User login
Pulmonary hypertension: An update of Dx and Tx guidelines
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.
Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
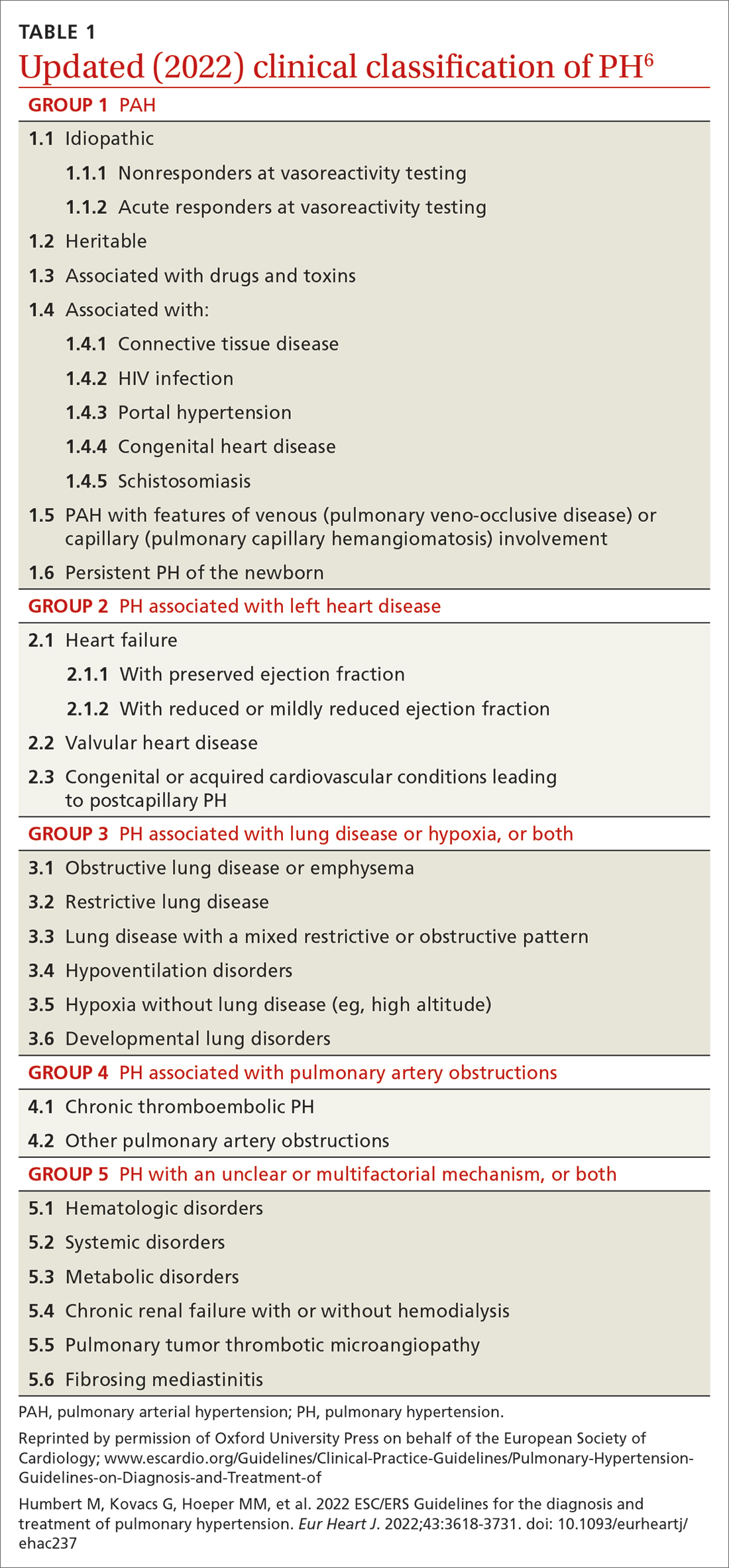
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
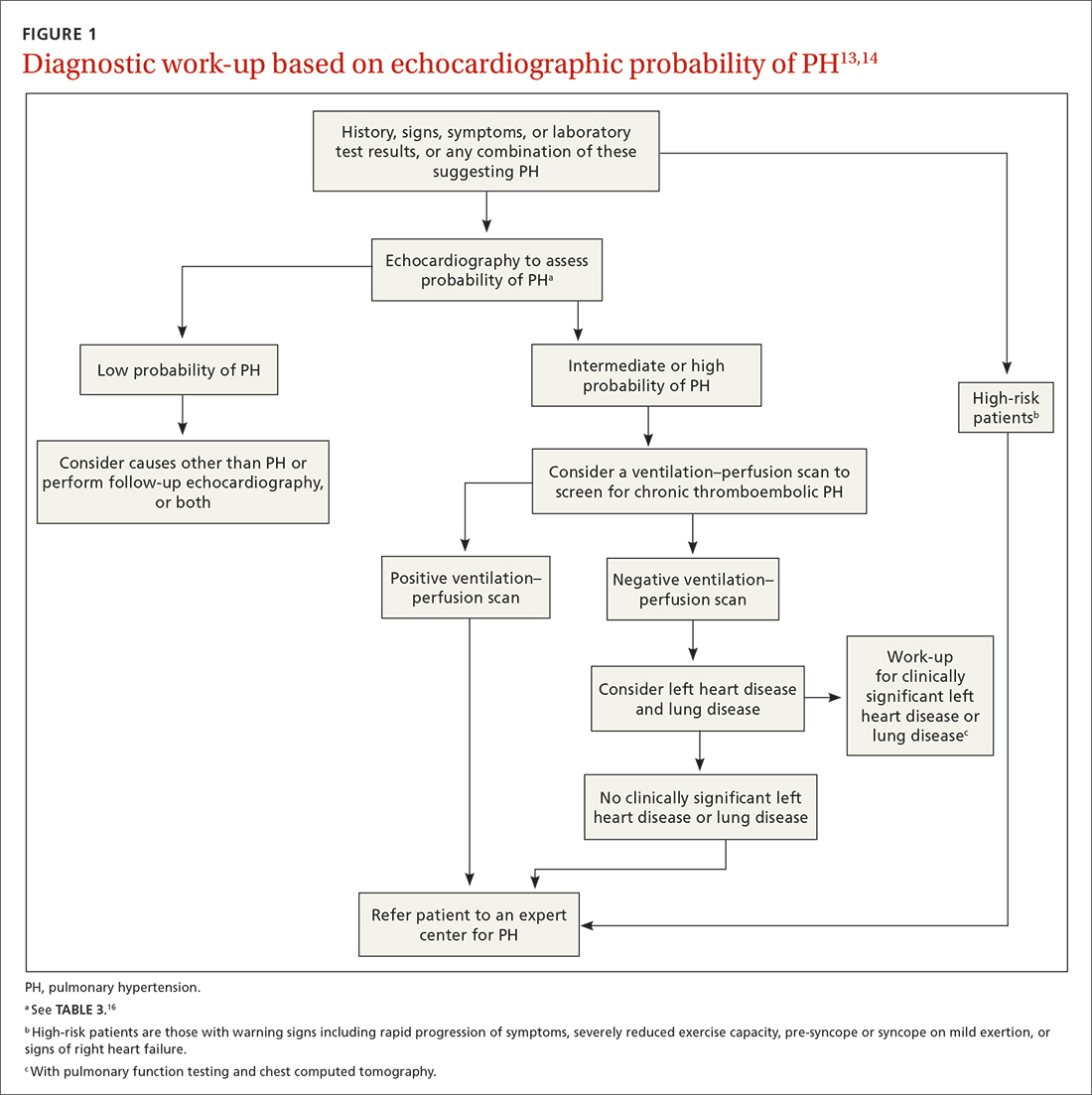
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.
Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7
Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6
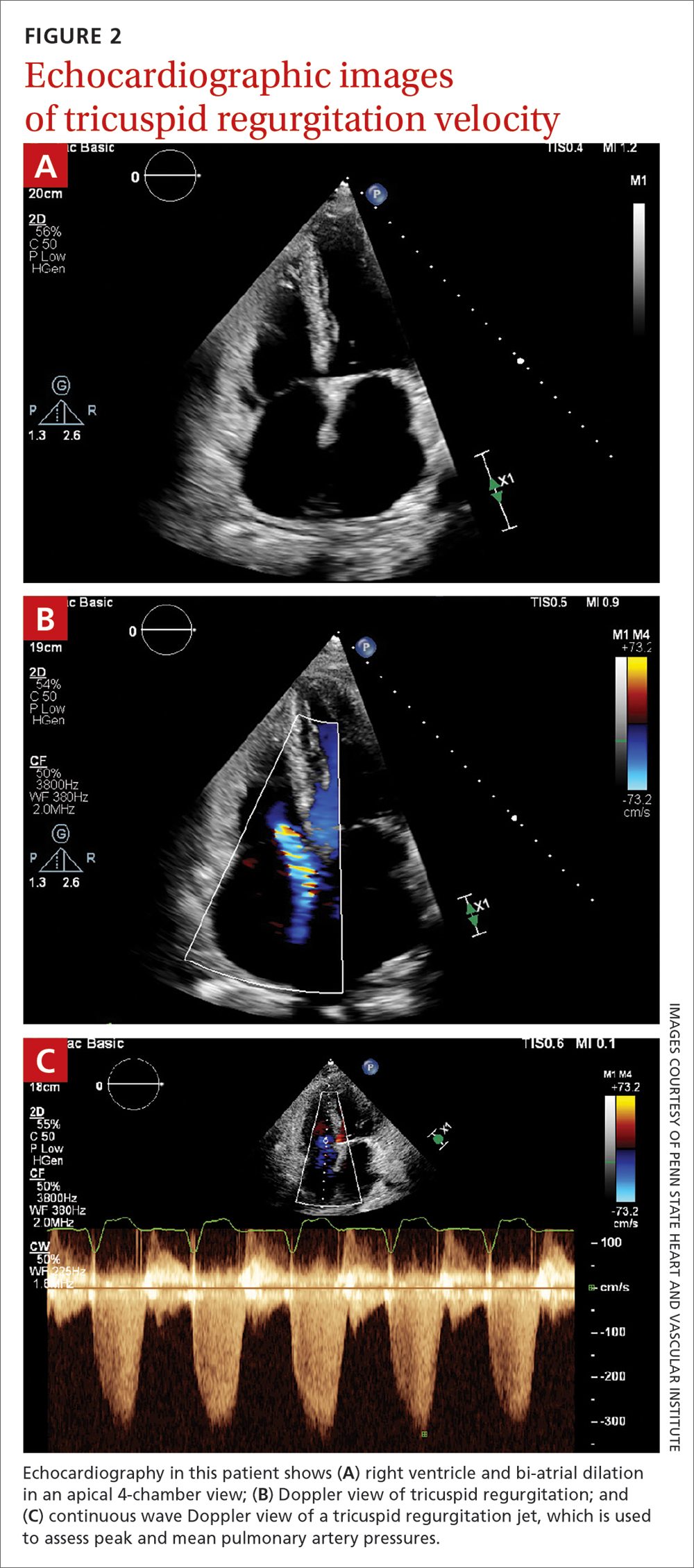
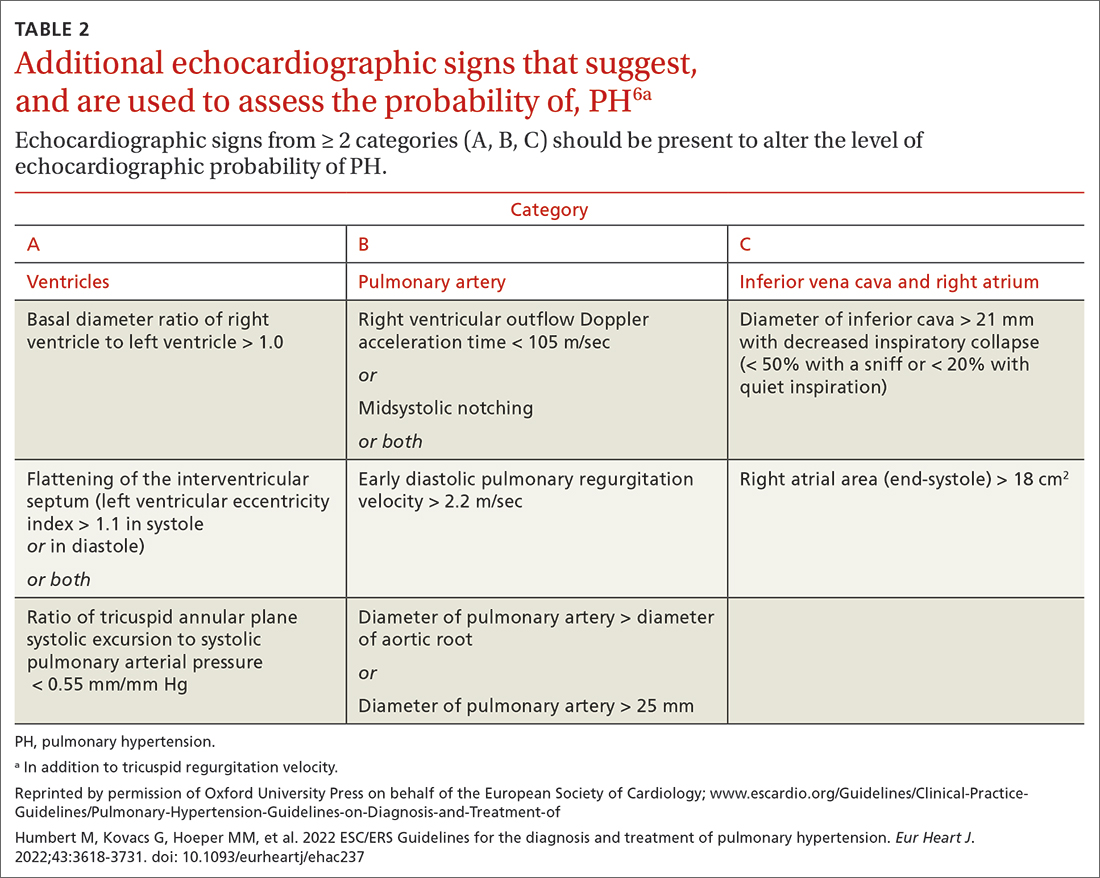
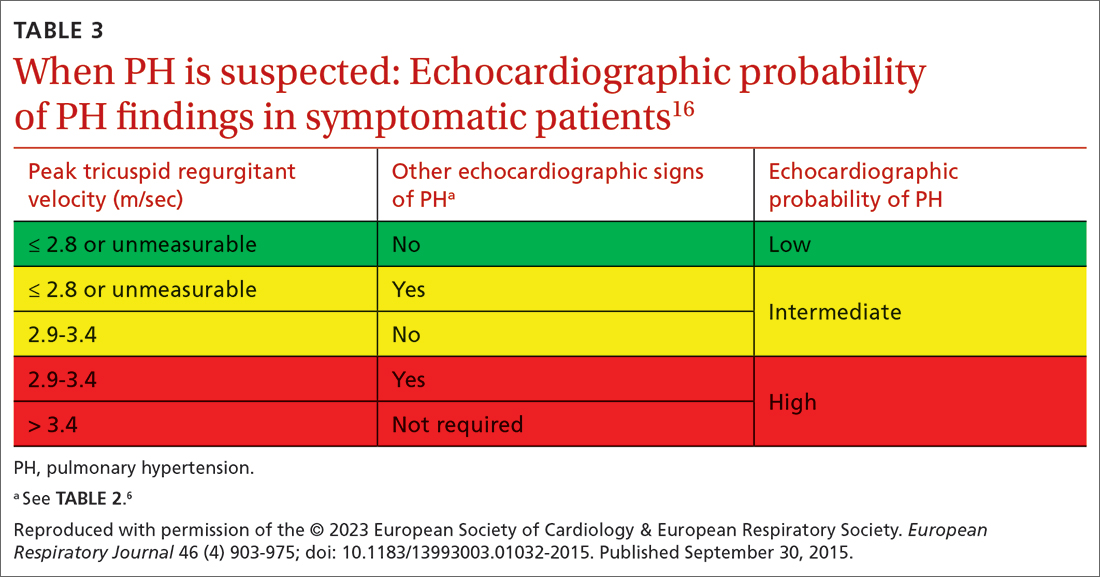
Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).
Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18
TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; [email protected]
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.

Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.

Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7

Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6

Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).

Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18

TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; [email protected]
New guidelines that redefine pulmonary hypertension (PH) by a lower mean pulmonary artery pressure (mPAP) have led to a reported increase in the number of patients given a diagnosis of PH. Although the evaluation and treatment of PH relies on the specialist, as we explain here, family physicians play a pivotal role in the diagnosis, reduction or elimination of risk factors for PH, and timely referral to a pulmonologist or cardiologist who has expertise in managing the disease. We also address the important finding that adult patients who have been evaluated, treated, and followed based on guidelines—updated just last year—have a longer life expectancy than patients who have not been treated properly or not treated at all.

Last, we summarize the etiology, evaluation, and management of PH in the pediatric population.
