User login
Hepatitis C: How To Fine-Tune Your Approach
› Screen at-risk patients and all those born between 1945 and 1965 for hepatitis C virus (HCV) infection. B
› Screen HCV-positive patients for level of fibrosis and for conditions that may accelerate liver disease, including alcohol use, hepatitis B virus, and human immunodeficiency virus. B
› Continuously monitor patients with chronic HCV for the development of cirrhosis and hepatocellular carcinoma. A
› Refer patients to specialty care for HCV treatment and, if they have cirrhosis, for potential transplant evaluation. C
› Counsel HCV-positive patients about how to avoid transmission to others. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease. Over the next few decades, the number of deaths per year due to complications of HCV such as liver failure and hepatocellular carcinoma (HCC) is predicted to more than triple to 36,000 by 2032.1
Fortunately, major advances in drug therapy have made it possible to cure patients of HCV, and treatment is now less complex, of shorter duration, and better tolerated than it once was. To help family physicians maximize the care they provide to these patients, we’ve summarized screening recommendations from the Centers for Disease Control and Prevention (CDC), innovative alternatives to biopsy for staging liver disease, and counseling points to cover with patients.
A common, usually silent infection with potentially fatal complications
According to the National Health and Nutrition Examination Survey (NHANES), an estimated 2.7 to 3.9 million people in the United States are chronically infected with HCV, about threefourths of whom were born between 1945 and 1965 (the “baby boomer” generation).2 However, by adding “unaccounted groups” (eg, incarcerated, homeless, and active duty military) to these estimates, the number of people with HCV is likely more than 5.2 million.3
HCV is a ribonucleic acid (RNA) virus capable of mutating at a high rate to escape detection and clearance by the host’s immune system.4 Most patients with HCV are asymptomatic during the acute and chronic phases of infection, and may have a silent infection for decades. In fact, 65% to 75% of patients with HCV are unaware of their infection.5
Approximately 20% of chronically infected patients develop cirrhosis after 20 years and, once they do, the annual rate of HCC and liver decompensation is about 5%.6-8 Risk factors for advancement to cirrhosis includes male sex, alcohol consumption, co-infection with human immunodeficiency virus (HIV) or hepatitis B virus (HBV), immunosuppression, having had HCV infection for a long time, becoming infected with HCV after age 40, and not having responded to previous treatment.9
Chronic HCV infection can lead to extrahepatic manifestations such as essential mixed cryoglobulinemia, porphyria cutanea tarda, membranoproliferative glomerulonephritis, lymphoma, and glucose intolerance.10 There is also growing evidence that HCV infection affects cognitive function in the absence of fibrosis and hepatic encephalopathy. Several studies show that HCV-infected patients score poorly on neuropsychological testing for verbal learning, attention, memory, and executive function.11 This may be related to the expression of receptors for HCV by the brain’s microvascular endothelial cells.12
Screening recommendations. Given the high prevalence of HCV infection among baby boomers, the CDC decided in 2012 to recommend one-time HCV screening for all patients born between 1945 and 1965.13 This is in addition to risk-based screening for all patients who have a history of injection drug use, those on long-term hemodialysis or with tattoos obtained in unregulated settings, offspring of HCV-infected mothers, and those with health-care associated exposures (TABLE13). In 2013, the US Preventive Services Task Force upgraded its recommendation to match those of the CDC.14
Despite these recommendations, which are expected to increase detection of HCV among asymptomatic persons who do not know they are infected, there remain significant barriers to HCV testing. These include poor access to primary care and preventive services, lack of knowledge and awareness of the disease among patients and providers, and a lack of studies that support a universal screening approach for HCV.5,15,16 One tool that might help overcome some of these barriers and aid family physicians in the screening process is automatic reminders or standing lab orders for HCV testing in electronic medical records systems.
Screening for HCV can be done using any of the US Food and Drug Administration (FDA)-approved tests for the anti-HCV antibody, which have sensitivities and specificities greater than 99%.17 A positive screening result should be confirmed with an HCV RNA test. However, for practical purposes, ordering the anti-HCV test with reflex to the HCV RNA test decreases the number of blood draws and office visits required of the patient. The reflex confirmation allows the physician to deliver the patient’s full diagnosis and reduces the psychological distress associated with waiting for confirmatory results. The HCV RNA test (alone) should be used, however, in immunocompromised patients, those who may have had exposure to HCV in the past 6 months, and those suspected of having an HCV re-infection after having cleared the virus.18
Look for the evidence of liver disease
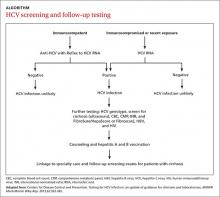
Family physicians should order several additional tests for patients found to have chronic HCV infection before referring such patients to a specialist (ALGORITHM). Work-up should include the complete blood count, HCV genotype (which will help guide treatment), liver function tests, international normalized ratio test, and ultrasound of the liver.18 In addition, all HCV-positive patients should be tested for HIV and HBV, because these co-infections may accelerate liver fibrosis.19,20
All patients with chronic HCV infection should also be screened for the presence of fibrosis and cirrhosis, as this will influence treatment choice and duration. Signs of cirrhosis that may be evident on physical exam include jaundice, spider angiomata, palmar erythema, encephalopathy with asterixis, and fluid overload, especially ascites. Cirrhosis can be classified clinically as compensated (stage 1 with no varices present and stage 2 with varices present) and decompensated (stages 3 and 4), which is defined as cirrhosis with signs of severe portal hypertension (bleeding varices, ascites, hepatic encephalopathy) or liver insufficiency (jaundice).21 Patients with decompensated cirrhosis should be managed by a liver transplant center. For more on cirrhosis, see “Cirrhosis complications: Keeping them under control” (J Fam Pract. 2015;64:338-342).
Several noninvasive alternatives to liver biopsy
Historically, liver biopsy has been the gold standard for staging liver disease. The Metavir scoring system is a histological assessment of the degree of inflammatory activity and the stage of fibrosis.22 The degree of inflammation activity, which is a precursor of fibrosis, is scored from A0 (no activity) to A3 (severe activity). The staging of fibrosis involves a 5-stage scoring system: F0 (chronic hepatitis without fibrosis); F1 (portal fibrosis without septae); F2 (portal fibrosis with rare septae); F3 (many septae without cirrhosis); or F4 (cirrhosis).
That said, noninvasive tests have largely supplanted liver biopsy for fibrosis screening.
For example, the FibroSure test uses the patient’s age, gender, and a combination of 6 serum markers of liver function in a computational algorithm to generate a quantitative indicator of liver fibrosis, with a score of 0.0 to 1.0 that corresponds to the Metavir fibrosis score (F0-F4), and an inflammatory activity score (A0-A3).23 Similarly, HepaScore uses several noninvasive markers to calculate a score from 0.00 to 1.00. A score ≤0.2 accurately excludes significant fibrosis. However, a score of ≥0.55 or higher corresponds to a Metavir score of at least F2, and in such cases further testing would be needed to evaluate for cirrhosis.24
FDA-approved in 2013, transient elastography (FibroScan) is another noninvasive alternative to liver biopsy for determining the stage of liver disease. This bedside test uses ultrasound technology to measure liver stiffness and provides a score ranging from 0 to 75 kPA that correlates with the Metavir score. Although not yet widely available in the United States, FibroScan is becoming increasingly popular as a rapid and noninvasive screening tool for cirrhosis.25
Identifying cirrhosis in patients who have HCV is crucial because such patients need prompt care from a specialist. In addition to receiving HCV treatment, patients with cirrhosis also need regular liver ultrasound exams to screen for HCC (every 6 months) and esophagogastroduodenoscopy to screen for esophageal and gastric varices.26
Advise patients to avoid alcohol, lose weight
Counsel patients who test positive for HCV infection about making lifestyle changes to avoid further liver damage and transmission of HCV to others. Infectious diseases and hepatology society guidelines recommend vaccination against hepatitis A and B for all HCV-infected patients who are not immune to these viruses because acute co-infection could lead to severe acute liver injury.18,27 Urge all HCV-infected patients to completely abstain from alcohol and, if necessary, refer them to an addiction specialist, because excess alcohol consumption is strongly associated with the development of cirrhosis and HCC.28,29
Comorbid conditions such as metabolic syndrome, obesity, and hyperlipidemia can worsen the prognosis for HCV-infected patients; therefore, intense counseling on weight loss is recommended.30 Statins are safe and beneficial for HCV patients with hypercholesterolemia and compensated cirrhosis.31
Teach patients that the primary mode of transmission of HCV is through infected blood. Sexual transmission of HCV has been well documented in HIV-positive men who have sex with men.32 Although the risk of transmission of HCV among heterosexual couples is extremely low, it is possible, and patients should be counseled accordingly.33 Transmission of HCV from mother to the baby occurs in up to 6% of births and most commonly occurs during delivery.34
Newer treatments are highly effective and well tolderated
HCV treatment has changed dramatically over the past few years. Previous treatments for HCV, particularly those containing interferon, were known for their poor tolerability due to adverse effects and low cure rates. Compared to previous therapies, the new interferon-free direct-acting antiviral (DAA) regimens are not only less complex but also shorter in duration, ranging from 8 to 24 weeks depending on the patient’s viral load, stage of liver disease, and previous treatment experience.18 The specific agents and dosages used in DAA regimens aren’t described here because these regimens are rapidly changing. However, continuously updated treatment recommendations from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America are available at http://www.hcvguidelines.org.
The goal of HCV treatment is cure as evidenced by a sustained virologic response (SVR), which is defined as the absence of HCV RNA 12 weeks or more after completing treatment.35,36 In general, for the most common genotypes of HCV, treatment with a DAA regimen results in a SVR in ≥95% of patients.18 Achieving SVR is associated with a 50% reduction in all-cause mortality, a 90% reduction in liver-associated mortality, and a >70% reduction in the risk of developing HCC.27,37,38 SVR also has been shown to have a significant effect on reducing extrahepatic manifestations of HCV infection, such as cryoglobulinemia and lymphoma.39-41
Current barriers to the newer, highly effective hepatitis C virus (HCV) infection treatments are largely financial. Although insurance companies have been able to negotiate substantial discounts from the high wholesale price of treatment, many insurance programs require prior authorizations and will approve treatment only for patients with advanced liver fibrosis. In our experience, many patients are left to wait for their liver disease to progress before their insurance company will agree to cover treatment.
In addition, many insurance companies have mandated that only subspecialists prescribe these medications. However, infectious diseases and hepatology specialists and their support staffs are often overburdened with paperwork and phone calls related to prior authorizations and justification of treatment, which can add to delays in treatment.
There is already evidence that treatment of all patients with HCV is cost-effective and leads to better healthcare outcomes42 and there are indications that these barriers will decrease over time, with prices already dropping significantly due to increasing competition between drug companies.
