population and the safety population were defined as all patients who were randomized and received at least 1 injection of study drug. All study results are based on the mITT population.
Categorical variables were summarized using frequency and percentage, while descriptive statistics for continuous variables included sample size, mean, median, standard deviation, and minimum and maximum values. All inferential statistical tests were 2‐tailed and used a tolerance for nominal type I error (alpha, ) of 0.05. There was no correction for multiplicity and no imputations were performed to account for missing data.
Fisher's exact test was used for comparisons between the proportion of patients with laxation within 2 hours and 4 hours of the first dose in the methylnaltrexone group versus the placebo group. The time to first laxation analysis was performed using the log‐rank test and Kaplan‐Meier method.
RESULTS
Patient Populations
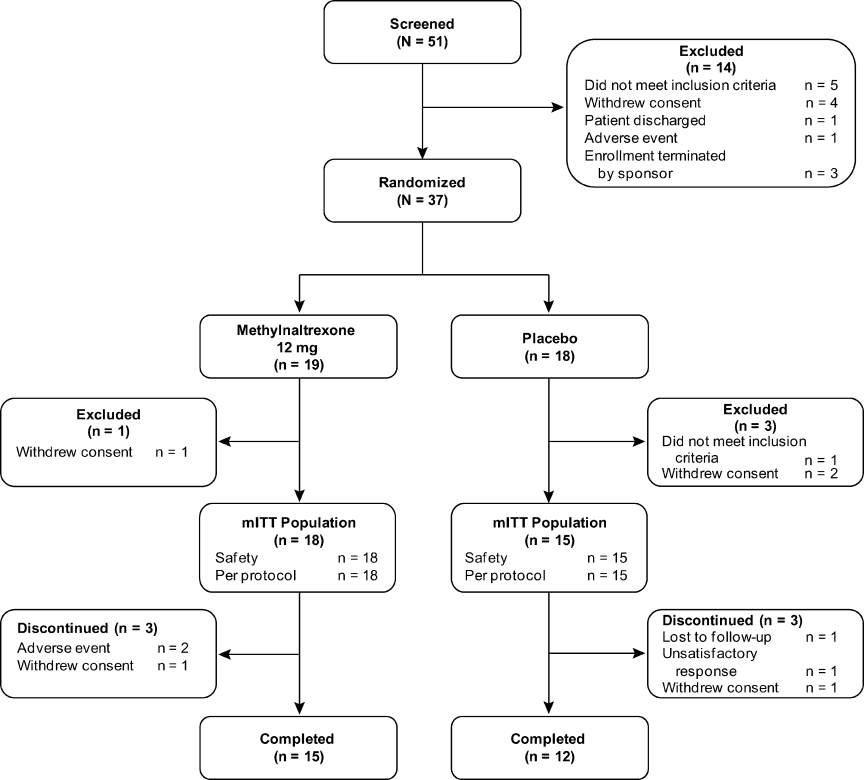
The flow of patients through the study is summarized in Figure 2. A total of 51 patients were enrolled. Of these, 33 received at least 1 dose of study treatment following double‐blind randomization and comprised both the mITT and safety populations. Seventeen of these patients were enrolled under the original protocol and could receive study drug for up to 7 days, while 16 patients enrolled under a subsequent protocol revision could receive study drug for up to 4 days. This change from a 7‐day to a 4‐day treatment protocol allowed for the capture of more study patients in view of the time pressures of short lengths of stay in postoperative settings. In total, 31 patients received at least 2 doses, and 26 patients received at least 4 doses of study drug. A total of 27 patients completed the study. Baseline demographics and prestudy surgical procedures were similar in both treatment groups (Table 1).
| Characteristic | Methylnaltrexone (n = 18) | Placebo (n = 15) |
|---|---|---|
| ||
| Mean age, yr (SD) | 64.2 (9.0) | 65.2 (11.6) |
| Mean weight, kg (SD) | 92.5 (22.5) | 91.0 (20.2) |
| Mean BMI, kg/m2 (SD) | 32.3 (7.2) | 34.2 (6.41) |
| Sex, n (%) | ||
| Female | 11 (61.1) | 11 (73.3) |
| Male | 7 (38.9) | 4 (26.7) |
| Race, n (%) | ||
| White | 14 (77.8) | 10 (66.7) |
| Black | 4 (22.2) | 5 (33.3) |
| Type of surgery, n (%) | ||
| Total knee replacement | 8 (44.4) | 7 (46.7) |
| Total hip replacement | 6 (33.3) | 6 (40.0) |
| Spinal fusion | 2 (11.1) | 0 |
| Fracture reduction | 2 (11.1) | 2 (13.3) |
| Median opioid use,* mg (range) | 28.00 (6.75‐168.01) | 25.00 (9.00‐75.00) |
| Median time from surgery to study drug administration, days (range) | 4 (3‐6) | 4 (3‐6) |
Efficacy
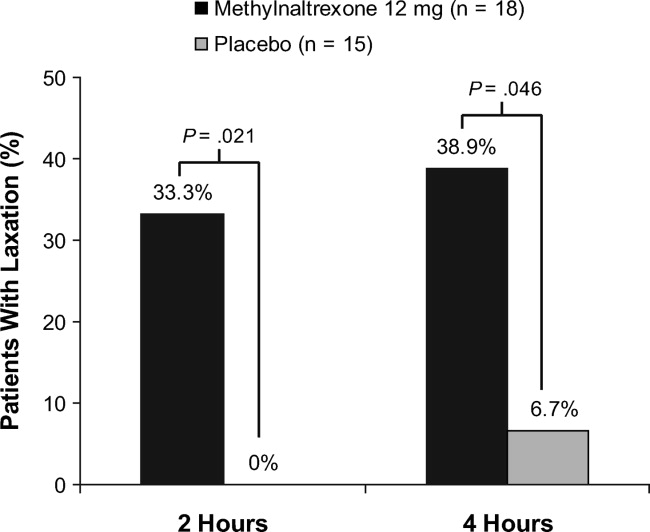
A significantly greater percentage of patients had a bowel movement within 2 hours (P = 0.021) and 4 hours (P = 0.046) of the first dose of methylnaltrexone compared with patients who received placebo (Figure 3). Within 2 hours, 6 patients (33.3%; 95% confidence interval [CI], 13.34‐59.01) who received methylnaltrexone achieved laxation, while laxation did not occur in any patient who received placebo. By 4 hours posttreatment, 7 patients (38.9%; 95% CI, 17.30‐64.25) in the methylnaltrexone group achieved laxation compared with only 1 patient (6.7%; 95% CI, 0.17‐31.95) on placebo. Three patients in each treatment group received rescue laxatives.
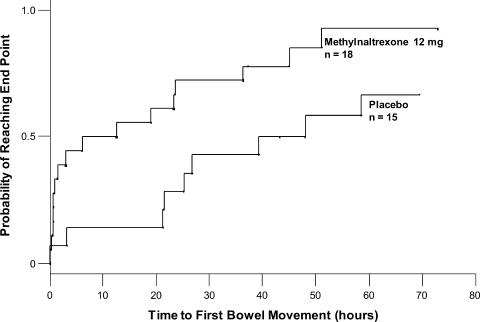
The time to first laxation (Figure 4) was significantly shorter in patients who received methylnaltrexone compared with those in the placebo group. Patients on methylnaltrexone achieved laxation in a median time of 15.8 hours, compared with a median time of 50.9 hours for patients in the placebo group (P = 0.02, log‐rank test). The median time to laxation was less than 1 hour in the 7 methylnaltrexone‐treated patients who experienced laxation within 4 hours following the first dose. Of the remaining 11 methylnaltrexone‐treated patients, one experienced no laxation after 6 doses, and the median time to laxation for the others was 29.9 hours (not shown in figure).
