User login
Management of Metastatic Gastric Cancer
INTRODUCTION
According to the Surveillance, Epidemiology and End Results database, in 2017 there were 28,000 new cases of gastric cancer, accounting for 1.8% of all malignancies in the United States, and an estimated 10,960 gastric cancer–related deaths.1 Worldwide, gastric cancer is the fifth most common malignancy and the third most common cause of death from any cancer.2 The incidence of gastric cancer varies significantly by geographic region, with countries in Eastern Asia (China, Japan), Eastern Europe, and Central and South America accounting for 50% of all new cases.3 Although the incidence of gastric cancer has declined in recent years, this decrease has not been observed consistently across all nations.2 In particular, the incidence of gastric cancers arising from the cardia has been increasing, which is perhaps due to a higher prevalence of obesity in Western societies.4
In this article, we review key aspects of management of metastatic gastric cancer, including selection of first- and second-line therapy, and discuss targeted agents and upcoming clinical trials.
EPIDEMIOLOGY AND RISK FACTORS
Chronic infection with Helicobacter pylori, a gram-negative bacterium, is a strong etiological factor for the development of gastric cancer, contributing to up to 70% of cases.2 The pathogen can colonize the gastric mucosa, leading to chronic inflammation. Although most patients remain asymptomatic, 1% to 3% develop gastric cancer and another 0.1% develop mucosa-associated lymphoid tissue lymphoma.5 H. pylori infection is more commonly associated with cancer of the gastric body than with cancer of the gastroesophageal junction (GEJ). The increased burden of gastric cancer in countries in Eastern Asia, Latin America, and Eastern Europe has been correlated to the prevalence of chronic H. pylori infection in these areas.
Carcinogenesis secondary to H. pylori infection may occur via several mechanisms. First, H. pylori can release virulence factors, such as cytotoxin-associated gene A, vacuolating cytotoxin, and outer membrane proteins, into the cytosol of host cells, leading to changes in patterns of cell proliferation and apoptosis.6 These virulence factors can modulate the host immune system, attenuating it to promote dysplasia. In addition, continued recognition of these factors by the immune system leads to a persistent inflammatory response, with the release of cytokines such as interleukin (IL) -1β, IL-6, and IL-8. This leads to chronic mucosal damage, further promoting dysplasia with eventual transformation into adenocarcinoma.7 In Japan and Korea, where screening for H. pylori infection is routinely performed, there have been improvements in overall survival (OS) rates for gastric cancer, with 5-year OS rates of 70%.8 The International Agency for Research on Cancer recommends further research into population-based screening and treatment programs for patients with chronic H. pylori infection. However, despite this recommendation, optimal screening strategies are not clearly defined.9
Other risk factors for the development of gastric cancer include chronic gastroesophageal reflux disease; smoking; alcohol use; exposure to radiation; diets high in fats, salt, and smoked items and low in fruits and vegetables; obesity; and exposure to chemotherapeutic agents such as procarbazine.10 Another pathogen suspected, but not proven, to be associated with increased risk for gastric cancer is the Epstein-Barr virus, a human herpesvirus found in 80% of all gastric carcinomas with lymphoid features.11 In addition, whether the use of medications such as statins and nonsteroidal anti-inflammatory drugs confers a decreased risk of gastric cancers remains unclear.10
EVALUATION
CASE PRESENTATION
A 55-year-old Caucasian man with a history of type 2 diabetes mellitus presents to the gastrointestinal (GI) clinic with a 6-month history of dysphagia. The dysphagia is worsened with ingestion of solids, particularly towards the end of the day. He states that the food often gets “stuck in the middle of the chest.” The patient denies any nausea or emesis but notes that he has a poor appetite. He reports having worsening mid-epigastric abdominal pain that is non-radiating, dull in character, and 6/10 in intensity. He also reports a 10-lb weight loss over the past 2 months. He has no previous history of reflux, chest pain, dyspnea, or cough. Review of systems is otherwise benign. Physical exam is within normal limits.
• Which tests should be conducted when gastric cancer is suspected?
Persistent epigastric abdominal pain and weight loss are the most common early symptoms of gastric cancer. Nausea, early satiety, dysphagia, and occult GI bleeding can be other presenting signs. Patients presenting with alarm symptoms of nausea, emesis, early satiety, abdominal pain, or weight loss should be fully evaluated with upper GI endoscopy. Early diagnosis of gastric cancer is essential in obtaining a curative resection. However, at least 40% of patients present with de novo metastatic disease at the time of initial diagnosis.12 Gastric cancer spreads by direct extension through the gastric wall, with the liver, peritoneum, and regional lymph nodes being the most common sites of metastatic deposits.13 Classically, Virchow’s node, the left supraclavicular lymph node, is involved with metastatic gastric cancer. Involvement of the left axillary lymph node (Irish node) or a periumbilical nodule (Sister Mary Joseph node) may also be observed. Other, less commonly noted sites of metastatic disease include the ovaries, central nervous system, bone, lung, and soft tissues.13
Upper GI endoscopy is the best method for determining tumor location and extent and obtaining a specimen for a definitive tissue diagnosis.14 It is essential to accurately identify the location of the tumor in the stomach and relative to the GEJ. The American Joint Committee on Cancer classification defines tumors involving the GEJ with an epicenter no more than 2 cm into the proximal stomach as esophageal cancers.15 Tumors of the GEJ with their epicenter more than 2 cm into the proximal stomach are defined as gastric cancers. If metastatic disease is suspected, computed tomography (CT) scan of the chest, abdomen, and pelvis with oral and intravenous contrast can be obtained to determine the extent of disease spread. In the absence of any metastatic disease, endoscopic ultrasound (EUS) should be conducted to determine the depth of tumor invasion (T staging) and lymph node status. In the era of targeted therapy, patients with metastatic disease should undergo testing for human epidermal growth factor-2 (HER-2) expression, microsatellite instability (MSI), and programmed death ligand 1 (PD-L1) expression. Patients should be staged according to the TNM staging system.
FIRST-LINE TREATMENT OPTIONS
CASE CONTINUED
The patient undergoes esophagoduodenoscopy (EGD) and is found to have a gastric cardia mass extending into the distal esophagus. EUS also demonstrates multiple abdominal and mediastinal lymph nodes. No gastric outlet obstruction is found. Biopsy shows poorly differentiated invasive adenocarcinoma. Warthin–Starry stain is negative for H. pylori organism. The tumor cells are positive for cytokeratin (CK7), CK19, and mucin-1 gene (MUC1); focally positive for CK20; and negative for MUC2. HER2 testing results are reported as immunohistochemistry (IHC) 3+, consistent with strongly positive HER2 protein expression. Further IHC testing for mismatch repair (MMR) proteins shows intact nuclear expression of MLH1, MSH2, MSH6, and PMS2 protein, consistent with a low probability of MSI-high tumor. The tumor is found to be PD-L1 positive. Imaging reveals abnormal mass-like nodular thickening of the gastric wall, with an infiltrative opacity within the pancreatico-duodenal groove, suspicious for tumor infiltration. Multiple metastatic deposits are noted in the liver, peritoneum, and bilateral lungs. There is extensive gastrohepatic ligament and periportal lymphadenopathy and mild enlargement of the pulmonary hilar lymph nodes. These findings are consistent with stage 4 (T4bN3aM1) gastric cancer. Given these findings, staging laparoscopy is deferred.
• What are the first-line treatment options for patients with metastatic gastric cancer?
Patients with metastatic gastric cancer have a poor prognosis, and management is stratified based on performance status (Figure). In patients with good performance status, systemic chemotherapy is the mainstay of treatment. The goal of therapy is not curative, but rather treatment focuses on palliation of symptoms arising from tumor spread. Given this treatment goal, there has been considerable interest in clarifying the utility of chemotherapy as opposed to best supportive care. In a recent Cochrane review of 64 randomized control trials involving 11,698 patients, chemotherapy was found to improve OS by 6.7 months as compared to best supportive care (hazard ratio [HR] 0.3 [95% confidence interval {CI} 0.24 to 0.55]).16 Five classes of cytotoxic chemotherapeutic agents have demonstrated activity in gastric cancer. These include fluoropyrimidine (either infusional fluorouracil or capecitabine), platinum agents (cisplatin or oxaliplatin), taxanes (docetaxel or paclitaxel), anthracyclines (epirubicin), and irinotecan.13 Treatment options are further divided based on whether the patient has HER2-overexpressing or non-expressing malignancy.
HER2-NEGATIVE DISEASE
For patients with HER2-negative disease, National Comprehensive Cancer Network (NCCN) guidelines recommend using 2-drug combination regimens rather than 3 drugs, given concern for increased toxicity with 3-drug regimens.17 For patients with a performance status of 0 to 1, utilization of a 3-drug regimen is a reasonable alternative. The combination of a fluoropyrimidine with a platinum agent is considered the standard of care, with regimens such as fluorouracil, leucovorin, and oxaliplatin (FOLFOX) being commonly used.
Epirubicin-containing regimens have also been extensively studied in advanced gastric cancer. In a study of 274 previously untreated patients with GEJ cancers, the combination of epirubicin, cisplatin, and fluorouracil (ECF) was compared to fluorouracil, doxorubicin, and methotrexate (FAMTX). There was an OS benefit favoring ECF (8.9 months versus 5.7 months) at 1 year (95% CI 27% to 45%, P = 0.0009). The ECF regimen was associated with an increased risk of nausea, emesis, and alopecia, while more hematologic toxicity and infections were noted with the FAMTX regimen.18 In addition, in a phase 3 trial, Van Cutsem and colleagues examined the role of docetaxel in combination with cisplatin and fluorouracil (DCF) compared to cisplatin and fluorouracil alone. Addition of docetaxel led to improved OS and time to progression (9.2 months versus 8.6 months for cisplatin and fluorouracil alone, P = 0.02) but with an increased risk of grade 3 and 4 toxicities (69% versus 59%). These adverse events included neutropenia (82% versus 57% of cisplatin and fluorouracil patients), diarrhea (19% versus 8%), stomatitis (21% versus 27%), and fatigue (19% versus 14%).19
The landmark phase 3 REAL-2 study compared 4 chemotherapy regimens in patients with untreated advanced esophagogastric cancer. This study was conducted to determine if the efficacy of cisplatin and oxaliplatin, a third-generation platinum agent, is equivalent to that of fluorouracil and capecitabine, an oral fluoropyrimidine. In this trial, a 2 × 2 design was used to compare 4 regimens: ECF versus epirubicin, cisplatin, and capecitabine (ECX) versus epirubicin, oxaliplatin, and fluorouracil (EOF) versus epirubicin, oxaliplatin, and capecitabine (EOX). The study found EOX to be noninferior to ECF, with a trend towards improved OS compared to other combination regimens (11.2 months versus 9.9 months, HR 0.80 [95% CI 0.66 to 0.97], P = 0.02).20 Thus, the study demonstrated that an oxaliplatin and capecitabine-based regimen could replace cisplatin and fluorouracil. Given that fluorouracil administration requires long continuous infusions, the oral-based capecitabine regimen is an attractive option for patients.
