User login
Evaluation and Management of Female Sexual Dysfunction

IN THIS ARTICLE
- Causes of pain
- Screening
- Multimodal treatment
Care of women with sexual disorders has made great strides since Masters and Johnson began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease devised the classification system for female sexual dysfunction, which was officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychologic components that require a detailed screening, history, and physical examination. Our goal in this review is to provide primary care providers with insights and practical advice to help screen, diagnose, and treat FSD, which can have a profound impact on patients’ most intimate relationships.
UNDERSTANDING THE TYPES OF FSD
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported to be as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only three female dysfunctions (as opposed to five in DSM-IV):
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than six months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the six-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
Continue to: A common symptom
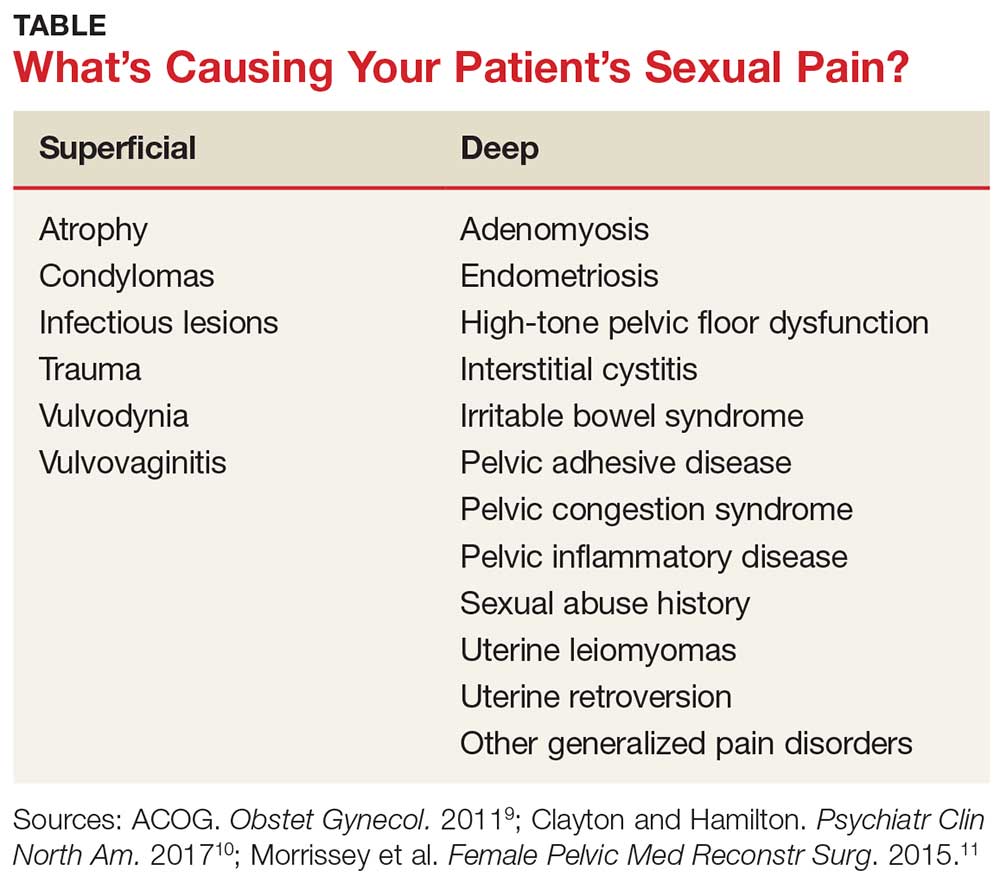
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychologic factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the Table.9-11
EVALUATING THE PATIENT
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation on Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists, the BSSC-W is not validated.9 The four items in the questionnaire ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and whether the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
Continue to: General exam: What to look for
General exam: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the Female Sexual Function Index (FSFI), compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and nonfunctioning.
Continue to: Normal PFMs
Normal PFMs are those that can voluntarily and involuntarily contract and relax.19,20
Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the exam, ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated.
Lukban et al have described a 0 to 4 numbered scale that evaluates tenderness in the pelvic floor.22 The scale denotes “1” as comfortable pressure associated with the exam, “2” as uncomfortable pressure associated with the exam, “3” as moderate pain associated with the exam that intensifies with contraction, and “4” indicating severe pain with the exam and inability to perform the contraction maneuver due to pain.
Continue to: EFFECTIVE TREATMENT INCLUDES MULTIPLE OPTIONS
EFFECTIVE TREATMENT INCLUDES MULTIPLE OPTIONS
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm.
Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain.26 Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy. For instance
Zestra, which contains a patented blend of botanical oils and extracts and is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn, a nonhormonal cream containing cutaneous lysate, has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients for three months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil, a prostaglandin E1 analogue that increases genital vasodilation when applied topically, is currently undergoing investigational trials.31,32
Patients can also choose from many OTC lubricants that contain water-based, oil-based, or silicone-based ingredients.
Continue to: Don't overlook physical therapy
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for FSD that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger-point identification and alleviation.
One pilot study examined use of transvaginal Thiele massage twice a week for five weeks in 21 symptomatic women with interstitial cystitis and high-tone pelvic floor dysfunction. The researchers found it decreased hypertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in six of 14 patients (43%).36
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of Kegel trainers and other insertion devices that may improve PFM coordination and strength.
Continue to: Pharmacotherapy has its place
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genitopelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used, including hormonal and nonhormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe, with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Nonhormonal drugs including flibanserin, a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Bupropion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants, such as nortriptyline and amitriptyline, may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to three times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines, such as oral clonazepam and intravaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al evaluated data for 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Continue to: Trigger point and Botox injections
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger-point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger-point injections in 18 women with levator ani muscle spasm using a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women showed improvement, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective six-month pilot study, 28 patients with pelvic pain for whom conservative treatment did not work received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients for whom conventional treatments fail.47,48
Addressing psychologic issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
CONCLUSION
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a woman’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al. Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior. Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5th ed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 pt 2):337-348.
13. Kegel A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual Behavior in the Human Female. Philadelphia, PA: WB Saunders; 1998.
16. Lowenstein L, Gruenwald I, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the Pelvic Floor Clinical Assessment Group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC, et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(suppl):22.
27. Farrell J, Cacchioni T. The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:CD007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010;21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.

IN THIS ARTICLE
- Causes of pain
- Screening
- Multimodal treatment
Care of women with sexual disorders has made great strides since Masters and Johnson began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease devised the classification system for female sexual dysfunction, which was officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychologic components that require a detailed screening, history, and physical examination. Our goal in this review is to provide primary care providers with insights and practical advice to help screen, diagnose, and treat FSD, which can have a profound impact on patients’ most intimate relationships.
UNDERSTANDING THE TYPES OF FSD
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported to be as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only three female dysfunctions (as opposed to five in DSM-IV):
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than six months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the six-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
Continue to: A common symptom
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychologic factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the Table.9-11

EVALUATING THE PATIENT
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation on Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists, the BSSC-W is not validated.9 The four items in the questionnaire ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and whether the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
Continue to: General exam: What to look for
General exam: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the Female Sexual Function Index (FSFI), compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and nonfunctioning.
Continue to: Normal PFMs
Normal PFMs are those that can voluntarily and involuntarily contract and relax.19,20
Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the exam, ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated.
Lukban et al have described a 0 to 4 numbered scale that evaluates tenderness in the pelvic floor.22 The scale denotes “1” as comfortable pressure associated with the exam, “2” as uncomfortable pressure associated with the exam, “3” as moderate pain associated with the exam that intensifies with contraction, and “4” indicating severe pain with the exam and inability to perform the contraction maneuver due to pain.
Continue to: EFFECTIVE TREATMENT INCLUDES MULTIPLE OPTIONS
EFFECTIVE TREATMENT INCLUDES MULTIPLE OPTIONS
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm.
Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain.26 Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy. For instance
Zestra, which contains a patented blend of botanical oils and extracts and is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn, a nonhormonal cream containing cutaneous lysate, has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients for three months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil, a prostaglandin E1 analogue that increases genital vasodilation when applied topically, is currently undergoing investigational trials.31,32
Patients can also choose from many OTC lubricants that contain water-based, oil-based, or silicone-based ingredients.
Continue to: Don't overlook physical therapy
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for FSD that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger-point identification and alleviation.
One pilot study examined use of transvaginal Thiele massage twice a week for five weeks in 21 symptomatic women with interstitial cystitis and high-tone pelvic floor dysfunction. The researchers found it decreased hypertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in six of 14 patients (43%).36
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of Kegel trainers and other insertion devices that may improve PFM coordination and strength.
Continue to: Pharmacotherapy has its place
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genitopelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used, including hormonal and nonhormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe, with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Nonhormonal drugs including flibanserin, a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Bupropion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants, such as nortriptyline and amitriptyline, may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to three times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines, such as oral clonazepam and intravaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al evaluated data for 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Continue to: Trigger point and Botox injections
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger-point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger-point injections in 18 women with levator ani muscle spasm using a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women showed improvement, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective six-month pilot study, 28 patients with pelvic pain for whom conservative treatment did not work received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients for whom conventional treatments fail.47,48
Addressing psychologic issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
CONCLUSION
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a woman’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.

IN THIS ARTICLE
- Causes of pain
- Screening
- Multimodal treatment
Care of women with sexual disorders has made great strides since Masters and Johnson began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease devised the classification system for female sexual dysfunction, which was officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychologic components that require a detailed screening, history, and physical examination. Our goal in this review is to provide primary care providers with insights and practical advice to help screen, diagnose, and treat FSD, which can have a profound impact on patients’ most intimate relationships.
UNDERSTANDING THE TYPES OF FSD
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported to be as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only three female dysfunctions (as opposed to five in DSM-IV):
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than six months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the six-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
Continue to: A common symptom
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychologic factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the Table.9-11

EVALUATING THE PATIENT
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation on Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists, the BSSC-W is not validated.9 The four items in the questionnaire ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and whether the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
Continue to: General exam: What to look for
General exam: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the Female Sexual Function Index (FSFI), compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and nonfunctioning.
Continue to: Normal PFMs
Normal PFMs are those that can voluntarily and involuntarily contract and relax.19,20
Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the exam, ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated.
Lukban et al have described a 0 to 4 numbered scale that evaluates tenderness in the pelvic floor.22 The scale denotes “1” as comfortable pressure associated with the exam, “2” as uncomfortable pressure associated with the exam, “3” as moderate pain associated with the exam that intensifies with contraction, and “4” indicating severe pain with the exam and inability to perform the contraction maneuver due to pain.
Continue to: EFFECTIVE TREATMENT INCLUDES MULTIPLE OPTIONS
EFFECTIVE TREATMENT INCLUDES MULTIPLE OPTIONS
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm.
Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain.26 Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy. For instance
Zestra, which contains a patented blend of botanical oils and extracts and is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn, a nonhormonal cream containing cutaneous lysate, has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients for three months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil, a prostaglandin E1 analogue that increases genital vasodilation when applied topically, is currently undergoing investigational trials.31,32
Patients can also choose from many OTC lubricants that contain water-based, oil-based, or silicone-based ingredients.
Continue to: Don't overlook physical therapy
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for FSD that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger-point identification and alleviation.
One pilot study examined use of transvaginal Thiele massage twice a week for five weeks in 21 symptomatic women with interstitial cystitis and high-tone pelvic floor dysfunction. The researchers found it decreased hypertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in six of 14 patients (43%).36
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of Kegel trainers and other insertion devices that may improve PFM coordination and strength.
Continue to: Pharmacotherapy has its place
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genitopelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used, including hormonal and nonhormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe, with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Nonhormonal drugs including flibanserin, a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Bupropion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants, such as nortriptyline and amitriptyline, may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to three times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines, such as oral clonazepam and intravaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al evaluated data for 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Continue to: Trigger point and Botox injections
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger-point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger-point injections in 18 women with levator ani muscle spasm using a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women showed improvement, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective six-month pilot study, 28 patients with pelvic pain for whom conservative treatment did not work received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients for whom conventional treatments fail.47,48
Addressing psychologic issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
CONCLUSION
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a woman’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al. Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior. Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5th ed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 pt 2):337-348.
13. Kegel A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual Behavior in the Human Female. Philadelphia, PA: WB Saunders; 1998.
16. Lowenstein L, Gruenwald I, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the Pelvic Floor Clinical Assessment Group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC, et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(suppl):22.
27. Farrell J, Cacchioni T. The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:CD007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010;21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al. Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior. Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5th ed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 pt 2):337-348.
13. Kegel A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual Behavior in the Human Female. Philadelphia, PA: WB Saunders; 1998.
16. Lowenstein L, Gruenwald I, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the Pelvic Floor Clinical Assessment Group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC, et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(suppl):22.
27. Farrell J, Cacchioni T. The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:CD007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010;21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
The evaluation and management of female sexual dysfunction
The care of women with female sexual disorders has made great strides since Masters and Johnson first began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease defined the classification system for female sexual dysfunction, which was eventually published and officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychological components that require a detailed screening, history, and physical examination. Our goal in this review is to provide family physicians with insights and practical advice to help screen, diagnose, and treat female sexual dysfunction, which can have a profound impact on patients’ most intimate relationships.
Understanding the types of female sexual dysfunction
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only 3 female dysfunctions as opposed to 5 in DSM-IV.
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than 6 months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the 6-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychological factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the TABLE.9-11
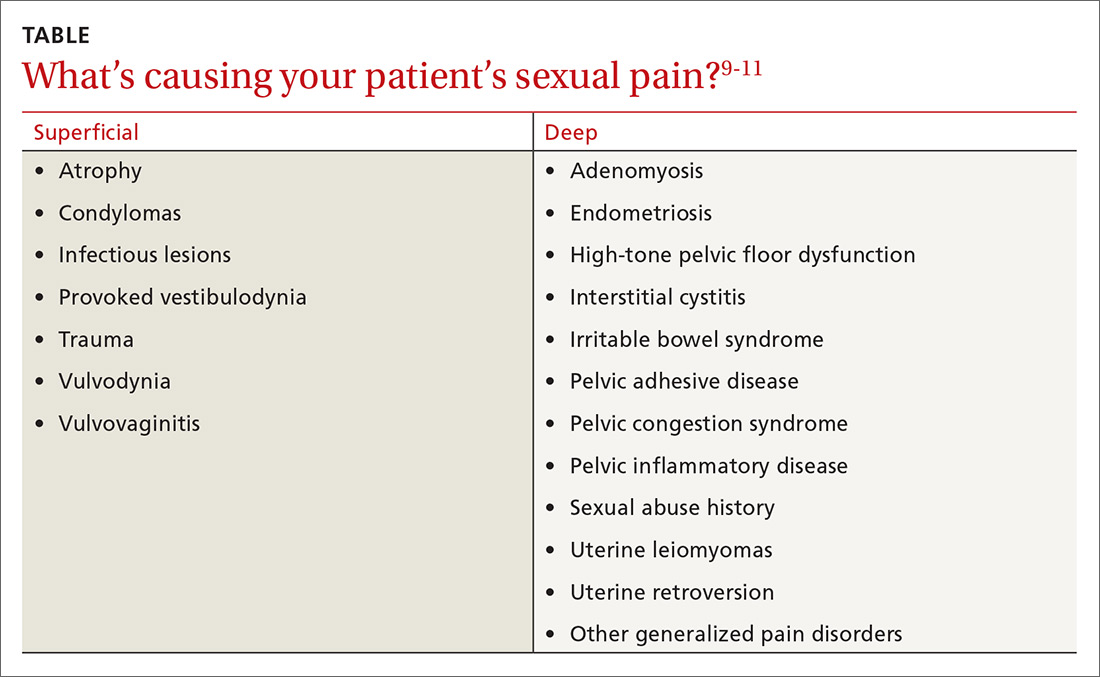
Evaluating the patient
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation in Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists,9 the BSSC-W is not validated. The questionnaire includes 4 questions that ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and if the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
General examination: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the female sexual function index (FSFI) compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and non-functioning.