What is pulmonary hypertension? A revised definition
Prior to 2018, PH was defined as mPAP (measured by right heart catheterization [RHC]) ≥ 25 mm Hg at rest. Now, based on guidelines developed at the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018, PH is defined as mPAP > 20 mm Hg.1,2 That change was based on studies in which researchers noted higher mortality in adults who had mPAP below the traditional threshold.3,4 There is no evidence, however, of increased mortality in the pediatric population in this lower mPAP range.5
PH is estimated to be present in approximately 1% of the population.6 PH due to other diseases—eg, cardiac disease, lung disease, or a chronic thromboembolic condition—reflects the prevalence of the causative disease.7
How is pulmonary hypertension classified?
Based on the work of a Task Force of the 6th WSPH, PH is classified by underlying pathophysiology, hemodynamics, and functional status. Clinical classification comprises 5 categories, or “groups,” based on underlying pathophysiology (TABLE 16).
Clinical classification
Group 1 PH includes patients with primary pulmonary hypertension, also referred to (including in this article) as pulmonary arterial hypertension (PAH). Hemodynamic criteria that define PAH include pulmonary vascular resistance (PVR) > 2 Woods unitsa and pulmonary capillary wedge pressure > 15 mm Hg. Idiopathic PAH is the most common diagnosis in this group.
The incidence of PAH is approximately 6 cases for every 1 million adults; prevalence is 48 to 55 cases for every 1 million adults. PAH is more common in women.6
Continue to: Less common causes...
Less common causes in Group 1 includ
Group 2 PH comprises patients whose disease results from left heart dysfunction, the most common cause of PH. This subgroup has an elevated pulmonary artery wedge pressure > 15 mm Hg.8 Patients have either isolated postcapillary PH or combined pre-capillary and postcapillary PH.
Group 3 PH comprises patients whose PH is secondary to chronic and hypoxic lung disease. Patients in this group have pre-capillary PH; even a modest elevation in mPAP (20-29 mm Hg) is associated with a poor prognosis. Group 3 patients have elevated PVR, even with mild PH.2 Exertional dyspnea disproportionate to the results of pulmonary function testing, low carbon monoxide diffusion capacity, and rapid decline of arterial oxygenation with exercise all point to severe PH in these patients.9
Group 4 PH encompasses patients with pulmonary artery obstruction, the most common cause of which is related to chronic thromboembolism. Other causes include obstruction of the pulmonary artery from an extrinsic source. Patients with chronic thromboembolic pulmonary hypertension (CTEPH) also have pre-capillary PH, resulting from elevated pulmonary pressures secondary to thromboembolic burden, as well as pulmonary remodeling in unobstructed small arterioles.
Group 5 PH is a miscellaneous group secondary to unclear or multiple causes, including chronic hematologic anemia (eg, sickle cell disease), systemic disorders (eg, sarcoidosis), and metabolic disorders (eg, glycogen storage disease). Patients in Group 5 can have both pre-capillary and postcapillary hypertension.
Classification by functional status
The World Health Organization (WHO) Functional Classification of Patients with Pulmonary Hypertension is divided into 4 classes.10 This system is used to guide treatment and for prognostic purposes:
Class I. Patients have no limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near-syncope.
Continue to: Class II
Class II. Patients have slight limitation of physical activity. They are comfortable at rest but daily physical activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class III. These patients have marked limitation of physical activity. They are comfortable at rest, but less-than-ordinary activity causes dyspnea, fatigue, chest pain, or near-syncope.
Class IV. Patients are unable to carry out any physical activity without symptoms. They manifest signs of right heart failure. Dyspnea or fatigue, or both, might be present even at rest.
How is the pathophysiology of PH described?
The term pulmonary hypertension refers to an elevation in PAP that can result from any number of causes. Pulmonary arterial hypertension is a subcategory of PH in which a rise in PAP is due to primary pathology in the arteries proper.
As noted, PH results from a variety of pathophysiologic mechanisms, reflected in the classification in TABLE 1.6
WSPH Group 1 patients are considered to have PAH; for most, disease is idiopathic. In small-caliber pulmonary arteries, hypertrophy of smooth muscle, endothelial cells, and adventitia leads to increased resistance. Production of nitric oxide and prostacyclins is also impaired in endothelial cells. Genetic mutation, environmental factors such as exposure to stimulant use, and collagen vascular disease have a role in different subtypes of PAH. Portopulmonary hypertension is a subtype of PAH in patients with portal hypertension.
WSPH Groups 2-5. Increased PVR can result from pulmonary vascular congestion due to left heart dysfunction; destruction of the alveolar capillary bed; chronic hypoxic vasoconstriction; and vascular occlusion from thromboembolism.
Continue to: Once approximately...
Once approximately 30% of the pulmonary vasculature is involved, pressure in the pulmonary circulation starts to rise. In all WSPH groups, this increase in PVR results in increased right ventricular afterload that, over time, leads to right ventricular dysfunction.7,11,12
How does PH manifest?
Patients who have PH usually present with dyspnea, fatigue, chest pain, near-syncope, syncope, or lower-extremity edema, or any combination of these symptoms. The nonspecificity of presenting symptoms can lead to a delay in diagnosis.
In addition, suspicion of PH should be raised when a patient:
- presents with skin discoloration (light or dark) or a telangiectatic rash
- presents with difficulty swallowing
- has a history of connective tissue disease or hemolytic anemia
- has risk factors for HIV infection or liver disease
- takes an appetite suppressant
- has been exposed to other toxins known to increase the risk of PH.
A detailed medical history—looking for chronic lung or heart disease, thromboembolism, sleep-disordered breathing, a thyroid disorder, chronic renal failure, or a metabolic disorder—should be obtained.
Common findings on the physical exam in PH include:
- an increased P2 heart sound (pulmonic closure)
- high-pitched holosystolic murmur from tricuspid regurgitation
- pulmonic insufficiency murmur
- jugular venous distension
- hepatojugular reflux
- peripheral edema.
These findings are not specific to PH but, again, their presence warrants consideration of PH.