The DAAs are well tolerated and have good safety profiles. In phase III clinical trials of today’s most commonly used DAA regimens, the discontinuation rate was <1% in non-cirrhotic patients and 2% in those with cirrhosis.18 The most commonly reported adverse effects were nausea, fatigue, and headache. DAAs may have drug-drug interactions; therefore, careful medication reconciliation should be performed before initiating treatment.18
Prioritizing treatment. Current evidence supports treatment for all patients with HCV except those with a life expectancy of <12 months.18 Evidence indicates that treatment becomes less effective as a patient’s liver injury progresses to cirrhosis. Due to the high cost of available treatments, however, many insurers have imposed strict criteria for coverage. (See “Barriers to HCV Treatment,” above.42)
The highest priority for treatment has been given to patients with advanced liver fibrosis, compensated cirrhosis, those who have received a liver transplant, and those with severe extrahepatic manifestations (eg, mixed cryoglobulinemia and end-organ disease such as nephropathy). Treatment is also prioritized for high-risk populations (eg, patients with HBV and HIV co-infection, diabetes mellitus) and patients who are at high risk of transmitting the virus (eg, individuals who inject drugs or are incarcerated, men who have sex with men, women of childbearing age, hemodialysis patients, and health care professionals who perform exposure-prone procedures).18
While it may eventually become feasible for family physicians to treat HCV-infected patients, the rapid evolution and significant cost of treatment, as well as the challenges in obtaining insurance coverage, have kept HCV treatment largely in the domain of specialists, at least for now. In the interim, family physicians play a crucial role by screening, diagnosing, and counseling patients with this infection, referring them to specialty care, and providing ongoing monitoring for signs of HCC and esophageal and gastric varices.
CORRESPONDENCE
Laura Wangensteen, MD, Department of Family Medicine, Drexel University, 3401 South Market Street #105 A, Philadelphia, PA 19104; [email protected]
1. Rein DB, Wittenborn JS, Weinbaum CM, et al. Forecasting the morbidity and mortality associated with prevalent cases of precirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66-72.
2. Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-714.
3. Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090-1101.
4. Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103-107.
5. Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729-733.
6. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
7. El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83.
8. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68.
9. McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut. 2004;53:318-321.
10. El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-1445.
11. Solinas A, Piras MR, Deplano A. Cognitive dysfunction and hepatitis C virus infection. World J Hepatol. 2015;7:922-925.
12. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142:634-643.e6.
13. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32.
14. US Preventive Services Task Force. Final recommendation statement on hepatitis C screening, June 2013. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-c-screening. Accessed on December 28, 2014.
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-2207.
16. Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754-758.
17. Shivkumar S, Peeling R, Jafari Y, et al. Accuracy of rapid and pointof- care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558-566.
18. American Association for the Study of Liver Diseases; Infectious Diseases Society of America; International Antiviral Society—USA. HCV guidance: Recommendations for testing, managing, and treating hepatitis C. HCV guidelines Web site. Available at: http://www.hcvguidelines.org. Accessed May 25, 2015.
19. Zarski JP, Bohn B, Bastie A, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27-33.
20. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562-569.
21. Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449.
22. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
23. Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896.
24. Becker L, Salameh W, Sferruzza A, et al. Validation of hepascore, compared with simple indices of fibrosis, in patients with chronic hepatitis C virus infection in United States. Clin Gastroenterol Hepatol. 2009;7:696-701.
25. Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16:372.
26. Garcia-Tsao G, Sanyal AJ, Grace ND, et al; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
27. Ghany MG, Strader DB, Thomas DL, et al; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
28. Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717-1722.
29. Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J Gastroenterol. 2009;15:3462-3471.
30. Ortiz V, Berenguer M, Rayón JM, et al. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408-2414.
31. Lewis JH, Mortensen ME, Zweig S, et al; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453-1463.
32. Gamage DG, Read TR, Bradshaw CS, et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis. 2011;11:39.
33. Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881-889.
34. Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223-229.
35. Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593-1601.
36. Thomas AM, Kattakuzhy S, Jones S, et al. SVR durability: HCV patients treated with IFN-free DAA regimens. Presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February, 2015; Seattle, Washington. Abstract 653.
37. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.e1.
38. Russo MW. Antiviral therapy for hepatitis C is associated with improved clinical outcomes in patients with advanced fibrosis. Expert Rev Gastroenterol Hepatol. 2010;4:535-539.
39. Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCVassociated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol. 2013;85:1019-1027.
40. Takahashi K, Nishida N, Kawabata H, et al. Regression of Hodgkin lymphoma in response to antiviral therapy for hepatitis C virus infection. Intern Med. 2012;51:2745-2747.
41. Gisbert JP, García-Buey L, Pajares JM, et al. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther. 2005;21:653-662.
42. Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407-419.
› Screen at-risk patients and all those born between 1945 and 1965 for hepatitis C virus (HCV) infection. B
› Screen HCV-positive patients for level of fibrosis and for conditions that may accelerate liver disease, including alcohol use, hepatitis B virus, and human immunodeficiency virus. B
› Continuously monitor patients with chronic HCV for the development of cirrhosis and hepatocellular carcinoma. A
› Refer patients to specialty care for HCV treatment and, if they have cirrhosis, for potential transplant evaluation. C
› Counsel HCV-positive patients about how to avoid transmission to others. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease. Over the next few decades, the number of deaths per year due to complications of HCV such as liver failure and hepatocellular carcinoma (HCC) is predicted to more than triple to 36,000 by 2032.1
Fortunately, major advances in drug therapy have made it possible to cure patients of HCV, and treatment is now less complex, of shorter duration, and better tolerated than it once was. To help family physicians maximize the care they provide to these patients, we’ve summarized screening recommendations from the Centers for Disease Control and Prevention (CDC), innovative alternatives to biopsy for staging liver disease, and counseling points to cover with patients.
A common, usually silent infection with potentially fatal complications
According to the National Health and Nutrition Examination Survey (NHANES), an estimated 2.7 to 3.9 million people in the United States are chronically infected with HCV, about threefourths of whom were born between 1945 and 1965 (the “baby boomer” generation).2 However, by adding “unaccounted groups” (eg, incarcerated, homeless, and active duty military) to these estimates, the number of people with HCV is likely more than 5.2 million.3
HCV is a ribonucleic acid (RNA) virus capable of mutating at a high rate to escape detection and clearance by the host’s immune system.4 Most patients with HCV are asymptomatic during the acute and chronic phases of infection, and may have a silent infection for decades. In fact, 65% to 75% of patients with HCV are unaware of their infection.5
Approximately 20% of chronically infected patients develop cirrhosis after 20 years and, once they do, the annual rate of HCC and liver decompensation is about 5%.6-8 Risk factors for advancement to cirrhosis includes male sex, alcohol consumption, co-infection with human immunodeficiency virus (HIV) or hepatitis B virus (HBV), immunosuppression, having had HCV infection for a long time, becoming infected with HCV after age 40, and not having responded to previous treatment.9
Chronic HCV infection can lead to extrahepatic manifestations such as essential mixed cryoglobulinemia, porphyria cutanea tarda, membranoproliferative glomerulonephritis, lymphoma, and glucose intolerance.10 There is also growing evidence that HCV infection affects cognitive function in the absence of fibrosis and hepatic encephalopathy. Several studies show that HCV-infected patients score poorly on neuropsychological testing for verbal learning, attention, memory, and executive function.11 This may be related to the expression of receptors for HCV by the brain’s microvascular endothelial cells.12
Screening recommendations. Given the high prevalence of HCV infection among baby boomers, the CDC decided in 2012 to recommend one-time HCV screening for all patients born between 1945 and 1965.13 This is in addition to risk-based screening for all patients who have a history of injection drug use, those on long-term hemodialysis or with tattoos obtained in unregulated settings, offspring of HCV-infected mothers, and those with health-care associated exposures (TABLE13). In 2013, the US Preventive Services Task Force upgraded its recommendation to match those of the CDC.14
Despite these recommendations, which are expected to increase detection of HCV among asymptomatic persons who do not know they are infected, there remain significant barriers to HCV testing. These include poor access to primary care and preventive services, lack of knowledge and awareness of the disease among patients and providers, and a lack of studies that support a universal screening approach for HCV.5,15,16 One tool that might help overcome some of these barriers and aid family physicians in the screening process is automatic reminders or standing lab orders for HCV testing in electronic medical records systems.
Screening for HCV can be done using any of the US Food and Drug Administration (FDA)-approved tests for the anti-HCV antibody, which have sensitivities and specificities greater than 99%.17 A positive screening result should be confirmed with an HCV RNA test. However, for practical purposes, ordering the anti-HCV test with reflex to the HCV RNA test decreases the number of blood draws and office visits required of the patient. The reflex confirmation allows the physician to deliver the patient’s full diagnosis and reduces the psychological distress associated with waiting for confirmatory results. The HCV RNA test (alone) should be used, however, in immunocompromised patients, those who may have had exposure to HCV in the past 6 months, and those suspected of having an HCV re-infection after having cleared the virus.18
Look for the evidence of liver disease
Family physicians should order several additional tests for patients found to have chronic HCV infection before referring such patients to a specialist (ALGORITHM). Work-up should include the complete blood count, HCV genotype (which will help guide treatment), liver function tests, international normalized ratio test, and ultrasound of the liver.18 In addition, all HCV-positive patients should be tested for HIV and HBV, because these co-infections may accelerate liver fibrosis.19,20
All patients with chronic HCV infection should also be screened for the presence of fibrosis and cirrhosis, as this will influence treatment choice and duration. Signs of cirrhosis that may be evident on physical exam include jaundice, spider angiomata, palmar erythema, encephalopathy with asterixis, and fluid overload, especially ascites. Cirrhosis can be classified clinically as compensated (stage 1 with no varices present and stage 2 with varices present) and decompensated (stages 3 and 4), which is defined as cirrhosis with signs of severe portal hypertension (bleeding varices, ascites, hepatic encephalopathy) or liver insufficiency (jaundice).21 Patients with decompensated cirrhosis should be managed by a liver transplant center. For more on cirrhosis, see “Cirrhosis complications: Keeping them under control” (J Fam Pract. 2015;64:338-342).
Several noninvasive alternatives to liver biopsy
Historically, liver biopsy has been the gold standard for staging liver disease. The Metavir scoring system is a histological assessment of the degree of inflammatory activity and the stage of fibrosis.22 The degree of inflammation activity, which is a precursor of fibrosis, is scored from A0 (no activity) to A3 (severe activity). The staging of fibrosis involves a 5-stage scoring system: F0 (chronic hepatitis without fibrosis); F1 (portal fibrosis without septae); F2 (portal fibrosis with rare septae); F3 (many septae without cirrhosis); or F4 (cirrhosis).
That said, noninvasive tests have largely supplanted liver biopsy for fibrosis screening.