- Normal PFMs are those that can voluntarily and involuntary contract and relax.19,20
- Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
- Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
- Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the examination, the physician should ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated. Lukban et al has described a zero
Effective treatment includes multiple options
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm. Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al26 found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain. Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy.
Zestra, for instance, which is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn is a non-hormonal cream containing cutaneous lysate and has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients over 3 months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil is a prostaglandin E1 analogue that increases genital vasodilation when applied topically and is currently undergoing investigational trials.31,32 Patients can also choose from many over-the-counter lubricants that contain water-based, oil-based, or silicone-based ingredients.
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for female sexual dysfunction that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger point identification and alleviation.
One pilot study, which involved transvaginal Thiele massage twice a week for 5 weeks on 21 symptomatic women with IC and high-tone pelvic floor dysfunction found it decreased hyptertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al36 investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in 6 out of 14 patients (43%).
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of kegal trainers and other insertion devices that may improve PFM coordination and strength.
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genito-pelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used including hormonal and non-hormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene (Osphena) is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Non-hormonal drugs including flibanserin (Addyi), a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events, and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Buproprion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants such as nortriptyline and amitriptyline may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to 3 times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines such as oral clonazepam and intra-vaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al reviewed 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger point injections in 18 women with levator ani muscle spasm with a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women improved, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective 6-month pilot study, 28 patients with pelvic pain who failed conservative treatment received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at Weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients who fail conventional treatments.47,48
Addressing psychological issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive-behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a women’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
CORRESPONDENCE
Melissa L. Dawson, DO, MS, Department of OB/GYN, Drexel University College of Medicine, 207 N Broad St. 4th Floor, Philadelphia, PA 19107; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren, JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al., Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior, Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual Dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5thed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009. 113:1124-1136.
9. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 2):337-348.
13. Kegel, A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The Role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual behavior in the human female. W. B. Saunders:Philadelphia, PA; 1998.
16. Lowenstein L, Gruenwald, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(Suppl):22.
27. Farrell J, Cacchioni T, The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014:11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:Cd007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(Suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014; 21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010:21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006.108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
The care of women with female sexual disorders has made great strides since Masters and Johnson first began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease defined the classification system for female sexual dysfunction, which was eventually published and officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychological components that require a detailed screening, history, and physical examination. Our goal in this review is to provide family physicians with insights and practical advice to help screen, diagnose, and treat female sexual dysfunction, which can have a profound impact on patients’ most intimate relationships.
Understanding the types of female sexual dysfunction
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only 3 female dysfunctions as opposed to 5 in DSM-IV.
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than 6 months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the 6-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychological factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the TABLE.9-11

Evaluating the patient
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation in Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists,9 the BSSC-W is not validated. The questionnaire includes 4 questions that ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and if the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
General examination: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the female sexual function index (FSFI) compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and non-functioning.
- Normal PFMs are those that can voluntarily and involuntary contract and relax.19,20
- Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
- Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
- Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the examination, the physician should ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated. Lukban et al has described a zero
Effective treatment includes multiple options
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm. Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al26 found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain. Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy.
Zestra, for instance, which is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn is a non-hormonal cream containing cutaneous lysate and has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients over 3 months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil is a prostaglandin E1 analogue that increases genital vasodilation when applied topically and is currently undergoing investigational trials.31,32 Patients can also choose from many over-the-counter lubricants that contain water-based, oil-based, or silicone-based ingredients.
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for female sexual dysfunction that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger point identification and alleviation.
One pilot study, which involved transvaginal Thiele massage twice a week for 5 weeks on 21 symptomatic women with IC and high-tone pelvic floor dysfunction found it decreased hyptertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al36 investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in 6 out of 14 patients (43%).
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of kegal trainers and other insertion devices that may improve PFM coordination and strength.
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genito-pelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used including hormonal and non-hormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene (Osphena) is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Non-hormonal drugs including flibanserin (Addyi), a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events, and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Buproprion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants such as nortriptyline and amitriptyline may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to 3 times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines such as oral clonazepam and intra-vaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al reviewed 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger point injections in 18 women with levator ani muscle spasm with a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women improved, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective 6-month pilot study, 28 patients with pelvic pain who failed conservative treatment received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at Weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients who fail conventional treatments.47,48
Addressing psychological issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive-behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a women’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
CORRESPONDENCE
Melissa L. Dawson, DO, MS, Department of OB/GYN, Drexel University College of Medicine, 207 N Broad St. 4th Floor, Philadelphia, PA 19107; [email protected].
The care of women with female sexual disorders has made great strides since Masters and Johnson first began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease defined the classification system for female sexual dysfunction, which was eventually published and officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychological components that require a detailed screening, history, and physical examination. Our goal in this review is to provide family physicians with insights and practical advice to help screen, diagnose, and treat female sexual dysfunction, which can have a profound impact on patients’ most intimate relationships.
Understanding the types of female sexual dysfunction
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only 3 female dysfunctions as opposed to 5 in DSM-IV.
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than 6 months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the 6-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychological factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the TABLE.9-11

Evaluating the patient
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation in Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists,9 the BSSC-W is not validated. The questionnaire includes 4 questions that ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and if the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
General examination: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the female sexual function index (FSFI) compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and non-functioning.
- Normal PFMs are those that can voluntarily and involuntary contract and relax.19,20
- Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
- Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
- Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the examination, the physician should ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated. Lukban et al has described a zero
Effective treatment includes multiple options
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm. Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al26 found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain. Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy.
Zestra, for instance, which is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn is a non-hormonal cream containing cutaneous lysate and has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients over 3 months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil is a prostaglandin E1 analogue that increases genital vasodilation when applied topically and is currently undergoing investigational trials.31,32 Patients can also choose from many over-the-counter lubricants that contain water-based, oil-based, or silicone-based ingredients.
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for female sexual dysfunction that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger point identification and alleviation.
One pilot study, which involved transvaginal Thiele massage twice a week for 5 weeks on 21 symptomatic women with IC and high-tone pelvic floor dysfunction found it decreased hyptertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al36 investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in 6 out of 14 patients (43%).
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of kegal trainers and other insertion devices that may improve PFM coordination and strength.
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genito-pelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used including hormonal and non-hormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene (Osphena) is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Non-hormonal drugs including flibanserin (Addyi), a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events, and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Buproprion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants such as nortriptyline and amitriptyline may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to 3 times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines such as oral clonazepam and intra-vaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al reviewed 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger point injections in 18 women with levator ani muscle spasm with a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women improved, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective 6-month pilot study, 28 patients with pelvic pain who failed conservative treatment received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at Weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients who fail conventional treatments.47,48
Addressing psychological issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive-behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a women’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
CORRESPONDENCE
Melissa L. Dawson, DO, MS, Department of OB/GYN, Drexel University College of Medicine, 207 N Broad St. 4th Floor, Philadelphia, PA 19107; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren, JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al., Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior, Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual Dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5thed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009. 113:1124-1136.
9. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 2):337-348.
13. Kegel, A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The Role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual behavior in the human female. W. B. Saunders:Philadelphia, PA; 1998.
16. Lowenstein L, Gruenwald, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(Suppl):22.
27. Farrell J, Cacchioni T, The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014:11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:Cd007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(Suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014; 21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010:21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006.108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren, JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al., Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior, Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual Dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5thed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009. 113:1124-1136.
9. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 2):337-348.
13. Kegel, A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The Role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual behavior in the human female. W. B. Saunders:Philadelphia, PA; 1998.
16. Lowenstein L, Gruenwald, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(Suppl):22.
27. Farrell J, Cacchioni T, The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014:11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:Cd007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(Suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014; 21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010:21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006.108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
PRACTICE RECOMMENDATIONS
› Obtain a detailed history and evaluate obstetric, gynecologic, sexually transmitted disease, sexual abuse, urinary and bowel complaint, and surgical history in women of all ages. B
› Consider a variety of lifestyle and pharmacologic approaches, as well as biofeedback in combination with pelvic floor physical therapy, to address your female patient’s sexual dysfunction. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vaginal dilation: When it’s indicated and tips on teaching it
Vaginal dilators are used to restore vaginal capacity, to expand the vagina in width and depth, to provide elasticity to the tissues, and to allow for comfortable sexual activity. Vaginal dilators are smooth plastic, rubber, or glass cylinder-shaped objects that come in a variety of graduated sizes and weights.
Several medical conditions may warrant the use of vaginal dilation, including superficial dyspareunia, high-tone pelvic floor dysfunction, vaginismus, provoked vestibulodynia, vaginal atrophy, vulvar dermatoses, vaginal agenesis, and postradiation adhesions. Dilation also can be used as deconditioning therapy for psychogenic dyspareunia.1-4 In addition, Masters and Johnson advocated the use of dilators for patients with female sexual dysfunction in order to interrupt the cycle of pain–fear–muscle spasm–more pain, and to build confidence “in the privacy of the marital bedroom.”5
Vaginal dilators often are sufficient to restore function, with dilator therapy considered successful if a woman is able to resume comfortable sexual intercourse or self-stimulation, as desired.1,6 Vaginal dilation also can be used as an adjunct to pelvic floor muscle physical therapy, psychotherapy, sex therapy, minimally absorbed local vaginal estrogen therapy, intravaginal muscle relaxants, lubricants, moisturizers, and vibrators.
Each patient in these case studies achieved success resuming sexual activity after several months of dilator therapy used in combination with other medical interventions.
CASE 1: Chronic vulvovaginal infection and pain
A 26-year-old G0P0 woman presented with a 2-year history of prohibitive penetrative dyspareunia. She had a history of chronic vulvovaginal candidiasis, treated by another clinician with multiple courses of intravaginal antifungal cream.
After extensive evaluation for sexual pain, a diagnosis of pelvic floor muscle spasm, sexual aversion, fear secondary to pain, and contact irritant dermatitis was reached. After vaginal fungal cultures indicated negative results, a size small dilator was introduced in the office using a hypoallergenic intravaginal moisturizer. After daily use of the vaginal dilator for 4 months, with progressed introduction of graduated sizes (small, medium, medium+, large), she was able to accommodate intravaginal intercourse with her partner.
CASE 2: Interstitial cystitis and fear of pain
A 58-year-old G3P3 postmenopausal woman presented with interstitial cystitis (IC), pelvic floor muscle hypertonus, vulvovaginal atrophy, and provoked vestibulodynia. Although her IC symptoms were well-controlled, she was fearful about reestablishing physical intimacy with her partner after 7 years of abstinence.
A program of intravaginal estrogen (Vagifem) 2 to 3 times per week, introital cutaneous lysate (Neogyn) vulvar soothing cream twice per day, and compounded muscle-relaxing intravaginal diazepam suppositories 2 to 3 times per week was initiated. After 2 months of treatment, she was taught in the office to use a size extra small vaginal dilator. She was delighted that use did not result in pain. Two months later, she was able to use a size small dilator, and 4 months later, a size medium dilator. At this point, the patient is confident that she can have sexual intercourse.
CASE 3: Lichen sclerosus
A 50-year-old G0P0 premenopausal woman had a history of IC and biopsy-proven lichen sclerosus. The white plaques surrounding her introitus had become so severe in the past year that she was no longer able to tolerate penile penetration without tearing. Nightly use of topical clobetasol cream and introital estrogen cream (Estrace) was recommended. After 30 days, the patient began twice-a-week maintenance with the creams and also began to use vaginal dilators. After success inserting a size extra large dilator following 5 months of dilator use, she was able to resume intercourse without tearing.
CASE 4: Vestibulodynia and vaginismus
A 25-year-old G0P0 woman underwent vestibulectomy for primary provoked vestibulodynia followed by pelvic floor muscle physical therapy for primary vaginismus. Her marriage of 6 years was unconsummated. Two weeks postoperatively, she began using a size small dilator daily and progressed to a size medium plus dilator after 6 weeks. She managed her chronic constipation and pelvic floor muscle hypertonus with daily fiber supplements, stool softeners, and self-transvaginal massage of the pelvic floor muscles. Seven weeks after surgery, she accomplished intercourse with her husband for the first time.
How to teach your patient to use vaginal dilation successfully
Before ordering vaginal dilation for your patient, 1) assess the levator ani muscle group for hypertonus or spasm and 2) choose the size dilator to start therapy that does not cause pain with insertion but enters with some resistance.
When beginning to teach your patient to use a vaginal dilator in the office, a mirror demonstration may be helpful. Be sure to instruct your patient regarding the following elements to help her achieve success with dilation therapy.
Relax and allow for privacy. About 10 to 15 minutes of privacy before vaginal dilation can help with the success of each individual therapy session. Relaxation can be facilitated with activities such as deep breathing, soaking in a warm bath, or using prescribed muscle relaxants 30 to 60 minutes prior to dilation.
Use proper positioning. Instruct the patient to lie on the bed with her knees bent and placed apart. Advise her to place the lubricated dilator in the vagina as far as it can go without causing any pain (FIGURE 1). It may be helpful for her to bear down when first inserting the dilator. An in-and-out motion is not necessary.

FIGURE 1 Inserting the dilator
Tell the patient to lie on the bed with her knees bent and to insert the lubricated dilator into her vagina as far as it will go without causing pain.Be sure to inform her to use a water-based lubricant—not lotion, petroleum jelly, or any non-water-based lubricant.
Dilate daily. The dilator should be used daily and left in place for 5 to 15 minutes. She may experience a small amount of spotting initially, but spotting should abate within 2 weeks of initiating dilator use. Each dilator size should be used for 3 to 4 weeks. When your patient is changing sizes, she should transition to the larger size over several days by dilating for the first few minutes with the smaller dilator, then changing to the larger dilator for the remainder of the time. If she experiences pain or heavy bleeding, she should cease dilation and follow-up with you.