How best to approach evaluation and diagnosis?
The work-up for PH is broad; FIGURE 113,14 provides an outline of how to proceed when there is a concern for PH. For the work-up of symptoms and signs listed earlier, chest radiography and electrocardiography are recommended.

Continue to: Radiographic findings
Radiographic findings that suggest PH include enlargement of central pulmonary arteries and the right ventricle and dilation of the right atrium. Pulmonary vascular congestion might also be seen, secondary to left heart disease.7
Electrocardiographic findings of PH are demonstrated by signs of left ventricular hypertrophy, especially in Group 2 PH. Upright R waves in V1-V2 with deeper S waves in V5-V6 might represent right ventricular hypertrophy or right heart strain. Frequent premature atrial contractions and multifocal atrial tachycardia are also associated with PH.7

Brain natriuretic peptide (BNP) or N-terminal (NT) proBNP. The level of BNP might be elevated in PH, but its role in the diagnostic process has not been established. BNP can, however, be used to monitor treatment effectiveness and prognosis.15 A normal electrocardiogram in tandem with a normal level of BNP or NT-proBNP is associated with a low likelihood of PH.6

Transthoracic echocardiography (TTE) is the initial evaluation tool whenever PH is suspected. Echocardiographic findings suggestive of PH include a combination of tricuspid regurgitation velocity > 2.8 m/s (FIGURE 2); estimated pulmonary artery systolic pressure > 35 mm Hg in younger adults and > 40 mm Hg in older adults; right ventricular hypertrophy or strain; or a combination of these. Other TTE findings suggestive of PH are related to the ventricles, pulmonary artery, inferior vena cava, and right atrium (TABLE 26). The probability of PH based on TTE findings is categorized as low, intermediate, or high (see TABLE 26 and TABLE 316 for details).

Older guidelines, still used by some, rely on the estimated pulmonary artery systolic pressure (ePASP) reading on echocardiography.13,17 However, studies have reported poor correlation between ePASP readings and values obtained from RHC.18

TTE also provides findings of left heart disease, such as left ventricular systolic and diastolic dysfunction and left-sided valvular pathology. Patients with suspected PH in whom evidence of left heart disease on TTE is insufficient for making the diagnosis should receive further evaluation for their possible status in Groups 3-5 PH.
Ventilation–perfusion (VQ) scan. If CTEPH is suspected, a VQ scan should be performed. The scan is highly sensitive for CTEPH; a normal VQ scan excludes CTEPH. Computed tomography (CT) of the chest is not helpful for identifying chronic thromboembolism.13
Continue to: Coagulation assays
Coagulation assays. When CTEPH is suspected, coagulopathy can be assessed by measuring anticardiolipin antibodies, lupus anticoagulant, and anti-b-2-glycoprotein antibodies.13
Chest CT will show radiographic findings in greater detail. An enlarged pulmonary artery (diameter ≥ 29 mm) or a ratio ≥ 1 of the diameter of the main pulmonary artery to the diameter of the ascending aorta is suggestive of PH.
Other tests. Overnight oximetry and testing for sleep-disordered breathing, performed in an appropriate setting, can be considered.13,14,19
Pulmonary function testing with diffusion capacity for carbon monoxide, high-resolution chest CT, and a 6-minute walk test (6MWT) can be considered in patients who have risk factors for chronic lung disease. Pulmonary function testing, including measurement of the diffusing capacity of the lungs for carbon monoxide, arterial blood gas analysis, and CT, is used to aid in interpreting echocardiographic findings in patients with lung disease in whom PH is suspected.
Testing for comorbidities. A given patient’s predisposing conditions for PH might already be known; if not, laboratory evaluation for conditions such as sickle cell disease, liver disease, thyroid dysfunction, connective tissue disorders (antibody tests of antinuclear antibody, rheumatoid factor, anticentromere, anti-topoisomerase, anti-RNA polymerase III, anti-double stranded DNA, anti-Ro, anti-La, and anti-U1-RNP), and vasculitis (anti-neutrophil cytoplasmic autoantibodies) should be undertaken.
Analysis of stool and urine for Schistosoma spp parasites can be considered in an appropriate clinical setting.13
Right heart catheterization. Once alternative diagnoses are excluded, RHC is recommended to make a definitive diagnosis and assess the contribution of left heart disease. Vasoreactivity—defined as a reduction in mPAP ≥ 10 mm Hg to reach an absolute value of mPAP ≤ 40 mm Hg with increased or unchanged cardiac output—is assessed during RHC by administering nitric oxide or another vasodilator. This definition of vasoreactivity helps guide medical management in patients with PAH.7,20
Continue to: 6MWT
6MWT. Once the diagnosis of PH is made, a 6MWT helps establish baseline functional performance and will help you to monitor disease progression.
Who can benefit from screening for PH?
Annual evaluation of the risk of PAH is recommended for patients with systemic sclerosis or portal hypertension13 and can be considered in patients who have connective tissue disease with overlap features of systemic sclerosis.
Assessment for CTEPH or chronic thromboembolic pulmonary disease is recommended for patients with persistent or new-onset dyspnea or exercise limitation after pulmonary embolism.
Screening echocardiography for PH is recommended for patients who have been referred for liver transplantation.6
How risk is stratified
Risk stratification is used to manage PH and assess prognosis.
At diagnosis. Application of a 3-strata model of risk assessment (low, intermediate, high) is recommended.6 Pertinent data to determine risk include signs of right heart failure, progression of symptoms and clinical manifestations, report of syncope, WHO functional class, 6MWT, cardiopulmonary exercise testing, biomarkers (BNP or NT-proBNP), echocardiography, presence of pericardial effusion, and cardiac magnetic resonance imaging.