Several trials have demonstrated the equivalency of oxaliplatin with cisplatin in combination regimens for the treatment of advanced gastric cancer. Oxaliplatin has the benefit of an improved toxicity profile as compared to cisplatin, with the major dose-limiting toxicity being peripheral neuropathy
Given previous evidence that DCF (docetaxel, cisplatin, fluorouracil) is superior to cisplatin and fluorouracil alone, there was interest in determining if the addition of docetaxel to a backbone of fluorouracil, oxaliplatin, and leucovorin (FLO) could elicit a higher response rate. This concept was investigated in a phase 2 trial that assigned 54 patients with metastatic gastric or GEJ adenocarcinoma to receive biweekly infusions of oxaliplatin, leucovorin, fluorouracil, and docetaxel.21 Median time to response was 1.54 months, and the overall response rate was 57.7%. Median progression-free survival (PFS) was 5.2 months, and OS was 11.1 months. The most common grade 3 or 4 toxicities included neutropenia (48%), leukopenia (27.8%), diarrhea (14.8%), and fatigue (11.1%).
Irinotecan-based regimens have also been extensively studied in the first-line treatment of metastatic gastric cancer, particularly as an alternative to platinum-based therapy, but superiority has not been established. The combination of fluorouracil, leucovorin, and irinotecan (FOLFIRI) was compared to ECX in a phase 3 trial.22 The study enrolled 416 patients with locally advanced or metastatic gastric or GEJ cancer. At a median follow up of 31 months, the time to progression was longer in the FOLFIRI arm as compared to the ECX arm (5.1 months versus 4.2 months, P = 0.008), but there was no difference in OS (9.5 months versus 9.7 months, P = 0.95), median PFS (5.3 months versus 5.8 months, P = 0.96), or response rate (39.2% versus 37.8%). However, the FOLFIRI regimen had an improved toxicity profile, with a lower overall rate of grade 3 or 4 toxicity (69% versus 84%, P < 0.001). Given these findings, the FOLFIRI regimen is an acceptable alternative to platinum-based therapy in suitable patients.22
HER2-POSITIVE DISEASE
The HER2 proto-oncogene, initially described in breast cancer, has been implicated in several malignancies, including gastric and esophageal cancer. Overexpression or amplification of HER2 can be found in up to 30% of gastric cancers.23 For these patients, adding trastuzumab to a standard regimen of platinum and fluoropyrimidine is the standard of care. The prospective phase 3 Trastuzumab for Gastric Cancer (ToGA) trial randomly assigned 594 patients with HER2-positive gastric cancer to receive either cisplatin and fluorouracil or capecitabine and cisplatin with trastuzumab (n = 294) or without (n = 290) trastuzumab every 3 weeks for a total of 6 cycles, followed by maintenance trastuzumab until disease progression was noted.24 HER2 positivity was defined as HER2 protein overexpression by IHC (cutoff of 3+) or gene amplification by fluorescence in situ hybridization (FISH); tumors with IHC 2+ patterns were followed with FISH studies to confirm positivity. The study found a higher incidence of HER2-positive tumors in patients with GEJ tumors compared to patients with distal gastric cancers (33% versus 20%).24 In this trial, the addition of trastuzumab was associated with an improvement in OS: 13.5 months in the trastuzumab cohort versus 11.1 months in those receiving chemotherapy alone (HR 0.74, P = 0.0048). There was not a significant difference in toxicities between the 2 cohorts, with nausea, emesis, and neutropenia being the most common adverse events. Rates of overall grade 3 or 4 events were similar as well (68% in each cohort). Further exploratory analysis was also conducted according to HER2 status by dividing patients into a “high-expressor” group (n = 446), defined as patients with IHC 3+ tumors or IHC 2+ and FISH positivity, and a “low-expressor” group (n = 131), which included patients with IHC 0 or 1+ tumors. Analysis of patients in the 2 subgroups demonstrated an improved OS with the addition of trastuzumab for the high-expressor cohort, with a median OS of 16 months (HR 0.65 [95% CI 0.51 to 0.83]) compared to 11.8 months in those receiving only chemotherapy.
Dual HER2 blockade has been investigated in metastatic gastric cancer. The phase 3 randomized JACOB trial assigned 780 patients to receive either trastuzumab with a cisplatin/fluoropyrimidine regimen with or without the addition of pertuzumab; the primary end point was OS.25 A non-statistically significant trend towards improvement in OS was found in the pertuzumab arm (17.5 months) as compared with the standard of care arm (14.2 months, HR 0.84, P = 0.0565). The pertuzumab/trastuzumab/chemotherapy cohort experienced a higher incidence of diarrhea (61.6% versus 35.1% in control arm). Cardiac toxicity was comparable in the 2 cohorts.
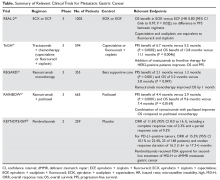
The Table provides a summary of relevant clinical trials in metastatic gastric cancer.
SECOND-LINE THERAPY
CASE CONTINUED
The patient receives capecitabine, oxaliplatin, and trastuzumab therapy for 6 cycles, followed by trastuzumab for another 3 cycles. While on therapy, he develops a painful right clavicular lesion. He undergoes magnetic resonance imaging of the right clavicle, which shows a lesion in the distal two-thirds of the right clavicle measuring 9.7 × 3.7 × 3.8 cm. The patient is started on palliative radiation to the clavicle. However, repeat CT imaging shows progressive liver metastases.
• What is the approach to second-line therapy for metastatic gastric cancer?
Improvements in our understanding of the molecular pathways that lead to tumorigenesis have contributed to the development of several targeted agents whose efficacy in gastric cancer is being investigated. The NCCN guidelines recommend that for all patients who progress on frontline therapy, second-line therapy consists of a combination of ramucirumab and paclitaxel. Other options include single-agent docetaxel, paclitaxel, irinotecan, or ramucirumab. Combination therapy using irinotecan with either docetaxel, fluorouracil, or cisplatin may also be used.
Ramucirumab, a human IgG1 monoclonal antibody that targets the vascular endothelial growth factor receptor 2 (VEGFR2), was initially approved in 2014 as monotherapy for patients who had previously progressed on first-line chemotherapy. Its approval was based on the results of the phase 3 randomized, double-blind placebo-controlled REGARD study.26 The trial randomly assigned 355 patients with advanced gastric or GEJ adenocarcinoma and disease progression after first-line platinum-containing or fluoropyrimidine-containing chemotherapy to receive best supportive care plus either ramucirumab (n = 238) or placebo (n = 117). Monotherapy with ramucirumab significantly improved median OS compared with placebo (5.2 months versus. 3.8 months; HR 0.776 [95% CI 0.6 to 0.99], P = 0.047). There was also an improvement in PFS of 2.1 months in the ramucirumab cohort, as compared to 1.3 months in the placebo cohort (P < 0.0001). Patients in the ramucirumab arm experienced a higher incidence of hypertension (16% versus 8%), but all other adverse events occurred at comparable rates. Five deaths in the ramucirumab group were thought to be secondary to the study drug, as compared to 2 deaths in the placebo group.
In the subsequent phase 3 RAINBOW trial, the addition of ramucirumab to paclitaxel was investigated, with 330 patients assigned to the combination group and 335 to the paclitaxel-only group.27 The trial again showed that combination therapy afforded patients a significant survival advantage compared to paclitaxel alone, with a median OS of 9.6 months versus 7.4 months for the monotherapy group (HR 0.807 [95% CI 0.678 to 0.962], P = 0.017). A PFS benefit of 4.4 months was observed in the combination therapy groups, as compared with 2.9 months in the monotherapy group (HR 0.635, P < 0.0001). The ramucirumab/paclitaxel group also had a higher overall response rate of 28% versus 16%. The combination cohort had an increased incidence of grade 3 or higher adverse hypertensive events (14% versus 2%) and neutropenia (41% versus 19%), while the incidence of grade 3 febrile neutropenic events was similar between the groups (3% versus 2%).
The addition of bevacizumab, another monoclonal antibody against VEGF, to standard chemotherapy regimens has been explored, but studies have failed to show a survival benefit with this agent in the first-line treatment of advanced gastric cancer. The phase 3 Avastin in Gastric Cancer (AVAGAST) trial was a multinational, randomized study where patients received either bevacizumab (n = 387) or placebo (n = 387) in addition to cisplatin and capecitabine.28 The substitution of fluorouracil for capecitabine was permitted for patients who were unable to tolerate oral medications. Cisplatin was administered for a maximum of 6 cycles, while capecitabine and bevacizumab were administered until disease progression. The study failed to show an improvement in OS, with a median OS of 12.1 months noted in the bevacizumab cohort, as compared to 10.1 months in the placebo arm (HR 0.87 [95% CI 0.73 to 1.03], P = 0.1002). However, there was a modest improvement in median PFS (6.7 months versus 5.3 months; HR 0.80 [95% CI 0.68 to 0.93], P = 0.0037) and overall response rate (46% versus 37.4%, P = 0.0315). The most commonly reported grade 3 to 5 adverse events included neutropenia (35%), anemia (10%), and loss of appetite (8%). Interestingly, in a follow-up report, higher serum levels of VEGF-A were thought to correlate with an enhanced response to bevacizumab.29 However, the routine use of biomarker analysis in selecting patients for treatment with bevacizumab in metastatic gastric cancer remains to be further clarified.
Use of other agents with anti-HER2 activity in the second-line treatment of patients who have experienced progression while on trastuzumab remains unclear. In the recent T-ACT trial, patients with disease refractory to frontline therapy with combination trastuzumab and fluoropyrimidine/platinum agents were randomly assigned to receive either weekly paclitaxel (n = 45) or weekly paclitaxel plus trastuzumab (n = 44).30 Patients in the combination cohort received an initial dose of trastuzumab 8 mg/kg followed by 6 mg/kg every 3 weeks until progression. The study did not find a difference in either PFS (3.19 months versus 3.68 months; HR 0.91 [95% CI 0.67 to 1.22], P = 0.33) or OS (9.95 months versus 10.2 months; HR 1.23 [95% CI 0.75 to 1.99], P = 0.20). The study thus failed to show a benefit to continuing trastuzumab after progression in the first-line setting.
Lapatinib in combination with paclitaxel has been compared to paclitaxel alone for the treatment of advanced HER2-positive gastric cancer in an Asian population in the phase 3 TyTAN trial.31 With a primary end point of OS, the study randomly assigned 129 patients to receive paclitaxel alone and 132 patients to receive paclitaxel with lapatinib. There was a nonsignificant trend towards improvement in OS in the combination group (11 months) as compared to the paclitaxel-only group(8.9 months, P = 0.1044), with no significant difference in median PFS (5.4 months versus 4.4 months). However, it is important to note that only 15 patients in this trial had previously been exposed to trastuzumab. Another trial, the phase 3 GATSBY study, examined the efficacy of trastuzumab emtansine in the second-line setting compared to taxanes alone and failed to show any improvement in PFS or OS.32 Given these results, no alternative anti-HER2 therapy has been proven to be efficacious for patients who are trastuzumab refractory. Therefore, including anti-HER2 therapy in the second-line treatment of HER2-positive gastric cancer is not recommended.