Analysis of the Global Satisfaction With Treatment Scale revealed that more patients expressed overall treatment satisfaction (defined as very satisfied, satisfied, or minimally satisfied) with methylnaltrexone assessed 4 hours ( 30 minutes) after the first dose, compared with patients on placebo (83.3% vs 60.0%, respectively). At the study endpoint, overall treatment satisfaction with methylnaltrexone remained high (83.3%), whereas satisfaction with placebo was 53.3%. Additionally, no patients in the methylnaltrexone group expressed any dissatisfaction with treatment (defined as minimally dissatisfied, dissatisfied, or very dissatisfied) at endpoint, compared with 26.7% of patients in the placebo group who expressed some degree of dissatisfaction.
Safety
Overall AE rates were similar between treatment groups (Table 2), with at least 1 treatment‐emergent AE reported in 6 patients (33.3%) in the methylnaltrexone group and 4 patients (26.7%) in the placebo group. The most common AEs reported during the study were classified as gastrointestinal in nature; 3 (nausea, abdominal pain, and diarrhea) were considered by the investigator to be possibly related to study medication. Two patients receiving methylnaltrexone discontinued the study because of AEs (one with moderate constipation, one with mild diarrhea) compared with none of the placebo group patients. No treatment‐emergent SAEs or deaths were reported during this study. Analysis of clinical laboratory parameters, vital signs, and ECGs revealed no safety signals and showed no pattern of concern related to methylnaltrexone exposure.
| Adverse Event* | Methylnaltrexone 12 mg (n = 18) n (%) | Placebo (n = 15) n (%) |
|---|---|---|
| ||
| Any | 6 (33.3) | 4 (26.7) |
| Anemia | 1 (5.6) | 0 |
| Gastrointestinal disorders | 3 (16.7) | 1 (6.7) |
| Abdominal discomfort | 0 | 1 (6.7) |
| Abdominal distension | 1 (5.6) | 0 |
| Abdominal pain | 1 (5.6) | 0 |
| Abdominal tenderness | 1 (5.6) | 0 |
| Constipation | 1 (5.6) | 0 |
| Diarrhea | 1 (5.6) | 0 |
| Nausea | 1 (5.6) | 0 |
| Headache | 1 (5.6) | 0 |
| Hypotension | 1 (5.6) | 0 |
| Joint swelling | 0 | 1 (6.7) |
| Peripheral edema | 0 | 2 (13.3) |
| Procedural pain | 0 | 1 (6.7) |
| Skin ulcer | 0 | 1 (6.7) |
| Somnolence | 0 | 1 (6.7) |
| Urinary tract infection | 1 (5.6) | 0 |
| Wound infection | 1 (5.6) | 0 |
Pain and Opioid Withdrawal
Results from the SOWS and OOWS measures indicated that signs and symptoms of withdrawal did not increase over time in patients treated with methylnaltrexone, and no discernable differences were found between study groups. Pain was assessed using a numeric rating scale ranging from 0 to 10, with higher scores indicating greater severity. Baseline pain scores were not significantly different between treatment groups, with a mean of 5.7 2.7 for placebo, and 5.4 3.0 for the methylnaltrexone group. At 1 day postdose, mean pain scores did not increase from baseline in the placebo (0.9 2.33) or methylnaltrexone group (0.5 2.5), and no significant between‐group differences were found. Similar results were observed at the end of the study. Thus, pain did not appear to increase in patients treated with methylnaltrexone, and changes in pain scores were indistinguishable between the 2 treatment groups.
DISCUSSION
This pilot study suggests that methylnaltrexone actively induces laxation and is generally well tolerated in patients receiving mu‐opioid analgesia, following orthopedic surgery, who develop OIC acutely. It was the first study, to our knowledge, to investigate the efficacy of methylnaltrexone for the treatment of OIC in an acute postoperative setting. The protocol amendment changing the duration of treatment from 7 days to 4 days did not materially affect the results of the study. The response to methylnaltrexone was rapid, with 33.3% experiencing laxation within 2 hours. The median time to laxation was nearly 1.5 days shorter in patients treated with methylnaltrexone compared with those receiving placebo. Correspondingly, overall patient satisfaction was high in the methylnaltrexone group. Efficacy was attained without diminishing opioid analgesia, and without inducing signs or symptoms of opioid withdrawal. The incidence of AEs was similar between groups, and no treatment‐emergent SAEs were reported in this study.
Previous clinical trials investigated the safety and efficacy of methylnaltrexone for the treatment of OIC in patients with advanced illness and with chronic, nonmalignant pain. The present study extends those findings to a population of patients experiencing acute OIC following orthopedic surgery. Previous studies showed that approximately 48% to 62% of advanced‐illness patients experienced laxation within 4 hours of receiving SC methylnaltrexone,17, 18 compared with 38.9% of acute OIC patients in this study. In a clinical trial of patients with chronic, nonmalignant pain, 34.2% of patients experienced laxation within 4 hours of SC methylnaltrexone injection (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA). The differences in laxation response between these trials may be attributable to differences in the patient populations or to methodologic differences between the studies.
Similar to findings demonstrated in a clinical study evaluating methylnaltrexone for OIC in a different patient population, those with advanced illness,22 this study supports the premise that future laxation response with prolonged use is most likely to occur when a laxation response was achieved after the first or second initial administrations of methylnaltrexone. In contradistinction, if laxation does not occur with these early doses, continued methylnaltrexone dosing is less likely to produce a response later.
This study has some limitations that must be considered. First, as this was a hypothesis‐generating study, all efficacy parameters investigated were exploratory in nature. The results reported herein warrant careful consideration, owing to a small sample size that may limit their generalizability, prior to replication in a more rigorously designed study with prespecified efficacy endpoints. Likewise, the assessment of health outcome parameters is limited. Another limitation is the small sample size utilized in this study, potentially resulting in a type II error.
Subcutaneous administration potentially offers a considerable benefit over oral therapies for OIC in this patient population post‐orthopedic surgery. Nausea and vomiting can occur as a consequence of anesthesia and of postoperative opioid analgesia, and may compromise adequate dosing of oral medications prescribed to treat OIC. Subcutaneous delivery of methylnaltrexone may circumvent this potential drawback while providing potentially rapid, effective treatment for OIC. Once‐daily dosing may also help to minimize caregiver burden and patient discomfort by preventing the need for more frequent or unpleasant treatments for OIC, such as enemas.
This study provides an initial positive signal for a broader, albeit off‐label use for methylnaltrexonethat being for the treatment of acute constipation that occurs as a consequence of postoperative opioid‐mediated analgesia in patients following orthopedic procedures. Adequate treatment of OIC, even in the acute postoperative setting, is likely to lead to better overall pain management and improved patient outcomes. Additionally, effective management of acute OIC is likely to be cost‐effective in terms of reducing the duration of hospital stays, reducing the need for nursing resources and the time spent administering rescue treatments for OIC (eg, enemas), and avoiding returns to an acute setting (eg, the emergency department) for treatment. The results presented herein suggest that methylnaltrexone may be effective and have a good safety profile in the treatment of acute OIC following orthopedic surgery. Validation of these results in larger well‐controlled trials would be welcome.
Acknowledgements
The authors thank the patients and clinical personnel involved in this study; John Charity, NP, for data collection and management, and John H. Simmons, MD, of Peloton Advantage, LLC, for assistance with manuscript preparation, which was funded by Pfizer Inc.
In addition to the authors, the following investigators participated in this trial: David Nathan Feldman, MD, Holy Name Hospital, Teaneck, NJ; Sam Hakki, MD, Bay Pines VA Healthcare System, Bay Pines, FL; Forrest A. Hanke, MD, Trover Health System, Madisonville, KY; William H. Horton, Jr, MD, Palmetto Clinical Research, Greenville, SC; M. Jay Jazayeri, MD, Pacific Hospital of Long Beach, Long Beach, CA; John F. Peppin, DO, The Pain Treatment Center of the Bluegrass, Lexington, KY; Bruce Pomeranz, MD, Kessler Institute for Rehabilitation, Saddle Brook, NJ, and Chester, NJ; Alan C. Schwartz, MD, Helping Hands Medical Associates, Santa Ana, CA; Michael J. Skyhar, MD, CORE Orthopaedic Medical Center, Encinitas, CA; Lex A. Simpson, MD, CORE Orthopaedic Medical Center, Encinitas, CA; James Slover, MD, New York University Hospital for Joint Disease, New York, NY; Dilip Tapadiya, MD, Fountain Valley Regional Hospital, Fountain Valley, CA; Stanley J. Waters, MD, PhD, Americana Orthopedics, Boise, ID.