At follow-up. Use of a 4-strata model (low, intermediate–low, intermediate–high, and high risk) is recommended. Data used are WHO functional class, 6MWT, and results of either BNP or NT-proBNP testing.6
Continue to: When to refer
When to refer
Specialty consultation21-23 is recommended for:
- all patients with PAH
- PH patients in clinical Groups 2 and 3 whose disease is disproportionate to the extent of their left heart disease or hypoxic lung disease
- patients in whom there is concern about CTEPH and who therefore require early referral to a specialist for definitive treatment
- patients in whom the cause of PH is unclear or multifactorial (ie, clinical Group 5).
What are the options for managing PH?
Management of PH is based on the cause and classification of the individual patient’s disease.
Treatment for WSPH Group 1
Patients require referral to a specialty clinic for diagnosis, treatment, and monitoring of progression.10
First, regrettably, none of the medications approved by the US Food and Drug Administration for treating PAH prevent progression.7
Patients with idiopathic, hereditary, or drug-induced PAH with positive vasoreactivity are treated with a calcium channel blocker (CCB). The dosage is titrated to optimize therapy for the individual patient.
The patient is then reassessed after 3 to 6 months of medical therapy. Current treatment is continued if the following goals have been met:
- WHO functional classification is I or II
- BNP < 50 ng/L or NT-proBNP < 300 ng/L
- hemodynamics are normal or near-normal (mPAP ≤ 30 mm Hg and PVR ≤ 4 WU).
If these goals have not been met, treatment is adjusted by following the algorithm described below.
Continue to: The treatment algorithm...
The treatment algorithm for idiopathic-, heritable-, drug-induced, and connective tissue disease–associated PAH highlights the importance of cardiopulmonary comorbidities and risk strata at the time treatment is initiated and then during follow-up.
Cardiopulmonary comorbidities are conditions associated with an increased risk of left ventricular diastolic dysfunction, including obesity, hypertension, diabetes, and coronary artery disease. Pulmonary comorbidities can include signs of mild parenchymal lung disease and are often associated with a low carbon monoxide diffusing capacity (< 45% of predicted value).
The management algorithm proceeds as follows:
- For patients without cardiopulmonary comorbidities and who are at low or intermediate risk, treatment of PAH with an endothelin receptor antagonist (ERA) plus a phosphodiesterase-5 (PDE5) inhibitor is recommended.
- For patients without cardiopulmonary comorbidities and who are at high risk, treatment with an ERA, a PDE5 inhibitor, and either an IV or subcutaneous prostacyclin analogue (PCA) can be considered.
- Patients in either of the preceding 2 categories should have regular follow-up assessment; at such follow-up, their risk should be stratified based on 4 strata (see “How risk is stratified”):
- Low risk: Continue initial therapy.
- Low-to-intermediate risk: Consider adding a prostacyclin receptor agonist to the initial regimen or switch to a PDE5 inhibitor or a soluble guanylate cyclase stimulator.
- Intermediate-to-high or high risk: Consider adding a PCA (IV epoprostenol or IV or subcutaneous treprostinil). In addition, or alternatively, have the patient evaluated for lung transplantation.
- For patients with cardiopulmonary comorbidity—in any risk category—consider oral monotherapy with a PDE5 inhibitor or an ERA. Provide regular follow-up and individualize therapy.6
Treatment for WSPH Groups 2 and 3
Treatment is focused on the underlying cause of PH:
- Patients who have left heart disease with either severe pre-capillary component PH or markers of right ventricular dysfunction, or both, should be referred to a PH center.
- Patients with combined pre-capillary and postcapillary PH in whom pre-capillary PH is severe should be considered for an individualized approach.
- Consider prescribing the ERA bosentan in specific scenarios (eg, the Eisenmenger syndrome of left-right shunting resulting from a congenital cardiac defect) to improve exercise capacity. If PAH persists after corrected adult congenital heart disease, follow the PAH treatment algorithm for Group 1 patients (described earlier).
- For patients in Group 3, those who have severe PH should be referred to a PH center.
- Consider prescribing inhaled treprostinil in PH with interstitial lung disease.
Treatment for WSPH Group 4
Patients with CTEPH are the only ones for whom pulmonary endarterectomy (PEA), the treatment of choice, might be curative. Balloon angioplasty can be considered for inoperable cases6; these patients should be placed on lifelong anticoagulant therapy.
Symptomatic patients who have inoperable CTEPH or persistent recurrent PH after PEA are medically managed; the agent of choice is riociguat. Patients who have undergone PEA or balloon angioplasty and those receiving pharmacotherapy should be followed long term.
Treatment for WSPH Group 5
Management of these patients focuses on associated conditions.
Continue to: Which medications for PAH?
Which medications for PAH?
CCBs. Four options in this class have shown utility, notably in patients who have had a positive vasoreactivity test (see “How best to approach evaluation and diagnosis?”):
- Nifedipine is started at 10 mg tid; target dosage is 20 to 60 mg, bid or tid.
- Diltiazem is started at 60 mg bid; target dosage is 120 to 360 mg bid.
- Amlodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
- Felodipine is started at 5 mg/d; target dosage is 15 to 30 mg/d.
Felodipine and amlodipine have longer half-lives than other CCBs and are well tolerated.
ERA. Used as vasodilators are ambrinsentan (starting dosage, 5 mg/d; target dosage, 10 mg/d), macitentan (starting and target dosage, 10 mg/d), and bosentan (starting dosage, 62.5 mg bid; target dosage, 125 mg bid).
Nitric oxide–cyclic guanosine monophosphate enhancers. These are the PDE5 inhibitors sildenafil (starting and target dosages, 20 mg tid) and tadalafil (starting dosage, 20 or 40 mg/d; target dosage, 40 mg/d), and the guanylate cyclase stimulant riociguat (starting dosage, 1 mg tid; target dosage, 2.5 mg tid). All 3 agents enhance production of the potent vasodilator nitric oxide, production of which is impaired in PH.
