User login
Improving your approach to nasal obstruction
Nasal obstruction is one of the most common reasons that patients visit their primary care providers.1,2 Often described by patients as nasal congestion or the inability to adequately breathe out of one or both nostrils during the day and/or night, nasal obstruction commonly interferes with a patient’s ability to eat, sleep, and function, thereby significantly impacting quality of life. Overlapping presentations can make discerning the exact cause of nasal obstruction difficult.
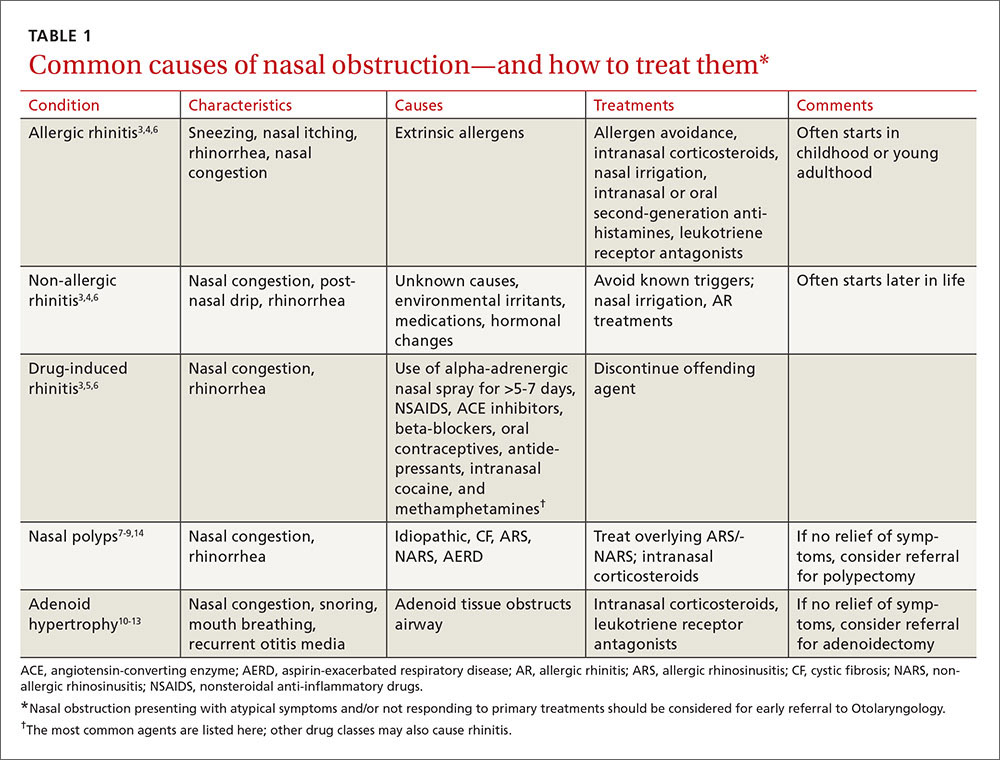
To improve diagnosis and treatment, we review here the evidence-based recommendations for the most common causes of nasal obstruction: rhinitis, rhinosinusitis (RS), drug-induced nasal obstruction, and mechanical/structural abnormalities (TABLE 13-14).
Rhinitis/rhinosinusitis: It all begins with inflammation
Sneezing, rhinorrhea, nasal congestion, and nasal itching are complaints that signal rhinitis, which affects 30 to 60 million people in the United States annually.3 Rhinitis can be allergic, non-allergic, infectious, hormonal, or occupational in nature. All forms of rhinitis share inflammation as the cause of the nasal obstruction. The most common form is allergic rhinitis (AR), which includes seasonal AR and perennial AR. Seasonal AR is typically caused by outdoor allergens and waxes and wanes with pollen seasons. Perennial AR is caused mostly by indoor allergens, such as dust mites, molds, cockroaches, and pet dander; it persists all or most of the year.6 Causes of non-allergic rhinitis (NAR) include environmental irritants such as cigarette smoke, perfume, and car exhaust; medications; and hormonal changes,6 but most causes of NAR are unknown.3,6
While AR can begin at any age, most people develop symptoms in childhood or as young adults, whereas NAR tends to begin later in life. Nasal itching can help to distinguish AR from NAR. NAR symptoms tend to be perennial and include postnasal drainage. If symptoms persist longer than 12 weeks despite treatment, the condition becomes known as chronic rhinosinusitis (CRS).
Treatment of rhinitis: Tiered and often continuous
Treatment of AR and NAR is similar and multitiered beginning with the avoidance of irritants and/or allergens whenever possible, moving on to pharmacotherapy, and, at least for AR, ending with allergen immunotherapy. Treatment is often an ongoing process and typically requires continuous therapy as opposed to treatment on an as-needed basis.3 It is unnecessary to perform allergy testing before making a presumed diagnosis of NAR and starting treatment.6
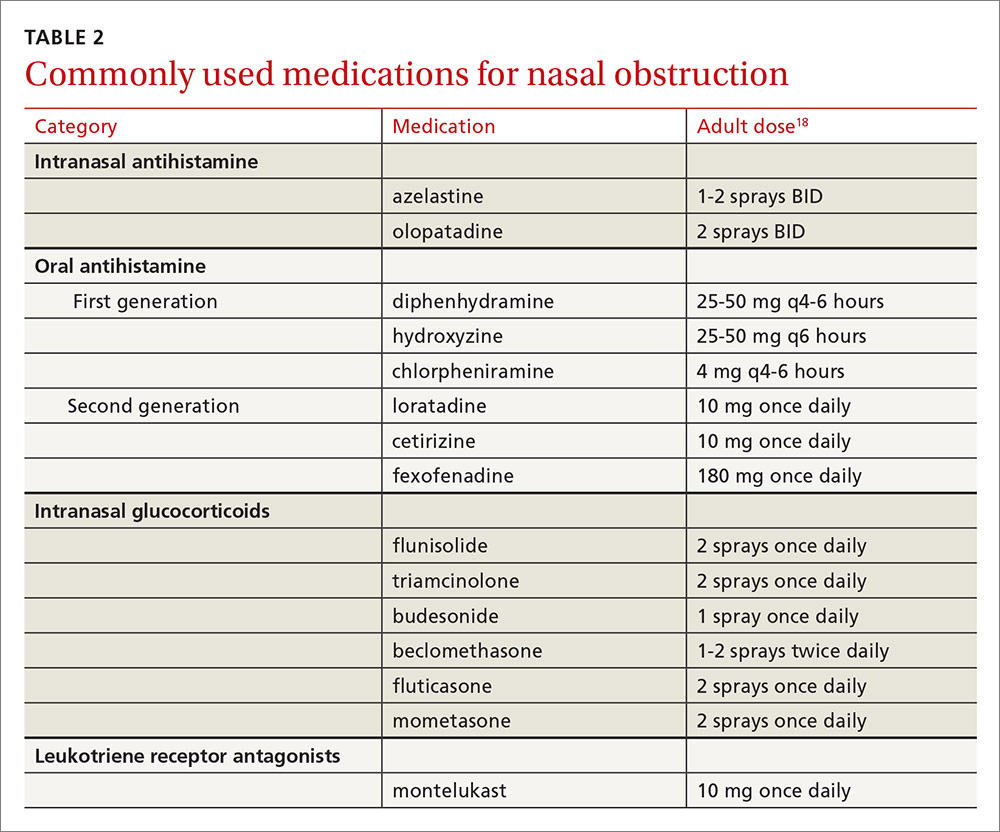
Intranasal corticosteroids. Currently, intranasal glucocorticosteroids (INGCs) are the most effective monotherapy for AR and NAR and have few adverse effects when used at prescribed doses.3,4 For mild to intermittent symptoms, begin with the maximum dosage of an INGC for the patient’s age and proceed with incremental reductions to identify the lowest effective dose.3 If INGCs alone are ineffective, studies have shown that the addition of an intranasal second-generation antihistamine can be of some benefit.3,4 In fact, an INGC and an intranasal antihistamine—along with saline nasal irrigation—is recommended for both AR and NAR resistant to single therapy.3,6,15 If intranasal antihistamines are not an option, oral therapy can be initiated.