- ,.Postoperative pain management.Chest Surg Clin N Am.1997;7:773–799.
- ,.Strategies for effective postoperative pain management.Minerva Anestesiol.2006;72:145–150.
- ,,.Are peripheral opioid antagonists the solution to opioid side effects?Anesth Analg.2004;98:116–122.
- ,.Neuroplasticity—an important factor in acute and chronic pain.Swiss Med Wkly.2002;132:273–278.
- ,,,,.The burden of acute postoperative pain and the potential role of the COX‐2‐specific inhibitors.Rheumatology (Oxford).2003;42(suppl 3):iii40–iii52.
- .Incidence, prevalence, and management of opioid bowel dysfunction.Am J Surg.2001;182(suppl 5A):11S–18S.
- ,.Management of common opioid‐induced adverse effects.Am Fam Physician.2006;74:1347–1354.
- ,.Antagonism of gastrointestinal opioid effects.Reg Anesth Pain Med.2000;25:639–642.
- .Methylnaltrexone mechanisms of action and effects on opioid bowel dysfunction and other opioid adverse effects.Ann Pharmacother.2007;41:984–993.
- ,.Methylnaltrexone: investigation of clinical applications.Drug Dev Res.2000;50:133–141.
- ,,,.Opioid‐induced delay in gastric emptying: a peripheral mechanism in humans.Anesthesiology.1997;87:765–770.
- ,,, et al.Effects of enteric‐coated methylnaltrexone in preventing opioid‐induced delay in oral‐cecal transit time.Clin Pharmacol Ther.2000;67:398–404.
- ,,,,,.Methylnaltrexone prevents morphine‐induced delay in oral‐cecal transit time without affecting analgesia: a double‐blind randomized placebo‐controlled trial.Clin Pharmacol Ther.1996;59:469–475.
- ,,,,,.Effects of intravenous methylnaltrexone on opioid‐induced gut motility and transit time changes in subjects receiving chronic methadone therapy: a pilot study.Pain.1999;83:631–635.
- ,,, et al.Methylnaltrexone for reversal of constipation due to chronic methadone use: a randomized controlled trial.JAMA.2000;283:367–372.
- ,.Oral methylnaltrexone for opioid‐induced constipation.JAMA.2000;284:1383–1384.
- ,,, et al.Methylnaltrexone for opioid‐induced constipation in advanced illness.N Engl J Med.2008;328:2332–2343.
- ,,, et al.Methylnaltrexone for treatment of opioid‐induced constipation in advanced illness patients.J Support Oncol.2009;7:39–46.
- Relistor [package insert].Philadelphia, PA, and Tarrytown, NY:Wyeth Pharmaceuticals Inc and Progenics Pharmaceuticals;2009.
- ,,, et al.Subcutaneous methylnaltrexone for treatment of opioid‐induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study.J Pain.2011;12:554–562.
- ,,,,,.Two new rating scales for opiate withdrawal.Am J Drug Alcohol Abuse.1987;13:293–308.
- ,,, et al.Methylnaltrexone treatment of opioid‐induced constipation in patients with advanced illness.J Pain Symptom Manage.2009;38:683–690.
The management of postoperative pain is essential to perioperative care, and adequate postoperative analgesia has been associated with several key clinical benefits, including fewer postoperative complications, earlier patient ambulation, reduced costs due to shorter hospital stays, and improved rehabilitation.1, 2 While opioids have long been central to postoperative analgesia, they have been associated with various adverse effects, including sedation, dizziness, nausea, vomiting, constipation, dependence, tolerance, and respiratory depression.2, 3 Constipation, one of the most common adverse effects resulting from opioid therapy, can be debilitating. Indeed, opioid effects on gut motility can occur even after a single dose.3 The consequences of opioid‐induced constipation (OIC) may be severe enough to warrant a dosage reduction of the opioid; however, this may lead to compromised analgesia, which can hinder recovery.4, 5 Thus, effective treatment of OIC is an important clinical consideration in patients undergoing pain management with opioids. Unfortunately, laxatives and other treatment strategies can have unpredictable or suboptimal results for many patients with OIC; therefore, other options are needed for the treatment of OIC.6, 7
Opioid receptor agonists cause constipation by adversely altering many aspects of intestinal function, including fluid dynamics, gastric emptying, propulsive motor activity, and transit time.3 Opioid receptors are widely distributed in the central nervous system and throughout the intestinal system. The mechanism of OIC may have both peripherally and centrally mediated components.8 Nonselective opioid receptor antagonists block the undesired effects on the gut, but because they cross the blood‐brain barrier, they also interfere with analgesia and may lead to symptoms of withdrawal. Methylnaltrexone is a selective, peripherally acting mu‐opioid receptor antagonist,9 formed by the addition of a methyl group to the amine ring of the mu‐opioid receptor antagonist naltrexone. The resulting quarternary amine has greater polarity, lower lipid solubility, and restricted ability to cross the blood‐brain barrier.10 Thus, methylnaltrexone was designed to decrease the peripheral adverse effects of opioids without interfering with centrally mediated analgesia.
Investigations of methylnaltrexone effects in healthy volunteers showed that methylnaltrexone attenuated morphine‐induced delays in gastric emptying and oral‐cecal transit without affecting analgesia.1113 Further studies of methylnaltrexone for the treatment of constipation due to methadone use demonstrated rapid laxation response.1416 Two randomized, double‐blind, placebo‐controlled studies of methylnaltrexone in 288 patients with advanced illness and OIC showed that methylnaltrexone rapidly induced laxation without compromising analgesia.17, 18 Methylnaltrexone is currently approved for the treatment of OIC in patients with advanced illness who are receiving palliative care, when response to laxative therapy has not been sufficient.19
Recently, the use of methylnaltrexone for the treatment of OIC in patients with chronic, nonmalignant pain was assessed in a randomized, double‐blind, placebo‐controlled trial of more than 400 patients. Investigators found that methylnaltrexone induced laxation and was generally well tolerated (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA; Duerden et al., 29th Annual Scientific Meeting of the American Pain Society, May 6‐10, 2010, Baltimore, MD), supporting the safety and efficacy of methylnaltrexone in the setting of OIC resulting from chronic opioid treatment. The present study aimed to assess the activity of methylnaltrexone in patients receiving mu‐agonist opioid analgesics during rehabilitation, following an orthopedic surgical procedure, who were experiencing acute OIC.
METHODS
Patients
Patients who had undergone orthopedic procedures within 4 to 10 days were screened for eligibility. Adults aged 18 years or older were considered eligible if they were acutely constipated, were receiving mu‐agonist opioid analgesics, and were expected to require daily opioid analgesics for at least 7 days following randomization. Acute constipation was defined as having no bowel movement for at least 48 hours prior to randomization, difficulty in having a spontaneous bowel movement (straining or sensation of incomplete evacuation or hard, lumpy stools), or the inability to have a spontaneous bowel movement. Exclusion criteria included fecal impaction, mechanical bowel obstruction, constipation not attributed to postprocedure opioid use, calculated creatinine clearance less than 50 mL/min, and corrected QT interval greater than 500 msec on a 12‐lead screening electrocardiogram (ECG). Patients with a known hypersensitivity to methylnaltrexone, naltrexone, or naloxone, who were pregnant or lactating, who had a history of alcohol or drug abuse within the past 2 years, or who had a spinal cord injury or gastrointestinal ostomy were also excluded. Any laxatives, enemas, and/or promotility agents being used must have been discontinued at least 48 hours prior to first dose of study medication and were not permitted during the study, but stool softener use was permitted if it had been administered at least 24 hours prior to screening and a stable dose was maintained throughout the study.