For example, the FibroSure test uses the patient’s age, gender, and a combination of 6 serum markers of liver function in a computational algorithm to generate a quantitative indicator of liver fibrosis, with a score of 0.0 to 1.0 that corresponds to the Metavir fibrosis score (F0-F4), and an inflammatory activity score (A0-A3).23 Similarly, HepaScore uses several noninvasive markers to calculate a score from 0.00 to 1.00. A score ≤0.2 accurately excludes significant fibrosis. However, a score of ≥0.55 or higher corresponds to a Metavir score of at least F2, and in such cases further testing would be needed to evaluate for cirrhosis.24
FDA-approved in 2013, transient elastography (FibroScan) is another noninvasive alternative to liver biopsy for determining the stage of liver disease. This bedside test uses ultrasound technology to measure liver stiffness and provides a score ranging from 0 to 75 kPA that correlates with the Metavir score. Although not yet widely available in the United States, FibroScan is becoming increasingly popular as a rapid and noninvasive screening tool for cirrhosis.25
Identifying cirrhosis in patients who have HCV is crucial because such patients need prompt care from a specialist. In addition to receiving HCV treatment, patients with cirrhosis also need regular liver ultrasound exams to screen for HCC (every 6 months) and esophagogastroduodenoscopy to screen for esophageal and gastric varices.26
Advise patients to avoid alcohol, lose weight
Counsel patients who test positive for HCV infection about making lifestyle changes to avoid further liver damage and transmission of HCV to others. Infectious diseases and hepatology society guidelines recommend vaccination against hepatitis A and B for all HCV-infected patients who are not immune to these viruses because acute co-infection could lead to severe acute liver injury.18,27 Urge all HCV-infected patients to completely abstain from alcohol and, if necessary, refer them to an addiction specialist, because excess alcohol consumption is strongly associated with the development of cirrhosis and HCC.28,29
Comorbid conditions such as metabolic syndrome, obesity, and hyperlipidemia can worsen the prognosis for HCV-infected patients; therefore, intense counseling on weight loss is recommended.30 Statins are safe and beneficial for HCV patients with hypercholesterolemia and compensated cirrhosis.31
Teach patients that the primary mode of transmission of HCV is through infected blood. Sexual transmission of HCV has been well documented in HIV-positive men who have sex with men.32 Although the risk of transmission of HCV among heterosexual couples is extremely low, it is possible, and patients should be counseled accordingly.33 Transmission of HCV from mother to the baby occurs in up to 6% of births and most commonly occurs during delivery.34
Newer treatments are highly effective and well tolderated
HCV treatment has changed dramatically over the past few years. Previous treatments for HCV, particularly those containing interferon, were known for their poor tolerability due to adverse effects and low cure rates. Compared to previous therapies, the new interferon-free direct-acting antiviral (DAA) regimens are not only less complex but also shorter in duration, ranging from 8 to 24 weeks depending on the patient’s viral load, stage of liver disease, and previous treatment experience.18 The specific agents and dosages used in DAA regimens aren’t described here because these regimens are rapidly changing. However, continuously updated treatment recommendations from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America are available at http://www.hcvguidelines.org.
The goal of HCV treatment is cure as evidenced by a sustained virologic response (SVR), which is defined as the absence of HCV RNA 12 weeks or more after completing treatment.35,36 In general, for the most common genotypes of HCV, treatment with a DAA regimen results in a SVR in ≥95% of patients.18 Achieving SVR is associated with a 50% reduction in all-cause mortality, a 90% reduction in liver-associated mortality, and a >70% reduction in the risk of developing HCC.27,37,38 SVR also has been shown to have a significant effect on reducing extrahepatic manifestations of HCV infection, such as cryoglobulinemia and lymphoma.39-41
Current barriers to the newer, highly effective hepatitis C virus (HCV) infection treatments are largely financial. Although insurance companies have been able to negotiate substantial discounts from the high wholesale price of treatment, many insurance programs require prior authorizations and will approve treatment only for patients with advanced liver fibrosis. In our experience, many patients are left to wait for their liver disease to progress before their insurance company will agree to cover treatment.
In addition, many insurance companies have mandated that only subspecialists prescribe these medications. However, infectious diseases and hepatology specialists and their support staffs are often overburdened with paperwork and phone calls related to prior authorizations and justification of treatment, which can add to delays in treatment.
There is already evidence that treatment of all patients with HCV is cost-effective and leads to better healthcare outcomes42 and there are indications that these barriers will decrease over time, with prices already dropping significantly due to increasing competition between drug companies.
The DAAs are well tolerated and have good safety profiles. In phase III clinical trials of today’s most commonly used DAA regimens, the discontinuation rate was <1% in non-cirrhotic patients and 2% in those with cirrhosis.18 The most commonly reported adverse effects were nausea, fatigue, and headache. DAAs may have drug-drug interactions; therefore, careful medication reconciliation should be performed before initiating treatment.18
Prioritizing treatment. Current evidence supports treatment for all patients with HCV except those with a life expectancy of <12 months.18 Evidence indicates that treatment becomes less effective as a patient’s liver injury progresses to cirrhosis. Due to the high cost of available treatments, however, many insurers have imposed strict criteria for coverage. (See “Barriers to HCV Treatment,” above.42)
The highest priority for treatment has been given to patients with advanced liver fibrosis, compensated cirrhosis, those who have received a liver transplant, and those with severe extrahepatic manifestations (eg, mixed cryoglobulinemia and end-organ disease such as nephropathy). Treatment is also prioritized for high-risk populations (eg, patients with HBV and HIV co-infection, diabetes mellitus) and patients who are at high risk of transmitting the virus (eg, individuals who inject drugs or are incarcerated, men who have sex with men, women of childbearing age, hemodialysis patients, and health care professionals who perform exposure-prone procedures).18
While it may eventually become feasible for family physicians to treat HCV-infected patients, the rapid evolution and significant cost of treatment, as well as the challenges in obtaining insurance coverage, have kept HCV treatment largely in the domain of specialists, at least for now. In the interim, family physicians play a crucial role by screening, diagnosing, and counseling patients with this infection, referring them to specialty care, and providing ongoing monitoring for signs of HCC and esophageal and gastric varices.
CORRESPONDENCE
Laura Wangensteen, MD, Department of Family Medicine, Drexel University, 3401 South Market Street #105 A, Philadelphia, PA 19104; [email protected]
› Screen at-risk patients and all those born between 1945 and 1965 for hepatitis C virus (HCV) infection. B
› Screen HCV-positive patients for level of fibrosis and for conditions that may accelerate liver disease, including alcohol use, hepatitis B virus, and human immunodeficiency virus. B
› Continuously monitor patients with chronic HCV for the development of cirrhosis and hepatocellular carcinoma. A
› Refer patients to specialty care for HCV treatment and, if they have cirrhosis, for potential transplant evaluation. C
› Counsel HCV-positive patients about how to avoid transmission to others. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease. Over the next few decades, the number of deaths per year due to complications of HCV such as liver failure and hepatocellular carcinoma (HCC) is predicted to more than triple to 36,000 by 2032.1
Fortunately, major advances in drug therapy have made it possible to cure patients of HCV, and treatment is now less complex, of shorter duration, and better tolerated than it once was. To help family physicians maximize the care they provide to these patients, we’ve summarized screening recommendations from the Centers for Disease Control and Prevention (CDC), innovative alternatives to biopsy for staging liver disease, and counseling points to cover with patients.
A common, usually silent infection with potentially fatal complications
According to the National Health and Nutrition Examination Survey (NHANES), an estimated 2.7 to 3.9 million people in the United States are chronically infected with HCV, about threefourths of whom were born between 1945 and 1965 (the “baby boomer” generation).2 However, by adding “unaccounted groups” (eg, incarcerated, homeless, and active duty military) to these estimates, the number of people with HCV is likely more than 5.2 million.3
HCV is a ribonucleic acid (RNA) virus capable of mutating at a high rate to escape detection and clearance by the host’s immune system.4 Most patients with HCV are asymptomatic during the acute and chronic phases of infection, and may have a silent infection for decades. In fact, 65% to 75% of patients with HCV are unaware of their infection.5
Approximately 20% of chronically infected patients develop cirrhosis after 20 years and, once they do, the annual rate of HCC and liver decompensation is about 5%.6-8 Risk factors for advancement to cirrhosis includes male sex, alcohol consumption, co-infection with human immunodeficiency virus (HIV) or hepatitis B virus (HBV), immunosuppression, having had HCV infection for a long time, becoming infected with HCV after age 40, and not having responded to previous treatment.9
Chronic HCV infection can lead to extrahepatic manifestations such as essential mixed cryoglobulinemia, porphyria cutanea tarda, membranoproliferative glomerulonephritis, lymphoma, and glucose intolerance.10 There is also growing evidence that HCV infection affects cognitive function in the absence of fibrosis and hepatic encephalopathy. Several studies show that HCV-infected patients score poorly on neuropsychological testing for verbal learning, attention, memory, and executive function.11 This may be related to the expression of receptors for HCV by the brain’s microvascular endothelial cells.12
Screening recommendations. Given the high prevalence of HCV infection among baby boomers, the CDC decided in 2012 to recommend one-time HCV screening for all patients born between 1945 and 1965.13 This is in addition to risk-based screening for all patients who have a history of injection drug use, those on long-term hemodialysis or with tattoos obtained in unregulated settings, offspring of HCV-infected mothers, and those with health-care associated exposures (TABLE13). In 2013, the US Preventive Services Task Force upgraded its recommendation to match those of the CDC.14
Despite these recommendations, which are expected to increase detection of HCV among asymptomatic persons who do not know they are infected, there remain significant barriers to HCV testing. These include poor access to primary care and preventive services, lack of knowledge and awareness of the disease among patients and providers, and a lack of studies that support a universal screening approach for HCV.5,15,16 One tool that might help overcome some of these barriers and aid family physicians in the screening process is automatic reminders or standing lab orders for HCV testing in electronic medical records systems.
Screening for HCV can be done using any of the US Food and Drug Administration (FDA)-approved tests for the anti-HCV antibody, which have sensitivities and specificities greater than 99%.17 A positive screening result should be confirmed with an HCV RNA test. However, for practical purposes, ordering the anti-HCV test with reflex to the HCV RNA test decreases the number of blood draws and office visits required of the patient. The reflex confirmation allows the physician to deliver the patient’s full diagnosis and reduces the psychological distress associated with waiting for confirmatory results. The HCV RNA test (alone) should be used, however, in immunocompromised patients, those who may have had exposure to HCV in the past 6 months, and those suspected of having an HCV re-infection after having cleared the virus.18
Look for the evidence of liver disease
Family physicians should order several additional tests for patients found to have chronic HCV infection before referring such patients to a specialist (ALGORITHM). Work-up should include the complete blood count, HCV genotype (which will help guide treatment), liver function tests, international normalized ratio test, and ultrasound of the liver.18 In addition, all HCV-positive patients should be tested for HIV and HBV, because these co-infections may accelerate liver fibrosis.19,20
All patients with chronic HCV infection should also be screened for the presence of fibrosis and cirrhosis, as this will influence treatment choice and duration. Signs of cirrhosis that may be evident on physical exam include jaundice, spider angiomata, palmar erythema, encephalopathy with asterixis, and fluid overload, especially ascites. Cirrhosis can be classified clinically as compensated (stage 1 with no varices present and stage 2 with varices present) and decompensated (stages 3 and 4), which is defined as cirrhosis with signs of severe portal hypertension (bleeding varices, ascites, hepatic encephalopathy) or liver insufficiency (jaundice).21 Patients with decompensated cirrhosis should be managed by a liver transplant center. For more on cirrhosis, see “Cirrhosis complications: Keeping them under control” (J Fam Pract. 2015;64:338-342).
Several noninvasive alternatives to liver biopsy
Historically, liver biopsy has been the gold standard for staging liver disease. The Metavir scoring system is a histological assessment of the degree of inflammatory activity and the stage of fibrosis.22 The degree of inflammation activity, which is a precursor of fibrosis, is scored from A0 (no activity) to A3 (severe activity). The staging of fibrosis involves a 5-stage scoring system: F0 (chronic hepatitis without fibrosis); F1 (portal fibrosis without septae); F2 (portal fibrosis with rare septae); F3 (many septae without cirrhosis); or F4 (cirrhosis).
That said, noninvasive tests have largely supplanted liver biopsy for fibrosis screening.