Proper cleaning. Instruct her to wash the dilator with antibacterial soap and water and to dry it thoroughly between uses.
Follow-up. When undergoing vaginal dilation therapy, your patient should be following up with you at regular intervals, usually once a month, to facilitate compliance with the program.
You can purchase dilators for your medical practice and resell them in your office. Patients also can be directed to purchase dilators directly from a manufacturer (such as Syracuse Medical Devices or AmeriMed Direct) or through a number of Internet sites, including Middlesexmd.com6 or Vaginismus.com.7 Both of these Web sites also offer educational materials, including videos and books and a private support forum or blog.

FIGURE 2 Vaginal dilators come in 8 different circumferences
Vaginal dilators range in size from Extra Small at 1/2 in (13 mm) to Large Plus at 1 1/2 in (39 mm). Each is 6 in (15 cm) long, has one rounded end, and is constructed of sterilizable, medical-grade plastic.
Printed with permission of Syracuse Medical Devices, Inc., Syracuse, New York.Most dilators used in the United States are 6 in (15 cm) long and are made from sterilizable, medical-grade, latex-free, rigid plastic with a smooth surface. Some dilators are made of softer material such as silicone, and others have a vibrating inner wand.6 They are available to purchase as single dilators or in sets of 5 to 8 graduated sizes (FIGURE 2). Some sets come with a storage bag and universal handles that lock-on for insertion. Graduated circumference sizes are fairly universal in the United States (TABLE).
Average dilator sizes and circumferences*
| Size | Circumference |
|---|---|
| Extra small | 1/2 in; 13 mm |
| Extra small plus | 11/16 in; 18 mm |
| Small | 7/8 in; 22 mm |
| Small plus | 1 in; 25 mm |
| Medium | 1 1/8 in; 29 mm |
| Medium plus | 1 1/4 in; 32 mm |
| Large | 1 3/8 in; 35 mm |
| Large plus | 1 5/8 in; 38 mm |
| *Based on Syracuse Medical Devices, Inc. product information. | |
Restoring her sexual health: Our goal
Sexual health is a vitally important quality-of-life issue; restoring that health should be our priority. We need to educate our patients on nonprescription methods to promote their vaginal and sexual health, as vaginal dilation therapy can result in the reduction or elimination of dyspareunia.
We want to hear from you! Tell us what you think.
Your age-based guide to comprehensive well-woman care
Robert L. Barbieri, MD (October 2012)
Sexual dysfunction
Barbara S. Levy, MD (Update, September 2012)
New study: ObGyns aren’t fully addressing their patients’ sexual function
(Web News, April 2012)
How to prepare your patient for the many nuances of postpartum sexuality
Roya Rezaee, MD, and Sheryl Kingsberg, PhD (January 2012)
Vaginal dilators are used to restore vaginal capacity, to expand the vagina in width and depth, to provide elasticity to the tissues, and to allow for comfortable sexual activity. Vaginal dilators are smooth plastic, rubber, or glass cylinder-shaped objects that come in a variety of graduated sizes and weights.
Several medical conditions may warrant the use of vaginal dilation, including superficial dyspareunia, high-tone pelvic floor dysfunction, vaginismus, provoked vestibulodynia, vaginal atrophy, vulvar dermatoses, vaginal agenesis, and postradiation adhesions. Dilation also can be used as deconditioning therapy for psychogenic dyspareunia.1-4 In addition, Masters and Johnson advocated the use of dilators for patients with female sexual dysfunction in order to interrupt the cycle of pain–fear–muscle spasm–more pain, and to build confidence “in the privacy of the marital bedroom.”5
Vaginal dilators often are sufficient to restore function, with dilator therapy considered successful if a woman is able to resume comfortable sexual intercourse or self-stimulation, as desired.1,6 Vaginal dilation also can be used as an adjunct to pelvic floor muscle physical therapy, psychotherapy, sex therapy, minimally absorbed local vaginal estrogen therapy, intravaginal muscle relaxants, lubricants, moisturizers, and vibrators.
Each patient in these case studies achieved success resuming sexual activity after several months of dilator therapy used in combination with other medical interventions.
CASE 1: Chronic vulvovaginal infection and pain
A 26-year-old G0P0 woman presented with a 2-year history of prohibitive penetrative dyspareunia. She had a history of chronic vulvovaginal candidiasis, treated by another clinician with multiple courses of intravaginal antifungal cream.
After extensive evaluation for sexual pain, a diagnosis of pelvic floor muscle spasm, sexual aversion, fear secondary to pain, and contact irritant dermatitis was reached. After vaginal fungal cultures indicated negative results, a size small dilator was introduced in the office using a hypoallergenic intravaginal moisturizer. After daily use of the vaginal dilator for 4 months, with progressed introduction of graduated sizes (small, medium, medium+, large), she was able to accommodate intravaginal intercourse with her partner.
CASE 2: Interstitial cystitis and fear of pain
A 58-year-old G3P3 postmenopausal woman presented with interstitial cystitis (IC), pelvic floor muscle hypertonus, vulvovaginal atrophy, and provoked vestibulodynia. Although her IC symptoms were well-controlled, she was fearful about reestablishing physical intimacy with her partner after 7 years of abstinence.
A program of intravaginal estrogen (Vagifem) 2 to 3 times per week, introital cutaneous lysate (Neogyn) vulvar soothing cream twice per day, and compounded muscle-relaxing intravaginal diazepam suppositories 2 to 3 times per week was initiated. After 2 months of treatment, she was taught in the office to use a size extra small vaginal dilator. She was delighted that use did not result in pain. Two months later, she was able to use a size small dilator, and 4 months later, a size medium dilator. At this point, the patient is confident that she can have sexual intercourse.
CASE 3: Lichen sclerosus
A 50-year-old G0P0 premenopausal woman had a history of IC and biopsy-proven lichen sclerosus. The white plaques surrounding her introitus had become so severe in the past year that she was no longer able to tolerate penile penetration without tearing. Nightly use of topical clobetasol cream and introital estrogen cream (Estrace) was recommended. After 30 days, the patient began twice-a-week maintenance with the creams and also began to use vaginal dilators. After success inserting a size extra large dilator following 5 months of dilator use, she was able to resume intercourse without tearing.
CASE 4: Vestibulodynia and vaginismus
A 25-year-old G0P0 woman underwent vestibulectomy for primary provoked vestibulodynia followed by pelvic floor muscle physical therapy for primary vaginismus. Her marriage of 6 years was unconsummated. Two weeks postoperatively, she began using a size small dilator daily and progressed to a size medium plus dilator after 6 weeks. She managed her chronic constipation and pelvic floor muscle hypertonus with daily fiber supplements, stool softeners, and self-transvaginal massage of the pelvic floor muscles. Seven weeks after surgery, she accomplished intercourse with her husband for the first time.
How to teach your patient to use vaginal dilation successfully
Before ordering vaginal dilation for your patient, 1) assess the levator ani muscle group for hypertonus or spasm and 2) choose the size dilator to start therapy that does not cause pain with insertion but enters with some resistance.
When beginning to teach your patient to use a vaginal dilator in the office, a mirror demonstration may be helpful. Be sure to instruct your patient regarding the following elements to help her achieve success with dilation therapy.
Relax and allow for privacy. About 10 to 15 minutes of privacy before vaginal dilation can help with the success of each individual therapy session. Relaxation can be facilitated with activities such as deep breathing, soaking in a warm bath, or using prescribed muscle relaxants 30 to 60 minutes prior to dilation.
Use proper positioning. Instruct the patient to lie on the bed with her knees bent and placed apart. Advise her to place the lubricated dilator in the vagina as far as it can go without causing any pain (FIGURE 1). It may be helpful for her to bear down when first inserting the dilator. An in-and-out motion is not necessary.

FIGURE 1 Inserting the dilator
Tell the patient to lie on the bed with her knees bent and to insert the lubricated dilator into her vagina as far as it will go without causing pain.Be sure to inform her to use a water-based lubricant—not lotion, petroleum jelly, or any non-water-based lubricant.
Dilate daily. The dilator should be used daily and left in place for 5 to 15 minutes. She may experience a small amount of spotting initially, but spotting should abate within 2 weeks of initiating dilator use. Each dilator size should be used for 3 to 4 weeks. When your patient is changing sizes, she should transition to the larger size over several days by dilating for the first few minutes with the smaller dilator, then changing to the larger dilator for the remainder of the time. If she experiences pain or heavy bleeding, she should cease dilation and follow-up with you.
Proper cleaning. Instruct her to wash the dilator with antibacterial soap and water and to dry it thoroughly between uses.
Follow-up. When undergoing vaginal dilation therapy, your patient should be following up with you at regular intervals, usually once a month, to facilitate compliance with the program.
You can purchase dilators for your medical practice and resell them in your office. Patients also can be directed to purchase dilators directly from a manufacturer (such as Syracuse Medical Devices or AmeriMed Direct) or through a number of Internet sites, including Middlesexmd.com6 or Vaginismus.com.7 Both of these Web sites also offer educational materials, including videos and books and a private support forum or blog.

FIGURE 2 Vaginal dilators come in 8 different circumferences
Vaginal dilators range in size from Extra Small at 1/2 in (13 mm) to Large Plus at 1 1/2 in (39 mm). Each is 6 in (15 cm) long, has one rounded end, and is constructed of sterilizable, medical-grade plastic.
Printed with permission of Syracuse Medical Devices, Inc., Syracuse, New York.Most dilators used in the United States are 6 in (15 cm) long and are made from sterilizable, medical-grade, latex-free, rigid plastic with a smooth surface. Some dilators are made of softer material such as silicone, and others have a vibrating inner wand.6 They are available to purchase as single dilators or in sets of 5 to 8 graduated sizes (FIGURE 2). Some sets come with a storage bag and universal handles that lock-on for insertion. Graduated circumference sizes are fairly universal in the United States (TABLE).
Average dilator sizes and circumferences*
| Size | Circumference |
|---|---|
| Extra small | 1/2 in; 13 mm |
| Extra small plus | 11/16 in; 18 mm |
| Small | 7/8 in; 22 mm |
| Small plus | 1 in; 25 mm |
| Medium | 1 1/8 in; 29 mm |
| Medium plus | 1 1/4 in; 32 mm |
| Large | 1 3/8 in; 35 mm |
| Large plus | 1 5/8 in; 38 mm |
| *Based on Syracuse Medical Devices, Inc. product information. | |
Restoring her sexual health: Our goal
Sexual health is a vitally important quality-of-life issue; restoring that health should be our priority. We need to educate our patients on nonprescription methods to promote their vaginal and sexual health, as vaginal dilation therapy can result in the reduction or elimination of dyspareunia.
We want to hear from you! Tell us what you think.
Your age-based guide to comprehensive well-woman care
Robert L. Barbieri, MD (October 2012)
Sexual dysfunction
Barbara S. Levy, MD (Update, September 2012)
New study: ObGyns aren’t fully addressing their patients’ sexual function
(Web News, April 2012)
How to prepare your patient for the many nuances of postpartum sexuality
Roya Rezaee, MD, and Sheryl Kingsberg, PhD (January 2012)
Vaginal dilators are used to restore vaginal capacity, to expand the vagina in width and depth, to provide elasticity to the tissues, and to allow for comfortable sexual activity. Vaginal dilators are smooth plastic, rubber, or glass cylinder-shaped objects that come in a variety of graduated sizes and weights.
Several medical conditions may warrant the use of vaginal dilation, including superficial dyspareunia, high-tone pelvic floor dysfunction, vaginismus, provoked vestibulodynia, vaginal atrophy, vulvar dermatoses, vaginal agenesis, and postradiation adhesions. Dilation also can be used as deconditioning therapy for psychogenic dyspareunia.1-4 In addition, Masters and Johnson advocated the use of dilators for patients with female sexual dysfunction in order to interrupt the cycle of pain–fear–muscle spasm–more pain, and to build confidence “in the privacy of the marital bedroom.”5
Vaginal dilators often are sufficient to restore function, with dilator therapy considered successful if a woman is able to resume comfortable sexual intercourse or self-stimulation, as desired.1,6 Vaginal dilation also can be used as an adjunct to pelvic floor muscle physical therapy, psychotherapy, sex therapy, minimally absorbed local vaginal estrogen therapy, intravaginal muscle relaxants, lubricants, moisturizers, and vibrators.
Each patient in these case studies achieved success resuming sexual activity after several months of dilator therapy used in combination with other medical interventions.
CASE 1: Chronic vulvovaginal infection and pain
A 26-year-old G0P0 woman presented with a 2-year history of prohibitive penetrative dyspareunia. She had a history of chronic vulvovaginal candidiasis, treated by another clinician with multiple courses of intravaginal antifungal cream.
After extensive evaluation for sexual pain, a diagnosis of pelvic floor muscle spasm, sexual aversion, fear secondary to pain, and contact irritant dermatitis was reached. After vaginal fungal cultures indicated negative results, a size small dilator was introduced in the office using a hypoallergenic intravaginal moisturizer. After daily use of the vaginal dilator for 4 months, with progressed introduction of graduated sizes (small, medium, medium+, large), she was able to accommodate intravaginal intercourse with her partner.
CASE 2: Interstitial cystitis and fear of pain
A 58-year-old G3P3 postmenopausal woman presented with interstitial cystitis (IC), pelvic floor muscle hypertonus, vulvovaginal atrophy, and provoked vestibulodynia. Although her IC symptoms were well-controlled, she was fearful about reestablishing physical intimacy with her partner after 7 years of abstinence.
A program of intravaginal estrogen (Vagifem) 2 to 3 times per week, introital cutaneous lysate (Neogyn) vulvar soothing cream twice per day, and compounded muscle-relaxing intravaginal diazepam suppositories 2 to 3 times per week was initiated. After 2 months of treatment, she was taught in the office to use a size extra small vaginal dilator. She was delighted that use did not result in pain. Two months later, she was able to use a size small dilator, and 4 months later, a size medium dilator. At this point, the patient is confident that she can have sexual intercourse.
CASE 3: Lichen sclerosus
A 50-year-old G0P0 premenopausal woman had a history of IC and biopsy-proven lichen sclerosus. The white plaques surrounding her introitus had become so severe in the past year that she was no longer able to tolerate penile penetration without tearing. Nightly use of topical clobetasol cream and introital estrogen cream (Estrace) was recommended. After 30 days, the patient began twice-a-week maintenance with the creams and also began to use vaginal dilators. After success inserting a size extra large dilator following 5 months of dilator use, she was able to resume intercourse without tearing.