Start with second-generation antihistamines and consider LRAs. For oral therapy, start with second-generation antihistamines (loratadine, cetirizine, fexofenadine). First-generation antihistamines (diphenhydramine, hydroxyzine, chlorpheniramine), although widely available at relatively low cost, can cause several significant adverse effects including sedation, impaired cognitive function, and agitation in children.3,4 Because second-generation antihistamines have fewer adverse effects, they are recommended as first-line therapy when oral antihistamine therapy is desired, such as for nasal congestion, sneezing, and itchy, watery eyes.
Of note: A 2014 meta-analysis found that a leukotriene receptor antagonist (LRA) (montelukast) had efficacy similar to oral antihistamines for symptom relief in AR, and that LRAs may be better suited to nighttime symptoms (difficulty falling asleep, nighttime awakenings, congestion on awakening), while antihistamines may provide better relief of daytime symptoms (pruritus, rhinorrhea, sneezing).16 Although further head-to-head, double-blind randomized controlled trials (RCTs) are needed to confirm the results and investigate possible gender differences in symptom response, consider an LRA for first-line therapy in patients with AR who have predominantly nighttime symptoms.
What about pregnant women and the elderly?
It is important to consider teratogenicity when selecting medications for pregnant patients, especially during the first trimester.3 Nasal cromolyn has the most reassuring safety profile in pregnancy. Cetirizine, chlorpheniramine, loratadine, diphenhydramine, and tripelennamine may be used in pregnancy. The US Food and Drug Administration considers them to have a low risk of fetal harm, based on human data, whereas it views many other antihistamines as probably safe, based on limited or no human data. Most INGCs are not expected to cause fetal harm, but limited human data are available. Avoid prescribing oral decongestants to women who are in the first trimester of pregnancy due to the risk of gastroschisis in newborns.17
Elderly patients represent another population for which adverse effects must be carefully considered. Allergies in individuals >65 years of age are uncommon. Rhinitis in this age group is often secondary to cholinergic hyperactivity, alpha-adrenergic hyperactivity, or rhinosinusitis. Given elderly patients’ increased susceptibility to the potential adverse central nervous system (CNS) and anticholinergic effects of antihistamines, non-sedating medications are recommended. Oral decongestants also should be used with caution in this population, not only because of CNS effects, but also because of heart and bladder effects3 (TABLE 218).
For drug-induced rhinitis, stop the offending drug and consider an INGC
Several types of medications, both oral and inhaled, are known to cause rhinitis. The use of alpha-adrenergic decongestant sprays for more than 5 to 7 days can induce rebound congestion on withdrawal, known as rhinitis medicamentosa.3 Repeated use of intranasal cocaine and methamphetamines can also result in rebound congestion. Oral medications that can result in rhinitis or congestion include angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, nonsteroidal anti-inflammatory drugs (NSAIDS), oral contraceptives, and even antidepressants.3
The treatment for drug-induced rhinitis is termination of the offending agent. INGCs can be used to help decrease inflammation and control symptoms once the offending agent is discontinued.
Mechanical/structural causes of obstruction are wide-ranging
Mechanical/structural causes of nasal obstruction range from foreign bodies to anatomical variations including nasal polyps, a deviated septum, adenoidal hypertrophy, foreign bodies, and tumors. Because more than one etiology may be at work, it is best to first treat any non-mechanical causes of obstruction, such as ARS or NARS.
Nasal polyposis often requires both a medical and surgical approach
Nasal polyps are benign growths arising from the mucosa of the nasal sinuses and nasal cavities and affecting up to 4% of the population.7 Their etiology is unclear, but we do know that nasal polyps result from underlying inflammation.7 Uncommon in children outside of those affected by cystic fibrosis,7 nasal polyposis can be associated with disease processes such as AR and sinusitis. Polyps are also associated with clinical syndromes such as aspirin-exacerbated respiratory disease (AERD) syndrome, which involves upper and lower respiratory tract symptoms in patients with asthma who have taken aspirin or other NSAIDs.9
Symptoms vary with the location and size of the polyps, but generally include nasal congestion, alteration in smell, and rhinorrhea. The goals of treatment are to restore or improve nasal breathing and olfaction and prevent recurrence.8 This often requires both a medical and surgical approach.
Topical corticosteroids are effective at reducing both the size of polyps and associated symptoms (rhinorrhea, rhinitis).8 And research has shown that steroids reduce the need for both primary and repeat surgical polypectomies.4 Other treatments to consider prior to surgery (if no symptom reduction occurs with INGCs) include systemic (oral) corticosteroids, intra-polyp steroid injections, macrolide antibiotics, and nasal washes.7,14
When symptoms of polyposis are refractory to medical management, functional endoscopic sinus surgery (FESS) is the surgical procedure of choice.3 In addition to refractory symptoms, indications for FESS include the need to correct anatomic deformities believed to be contributing to the persistence of disease and the need to debulk advanced nasal polyposis.3 The principal goal is to restore patency to the ostiomeatal unit.3
Several studies have reported a high success rate for FESS in improving the symptoms of CRS.3,19-23 In a 1992 study, for example, 98% of patients reported improvement following surgery,19 and in a follow-up report approximately 6 years later, 98% of patients continued to report subjective improvement.22
For septal etiologies, consider septoplasty
Deviation of the nasal septum is a common structural etiology for nasal obstruction arising primarily from congenital, genetic, or traumatic causes.24 Turbulent airflow from the septal deviation often causes turbinate hypertrophy, which creates (or exacerbates) the obstructive symptoms from the septal deviation.25
Septoplasty is the most common ear, nose, and throat operation in adults.26 Reduction of nasal symptoms has been reported in up to 89% of patients who receive this surgery, according to one single-center, non-randomized trial.27 Currently, at least one multicenter, randomized trial is underway that aims to develop evidence-based guidelines for septoplasty.26
Septal perforation is another etiology that can present with nasal obstruction symptoms. Causes include traumatic perforation, inflammatory or collagen vascular diseases, infections, overuse of vasoconstrictive medications, and malignancy.28,29 A careful inspection of the nasal septum is necessary to identify a perforation; this may require nasal endoscopy.
Anterior, rather than posterior, perforations are more likely to cause symptoms of nasal obstruction. Posterior perforations rarely require treatment unless malignancy is suspected, in which case referral for biopsy is recommended. Anterior perforations are treated initially with avoidance of any causative agent if, for example, the problem is drug- or medication-induced, and then with humidification and emollients.28,29
For anterior perforations, septal silicone buttons can be used for recalcitrant symptoms. However, observational studies indicate that for long-term symptom resolution, silicone buttons are effective in only about one-third of patients.29
For patients with persistent symptoms despite the above measures, surgical repair with various flap techniques is an option. A meta-analysis of case studies involving various techniques concluded that there is a wide variety of options, and that surgeons must weigh factors such as the characteristics and etiology of the perforation and their own experience and expertise when choosing from among available methods.30 Additional good quality research is necessary before clear recommendations regarding technique can be made.