Study Design
This randomized, double‐blind, placebo‐controlled, parallel‐group, hypothesis‐generating phase 2 study was conducted from October 2007 to January 2009 at 16 US hospitals and rehabilitation facilities in accordance with the International Conference on Harmonisation Good Clinical Practice Guidelines and the Declaration of Helsinki, and was approved by the Institutional Review Board and/or Independent Ethics Committee at each of the participating investigational centers. All patients provided written informed consent prior to study participation.
Eligible patients were randomized by interactive voice response system in a 1:1 ratio to receive once‐daily subcutaneous (SC) injections of either 12 mg methylnaltrexone or placebo (Figure 1). The chosen 12‐mg unit dosing corresponds to approximately 0.15 mg/kg (assuming an 80‐kg patient) and was found to be both efficacious and well tolerated in the treatment of OIC in prior studies, including studies in advanced‐illness patients17, 18 and in patients with chronic, nonmalignant pain (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA; Duerden et al., 29th Annual Scientific Meeting of the American Pain Society, May 6‐10, 2010, Baltimore, MD.20 The first dose of study medication was administered on the day of randomization or on the next calendar day. Once enrolled, the patient received once‐daily doses of methylnaltrexone for up to 4 or 7 days. Dosing continued until the patient received the maximum number of doses allowed, no longer needed opioid medication, or was discharged from the medical facility. Each patient completed a follow‐up safety visit at 14 3 days following the last dose.

Evaluations
All efficacy variables were considered exploratory and included the occurrence of laxation within 2 and 4 hours of the first dose of study drug, time to laxation, and a questionnaire assessing patient global satisfaction. Patients recorded the date, time, and assessment of each bowel movement in diaries.
Safety variables included adverse events (AEs), serious AEs (SAEs), clinical laboratory parameters, physical examinations, vital signs, ECGs, concomitant medications, Objective and Subjective Opioid Withdrawal Scales (OOWS and SOWS),21 and Numeric Rating Scales for Pain ([NRSP] 0 = no pain, 10 = worst pain possible).
Statistical Analysis
Enrolled patients were defined as all patients who consented to participate in the study. Both the modified intent‐to‐treat (mITT) population and the safety population were defined as all patients who were randomized and received at least 1 injection of study drug. All study results are based on the mITT population.
Categorical variables were summarized using frequency and percentage, while descriptive statistics for continuous variables included sample size, mean, median, standard deviation, and minimum and maximum values. All inferential statistical tests were 2‐tailed and used a tolerance for nominal type I error (alpha, ) of 0.05. There was no correction for multiplicity and no imputations were performed to account for missing data.
Fisher's exact test was used for comparisons between the proportion of patients with laxation within 2 hours and 4 hours of the first dose in the methylnaltrexone group versus the placebo group. The time to first laxation analysis was performed using the log‐rank test and Kaplan‐Meier method.
RESULTS
Patient Populations
The flow of patients through the study is summarized in Figure 2. A total of 51 patients were enrolled. Of these, 33 received at least 1 dose of study treatment following double‐blind randomization and comprised both the mITT and safety populations. Seventeen of these patients were enrolled under the original protocol and could receive study drug for up to 7 days, while 16 patients enrolled under a subsequent protocol revision could receive study drug for up to 4 days. This change from a 7‐day to a 4‐day treatment protocol allowed for the capture of more study patients in view of the time pressures of short lengths of stay in postoperative settings. In total, 31 patients received at least 2 doses, and 26 patients received at least 4 doses of study drug. A total of 27 patients completed the study. Baseline demographics and prestudy surgical procedures were similar in both treatment groups (Table 1).

| Characteristic | Methylnaltrexone (n = 18) | Placebo (n = 15) |
|---|---|---|
| ||
| Mean age, yr (SD) | 64.2 (9.0) | 65.2 (11.6) |
| Mean weight, kg (SD) | 92.5 (22.5) | 91.0 (20.2) |
| Mean BMI, kg/m2 (SD) | 32.3 (7.2) | 34.2 (6.41) |
| Sex, n (%) | ||
| Female | 11 (61.1) | 11 (73.3) |
| Male | 7 (38.9) | 4 (26.7) |
| Race, n (%) | ||
| White | 14 (77.8) | 10 (66.7) |
| Black | 4 (22.2) | 5 (33.3) |
| Type of surgery, n (%) | ||
| Total knee replacement | 8 (44.4) | 7 (46.7) |
| Total hip replacement | 6 (33.3) | 6 (40.0) |
| Spinal fusion | 2 (11.1) | 0 |
| Fracture reduction | 2 (11.1) | 2 (13.3) |
| Median opioid use,* mg (range) | 28.00 (6.75‐168.01) | 25.00 (9.00‐75.00) |
| Median time from surgery to study drug administration, days (range) | 4 (3‐6) | 4 (3‐6) |
Efficacy
A significantly greater percentage of patients had a bowel movement within 2 hours (P = 0.021) and 4 hours (P = 0.046) of the first dose of methylnaltrexone compared with patients who received placebo (Figure 3). Within 2 hours, 6 patients (33.3%; 95% confidence interval [CI], 13.34‐59.01) who received methylnaltrexone achieved laxation, while laxation did not occur in any patient who received placebo. By 4 hours posttreatment, 7 patients (38.9%; 95% CI, 17.30‐64.25) in the methylnaltrexone group achieved laxation compared with only 1 patient (6.7%; 95% CI, 0.17‐31.95) on placebo. Three patients in each treatment group received rescue laxatives.

The time to first laxation (Figure 4) was significantly shorter in patients who received methylnaltrexone compared with those in the placebo group. Patients on methylnaltrexone achieved laxation in a median time of 15.8 hours, compared with a median time of 50.9 hours for patients in the placebo group (P = 0.02, log‐rank test). The median time to laxation was less than 1 hour in the 7 methylnaltrexone‐treated patients who experienced laxation within 4 hours following the first dose. Of the remaining 11 methylnaltrexone‐treated patients, one experienced no laxation after 6 doses, and the median time to laxation for the others was 29.9 hours (not shown in figure).

Analysis of the Global Satisfaction With Treatment Scale revealed that more patients expressed overall treatment satisfaction (defined as very satisfied, satisfied, or minimally satisfied) with methylnaltrexone assessed 4 hours ( 30 minutes) after the first dose, compared with patients on placebo (83.3% vs 60.0%, respectively). At the study endpoint, overall treatment satisfaction with methylnaltrexone remained high (83.3%), whereas satisfaction with placebo was 53.3%. Additionally, no patients in the methylnaltrexone group expressed any dissatisfaction with treatment (defined as minimally dissatisfied, dissatisfied, or very dissatisfied) at endpoint, compared with 26.7% of patients in the placebo group who expressed some degree of dissatisfaction.
Safety
Overall AE rates were similar between treatment groups (Table 2), with at least 1 treatment‐emergent AE reported in 6 patients (33.3%) in the methylnaltrexone group and 4 patients (26.7%) in the placebo group. The most common AEs reported during the study were classified as gastrointestinal in nature; 3 (nausea, abdominal pain, and diarrhea) were considered by the investigator to be possibly related to study medication. Two patients receiving methylnaltrexone discontinued the study because of AEs (one with moderate constipation, one with mild diarrhea) compared with none of the placebo group patients. No treatment‐emergent SAEs or deaths were reported during this study. Analysis of clinical laboratory parameters, vital signs, and ECGs revealed no safety signals and showed no pattern of concern related to methylnaltrexone exposure.