CASE 4: Vestibulodynia and vaginismus
A 25-year-old G0P0 woman underwent vestibulectomy for primary provoked vestibulodynia followed by pelvic floor muscle physical therapy for primary vaginismus. Her marriage of 6 years was unconsummated. Two weeks postoperatively, she began using a size small dilator daily and progressed to a size medium plus dilator after 6 weeks. She managed her chronic constipation and pelvic floor muscle hypertonus with daily fiber supplements, stool softeners, and self-transvaginal massage of the pelvic floor muscles. Seven weeks after surgery, she accomplished intercourse with her husband for the first time.
How to teach your patient to use vaginal dilation successfully
Before ordering vaginal dilation for your patient, 1) assess the levator ani muscle group for hypertonus or spasm and 2) choose the size dilator to start therapy that does not cause pain with insertion but enters with some resistance.
When beginning to teach your patient to use a vaginal dilator in the office, a mirror demonstration may be helpful. Be sure to instruct your patient regarding the following elements to help her achieve success with dilation therapy.
Relax and allow for privacy. About 10 to 15 minutes of privacy before vaginal dilation can help with the success of each individual therapy session. Relaxation can be facilitated with activities such as deep breathing, soaking in a warm bath, or using prescribed muscle relaxants 30 to 60 minutes prior to dilation.
Use proper positioning. Instruct the patient to lie on the bed with her knees bent and placed apart. Advise her to place the lubricated dilator in the vagina as far as it can go without causing any pain (FIGURE 1). It may be helpful for her to bear down when first inserting the dilator. An in-and-out motion is not necessary.

FIGURE 1 Inserting the dilator
Tell the patient to lie on the bed with her knees bent and to insert the lubricated dilator into her vagina as far as it will go without causing pain.Be sure to inform her to use a water-based lubricant—not lotion, petroleum jelly, or any non-water-based lubricant.
Dilate daily. The dilator should be used daily and left in place for 5 to 15 minutes. She may experience a small amount of spotting initially, but spotting should abate within 2 weeks of initiating dilator use. Each dilator size should be used for 3 to 4 weeks. When your patient is changing sizes, she should transition to the larger size over several days by dilating for the first few minutes with the smaller dilator, then changing to the larger dilator for the remainder of the time. If she experiences pain or heavy bleeding, she should cease dilation and follow-up with you.
Proper cleaning. Instruct her to wash the dilator with antibacterial soap and water and to dry it thoroughly between uses.
Follow-up. When undergoing vaginal dilation therapy, your patient should be following up with you at regular intervals, usually once a month, to facilitate compliance with the program.
You can purchase dilators for your medical practice and resell them in your office. Patients also can be directed to purchase dilators directly from a manufacturer (such as Syracuse Medical Devices or AmeriMed Direct) or through a number of Internet sites, including Middlesexmd.com6 or Vaginismus.com.7 Both of these Web sites also offer educational materials, including videos and books and a private support forum or blog.

FIGURE 2 Vaginal dilators come in 8 different circumferences
Vaginal dilators range in size from Extra Small at 1/2 in (13 mm) to Large Plus at 1 1/2 in (39 mm). Each is 6 in (15 cm) long, has one rounded end, and is constructed of sterilizable, medical-grade plastic.
Printed with permission of Syracuse Medical Devices, Inc., Syracuse, New York.Most dilators used in the United States are 6 in (15 cm) long and are made from sterilizable, medical-grade, latex-free, rigid plastic with a smooth surface. Some dilators are made of softer material such as silicone, and others have a vibrating inner wand.6 They are available to purchase as single dilators or in sets of 5 to 8 graduated sizes (FIGURE 2). Some sets come with a storage bag and universal handles that lock-on for insertion. Graduated circumference sizes are fairly universal in the United States (TABLE).
Average dilator sizes and circumferences*
| Size | Circumference |
|---|---|
| Extra small | 1/2 in; 13 mm |
| Extra small plus | 11/16 in; 18 mm |
| Small | 7/8 in; 22 mm |
| Small plus | 1 in; 25 mm |
| Medium | 1 1/8 in; 29 mm |
| Medium plus | 1 1/4 in; 32 mm |
| Large | 1 3/8 in; 35 mm |
| Large plus | 1 5/8 in; 38 mm |
| *Based on Syracuse Medical Devices, Inc. product information. | |
Restoring her sexual health: Our goal
Sexual health is a vitally important quality-of-life issue; restoring that health should be our priority. We need to educate our patients on nonprescription methods to promote their vaginal and sexual health, as vaginal dilation therapy can result in the reduction or elimination of dyspareunia.
We want to hear from you! Tell us what you think.
Your age-based guide to comprehensive well-woman care
Robert L. Barbieri, MD (October 2012)
Sexual dysfunction
Barbara S. Levy, MD (Update, September 2012)
New study: ObGyns aren’t fully addressing their patients’ sexual function
(Web News, April 2012)
How to prepare your patient for the many nuances of postpartum sexuality
Roya Rezaee, MD, and Sheryl Kingsberg, PhD (January 2012)
When to suspect interstitial cystitis
• Suspect interstitial cystitis (IC) in a patient who has had suprapubic pain, pressure, or discomfort and frequency of urination for >3 months in the absence of a urinary tract infection or other pelvic condition with similar symptoms. A
• Mild symptoms of IC can be largely contained with dietary changes, off-label oral agents such as amitriptyline or hydroxyzine, and muscle relaxants to reduce pelvic floor muscle spasm. B
• Use pentosan polysulfate with caution; although the drug is approved for the treatment of IC, recent studies indicate it has little benefit. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jan D, a 27-year-old woman, comes in requesting treatment for pelvic pain and urinary frequency, symptoms she’s had for about 6 months. She describes a feeling of pressure over the suprapubic area that’s relieved by voiding, sensitivity over the vulvar area, and both daytime frequency and nocturia. The patient has a history of allergies and chronic fatigue syndrome (CFS) of 2 years’ duration. When you inquire about her prior medical history, Jan reports that she had frequent urinary tract infections (UTIs) during adolescence.
You order a urinalysis and culture, both of which are negative. If Jan were your patient, what would your next step be?
Interstitial cystitis (IC) is a painful bladder disorder that predominantly affects young and middle-aged women, with an average age of onset of 40 years.1,2 But men can also develop IC, as can women of any age.2 Estimates of prevalence among US women range from less than 1% to more than 6%.2,3 In recent years, however, the number of cases reported has multiplied, the combined result of greater awareness of IC and population surveys based on symptoms rather than on established criteria alone.4
Because the disorder is recognized as a major source of chronic pelvic pain and disability, the term interstitial cystitis/bladder pain syndrome (IC/BPS) is now used by the American Urological Association and many experts to describe it.1,5
Early diagnosis and management of IC/BPS are keys to substantial symptom reduction and improved quality of life. Yet it is often under- or misdiagnosed, both because of the many comorbidities found in patients with the disorder and because its symptoms overlap with those of other common conditions.5
Family physicians are often the first practitioners whom patients with IC/BPS turn to for help. Yet a recent survey of physician practices found significant knowledge gaps with regard to IC/BPS among primary care physicians.6 This evidence-based review is designed to raise awareness of this chronic condition and better prepare you to diagnose and treat it.
IC/BPS: An overview
IC/BPS is characterized by at least 3 to 6 months of pain, pressure, or discomfort over the suprapubic area or the bladder, accompanied by frequency of urination during the day and night in a patient who does not have a UTI.1 There is no known etiology or cure. While evidence suggests that about 90% of those affected are female, some urologists consider chronic bacterial prostatitis to be the male equivalent of IC/BPS, and therefore maintain that the proportion of men with IC/BPS may be considerably higher. 2,3
Chronic pain—the most common symptom—is regional and diffuse over the lower pelvic area, and can be severe. In one study of more than 600 patients with IC/BPS, the most common locations of the pain were the lower abdomen, cited by 80% of those surveyed; the urethral area, cited by 74%; and the low back, by 65%.7 (Dyspareunia is also common, and contributes to the poor quality of life associated with this condition.8)
For about 40% of female patients, the pain and urinary frequency are highest perimenstrually.8 Physical or psychological stress, spicy foods, and smoking can exacerbate symptoms.9,10
Genetics may play a role. Some evidence suggests a genetic predisposition to IC/BPS. In one study, 5 of 8 monozygotic twins of patients with the condition (but 0 of 8 dizygotic twins) were found to have either probable or confirmed IC/BPS. In addition, IC/BPS was 17 times more common in first-degree relatives of patients with the disorder than in the general population.11
There is no established pathogenesis. No infectious organism (bacterial, fungal, or viral) has been identified as a cause for IC/BPS.12 However, an increased number of activated bladder mast cells has been documented in patients with IC/BPS—a possible reason for the pain and some of the histology associated with the condition.9 Inflammation is present to variable degrees and not in all patients.
Some studies suggest that bladder glycosaminoglycans—which form a coating on the luminal surface of the bladder that creates an impermeable, protective barrier—may be compromised in patients with IC/BPS,13 which makes it possible for noxious molecules in the urine to activate sensory nerve endings and lead to chronic pelvic pain.
Overlapping symptoms, comorbidities are common
Symptoms associated with IC/BPS overlap with those of a number of other conditions, including UTIs, sensory urgency, recurrent cystitis, and overactive bladder (OAB), as well as chronic nonbacterial prostatitis in men.4 Comorbidities further complicate the picture.14,15
IC/BPS patients often have a history of allergies,14,15 although they may have negative results on radioallergosorbent (RAST) or skin prick tests, and a number of other comorbidities (TABLE 1). Studies have shown a high correlation between IC/BPS and chronic fatigue syndrome, irritable bowel syndrome, vulvodynia, fibromyalgia, endometriosis, and panic disorder.16-20
TABLE 1
Interstitial cystitis/bladder pain syndrome: Common comorbidities14-20
| Condition | Frequency of comorbidity |
|---|---|
| Allergies | 40%-60% |
| Chronic fatigue syndrome | 35% |
| Endometriosis | 50% |
| Fibromyalgia | 35% |
| Irritable bowel syndrome | 35% |
| Vulvodynia | 20% |
Rule out UTIs and overactive bladder
IC/BPS is largely a diagnosis of exclusion: When a patient presents with suprapubic pain, pressure, or discomfort related to bladder filling and increased urinary frequency lasting for several months, other related conditions—most notably, UTI and OAB—must be ruled out. Often, this can be done with urinalysis and culture, a complete medical history, and symptom assessment. But when doubt remains, a trial of antibiotics (for a UTI) or an anticholinergic agent (for OAB) may be appropriate.
A targeted history and symptom assessment
A history of allergic, gastrointestinal, gynecologic, and/or musculoskeletal disease is often significant.4 In addition, bladder problems in childhood and adolescence are notable, as they are far more common in women with IC/BPS than in the general population.21,22
Identify voiding problems. Question the patient not only about how often she voids, but also about the extent to which the frequency is affecting her life. The severity of the persistent need to void is more significant for an IC/BPS diagnosis than the sudden urge to void for fear of leakage, which is typical of OAB.23
Ask about abuse. Evidence suggests that 50% of women with IC/BPS have been abused, half of them sexually,24 so it is important to include questions about past and present physical, emotional, and sexual abuse in the medical history. Physical or sexual trauma in childhood appears to increase an individual’s lifetime risk for chronic pain syndromes.25
Use these tools to gauge symptoms and severity. Two tools that can aid in diagnosing—or ruling out—IC/BPS are the O’Leary-Sant Symptom and Problem Index, and the Pelvic Pain and Urgency/Frequency (PUF) questionnaire. Both are available at http://www.ichelp.org/Page.aspx?pid=444.
The O’Leary-Sant Index is a measure of urinary and pain symptoms, and of how problematic the symptoms are for the patient.26 The PUF questionnaire also incorporates an assessment of sexual function and the impact of the pain and urinary symptoms,27 but it has not been validated.
The medical work-up
Perform a full gynecologic evaluation of female patients and a rectal examination of men. Include the following laboratory tests in your evaluation: complete blood count (CBC) with differential, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), total immunoglobulin E, and liver and thyroid function; test leuteinizing hormone and follicle-stimulating hormone levels for women, as well. Also include urine culture and sensitivity tests in the work-up.
Referral to a urologist is indicated if microscopic hematuria and pyuria are present or the patient’s symptoms are severe. The urologist may conduct a number of tests, for further evaluation or to confirm an IC/BPS diagnosis. These include:
Digital and manometric pelvic floor muscle examination. Manometry is performed using a vaginal or rectal pressure-sensitive probe that measures the strength of contraction and the ability to relax the pelvic floor muscles. In one study, 87% of women with IC/BPS were found to have levator muscle pain described as “consistent with pelvic floor dysfunction.”28
Kaufman Q-tip touch sensitivity test. This involves touching all 4 quadrants of the vulvar and vestibular Skene’s gland ostia to evaluate for vestibulodynia, using a visual analog scale to document the level of pain and sensitivity the patient is experiencing.
Potassium sensitivity test. The physician instills a high concentration of potassium chloride into the bladder to evaluate how much pain it elicits.27 (Although this test is frequently included in the evaluation of patients suspected of having IC/BPS, its use is controversial because it is unnecessarily painful, while its sensitivity and specificity are low.1)
Cystoscopy after hydrodistension, preferably with isotonic saline or isotonic glycine to avoid hypotonic damage to the urothelium, is performed under anesthesia (FIGURE 1). This is the most common procedure performed on patients with IC/BPS29 because it allows visualization of the urothelial glomerulations, or petechiae, and submucosal hemorrhages, found in most patients with this condition. The test would also reveal the presence of the mucosal lesions (Hunner’s ulcers) found in those with “classic” IC/BPS, which represents 5% to 15% of all cases.4
FIGURE 1
IC/BPS: A cystoscopic image
Visualization of the bladder during cystoscopy after hydrodistension reveals submucosal hemorrhages (black arrow) and glomerulations (white arrow), found in most patients with IC/BPS.
Glomerulations can occur in other bladder conditions, however, and may even be found in normal bladders.30 Thus, glomerulations are not diagnostic of IC/BPS in and of themselves, but this finding is often used to confirm the diagnosis.
CASE After Jan’s initial urinalysis, culture, and sensitivity tests, you follow up with a number of other laboratory tests, including CBC with differential, ESR, CRP, and hormonal and immune indexes. All are within the normal range. You also perform a gynecologic examination and use the O’Leary-Sant Symptom and Problem Index to diagnose IC/BPS, and refer the patient to a urologist for further evaluation. The urologist performs cystoscopy with bladder hydrodistension, which reveals multiple submucosal hemorrhages and glomerulations.
Treatment of IC/BPS is multimodal
In January 2011, the American Urological Association (AUA) approved diagnostic and treatment recommendations for IC/BPS (available at http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic-bps). The AUA recommends starting with the most conservative treatments, such as stress management, patient education, and self-care. Interventions may include dietary modification (eliminating bladder irritants such as caffeine and alcohol), self-guided imagery, meditation, yoga, deep breathing, self-hypnosis, and manual physical therapy to the pelvic floor myofascial trigger points, with oral or intravesical medications and other procedures added, as needed (FIGURE 2). Pain management, the AUA notes, should be considered throughout the course of therapy.31
FIGURE 2
Treatment algorithm for interstitial cystitis/bladder pain syndrome (IC/BPS)
DMSO, dimethylsulfoxide; IBS, irritable bowel syndrome; PPS, pentosan polysulfate; UTI, urinary tract infection.