Adenoid hypertrophy: Consider corticosteroid nasal drops
Adenoid hypertrophy is a common cause of chronic nasal obstruction in children. Although adenoidectomy is commonly performed to correct the problem, current evidence regarding the efficacy of the procedure is inconclusive.10 Evidence demonstrates corticosteroid nasal drops significantly reduce symptoms of nasal obstruction in children and may provide an effective alternative to surgical resection.18 Studies have also demonstrated that treatment with oral LRAs significantly reduces adenoid size and nasal obstruction symptoms.12,13
Foreign bodies: Don’t forget “a mother’s kiss”
Foreign bodies are the most common cause of nasal obstruction in the pediatric population. There is a paucity of high-quality evidence on removal of these objects; however, a number of retrospective reviews and case series support that most objects can be removed in the office or emergency department without otolaryngologic referral.31,32
Techniques for removal include positive pressure, which is best used for smooth or soft objects. Positive pressure techniques include having the patient blow their own nose or having a parent use a mouth-to-mouth–type blowing technique (ie, the “mother’s kiss” method).32 Refer patients to Otolaryngology if the obstruction involves:31
- objects not easily visualized by anterior rhinoscopy
- chronic or impacted objects
- button batteries or magnets
- penetrating or hooked objects
- any object that cannot be removed during an initial attempt.
Nasal tumors: More common in older men
Nasal tumors occur most often in the nasal cavity itself and are more common in men ≥60 years.33 There is no notable racial predominance.33 Other risk factors include human papillomavirus (HPV) infection, tobacco smoke, and occupational exposure to inhaled wood dust, glues, and adhesives.34-37
Benign tumors occurring in the nasal cavity are a diverse group of disorders, including inverted papillomas, squamous papillomas, pyogenic granulomas, and other less common lesions, all of which typically present with nasal obstruction as a symptom. Many of these lesions cause local tissue destruction or have a high incidence of recurrence. These tumors are treated universally with nasoendoscopic resection.38
Malignant nasal tumors are rare but serious causes of nasal obstruction, making up 3% of all head and neck cancers.39 Most nasal cancers present when they are locally advanced and cause unilateral nasal obstruction, lacrimation, and epistaxis. These symptoms are typically refractory to initial medical management and present as CRS. This diagnosis should be suspected in certain patient groups, such as those who have been exposed to wood dust (eg, construction workers or those who work in wood mills).36
Computed tomography is the gold standard imaging method for CRS; however, if nasal cancer is suspected, referral for biopsy and histopathologic examination is necessary for a final diagnosis.39 Because of the nonspecific nature of their initial presentation, many nasal tumors are at an advanced stage and carry a poor prognosis by the time they are diagnosed.39
CORRESPONDENCE
Margaret A. Bayard, MD, MPH, FAAFP, Naval Hospital Camp Pendleton, 200 Mercy Circle, Camp Pendleton, CA 92005; [email protected].
1. Centers for Disease Control and Prevention. CDC’s FastStats on Ambulatory Care Use and Physician Office Visits. Selected patient and provider characteristics for ambulatory care visits to physician offices and hospital outpatient and emergency departments: United States, 2009-2010. Available at: http://www.cdc.gov/nchs/data/ahcd/combined_tables/AMC_2009-2010_combined_web_table01.pdf. Accessed November 9, 2016.
2. US Census Bureau. Table 158. Visits to office-based physician and hospital outpatient departments, 2006. Available at: http://www2.census.gov/library/publications/2006/compendia/statab/126ed/tables/07s0158.xls. Accessed November 13, 2016.
3. Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103-S115.
4. Wallace DV, Dykewicz MS. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1-S84.
5. Krouse J, Lund V, Fokkens W, et al. Diagnostic strategies in nasal congestion. Int J Gen Med. 2010;3:59-67.
6. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2-S8.
7. Newton JR, Ah-See KW. A review of nasal polyposis. Ther Clin Risk Manag. 2008;4:507-512.
8. Badia L, Lund V. Topical corticosteroids in nasal polyposis. Drugs. 2001;61:573-578.
9. Fahrenholz JM. Natural history and clinical features of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol. 2003;24:113-124.
10. van den Aardweg MT, Schilder AG, Herket E, et al. Adenoidectomy for recurrent or chronic nasal symptoms in children. Cochrane Database Syst Rev. 2010;CD008282.
11. Demirhan H, Aksoy F, Ozturan O, et al. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int J Pediatr Otorhinolaryngol. 2010;74:773-776.
12. Shokouhi F, Jahromi AM, Majidi MR, et al. Montelukast in adenoid hypertrophy: its effect on size and symptoms. Iran J Otorhinolaryngol. 2015;27:433-448.
13. Goldbart AD, Greenberg-Dotan S, Tai A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130:e575-e580.
14. Moss WJ, Kjos KB, Karnezis TT, et al. Intranasal steroid injections and blindness: our personal experience and a review of the past 60 years. Laryngoscope. 2015;125:796-800.
15. Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis. Laryngoscope. 2013;123:53-56.
16. Xu Y, Zhang J, Wang J. The efficacy and safety of selective H1-antihistamine versus leukotriene receptor antagonist for seasonal allergic rhinitis: a meta-analysis. PLoS One. 2014;9:e112815.
17. Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
18. Lexi-Comp Online. Available at: online.lexi.com/crlsql/servlet/crlonline. Accessed November 9, 2016.
19. Kennedy DW. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope.1992;102:1-18.
20. Khalil HS, Nunez DA. Functional endoscopic sinus surgery for chronic rhinosinusitis. Cochrane Database Syst Rev. 2006;CD004458.
21. Chambers DW, Davis WE, Cook PR, et al. Long-term outcome analysis of functional endoscopic sinus surgery: correlation of symptoms with endoscopic examination findings and potential prognostic variables. Laryngoscope. 1997;107:504-510.
22. Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151-157.
23. Jakobsen J, Svendstrup F. Functional endoscopic sinus surgery in chronic sinusitis—a series of 237 consecutively operated patients. Acta Otolaryngol Suppl. 2000;543:158-161.
24. Aziz T, Biron VL, Ansari K, et al. Measurement tools for the diagnosis of nasal septal deviation: a systematic review. J Otolaryngol Head Neck Surg. 2014;43:11.
25. Grutzenmacher S, Robinson DM, Grafe K, et al. First findings concerning airflow in noses with septal deviation and compensatory turbinate hypertrophy—a model study. ORL J Otorhinolaryngol Relat Spec. 2006;68:199-205.
26. van Egmond MMHT, Rovers MM, Hendriks CTM, et al. Effectiveness of septoplasty versus non-surgical management for nasal obstruction due to deviated nasal septum in adults: study protocol for a randomized controlled trial. Trials. 2015;16:500.
27. Gandomi B, Bayat A, Kazemei T. Outcomes of septoplasty in young adults: the Nasal Obstruction Septoplasty Effectiveness study. Am J Otolaryngol. 2010;31:189-192.
28. Døsen L, Have R. Silicone button in nasal septal perforation. Long term observations. Rhinology. 2008;46:324-327.
29. Kridel RW. Considerations in the etiology, treatment and repair of septal perforations. Facial Plast Surg Clin North Am. 2004;12:435-450.
30. Goh AY, Hussain SS. Different surgical treatments for nasal septal perforation and their outcomes. J Laryngol Otol. 2007;121:419-426.
31. Mackle T, Conlon B. Foreign bodies of the nose and ears in children. Should these be managed in the accident and emergency setting? Int J Pediatr Otorhinolaryngol. 2006;70:425-428.