For example, the FibroSure test uses the patient’s age, gender, and a combination of 6 serum markers of liver function in a computational algorithm to generate a quantitative indicator of liver fibrosis, with a score of 0.0 to 1.0 that corresponds to the Metavir fibrosis score (F0-F4), and an inflammatory activity score (A0-A3).23 Similarly, HepaScore uses several noninvasive markers to calculate a score from 0.00 to 1.00. A score ≤0.2 accurately excludes significant fibrosis. However, a score of ≥0.55 or higher corresponds to a Metavir score of at least F2, and in such cases further testing would be needed to evaluate for cirrhosis.24
FDA-approved in 2013, transient elastography (FibroScan) is another noninvasive alternative to liver biopsy for determining the stage of liver disease. This bedside test uses ultrasound technology to measure liver stiffness and provides a score ranging from 0 to 75 kPA that correlates with the Metavir score. Although not yet widely available in the United States, FibroScan is becoming increasingly popular as a rapid and noninvasive screening tool for cirrhosis.25
Identifying cirrhosis in patients who have HCV is crucial because such patients need prompt care from a specialist. In addition to receiving HCV treatment, patients with cirrhosis also need regular liver ultrasound exams to screen for HCC (every 6 months) and esophagogastroduodenoscopy to screen for esophageal and gastric varices.26
Advise patients to avoid alcohol, lose weight
Counsel patients who test positive for HCV infection about making lifestyle changes to avoid further liver damage and transmission of HCV to others. Infectious diseases and hepatology society guidelines recommend vaccination against hepatitis A and B for all HCV-infected patients who are not immune to these viruses because acute co-infection could lead to severe acute liver injury.18,27 Urge all HCV-infected patients to completely abstain from alcohol and, if necessary, refer them to an addiction specialist, because excess alcohol consumption is strongly associated with the development of cirrhosis and HCC.28,29
Comorbid conditions such as metabolic syndrome, obesity, and hyperlipidemia can worsen the prognosis for HCV-infected patients; therefore, intense counseling on weight loss is recommended.30 Statins are safe and beneficial for HCV patients with hypercholesterolemia and compensated cirrhosis.31
Teach patients that the primary mode of transmission of HCV is through infected blood. Sexual transmission of HCV has been well documented in HIV-positive men who have sex with men.32 Although the risk of transmission of HCV among heterosexual couples is extremely low, it is possible, and patients should be counseled accordingly.33 Transmission of HCV from mother to the baby occurs in up to 6% of births and most commonly occurs during delivery.34
Newer treatments are highly effective and well tolderated
HCV treatment has changed dramatically over the past few years. Previous treatments for HCV, particularly those containing interferon, were known for their poor tolerability due to adverse effects and low cure rates. Compared to previous therapies, the new interferon-free direct-acting antiviral (DAA) regimens are not only less complex but also shorter in duration, ranging from 8 to 24 weeks depending on the patient’s viral load, stage of liver disease, and previous treatment experience.18 The specific agents and dosages used in DAA regimens aren’t described here because these regimens are rapidly changing. However, continuously updated treatment recommendations from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America are available at http://www.hcvguidelines.org.
The goal of HCV treatment is cure as evidenced by a sustained virologic response (SVR), which is defined as the absence of HCV RNA 12 weeks or more after completing treatment.35,36 In general, for the most common genotypes of HCV, treatment with a DAA regimen results in a SVR in ≥95% of patients.18 Achieving SVR is associated with a 50% reduction in all-cause mortality, a 90% reduction in liver-associated mortality, and a >70% reduction in the risk of developing HCC.27,37,38 SVR also has been shown to have a significant effect on reducing extrahepatic manifestations of HCV infection, such as cryoglobulinemia and lymphoma.39-41
Current barriers to the newer, highly effective hepatitis C virus (HCV) infection treatments are largely financial. Although insurance companies have been able to negotiate substantial discounts from the high wholesale price of treatment, many insurance programs require prior authorizations and will approve treatment only for patients with advanced liver fibrosis. In our experience, many patients are left to wait for their liver disease to progress before their insurance company will agree to cover treatment.
In addition, many insurance companies have mandated that only subspecialists prescribe these medications. However, infectious diseases and hepatology specialists and their support staffs are often overburdened with paperwork and phone calls related to prior authorizations and justification of treatment, which can add to delays in treatment.
There is already evidence that treatment of all patients with HCV is cost-effective and leads to better healthcare outcomes42 and there are indications that these barriers will decrease over time, with prices already dropping significantly due to increasing competition between drug companies.
The DAAs are well tolerated and have good safety profiles. In phase III clinical trials of today’s most commonly used DAA regimens, the discontinuation rate was <1% in non-cirrhotic patients and 2% in those with cirrhosis.18 The most commonly reported adverse effects were nausea, fatigue, and headache. DAAs may have drug-drug interactions; therefore, careful medication reconciliation should be performed before initiating treatment.18
Prioritizing treatment. Current evidence supports treatment for all patients with HCV except those with a life expectancy of <12 months.18 Evidence indicates that treatment becomes less effective as a patient’s liver injury progresses to cirrhosis. Due to the high cost of available treatments, however, many insurers have imposed strict criteria for coverage. (See “Barriers to HCV Treatment,” above.42)
The highest priority for treatment has been given to patients with advanced liver fibrosis, compensated cirrhosis, those who have received a liver transplant, and those with severe extrahepatic manifestations (eg, mixed cryoglobulinemia and end-organ disease such as nephropathy). Treatment is also prioritized for high-risk populations (eg, patients with HBV and HIV co-infection, diabetes mellitus) and patients who are at high risk of transmitting the virus (eg, individuals who inject drugs or are incarcerated, men who have sex with men, women of childbearing age, hemodialysis patients, and health care professionals who perform exposure-prone procedures).18
While it may eventually become feasible for family physicians to treat HCV-infected patients, the rapid evolution and significant cost of treatment, as well as the challenges in obtaining insurance coverage, have kept HCV treatment largely in the domain of specialists, at least for now. In the interim, family physicians play a crucial role by screening, diagnosing, and counseling patients with this infection, referring them to specialty care, and providing ongoing monitoring for signs of HCC and esophageal and gastric varices.
CORRESPONDENCE
Laura Wangensteen, MD, Department of Family Medicine, Drexel University, 3401 South Market Street #105 A, Philadelphia, PA 19104; [email protected]
1. Rein DB, Wittenborn JS, Weinbaum CM, et al. Forecasting the morbidity and mortality associated with prevalent cases of precirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66-72.
2. Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-714.
3. Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090-1101.
4. Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103-107.
5. Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729-733.
6. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
7. El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83.
8. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68.
9. McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut. 2004;53:318-321.
10. El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-1445.
11. Solinas A, Piras MR, Deplano A. Cognitive dysfunction and hepatitis C virus infection. World J Hepatol. 2015;7:922-925.
12. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142:634-643.e6.
13. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32.
14. US Preventive Services Task Force. Final recommendation statement on hepatitis C screening, June 2013. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-c-screening. Accessed on December 28, 2014.
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-2207.
16. Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754-758.
17. Shivkumar S, Peeling R, Jafari Y, et al. Accuracy of rapid and pointof- care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558-566.
18. American Association for the Study of Liver Diseases; Infectious Diseases Society of America; International Antiviral Society—USA. HCV guidance: Recommendations for testing, managing, and treating hepatitis C. HCV guidelines Web site. Available at: http://www.hcvguidelines.org. Accessed May 25, 2015.
19. Zarski JP, Bohn B, Bastie A, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27-33.
20. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562-569.
21. Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449.
22. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
23. Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896.
24. Becker L, Salameh W, Sferruzza A, et al. Validation of hepascore, compared with simple indices of fibrosis, in patients with chronic hepatitis C virus infection in United States. Clin Gastroenterol Hepatol. 2009;7:696-701.
25. Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16:372.
26. Garcia-Tsao G, Sanyal AJ, Grace ND, et al; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
27. Ghany MG, Strader DB, Thomas DL, et al; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
28. Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717-1722.
29. Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J Gastroenterol. 2009;15:3462-3471.
30. Ortiz V, Berenguer M, Rayón JM, et al. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408-2414.
31. Lewis JH, Mortensen ME, Zweig S, et al; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453-1463.
32. Gamage DG, Read TR, Bradshaw CS, et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis. 2011;11:39.
33. Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881-889.
34. Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223-229.
35. Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593-1601.
36. Thomas AM, Kattakuzhy S, Jones S, et al. SVR durability: HCV patients treated with IFN-free DAA regimens. Presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February, 2015; Seattle, Washington. Abstract 653.
37. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.e1.
38. Russo MW. Antiviral therapy for hepatitis C is associated with improved clinical outcomes in patients with advanced fibrosis. Expert Rev Gastroenterol Hepatol. 2010;4:535-539.
39. Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCVassociated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol. 2013;85:1019-1027.
40. Takahashi K, Nishida N, Kawabata H, et al. Regression of Hodgkin lymphoma in response to antiviral therapy for hepatitis C virus infection. Intern Med. 2012;51:2745-2747.
41. Gisbert JP, García-Buey L, Pajares JM, et al. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther. 2005;21:653-662.
42. Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407-419.
1. Rein DB, Wittenborn JS, Weinbaum CM, et al. Forecasting the morbidity and mortality associated with prevalent cases of precirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66-72.
2. Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-714.
3. Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090-1101.
4. Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103-107.
5. Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729-733.
6. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
7. El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83.
8. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68.
9. McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut. 2004;53:318-321.
10. El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-1445.
11. Solinas A, Piras MR, Deplano A. Cognitive dysfunction and hepatitis C virus infection. World J Hepatol. 2015;7:922-925.
12. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142:634-643.e6.
13. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32.
14. US Preventive Services Task Force. Final recommendation statement on hepatitis C screening, June 2013. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-c-screening. Accessed on December 28, 2014.
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-2207.
16. Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754-758.
17. Shivkumar S, Peeling R, Jafari Y, et al. Accuracy of rapid and pointof- care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558-566.
18. American Association for the Study of Liver Diseases; Infectious Diseases Society of America; International Antiviral Society—USA. HCV guidance: Recommendations for testing, managing, and treating hepatitis C. HCV guidelines Web site. Available at: http://www.hcvguidelines.org. Accessed May 25, 2015.
19. Zarski JP, Bohn B, Bastie A, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27-33.
20. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562-569.
21. Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449.
22. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
23. Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896.
24. Becker L, Salameh W, Sferruzza A, et al. Validation of hepascore, compared with simple indices of fibrosis, in patients with chronic hepatitis C virus infection in United States. Clin Gastroenterol Hepatol. 2009;7:696-701.
25. Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16:372.
26. Garcia-Tsao G, Sanyal AJ, Grace ND, et al; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
27. Ghany MG, Strader DB, Thomas DL, et al; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
28. Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717-1722.
29. Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J Gastroenterol. 2009;15:3462-3471.
30. Ortiz V, Berenguer M, Rayón JM, et al. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408-2414.
31. Lewis JH, Mortensen ME, Zweig S, et al; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453-1463.
32. Gamage DG, Read TR, Bradshaw CS, et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis. 2011;11:39.
33. Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881-889.
34. Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223-229.
35. Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593-1601.
36. Thomas AM, Kattakuzhy S, Jones S, et al. SVR durability: HCV patients treated with IFN-free DAA regimens. Presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February, 2015; Seattle, Washington. Abstract 653.
37. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.e1.
38. Russo MW. Antiviral therapy for hepatitis C is associated with improved clinical outcomes in patients with advanced fibrosis. Expert Rev Gastroenterol Hepatol. 2010;4:535-539.
39. Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCVassociated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol. 2013;85:1019-1027.
40. Takahashi K, Nishida N, Kawabata H, et al. Regression of Hodgkin lymphoma in response to antiviral therapy for hepatitis C virus infection. Intern Med. 2012;51:2745-2747.