Sources: American Urological Association31; International Incontinence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494).
The other professional society with treatment recommendations for IC/BPS is the International Continence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494). Because there is no known cure for IC/BPS, the Society focuses on alleviating symptoms and improving the patient’s quality of life. Treatment is highly individual, the Society states, and may consist of diet modification, oral drugs, bladder instillations or injections, and neuromodulation or surgical interventions, as a last resort. Multiple approaches may be used, often together. 29
Both mild discomfort/pain and urinary frequency in newly diagnosed patients may be treated with a number of oral drugs used off label (TABLE 2), as well as with muscle relaxants, such as diazepam. Pentosan polysulfate—the only oral drug with US Food and Drug Administration approval for treatment of IC/BPS—was initially shown to be “modestly beneficial.”32 However, in 2 recent randomized studies, including one multicenter trial funded by the National Institute of Diabetes and Digestive and Kidney Diseases, it was found to be little (or no) better than placebo.33,34 Thus, we recommend that the drug be tried for no more than 4 to 6 months. If there is no benefit or adverse effects such as GI problems or hair loss develop, the drug should be discontinued.
TABLE 2
Frequently used medications for mild to moderate symptoms
| Agent | Usual dose |
|---|---|
| Amitriptyline | 50-75 mg at bedtime* |
| Diazepam | 2-5 mg up to 4 times per day prn |
| Hydroxyzine | 50-75 mg at bedtime* |
| Pentosan polysulfate | 100 mg tid |
| Pain control | |
| Doxepin cream† | Apply 2-3 times per day |
| Gabapentin | 200 mg tid (starting dose) |
| Tramadol | 100 mg bid |
| *Titrated up over 3-4 weeks. †For vulvodynia. | |
The tricyclic antidepressant amitriptyline is often used to relieve symptoms—both urinary frequency and pain/discomfort—that are mild to moderate. In one small clinical trial, amitriptyline (self-titrated up to 100 mg/d for 4 months) produced a 64% response rate. But nearly a third of the patients in the intervention group (and many more on placebo) dropped out due to nonresponse.35 A recent multicenter, randomized, placebo-controlled trial of amitriptyline showed that only patients who took >50 mg daily had a significantly higher response rate (P=.01) compared with the placebo group.36
Amitriptyline may also be combined with hydroxyzine, especially in patients with allergies, but the combination could result in considerable sedation. The antidepressant doxepin, which is both a histamine-1 (H1) and histamine-2 (H2) receptor antagonist (RA), is an alternative that has been shown to reduce chronic neuropathic pain.37 A doxepin cream may also be used locally for vulvodynia.
Although no comparative studies have been conducted, nontricyclic antidepressants do not appear to have the same benefit for IC symptoms. In an open-label study of 48 women with IC/BPS, the antidepressant duloxetine (titrated to 40 mg bid for 5 weeks) showed no significant improvement of symptoms.38
Hydroxyzine is an H1 RA with mild anxiolytic and antiallergy properties. In an open-label study of IC/BPS patients with allergies (n=37), it was found to reduce symptoms by 55%.39
Is there a role for dietary supplements? In developing its new recommendations for IC/BPS, the AUA did not review studies of dietary supplements.31 The Interstitial Cystitis Association (ICA), however, includes information on its Web site about a number of dietary supplements that may be helpful in controlling symptoms (http://www.ichelp.org/Page.aspx?pid=635).
One such product contains aloe vera, which the ICA describes as having anti-inflammatory actions that have been found to reduce IC/BPS symptoms.40 Another is a dietary supplement that the author (TCT) developed, which contains quercetin, a flavonoid with anti-inflammatory properties, as well as chondroitin and hyaluronate—components of the glycosaminoglycan protective layer in the bladder that may be damaged in patients with ICS. In an open label study of 127 patients with refractory symptoms of IC/BPS, this supplement produced a 51% response rate (P<.0001).41
Pain management is particularly challenging
Intense chronic pain is the most difficult aspect of IC/BPS to treat. Tramadol, an opioid with weaker addiction potential and fewer adverse effects than morphine, is often helpful. Gabapentin, an antiseizure drug, and pregabalin, a similar drug recently approved for fibromyalgia, may also be useful. A fentanyl patch, as well as belladonna and opium suppositories, may be used under the care of a pain management specialist.
If these pain regimens fail, urologists often try intravesical approaches, such as bladder hydrodistension under anesthesia, which has been found to provide short-term (up to 5.3 months) symptom relief in 30% to 50% of patients.4,42,43 Intravesical treatments, in which medication is directly instilled into the bladder, are frequently used, especially in patients with severe symptoms.44
Intravesical dimethylsulfoxide (DMSO) may be given once a week for 6 weeks, but instillation often hurts and DMSO causes the patients to smell of garlic, which severely limits compliance. In one randomized double-blind study involving 11 patients with classic IC/BPS (ie, with Hunner’s ulcers) and 10 IC/BPS patients without Hunner’s ulcers, DMSO reduced urinary frequency and pain only in those with classic cases.45
Intravesical hyaluronate sodium, given in weekly instillations for 4 weeks, resulted in some pain reduction in 2 open-label studies,4 but in a subsequent randomized, double-blind, placebo-controlled, multicenter study, instilling 10 times the amount of hyaluronate failed to show any benefit and was terminated by the sponsor (Seikagaku Corp., written correspondence, March 2004). In one small study, intravesical hyaluronate and chondroitin, given weekly for 20 weeks and then monthly for 3 months, led to significant improvement in frequency, urgency, and pain.46
In a multicenter trial, intravesical instillation of lidocaine, together with sodium bicarbonate, led to 30% improvement, compared with the controls.47 (Such intravesical “cocktails” are often supplemented with other agents, such as heparin or hydrocortisone.)
Other options for refractory pain include intravesical laser ablation, fulguration of bladder lesions, intravesical injections of botulinum toxin, and neuromodulation of the sacral or pudendal nerve via an implanted impulse generator.
CASE After the urologist confirmed Jan’s diagnosis, she returned to your office for treatment. Jan was started on dietary modification, hydroxyzine (50 mg at bedtime), and physical therapy. She had a 50% reduction in symptoms after 3 months of therapy.
CORRESPONDENCE
Theoharis C. Theoharides, MD, PhD, FAAAAI, Tufts University School of Medicine, 136 Harrison Avenue, Boston, MA 02111; [email protected]
1. Hanno P, Lin A, Nordling J, et al. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn. 2010;29:191-198.
2. Association of Reproductive Health Professionals. Screening, tratment, and management of IC/PBS. May 2008. Available at: http://www.arhp.org/Publications-and-Resources/Clinical-Proceedings/Screening-Treatment-and-Management-of-ICPBS/Definition. Accessed May 9, 2011.
3. Interstitial Cystitis Association. 4 to 12 million may have IC. http://www.ichelp.org/Page.aspx?pid=917. Posted January 12, 2010. Accessed May 9, 2011.
4. Hanno PM. Painful bladder syndrome. In: Wein AJ, Kavossi LR, Novick AC, et al, eds. Campbell’s Urology. 9th ed. Philadelphia, Pa: Elsevier; 2007:330-370.
5. American Urological Association. First-ever clinical guidance on interstitial cystitis/bladder pain syndrome released [press release]. March 1, 2011. Available at: http://www.auanet.org/content/press/press_releases/article.cfm?articleNo=224. Accessed May 9, 2011.
6. Clemens JQ, Calhoun EA, Litwin MS, et al. A survey of primary care physician practices in the diagnosis and management of women with interstitial cystitis/painful bladder syndrome. Urology. 2010;76:323-328.
7. FitzGerald MP, Brensinger C, Brubaker L, et al. What is the pain of interstitial cystitis like? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:69-72.
8. Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832-1836.
9. Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1-12.
10. Shorter B, Lesser M, Moldwin RM, et al. Effect of comestibles on symptoms of interstitial cystitis. J Urol. 2007;178:145-152.
11. Warren JW, Jackson TL, Langenberg P, et al. Prevalence of interstitial cystitis in first-degree relatives of patients with interstitial cystitis. Urology. 2004;63:17-21.
12. Al-Hadithi HN, Williams H, Hart CA, et al. Absence of bacterial and viral DNA in bladder biopsies from patients with interstitial cystitis/chronic pelvic pain syndrome. J Urol. 2005;174:151-154.
13. Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J Urol. 1991;145:732-735.
14. Erickson DR, Morgan KC, Ordille S, et al. Nonbladder related symptoms in patients with interstitial cystitis. J Urol. 2001;166:557-562.
15. Theoharides TC, Whitmore K, Stanford E, et al. Interstitial cystitis: bladder pain and beyond. Expert Opin Pharmacother. 2008;9:2979-2994.
16. Nickel JC, Tripp DA, Pontari M, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010;184:1358-1363.
17. Novi JM, Jeronis S, Srinivas S, et al. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: a case-control study. J Urol. 2005;174:937-940.
18. Weissman MM, Gross R, Fyer A, et al. Interstitial cystitis and panic disorder: a potential genetic syndrome. Arch Gen Psychiatr. 2004;61:273-279.
19. Wu EQ, Birnbaum H, Mareva M, et al. Interstitial cystitis: cost, treatment and comorbidities in an employed population. Pharmacoeconomics. 2006;24:55-65.
20. Stanford EJ, Koziol J, Feng A. The prevalence of interstitial cystitis, endometriosis, adhesions, and vulvar pain in women with chronic pelvic pain. J Minim Invasive Gynecol. 2005;12:43-49.
21. Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258-262.
22. Rackow BW, Novi JM, Arya LA, Pfeifer SM. Interstitial cystitis is an etiology of chronic pelvic pain in young women. J Pediatr Adolesc Gynecol. 2009;22:181-185.
23. Diggs C, Meyer WA, Langenberg P, et al. Assessing urgency in interstitial cystitis/painful bladder syndrome. Urology. 2007;69:210-214.
24. Peters KM, Kalinowski SE, Carrico DJ, et al. Fact or fiction—is abuse prevalent in patients with interstitial cystitis? Results from a community survey and clinic population. J Urol. 2007;178(3 Pt 1):891-895.
25. Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2009;10:441-447.
26. O’Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49(suppl 5A):S58-S63.
27. Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573-578.
28. Peters KM, Carrico DJ, Kalinowski SE, et al. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70:16-18.
29. Nordling J, Anjum FH, Bade JJ, et al. Primary evaluation of patients suspected of having interstitial cystitis (IC). Eur Urol. 2004;45:662-669.
30. Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol. 1998;160:1663-1667.
31. American Urological Association. Guideline on the diagnosis and treatment of interstitial cystitis/bladder pain syndrome (2011). Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic.bps. Accessed May 10, 2011.
32. Dimitrakov J, Kroenke K, Steers WD, et al. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922-1929.
33. Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810-815.
34. Buffington CA. Re: cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2006;176:838.-
35. van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
36. Foster HE, Jr, Hanno PM, Nickel JC, et al. Effect of amitriptyline on symptoms in treatment naive patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1853-1858.
37. Hameroff SR, Weiss JL, Lerman JC, et al. Doxepin’s effects on chronic pain and depression: a controlled study. J Clin Psychiatry. 1984;45(3 Pt 2):PMID 6321454.-
38. van Ophoven A, Hertle L. The dual serotonin and noradrenaline reuptake inhibitor duloxetine for the treatment of interstitial cystitis: results of an observational study. J Urol. 2007;177:552-555.
39. Theoharides TC, Sant GR. Hydroxyzine therapy for interstitial cystitis. Urology. 1997;49(suppl):S108-S110.
40. Interstitial Cystitis Association. IC supplements. Revised April 11, 2011. Available at: http://www.ichelp.org/Page.aspx?pid=635. Accessed May 9, 2011.
41. Theoharides TC, Kempuraj D, Vakali S, et al. Treatment of refractory interstitial cystitis/painful bladder syndrome with CystoProtek—an oral multi-agent natural supplement. Can J Urol. 2008;15:4410-4414.
42. Phatak S, Foster HE, Jr. The management of interstitial cystitis: an update. Nat Clin Pract Urol. 2006;3:45-53.
43. Erickson DR, Kunselman AR, Bentley CM, et al. Changes in urine markers and symptoms after bladder distension for interstitial cystitis. J Urol. 2007;117:556-560.
44. Dawson TE, Jamison J. Intravesical treatments for painful bladder syndrome/interstitial cystitis. Cochrane Database Syst Rev. 2007;(4):CD006113.-
45. Peeker R, Haghsheno MA, Holmang S, et al. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis:a prospective, randomized double-blind study. J Urol. 2000;164:1912-1916.
46. Cervigni M, Natale F, Nasta L, et al. A combined intravesical therapy with hyaluronic acid and chondroitin for refractory painful bladder syndrome/interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:943-947.
47. Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103:910-918.
• Suspect interstitial cystitis (IC) in a patient who has had suprapubic pain, pressure, or discomfort and frequency of urination for >3 months in the absence of a urinary tract infection or other pelvic condition with similar symptoms. A
• Mild symptoms of IC can be largely contained with dietary changes, off-label oral agents such as amitriptyline or hydroxyzine, and muscle relaxants to reduce pelvic floor muscle spasm. B
• Use pentosan polysulfate with caution; although the drug is approved for the treatment of IC, recent studies indicate it has little benefit. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jan D, a 27-year-old woman, comes in requesting treatment for pelvic pain and urinary frequency, symptoms she’s had for about 6 months. She describes a feeling of pressure over the suprapubic area that’s relieved by voiding, sensitivity over the vulvar area, and both daytime frequency and nocturia. The patient has a history of allergies and chronic fatigue syndrome (CFS) of 2 years’ duration. When you inquire about her prior medical history, Jan reports that she had frequent urinary tract infections (UTIs) during adolescence.
You order a urinalysis and culture, both of which are negative. If Jan were your patient, what would your next step be?
Interstitial cystitis (IC) is a painful bladder disorder that predominantly affects young and middle-aged women, with an average age of onset of 40 years.1,2 But men can also develop IC, as can women of any age.2 Estimates of prevalence among US women range from less than 1% to more than 6%.2,3 In recent years, however, the number of cases reported has multiplied, the combined result of greater awareness of IC and population surveys based on symptoms rather than on established criteria alone.4
Because the disorder is recognized as a major source of chronic pelvic pain and disability, the term interstitial cystitis/bladder pain syndrome (IC/BPS) is now used by the American Urological Association and many experts to describe it.1,5
Early diagnosis and management of IC/BPS are keys to substantial symptom reduction and improved quality of life. Yet it is often under- or misdiagnosed, both because of the many comorbidities found in patients with the disorder and because its symptoms overlap with those of other common conditions.5
Family physicians are often the first practitioners whom patients with IC/BPS turn to for help. Yet a recent survey of physician practices found significant knowledge gaps with regard to IC/BPS among primary care physicians.6 This evidence-based review is designed to raise awareness of this chronic condition and better prepare you to diagnose and treat it.