32. Cook S, Burton M, Glasziou P. Efficacy and safety of the “mother’s kiss” technique: a systematic review of case reports and case series. CMAJ. 2012;184:E904-E912.
33. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
34. Benninger MS. The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999:13:435-438.
35. Luce D, Gerin M, Leclerc A, et al. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int J Cancer. 1993;53:224-231.
36. Mayr SI, Hafizovic K, Waldfahrer F, et al. Characterization of initial clinical symptoms and risk factors for sinonasal adenocarcinomas: results of a case-control study. Int Arch Occup Environ Health. 2010;83;631-638.
37. Syrjänen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174-181.
38. Wood JW, Casiano RR. Inverted papillomas and benign nonneoplastic lesions of the nasal cavity. Am J Rhinol Allergy. 2012;26:157-163.
39. Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging. 2012;12:136-152.
Nasal obstruction is one of the most common reasons that patients visit their primary care providers.1,2 Often described by patients as nasal congestion or the inability to adequately breathe out of one or both nostrils during the day and/or night, nasal obstruction commonly interferes with a patient’s ability to eat, sleep, and function, thereby significantly impacting quality of life. Overlapping presentations can make discerning the exact cause of nasal obstruction difficult.
To improve diagnosis and treatment, we review here the evidence-based recommendations for the most common causes of nasal obstruction: rhinitis, rhinosinusitis (RS), drug-induced nasal obstruction, and mechanical/structural abnormalities (TABLE 13-14).

Rhinitis/rhinosinusitis: It all begins with inflammation
Sneezing, rhinorrhea, nasal congestion, and nasal itching are complaints that signal rhinitis, which affects 30 to 60 million people in the United States annually.3 Rhinitis can be allergic, non-allergic, infectious, hormonal, or occupational in nature. All forms of rhinitis share inflammation as the cause of the nasal obstruction. The most common form is allergic rhinitis (AR), which includes seasonal AR and perennial AR. Seasonal AR is typically caused by outdoor allergens and waxes and wanes with pollen seasons. Perennial AR is caused mostly by indoor allergens, such as dust mites, molds, cockroaches, and pet dander; it persists all or most of the year.6 Causes of non-allergic rhinitis (NAR) include environmental irritants such as cigarette smoke, perfume, and car exhaust; medications; and hormonal changes,6 but most causes of NAR are unknown.3,6
While AR can begin at any age, most people develop symptoms in childhood or as young adults, whereas NAR tends to begin later in life. Nasal itching can help to distinguish AR from NAR. NAR symptoms tend to be perennial and include postnasal drainage. If symptoms persist longer than 12 weeks despite treatment, the condition becomes known as chronic rhinosinusitis (CRS).
Treatment of rhinitis: Tiered and often continuous
Treatment of AR and NAR is similar and multitiered beginning with the avoidance of irritants and/or allergens whenever possible, moving on to pharmacotherapy, and, at least for AR, ending with allergen immunotherapy. Treatment is often an ongoing process and typically requires continuous therapy as opposed to treatment on an as-needed basis.3 It is unnecessary to perform allergy testing before making a presumed diagnosis of NAR and starting treatment.6
Intranasal corticosteroids. Currently, intranasal glucocorticosteroids (INGCs) are the most effective monotherapy for AR and NAR and have few adverse effects when used at prescribed doses.3,4 For mild to intermittent symptoms, begin with the maximum dosage of an INGC for the patient’s age and proceed with incremental reductions to identify the lowest effective dose.3 If INGCs alone are ineffective, studies have shown that the addition of an intranasal second-generation antihistamine can be of some benefit.3,4 In fact, an INGC and an intranasal antihistamine—along with saline nasal irrigation—is recommended for both AR and NAR resistant to single therapy.3,6,15 If intranasal antihistamines are not an option, oral therapy can be initiated.
Start with second-generation antihistamines and consider LRAs. For oral therapy, start with second-generation antihistamines (loratadine, cetirizine, fexofenadine). First-generation antihistamines (diphenhydramine, hydroxyzine, chlorpheniramine), although widely available at relatively low cost, can cause several significant adverse effects including sedation, impaired cognitive function, and agitation in children.3,4 Because second-generation antihistamines have fewer adverse effects, they are recommended as first-line therapy when oral antihistamine therapy is desired, such as for nasal congestion, sneezing, and itchy, watery eyes.
Of note: A 2014 meta-analysis found that a leukotriene receptor antagonist (LRA) (montelukast) had efficacy similar to oral antihistamines for symptom relief in AR, and that LRAs may be better suited to nighttime symptoms (difficulty falling asleep, nighttime awakenings, congestion on awakening), while antihistamines may provide better relief of daytime symptoms (pruritus, rhinorrhea, sneezing).16 Although further head-to-head, double-blind randomized controlled trials (RCTs) are needed to confirm the results and investigate possible gender differences in symptom response, consider an LRA for first-line therapy in patients with AR who have predominantly nighttime symptoms.
What about pregnant women and the elderly?
It is important to consider teratogenicity when selecting medications for pregnant patients, especially during the first trimester.3 Nasal cromolyn has the most reassuring safety profile in pregnancy. Cetirizine, chlorpheniramine, loratadine, diphenhydramine, and tripelennamine may be used in pregnancy. The US Food and Drug Administration considers them to have a low risk of fetal harm, based on human data, whereas it views many other antihistamines as probably safe, based on limited or no human data. Most INGCs are not expected to cause fetal harm, but limited human data are available. Avoid prescribing oral decongestants to women who are in the first trimester of pregnancy due to the risk of gastroschisis in newborns.17
Elderly patients represent another population for which adverse effects must be carefully considered. Allergies in individuals >65 years of age are uncommon. Rhinitis in this age group is often secondary to cholinergic hyperactivity, alpha-adrenergic hyperactivity, or rhinosinusitis. Given elderly patients’ increased susceptibility to the potential adverse central nervous system (CNS) and anticholinergic effects of antihistamines, non-sedating medications are recommended. Oral decongestants also should be used with caution in this population, not only because of CNS effects, but also because of heart and bladder effects3 (TABLE 218).
For drug-induced rhinitis, stop the offending drug and consider an INGC
Several types of medications, both oral and inhaled, are known to cause rhinitis. The use of alpha-adrenergic decongestant sprays for more than 5 to 7 days can induce rebound congestion on withdrawal, known as rhinitis medicamentosa.3 Repeated use of intranasal cocaine and methamphetamines can also result in rebound congestion. Oral medications that can result in rhinitis or congestion include angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, nonsteroidal anti-inflammatory drugs (NSAIDS), oral contraceptives, and even antidepressants.3
The treatment for drug-induced rhinitis is termination of the offending agent. INGCs can be used to help decrease inflammation and control symptoms once the offending agent is discontinued.

Mechanical/structural causes of obstruction are wide-ranging
Mechanical/structural causes of nasal obstruction range from foreign bodies to anatomical variations including nasal polyps, a deviated septum, adenoidal hypertrophy, foreign bodies, and tumors. Because more than one etiology may be at work, it is best to first treat any non-mechanical causes of obstruction, such as ARS or NARS.
Nasal polyposis often requires both a medical and surgical approach
Nasal polyps are benign growths arising from the mucosa of the nasal sinuses and nasal cavities and affecting up to 4% of the population.7 Their etiology is unclear, but we do know that nasal polyps result from underlying inflammation.7 Uncommon in children outside of those affected by cystic fibrosis,7 nasal polyposis can be associated with disease processes such as AR and sinusitis. Polyps are also associated with clinical syndromes such as aspirin-exacerbated respiratory disease (AERD) syndrome, which involves upper and lower respiratory tract symptoms in patients with asthma who have taken aspirin or other NSAIDs.9
Symptoms vary with the location and size of the polyps, but generally include nasal congestion, alteration in smell, and rhinorrhea. The goals of treatment are to restore or improve nasal breathing and olfaction and prevent recurrence.8 This often requires both a medical and surgical approach.