| Adverse Event* | Methylnaltrexone 12 mg (n = 18) n (%) | Placebo (n = 15) n (%) |
|---|---|---|
| ||
| Any | 6 (33.3) | 4 (26.7) |
| Anemia | 1 (5.6) | 0 |
| Gastrointestinal disorders | 3 (16.7) | 1 (6.7) |
| Abdominal discomfort | 0 | 1 (6.7) |
| Abdominal distension | 1 (5.6) | 0 |
| Abdominal pain | 1 (5.6) | 0 |
| Abdominal tenderness | 1 (5.6) | 0 |
| Constipation | 1 (5.6) | 0 |
| Diarrhea | 1 (5.6) | 0 |
| Nausea | 1 (5.6) | 0 |
| Headache | 1 (5.6) | 0 |
| Hypotension | 1 (5.6) | 0 |
| Joint swelling | 0 | 1 (6.7) |
| Peripheral edema | 0 | 2 (13.3) |
| Procedural pain | 0 | 1 (6.7) |
| Skin ulcer | 0 | 1 (6.7) |
| Somnolence | 0 | 1 (6.7) |
| Urinary tract infection | 1 (5.6) | 0 |
| Wound infection | 1 (5.6) | 0 |
Pain and Opioid Withdrawal
Results from the SOWS and OOWS measures indicated that signs and symptoms of withdrawal did not increase over time in patients treated with methylnaltrexone, and no discernable differences were found between study groups. Pain was assessed using a numeric rating scale ranging from 0 to 10, with higher scores indicating greater severity. Baseline pain scores were not significantly different between treatment groups, with a mean of 5.7 2.7 for placebo, and 5.4 3.0 for the methylnaltrexone group. At 1 day postdose, mean pain scores did not increase from baseline in the placebo (0.9 2.33) or methylnaltrexone group (0.5 2.5), and no significant between‐group differences were found. Similar results were observed at the end of the study. Thus, pain did not appear to increase in patients treated with methylnaltrexone, and changes in pain scores were indistinguishable between the 2 treatment groups.
DISCUSSION
This pilot study suggests that methylnaltrexone actively induces laxation and is generally well tolerated in patients receiving mu‐opioid analgesia, following orthopedic surgery, who develop OIC acutely. It was the first study, to our knowledge, to investigate the efficacy of methylnaltrexone for the treatment of OIC in an acute postoperative setting. The protocol amendment changing the duration of treatment from 7 days to 4 days did not materially affect the results of the study. The response to methylnaltrexone was rapid, with 33.3% experiencing laxation within 2 hours. The median time to laxation was nearly 1.5 days shorter in patients treated with methylnaltrexone compared with those receiving placebo. Correspondingly, overall patient satisfaction was high in the methylnaltrexone group. Efficacy was attained without diminishing opioid analgesia, and without inducing signs or symptoms of opioid withdrawal. The incidence of AEs was similar between groups, and no treatment‐emergent SAEs were reported in this study.
Previous clinical trials investigated the safety and efficacy of methylnaltrexone for the treatment of OIC in patients with advanced illness and with chronic, nonmalignant pain. The present study extends those findings to a population of patients experiencing acute OIC following orthopedic surgery. Previous studies showed that approximately 48% to 62% of advanced‐illness patients experienced laxation within 4 hours of receiving SC methylnaltrexone,17, 18 compared with 38.9% of acute OIC patients in this study. In a clinical trial of patients with chronic, nonmalignant pain, 34.2% of patients experienced laxation within 4 hours of SC methylnaltrexone injection (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA). The differences in laxation response between these trials may be attributable to differences in the patient populations or to methodologic differences between the studies.
Similar to findings demonstrated in a clinical study evaluating methylnaltrexone for OIC in a different patient population, those with advanced illness,22 this study supports the premise that future laxation response with prolonged use is most likely to occur when a laxation response was achieved after the first or second initial administrations of methylnaltrexone. In contradistinction, if laxation does not occur with these early doses, continued methylnaltrexone dosing is less likely to produce a response later.
This study has some limitations that must be considered. First, as this was a hypothesis‐generating study, all efficacy parameters investigated were exploratory in nature. The results reported herein warrant careful consideration, owing to a small sample size that may limit their generalizability, prior to replication in a more rigorously designed study with prespecified efficacy endpoints. Likewise, the assessment of health outcome parameters is limited. Another limitation is the small sample size utilized in this study, potentially resulting in a type II error.
Subcutaneous administration potentially offers a considerable benefit over oral therapies for OIC in this patient population post‐orthopedic surgery. Nausea and vomiting can occur as a consequence of anesthesia and of postoperative opioid analgesia, and may compromise adequate dosing of oral medications prescribed to treat OIC. Subcutaneous delivery of methylnaltrexone may circumvent this potential drawback while providing potentially rapid, effective treatment for OIC. Once‐daily dosing may also help to minimize caregiver burden and patient discomfort by preventing the need for more frequent or unpleasant treatments for OIC, such as enemas.
This study provides an initial positive signal for a broader, albeit off‐label use for methylnaltrexonethat being for the treatment of acute constipation that occurs as a consequence of postoperative opioid‐mediated analgesia in patients following orthopedic procedures. Adequate treatment of OIC, even in the acute postoperative setting, is likely to lead to better overall pain management and improved patient outcomes. Additionally, effective management of acute OIC is likely to be cost‐effective in terms of reducing the duration of hospital stays, reducing the need for nursing resources and the time spent administering rescue treatments for OIC (eg, enemas), and avoiding returns to an acute setting (eg, the emergency department) for treatment. The results presented herein suggest that methylnaltrexone may be effective and have a good safety profile in the treatment of acute OIC following orthopedic surgery. Validation of these results in larger well‐controlled trials would be welcome.
Acknowledgements
The authors thank the patients and clinical personnel involved in this study; John Charity, NP, for data collection and management, and John H. Simmons, MD, of Peloton Advantage, LLC, for assistance with manuscript preparation, which was funded by Pfizer Inc.
In addition to the authors, the following investigators participated in this trial: David Nathan Feldman, MD, Holy Name Hospital, Teaneck, NJ; Sam Hakki, MD, Bay Pines VA Healthcare System, Bay Pines, FL; Forrest A. Hanke, MD, Trover Health System, Madisonville, KY; William H. Horton, Jr, MD, Palmetto Clinical Research, Greenville, SC; M. Jay Jazayeri, MD, Pacific Hospital of Long Beach, Long Beach, CA; John F. Peppin, DO, The Pain Treatment Center of the Bluegrass, Lexington, KY; Bruce Pomeranz, MD, Kessler Institute for Rehabilitation, Saddle Brook, NJ, and Chester, NJ; Alan C. Schwartz, MD, Helping Hands Medical Associates, Santa Ana, CA; Michael J. Skyhar, MD, CORE Orthopaedic Medical Center, Encinitas, CA; Lex A. Simpson, MD, CORE Orthopaedic Medical Center, Encinitas, CA; James Slover, MD, New York University Hospital for Joint Disease, New York, NY; Dilip Tapadiya, MD, Fountain Valley Regional Hospital, Fountain Valley, CA; Stanley J. Waters, MD, PhD, Americana Orthopedics, Boise, ID.
The management of postoperative pain is essential to perioperative care, and adequate postoperative analgesia has been associated with several key clinical benefits, including fewer postoperative complications, earlier patient ambulation, reduced costs due to shorter hospital stays, and improved rehabilitation.1, 2 While opioids have long been central to postoperative analgesia, they have been associated with various adverse effects, including sedation, dizziness, nausea, vomiting, constipation, dependence, tolerance, and respiratory depression.2, 3 Constipation, one of the most common adverse effects resulting from opioid therapy, can be debilitating. Indeed, opioid effects on gut motility can occur even after a single dose.3 The consequences of opioid‐induced constipation (OIC) may be severe enough to warrant a dosage reduction of the opioid; however, this may lead to compromised analgesia, which can hinder recovery.4, 5 Thus, effective treatment of OIC is an important clinical consideration in patients undergoing pain management with opioids. Unfortunately, laxatives and other treatment strategies can have unpredictable or suboptimal results for many patients with OIC; therefore, other options are needed for the treatment of OIC.6, 7
Opioid receptor agonists cause constipation by adversely altering many aspects of intestinal function, including fluid dynamics, gastric emptying, propulsive motor activity, and transit time.3 Opioid receptors are widely distributed in the central nervous system and throughout the intestinal system. The mechanism of OIC may have both peripherally and centrally mediated components.8 Nonselective opioid receptor antagonists block the undesired effects on the gut, but because they cross the blood‐brain barrier, they also interfere with analgesia and may lead to symptoms of withdrawal. Methylnaltrexone is a selective, peripherally acting mu‐opioid receptor antagonist,9 formed by the addition of a methyl group to the amine ring of the mu‐opioid receptor antagonist naltrexone. The resulting quarternary amine has greater polarity, lower lipid solubility, and restricted ability to cross the blood‐brain barrier.10 Thus, methylnaltrexone was designed to decrease the peripheral adverse effects of opioids without interfering with centrally mediated analgesia.