41. Gisbert JP, García-Buey L, Pajares JM, et al. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther. 2005;21:653-662.
42. Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407-419.
Hepatitis C: How to fine-tune your approach
› Screen at-risk patients and all those born between 1945 and 1965 for hepatitis C virus (HCV) infection. B
› Screen HCV-positive patients for level of fibrosis and for conditions that may accelerate liver disease, including alcohol use, hepatitis B virus, and human immunodeficiency virus. B
› Continuously monitor patients with chronic HCV for the development of cirrhosis and hepatocellular carcinoma. A
› Refer patients to specialty care for HCV treatment and, if they have cirrhosis, for potential transplant evaluation. C
› Counsel HCV-positive patients about how to avoid transmission to others. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease. Over the next few decades, the number of deaths per year due to complications of HCV such as liver failure and hepatocellular carcinoma (HCC) is predicted to more than triple to 36,000 by 2032.1
Fortunately, major advances in drug therapy have made it possible to cure patients of HCV, and treatment is now less complex, of shorter duration, and better tolerated than it once was. To help family physicians maximize the care they provide to these patients, we’ve summarized screening recommendations from the Centers for Disease Control and Prevention (CDC), innovative alternatives to biopsy for staging liver disease, and counseling points to cover with patients.
A common, usually silent infection with potentially fatal complications
According to the National Health and Nutrition Examination Survey (NHANES), an estimated 2.7 to 3.9 million people in the United States are chronically infected with HCV, about threefourths of whom were born between 1945 and 1965 (the “baby boomer” generation).2 However, by adding “unaccounted groups” (eg, incarcerated, homeless, and active duty military) to these estimates, the number of people with HCV is likely more than 5.2 million.3
HCV is a ribonucleic acid (RNA) virus capable of mutating at a high rate to escape detection and clearance by the host’s immune system.4 Most patients with HCV are asymptomatic during the acute and chronic phases of infection, and may have a silent infection for decades. In fact, 65% to 75% of patients with HCV are unaware of their infection.5
Approximately 20% of chronically infected patients develop cirrhosis after 20 years and, once they do, the annual rate of HCC and liver decompensation is about 5%.6-8 Risk factors for advancement to cirrhosis includes male sex, alcohol consumption, co-infection with human immunodeficiency virus (HIV) or hepatitis B virus (HBV), immunosuppression, having had HCV infection for a long time, becoming infected with HCV after age 40, and not having responded to previous treatment.9
Chronic HCV infection can lead to extrahepatic manifestations such as essential mixed cryoglobulinemia, porphyria cutanea tarda, membranoproliferative glomerulonephritis, lymphoma, and glucose intolerance.10 There is also growing evidence that HCV infection affects cognitive function in the absence of fibrosis and hepatic encephalopathy. Several studies show that HCV-infected patients score poorly on neuropsychological testing for verbal learning, attention, memory, and executive function.11 This may be related to the expression of receptors for HCV by the brain’s microvascular endothelial cells.12
Screening recommendations. Given the high prevalence of HCV infection among baby boomers, the CDC decided in 2012 to recommend one-time HCV screening for all patients born between 1945 and 1965.13 This is in addition to risk-based screening for all patients who have a history of injection drug use, those on long-term hemodialysis or with tattoos obtained in unregulated settings, offspring of HCV-infected mothers, and those with health-care associated exposures (TABLE13). In 2013, the US Preventive Services Task Force upgraded its recommendation to match those of the CDC.14
Despite these recommendations, which are expected to increase detection of HCV among asymptomatic persons who do not know they are infected, there remain significant barriers to HCV testing. These include poor access to primary care and preventive services, lack of knowledge and awareness of the disease among patients and providers, and a lack of studies that support a universal screening approach for HCV.5,15,16 One tool that might help overcome some of these barriers and aid family physicians in the screening process is automatic reminders or standing lab orders for HCV testing in electronic medical records systems.
Screening for HCV can be done using any of the US Food and Drug Administration (FDA)-approved tests for the anti-HCV antibody, which have sensitivities and specificities greater than 99%.17 A positive screening result should be confirmed with an HCV RNA test. However, for practical purposes, ordering the anti-HCV test with reflex to the HCV RNA test decreases the number of blood draws and office visits required of the patient. The reflex confirmation allows the physician to deliver the patient’s full diagnosis and reduces the psychological distress associated with waiting for confirmatory results. The HCV RNA test (alone) should be used, however, in immunocompromised patients, those who may have had exposure to HCV in the past 6 months, and those suspected of having an HCV re-infection after having cleared the virus.18
Look for the evidence of liver disease
Family physicians should order several additional tests for patients found to have chronic HCV infection before referring such patients to a specialist (ALGORITHM). Work-up should include the complete blood count, HCV genotype (which will help guide treatment), liver function tests, international normalized ratio test, and ultrasound of the liver.18 In addition, all HCV-positive patients should be tested for HIV and HBV, because these co-infections may accelerate liver fibrosis.19,20
All patients with chronic HCV infection should also be screened for the presence of fibrosis and cirrhosis, as this will influence treatment choice and duration. Signs of cirrhosis that may be evident on physical exam include jaundice, spider angiomata, palmar erythema, encephalopathy with asterixis, and fluid overload, especially ascites. Cirrhosis can be classified clinically as compensated (stage 1 with no varices present and stage 2 with varices present) and decompensated (stages 3 and 4), which is defined as cirrhosis with signs of severe portal hypertension (bleeding varices, ascites, hepatic encephalopathy) or liver insufficiency (jaundice).21 Patients with decompensated cirrhosis should be managed by a liver transplant center. For more on cirrhosis, see “Cirrhosis complications: Keeping them under control” (J Fam Pract. 2015;64:338-342).
Several noninvasive alternatives to liver biopsy
Historically, liver biopsy has been the gold standard for staging liver disease. The Metavir scoring system is a histological assessment of the degree of inflammatory activity and the stage of fibrosis.22 The degree of inflammation activity, which is a precursor of fibrosis, is scored from A0 (no activity) to A3 (severe activity). The staging of fibrosis involves a 5-stage scoring system: F0 (chronic hepatitis without fibrosis); F1 (portal fibrosis without septae); F2 (portal fibrosis with rare septae); F3 (many septae without cirrhosis); or F4 (cirrhosis).
That said, noninvasive tests have largely supplanted liver biopsy for fibrosis screening.
For example, the FibroSure test uses the patient’s age, gender, and a combination of 6 serum markers of liver function in a computational algorithm to generate a quantitative indicator of liver fibrosis, with a score of 0.0 to 1.0 that corresponds to the Metavir fibrosis score (F0-F4), and an inflammatory activity score (A0-A3).23 Similarly, HepaScore uses several noninvasive markers to calculate a score from 0.00 to 1.00. A score ≤0.2 accurately excludes significant fibrosis. However, a score of ≥0.55 or higher corresponds to a Metavir score of at least F2, and in such cases further testing would be needed to evaluate for cirrhosis.24
FDA-approved in 2013, transient elastography (FibroScan) is another noninvasive alternative to liver biopsy for determining the stage of liver disease. This bedside test uses ultrasound technology to measure liver stiffness and provides a score ranging from 0 to 75 kPA that correlates with the Metavir score. Although not yet widely available in the United States, FibroScan is becoming increasingly popular as a rapid and noninvasive screening tool for cirrhosis.25
Identifying cirrhosis in patients who have HCV is crucial because such patients need prompt care from a specialist. In addition to receiving HCV treatment, patients with cirrhosis also need regular liver ultrasound exams to screen for HCC (every 6 months) and esophagogastroduodenoscopy to screen for esophageal and gastric varices.26
Advise patients to avoid alcohol, lose weight
Counsel patients who test positive for HCV infection about making lifestyle changes to avoid further liver damage and transmission of HCV to others. Infectious diseases and hepatology society guidelines recommend vaccination against hepatitis A and B for all HCV-infected patients who are not immune to these viruses because acute co-infection could lead to severe acute liver injury.18,27 Urge all HCV-infected patients to completely abstain from alcohol and, if necessary, refer them to an addiction specialist, because excess alcohol consumption is strongly associated with the development of cirrhosis and HCC.28,29
Comorbid conditions such as metabolic syndrome, obesity, and hyperlipidemia can worsen the prognosis for HCV-infected patients; therefore, intense counseling on weight loss is recommended.30 Statins are safe and beneficial for HCV patients with hypercholesterolemia and compensated cirrhosis.31
Teach patients that the primary mode of transmission of HCV is through infected blood. Sexual transmission of HCV has been well documented in HIV-positive men who have sex with men.32 Although the risk of transmission of HCV among heterosexual couples is extremely low, it is possible, and patients should be counseled accordingly.33 Transmission of HCV from mother to the baby occurs in up to 6% of births and most commonly occurs during delivery.34
Newer treatments are highly effective and well tolderated
HCV treatment has changed dramatically over the past few years. Previous treatments for HCV, particularly those containing interferon, were known for their poor tolerability due to adverse effects and low cure rates. Compared to previous therapies, the new interferon-free direct-acting antiviral (DAA) regimens are not only less complex but also shorter in duration, ranging from 8 to 24 weeks depending on the patient’s viral load, stage of liver disease, and previous treatment experience.18 The specific agents and dosages used in DAA regimens aren’t described here because these regimens are rapidly changing. However, continuously updated treatment recommendations from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America are available at http://www.hcvguidelines.org.
The goal of HCV treatment is cure as evidenced by a sustained virologic response (SVR), which is defined as the absence of HCV RNA 12 weeks or more after completing treatment.35,36 In general, for the most common genotypes of HCV, treatment with a DAA regimen results in a SVR in ≥95% of patients.18 Achieving SVR is associated with a 50% reduction in all-cause mortality, a 90% reduction in liver-associated mortality, and a >70% reduction in the risk of developing HCC.27,37,38 SVR also has been shown to have a significant effect on reducing extrahepatic manifestations of HCV infection, such as cryoglobulinemia and lymphoma.39-41
Current barriers to the newer, highly effective hepatitis C virus (HCV) infection treatments are largely financial. Although insurance companies have been able to negotiate substantial discounts from the high wholesale price of treatment, many insurance programs require prior authorizations and will approve treatment only for patients with advanced liver fibrosis. In our experience, many patients are left to wait for their liver disease to progress before their insurance company will agree to cover treatment.
In addition, many insurance companies have mandated that only subspecialists prescribe these medications. However, infectious diseases and hepatology specialists and their support staffs are often overburdened with paperwork and phone calls related to prior authorizations and justification of treatment, which can add to delays in treatment.
There is already evidence that treatment of all patients with HCV is cost-effective and leads to better healthcare outcomes42 and there are indications that these barriers will decrease over time, with prices already dropping significantly due to increasing competition between drug companies.