Topical corticosteroids are effective at reducing both the size of polyps and associated symptoms (rhinorrhea, rhinitis).8 And research has shown that steroids reduce the need for both primary and repeat surgical polypectomies.4 Other treatments to consider prior to surgery (if no symptom reduction occurs with INGCs) include systemic (oral) corticosteroids, intra-polyp steroid injections, macrolide antibiotics, and nasal washes.7,14
When symptoms of polyposis are refractory to medical management, functional endoscopic sinus surgery (FESS) is the surgical procedure of choice.3 In addition to refractory symptoms, indications for FESS include the need to correct anatomic deformities believed to be contributing to the persistence of disease and the need to debulk advanced nasal polyposis.3 The principal goal is to restore patency to the ostiomeatal unit.3
Several studies have reported a high success rate for FESS in improving the symptoms of CRS.3,19-23 In a 1992 study, for example, 98% of patients reported improvement following surgery,19 and in a follow-up report approximately 6 years later, 98% of patients continued to report subjective improvement.22
For septal etiologies, consider septoplasty
Deviation of the nasal septum is a common structural etiology for nasal obstruction arising primarily from congenital, genetic, or traumatic causes.24 Turbulent airflow from the septal deviation often causes turbinate hypertrophy, which creates (or exacerbates) the obstructive symptoms from the septal deviation.25
Septoplasty is the most common ear, nose, and throat operation in adults.26 Reduction of nasal symptoms has been reported in up to 89% of patients who receive this surgery, according to one single-center, non-randomized trial.27 Currently, at least one multicenter, randomized trial is underway that aims to develop evidence-based guidelines for septoplasty.26
Septal perforation is another etiology that can present with nasal obstruction symptoms. Causes include traumatic perforation, inflammatory or collagen vascular diseases, infections, overuse of vasoconstrictive medications, and malignancy.28,29 A careful inspection of the nasal septum is necessary to identify a perforation; this may require nasal endoscopy.
Anterior, rather than posterior, perforations are more likely to cause symptoms of nasal obstruction. Posterior perforations rarely require treatment unless malignancy is suspected, in which case referral for biopsy is recommended. Anterior perforations are treated initially with avoidance of any causative agent if, for example, the problem is drug- or medication-induced, and then with humidification and emollients.28,29
For anterior perforations, septal silicone buttons can be used for recalcitrant symptoms. However, observational studies indicate that for long-term symptom resolution, silicone buttons are effective in only about one-third of patients.29
For patients with persistent symptoms despite the above measures, surgical repair with various flap techniques is an option. A meta-analysis of case studies involving various techniques concluded that there is a wide variety of options, and that surgeons must weigh factors such as the characteristics and etiology of the perforation and their own experience and expertise when choosing from among available methods.30 Additional good quality research is necessary before clear recommendations regarding technique can be made.
Adenoid hypertrophy: Consider corticosteroid nasal drops
Adenoid hypertrophy is a common cause of chronic nasal obstruction in children. Although adenoidectomy is commonly performed to correct the problem, current evidence regarding the efficacy of the procedure is inconclusive.10 Evidence demonstrates corticosteroid nasal drops significantly reduce symptoms of nasal obstruction in children and may provide an effective alternative to surgical resection.18 Studies have also demonstrated that treatment with oral LRAs significantly reduces adenoid size and nasal obstruction symptoms.12,13
Foreign bodies: Don’t forget “a mother’s kiss”
Foreign bodies are the most common cause of nasal obstruction in the pediatric population. There is a paucity of high-quality evidence on removal of these objects; however, a number of retrospective reviews and case series support that most objects can be removed in the office or emergency department without otolaryngologic referral.31,32
Techniques for removal include positive pressure, which is best used for smooth or soft objects. Positive pressure techniques include having the patient blow their own nose or having a parent use a mouth-to-mouth–type blowing technique (ie, the “mother’s kiss” method).32 Refer patients to Otolaryngology if the obstruction involves:31
- objects not easily visualized by anterior rhinoscopy
- chronic or impacted objects
- button batteries or magnets
- penetrating or hooked objects
- any object that cannot be removed during an initial attempt.
Nasal tumors: More common in older men
Nasal tumors occur most often in the nasal cavity itself and are more common in men ≥60 years.33 There is no notable racial predominance.33 Other risk factors include human papillomavirus (HPV) infection, tobacco smoke, and occupational exposure to inhaled wood dust, glues, and adhesives.34-37
Benign tumors occurring in the nasal cavity are a diverse group of disorders, including inverted papillomas, squamous papillomas, pyogenic granulomas, and other less common lesions, all of which typically present with nasal obstruction as a symptom. Many of these lesions cause local tissue destruction or have a high incidence of recurrence. These tumors are treated universally with nasoendoscopic resection.38
Malignant nasal tumors are rare but serious causes of nasal obstruction, making up 3% of all head and neck cancers.39 Most nasal cancers present when they are locally advanced and cause unilateral nasal obstruction, lacrimation, and epistaxis. These symptoms are typically refractory to initial medical management and present as CRS. This diagnosis should be suspected in certain patient groups, such as those who have been exposed to wood dust (eg, construction workers or those who work in wood mills).36
Computed tomography is the gold standard imaging method for CRS; however, if nasal cancer is suspected, referral for biopsy and histopathologic examination is necessary for a final diagnosis.39 Because of the nonspecific nature of their initial presentation, many nasal tumors are at an advanced stage and carry a poor prognosis by the time they are diagnosed.39
CORRESPONDENCE
Margaret A. Bayard, MD, MPH, FAAFP, Naval Hospital Camp Pendleton, 200 Mercy Circle, Camp Pendleton, CA 92005; [email protected].
Nasal obstruction is one of the most common reasons that patients visit their primary care providers.1,2 Often described by patients as nasal congestion or the inability to adequately breathe out of one or both nostrils during the day and/or night, nasal obstruction commonly interferes with a patient’s ability to eat, sleep, and function, thereby significantly impacting quality of life. Overlapping presentations can make discerning the exact cause of nasal obstruction difficult.
To improve diagnosis and treatment, we review here the evidence-based recommendations for the most common causes of nasal obstruction: rhinitis, rhinosinusitis (RS), drug-induced nasal obstruction, and mechanical/structural abnormalities (TABLE 13-14).

Rhinitis/rhinosinusitis: It all begins with inflammation
Sneezing, rhinorrhea, nasal congestion, and nasal itching are complaints that signal rhinitis, which affects 30 to 60 million people in the United States annually.3 Rhinitis can be allergic, non-allergic, infectious, hormonal, or occupational in nature. All forms of rhinitis share inflammation as the cause of the nasal obstruction. The most common form is allergic rhinitis (AR), which includes seasonal AR and perennial AR. Seasonal AR is typically caused by outdoor allergens and waxes and wanes with pollen seasons. Perennial AR is caused mostly by indoor allergens, such as dust mites, molds, cockroaches, and pet dander; it persists all or most of the year.6 Causes of non-allergic rhinitis (NAR) include environmental irritants such as cigarette smoke, perfume, and car exhaust; medications; and hormonal changes,6 but most causes of NAR are unknown.3,6
While AR can begin at any age, most people develop symptoms in childhood or as young adults, whereas NAR tends to begin later in life. Nasal itching can help to distinguish AR from NAR. NAR symptoms tend to be perennial and include postnasal drainage. If symptoms persist longer than 12 weeks despite treatment, the condition becomes known as chronic rhinosinusitis (CRS).