IMMUNOTHERAPY AND OTHER TARGETED THERAPIES
Several other targeted therapies have been studied in advanced gastric cancer, without any demonstrable survival benefit. The PI3K/AKT/mTOR pathway is known to be involved in regulation of cell growth and angiogenesis, and the mTOR inhibitor everolimus is widely used to treat other malignancies, including breast cancer. The use of everolimus in the second-line setting was studied in the phase 3 GRANITE-1 trial, where it was compared to best supportive care and failed to provide any survival benefit.33 Cetuximab, a recombinant human and mouse chimeric monoclonal antibody, and panitumumab, a recombinant human antibody against the epidermal growth factor receptor (EGFR), have also been examined in gastric and GEJ cancer patients. However, the large phase 3 EXPAND and REAL-3 trials did not show a survival benefit when these agents were added to standard chemotherapy.34,35
Overexpression of MET, a proto-oncogene and tyrosine kinase receptor, has also been implicated in gastric cancer progression. The ligand for MET is the hepatocyte growth factor (HGF), and aberrant signaling of this pathway has been shown to correlate with an aggressive gastric cancer phenotype and poorer OS by promoting tumor growth and angiogenesis. However, no MET inhibitors thus far have been found to be clinically effective. RILOMET-1 and RILOMET-2 were phase 3 trials examining the efficacy of rilotumumab, a humanized anti-HGF antibody, in combination with chemotherapy (ECX and cisplatin with capecitabine, respectively) for the frontline treatment of MET-positive GEJ and gastric cancers. Both studies were discontinued due to a higher treatment-related mortality in patients receiving rilotumumab, with a higher incidence of adverse events due to disease progression being noted.36 Similarly, onartuzumab, a monovalent monoclonal antibody against the MET receptor, was investigated in the phase 3 METGastric trial in combination with modified FOLFOX6 as first-line therapy for HER2-negative, MET-positive metastatic GEJ and gastric cancers. The study did not demonstrate any significant improvements in OS or PFS.37
There has been significant interest in incorporating immunotherapy in the treatment of early and metastatic gastric cancer. Pembrolizumab is the first programmed death receptor (PD-1) inhibitor to be approved for treatment of patients with PD-L1−positive advanced gastric cancer who had previously received 2 or more lines of chemotherapy. Although earlier studies of pembrolizumab in lung cancer utilized the tumor proportion score (TPS) to determine PD-L1 positivity, this was not found to be applicable to gastric cancer. Instead, the combined positive score (CPS) is used in gastric cancer. The CPS evaluates the number of tumor cells and immune cells (macrophages and lymphocytes) that stain positive for PD-L1 relative to all viable tumor cells. Comparatively, the TPS only examines the percentage of viable tumor cells that show complete or partial positive staining for PD-L1. A CPS score of 1 or greater identifies patients who would be suitable candidates for pembrolizumab.
The approval of pembrolizumab was based on the positive findings from the recent KEYNOTE-059 trial.38 The study included 259 patients who had previously received either fluoropyrimidine, cisplatin, or anti-HER2 therapy, with 148 patients (55%) of these patients having PD-L1−positive tumors. The PD-L1 status was determined using a pharmDx Kit, which is now approved by the US Food and Drug Administration to select patients who could benefit from pembrolizumab treatment. CPS was calculated as the number of PD-L1−staining cells divided by the total number of evaluated cells. The study included patients with microsatellite stable (MSI-S), undetermined, or deficient MMR status. The overall response rate to pembrolizumab across all patients was 11.6%, median PFS was 2 months, and the 12-month OS rate was 23.4%. In the subset of patients with MSI-H tumors, the overall response rate was 57.1%, with a complete response rate of 14.3%; in those with MSI-S tumors, the overall response rate was 9% and the complete response rate was 2.4%. Among patients with PD-L1–positive tumors, the overall response rate was 15.5% (95% CI 10.1% to 22.4). Common adverse events included fatigue, hypothyroidism, nausea, diarrhea, and arthralgia.38
CASE CONCLUSION
This patient with metastatic gastric cancer receives second-line chemotherapy with ramucirumab and paclitaxel. Follow-up imaging shows persistent liver metastases and new lung metastasis. Because the tumor is PD-L1–positive, the patient receives 4 cycles of pembrolizumab, with no significant change noted in disease burden. He notes a significant decline in functional status with increased weight loss, nausea, emesis, and fatigue. The patient opts to forego any further therapy and instead chooses to pursue supportive care only.
SUMMARY
Gastric cancer is the third most common cause of cancer death worldwide. Common risk factors for developing gastric cancer include H. pylori infection, smoking, alcohol abuse, radiation exposure, high-fat diet, and obesity. Patients presenting with alarm symptoms of nausea, emesis, early satiety, abdominal pain, or weight loss should be fully evaluated with upper GI endoscopy. If there is suspicion for metastatic disease, CT evaluation of the chest, abdomen, and pelvis with oral and intravenous contrast should be obtained. Treatment of patients with metastatic gastric cancer is guided by their performance status at presentation. For patients with good performance status, a combination of platinum and fluoropyrimidine therapy, such as FOLFOX, can be considered. Doublet chemotherapy regimens are preferred over triplet chemotherapy regimens given their better tolerability. For patients with HER2-positive disease, the addition of trastuzumab to the platinum and fluoropyrimidine backbone is the standard of care in the first line.
Several targeted agents have been studied in patients progressing on initial therapy, with ramucirumab and paclitaxel being considered the regimen of choice in the second line. No anti-HER2 therapy has been approved for patients who are refractory to trastuzumab. Pembrolizumab is approved for use in patients who are PD-L1–positive and have previously progressed on at least 2 lines of chemotherapy. Pembrolizumab is also approved for the treatment of patients with unresectable or metastatic, MSI-H or MMR-deficient gastric cancers that have progressed after prior treatment and who have no satisfactory alternative treatment options.
1. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–41
2. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524–8.
3. Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 2018;10:239–48.
4. Olefson S, Moss SF. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer 2015;18:23–32.
5. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 2014;345:196–202.
6. Espinoza JL, Matsumoto A, Tanaka H, Matsumura I. Gastric microbiota: An emerging player in Helicobacter pylori-induced gastric malignancies. Cancer Lett 2018;414:147–52.
7. Chmiela M, Gonciarz W. Molecular mimicry in Helicobacter pylori infections. World J Gastroenterol 2017;23:3964–77.
8. Isobe Y, Nashimoto A, Akazawa K, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer 2011;14:301–16.
9. Ford A, Gurusamy KS, Delaney B, et al. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive patients. Cochrane Database Syst Rev 2004(4):CD003840.
10. Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–13.
11. Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016;388:2654–64.
12. Chan BA , Sim HW, Natori A, et al. Survival outcomes for de novo versus relapsed stage IV gastric and gastroesophageal junction (GEJ) adenocarcinoma [abstract]. J Clin Oncol 2018;36(no. 4 suppl):148.
13. DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology. 9th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011.
14. Siewert JR, Hölscher AH, Becker K, Gössner W. [Cardia cancer: attempt at a therapeutically relevant classification]. [Article in German.] Chirurg 1987;58:25–32.
15. Amin MB, Edge SB, Greene FL, et al, eds. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
16. Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2017;8:CD004064.
17. Qiu H, Zhou Z. [Updates and interpretation on NCCN clinical practice guidelines for gastric cancer 2017 version 5]. [Article in Chinese.] Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:160–4.
18. Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997;15:261–7.
19. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991–7.
20. Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010;362:858–9.
21. Al-Batran SE, Hartmann JT, Hofheinz R, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2008;19:1882–7.
22. Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Federation Francophone de Cancerologie Digestive, Federation Nationale des Centres de Lutte Contre le Cancer, and Groupe Cooperateur Multidisciplinaire en Oncologie) study. J Clin Oncol 2014;32:3520–6.
23. Boku N. HER2-positive gastric cancer. Gastric Cancer 2014;17:1–12.
24. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97.
25. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab + trastuzumab + chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer: Final analysis of a Phase III study (JACOB) [abstract]. Ann Oncol 2017;28(suppl 5):6160.
26. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–9.
27. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35.
28. Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968–76.
29. Van Cutsem E, de Haas S, Kang YK, et al, Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119–27.
30. Makiyama A, Sagara K, Kawada J, et al. A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT) [abstract]. J Clin Oncol 2018;36(no. 15 suppl):4011.
31. Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039–49.
32. Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017;18:640–53.
33. Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013;31:3935–43.
34. Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490–9.
35. Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481–9.
36. Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1467–82.
37. Shah MA, Bang YJ, Lordick F, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol 2017;3:620–7.
38. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4(5):e180013.
INTRODUCTION
According to the Surveillance, Epidemiology and End Results database, in 2017 there were 28,000 new cases of gastric cancer, accounting for 1.8% of all malignancies in the United States, and an estimated 10,960 gastric cancer–related deaths.1 Worldwide, gastric cancer is the fifth most common malignancy and the third most common cause of death from any cancer.2 The incidence of gastric cancer varies significantly by geographic region, with countries in Eastern Asia (China, Japan), Eastern Europe, and Central and South America accounting for 50% of all new cases.3 Although the incidence of gastric cancer has declined in recent years, this decrease has not been observed consistently across all nations.2 In particular, the incidence of gastric cancers arising from the cardia has been increasing, which is perhaps due to a higher prevalence of obesity in Western societies.4
In this article, we review key aspects of management of metastatic gastric cancer, including selection of first- and second-line therapy, and discuss targeted agents and upcoming clinical trials.
EPIDEMIOLOGY AND RISK FACTORS
Chronic infection with Helicobacter pylori, a gram-negative bacterium, is a strong etiological factor for the development of gastric cancer, contributing to up to 70% of cases.2 The pathogen can colonize the gastric mucosa, leading to chronic inflammation. Although most patients remain asymptomatic, 1% to 3% develop gastric cancer and another 0.1% develop mucosa-associated lymphoid tissue lymphoma.5 H. pylori infection is more commonly associated with cancer of the gastric body than with cancer of the gastroesophageal junction (GEJ). The increased burden of gastric cancer in countries in Eastern Asia, Latin America, and Eastern Europe has been correlated to the prevalence of chronic H. pylori infection in these areas.
Carcinogenesis secondary to H. pylori infection may occur via several mechanisms. First, H. pylori can release virulence factors, such as cytotoxin-associated gene A, vacuolating cytotoxin, and outer membrane proteins, into the cytosol of host cells, leading to changes in patterns of cell proliferation and apoptosis.6 These virulence factors can modulate the host immune system, attenuating it to promote dysplasia. In addition, continued recognition of these factors by the immune system leads to a persistent inflammatory response, with the release of cytokines such as interleukin (IL) -1β, IL-6, and IL-8. This leads to chronic mucosal damage, further promoting dysplasia with eventual transformation into adenocarcinoma.7 In Japan and Korea, where screening for H. pylori infection is routinely performed, there have been improvements in overall survival (OS) rates for gastric cancer, with 5-year OS rates of 70%.8 The International Agency for Research on Cancer recommends further research into population-based screening and treatment programs for patients with chronic H. pylori infection. However, despite this recommendation, optimal screening strategies are not clearly defined.9
Other risk factors for the development of gastric cancer include chronic gastroesophageal reflux disease; smoking; alcohol use; exposure to radiation; diets high in fats, salt, and smoked items and low in fruits and vegetables; obesity; and exposure to chemotherapeutic agents such as procarbazine.10 Another pathogen suspected, but not proven, to be associated with increased risk for gastric cancer is the Epstein-Barr virus, a human herpesvirus found in 80% of all gastric carcinomas with lymphoid features.11 In addition, whether the use of medications such as statins and nonsteroidal anti-inflammatory drugs confers a decreased risk of gastric cancers remains unclear.10
EVALUATION
CASE PRESENTATION
A 55-year-old Caucasian man with a history of type 2 diabetes mellitus presents to the gastrointestinal (GI) clinic with a 6-month history of dysphagia. The dysphagia is worsened with ingestion of solids, particularly towards the end of the day. He states that the food often gets “stuck in the middle of the chest.” The patient denies any nausea or emesis but notes that he has a poor appetite. He reports having worsening mid-epigastric abdominal pain that is non-radiating, dull in character, and 6/10 in intensity. He also reports a 10-lb weight loss over the past 2 months. He has no previous history of reflux, chest pain, dyspnea, or cough. Review of systems is otherwise benign. Physical exam is within normal limits.