Prostanoids. Several options are available:
- Beraprost sodium. For this oral prostacyclin analogue, starting dosage is 20 μg tid; target dosage is the maximum tolerated dosage (as high as 40 μg tid).
- Extended-release beraprost. Starting dosage is 60 μg bid; target dosage is the maximum tolerated dosage (as high as 180 μg bid).
- Oral treprostinil. Starting dosage is 0.25 mg bid or 0.125 mg tid; target dosage is the maximum tolerated dosage.
- Inhaled iloprost. Starting dosage of this prostacyclin analogue is 2.5 μg, 6 to 9 times per day; target dosage is 5 μg, 6 to 9 times per day.
- Inhaled treprostinil. Starting dosage is 18 μg qid; target dosage is 54 to 72 μg qid.
- Eproprostenol is administered by continuous IV infusion, at a starting dosage of 2 ng/kg/min; target dosage is determined by tolerability and effectiveness (typically, 30 ng/kg/min).
- IV treprostinil. Starting dosage 1.25 ng/kg/min; target dosage is determined by tolerability and effectiveness, with a typical dosage of 60 ng/kg/min.
Combination treatment with the agents listed above is often utilized.
Selexipag. This oral selective nonprostainoid prostacyclin receptor agonist is started at 200 μg bid; target dosage is the maximum tolerated, as high as 1600 μg bid.
Continue to: Supportive therapy
Supportive therapy
The need for oxygen should be addressed in patients with hypoxia in any setting—resting, exercise induced, and nocturnal.24 Patients with an arterial blood oxygen pressure < 60 mm Hg (SaO2 < 90 mm Hg) should be on long-term oxygen therapy.6
Diuretics are beneficial in patients with chronic fluid retention from PH that is related to right ventricular failure.24
Pulmonary rehabilitation and exercise. Contrary to common belief that exercise training is contraindicated in patients with PH, exercise training has emerged in the past decade as an effective tool to improve exercise capacity, ventilatory efficiency, and quality of life. While a patient is training, oxygen saturation, measured by pulse oximetry, should be maintained at > 90% throughout the exercise session to avoid hypoxic pulmonary artery vasoconstriction.25
A patient who does not qualify for pulmonary or cardiac rehabilitation should be referred for physical therapy.24
Ongoing follow-up in primary care
Instruct patients not to abruptly discontinue medications that have been prescribed for PH. Ongoing follow-up and monitoring involves assessing right heart function, exercise tolerance, and resting and ambulatory oximetry. Testing for the level of BNP provides prognostic information and allows assessment of treatment response.15 The frequency of 6MWT, echocardiography, and RHC is decided on a case-by-case basis.
Other considerations
Pregnancy. PAH often affects patients of childbearing age. Because PAH-associated maternal mortality and the risk to the fetus during pregnancy are high, pregnancy is not recommended for patients with PAH. After a diagnosis of PAH in a patient of childbearing age, counseling should be offered at an expert center. Advice on effective contraception methods should be given early on.10,26-29
Surgery. Every patient with clinically significant PH is at increased risk of perioperative morbidity and death.30,31 Guidelines recommend that these patients avoid nonessential surgery; if surgery is necessary, care should be provided at a PH expert center.10
Continue to: Patients with severe PH...
Patients with severe PH should consider surgery for any indication carefully, discussing with the care team their risk and exploring nonsurgical options. Cardiothoracic surgical and liver transplantation services might have highly specific criteria for treating patients with PH, but other essential and nonessential surgeries require individualized risk stratification. Surgery for patients with severe PH and right ventricular dysfunction should be performed at a center equipped to handle high-risk patients.
Other preventive measures. Patients with PAH should6,10:
- remain current with immunization against influenza virus, SARS-CoV-2, and pneumococcal pneumonia
- avoid high altitudes
- use supplemental oxygen during air travel to keep arterial oxygen saturation > 91%.
Lung transplantation. Patients eligible for transplantation who (1) are at intermediate-to-high risk or high risk or (2) have a REVEAL (Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management) risk score > 7, and who have had an inadequate response to oral combination therapy, should be referred for evaluation for lung transplantation. Placement on the list for lung transplantation is also recommended for patients at high risk of death and who have a REVEAL risk score ≥ 10 despite medical therapy, including a subcutaneous or IV prostacyclin analogue.6
PH in infants and children
The Pediatric Task Force of the 6th WSPH has applied the new definition proposed for adult PH (> 20 mm Hg mPAP) to children and infants > 3 months of age (see “Pulmonary hypertension in the pediatric population,” at left32-36).
SIDEBAR
Pulmonary hypertension in the pediatric population
The onset of pulmonary hypertension (PH) in children can occur at any age and be of quite different causes than in adults. In newborns, pulmonary pressure drops rapidly during the week after delivery; in some cases, however, pressures remain elevated (> 20 mm Hg) despite healthy lungs. These asymptomatic newborns require close monitoring.32
Etiology. Pediatric PH can be persistent or transient. Prominent causes of persistent or progressive PH in children are pulmonary arterial hypertension (PAH) associated with congenital heart disease and developmental lung disease, such as bronchopulmonary dysplasia and idiopathic PAH. Major categories of congenital heart disease that cause PH are shunting lesions and left heart disease associated with elevated atrial pressure. Other causes are rare.33
Persistent PH of the newborn (PPHN) and PH due to diaphragmatic hernia are common causes of transient PH.34 In PPHN, pulmonary vascular resistance remains abnormally high after birth, resulting in right-to-left shunting of the circulation that, in turn, leads to hypoxemia unresponsive to usual measures. In most cases, signs of respiratory distress and hypoxia are noted within the first 24 hours of life. The most common cause of PPHN is infection.35
Evaluation. The typical diagnostic work-up of suspected pediatric PH is similar to what is undertaken in the adult population—varying, however, according to the specific suspected cause. As in adults, right heart catheterization remains the gold standard of diagnosis, and should be conducted at a pediatric PH expert center. As with adult patients, infants and children with PH should be managed by a multidisciplinary expert team.