Investigations of methylnaltrexone effects in healthy volunteers showed that methylnaltrexone attenuated morphine‐induced delays in gastric emptying and oral‐cecal transit without affecting analgesia.1113 Further studies of methylnaltrexone for the treatment of constipation due to methadone use demonstrated rapid laxation response.1416 Two randomized, double‐blind, placebo‐controlled studies of methylnaltrexone in 288 patients with advanced illness and OIC showed that methylnaltrexone rapidly induced laxation without compromising analgesia.17, 18 Methylnaltrexone is currently approved for the treatment of OIC in patients with advanced illness who are receiving palliative care, when response to laxative therapy has not been sufficient.19
Recently, the use of methylnaltrexone for the treatment of OIC in patients with chronic, nonmalignant pain was assessed in a randomized, double‐blind, placebo‐controlled trial of more than 400 patients. Investigators found that methylnaltrexone induced laxation and was generally well tolerated (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA; Duerden et al., 29th Annual Scientific Meeting of the American Pain Society, May 6‐10, 2010, Baltimore, MD), supporting the safety and efficacy of methylnaltrexone in the setting of OIC resulting from chronic opioid treatment. The present study aimed to assess the activity of methylnaltrexone in patients receiving mu‐agonist opioid analgesics during rehabilitation, following an orthopedic surgical procedure, who were experiencing acute OIC.
METHODS
Patients
Patients who had undergone orthopedic procedures within 4 to 10 days were screened for eligibility. Adults aged 18 years or older were considered eligible if they were acutely constipated, were receiving mu‐agonist opioid analgesics, and were expected to require daily opioid analgesics for at least 7 days following randomization. Acute constipation was defined as having no bowel movement for at least 48 hours prior to randomization, difficulty in having a spontaneous bowel movement (straining or sensation of incomplete evacuation or hard, lumpy stools), or the inability to have a spontaneous bowel movement. Exclusion criteria included fecal impaction, mechanical bowel obstruction, constipation not attributed to postprocedure opioid use, calculated creatinine clearance less than 50 mL/min, and corrected QT interval greater than 500 msec on a 12‐lead screening electrocardiogram (ECG). Patients with a known hypersensitivity to methylnaltrexone, naltrexone, or naloxone, who were pregnant or lactating, who had a history of alcohol or drug abuse within the past 2 years, or who had a spinal cord injury or gastrointestinal ostomy were also excluded. Any laxatives, enemas, and/or promotility agents being used must have been discontinued at least 48 hours prior to first dose of study medication and were not permitted during the study, but stool softener use was permitted if it had been administered at least 24 hours prior to screening and a stable dose was maintained throughout the study.
Study Design
This randomized, double‐blind, placebo‐controlled, parallel‐group, hypothesis‐generating phase 2 study was conducted from October 2007 to January 2009 at 16 US hospitals and rehabilitation facilities in accordance with the International Conference on Harmonisation Good Clinical Practice Guidelines and the Declaration of Helsinki, and was approved by the Institutional Review Board and/or Independent Ethics Committee at each of the participating investigational centers. All patients provided written informed consent prior to study participation.
Eligible patients were randomized by interactive voice response system in a 1:1 ratio to receive once‐daily subcutaneous (SC) injections of either 12 mg methylnaltrexone or placebo (Figure 1). The chosen 12‐mg unit dosing corresponds to approximately 0.15 mg/kg (assuming an 80‐kg patient) and was found to be both efficacious and well tolerated in the treatment of OIC in prior studies, including studies in advanced‐illness patients17, 18 and in patients with chronic, nonmalignant pain (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA; Duerden et al., 29th Annual Scientific Meeting of the American Pain Society, May 6‐10, 2010, Baltimore, MD.20 The first dose of study medication was administered on the day of randomization or on the next calendar day. Once enrolled, the patient received once‐daily doses of methylnaltrexone for up to 4 or 7 days. Dosing continued until the patient received the maximum number of doses allowed, no longer needed opioid medication, or was discharged from the medical facility. Each patient completed a follow‐up safety visit at 14 3 days following the last dose.

Evaluations
All efficacy variables were considered exploratory and included the occurrence of laxation within 2 and 4 hours of the first dose of study drug, time to laxation, and a questionnaire assessing patient global satisfaction. Patients recorded the date, time, and assessment of each bowel movement in diaries.
Safety variables included adverse events (AEs), serious AEs (SAEs), clinical laboratory parameters, physical examinations, vital signs, ECGs, concomitant medications, Objective and Subjective Opioid Withdrawal Scales (OOWS and SOWS),21 and Numeric Rating Scales for Pain ([NRSP] 0 = no pain, 10 = worst pain possible).
Statistical Analysis
Enrolled patients were defined as all patients who consented to participate in the study. Both the modified intent‐to‐treat (mITT) population and the safety population were defined as all patients who were randomized and received at least 1 injection of study drug. All study results are based on the mITT population.
Categorical variables were summarized using frequency and percentage, while descriptive statistics for continuous variables included sample size, mean, median, standard deviation, and minimum and maximum values. All inferential statistical tests were 2‐tailed and used a tolerance for nominal type I error (alpha, ) of 0.05. There was no correction for multiplicity and no imputations were performed to account for missing data.
Fisher's exact test was used for comparisons between the proportion of patients with laxation within 2 hours and 4 hours of the first dose in the methylnaltrexone group versus the placebo group. The time to first laxation analysis was performed using the log‐rank test and Kaplan‐Meier method.
RESULTS
Patient Populations
The flow of patients through the study is summarized in Figure 2. A total of 51 patients were enrolled. Of these, 33 received at least 1 dose of study treatment following double‐blind randomization and comprised both the mITT and safety populations. Seventeen of these patients were enrolled under the original protocol and could receive study drug for up to 7 days, while 16 patients enrolled under a subsequent protocol revision could receive study drug for up to 4 days. This change from a 7‐day to a 4‐day treatment protocol allowed for the capture of more study patients in view of the time pressures of short lengths of stay in postoperative settings. In total, 31 patients received at least 2 doses, and 26 patients received at least 4 doses of study drug. A total of 27 patients completed the study. Baseline demographics and prestudy surgical procedures were similar in both treatment groups (Table 1).

| Characteristic | Methylnaltrexone (n = 18) | Placebo (n = 15) |
|---|---|---|
| ||
| Mean age, yr (SD) | 64.2 (9.0) | 65.2 (11.6) |
| Mean weight, kg (SD) | 92.5 (22.5) | 91.0 (20.2) |
| Mean BMI, kg/m2 (SD) | 32.3 (7.2) | 34.2 (6.41) |
| Sex, n (%) | ||
| Female | 11 (61.1) | 11 (73.3) |
| Male | 7 (38.9) | 4 (26.7) |
| Race, n (%) | ||
| White | 14 (77.8) | 10 (66.7) |
| Black | 4 (22.2) | 5 (33.3) |
| Type of surgery, n (%) | ||
| Total knee replacement | 8 (44.4) | 7 (46.7) |
| Total hip replacement | 6 (33.3) | 6 (40.0) |
| Spinal fusion | 2 (11.1) | 0 |
| Fracture reduction | 2 (11.1) | 2 (13.3) |
| Median opioid use,* mg (range) | 28.00 (6.75‐168.01) | 25.00 (9.00‐75.00) |
| Median time from surgery to study drug administration, days (range) | 4 (3‐6) | 4 (3‐6) |
Efficacy
A significantly greater percentage of patients had a bowel movement within 2 hours (P = 0.021) and 4 hours (P = 0.046) of the first dose of methylnaltrexone compared with patients who received placebo (Figure 3). Within 2 hours, 6 patients (33.3%; 95% confidence interval [CI], 13.34‐59.01) who received methylnaltrexone achieved laxation, while laxation did not occur in any patient who received placebo. By 4 hours posttreatment, 7 patients (38.9%; 95% CI, 17.30‐64.25) in the methylnaltrexone group achieved laxation compared with only 1 patient (6.7%; 95% CI, 0.17‐31.95) on placebo. Three patients in each treatment group received rescue laxatives.