• Which tests should be conducted when gastric cancer is suspected?
Persistent epigastric abdominal pain and weight loss are the most common early symptoms of gastric cancer. Nausea, early satiety, dysphagia, and occult GI bleeding can be other presenting signs. Patients presenting with alarm symptoms of nausea, emesis, early satiety, abdominal pain, or weight loss should be fully evaluated with upper GI endoscopy. Early diagnosis of gastric cancer is essential in obtaining a curative resection. However, at least 40% of patients present with de novo metastatic disease at the time of initial diagnosis.12 Gastric cancer spreads by direct extension through the gastric wall, with the liver, peritoneum, and regional lymph nodes being the most common sites of metastatic deposits.13 Classically, Virchow’s node, the left supraclavicular lymph node, is involved with metastatic gastric cancer. Involvement of the left axillary lymph node (Irish node) or a periumbilical nodule (Sister Mary Joseph node) may also be observed. Other, less commonly noted sites of metastatic disease include the ovaries, central nervous system, bone, lung, and soft tissues.13
Upper GI endoscopy is the best method for determining tumor location and extent and obtaining a specimen for a definitive tissue diagnosis.14 It is essential to accurately identify the location of the tumor in the stomach and relative to the GEJ. The American Joint Committee on Cancer classification defines tumors involving the GEJ with an epicenter no more than 2 cm into the proximal stomach as esophageal cancers.15 Tumors of the GEJ with their epicenter more than 2 cm into the proximal stomach are defined as gastric cancers. If metastatic disease is suspected, computed tomography (CT) scan of the chest, abdomen, and pelvis with oral and intravenous contrast can be obtained to determine the extent of disease spread. In the absence of any metastatic disease, endoscopic ultrasound (EUS) should be conducted to determine the depth of tumor invasion (T staging) and lymph node status. In the era of targeted therapy, patients with metastatic disease should undergo testing for human epidermal growth factor-2 (HER-2) expression, microsatellite instability (MSI), and programmed death ligand 1 (PD-L1) expression. Patients should be staged according to the TNM staging system.
FIRST-LINE TREATMENT OPTIONS
CASE CONTINUED
The patient undergoes esophagoduodenoscopy (EGD) and is found to have a gastric cardia mass extending into the distal esophagus. EUS also demonstrates multiple abdominal and mediastinal lymph nodes. No gastric outlet obstruction is found. Biopsy shows poorly differentiated invasive adenocarcinoma. Warthin–Starry stain is negative for H. pylori organism. The tumor cells are positive for cytokeratin (CK7), CK19, and mucin-1 gene (MUC1); focally positive for CK20; and negative for MUC2. HER2 testing results are reported as immunohistochemistry (IHC) 3+, consistent with strongly positive HER2 protein expression. Further IHC testing for mismatch repair (MMR) proteins shows intact nuclear expression of MLH1, MSH2, MSH6, and PMS2 protein, consistent with a low probability of MSI-high tumor. The tumor is found to be PD-L1 positive. Imaging reveals abnormal mass-like nodular thickening of the gastric wall, with an infiltrative opacity within the pancreatico-duodenal groove, suspicious for tumor infiltration. Multiple metastatic deposits are noted in the liver, peritoneum, and bilateral lungs. There is extensive gastrohepatic ligament and periportal lymphadenopathy and mild enlargement of the pulmonary hilar lymph nodes. These findings are consistent with stage 4 (T4bN3aM1) gastric cancer. Given these findings, staging laparoscopy is deferred.
• What are the first-line treatment options for patients with metastatic gastric cancer?
Patients with metastatic gastric cancer have a poor prognosis, and management is stratified based on performance status (Figure). In patients with good performance status, systemic chemotherapy is the mainstay of treatment. The goal of therapy is not curative, but rather treatment focuses on palliation of symptoms arising from tumor spread. Given this treatment goal, there has been considerable interest in clarifying the utility of chemotherapy as opposed to best supportive care. In a recent Cochrane review of 64 randomized control trials involving 11,698 patients, chemotherapy was found to improve OS by 6.7 months as compared to best supportive care (hazard ratio [HR] 0.3 [95% confidence interval {CI} 0.24 to 0.55]).16 Five classes of cytotoxic chemotherapeutic agents have demonstrated activity in gastric cancer. These include fluoropyrimidine (either infusional fluorouracil or capecitabine), platinum agents (cisplatin or oxaliplatin), taxanes (docetaxel or paclitaxel), anthracyclines (epirubicin), and irinotecan.13 Treatment options are further divided based on whether the patient has HER2-overexpressing or non-expressing malignancy.
HER2-NEGATIVE DISEASE
For patients with HER2-negative disease, National Comprehensive Cancer Network (NCCN) guidelines recommend using 2-drug combination regimens rather than 3 drugs, given concern for increased toxicity with 3-drug regimens.17 For patients with a performance status of 0 to 1, utilization of a 3-drug regimen is a reasonable alternative. The combination of a fluoropyrimidine with a platinum agent is considered the standard of care, with regimens such as fluorouracil, leucovorin, and oxaliplatin (FOLFOX) being commonly used.
Epirubicin-containing regimens have also been extensively studied in advanced gastric cancer. In a study of 274 previously untreated patients with GEJ cancers, the combination of epirubicin, cisplatin, and fluorouracil (ECF) was compared to fluorouracil, doxorubicin, and methotrexate (FAMTX). There was an OS benefit favoring ECF (8.9 months versus 5.7 months) at 1 year (95% CI 27% to 45%, P = 0.0009). The ECF regimen was associated with an increased risk of nausea, emesis, and alopecia, while more hematologic toxicity and infections were noted with the FAMTX regimen.18 In addition, in a phase 3 trial, Van Cutsem and colleagues examined the role of docetaxel in combination with cisplatin and fluorouracil (DCF) compared to cisplatin and fluorouracil alone. Addition of docetaxel led to improved OS and time to progression (9.2 months versus 8.6 months for cisplatin and fluorouracil alone, P = 0.02) but with an increased risk of grade 3 and 4 toxicities (69% versus 59%). These adverse events included neutropenia (82% versus 57% of cisplatin and fluorouracil patients), diarrhea (19% versus 8%), stomatitis (21% versus 27%), and fatigue (19% versus 14%).19
The landmark phase 3 REAL-2 study compared 4 chemotherapy regimens in patients with untreated advanced esophagogastric cancer. This study was conducted to determine if the efficacy of cisplatin and oxaliplatin, a third-generation platinum agent, is equivalent to that of fluorouracil and capecitabine, an oral fluoropyrimidine. In this trial, a 2 × 2 design was used to compare 4 regimens: ECF versus epirubicin, cisplatin, and capecitabine (ECX) versus epirubicin, oxaliplatin, and fluorouracil (EOF) versus epirubicin, oxaliplatin, and capecitabine (EOX). The study found EOX to be noninferior to ECF, with a trend towards improved OS compared to other combination regimens (11.2 months versus 9.9 months, HR 0.80 [95% CI 0.66 to 0.97], P = 0.02).20 Thus, the study demonstrated that an oxaliplatin and capecitabine-based regimen could replace cisplatin and fluorouracil. Given that fluorouracil administration requires long continuous infusions, the oral-based capecitabine regimen is an attractive option for patients.
Several trials have demonstrated the equivalency of oxaliplatin with cisplatin in combination regimens for the treatment of advanced gastric cancer. Oxaliplatin has the benefit of an improved toxicity profile as compared to cisplatin, with the major dose-limiting toxicity being peripheral neuropathy
Given previous evidence that DCF (docetaxel, cisplatin, fluorouracil) is superior to cisplatin and fluorouracil alone, there was interest in determining if the addition of docetaxel to a backbone of fluorouracil, oxaliplatin, and leucovorin (FLO) could elicit a higher response rate. This concept was investigated in a phase 2 trial that assigned 54 patients with metastatic gastric or GEJ adenocarcinoma to receive biweekly infusions of oxaliplatin, leucovorin, fluorouracil, and docetaxel.21 Median time to response was 1.54 months, and the overall response rate was 57.7%. Median progression-free survival (PFS) was 5.2 months, and OS was 11.1 months. The most common grade 3 or 4 toxicities included neutropenia (48%), leukopenia (27.8%), diarrhea (14.8%), and fatigue (11.1%).
Irinotecan-based regimens have also been extensively studied in the first-line treatment of metastatic gastric cancer, particularly as an alternative to platinum-based therapy, but superiority has not been established. The combination of fluorouracil, leucovorin, and irinotecan (FOLFIRI) was compared to ECX in a phase 3 trial.22 The study enrolled 416 patients with locally advanced or metastatic gastric or GEJ cancer. At a median follow up of 31 months, the time to progression was longer in the FOLFIRI arm as compared to the ECX arm (5.1 months versus 4.2 months, P = 0.008), but there was no difference in OS (9.5 months versus 9.7 months, P = 0.95), median PFS (5.3 months versus 5.8 months, P = 0.96), or response rate (39.2% versus 37.8%). However, the FOLFIRI regimen had an improved toxicity profile, with a lower overall rate of grade 3 or 4 toxicity (69% versus 84%, P < 0.001). Given these findings, the FOLFIRI regimen is an acceptable alternative to platinum-based therapy in suitable patients.22
HER2-POSITIVE DISEASE
The HER2 proto-oncogene, initially described in breast cancer, has been implicated in several malignancies, including gastric and esophageal cancer. Overexpression or amplification of HER2 can be found in up to 30% of gastric cancers.23 For these patients, adding trastuzumab to a standard regimen of platinum and fluoropyrimidine is the standard of care. The prospective phase 3 Trastuzumab for Gastric Cancer (ToGA) trial randomly assigned 594 patients with HER2-positive gastric cancer to receive either cisplatin and fluorouracil or capecitabine and cisplatin with trastuzumab (n = 294) or without (n = 290) trastuzumab every 3 weeks for a total of 6 cycles, followed by maintenance trastuzumab until disease progression was noted.24 HER2 positivity was defined as HER2 protein overexpression by IHC (cutoff of 3+) or gene amplification by fluorescence in situ hybridization (FISH); tumors with IHC 2+ patterns were followed with FISH studies to confirm positivity. The study found a higher incidence of HER2-positive tumors in patients with GEJ tumors compared to patients with distal gastric cancers (33% versus 20%).24 In this trial, the addition of trastuzumab was associated with an improvement in OS: 13.5 months in the trastuzumab cohort versus 11.1 months in those receiving chemotherapy alone (HR 0.74, P = 0.0048). There was not a significant difference in toxicities between the 2 cohorts, with nausea, emesis, and neutropenia being the most common adverse events. Rates of overall grade 3 or 4 events were similar as well (68% in each cohort). Further exploratory analysis was also conducted according to HER2 status by dividing patients into a “high-expressor” group (n = 446), defined as patients with IHC 3+ tumors or IHC 2+ and FISH positivity, and a “low-expressor” group (n = 131), which included patients with IHC 0 or 1+ tumors. Analysis of patients in the 2 subgroups demonstrated an improved OS with the addition of trastuzumab for the high-expressor cohort, with a median OS of 16 months (HR 0.65 [95% CI 0.51 to 0.83]) compared to 11.8 months in those receiving only chemotherapy.
Dual HER2 blockade has been investigated in metastatic gastric cancer. The phase 3 randomized JACOB trial assigned 780 patients to receive either trastuzumab with a cisplatin/fluoropyrimidine regimen with or without the addition of pertuzumab; the primary end point was OS.25 A non-statistically significant trend towards improvement in OS was found in the pertuzumab arm (17.5 months) as compared with the standard of care arm (14.2 months, HR 0.84, P = 0.0565). The pertuzumab/trastuzumab/chemotherapy cohort experienced a higher incidence of diarrhea (61.6% versus 35.1% in control arm). Cardiac toxicity was comparable in the 2 cohorts.