IC/BPS: An overview
IC/BPS is characterized by at least 3 to 6 months of pain, pressure, or discomfort over the suprapubic area or the bladder, accompanied by frequency of urination during the day and night in a patient who does not have a UTI.1 There is no known etiology or cure. While evidence suggests that about 90% of those affected are female, some urologists consider chronic bacterial prostatitis to be the male equivalent of IC/BPS, and therefore maintain that the proportion of men with IC/BPS may be considerably higher. 2,3
Chronic pain—the most common symptom—is regional and diffuse over the lower pelvic area, and can be severe. In one study of more than 600 patients with IC/BPS, the most common locations of the pain were the lower abdomen, cited by 80% of those surveyed; the urethral area, cited by 74%; and the low back, by 65%.7 (Dyspareunia is also common, and contributes to the poor quality of life associated with this condition.8)
For about 40% of female patients, the pain and urinary frequency are highest perimenstrually.8 Physical or psychological stress, spicy foods, and smoking can exacerbate symptoms.9,10
Genetics may play a role. Some evidence suggests a genetic predisposition to IC/BPS. In one study, 5 of 8 monozygotic twins of patients with the condition (but 0 of 8 dizygotic twins) were found to have either probable or confirmed IC/BPS. In addition, IC/BPS was 17 times more common in first-degree relatives of patients with the disorder than in the general population.11
There is no established pathogenesis. No infectious organism (bacterial, fungal, or viral) has been identified as a cause for IC/BPS.12 However, an increased number of activated bladder mast cells has been documented in patients with IC/BPS—a possible reason for the pain and some of the histology associated with the condition.9 Inflammation is present to variable degrees and not in all patients.
Some studies suggest that bladder glycosaminoglycans—which form a coating on the luminal surface of the bladder that creates an impermeable, protective barrier—may be compromised in patients with IC/BPS,13 which makes it possible for noxious molecules in the urine to activate sensory nerve endings and lead to chronic pelvic pain.
Overlapping symptoms, comorbidities are common
Symptoms associated with IC/BPS overlap with those of a number of other conditions, including UTIs, sensory urgency, recurrent cystitis, and overactive bladder (OAB), as well as chronic nonbacterial prostatitis in men.4 Comorbidities further complicate the picture.14,15
IC/BPS patients often have a history of allergies,14,15 although they may have negative results on radioallergosorbent (RAST) or skin prick tests, and a number of other comorbidities (TABLE 1). Studies have shown a high correlation between IC/BPS and chronic fatigue syndrome, irritable bowel syndrome, vulvodynia, fibromyalgia, endometriosis, and panic disorder.16-20
TABLE 1
Interstitial cystitis/bladder pain syndrome: Common comorbidities14-20
| Condition | Frequency of comorbidity |
|---|---|
| Allergies | 40%-60% |
| Chronic fatigue syndrome | 35% |
| Endometriosis | 50% |
| Fibromyalgia | 35% |
| Irritable bowel syndrome | 35% |
| Vulvodynia | 20% |
Rule out UTIs and overactive bladder
IC/BPS is largely a diagnosis of exclusion: When a patient presents with suprapubic pain, pressure, or discomfort related to bladder filling and increased urinary frequency lasting for several months, other related conditions—most notably, UTI and OAB—must be ruled out. Often, this can be done with urinalysis and culture, a complete medical history, and symptom assessment. But when doubt remains, a trial of antibiotics (for a UTI) or an anticholinergic agent (for OAB) may be appropriate.
A targeted history and symptom assessment
A history of allergic, gastrointestinal, gynecologic, and/or musculoskeletal disease is often significant.4 In addition, bladder problems in childhood and adolescence are notable, as they are far more common in women with IC/BPS than in the general population.21,22
Identify voiding problems. Question the patient not only about how often she voids, but also about the extent to which the frequency is affecting her life. The severity of the persistent need to void is more significant for an IC/BPS diagnosis than the sudden urge to void for fear of leakage, which is typical of OAB.23
Ask about abuse. Evidence suggests that 50% of women with IC/BPS have been abused, half of them sexually,24 so it is important to include questions about past and present physical, emotional, and sexual abuse in the medical history. Physical or sexual trauma in childhood appears to increase an individual’s lifetime risk for chronic pain syndromes.25
Use these tools to gauge symptoms and severity. Two tools that can aid in diagnosing—or ruling out—IC/BPS are the O’Leary-Sant Symptom and Problem Index, and the Pelvic Pain and Urgency/Frequency (PUF) questionnaire. Both are available at http://www.ichelp.org/Page.aspx?pid=444.
The O’Leary-Sant Index is a measure of urinary and pain symptoms, and of how problematic the symptoms are for the patient.26 The PUF questionnaire also incorporates an assessment of sexual function and the impact of the pain and urinary symptoms,27 but it has not been validated.
The medical work-up
Perform a full gynecologic evaluation of female patients and a rectal examination of men. Include the following laboratory tests in your evaluation: complete blood count (CBC) with differential, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), total immunoglobulin E, and liver and thyroid function; test leuteinizing hormone and follicle-stimulating hormone levels for women, as well. Also include urine culture and sensitivity tests in the work-up.
Referral to a urologist is indicated if microscopic hematuria and pyuria are present or the patient’s symptoms are severe. The urologist may conduct a number of tests, for further evaluation or to confirm an IC/BPS diagnosis. These include:
Digital and manometric pelvic floor muscle examination. Manometry is performed using a vaginal or rectal pressure-sensitive probe that measures the strength of contraction and the ability to relax the pelvic floor muscles. In one study, 87% of women with IC/BPS were found to have levator muscle pain described as “consistent with pelvic floor dysfunction.”28
Kaufman Q-tip touch sensitivity test. This involves touching all 4 quadrants of the vulvar and vestibular Skene’s gland ostia to evaluate for vestibulodynia, using a visual analog scale to document the level of pain and sensitivity the patient is experiencing.
Potassium sensitivity test. The physician instills a high concentration of potassium chloride into the bladder to evaluate how much pain it elicits.27 (Although this test is frequently included in the evaluation of patients suspected of having IC/BPS, its use is controversial because it is unnecessarily painful, while its sensitivity and specificity are low.1)
Cystoscopy after hydrodistension, preferably with isotonic saline or isotonic glycine to avoid hypotonic damage to the urothelium, is performed under anesthesia (FIGURE 1). This is the most common procedure performed on patients with IC/BPS29 because it allows visualization of the urothelial glomerulations, or petechiae, and submucosal hemorrhages, found in most patients with this condition. The test would also reveal the presence of the mucosal lesions (Hunner’s ulcers) found in those with “classic” IC/BPS, which represents 5% to 15% of all cases.4
FIGURE 1
IC/BPS: A cystoscopic image
Visualization of the bladder during cystoscopy after hydrodistension reveals submucosal hemorrhages (black arrow) and glomerulations (white arrow), found in most patients with IC/BPS.
Glomerulations can occur in other bladder conditions, however, and may even be found in normal bladders.30 Thus, glomerulations are not diagnostic of IC/BPS in and of themselves, but this finding is often used to confirm the diagnosis.
CASE After Jan’s initial urinalysis, culture, and sensitivity tests, you follow up with a number of other laboratory tests, including CBC with differential, ESR, CRP, and hormonal and immune indexes. All are within the normal range. You also perform a gynecologic examination and use the O’Leary-Sant Symptom and Problem Index to diagnose IC/BPS, and refer the patient to a urologist for further evaluation. The urologist performs cystoscopy with bladder hydrodistension, which reveals multiple submucosal hemorrhages and glomerulations.
Treatment of IC/BPS is multimodal
In January 2011, the American Urological Association (AUA) approved diagnostic and treatment recommendations for IC/BPS (available at http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic-bps). The AUA recommends starting with the most conservative treatments, such as stress management, patient education, and self-care. Interventions may include dietary modification (eliminating bladder irritants such as caffeine and alcohol), self-guided imagery, meditation, yoga, deep breathing, self-hypnosis, and manual physical therapy to the pelvic floor myofascial trigger points, with oral or intravesical medications and other procedures added, as needed (FIGURE 2). Pain management, the AUA notes, should be considered throughout the course of therapy.31
FIGURE 2
Treatment algorithm for interstitial cystitis/bladder pain syndrome (IC/BPS)
DMSO, dimethylsulfoxide; IBS, irritable bowel syndrome; PPS, pentosan polysulfate; UTI, urinary tract infection.
Sources: American Urological Association31; International Incontinence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494).
The other professional society with treatment recommendations for IC/BPS is the International Continence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494). Because there is no known cure for IC/BPS, the Society focuses on alleviating symptoms and improving the patient’s quality of life. Treatment is highly individual, the Society states, and may consist of diet modification, oral drugs, bladder instillations or injections, and neuromodulation or surgical interventions, as a last resort. Multiple approaches may be used, often together. 29
Both mild discomfort/pain and urinary frequency in newly diagnosed patients may be treated with a number of oral drugs used off label (TABLE 2), as well as with muscle relaxants, such as diazepam. Pentosan polysulfate—the only oral drug with US Food and Drug Administration approval for treatment of IC/BPS—was initially shown to be “modestly beneficial.”32 However, in 2 recent randomized studies, including one multicenter trial funded by the National Institute of Diabetes and Digestive and Kidney Diseases, it was found to be little (or no) better than placebo.33,34 Thus, we recommend that the drug be tried for no more than 4 to 6 months. If there is no benefit or adverse effects such as GI problems or hair loss develop, the drug should be discontinued.
TABLE 2
Frequently used medications for mild to moderate symptoms
| Agent | Usual dose |
|---|---|
| Amitriptyline | 50-75 mg at bedtime* |
| Diazepam | 2-5 mg up to 4 times per day prn |
| Hydroxyzine | 50-75 mg at bedtime* |
| Pentosan polysulfate | 100 mg tid |
| Pain control | |
| Doxepin cream† | Apply 2-3 times per day |
| Gabapentin | 200 mg tid (starting dose) |
| Tramadol | 100 mg bid |
| *Titrated up over 3-4 weeks. †For vulvodynia. | |
The tricyclic antidepressant amitriptyline is often used to relieve symptoms—both urinary frequency and pain/discomfort—that are mild to moderate. In one small clinical trial, amitriptyline (self-titrated up to 100 mg/d for 4 months) produced a 64% response rate. But nearly a third of the patients in the intervention group (and many more on placebo) dropped out due to nonresponse.35 A recent multicenter, randomized, placebo-controlled trial of amitriptyline showed that only patients who took >50 mg daily had a significantly higher response rate (P=.01) compared with the placebo group.36
Amitriptyline may also be combined with hydroxyzine, especially in patients with allergies, but the combination could result in considerable sedation. The antidepressant doxepin, which is both a histamine-1 (H1) and histamine-2 (H2) receptor antagonist (RA), is an alternative that has been shown to reduce chronic neuropathic pain.37 A doxepin cream may also be used locally for vulvodynia.
Although no comparative studies have been conducted, nontricyclic antidepressants do not appear to have the same benefit for IC symptoms. In an open-label study of 48 women with IC/BPS, the antidepressant duloxetine (titrated to 40 mg bid for 5 weeks) showed no significant improvement of symptoms.38
Hydroxyzine is an H1 RA with mild anxiolytic and antiallergy properties. In an open-label study of IC/BPS patients with allergies (n=37), it was found to reduce symptoms by 55%.39
Is there a role for dietary supplements? In developing its new recommendations for IC/BPS, the AUA did not review studies of dietary supplements.31 The Interstitial Cystitis Association (ICA), however, includes information on its Web site about a number of dietary supplements that may be helpful in controlling symptoms (http://www.ichelp.org/Page.aspx?pid=635).
One such product contains aloe vera, which the ICA describes as having anti-inflammatory actions that have been found to reduce IC/BPS symptoms.40 Another is a dietary supplement that the author (TCT) developed, which contains quercetin, a flavonoid with anti-inflammatory properties, as well as chondroitin and hyaluronate—components of the glycosaminoglycan protective layer in the bladder that may be damaged in patients with ICS. In an open label study of 127 patients with refractory symptoms of IC/BPS, this supplement produced a 51% response rate (P<.0001).41
Pain management is particularly challenging
Intense chronic pain is the most difficult aspect of IC/BPS to treat. Tramadol, an opioid with weaker addiction potential and fewer adverse effects than morphine, is often helpful. Gabapentin, an antiseizure drug, and pregabalin, a similar drug recently approved for fibromyalgia, may also be useful. A fentanyl patch, as well as belladonna and opium suppositories, may be used under the care of a pain management specialist.
If these pain regimens fail, urologists often try intravesical approaches, such as bladder hydrodistension under anesthesia, which has been found to provide short-term (up to 5.3 months) symptom relief in 30% to 50% of patients.4,42,43 Intravesical treatments, in which medication is directly instilled into the bladder, are frequently used, especially in patients with severe symptoms.44
Intravesical dimethylsulfoxide (DMSO) may be given once a week for 6 weeks, but instillation often hurts and DMSO causes the patients to smell of garlic, which severely limits compliance. In one randomized double-blind study involving 11 patients with classic IC/BPS (ie, with Hunner’s ulcers) and 10 IC/BPS patients without Hunner’s ulcers, DMSO reduced urinary frequency and pain only in those with classic cases.45
Intravesical hyaluronate sodium, given in weekly instillations for 4 weeks, resulted in some pain reduction in 2 open-label studies,4 but in a subsequent randomized, double-blind, placebo-controlled, multicenter study, instilling 10 times the amount of hyaluronate failed to show any benefit and was terminated by the sponsor (Seikagaku Corp., written correspondence, March 2004). In one small study, intravesical hyaluronate and chondroitin, given weekly for 20 weeks and then monthly for 3 months, led to significant improvement in frequency, urgency, and pain.46
In a multicenter trial, intravesical instillation of lidocaine, together with sodium bicarbonate, led to 30% improvement, compared with the controls.47 (Such intravesical “cocktails” are often supplemented with other agents, such as heparin or hydrocortisone.)
Other options for refractory pain include intravesical laser ablation, fulguration of bladder lesions, intravesical injections of botulinum toxin, and neuromodulation of the sacral or pudendal nerve via an implanted impulse generator.
CASE After the urologist confirmed Jan’s diagnosis, she returned to your office for treatment. Jan was started on dietary modification, hydroxyzine (50 mg at bedtime), and physical therapy. She had a 50% reduction in symptoms after 3 months of therapy.