Treatment of rhinitis: Tiered and often continuous
Treatment of AR and NAR is similar and multitiered beginning with the avoidance of irritants and/or allergens whenever possible, moving on to pharmacotherapy, and, at least for AR, ending with allergen immunotherapy. Treatment is often an ongoing process and typically requires continuous therapy as opposed to treatment on an as-needed basis.3 It is unnecessary to perform allergy testing before making a presumed diagnosis of NAR and starting treatment.6
Intranasal corticosteroids. Currently, intranasal glucocorticosteroids (INGCs) are the most effective monotherapy for AR and NAR and have few adverse effects when used at prescribed doses.3,4 For mild to intermittent symptoms, begin with the maximum dosage of an INGC for the patient’s age and proceed with incremental reductions to identify the lowest effective dose.3 If INGCs alone are ineffective, studies have shown that the addition of an intranasal second-generation antihistamine can be of some benefit.3,4 In fact, an INGC and an intranasal antihistamine—along with saline nasal irrigation—is recommended for both AR and NAR resistant to single therapy.3,6,15 If intranasal antihistamines are not an option, oral therapy can be initiated.
Start with second-generation antihistamines and consider LRAs. For oral therapy, start with second-generation antihistamines (loratadine, cetirizine, fexofenadine). First-generation antihistamines (diphenhydramine, hydroxyzine, chlorpheniramine), although widely available at relatively low cost, can cause several significant adverse effects including sedation, impaired cognitive function, and agitation in children.3,4 Because second-generation antihistamines have fewer adverse effects, they are recommended as first-line therapy when oral antihistamine therapy is desired, such as for nasal congestion, sneezing, and itchy, watery eyes.
Of note: A 2014 meta-analysis found that a leukotriene receptor antagonist (LRA) (montelukast) had efficacy similar to oral antihistamines for symptom relief in AR, and that LRAs may be better suited to nighttime symptoms (difficulty falling asleep, nighttime awakenings, congestion on awakening), while antihistamines may provide better relief of daytime symptoms (pruritus, rhinorrhea, sneezing).16 Although further head-to-head, double-blind randomized controlled trials (RCTs) are needed to confirm the results and investigate possible gender differences in symptom response, consider an LRA for first-line therapy in patients with AR who have predominantly nighttime symptoms.
What about pregnant women and the elderly?
It is important to consider teratogenicity when selecting medications for pregnant patients, especially during the first trimester.3 Nasal cromolyn has the most reassuring safety profile in pregnancy. Cetirizine, chlorpheniramine, loratadine, diphenhydramine, and tripelennamine may be used in pregnancy. The US Food and Drug Administration considers them to have a low risk of fetal harm, based on human data, whereas it views many other antihistamines as probably safe, based on limited or no human data. Most INGCs are not expected to cause fetal harm, but limited human data are available. Avoid prescribing oral decongestants to women who are in the first trimester of pregnancy due to the risk of gastroschisis in newborns.17
Elderly patients represent another population for which adverse effects must be carefully considered. Allergies in individuals >65 years of age are uncommon. Rhinitis in this age group is often secondary to cholinergic hyperactivity, alpha-adrenergic hyperactivity, or rhinosinusitis. Given elderly patients’ increased susceptibility to the potential adverse central nervous system (CNS) and anticholinergic effects of antihistamines, non-sedating medications are recommended. Oral decongestants also should be used with caution in this population, not only because of CNS effects, but also because of heart and bladder effects3 (TABLE 218).
For drug-induced rhinitis, stop the offending drug and consider an INGC
Several types of medications, both oral and inhaled, are known to cause rhinitis. The use of alpha-adrenergic decongestant sprays for more than 5 to 7 days can induce rebound congestion on withdrawal, known as rhinitis medicamentosa.3 Repeated use of intranasal cocaine and methamphetamines can also result in rebound congestion. Oral medications that can result in rhinitis or congestion include angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, nonsteroidal anti-inflammatory drugs (NSAIDS), oral contraceptives, and even antidepressants.3
The treatment for drug-induced rhinitis is termination of the offending agent. INGCs can be used to help decrease inflammation and control symptoms once the offending agent is discontinued.

Mechanical/structural causes of obstruction are wide-ranging
Mechanical/structural causes of nasal obstruction range from foreign bodies to anatomical variations including nasal polyps, a deviated septum, adenoidal hypertrophy, foreign bodies, and tumors. Because more than one etiology may be at work, it is best to first treat any non-mechanical causes of obstruction, such as ARS or NARS.
Nasal polyposis often requires both a medical and surgical approach
Nasal polyps are benign growths arising from the mucosa of the nasal sinuses and nasal cavities and affecting up to 4% of the population.7 Their etiology is unclear, but we do know that nasal polyps result from underlying inflammation.7 Uncommon in children outside of those affected by cystic fibrosis,7 nasal polyposis can be associated with disease processes such as AR and sinusitis. Polyps are also associated with clinical syndromes such as aspirin-exacerbated respiratory disease (AERD) syndrome, which involves upper and lower respiratory tract symptoms in patients with asthma who have taken aspirin or other NSAIDs.9
Symptoms vary with the location and size of the polyps, but generally include nasal congestion, alteration in smell, and rhinorrhea. The goals of treatment are to restore or improve nasal breathing and olfaction and prevent recurrence.8 This often requires both a medical and surgical approach.
Topical corticosteroids are effective at reducing both the size of polyps and associated symptoms (rhinorrhea, rhinitis).8 And research has shown that steroids reduce the need for both primary and repeat surgical polypectomies.4 Other treatments to consider prior to surgery (if no symptom reduction occurs with INGCs) include systemic (oral) corticosteroids, intra-polyp steroid injections, macrolide antibiotics, and nasal washes.7,14
When symptoms of polyposis are refractory to medical management, functional endoscopic sinus surgery (FESS) is the surgical procedure of choice.3 In addition to refractory symptoms, indications for FESS include the need to correct anatomic deformities believed to be contributing to the persistence of disease and the need to debulk advanced nasal polyposis.3 The principal goal is to restore patency to the ostiomeatal unit.3
Several studies have reported a high success rate for FESS in improving the symptoms of CRS.3,19-23 In a 1992 study, for example, 98% of patients reported improvement following surgery,19 and in a follow-up report approximately 6 years later, 98% of patients continued to report subjective improvement.22
For septal etiologies, consider septoplasty
Deviation of the nasal septum is a common structural etiology for nasal obstruction arising primarily from congenital, genetic, or traumatic causes.24 Turbulent airflow from the septal deviation often causes turbinate hypertrophy, which creates (or exacerbates) the obstructive symptoms from the septal deviation.25
Septoplasty is the most common ear, nose, and throat operation in adults.26 Reduction of nasal symptoms has been reported in up to 89% of patients who receive this surgery, according to one single-center, non-randomized trial.27 Currently, at least one multicenter, randomized trial is underway that aims to develop evidence-based guidelines for septoplasty.26
Septal perforation is another etiology that can present with nasal obstruction symptoms. Causes include traumatic perforation, inflammatory or collagen vascular diseases, infections, overuse of vasoconstrictive medications, and malignancy.28,29 A careful inspection of the nasal septum is necessary to identify a perforation; this may require nasal endoscopy.