The Table provides a summary of relevant clinical trials in metastatic gastric cancer.
SECOND-LINE THERAPY
CASE CONTINUED
The patient receives capecitabine, oxaliplatin, and trastuzumab therapy for 6 cycles, followed by trastuzumab for another 3 cycles. While on therapy, he develops a painful right clavicular lesion. He undergoes magnetic resonance imaging of the right clavicle, which shows a lesion in the distal two-thirds of the right clavicle measuring 9.7 × 3.7 × 3.8 cm. The patient is started on palliative radiation to the clavicle. However, repeat CT imaging shows progressive liver metastases.
• What is the approach to second-line therapy for metastatic gastric cancer?
Improvements in our understanding of the molecular pathways that lead to tumorigenesis have contributed to the development of several targeted agents whose efficacy in gastric cancer is being investigated. The NCCN guidelines recommend that for all patients who progress on frontline therapy, second-line therapy consists of a combination of ramucirumab and paclitaxel. Other options include single-agent docetaxel, paclitaxel, irinotecan, or ramucirumab. Combination therapy using irinotecan with either docetaxel, fluorouracil, or cisplatin may also be used.
Ramucirumab, a human IgG1 monoclonal antibody that targets the vascular endothelial growth factor receptor 2 (VEGFR2), was initially approved in 2014 as monotherapy for patients who had previously progressed on first-line chemotherapy. Its approval was based on the results of the phase 3 randomized, double-blind placebo-controlled REGARD study.26 The trial randomly assigned 355 patients with advanced gastric or GEJ adenocarcinoma and disease progression after first-line platinum-containing or fluoropyrimidine-containing chemotherapy to receive best supportive care plus either ramucirumab (n = 238) or placebo (n = 117). Monotherapy with ramucirumab significantly improved median OS compared with placebo (5.2 months versus. 3.8 months; HR 0.776 [95% CI 0.6 to 0.99], P = 0.047). There was also an improvement in PFS of 2.1 months in the ramucirumab cohort, as compared to 1.3 months in the placebo cohort (P < 0.0001). Patients in the ramucirumab arm experienced a higher incidence of hypertension (16% versus 8%), but all other adverse events occurred at comparable rates. Five deaths in the ramucirumab group were thought to be secondary to the study drug, as compared to 2 deaths in the placebo group.
In the subsequent phase 3 RAINBOW trial, the addition of ramucirumab to paclitaxel was investigated, with 330 patients assigned to the combination group and 335 to the paclitaxel-only group.27 The trial again showed that combination therapy afforded patients a significant survival advantage compared to paclitaxel alone, with a median OS of 9.6 months versus 7.4 months for the monotherapy group (HR 0.807 [95% CI 0.678 to 0.962], P = 0.017). A PFS benefit of 4.4 months was observed in the combination therapy groups, as compared with 2.9 months in the monotherapy group (HR 0.635, P < 0.0001). The ramucirumab/paclitaxel group also had a higher overall response rate of 28% versus 16%. The combination cohort had an increased incidence of grade 3 or higher adverse hypertensive events (14% versus 2%) and neutropenia (41% versus 19%), while the incidence of grade 3 febrile neutropenic events was similar between the groups (3% versus 2%).
The addition of bevacizumab, another monoclonal antibody against VEGF, to standard chemotherapy regimens has been explored, but studies have failed to show a survival benefit with this agent in the first-line treatment of advanced gastric cancer. The phase 3 Avastin in Gastric Cancer (AVAGAST) trial was a multinational, randomized study where patients received either bevacizumab (n = 387) or placebo (n = 387) in addition to cisplatin and capecitabine.28 The substitution of fluorouracil for capecitabine was permitted for patients who were unable to tolerate oral medications. Cisplatin was administered for a maximum of 6 cycles, while capecitabine and bevacizumab were administered until disease progression. The study failed to show an improvement in OS, with a median OS of 12.1 months noted in the bevacizumab cohort, as compared to 10.1 months in the placebo arm (HR 0.87 [95% CI 0.73 to 1.03], P = 0.1002). However, there was a modest improvement in median PFS (6.7 months versus 5.3 months; HR 0.80 [95% CI 0.68 to 0.93], P = 0.0037) and overall response rate (46% versus 37.4%, P = 0.0315). The most commonly reported grade 3 to 5 adverse events included neutropenia (35%), anemia (10%), and loss of appetite (8%). Interestingly, in a follow-up report, higher serum levels of VEGF-A were thought to correlate with an enhanced response to bevacizumab.29 However, the routine use of biomarker analysis in selecting patients for treatment with bevacizumab in metastatic gastric cancer remains to be further clarified.
Use of other agents with anti-HER2 activity in the second-line treatment of patients who have experienced progression while on trastuzumab remains unclear. In the recent T-ACT trial, patients with disease refractory to frontline therapy with combination trastuzumab and fluoropyrimidine/platinum agents were randomly assigned to receive either weekly paclitaxel (n = 45) or weekly paclitaxel plus trastuzumab (n = 44).30 Patients in the combination cohort received an initial dose of trastuzumab 8 mg/kg followed by 6 mg/kg every 3 weeks until progression. The study did not find a difference in either PFS (3.19 months versus 3.68 months; HR 0.91 [95% CI 0.67 to 1.22], P = 0.33) or OS (9.95 months versus 10.2 months; HR 1.23 [95% CI 0.75 to 1.99], P = 0.20). The study thus failed to show a benefit to continuing trastuzumab after progression in the first-line setting.
Lapatinib in combination with paclitaxel has been compared to paclitaxel alone for the treatment of advanced HER2-positive gastric cancer in an Asian population in the phase 3 TyTAN trial.31 With a primary end point of OS, the study randomly assigned 129 patients to receive paclitaxel alone and 132 patients to receive paclitaxel with lapatinib. There was a nonsignificant trend towards improvement in OS in the combination group (11 months) as compared to the paclitaxel-only group(8.9 months, P = 0.1044), with no significant difference in median PFS (5.4 months versus 4.4 months). However, it is important to note that only 15 patients in this trial had previously been exposed to trastuzumab. Another trial, the phase 3 GATSBY study, examined the efficacy of trastuzumab emtansine in the second-line setting compared to taxanes alone and failed to show any improvement in PFS or OS.32 Given these results, no alternative anti-HER2 therapy has been proven to be efficacious for patients who are trastuzumab refractory. Therefore, including anti-HER2 therapy in the second-line treatment of HER2-positive gastric cancer is not recommended.
IMMUNOTHERAPY AND OTHER TARGETED THERAPIES
Several other targeted therapies have been studied in advanced gastric cancer, without any demonstrable survival benefit. The PI3K/AKT/mTOR pathway is known to be involved in regulation of cell growth and angiogenesis, and the mTOR inhibitor everolimus is widely used to treat other malignancies, including breast cancer. The use of everolimus in the second-line setting was studied in the phase 3 GRANITE-1 trial, where it was compared to best supportive care and failed to provide any survival benefit.33 Cetuximab, a recombinant human and mouse chimeric monoclonal antibody, and panitumumab, a recombinant human antibody against the epidermal growth factor receptor (EGFR), have also been examined in gastric and GEJ cancer patients. However, the large phase 3 EXPAND and REAL-3 trials did not show a survival benefit when these agents were added to standard chemotherapy.34,35
Overexpression of MET, a proto-oncogene and tyrosine kinase receptor, has also been implicated in gastric cancer progression. The ligand for MET is the hepatocyte growth factor (HGF), and aberrant signaling of this pathway has been shown to correlate with an aggressive gastric cancer phenotype and poorer OS by promoting tumor growth and angiogenesis. However, no MET inhibitors thus far have been found to be clinically effective. RILOMET-1 and RILOMET-2 were phase 3 trials examining the efficacy of rilotumumab, a humanized anti-HGF antibody, in combination with chemotherapy (ECX and cisplatin with capecitabine, respectively) for the frontline treatment of MET-positive GEJ and gastric cancers. Both studies were discontinued due to a higher treatment-related mortality in patients receiving rilotumumab, with a higher incidence of adverse events due to disease progression being noted.36 Similarly, onartuzumab, a monovalent monoclonal antibody against the MET receptor, was investigated in the phase 3 METGastric trial in combination with modified FOLFOX6 as first-line therapy for HER2-negative, MET-positive metastatic GEJ and gastric cancers. The study did not demonstrate any significant improvements in OS or PFS.37
There has been significant interest in incorporating immunotherapy in the treatment of early and metastatic gastric cancer. Pembrolizumab is the first programmed death receptor (PD-1) inhibitor to be approved for treatment of patients with PD-L1−positive advanced gastric cancer who had previously received 2 or more lines of chemotherapy. Although earlier studies of pembrolizumab in lung cancer utilized the tumor proportion score (TPS) to determine PD-L1 positivity, this was not found to be applicable to gastric cancer. Instead, the combined positive score (CPS) is used in gastric cancer. The CPS evaluates the number of tumor cells and immune cells (macrophages and lymphocytes) that stain positive for PD-L1 relative to all viable tumor cells. Comparatively, the TPS only examines the percentage of viable tumor cells that show complete or partial positive staining for PD-L1. A CPS score of 1 or greater identifies patients who would be suitable candidates for pembrolizumab.
The approval of pembrolizumab was based on the positive findings from the recent KEYNOTE-059 trial.38 The study included 259 patients who had previously received either fluoropyrimidine, cisplatin, or anti-HER2 therapy, with 148 patients (55%) of these patients having PD-L1−positive tumors. The PD-L1 status was determined using a pharmDx Kit, which is now approved by the US Food and Drug Administration to select patients who could benefit from pembrolizumab treatment. CPS was calculated as the number of PD-L1−staining cells divided by the total number of evaluated cells. The study included patients with microsatellite stable (MSI-S), undetermined, or deficient MMR status. The overall response rate to pembrolizumab across all patients was 11.6%, median PFS was 2 months, and the 12-month OS rate was 23.4%. In the subset of patients with MSI-H tumors, the overall response rate was 57.1%, with a complete response rate of 14.3%; in those with MSI-S tumors, the overall response rate was 9% and the complete response rate was 2.4%. Among patients with PD-L1–positive tumors, the overall response rate was 15.5% (95% CI 10.1% to 22.4). Common adverse events included fatigue, hypothyroidism, nausea, diarrhea, and arthralgia.38
CASE CONCLUSION
This patient with metastatic gastric cancer receives second-line chemotherapy with ramucirumab and paclitaxel. Follow-up imaging shows persistent liver metastases and new lung metastasis. Because the tumor is PD-L1–positive, the patient receives 4 cycles of pembrolizumab, with no significant change noted in disease burden. He notes a significant decline in functional status with increased weight loss, nausea, emesis, and fatigue. The patient opts to forego any further therapy and instead chooses to pursue supportive care only.
SUMMARY
Gastric cancer is the third most common cause of cancer death worldwide. Common risk factors for developing gastric cancer include H. pylori infection, smoking, alcohol abuse, radiation exposure, high-fat diet, and obesity. Patients presenting with alarm symptoms of nausea, emesis, early satiety, abdominal pain, or weight loss should be fully evaluated with upper GI endoscopy. If there is suspicion for metastatic disease, CT evaluation of the chest, abdomen, and pelvis with oral and intravenous contrast should be obtained. Treatment of patients with metastatic gastric cancer is guided by their performance status at presentation. For patients with good performance status, a combination of platinum and fluoropyrimidine therapy, such as FOLFOX, can be considered. Doublet chemotherapy regimens are preferred over triplet chemotherapy regimens given their better tolerability. For patients with HER2-positive disease, the addition of trastuzumab to the platinum and fluoropyrimidine backbone is the standard of care in the first line.