The DAAs are well tolerated and have good safety profiles. In phase III clinical trials of today’s most commonly used DAA regimens, the discontinuation rate was <1% in non-cirrhotic patients and 2% in those with cirrhosis.18 The most commonly reported adverse effects were nausea, fatigue, and headache. DAAs may have drug-drug interactions; therefore, careful medication reconciliation should be performed before initiating treatment.18
Prioritizing treatment. Current evidence supports treatment for all patients with HCV except those with a life expectancy of <12 months.18 Evidence indicates that treatment becomes less effective as a patient’s liver injury progresses to cirrhosis. Due to the high cost of available treatments, however, many insurers have imposed strict criteria for coverage. (See “Barriers to HCV Treatment,” above.42)
The highest priority for treatment has been given to patients with advanced liver fibrosis, compensated cirrhosis, those who have received a liver transplant, and those with severe extrahepatic manifestations (eg, mixed cryoglobulinemia and end-organ disease such as nephropathy). Treatment is also prioritized for high-risk populations (eg, patients with HBV and HIV co-infection, diabetes mellitus) and patients who are at high risk of transmitting the virus (eg, individuals who inject drugs or are incarcerated, men who have sex with men, women of childbearing age, hemodialysis patients, and health care professionals who perform exposure-prone procedures).18
While it may eventually become feasible for family physicians to treat HCV-infected patients, the rapid evolution and significant cost of treatment, as well as the challenges in obtaining insurance coverage, have kept HCV treatment largely in the domain of specialists, at least for now. In the interim, family physicians play a crucial role by screening, diagnosing, and counseling patients with this infection, referring them to specialty care, and providing ongoing monitoring for signs of HCC and esophageal and gastric varices.
CORRESPONDENCE
Laura Wangensteen, MD, Department of Family Medicine, Drexel University, 3401 South Market Street #105 A, Philadelphia, PA 19104; [email protected]
1. Rein DB, Wittenborn JS, Weinbaum CM, et al. Forecasting the morbidity and mortality associated with prevalent cases of precirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66-72.
2. Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-714.
3. Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090-1101.
4. Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103-107.
5. Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729-733.
6. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
7. El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83.
8. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68.
9. McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut. 2004;53:318-321.
10. El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-1445.
11. Solinas A, Piras MR, Deplano A. Cognitive dysfunction and hepatitis C virus infection. World J Hepatol. 2015;7:922-925.
12. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142:634-643.e6.
13. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32.
14. US Preventive Services Task Force. Final recommendation statement on hepatitis C screening, June 2013. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-c-screening. Accessed on December 28, 2014.
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-2207.
16. Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754-758.
17. Shivkumar S, Peeling R, Jafari Y, et al. Accuracy of rapid and pointof- care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558-566.
18. American Association for the Study of Liver Diseases; Infectious Diseases Society of America; International Antiviral Society—USA. HCV guidance: Recommendations for testing, managing, and treating hepatitis C. HCV guidelines Web site. Available at: http://www.hcvguidelines.org. Accessed May 25, 2015.
19. Zarski JP, Bohn B, Bastie A, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27-33.
20. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562-569.
21. Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449.
22. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
23. Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896.
24. Becker L, Salameh W, Sferruzza A, et al. Validation of hepascore, compared with simple indices of fibrosis, in patients with chronic hepatitis C virus infection in United States. Clin Gastroenterol Hepatol. 2009;7:696-701.
25. Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16:372.
26. Garcia-Tsao G, Sanyal AJ, Grace ND, et al; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
27. Ghany MG, Strader DB, Thomas DL, et al; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
28. Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717-1722.
29. Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J Gastroenterol. 2009;15:3462-3471.
30. Ortiz V, Berenguer M, Rayón JM, et al. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408-2414.
31. Lewis JH, Mortensen ME, Zweig S, et al; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453-1463.
32. Gamage DG, Read TR, Bradshaw CS, et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis. 2011;11:39.
33. Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881-889.
34. Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223-229.
35. Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593-1601.
36. Thomas AM, Kattakuzhy S, Jones S, et al. SVR durability: HCV patients treated with IFN-free DAA regimens. Presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February, 2015; Seattle, Washington. Abstract 653.
37. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.e1.
38. Russo MW. Antiviral therapy for hepatitis C is associated with improved clinical outcomes in patients with advanced fibrosis. Expert Rev Gastroenterol Hepatol. 2010;4:535-539.
39. Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCVassociated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol. 2013;85:1019-1027.
40. Takahashi K, Nishida N, Kawabata H, et al. Regression of Hodgkin lymphoma in response to antiviral therapy for hepatitis C virus infection. Intern Med. 2012;51:2745-2747.
41. Gisbert JP, García-Buey L, Pajares JM, et al. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther. 2005;21:653-662.
42. Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407-419.
› Screen at-risk patients and all those born between 1945 and 1965 for hepatitis C virus (HCV) infection. B
› Screen HCV-positive patients for level of fibrosis and for conditions that may accelerate liver disease, including alcohol use, hepatitis B virus, and human immunodeficiency virus. B
› Continuously monitor patients with chronic HCV for the development of cirrhosis and hepatocellular carcinoma. A
› Refer patients to specialty care for HCV treatment and, if they have cirrhosis, for potential transplant evaluation. C
› Counsel HCV-positive patients about how to avoid transmission to others. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease. Over the next few decades, the number of deaths per year due to complications of HCV such as liver failure and hepatocellular carcinoma (HCC) is predicted to more than triple to 36,000 by 2032.1
Fortunately, major advances in drug therapy have made it possible to cure patients of HCV, and treatment is now less complex, of shorter duration, and better tolerated than it once was. To help family physicians maximize the care they provide to these patients, we’ve summarized screening recommendations from the Centers for Disease Control and Prevention (CDC), innovative alternatives to biopsy for staging liver disease, and counseling points to cover with patients.
A common, usually silent infection with potentially fatal complications
According to the National Health and Nutrition Examination Survey (NHANES), an estimated 2.7 to 3.9 million people in the United States are chronically infected with HCV, about threefourths of whom were born between 1945 and 1965 (the “baby boomer” generation).2 However, by adding “unaccounted groups” (eg, incarcerated, homeless, and active duty military) to these estimates, the number of people with HCV is likely more than 5.2 million.3
HCV is a ribonucleic acid (RNA) virus capable of mutating at a high rate to escape detection and clearance by the host’s immune system.4 Most patients with HCV are asymptomatic during the acute and chronic phases of infection, and may have a silent infection for decades. In fact, 65% to 75% of patients with HCV are unaware of their infection.5
Approximately 20% of chronically infected patients develop cirrhosis after 20 years and, once they do, the annual rate of HCC and liver decompensation is about 5%.6-8 Risk factors for advancement to cirrhosis includes male sex, alcohol consumption, co-infection with human immunodeficiency virus (HIV) or hepatitis B virus (HBV), immunosuppression, having had HCV infection for a long time, becoming infected with HCV after age 40, and not having responded to previous treatment.9
Chronic HCV infection can lead to extrahepatic manifestations such as essential mixed cryoglobulinemia, porphyria cutanea tarda, membranoproliferative glomerulonephritis, lymphoma, and glucose intolerance.10 There is also growing evidence that HCV infection affects cognitive function in the absence of fibrosis and hepatic encephalopathy. Several studies show that HCV-infected patients score poorly on neuropsychological testing for verbal learning, attention, memory, and executive function.11 This may be related to the expression of receptors for HCV by the brain’s microvascular endothelial cells.12
Screening recommendations. Given the high prevalence of HCV infection among baby boomers, the CDC decided in 2012 to recommend one-time HCV screening for all patients born between 1945 and 1965.13 This is in addition to risk-based screening for all patients who have a history of injection drug use, those on long-term hemodialysis or with tattoos obtained in unregulated settings, offspring of HCV-infected mothers, and those with health-care associated exposures (TABLE13). In 2013, the US Preventive Services Task Force upgraded its recommendation to match those of the CDC.14
Despite these recommendations, which are expected to increase detection of HCV among asymptomatic persons who do not know they are infected, there remain significant barriers to HCV testing. These include poor access to primary care and preventive services, lack of knowledge and awareness of the disease among patients and providers, and a lack of studies that support a universal screening approach for HCV.5,15,16 One tool that might help overcome some of these barriers and aid family physicians in the screening process is automatic reminders or standing lab orders for HCV testing in electronic medical records systems.
Screening for HCV can be done using any of the US Food and Drug Administration (FDA)-approved tests for the anti-HCV antibody, which have sensitivities and specificities greater than 99%.17 A positive screening result should be confirmed with an HCV RNA test. However, for practical purposes, ordering the anti-HCV test with reflex to the HCV RNA test decreases the number of blood draws and office visits required of the patient. The reflex confirmation allows the physician to deliver the patient’s full diagnosis and reduces the psychological distress associated with waiting for confirmatory results. The HCV RNA test (alone) should be used, however, in immunocompromised patients, those who may have had exposure to HCV in the past 6 months, and those suspected of having an HCV re-infection after having cleared the virus.18
Look for the evidence of liver disease
Family physicians should order several additional tests for patients found to have chronic HCV infection before referring such patients to a specialist (ALGORITHM). Work-up should include the complete blood count, HCV genotype (which will help guide treatment), liver function tests, international normalized ratio test, and ultrasound of the liver.18 In addition, all HCV-positive patients should be tested for HIV and HBV, because these co-infections may accelerate liver fibrosis.19,20
All patients with chronic HCV infection should also be screened for the presence of fibrosis and cirrhosis, as this will influence treatment choice and duration. Signs of cirrhosis that may be evident on physical exam include jaundice, spider angiomata, palmar erythema, encephalopathy with asterixis, and fluid overload, especially ascites. Cirrhosis can be classified clinically as compensated (stage 1 with no varices present and stage 2 with varices present) and decompensated (stages 3 and 4), which is defined as cirrhosis with signs of severe portal hypertension (bleeding varices, ascites, hepatic encephalopathy) or liver insufficiency (jaundice).21 Patients with decompensated cirrhosis should be managed by a liver transplant center. For more on cirrhosis, see “Cirrhosis complications: Keeping them under control” (J Fam Pract. 2015;64:338-342).
Several noninvasive alternatives to liver biopsy
Historically, liver biopsy has been the gold standard for staging liver disease. The Metavir scoring system is a histological assessment of the degree of inflammatory activity and the stage of fibrosis.22 The degree of inflammation activity, which is a precursor of fibrosis, is scored from A0 (no activity) to A3 (severe activity). The staging of fibrosis involves a 5-stage scoring system: F0 (chronic hepatitis without fibrosis); F1 (portal fibrosis without septae); F2 (portal fibrosis with rare septae); F3 (many septae without cirrhosis); or F4 (cirrhosis).
That said, noninvasive tests have largely supplanted liver biopsy for fibrosis screening.