CORRESPONDENCE
Theoharis C. Theoharides, MD, PhD, FAAAAI, Tufts University School of Medicine, 136 Harrison Avenue, Boston, MA 02111; [email protected]
• Suspect interstitial cystitis (IC) in a patient who has had suprapubic pain, pressure, or discomfort and frequency of urination for >3 months in the absence of a urinary tract infection or other pelvic condition with similar symptoms. A
• Mild symptoms of IC can be largely contained with dietary changes, off-label oral agents such as amitriptyline or hydroxyzine, and muscle relaxants to reduce pelvic floor muscle spasm. B
• Use pentosan polysulfate with caution; although the drug is approved for the treatment of IC, recent studies indicate it has little benefit. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jan D, a 27-year-old woman, comes in requesting treatment for pelvic pain and urinary frequency, symptoms she’s had for about 6 months. She describes a feeling of pressure over the suprapubic area that’s relieved by voiding, sensitivity over the vulvar area, and both daytime frequency and nocturia. The patient has a history of allergies and chronic fatigue syndrome (CFS) of 2 years’ duration. When you inquire about her prior medical history, Jan reports that she had frequent urinary tract infections (UTIs) during adolescence.
You order a urinalysis and culture, both of which are negative. If Jan were your patient, what would your next step be?
Interstitial cystitis (IC) is a painful bladder disorder that predominantly affects young and middle-aged women, with an average age of onset of 40 years.1,2 But men can also develop IC, as can women of any age.2 Estimates of prevalence among US women range from less than 1% to more than 6%.2,3 In recent years, however, the number of cases reported has multiplied, the combined result of greater awareness of IC and population surveys based on symptoms rather than on established criteria alone.4
Because the disorder is recognized as a major source of chronic pelvic pain and disability, the term interstitial cystitis/bladder pain syndrome (IC/BPS) is now used by the American Urological Association and many experts to describe it.1,5
Early diagnosis and management of IC/BPS are keys to substantial symptom reduction and improved quality of life. Yet it is often under- or misdiagnosed, both because of the many comorbidities found in patients with the disorder and because its symptoms overlap with those of other common conditions.5
Family physicians are often the first practitioners whom patients with IC/BPS turn to for help. Yet a recent survey of physician practices found significant knowledge gaps with regard to IC/BPS among primary care physicians.6 This evidence-based review is designed to raise awareness of this chronic condition and better prepare you to diagnose and treat it.
IC/BPS: An overview
IC/BPS is characterized by at least 3 to 6 months of pain, pressure, or discomfort over the suprapubic area or the bladder, accompanied by frequency of urination during the day and night in a patient who does not have a UTI.1 There is no known etiology or cure. While evidence suggests that about 90% of those affected are female, some urologists consider chronic bacterial prostatitis to be the male equivalent of IC/BPS, and therefore maintain that the proportion of men with IC/BPS may be considerably higher. 2,3
Chronic pain—the most common symptom—is regional and diffuse over the lower pelvic area, and can be severe. In one study of more than 600 patients with IC/BPS, the most common locations of the pain were the lower abdomen, cited by 80% of those surveyed; the urethral area, cited by 74%; and the low back, by 65%.7 (Dyspareunia is also common, and contributes to the poor quality of life associated with this condition.8)
For about 40% of female patients, the pain and urinary frequency are highest perimenstrually.8 Physical or psychological stress, spicy foods, and smoking can exacerbate symptoms.9,10
Genetics may play a role. Some evidence suggests a genetic predisposition to IC/BPS. In one study, 5 of 8 monozygotic twins of patients with the condition (but 0 of 8 dizygotic twins) were found to have either probable or confirmed IC/BPS. In addition, IC/BPS was 17 times more common in first-degree relatives of patients with the disorder than in the general population.11
There is no established pathogenesis. No infectious organism (bacterial, fungal, or viral) has been identified as a cause for IC/BPS.12 However, an increased number of activated bladder mast cells has been documented in patients with IC/BPS—a possible reason for the pain and some of the histology associated with the condition.9 Inflammation is present to variable degrees and not in all patients.
Some studies suggest that bladder glycosaminoglycans—which form a coating on the luminal surface of the bladder that creates an impermeable, protective barrier—may be compromised in patients with IC/BPS,13 which makes it possible for noxious molecules in the urine to activate sensory nerve endings and lead to chronic pelvic pain.
Overlapping symptoms, comorbidities are common
Symptoms associated with IC/BPS overlap with those of a number of other conditions, including UTIs, sensory urgency, recurrent cystitis, and overactive bladder (OAB), as well as chronic nonbacterial prostatitis in men.4 Comorbidities further complicate the picture.14,15
IC/BPS patients often have a history of allergies,14,15 although they may have negative results on radioallergosorbent (RAST) or skin prick tests, and a number of other comorbidities (TABLE 1). Studies have shown a high correlation between IC/BPS and chronic fatigue syndrome, irritable bowel syndrome, vulvodynia, fibromyalgia, endometriosis, and panic disorder.16-20
TABLE 1
Interstitial cystitis/bladder pain syndrome: Common comorbidities14-20
| Condition | Frequency of comorbidity |
|---|---|
| Allergies | 40%-60% |
| Chronic fatigue syndrome | 35% |
| Endometriosis | 50% |
| Fibromyalgia | 35% |
| Irritable bowel syndrome | 35% |
| Vulvodynia | 20% |
Rule out UTIs and overactive bladder
IC/BPS is largely a diagnosis of exclusion: When a patient presents with suprapubic pain, pressure, or discomfort related to bladder filling and increased urinary frequency lasting for several months, other related conditions—most notably, UTI and OAB—must be ruled out. Often, this can be done with urinalysis and culture, a complete medical history, and symptom assessment. But when doubt remains, a trial of antibiotics (for a UTI) or an anticholinergic agent (for OAB) may be appropriate.
A targeted history and symptom assessment
A history of allergic, gastrointestinal, gynecologic, and/or musculoskeletal disease is often significant.4 In addition, bladder problems in childhood and adolescence are notable, as they are far more common in women with IC/BPS than in the general population.21,22
Identify voiding problems. Question the patient not only about how often she voids, but also about the extent to which the frequency is affecting her life. The severity of the persistent need to void is more significant for an IC/BPS diagnosis than the sudden urge to void for fear of leakage, which is typical of OAB.23
Ask about abuse. Evidence suggests that 50% of women with IC/BPS have been abused, half of them sexually,24 so it is important to include questions about past and present physical, emotional, and sexual abuse in the medical history. Physical or sexual trauma in childhood appears to increase an individual’s lifetime risk for chronic pain syndromes.25
Use these tools to gauge symptoms and severity. Two tools that can aid in diagnosing—or ruling out—IC/BPS are the O’Leary-Sant Symptom and Problem Index, and the Pelvic Pain and Urgency/Frequency (PUF) questionnaire. Both are available at http://www.ichelp.org/Page.aspx?pid=444.
The O’Leary-Sant Index is a measure of urinary and pain symptoms, and of how problematic the symptoms are for the patient.26 The PUF questionnaire also incorporates an assessment of sexual function and the impact of the pain and urinary symptoms,27 but it has not been validated.
The medical work-up
Perform a full gynecologic evaluation of female patients and a rectal examination of men. Include the following laboratory tests in your evaluation: complete blood count (CBC) with differential, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), total immunoglobulin E, and liver and thyroid function; test leuteinizing hormone and follicle-stimulating hormone levels for women, as well. Also include urine culture and sensitivity tests in the work-up.
Referral to a urologist is indicated if microscopic hematuria and pyuria are present or the patient’s symptoms are severe. The urologist may conduct a number of tests, for further evaluation or to confirm an IC/BPS diagnosis. These include:
Digital and manometric pelvic floor muscle examination. Manometry is performed using a vaginal or rectal pressure-sensitive probe that measures the strength of contraction and the ability to relax the pelvic floor muscles. In one study, 87% of women with IC/BPS were found to have levator muscle pain described as “consistent with pelvic floor dysfunction.”28
Kaufman Q-tip touch sensitivity test. This involves touching all 4 quadrants of the vulvar and vestibular Skene’s gland ostia to evaluate for vestibulodynia, using a visual analog scale to document the level of pain and sensitivity the patient is experiencing.
Potassium sensitivity test. The physician instills a high concentration of potassium chloride into the bladder to evaluate how much pain it elicits.27 (Although this test is frequently included in the evaluation of patients suspected of having IC/BPS, its use is controversial because it is unnecessarily painful, while its sensitivity and specificity are low.1)
Cystoscopy after hydrodistension, preferably with isotonic saline or isotonic glycine to avoid hypotonic damage to the urothelium, is performed under anesthesia (FIGURE 1). This is the most common procedure performed on patients with IC/BPS29 because it allows visualization of the urothelial glomerulations, or petechiae, and submucosal hemorrhages, found in most patients with this condition. The test would also reveal the presence of the mucosal lesions (Hunner’s ulcers) found in those with “classic” IC/BPS, which represents 5% to 15% of all cases.4
FIGURE 1
IC/BPS: A cystoscopic image
Visualization of the bladder during cystoscopy after hydrodistension reveals submucosal hemorrhages (black arrow) and glomerulations (white arrow), found in most patients with IC/BPS.
Glomerulations can occur in other bladder conditions, however, and may even be found in normal bladders.30 Thus, glomerulations are not diagnostic of IC/BPS in and of themselves, but this finding is often used to confirm the diagnosis.
CASE After Jan’s initial urinalysis, culture, and sensitivity tests, you follow up with a number of other laboratory tests, including CBC with differential, ESR, CRP, and hormonal and immune indexes. All are within the normal range. You also perform a gynecologic examination and use the O’Leary-Sant Symptom and Problem Index to diagnose IC/BPS, and refer the patient to a urologist for further evaluation. The urologist performs cystoscopy with bladder hydrodistension, which reveals multiple submucosal hemorrhages and glomerulations.
Treatment of IC/BPS is multimodal
In January 2011, the American Urological Association (AUA) approved diagnostic and treatment recommendations for IC/BPS (available at http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic-bps). The AUA recommends starting with the most conservative treatments, such as stress management, patient education, and self-care. Interventions may include dietary modification (eliminating bladder irritants such as caffeine and alcohol), self-guided imagery, meditation, yoga, deep breathing, self-hypnosis, and manual physical therapy to the pelvic floor myofascial trigger points, with oral or intravesical medications and other procedures added, as needed (FIGURE 2). Pain management, the AUA notes, should be considered throughout the course of therapy.31
FIGURE 2
Treatment algorithm for interstitial cystitis/bladder pain syndrome (IC/BPS)
DMSO, dimethylsulfoxide; IBS, irritable bowel syndrome; PPS, pentosan polysulfate; UTI, urinary tract infection.
Sources: American Urological Association31; International Incontinence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494).
The other professional society with treatment recommendations for IC/BPS is the International Continence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494). Because there is no known cure for IC/BPS, the Society focuses on alleviating symptoms and improving the patient’s quality of life. Treatment is highly individual, the Society states, and may consist of diet modification, oral drugs, bladder instillations or injections, and neuromodulation or surgical interventions, as a last resort. Multiple approaches may be used, often together. 29
Both mild discomfort/pain and urinary frequency in newly diagnosed patients may be treated with a number of oral drugs used off label (TABLE 2), as well as with muscle relaxants, such as diazepam. Pentosan polysulfate—the only oral drug with US Food and Drug Administration approval for treatment of IC/BPS—was initially shown to be “modestly beneficial.”32 However, in 2 recent randomized studies, including one multicenter trial funded by the National Institute of Diabetes and Digestive and Kidney Diseases, it was found to be little (or no) better than placebo.33,34 Thus, we recommend that the drug be tried for no more than 4 to 6 months. If there is no benefit or adverse effects such as GI problems or hair loss develop, the drug should be discontinued.
TABLE 2
Frequently used medications for mild to moderate symptoms
| Agent | Usual dose |
|---|---|
| Amitriptyline | 50-75 mg at bedtime* |
| Diazepam | 2-5 mg up to 4 times per day prn |
| Hydroxyzine | 50-75 mg at bedtime* |
| Pentosan polysulfate | 100 mg tid |
| Pain control | |
| Doxepin cream† | Apply 2-3 times per day |
| Gabapentin | 200 mg tid (starting dose) |
| Tramadol | 100 mg bid |
| *Titrated up over 3-4 weeks. †For vulvodynia. | |
The tricyclic antidepressant amitriptyline is often used to relieve symptoms—both urinary frequency and pain/discomfort—that are mild to moderate. In one small clinical trial, amitriptyline (self-titrated up to 100 mg/d for 4 months) produced a 64% response rate. But nearly a third of the patients in the intervention group (and many more on placebo) dropped out due to nonresponse.35 A recent multicenter, randomized, placebo-controlled trial of amitriptyline showed that only patients who took >50 mg daily had a significantly higher response rate (P=.01) compared with the placebo group.36
Amitriptyline may also be combined with hydroxyzine, especially in patients with allergies, but the combination could result in considerable sedation. The antidepressant doxepin, which is both a histamine-1 (H1) and histamine-2 (H2) receptor antagonist (RA), is an alternative that has been shown to reduce chronic neuropathic pain.37 A doxepin cream may also be used locally for vulvodynia.
Although no comparative studies have been conducted, nontricyclic antidepressants do not appear to have the same benefit for IC symptoms. In an open-label study of 48 women with IC/BPS, the antidepressant duloxetine (titrated to 40 mg bid for 5 weeks) showed no significant improvement of symptoms.38
Hydroxyzine is an H1 RA with mild anxiolytic and antiallergy properties. In an open-label study of IC/BPS patients with allergies (n=37), it was found to reduce symptoms by 55%.39
Is there a role for dietary supplements? In developing its new recommendations for IC/BPS, the AUA did not review studies of dietary supplements.31 The Interstitial Cystitis Association (ICA), however, includes information on its Web site about a number of dietary supplements that may be helpful in controlling symptoms (http://www.ichelp.org/Page.aspx?pid=635).
One such product contains aloe vera, which the ICA describes as having anti-inflammatory actions that have been found to reduce IC/BPS symptoms.40 Another is a dietary supplement that the author (TCT) developed, which contains quercetin, a flavonoid with anti-inflammatory properties, as well as chondroitin and hyaluronate—components of the glycosaminoglycan protective layer in the bladder that may be damaged in patients with ICS. In an open label study of 127 patients with refractory symptoms of IC/BPS, this supplement produced a 51% response rate (P<.0001).41
Pain management is particularly challenging
Intense chronic pain is the most difficult aspect of IC/BPS to treat. Tramadol, an opioid with weaker addiction potential and fewer adverse effects than morphine, is often helpful. Gabapentin, an antiseizure drug, and pregabalin, a similar drug recently approved for fibromyalgia, may also be useful. A fentanyl patch, as well as belladonna and opium suppositories, may be used under the care of a pain management specialist.