Several targeted agents have been studied in patients progressing on initial therapy, with ramucirumab and paclitaxel being considered the regimen of choice in the second line. No anti-HER2 therapy has been approved for patients who are refractory to trastuzumab. Pembrolizumab is approved for use in patients who are PD-L1–positive and have previously progressed on at least 2 lines of chemotherapy. Pembrolizumab is also approved for the treatment of patients with unresectable or metastatic, MSI-H or MMR-deficient gastric cancers that have progressed after prior treatment and who have no satisfactory alternative treatment options.
INTRODUCTION
According to the Surveillance, Epidemiology and End Results database, in 2017 there were 28,000 new cases of gastric cancer, accounting for 1.8% of all malignancies in the United States, and an estimated 10,960 gastric cancer–related deaths.1 Worldwide, gastric cancer is the fifth most common malignancy and the third most common cause of death from any cancer.2 The incidence of gastric cancer varies significantly by geographic region, with countries in Eastern Asia (China, Japan), Eastern Europe, and Central and South America accounting for 50% of all new cases.3 Although the incidence of gastric cancer has declined in recent years, this decrease has not been observed consistently across all nations.2 In particular, the incidence of gastric cancers arising from the cardia has been increasing, which is perhaps due to a higher prevalence of obesity in Western societies.4
In this article, we review key aspects of management of metastatic gastric cancer, including selection of first- and second-line therapy, and discuss targeted agents and upcoming clinical trials.
EPIDEMIOLOGY AND RISK FACTORS
Chronic infection with Helicobacter pylori, a gram-negative bacterium, is a strong etiological factor for the development of gastric cancer, contributing to up to 70% of cases.2 The pathogen can colonize the gastric mucosa, leading to chronic inflammation. Although most patients remain asymptomatic, 1% to 3% develop gastric cancer and another 0.1% develop mucosa-associated lymphoid tissue lymphoma.5 H. pylori infection is more commonly associated with cancer of the gastric body than with cancer of the gastroesophageal junction (GEJ). The increased burden of gastric cancer in countries in Eastern Asia, Latin America, and Eastern Europe has been correlated to the prevalence of chronic H. pylori infection in these areas.
Carcinogenesis secondary to H. pylori infection may occur via several mechanisms. First, H. pylori can release virulence factors, such as cytotoxin-associated gene A, vacuolating cytotoxin, and outer membrane proteins, into the cytosol of host cells, leading to changes in patterns of cell proliferation and apoptosis.6 These virulence factors can modulate the host immune system, attenuating it to promote dysplasia. In addition, continued recognition of these factors by the immune system leads to a persistent inflammatory response, with the release of cytokines such as interleukin (IL) -1β, IL-6, and IL-8. This leads to chronic mucosal damage, further promoting dysplasia with eventual transformation into adenocarcinoma.7 In Japan and Korea, where screening for H. pylori infection is routinely performed, there have been improvements in overall survival (OS) rates for gastric cancer, with 5-year OS rates of 70%.8 The International Agency for Research on Cancer recommends further research into population-based screening and treatment programs for patients with chronic H. pylori infection. However, despite this recommendation, optimal screening strategies are not clearly defined.9
Other risk factors for the development of gastric cancer include chronic gastroesophageal reflux disease; smoking; alcohol use; exposure to radiation; diets high in fats, salt, and smoked items and low in fruits and vegetables; obesity; and exposure to chemotherapeutic agents such as procarbazine.10 Another pathogen suspected, but not proven, to be associated with increased risk for gastric cancer is the Epstein-Barr virus, a human herpesvirus found in 80% of all gastric carcinomas with lymphoid features.11 In addition, whether the use of medications such as statins and nonsteroidal anti-inflammatory drugs confers a decreased risk of gastric cancers remains unclear.10
EVALUATION
CASE PRESENTATION
A 55-year-old Caucasian man with a history of type 2 diabetes mellitus presents to the gastrointestinal (GI) clinic with a 6-month history of dysphagia. The dysphagia is worsened with ingestion of solids, particularly towards the end of the day. He states that the food often gets “stuck in the middle of the chest.” The patient denies any nausea or emesis but notes that he has a poor appetite. He reports having worsening mid-epigastric abdominal pain that is non-radiating, dull in character, and 6/10 in intensity. He also reports a 10-lb weight loss over the past 2 months. He has no previous history of reflux, chest pain, dyspnea, or cough. Review of systems is otherwise benign. Physical exam is within normal limits.
• Which tests should be conducted when gastric cancer is suspected?
Persistent epigastric abdominal pain and weight loss are the most common early symptoms of gastric cancer. Nausea, early satiety, dysphagia, and occult GI bleeding can be other presenting signs. Patients presenting with alarm symptoms of nausea, emesis, early satiety, abdominal pain, or weight loss should be fully evaluated with upper GI endoscopy. Early diagnosis of gastric cancer is essential in obtaining a curative resection. However, at least 40% of patients present with de novo metastatic disease at the time of initial diagnosis.12 Gastric cancer spreads by direct extension through the gastric wall, with the liver, peritoneum, and regional lymph nodes being the most common sites of metastatic deposits.13 Classically, Virchow’s node, the left supraclavicular lymph node, is involved with metastatic gastric cancer. Involvement of the left axillary lymph node (Irish node) or a periumbilical nodule (Sister Mary Joseph node) may also be observed. Other, less commonly noted sites of metastatic disease include the ovaries, central nervous system, bone, lung, and soft tissues.13
Upper GI endoscopy is the best method for determining tumor location and extent and obtaining a specimen for a definitive tissue diagnosis.14 It is essential to accurately identify the location of the tumor in the stomach and relative to the GEJ. The American Joint Committee on Cancer classification defines tumors involving the GEJ with an epicenter no more than 2 cm into the proximal stomach as esophageal cancers.15 Tumors of the GEJ with their epicenter more than 2 cm into the proximal stomach are defined as gastric cancers. If metastatic disease is suspected, computed tomography (CT) scan of the chest, abdomen, and pelvis with oral and intravenous contrast can be obtained to determine the extent of disease spread. In the absence of any metastatic disease, endoscopic ultrasound (EUS) should be conducted to determine the depth of tumor invasion (T staging) and lymph node status. In the era of targeted therapy, patients with metastatic disease should undergo testing for human epidermal growth factor-2 (HER-2) expression, microsatellite instability (MSI), and programmed death ligand 1 (PD-L1) expression. Patients should be staged according to the TNM staging system.
FIRST-LINE TREATMENT OPTIONS
CASE CONTINUED
The patient undergoes esophagoduodenoscopy (EGD) and is found to have a gastric cardia mass extending into the distal esophagus. EUS also demonstrates multiple abdominal and mediastinal lymph nodes. No gastric outlet obstruction is found. Biopsy shows poorly differentiated invasive adenocarcinoma. Warthin–Starry stain is negative for H. pylori organism. The tumor cells are positive for cytokeratin (CK7), CK19, and mucin-1 gene (MUC1); focally positive for CK20; and negative for MUC2. HER2 testing results are reported as immunohistochemistry (IHC) 3+, consistent with strongly positive HER2 protein expression. Further IHC testing for mismatch repair (MMR) proteins shows intact nuclear expression of MLH1, MSH2, MSH6, and PMS2 protein, consistent with a low probability of MSI-high tumor. The tumor is found to be PD-L1 positive. Imaging reveals abnormal mass-like nodular thickening of the gastric wall, with an infiltrative opacity within the pancreatico-duodenal groove, suspicious for tumor infiltration. Multiple metastatic deposits are noted in the liver, peritoneum, and bilateral lungs. There is extensive gastrohepatic ligament and periportal lymphadenopathy and mild enlargement of the pulmonary hilar lymph nodes. These findings are consistent with stage 4 (T4bN3aM1) gastric cancer. Given these findings, staging laparoscopy is deferred.
• What are the first-line treatment options for patients with metastatic gastric cancer?
Patients with metastatic gastric cancer have a poor prognosis, and management is stratified based on performance status (Figure). In patients with good performance status, systemic chemotherapy is the mainstay of treatment. The goal of therapy is not curative, but rather treatment focuses on palliation of symptoms arising from tumor spread. Given this treatment goal, there has been considerable interest in clarifying the utility of chemotherapy as opposed to best supportive care. In a recent Cochrane review of 64 randomized control trials involving 11,698 patients, chemotherapy was found to improve OS by 6.7 months as compared to best supportive care (hazard ratio [HR] 0.3 [95% confidence interval {CI} 0.24 to 0.55]).16 Five classes of cytotoxic chemotherapeutic agents have demonstrated activity in gastric cancer. These include fluoropyrimidine (either infusional fluorouracil or capecitabine), platinum agents (cisplatin or oxaliplatin), taxanes (docetaxel or paclitaxel), anthracyclines (epirubicin), and irinotecan.13 Treatment options are further divided based on whether the patient has HER2-overexpressing or non-expressing malignancy.
HER2-NEGATIVE DISEASE
For patients with HER2-negative disease, National Comprehensive Cancer Network (NCCN) guidelines recommend using 2-drug combination regimens rather than 3 drugs, given concern for increased toxicity with 3-drug regimens.17 For patients with a performance status of 0 to 1, utilization of a 3-drug regimen is a reasonable alternative. The combination of a fluoropyrimidine with a platinum agent is considered the standard of care, with regimens such as fluorouracil, leucovorin, and oxaliplatin (FOLFOX) being commonly used.
Epirubicin-containing regimens have also been extensively studied in advanced gastric cancer. In a study of 274 previously untreated patients with GEJ cancers, the combination of epirubicin, cisplatin, and fluorouracil (ECF) was compared to fluorouracil, doxorubicin, and methotrexate (FAMTX). There was an OS benefit favoring ECF (8.9 months versus 5.7 months) at 1 year (95% CI 27% to 45%, P = 0.0009). The ECF regimen was associated with an increased risk of nausea, emesis, and alopecia, while more hematologic toxicity and infections were noted with the FAMTX regimen.18 In addition, in a phase 3 trial, Van Cutsem and colleagues examined the role of docetaxel in combination with cisplatin and fluorouracil (DCF) compared to cisplatin and fluorouracil alone. Addition of docetaxel led to improved OS and time to progression (9.2 months versus 8.6 months for cisplatin and fluorouracil alone, P = 0.02) but with an increased risk of grade 3 and 4 toxicities (69% versus 59%). These adverse events included neutropenia (82% versus 57% of cisplatin and fluorouracil patients), diarrhea (19% versus 8%), stomatitis (21% versus 27%), and fatigue (19% versus 14%).19
The landmark phase 3 REAL-2 study compared 4 chemotherapy regimens in patients with untreated advanced esophagogastric cancer. This study was conducted to determine if the efficacy of cisplatin and oxaliplatin, a third-generation platinum agent, is equivalent to that of fluorouracil and capecitabine, an oral fluoropyrimidine. In this trial, a 2 × 2 design was used to compare 4 regimens: ECF versus epirubicin, cisplatin, and capecitabine (ECX) versus epirubicin, oxaliplatin, and fluorouracil (EOF) versus epirubicin, oxaliplatin, and capecitabine (EOX). The study found EOX to be noninferior to ECF, with a trend towards improved OS compared to other combination regimens (11.2 months versus 9.9 months, HR 0.80 [95% CI 0.66 to 0.97], P = 0.02).20 Thus, the study demonstrated that an oxaliplatin and capecitabine-based regimen could replace cisplatin and fluorouracil. Given that fluorouracil administration requires long continuous infusions, the oral-based capecitabine regimen is an attractive option for patients.