For example, the FibroSure test uses the patient’s age, gender, and a combination of 6 serum markers of liver function in a computational algorithm to generate a quantitative indicator of liver fibrosis, with a score of 0.0 to 1.0 that corresponds to the Metavir fibrosis score (F0-F4), and an inflammatory activity score (A0-A3).23 Similarly, HepaScore uses several noninvasive markers to calculate a score from 0.00 to 1.00. A score ≤0.2 accurately excludes significant fibrosis. However, a score of ≥0.55 or higher corresponds to a Metavir score of at least F2, and in such cases further testing would be needed to evaluate for cirrhosis.24
FDA-approved in 2013, transient elastography (FibroScan) is another noninvasive alternative to liver biopsy for determining the stage of liver disease. This bedside test uses ultrasound technology to measure liver stiffness and provides a score ranging from 0 to 75 kPA that correlates with the Metavir score. Although not yet widely available in the United States, FibroScan is becoming increasingly popular as a rapid and noninvasive screening tool for cirrhosis.25
Identifying cirrhosis in patients who have HCV is crucial because such patients need prompt care from a specialist. In addition to receiving HCV treatment, patients with cirrhosis also need regular liver ultrasound exams to screen for HCC (every 6 months) and esophagogastroduodenoscopy to screen for esophageal and gastric varices.26
Advise patients to avoid alcohol, lose weight
Counsel patients who test positive for HCV infection about making lifestyle changes to avoid further liver damage and transmission of HCV to others. Infectious diseases and hepatology society guidelines recommend vaccination against hepatitis A and B for all HCV-infected patients who are not immune to these viruses because acute co-infection could lead to severe acute liver injury.18,27 Urge all HCV-infected patients to completely abstain from alcohol and, if necessary, refer them to an addiction specialist, because excess alcohol consumption is strongly associated with the development of cirrhosis and HCC.28,29
Comorbid conditions such as metabolic syndrome, obesity, and hyperlipidemia can worsen the prognosis for HCV-infected patients; therefore, intense counseling on weight loss is recommended.30 Statins are safe and beneficial for HCV patients with hypercholesterolemia and compensated cirrhosis.31
Teach patients that the primary mode of transmission of HCV is through infected blood. Sexual transmission of HCV has been well documented in HIV-positive men who have sex with men.32 Although the risk of transmission of HCV among heterosexual couples is extremely low, it is possible, and patients should be counseled accordingly.33 Transmission of HCV from mother to the baby occurs in up to 6% of births and most commonly occurs during delivery.34
Newer treatments are highly effective and well tolderated
HCV treatment has changed dramatically over the past few years. Previous treatments for HCV, particularly those containing interferon, were known for their poor tolerability due to adverse effects and low cure rates. Compared to previous therapies, the new interferon-free direct-acting antiviral (DAA) regimens are not only less complex but also shorter in duration, ranging from 8 to 24 weeks depending on the patient’s viral load, stage of liver disease, and previous treatment experience.18 The specific agents and dosages used in DAA regimens aren’t described here because these regimens are rapidly changing. However, continuously updated treatment recommendations from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America are available at http://www.hcvguidelines.org.
The goal of HCV treatment is cure as evidenced by a sustained virologic response (SVR), which is defined as the absence of HCV RNA 12 weeks or more after completing treatment.35,36 In general, for the most common genotypes of HCV, treatment with a DAA regimen results in a SVR in ≥95% of patients.18 Achieving SVR is associated with a 50% reduction in all-cause mortality, a 90% reduction in liver-associated mortality, and a >70% reduction in the risk of developing HCC.27,37,38 SVR also has been shown to have a significant effect on reducing extrahepatic manifestations of HCV infection, such as cryoglobulinemia and lymphoma.39-41
Current barriers to the newer, highly effective hepatitis C virus (HCV) infection treatments are largely financial. Although insurance companies have been able to negotiate substantial discounts from the high wholesale price of treatment, many insurance programs require prior authorizations and will approve treatment only for patients with advanced liver fibrosis. In our experience, many patients are left to wait for their liver disease to progress before their insurance company will agree to cover treatment.
In addition, many insurance companies have mandated that only subspecialists prescribe these medications. However, infectious diseases and hepatology specialists and their support staffs are often overburdened with paperwork and phone calls related to prior authorizations and justification of treatment, which can add to delays in treatment.
There is already evidence that treatment of all patients with HCV is cost-effective and leads to better healthcare outcomes42 and there are indications that these barriers will decrease over time, with prices already dropping significantly due to increasing competition between drug companies.
The DAAs are well tolerated and have good safety profiles. In phase III clinical trials of today’s most commonly used DAA regimens, the discontinuation rate was <1% in non-cirrhotic patients and 2% in those with cirrhosis.18 The most commonly reported adverse effects were nausea, fatigue, and headache. DAAs may have drug-drug interactions; therefore, careful medication reconciliation should be performed before initiating treatment.18
Prioritizing treatment. Current evidence supports treatment for all patients with HCV except those with a life expectancy of <12 months.18 Evidence indicates that treatment becomes less effective as a patient’s liver injury progresses to cirrhosis. Due to the high cost of available treatments, however, many insurers have imposed strict criteria for coverage. (See “Barriers to HCV Treatment,” above.42)
The highest priority for treatment has been given to patients with advanced liver fibrosis, compensated cirrhosis, those who have received a liver transplant, and those with severe extrahepatic manifestations (eg, mixed cryoglobulinemia and end-organ disease such as nephropathy). Treatment is also prioritized for high-risk populations (eg, patients with HBV and HIV co-infection, diabetes mellitus) and patients who are at high risk of transmitting the virus (eg, individuals who inject drugs or are incarcerated, men who have sex with men, women of childbearing age, hemodialysis patients, and health care professionals who perform exposure-prone procedures).18
While it may eventually become feasible for family physicians to treat HCV-infected patients, the rapid evolution and significant cost of treatment, as well as the challenges in obtaining insurance coverage, have kept HCV treatment largely in the domain of specialists, at least for now. In the interim, family physicians play a crucial role by screening, diagnosing, and counseling patients with this infection, referring them to specialty care, and providing ongoing monitoring for signs of HCC and esophageal and gastric varices.
CORRESPONDENCE
Laura Wangensteen, MD, Department of Family Medicine, Drexel University, 3401 South Market Street #105 A, Philadelphia, PA 19104; [email protected]
› Screen at-risk patients and all those born between 1945 and 1965 for hepatitis C virus (HCV) infection. B
› Screen HCV-positive patients for level of fibrosis and for conditions that may accelerate liver disease, including alcohol use, hepatitis B virus, and human immunodeficiency virus. B
› Continuously monitor patients with chronic HCV for the development of cirrhosis and hepatocellular carcinoma. A
› Refer patients to specialty care for HCV treatment and, if they have cirrhosis, for potential transplant evaluation. C
› Counsel HCV-positive patients about how to avoid transmission to others. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease. Over the next few decades, the number of deaths per year due to complications of HCV such as liver failure and hepatocellular carcinoma (HCC) is predicted to more than triple to 36,000 by 2032.1
Fortunately, major advances in drug therapy have made it possible to cure patients of HCV, and treatment is now less complex, of shorter duration, and better tolerated than it once was. To help family physicians maximize the care they provide to these patients, we’ve summarized screening recommendations from the Centers for Disease Control and Prevention (CDC), innovative alternatives to biopsy for staging liver disease, and counseling points to cover with patients.
A common, usually silent infection with potentially fatal complications
According to the National Health and Nutrition Examination Survey (NHANES), an estimated 2.7 to 3.9 million people in the United States are chronically infected with HCV, about threefourths of whom were born between 1945 and 1965 (the “baby boomer” generation).2 However, by adding “unaccounted groups” (eg, incarcerated, homeless, and active duty military) to these estimates, the number of people with HCV is likely more than 5.2 million.3
HCV is a ribonucleic acid (RNA) virus capable of mutating at a high rate to escape detection and clearance by the host’s immune system.4 Most patients with HCV are asymptomatic during the acute and chronic phases of infection, and may have a silent infection for decades. In fact, 65% to 75% of patients with HCV are unaware of their infection.5
Approximately 20% of chronically infected patients develop cirrhosis after 20 years and, once they do, the annual rate of HCC and liver decompensation is about 5%.6-8 Risk factors for advancement to cirrhosis includes male sex, alcohol consumption, co-infection with human immunodeficiency virus (HIV) or hepatitis B virus (HBV), immunosuppression, having had HCV infection for a long time, becoming infected with HCV after age 40, and not having responded to previous treatment.9
Chronic HCV infection can lead to extrahepatic manifestations such as essential mixed cryoglobulinemia, porphyria cutanea tarda, membranoproliferative glomerulonephritis, lymphoma, and glucose intolerance.10 There is also growing evidence that HCV infection affects cognitive function in the absence of fibrosis and hepatic encephalopathy. Several studies show that HCV-infected patients score poorly on neuropsychological testing for verbal learning, attention, memory, and executive function.11 This may be related to the expression of receptors for HCV by the brain’s microvascular endothelial cells.12
Screening recommendations. Given the high prevalence of HCV infection among baby boomers, the CDC decided in 2012 to recommend one-time HCV screening for all patients born between 1945 and 1965.13 This is in addition to risk-based screening for all patients who have a history of injection drug use, those on long-term hemodialysis or with tattoos obtained in unregulated settings, offspring of HCV-infected mothers, and those with health-care associated exposures (TABLE13). In 2013, the US Preventive Services Task Force upgraded its recommendation to match those of the CDC.14
Despite these recommendations, which are expected to increase detection of HCV among asymptomatic persons who do not know they are infected, there remain significant barriers to HCV testing. These include poor access to primary care and preventive services, lack of knowledge and awareness of the disease among patients and providers, and a lack of studies that support a universal screening approach for HCV.5,15,16 One tool that might help overcome some of these barriers and aid family physicians in the screening process is automatic reminders or standing lab orders for HCV testing in electronic medical records systems.
Screening for HCV can be done using any of the US Food and Drug Administration (FDA)-approved tests for the anti-HCV antibody, which have sensitivities and specificities greater than 99%.17 A positive screening result should be confirmed with an HCV RNA test. However, for practical purposes, ordering the anti-HCV test with reflex to the HCV RNA test decreases the number of blood draws and office visits required of the patient. The reflex confirmation allows the physician to deliver the patient’s full diagnosis and reduces the psychological distress associated with waiting for confirmatory results. The HCV RNA test (alone) should be used, however, in immunocompromised patients, those who may have had exposure to HCV in the past 6 months, and those suspected of having an HCV re-infection after having cleared the virus.18
Look for the evidence of liver disease
Family physicians should order several additional tests for patients found to have chronic HCV infection before referring such patients to a specialist (ALGORITHM). Work-up should include the complete blood count, HCV genotype (which will help guide treatment), liver function tests, international normalized ratio test, and ultrasound of the liver.18 In addition, all HCV-positive patients should be tested for HIV and HBV, because these co-infections may accelerate liver fibrosis.19,20
All patients with chronic HCV infection should also be screened for the presence of fibrosis and cirrhosis, as this will influence treatment choice and duration. Signs of cirrhosis that may be evident on physical exam include jaundice, spider angiomata, palmar erythema, encephalopathy with asterixis, and fluid overload, especially ascites. Cirrhosis can be classified clinically as compensated (stage 1 with no varices present and stage 2 with varices present) and decompensated (stages 3 and 4), which is defined as cirrhosis with signs of severe portal hypertension (bleeding varices, ascites, hepatic encephalopathy) or liver insufficiency (jaundice).21 Patients with decompensated cirrhosis should be managed by a liver transplant center. For more on cirrhosis, see “Cirrhosis complications: Keeping them under control” (J Fam Pract. 2015;64:338-342).
Several noninvasive alternatives to liver biopsy
Historically, liver biopsy has been the gold standard for staging liver disease. The Metavir scoring system is a histological assessment of the degree of inflammatory activity and the stage of fibrosis.22 The degree of inflammation activity, which is a precursor of fibrosis, is scored from A0 (no activity) to A3 (severe activity). The staging of fibrosis involves a 5-stage scoring system: F0 (chronic hepatitis without fibrosis); F1 (portal fibrosis without septae); F2 (portal fibrosis with rare septae); F3 (many septae without cirrhosis); or F4 (cirrhosis).
That said, noninvasive tests have largely supplanted liver biopsy for fibrosis screening.