The time to first laxation (Figure 4) was significantly shorter in patients who received methylnaltrexone compared with those in the placebo group. Patients on methylnaltrexone achieved laxation in a median time of 15.8 hours, compared with a median time of 50.9 hours for patients in the placebo group (P = 0.02, log‐rank test). The median time to laxation was less than 1 hour in the 7 methylnaltrexone‐treated patients who experienced laxation within 4 hours following the first dose. Of the remaining 11 methylnaltrexone‐treated patients, one experienced no laxation after 6 doses, and the median time to laxation for the others was 29.9 hours (not shown in figure).

Analysis of the Global Satisfaction With Treatment Scale revealed that more patients expressed overall treatment satisfaction (defined as very satisfied, satisfied, or minimally satisfied) with methylnaltrexone assessed 4 hours ( 30 minutes) after the first dose, compared with patients on placebo (83.3% vs 60.0%, respectively). At the study endpoint, overall treatment satisfaction with methylnaltrexone remained high (83.3%), whereas satisfaction with placebo was 53.3%. Additionally, no patients in the methylnaltrexone group expressed any dissatisfaction with treatment (defined as minimally dissatisfied, dissatisfied, or very dissatisfied) at endpoint, compared with 26.7% of patients in the placebo group who expressed some degree of dissatisfaction.
Safety
Overall AE rates were similar between treatment groups (Table 2), with at least 1 treatment‐emergent AE reported in 6 patients (33.3%) in the methylnaltrexone group and 4 patients (26.7%) in the placebo group. The most common AEs reported during the study were classified as gastrointestinal in nature; 3 (nausea, abdominal pain, and diarrhea) were considered by the investigator to be possibly related to study medication. Two patients receiving methylnaltrexone discontinued the study because of AEs (one with moderate constipation, one with mild diarrhea) compared with none of the placebo group patients. No treatment‐emergent SAEs or deaths were reported during this study. Analysis of clinical laboratory parameters, vital signs, and ECGs revealed no safety signals and showed no pattern of concern related to methylnaltrexone exposure.
| Adverse Event* | Methylnaltrexone 12 mg (n = 18) n (%) | Placebo (n = 15) n (%) |
|---|---|---|
| ||
| Any | 6 (33.3) | 4 (26.7) |
| Anemia | 1 (5.6) | 0 |
| Gastrointestinal disorders | 3 (16.7) | 1 (6.7) |
| Abdominal discomfort | 0 | 1 (6.7) |
| Abdominal distension | 1 (5.6) | 0 |
| Abdominal pain | 1 (5.6) | 0 |
| Abdominal tenderness | 1 (5.6) | 0 |
| Constipation | 1 (5.6) | 0 |
| Diarrhea | 1 (5.6) | 0 |
| Nausea | 1 (5.6) | 0 |
| Headache | 1 (5.6) | 0 |
| Hypotension | 1 (5.6) | 0 |
| Joint swelling | 0 | 1 (6.7) |
| Peripheral edema | 0 | 2 (13.3) |
| Procedural pain | 0 | 1 (6.7) |
| Skin ulcer | 0 | 1 (6.7) |
| Somnolence | 0 | 1 (6.7) |
| Urinary tract infection | 1 (5.6) | 0 |
| Wound infection | 1 (5.6) | 0 |
Pain and Opioid Withdrawal
Results from the SOWS and OOWS measures indicated that signs and symptoms of withdrawal did not increase over time in patients treated with methylnaltrexone, and no discernable differences were found between study groups. Pain was assessed using a numeric rating scale ranging from 0 to 10, with higher scores indicating greater severity. Baseline pain scores were not significantly different between treatment groups, with a mean of 5.7 2.7 for placebo, and 5.4 3.0 for the methylnaltrexone group. At 1 day postdose, mean pain scores did not increase from baseline in the placebo (0.9 2.33) or methylnaltrexone group (0.5 2.5), and no significant between‐group differences were found. Similar results were observed at the end of the study. Thus, pain did not appear to increase in patients treated with methylnaltrexone, and changes in pain scores were indistinguishable between the 2 treatment groups.
DISCUSSION
This pilot study suggests that methylnaltrexone actively induces laxation and is generally well tolerated in patients receiving mu‐opioid analgesia, following orthopedic surgery, who develop OIC acutely. It was the first study, to our knowledge, to investigate the efficacy of methylnaltrexone for the treatment of OIC in an acute postoperative setting. The protocol amendment changing the duration of treatment from 7 days to 4 days did not materially affect the results of the study. The response to methylnaltrexone was rapid, with 33.3% experiencing laxation within 2 hours. The median time to laxation was nearly 1.5 days shorter in patients treated with methylnaltrexone compared with those receiving placebo. Correspondingly, overall patient satisfaction was high in the methylnaltrexone group. Efficacy was attained without diminishing opioid analgesia, and without inducing signs or symptoms of opioid withdrawal. The incidence of AEs was similar between groups, and no treatment‐emergent SAEs were reported in this study.
Previous clinical trials investigated the safety and efficacy of methylnaltrexone for the treatment of OIC in patients with advanced illness and with chronic, nonmalignant pain. The present study extends those findings to a population of patients experiencing acute OIC following orthopedic surgery. Previous studies showed that approximately 48% to 62% of advanced‐illness patients experienced laxation within 4 hours of receiving SC methylnaltrexone,17, 18 compared with 38.9% of acute OIC patients in this study. In a clinical trial of patients with chronic, nonmalignant pain, 34.2% of patients experienced laxation within 4 hours of SC methylnaltrexone injection (Blonsky et al., 28th Annual Scientific Meeting of the American Pain Society, May 7‐9, 2009, San Diego, CA). The differences in laxation response between these trials may be attributable to differences in the patient populations or to methodologic differences between the studies.
Similar to findings demonstrated in a clinical study evaluating methylnaltrexone for OIC in a different patient population, those with advanced illness,22 this study supports the premise that future laxation response with prolonged use is most likely to occur when a laxation response was achieved after the first or second initial administrations of methylnaltrexone. In contradistinction, if laxation does not occur with these early doses, continued methylnaltrexone dosing is less likely to produce a response later.
This study has some limitations that must be considered. First, as this was a hypothesis‐generating study, all efficacy parameters investigated were exploratory in nature. The results reported herein warrant careful consideration, owing to a small sample size that may limit their generalizability, prior to replication in a more rigorously designed study with prespecified efficacy endpoints. Likewise, the assessment of health outcome parameters is limited. Another limitation is the small sample size utilized in this study, potentially resulting in a type II error.
Subcutaneous administration potentially offers a considerable benefit over oral therapies for OIC in this patient population post‐orthopedic surgery. Nausea and vomiting can occur as a consequence of anesthesia and of postoperative opioid analgesia, and may compromise adequate dosing of oral medications prescribed to treat OIC. Subcutaneous delivery of methylnaltrexone may circumvent this potential drawback while providing potentially rapid, effective treatment for OIC. Once‐daily dosing may also help to minimize caregiver burden and patient discomfort by preventing the need for more frequent or unpleasant treatments for OIC, such as enemas.
This study provides an initial positive signal for a broader, albeit off‐label use for methylnaltrexonethat being for the treatment of acute constipation that occurs as a consequence of postoperative opioid‐mediated analgesia in patients following orthopedic procedures. Adequate treatment of OIC, even in the acute postoperative setting, is likely to lead to better overall pain management and improved patient outcomes. Additionally, effective management of acute OIC is likely to be cost‐effective in terms of reducing the duration of hospital stays, reducing the need for nursing resources and the time spent administering rescue treatments for OIC (eg, enemas), and avoiding returns to an acute setting (eg, the emergency department) for treatment. The results presented herein suggest that methylnaltrexone may be effective and have a good safety profile in the treatment of acute OIC following orthopedic surgery. Validation of these results in larger well‐controlled trials would be welcome.