Several trials have demonstrated the equivalency of oxaliplatin with cisplatin in combination regimens for the treatment of advanced gastric cancer. Oxaliplatin has the benefit of an improved toxicity profile as compared to cisplatin, with the major dose-limiting toxicity being peripheral neuropathy
Given previous evidence that DCF (docetaxel, cisplatin, fluorouracil) is superior to cisplatin and fluorouracil alone, there was interest in determining if the addition of docetaxel to a backbone of fluorouracil, oxaliplatin, and leucovorin (FLO) could elicit a higher response rate. This concept was investigated in a phase 2 trial that assigned 54 patients with metastatic gastric or GEJ adenocarcinoma to receive biweekly infusions of oxaliplatin, leucovorin, fluorouracil, and docetaxel.21 Median time to response was 1.54 months, and the overall response rate was 57.7%. Median progression-free survival (PFS) was 5.2 months, and OS was 11.1 months. The most common grade 3 or 4 toxicities included neutropenia (48%), leukopenia (27.8%), diarrhea (14.8%), and fatigue (11.1%).
Irinotecan-based regimens have also been extensively studied in the first-line treatment of metastatic gastric cancer, particularly as an alternative to platinum-based therapy, but superiority has not been established. The combination of fluorouracil, leucovorin, and irinotecan (FOLFIRI) was compared to ECX in a phase 3 trial.22 The study enrolled 416 patients with locally advanced or metastatic gastric or GEJ cancer. At a median follow up of 31 months, the time to progression was longer in the FOLFIRI arm as compared to the ECX arm (5.1 months versus 4.2 months, P = 0.008), but there was no difference in OS (9.5 months versus 9.7 months, P = 0.95), median PFS (5.3 months versus 5.8 months, P = 0.96), or response rate (39.2% versus 37.8%). However, the FOLFIRI regimen had an improved toxicity profile, with a lower overall rate of grade 3 or 4 toxicity (69% versus 84%, P < 0.001). Given these findings, the FOLFIRI regimen is an acceptable alternative to platinum-based therapy in suitable patients.22
HER2-POSITIVE DISEASE
The HER2 proto-oncogene, initially described in breast cancer, has been implicated in several malignancies, including gastric and esophageal cancer. Overexpression or amplification of HER2 can be found in up to 30% of gastric cancers.23 For these patients, adding trastuzumab to a standard regimen of platinum and fluoropyrimidine is the standard of care. The prospective phase 3 Trastuzumab for Gastric Cancer (ToGA) trial randomly assigned 594 patients with HER2-positive gastric cancer to receive either cisplatin and fluorouracil or capecitabine and cisplatin with trastuzumab (n = 294) or without (n = 290) trastuzumab every 3 weeks for a total of 6 cycles, followed by maintenance trastuzumab until disease progression was noted.24 HER2 positivity was defined as HER2 protein overexpression by IHC (cutoff of 3+) or gene amplification by fluorescence in situ hybridization (FISH); tumors with IHC 2+ patterns were followed with FISH studies to confirm positivity. The study found a higher incidence of HER2-positive tumors in patients with GEJ tumors compared to patients with distal gastric cancers (33% versus 20%).24 In this trial, the addition of trastuzumab was associated with an improvement in OS: 13.5 months in the trastuzumab cohort versus 11.1 months in those receiving chemotherapy alone (HR 0.74, P = 0.0048). There was not a significant difference in toxicities between the 2 cohorts, with nausea, emesis, and neutropenia being the most common adverse events. Rates of overall grade 3 or 4 events were similar as well (68% in each cohort). Further exploratory analysis was also conducted according to HER2 status by dividing patients into a “high-expressor” group (n = 446), defined as patients with IHC 3+ tumors or IHC 2+ and FISH positivity, and a “low-expressor” group (n = 131), which included patients with IHC 0 or 1+ tumors. Analysis of patients in the 2 subgroups demonstrated an improved OS with the addition of trastuzumab for the high-expressor cohort, with a median OS of 16 months (HR 0.65 [95% CI 0.51 to 0.83]) compared to 11.8 months in those receiving only chemotherapy.
Dual HER2 blockade has been investigated in metastatic gastric cancer. The phase 3 randomized JACOB trial assigned 780 patients to receive either trastuzumab with a cisplatin/fluoropyrimidine regimen with or without the addition of pertuzumab; the primary end point was OS.25 A non-statistically significant trend towards improvement in OS was found in the pertuzumab arm (17.5 months) as compared with the standard of care arm (14.2 months, HR 0.84, P = 0.0565). The pertuzumab/trastuzumab/chemotherapy cohort experienced a higher incidence of diarrhea (61.6% versus 35.1% in control arm). Cardiac toxicity was comparable in the 2 cohorts.
The Table provides a summary of relevant clinical trials in metastatic gastric cancer.
SECOND-LINE THERAPY
CASE CONTINUED
The patient receives capecitabine, oxaliplatin, and trastuzumab therapy for 6 cycles, followed by trastuzumab for another 3 cycles. While on therapy, he develops a painful right clavicular lesion. He undergoes magnetic resonance imaging of the right clavicle, which shows a lesion in the distal two-thirds of the right clavicle measuring 9.7 × 3.7 × 3.8 cm. The patient is started on palliative radiation to the clavicle. However, repeat CT imaging shows progressive liver metastases.
• What is the approach to second-line therapy for metastatic gastric cancer?
Improvements in our understanding of the molecular pathways that lead to tumorigenesis have contributed to the development of several targeted agents whose efficacy in gastric cancer is being investigated. The NCCN guidelines recommend that for all patients who progress on frontline therapy, second-line therapy consists of a combination of ramucirumab and paclitaxel. Other options include single-agent docetaxel, paclitaxel, irinotecan, or ramucirumab. Combination therapy using irinotecan with either docetaxel, fluorouracil, or cisplatin may also be used.
Ramucirumab, a human IgG1 monoclonal antibody that targets the vascular endothelial growth factor receptor 2 (VEGFR2), was initially approved in 2014 as monotherapy for patients who had previously progressed on first-line chemotherapy. Its approval was based on the results of the phase 3 randomized, double-blind placebo-controlled REGARD study.26 The trial randomly assigned 355 patients with advanced gastric or GEJ adenocarcinoma and disease progression after first-line platinum-containing or fluoropyrimidine-containing chemotherapy to receive best supportive care plus either ramucirumab (n = 238) or placebo (n = 117). Monotherapy with ramucirumab significantly improved median OS compared with placebo (5.2 months versus. 3.8 months; HR 0.776 [95% CI 0.6 to 0.99], P = 0.047). There was also an improvement in PFS of 2.1 months in the ramucirumab cohort, as compared to 1.3 months in the placebo cohort (P < 0.0001). Patients in the ramucirumab arm experienced a higher incidence of hypertension (16% versus 8%), but all other adverse events occurred at comparable rates. Five deaths in the ramucirumab group were thought to be secondary to the study drug, as compared to 2 deaths in the placebo group.
In the subsequent phase 3 RAINBOW trial, the addition of ramucirumab to paclitaxel was investigated, with 330 patients assigned to the combination group and 335 to the paclitaxel-only group.27 The trial again showed that combination therapy afforded patients a significant survival advantage compared to paclitaxel alone, with a median OS of 9.6 months versus 7.4 months for the monotherapy group (HR 0.807 [95% CI 0.678 to 0.962], P = 0.017). A PFS benefit of 4.4 months was observed in the combination therapy groups, as compared with 2.9 months in the monotherapy group (HR 0.635, P < 0.0001). The ramucirumab/paclitaxel group also had a higher overall response rate of 28% versus 16%. The combination cohort had an increased incidence of grade 3 or higher adverse hypertensive events (14% versus 2%) and neutropenia (41% versus 19%), while the incidence of grade 3 febrile neutropenic events was similar between the groups (3% versus 2%).
The addition of bevacizumab, another monoclonal antibody against VEGF, to standard chemotherapy regimens has been explored, but studies have failed to show a survival benefit with this agent in the first-line treatment of advanced gastric cancer. The phase 3 Avastin in Gastric Cancer (AVAGAST) trial was a multinational, randomized study where patients received either bevacizumab (n = 387) or placebo (n = 387) in addition to cisplatin and capecitabine.28 The substitution of fluorouracil for capecitabine was permitted for patients who were unable to tolerate oral medications. Cisplatin was administered for a maximum of 6 cycles, while capecitabine and bevacizumab were administered until disease progression. The study failed to show an improvement in OS, with a median OS of 12.1 months noted in the bevacizumab cohort, as compared to 10.1 months in the placebo arm (HR 0.87 [95% CI 0.73 to 1.03], P = 0.1002). However, there was a modest improvement in median PFS (6.7 months versus 5.3 months; HR 0.80 [95% CI 0.68 to 0.93], P = 0.0037) and overall response rate (46% versus 37.4%, P = 0.0315). The most commonly reported grade 3 to 5 adverse events included neutropenia (35%), anemia (10%), and loss of appetite (8%). Interestingly, in a follow-up report, higher serum levels of VEGF-A were thought to correlate with an enhanced response to bevacizumab.29 However, the routine use of biomarker analysis in selecting patients for treatment with bevacizumab in metastatic gastric cancer remains to be further clarified.
Use of other agents with anti-HER2 activity in the second-line treatment of patients who have experienced progression while on trastuzumab remains unclear. In the recent T-ACT trial, patients with disease refractory to frontline therapy with combination trastuzumab and fluoropyrimidine/platinum agents were randomly assigned to receive either weekly paclitaxel (n = 45) or weekly paclitaxel plus trastuzumab (n = 44).30 Patients in the combination cohort received an initial dose of trastuzumab 8 mg/kg followed by 6 mg/kg every 3 weeks until progression. The study did not find a difference in either PFS (3.19 months versus 3.68 months; HR 0.91 [95% CI 0.67 to 1.22], P = 0.33) or OS (9.95 months versus 10.2 months; HR 1.23 [95% CI 0.75 to 1.99], P = 0.20). The study thus failed to show a benefit to continuing trastuzumab after progression in the first-line setting.
Lapatinib in combination with paclitaxel has been compared to paclitaxel alone for the treatment of advanced HER2-positive gastric cancer in an Asian population in the phase 3 TyTAN trial.31 With a primary end point of OS, the study randomly assigned 129 patients to receive paclitaxel alone and 132 patients to receive paclitaxel with lapatinib. There was a nonsignificant trend towards improvement in OS in the combination group (11 months) as compared to the paclitaxel-only group(8.9 months, P = 0.1044), with no significant difference in median PFS (5.4 months versus 4.4 months). However, it is important to note that only 15 patients in this trial had previously been exposed to trastuzumab. Another trial, the phase 3 GATSBY study, examined the efficacy of trastuzumab emtansine in the second-line setting compared to taxanes alone and failed to show any improvement in PFS or OS.32 Given these results, no alternative anti-HER2 therapy has been proven to be efficacious for patients who are trastuzumab refractory. Therefore, including anti-HER2 therapy in the second-line treatment of HER2-positive gastric cancer is not recommended.