Management. PAH-targeted medications (see “What are the options for managing PH?”) are used to treat PAH in children.36
CORRESPONDENCE
Madhavi Singh, MD, 1850 East Park Ave., Suite 207, State College, PA 16803; [email protected]
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
1. Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018
2. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018
3. Kolte D, Lakshmanan S, Jankowich MD, et al. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:e009729. doi: 10.1161/JAHA.118.009729
4. Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509-516. doi: 10.1164/rccm.201706-1215OC
5. Lammers AE, Apitz C. Update from the World Symposium on Pulmonary Hypertension 2018: does the new hemodynamic definition of pediatric pulmonary hypertension have an impact on treatment strategies? Cardiovasc Diagn Ther. 2021;11:1048-1051. doi: 10.21037/cdt-20-412
6. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618-3731. doi: 10.1093/eurheartj/ehac237
7. Oldroyd SH, Manek G, Bhardwaj A. Pulmonary hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK482463/?report=classic
8. Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018
9. Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 suppl):D109-D116. doi: 10.1016/j.jacc.2013.10.036
10. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146:449-475. doi: 10.1378/chest.14-0793
11. Krowl L, Anjum F, Kaul P. Pulmonary idiopathic hypertension. In: StatPearls [Internet]. StatPearls Publishing. Updated August 8, 2022. Accessed November 27, 2022. www.ncbi.nlm.nih.gov/books/NBK519041/#_NBK519041_pubdet_
12. Bartolome SD. Portopulmonary hypertension: diagnosis, clinical features, and medical therapy. Clin Liver Dis (Hoboken). 2014;4:42-45. doi: 10.1002/cld.401
13. Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/ 13993003.01904-2018
14. Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. doi: 10.1136/jim-2020-001291
15. Chin KM, Rubin LJ, Channick R, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019;139:2440-2450. doi: 10.1161/CIRCULATIONAHA.118.039360
16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903-975. doi: 10.1183/13993003.01032-2015
17. N,, , et al; Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263. doi: 10.1183/09031936.00139009
18. Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139:988-993. doi: 10.1378/chest.10-1269
19. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97:612-622. doi: 10.1136/hrt.2010.212084
20. Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail. 2011;17:56-63. doi: 10.1111/j.1751-7133.2010.00202.x
21. Suntharalingam J, Ross RM, Easaw J, et al. Who should be referred to a specialist pulmonary hypertension centre—a referrer’s guide. Clin Med (Lond). 2016;16:135-141. doi: 10.7861/clinmedicine.16-2-135
22. Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral Study. JAMA Intern Med. 2013;173:887-893. doi: 10.1001/jamainternmed.2013.319
23. Guidelines for referring patients with pulmonary hypertension. Royal Papworth Hospital, NHS Foundation Trust. Updated February 2019. Accessed November 27, 2022. https://royalpapworth.nhs.uk/application/files/9015/5014/6935/PVDU-Referral-guidelines-2019.pdf
24. Yuan P, Yuan X-T, Sun X-Y, et al. Exercise training for pulmonary hypertension: a systematic review and meta-analysis. Int J Cardiol. 2015;178:142-146. doi: 10.1016/j.ijcard.2014.10.161
25. Spruit MA, Singh SJ, Garvey C, et al; . An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64. doi: 10.1164/rccm.201309-1634ST
26. Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431-437. doi: 10.1183/ 16000617.0079-2016
27. Weiss BM, Zemp L, Swifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996; J Am Coll Cardiol. 1998;31:1650-1657. doi: 10.1016/s0735-1097(98)00162-4
28. Qiangqiang Li, Dimopoulos K, Liu T, et al, Peripartum outcomes in a large population of women with pulmonary arterial hypertension associated with congenital heart disease, Euro J Prev Cardiol. 2019;26:1067-1076. doi: 10.1177/2047487318821246
29. Olsson KM, Jaïs X. Birth control and pregnancy management in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:681-688. doi: 10.1055/s-0033-1355438
30. Price LC, Montani D, Jaïs X, et al. Noncardiothoracic nonobstetric surgery in mild-to-moderate pulmonary hypertension. Eur Respir J. 2010;35:1294-1302. doi: 10.1183/09031936.00113009
31. Memtsoudis SG, Ma Y, Chiu YL, et al. Perioperative mortality in patients with pulmonary hypertension undergoing major joint replacement. Anesth Analg. 2010;111:1110-1116. doi: 10.1213/ANE.0b013e3181f43149
32. Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018
33. Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537-546. doi: 10.1016/S0140-6736(11)61621-8
34. van Loon RL, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755-1764. doi: 10.1161/CIRCULATIONAHA.110.969584
35. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. 2017;139:e20161165. doi: 10.1542/peds.2016-1165
36. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879-901. doi: 10.1016/j.healun.2019.06.022
PRACTICE RECOMMENDATIONS
› Employ echocardiography as the first-line diagnostic test when pulmonary hypertension (PH) is suspected. C
› Order a ventilation– perfusion scan in patients with unexplained PH to exclude chronic thromboembolic PH. C
› Order lung function testing with diffusion capacity for carbon monoxide as part of the initial evaluation of PH. C
› Use right heart catheterization to confirm the diagnosis of pulmonary arterial hypertension. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A guide to managing disorders of the ear pinna and canal
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13
How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; [email protected]
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13

How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; [email protected]
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13

How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; [email protected]
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
PRACTICE RECOMMENDATIONS
› Prescribe topical antibiotics for uncomplicated otitis externa, reserving systemic agents for infection extending outside the ear canal, necrotizing otitis externa, or patients who are immunodeficient. C
› Avoid clearing cerumen if a patient is asymptomatic and advise patients/parents on Do’s and Don’ts for ear wax accumulation. C
› Consider flooding the ear canal with xylocaine, alcohol, or mineral oil before attempting insect removal. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series