For example, the FibroSure test uses the patient’s age, gender, and a combination of 6 serum markers of liver function in a computational algorithm to generate a quantitative indicator of liver fibrosis, with a score of 0.0 to 1.0 that corresponds to the Metavir fibrosis score (F0-F4), and an inflammatory activity score (A0-A3).23 Similarly, HepaScore uses several noninvasive markers to calculate a score from 0.00 to 1.00. A score ≤0.2 accurately excludes significant fibrosis. However, a score of ≥0.55 or higher corresponds to a Metavir score of at least F2, and in such cases further testing would be needed to evaluate for cirrhosis.24
FDA-approved in 2013, transient elastography (FibroScan) is another noninvasive alternative to liver biopsy for determining the stage of liver disease. This bedside test uses ultrasound technology to measure liver stiffness and provides a score ranging from 0 to 75 kPA that correlates with the Metavir score. Although not yet widely available in the United States, FibroScan is becoming increasingly popular as a rapid and noninvasive screening tool for cirrhosis.25
Identifying cirrhosis in patients who have HCV is crucial because such patients need prompt care from a specialist. In addition to receiving HCV treatment, patients with cirrhosis also need regular liver ultrasound exams to screen for HCC (every 6 months) and esophagogastroduodenoscopy to screen for esophageal and gastric varices.26
Advise patients to avoid alcohol, lose weight
Counsel patients who test positive for HCV infection about making lifestyle changes to avoid further liver damage and transmission of HCV to others. Infectious diseases and hepatology society guidelines recommend vaccination against hepatitis A and B for all HCV-infected patients who are not immune to these viruses because acute co-infection could lead to severe acute liver injury.18,27 Urge all HCV-infected patients to completely abstain from alcohol and, if necessary, refer them to an addiction specialist, because excess alcohol consumption is strongly associated with the development of cirrhosis and HCC.28,29
Comorbid conditions such as metabolic syndrome, obesity, and hyperlipidemia can worsen the prognosis for HCV-infected patients; therefore, intense counseling on weight loss is recommended.30 Statins are safe and beneficial for HCV patients with hypercholesterolemia and compensated cirrhosis.31
Teach patients that the primary mode of transmission of HCV is through infected blood. Sexual transmission of HCV has been well documented in HIV-positive men who have sex with men.32 Although the risk of transmission of HCV among heterosexual couples is extremely low, it is possible, and patients should be counseled accordingly.33 Transmission of HCV from mother to the baby occurs in up to 6% of births and most commonly occurs during delivery.34
Newer treatments are highly effective and well tolderated
HCV treatment has changed dramatically over the past few years. Previous treatments for HCV, particularly those containing interferon, were known for their poor tolerability due to adverse effects and low cure rates. Compared to previous therapies, the new interferon-free direct-acting antiviral (DAA) regimens are not only less complex but also shorter in duration, ranging from 8 to 24 weeks depending on the patient’s viral load, stage of liver disease, and previous treatment experience.18 The specific agents and dosages used in DAA regimens aren’t described here because these regimens are rapidly changing. However, continuously updated treatment recommendations from the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America are available at http://www.hcvguidelines.org.
The goal of HCV treatment is cure as evidenced by a sustained virologic response (SVR), which is defined as the absence of HCV RNA 12 weeks or more after completing treatment.35,36 In general, for the most common genotypes of HCV, treatment with a DAA regimen results in a SVR in ≥95% of patients.18 Achieving SVR is associated with a 50% reduction in all-cause mortality, a 90% reduction in liver-associated mortality, and a >70% reduction in the risk of developing HCC.27,37,38 SVR also has been shown to have a significant effect on reducing extrahepatic manifestations of HCV infection, such as cryoglobulinemia and lymphoma.39-41
Current barriers to the newer, highly effective hepatitis C virus (HCV) infection treatments are largely financial. Although insurance companies have been able to negotiate substantial discounts from the high wholesale price of treatment, many insurance programs require prior authorizations and will approve treatment only for patients with advanced liver fibrosis. In our experience, many patients are left to wait for their liver disease to progress before their insurance company will agree to cover treatment.
In addition, many insurance companies have mandated that only subspecialists prescribe these medications. However, infectious diseases and hepatology specialists and their support staffs are often overburdened with paperwork and phone calls related to prior authorizations and justification of treatment, which can add to delays in treatment.
There is already evidence that treatment of all patients with HCV is cost-effective and leads to better healthcare outcomes42 and there are indications that these barriers will decrease over time, with prices already dropping significantly due to increasing competition between drug companies.
The DAAs are well tolerated and have good safety profiles. In phase III clinical trials of today’s most commonly used DAA regimens, the discontinuation rate was <1% in non-cirrhotic patients and 2% in those with cirrhosis.18 The most commonly reported adverse effects were nausea, fatigue, and headache. DAAs may have drug-drug interactions; therefore, careful medication reconciliation should be performed before initiating treatment.18
Prioritizing treatment. Current evidence supports treatment for all patients with HCV except those with a life expectancy of <12 months.18 Evidence indicates that treatment becomes less effective as a patient’s liver injury progresses to cirrhosis. Due to the high cost of available treatments, however, many insurers have imposed strict criteria for coverage. (See “Barriers to HCV Treatment,” above.42)
The highest priority for treatment has been given to patients with advanced liver fibrosis, compensated cirrhosis, those who have received a liver transplant, and those with severe extrahepatic manifestations (eg, mixed cryoglobulinemia and end-organ disease such as nephropathy). Treatment is also prioritized for high-risk populations (eg, patients with HBV and HIV co-infection, diabetes mellitus) and patients who are at high risk of transmitting the virus (eg, individuals who inject drugs or are incarcerated, men who have sex with men, women of childbearing age, hemodialysis patients, and health care professionals who perform exposure-prone procedures).18
While it may eventually become feasible for family physicians to treat HCV-infected patients, the rapid evolution and significant cost of treatment, as well as the challenges in obtaining insurance coverage, have kept HCV treatment largely in the domain of specialists, at least for now. In the interim, family physicians play a crucial role by screening, diagnosing, and counseling patients with this infection, referring them to specialty care, and providing ongoing monitoring for signs of HCC and esophageal and gastric varices.
CORRESPONDENCE
Laura Wangensteen, MD, Department of Family Medicine, Drexel University, 3401 South Market Street #105 A, Philadelphia, PA 19104; [email protected]
1. Rein DB, Wittenborn JS, Weinbaum CM, et al. Forecasting the morbidity and mortality associated with prevalent cases of precirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66-72.
2. Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-714.
3. Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090-1101.
4. Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103-107.
5. Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729-733.
6. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
7. El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83.
8. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68.
9. McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut. 2004;53:318-321.
10. El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-1445.
11. Solinas A, Piras MR, Deplano A. Cognitive dysfunction and hepatitis C virus infection. World J Hepatol. 2015;7:922-925.
12. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142:634-643.e6.
13. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32.
14. US Preventive Services Task Force. Final recommendation statement on hepatitis C screening, June 2013. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-c-screening. Accessed on December 28, 2014.
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-2207.
16. Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754-758.
17. Shivkumar S, Peeling R, Jafari Y, et al. Accuracy of rapid and pointof- care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558-566.
18. American Association for the Study of Liver Diseases; Infectious Diseases Society of America; International Antiviral Society—USA. HCV guidance: Recommendations for testing, managing, and treating hepatitis C. HCV guidelines Web site. Available at: http://www.hcvguidelines.org. Accessed May 25, 2015.
19. Zarski JP, Bohn B, Bastie A, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27-33.
20. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562-569.
21. Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449.
22. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
23. Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896.
24. Becker L, Salameh W, Sferruzza A, et al. Validation of hepascore, compared with simple indices of fibrosis, in patients with chronic hepatitis C virus infection in United States. Clin Gastroenterol Hepatol. 2009;7:696-701.
25. Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16:372.
26. Garcia-Tsao G, Sanyal AJ, Grace ND, et al; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
27. Ghany MG, Strader DB, Thomas DL, et al; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
28. Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717-1722.
29. Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J Gastroenterol. 2009;15:3462-3471.
30. Ortiz V, Berenguer M, Rayón JM, et al. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408-2414.
31. Lewis JH, Mortensen ME, Zweig S, et al; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453-1463.
32. Gamage DG, Read TR, Bradshaw CS, et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis. 2011;11:39.
33. Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881-889.
34. Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223-229.
35. Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593-1601.
36. Thomas AM, Kattakuzhy S, Jones S, et al. SVR durability: HCV patients treated with IFN-free DAA regimens. Presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February, 2015; Seattle, Washington. Abstract 653.
37. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.e1.
38. Russo MW. Antiviral therapy for hepatitis C is associated with improved clinical outcomes in patients with advanced fibrosis. Expert Rev Gastroenterol Hepatol. 2010;4:535-539.
39. Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCVassociated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol. 2013;85:1019-1027.
40. Takahashi K, Nishida N, Kawabata H, et al. Regression of Hodgkin lymphoma in response to antiviral therapy for hepatitis C virus infection. Intern Med. 2012;51:2745-2747.
41. Gisbert JP, García-Buey L, Pajares JM, et al. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther. 2005;21:653-662.
42. Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407-419.
1. Rein DB, Wittenborn JS, Weinbaum CM, et al. Forecasting the morbidity and mortality associated with prevalent cases of precirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66-72.
2. Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-714.
3. Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090-1101.
4. Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103-107.
5. Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729-733.
6. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35.
7. El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83.
8. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68.
9. McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut. 2004;53:318-321.
10. El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439-1445.
11. Solinas A, Piras MR, Deplano A. Cognitive dysfunction and hepatitis C virus infection. World J Hepatol. 2015;7:922-925.
12. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142:634-643.e6.
13. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1-32.
14. US Preventive Services Task Force. Final recommendation statement on hepatitis C screening, June 2013. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-c-screening. Accessed on December 28, 2014.
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-2207.
16. Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754-758.
17. Shivkumar S, Peeling R, Jafari Y, et al. Accuracy of rapid and pointof- care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558-566.
18. American Association for the Study of Liver Diseases; Infectious Diseases Society of America; International Antiviral Society—USA. HCV guidance: Recommendations for testing, managing, and treating hepatitis C. HCV guidelines Web site. Available at: http://www.hcvguidelines.org. Accessed May 25, 2015.
19. Zarski JP, Bohn B, Bastie A, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27-33.
20. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562-569.
21. Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449.
22. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293.
23. Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887-1896.
24. Becker L, Salameh W, Sferruzza A, et al. Validation of hepascore, compared with simple indices of fibrosis, in patients with chronic hepatitis C virus infection in United States. Clin Gastroenterol Hepatol. 2009;7:696-701.
25. Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16:372.
26. Garcia-Tsao G, Sanyal AJ, Grace ND, et al; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938.
27. Ghany MG, Strader DB, Thomas DL, et al; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
28. Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717-1722.
29. Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J Gastroenterol. 2009;15:3462-3471.
30. Ortiz V, Berenguer M, Rayón JM, et al. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408-2414.
31. Lewis JH, Mortensen ME, Zweig S, et al; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453-1463.
32. Gamage DG, Read TR, Bradshaw CS, et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis. 2011;11:39.
33. Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881-889.
34. Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223-229.
35. Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139:1593-1601.
36. Thomas AM, Kattakuzhy S, Jones S, et al. SVR durability: HCV patients treated with IFN-free DAA regimens. Presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February, 2015; Seattle, Washington. Abstract 653.
37. Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.e1.
38. Russo MW. Antiviral therapy for hepatitis C is associated with improved clinical outcomes in patients with advanced fibrosis. Expert Rev Gastroenterol Hepatol. 2010;4:535-539.
39. Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCVassociated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol. 2013;85:1019-1027.
40. Takahashi K, Nishida N, Kawabata H, et al. Regression of Hodgkin lymphoma in response to antiviral therapy for hepatitis C virus infection. Intern Med. 2012;51:2745-2747.
41. Gisbert JP, García-Buey L, Pajares JM, et al. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther. 2005;21:653-662.
42. Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407-419.