Acknowledgements
The authors thank the patients and clinical personnel involved in this study; John Charity, NP, for data collection and management, and John H. Simmons, MD, of Peloton Advantage, LLC, for assistance with manuscript preparation, which was funded by Pfizer Inc.
In addition to the authors, the following investigators participated in this trial: David Nathan Feldman, MD, Holy Name Hospital, Teaneck, NJ; Sam Hakki, MD, Bay Pines VA Healthcare System, Bay Pines, FL; Forrest A. Hanke, MD, Trover Health System, Madisonville, KY; William H. Horton, Jr, MD, Palmetto Clinical Research, Greenville, SC; M. Jay Jazayeri, MD, Pacific Hospital of Long Beach, Long Beach, CA; John F. Peppin, DO, The Pain Treatment Center of the Bluegrass, Lexington, KY; Bruce Pomeranz, MD, Kessler Institute for Rehabilitation, Saddle Brook, NJ, and Chester, NJ; Alan C. Schwartz, MD, Helping Hands Medical Associates, Santa Ana, CA; Michael J. Skyhar, MD, CORE Orthopaedic Medical Center, Encinitas, CA; Lex A. Simpson, MD, CORE Orthopaedic Medical Center, Encinitas, CA; James Slover, MD, New York University Hospital for Joint Disease, New York, NY; Dilip Tapadiya, MD, Fountain Valley Regional Hospital, Fountain Valley, CA; Stanley J. Waters, MD, PhD, Americana Orthopedics, Boise, ID.
- ,.Postoperative pain management.Chest Surg Clin N Am.1997;7:773–799.
- ,.Strategies for effective postoperative pain management.Minerva Anestesiol.2006;72:145–150.
- ,,.Are peripheral opioid antagonists the solution to opioid side effects?Anesth Analg.2004;98:116–122.
- ,.Neuroplasticity—an important factor in acute and chronic pain.Swiss Med Wkly.2002;132:273–278.
- ,,,,.The burden of acute postoperative pain and the potential role of the COX‐2‐specific inhibitors.Rheumatology (Oxford).2003;42(suppl 3):iii40–iii52.
- .Incidence, prevalence, and management of opioid bowel dysfunction.Am J Surg.2001;182(suppl 5A):11S–18S.
- ,.Management of common opioid‐induced adverse effects.Am Fam Physician.2006;74:1347–1354.
- ,.Antagonism of gastrointestinal opioid effects.Reg Anesth Pain Med.2000;25:639–642.
- .Methylnaltrexone mechanisms of action and effects on opioid bowel dysfunction and other opioid adverse effects.Ann Pharmacother.2007;41:984–993.
- ,.Methylnaltrexone: investigation of clinical applications.Drug Dev Res.2000;50:133–141.
- ,,,.Opioid‐induced delay in gastric emptying: a peripheral mechanism in humans.Anesthesiology.1997;87:765–770.
- ,,, et al.Effects of enteric‐coated methylnaltrexone in preventing opioid‐induced delay in oral‐cecal transit time.Clin Pharmacol Ther.2000;67:398–404.
- ,,,,,.Methylnaltrexone prevents morphine‐induced delay in oral‐cecal transit time without affecting analgesia: a double‐blind randomized placebo‐controlled trial.Clin Pharmacol Ther.1996;59:469–475.
- ,,,,,.Effects of intravenous methylnaltrexone on opioid‐induced gut motility and transit time changes in subjects receiving chronic methadone therapy: a pilot study.Pain.1999;83:631–635.
- ,,, et al.Methylnaltrexone for reversal of constipation due to chronic methadone use: a randomized controlled trial.JAMA.2000;283:367–372.
- ,.Oral methylnaltrexone for opioid‐induced constipation.JAMA.2000;284:1383–1384.
- ,,, et al.Methylnaltrexone for opioid‐induced constipation in advanced illness.N Engl J Med.2008;328:2332–2343.
- ,,, et al.Methylnaltrexone for treatment of opioid‐induced constipation in advanced illness patients.J Support Oncol.2009;7:39–46.
- Relistor [package insert].Philadelphia, PA, and Tarrytown, NY:Wyeth Pharmaceuticals Inc and Progenics Pharmaceuticals;2009.
- ,,, et al.Subcutaneous methylnaltrexone for treatment of opioid‐induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study.J Pain.2011;12:554–562.
- ,,,,,.Two new rating scales for opiate withdrawal.Am J Drug Alcohol Abuse.1987;13:293–308.
- ,,, et al.Methylnaltrexone treatment of opioid‐induced constipation in patients with advanced illness.J Pain Symptom Manage.2009;38:683–690.
- ,.Postoperative pain management.Chest Surg Clin N Am.1997;7:773–799.
- ,.Strategies for effective postoperative pain management.Minerva Anestesiol.2006;72:145–150.
- ,,.Are peripheral opioid antagonists the solution to opioid side effects?Anesth Analg.2004;98:116–122.
- ,.Neuroplasticity—an important factor in acute and chronic pain.Swiss Med Wkly.2002;132:273–278.
- ,,,,.The burden of acute postoperative pain and the potential role of the COX‐2‐specific inhibitors.Rheumatology (Oxford).2003;42(suppl 3):iii40–iii52.
- .Incidence, prevalence, and management of opioid bowel dysfunction.Am J Surg.2001;182(suppl 5A):11S–18S.
- ,.Management of common opioid‐induced adverse effects.Am Fam Physician.2006;74:1347–1354.
- ,.Antagonism of gastrointestinal opioid effects.Reg Anesth Pain Med.2000;25:639–642.
- .Methylnaltrexone mechanisms of action and effects on opioid bowel dysfunction and other opioid adverse effects.Ann Pharmacother.2007;41:984–993.
- ,.Methylnaltrexone: investigation of clinical applications.Drug Dev Res.2000;50:133–141.
- ,,,.Opioid‐induced delay in gastric emptying: a peripheral mechanism in humans.Anesthesiology.1997;87:765–770.
- ,,, et al.Effects of enteric‐coated methylnaltrexone in preventing opioid‐induced delay in oral‐cecal transit time.Clin Pharmacol Ther.2000;67:398–404.
- ,,,,,.Methylnaltrexone prevents morphine‐induced delay in oral‐cecal transit time without affecting analgesia: a double‐blind randomized placebo‐controlled trial.Clin Pharmacol Ther.1996;59:469–475.
- ,,,,,.Effects of intravenous methylnaltrexone on opioid‐induced gut motility and transit time changes in subjects receiving chronic methadone therapy: a pilot study.Pain.1999;83:631–635.
- ,,, et al.Methylnaltrexone for reversal of constipation due to chronic methadone use: a randomized controlled trial.JAMA.2000;283:367–372.
- ,.Oral methylnaltrexone for opioid‐induced constipation.JAMA.2000;284:1383–1384.
- ,,, et al.Methylnaltrexone for opioid‐induced constipation in advanced illness.N Engl J Med.2008;328:2332–2343.
- ,,, et al.Methylnaltrexone for treatment of opioid‐induced constipation in advanced illness patients.J Support Oncol.2009;7:39–46.
- Relistor [package insert].Philadelphia, PA, and Tarrytown, NY:Wyeth Pharmaceuticals Inc and Progenics Pharmaceuticals;2009.
- ,,, et al.Subcutaneous methylnaltrexone for treatment of opioid‐induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study.J Pain.2011;12:554–562.
- ,,,,,.Two new rating scales for opiate withdrawal.Am J Drug Alcohol Abuse.1987;13:293–308.
- ,,, et al.Methylnaltrexone treatment of opioid‐induced constipation in patients with advanced illness.J Pain Symptom Manage.2009;38:683–690.
Copyright © 2011 Society of Hospital Medicine