Anterior, rather than posterior, perforations are more likely to cause symptoms of nasal obstruction. Posterior perforations rarely require treatment unless malignancy is suspected, in which case referral for biopsy is recommended. Anterior perforations are treated initially with avoidance of any causative agent if, for example, the problem is drug- or medication-induced, and then with humidification and emollients.28,29
For anterior perforations, septal silicone buttons can be used for recalcitrant symptoms. However, observational studies indicate that for long-term symptom resolution, silicone buttons are effective in only about one-third of patients.29
For patients with persistent symptoms despite the above measures, surgical repair with various flap techniques is an option. A meta-analysis of case studies involving various techniques concluded that there is a wide variety of options, and that surgeons must weigh factors such as the characteristics and etiology of the perforation and their own experience and expertise when choosing from among available methods.30 Additional good quality research is necessary before clear recommendations regarding technique can be made.
Adenoid hypertrophy: Consider corticosteroid nasal drops
Adenoid hypertrophy is a common cause of chronic nasal obstruction in children. Although adenoidectomy is commonly performed to correct the problem, current evidence regarding the efficacy of the procedure is inconclusive.10 Evidence demonstrates corticosteroid nasal drops significantly reduce symptoms of nasal obstruction in children and may provide an effective alternative to surgical resection.18 Studies have also demonstrated that treatment with oral LRAs significantly reduces adenoid size and nasal obstruction symptoms.12,13
Foreign bodies: Don’t forget “a mother’s kiss”
Foreign bodies are the most common cause of nasal obstruction in the pediatric population. There is a paucity of high-quality evidence on removal of these objects; however, a number of retrospective reviews and case series support that most objects can be removed in the office or emergency department without otolaryngologic referral.31,32
Techniques for removal include positive pressure, which is best used for smooth or soft objects. Positive pressure techniques include having the patient blow their own nose or having a parent use a mouth-to-mouth–type blowing technique (ie, the “mother’s kiss” method).32 Refer patients to Otolaryngology if the obstruction involves:31
- objects not easily visualized by anterior rhinoscopy
- chronic or impacted objects
- button batteries or magnets
- penetrating or hooked objects
- any object that cannot be removed during an initial attempt.
Nasal tumors: More common in older men
Nasal tumors occur most often in the nasal cavity itself and are more common in men ≥60 years.33 There is no notable racial predominance.33 Other risk factors include human papillomavirus (HPV) infection, tobacco smoke, and occupational exposure to inhaled wood dust, glues, and adhesives.34-37
Benign tumors occurring in the nasal cavity are a diverse group of disorders, including inverted papillomas, squamous papillomas, pyogenic granulomas, and other less common lesions, all of which typically present with nasal obstruction as a symptom. Many of these lesions cause local tissue destruction or have a high incidence of recurrence. These tumors are treated universally with nasoendoscopic resection.38
Malignant nasal tumors are rare but serious causes of nasal obstruction, making up 3% of all head and neck cancers.39 Most nasal cancers present when they are locally advanced and cause unilateral nasal obstruction, lacrimation, and epistaxis. These symptoms are typically refractory to initial medical management and present as CRS. This diagnosis should be suspected in certain patient groups, such as those who have been exposed to wood dust (eg, construction workers or those who work in wood mills).36
Computed tomography is the gold standard imaging method for CRS; however, if nasal cancer is suspected, referral for biopsy and histopathologic examination is necessary for a final diagnosis.39 Because of the nonspecific nature of their initial presentation, many nasal tumors are at an advanced stage and carry a poor prognosis by the time they are diagnosed.39
CORRESPONDENCE
Margaret A. Bayard, MD, MPH, FAAFP, Naval Hospital Camp Pendleton, 200 Mercy Circle, Camp Pendleton, CA 92005; [email protected].
1. Centers for Disease Control and Prevention. CDC’s FastStats on Ambulatory Care Use and Physician Office Visits. Selected patient and provider characteristics for ambulatory care visits to physician offices and hospital outpatient and emergency departments: United States, 2009-2010. Available at: http://www.cdc.gov/nchs/data/ahcd/combined_tables/AMC_2009-2010_combined_web_table01.pdf. Accessed November 9, 2016.
2. US Census Bureau. Table 158. Visits to office-based physician and hospital outpatient departments, 2006. Available at: http://www2.census.gov/library/publications/2006/compendia/statab/126ed/tables/07s0158.xls. Accessed November 13, 2016.
3. Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103-S115.
4. Wallace DV, Dykewicz MS. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1-S84.
5. Krouse J, Lund V, Fokkens W, et al. Diagnostic strategies in nasal congestion. Int J Gen Med. 2010;3:59-67.
6. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2-S8.
7. Newton JR, Ah-See KW. A review of nasal polyposis. Ther Clin Risk Manag. 2008;4:507-512.
8. Badia L, Lund V. Topical corticosteroids in nasal polyposis. Drugs. 2001;61:573-578.
9. Fahrenholz JM. Natural history and clinical features of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol. 2003;24:113-124.
10. van den Aardweg MT, Schilder AG, Herket E, et al. Adenoidectomy for recurrent or chronic nasal symptoms in children. Cochrane Database Syst Rev. 2010;CD008282.
11. Demirhan H, Aksoy F, Ozturan O, et al. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int J Pediatr Otorhinolaryngol. 2010;74:773-776.
12. Shokouhi F, Jahromi AM, Majidi MR, et al. Montelukast in adenoid hypertrophy: its effect on size and symptoms. Iran J Otorhinolaryngol. 2015;27:433-448.
13. Goldbart AD, Greenberg-Dotan S, Tai A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130:e575-e580.
14. Moss WJ, Kjos KB, Karnezis TT, et al. Intranasal steroid injections and blindness: our personal experience and a review of the past 60 years. Laryngoscope. 2015;125:796-800.
15. Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis. Laryngoscope. 2013;123:53-56.
16. Xu Y, Zhang J, Wang J. The efficacy and safety of selective H1-antihistamine versus leukotriene receptor antagonist for seasonal allergic rhinitis: a meta-analysis. PLoS One. 2014;9:e112815.
17. Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
18. Lexi-Comp Online. Available at: online.lexi.com/crlsql/servlet/crlonline. Accessed November 9, 2016.
19. Kennedy DW. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope.1992;102:1-18.
20. Khalil HS, Nunez DA. Functional endoscopic sinus surgery for chronic rhinosinusitis. Cochrane Database Syst Rev. 2006;CD004458.
21. Chambers DW, Davis WE, Cook PR, et al. Long-term outcome analysis of functional endoscopic sinus surgery: correlation of symptoms with endoscopic examination findings and potential prognostic variables. Laryngoscope. 1997;107:504-510.
22. Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151-157.
23. Jakobsen J, Svendstrup F. Functional endoscopic sinus surgery in chronic sinusitis—a series of 237 consecutively operated patients. Acta Otolaryngol Suppl. 2000;543:158-161.
24. Aziz T, Biron VL, Ansari K, et al. Measurement tools for the diagnosis of nasal septal deviation: a systematic review. J Otolaryngol Head Neck Surg. 2014;43:11.
25. Grutzenmacher S, Robinson DM, Grafe K, et al. First findings concerning airflow in noses with septal deviation and compensatory turbinate hypertrophy—a model study. ORL J Otorhinolaryngol Relat Spec. 2006;68:199-205.
26. van Egmond MMHT, Rovers MM, Hendriks CTM, et al. Effectiveness of septoplasty versus non-surgical management for nasal obstruction due to deviated nasal septum in adults: study protocol for a randomized controlled trial. Trials. 2015;16:500.