IMMUNOTHERAPY AND OTHER TARGETED THERAPIES
Several other targeted therapies have been studied in advanced gastric cancer, without any demonstrable survival benefit. The PI3K/AKT/mTOR pathway is known to be involved in regulation of cell growth and angiogenesis, and the mTOR inhibitor everolimus is widely used to treat other malignancies, including breast cancer. The use of everolimus in the second-line setting was studied in the phase 3 GRANITE-1 trial, where it was compared to best supportive care and failed to provide any survival benefit.33 Cetuximab, a recombinant human and mouse chimeric monoclonal antibody, and panitumumab, a recombinant human antibody against the epidermal growth factor receptor (EGFR), have also been examined in gastric and GEJ cancer patients. However, the large phase 3 EXPAND and REAL-3 trials did not show a survival benefit when these agents were added to standard chemotherapy.34,35
Overexpression of MET, a proto-oncogene and tyrosine kinase receptor, has also been implicated in gastric cancer progression. The ligand for MET is the hepatocyte growth factor (HGF), and aberrant signaling of this pathway has been shown to correlate with an aggressive gastric cancer phenotype and poorer OS by promoting tumor growth and angiogenesis. However, no MET inhibitors thus far have been found to be clinically effective. RILOMET-1 and RILOMET-2 were phase 3 trials examining the efficacy of rilotumumab, a humanized anti-HGF antibody, in combination with chemotherapy (ECX and cisplatin with capecitabine, respectively) for the frontline treatment of MET-positive GEJ and gastric cancers. Both studies were discontinued due to a higher treatment-related mortality in patients receiving rilotumumab, with a higher incidence of adverse events due to disease progression being noted.36 Similarly, onartuzumab, a monovalent monoclonal antibody against the MET receptor, was investigated in the phase 3 METGastric trial in combination with modified FOLFOX6 as first-line therapy for HER2-negative, MET-positive metastatic GEJ and gastric cancers. The study did not demonstrate any significant improvements in OS or PFS.37
There has been significant interest in incorporating immunotherapy in the treatment of early and metastatic gastric cancer. Pembrolizumab is the first programmed death receptor (PD-1) inhibitor to be approved for treatment of patients with PD-L1−positive advanced gastric cancer who had previously received 2 or more lines of chemotherapy. Although earlier studies of pembrolizumab in lung cancer utilized the tumor proportion score (TPS) to determine PD-L1 positivity, this was not found to be applicable to gastric cancer. Instead, the combined positive score (CPS) is used in gastric cancer. The CPS evaluates the number of tumor cells and immune cells (macrophages and lymphocytes) that stain positive for PD-L1 relative to all viable tumor cells. Comparatively, the TPS only examines the percentage of viable tumor cells that show complete or partial positive staining for PD-L1. A CPS score of 1 or greater identifies patients who would be suitable candidates for pembrolizumab.
The approval of pembrolizumab was based on the positive findings from the recent KEYNOTE-059 trial.38 The study included 259 patients who had previously received either fluoropyrimidine, cisplatin, or anti-HER2 therapy, with 148 patients (55%) of these patients having PD-L1−positive tumors. The PD-L1 status was determined using a pharmDx Kit, which is now approved by the US Food and Drug Administration to select patients who could benefit from pembrolizumab treatment. CPS was calculated as the number of PD-L1−staining cells divided by the total number of evaluated cells. The study included patients with microsatellite stable (MSI-S), undetermined, or deficient MMR status. The overall response rate to pembrolizumab across all patients was 11.6%, median PFS was 2 months, and the 12-month OS rate was 23.4%. In the subset of patients with MSI-H tumors, the overall response rate was 57.1%, with a complete response rate of 14.3%; in those with MSI-S tumors, the overall response rate was 9% and the complete response rate was 2.4%. Among patients with PD-L1–positive tumors, the overall response rate was 15.5% (95% CI 10.1% to 22.4). Common adverse events included fatigue, hypothyroidism, nausea, diarrhea, and arthralgia.38
CASE CONCLUSION
This patient with metastatic gastric cancer receives second-line chemotherapy with ramucirumab and paclitaxel. Follow-up imaging shows persistent liver metastases and new lung metastasis. Because the tumor is PD-L1–positive, the patient receives 4 cycles of pembrolizumab, with no significant change noted in disease burden. He notes a significant decline in functional status with increased weight loss, nausea, emesis, and fatigue. The patient opts to forego any further therapy and instead chooses to pursue supportive care only.
SUMMARY
Gastric cancer is the third most common cause of cancer death worldwide. Common risk factors for developing gastric cancer include H. pylori infection, smoking, alcohol abuse, radiation exposure, high-fat diet, and obesity. Patients presenting with alarm symptoms of nausea, emesis, early satiety, abdominal pain, or weight loss should be fully evaluated with upper GI endoscopy. If there is suspicion for metastatic disease, CT evaluation of the chest, abdomen, and pelvis with oral and intravenous contrast should be obtained. Treatment of patients with metastatic gastric cancer is guided by their performance status at presentation. For patients with good performance status, a combination of platinum and fluoropyrimidine therapy, such as FOLFOX, can be considered. Doublet chemotherapy regimens are preferred over triplet chemotherapy regimens given their better tolerability. For patients with HER2-positive disease, the addition of trastuzumab to the platinum and fluoropyrimidine backbone is the standard of care in the first line.
Several targeted agents have been studied in patients progressing on initial therapy, with ramucirumab and paclitaxel being considered the regimen of choice in the second line. No anti-HER2 therapy has been approved for patients who are refractory to trastuzumab. Pembrolizumab is approved for use in patients who are PD-L1–positive and have previously progressed on at least 2 lines of chemotherapy. Pembrolizumab is also approved for the treatment of patients with unresectable or metastatic, MSI-H or MMR-deficient gastric cancers that have progressed after prior treatment and who have no satisfactory alternative treatment options.
1. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–41
2. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524–8.
3. Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 2018;10:239–48.
4. Olefson S, Moss SF. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer 2015;18:23–32.
5. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 2014;345:196–202.
6. Espinoza JL, Matsumoto A, Tanaka H, Matsumura I. Gastric microbiota: An emerging player in Helicobacter pylori-induced gastric malignancies. Cancer Lett 2018;414:147–52.
7. Chmiela M, Gonciarz W. Molecular mimicry in Helicobacter pylori infections. World J Gastroenterol 2017;23:3964–77.
8. Isobe Y, Nashimoto A, Akazawa K, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer 2011;14:301–16.
9. Ford A, Gurusamy KS, Delaney B, et al. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive patients. Cochrane Database Syst Rev 2004(4):CD003840.
10. Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–13.
11. Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016;388:2654–64.
12. Chan BA , Sim HW, Natori A, et al. Survival outcomes for de novo versus relapsed stage IV gastric and gastroesophageal junction (GEJ) adenocarcinoma [abstract]. J Clin Oncol 2018;36(no. 4 suppl):148.
13. DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology. 9th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011.
14. Siewert JR, Hölscher AH, Becker K, Gössner W. [Cardia cancer: attempt at a therapeutically relevant classification]. [Article in German.] Chirurg 1987;58:25–32.
15. Amin MB, Edge SB, Greene FL, et al, eds. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
16. Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2017;8:CD004064.
17. Qiu H, Zhou Z. [Updates and interpretation on NCCN clinical practice guidelines for gastric cancer 2017 version 5]. [Article in Chinese.] Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:160–4.
18. Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997;15:261–7.
19. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991–7.
20. Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010;362:858–9.
21. Al-Batran SE, Hartmann JT, Hofheinz R, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2008;19:1882–7.
22. Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Federation Francophone de Cancerologie Digestive, Federation Nationale des Centres de Lutte Contre le Cancer, and Groupe Cooperateur Multidisciplinaire en Oncologie) study. J Clin Oncol 2014;32:3520–6.
23. Boku N. HER2-positive gastric cancer. Gastric Cancer 2014;17:1–12.
24. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97.
25. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab + trastuzumab + chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer: Final analysis of a Phase III study (JACOB) [abstract]. Ann Oncol 2017;28(suppl 5):6160.
26. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–9.
27. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35.
28. Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968–76.
29. Van Cutsem E, de Haas S, Kang YK, et al, Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119–27.
30. Makiyama A, Sagara K, Kawada J, et al. A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT) [abstract]. J Clin Oncol 2018;36(no. 15 suppl):4011.
31. Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039–49.
32. Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017;18:640–53.
33. Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013;31:3935–43.
34. Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490–9.
35. Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481–9.
36. Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1467–82.
37. Shah MA, Bang YJ, Lordick F, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol 2017;3:620–7.
38. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4(5):e180013.
1. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–41
2. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524–8.
3. Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 2018;10:239–48.
4. Olefson S, Moss SF. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer 2015;18:23–32.
5. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 2014;345:196–202.
6. Espinoza JL, Matsumoto A, Tanaka H, Matsumura I. Gastric microbiota: An emerging player in Helicobacter pylori-induced gastric malignancies. Cancer Lett 2018;414:147–52.
7. Chmiela M, Gonciarz W. Molecular mimicry in Helicobacter pylori infections. World J Gastroenterol 2017;23:3964–77.
8. Isobe Y, Nashimoto A, Akazawa K, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer 2011;14:301–16.
9. Ford A, Gurusamy KS, Delaney B, et al. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive patients. Cochrane Database Syst Rev 2004(4):CD003840.
10. Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–13.
11. Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet 2016;388:2654–64.
12. Chan BA , Sim HW, Natori A, et al. Survival outcomes for de novo versus relapsed stage IV gastric and gastroesophageal junction (GEJ) adenocarcinoma [abstract]. J Clin Oncol 2018;36(no. 4 suppl):148.
13. DeVita VT, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology. 9th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011.
14. Siewert JR, Hölscher AH, Becker K, Gössner W. [Cardia cancer: attempt at a therapeutically relevant classification]. [Article in German.] Chirurg 1987;58:25–32.
15. Amin MB, Edge SB, Greene FL, et al, eds. AJCC cancer staging manual. 8th ed. New York: Springer; 2017.
16. Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2017;8:CD004064.
17. Qiu H, Zhou Z. [Updates and interpretation on NCCN clinical practice guidelines for gastric cancer 2017 version 5]. [Article in Chinese.] Zhonghua Wei Chang Wai Ke Za Zhi 2018;21:160–4.
18. Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997;15:261–7.
19. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991–7.
20. Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010;362:858–9.
21. Al-Batran SE, Hartmann JT, Hofheinz R, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2008;19:1882–7.
22. Guimbaud R, Louvet C, Ries P, et al. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Federation Francophone de Cancerologie Digestive, Federation Nationale des Centres de Lutte Contre le Cancer, and Groupe Cooperateur Multidisciplinaire en Oncologie) study. J Clin Oncol 2014;32:3520–6.
23. Boku N. HER2-positive gastric cancer. Gastric Cancer 2014;17:1–12.
24. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97.
25. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab + trastuzumab + chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer: Final analysis of a Phase III study (JACOB) [abstract]. Ann Oncol 2017;28(suppl 5):6160.
26. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–9.
27. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35.
28. Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968–76.
29. Van Cutsem E, de Haas S, Kang YK, et al, Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119–27.
30. Makiyama A, Sagara K, Kawada J, et al. A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT) [abstract]. J Clin Oncol 2018;36(no. 15 suppl):4011.
31. Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039–49.
32. Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017;18:640–53.
33. Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013;31:3935–43.
34. Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490–9.
35. Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481–9.
36. Catenacci DVT, Tebbutt NC, Davidenko I, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1467–82.
37. Shah MA, Bang YJ, Lordick F, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol 2017;3:620–7.
38. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4(5):e180013.