If these pain regimens fail, urologists often try intravesical approaches, such as bladder hydrodistension under anesthesia, which has been found to provide short-term (up to 5.3 months) symptom relief in 30% to 50% of patients.4,42,43 Intravesical treatments, in which medication is directly instilled into the bladder, are frequently used, especially in patients with severe symptoms.44
Intravesical dimethylsulfoxide (DMSO) may be given once a week for 6 weeks, but instillation often hurts and DMSO causes the patients to smell of garlic, which severely limits compliance. In one randomized double-blind study involving 11 patients with classic IC/BPS (ie, with Hunner’s ulcers) and 10 IC/BPS patients without Hunner’s ulcers, DMSO reduced urinary frequency and pain only in those with classic cases.45
Intravesical hyaluronate sodium, given in weekly instillations for 4 weeks, resulted in some pain reduction in 2 open-label studies,4 but in a subsequent randomized, double-blind, placebo-controlled, multicenter study, instilling 10 times the amount of hyaluronate failed to show any benefit and was terminated by the sponsor (Seikagaku Corp., written correspondence, March 2004). In one small study, intravesical hyaluronate and chondroitin, given weekly for 20 weeks and then monthly for 3 months, led to significant improvement in frequency, urgency, and pain.46
In a multicenter trial, intravesical instillation of lidocaine, together with sodium bicarbonate, led to 30% improvement, compared with the controls.47 (Such intravesical “cocktails” are often supplemented with other agents, such as heparin or hydrocortisone.)
Other options for refractory pain include intravesical laser ablation, fulguration of bladder lesions, intravesical injections of botulinum toxin, and neuromodulation of the sacral or pudendal nerve via an implanted impulse generator.
CASE After the urologist confirmed Jan’s diagnosis, she returned to your office for treatment. Jan was started on dietary modification, hydroxyzine (50 mg at bedtime), and physical therapy. She had a 50% reduction in symptoms after 3 months of therapy.
CORRESPONDENCE
Theoharis C. Theoharides, MD, PhD, FAAAAI, Tufts University School of Medicine, 136 Harrison Avenue, Boston, MA 02111; [email protected]
1. Hanno P, Lin A, Nordling J, et al. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn. 2010;29:191-198.
2. Association of Reproductive Health Professionals. Screening, tratment, and management of IC/PBS. May 2008. Available at: http://www.arhp.org/Publications-and-Resources/Clinical-Proceedings/Screening-Treatment-and-Management-of-ICPBS/Definition. Accessed May 9, 2011.
3. Interstitial Cystitis Association. 4 to 12 million may have IC. http://www.ichelp.org/Page.aspx?pid=917. Posted January 12, 2010. Accessed May 9, 2011.
4. Hanno PM. Painful bladder syndrome. In: Wein AJ, Kavossi LR, Novick AC, et al, eds. Campbell’s Urology. 9th ed. Philadelphia, Pa: Elsevier; 2007:330-370.
5. American Urological Association. First-ever clinical guidance on interstitial cystitis/bladder pain syndrome released [press release]. March 1, 2011. Available at: http://www.auanet.org/content/press/press_releases/article.cfm?articleNo=224. Accessed May 9, 2011.
6. Clemens JQ, Calhoun EA, Litwin MS, et al. A survey of primary care physician practices in the diagnosis and management of women with interstitial cystitis/painful bladder syndrome. Urology. 2010;76:323-328.
7. FitzGerald MP, Brensinger C, Brubaker L, et al. What is the pain of interstitial cystitis like? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:69-72.
8. Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832-1836.
9. Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1-12.
10. Shorter B, Lesser M, Moldwin RM, et al. Effect of comestibles on symptoms of interstitial cystitis. J Urol. 2007;178:145-152.
11. Warren JW, Jackson TL, Langenberg P, et al. Prevalence of interstitial cystitis in first-degree relatives of patients with interstitial cystitis. Urology. 2004;63:17-21.
12. Al-Hadithi HN, Williams H, Hart CA, et al. Absence of bacterial and viral DNA in bladder biopsies from patients with interstitial cystitis/chronic pelvic pain syndrome. J Urol. 2005;174:151-154.
13. Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J Urol. 1991;145:732-735.
14. Erickson DR, Morgan KC, Ordille S, et al. Nonbladder related symptoms in patients with interstitial cystitis. J Urol. 2001;166:557-562.
15. Theoharides TC, Whitmore K, Stanford E, et al. Interstitial cystitis: bladder pain and beyond. Expert Opin Pharmacother. 2008;9:2979-2994.
16. Nickel JC, Tripp DA, Pontari M, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010;184:1358-1363.
17. Novi JM, Jeronis S, Srinivas S, et al. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: a case-control study. J Urol. 2005;174:937-940.
18. Weissman MM, Gross R, Fyer A, et al. Interstitial cystitis and panic disorder: a potential genetic syndrome. Arch Gen Psychiatr. 2004;61:273-279.
19. Wu EQ, Birnbaum H, Mareva M, et al. Interstitial cystitis: cost, treatment and comorbidities in an employed population. Pharmacoeconomics. 2006;24:55-65.
20. Stanford EJ, Koziol J, Feng A. The prevalence of interstitial cystitis, endometriosis, adhesions, and vulvar pain in women with chronic pelvic pain. J Minim Invasive Gynecol. 2005;12:43-49.
21. Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258-262.
22. Rackow BW, Novi JM, Arya LA, Pfeifer SM. Interstitial cystitis is an etiology of chronic pelvic pain in young women. J Pediatr Adolesc Gynecol. 2009;22:181-185.
23. Diggs C, Meyer WA, Langenberg P, et al. Assessing urgency in interstitial cystitis/painful bladder syndrome. Urology. 2007;69:210-214.
24. Peters KM, Kalinowski SE, Carrico DJ, et al. Fact or fiction—is abuse prevalent in patients with interstitial cystitis? Results from a community survey and clinic population. J Urol. 2007;178(3 Pt 1):891-895.
25. Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2009;10:441-447.
26. O’Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49(suppl 5A):S58-S63.
27. Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573-578.
28. Peters KM, Carrico DJ, Kalinowski SE, et al. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70:16-18.
29. Nordling J, Anjum FH, Bade JJ, et al. Primary evaluation of patients suspected of having interstitial cystitis (IC). Eur Urol. 2004;45:662-669.
30. Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol. 1998;160:1663-1667.
31. American Urological Association. Guideline on the diagnosis and treatment of interstitial cystitis/bladder pain syndrome (2011). Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic.bps. Accessed May 10, 2011.
32. Dimitrakov J, Kroenke K, Steers WD, et al. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922-1929.
33. Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810-815.
34. Buffington CA. Re: cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2006;176:838.-
35. van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
36. Foster HE, Jr, Hanno PM, Nickel JC, et al. Effect of amitriptyline on symptoms in treatment naive patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1853-1858.
37. Hameroff SR, Weiss JL, Lerman JC, et al. Doxepin’s effects on chronic pain and depression: a controlled study. J Clin Psychiatry. 1984;45(3 Pt 2):PMID 6321454.-
38. van Ophoven A, Hertle L. The dual serotonin and noradrenaline reuptake inhibitor duloxetine for the treatment of interstitial cystitis: results of an observational study. J Urol. 2007;177:552-555.
39. Theoharides TC, Sant GR. Hydroxyzine therapy for interstitial cystitis. Urology. 1997;49(suppl):S108-S110.
40. Interstitial Cystitis Association. IC supplements. Revised April 11, 2011. Available at: http://www.ichelp.org/Page.aspx?pid=635. Accessed May 9, 2011.
41. Theoharides TC, Kempuraj D, Vakali S, et al. Treatment of refractory interstitial cystitis/painful bladder syndrome with CystoProtek—an oral multi-agent natural supplement. Can J Urol. 2008;15:4410-4414.
42. Phatak S, Foster HE, Jr. The management of interstitial cystitis: an update. Nat Clin Pract Urol. 2006;3:45-53.
43. Erickson DR, Kunselman AR, Bentley CM, et al. Changes in urine markers and symptoms after bladder distension for interstitial cystitis. J Urol. 2007;117:556-560.
44. Dawson TE, Jamison J. Intravesical treatments for painful bladder syndrome/interstitial cystitis. Cochrane Database Syst Rev. 2007;(4):CD006113.-
45. Peeker R, Haghsheno MA, Holmang S, et al. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis:a prospective, randomized double-blind study. J Urol. 2000;164:1912-1916.
46. Cervigni M, Natale F, Nasta L, et al. A combined intravesical therapy with hyaluronic acid and chondroitin for refractory painful bladder syndrome/interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:943-947.
47. Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103:910-918.
1. Hanno P, Lin A, Nordling J, et al. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn. 2010;29:191-198.
2. Association of Reproductive Health Professionals. Screening, tratment, and management of IC/PBS. May 2008. Available at: http://www.arhp.org/Publications-and-Resources/Clinical-Proceedings/Screening-Treatment-and-Management-of-ICPBS/Definition. Accessed May 9, 2011.
3. Interstitial Cystitis Association. 4 to 12 million may have IC. http://www.ichelp.org/Page.aspx?pid=917. Posted January 12, 2010. Accessed May 9, 2011.
4. Hanno PM. Painful bladder syndrome. In: Wein AJ, Kavossi LR, Novick AC, et al, eds. Campbell’s Urology. 9th ed. Philadelphia, Pa: Elsevier; 2007:330-370.
5. American Urological Association. First-ever clinical guidance on interstitial cystitis/bladder pain syndrome released [press release]. March 1, 2011. Available at: http://www.auanet.org/content/press/press_releases/article.cfm?articleNo=224. Accessed May 9, 2011.
6. Clemens JQ, Calhoun EA, Litwin MS, et al. A survey of primary care physician practices in the diagnosis and management of women with interstitial cystitis/painful bladder syndrome. Urology. 2010;76:323-328.
7. FitzGerald MP, Brensinger C, Brubaker L, et al. What is the pain of interstitial cystitis like? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:69-72.
8. Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832-1836.
9. Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1-12.
10. Shorter B, Lesser M, Moldwin RM, et al. Effect of comestibles on symptoms of interstitial cystitis. J Urol. 2007;178:145-152.
11. Warren JW, Jackson TL, Langenberg P, et al. Prevalence of interstitial cystitis in first-degree relatives of patients with interstitial cystitis. Urology. 2004;63:17-21.
12. Al-Hadithi HN, Williams H, Hart CA, et al. Absence of bacterial and viral DNA in bladder biopsies from patients with interstitial cystitis/chronic pelvic pain syndrome. J Urol. 2005;174:151-154.
13. Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J Urol. 1991;145:732-735.
14. Erickson DR, Morgan KC, Ordille S, et al. Nonbladder related symptoms in patients with interstitial cystitis. J Urol. 2001;166:557-562.
15. Theoharides TC, Whitmore K, Stanford E, et al. Interstitial cystitis: bladder pain and beyond. Expert Opin Pharmacother. 2008;9:2979-2994.
16. Nickel JC, Tripp DA, Pontari M, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010;184:1358-1363.
17. Novi JM, Jeronis S, Srinivas S, et al. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: a case-control study. J Urol. 2005;174:937-940.
18. Weissman MM, Gross R, Fyer A, et al. Interstitial cystitis and panic disorder: a potential genetic syndrome. Arch Gen Psychiatr. 2004;61:273-279.
19. Wu EQ, Birnbaum H, Mareva M, et al. Interstitial cystitis: cost, treatment and comorbidities in an employed population. Pharmacoeconomics. 2006;24:55-65.
20. Stanford EJ, Koziol J, Feng A. The prevalence of interstitial cystitis, endometriosis, adhesions, and vulvar pain in women with chronic pelvic pain. J Minim Invasive Gynecol. 2005;12:43-49.
21. Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258-262.
22. Rackow BW, Novi JM, Arya LA, Pfeifer SM. Interstitial cystitis is an etiology of chronic pelvic pain in young women. J Pediatr Adolesc Gynecol. 2009;22:181-185.
23. Diggs C, Meyer WA, Langenberg P, et al. Assessing urgency in interstitial cystitis/painful bladder syndrome. Urology. 2007;69:210-214.
24. Peters KM, Kalinowski SE, Carrico DJ, et al. Fact or fiction—is abuse prevalent in patients with interstitial cystitis? Results from a community survey and clinic population. J Urol. 2007;178(3 Pt 1):891-895.
25. Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2009;10:441-447.
26. O’Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49(suppl 5A):S58-S63.
27. Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573-578.
28. Peters KM, Carrico DJ, Kalinowski SE, et al. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70:16-18.
29. Nordling J, Anjum FH, Bade JJ, et al. Primary evaluation of patients suspected of having interstitial cystitis (IC). Eur Urol. 2004;45:662-669.
30. Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol. 1998;160:1663-1667.
31. American Urological Association. Guideline on the diagnosis and treatment of interstitial cystitis/bladder pain syndrome (2011). Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic.bps. Accessed May 10, 2011.
32. Dimitrakov J, Kroenke K, Steers WD, et al. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922-1929.
33. Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810-815.
34. Buffington CA. Re: cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2006;176:838.-
35. van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
36. Foster HE, Jr, Hanno PM, Nickel JC, et al. Effect of amitriptyline on symptoms in treatment naive patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1853-1858.
37. Hameroff SR, Weiss JL, Lerman JC, et al. Doxepin’s effects on chronic pain and depression: a controlled study. J Clin Psychiatry. 1984;45(3 Pt 2):PMID 6321454.-
38. van Ophoven A, Hertle L. The dual serotonin and noradrenaline reuptake inhibitor duloxetine for the treatment of interstitial cystitis: results of an observational study. J Urol. 2007;177:552-555.
39. Theoharides TC, Sant GR. Hydroxyzine therapy for interstitial cystitis. Urology. 1997;49(suppl):S108-S110.
40. Interstitial Cystitis Association. IC supplements. Revised April 11, 2011. Available at: http://www.ichelp.org/Page.aspx?pid=635. Accessed May 9, 2011.
41. Theoharides TC, Kempuraj D, Vakali S, et al. Treatment of refractory interstitial cystitis/painful bladder syndrome with CystoProtek—an oral multi-agent natural supplement. Can J Urol. 2008;15:4410-4414.
42. Phatak S, Foster HE, Jr. The management of interstitial cystitis: an update. Nat Clin Pract Urol. 2006;3:45-53.
43. Erickson DR, Kunselman AR, Bentley CM, et al. Changes in urine markers and symptoms after bladder distension for interstitial cystitis. J Urol. 2007;117:556-560.
44. Dawson TE, Jamison J. Intravesical treatments for painful bladder syndrome/interstitial cystitis. Cochrane Database Syst Rev. 2007;(4):CD006113.-
45. Peeker R, Haghsheno MA, Holmang S, et al. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis:a prospective, randomized double-blind study. J Urol. 2000;164:1912-1916.
46. Cervigni M, Natale F, Nasta L, et al. A combined intravesical therapy with hyaluronic acid and chondroitin for refractory painful bladder syndrome/interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:943-947.
47. Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103:910-918.