27. Gandomi B, Bayat A, Kazemei T. Outcomes of septoplasty in young adults: the Nasal Obstruction Septoplasty Effectiveness study. Am J Otolaryngol. 2010;31:189-192.
28. Døsen L, Have R. Silicone button in nasal septal perforation. Long term observations. Rhinology. 2008;46:324-327.
29. Kridel RW. Considerations in the etiology, treatment and repair of septal perforations. Facial Plast Surg Clin North Am. 2004;12:435-450.
30. Goh AY, Hussain SS. Different surgical treatments for nasal septal perforation and their outcomes. J Laryngol Otol. 2007;121:419-426.
31. Mackle T, Conlon B. Foreign bodies of the nose and ears in children. Should these be managed in the accident and emergency setting? Int J Pediatr Otorhinolaryngol. 2006;70:425-428.
32. Cook S, Burton M, Glasziou P. Efficacy and safety of the “mother’s kiss” technique: a systematic review of case reports and case series. CMAJ. 2012;184:E904-E912.
33. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
34. Benninger MS. The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999:13:435-438.
35. Luce D, Gerin M, Leclerc A, et al. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int J Cancer. 1993;53:224-231.
36. Mayr SI, Hafizovic K, Waldfahrer F, et al. Characterization of initial clinical symptoms and risk factors for sinonasal adenocarcinomas: results of a case-control study. Int Arch Occup Environ Health. 2010;83;631-638.
37. Syrjänen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174-181.
38. Wood JW, Casiano RR. Inverted papillomas and benign nonneoplastic lesions of the nasal cavity. Am J Rhinol Allergy. 2012;26:157-163.
39. Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging. 2012;12:136-152.
1. Centers for Disease Control and Prevention. CDC’s FastStats on Ambulatory Care Use and Physician Office Visits. Selected patient and provider characteristics for ambulatory care visits to physician offices and hospital outpatient and emergency departments: United States, 2009-2010. Available at: http://www.cdc.gov/nchs/data/ahcd/combined_tables/AMC_2009-2010_combined_web_table01.pdf. Accessed November 9, 2016.
2. US Census Bureau. Table 158. Visits to office-based physician and hospital outpatient departments, 2006. Available at: http://www2.census.gov/library/publications/2006/compendia/statab/126ed/tables/07s0158.xls. Accessed November 13, 2016.
3. Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103-S115.
4. Wallace DV, Dykewicz MS. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1-S84.
5. Krouse J, Lund V, Fokkens W, et al. Diagnostic strategies in nasal congestion. Int J Gen Med. 2010;3:59-67.
6. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2-S8.
7. Newton JR, Ah-See KW. A review of nasal polyposis. Ther Clin Risk Manag. 2008;4:507-512.
8. Badia L, Lund V. Topical corticosteroids in nasal polyposis. Drugs. 2001;61:573-578.
9. Fahrenholz JM. Natural history and clinical features of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol. 2003;24:113-124.
10. van den Aardweg MT, Schilder AG, Herket E, et al. Adenoidectomy for recurrent or chronic nasal symptoms in children. Cochrane Database Syst Rev. 2010;CD008282.
11. Demirhan H, Aksoy F, Ozturan O, et al. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int J Pediatr Otorhinolaryngol. 2010;74:773-776.
12. Shokouhi F, Jahromi AM, Majidi MR, et al. Montelukast in adenoid hypertrophy: its effect on size and symptoms. Iran J Otorhinolaryngol. 2015;27:433-448.
13. Goldbart AD, Greenberg-Dotan S, Tai A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130:e575-e580.
14. Moss WJ, Kjos KB, Karnezis TT, et al. Intranasal steroid injections and blindness: our personal experience and a review of the past 60 years. Laryngoscope. 2015;125:796-800.
15. Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis. Laryngoscope. 2013;123:53-56.
16. Xu Y, Zhang J, Wang J. The efficacy and safety of selective H1-antihistamine versus leukotriene receptor antagonist for seasonal allergic rhinitis: a meta-analysis. PLoS One. 2014;9:e112815.
17. Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
18. Lexi-Comp Online. Available at: online.lexi.com/crlsql/servlet/crlonline. Accessed November 9, 2016.
19. Kennedy DW. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope.1992;102:1-18.
20. Khalil HS, Nunez DA. Functional endoscopic sinus surgery for chronic rhinosinusitis. Cochrane Database Syst Rev. 2006;CD004458.
21. Chambers DW, Davis WE, Cook PR, et al. Long-term outcome analysis of functional endoscopic sinus surgery: correlation of symptoms with endoscopic examination findings and potential prognostic variables. Laryngoscope. 1997;107:504-510.
22. Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151-157.
23. Jakobsen J, Svendstrup F. Functional endoscopic sinus surgery in chronic sinusitis—a series of 237 consecutively operated patients. Acta Otolaryngol Suppl. 2000;543:158-161.
24. Aziz T, Biron VL, Ansari K, et al. Measurement tools for the diagnosis of nasal septal deviation: a systematic review. J Otolaryngol Head Neck Surg. 2014;43:11.
25. Grutzenmacher S, Robinson DM, Grafe K, et al. First findings concerning airflow in noses with septal deviation and compensatory turbinate hypertrophy—a model study. ORL J Otorhinolaryngol Relat Spec. 2006;68:199-205.
26. van Egmond MMHT, Rovers MM, Hendriks CTM, et al. Effectiveness of septoplasty versus non-surgical management for nasal obstruction due to deviated nasal septum in adults: study protocol for a randomized controlled trial. Trials. 2015;16:500.
27. Gandomi B, Bayat A, Kazemei T. Outcomes of septoplasty in young adults: the Nasal Obstruction Septoplasty Effectiveness study. Am J Otolaryngol. 2010;31:189-192.
28. Døsen L, Have R. Silicone button in nasal septal perforation. Long term observations. Rhinology. 2008;46:324-327.
29. Kridel RW. Considerations in the etiology, treatment and repair of septal perforations. Facial Plast Surg Clin North Am. 2004;12:435-450.
30. Goh AY, Hussain SS. Different surgical treatments for nasal septal perforation and their outcomes. J Laryngol Otol. 2007;121:419-426.
31. Mackle T, Conlon B. Foreign bodies of the nose and ears in children. Should these be managed in the accident and emergency setting? Int J Pediatr Otorhinolaryngol. 2006;70:425-428.
32. Cook S, Burton M, Glasziou P. Efficacy and safety of the “mother’s kiss” technique: a systematic review of case reports and case series. CMAJ. 2012;184:E904-E912.
33. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
34. Benninger MS. The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999:13:435-438.
35. Luce D, Gerin M, Leclerc A, et al. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int J Cancer. 1993;53:224-231.
36. Mayr SI, Hafizovic K, Waldfahrer F, et al. Characterization of initial clinical symptoms and risk factors for sinonasal adenocarcinomas: results of a case-control study. Int Arch Occup Environ Health. 2010;83;631-638.
37. Syrjänen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174-181.
38. Wood JW, Casiano RR. Inverted papillomas and benign nonneoplastic lesions of the nasal cavity. Am J Rhinol Allergy. 2012;26:157-163.
39. Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging. 2012;12:136-152.
PRACTICE RECOMMENDATIONS
› Consider intranasal corticosteroids for patients with nasal polyps, as they are effective at reducing the size of the polyps and associated symptoms of obstruction, rhinorrhea, and rhinitis. A
› Prescribe intranasal corticosteroids for patients with adenoid hypertrophy. A
› Refer patients with chronic refractory rhinosinusitis for functional endoscopic sinus surgery. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
